- 110th Department - Oncology, “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 2Clinical Municipal Hospital Cluj-Napoca, Cluj-Napoca, Romania
- 3Department of Pharmacy and Biotechnology (FABIT), University of Bologna, Bologna, Italy
- 4Clinical Pharmacology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 5Institute of Oncology “Prof. Dr. Ion Chiricuţă”, Cluj-Napoca, Romania
- 6Solid Tumor Molecular Pathology Laboratory, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 7Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy
- 8Faculty of Medicine, University of Medicine and Pharmacy “Iuliu Haţieganu”, Cluj-Napoca, Romania
- 9Department of Morpho-functional Sciences, University of Medicine and Pharmacy “Iuliu Haţieganu”, Cluj-Napoca, Romania
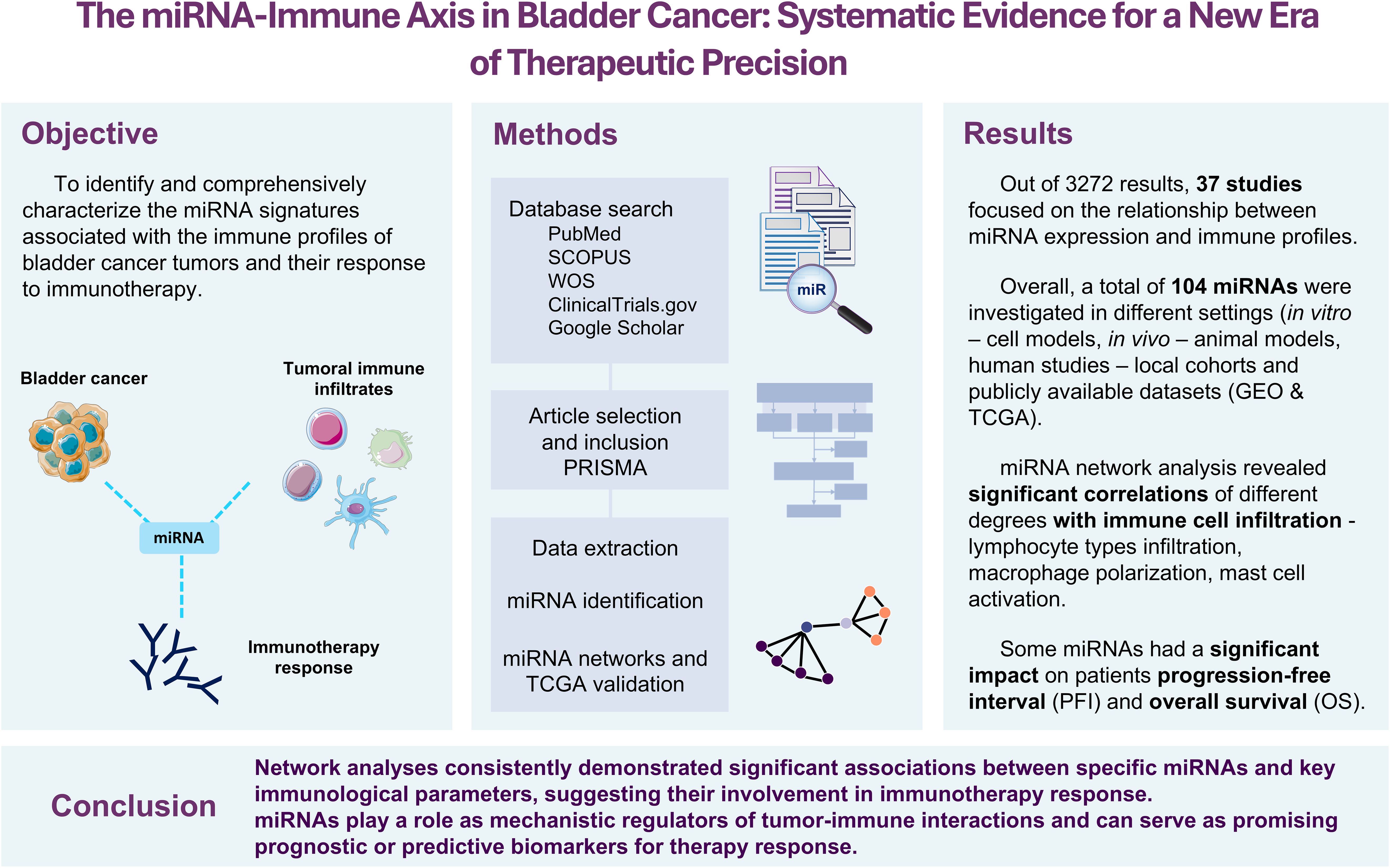
Introduction: Bladder cancer (BC) is a complex disease with patients showing widely variable responses to treatment. While immunotherapy has recently emerged as a promising alternative to the standard platinum-based chemotherapy, especially for platinum-resistant tumors, clinicians still lack reliable biomarkers to predict which patients will truly benefit from immunotherapy.
Aim: This systematic review aimed to explore whether miRNAs could help decode the immune landscape of BC and serve as predictive biomarkers for immunotherapy response.
Methods: A total of 3,272 articles were systematically screened on medical databases and narrowed down to 37 studies that examined the relationship between miRNAs, immune cell infiltration, and patient outcomes in BC. To further strengthen and validate our findings, we analyzed large-scale genomic data from The Cancer Genome Atlas (TCGA-BLCA).
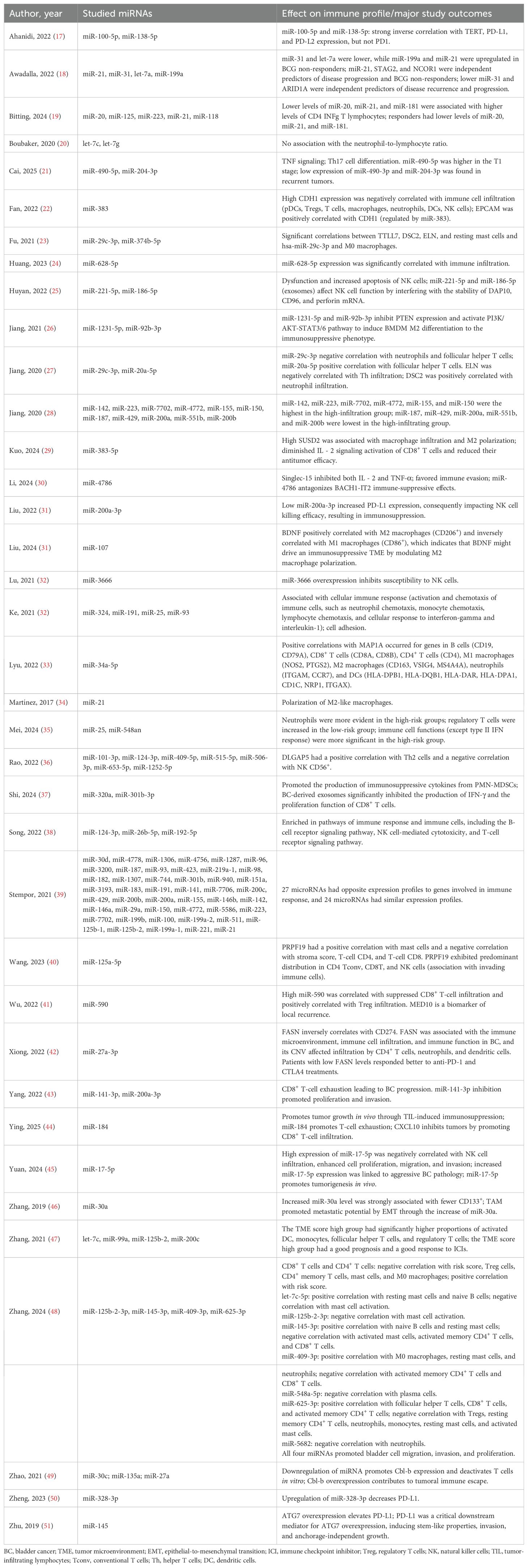
Results: A total of 104 different miRNAs appeared to shape the BC immune microenvironment. Some studies also linked miRNA expression with clinical outcomes such as BCG therapy response and prognosis, while others dissected the molecular pathways. Further analyses established miR-155, miR-142, and miR-146b as key factors for CD4+ memory T-cell and M1 macrophage infiltration. Notably, 49 miRNAs showed stage-specific expression differences in TCGA data, with 25 of them also significantly associated with progression-free interval or overall survival.
Conclusion: miRNAs are emerging as powerful regulators of the immune microenvironment of BC. However, despite growing evidence, to date, no studies have directly explored miRNA profiles in driving immunotherapeutic decisions. Our findings highlight the need for prospective studies to translate these molecular insights into personalized treatment strategies.
Introduction
Bladder cancer remains one of the most prevalent malignancies of the urinary tract, with urothelial carcinoma accounting for over 90% of cases (1). Approximately 25% of patients are diagnosed with muscle-invasive bladder cancer (MIBC), a form associated with a worse prognosis (2). For these patients, the current standard treatment is represented by cisplatin-based neoadjuvant chemotherapy followed by cystectomy (2). Unfortunately, many patients experience disease progression or do not tolerate the treatment due to limited renal function, underscoring the urgent need for more effective therapeutic strategies (3). Recently, immunotherapy, such as immune checkpoint inhibitors (ICIs), has emerged as an alternative treatment for bladder cancer (in both neoadjuvant and adjuvant settings), with clinical trials evaluating the efficacy either alone or in combination with chemotherapy (4, 5). Despite the growing body of literature on this topic, no validated biomarkers are currently available in the clinical setting to identify bladder cancer patients who are most likely to benefit from immunotherapy, and a substantial proportion of patients exhibit limited or no durable response (4, 5).
MicroRNAs (miRNAs), a class of small non-coding RNAs that regulate gene expression at the post-transcriptional level, are well known for their modulating role in cancer biology (6). In bladder cancer, miRNAs have been implicated in key oncogenic processes, including proliferation, invasion, and epithelial-to-mesenchymal transition (7). Moreover, an increasing body of research is showing that miRNAs also play a role in regulating components of the tumoral microenvironment (TME), such as immune cell infiltration, expression of immune checkpoints, regulation of PD - 1/PD-L1 and CTLA - 4, and antigen presentation (2). These regulatory functions make miRNAs potential candidates as biomarkers for immune responsiveness as well as therapeutic agents.
The interplay between miRNAs and tumor immunity in bladder cancer remains largely incompletely understood. To date, a comprehensive characterization of miRNA-mediated immune regulation in the process of resistance to ICIs in bladder cancer is lacking.
In this study, we conducted a systematic review of available literature investigating the role of miRNAs in modulating the immune microenvironment within bladder cancer, as well as its response to immunotherapy, with the aim of synthesizing the current state-of-the-art knowledge on this topic. Furthermore, we comprehensively analyzed the relationship between the identified miRNAs, bladder cancer TME, and clinical profiles, to further strengthen the available level of evidence as well as to unravel novel, yet unexplored relationships that may lay the groundwork for precision immuno-oncology guided by miRNAs.
Materials and methods
Database search and study identification
A systematic literature search was performed to identify relevant studies investigating the relationship between bladder cancer, tumoral immune infiltration, immunotherapy, and miRNA. The search strategy employed a combination of targeted keywords, including “bladder cancer,” “urothelial carcinoma,” “immunotherapy,” “checkpoint inhibitor,” “immune response,” “immune evasion,” “immune infiltration,” “miR,” “microRNA,” “miRNA,” and “non-coding RNA.”
The databases used for the identification of relevant studies were PubMed, SCOPUS, Web of Science, ClinicalTrials.gov, and Google Scholar. Literature retrieval from Google Scholar was done via the Publish or Perish software (8), which facilitates structured bibliographic data extraction. The last updated search was conducted in March 2025.
Study screening and inclusion criteria
The inclusion criteria were peer-reviewed original research articles published in English that addressed the role of miRNAs in the context of immunotherapy and/or immune mechanisms in bladder cancer. We considered studies including patients and animal models, as well as those conducted on cell lines. Case reports, editorials, and book chapters were excluded. The results from all databases were imported into Rayyan (9). Duplicates were removed, and titles and abstracts were screened manually by three authors for relevance. Full texts of eligible studies were downloaded and read in detail. Disagreements were resolved by discussion with a fourth researcher. Relevant data from each study were extracted and summarized in tables.
TCGA data retrieval and analysis
Expression data (mRNA and miRNA) and clinical data for muscle-invasive urothelial carcinoma (pT2 or above) were obtained from The Cancer Genome Atlas (TCGA-BLCA) project using the FirebrowseR R package, via the Broad Institute’s Firehose pipeline. Only primary tumor samples with complete expression and clinical annotation were included in the subsequent analyses (10). Corresponding expression and clinical annotation files were integrated based on TCGA patient barcodes. Clinical data for survival analysis were obtained from the TCGA Pan-Cancer Clinical Data Resource (TCGA-CDR) (11).
Tumoral immune cell populations and miRNA expression analysis
Immune cell infiltration estimates were obtained from the Tumor Immune Estimation Resource (TIMER) (7), a computational framework that deconvolutes the composition of the tumor immune microenvironment using bulk RNA-sequencing data. TIMER provides robust quantification of six key immune cell populations: B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells. To ensure a comprehensive evaluation of immune infiltration, we utilized multiple established deconvolution algorithms that estimate immune cell abundance from the transcriptome data. The R packages igraph and ggraph were used for the construction of the networks between TME populations and miRNAs (12, 13).
Statistical analysis
All statistical analyses were conducted in R version 4.4.3 (2025 - 02–28 ucrt) - “Trophy Case” (14). For correlation analyses, the Spearman rank correlation test was used. To enhance the strength of the findings and reduce the likelihood of false-positive results due to weak associations between variables of interest, a minimum correlation coefficient threshold of 0.3 was set to ensure a moderate to strong association. This cutoff was used to prioritize miRNAs with a potentially higher influence on the TME. All p-values were adjusted for multiple comparisons using the Benjamini–Hochberg procedure to control the false discovery rate (15).
For clinical analyses, one-way ANOVA tests were performed to compare miRNA expression levels between patient groups. To evaluate the prognostic impact of miRNAs, patients were stratified into high- and low-expression groups based on the median expression level of each miRNA. Progression-free interval (PFI) and overall survival (OS) were assessed using Kaplan–Meier survival curves and compared with the log-rank tests. Cox proportional hazards regression models were used to estimate the degree and significance of the association between miRNA expression and patient outcomes. A p-value of 0.05 was set for statistical significance.
Results
A total of 3,272 records were identified through the initial search. After removing 574 duplicates, 2,780 records remained for title and abstract screening. Following this step, 161 articles were deemed potentially eligible and retrieved for full-text review. Of these, 37 studies met the inclusion criteria and were included in the final analysis (Figure 1). Overall, the studies investigated a total of 104 different miRNAs (Table 1) using diverse cohorts and model systems. Several studies investigated the association between the expression levels of specific miRNAs and clinical patient characteristics such as OS and response to immunotherapy. Other studies focused on elucidating the molecular mechanisms by which these miRNAs regulate the TME, providing insights into their immunoregulatory functions and potential therapeutic relevance (Table 1, Supplementary Table 1).

Figure 1. PRISMA chart showing the selection process of the articles included in this systematic review.
Of the 104 studies in the literature, 20 have been classified by the Cancer miRNA Census (16) as oncomiRNAs (miR-155, miR-182, miR-183, miR-20a, miR-21, miR-25, miR-93, miR-96, and miR-17) and tumor suppressor miRNAs (miR-100, miR-138, miR-145, miR-204, miR-99a, let-7c, miR-101, miR-125a, miR-29c, miR-30a, and miR-30c) (Supplementary Table 2). Furthermore, these miRNAs have been demonstrated to be involved not only in immune evasion but also in other cancer hallmarks such as the p53 pathway, angiogenesis, apoptosis, epithelial-to-mesenchymal transition (EMT), and various signaling pathways such as KRAS and PI3K-AKT-mTOR (Supplementary Table 2).
miRNA expression profiles associated with tumoral immune infiltration in the TCGA-bladder cancer cohort
We identified a robust set of positive correlations between miRNA expression and immune cell infiltration in bladder cancer, highlighting potential immunoregulatory roles for these miRNAs. Among the most significant results, miR-155 had the strongest associations, showing high positive correlations with CD4+ T cells (r = 0.568) and M1 macrophages (r = 0.537). Similarly, miR-142, miR-146b, and miR-511 were strongly associated with CD4+ memory-activated T cells (r = 0.483, r = 0.477, and r = 0.452, respectively). miR-146b and miR-142 also significantly correlated with M1 macrophage infiltration (r = 0.453 and r = 0.441, respectively) (Figure 2, Supplementary Table 3).
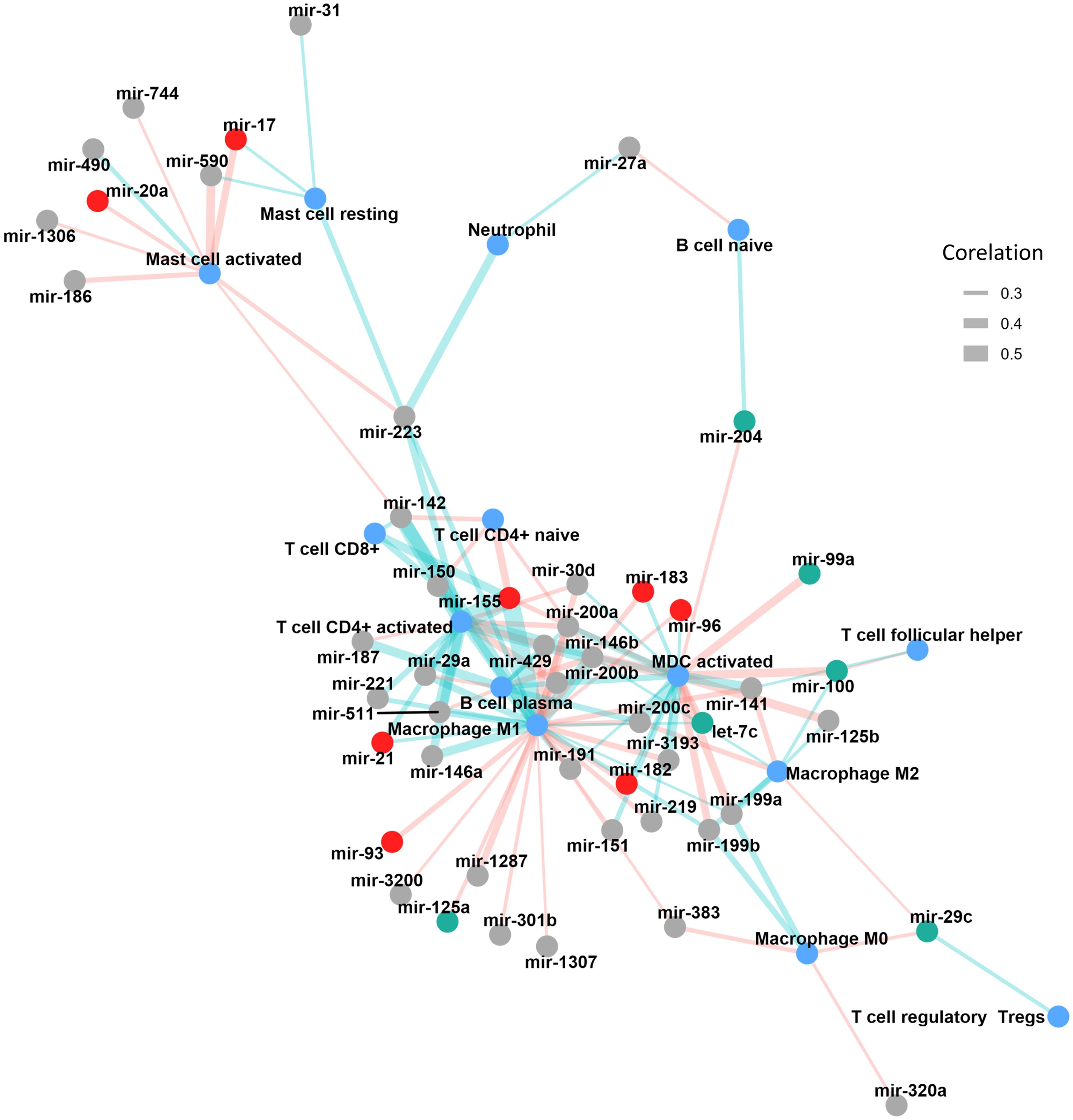
Figure 2. Correlation network illustrating the associations between miRNA expression and immune cell infiltration in TCGA-BLCA tumors. miRNAs are represented as nodes, with red indicating oncomiRs and green indicating tumor suppressor miRNAs. Immune cell populations are shown in blue. The thickness of the connecting edges reflects the absolute strength of the correlation, while the edge color denotes direction, light red for negative and light green for positive correlations. MDC, myeloid dendritic cells.
Additional miRNAs, including members of the miR-200 family (miR-200c, miR-200a, miR-200b, and miR-429), demonstrated moderate correlations with activated myeloid dendritic cells (r ranging from 0.338 to 0.42), suggesting a role in dendritic cell-mediated immune responses. Other miRNAs such as miR-223, miR-29a, miR-150, and miR-146a showed moderate associations with various immune subsets, including neutrophils, T cells, and macrophages (Figure 2, Supplementary Table 3).
In contrast, a substantial number of miRNAs demonstrated moderate to strong negative correlations with immune cell subsets. Namely, miR-146b, miR-125b, and miR-100 exhibited strong inverse correlations with activated myeloid dendritic cells (correlation coefficients ranging from −0.39 to −0.37), while miR-200b, miR-200a, and miR-429 were negatively associated with M1 macrophages and activated CD4+ memory T cells. miR-590 and miR-17 also showed consistent negative correlations with activated mast cells. Interestingly, several miRNAs such as miR-200c, miR-141, miR-155, and miR-142 were negatively correlated with multiple immune cell types, highlighting their possible involvement in broader immune regulatory networks within the TME. This multiple regulation was also seen when analyzing how many different miRNAs correlated with each immune population (Figure 2, Table 2). M1 macrophages were the most frequently targeted immune cells, with 10 miRNAs being positively correlated with their degree of infiltration in the tumors and 13 miRNAs showing a negative correlation. CD4+ T cells were also correlated with multiple miRNAs (10 positively and 4 negatively correlated) (Figure 2, Table 2).

Table 2. Number of miRNAs with a significant correlation (r ≥ |0.3|, adjusted p-value < 0.05) with tumoral immune infiltrates in the TCGA-BLCA cohort.
Impact of miRNA levels on the TCGA bladder cancer patients’ outcomes
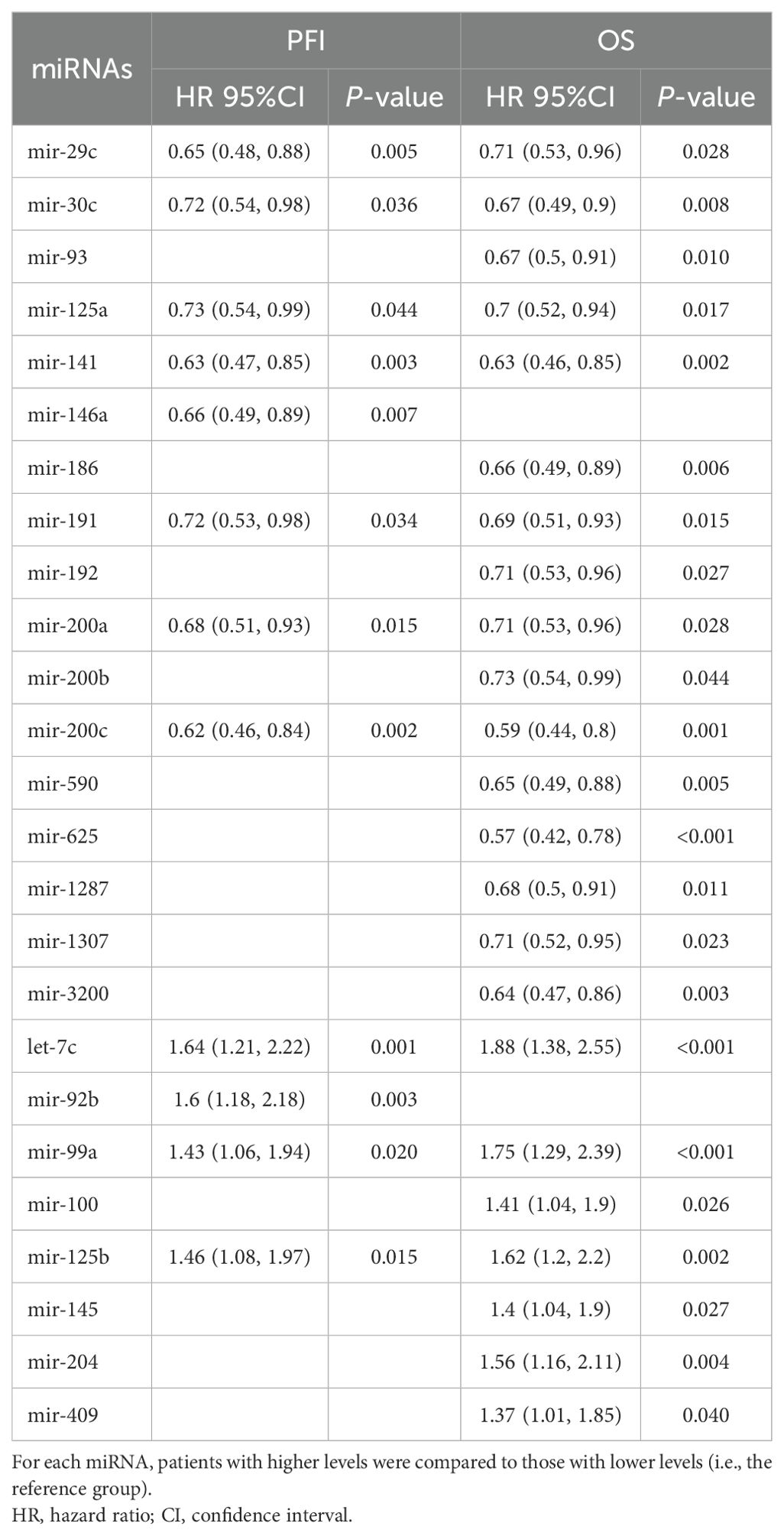
Analysis of miRNA expression across different tumor stages yielded a total of 49 miRNAs with significant differences (Supplementary Table 4). Of these, 13 were also significantly associated with patients’ prognosis (Supplementary Tables 5, 6). Notably, six miRNAs (miR-1278, miR-29c, miR-30c, miR-93, miR-191, and miR-141) exhibited a decreasing expression trend, with the highest levels observed in stage II and the lowest in stage IV. Conversely, six other miRNAs (miR-125b, miR-100, miR-99a, let-7c, miR-145, and miR-409) showed increasing expression levels from stage II to the more advanced stages (Figure 3, Supplementary Table 4).
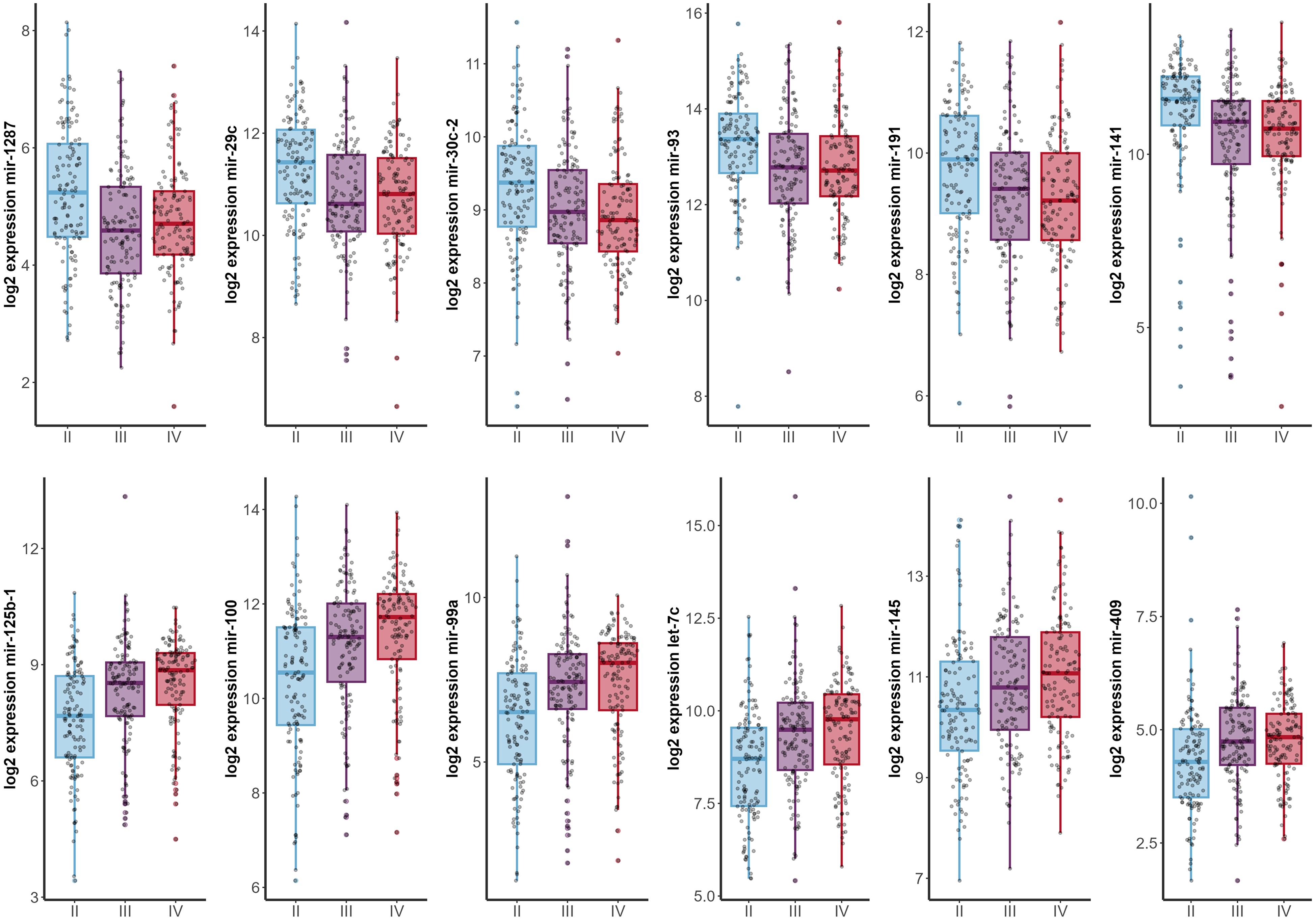
Figure 3. Expression profiles of highly significant miRNAs (p < 0.001) across different tumor stages (x-axis). Stage I patients were excluded from the analysis due to insufficient sample size (n=2).
Since only nine studies evaluated the impact of miRNAs on OS and only one on PFS, we sought to analyze these data on the TCGA-BLCA cohort to further evaluate the clinical relevance of these miRNAs (Figures 4, 5, Supplementary Tables 5, 6). Out of all miRNAs analyzed, 12 were significantly associated with PFI, and 23 were significantly associated with OS. Specifically, elevated expression levels of miR-200c, miR-141, miR-30c, miR-191, miR-125a, miR-200a, and miR-29c were associated with improved PFI and OS. Additionally, higher levels of miR-625, miR-3200, miR-590, miR-186, miR-93, miR-1287, miR-1307, miR-192, and miR-200b were significantly associated with better OS. Conversely, increased expression of let-7c, miR-99a, and miR-125b correlated with poorer PFI and OS. Higher levels of miR-204, miR-100, miR-145, and miR-409 were linked to worse OS. Notably, elevated miR-92b expression was associated with a shorter PFI, although its association with OS did not reach statistical significance (Table 3, Supplementary Tables 5, 6).
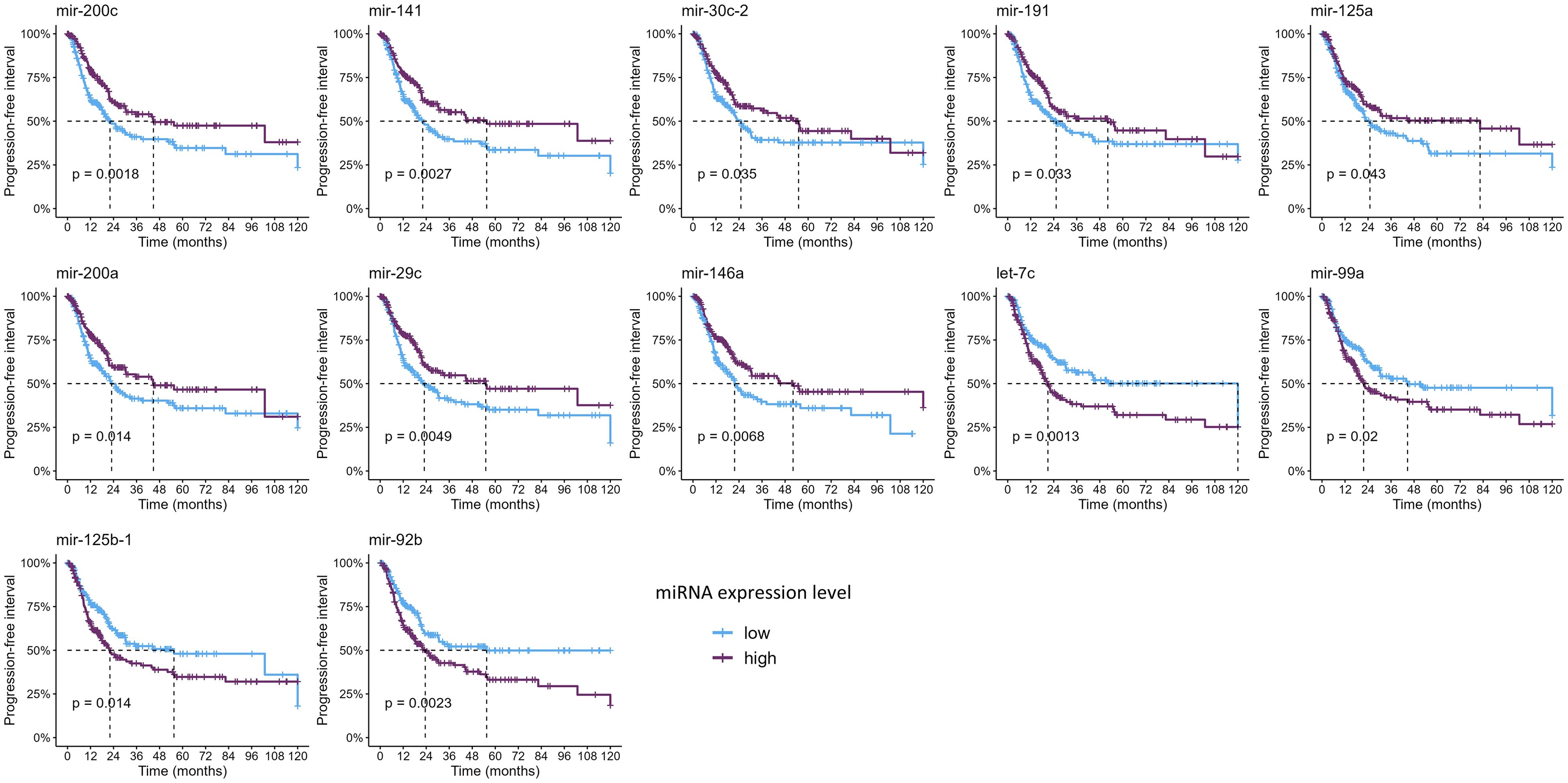
Figure 4. Kaplan–Meier curves showing the miRNAs significantly associated with progression-free interval (p-values were computed with the log-rank test).
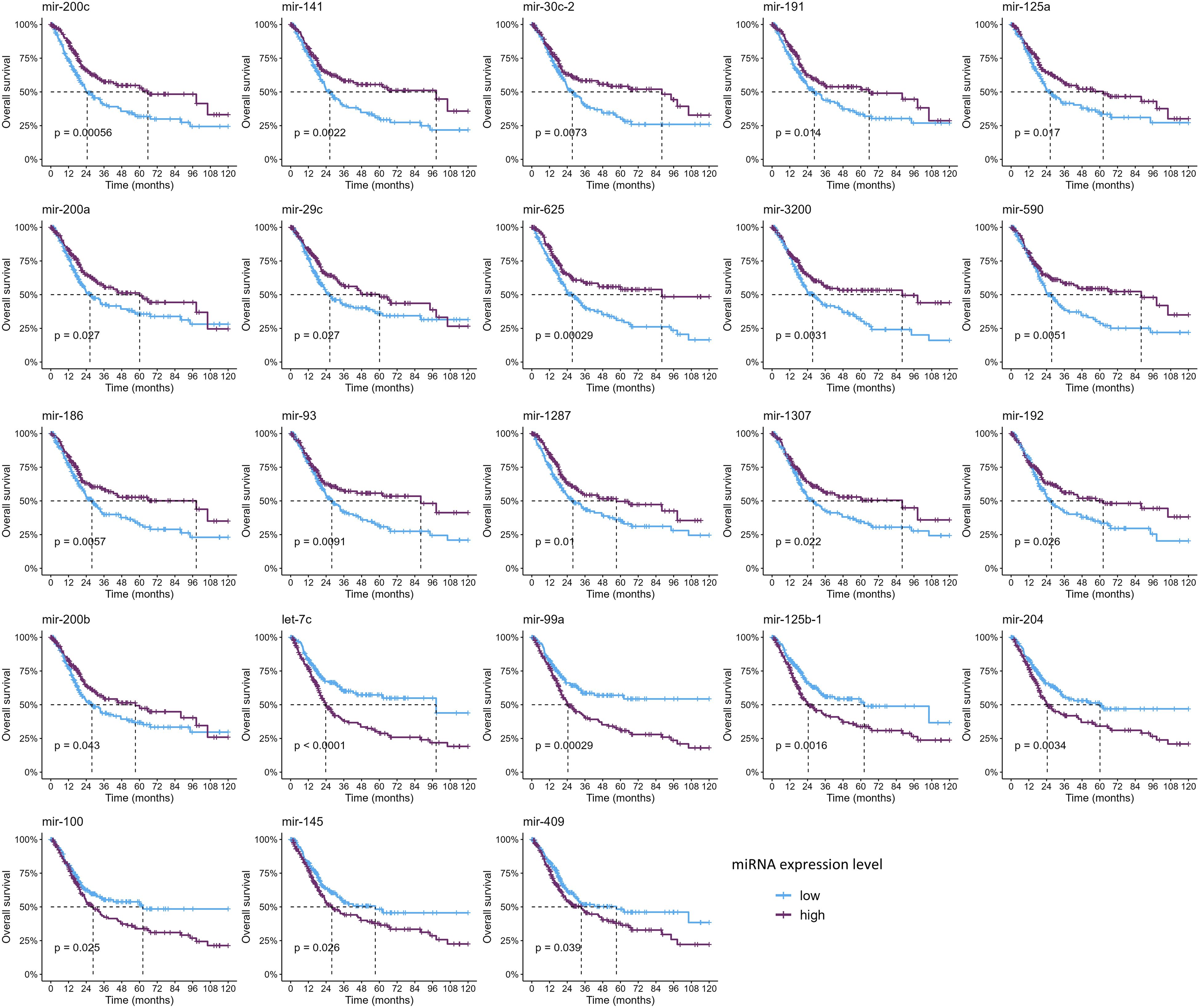
Figure 5. Kaplan–Meier curves showing the miRNAs significantly associated with overall survival (p-values were computed with the log-rank test).
Discussion
Summary of main findings
The results of this systematic review and comprehensive analysis highlight a complex network of interactions between specific miRNAs and components of the bladder cancer TME, which is yet to be fully explored and understood. These findings, completed by the bioinformatic analyses aimed at filling the literature gaps, support their potential as modulators of immune infiltration in bladder cancer. Although different perspectives have been explored, there are still insufficient data to definitively demonstrate the value of miRNAs in the selection of patients undergoing immunotherapy.
While numerous studies have investigated the role of individual miRNAs in bladder cancer immunity, their focus has often been fragmented, with different studies examining different miRNAs and distinct immune cell populations, very few on checkpoint molecules, and with limited clinical endpoints. For instance, miR-100-5p and miR-138-5p were linked to PD-L1/PD-L2 regulation (17), while others, such as miR-21 and miR-31, were associated with BCG response, disease progression, and recurrence (18), but no validation studies to confirm these results were found. Additional studies explored the interplay between select miRNAs and specific immune subsets, including CD4+ IFN-γ+ T cells, NK cells, macrophages, and regulatory T cells (19, 22, 26, 28). However, these investigations frequently lacked integration across cell types, mechanisms, and clinical impact. Moreover, only a few studies examined the prognostic significance of immune-related miRNAs systematically and comparatively.
Our study addresses these gaps by providing a comprehensive synthesis of the roles of the 104 miRNAs implicated in shaping the immune microenvironment of bladder cancer. By integrating findings from the published experimental models and validating them with large-scale data (TCGA-BLCA), we connected the dots between immune modulation and clinical outcomes such as PFI and OS. This integrative approach summarizes the current state-of-the-art on the immunoregulatory role of miRNAs in bladder cancer and offers a more unified view for understanding their potential impact on immunotherapy response. Moreover, this work lays the ground for their future use as prognostic and potentially predictive biomarkers.
miRNAs as modulators of the immune microenvironment and response
The differential expression of specific miRNAs and their association with immune cell infiltration patterns highlight the potential of miRNAs as both biomarkers and functional regulators of immune dynamics (52). These observations align with the broader framework of tumor immunoediting, a dynamic process encompassing the phases of elimination, equilibrium, and escape, through which cancer cells continuously evolve to evade immune surveillance (53).
In the escape phase, immune evasion becomes dominant, and tumors acquire multiple mechanisms to suppress antitumor immunity. Many of the miRNAs identified in this review are likely active participants in this phase. For instance, expression of miR-21 and miR-141-3p correlates with diminished cytotoxic immune cell infiltration (e.g., CD8+ T cells, NK cells), suggesting a role in establishing immune exclusion or immune suppression (18, 19, 34, 39, 43). Similarly, downregulation of miRNAs such as miR-29c-3p and miR-186-5p, which have been linked to immune activation, may further support the development of an immunosuppressive TME (23, 25) (Figure 6).
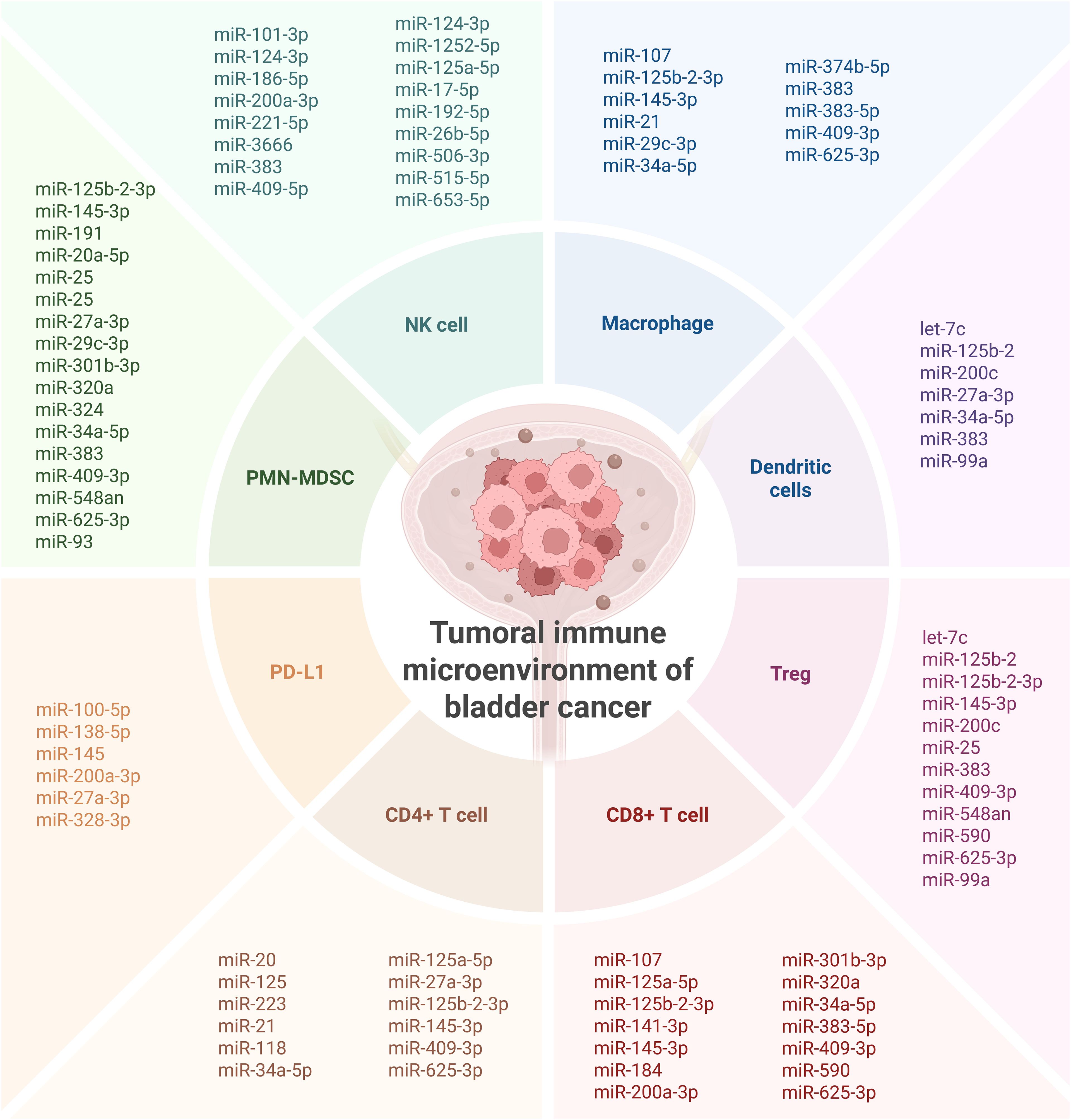
Figure 6. Overall presentation of the investigated miRNAs involved in shaping the immune microenvironment of bladder cancer. Created in BioRender. Coada, C. (2025) https://BioRender.com/dknj33y.
The heterogeneity of miRNA expression patterns observed across patient cohorts also reflects the broader variability in immune infiltration and response observed in bladder tumors. The bladder cancer TME frequently harbors immunosuppressive components, including tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), all of which can be recruited and sustained via miRNA-regulated signaling pathways (54). Moreover, the enrichment of immune checkpoint ligands such as PD-L1 and PD-L2 in miRNA-associated gene networks suggests a role for miRNAs in modulating immune checkpoint pathways, which are central to current immunotherapeutic strategies. High expression of PD-L1, often driven by oncogenic or hypoxia-induced signaling, is further modulated by miRNAs such as miR-138-5p and miR-100-5p (54). Loss or suppression of these miRNAs may contribute to the upregulation of immune inhibitory signals, allowing tumor cells to escape T-cell-mediated cytotoxicity (Figure 6).
The immunosuppressive TME interferes with normal immune responses and, thus, can reduce the efficacy of immune checkpoint blockade, which has been recently approved for bladder cancer treatment (55). Our review suggests that miRNA expression profiles could potentially serve as predictive biomarkers for immunotherapy response, as evidenced, for example, by their association with BCG failure (e.g., miR-21, miR-31) (18) (Figure 6).
Importantly, the functional links between miRNAs and immune regulation open up new opportunities for therapeutic intervention. Targeting specific miRNAs could complement existing immunotherapies by reversing immunosuppressive programming or restoring antitumor immunity. For example, miRNA mimics or antagomirs could be employed to modulate key pathways associated with T-cell recruitment, macrophage polarization, or checkpoint expression, thus enhancing the depth and durability of immune responses (54).
Implications for current medical practice and future directions
With the highly positive results obtained by immunotherapy in other cancers (54) and the approval of novel therapies for bladder cancer, research focusing on identifying the most promising candidates who would benefit from it is highly needed. Significant progress has been made in the use of miRNAs as biomarkers in bladder cancer, including the validation of a panel capable of diagnosing and stratifying patients (7). However, whether these panels or similar ones can also identify responders to treatment remains an open question. Of note, several of the miRNAs (miR-221-3p, miR-93-5p, miR-191-5p, miR-200c-3p, miR-192-5p, miR-21-5p) included in this panel have already been investigated for their roles in tumor immunity, suggesting that the development of miRNA-based tools to predict immunotherapy response is a feasible and promising avenue.
Emerging evidence highlights the critical role of miRNAs (often transported via tumor-derived exosomes) in preparing the pre-metastatic niche, particularly by modulating immune landscapes at distant sites (56). These miRNA-loaded exosomes act as messengers that condition the future metastatic environment, promoting immune evasion, suppressing cytotoxic T-cell activity, and recruiting immunosuppressive cells such as regulatory T cells, M2 macrophages, and myeloid-derived suppressor cells (56, 57). For example, certain miRNAs can inhibit antigen presentation by downregulating MHC class I molecules or modulate cytokine production to create a tolerogenic or “immune-silent” microenvironment that favors metastatic colonization. This immune reprogramming not only facilitates tumor cell seeding and outgrowth at distant sites but also contributes to resistance against immunotherapies such as immune checkpoint blockade (56, 57). By converting immunologically “hot” environments into “cold” niches, these miRNAs help shield disseminated tumor cells from T-cell-mediated clearance. Understanding and targeting these miRNA-mediated pathways offer a novel opportunity for disrupting the metastatic cascade and enhancing immunotherapeutic efficacy through restoration of immune surveillance in both primary and secondary tumor sites (53, 58).
miRNAs play multifaceted roles in modulating immune infiltration in bladder cancer and are increasingly recognized as promising biomarkers. Although increasing evidence shows their potential clinical relevance in immune-oncology, no studies to date have directly assessed miRNA profiles in patients receiving immunotherapy. Prospective studies and robust clinical validation are urgently needed to assess their utility as reliable biomarkers of immunotherapy response in bladder cancer patients.
Study limitations
While this systematic review and integrated TCGA analysis provide a comprehensive overview of the miRNA–immune axis in bladder cancer, several limitations should be mentioned to contextualize our findings and guide future research. The primary studies included in this systematic review had considerable heterogeneity. This was evident in the diversity of miRNAs investigated, the variety of experimental models used (ranging from in vitro cell lines to animal models and human tumor tissues), the different immune cell populations assessed, and the clinical or biological endpoints reported. Such heterogeneity, while reflective of the exploratory nature of much of the research on this topic, prevented any meaningful meta-analysis and therefore requires caution when concluding from this combined literature.
Moreover, our TCGA-BLCA data analysis, while robust, has some limitations as well. The use of bulk RNA-sequencing data for immune cell deconvolution provides estimates of immune cell abundance but cannot fully show the spatial organization or functional state of these cells within the tumor microenvironment. The associations identified between miRNA expression, immune infiltration, and clinical outcomes are also correlational and do not imply causality. While these findings provide valuable hypotheses, they require experimental validation on other cohorts.
Despite the promising associations identified, a significant translational gap remains. The gap from identifying significant miRNA expression patterns or in vitro/in vivo mechanistic links to clinically validated biomarkers predictive of immunotherapy response is quite wide. The current body of evidence, as synthesized here, highlights the need for prospective, well-designed clinical trials to validate the utility of these miRNAs in guiding therapeutic decisions in bladder cancer.
Conclusion
This systematic review shows the emerging and multifaceted role of miRNAs in modulating the immune landscape of cancer.
Network analyses consistently demonstrated significant associations between specific miRNAs and key immunological parameters, including lymphocyte infiltration, macrophage polarization, and mast cell activation. Several miRNAs were linked to oncological endpoints, showing a strong impact on PFI and OS.
These findings underscore the potential of miRNAs as both mechanistic regulators of tumor–immune interactions and as promising prognostic and/or predictive biomarkers. However, further studies are required to evaluate their potential role as predictors for immunotherapy response.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DVD: Writing – original draft, Project administration. GR: Conceptualization, Methodology, Validation, Investigation, Supervision, Funding acquisition, Writing – review & editing. FMG: Conceptualization, Formal analysis, Investigation, Resources, Visualization, Funding acquisition, Writing – review & editing. ALB: Investigation, Data curation, Writing – review & editing. FG: Validation, Investigation, Data curation, Writing – review & editing. ADL: Methodology, Writing – review & editing. HL: Investigation, Writing – review & editing. LO: Data Curation, Writing – review & editing. CT: Investigation, Writing – review & editing. TEM: Supervision, Writing – review & editing. CAC: Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The work reported in this publication was funded by the Italian Ministry of Health, RC - 2025-2797531.
Acknowledgments
The graphical abstract was made using icons from https://bioicons.com/.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used to improve the English syntax and clarity of the manuscript (ChatGPT and Gemini). Text was revised by the authors to ensure that the text meaning remained the same.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1639334/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Holzbeierlein J, Bixler BR, Buckley DI, Chang SS, Holmes RS, James AC, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/SUO guideline (2017; amended 2020, 2024). J Urol. (2024) 212:3–10. doi: 10.1097/JU.0000000000003981
3. Ho MD, Black AJ, Zargar H, Fairey AS, Mertens LS, Dinney CP, et al. The effect of cisplatin-based neoadjuvant chemotherapy on the renal function of patients undergoing radical cystectomy. Can Urological Assoc J. (2023) 17:301–9. doi: 10.5489/cuaj.8570
4. Giannakodimos I, Ziogou A, Giannakodimos A, Tzelepis K, Kratiras Z, Fragkiadis E, et al. Neoadjuvant immunotherapy for muscle-invasive bladder cancer: A 2025 update. Immunotherapy. (2025) 1–9. doi: 10.1080/1750743X.2025.2501929
5. Narain TA, Tosh JM, Gautam G, Talwar HS, Panwar VK, Mittal A, et al. Neoadjuvant therapy for cisplatin ineligible muscle invasive bladder cancer patients: A review of available evidence. Urology. (2021) 154:8–15. doi: 10.1016/j.urology.2021.03.010
6. Si W, Shen J, Zheng H, and Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenet. (2019) 11:25. doi: 10.1186/s13148-018-0587-8
7. Oto J, Herranz R, Plana E, Pérez-Ardavín J, Hervás D, Cana F, et al. Validation of a microRNA Profile in Urine Liquid Biopsy with Diagnostic and Stratification Value for Bladder Cancer Classification, Available through the Open App BladdermiRaCan. Exp Hematol Oncol. (2025) 14:58. doi: 10.1186/s40164-025-00649-0
9. Ouzzani M, Hammady H, Fedorowicz Z, and Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
10. Deng M, Brägelmann J, Kryukov I, Saraiva-Agostinho N, and Perner S. FirebrowseR: an R client to the broad institute’s firehose pipeline. Database. (2017) 2017:baw160. doi: 10.1093/database/baw160
11. Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. (2018) 173:400–416.e11. doi: 10.1016/j.cell.2018.02.052
13. Csárdi G, Nepusz T, Müller K, Horvát S, Traag V, Zanini F, et al. Igraph for R: R interface of the igraph library for graph theory and network analysis. (2025).
14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2021).
15. Benjamini Y and Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Society Ser B (Methodological). (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
16. Suszynska M, Machowska M, Fraszczyk E, Michalczyk M, Philips A, Galka-Marciniak P, et al. CMC: Cancer miRNA Census–a List of Cancer-Related miRNA Genes. Nucleic Acids Res. (2024) 52:1628–44. doi: 10.1093/nar/gkae017
17. El Ahanidi H, El Azzouzi M, Hafidi Alaoui C, Tetou M, Bensaid M, Chaoui I, et al. Immune checkpoint and telomerase crosstalk is mediated by miRNA-138 in bladder cancer. Front Oncol. (2022) 11:795242. doi: 10.3389/fonc.2021.795242
18. Awadalla A, Zahran MH, Abol-Enein H, Zekri A-RN, Elbaset MA, Ahmed AE, et al. Identification of Different miRNAs and Their Relevant miRNA Targeted Genes Involved in Sister Chromatid Cohesion and Segregation (SCCS)/Chromatin Remodeling Pathway on T1G3 Urothelial Carcinoma (UC) Response to BCG Immunotherapy. Clin Genitourinary Cancer. (2022) 20:e181–9. doi: 10.1016/j.clgc.2021.12.001
19. Bitting RL, Tooze JA, Goodman M, Vile DC, Brown JM, Thomas CY, et al. Low-dose paclitaxel with pembrolizumab enhances clinical and immunologic responses in platinum-refractory urothelial carcinoma. Cancer Res Commun. (2024) 4:530–9. doi: 10.1158/2767-9764.CRC-23-0436
20. Boubaker NS, Gurtner A, Trabelsi N, Manni I, Said R, Ayed H, et al. Evaluating prognostic utility of preoperative neutrophil to lymphocyte ratio and hsa-let-7g/c up-regulation in patients with urinary bladder cancer. Cancer Biomarkers. (2020) 27:63–73. doi: 10.3233/CBM-190483
21. Cai J, Yan Z, Zhong Y, Li Y, Huang J, Hu H, et al. Small non-coding RNA profiling in patients with non-muscle invasive bladder cancer. BMC Cancer. (2025) 25:319. doi: 10.1186/s12885-025-13672-5
22. Fan T, Xue L, Dong B, He H, Zhang W, Hao L, et al. CDH1 overexpression predicts bladder cancer from early stage and inversely correlates with immune infiltration. BMC Urol. (2022) 22:156. doi: 10.1186/s12894-022-01103-7
23. Fu Y, Sun S, Bi J, Kong C, and Yin L. Construction and analysis of a ceRNA network and patterns of immune infiltration in bladder cancer. Trans Andrology Urol. (2021) 10:1939955–1931955. doi: 10.21037/tau-20-1250
24. Huang H, Xu Q, Zhang Y, Zhou Y, Ma K, and Luo Y. miR-628-5p is a potential novel prognosis biomarker, associated with immune infiltration in bladder urothelial carcinoma. Curr Pharm Design. 29:2477–88. doi: 10.2174/0113816128254621231017062923
25. Huyan T, Gao L, Gao N, Wang C, Guo W, Zhou X, et al. miR-221-5p and miR-186-5p Are the Critical Bladder Cancer Derived Exosomal miRNAs in Natural Killer Cell Dysfunction. Int J Mol Sci. (2022) 23:15177. doi: 10.3390/ijms232315177
26. Jiang Z, Zhang Y, Zhang Y, Jia Z, Zhang Z, and Yang J. Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Communication Signaling. (2021) 19:93. doi: 10.1186/s12964-021-00768-1
27. Jiang A, Liu N, Bai S, Wang J, Gao H, Zheng X, et al. The construction and analysis of tumor-infiltrating immune cells and ceRNA networks in bladder cancer. Front Genet. (2020) 11:605767. doi: 10.3389/fgene.2020.605767
28. Jiang W, Zhu D, Wang C, and Zhu Y. An immune relevant signature for predicting prognoses and immunotherapeutic responses in patients with muscle-invasive bladder cancer (MIBC). Cancer Med. (2020) 9:2774–90. doi: 10.1002/cam4.2942
29. Kuo W-T, Lee Y-C, Yang Y-F, Cheng C-F, Tseng C-J, and Tsai K-W. Sushi domain containing 2 dysfunction contributes to cancer progression in patients with bladder cancer. J Cancer. (2024) 15:5318–28. doi: 10.7150/jca.97537
30. Li X, Liang Z, Pan J, Zhang M, Liu J, Hu R, et al. Identification of BACH1-IT2-miR-4786-siglec-15 immune suppressive axis in bladder cancer. BMC Cancer. (2024) 24:328. doi: 10.1186/s12885-024-12061-8
31. Liu Q, You B, Meng J, Huang C-P, Dong G, Wang R, et al. Targeting the androgen receptor to enhance NK cell killing efficacy in bladder cancer by modulating ADAR2/circ_0001005/PD-L1 signaling. Cancer Gene Ther. (2022) 29:1988–2000. doi: 10.1038/s41417-022-00506-w
32. Ke H, Zhang J, Wang F, and Xiong Y. ZNF652-Induced circRHOT1 Promotes SMAD5 Expression to Modulate Tumorigenic Properties and Nature Killer Cell-Mediated Toxicity in Bladder Cancer via Targeting miR-3666. J Immunol Res. (2021) 2021:7608178. doi: 10.1155/2021/7608178
33. Lyu X, Qiang Y, Zhang B, Xu W, Cui Y, and Ma L. Identification of immuno-infiltrating MAP1A as a prognosis-related biomarker for bladder cancer and its ceRNA network construction. Front Oncol. (2022) 12:1016542. doi: 10.3389/fonc.2022.1016542
34. Martínez VG, Rubio C, Martínez-Fernández M, Segovia C, López-Calderón F, Garín MI, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. (2017) 23:7388–99. doi: 10.1158/1078-0432.CCR-17-1004
35. Mei K, Chen Z, Huang L, Wang J, and Wei Y. Correlation between the immune microenvironment and bladder cancer based on a prognostic miRNA risk model. Cancer Insight. (2024) 3:14–25. doi: 10.58567/ci03020002
36. Rao X, Cao H, Yu Q, Ou X, Deng R, and Huang J. NEAT1/MALAT1/XIST/PKD–hsa-mir-101-3p–DLGAP5 axis as a novel diagnostic and prognostic biomarker associated with immune cell infiltration in bladder cancer. Front Genet. (2022) 13:892535. doi: 10.3389/fgene.2022.892535
37. Shi X, Pang S, Zhou J, Yan G, Gao R, Wu H, et al. Bladder-Cancer-Derived Exosomal circRNA_0013936 Promotes Suppressive Immunity by up-Regulating Fatty Acid Transporter Protein 2 and down-Regulating Receptor-Interacting Protein Kinase 3 in PMN-MDSCs. Mol Cancer. (2024) 23:52. doi: 10.1186/s12943-024-01968-2
38. Song Y, Du Y, Qin C, Liang H, Yang W, Lin J, et al. Gemcitabine-resistant biomarkers in bladder cancer are associated with tumor-immune microenvironment. Front Cell Dev Biol. (2022) 9:809620. doi: 10.3389/fcell.2021.809620
39. Stempor PA, Avni D, Leibowitz R, Sidi Y, Stępień M, Dzieciątkowski T, et al. Comprehensive analysis of correlations in the expression of miRNA genes and immune checkpoint genes in bladder cancer cells. Int J Mol Sci. (2021) 22:2553. doi: 10.3390/ijms22052553
40. Wang Y, Wang J, He J, Ji B, Pang Z, Wang J, et al. Comprehensive analysis of PRPF19 immune infiltrates, DNA methylation, senescence-associated secretory phenotype and ceRNA network in bladder cancer. Front Immunol. (2023) 14:1289198. doi: 10.3389/fimmu.2023.1289198
41. Wu C-C, Wang Y-H, Hu S-W, Wu W-L, Yeh C-T, and Bamodu OA. MED10 drives the oncogenicity and refractory phenotype of bladder urothelial carcinoma through the upregulation of hsa-miR-590. Front Oncol. (2022) 11:744937. doi: 10.3389/fonc.2021.744937
42. Xiong Q, Feng D, Wang Z, Ying Y, Xu C, Wei Q, et al. Fatty acid synthase is the key regulator of fatty acid metabolism and is related to immunotherapy in bladder cancer. Front Immunol. (2022) 13:836939. doi: 10.3389/fimmu.2022.836939
43. Yang C, Wu S, Mou Z, Zhou Q, Dai X, Ou Y, et al. Exosome-derived circTRPS1 promotes Malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol Ther. (2022) 30:1054–70. doi: 10.1016/j.ymthe.2022.01.022
44. Ying W, Zhao Y, He Y, Deng Y, Gan X, Li P, et al. Exosomal miR-184 Facilitates Bladder Cancer Progression by Targeting AKR1C3 and Inducing Immune Escape via IRF2-CXCL10 Axis. Biochim Biophys Acta (BBA) - Mol Basis Dis. (2025) 1871:167627. doi: 10.1016/j.bbadis.2024.167627
45. Yuan H, Wang T, Peng P, Xu Z, Feng F, Cui Y, et al. Urinary exosomal miR-17-5p accelerates bladder cancer invasion by repressing its target gene ARID4B and regulating the immune microenvironment. Clin Genitourinary Cancer. (2024) 22:569–579.e1. doi: 10.1016/j.clgc.2024.01.012
46. Zhang Q, Mao Z, and Sun J. NF-κB Inhibitor, BAY11 - 7082, Suppresses M2 Tumor-Associated Macrophage Induced EMT Potential via miR-30a/NF-κB/Snail Signaling in Bladder Cancer Cells. Gene. (2019) 710:91–7. doi: 10.1016/j.gene.2019.04.039
47. Zhang H, Song J, Dong J, Liu Z, Lin L, Wang B, et al. Tumor microenvironment analysis identified subtypes associated with the prognosis and the tumor response to immunotherapy in bladder cancer. Front Genet. (2021) 12:551605. doi: 10.3389/fgene.2021.551605
48. Zhang Z, Liu F, Yu Y, Xie F, and Zhu T. Prognosis and immune landscape of bladder cancer can be predicted using a novel miRNA signature associated with cuproptosis. PeerJ. (2024) 12:e18530. doi: 10.7717/peerj.18530
49. Zhao Y, Shi Z, Hao Z, Zhou J, Han C, Li R, et al. Hypoxia-Mediated down-Regulation of miRNAs’ Biogenesis Promotes Tumor Immune Escape in Bladder Cancer. Clin Transl Oncol. (2021) 23:1678–87. doi: 10.1007/s12094-021-02569-x
50. Zheng R, Gao F, Mao Z, Xiao Y, Yuan L, Huang Z, et al. LncRNA BCCE4 genetically enhances the PD-L1/PD-1 interaction in smoking-related bladder cancer by modulating miR-328-3p-USP18 signaling. Advanced Sci. (2023) 10:2303473. doi: 10.1002/advs.202303473
51. Zhu J, Li Y, Luo Y, Xu J, Liufu H, Tian Z, et al. Feedback loop formed by ATG7/autophagy, FOXO3a/miR-145 and PD-L1 regulates stem-like properties and invasion in human bladder cancer. Cancers. (2019) 11:349. doi: 10.3390/cancers11030349
52. Martino MTD, Tagliaferri P, and Tassone P. MicroRNA in cancer therapy: breakthroughs and challenges in early clinical applications. J Exp Clin Cancer Res. (2025) 44:126. doi: 10.1186/s13046-025-03391-x
53. Cha J-H, Chan L-C, Song MS, and Hung M-C. New approaches on cancer immunotherapy. Cold Spring Harb Perspect Med. (2020) 10:a036863. doi: 10.1101/cshperspect.a036863
54. Zabeti Touchaei A and Vahidi S. MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: targeting PD - 1/PD-L1 and CTLA - 4 pathways. Cancer Cell Int. (2024) 24:102. doi: 10.1186/s12935-024-03293-6
55. Matsuo T, Miyata Y, Harada J, Itoh I, Matsuda T, Asai A, et al. Efficacy of pembrolizumab as second or third-line therapy for local advanced and metastatic urothelial cancer. Anticancer Res. (2025) 45:2563–73. doi: 10.21873/anticanres.17628
56. Bayat M and Sadri Nahand J. Exosomal miRNAs: the tumor’s trojan horse in selective metastasis. Mol Cancer. (2024) 23:167. doi: 10.1186/s12943-024-02081-0
57. Zhang Z, Huang Q, Yu L, Zhu D, Li Y, Xue Z, et al. The Role of miRNA in Tumor Immune Escape and miRNA-Based Therapeutic Strategies. Front Immunol. (2022) 12:807895. doi: 10.3389/fimmu.2021.807895
Keywords: immune checkpoint inhibitor, immunotherapy, tumor immune infiltration, PD-L1, prognosis, urothelial carcinoma, personalized therapy, microRNA
Citation: Dulf D-V, Ravegnini G, Giorgi FM, Burnar AL, Gorini F, De Leo A, Luţichievici H, Opriţa C-L, Todiruţ C-N, Ciuleanu T-E and Coadă CA (2025) The miRNA–immune axis in bladder cancer: systematic evidence for a new era of immunotherapy precision. Front. Immunol. 16:1639334. doi: 10.3389/fimmu.2025.1639334
Received: 01 June 2025; Accepted: 18 August 2025;
Published: 19 September 2025.
Edited by:
Assunta Sellitto, Italian Institute of Technology, ItalyReviewed by:
María Marcela Barrio, Fundación Cáncer, ArgentinaPaula Dobosz, Poznan University of Medical Sciences, Poland
YaXuan Wang, First Affiliated Hospital of Harbin Medical University, China
Copyright © 2025 Dulf, Ravegnini, Giorgi, Burnar, Gorini, De Leo, Luţichievici, Opriţa, Todiruţ, Ciuleanu and Coadă. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gloria Ravegnini, Z2xvcmlhLnJhdmVnbmluaTJAdW5pYm8uaXQ=
 Daniel-Vasile Dulf1,2
Daniel-Vasile Dulf1,2 Gloria Ravegnini
Gloria Ravegnini Federico Manuel Giorgi
Federico Manuel Giorgi Francesca Gorini
Francesca Gorini Antonio De Leo
Antonio De Leo Constantin-Lucian Opriţa
Constantin-Lucian Opriţa Camelia Alexandra Coadă
Camelia Alexandra Coadă