- 1Department of Data Science, The Catholic University of Korea, Gyeonggi-do, Republic of Korea
- 2Department of Surgery, Uijeongbu St. Mary Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 3Department of Hospital Pathology, Eunpyeong St. Mary Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
- 4Department of Hospital Pathology, Uijeongbu St. Mary Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Background: Neutrophil extracellular traps (NETs) are fibrous, web like chromatin structures released by activated neutrophils that entrap and immobilize pathogens through histones, granule derived proteolytic enzymes, and myeloperoxidase (MPO) dependent mechanisms. Beyond host defense, NETs have been implicated in tumor progression; yet anticancer activity also has been reported, and findings vary across specimen types (tumor tissue versus blood) and detection methods, antibody panels, leaving their role in oncogenesis uncertain. We performed a systematic review and meta-analysis to define the prognostic significance of NETs in cancer, stratified by specimen type, detection technique, and antibody panels.
Methods: Following PRISMA guidelines, we searched PubMed, EMBASE, and the Cochrane Library for studies published through August 10, 2023, that reported quantitative NET measurements linked to oncologic outcomes.
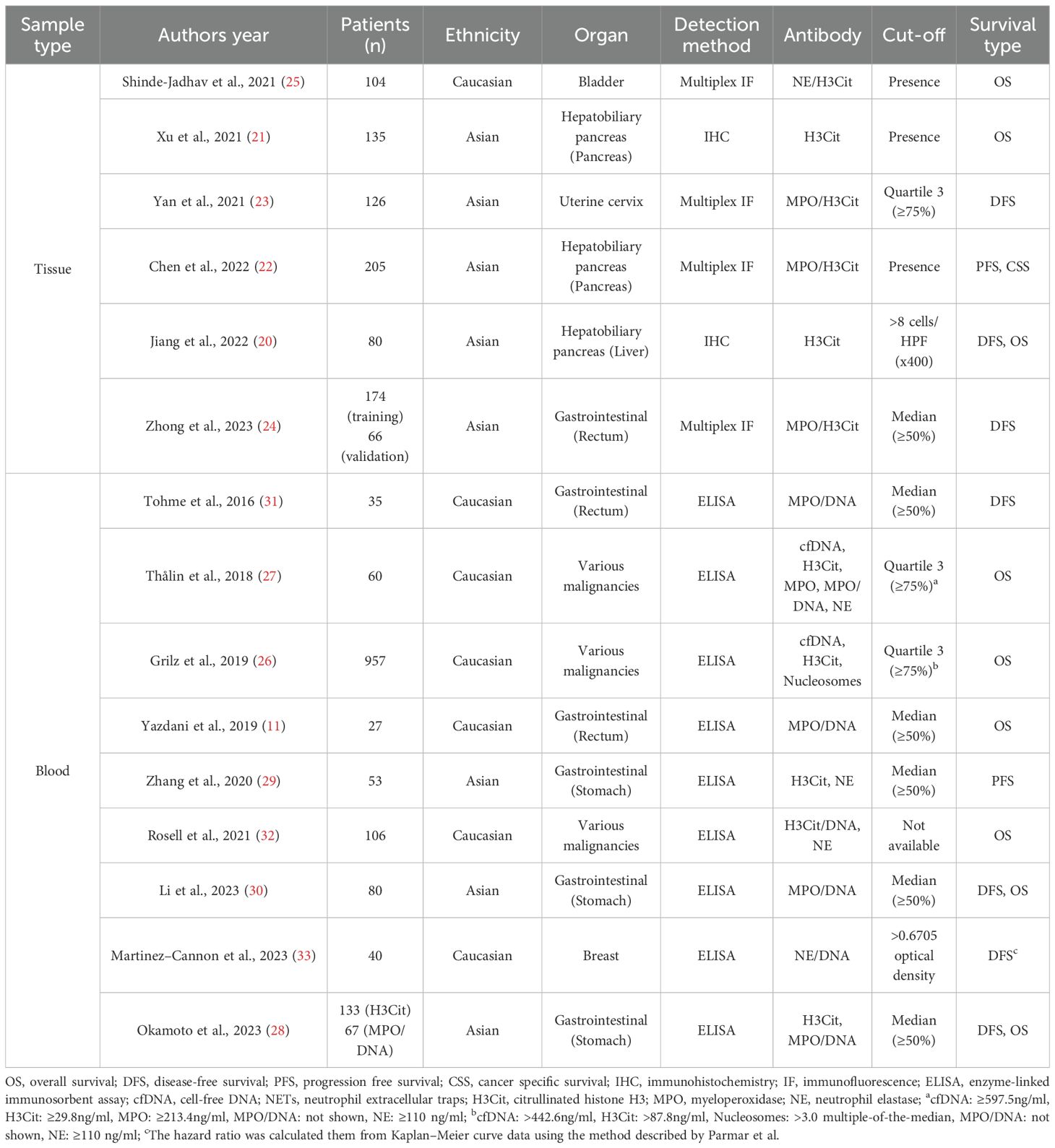
Results: Fifteen studies (5,202 patients; publication years 2016–2023) reporting hazard ratios (HRs) for overall survival (OS) and disease free survival (DFS) relative to NET levels met inclusion criteria. Six studies evaluated tumor derived NETs in tissue and nine assessed circulating NETs in blood. Among tissue studies, two used immunohistochemistry for citrullinated histone H3 (H3Cit) alone, and four applied multiplex immunofluorescence for MPO/H3Cit or neutrophil elastase (NE)/H3Cit. Among blood studies, enzyme linked immunosorbent assays targeting MPO/DNA predominated, followed by H3Cit assays. Higher NET levels were significantly associated with worse OS (HR 1.80; 95% CI 1.35–2.41) and DFS (HR 2.26; 95% CI 1.82–2.82), irrespective of tissue or blood based measurement. Prognostic associations were robust for MPO/DNA, H3Cit, and NE, but not for cell free DNA.
Conclusion: Elevated NET levels predict poorer outcomes in patients with cancer independent of specimen source and most analytic modalities (except cell free DNA), supporting NETs as a promising biomarker for risk stratification and precision oncologic decision making.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025596821.
Introduction
Neutrophils are the first responders of the innate immune system, and they play a pivotal role not only in defending the host against invading pathogens (1, 2) but also in modulating the tumor microenvironment and influencing cancer progression (3). In addition to their conventional antimicrobial functions, recent attention has been focused on neutrophil extracellular traps (NETs) and fibrous web-like chromatin structures released by activated neutrophils (4–7). NETs contribute to host defense by entrapping and immobilizing pathogens through a process that relies on histones, granule-derived proteolytic enzymes, and myeloperoxidase (MPO) (6, 8).
Emerging evidence has highlighted the pro-tumorigenic role of NETs in various malignancies (8–10). This role is primarily attributed to their involvement in cellular injury and tissue regeneration, which in turn trigger excessive inflammatory responses (8–10). NETs have been reported to facilitate tumor cell proliferation (11), metastatic dissemination (12–14), immune evasion (15), and cancer-associated thrombosis (16–18).
Nevertheless, NETs have also been reported to exert antitumor effects in certain contexts, and their functional outcomes appear to vary according to tumor type and microenvironmental conditions (19). Moreover, studies investigating the prognostic effect of NETs in cancer have used different sample sources, including blood and tumor tissues. A wide range of detection methods, such as immunohistochemistry (IHC) (20, 21), immunofluorescence (IF) (22–25), and enzyme-linked immunosorbent assay (ELISA) (11, 26–33) have been employed using diverse antibodies, including citrullinated histone H3 (H3Cit) (20, 21, 26–29), MPO/H3Cit (22–24), neutrophil elastase (NE)/H3Cit (25), MPO/DNA (11, 27, 28, 30, 31), NE (27, 29, 32), and cell-free DNA (cfDNA) (26, 27). This methodological variability significantly contributes to heterogeneity in results, complicating the interpretation and comparison of results across studies.
We aimed to address the current literature gaps by systematically analyzing the prognostic relevance of NETs in cancer. We specifically evaluated the heterogeneous findings of NET-related studies by stratifying our analyses based on sample source (tissue vs. blood), detection methodologies, and antibody selection. Through this comprehensive meta-analysis, we aimed to deepen our understanding of the role of NETs in cancer progression and contribute to future clinical applications, including the development of NET-targeted therapeutic strategies.
Methods
Search strategy
This meta-analysis was prospectively submitted to PROSPERO (CRD42025596821) and was approved by the Institutional Review Board of the Catholic University of Korea, College of Medicine (UC22ZASI0033). A comprehensive literature search of relevant English-language articles published up to August 10, 2023, was conducted across three major electronic databases (PubMed, EMBASE, and the Cochrane Library) using the search strategy outlined in Supplementary Table S1. Additionally, a manual search was performed by screening the reference list of a key article (10). Potentially relevant titles were cross-checked with records from the database search, and any unmatched studies underwent full-text review in accordance with the predefined inclusion and exclusion criteria. EndNote X20 (Build 10136; Thomson Reuters, New York, NY, USA) was used to manage the retrieved studies.
Inclusion and exclusion criteria
This meta-analysis applied the following inclusion criteria: 1) studies on the relationship between NETs and prognosis of patients with cancer was assessed; 2) NETs identified with accurate examination; 3) studies that provided sufficient information on hazard ratios (HRs) of patient survival; 4) studies that demonstrated an association between NETs and clinicopathological features; and 5) articles written in English language. The following exclusion criteria were applied: 1) duplicate studies, reviews, case reports, letters, and conference proceedings; 2) studies that did not show an association between NETs and survival or clinicopathological parameters; 3) studies related to cancer cell lines and animal models; and 4) studies with insufficient data on HRs and 95% confidence intervals (CIs) that could be extracted or calculated.
Data extraction and assessment of study quality
Data extraction was performed by five independent reviewers (S. L., E.Y.K., W.P., Y.S.L., and K.Y.). In cases of disagreement, consensus was reached among them. The following data were extracted from all included studies: author, year, ethnicity, number of patients, antibody, detection method, organ, sample type, pathological stage, and survival outcomes such as overall survival (OS) or disease-free survival (DFS). Risk of bias was assessed, and studies that met the inclusion criteria were selected using the Quality in Prognostic Studies tool. In studies without HRs, we used data on the Kaplan–Meier curve to calculate the HR using the method described by Parmar et al. (34).
Statistical analysis
Statistical analysis was conducted using Review Manager Software (version 5.4.1; Cochrane Collaboration, Copenhagen, Denmark). Pooled HRs with 95% CIs were used to assess the association between NETs and OS. HRs >1 indicated poor survival, whereas those <1 indicated better survival. The association between NETs and other clinicopathological parameters was analyzed using the Mantel–Haenszel pooled odds ratio (OR) with 95% CIs and combined effective value. An I2 value of <50% indicated no heterogeneity among the studies. A subgroup analysis was conducted to explore potential sources of heterogeneity. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram and forest plots were generated using Review Manager software.
Results
Eligible studies
An initial literature search included 3,130 articles from PubMed, EMBASE, and the Cochrane Library (Figure 1). From the reference list, several articles were initially considered potentially relevant, but nearly all had already been captured through our database searches, supporting the robustness of our strategy. One additional article mentioned “angiogenesis,” yet full-text assessment revealed that it was study on cancer cell lines and therefore did not meet our inclusion criteria. After removing 862 duplicate articles, the remaining 2,268 articles were screened based on the reference type criteria. The study by Wang et al. (35) was excluded from the systematic review owing to discrepancies among in figure legends, corresponding graphical data, and main text descriptions. Considering available data on prognosis, clinicopathological parameters, evaluation methods, and their association with NETs, 15 articles met the inclusion criteria (Figure 1). Most of the studies showed a low risk of bias (Supplementary Figure S1).
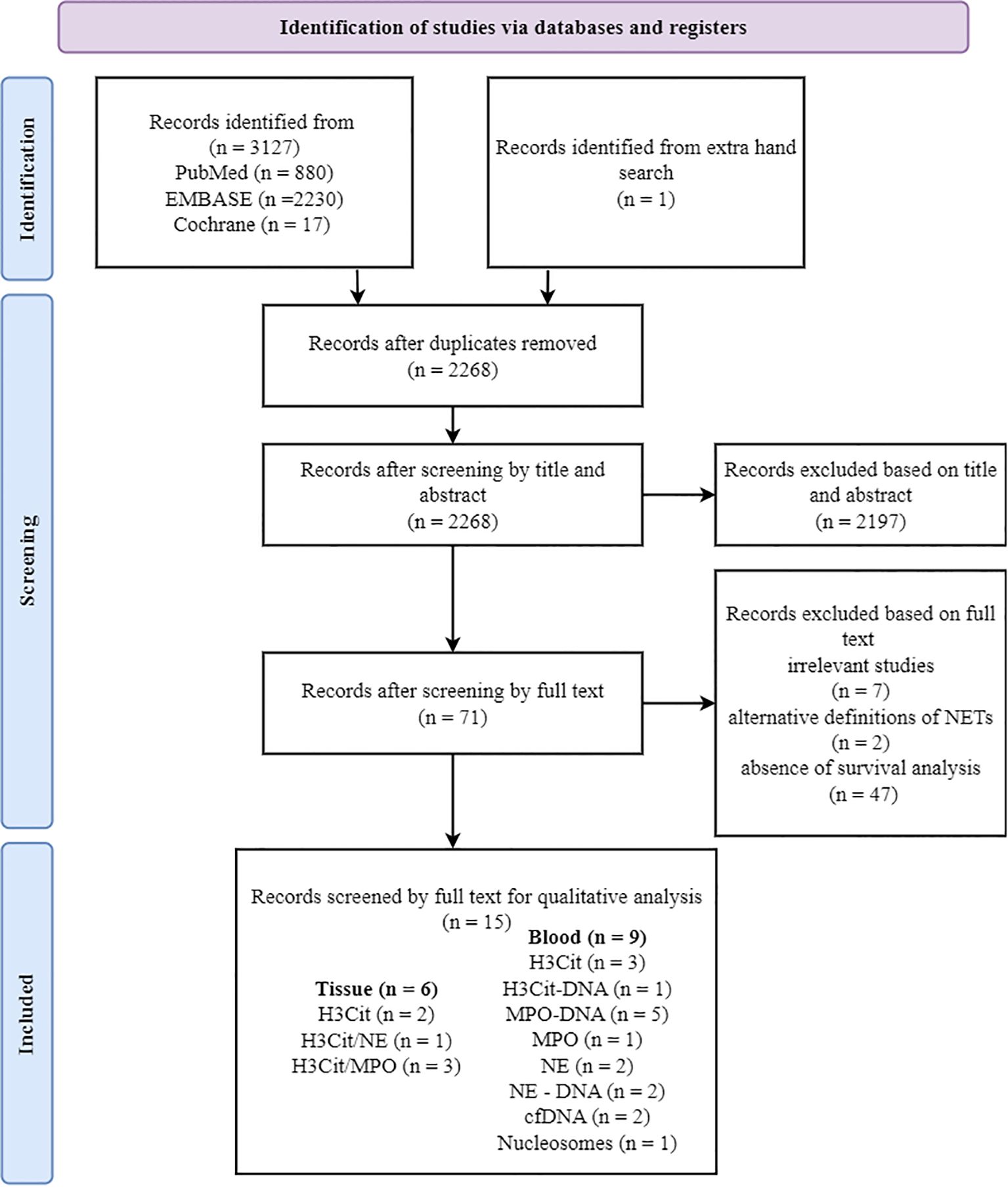
Figure 1. PRISMA flow diagram showing the study selection process. Of 15 studies included in the qualitative analysis, some utilized co-staining approaches (e.g., H3Cit/NE and H3Cit/MPO). The total number of markers exceeds the number of studies, as some studies analyzed multiple antibodies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; H3Cit, citrullinated histone H3; MPO, myeloperoxidase; NE, neutrophil elastase.
Study characteristics
Fifteen studies were selected for the final analysis that investigated the relationship between NETs and survival rates. These studies were conducted in seven different countries and published between 2016 and 2023 (Table 1; Supplementary Table S2). Among them, studies analyzing progression-free survival (PFS) (22, 29) and cancer-specific survival (CSS) (22) were limited to two and one, respectively, making further analysis challenging (Table 1). The total number of patients included was 5,202, with individual study sizes ranging between 27–954 (Table 1; Supplementary Table S2). The patients were divided into groups with high and low NET levels for comparative analysis.
High NETs levels and prognosis in patients with solid cancer
We evaluated the correlation between NETs and prognosis of patients with solid cancers. Pooled HR for OS and DFS demonstrated that high NETs levels were significantly associated with poor OS (HR: 1.80, 95% CI: 1.35–2.41, P < 0.0001) (11, 20, 25–28, 30, 32) and DFS (HR: 2.26, 95% CI: 1.82–2.82, P < 0.00001) (Figure 2).
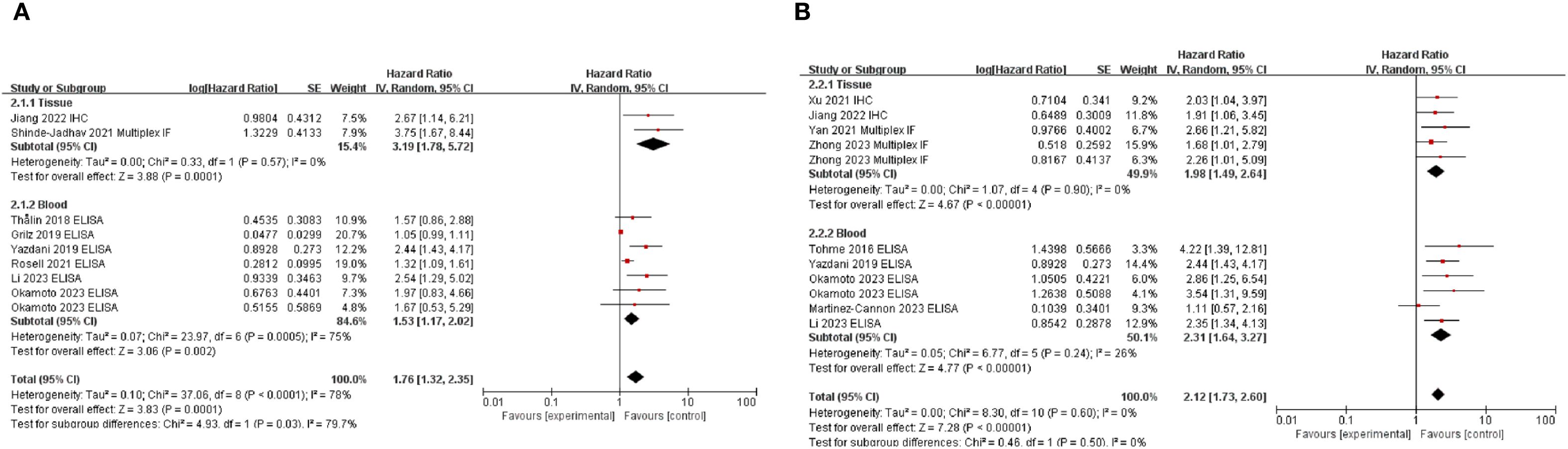
Figure 2. Subgroup analysis of neutrophil extracellular traps according to sample source: overall survival (A) and disease-free survival (B) in patients with cancer. Zhong et al. provided multivariate analysis results for both training and validation cohorts, whereas Okamoto et al. analyzed patient groups using H3Cit and MPO-DNA antibodies. Additionally, data from studies by Yazdani et al., Okamoto et al., Li et al., and Jiang et al., who reported OS and DFS outcomes, were included in the analysis. High NET levels of neutrophil extracellular traps were associated with poor survival outcomes in both OS and DFS. OS, overall survival; DFS, disease-free survival; NETs, neutrophil extracellular traps.
Subgroup analysis based on sample type demonstrated that the association between high NETs levels and poor prognosis was consistent, irrespective of sample type (Figure 2). When subgroup analyses were conducted according to the primary organ site (Supplementary Figure S2), the included studies were classified into gastrointestinal cancers [stomach (29, 30), n = 3; rectum (11, 24, 31), n = 3], hepatobiliary cancers [pancreas (21, 22), n = 2; liver (20), n = 1], and other malignancies [breast (33), urinary bladder (25), and uterine cervix (23), n = 1 each]. Within these categories, elevated levels of NETs were consistently associated with poorer prognosis. In contrast, three studies (26, 27, 32) evaluated the prognostic role of NETs across a broad spectrum of malignancies. In this heterogeneous cohort, no significant association between NETs and prognosis was identified. Except for the study by Martinez–Cannon et al. (33), all the included studies conducted multivariate analyses and demonstrated a significant association between high NET levels and pooled HRs (Figure 2; Supplementary Figure S3). As Martinez–Cannon et al. (33) did not report HR, data from their Kaplan–Meier curves were extracted and analyzed using the method described by Parmar et al. (34). The analysis revealed no significant association between high NETs and prognosis (Supplementary Figure S3). Subgroup analyses stratified according to ethnicity consistently showed that high NET levels were associated with a poor prognosis across all ethnic groups (Supplementary Figure S4).
Subgroup analyses by detection methods and antibodies used for NETs analysis
For tissue samples, IHC with H3Cit alone or multiplex IF with co-staining with MPO/H3Cit or NE/H3Cit was utilized. The most frequently used assay for blood samples was ELISA for the MPO/DNA complex, with H3Cit measurement being the next most common method. In the OS analysis, MPO/DNA (HR: 2.04, 95% CI: 1.43–2.92, P < 0.0001), H3Cit (HR: 2.37, 95% CI: 1.48–3.78, P = 0.0003), NE (HR: 1.79, 95% CI: 1.32–2.44, P = 0.0002), and H3Cit/NE co-staining (HR: 3.75, 95% CI: 1.67–8.44, P = 0.001) demonstrated significant associations, while for DFS, MPO/DNA (HR: 2.65, 95% CI: 1.88–3.73, P < 0.00001), H3Cit (HR: 2.14, 95% CI: 1.45–3.15, P = 0.0001), and H3Cit/MPO co-staining (HR: 1.99, 95% CI: 1.36–2.91, P = 0.0004) showed significant associations. Notably, MPO-DNA and H3Cit levels were associated with both OS and DFS, indicating their potential as key biomarkers. In contrast, NE-DNA (P = 0.97) and cfDNA (P = 0.44) levels were not significantly different. Furthermore, MPO, H3Cit-DNA, nucleosomes, and NE-DNA were evaluated in only one study, limiting the feasibility of further analyses (Figure 3).
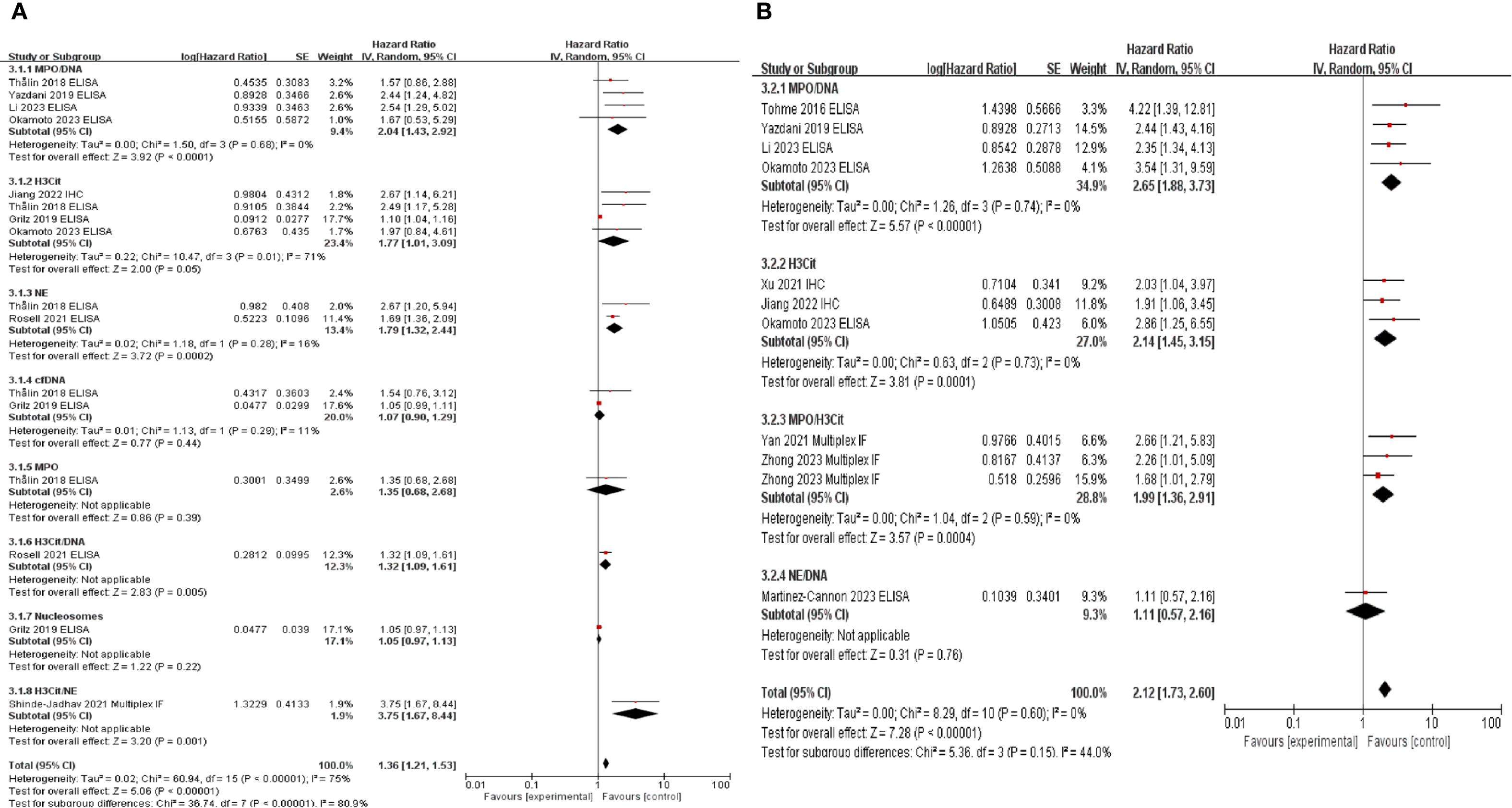
Figure 3. Subgroup analysis according to neutrophil extracellular traps detecting antibodies. Subgroup analysis was performed to evaluate overall survival (A) and disease-free survival (B) based on the antibodies used to detect neutrophil extracellular traps.
High NETs levels and clinicopathological parameters
Principal clinicopathological parameters associated with elevated NETs levels, as reported across all studies included in the meta-analysis, are summarized in Table 2; Supplementary Table S2; Figure 4. The pooled analysis indicated that larger tumor size (OR: 2.17; 95% CI: 1.28–5.74; P = 0.02) and advanced TNM stage (OR: 1.63; 95% CI: 1.07–2.50; P = 0.0003) were significantly associated with high NETs levels (Table 2).
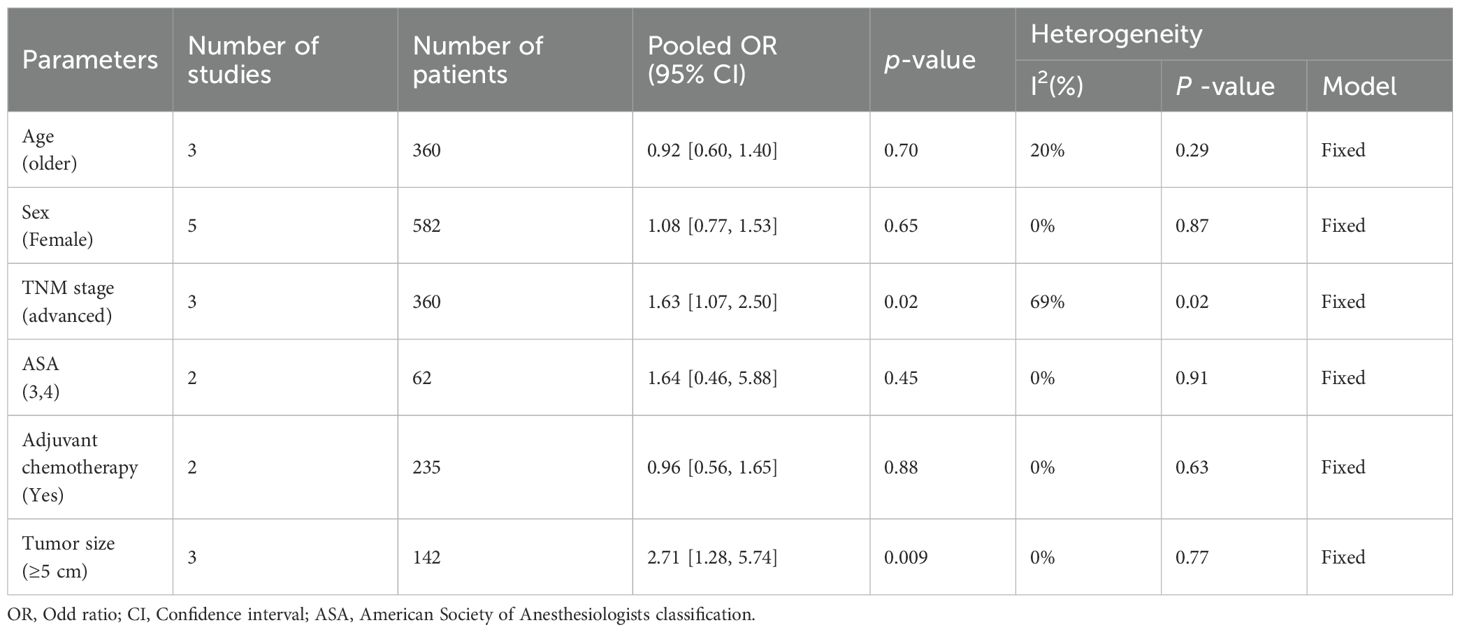
Table 2. Summary of the meta-analysis evaluating the relationship between neutrophil extracellular traps and clinicopathological parameters.
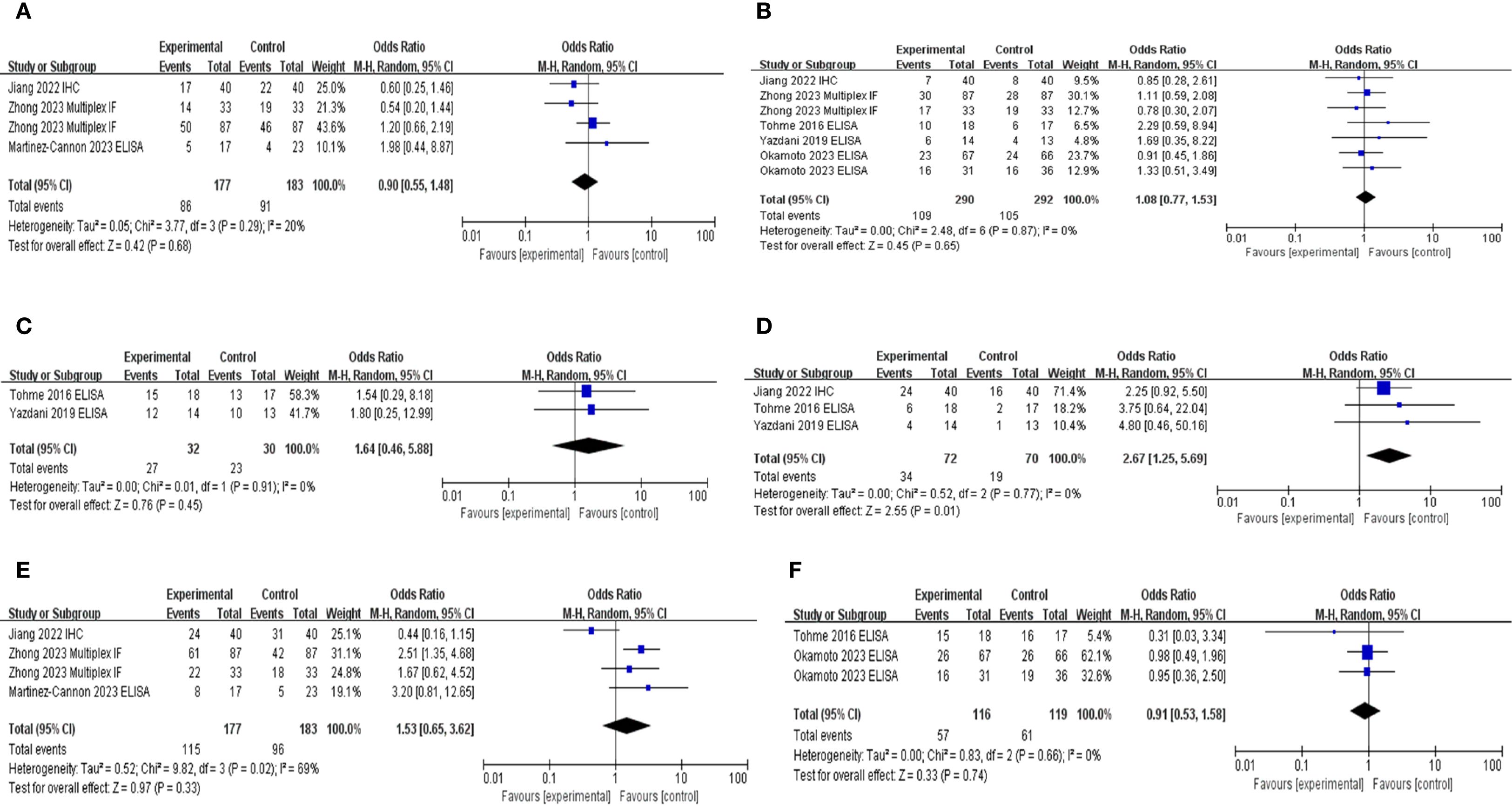
Figure 4. Subgroup hazard ratio analysis of neutrophil extracellular traps and pathological parameters in patients with cancer: (A) age, (B) sex, (C) American Society of Anesthesiologists classification, (D) tumor size, (E) TNM stage, and (F) adjuvant chemotherapy.
Discussion
Our analysis confirmed that elevated NETs levels were associated with poor prognosis in patients with cancer, regardless of whether tissue or blood samples were analyzed (Figure 2). This association remained consistent across various detection methods and most antibodies used, with the exception of cfDNA-based approaches, thereby underscoring the prognostic relevance of NETs in oncology (Figure 3; Supplementary Figure S5). To the best of our knowledge, this is the first comprehensive evaluation of the association between NETs and cancer outcomes, suggesting their potential clinical utility in cancer treatment and management.
The objective of this review was to comprehensively identify studies that have investigated NETs across all cancer types. The low inclusion rate (0.48%) following literature screening reflects the rigorous selection criteria applied to ensure the inclusion of studies specifically addressing the role of NETs in various malignancies. Despite extensive efforts, the limited number of studies reporting PFS (22, 29) and CSS (22) outcomes, likely reflecting the early stages of NET studies in oncology, has restricted our ability to conduct detailed subgroup analyses. Additionally, an insufficient number of studies investigating MPO (27), H3Cit/DNA (32), nucleosomes (26), NE/H3Cit (25), and NE/DNA (33) were available, precluding meaningful subgroup analyses for these markers (Figure 3; Supplementary Figure S5). Therefore, expanding the scope of future studies will be essential in providing a more comprehensive understanding of the role of NETs in cancer progression.
NETs are web-like structures composed of chromatin filaments coated with histones, proteases, and various granular and cytosolic proteins. NETosis is the process in which neutrophils generate and release NETs. This mechanism facilitates the immobilization and capture of pathogens, including bacteria, fungi, and viruses, thereby enhancing the efficiency of host antimicrobial defense (4–7). Recently, the role of NETs in various cancers has garnered increasing attention (6–8). Investigations of the antitumor functions of NETs have been conducted in colorectal cancer (36), head and neck squamous cell carcinoma (37), and malignant melanoma (38), predominantly using in vitro experimental studies. These studies suggested that NETs exert their antitumor effects by inducing apoptosis (36, 37) and necrosis (38).
In the present meta-analysis, we confirmed that elevated NET levels in both human tissues and blood samples were consistently associated with poor patient survival (Figure 2). This is likely due to the direct role of NETs in promoting tumor cell proliferation (11) and metastasis (12–14). Tumor-derived cytokines (e.g., interleukin [IL]-8, IL-17, granulocyte colony-stimulating factor, and CXCL6) recruit neutrophils and induce NETosis, thereby promoting tumor proliferation (39, 40). High mobility group box 1 (HMGB1), a NETs component, enhances proliferation by activating mitogen-activated protein kinase via toll-like receptor 9 (TLR9) and stimulating nuclear factor kappa B signaling (31) and IL-8 secretion through Receptor for Advanced Glycation End products (41). Additionally, NETs promote metastatic progression by degrading vascular endothelial-cadherin, thereby activating the Wnt/β-catenin signaling pathway and inducing the expression of epithelial–mesenchymal transition-related genes such as ZEB1 and Snail (42, 43). In parallel, HMGB1, a NET-associated component, facilitates tumor metastasis by activating TLR9, which in turn stimulates p38 and JNK signaling cascades, enhancing cancer cell migration and invasion (31).
Circulating NETs also enhance tumor cell survival by suppressing the cytotoxic activity of infiltrating CD8+ T (15). Additionally, NETs have emerged as key mediators of cancer-associated thrombosis, the second leading cause of death in patients with cancer having hypercoagulable states (16). Emerging evidence indicates that NETs promote cancer-associated thrombosis by enhancing the adhesion, activation, and aggregation of platelets and erythrocytes, leading to fibrin deposition and clot formation (17). This process is partially mediated by neutrophil-derived histones via TLR2- and TLR4-dependent platelet activation (18).
High-grade NETs have been associated with poor prognosis in studies that used various antibodies in tissue samples (20–25). Our analysis demonstrated that H3Cit (26–28), MPO-DNA (11, 27, 28, 30, 31), and NE (27, 32) were associated with poor prognosis, whereas cfDNA (26, 27) showed no such association. H3Cit, MPO-DNA, and NE are the key markers of NETs formation (8). H3Cit is produced by PAD4-mediated citrullination of histone H3, promoting chromatin decondenzation (44). MPO-DNA reflects NETs activity and contributes to metastasis and inflammation (45). NE released from neutrophil granules facilitate DNA decondenzation by cleaving histones (8, 44). However, cfDNA is a non-specific marker for NETs, as it detects extracellular DNA, regardless of origin (46). Although cfDNA can arise from NETosis (8), it is also released during apoptosis, necrosis, and erythroid precursor enucleation, and also from NET-like structures produced by eosinophils and macrophages (46).
This study has several limitations. First, non-English publications were excluded, potentially introducing a selection bias. Second, one study lacking HRs with 95% CIs required indirect data extraction, which may have affected accuracy. Third, limited data were available on the association between high NETs and CSS or PFS, warranting further investigation. Despite these limitations, our meta-analysis supports the prognostic significance of elevated NETs in patients with cancer.
Our analysis confirmed that elevated NETs levels were associated with poor prognosis in patients with cancer, irrespective of sample type (tissue or blood). This association was consistent across most detection methods and antibodies except for cfDNA-based approaches, highlighting the prognostic relevance of NETs in oncology. We believe that elevated NETs levels have potential as a prognostic biomarker and may contribute to risk stratification and personalized therapeutic approaches in precision oncology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SL: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. EK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YL: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing, Funding acquisition. WP: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. KY: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing, Conceptualization, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (RS-2022-00166635). The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the 2025-year program under grant No. 5-2025-B0001-00061. The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2023.
Acknowledgments
We appreciate NaJin Kim for performing the literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1676854/full#supplementary-material
References
1. Nauseef WM. Identification and quantitation of superoxide anion: essential steps in elucidation of the phagocyte "respiratory burst. J Immunol. (2014) 193:5357–8. doi: 10.4049/jimmunol.1402580
2. Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, et al. Neutrophils: New insights and open questions. Sci Immunol. (2018) 3:eaat4579. doi: 10.1126/sciimmunol.aat4579
3. Kim EY, Abdul-Ghafar J, Chong Y, and Yim K. Calculated tumor-associated neutrophils are associated with the tumor-stroma ratio and predict a poor prognosis in advanced gastric cancer. Biomedicines. (2022) 10:708. doi: 10.3390/biomedicines10030708
4. Sørensen OE and Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. (2016) 126:1612–20. doi: 10.1172/jci84538
5. Wigerblad G and Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. (2023) 23:274–88. doi: 10.1038/s41577-022-00787-0
6. Wang H, Kim SJ, Lei Y, Wang S, Wang H, Huang H, et al. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct Target Ther. (2024) 9:235. doi: 10.1038/s41392-024-01933-x
7. Jorch SK and Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. (2017) 23:279–87. doi: 10.1038/nm.4294
8. Chen Y, Hu H, Tan S, Dong Q, Fan X, Wang Y, et al. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp Hematol Oncol. (2022) 11:99. doi: 10.1186/s40164-022-00345-3
9. De Meo ML and Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. (2021) 57:101595. doi: 10.1016/j.smim.2022.101595
10. Zhao J and Jin J. Neutrophil extracellular traps: New players in cancer research. Front Immunol. (2022) 13:937565. doi: 10.3389/fimmu.2022.937565
11. Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. (2019) 79:5626–39. doi: 10.1158/0008-5472.Can-19-0800
12. Kaltenmeier C, Simmons RL, Tohme S, and Yazdani HO. Neutrophil extracellular traps (NETs) in cancer metastasis. Cancers (Basel). (2021) 13:6131. doi: 10.3390/cancers13236131
13. Yang D and Liu J. Neutrophil extracellular traps: A new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res. (2021) 40:233. doi: 10.1186/s13046-021-02013-6
14. Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. (2020) 13:3. doi: 10.1186/s13045-019-0836-0
15. Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. (2020) 52:856–71 e8. doi: 10.1016/j.immuni.2020.03.001
16. Moschonas IC and Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. (2019) 288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919
17. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U.S.A. (2010) 107:15880–5. doi: 10.1073/pnas.1005743107
18. Zhou Y, Tao W, Shen F, Du W, Xu Z, and Liu Z. The emerging role of neutrophil extracellular traps in arterial, venous and cancer-associated thrombosis. Front Cardiovasc Med. (2021) 8:786387. doi: 10.3389/fcvm.2021.786387
19. Liu Y and Liu L. The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps. Biosci Trends. (2020) 13:469–75. doi: 10.5582/bst.2019.01326
20. Jiang ZZ, Peng ZP, Liu XC, Guo HF, Zhou MM, Jiang D, et al. Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells. Oncoimmunology. (2022) 11:2052418. doi: 10.1080/2162402x.2022.2052418
21. Xu SS, Li H, Li TJ, Li S, Xia HY, Long J, et al. Neutrophil extracellular traps and macrophage extracellular traps predict postoperative recurrence in resectable nonfunctional pancreatic neuroendocrine tumors. Front Immunol. (2021) 12:577517. doi: 10.3389/fimmu.2021.577517
22. Chen X, Ma H, Mo S, Yu S, Lu Z, and Chen J. Intratumoral neutrophil extracellular traps are associated with unfavorable clinical outcomes and immunogenic context in pancreatic ductal adenocarcinoma. Front Immunol. (2022) 13:1027459. doi: 10.3389/fimmu.2022.1027459
23. Yan B, Dai X, Ma Q, and Wu X. Stromal neutrophil extracellular trap density is an independent prognostic factor for cervical cancer recurrence. Front Oncol. (2021) 11:659445. doi: 10.3389/fonc.2021.659445
24. Zhong W, Wang Q, Shen X, Lv Y, Sun L, An R, et al. Neutrophil extracellular trap is surrogate biomarker for prognosis and response to neoadjuvant therapy in locally advanced rectal cancer. J Inflammation Res. (2023) 16:6443–55. doi: 10.2147/jir.S441981
25. Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. (2021) 12:2776. doi: 10.1038/s41467-021-23086-z
26. Grilz E, Mauracher LM, Posch F, Königsbrügge O, Zöchbauer-Müller S, Marosi C, et al. Citrullinated histone H3, a biomarker for neutrophil extracellular trap formation, predicts the risk of mortality in patients with cancer. Br J Haematol. (2019) 186:311–20. doi: 10.1111/bjh.15906
27. Thålin C, Lundström S, Seignez C, Daleskog M, Lundström A, Henriksson P, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PloS One. (2018) 13:e0191231. doi: 10.1371/journal.pone.0191231
28. Okamoto M, Mizuno R, Kawada K, Itatani Y, Kiyasu Y, Hanada K, et al. Neutrophil extracellular traps promote metastases of colorectal cancers through activation of ERK signaling by releasing neutrophil elastase. Int J Mol Sci. (2023) 24:1118. doi: 10.3390/ijms24021118
29. Zhang Y, Hu Y, Ma C, Sun H, Wei X, Li M, et al. Diagnostic, therapeutic predictive, and prognostic value of neutrophil extracellular traps in patients with gastric adenocarcinoma. Front Oncol. (2020) 10:1036. doi: 10.3389/fonc.2020.01036
30. Li J, Xia Y, Sun B, Zheng N, Li Y, Pang X, et al. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell Commun Signal. (2023) 21:86. doi: 10.1186/s12964-023-01112-5
31. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. (2016) 76:1367–80. doi: 10.1158/0008-5472.Can-15-1591
32. Rosell A, Aguilera K, Hisada Y, Schmedes C, Mackman N, Wallén H, et al. Prognostic value of circulating markers of neutrophil activation, neutrophil extracellular traps, coagulation and fibrinolysis in patients with terminal cancer. Sci Rep. (2021) 11:5074. doi: 10.1038/s41598-021-84476-3
33. Martinez-Cannon BA, Garcia-Ronquillo K, Rivera-Franco MM, and Leon-Rodriguez E. Do circulating neutrophil extracellular traps predict recurrence in early breast cancer? Front Oncol. (2022) 12:1044611. doi: 10.3389/fonc.2022.1044611
34. Parmar MKB, Torri V, and Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::Aid-sim110>3.0.Co;2-8
35. Wang H, Wang Y, Zhang D, and Li P. Circulating nucleosomes as potential biomarkers for cancer diagnosis and treatment monitoring. Int J Biol Macromol. (2024) 262:130005. doi: 10.1016/j.ijbiomac.2024.130005
36. Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PloS One. (2016) 11:e0154484. doi: 10.1371/journal.pone.0154484
37. Millrud CR, Kagedal A, Kumlien Georen S, Winqvist O, Uddman R, Razavi R, et al. NET-producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. (2017) 140:2557–67. doi: 10.1002/ijc.30671
38. Schedel F, Mayer-Hain S, Pappelbaum KI, Metze D, Stock M, Goerge T, et al. Evidence and impact of neutrophil extracellular traps in Malignant melanoma. Pigment Cell Melanoma Res. (2020) 33:63–73. doi: 10.1111/pcmr.12818
39. Gonzalez-Aparicio M and Alfaro C. Influence of interleukin-8 and neutrophil extracellular trap (NET) formation in the tumor microenvironment: is there a pathogenic role? J Immunol Res. (2019) 2019:6252138. doi: 10.1155/2019/6252138
40. Mao Z, Zhang J, Shi Y, Li W, Shi H, Ji R, et al. CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis. (2020) 9:63. doi: 10.1038/s41389-020-00249-z
41. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. (2020) 17:154–68. doi: 10.20892/j.issn.2095-3941.2019.0353
42. Martins-Cardoso K, Almeida VH, Bagri KM, Rossi MID, Mermelstein CS, König S, et al. Neutrophil extracellular traps (NETs) promote pro-metastatic phenotype in human breast cancer cells through epithelial-mesenchymal transition. Cancers (Basel). (2020) 12:1542. doi: 10.3390/cancers12061542
43. Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol. (2017) 37:1371–9. doi: 10.1161/atvbaha.117.309002
44. Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. (2012) 3:307. doi: 10.3389/fimmu.2012.00307
45. Efrimescu CI, Buggy PM, and Buggy DJ. Neutrophil extracellular trapping role in cancer, metastases, and cancer-related thrombosis: a narrative review of the current evidence base. Curr Oncol Rep. (2021) 23:118. doi: 10.1007/s11912-021-01103-0
Keywords: neutrophil extracellular traps, neoplasms, prognosis, systematic review as topic, meta-analysis as topic
Citation: Lee S, Kim EY, Park W, Lee YS and Yim K (2025) Neutrophil extracellular traps predict poor survival in cancer: a systematic review and meta-analysis of studies on tissue and circulating biomarkers. Front. Immunol. 16:1676854. doi: 10.3389/fimmu.2025.1676854
Received: 31 July 2025; Accepted: 22 September 2025;
Published: 03 October 2025.
Edited by:
Alexey Stepanov, The Scripps Research Institute, United StatesReviewed by:
Ding Zhang, The Scripps Research Institute, United StatesYingqin Hou, The Scripps Research Institute, United States
Copyright © 2025 Lee, Kim, Park, Lee and Yim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwangil Yim, a2FuZ3NlX21hbnNlQGNhdGhvbGljLmFjLmty
†These authors have contributed equally to this work and share first authorship
 Seungwoo Lee1†
Seungwoo Lee1† Eun Young Kim
Eun Young Kim Woohyun Park
Woohyun Park Young Sub Lee
Young Sub Lee Kwangil Yim
Kwangil Yim