- 1Department of Neurology, Wenzhou Central Hospital & Dingli Clinical Institute of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Department of Neurology, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China
- 3National Center for Neurological Disorders, Shanghai, China
- 4Rare Disease Center, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China
- 5Department of Ophthalmology, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, China
- 6Eye Institute and Department of Ophthalmology, Eye & ENT Hospital, Shanghai Medical College, Fudan University, Shanghai, China
Objective: To assess clinical outcomes in patients treated with eculizumab for acute attacks of aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (NMOSD).
Methods: We retrospectively analyzed prospectively collected data from the Huashan NMOSD registry cohort, and included patients who received eculizumab within 30 days of attack onset. Eculizumab was administered at 900 mg weekly for four weeks, followed by an eight-week observation period. Primary outcomes included visual acuity and visual field for optic neuritis (ON) and muscle strength, assessed using the Medical Research Council (MRC) scale for longitudinally extensive transverse myelitis (LETM). Serum neurofilament light chain (sNfL) and glial fibrillary acidic protein (sGFAP) levels were also monitored.
Results: Nine patients (seven with ON, two with LETM) were included. All patients received high-dose intravenous methylprednisolone prior to eculizumab treatment. Following eculizumab, six of the seven ON patients showed significant improvements in visual acuity and visual fields, with five recovering to near-normal or pre-attack vision. Visual field mean deviation improved from −22.4 dB to −2.0 dB (p = 0.008). Among LETM patients, one regained substantial lower limb strength (MRC grade 0 to 3 proximally, 4 distally), while the other showed improvement in distal strength (MRC grade 0 to 3). Serum sGFAP decreased from 278.0 to 130 pg/mL (p = 0.027), while sNfL levels transiently increased before stabilizing. One LETM patient developed a urinary tract infection, and another had Klebsiella pneumoniae pneumonia; all infections were effectively treated.
Conclusion: Eculizumab may yield favorable outcomes in acute NMOSD attacks, with infection monitoring being particularly important in severe cases.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune inflammatory demyelinating disease of the central nervous system (CNS), mediated by anti-aquaporin-4 immunoglobulin G (AQP4-IgG) antibodies (1). NMOSD is characterized by recurrent attacks of optic neuritis (ON) and longitudinally extensive transverse myelitis (LETM), frequently resulting in permanent vision loss and motor paralysis (2). Current therapeutic interventions for acute attacks primarily include high-dose intravenous methylprednisolone pulse therapy (IVMP) and therapeutic plasma exchange (TPE) (3). However, clinical outcome data indicate that only approximately 21.6% of patients attain complete recovery post-relapse, while 6% show no improvement at all (4). These findings underscore the critical need for therapies capable of rapidly attenuating acute tissue injury.
Complement-mediated enhancement of local inflammation and cytotoxicity are key elements in the pathophysiology of NMOSD. Upon binding to extracellular epitopes of AQP4, AQP4-IgG activates the complement system (5, 6) and ultimately forming C5b-9 membrane attack complex (MAC), driving astrocyte lysis, blood-brain barrier disruption and neuronal injury (7). Histopathological analyses have revealed that the sites of AQP4 loss within lesions correspond to regions of immunoglobulin and complement activation (8, 9). In the cerebrospinal fluid (CSF) of untreated NMOSD patients during the acute phase, increased levels of C5a and C5b-9 have been observed (10, 11).
Eculizumab, a humanized monoclonal antibody inhibiting complement C5, exerts its therapeutic effect by preventing the generation of C5a and MAC formation, thereby abrogating the pathological consequences of complement cascade activation (12). The phase III PREVENT trial (NCT01892345) established eculizumab as a first-line therapy for relapse prevention in AQP4-IgG-positive NMOSD (13). Rationales also exist for using eculizumab as an acute therapy for NMOSD relapse, as its rapid onset of action and continuous near-complete inhibition of C5 activity upon the first infusion (14).
Eculizumab has been approved for NMOSD in China since October 2023. The current study included nine patients with AQP4-IgG-positive NMOSD who were treated with eculizumab during the acute relapse phase. Changes in vision and motor functions, serological biomarkers, and retina measurements by optical coherence tomography (OCT) were assessed over an eight-week observation period.
Methods
Study design and participants
This is a retrospective analysis of prospectively collected data from the NMOSD cohort at Huashan Hospital. The study involved patients who were part of the cohort followed prospectively from their NMOSD diagnosis. Between October 2023 (when eculizumab was approved for NMOSD in China) and February 2025, 26 consecutive patients with AQP4-IgG-positive NMOSD received eculizumab, of whom 9 were included in this analysis. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) diagnosed with AQP4-IgG-positive NMOSD according to the 2015 revised criteria (15); (3) received eculizumab during an acute phase of NMOSD, defined as within 30 days of attack onset, and (4) adherence to an 8-week follow-up from eculizumab initiation.
The decision to initiate eculizumab “early” was based on the aim of preventing complement-mediated neuronal damage and alleviating neurological deficits. This decision was made collaboratively between patients and physicians. As eculizumab is not reimbursed by national insurance, the decision was also influenced by the patients’ financial capacity. The cost of eculizumab in China is 351.26 USD per vial, with each vial containing 300 mg.
Eculizumab was administered at a dosage of 900 mg weekly for four consecutive weeks. Since the drug is not covered by China’s national insurance, patients autonomously decided whether to continue eculizumab after receiving the four doses. Patients were advised to receive meningococcal vaccination at the earliest opportunity. For those who were vaccinated, oral antibiotics were continued for two weeks following vaccination completion. In patients who did not receive vaccination, oral antibiotic prophylaxis was maintained for two weeks after the completion of eculizumab treatment.
Attacks were defined as followings and confirmed by a neurologist: (1) new neurological symptoms related to NMOSD lasting longer than 24 hours and occurring > 30 days after the previous attack; (2) no other underlying aetiology (e.g., fever or infection); (3) accompanying objective change in neurological examination, such as decreased muscle strength for myelitis and reduction in visual acuity or visual field defect for ON.
Data collection
Data were extracted from the Huashan NMOSD prospective registry cohort, which includes regular follow-ups every six months during the remission phase, along with more intensive follow-ups during acute relapses. Demographic data and disease history prior to eculizumab treatment were collected, including age, gender, disease duration from the initial attack to the time of study inclusion, history of previous relapses, comorbidities, AQP4-IgG titers, and use of medications prior to the current attack. The time from the onset of the current attack to the initiation of IVMP, as well as the time from current attack onset to the initiation of eculizumab, were also recorded.
Clinical, serological, and ophthalmologic assessments were conducted for acute relapse treated with eculizumab, according to the protocol predefined in the NMOSD hospital registry. Specifically, assessments were conducted on the day of eculizumab initiation (W0), followed by weekly assessments for the first four weeks (Weeks 1, 2, 3, and 4) and again at Week 8 after eculizumab initiation. Each evaluation included vital signs, adverse event, the Expanded Disability Status Scale (EDSS) score, muscle strength in patients with myelitis attack, best corrected visual acuity (BCVA), visual fields, Visual Functional System Score (VFSS), and OCT measurements including peripapillary retinal nerve fiber layer (pRNFL) and macular ganglion cell-inner plexiform layer (mGCIPL) in patients with ON attack. Serum neurofilament light chain (sNfL), serum glial fibrillary acidic protein (sGFAP), complete blood count, C-reactive protein, and hepatorenal functions were determined on the day of eculizumab initiation (W0) and at Weeks 0, 2, 4 and 8.
Outcome measures
The primary outcome was the change in disability status, including muscle strength measured by the Medical Research Council (MRC) scale for patients with LETM, BCVA and visual field for patients with ON. The secondary outcome was EDSS, VFSS, and adverse event. The exploratory outcomes were changes of serological biomarkers (sGFAP and sNfL) and OCT measurements (pRNFL and mGCIPL).
AQP4-IgG
AQP4-IgG detection was conducted using a fixed-cell-based assay with indirect immunofluorescence, utilizing HEK-293 cells transfected with M1-AQP4 at Huashan Hospital.
sGFAP and sNfL
The collected serum samples were immediately separated by centrifugation and frozen at −80 °C until analysis. sGFAP and sNfL concentrations were measured in duplicates via ultra-sensitive single-molecule arrays (SIMOA). Neurology 2-plex B kits were used with an HD-1 immunoassay analyzer (Quanterix, Boston, Massachusetts, USA) as per the manufacturer’s instructions.
BCVA and visual field
BCVA was assessed using the Standard Logarithmic Visual Acuity Chart (GB/T 11533-2011) in accordance with Chinese national standard (16), and a BCVA below 0.01 was documented with count fingers (CF) or hand movements (HM).
The central visual field was assessed using a Humphrey visual field analyzer (Carl Zeiss, Germany). Swedish Interactive Thresholding Algorithm strategy 30-2, No. III white visual label was selected as the detection program, and the visual field mean deviation (MD) was automatically calculated. The reliability criteria were false-positive and false-negative rates of < 33% and fixation losses of < 20%.
OCT
TOPCON’s sweep OCT Triton (Topcon Healthcare, Tokyo, Japan) was used with a speed of 100000 A-scans per second. The peripapillary pRNFL thickness was measured using the “3D Disc 6.0*6.0mm” mode, which scans a circular area with a 6-mm diameter centered on the optic disc. The ganglion cell complex thickness was acquired using the “3D Macula 7.0*7.0mm” mode, which captures data from a square grid (7 mm × 7 mm) centered on the macula. The device automatically segmented the measurements into three components: macular retinal nerve fiber layer (mRNFL), mGCIPL, and mGCIPL plus mRNFL. Only high-quality images with signal strength index ≥ 40 were accepted according to the OSCAR-IB criteria (17).
Statistical analysis
Quantitative data are presented as median and range. Categorical variables were described by counts and percentages. The comparison between pre- and post-treatment was conducted using the paired Wilcoxon signed-rank test. All statistical analyses were performed using R version 4.4.1(R Foundation). Graphs were generated using R version 4.4.1 and adjusted using Adobe Illustrator version 27.0 (Adobe Inc.). The level of statistical significance was set at p < 0.05.
Result
Patient characteristics
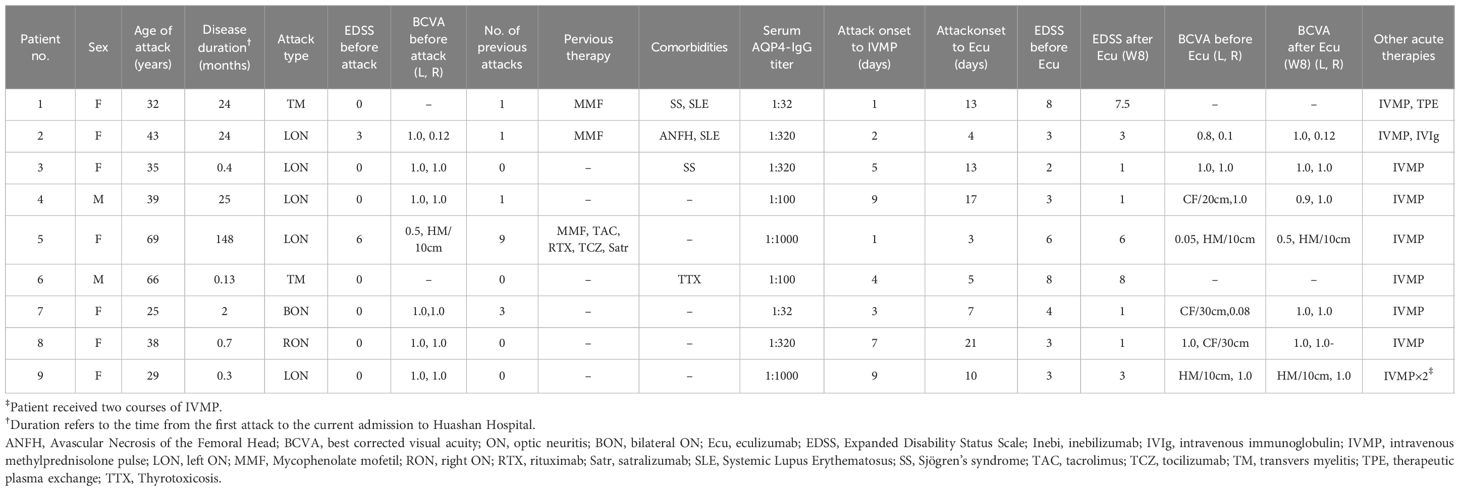
This study included nine patients diagnosed with AQP4-IgG-positive NMOSD, comprising seven females and two males, with ages ranging from 25 to 69. Among them, four patients experienced their first NMOSD attack, while the remaining had a history of 1 to 9 previous attacks (Table 1). Notably, three patients had prior immunomodulatory treatment before the current attack. Patients 1 and 2 had been receiving mycophenolate mofetil therapy and remained stable for two years prior to the current relapse. Patient 5, who experienced nine relapses over 12 years, had previously been treated with mycophenolate mofetil, tacrolimus, and rituximab, all of which failed to prevent subsequent attacks. Upon transitioning to tocilizumab, the patient remained relapse-free; however, she reported significant systemic pain and discomfort. Subsequently, she opted to switch to satralizumab, which was administered regularly until the current relapse, following a COVID-19 infection reported approximately two weeks prior to the relapse.
Current attack
Two patients (Patients 1 and 6) presented with LETM. Patient 1 had spinal cord lesions extending from C3 to T7, whereas Patient 6 had involvement from C1 to T2. The T2 sequence of magnetic resonance imaging (MRI) in both patients revealed hyperintense spinal cord signals accompanied by cord swelling.
Eight eyes from seven patients (Patients 2–5 and 7–9) were affected by ON. During the follow-up, ophthalmological examination data were missing for Patients 2 and 9 at Week 4. Orbital MRI was performed on five of these seven patients, revealing abnormal optic nerve signals with enhancement. Patient 7 exhibited bilateral involvement, while the other patients had unilateral ON.
Among all nine patients, the median nadir EDSS score prior to eculizumab treatment was 3 (range 2–8). Of the eight affected eyes, six exhibited a nadir BCVA below 0.1.
All nine patients received IVMP with an initial dose of 1000 mg, with two also undergoing TPE (Patient 1) or intravenous immunoglobulin (IVIg, Patient 2) before starting eculizumab (Figure 1). Patient 9 received two rounds of IVMP because of unsatisfactory visual recovery. The median time from symptom onset to eculizumab initiation was 10 (range 3–21) days.
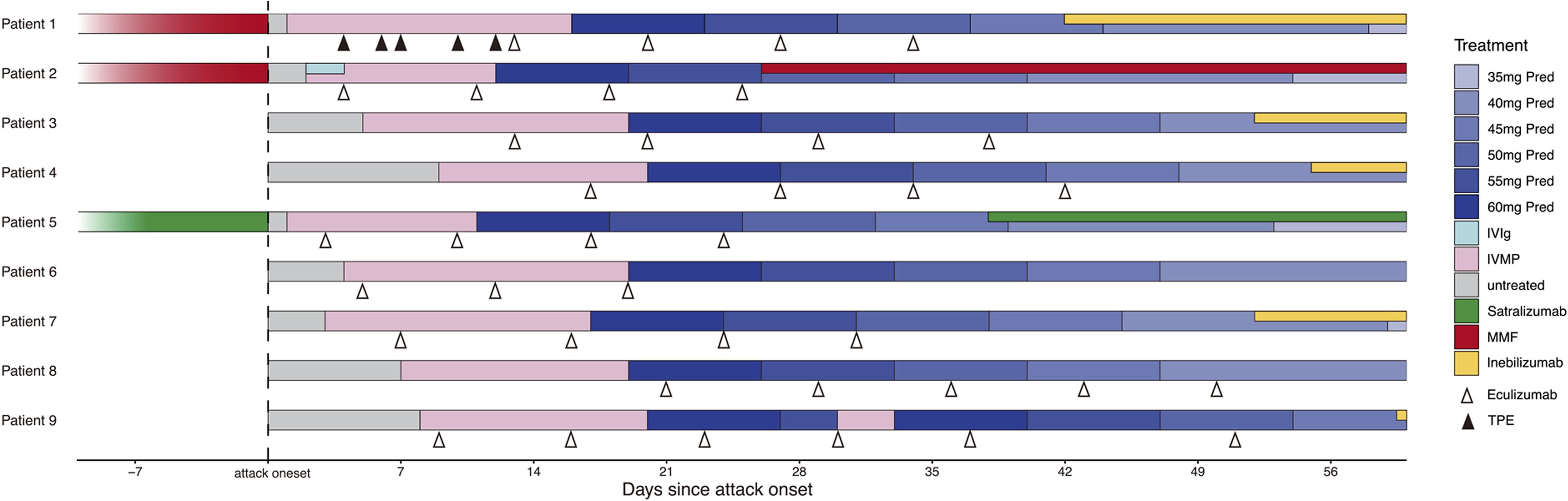
Figure 1. Treatment of the nine patients before and during NMOSD acute attack. Pred, prednisone; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone pulse; MMF, Mycophenolate mofetil; TPE, therapeutic plasma exchange.
Improvement in neurological function
Eight out of nine patients completed the full course of four weekly doses of eculizumab, while one patient (Patient 6) discontinued treatment after receiving three doses.
Myelitis
Patient 1, a 32-year-old female with LETM, began IVMP therapy one day after symptom onset, followed by five sessions of TPE. However, her lower extremity muscle strength remained at grade 0 after the final TPE session. Eculizumab therapy was initiated 13 days after symptom onset, leading to a gradual recovery of muscle strength. She received a total of four weekly doses of eculizumab, and by week 8, her lower extremity strength had improved to grade 3 proximally and grade 4 distally (Table 2). She was subsequently transitioned to the inebilizumab regimen. During a follow-up visit six months after the final dose of eculizumab, despite being beyond the observation window of this study, the patient was able to walk 500 meters independently.
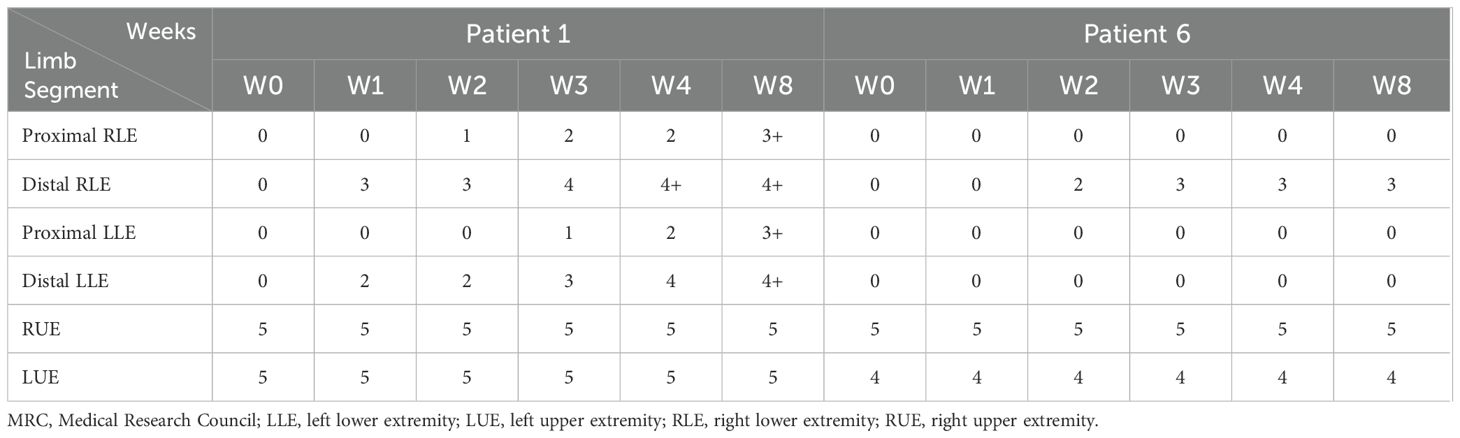
Table 2. Muscle strength changes by MRC scale score of Patient 1 and 6 after eculizumab treatment initiation.
Patient 6, a 66-year-old male, developed ascending myelitis involving nearly the entire cervical cord. He experienced complete paralysis of the lower limbs, left upper limb weakness, and a gradual ascension of the sensory level. His baseline sGFAP level was markedly elevated at 26,360.0 pg/mL (Figure 2A). IVMP therapy was initiated four days after symptom onset, followed by eculizumab on the second day after IVMP initiation. After starting eculizumab, no further progression of disability was observed. By week 2 of treatment, he regained movement in the distal right lower limb, coinciding with a sharp decline in sGFAP levels to 292.0 pg/mL. He continued weekly eculizumab treatments for three weeks. However, despite prophylactic antibiotic coverage, he developed a Klebsiella pneumoniae pneumonia, leading to the discontinuation of eculizumab therapy. Intravenous antibiotics were initiated and effectively controlled the infection. By week 8, muscle strength in his distal right extremities stabilized at grade 3 (Table 2).
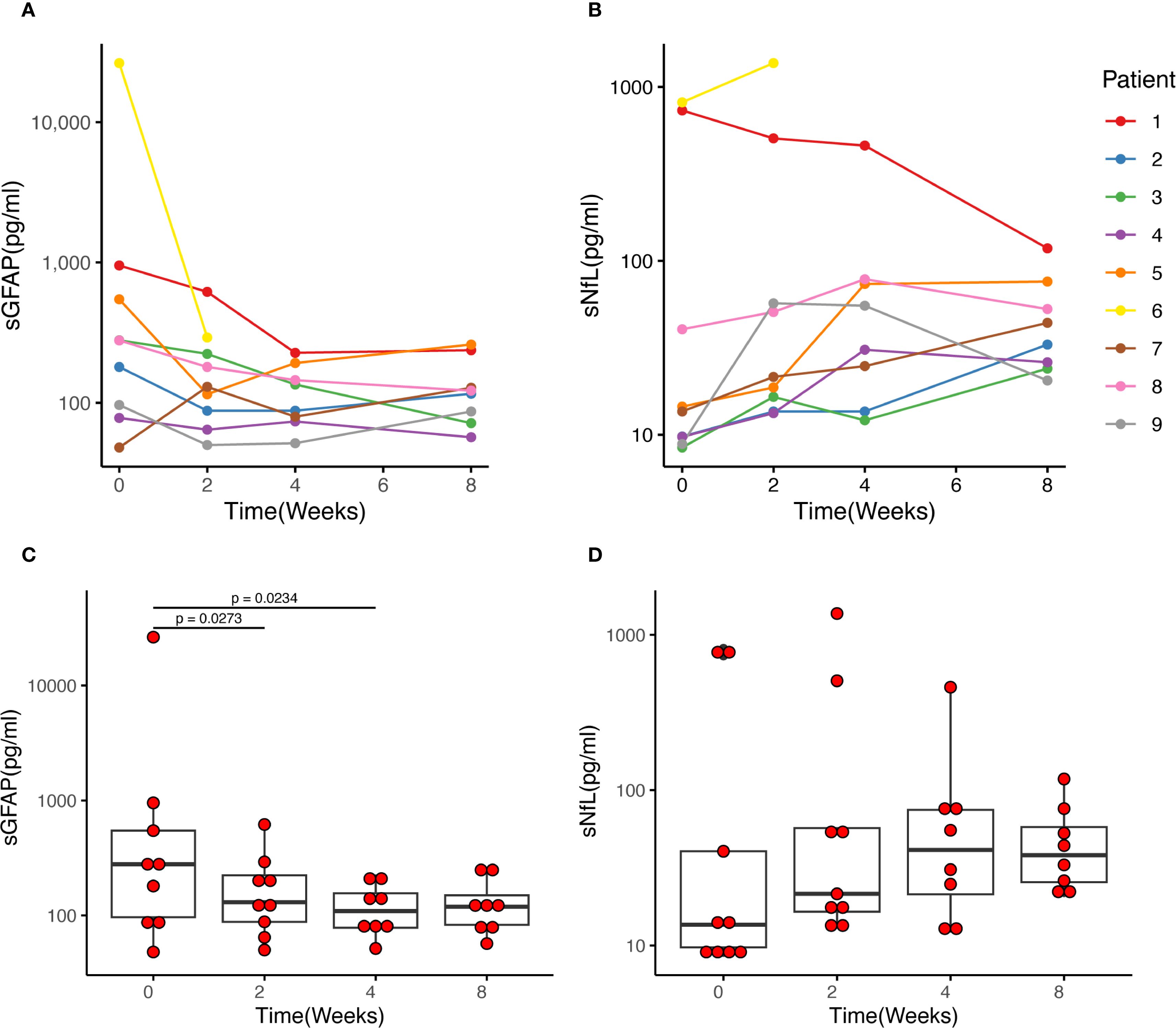
Figure 2. Changes in sGFAP and sNfL levels of each patient after eculizumab initiation. (A, C) The median sGFAP level was 278.0 pg/ml before eculizumab treatment and decreased to 130.0 pg/ml after 2 weeks of treatment (p = 0.027). Then the sGFAP levels tended to be stable, and there was no significant difference between Week 2 and Week 4, and week 4 and week 8. (B, D) The middle horizontal line represents the median, and the box represents the interquartile range. The sNfL levels showed an upward trend after the initiation of eculizumab treatment, reaching its peak at Week 4, then slightly decreased at Week 8, yet there was no statistically significant difference between each week. sGFAP, serum glial fibrillary acidic protein; sNfL, serum neurofilament light chain.
ON
Seven patients received eculizumab treatment during an ON attack, affecting eight eyes. Among them, four patients (Patients 2, 3, 7, and 8) demonstrated prominent visual recovery in five eyes, with BCVA improving to 1.0 and visual fields nearly returning to normal by Week 8. Notably, Patient 5, who had a history of recurrent ON attacks, also experienced restoration of both BCVA and the visual field in her left eye to baseline levels prior to this attack. In Patient 4, BCVA improved to 0.9, though some residual visual field defects persisted (Figure 3). Altogether, among the 8 affected eyes of the seven patients, the median visual field MD significantly improved from −22.4 dB (range −32.8 to −4.8) at Week 0 to −2.0 dB (range −30.7 to 1.4) at Week 8 (p = 0.008) (Figure 4A). The VFSS decreased from 5 (range 3 to 6) at Week 0 to 1 (rang 1 to 5) at Week 8 after treatment (p = 0.042) (Figure 5B).
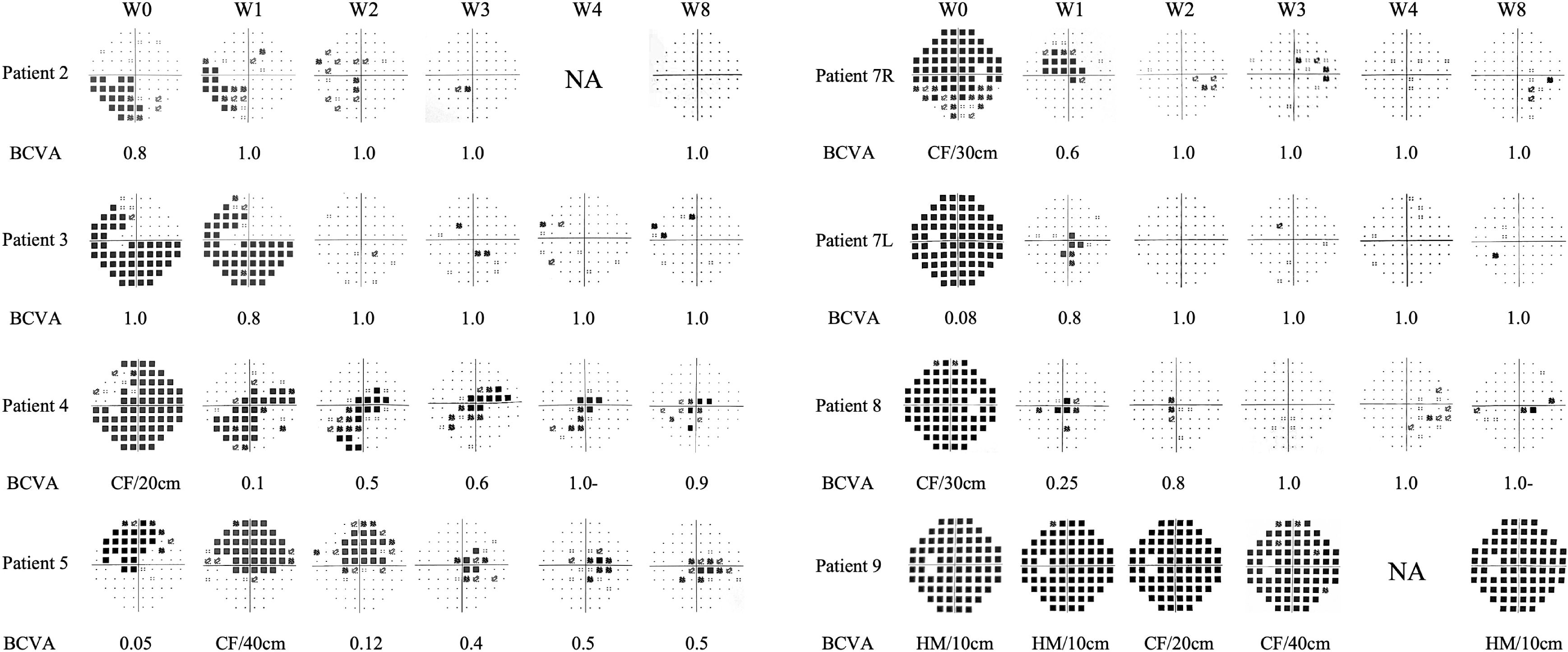
Figure 3. Changes of visual fields and BCVA in the seven patients with optic neuritis attack treated with eculizumab. The visual fields are primarily depicted using pattern deviation. For visual fields with severe depression where pattern deviation cannot be reliably calculated, total deviation is shown instead (for Patient 4, Patient 7L, Patient 7R, Patient 8 at W0, and Patient 9 at all time points). BCVA, best corrected visual acuity; CF, count finger; HM, hand movement; L, left eye; NA, not performed; R, right eye.
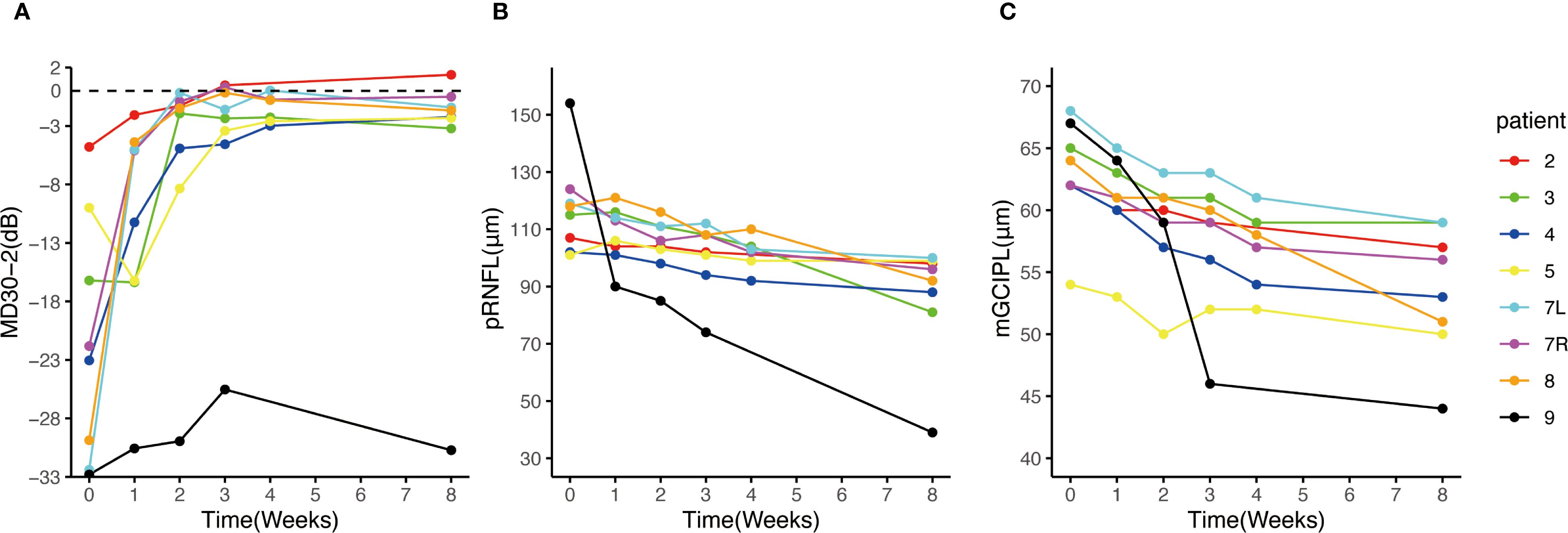
Figure 4. Change of visual field MD30-2, pRNFL and mGCIPL after eculizumab treatment initiation. (A) The MDs of the 8 eyes of the 7 patients with ON attack improved prominently after eculizumab treatment initiation; (B, C) the thickness of pRNFL and mGCIPL decreased over the 8-week observation period. Patient 9 exhibited faster pRNFL and mGCIPL atrophy compared to other patients. MD, mean deviation; mGCIPL, macular ganglion cell-inner plexiform layer; pRNFL, peripapillary retinal nerve fiber layer.
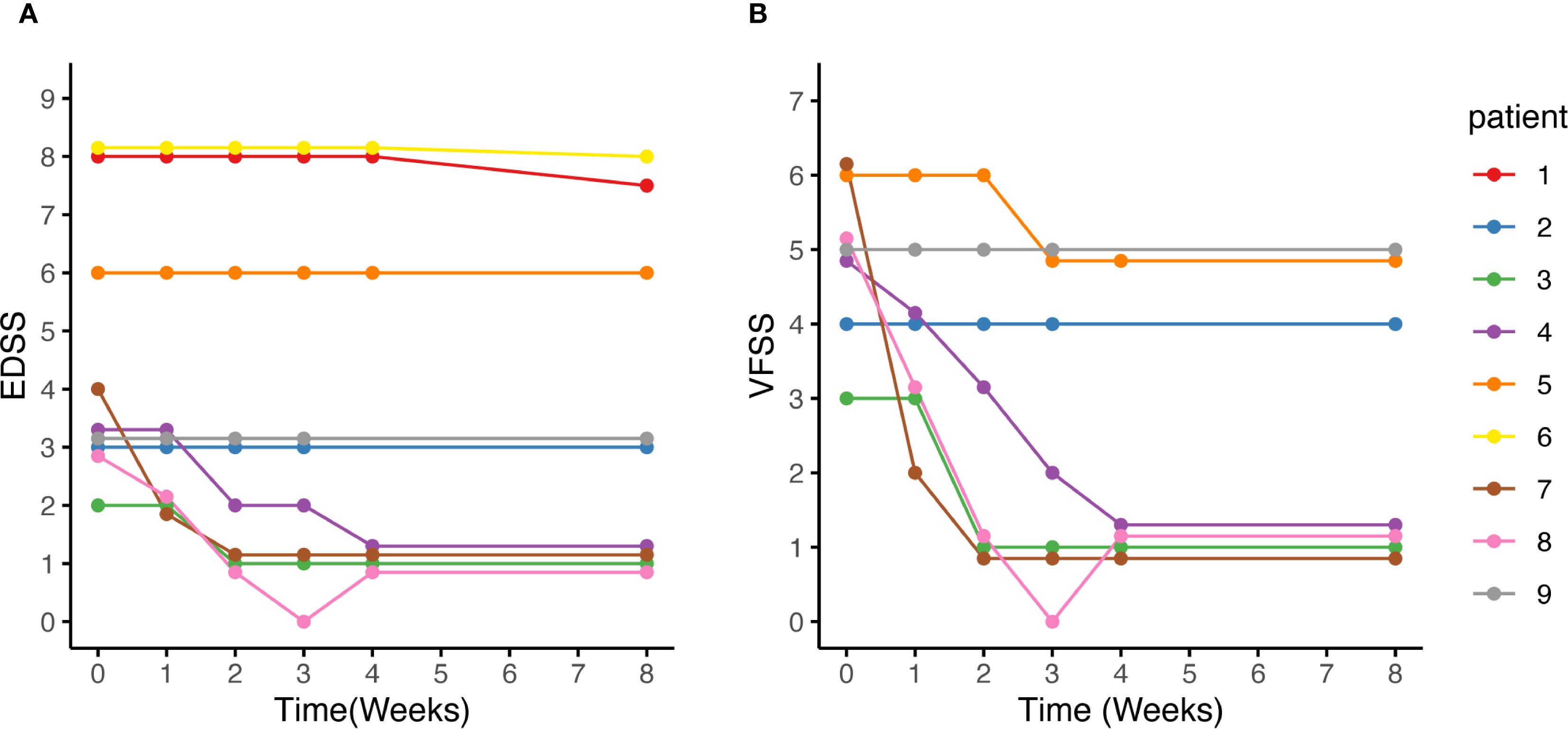
Figure 5. EDSS score, and VFSS changes after eculizumab initiation. (A, B) After eculizumab initiation, both the EDSS and VFSS scores demonstrated either improvement or stability. (B) Among the 7 patients with optic neuritis, Patient 2 had severe visual field defects and a BCVA of 0.12 in the contralateral eye due to a previous optic neuritis, while Patient 5 had only HM/10cm in the contralateral eye due to a previous optic neuritis. Consequently, no obvious changes were observed in the VFSS for the two patients. Patient 9 did not experience visual improvement, while the VFSS of the remaining patients decreased to 1. BCVA, best corrected visual acuity; EDSS, Expanded Disability Status Scale; HM: hand movements; VFSS, Visual Functional System Score.
Patient 9 was the only patient whose visual function did not recover. She began IVMP therapy on day 9 after symptom onset and started eculizumab on day 10. Prior to this, she underwent extensive diagnostic evaluations, including waiting for optic nerve MRI and AQP4 antibody results, during which her visual field continued to deteriorate (Supplementary Figure S1) and her visual acuity decreased to HM. IVMP was only initiated after she presented to our center. Her serum AQP4-IgG titer was very high (>1:1000), and MRI revealed a strikingly long-segment enhancement of the left optic nerve in the intraorbital region, with pronounced nerve thickening and tortuosity (Supplementary Figure S1). Her baseline pRNFL thickness was also greater than that of other patients. Despite two rounds of IVMP and eculizumab therapy, her visual function did not improve at week 8, and the improvement in MD was also unsatisfactory (Figure 3).
pRNFL and mGCIPL alteration
Through the initial weekly OCT examinations and a final examination on week 8, it was observed that despite all seven patients with an ON attack receiving IVMP and eculizumab treatment, both the mGCIPL and pRNFL exhibited progressive thinning over the course of eight weeks (Figure 4B, C). Specifically, the pRNFL thickness decreased from 116.5 μm (range 101 to 154) at Week 0 to 94 μm (range 39 to 100) at Week 8 (p = 0.008), while the mGCIPL thickness decreased from 63 μm (range 54 to 68) at Week 0 to 54.5 μm (range 44 to 59) at Week 8 (p = 0.014). In Patient 9, who showed poor visual recovery, a more rapid progression of retinal thinning was observed, with the rate faster than that of the other patients (Figure 4B, C). Compared to other patients, Patient 8 exhibited a faster rate of retinal atrophy during weeks 4–8. Correspondingly, we observed a slight decline in her vision and visual field at week 8 compared to week 4.
sGFAP and sNfL
Baseline sGFAP and sNfL levels in the two patients with acute myelitis were remarkably higher than those with ON. In the patients with acute myelitis, sGFAP levels were measured at 26,360.0 pg/ml (Patient 6) and 952.0 pg/ml (Patient 1), while sNfL levels were 816.0 pg/ml (Patient 6) and 733.0 pg/ml (Patient 1). The seven patients with ON exhibited a median sGFAP level of 180.0 (range 48.0–546.0) pg/ml and a median sNfL level of 9.8 (range 8.4–40.4) pg/ml.
Following eculizumab treatment, sGFAP levels showed a notable decline within four weeks. Statistically significant reductions were observed between Week 0 and Week 2 (median 278.0 vs 130.0 pg/ml, p = 0.027) and between Week 0 and Week 4 (median 278.0 vs 111.4 pg/ml, p = 0.023). However, no significant differences were noted between Week 2 and Week 4 (median 130.0 vs 111.4 pg/ml, p = 0.311), or Week 4 and Week 8 (median 111.4 vs 119.0 pg/ml, p = 0.461) (Figure 2A, C).
Conversely, sNfL levels exhibited an upward trend, peaking at Week 4 of eculizumab treatment and then stabilized. Despite this increase, no statistically significant differences were detected across various time points (Figure 2B, D).
Concomitant autoimmune condition
Patients 1 and 2 were diagnosed with systemic lupus erythematosus (SLE), while Patients 1 and 3 also had Sjögren’s syndrome (SS). Furthermore, Patient 6 developed thyrotoxicosis (TTX), which was likely induced by amiodarone therapy rather than being a primary autoimmune disorder. Throughout the treatment for NMOSD, all patients with comorbid autoimmune conditions demonstrated stable disease activity. No clinical deterioration or new exacerbations of their concurrent autoimmune diseases were observed during the follow-up period.
Safety
At the initiation of eculizumab therapy, oral antibiotic prophylaxis was administered to all nine patients. Specifically, Patients 1–5 and 7, who tested negative on penicillin skin testing, received phenoxymethylpenicillin potassium tablets. Patients 6 and 8, with positive penicillin skin tests, were prescribed oral cephalosporin antibiotics. Patient 9, with a documented cephalosporin allergy, was administered oral levofloxacin. Meningococcal vaccination was administered to four patients (Patients 1, 3-5) within two weeks following the first dose of eculizumab. The remaining four unvaccinated patients continued oral penicillin prophylaxis for two weeks after the completion of eculizumab therapy. No cases of meningococcal infection were observed during the study period.
Patient 1, who had spinal cord involvement and required urinary catheterization, developed a urinary tract infection during the fourth week of eculizumab therapy. Patient 6, who experienced a myelitis flare, developed Klebsiella pneumoniae pneumonia during the third week of eculizumab treatment and subsequently discontinued therapy. Following appropriate intravenous anti-biotics, the infections in both patients were effectively controlled. No adverse reactions were observed in patients with ON, and their vital signs, routine blood test results, and liver and renal function parameters remained within normal limits. None of the patients experienced hypertension, headache, diarrhea, nausea and vomiting, skin rash, anemia, or thrombocytopenia.
Maintenance therapy after eculizumab
Following acute treatment with eculizumab, five patients switched to inebilizumab as their maintenance therapy, while one patient received satralizumab, and another was treated with mycophenolate mofetil. Only one patient (Patient 8) continued eculizumab biweekly for recurrence prevention. One patient did not receive any additional preventive treatment aside from oral steroids (Figure 1). No relapses were recorded after eculizumab discontinuation.
Discussion
Traditional therapies for acute NMOSD attacks primarily involve IVMP, which works by suppressing immune activation, inhibiting cytokine production, stabilizing the blood-brain barrier, and reducing edema in the spinal cord and optic nerve. The peak anti-inflammatory effects typically occur within the first few days following administration. If minimal or no improvement is observed with IVMP, TPE is recommended. TPE functions by physically removing circulating pathogenic autoantibodies (e.g., anti-AQP4-IgG), complement factors, and pro-inflammatory mediators from the plasma, providing rapid immunological relief, usually with effects seen within the first few days to a week after treatment completion (18–20). However, structural recovery and functional improvements often take several weeks to months as local inflammation subsides, and remyelination or neuronal repair processes occur (21, 22).
Although solid evidences support eculizumab as a preventive therapy for NMOSD (13, 23), its potential role in treating acute attacks of NMOSD remains underexplored. Since complement activation plays a key role in driving neuroinflammation and tissue damage during NMOSD attacks, and eculizumab rapidly lowers free C5 concentrations below the threshold required for complete terminal complement inhibition within just 60 minutes of infusion (24), it is anticipated that eculizumab could also be beneficial in the acute phase of NMOSD.
To date, 13 cases have been reported in which eculizumab was administered early after the onset of an NMOSD attack, demonstrating its potential in mitigating the worsening of neurological disability associated with these attacks (Supplementary Table S1) (25–31). However, among these cases, only six received eculizumab within 30 days of attack onset. Moreover, key supportive evidence of effectiveness, such as dynamic changes in visual fields and retinal thickness, or neuroinjury-related biomarkers like sGFAP and sNfL were not investigated in these cases, leaving critical gaps in our understanding of eculizumab’s therapeutic potential in the acute phase.
In the current study, nine patients receiving eculizumab after 10 (range 3-21) days from the onset of acute attacks were analyzed. Seven with ON and two with myelitis attack. Over the subsequent 8-week observation period, the majority of cases showed improvement in disability.
Seven patients experiencing an ON attack initiated eculizumab treatment between the 3rd and 21st day after onset. Six of them demonstrated remarkable recovery of visual function. Among these, five patients regained visual acuity ranging from 0.9 to 1.0, with their visual fields largely or nearly fully restored. Additionally, one patient with a history of multiple ON episodes recovered both BCVA and visual field to their pre-attack status.
The dynamic changes in the pRNFL and mGCIPL following acute ON remain incompletely understood. In this acute-phase study, we longitudinally monitored these changes over time. Despite all patients receiving eculizumab in combination with high-dose corticosteroid pulse therapy, a progressive trend of retinal atrophy was still observed. Notably, in the only patient with poor visual recovery (Patient 9), both the rate and extent of retinal atrophy were more pronounced. This suggests that rapid and substantial retinal atrophy during the acute phase correlates with unfavorable visual outcomes. Furthermore, it indicates that once inflammation is triggered, secondary tissue degeneration appears to be inevitable, regardless of the intensity of therapeutic intervention. The current 8-week observation period may be insufficient to determine when retinal atrophy stabilizes (32). Future studies are warranted to further investigate this issue.
Patient 9, who exhibited poor visual recovery, highlights the necessity of timely intervention. Corticosteroid therapy was initiated on day 9 post-symptom onset, followed by eculizumab on day 10. Delayed treatment due to prolonged waiting for optic nerve MRI and AQP4-IgG results allowed progressive visual field deterioration, culminating in complete visual field loss upon arrival at our center. Her markedly elevated AQP4-IgG titer (>1:1000), pronounced optic nerve enhancement and swelling on MRI, and pRNFL thickening indicated severe inflammatory activity. We speculate that such aggressive inflammation has a critically narrow therapeutic window. Despite two courses of 1000 mg IVMP and eculizumab therapy, visual recovery remained poor, emphasizing the imperative for early intervention to improve outcomes.
Following treatment initiation, patients with myelitis exhibited varying degrees of muscle strength improvement. Patient 1 showed a reduction in EDSS score from 8 to 7.5. Patient 6 achieved disease stabilization, with no further sensory level ascension. Although the overall improvement in Patient 6 was modest, it is noteworthy that this elderly patient presented with potentially life-threatening LETM. The attainment of a favorable trajectory within the 8-week observation period is therefore clinically meaningful.
The speed of recovery from myelitis within the 8-week period appeared slower than that observed in ON, likely due to the greater lesion burden. However, beyond the initial phase of recovery, primarily driven by the resolution of inflammation and edema, it is anticipated that a prolonged period of neurological repair will occur over 6–24 months. During this phase, the optimization of alternative neural circuits through physical and occupational therapy may facilitate further functional improvement (22). Therefore, an 8-week observation period may be insufficient to fully assess recovery following acute myelitis. At six months post-attack, Patient 1 regained the ability to ambulate independently for 500 meters, although the effects of steroids and subsequent inebilizumab treatment cannot be excluded. It is important to acknowledge that while steroids, TPE, or eculizumab have relatively short-term immunological actions, their impact on functional recovery can unfold over months or even longer, highlighting the importance of adequate waiting time and follow-up. Unfortunately, Patient 6 was lost to follow-up.
Through this study, we were able to characterize the dynamics of sGFAP and sNfL following an acute attack. A distinct pattern emerged: sGFAP levels demonstrated a significant decline within the first two weeks of eculizumab treatment, after which the rate of decline slowed. In contrast, sNfL levels showed a gradual upward trend despite eculizumab treatment, suggesting the occurrence of delayed neuro-axonal damage secondary to astrocytopathy. This finding aligns with a recent report indicating that sGFAP levels peak within the first week, whereas sNfL levels peak at five weeks post-attack (33). However, due to the absence of a control group, we cannot confirm whether eculizumab attenuated the extent of the increase in sGFAP and sNfL levels.
No new safety concerns were identified in this cohort of acute attacks. This study demonstrated that eculizumab was generally well-tolerated for the treatment of NMOSD patients with acute ON. It is important to note that two patients with acute LETM developed urinary tract infections, and one patient developed a pulmonary infection. However, the potential association between these infections and NMOSD itself should also be considered, as severe myelitis is known to increase the risk of both pulmonary and urinary tract infections, especially in elderly patients. Notably, no cases of meningococcal infection were observed in this study.
In our cohort, only one patient underwent TPE prior to eculizumab administration, while the remainder received eculizumab alone. This pattern may reflect a clinical challenge: although TPE is supported by class I evidence particularly in corticosteroid-refractory myelitis (20), it presents logistical complexities when used alongside eculizumab. Specifically, TPE can remove circulating monoclonal antibodies such as eculizumab from plasma, necessitating a delay in eculizumab initiation if TPE is anticipated. Conversely, when TPE is required following eculizumab administration, re-dosing of eculizumab may be necessary to maintain therapeutic drug levels. Notably, substituting eculizumab for TPE may entail potential risk, particularly in severe or refractory cases where TPE has demonstrated benefit. Further comparative studies are needed to determine the optimal sequencing or combination of these therapies, given their potential pharmacokinetic interactions.
In this study, no relapse occurred following eculizumab discontinuation. However, prior evidence has highlighted a significant risk of rebound disease activity after discontinuation. For instance, Maillart et al. reported a severe relapse with tetraplegia in a patient who had been stable on eculizumab for four years but experienced a major attack 11 weeks after the last infusion during a switch to satralizumab (34). This observation is consistent with earlier studies: in an open-label trial, 5 out of 14 patients relapsed after stopping eculizumab, with two attacks occurring within three months (23). Additionally, another real-world report documented four early relapses in six patients who stopped eculizumab and switched to rituximab, despite initiating new therapies within six weeks (35). The proposed mechanism behind this rebound is the formation of the membrane attack complex, as complement activity resumes once eculizumab’s effect wanes—approximately 39 to 74 days after cessation (5 × half-life of 11.3 days). This “at-risk window” necessitates careful planning. These findings emphasize the importance of caution when discontinuing eculizumab, recommending immediate initiation of a new NMOSD therapy, ideally one with a rapid onset of effect, to mitigate rebound disease activity.
The primary limitation of this study is the absence of a control group, which complicates the exclusion of confounding factors, including therapeutic effects from concomitant acute treatments (corticosteroids, TPE, IVIg) and maintenance biologics (inebilizumab/satralizumab), as well as potential spontaneous improvement. Critically, the intermingled effects of these combined therapies challenge the isolation of eculizumab’s specific contribution to observed outcomes. Additional constraints include the small sample size, which limits generalizability, the short follow-up duration, which may miss long-term events, and possible selection bias from consent-driven decisions. Therefore, prospective randomized trials are needed to rigorously assess eculizumab’s efficacy and safety for acute AQP4-positive NMOSD attacks. If confirmed, early initiation could serve dual purposes: aiding recovery from the current attack and preventing future relapses. Additionally, ravulizumab, which has recently demonstrated efficacy in AQP4-positive NMOSD (36), may also emerge as a promising candidate for acute intervention, pending confirmation of a sufficiently rapid onset of action.
Conclusion
In conclusion, this study observed potentially favorable outcomes in patients treated with eculizumab during acute NMOSD attacks. However, careful monitoring for infection is warranted, particularly in elderly patients with severe myelitis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The current analysis (HIRB-KY2025-062), as well as the prospective NMOSD hospital registry (HIRB-KY2020-007) were approved by the Medical Ethics Committee of Huashan Hospital, respectively. The participants provided their written informed consent to participate in this study.
Author contributions
QZ: Formal Analysis, Writing – original draft, Investigation, Writing – review & editing, Data curation, Methodology. WH: Formal Analysis, Writing – review & editing, Writing – original draft, Data curation. JZ: Writing – review & editing, Writing – original draft, Data curation, Formal Analysis. ZW: Writing – original draft, Data curation, Writing – review & editing. LZ: Writing – original draft, Writing – review & editing, Data curation. XL: Writing – review & editing. YF: Writing – review & editing. LW: Writing – review & editing. YX: Writing – review & editing. JY: Writing – review & editing. MW: Writing – review & editing. HT: Project administration, Conceptualization, Formal Analysis, Supervision, Writing – review & editing, Investigation. CQ: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – review & editing, Investigation, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by the national natural science foundation of China (Grant No. 82171341).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1645401/full#supplementary-material
References
1. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, and Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. (2007) 6:805–15. doi: 10.1016/S1474-4422(07)70216-8
2. Wingerchuk DM and Lucchinetti CF. Neuromyelitis optica spectrum disorder. N Engl J Med. (2022) 387:631–9. doi: 10.1056/NEJMra1904655
3. Carnero Contentti E and Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammation. (2021) 18:208. doi: 10.1186/s12974-021-02249-1
4. Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Wegner B, et al. Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. (2016) 79:206–16. doi: 10.1002/ana.24554
5. Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. (2007) 69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce
6. Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. (2008) 65:913–9. doi: 10.1001/archneur.65.7.913
7. Miyamoto K, Minamino M, Kuwahara M, Tsujimoto H, Ohtani K, Wakamiya N, et al. Complement biomarkers reflect the pathological status of neuromyelitis optica spectrum disorders. Front Immunol. (2023) 14:1090548. doi: 10.3389/fimmu.2023.1090548
8. Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. (2007) 130:1194–205. doi: 10.1093/brain/awl371
9. Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. (2007) 130:1224–34. doi: 10.1093/brain/awm047
10. Kuroda H, Fujihara K, Takano R, Takai Y, Takahashi T, Misu T, et al. Increase of complement fragment C5a in cerebrospinal fluid during exacerbation of neuromyelitis optica. J Neuroimmunol. (2013) 254:178–82. doi: 10.1016/j.jneuroim.2012.09.002
11. Wang H, Wang K, Wang C, Qiu W, Lu Z, and Hu X. Increased soluble C5b-9 in CSF of neuromyelitis optica. Scand J Immunol. (2014) 79:127–30. doi: 10.1111/sji.12132
12. Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. (1996) 33:1389–401. doi: 10.1016/s0161-5890(96)00078-8
13. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. (2019) 381:614–25. doi: 10.1056/NEJMoa1900866
14. Singh P, Gao X, Kleijn HJ, Bellanti F, and Pelto R. Eculizumab pharmacokinetics and pharmacodynamics in patients with neuromyelitis optica spectrum disorder. Front Neurol. (2021) 12:696387. doi: 10.3389/fneur.2021.696387
15. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
16. General Administration of Standardization of China. Standard Logarithmic Vision Chart (GB/T 11533-2011). Beijing: China Standard Press (2011).
17. Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PloS One. (2012) 7:e34823. doi: 10.1371/journal.pone.0034823
18. Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, and Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. (2009) 15:487–92. doi: 10.1177/1352458508100837
19. Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, and Levy M. Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler. (2016) 22:185–92. doi: 10.1177/1352458515581438
20. Weinshenker BG, O’Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. (1999) 46:878–86. doi: 10.3310/hta21310
21. Calis M, Kirnap M, Calis H, Mistik S, and Demir H. Rehabilitation results of patients with acute transverse myelitis. Bratisl Lek Listy. (2011) 112:154–6.
22. Kessler RA, Mealy MA, and Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol. (2016) 18:2. doi: 10.1007/s11940-015-0387-9
23. Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. (2013) 12:554–62. doi: 10.1016/S1474-4422(13)70076-0
24. Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, Xhaard A, Rosain J, Castaneda DC, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. (2015) 125:775–83. doi: 10.1182/blood-2014-03-560540
25. Chatterton S, Parratt JDE, and Ng K. Eculizumab for acute relapse of neuromyelitis optica spectrum disorder: Case report. Front Neurol. (2022) 13:951423. doi: 10.3389/fneur.2022.951423
26. Kaneko K, Namioka Y, Ohyama A, Takai Y, Kuramori N, Nakazawa T, et al. Two cases of anti-aquaporin 4 antibody-positive neuromyelitis optica treated with eculizumab early after relapse. Neurol Ther. (2022) 39:731–5. doi: 10.15082/jsnt.39.4_731
27. Enriquez M, Rosenthal S, McLendon LA, Bennett JL, Piquet AL, and Kammeyer R. Efficacy of eculizumab in acute refractory pediatric neuromyelitis optica: A case report. Neuroimmunol Rep. (2024) 5:100213. doi: 10.1016/j.nerep.2024.100213
28. Gorriz D, Pérez-Miralles FC, Quintanilla-Bordás C, Alcalá C, Frasquet M, and Casanova B. Eculizumab for a catastrophic relapse in NMOSD: case report. Neurol Sci. (2024) 45:249–51. doi: 10.1007/s10072-023-06971-x
29. San-Galli A, Chaumont H, Bourgeois Q, Roge J, Lobjois Q, and Cabre P. Eculizumab as rescue therapy in a context of dramatic NMOSD attack: Report of two cases. Rev Neurol (Paris). (2024) 180:995–7. doi: 10.1016/j.neurol.2024.09.001
30. Watanabe M, Masaki K, Tanaka E, Matsushita T, and Isobe N. The efficacy of eculizumab in the acute phase of neuromyelitis optica spectrum disorder: A case series study. Cureus. (2024) 16:e73205. doi: 10.7759/cureus.73205
31. Soni RH, Garcia M, Oak E, Applbaum EJ, Rajagopalan L, Krupp LB, et al. Acute eculizumab treatment in a pediatric patient with AQP4-IgG+ NMOSD. Mult Scler. (2025) 31:612–4. doi: 10.1177/13524585241283650
32. Oertel FC, Zimmermann HG, Motamedi S, Bereuter C, Asseyer ES, Chien C, et al. Retinal changes after acute and late optic neuritis in aquaporin-4 antibody seropositive NMOSD. J Neuroophthalmol. (2024) 44:e554–e7. doi: 10.1097/WNO.0000000000001991
33. Kim SH, Gomes A, Schindler P, Hyun JW, Kim KH, Lee DE, et al. Blood-based biomarkers for identifying disease activity in AQP4-igG-positive neuromyelitis optica spectrum disorder. JAMA Neurol. (2025) 82:168–75. doi: 10.1001/jamaneurol.2024.4400
34. Maillart E, Dubessy AL, Shor N, Piljan M, Decombe R, Lubetzki C, et al. Severe relapse after switching from eculizumab to satralizumab in neuromyelitis optica spectrum disorder. Neurology. (2025) 104:e213399. doi: 10.1212/WNL.0000000000213399
35. Sen S, Tuncer A, Terzi M, Bunul SD, Ozen-Acar P, Altunrende B, et al. Severe disease reactivation in seropositive neuromyelitis optica spectrum disorders patients after stopping eculizumab treatment. Mult Scler Relat Disord. (2023) 79:104949. doi: 10.1016/j.msard.2023.104949
Keywords: eculizumab, neuromyelitis optica spectrum disorder, acute attack, neurofilament light, glial fibrillary acidic protein
Citation: Zhuang Q, Huang W, ZhangBao J, Wang Z, Zhou L, Li X, Fan Y, Wang L, Xiao Y, Yu J, Wang M, Tan H and Quan C (2025) Eculizumab for the acute attack of neuromyelitis optica spectrum disorder. Front. Immunol. 16:1645401. doi: 10.3389/fimmu.2025.1645401
Received: 11 June 2025; Accepted: 01 September 2025;
Published: 25 September 2025.
Edited by:
Rosaria Talarico, University of Pisa, ItalyReviewed by:
Victor M. Rivera, Baylor College of Medicine, United StatesFrancesco Patti, University of Catania, Italy
Amirali Ghahremani, North Khorasan University of Medical Sciences, Iran
Sedat Şen, Ondokuz Mayıs University, Türkiye
Copyright © 2025 Zhuang, Huang, ZhangBao, Wang, Zhou, Li, Fan, Wang, Xiao, Yu, Wang, Tan and Quan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Tan, aG9uZ21laV90YW4wMjNAMTYzLmNvbQ==; Chao Quan, Y2hhb19xdWFuQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Qingqing Zhuang
Qingqing Zhuang Wenjuan Huang
Wenjuan Huang Jingzi ZhangBao
Jingzi ZhangBao Zhouzhou Wang2,3
Zhouzhou Wang2,3 Lei Zhou
Lei Zhou Liang Wang
Liang Wang Yiqin Xiao
Yiqin Xiao Jian Yu
Jian Yu Hongmei Tan
Hongmei Tan Chao Quan
Chao Quan