- 1Department of Respiratory and Critical Care Medicine, The Affiliated Xuzhou Municipal Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of Respiratory and Critial Care Medicine, Xuzhou First People's Hospital, Xuzhou, Jiangsu, China
Non-small cell lung cancer (NSCLC) exhibits profound immune dysregulation, driven in part by the opposing roles of regulatory T cells (Tregs) and T helper 17 (Th17) cells. Tregs facilitate tumor progression through immune suppression, angiogenesis, and checkpoint engagement, while Th17 cells display dual effects depending on the tumor microenvironment, either promoting anti-tumor responses or enhancing malignancy. Importantly, plasticity between these subsets, orchestrated by cytokines such as TGF-β, IL-6, and IL-1β, allows dynamic interconversion that shapes immune outcomes. This review comprehensively summarizes the differentiation, molecular mechanisms, and functions of Tregs and Th17 cells in NSCLC. We highlight recent advances in targeting the Th17/Treg axis via immune checkpoint inhibitors, Treg depletion, and metabolic reprogramming. Understanding this immunological balance offers promising avenues for restoring anti-tumor immunity and improving therapeutic efficacy in NSCLC patients.
1 Introduction
Globally, NSCLC continues to be the predominant contributor to cancer-associated deaths, imposing a significant strain on healthcare infrastructures worldwide (1, 2). Recent research has focused on the immunological milieu within tumors, where compromised immune surveillance mechanisms play a pivotal role in oncogenesis and disease advancement (3, 4). Of the diverse immune cell populations, Tregs have been identified as central mediators of immune escape by tumors and are involved in NSCLC (5). Immunosuppression mediated by Tregs occurs via cytokine release, interference with metabolic pathways, and direct cytotoxic actions against effector immune cells (6). Conversely, Th17 cells display functional ambivalence, either enhancing or restraining tumor growth contingent upon the inflammatory context (7, 8). This duality is further obscured by the interconversion potential of these subsets, regulated by pivotal cytokines including TGF-β, IL-6, and IL-1β (9).
Beyond their involvement in tumorigenesis, proliferation, dissemination, and metastatic spread, Tregs also collaborate with Th17 cells in the progression of infections, autoimmune conditions, and neoplastic diseases (10). Within NSCLC, accumulating data reveal a complex interaction between Tregs and Th17 cells, both of which significantly influence the immunological profile of tumors (11). Deciphering the equilibrium between Th17 and Treg populations, along with their flexibility, is essential for devising successful immunotherapeutic strategies that reestablish immune homeostasis in NSCLC (12). This review systematically summarizes the differentiation processes, underlying molecular mechanisms, and functional contributions of Th17 and Treg cells in NSCLC. Moreover, it outlines recent breakthroughs in immunomodulatory therapies directed at the Th17/Treg axis, encompassing Treg elimination, inhibition of immune checkpoints, and alterations in cellular metabolism (13), offering novel insights into strategies for overcoming immune suppression and improving clinical outcomes in NSCLC patients.
2 Overview of regulatory T cells (Tregs)
2.1 Historical identification of Treg lineages
The seminal discovery of regulatory T cells dates to 1995, when Sakaguchi and colleagues demonstrated that selective removal of CD4+CD25+ T cell populations in murine models triggered systemic autoimmunity, while adoptive transfer of these cells conferred protection, establishing their immunoregulatory function (14). While CD25 serves as an operational surface marker, its expression is not exclusive to this subset (15). The transcription factor FOXP3 has since been identified as both a definitive molecular signature and a master regulator of Treg identity (16). Evidence from genetic analyses underscores FOXP3's non-redundant role in maintaining immunological tolerance, given that loss-of-function mutations precipitate multiorgan inflammatory syndromes across species. The molecular circuitry governing FOXP3 expression involves multiple regulatory layers: The COX-2/PGE2 signaling axis modulates its transcriptional activity, whereas TCR stimulation coupled with CD28-mediated co-signaling induces chromatin reorganization at the Foxp3 gene locus, predominantly through the NF-κB transcription factor c-Rel (17–20). Notably, STAT5-mediated signaling represents an indispensable pathway for the terminal differentiation of FOXP3-expressing Tregs from progenitor populations (21, 22). These molecular mechanisms collectively define the developmental paradigm of Treg specification and continue to inform contemporary models of immune homeostasis.
2.2 Functional heterogeneity of Treg populations
Tregs play a pivotal role in sustaining immune tolerance and preventing aberrant inflammatory responses, including those associated with tumorigenesis (23, 24). Unlike antigen-specific immune effectors, Tregs mediate broad immunosuppression, and their functional impairment is linked to autoimmune pathogenesis (25). The transcription factor FOXP3 serves as a critical determinant of their lineage commitment and functional maturation (26). Phenotypically, Tregs are characterized by co-expression of CD25 alongside inhibitory receptors such as CTLA-4, GITR, and LAG-3, as well as membrane-associated TGF-β (27, 28). Further subclassification is possible based on CD45RA expression, distinguishing naïve (CD45RA+) from antigen-experienced (CD45RA-) subsets (29). Although Neuropilin-1 has been proposed as a potential surface marker, no single definitive identifier currently exists for this population (30). The induced Tregs (iTregs) arise extrathymically from conventional CD4+ T cells under specific cytokine milieus, particularly within tumor microenvironments where they paradoxically facilitate immune evasion and malignant progression (31). Among these, Type 1 regulatory T (Tr1) cells—enriched in intestinal mucosa—do not express FOXP3 but instead mediate suppression via copious secretion of IL-10 and TGF-β (32, 33). Beyond classical CD4+ Tregs, regulatory function extends to multiple lymphocyte lineages. Certain CD4+ T cells acquire suppressive properties upon stimulation with autologous dendritic cells, upregulating FOXP3, CTLA-4, and immunomodulatory cytokines (IL-10, TGF-β) (34). Additionally, regulatory activity is observed in innate-like lymphocytes, including IL-10-producing NKT and γδ T cells, as well as CD8+CD28- and CD8+FOXP3+ T cells. Double-negative (CD3+CD4-CD8-) T cells further contribute to immune regulation through analogous mechanisms (35).
2.3 Immunosuppressive functions of Treg cells
Tregs mediate immune suppression through diverse mechanisms. Central pathways include the secretion of immunosuppressive cytokines such as TGF-β and IL-10, and the expression of high-affinity IL-2 receptors that deplete IL-2, thereby restricting effector T cell proliferation (36, 37). Soluble factors like IL-10 and TGF-β act in a contact-independent manner. Activated human Tregs also express granzyme A (GZ-A) and utilize the perforin pathway to induce apoptosis in antigen-presenting cells (APCs), while granzyme B contributes to effector T cell suppression (38). The cell-surface repertoire of Tregs features several co-inhibitory molecules essential for their function. Notably, CTLA-4 and GITR engage cognate receptors on target cells to transmit inhibitory signals, with CTLA-4 additionally facilitating the induction of regulatory phenotypes in CD4+ T cell precursors (39, 40). Besides, other critical regulators include PD-1, LAG-3, and CD39. LAG-3 modulates APC activity through MHC class II interaction, PD-1/PD-L1/PD-L2 signaling promotes Foxp3+ Treg development (41), while CD39 generates immunoregulatory adenosine via nucleotide catabolism (42). The suppressive arsenal of Tregs extends to metabolic interference through IDO-dependent tryptophan degradation, cytotoxic effector mechanisms involving perforin/granzyme systems (43, 44), and suppression of NK cell-mediated cytotoxicity by interfering with NKG2D signaling pathways (45). Their multifaceted regulation operates across immunological contexts through spatial competition with naïve T cells for APC engagement via chemokine gradients, and functional impairment of dendritic cell maturation; dynamic secretion of IL-10, IL-35, and cytotoxic mediators tailored to microenvironmental cues.
3 Properties of Th17 and Treg cells
Th17 cells constitute a unique CD4+ T helper subset, distinct from classical Th1 and Th2 lineages. Harrington identified IL-17–producing CD4+ T cells in mice, which were subsequently termed Th17 cells (46). Lineage-defining transcriptional regulators RORγt and STAT3 govern both their developmental program and functional stability (47, 48). Th17 lineage commitment is highly dependent on the cytokine environment. IL-6 and TGF-β act cooperatively to promote Th17 polarization (49). The concentration of TGF-β is crucial in determining CD4+ T cell fate, lower levels favor RORγt expression and Th17 differentiation, while higher levels suppress RORγt and induce Foxp3, promoting Treg development (50, 51). Notably, IL-21 can substitute for IL-6 in the presence of TGF-β to induce RORγt and inhibit Foxp3, further facilitating Th17 differentiation (51). Functionally, Th17 cells are pro-inflammatory, primarily through the secretion of IL-17, their hallmark cytokine (52, 53). The Th17/IL-17 axis has been implicated in autoimmune diseases such as asthma, systemic lupus erythematosus, and rheumatoid arthritis, although its role in tumor biology remains controversial and under active investigation (53–55).
Tregs are essential mediators of immune tolerance and immune suppression in both physiological and pathological contexts, including tumor immunity (56). Although Tregs constitute a minor fraction of CD4+ T lymphocytes, their capacity to suppress effector T cell responses enables tumors to evade immune surveillance in NSCLC (57, 58). The transcription factor FOXP3 remains a key determinant of Treg identity and function, exerting transcriptional repression of pro-inflammatory genes to encode inflammatory mediators such as IFN-γ, IL-13, and GM-CSF (59–61). In addition, Tregs modulate dendritic cell activity by secreting immunosuppressive cytokines such as IL-10, which promotes DC apoptosis and impairs their antigen-presenting capacity by downregulating co-stimulatory molecules like CD80 and CD86 (62, 63). These effects reduce effective T cell priming and promote an immunosuppressive microenvironment (64). Importantly, in NSCLC, Tregs express high levels of PD-1, CTLA-4, and CD39, which contribute to immune checkpoint-mediated suppression and adenosine production that further dampens effector cell functions (65–67). By shaping the tumor immune landscape through direct suppression and immune modulation, Tregs play a pivotal role in promoting tumor progression and resistance to immunotherapy.
4 Roles of Th17 and Treg cells in NSCLC
The immunosuppressive TME in NSCLC is particularly pronounced, characterized by high infiltration of Tregs, chronic inflammation, and resistance to immune checkpoint blockade therapies (68). Notably, NSCLC has been extensively studied in Th17 and Treg cell dynamics, offering a well-established framework to investigate their functional plasticity and therapeutic implications (69). Given these features, NSCLC represents a clinically relevant and immunologically tractable model for dissecting the Th17/Treg axis.
4.1 Roles of Th17 cells in NSCLC
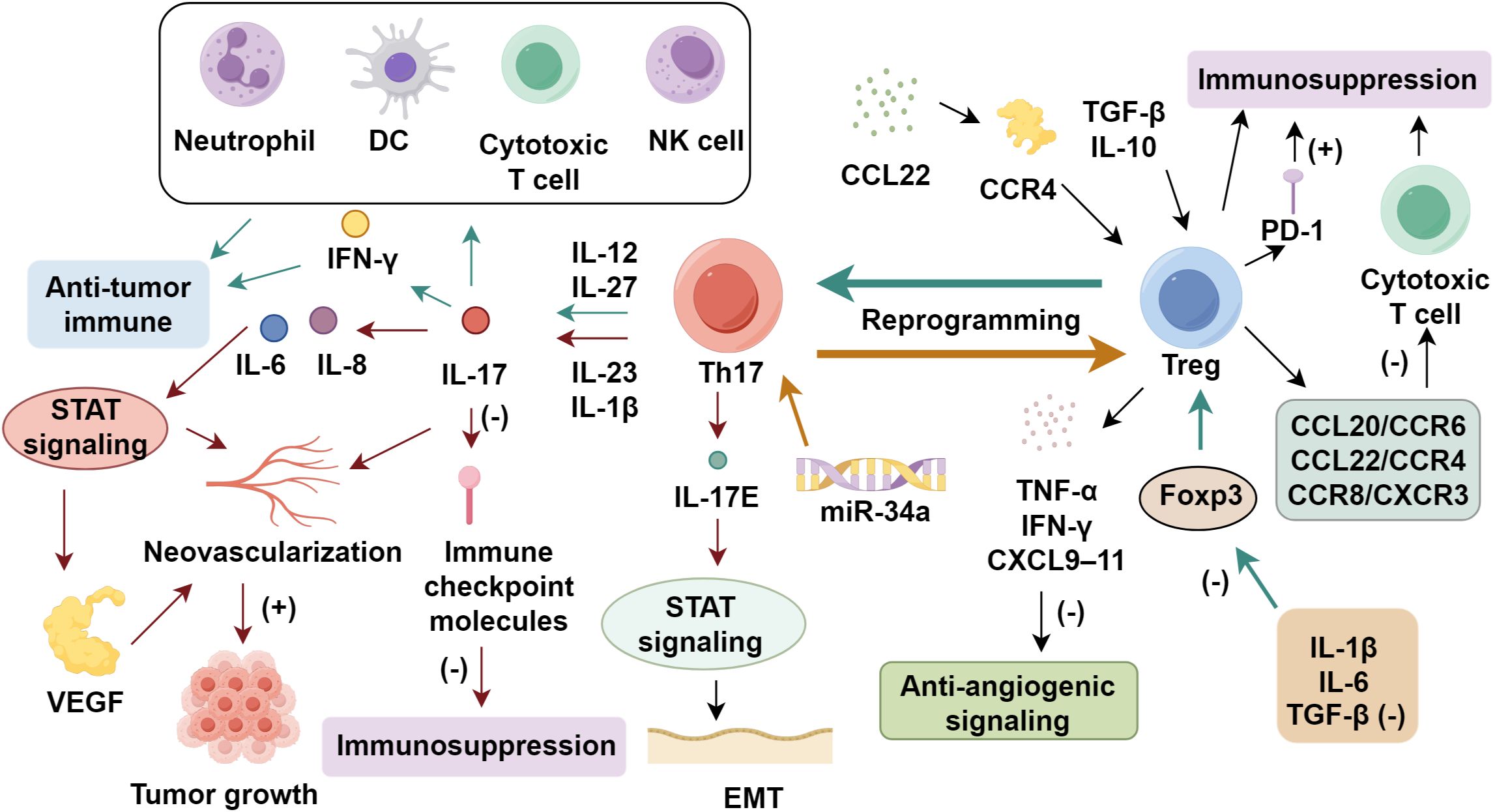
The functional dichotomy of Th17 cells in NSCLC continues to be a subject of controversy, as these lymphocytes exhibit both pro-tumorigenic and anti-tumor activities (70). Elevated concentrations of IL-17, a key Th17-derived cytokine, correlate with augmented neovascularization in multiple malignancies, suggesting a role in facilitating tumor growth (71). Huang et al. (72) reported that IL-17 increased microvessel density and VEGF via STAT signaling, upregulating IL-6 and IL-8. IL-17 administration accelerated tumor growth in mice (73). In contrast, increased Th17 infiltration within the tumor microenvironment has been shown to coincide with elevated neutrophil recruitment alongside heightened IFN-γ secretion, implying a capacity to bolster anti-tumor immune responses (74, 75). Ye et al. (76) revealed a marked enrichment of Th17 cells in NSCLC-associated malignant pleural effusions compared to peripheral blood, with higher Th17 frequencies predicting improved patient outcomes. The lineage-defining transcription factor RORγt orchestrates Th17 differentiation and may facilitate their transdifferentiation into cytotoxic CD8+ T lymphocytes. Additionally, RORγt-driven IL-17 production has been implicated in the suppression of immune checkpoint molecules, potentially mitigating tumor-induced immunosuppression (77). In NSCLC, IL-17E facilitates cell proliferation and epithelial-mesenchymal transition in A549 cells by regulating the NF-κB pathway (78). Th17 cells activate dendritic cells, enhance effector and cytotoxic T cell responses, and promote NK cell infiltration, collectively strengthening anti-tumor immunity (79, 80). The dual behavior of Th17 cells is largely shaped by the upstream cytokine milieu and the tumor microenvironmental context. IL-23 and IL-1β are critical determinants of Th17 pathogenicity (81, 82). IL-23 stabilizes the Th17 phenotype and promotes expression of pro-tumor mediators such as IL-17A, IL-22, and GM-CSF, while inhibiting anti-tumor features such as IFN-γ production (83–87). IL-1β, in cooperation with IL-6 and low-dose TGF-β, biases Th17 cells toward a pathogenic profile that favors inflammation, angiogenesis, and tumor progression (13, 88). In contrast, IL-12 or IL-27 exposure can redirect Th17 cells toward an IFN-γ–producing, tumoricidal phenotype (89) (Figure 1).
4.2 Roles of Tregs in NSCLC
4.2.1 Tregs in NSCLC initiation and progression
In early stage, the immune system maintains equilibrium by eliminating spontaneously arising tumor cells through coordinated innate and adaptive immune responses (90). However, when malignant cells proliferate beyond the capacity of immunological control mechanisms, this homeostatic balance is disrupted, leading to impaired immune surveillance and functional deficits (91). Such disruption enables immune escape and promotes malignant phenotypes, including unchecked proliferation, genomic instability, and metastasis. Among these, CD4+CD25+ Tregs have been increasingly recognized as critical mediators of immune suppression in NSCLC (14). Clinically, increased Treg frequencies are consistently observed in both tumor sites and peripheral blood of lung cancer patients (92). This expansion is orchestrated by the tumor microenvironment, where immunosuppressive cytokines such as TGF-β and IL-10 induce naïve T cell conversion into Tregs, and chemokines like CCL22 mediate recruitment via CCR4 signaling (93). Infiltrating Tregs then reinforce immunosuppression, forming a feedback loop that accelerates immune escape (94). Moreover, TGF-β–induced Treg infiltration suppresses cytotoxic T cell activity in NSCLC. These include chemokine-mediated recruitment via CCL20/CCR6, CCL22/CCR4, CCR8, and CXCR3 (95, 96), antigen-driven clonal expansion facilitated by dendritic cell presentation and TGF-β–dependent polarization (96), metabolic reprogramming favoring glycolytic and lipid oxidation pathways to support Treg survival (97, 98), and the contribution of tumor-derived extracellular vesicles that enhance Treg proliferation and confer resistance to apoptosis (99, 100).
4.2.2 Tregs in invasion and metastasis of NSCLC
Elevated Treg levels are strongly associated with advanced clinical stage, poor differentiation, and enhanced metastatic potential in lung cancer (68). Prognostic analyses consistently identify tumor-infiltrating Treg abundance as an independent predictor of unfavorable clinical outcomes. These immunosuppressive cells promote metastatic progression through diverse biological pathways (101, 102). Tregs disrupt anti-angiogenic signaling by inhibiting Th1-cell derived mediators including TNF-α, IFN-γ, and CXCL9–11 (103, 104). Hypoxia further induce the VEGF production, fostering tumor vascularization (105, 106). The stromal compartment contributes to therapy resistance through elevated COX-2/PGE2 pathway activity, which simultaneously enhances Treg differentiation and metastatic potential (107, 108). Functionally, Treg-mediated immune suppression manifests through impaired CD8+ T cell cytotoxic activity, with experimental depletion studies demonstrating restored expression of effector molecules (perforin, granzyme) and Th1 cytokines (109, 110). Clinically, elevated TGF-β and IL-10 in circulation and tumor tissues reflect Treg-mediated immunosuppression (111). Foxp3+ Tregs are increased in patient blood and decline postoperatively, implicating them in tumor development (112). Notably, Tregs engage in functional crosstalk with immune checkpoint pathways, particularly through their high PD-1 expression, which appears to amplify immunosuppressive activity and promote immune evasion (113). This mechanistic insight has spurred the development of several Treg-targeted therapeutic strategies. These include novel anti-CD25 antibodies like RG6292, which is engineered to deplete immunosuppressive Tregs while sparing IL-2 signaling in effector T cells, as well as combination approaches that integrate immune checkpoint inhibitors with Treg-targeting agents, currently under evaluation (114). These next-generation approaches demonstrate improved specificity and reduced toxicity profiles compared to earlier agents such as diftitox (115). Additionally, metabolic reprogramming remains a promising adjunctive strategy, with S-adenosylmethionine (SAM) showing potential to modulate Treg plasticity by downregulating Foxp3 and IL-10 while simultaneously enhancing IFN-γ production (116).
4.3 Dynamic interplay between Th17 and Treg cells in NSCLC pathogenesis
The functional plasticity between Th17 and Treg populations represents a critical immunoregulatory mechanism in NSCLC, with these cell subsets demonstrating capacity for bidirectional conversion that dynamically shapes tumor immunity (13, 69). Cytokines such as IL-1β and IL-6, secreted predominantly by tumor-associated macrophages and stromal cells, in concert with suboptimal concentrations of TME-derived TGF-β, drives Treg-to-Th17 reprogramming through Foxp3 suppression and impairment of regulatory function (117, 118). This phenotypic switching involves Treg acquisition of c-like properties, characterized by ROR-γt upregulation, Foxp3 loss, and development of IL-17 secretory capacity (119, 120). Post-transcriptional regulation further modulates this plasticity, as demonstrated by miR-34a-mediated enhancement of Th17 differentiation coupled with Treg functional inhibition (121). Clinically, NSCLC patients exhibit concurrent elevation of both subsets in circulation, with the Th17/Treg ratio serving as a more informative immunological parameter than absolute cell counts (13, 122). For example, Li et al. demonstrated that NSCLC patients displayed a significant increase in the Th17/Treg ratio post-treatment, suggesting its potential utility as a predictive marker of therapeutic efficacy (13). This ratio demonstrates stage-dependent progression, showing positive correlation with advancing tumor burden (123). Notably, this balance shifts throughout tumor progression: early-stage NSCLC, characterized by low TGF-β and high IL-6, favors Th17 polarization, whereas advanced stages, enriched in TGF-β, promote Foxp3 expression and Treg dominance (124–126). Crucially, the immunological impact of Th17 cells is not defined by their absolute numbers alone but by their dynamic balance with Treg cells. This Th17/Treg interplay determines the net immune response toward either tumor suppression or promotion in NSCLC (Supplementary Table S1).
5 Conclusion
The intricate interplay between Tregs and Th17 cells represents a central axis of immune regulation in non-small cell lung cancer (NSCLC). Tregs suppress anti-tumor immunity through cytokine secretion, checkpoint engagement, metabolic modulation, and inhibition of cytotoxic effector cells, thereby promoting tumor immune evasion, angiogenesis, and metastasis. In contrast, Th17 cells display context-dependent functions—exerting either tumor-promoting or tumor-inhibiting effects depending on the cytokine milieu, tumor stage, and metabolic cues within the tumor microenvironment. The dynamic balance and plasticity between these two subsets, particularly their bidirectional interconversion mediated by TGF-β, IL-6, and IL-1β, critically shape the immune landscape of NSCLC.
Targeting the Th17/Treg axis offers a promising strategy to restore immune surveillance and improve therapeutic responses in NSCLC. Advances in Treg-selective depletion, immune checkpoint inhibition, and modulation of T cell differentiation through metabolic or epigenetic interventions provide novel avenues for immunotherapy. Future research should prioritize refining these approaches, optimizing combination regimens, and identifying predictive biomarkers such as the Th17/Treg ratio to guide individualized treatment. A better understanding of the functional plasticity between Tregs and Th17 cells will be essential to overcoming immunosuppression and enhancing durable responses in NSCLC patients.
Author contributions
SZ: Writing – original draft. NZ: Writing – original draft. QL: Writing – original draft. XL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1658848/full#supplementary-material
References
1. Rahal Z, El Darzi R, Moghaddam SJ, Cascone T, and Kadara H. Tumour and microenvironment crosstalk in NSCLC progression and response to therapy. Nat Rev Clin Oncol. (2025) 22:463–82. doi: 10.1038/s41571-025-01021-1
2. Jeon H, Wang S, Song J, Gill H, and Cheng H. Update 2025: management of non−Small-cell lung cancer. Lung. (2025) 203:53. doi: 10.1007/s00408-025-00801-x
3. Herzog BH, Baer JM, Borcherding N, Kingston NL, Belle JI, Knolhoff BL, et al. Tumor-associated fibrosis impairs immune surveillance and response to immune checkpoint blockade in non-small cell lung cancer. Sci Transl Med. (2023) 15:eadh8005. doi: 10.1126/scitranslmed.adh8005
4. Madeddu C, Donisi C, Liscia N, Lai E, Scartozzi M, and Macciò A. EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int J Mol Sci. (2022) 23:6489. doi: 10.3390/ijms23126489
5. Xie M, Wei J, and Xu J. Inducers, attractors and modulators of CD4(+) treg cells in non-small-cell lung cancer. Front Immunol. (2020) 11:676. doi: 10.3389/fimmu.2020.00676
6. Fortunato O, Belisario DC, Compagno M, Giovinazzo F, Bracci C, Pastorino U, et al. CXCR4 inhibition counteracts immunosuppressive properties of metastatic NSCLC stem cells. Front Immunol. (2020) 11:02168. doi: 10.3389/fimmu.2020.02168
7. Peng DH, Rodriguez BL, Diao L, Gaudreau PO, Padhye A, Konen JM, et al. Th17 cells contribute to combination MEK inhibitor and anti-PD-L1 therapy resistance in KRAS/p53 mutant lung cancers. Nat Commun. (2021) 12:2606. doi: 10.1038/s41467-021-22875-w
8. Ceylan A, Artac M, Kocak MZ, and Artac H. Epidermal growth factor receptor and programmed cell death-1 expression levels in peripheral T cell subsets of patients with non-small cell lung cancer. Scand J Immunol. (2024) 100:e13398. doi: 10.1111/sji.13398
9. Niu Y and Zhou Q. Th17 cells and their related cytokines: vital players in progression of Malignant pleural effusion. Cell Mol Life Sci. (2022) 79:194. doi: 10.1007/s00018-022-04227-z
10. Wang J, Zhao X, and Wan YY. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell Mol Immunol. (2023) 20:1002–22. doi: 10.1038/s41423-023-01036-7
11. Wang X, She X, Gao W, Liu X, and Shi B. Correlation between the treg/thl7 index and the efficacy of PD-1 monoclonal antibody in patients with advanced non-small-cell lung cancer complicated with chronic obstructive pulmonary disease. Comput Math Methods Med. (2022) 2022:2923998. doi: 10.1155/2022/2923998
12. Duan MC, Zhong XN, Liu GN, and Wei JR. The Treg/Th17 paradigm in lung cancer. J Immunol Res. (2014) 2014:730380. doi: 10.1155/2014/730380
13. Duan MC, Han W, Jin PW, Wei YP, Wei Q, Zhang LM, et al. Disturbed th17/treg balance in patients with non-small cell lung cancer. Inflammation. (2015) 38:2156–65. doi: 10.1007/s10753-015-0198-x
14. Zheng G, Ye H, Bai J, and Zhang X. Downregulation of lncRNA MIR17HG reduced tumorigenicity and Treg-mediated immune escape of non-small-cell lung cancer cells through targeting the miR-17-5p/RUNX3 axis. J Biochem Mol Toxicol. (2024) 38:e23715. doi: 10.1002/jbt.23715
15. Chandnani N, Choudhari VS, Talukdar R, Rakshit S, Shanmugam G, Guchait S, et al. Depletion of enhancer zeste homolog 2 (EZH2) directs transcription factors associated with T cell differentiation through epigenetic regulation of Yin Yang 1(YY1) in combating non-small cell lung cancer (NSCLC). Med Oncol. (2023) 40:185. doi: 10.1007/s12032-023-02053-2
16. Wen Z, Liu T, Zhang Y, Yue Q, Meng H, He Y, et al. Salidroside regulates tumor microenvironment of non-small cell lung cancer via Hsp70/Stub1/Foxp3 pathway in Tregs. BMC Cancer. (2023) 23:717. doi: 10.1186/s12885-023-11036-5
17. Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. (2005) 65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141
18. Chen S, McMiller TL, Soni A, Succaria F, Sidhom JW, Cappelli LC, et al. Comparing anti-tumor and anti-self immunity in a patient with melanoma receiving immune checkpoint blockade. J Transl Med. (2024) 22:241. doi: 10.1186/s12967-024-04973-7
19. Mikami N, Kawakami R, Chen KY, Sugimoto A, Ohkura N, and Sakaguchi S. Epigenetic conversion of conventional T cells into regulatory T cells by CD28 signal deprivation. Proc Natl Acad Sci U.S.A. (2020) 117:12258–68. doi: 10.1073/pnas.1922600117
20. Vang KB, Yang J, Pagán AJ, Li LX, Wang J, Green JM, et al. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol. (2010) 184:4074–7. doi: 10.4049/jimmunol.0903933
21. Zhang S, Gan X, Qiu J, Ju Z, Gao J, Zhou J, et al. IL-10 derived from Hepatocarcinoma cells improves human induced regulatory T cells function via JAK1/STAT5 pathway in tumor microenvironment. Mol Immunol. (2021) 133:163–72. doi: 10.1016/j.molimm.2021.02.014
22. Zhu J, Li Z, Chen J, Li W, Wang H, Jiang T, et al. A comprehensive bioinformatics analysis of FOXP3 in nonsmall cell lung cancer. Med (Baltimore). (2022) 101:e32102. doi: 10.1097/MD.0000000000032102
23. Stüve P, Godoy GJ, Ferreyra FN, Hellriegel F, Boukhallouk F, Kao YS, et al. ACC1 is a dual metabolic-epigenetic regulator of Treg stability and immune tolerance. Mol Metab. (2025) 94:102111. doi: 10.1016/j.molmet.2025.102111
24. Fisher MS and Sennikov SV. T-regulatory cells for the treatment of autoimmune diseases. Front Immunol. (2025) 16:1511671. doi: 10.3389/fimmu.2025.1511671
25. Liu K, Chen B, Lin X, Zhou Q, Ben T, Xu J, et al. α1,3 fucosyltransferase VII improves intestinal immune homeostasis in inflammatory bowel disease by enhancing regulatory T-cell intestinal homing and immunosuppression. Gastroenterology. (2025) 169:632–46. doi: 10.1053/j.gastro.2025.02.041
26. Liu Z, Lee DS, Liang Y, Zheng Y, and Dixon JR. Foxp3 orchestrates reorganization of chromatin architecture to establish regulatory T cell identity. Nat Commun. (2023) 14:6943. doi: 10.1038/s41467-023-42647-y
27. Ho TH, Pfeffer K, Weiss GJ, Ruiz Y, and Lake DF. Identification of a CD4(+) T cell line with Treg-like activity. Hum Immunol. (2022) 83:281–94. doi: 10.1016/j.humimm.2022.01.008
28. Wang M, Feng Y, Zhang P, Shen K, Su J, Zhong Y, et al. Jiawei Maxing Shigan Tang alleviates radiation-induced lung injury via TGF-β1/Smad signaling pathway mediated by regulatory T cells. J Ethnopharmacol. (2024) 320:117389. doi: 10.1016/j.jep.2023.117389
29. Phillips JD, Knab LM, Blatner NR, Haghi L, DeCamp MM, Meyerson SL, et al. Preferential expansion of pro-inflammatory Tregs in human non-small cell lung cancer. Cancer Immunol Immunother. (2015) 64:1185–91. doi: 10.1007/s00262-015-1725-1
30. Chen W, Huang W, Xue Y, Chen Y, Qian W, Ma J, et al. Neuropilin-1 identifies a new subpopulation of TGF-β-induced foxp3(+) regulatory T cells with potent suppressive function and enhanced stability during inflammation. Front Immunol. (2022) 13:900139. doi: 10.3389/fimmu.2022.900139
31. Arroyo-Olarte RD, Flores-Castelán JC, Armas-López L, Escobedo G, Terrazas LI, Ávila-Moreno F, et al. Targeted demethylation of FOXP3-TSDR enhances the suppressive capacity of STAT6-deficient inducible T regulatory cells. Inflammation. (2024) 47:2159–72. doi: 10.1007/s10753-024-02031-4
32. Heinl PV, Graulich E, Weigmann B, Wangorsch A, Ose R, Bellinghausen I, et al. IL-10-modulated dendritic cells from birch pollen- and hazelnut-allergic patients facilitate Treg-mediated allergen-specific and cross-reactive tolerance. Allergy. (2024) 79:2826–39. doi: 10.1111/all.16255
33. Sayitoglu EC, Freeborn RA, and Roncarolo MG. The yin and yang of type 1 regulatory T cells: from discovery to clinical application. Front Immunol. (2021) 12:693105. doi: 10.3389/fimmu.2021.693105
34. Lang C, Wang J, and Chen L. CD25-expressing Th17 cells mediate CD8(+) T cell suppression in CTLA-4 dependent mechanisms in pancreatic ductal adenocarcinoma. Exp Cell Res. (2017) 360:384–9. doi: 10.1016/j.yexcr.2017.09.030
35. Kanamori M, Nakatsukasa H, Okada M, Lu Q, and Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. (2016) 37:803–11. doi: 10.1016/j.it.2016.08.012
36. Tuomela K, Salim K, and Levings MK. Eras of designer Tregs: Harnessing synthetic biology for immune suppression. Immunol Rev. (2023) 320:250–67. doi: 10.1111/imr.13254
37. Cohen JN, Gouirand V, Macon CE, Lowe MM, Boothby IC, Moreau JM, et al. Regulatory T cells in skin mediate immune privilege of the hair follicle stem cell niche. Sci Immunol. (2024) 9:eadh0152. doi: 10.1126/sciimmunol.adh0152
38. Sula Karreci E, Eskandari SK, Dotiwala F, Routray SK, Kurdi AT, Assaker JP, et al. Human regulatory T cells undergo self-inflicted damage. via granzyme pathways upon activation. JCI Insight. (2017) 2:e91599. doi: 10.1172/jci.insight.91599
39. Mok S, Ağaç Çobanoğlu D, Liu H, Mancuso JJ, and Allison JP. Post-immunotherapy CTLA-4 Ig treatment improves antitumor efficacy. Proc Natl Acad Sci U.S.A. (2024) 121:e2404661121. doi: 10.1073/pnas.2404661121
40. Han J, Yin H, He T, He J, Jiang Z, Chen Q, et al. The evolutionary trajectory and prognostic value of GITR+ Tregs reprogramed by tumor-intrinsic PD-1/c-MET signaling in pancreatic cancer. Adv Sci (Weinh). (2025) 12:e00806. doi: 10.1002/advs.202500806
41. Nowicka D, Grywalska E, Surdacka A, Grafka A, and Roliński J. Frequencies of PD-1- and PD-L1- positive T CD3(+)CD4(+), T CD3(+)CD8(+) and B CD19(+) lymphocytes and its correlations with other immune cells in patients with recurrent furunculosis. Microb Pathog. (2019) 126:85–91. doi: 10.1016/j.micpath.2018.10.019
42. Jenabian MA, Seddiki N, Yatim A, Carriere M, Hulin A, Younas M, et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PloS Pathog. (2013) 9:e1003319. doi: 10.1371/journal.ppat.1003319
43. Hakak R, Poopak B, and Majd A. Increased IDO expression and regulatory T cells in acute myeloid leukemia: implications for immune escape and therapeutic targeting. Blood Res. (2024) 59:42. doi: 10.1007/s44313-024-00048-0
44. Chitnis T, Kaskow BJ, Case J, Hanus K, Li Z, Varghese JF, et al. Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects. Front Immunol. (2022) 13:956907. doi: 10.3389/fimmu.2022.956907
45. Vourc'h M, David G, Gaborit B, Broquet A, Jacqueline C, Chaumette T, et al. Pseudomonas aeruginosa Infection Impairs NKG2D-Dependent NK Cell Cytotoxicity through Regulatory T-Cell Activation. Infect Immun. (2020) 88:e00363–20. doi: 10.1128/IAI.00363-20
46. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. (2005) 6:1123–32. doi: 10.1038/ni1254
47. Jia M, Jia X, Zhang D, Liu W, Yi S, Li Z, et al. CD2(+) T-helper 17-like cells differentiated from a CD133(+) subpopulation of non-small cell lung carcinoma cells promote the growth of lung carcinoma. Ann Transl Med. (2021) 9:687. doi: 10.21037/atm-21-980
48. Wang R, Yang L, Zhang C, Wang R, Zhang Z, He Q, et al. Th17 cell-derived IL-17A promoted tumor progression via STAT3/NF-κB/Notch1 signaling in non-small cell lung cancer. Oncoimmunology. (2018) 7:e1461303. doi: 10.1080/2162402X.2018.1461303
49. Yu XY, Zhang ZQ, Huang JC, Lin JY, Cai XP, and Liu CF. IL-7-treated periodontal ligament cells regulate local immune homeostasis by modulating treg/th17 cell polarization. Front Med (Lausanne). (2022) 9:754341. doi: 10.3389/fmed.2022.754341
50. Wu B, Zhang S, Guo Z, Bi Y, Zhou M, Li P, et al. The TGF-β superfamily cytokine Activin-A is induced during autoimmune neuroinflammation and drives pathogenic Th17 cell differentiation. Immunity. (2021) 54:308–323.e306. doi: 10.1016/j.immuni.2020.12.010
51. Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORγt(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. (2022) 610:737–43. doi: 10.1038/s41586-022-05089-y
52. Joerger M, Finn SP, Cuffe S, Byrne AT, and Gray SG. The IL-17-Th1/Th17 pathway: an attractive target for lung cancer therapy? Expert Opin Ther Targets. (2016) 20:1339–56. doi: 10.1080/14728222.2016.1206891
53. Chen G, Zhang PG, Li JS, Duan JJ, Su W, Guo SP, et al. Th17 cell frequency and IL-17A production in peripheral blood of patients with non-small-cell lung cancer. J Int Med Res. (2020) 48:300060520925948. doi: 10.1177/0300060520925948
54. Song ZC, Liu ST, Xia XY, Hu JJ, Leng RX, and Zhao W. In vitro silencing of RIP2 in naive CD4(+) T cells from lupus-prone mice promotes pathogenic Th17 cell differentiation. Clin Rheumatol. (2024) 43:3515–23. doi: 10.1007/s10067-024-07124-x
55. Liu D, Tan Y, Bajinka O, Wang L, and Tang Z. Th17/IL-17 axis regulated by airway microbes get involved in the development of asthma. Curr Allergy Asthma Rep. (2020) 20:11. doi: 10.1007/s11882-020-00903-x
56. Sakaguchi S, Kawakami R, and Mikami N. Treg-based immunotherapy for antigen-specific immune suppression and stable tolerance induction: a perspective. Immunother Adv. (2023) 3:ltad007. doi: 10.1093/immadv/ltad007
57. Gao W, Yang N, Ji S, and Zeng Y. Frequency of CD4(+) regulatory T cells and modulation of CD4(+)T lymphocyte activation in pleural tuberculoma. Tuberculosis (Edinb). (2022) 134:102210. doi: 10.1016/j.tube.2022.102210
58. Monkman J, Moradi A, Yunis J, Ivison G, Mayer A, Ladwa R, et al. Spatial insights into immunotherapy response in non-small cell lung cancer (NSCLC) by multiplexed tissue imaging. J Transl Med. (2024) 22:239. doi: 10.1186/s12967-024-05035-8
59. Ichiyama K, Long J, Kobayashi Y, Horita Y, Kinoshita T, Nakamura Y, et al. Transcription factor Ikzf1 associates with Foxp3 to repress gene expression in Treg cells and limit autoimmunity and anti-tumor immunity. Immunity. (2024) 57:2043–2060.e2010. doi: 10.1016/j.immuni.2024.07.010
60. Huang K, Ren HY, Lin BY, Liu YY, and Guo QF. Protective effects of Wuwei Xiaodu Drink against chronic osteomyelitis through Foxp3(+)CD25(+)CD4(+) Treg cells via the IL-2/STAT5 signaling pathway. Chin J Nat Med. (2022) 20:185–93. doi: 10.1016/S1875-5364(22)60146-8
61. Wang T, Feng W, Ju M, Yu H, Guo Z, Sun X, et al. 27-hydroxycholesterol causes cognitive deficits by disturbing Th17/Treg balance and the related immune responses in mild cognitive impairment patients and C57BL/6J mice. J Neuroinflamm. (2023) 20:305. doi: 10.1186/s12974-023-02986-5
62. Morali K, Giacomello G, Vuono M, and Gregori S. Leveraging current insights on IL-10-producing dendritic cells for developing effective immunotherapeutic approaches. FEBS Lett. (2025) 599:2025–47. doi: 10.1002/1873-3468.15017
63. Upadhaya P, Lamenza FF, Shrestha S, Roth P, Jagadeesha S, Pracha H, et al. Berry extracts and their bioactive compounds mitigate LPS and DNFB-mediated dendritic cell activation and induction of antigen specific T-cell effector responses. Antioxidants (Basel). (2023) 12:1667. doi: 10.3390/antiox12091667
64. Dykema AG, Zhang J, Cheung LS, Connor S, Zhang B, Zeng Z, et al. Lung tumor-infiltrating T(reg) have divergent transcriptional profiles and function linked to checkpoint blockade response. Sci Immunol. (2023) 8:eadg1487. doi: 10.1126/sciimmunol.adg1487
65. Liu Z, Yang Z, Wu J, Zhang W, Sun Y, Zhang C, et al. A single-cell atlas reveals immune heterogeneity in anti-PD-1-treated non-small cell lung cancer. Cell. (2025) 188:3081–3096.e3019. doi: 10.1016/j.cell.2025.03.018
66. Li JF, Niu YY, Xing YL, and Liu F. A novel bispecific c-MET/CTLA-4 antibody targetting lung cancer stem cell-like cells with therapeutic potential in human non-small-cell lung cancer. Biosci Rep. (2019) 39:BSR20171278. doi: 10.1042/BSR20171278
67. Huang SW, Jiang W, Xu S, Zhang Y, Du J, Wang YQ, et al. Systemic longitudinal immune profiling identifies proliferating Treg cells as predictors of immunotherapy benefit: biomarker analysis from the phase 3 CONTINUUM and DIPPER trials. Signal Transduct Target Ther. (2024) 9:285. doi: 10.1038/s41392-024-01988-w
68. Exposito F, Redrado M, Houry M, Hastings K, Molero-Abraham M, Lozano T, et al. PTEN loss confers resistance to anti-PD-1 therapy in non-small cell lung cancer by increasing tumor infiltration of regulatory T cells. Cancer Res. (2023) 83:2513–26. doi: 10.1158/0008-5472.CAN-22-3023
69. Luo Y, Liu G, and Hou P. Synergism effect of dendrobine on cisplatin in treatment of H1299 by modulating the balance of treg/th17. Anticancer Agents Med Chem. (2023) 23:105–12. doi: 10.2174/1871520622666220520093837
70. Fu K, Wang X, Li J, and Wang J. Role of peripheral blood T helper 17 cells in non-small cell lung cancer of different clinical stages, pathological types and differentiation degrees. Clin Lab. (2023) 69:71. doi: 10.7754/Clin.Lab.2022.220116
71. Liao H, Chang X, Gao L, Ye C, Qiao Y, Xie L, et al. IL-17A promotes tumorigenesis and upregulates PD-L1 expression in non-small cell lung cancer. J Transl Med. (2023) 21:828. doi: 10.1186/s12967-023-04365-3
72. Huang Q, Duan L, Qian X, Fan J, Lv Z, Zhang X, et al. IL-17 promotes angiogenic factors IL-6, IL-8, and vegf production via stat1 in lung adenocarcinoma. Sci Rep. (2016) 6:36551. doi: 10.1038/srep36551
73. Moadab A, Valizadeh MR, Nazari A, and Khorramdelazad H. Association of interleukin-17A and chemokine/vascular endothelial growth factor-induced angiogenesis in newly diagnosed patients with bladder cancer. BMC Immunol. (2024) 25:20. doi: 10.1186/s12865-024-00612-4
74. Nakae S, Suto H, Berry GJ, and Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. (2007) 109:3640–8. doi: 10.1182/blood-2006-09-046128
75. Ding FM, Liao RM, Chen YQ, Xie GG, Zhang PY, Shao P, et al. Upregulation of SOCS3 in lung CD4+ T cells in a mouse model of chronic PA lung infection and suppression of Th17−mediated neutrophil recruitment in exogenous SOCS3 transfer. vitro. Mol Med Rep. (2017) 16:778–86. doi: 10.3892/mmr.2017.6630
76. Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, et al. Generation and differentiation of IL-17-producing CD4+ T cells in Malignant pleural effusion. J Immunol. (2010) 185:6348–54. doi: 10.4049/jimmunol.1001728
77. Chang MR, Dharmarajan V, Doebelin C, Garcia-Ordonez RD, Novick SJ, Kuruvilla DS, et al. Synthetic RORγt agonists enhance protective immunity. ACS Chem Biol. (2016) 11:1012–8. doi: 10.1021/acschembio.5b00899
78. Li C, Zhao Y, He C, Wang X, Ren Q, Gai X, et al. IL-17E facilitates cell proliferation and epithelial-mesenchymal transition in A549 NSCLC cells by regulating the NF-κB pathway. Pathol Res Pract. (2025) 266:155792. doi: 10.1016/j.prp.2024.155792
79. Son S, Nam J, Kim AS, Ahn J, Park KS, Phoo MT, et al. Induction of T-helper-17-cell-mediated anti-tumour immunity by pathogen-mimicking polymer nanoparticles. Nat BioMed Eng. (2023) 7:72–84. doi: 10.1038/s41551-022-00973-4
80. Perrone C, Bozzano F, Dal Bello MG, Del Zotto G, Antonini F, Munari E, et al. CD34(+)DNAM-1(bright)CXCR4(+) haemopoietic precursors circulate after chemotherapy, seed lung tissue and generate functional innate-like T cells and NK cells. Front Immunol. (2024) 15:1332781. doi: 10.3389/fimmu.2024.1332781
81. Liu S, Rivero SL, Zhang B, Shen K, Li Z, Niu T, et al. BATF-dependent Th17 cells act through the IL-23R pathway to promote prostate adenocarcinoma initiation and progression. J Natl Cancer Inst. (2024) 116:1598–611. doi: 10.1093/jnci/djae120
82. Voigt C, May P, Gottschlich A, Markota A, Wenk D, Gerlach I, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U.S.A. (2017) 114:12994–9. doi: 10.1073/pnas.1705165114
83. Liu D, Xing S, Wang W, Huang X, Lin H, Chen Y, et al. Prognostic value of serum soluble interleukin-23 receptor and related T-helper 17 cell cytokines in non-small cell lung carcinoma. Cancer Sci. (2020) 111:1093–102. doi: 10.1111/cas.14343
84. Nicola S, Ridolfi I, Rolla G, Filosso P, Giobbe R, Boita M, et al. IL-17 promotes nitric oxide production in non-small-cell lung cancer. J Clin Med. (2021) 10:4572. doi: 10.3390/jcm10194572
85. Komuczki J, Tuzlak S, Friebel E, Hartwig T, Spath S, Rosenstiel P, et al. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1β. Immunity. (2019) 50:1289–1304.e1286. doi: 10.1016/j.immuni.2019.04.006
86. Döring Y. Not growth but death: GM-CSF/IL-23 axis drives atherosclerotic plaque vulnerability by enhancing macrophage and DC apoptosis. Circ Res. (2015) 116:222–4. doi: 10.1161/CIRCRESAHA.114.305674
87. Liu JM, Jin QX, Fujimoto M, Li FF, Jin LB, Yu R, et al. Dihydroartemisinin alleviates imiquimod-induced psoriasis-like skin lesion in mice involving modulation of IL-23/th17 axis. Front Pharmacol. (2021) 12:704481. doi: 10.3389/fphar.2021.704481
88. Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang Y, et al. Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine. (2021) 84:153519. doi: 10.1016/j.phymed.2021.153519
89. Núñez C, Lozada-Requena I, Ysmodes T, Zegarra D, Saldaña F, and Aguilar J. Immunomodulation of Uncaria tomentosa over dendritic cells, il-12 and profile TH1/TH2/TH17 in breast cancer. Rev Peru Med Exp Salud Publica. (2015) 32:643–51.
90. Cascone T, Fradette J, Pradhan M, and Gibbons DL. Tumor immunology and immunotherapy of non-small-cell lung cancer. Cold Spring Harb Perspect Med. (2022) 12:a037895. doi: 10.1101/cshperspect.a037895
91. Zhou H, Zheng Z, Fan C, and Zhou Z. Mechanisms and strategies of immunosenescence effects on non-small cell lung cancer (NSCLC) treatment: A comprehensive analysis and future directions. Semin Cancer Biol. (2025) 109:44–66. doi: 10.1016/j.semcancer.2025.01.001
92. De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. (2016) 45:1135–47. doi: 10.1016/j.immuni.2016.10.021
93. Liu W, Wei X, Li L, Wu X, Yan J, Yang H, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem Biophys Res Commun. (2017) 488:196–203. doi: 10.1016/j.bbrc.2017.05.034
94. Heim L, Yang Z, Tausche P, Hohenberger K, Chiriac MT, Koelle J, et al. IL-9 producing tumor-infiltrating lymphocytes and treg subsets drive immune escape of tumor cells in non-small cell lung cancer. Front Immunol. (2022) 13:859738. doi: 10.3389/fimmu.2022.859738
95. Zhang CY, Qi Y, Li XN, Yang Y, Liu DL, Zhao J, et al. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. BioMed Pharmacother. (2015) 69:242–8. doi: 10.1016/j.biopha.2014.12.008
96. Chao JL and Savage PA. Unlocking the complexities of tumor-associated regulatory T cells. J Immunol. (2018) 200:415–21. doi: 10.4049/jimmunol.1701188
97. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2
98. Zhang C, Zhou L, Li S, Zhao J, Meng X, Ma L, et al. Obesity accelerates immune evasion of non-small cell lung carcinoma via TFEB-dependent upregulation of Siglec-15 and glycolytic reprogramming. Cancer Lett. (2022) 550:215918. doi: 10.1016/j.canlet.2022.215918
99. Shi Z, Pu W, Li M, Aihemaitijiang M, Li S, Zhang X, et al. Prostate cancer cell-derived exosomes ZNF667-AS1 reduces TGFBR1 mRNA stability to inhibit Treg expansion and DTX resistance by binding to U2AF1. Mol Med. (2024) 30:179. doi: 10.1186/s10020-024-00947-z
100. Olejarz W, Dominiak A, Żołnierzak A, Kubiak-Tomaszewska G, and Lorenc T. Tumor-Derived exosomes in immunosuppression and immunotherapy. J Immunol Res. (2020) 2020:6272498. doi: 10.1155/2020/6272498
101. Shiri AM, Fard-Aghaie M, Bedke T, Papazoglou ED, Sabihi M, Zazara DE, et al. Foxp3 + Treg-derived IL-10 promotes colorectal cancer-derived lung metastasis. Sci Rep. (2024) 14:30483. doi: 10.1038/s41598-024-80437-8
102. Huang Q, Tian N, Zhang J, Song S, Cheng H, Liu X, et al. Nonclassical action of Ku70 promotes Treg-suppressive function through a FOXP3-dependent mechanism in lung adenocarcinoma. J Clin Invest. (2024) 134:e178079. doi: 10.1172/JCI178079
103. Tuomela K, Garcia RV, Boardman DA, Tavakoli P, Ancheta-Schmit M, Sham HP, et al. TYK2 inhibition enhances Treg differentiation and function while preventing Th1 and Th17 differentiation. Cell Rep Med. (2025) 16:102303. doi: 10.1016/j.xcrm.2025.102303
104. Kewang L, Wei Y, Meiye L, Tianyong H, and Baohui C. The inhibitory effects of modified HSJZ decoction on NSCLC by regulating regulatory T cells via downregulation of EZH2 and PI3K/AKT pathway. J Ethnopharmacol. (2025) 348:119802. doi: 10.1016/j.jep.2025.119802
105. Sun CC, Zhu W, Li SJ, Hu W, Zhang J, Zhuo Y, et al. FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway. Genome Med. (2020) 12:77. doi: 10.1186/s13073-020-00773-y
106. Li H. Melittin inactivates YAP/HIF-1α pathway via up-regulation of LATS2 to inhibit hypoxia-induced proliferation, glycolysis and angiogenesis in NSCLC. Clinics (Sao Paulo). (2024) 79:100407. doi: 10.1016/j.clinsp.2024.100407
107. Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. (2018) 93:814–25. doi: 10.1016/j.kint.2017.08.030
108. Prasetya RA, Metselaar-Albers M, and Engels F. Concomitant use of analgesics and immune checkpoint inhibitors in non-small cell lung cancer: A pharmacodynamics perspective. Eur J Pharmacol. (2021) 906:174284. doi: 10.1016/j.ejphar.2021.174284
109. Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8(+) T cell antitumor immunity. Immunity. (2023) 56:1613–1630.e1615. doi: 10.1016/j.immuni.2023.06.003
110. Torres-Mejia E, Weng S, Whittaker CA, Nguyen KB, Duong E, Yim L, et al. Lung cancer-intrinsic SOX2 expression mediates resistance to checkpoint blockade therapy by inducing treg-dependent CD8+ T-cell exclusion. Cancer Immunol Res. (2025) 13:496–516. doi: 10.1158/2326-6066.CIR-24-0184
111. Karlicic V, Vukovic J, Stanojevic I, Sotirovic J, Peric A, Jovic M, et al. Association of locally produced IL10 and TGFb1 with tumor size, histological type and presence of metastases in patients with lung carcinoma. J buon. (2016) 21:1210–8.
112. Chen C, Chen Z, Chen D, Zhang B, Wang Z, and Le H. Suppressive effects of gemcitabine plus cisplatin chemotherapy on regulatory T cells in nonsmall-cell lung cancer. J Int Med Res. (2015) 43:180–7. doi: 10.1177/0300060514561504
113. Pal K, Hussain T, Xie H, Li S, Yang P, Mansfield A, et al. Expression, correlation, and prognostic significance of different nicotinic acetylcholine receptors, programed death ligand 1, and dopamine receptor D2 in lung adenocarcinoma. Front Oncol. (2022) 12:959500. doi: 10.3389/fonc.2022.959500
114. Belli S, Amann M, Hutchinson L, Pousse L, Abdolzade-Bavil A, Justies N, et al. Optimizing early clinical investigations in cancer immunotherapy: the translational journey of RG6292, a novel, selective treg-depleting antibody. Clin Pharmacol Ther. (2024) 116:834–46. doi: 10.1002/cpt.3303
115. Thibodeaux SR, Barnett BB, Pandeswara S, Wall SR, Hurez V, Dao V, et al. IFNα Augments clinical efficacy of regulatory T-cell depletion with denileukin diftitox in ovarian cancer. Clin Cancer Res. (2021) 27:3661–73. doi: 10.1158/1078-0432.CCR-20-4594
116. Sahin E and Sahin M. Epigenetical targeting of the FOXP3 gene by S-adenosylmethionine diminishes the suppressive capacity of regulatory T cells ex vivo and alters the expression profiles. J Immunother. (2019) 42:11–22. doi: 10.1097/CJI.0000000000000247
117. Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. (2020) 10:5225–41. doi: 10.7150/thno.43716
118. Rice SJ, Liu X, Zhang J, Jia B, Zheng H, and Belani CP. Advanced NSCLC patients with high IL-6 levels have altered peripheral T cell population and signaling. Lung Cancer. (2019) 131:58–61. doi: 10.1016/j.lungcan.2019.03.014
119. Hanna BS, Wang G, Galván-Peña S, Mann AO, Ramirez RN, Muñoz-Rojas AR, et al. The gut microbiota promotes distal tissue regeneration via RORγ(+) regulatory T cell emissaries. Immunity. (2023) 56:829–846.e828. doi: 10.1016/j.immuni.2023.01.033
120. Paz Del Socorro T, Oka K, Boulard O, Takahashi M, Poulin LF, Hayashi A, et al. The biotherapeutic Clostridium butyricum MIYAIRI 588 strain potentiates enterotropism of Rorγt(+)Treg and PD-1 blockade efficacy. Gut Microbes. (2024) 16:2315631. doi: 10.1080/19490976.2024.2315631
121. Ghadiri N, Emamnia N, Ganjalikhani-Hakemi M, Ghaedi K, Etemadifar M, Salehi M, et al. Analysis of the expression of mir-34a, mir-199a, mir-30c and mir-19a in peripheral blood CD4+T lymphocytes of relapsing-remitting multiple sclerosis patients. Gene. (2018) 659:109–17. doi: 10.1016/j.gene.2018.03.035
122. Zhao L, Yang J, Wang HP, and Liu RY. Imbalance in the Th17/Treg and cytokine environment in peripheral blood of patients with adenocarcinoma and squamous cell carcinoma. Med Oncol. (2013) 30:461. doi: 10.1007/s12032-013-0461-7
123. Lin W, Zhang HL, Niu ZY, Wang Z, Kong Y, Yang XS, et al. The disease stage-associated imbalance of Th1/Th2 and Th17/Treg in uterine cervical cancer patients and their recovery with the reduction of tumor burden. BMC Womens Health. (2020) 20:126. doi: 10.1186/s12905-020-00972-0
124. Chen W. TGF-β Regulation of T cells. Annu Rev Immunol. (2023) 41:483–512. doi: 10.1146/annurev-immunol-101921-045939
125. Gao Y, Wang Y, Chauss D, Villarino AV, Link VM, Nagashima H, et al. Transcription factor EGR2 controls homing and pathogenicity of T(H)17 cells in the central nervous system. Nat Immunol. (2023) 24:1331–44. doi: 10.1038/s41590-023-01553-7
Keywords: NSCLC, regulatory T cells, Th17 cells, immunotherapy, tumor immune microenvironment, immune plasticity
Citation: Zhu S, Zhou N, Li Q and Liu X (2025) Rewiring immune suppression in NSCLC: Roles and plasticity of Tregs and Th17 cells. Front. Immunol. 16:1658848. doi: 10.3389/fimmu.2025.1658848
Received: 03 July 2025; Accepted: 30 September 2025;
Published: 16 October 2025.
Edited by:
Raquel Alarcon Rodriguez, University of Almeria, SpainReviewed by:
Xiaolong Tian, Yale University, United StatesCopyright © 2025 Zhu, Zhou, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxing Liu, MTU2NjUxNjkxNzdAMTYzLmNvbQ==
 Shasha Zhu1,2
Shasha Zhu1,2 Xiaoxing Liu
Xiaoxing Liu