- The Clinical Medical Research Center of Breast and Thyroid Tumor in Xinjiang, Tumor Hospital Affiliated to Xinjiang Medical University, Urumqi, China
Background: The platelet to lymphocyte ratio(PLR) is widely recognized as an important biomarker of systemic inflammation and has been associated with treatment responses in breast cancer (BC) patients undergoing neoadjuvant therapy. However, existing evidence remains inconsistent. This meta-analysis aims to systematically investigate the prognostic value of PLR in BC patients receiving neoadjuvant chemotherapy (NACT).
Methods: A broad and systematic search of the literature was carried out using PubMed, Embase, Web of Science, and the Cochrane Library, covering all available records from the inception of each database through April 7, 2025. Study selection was guided by a set of predetermined inclusion and exclusion parameters. Primary outcomes included overall survival (OS), disease-free survival (DFS), and pathological complete response (pCR), assessed through hazard ratios (HRs) or odds ratios (ORs) with corresponding 95% confidence intervals (CIs).
Results: Twenty-four studies involving 7,557 BC patients receiving NACT were included. Elevated PLR was significantly associated with reduced pCR rates (HR = 1.51; 95% CI: 1.24–1.84; p < 0.0001; I² = 70%), shorter OS (HR = 1.64; 95% CI: 1.27–2.11; p = 0.0002; I² = 0%), and decreased DFS (HR = 2.29; 95% CI: 1.54–3.39; p < 0.0001; I² = 44%). Subgroup analyses indicated that PLR’s prognostic value varied by timing of PLR measurement, geographic location, and PLR cutoff values.
Conclusions: Elevated PLR is significantly correlated with poorer clinical outcomes in BC patients undergoing NACT, suggesting its potential as a predictive biomarker for treatment efficacy. However, due to methodological limitations of the included studies, further prospective investigations are required to confirm these findings across diverse populations.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420251064051.
1 Introduction
Breast cancer constitutes the globe’s second most frequently diagnosed malignancy, as reported in the 2022 Global Cancer Statistics compiled by the World Health Organization’s International Agency for Research on Cancer. Breast cancer is responsible for an estimated 2.297 million newly diagnosed cases each year, maintaining its status as the leading cancer type affecting women globally. In China alone, the yearly incidence is projected to be around 357,000 cases. This figure accounts for 15.6% of all newly diagnosed malignancies among women (1). With the continuous advancement of therapeutic approaches, such as surgical intervention, radiotherapy, chemotherapy, endocrine therapy, immunotherapy, and targeted therapy—the survival outcomes for patients with BC have improved substantially (2). At the point of initial diagnosis, an estimated 5% to 15% of individuals are found to have breast cancer that has progressed to a locally advanced stage. For this subgroup, the five-year survival rate remains low, estimated at only around 29% (3). NACT is currently recognized as a standard therapeutic approach for the management of locally advanced breast cancer. It is widely regarded as both an effective and evidence based treatment strategy (4). The main goals of NACT are multifaceted. These include reducing the overall tumor burden, downstaging axillary lymph node involvement, and increasing the feasibility of surgical resection. Additionally, NACT aims to enhance the chances of achieving successful breast-conserving surgery (5). Achieving a pCR is regarded as the most desirable outcome of neoadjuvant therapy, serving as a surrogate indicator of improved long-term prognosis. Nonetheless, current research has highlighted significant discrepancies in pCR rates among the various molecular subtypes of breast cancer. Notably, individuals diagnosed with HER2-positive breast cancer tend to achieve a pathological complete response in approximately 30% of cases following treatment with NACT. For patients diagnosed with triple-negative breast cancer, the documented rates of pathological complete response vary between 30% and 50%. Conversely, individuals with tumors that are positive for estrogen receptor (ER) expression but lack HER2 amplification tend to have markedly reduced pathological complete response rates, generally falling below 10% (6, 7). These observed differences in treatment response may be explained by distinct molecular alterations within the tumor microenvironment. Various elements, including the density of stromal tumor-infiltrating lymphocytes (TILs), the expression of cyclin-dependent kinases, and the activity of non-coding RNA transcripts, have been shown to influence how breast cancer patients respond to NACT (8). However, these predictive factors are often difficult to obtain in clinical settings. Thus, there is a pressing need for a cost-effective, practical, and easily accessible method to predict the response to NACT.
Systemic inflammatory responses are widely recognized as key contributors to the progression of BC (9). Research has shown that systemic inflammatory indicators, most notably the platelet to lymphocyte ratio(PLR), are linked to clinical outcomes and therapeutic efficacy in various types of cancer (9–11). As a readily obtainable and cost-effective blood-based marker, an elevated PLR has been associated with poorer prognostic outcomes in breast cancer and may act as an indicator of diminished responsiveness to NACT (12). In a single-center study by Li et al, 215 breast cancer patients who received NACT followed by surgery were enrolled. After a ten-year follow-up, a higher pre-NACT PLR was found to be predictive of reduced OS (13). In another multicenter study, 63 breast cancer patients who underwent NACT between 2018 and 2024 were retrospectively analyzed by Fiste et al. A higher pre-NACT PLR was found to be predictive of a lower pCR (14). Despite emerging evidence, the utility of PLR as a predictive biomarker for tailoring neoadjuvant therapeutic approaches in breast cancer remains insufficiently defined and warrants further comprehensive investigation. While earlier meta-analyses have validated the prognostic relevance of PLR in forecasting OS, DFS, and pCR among breast cancer patients receiving NACT, further investigation is still warranted (12), they included only studies published before 2022. Subsequently, a considerable volume of additional clinical research has emerged in the literature, yet their findings remain inconclusive. Accordingly, this meta-analysis incorporates an additional nine studies published between 2022 and 2025 (13–21). In light of the ongoing discrepancies among recent findings, the present analysis seeks to extend prior research by incorporating up-to-date evidence to more comprehensively assess the prognostic significance of PLR in breast cancer patients undergoing NACT.
2 Materials and methods
2.1 Literature search
This meta-analysis was performed in alignment with the methodological framework detailed in the 2020 update of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22). Moreover, the research protocol was submitted in advance and formally recorded in the International Prospective Register of Systematic Reviews (PROSPERO), bearing the registration ID CRD420251064051.
Two researchers (ZQZ and CD) independently formulated the search methodology used in this study. Both investigators identified and selected relevant subject headings and keywords to conduct a comprehensive literature search across PubMed, Embase, Web of Science, and the Cochrane Library, covering publications from database inception to April 7, 2025. The search terms included “Blood Platelet,” “Platelets,” “Platelet,” “Thrombocytes,” “Thrombocyte,” “Lymphocyte,” “Lymphoid Cells,” “Lymphoid Cell,” “Breast Neoplasm,” “Breast Tumor,” “Breast Cancer,” “Breast Malignant Neoplasm,” “Mammary Cancers,” “Neoadjuvant Therapies,” “Neoadjuvant Chemotherapy Treatments,” “Neoadjuvant Systemic Therapy,” “Neoadjuvant Radiation,” and “Neoadjuvant Radiation Treatments.” A comprehensive description of the search methodology can be found in S1.
2.2 Study selection
Eligibility for study inclusion was determined according to the following predefined criteria (1): pathological confirmation of breast cancer (2); receipt of neoadjuvant therapy (3); evaluation of the prognostic value of PLR concerning OS, DFS, or pCR (4); availability or calculability of HRs, ORs, and corresponding 95% CIs (5); classification into elevated and reduced PLR cohorts based on explicitly stated threshold values; and (6) full-text availability.
Exclusion criteria included (1): secondary literature and non-primary sources, including review articles, editorial commentaries, conference summaries, individual case studies, and correspondence pieces (2); studies that did not provide adequate information to calculate HRs or corresponding 95% CIs (3); studies not reporting relevant survival outcomes; and (4) duplicate or overlapping publications.
Titles and abstracts were independently reviewed by two authors (ZQZ and CD), who also evaluated the full texts of potentially eligible for inclusion in the analysis. Any discrepancies were addressed and resolved through consensus-based discussion.
2.3 Data extraction
Two reviewers (ZQZ and CD) independently carried out the process of data extraction. Any conflicts in interpretation were addressed collaboratively through collective discussion, with all contributing authors participating to reach a unified agreement. The collected data encompassed various study characteristics, including first author’s name, publication year, study location, design, sample size, patient demographics, duration of study, treatment approach, TNM stage, PLR cutoff values, timing of PLR measurement, and reported HRs or ORs with 95% CIs for OS, DFS, and pCR.
2.4 Quality assessment
Study quality was assessed using the Newcastle–Ottawa Scale (NOS), which validates methodological rigor across three key domains: cohort selection, cohort comparability, and the determination of outcomes. The highest possible rating that can be assigned using the Newcastle–Ottawa Scale is 9 points (23).
2.5 Statistical analysis
Pooled HRs or ORs with corresponding 95% CIs were calculated to assess the prognostic significance of PLR in breast cancer patients undergoing NACT. Statistical heterogeneity was evaluated using Cochran’s Q test and Higgins’ I² statistic (24), with significant heterogeneity defined as I² >50% or P < 0.1. All analyses utilized a random-effects model to account for between-study variability. To test the stability of the aggregated outcomes, sensitivity analyses were performed. Additionally, subgroup analyses were carried out to identify possible heterogeneity sources and verify findings related specifically to OS, DFS and pCR. A separate random-effects model was applied to each subgroup to obtain subgroup-specific hazard ratios and odds ratios. Publication bias was visually assessed through funnel plots and statistically evaluated using Egger’s regression test, considering a p <0.05 indicative of significant bias. All statistical procedures were executed using STATA version 15.0 and Review Manager (Rev Man) version 5.4. The strength of evidence for each outcome was appraised following the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework, with results categorized into one of four tiers: high, moderate, low, or very low (25).
3 Results
3.1 Study characteristics
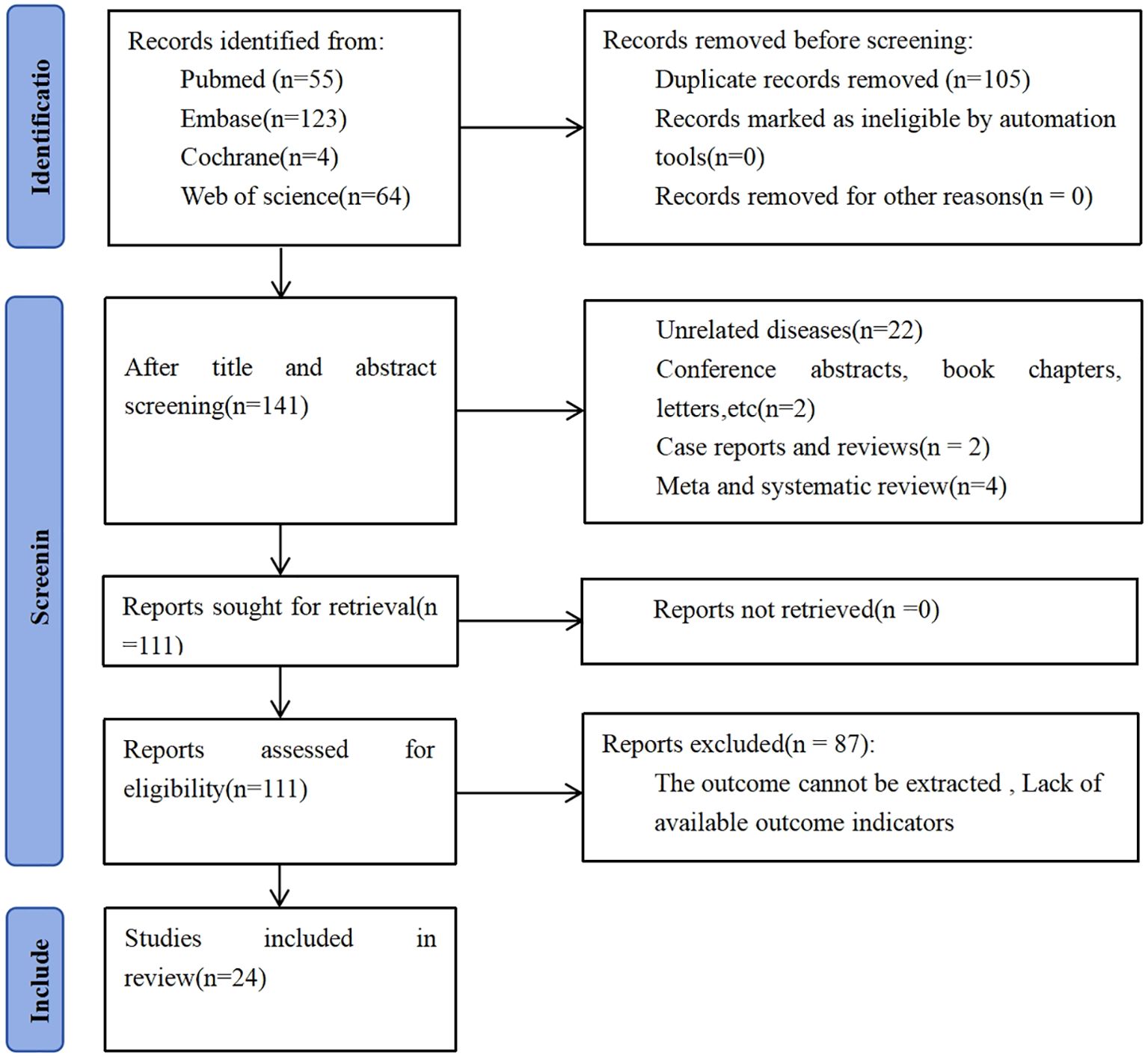
An initial total of 246 records were retrieved from database searches. Following the elimination of 105 duplicate entries, 141 distinct studies were retained for further evaluation. Screening titles and abstracts resulted in excluding 30 articles. Detailed assessment of the full texts from the remaining 111 studies led to the exclusion of 87 articles, primarily due to inadequate data for survival analysis. In the end, 24 studies satisfied the inclusion criteria and were incorporated into the meta-analysis, encompassing a combined cohort of 7,557 individuals diagnosed with breast cancer. Participant enrollment across individual studies ranged from a minimum of 55 to a maximum of 1,994 individuals, as illustrated in Figure 1.
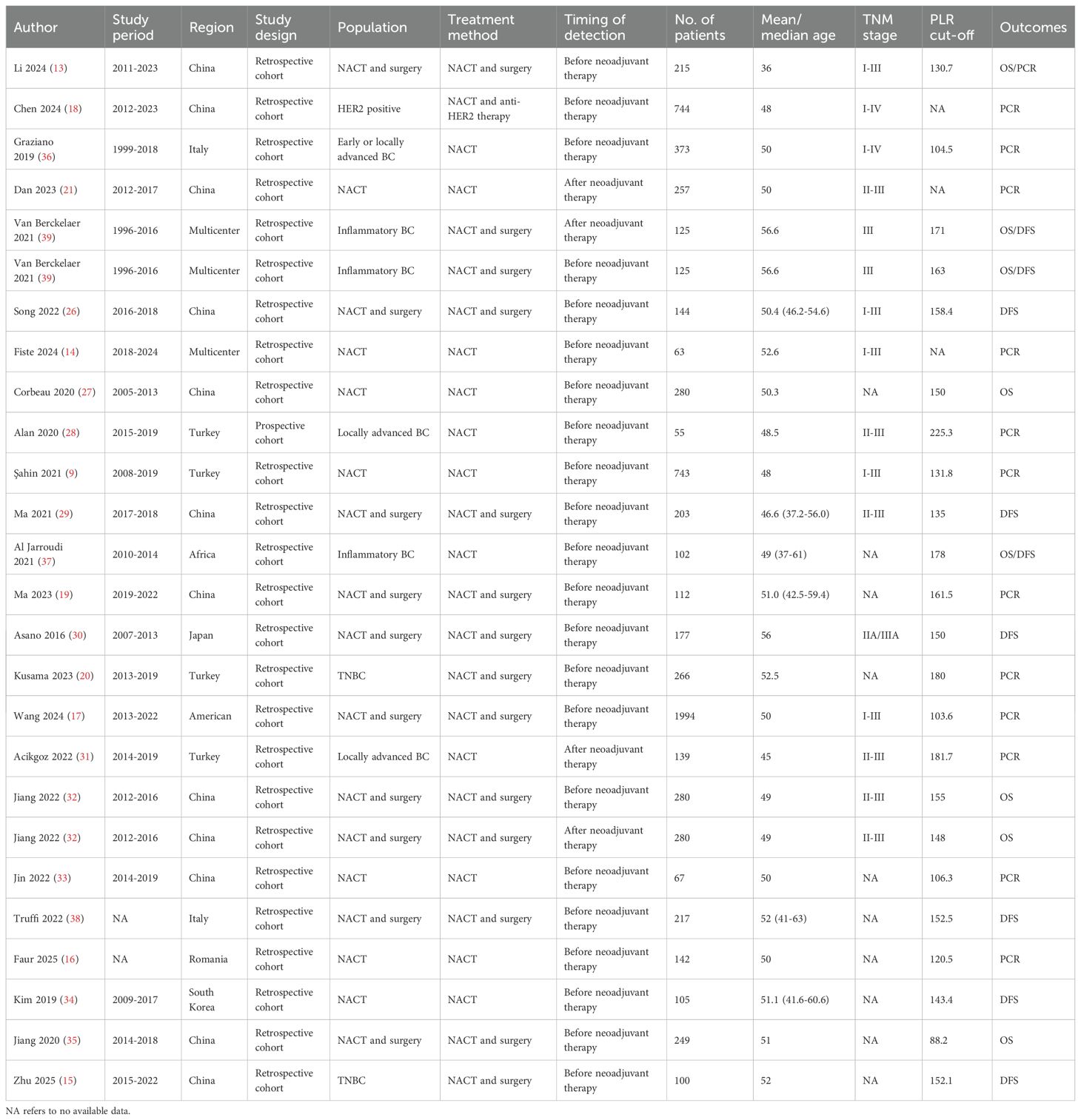
From the 24 studies published between 2016 and 2025, a total of 26 distinct comparison cohorts were derived for analysis. Of these, 18 comparison groups were conducted in Asia (9, 13, 15, 18–21, 26–35), 5 in Europe (16, 17, 36–38), and the remaining 3 were multicenter studies (14, 39). Among them, 25 comparison groups were retrospective in design (9, 13–21, 26, 27, 29–39), while the remaining 1 was prospective (28). All identified comparison cohorts were reported in English-language publications, with study durations spanning from 1996 to 2024. Two comparison groups did not report the study period (16, 38). The median age across these comparison groups ranged from 36 to 56.6 years. All patients received NACT, including 11 groups that received NACT alone, 14 groups that received NACT followed by surgery, and 1 group that received NACT combined with anti-HER2 therapy. For analytical purposes, patients were stratified into two cohorts based on PLR levels: those with elevated PLR and those with lower PLR values. Regarding PLR measurement, 22 comparison groups assessed PLR before NACT, while 4 evaluated PLR after NACT. Based on PLR assessment, 8 groups investigated its prognostic impact on OS, 9 on DFS, and 13 on pCR. Table 1 presents a comprehensive overview of the key characteristics associated with each included comparison group.
3.2 Study quality
All 26 comparison groups had NOS scores ranging from 6 to 9 (Supplementary Table S2).
3.3 Meta-analysis results
3.3.1 PLR and OS
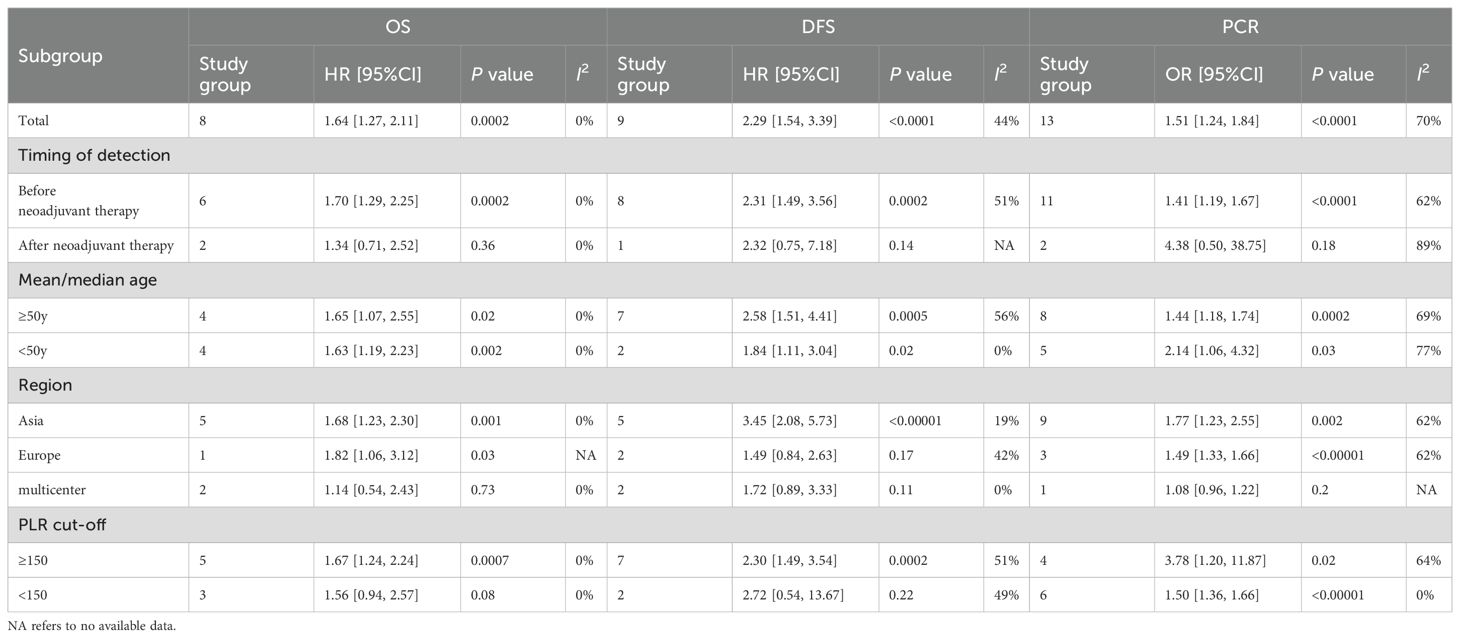
To examine the relationship between PLR and OS, eight comparison cohorts comprising a total of 1,656 patients were evaluated. Six studies evaluated PLR before NACT, while two studies measured PLR afterward. Overall, elevated PLR significantly correlated with reduced OS (HR = 1.64; 95% CI: 1.27–2.11; p = 0.0002). Subgroup analyses indicated significant prognostic value for pre-NACT PLR (HR = 1.70; 95% CI: 1.29–2.25; p = 0.0002; Figure 2A), whereas post-NACT PLR showed no significant association (HR = 1.34; 95% CI: 0.71–2.52; p = 0.36). Further stratification by median age demonstrated significant associations in both age groups: ≥50 years (HR = 1.65; 95% CI: 1.07–2.55; p = 0.02) and <50 years (HR = 1.63; 95% CI: 1.19–2.23; p = 0.002). Geographic subgroup analyses showed elevated PLR significantly predicted poorer OS in Asian (HR = 1.68; 95% CI: 1.23–2.30; p = 0.001) and European populations (HR = 1.82; 95% CI: 1.06–3.12; p = 0.03), but not in multicenter groups (HR = 1.14; 95% CI: 0.54–2.43; p = 0.73). Analysis by PLR cutoff values revealed significant prognostic value at thresholds ≥150 (HR = 1.67; 95% CI: 1.24–2.24; p = 0.0007), whereas lower thresholds did not yield significant results (HR = 1.56; 95% CI: 0.94–2.57; p = 0.08). Detailed results are summarized in Table 2.
![Forest plots showing three meta-analyses labeled A, B, and C. Plot A shows hazard ratios for eight studies with a total effect size of 1.64 [1.27, 2.11]. Plot B shows hazard ratios for nine studies with a total effect size of 2.29 [1.54, 3.39]. Plot C shows odds ratios for thirteen studies with a total effect size of 1.51 [1.24, 1.84]. Each plot includes individual study statistics, overall effect sizes, and confidence intervals, displayed with horizontal lines and red squares, and an overall effect diamond.](https://www.frontiersin.org/files/Articles/1658571/fimmu-16-1658571-HTML/image_m/fimmu-16-1658571-g002.jpg)
Figure 2. (A) Forest plots for the association between PLR and OS; (B) Forest plots for the association between PLR and DFS; (C) Forest plots for the association between PLR and pCR.
3.3.2 PLR and DFS
PLR data pertaining to disease-free survival (DFS) were available in nine studies; eight of these evaluated PLR levels prior to NACT, while one study assessed PLR following NACT. An increased PLR demonstrated a strong inverse association with disease-free survival (DFS), with a pooled hazard ratio of 2.29 (95% CI: 1.54–3.39; p < 0.0001; Figure 2B). Subgroup analyses revealed significant associations only for pre-NACT PLR (HR = 2.31; 95% CI: 1.49–3.56; p = 0.0002), not post-NACT PLR (HR = 2.32; 95% CI: 0.75–7.18; p = 0.14). Significant prognostic value was found across median age groups (≥50 years: HR = 2.58; 95% CI: 1.51–4.41; p = 0.0005; <50 years: HR = 1.84; 95% CI: 1.11–3.04; p = 0.02). Subgroup analysis based on geographic region demonstrated a statistically significant association within Asian populations (HR = 3.45; 95% CI: 2.08–5.73; p < 0.00001), whereas no meaningful correlation was observed in European cohorts (HR = 1.49; 95% CI: 0.84–2.63; p = 0.17) or in studies conducted across multiple centers (HR = 1.72; 95% CI: 0.89–3.33; p = 0.11). PLR cutoff analysis showed significance at ≥150 (HR = 2.30; 95% CI: 1.49–3.54; p = 0.0002) but not at lower cutoffs (HR = 2.72; 95% CI: 0.54–13.67; p = 0.22). Results are presented in Table 2.
3.3.3 PLR and pCR
Thirteen studies (5,170 patients) assessed PLR’s relationship with pCR, showing significantly lower pCR rates associated with elevated PLR (HR = 1.51; 95% CI: 1.24–1.84; p < 0.0001; Figure 2C). Pre-NACT PLR significantly correlated with reduced pCR (HR = 1.41; 95% CI: 1.19–1.67; p < 0.0001), while post-NACT PLR showed no significant effect (HR = 4.38; 95% CI: 0.50–38.75; p = 0.18). Subgroup analyses by median age confirmed significant associations across both age groups (≥50 years: HR = 1.44; 95% CI: 1.18–1.74; p = 0.0002; <50 years: HR = 2.14; 95% CI: 1.06–4.32; p = 0.03). PLR significantly predicted lower pCR rates in Asian (HR = 1.77; 95% CI: 1.23–2.55; p = 0.002) and European groups (HR = 1.49; 95% CI: 1.33–1.66; p < 0.00001), but not in multicenter groups (HR = 1.08; 95% CI: 0.96–1.22; p = 0.20). High PLR cutoff values (≥150) significantly correlated with lower pCR rates (HR = 3.78; 95% CI: 1.20–11.87; p = 0.02), whereas lower cutoffs did not yield significant results (HR = 1.50; 95% CI: 1.36–1.66; p < 0.00001). Results are detailed in Table 2.
3.4 Sensitivity analysis
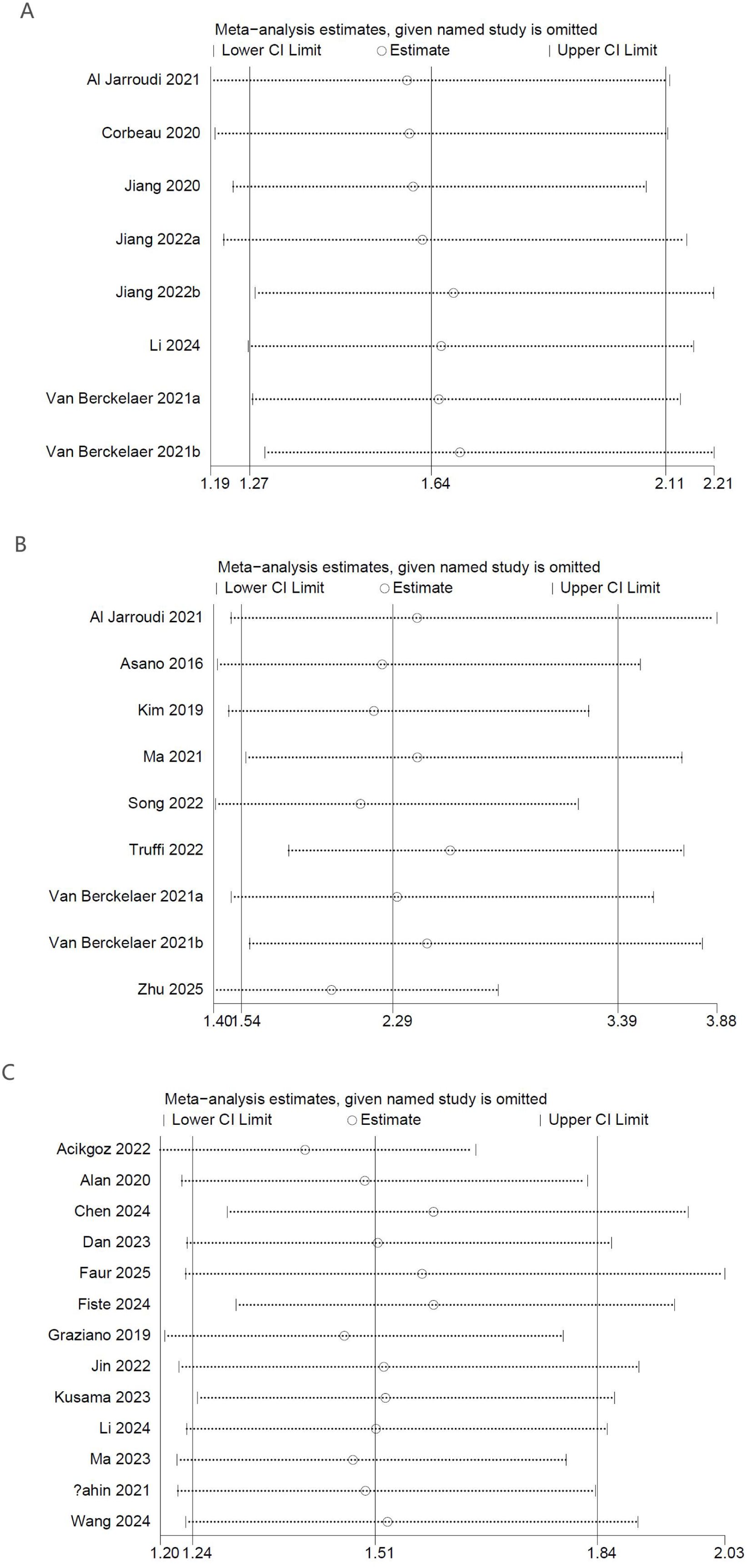
To evaluate the reliability of the results related to PLR levels measured before NACT, a sensitivity analysis was performed. The stepwise removal of individual studies from the analysis had minimal impact on the overall pooled estimates, which consistently fell within the bounds of the original confidence intervals. The findings suggest that no individual comparison group exerted an undue influence on the results for OS (Figure 3A), DFS (Figure 3B), or pCR (Figure 3C), thereby supporting the consistency and robustness of the overall analysis.
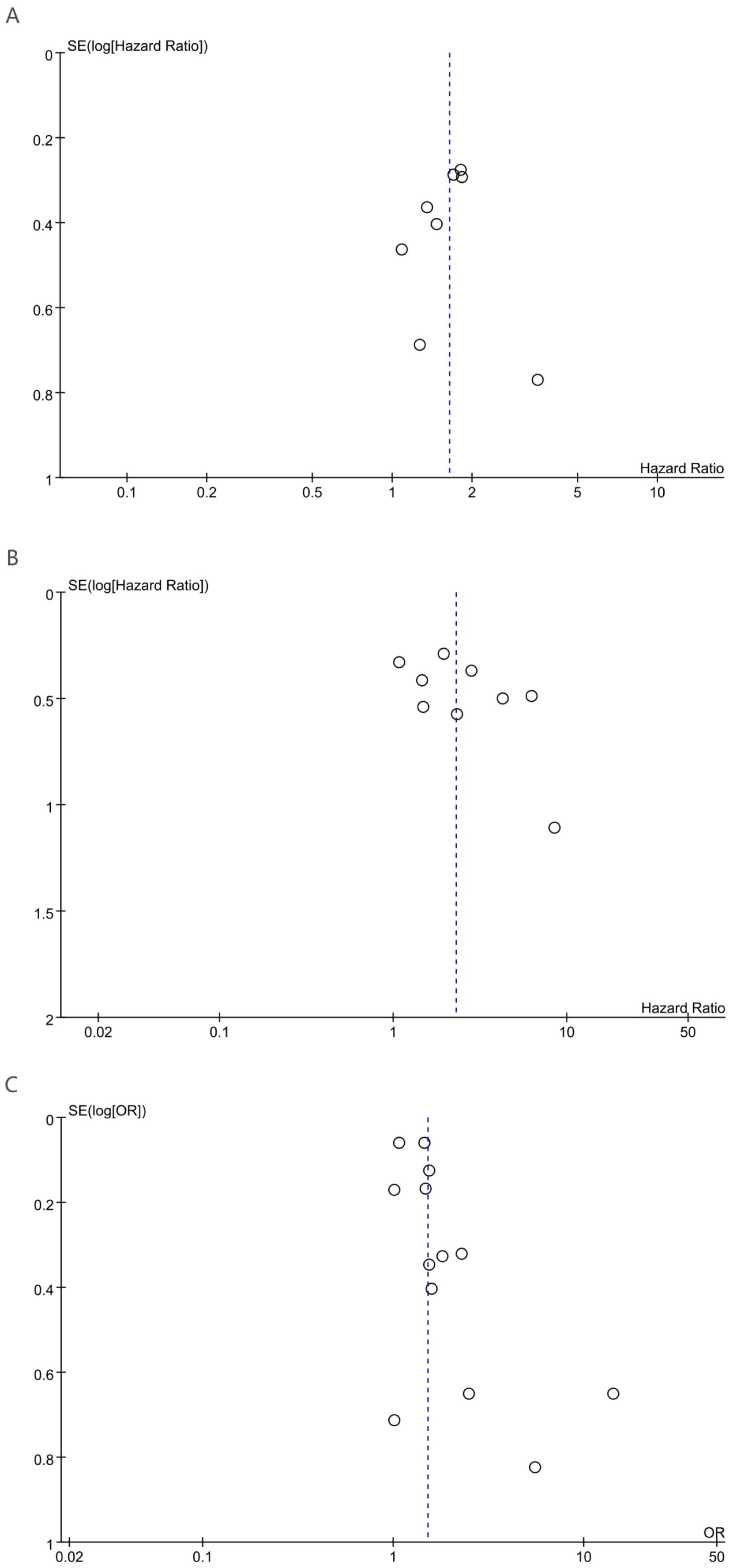
3.5 Publication bias
Potential publication bias was evaluated through visual inspection of funnel plots alongside statistical assessment using Egger’s regression analysis. Egger’s regression analysis indicated no statistically significant signs of publication bias for OS (p = 0.85), DFS (p = 0.146), or pCR (p = 0.053). Additionally, funnel plot symmetry supported the absence of substantial publication bias for OS (Figure 4A), DFS (Figure 4B), and pCR (Figure 4C).
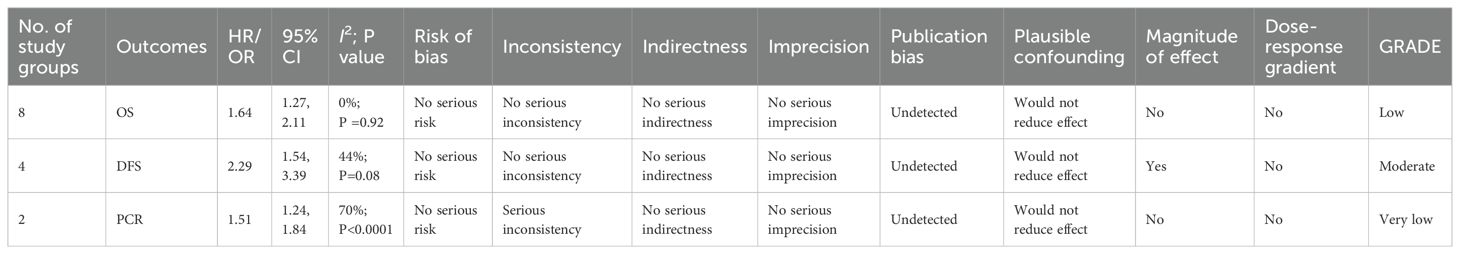
3.6 GRADE approach
Using the GRADE methodology, the quality of evidence was rated as low for OS, moderate for DFS, and very low for pCR. Detailed GRADE assessments are summarized in Table 3.
4 Discussion
Inflammation plays a critical role in all stages of carcinogenesis, tumor progression, and resistance to anticancer therapies (40). Extensive evidence indicates that systemic inflammation is associated with poor survival in cancer patients, thereby supporting the clinical application of inflammatory markers as prognostic indicators (41). As a commonly used indicator of systemic inflammation, the PLR has been shown to be significantly associated with survival outcomes in colorectal, gastric, and hepatocellular cancers (42, 43). However, its prognostic value in BC remains controversial. Xue et al. reported that elevated PLR is associated with poorer OS, DFS, and pCR in BC patients (12), whereas Yuce et al. found no significant association between PLR and pCR (44).
Therefore, a meta-analysis involving 7,557 patients is conducted to evaluate the prognostic value of the PLR in breast cancer patients undergoing NACT. Our findings indicate that elevated PLR levels are significantly associated with shorter OS, reduced DFS, and lower pCR rates. In addition, sensitivity analyses further confirm the stability of these results. Egger’s test also reveals no evidence of publication bias. This finding is consistent with previous meta-analyses, which have shown that high PLR is significantly associated with lower pCR rates and poorer OS and DFS in breast cancer patients receiving NACT. Therefore, our study, based on a larger sample size, further validates these earlier findings and supports the potential role of PLR as an important biomarker for predicting treatment response to NACT in breast cancer patients.
In the subgroup analysis, PLR measured before NACT demonstrates greater prognostic value, whereas post-NACT PLR shows no significant association with breast cancer outcomes. Pre-NACT PLR reflects the intrinsic inflammatory state of the tumor microenvironment. Such chronic inflammation potentially influences tumor aggressiveness directly through mechanisms such as promoting angiogenesis and suppressing immune surveillance, thereby maintaining stable prognostic efficacy (45). Conversely, post-NACT PLR changes are influenced by chemotherapy and confounding factors, reflecting acute treatment-related stress responses, such as chemotherapy-induced neutropenia. These acute alterations have limited relevance to long-term prognosis, thus diminishing the predictive value of post-treatment PLR (46, 47). Therefore, clinical practice should prioritize pre-NACT PLR measurements for outcome prediction. In our meta-analysis, subgroup analyses by geographic region reveal no significant association between PLR and breast cancer prognosis in European or multicenter groups. The lack of statistical significance might result from the limited number of included studies. Additionally, genetic heterogeneity in populations outside Asia, such as differences between Northern and Southern European genetic backgrounds, may influence inflammatory responses. Furthermore, multicenter studies typically adhere to highly standardized treatment protocols, potentially masking the effects of PLR and generating false-negative results. Future international multicenter studies with standardized PLR measurement procedures are necessary to further validate the consistency of PLR’s prognostic value across diverse populations. Subgroup analysis based on PLR cutoff values indicates superior predictive efficacy in NACT-treated breast cancer patients when the cutoff value is ≥150 compared to <150. This finding suggests that future predictive models should ideally set PLR cutoff values at 150 or higher, or adjust them comprehensively based on specific patient characteristics such as tumor staging, treatment efficacy, therapeutic response, and age. Our findings indicate that cutoff values might contribute to heterogeneity observed in these analyses.
Although the exact biological pathways through which PLR influences breast cancer prognosis have yet to be fully elucidated, PLR has nonetheless shown promise as a predictive marker of responsiveness to NACT. PLR depends on platelet and lymphocyte levels, serving respectively as pro-tumor and anti-tumor indicators (21). Lymphocytes suppress breast cancer progression through immune surveillance mechanisms, including mediating cytotoxic apoptosis of tumor cells (48) and secreting anti-tumor factors such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) (49). High levels of TILs not only improve patient prognosis but also predict responses to NACT (50–53). Conversely, platelets accelerate cancer progression through a triple pro-tumor mechanism. First, platelets aggregate around tumor cells, forming a physical barrier against blood shear stress and immune attacks (54). Second, platelets secrete pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), promoting tumor vascularization (55–57). Lastly, platelets trigger epithelial–mesenchymal transition (EMT), facilitating metastasis and hindering immune clearance (58). The disruption of lymphocyte–platelet balance elevates PLR, ultimately signaling poor breast cancer prognosis.
Although this meta-analysis offers a comprehensive summary of existing data, several limitations merit careful consideration. To begin with, most of the studies meeting the inclusion criteria for our analysis were conducted at single institutions and were predominantly based in Asian populations. Consequently, the findings should be interpreted cautiously within this specific geographic context. Generalization to patients in Europe, Africa, the Americas, or other regions may be inappropriate without further validation. Indeed, additional investigations are required to verify the prognostic value of PLR among breast cancer patients from non-Asian populations undergoing NACT. Secondly, the majority of the included investigations adopt a retrospective study design, as opposed to a prospective approach. Retrospective study designs are intrinsically susceptible to confounding variables, which may undermine both the accuracy and interpretability of the findings. Furthermore, inconsistency in PLR cutoff values across different studies poses another limitation. Some studies established cutoff values based on previous literature rather than employing ROC curve analyses. Even in studies utilizing ROC curve analysis, variability in blood sampling protocols, baseline hematological parameters, or timing of assessments may have resulted in inconsistent cutoff thresholds. Such variability could introduce selection bias into the meta-analysis. Therefore, future research would benefit from establishing standardized and universally accepted cutoff values for PLR to improve consistency and comparability across studies.
While our meta-analysis confirms PLR’s prognostic value in NACT-treated breast cancer, its biological interpretation warrants caution. The term inflammation oversimplifies a multifactorial process: elevated PLR may concurrently reflect platelet-mediated pro-tumorigenic pathways (e.g., VEGF-driven angiogenesis, EMT facilitation) and impaired lymphocyte-dependent immune surveillance (59–63). This mechanistic complexity underscores why PLR should not yet guide definitive clinical actions.
However, in the specific context of NACT, PLR offers practical utility. As pCR strongly correlates with survival (64, 65), a readily accessible biomarker predicting pCR failure (PLR ≥150) could help triage high-risk patients for advanced imaging or molecular profiling. This is particularly relevant in resource-constrained regions where genomic testing remains inaccessible. Future studies should integrate PLR with established biomarkers to build multimodal risk models rather than relying on isolated metrics.
5 Conclusion
Our meta-analysis demonstrates that elevated PLR significantly correlates with worse outcomes in breast cancer patients undergoing NACT, including reduced OS, shorter DFS, and lower pCR rates. These findings suggest PLR as a potentially valuable independent prognostic biomarker for informing clinical decisions regarding neoadjuvant treatment strategies. However, considering the limitations inherent in the included studies, further prospective research across diverse ethnic and geographical populations is necessary to validate these results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZZ: Project administration, Supervision, Conceptualization, Methodology, Writing – review & editing, Data curation, Formal analysis, Investigation, Writing – original draft, Software. HX: Investigation, Methodology, Writing – review & editing, Software, Conceptualization, Writing – original draft, Supervision, Data curation, Formal analysis, Project administration. BM: Supervision, Conceptualization, Methodology, Software, Project administration, Investigation, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. CD: Project administration, Visualization, Funding acquisition, Resources, Data curation, Validation, Formal analysis, Conceptualization, Methodology, Supervision, Software, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Tianshan Innovation Team Project by Xinjiang Uygur Autonomous Region Science and Technology Department (Grant Number: 2022TSYCTD0017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1658571/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, AlHilli Z, et al. NCCN guidelines® Insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2024. J Natl Compr Canc Netw. (2023) 21:1000–10. doi: 10.6004/jnccn.2023.0051
3. Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J, et al. Breast cancer statistics 2024. CA Cancer J Clin. (2024) 74:477–95. doi: 10.3322/caac.21863
4. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res. (2020) 26:2838–48. doi: 10.1158/1078-0432.Ccr-19-3492
5. Le-Petross HT, McCall LM, Hunt KK, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Axillary ultrasound identifies residual nodal disease after chemotherapy: results from the American college of surgeons oncology group Z1071 trial (Alliance). AJR Am J Roentgenol. (2018) 210:669–76. doi: 10.2214/ajr.17.18295
6. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. (2012) 30:1796–804. doi: 10.1200/jco.2011.38.8595
7. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. (2014) 384:164–72. doi: 10.1016/s0140-6736(13)62422-8
8. Xu W, Chen X, Deng F, Zhang J, and Zhang W. and J tang predictors of neoadjuvant chemotherapy response in breast cancer: A review. Onco Targets Ther. (2020) 13:5887–99. doi: 10.2147/ott.S253056
9. Şahin AB, Cubukcu E, Ocak B, Deligonul A, Oyucu Orhan S, Tolunay S, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep. (2021) 11:14662. doi: 10.1038/s41598-021-94184-7
10. Zhang X, Zhao W, Yu Y, Qi X, Song L, Zhang C, et al. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol. (2020) 18:191. doi: 10.1186/s12957-020-01952-2
11. Kubota K, Shimizu A, Notake T, Masuo H, Hosoda K, Yasukawa K, et al. Preoperative peripheral blood lymphocyte-to-monocyte ratio predicts long-term outcome for patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. (2022) 29:1437–48. doi: 10.1245/s10434-021-10848-8
12. Qi X, Chen J, Wei S, Ni J, Song L, Jin C, et al. Prognostic significance of platelet-to-lymphocyte ratio (PLR) in patients with breast cancer treated with neoadjuvant chemotherapy: a meta-analysis. BMJ Open. (2023) 13:e074874. doi: 10.1136/bmjopen-2023-074874
13. Li F, Wang Y, Dou H, Chen X, and Wang J. and M Xiao Association of immune inflammatory biomarkers with pathological complete response and clinical prognosis in young breast cancer patients undergoing neoadjuvant chemotherapy. Front Oncol. (2024) 14:1349021. doi: 10.3389/fonc.2024.1349021
14. Fiste O, Mavrothalassitis E, Kokkalis A, Anagnostakis M, Gomatou G, Kontogiannis A, et al. Inflammation-related biomarkers as predictors of pathological complete response in early-stage breast cancer. Clin Transl Oncol. (2024) 27:2453–60. doi: 10.1007/s12094-024-03814-9
15. Zhu J, Cheng J, Ma Y, Wang Y, Zou Z, Wang W, et al. The value of inflammation-related indicators in chemotherapy efficacy and disease-free survival of triple-negative breast cancer. Eur J Med Res. (2025) 30:77. doi: 10.1186/s40001-025-02328-6
16. Faur IF, Dobrescu A, Clim IA, Pasca P, Burta C, Tarta C, et al. Prognostic significance of peripheral blood parameters as predictor of neoadjuvant chemotherapy response in breast cancer. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26062541
17. Wang H, Huang Z, Xu B, Zhang J, He P, Gao F, et al. The predictive value of systemic immune-inflammatory markers before and after treatment for pathological complete response in patients undergoing neoadjuvant therapy for breast cancer: a retrospective study of 1994 patients. Clin Transl Oncol. (2024) 26:1467–79. doi: 10.1007/s12094-023-03371-7
18. Chen X, Cai Q, Deng L, Chen M, Xu M, Chen L, et al. Association of inflammatory blood markers and pathological complete response in HER2-positive breast cancer: a retrospective single-center cohort study. Front Immunol. (2024) 15:1465862. doi: 10.3389/fimmu.2024.1465862
19. Ma R, Wei W, Ye H, Dang C, and Li K. and D Yuan A nomogram based on platelet-to-lymphocyte ratio for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy. BMC Cancer. (2023) 23:245. doi: 10.1186/s12885-023-10703-x
20. Kusama H, Kittaka N, Soma A, Taniguchi A, Kanaoka H, Nakajima S, et al. Predictive factors for response to neoadjuvant chemotherapy: inflammatory and immune markers in triple-negative breast cancer. Breast Cancer. (2023) 30:1085–93. doi: 10.1007/s12282-023-01504-y
21. Dan J, Tan J, Huang J, Yuan Z, and Guo Y. Early changes of platelet−lymphocyte ratio correlate with neoadjuvant chemotherapy response and predict pathological complete response in breast cancer. Mol Clin Oncol. (2023) 19:90. doi: 10.3892/mco.2023.2686
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23. Aanesen F, Berg RC, Jørgensen IL, Mohr B, and Proper K. and L K Lunde Employment and mental health in the working age population: a protocol for a systematic review of longitudinal studies. Syst Rev. (2024) 13:197. doi: 10.1186/s13643-024-02613-1
24. Higgins JP, Thompson SG, and Deeks JJ. and D G Altman Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
26. Song D, Li X, and Zhang X. Expression and prognostic value of ratios of platelet lymphocyte, neutrophil lymphocyte and lymphocyte monocyte in breast cancer patients. Am J Transl Res. (2022) 14:3233–9.
27. Corbeau I, Thezenas S, Maran-Gonzalez A, Colombo PE, and Jacot W. and S guiu inflammatory blood markers as prognostic and predictive factors in early breast cancer patients receiving neoadjuvant chemotherapy. Cancers (Basel). (2020) 12:3–8. doi: 10.3390/cancers12092666
28. Alan O, Akin Telli T, Aktas B, Koca S, Ökten IN, Hasanov R, et al. Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J Surg Oncol. (2020) 18:242. doi: 10.1186/s12957-020-02019-y
29. Ma Y and Zhang J. and X chen lymphocyte-to-monocyte ratio is associated with the poor prognosis of breast cancer patients receiving neoadjuvant chemotherapy. Cancer Manag Res. (2021) 13:1571–80. doi: 10.2147/cmar.S292048
30. Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, et al. Platelet-lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS One. (2016) 11:e0153459. doi: 10.1371/journal.pone.0153459
31. Acikgoz O, Yildiz A, Bilici A, Olmez OF, and Basim P. and A Cakir Pretreatment platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as a predictor of pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: single center experience from Turkey. Anticancer Drugs. (2022) 33:1150–5. doi: 10.1097/cad.0000000000001389
32. Jiang C, Zhang S, Qiao K, Xiu Y, and Yu X. and Y huang the pretreatment systemic inflammation response index as a useful prognostic factor is better than lymphocyte to monocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy. Clin Breast Cancer. (2022) 22:424–38. doi: 10.1016/j.clbc.2022.03.003
33. Jin X, Wang K, and Shao X. and J Huang Prognostic implications of the peripheral platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in predicting pathologic complete response after neoadjuvant chemotherapy in breast cancer patients. Gland Surg. (2022) 11:1057–66. doi: 10.21037/gs-22-244
34. Kim HY, Kim TH, and Yoon HK. and A lee the role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J Breast Cancer. (2019) 22:425–38. doi: 10.4048/jbc.2019.22.e41
35. Jiang C, Lu Y, and Zhang S. and Y huang systemic immune-inflammation index is superior to neutrophil to lymphocyte ratio in prognostic assessment of breast cancer patients undergoing neoadjuvant chemotherapy. BioMed Res Int. (2020) 2020:7961568. doi: 10.1155/2020/7961568
36. Graziano V, Grassadonia A, Iezzi L, Vici P, Pizzuti L, Barba M, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. (2019) 44:33–8. doi: 10.1016/j.breast.2018.12.014
37. Al Jarroudi O, El Bairi K, Abda N, Zaimi A, Jaouani L, Chibani H, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in inflammatory breast cancer. biomark Med. (2021) 15:1289–98. doi: 10.2217/bmm-2020-0717
38. Truffi M, Sottotetti F, Gafni N, Albasini S, Piccotti F, Morasso C, et al. Prognostic potential of immune inflammatory biomarkers in breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14215287
39. Van Berckelaer C, Vermeiren I, Vercauteren L, Rypens C, Oner G, Trinh XB, et al. The evolution and prognostic role of tumour-infiltrating lymphocytes and peripheral blood-based biomarkers in inflammatory breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13184656
40. Crusz SM and Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. (2015) 12:584–96. doi: 10.1038/nrclinonc.2015.105
41. Zhou Q, Dong J, Sun Q, Lu N, and Pan Y. and X Han Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker in patients with breast cancer receiving neoadjuvant chemotherapy: a meta-analysis. BMJ Open. (2021) 11:e047957. doi: 10.1136/bmjopen-2020-047957
42. Mei P, Feng W, and Zhan Y. and X Guo Prognostic value of lymphocyte-to-monocyte ratio in gastric cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1321584. doi: 10.3389/fimmu.2023.1321584
43. Yang XC, Liu H, Liu DC, Tong C, and Liang XW. and R H Chen Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front Oncol. (2022) 12:1036890. doi: 10.3389/fonc.2022.1036890
44. Yuce E, Karakullukcu S, Bulbul H, Alandag C, and Saygin I. and H Kavgaci The effect of the change in hemoglobin-albumin-lymphocyte-platelet scores occurring with neoadjuvant chemotherapy on clinical and pathological responses in breast cancer. Bratisl Lek Listy. (2023) 124:59–63. doi: 10.4149/bll_2023_009
45. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, and Clarke The systemic inflammation-based neutrophil-lymphocyte ratio SJ. experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. doi: 10.1016/j.critrevonc.2013.03.010
46. Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. (2011) 25:1441–8. doi: 10.1096/fj.11-0502ufm
47. Gajewski TF and Schreiber H. and Y X Fu Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. doi: 10.1038/ni.2703
48. Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. (2006) 94:275–80. doi: 10.1038/sj.bjc.6602934
49. Mijic S. and C dabrosin platelet activation in situ in breasts at high risk of cancer: relationship with mammographic density and estradiol. J Clin Endocrinol Metab. (2021) 106:485–500. doi: 10.1210/clinem/dgaa820
50. Kotoula V, Chatzopoulos K, Lakis S, Alexopoulou Z, Timotheadou E, Zagouri F, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget. (2016) 7:5074–87. doi: 10.18632/oncotarget.6231
51. Ibrahim EM, Al-Foheidi ME, and Al-Mansour MM. and G A Kazkaz The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. (2014) 148:467–76. doi: 10.1007/s10549-014-3185-2
52. Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. (2013) 109:2705–13. doi: 10.1038/bjc.2013.634
53. Mao Y, Qu Q, Zhang Y, Liu J, and Chen X. and K Shen The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. (2014) 9:e115103. doi: 10.1371/journal.pone.0115103
54. Yang R, Chang Q, Meng X, and Gao N. and W Wang Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer. (2018) 9:3295–302. doi: 10.7150/jca.25691
55. Egan K, Crowley D, Smyth P, O’Toole S, Spillane C, Martin C, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS One. (2011) 6:e26125. doi: 10.1371/journal.pone.0026125
56. Kono SA, Heasley LE, and Doebele RC. and D R Camidge Adding to the mix: fibroblast growth factor and platelet-derived growth factor receptor pathways as targets in non-small cell lung cancer. Curr Cancer Drug Targets. (2012) 12:107–23. doi: 10.2174/156800912799095144
57. Klinger MH. and W Jelkmann Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. (2002) 22:913–22. doi: 10.1089/10799900260286623
58. Floris G, Richard F, Hamy AS, Jongen L, Wildiers H, Ardui J, et al. Body mass index and tumor-infiltrating lymphocytes in triple-negative breast cancer. J Natl Cancer Inst. (2021) 113:146–53. doi: 10.1093/jnci/djaa090
59. Salima S, Sampeliling DG, Permadi W, Sasotya RMS, Aziz MA, Kurniadi A, et al. Analysis of inflammation parameter value lymphocyte monocyte ratio (LMR), platelet lymphocyte ratio (PLR), and systemic inflammation response index (SIRI) to differentiate Malignant and benign ovarian tumors. BMC Res Notes. (2025) 18:328. doi: 10.1186/s13104-025-07330-z
60. Maloney S, Pavlakis N, Itchins M, Arena J, Mittal A, Hudson A, et al. The prognostic and predictive role of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) as biomarkers in resected pancreatic cancer. J Clin Med. (2023) 12. doi: 10.3390/jcm12051989
61. Modica R, Minotta R, Liccardi A, Cannavale G, and Benevento E. and A colao evaluation of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) as potential biomarkers in patients with sporadic medullary thyroid cancer (MTC). J Pers Med. (2023) 13. doi: 10.3390/jpm13060953
62. Kim DH and Jang SY. and B Keam Predictive value of early dynamic changes of NLR and PLR for the efficacy of immune checkpoint inhibitor in head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. (2024) 138:763–71. doi: 10.1016/j.oooo.2024.07.014
63. Knetki-Wróblewska M, Grzywna A, Krawczyk P, Wojas-Krawczyk K, Chmielewska I, Jankowski T, et al. Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in second-line immunotherapy for patients with non-small cell lung cancer. Transl Lung Cancer Res. (2025) 14:749–60. doi: 10.21037/tlcr-24-675
64. Moldoveanu D, Hoskin TL, Day CN, Schulze AK, and Goetz MP. and J C Boughey Nodal pCR and overall survival following neoadjuvant chemotherapy for node positive ER+/Her2- breast cancer. Breast Cancer Res Treat. (2024) 203:419–28. doi: 10.1007/s10549-023-07152-2
65. Pouwer AW, Te Grootenhuis NC, Hinten F, de Bock GH, van der Zee AGJ, Melchers WJG, et al. Prognostic value of HPV-PCR, p16 and p53 immunohistochemical status on local recurrence rate and survival in patients with vulvar squamous cell carcinoma. Virchows Arch. (2024) 484:985–94. doi: 10.1007/s00428-023-03690-8
Keywords: platelet to lymphocyte ratio (PLR), breast cancer, NACT, prognostic value of survival, meta-analysis
Citation: Zhao Z, Xu H, Ma B and Dong C (2025) Prognostic value of platelet to lymphocyte ratio (PLR) in breast cancer patients receiving neoadjuvant therapy: a systematic review and meta-analysis. Front. Immunol. 16:1658571. doi: 10.3389/fimmu.2025.1658571
Received: 02 July 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
Behjatolah Monzavi-Karbassi, University of Arkansas for Medical Sciences, United StatesReviewed by:
Eric Siegel, University of Arkansas for Medical Sciences, United StatesA. Mazin Safar, United States Department of Veterans Affairs, United States
Copyright © 2025 Zhao, Xu, Ma and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Dong, ZG9uZ2NoYW9AeGptdS5lZHUuY24=; Binlin Ma, bWFiaW5saW5tYmxAMjFjbi5jb20=
 Ziqian Zhao
Ziqian Zhao Haoyi Xu
Haoyi Xu