- 1Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 2First College of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Institute for Brain Disorders, Beijing University of Chinese Medicine, Beijing, China
- 4Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Beijing University of Chinese Medicine, Beijing, China
Background: Vascular cognitive impairment (VCI) exhibits particularly high prevalence in East Asian populations. However, its pathogenesis remains elusive due to its multifactorial and complex nature. Emerging evidence highlights the microbiota-gut-brain axis as a novel and promising paradigm for elucidating VCI mechanisms and developing therapeutic interventions. This systematic review aims to synthesize recent advances in this field, offering critical perspectives to guide future research on VCI through the lens of gut-brain interactions. Notably, given Traditional Chinese Medicine’s (TCM) holistic and multi-target therapeutic advantages, we incorporate TCM studies to complement conventional approaches.
Methods: We systematically searched PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP), and Wanfang database for relevant studies from their inception to March 31, 2025, and conducted a comprehensive review.
Results: A total of 22 relevant studies were included in the final review. Current research primarily focused on analyzing the altered gut microbiota in VCI patients, with findings indicating significant changes in both the structure and abundance of gut microbiota. Enterobacteriaceae exhibited potential as a diagnostic biomarker for post-stroke cognitive impairment (PSCI) (AUC=0.629), while distinct microbial signatures involving Bifidobacterium, Lactobacillus gasseri, and Anaerostipes hadrus may effectively differentiated PSCI patients from stroke survivors without cognitive deficits (AUC values of 0.785, 0.792, and 0.750, respectively). Furthermore, multiple interventional studies from both basic and clinical research systematically explored the microbiota-gut-brain axis as a promising therapeutic target for VCI. They evaluated the efficacy of diverse approaches—such as fecal microbiota transplantation, aerobic exercise, pharmacological interventions, and acupuncture—on key outcome including gut microbiota composition, cognitive function, hippocampal integrity, and inflammatory markers. Basic experimental studies revealed that Prevotella histicola, Clostridium butyricum, aerobic exercise, and TCM improved cognitive function, whereas trimethylamine N-oxide exacerbated cognitive impairment. The efficacy of TCM was further confirmed by clinical studies.
Conclusion: Research is in its early stages, but the microbiota-gut-brain axis already offers promising prospects for a deeper understanding and discovery of potential new therapeutic targets for VCI.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42024560293.
1 Introduction
Vascular cognitive impairment (VCI) refers to cognitive dysfunction caused by cerebrovascular pathologies and their risk factors, encompassing the full disease spectrum from mild cognitive impairment to dementia (1). The disorder is typically characterized by impairments in reasoning, planning, judgment and other cognitive functions, with particularly notable executive dysfunction, and may be accompanied by gait abnormalities (2). Although the exact prevalence of VCI remains undetermined, epidemiological studies show its subtype vascular dementia (VaD) ranks as the second leading cause of dementia worldwide, accounting for approximately 20-40% of all dementia cases, and appears to be the predominant dementia subtype in Southeast Asian populations (3, 4). Despite its significant clinical burden, effective therapies for VCI remain limited.
Chronic cerebral hypoperfusion serves as a primary driver of VCI. Numerous risk factors for VCI have been identified, particularly vascular risk factors including hypertension, obesity, diabetes mellitus, dyslipidemia, hyperhomocysteinemia, and smoking (5). However, the precise pathogenesis of VCI remains incompletely understood and may involve multiple interrelated mechanisms: neurovascular dysfunction, blood-brain barrier disruption, white matter damage, oxidative stress, neuroinflammation, and alterations in the gut microbiota (1, 4, 6). Among these factors, the gut microbiota has recently emerged as a crucial research focus in VCI. The gut microbiota refers to the microorganisms residing in the gastrointestinal tract, including bacteria, archaea, and eukaryotes (7). It may influence physiological, behavioral, and cognitive brain functions through the gut-brain axis via neural, immune, endocrine, and metabolic pathways (6, 8).
The gut microbiota, as a key component of the gastrointestinal tract, and emerging research suggests that the classic bidirectional interaction between the gut and the brain (brain-gut axis) should be expanded to include the gut microbiota, forming what is now termed the microbiota-gut-brain axis (9, 10). On the one hand, the brain regulates various physiological processes in the intestine through the hypothalamus-pituitary-adrenal (HPA) axis and the autonomic nervous system; on the other hand, the intestine regulates brain function through various microorganisms and their derived metabolites and products, as well as neuroactive substances. These metabolites and products pass through the enteric nervous system, vagus nerve, circulatory system and immune system to reach the brain (11, 12). Gut microbiota-derived components such as microbial antigens, cytokines, and prostaglandins can traverse the blood-brain barrier to activate the HPA axis, whereas certain microbial metabolites like short-chain fatty acids demonstrate the capacity to attenuate HPA axis responses; moreover, bacterial-derived neurotransmitters can directly interact with vagal afferent nerves (13–15). The hepatic and celiac branches of the vagus nerve exhibit particular susceptibility to stimulation by gut microbiota-derived molecules (including nitric oxide and bile acids), metabolites (such as short-chain fatty acids and trimethylamine N-oxide), and enteroendocrine hormones, which upon entering systemic circulation can exert central nervous system effects either by crossing the blood-brain barrier or via neural pathways (16–18). Through these concerted mechanisms, the gut microbiota collectively regulates neuronal activity, astrocytic function, microglial polarization states, and blood-brain barrier integrity, thereby contributing to neuroinflammatory processes, cerebrovascular dysfunction, and neuronal injury.
In recent years, a series of studies on the relationship between the microbiota-gut-brain axis and VCI have been conducted, including investigations into the characteristics of gut microbiota in VCI and the mechanisms by which the microbiota and its metabolites influence VCI progression (Figure 1) (19–23). The microbiota-gut-brain axis offers a promising framework for understanding and potentially treating VCI. This framework not only facilitates a deeper understanding of VCI pathogenesis but may also identify novel therapeutic targets to delay disease progression and reduce VCI risk, which is critical for advancing drug development.
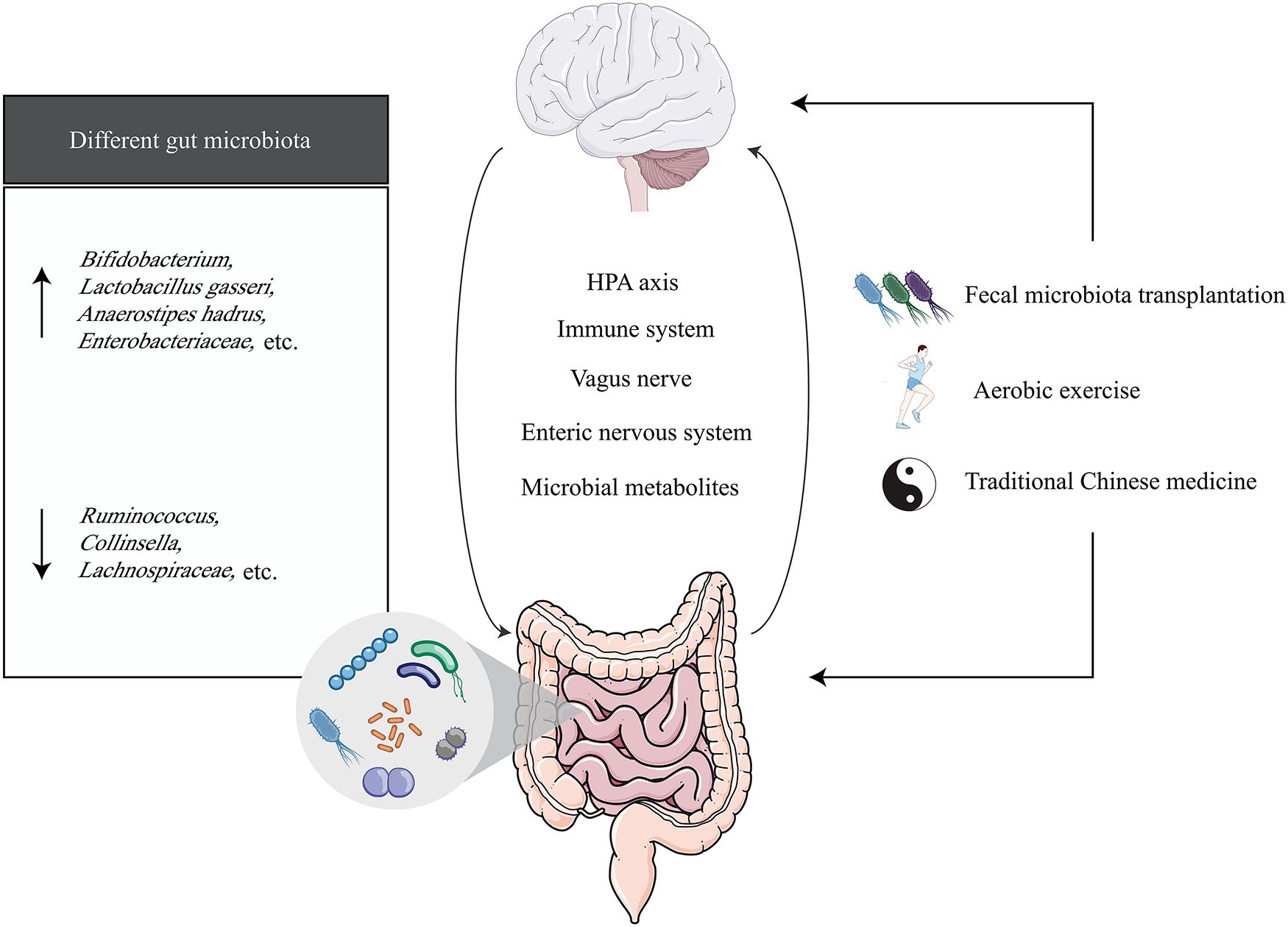
Figure 1. The microbiota-gut-brain axis in vascular cognitive impairment. The black arrow (↑) represents the elevated gut microbiota expressed in vascular cognitive impairment, and the black arrow (↓) represents the declining expression of the gut microbiota expressed in vascular cognitive impairment.
Recognizing the current lack of systematic reviews addressing the relationship between the microbiota-gut-brain axis and VCI, we conducted the first systematic review to summarize recent progress and highlight the challenges that must be addressed. Furthermore, given the multifaceted pathological mechanisms underlying VCI—where conventional single-target therapies frequently demonstrate restricted clinical efficacy—our investigation intentionally incorporated TCM research due to its distinctive holistic approach and multi-target therapeutic potential. This systematic review aims to provide important insights for future research on VCI through the lens of the microbiota-gut-brain axis.
2 Methods
2.1 Search strategy and selection criteria
The study protocol was registered on PROSPERO (CRD42024560293) on June 20, 2024, and this systematic review strictly adhered to the registered protocol. Seven electronic databases were searched without language limitation (from their inception to March 31, 2025), including PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP), and Wanfang database. All searches were performed by combining free-text and MESH terms, containing vascular cognitive impairment, vascular dementia, multiinfarct dementia, post stroke cognitive impairment, cerebral small vessel disease, brain-gut axis, gut microbiome, and intestine flora.
Two reviewers (Y.L. and X.X.) independently screened titles, abstracts and selected potential full-texts for further analysis. Those studies fulfilling our pre-defined eligibility criteria were included in the review. Any disagreements were resolved by discussion or consultation with a third reviewer (X.X.). The detailed inclusion criteria were: (a) VCI patients or VCI animals, (b) application of microbiota-gut-brain axis to study VCI, and (c) experimental or observational studies. Exclusion criteria included abstracts, editorials, letters, reviews, case reports, and review papers.
2.2 Data extraction
Data were independently extracted by 2 reviewers (Y.L. and X.X.) using a preformulated data collection form. A narrative summary of the results was produced according to specific data subjects, basically including: (a) the article’s author and publication year; (b) study characteristics, involving study location, VCI type, sample size, differential gut microbiota (or metabolites) and their effects, intervention methods and their effects on gut microbiota and cognitive function. For each study, all relevant data were extracted from tables, figures, text, and Supplementary Material.
2.3 Quality assessment
The risk of bias assessment for included studies was conducted independently by two reviewers (Y.L. and X.X.): randomized controlled trials were evaluated using the Cochrane Risk of Bias Tool version 2.0, while other clinical studies were assessed with the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies developed by the National Heart, Lung, and Blood Institute of the National Institutes of Health, with quality thresholds defined as ≥ 75% (good), 50-74% (fair), and < 50% (poor) (24, 25). Animal studies were evaluated using the SYRCLE’s Risk of Bias Tool (26).
3 Results
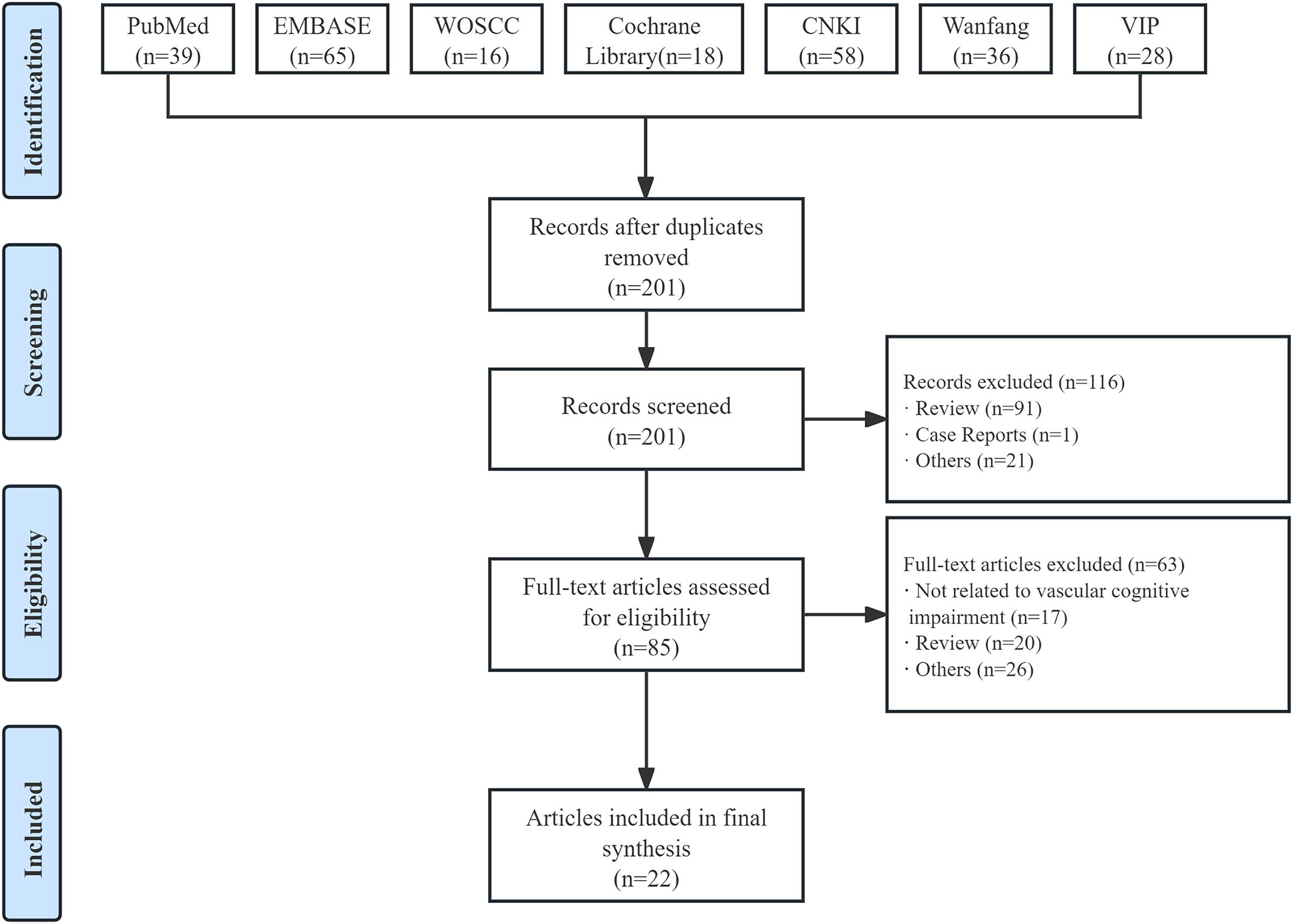
A total of 260 studies were retrieved through seven electronic database systems, with 59 excluded due to duplication. After screening titles and abstracts, 85 potentially relevant full-text articles were identified. Ultimately, 22 studies (19, 20, 22, 23, 27–44) were included in the final analysis (Figure 2).
Of the 22 studies on VCI, 11 were basic research experiments (20, 23, 27, 29–32, 38, 42–44), and 11 were clinical trials (19, 22, 28, 33–37, 39–41). A total of 527 rats and 129 mice were included in the basic research experiment, and a total of 787 human subjects were included in the clinical trial. In the clinical trial, there were 252 women except for two studies that did not report gender-specific data. Seven studies focused on the gut microbial characteristics of VCI, and 15 examined interventions targeting the microbiota-gut-brain axis in the context of VCI.
3.1 The close relationship between gut microbiota and VCI
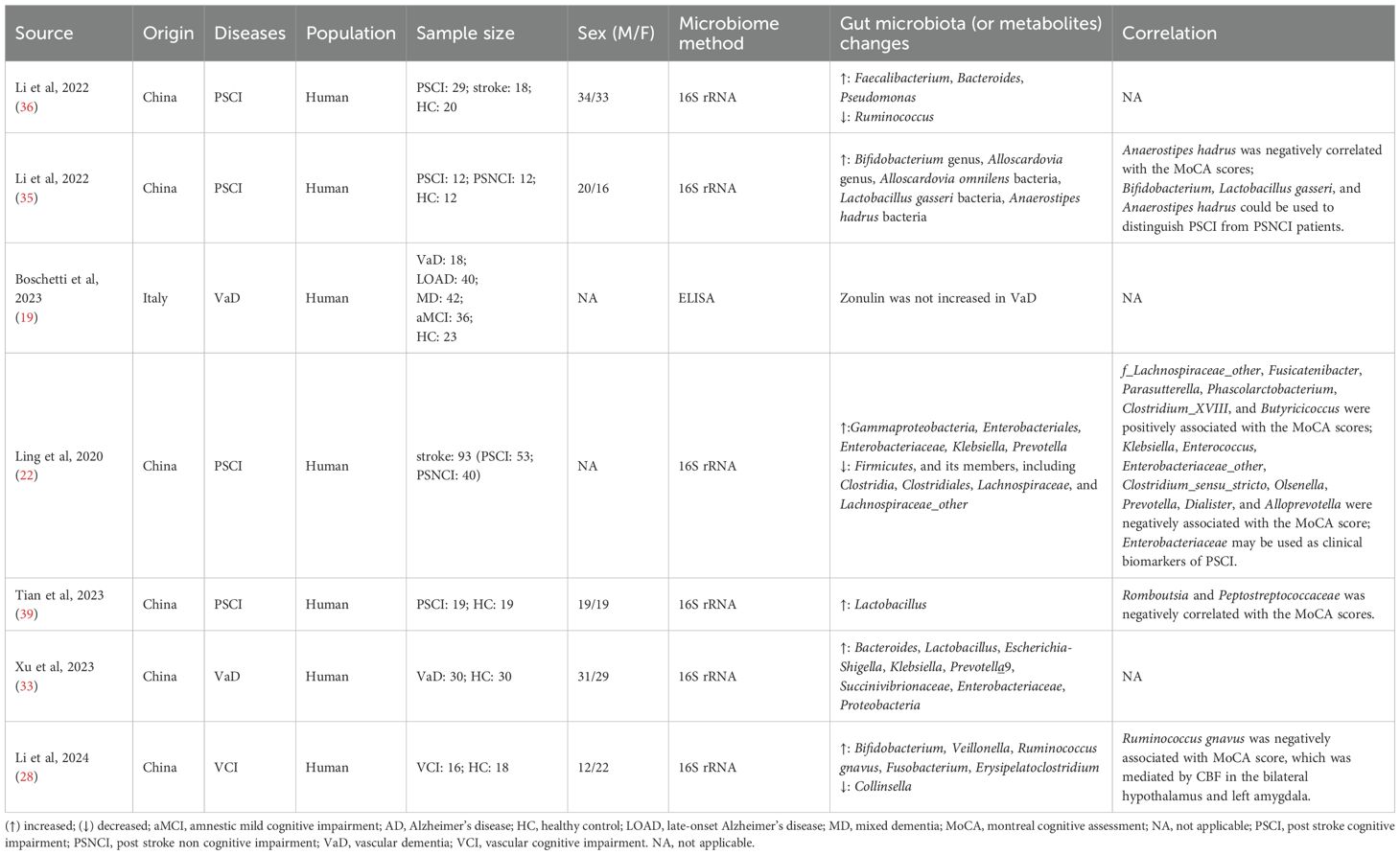
This section included a total of seven studies, six of which analyzed the gut microbiota characteristics of VCI patients (22, 28, 33, 35, 36, 39), and one study explored the relationship between gut proteins and VCI (Table 1) (19). All studies demonstrated fair methodological quality (Supplementary Table S1).
Four studies focused on the characteristics of gut microbiota in Chinese patients with post-stroke cognitive impairment (PSCI) and its relationship with cognitive function (22, 35, 36, 39). Through 16S rRNA sequencing of fecal samples, these studies consistently demonstrated significant structural alterations in the gut microbiome of PSCI patients. The timing of assessment varied across studies, with Li et al. and Tian et al. evaluating patients within one month post-stroke (35, 39), while Ling et al. extended the observation window to three months post-stroke (22). Notably, Li et al.’s study of 12 PSCI patients (5 females; mean age 60.75 ± 12.45 years) revealed increased abundance of Actinobacteria (Bifidobacterium, Alloscardovia, Alloscardovia omnilens) and Firmicutes (Lactobacillus gasseri, Anaerostipes hadrus) compared to post-stroke non-cognitive impairment (PSNCI) and control groups, with Anaerostipes hadrus showing a negative correlation with Montreal Cognitive Assessment (MoCA) scores (35). Tian et al.’s study of 19 PSCI patients (7 females; mean age 58.16 ± 7.81 years) demonstrated elevated Lactobacillus levels versus healthy controls, while Romboutsia and Peptostreptococcaceae were negatively associated with MoCA performance (39). Another study by Li et al. involving 29 PSCI patients (15 females; mean age 68.5 ± 9.9 years) observed increased conditional pathogens (Faecalibacterium, Bacteroides, Pseudomonas) alongside decreased beneficial bacteria like Ruminococcus when compared to stroke patients without cognitive impairment and healthy controls (36). Interestingly, Ling et al.’s longitudinal study of 135 ischemic stroke patients (with 93 analyzable at 3 months: 53 PSCI vs 40 PSNCI) found significantly reduced Firmicutes (Clostridia, Clostridiales, Lachnospiraceae, and Lachnospiraceae_other) and markedly increased Enterobacteriaceae abundance in the PSCI group (22). These distinct microbial patterns show promise as potential diagnostic biomarkers for PSCI monitoring and clinical management.
Similar gut microbiota alterations were observed in both VaD and VCI patients. The VaD study included 30 patients (12 females; mean age 68.17 ± 10.249 years) showing significantly increased abundances of Bacteroides, Lactobacillus, and Escherichia-Shigella compared to healthy controls (33). In parallel, the VCI investigation of 16 patients (9 females; mean age 69.75 ± 6.44 years) revealed elevated levels of Bifidobacterium, Veillonella, Ruminococcus gnavus, Fusobacterium, and Erysipelatoclostridium, alongside significantly reduced Collinsella abundance versus controls (28). Of particular clinical significance, Ruminococcus gnavus levels demonstrated a negative correlation with MoCA scores. Neuroimaging analyses further identified markedly diminished cerebral blood flow in bilateral hypothalamic and left amygdalar regions, suggesting these nutrient-sensitive brain areas may critically contribute to VCI pathogenesis through gut-brain axis interactions.
Current understanding indicated that serum zonulin plays a crucial regulatory role in maintaining intestinal and blood-brain barrier function through its modulation of tight junction proteins (45). However, emerging clinical evidence failed to demonstrate elevated zonulin levels in patients with vascular dementia (19).
3.2 Microbiota-gut-brain axis as a potential therapeutic target for VCI
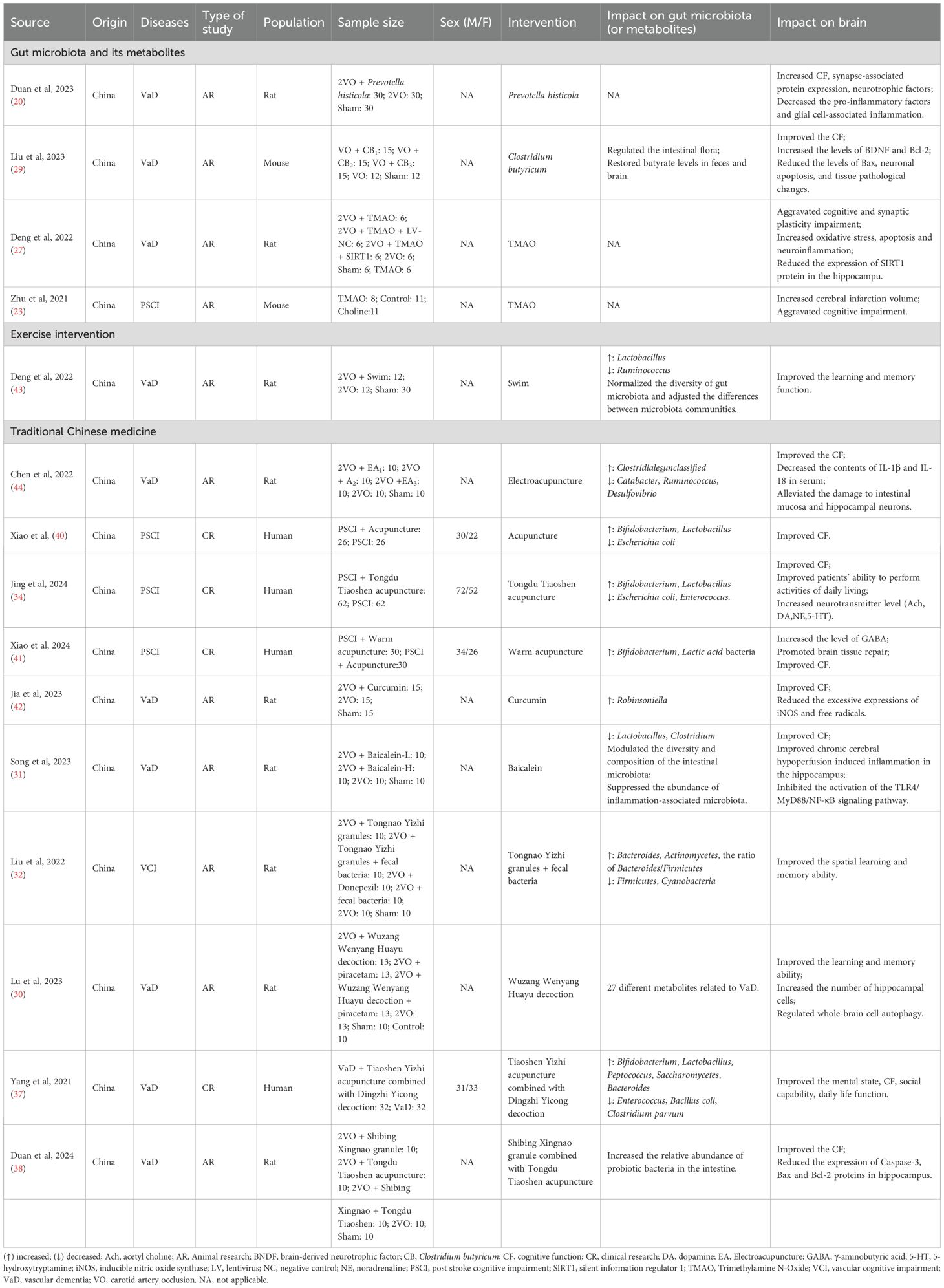
Currently, there are no effective and therapeutic approaches for VCI. Given the well-established brain-gut connection and particularly the crucial role of gut microbiota in VCI pathogenesis, growing research attention has been directed toward targeting the microbiota-gut-brain axis as a potential treatment strategy (20, 23, 27, 29). This section includes 15 studies, comprising four clinical trials and 11 experimental research. The overall risk of bias for clinical trials was rated as moderate (Supplementary Table S2). With the exception of one animal study that was judged to have an unclear risk of bias, all others were assessed as high risk (Supplementary Table S3). These studies explored diverse intervention approaches, including gut microbiota manipulation, exercise interventions, and traditional Chinese medicine (Table 2).
3.2.1 Gut microbiota and its metabolites
Prevotella histicola, an obligate anaerobic species within the Prevotella genus, demonstrated significant neuroprotective effects in a rat model of vascular dementia induced by bilateral common carotid artery ligation. Thirty 8-week-old rats receiving six-week oral administration of Prevotella histicola showed improved cognitive performance compared to the VaD model group, as evidenced by reduced escape latency and increased target quadrant duration in Morris water maze tests. Additionally, the treatment modulated multiple molecular markers, including upregulated synaptic proteins (MAP2, SYP, PSD-95) and downregulated pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) (20).
Clostridium butyricum, a natural commensal organism in the gastrointestinal tracts of healthy humans and animals, has well-documented gastrointestinal benefits supported by extensive in vivo and in vitro studies (46). In a study using a murine vascular dementia model established by unilateral carotid artery occlusion, six-week administration of Clostridium butyricum induced multidimensional neuroprotective effects in 15 six-week-old mice compared to the VaD model group. Specifically, the treated mice exhibited enhanced spatial learning ability in behavioral tests alongside alleviated cognitive impairment. At the histological and molecular levels, the treatment improved hippocampal morphology and regulated apoptotic signaling pathways—evidenced by increased BDNF and Bcl-2 expression, decreased Bax levels, and enhanced Akt phosphorylation—thereby attenuating neuronal apoptosis. Furthermore, the therapy restored gut microbial homeostasis and normalized butyrate concentrations in both fecal samples and brain tissue brain tissue (29).
Furthermore, two independent studies investigated the role of trimethylamine N-oxide (TMAO), a gut microbiota-derived metabolite generated from dietary choline, betaine, and carnitine (23, 27). The first study demonstrated that compared to normal diet controls, three-week choline supplementation prior to stroke induction in mice significantly elevated plasma TMAO levels, exacerbating both cerebral infarct volume and cognitive impairment as evidenced by impaired performance in Y-maze and Barnes maze tests (23). In the second study, rats received TMAO administration beginning four weeks prior to VaD model induction and continuing for four weeks post-surgery, starting from the second postoperative day. Compared to the VaD model group, TMAO-treated animals exhibited more severe cognitive deficits as demonstrated by Morris water maze testing, along with aggravated oxidative stress markers, neuroinflammation (NLRP3 inflammasome activation), and neuronal apoptosis (27). Mechanistically, TMAO treatment activated the NLRP3 inflammasome while suppressing hippocampal expression of silent information regulator 1 (SIRT1).
3.2.2 Exercise intervention
Aerobic exercise training has demonstrated efficacy in enhancing aerobic endurance, promoting vascular health, and improving quality of life, while simultaneously repairing neurovascular damage induced by ischemia and modulating synaptic plasticity (47–49). Emerging evidence further indicates that exercise exerts beneficial effects on gut microbiota metabolic function, augmenting microbial diversity and reinforcing the microbiota-gut-brain axis (50, 51).
In a VaD rat model induced by permanent bilateral common carotid artery occlusion, a four-week aerobic exercise intervention consisting of daily 20-minute non-loaded swimming sessions was implemented post-model establishment (43). Exercise intervention induced a substantial remodeling of gut microbial composition, characterized by a significant reduction in Ruminococcus abundance concomitant with increased Lactobacillus colonization. These microbial changes were paralleled by marked enhancements in cognitive function, as evidenced by improved learning and memory performance in behavioral assessments. Furthermore, the aerobic exercise effectively restored gut microbial diversity and induced significant modifications in inter-community microbiota structure, suggesting a comprehensive exercise-mediated modulation of the gut ecosystem in the vascular dementia model.
3.2.3 Traditional Chinese medicine
Given the complex pathological mechanisms underlying VCI, conventional single-target therapeutic strategies often demonstrate limited clinical efficacy. In contrast, TCM employs a holistic, multi-target approach, positioning it as a potentially valuable intervention for VCI prevention and management (52, 53). Current TCM research on VCI primarily focuses on acupuncture and herbal therapies. This section systematically reviews 10 studies evaluating various TCM modalities, including acupuncture, moxibustion, herbal formulations, and combined acupuncture-herbal regimens.
(i)Acupuncture
Acupuncture, a well-established external therapy in TCM, demonstrates notable safety, reliability, and clinical efficacy. By stimulating specific acupoints, this intervention achieves therapeutic effects while exhibiting significantly fewer adverse reactions compared to conventional pharmaceutical treatments (54, 55).
Experimental studies utilizing a vascular dementia rat model demonstrated that electroacupuncture stimulation at key acupoints (Baihui [GV20], Dazhui [GV14], Shenshu [BL23], and Zusanli [ST36]) produced significant therapeutic effects (44). The treatment modulated gut microbiota composition by increasing Clostridiales̲unclassified abundance while reducing Catabacter, Ruminococcus, and Desulfovibrio populations. Furthermore, electroacupuncture intervention decreased serum levels of pro-inflammatory cytokines IL-1β and IL-18, protected against intestinal mucosal and hippocampal neuronal damage, and ameliorated cognitive dysfunction in the animal model.
Clinical investigations involving PSCI patients across three studies consistently demonstrated the therapeutic benefits of acupuncture interventions (34, 40, 41). The first trial employed a combined approach of acupuncture (targeting Fengfu [DU16], Dazhui [GV14], Shendao [DU11], Baihui [GV20], and Shenting [DU24]) with cognitive training, resulting in enhanced cognitive scale performance, elevated serum neurotransmitter levels (acetylcholine, dopamine, norepinephrine, and 5-hydroxytryptamine), and favorable gut microbiota alterations characterized by increased Bifidobacterium and Lactobacillus populations alongside decreased Escherichia coli and Enterococcus abundance (34). A second study incorporating warm needle therapy at Zhongwan (RN12), Tianshu (ST25), Zusanli (ST36), and Shangjuxu (ST37) with cognitive rehabilitation showed increased Bifidobacteria and Lactobacilli counts, elevated plasma γ-aminobutyric acid (GABA) concentrations, and associated cognitive improvements. A third study applied scalp acupuncture combined with body acupuncture to treat PSCI patients using the same acupoints: Zhongwan (RN12), Tianshu (ST25), Zusanli (ST36), and Shangjuxu (ST37). The results indicated that after treatment, the number of Bifidobacteria and Lactobacilli increased significantly, cognitive function improved, and the abundance of Escherichia coli decreased (40).
(ii)Chinese herbs
Both curcumin, a bioactive phenolic compound from Curcuma longa (turmeric), and baicalein, a key flavonoid in Scutellaria baicalensis roots, demonstrate potent anti-inflammatory, antioxidant, and free radical-scavenging properties (56–58). Experimental studies in vascular dementia models revealed distinct neuroprotective mechanisms: curcumin administration significantly increased Robinsoniella abundance while reducing inducible nitric oxide synthase (iNOS) expression and oxidative stress markers (free radicals and hydroxyl radicals), ultimately improving memory function (42); whereas baicalein treatment modulated gut microbiota by decreasing Lactobacillus and Clostridium populations, while concurrently attenuating neuroinflammation through reduced glial activation, suppressed proinflammatory cytokine release, and inhibition of the TLR4/MyD88/NF-κB pathway, accompanied by preserved CA1 hippocampal neuronal integrity and enhanced cognitive performance (31).
(iii)Chinese herbal compound
Wuzang Wenyang Huayu decoction, composed of Ganjiang (Zingiberis rhizoma), Fuzi (Aconiti radix lateralis praeparata), Guizhi (Cinnamomi ramulus), Bajitian (Morindae officinalis radix), Yinyanghuo (Epimedii herba), Banxia (Pinelliae rhizoma), Sanqi (Notoginseng radix et rhizoma), Shichangpu (Acori tatarinowii rhizoma), and Dahuang (Rhei radix et rhizoma), was found to improve learning and memory abilities in rats with vascular dementia (30). Mechanistic studies revealed this formulation promoted hippocampal neurogenesis, suppressed Caspase-3-mediated apoptosis, and enhanced autophagic activity through upregulation of APG5L/ATG5, Beclin-1, and LC3A/B protein expression. Fecal metabolomic analysis identified 27 dementia-associated metabolites, with pathway enrichment analysis implicating vitamin B metabolism, purine metabolism, pyrimidine metabolism, and histidine metabolic pathways.
Tongnao Yizhi granules, composed of Dahuang (Rhei radix et rhizoma), Yujin (Curcumae radix), Huangqi (Astragali radix), Chuanxiong (Chuanxiong rhizoma), Shichangpu (Acori tatarinowii rhizoma), Gouqi (Lycii fructus), Shuizhi (Hirudo), Yizhiren (Alpiniae oxyphyllae fructus), and Xianhecao (Agrimoniae herba), were combined with fecal bacteria capsules prepared from the fresh feces of healthy rats (32). This treatment significantly altered gut microbial ecology, characterized by increased of Bacteroides abundance, elevated Bacteroides/Firmicutes ratio, and enriched Actinomycetes populations in VCI rats, while reducing the abundance of Firmicutes and Cyanobacteria. The intervention improved spatial learning and memory abilities in behavioral tests.
(iv)Combination of acupuncture and Chinese medicine
The Tongdu Tiaoshen acupuncture protocol involved acupoints such as Shenting (DU24), Baihui (GV20), Dazhui (DU14), Zhiyang (DU9), and Yaoyangguan (DU3). Shibing Xingnao granules, composed of Huangqi (Astragali radix), Shichangpu (Acori tatarinowii rhizoma), Yuanzhi (Polygalae radix), Chuanxiong (Chuanxiong rhizoma), and Bingpian (Borneolum Syntheticum), were combined with acupuncture in vascular dementia rats (38). This integrated treatment demonstrated behavioral improvements through reduced escape latency and enhanced cognitive function, while also exerting neuroprotective effects via increased Bcl-2 expression and decreased Caspase-3/Bax protein levels. Furthermore, the intervention modulated gut microbiota composition by promoting beneficial bacterial populations.
The Tiaoshen Yizhi acupuncture method targeted acupoints including Shenting (DU24), Sishencong (EX-HN1), Renzhong (DU26), Neiguan (PC6), Daling (PC7), Rangu (KI2), Xuehai (SP10), and Taichong (LR03). Dingzhi Yicong formula was composed of Dangshen (Codonopsis radix), Shichangpu (Acori tatarinowii rhizoma), Yuanzhi (Polygalae radix), Yizhiren (Alpiniae oxyphyllae fructus), Danggui (Angelicae sinensis radix), Shudihuang (Rehmanniae radix), Chishao (Paeoniae radix rubra), Fuling (Poria), Gouqi (Lycii fructus), Chuanxiong (Chuanxiong rhizoma), Taoren (Persicae semen), and Honghua (Carthami flos). These two methods were combined to treat vascular dementia patients (37). The combined therapy improved cognitive performance and restored gut microbial balance, as evidenced by increased abundance of BBifidobacterium, Lactobacillus, Peptococcus, Saccharomycetes, and Bacteroides, alongside decreased levels of Enterococcus, Escherichia coli, and Clostridium parvum.
4 Discussion
This systematic review provides the first comprehensive synthesis of the microbiota-gut-brain axis in VCI, encompassing both pathophysiological mechanisms and therapeutic interventions. Our analysis of 22 studies reveal several key findings that advance our understanding of VCI pathogenesis and highlight promising treatment approaches targeting gut-brain interactions.
The observed gut microbiota dysbiosis across VCI subtypes provides robust evidence for the involvement of the microbiota-gut-brain axis in disease pathogenesis. The identification of specific microbial signatures, particularly the increased abundance of Enterobacteriaceae in PSCI, suggests its potential as diagnostic biomarkers. Furthermore, the negative correlations observed between certain bacterial taxa (e.g., Anaerostipes hadrus and Ruminococcus gnavus) and cognitive performance underscore the functional significance of these microbial alterations.
The reviewed studies primarily focus on three principal intervention strategies targeting the gut-brain axis in VCI management. First, direct modulation of gut microbiota through probiotic administration or fecal microbiota transplantation has demonstrated significant neuroprotective effects, including cognitive improvement, neuroinflammation reduction, and synaptic plasticity enhancement. Second, aerobic exercise has emerged as an effective modulator of gut-brain interactions, demonstrating the capacity to restore microbial diversity, mitigate pro-inflammatory cytokines, and enhance cognitive outcomes. Third, TCM approaches, incorporating both acupuncture and herbal formulations, exhibit multi-target effects on gut microbiota composition, inflammatory markers, and neuronal protection, reflecting the potential advantages of holistic interventions for addressing VCI’s complex pathophysiology. The consistent observations of improved gut barrier function and reduced inflammation across various intervention modalities further substantiate the critical role of gut-brain communication in VCI.
The current evidence also reveals important gaps and limitations in the field. Although differential gut microbiota and their metabolites have been preliminarily identified, the research remains exploratory and requires large-scale validation. More importantly, the precise mechanistic relationships between specific microbial alterations and VCI pathogenesis need further elucidation, as only by clarifying these specific mechanisms can more targeted therapeutic strategies be developed. Furthermore, existing studies exhibit significant heterogeneity in study design, including variations in cognitive impairment severity and methodological differences in microbial analysis, which complicate direct comparisons across studies.
In future studies, first, it is essential to implement a well-designed study framework and adopt standardized inclusion and exclusion criteria as much as possible to facilitate the integration and analysis of results across different studies. For studies with relatively small sample sizes, strict matching of variables such as age, gender, and underlying diseases is essential to minimize the influence of confounding factors. Alternatively, consideration should be given to conducting large-scale prospective cohort studies similar to the “Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on RecoverY (DISCOVERY) study” (59). Currently, a multicenter cohort study evaluating the predictive value of gut microbiota and serum biomarkers for cognitive impairment and poor prognosis after ischemic stroke is underway (NCT:04688138, ClinicalTrials.gov). Second, all differential gut microbiota identified must undergo external validation before clinical applications, which will help objectively identify VCI and simplify diagnostic procedures. Third, more in-depth mechanistic research on the discovered differential gut microbiota is necessary to lay the foundation for developing effective VCI treatment strategies. Fourth, therapeutic interventions such as aerobic exercise, microbiota transplantation, and TCM still need to be further evaluated in standardized, well-designed clinical trials. Probiotics also represent a treatment approach targeting, with an ongoing clinical trial evaluating the efficacy of Bifidobacterium lactis Probio-M8 for post-stroke cognitive impairment (ChiCTR2400079870, Chinese Clinical Trial Registry). Fifth, to ensure data quality, strict standard operating procedures must be established for sample collection, preservation, processing, and testing (60). The standardization of these procedures is crucial for maintaining consistency and accuracy across studies. Additionally, future research should incorporate neuroimaging assessments. As an indispensable tool in VCI research, neuroimaging not only reveals pathophysiological mechanisms but also evaluates intervention effects, demonstrating increasingly prominent value in both clinical and research applications (61).
5 Conclusion
This systematic review establishes the microbiota-gut-brain axis as a critical framework for understanding VCI pathogenesis and developing novel treatment strategies. Although characteristic alterations in gut microbiota composition and abundance have been consistently documented in VCI patients, these findings require further validation. Furthermore, the mechanistic interplay between gut microbial dysbiosis, microbial-derived metabolites, and VCI pathobiology remains to be fully delineated, representing a crucial knowledge gap that demands systematic investigation through integrated multi-omics approaches and longitudinal study designs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XQX: Writing – review & editing, Project administration, Data curation, Conceptualization, Supervision. TL: Resources, Investigation, Writing – original draft, Software, Funding acquisition, Visualization, Validation, Formal Analysis, Conceptualization, Data curation, Methodology, Project administration, Supervision. YL: Supervision, Visualization, Project administration, Data curation, Writing – original draft, Investigation, Validation, Methodology. XJX: Validation, Methodology, Data curation, Writing – original draft, Software, Investigation. XL: Supervision, Investigation, Conceptualization, Writing – review & editing, Project administration, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1648800/full#supplementary-material
Abbreviations
BDNF, brain-derived neurotrophic factor; CNKI, China National Knowledge Infrastructure; GABA, γ-aminobutyric acid; HPA, hypothalamus-pituitary-adrenal; iNOS, inducible nitric oxide synthase; MoCA, montreal cognitive assessment; PSCI, post-stroke cognitive impairment; PSNCI, post-stroke non-cognitive impairment; SIRT1, silent information regulator 1; TCM, traditional Chinese medicine; TMAO, trimethylamine N-oxide; VaD, vascular dementia; VCI, vascular cognitive impairment; VIP, Chinese Science and Technology Periodical Database.
References
2. Biesbroek JM and Biessels GJ. Diagnosing vascular cognitive impairment: Current challenges and future perspectives. Int J Stroke. (2023) 18:36–43. doi: 10.1177/17474930211073387
3. Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. (2019) 73:3326–44. doi: 10.1016/j.jacc.2019.04.034
4. Rundek T, Tolea M, Ariko T, Fagerli EA, and Camargo CJ. Vascular cognitive impairment (VCI). Neurotherapeutics. (2022) 19:68–88. doi: 10.1007/s13311-021-01170-y
5. van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, et al. Vascular cognitive impairment. Nat Rev Dis Primers. (2018) 4:18003. doi: 10.1038/nrdp.2018.3
6. Yang T and Zhang F. Targeting transcription factor nrf2 (Nuclear factor erythroid 2-related factor 2) for the intervention of vascular cognitive impairment and dementia. Arterioscler Thromb Vasc Biol. (2021) 41:97–116. doi: 10.1161/ATVBAHA.120.314804
7. Mayer EA, Knight R, Mazmanian SK, Cryan JF, and Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. (2014) 34:15490–6. doi: 10.1523/JNEUROSCI.3299-14.2014
8. Li S, Shao Y, Li K, HuangFu C, Wang W, Liu Z, et al. Vascular cognitive impairment and the gut microbiota. J Alzheimers Dis. (2018) 63:1209–22. doi: 10.3233/JAD-171103
9. Doroszkiewicz J, Groblewska M, and Mroczko B. The role of gut microbiota and gut-brain interplay in selected diseases of the central nervous system. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms221810028
10. Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. (2024) 9:37. doi: 10.1038/s41392-024-01743-1
11. Foster JA, Rinaman L, and Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
12. Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. (2021) 172:105840. doi: 10.1016/j.phrs.2021.105840
13. Farzi A, Fröhlich EE, and Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. (2018) 15:5–22. doi: 10.1007/s13311-017-0600-5
14. Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. (2017) 7:489. doi: 10.3389/fcimb.2017.00489
15. Misiak B, Łoniewski I, Marlicz W, Frydecka D, Szulc A, Rudzki L, et al. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog Neuropsychopharmacol Biol Psychiatry. (2020) 102:109951.
16. Chidambaram SB, Rathipriya AG, Mahalakshmi AM, Sharma S, Hediyal TA, Ray B, et al. The influence of gut dysbiosis in the pathogenesis and management of ischemic stroke. Cells. (2022) 11. doi: 10.3390/cells11071239
17. Kuijer EJ and Steenbergen L. The microbiota-gut-brain axis in hippocampus-dependent learning and memory: current state and future challenges. Neurosci Biobehav Rev. (2023) 152:105296. doi: 10.1016/j.neubiorev.2023.105296
18. Rusch JA, Layden BT, and Dugas LR. Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis. Front Endocrinol (Lausanne). (2023) 14:1130689. doi: 10.3389/fendo.2023.1130689
19. Boschetti E, Caio G, Cervellati C, Costanzini A, Rosta V, Caputo F, et al. Serum zonulin levels are increased in Alzheimer's disease but not in vascular dementia. Aging Clin Exp Res. (2023) 35:1835–43. doi: 10.1007/s40520-023-02463-2
20. Duan R, Hou J, Wang X, Huang Z, Cao H, Hu J, et al. Prevotella histicola transplantation ameliorates cognitive impairment and decreases oxidative stress in vascular dementia rats. Brain Sci. (2023) 13. doi: 10.3390/brainsci13081136
21. Kaur N, LaForce G, Mallela DP, Saha PP, Buffa J, Li XS, et al. Exploratory transcriptomic profiling reveals the role of gut microbiota in vascular dementia. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24098091
22. Ling Y, Gong T, Zhang J, Gu Q, Gao X, Weng X, et al. Gut microbiome signatures are biomarkers for cognitive impairment in patients with ischemic stroke. Front Aging Neurosci. (2020) 12:511562. doi: 10.3389/fnagi.2020.511562
23. Zhu W, Romano KA, Li L, Buffa JA, Sangwan N, Prakash P, et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe. (2021) 29:1199–1208.e5. doi: 10.1016/j.chom.2021.05.002
24. Mulder D, Aarts E, Arias Vasquez A, and Bloemendaal M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol Psychiatry. (2023) 28:5037–61. doi: 10.1038/s41380-023-02146-4
25. National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies . Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
26. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, and Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
27. Deng Y, Zou J, Hong Y, Peng Q, Fu X, Duan R, et al. Higher circulating trimethylamine N-oxide aggravates cognitive impairment probably via downregulating hippocampal SIRT1 in vascular dementia rats. Cells. (2022) 11. doi: 10.3390/cells11223650
28. Li W, Jiang J, Yin X, Zhang Y, Zou X, Sun M, et al. Mediation of regional cerebral blood flow in the relationship between specific gut microbiota and cognition in vascular cognitive impairment. J Alzheimers Dis. (2024) 97:435–45. doi: 10.3233/JAD-230709
29. Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. BioMed Res Int. (2015) 2015:412946. doi: 10.1155/2015/412946
30. Lu Y-C, Li M-Q, Zhang L, Tang Y-L, Zeng Y-F, Li Y-C, et al. Wuzang wenyang huayu tang promoting learning-memory ability in vascular dementia rats via brain-gut-microbiome axis. Pharmacol Res - Modern Chin Med. (2023) 7:100259. doi: 10.1016/j.prmcm.2023.100259
31. Song J, Li M, Kang N, Jin W, Xiao Y, Li Z, et al. Baicalein ameliorates cognitive impairment of vascular dementia rats via suppressing neuroinflammation and regulating intestinal microbiota. Brain Res Bull. (2024) 208:110888. doi: 10.1016/j.brainresbull.2024.110888
32. Liu H, Yan X, Zhang Q, Meng T, Liu J, and Chang C. Effects of Tongnao Yizhi granules combined with fecal microbiota transplantation on learning and memory ability and gut microbiota of rats with vascular cognitive impairment. Modern J Integrated Traditional Chin Western Med. (2022) 31:2786–2792+2862.
33. Xu M, Liu Y, Li D, Zhang Y, Zhang Y, Xu B, et al. Differences in intestinal flora abundance in patients with vascular dementia. Chin J Alzheimer's Dis Related Disord. (2023) 6:206–14.
34. Jing Y, Gu C, Wei K, Liu J, and Wang L. Effect of tongdu tiaoshen acupuncture combined with coognitive function training on intestinal flora and neurotransmitters in patients with post stroke cognitive impairment. Prog Modern Biomedicine. (2024) 24:167–70.
35. Li Y, Yu Q, Huang L, Fu J, and Feng R. Correlations between gut microbiota changes and cognitive function in patients with post-stroke cognitive impairment in the early stage. J Sichuan Univ (Medical Sciences). (2022) 53:857–65.
36. Li Y and Lu S. Intestinal flora of patients with post-stroke cognitive impairment. J Binzhou Med Univ. (2022) 45:5–8.
37. Yang Y, Li S, Ji X, Zhang P, Yang X, and Zhang Z. Clinical observation of the cognitive function and intestinal microecology of patients with vascular dementia treated with Tiaoshen Yizhi acupuncture combined with Dingzhi Yicong Fang. J Beijing Univ Traditional Chin Med. (2021) 44:562–8.
38. Duan T, Li M, Li P, Wu J, He C, Liu Q, et al. Effect of shibing xingnao granule combined with tongdu tiaoshen acupuncture on gut microbiota in rats with vascular dementia. J Anhui Univ Chin Med. (2024) 43:53–9.
39. Tian Y, Xu L, Zhan D, Cui W, Huang L, Feng R, et al. The correlation between gut microbiota and cognitive function in post-stroke cognitive impairment patients. Sichuan Med J. (2023) 44:604–9.
40. Xiao Y, Li X, Wu Y, Miao Z, Niu X, and Zhou Y. Observation of clinical efficacy of he-sea and front-mu points in treatment of post-stroke cognitive impairment based on theory of intestinal flora. Xinjiang J Traditional Chin Med. (2023) 41:12–5.
41. Xiao Y, Li X, Wu Y, Miao Z, Niu X, and Zhou Y. Based on intestinal flora theory to explore the effect of warm acupuncture on cognitive impairment after stroke. Int J Traditional Chin Med. (2024) 46:37–41.
42. Jia R, Ma L, Ma X, Hao R, and Yan X. Mechanism of curcumin on improvement of intestinal flora metabolism and memory dysfunction in rats with vascular dementia. Chin Arch Traditional Chin Med. (2023) 41:174–8.
43. Deng C, Chen D, Qiu R, Zhang H, Zou Y, Xu Y, et al. Effects of aerobic exercise training on intestinal flora constructures of rats with vascular dementia. Chin J Rehabil Med. (2022) 37:443–50.
44. Chen D, Zhang H, Xie J, Deng C, Qiu R, Xu Y, et al. Effect of electroacupuncture on gut microbiota and serum IL-1β and IL-18 in rats with vascular dementia based on principle of curing brain disorders by treating intestines. Acupuncture Res. (2022) 47:216–23.
45. Veres-Székely A, Szász C, Pap D, Szebeni B, Bokrossy P, and Vannay Á. Zonulin as a potential therapeutic target in microbiota-gut-brain axis disorders: encouraging results and emerging questions. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24087548
46. Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. (2021) 13:1–28. doi: 10.1080/19490976.2021.1907272
47. Hillman CH, Erickson KI, and Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. (2008) 9:58–65. doi: 10.1038/nrn2298
48. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for americans. Jama. (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
49. Seals DR, Nagy EE, and Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol. (2019) 597:4901–14. doi: 10.1113/JP277764
50. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) 63:1913–20. doi: 10.1136/gutjnl-2013-306541
51. Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M, and Santacroce L. The connection between physical exercise and gut microbiota: implications for competitive sports athletes. Sports Med. (2022) 52:2355–69. doi: 10.1007/s40279-022-01696-x
52. Bai X and Zhang M. Traditional chinese medicine intervenes in vascular dementia: traditional medicine brings new expectations. Front Pharmacol. (2021) 12:689625. doi: 10.3389/fphar.2021.689625
53. Lv S, Wang Q, Zhang X, Ning F, Liu W, Cui M, et al. Mechanisms of multi-omics and network pharmacology to explain traditional chinese medicine for vascular cognitive impairment: A narrative review. Phytomedicine. (2024) 123:155231. doi: 10.1016/j.phymed.2023.155231
54. Wen J, Chen X, Yang Y, Liu J, Li E, Liu J, et al. Acupuncture medical therapy and its underlying mechanisms: A systematic review. Am J Chin Med. (2021) 49:1–23. doi: 10.1142/S0192415X21500014
55. Yang A, Wu HM, Tang JL, Xu L, Yang M, and Liu GJ. Acupuncture for stroke rehabilitation. Cochrane Database Syst Rev. (2016) 2016:Cd004131. doi: 10.1002/14651858.CD004131.pub3
56. Amalraj A, Pius A, Gopi S, and Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Tradit Complement Med. (2017) 7:205–33. doi: 10.1016/j.jtcme.2016.05.005
57. Sowndhararajan K, Deepa P, Kim M, Park SJ, and Kim S. Baicalein as a potent neuroprotective agent: A review. BioMed Pharmacother. (2017) 95:1021–32. doi: 10.1016/j.biopha.2017.08.135
58. Zia A, Farkhondeh T, Pourbagher-Shahri AM, and Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. BioMed Pharmacother. (2021) 134:111119. doi: 10.1016/j.biopha.2020.111119
59. Rost NS, Meschia JF, Gottesman R, Wruck L, Helmer K, and Greenberg SM. Cognitive impairment and dementia after stroke: design and rationale for the DISCOVERY study. Stroke. (2021) 52:e499–516. doi: 10.1161/STROKEAHA.120.031611
60. Su W, Du Y, Lian F, Wu H, Zhang X, Yang W, et al. Standards for collection, preservation, and transportation of fecal samples in TCM clinical trials. Front Cell Infect Microbiol. (2022) 12:783682. doi: 10.3389/fcimb.2022.783682
Keywords: vascular cognitive impairment, microbiota-gut-brain axis, pathogenesis, treatment, systematic review
Citation: Liu T, Li Y, Xiong X, Lai X and Xu X (2025) The Microbiota-gut-brain axis in vascular cognitive impairment: unraveling the mysterious link and therapeutic prospects. Front. Immunol. 16:1648800. doi: 10.3389/fimmu.2025.1648800
Received: 17 June 2025; Accepted: 22 September 2025;
Published: 07 October 2025.
Edited by:
Amélia M. Sarmento, Fernando Pessoa University, PortugalReviewed by:
Wencan He, Sun Yat-Sen University, ChinaDavid Kaulmann, Weizmann Institute of Science, Israel
Copyright © 2025 Liu, Li, Xiong, Lai and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangqing Xu, aGFwcHl4aWFuZ3FpbmdAMTYzLmNvbQ==; Xinxing Lai, bmV3LXN0YXJAMTYzLmNvbQ==
†These authors share first authorship
 Tingting Liu
Tingting Liu Ying Li1,2†
Ying Li1,2† Xuejiao Xiong
Xuejiao Xiong Xinxing Lai
Xinxing Lai Xiangqing Xu
Xiangqing Xu