- 1Department of General Medicine, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Department of Pathology, Affiliated Rehabilitation Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
- 3Tumor Immunology Institute, Nanchang University, Nanchang, Jiangxi, China
- 4Department of Radiotherapy, First Hospital of Tsinghua University, Beijing, China
- 5The Ministry of Education (MOE) Basic Research and Innovation Center for the Targeted Therapeutics of Solid Tumors, Affiliated Rehabilitation Hospital, Jiangxi Medical College, Nanchang University, Nanchang, China
Background: Chronic stress and gut dysbiosis are established risk factors for colorectal adenocarcinoma, yet their synergistic effects on the development of intestinal precancerous lesions remain poorly understood.
Methods: This study investigates the molecular mechanisms through which chronic stress interacts with opportunistic pathogen Listeria monocytogenes to drive intestinal tumorigenesis in ApcMin/+ mice, with particular focus on the involvement of tumor immune microenvironment remodeling.
Results: The combination of L. monocytogenes infection and chronic stress, rather than bacterial infection alone, significantly increased colonic adenoma burden and epithelial dysplasia, suggesting that chronic stress establishes a permissive microenvironment for opportunistic pathogens to exert pro-tumorigenic effects. Mechanistically, chronic stress downregulated intestinal epithelial Muc-2 expression and reduced microbial diversity, thereby compromising mucus/microbial barrier integrity and enhancing L. monocytogenes colonization. Under dual stress-pathogen exposure, we observed the expansion of myeloid-derived suppressor cells (MDSCs) in spleen and the upregulation of IL-6 in colonic mucosa, which facilitated MDSCs recruitment to tumor sites. Infiltrating MDSCs driven CD8+ T cell depletion through cAMP/PKA/CREB signaling, leading to the establishment of immunosuppressive microenvironment.
Conclusion: Our results propose that chronic stress-induced gut barrier disruption may serve as a prerequisite for opportunistic pathogens to accelerate the development of precancerous lesions. Their synergistic effects reshape systemic/local immune responses, creating a microenvironment conducive to malignant transformation and tumor cell survival. These preliminary findings highlight potential clinical applications of psychological interventions and immune modulation strategies in preventing intestinal carcinogenesis.
Introduction
Colorectal adenocarcinoma (CRC) ranks as the third most prevalent malignancy and represents the second leading cause of cancer-related mortality globally (1–3). The colorectal adenoma, a benign glandular tumor, is a precursor lesion of CRC (2). As the only currently effective treatment for colorectal adenoma, colonoscopic resection has been proven to significantly reduce the incidence of CRC. However, high recurrence rates of adenoma remain the biggest obstacle to progress in complete healing (4–6).
Insufficient understanding of pathogenesis represents an important factor limiting our efforts to develop therapeutic interventions against adenoma. Considering the pathological continuum from chronic inflammation to tumor, intestinal dysbiosis may stand at the crossroad and contribute to the colonization of opportunistic pathogen (7). Opportunistic pathogens such as Fusobacterium nucleatum and Streptococcus gallolyticus can promote the development of intestinal adenoma through various mechanisms, including disrupting intestinal barrier function, inducing chronic inflammatory responses, and modulating the immune microenvironment (7–9).
Listeria monocytogenes (L. monocytogenes), a common opportunistic pathogen, is widely distributed in natural environments and food products (10). Clinical studies have revealed that L. monocytogenes is significantly enriched in tumor tissues of CRC patients compared to adjacent normal tissues (11), suggesting a potential spatiotemporal association between L. monocytogenes and CRC. However, there have been no reports addressing whether L. monocytogenes participates in driving colorectal precancerous lesions, considering the pathological continuum from adenoma to adenocarcinoma. Notably, as L. monocytogenes is more prone to cause systemic infections in immunocompromised individuals, it has been suggested to preferentially colonize tissues under the immunosuppressive microenvironment (12, 13). Moreover, unlike common CRC-associated pathogens such as Fusobacterium nucleatum, L. monocytogenes preferentially colonizes in immunosuppressive microenvironments. Surface proteins of L. monocytogenes have been shown to disrupt intestinal epithelial tight junctions (TJs) and facilitate trans-epithelial transport within 24 hours (14). Critically, L. monocytogenes can induce IL-6 production via the TLR2/NF-κB and MAPK pathways, thereby recruiting myeloid-derived suppressor cells (MDSCs) (15). However, the specific pathophysiological contexts through which L. monocytogenes contributes to adenomagenesis require systematic investigation.
Recent studies have increasingly recognized the fact that mental disorders have etiologic impact on the pathogenesis of chronic diseases and tumors (16). Both direct such as neuroendocrine pathways and indirect mechanisms, including gut microbiota dysbiosis, impaired intestinal barrier function, and tumor immune microenvironment dysregulation, are involved in this process (17, 18). However, direct experimental evidence linking these mechanisms to preneoplastic lesions remains conspicuously absent (19, 20). The immune system and mental disorders are intricately linked. Recent research suggests that MDSCs, a heterogeneous population of bone marrow-derived cells with significant immunosuppressive functions, may also play a central role in the immune dysregulation associated with mental disorders, such as major depression (21). Accumulating evidence has documented that MDSCs exhibit marked infiltration within the tumor microenvironment (TME) of colonic adenoma, where they potently suppress CD4+/CD8+ T cells directly or through Tregs, thereby facilitating immune evasion of neoplastic cells (22, 23). Moreover, preclinical studies across multiple tumor models have corroborated that psychological stress potentiates both recruitment and functional activation of MDSCs via sympathetic nervous pathway (24–26). However, the potential involvement of opportunistic pathogens in remodeling the immune microenvironment to impact intestinal adenomagenesis under chronic stress conditions remains a mechanistically unresolved paradigm.
In this study, we established a dual-perturbation model integrating chronic stress and L. monocytogenes infection in ApcMin/+ mice to mechanistically delineate how the neuro-microbe-immune triad orchestrates molecular pathways governing premalignant lesion initiation and development within the colonic microenvironment. The findings demonstrate that chronic stress compromises intestinal barrier integrity and enhances mucosal colonization capacity of L. monocytogenes. These dual perturbations synergistically drive splenic expansion and colonic recruitment of MDSCs. Infiltrating MDSCs impair CD8+ T cell functions via the cAMP/PKA/CREB signaling axis, thereby enhancing the immunosuppressive capacity of TME to facilitate adenomagenesis. This study elucidates the mechanistic interplay between neuropsychological stress and opportunistic pathogens in orchestrating tumor-permissive microenvironments, providing novel molecular basis and attractive targets for the diagnosis and treatment of intestinal adenoma.
Materials and methods
Mice
C57BL/6 background ApcMin/+ mice have been described previously (27). Mice were observed carefully by laboratory staff and veterinarian personnel for health and activity. Mice were monitored to ensure that food and fluid intake meets their nutritional needs. Body weights were recorded at minimum weekly, and more often for animals requiring greater attention. Mice were maintained on wood chip bedding, and given ad libitum access to water and standard mouse chow, with 12-hour light/dark phase cycles. The colonies were specific pathogen free (SPF) and tested quarterly for known pathogens. Mice in the barrier facilities are housed in cages with microisolator tops on ventilated or static racks. All caging materials and bedding are autoclaved. Food is irradiated and water is either RO, autoclaved or acidified, depending on the barrier. All manipulations are performed in laminar flow hoods. Once animals are removed from a barrier, they are not returned. All personnel wear shoe covers, gloves, hair bonnets and gowns. All mouse studies were approved by the Nanchang University Institutional Animal Care and Use Committee (No. SYXK2021-0004).
Chronic stress model
Adenoma formation in ApcMin/+ mice can be detected as early as 5 weeks of age (28). In this study, 7-week-old ApcMin/+ mice were randomly assigned to a control group (Ctrl) and a chronic stress group (CS). Mice in the CS group underwent 4-hour daily restraint stress for 10 consecutive days, while the control group was maintained under standard environmental exposure conditions (29). Body weight was monitored daily throughout the experimental period. On day 11, anxiety-like behaviors induced by chronic stress were evaluated using the open field test (OFT) and elevated plus maze (EPM), which exploit rodents’ innate aversion to exposed spaces, where unprotected or open areas function as anxiogenic stimuli. In these paradigms, anxiety-like behavior and exploratory activity are quantified by measuring time spent in each zone and total distance traveled during the test sessions (30, 31).
OFT and EPM were utilized to assess chronic stress-induced anxiety-like behaviors in mice. Behavioral parameters in OFT, including total distance traveled, locomotor trajectories, and duration in the central zone, were automatically tracked and analyzed using Any-Maze software. The EPM apparatus consisted of two open arms (27 × 5 cm), two closed arms (27 × 5 cm), and a central platform (5 × 5 cm). During testing, mice were gently placed on the central platform facing an open arm and allowed to freely explore the maze for 10 minutes. Behavioral metrics for EPM, such as total distance traveled, movement trajectories, and time spent in the open arms, were subsequently quantified. Behavioral metrics in EPM, including movement trajectories, their entries into the open arms, and time spent in the open arms, were quantified.
L. monocytogenes infection
L. monocytogenes (strain ATCC 19115), kindly provided by Dr. Hengyi Xu, was revived overnight on PALCAM agar plates (HopeBiol, Qingdao, China) at 37°C. A single colony was inoculated into Tryptic Soy Broth (TSB) supplemented with 0.6% yeast extract (HopeBiol, Qingdao, China) and incubated under shaking (200 rpm) at 37°C overnight. The bacterial culture was centrifuged (8,000 ×g, 10 min, 4°C) the following day, and the pellet was resuspended in sterile phosphate-buffered saline (PBS) to adjust the optical density to OD600 = 1.0.
Following successful establishment of the chronic stress model, mice were stratified into 4 groups based on L. monocytogenes infection status: Ctrl (control), LM (L. monocytogenes infection), CS (chronic stress), and CS+LM (chronic stress and L. monocytogenes infection). On day 12 of the experiment, mice in the LM and CS+LM groups were orally gavaged with 200 μL of L. monocytogenes suspension (OD600 = 1.0, ~10^8 CFU), while Ctrl and CS groups received an equal volume of sterile PBS. After 24 hours of bacterial colonization, all mice were euthanized by cervical dislocation. Spleen, colon, and fecal samples were collected for analyses.
Histopathological analysis
Colon mucosal and adenoma tissues were harvested and fixed overnight in 4% paraformaldehyde solution. The tissues were subsequently dehydrated through graded alcohol solutions. Following dehydration, the tissues were embedded in paraffin wax. Sections of 5 μm thickness were prepared using a microtome and subjected to hematoxylin and eosin (HE) staining (Solarbio, Beijing, China). Epithelial cell morphology, glandular architecture, and crypt structures were observed and analyzed under an optical microscope by two independent pathologists (Dr. Mei Li and Qiong Feng).
Flow cytometry analysis
Single-cell suspensions from mouse spleen, colon, and adenoma tissues were prepared to assess the proportions of different immune cell populations using flow cytometry. Spleen tissues were mechanically homogenized, subjected to red blood cell lysis, and filtered through a 70 μm nylon mesh to obtain single-cell suspensions. Colon tissues were minced and digested in RPMI 1640 medium (Thermo Fisher, Massachusetts, USA) containing collagenase I (Beyotime, Shanghai, China) and DNase I (Sangon Biotech, Shanghai, China) at 37°C in a shaking incubator for 30 minutes, followed by filtration through a 70 μm nylon mesh. To reduce nonspecific binding and background fluorescence, Fc receptor blocking reagent (BD Biosciences, New Jersey, USA) was added to the single-cell suspensions (≤1×106 cells). Antibodies targeting specific immune markers (Supplementary Table S1) were then incubated with the cells based on their immunophenotypes: MDSCs: Identified as CD45+CD11b+GR1+. CD8+ T cells: Identified as CD3+CD8+CD4−. Samples were analyzed using a Beckman CytoFLEX flow cytometer (CA, USA), and data were processed with FlowJo 10.6.2 software.
ELISA detection
Fresh mouse colon tissues were homogenized in RIPA lysis buffer (Sangon Biotech, Shanghai, China) containing protease inhibitors (Ncmbio, Suzhou, China) using a cryogenic grinder (Jingxin, Shanghai, China). After low-temperature centrifugation, supernatants were collected, and protein concentrations were quantified using a BCA assay (Beyotime, Shanghai, China). Levels of interleukin-6 (IL-6; Beyotime, Shanghai, China) and cyclic adenosine monophosphate (cAMP; Cayman Chem, Michigan, USA) in colon tissues were measured according to the manufacturers’ protocols.
RNA-seq and analysis
Total RNA was extracted from mouse colon tissues using the Trizol method. RNA quality and concentration were assessed via NanoDrop spectrophotometry (Thermo Fisher, Massachusetts, USA). Eukaryotic transcriptome libraries were constructed using the Hieff NGS® Ultima Dual-mode mRNA Library Prep Kit (12309ES, Yeasen, Shanghai, China). Library quality was verified with a DNA 1000 Assay Kit (Agilent Technologies, 5067-1504) or High Sensitivity DNA Assay Kit (Agilent Technologies, 5067-4626). Sequencing data were analyzed on the Omicsmart platform (www.omicsmart.com). Sequencing services were provided by Gene Denovo Co., Ltd (Guangzhou, China).
Principal Component Analysis (PCA) evaluated intra-group reproducibility and inter-group differences. Differentially expressed genes (DEGs) were identified using volcano plots with thresholds of |log2FoldChange| > 2 and FDR < 0.05. Venn diagrams visualized shared and unique DEGs across groups. KEGG pathway analysis was performed to annotate biological functions of DEGs.
Western blotting analysis
Total protein was extracted from colon tissues, and concentrations were determined using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 5% skimmed milk, incubated with primary antibodies, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. Signals were detected using a ChemiDoc TM XRS+ imaging system (Bio-Rad, USA) with chemiluminescent substrate (Uelandy, Suzhou, China). Band intensities were quantified via ImageJ software and normalized to β-tubulin. Antibodies included: anti-PKA (1:3000), anti-CREB (1:3000), and anti-β-tubulin (1:7000) (Immunoway, Texas, USA).
Quantitative RT-PCR
Total RNA was extracted from colon tissues using Trizol reagent. cDNA was synthesized using Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR (Yeasen, Shanghai, China). Gene expression levels of Mucin-2, Occludin, Zonula Occludens-1, and Claudin3 were amplified using Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix (Yeasen, Shanghai, China). mRNA levels were normalized to GCRSPH (primers synthesized by Sangon Biotech, Shanghai, China; see Supplementary Table S2).
16S rDNA sequencing
Total bacterial DNA from mouse fecal samples was extracted using the HiPurA Stool DNA Purification Kit (Magen, Guangzhou, China). DNA quality and concentration were assessed via NanoDrop spectrophotometry. The V3-V4 hypervariable regions of bacterial 16S rRNA genes were amplified with primers 341F (CCTACGGGNGGCWGCAG) and 806R (GGACTACHVGGGTATCTAAT). Valid tags were clustered into operational taxonomic units (OTUs) at 97% similarity using the UPARSE pipeline (v9.2.64). Sequencing was performed on an Illumina HiSeq 2500 platform (PE250; Gene Denovo Co., Ltd, Guangzhou, China). Data analysis was conducted on the Omicsmart platform. Alpha diversity indices (ACE, Sob, Shannon) were calculated based on OTU richness. Beta diversity was visualized via PCoA and NMDS using unweighted UniFrac distances. Taxonomic composition (phylum and genus levels) was displayed as stacked bar plots. Welch’s t-test identified species with significant abundance differences between groups.
Intestinal permeability analysis
After fasting for 6 hours, mice were orally gavaged with 200 μL of 600 mg/mL fluorescein isothiocyanate-dextran (FITC-dextran; Sigma-Aldrich, Missouri, USA). Blood was collected 4 hours post-administration. Serum FITC-dextran concentrations were measured after centrifugation.
Statistical analysis
Data were analyzed and visualized using GraphPad Prism 9.5. Results are expressed as mean ± SEM. Two-group comparisons: Unpaired Student’s t-test (normally distributed data with equal variance) or Mann-Whitney U test (nonparametric data). Multi-group comparisons: Kruskal-Wallis H test (nonparametric data). Significance was defined as P < 0.05.
Results
CS/LM synergistically promotes colonic adenomagenesis
Although prior studies have demonstrated the individual roles of chronic stress and gut microbiota in intestinal tumorigenesis (32–34), their synergistic effects remain unclear. We established a chronic stress model in ApcMin/+ mice and validated its success through behavioral tests, including OFT and EPM (Figure 1A). Results showed that CS-treated mice exhibited significant weight loss, reduced time spent in the central zone of the OFT, and diminished exploration in the open arms of the EPM, indicating pronounced chronic stress-like behaviors (Figures 1B–F).
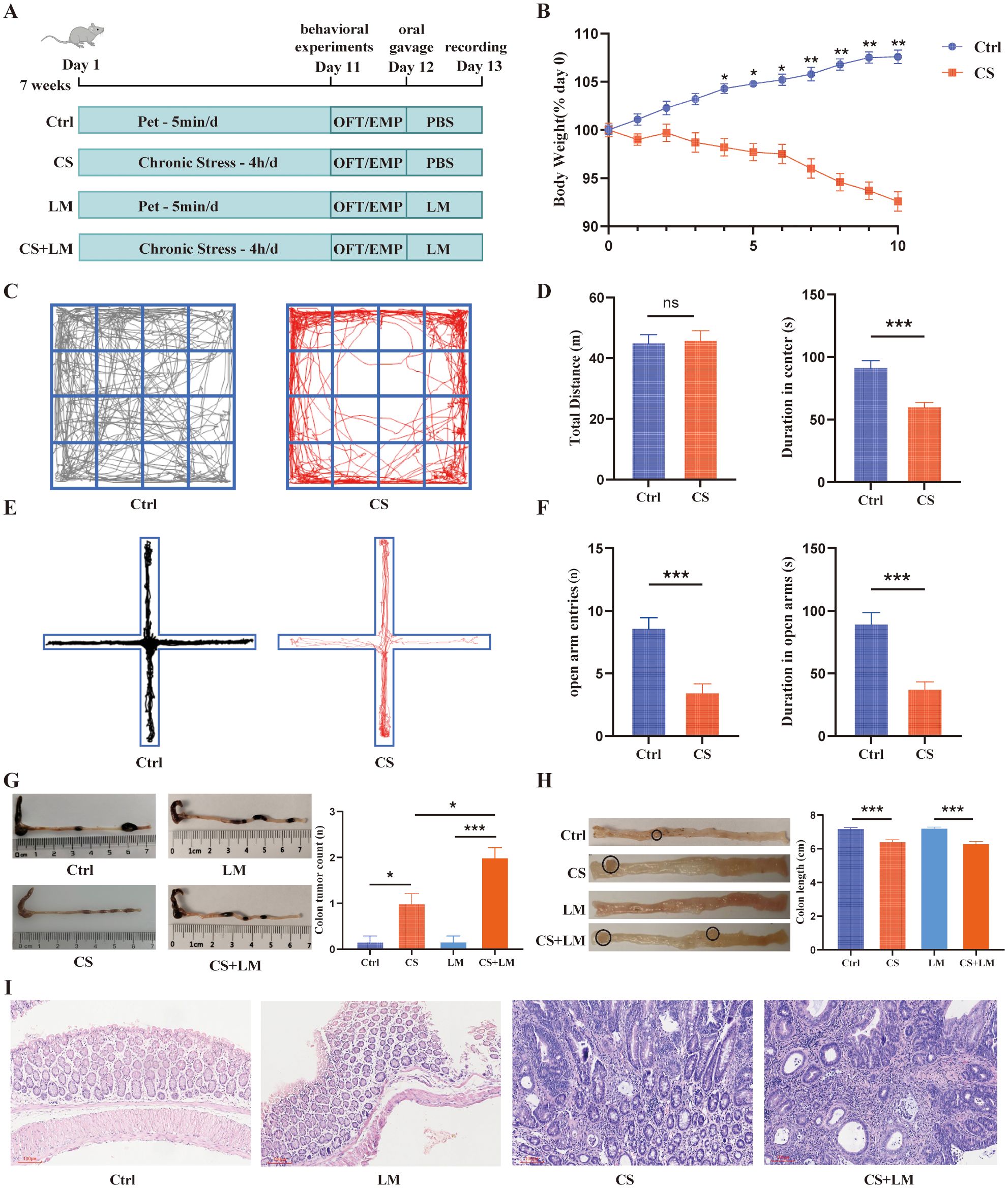
Figure 1. L. monocytogenes cooperates with chronic stress to promote intestinal adenoma formation. (A) Schematic diagram of the chronic stress and LM infection model. (B) Chronic stress caused a significant reduction in mouse body weight (n=6). (C) Representative locomotion traces from the open field test. (D) Spontaneous locomotor activity assessed by the open field test, comparing the total distance traveled (left) and time spent in the central zone (right) (n=12). (E) Locomotion traces from the elevated plus maze test. The horizontal arms are open, while the vertical arms are closed. (F) Spontaneous locomotor activity assessed by the elevated plus maze test, comparing the entries into the open arms (left) and time spent in the open arms (right) (n=12). (G) Colon length obtained from mice in the CS and CS+LM groups was significantly shorter than that in the Ctrl and LM groups (n=6). (H) Chronic stress synergized with L. monocytogenes to increase the number of colonic adenomas (n=6). (I) Chronic stress alone or in combination with L. monocytogenes induced intestinal epithelial atypia (original magnification ×100). n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. n indicates biological replicates.
Upon L. monocytogenes infection in CS-exposed mice (Figure 1A), we observed that both CS and CS+LM groups displayed markedly shortened colon lengths and increased colonic adenoma counts compared to Ctrl and LM-only groups (Figures 1G, H). Notably, while CS+LM group developed more adenomas than CS group, polyp lesions in both groups were histologically classified as high-grade tubular adenomas (Figure 1I). These findings suggest that L. monocytogenes exacerbates colonic adenoma burden in ApcMin/+ mice only under chronic stress conditions, revealing a synergistic tumor-promoting effect between CS and L. monocytogenes.
MDSCs expansion and recruitment
A higher proportion of MDSCs has been detected in tumor tissues of patients with intestinal adenomas and adenocarcinomas (35, 36). Consistent with previous findings (37), flow cytometry revealed that the proportions of CD11b+Gr1+ MDSCs in both the spleen and colon of CS-treated mice were significantly elevated compared to the Ctrl group (Figures 2A, B). Notably, CS/LM co-exposure further increased the proportion of MDSCs within splenic and colonic immune cell populations (Figures 2A, B). However, L. monocytogenes alone only increased MDSCs in the spleen, not the colon, suggesting that chronic stress is a prerequisite for L. monocytogenes to modulate the colonic mucosal microenvironment (Figures 2C, D).
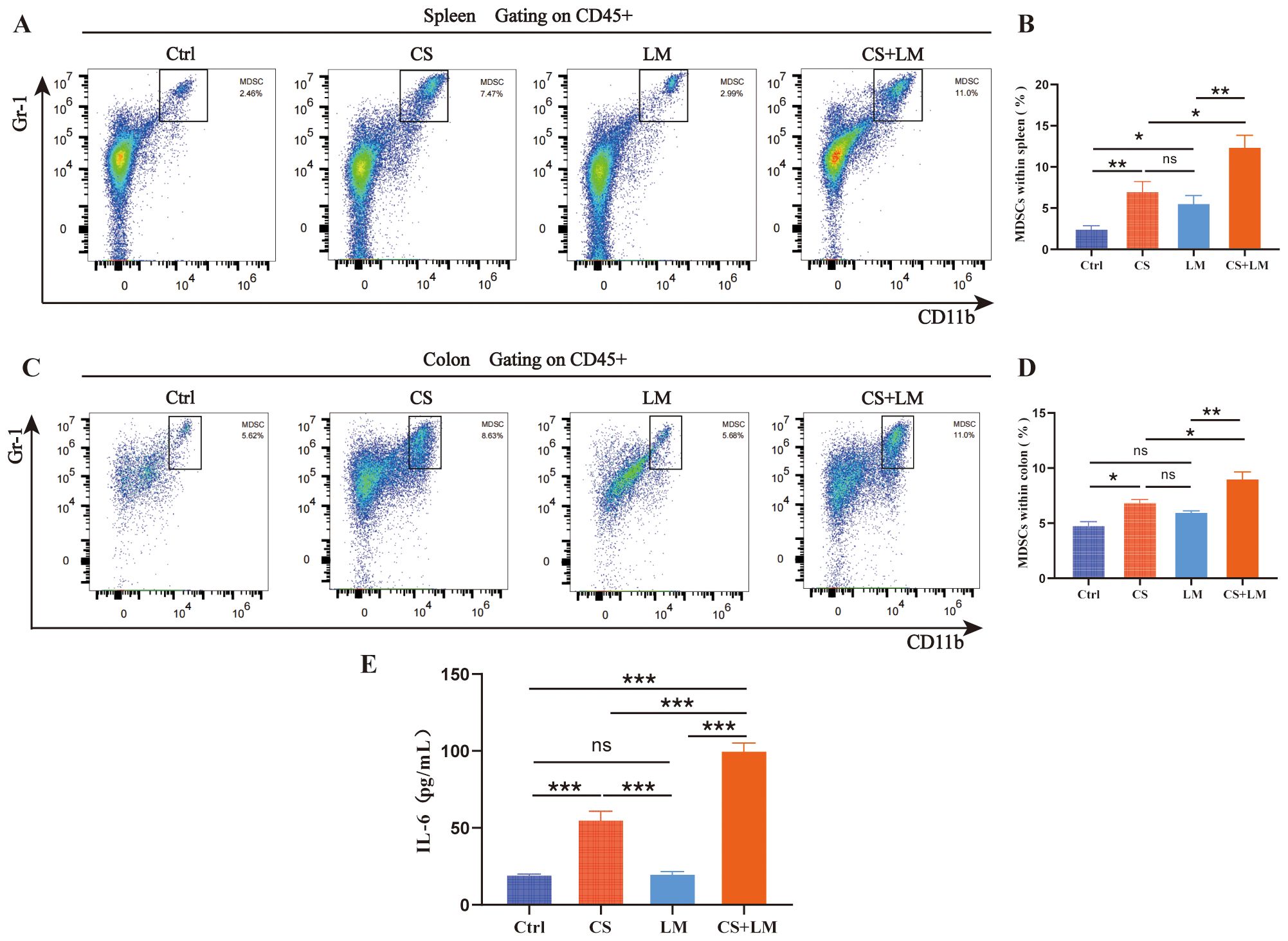
Figure 2. Expansion and recruitment of MDSCs. (A, B) Chronic stress, L. monocytogenes, or their combination promoted an increase in the proportion of MDSCs within the spleen (n=6). (C, D) Chronic stress alone or in combination with L. monocytogenes, but not L. monocytogenes alone, promoted the infiltration of MDSCs (n=6). (E) Chronic stress alone or in combination with L. monocytogenes promoted an increase in IL-6 level within the intestinal mucosa (n=6). n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. n indicates biological replicates.
IL-6, a key regulator of MDSCs recruitment and activation, exhibits a positive correlation with immunosuppressive cell infiltration in the tumor microenvironment (38, 39). To investigate the molecular mechanism underlying MDSCs recruitment to the colon, ELISA analysis confirmed that CS alone, but not LM, significantly upregulated colonic mucosal IL-6 levels. This aligns with the observation that L. monocytogenes-induced MDSCs accumulation in the colon only in the setting of chronic stress. The highest IL-6 levels were detected in the CS+LM group, further supporting the synergistic effect of CS and L. monocytogenes in promoting MDSCs expansion and infiltration into the colon (Figure 2E).
cAMP/PKA/CREB pathway enhances the function of MDSCs
The core function of MDSCs lies in their potent immunosuppressive activity, particularly in promoting regulatory T cells (Tregs) and suppressing effector T cells (23, 40). To investigate changes in MDSCs roles under CS and L. monocytogenes infection conditions, we examined CD8+ T cell populations in the colon mucosa. Results showed that CS alone, but not LM alone, reduced the proportion of CD8+ T cells in both the spleen and colon mucosa, with a more pronounced reduction in the CS+LM group (Figures 3A, B). These findings suggest that L. monocytogenes infection under chronic stress suppresses CD8+ T cell proliferation and infiltration.
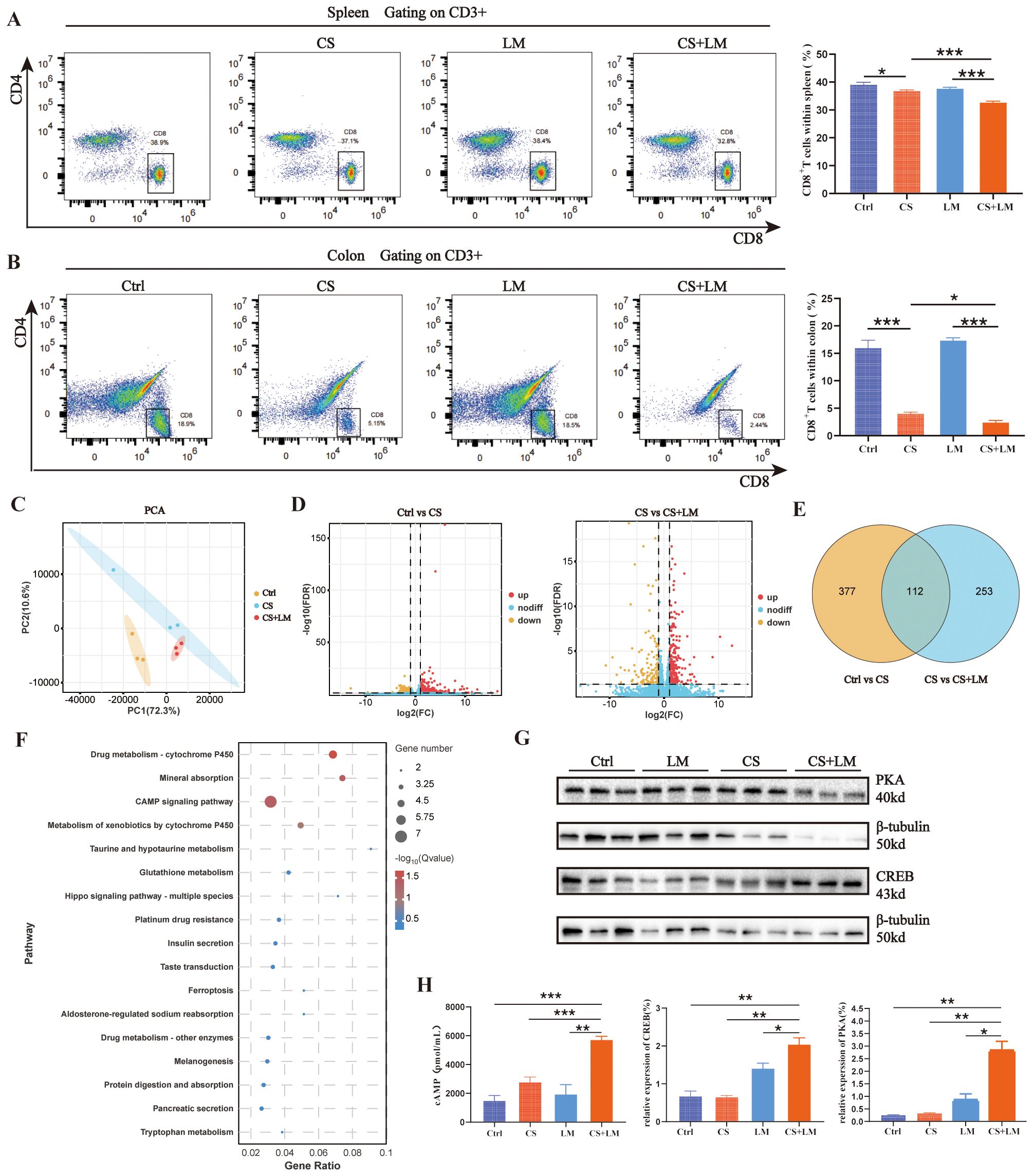
Figure 3. The cAMP/PKA/CREB pathway regulates the immunosuppressive function of MDSCs. (A) Chronic stress and L. monocytogenes reduce the proportion of CD8+ T cells in the spleen (n=6). (B) Chronic stress, but not L. monocytogenes, decreases the proportion of CD8+ T cells in colonic mucosa across groups (n=6). (C) PCA results show significant differences between Ctrl and the other two groups, while CS and CS+LM exhibit similar sample composition (n=3). (D) Volcano plots of differentially expressed genes (DEGs) between groups. Left: Ctrl vs CS; Right: CS vs CS+LM (n=3). (E) Venn diagram displays 112 common DEGs between the two comparisons (Ctrl vs CS and CS vs CS+LM). (F) Bubble plot of KEGG pathway enrichment analysis for significant DEGs. (G) Western blot analysis of PKA and CREB proteins in colonic tissues (n=3). (H) Chronic stress and L. monocytogenes induce elevated expression of cAMP, PKA, and CREB in colonic mucosa (n=3 or 4). n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. n indicates biological replicates.
We further explored the correlation between MDSCs and T cell behaviors in lesion tissues using transcriptomics. Principal component analysis (PCA) revealed closer clustering between CS and CS+LM groups, indicating similar sample compositions, while both groups diverged significantly from the Ctrl group (Figure 3C). Volcano plot analysis identified 489 DEGs in the CS group versus Ctrl, with 365 additional DEGs induced by CS+LM compared to CS alone (Figure 3D). A Venn diagram highlighted 112 overlapping DEGs between the two comparison groups (Figure 3E). KEGG pathway enrichment analysis showed these DEGs were primarily associated with tumor metabolism, inflammation, and immune-related pathways, including the cyclic adenosine monophosphate (cAMP) signaling pathway, ferroptosis, glutathione metabolism, and mineral absorption. Notably, the cAMP pathway exhibited the highest number of DEGs (Figure 3F).
The cAMP signaling pathway is implicated in regulating the immunosuppressive effects of MDSCs within tumors (41, 42). To investigate whether L. monocytogenes activates this pathway to remodel the TME via MDSCs in precancerous lesions under chronic stress, we measured key components of the cAMP pathway, including cAMP, protein kinase A (PKA), and cAMP-response element binding protein (CREB), in the colon mucosa using ELISA and Western blotting. Results demonstrated significantly elevated expression of cAMP, PKA, and CREB in the CS+LM group compared to other groups (Figures 3G, H). These data suggest that LM infection under chronic stress activates the cAMP/PKA/CREB pathway, thereby enhancing MDSCs immunosuppressive function, inducing CD8+ T cell depletion, and accelerating adenoma progression.
Chronic stress and L. monocytogenes compromise intestinal barrier integrity
As the primary defense of the digestive system, intestinal barrier dysfunction plays a pivotal role in the pathogenesis of chronic inflammation-related CRC (43). The intestinal barrier is collectively composed of mechanical, immune, chemical, and microbial barriers (43). The up-mentioned results suggest that CS and L. monocytogenes infection may exert distinct effects on the intestinal mucosa. To further explore the underlying mechanisms, we first examined the expression of key genes associated with the chemical and mechanical barriers. Mucin-2, a major component of the intestinal mucus layer, contributes to the chemical barrier in the colon (44). RT-qPCR results revealed that the expression levels of MUC-2 (the gene encoding Mucin-2) in the colon tissues of CS+LM and CS group mice were significantly lower than those in the Ctrl group. However, L. monocytogenes infection alone did not exhibit a significant impact on MUC-2 expression (Figure 4A).
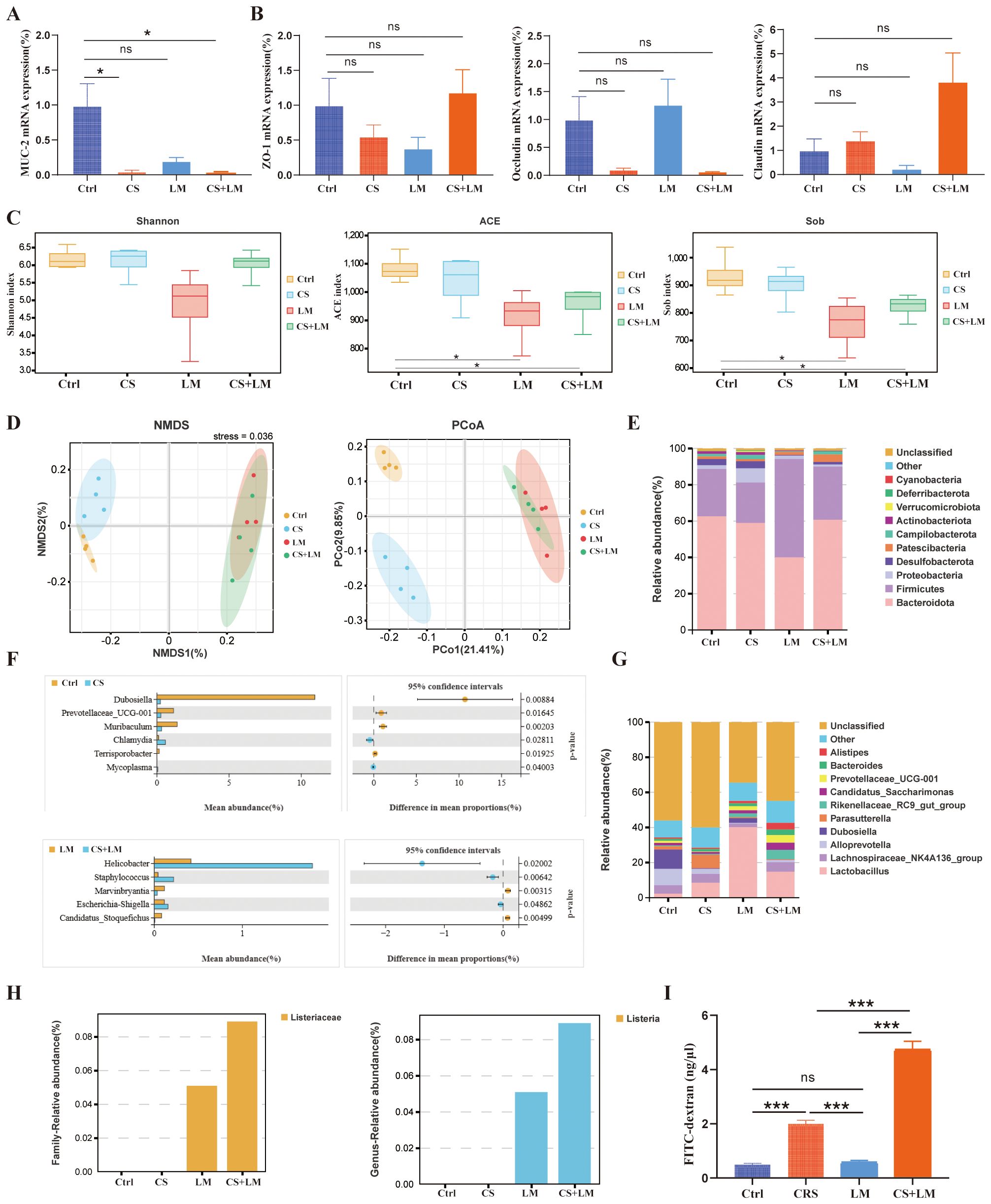
Figure 4. Chronic stress and L. monocytogenes compromise the intestinal barrier. (A, B) Effects of chronic stress and L. monocytogenes on the expression of MUC-2, Occludin, ZO-1, and Claudin3 in colonic tissues (n=4). (C) Alpha diversity indices (ACE, Sob, Shannon) assessing community diversity (n=4). (D) Differences in fecal microbiome composition among groups evaluated by NMDS (non-metric multidimensional scaling) and PCoA (principal coordinate analysis) (n=4). (E) Stacked bar plot showing the cumulative relative abundance of the top 10 bacterial phyla across groups (n=4). (F) Differential abundance analysis of bacterial genera between comparison groups (n=4). (G) Stacked bar plot showing the cumulative relative abundance of the top 10 bacterial genera (n=4). (H) Relative abundance of Lactobacillus at the family (left) and genus (right) levels (n=4). (I) Intestinal permeability assessed by FITC-dextran assay (n=4). n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001. n indicates biological replicates.
TJs between colonic epithelial cells serve as the physical foundation of the colonic mechanical barrier. Among these, Occludin, Zonula Occludens-1 (ZO-1), and Claudin-3 are the most crucial protein structures of TJs (43). RT-qPCR results indicated no significant differences in the expression of TJ-related genes compared to the Ctrl group (Figure 4B). These findings suggest that CS and L. monocytogenes primarily affect the intestinal chemical barrier, rather than the mechanical barrier.
To investigate the status of the intestinal microbial barrier, we collected fecal samples from each group of mice for 16S rDNA gene sequencing. The results revealed that chronic stress had minimal impact on the α-diversity of the gut microbiota, while L. monocytogenes infection led to a significant decrease in the richness component of gut microbiota α-diversity (Figure 4C). Analyses of β-diversity, including Non-metric Multidimensional Scaling (NMDS) and Principal Coordinates Analysis (PCoA), demonstrated significant separation among the 4 groups, indicating distinct differences in species composition between the groups (Figure 4D).
At the phylum level, CS led to a decrease in the abundance of Firmicutes. Following L. monocytogenes infection, the abundance of Actinobacteria decreased (Figure 4E). At the genus level, the abundances of Gluconobacter, Bacteroides, and Alistipes increased under chronic stress, whereas L. monocytogenes infection did not significantly alter the abundance of these bacterial groups (Figure 4G). To further investigate the impact of chronic stress on the microbiota, we performed Welch’s t-test to identify differentially abundant genera (Figure 4F). Compared to the Ctrl group, CS mice exhibited significantly increased abundances of Dubosiella, Prevotellaceae UCG-001, Muribaculum, and Terrisporobacter in their feces, alongside significantly decreased abundances of Chlamydia and Mycoplasma.
Compared to the LM group, CS+LM mice showed significantly increased abundances of Helicobacter, Staphylococcus, and Escherichia-Shigella, while abundances of Marvinbryantia and Candidatus Saccharimonas were significantly decreased. Furthermore, analysis of L. monocytogenes-affected bacterial families and genera revealed that although normal mice and chronic stress mice were gavaged with an equivalent number of L. monocytogenes suspension, the intestinal colonization load of L. monocytogenes was significantly higher under chronic stress conditions (Figure 4H).
Additionally, intestinal barrier integrity analysis showed the highest serum concentration of FITC-D4000 in the CS+LM group, indicating the greatest intestinal permeability. The CS group had the next highest level, while the Ctrl and LM groups showed the lowest levels with no significant difference between them (Figure 4I). These results indicate that CRS primarily affects the integrity of the epithelial cell barrier. On this basis, L. monocytogenes synergizes with CS to further compromise the normal function of the intestinal barrier.
Discussion
The significant roles of chronic stress and enteric pathogens in gastrointestinal diseases have garnered increasing attention (22, 32, 34). However, the synergistic mechanisms between these factors remain elusive. Clinical studies demonstrate that L. monocytogenes causes severe infections exclusively in immunocompromised patients and is enriched in tumor tissues of CRC patients (11–13), suggesting that this pathogen may exploit immunosuppressive environments to establish colonization and promote tumorigenesis through dysbiosis. Given the pathological progression from chronic inflammation to CRC, whether L. monocytogenes participates in the precancerous stage of CRC under specific host conditions remains systematically unexplored. This study provides experimental evidence in animal models that chronic stress facilitates L. monocytogenes-driven tumorigenesis by disrupting intestinal barrier integrity and recruiting MDSCs to the gut mucosa, thereby establishing a pro-tumorigenic immune microenvironment.
The expansion and recruitment of MDSCs are recognized as critical mechanisms underlying tumor immune evasion in multiple cancers (22, 24, 45). Under chronic stress stimulation, the spleen, the largest peripheral immune organ, exhibits significantly increased MDSCs proportions (45). We demonstrate that L. monocytogenes infection further amplifies chronic stress-induced generation of immature immune cells, indicating their synergistic modulation of systemic immunity.
The distribution of immune cells within hosts is influenced by multifactorial determinants. In tumor-bearing murine models, splenic MDSCs migrate to lesion sites via inflammatory signaling pathways, thereby promoting tumor progression (46). In this study, chronic stress and opportunistic pathogen infection recruited MDSCs to colonic tissues through elevated interleukin-6 (IL-6) levels. Notably, MDSCs proportion alterations in the spleen and colon displayed asynchronous dynamics. Splenic MDSCs did not traffic to the colon in the setting of L. monocytogenes infection alone. We propose that L. monocytogenes ruins microbial barrier function, while intact mucus layers, epithelial integrity, and TJs prevent microbiota and their metabolites from penetrating into the mucosa, thus failing to trigger MDSCs recruitment.
In TME, IL-6 secreted by stromal cells (e.g., T lymphocytes, macrophages, fibroblasts) binds MDSCs receptors to drive their recruitment (25, 38, 47, 48). Our data revealing synchronized trends between colonic IL-6 concentrations and MDSCs proportions, providing evidence supporting IL-6 as a pivotal mediator of MDSCs recruitment under chronic stress conditions.
MDSCs within TME exhibit potent immunosuppressive capabilities, primarily mediated through T-cell functional modulation (49). In colorectal tumor models, MDSCs suppress infiltration of CD8+ T cells and NK cells by upregulating arginase-1 (Arg1) activity (50, 51). Our prior work demonstrated that MDSCs stimulate Foxp3+ Treg proliferation via phosphorylation of the Stat3 pathway, thereby promoting colorectal carcinogenesis (23).
This study confirms that chronic stress combined with L. monocytogenes infection reduces CD8+ T-cell populations through enhanced MDSCs immunosuppressive function. Notably, L. monocytogenes infection alone did not induce significant local T-cell suppression in intestinal mucosal lesions. This may be attributed to the primary localization of L. monocytogenes-induced alterations within the intestinal lumen, where low-molecular-weight metabolites or inflammatory cytokines traverse the gut barrier to stimulate splenic MDSC expansion without activating mucosal recruitment mechanisms that require direct bacterial invasion or high-molecular-weight metabolite penetration.
Transcriptomic profiling revealed differential gene expression in MDSCs across experimental groups, with significant enrichment in tumor metabolism and immune-related signaling pathways. The cAMP pathway participates in regulating MDSCs immunosuppression across diverse malignancies (42). MDSCs in multiple cancer models overexpress cAMP signaling to activate STAT3-dependent T-cell inhibition. Furthermore, netrin-1 selectively secreted by colon cancer cells activates the cAMP/PKA cascade in MDSCs, amplifying their immunosuppressive activity (41). Notably, the significant enrichment of the “glutathione metabolism” and “mineral absorption” pathways suggests that chronic stress and L. monocytogenes infection disrupt immune homeostasis, potentially through modulation of MDSCs function. Specifically, the glutathione metabolism pathway is known to enhance the immunosuppressive capacity of MDSCs via glutamate signaling at metabotropic glutamate receptor 2/3 (52). Moreover, while minerals such as selenium and selenoprotein metabolites are established contributors to immune and inflammatory responses, the precise mechanisms underlying their beneficial effects remain incompletely defined (53).
We provide evidence that under sustained chronic stress, opportunistic pathogens can reshape immune responses to establish a pro-tumorigenic microenvironment conducive to intestinal adenoma development. Given MDSCs heterogeneity and interspecies variations, future investigations delineating human MDSCs subtype functions could advance targeted therapeutic strategies.
We demonstrate that the pro-tumorigenic effects of L. monocytogenes critically depend on chronic stress-induced disruption of barrier integrity. The outermost chemical barrier not only protects against microbial invasion but also modulates immune tolerance in the lamina propria (44). Our findings further indicate that chronic stress combined with L. monocytogenes, rather than pathogenic bacterium alone, primarily damages the mucus barrier.
Only after chronic stress compromises mucosal barrier integrity can L. monocytogenes and its pathogenic metabolites inflict subsequent damage (54). Moreover, although the microbial barrier formed by commensal flora adherent to the intestinal mucosa prevents colonization by exogenous pathogens under physiological conditions, its intimate contact with the gut wall may paradoxically increase invasion risks (55, 56). Published data confirm that gut dysbiosis disrupts host-microbiota interactions, promoting secretion of bacterial toxins and carcinogenic metabolites while reducing beneficial metabolites. This cascade impairs barrier function, induces immune dysregulation and cellular hyperproliferation, ultimately triggering CRC (57). Histopathological analyses corroborated that L. monocytogenes infection alone does not elicit epithelial dysplasia in the intestinal mucosa.
Previous study by our group revealed intestinal dysbiosis in patients with recurrent colorectal polyps, even in histologically normal mucosa 10 cm away from the lesions. This suggests that gut microbiota dysregulation may be an early event in the formation of preneoplastic lesions (27). In the current study, we observed that chronic stress led to a reduction in beneficial bacteria (e.g., Dubosiella, Prevotella UCG-001, Muribaculum) and an increase in potentially harmful bacteria (e.g., Helicobacter, L. monocytogenes). The decline in the beneficial genus Prevotella is consistent with findings from prior clinical studies (27). Combined with elevated intestinal permeability, these results indicate that chronic stress, synergizing with L. monocytogenes infection, rather than opportunistic pathogen alone, significantly disrupts both the microbial and intestinal epithelial barrier, thereby exacerbating local immune responses (58–62) (Figure 5). This cascade of alterations creates a favorable microenvironment for the adhesion, colonization, and tumor-promoting effects of L. monocytogenes, further elucidating the mechanisms underlying the synergistic tumor-promoting effects of chronic stress and opportunistic pathogens.
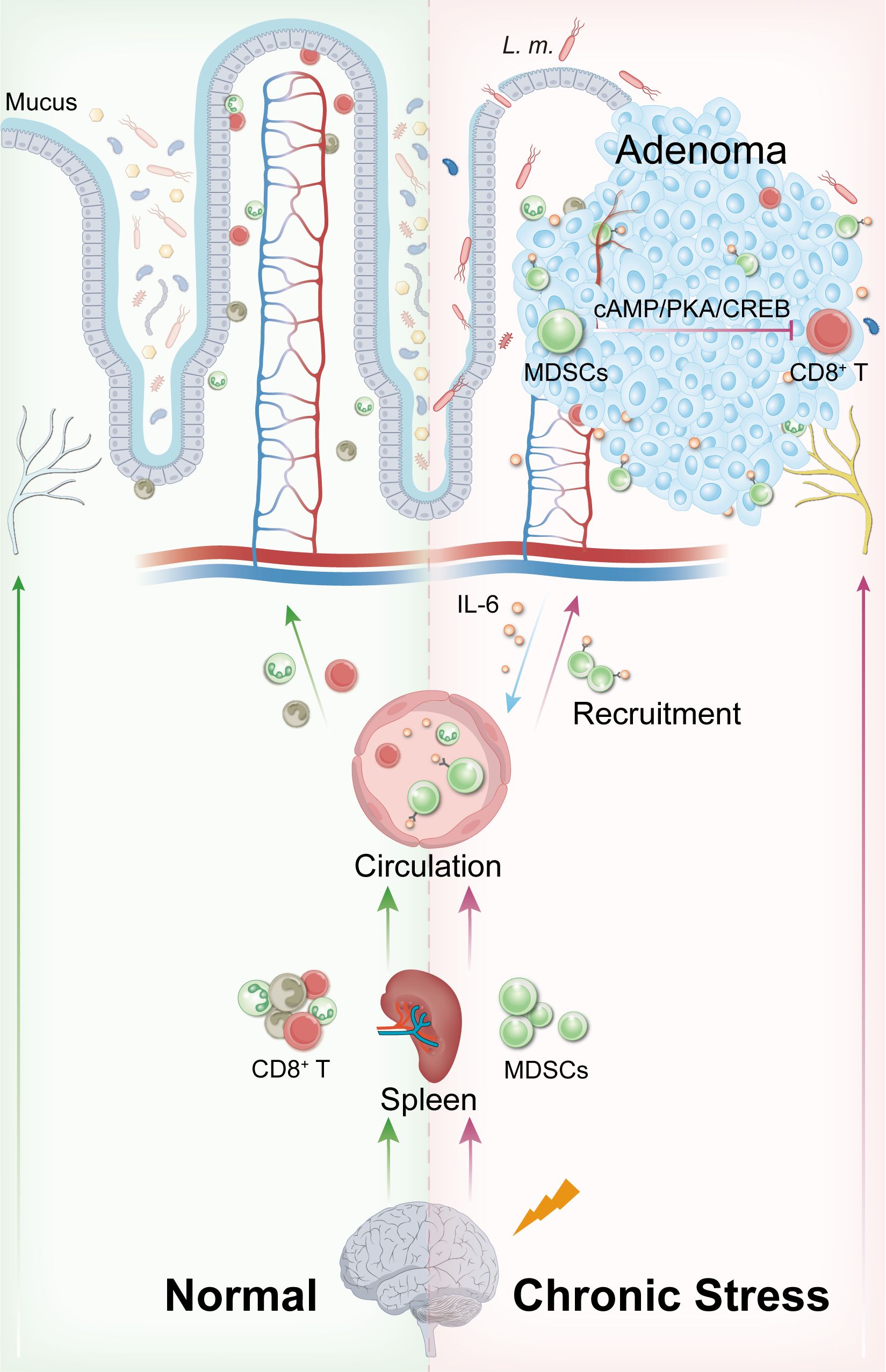
Figure 5. Schematic mechanism of chronic stress and L. monocytogenes synergistically promoting intestinal tumorigenesis via MDSCs.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1305642?reviewer=q5mmjlgsn27tgpve1slpej7ifu.
Ethics statement
The animal studies were approved by Nanchang University Institutional Animal Care and Use Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PQ: Data curation, Visualization, Writing – original draft. LY: Formal Analysis, Methodology, Writing – original draft. RW: Data curation, Formal Analysis, Writing – original draft. SY: Conceptualization, Resources, Software, Writing – original draft. ZL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. PH: Investigation, Methodology, Writing – original draft. QY: Formal Analysis, Writing – original draft. SX: Methodology, Writing – original draft. MW: Data curation, Methodology, Writing – original draft. YD: Formal Analysis, Methodology, Writing – original draft. JH: Investigation, Software, Writing – original draft. LZ: Data curation, Methodology, Writing – original draft. RH: Methodology, Writing – original draft. HD: Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. YX: Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Science Foundation of China, No.82160546 and 82460116; the Science Foundation of Jiangxi Province, No. 20202BBG73027, 20202ACBL206017, and 20242BAB26116; the Foundation of Jiangxi Province for Distinguished Scholars No. JXSQ2023201020;the Foundation of Education Department of Jiangxi Province No. GJJ210185.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1653548/full#supplementary-material
Supplementary Table 1 | Antibody details.
Supplementary Table 2 | Primer sequences.
References
1. Choi CR, Al Bakir I, Ding NJ, Lee GH, Askari A, Warusavitarne J, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. (2019) 68:414–22. doi: 10.1136/gutjnl-2017-314190
2. Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, et al. Endoscopic recognition and management strategies for Malignant colorectal polyps: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology. (2020) 159:1916–1934.e1912. doi: 10.1053/j.gastro.2020.08.050
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
4. García Sánchez J. Colonoscopic polypectomy and long-term prevention of colorectal cancer deaths]. Rev Clin Esp. (2012) 212:408.
5. Ladabaum U, Dominitz JA, Kahi C, and Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. (2020) 158:418–32. doi: 10.1053/j.gastro.2019.06.043
6. Zauber AG, Winawer SJ, O’brien MJ, Ballegooijen MV, Hankey BF, Shi W, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. (2012) 366:687–96. doi: 10.1056/NEJMoa1100370
7. He Z, Tian W, Wei Q, and Xu J. Involvement of Fusobacterium nucleatum in Malignancies except for colorectal cancer: A literature review. Front Immunol. (2022) 13:968649. doi: 10.3389/fimmu.2022.968649
8. Pasquereau-Kotula E, Martins M, Aymeric L, and Dramsi S. Significance of Streptococcus gallolyticus subsp. gallolyticus Association With Colorectal Cancer. Front Microbiol. (2018) 9:614. doi: 10.3389/fmicb.2018.00614
9. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. (2013) 14:207–15. doi: 10.1016/j.chom.2013.07.007
10. Quereda JJ, Moron-Garca A, Palacios-Gorba C, Dessaux C, Portillo FG, Pucciarelli MG, et al. Pathogenicity and virulence of Listeria monocytogenes: A trip from environmental to medical microbiology. Virulence. (2021) 12:2509–45. doi: 10.1080/21505594.2021.1975526
11. Zuo Y, Lu Y, Pang J, Jin S, Zhang X, Zhao E, et al. Detection and comparison of tumor cell-associated microbiota from different compartments of colorectal cancer. Front Oncol. (2024) 14:1374769. doi: 10.3389/fonc.2024.1374769
12. Mclauchlin J. Human listeriosis in Britain, 1967-85, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol Infect. (1990) 104:191–201. doi: 10.1017/S0950268800059355
13. Schlech WF3rd, Lavigne PM, Bortolussi RA, Haldane EV, Wort AJ, Hightower AW, et al. Epidemic listeriosis–evidence for transmission by food. N Engl J Med. (1983) 308:203–6. doi: 10.1056/NEJM198301273080407
14. Drolia R, Bryant DB, Tenguria S, Jules-Culver ZA, Thind J, Amelunke B, et al. Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for Listeria monocytogenes translocation. mBio. (2024) 15:e0282123. doi: 10.1128/mbio.02821-23
15. Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. (2007) 67:4346–52. doi: 10.1158/0008-5472.CAN-06-4067
16. Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. (2020) 25:1487–99. doi: 10.1038/s41380-019-0595-x
17. Al Omran Y and Aziz Q. The brain-gut axis in health and disease. Adv Exp Med Biol. (2014) 817:135–53. doi: 10.1007/978-1-4939-0897-4_6
18. Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U.S.A. (2018) 115:E2960–e2969. doi: 10.1073/pnas.1720696115
19. Zhou Z, Shu Y, Bao H, Han S, Liu Z, Zhao N, et al. Stress-induced epinephrine promotes epithelial-to-mesenchymal transition and stemness of CRC through the CEBPB/TRIM2/P53 axis. J Transl Med. (2022) 20:262. doi: 10.1186/s12967-022-03467-8
20. Guan Y, Yao W, Yu H, Feng Y, Zhao Y, Zhan X, et al. Chronic stress promotes colorectal cancer progression by enhancing glycolysis through β2-AR/CREB1 signal pathway. Int J Biol Sci. (2023) 19:2006–19. doi: 10.7150/ijbs.79583
21. Wei J, Zhang M, and Zhou J. Myeloid-derived suppressor cells in major depression patients suppress T-cell responses through the production of reactive oxygen species. Psychiatry Res. (2015) 228:695–701. doi: 10.1016/j.psychres.2015.06.002
22. Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, et al. CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid-derived suppressor cell population and function. Cell Rep. (2015) 12:244–57. doi: 10.1016/j.celrep.2015.06.024
23. Chen X, Takemoto Y, Deng H, Middelhoff M, Friedman RA, Chu TH, et al. Histidine decarboxylase (HDC)-expressing granulocytic myeloid cells induce and recruit Foxp3(+) regulatory T cells in murine colon cancer. Oncoimmunology. (2017) 6:e1290034. doi: 10.1080/2162402X.2017.1290034
24. Liu X, Tang R, Xu J, Tan Z, Liang C, Meng Q, et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. (2023) 72:2329–43. doi: 10.1136/gutjnl-2022-329349
25. Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. (2009) 15:103–13. doi: 10.1016/j.ccr.2009.01.001
26. Cao M, Huang W, Chen Y, Li G, Liu N, Wu Y, et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int J Cancer. (2021) 149:460–72. doi: 10.1002/ijc.33552
27. Yin LL, Qi PQ, Hu YF, Fu XJ, He RS, Wang MM, et al. Dysbiosis promotes recurrence of adenomatous polyps in the distal colorectum. World J Gastrointest Oncol. (2024) 16:3600–23. doi: 10.4251/wjgo.v16.i8.3600
28. Hata K, Tanaka T, Kohno H, Suzuki R, Qiang SH, Yamada Y, et al. beta-Catenin-accumulated crypts in the colonic mucosa of juvenile ApcMin/+ mice. Cancer Lett. (2006) 239:123–8. doi: 10.1016/j.canlet.2005.07.033
29. Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. (2020) 11:2221. doi: 10.1038/s41467-020-15920-7
30. Seibenhener ML and Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. (2015) 96):e52434. doi: 10.3791/52434
31. Komada M, Takao K, and Miyakawa T. Elevated plus maze for mice. J Vis Exp. (2008) 22:1088. doi: 10.3791/1088-v
32. Cao W, Wang C, Chin Y, Chen X, Gao Y, Yuan S, et al. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. (2019) 10:277–88. doi: 10.1039/C8FO01404C
33. Kumar R, Herold JL, Schady D, Davis J, Kopetz S, Martinez-Moczygemba M, et al. Streptococcus gallolyticus subsp. gallolyticus promotes colorectal tumor development. PloS Pathog. (2017) 13:e1006440. doi: 10.1371/journal.ppat.1006440
34. Zhang Y, Feng Y, Zhao Y, Feng Y, Li M, Wang W, et al. Single-cell RNA sequencing reveals that the immunosuppression landscape induced by chronic stress promotes colorectal cancer metastasis. Heliyon. (2024) 10:e23552. doi: 10.1016/j.heliyon.2023.e23552
35. Sasidharan Nair V, Saleh R, Toor SM, Alajez NM, and Elkord E. Transcriptomic analyses of myeloid-derived suppressor cell subsets in the circulation of colorectal cancer patients. Front Oncol. (2020) 10:1530. doi: 10.3389/fonc.2020.01530
36. Guo B, Zheng Y, Fan Y, Yang Y, Wang Y, Qin L, et al. Enhanced Apc(Min/+) adenoma formation after epithelial CUL4B deletion by recruitment of myeloid-derived suppressor cells. Neoplasia. (2024) 53:101005. doi: 10.1016/j.neo.2024.101005
37. Yang S, Li Y, Zhang Y, and Wang Y. Impact of chronic stress on intestinal mucosal immunity in colorectal cancer progression. Cytokine Growth Factor Rev. (2024) 80:24–36. doi: 10.1016/j.cytogfr.2024.10.007
38. Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, and Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. (2014) 110:469–78. doi: 10.1038/bjc.2013.748
39. Yamamoto T, Tsunedomi R, Nakajima M, Suzuki N, Yoshida S, Tomochika S, et al. IL-6 levels correlate with prognosis and immunosuppressive stromal cells in patients with colorectal cancer. Ann Surg Oncol. (2023) 30:5267–77. doi: 10.1245/s10434-023-13527-y
40. Zeng S, Hu H, Li Z, Hu Q, Shen R, Li M, et al. Local TSH/TSHR signaling promotes CD8(+) T cell exhaustion and immune evasion in colorectal carcinoma. Cancer Commun (Lond). (2024) 44:1287–310. doi: 10.1002/cac2.12605
41. Xia X, Mao Z, Wang W, Ma J, Tian J, Wang S, et al. Netrin-1 promotes the immunosuppressive activity of MDSCs in colorectal cancer. Cancer Immunol Res. (2023) 11:600–13. doi: 10.1158/2326-6066.CIR-22-0658
42. Sarkar OS, Donninger H, Al Rayyan N, Chew LC, Stamp B, Zhang X, et al. Monocytic MDSCs exhibit superior immune suppression via adenosine and depletion of adenosine improves efficacy of immunotherapy. Sci Adv. (2023) 9:eadg3736. doi: 10.1126/sciadv.adg3736
43. Aburto MR and Cryan JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol. (2024) 21:222–47. doi: 10.1038/s41575-023-00890-0
44. Le Berre M, Gerlach JQ, Gallagher ME, Joshi L, Carrington SD, and Kilcoyne M. Mucin purification and printing natural mucin microarrays. Methods Mol Biol. (2022) 2460:127–46. doi: 10.1007/978-1-0716-2148-6_8
45. Jiang W, Li Y, Li ZZ, Sun J, Li JW, Wei W, et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav Immun. (2019) 80:825–38. doi: 10.1016/j.bbi.2019.05.031
46. Mohammadpour H, Macdonald CR, Mccarthy PL, Abrams S, and Repasky EA. β2-adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. (2021) 37:109883. doi: 10.1016/j.celrep.2021.109883
47. Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. (2000) 6:583–8. doi: 10.1038/75068
48. Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. (2005) 7:545–55. doi: 10.1593/neo.04571
49. Shirasuna K, Ito M, Matsuda T, Enomoto T, Ohara Y, Yamamoto M, et al. Correlation analysis of the proportion of monocytic myeloid-derived suppressor cells in colorectal cancer patients. PloS One. (2020) 15:e0243643. doi: 10.1371/journal.pone.0243643
50. Oberlies J, Watzl C, Giese T, Luckner C, Kropf P, Müller I, et al. Regulation of NK cell function by human granulocyte arginase. J Immunol. (2009) 182:5259–67. doi: 10.4049/jimmunol.0803523
51. Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. (2017) 5:101. doi: 10.1186/s40425-017-0308-4
52. Morikawa N, Tachibana M, Ago Y, Goda H, Sakurai F, and Mizuguchi H. LY341495, an mGluR2/3 antagonist, regulates the immunosuppressive function of myeloid-derived suppressor cells and inhibits melanoma tumor growth. Biol Pharm Bull. (2018) 41:1866–9. doi: 10.1248/bpb.b18-00055
53. Mehdi Y, Hornick JL, Istasse L, and Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. (2013) 18:3292–311. doi: 10.3390/molecules18033292
54. Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. (2001) 14:584–640. doi: 10.1128/CMR.14.3.584-640.2001
55. Brennan CA and Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. (2016) 70:395–411. doi: 10.1146/annurev-micro-102215-095513
56. Janney A, Powrie F, and Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature. (2020) 585:509–17. doi: 10.1038/s41586-020-2729-3
57. Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. (2017) 153:1621–1633.e1626. doi: 10.1016/S0016-5085(17)30864-8
58. Li Y, Wang W, Liu Y, Li S, Wang J, and Hou L. Diminished immune response and elevated abundance in gut microbe dubosiella in mouse models of chronic colitis with GBP5 deficiency. Biomolecules. (2024) 14:873. doi: 10.3390/biom14070873
59. Li R, Yang P, Liu B, Ye Z, Zhang P, Li M, et al. Lycium barbarum polysaccharide remodels colon inflammatory microenvironment and improves gut health. Heliyon. (2024) 10:e30594. doi: 10.1016/j.heliyon.2024.e30594
60. Xu S, Kong J, Dai Y, and Li H. Prevotellaceae modulates colorectal cancer immune microenvironment to assist anti-PD-L1 immunotherapy. Turk J Gastroenterol. (2024) 35:909–21. doi: 10.5152/tjg.2024.23683
61. Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD, et al. Genomic characterization of the uncultured Bacteroidales family S24–7 inhabiting the guts of homeothermic animals. Microbiome. (2016) 4:36. doi: 10.1186/s40168-016-0181-2
Keywords: Listeria monocytogenes, MDSCs, adenoma, intestinal barrier, chronic stress
Citation: Qi P, Yin L, Wei R, Yang S, Liu Z, Huang P, Yu Q, Xiong S, Wang M, Deng Y, Hu J, Zhou L, He R, Deng H and Xiong Y (2025) Chronic stress synergizes with Listeria monocytogenes to promote intestinal adenomagenesis via myeloid-derived suppressor cells. Front. Immunol. 16:1653548. doi: 10.3389/fimmu.2025.1653548
Received: 25 June 2025; Accepted: 28 July 2025;
Published: 03 September 2025.
Edited by:
Li-Shang Dai, Wenzhou Medical University, ChinaReviewed by:
Jiansong Huang, Zhejiang University, ChinaHua Hao, Tongji University, China
Yan Guo, Histo Pathology Diagnostic Center, China
Copyright © 2025 Qi, Yin, Wei, Yang, Liu, Huang, Yu, Xiong, Wang, Deng, Hu, Zhou, He, Deng and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Deng, YmVhbmRlbmdAbmN1LmVkdS5jbg==; Ying Xiong, bmRlZnkwNjA1MEBuY3UuZWR1LmNu
†These authors have contributed equally to this work
 Pingqian Qi1,2,3†
Pingqian Qi1,2,3† Lili Yin
Lili Yin Huan Deng
Huan Deng