- 1Division of Biliary Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Research Center for Biliary Diseases, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Intrahepatic cholangiocarcinoma (iCC) is an aggressive hepatobiliary malignancy with limited therapeutic options and poor survival outcomes. Hepatic arterial infusion chemotherapy (HAIC) has emerged as a promising treatment alternative to systemic chemotherapy, but its clinical benefits require comprehensive evaluation.
Methods: A systematic review and meta-analysis were conducted, including 10 studies with 1,493 patients. Data on overall survival (OS), progression-free survival (PFS), and key prognostic factors were extracted. Pooled hazard ratios (HR) were calculated using a random-effects model.
Results: HAIC significantly improved OS (HR = 0.51, p < 0.001) and PFS (HR = 0.58, p < 0.001) compared to systemic chemotherapy. Subgroup analyses revealed consistent benefits across various patient characteristics, including age, tumor stage, and baseline liver function. Patients with lower tumor burden (HR = 0.45) and ECOG performance status ≤1 (HR = 0.50) derived the greatest benefit. Additionally, patients with CA 19–9 levels <1,000 U/mL showed significantly improved OS (HR = 0.48).
Conclusion: HAIC prolongs survival and improves disease control in advanced iCC patients compared to systemic chemotherapy. These findings support the adoption of HAIC as a valuable treatment strategy for selected patients, particularly those with lower tumor burden and favorable performance status.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024615752.
1 Introduction
Intrahepatic cholangiocarcinoma (iCC) is a highly lethal hepatobiliary neoplasm whose incidence is increasing (1). Risk factors for intrahepatic cholangiocarcinoma (iCC) include fibroinflammatory biliary tract diseases such as primary sclerosing cholangitis, Caroli’s disease, hepatolithiasis, and liver fluke infections, as well as systemic conditions like non-alcoholic steatohepatitis and hepatitis (2, 3) Patients with iCC often remain asymptomatic for a long time, leading to late diagnoses when most patients already have advanced, unresectable, or metastatic disease (4). For those who undergo curative-intent resection, the cure rate remains low, with up to 60% experiencing recurrence (5).
The current standard systemic therapy for intrahepatic cholangiocarcinoma (ICC) is platinum-based chemotherapy combined with gemcitabine. The addition of cisplatin to gemcitabine improves median overall survival (OS) from 8.1 months with gemcitabine alone to 11.7 months (6). Similarly, the combination of gemcitabine and oxaliplatin has shown comparable efficacy (7). However, the optimal chemotherapy regimen for patients with advanced BTC refractory to GC has not yet been established (8).
Most patients with advanced ICC present with disease confined to the liver that is unresectable owing to tumor location and/or multifocal involvement (9). A continuous flow of intra-arterial chemotherapy is delivered in the hepatic artery via a surgically implantable subcutaneous pump with a catheter in the gastroduodenal artery (GDA), which is a side branch of the hepatic artery (10). Hepatic arterial infusion chemotherapy (HAIC) delivers high doses of chemotherapy directly to the tumor-rich hepatic artery circulation. Meanwhile, the portal vein maintains the health of the surrounding non-tumorous liver tissue (11). This targeted approach maximizes drug concentration at the tumor site while minimizing systemic toxicity, thanks to the liver’s efficient clearance of chemotherapy through first-pass metabolism (12). Selective hepatic arterial infusion chemotherapy (HAIC) has been used for over four decades and is currently being evaluated for various tumor types and clinical settings (13, 14). In Japan, HAIC has demonstrated effectiveness in treating unresectable hepatocellular carcinoma (15), with studies highlighting its safety and ability to enhance drug delivery to tumors (16).
Hepatic arterial infusion chemotherapy (HAIC) is not widely used as a treatment for intrahepatic cholangiocarcinoma (ICC) worldwide. However, some studies have reported its effectiveness in patients with advanced ICC (17). On the other hand, chemotherapy regimens represented by gemcitabine and platinum-based therapies are more commonly recognized as the standard treatment for ICC in other studies (18, 19). This suggests that the role of HAIC in ICC treatment remains uncertain and requires further investigation. The aim of this meta-analysis is to investigate the therapeutic efficacy of hepatic arterial infusion chemotherapy (HAIC) in the treatment of advanced intrahepatic cholangiocarcinoma (ICC). Specifically, it seeks to determine whether HAIC, compared to traditional systemic chemotherapy regimens, can improve patient survival outcomes. Furthermore, this study aims to identify key prognostic factors that influence survival in ICC patients receiving HAIC and to explore the prognostic characteristics of patients who may derive greater benefit from HAIC than from standard chemotherapy alone.
2 Methods
2.1 Literature search
This systematic review, registered with the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD 42024615752, was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Two independent reviewers, Zeng D and Wang YQ, carried out a comprehensive literature search across PubMed, Embase, and Web of Science databases, covering all records up to April 2024 and limited to English-language studies. Any disagreements between the reviewers were resolved through discussion with a third reviewer, Wang SF, to reach a consensus.
2.2 Inclusion and exclusion criteria
Inclusion Criteria:
1. Studies involving patients diagnosed with advanced intrahepatic cholangiocarcinoma (ICC) through pathological confirmation.
2. Studies evaluating the therapeutic efficacy of hepatic arterial infusion chemotherapy (HAIC) alone or in combination with systemic chemotherapy, specifically in terms of survival outcomes such as overall survival (OS), progression-free survival (PFS), or disease-free survival (DFS).
3. Studies reporting survival data with hazard ratios (HR) and 95% confidence intervals (CI) or providing sufficient statistical data to evaluate the impact of HAIC on survival outcomes.
4. Studies reporting survival data with hazard ratios (HR) and 95% confidence intervals (CI) or providing sufficient statistical data to evaluate the prognostic factors affecting survival outcomes in patients receiving HAIC treatment.
5. Studies focusing on locoregional treatment strategies for ICC, including comparisons between HAIC and standard systemic chemotherapy.
Exclusion Criteria:
1. Studies focusing on other biliary tract malignancies or benign liver conditions, such as extrahepatic cholangiocarcinoma, gallbladder cancer, or hepatocellular carcinoma, without separate data for intrahepatic cholangiocarcinoma.
2. Studies that do not specifically assess the efficacy of HAIC or compare it with traditional systemic chemotherapy in advanced ICC patients.
3. Studies lacking sufficient survival data or essential statistics (e.g., HR, odds ratios [OR], or relative risks [RR]) to evaluate outcomes.
4. Case reports, review articles, conference abstracts, or studies with fewer than ten participants.
5. Studies involving patients without advanced or unresectable ICC, or those focusing solely on surgical or other non-chemotherapy-based treatments.
2.3 Statistical analysis
Survival data were analyzed using multivariate regression techniques, with hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) as the primary measures. Categorical variables were assessed through odds ratios (ORs). Statistical heterogeneity among studies was evaluated using Cochrane’s Q-test and I² statistics, categorizing heterogeneity as low, moderate, or high at thresholds of 25%, 50%, and 75%, respectively. A random-effects model was applied consistently to account for variability across studies, regardless of the heterogeneity level.
To evaluate publication bias, funnel plots were used for visual inspection of asymmetry, which may suggest bias in study selection or reporting. This was further supported by Egger’s test, a statistical method used to quantify and confirm the presence of publication bias. A significant result (P < 0.05) from Egger’s test would indicate potential bias, requiring cautious interpretation of the pooled results.
To assess the robustness of the findings, sensitivity analyses were conducted by sequentially excluding each study and reanalyzing the remaining data. This approach helped evaluate whether any single study had a disproportionate impact on the overall estimates. These steps ensured that the conclusions were stable and not overly influenced by individual datasets. A p-value < 0.05 (two-tailed) was considered statistically significant.
2.4 Quality assessment of studies
The quality of the included studies was assessed independently by two investigators, Zeng D and Wang SF, using the Newcastle-Ottawa Scale (NOS). This tool evaluates studies across three main domains: selection of study groups, comparability of cohorts, and outcome assessment. Each study was assigned a score out of nine, with a score of six or higher indicating acceptable quality for inclusion. Discrepancies between the investigators were resolved through discussion or consultation with a third reviewer to ensure consistency and reliability in the quality assessment. Detailed NOS scoring results are provided in Supplementary Table 1.
3 Results
3.1 Literature search
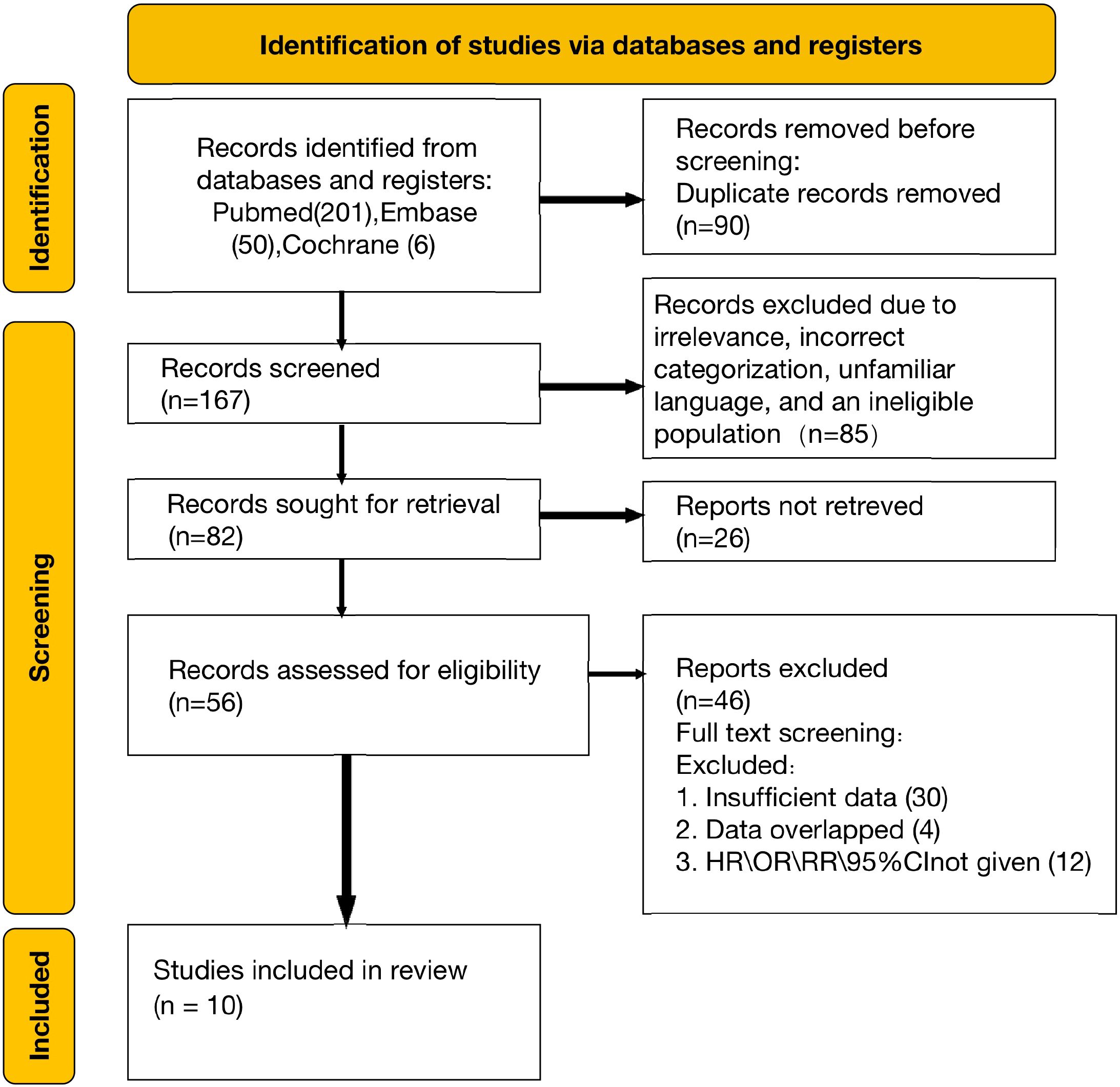
A total of 257 articles were initially retrieved from electronic databases, including PubMed, Embase, and Web of Science. After the removal of duplicates and screening for relevance, 167 full-text articles were reviewed for eligibility. Upon detailed evaluation, 10 studies satisfied the inclusion criteria and were incorporated into the qualitative analysis (8, 10, 20–27). The selection process is depicted in the PRISMA flowchart (Figure 1).
3.2 Study characteristics and quality assessment
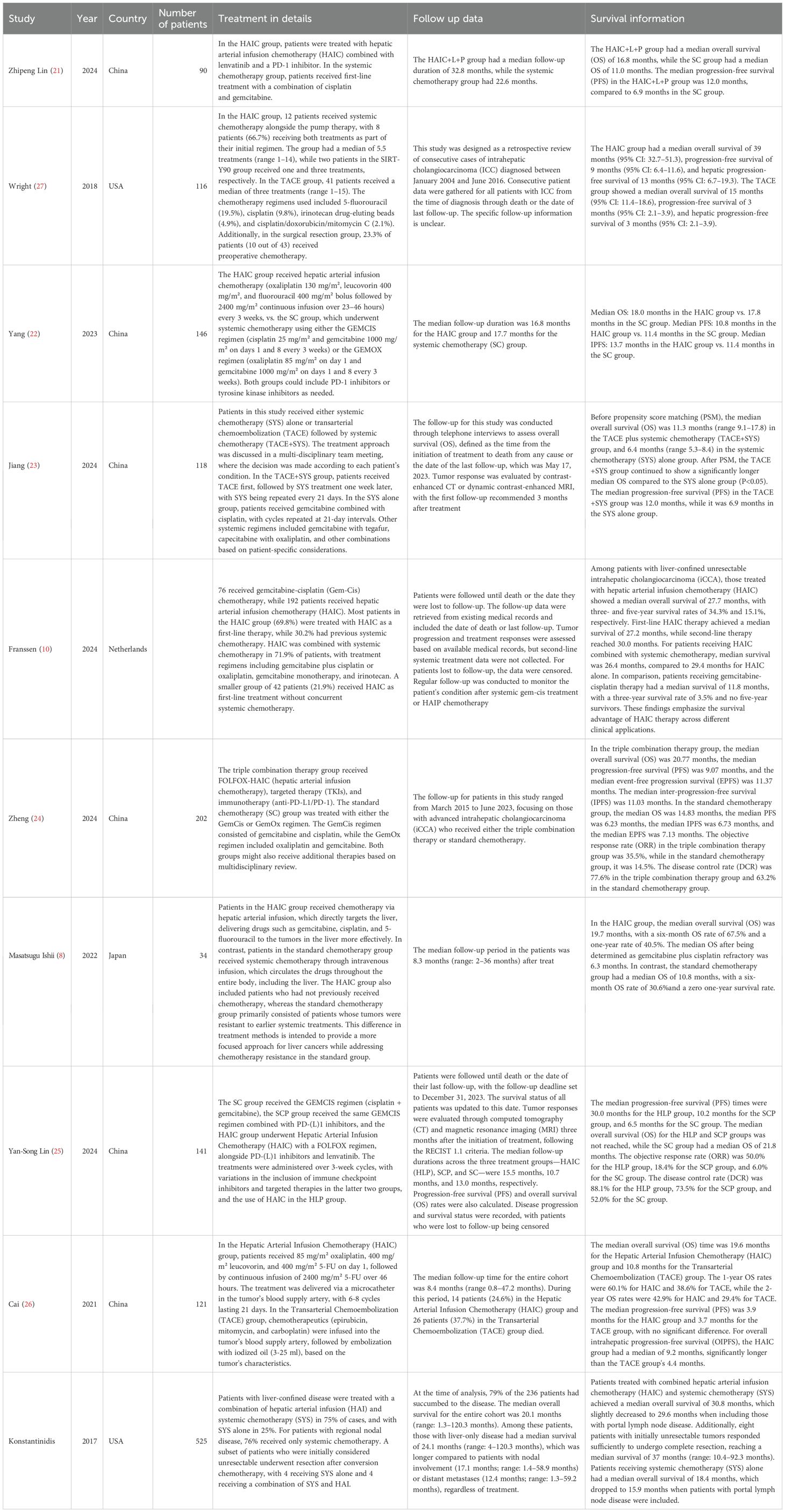
This meta-analysis included a total of 1493 patients diagnosed with intrahepatic cholangiocarcinoma (ICC) from studies published between 2018 and 2024. Among the 10 included studies, all compared the effects of hepatic arterial infusion chemotherapy (HAIC) versus systemic chemotherapy on patient survival outcomes. Five studies focused on identifying prognostic factors that influence outcomes in patients treated with HAIC. Additionally, three studies analyzed which prognostic factors determined greater benefit from HAIC compared to chemotherapy, or vice versa. Among the 10 included studies, the Newcastle-Ottawa Scale (NOS) quality assessment demonstrated that most were of high quality, with nine achieving a score of seven or higher (detailed NOS evaluations are available in Supplementary Table 1). The PRISMA diagram (Figure 1) provides an overview of the study selection process, while the detailed characteristics of these studies are summarized in Table 1.
3.3 HAIC vs systemic chemotherapy: impact on overall survival in advanced intrahepatic cholangiocarcinoma
Ten studies evaluated the comparative impact of HAIC and systemic chemotherapy on overall survival in advanced intrahepatic cholangiocarcinoma. The pooled analysis of these studies demonstrated that HAIC significantly improved overall survival, with a hazard ratio (HR) of 0.51 (95% CI: 0.38–0.70, p < 0.001) (Figure 2).
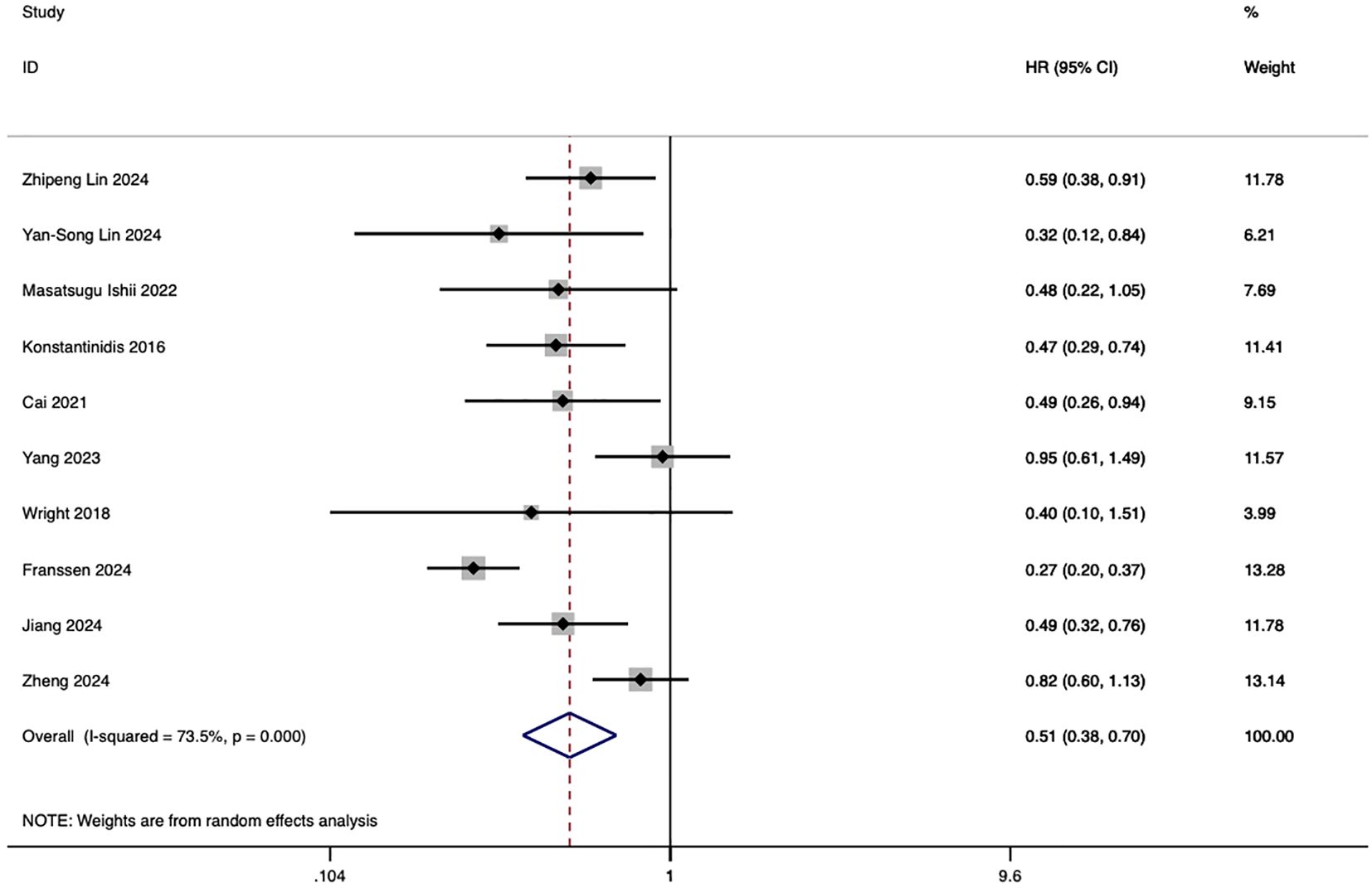
Figure 2. Forest plot of overall survival comparing HAIC and systemic chemotherapy in advanced intrahepatic cholangiocarcinoma (ICC).
3.4 HAIC vs systemic chemotherapy: impact on progression-free survival in advanced intrahepatic cholangiocarcinoma
Seven studies investigated the comparative impact of HAIC and systemic chemotherapy on progression-free survival in advanced intrahepatic cholangiocarcinoma. The pooled analysis demonstrated that HAIC significantly improved overall survival, with a hazard ratio (HR) of 0.58 (95% CI: 0.48, 0.69; p < 0.001) (Figure 3).
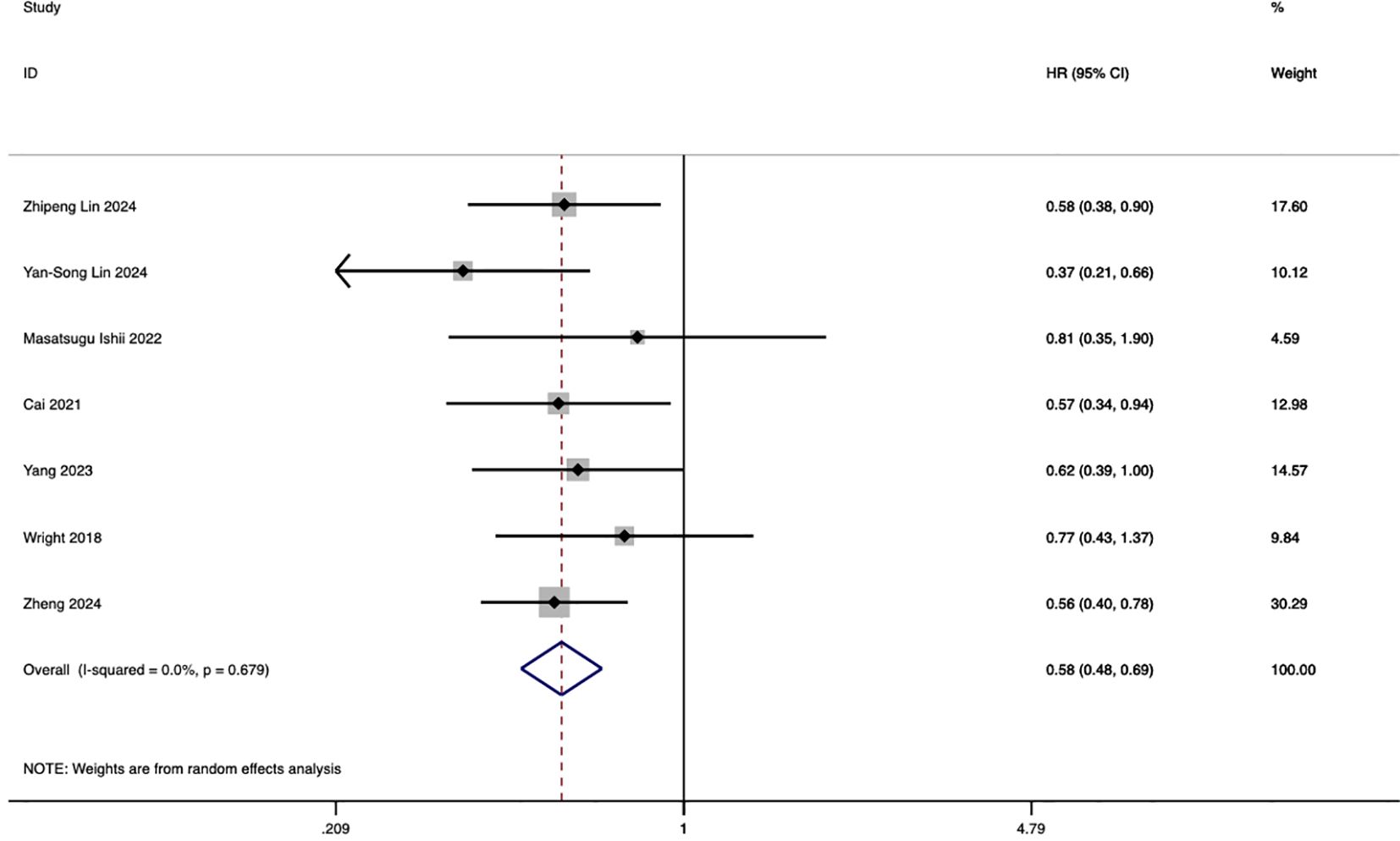
Figure 3. Forest plot of progression-free survival comparing HAIC and systemic chemotherapy in advanced ICC.
3.5 Prognostic factors influencing survival in intrahepatic cholangiocarcinoma patients undergoing HAIC therapy
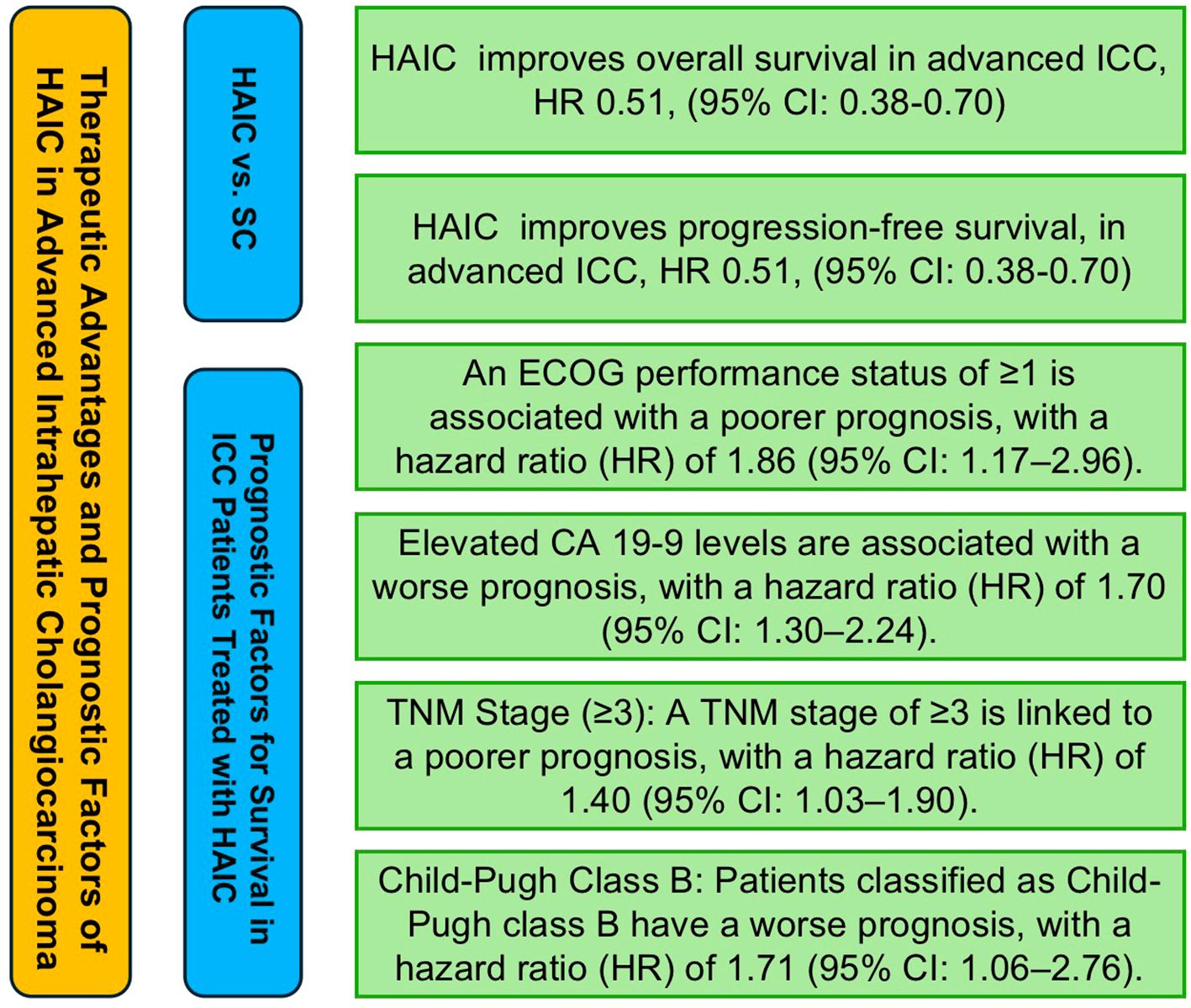
In five studies analyzing prognostic factors influencing survival in intrahepatic cholangiocarcinoma patients undergoing hepatic arterial infusion chemotherapy (HAIC), a total of 15 factors were evaluated for their potential impact on survival outcomes. These factors included age (≥50/60 years), gender (male vs. female), Eastern Cooperative Oncology Group (ECOG) performance status (≥1), multifocal tumor (yes vs. no), tumor location (bilobar vs. unilobar), largest tumor size (>5/10 cm), carbohydrate antigen 19-9 (CA 19-9) levels (elevated levels), CEA levels (>5 ng/mL), TNM stage (stage ≥3), vascular invasion (yes vs. no), lymph node metastasis (yes vs. no), Child-Pugh class (B vs. A), albumin (ALB) levels (<35 g/L vs. >35 g/L), and HBV status (positive vs. negative).
Among these factors, age (≥50/60 years), gender (male), ECOG performance status (≥1), multifocal tumor (yes vs. no), CA 19–9 levels (elevated), CEA levels (>5 ng/mL), TNM stage (stage ≥3), vascular invasion (yes vs. no), lymph node metastasis (yes vs. no), Child-Pugh class (B vs. A), albumin levels (<35 g/L), and HBV status (positive vs. negative) all demonstrated a negative association with prognosis, although some analyses did not show full statistical significance. Specifically, gender (male) had a hazard ratio (HR) of 1.49 (95% CI: 1.18, 1.88), ECOG performance status (≥1) had an HR of 1.86 (95% CI: 1.17, 2.96), CA 19–9 levels (elevated) had an HR of 1.70 (95% CI: 1.30, 2.24), TNM stage (stage ≥3) had an HR of 1.40 (95% CI: 1.03, 1.90), and Child-Pugh class (B vs. A) had an HR of 1.71 (95% CI: 1.06, 2.76), all of which were significantly associated with poorer prognosis. The results of the above analysis are presented in Figure 4.
3.6 Prognostic factors influencing the differences between HAIC and systemic chemotherapy in advanced intrahepatic cholangiocarcinoma patients
Three original studies evaluated 24 prognostic factors that may influence the differences in survival outcomes between hepatic arterial infusion chemotherapy (HAIC) and systemic chemotherapy in patients with advanced intrahepatic cholangiocarcinoma. These factors included age (>60 vs. ≤60), gender (male vs. female), ECOG performance status (0 vs. ≥1), Child-Pugh class (A vs. B), CEA levels (<5 vs. ≥5 ng/mL), CA 19–9 levels (<40/100 vs. ≥40/100), lymph node metastasis (present vs. absent), distant metastasis (present vs. absent), TNM stage (<3 vs. ≥3), tumor size (<10 cm vs. ≥10 cm), portal vein invasion (present vs. absent), and liver cirrhosis (present vs. absent). These factors were systematically analyzed to identify their potential impact on the efficacy of the two treatments. In nearly all prognostic factor subgroups, hepatic arterial infusion chemotherapy (HAIC) demonstrated better efficacy compared to traditional systemic chemotherapy in advanced intrahepatic cholangiocarcinoma patients. The only exception was observed in patients with CA 19–9 levels <40/100, where HAIC showed slightly worse outcomes with a hazard ratio (HR) of 1.14 (95% CI: 0.66–2.03). Notably, HAIC achieved statistically significant better outcomes in specific subgroups, including patients aged >60 (HR: 0.58, 95% CI: 0.41–0.82), those with an ECOG performance status of 0 (HR: 0.53, 95% CI: 0.36–0.79), Child-Pugh class A (HR: 0.49, 95% CI: 0.34–0.71), lymph node metastasis present (HR: 0.54, 95% CI: 0.40–0.74), and distant metastasis absent (HR: 0.54, 95% CI: 0.38–0.75). The results of the above analysis are shown in Figure 5.
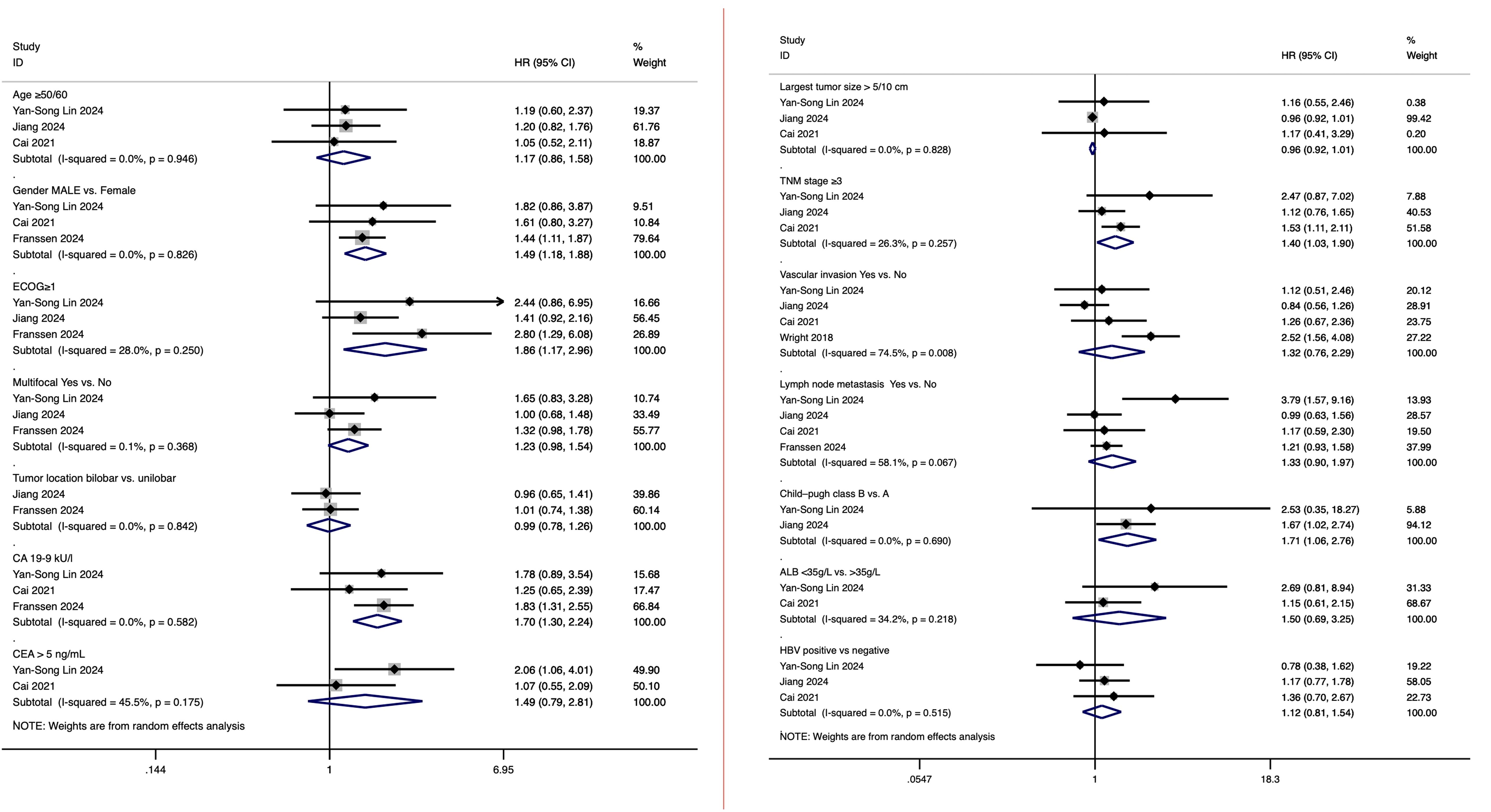
Figure 5. Subgroup analysis of prognostic factors influencing the comparative efficacy of HAIC versus systemic chemotherapy in advanced ICC.
3.7 Sensitivity analyses
In the sensitivity analyses, a random-effects model was applied, and each study was systematically excluded in turn to evaluate the robustness of the prognostic role of hepatic arterial infusion chemotherapy (HAIC) compared to systemic chemotherapy in advanced intrahepatic cholangiocarcinoma (ICC). Specifically, sensitivity analyses were conducted for studies related to HAIC vs. Systemic Chemotherapy: Impact on Overall Survival in Advanced Intrahepatic Cholangiocarcinoma and HAIC vs. Systemic Chemotherapy: Impact on Progression-Free Survival in Advanced Intrahepatic Cholangiocarcinoma. The analyses demonstrated consistent results, further confirming the reliability of the findings and indicating that the conclusions are robust. Additionally, recalculations performed after excluding studies with smaller sample sizes or lower Newcastle-Ottawa Scale (NOS) scores produced consistent results, further supporting the reliability of the findings. These analyses suggest that the conclusions drawn from this meta-analysis are robust and not unduly influenced by any single dataset. Sensitivity analyses were conducted using StataMP 17 software (StataCorp. 2022. Stata Statistical Software: Release 17), and the results confirmed the robustness of the conclusions. Comprehensive details of the sensitivity analyses for each type of adjuvant therapy are provided in the supplementary materials. The results of the above analysis are shown in Supplementary Figures 1, 2.
3.8 Publication bias
In studies comparing hepatic arterial infusion chemotherapy (HAIC) and systemic chemotherapy for overall survival in advanced intrahepatic cholangiocarcinoma, the symmetrical distribution of the funnel plots indicated no significant risk of publication bias. Additionally, Egger’s regression test confirmed this finding, demonstrating an insignificant presence of publication bias with a p-value of 0.937. The results of the above analysis are shown in Supplementary Figures 3, 4.
In studies comparing hepatic arterial infusion chemotherapy (HAIC) and systemic chemotherapy for progression-free survival (PFS) in advanced intrahepatic cholangiocarcinoma, the symmetrical distribution of the funnel plots indicated no significant risk of publication bias. Furthermore, Egger’s regression test confirmed this finding, demonstrating an insignificant presence of publication bias with a p-value of 0.585. The results of the above analysis are shown in Supplementary Figures 5, 6.
As for Prognostic Factors Influencing Survival in Intrahepatic Cholangiocarcinoma Patients Undergoing HAIC Therapy and Prognostic Factors Influencing the Differences Between HAIC and Systemic Chemotherapy in Advanced Intrahepatic Cholangiocarcinoma Patients, no assessment of publication bias was performed due to the limited number of original studies focusing on individual factors. A figure abstract has been created to succinctly illustrate the main findings of this study (Figure 6).
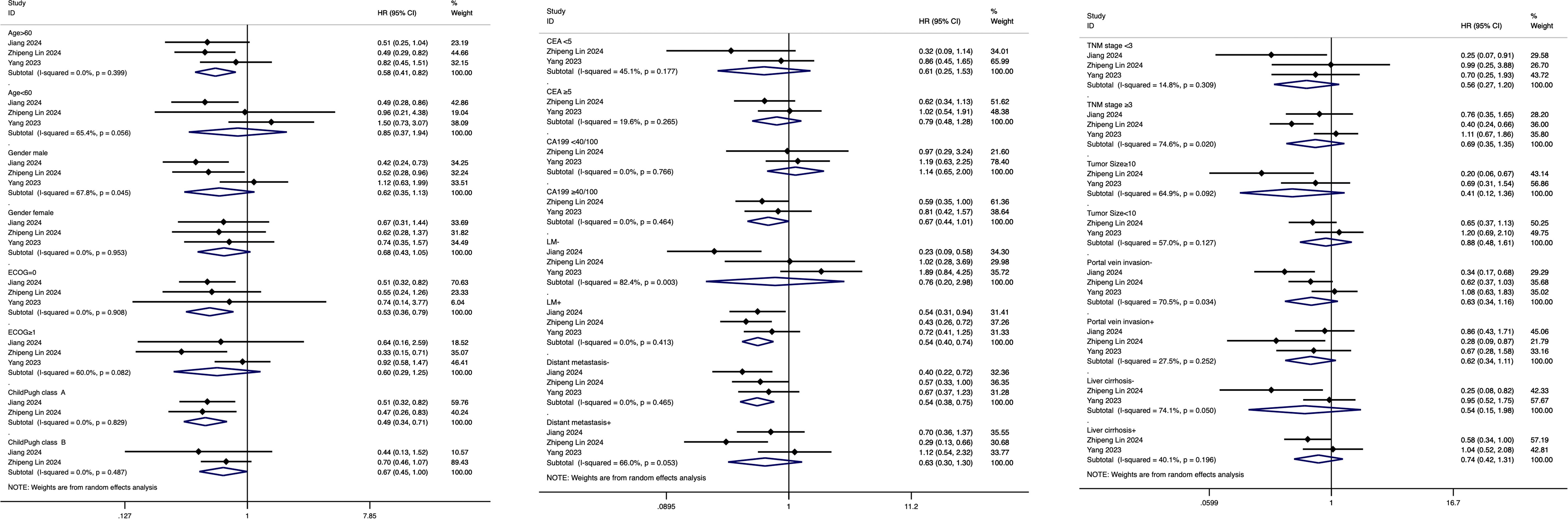
Figure 6. Figure abstract of hepatic arterial infusion chemotherapy in advanced intrahepatic cholangiocarcinoma.
4 Discussions
This meta-analysis reveals that hepatic arterial infusion chemotherapy (HAIC) demonstrates significant survival benefits over systemic chemotherapy in the treatment of advanced intrahepatic cholangiocarcinoma (ICC). HAIC was associated with improved overall survival (OS) and progression-free survival (PFS), with hazard ratios indicating a marked reduction in mortality and disease progression risk. Prognostic analyses identified factors such as ECOG performance status, CA 19–9 levels, and TNM stage as critical determinants of survival in ICC patients undergoing HAIC. Furthermore, subgroup evaluations suggest that specific patient characteristics, including older age, better liver function (Child-Pugh class A), and the absence of distant metastasis, may predict enhanced outcomes with HAIC compared to systemic chemotherapy. These findings underscore HAIC’s potential as an effective locoregional treatment strategy and highlight the importance of individualized therapeutic approaches in managing advanced ICC. To our knowledge, this is the first meta-analysis specifically comparing hepatic arterial infusion chemotherapy (HAIC) with systemic chemotherapy in intrahepatic cholangiocarcinoma (ICC).
An increasing body of evidence supports the therapeutic benefits of hepatic arterial infusion chemotherapy (HAIC). A review of nine studies, encompassing 478 patients with unresectable intrahepatic cholangiocarcinoma (iCCA) treated with hepatic arterial infusion chemotherapy (HAIC) using floxuridine, often in combination with systemic chemotherapy, demonstrated the efficacy of HAIC. The review reported a favorable overall survival (OS) rate, with a pooled 3-year OS of 39.5%. This outcome surpasses that observed with systemic chemotherapy in the ABC trials, where no patient survived beyond 3 years, underscoring the potential of HAIC as an effective treatment option for iCCA (28). In addition, Ghiringhelli et al. reported that the median OS of patients who received HAIC using gemcitabine and oxaliplatin as the second-line treatment was 20.3 months (29). A possible explanation is that hepatic arterial infusion chemotherapy (HAIC) achieves higher concentrations of chemotherapeutic agents in the liver compared to systemic chemotherapy (SC), enhancing its ability to control tumors in this region. The liver’s unique dual blood supply plays a critical role in this process. Specifically, the hepatic artery supplies nearly all the blood flow to the tumor, while the portal vein primarily supplies the non-neoplastic liver parenchyma. By preferentially delivering chemotherapeutic agents via the hepatic artery, HAIC more effectively targets and controls liver tumors (22). In addition to advanced intrahepatic cholangiocarcinoma (iCCA), hepatic arterial infusion (HAI) has demonstrated significant benefits in the treatment of extensive colorectal cancer liver metastases. Compared to systemic chemotherapy alone, HAI is associated with improved treatment responses, reduced toxicity, and a potential survival advantage, highlighting its efficacy in managing liver-dominant metastatic disease (20).
There is growing evidence that combining hepatic arterial infusion chemotherapy (HAIC) with other therapeutic strategies offers benefits for advanced intrahepatic cholangiocarcinoma (iCCA). For instance, patients treated with HAIC combined with lenvatinib and PD-(L)1 inhibitors (HLP) demonstrated significantly improved progression-free survival (PFS) and objective response rate (ORR) compared to those receiving systemic chemotherapy (SC), either with or without PD-(L)1 inhibitors (30, 31). While the overall survival (OS) in the HLP group was not statistically superior to the SCP group, it was notably better than the SC group. Widely endorsed in Asia as a treatment for advanced hepatocellular carcinoma (HCC), HAIC has also shown remarkable efficacy when combined with sorafenib or the combination of PD-1 inhibitors and lenvatinib (32). The observed benefits of HAIC in combination therapy may be attributed to synergistic mechanisms, including the ability of chemotherapy to induce tumor apoptosis through DNA damage and immunogenic cell death, thereby enhancing antitumor immune responses and boosting the effectiveness of immunotherapy (33). Additionally, small-molecule tyrosine kinase inhibitors, such as lenvatinib, improve the tumor microenvironment by targeting VEGFR1–3 and fibroblast growth factor receptor 1 (FGFR1), while regulating colony-stimulating factor 1 receptor (CSF1R) to reduce M2-type macrophage infiltration and suppress regulatory T cells (Tregs) (34). These effects weaken immunosuppressive responses, enhance the activity of PD-1 and PD-L1 inhibitors, and further promote robust immune responses, highlighting the potential of HAIC-based combination therapies in advanced iCCA.
Hepatic arterial infusion chemotherapy (HAIC) has not been directly assessed in a meta-analysis for safety and adverse events, but existing studies suggest that HAIC has certain advantages over systemic chemotherapy (SC) in these aspects. The target lesions in intrahepatic cholangiocarcinoma (iCCA) are predominantly located within the liver. Hepatic arterial infusion chemotherapy (HAIC) delivers chemotherapy drugs directly to the intrahepatic target lesion via the hepatic artery, leveraging the liver’s first-pass effect to significantly lower systemic drug levels. This approach reduces systemic toxicity and minimizes adverse reactions. In contrast, systemic chemotherapy is administered intravenously, requiring higher drug concentrations in the systemic circulation to achieve therapeutic effects at the target site. This can lead to increased toxicity, harm to multiple organ systems, and a higher likelihood of adverse events. A 2023 study found that the overall incidence of adverse events (AEs), including rash, vomiting, fatigue, leukopenia, anemia, and sensory neuropathy, was lower in patients receiving hepatic arterial infusion chemotherapy (HAIC) compared to those undergoing systemic chemotherapy (SC). Additionally, grade 3–4 AEs, such as hematologic toxicity and liver function damage, were less frequent in the HAIC group (22). LIN ET AL found similar results, the HAIC+L+P group experienced fewer TRAEs than the SC group (21). However, specific complications such as biliary toxicity and liver-related effects require vigilant management. The combination of HAIC with systemic agents, such as bevacizumab, has been explored. While such combinations showed efficacy, they sometimes led to increased biliary toxicity without significant improvements in outcomes, emphasizing the need for careful patient selection and monitoring (35). Future trials focusing on optimizing HAIC protocols and exploring its integration with systemic treatments are necessary to establish its broader applicability.
HAIC has shown promise in improving overall and progression-free survival compared to systemic chemotherapy in advanced intrahepatic cholangiocarcinoma (ICC). Its clinical application lies in leveraging these survival benefits to develop tailored treatment approaches for patients based on their individual prognostic profiles. This analysis highlights its potential role by identifying key prognostic factors and tailoring treatment strategies accordingly. Patients with unfavorable indicators—such as advanced TNM stage, multifocal or large tumors, elevated CA 19–9 or CEA levels, and ECOG =0—may require more intensive treatment regimens and closer follow-up. Conversely, those with localized disease, preserved liver function, and robust performance status may derive greater benefits from HAIC, allowing for more focused and potentially less invasive management plans. The findings also underscore the importance of subgroup analysis in clinical decision-making. For instance, patients aged >60, those with lymph node metastasis, or without distant metastases tend to achieve better outcomes with HAIC compared to systemic chemotherapy. Meanwhile, systemic chemotherapy may remain a viable option for patients with widespread disease or specific biomarker profiles where HAIC’s efficacy could be limited. This study offers valuable insights into the clinical application of HAIC by integrating prognostic factors to enhance personalized treatment strategies for advanced ICC. These results emphasize the need to balance treatment intensity with patient-specific characteristics to optimize outcomes while minimizing unnecessary interventions. Nonetheless, further well-designed, large-scale studies are essential to validate these conclusions and refine clinical protocols.
This meta-analysis is the first to explore the efficacy of hepatic arterial infusion chemotherapy (HAIC) compared to traditional systemic chemotherapy in intrahepatic cholangiocarcinoma (ICC), providing a meaningful addition to the existing body of research. However, several limitations should be noted. The small number of studies included in this analysis increases the potential for heterogeneity, which may limit the generalizability of the findings. Additionally, the restricted data set hampers the ability to conduct more detailed subgroup analyses, which could provide deeper insights into specific patient populations or treatment scenarios. Another limitation is the absence of a comprehensive evaluation of important clinical factors, such as patient quality of life, toxicity management, and treatment costs, which are essential for informed clinical decision-making. Furthermore, variations in HAIC protocols across the included studies, including differences in treatment regimens, may have introduced heterogeneity, potentially affecting the reliability and consistency of the results.
5 Conclusion
This meta-analysis indicates that hepatic arterial infusion chemotherapy may offer survival benefits over systemic chemotherapy in advanced intrahepatic cholangiocarcinoma, with potential improvements in overall survival and progression-free survival. Key prognostic factors influencing treatment outcomes include gender, ECOG performance status, elevated CA 19–9 levels, advanced TNM stage, and poorer liver function. Future large-scale, well-designed studies are essential to validate these findings, optimize protocols, and enhance clinical decision-making for advanced intrahepatic cholangiocarcinoma management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
DZ: Project administration, Writing – review & editing, Formal Analysis, Data curation, Visualization, Methodology, Software, Validation, Investigation, Writing – original draft, Conceptualization. ZC: Conceptualization, Validation, Formal Analysis, Writing – review & editing, Data curation, Writing – original draft. GL: Resources, Visualization, Writing – review & editing, Funding acquisition, Investigation, Software, Writing – original draft. JL: Project administration, Writing – original draft, Software, Writing – review & editing, Resources. BL: Investigation, Writing – review & editing, Resources, Data curation, Writing – original draft, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Science and Technology Program (2024NSFSC0642 to GL; 2024NSFSC0755 to Nansheng Cheng) and Sichuan University Innovation Research Project (2023SCUH0041) to GL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1640970/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis for overall survival comparing HAIC and systemic chemotherapy in advanced ICC.
Supplementary Figure 2 | Sensitivity analysis for progression-free survival comparing HAIC and systemic chemotherapy in advanced ICC.
Supplementary Figure 3 | Funnel plot for publication bias in overall survival analysis between HAIC and systemic chemotherapy.
Supplementary Figure 4 | Funnel plot for publication bias in progression-free survival analysis between HAIC and systemic chemotherapy.
Supplementary Figure 5 | Egger’s test results for publication bias in overall survival analysis.
Supplementary Figure 6 | Egger’s test results for publication bias in progression-free survival analysis.
Supplementary Table 1 | Newcastle-Ottawa Scale (NOS) quality assessment scores for the included studies. The table provides a breakdown of scores across three domains: selection, comparability, and outcome, demonstrating the high quality of the majority of included studies.
BTC, Biliary Tract Cancer; CA 19–9, Carbohydrate Antigen 19-9; CEA, Carcinoembryonic Antigen; DFS, Disease-Free Survival; ECOG, Eastern Cooperative Oncology Group; GDA, Gastroduodenal Artery; GC, Gemcitabine and Cisplatin; HAIC, Hepatic Arterial Infusion Chemotherapy; HBV, Hepatitis B Virus; HR, Hazard Ratio; ICC, Intrahepatic Cholangiocarcinoma; iCC, Intrahepatic Cholangiocarcinoma; NOS, Newcastle-Ottawa Scale; OR, Odds Ratio; OS, Overall Survival; PFS, Progression-Free Survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International Prospective Register of Systematic Reviews; RR, Relative Risk; TNM, Tumor, Node, Metastasis.
References
1. Kelley RK, Bridgewater J, Gores GJ, and Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. (2020) 72:353–63. doi: 10.1016/j.jhep.2019.10.009
2. Razumilava N and Gores GJ. Cholangiocarcinoma. Lancet. (2014) 383:2168–79. doi: 10.1016/s0140-6736(13)61903-0
3. Mavros MN, Economopoulos KP, Alexiou VG, and Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. (2014) 149:565–74. doi: 10.1001/jamasurg.2013.5137
4. Tan JC, Coburn NG, Baxter NN, Kiss A, and Law CH. Surgical management of intrahepatic cholangiocarcinoma–a population-based study. Ann Surg Oncol. (2008) 15:600–8. doi: 10.1245/s10434-007-9627-x
5. Yamashita S, Koay EJ, Passot G, Shroff R, Raghav KP, Conrad C, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer. (2017) 123:1354–62. doi: 10.1002/cncr.30488
6. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
7. André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. (2004) 15:1339–43. doi: 10.1093/annonc/mdh351
8. Ishii M, Itano O, Morinaga J, Shirakawa H, and Itano S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PloS One. (2022) 17:e0266707. doi: 10.1371/journal.pone.0266707
9. Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: A phase 2 clinical trial. JAMA Oncol. (2020) 6:60–7. doi: 10.1001/jamaoncol.2019.3718
10. Franssen S, Holster JJ, Jolissaint JS, Nooijen LE, Cercek A, D’Angelica MI, et al. Gemcitabine with cisplatin versus hepatic arterial infusion pump chemotherapy for liver-confined unresectable intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2024) 31:115–24. doi: 10.1245/s10434-023-14409-z
11. Lorenz M and Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. (2000) 18:243–54. doi: 10.1200/jco.2000.18.2.243
12. Kemeny N and Fata F. Hepatic-arterial chemotherapy. Lancet Oncol. (2001) 2:418–28. doi: 10.1016/s1470-2045(00)00419-8
13. Pernot S, Velut G, Kourie RH, Amouyal G, Sapoval M, Pointet AL, et al. 5-FU or mitomycin C hepatic arterial infusion after failure of arterial oxaliplatin in patients with colorectal cancer unresectable liver metastases. Clin Res Hepatol Gastroenterol. (2018) 42:255–60. doi: 10.1016/j.clinre.2017.11.004
14. Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, Yamaguchi K, et al. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer. (2017) 6:275–86. doi: 10.1159/000473887
15. Yamagami T, Kato T, Iida S, Tanaka O, and Nishimura T. Value of transcatheter arterial embolization with coils and n-butyl cyanoacrylate for long-term hepatic arterial infusion chemotherapy. Radiology. (2004) 230:792–802. doi: 10.1148/radiol.2303021564
16. Vogl TJ, Zangos S, Eichler K, Selby JB, and Bauer RW. Palliative hepatic intraarterial chemotherapy (HIC) using a novel combination of gemcitabine and mitomycin C: results in hepatic metastases. Eur Radiol. (2008) 18:468–76. doi: 10.1007/s00330-007-0781-0
17. Konstantinidis IT, Do RK, Gultekin DH, Gönen M, Schwartz LH, Fong Y, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol. (2014) 21:2675–83. doi: 10.1245/s10434-014-3649-y
18. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. (2010) 103:469–74. doi: 10.1038/sj.bjc.6605779
19. Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. (2021) 22:1560–72. doi: 10.1016/s1470-2045(21)00486-1
20. Konstantinidis IT, Groot Koerkamp B, Do RK, Gönen M, Fong Y, Allen PJ, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. (2016) 122:758–65. doi: 10.1002/cncr.29824
21. Lin Z, Zou X, Hu X, Huang D, Chen Y, Lin J, et al. Efficacy analysis of HAIC combined with lenvatinib plus PD1 inhibitor vs. first-line systemic chemotherapy for advanced intrahepatic cholangiocarcinoma. Sci Rep. (2024) 14:23961. doi: 10.1038/s41598-024-75102-z
22. Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, et al. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front Pharmacol. (2023) 14:1234342. doi: 10.3389/fphar.2023.1234342
23. Jiang N, Zhang Z, Yin X, Qiu H, Yan W, Hao Y, et al. Systemic chemotherapy plus transarterial chemoembolization versus systemic chemotherapy alone for unresectable intrahepatic cholangiocarcinoma: a multicenter retrospective cohort study. Radiol Med. (2024) 129:631–42. doi: 10.1007/s11547-024-01781-3
24. Zheng Z, Wang J, Wu T, He M, Pan Y, Wang J, et al. Hepatic arterial infusion chemotherapy plus targeted therapy and immunotherapy versus systemic chemotherapy for advanced intrahepatic cholangiocarcinoma: a retrospective cohort study. Int J Surg. (2024). doi: 10.1097/js9.0000000000002013
25. Lin YS, Li S, Yang X, Guo RP, Huang YH, Bai KH, et al. First-line hepatic arterial infusion chemotherapy plus lenvatinib and PD-(L)1 inhibitors versus systemic chemotherapy alone or with PD-(L)1 inhibitors in unresectable intrahepatic cholangiocarcinoma. J Cancer Res Clin Oncol. (2024) 150:309. doi: 10.1007/s00432-024-05795-2
26. Cai Z, He C, Zhao C, and Lin X. Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front Oncol. (2021) 11:611118. doi: 10.3389/fonc.2021.611118
27. Wright GP, Perkins S, Jones H, Zureikat AH, Marsh JW, Holtzman MP, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: A comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. (2018) 25:83–90. doi: 10.1245/s10434-017-6110-1
28. Holster JJ, El Hassnaoui M, Franssen S, JNM IJ, de Jonge J, Mostert B, et al. Hepatic arterial infusion pump chemotherapy for unresectable intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Ann Surg Oncol. (2022) 29:5528–38. doi: 10.1245/s10434-022-11439-x
29. Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, and Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. (2013) 59:354–60. doi: 10.1159/000362223
30. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. doi: 10.1159/000343875
31. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. (2021) 11:618206. doi: 10.3389/fonc.2021.618206
32. Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. (2020) 31:334–51. doi: 10.1016/j.annonc.2019.12.001
33. Cheu JW and Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. (2021) 74:2264–76. doi: 10.1002/hep.31840
34. Moreno-Cubero E and Larrubia JR. Specific CD8(+) T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J Gastroenterol. (2016) 22:6469–83. doi: 10.3748/wjg.v22.i28.6469
Keywords: intrahepatic cholangiocarcinoma (ICC), hepatic arterial infusion chemotherapy (HAIC), survival outcomes, meta-analysis, prognostic factors (PF)
Citation: Zeng D, Cheng Z, Liu G, Lu J and Li B (2025) Hepatic arterial infusion chemotherapy versus systemic chemotherapy for advanced intrahepatic cholangiocarcinoma: a meta-analysis of survival outcomes. Front. Immunol. 16:1640970. doi: 10.3389/fimmu.2025.1640970
Received: 04 June 2025; Accepted: 24 June 2025;
Published: 16 July 2025.
Edited by:
Rongce Zhao, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Jie Mei, Sun Yat-sen University Cancer Center (SYSUCC), ChinaXinhao Xiong, Sun Yat-sen University Cancer Center (SYSUCC), China
Shaohua Li, First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2025 Zeng, Cheng, Liu, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Lu, bHVqaW9uZ0BzY3UuZWR1LmNu; Bei Li, bGliZWlAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Di Zeng1,2†
Di Zeng1,2† Bei Li
Bei Li