- 1School of Life Sciences, Guangzhou University, Guangzhou, China
- 2Guangdong Metabolic Diseases Research Center of Integrated Chinese and Western Medicine, Guangzhou, Guangdong, China
- 3Key Laboratory of Glucolipid Metabolic Disorder, Ministry of Education of China, Guangzhou, Guangdong, China
- 4Guangdong Key Laboratory of Metabolic Disease Prevention and Treatment of Traditional Chinese Medicine, Guangzhou, Guangdong, China
- 5Institute of Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong, China
- 6Beijing Key Laboratory of Maternal-Fetal Medicine and Fetal Heart Disease & Echocardiography Department, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 7School of Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, China
As ubiquitous innate immune cells, macrophages are crucial for tissue homeostasis and disease pathogenesis. Although our understanding of macrophage subsets and functions has advanced, no effective strategies are available for targeting macrophages to treat diseases in clinical settings due to their heterogeneity. Transcription factors that regulate macrophage function have received increasing attention. CCAAT/enhancer-binding protein delta (CEBPD), an inflammation-associated transcription factor characterized by low basal expression but rapid induction by stimuli, has emerged as a key regulator of macrophages. CEBPD governs diverse biological processes in macrophages through its target genes. Furthermore, macrophage CEBPD significantly contributes to various pathologies. Modulating CEBPD expression or activity in macrophages could regulate various molecular processes to improve disease progression and alleviate organ damage; therefore, novel CEBPD-based therapeutic methods for treating diseases have attracted attention. In this review, we describe the factors upstream and downstream of CEBPD in macrophages. We then summarize recent advances in the regulation of macrophage biological processes by CEBPD. Finally, we discuss the contribution of macrophage CEBPD to various diseases and highlight strategies for developing novel therapies to modulate macrophage function by targeting CEBPD.
1 Introduction
Macrophages are crucial innate immune cells present in essentially all tissues and play vital roles in tissue development, homeostasis, and pathogenesis (1). In the past decade, macrophages have been established as two broad populations originating from either embryonic or definitive bone marrow-derived hematopoiesis, and macrophages in most tissues consist of these two populations (2, 3). Embryo-derived macrophages, termed tissue-resident macrophages (TRMs), are characterized by their persistence in adults and their stable and close association with tissue cells (3). Monocyte-derived macrophages (MDMs) originating from bone marrow hematopoietic stem cells are short-lived, rely on circulating monocytes for renewal, and can expand significantly in response to multiple stimuli (4). In certain tissues such as the brain, epidermis, and liver, TRMs maintain themselves by self-renewal, whereas TRMs in the gut, dermis, lung, and spleen are replaced by monocytes at tissue-specific levels (5, 6). Functionally, TRMs integrate signals from environmental sensors to orchestrate adaptive cellular responses critical for the growth and homeostasis of tissue cells, whereas MDMs play an important role in pathological conditions including inflammation, fibrosis, infection, and malignant remodeling (3, 4, 6). Recent advances in single-cell RNA sequencing have revealed that these two types of macrophages have different transcriptomes (7–9), which may underlie their different functions.
Although our knowledge of macrophage subsets and functions has advanced significantly, no effective strategies are available for targeting macrophages to treat diseases in clinical settings. Existing strategies mainly target neutralization of factors secreted by macrophages, including interleukin-1 beta, but this is not directly related to targeting macrophages (8, 9). This may be attributed to the following reasons: (1) Macrophage plasticity changes according to the surroundings, making the targeting of a particular subset of macrophages for disease treatment much less feasible. Increasing data show that macrophage types and compositions change considerably at different times or stages of the disease process (7, 10, 11). (2) The identification of macrophage phenotypes relies on multiple rather than single markers (12, 13). Further, the relationship between these markers and macrophage functions is unclear and requires further study. (3) Technically, protein engineering techniques and targeted delivery systems have a long way to go before diseases can be treated by targeting macrophages. These techniques play an increasingly important role in tumor therapy; however, they mostly induce immune cells to kill tumor cells rather than target the immune cells themselves (14, 15). In mice and humans, genetically inherited macrophage defects often result in severe or even fatal disorders, indicating that targeting macrophages should involve modulating, not just inhibiting their function (3, 16, 17).
CCAAT/enhancer-binding protein delta (CEBPD) belongs to the CEBP family of the basic-leucine zipper (bZIP) class of transcription factors. Six members of this family share a highly conserved C-terminal region comprising a basic amino acid-rich DNA-binding motif, followed by a bZIP domain responsible for dimerization (Figure 1A) (18). Under normal physiological conditions, CEBPD expression is low in various cells including macrophages, but it can be rapidly induced by external stimuli (19).
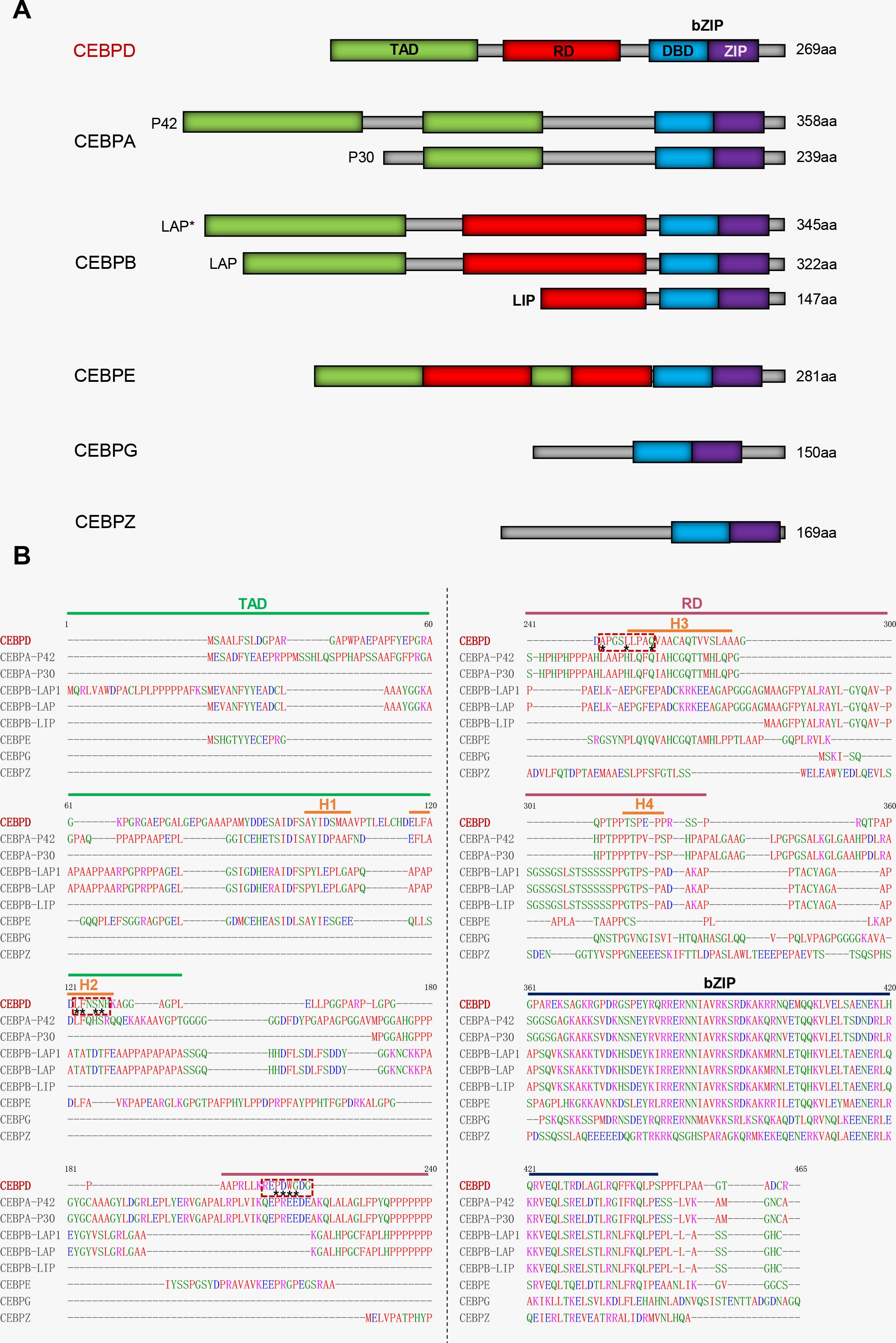
Figure 1. Comparative analysis of protein sequence between CEBPD and other CEBP family members. (A) The cartoon of domains among CEBP family members. (B) The red dashed box indicates the main region on CEBPD protein where the compound binds to. Asterisks indicate main points of contact of CEBPD. H, helix; TAD, Transcriptional activation domain; RD, Regulatory domain; bZIP, Basic-leucine zipper domain; DBD, DNA-binding domain; ZIP, Leucine zipper.
Many studies have shown that CEBPD plays a crucial role in pathological processes including tumorigenesis and inflammatory responses, as well as neurological and fibrotic diseases, and these effects have been excellently reviewed elsewhere (19–22). CEBPD is involved in these pathological processes mainly by regulating its target genes. Recent studies have shown that macrophage CEBPD participates in the progression of various diseases including osteoporosis, rheumatoid arthritis (RA), atherosclerosis, lung injury, and cancers (23–27). Overexpression of CEBPD in macrophages, caused by mitochondrial dysfunction, directly upregulates the expression of cathepsin K (CTSK), which induces macrophage differentiation into osteoclasts, leading to bone loss (28). In RA, CEBPD is induced and upregulated in macrophages, enhancing the tube formation of endothelial cells and the migration and proliferation of synoviocytes to promote RA progression (24, 29). Moreover, activated macrophage CEBPD promotes tumor progression and atherosclerosis (25, 27). In response to external stimuli, upregulated CEBPD in macrophages can modify various cellular biological processes, including inflammatory response, oxidative stress, phagocytosis, and pyrimidine metabolism (25, 30–32). Furthermore, CEBPD plays a crucial role in macrophage polarization (30, 33). These studies indicate that macrophage CEBPD may be a potential target for disease treatment.
In this review, we first discuss the factors upstream and downstream of CEBPD in macrophages and the regulation of macrophage biological processes by CEBPD. We then describe the role of macrophage CEBPD in various diseases. We also discuss the feasibility of targeting macrophage CEBPD for disease treatment.
2 The upstream and downstream factors of CEBPD in macrophages
As a transcription factor, the main role of CEBPD is to regulate the expression of downstream target genes involved in various cellular biological processes. The transcriptional function of CEBPD also depends on the regulation of its expression and activity by upstream factors. In this section, we describe the upstream and downstream factors of CEBPD in macrophages.
2.1 Upstream regulators of CEBPD
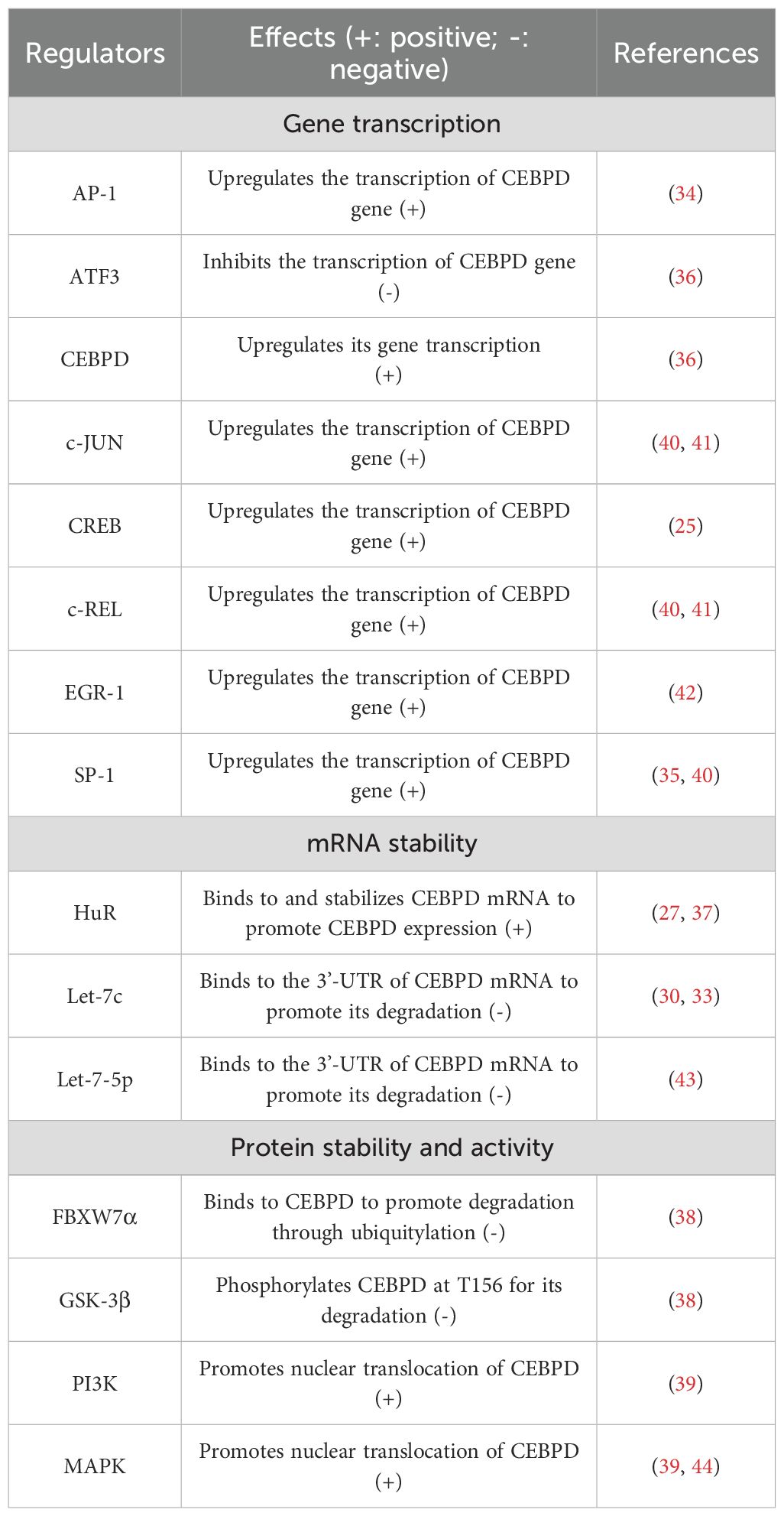
Previous studies indicate that CEBPD expression in macrophages is regulated at three levels: gene transcription, mRNA stability, and protein expression. Recent studies have shown that some transcription factors, such as activator protein 1 (AP-1), cAMP-response element binding protein (CREB), and specificity protein-1 (SP-1), can bind to the CEBPD gene promoter and directly upregulate its expression in macrophages (25, 34, 35). Further, CEBPD regulates its expression in a positive feedback manner by binding to its own promoter (36). In contrast, activating transcription factor 3 (ATF3) directly binds to the CEBPD gene promoter and represses its expression in macrophages (36). Regarding CEBPD mRNA stability, the RNA-binding protein Hu antigen R (HuR) can bind to the 3’-untranslated region (3’-UTR) of CEBPD mRNA and stabilize it, thereby increasing its expression in macrophages (37). MicroRNAs (miRNAs) form a class of small noncoding RNAs that regulate gene expression through degradation or inhibition of specific mRNA targets by binding to their 3’-UTR (18). MiRNA let-7c directly inhibits CEBPD expression by promoting its mRNA degradation (30). At the protein level, ubiquitin-ligase F-box and WD repeat domain containing 7 α (FBXW7α) can bind to CEBPD to promote its degradation through ubiquitylation in macrophages (38). In addition, phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinases (MAPK) promote the nuclear translocation of CEBPD to activate its transcriptional function (39). The upstream regulators of CEBPD in macrophages are presented in detail in Table 1.
2.2 Downstream targets of CEBPD
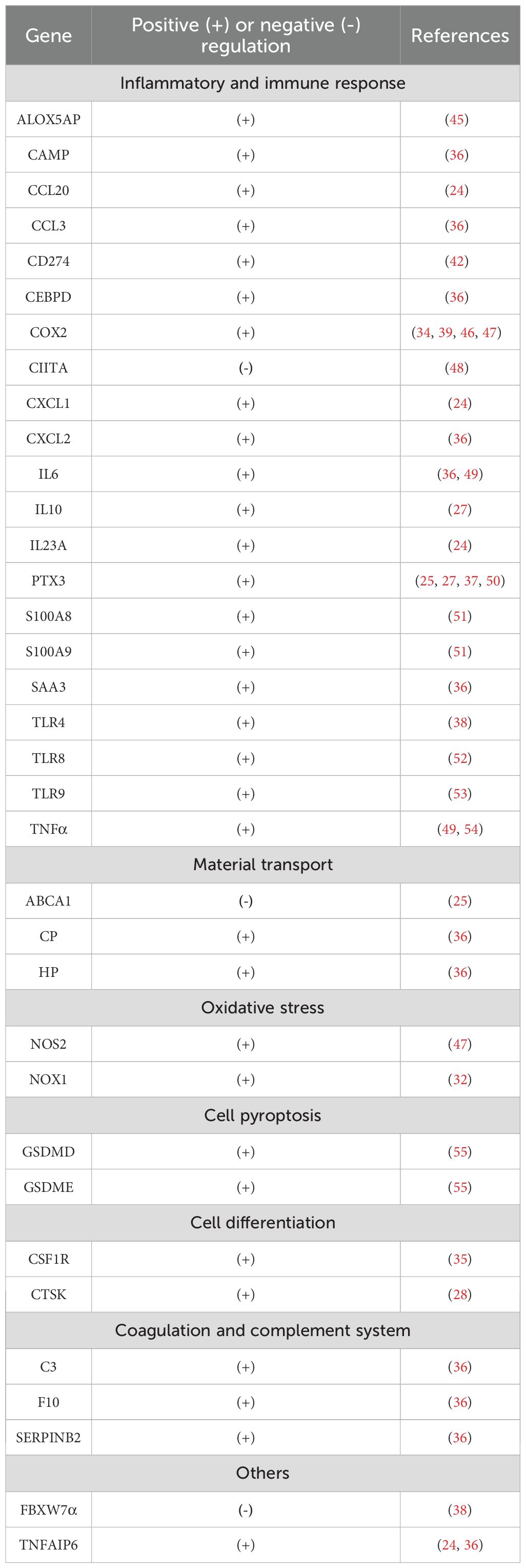
To date, 35 target genes of CEBPD have been identified in macrophages (Table 2). Based on their function, these genes can be divided into 9 categories: inflammatory and immune response, material transport, oxidative stress, cell pyroptosis, differentiation, blood coagulation, coagulation and complement system, extracellular matrix, and ubiquitination. Of these, 60% (21/35) are associated with inflammatory and immune responses (Table 2). CEBPD binds to the promoter of most target genes and upregulates their expression. However, CEBPD binding to the promoter of ATP-binding cassette subfamily A member 1 (ABCA1), major histocompatibility complex class II trans-activator (CIITA), and FBXW7α inhibits expression of these genes in macrophages (25, 38, 48). The inhibitory effects of CEBPD may be due to its formation of heterodimers with negative regulatory factors such as its CEBP family member CCAAT/enhancer-binding protein gamma (CEBPG) (49). In addition to the well-defined target genes in macrophages, many additional targets of CEBPD have been reported in other cells (18). In fact, the number of CEBPD target genes is extensive, as shown in target gene online analysis databases (such as Transcription Factor Target Gene Database, Gene Transcription Regulation Database, Harmonizonme, and Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining). Further investigations will be required to determine whether these genes are regulated by CEBPD in macrophages.
3 Regulatory roles of CEBPD in macrophage biological processes
Macrophages are among the first responders of the immune system. In this role, they have both effector functions for neutralizing pathogens and sentinel functions for alerting other immune cells to diverse pathological threats, thereby initiating and coordinating a multipronged immune response through the secretion of factors (56). CEBPD participates in regulating the multiple biological processes in macrophages under external stimuli (Figure 2, Supplementary Table S1). In this section, we discuss the roles of CEBPD in macrophage polarization, phagocytosis, inflammatory responses, oxidative stress, cell pyroptosis, and differentiation.
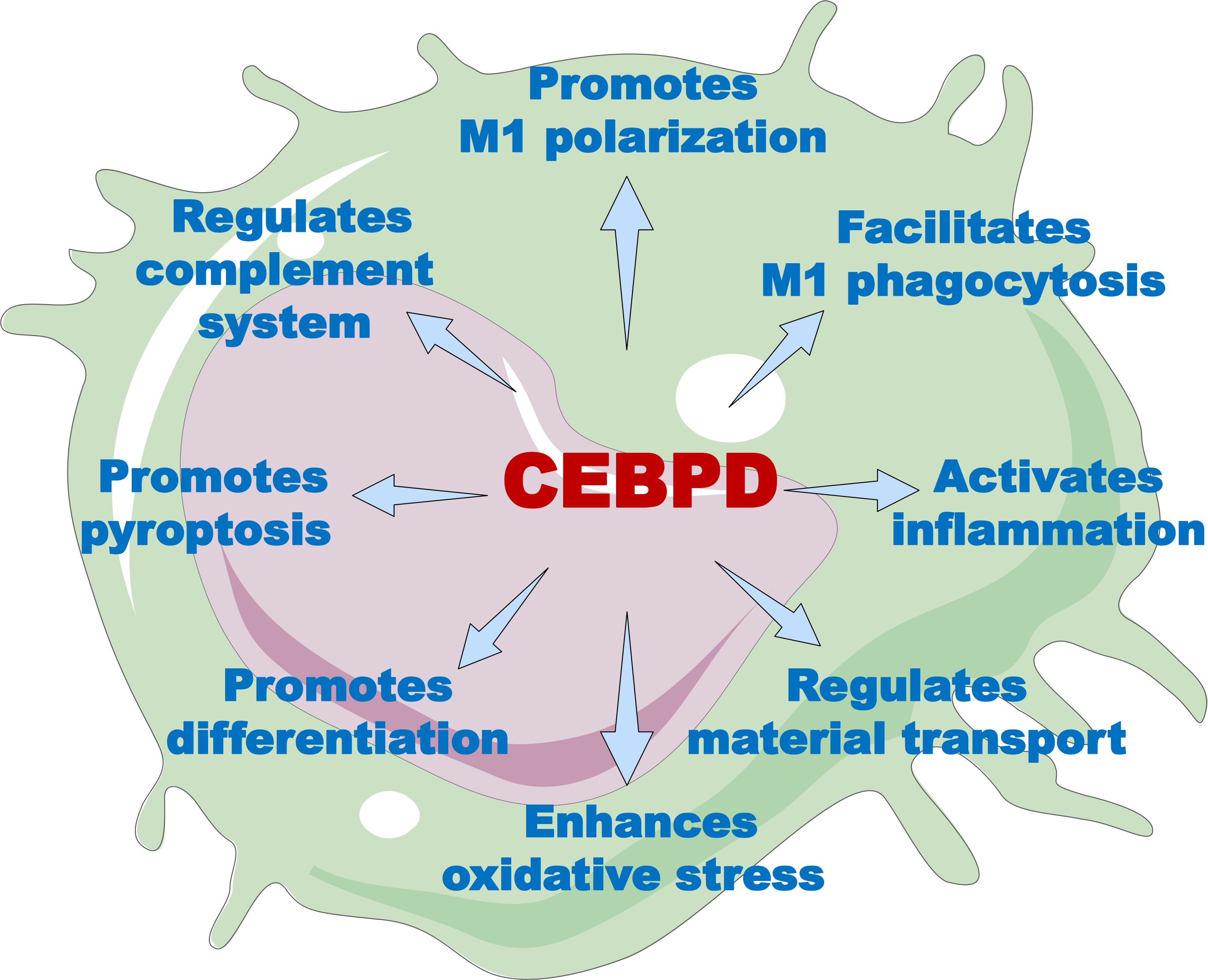
Figure 2. The overview of CEBPD functions in macrophages. Through its target genes, CEBPD can modulate a variety of macrophage functions, including polarization, phagocytosis, inflammatory response, substance transport, oxidative stress, cell differentiation, cellular pyroptosis, and complement activity.
3.1 Macrophage polarization
Macrophage polarization is a process in which macrophages acquire distinct effector states based on surrounding microenvironmental stimuli to perform multiple and sometimes opposite functions. Traditionally, macrophages were thought to be polarized into two opposite types: classically activated or pro-inflammatory macrophages (M1) and alternatively activated or anti-inflammatory macrophages (M2) (12). However, recent advances have revealed a spectrum of macrophage activation states in vivo that extend beyond this simple dichotomy (57). Nevertheless, this dichotomy still reflects, to some extent, the relationship between different polarization states (M1 or M2) and the disease process.
Various studies have shown that CEBPD mRNA and protein levels are increased in macrophages following lipopolysaccharide (LPS) treatment in vitro, suggesting that CEBPD participates in macrophage M1 polarization (46, 58). Furthermore, CEBPD upregulation caused by circular RNA circCdyl (M1-enriched) overexpression is positively correlated with M1 polarization (30). On the other hand, inhibition of CEBPD by let-7c or interfering RNA induces macrophage polarization towards M2 but not M1 (33, 43, 59). Together, these studies suggest that CEBPD is a key factor in macrophage polarization, where elevated CEBPD levels induce M1 polarization, and CEBPD inhibition promotes M2 polarization. However, only inflammatory factors and polarization markers were assessed in these studies, while other biological processes that play an important role in macrophage polarization, such as efferocytosis, migration, material and energy metabolism, were not examined. Whether these biological processes are also directly regulated by CEBPD requires further investigation.
3.2 Phagocytosis
As a core macrophage function, phagocytosis serves as a defense mechanism to eliminate pathogens, apoptotic cells, and cellular debris to maintain tissue homeostasis and respond to injury (60). Phagocytosis is a complex receptor-mediated process that involves the recognition, engulfment, and degradation of particles larger than 0.5 μm (61). As professional phagocytes, macrophages express two classes of phagocytic receptors that activate signaling pathways resulting in phagocytosis. Opsonic receptors detect host-derived serum proteins bound to target particles. These proteins, known as opsonins, include antibodies, fibronectin, complement, and mannose-binding lectin, which enhance the clearance of particles (61). Non-opsonic receptors, including C-type lectins, lectin-like recognition molecules, and pathogen-associated molecular patterns receptors (such as CD36 and scavenger receptors), directly identify distinct molecular patterns on the particle to be ingested (61). The process of phagocytosis typically involves four phases: (1) detection of the particle, (2) activation of the internalization process, (3) formation of a phagosome, and (4) transformation of the phagosome into phagolysosome (61). For exogenous particles such as pathogens, macrophages typically clear them through non-opsonic receptors-mediated phagocytosis. However, macrophages usually clear endogenous particles such as immune complexes through opsonic receptor-mediated phagocytosis. Low-density lipoprotein (LDL) and liposomes appear to be cleared through opsonic receptor-mediated phagocytosis at low concentrations and non-opsonic receptor-mediated phagocytosis at high concentrations (62, 63).
Recent studies have shown that CEBPD is associated with macrophage phagocytosis. In one study, macrophages with inhibited CEBPD show enhanced phagocytosis (27), indicating that CEBPD is negatively correlated with macrophage phagocytosis. However, another study has shown that CEBPD promotes lipid accumulation in M1 macrophages, but not in M2 macrophages (25). Excessive lipid accumulation in macrophages results from increased uptake of LDL or impaired cholesterol efflux, which causes the formation of lipid-laden foam cells (25). Mechanistically, CEBPD upregulates the prototypic humoral pattern recognition molecule pentraxin 3 (PTX3) expression in M1 macrophages, and PTX3 promotes the opsonic receptor-mediated phagocytosis of LDL by macrophages through binding to C1q or Ficolin-1 (25, 61, 64). In addition, a recent study showed that CEBPD mediates phagocytosis of Aspergillus fumigatus by macrophages through PTX3 (37), but the exact mechanism remains to be further investigated. These data indicate that the CEBPD-PTX3 axis plays an important role in macrophage phagocytosis.
3.3 Inflammatory and immune response
CEBPD expression is low under normal physiological conditions and can be rapidly induced by external stimuli (28, 58), suggesting that CEBPD plays an important role in the inflammatory and immune responses to these stimuli. In macrophages, CEBPD directly binds to the promoter and upregulates the expression of inflammatory factors, including C-C motif chemokine ligand 20 (CCL20), CCL3, cyclooxygenase 2 (COX2), C-X-C motif chemokine ligand 1 (CXCL1), interleukin-6 (IL-6), PTX3, S100 calcium-binding protein a8/9 (S100a8/9), and tumor necrosis factor alpha (TNFα) in response to inflammatory stimuli (24, 36, 37, 49, 51). Serum amyloid A 3 (SAA3), an acute-phase protein, is primarily expressed in macrophages and is positively regulated by CEBPD (36). CEBPD directly upregulates the expression of component C3 in macrophages, which plays an important role in the complement system (36). In addition, CEBPD directly upregulates the expression of Toll-like receptors (TLR) 4, 8, and 9 in macrophages to maintain and enhance inflammatory responses (38, 52, 53). On the other hand, CEBPD mediates immunosuppression by directly upregulating the expression of CD274 (also called programmed death ligand 1, PD-L1) and downregulating the expression of the class II major histocompatibility complex transactivator CIITA in macrophages (42, 48). Besides, elevated macrophage CEBPD can directly upregulate the expression of complement C3, coagulation factor X (F10), and serpin family B member 2 (PAI2) to activate the coagulation and complement system, in coordination with inflammation (36). The detailed target genes of CEBPD in macrophages are listed in Table 2.
3.4 Other biological processes
CEBPD also plays an important role in other biological processes involving macrophages. Macrophage cholesterol efflux substantially protects against the progression of atherosclerosis. In M1 macrophages, increased CEBPD downregulates transmembrane transporter ABCA1 expression, impairing the cholesterol efflux from macrophages and accelerating the development of atherosclerotic lesions (25, 65). Further, CEBPD directly upregulates the expression of NADPH oxidase 1 (NOX-1) and nitric oxide synthase-2 (NOS2) in macrophages to mediate LPS-induced oxidative stress (32, 47). During pyroptosis, activated CEBPD upregulates the expression of gasdermin E (GSDME) and GSDMD to promote macrophage pyroptosis caused by immunoglobulin G (IgG) immune complex treatment (55). In addition, elevated CEBPD binds to the promoter of the osteoclast differentiation marker CTSK gene and upregulates its expression, promoting macrophage differentiation into osteoclasts (28). Recent studies have shown that CEBPD regulates macrophage metabolism. In CEBPD-deficient macrophages, the pyrimidine metabolism pathway is significantly downregulated (31, 66), indicating that CEBPD participates in pyrimidine metabolism. However, the underlying regulatory mechanisms still require clarification.
4 Roles of macrophage CEBPD in disease
Macrophages participate in tissue homeostasis and disease pathogenesis, whereas macrophage CEBPD is mainly involved in the progression of pathological processes. In this section, we discuss the role of macrophage CEBPD in lung disease, osteoporosis, RA, vascular disease, and tumor biology (Figure 3).
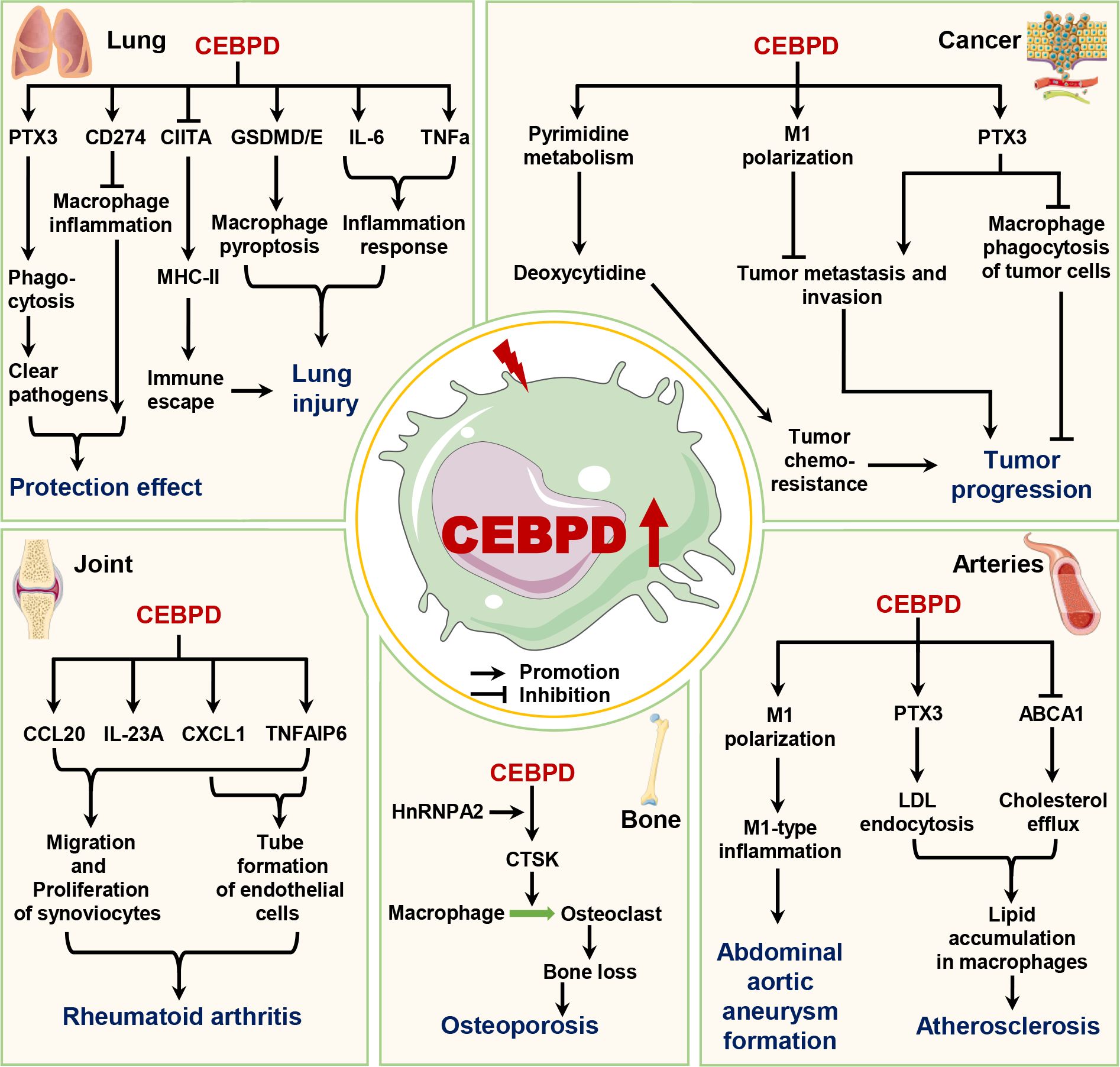
Figure 3. The roles of macrophage CEBPD in diseases. Macrophage CEBPD has different roles in lung diseases and cancers, whereas it can promote the progression of osteoporosis, rheumatoid arthritis, atherosclerosis, and abdominal aortic aneurysm. ABCA1, ATP binding cassette subfamily A member 1; CCL20, C-C motif chemokine ligand 20; CD274, Cluster of differentiation 274; CIITA, Major histocompatibility complex class II trans-activator; CTSK, Cathepsin K; CXCL1, C-X-C motif chemokine ligand 1; GSDMD/E, Gasdermin D/E; HnRNPA2, Heterogeneous nuclear ribonucleoprotein A2; IL-6, Interleukin-6; IL-23A, Interleukin-23A; LDL, Low-density lipoprotein; MHC-II, Major histocompatibility complex class II; PTX3, Pentraxin 3; TNFa, Tumor necrosis factor alpha; TNFAIP6, TNF alpha induced protein 6.
4.1 Lung diseases
Recent studies have shown that Klebsiella pneumoniae (Kp) infection, Aspergillus fumigatus infection, and acute lung injury caused by LPS all induce CEBPD expression in lung tissue (37, 54, 67). The bacterial load is increased in the blood of CEBPD-deficient mice compared to wild-type mice after Kp infection, indicating that CEBPD has a role in preventing bacterial spread in mice (67). Further findings indicate that elevated CEBPD caused by both these infections and injury is mainly expressed in macrophages (26, 37, 54, 67). Mechanistically, pathogenic infections induce HuR expression in macrophages to upregulate CEBPD expression, which activates PTX3 by directly binding to its promoter, thereby promoting the phagocytic ability of macrophages toward pathogens (37). Furthermore, in murine lung macrophages with LPS-induced acute respiratory distress syndrome, increased CEBPD upregulates the expression of PD-L1, which inhibits the macrophage-associated inflammatory response to alleviate disease (42).
However, some studies have shown that elevated CEBPD levels in macrophages can exacerbate the progression of lung disease. In acute lung injury caused by LPS, IgG immune complex, or LPS + IgG immune complex, these stimuli increase CEBPD expression via p38/ERK signaling, PI3K/AKT1 signaling, or suppressors of cytokine signaling 3 (SOCS3) pathways (26, 42, 49, 54). High CEBPD levels in macrophages both activate the inflammatory response by directly upregulating the expression of inflammatory factors, such as TNFα, IL-6, and MIP-1/2, and induce macrophage pyroptosis by upregulating the expression of GSDMD/E, which exacerbates the disease process (44, 49, 55). Further, Mycobacterium tuberculosis (Mtb) infection or exposure to 19-kDa lipoprotein Mtb components increase CEBPD expression in macrophages (48, 68). Elevated CEBPD levels directly inhibit the CIITA expression that suppresses MHC-II transcription and inhibits macrophage MHC-II Ag presentation, allowing Mtb to evade immune surveillance (48, 69). These studies show that the levels of lung macrophage CEBPD are elevated in response to infection and injury. Elevated macrophage CEBPD facilitates clearance of pathogenic bacteria during acute lung pathogenic infection (e.g., Kp infection). In contrast, in persistent bacterial infections (e.g., Mtb infection), toxins, and immunologic injury, elevated macrophage CEBPD levels exacerbate lung disease progression.
4.2 Osteoporosis
A recent study has shown that CEBPD plays vital roles in osteoclastogenesis and bone loss during aging (70). Mitochondrial dysfunction has emerged as an important factor in a wide range of pathologies including aging and osteoporosis. The mitochondria-to-nucleus retrograde signaling (MtRS) pathway originates from dysfunctional mitochondria and causes global nuclear transcriptional reprogramming as its end point, which aids in cellular adaptation to stress (23, 28). Notably, CEBPD is an important factor in the MtRS pathway (23). During aging, hypoxia, or cytochrome c oxidase dysfunction, dysfunctional mitochondria activate the MtRS pathway in macrophages, thereby inducing the expression of heterogeneous ribonucleoprotein A2 (hnRNPA2) and CEBPD (23, 28). HnRNPA2 acts as a co-activator with CEBPD to bind the CTSK promoter, upregulating its expression under hypoxic conditions and promoting macrophage differentiation into osteoclasts (28). However, it should be noted that this enhanced osteoclastogenesis results in bone loss and osteoporosis. In addition, mitochondrial dysfunction caused by cytochrome c oxidase inactivation in macrophages leads to higher levels of cellular and mitochondrial reactive oxygen species, enhanced phagocytosis, boosted inflammatory response, and increased glycolysis (23). This study did not provide evidence of whether these effects were related to elevated CEBPD levels, indicating the need for further research. These studies indicate that elevated macrophage CEBPD promotes the onset and progression of osteoporosis, and targeting macrophage CEBPD is expected to attenuate osteoporosis.
4.3 Vascular diseases
CEBPD is known to be involved in the progression of vascular diseases. Lai et al. have reported that CEBPD is mainly expressed in macrophages of atherosclerotic plaques and that CEBPD deficiency in bone marrow cells suppresses atherosclerotic lesions in ApoE-/- mice, indicating that macrophage CEBPD plays a functional role in the pathogenesis of atherosclerosis (25). Further, both M1 and M2 macrophages phagocytose modified LDL; however, CEBPD deficiency reduces the phagocytic ability of M1 macrophages, suggesting that CEBPD is only involved in lipid phagocytosis by M1 macrophages. Further studies have shown that increased CEBPD enhances the lipid accumulation in M1 macrophages through two pathway: (1) directly upregulating PTX3 expression to promote the macropinocytosis of LDL, and (2) downregulating the expression of ABCA1, which impairs the intracellular cholesterol efflux from M1 macrophages (25). In addition, macrophage CEBPD plays a crucial role in the initial inflammatory phase and dominates the key pathogenesis of abdominal aortic aneurysm (AAA) (18). A recent report showed that elevated circular RNA Cdyl promotes AAA formation by inducing M1 macrophage polarization and M1-type inflammation in an angiotensin II (Ang II)- and calcium chloride (CaCl2)-induced mouse model of AAA (30). Furthermore, the circular RNA Cdyl acts as a sponge of let-7c to inhibit its expression in macrophages (30). CEBPD is a target of let-7c and plays a vital role in macrophage polarization caused by let-7c (59). Mechanistically, increased CEBPD caused by the upregulated circular RNA Cdyl promotes macrophage M1 polarization and maintains the M1 inflammatory state, which accelerates AAA progression (30). Available research evidence suggests that elevated levels of macrophage CEBPD in vascular tissue can exacerbate the progression of vascular diseases. These studies also suggest that the role of macrophage CEBPD should be considered in studies of other vascular diseases.
4.4 Rheumatoid arthritis
CEBPD also plays an important role in RA progression. Chang et al. have reported decreased pannus proliferation and angiogenesis in CEBPD-knockout mice compared with those in WT mice under the collagen-induced RA model (24). In addition, CEBPD is reported to be activated in human RA macrophages (29). Activated CEBPD in macrophages promotes tube formation by endothelial cells and the migration and proliferation of synoviocytes (24). Mechanistically, elevated CEBPD upregulates CXCL1, TNF-alpha-induced protein 6 (TNFAIP6), CCL20, and IL-32A gene transcripts by directly binding to their promoter regions (24). CXCL1 and TNFAIP6 enhance tube formation by endothelial cells, and all four proteins contribute to the migration and proliferation of synoviocytes. Thus, inhibiting macrophage CEBPD expression or activity in the synovial cavity could be a feasible and valuable strategy for RA treatment.
4.5 Tumors
Accumulating evidence has shown that macrophage CEBPD plays crucial roles in tumor escape, drug resistance, metastasis, and invasion. Among the cell types present in the tumor microenvironment, tumor-associated macrophages (TAMs) and their precursors account for the largest fraction of the myeloid infiltrates in most solid tumors (71). TAMs produce both tumor-supportive factors and anti-tumor factors that impact tumor growth, angiogenesis, invasion, and metastasis (72). Hsiao et al. have reported that the immunosuppressive prostaglandin E2 increases the expression and activity of CEBPD by stimulating the nucleocytoplasmic shuttling of the RNA-binding protein HuR, which binds to and stabilizes CEBPD mRNA in macrophages (27). Then, elevated CEBPD directly upregulates IL-10 and PTX3, which suppresses the ability of macrophages to phagocytose nasopharyngeal carcinoma cells via an autocrine mode of regulation (27). Additionally, antitumor drugs can lead to elevated CEBPD levels in macrophages (31, 50). Increased macrophage CEBPD promotes tumor resistance in two ways: (1) activating the pyrimidine metabolism pathway to produce the low molecular weight free metabolites in macrophages, as in the induction of gemcitabine resistance in pancreatic cancer cells (31); and (2) upregulating PTX3 expression, which enhances the resistance of breast cancer cells to cisplatin and 5-fluorouracil (50). In addition, the macrophage CEPBD/PTX3 axis contributes to metastasis, invasion, and stemness in breast cancer cells (50). Besides, given the important role of macrophage-associated tumor fibrosis in tumor resistance and the association of CEBPD with fibrotic disease (22), additional research is needed to assess whether macrophage CEBPD influences tumor resistance by regulating tumor fibrosis. These studies suggest that macrophage CEPBD plays several important roles in tumor chemoresistance, invasion, metastasis, and stemness maintenance, and can be an effective target for solid tumor therapy.
5 Developing therapeutic modulators of CEBPD
As CEBPD plays an important role in a variety of macrophage functions, modifying CEBPD expression or activity may be a promising strategy to treat various diseases. The endogenous CEBPD antagonists ATF3 and FBXW7 were found to inhibit CEBPD expression during monocyte-to-macrophage differentiation at the transcriptional and post-translational levels, respectively, with the inhibitory effect of FBXW7 being stronger (51). Further, CEBPG overexpression in macrophages results in the formation of a heterodimer with CEBPD that inhibits its transcriptional activity (49). However, unlike CEBPD, these proteins are more highly expressed and essential for maintaining normal cellular function, so are not viable therapeutic targets.
5.1 Known inhibitors
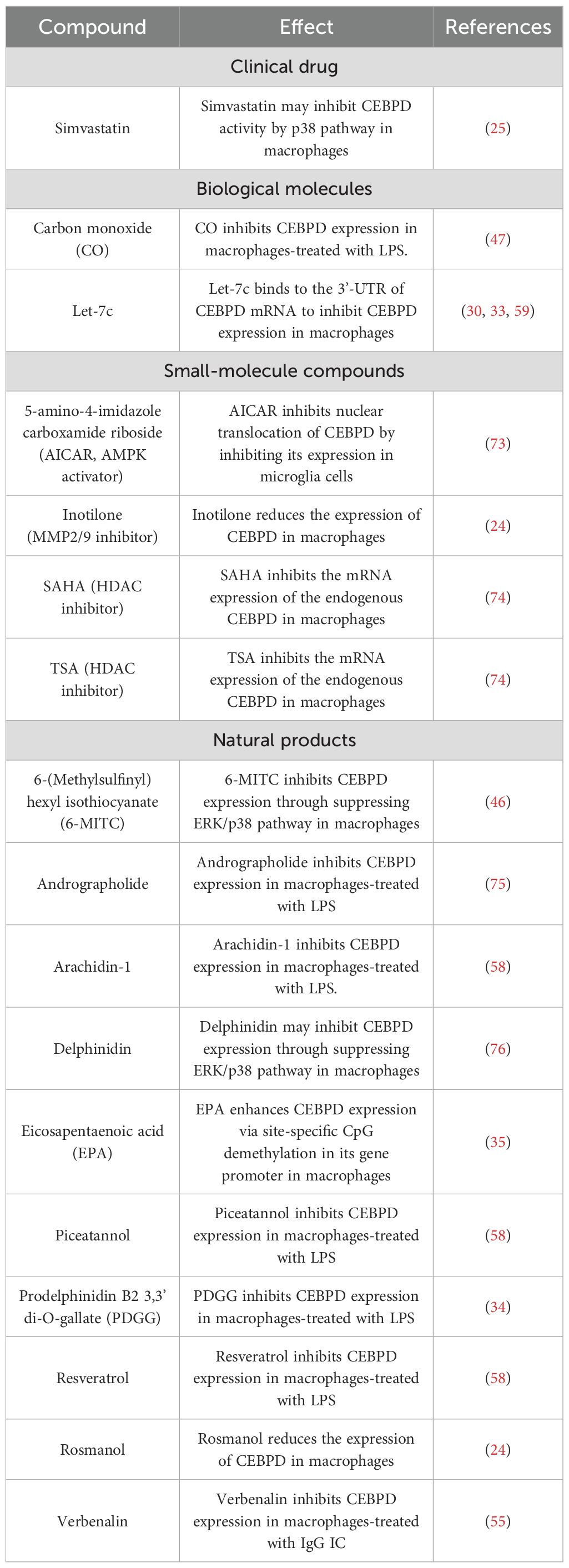
A growing body of evidence has shown that various compounds inhibit CEBPD expression in macrophages. These compounds are divided into 4 groups: clinical drugs, biological molecules, small-molecule compounds, and natural products (Table 3). The antihyperlipidemic drug simvastatin can inhibit CEBPD activity in macrophages to block lipid accumulation, and this inhibitory effect may be mediated by affecting the p38/CEBPD pathway (25). However, the bioavailability and biological half-life of simvastatin are 5% and 3 h, respectively, suggesting a significant rapid pass metabolism in the liver (77). Biological molecules such as carbon monoxide (CO) and miRNA let-7c inhibit CEBPD expression in macrophages treated with LPS (47, 59). Let-7c binds to the 3’-UTR of CEBPD mRNA to inhibit CEBPD expression, but the inhibition mechanism of CO remains unclear. Small-molecule compounds such as the AMPK activator 5-amino-4-imidazole carboxamide riboside (AICAR), MMP2/9 inhibitor inotilone, and HDAC inhibitors SAHA and TSA also inhibit CEBPD expression in macrophages, but the underlying mechanisms require further study (24, 73, 74). Various studies have also shown that treatment with natural products, such as andrographolide, delphinidin, and rosmanol, can inhibit CEBPD expression or activity in macrophages (24, 75, 76).
5.2 Using molecular modeling to identify putative binding sites
We have analyzed the ability of these inhibitors to bind to the CEBPD protein using molecular docking software (Discovery Studio). All compounds, except prodelphinidin B2 3,3’ di-O-gallate (PDGG) and CO, are predicted to bind to CEBPD protein (human), with binding energies of less than -18 kcal/mol (Figure 4, Supplementary Figure S1). Binding energy < 0 indicates that a compound can spontaneously bind to the target protein, and increasingly negative free binding energies result in the formation of stronger complexes between the compound and the target protein (78). Further, we predicted that these compounds bind to the helix 2 (H2) in N-terminal transcriptional activation domain (TAD) and H3 in middle regulatory domain (RD) of CEBPD (Figure 1B). The 11 main points of contact of CEBPD are Leu 85, Phe 86, Ser 88, Asn 89, Pro 123, Asp 124, Trp 125, Gly 126, Ala 130, Leu 134, and Gln 138 (Figure 4, Supplementary Figure S1). We comparatively analyzed the protein sequences of human CEBP family members with Clustal Omega (Figure 1B). All members of the CEBP family possess a variable N-terminal region and a highly conserved C-terminal (>90% sequence identity) containing a bZIP domain (22). The inhibitors we evaluated are predicted to bind to target the TAD and RD domain, not the bZIP domain of CEBPD (Figure 4, Supplementary Figure S1), suggesting that these compounds are less likely to bind other CEBP family members. Moreover, among CEBP family members, only four of the 11 critical binding residues predicted for CEBPD (Leu 85, Phe 86, Pro 123, and Gln 138) are also present only on CEBPA (Figure 1B). Among these compounds, only eicosapentaenoic acid (EPA) binds both Leu 85 and Phe 86 when it binds H2 in the TAD domain (Figures 1B, 4). These results suggest that with the exception of EPA which may have some effect on CEBPA activity, the remaining compounds have no effect on other members of the CEBP family. Further, we found that these 14 compounds do not bind to CEBPG and CEBPZ. In these 14 compounds, only simvastatin, 6-(methylsulfinyl) hexyl isothiocyanate (6-MITC), and verbenalin have only one binding site with CEBPA, CEBP, and CEBPE and their binding energies with these members are all lower than that with CEBPD (Supplementary Figure S2). The above results indicate that these compounds are predicted to inhibition of primarily affect CEBPD activity in macrophages. The exact mechanisms of these compounds require further study.
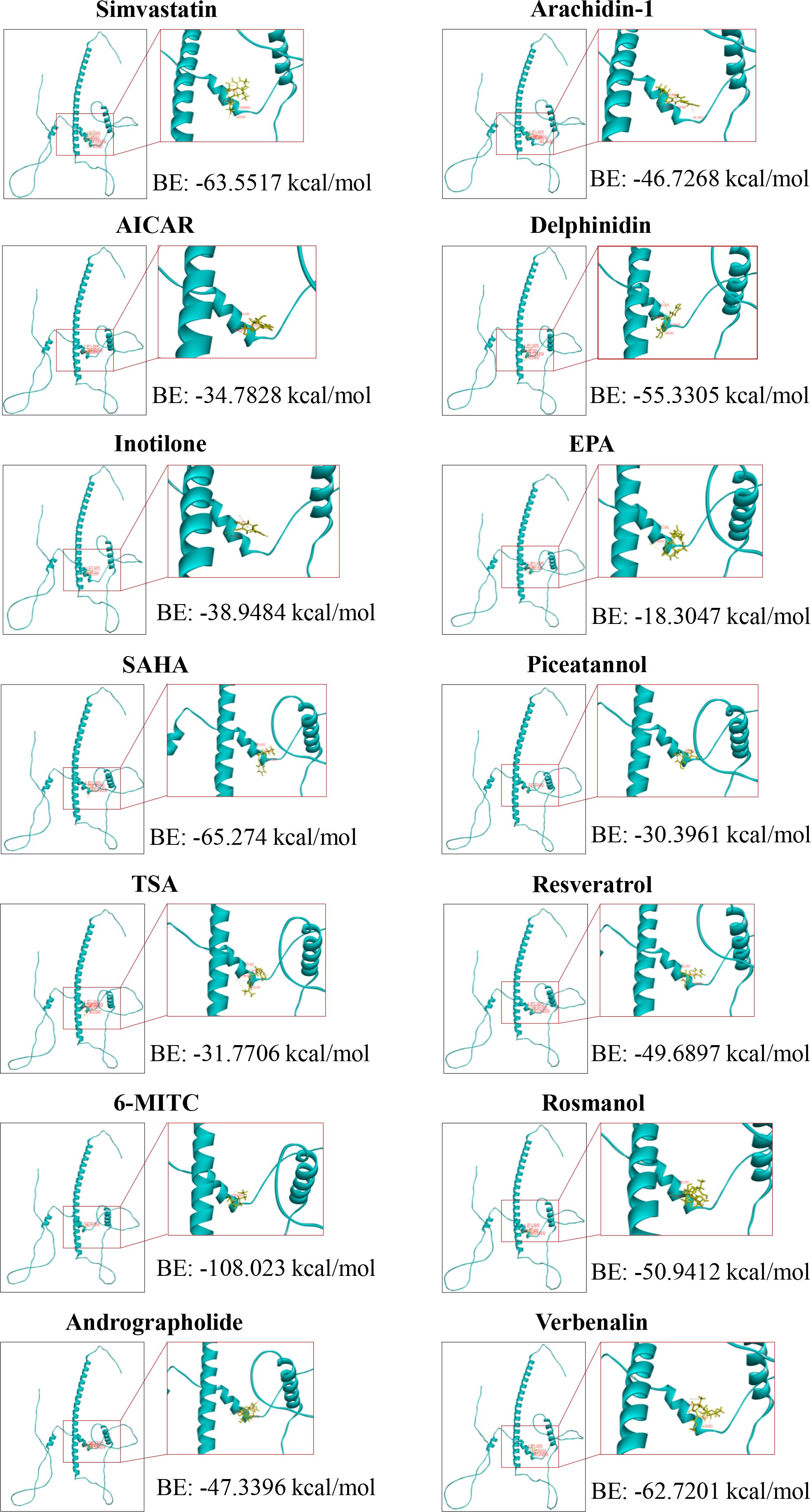
Figure 4. Molecular docking between the transcriptional activation domain of human CEPBD protein and compounds. 6-MITC, 6-(Methylsulfinyl) hexyl Isothiocyanate; AICAR, 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; EPA, Eicosapentaenoic acid; SAHA, Suberoylanilide hydroxamic acid; TSA, Trichostatin A.
5.3 Towards macrophage-specific delivery of inhibitors
When used, these drugs often inhibit CEBPD and other proteins in other types of cells, causing side effects, indicating the importance of designing macrophage-specific delivery systems. One such delivery system is clodronate liposomes, which are used to deplete macrophages in a temporally controlled manner in many research laboratories worldwide, suggesting that liposomes may be ideal vehicles for macrophage phagocytosis (79). Liposomes are spherical vesicles comprising one or more concentric phospholipid bilayers enclosing an aqueous core, and have been used as nontoxic and biodegradable delivery systems for several drugs, such as amphotericin B, doxorubicin, and siRNA, to treat fungal infections, Kaposi’s sarcoma, and ovarian cancer, respectively (80, 81). Mechanistically, liposomes are recognized as foreign particles by phagocytes, especially macrophages, and are ingested as an internal vesicle called phagosome. The ingestion is followed by a fusion of the phagosome with lysosomes containing phospholipases, which disrupt the phospholipid bilayer of liposomes, releasing its contents inside the cells/macrophages (82). Therefore, biological molecules or small-molecule compounds such as let-7c or andrographolide can be encapsulated into liposome vesicles. After liposomes are phagocytosed by macrophages, these molecules or compounds could regulate CEBPD expression or activity, thereby modulating macrophage function. However, accelerated blood clearance and non-specific targeting are the main drawbacks of the liposome delivery system. Despite years of in-depth research in these areas, progress has been slow.
Alternatively, the specificity of other drug delivery systems, such as lipid nanoparticles (LNP), has shown promising applications in targeting macrophage regulation (83, 84). LNPs are a class of lipid vesicles that have gained recognition as highly effective carriers for delivering diverse therapeutic agents due to their biocompatibility, loading capacity, and customizable features (85). LNPs consist of four main lipid components with essential functions: ionizable lipids, helper lipids, cholesterol, and polyethylene glycol (PEG) lipids (85). In recent years, LNPs have emerged as a highly promising delivery platform for gene-based therapies in various diseases, such as cardiovascular disease (85, 86). Recently, Du et al. formulated 1,2-dioleoyl-sn-glycero-3-phospho-l-serine-doping ALC-0315 (DOPS, an ionizable lipid) LNP that successfully generated chimeric antigen receptor-macrophages capable of selectively engaging and phagocytosing activated cardiac fibroblasts in ischemia-reperfusion mouse hearts (86). To target macrophages, DOPS is recognized by scavenger receptors highly expressed on the surface of macrophages (87). These studies suggest that DOPS-LNP containing CEBPD inhibitors is an effective potential delivery vehicle for regulating macrophage function.
6 Summary and perspectives
Recent technological advances in high-throughput sequencing have allowed us to understand macrophage heterogeneity across development, health, and different disease conditions with unprecedented depth and perspective, highlighting the irreplaceable role of macrophages in physiology and disease progression. However, there are no effective strategies for targeting macrophages to treat diseases. Despite the identification of many distinct cellular states of macrophages, we are only beginning to understand whether these states represent functionally distinct macrophage subsets determined by their ontogeny or whether plasticity allows them to switch between functional states (61). In this review, we explored the feasibility of targeting macrophages for disease treatment, using CEBPD as an example. CEBPD expression is low in macrophages under physiological conditions, but it can be rapidly induced by external stimuli (35, 37, 42, 46). We have summarized a broad range of recent advances in research on macrophage CEBPD and its roles in various diseases. These studies suggest that modulating macrophage function by targeting CEBPD is an attractive strategy for disease treatment.
Despite the encouraging progress in exploring the relationship between CEBPD and macrophages, some critical questions must be answered before targeting macrophage CEBPD for clinical use in disease treatment. Macrophages used in the aforementioned studies were derived from the mouse macrophage cell line RAW264.7, the human monocytic leukemia cell line THP-1, the human peripheral blood mononuclear cells, or the mouse bone marrow. These macrophages are either immortalized or induced rather than isolated from tissues. Although in vitro methods are easy to evaluate and readily provide data related to biological mechanisms, they lack the ability to in vivo biokinetics and cross-talk between tissues, potentially leading to a misinterpretation of in vitro data (88). It is possible that in in vivo systems, the cells are exposed to lower concentrations, since chemical compounds cannot directly reach the target cells. On the other hand, drugs may accumulate in certain organs, tissues, or cells, resulting in a prolonged or enhanced exposure of the target tissue. Another consideration is that macrophages respond differently to drugs or stimuli under the influence of other cells in the tissue microenvironment. Further studies should focus on testing and validating the role of macrophage CEBPD in the tissues of patients and/or model animals. Additionally, although evidence shows that CEBPD levels are elevated in macrophages under in vitro stimuli, the expression patterns of CEBPD in distinct macrophage subsets during physiological states and different disease states remain unclear. Furthermore, Tanaka et al. have reported that 35% of CEBPB-/- mice and 85% of CEBPB-/-; CEBPD-/- mice die soon after birth, suggesting that CEBPD and CEBPB have overlapping functions to some degree (89). However, Spek et al. have shown that the expression of other CEBP family members, except for an increase of CEBPA, shows no changes in CEBPD knockout RAW264.7 cells under LPS stimulation, compared to that in wild type cells (66). We treated bone marrow-derived macrophages from macrophage-specific knockout CEBPD mice with LPS and the results were similar to those of the aforementioned study (Unpublished). Further investigations are thus required to determine whether CEBPA and CEBPD are functionally complementary in macrophages. The evidence summarized in this review strengthens the hypothesis that CEBPD may be an effective target for modulating macrophage function to treat disease and serves as a reference for further investigation in this field.
Author contributions
TF: Writing – review & editing, Writing – original draft, Visualization, Investigation. SL: Writing – review & editing, Visualization. JZ: Conceptualization, Project administration, Writing – review & editing. JC: Writing – review & editing, Project administration. LW: Funding acquisition, Project administration, Visualization, Writing – review & editing, Conceptualization, Supervision, Investigation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the basic and applied basic research project of Guangdong province of China (2024A1515013218), the research team project of prevention and treatment of diabetic cardiomyopathy with integrated Chinese and Western medicine (2024ZZ06), and the research program of Guangdong provincial administration of traditional Chinese medicine (20244045).
Acknowledgments
We thanked Siyu Dong for her contribution to the Supplementary Figure 2. Figures 2 and 3 were modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License. (https://creativecommons.org/licenses/by/3.0/). We apologize to researchers whose work could not be cited in this review because of space limitations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1650161/full#supplementary-material
References
1. Tiwari SK, Wong WJ, Moreira M, Pasqualini C, and Ginhoux F. Induced pluripotent stem cell-derived macrophages as a platform for modelling human disease. Nat Rev Immunol. (2025) 25:108–24. doi: 10.1038/s41577-024-01081-x
2. Guan F, Wang R, Yi Z, Luo P, Liu W, Xie Y, et al. Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct Target Ther. (2025) 10:93. doi: 10.1038/s41392-025-02124-y
3. Lazarov T, Juarez-Carreno S, Cox N, and Geissmann F. Physiology and diseases of tissue-resident macrophages. Nature. (2023) 618:698–707. doi: 10.1038/s41586-023-06002-x
4. T’Jonck W and Bain CC. The role of monocyte-derived macrophages in the lung: it’s all about context. Int J Biochem Cell Biol. (2023) 159:106421. doi: 10.1016/j.biocel.2023.106421
5. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. (2019) 363:eaau0964. doi: 10.1126/science.aau0964
6. Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell. (2019) 178:1509–25.e19. doi: 10.1016/j.cell.2019.08.009
7. Li W, Yang Y, Yang L, Chang N, and Li L. Monocyte-derived Kupffer cells dominate in the Kupffer cell pool during liver injury. Cell Rep. (2023) 42:113164. doi: 10.1016/j.celrep.2023.113164
8. Amrute JM, Luo X, Penna V, Yang S, Yamawaki T, Hayat S, et al. Targeting immune-fibroblast cell communication in heart failure. Nature. (2024) 635:423–33. doi: 10.1038/s41586-024-08008-5
9. Alexanian M, Padmanabhan A, Nishino T, Travers JG, Ye L, Pelonero A, et al. Chromatin remodelling drives immune cell-fibroblast communication in heart failure. Nature. (2024) 635:434–43. doi: 10.1038/s41586-024-08085-6
10. Zhang K, Wang Y, Chen S, Mao J, Jin Y, Ye H, et al. Trem2(Hi) resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis. Nat Metab. (2023) 5:129–46. doi: 10.1038/s42255-022-00715-5
11. Ganguly S, Rosenthal SB, Ishizuka K, Troutman TD, Rohm TV, Khader N, et al. Lipid-associated macrophages’ Promotion of fibrosis resolution during mash regression requires Trem2. Proc Natl Acad Sci U.S.A. (2024) 121:e2405746121. doi: 10.1073/pnas.2405746121
12. Wang LX, Zhang SX, Wu HJ, Rong XL, and Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. (2019) 106:345–58. doi: 10.1002/JLB.3RU1018-378RR
13. Wei Q, Deng Y, Yang Q, Zhan A, and Wang L. The markers to delineate different phenotypes of macrophages related to metabolic disorders. Front Immunol. (2023) 14:1084636. doi: 10.3389/fimmu.2023.1084636
14. Goebeler ME, Stuhler G, and Bargou R. Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nat Rev Clin Oncol. (2024) 21:539–60. doi: 10.1038/s41571-024-00905-y
15. Liu W, Cheng G, Cui H, Tian Z, Li B, Han Y, et al. Theoretical basis, state and challenges of living cell-based drug delivery systems. Theranostics. (2024) 14:5152–83. doi: 10.7150/thno.99257
16. Oosterhof N, Chang IJ, Karimiani EG, Kuil LE, Jensen DM, Daza R, et al. Homozygous mutations in csf1r cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am J Hum Genet. (2019) 104:936–47. doi: 10.1016/j.ajhg.2019.03.010
17. Mirza R, DiPietro LA, and Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. (2009) 175:2454–62. doi: 10.2353/ajpath.2009.090248
18. Li T, Lin S, Zhu Y, Ye D, Rong X, and Wang L. Basic biology and roles of Cebpd in cardiovascular disease. Cell Death Discov. (2025) 11:102. doi: 10.1038/s41420-025-02357-4
19. Pulido-Salgado M, Vidal-Taboada JM, and Saura J. C/Ebpbeta and C/Ebpdelta transcription factors: basic biology and roles in the Cns. Prog Neurobiol. (2015) 132:1–33. doi: 10.1016/j.pneurobio.2015.06.003
20. Hartl L, Duitman J, Bijlsma MF, and Spek CA. The dual role of C/Ebpdelta in cancer. Crit Rev Oncol Hematol. (2023) 185:103983. doi: 10.1016/j.critrevonc.2023.103983
21. Ko CY, Chang WC, and Wang JM. Biological roles of Ccaat/enhancer-binding protein delta during inflammation. J BioMed Sci. (2015) 22:6. doi: 10.1186/s12929-014-0110-2
22. Wang L, Feng J, Deng Y, Yang Q, Wei Q, Ye D, et al. Ccaat/enhancer-binding proteins in fibrosis: complex roles beyond conventional understanding. Res (Wash D C). (2022) 2022:9891689. doi: 10.34133/2022/9891689
23. Angireddy R, Kazmi HR, Srinivasan S, Sun L, Iqbal J, Fuchs SY, et al. Cytochrome C oxidase dysfunction enhances phagocytic function and osteoclast formation in macrophages. FASEB J. (2019) 33:9167–81. doi: 10.1096/fj.201900010RR
24. Chang LH, Huang HS, Wu PT, Jou IM, Pan MH, Chang WC, et al. Role of macrophage Ccaat/enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice. PloS One. (2012) 7:e45378. doi: 10.1371/journal.pone.0045378
25. Lai HY, Hsu LW, Tsai HH, Lo YC, Yang SH, Liu PY, et al. Ccaat/enhancer-binding protein delta promotes intracellular lipid accumulation in M1 macrophages of vascular lesions. Cardiovasc Res. (2017) 113:1376–88. doi: 10.1093/cvr/cvx134
26. Yan C, Liu Y, Gao H, and Wang X. Suppressors of cytokine signaling 3 is essential for Fcgammar-mediated inflammatory response via enhancing Ccaat/enhancer-binding protein delta transcriptional activity in macrophages. Exp Cell Res. (2015) 337:120–7. doi: 10.1016/j.yexcr.2015.07.017
27. Hsiao YW, Li CF, Chi JY, Tseng JT, Chang Y, Hsu LJ, et al. Ccaat/enhancer binding protein delta in macrophages contributes to immunosuppression and inhibits phagocytosis in nasopharyngeal carcinoma. Sci Signal. (2013) 6:ra59. doi: 10.1126/scisignal.2003648
28. Guha M, Srinivasan S, Koenigstein A, Zaidi M, and Avadhani NG. Enhanced osteoclastogenesis by mitochondrial retrograde signaling through transcriptional activation of the cathepsin K gene. Ann N Y Acad Sci. (2016) 1364:52–61. doi: 10.1111/nyas.12709
29. Nishioka K, Ohshima S, Umeshita-Sasai M, Yamaguchi N, Mima T, Nomura S, et al. Enhanced expression and DNA binding activity of two Ccaat/enhancer-binding protein isoforms, C/Ebpbeta and C/Ebpdelta, in rheumatoid synovium. Arthritis Rheum. (2000) 43:1591–6. doi: 10.1002/1529-0131(200007)43:7<1591::AID-ANR24>3.0.CO;2-9
30. Song H, Yang Y, Sun Y, Wei G, Zheng H, Chen Y, et al. Circular Rna Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol Ther. (2022) 30:915–31. doi: 10.1016/j.ymthe.2021.09.017
31. Spek CA, Aberson HL, and Duitman J. Macrophage C/Ebpdelta drives gemcitabine, but not 5-Fu or paclitaxel, resistance of pancreatic cancer cells in a deoxycytidine-dependent manner. Biomedicines. (2022) 10:219. doi: 10.3390/biomedicines10020219
32. Maitra U, Singh N, Gan L, Ringwood L, and Li L. Irak-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing Nox-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem. (2009) 284:35403–11. doi: 10.1074/jbc.M109.059501
33. Hao X, Guo Y, Wang R, Yu X, He L, and Shu M. Exosomes from adipose-derived mesenchymal stem cells promote survival of fat grafts by regulating macrophage polarization via let-7c. Acta Biochim Biophys Sin (Shanghai). (2021) 53:501–10. doi: 10.1093/abbs/gmab018
34. Hou DX, Masuzaki S, Hashimoto F, Uto T, Tanigawa S, Fujii M, et al. Green tea proanthocyanidins inhibit cyclooxygenase-2 expression in Lps-activated mouse macrophages: molecular mechanisms and structure-activity relationship. Arch Biochem Biophys. (2007) 460:67–74. doi: 10.1016/j.abb.2007.01.009
35. Ceccarelli V, Racanicchi S, Martelli MP, Nocentini G, Fettucciari K, Riccardi C, et al. Eicosapentaenoic acid demethylates a single cpg that mediates expression of tumor suppressor Ccaat/enhancer-binding protein delta in U937 leukemia cells. J Biol Chem. (2011) 286:27092–102. doi: 10.1074/jbc.M111.253609
36. Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, et al. Function of C/Ebpdelta in a regulatory circuit that discriminates between transient and persistent Tlr4-induced signals. Nat Immunol. (2009) 10:437–43. doi: 10.1038/ni.1721
37. Liu L, Zhong J, Chen B, Wang W, Xi H, and Su X. Ccaat/enhancer binding protein (C/Ebp) delta promotes the expression of Ptx3 and macrophage phagocytosis during A. Fumigatus infection. J Leukoc Biol. (2022) 111:1225–34. doi: 10.1002/JLB.4MA1121-451RR
38. Balamurugan K, Sharan S, Klarmann KD, Zhang Y, Coppola V, Summers GH, et al. Fbxw7alpha attenuates inflammatory signalling by downregulating C/Ebpdelta and its target gene Tlr4. Nat Commun. (2013) 4:1662. doi: 10.1038/ncomms2677
39. Cho MK, Cho YH, Lee GH, and Kim SG. Induction of cyclooxygenase-2 by bovine type I collagen in macrophages via C/Ebp and Creb activation by multiple cell signaling pathways. Biochem Pharmacol. (2004) 67:2239–50. doi: 10.1016/j.bcp.2004.02.024
40. Liu YW, Chen CC, Wang JM, Chang WC, Huang YC, Chung SY, et al. Role of transcriptional factors sp1, C-Rel, and C-Jun in Lps-induced C/Ebpdelta gene expression of mouse macrophages. Cell Mol Life Sci. (2007) 64:3282–94. doi: 10.1007/s00018-007-7375-5
41. Huang YC, Chang WC, Su JG, Cai JL, Chen CC, Hung JJ, et al. Peptidoglycan enhances transcriptional expression of Ccaat/enhancer-binding protein delta gene in mouse macrophages. J BioMed Sci. (2007) 14:407–18. doi: 10.1007/s11373-007-9146-6
42. Yan C, Chen J, Wang B, Wang J, Luo M, Tong J, et al. Pd-L1 expression is increased in Lps-induced acute respiratory distress syndrome by Pi3k-Akt-Egr-1/C/Ebpdelta signaling pathway. Inflammation. (2024) 47:1459–78. doi: 10.1007/s10753-024-01988-6
43. Wang L, Liu T, Chen G, Li Y, Zhang S, Mao L, et al. Exosomal microrna let-7-5p from taenia pisiformis cysticercus prompted macrophage to M2 polarization through inhibiting the expression of C/Ebp delta. Microorganisms. (2021) 9:1403. doi: 10.3390/microorganisms9071403
44. Yan C, Zhu M, Staiger J, Johnson PF, and Gao H. C5a-regulated ccaat/enhancer-binding proteins beta and delta are essential in Fcgamma receptor-mediated inflammatory cytokine and chemokine production in macrophages. J Biol Chem. (2012) 287:3217–30. doi: 10.1074/jbc.M111.280834
45. Serio KJ, Reddy KV, and Bigby TD. Lipopolysaccharide induces 5-lipoxygenase-activating protein gene expression in Thp-1 cells via a Nf-Kappab and C/Ebp-mediated mechanism. Am J Physiol Cell Physiol. (2005) 288:C1125–33. doi: 10.1152/ajpcell.00296.2004
46. Uto T, Fujii M, and Hou DX. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 transcription by 6-(Methylsulfinyl) hexyl isothiocyanate, a chemopreventive compound from Wasabia Japonica (Miq.) Matsumura, in mouse macrophages. Biochem Pharmacol. (2005) 70:1772–84. doi: 10.1016/j.bcp.2005.09.023
47. Suh GY, Jin Y, Yi AK, Wang XM, and Choi AM. Ccaat/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. (2006) 35:220–6. doi: 10.1165/rcmb.2005-0154OC
48. Pennini ME, Liu Y, Yang J, Croniger CM, Boom WH, and Harding CV. Ccaat/enhancer-binding protein beta and delta binding to ciita promoters is associated with the inhibition of ciita expression in response to mycobacterium tuberculosis 19-Kda lipoprotein. J Immunol. (2007) 179:6910–8. doi: 10.4049/jimmunol.179.10.6910
49. Yan C, Zhang L, Yang L, Zhang Q, and Wang X. C/Ebpgamma is a critical negative regulator of Lps-/Igg immune complex-induced acute lung injury through the downregulation of C/Ebpbeta-/C/Ebpdelta-dependent C/Ebp transcription activation. FASEB J. (2020) 34:13696–710. doi: 10.1096/fj.202001402R
50. Chi JY, Hsiao YW, Li CF, Lo YC, Lin ZY, Hong JY, et al. Targeting chemotherapy-induced Ptx3 in tumor stroma to prevent the progression of drug-resistant cancers. Oncotarget. (2015) 6:23987–4001. doi: 10.18632/oncotarget.4364
51. Jauch-Speer SL, Herrera-Rivero M, Ludwig N, Veras De Carvalho BC, Martens L, Wolf J, et al. C/Ebpdelta-induced epigenetic changes control the dynamic gene transcription of S100a8 and S100a9. Elife. (2022) 11:e75594. doi: 10.7554/eLife.75594
52. Zannetti C, Bonnay F, Takeshita F, Parroche P, Menetrier-Caux C, Tommasino M, et al. C/ebpdelta and Stat-1 are required for Tlr8 transcriptional activity. J Biol Chem. (2010) 285:34773–80. doi: 10.1074/jbc.M110.133884
53. Takeshita F, Suzuki K, Sasaki S, Ishii N, Klinman DM, and Ishii KJ. Transcriptional regulation of the human Tlr9 gene. J Immunol. (2004) 173:2552–61. doi: 10.4049/jimmunol.173.4.2552
54. Yan C, Johnson PF, Tang H, Ye Y, Wu M, and Gao H. Ccaat/enhancer-binding protein delta is a critical mediator of lipopolysaccharide-induced acute lung injury. Am J Pathol. (2013) 182:420–30. doi: 10.1016/j.ajpath.2012.10.013
55. Yang L, Liu T, Zhuo Y, Li D, Li D, Liu J, et al. Verbenalin alleviates acute lung injury induced by sepsis and igg immune complex through Gpr18 receptor. Cell Signal. (2023) 109:110768. doi: 10.1016/j.cellsig.2023.110768
56. Sheu KM and Hoffmann A. Functional hallmarks of healthy macrophage responses: their regulatory basis and disease relevance. Annu Rev Immunol. (2022) 40:295–321. doi: 10.1146/annurev-immunol-101320-031555
57. Hume DA, Millard SM, and Pettit AR. Macrophage heterogeneity in the single-cell era: facts and artifacts. Blood. (2023) 142:1339–47. doi: 10.1182/blood.2023020597
58. Djoko B, Chiou RY, Shee JJ, and Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of raw 264.7 macrophages. J Agric Food Chem. (2007) 55:2376–83. doi: 10.1021/jf062741a
59. Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, et al. Microrna let-7c regulates macrophage polarization. J Immunol. (2013) 190:6542–9. doi: 10.4049/jimmunol.1202496
60. Mass E, Nimmerjahn F, Kierdorf K, and Schlitzer A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat Rev Immunol. (2023) 23:563–79. doi: 10.1038/s41577-023-00848-y
61. Uribe-Querol E and Rosales C. Phagocytosis: Our current understanding of a universal biological process. Front Immunol. (2020) 11:1066. doi: 10.3389/fimmu.2020.01066
62. Hoff HF, O’Neil J, Pepin JM, and Cole TB. Macrophage uptake of cholesterol-containing particles derived from LDL and isolated from atherosclerotic lesions. Eur Heart J. (1990) 11 Suppl:E:105–15. doi: 10.1093/eurheartj/11.suppl_e.105
63. Ishida T, Harashima H, and Kiwada H. Liposome clearance. Biosci Rep. (2002) 22:197–224. doi: 10.1023/a:1020134521778
64. Erreni M, Manfredi AA, Garlanda C, Mantovani A, and Rovere-Querini P. The long pentraxin PTX3: A prototypical sensor of tissue injury and a regulator of homeostasis. Immunol Rev. (2017) 280:112–25. doi: 10.1111/imr.12570
65. Nyandwi JB, Ko YS, Jin H, Yun SP, Park SW, and Kim HJ. Rosmarinic acid increases macrophage cholesterol efflux through regulation of abca1 and abcg1 in different mechanisms. Int J Mol Sci. (2021) 22:8791. doi: 10.3390/ijms22168791
66. Spek CA, Aberson HL, Butler JM, de Vos AF, and Duitman J. Cebpd potentiates the macrophage inflammatory response but cebpd knock-out macrophages fail to identify cebpd-dependent pro-inflammatory transcriptional programs. Cells. (2021) 10:2233. doi: 10.3390/cells10092233
67. Duitman J, Hoogendijk AJ, Groot AP, Ruela de Sousa RR, van der Poll T, Florquin S, et al. Ccaat-enhancer binding protein delta (C/Ebpdelta) protects against klebsiella pneumoniae-induced pulmonary infection: potential role for macrophage migration. J Infect Dis. (2012) 206:1826–35. doi: 10.1093/infdis/jis615
68. Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, and Harding CV. Prolonged toll-like receptor signaling by mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect Immun. (2004) 72:6603–14. doi: 10.1128/IAI.72.11.6603-6614.2004
69. Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, and Boom WH. The mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated Hla-Dr and Fc gamma R1 on human macrophages through toll-like receptor 2. Infect Immun. (2003) 71:4487–97. doi: 10.1128/IAI.71.8.4487-4497.2003
70. Li H, Xu WX, Tan JC, Hong YM, He J, Zhao BP, et al. Single-cell multi-omics identify novel regulators required for osteoclastogenesis during aging. iScience. (2024) 27:110734. doi: 10.1016/j.isci.2024.110734
71. Cassetta L and Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. (2023) 23:238–57. doi: 10.1038/s41568-022-00547-1
72. Zhang Q and Sioud M. Tumor-associated macrophage subsets: shaping polarization and targeting. Int J Mol Sci. (2023) 24:7493. doi: 10.3390/ijms24087493
73. Giri S, Nath N, Smith B, Viollet B, Singh AK, and Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: A possible role of amp-activated protein kinase. J Neurosci. (2004) 24:479–87. doi: 10.1523/JNEUROSCI.4288-03.2004
74. Ullmann T, Luckhardt S, Wolf M, Parnham MJ, and Resch E. High-throughput screening for Cebpd-modulating compounds in Thp-1-derived reporter macrophages identifies anti-inflammatory Hdac and bet inhibitors. Int J Mol Sci. (2021) 22:3022. doi: 10.3390/ijms22063022
75. Lee KC, Chang HH, Chung YH, and Lee TY. Andrographolide acts as an anti-inflammatory agent in lps-stimulated raw264.7 macrophages by inhibiting Stat3-mediated suppression of the Nf-Kappab pathway. J Ethnopharmacol. (2011) 135:678–84. doi: 10.1016/j.jep.2011.03.068
76. Hou DX, Yanagita T, Uto T, Masuzaki S, and Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in Lps-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. (2005) 70:417–25. doi: 10.1016/j.bcp.2005.05.003
77. Rahamathulla M, Gangadharappa HV, Veerapu G, Hani U, Alhamhoom Y, Alqahtani A, et al. Characterization, optimization, in vitro and in vivo evaluation of simvastatin proliposomes, as a drug delivery. AAPS PharmSciTech. (2020) 21:129. doi: 10.1208/s12249-020-01666-4
78. Lin S, Zhang S, Zhan A, Feng J, Yang Q, Li T, et al. Palmatine alleviates cardiac fibrosis by inhibiting fibroblast activation through the STAT3 pathway. Eur J Pharmacol. (2024) 967:176395. doi: 10.1016/j.ejphar.2024.176395
80. Guimaraes D, Cavaco-Paulo A, and Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. (2021) 601:120571. doi: 10.1016/j.ijpharm.2021.120571
81. Bozzuto G and Molinari A. Liposomes as nanomedical devices. Int J Nanomed. (2015) 10:975–99. doi: 10.2147/IJN.S68861
82. Moreno SG. Depleting macrophages in vivo with clodronate-liposomes. Methods Mol Biol. (2018) 1784:259–62. doi: 10.1007/978-1-4939-7837-3_23
83. Huang L, Huang XH, Yang X, Hu JQ, Zhu YZ, Yan PY, et al. Novel nano-drug delivery system for natural products and their application. Pharmacol Res. (2024) 201:107100. doi: 10.1016/j.phrs.2024.107100
84. Chuang ST, Stein JB, Nevins S, Bektas CK, Choi HK, Ko W, et al. Enhancing CAR macrophage efferocytosis via surface engineered lipid nanoparticles targeting LXR signaling. Adv Mater. (2024) 36:e2308377. doi: 10.1002/adma.202308377
85. Soroudi S, Jaafari MR, and Arabi L. Lipid nanoparticle (LNP) mediated mRNA delivery in cardiovascular diseases: Advances in genome editing and CAR T cell therapy. J Control Release. (2024) 372:113–40. doi: 10.1016/j.jconrel.2024.06.023
86. Du H, You X, Zhang J, Liu S, Zhou Y, Wang Y, et al. CAR macrophages engineered in vivo for attenuating myocardial ischemia-reperfusion injury. Circ Res. (2025) 137:846–59. doi: 10.1161/CIRCRESAHA.125.326716
87. Alquraini A and Khoury JE. Scavenger receptors. Curr Biol. (2020) 30:R790–5. doi: 10.1016/j.cub.2020.05.051
88. Saeidnia S, Manayi A, and Abdollahi M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr Drug Discov Technol. (2015) 12:218–24. doi: 10.2174/1570163813666160114093140
Keywords: macrophage, CEBPD, polarization, phagocytosis, atherosclerosis, osteoporosis
Citation: Fan T, Lin S, Zhou J, Chen J and Wang L (2025) The feasibility of targeting macrophage for disease treatment: roles of CEBPD. Front. Immunol. 16:1650161. doi: 10.3389/fimmu.2025.1650161
Received: 19 June 2025; Accepted: 25 August 2025;
Published: 08 September 2025.
Edited by:
Alok Agrawal, Retired, United StatesReviewed by:
Susan Taylor Yeyeodu, OUHSC Stephenson Cancer Center, United StatesZhongnan Ma, Johns Hopkins University, United States
Copyright © 2025 Fan, Lin, Zhou, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Fan, YmlvZmFudGlhbkBnemh1LmVkdS5jbg==; Lexun Wang, d2FuZ2xleHVuQGdkcHUuZWR1LmNu
 Tian Fan1*
Tian Fan1* Jingjing Zhou
Jingjing Zhou Lexun Wang
Lexun Wang