- 1Department of Chronic Diseases and Metabolism, Laboratory of Respiratory Diseases and Thoracic Surgery, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 2Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Group, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 3Clinical Division of Paediatrics, UZ Leuven, Leuven, Belgium
- 4Clinical Division of Respiratory Medicine, UZ Leuven, Leuven, Belgium
- 5Department of Public Health and Primary Care, Environment and Health, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
Background: Cold air and air pollution are known triggers to induce symptoms in exercise-induced bronchoconstriction (EIB). Mast cells are hypothesized to be a key player in the pathogenesis of EIB. This study aims to investigate the role of mast cells using mast cell deficient (Cpa3cre/+) mice and with mast cell stabilizers (Doxantrazole) in an exposure-associated mouse model of EIB.
Methods: Male Cpa3cre/+ mice and wild type littermates or BALB/c mice were exposed to a submaximal running protocol in cold environment (4°C) or resting period (room temperature) 5 days for 3 weeks after oropharyngeal challenge with saline or 0.1 mg/ml diesel exhaust particles (DEP). BALB/c mice were intraperitoneally injected with 16.5 mg/kg Doxantrazole or placebo (0.5% NaHCO3) during the last week. Twenty-four hours after the last running or resting session, lung function, lung inflammation and immune mediated response was determined.
Results: Inhibition of mast cells by Doxantrazole or mice lacking functional mast cells (Cpa3cre/+), resulted in blunting of bronchial hyperreactivity, both in acute breathing pattern and in hyperreactivity to increasing doses of methacholine. Neutrophilic inflammation was still present after Doxantrazole treatment, but not in Cpa3cre/+ mice. These results were similar in neutrophil extracellular traps and neutrophil-linked cytokines and chemokines. Macrophage activaty was also reduced in the absence of functional mast cells.
Conclusion: Mast cells are crucial in the development of bronchial hyperreactivity and macrophage activation. Additionally, they have a complex interplay with neutrophilic inflammation. These findings highlight the potential of mast cell modulation as therapeutic strategy in exposure-associated EIB.
Introduction
Exercise-induced bronchoconstriction (EIB) is defined as the reversible and transient narrowing of the airways during or shortly after physical exertion (1). Typically experienced symptoms include cough, chest tightness, shortness of breath and wheezing. Although the prevalence is high in both athletes and patients with asthma, with an average of 30 – 70% and 40 – 90%, respectively, the pathophysiological mechanisms are still not fully understood (2).
Couto et al. suggests that mast cells are a key player in EIB, next to neutrophils and TRP-channels (3). Mast cells are bone-marrow derived cells that mature under the influence of stem cell factor, the ligand of the c-kit receptor. Mast cell precursors migrate from the bone marrow to epithelial and mucosal tissues, such as the gastrointestinal tract, skin and the respiratory epithelium, where they complete maturation (4). Different subtypes of mast cells have been identified in both humans and mice, distinguished by their granule content, tissue localisation and functional characteristics (5).
In the lung, mast cells can promote airway smooth muscle contraction through the release of contractile agonists such as histamine and cysteinyl leukotriene (6). While IgE/FcϵRI-dependent mast cell activation is most studied and understood (7), IgE/FcϵRI-independent routes also contribute to bronchoconstriction. The mast cell receptor MAS-related G protein-coupled receptor X2 (MRGPRX2) was over 15 years ago identified in humans to play an important role in IgE-independent mast cell activation (8, 9). Although several studies report increased numbers of MRGPRX2-positive mast cells in lung tissue from patients with asthma (10, 11), its exact role in the pathogenesis of asthma remains incompletely understood and difficult to explore (8). Thus, MRGPRX2 may contribute to mast cell–mediated airway responses under certain conditions, but its precise role in asthma pathogenesis requires further clarification.
Different mouse models lacking functional mast cells have been developed and used to study allergic and non-allergic asthma (12–15). KitW-sh/W-sh mice have the W-sash inversion mutation in the regulatory elements upstream of the c-kit transcription site (16), whereas the Cpa3cre/+ mice exhibit mast cell deficiency as a result of Cre-mediated toxicity driven by the carboxypeptidase A3 promotor (17). Both models lack mast cells in all tissues, while maintaining normal levels of other immunological cells, such as neutrophils and erythrocytes (13, 18). Cpa3cre/+ mice have an additional reduction in the number of basophils compared to wild type mice (18).
In this study, we aim to evaluate mast cell involvement in the development of bronchial hyperresponsiveness (BHR) and airway inflammation in an EIB-mouse model exposed to cold air and/or diesel exhaust particles (DEP). To achieve this, mice lacking functional mast cells (Cpa3cre/+ mice) and wild type (WT) littermates were compared. Additionally, therapeutic inhibition of BHR and inflammation was evaluated with a mast cell stabilizer [doxantrazole (19)] in WT BALB/c mice.
Methods
Reagents
Diesel exhaust particles (DEP, NIST2975), which was characterized by the National Institute of Standards and Technology (NIST), Tween®20 and doxantrazole (3-(1H-tetrazol-5-YL)-9H-thioxanthen-9-one 10,10-dioxide monohydrate) were obtained from Merck (Belgium). Doxantrazole was dissolved in 0.5% NAHCO3 and sonicated for 25 minutes. Pentobarbital (Dolethal®) and Isoflurane (Iso-Vet 1000 mg/g) were obtained via the animal facility from KU Leuven.
Animals
Eight- to ten-week old male BALB/c mice (n = 80) were obtained from Charles River Laboratories (Belgium). Cpa3Cre/+ gene-targeted mice were kindly provided by Prof. Hans-Reimer Rodewald (17) and were bred on a BALB/c background. Cpa3cre/+ mice (n = 64) and wild type littermates (n = 53) were used in the experimental set-up. To ensure an adequate sample size, littermates were combined with wild type BALB/c mice (Charles River Laboratories, n = 64) after an evaluation of potential differences between experiments. Schematic overview of the experiments can be found in Supplementary Figure S1.
Mice were housed in individually ventilated cages per experimental group with ad libitum access to food and water. Environmental conditions were standardized (12h dark/light cycles, 22 – 24°C and relative humidity of 50-60%). All experiments were approved by the local Ethical Committee for animal experiments of KU Leuven, Belgium (P118/2021) and comply with the ARRIVE guidelines.
Experimental running and instillation protocol
A previously optimized sub-maximal running protocol of 3 weeks in combination with DEP and cold exposure was used to induce EIB as described earlier (20, 21). Immediately before each running or resting session, animals were oropharyngeally instilled with 50 µl 0.1 mg/ml DEP, dissolved in vehicle (saline + 0.2% Tween20) or with 50 µl vehicle.
Mice were divided into 8 groups based on 3 variables (1): Exercise (E) or No Exercise (NE) (2); Room temperature (RT) or cold air (4°C) and (3) DEP or Saline (Sal). During the first four days of the last week of the protocol, mice in the mast cell stabilizer experiment were intraperitoneally injected with doxantrazole (16.5 mg/kg) or placebo (50 µl NaHCO3) one hour before oropharyngeal instillation with saline or DEP. In this experimental set-up, only RT resting mice (NE/RT) and 4°C exercising (E/4°C) mice were evaluated, with saline or DEP.
Lung function assessment
Every first day of the week, respiratory pattern was measured non-invasively using Double Chamber Plethysmography (DCP, Emka Technologies, Paris, France) in conscious mice as described earlier (22).
Lung function measurements were performed 24 hours after the final running session, following the methodology previously described (17, 18) using the flexiVent FX system (SCIREQ, Montreal, Canada, version 7.6) equipped with a Negative Pressure Forced Expiration (NPFE) and FX1 module. Baseline lung function was evaluated using deep inflation (inspiratory capacity), Quick-Prime 3 (QP3) pertubations (airway resistance (Rn), tissue damping (G), tissue elastance (H)) and NPFE pertubations (forced volumes). Subsequently, airway resistance and forced expiratory volume in 0.1 seconds (FEV0.1) were measured in response to increasing doses of nebulized methacholine (baseline, 0, 2.5, 5, 10, 20 and 40 mg/ml).
Blood analysis
Immediately after lung function assessment, an overdose of pentobarbital was administered and blood was collected from the retro-orbital plexus. Blood was centrifuged (1400g, 4°C, 10 min), serum was collected and stored at -80°C until further analyses. Serum surfactant protein D (SpD) was determined using the Mouse SP-D DuoSet ELISA D DuoSet ELISA (R&D Systems, Minneapolis, US, detection limit 62.5 µg) according to manufacturer’s instructions.
Bronchoalveolar lavage fluid analyses
Bronchoalveolar lavage fluid (BALF) was collected by three washes of the lungs with 700 µl saline (0.9% NaCl). Pooled fluid was centrifuged (1000 g, 10 min, RT) and resuspended cells were counted using an automated cell counter (NanoEnTek, Seoul, Korea). Cytospins were made (300 g, 6 min, Cytospin 3, Shandon, TechGen, Zellik, Belgium) and stained with the Diff-Quick method (ThermoFisher Scientific, Massachusetts, US). 250 cells per slide were counted to determine the proportion of neutrophils, macrophages, eosinophils and lymphocytes.
DEP-uptake in macrophages was evaluated as percentage of loaded macrophages, determined by manually counting 250 cells. The DEP-covered area within the macrophage was calculated using the ImageJ software (NIH, Maryland, US) following the method of Bai et al. (23). Twenty-five macrophages per mouse were manually delineated, DEP-covered area was calculated by the software.
Cytokine and chemokine concentrations of granulocyte-macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-10, IL-33, keratinocyte-derived chemokine (KC) and monocyte chemoattractant protein-1 (MCP-1) were measured using a U-plex Assay (Meso Scale Diagnostics, Maryland, USA) according to manufacturer’s instructions. Neutrophil elastase concentration (Mouse Neutrophil Elastase/ELA2 DuoSet ELISA, R&D Systems, Minneapolis, US, detection limit 12.5 pg/ml) and dsDNA (Quant-iT PicoGreen dsDNA assay kit, ThermoFisher Scientific, Massachusetts, USA) were determined according to manufacturer’s instructions.
Flow cytometric analyses
Left lung was collected during harvest at the end of the protocol and processed to a single cell suspension. Cells were stained for viability and labelled with different anti-mouse fluorochrome-conjugated monoclonal antibodies to identify dendritic cell as previously described (22). Scatter plots of dendritic cell subpopulations are available in Supplementary Figure S2. Overview of used markers for flow cytometric analyses can be found in Supplementary Table S1.
Data analysis
Data are shown as individual mice and group mean or as mean with standard deviation (SD). Normality of the data was assessed using Shapiro-Wilk test. Non-parametric Wilcoxon matched-pairs rank test and parametric unpaired t-test were used to compare two groups. Intergroup differences were evaluated using a one-way parametric ANOVA combined with a Bonferroni multiple comparison post hoc test or a non-parametric Kruskal-Wallis test with a Dunn’s multiple comparison post hoc test. To evaluate combination effects, two-way ANOVA with a Bonferroni multiple comparison post hoc test was used. Data was evaluated using GraphPad Prism (9.3.0, GraphPad Software Inc., San Diego, USA). A level of p < 0.05 was considered significant. To enhance clarity, we restricted group comparisons to RT resting groups (NE/RT) and 4°C exercising groups (E/4°C). Additional groups were included to maintain consistency in the experimental model; however, no comparisons were made between WT and Cpa3cre/+ mice in this context. The same strategy and groups were used to compare the data of the doxantrazole experiment.
Results
Mast cells play a major role in breathing pattern and lung function
Previously, we showed significantly changes in the breathing pattern of animals exercising in cold environment (E/4°C) (20). One of the parameters which was significantly increased in the E/4°C groups, was the expiratory time (Te). Mice lacking functional mast cells (Cpa3cre/+) did not show an increased Te after exercise at 4°C, both when challenged with saline (Figure 1A) and with diesel exhaust particles (DEP, Figure 1B). WT DEP cold running mice showed a significantly more increased Te compared to Cpa3cre/+ mice. Wild type (WT) mice in the doxantrazole experiment all showed a significantly increased Te compared to non-exercising room temperature (RT/NE) mice (Figures 1C, D). The effect of doxantrazole could not be assessed in this setting due to the study design, in which mast cells were only inhibited during the final days. However, this indicates that the mice in the doxantrazole experiment also developed an altered breathing pattern resembling EIB in the first weeks of exercise at 4°C.
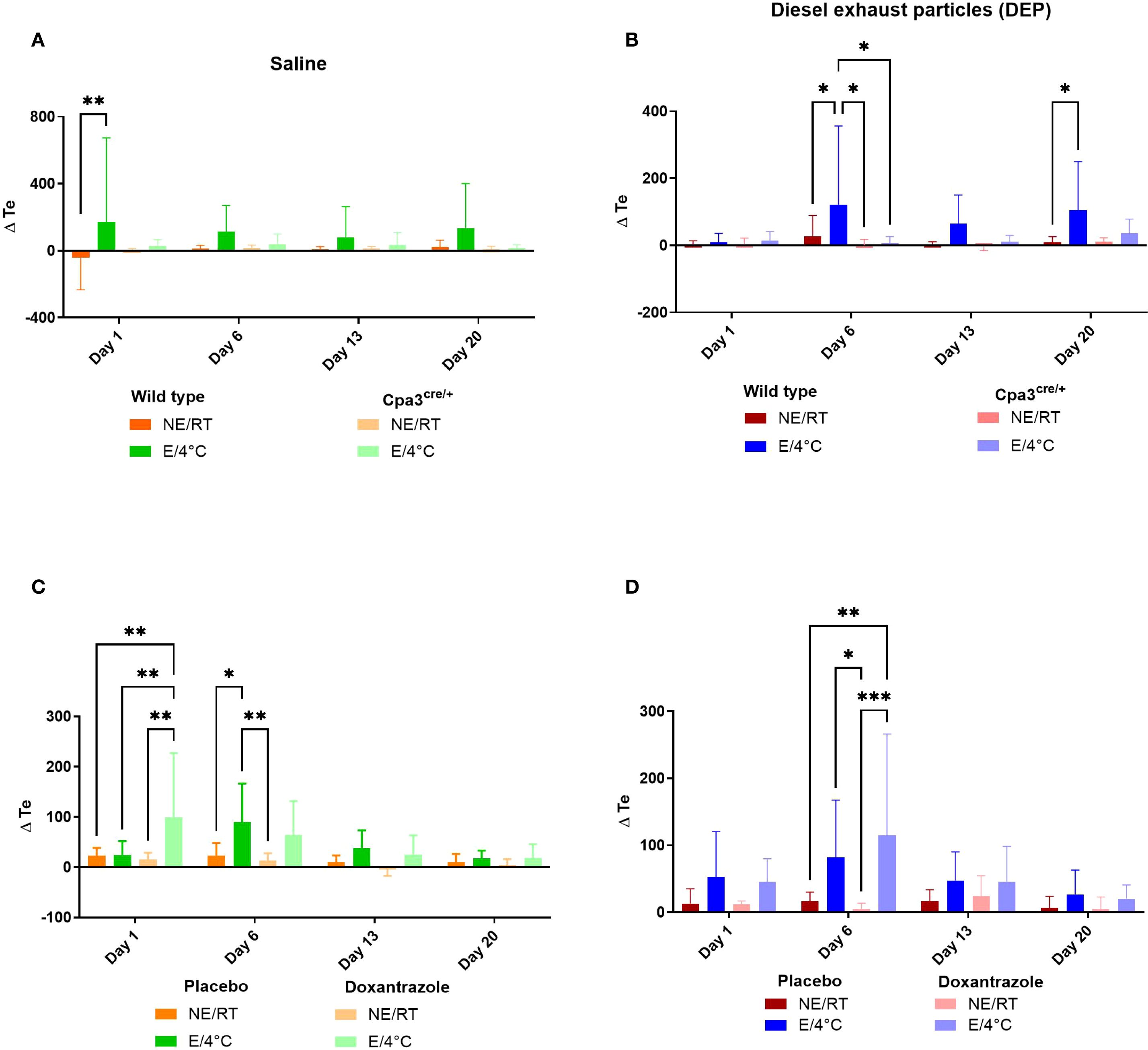
Figure 1. Role of mast cells in responses to cold air, exercise and DEP exposure on expiratory time. Expiratory time (Te) was measured on day 1, 6, 13 and 20 before and immediately after exercise or resting session with double chamber plethysmography, as outlined in Supplementary Figure S1. Changes were calculated as differences between pre- and post-exposure. This was assessed in saline-exposed wild type (WT) compared to mast cell deficient (Cpa3cre/+) mice (A), in diesel-exposed WT compared Cpa3cre/+ mice (B), in saline-exposed placebo-treated mice compared to doxantrazole-treated mice (C) and in diesel-exposed placebo-treated mice compared to doxantrazole-treated mice (D). Data are evaluated using two-way ANOVA with Bonferroni multiple comparison post hoc test and represented as mean ± SD. Levels of significance were *p < 0.05, **p < 0.01, ***p < 0.001. n = 7–8 per group. NE, no exercise; E, exercise; RT, room temperature.
Similar changes were seen in end-expiratory pause (EEP). The increase in EEP in cold air exercising mice exposed to DEP (DEP/E/4°C) as seen in WT animals was no longer present in Cpa3cre/+ mice (Figure 2B). Similar trends were seen after saline challenge, yet not significant (Figure 2A). In the doxantrazole experiment, the mice of the Sal/E(/4°C) also exhibited an increased EEP on day 1 (Figure 2C) and mice of the DEP/E(/4°C) group showed an increased EEP on day 6 (Figure 2D).
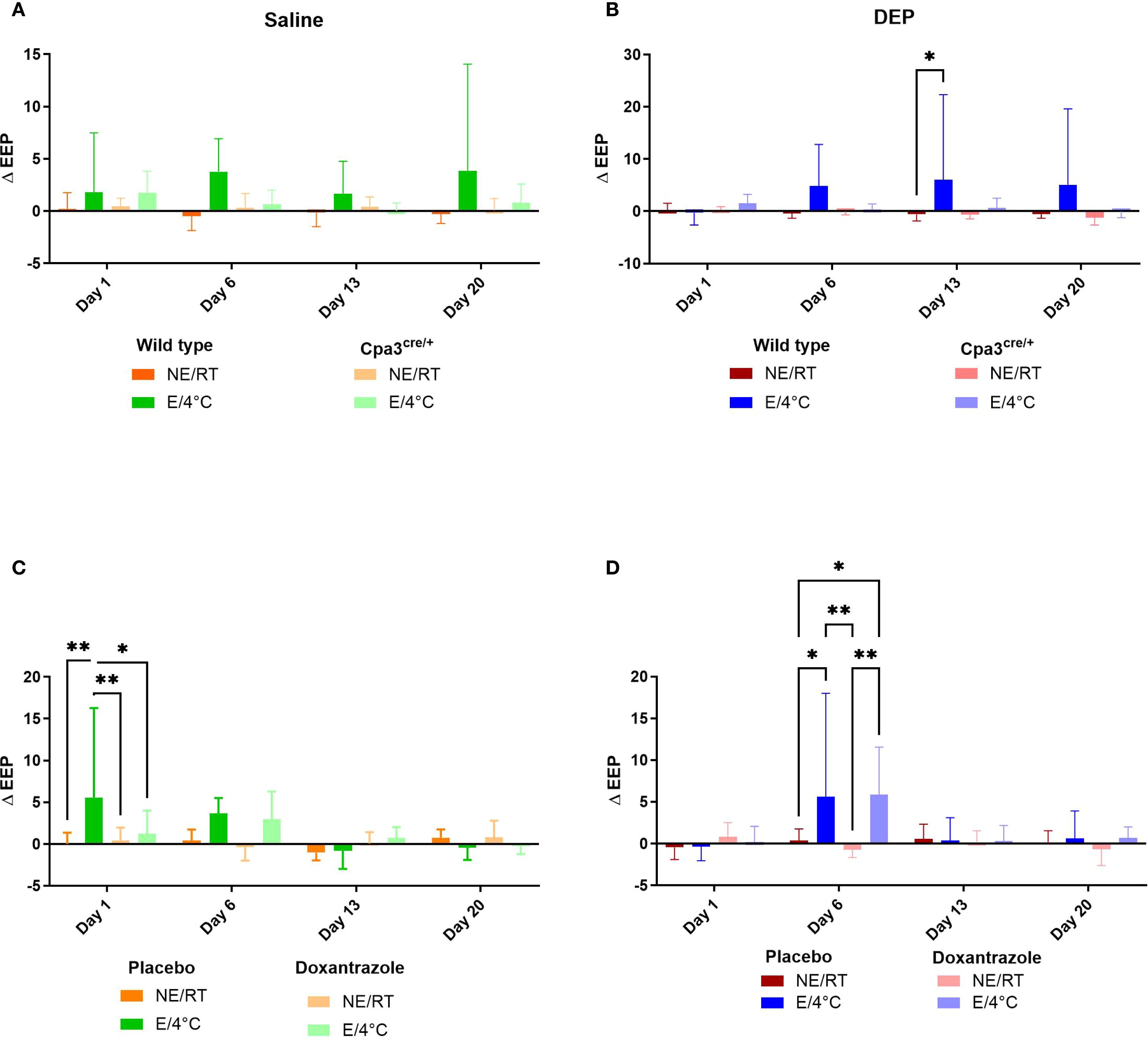
Figure 2. Role of mast cells in responses to cold air, exercise and DEP exposure on end-expiratory pause. End-expiratory pause (EEP) was measured on day 1, 6, 13 and 20 before and immediately after exercise or resting session with double chamber plethysmography based on the experimental protocol (Supplementary Figure S1). Changes were calculated as differences between pre- and post-exposure. This was measured in saline-exposed wild type (WT) compared to mast cell deficient (Cpa3cre/+) mice (A), in diesel-exposed WT compared Cpa3cre/+ mice (B), in saline-exposed placebo-treated mice compared to doxantrazole-treated mice (C) and in diesel-exposed placebo-treated mice compared to doxantrazole-treated mice (D). Data are evaluated using two-way ANOVA with Bonferroni multiple comparison post hoc test and represented as mean ± SD. Levels of significance were *p < 0.05, **p < 0.01, ***p < 0.001. n = 7–8 per group. NE, no exercise; E, exercise; RT, room temperature.
Twenty-four hours after the last running or resting session, lung function was measured invasively. No differences between WT and Cpa3cre/+ saline resting RT mice were seen in baseline volumes (FVC, FEV0.1, FEV0.2), baseline airway resistance (Rn), tissue elastance (H) or tissue damping (G) (data not shown). There were also no baseline differences between the groups in the Cpa3cre/+ experiment (data not shown).
To investigate the role of mast cells in exercise-induced bronchoconstriction in response to cold air, we assessed the reduction in forced expiratory volume in 0.1 second (FEV0.1) to increasing doses of methacholine. In WT mice, exercise in cold air induced a dose-dependent decrease in FEV0.1, whereas this response was absent in Cpa3cre/+ mice (Figure 3A). The reduction in FEV0.1 was significantly more pronounced in WT cold exercising mice compared to Cpa3cre/+ cold exercising mice. No differences were seen between the Cpa3cre/+ mice groups (Supplementary Figure S3A). One week of doxantrazole treatment was sufficient to inhibit the bronchoconstrictive effect, resulting in a significant lower reduction in FEV0.1 compared to placebo (Figure 3B). The dose response curves for airway resistance (Rn) showed similar results as the ones for FEV0.1 in both experiments, with no significantly increased airway resistance upon methacholine challenge in either Cpa3cre/+ exercising mice (Figure 3C, Supplementary Figure S3B) or doxantrazole-treated exercising mice (Figure 3D).
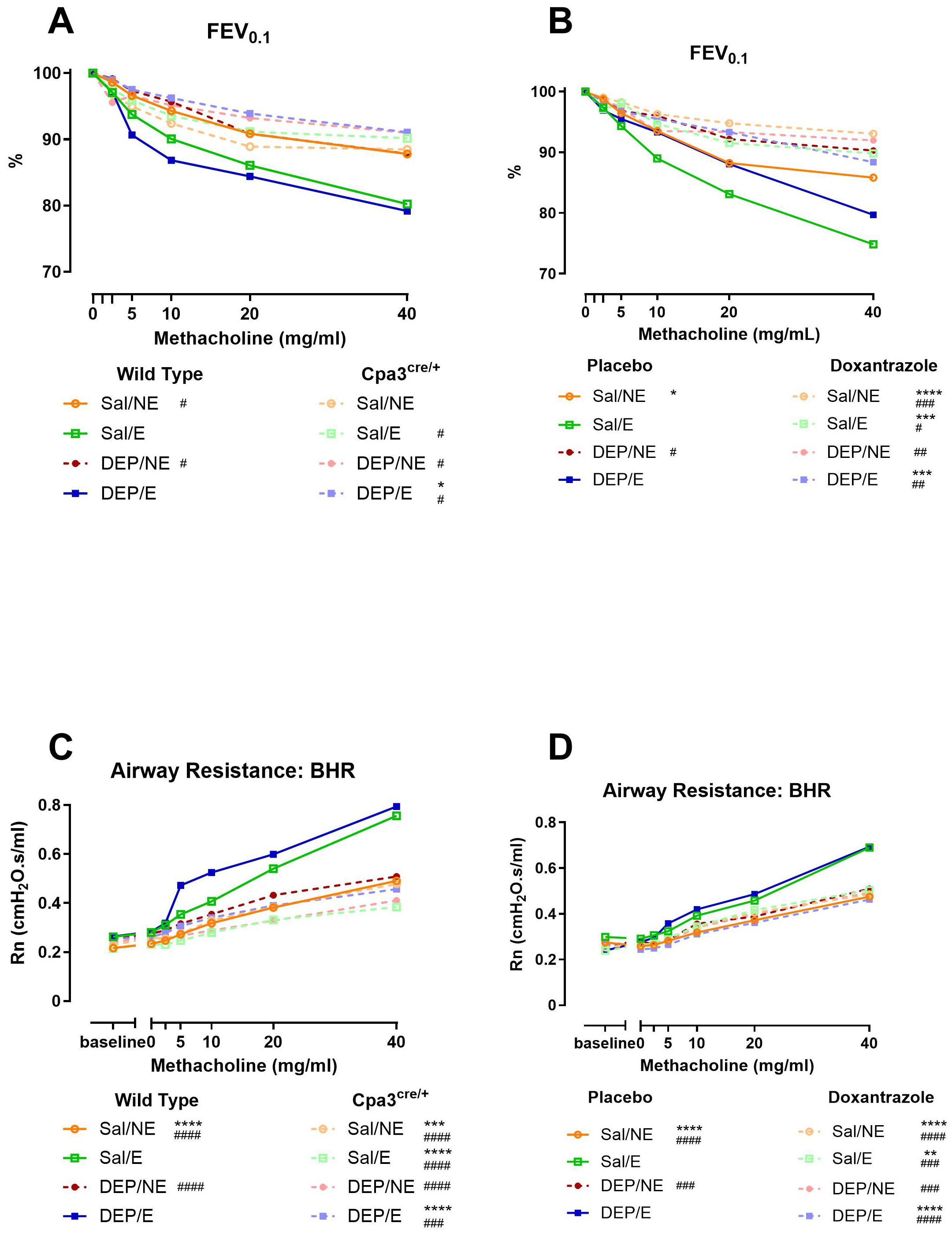
Figure 3. Role of mast cells in bronchial hyperreactivity to cold air, exercise and DEP exposure. Twenty-four hours after the last running or resting session (Supplementary Figure S1), animals were sacrificed and reactivity to methacholine was measured using the flexiVent. Dose-response curve in FEV0.1 (%) to increasing doses of methacholine (0–40 mg/ml) in WT compared to Cpa3cre/+ mice (A) and in placebo- compared to Doxantrazole-treated mice (B). Dose-response curve in airway resistance (Rn) to increasing doses of methacholine (0–40 mg/ml) in WT compared to Cpa3cre/+ mice (C) and in placebo- compared to Doxantrazole-treated mice (D). Data was acquired through negative pressure forced expiration (NPFE) manoeuvre and QP3 forced oscillation technique, respectively, with the flexiVent. Data are represented as group average and evaluated using two-way ANOVA with Bonferroni multiple comparison post hoc test, n = 7–16 per group. Levels of significance for groups were * compared to Sal/E (4°C, WT [A&C] or placebo [B&D] and # compared to DEP/E (4°C, WT [A&C] or placebo [C&D]). *, #p < 0.05, **, ##p < 0.01, ***, ###p < 0.001, ****, ####p < 0.0001. n = 7–8 per group. Sal, saline; DEP, diesel exhaust particles; NE, no exercise; E, exercise; RT, room temperature; FEV0.1, forced expiratory volume in 0.1 second; BHR, bronchial hyperresponsiveness.
Neutrophilic airway inflammation is not completely inhibited by mast cell inhibition
WT cold exercising mice showed significantly more neutrophils in the bronchoalveolar lavage fluid (BALF) compared to resting (RT) mice, with a more pronounced effect in DEP-challenged animals. This effect was entirely absent in Cpa3cre/+ mice. A significantly lower percentage of neutrophils was present in Cpa3cre/+ DEP/E/4°C mice compared to WT DEP/E/4°C mice (Figure 4A). Additionally, no differences were seen between the different Cpa3cre/+ groups (Supplementary Figure S4).
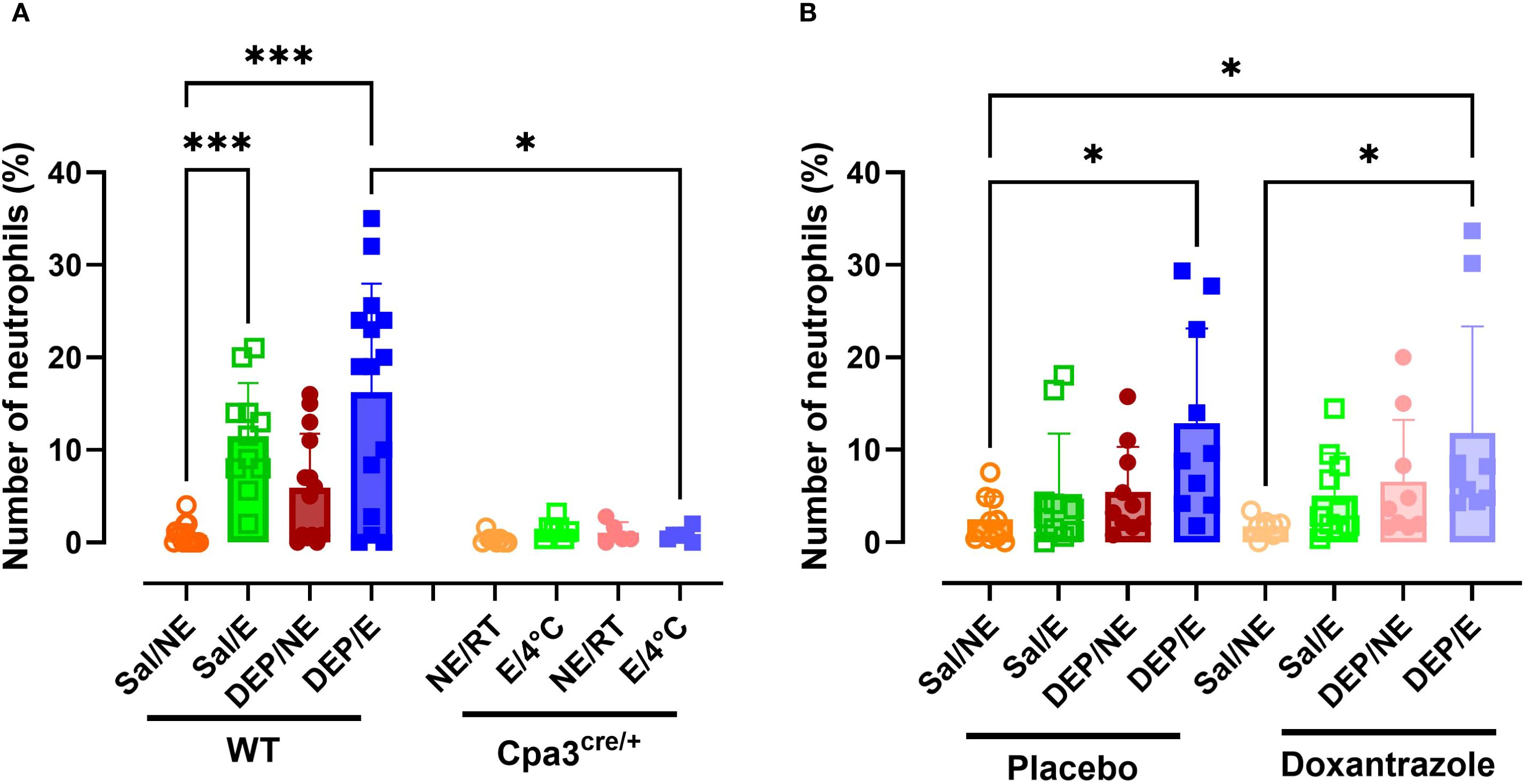
Figure 4. Response of mast cell inhibition on neutrophilic inflammation in the bronchoalveolar lavage fluid. After the experimental running protocol (Supplementary Figure S1), animals were sacrificed and bronchoalveolar lavage fluid (BAL) was collected to evaluate (neutrophilic) inflammation. Percentual numbers of neutrophils among total inflammatory cells present in the BAL of Cpa3cre/+ mice compared to WT mice (A) and placebo- compared to Doxantrazole treated mice. Data are represented as mean ± SD with individual values and were evaluated using Kruskal–Wallis test with Dunn’s post hoc testing. Levels of significance were Levels of significance were *p < 0.05, ***p < 0.001. n = 7–16 per group. Sal, saline; DEP, diesel exhaust particles; NE, no exercise; E, exercise; RT, room temperature; WT, wild type.
Both placebo and doxantrazole treated DEP/E/4°C mice showed significantly higher percentages of neutrophils in the BALF compared to Sal/NE/RT mice (Figure 4B). No differences were observed between doxantrazole and placebo treated animals.
Neutrophil elastase (NE) and double stranded DNA (dsDNA) were assessed as markers for neutrophil extracellular traps (NETs) in BAL. WT DEP/E(/4°C) mice showed significantly more NE in BAL compared to WT DEP/NE(/RT) and Cpa3cre/+ DEP/E(/4°C) mice (Figure 5A). This effect was not seen in doxantrazole-treated animals compared to resting nor placebo-treated animals (Figure 5B). Levels of dsDNA did not differ significantly between groups in either the Cpa3cre/+ knockout or the doxantrazole experiment (Supplementary Figures S5A, B).
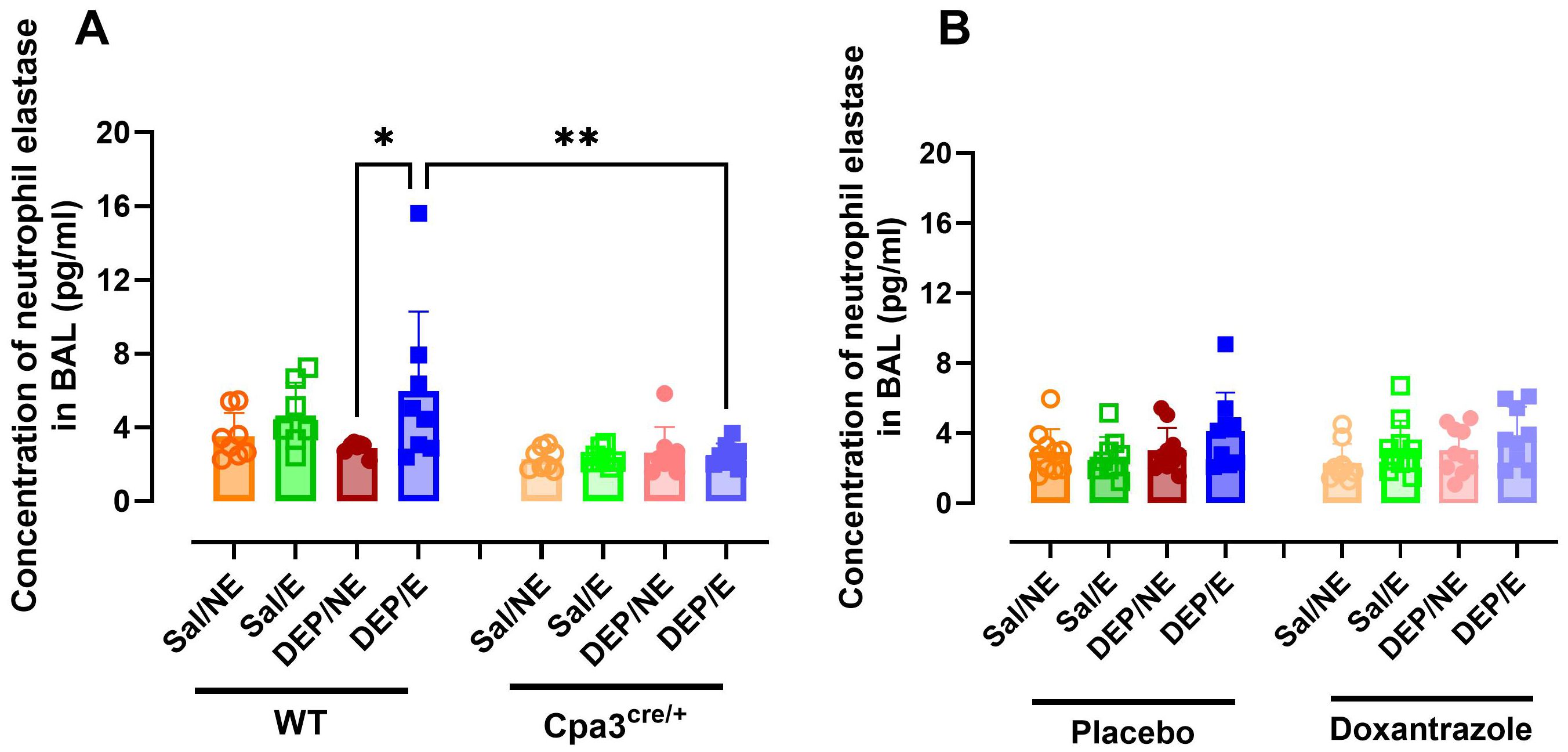
Figure 5. Response of mast cell inhibition on neutrophil extracellular traps in the bronchoalveolar lavage fluid. After the experimental running protocol (Supplementary Figure S1), animals were sacrificed and bronchoalveolar lavage fluid (BAL) was collected to evaluate presence of neutrophil extracellular traps (NETs, measured as neutrophil elastase). Concentration of neutrophil elastase present in the BAL of Cpa3cre/+ mice compared to WT mice (A) and placebo- compared to Doxantrazole treated mice (B). Data are represented as mean ± SD with individual values and were evaluated using Kruskal–Wallis test with Dunn’s post hoc testing. Levels of significance were *p < 0.05, **p < 0.01. n = 7–16 per group. Sal, saline; DEP, diesel exhaust particles; NE, no exercise; E, exercise; RT, room temperature; WT, wild type.
The concentration of different cytokines and chemokines was assessed in BAL fluid. Concentration of keratinocyte chemoattractant (KC) was significantly higher in WT DEP/E(/4°C) mice compared to WT Sal/E(/4°C) and Cpa3cre/+ DEP/E(/4°C) mice (Figure 6A). No differences were seen between Cpa3cre/+ groups (Supplementary Figure S6). Similarly, DEP/E(/4°C) mice in the doxantrazole experiment had a significantly higher concentration of KC in the BAL fluid compared to placebo-treated Sal/NE(/RT) and Sal/E(/4°C) mice (Figure 6B). However, in this experiment, no differences were seen between placebo-treated and doxantrazole-treated DEP/E(/4°C) mice.
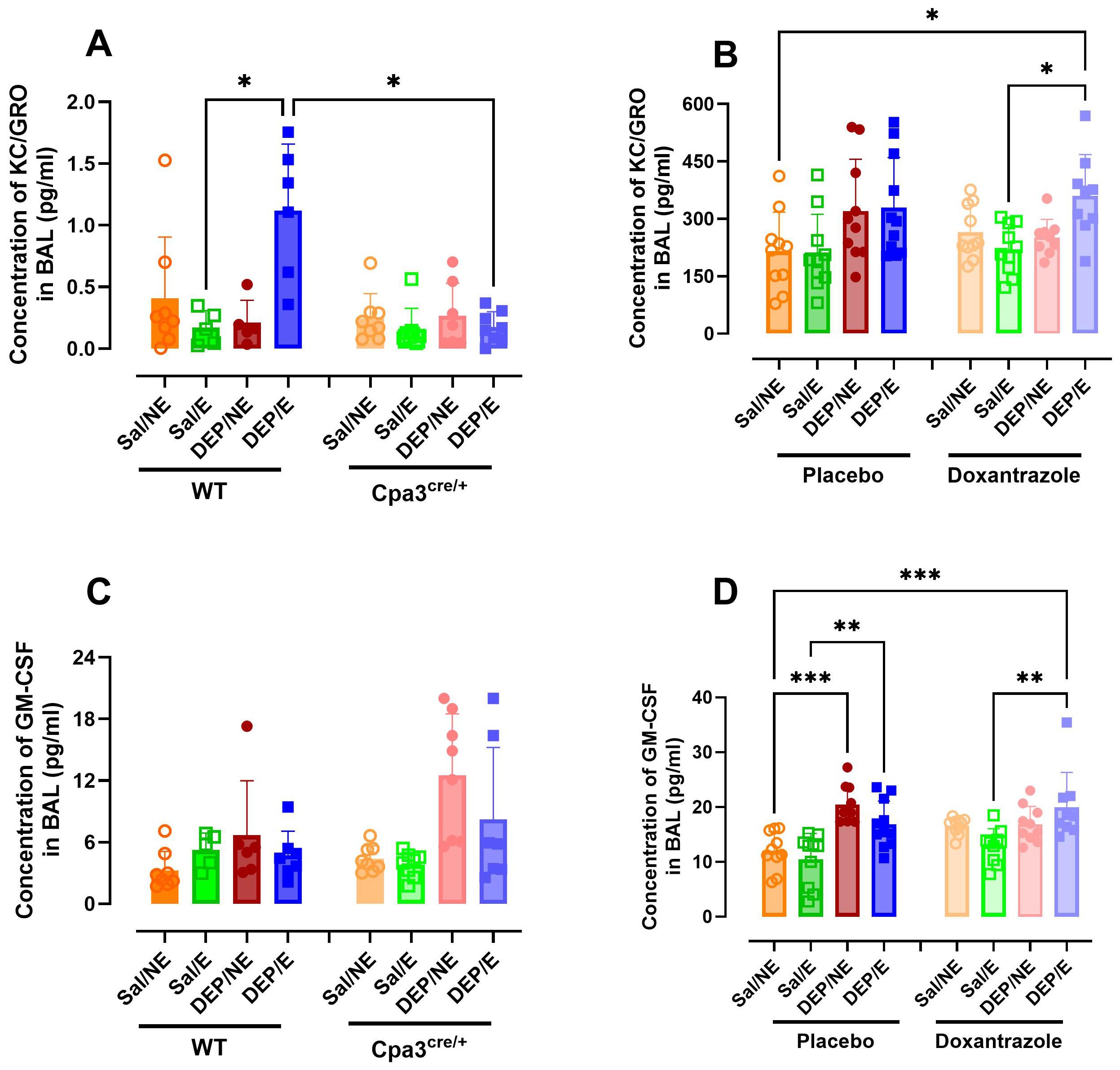
Figure 6. Effect of mast cell inhibition on inflammatory cytokine and chemokine response in the airways to cold air, exercise and diesel exhaust particles. After the experimental running protocol (Supplementary Figure S1), animals were sacrificed and bronchoalveolar lavage fluid (BAL) was collected to evaluate airway inflammation through cytokine concentration. Concentration of keratinocyte-derived chemokine (KC) (A) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (C) present in the BAL of Cpa3cre/+ mice compared to WT mice and of KC (B) and GM-CSF (D) placebo- compared to Doxantrazole treated mice. Data are represented as mean ± SD with individual values and were evaluated using Kruskal–Wallis test with Dunn’s post hoc testing. Levels of significance were *p < 0.05, **p < 0.01, ***p < 0.001. n = 7–16 per group. Salll, saline, DEP, diesel exhaust particles, NE, no exercise; E, exercise; RT, room temperature; WT, wild type.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) concentration was not influenced by mast cell inhibition. Only in the doxantrazole experiment, a significant increase in GM-CSF concentration was visible after administering DEP. Yet, no difference was seen between placebo- and Doxantrazole treated mice (Figure 6D). This difference was not present in the Cpa3cre/+ mice and WT littermates (Figure 6C).
TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-33, and MCP-1 concentrations did not differ between groups in the Cpa3cre/+ knockout or the doxantrazole experiment (Supplementary Table S2).
Mast cell inhibition affects macrophage count and function
The total number of macrophages did not differ between groups in WT mice and Cpa3cre/+ mice. While not statistically significant between Cpa3cre/+ and WT mice, the increase in macrophages as seen in WT DEP/E(/4°C) mice was absent in Cpa3cre/+ DEP/E(/4°C) mice (Figure 7A). In contrast, doxantrazole treatment induced a significantly prevented influx of macrophages compared to placebo in DEP/E(/4°C) mice (Figure 7B).
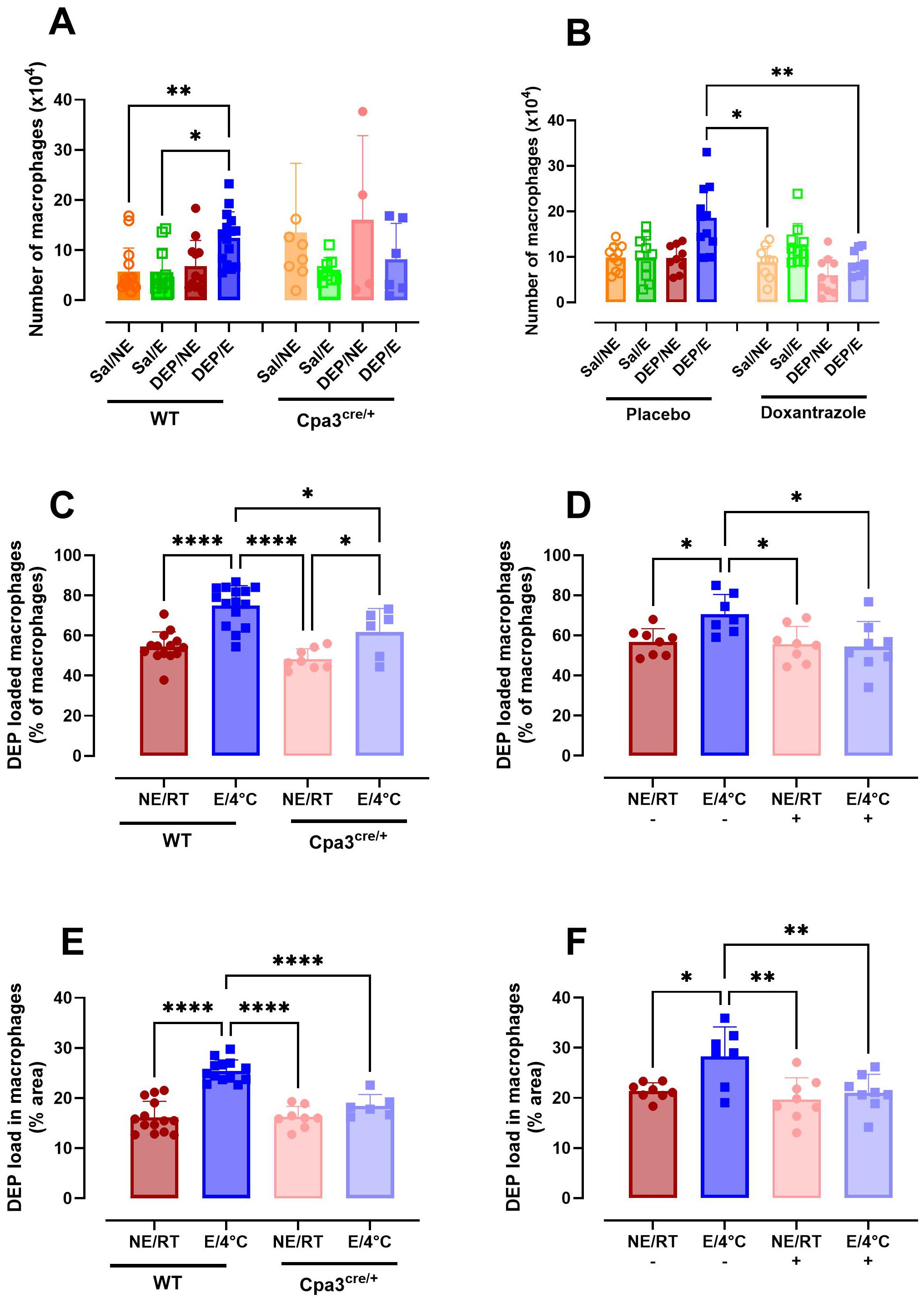
Figure 7. Role of mast cells in macrophage population and particle uptake in the airways in response to cold air, exercise and diesel exhaust particles. After the experimental running protocol (Supplementary Figure S1), animals were sacrificed and bronchoalveolar lavage fluid (BAL) was collected to evaluate presence and activity of macrophages. Absolute number of macrophages present in BAL of Cpa3cre/+ mice compared to WT mice (A) and placebo- compared to Doxantrazole-treated mice (B). The percentage of macrophages loaded with diesel exhaust particles (DEP) as manually counted in Cpa3cre/+ mice compared to WT mice (C) and placebo- compared to Doxantrazole-treated mice (D) and the percentage area of the macrophage covered with particles as calculated with the ImageJ software of Cpa3cre/+ mice compared to WT mice (E) and placebo- compared to Doxantrazole-treated mice (F). Data are represented as mean ± SD with individual values and were evaluated using Kruskal–Wallis test with Dunn’s post hoc testing. Levels of significance were *p < 0.05, **p < 0.01, ****p < 0.001. n = 7–16 per group. Sal, saline; DEP, diesel exhaust particles; NE, no exercise; E, exercise; RT, room temperature; -, placebo; +, Doxantrazole; WT, wild type.
The number of macrophages taking up DEP was significantly higher in WT DEP/E(/4°C) mice compared to non-exercising (RT) mice. Cpa3cre/+ mice also had an increased number of macrophages containing particles in their cytoplasm when exercising in cold (DEP/E/4°C) compared to resting Cpacre/+ mice, however the number was still significantly lower compared to WT DEP/E/4°C mice. Doxantrazole treatment prevented particle uptake in the E/4°C group (Figures 7C, D). Similar significant differences were seen when evaluating the with DEP covered area of the macrophages (Figures 7E, F). Representative image of DEP loading of all groups is available in Supplementary Figure S7.
No effects of mast cells on dendritic subpopulations
No differences were seen in the different subsets of dendritic cells (DC, Supplementary Table S3). Wild type mice did not show an increased presence of DC subsets when challenged with DEP and exercise in cold compared to resting saline-challenged mice. Similarly, no differences were seen in the different groups treated with placebo or doxantrazole (data not shown).
Discussion
This study investigated the role of mast cells in bronchial hyperreactivity and airway inflammation induced by exercise in combination with DEP and cold air in mice. We found that mast cells play an essential role in the development of bronchial hyperreactivity and seem to be involved in the development of neutrophilic inflammation in the lungs.
Cpa3cre/+ mice were chosen over other mast cell-deficient mouse models due to their more specific targeting of mast cells. Unlike Kit-dependent models, which affect multiple cell lineages such as erythrocytes and neutrophils (24), Cpa3cre/+ mice have more selective depletion of mast cells and basophils. Cpa3cre/+ mast cell-deficient mice showed no signs of BHR nor airway inflammation, thereby suggesting that there is a crucial role for mast cells early in the development of exercise-induced bronchoconstriction in association to cold air and DEP exposure. This is in line with the hypotheses that has been summarized by Couto et al., where mast cells are an important player in the development of EIB (3).
Our findings are in line with previous research that showed an important role for mast cells in non-allergic asthma (25), especially in the phenotypes exacerbated by environmental triggers such as cold air and air pollution (26). In allergen-driven models, mast cell deficient mice demonstrated attenuated BHR and inflammation, which could be restored by mast cell reconstitution (27), and mast cell-derived TNF was shown to be critical for BHR development (28).In contrast, we did not observe TNF differences in our model, despite consistent BHR. Other studies reported increased mast cell activation and mediator release in response to air pollution, mainly in an IgE-dependent manner (29–31). Human provocation studies further supported the role of mast cells in airway narrowing, where mast cell-derived prostaglandin D2 and leukotriene C4 metabolites were increased after mannitol challenges (32). Currently, we were unable to identify the exact mechanism through which mast cells are activated in our model to induce BHR and airway inflammation. Further research with e.g. FcϵRI knockout mice or anti-IgE neutralisation could provide indispensable insights.
To evaluate in which phase of the development of EIB mast cells play a role, WT mice were treated with the mast cell stabilizer doxantrazole, otherwise known as 3-(5-tetrazolyl) thioxanthene-9-one 10, 10-dioxide (33). Although the exact mode of action of doxantrazole remains unclear, it has been suggested that the compound may inhibit 3’-5’-cyclic adenosine monophosphate phosphodiesterase (34), thereby reducing intracellular calcium influx and thus preventing degranulation of mast cells (35, 36). Although doxantrazole is currently not used in clinical practice and may not be the most potent available mast cell stabilizers, its use here was justified based on prior dose optimization and standardized intraperitoneal administration (37). These findings raise the question whether mast cell stabilizers could be used in addition to β2-agonists for the treatment of bronchoconstriction in asthma and EIB. However, further research is required to confirm this in humans.
Doxantrazole treatment was limited to the final week of the experimental to allow development of EIB. Despite the short treatment duration, mast cell stabilization was sufficient to significantly reduce the manifestation of BHR, thereby suggesting that mast cells have an important role in the later stages of EIB and its characteristic bronchial hyperresponsiveness. Yet, the infiltration of neutrophils could not be reversed by doxantrazole treatment, indicating that mast cell inhibition alone does not fully abrogate airway inflammation.
These findings suggest a complex interplay between mast cells, neutrophils and BHR. While mast cells consistently modulate BHR, neutrophilic inflammation appears to be context dependent. This indicates that BHR can occur in the absence and in the presence of neutrophils and that mast cells contributing to BHR partly independent of neutrophil infiltration. Mast cells likely induce bronchoconstriction through the release of early-phase mediators such as histamine and leukotrienes (38). Therefore, the neutrophilia likely represents a parallel or secondary inflammatory process. This is supported by previous studies showing that depletion of neutrophils does not consistently prevent BHR (39). However, future studies investigating direct mast cell-neutrophil interactions in vitro or in vivo are required to elucidate the mechanisms underlying this complex relationship.
The influence of mast cells on the neutrophils in the airways were further reflected in the neutrophil extracellular traps (neutrophil elastase) and in the concentration of KC/GRO in BAL. Only in the mast cell deficient mice, a significantly lower concentration of neutrophil elastase and KC/GRO in BAL was seen. This difference was not present in the doxantrazole-treated animals. To further elucidate the role of neutrophils in this exposure-associated EIB model, future studies using specific neutrophil depletion strategies or a neutrophil-deficient mouse models should be used. GM-CSF concentrations showed inconsistent increases. In Cpa3cre/+ mice and their littermates, cold, exercise, and/or DEP did not affect GM-CSF levels. In contrast, in both doxantrazole- and placebo-treated mice, DEP exposure led to increased concentrations. This pattern suggests that the effect is primarily DEP-induced rather than EIB-related.”
In this study, we showed that macrophages were mildly affected by inhibition of mast cells. Mast cell deficient mice showed a decreased trend in number of macrophages present in the airways. This difference reached significance in doxantrazole-treated mice. Additionally, our findings point to an association between absence of mast cells and lower DEP uptake by macrophages, as well as lower amounts of macrophages taking up DEP. This could indicate that macrophages are less active in the absence of mast cells. Despite that there is no direct link, this aligns with literature showing that macrophages can become more activated in the presence of mast cells (40, 41).
A limitation of this study is the absence of functional mast cell assays, such as a hexosaminidase assay. This would strengthen the mechanistic link between mast cell activation and bronchial hyperresponsiveness and/or airway inflammation. Likewise, macrophage activity could not be objectified with a phagocytotic assay, such as a zymosan uptake assay (42). Future studies should incorporate these analyses to make a stronger mechanistic evaluation of the underlying processes. Additionally, other hypotheses, including neuronal activation (3), should be assessed using inhibitors of TRP-channels or deficient mouse models. In our study, we specifically focused on inflammatory processes detectable in the airway lumen rather than the lung parenchyma. While we assessed neutrophils and NET markers in BALF, we did not perform histological analyses to evaluate neutrophilic infiltration or direct mast cell–neutrophil interactions in lung tissue. Targeting the parenchymal compartment is necessary to define these cellular interactions and to establish a more comprehensive mechanistic link between mast cells and neutrophilic inflammation.
Conclusion
In conclusion, this study evaluated the role of mast cells in (early) bronchial hyperresponsiveness (BHR) and airway inflammation in an EIB-mouse model exposed to cold air and/or diesel exhaust particles (DEP). We found that mast cells are crucial in the development of BHR and has a complex interplay with neutrophilic inflammation. These findings suggest that mast cells are a promising therapeutic target and may contribute to improved treatment outcomes in patients with exposure-associated EIB.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by local Ethical Committee for animal experiments of KU Leuven, Belgium. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NM: Investigation, Writing – original draft, Conceptualization, Formal Analysis, Visualization. FB: Writing – review & editing, Investigation. MC: Investigation, Writing – review & editing. ED: Writing – review & editing, Resources. JC: Writing – review & editing, Resources. DB: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. LD: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. JV: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We want to thank Rodewald HR and co-workers for providing the Cpa3cre/+ mice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1650057/full#supplementary-material
References
1. Bonini M and Silvers W. Exercise-induced bronchoconstriction: background, prevalence, and sport considerations. Immunol Allergy Clin North Am. (2018) 38:205–14. doi: 10.1016/j.iac.2018.01.007
2. Gerow M and Bruner PJ. Exercise-induced bronchoconstriction. Treasure Island, Florida, USA: StatPearls (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK557554/ (Accessed November 26, 2024).
3. Couto M, Kurowski M, Moreira A, Bullens DMA, Carlsen KH, Delgado L, et al. Mechanisms of exercise-induced bronchoconstriction in athletes: Current perspectives and future challenges. Allergy. (2018) 73:8–16. doi: 10.1111/all.13224
4. Krystel-Whittemore M, Dileepan KN, and Wood JG. Mast cell: A multi-functional master cell. Front Immunol. (2016) 6:165675. doi: 10.3389/fimmu.2015.00620
5. Tauber M, Basso L, Martin J, Bostan L, Pinto MM, Thierry GR, et al. Landscape of mast cell populations across organs in mice and humans. J Exp Med. (2023) 220:e20230570. doi: 10.1084/jem.20230570
6. Penn RB. Mast cells in asthma: Here I am, stuck in the middle with you. Eur Respir J. (2020) 56(1):2001337. doi: 10.1183/13993003.01337-2020
7. Bradding P and Arthur G. Mast cells in asthma–state of the art. Clin Exp Allergy. (2016) 46:194–263. doi: 10.1111/cea.12675
8. Kumar M, Duraisamy K, and Chow BKC. Unlocking the non-igE-mediated pseudo-allergic reaction puzzle with mas-related G-protein coupled receptor member X2 (MRGPRX2). Cells. (2021) 10(5):1033. doi: 10.3390/cells10051033
9. Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. (2006) 349:1322–8. doi: 10.1016/j.bbrc.2006.08.177
10. Manorak W, Idahosa C, Gupta K, Roy S, Panettieri R, and Ali H. Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir Res. (2018) 19:1. doi: 10.1186/s12931-017-0698-3
11. Zhang Y, Bai H, Zhang W, Gao J, Gao C, Deng T, et al. miR-212/132 attenuates OVA-induced airway inflammation by inhibiting mast cells activation through MRGPRX2 and ASAP1. Exp Cell Res. (2023) 433:113828. doi: 10.1016/j.yexcr.2023.113828
12. Hox V, Vanoirbeek JA, Alpizar YA, Voedisch S, Callebaut I, Bobic S, et al. Crucial role of transient receptor potential ankyrin 1 and mast cells in induction of nonallergic airway hyperreactivity in mice. Am J Respir Crit Care Med. (2013) 187:486–93. doi: 10.1164/rccm.201208-1358OC
13. Pohlmeier L, Sonar SS, Rodewald HR, Kopf M, and Tortola L. Comparative analysis of the role of mast cells in murine asthma models using Kit-sufficient mast cell-deficient animals. Allergy. (2021) 76:2030–43. doi: 10.1111/all.14765
14. Yu M, Tsai M, Tam SY, Jones C, Zehnder J, and Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. (2006) 116:1633–41. doi: 10.1172/JCI25702
15. Mendez-Enriquez E, Alvarado-Vazquez PA, Abma W, Simonson OE, Rodin S, Feyerabend TB, et al. Mast cell-derived serotonin enhances methacholine-induced airway hyperresponsiveness in house dust mite-induced experimental asthma. Allergy. (2021) 76:2057–69. doi: 10.1111/all.14748
16. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, and Galli SJ. Mast cell-deficient W-sash c-kit mutant kitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. (2005) 167:835–48. doi: 10.1016/S0002-9440(10)62055-X
17. Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. (2011) 35:832–44. doi: 10.1016/j.immuni.2011.09.015
18. Katz HR and Austen KF. Mast cell deficiency, a game of kit and mouse. Immunity. (2011) 35:668–70. doi: 10.1016/j.immuni.2011.11.004
19. Batchelor JF, Garland LG, Green AF, Hughes DTD, Follenfant MJ, Gorvin JH, et al. DOXANTRAZOLE, AN ANTIALLERGIC AGENT ORALLY EFFECTIVE IN MAN. Lancet. (1975) 305:1169–70. doi: 10.1016/s0140-6736(75)93141-4
20. Marain NF, Dilissen E, Cremer J, Vanstapel A, Bullens DM, Dupont LJ, et al. Cold air and air pollution induce bronchial hyperresponsiveness and inflammation in a murine model. Sci Total Environ. (2025) 989:989. doi: 10.1016/j.scitotenv.2025.179828
21. Marain NF, Jonckheere AC, Dilissen E, Cremer J, Roskams T, Colemont M, et al. Combined exercise and diet induce airway hyperreactivity while reducing liver steatosis in mice with diet-induced obesity. Nutrients. (2024) 16:2129. doi: 10.3390/nu16132129
22. Decaesteker T, Vanhoffelen E, Trekels K, Jonckheere AC, Cremer J, Vanstapel A, et al. Differential effects of intense exercise and pollution on the airways in a murine model. Part Fibre Toxicol. (2021) 18(1):12. doi: 10.1186/s12989-021-00401-6
23. Bai Y, Bové H, Nawrot TS, and Nemery B. Carbon load in airway macrophages as a biomarker of exposure to particulate air pollution; a longitudinal study of an international Panel. Part Fibre Toxicol. (2018) 15(1):14. doi: 10.1186/s12989-018-0250-8
24. Jachetti E, D’Incà F, Danelli L, Magris R, Dal Secco C, Vit F, et al. Frontline Science: Mast cells regulate neutrophil homeostasis by influencing macrophage clearance activity. J Leukoc Biol. (2019) 105:633–44. doi: 10.1002/JLB.4HI1018-390R
25. Kraneveld AD, van der Kleij HPM, Kool M, van Houwelingen AH, Weitenberg ACD, Redegeld FAM, et al. Key role for mast cells in nonatopic asthma. J Immunol. (2002) 169:2044–53. doi: 10.4049/jimmunol.169.4.2044
26. Huang SK, Zhang Q, Qiu Z, and Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J Thorac Dis. (2015) 7:23. doi: 10.3978/j.issn.2072-1439.2014.12.13
27. Williams CMM and Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. (2000) 192:455–62. doi: 10.1084/jem.192.3.455
28. Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. (2007) 120:48–55. doi: 10.1016/j.jaci.2007.02.046
29. Jin Y, Zhu M, Guo Y, Foreman D, Feng F, Duan G, et al. Fine particulate matter (PM2.5) enhances FcϵRI-mediated signaling and mast cell function. Cell Signal. (2019) 57:102–9. doi: 10.1016/j.cellsig.2019.01.010
30. González-Díaz SN, Arias-Cruz A, Macouzet-Sánchez C, and Partida-Ortega AB. Impact of air pollution in respiratory allergic diseases. Medicina Universitaria. (2017) 19(75):35–42. doi: 10.1016/j.rmu.2017.04.006
31. Wang Y, Tang N, Mao M, Zhou Y, Wu Y, Li J, et al. Fine particulate matter (PM2.5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. J Dermatol Sci. (2021) 102:47–57. doi: 10.1016/j.jdermsci.2021.02.004
32. Gulliksson M, Palmberg L, Nilsson G, Ahlstedt S, and Kumlin M. Release of prostaglandin D2 and leukotriene C4 in response to hyperosmolar stimulation of mast cells. Allergy: Eur J Allergy Clin Immunol. (2006) 61:1473–9. doi: 10.1111/j.1398-9995.2006.01213.x
33. Pauwels R, Lamont H, and van der Straeten M. The effect of oral doxantrazole on allergen-induced bronchospasm. Acta Allergol. (1976) 31:239–2444. doi: 10.1111/j.1398-9995.1976.tb01684.x
34. Haydu SP, Bradley JL, and Hughes DTD. Inhibitory effect of oral doxantrazole on asthma induced by allergen inhalation. Br Med J. (1975) 3:283. doi: 10.1136/bmj.3.5978.283-a
35. Foreman JC, Hallett MB, and Mongar JL. Site of action of the antiallergic drugs cromoglycate and doxantrazole [proceedings. Br J Pharmacol. (1977) 59:473.
36. Cochrane DE and Douglas WW. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48–80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U.S.A. (1974) 71:408–12. doi: 10.1073/pnas.71.2.408
37. Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, Appeltans I, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. (2021) 590(7844):151–6. doi: 10.1038/s41586-020-03118-2
38. Bradding P. Mast cell regulation of airway smooth muscle function in asthma. Eur Respir J. (2007) 29:827–30. doi: 10.1183/09031936.00017707
39. Phipps S, Chuan EL, Kaiko GE, Shen YF, Collison A, Mattes J, et al. Toll/IL-1 signaling is critical for house dust mite-specific Th1 and Th2 responses. Am J Respir Crit Care Med. (2009) 179:883–93. doi: 10.1164/rccm.200806-974OC?download=true
40. Granata F, Nardicchi V, Loffredo S, Frattini A, Ilaria Staiano R, Agostini C, et al. Secreted phospholipases A2: A proinflammatory connection between macrophages and mast cells in the human lung. Immunobiology. (2009) 214:811–21. doi: 10.1016/j.imbio.2009.06.006
41. Rock MJ, Despot J, and Lemanske RF. Mast cell granules modulate alveolar macrophage respiratory-burst activity and eicosanoid metabolism. J Allergy Clin Immunol. (1990) 86:452–61. doi: 10.1016/S0091-6749(05)80199-X
Keywords: mast cells, exercise-induced bronchoconstriction, cold air, diesel exhaust particles, doxantrazole
Citation: Marain NF, Byttebier F, Colemont M, Dilissen E, Cremer J, Bullens DMA, Dupont LJ and Vanoirbeek JA (2025) Mast cells are essential in the development of exposure-associated exercise-induced bronchoconstriction in a mouse model. Front. Immunol. 16:1650057. doi: 10.3389/fimmu.2025.1650057
Received: 19 June 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Karin Vonbank, Medical University of Vienna, AustriaReviewed by:
Yu Fang, Affiliated Hospital of Guizhou Medical University, ChinaAnna-Karin Johnsson, Karolinska Institutet (KI), Sweden
Copyright © 2025 Marain, Byttebier, Colemont, Dilissen, Cremer, Bullens, Dupont and Vanoirbeek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeroen A. Vanoirbeek, amVyb2VuLnZhbm9pcmJlZWtAa3VsZXV2ZW4uYmU=
 Nora F. Marain
Nora F. Marain Fien Byttebier1
Fien Byttebier1 Ellen Dilissen
Ellen Dilissen Dominique MA Bullens
Dominique MA Bullens Jeroen A. Vanoirbeek
Jeroen A. Vanoirbeek