- 1Guangdong Medical University, Zhanjiang, Guangdong, China
- 2Department of Breast Surgery, Huizhou First Hospital, Guangdong Medical University, Huizhou, Guangdong, China
Triple-negative breast cancer (TNBC) is an aggressive subtype characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. It is associated with a high risk of recurrence, metastasis, and limited therapeutic options. Tumor-associated macrophages (TAMs) play a central role in TNBC progression by shaping an immunosuppressive tumor microenvironment. Primarily polarized toward an M2-like phenotype under the influence of cytokines such as IL-10 and TGF-β, TAMs facilitate tumor growth, angiogenesis, metastasis, and immune evasion through multiple mechanisms. This review summarizes current understanding of TAM recruitment, polarization, and pro-tumoral functions in TNBC, and outlines emerging therapeutic strategies aimed at depleting TAMs, reprogramming them to an anti-tumor M1-like state, or blocking the CD47-SIRPα phagocytosis checkpoint. These approaches offer promising avenues for reprogramming the TNBC microenvironment and improving clinical outcomes.
1 Introduction
Triple-negative breast cancer (TNBC) is a clinically aggressive subtype marked by a high propensity for recurrence and distant metastasis (1, 2). Despite standard regimens, many patients rapidly relapse (3, 4). Recent advances have shifted the focus from tumor-intrinsic traits to the tumor microenvironment (TME), a dynamic niche that orchestrates cancer initiation and progression in concert with tumor cells (5–9). Among TME components, tumor-associated macrophages (TAMs)—the most prevalent innate immune cells—can constitute up to 71.4% of the immune infiltrate in TNBC, far surpassing that in other malignancies (10).
TAMs are implicated across the entire course of TNBC development, from early tumorigenesis to metastatic dissemination, and correlate with poor clinical outcomes (11–13). In TNBC, TAMs adopt either pro-inflammatory M1 or immunosuppressive M2 phenotypes in response to cytokine cues (14). M1 macrophages enhance anti-tumor immunity via inflammatory mediator release, antigen presentation, and tumor cell phagocytosis, whereas M2 macrophages facilitate tumor progression by promoting proliferation, angiogenesis, immune evasion, and metastatic potential (15, 16). During tumor progression, TAMs predominantly exhibit an M2-like phenotype, thereby facilitating TNBC initiation and advancement (17). This review provides a comprehensive overview of the ontogeny, recruitment mechanisms, and polarization dynamics of TAMs in the TNBC microenvironment, and delineates their multifaceted roles in disease progression. In addition, we summarize recent advances in TAM-targeted therapeutic strategies aimed at improving outcomes in TNBC patients.
2 Origin, recruitment, and polarization of TAMs
2.1 Origin and recruitment of TAMs
Current evidence indicates that TAMs originate from two principal sources. The first comprises bone marrow–derived myeloid progenitors that differentiate into circulating monocytes, which infiltrate the TME and mature into macrophages (18). The second involves embryonic progenitors from the yolk sac or fetal liver that give rise to tissue-resident macrophages (TRMs), which are seeded into organs during development and sustained independently of hematopoietic input through local proliferation (19). Notably, both embryonically derived and monocyte-derived TAMs have been documented in several malignancies, including breast cancer (20). The recruitment of TAMs into the TME of TNBC is primarily mediated by tumor-secreted growth factors and chemokines. Colony-stimulating factor 1 (CSF-1), through binding to its receptor CSF-1R, plays a pivotal role in the recruitment and differentiation of peripheral blood monocytes into TAMs (21). TNBC cells produce substantially higher levels of CSF-1 than non-TNBC subtypes, promoting robust TAM infiltration (22, 23). In parallel, CC chemokine ligand 2 (CCL2) drives monocyte chemotaxis via CCR2 signaling (24), while CCL5 contributes to TAM aggregation and enhances tumor invasion. Importantly, CCL5-CCR3 signaling in tumor cells correlates with poor prognosis in TNBC (25). Immunohistochemical analyses of tumors from 40 TNBC patients further demonstrate that CCL5 production by peritumoral adipose tissue potentiates invasion and metastasis (26). Moreover, VEGF has also been implicated in TAM recruitment, with elevated VEGF levels in TNBC strongly associated with increased macrophage infiltration (27).
2.2 Polarization of TAMs
Upon recruitment into the TME, TAMs acquire distinct functional phenotypes shaped by local cues (28). Exposure to lipopolysaccharide (LPS) and IFN-γ drives macrophages toward a classically activated, pro-inflammatory M1-like state, whereas anti-inflammatory cytokines such as IL-10 and TGF-β promote an alternatively activated, immunosuppressive M2-like phenotype (29). M1-like TAMs exert antitumor functions via the production of reactive oxygen species (ROS), nitrogen intermediates, and enhaantigen presentation to T cellnced s (30). By contrast, M2-like TAMs facilitate tumor progression by mediating tissue remodeling, angiogenesis, and immune suppression (28). The TME promotes a phenotypic shift from M1 to M2 polarization through sustained exposure to IL-10, TGF-β, and other tumor-derived factors (31). Additionally, interactions with the extracellular matrix and neoplastic signals further reinforce M2-skewed polarization in TAMs, particularly in TNBC, where such phenotypes dominate the immune landscape (32). This biased polarization underpins the immunosuppressive and pro-tumoral roles of TAMs in TNBC progression. Besides, cytokines activate downstream intracellular signaling pathways that orchestrate M2 polarization. IL-10 predominantly signals through the JAK1/STAT3 axis, where phosphorylated STAT3 translocates to the nucleus and induces the expression of M2-associated genes such as IL-10 and arginase-1 (33, 34). Similarly, IL-4 and IL-13 activate the STAT6 pathway, which promotes transcription of M2 markers including CD206 (35, 36). In addition, TGF-β signaling induces M2 polarization through activation of the PI3K/Akt and SMAD pathways, enhancing the expression of anti-inflammatory and pro-tumoral mediators (37, 38). Notably, activation of the PI3K/Akt axis has also been implicated in metabolic reprogramming of TAMs toward an oxidative phosphorylation (OXPHOS)-dominant state, further supporting their M2-like phenotype and immunosuppressive functions (39, 40).
TNBC progression has been modeled by co-injecting RAW264.7 macrophages and 4T1 TNBC cells into murine mammary ducts (40). During the transition from in situ carcinoma to invasive statue, this co-injection approach resulted in suppressed expression of the M1-associated cytokine IL-12 and elevated levels of the M2-associated cytokine TGF-β1 (41). These immunological alterations were accompanied by both lymphatic and pulmonary metastases (42). Additionally, increased concentrations of MMP-8 and VEGF were detected in peripheral blood—both recognized modulators of macrophage polarization (43). These findings suggest that tumor-induced M2 polarization of TAMs may operate through a reinforcing positive feedback loop. MicroRNAs (miRNAs), a class of non-coding single-stranded RNAs with dual oncogenic and tumor-suppressive roles, have emerged as key regulators of TAM polarization (44, 45). For instance, co-culturing miR-200c–overexpressing MDA-MB-231 TNBC cells with RAW264.7 macrophages enhanced expression of M2 markers such as CD206 and IL-10, indicating a role for miR-200c in promoting M2-like phenotypes (46). Conversely, miR-34a has been implicated in facilitating M1 polarization. Using viral transduction to manipulate miR-34a expression in MDA-MB-231 cells followed by co-culture with THP-1 monocytes, it was observed that tumor cells expressing miR-34a more effectively induced M1 polarization compared to miR-34a–silenced controls (17). In addition to miR-200c and miR-34a, other miRNAs such as miR-21 and miR-155 have also been implicated in TAM regulation within the breast cancer microenvironment. miR-21, commonly upregulated in breast cancer, promotes M2 polarization by targeting PTEN and enhancing PI3K/Akt signaling, thereby reinforcing the immunosuppressive phenotype of TAMs (47, 48). Conversely, miR-155 facilitates M1 polarization by inhibiting suppressor of cytokine signaling 1 (SOCS1), leading to enhanced pro-inflammatory cytokine production and tumoricidal activity (49, 50).
3 The role of TAMs in TNBC progression
3.1 Promotion of tumor cell proliferation
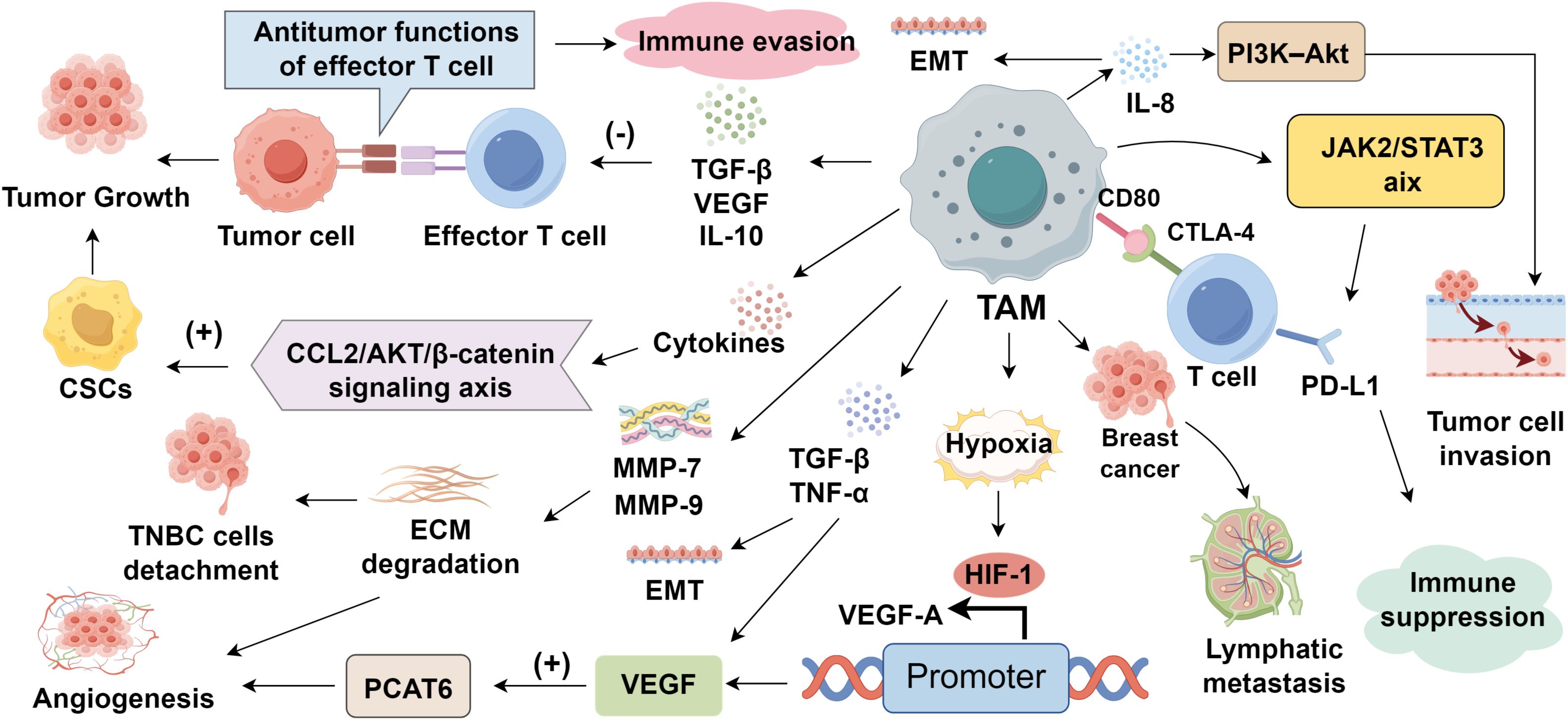
The infiltration of TAMs in the TNBC microenvironment is tightly associated with enhanced tumor cell proliferation (51). TAMs secrete various signaling molecules, including TGF-β, VEGF, and IL-10, which suppress the antitumor functions of effector T cells, thereby facilitating tumor cell growth (52, 53). Notably, TAMs also support the maintenance and expansion of cancer stem cells (CSCs), a subpopulation endowed with self-renewal and tumor-initiating capacity, through a variety of paracrine signaling pathways (54, 55). For instance, TAM-derived IL-6 activates STAT3 signaling in TNBC cells, reinforcing stem-like traits and contributing to chemoresistance (56). Similarly, IL-8 promotes the CSC phenotype by upregulating ALDH1, while concurrently activating PI3K/AKT/mTOR and NF-κB signaling cascades (57–59). Moreover, cytokine-driven activation of the CCL2/AKT/β-catenin axis by TAMs further potentiates CSC maintenance and tumor aggressiveness, ultimately fostering TNBC progression and resistance to therapy (60) (Figure 1).
3.2 Induction of angiogenesis and lymphangiogenesis
The vascular network plays a pivotal role in sustaining tumor growth by delivering oxygen and nutrients, while also serving as a conduit for metastatic dissemination (61). In TNBC, TAMs drive angiogenesis via several key mechanisms (62). First, TAMs secrete matrix metalloproteinases (MMPs), notably MMP-7 and MMP-9, along with cathepsins, to degrade the extracellular matrix (ECM), thereby facilitating endothelial cell invasion and neovessel formation (63). Second, the hypoxic tumor microenvironment polarizes macrophages toward a pro-tumoral TAM phenotype. These cells accumulate in hypoxic niches and upregulate hypoxia-inducible factor-1 (HIF-1), which transcriptionally activates proangiogenic genes such as VEGF-A (64). Third, TAMs amplify VEGF expression through the secretion of TGF-β and TNF-α, further enhancing neovascularization (65). Recent studies have shown that TAM-derived VEGF promotes angiogenesis in TNBC by upregulating prostate cancer-associated transcript 6 (PCAT6) (66). In addition to angiogenesis, TAMs promote lymphangiogenesis, a process critical for lymphatic metastasis (67). Tumor-induced expression of integrin β4 in macrophages enhances their chemotactic aggregation and adhesion to lymphatic vessels, where they secrete TNF-β1, triggering lymphatic endothelial contraction. These macrophages also elevate vascular permeability and disrupt perivascular structures, collectively fostering lymphatic remodeling and tumor cell dissemination through lymphatic routes (68).
3.3 Facilitation of metastasis
Metastasis remains the leading cause of death in patients with TNBC, with TAMs playing a pivotal role in promoting tumor cell invasion and dissemination (69). Through secretion of MMPs, TAMs degrade and remodel the ECM, thereby facilitating detachment of tumor cells from the primary site (70). A hallmark of this invasive transition is epithelial-to-mesenchymal transition (EMT), wherein epithelial cells acquire mesenchymal properties and heightened motility (71). TAMs induce EMT via cytokine secretion, including TGF-β (38), TNF-α (12), and IL-4 (72). TGF-β, in particular, triggers EMT-associated transcriptional programs by engaging tumor cell receptors and activating downstream pathways (73). In TNBC, IL-8 further promotes EMT and invasiveness through PI3K–Akt signaling (74). Beyond soluble mediators, TAM-derived exosomes are potent effectors of EMT and metastatic reprogramming (75). Notably, M2-like TAMs release exosomes enriched in miR-223, which activates β-catenin signaling and suppresses epithelial markers such as E-cadherin, thereby enhancing cellular plasticity and invasive capacity (76, 77). TAM-derived exosomal MMPs, particularly MMP-9, degrade ECM components and compromise basement membrane integrity (78, 79). Within the pre-metastatic niche (PMN), these exosomes further contribute to stromal cell recruitment, vascular leakage, and the establishment of a supportive microenvironment for metastatic colonization (80).
3.4 Induction of immunosuppression and immune evasion
Immunosuppression is a fundamental prerequisite for tumor initiation and sustained progression (81–83). In TNBC, TAMs exert profound immunoregulatory functions that suppress anti-tumor immunity (84). One key mechanism involves the expression of CD80 and CD86 on TAMs, which engage cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) on T cells to block activation and cell cycle progression, thereby promoting T cell anergy (85). Additionally, TAMs secrete immunosuppressive cytokines such as TGF-β and IL-10 within the TNBC microenvironment, directly impairing the cytotoxic capacity of effector T cells and facilitating immune evasion (86). A central axis in TAM-mediated immunosuppression is the PD-1/PD-L1 pathway. PD-1 ligation by PD-L1 inhibits T cell effector function, and TNBC tissues show markedly elevated PD-L1 expression compared to other subtypes, contributing to enhanced T cell suppression (84). Notably, TNBC cells induce TAMs to upregulate PD-L1 via the JAK2/STAT3 pathway, which further inhibits CD8+ T cell–mediated cytotoxicity (87). Moreover, PD-1 is also expressed on TAMs themselves, particularly those with an M2-like phenotype. High PD-1 expression on TAMs is associated with diminished phagocytosis and impaired anti-tumor responses. Blocking PD-1/PD-L1 signaling not only restores macrophage function but also suppresses tumor growth and prolongs survival in murine models (88). In addition to PD-1/PD-L1, other immune checkpoints such as T cell immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and V-domain Ig suppressor of T cell activation (VISTA) are increasingly recognized in TNBC immunotherapy (89–91). These molecules are also regulated by TAMs and contribute to the suppression of T cell activity and immune evasion. For example, TIM-3 and LAG-3 are frequently co-expressed with PD-1 on exhausted T cells in the TNBC microenvironment, and their ligands, including galectin-9 and MHC class II, can be upregulated by TAMs (92–94). VISTA, predominantly expressed on myeloid cells such as TAMs, mediates immune suppression by dampening T cell proliferation and cytokine production (95, 96). Collectively, these findings highlight the role of TAMs as critical regulators of immunosuppression and immune escape in TNBC. Targeting these alternative checkpoints alongside PD-1/PD-L1 may offer synergistic immunotherapeutic benefits in TNBC.
4 Therapeutic strategies targeting TAMs
4.1 Depleting TAM populations
Given the critical role of TAMs in orchestrating immunosuppressive TME and promoting cancer progression, substantial preclinical and clinical efforts have focused on TAM-targeted interventions (13, 97). These strategies fall into three principal categories: depletion of TAMs, reprogramming toward anti-tumor phenotypes, and blockade of the CD47–SIRPα axis (98–100). Colony-stimulating factor 1 (CSF-1) facilitates the recruitment of TAMs into the TME of breast cancer, where they promote tumor invasion and metastasis. CSF-1 binds its receptor CSF-1R to regulate macrophage survival and trafficking. Pharmacological inhibition of CSF-1R not only reduces TAM infiltration but also delays tumor growth and dissemination (101). Emactuzumab (RG7155), a monoclonal antibody targeting CSF-1R, depletes TAMs by blocking receptor activation (102). Preclinical studies revealed that RG7155 markedly suppressed TAM levels and enhanced T cell infiltration (103). However, a Phase I clinical trial evaluating RG7155 in breast cancer demonstrated no significant clinical benefit when administered alone or in combination with paclitaxel, despite successful TAM suppression (104). These findings highlight the necessity of thoroughly evaluating the TME before initiating CSF-1R-targeted therapies.
Chemokines also critically regulate TAM recruitment (105). CCL2 recruits circulating monocytes that differentiate into TAMs via its receptor CCR2 (106). Inhibition of CCL2 has been shown to attenuate TAM infiltration and impair cancer stem cell renewal, thereby restraining TNBC progression (60, 107). However, abrupt withdrawal of CCL2 blockade can trigger a rebound effect, marked by increased TAM accumulation, enhanced metastasis, and reduced survival in preclinical breast cancer models (108). This underscores the limitations of monotherapy targeting CCL2 in metastatic disease and emphasizes the importance of understanding TME composition and antitumor dynamics. CCL5 is a key modulator of tumor growth and metastatic dissemination, and its receptor CCR5 is frequently overexpressed in TNBC (109). Elevated CCL5 levels correlate with increased tumor burden following neoadjuvant chemotherapy (110), and gene expression profiling of residual tumors reveals enrichment of CCL5, suggesting its role in recruiting macrophages and fostering recurrence (111). Thus, targeting the CCL5/CCR5 axis emerges as a promising approach to limit TAM-driven relapse in TNBC.
4.2 Reprogramming TAMs toward an anti-tumor phenotype
Macrophages exhibit remarkable plasticity and can dynamically shift their phenotype in response to environmental cues. Reprogramming TAMs from a tumor-promoting (M2-like) to an inflammatory, tumoricidal (M1-like) phenotype offers a promising avenue for TNBC therapy (112, 113). CD40, a member of the TNF receptor superfamily, is expressed on antigen-presenting cells, including macrophages and B cells (114). Engagement of CD40 by CD40L triggers the production of TNF, ROS, and nitric oxide (NO), and promotes T cell activation and antitumor immunity (115). In preclinical studies, CD40 agonists have successfully reprogrammed TAMs into M1-like macrophages with enhanced tumoricidal activity, thereby restoring immune surveillance and delaying tumor progression (116). Additionally, Toll-like receptor (TLR) agonists have demonstrated the capacity to re-educate TAMs, further supporting their therapeutic potential in TNBC (55). Notably, ATM gene deficiency in murine breast cancer cells has been shown to facilitate macrophage repolarization from M2- to M1-like phenotypes within the TME, leading to reduced tumor growth, angiogenesis, and metastatic burden (117). Another axis of interest is the CD47–SIRPα signaling pathway. CD47, a transmembrane protein overexpressed in many malignancies including TNBC, is associated with immune escape and poor prognosis (118). Its interaction with SIRPα, expressed on macrophages, delivers a “don’t eat me” signal that suppresses phagocytosis. This mechanism enables tumor cells to evade immune clearance. Blocking the CD47–SIRPα interaction reactivates macrophage-mediated phagocytosis and enhances anti-tumor responses. Notably, CD47-targeted agents are currently in clinical trials, with encouraging evidence supporting CD47 blockade as an effective strategy for suppressing TNBC development and metastasis (119).
4.3 TAM-targeted nanoengineering strategies
Tumor-targeted nanoparticles (NPs) offer a promising platform for precision drug delivery due to their enhanced specificity, penetrability, and biocompatibility, which improve intratumoral drug accumulation while reducing systemic toxicity (120). Haney et al. (121) demonstrated that EVs loaded with paclitaxel or doxorubicin effectively suppressed tumor growth in vitro and in vivo. In TNBC, where residual cancer stem cells and inflammatory cues persist post-surgery, TAMs are preferentially recruited to tumor margins. Leveraging this, dual-loaded R8-modified liposomes co-encapsulating paclitaxel and resveratrol were developed for macrophage-mediated delivery, effectively suppressing recurrence and downregulating pro-tumor cytokines (122). Additionally, hybrid membranes composed of macrophage and tumor cell membranes were employed to coat DOX-loaded PLGA NPs, markedly enhancing tumor homing and systemic stability, achieving a metastasis-targeting rate of 88.9% (123). Beyond delivery efficiency, TAM-targeted nanoplatforms are increasingly tailored to modulate the tumor immune microenvironment (124). For instance, dextran-coated iron oxide NPs catalyze Fenton-like reactions to generate ROS, promoting TAM polarization toward an M1 phenotype and inhibiting metastasis (125–127). Moreover, MnO-doped DOX nanospheres encapsulated in macrophages enable laser-triggered release at tumor sites, locally decomposing H2O2 to relieve hypoxia while MnO reduction liberates Mn2+ and DOX, amplifying cytotoxicity through enhanced ROS production (128). Together, these studies highlight the transformative potential of TAM-targeted nanoengineering in overcoming drug delivery barriers, modulating immune responses, and improving therapeutic outcomes. Continued innovation in macrophage-based nanotechnology offers promising translational avenues for cancer immunotherapy.
5 Conclusion
Tumor-associated macrophages (TAMs) are key orchestrators of triple-negative breast cancer (TNBC) progression, contributing to immunosuppression, angiogenesis, metastasis, and therapeutic resistance. Recent advances highlight various strategies targeting TAMs, including depletion via CSF-1R inhibition, repolarization toward M1-like phenotypes, blockade of the CD47–SIRPα axis, and macrophage-mediated nano-drug delivery. These approaches hold substantial potential to reshape the tumor immune microenvironment and enhance treatment responses.
However, translating TAM targeted therapies into clinical success remains challenging. The functional heterogeneity of TAMs, shaped by ontogeny, spatial localization, and cytokine context, complicates precise targeting. Additionally, the dynamic plasticity of TAM polarization hinders real time monitoring, while the absence of robust biomarkers limits patient stratification and treatment evaluation. Addressing these obstacles will require integrative strategies incorporating single cell technologies, spatial profiling, and biomarker guided trial designs to identify responsive TNBC subgroups. Only through overcoming these translational barriers can TAM directed interventions be effectively implemented to improve outcomes in TNBC patients.
Author contributions
TW: Writing – original draft. YL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun Z, Li Z, Wei Y, Xu L, Hang X, and Kang Y. SMARCA4 inhibits breast cancer progression and metastasis through RHOA suppression. Cancer Res. (2025) 85:1803–18. doi: 10.1158/0008-5472.CAN-24-2801
2. Zhang Z, Li F, Dai X, Deng J, Wang Y, Zhang S, et al. A novel micropeptide miPEP205 suppresses the growth and metastasis of TNBC. Oncogene. (2025) 44:513–29. doi: 10.1038/s41388-024-03240-9
3. Turner NC, Swift C, Jenkins B, Kilburn L, Coakley M, Beaney M, et al. Results of the c-TRAK TN trial: a clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate- and high-risk early-stage triple-negative breast cancer. Ann Oncol. (2023) 34:200–11. doi: 10.1016/j.annonc.2022.11.005
4. Bai X, Ni J, Beretov J, Graham P, and Li Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles' heel? Cancer Lett. (2021) 497:100–11. doi: 10.1016/j.canlet.2020.10.016
5. Harris MA, Savas P, Virassamy B, O'Malley MMR, Kay J, Mueller SN, et al. Towards targeting the breast cancer immune microenvironment. Nat Rev Cancer. (2024) 24:554–77. doi: 10.1038/s41568-024-00714-6
6. Zhou L and Yu CW. Epigenetic modulations in triple-negative breast cancer: Therapeutic implications for tumor microenvironment. Pharmacol Res. (2024) 204:107205. doi: 10.1016/j.phrs.2024.107205
7. Xie H, Xi X, Lei T, Liu H, and Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
8. Chen Y, Yan H, Xu Y, Chen K, Yang R, Yang J, et al. Analysis of the predictive value of the prostate-specific antigen-to-neutrophil ratio for the diagnosis of prostate cancer. Discov Oncol. (2025) 16:13. doi: 10.1007/s12672-025-01760-8
9. Chi H, Jiang L, Zhou X, Wang L, Yang G, Luo H, et al. Editorial: Immune cell exhaustion: new challenges and opportunities in cancer therapy. Front Immunol. (2024) 15:1527428. doi: 10.3389/fimmu.2024.1527428
10. Zhang WJ, Wang XH, Gao ST, Chen C, Xu XY, Sun Q, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. (2018) 222:93–101. doi: 10.1016/j.jss.2017.09.035
11. Wang Z, Wang F, Ding XY, Li TE, Wang HY, Gao YH, et al. Hippo/YAP signaling choreographs the tumor immune microenvironment to promote triple negative breast cancer progression via TAZ/IL-34 axis. Cancer Lett. (2022) 527:174–90. doi: 10.1016/j.canlet.2021.12.016
12. Li X, Guo Y, Deng X, Jiao Y, Hao H, Dong Q, et al. Taraxacum mongolicum Hand.-Mazz. extract disrupts the interaction between triple-negative breast cancer cells and tumor-associated macrophages by inhibiting RAC2/NF-κB p65/p38 MAPK pathway. J Ethnopharmacol. (2025) 347:119757. doi: 10.1016/j.jep.2025.119757
13. O'Connell BC, Hubbard C, Zizlsperger N, Fitzgerald D, Kutok JL, Varner J, et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J Immunother Cancer. (2024) 12:e009160. doi: 10.1136/jitc-2024-009160
14. Pe KCS, Saetung R, Yodsurang V, Chaotham C, Suppipat K, Chanvorachote P, et al. Triple-negative breast cancer influences a mixed M1/M2 macrophage phenotype associated with tumor aggressiveness. PloS One. (2022) 17:e0273044. doi: 10.1371/journal.pone.0273044
15. Xu B, Sun H, Song X, Liu Q, and Jin W. Mapping the tumor microenvironment in TNBC and deep exploration for M1 macrophages-associated prognostic genes. Front Immunol. (2022) 13:923481. doi: 10.3389/fimmu.2022.923481
16. Shen HY, Xu JL, Zhang W, Chen QN, Zhu Z, and Mao Y. Exosomal circRHCG promotes breast cancer metastasis via facilitating M2 polarization through TFEB ubiquitination and degradation. NPJ Precis Oncol. (2024) 8:22. doi: 10.1038/s41698-024-00507-y
17. Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. (2019) 18:42. doi: 10.1186/s12943-019-0988-0
18. Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, and Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. (2017) 316:1–10. doi: 10.1016/j.cellimm.2017.04.005
19. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
20. Zhang XM, Chen DG, Li SC, Zhu B, and Li ZJ. Embryonic origin and subclonal evolution of tumor-associated macrophages imply preventive care for cancer. Cells. (2021) 10:903. doi: 10.3390/cells10040903
21. Tkach M, Thalmensi J, Timperi E, Gueguen P, Névo N, Grisard E, et al. Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc Natl Acad Sci U.S.A. (2022) 119:e2107394119. doi: 10.1073/pnas.2107394119
22. Ding J, Guo C, Hu P, Chen J, Liu Q, Wu X, et al. CSF1 is involved in breast cancer progression through inducing monocyte differentiation and homing. Int J Oncol. (2016) 49:2064–74. doi: 10.3892/ijo.2016.3680
23. Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, and Ch'ng ES. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol. (2019) 9:1512. doi: 10.3389/fonc.2019.01512
24. Zhao L, Wang G, Qi H, Yu L, Yin H, Sun R, et al. LINC00330/CCL2 axis-mediated ESCC TAM reprogramming affects tumor progression. Cell Mol Biol Lett. (2024) 29:77. doi: 10.1186/s11658-024-00592-8
25. Liubomirski Y, Lerrer S, Meshel T, Rubinstein-Achiasaf L, Morein D, Wiemann S, et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Front Immunol. (2019) 10:757. doi: 10.3389/fimmu.2019.00757
26. Liu C, Yao Z, Wang J, Zhang W, Yang Y, Zhang Y, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. (2020) 27:1765–81. doi: 10.1038/s41418-019-0460-0
27. Wang L, Zhang L, Zhao L, Shao S, Ning Q, Jing X, et al. VEGFA/NRP-1/GAPVD1 axis promotes progression and cancer stemness of triple-negative breast cancer by enhancing tumor cell-macrophage crosstalk. Int J Biol Sci. (2024) 20:446–63. doi: 10.7150/ijbs.86085
28. Bao X, Shi R, Zhao T, Wang Y, Anastasov N, Rosemann M, et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq unravels tumour heterogeneity plus M2-like tumour-associated macrophage infiltration and aggressiveness in TNBC. Cancer Immunol Immunother. (2021) 70:189–202. doi: 10.1007/s00262-020-02669-7
29. Wang S, Liu G, Li Y, and Pan Y. Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front Immunol. (2022) 13:840029. doi: 10.3389/fimmu.2022.840029
30. Chen M, Song L, Zhou Y, Xu T, Sun T, Liu Z, et al. Promotion of triple negative breast cancer immunotherapy by combining bioactive radicals with immune checkpoint blockade. Acta Biomater. (2025) 194:305–22. doi: 10.1016/j.actbio.2025.01.015
31. Fu J, Wei C, Chen Y, He X, and Zhang K. RBM15 enhances paclitaxel resistance in triple-negative breast cancer by targeting m(6)A methylation of TNFSF9 and inducing polarization of tumor-associated macrophages to M2 phenotype. Hereditas. (2025) 162:167. doi: 10.1186/s41065-025-00534-0
32. Zhao Q, He X, Qin X, Liu Y, Jiang H, Wang J, et al. Enhanced therapeutic efficacy of combining losartan and chemo-immunotherapy for triple negative breast cancer. Front Immunol. (2022) 13:938439. doi: 10.3389/fimmu.2022.938439
33. Lu R, Zhang L, Wang H, Li M, Feng W, and Zheng X. EChinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway. Front Pharmacol. (2022) 13:993483. doi: 10.3389/fphar.2022.993483
34. Choi JY, Seok HJ, Lee DH, Lee E, Kim TJ, Bae S, et al. Tumor-derived miR-6794-5p enhances cancer growth by promoting M2 macrophage polarization. Cell Commun Signal. (2024) 22:190. doi: 10.1186/s12964-024-01570-5
35. Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. (2018) 100:1034–43. doi: 10.1016/j.ijrobp.2017.11.043
36. Shi JH, Liu LN, Song DD, Liu WW, Ling C, Wu FX, et al. TRAF3/STAT6 axis regulates macrophage polarization and tumor progression. Cell Death Differ. (2023) 30:2005–16. doi: 10.1038/s41418-023-01194-1
37. Zhao HY, Zhang YY, Xing T, Tang SQ, Wen Q, Lyu ZS, et al. M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity. Signal Transduct Target Ther. (2021) 6:234. doi: 10.1038/s41392-021-00627-y
38. Seok Han B, Ko S, Seok Park M, Ji Lee Y, Eun Kim S, Lee P, et al. Lidocaine combined with general anesthetics impedes metastasis of breast cancer cells via inhibition of TGF-β/Smad-mediated EMT signaling by reprogramming tumor-associated macrophages. Int Immunopharmacol. (2024) 142:113207. doi: 10.1016/j.intimp.2024.113207
39. Feng L, Wang Y, Wang X, Li B, Liu Y, Zhang W, et al. Exosomal lncRNA MIR210HG derived from hypoxic TAMs promotes the metastasis of triple negative breast cancer. Int Immunopharmacol. (2025) 165:115416. doi: 10.1016/j.intimp.2025.115416
40. Steenbrugge J, Breyne K, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, et al. Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer. J Exp Clin Cancer Res. (2018) 37:191. doi: 10.1186/s13046-018-0860-x
41. Yao J, Du Z, Li Z, Zhang S, Lin Y, Li H, et al. 6-Gingerol as an arginase inhibitor prevents urethane-induced lung carcinogenesis by reprogramming tumor supporting M2 macrophages to M1 phenotype. Food Funct. (2018) 9:4611–20. doi: 10.1039/C8FO01147H
42. Lee C, Kim S, Jeong C, Cho I, Jo J, Han IH, et al. TAMpepK suppresses metastasis through the elimination of M2-like tumor-associated macrophages in triple-negative breast cancer. Int J Mol Sci. (2022) 23:2157. doi: 10.3390/ijms23042157
43. Chen J, Huang ZB, Liao CJ, Hu XW, Li SL, Qi M, et al. LncRNA TP73-AS1/miR-539/MMP-8 axis modulates M2 macrophage polarization in hepatocellular carcinoma via TGF-β1 signaling. Cell Signal. (2020) 75:109738. doi: 10.1016/j.cellsig.2020.109738
44. Qiao L, Dong C, Jia W, and Sun G. RAB5A in triple-negative breast cancer: a critical role in macrophage reshaping in an exosomal miR-21-dependent manner. Endocr Relat Cancer. (2024) 31:e230257. doi: 10.1530/ERC-23-0257
45. Yan J, Ye G, Jin Y, Miao M, Li Q, and Zhou H. Identification of novel prognostic circRNA biomarkers in circRNA-miRNA-mRNA regulatory network in gastric cancer and immune infiltration analysis. BMC Genomics. (2023) 24:323. doi: 10.1186/s12864-023-09421-2
46. Meng Z, Zhang R, Wang Y, Zhu G, Jin T, Li C, et al. miR-200c/PAI-2 promotes the progression of triple negative breast cancer via M1/M2 polarization induction of macrophage. Int Immunopharmacol. (2020) 81:106028. doi: 10.1016/j.intimp.2019.106028
47. Zhang Z, Hu J, Ishihara M, Sharrow AC, Flora K, He Y, et al. The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. Int J Mol Sci. (2022) 23:3005. doi: 10.3390/ijms23063005
48. Fang H, Chi X, Wang M, Liu J, Sun M, Zhang J, et al. M2 macrophage-derived exosomes promote cell proliferation, migration and EMT of non-small cell lung cancer by secreting miR-155-5p. Mol Cell Biochem. (2025) 480:3019–32. doi: 10.1007/s11010-024-05161-3
49. Ling Q, Fang J, Zhai C, Huang W, Chen Y, Zhou T, et al. Berberine induces SOCS1 pathway to reprogram the M1 polarization of macrophages via miR-155-5p in colitis-associated colorectal cancer. Eur J Pharmacol. (2023) 949:175724. doi: 10.1016/j.ejphar.2023.175724
50. Chen X, Wang X, Cui Z, Luo Q, Jiang Z, Huang Y, et al. M1 microglia-derived exosomes promote activation of resting microglia and amplifies proangiogenic effects through irf1/miR-155-5p/socs1 axis in the retina. Int J Biol Sci. (2023) 19:1791–812. doi: 10.7150/ijbs.79784
51. Lin Y, Li L, Huang H, Wen X, Zhang Y, Zhang R, et al. Vitexin inhibits TNBC progression and metastasis by modulating macrophage polarization through EGFR signaling. J Immunother. (2024) 47:303–12. doi: 10.1097/CJI.0000000000000519
52. Deswal B, Bagchi U, Santra MK, Garg M, and Kapoor S. Inhibition of STAT3 by 2-Methoxyestradiol suppresses M2 polarization and protumoral functions of macrophages in breast cancer. BMC Cancer. (2024) 24:1129. doi: 10.1186/s12885-024-12871-w
53. Lin Y, Xu J, and Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. (2019) 12:76. doi: 10.1186/s13045-019-0760-3
54. Fang WB, Yao M, Brummer G, Acevedo D, Alhakamy N, Berkland C, et al. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. (2016) 7:49349–67. doi: 10.18632/oncotarget.9885
55. Zhang L, Wang L, Xu Z, Zhang X, Guan S, Liu Z, et al. eNAMPT/ac-STAT3/DIRAS2 axis promotes development and cancer stemness in triple-negative breast cancer by enhancing cytokine crosstalk between tumor-associated macrophages and cancer cells. Int J Biol Sci. (2025) 21:2027–47. doi: 10.7150/ijbs.103723
56. You Y, Chen X, Chen X, Li H, Zhou R, Zhou J, et al. Jiawei Yanghe Decoction suppresses breast cancer by regulating immune responses via JAK2/STAT3 signaling pathway. J Ethnopharmacol. (2023) 316:116358. doi: 10.1016/j.jep.2023.116358
57. Ning Y, Cui Y, Li X, Cao X, Chen A, Xu C, et al. Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. BioMed Pharmacother. (2018) 103:262–71. doi: 10.1016/j.biopha.2018.04.022
58. Fleisher B, Clarke C, and Ait-Oudhia S. Current advances in biomarkers for targeted therapy in triple-negative breast cancer. Breast Cancer (Dove Med Press). (2016) 8:183–97. doi: 10.2147/BCTT.S114659
59. Yang Z, Chen Y, Miao Y, Yan H, Chen K, Xu Y, et al. Elucidating stearoyl metabolism and NCOA4-mediated ferroptosis in gastric cancer liver metastasis through multi-omics single-cell integrative mendelian analysis: advancing personalized immunotherapy strategies. Discov Oncol. (2025) 16:46. doi: 10.1007/s12672-025-01769-z
60. Chen X, Yang M, Yin J, Li P, Zeng S, Zheng G, et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. (2022) 20:92. doi: 10.1186/s12964-022-00888-2
61. Alemu BK, Tommasi S, Hulin JA, Meyers J, and Mangoni AA. Current knowledge on the mechanisms underpinning vasculogenic mimicry in triple negative breast cancer and the emerging role of nitric oxide. BioMed Pharmacother. (2025) 186:118013. doi: 10.1016/j.biopha.2025.118013
62. Li Y, Zhang MZ, Zhang SJ, Sun X, Zhou C, Li J, et al. HIF-1α inhibitor YC-1 suppresses triple-negative breast cancer growth and angiogenesis by targeting PlGF/VEGFR1-induced macrophage polarization. BioMed Pharmacother. (2023) 161:114423. doi: 10.1016/j.biopha.2023.114423
63. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, and Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. (2020) 353:104119. doi: 10.1016/j.cellimm.2020.104119
64. Szebeni GJ, Vizler C, Kitajka K, and Puskas LG. Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflammation. (2017) 2017:9294018. doi: 10.1155/2017/9294018
65. Owen JL and Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front Physiol. (2013) 4:159. doi: 10.3389/fphys.2013.00159
66. Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X, et al. Correction: M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. (2022) 13:752. doi: 10.1038/s41419-022-05203-y
67. Huang Z, Li Y, Qian Y, Ye L, Zhang T, Cheng Y, et al. An LCN2-dependent positive-feedback loop between gastric cancer cells and tumor-associated-macrophages mediates lymphangiogenesis and lymphatic metastasis. Adv Sci (Weinh). (2025) 30:e08352. doi: 10.1002/advs.202508352
68. Evans R, Flores-Borja F, Nassiri S, Miranda E, Lawler K, Grigoriadis A, et al. Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Rep. (2019) 27:1967–1978.e1964. doi: 10.1016/j.celrep.2019.04.076
69. Li X, Chen M, Lu W, Tang J, Deng L, Wen Q, et al. Targeting FAPα-expressing tumor-associated mesenchymal stromal cells inhibits triple-negative breast cancer pulmonary metastasis. Cancer Lett. (2021) 503:32–42. doi: 10.1016/j.canlet.2021.01.013
70. Liu P, Liu Y, Chen L, Fan Z, Luo Y, and Cui Y. Anemoside A3 inhibits macrophage M2-like polarization to prevent triple-negative breast cancer metastasis. Molecules. (2023) 28:1611. doi: 10.3390/molecules28041611
71. Dang Cao TL, Kawanishi K, Hashimoto S, Hengphasatporn K, Nagai-Okatani C, Kimura T, et al. Tumor-expressed GPNMB orchestrates Siglec-9(+) TAM polarization and EMT to promote metastasis in triple-negative breast cancer. Proc Natl Acad Sci U.S.A. (2025) 122:e2503081122. doi: 10.1073/pnas.2503081122
72. Lin X, Wang S, Sun M, Zhang C, Wei C, Yang C, et al. Retraction Note: miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J Hematol Oncol. (2023) 16:41. doi: 10.1186/s13045-023-01438-0
73. Zhang J, Zhang Z, Huang Z, Li M, Yang F, Wu Z, et al. Isotoosendanin exerts inhibition on triple-negative breast cancer through abrogating TGF-β-induced epithelial-mesenchymal transition via directly targeting TGFβR1. Acta Pharm Sin B. (2023) 13:2990–3007. doi: 10.1016/j.apsb.2023.05.006
74. Deng F, Weng Y, Li X, Wang T, Fan M, and Shi Q. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer. Pathol Res Pract. (2021) 223:152824. doi: 10.1016/j.prp.2020.152824
75. Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang Q, et al. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell Commun Signal. (2023) 21:299. doi: 10.1186/s12964-022-00872-w
76. Wang Z, Zhang C, Guo J, Wang W, Si Q, Chen C, et al. Exosomal miRNA-223-3p derived from tumor associated macrophages promotes pulmonary metastasis of breast cancer 4T1 cells. Transl Oncol. (2023) 35:101715. doi: 10.1016/j.tranon.2023.101715
77. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. (2019) 38:81. doi: 10.1186/s13046-019-1095-1
78. Ye M, Lu F, Gu D, Xue B, Xu L, Hu C, et al. Hypoxia exosome derived CEACAM5 promotes tumor-associated macrophages M2 polarization to accelerate pancreatic neuroendocrine tumors metastasis via MMP9. FASEB J. (2024) 38:e23762. doi: 10.1096/fj.202302489RRR
79. Palmulli R, Jackson HK, and Edgar JR. Tethered exosomes containing the matrix metalloproteinase MT1-MMP contribute to extracellular matrix degradation. J Extracell Vesicles. (2025) 14:e70122. doi: 10.1002/jev2.70122
80. Li Y, You J, Zou Z, Sun G, Shi Y, Sun Y, et al. Decoding the tumor microenvironment: exosome-mediated macrophage polarization and therapeutic frontiers. Int J Biol Sci. (2025) 21:4187–214. doi: 10.7150/ijbs.114222
81. Yu L, Liebenberg K, Shen Y, Liu F, Xu Z, Hao X, et al. Tumor-derived arachidonic acid reprograms neutrophils to promote immune suppression and therapy resistance in triple-negative breast cancer. Immunity. (2025) 58:909–925.e907. doi: 10.1016/j.immuni.2025.03.002
82. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X, et al. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
83. Jiang L, Jiang Y, Zhou X, Wang L, Zhang S, Jiang C, et al. The key role of COA6 in pancreatic ductal adenocarcinoma: metabolic reprogramming and regulation of the immune microenvironment. J Cell Mol Med. (2025) 29:e70685. doi: 10.1111/jcmm.70685
84. Xia J, Zhang L, Peng X, Tu J, Li S, He X, et al. IL1R2 blockade alleviates immunosuppression and potentiates anti-PD-1 efficacy in triple-negative breast cancer. Cancer Res. (2024) 84:2282–96. doi: 10.1158/0008-5472.CAN-23-3429
85. Williams CB, Yeh ES, and Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer Malignancy. NPJ Breast Cancer. (2016) 2:15025–. doi: 10.1038/npjbcancer.2015.25
86. Liu B, Huang J, Xiao J, Xu W, Zhang H, Yuan Y, et al. The Streptococcus virulence protein PepO triggers anti-tumor immune responses by reprograming tumor-associated macrophages in a mouse triple negative breast cancer model. Cell Biosci. (2023) 13:198. doi: 10.1186/s13578-023-01153-w
87. Jing W, Guo X, Wang G, Bi Y, Han L, Zhu Q, et al. Breast cancer cells promote CD169(+) macrophage-associated immunosuppression through JAK2-mediated PD-L1 upregulation on macrophages. Int Immunopharmacol. (2020) 78:106012. doi: 10.1016/j.intimp.2019.106012
88. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. (2017) 545:495–9. doi: 10.1038/nature22396
89. Núñez SY, Trotta A, Regge MV, Amarilla MS, Secchiari F, Sierra JM, et al. Tumor-associated macrophages impair NK cell IFN-γ production and contribute to tumor progression in clear cell renal cell carcinoma. Eur J Immunol. (2024) 54:e2350878. doi: 10.1002/eji.202350878
90. Hensler M, Kasikova L, Fiser K, Rakova J, Skapa P, Laco J, et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J Immunother Cancer. (2020) 8:e000979. doi: 10.1136/jitc-2020-000979
91. Low JT, Ho PC, and Matsushita M. TAM-tastic: from resistance to resilience in cancer. Trends Pharmacol Sci. (2024) 45:953–4. doi: 10.1016/j.tips.2024.09.006
92. Saleh R, Toor SM, Khalaf S, and Elkord E. Breast cancer cells and PD-1/PD-L1 blockade upregulate the expression of PD-1, CTLA-4, TIM-3 and LAG-3 immune checkpoints in CD4(+) T cells. Vaccines (Basel). (2019) 7:149. doi: 10.3390/vaccines7040149
93. Lerévérend C, Kotaich N, Cartier L, De Boni M, Lahire S, Fichel C, et al. Enhanced expression of galectin-9 in triple negative breast cancer cells following radiotherapy: Implications for targeted therapy. Int J Cancer. (2025) 156:229–42. doi: 10.1002/ijc.35107
94. Sun X, Kennedy LC, Gonzalez-Ericsson PI, Sanchez V, Sanders M, Perou CM, et al. Associations of immune checkpoint predictive biomarkers (MHC-I and MHC-II) with clinical and molecular features in a diverse breast cancer cohort. Clin Cancer Res. (2024) 30:4077–81. doi: 10.1158/1078-0432.CCR-24-1286
95. Lin Y, Choukrani G, Dubbel L, Rockstein L, Freile JA, Qi Y, et al. VISTA drives macrophages towards a pro-tumoral phenotype that promotes cancer cell phagocytosis yet down-regulates T cell responses. Exp Hematol Oncol. (2024) 13:35. doi: 10.1186/s40164-024-00501-x
96. Abudula M, Astuti Y, Raymant M, Sharma V, Schmid MC, and Mielgo A. Macrophages suppress CD8 + T cell cytotoxic function in triple negative breast cancer via VISTA. Br J Cancer. (2025) 133:40–51. doi: 10.1038/s41416-025-03013-5
97. Wang R, Li G, Gao F, Xu F, Li X, Zhang J, et al. Ultrasound-responsive spherical nucleic acid against c-Myc/PD-L1 to enhance anti-tumoral macrophages in triple-negative breast cancer progression. Sci China Life Sci. (2024) 67:698–710. doi: 10.1007/s11427-023-2433-y
98. Sami E, Paul BT, Koziol JA, and ElShamy WM. The immunosuppressive microenvironment in BRCA1-IRIS-overexpressing TNBC tumors is induced by bidirectional interaction with tumor-associated macrophages. Cancer Res. (2020) 80:1102–17. doi: 10.1158/0008-5472.CAN-19-2374
99. Takizawa H and Manz MG. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol. (2007) 8:1287–9. doi: 10.1038/ni1207-1287
100. Yang H, Shao R, Huang H, Wang X, Rong Z, and Lin Y. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPα axis. Cancer Med. (2019) 8:4245–53. doi: 10.1002/cam4.2332
101. Riaz N, Burugu S, Cheng AS, Leung SCY, Gao D, and Nielsen TO. Prognostic significance of CSF-1R expression in early invasive breast cancer. Cancers (Basel). (2021) 13:5769. doi: 10.3390/cancers13225769
102. Gomez-Roca C, Cassier P, Zamarin D, Machiels JP, Perez Gracia JL, Stephen Hodi F, et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naïve or experienced for immune checkpoint blockade. J Immunother Cancer. (2022) 10:e004076. doi: 10.1136/jitc-2021-004076
103. Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med. (2018) 10:eaan3311. doi: 10.1126/scitranslmed.aan3311
104. Gomez-Roca CA, Italiano A, Le Tourneau C, Cassier PA, Toulmonde M, D'Angelo SP, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol. (2019) 30:1381–92. doi: 10.1093/annonc/mdz163
105. Tang J, Wei W, Xu Y, Chen K, Miao Y, Fan W, et al. CXC chemokine receptor 4 - mediated immune modulation and tumor microenvironment heterogeneity in gastric cancer: Utilizing multi-omics approaches to identify potential therapeutic targets. Biofactors. (2025) 51:e2130. doi: 10.1002/biof.2130
106. Wan Z, Huang H, West RE 3rd, Zhang M, Zhang B, Cai X, et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater Today (Kidlington). (2023) 62:33–50. doi: 10.1016/j.mattod.2022.11.008
107. Liu Y, Tiruthani K, Wang M, Zhou X, Qiu N, Xiong Y, et al. Tumor-targeted gene therapy with lipid nanoparticles inhibits tumor-associated adipocytes and remodels the immunosuppressive tumor microenvironment in triple-negative breast cancer. Nanoscale Horiz. (2021) 6:319–29. doi: 10.1039/D0NH00588F
108. Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. (2014) 515:130–3. doi: 10.1038/nature13862
109. Augimeri G, Fiorillo M, Morelli C, Panza S, Giordano C, Barone I, et al. The omega-3 docosahexaenoyl ethanolamide reduces CCL5 secretion in triple negative breast cancer cells affecting tumor progression and macrophage recruitment. Cancers (Basel). (2023) 15:819. doi: 10.3390/cancers15030819
110. Araujo JM, Gomez AC, Aguilar A, Salgado R, Balko JM, Bravo L, et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci Rep. (2018) 8:4899. doi: 10.1038/s41598-018-23099-7
111. Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, et al. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res. (2015) 75:4063–73. doi: 10.1158/0008-5472.CAN-14-3394
112. Lepland A, Peranzoni E, Haljasorg U, Asciutto EK, Crespí-Amer M, Modesti L, et al. Peptide-drug conjugate for therapeutic reprogramming of tumor-associated macrophages in breast cancer. Adv Sci (Weinh). (2025) 12:e2410288. doi: 10.1002/advs.202410288
113. Dong X, Wang X, Zheng X, Jiang H, Liu L, Ma N, et al. Targeted nanoparticle delivery system for tumor-associated macrophage reprogramming to enhance TNBC therapy. Cell Biol Toxicol. (2025) 41:58. doi: 10.1007/s10565-025-10001-1
114. Rodriguez-Perdigon M, Haeni L, Rothen-Rutishauser B, and Rüegg C. Dual CSF1R inhibition and CD40 activation demonstrates anti-tumor activity in a 3D macrophage- HER2(+) breast cancer spheroid model. Front Bioeng Biotechnol. (2023) 11:1159819. doi: 10.3389/fbioe.2023.1159819
115. Yi J, Chen Q, Liu X, Mao Y, Wang Y, Lv M, et al. Genetic evidence from Mendelian randomization links CD40 levels to increased risk of estrogen receptor-positive breast cancer. Sci Rep. (2025) 15:14892. doi: 10.1038/s41598-025-99410-0
116. Jardim C, Bica M, Reis-Sobreiro M, Teixeira da Mota A, Lopes R, Pinto MF, et al. Sustained macrophage reprogramming is required for CD8+ T cell-dependent long-term tumor eradication. Cancer Immunol Res. (2025) 13:1207–25. doi: 10.1158/2326-6066.CIR-24-0797
117. Cai XJ, Wang Z, Cao JW, Ni JJ, Xu YY, Yao J, et al. Anti-angiogenic and anti-tumor effects of metronomic use of novel liposomal zoledronic acid depletes tumor-associated macrophages in triple negative breast cancer. Oncotarget. (2017) 8:84248–57. doi: 10.18632/oncotarget.20539
118. Yuan J, Shi X, Chen C, He H, Liu L, Wu J, et al. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol Lett. (2019) 18:3249–55. doi: 10.3892/ol.2019.10618
119. Cao X, Li B, Chen J, Dang J, Chen S, Gunes EG, et al. Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer. J Immunother Cancer. (2021) 9:e002022. doi: 10.1136/jitc-2020-002022
120. Moon Y, Cho H, Kim J, Song S, Yeon Park J, Young Min J, et al. Self-assembled peptide-derived proteolysis-targeting chimera (PROTAC) nanoparticles for tumor-targeted and durable PD-L1 degradation in cancer immunotherapy. Angew Chem Int Ed Engl. (2025) 64:e202414146. doi: 10.1002/anie.202414146
121. Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol. (2020) 15:487–500. doi: 10.1007/s11481-019-09884-9
122. Qiu Y, Ren K, Zhao W, Yu Q, Guo R, He J, et al. A "dual-guide" bioinspired drug delivery strategy of a macrophage-based carrier against postoperative triple-negative breast cancer recurrence. J Control Release. (2021) 329:191–204. doi: 10.1016/j.jconrel.2020.11.039
123. Gong C, Yu X, You B, Wu Y, Wang R, Han L, et al. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnol. (2020) 18:92. doi: 10.1186/s12951-020-00649-8
124. Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. (2020) 32:e2004853. doi: 10.1002/adma.202004853
125. Shi L and Gu H. Emerging nanoparticle strategies for modulating tumor-associated macrophage polarization. Biomolecules. (2021) 11:1912. doi: 10.3390/biom11121912
126. Hu Y, Nie W, Lyu L, Zhang X, Wang W, Zhang Y, et al. Tumor-microenvironment-activatable nanoparticle mediating immunogene therapy and M2 macrophage-targeted inhibitor for synergistic cancer immunotherapy. ACS Nano. (2024) 18:3295–312. doi: 10.1021/acsnano.3c10037
127. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. (2016) 11:986–94. doi: 10.1038/nnano.2016.168
Keywords: triple-negative breast cancer, tumor-associated macrophages, tumor microenvironment, immune evasion, TAM
Citation: Wu T and Liao Y (2025) Roles of tumor-associated macrophages in triple-negative breast cancer progression. Front. Immunol. 16:1677363. doi: 10.3389/fimmu.2025.1677363
Received: 31 July 2025; Accepted: 26 September 2025;
Published: 09 October 2025.
Edited by:
Shengshan Xu, Jiangmen Central Hospital, ChinaReviewed by:
Lexin Wang, Ningxia Medical University, ChinaCopyright © 2025 Wu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuling Liao, dzEzMDc4MjY0QDE2My5jb20=
†These authors have contributed equally to this work
 Tianhai Wu
Tianhai Wu Yuling Liao
Yuling Liao