- 1Department of Pathology, Affiliated Cancer Hospital of Dalian University of Technology (Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University), Shenyang, China
- 2Department of Thoracic Surgery, Affiliated Cancer Hospital of Dalian University of Technology (Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University), Shenyang, China
- 3Department of Central Laboratory, Affiliated Cancer Hospital of Dalian University of Technology (Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University), Shenyang, China
Cancer-associated fibroblasts (CAFs) are key components of the tumor microenvironment (TME) which promote drug resistance by remodeling the extracellular matrix, generating an immunosuppressive microenvironment, and activating metabolic signaling pathways. Nanomaterials provide an effective method for specifically targeting CAF-mediated drug resistance because of their unique targeted delivery capabilities, responsive release characteristics, and multifunctional integration. Here, we describe the mechanisms underlying the role of CAFs in drug resistance. The types of materials used and design principles are described, and examples of the application of nanomaterials for targeting CAFs are provided. Current challenges and future directions of nanomaterials targeting CAFs for reversing tumor drug resistance are also discussed to provide theoretical support for the development of effective nanotherapies aimed at reversing drug resistance in cancer.
1 Introduction
Tumor drug resistance is closely related to the dynamic regulation of the tumor microenvironment (TME) (1). The TME includes tumor cells and surrounding stromal cells, immune cells, and secreted factors, which together affect tumor growth, invasion and the response to treatment (1). Dynamic changes in the TME can promote tumor growth, hinder immune surveillance, and lead to treatment resistance in targeted therapy and immunotherapy (2). Cancer-associated fibroblasts (CAFs) are a heterogeneous group of cells that are abnormally activated in the tumor stroma and play a crucial role in the TME. Because of their heterogeneity, CAFs confer a drug resistance phenotype in tumor cells through many mechanisms, such as by acting as a physical barrier, affecting signaling pathways, or by affecting metabolic support and the immune microenvironment. Complex CAF-mediated drug resistance networks are challenging for traditional single-target therapy strategies.
Nanomaterials provide an innovative solution to this problem by virtue of their unique size effect, surface modification and functional integration advantages. Recent reviews highlight the critical role of CAFs in mediating tumor drug resistance and the promising applications of nanomaterials in targeting CAFs. Nanotherapeutic strategies include surface modification with CAF-specific ligands (e.g., FAP antibodies, peptide conjugates) for precision delivery, stimuli-responsive systems (pH/enzyme-sensitive nanoparticles) for controlled drug release, and multimodal platforms co-loading CAF inhibitors and chemotherapeutics (3).
Emerging evidence demonstrates that nanomaterials can normalize the tumor stroma, enhance drug penetration, and synergize with immunotherapy by reversing CAF-mediated immune suppression (4). Nanomaterials possess significant advantages, such as improved pharmacokinetics and biodistribution, long circulation, targeting, and controlled release. Improved pharmacokinetics/biodistribution: Nanomaterials (e.g., PEGylated polymeric micelles, lipid nanoparticles) overcome small-molecule limitations (short circulation, non-specific accumulation). They leverage the enhanced permeability and retention(EPR) effect to accumulate in CAF-rich tumor stroma, achieving a 40% higher tumor-to-plasma concentration ratio than free drugs (5). Long circulation: PEGylation reduces reticuloendothelial system(RES) clearance, extending nanocarrier circulation to 10–24 h (vs. 1–2 h for free drugs). This prolongs exposure, increasing tumor targeting chances (5, 6). Precision targeting: Ligand-modified nanocarriers (e.g., FAP antibody, RGD peptide) bind CAF markers (FAP, α-SMA), cutting liver/spleen accumulation by 50% vs. non-targeted carriers and avoiding normal fibroblast damage (5, 7). Controlled release: Stimuli-responsive systems (pH/ROS-sensitive micelles) release drugs (doxorubicin, TGF-β inhibitors) over 24–48 h in CAF-rich TME, maintaining effective concentrations to inhibit CAF activation/ECM synthesis (5, 7).
2 Biological characteristics of CAFs and mechanisms of CAF-mediated tumor drug resistance
2.1 Origin and heterogeneity of CAFs
CAFs are a group of abnormally activated fibroblasts in the TME that arise from a diversity of cells, including tissue-resident fibroblasts (8), mesenchymal stem cells (9, 10), epithelial cells (through epithelial-mesenchymal transition, EMT) (11), endothelial cells (through endothelial-mesenchymal transition), adipocytes and perivascular cells (12, 13). These precursor cells are transformed into activated CAFs in response to specific signaling factors in the TME, such as transforming growth factor (TGF)-β, stromal cell-derived factor 1, and interleukin (IL-6) (14, 15).
CAFs constitute a heterogeneous cell population that is divided into subtypes according to phenotype and function. Myofibroblast-like CAFs (myCAFs), which express high levels of α-SMA and collagen fibers, regulate extracellular matrix (ECM) remodeling and tumor mechanical stiffness (16, 17); they play an important role in pancreatic cancer, breast cancer, and other stromal tumors. Inflammatory CAFs (iCAFs) secrete pro-inflammatory factors such as IL-6 and CXCL1, activate the JAK/STAT3 pathway, recruit immunosuppressive cells such as MDSCs and M2 macrophages, and form an immune escape microenvironment (18). Antigen-presenting CAFs (apCAFs) express high levels of MHCII, which interacts with T cells and is involved in the responsiveness to immunotherapy (19). Lipid-rich CAFs, which promote tumor growth by transporting lipids, are induced in SETD2-deficient pancreatic cancer cells (20). SETD2 is a histone lysine methyltransferase whose deficiency leads to metabolic reprogramming of tumor cells and immune escape (21). Vascular CAFs localize predominantly to the core area of tumors and promote angiogenesis by secreting various factors such as vascular endothelial growth factor to support tumor growth and spread (22, 23).
2.2 Key mechanisms of CAF-mediated tumor drug resistance
CAFs are important components of the TME that promote drug resistance through multi-dimensional mechanisms, including the formation of physical barriers, activation of drug resistance pathways, activation of signaling pathways promoting dryness and invasion, generation of an immune escape microenvironment, maintenance of the drug resistance phenotype, and tumor cell resistance memory.
Physical barriers and drug delivery obstacles: CAFs secrete a large number of ECM components such as collagen, thereby increasing the stiffness of the matrix by regulating the composition and structure of the ECM. This results in the formation of a physical barrier that limits the penetration of chemotherapeutic drugs, which is one of the key causes of chemotherapy resistance in cancer (24, 25). CAF-secreted collagen decreases the concentration of doxorubicin in the tumor core; the resulting increase in matrix stiffness hinders drug diffusion, resulting in partial survival of tumor cells and induction of drug resistance (26, 27).
Metabolic reprogramming and energy support: lipid-rich CAFs provide fatty acids to tumor cells via ATP-binding cassette subfamily A member 8a (ABCA8a), enhancing their mitochondrial function, anti-metabolic drugs and mitochondrial targeting drugs (28). iCAFs and myCAFs upregulate GLUT1 and LDH, transport lactate to tumor cells through MCT4 (29), activate the HIF-1α pathway, induce the expression of drug resistance genes such as MDR1, and increase drug resistance in tumor cells (30, 31). CAFs increase glutathione (GSH) levels in cancer cells through secretion mediators, decrease drug-induced reactive oxygen species production and DNA damage, and promote chemotherapy resistance (32).
Signaling pathway activation and tumor cell survival: hepatocyte growth factor (HGF) secreted by CAFs activates the c-Met receptor and restores PI3K/Akt and MAPK/ERK signaling, leading to epidermal growth factor (EGFR)-tyrosine kinase inhibitor (TKI) resistance (33). Combination treatment with c-Met inhibitors increases the efficacy of EGFR-TKIs, thereby inhibiting tumor cell growth and proliferation (34) and delaying relapse in drug-resistant tumors (35). C-X-C Motif Chemokine Ligand 12 (CXCL12) secreted by CAFs binds to tumor cell CXCR4, thereby activating downstream signaling that enhances cancer stem cell (CSC) drying and invasion, leading to tumor progression and metastasis (36–38). Drug resistance in CSCs is acquired through multiple mechanisms, including overexpression of molecules such as aldehyde dehydrogenase 1 (ALDH1) and ATP-binding cassette subfamily G member 2 (ABCG2). ALDH1 is an enzyme involved in cellular redox reactions, and its high activity is closely related to tumor invasiveness and drug resistance (39, 40). ABCG2 is a transmembrane protein that pumps chemotherapy drugs out of cells, thereby reducing their cytotoxicity (41, 42). CAFs promote CSC enrichment by activating the CXCL12/CXCR4 axis and increase the migratory ability and drug resistance of tumor cells by inducing EMT, further aggravating the malignant progression of tumors (43, 44).
Immunosuppressive microenvironment and immunotherapy resistance: iCAFs secrete CCL2 to recruit MDSCs and inhibit T cell activity by secreting multiple inhibitory factors in the TME (45). IL-6 secreted by iCAFs promotes M2 macrophage polarization and inhibits CD8 +T cell function through the inhibitory factor IL-10 (46, 47). Although apCAFs express MHCII, they often cannot activate T cells effectively because of the expression of co-inhibitory molecules (PD-L1, CTLA-4) or defects in antigen processing, and they may even induce immune tolerance. For example, the combination of PD-L1 and PD-1 can lead to T cell failure and inhibit immune responses (48, 49). ApCAFs may be deficient in antigen processing. Although some cell types can continue to synthesize MHCII molecules after activation, they lack effective antigen processing mechanisms, resulting in ineffective presentation of exogenous antigens (50). Hypoxic conditions in the TME may also help tumor cells evade immune surveillance by downregulating the expression of MHCI and MHCII to limit antigen presentation (51).
Epigenetic regulation and drug resistance memory: the involvement of CAFs in drug resistance is mediated by several mechanisms including epigenetic reprogramming (DNA methylation and histone modification) (52). For example, activation of the YAP/TAZ signaling pathway can promote tumor cell growth (53), whereas histone deacetylase (HDAC) inhibitors increase histone acetylation by inhibiting HDAC activity, thereby affecting epigenetic reprogramming of CAFs (YAP/TAZ signaling pathway) and interfering with their role in promoting drug resistance (54). However, this process may trigger a compensatory mechanism of CAFs (through YAP/TAZ activation to counteract drug action) and conserve the drug resistance-promoting function (55). These processes highlight the complexity of CAF drug resistance mechanisms.
The involvement of CAFs in drug resistance suggests that targeting CAFs could be an important strategy to improve the efficacy of cancer treatment.
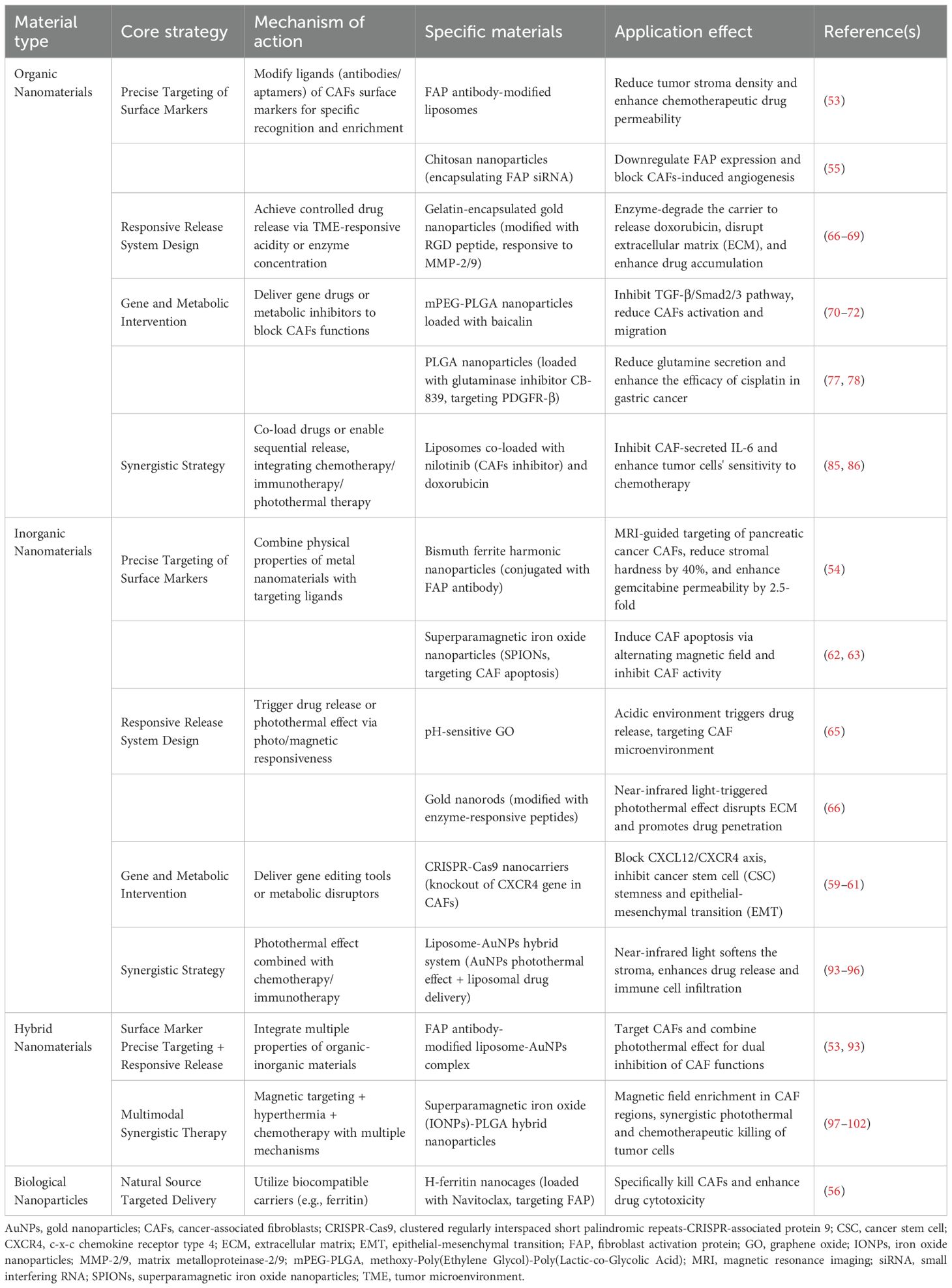
3 Types and core strategies of nanomaterials targeting CAFs
Nanomaterials are optimal carriers for targeting CAFs because of their unique physicochemical properties and biocompatibility. Nanomaterials can be divided into organic nanomaterials and inorganic nanomaterials according to their chemical composition and functional properties. Organic nanomaterials are carbon-based molecules including lipid-based nanoparticles, polymer nanoparticles, and dendrimers. They are biocompatible and can be designed to specifically recognize and bind to CAFs, which increases the efficacy of drug delivery and optimizes the therapeutic effects (56). Inorganic nanomaterials do not contain carbon and can modify the CAF microenvironment through optical and magnetic effects, such as metal nanomaterials and carbon-based nanomaterials. They can be developed into effective tools for targeting CAFs due to their excellent stability and easily modified surface characteristics (57). Other types of nanomaterials include biological nanoparticles and hybrid nanomaterials. Nanomaterial-based CAF targeting strategies aim to modulate CAF function and reverse tumor drug resistance through multiple pathways, including clearing and killing CAFs, inhibiting CAF activation and reprogramming, and disrupting CAF function.Detailed information regarding the types and core strategies of CAFs-targeting nanomaterials is shown in Table 1.
3.1 Precision targeting strategies for surface markers
CAF surface-specific markers (such as FAP, α-SMA, and PDGFRβ) are used to accurately identify and enrich CAFs through nanomaterial surface modification using targeting ligands (antibodies, aptamers, peptides). FAP antibody-modified liposomes can specifically target CAFs and decrease the density of the tumor stroma, thereby increasing membrane permeability and the accumulation of chemotherapeutic drugs (58). Bismuth ferrite harmonic nanoparticles conjugated with FAP antibody can be designed to target pancreatic cancer CAFs and release chemotherapy drugs, decreasing tumor interstitial hardness by 40%, and resulting in a 2.5-fold increase in gemcitabine penetration (59). FAP siRNA encapsulated in chitosan nanoparticles can be enriched in the tumor stroma through mucosal adhesion, where it downregulates FAP expression in CAFs and blocks angiogenesis (60). Navitoclax loaded with H-ferritin nanocages targets CAFs via FAP antibody fragments, which markedly increases the cytotoxicity of the drug (61). Navitoclax loaded nanoliposomes can also achieve selective apoptosis of CAFs by specifically binding to the tenascin C protein secreted by CAFs (62). A CRISPR-Cas9 nanovector designed to knock out the CXCR4 gene in CAFs blocks the effect of the CXCL12/CXCR4 axis on maintaining stem cell dryness and EMT induction (63–65). Some nanoparticles have the ability to kill CAFs, such as superparamagnetic iron oxide nanoparticles (SPIONs) that induce CAF apoptosis under the action of alternating magnetic fields (66), effectively inhibiting CAF activity and altering the TME (67). The precise targeting strategy of nanomaterials against CAF surface markers is illustrated in Figure 1A and summarized in Supplementary Table 1.
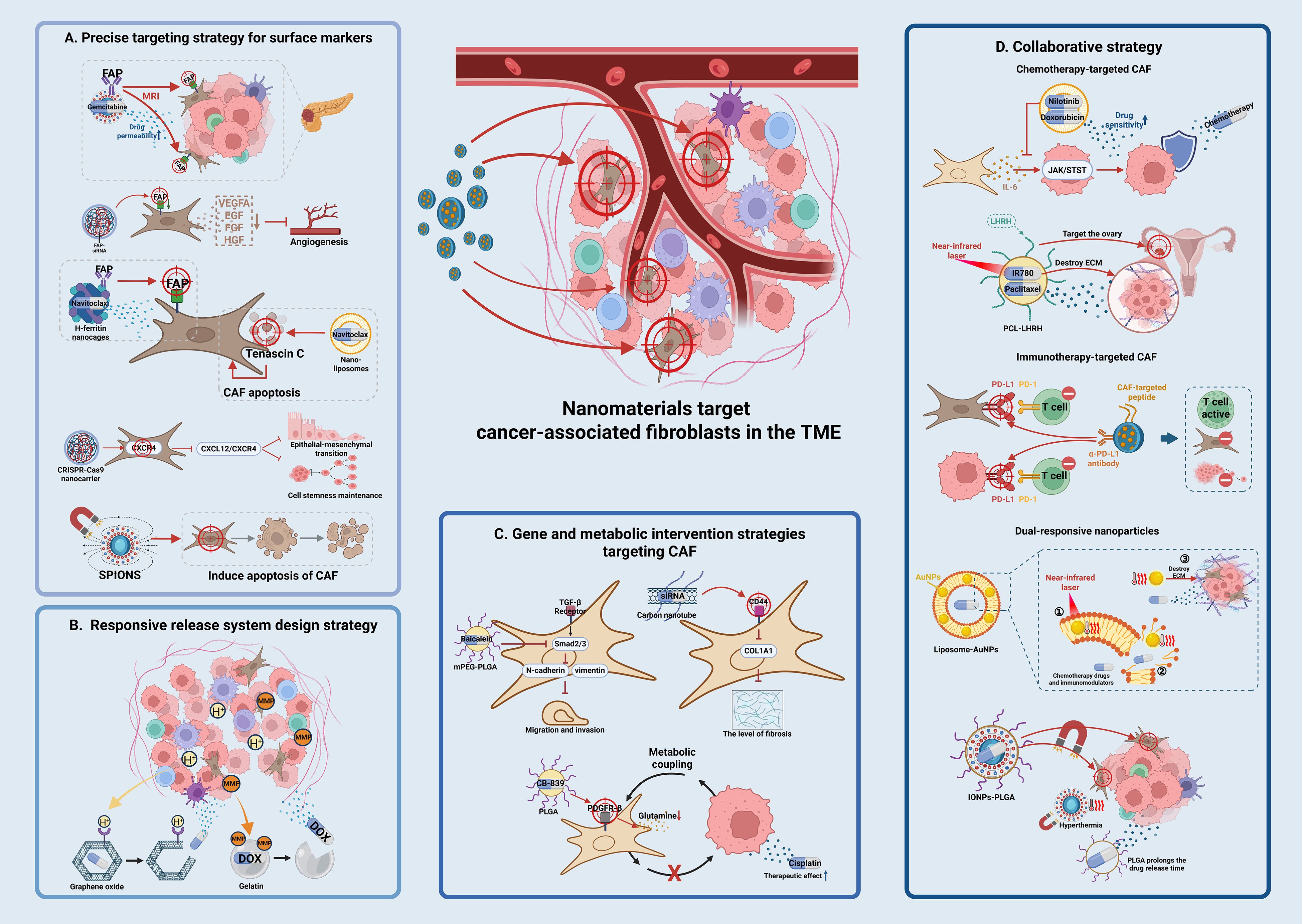
Figure 1. Schematic illustration of nanomaterial-based targeting strategies for CAFs in reversing tumor drug resistance. (A) Precision targeting strategies for surface markers nanomaterials are modified with ligands (e.g., antibodies, aptamers) to recognize CAF-specific surface markers such as FAP, α-SMA, or PDGFRβ. For example, FAP antibody-conjugated liposomes and bismuth ferrite nanoparticles enable specific binding to CAFs, reducing stromal density and enhancing drug penetration. CRISPR-Cas9 nanovectors are used to knock out CXCR4 in CAFs, disrupting the CXCL12/CXCR4 axis and inhibiting cancer stem cell properties. (B) Responsive release system design pH-sensitive nanoparticles (e.g., graphene oxide, GO) exploit the acidic tumor microenvironment (TME) to trigger drug release. Enzyme-responsive systems (e.g., MMP-2/9-degradable gelatin-coated gold nanoparticles) specifically degrade in the TME, releasing drugs like doxorubicin. These strategies enhance drug accumulation in tumors while minimizing systemic side effects. (C) Genetic and metabolic intervention strategies nanomaterials deliver gene drugs (e.g., siRNA, CRISPR-Cas9) or metabolic inhibitors to disrupt CAF functions. For instance, mPEG-PLGA nanoparticles loaded with baicalein inhibit TGF-β signaling, while PLGA nanoparticles carrying glutamine inhibitors (CB-839) target PDGFR-β to deplete energy supply for tumor cells. Carbon nanotubes with hyaluronic acid modification silence fibrosis-related genes (e.g., COL1A1) via CD44 receptor targeting. (D) Synergy strategies. Multifunctional nanosystems integrate chemotherapy, photothermal therapy, and immunotherapy. Liposomes co-loading nilotinib and doxorubicin suppress CAF-secreted IL-6 to enhance tumor cell sensitivity. Photothermal agents (e.g., IR780-loaded PCL nanoparticles) combined with near-infrared light disrupt the extracellular matrix, improving drug penetration. Dual-responsive nanoparticles sequentially release FAP inhibitors and chemotherapeutics, remodeling the TME and enhancing antitumor efficacy.
Current marker-based strategies (targeting FAP, α-SMA, PDGFRβ) face two core challenges due to CAF heterogeneity: non-universal expression across pro-tumor CAF subtypes and off-target expression in normal stromal cells. For non-universality, FAP, a classic marker, is primarily enriched in myCAFs but barely expressed in iCAFs (which secrete IL-6) or apCAFs (which express MHCII) (28). FAP-targeted nanocarriers only reduce stromal stiffness but fail to inhibit iCAF-mediated immunosuppression, leaving a 45% gemcitabine resistance rate in tumor cells (68). For off-target risks, α-SMA (a myCAF marker) is highly conserved in normal vascular smooth muscle and cardiac fibroblasts, leading to nanocarrier accumulation in the liver/spleen (16). PDGFRβ, expressed in multiple CAF subtypes, also exists in bone marrow mesenchymal stem cells and pericytes, preventing distinction between pro-tumor CAFs and normal stromal cells (22). Thus, single-marker strategies are insufficient, and future designs should use single-cell omics-identified subtype-specific markers (e.g., IL-6R for iCAFs) for “combinatorial marker recognition” to enhance specificity (61).
3.2 Responsive release system design strategy
Nanoparticles can utilize the pH, enzyme concentration, and other characteristics of the TME to achieve controlled drug release and improve targeting. pH-sensitive nanoparticles can exploit the acidic nature of the TME to achieve efficient drug delivery and release, thereby overcoming chemotherapy resistance and reducing side effects (69). The pH response characteristics of graphene oxide (GO) are particularly important. pH-sensitive prodrug molecules on the GO surface can be modified to achieve controlled release under acidic conditions, which is important for the CAF microenvironment in cancer therapy (70).
Matrix metalloproteinases (MMP-2/9) that are expressed at high levels in CAFs are used for specific drug delivery and targeted therapy in the TME. This strategy involves coating gold nanoparticles with enzyme-degradable carriers, such as gelatin modified with RGD peptides, to target CAFs and disrupt the ECM (71, 72). Gelatin-based nanoparticles can achieve controlled drug release through the action of MMP-2/9. These nanoparticles can be specifically degraded in the TME to release loaded anticancer drugs such as doxorubicin (DOX) and increase drug accumulation, thereby improving therapeutic efficacy in tumor cells (73, 74). In the MMP-2/9-responsive delivery system, the nanoparticle carrier does not directly disrupt the ECM; instead, its ECM-modulating effect follows an indirect chain: MMP-2/9 (highly expressed by CAFs) degrades the enzyme-sensitive carrier (gelatin-coated gold nanoparticles modified with RGD peptides), triggering the release of loaded drugs (DOX) (75). The released drugs then inhibit CAF activation/proliferation (e.g., downregulating collagen-synthesis gene COL1A1 in CAFs), reducing CAF-secreted ECM components (collagen, fibronectin) (76). Simultaneously, targeted elimination of CAFs impairs their ECM-remodeling ability, decreasing tumor stromal density, and ultimately achieving indirect ECM modulation. Responsive release system design strategy is illustrated in Figure 1B and summarized in Supplementary Table 2.
3.3 Genetic and metabolic intervention strategies
Nanomaterials can deliver gene drugs or metabolic inhibitors to block the ability of CAFs to promote drug resistance at the molecular level. For example, mPEG-PLGA loaded baicalein can block TGF-β signaling by inhibiting the phosphorylation of key proteins in the TGF-β signaling pathway such as Smad2/3, thereby inhibiting CAF activation (77, 78). Furthermore, baicalein can inhibit CAF migration and invasion by downregulating TGF-β expression and its downstream target genes such as N-cadherin and vimentin (77, 79). SPIONs delivering fibroblast growth factor 2 interferes with TGF-β1-induced CAF activation (68). Nanomaterials target myCAFs through “multivalent recognition-efficient endocytosis”, blocking the communication between CAFs and tumor cells and simultaneously loading gemcitabine and CXCL12 antagonists to block the drug resistance signaling pathway between CAFs and tumor cells (80, 81). The hollow structure of carbon nanotubes is suitable for carrying gene drugs such as siRNAs. Surface modification with hyaluronic acid is performed to target the CD44 receptor on the surface of CAFs; this achieves specific silencing of fibrosis-promoting genes such as COL1A1, thereby improving the delivery of siRNAs and increasing stability and targeting in vivo (82).
To interfere with the metabolic coupling between CAFs and tumor cells (such as lactic acid shuttle and glutamine metabolism) and deprive tumor cells of energy sources, PLGA nanoparticles loaded with glutamine inhibitor (CB-839) can target PDGFR-β on the CAF surface, decreasing glutamine secretion and increasing the efficacy of cisplatin in gastric cancer (83, 84). Functional nucleic acid (FNA)-based nanoplatforms combined with ZnO nanoparticles can deplete GSH in the TME, activate ferroptosis, and inhibit P-gp mediated drug efflux, thus overcoming multiple drug resistance (85–90). Genetic and metabolic intervention strategies is illustrated in Figure 1C and summarized in Supplementary Table 3.
3.4 Synergy strategies
Multiple therapeutic approaches can be integrated to synergistically reverse CAF-mediated resistance networks through co-loading or sequential release of nanomaterials. Combination treatments based on chemotherapy targeting CAFs inhibit CAF function and kill tumor cells simultaneously, blocking the protective effect of CAFs on tumor cells. For example, liposomes co-loaded with nilotinib (CAF activation inhibitor) and doxorubicin inhibit the secretion of IL-6 by CAFs, thereby impairing the protective effect of CAFs on tumor cells (91). IL-6 secreted by CAFs can inhibit tumor cell sensitivity to chemotherapy drugs through the JAK/STAT signaling pathway, and nilotinib enhances tumor cell sensitivity to doxorubicin by blocking the corresponding signaling pathway (92). PCL-luteinizing hormone-releasing hormone (LHRH) nanoparticles are a novel drug delivery system capable of loading paclitaxel and the photothermal agent IR780; this system targets ovarian cancer by modifying LHRH peptides. The system utilizes the photothermal effect of near-infrared light to destroy the tumor ECM, which increases drug penetration and release and improves the inhibitory rate in drug-resistant tumors (93). Dual-responsive nanoparticles can release FAP inhibitors in the TME to decrease ECM stiffness, followed by the release of chemotherapeutic drugs such as paclitaxel to kill tumor cells. This strategy is particularly applicable to paclitaxel-resistant ovarian cancer models because it can overcome drug resistance by altering the TME (94, 95).
Immune-targeting CAF combinations aim to reverse the CAF-mediated immunosuppressive microenvironment and enhance immune cell infiltration and function. PD-L1 is expressed on tumor cells and in CAFs, providing a theoretical basis for combined blockade of PD-L1/PD-1 signaling (96). α-PD-L1 antibodies are co-loaded with CAF targeting peptides in nanoparticles to specifically attack tumors and their microenvironments, ultimately improving antitumor immune responses (97). This combination strategy not only enhances CD8+T cell infiltration and activation, but also impairs tumor immune escape by inhibiting CAF function (98).
Dual-response nanoparticles (such as pH/enzyme dual response) and multi-drug co-loading with intelligent response synergy mediate the sequential release of CAF inhibitors and chemotherapy drugs to achieve “microenvironment remodeling and tumor killing”. In the liposome-AuNP hybrid system, the photothermal effect of AuNPs (gold nanoparticles) generates local high temperatures under near infrared light irradiation; this softens the tumor matrix and destroys its physical barrier, improving drug release and penetration (99, 100). Liposomes can carry chemotherapeutic drugs and immunomodulators, and drug release is triggered through a photothermal effect. The liposome-AuNP hybrid system is a promising cancer treatment strategy based on different synergy mechanisms that can effectively overcome physical and physiological obstacles in the TME to improve drug delivery and therapeutic effects (101, 102). Superparamagnetic iron oxide nanoparticles (IONPs)-poly (lactic acid-co-glycolic acid)(PLGA) hybrid nanoparticles are a novel nano-drug delivery system that combines magnetic targeting with sustained release of chemotherapeutic drugs; it can enrich in CAF regions and achieve synergistic killing via hyperthermia and chemotherapy (103, 104). PLGA acts as a drug carrier and prolongs the release time of chemotherapeutic drugs; the sustained release decreases the systemic toxicity of drugs (105, 106). With the introduction of IONPs, nanoparticles produce a thermal effect under an applied magnetic field, and the resulting synergistic effect of hyperthermia and chemotherapy improves the killing efficacy in tumor cells (107, 108). The synergistic strategies combining multiple therapeutic approaches is illustrated in Figure 1D and summarized in Supplementary Table 4.
5 Discussion
Targeting CAFs with nanomaterials provides an innovative pathway to reverse tumor drug resistance; however, its clinical translation is limited by a series of challenges. First, because of the heterogeneity of CAFs, identifying a single marker for all drug resistance subgroups is difficult, and there is overlap with normal fibroblasts, resulting in off-target risk. Second, inorganic materials are associated with liver and spleen toxicity; the biocompatibility of the degradation products of organic materials needs to be verified, and targeting ligands may cause immunogenicity. Third, uniformity and stability are difficult to achieve in the large-scale production of nanomaterials; real-time monitoring technology of in vivo targeting efficiency is insufficient, and elucidating the mechanisms underlying the effect of combination therapy requires additional study and biomarker guidance.
Among the nanomaterial-based strategies targeting CAFs, stimuli-responsive release systems and multimodal synergy strategies exhibit the most translational promise, and the reasons are as follows. First, unlike surface marker-based precision targeting limited by CAF heterogeneity and non-specific markers, these two strategies adapt to the dynamic TME. Stimuli-responsive systems (e.g., pH/MMP-2/9-sensitive nanoparticles) utilize inherent TME features for controlled drug release, avoiding off-target risks from static markers. Multimodal synergy (e.g., co-loading CAF inhibitors with chemotherapeutics/immunomodulators) remodels the TME while killing tumors, addressing single-target therapy limitations. Second, they align with clinical needs: some stimuli-responsive systems (e.g., pH-sensitive epirubicin micelles NC-6300) have entered Phase I trials (109), and multimodal strategies can combine with existing chemo/immunotherapies, reducing clinical transformation barriers.
The perspective on key hurdles and paths forward: The core bottleneck is not just “insufficient specificity” or “weak translational evidence”, but the overreliance on “static, single-target” thinking in traditional strategies. Future breakthroughs lie in shifting to “dynamic, subtype-specific intervention”. Leveraging single-cell omics to identify subtype markers (e.g., IL-6R for iCAFs) and AI to design “combinatorial marker-recognition” multivalent nanocarriers will solve heterogeneity issues. Meanwhile, using patient-derived organoids instead of animal models to verify targeting efficiency and safety can fill the “translational gap” between preclinical and clinical studies, which is a more debatable yet transformative path than incremental improvements to existing strategies. Only in this way can CAF-targeted nanodrugs be advanced from basic research to clinical application, truly providing a transformative therapeutic approach for overcoming tumor drug resistance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XG: Funding acquisition, Writing – original draft. LS: Writing – original draft. TL: Visualization, Writing – original draft. PL: Writing – original draft. CL: Funding acquisition, Writing – review & editing. QL: Writing – review & editing. YZ: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grants from the Liaoning Cancer Hospital and Institute, Dalian University of Technology “Medical industrial interdisciplinary research fund” (LD2023017, LD2023010, LD2023019), Liaoning Provincial Natural Science Foundation (2024-MS-271,2025-MS-336), Liaoning Province Science and Technology Plan Project (reference: 2023-MS-060, 2023021045-JH2/1017), Wu Jieping Medical Foundation Scientific Research Fund (reference: 320.6750.2024-03-29) and The Liaoning Provincial Medical Education Research Project (No. 2024-N002-04).
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1647988/full#supplementary-material
References
1. Xiao Y, Hassani M, Moghaddam MB, Fazilat A, Ojarudi M, and Valilo M. Contribution of tumor microenvironment (TME) to tumor apoptosis, angiogenesis, metastasis, and drug resistance. Med Oncol (Northwood London England). (2025) 42:108. doi: 10.1007/s12032-025-02675-8
2. Chou MY and Yang MH. Immunomodulation on tumor immune microenvironment in acquired targeted therapy resistance and implication for immunotherapy resistance. Trans Oncol. (2025) 54:102353. doi: 10.1016/j.tranon.2025.102353
3. Tang L, Mei Y, Shen Y, He S, Xiao Q, Yin Y, et al. Nanoparticle-mediated targeted drug delivery to remodel tumor microenvironment for cancer therapy. Int J Nanomedicine. (2021) 16:5811–29. doi: 10.2147/IJN.S321416
4. Feng B, Wu J, Shen B, Jiang F, and Feng J. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell Int. (2022) 22:166. doi: 10.1186/s12935-022-02599-7
5. Wen P, Ke W, Dirisala A, Toh K, Tanaka M, Li J, et al. Stealth and pseudo-stealth nanocarriers. Advanced Drug Delivery Rev. (2023) 198:114895. doi: 10.1016/j.addr.2023.114895
6. Cabral H, Li JJ, Miyata K, and Kataoka K. Controlling the biodistribution and clearance of nanomedicines. Nat Rev Bioeng. (2024) 2:214–32. doi: 10.1038/s44222-023-00138-1
7. Li J and Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation. J Am Chem Soc. (2021) 143:538–59. doi: 10.1021/jacs.0c09029
8. Xouri G and Christian S. Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol. (2010) 21:40–6. doi: 10.1016/j.semcdb.2009.11.017
9. Miyazaki Y, Oda T, Inagaki Y, Kushige H, Saito Y, Mori N, et al. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci Rep. (2021) 11:4690. doi: 10.1038/s41598-021-84058-3
10. Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. (2011) 19:257–72. doi: 10.1016/j.ccr.2011.01.020
11. Wang X, Sun X, Mu L, and Chen W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition in endometrial cancer cells by regulating pituitary tumor transforming gene. Cancer Invest. (2019) 37:134–43. doi: 10.1080/07357907.2019.1575969
12. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. (2013) 153:139–52. doi: 10.1016/j.cell.2013.02.021
13. Huang M, Liu M, Huang D, Ma Y, Ye G, Wen Q, et al. Tumor perivascular cell-derived extracellular vesicles promote angiogenesis via the Gas6/Axl pathway. Cancer Lett. (2022) 524:131–43. doi: 10.1016/j.canlet.2021.10.023
14. Dunér S, Lopatko Lindman J, Ansari D, Gundewar C, and Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. (2010) 10:673–81. doi: 10.1159/000320711
15. Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, and Apte MV. Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. (2016) 381:194–200. doi: 10.1016/j.canlet.2015.10.035
16. Li JP, Liu YJ, Wang SS, Lu ZH, Ye QW, Zhou JY, et al. EBF1-COX4I2 signaling axis promotes a myofibroblast-like phenotype in cancer-associated fibroblasts (CAFs) and is associated with an immunosuppressive microenvironment. Int Immunopharmacol. (2024) 139:112666. doi: 10.1016/j.intimp.2024.112666
17. Mieulet V, Garnier C, Kieffer Y, Guilbert T, Nemati F, Marangoni E, et al. Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer. Sci Rep. (2021) 11:4219. doi: 10.1038/s41598-021-83685-0
18. Jeong H, Koh J, Kim S, Yim J, Song SG, Kim H, et al. Cell-intrinsic PD-L1 signaling drives immunosuppression by myeloid-derived suppressor cells through IL-6/Jak/Stat3 in PD-L1-high lung cancer. J Immunotherapy Cancer. (2025) 13(3):e010612. doi: 10.1136/jitc-2024-010612
19. Song J, Wei R, Liu C, Zhao Z, Liu X, Wang Y, et al. Antigen-presenting cancer associated fibroblasts enhance antitumor immunity and predict immunotherapy response. Nat Commun. (2025) 16:2175. doi: 10.1038/s41467-025-57465-7
20. Papavassiliou KA and Papavassiliou AG. Hungry for fat: Metabolic crosstalk with lipid-rich CAFs fuels pancreatic cancer. Cell Metab. (2024) 36:1172–4. doi: 10.1016/j.cmet.2024.05.007
21. Niu N, Shen X, Zhang L, Chen Y, Lu P, Yang W, et al. Tumor cell-intrinsic SETD2 deficiency reprograms neutrophils to foster immune escape in pancreatic tumorigenesis. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2023) 10:e2202937. doi: 10.1002/advs.202202937
22. Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med (Cambridge Mass). (2011) 17:579–87. doi: 10.2119/molmed.2010.00157
23. Wang K, Andresen Eguiluz RC, Wu F, Seo BR, Fischbach C, and Gourdon D. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials. (2015) 54:63–71. doi: 10.1016/j.biomaterials.2015.03.019
24. Lovitt CJ, Shelper TB, and Avery VM. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer. (2018) 18:41. doi: 10.1186/s12885-017-3953-6
25. Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A, et al. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. (2014) 74:4239–46. doi: 10.1158/0008-5472.CAN-13-3494
26. Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, Affo S, et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest. (2021) 131(11):e146987. doi: 10.1172/JCI146987
27. Zhao C, Liu S, Gao F, Zou Y, Ren Z, and Yu Z. The role of tumor microenvironment reprogramming in primary liver cancer chemotherapy resistance. Front Oncol. (2022) 12:1008902. doi: 10.3389/fonc.2022.1008902
28. Niu N, Shen X, Wang Z, Chen Y, Weng Y, Yu F, et al. Tumor cell-intrinsic epigenetic dysregulation shapes cancer-associated fibroblasts heterogeneity to metabolically support pancreatic cancer. Cancer Cell. (2024) 42:869–884.e869. doi: 10.1016/j.ccell.2024.03.005
29. Jena BC, Das CK, Banerjee I, Bharadwaj D, Majumder R, Das S, et al. TGF-β1 induced autophagy in cancer associated fibroblasts during hypoxia contributes EMT and glycolysis via MCT4 upregulation. Exp Cell Res. (2022) 417:113195. doi: 10.1016/j.yexcr.2022.113195
30. Ke X, Fei F, Chen Y, Xu L, Zhang Z, Huang Q, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis. (2012) 33:1598–607. doi: 10.1093/carcin/bgs196
31. Luo F, Zou Z, Liu X, Ling M, Wang Q, Wang Q, et al. Enhanced glycolysis, regulated by HIF-1α via MCT-4, promotes inflammation in arsenite-induced carcinogenesis. Carcinogenesis. (2017) 38:615–26. doi: 10.1093/carcin/bgx034
32. Cheteh EH, Augsten M, Rundqvist H, Bianchi J, Sarne V, Egevad L, et al. Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death. Cell Death Dis. (2017) 8:e2848. doi: 10.1038/cddis.2017.225
33. Zhao Y, Wang H, and He C. Drug resistance of targeted therapy for advanced non-small cell lung cancer harbored EGFR mutation: from mechanism analysis to clinical strategy. J Cancer Res Clin Oncol. (2021) 147:3653–64. doi: 10.1007/s00432-021-03828-8
34. Wu YL, Soo RA, Locatelli G, Stammberger U, Scagliotti G, Park K, et al. Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non-small cell lung cancer? Cancer Treat Rev. (2017) 61:70–81. doi: 10.1016/j.ctrv.2017.10.003
35. Nakade J, Takeuchi S, Nakagawa T, Ishikawa D, Sano T, Nanjo S, et al. Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR mutation. J Thorac Oncol. (2014) 9:775–83. doi: 10.1097/JTO.0000000000000170
36. Chen CY, Yang SH, Chang PY, Chen SF, Nieh S, Huang WY, et al. Cancer-associated-fibroblast-mediated paracrine and autocrine SDF-1/CXCR4 signaling promotes stemness and aggressiveness of colorectal cancers. Cells. (2024) 13(16):1334. doi: 10.3390/cells13161334
37. Izumi D, Ishimoto T, Miyake K, Sugihara H, Eto K, Sawayama H, et al. CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer. Int J Cancer. (2016) 138:1207–19. doi: 10.1002/ijc.29864
38. López-Gil JC, Martin-Hijano L, Hermann PC, and Sainz Jr B. The CXCL12 crossroads in cancer stem cells and their niche. Cancers. (2021) 13(3):469. doi: 10.3390/cancers13030469
39. Kim N, Choung HK, Lee MJ, Khwarg SI, and Kim JE. Cancer stem cell markers in eyelid sebaceous gland carcinoma: high expression of ALDH1, CD133, and ABCG2 correlates with poor prognosis. Invest Ophthalmol Visual Sci. (2015) 56:1813–9. doi: 10.1167/iovs.14-15547
40. Martinez-Cruzado L, Tornin J, Santos L, Rodriguez A, García-Castro J, Morís F, et al. Aldh1 expression and activity increase during tumor evolution in sarcoma cancer stem cell populations. Sci Rep. (2016) 6:27878. doi: 10.1038/srep27878
41. Hong WC, Kim M, Kim JH, Kang HW, Fang S, Jung HS, et al. The FOXP1-ABCG2 axis promotes the proliferation of cancer stem cells and induces chemoresistance in pancreatic cancer. Cancer Gene Ther. (2025) 32(5):563–72. doi: 10.1038/s41417-025-00896-7
42. Ramović Hamzagić A, Cvetković D, Gazdić Janković M, et al. Modeling 5-FU-induced chemotherapy selection of a drug-resistant cancer stem cell subpopulation. Curr Oncol (Toronto Ont). (2024) 31:1221–34. doi: 10.3390/curroncol31030091
43. Ko YS, Jin H, Lee JS, Park SW, Chang KC, Kang KM, et al. Radioresistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol Rep. (2018) 40:3752–62. doi: 10.3892/or.2018.6714
44. Zhao Z and Zhu Y. FAP, CD10, and GPR77-labeled CAFs cause neoadjuvant chemotherapy resistance by inducing EMT and CSC in gastric cancer. BMC Cancer. (2023) 23:507. doi: 10.1186/s12885-023-11011-0
45. Oo MW, Kawai H, Takabatake K, Tomida S, Eguchi T, Ono K, et al. Resident stroma-secreted chemokine CCL2 governs myeloid-derived suppressor cells in the tumor microenvironment. JCI Insight. (2022) 7(1):e148960. doi: 10.1172/jci.insight.148960
46. Huang J, Xiao R, Shi S, Li Q, Li M, Xiao M, et al. Circulating IL6 is involved in the infiltration of M2 macrophages and CD8+ T cells. Sci Rep. (2025) 15:8681. doi: 10.1038/s41598-025-92817-9
47. Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, and Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. (2009) 284:34342–54. doi: 10.1074/jbc.M109.042671
48. Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R, et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell. (2022) 40:656–673.e657. doi: 10.1016/j.ccell.2022.04.011
49. Tekguc M, Wing JB, Osaki M, Long J, and Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci United States America. (2021) 118(30):e2023739118. doi: 10.1073/pnas.2023739118
50. Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. (2008) 9:1244–52. doi: 10.1038/ni.1665
51. Sethumadhavan S and Silva M. Philbrook P et al: Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PloS One. (2017) 12:e0187314. doi: 10.1371/journal.pone.0187314
52. Mishra R, Haldar S, Suchanti S, and Bhowmick NA. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocrine-Related Cancer. (2019) 26:R673–88. doi: 10.1530/ERC-19-0347
53. Pocaterra A, Romani P, and Dupont S. YAP/TAZ functions and their regulation at a glance. J Cell Sci. (2020) 133(2):jcs230425. doi: 10.1242/jcs.230425
54. Hu Z, Wei F, Su Y, Wang Y, Shen Y, Fang Y, et al. Histone deacetylase inhibitors promote breast cancer metastasis by elevating NEDD9 expression. Signal Transduction Targeted Ther. (2023) 8:11. doi: 10.1038/s41392-022-01221-6
55. Zanconato F, Battilana G, Cordenonsi M, and Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. (2016) 29:26–33. doi: 10.1016/j.coph.2016.05.002
56. Zhang Z, Wang R, and Chen L. Drug delivery system targeting cancer-associated fibroblast for improving immunotherapy. Int J Nanomedicine. (2025) 20:483–503. doi: 10.2147/IJN.S500591
57. Pei Z, Lei H, and Cheng L. Bioactive inorganic nanomaterials for cancer theranostics. Chem Soc Rev. (2023) 52:2031–81. doi: 10.1039/D2CS00352J
58. Duan H, Liu C, Hou Y, Liu Y, Zhang Z, Zhao H, et al. Sequential delivery of quercetin and paclitaxel for the fibrotic tumor microenvironment remodeling and chemotherapy potentiation via a dual-targeting hybrid micelle-in-liposome system. ACS Appl Materials Interfaces. (2022) 14:10102–16. doi: 10.1021/acsami.1c23166
59. Rajaee A, Wensheng X, Zhao L, Wang S, Liu Y, Wu Z, et al. Multifunctional bismuth ferrite nanoparticles as magnetic localized dose enhancement in radiotherapy and imaging. J Biomed Nanotechnology. (2018) 14:1159–68. doi: 10.1166/jbn.2018.2553
60. Zhou S, Zhen Z, Paschall AV, Xue L, Yang X, Bebin-Blackwell AG, et al. FAP-targeted photodynamic therapy mediated by ferritin nanoparticles elicits an immune response against cancer cells and cancer associated fibroblasts. Advanced Funct Materials. (2021) 31(7):2007017. doi: 10.1002/adfm.202007017
61. Sitia L, Bonizzi A, Mazzucchelli S, Negri S, Sottani C, Grignani E, et al. Selective targeting of cancer-associated fibroblasts by engineered H-ferritin nanocages loaded with navitoclax. Cells. (2021) 10(2):328. doi: 10.3390/cells10020328
62. Chen B, Wang Z, Sun J, Song Q, He B, Zhang H, et al. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine: Nanotechnology Biology Med. (2016) 12:131–41. doi: 10.1016/j.nano.2015.10.001
63. Teicher BA and Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. (2010) 16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329
64. Wang X, Zhang W, Ding Y, Guo X, Yuan Y, and Li D. CRISPR/Cas9-mediated genome engineering of CXCR4 decreases the Malignancy of hepatocellular carcinoma cells in vitro and in vivo. Oncol Rep. (2017) 37:3565–71. doi: 10.3892/or.2017.5601
65. Zhou Y, Cao HB, Li WJ, and Zhao L. The CXCL12 (SDF-1)/CXCR4 chemokine axis: Oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Natural Medicines. (2018) 16:801–10. doi: 10.1016/S1875-5364(18)30122-5
66. Teo P, Wang X, Chen B, Zhang H, Yang X, Huang Y, et al. Complex of TNF-α and modified fe(3)O(4) nanoparticles suppresses tumor growth by magnetic induction hyperthermia. Cancer Biotherapy Radiopharmaceuticals. (2017) 32:379–86. doi: 10.1089/cbr.2017.2404
67. Sun H, Wang X, Guo Z, Hu Z, Yin Y, Duan S, et al. Fe(3)O(4) nanoparticles that modulate the polarisation of tumor-associated macrophages synergize with photothermal therapy and immunotherapy (PD-1/PD-L1 inhibitors) to enhance anti-tumor therapy. Int J Nanomedicine. (2024) 19:7185–200. doi: 10.2147/IJN.S459400
68. Mardhian DF, Vrynas A, Storm G, Bansal R, and Prakash J. FGF2 engineered SPIONs attenuate tumor stroma and potentiate the effect of chemotherapy in 3D heterospheroidal model of pancreatic tumor. Nanotheranostics. (2020) 4:26–39. doi: 10.7150/ntno.38092
69. Xu Z, Yang D, Long T, Yuan L, Qiu S, Li D, et al. pH-Sensitive nanoparticles based on amphiphilic imidazole/cholesterol modified hydroxyethyl starch for tumor chemotherapy. Carbohydr Polymers. (2022) 277:118827. doi: 10.1016/j.carbpol.2021.118827
70. Zaboli A, Raissi H, Hashemzadeh H, and Farzad F. Graphene Oxide Hosting a pH-Sensitive Prodrug: An In Silico Investigation of Graphene Oxide-Based Nanovehicle toward Cancer Therapy. ACS Appl Bio Materials. (2023) 6:2826–36. doi: 10.1021/acsabm.3c00276
71. Wu L, Lin B, Yang H, Chen J, Mao Z, Wang W, et al. Enzyme-responsive multifunctional peptide coating of gold nanorods improves tumor targeting and photothermal therapy efficacy. Acta Biomaterialia. (2019) 86:363–72. doi: 10.1016/j.actbio.2019.01.026
72. Zhou J, Wang M, Ying H, Su D, Zhang H, Lu G, et al. Extracellular matrix component shelled nanoparticles as dual enzyme-responsive drug delivery vehicles for cancer therapy. ACS Biomaterials Sci Eng. (2018) 4:2404–11. doi: 10.1021/acsbiomaterials.8b00327
73. Qi A, Deng L, Liu X, Wang S, Zhang X, Wang B, et al. Gelatin-encapsulated magnetic nanoparticles for pH, redox, and enzyme multiple stimuli-responsive drug delivery and magnetic resonance imaging. J Biomed Nanotechnology. (2017) 13:1386–97. doi: 10.1166/jbn.2017.2433
74. Vaghasiya K, Ray E, Singh R, Jadhav K, Sharma A, Khan R, et al. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Materials Sci Eng C Materials Biol Appl. (2021) 123:112027. doi: 10.1016/j.msec.2021.112027
75. Yao H, Guo X, Zhou H, Ren J, Li Y, Duan S, et al. Mild acid-responsive "Nanoenzyme capsule" Remodeling of the tumor microenvironment to increase tumor penetration. ACS Appl Materials Interfaces. (2020) 12:20214–27. doi: 10.1021/acsami.0c03022
76. Mishra M, Mishra M, and Dutta S. Dual enzyme-encapsulated materials for biological cascade chemistry and synergistic tumor starvation. Chem (Weinheim an der Bergstrasse Germany). (2024) 30:e202400195. doi: 10.1002/chem.202400195
77. Chen F, Zhuang M, Peng J, Wang X, Huang T, Li S, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol Med Rep. (2014) 10:1999–2003. doi: 10.3892/mmr.2014.2452
78. Zhang YF, Zhou SZ, Cheng XY, Yi B, Shan SZ, Wang J, et al. Baicalein attenuates hypertrophic scar formation via inhibition of the transforming growth factor-β/Smad2/3 signalling pathway. Br J Dermatol. (2016) 174:120–30. doi: 10.1111/bjd.14108
79. Li J, Liu H, Lin Q, Chen H, Liu L, Liao H, et al. Baicalin suppresses the migration and invasion of breast cancer cells via the TGF-β/lncRNA-MALAT1/miR-200c signaling pathway. Medicine. (2022) 101:e29328. doi: 10.1097/MD.0000000000029328
80. Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, and Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. (2011) 17:2074–80. doi: 10.1158/1078-0432.CCR-10-2636
81. Li X, Bu W, Meng L, Liu X, Wang S, Jiang L, et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp Cell Res. (2019) 378:131–8. doi: 10.1016/j.yexcr.2019.03.013
82. Varshosaz J and Taymouri S. Hollow inorganic nanoparticles as efficient carriers for siRNA delivery: A comprehensive review. Curr Pharm Design. (2015) 21:4310–28. doi: 10.2174/1381612821666150901103937
83. Li Z, Li X, Lu Y, Zhu X, Zheng W, Chen K, et al. Improved photodynamic therapy based on glutaminase blockage via tumor membrane coated CB-839/IR-780 nanoparticles. Small (Weinheim an der Bergstrasse Germany). (2024) 20:e2305174. doi: 10.1002/smll.202305174
84. Usart M, Hansen N, Stetka J, Almeida Fonseca T, Guy A, Kimmerlin Q, et al. The glutaminase inhibitor CB-839 targets metabolic dependencies of JAK2-mutant hematopoiesis in MPN. Blood Adv. (2024) 8:2312–25. doi: 10.1182/bloodadvances.2023010950
85. Jiao X, Wang Z, Wang F, and Wen Y. Dual stimuli-responsive controlled release nanocarrier for multidrug resistance cancer therapy. Chemphyschem. (2019) 20:3271–5. doi: 10.1002/cphc.201900935
86. Wang M, Zhang Z, Li Q, Liu R, Li J, and Wang X. Multifunctional nanoplatform with near-infrared triggered nitric-oxide release for enhanced tumor ferroptosis. J Nanobiotechnology. (2024) 22:656. doi: 10.1186/s12951-024-02942-2
87. Wang X, Zhao Y, Hu Y, Fei Y, Zhao Y, Xue C, et al. Activatable biomineralized nanoplatform remodels the intracellular environment of multidrug-resistant tumors for enhanced ferroptosis/apoptosis therapy. Small (Weinheim an der Bergstrasse Germany). (2021) 17:e2102269. doi: 10.1002/smll.202102269
88. Wei Y, Xia H, Zhang F, Wang K, Luo P, Wu Y, et al. Theranostic nanoprobe mediated simultaneous monitoring and inhibition of P-glycoprotein potentiating multidrug-resistant cancer therapy. Analytical Chem. (2019) 91:11200–8. doi: 10.1021/acs.analchem.9b02118
89. Zhang M, Xu H, Wu X, Chen B, Gong X, and He Y. Engineering dual-responsive nanoplatform achieves copper metabolism disruption and glutathione consumption to provoke cuproptosis/ferroptosis/apoptosis for cancer therapy. ACS Appl Materials Interfaces. (2025) 17(14):20726–40. doi: 10.1021/acsami.4c22546
90. Zhi S, Zhang X, Zhang J, Wang XY, and Bi S. Functional nucleic acids-engineered bio-barcode nanoplatforms for targeted synergistic therapy of multidrug-resistant cancer. ACS Nano. (2023) 17:13533–44. doi: 10.1021/acsnano.3c02009
91. Lee YE, Go GY, Koh EY, Yoon HN, Seo M, Hong SM, et al. Synergistic therapeutic combination with a CAF inhibitor enhances CAR-NK-mediated cytotoxicity via reduction of CAF-released IL-6. J Immunotherapy Cancer. (2023) 11(2):e006130. doi: 10.1136/jitc-2022-006130
92. Cheteh EH, Sarne V, Ceder S, Bianchi J, Augsten M, Rundqvist H, et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. (2020) 6:42. doi: 10.1038/s41420-020-0272-5
93. Pan Q, Tian J, Zhu H, Hong L, Mao Z, Oliveira JM, et al. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomaterials Sci Eng. (2020) 6:2175–85. doi: 10.1021/acsbiomaterials.0c00163
94. Coluccia M, Parisse V, Guglielmi P, Giannini G, and Secci D. Metal-organic frameworks (MOFs) as biomolecules drug delivery systems for anticancer purposes. Eur J Medicinal Chem. (2022) 244:114801. doi: 10.1016/j.ejmech.2022.114801
95. Li M, Zhang F, Su Y, Zhou J, and Wang W. Nanoparticles designed to regulate tumor microenvironment for cancer therapy. Life Sci. (2018) 201:37–44. doi: 10.1016/j.lfs.2018.03.044
96. Tanaka T, Koga H, Suzuki H, Iwamoto H, Sakaue T, Masuda A, et al. Anti-PD-L1 antibodies promote cellular proliferation by activating the PD-L1-AXL signal relay in liver cancer cells. Hepatol Int. (2024) 18:984–97. doi: 10.1007/s12072-023-10572-3
97. Wang X, Jing Z, Huang X, Liu X, Zhang Y, Wang Z, et al. PD-L1 antibody conjugated dihydrotanshinone I-loaded polymeric nanoparticle for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Int J Pharmaceutics. (2024) 667:125004. doi: 10.1016/j.ijpharm.2024.125004
98. Jeon SH, You G, Park J, Chung Y, Park K, Kim H, et al. Anti-4-1BB×PDL1 bispecific antibody reinvigorates tumor-specific exhausted CD8+ T cells and enhances the efficacy of anti-PD1 blockade. Clin Cancer Res. (2024) 30:4155–66. doi: 10.1158/1078-0432.CCR-23-2864
99. An X, Zhan F, and Zhu Y. Smart photothermal-triggered bilayer phase transition in AuNPs-liposomes to release drug. Langmuir: ACS J Surfaces Colloids. (2013) 29:1061–8. doi: 10.1021/la304692h
100. Singh SP, Alvi SB, Pemmaraju DB, Singh AD, Manda SV, Srivastava R, et al. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int J Biol Macromolecules. (2018) 110:375–82. doi: 10.1016/j.ijbiomac.2017.11.163
101. De Leo V, Milano F, Agostiano A, and Catucci L. Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles. Polymers. (2021) 13(7):1027. doi: 10.3390/polym13071027
102. Wang J, Liu J, Liu Y, Wang L, Cao M, Ji Y, et al. Gd-hybridized plasmonic au-nanocomposites enhanced tumor-interior drug permeability in multimodal imaging-guided therapy. Advanced Materials (Deerfield Beach Fla). (2016) 28:8950–8. doi: 10.1002/adma.201603114
103. Fu X, Wang X, Zhou S, and Zhang Y. IONP-doped nanoparticles for highly effective NIR-controlled drug release and combination tumor therapy. Int J Nanomedicine. (2017) 12:3751–66. doi: 10.2147/IJN.S113963
104. Huang SJ, Wang TH, Chou YH, Wang HD, Hsu TC, Yow JL, et al. Hybrid PEGylated chitosan/PLGA nanoparticles designed as pH-responsive vehicles to promote intracellular drug delivery and cancer chemotherapy. Int J Biol Macromolecules. (2022) 210:565–78. doi: 10.1016/j.ijbiomac.2022.04.209
105. Scheeren LE, Nogueira-Librelotto DR, Mathes D, Pillat MM, Macedo LB, Mitjans M, et al. Multifunctional PLGA nanoparticles combining transferrin-targetability and pH-stimuli sensitivity enhanced doxorubicin intracellular delivery and in vitro antineoplastic activity in MDR tumor cells. Toxicol Vitro. (2021) 75:105192. doi: 10.1016/j.tiv.2021.105192
106. Tonbul H, Şahin A, Öztürk SC, Ultav G, Tavukçuoğlu E, Akbaş S, et al. An all-in-one nanoparticle for overcoming drug resistance: doxorubicin and elacridar co-loaded folate receptor targeted PLGA/MSN hybrid nanoparticles. J Drug Targeting. (2024) 32:1101–10. doi: 10.1080/1061186X.2024.2374034
107. Liu X, Yang Y, Wang X, Liu X, Cheng H, Wang P, et al. Self-assembled Au(4)Cu(4)/Au(25) NCs@liposome tumor nanotheranostics with PT/fluorescence imaging-guided synergetic PTT/PDT. J Materials Chem B. (2021) 9:6396–405. doi: 10.1039/D1TB01092A
108. Zhong Y, Su T, Shi Q, Feng Y, Tao Z, Huang Q, et al. Co-administration of iRGD enhances tumor-targeted delivery and anti-tumor effects of paclitaxel-loaded PLGA nanoparticles for colorectal cancer treatment. Int J Nanomedicine. (2019) 14:8543–60. doi: 10.2147/IJN.S219820
Keywords: nanomaterials, cancer-associated fibroblasts, tumor microenvironment, tumor drug resistance, reverse tumor drug
Citation: Gao X, Sun L, Li T, Li P, Liu C, Li Q and Zhu Y (2025) Advances in the study of reversing tumor drug resistance by targeting cancer-associated fibroblasts with nanomaterials. Front. Immunol. 16:1647988. doi: 10.3389/fimmu.2025.1647988
Received: 16 June 2025; Accepted: 31 October 2025;
Published: 19 November 2025.
Edited by:
Junjie Li, Kyushu University, JapanReviewed by:
Panyue Wen, University of Science and Technology of China, ChinaShevanuja Theivendran, The University of Queensland, Australia
Copyright © 2025 Gao, Sun, Li, Li, Liu, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanmei Zhu, emh1eWFubWVpQGNhbmNlcmhvc3AtbG4tY211LmNvbQ==; Chang Liu, bGl1Y2hhbmdAY211LmVkdS5jbg==; Qiang Li, bGlxaWFuZ0BjYW5jZXJob3NwLWxuLWNtdS5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaozhuo Gao1†
Xiaozhuo Gao1† Chang Liu
Chang Liu Yanmei Zhu
Yanmei Zhu