- 1Clinical Diagnosis and Treatment Center of Oncology, The Second Affiliated Hospital of Soochow University, Suzhou, China
- 2Institute of Radiotherapy & Oncology, Soochow University, Suzhou, China
- 3Department of Radiation Oncology, The Affiliated Cancer Hospital of Nanjing Medical University and Jiangsu Cancer Hospital and Jiangsu Institute of Cancer Research, Nanjing, China
- 4Department of Otolaryngology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 5Department of Radiation Oncology, Fujian Medical University Union Hospital, Fuzhou, China
- 6Department of Basic Medical Sciences, 960th Hospital of People's Liberation Army Joint Logistic Support Force, Jinan, China
- 7Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
Immune checkpoint blockade (ICB) and combination treatment paradigms have gradually improved the prognosis of head and neck cancer (HNC) patients. However, HNC survivors struggle with anxiety and depression because of the large variety of persistent cancer-related or treatment-related symptoms. Recent studies have suggested that emotional distress is closely related to the therapeutic efficacy of ICB. In this study, 232 advanced HNC patients were recruited and their anxiety and depression status were assessed by the Hospital Anxiety and Depression Scale (HADS). Propensity score matching (PSM) was applied to balance confounders. The nearest-neighbor matching method was used for PSM matching to perform 1:1 matching. By comparing the anxiety and depression status of HNC patients after PSM, we showed that HNC patients receiving chemoradiotherapy (CRT) combined with ICB, both concurrent radiotherapy (RT) and sequential RT, had lower depression levels than did those receiving CRT alone. These findings suggest that ICB treatment may be associated with emotional status, which may could offer insights into ameliorate quality of life, both physically and psychologically, of HNC patients.
Introduction
The incidence of head and neck cancer (HNC) has risen to sixth place among all tumors worldwide (1, 2). Radiotherapy (RT), which is a traditional antitumor method, plays an irreplaceable role in HNC treatment. The role of immune checkpoint blockade (ICB) in the treatment of HNC is increasing. The combination of RT and ICB has been hailed as a new dawn for antitumor treatment (3). The long-term effects of treatment are of concern in survivors. Most studies have shown that HNC survivors struggle with distress and depression in addition to a large variety of persistent cancer-related symptoms (4, 5). Higher rates of anxiety and depression symptoms are found in patients with HNC because of their unique tumor location (6). Integrated supportive care, especially cognitive-behavioral therapy (CBT) and mindfulness-based interventions, could help patients identify and modify negative thought patterns and behaviors and thus alleviating psychological distress (7, 8). Notably, anxiety and depression in these patients not only diminish quality of life but also might affect antitumor therapeutic efficacy. For example, Zeng et al. and Fraterman et al. reported an association between emotional distress and worse clinical outcomes in advanced non-small cell lung cancer and melanoma patients receiving ICB treatment (9, 10). This finding indicated an intrinsic link between emotional distress and ICB treatment. On the one hand, the undifferentiated attack of ICB often induces immune-related adverse events. On the other hand, immune checkpoint blockade might activate common immune-dependent repair mechanisms. However, to our knowledge, no studies have investigated the associations of the combination of RT and ICB with depression and anxiety in HNC patients.
Methods
Between October 9, 2020, and December 6, 2023, stage III-IV HNC patients undergoing intensity-modulated radiotherapy (IMRT) were recruited from Shandong Cancer Hospital and Institute. All male and female participants provided informed consent. The inclusion criteria were as follows: (1) new diagnosis of stage III-IV stage head and neck cancer (nasopharynx, larynx, oropharynx, or hypopharynx) according to the eighth AJCC and pathological staging; and (2) concurrent chemotherapy and radiotherapy treatment. (3) Combined ICB patients must receive ICB therapy (PD-1 and PD-L1 inhibitors) during or before RT. (4) Control patients must never receive ICB therapy before or after RT. The exclusion criteria were as follows: (1) had multiple primary cancers, (2) had prior irradiation, and (3) could not cooperate with the relevant evaluation and follow-up of the study.
All patients received daily IMRT treatment using 2.0 Gy fractions. The total dose range was 48–76 Gy. Patients were divided into 3 groups: RT + chemotherapy ± targeted drug group (control group), ICB + sequential RT + chemotherapy ± targeted drug group (ICB + sRT group) and RT + chemotherapy ± targeted drug + concurrent ICB group (ICB + cRT group). ICB drugs include PD-1 inhibitors (camrelizumab, pembrolizumab, toripalimab, sintilimab, tislelizumab) and a PD-L1 inhibitor (adebrelimab). The main outcomes considered were anxiety and depression scores on the Hospital Anxiety and Depression Scale (HADS) (11). Originally designed as a self-assessment tool for detecting depression and anxiety, the HADS is now extensively utilized in both nonhospital settings and research studies. The assessment includes 14 questions, with seven evaluating anxiety and seven evaluating depression, resulting in two subscale scores. Each question is rated on a scale from 0 to 3, where a higher score signifies a greater severity of mood disorder. No missing data were present for HADS scores or covariates, and a complete-case approach was naturally applied. Patients completed questionnaires by email, postal mail or phone on Feb. 01, 2024. We adopted 1:1 propensity score matching (PSM) via the nearest-neighbor matching method to minimize between-group heterogeneity and selection bias for the control group vs. the ICB + sRT group, the control group vs. the ICB + cRT group, and the ICB + sRT group vs. the ICB + cRT group. The caliper width was set at 0.2 standard deviations, with no replacement applied. The study’s propensity score incorporated the following variables: time since radiotherapy, DT, tumor type, gender, age, KPS score, smoking status, alcohol consumption, BMI, coronary artery disease (CAD), hypertension, diabetes, T stage, N stage, M stage, and target drug. Pearson’s chi-square test (or Fisher’s exact test for sparse data) was used to compare categorical variables between the two groups. Multiple comparisons were corrected using the Bonferroni-Holm correction. The effect size (such as r value), power (80%), and significance level (α = 0.05) used to determine the required sample size. Independent samples t tests or Wilcoxon rank-sum tests (depending on distributional assumptions) were used to compare the differences in anxiety and depression scores between two groups. p-values were adjusted using the Holm-Bonferroni correction (Holm, 1979). The steps for the power analysis are as follows: convert the effect size r value to the corresponding Z-value, calculate the noncentrality parameter by incorporating the sample size and significance level, and then back-calculate the power. Quantitative data with a normal distribution are shown as the means ± SDs; if not normally distributed, they are represented as medians (Q1, Q3). The date were analyzed using SPSS (v26.0) and R software (v4.2.3). Graphs were created using GraphPad Prism (v10.1.2). P <0.05 was considered statistically significant.
Results
Demographics
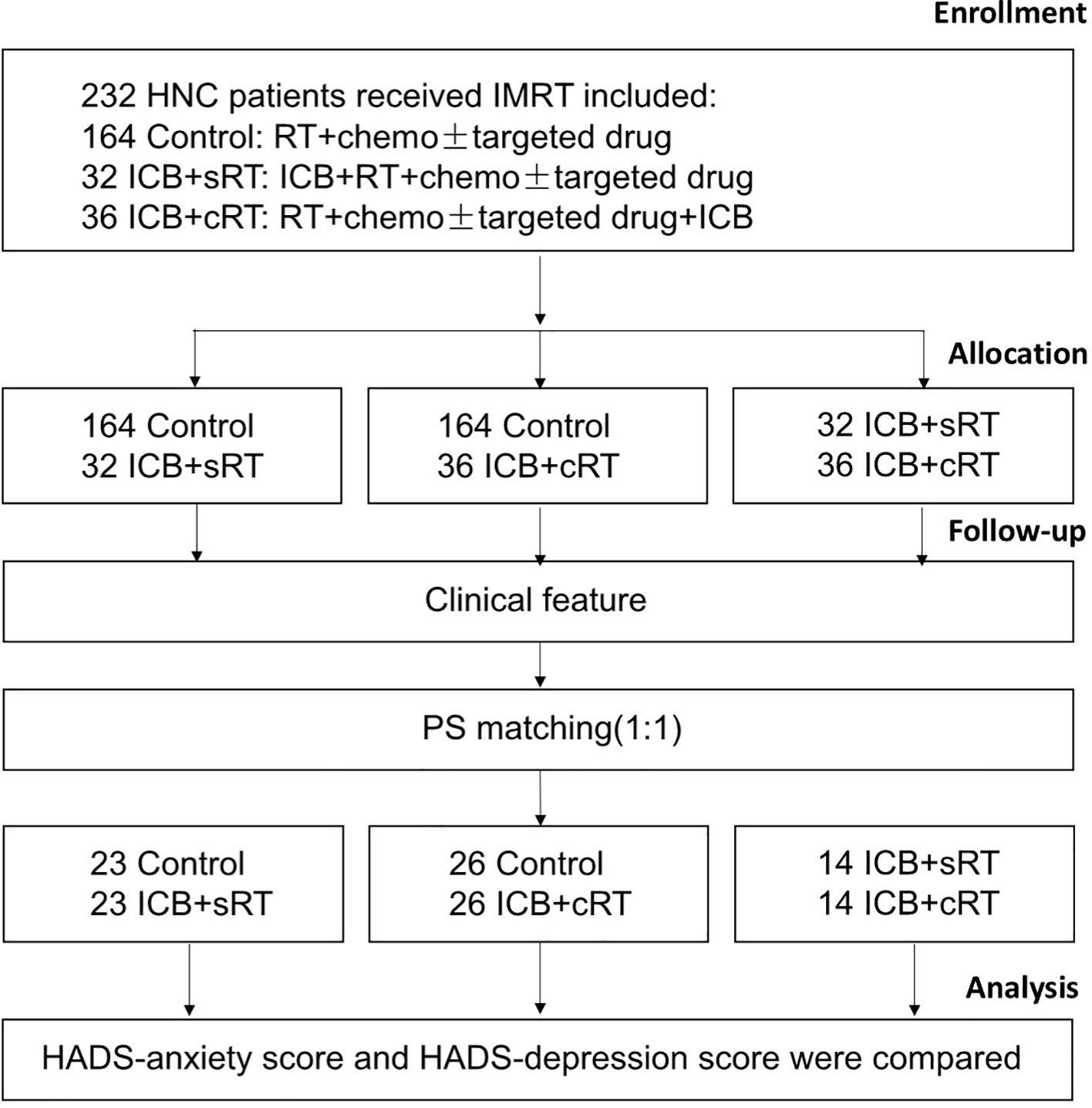
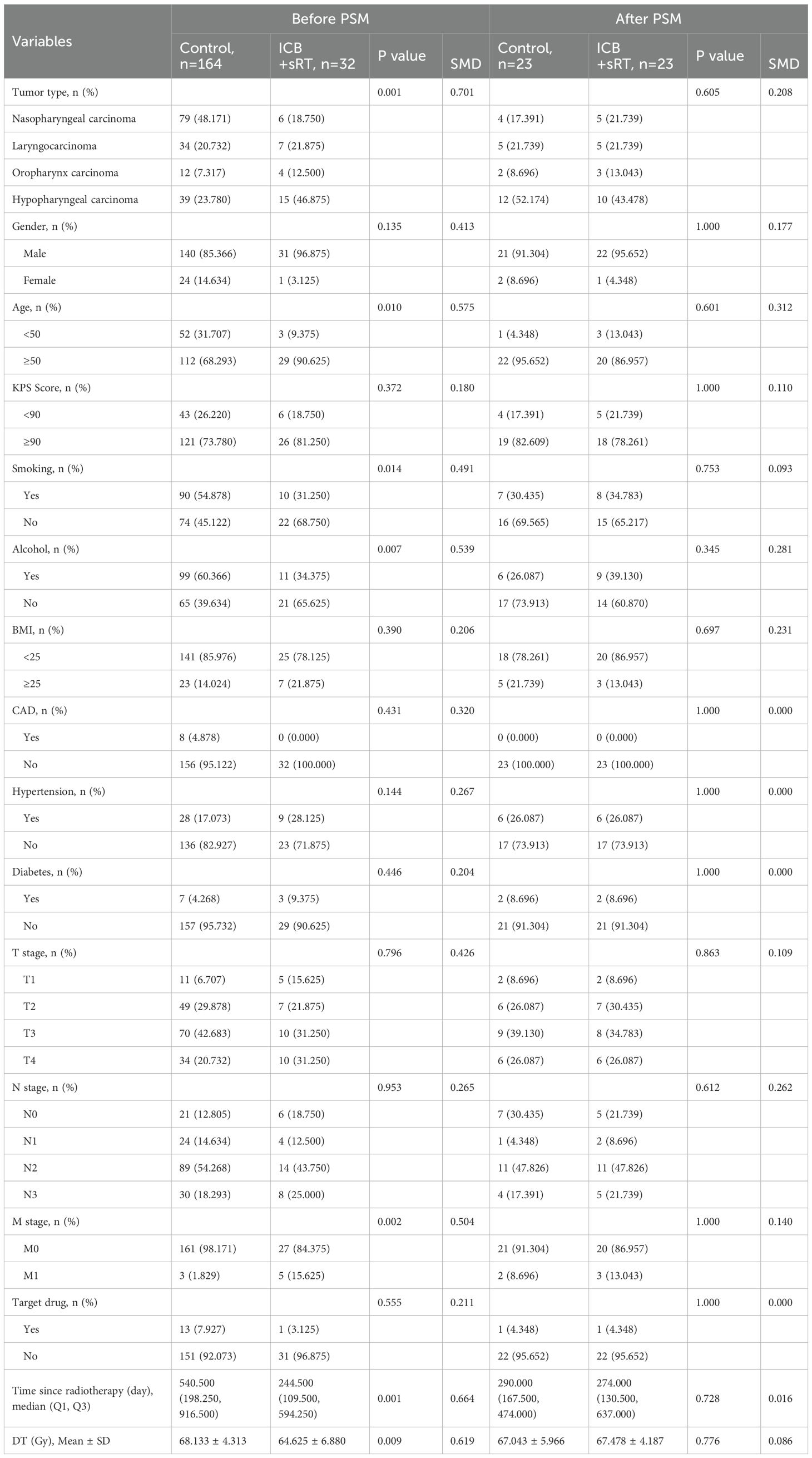
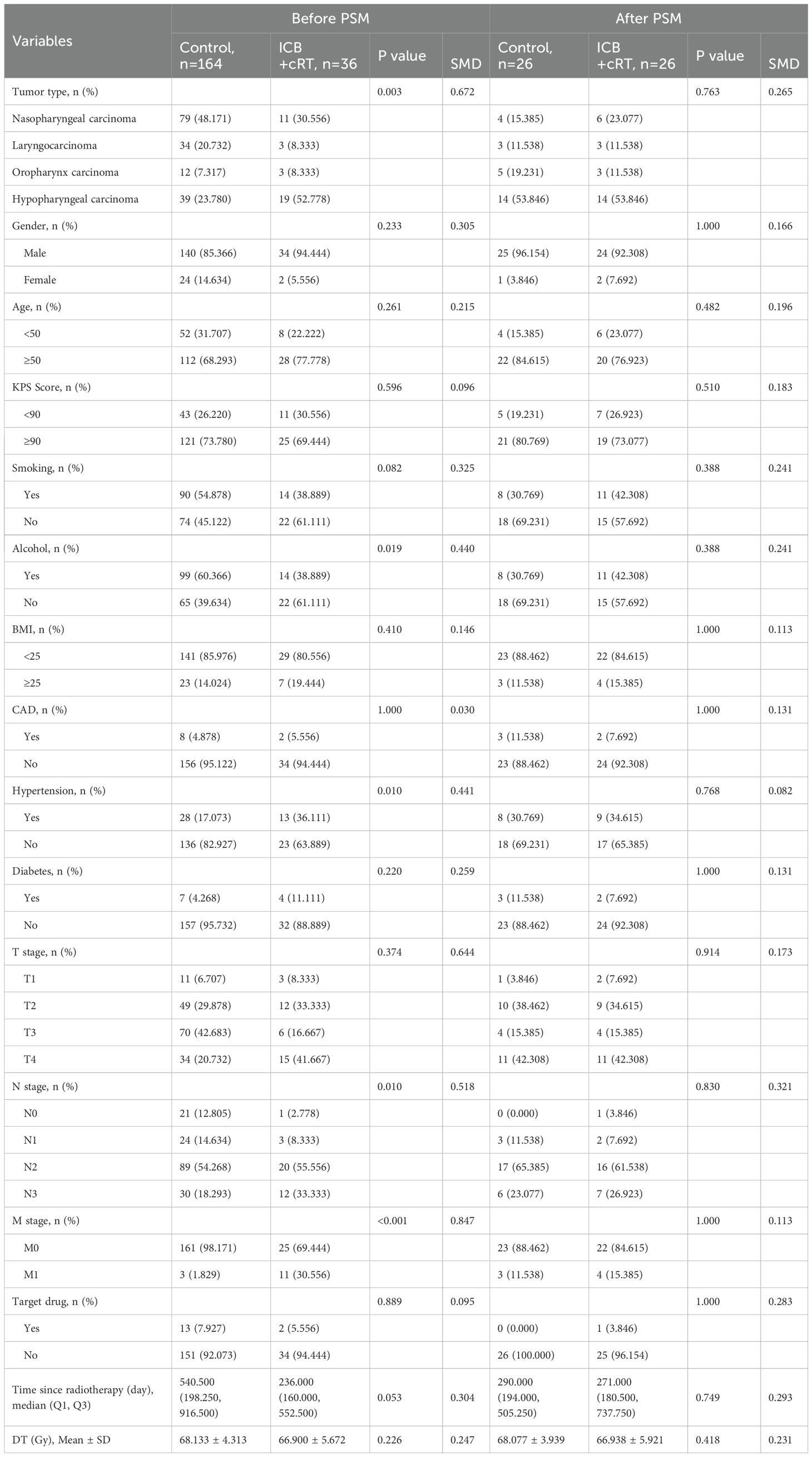
The research flowchart and PSM protocol are shown in Figure 1. After PSM, no significant difference remained in the baseline characteristics of the matched patients between the two groups (Tables 1–3). Twenty-three patients in the control group were matched with 23 patients in the ICB + sRT group (Table 1). Twenty-six patients in the control group were matched with 26 patients in the ICB + cRT group (Table 2). Fourteen patients in the ICB + sRT group were matched with 14 patients in the ICB + cRT group (Table 3). Love plots to demonstrate covariate balance were shown in the Supplementary Figure 1.
HADS score
A total of 232 patients were included, and the numbers of patients with anxiety in the control group, ICB + sRT group, and ICB + cRT group were 6, 1, and 2, respectively; the numbers of patients with depression were 36, 4, and 5, respectively; the numbers of patients with both anxiety and depression were 29, 6, and 6, respectively; and the occurrence rates of anxiety or depression were 43.29% (71/164), 34.38% (11/32), and 36.11% (13/36), respectively (Figure 2A). After PSM, the occurrence rates of anxiety or depression were 39.13% (9/23) and 26.09% (6/23) in the control group and the ICB + sRT group, respectively (Figure 2B). The occurrence rates of anxiety or depression were 46.15% (12/26) and 42.31% (11/26) in the control group and the ICB + cRT group, respectively (Figure 2C). The occurrence rates of anxiety or depression were 21.43% (3/14) and 28.57% (4/14) in the ICB + sRT group and the ICB + cRT group, respectively (Figure 2D).
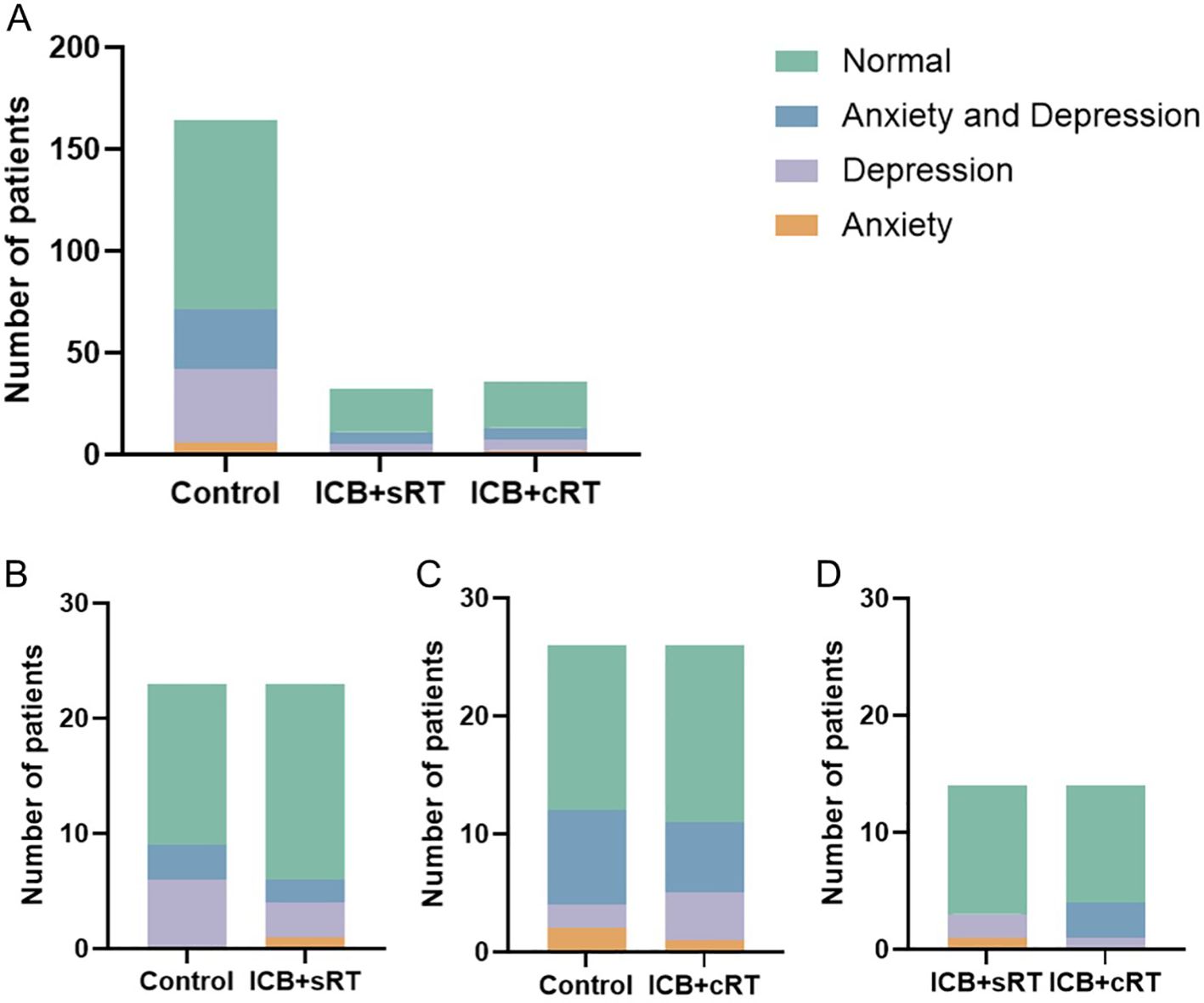
Figure 2. The distribution of patients with anxiety and depression. (A) The distribution of patients with anxiety and depression in the control group (n = 164), the ICB + sRT group (n = 32), and the ICB + cRT group (n = 36) before PSM. (B–D) The distribution of patients with anxiety and depression between the control group and the ICB + sRT group (n = 23), the control group and the ICB + cRT group (n = 26), and the ICB + sRT group and the ICB + cRT group (n = 14) after PSM.
Association of ICB with HADS score
After PSM, compared with those of the control group, the depression scores of the ICB + sRT group were significantly lower than those of the control group [2.00 (1.00, 6.00) vs. 7.00 (3.00, 9.00), P = 0.009; r = 0.386, 95% CI (0.121-0.624), power = 0.82; Figure 3A]. However, the anxiety scores of the ICB + sRT group did not differ from those of the control group [3.00 (0.00, 7.00) vs. 5.00 (2.00, 7.00), P = 0.124; r = 0.227, 95% CI (-0.053-0.499), power = 0.35; Figure 3B]. Similarly, the depression scores of the combined ICB + cRT group were significantly lower [2.50 (1.00, 8.00) vs. 7.00 (4.75, 8.25), P = 0.018; r = 0.327, 95% CI (0.041-0.562), power = 0.68; Figure 3C]. However, no difference was detected in the anxiety scores between the two groups [5.50 (1.00, 8.25) vs. 5.50 (2.75, 8.00), P = 0.473; r = 0.099, 95% CI (-0.179-0.374), power = 0.15; Figure 3D]. For the ICB + sRT group and the ICB + cRT group, no differences in depression scores [2.00 (1.00, 6.25) vs. 2.00 (1.00, 8.00), P = 0.708; r = 0.071, 95% CI (-0.312-0.460), power = 0.09; Figure 3E] or anxiety scores [3.50 (0.00, 5.25) vs. 2.50 (1.00, 7.00), P = 0.548; r = 0.114, 95% CI (-0.267-0.488); power = 0.12, Figure 3F] were detected.
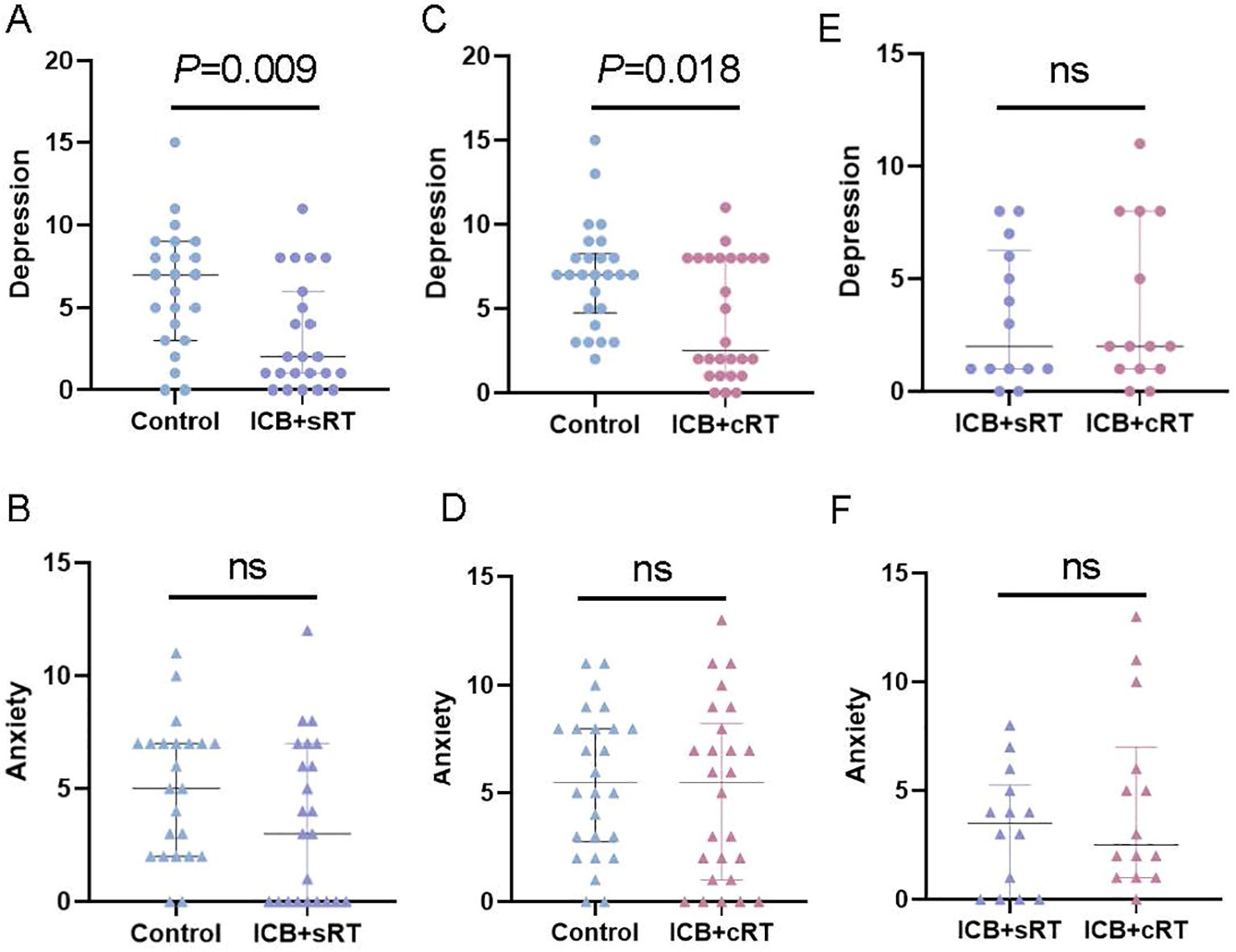
Figure 3. Association of ICB with HADS scores. (A–E) Comparison of depression status between the control group and the ICB + sRT group, the control group and the ICB + cRT group, and the ICB + sRT group and the ICB + cRT group. (B–F) Comparison of anxiety status between the control group and the ICB + sRT group, the control group and the ICB + cRT group, and the ICB + sRT group and the ICB + cRT group. The median and interquartile range of anxiety and depression scores are shown by bars. ns, not significant.
Discussion
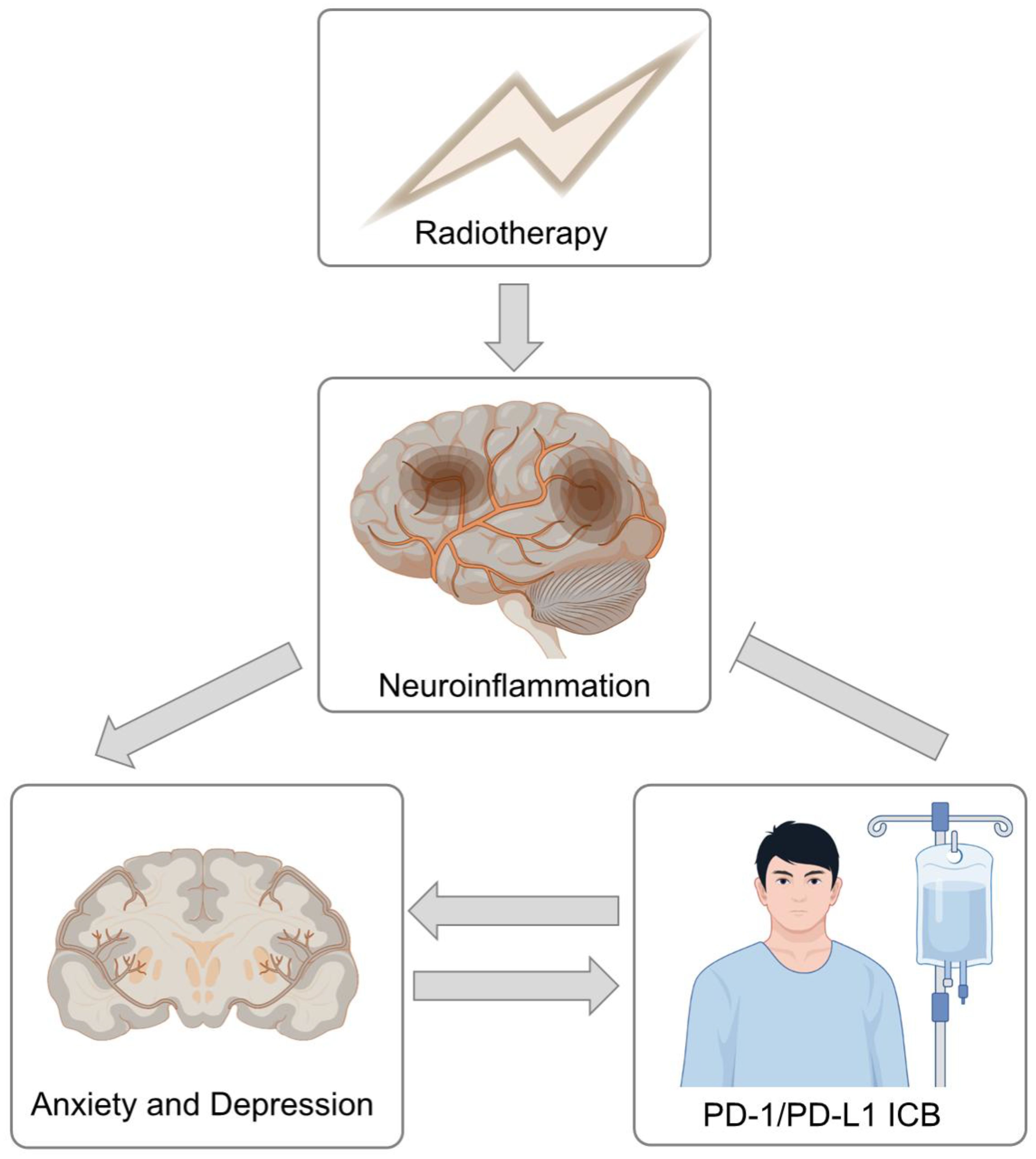
This is the first study to explore the effect of combined ICB on depression and anxiety in stage III–IV HNC patients after RT. We found that HNC patients receiving CRT combined with ICB, both concurrent RT and sequential RT, had lower depression levels than did patients receiving CRT alone. As previously mentioned, two studies have reported the negative roles of anxiety and depression in the efficacy of ICB. It looks like a closed loop (graphical abstract) (9, 10). There is currently no direct evidence to explain this result. It has previously been proposed that increased depression is independently linked to worsened neurocognition among primary brain tumor patients receiving RT (12). For late-stage HNC patients, the target area of RT is relatively large and normal brain tissue is inevitably inevitable exposed to radiation. Clinical manifestations may include mental disorders, focal neurological deficits, and the progressive degeneration of learning and memory functions due to hippocampal damage (13–16). Therefore, one of the possible explanations might be related to the effect of PD-1/PD-L1 inhibitors on the cognitive function of patients. Alzheimer’s disease (AD) is clinically characterized by memory loss and cognitive decline (17). In AD and dementia mouse models, Baruch et al. and Rosenzweig et al. demonstrated that a PD-1/PD-L1 inhibitor evokes an IFNγ-dependent systemic immune response that recruits monocyte-derived macrophages to the brain. This immunological response can improve cognitive performance (18, 19). However, a more likely explanation is that, PD-1 inhibitors can reduce neuroinflammatory responses and may also regulate neuronal activity (19–21). Direct damage to neurons and chronic inflammation induced by RT are well recognized (22), and depressive-like behavior in vivo occurs mainly via neuroinflammatory response activation (23). It is reasonable to speculate that PD-1/PD-L1 inhibitors can also reduce the RT-induced decrease in cognitive function. However, owing to the lack of brain structure-specific dosimetry data, especially for hippocampal radiation exposure, we cannot directly assess the relationships among regional radiation doses, neuroinflammation, and changes in anxiety or depression. These findings should be further validated in HNC patients.
Additionally, although our findings revealed a statistically detectable association between radiotherapy sequencing data and HADS scores, the magnitude of these differences may not reach the threshold for meaningful clinical changes in most patients. The clinical implications of these unique findings merit further evaluation, and pretreatment mood assessment should be included to assess treatment-attributable changes in future studies. Notably, power analysis revealed that after PSM, the statistical power for some comparisons fell below 0.8, indicating that our ability to detect true differences in these cases was limited, with an increased risk of Type II error. Future studies with larger sample sizes would help strengthen the evidentiary basis and confirm the robustness of these findings.
Limitations
This study had several limitations. First, our study is subject to potential survivorship bias and immortal-time bias, which may have excluded those with worse clinical or psychological outcomes, potentially skewing our findings toward more favorable mood profiles. Second, this was a single-center study without independent validation. Third, the types of anti-PD-1/PD-L1 antibodies used for treatment were heterogeneous, subgroup analyses were not feasible here given the individualized nature of drug selection and small sample sizes for certain agents, which would compromise statistical robustness. Furthermore, the omission of key confounders, including socioeconomic status, education, social support, psychotropic medication use, and prior psychiatric history, may introduce residual confounding, as they could independently influence anxiety or depression scores and thus affect the interpretation of our findings. Multicenter and prospective studies are needed to confirm these findings.
Conclusion
In this study, HNC patients receiving CRT combined with ICB, both concurrent RT and sequential RT, had lower depression levels than did those receiving CRT alone. Conversely, no significant differences in anxiety levels were noted across these treatment groups. This study is the first to show that patients who received ICB tended to have lower HADS scores over time. This information may have important implications for personalized approaches to preventing anxiety or depression in HNC patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Shandong Cancer Hospital and Institute. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Data curation, Writing – original draft, Formal analysis. YC: Data curation, Writing – original draft, Formal analysis. XZ: Writing – review & editing, Methodology. YH: Methodology, Writing – review & editing. ZZ: Conceptualization, Writing – review & editing. XD: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by National Natural Science Foundation of China (No. 82202958), Higher Educational Institutions Youth Innovation Science and Technology Support Program of Shandong Province (2023KJL004), Science Technology Program of Jinan (No. 202225013) and China Postdoctoral Science Foundation (2023M734299).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1649486/full#supplementary-material
Supplementary Figure 1 | The Love plots before and after PSM. (A, B): Love plots of the control group and the ICB + sRT group before and after PSM. (C, D): Love plots of the control group and the ICB + cRT group before and after PSM. (E, F): Love plots of the ICB + sRT group and the ICB + cRT group before and after PSM. Group 1: The control group. Group 2: The ICB + sRT group. Group 3: the ICB + cRT group.
References
1. De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, and Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Dev (Cambridge England). (2000) 127:483–92. doi: 10.1242/dev.127.3.483
2. Kang H, Kiess A, and Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat Rev Clin Oncol. (2015) 12:11–26. doi: 10.1038/nrclinonc.2014.192
3. Zhang Z, Liu X, Chen D, and Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal transduction targeted Ther. (2022) 7:258. doi: 10.1038/s41392-022-01102-y
4. Oskam IM, Verdonck-de Leeuw IM, Aaronson NK, Kuik DJ, de Bree R, Doornaert P, et al. Quality of life as predictor of survival: a prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiotherapy oncology: J Eur Soc Ther Radiol Oncol. (2010) 97:258–62. doi: 10.1016/j.radonc.2010.02.005
5. Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, and Duffy SA. Quality of life scores predict survival among patients with head and neck cancer. J Clin oncology: Off J Am Soc Clin Oncol. (2008) 26:2754–60. doi: 10.1200/JCO.2007.12.9510
6. Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, and Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. (2009) 50:383–91. doi: 10.1176/appi.psy.50.4.383
7. Olatunji BO, Cisler JM, and Deacon BJ. Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings. Psychiatr Clinics North America. (2010) 33:557–77. doi: 10.1016/j.psc.2010.04.002
8. Suleiman-Martos N, Gomez-Urquiza JL, Aguayo-Estremera R, Cañadas-De La Fuente GA, de la Fuente-Solana EI, and Albendín-García L. The effect of mindfulness training on burnout syndrome in nursing: A systematic review and meta-analysis. J advanced nursing. (2020) 76:1124–40. doi: 10.1111/jan.14318
9. Zeng Y, Hu CH, Li YZ, Zhou JS, Wang SX, Liu MD, et al. Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer. Nat Med. (2024) 30:1680–8. doi: 10.1038/s41591-024-02929-4
10. Fraterman I, Reijers ILM, Dimitriadis P, Broeks A, Gonzalez M, Menzies AMM, et al. Association between pretreatment emotional distress and neoadjuvant immune checkpoint blockade response in melanoma. Nat Med. (2023) 29:3090–9. doi: 10.1038/s41591-023-02631-x
11. Zigmond AS and Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
12. Tibbs MD, Huynh-Le MP, Reyes A, Macari AC, Karunamuni R, Tringale K, et al. Longitudinal analysis of depression and anxiety symptoms as independent predictors of neurocognitive function in primary brain tumor patients. Int J Radiat oncology biology physics. (2020) 108:1229–39. doi: 10.1016/j.ijrobp.2020.07.002
13. Roman DD and Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat oncology biology physics. (1995) 31:983–98. doi: 10.1016/0360-3016(94)00550-8
14. Li Y, Shi X, Rong X, Peng Y, and Tang Y. Neurosurgery and prognosis in patients with radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy: a follow-up study. Radiat Oncol (London England). (2013) 8:88. doi: 10.1186/1748-717X-8-88
15. Huang X, Zhang X, Wang X, Rong X, Li Y, Li H, et al. A nomogram to predict symptomatic epilepsy in patients with radiation-induced brain necrosis. Neurology. (2020) 95:e1392–e403. doi: 10.1212/WNL.0000000000010190
16. Cai J, Cheng J, Li H, Lin WJ, Li Y, Zhuo X, et al. A nomogram for the prediction of cerebrovascular disease among patients with brain necrosis after radiotherapy for nasopharyngeal carcinoma. Radiotherapy Oncol. (2019) 132:34–41. doi: 10.1016/j.radonc.2018.11.008
17. Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, et al. O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell. (2016) 15:455–64. doi: 10.1111/acel.12449
18. Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. (2016) 22:135–7. doi: 10.1038/nm.4022
19. Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, et al. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun. (2019) 10:465. doi: 10.1038/s41467-019-08352-5
20. Zhao S, Li F, Leak RK, Chen J, and Hu X. Regulation of neuroinflammation through programed death-1/programed death ligand signaling in neurological disorders. Front Cell Neurosci. (2014) 8:271. doi: 10.3389/fncel.2014.00271
21. Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci. (2017) 20:917–26. doi: 10.1038/nn.4571
22. Gan C, Li W, Xu J, Pang L, Tang L, Yu S, et al. Advances in the study of the molecular biological mechanisms of radiation-induced brain injury. Am J Cancer Res. (2023) 13:3275–99.
Keywords: immune checkpoint blockade, head and neck cancer, chemoradiotherapy, depression, anxiety
Citation: Zhang Y, Chen Y, Zhang X, Hong Y, Zhou Z and Ding X (2025) Associations between chemoradiotherapy-based immune checkpoint blockade and posttreatment depression and anxiety in head and neck cancer patients: a cross-sectional study. Front. Immunol. 16:1649486. doi: 10.3389/fimmu.2025.1649486
Received: 18 June 2025; Accepted: 18 August 2025;
Published: 01 September 2025.
Edited by:
Claire J. Han, The Ohio State University, United StatesReviewed by:
Pramod G. V., Bapuji Dental College and Hospital, IndiaDaniel Thomas Jones, HCA Healthcare, United States
Copyright © 2025 Zhang, Chen, Zhang, Hong, Zhou and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingchen Ding, U0RkaW5neGluZ2NoZW5AMTI2LmNvbQ==; Zihan Zhou, emloYW56aG91MTIzQDE2My5jb20=
†These authors have contributed equally to this work
 Yang Zhang1,2†
Yang Zhang1,2† Yunhao Chen
Yunhao Chen Xingchen Ding
Xingchen Ding