- 1Mirakle Integrated Health Centre, Pollachi, India
- 2International Institute of Yoga and Naturopathy Medical Sciences, Chengalpattu, India
- 3Swami Vivekananda Yoga Anusandhana Samsthana, Bengaluru, India
- 4SDM College of Naturopathy and Yogic Sciences, Ujire, India
- 5Sant Hirdaram Medical College of Naturopathy & Yogic Sciences for Women, Bhopal, India
Background: India, has a rich ethnomedicinal tradition where numerous herbs are used in cancer care. However, scientific validation of these practices remains limited. This narrative review explores the phytochemical mechanisms underlying their anti-cancer effects of 32 herbs identified by herbal activists, including physicians and traditional healers from Tamil Nadu, India, for their purported anticancer properties.
Methods: A narrative review was conducted using PubMed, Scopus, and Google Scholar to identify studies published between 2014 and January 2025 on 32 selected anecdotal herbs. Eligible studies included in vitro, in vivo, clinical, and in silico investigations. Data extraction by five independent reviewers focused on botanical and common names, bioactive compounds, mechanisms of anticancer activity, cancer types studied, and evidence level (preclinical vs. clinical).
Results: Herbs such as Withania somnifera, Curcuma longa, and Annona muricata exhibited strong preclinical and limited clinical anticancer activity through apoptosis induction, inhibition of angiogenesis and metastasis, immune modulation, and synergy with standard therapies. Most other herbs remain at the preclinical stage, with minimal clinical data. Only Catharanthus roseus (purified) and Curcuma longa (formulations) have limited clinical application. Challenges including poor bioavailability, lack of standardization, safety concerns, and toxicity (e.g., Annona muricata, Gloriosa superba) hinder clinical translation, underscoring the need for rigorous evaluation.
Conclusion: Traditional herbs demonstrate notable experimental anticancer potential, yet clinical validation is limited. Integrating ethnomedicinal knowledge with systematic research could guide future cancer therapies and inform policy development in integrative oncology.
Introduction
Cancer remains a leading cause of morbidity and mortality worldwide, accounting for nearly one in six deaths globally. Despite significant advances in diagnostics and therapeutics, conventional cancer treatments such as chemotherapy, radiation, and surgery are often associated with adverse effects, resistance, and high economic burden. These limitations have prompted growing interest in complementary and integrative approaches, including the use of plant-based therapies rooted in traditional medicine systems (1).
India, with its rich biodiversity and longstanding traditions of ethnomedicine, offers a vast pharmacopeia of medicinal plants with therapeutic potential (2). In particular, the southern state of Tamil Nadu harbors a deep legacy of herbal healing practices, sustained by intergenerational knowledge among Yoga & Naturopathy, Siddha physicians, local vaidyas, and indigenous communities. While many of these herbs have been used for centuries to manage a variety of ailments including tumors and growths their scientific validation in the context of cancer remains incomplete.
This narrative review aims to bridge the gap between traditional herbal knowledge and modern scientific inquiry by examining 32 herbs widely used or recommended for their purported anticancer effects by a collective of herbal activists, including physicians and traditional healers from Tamil Nadu. The primary objective is to evaluate the scientific evidence regarding their efficacy in cancer prevention and treatment, both as standalone interventions and as adjuncts to standard therapies. The secondary objective is to elucidate the underlying mechanisms of action, focusing on phytochemical constituents and their interactions with cancer cell biology.
Indigenous people in India have long used medicinal plants to fight cancer. However, most such herbal treatments remain anecdotal and under-researched. Tavakoli et al. reported that many herbal cancer therapies have not undergone systematic scientific evaluation (3). By beginning with community‐endorsed remedies rather than familiar botanicals, this review inverts the usual research paradigm and systematically examines what evidence exists for each plant. This practice to literature approach sets this review apart, highlighting unique leads from Tamil Nadu’s tradition while exposing critical gaps in the scientific validation of these anticancer claims. In doing so, it emphasizes the critical need to integrate ethnobotanical knowledge with rigorous biomedical research, thereby contributing to a more inclusive and evidence-informed framework for integrative oncology.
Methods
Study design
This narrative review employed a comprehensive approach to examine 32 herbs with purported anticancer properties, identified by a group of herbal activists, including physicians and traditional healers from Tamil Nadu, India. The term “herbal activists” refers to a collaborative group comprising physicians trained in traditional medicine, local healers, and community practitioners actively engaged in preserving and promoting indigenous herbal knowledge. Their collective input was used as a pragmatic starting point to identify frequently utilized herbs warranting scientific validation. The selection of these herbs was based on anecdotal evidence, popular use, and their anthropological prevalence. The primary objective was to evaluate existing literature on the efficacy of these herbs, used either independently or alongside standard cancer treatments. The secondary objective was to explore the mechanisms by which their phytochemicals influence cancer metabolism.
Database and search strategy
A literature review was conducted across PubMed, Scopus, and Google Scholar, covering the period from 2014 to January 2025. Manual screening of references from key studies complemented the database search. The search aimed to identify at least five published articles per herb, irrespective of publication type. Keywords combined scientific and common names of herbs with terms such as “anticancer properties,” “cytotoxicity,” “traditional medicine,” “phytochemicals,” “tumor inhibition,” “herbal oncology,” “integrative cancer therapies,” and “bioactive compounds.” Boolean operators were applied to optimize search precision: AND linked different concepts (e.g., “herb name AND anticancer activity AND cytotoxicity”), OR connected synonyms (e.g., “phytochemicals OR bioactive compounds”), and NOT excluded irrelevant results (e.g., “traditional medicine NOT Ayurveda”).
Eligibility criteria
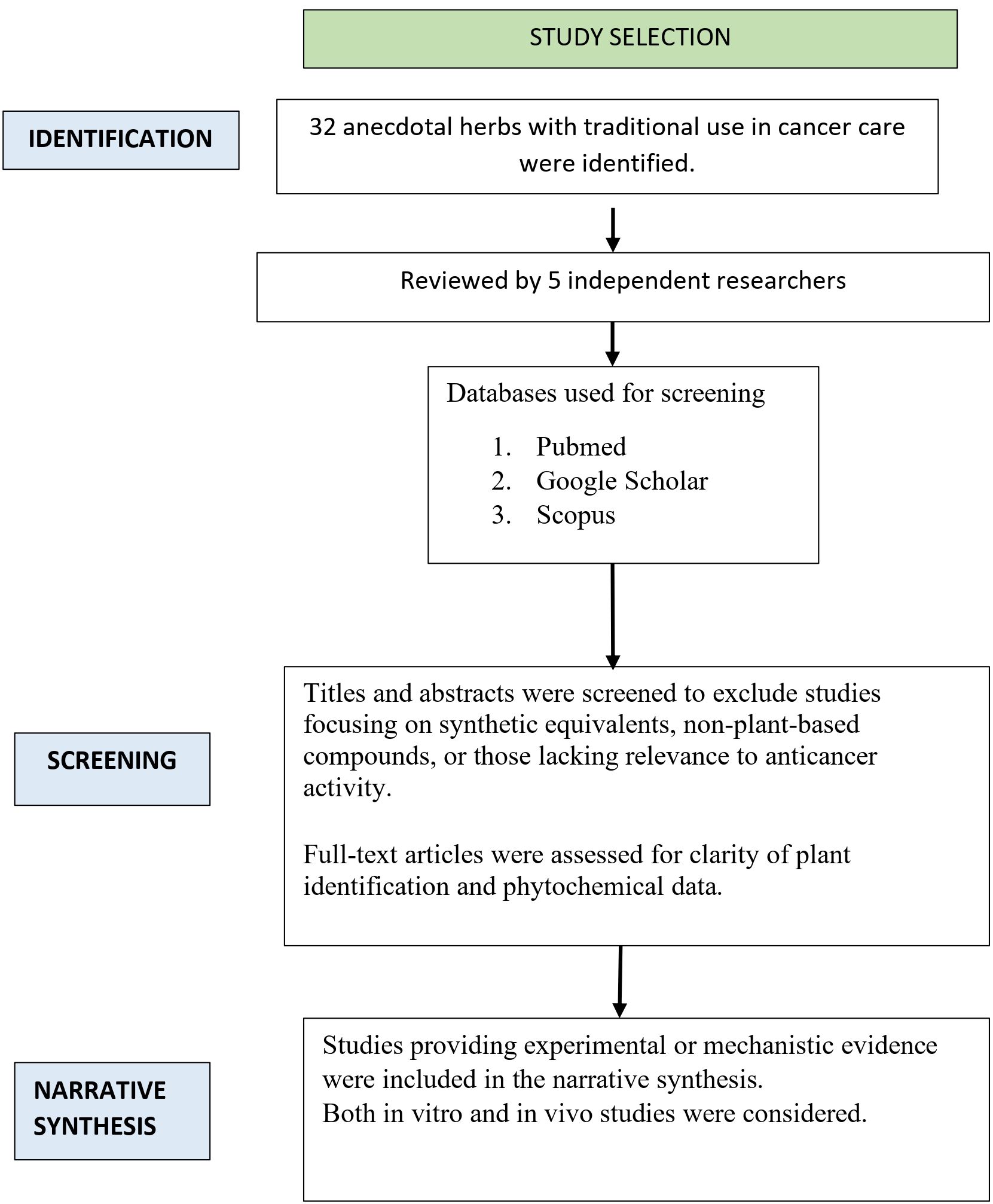
Studies published between 2014 and January 2025, available in full text and written in English, were included. Eligible studies specifically investigated the anticancer effects of the 32 identified herbal plants or their bioactive constituents. Both preclinical (in vitro, in vivo, and in silico) and clinical studies were considered. Exclusion criteria included studies unrelated to cancer, duplicate publications, and literature reviews. Figure 1 illustrates the flow of the literature review used for this narrative review.
Data extraction
Five independent reviewers conducted a two-stage evaluation of selected studies, beginning with title and abstract screening, followed by full-text review. Discrepancies were resolved through discussion or consultation with an additional reviewer. Extracted data included study characteristics such as the investigated herbs, cancer types and corresponding cell lines, and strength of evidence (preclinical or clinical). Additional details encompassed the botanical and local (Hindi) names of herbs, plant parts used, mode of administration, major bioactive phytochemicals with reported anticancer activity, mechanisms of action (e.g., apoptosis induction, inhibition of angiogenesis and metastasis), cancer models employed (in vitro, in vivo, or in silico), and main study outcomes.
Data charting process
A standardized data-charting template was developed collaboratively by five reviewers using Microsoft Excel to ensure systematic extraction. Each reviewer independently entered and verified study data, and the template was refined through iterative rounds of review and consensus to enhance accuracy, completeness, and consistency across all variables.
Results
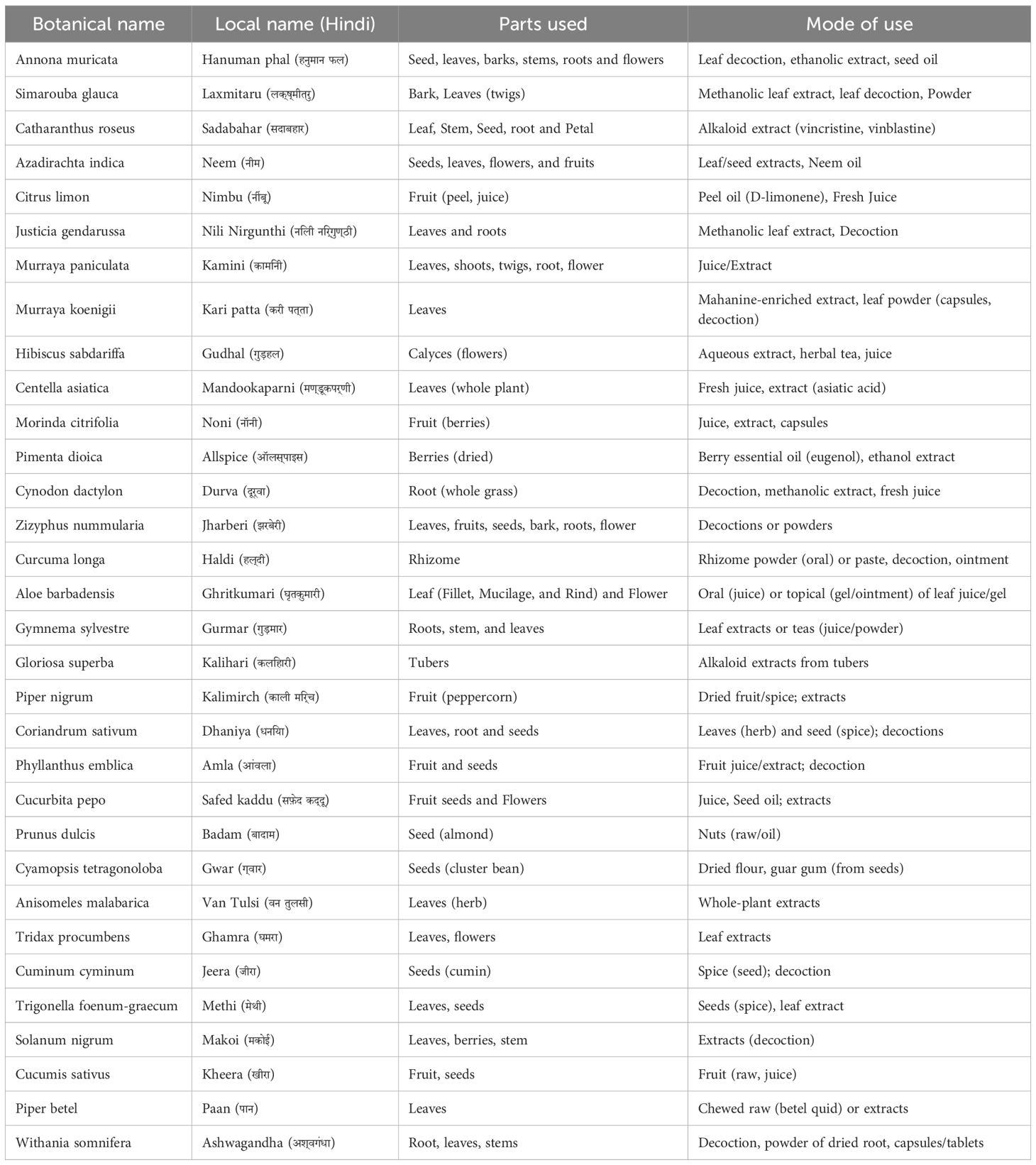
Table 1 summarize the general overview of the herbs discussed in this review. This review analyzed preclinical, in vivo, and clinical studies related to 32 traditionally used anecdotal herbs to determine whether their applications are supported by evidence-based research. Supplementary Table 1 summarizes the available preclinical and clinical evidence regarding the anticancer potential of these herbs. The independent effects of each plant on cancer biology are detailed below.
Annona muricata
Annona muricata (soursop) has shown notable anticancer effects in preclinical studies, primarily attributed to phytochemicals like annonaceous acetogenins and alkaloids (4). Leaf extracts, along with seed and bark of soursop in some studies, have demonstrated activity against cancer cell lines such as MCF-7 (breast), HT-29 (colorectal), PC-3 (prostate), and PANC-1 (pancreatic) (5–11). The main mechanism involves inhibition of mitochondrial Complex I, leading to ATP depletion and selective cancer cell death; apoptosis is triggered via upregulation of Bax, caspase-3, and caspase-9, and downregulation of Bcl-2 (6, 12–15). The extracts of soursop leaves also induce cell cycle arrest at G0/G1 or G2/M phases, likely through cyclin-CDK regulation (16–19), and promote oxidative stress via ROS generation, causing DNA damage (7, 20–22). Furthermore, inhibition of NF-κB, PI3K/Akt, and EGFR pathways reduces proliferation, invasion, and angiogenesis (23–25).
Simarouba glauca
Simarouba glauca (paradise tree) has demonstrated broad-spectrum anticancer activity, primarily in in vitro models. Extracts from leaves, bark, and seeds, prepared using methanol, petroleum ether, or chloroform contain phytochemicals such as alkaloids, flavonoids, terpenoids, glycosides, triterpenoids, and quassinoids, exhibiting cytotoxic, antiproliferative, and pro-apoptotic effects. Leaf extracts of S. glauca showed potent cytotoxicity in leukemia and non-small cell lung cancer cells via caspase-dependent apoptosis, activating both mitochondrial and death receptor pathways (26, 27). In breast cancer models, ethanolic and methanolic extracts of S. glauca displayed low half-maximal inhibitory concentration (IC50) values and similar apoptotic mechanisms (28).Bark extracts of S. glauca disrupted oxidative phosphorylation and induced apoptosis in triple-negative breast cancer cells (29), while chloroform and ethyl acetate fractions suppressed cell proliferation in cervical, colorectal, and mammary cancer cell lines via oxidative stress and apoptotic pathway activation (30). In vivo, Yeo et al., reported synergism between gemcitabine and glaucarubinone from S. glauca seeds, suppressing pancreatic tumor growth through HIF-1α and β-catenin regulation (31). Additional studies in bladder, prostate, and colorectal cancers revealed mechanisms such as p53/p21 upregulation, G0/G1 arrest, and mitochondrial depolarization (32–34), affirming S. glauca’s anticancer potential.
Catharanthus roseus
Catharanthus roseus is a well-documented medicinal plant known for its anticancer alkaloids vincristine and vinblastine, widely used in chemotherapy for leukemia, Hodgkin’s lymphoma, and breast cancer. These compounds act by inhibiting mitosis, disrupting microtubule formation, and inducing apoptosis (35, 36). An in vitro study reported the cytotoxicity effects of methanolic leaf extracts of C. roseus against MCF-7 breast cancer cells indicating potential anti-carcinogenic effect of C. roseus (37). Endophytic fungi such as Talaromyces radicus and Eutypella spp., isolated from Catharanthus roseus, have been shown to produce vincristine and vinblastine like compounds. These fungi induce reactive oxygen species (ROS) generation and disrupt mitochondrial membrane potential, thereby mimicking the apoptotic effects of the parent alkaloids (38, 39). A 2023 in vivo–in vitro–in silico study using a nanoemulsion formulation of incensole acetate from C. roseus essential oil demonstrated significant anticancer activity against breast cancer, with improved bioavailability and reduced toxicity (40). Similarly, an in vitro study on lung adenocarcinoma cells confirmed C. roseus induced cytotoxic and apoptotic effects (41). Furthermore, an in vivo study combining C. roseus with Phyllanthus niruri showed enhanced macrophage polarization and immune response in mice with induced breast cancer (42).
Azadirachta indica
Azadirachta indica (neem) is a medicinal plant rich in over 300 bioactive compounds, including azadirachtin, gedunin, nimbin, nimbolide, and quercetin, with established antineoplastic properties. Extracts from various parts of the plant exhibit cytotoxic, anti-proliferative, and pro-apoptotic effects across multiple cancers (43). Methanolic bark extracts of neem modulate migration-related genes (ZO-1, MMP2, FAK, N-cadherin) in cervical cancer (44), while specific compounds such as phthalic acid and 4-ethylbenzamide show cytotoxicity in breast cancer (45). Nimbolide, another bioactive compound in neem has shown to inhibit PI3K/Akt/mTOR and ERK pathways in pancreatic cancer, suppresses epithelial-mesenchymal transition, and induces mitochondrial-mediated apoptosis (46). Other compounds like desacetyl nimbinene and epoxyazadiradione modulate MAPK and PI3K/Akt signaling, leading to apoptosis and reduced tumor growth (47). Gedunin targets the Hedgehog pathway in pancreatic cancer and promotes apoptosis via p53 and Bax in teratocarcinoma (48, 49). Neem-derived flavonoids and limonoids also inhibit STAT3, BCL-2, and enhance BAX, Caspase-3, and mitochondrial depolarization in hepatocellular and prostate cancers (50, 51). Additionally, neem silver nanoparticles downregulate VEGF and Cyclin D1 in lung cancer (52), and combined extracts show apoptotic effects in breast, prostate, and colorectal cancers through p53/Bax activation and BCL2 inhibition (50).
Citrus limonum
Citrus limonum (lemon) contains diverse bioactive compounds including flavonoids (naringenin, naringin, quercetin, nobiletin, 5-demethylnobiletin, 2′-hydroxyflavanone, tangeretin), alkaloids (synephrine, N-methyltyramine, hordenine), terpenoids (limonene, geranial, neral, obacunone), and secondary metabolites like tannins, saponins, and modified citrus pectin (MCP). MCP inhibits STAT3 phosphorylation and galectin-3, suppressing M2 macrophages and STAT3 signaling in breast, prostate, and ovarian cancers (53). Lemon juice–derived nanovesicles show antileukemic effects via TRAIL and anti-angiogenesis mechanisms (54). Flavonoids modulate estrogen signaling, inhibit aromatase, reduce BCL-2, and activate caspases in breast cancer (55). Nobiletin and its derivatives regulate G0/G1 arrest, apoptosis, and multiple signaling pathways across cholangiocarcinoma, colorectal, lung, gastric, and myeloid cancers (56). Other compounds like 2′-hydroxyflavanone, tangeretin, and obacunone exhibit anti-STAT3, anti-inflammatory, and antiproliferative effects (57), while alkaloids and terpenoids contribute to cytotoxicity in hepatocellular, cervical, and melanoma models via redox modulation (58).
Justica gendarussa
Justica gendarussa is a member of Acanthaceae family, which contains bioactive compounds such as flavonoids (including kaempferol and naringenin), alkaloids, triterpenoidal saponins, amino acids, aromatic amines, stigmasterol and lupeol. Kaempferol and naringenin have shown cytotoxic effects against mammary carcinoma (59), while methanolic leaf extract exhibits both proapoptotic and cytotoxic activity in lung cancer (60). Gendarusin A is likely to activate multiple cell signaling pathways related to programmed cell death, including the expression of proteins such as BID, BAX and BCL-2 in T-lymphocyte cancer (61).
Murraya paniculata
Murraya paniculata (Orange Jessamine) contains a wide range of bioactive compounds including flavonoids, phenols, alkaloids, coumarins, polysaccharides, and essential oils rich in sesquiterpenes like β-caryophyllene, limonene, and spathulenol (62). Flavonoids and coumarins inhibit metastasis by suppressing cancer cell adhesion and invasion (63). A specific flavonoid glycoside modulates integrin β1, EGFR, COX-2, MMPs, EMT markers, and STAT3/NF-κB/PI3K pathways in lung cancer (64). Compounds such as 6′-O-β-d-apiofuranosylapterin and coumarins showed cytotoxicity against leukemia, lung, liver, breast, and colon cancer cells (65). Sesquiterpenes showed hepatotoxicity in liver cancer models (62), while certain coumarins (e.g., murpanidin, murralongin) inhibited colon cancer metastasis by downregulating EpCAM without cytotoxicity (66). Alkaloids, saponins, phenols, and volatile oils induced apoptosis and inhibited growth in breast cancer (67). Coumarin derivatives like auraptene and scopolin exhibited antiproliferative activity via estrogen receptor binding (68). Nanoparticle formulations enhanced cytotoxicity by targeting DNA replication in MCF-7 cells (69).
Murraya koenigii
Murraya koenigii (curry leaf plant) contains potent bioactive compounds, especially carbazole alkaloids such as mahanine, mahanimbine, and koenimbine, which exhibit cytotoxic, anti-proliferative, apoptotic, anti-inflammatory, and anti-metastatic properties across various cancer types. Mahanine induces apoptosis via mitochondrial pathways, increases ROS, activates caspases 3, 7, and 9, upregulates Bax, downregulates Bcl-2, and targets estrogen receptor alpha and CDKs in leukemia and breast cancer cells (70). It also interferes with STAT3, PI3K/AKT, and Wnt/β-catenin signaling, contributing to cell cycle arrest and tumor suppression in lung, ovarian, and prostate cancers (71–73). Grinimbine and other alkaloids such as koenimbine and mukonal induce G0/G1 arrest and apoptosis, demonstrating selective cytotoxicity in colorectal (HT-29), breast (MCF-7, MDA-MB-231), and glioblastoma cells (U373MG) (74).These compounds also reduce cell viability, disrupt mitochondrial membrane potential, deplete glutathione, modulate heat shock proteins (HSP70/90), and increase DNA damage in hepatocellular carcinoma (HepG2) (75).
Hibiscus sabdariffa
Hibiscus sabdariffa, a member of the Malvaceae family, is rich in diverse bioactive compounds including gossypetin, oleuropein, hydroxytyrosol, flavonoids, anthocyanins (such as cyanidin and delphinidin), catechin, ellagic acid, protocatechuic acid, quercetin, hibiscus acid, rutin, oleanolic acid derivatives, and polysaccharides. These compounds exert anticancer effects across various cancer types through multiple mechanisms. Gossypetin induces cell cycle arrest in oral cancer cells by upregulating p21 (76), while oleuropein forms copper complexes that trigger apoptosis in neuroblastoma cells (77). Polyphenol-rich extracts downregulate ERα and BRCA1, promote autophagy, and inhibit proteasome activity in breast cancer cells (78). Fatty acid esters selectively induce apoptosis in colon cancer by targeting the 2HQ6 protein (79), and hibiscus acid along with anthocyanins inhibits the proteasome in multiple myeloma cells (80, 81). Catechins and flavonoids suppress prostate cancer progression by inhibiting Akt/NF-κB/MMP-9 signaling (82). In breast and oral cancers, anthocyanins and rutin reduce oxidative stress, protect against DNA damage, and induce cytotoxicity (83, 84).
Centella asiatica
Centella asiatica (mandukparni/gotu kola) contains triterpenoids (asiaticoside, madecassoside, asiatic acid) and flavonoids (quercetin, kaempferol) that exhibit anticancer, anti-angiogenic, cytotoxic, and apoptotic effects across multiple cancers. These compounds inhibit VEGF165-induced angiogenesis and modulate key signaling pathways like PI3K/Akt, PD-1, RAGE, and AGE-1. In oral, liver, and melanoma models, they induce apoptosis via Bax/Bcl-2 regulation, caspase activation, and suppression of cAMP-PKA-MITF and migration pathways (85). Asiatic acid enhances mitochondrial damage, modulates YAP1, VEGFA, STAT3, and inhibits EMT (86, 87), while also suppressing PI3K/Akt/mTOR signaling to promote autophagy and apoptosis in lung, colon, and ovarian cancers [88–94].Phytochemicals such as alkaloids, glycosides, and flavonoids induce mitochondrial dysfunction, oxidative stress, and caspase-mediated apoptosis in MCF-7, A549, HeLa, and Caco-2 cells (95).
Morinda citrifolia
Morinda citrifolia (noni), from the Rubiaceae family, is rich in bioactive compounds such as scopoletin, epicatechin, phytosterols, nordamnacanthal, and damnacanthal, contributing to its antioxidative, immunostimulatory, hepatoprotective, anti-inflammatory, and anticancer activities. Epicatechin and scopoletin induce apoptosis in leukemia (Jurkat, WEHI-3b) and lung adenocarcinoma (A549) through caspase-3/8 activation, G0/G1 arrest, and downregulation of tumor proliferation genes (EGFR, MDM2, RAF1, mTOR), while enhancing immune responses and reducing COX-2 expression (96). Nordamnacanthal and damnacanthal exhibit apoptotic and anti-proliferative effects in breast cancer (MCF-7, MDA-MB231, 4T1), promoting annexin V+ expression, G1 arrest, and modulation of apoptotic regulators such as p53, Bax, Bcl-2, XIAP, and ER-α (97–99). Other phytoconstituents like asperuloside, asperulosidic acid, deacetylasperulosidic acid, eugenol, rutin, and morindone target MAPK6 and MDM2 pathways, suppressing tumor growth and promoting p53-mediated apoptosis in colorectal and liver cancers (100, 101).Collectively, Morinda citrifolia exerts anti-cancer effects via modulation of multiple apoptotic and inflammatory pathways including VEGF/EGFR/NF-κB, AKT1, MAPK, JAK2/STAT3/STAT5A, contributing to reduced tumor growth and angiogenesis (102).
Pimenta dioica
Commonly known as allspice, the aqueous extract of Pimenta dioica has been identified as an anti-cancer agent against prostate cancer and identified a potent novel anti-proliferative compound Ericifolin (eugenol 5-O-beta-galloylglucopyranoside. Ericifolin, gallic acid and eugenol derivatives have demonstrated anti-tumor, apoptotic, cytotoxic, chempreventive and anti-proliferative properties by increasing the levels of autophagy markers LC3B and LC3B-positive puncta and downregulating Akt and mTOR phosphorylation in breast cancer (triple negative breast cancer, TNBC – MDA-MB231 cells) (103).
Cynodon dactylon
Cynodon dactylon, commonly known as Bermuda grass is valued in Indian traditional medicine for treating cancer, with studies identifying 22 compounds including hydroquinone, levoglucosenone and furfural as major constituents responsible for its medicinal properties. The methanolic extract from root exhibited anti-tumor, anti-inflammatory and chemo preventive effects by modulating the levels of liver detoxification enzymes and by decreasing the levels of serum glutamate pyruvate transaminase and serum glutamate oxaloacetate transaminase in Liver cancer (Hepatocellular carcinoma induced by diethyl nitrosamine) (104). The petroleum ether extract from leaf, stem and root exhibited cytotoxic, apoptotic, antitumor and antiproliferative effects confirmed via DNA fragmentation assay in Laryngeal cancer (Hep-2) Cervical cancer (HeLa) and Breast cancer (MCF-7) (105). Hydroxycinnamic acid, alkaloids, flavonoids, phenolic compounds, tannins, steroids and vitamin A extracted from the leaves exhibited cytotoxicity through apoptosis, antiproliferative, antitumor and antioxidant activity likely contributing to chemo preventive effects in chronic lymphocytic leukemia (K-562 cells line) (106).
Zizyphus nummularia
Commonly known as Indian Jujube, exhibits significant anti-cancer activity against multiple cell lines including human colon adenocarcinoma (HT-29), breast cancer (MCF-7), ovarian cancer (OVCAR-3), leukemia (K-562), human kidney carcinoma (A-498) and pancreatic Capan-2 cancer cells. Its ethanolic extract contains compounds like Lupeol, Rutin, Caryophyllene, Procyanidin B1, Nummularine R, 2-Isobutyl-3-methoxypyrazine, Nummularine A, Luteolin-7-O-glucoside. The ethyl Acetate Extract includes Guaifenesin, Nummularine R, Quercetin, Procyanidin B1, 2-Methoxy-4-vinylphenol, Coumaroylquinic acid, Chlorogenic acid. These constituents demonstrate anti-tumor, apoptotic, cytotoxic and chemopreventive effects by destabilizing the microtubule network in cancer cells (KAIMRC2) and upregulated phosphorylated mTOR and AKT proteins which indicates a possible involvement in signaling pathways related to cell growth and survival (107). Quercetin and kaempferol further enhance ROS generation, leading to apoptosis, while upregulating p38 MAPK, p21 and p27 and downregulating MMP-9 to inhibit metastasis (108). The ethanolic extract also suppresses ERK1/2 (MAPK) and NF-KB signaling pathways, downregulating alpha 2 integrin expression, decreasing VEGF production, reducing nitric oxide levels and upregulating E-cadherin which is a tumor suppressor protein (109).
Curcuma longa
Turmeric (Curcuma longa), extensively mentioned in Ayurvedic literature, demonstrates wide-ranging therapeutic effects due to bioactive constituents like curcumin, demethoxycurcumin, bisdemethoxycurcumin, tumerone, ar-tumerone, and quercetin (110). Curcumin modulates key oncogenic pathways such as RAS-ERK, PI3K/AKT/mTOR, COX-2, NF-κB, and JAK/STAT, showing efficacy in neurofibromatosis type 1, breast, cervical, lung, esophageal, uterine, prostate, pancreatic, hepatocellular, and colorectal cancers (111). Tumerone enhances curcumin bioavailability and ar-tumerone induces apoptosis in cervical cancer cell lines (110). Curcumin also potentiates the effects of bortezomib, paclitaxel, and FOLFOX chemotherapy, and suppresses tumor-associated inflammation and angiogenesis (112). Clinical studies report tolerability and efficacy of curcumin-based interventions in breast, prostate, and colorectal cancers, including oral doses up to 6000 mg/day and topical application for radiation-induced oral mucositis (113). Quercetin, an additional bioactive, shows promise for further exploration due to its high binding affinity in silico and anti-cancer potential (114). Collectively, turmeric exerts its anti-cancer properties by disrupting cell proliferation, inducing apoptosis, modulating immune responses, and targeting angiogenesis, EMT, and cancer stem cell pathways.
Aloe barbadensis
A. barbadensis Miller (Aloe vera), commonly studied species of Aloe (115), is known for many properties like cytotoxicity (115–118), anticancer (115, 117–120), antitumor (115, 118), genotoxic (118), antiproliferative and apoptotic (120). Aloctin, a lectin from Aloe vera (AV), has been reported to influence autophagy and apoptosis in various human cancer cell lines, including AGS (gastric adenocarcinoma), HCT116 (colon cancer), HEP3B (hepatoma), HL60 (acute promyelocytic leukemia), K562 (chronic myelogenous leukemia), and Saos-2 (osteosarcoma) (115). A synergistic effect of A. vera and A. arborescens displayed antiproliferative activity against HT-29 cells via MMP-2 and MMP-9, underlining the need to explore other species (119). Aloin downregulated cyclin B1 in MCF-7 cells and inhibited topo IIα expression in both MCF-7 and SKBR-3 cell lines (116). A preclinical study showed that A. vera gel exhibited stronger anticancer effects against melanoma cells than its purified form, suggesting greater potency of the natural extract and supporting its use as a protective and skin-healing agent (117). Lyophilized A. vera extract induced cytotoxicity in HepG2 cells by upregulating p53 and downregulating Bcl-2 (118). In A549 and HT-29 cells, combining royal jelly with A. vera enhanced efficacy and reduced AV toxicity (120).
Gymnema sylvestre
Gymnema Sylvestre (GS) or cowplant exhibits diverse properties including hepatoprotection (121), antioxidant (121–123), anticancer (124–130), immunomodulation (121, 125), apoptotic (122, 129, 130), autophagy (128), antiproliferation (126, 130), cytostatic (128), chemoprevention (123), cytotoxic (50, 122, 124, 126), and genotoxicity (124). These effects are mediated by multiple components like alkaloids, steroids, flavonoids (127), saponins (122, 127), triterpines (121, 127), gymnemagenol, dasyscyphin C (126) and gymnenic acid (125). An in vivo study demonstrated that gavage with GS triterpenoid saponin extract (GST) could upregulate IL-2 and IL-4 mRNA expression and reduce TNF-α expression indicating immunomodulation in breast cancer cell lines- MCF-7, MDA-MB-231 (121) along with tumor weight reduction at dose 200mg/kg body weight per day in MCF-7 and MDA-MB-468 cell lines (122). The ethanolic extract displayed apoptotic activity against A375 cells by activating mitochondria- dependent cell death pathway shown by increase in cytochrome c, caspase 3, PARP, Bax and reduced ICAD, EGFR and Bcl2 (130). Silver nanoparticles of GS demonstrated inhibition of cell growth in HT29 human colon adenocarcinoma cells which could have been by intracellular reactive oxygen species generation (127) or by cell cycle arrest (129). GS extract also displayed considerable cytotoxic effect and autophagy in Human continuous Glioblastoma cell line U87Mg (128) and chemoprevention in papillomas (123). It presented a significant synergy with neem and Moringa Oliefera on A549 lung cancer cells (50).An active principle of GS, gymnemagenol highly inhibited growth of HepG2 cells, while some compounds inhibited by inducing apoptosis (124, 126). GS leaf extract, via gymnemic acid, enhances macrophage activity and supports myeloid and lymphoid immune components, aiding in restoring innate immune function (125).
Gloriosa superba
Gloriosa superba, or kanivalu kilangu, contains phytochemicals like colchicine, gloriosine, and thiocolcoside that display various bioactivities, including anticancer, apoptotic, and anti-inflammatory effects. Colchicine exhibits strong anticancer properties in lung, breast, and liver cancers in vitro, and promotes IL-8 activity in pancreatic cancer (131). Gloriosine inhibits migration and induces apoptosis in cancer cells, such as A549 lung cancer cells (132). Synthetic derivatives selectively inhibit tumor cells and induce apoptosis, while suppressing NF-κB activity. Purified colchicine is minimally cytotoxic at low doses but shows resistance at higher concentrations (133). It affects MDA-MB231 cells at 40 nM, and other cancers at 80 nM (134). G. superba’s rhizome enhances anticolon activity through p53 upregulation and NF-κB downregulation (135). Phytogenic platinum and palladium nanoparticles from G. superba tuber extracts show significant anticancer effects, especially in MCF-7 breast cancer cells, causing apoptosis and promoting free radical formation (136). Thiocolchicoside from the seeds inhibits osteoclastogenesis by blocking NF-κB activation, offering potential in treating metastatic bone disease (137). The plant’s methanolic extracts have strong antioxidant properties and effectively inhibit Hep-G2 liver cancer cells. Colchicine disrupts the mitotic spindle apparatus, impacting cells with high metabolic rates (88). Thereby, Gloriosa superba shows anticancer potential by promoting apoptosis, reducing NF-κB activity, and inducing oxidative stress in cancer cells.
Piper nigrum
Piper nigrum or commonly known as pepper or “King of Spices” elicits beneficial effects on various conditions due to the presence of active principles like piperine, Piperettine, Trichostachine, Piperine, Piperolein A, Piperolein B (89). Pepper displayed apoptotic, anticancer, antiproliferative activity through JNK/p38 MAPK-mediated intrinsic apoptotic pathway in A2780 cells- human ovarian cancer cells (90). Cytotoxicity enhanced when combined with turmeric on lung cancer cell lines (91), while in metastatic 4T1 breast cancer and B6-F10 melanoma, PN enhanced antitumor responses by promoting CD45+ hematopoietic cell infiltration and modulating the Th1/Th2/Treg ratio (92). A further aspect worth noting is that PN extract revealed apoptotic pathways in colorectal cancer cell line, which produced antitumor activity by mitigating Matrix metalloproteinases(MMP). The mode of administration notably intragastric with doses- 25, 50, 100mg/kg/day paves a way for natural anti-colorectal cancer drug formulations (93). A preclinical study depicted anticancer activity by suppressing TYR and TRP-1 genes in melanoma cells (94). Piperine also helps enhance the effect of anticancer drugs by suppressing level of ABCB1, ABCC1 and ABCG2 genes which encode P-gp, MRP1 and BCRP respectively in line MCF-7 cell line and its doxorubicin resistant subline MCF-7/DOX and A-549 cell line and its resistant subline A-549/DDP which suggests that Piperine may reverse multi-drug resistance (138). Further, Piperine inhibited H. pylori growth and motility and adhesion to gastric adenocarcinoma by suppressing Flha and flge expression (139).
Coriandrum sativum
Coriander sativum also known as coriander is an aromatic, edible plant, commonly used as a spice and used chiefly in traditional medicines (140, 141). It has an umbrella of active principles including polyphenols like catechin, epicatechin, epicatechin gallate, vanillic acid, components like adenine, adenosine, tryptophan, coriandrin, alkaloids, flavonoids, tannins, saponins and alpha linalool among many others (141–144). Coriander seeds oil and nanoemulgel along with doxorubicin were reported to effectively inhibit cancer cell lines like MCF-7, Hep3B and HeLa, thus exhibiting its cytotoxicty (140). Antileukemic activity was explicitly seen as synergestic action of catechin and rutein components in a preclinical study with in vivo dosage of 2000mg/kg per oral (141). A intriguing study with various concentrations of 1%, 3%, and 5% w/w dietary coriander powder showed significant anticancer effects in HepG2 and B16F10 cells by reducing migration, invasion, and inhibiting MMP-2 and u-PA activities (145). CS induced apoptosis in SH-SY5Y neuroblastoma cells via the mitochondrial apoptotic pathway by increasing Bax and reducing Bcl-2 expression (142). In human colon cancer HT-29 cell line, the extract reduced the viability of cancer cells in concentration dependent manner. 267 Alpha-linalool from decoction of coriander root displayed reduction in β-catenin and TGF-β/SMAD pathway genes like P-GSK-3β, TGF-β, and P-SMAD2/3 and reduction in tumor growth (144).
Phyllanthus emblica
Phyllanthus emblica L. (Indian gooseberry or amla) is a nutrient‐dense functional fruit whose vitamin C, polyphenols (e.g. gallic/ellagic acids, flavonoids, tannins) and other phytoconstituents exert broad anticancer effects. Bioactive compounds such as Trigonelline, Naringin, Kaempferol, Catechin, Quercetin, Embinin, Isorhamnetin, Apigenindin and Colchicine downregulated c-Myc and cyclin D1, suppressed beta-catenin signaling and caused p53-independent apoptosis (increased Bax/Bcl-2) in colon cancer (colon cancer stem cells, HCT116) (146). Likewise, compounds like Gallic acid, syringic acid, ellagic acid, catechin, epicatechin, trans-cinnamic acid, rutin, condensed and hydrolysable tannins, polyphenols and flavonoids inhibited preneoplastic lesions, enhanced antioxidant enzymes (catalase, GPx), reduced oxidative stress, downregulated PCNA+ cell proliferation and modulated xenobiotic metabolism in liver and colon (dual carcinogenesis model) (147). Gallic acid, corilagin and ellagic acid induced apoptosis, inhibited tumor volume and weight, sustained drug release and enhanced bioavailability via SLNs in lung cancer (Lewis lung carcinoma model) (148). Extracts from the fruit increased ROS production, enhanced mitochondrial membrane potential, upregulated apoptotic genes (BAX, CASP3), modulated antioxidant (SOD2, GPX3) and inflammatory genes (IL6, IL-1 beta, TNF-alpha, TGF-beta) in chronic lymphoblastic leukemia (CLL) (149). Collectively, these findings indicate that P. emblica’s bioactives inhibit tumor progression and recurrence across multiple cancers by scavenging ROS, modulating redox and inflammatory pathways, and reprogramming oncogenic and epigenetic signals to induce apoptosis and block proliferation.
Cucurbita pepo
Curcubita pepo, commonly known as Pumpkin is native to northern Mexico and USA. Bioactive compounds such as flavonoids, triterpenoids, steroids, tannins, phytosterols, and saponins have been shown to modulate oxidative stress markers (↑SOD, CAT, GSH; ↓MDA, nitrite), reduce aberrant crypt foci (ACF) counts, and histological restoration in colon cancer thereby demonstrating anti-tumor, chemopreventive and antioxidant properties (150). Ribosome-inactivating proteins (cucurmosins) and cucurbitacins from pumpkins induce cell-cycle arrest (G0/G1 or G2/M) and caspase-dependent apoptosis in various tumor cells (e.g. HER2+ breast, NSCLC, colorectal), while inhibiting oncogenic pathways (Notch–Hes1, PI3K/Akt/mTOR, STAT3) and activating AMPK (151, 152). Likewise, major fatty acids from pumpkin; Oleic acid, Stigmasta-7,25-dien-3-ol and Linoleic acid mediated apoptosis via chromatin condensation, membrane blebbing, nuclear fragmentation and modulates antioxidants stress in papillary thyroid carcinoma (153).Pumpkin carotenoids such as Beta-carotene, alpha-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin and esterified carotenoids induced cell death at high concentration possibly mediated by ROS cytotoxicity in neuroblastoma (SH-SYS cells) (154). Emerging formulations also show promise: green-synthesized Cu–Mn nanoparticles (using pumpkin seed extract) induced DNA damage, cell migration inhibition and lysosomal integrity loss in colon adenocarcinoma (HT-29) (155). Together, these studies demonstrate that pumpkin-derived bioactives exert multi-targeted anticancer, chemo preventive and pro-apoptotic effects in diverse tumor models.
Prunus dulcis
Prunus dulcis, commonly known as almond, produces seeds rich in fixed oils, phenolic compounds, vitamins, minerals and unsaturated fatty acids like oleic and linoleic acids (156). Oleic acid, linoleic acid and palmitic acid have been shown to modulate BMP-2, beta-catenin, LGR-5, Jagged 1, Ki-67 expression; affects Wnt, Notch and BMP signaling pathways in colon cancer (156). Quercetin-3-o-rutinoside, kaempferol-3-o-rutinoside, isorhamnetin-3-o-galactoside, quercetin-3-o-galactoside and kaempferol-3-o-glucoside further caused selective inhibition of CYP17a1 lyase in Castration-resistant prostate cancer (CRPC) (157). Amygdalin downregulated PI3K-AKT-mTOR pathway and indirectly affected Ras thus displaying anti-tumor and anti-proliferative effects (158). Together, these findings highlight the multi-targeted anti-cancer potential of almonds through modulation of signaling pathways and enzyme activity.
Cyamopsis tetragonoloba
Cyamopsis Tetragonoloba is commonly known as cluster bean and contains flavonoids and isoflavonoids such as daidzein, genistein and quercetin, which have anti-cancer properties. Flavonoid-enriched fractions (FEFs) have cytotoxic and anti-proliferative effects through induction of apoptosis, reduced cell viability and apoptosis or necrosis in hepatocellular carcinoma (Huh7) cells (159).
Anisomeles malabarica
Anisomeles Malabarica, commonly known as pei viratti, contains active components such as anisomelic acid, quinones, flavonoids, phenols, terpenoids and beta-sitosterol. Anisomeles malabarica has anti-tumor, cytotoxic, apoptotic and anti-proliferative effects in various cancer models (160). Anisomelic acid isolated from the aerial parts of the herb was found effective in inducing DNA strand breaks and apoptosis in breast and cervical cancer cells (MCF-7, MDA-MB-231, SiHa, ME-180) (160). In a related study, it was found that n-hexane and chloroform extracts of the whole plant and Phytochemicals like ovatodiolide and citral induced apoptosis through mitochondrial membrane depolarization, DNA fragmentation, and cell cycle arrest at S and G2/M phases in HPV16-positive cervical cancer cells (161). The aqueous lead extract of the herb demonstrated cytotoxic effects on HepG2 liver cancer cells, observing a concentration-dependent inhibition and apoptosis induction (162).
Tridax procumbens
Tridax procumbens, commonly known as Vettukaya Poondu, contains Phytochemicals which includes flavonoids, terpenoids, essential oils, saponins, and other secondary metabolites many of which are associated potential anticancer benefits, including cytotoxic, anti-tumor, anti-proliferative, and apoptotic effects (163).The computational in-silico study conducted by Shradha et al. investigated the active component of Tridax procumbens, luteolin, as a potential anticancer agent, demonstrating its binding capacity with MCM7 protein and predicting its anti-tumor, cytotoxic and anti-proliferative activities (164). Another study focused on green synthesis of silver nanoparticles using Tridax procumbens plant extract, showing cytotoxic and anti-proliferative action of polyphenols and peptides and their effects on A549 lung cancer cells (165). Tridax procumbens and Curcuma longa powders in combination showed synergistic cytotoxic and anti-tumor effects on A549 lung cancer cells (166). An in vitro study found that methanolic crude extracts and fractions exhibited cytotoxic and antioxidant effects on breast, lung, colon and leukemia cell lines (167).
Cuminum cyminum
Cuminum cyminum, commonly known as Cumin and Seeragam, contains active components such as cuminaldehyde, cymene, and other terpenes showing anti-tumor, anti-proliferative, chemopreventive and apoptotic effects (168). An in vitro study using SAS cell lines showed anti-proliferative and apoptotic effects of nano-emulsion of the essential oil of Cuminum cyminum (169). Another study found that the hexane extract of cumin seeds showed cytotoxic, apoptotic and anti-proliferative effects on MG63 bone cancer cells (170). An in-silico study with a molecular modeling approach showed apigetrin, cyanaroside, and cuminum compounds might inhibit CDK8 and PR receptors, indicating potential anti-tumor, anti-proliferative and enzyme inhibitory effects (171). Further, a study demonstrated chemopreventive and anti-proliferative actions through dietary administration of ethanolic extract of cumin powder by reversing miRNA-mediated oncogenic pathways and modulating CYP1A1 (172). These findings suggest the promising anticancer potential through modulation of oncogenic pathways.
Trigonella foenum graecum
Trigonella foenum graecum, commonly known as Fenugreek, is known for active components like diosgenin, saponins, polyphenols and various flavonoids, exhibiting effects like anti-tumor, cytotoxic, apoptotic, anti-proliferative and antioxidant properties (173). An in vitro study showed diosgenins’ anti-tumor and apoptotic effects in PC-3 prostate cancer via downregulation of NEDD4 and modulation of pAkt, P73 and LATS1. 316 Another study demonstrated anti-angiogenic and cytotoxic actions and inhibition of endothelial cell viability, tube formation, and neovascularization, potential suppression of VEGF and NF-κB signaling pathways by using ethanolic extract of fenugreek seeds (174). Fenugreek showed potential anti-tumor effects in breast cancer by high binding affinity of galactomannan to breast cancer protein (PDB ID: 3EQM), forming multiple hydrogen bonds and a stable molecular dynamics profile (175). Protein hydrolysates from fenugreek seeds showed anti-proliferative and apoptotic effects on Caco2/TC7 colon cancer cells via the G1 phase arrest and Caspase-3 activation (176). Mahmoud et al. reported that fenugreek induced apoptosis in hepatocellular carcinoma cell line HepG2 mediated by upregulation of p53 and PCNA, with anti-tumor effects (177). The active component of fenugreek, diosgenin, downregulates NEDD4 and induces apoptosis in PC-3 prostate cancer cells through pAkt suppression and p73 activation (178). Al Asmari et al. reported cytotoxicity in HepG2 and MCF-7, via apoptosis induction and caspase activation by aqueous extract of fenugreek (179). Active components like saponins in fenugreek induced apoptosis in colorectal cancer cells via ROS generation and caspase activation (180). In sum, these primary studies indicate that fenugreek-derived compounds can inhibit cancer cell viability, angiogenesis and proliferation while promoting apoptotic pathways (often via caspases and tumor-suppressor signaling) in diverse cancer types.
Solanum nigrum
Solanum nigrum (black nightshade) is a medicinal herb rich in diverse bioactive compounds – notably steroidal saponins and alkaloids (e.g. solanine, solasonine, solamargine) as well as flavonoids, polyphenols, glycoproteins and polysaccharides (181). Unripe berries in particular contain high levels of these glycosides, which underlie the plant’s pharmacological potency. Extracts of S. nigrum (aqueous, ethanolic, etc.) from whole plant, leaves or fruits have demonstrated broad anticancer activity: they inhibit proliferation of many cancer cell lines (e.g. liver HepG2, cervical HeLa, breast MCF-7, ovarian, prostate, etc.) and induce apoptosis via mitochondrial and death-receptor pathways. Mechanistically, S. nigrum treatments upregulate pro-apoptotic factors (Bax, cleaved caspases, p21) and downregulate anti-apoptotic/cell-cycle proteins (Bcl-2, cyclin B1, CDK1), often through increases in ROS and caspase-9/3 activation (181, 182). The extracts also disrupt tumor-promoting signaling (e.g. AKT, STAT3, NF-κB, MAPK, VEGF/VEGFR) and metastasis-related factors (e.g. MMPs, E-cadherin) and can synergize with chemotherapies (cisplatin, doxorubicin, docetaxel) to enhance cancer cell killing (181, 182). In summary, S. nigrum’s rich mix of glycoalkaloids, saponins and polyphenols confers multi-targeted anticancer, anti-inflammatory and immunoregulatory properties, making it a promising source of therapeutic agents (181, 182).
Cucumis sativus
Cucumis sativus, or cucumber, shows promising therapeutic potential against prostate carcinoma and benign prostatic hyperplasia (BPH) (183).In studies with Wistar rats, raw cucumber seeds and oil treatment over 28 days led to significant improvements in prostate health, including reduced prostate weight and volume, decreased total protein levels, and lower PSA which inhibit prostate hyperplasia through the inhibition of 5α-reductase (184). Additionally, C. sativus exhibits analgesic, antioxidant, antibacterial, anti-inflammatory, anti-androgenic, weak estrogenic activities, antiproliferative, anticancer properties and cytotoxic, resulting in growth arrest and apoptosis in various cancer types (183–185). Further analysis demonstrated that CuS-CuC significantly affects cell cycle regulators and apoptosis mediators in prostate, bladder, and HepG2 cells, reducing colony formation (184). In LNCaP cells, there was an accumulation of cleaved caspase-3, and in HepG2 cells, increased cleaved caspase-9 levels indicated apoptosis, while Bcl-2 levels remained steady (184). CuC was found to inhibit Akt signaling by blocking phosphorylation at Ser473, inducing apoptosis without necrosis in breast cancer cells (184). Cucumis sativus methanol extract (CSME) treatment in breast cancer cells led to significant morphological changes, including cell shrinkage, blebbing, and reduced cell population compared to untreated cells (185). The study also investigated the impact of C. sativus seed oil in combination with estrogen and letrozole prostatic cancer cell lines.
Piper betel leaves
Piper betel, or betel, contains various bioactive compounds, including chavicol, cineol, eugenol, alkaloids, flavonoids, steroids, saponins, chavibetol, chavibetol acetate, caryophyllene, allyl pyrocatechol diacetate, camphene, chavibetol methyl ether, eugenol, a-pinene, f-pinene, u-Limonene, saprobe, 1–8- cineol, and allyl pyrocatechol monoacetate and tannins, along with sugars and essential oils (186–189). In vitro, hydroxychavicol‐enriched extracts induce cell-cycle arrest and apoptosis in diverse cancer cells: for example, treatment of human prostate cancer cells caused G1‐phase accumulation, loss of mitochondrial membrane potential, ROS overproduction and activation of caspase-3/PARP (190).These insults activate stress pathways (JNK/MAPK) and DNA damage responses leading to caspase-dependent apoptosis, while concomitantly suppressing epithelial–mesenchymal transition (EMT) and migration (190, 191). By rebalancing redox homeostasis, betel phenolics both prevent oxidative DNA damage and trigger ROS‐driven cancer cell death; notably, increased MnSOD activity induced by these compounds can suppress NF-κB/AP-1 signaling in tumor cells (192). It has a cytotoxicity and genotoxicity range from 34.91 mg/ml to 101.79 mg/ml (188). The essential oils of P. betel exhibit higher toxicity compared to crude extracts on leukocytes (188). P. betel exhibits anticancer, anti-allergic, antimicrobial, anti-platelet, and immunomodulatory actions, with potential applications against breast cancer, colon cancer, and human cervical cell lines (186–188).
Withania somnifera
Withania somnifera (WS), popularly called as Ashwagandha or Indian ginseng possesses bioactive components like Withaferin A (193, 194), withanone (193), L-asparginase (195), withanolide D, withanolide O, Kaempferol (196). Withaferin A and Withanone from ashwagandha leaves displayed anti-inflammatory, anticancer, apoptotic and cytotoxic effects by binding to and preventing the homodimerization of Survivin in several cancer cell lines, including Human normal lung fibroblasts (MRC-5, TIG-3, and WI-38) and a variety of cancer cells, including colon cancer (HCT116), breast cancer (MDA-MB-231, MCF-7 and T-47D), fibrosarcoma (HT1080), non-small lung cancer (A549), cervical cancer (HeLa, ME-180, SKG-II, and CaSki), osteosarcoma (U2OS and Saos-2), and melanoma (G361) (193). Decrease in C6 glioma cells in dose dependent manner was reported in an in vitro study when cells were treated with doses ranging 50, 100, 200 and 500 μg/mL with downregulation of Bcl-2, upregulation of Bax, and increased expression of apoptotic markers Caspase-3 and -9 (197). A preclinical study of oral carcinoma cell lines, Ca9-22, HSC-2, HSC-3, HSC-4, demonstrated that Kaempferol and withanolide D kaempferol and withanolide D effectively inhibited oncogenic proteins, CDK2, and BRD3, inducing cytotoxicity via autophagy and caspase activation (196). L-asparaginase derived from the leaves, unripe, and ripe fruits of WS selectively deaminates asparagine, promoting cytotoxicity in acute and chronic lymphoblastic leukemia (195).
Discussion
This narrative review provides an integrative analysis of 32 herbs with claimed anticancer properties, rooted in traditional use and ethnobotanical knowledge from Tamil Nadu, India. By synthesizing data from peer-reviewed literatures, the review highlights both the depth and limitations of existing evidence supporting these herbs. Figure 2 provides an overview of the key mechanisms underlying the anticancer potential of the herbs discussed in this study.
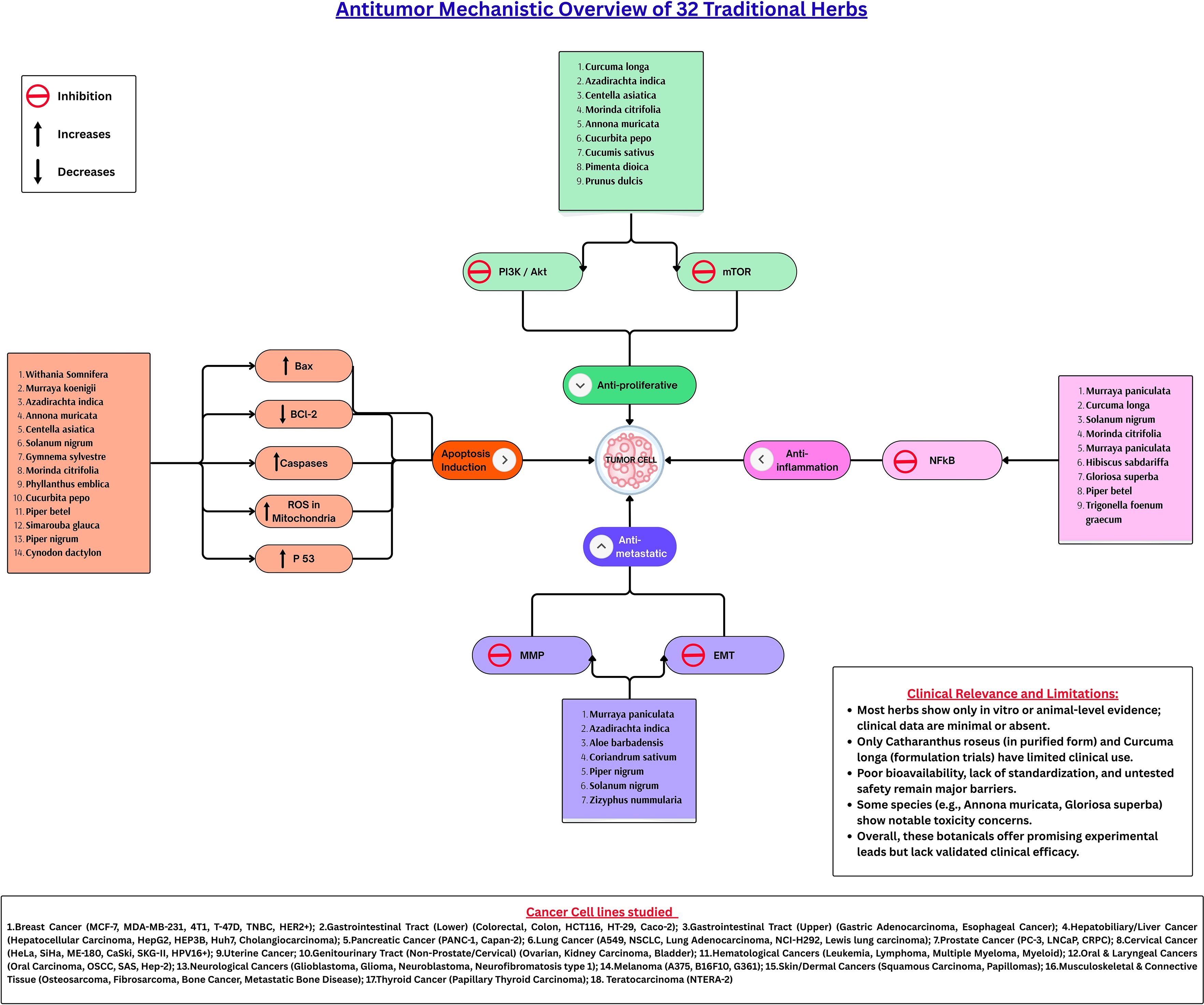
Figure 2. Antitumor mechanistic overview of 32 traditional herbs. The 32 traditional medicinal herbs exert their antitumor effects through multiple converging pathways. These herbs modulate four principal mechanisms: induction of apoptosis, suppression of proliferation, inhibition of inflammation, and blockade of metastasis. Apoptosis is facilitated by upregulating Bax, p53, and caspases while downregulating Bcl-2 and enhancing mitochondrial ROS activity. The anti-proliferative effect is primarily achieved through inhibition of the PI3K/Akt/mTOR axis, whereas NF-κB suppression mediates the anti-inflammatory response. Concurrently, inhibition of MMPs and reversal of EMT signaling prevent cancer cell migration and invasion. Collectively, these pathways underscore the multi-targeted and synergistic potential of phytochemicals in regulating tumor progression. Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; p53, Tumor Protein p53; ROS, Reactive Oxygen Species; PI3K, Phosphoinositide 3-kinase; Akt, Protein Kinase B; mTOR, Mechanistic Target of Rapamycin; NF-κB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; MMPs, Matrix Metalloproteinases; EMT, Epithelial-Mesenchymal Transition.
Herbs with reported anticancer effects show differences in the depth and quality of evidence. For example, Withania somnifera, Curcuma longa, and Annona muricata, have been extensively studied in vitro and in animal models with identified mechanisms (apoptosis induction, angiogenesis inhibition, anti-metastatic effects, and immune modulation (4, 198, 199). By contrast, many other traditionally used herbs have virtually no preclinical animal or clinical data. This disparity highlights critical gaps, some high-use plants lack validation. This discrepancy underscores a critical research gap several high-use herbs in folk medicine continue to lack systematic validation of their anticancer efficacy or safety.
Several herbs identified in this review appear to be promising candidates for future clinical research and potential therapeutic application. Withania somnifera contains withanolides that show broad anti-cancer activity and immunomodulatory effects. Its safety record is favorable in small trials, and it alleviated chemotherapy-induced fatigue (200). Curcuma longa’s curcuminoids modulate multiple cancer pathways (NF-κB, STAT3, PI3K/Akt etc.) and enhance immune responses. Curcumin is well-tolerated in humans (numerous Phase I/II trials) though its low bioavailability is a hurdle (201). Other promising candidates include Morinda citrifolia with Phase I data showing no dose-limiting toxicity (202).
By contrast, Annona muricata despite potent anticancer acetogenins must be approached cautiously its acetogenin annonacin is a potent mitochondrial toxin linked to Parkinsonian neurodegeneration (203).Likewise, the vinca alkaloids from Catharanthus roseus are clinically active but highly toxic (peripheral neuropathy and marrow suppression) (204), underscoring that efficacy must be balanced with safety. Further, systematic validation through controlled in vivo and clinical studies will be essential to distinguish promising candidates from those posing unacceptable risks, ultimately guiding their responsible integration into evidence-based cancer care.
Studies suggest that some herbal compounds can potentiate conventional cancer therapies. Curcumin, for instance, enhances chemotherapy efficacy its combination with cisplatin in papillary thyroid cancer cells produced stronger STAT3 inhibition and apoptosis than either treatment alone (201). Likewise, Withania somnifera’s active constituent withaferin A increases radio and chemosensitivity, showing synergistic effects with sorafenib through apoptosis induction and suppression of oncogenic signaling (200). Despite these promising findings, clinical translation remains limited, as large randomized trials integrating such herbs with standard cancer therapies are still lacking.
Safety
A critical gap in the current evidence is safety. Many studies overlook toxicity or use non-standardized extracts. For instance, Annona muricata preparations vary widely in acetogenin content, and chronic exposure is neurotoxic (203). Catharanthus roseus derivatives vincristine/vinblastine are clinically potent but have neuropathy and myelosuppression (204).Curcumin is generally safe at dietary levels, but recent reports of liver toxicity from adulterated supplements highlight regulatory risks (201).For most herbs, human safety data are non-existent. Therefore, regulatory authorities should mandate rigorous phytochemical characterization and adherence to good manufacturing practices, even for traditional remedies. This review emphasizes that traditional use does not equate to proven safety, and scientific validation is essential before clinical application.
From a policy perspective, preserving traditional plant knowledge and supporting collaborative research is essential. Regulatory frameworks should ensure intellectual property protection, quality control, and ethical study designs. Multidisciplinary teams including oncologists, pharmacognosists, ethnobotanists, and AYUSH practitioners can accelerate translation of traditional remedies into evidence-based integrative oncology. The findings from this review aligns with the earlier evidence based reviews that call for integration of herbal/plant-based remedies with conventional cancer care (205).
Limitations
This narrative review has several methodological constraints. First, it did not employ systematic criteria to assess the quality, risk of bias, or methodological rigor of the included studies, which limits the strength of inferences. Second, as a narrative synthesis, it lacks quantitative synthesis or meta-analysis, making it susceptible to subjective interpretation. Third, many included studies rely on preclinical models, small pilot trials, or non-standardized herbal preparations, which reduces generalizability and reproducibility. Finally, publication bias and selective reporting cannot be excluded, further constraining the certainty of the results discussed. Another key limitation of this review is the potential selection bias arising from the inclusion of herbs based on anecdotal evidence, popular traditional use, and reports from herbal activists, rather than through a systematic ethnobotanical survey; however, this approach was intended to capture herbs of real-world relevance that are actively used in traditional cancer care settings or popularly believed to be anti-cancerous. Future studies should adopt a systematic ethnobotanical or participatory rural appraisal framework to minimize potential bias and enhance reproducibility.
Conclusion
This review identifies several promising herbal candidates with anticancer and immunomodulatory potential particularly Withania somnifera, Curcuma longa, Azadirachta indica, and Morinda citrifolia. These herbs merit priority in future clinical trials due to their multi-target mechanisms and preliminary safety data. However, some plants with known or uncertain toxicity, (e.g.: Annona muricata), require caution. Future research should focus on standardized extracts, defined dosing, and rigorous safety monitoring, while testing potential synergies with chemotherapy and immunotherapy. Policy support is essential to enable well-designed clinical trials that respect traditional knowledge and ensure quality control. In summary, traditional herbs offer significant promise for integrative oncology, but their translation into clinical use demands robust pharmacological validation, ethical testing, and interdisciplinary collaboration bridging ethnomedicine with modern cancer science.
Author contributions
PN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. VM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. ST: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. AS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. MM: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. KS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. OpenAI’s ChatGPT was used solely to improve the grammar and language of this manuscript. All scientific content and interpretations are the authors’ own.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1680062/full#supplementary-material
References
1. Pandey L, Pasricha R, Joseph D, Ahuja R, Yanthan Y, Garg PK, et al. Use of complementary and alternative medicine among patients with cancer in a sub-Himalayan state in India: An exploratory study. J Ayurveda Integr Med. (2021) 12:126–30. doi: 10.1016/J.JAIM.2021.01.001
2. Sidhu JS and Zafar TA. Indian herbal medicine and their functional components in cancer therapy and prevention. In: Functional foods in Cancer Prevention and Therapy. London, United Kingdom: Academic Press (2020). p. 169–94. doi: 10.1016/B978-0-12-816151-7.00010-7
3. Tavakoli J, Miar S, Zadehzare MM, and Akbari H. Evaluation of Effectiveness of herbal medication in cancer care: A review study. Iran J Cancer Prev. (2012) 5:144.
4. Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, et al. A review on annona muricata and its anticancer activity. Cancers (Basel). (2022) 14:4539. doi: 10.3390/CANCERS14184539
5. Pathirana OC, Paranagama MP, Wijesundera KK, Mahakapuge TAN, Abeykoon AMAU, and Rajapakse J. Elucidating the potential of Annona muricata L. grown in Sri Lanka to be used in developing an anticancer drug against colorectal and breast cancers. BMC Complement Med Ther. (2024) 24:410. doi: 10.1186/S12906-024-04712-X
6. Yang C, Gundala SR, Mukkavilli R, Vangala S, Reid MD, and Aneja R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis. (2015) 36:656. doi: 10.1093/CARCIN/BGV046
7. Syed Najmuddin SUF, Romli MF, Hamid M, Alitheen NB, and Abd Rahman NMAN. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement Altern Med. (2016) 16:311. doi: 10.1186/S12906-016-1290-Y
8. Indrawati L, Ascobat P, Bela B, Abdullah M, and Surono IS. The effect of an Annona muricata leaf extract on nutritional status and cytotoxicity in colorectal cancer: a randomized controlled trial. Asia Pac J Clin Nutr. (2017) 26:606–12. doi: 10.6133/APJCN.062016.02
9. Rojas-Armas JP, Arroyo-Acevedo JL, Palomino-Pacheco M, Ortiz-Sánchez JM, Calva J, Justil-Guerrero HJ, et al. Phytochemical constituents and ameliorative effect of the essential oil from annona muricata L. Leaves in a murine model of breast cancer. Molecules. (2022) 27:1818. doi: 10.3390/MOLECULES27061818
10. Serretta V, Berardinis E, Simonato A, Guarneri A, Dispensa N, Pavone C, et al. A prospective observational study on oral administration of Ellagic Acid and Annona Muricata in patients affected by non-muscle invasive bladder cancer not undergoing maintenance after 6-week intravesical prophylaxis. Urol J. (2022) 89:49–52. doi: 10.1177/03915603211022285
11. Endang A, Hasan Z, Bermawie N, Rianti EI, and Arifni FR. Phytochemical screening and anti-breastcancer activities of annona muricata (L.) leaf extracts. (2020) 2:23–30. doi: 10.1016/S1995-7645(14)60258-3
12. El Tawiil GA, Noaman EA, Askar MA, El Fatih NM, and Mohamed HE. Anticancer and apoptogenic effect of graviola and low-dose radiation in tumor xenograft in mice. Integr Cancer Ther. (2020) 19:1534735419900930. doi: 10.1177/1534735419900930/ASSET/769D7429-E3EE-4EF8-89C9-F0D09674560A/ASSETS/IMAGES/LARGE/10.1177_1534735419900930-FIG4.JPG
13. Foster K, Oyenihi O, Rademan S, Erhabor J, Matsabisa M, Barker J, et al. Selective cytotoxic and anti-metastatic activity in DU-145 prostate cancer cells induced by Annona muricata L. bark extract and phytochemical, annonacin. BMC Complement Med Ther. (2020) 20:1–15. doi: 10.1186/S12906-020-03130-Z/FIGURES/11
14. Chan WJJ, Beale P, McLachlan AJ, Hanrahan JR, and Harnett JE. The safety and tolerability of Annona muricata leaf product in people living with cancer: Study protocol. Adv Integr Med. (2024) 11:143–8. doi: 10.1016/J.AIMED.2024.06.004
15. Swapna B, Rao R, Kotha S, Sagar S, Setty R, and Setty R. In vitro cytotoxic activity using fruits of Annona muricata and aerial parts of Euphorbia tirucalli against lung and oral cancer cell lines. Pharmacogn Magazine. (2022) 18:66. doi: 10.4103/pm.pm_357_21
16. Drishya G, Nambiar J, Shaji SK, Vanuopadath M, Achuthan A, Kumar A, et al. RECK and TIMP-2 mediate inhibition of MMP-2 and MMP-9 by Annona muricata. J Biosci. (2020) 45:1–11. doi: 10.1007/S12038-020-00056-Z/METRICS
17. Zorofchian Moghadamtousi S, Karimian H, Rouhollahi E, Paydar M, Fadaeinasab M, and Abdul Kadir H. Annona muricata leaves induce G1 cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. J Ethnopharmacol. (2014) 156:277–89. doi: 10.1016/J.JEP.2014.08.011
18. Prasad SK, Veeresh PM, Ramesh PS, Natraj SM, Madhunapantula SRV, and Devegowda D. Phytochemical fractions from Annona muricata seeds and fruit pulp inhibited the growth of breast cancer cells through cell cycle arrest at G0/G1phase. J Cancer Res Ther. (2020) 16:1235–49. doi: 10.4103/JCRT.JCRT_494_19
19. Desmarini D, Abdullah M, Sari P, Yunaini L, Fadilah F, and Murdani Abdullah C. Effects of ethanolic leave extract of soursop (Annona muricata L.) on human colorectal cancer line: cell viability and in silico study to cyclin d1 protein. J Pharmacogn Phytochem. (2019) 8(2):232–7.
20. Naik AV and Sellappan K. In vitro evaluation of Annona muricata L. (Soursop) leaf methanol extracts on inhibition of tumorigenicity and metastasis of breast cancer cells. Biomarkers. (2020) 25:701–10. doi: 10.1080/1354750X.2020.1836025
21. Hadisaputri YE, Habibah U, Abdullah FF, Halimah E, Mutakin M, Megantara S, et al. Antiproliferation activity and apoptotic mechanism of soursop (Annona muricata L.) leaves extract and fractions on MCF7 breast cancer cells. Breast Cancer: Targets Ther. (2021) 13:447–57. doi: 10.2147/BCTT.S317682
22. Ismail N, Rajaratinam H, Ghazali KC, Ibrahim W, and Yahya MM. The Phytochemical Components of Kelantan Grown Annona muricata Leaves and Its Anti-Proliferative Properties on MCF-7 Breast Cancer Cells (Komponen Fitokimia Daun Annona muricata Ditanam di Kelantan dan Sifat Anti-Proliferatifnya terhadap Sel Kanser Payudara MCF-7). Sains Malays. (2023) 52:2785–801. doi: 10.17576/jsm-2023-5210-05
23. Zein N, Yassin F, and Hassan A. The potential effect of Annona muricata and Cisplatin as antioxidant and antitumor in rat with liver cancer by induction of apoptosis through P13K \ AKT signaling pathway. Biochem Lett. (2023) 19:37–51. doi: 10.21608/BLJ.2023.314428
24. Silihe KK, Mbou WD, Ngo Pambe JC, Kenmongne LV, Maptouom LF, Sipping MTK, et al. Comparative anticancer effects of Annona muricata Linn (Annonaceae) leaves and fruits on DMBA-induced breast cancer in female rats. BMC Complement Med Ther. (2023) 23:1–16. doi: 10.1186/S12906-023-04073-X/TABLES/6
25. Hashem SA, Abu-Qatouseh L, Mallah E, Mansoor K, Darwish F, Hajji EL, et al. The Effect of Graviola Leaves Extract (Annona muricata L.) on Pharmacokinetic of Metformin in Rats’ Plasma and Pharmacological Activity of their Combination on Breast and Prostate Cancer Cell Lines. Biomed Pharmacol J. (2023) 16:319–27. doi: 10.13005/BPJ/2613
26. Vikas B, Kunjiraman S, Rajam SSN, and Anil S. The Apoptotic Properties of Leaf Extracts of Simarouba glauca against Human Leukemic Cancer Cells. Asian Pac J Cancer Prev. (2021) 22:1305–12. doi: 10.31557/APJCP.2021.22.4.1305
27. Vikas B, Sujathan K, Rajam SSN, and Anil S. Caspase-dependent apoptosis induced by simarouba glauca on human non-small-cell lung cancer, A549 cells. Asian Pac J Cancer Prev. (2022) 23:1867–72. doi: 10.31557/APJCP.2022.23.6.1867
28. Ramasamy SP, Rajendran A, Pallikondaperumal M, Sundararajan P, Husain FM, Khan A, et al. Broad-spectrum antimicrobial, antioxidant, and anticancer studies of leaf extract of simarouba glauca DC in vitro. Antibiotics. (2022) 11:59. doi: 10.3390/ANTIBIOTICS11010059
29. Pandhari RMR and Taranath TC. In-vitro antioxidant activity and flow cytometric analysis of simarouba glauca DC bark extract induced apoptosis in triple negative breast cancer cells. Asian Pac J Cancer Prev. (2024) 25:201–10. doi: 10.31557/APJCP.2024.25.1.20
30. Jose A, Kannan E, and Madhunapantula SRV. Anti-proliferative potential of phytochemical fractions isolated from Simarouba glauca DC leaf. Heliyon. (2020) 6:e03836. doi: 10.1016/J.HELIYON.2020.E03836/ASSET/CC741B27-824D-4292-803B-12733B4DCC81/MAIN.ASSETS/GR9.JPG
31. Yeo D, Huynh N, Beutler JA, Baldwin GS, He H, and Nikfarjam M. Glaucarubinone combined with gemcitabine improves pancreatic cancer survival in an immunocompetent orthotopic murine model. J Invest Surg. (2016) 29:366–72. doi: 10.3109/08941939.2016.1160167
32. Puranik SI, Ghagane SC, Nerli RB, Jalalpure SS, and Hiremath MB. Evaluation of in vitro Antioxidant and Anticancer Activity of Simarouba glauca Leaf Extracts on T-24 Bladder Cancer Cell Line. Pharmacogn J. (2017) 9:906–12. doi: 10.5530/pj.2017.6.142
33. Shalal OS and Irayyif SM. Evaluation of cytotoxicity and Apoptotic effects of Simarouba glauca on the Prostate Cancer Cell Lines PC3. J Pak Med Assoc. (2023) 73:S113–8. doi: 10.47391/JPMA.IQ-24
34. Jose A, Elango K, Madhunapantula SRV, and Raghavamenon AC. Tricaproin isolated from Simarouba glauca inhibit colorectal cancer cell growth: A mechanistic approach in vitro and in vivo. Mater Today Proc. (2020) 33:2193–202. doi: 10.1016/J.MATPR.2020.04.015
35. Banyal A, Tiwari S, Sharma A, Chanana I, Patel SKS, Kulshrestha S, et al. Vinca alkaloids as a potential cancer therapeutics: recent update and future challenges. 3 Biotech. (2023) 13:211. doi: 10.1007/S13205-023-03636-6
36. Goswami S, Ali A, Prasad ME, and Singh P. Pharmacological significance of Catharanthus roseus in cancer management: A review. Pharmacol Res - Modern Chin Med. (2024) 11:100444. doi: 10.1016/J.PRMCM.2024.100444
37. Rajashekara S, Reena D, Mainavi MV, Sandhya LS, and Baro U. Biological isolation and characterization of Catharanthus roseus (L.) G. Don methanolic leaves extracts and their assessment for antimicrobial, cytotoxic, and apoptotic activities. BMC Complement Med Ther. (2022) 22:1–18. doi: 10.1186/S12906-022-03810-Y/TABLES/6
38. Kuriakose GC, Palem PPC, and Jayabaskaran C. Fungal vincristine from Eutypella spp - CrP14 isolated from Catharanthus roseus induces apoptosis in human squamous carcinoma cell line -A431. BMC Complement Altern Med. (2016) 16:1–8. doi: 10.1186/S12906-016-1299-2/FIGURES/4
39. Palem PPC, Kuriakose GC, and Jayabaskaran C. An Endophytic Fungus, Talaromyces radicus, Isolated from Catharanthus roseus, Produces Vincristine and Vinblastine, Which Induce Apoptotic Cell Death. PloS One. (2015) 10:e0144476. doi: 10.1371/JOURNAL.PONE.0144476
40. Nayila I, Sharif S, Lodhi MS, Rehman MFU, and Aman F. Synthesis, characterization and anti-breast cancer potential of an incensole acetate nanoemulsion from Catharanthus roseus essential oil; in silico, in vitro, and in vivo study. RSC Adv. (2023) 13:32335–62. doi: 10.1039/D3RA06335F
41. Shalal OS and Sevastre AS. Evaluation of cytotoxicity and apoptotic effects of Catharanthus roseus on the human lung cancer cell lines CaLu-6. Rev Rom Med Lab. (2024) 32:177–84. doi: 10.2478/RRLM-2024-0017
42. Sakti SP, Sari FN, Rachmawati F, Widyarti S, Rahayu S, Soewando A, et al. The effect of Phyllanthus niruri and Catharanthus roseus on Macrophage Polarization in Breast Cancer Mice Model: The Effect of P. niruri and C. roseus in Breast Cancer Mice Model. J Trop Life Sci. (2024) 14:21–6. doi: 10.11594/JTLS.14.01.03
43. Islas JF, Acosta E, G-Buentello Z, Moreno-Treviño MG, Escalante BA, Moreno-Cuevas JE, et al. An overview of Neem (Azadirachta indica) and its potential impact on health. J Funct Foods. (2020) 74:104171. doi: 10.1016/J.JFF.2020.104171
44. Kumar S, Mulchandani V, and Das Sarma J. Methanolic neem (Azadirachta indica) stem bark extract induces cell cycle arrest, apoptosis and inhibits the migration of cervical cancer cells in vitro. BMC Complement Med Ther. (2022) 22:239. doi: 10.1186/S12906-022-03718-7
45. Guchhait KC, Manna T, Barai M, Karmakar M, Nandi SK, Jana D, et al. Antibiofilm and anticancer activities of unripe and ripe Azadirachta indica (neem) seed extracts. BMC Complement Med Ther. (2022) 22:42. doi: 10.1186/S12906-022-03513-4
46. Subramani R, Gonzalez E, Arumugam A, Nandy S, Gonzalez V, Medel J, et al. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci Rep. (2016) 6:19819. doi: 10.1038/SREP19819
47. Arumugam A, Subramani R, Nandy S, Powell S, Velazquez M, Orozco A, et al. Desacetyl nimbinene inhibits breast cancer growth and metastasis through reactive oxygen species mediated mechanisms. Tumor Biol. (2016) 37:6527–37. doi: 10.1007/S13277-015-4468-X
48. Subramani R, Gonzalez E, Nandy SB, Arumugam A, Camacho F, Medel J, et al. Gedunin inhibits pancreatic cancer by altering sonic hedgehog signaling pathway. Oncotarget. (2017) 8:10891–904. doi: 10.18632/ONCOTARGET.8055
49. Tharmarajah L, Samarakoon SR, Ediriweera MK, Piyathilaka P, Tennekoon KH, Senathilake KS, et al. In vitro anticancer effect of gedunin on human teratocarcinomal (NTERA-2) cancer stem-like cells. BioMed Res Int. (2017) 2017:2413197. doi: 10.1155/2017/2413197
50. Muthu T, Adusumalli R, Vemuri SK, Indira Devi M, Pavan Kumar P, Banala RR, et al. Eco-biofabrication of silver nanoparticles from Azadirachta indica, Gymnema sylvestre, and Moringa oleifera for lung cancer treatment. J Egypt Natl Canc Inst. (2025) 37:1–13. doi: 10.1186/S43046-024-00252-0/FIGURES/6
51. Khalid H, Shityakov S, Förster CY, and Song Y. Exploring the anticancer properties of Azadirachta indica: In silico and in vitro study of its phytochemicals against hepatocellular carcinoma. J Mol Struct. (2024) 1317:138962. doi: 10.1016/j.molstruc.2024.138962
52. Azhagu M. Phytochemical analysis and anticancer activity of azadirachta indica ethanolic extract against A549 human lung cancer cell line. J Biomed Res Environ Sci. (2021) 2:280–5. doi: 10.37871/JBRES1225
53. Wang L, Zhao L, Gong lian F, Sun C, Du dan D, Yang xia X, et al. Modified citrus pectin inhibits breast cancer development in mice by targeting tumor-associated macrophage survival and polarization in hypoxic microenvironment. Acta Pharmacol Sin. (2022) 43:1556–67. doi: 10.1038/S41401-021-00748-8
54. Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. (2015) 6:19514–27. doi: 10.18632/ONCOTARGET.4004
55. El-Kersh DM, Ezzat SM, Salama MM, Mahrous EA, Attia YM, Ahmed MS, et al. Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer. Sci Rep. (2021) 11:7121. doi: 10.1038/S41598-021-86599-Z
56. You L, Lin J, Yu Z, Qian Y, Bi Y, Wang F, et al. Nobiletin suppresses cholangiocarcinoma proliferation via inhibiting GSK3β. Int J Biol Sci. (2022) 18:5698–712. doi: 10.7150/IJBS.78345
57. Yue Y, Qian W, Li J, Wu S, Zhang M, Wu Z, et al. 2′-Hydroxyflavanone inhibits the progression of pancreatic cancer cells and sensitizes the chemosensitivity of EGFR inhibitors via repressing STAT3 signaling. Cancer Lett. (2020) 471:135–46. doi: 10.1016/j.canlet.2019.11.041
58. Boye A, Asiamah EA, Martey O, and Ayertey F. Citrus limon (L.) osbeck fruit peel extract attenuates carbon tetrachloride-induced hepatocarcinogenesis in sprague-dawley rats. BioMed Res Int. (2024) 2024:6673550. doi: 10.1155/2024/6673550
59. Ayob Z, Mohd Bohari SP, Abd Samad A, and Jamil S. Cytotoxic activities against breast cancer cells of local Justicia gendarussa crude extracts. Evidence-Based Complement Altern Med. (2014) 2014:732980. doi: 10.1155/2014/732980
60. Lavanya R and Vimal S. Studies on anticancer effect of methanolic leaf extract of justicia gendarussa on lung cancer cell line. J Pharm Bioallied Sci. (2024) 16:S1207–10. doi: 10.4103/JPBS.JPBS_543_23
61. Widiyanti P, Prajogo B, and Hikmawati NPE. Cytotoxicity of justicia gendarussa burm F. Leaf extracts on molt-4 cell. Indonesian J Trop Infect Dis. (2016) 6:24. doi: 10.20473/IJTID.V6I1.1207
62. Neta MCS, Vittorazzi C, Guimarães AC, Martins JDL, Fronza M, Endringer DC, et al. Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm Biol. (2017) 55:190–7. doi: 10.1080/13880209.2016.1254251
63. Jiang Z, Pang Y, Yu X, Zhou S, Qian J, Zheng N, et al. The paradigm-shifting idea and its practice: From traditional abortion Chinese medicine Murraya paniculata to safe and effective cancer metastatic chemopreventives. Oncotarget. (2016) 7:21699–712. doi: 10.18632/ONCOTARGET.7932
64. Shi Q, Jiang Z, Yang J, Cheng Y, Pang Y, Zheng N, et al. A flavonoid glycoside compound from murraya paniculata (L.) interrupts metastatic characteristics of A549 cells by regulating STAT3/NF-κB/COX-2 and EGFR signaling pathways. AAPS J. (2017) 19:1779–90. doi: 10.1208/S12248-017-0134-0
65. Li XM, Jiang XJ, Yang K, Wang LX, Wen SZ, and Wang F. Prenylated Coumarins from Heracleum stenopterum, Peucedanum praeruptorum, Clausena lansium, and Murraya paniculata. Nat Prod Bioprospect. (2016) 6:233–7. doi: 10.1007/S13659-016-0107-5/TABLES/4
66. Shao J, Zhou S, Jiang Z, Chi T, Ma J, Kuo M, et al. Warfarin and coumarin-like Murraya paniculata extract down-regulate EpCAM-mediated cell adhesion: Individual components versus mixture for studying botanical metastatic chemopreventives. Sci Rep. (2016) 6:1–11. doi: 10.1038/SREP30549
67. Phytochemical analysis and anticancer activity of Murraya paniculata leaves . Available online at: https://www.wisdomlib.org/science/journal/world-journal-of-pharmaceutical-research/d/doc1383538.html (Accessed June 4, 2025).
68. Maharajan KK, Nallaperumal P, Ramaswamy UM, Sakthivel B, Kannika Maharajan K, and Sakthivel BM. Murraya paniculata for anti-breast cancer activity-A Molecular docking and dynamics simulation approaches Virtual screening and identifying the potent phytochemicals fromMurraya paniculata for anti-breast cancer activity-A Molecular docking and dynamics simulation approaches. (2024) doi: 10.22541/au.173129045.51625109/v1
69. Adinarayana D, Annapurna N, Pavan Kumar G, Douglas P, and Author C. Murraya paniculata mediated synthesis of cdS nanoparticles for potent biomedical applications. J Adv Zool. (2023) 44:430–6. doi: 10.53555/JAZ.V44IS6.2210
70. Samanta SK, Choudhury P, Kandimalla R, Aqil F, Moholkar DN, Gupta RC, et al. Mahanine mediated therapeutic inhibition of estrogen receptor-α and CDK4/6 expression, decipher the chemoprevention-signaling cascade in preclinical model of breast cancer. J Ethnopharmacol. (2024) 319:117235. doi: 10.1016/j.jep.2023.117235
71. Kandimalla R, Moholkar DN, Samanta SK, Tyagi N, Aqil F, and Gupta R. Oncogene downregulation by mahanine suppresses drug-sensitive and drug-resistant lung cancer and inhibits orthotopic tumor progression. Cancers (Basel). (2024) 16:3572. doi: 10.3390/CANCERS16213572
72. Chatterjee P, Seal S, Mukherjee S, Kundu R, Bhuyan M, Barua NC, et al. A carbazole alkaloid deactivates mTOR through the suppression of rictor and that induces apoptosis in lung cancer cells. Mol Cell Biochem. (2015) 405:149–58. doi: 10.1007/S11010-015-2406-2
73. Mondal P, Natesh J, Abdul Salam AA, and Meeran SM. Mahanimbine isolated from Murraya koenigii inhibits P-glycoprotein involved in lung cancer chemoresistance. Bioorg Chem. (2022) 129:106170. doi: 10.1016/j.bioorg.2022.106170
74. Iman V, Mohan S, Abdelwahab SI, Karimian H, Nordin N, Fadaeinasab M, et al. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des Devel Ther. (2017) 11:103–21. doi: 10.2147/DDDT.S115135
75. Hobani YH. The role of oxidative stress in koenimbine-induced DNA damage and heat shock protein modulation in hepG2 cells. Integr Cancer Ther. (2017) 16:563–71. doi: 10.1177/1534735416678982
76. Huang K, Liu Z, Kim MO, and Kim KR. Anticancer effects of gossypetin from Hibiscus sabdariffa in oral squamous cell carcinoma. J Appl Oral Sci. (2023) 31:e20230243. doi: 10.1590/1678-7757-2023-0243
77. Chiaino E, Micucci M, Budriesi R, Mattioli LB, Marzetti C, Corsini M, et al. Hibiscus flower and olive leaf extracts activate apoptosis in SH-SY5Y cells. Antioxidants. (2021) 10:1962. doi: 10.3390/ANTIOX10121962/S1
78. Bassong TR, Kenmogne LV, Awounfack CF, Ndinteh DT, Njamen D, and Zingue S. Effects of hibiscus sabdariffa calyxes aqueous extract on antioxidant status and histopathology in mammary tumor-induced in rats. Evidence-Based Complement Altern Med. (2022) 2022:9872788. doi: 10.1155/2022/9872788
79. Amin BE, Yosri M, Salama HM, Alkhalifah DH, Alwaili MA, Abd RY, et al. GC-MS analysis, antibacterial, and anticancer activities of hibiscus sabdariffa L. Methanolic extract: in vitro and in silico studies. Microorganisms. (2023) 11:1601. doi: 10.3390/MICROORGANISMS11061601
80. Malacrida A, Cavalloro V, Martino E, Cassetti A, Nicolini G, Rigolio R, et al. Anti-multiple myeloma potential of secondary metabolites from hibiscus sabdariffa. Molecules. (2019) 24:2500. doi: 10.3390/MOLECULES24132500
81. Malacrida A, Cavalloro V, Martino E, Costa G, Ambrosio FA, Alcaro S, et al. Anti-multiple myeloma potential of secondary metabolites from hibiscus sabdariffa—part 2. Molecules. (2021) 26:6596. doi: 10.3390/MOLECULES26216596
82. Chiu CT, Chen JH, Chou FP, and Lin HH. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-κB/MMP-9 Pathway. Nutrients. (2015) 7:5065. doi: 10.3390/NU7075065
83. Akram HM, Haleem AM, and Salah R. Antioxidant and Antineoplastic Activities of Hibiscus sabdariffa Linn. Petal Extracts against Oral Squamous Cell Carcinoma Cell Line. Oral Health Prev Dent. (2024) 22:131–8. doi: 10.3290/J.OHPD.B4997059
84. Amran N, Rani A, Mahmud R, and Yin K. Antioxidant and cytotoxic effect of Barringtonia racemosa and Hibiscus sabdariffa fruit extracts in MCF-7 human breast cancer cell line. Pharmacogn Res. (2016) 8:66–70. doi: 10.4103/0974-8490.171104
85. Huang JY, Cao XY, Wu WF, Han L, and Wang FY. Investigating the proliferative inhibition of HepG2 cells by exosome-like nanovesicles derived from Centella asiatica extract through metabolomics. Biomed Pharmacother. (2024) 176:116855. doi: 10.1016/J.BIOPHA.2024.116855
86. Guo M, Ying Y, Chen Y, Miao X, and Yu Z. Asiaticoside inhibits breast cancer progression and tumor angiogenesis via YAP1/VEGFA signal pathway. Heliyon. (2024) 10:e37169. doi: 10.1016/j.heliyon.2024.e37169
87. Al-Saeedi FJ. Study of the cytotoxicity of asiaticoside on rats and tumour cells. BMC Cancer. (2014) 14:220. doi: 10.1186/1471-2407-14-220
88. Patil P, Shalavadi M, and V.M C. Anticancer activity of Gloriosa superba Linn in Ehrlich ascites carcinoma tumor-bearing Balb/c mice. TMR Cancer (2021) 4:11. doi: 10.12032/TMRC201800101
89. Rao VRS, Raju SS, Sarma VU, Sabine F, Babu KH, Babu KS, et al. Simultaneous determination of bioactive compounds in Piper nigrum L. and a species comparison study using HPLC-PDA. Nat Prod Res. (2011) 25:1288–94. doi: 10.1080/14786419.2010.535158
90. Si L, Yang R, Lin R, and Yang S. Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci Rep. (2018) 38:BSR20180503. doi: 10.1042/BSR20180503
91. Cho HK, Park CG, and Lim HB. Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells. Curr Issues Mol Biol. (2024) 46:5551–60. doi: 10.3390/CIMB46060332
92. Lasso P, Rojas L, Arévalo C, Urueña C, Murillo N, Nossa P, et al. Piper nigrum extract suppresses tumor growth and enhances the antitumor immune response in murine models of breast cancer and melanoma. Cancer Immunol Immunother. (2023) 72:3279–92. doi: 10.1007/S00262-023-03487-3
93. Wu R, Zhao J, Wei P, Tang M, Ma Z, Zhao Y, et al. Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis. Pharmaceuticals. (2023) 16:1325. doi: 10.3390/PH16091325
94. Khongkarat P, Sadangrit P, Puthong S, Meemongkolkiat T, Phuwapraisirisan P, and Chanchao C. Anti-tyrosinase and anti-melanogenic effects of piperine isolated from Piper nigrum on B16F10 mouse melanoma cells. Heliyon. (2024) 10:e33423. doi: 10.1016/j.heliyon.2024.e33423
95. Fard SE, Tafvizi F, and Torbati MB. Silver nanoparticles biosynthesised using Centella asiatica leaf extract: Apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. (2018) 12:994–1002. doi: 10.1049/IET-NBT.2018.5069
96. Ahmadi N, Mohamed S, Sulaiman Rahman H, and Rosli R. Epicatechin and scopoletin-rich Morinda citrifolia leaf ameliorated leukemia via anti-inflammatory, anti-angiogenesis, and apoptosis pathways in vitro and in vivo. J Food Biochem. (2019) 43:e12868. doi: 10.1111/JFBC.12868
97. Abu N, Zamberi NR, Yeap SK, Nordin N, Mohamad NE, Romli MF, et al. Subchronic toxicity, immunoregulation and anti-breast tumor effect of Nordamnacantal, an anthraquinone extracted from the stems of Morinda citrifolia L. BMC Complement Altern Med. (2018) 18:31–1. doi: 10.1186/S12906-018-2102-3
98. Aziz MYA, Omar AR, Subramani T, Yeap SK, Ho WY, Ismail NH, et al. Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol Lett. (2014) 7:1479–84. doi: 10.3892/OL.2014.1898/DOWNLOAD
99. Aziz MYA, Abu N, Yeap SK, Ho WY, Omar AR, Ismail NH, et al. Combinatorial cytotoxic effects of damnacanthal and doxorubicin against human breast cancer MCF-7 cells in vitro. Molecules. (2016) 21:1228. doi: 10.3390/MOLECULES21091228
100. Chandran K, Shane DI, Zochedh A, Sultan AB, and Kathiresan T. Docking simulation and ADMET prediction based investigation on the phytochemical constituents of Noni (Morinda citrifolia) fruit as a potential anticancer drug. In Silico Pharmacol. (2022) 10:14. doi: 10.1007/S40203-022-00130-4
101. Chee CW, Zamakshshari NH, Lee VS, Abdullah I, Othman R, Lee YK, et al. Morindone from Morinda citrifolia as a potential antiproliferative agent against colorectal cancer cell lines. PloS One. (2022) 17:e0270970. doi: 10.1371/JOURNAL.PONE.0270970
102. Lim SL, Mustapha N, Goh YM, Rahman H, and Mohamed S. Morinda citrifolia Leaf Extract Suppressed Metastasised Cancer Progression via EGFR and MAPK Pathways. Planta Med Int Open. (2017) 4:e8–e16. doi: 10.1055/S-0043-107030
103. Zhang L, Shamaladevi N, Jayaprakasha G, Patil BS, and Lokeshwar BL. Polyphenol-rich extract of Pimenta dioica berries (Allspice) kills breast cancer cells by autophagy and delays growth of triple negative breast cancer in athymic mice. Oncotarget. (2015) 6:16379–95. doi: 10.18632/ONCOTARGET.3834
104. Kowsalya R, Kaliaperumal J, Vaishnavi M, and Namasivayam E. Anticancer activity of Cynodon dactylon L. root extract against diethyl nitrosamine induced hepatic carcinoma. South Asian J Cancer. (2015) 4:83. doi: 10.4103/2278-330X.155691
105. (PDF) In-vitro anticancer activity of petroleum ether extract of Cynodon dactylon . Available online at: https://www.researchgate.net/publication/284419163_In-vitro_anticancer_activity_of_petroleum_ether_extract_of_Cynodon_dactylon (Accessed June 9, 2025).
106. Koushik OS and Babu PS. Isolation of anticancer bioactive and in vitro evaluation of antioxidant and anticancer activity of cynodon dactylon (L). Pers. Transl BioMed. (2015) 6. doi: 10.21767/2172-0479.100023
107. Alghamdi SS, Alghashem SA, Ali R, Alsubait A, Suliman RS, Mohammed AE, et al. Exploring the potential of Ziziphus nummularia and luteolin-7-O-glucoside as tubulin inhibitors in cancer therapy and survival. Sci Rep. (2024) 14:7202. doi: 10.1038/S41598-024-57680-0
108. Abdallah R, Shaito AA, Badran A, Baydoun S, Sobeh M, Ouchari W, et al. Fractionation and phytochemical composition of an ethanolic extract of Ziziphus nummularia leaves: antioxidant and anticancerous properties in human triple negative breast cancer cells. Front Pharmacol. (2024) 15:1331843/XML/NLM. doi: 10.3389/FPHAR.2024.1331843/XML/NLM
109. Mesmar J, Fardoun MM, Abdallah R, Al Dhaheri Y, Yassine HM, Iratni R, et al. Ziziphus nummularia attenuates the Malignant phenotype of human pancreatic cancer cells: Role of ros. Molecules. (2021) 26:4295. doi: 10.3390/MOLECULES26144295
110. Paradkar PH, Juvekar AS, Barkume MS, Amonkar AJ, Joshi JV, Soman G, et al. In vitro and in vivo evaluation of a standardized Curcuma longa Linn formulation in cervical cancer. J Ayurveda Integr Med. (2021) 12:616–22. doi: 10.1016/j.jaim.2021.06.002
111. Esposito T, Schettino C, Polverino P, Allocca S, Adelfi L, D’Amico A, et al. Synergistic interplay between curcumin and polyphenol-rich foods in the mediterranean diet: Therapeutic prospects for neurofibromatosis 1 patients. Nutrients. (2017) 9:783. doi: 10.3390/NU9070783
112. Park J, Ayyappan V, Bae EK, Lee C, Kim BS, Kim BK, et al. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol Oncol. (2008) 2:317–26. doi: 10.1016/J.MOLONC.2008.09.006
113. Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. (2019) 149:1133. doi: 10.1093/JN/NXZ029
114. Tamam MB, Aini NS, Murtadlo AAA, Turista DDR, Naw SW, and Ullah M. Antiviral and Anticancer Activity from Curcuma longa L. and Tamarindus indica Bioactive Compounds through In Silico Analysis. SAINSTEK Int J Appl Sci Adv Technol Inf. (2023) 2:12–7. doi: 10.24036/SAINSTEK/VOL2-ISS01/21
115. Akev N, Candoken E, and Kuruca SE. Comparative study on the anticancer drug potential of a lectin purifid from Aloe Vera and aloe-emodin. Asian Pac J Cancer Prev. (2020) 21:99–106. doi: 10.31557/APJCP.2020.21.1.99
116. Esmat AY, Tomasetto C, and Rio MC. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: Topoisomerase II-alpha coamplification. Cancer Biol Ther. (2006) 5:97–103. doi: 10.4161/CBT.5.1.2347
117. Candoken E, Erdem Kuruca S, and Akev N. Evaluation of the anticancer effects of Aloe vera and aloe emodin on B16F10 murine melanoma and NIH3T3 mouse embryogenic fibroblast cells. Istanbul J Pharm. (2017) 47(3):77–83. doi: 10.5152/ISTANBULJPHARM.2017.0013
118. Shalabi M, Khilo K, Zakaria MM, Elsebaei MG, Abdo W, and Awadin W. Anticancer activity of Aloe vera and Calligonum comosum extracts separetely on hepatocellular carcinoma cells. Asian Pac J Trop BioMed. (2015) 5:375–81. doi: 10.1016/S2221-1691(15)30372-5
119. Lima A, Batista-Santos P, Veríssimo E, Rebelo P, and Ferreira RB. Differential inhibition of gelatinase activity in human colon adenocarcinoma cells by Aloe vera and Aloe arborescens extracts. BMC Complement Med Ther. (2020) 20:1–11. doi: 10.1186/S12906-020-03134-9/FIGURES/5
120. Tu L. Enhanced anticancer potency of Aloe vera in combination with Royal jelly in non-small cell lung cancer and colorectal adenocarcinoma. Ann Med Res. (2023) 30:1. doi: 10.5455/ANNALSMEDRES.2023.08.207
121. Netala VR, Hou T, Devarapogu R, Bethu MS, Zhang Z, and Vijaya T. Exploring the therapeutic potential of triterpenoid saponins from Gymnema sylvestre: Mechanistic insights into hepatoprotection, immunomodulation, anticancer activities, molecular docking, and pharmacokinetics. Heliyon. (2024) 10:e40850. doi: 10.1016/J.HELIYON.2024.E40850
122. Ghosh AR, Alsayari A, Habib AH, Wahab S, Nadig APR, Rafeeq MM, et al. Anti-tumor potential of gymnema sylvestre saponin rich fraction on in vitro breast cancer cell lines and in vivo tumor-bearing mouse models. Antioxidants. (2023) 12:134. doi: 10.3390/ANTIOX12010134
123. Agrawal RC and Maheshwari SK. Chemopreventive effect of Gymnema sylvestre in Swiss albino mice. Int J Sci Res Publ. (2016) 6:78. Available online at: www.ijsrp.org.
124. Vannini S, Villarini M, Levorato S, Salvatori T, Fatigoni C, Pagiotti R, et al. In vitro evalutation of cytotoxic, genotoxic and apoptotic properties of herbal products from leaves of Gymnema sylvestre. Int J Herbal Med. (2017) 5:33–8.
125. Singh VK, Dwivedi P, Chaudhary BR, and Singh R. Immunomodulatory effect of gymnema sylvestre (R.Br.) leaf extract: an in vitro study in rat model. PloS One. (2015) 10:e0139631. doi: 10.1371/JOURNAL.PONE.0139631
126. Khanna VG, Kannabiran K, and Kannabiran K. Non-proliferative activity of saponins isolated from the leaves of gymnema sylvestre and eclipta prostrata on hepg2 cells-in vitro study. IJPSR. (2010) 1:38–42. Available online at: www.ijpsr.com.
127. Arunachalam KD, Arun LB, Annamalai SK, and Arunachalam AM. Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed. (2014) 10:31–41. doi: 10.2147/IJN.S71182
128. Rotondo R, Castaldo S, Oliva MA, Arcella A, Tuccari G, Caruso RA, et al. Gymnema sylvestre extract restores the autophagic pathway in human glioblastoma cells U87Mg. Biology. (2021) 10:870. doi: 10.3390/BIOLOGY10090870
129. Arumugam S, Yadav SA, Krishnan A, Manoharan SP, and Gnanaselvan S. In vitro cytotoxic potential of Gymnema sylvestre fractions on HT-29 cell line. J Phytol. (2022) 14:121–6. doi: 10.25081/JP.2022.V14.7942
130. Chakraborty D, Ghosh S, Bishayee K, Mukherjee A, Sikdar S, and Khuda-Bukhsh AR. Antihyperglycemic drug gymnema sylvestre also shows anticancer potentials in human melanoma A375 cells via reactive oxygen species generation and mitochondria-dependent caspase pathway. Integr Cancer Ther. (2013) 12:433–41. doi: 10.1177/1534735413485419/ASSET/A21297B5-FD50-486B-81F3-2B2460D98EF5/ASSETS/IMAGES/LARGE/10.1177_1534735413485419-FIG7.JPG
131. Sivakumar G, Alba K, and Phillips GC. Biorhizome: A biosynthetic platform for colchicine biomanufacturing. Front Plant Sci. (2017) 8:1137. doi: 10.3389/fpls.2017.01137
132. Goel B, Dey B, Chatterjee E, Tripathi N, Bhardwaj N, Kumar S, et al. Antiproliferative potential of gloriosine: A lead for anticancer drug development. ACS Omega. (2022) 7:28994–9001. doi: 10.1021/acsomega.2c02688
133. Reuter S, Prasad S, Phromnoi K, Ravindran J, Sung B, Yadav VR, et al. Thiocolchicoside exhibits anticancer effects through downregulation of NF-κB pathway and its regulated gene products linked to inflammation and cancer. Cancer Prev Res. (2010) 3:1462–72. doi: 10.1158/1940-6207.CAPR-10-0037
134. Zhai Z, Qu X, Li H, Ouyang Z, Yan W, Liu G, et al. Inhibition of MDA-MB-231 breast cancer cell migration and invasion activity by andrographolide via suppression of nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Mol Med Rep. (2015) 11(2):1139–45. doi: 10.3892/mmr.2014.2872
135. Budchart P, Khamwut A, Sinthuvanich C, Ratanapo S, Poovorawan Y, and T-Thienprasert NP. Partially Purified Gloriosa superba Peptides Inhibit Colon Cancer Cell Viability by Inducing Apoptosis Through p53 Upregulation. Am J Med Sci. (2017) 354:423–9. doi: 10.1016/j.amjms.2017.06.005
136. Rokade SS, Joshi KA, Mahajan K, Patil S, Tomar G, Dubal DS, et al. Gloriosa superba mediated synthesis of platinum and palladium nanoparticles for induction of apoptosis in breast cancer. Bioinorg Chem Appl. (2018) 2018:4924186. doi: 10.1155/2018/4924186
137. Micheau O, Dufour F, and Walczak H. Thiocolchicoside a semi-synthetic derivative of the Glory Lily: a new weapon to fight metastatic bone resorption? Br J Pharmacol. (2012) 165:2124–6. doi: 10.1111/j.1476-5381.2011.01792.x
138. Li S, Lei Y, Jia Y, Li N, Wink M, and Ma Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine. (2011) 19:83–7. doi: 10.1016/J.PHYMED.2011.06.031
139. Tharmalingam N, Kim SH, Park M, Woo HJ, Kim HW, Yang JY, et al. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect Agent Cancer. (2014) 9:43. doi: 10.1186/1750-9378-9-43
140. Eid AM, Issa L, Al-Kharouf O, Jaber R, and Hreash F. Development of coriandrum sativum oil nanoemulgel and evaluation of its antimicrobial and anticancer activity. BioMed Res Int. (2021) 2021:5247816. doi: 10.1155/2021/5247816
141. Mechchate H, de Oliveira RC, Es-Safi I, Vasconcelos Mourão EM, Bouhrim M, Kyrylchuk A, et al. Antileukemic activity and molecular docking study of a polyphenolic extract from coriander seeds. Pharmaceuticals. (2021) 14:770. doi: 10.3390/PH14080770
142. Marcucci MC, Oliveira CR, Spindola D, Antunes AA, Santana LYK, Cavalaro V, et al. Molecular Dereplication and In Vitro and In Silico Pharmacological Evaluation of Coriandrum sativum against Neuroblastoma Cells. Molecules. (2022) 27:5389. doi: 10.3390/MOLECULES27175389
143. Sumalatha D and Nithya TG. Invitro anti-oxidant and anticancer activity of Murraya koenigii against human colon cancer HT-29 cell lines. Asian Journal of Pharmaceutical and Clinical Research. (2014) 7:83–6.
144. Xie L, Wu Z, Liu Y, Tang J, Lu C, and Wang H. α-linalool from coriander root inhibits the proliferation and invasion of a human gastric cancer cell line. Clin Cancer Investig J. (2023) 12:6–14. doi: 10.51847/OQ9XFTWACJ
145. Huang H, Nakamura T, Yasuzawa T, and Ueshima S. Effects of coriandrum sativum on migration and invasion abilities of cancer cells. J Nutr Sci Vitaminol (Tokyo). (2020) 66:468–77. doi: 10.3177/JNSV.66.468
146. Vadde R, Radhakrishnan S, Eranda Karunathilake Kurundu H, Reddivari L, and Vanamala JKP. Indian gooseberry (Emblica officinalis Gaertn.) suppresses cell proliferation and induces apoptosis in human colon cancer stem cells independent of p53 status via suppression of c-Myc and cyclin D1. J Funct Foods. (2016) 25:267–78. doi: 10.1016/J.JFF.2016.06.007
147. Singai C, Pitchakarn P, Taya S, Phannasorn W, Wongpoomchai R, and Wongnoppavich A. Chemopreventive Potential of Phyllanthus emblica Fruit Extract against Colon and Liver Cancer Using a Dual-Organ Rat Carcinogenesis Model. Pharmaceuticals. (2024) 17:818. doi: 10.3390/PH17070818
148. Wang B, Wu K, Liu R, Huang Y, Chang Z, Gao Y, et al. Phyllanthi tannin loaded solid lipid nanoparticles for lung cancer therapy: preparation, characterization, pharmacodynamics and safety evaluation. Molecules. (2023) 28:7399. doi: 10.3390/MOLECULES28217399
149. Singh L, Atilano S, Chwa M, Singh MK, Ozgul M, Nesburn A, et al. Using human ‘Personalized’ Cybrids to identify drugs/agents that can regulate chronic lymphoblastic leukemia mitochondrial dysfunction. Int J Mol Sci. (2023) 24:11025. doi: 10.3390/IJMS241311025
150. Chari KY, Polu PR, and Shenoy RR. An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine induced colon cancer in wistar rats. J Toxicol. (2018) 2018:6086490. doi: 10.1155/2018/6086490
151. Khan N, Jajeh F, Khan MI, Mukhtar E, Shabana SM, and Mukhtar H. Sestrin-3 modulation is essential for therapeutic efficacy of cucurbitacin B in lung cancer cells. Carcinogenesis. (2016) 38:184. doi: 10.1093/CARCIN/BGW124
152. Dandawate P, Subramaniam D, Panovich P, Standing D, Krishnamachary B, Kaushik G, et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci Rep. (2020) 10:1–15. doi: 10.1038/s41598-020-57940-9
153. Bahadori MH, Azari Z, Zaminy A, Dabirian S, Mehrdad SM, and Kondori BJ. Anti-proliferative and apoptotic effects of hull-less pumpkin extract on human papillary thyroid carcinoma cell line. Anat Cell Biol. (2021) 54:104–11. doi: 10.5115/ACB.20.228
154. Pinna N, Ianni F, Conte C, Codini M, di Vito R, Urbani S, et al. Carotenoids from different pumpkin varieties exert a cytotoxic effect on human neuroblastoma SH-SY5Y cells. Nutrients. (2024) 16:3043. doi: 10.3390/NU16173043
155. Alafaleq NO, Zughaibi TA, Jabir NR, Khan AU, Khan MS, and Tabrez S. Biogenic synthesis of cu-mn bimetallic nanoparticles using pumpkin seeds extract and their characterization and anticancer efficacy. Nanomaterials. (2023) 13:1201. doi: 10.3390/NANO13071201
156. Mericli F, Becer E, Kabadayi H, Hanoglu A, Hanoglu DY, Yavuz DO, et al. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm Biol. (2017) 55:1239–48. doi: 10.1080/13880209.2017.1296003
157. Omoboyowa DA, Balogun TA, Saibu OA, Chukwudozie OS, Alausa A, Olubode SO, et al. Structure-based discovery of selective CYP17A1inhibitors for Castration-resistant prostate cancer treatment. Biol Methods Protoc. (2022) 7:bpab026. doi: 10.1093/BIOMETHODS/BPAB026
158. Ayaz Z, Zainab B, Khan S, Abbasi AM, Elshikh MS, Munir A, et al. In silico authentication of amygdalin as a potent anticancer compound in the bitter kernels of family Rosaceae. Saudi J Biol Sci. (2020) 27:2444–51. doi: 10.1016/J.SJBS.2020.06.041
159. Asati V, Srivastava A, Mukherjee S, and Sharma PK. Comparative analysis of antioxidant and antiproliferative activities of crude and purified flavonoid enriched fractions of pods/seeds of two desert legumes Prosopis cineraria and Cyamopsis tetragonoloba. Heliyon. (2021) 7:e07304. doi: 10.1016/j.heliyon.2021.e07304
160. Preethy CP, Alshatwi AA, Gunasekaran M, and Akbarsha MA. Analysis of the cytotoxic potential of anisomelic acid isolated from anisomeles malabarica. Sci Pharm. (2013) 81:559–66. doi: 10.3797/SCIPHARM.1210-15
161. Preethy C, Padmapriya R, Periasamy V, Riyasdeen A, Srinag S, Krishnamurthy H, et al. Antiproliferative property of n-hexane and chloroform extracts of Anisomeles malabarica (L). R. Br. in HPV16-positive human cervical cancer cells. J Pharmacol Pharmacother. (2012) 3:26–34. doi: 10.4103/0976-500X.92500
162. Jansi PCJP and Padua JC. In vitro Antibacterial and Cytotoxic Activity of The Leaf Extract of Anisomeles malabarica against HepG2 Cancer Cell Lines. Egypt Acad J Biol Sci B Zool. (2022) 14:349–10. doi: 10.21608/EAJBSZ.2022.271230
163. Ingole VV, Mhaske PC, and Katade SR. Phytochemistry and pharmacological aspects of Tridax procumbens (L.): A systematic and comprehensive review. Phytomed Plus. (2022) 2:100199. doi: 10.1016/J.PHYPLU.2021.100199
164. Lakhera S, Rana M, Devlal K, Celik I, and Yadav R. A comprehensive exploration of pharmacological properties, bioactivities and inhibitory potentiality of luteolin from Tridax procumbens as anticancer drug by in-silico approach. Struct Chem. (2022) 33:703–19. doi: 10.1007/S11224-022-01882-7
165. Pungle R, Nile SH, Makwana N, Singh R, Singh RP, and Kharat AS. Green synthesis of silver nanoparticles using the tridax procumbens plant extract and screening of its antimicrobial and anticancer activities. Oxid Med Cell Longev. (2022) 2022:9671594. doi: 10.1155/2022/9671594
166. Sundhari T, Soundarya R, Jansirani D, and Lavanya P. Anticancer activity of Vettukaaya Poondu (Tridax procumbens) as evidenced by Cytotoxic assay . Available online at: https://www.researchgate.net/profile/Thomas-M-Walter/publication/333918728_Anticancer_activity_of_Vettukaaya_Poondu_Tridax_procumbens_as_evidenced_by_Cytotoxic_assay/links/5d0cc4a0458515c11ceb4f50/Anticancer-activity-of-Vettukaaya-Poondu-Tridax-procumbens-as-evidenced-by-Cytotoxic-assay.pdf (Accessed June 14, 2025). researchgate.netTV Sundhari, R Soundarya, D Jansirani, P Lavanya, TM Walterresearchgate.net.
167. Sagheer R, Gupta A, Luqman S, Kaur H, and Srivastava K. Antiproliferative and antioxidant potential of Tridax procumbens extracts against various human cancer cell lines: An insight for medicines from weeds. J King Saud University-Sci. (2024) 36:103474. Available online at: https://www.sciencedirect.com/science/article/pii/S1018364724003860.
168. Mohammed FS, Sevindik M, Uysal İ, Çesko C, and Koraqi H. Chemical composition, biological activities, uses, nutritional and mineral contents of cumin (Cuminum cyminum). Measurement: Food. (2024) 14:100157. doi: 10.1016/J.MEAFOO.2024.100157
169. Nirmala MJ, Durai L, Rao KA, and Nagarajan R. Ultrasonic nanoemulsification of cuminum cyminum essential oil and its applications in medicine. Int J Nanomed. (2020) 15:795–807. doi: 10.2147/IJN.S230893
170. Chandrasekaran R, Krishnan M, Chacko S, Gawade O, Hasan S, Joseph J, et al. Assessment of anticancer properties of cumin seed (Cuminum cyminum) against bone cancer. Front Oncol. (2023) 13:1322875/PDF. doi: 10.3389/FONC.2023.1322875/PDF
171. Hazra S, Ray AS, and Rahaman CH. Natural phytocompounds from common Indian spices for identification of three potential inhibitors of breast cancer: A molecular modelling approach. Molecules. (2022) 27:6590. doi: 10.3390/MOLECULES27196590
172. Aqil F, Jeyabalan J, Munagala R, Ahmad I, Schultz DJ, and Gupta RC. Cumin prevents 17β-estradiol-associated breast cancer in aci rats. Int J Mol Sci. (2021) 22:6194. doi: 10.3390/IJMS22126194
173. Ruwali P, Pandey N, Jindal K, and Singh RV. Fenugreek (Trigonella foenum-graecum): Nutraceutical values, phytochemical, ethnomedicinal and pharmacological overview. South Afr J Bot. (2022) 151:423–31. doi: 10.1016/J.SAJB.2022.04.014
174. Iranmanesh M, Mohebbati R, Forouzanfar F, Roshan MK, Ghorbani A, Nik MJ, et al. In vivo and In vitro effects of ethanolic extract of Trigonella foenum-graecum L. seeds on proliferation, angiogenesis and tube formation of endothelial cells. Res Pharm Sci. (2018) 13:343. doi: 10.4103/1735-5362.235161
175. Rampogu S, Parameswaran S, Lemuel MR, and Lee KW. Exploring the therapeutic ability of fenugreek against type 2 diabetes and breast cancer employing molecular docking and molecular dynamics simulations. Evidence-Based Complement Altern Med. (2018) 2018:1943203. doi: 10.1155/2018/1943203
176. Allaoui A, Gascón S, Benomar S, Quero J, Osada J, Nasri M, et al. Protein hydrolysates from fenugreek (Trigonella foenum graecum) as nutraceutical molecules in colon cancer treatment. Nutrients. (2019) 11:724. doi: 10.3390/NU11040724
177. Khalil MIM, Ibrahim MM, El-Gaaly GA, and Sultan AS. Trigonella foenum (Fenugreek) Induced Apoptosis in Hepatocellular Carcinoma Cell Line, HepG2, Mediated by Upregulation of p53 and Proliferating Cell Nuclear Antigen. BioMed Res Int. (2015) 2015:914645. doi: 10.1155/2015/914645
178. Goyal S, Gupta N, Kumar A, Chatterjee S, and Nimesh S. Antibacterial, anticancer and antioxidant potential of silver nanoparticles engineered using Trigonella foenum-graecum seed extract. IET Nanobiotechnol. (2018) 12:526–33. doi: 10.1049/IET-NBT.2017.0089
179. Alsemari A, Alkhodairy F, Aldakan A, Al-Mohanna M, Bahoush E, Shinwari Z, et al. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum. BMC Complement Altern Med. (2014) 14:1–9. doi: 10.1186/1472-6882-14-114/COMMENTS
180. de Cedrón MG, del Hierro JN, Reguero M, Wagner S, Bouzas A, Quijada-Freire A, et al. Saponin-rich extracts and their acid hydrolysates differentially target colorectal cancer metabolism in the frame of precision nutrition. Cancers. (2020) 12:3399. doi: 10.3390/CANCERS12113399
181. Zhang H, Lv JL, Zheng QS, and Li J. Active components of Solanum nigrum and their antitumor effects: a literature review. Front Oncol. (2023) 13:1329957. doi: 10.3389/FONC.2023.1329957
182. Chen X, Dai X, Liu Y, Yang Y, Yuan L, He X, et al. Solanum nigrum Linn.: An Insight into Current Research on Traditional Uses. Phytochem Pharmacol Front Pharmacol. (2022) 13:918071. doi: 10.3389/FPHAR.2022.918071
183. Yengwa Bakam B, Fosso RU, Grein T, Ndinteh DT, Maxeiner S, Zingue S, et al. Cucumis sativus (Curcubitaceae) inhibits prostate carcinoma cell growth and prevents the testosterone-induced benign prostatic hyperplasia in Wistar rat. J Funct Foods. (2024) 114:106088. doi: 10.1016/j.jff.2024.106088
184. Wu D, Wang Z, Lin M, Shang Y, Wang F, Zhou JY, et al. In vitro and in vivo antitumor activity of cucurbitacin C, a novel natural product from cucumber. Front Pharmacol. (2019) 10:1287. doi: 10.3389/FPHAR.2019.01287
185. Tuama AA and Mohammed AA. Phytochemical screening and in vitro antibacterial and anticancer activities of the aqueous extract of Cucumis sativus. Saudi J Biol Sci. (2019) 26:600–4. doi: 10.1016/j.sjbs.2018.07.012
186. Maslikah SI, Lestari SR, Handayani N, Malek N, Jemon KB, Amalia A, et al. docking of Red Betel (Piper crocatum Ruiz & Pav.) active compound and tamoxifen drug as an inhibitor of Estrogen receptor-α (ER-α) that plays a role in breast cancer. AIP Conf. Proc. (2020) 2231:040001. doi: 10.1063/5.0002556/14211895/040001_1_ONLINE.PDF
187. Kangralkar V. Evaluation of effect of Piper betel, Centella asiatica and Aristolochia indica extracts on bacterial enzymes in 1, 2-dimethyl hydrazine induced colon cancer in wistar rats. Research J. Pharm. and Tech. (2014) 7:151–4.
188. Sanubol A, Chaveerach A, and Tanee T. Pre-clinical evaluation of extracts and essential oils from betel-like scent Piper species identified potential cancer treatment. Afr J Tradit Complement Altern Med. (2017) 14:89–102. doi: 10.21010/ajtcam.v14i1.10
189. Aara A, Chappidi V, and Ramadas M. Antioxidant activity of eugenol in Piper betel leaf extract. J Family Med Prim Care. (2020) 9:327. doi: 10.4103/JFMPC.JFMPC_809_19
190. Mohamad NA, Rahman AA, and Sheikh Abdul Kadir SH. Hydroxychavicol as a potential anticancer agent (Review). Oncol Lett. (2022) 25(1):34. doi: 10.3892/ol.2022.13620
191. Biswas P, Anand U, Saha SC, Kant N, Mishra T, Masih H, et al. Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J Cell Mol Med. (2022) 26:3083. doi: 10.1111/JCMM.17323
192. Abrahim NN, Kanthimathi MS, and Abdul-Aziz A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement Altern Med. (2012) 12:220. doi: 10.1186/1472-6882-12-220
193. Wadhwa R, Wang J, Shefrin S, Zhang H, Sundar D, and Kaul SC. Molecular insights into the anticancer activity of withaferin-A: the inhibition of survivin signaling. Cancers (Basel). (2024) 16:3090. doi: 10.3390/CANCERS16173090/S1
194. Zhang HL and Zhang H. Withaferin-A induces apoptosis in osteosarcoma U2OS cell line via generation of ROS and disruption of mitochondrial membrane potential. Pharmacogn Mag. (2017) 13:523. doi: 10.4103/0973-1296.211042
195. Nada SM, Ahmed NS, Hadi YA, Saif Z, Mohammed AD, and Al Rikabi ZHA. In vitro cytotoxic study for partially purified Lasparaginase from fresh leaves, unripe and ripe fruits of Withania somnifera plant. Afr J Biotechnol. (2014) 13:957–61. doi: 10.5897/AJB2013.13263
196. Al Awadh AA, Sakagami H, Amano S, Sayed AM, Abouelela ME, Alhasaniah AH, et al. In vitro cytotoxicity of Withania somnifera (L.) roots and fruits on oral squamous cell carcinoma cell lines: a study supported by flow cytometry, spectral, and computational investigations. Front Pharmacol. (2024) 15:1325272. doi: 10.3389/FPHAR.2024.1325272
197. Hou WC, Miao XH, Ma LJ, Bai XX, Liu Q, and Song L. WITHAFERIN A INDUCES APOPTOSIS IN RAT C6 GLIOMA CELLS THROUGH REGULATING NF-KB NUCLEAR TRANSLOCATION AND ACTIVATION OF CASPASE CASCADE. Afr J Tradit Complement Altern Medicines. (2017) 14:319. doi: 10.21010/AJTCAM.V14I2.33
198. Singh N, Yadav SS, Rao AS, Nandal A, Kumar S, Ganaie SA, et al. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J Ethnopharmacol. (2021) 270:113704. doi: 10.1016/J.JEP.2020.113704
199. Chemical Constituents and Experimental Pharmacology of Curcuma longa: A Comprehensive Review . Auctores. Available online at: https://www.auctoresonline.org/article/chemical-constituents-and-experimental-pharmacology-of-curcuma-longa-a-comprehensive-review (Accessed July 28, 2025).
200. Dutta R, Khalil R, Green R, Mohapatra SS, and Mohapatra S. Withania somnifera (Ashwagandha) and withaferin A: potential in integrative oncology. Int J Mol Sci. (2019) 20:5310. doi: 10.3390/IJMS20215310
201. Ameer SF, Mohamed MY, Elzubair QA, Sharif EAM, and Ibrahim WN. Curcumin as a novel therapeutic candidate for cancer: can this natural compound revolutionize cancer treatment? Front Oncol. (2024) 14:1438040/BIBTEX. doi: 10.3389/FONC.2024.1438040/BIBTEX
202. Issell BF, Gotay CC, Pagano I, and Franke AA. Using quality of life measures in a phase I clinical trial of noni in patients with advanced cancer to select a phase II dose. J Diet Suppl. (2009) 6:347–59. doi: 10.3109/19390210903280272
203. Cleret de Langavant L, Roze E, Petit A, Tressières B, Gharbi-Meliani A, Chaumont H, et al. Annonaceae consumption worsens disease severity and cognitive deficits in degenerative parkinsonism. Movement Disord. (2022) 37:2355. doi: 10.1002/MDS.29222
204. Mora E, Lavoie Smith EM, Donohoe C, and Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. (2016) 6:2416.
Keywords: traditional medicine, anticancer herbs, phytochemicals, integrative oncology, herbal medicine, ethnomedicine
Citation: Nair PMK, Pandian AR, Mathapati V, B. ST, Sai A, Pai N, Sudarshan S, Mahalingam M and Silwal K (2025) Exploring the anticancer potential of traditional herbs from Tamil Nadu: a narrative review of ethnomedicinal insights and scientific evidence. Front. Immunol. 16:1680062. doi: 10.3389/fimmu.2025.1680062
Received: 05 August 2025; Accepted: 03 November 2025;
Published: 19 November 2025.
Edited by:
Mohamad Taleuzzaman, Maulana Azad University, IndiaReviewed by:
Kiran George, University of Oklahoma Health Sciences Center, United StatesZeena Pillai, Amrita Vishwa Vidyapeetham (Amritapuri Campus), India
Copyright © 2025 Nair, Pandian, Mathapati, B., Sai, Pai, Sudarshan, Mahalingam and Silwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep M. K. Nair, ZHJwcmFkZWVwMThibnlzQGdtYWlsLmNvbQ==
 Pradeep M. K. Nair
Pradeep M. K. Nair Ayswarya Rohini Pandian
Ayswarya Rohini Pandian Vaishali Mathapati
Vaishali Mathapati Shobhitha Tantry B.
Shobhitha Tantry B. Abhay Sai
Abhay Sai Navya Pai
Navya Pai Shanmugam Sudarshan
Shanmugam Sudarshan Manickam Mahalingam1
Manickam Mahalingam1 Karishma Silwal
Karishma Silwal