- 1Postgraduate Cultivation Base of Guangzhou University of Chinese Medicine, Panyu Central Hospital, Guangzhou, Guangdong, China
- 2Department of Radiology, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
- 3School of Life Sciences, South China Normal University, Guangzhou, Guangdong, China
- 4Department of Gynecology, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, Guangdong, China
The respiratory microbiome, as an integral component of the lung cancer microenvironment, exerts pivotal influences on tumorigenesis, immune homeostasis, and therapeutic response through intricate crosstalk with host immunity. Despite advancements, current limitations in lung cancer immunotherapy persist, including heterogeneous therapeutic responses, immune-related adverse events, and the lack of predictive biomarkers. These unmet clinical needs underscore the imperative to delineate the complex immune landscape of respiratory microbiome in lung cancer pathogenesis. This review systematically analyzes the hallmarks of respiratory dysbiosis (reduced α-diversity and enrichment of Streptococcus and Veillonella) and their associations with lung cancer staging, histological subtypes, and prognosis. We further elucidate how these microbial alterations influence tumor progression via metabolic-epigenetic-immune pathways. Additionally, we establish clinical correlations between microbiome signatures and both immune checkpoint inhibitor therapeutic efficacy/toxicity profiles, while examining the paradoxical effects of antibiotic exposure during immunotherapy. Emerging intervention strategies targeting the respiratory microbiome, such as aerosolized probiotics, engineered bacteria (e.g., Escherichia coli), and microbiota-derived nanomaterials, showcase potential in remodeling antitumor immunity and improving therapeutic outcomes. Our findings highlight the double-edged sword effect of the respiratory microbiota as biomarkers and therapeutic targets in lung cancer management, providing critical insights for clinical translation.
1 Introduction
Lung cancer represents the leading cause of cancer-related mortality worldwide, accounting for 18.7% of all deaths across all cancer types. Its pathogenesis is complex, involving genetic predisposition, environmental exposures, and microbiome dysbiosis (1). Due to the insidious nature of early-stage symptoms, approximately 60% of lung cancer patients are diagnosed at advanced stages (2). Immune checkpoint inhibitors (ICIs), which disrupt inhibitory pathways such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1) protein, and its ligand PD-L1 to potentiate antitumor immunity, have become a cornerstone of treatment for advanced non-small cell lung cancer(NSCLC), substantially improving 5-year survival rates (3, 4). Nevertheless, three unresolved scientific issues hinder progress (1): Heterogeneous therapeutic responses limit durable clinical benefits to 20–50% of patients (5) (2); Immune-related adverse events (irAEs) affect nearly half of the treated individuals (incidence: 43%) (6) (3); Existing predictive biomarkers, including PD-L1 expression and tumor mutational burden, exhibit suboptimal specificity for identifying true responders (7). Therefore, advancing systematic biomarker identification and optimizing clinical therapeutic efficacy remain critical scientific challenges requiring breakthroughs in lung cancer immunotherapy research.
In recent years, the synergistic development of multi-omics technologies—including metagenomic sequencing, single-cell transcriptomics, and spatial transcriptomics—has overcome the spatiotemporal resolution limitations of traditional microbiome research. These advances have not only confirmed the existence of low-biomass dynamic microbial communities in the respiratory tract under physiological conditions but also revealed their dynamic interactions with the lung cancer immune microenvironment (8). Clinically, lung cancer patients exhibit marked shifts in commensal microbial diversity and taxonomic abundance, where relative abundance of signature taxa (e.g., Streptococcus) correlates with disease trajectory and survival outcomes (9). Emerging preclinical evidence highlights beneficial microbiota transplantation and metabolic intervention (e.g., short-chain fatty acids and tryptophan derivatives) as potent adjuvants to PD-1/PD-L1 blockade, achieving near-abrogation of tumor growth in murine models (10, 11). Notably, microbiome dysbiosis may conversely exacerbate tumor progression and diminish therapeutic responses, particularly to immunotherapy (12). Mechanistically, commensal microbiota can influence immunotherapy efficacy by modulating immune cell activity and remodeling the tumor immune microenvironment (TIME) (including fostering an immunosuppressive environment or enhancing immune surveillance) (13). Consequently, restoring microbial homeostasis through probiotics, prebiotics, and postbiotics has emerged as a novel research paradigm in comprehensive cancer care, providing critical insights into the heterogeneity of immunotherapy outcomes and guiding personalized treatment strategies (14).
In this review, we systematically explore the multidimensional interaction network between the respiratory microbiome and lung cancer pathogenesis and immunotherapy efficacy, with a focus on the molecular mechanisms underlying microbiome-mediated modulation of immunotherapy outcomes and its translational clinical value. Finally, through critical evaluation of current preclinical limitations, we propose targeted future research directions and optimization strategies for leveraging the respiratory microbiome in precision lung cancer therapy.
2 Relationship between respiratory microbiome and clinical features of lung cancer
The pathogenesis of lung cancer constitutes a multifactorial, multistage process wherein microbiome-tumor microenvironment interactions play a critical regulatory role. Emerging evidence highlights that the compositional and functional heterogeneity of microbial communities across distinct anatomical regions of the respiratory tract may hold unique biological significance in lung carcinogenesis (15). Salivary microbiota is postulated as a primary source for pulmonary microbial colonization (16). The dysbiosis of salivary microbiota, which is characterized by diminished alpha diversity(95% CI 0.84-0.96) and Streptococcus-dominant enrichment(95% CI 1.06-1.22), correlates with heightened malignancy risk (17). Notably, lung cancer patients demonstrate markedly lower salivary microbial diversity and richness compared to healthy controls, while specific microbial taxa such as Capnocytophaga, Veillonella, Sphingomonas, and Blastomonas display significant enrichment (8, 18), implicating salivary microbiota as potential biomarkers for lung cancer. Sputum-derived microbial profiles provide more direct insights into lower respiratory tract ecology. Leng et al. (19) revealed that the abundance of Acidovorax and Veillonella was significantly increased in sputum of NSCLC patients by Droplet digital PCR, underscoring their biomarker potential for early detection and tumor classification. Metagenomic signatures further implicate Streptococcus viridans overabundance in sputum as a progression-associated indicator of lung cancer (20). Bronchoalveolar lavage fluid (BALF), the gold standard for detecting the lung microbiome in clinical settings, enables precise characterization of the peritumoral microenvironment. BALF microbial alterations not only associate closely with lung cancer development, progression, and histological subtypes (21), but also offer novel approaches for early detection (22). Multiple studies demonstrate reduced species diversity and richness in BALF microbiota from lung cancer patients compared to healthy controls, dominated by Bacillota, Pseudomonadota, Bacteroidota, Actinomycetota, and Fusobacteriota, with additional enrichment of Cyanobacteriota, Saccharibacteria, and genera including Prevotella, Streptococcus, Veillonella, Neisseria, Haemophilus, Clostridium, and Actinobacillus (23, 24). Wang et al. report diminished microbial diversity in both saliva and BALF samples from lung cancer patients, identifying Treponema and Filifactor in BALF as potential diagnostic biomarkers (25). Furthermore, significant differences in BALF microbiota exist between lung squamous cell carcinoma(LUSC) and adenocarcinoma, with Pseudomonadota enrichment in LUSC patients—particularly among males and heavy smokers—potentially linked to tumor invasiveness and metastatic potential (26).
Lung cancer exhibits significant spatial heterogeneity in microbial composition between intratumoral and peritumoral tissues. Bingula et al. (27) characterized unique microbiome signatures in saliva, BALF, peritumoral lung tissues, and tumor tissues, which showed associations with tumor localization, histological subtype, and immune activation. Notably, compared to peritumoral tissue microbiomes, the intratumoral microbiome is least influenced by anatomical location. Furthermore, Peters et al. (28) revealed that the microbial diversity in peritumoral tissues is significantly associated with the prognosis of NSCLC patients. Subsequent investigations have delineated specific relationships between intratumoral microbial diversity, abundance shifts of particular taxa, and oncogenesis. For instance, Yu et al. (29) reported increased Thermus abundance in advanced-stage patients and Legionella enrichment in metastatic cases. Li et al. (30) identified marked microbiome differences between malignant and non-malignant lung tissues in advanced NSCLC, particularly enrichment of Pseudomonadota (predominantly Acinetobacter and Acidovorax), Bacillota, and Actinomycetota. Smoking-related lung cancer tissues showed a correlation between Acidovorax enrichment and TP53 mutations (31), while Apopa et al. (32) detected Cyanobacteriota prevalence in lung adenocarcinomas with microcystin levels linked to PARP1 overexpression. Collectively, these findings support the existence of tumor-associated microbiome patterns in lung carcinogenesis and progression. Prognostically, intratumoral microbiota features show significant associations with recurrence and metastasis. Zhou et al. (33) demonstrated the predictive value of intratumoral microbiota for recurrence/metastasis risk in LUSC, with microbial risk scores correlating with survival outcomes. Patnaik et al. (34) established associations between preoperative lower respiratory tract microbiota and early NSCLC recurrence. Deng et al. (35) developed a translational prognostic model integrating 18 microbial taxa with a 19-gene glycolysis-lactate signature. Ma et al. (36) identified butyrate-producing bacteria (e.g., Roseburia) enrichment in recurrent cases, mechanistically linking butyrate-mediated HDAC2 inhibition to H3K27 hyperacetylation, upregulation of H19 expression, and M2 macrophage polarization-driven metastasis. These advancements underscore the diagnostic and prognostic potential of respiratory microbiota in lung cancer management (Table 1).
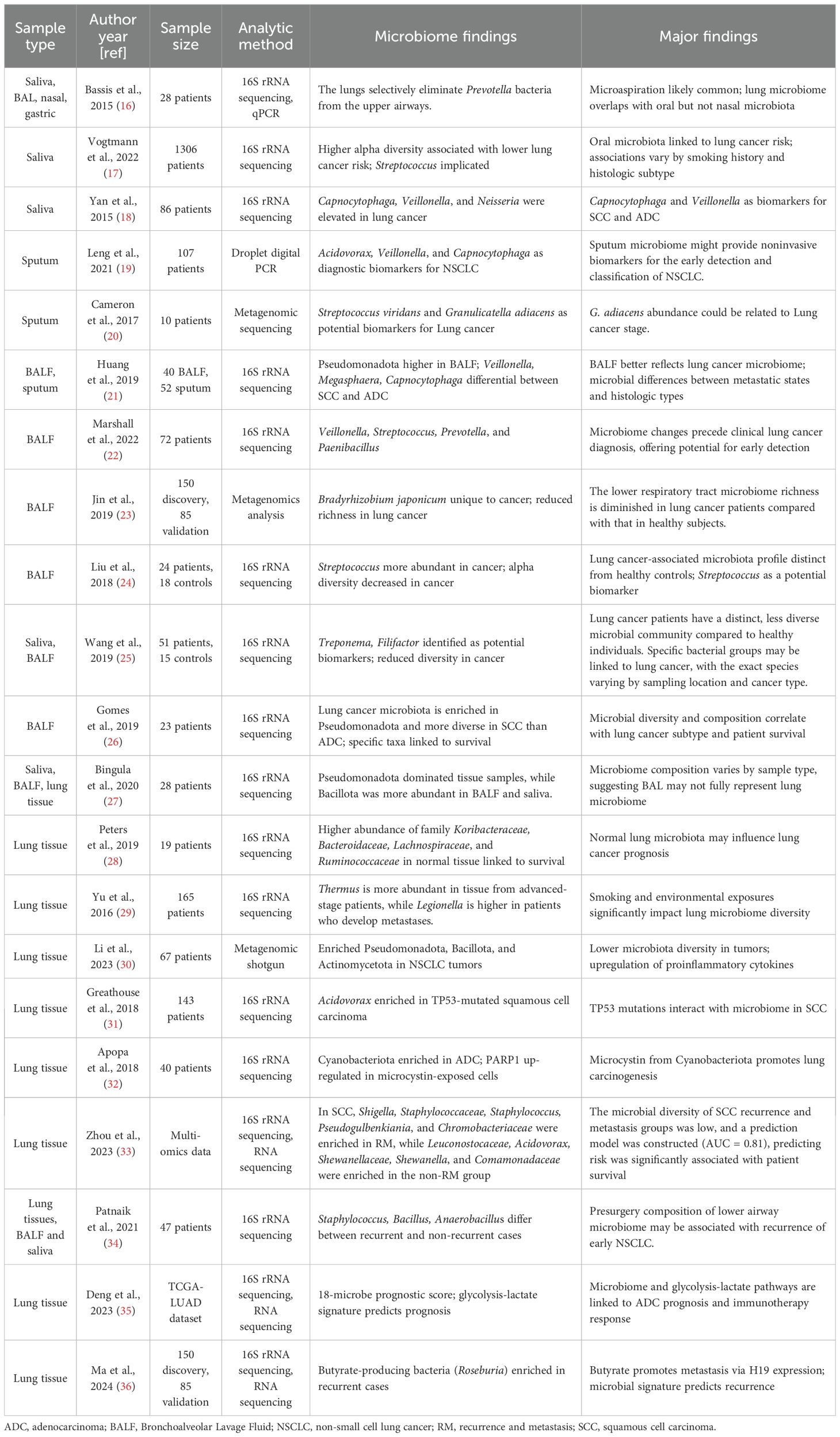
Table 1. Studies investigating the composition of the microbiome in different sample types obtained from patients with NSCLC.
3 Possible mechanisms of the microbiome on lung cancer pathogenesis
The dual role of the respiratory microbiome in lung cancer progression: Maintaining immune homeostasis versus driving inflammation during dysbiosis. As a pivotal regulator of the TIME, the respiratory microbiota exerts significant influence on pulmonary immune equilibrium and oncogenesis through multidimensional modulation of innate and adaptive immune networks (37). These biological effects appear contingent upon the immunogenicity and colonization patterns of specific microbial species (38). Within the adaptive immune system, a sophisticated regulatory network emerges through the Treg/Th17 cell balance. Research reveals intricate connections between neonatal lung microbial colonization, reduced airway hyperresponsiveness, and Treg cell subset (39). Healthy lower airways exhibit a characteristic microbial profile dominated by stable colonization of oropharyngeal commensals, including Prevotella, Veillonella, and Streptococcus (40). This colonization primarily occurs through microaspiration pathways, establishing a dynamic equilibrium with the respiratory epithelium (41). These commensal communities demonstrate significant interactions with Th17-mediated mucosal immunity, crucially modulating the balance between pulmonary immune surveillance and pathological inflammatory responses (42). The pulmonary innate immune defense comprises alveolar macrophages and γδT cell populations. Through BALF multi-omics analysis, Zheng’s team revealed that altered lung microbiota in NSCLC patients associates with suppressed tumor growth via M2 macrophage reduction and enhanced CD3+/CD8+T cell infiltration (43). Mechanistic investigations further demonstrate that lung commensals sustain γδT cell-mediated antitumor responses through alveolar macrophage regulation of CCL24 chemokine production (44). Crucially, pulmonary microbiota orchestrates tumor immune surveillance through γδT17-dependent mechanisms, playing indispensable roles in immune cell regulation, barrier maintenance, and host antitumor immunity coordination (45, 46).
Emerging evidence establishes that respiratory microbiome dysbiosis promotes lung carcinogenesis through multifactorial immunomodulatory pathways, which can be systematically categorized into three principal biological mechanisms (1): chronic inflammation secondary to immune homeostasis disruption (2), epigenetic modulation via microbial metabolites, and (3) genetic mutation/signaling pathway activation through host-microbe interactions (47) (Figure 1). At the immunomodulatory level, pulmonary dysbiosis directly drives tumorigenesis by inducing Th17/γδ T cell-mediated inflammatory responses (48). Clinical studies have demonstrated an association between pulmonary dysbiosis in NSCLC patients and Th17-mediated pulmonary inflammation, where IL-17 secretion by these cells perpetuates chronic inflammation and accelerates malignant progression (49). Preclinical investigations further reveal that lung commensal bacteria activate γδ T cells to initiate inflammation linked to adenocarcinoma. Notably, germ-free or antibiotic-treated mice exhibit significant protection against Kras mutation- and p53 deletion-driven lung carcinogenesis (38), suggesting γδ T cell hyperactivation as a critical microbiome-dependent mechanism in inflammation-driven malignancy. Microbial metabolites exhibit dual regulatory roles in epigenetic modulation (50). Reduced microbial diversity and increased Streptococcus abundance characterize the lower respiratory tract microbiome in lung cancer patients. Streptococcus pneumoniae-derived pneumolysin and pyruvate oxidase may promote carcinogenesis by disrupting host cell metabolism and apoptosis (24, 51). Cyanobacteriota-derived microcystins correlate with reduced CD36 and elevated PARP1 levels, suggesting therapeutic potential through microcystin transport inhibition or PARP1 targeting (32). Conversely, beneficial metabolites like butyrate (a short-chain fatty acid) and indole-3-aldehyde (I3A) demonstrate anticancer properties. Butyrate modulates miRNA expression in NSCLC A549 cells to suppress proliferation (52) and inhibits HDAC3 to drive monocyte-to-macrophage differentiation, thereby reducing inflammatory mediators and enhancing antimicrobial activity (53). The interaction between probiotic-derived I3A and tumor-infiltrating CD8+ T cells further enhances antitumor immunity (54). Aspects of genetic mutations and signaling pathways, microbiome-smoking interactions correlate with TP53 mutations in LUSC, particularly the enrichment of polycyclic aromatic hydrocarbon-degrading genera (Acidovorax, Massilia) in smokers’ tumor microbiota (31). Lower airway enrichment of Streptococcus and Veillonella in lung cancer patients associates with ERK/PI3K pathway activation, potentially fostering tumor progression (55). Intriguingly, these bidirectional regulatory mechanisms may extend to immunotherapy responses, though mechanistic details remain to be elucidated.
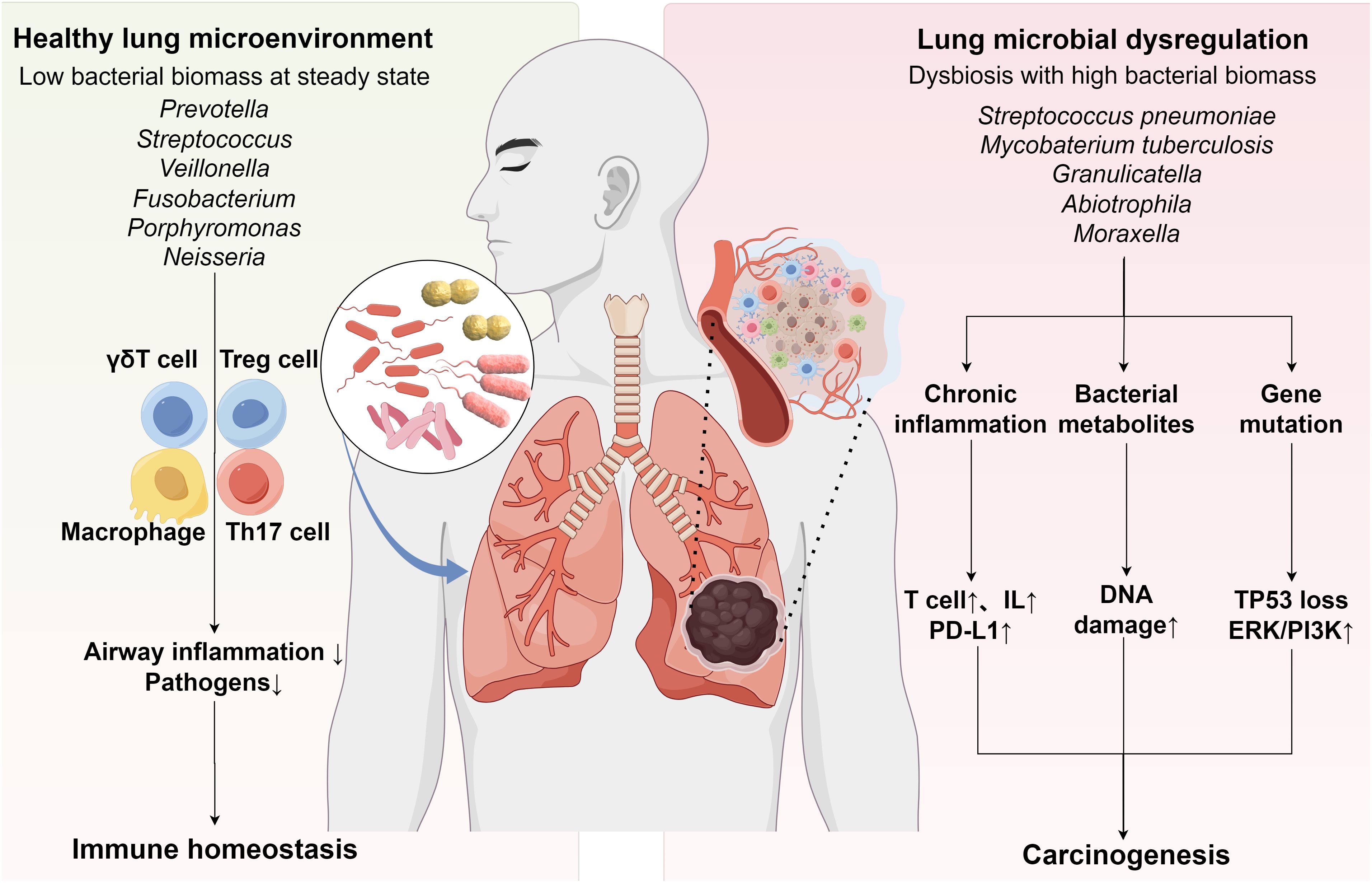
Figure 1. Interactions among the Respiratory Microbiota, Immune Homeostasis, and Lung Cancer. Under physiological conditions, commensal microbiota establishes multi-tiered immunoregulatory mechanisms by dynamically coordinating innate immunity (via Treg/Th17 balance regulation) and adaptive immunity (through alveolar macrophage polarization and γδT cell functional differentiation). This synergistic interaction prevents excessive inflammation while maintaining pulmonary immune homeostasis (Left panel). Respiratory dysbiosis may promote lung carcinogenesis through three interconnected mechanisms: chronic inflammation triggered by immune dysregulation, microbiota-derived metabolite-mediated epigenetic modifications, and host-microbe interaction-associated genetic mutations coupled with aberrant activation of oncogenic signaling pathways (Right panel).
4 Microbiota and lung cancer immunotherapy
4.1 Microbiota and efficacy of immunotherapy in lung cancer
The microbiome exerts dual regulatory effects on immunotherapy efficacy through distinct immunomodulatory pathways mediated by respiratory and gut microbiota (56). First, respiratory microbiota (particularly the abundance of specific bacteria) directly interact with the tumor immune microenvironment through local immunomodulation (57). For instance, Jang et al. (58) demonstrated that elevated Veillonella dispar abundance positively correlates with PD-L1 upregulation and enhanced immunotherapy responsiveness, whereas Gammapseudomonadota predominance associates with PD-L1 suppression and unfavorable prognosis. Furthermore, Zapata et al. (59) identified Gemella abundance in respiratory microbiota as a potential predictor of ICI resistance, while Lachnoanaerobaculum abundance shows potential as a biomarker for favorable ICI response. Notably, intratumoral microbial heterogeneity in lung cancer, particularly Fusobacterium enrichment, has been linked to immunotherapy resistance (60). Second, gut microbiota mediates systemic immunomodulation through the gut-lung axis, significantly impacting treatment outcomes (61). Substantial evidence reveals that ICI-responsive NSCLC patients typically exhibit enriched populations of Bifidobacterium (9), Akkermansia muciniphila(AKK) (62), Bacteroides (63), and Ruminococcus (64) in gut microbiota, with these microbial profiles correlating with improved PFS following ICI therapy (65).
With the widespread application of ICIs in lung cancer treatment, approximately 40% of patients experience irAEs affecting multiple organ systems, including the skin, gastrointestinal tract, and cardiopulmonary systems (66). Notably, irAEs exhibit a complex double-edged sword effect of clinical efficacy: while their occurrence correlates with improved patient survival (6), severe immune toxicities induced by ICIs may lead to treatment discontinuation or life-threatening complications (67). Consequently, identifying early predictive biomarkers for severe irAEs has become a priority in ICI patient management (68). In recent years, the microbiome has gained attention as a potential biomarker for predicting ICI efficacy and toxicity. The MITRE trial pioneered the evaluation of microbiome signatures as biomarkers to assess treatment response and toxicity in cancer patients receiving ICIs, aiming to elucidate dynamic associations between microbial features and clinical outcomes (69). Chau et al. (70) investigated correlations among nasal, oral, and gut microbiomes, treatment response, and irAEs in lung cancer patients undergoing ICI therapy, revealing significantly reduced gut microbiome alpha-diversity in these patients, which was strongly associated with both therapeutic response and irAEs. The Liu team further demonstrated that gut microbiota alterations in anti-PD-1-treated lung cancer patients were linked to immune-related diarrhea (71). Regarding microbial intervention strategies, clinical studies indicate that NSCLC patients receiving probiotics during immunotherapy exhibit significantly prolonged progression-free survival (PFS) and overall survival (OS), correlating with favorable clinical outcomes (72). Shaikh et al. (73) proposed that modulating the microbiome during treatment or applying microbiota transplantation might optimize therapeutic effects and mitigate irAEs. Mechanistic studies suggest that Clostridium butyricum supplementation enhances survival rates and ICI responsiveness in lung cancer patients (74), while AKK potentiates the anti-tumor efficacy of IL-2 immunotherapy (75). Additionally, Lactobacillus rhamnosus Probio-M9 has been shown to reverse antibiotic-induced dysbiosis and improve ICI efficacy (76). Chen et al. (77) reported that the postbiotic JK5G alleviates irAEs in advanced NSCLC patients receiving ICIs, demonstrating potential to enhance treatment outcomes while reducing adverse events. Collectively, these findings position the lung cancer microbiome as a promising diagnostic and predictive biomarker platform, potentially enabling patient stratification and treatment optimization.
4.2 Influence of antibiotics on immunotherapy efficacy in patients with lung cancer
The impact of antibiotic use on ICI efficacy in NSCLC has garnered significant attention in recent years. While current research generally suggests that antibiotics may compromise immunotherapy outcomes by disrupting commensal microbiota, current findings demonstrate notable heterogeneity. Substantial clinical evidence indicates that antibiotic exposure correlates with adverse prognostic outcomes and reduced ICI efficacy, with dose-dependent effects (59). Notably, Derosa et al. (78) documented a 5.5-month reduction in median PFS and 14.3-month decrease in OS among NSCLC patients receiving β-lactams or quinolones versus controls (P < 0.05). These findings gain support from a comprehensive meta-analysis by Abdelhamid et al. (79) (19 studies, n=2,932), demonstrating significant associations between antibiotic use and inferior survival outcomes (PFS HR = 1.64, OS HR = 1.67) in ICI-treated cohorts. Notably, antibiotic administration within ±60 days of treatment initiation correlates with diminished survival outcomes (80). Castello et al. (81) identified correlations between antibiotic exposure, elevated tumor metabolic burden, and accelerated disease progression. Beyond therapeutic efficacy, antibiotics may exacerbate irAEs. Jing et al. (82) reported a 1.39-fold higher risk of irAEs (95% CI 1.21-1.59) in antibiotic-exposed NSCLC patients, particularly evident in those receiving anti-PD-1/PD-L1 therapies. Preclinical models reinforce these clinical patterns: Antibiotic-treated mice exhibited enhanced Lewis lung carcinoma progression with reduced survival and increased pulmonary tumor burden (46). Routy et al. (83) revealed that oral antibiotics impair ICI efficacy, while fecal microbiota transplantation from ICI-responsive patients (enriched with AKK) restored anti-tumor responses to PD-1 blockade in germ-free or antibiotic-pretreated mice. Furthermore, Tan et al. (84) observed that although antibiotics aggravated ICI-associated colitis in murine models, specific probiotic supplementation mitigated these effects. Recent mechanistic insights highlight the microbiota-immune axis as a critical therapeutic determinant. An intact gut microbiome facilitates optimal treatment responses by modulating myeloid-derived cell functions within the tumor microenvironment (85). Conversely, antibiotic-induced dysbiosis promotes the migration of immunosuppressive intestinal Treg/Th17 cells to tumors via the MAdCAM-1-α4β7 axis, establishing an immunoinhibitory microenvironment that compromises PD-1 blockade efficacy (86).
However, other studies have failed to establish statistically significant associations between antibiotic exposure and survival outcomes in lung cancer patients. While Hakozaki et al. (87) reported a potential association between antibiotic use and reduced PFS in NSCLC patients through univariate analysis (P = 0.04), this finding lost statistical significance in multivariate models. Similarly, Nyein et al. (88) identified a non-significant trend toward worse OS in antibiotic-exposed NSCLC patients receiving immunotherapy (HR = 1.35, P = 0.145). In epidemiological investigations, Zhang et al. (89) observed an attenuated association between frequent antibiotic prescriptions (≥10 courses) and lung cancer risk after covariate adjustment (RR = 2.52 vs 1.31), concluding insufficient evidence for antibiotic-induced carcinogenesis. Preclinical findings by Noci et al. (90) revealed that aerosolized antibiotic treatment reduced pulmonary bacterial load in mice, decreased Treg cell populations, and enhanced T-cell/NK cell activity, thereby significantly inhibiting B16 melanoma lung metastasis and potentiating antitumor immunity. The divergent outcomes between aerosolized and oral antibiotic administration may stem from their distinct microbial targets: Systemic oral antibiotics primarily disrupt gut microbiota, potentially impairing the gut-lung axis and systemic antitumor immunity, whereas localized aerosol therapy selectively modulates respiratory microbiota to reshape the immunosuppressive pulmonary microenvironment. These findings underscore the need for judicious consideration of infection management strategies and anatomical site-specific microbiome modulation in lung cancer therapeutics.
5 The translational medical value of respiratory microbiota modulation in lung cancer immunotherapy
5.1 Aerosolized probiotics
Targeted drug delivery strategies leveraging respiratory tract anatomy have revolutionized lung cancer therapy. Aerosolized inhalation systems, distinguished by their favorable pharmacokinetic profiles, enable non-invasive, site-specific delivery of therapeutics across the air-blood barrier. This approach enhances localized drug deposition in tumor microenvironments while mitigating systemic toxicity (91). Notably, the Le Noci team demonstrated that aerosolized immunostimulants enable repeatable dosing in metastatic lung cancer patients, curtailing M2 macrophage polarization and boosting anti-tumor effects (92). Subsequent work established that aerosolized antibiotics/probiotics remodel pulmonary niches to foster anti-metastatic immunity (90). Further investigations showed that aerosolized live or inactivated Lactobacillus rhamnosus impeded murine lung tumorigenesis, marked by decreased tumor burden, reduced Treg infiltration, and elevated IgA titers (93). Aerosolized probiotic formulations may reverse immunosuppressive pulmonary microenvironments through dual mechanisms of microbial community restoration and immune tolerance modulation, thereby enhancing antitumor efficacy in lung cancer (94). Supporting evidence includes Zheng et al.’s findings (43) showing that inhalation of NSCLC patients’ lung microbiota induces significant compositional shifts in murine pulmonary microbiomes (with Pasteurella replacing Delftia as the dominant genus), subsequently inhibiting lung cancer cell proliferation. Youn et al. (95) revealed that the intranasal administration of viable Lactobacillus conferred stronger protection against murine influenza infection than oral delivery, with live bacteria exhibiting superior efficacy to inactivated counterparts.
5.2 Engineered bacteria
Engineered bacterial systems utilizing synthetic biology have emerged as a promising frontier in lung cancer therapeutics, particularly excelling in targeted drug delivery and immunomodulation (96). Current research bifurcates into two primary strategies: engineering bacterial outer membrane vesicles (OMVs) and developing programmable live bacteria. Distinct from conventional treatments, this technology platform combines three fundamental advantages: 1) Coordinated activation of innate and adaptive immunity to enhance therapeutic efficacy while reducing off-target toxicity; 2) Tumor-specific colonization through bacteria’s inherent immunogenicity; 3) Implementation of tumor microenvironment-responsive drug release through genetic engineering (97). Notably, engineered OMVs demonstrate enhanced immunotherapeutic specificity (98). Chen et al. (99) innovatively integrated OMV-coated drug-loaded polymeric micelles, where OMVs activate immune responses while micellar components simultaneously execute chemotherapy and immune sensitization of cancer cells to cytotoxic T lymphocytes. Kuerban et al. (100) developed attenuated Klebsiella pneumoniae-derived OMVs loaded with doxorubicin (DOX-OMV), demonstrating superior cell targeting and cytotoxicity in A549 lung adenocarcinoma models, coupled with potent tumor suppression in vivo. Parallel advancements include Gurbatri et al. ‘s probiotic system for localized PD-L1/CTLA-4 nanobody delivery (101) and Chowdhury et al.’s tumor microenvironment-responsive E. coli strain releasing CD47-blocking nanobodies, which collectively enhance T cell infiltration, induce tumor regression, and inhibit metastasis in preclinical models (102).
Specific engineered bacterial strains have demonstrated dual functionality as both delivery vectors for antitumor drugs and active modulators of tumor immunity. These strains stimulate the host immune system, enhance the presentation of tumor-associated antigens, and amplify effector T cell activity to achieve therapeutic effects (102). For example, engineered commensal microbes show promise in preventing cancer initiation and inducing regression in colorectal cancer (CRC) (103). Among these, Escherichia coli Nissle 1917 (EcN) is the most extensively studied engineered strain. Leveraging its intrinsic tumor-colonizing capability and well-established safety profile in humans, EcN has become a premier platform for synthetic biology applications (104). The Canale research team developed metabolically engineered EcN strains that continuously convert ammonia to L-arginine within the tumor microenvironment. This metabolic reprogramming markedly improved mitochondrial function and survival of CD8+ T cells, enhanced tumor infiltration depth and cytotoxic activity of effector T cells, and ultimately elevated therapeutic response rates to ICIs (105). To optimize EcN’s bioavailability, Xie et al. engineered a prebiotic-based “barrier” system that not only increased EcN’s survival in simulated gastric acid but also extended its intestinal retention time. Mechanistic investigations revealed that this prebiotic-EcN synergy reshapes gut microbiota composition and stimulates the production of SCFAs, particularly butyrate, offering an innovative strategy for managing inflammation-associated CRC (106). Further studies combining probiotics with prebiotics demonstrated that orally administered prebiotic-coated probiotic spores (spores-dex) modulate the gut microbiome, enrich SCFA-producing bacteria (e.g., Eubacterium and Roseburia), and significantly boost overall microbial diversity. Notably, these spores exhibited specific enrichment within CRC cells, where they locally produced anticancer SCFAs, effectively suppressing tumor growth (107).
5.3 Microbiota-derived nanomaterials
Advances in nanotechnology have positioned probiotic-derived nanomaterials as promising agents for personalized medicine. In drug delivery systems, these materials enable precision-targeted tumor therapies. For instance, Li et al. (108) employed the anaerobic probiotic Bifidobacterium infantis as a pre-implanted carrier to recruit bacteria, achieving localized enrichment of nano-drug missiles in hypoxic tumor regions of lung cancer. This approach enhances probiotic stability and bioavailability while amplifying therapeutic outcomes and minimizing adverse effects (109). In selective anti-tumor research, metabolite-driven nanosynthesis techniques offer distinct advantages. The Repotente group engineered gold nanoparticles using Lactobacillus acidophilus metabolites, which selectively targeted breast cancer MCF7 and lung cancer A549 cells (IC50: 0.075 mM and 0.07 mM, respectively) in vitro but remained nontoxic to normal cells and myoblasts (110). Shehata et al. (111) synthesized exopolysaccharide-coated selenium nanoparticles, demonstrating their antioxidant and anti-lung cancer potential. However, the IC50 against A549 cells (5.324 µg/mL) highlighted efficacy disparities across nanomaterial systems. Nanomaterials can also enhance the host immune response by modulating the microbiome (112). Zheng et al. (113) further showed that silver nanoparticle-embedded mucoadhesive hydrogels, by modulating oral microbiota (Peptostreptococcus), potentiated PD-1 blockade efficacy in a mouse model of oral squamous cell carcinoma (Figure 2).
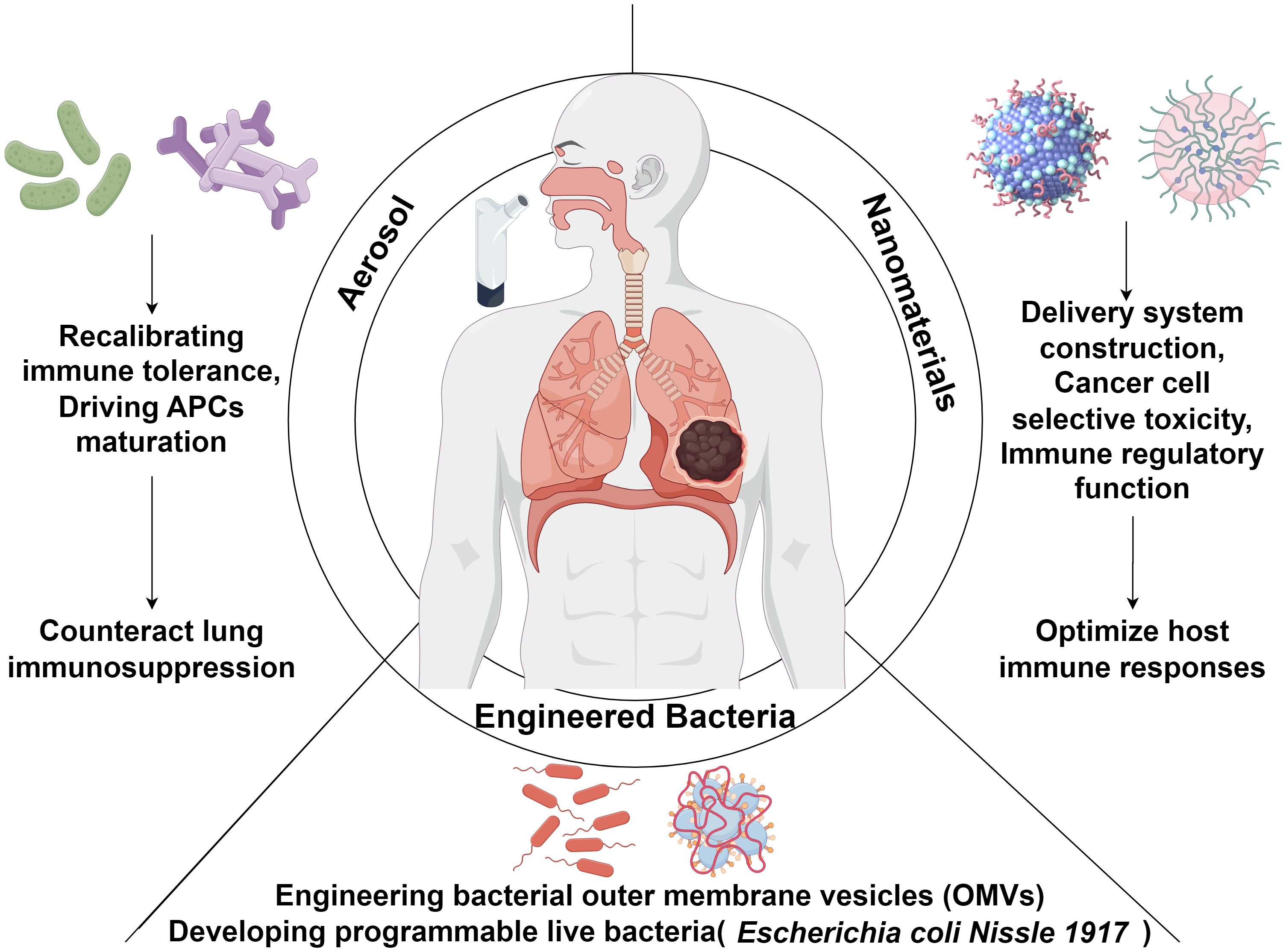
Figure 2. Schematic overview of probiotic-based strategies for modulating host immunity in lung cancer immunotherapy. Probiotic interventions, delivered via aerosolization, nanomaterial platforms, or bacterial engineering, offer novel avenues for enhancing antitumor immune responses in lung cancer. Aerosol delivery enables localized immune modulation by recalibrating immune tolerance and promoting the maturation of antigen-presenting cells (APCs), thereby mitigating the immunosuppressive tumor microenvironment within the lung. Nanomaterials can be tailored for targeted delivery, tumor-selective cytotoxicity, and immune-regulatory functions to potentiate host antitumor immunity. Advances in synthetic biology have enabled the engineering of bacterial outer membrane vesicles (OMVs) and programmable live microorganisms, such as Escherichia coli Nissle 1917 (EcN), further augmenting the efficacy and precision of lung cancer immunotherapeutic strategies.
6 Conclusion
This review synthesizes current evidence on the interplay between the pulmonary microbiome and lung carcinogenesis, highlighting its therapeutic relevance in immunotherapy. Nevertheless, existing research exhibits critical limitations (1): Predominantly cross-sectional designs restrict insights into microbial dynamic evolution (2); Methodological variability in sampling sites (e.g., sputum, BALF, tissue biopsies) and sequencing protocols compromises reproducibility and cross-study comparability (3); While correlative evidence underscores associations between lung microbiota composition/diversity and cancer outcomes, causative mechanisms remain poorly characterized (4); Greater attention must be paid to the interaction between airway microbiota and the host, as well as the potentially distinct effects and mechanisms of antibiotics in the gut versus the lungs; Moving forward, multidisciplinary approaches integrating single-cell spatial transcriptomics, metabolomics, and multi-omics are imperative to decode the microbiome-tumor-immune axis. Rigorous multi-center trials are needed to validate microbial biomarkers for clinical translation, alongside innovative therapies to enhance efficacy and minimize toxicity (46). Additionally, the safety and ethical implications of microbiome modulation demand thorough consideration to ensure clinical feasibility and safety.
Author contributions
PW: Investigation, Writing – original draft. CG: Writing – original draft, Methodology. QH: Writing – original draft, Software. XJ: Writing – original draft, Formal Analysis. MW: Writing – original draft, Resources. MH: Methodology, Writing – review & editing. ZX: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the China Postdoctoral Science Foundation (Grant No. 2024M750642) and National Natural Science Foundation of China (No.82171931).
Acknowledgments
Figures were created with Biorender (https://biorender.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1676302/full#supplementary-material
Abbreviations
AKK, Akkermansia muciniphila; BALF, Bronchoalveolar Lavage Fluid; CRC, Colorectal Cancer; CTLA-4, Cytotoxic T Lymphocyte-Associated Antigen 4; DCs, Dendritic Cells; DOX-OMV, Doxorubicin-Loaded Outer Membrane Vesicles; EcN, Escherichia coli Nissle 1917; ERK, Extracellular Signal-Regulated Kinase; HDAC3, Histone Deacetylase 3; ICIs, Immune Checkpoint Inhibitors; irAEs, Immune-Related Adverse Events; LUSC, Lung Squamous Cell Carcinoma; MDSCs, Myeloid-Derived Suppressor Cells; NK cells, Natural Killer Cells; NSCLC, Non-Small Cell Lung Cancer; OMVs, Outer Membrane Vesicles; OS, Overall Survival; PD-1, Programmed Cell Death-1; PD-L1, Programmed Death-Ligand 1; PFS, Progression-Free Survival; PI3K, Phosphoinositide 3-Kinase; ROS, Reactive Oxygen Species; SCFA, Short-Chain Fatty Acid; Th17, T Helper 17; Treg, Regulatory T Cell; TMB, Tumor Mutational Burden; IME, Tumor Immune Microenvironment
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-l1 expression in non–small cell lung cancer. JAMA Oncol. (2017) 3:1051. doi: 10.1001/jamaoncol.2017.0013
3. Galluzzi L, Chan TA, Kroemer G, Wolchok JD, and López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. (2018) 10:eaat7807. doi: 10.1126/scitranslmed.aat7807
4. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: Two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. (2017) 35:3924–33. doi: 10.1200/JCO.2017.74.3062
5. Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
6. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. (2020) 6:1952–6. doi: 10.1001/jamaoncol.2020.5012
7. Thai AA, Solomon BJ, Sequist LV, Gainor JF, and Heist RS. Lung cancer. Lancet. (2021) 398:535–54. doi: 10.1016/s0140-6736(21)00312-3
8. Yang J, Mu X, Wang Y, Zhu D, Zhang J, Liang C, et al. Dysbiosis of the salivary microbiome is associated with non-smoking female lung cancer and correlated with immunocytochemistry markers. Front Oncol. (2018) 8:520. doi: 10.3389/fonc.2018.00520
9. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-l1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
10. Zitvogel L, Daillère R, Roberti MP, Routy B, and Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. (2017) 15:465–78. doi: 10.1038/nrmicro.2017.44
11. Ramírez-Labrada AG, Isla D, Artal A, Arias M, Rezusta A, Pardo J, et al. The influence of lung microbiota on lung carcinogenesis, immunity, and immunotherapy. Trends Cancer. (2020) 6:86–97. doi: 10.1016/j.trecan.2019.12.007
12. McLean AEB, Kao SC, Barnes DJ, Wong KKH, Scolyer RA, Cooper WA, et al. The emerging role of the lung microbiome and its importance in non-small cell lung cancer diagnosis and treatment. Lung Cancer. (2022) 165:124–32. doi: 10.1016/j.lungcan.2022.01.011
13. Dong Q, Chen ES, Zhao C, and Jin C. Host-microbiome interaction in lung cancer. Front Immunol. (2021) 12:679829. doi: 10.3389/fimmu.2021.679829
14. Pennell NA. Strategies and end points in the development of novel immunotherapy trials for patients with unresectable, locally advanced non-small-cell lung cancer. J Clin Oncol. (2022) 40:3353–6. doi: 10.1200/JCO.22.00827
15. Kennedy MS and Chang EB. The microbiome: Composition and locations. Prog Mol Biol Transl Sci. (2020). 176:1–42. doi: 10.1016/bs.pmbts.2020.08.013
16. Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. (2015) 6:e00037. doi: 10.1128/mBio.00037-15
17. Vogtmann E, Hua X, Yu G, Purandare V, Hullings AG, Shao D, et al. The oral microbiome and lung cancer risk: An analysis of 3 prospective cohort studies. J Natl Cancer Inst. (2022) 114:1501–10. doi: 10.1093/jnci/djac149
18. Yan X, Yang M, Liu J, Gao R, Hu J, Li J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. (2015) 5:3111–22.
19. Leng Q, Holden VK, Deepak J, Todd NW, and Jiang F. Microbiota biomarkers for lung cancer. Diagnostics (Basel Switzerland). (2021) 11:407. doi: 10.3390/diagnostics11030407
20. Cameron SJS, Lewis KE, Huws SA, Hegarty MJ, Lewis PD, Pachebat JA, et al. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PloS One. (2017) 12:e0177062. doi: 10.1371/journal.pone.0177062
21. Huang D, Su X, Yuan M, Zhang S, He J, Deng Q, et al. The characterization of lung microbiome in lung cancer patients with different clinicopathology. Am J Cancer Res. (2019) 9:2047–63.
22. Marshall EA, Filho FSL, Sin DD, Lam S, Leung JM, and Lam WL. Distinct bronchial microbiome precedes clinical diagnosis of lung cancer. Mol Cancer. (2022) 21:68. doi: 10.1186/s12943-022-01544-6
23. Jin J, Gan Y, Liu H, Wang Z, Yuan J, Deng T, et al. Diminishing microbiome richness and distinction in the lower respiratory tract of lung cancer patients: A multiple comparative study design with independent validation. Lung Cancer (Amsterdam Netherlands). (2019) 136:129–35. doi: 10.1016/j.lungcan.2019.08.022
24. Liu H-X, Tao L-L, Zhang J, Zhu Y-G, Zheng Y, Liu D, et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int J Cancer. (2018) 142:769–78. doi: 10.1002/ijc.31098
25. Wang K, Huang Y, Zhang Z, Liao J, Ding Y, Fang X, et al. A preliminary study of microbiota diversity in saliva and bronchoalveolar lavage fluid from patients with primary bronchogenic carcinoma. Med Sci Monit. (2019) 25:2819–34. doi: 10.12659/MSM.915332
26. Gomes S, Cavadas B, Ferreira JC, Marques PI, Monteiro C, Sucena M, et al. Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci Rep. (2019) 9:12838. doi: 10.1038/s41598-019-49195-w
27. Bingula R, Filaire E, Molnar I, Delmas E, Berthon J-Y, Vasson M-P, et al. Characterisation of microbiota in saliva, bronchoalveolar lavage fluid, non-malignant, peritumoural and tumour tissue in non-small cell lung cancer patients: a cross-sectional clinical trial. Respir Res. (2020) 21:129. doi: 10.1186/s12931-020-01392-2
28. Peters BA, Hayes RB, Goparaju C, Reid C, Pass HI, and Ahn J. The microbiome in lung cancer tissue and recurrence-free survival. Cancer epidemiology Biomarkers prevention: Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol. (2019) 28:731–40. doi: 10.1158/1055-9965.EPI-18-0966
29. Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC, et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. (2016) 17:163. doi: 10.1186/s13059-016-1021-1
30. Li Y, Rao G, Zhu G, Cheng C, Yuan L, Li C, et al. Dysbiosis of lower respiratory tract microbiome are associated with proinflammatory states in non-small cell lung cancer patients. Thorac Cancer. (2023) 15:111–21. doi: 10.1111/1759-7714.15166
31. Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, Von Muhlinen N, et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. (2018) 19:123. doi: 10.1186/s13059-018-1501-6
32. Apopa PL, Alley L, Penney RB, Arnaoutakis K, Steliga MA, Jeffus S, et al. PARP1 is up-regulated in non-small cell lung cancer tissues in the presence of the Cyanobacteriotal toxin microcystin. Front Microbiol. (2018) 9:1757. doi: 10.3389/fmicb.2018.01757
33. Zhou X, Ji L, Ma Y, Tian G, Lv K, and Yang J. Intratumoral microbiota-host interactions shape the variability of lung adenocarcinoma and lung squamous cell carcinoma in recurrence and metastasis. Microbiol Spectr. (2023) 11:e03738–22. doi: 10.1128/spectrum.03738-22
34. Patnaik SK, Cortes EG, Kannisto ED, Punnanitinont A, Dhillon SS, Liu S, et al. Lower airway bacterial microbiome may influence recurrence after resection of early-stage non–small cell lung cancer. J Thorac Cardiovasc Surg. (2021) 161:419–429.e16. doi: 10.1016/j.jtcvs.2020.01.104
35. Deng X, Chen X, Luo Y, Que J, and Chen L. Intratumor microbiome derived glycolysis-lactate signatures depicts immune heterogeneity in lung adenocarcinoma by integration of microbiomic, transcriptomic, proteomic and single-cell data. Front Microbiol. (2023) 14:1202454. doi: 10.3389/fmicb.2023.1202454
36. Ma Y, Chen H, Li H, Zheng M, Zuo X, Wang W, et al. Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep Med. (2024) 5:101488. doi: 10.1016/j.xcrm.2024.101488
37. Maman S and Witz IP. A history of exploring cancer in context. Nat Rev Cancer. (2018) 18:359–76. doi: 10.1038/s41568-018-0006-7
38. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. (2019) 176:998–1013.e16. doi: 10.1016/j.cell.2018.12.040
39. Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-l1. Nat Med. (2014) 20:642–7. doi: 10.1038/nm.3568
40. Pattaroni C, Watzenboeck ML, Schneidegger S, Kieser S, Wong NC, Bernasconi E, et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe. (2018) 24:857–865.e4. doi: 10.1016/j.chom.2018.10.019
41. Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. mBio. (2017) 8:e02287–16. doi: 10.1128/mBio.02287-16
42. Segal LN, Clemente JC, Tsay J-CJ, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a th17 phenotype. Nat Microbiol. (2016) 1:16031. doi: 10.1038/nmicrobiol.2016.31
43. Zheng L, Xu J, Sai B, Zhu Y, Wang L, Yin N, et al. Microbiome related cytotoxically active CD8+ TIL are inversely associated with lung cancer development. Front Oncol. (2020) 10:531131. doi: 10.3389/fonc.2020.531131
44. Cheng M, Chen Y, Wang L, Chen W, Yang L, Shen G, et al. Commensal microbiota maintains alveolar macrophages with a low level of CCL24 production to generate anti-metastatic tumor activity. Sci Rep. (2017) 7:7471. doi: 10.1038/s41598-017-08264-8
45. Invernizzi R, Lloyd CM, and Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. (2020) 160:171–82. doi: 10.1111/imm.13195
46. Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, et al. Microbiota modulate tumoral immune surveillance in lung through a γδt17 immune cell-dependent mechanism. Cancer Res. (2014) 74:4030–41. doi: 10.1158/0008-5472.CAN-13-2462
47. Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J, et al. Role of lung and gut microbiota on lung cancer pathogenesis. J Cancer Res Clin Oncol. (2021) 147:2177–86. doi: 10.1007/s00432-021-03644-0
48. Goto T. Airway microbiota as a modulator of lung cancer. Int J Mol Sci. (2020) 21:3044. doi: 10.3390/ijms21093044
49. Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U.S.A. (2014) 111:5664–9. doi: 10.1073/pnas.1319051111
50. Khan FH, Bhat BA, Sheikh BA, Tariq L, Padmanabhan R, Verma JP, et al. Microbiome dysbiosis and epigenetic modulations in lung cancer: From pathogenesis to therapy. Semin Cancer Biol. (2022) 86:732–42. doi: 10.1016/j.semcancer.2021.07.005
51. Bryant JC, Dabbs RC, Oswalt KL, Brown LR, Rosch JW, Seo KS, et al. Pyruvate oxidase of Streptococcus pneumoniae contributes to pneumolysin release. BMC Microbiol. (2016) 16:271. doi: 10.1186/s12866-016-0881-6
52. Xiao X, Cao Y, and Chen H. Profiling and characterization of microRNAs responding to sodium butyrate treatment in a549 cells. J Cell Biochem. (2018) 119:3563–73. doi: 10.1002/jcb.26547
53. Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. (2019) 50:432–445.e7. doi: 10.1016/j.immuni.2018.12.018
54. Bender MJ, McPherson AC, Phelps CM, Pandey SP, Laughlin CR, Shapira JH, et al. Dietary tryptophan metabolite released by intratumoral lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. (2023) 186:1846–1862.e26. doi: 10.1016/j.cell.2023.03.011
55. Tsay J-CJ, Wu BG, Badri MH, Clemente JC, Shen N, Meyn P, et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med. (2018) 198:1188–98. doi: 10.1164/rccm.201710-2118OC
56. Shi R, Li Y, Liu H, and Chen J. Lung microbiota: Unexploited treasure hidden in the immune microenvironment of lung cancer. Thorac Cancer. (2021) 12:2964–6. doi: 10.1111/1759-7714.14159
57. Boesch M, Baty F, Albrich WC, Flatz L, Rodriguez R, Rothschild SI, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. OncoImmunology. (2021) 10:1988403. doi: 10.1080/2162402X.2021.1988403
58. Jang HJ, Choi JY, Kim K, Yong SH, Kim YW, Kim SY, et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir Res. (2021) 22:322. doi: 10.1186/s12931-021-01919-1
59. Zapata-García M, Moratiel-Pellitero A, Isla D, Gálvez E, Gascón-Ruiz M, Sesma A, et al. Impact of antibiotics, corticosteroids, and microbiota on immunotherapy efficacy in patients with non-small cell lung cancer. Heliyon. (2024) 10:e33684. doi: 10.1016/j.heliyon.2024.e33684
60. Chu S, Cheng Z, Yin Z, Xu J, Wu F, Jin Y, et al. Airway Fusobacterium is associated with poor response to immunotherapy in lung cancer. OTT. (2022) 15:201–13. doi: 10.2147/OTT.S348382
61. Bingula R, Filaire M, Radosevic-Robin N, Berthon J-Y, Bernalier-Donadille A, Vasson M-P, et al. Characterisation of gut, lung, and upper airways microbiota in patients with non-small cell lung carcinoma: Study protocol for case-control observational trial. Med (baltimore). (2018) 97:e13676. doi: 10.1097/MD.0000000000013676
62. He D, Li X, An R, Wang L, Wang Y, Zheng S, et al. Response to PD-1-based immunotherapy for non-small cell lung cancer altered by gut microbiota. Oncol Ther. (2021) 9:647–57. doi: 10.1007/s40487-021-00171-3
63. Grenda A, Iwan E, Krawczyk P, Frąk M, Chmielewska I, Bomba A, et al. Attempting to identify bacterial allies in immunotherapy of NSCLC patients. Cancers (Basel). (2022) 14:6250. doi: 10.3390/cancers14246250
64. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. (2020) 8:1243–50. doi: 10.1158/2326-6066.CIR-20-0196
65. Katayama Y, Yamada T, Shimamoto T, Iwasaku M, Kaneko Y, Uchino J, et al. The role of the gut microbiome on the efficacy of immune checkpoint inhibitors in Japanese responder patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. (2019) 8:847–53. doi: 10.21037/tlcr.2019.10.23
66. Les I, Martinez M, Perez-Francisco I, Cabero M, Teijeira L, Arrazubi V, et al. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. CANCERS. (2023) 15:1629. doi: 10.3390/cancers15051629
67. Kennedy LB and Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. (2020) 70:86–104. doi: 10.3322/caac.21596
68. Yin J, Wu Y, Yang X, Gan L, and Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-l1 therapy in non-small-cell lung cancer: Occurrence and mechanism. Front Immunol. (2022) 13:830631. doi: 10.3389/fimmu.2022.830631
69. Thompson NA, Stewart GD, Welsh SJ, Doherty GJ, Robinson MJ, Neville BA, et al. The MITRE trial protocol: A study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer. (2022) 22:99. doi: 10.1186/s12885-021-09156-x
70. Chau J, Yadav M, Liu B, Furqan M, Dai Q, Shahi S, et al. Prospective correlation between the patient microbiome with response to and development of immune-mediated adverse effects to immunotherapy in lung cancer. BMC Cancer. (2021) 21:808. doi: 10.1186/s12885-021-08530-z
71. Liu T, Xiong Q, Li L, and Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy. (2019) 11:385–96. doi: 10.2217/imt-2018-0144
72. Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: A multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer. (2021) 149:473–82. doi: 10.1002/ijc.33557
73. Shaikh FY, Gills JJ, and Sears CL. Impact of the microbiome on checkpoint inhibitor treatment in patients with non-small cell lung cancer and melanoma. EBioMedicine. (2019) 48:642–7. doi: 10.1016/j.ebiom.2019.08.076
74. Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, et al. Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. (2020) 8:1236–42. doi: 10.1158/2326-6066.CIR-20-0051
75. Shi L, Sheng J, Chen G, Zhu P, Shi C, Li B, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer. (2020) 8:e000973. doi: 10.1136/jitc-2020-000973
76. Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok L-Y, et al. Adjunctive probiotic lactobacillus rhamnosus probio-m9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol. (2021) 12:772532. doi: 10.3389/fimmu.2021.772532
77. Chen M, Ma L, Yu H, Huang S, Zhang J, Gong J, et al. JK5G postbiotics attenuate immune-related adverse events in NSCLC patients by regulating gut microbiota: A randomized controlled trial in China. Front Oncol. (2023) 13:1155592. doi: 10.3389/fonc.2023.1155592
78. Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. (2018) 29:1437–44. doi: 10.1093/annonc/mdy103
79. Abdelhamid A, Tuminello S, Ivic-Pavlicic T, Flores R, and Taioli E. Antibiotic treatment and survival in non-small cell lung cancer patients receiving immunotherapy: A systematic review and meta-analysis. Transl Lung Cancer Res. (2023) 12:2427–39. doi: 10.21037/tlcr-23-597
80. Lurienne L, Cervesi J, Duhalde L, de Gunzburg J, Andremont A, Zalcman G, et al. NSCLC immunotherapy efficacy and antibiotic use: A systematic review and meta-analysis. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2020) 15:1147–59. doi: 10.1016/j.jtho.2020.03.002
81. Castello A, Rossi S, Toschi L, and Lopci E. Impact of antibiotic therapy and metabolic parameters in non-small cell lung cancer patients receiving checkpoint inhibitors. J Clin Med. (2021) 10:1251. doi: 10.3390/jcm10061251
82. Jing Y, Chen X, Li K, Liu Y, Zhang Z, Chen Y, et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J Immunother Cancer. (2022) 10:e003779. doi: 10.1136/jitc-2021-003779
83. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
84. Tan B, Liu Y-X, Tang H, Chen D, Xu Y, Chen M-J, et al. Gut microbiota shed new light on the management of immune-related adverse events. Thorac Cancer. (2022) 13:2681–91. doi: 10.1111/1759-7714.14626
85. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. (2013) 342:967–70. doi: 10.1126/science.1240527
86. Fidelle M, Rauber C, Alves Costa Silva C, Tian A-L, Lahmar I, de la Varende A-LM, et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science. (2023) 380:eabo2296. doi: 10.1126/science.abo2296
87. Hakozaki T, Okuma Y, Omori M, and Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non−small cell lung cancer. Oncol Lett. (2019) 17:2946–52. doi: 10.3892/ol.2019.9899
88. Nyein AF, Bari S, Hogue S, Zhao Y, Maller B, Sha S, et al. Effect of prior antibiotic or chemotherapy treatment on immunotherapy response in non-small cell lung cancer. BMC Cancer. (2022) 22:101. doi: 10.1186/s12885-022-09210-2
89. Zhang H, García Rodríguez LA, and Hernández-Díaz S. Antibiotic use and the risk of lung cancer. Cancer epidemiology Biomarkers prevention: Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol. (2008) 17:1308–15. doi: 10.1158/1055-9965.EPI-07-2817
90. Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, et al. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: A strategy to promote immunosurveillance against lung metastases. Cell Rep. (2018) 24:3528–38. doi: 10.1016/j.celrep.2018.08.090
91. Traversi L, Perez-Miranda J, and Polverino E. Inhaled treatments and the future of respiratory diseases: Holding our breath. Respiration. (2019) 97:498–500. doi: 10.1159/000496357
92. Le Noci V, Tortoreto M, Gulino A, Storti C, Bianchi F, Zaffaroni N, et al. Poly(I:C) and CpG-ODN combined aerosolization to treat lung metastases and counter the immunosuppressive microenvironment. Oncoimmunology. (2015) 4:e1040214. doi: 10.1080/2162402X.2015.1040214
93. Le Noci V, Bernardo G, Manenti G, Infante G, Khaleghi Hashemian D, Minoli L, et al. Live or heat-killed lactobacillus rhamnosus aerosolization decreases adenomatous lung cancer development in a mouse carcinogen-induced tumor model. Int J Mol Sci. (2022) 23:12748. doi: 10.3390/ijms232112748
94. Wang D, Cheng J, Zhang J, Zhou F, He X, Shi Y, et al. The role of respiratory microbiota in lung cancer. Int J Biol Sci. (2021) 17:3646–58. doi: 10.7150/ijbs.51376
95. Youn H-N, Lee D-H, Lee Y-N, Park J-K, Yuk S-S, Yang S-Y, et al. Intranasal administration of live lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. (2012) 93:138–43. doi: 10.1016/j.antiviral.2011.11.004
96. Li W, Zhang Z, Liu J, Wang B, Pu G, Li J, et al. Nanodrug-loaded Bifidobacterium bifidum conjugated with anti-death receptor antibody for tumor-targeted photodynamic and sonodynamic synergistic therapy. Acta Biomater. (2022) 146:341–56. doi: 10.1016/j.actbio.2022.05.016
97. Zhou S, Gravekamp C, Bermudes D, and Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. (2018) 18:727–43. doi: 10.1038/s41568-018-0070-z
98. Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. (2017) 8:626. doi: 10.1038/s41467-017-00729-8
99. Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, et al. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. (2020) 20:11–21. doi: 10.1021/acs.nanolett.9b02182
100. Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. (2020) 10:1534–48. doi: 10.1016/j.apsb.2020.02.002
101. Gurbatri CR, Lia I, Vincent R, Coker C, Castro S, Treuting PM, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med. (2020) 12:eaax0876. doi: 10.1126/scitranslmed.aax0876
102. Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, and Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. (2019) 25:1057–63. doi: 10.1038/s41591-019-0498-z
103. Ho CL, Tan HQ, Chua KJ, Kang A, Lim KH, Ling KL, et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat BioMed Eng. (2018) 2:27–37. doi: 10.1038/s41551-017-0181-y
104. Lynch JP, Goers L, and Lesser CF. Emerging strategies for engineering escherichia coli nissle 1917-based therapeutics. Trends Pharmacol Sci. (2022) 43:772–86. doi: 10.1016/j.tips.2022.02.002
105. Canale FP, Basso C, Antonini G, Perotti M, Li N, Sokolovska A, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. (2021) 598:662–6. doi: 10.1038/s41586-021-04003-2
106. Xie A, Ji H, Liu Z, Wan Y, Zhang X, Xiong H, et al. Modified prebiotic-based “shield” armed probiotics with enhanced resistance of gastrointestinal stresses and prolonged intestinal retention for synergistic alleviation of colitis. ACS Nano. (2023) 17:14775–91. doi: 10.1021/acsnano.3c02914
107. Zheng D-W, Li R-Q, An J-X, Xie T-Q, Han Z-Y, Xu R, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer. Adv Mater. (2020) 32:e2004529. doi: 10.1002/adma.202004529
108. Li Y, Leng Q, Zhang Y, Lin S, Wen Q, Lu Y, et al. Anaerobic bacteria mediated ‘smart missile’ targeting tumor hypoxic area enhances the therapeutic outcome of lung cancer. Chem Eng J. (2022) 438:135566. doi: 10.1016/j.cej.2022.135566
109. Patarroyo JL, Fonseca E, Cifuentes J, Salcedo F, Cruz JC, and Reyes LH. Gelatin-graphene oxide nanocomposite hydrogels for kluyveromyces lactis encapsulation: Potential applications in probiotics and bioreactor packings. Biomolecules. (2021) 11:922. doi: 10.3390/biom11070922
110. Repotente EC, Carreon AJ, Devanadera MK, Esmalla MS, and Santiago-Bautista M. Cytotoxic potential on human breast and lung cancer cells of the biosynthesized gold nanoparticles from the reduction of chloroauric acid by lactic acid isolated from lactobacillus acidophilus. Front Mater. (2022) 9:933749. doi: 10.3389/fmats.2022.933749
111. Shehata NS, Elwakil BH, Elshewemi SS, Ghareeb DA, and Olama ZA. Selenium nanoparticles coated bacterial polysaccharide with potent antimicrobial and anti-lung cancer activities. Sci Rep. (2023) 13:21871. doi: 10.1038/s41598-023-48921-9
112. Molina-Tijeras JA, Gálvez J, and Rodríguez-Cabezas ME. The immunomodulatory properties of extracellular vesicles derived from probiotics: A novel approach for the management of gastrointestinal diseases. Nutrients. (2019) 11:1038. doi: 10.3390/nu11051038
Keywords: respiratory microbiome, non-small cell lung cancer, immunity, immunotherapy, antibiotics
Citation: Wang P, Ge C, Jing X, Han Q, Wang M, Huang M and Xiang Z (2025) Respiratory microbiome-host interaction on lung carcinogenesis, immunity, and immunotherapy. Front. Immunol. 16:1676302. doi: 10.3389/fimmu.2025.1676302
Received: 30 July 2025; Accepted: 10 November 2025; Revised: 05 November 2025;
Published: 28 November 2025.
Edited by:
Donald O. Natvig, University of New Mexico, United StatesReviewed by:
Kaiser Jamil, Bhagwan Mahavir Medical Research Centre, IndiaDorota Pastuszak-Lewandoska, Medical University of Lodz, Poland
Copyright © 2025 Wang, Ge, Jing, Han, Wang, Huang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghua Huang, aHVhbmdtaW5odWFAemp1LmVkdS5jbg==; Zhiming Xiang, eGlhbmd6aGltaW5nQHB5aG9zcGl0YWwuY29tLmNu
†These authors have contributed equally to this work
 Peng Wang1,2†
Peng Wang1,2† Qijia Han
Qijia Han Zhiming Xiang
Zhiming Xiang