- 1Department of Burns and Cutaneous Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, China
- 2Department of Dermatology, The 989th Hospital of the Chinese People's Liberation Army Joint Logistic Support Force, Luoyang, China
- 3Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
- 4Dermatology Hospital, Southern Medical University, Guangzhou, China
Background: Regional lymphadenectomy was once considered as the standard treatment for melanoma patients with positive SLNB result, aimed at reducing the risk of recurrence and metastasis. However, recent researches have suggested multiple key roles of regional lymph node clusters in anti-tumor immune responses. Therefore, in the era of immunotherapy, whether lymph node dissection ultimately benefits patients remains to be determined, especially in acral melanoma, the leading subtype in east Asian population, which has a poorer response to immune checkpoint inhibitors (ICIs).
Methods: We retrospectively analyzed 172 patients with resectable advanced melanoma (stage III-IV, M1a) from a tertiary center and categorized them into four groups based on treatment regimens: immunotherapy group, observation group, neoadjuvant group, adjuvant group. In patients receiving immunotherapy (including immunotherapy group, neoadjuvant group, and adjuvant group), anti-PD-1 antibody was given accompanied with interferon-α1b.
Results: With a median follow-up of 18.87 months, multivariable analysis confirmed significantly longer RFS for neoadjuvant versus adjuvant group. The adjusted HR was 2.02 (95% Cl 1.05–3.89, p= 0.035). Numerical improvements over immunotherapy and observation groups did not reach statistical significance, with p-values of 0.120 and 0.073 respectively. The 2-year RFS rate was significantly higher at 39.5% versus 13.8%. Notably, surgery-related adverse events occurred in 50.0% of patients (12.5% grade ≥3), with no significant differences observed among groups.
Conclusions: This study demonstrates that in Chinese patients with advanced melanoma, neoadjuvant therapy is associated with significantly prolonged RFS compared to adjuvant therapy, with suggestive but non-significant advantages against other approaches.
1 Introduction
Although immediate completely lymph node dissection (CLND) failed to improve overall survival (OS) in melanoma patients with positive SLNB, it still showed benefits in disease-free survival/melanoma-specific survival (DFS/MSS) for patients with Breslow thickness 1.2–3.5 mm (1, 2). These findings once established CLND as the standard treatment for SLNB-positive patients (3). However, subsequent MSLT-II and DeCOG-SLT trials confirmed that close observation achieved comparable survival outcomes to CLND in SLNB-positive patients (4, 5), while CLND was associated with higher complication rates (6). Based on this evidence, the NCCN guidelines progressively de-escalated their recommendation for CLND, with the 2025 version explicitly listing “active surveillance with regional lymph node ultrasound or imaging alternatives to immediate surgery” as the preferred approach (7).
In the era of immunotherapy, the clinical benefit of CLND for patients with advanced melanoma has been further questioned. Recently, Fransen MF and colleagues proved that resection of tumor-draining lymph nodes significantly impairs antitumor immune responses using in vivo model (8). These findings also appear to corroborate some clinical observations: in postoperative recurrent non-small cell lung cancer patients, an elevated dissected lymph node count (cutoff: 16) was associated with poorer immunotherapy efficacy (9). Current evidence suggests that draining lymph nodes serve as a critical foundation for antitumor immunity (10), necessitating a fundamental reevaluation of conventional lymph node dissection—shifting from “questionable clinical benefit” to “potentially detrimental to immunotherapy efficacy. In the PRADO trial, patients who achieved a major pathologic response (MPR) after ipilimumab plus nivolumab therapy maintained a 2-year recurrence-free survival (RFS) of 93% despite omitting therapeutic lymph node dissection (TLND), further supporting the trend toward surgical de-escalation in melanoma management (11).
The Asian population exhibits distinct clinicopathological features: acral melanoma accounts for of cases 50–60% (vs. <5% in Caucasians) (12), which results in earlier lymph node metastasis, inferior survival outcomes (12, 13), and attenuated responses to immunotherapy (14–16). Consequently, the generalizability of cutaneous melanoma-based nodal management to Asian populations warrants further investigation. Here, we assess how different treatment strategies involving lymph node management influence immunotherapy outcomes in Chinese patients with advanced melanoma, addressing this critical knowledge gap to provide evidence for optimized surgical timing in precision management.
2 Methods
2.1 Study design and patients
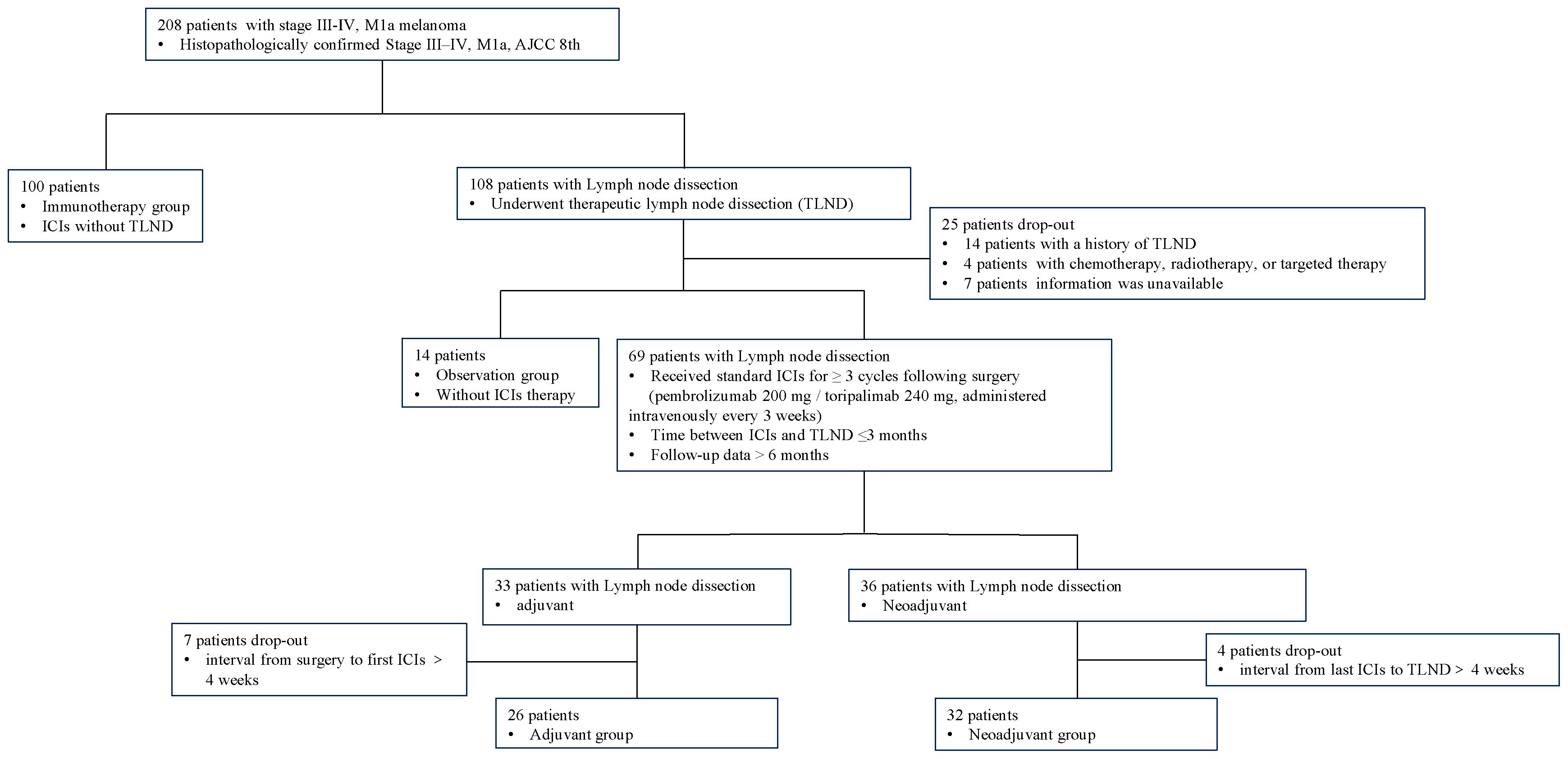
This retrospective study included patients with stage III–IV, M1a resectable melanoma (AJCC 8th edition) at Xijing Hospital, Fourth Military Medical University between July 1, 2019 and September 30, 2024. Inclusion criteria: (1) histopathologically confirmed melanoma, staging III–IV, M1a; (2) receive TLND in Department of Burns and Cutaneous Surgery, Xijing Hospital; (3) follow-up duration >6 months; (4) complete medical records available. Exclusion criteria: (1) with a previous history of lymph node dissection; (2) combination management including chemotherapy, radiotherapy, or targeted therapy.
The eligibility criteria and study flow are detailed in Figure 1. This cohort study included four treatment groups: (1)immunotherapy group: patients received immunotherapy only, without TLND; (2)observation group: patients underwent TLND, followed by observation; (3)neoadjuvant group: patient received 3 cycles of immunotherapy before TLND, followed by at least 3 additional postoperative cycles of immunotherapy, the surgery was performed within 4 weeks after the last immunotherapy administration; (4)adjuvant group: patient received at least 3 cycles of immunotherapy after TLND (17).
All patients included in this study were initially diagnosed with technically resectable disease. Resectability was defined as the anatomical feasibility of achieving a complete macroscopic resection (R0) with negative margins, as confirmed by a multidisciplinary team (MDT) based on pretreatment radiological imaging (18). The final treatment is determined through joint decision-making between the physicians and the patients, influenced by factors including clinical practice guideline recommendations, MDT discussion outcomes, the patient’s health insurance coverage, financial capacity, and patients’ personal preferences. In patients receiving immunotherapy (including immunotherapy group, neoadjuvant group, and adjuvant group), ICIs were given accompanied with interferon-α1b (600 ug, subcutaneously administered every other day).
All lymph node dissection procedures were performed by certified and experienced surgeons using standardized techniques, with removal of the involved nodal basins. All excised lymph node specimens were sent for pathological examination and evaluated by at least two board-certified pathologists trained in standardized diagnostic practices. All specimens were independently reviewed by two pathologists. Detailed surgical data are presented in Supplementary Table 1.
2.2 Efficacy and safety assessment
The primary endpoint was RFS, which was defined as the time from treatment initiation (date of first anti-PD-1 monoclonal antibody infusion for the immunotherapy group or surgical date for the therapeutic lymph node dissection group) to the first documented recurrence (local/lymph nodal/distant) or death from any cause. Data were retrospectively collected through hospital electronic medical records, institutional databases, and structured telephone follow-ups. Per clinical protocols, patients underwent regular clinical/imaging assessments (contrast-enhanced CT/MRI or PET-CT) every 3–6 months post-treatment, with extended intervals permitted for long-term recurrence-free cases; all data were systematically recorded. Recurrence required meeting ≥1 criterion (prioritizing histologic confirmation when feasible): (1) radiographic progression per RECIST 1.1 (new lesions or ≥20% increase in target lesions), (2) biopsy-confirmed metastasis (unless contraindicated), or (3) multidisciplinary consensus (≥1 medical oncologist, radiologist, and pathologist) for equivocal cases.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). This study was conducted in compliance with the Declaration of Helsinki, approved by the Ethics Committee of the Fourth Military Medical University (Approval No: KY20242252-F-1).
2.3 Statistical analyses
Continuous variables were summarized as medians (interquartile ranges), and categorical variables as frequencies (percentages). Intergroup differences were assessed using Pearson’s χ² test (or Fisher’s exact test for expected cell counts <5) for categorical variables and the Mann-Whitney U test for continuous variables, as appropriate. RFS was estimated via the Kaplan-Meier method, with between-group comparisons performed using the log-rank test. Multivariable Cox proportional hazards regression was employed to adjust for prespecified confounders. All statistical tests were two-sided, with p < 0.05 considered significant. Analyses were conducted using IBM SPSS Statistics (Version 26.0) and R 4.4.3.
A multivariable Cox proportional hazards model was used to compare RFS between groups. The model adjusted for the following pre-specified covariates: age, sex, primary site, stage, Breslow thickness, ulceration, gene mutation, and ICIs type. The proportional hazards assumption was verified using Schoenfeld residuals. The global test yielded a p-value of 0.098. Specifically, the treatment variable yielded a p-value of 0.370, indicating no significant violation of the proportional hazards assumption for any covariate in the model (see Supplementary Figure 1).
3 Results
3.1 Baseline characteristics
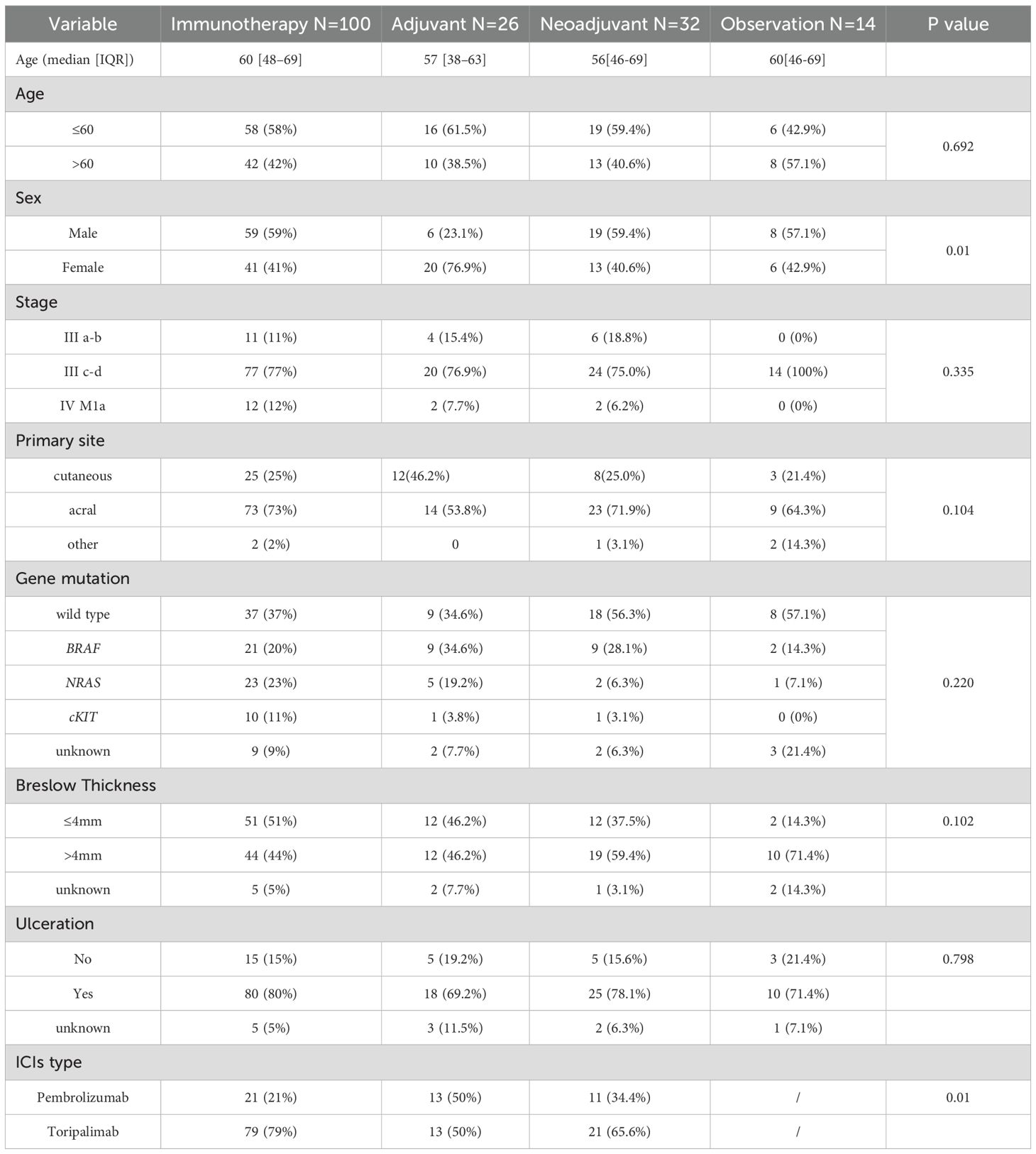
This study enrolled a total of 172 patients with stage III to IV, M1a melanoma, who were categorized into four groups according to different treatments. One hundred patients received immunotherapy without surgery, 32 underwent neoadjuvant therapy while 26 received adjuvant therapy, 14 patients received close observation after surgery. Baseline characteristics including age, primary site, clinical stage, Breslow thickness, ulceration status, and driver gene mutations were comparable across all groups (all P>0.05). Significant differences were observed in sex distribution (P<0.01) and types of ICIs(P<0.01) (Table 1).
3.2 Efficacy
3.2.1 RFS outcomes
3.2.1.1 Impact of lymph node dissection on immunotherapy efficacy
As of April 30, 2025, the median follow-up time for the entire cohort was 18.87 months (IQR: 11.83-31.21). Firstly, we compared the RFS of patients who received immunotherapy alone (n=100) with those who received a combination of immunotherapy and lymph node dissection (neoadjuvant and adjuvant group, n=58). In a univariate, unadjusted analysis, the Kaplan-Meier curve and log-rank test demonstrated no significant difference in RFS between these two strategies (p = 0.298, Log-rank). The median RFS was 8.27 months (95% CI, 4.13–12.41) in the immunotherapy group versus 12.97 months (95% CI, 6.53–19.41) in the combination therapy group (Figure 2).
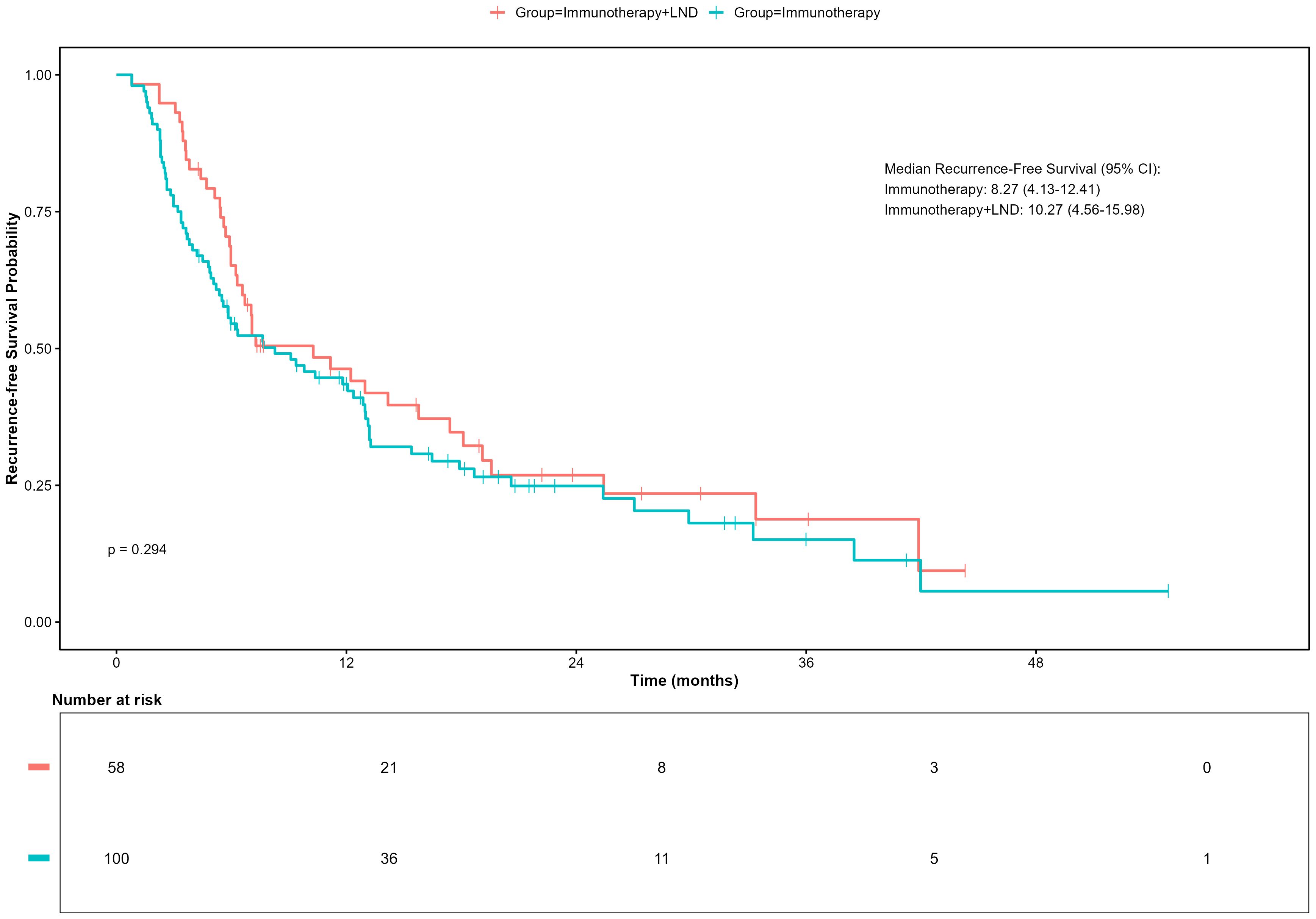
Figure 2. Recurrence-free survival (RFS) in patients receiving immunotherapy with or without lymph node dissection (LND).
3.2.1.2 Impact of surgical timing in patients undergoing dissection
We next focused on the cohort of patients who underwent lymph node dissection to evaluate the impact of surgical timing on the treatment efficacy.
Kaplan-Meier analysis indicated a clear difference in RFS among the treatment groups, though it did not reach statistical significance (p=0.051, Figure 3). In unadjusted analyses, the neoadjuvant-adjuvant group showed the most favorable clinical outcomes, with a median RFS of 12.97 months (95% CI 6.53–19.41). This was significantly longer than that observed in the adjuvant group (5.97 months; 95% CI 3.75–8.19; p=0.012). Numerical trends suggesting improved RFS were also observed when compared to the immunotherapy group (8.27 months; 95% CI 4.13–12.41; p=0.037) and the observation group (6.13 months; 95% CI 4.53–7.73; p=0.025). No other pairwise comparisons showed statistically significant differences.
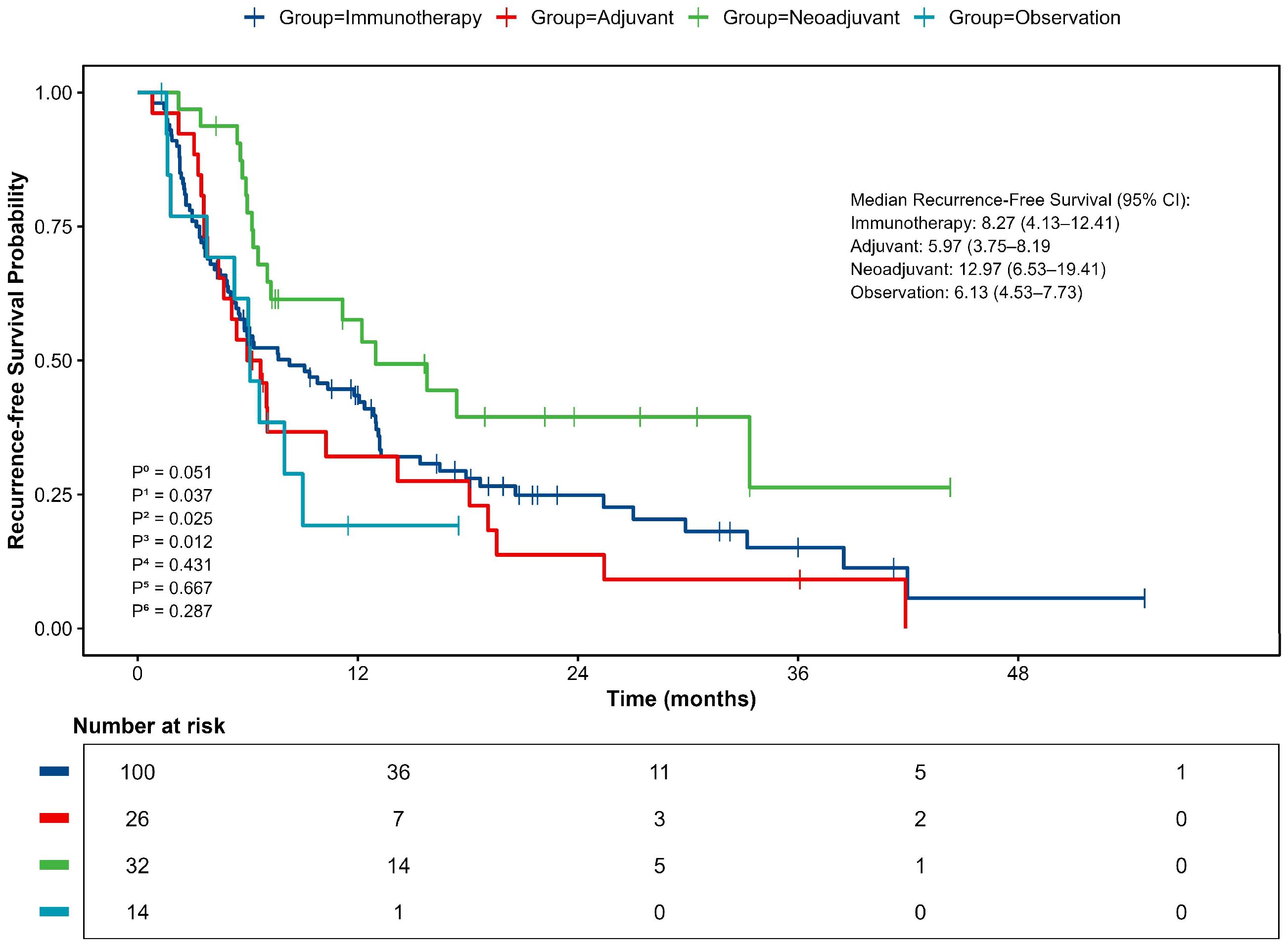
Figure 3. Kaplan–Meier curve for Recurrence-free survival (RFS). P0, comparison between 4 groups; P1, neoadjuvant vs immunotherapy; P2, neoadjuvant vs observation; P3, neoadjuvant vs adjuvant; P4, adjuvant vs immunotherapy; P5, adjuvant vs observation; P6, observation vs immunotherapy.
Consistent with the median RFS, analysis of survival rates at specific timepoints showed that the neoadjuvant-adjuvant group had a 1-year RFS rate of 57.6%. This was significantly higher than the observation group (19.2%, p = 0.009, z-test) and showed a strong trend toward significance compared to the adjuvant group (32.1%, p=0.053, z-test). The 2-year RFS rate was 39.5% for the neoadjuvant group, which was significantly higher than that of the adjuvant group (13.8%, p=0.035, z-test). All patients in the observation group experienced recurrence within 2 years.
3.2.2 Multivariable Cox regression analysis
Multivariable Cox proportional hazards analysis adjusting for age, sex, primary site, stage, Breslow thickness, ulceration, gene alterations, ICIs type, and treatment demonstrated that advanced disease stage and treatment modality were independent risk factors for RFS in advanced melanoma (Figure 4). Compared to stage IIIa-b disease, stage IIIc-d was associated with a significantly worse RFS (HR = 2.16, 95% CI: 1.09-4.31, P = 0.028), while stage IV, M1a patients exhibited an even higher risk (HR = 4.78, 95% CI: 1.96-11.65, P<0.001). Compared with the other three treatment approaches, the neoadjuvant group showed longer RFS, although statistical significance was achieved only in the comparison with the adjuvant group (HR = 2.02, 95% CI: 1.05-3.89, P = 0.035). Other examined factors did not show statistically significant association with RFS in the multivariate model (all P>0.050).
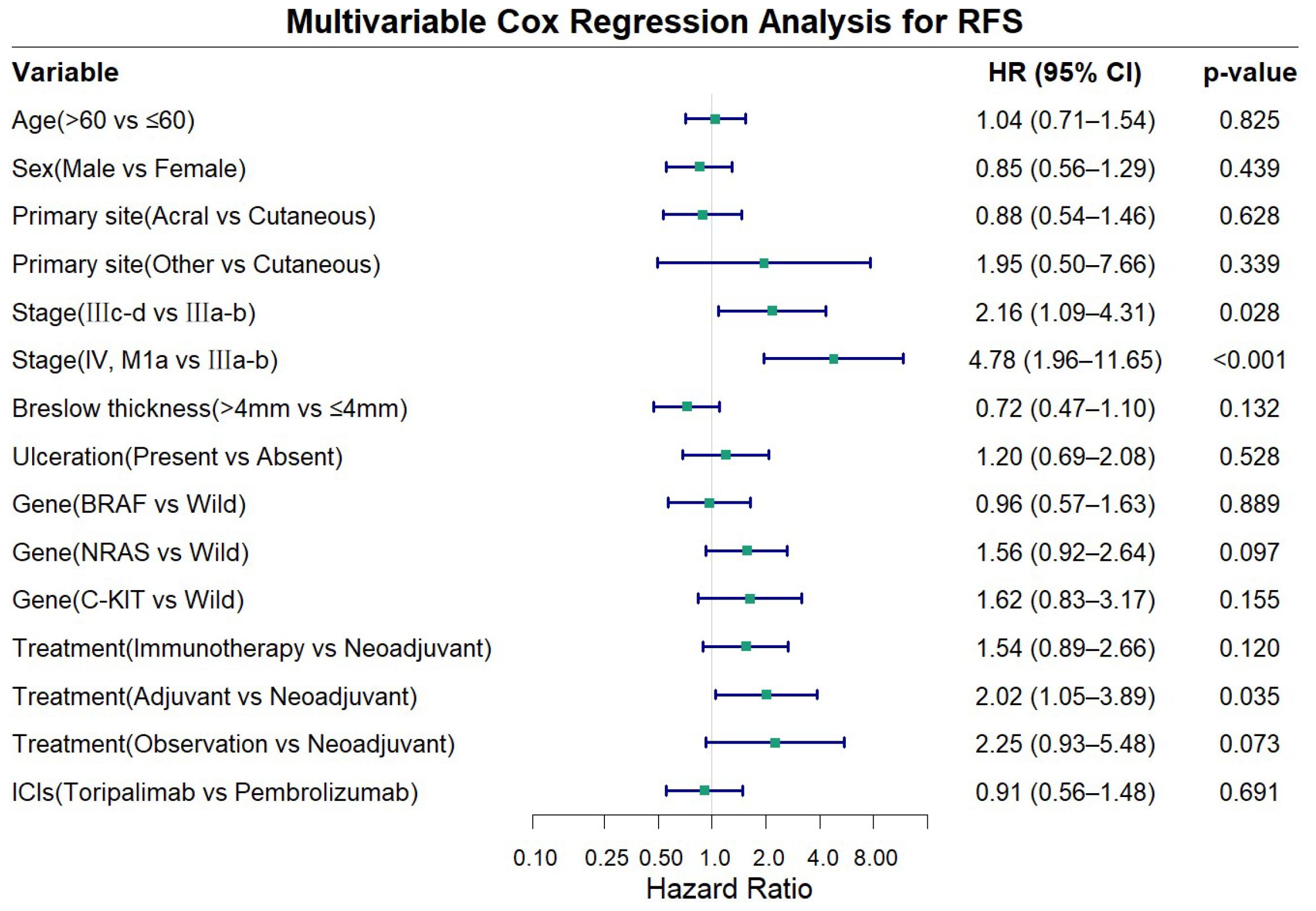
Figure 4. Forest plot showing multivariable cox regression analysis for recurrence-free survival (RFS).
3.2.3 Pathological response in the neoadjuvant therapy group
Among the 32 melanoma patients who received neoadjuvant therapy followed by lymph node dissection, the pathological responses were as follows: Pathological Complete Response (pCR): 6 cases (18.8%). Pathological Partial Response (pPR): 4 cases (12.5%). Pathological No Response (PNR): 22 cases (68.8%). Based on these data, the Pathological Response (PR) rate—defined as the proportion of patients with ≤50% viable tumor cells (pCR + pPR)—was 31.3% (10/32). Interestingly, the pathologically confirmed positive lymph node rate in patients who did not receive immunotherapy was as high as 90% (36/40). Detailed lymph node dissection-related data, including the anatomical extent of dissection, number of lymph nodes harvested, and the ratio of positive lymph nodes, are provided in Supplementary Table 2.
3.3 Safety
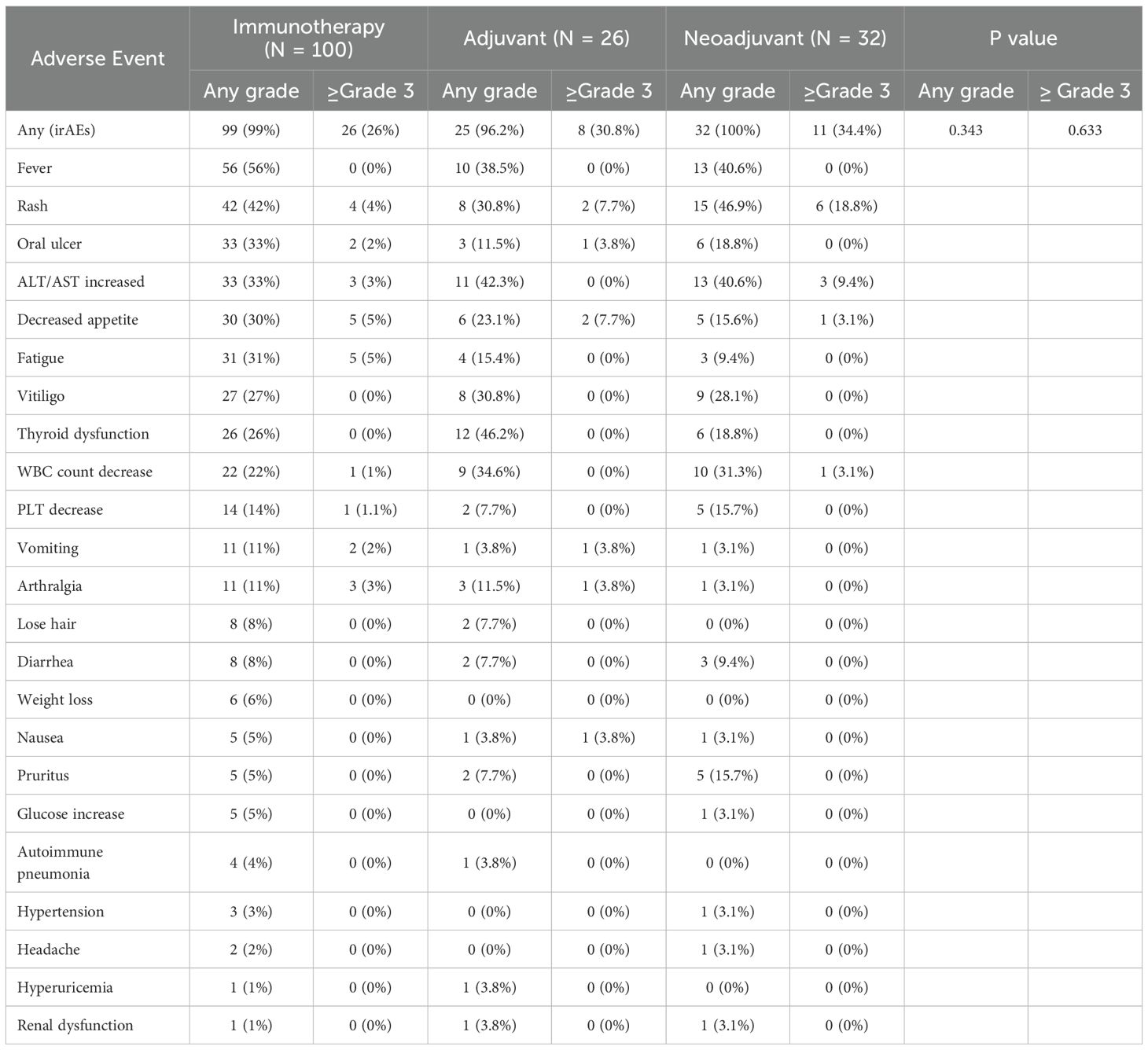
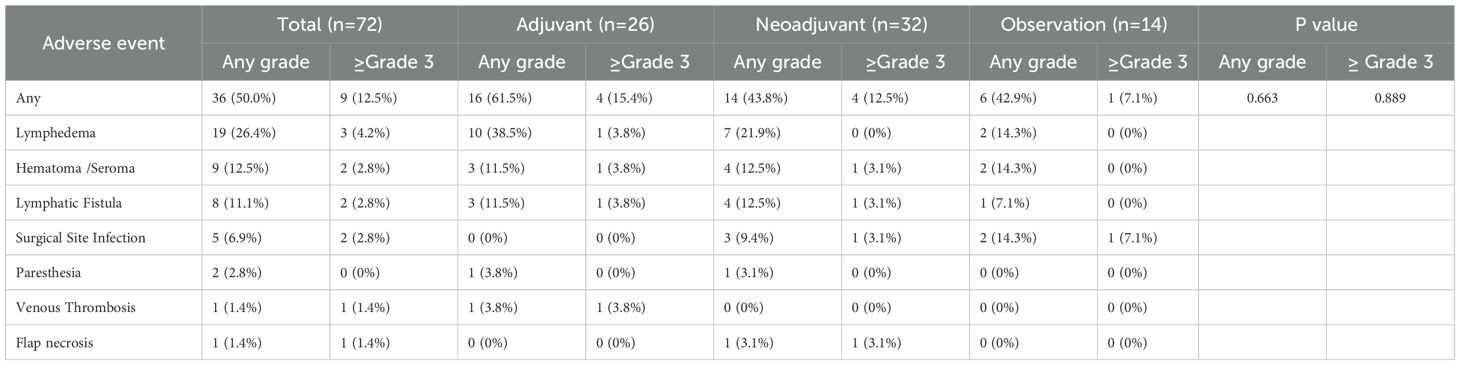
The incidence of immune-related adverse events (irAEs) was evaluated in the immunotherapy, neoadjuvant, and adjuvant groups while the surgery-related adverse events were analyzed in patients underwent TLND. The results demonstrated no statistically significant differences in the occurrence of either any-grade irAEs or grade ≥3 irAEs between treatment groups (Fisher’s exact test, Table 2). Among the 72 patients who underwent TLND, the overall incidence of surgery-related adverse events was 50.0%, with grade ≥3 surgery-related adverse events occurring in 12.5% of cases (as graded by CTCAE v5.0 criteria). No significant differences in surgery-related adverse events rates were observed among the different surgical groups (observation vs adjuvant vs neoadjuvant; all p>0.05). Lymphoedema following TLND emerged as the most frequent surgical complication (incidence 26.4%), with detailed characteristics of other complications presented in Table 3.
4 Discussion
Completion lymph node dissection has been shown to bring little improvement to advanced melanoma patients in long-term outcomes (4, 5), and may even compromise the efficacy of immunotherapy (19). Therefore, de-escalation of lymph node dissection has become a reasonable therapeutic approach (7, 20). However, while TLND is undergoing de-escalation globally (21), the unique characteristics of Chinese melanoma patients—including their higher nodal metastasis rates (12, 13, 19) and distinct immunotherapy responses (14–16, 21)—suggest retained therapeutic value of lymph node dissection in this population., compel us to re-evaluate this approach in our population. Therefore, further investigation into the impact of lymph node dissection in the era of immunotherapy is critically important for developing individualized and precision-based treatment strategies for Chinese melanoma patients.
The key finding is that while adding TLND to immunotherapy did not significantly improve RFS, the timing of surgery relative to immunotherapy proved critical. Biologically, although resection may reduce tumor burden, extensive lymph node dissection risks removing crucial sites for immune cell activation—impairing systemic antitumor responses. Our results support this latter mechanism, suggesting that TLND’s theoretical benefits did not translate into survival gains, possibly due to this immunological compromise or confounding prognostic factors. The observed pathological response rate after neoadjuvant therapy further supports the role of immunotherapy underlying the clinical benefit of sequenced treatment.
The PRADO trial provides compelling evidence for de-escalating therapeutic lymph node dissection (TLND) in patients who achieve a major pathologic response to immunotherapy. However, the generalizability of this strategy to our patient population remains uncertain. In our cohort, a substantial proportion of patients did not achieve a sufficient pathologic response. This finding underscores the distinct tumor biology and differential treatment outcomes in this demographic. Consequently, TLND may retain clinical utility for regional disease control in Chinese patients with melanoma, necessitating further investigation within the context of this specific subtype.
This study is the first to demonstrate in Chinese melanoma patients that the clinical outcomes of the neoadjuvant group were significantly better than those of the adjuvant group, align with the SWOG 1801 findings that who received pembrolizumab both before and after surgery demonstrated superior event-free survival (EFS) over adjuvant pembrolizumab alone - although acral melanomas comprised <3% (9/313) of cases in that trial. Importantly, our study confirmed that this therapeutic advantage persists even in Chinese melanoma patients with traditionally lower response rates to immune checkpoint inhibitors (22).
We speculate that this discrepancy may stem from the high proportion of acral melanoma in the Chinese population, its aggressive nature, and early metastatic tendency. Interestingly, the pathologically confirmed positive lymph node rate in patients who did not receive immunotherapy was as high as 90% (36/40), significantly exceeding previously reported rates (23–25). On one hand, this reflects the early metastatic characteristics of acral melanoma; on the other hand, it is closely related to the patient population in this study—over 80% were stage IIIc or later, indicating that patients underwent “therapeutic lymph node dissection”, which aligns more closely with real-world clinical practice.
This phenomenon may have an immunological basis: the antitumor effect of ICIs relies on T-cell activation within tumor-draining lymph nodes (8). As the central hub of antitumor immune responses (26), intact draining lymph nodes facilitate more effective expansion of tumor-reactive T-cell clones (27). Conversely, performing TLND before immunotherapy not only removes this critical immune response site but may also impair the efficacy of subsequent ICIs due to excessive lymph node clearance (9). For Chinese melanoma patients, the neoadjuvant immunotherapy strategy may represent the current optimal choice. Immunotherapy provides powerful systemic control, while subsequent surgery may add value in the following ways: addressing potentially immunotherapy-resistant clones in the primary lesion and regional lymph nodes. Providing critical rescue treatment for patients with a poor pathological response to neoadjuvant therapy. Therefore, the “immunotherapy-first, TLND-second” sequence not only has a theoretical immunological basis but is also supported by clinical data in this Chinese cohort, providing important evidence for optimizing the timing of TLND in melanoma patients.
This conclusion appears to differ from some reported literature (28, 29), which suggests that TLND does not affect the efficacy of adjuvant therapy. We hypothesize that this discrepancy may arise from the failure to distinguish immunotherapy from other adjuvant treatments (e.g., targeted therapy), which is why our study excluded patients who received targeted therapy, radiation, or other non-immunotherapy treatments before recurrence to ensure study homogeneity.
Regarding safety, TLND did not significantly increase the risk of irAEs. The incidence of surgery-related adverse events was 50.0%, with lymphedema (26.4%) being the most common complication. However, as this was a retrospective study, some mild adverse events (e.g., asymptomatic effusions or those not requiring intervention) may have been incompletely recorded, potentially leading to an underestimation of surgery-related adverse events rates.
This study has several limitations that should be considered when interpreting the results. As a single-center retrospective study conducted in Northwest China, our cohort may not be fully representative of the broader melanoma population, thereby limiting the generalizability of our findings. The sample size, particularly in subgroup analyses, was relatively small, reducing statistical power and increasing the risk of Type II error. Significant selection bias may also have been introduced through non-random treatment allocation, influenced by regional healthcare policies, socioeconomic factors, and patient preferences. A substantial proportion of patients chose immunotherapy alone due to concerns about surgical complications, preferences for minimally invasive treatment, financial constraints, or insurance coverage limitations—reflecting underlying disparities in access to multidisciplinary care.
The median follow-up period of 18.9 months was insufficient to evaluate overall survival (OS), so recurrence-free survival (RFS) was used as the primary endpoint. The retrospective design also prevented systematic collection of serial biomarker data, such as paired tissue or blood samples across treatment timepoints, limiting assessment of dynamic changes in the tumor immune microenvironment or identification of predictive biomarkers. The higher rate of pathologically positive lymph nodes observed in our cohort compared to other studies requires further validation in independent datasets. These limitations highlight the need for future multi-institutional prospective studies with broader geographic representation and integrated translational components to validate the impact of lymph node dissection timing and better control for socioeconomic, clinical, and immunologic variables. Such studies should include standardized biomarker collection protocols and extended follow-up to facilitate robust evaluation of survival and correlative biological endpoints.
In summary, our findings suggest that performing TLND after ICI therapy is associated with improved RFS in Chinese melanoma patients, indicating that preservation of tumor-draining lymph node architecture prior to immunotherapy may enhance treatment efficacy. In contrast, upfront TLND before ICI initiation appears to be associated with poorer outcomes. These results are supported by clinical observations and immunological mechanisms. The relatively high incidence of surgery-related adverse events further underscores the need for caution when considering TLND prior to immunotherapy. Additionally, differential treatment responses may be influenced not only by TLND timing but also by distinct biological features of acral melanoma, which is predominant in the Chinese population.
Collectively, these results emphasize the critical role of immunotherapy and lymph node dissection sequencing in treatment outcomes, particularly in populations with unique melanoma subtypes and disease biology. Further validation through larger, multicenter prospective studies is essential to better inform individualized treatment strategies for melanoma patients in China and other Asian populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Fourth Military Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. BLZ: Formal Analysis, Investigation, Supervision, Writing – review & editing. BBZ: Data curation, Investigation, Writing – original draft. LZ: Data curation, Formal Analysis, Writing – original draft. HZ: Investigation, Supervision, Writing – review & editing. YC: Data curation, Supervision, Writing – review & editing. WZ: Supervision, Writing – review & editing. GZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing – review & editing. HG: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82272268, 82172607), and the Key Research and Development Projects of Shaanxi Province (Grant No. 2024SF-ZDCYL-04-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1673308/full#supplementary-material
Abbreviations
RFS, recurrence-free survival; ICIs, Immune Checkpoint Inhibitors; CLND, completely lymph node dissection; TLND, therapeutic lymph node dissection; irAEs, immune-related adverse events.
References
1. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. (2014) 370:599–609. doi: 10.1056/NEJMoa1310460
2. Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. (2006) 355:1307–17. doi: 10.1056/NEJMoa060992
3. Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE, et al. Melanoma, version 2.2016, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2016) 14:450–73. doi: 10.6004/jnccn.2016.0051
4. Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. (2017) 376:2211–22. doi: 10.1056/NEJMoa1613210
5. Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer NH, Berking C, et al. Final analysis of decog-slt trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. (2019) 37:3000–08. doi: 10.1200/JCO.18.02306
6. Faries MB, Thompson JF, Cochran A, Elashoff R, Glass EC, Mozzillo N, et al. The impact on morbidity and length of stay of early versus delayed complete lymphadenectomy in melanoma: results of the multicenter selective lymphadenectomy trial (i). Ann Surg Oncol. (2010) 17:3324–29. doi: 10.1245/s10434-010-1203-0
7. NCCN. Nccn guidelines for patients®: melanoma (2025). Available online at: https://www.nccn.org/patients/guidelines/content/PDF/melanoma-patient.pdf (Accessed July 25, 2025).
8. Fransen MF, Schoonderwoerd M, Knopf P, Camps MGM, Hawinkels LJAC, Kneilling M, et al. Tumor-draining lymph nodes are pivotal in pd-1/pd-l1 checkpoint therapy. JCI Insight. (2018) 3:e124507. doi: 10.1172/jci.insight.124507
9. Deng H, Zhou J, Chen H, Cai X, Zhong R, Li F, et al. Impact of lymphadenectomy extent on immunotherapy efficacy in post-resectional recurred non-small cell lung cancer: a multi-institutional retrospective cohort study. Int J Surg. (2024) 110:238–52. doi: 10.1097/JS9.0000000000000774
10. Wei J, Li D, Long H, and Han M. Immune microenvironment of tumor-draining lymph nodes: insights for immunotherapy. Front Immunol. (2025) 16:1562797. doi: 10.3389/fimmu.2025.1562797
11. Reijers ILM, Menzies AM, van Akkooi ACJ, Versluis JM, van den Heuvel NMJ, Saw RPM, et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage iii melanoma: the prado trial. Nat Med. (2022) 28:1178–88. doi: 10.1038/s41591-022-01851-x
12. Mandalà M, Rutkowski P, Galli F, Patuzzo R, De Giorgi V, Rulli E, et al. Acral lentiginous melanoma histotype predicts outcome in clinical stage i-ii melanoma patients: an international multicenter study. Esmo Open. (2022) 7:100469. doi: 10.1016/j.esmoop.2022.100469
13. Huang K, Fan J, and Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015, an analysis of the seer registry. J Surg Res. (2020) 251:329–39. doi: 10.1016/j.jss.2020.02.010
14. Hu R, Han Q, and Zhang J. Stat3: a key signaling molecule for converting cold to hot tumors. Cancer Lett. (2020) 489:29–40. doi: 10.1016/j.canlet.2020.05.035
15. Ogata D, Haydu LE, Glitza IC, Patel SP, Tawbi HA, McQuade JL, et al. The efficacy of anti-programmed cell death protein 1 therapy among patients with metastatic acral and metastatic mucosal melanoma. Cancer Med. (2021) 10:2293–99. doi: 10.1002/cam4.3781
16. Koizumi S, Yamazaki N, Ichigozaki Y, Kitagawa H, Kiniwa Y, Sato S, et al. Adjuvant anti–pd-1 monotherapy versus observation for stage iii acral melanoma of the sole: a multicenter retrospective study in Japanese patients. Jco Glob Oncol. (2025) 11:e2400644. doi: 10.1200/GO-24-00644
17. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage iii melanoma. N Engl J Med. (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
18. Chen Y, Zhong N, Cao L, Liu B, and Bu L. Surgical margins in head and neck squamous cell carcinoma: a narrative review. Int J Surg. (2024) 110:3680–700. doi: 10.1097/JS9.0000000000001306
19. Kirsch MJ, Yee EJ, Hosokawa P, Robinson W, Medina T, Mantle L, et al. Real world experience with omission of therapeutic lymph node dissection in clinical stage iii Malignant melanoma treated with checkpoint or kinase inhibition systemic therapy. Surg Oncol Insight. (2025) 2:100131. doi: 10.1016/j.soi.2025.100131
20. Ogata D, Tanese K, Nakamura Y, Otsuka M, Namikawa K, Funakoshi T, et al. Impact of the changes in the completion lymph node dissection criteria and approval of adjuvant therapies on the real-world outcomes of Japanese stage iii melanoma patients. Int J Clin Oncol. (2021) 26:2338–46. doi: 10.1007/s10147-021-02029-0
21. Reijers ILM, Menzies AM, Lopez-Yurda M, Versluis JM, Rozeman EA, Saw RPM, et al. Impact of personalized response-directed surgery and adjuvant therapy on survival after neoadjuvant immunotherapy in stage iii melanoma: comparison of 3-year data from prado and opacin-neo. Eur J Cancer. (2025) 214:115141. doi: 10.1016/j.ejca.2024.115141
22. Patel SP, Othus M, Chen Y, Wright GPJ, Yost KJ, Hyngstrom JR, et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
23. Rutkowski P, Szydlowski K, Nowecki ZI, Salamacha M, Goryn T, Mitrega-Korab B, et al. The long-term results and prognostic significance of cutaneous melanoma surgery using sentinel node biopsy with triple technique. World J Surg Oncol. (2015) 13:299. doi: 10.1186/s12957-015-0701-8
24. Kettlewell S, Moyes C, Bray C, Soutar D, MacKay A, Byrne D, et al. Value of sentinel node status as a prognostic factor in melanoma: prospective observational study. Bmj. (2006) 332:1423. doi: 10.1136/bmj.38849.680509.AE
25. Pasquali S, Mocellin S, Mozzillo N, Maurichi A, Quaglino P, Borgognoni L, et al. Nonsentinel lymph node status in patients with cutaneous melanoma: results from a multi-institution prognostic study. J Clin Oncol. (2014) 32:935–41. doi: 10.1200/JCO.2013.50.7681
26. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The pd-1/pd-l1-checkpoint restrains t cell immunity in tumor-draining lymph nodes. Cancer Cell. (2020) 38:685–700. doi: 10.1016/j.ccell.2020.09.001
27. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage iii melanoma. Nat Med. (2018) 24:1655–61. doi: 10.1038/s41591-018-0198-0
28. Placzke J, Rosińska M, Sobczuk P, Ziętek M, Kempa-Kamińska N, Cybulska-Stopa B, et al. Modern approach to melanoma adjuvant treatment with anti-pd1 immune check point inhibitors or braf/mek targeted therapy: multicenter real-world report. Cancers (Basel). (2023) 15:4384. doi: 10.3390/cancers15174384
Keywords: acral melanoma, lymph node dissection, immunotherapy, adjuvant, neoadjuvant
Citation: Hou S, Zhao B, Zhang B, Zhao L, Zhang H, Chen Y, Zhang W, Zhu G and Guan H (2025) Neoadjuvant outperforms adjuvant regimens in resectable advanced Chinese melanoma patients: lymph node preservation as a key immunological advantage. Front. Immunol. 16:1673308. doi: 10.3389/fimmu.2025.1673308
Received: 25 July 2025; Accepted: 19 September 2025;
Published: 01 October 2025.
Edited by:
Jorge B. Aquino, Universidad Austral, ArgentinaReviewed by:
Takaya Komori, Saitama Medical University International Medical Center, JapanJingqin Zhong, Fudan University, China
Copyright © 2025 Hou, Zhao, Zhang, Zhao, Zhang, Chen, Zhang, Zhu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Guan, Z3VhbmhhbzIwMjBAeWVhaC5uZXQ=; Guannan Zhu, emh1Z3Vhbm5hbkBzbXUuZWR1LmNu
†These authors have contributed equally to this work
 Shuguang Hou1,2†
Shuguang Hou1,2† Guannan Zhu
Guannan Zhu Hao Guan
Hao Guan