- 1Department of Nephro-Urology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
- 2Department of Clinical Pharmaceutics, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
- 3Department of Urology, Kainan Hospital, Yatomi, Japan
- 4Department of Urology, Anjo Kosei Hospital, Anjo, Japan
- 5Department of Urology, Toyota Kosei Hospital, Toyota, Japan
- 6Department of Urology, Konan Kosei Hospital, Konan, Japan
Introduction: Immune checkpoint inhibitor (ICI)-based combination therapy has revolutionized first-line treatment outcomes for metastatic renal cell carcinoma (mRCC). In this study, we aimed to retrospectively analyze real-world clinical outcomes and toxicities of first-line ICI-based combination therapies, specifically nivolumab plus ipilimumab (IO+IO) and ICIs plus tyrosine kinase inhibitors (IO+TKI), in Japanese patients with mRCC aged ≥ 75 years compared with non-older adult patients.
Methods: We retrospectively enrolled 156 patients with mRCC who received first-line IO+IO or IO+TKI between September 2018 and June 2024 at eight Japanese institutions. Patients were categorized into an older adult group (≥ 75 years, n=49) and a non-older adult group (< 75 years, n=107). We evaluated objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs).
Results: The overall ORR (47% vs. 59%, p=0.43) and DCR (86% vs. 83%, p=0.65) were comparable between groups. No significant differences were observed in PFS (median: 15.5 vs. 17.0 months, p=0.78) or OS (NA vs. 52.2 months, p=0.61). In the IO+IO regimen, the ORR, DCR, PFS, OS, and AE rates were comparable across age groups. However, in the IO+TKI regimen, the ORR was significantly lower in older adults (55% vs. 81%, p=0.04), and treatment discontinuation due to AEs was significantly higher in older adults (60% vs. 32%, p=0.02), with a shorter time to discontinuation despite no difference in the initial TKI dose and RDI. The non-older adult group showed significantly better PFS with IO+TKI compared with IO+IO (hazard ratio: 2.37, p=0.02). In contrast, in the older adult group, PFS and OS were approximately equivalent between the two regimens.
Conclusion: Our real-world data indicated that ICI-based combination therapies are effective in patients with mRCC aged ≥75 years, with outcomes largely non-inferior to non-older adult patients. However, the comparable efficacy of IO+TKI and IO+IO in the older adult group, which may differs from that in the non-older adult group, highlights the importance of understanding the distinct characteristics of each regimen for individualized treatment selection and careful management, particularly regarding AE monitoring and dose adjustment in older adult patients receiving IO+TKI.
1 Introduction
Globally, population aging is progressing rapidly, with a proportion of individuals ≥ 65 years old expected to nearly double by 2050 (1). For example, in Japan, 29.3% of the population will be ≥ 65 years old, and 16.8% will be ≥ 75 years old by 2024 (2), making it one of the most aged societies in the world. The Japanese Cancer Registry estimated that approximately 29,000 new cases of renal cancer were diagnosed in 2020, with renal cell carcinoma (RCC) accounting for most. The proportion of patients ≥ 75 years old is 44.4% (3). Therefore, carefully considering treatment options for older adult patients with RCC is essential.
Immune checkpoint inhibitor (ICI)-based combination therapy has revolutionized first-line treatment outcomes by improving the survival and response rates of patients with metastatic RCC (mRCC) (4, 5). The CheckMate 214 trial demonstrated that nivolumab and ipilimumab combination therapy (IO+IO) showed superior efficacy compared with sunitinib (6, 7). Furthermore, the survival benefits of first-line ICI plus tyrosine kinase inhibitor (IO+TKI) therapies have been widely reported, and several regimens have been established as treatment options (8–11). However, the safety and efficacy of ICI-based combination therapies in older adult patients remain controversial. The proportion of older adult patients enrolled in clinical trials is relatively low, which limits the comprehensive evaluation of treatment safety and efficacy. This is primarily due to older adult patients often meeting the exclusion criteria, including multiple primary cancers, impaired renal function, or reduced cardiac function. For example, patients aged ≥ 70 years accounted for only 17.4% of the total population in the CheckMate 214 trial (6), and in the KEYNOTE-426 trial, patients aged ≥ 65 years comprised approximately 40% of the total population (9). There have been reports that the average age of patients with RCC in real-world settings is 6.49 years older than those included in clinical trials (12), indicating a discrepancy between real-world and clinical trial data regarding patient age. Furthermore, immune aging is characterized by impaired T cell function and altered inflammatory environments, potentially causing reduced ICI effectiveness (13, 14). However, it is unclear whether these treatment strategies offer comparable efficacy and safety in older adult and non-older adult patients.
Therefore, evaluating the treatment outcomes in older adult patients and comparing them with those of non-older adult patients is crucial. In this study, we aimed to retrospectively analyze the clinical outcomes and toxicities of IO+IO and IO+TKI as first-line combination therapies in Japanese patients with mRCC aged ≥ 75 years.
2 Materials and methods
2.1 Patients and methods
The Ethics Review Board of the Nagoya City University Graduate School of Medical Sciences approved this study (Approval Number: 60-19-0196), and it was conducted in accordance with the guidelines of the Declaration of Helsinki. In this retrospective study, we enrolled 156 patients diagnosed with mRCC who received first-line IO+IO or IO+TKI therapy between September 2018 and June 2024 at Nagoya City University Hospital and seven affiliated institutions. We classified patients aged ≥ 75 years as the older adult group and those aged < 75 years as the non-older adult group using a previous report showing that the global median age at diagnosis of kidney cancer is 75 years (15). RCC diagnosis was confirmed by experienced pathologists through histological analysis. The choice of ICI-based combination therapy was made after discussion between the attending physician and other urologists, and after considering patient characteristics using the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk classification. All favorable-risk patients were assigned to IO+TKI therapy. Informed consent was obtained from all patients, and treatment regimens were determined with their full agreement. Baseline and on-treatment assessments included medical history, demographic and physical examinations, Karnofsky Performance Status (KPS), age-unadjusted Charlson Comorbidity Index (CCI) (16), and blood and urine tests, all conducted at the discretion of the attending physician. Treatment response after initiating ICI-based combination therapy was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (17) at physician-scheduled intervals. Toxicity was graded using the Common Terminology Criteria for Adverse Events version 5.0. Adverse events (AEs) occurring within the first two months after treatment initiation were specifically used in the analysis. Blood test values were obtained on the day before or the day of treatment initiation. The dosage and duration of TKI administration were assessed in all the patients undergoing IO+TKI treatment. Relative dose intensity (RDI) was calculated as the ratio of the actual administered dose to the planned maximum dose from treatment initiation to discontinuation.
2.2 Statistical analysis
The chi-square test or Fisher’s exact test (for stratification factors) and the Mann–Whitney U test (for continuous variables) were used to compare patient characteristics. In addition, the interquartile range (IQR) was used to report continuous variables. progression-free survival (PFS) and overall survival (OS) were stratified using the Kaplan–Meier method and analyzed using the log-rank test. The Cox proportional hazards model was used to analyze the hazard ratios (HR) and 95% confidence intervals (CI). All reported p-values were two-sided, with statistical significance set at p < 0.05. Statistical analyses were performed using the EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (18). Patients with favorable IMDC risk were excluded to ensure balanced baseline conditions and to compare the treatment outcomes between the IO+IO and IO+TKI groups.
3 Results
3.1 Patient characteristics
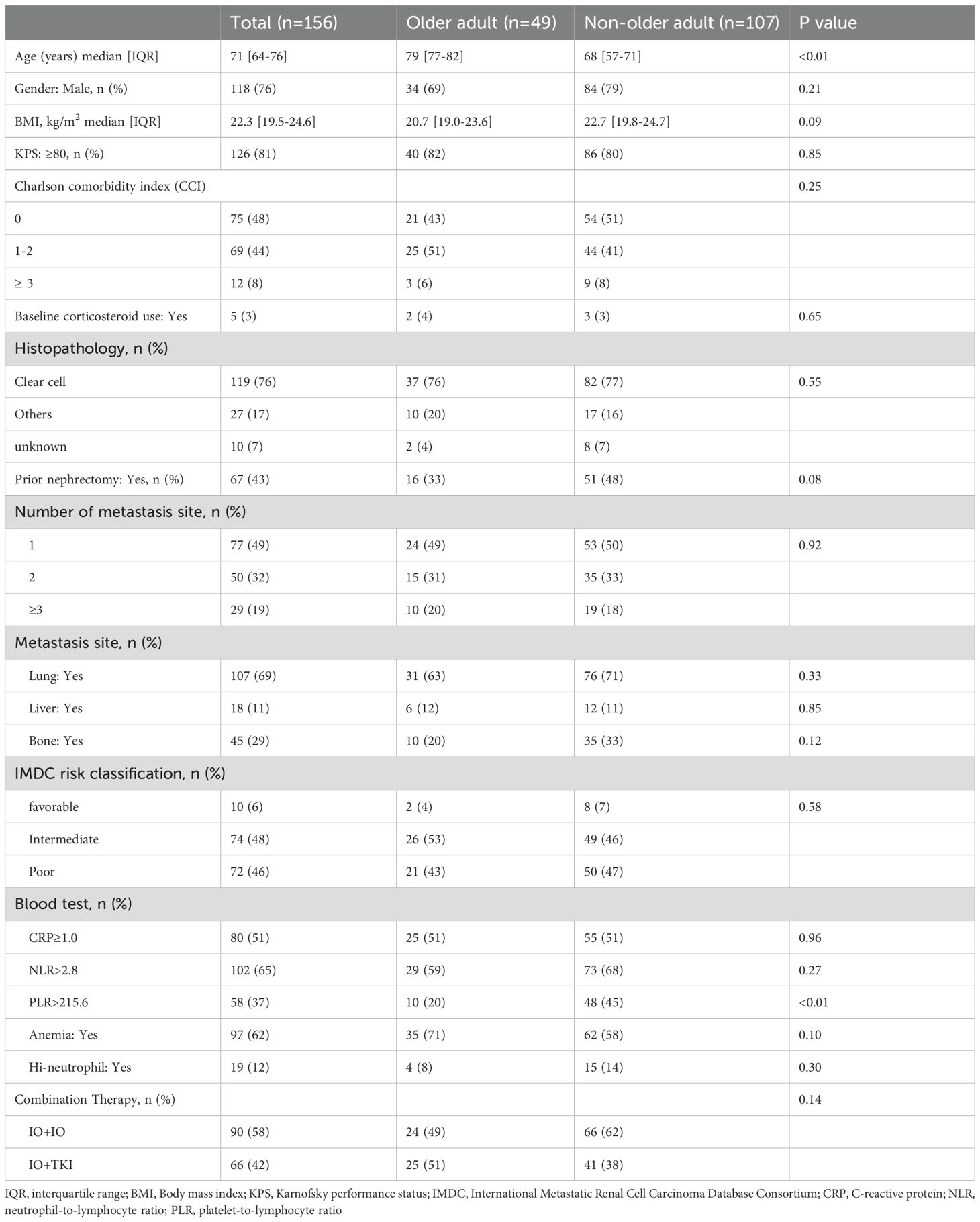
Table 1 presents the baseline characteristics of patients before the first course of ICI-based combination therapy, categorized from the total (n = 156) into the older adult group (n = 49, 31.4%) and the non-older adult group (n = 107, 68.6%). The median follow-up period following initiation of the ICI-based combination therapy was 13.9 and 25.9 months (IQR: 6.5–32.2 and 8.1–34.3 months, respectively) in the older adult and non-older adult groups (p=0.19). In the older adult group, 24 patients (49%) received IO+IO, and 25 patients (51%) received IO+TKI. In the non-older adult group, 66 and 41 patients (62 and 38%) received IO+IO and IO+TKI, respectively. There was no significant difference between the groups (p=0.14). Details of each IO+TKI regimen are provided in Supplementary Table S1.
3.2 Antitumor efficacy
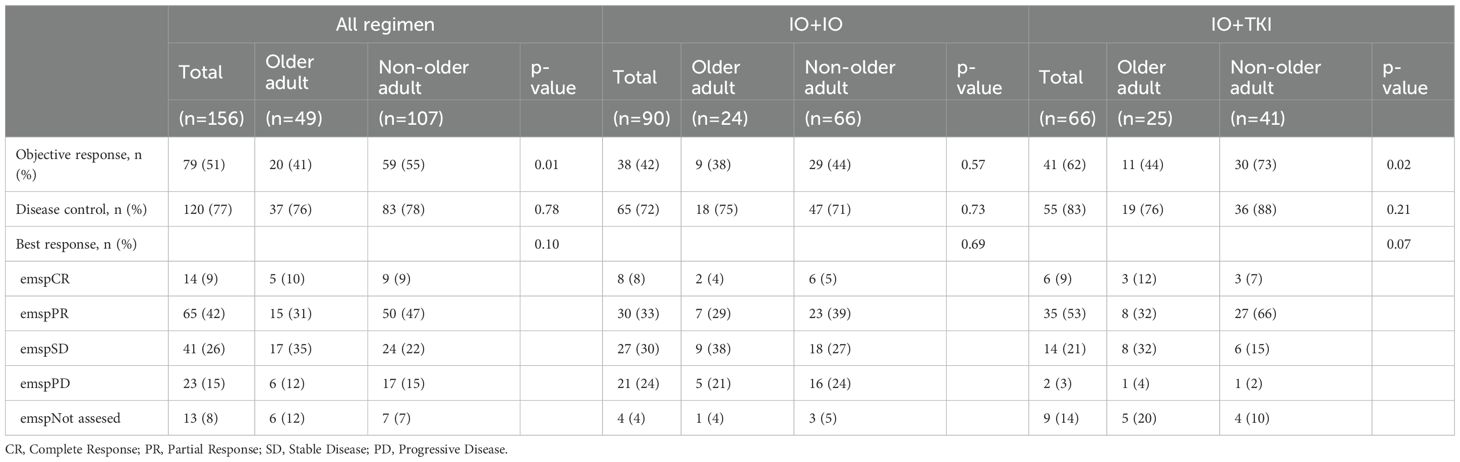
Table 2 presents the objective response rate (ORR), disease control rate (DCR), and best treatment response for each group. The ORR and DCR were comparable between both groups (ORR: 47% vs. 59%, p=0.43; DCR: 86% vs. 83%, p=0.65). The best responses were not significantly different between the two groups (p=0.10).
We compared IO+IO and IO+TKI separately and analyzed the data. In the IO+IO regimen, ORR (39% vs. 46%, p=0.57) and DCR (76% vs. 78%, p=0.73) did not differ significantly between the two regimens. In contrast, the ORR (55% vs. 81%, p=0.04) in the IO+TKI regimen was significantly lower in the older adult group. In the older adult group, the response rates of IO+IO and IO+TKI were comparable (ORR: 39% vs. 55%, p=0.30; DCR: 78% vs. 95%, p=0.11).
No significant differences in PFS (median: 15.5 vs. 17.0 months; HR: 1.07; 95% CI: 0.66–1.75, p=0.78, Figure 1A) and OS (Not reached vs. 52.2 months; HR: 1.17; 95% CI: 0.64–2.16, p=0.61, Figure 1B) were observed between the older adult and the non-older adult groups. The comparison of the two groups, excluding favorable-risk patients, is shown in Supplementary Figure S1 which also presents no significant differences between two groups. PFS and OS were comparable between the two groups when stratified by the treatment regimen (Supplementary Figure S2). Figure 2 presents the results of the subgroup analysis. In the older adult group, there was no significant difference in PFS (HR: 1.49; 95% CI: 0.61–3.64, p=0.38, Figure 2A) or OS (HR: 1.14; 95% CI: 0.41–3.16, p=0.65, Figure 2B) between the IO+IO and IO+TKI regimens. In contrast, in the subgroup analysis of the non-older adult group, PFS was significantly better in the IO+TKI regimen (HR: 2.37; 95% CI: 1.11–5.10, p=0.03, Figure 2C); however, no significant difference was observed in OS (HR: 1.23; 95% CI: 0.52–2.90, p=0.65, Figure 2D).
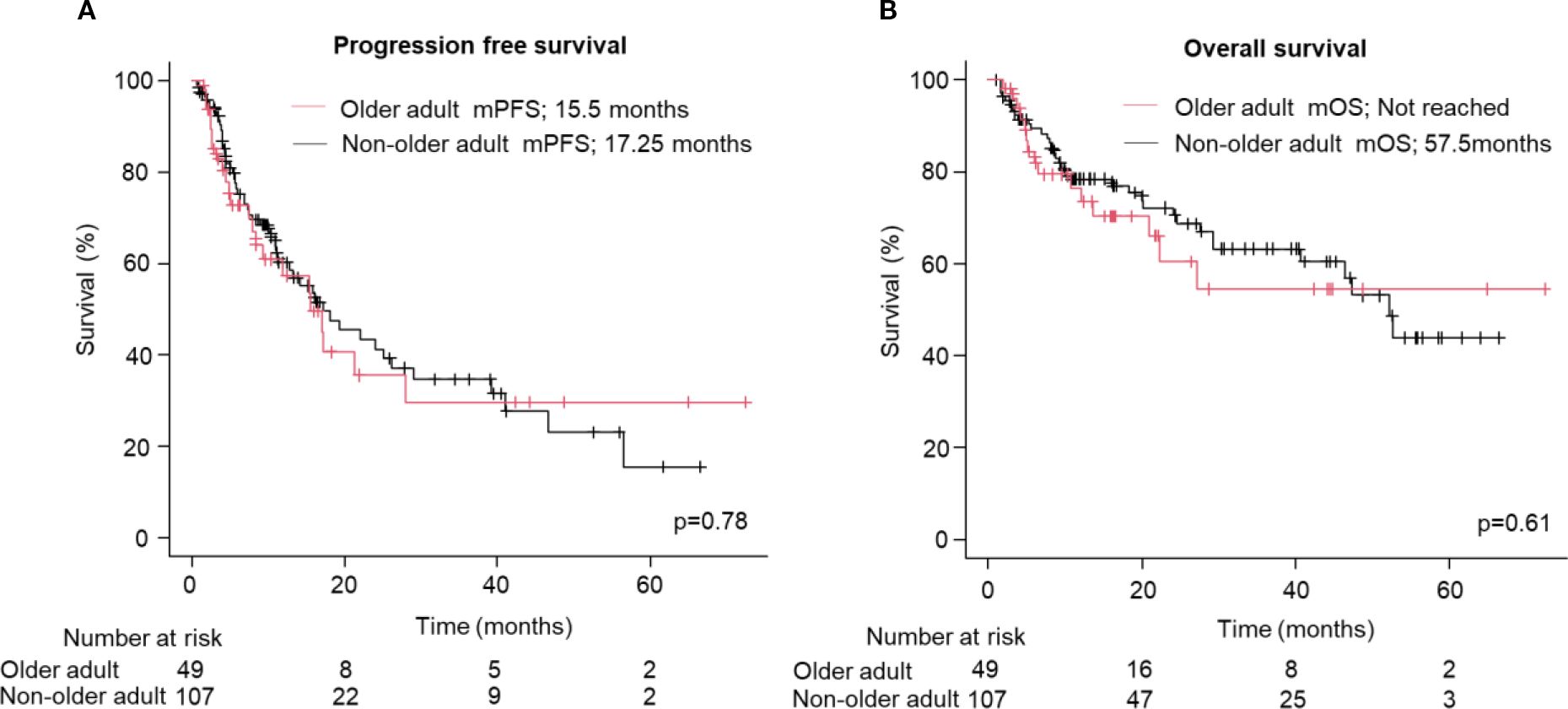
Figure 1. Progression free and overall survival following treatment with ICI-based combination therapy. (A, B) Kaplan-Meier survival curves for (A) progression free survival (Older adult group: n = 49; Non-older adult group: n = 107) and (B) overall survival (Older adult group: n = 49; Non-older adult group: n = 107) in patients. (A, B) Log-rank test. mPFS, median progression free survival; mOS, median overall survival.
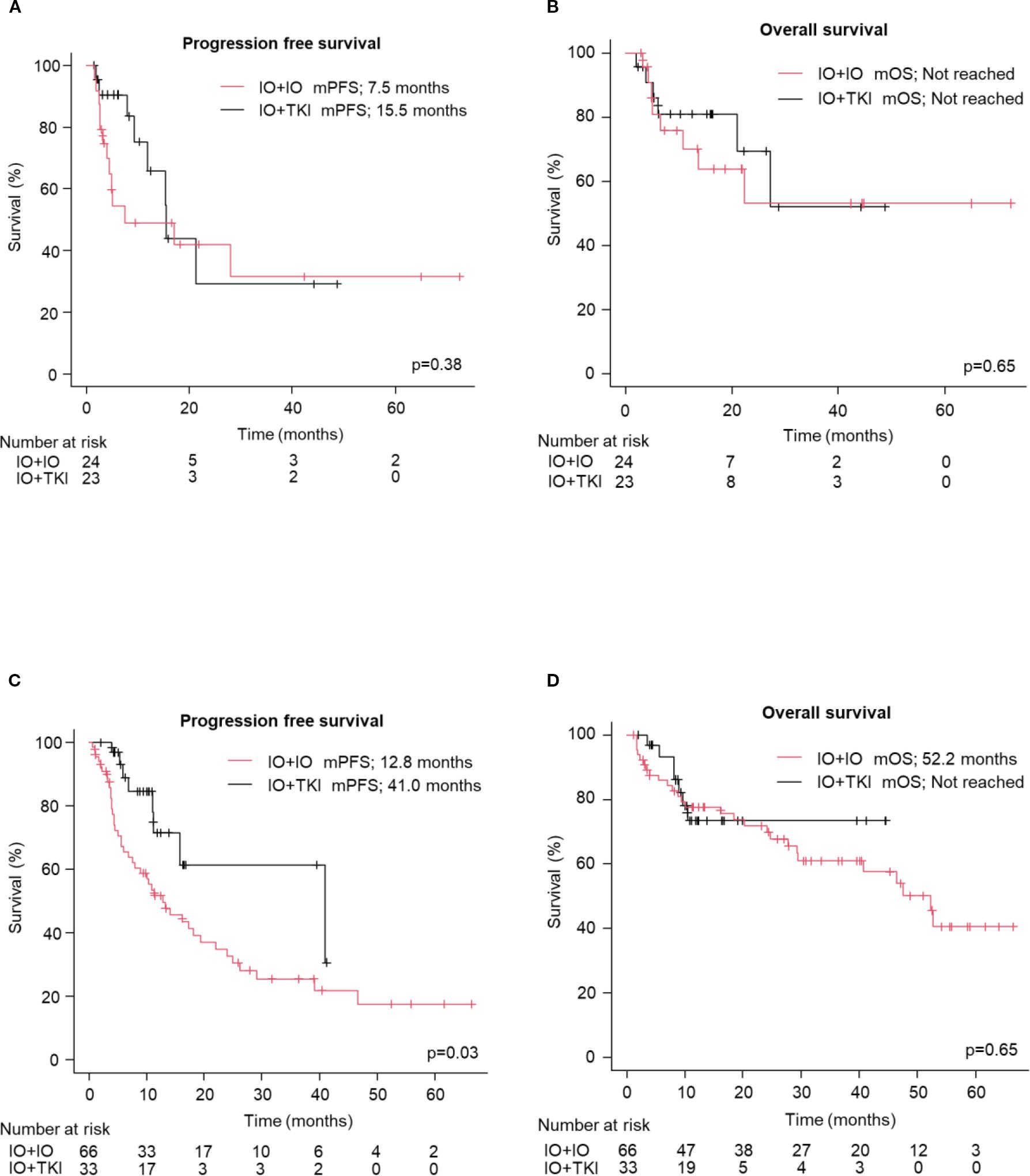
Figure 2. Progression free and overall survival following treatment with ICI-based combination therapy (except for IMDC: favorable). (A, B) Kaplan-Meier survival curves in the older adult group for (A) progression free survival (IO+IO: n = 24; IO+TKI: n = 23) and (B) overall survival (IO+IO: n = 24; IO+TKI: n = 23) in patients. (C, D) Kaplan-Meier survival curves in the Non-older adult group for (C) progression free survival (IO+IO: n = 66; IO+TKI: n = 33) and (D) overall survival (IO+IO: n = 66; IO+TKI: n = 33) in patients. (A–D) Log-rank test. mPFS, median progression free survival; mOS, median overall survival; ICI, immune checkpoint inhibitors; TKI, tyrosine kinase.
3.3 Adverse events
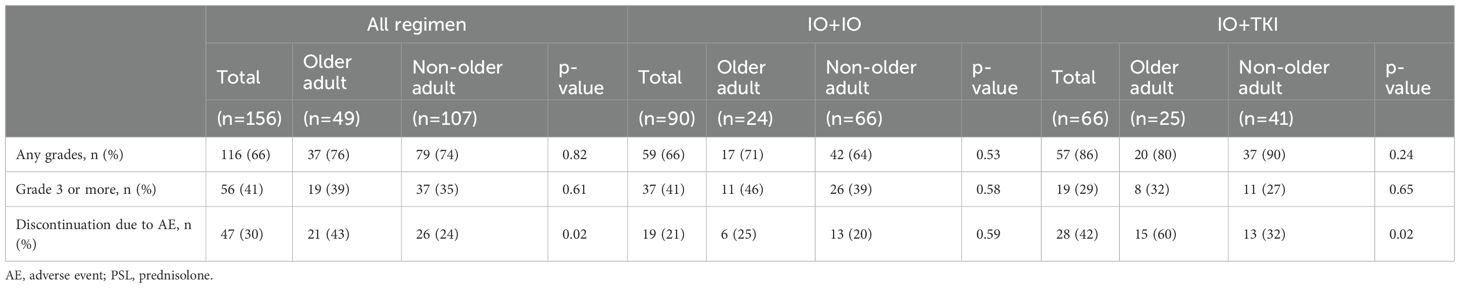
Table 3 shows the frequencies of AEs. AEs occurred in 76% and 74% of the older adult and non-older adult groups, respectively (p=0.82). Grade ≥ 3 AEs occurred in 39% vs. 35%. (p=0.61). No significant differences were observed in treatment discontinuation due to AEs (25% vs. 20%, p=0.59) or steroid administration (29 vs. 30%, p=0.92) between the older adult and non-older adult groups in the IO+IO regimen. In contrast, older adult patients in the IO+TKI regimen had significantly higher treatment discontinuation rates owing to AEs (60% vs. 32%, p=0.02). Figure 3 presents a comparison between the initial TKI dose and RDI, the duration of IO+TKI administration. No significant difference was observed in the initial TKI dose (p=0.13, Figure 3A) and RDI (p=0.92, Figure 3B) between the two groups. However, the time from initiating the first therapy to discontinuation due to AEs was significantly shorter in the older adult group (median: 10.4 vs. 68.9 months; HR: 2.53; 95% CI: 1.18–5.43, p=0.78, Figure 3C). Among the 15 older adult patients who discontinued treatment, 12 (80%) experienced multiple AEs. The detailed AEs profiles are summarized in Supplementary Table S2.
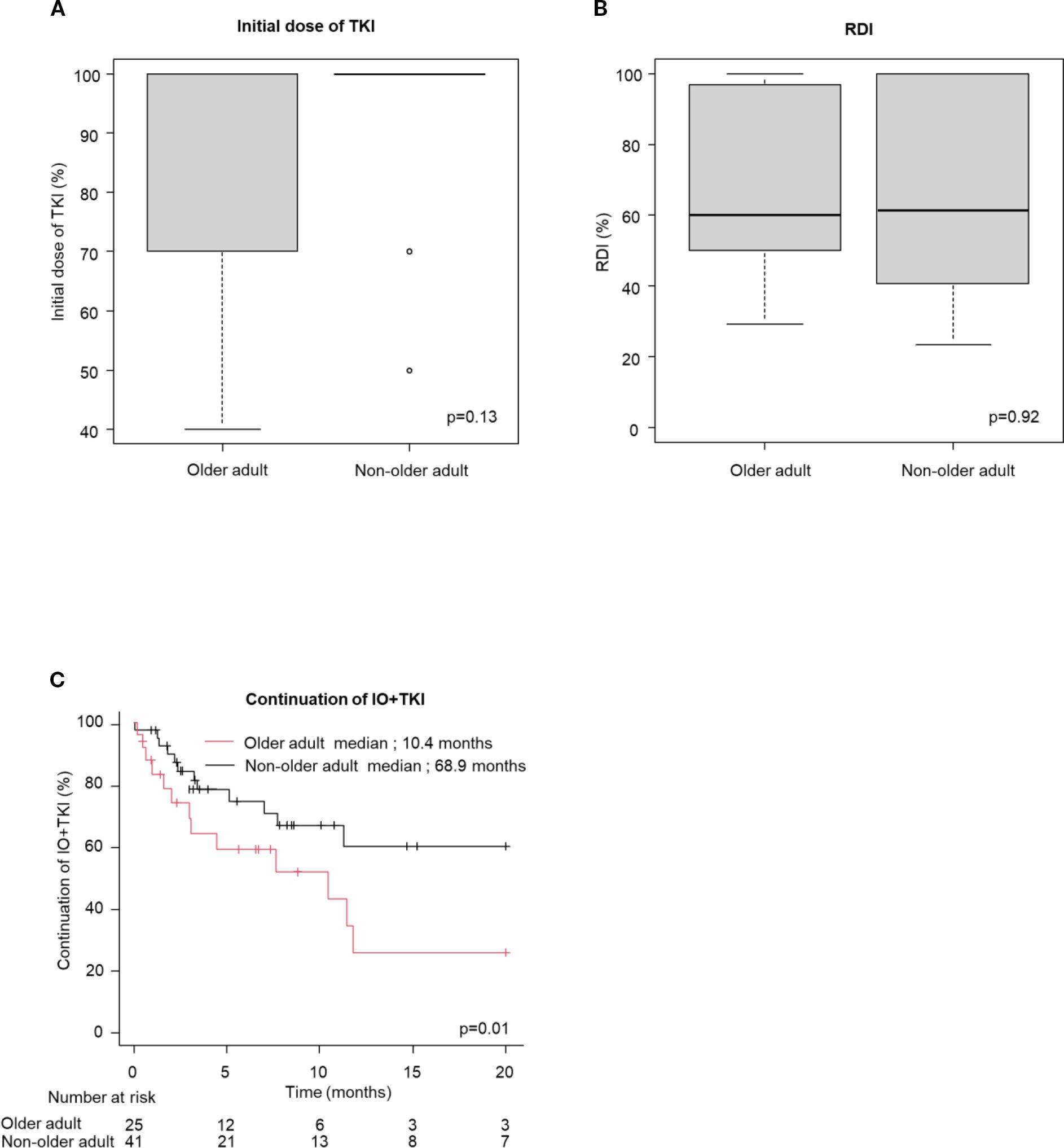
Figure 3. Initial dose of TKI and RDI, the time from initiating the first therapy to discontinuation owing to AEs (continuation of IO+TKI) (A, B) box plot for (A) initial dose of TKI (Older adult group: n = 22; Non-older adult group: n = 34) and (B) RDI (Older adult group: n = 25; Non-older adult group: n = 41), (C) Kaplan-Meier survival curves for continuation of IO+TKI (older adult group: n = 25; Non-older adult group: n = 41) in IO+TKI. (A, B) Mann-Whitney U test. (C) Log-rank test.
We analyzed the association between early AEs, occurring within the first two months of treatment, and OS for each regimen. The presence of early immune-related AEs (irAEs) or AEs was associated with longer OS in the IO+IO regimen (HR: 0.41, 95% CI: 0.20–0.81, p=0.01, Supplementary Figure S3A), but no significant association was found in the IO+TKI regimen (HR: 0.64, 95% CI: 0.24-1.72, p=0.38, Supplementary Figure S3B). A similar trend was observed in older patients, although the association did not reach statistical significance in either the IO+IO (HR: 0.39, 95% CI: 0.09–1.67, p=0.19, Figure 4A) or IO+TKI groups (HR: 0.52, 95% CI: 0.13–2.10, p=0.36, Figure 4B).
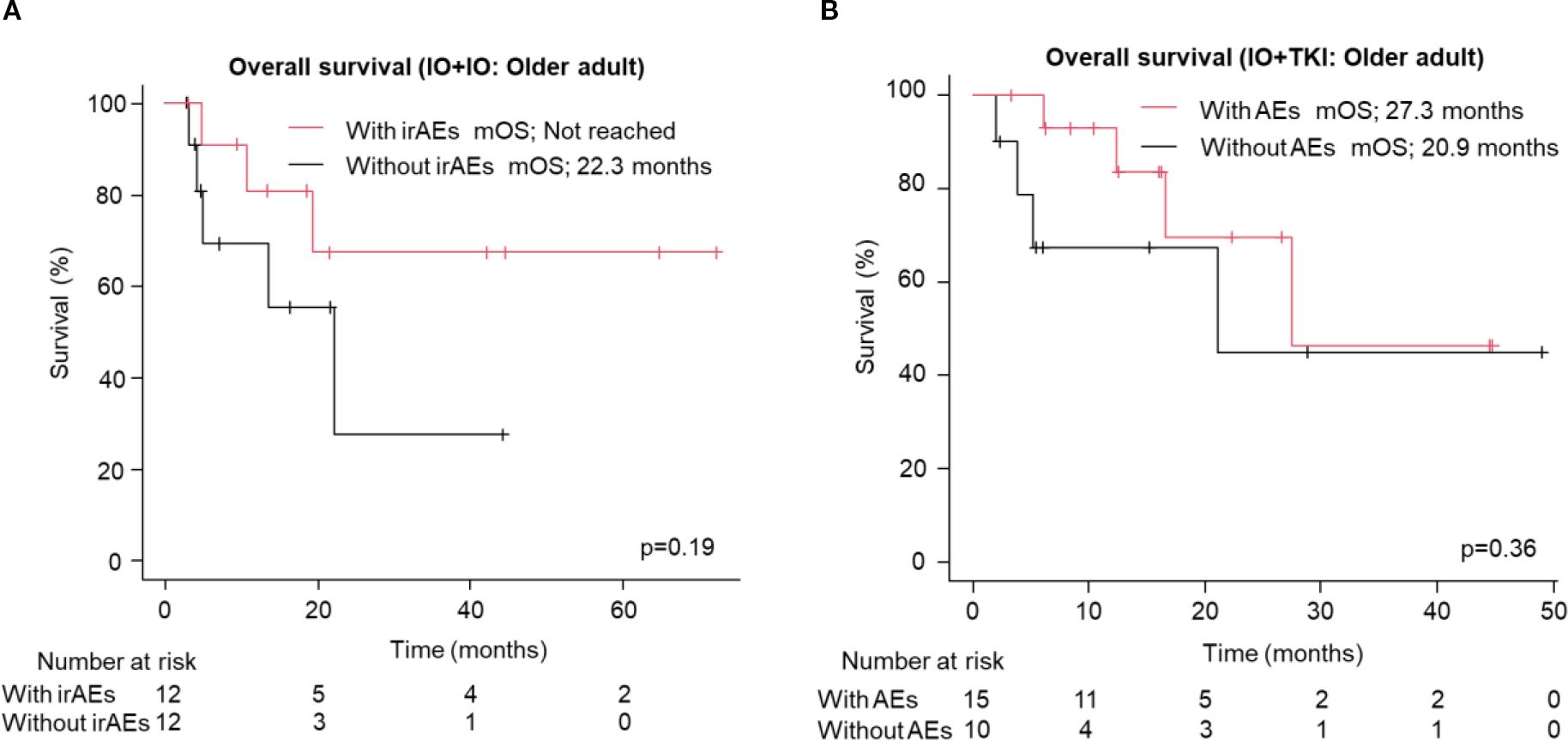
Figure 4. Overall survival in the older adult group following treatment with ICI-based combination therapies. (A, B) Kaplan-Meier survival curves for (A) IO+IO (With irAEs: N = 12; Without irAEs: N = 12) and (B) IO+TKI (With AEs: N = 15; Without AEs: N = 10) in patients. AEs, adverse events.
4 Discussion
In this study, we revealed no significant differences in the PFS, OS, or AEs frequency between the older adult and non-older adult groups. Our findings indicated that ICI-based combination therapies, when managed appropriately, can be effective in older adult patients with mRCC. We propose that treatment decisions should not be based solely on chronological age; rather, the characteristics of each regimen should be considered.
Focusing on IO+IO, we found similar response rates (ORR and DCR) across both age groups. Crucially, the incidence and severity of irAEs, alongside the rates of treatment discontinuation owing to irAEs, were comparable between the older adults and non-older adult groups.
These real-world observations regarding IO+IO in older adults are consistent with a growing body of evidence. The CheckMate 214 trial’s age-stratified analysis of OS did not show the superiority of IO+IO over sunitinib in older adult patients definitively (6); however, its conditional OS was favorable even in this group, suggesting its contribution to long-term outcomes (7). Furthermore, real-world data from the IMDC stated that survival benefits from ICI-based combination therapies are maintained even in patients aged ≥ 70 years, emphasizing that only chronological age should not dictate treatment selection (19). In addition, several studies have demonstrated non-inferior IO+IO treatment outcomes in older adult patients compared with younger patients, reinforcing our findings (20–23).
In the IO+TKI cohort, the older adult group showed a lower ORR trend than the non-older adult group. Nevertheless, the DOR was nearly identical, and no significant differences were observed in OS or PFS. The overall incidence of AEs was similar between groups; however, treatment discontinuation due to AEs was significantly higher. Moreover, the time to discontinuation was shorter in older adults, despite no differences in the initial TKI dose or RDI. These findings suggest that, in the IO+TKI regimen, older adults may discontinue treatment earlier due to AEs even when receiving the same initial TKI dose as non-older adults, despite experiencing a similar overall frequency of AEs. Therefore, a greater reduction in the TKI dose than in non-older adults may help maintain treatment in older adults. Several subgroup analyses have been conducted in clinical trials on IO+TKI therapy. Tomita et al. suggested that patients > 75 years old who underwent avelumab plus axitinib treatment would have a similar survival benefit as those aged 65–74 years (24). Varkaris et al. reported that treatment comprising pembrolizumab and axitinib prolonged PFS and OS compared with sunitinib even in patients aged ≥ 65 years (25). While there are a few real-world reports, Iinuma et al. reported that the median PFS for patients aged ≥ 70 years was significantly shorter than for patients aged < 70 years in their study utilizing various IO+TKI regimens (26). However, these findings were based on a relatively small cohort of 51 patients. More studies are needed to clarify the optimal management and outcomes of IO+TKI therapy in older adults.
Kaymakcalan et al. and Donskov et al. identified older age as a predictive factor for treatment discontinuation due to AEs among patients treated with TKI monotherapies (27, 28). However, Carina et al. reported that the increased incidence of treatment interruptions and dose reductions among older adult patients did not affect the overall efficacy despite the significantly lower TKI doses observed. Sustained drug exposure in each patient, rather than the absolute dose in milligrams, contributes to the clinical benefit of targeted therapies (29). These studies focused on TKI monotherapy; however, their findings share commonalities with our results in IO+TKI combination therapy, highlighting the importance of dose management, including TKI dose reduction. A recent report proposed the “start-low, go-slow” (SLGS) strategy, which involves initiating cancer treatment at lower-than-standard doses in older patients with solid tumors to reduce toxicity without affecting survival (30). Our findings suggest that in IO+TKI treatment of mRCC, SLGS strategy may help mitigate toxicity and prolong the duration of first-line therapy.
In previous reports, the occurrence of irAEs or treatment discontinuation due to irAEs did not negatively impact OS. The early occurrence of irAEs has been suggested to reflect immune activation, and in some cases, may even prolong OS (31–33). In our study, the results for the IO+IO regimen support this finding. Although the data for older patients were limited and the difference was not statistically significant, the presence of irAEs suggested a potential benefit to OS. In contrast, the lack of a significant association in the IO+TKI regimen may be due to the high number of TKI-related AEs. These results highlight the clinical significance of AEs and the need for further investigation.
Previous studies have shown that while IO+TKI therapy offers superior PFS compared with IO+IO, OS remains similar (34–36). The favorable PFS of IO+TKI is thought to result from the interaction between tumor immunity and angiogenesis, as anti-angiogenic therapy may enhance ICI efficacy (37). However, while IO+IO demonstrates inferior PFS compared with IO+TKI, it is considered non-inferior in OS due to several factors: the potential for durable long-term responses and better treatment-free survival (38), the sustained efficacy of vascular endothelial growth factor-TKIs even after ICI-based combination therapy (39, 40), and the fact that PFS is not a perfect surrogate for OS (41). Our study showed similar results in the non-older adult cohort; however, we suggest that this trend may be attenuated in older adults. In addition, the attenuated trend may be attributable to the higher frequency of treatment discontinuation due to AEs with IO+TKI therapy in the older adult group. These findings may have important implications for treatment decision-making in older adults. However, the small sample size limits the statistical power of this analysis, and the results should be interpreted with caution.
This study has some limitations. First, the participants were few, and this was a retrospective study. While the number of patients is especially limited for each IO+TKI regimen, real-world data on IO+TKI therapy in older adult patients remain scarce, underscoring the significance of our findings. Second, there were no explicit criteria for regimen selection, and the choice was left to the treating physician’s discretion. Particularly, the selection of IO+TKI regimens lacked uniformity. Such biases inherent in retrospective, real-world studies were recognized in other analyses concerning IO+IO by our group (42, 43). Third, data regarding geriatric assessment were insufficient, so the KPS and CCI were used as surrogate indicators. Fourth, the participants in our study with mRCC were all Japanese, and the clinical outcomes of ICI combination therapy could have been influenced by geographic region and ethnicity. Therefore, patient bias could not be controlled. Larger-scale studies are warranted.
In conclusion, our findings suggest that the treatment outcomes of immune combination therapy in patients with mRCC aged ≥ 75 years are not inferior to those in patients younger than 75. IO+IO and IO+TKI are distinct treatment modalities; therefore, understanding their individual characteristics is crucial for appropriate treatment selection and management. ICI-based combination therapy may be an effective treatment option, even in older adult patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Review Board of the Nagoya City University Graduate School of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is retrospective study.
Author contributions
HI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. SH: Conceptualization, Data curation, Project administration, Writing – review & editing. YTa: Data curation, Investigation, Project administration, Writing – review & editing. MT: Data curation, Investigation, Writing – review & editing. TS: Data curation, Investigation, Writing – review & editing. HS: Data curation, Investigation, Writing – review & editing. YN: Data curation, Investigation, Writing – review & editing. MU: Data curation, Investigation, Writing – review & editing. YTs: Data curation, Investigation, Writing – review & editing. YM: Data curation, Investigation, Writing – review & editing. TM: Data curation, Investigation, Writing – review & editing. TNag: Data curation, Investigation, Writing – review & editing. RU: Data curation, Investigation, Writing – review & editing. TE: Data curation, Investigation, Writing – review & editing. TNai: Data curation, Investigation, Writing – review & editing. YS: Data curation, Investigation, Writing – review & editing. TY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1668406/full#supplementary-material
References
1. United Nations Department of Economic and Social Affairs. World Social Report 2023: Leaving No One Behind in an Ageing World. New York, United States of America: World Social Report (2023). Available online at: https://www.un-ilibrary.org/content/books/9789210019682 (Accessed May 22, 2025).
2. Statistics Bureau Home Page/Population Estimates/Current Population Estimates as of October 1, 2024 . Available online at: https://www.stat.go.jp/english/data/jinsui/2024np/index.html accessed (Accessed May 22, 2025).
3. Cancer Statistics. Cancer Information Service, National Cancer Center, Tokyo, Japan . Available online at: https://ganjoho.jp/reg_stat/statistics/stat/cancer/22_renal.html accessed (Accessed May 22, 2025).
4. Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, et al. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer. (2021) :154:120–7. doi: 10.1016/j.ejca.2021.06.015
5. Rizzo A, Mollica V, Santoni M, Ricci AD, Rosellini M, Marchetti A, et al. Impact of clinicopathological features on survival in patients treated with first-line immune checkpoint inhibitors plus tyrosine kinase inhibitors for renal cell carcinoma: A meta-analysis of randomized clinical trials. Eur Urol Focus. (2022) 8:514–21. doi: 10.1016/j.euf.2021.03.001
6. Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
7. Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. (2022) 128:2085–97. doi: 10.1002/cncr.34180
8. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. (2021) 384:1289–300. doi: 10.1056/NEJMoa2035716
9. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
10. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
11. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) 384:829–41. doi: 10.1056/NEJMoa2026982
12. Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. (2019) 5:1769. doi: 10.1001/jamaoncol.2019.2055
13. Hou C, Wang Z, and Lu X. Impact of immunosenescence and inflammaging on the effects of immune checkpoint inhibitors. Cancer Pathogenesis Ther. (2024) 2:24–30. doi: 10.1016/j.cpt.2023.08.001
14. Kao C, Charmsaz S, Tsai HL, Aziz K, Shu DH, Munjal K, et al. Age-related divergence of circulating immune responses in patients with solid tumors treated with immune checkpoint inhibitors. Nat Commun. (2025) 16:3531. doi: 10.1038/s41467-025-58512-z
15. Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urology. (2022) 82:529–42. doi: 10.1016/j.eururo.2022.08.019
16. Charlson ME, Pompei P, Ales KL, and MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Diseases. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
18. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
19. Araujo DV, Wells C, Hansen AR, Dizman N, Pal SK, Beuselinck B, et al. Efficacy of immune-checkpoint inhibitors (ICI) in the treatment of older adults with metastatic renal cell carcinoma (mRCC): An international mRCC database consortium (IMDC) analysis. JCO. (2020) 38:5068–8. doi: 10.1200/JCO.2020.38.15_suppl.5068
20. Kobayashi M, Numakura K, Hatakeyama S, Ishida T, Koizumi A, Tadachi K, et al. Real clinical outcomes of nivolumab plus ipilimumab for renal cell carcinoma in patients over 75 years old. Int J Clin Oncol. (2023) 28:1530–7. doi: 10.1007/s10147-023-02394-y
21. Ueda K, Ito N, Sakai Y, Ohnishi S, Hirano T, Kurose H, et al. Efficacy and safety of nivolumab with ipilimumab in elderly patients with advanced renal cell carcinoma. Anticancer Res. (2024) 44:5087–93. doi: 10.21873/anticanres.17333
22. Nemoto Y, Ishihara H, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Efficacy and safety of immune checkpoint inhibitors in elderly patients with metastatic renal cell carcinoma. Int Urol Nephrol. (2022) 54:47–54. doi: 10.1007/s11255-021-03042-y
23. Shani Shrem N, Beltran-Bless AA, Ghosh S, Tajzler C, Wood LA, Kollmannsberger C, et al. Real-world efficacy and toxicity of ipilimumab and nivolumab as first-line treatment of metastatic renal cell carcinoma (mRCC) in a subpopulation of elderly and poor performance status patients. Cancers. (2025) 17:522. doi: 10.3390/cancers17030522
24. Tomita Y, Motzer RJ, Choueiri TK, Rini BI, Miyake H, Uemura H, et al. Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN Renal 101. ESMO Open. (2022) 7:100450. doi: 10.1016/j.esmoop.2022.100450
25. Varkaris A, Xu W, Davis RB, Healy B, and McDermott DF. Combining immune checkpoint and VEGFR inhibition in favorable-risk and elderly patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. (2020) 18:179–184.e3. doi: 10.1016/j.clgc.2019.11.016
26. Iinuma K, Yamada T, Kameyama K, Taniguchi T, Kawada K, Ishida T, et al. The efficacy and safety of immune checkpoint inhibitor and tyrosine kinase inhibitor combination therapy for advanced or metastatic renal cell carcinoma: A multicenter retrospective real-world cohort study. Cancers (Basel). (2023) 15:947. doi: 10.3390/cancers15030947
27. Kaymakcalan MD, Xie W, Albiges L, North SA, Kollmannsberger CK, Smoragiewicz M, et al. Risk factors and model for predicting toxicity-related treatment discontinuation in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted therapy: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer. (2016) 122:411–9. doi: 10.1002/cncr.29773
28. Donskov F, Motzer RJ, Voog E, Hovey E, Grüllich C, Nott LM, et al. Outcomes based on age in the phase III METEOR trial of cabozantinib versus everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. (2020) 126:1–10. doi: 10.1016/j.ejca.2019.10.032
29. Hermansen CK and Donskov F. Outcomes based on age in patients with metastatic renal cell carcinoma treated with first line targeted therapy or checkpoint immunotherapy: Older patients more prone to toxicity. J Geriatric Oncol. (2021) 12:827–33. doi: 10.1016/j.jgo.2020.12.008
30. Aleixo G, Patel T, Ani J, Ferrell WJ, Dotan E, Takvorian SU, et al. Start low, go slow,” a strategy to tailor treatment dosing in older or vulnerable adults with advanced solid cancer: A systematic review and meta-analysis. J Geriatric Oncol. (2025) 16:102153. doi: 10.1016/j.jgo.2024.102153
31. Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. (2021) 8:e000891. doi: 10.1136/jitc-2020-000891
32. Grimm MO, Leucht K, Grünwald V, and Foller S. New first line treatment options of clear cell renal cell cancer patients with PD-1 or PD-L1 immune-checkpoint inhibitor-based combination therapies. J Clin Med. (2020) 9:565. doi: 10.3390/jcm9020565
33. Martini DJ, Goyal S, Liu Y, Evans ST, Olsen TA, Case K, et al. Immune-related adverse events as clinical biomarkers in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist. (2021) 26:e1742–50. doi: 10.1002/onco.13868
34. Ishihara H, Omae K, Nemoto Y, Ishiyama R, Tachibana H, Nishimura K, et al. First-line dual immune checkpoint inhibitor therapies versus combination therapies comprising immune checkpoint inhibitors and tyrosine kinase inhibitors for advanced renal cell carcinoma: a comparative analysis of the effectiveness using real-world data. Int J Clin Oncol. (2024) 29:473–80. doi: 10.1007/s10147-024-02471-w
35. Haack M, Neuberger S, Boerner JH, Ziewers S, Duwe G, Dotzauer R, et al. Real-world comparison of the efficacy of first-line therapies and the influence of risk factors in advanced renal cell carcinoma. Discov Onc. (2025) 16:359. doi: 10.1007/s12672-025-02131-z
36. Esterberg E, Iyer S, Nagar SP, Davis KL, and Tannir NM. Real-world treatment patterns and clinical outcomes among patients with advanced renal cell carcinoma. Clin Genitourin Cancer. (2024) 22:115–125.e3. doi: 10.1016/j.clgc.2023.09.009
37. Yi M, Jiao D, Qin S, Chu Q, Wu K, and Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. (2019) 18:60. doi: 10.1186/s12943-019-0974-6
38. O’Rourke K. CheckMate-214 trial results show longer treatment-free survival with immunotherapy. Cancer. (2022) 128:1156–6. doi: 10.1002/cncr.34139
39. Heng DYC, Doshi GK, Dutailly P, Houchard A, Lothgren M, Monnette A, et al. Real-world outcomes in patients with advanced/metastatic renal cell carcinoma receiving cabozantinib or other tyrosine kinase inhibitors after checkpoint inhibitor-based therapy. Kidney Cancer. (2024) 8:99–114. doi: 10.3233/KCA-240005
40. Albiges L, McGregor BA, Heng DYC, Procopio G, de Velasco G, Taguieva-Pioger N, et al. Vascular endothelial growth factor-targeted therapy in patients with renal cell carcinoma pretreated with immune checkpoint inhibitors: A systematic literature review. Cancer Treat Rev. (2024) 122:102652. doi: 10.1016/j.ctrv.2023.102652
41. Shahnam A, Hitchen N, Nindra U, Manoharan S, Desai J, Tran B, et al. Objective response rate and progression-free survival as surrogates for overall survival treatment effect: A meta-analysis across diverse tumour groups and contemporary therapies. Eur J Cancer. (2024) 198:113503. doi: 10.1016/j.ejca.2023.113503
42. Hamamoto S, Tasaki Y, Yamashita S, Furukawa J, Fujita K, Tomida R, et al. External validation of hemoglobin and neutrophil levels as predictors of the effectiveness of ipilimumab plus nivolumab for treating renal cell carcinoma. Front Oncol. (2024) 14:1400041/full. doi: 10.3389/fonc.2024.1400041/full
43. Tasaki Y, Hamamoto S, Yamashita S, Furukawa J, Fujita K, Tomida R, et al. Eosinophil is a predictor of severe immune-related adverse events induced by ipilimumab plus nivolumab therapy in patients with renal cell carcinoma: a retrospective multicenter cohort study. Front Immunol. (2025) 15:1483956. doi: 10.3389/fimmu.2024.1483956
Keywords: metastatic renal cell carcinoma, immune checkpoint inhibitor, tyrosine kinase inhibitor, older adult patient, adverse event
Citation: Ikoma H, Hamamoto S, Tasaki Y, Tomita M, Sakata T, Suzuki H, Noda Y, Usami M, Tsubouchi Y, Mimura Y, Morikawa T, Nagai T, Unno R, Etani T, Naiki T, Sugiyama Y and Yasui T (2025) Real-world outcomes of immune checkpoint inhibitor-based combination therapy in older adult patients with metastatic renal cell carcinoma: a multi-center, retrospective analysis. Front. Immunol. 16:1668406. doi: 10.3389/fimmu.2025.1668406
Received: 17 July 2025; Accepted: 09 September 2025;
Published: 25 September 2025; Corrected: 15 October 2025.
Edited by:
Amorette Barber, Longwood University, United StatesReviewed by:
Lingxiang Ran, Peking University, ChinaTomokazu Sazuka, Chiba University Hospital, Japan
Copyright © 2025 Ikoma, Hamamoto, Tasaki, Tomita, Sakata, Suzuki, Noda, Usami, Tsubouchi, Mimura, Morikawa, Nagai, Unno, Etani, Naiki, Sugiyama and Yasui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuzo Hamamoto, aGFtYW1vMTBAbWVkLm5hZ295YS1jdS5hYy5qcA==
 Hiroaki Ikoma
Hiroaki Ikoma Shuzo Hamamoto
Shuzo Hamamoto Yoshihiko Tasaki
Yoshihiko Tasaki Misato Tomita2
Misato Tomita2 Yoshihisa Mimura
Yoshihisa Mimura Toshiharu Morikawa
Toshiharu Morikawa Rei Unno
Rei Unno Taku Naiki
Taku Naiki Yosuke Sugiyama
Yosuke Sugiyama Takahiro Yasui
Takahiro Yasui