- 1Department of Breast Surgery, Honghezhou Third People’s Hospital, Honghezhou Cancer Hospital, Gejiu, China
- 2Charité-Universitätsmedizin Humboldt University, Berlin, Germany
- 3Department of Oncology, the Fifth Affiliated Hospital of Kunming Medical University, Gejiu, China
- 4Department of Oncology, the Sixth Affiliated Hospital of Kunming Medical University, YuXi, China
Oncolytic viruses (OVs) possess dual advantages in cancer immunotherapy: they selectively replicate within and lyse tumor cells while simultaneously releasing tumor-associated antigens to recruit and activate immune cells within the local tumor microenvironment (TME), thereby inducing robust and sustained antitumor immunity. Furthermore, these viruses can serve as tumor-targeting vectors for immunomodulation and synergize with other immunotherapeutic approaches. As such, oncolytic virotherapy holds significant potential to overcome the low response rates of breast cancer to existing immunotherapies and expand the therapeutic arsenal. This review systematically elucidates the application and mechanisms of this emerging immunotherapy in addressing the challenges of conventional breast cancer treatments. It also discusses engineering strategies to enhance antitumor immunity, highlights recent preclinical and clinical studies on rational combinations of OVs with other therapies, and outlines current challenges and future prospects.
Introduction
Breast cancer remains a leading cause of cancer-related mortality among women (1), Oncolytic virotherapy (OV), which combines the selective infection and destruction of cancer cells with the induction of adaptive immune responses against tumors, has emerged as a promising frontier in the fight against various cancers, including breast cancer (2–4). Additionally, the integration of OVs with other breast cancer treatments to leverage the strengths of each modality has garnered significant attention as a strategy to address tumor heterogeneity (5–7).
Mechanisms of oncolytic viruses in breast cancer
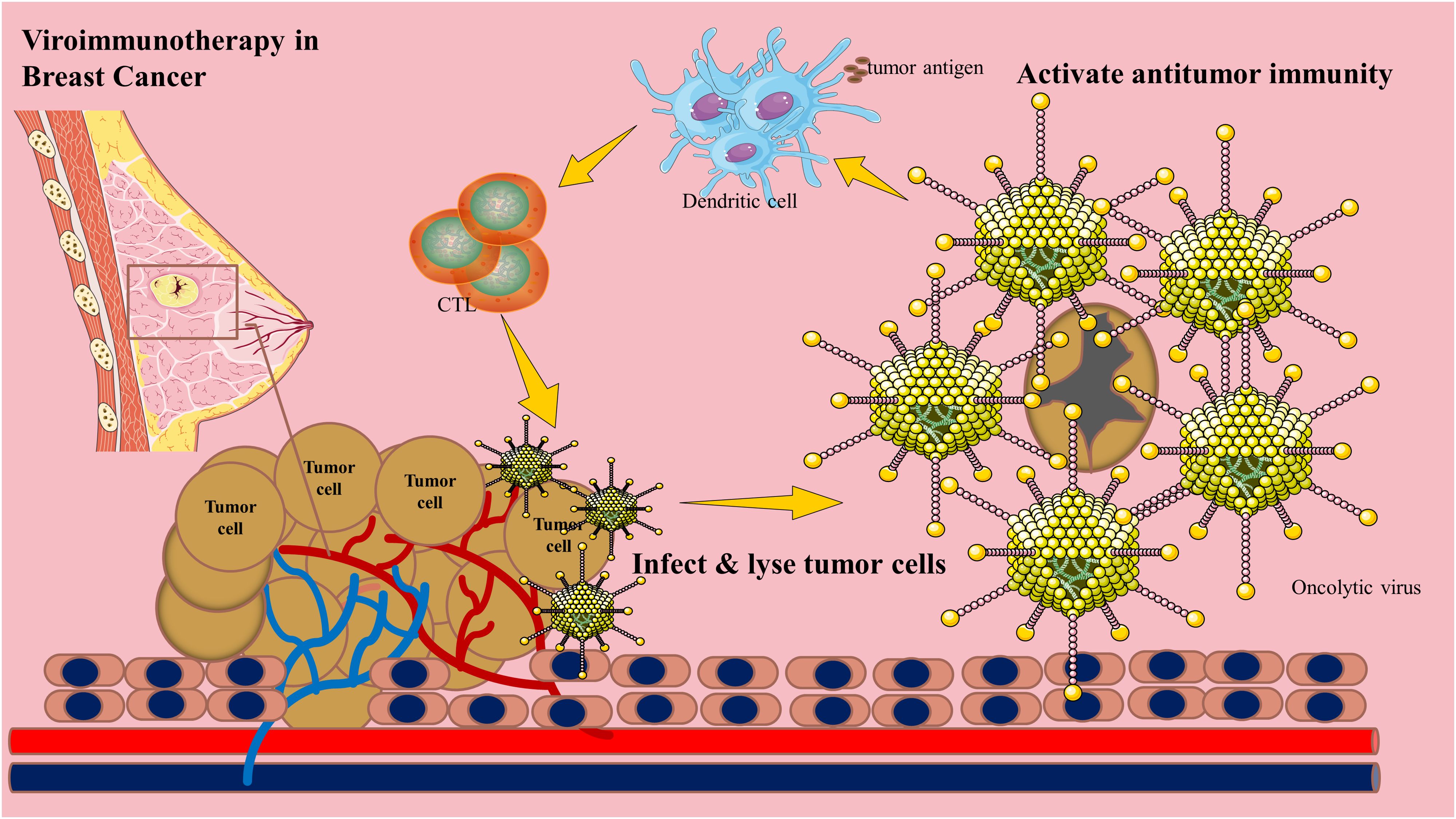
Oncolytic viruses are naturally occurring or genetically engineered immunotherapeutic agents that preferentially replicate in tumors, promoting immunogenic cell death. For instance, the oncolytic mumps virus exhibits potent cytotoxic activity against breast cancer xenografts, and oncolytic peptides demonstrate remarkable antimetastatic properties (8–10). Recombinant OVs have also shown efficacy in triple-negative breast cancer (TNBC) mouse models, a highly aggressive subtype with limited treatment options (11). Beyond direct tumor lysis, OVs primarily exert antitumor effects by activating immune responses (12) Figure 1.
The immunosuppressive tumor microenvironment (TME) is a major barrier to cancer immunotherapy (13). By recruiting inhibitory immune cells and upregulating immune checkpoint ligands, the TME fosters immunosuppression and restricts antitumor immunity, facilitating tumor progression and metastasis. OVs remodel the TME by inducing immunogenic cell death, disrupting the dense tumor stroma, and triggering the release of danger signals and tumor-associated antigens. This process attracts antigen-presenting cells, activates and expands lymphocyte populations, and enhances their infiltration into the tumor bed, transforming the TME from an immunologically “cold” to a “hot” state (14–17). T lymphocytes play a pivotal role in this transition.
Insufficient T cell activation limits the efficacy of immunotherapies. The oncolytic parapoxvirus ORFV and its derivatives induce pyroptosis in breast cancer cells and increase intratumoral cytotoxic T lymphocyte (CTL) populations (18, 19). Similarly, the oncolytic vaccinia virus CF33-hNIS-ΔF14.5 enhances CD8+ T cell infiltration in TNBC models (20). A virus-like nanoplatform (PolyIC@ZIF-8) degrades in the acidic TME, releasing PolyIC to induce apoptosis and promote T cell recruitment and activation in an antigen-dependent manner (21). Dendritic cell (DC) activation is also critical for OV-mediated antitumor immunity (22). A GFP-transgenic Newcastle disease virus (NDV-GFP) matures monocyte-derived DCs, priming antigen-specific T cell responses against breast cancer cells (23). Notably, combining high-dose vitamin C with oncolytic adenoviruses (oAds) amplifies T cell activation (24).
Natural killer (NK) cells also contribute to antitumor immunity. An oncolytic vesicular stomatitis virus (VSV)-based vaccine enhances NK cell activity and improves TNBC outcomes (25). OVs engineered to express CCL5 recruit NK cells to tumor sites, synergizing with NK cell-based therapies (26). Moreover, tumor-tropic NK cells can serve as carriers for systemic OV delivery (27) Combining NKT cell immunotherapy with engineered OVs further enhances tumor targeting (28).
Challenges and optimization strategies for oncolytic virotherapy in breast cancer
Limited tumor targeting after systemic administration
Enhancing OV specificity for breast cancer cells is a key research focus. For HER2-positive breast cancer, the HSV-based OV R-LM249 selectively infects and kills HER2-overexpressing cells (29) For HER2-negative tumors, mesothelin (MSLN) is a promising target (30). The recombinant measles virus rMV-SLAMblind suppresses Nectin-4-positive TNBC cells (31). Bispecific T cell engagers (BiTEs) can redirect T cells to tumor antigens, and OV-BiTE combinations represent a novel targeting strategy (32). For instance, a PD-L1-targeting BiTE-armed oHSV-1 selectively kills PD-L1+ tumor cells and macrophages while sparing T cells (33). Intratumoral OV delivery also minimizes off-target toxicity (34).
Host antiviral immune responses
Neutralizing antibodies pose a major challenge to systemic OV delivery. Strategies to evade or repurpose these antibodies are under investigation (35, 36). Antibody retargeting improves intratumoral adenovirus efficacy (37). Magnetic nanoparticles conjugated to HSV1716 shield the virus from neutralizing antibodies and enable magnetic tumor targeting (38). Mesenchymal stem cells (MSCs) serve as effective OV carriers, enhancing tumor delivery and infiltration (39, 40). A liposome-encapsulated NDV expressing MIP-3α stimulates antitumor immunity and inhibits angiogenesis (41). Exosome-based delivery systems also show promise in TNBC (42).
Engineering multifunctional OVs
Genetic modifications enhance OV specificity and potency. A miR-145/143-modified coxsackievirus B3 (miR-CVB3-1.1) selectively lyses breast cancer cells (43). Combining miR-CVB3 with CpG-melittin suppresses primary and metastatic tumor growth (44). The oncolytic adenovirus AdSVP-lncRNAi9 silences oncogenic miRNAs to inhibit TNBC proliferation and migration (45).
Immunomodulatory OVs amplify antitumor immunity. IL-21- or IL-23-armed vaccinia viruses induce potent immune responses (46, 47). A TK/N1L-deleted vaccinia virus (VVΔTKΔN1L) prevents postoperative recurrence and metastasis (48) TGF-β inhibition synergizes with OVs (49)., and deleting immune evasion genes enhances efficacy (50). Besides, OVs expressing PD-1/IL-12 remodel the immunosuppressive TME (51).
Immune checkpoint-armed OVs combine virotherapy with checkpoint blockade. An adenovirus suppressing PD-L1 improves checkpoint inhibitor safety and efficacy (52). A TIGIT-targeting scFv-armed vaccinia virus (VV-scFv-TIGIT) synergizes with PD-1 blockade (53). Another engineered VV-α-TIGIT enhances T cell recruitment and activation (54).
Combination therapies with oncolytic viruses
OVs are potent modulators of the TME, and their combination with immune checkpoint inhibitors (ICIs), CAR-T cells, or other immunotherapies represents a highly promising strategy (55–58). For instance, the combination of reovirus and CD3-bispecific antibodies enhances interferon-mediated responses and promotes T cell infiltration, leading to tumor regression in HER2+ breast cancer models (59). Additionally, genome-wide CRISPR-Cas9 screening has identified PARP1 as a key cellular factor that restricts viral replication; accordingly, PARP inhibition sensitizes TNBC to OV-ICI combination therapy (60) Neoadjuvant OV treatment has also been shown to improve surgical outcomes and reduce recurrence rates (61). It is critical to optimize dosing and timing in these combinational approaches, as the synergistic effects between OVs and ICIs are both dose- and schedule-dependent (62, 63) Therefore, establishing optimal dosing regimens and treatment sequences is paramount for the rational design of clinical trials investigating OV-based combination immunotherapies.
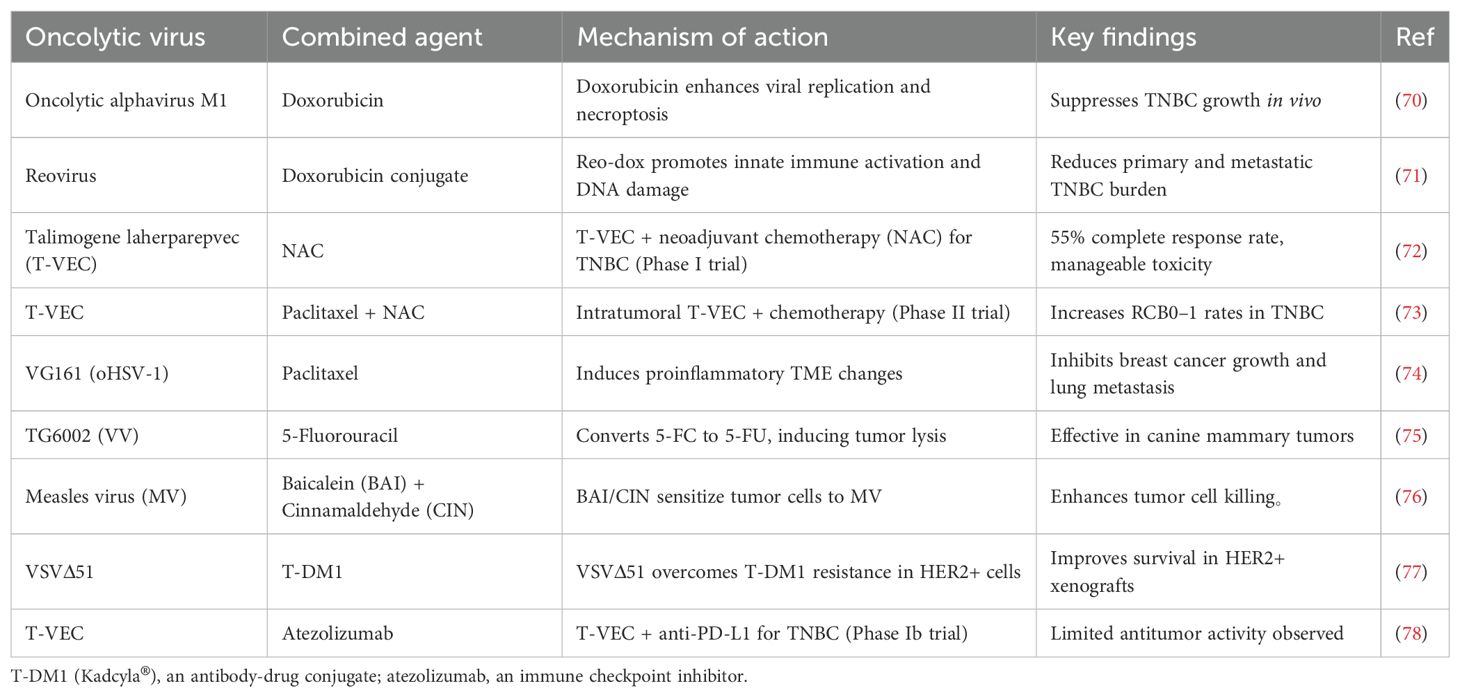
OVs also synergize with chemotherapy, radiotherapy, and targeted therapies (64). Table 1 Stereotactic body radiotherapy (SBRT) enhances OV-induced immunogenic cell death (65). Kinase inhibitors, such as BRAF inhibitors, improve OV efficacy (66, 67). Epigenetic modifiers like entinostat augment OV-IL-15 superagonist combinations (68, 69).
Development of potent oncolytic virus cancer vaccines
The development of robust oncolytic virus (OV)-based cancer vaccines relies on the rational design of tumor-selective viruses and the strategic exploitation of their immunostimulatory properties. Utilizing OVs as an adjuvant platform for therapeutic cancer vaccines is particularly attractive for personalized immunotherapy targeting patient-specific neoantigens (79, 80). High-throughput sequencing technologies can be leveraged to optimize viral design, modulate immune responses, and identify predictive biomarkers of clinical efficacy (81). Furthermore, direct imaging and automated analysis using tumor-on-chip systems have elucidated the cooperative antitumor activity between immune cells and oncolytic vaccinia virus, providing novel insights into the mechanisms of action of oncolytic vaccines (82).
Discussion
Oncolytic virotherapy represents a novel multimodal approach bridging virology, oncology, and immunology. While preclinical and clinical studies validate the antitumor effects of several OVs, their clinical translation faces challenges, including immune and physical barriers that limit intratumoral delivery, replication, and spread. Beyond improving OV bioavailability and efficacy, developing platforms that synergize with existing therapies is crucial. A deeper understanding of host-virus interactions, particularly in metabolically relevant models, will help bridge the gap between bench and bedside.
Author contributions
KX: Writing – original draft, Formal Analysis, Methodology, Data curation, Writing – review & editing, Software, Conceptualization. LZ: Writing – review & editing, Project administration, Resources. DJ: Writing – review & editing, Software, Investigation. PQ: Methodology, Writing – review & editing. ZL: Validation, Visualization, Writing – review & editing, Conceptualization, Supervision, Writing – original draft. ZF: Writing – original draft, Supervision, Writing – review & editing. SX: Data curation, Resources, Supervision, Writing – original draft, Formal Analysis, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Ma XY, Hill BD, Hoang T, and Wen F. Virus-inspired strategies for cancer therapy. Semin Cancer Biol. (2022) 86:1143–57. doi: 10.1016/j.semcancer.2021.06.021
3. Javanbakht M, Tahmasebzadeh S, Cegolon L, Gholami N, Kashaki M, Nikoueinejad H, et al. Oncolytic viruses: A novel treatment strategy for breast cancer. Genes Dis. (2023) 10:430–46. doi: 10.1016/j.gendis.2021.11.011
4. Li Z, Feiyue Z, Gaofeng L, and Haifeng L. Lung cancer and oncolytic virotherapy–enemy’s enemy. Transl Oncol. (2023) 27:101563. doi: 10.1016/j.tranon.2022.101563
5. Bahreyni A, Mohamud Y, and Luo H. Oncolytic virus-based combination therapy in breast cancer. Cancer Lett. (2024) 585:216634. doi: 10.1016/j.canlet.2024.216634
6. Lin W, Zhao Y, and Zhong L. Current strategies of virotherapy in clinical trials for cancer treatment. J Med Virol. (2021) 93:4668–92. doi: 10.1002/jmv.26947
7. de Graaf JF, Huberts M, Fouchier RAM, and van den Hoogen BG. Determinants of the efficacy of viro-immunotherapy: A review. Cytokine Growth Factor Rev. (2020) 56:124–32. doi: 10.1016/j.cytogfr.2020.07.001
8. Hartley A, Kavishwar G, Salvato I, and Marchini A. A roadmap for the success of oncolytic parvovirus-based anticancer therapies. Annu Rev Virol. (2020) 7:537–57. doi: 10.1146/annurev-virology-012220-023606
9. Behrens MD, Stiles RJ, Pike GM, Sikkink LA, Zhuang Y, Yu J, et al. Oncolytic Urabe mumps virus: A promising virotherapy for triple-negative breast cancer. Mol Ther Oncolytics. (2022) 27:239–55. doi: 10.1016/j.omto.2022.11.002
10. Tang T, Huang X, Zhang G, and Liang T. Oncolytic immunotherapy: multiple mechanisms of oncolytic peptides to confer anticancer immunity. J Immunother Cancer. (2022) 10:e005065. doi: 10.1136/jitc-2022-005065
11. Thomas RJ, Bartee MY, Valenzuela-Cardenas M, and Bartee E. Oncolytic myxoma virus is effective in murine models of triple negative breast cancer despite poor rates of infection. Mol Ther Oncolytics. (2023) 30:316–9. doi: 10.1016/j.omto.2023.08.014
12. Feola S, Russo S, Ylösmäki E, and Cerullo V. Oncolytic ImmunoViroTherapy: A long history of crosstalk between viruses and immune system for cancer treatment. Pharmacol Ther. (2022) 236:108103. doi: 10.1016/j.pharmthera.2021.108103
13. Hemminki O, Dos Santos JM, and Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. (2020) 13:84. doi: 10.1186/s13045-020-00922-1
14. Jamieson TR, Poutou J, and Ilkow CS. Redirecting oncolytic viruses: Engineering opportunists to take control of the tumour microenvironment. Cytokine Growth Factor Rev. (2020) 56:102–14. doi: 10.1016/j.cytogfr.2020.07.004
15. DePeaux K and Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. (2021) 21:785–97. doi: 10.1038/s41577-021-00541-y
16. Zhang B and Cheng P. Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy. Mol Cancer. (2020) 19:158. doi: 10.1186/s12943-020-01275-6
17. Pol JG, Workenhe ST, Konda P, Gujar S, and Kroemer G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. (2020) 56:4–27. doi: 10.1016/j.cytogfr.2020.10.007
18. Su W, Qiu W, Li SJ, Wang S, Xie J, Yang QC, et al. A dual-responsive STAT3 inhibitor nanoprodrug combined with oncolytic virus elicits synergistic antitumor immune responses by igniting pyroptosis. Adv Mater. (2023) 35:e2209379. doi: 10.1002/adma.202209379
19. Lin J, Sun S, Zhao K, Gao F, Wang R, Li Q, et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. (2023) 14:224. doi: 10.1038/s41467-023-35917-2
20. Chaurasiya S, Yang A, Kang S, Lu J, Kim SI, Park AK, et al. Oncolytic poxvirus CF33-hNIS-ΔF14.5 favorably modulates tumor immune microenvironment and works synergistically with anti-PD-L1 antibody in a triple-negative breast cancer model. Oncoimmunology. (2020) 9:1729300. doi: 10.1080/2162402X.2020.1729300
21. Wu F, Li Y, Meng Y, Cai X, Shi J, Li J, et al. An ion-enhanced oncolytic virus-like nanoparticle for tumor immunotherapy. Angew Chem Int Ed Engl. (2022) 61:e202210487.
22. Wang W, Liu S, Dai P, Yang N, Wang Y, Giese RA, et al. Elucidating mechanisms of antitumor immunity mediated by live oncolytic vaccinia and heat-inactivated vaccinia. J Immunother Cancer. (2021) 9:e002569. doi: 10.1136/jitc-2021-002569
23. Xu Q, Rangaswamy US, Wang W, Robbins SH, Harper J, Jin H, et al. Evaluation of Newcastle disease virus mediated dendritic cell activation and cross-priming tumor-specific immune responses ex vivo. Int J Cancer. (2020) 146:531–41. doi: 10.1002/ijc.32694
24. Ma J, Zhang C, Shi G, Yue D, Shu Y, Hu S, et al. High-dose VitC plus oncolytic adenoviruses enhance immunogenic tumor cell death and reprogram tumor immune microenvironment. Mol Ther. (2022) 30:644–61. doi: 10.1016/j.ymthe.2021.09.015
25. Niavarani SR, Lawson C, Boudaud M, Simard C, and Tai LH. Oncolytic vesicular stomatitis virus-based cellular vaccine improves triple-negative breast cancer outcome by enhancing natural killer and CD8(+) T-cell functionality. J Immunother Cancer. (2020) 8:e000465. doi: 10.1136/jitc-2019-000465
26. Li F, Sheng Y, Hou W, Sampath P, Byrd D, Thorne S, et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J Immunother Cancer. (2020) 8:e000131. doi: 10.1136/jitc-2019-000131
27. Ding L, Gao Q, Xu Z, Cai L, Chen S, Zhang X, et al. An inter-supplementary biohybrid system based on natural killer cells for the combinational immunotherapy and virotherapy of cancer. Adv Sci (Weinh). (2022) 9:e2103470. doi: 10.1002/advs.202103470
28. Nelson A, McMullen N, Gebremeskel S, De Antueno R, Mackenzie D, Duncan R, et al. Fusogenic vesicular stomatitis virus combined with natural killer T cell immunotherapy controls metastatic breast cancer. Breast Cancer Res. (2024) 26:78. doi: 10.1186/s13058-024-01818-5
29. Nanni P, Gatta V, Menotti L, De Giovanni C, Ianzano M, Palladini A, et al. Preclinical therapy of disseminated HER-2+ ovarian and breast carcinomas with a HER-2-retargeted oncolytic herpesvirus. PLoS Pathog. (2013) 9:e1003155. doi: 10.1371/journal.ppat.1003155
30. Froechlich G, Gentile C, Infante L, Caiazza C, Pagano P, Scatigna S, et al. Generation of a novel mesothelin-targeted oncolytic herpes virus and implemented strategies for manufacturing. Int J Mol Sci. (2021) 22:477. doi: 10.3390/ijms22020477
31. Fujiyuki T, Amagai Y, Shoji K, Kuraishi T, Sugai A, Awano M, et al. Recombinant SLAMblind measles virus is a promising candidate for nectin-4-positive triple negative breast cancer therapy. Mol Ther Oncolytics. (2020) 19:127–35. doi: 10.1016/j.omto.2020.09.007
32. Heidbuechel JPW and Engeland CE. Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J Hematol Oncol. (2021) 14:63. doi: 10.1186/s13045-021-01075-5
33. Khalique H, Baugh R, Dyer A, Scott EM, Frost S, Larkin S, et al. Oncolytic herpesvirus expressing PD-L1 BiTE for cancer therapy: exploiting tumor immune suppression as an opportunity for targeted immunotherapy. J Immunother Cancer. (2021) 9:e001292. doi: 10.1136/jitc-2020-001292
34. Hong WX, Haebe S, Lee AS, Westphalen CB, Norton JA, Jiang W, et al. Intratumoral immunotherapy for early-stage solid tumors. Clin Cancer Res. (2020) 26:3091–9. doi: 10.1158/1078-0432.CCR-19-3642
35. Ma R, Li Z, Chiocca EA, Caligiuri MA, and Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. (2023) 9:122–39. doi: 10.1016/j.trecan.2022.10.003
36. Shin DH, Nguyen T, Ozpolat B, Lang F, Alonso M, Gomez-Manzano C, et al. Current strategies to circumvent the antiviral immunity to optimize cancer virotherapy. J Immunother Cancer. (2021) 9:e002086. doi: 10.1136/jitc-2020-002086
37. Niemann J, Woller N, Brooks J, Fleischmann-Mundt B, Martin NT, Kloos A, et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy. Nat Commun. (2019) 10:3236. doi: 10.1038/s41467-019-11137-5
38. Howard FHN, Al-Janabi H, Patel P, Cox K, Smith E, Vadakekolathu J, et al. Nanobugs as drugs: bacterial derived nanomagnets enhance tumor targeting and oncolytic activity of HSV-1 virus. Small. (2022) 18:e2104763. doi: 10.1002/smll.202104763
39. Moreno R. Mesenchymal stem cells and oncolytic viruses: joining forces against cancer. J Immunother Cancer. (2021) 9:e001684. doi: 10.1136/jitc-2020-001684
40. Seyed-Khorrami SM, Soleimanjahi H, Soudi S, and Habibian A. MSCs loaded with oncolytic reovirus: migration and in vivo virus delivery potential for evaluating anti-cancer effect in tumor-bearing C57BL/6 mice. Cancer Cell Int. (2021) 21:244. doi: 10.1186/s12935-021-01848-5
41. Wang JY, Chen H, Dai SZ, Huang FY, Lin YY, Wang CC, et al. Immunotherapy combining tumor and endothelium cell lysis with immune enforcement by recombinant MIP-3α Newcastle disease virus in a vessel-targeting liposome enhances antitumor immunity. J Immunother Cancer. (2022) 10:e003950. doi: 10.1136/jitc-2021-003950
42. Zhang C, Tang S, Wang M, Li L, Li J, Wang D, et al. Triple-punch” Strategy exosome-mimetic nanovesicles for triple negative breast cancer therapy. ACS Nano. (2024), 3c10568. doi: 10.1021/acsnano.3c10568
43. Bahreyni A, Liu H, Mohamud Y, Xue YC, Zhang J, and Luo H. A new miRNA-Modified coxsackievirus B3 inhibits triple negative breast cancer growth with improved safety profile in immunocompetent mice. Cancer Lett. (2022) 548:215849. doi: 10.1016/j.canlet.2022.215849
44. Bahreyni A, Liu H, Mohamud Y, Xue YC, Fan YM, Zhang YL, et al. A combination of genetically engineered oncolytic virus and melittin-CpG for cancer viro-chemo-immunotherapy. BMC Med. (2023) 21:193. doi: 10.1186/s12916-023-02901-y
45. Ang L, Guo L, Wang J, Huang J, Lou X, and Zhao M. Oncolytic virotherapy armed with an engineered interfering lncRNA exhibits antitumor activity by blocking the epithelial mesenchymal transition in triple-negative breast cancer. Cancer Lett. (2020) 479:42–53. doi: 10.1016/j.canlet.2020.03.012
46. Chen T, Ding X, Liao Q, Gao N, Chen Y, Zhao C, et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J Immunother Cancer. (2021) 9:e001647. doi: 10.1136/jitc-2020-001647
47. Chen L, Chen H, Ye J, Ge Y, Wang H, Dai E, et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics. (2021) 11:6668–81. doi: 10.7150/thno.56494
48. Ahmed J, Chard LS, Yuan M, Wang J, Howells A, Li Y, et al. A new oncolytic Vacciniavirus augments antitumor immune responses to prevent tumor recurrence and metastasis after surgery. J Immunother Cancer. (2020) 8:e000415.
49. Groeneveldt C, van Hall T, van der Burg SH, Ten Dijke P, and van Montfoort N. Immunotherapeutic potential of TGF-β Inhibition and oncolytic viruses. Trends Immunol. (2020) 41:406–20. doi: 10.1016/j.it.2020.03.003
50. Umer BA, Noyce RS, Franczak BC, Shenouda MM, Kelly RG, Favis NA, et al. Deciphering the immunomodulatory capacity of oncolytic vaccinia virus to enhance the immune response to breast cancer. Cancer Immunol Res. (2020) 8:618–31. doi: 10.1158/2326-6066.CIR-19-0703
51. Valenzuela-Cardenas M, Gowan C, Dryja P, Bartee MY, and Bartee E. TNF blockade enhances the efficacy of myxoma virus-based oncolytic virotherapy. J Immunother Cancer. (2022) 10:e004770. doi: 10.1136/jitc-2022-004770
52. Hamdan F, Ylösmäki E, Chiaro J, Giannoula Y, Long M, Fusciello M, et al. Novel oncolytic adenovirus expressing enhanced cross-hybrid IgGA Fc PD-L1 inhibitor activates multiple immune effector populations leading to enhanced tumor killing in vitro, in vivo and with patient-derived tumor organoids. J Immunother Cancer. (2021) 9:e003000. doi: 10.1136/jitc-2021-003000
53. Zuo S, Wei M, Xu T, Kong L, He B, Wang S, et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J Immunother Cancer. (2021) 9:e002843. doi: 10.1136/jitc-2021-002843
54. Zuo S, Wei M, He B, Chen A, Wang S, Kong L, et al. Enhanced antitumor efficacy of a novel oncolytic vaccinia virus encoding a fully monoclonal antibody against T-cell immunoglobulin and ITIM domain (TIGIT). EBioMedicine. (2021) 64:103240. doi: 10.1016/j.ebiom.2021.103240
55. Zhu Z, McGray AJR, Jiang W, Lu B, Kalinski P, and Guo ZS. Improving cancer immunotherapy by rationally combining oncolytic virus with modulators targeting key signaling pathways. Mol Cancer. (2022) 21:196. doi: 10.1186/s12943-022-01664-z
56. Melcher A, Harrington K, and Vile R. Oncolytic virotherapy as immunotherapy. Science. (2021) 374:1325–6. doi: 10.1126/science.abk3436
57. Tian Y, Xie D, and Yang L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct Target Ther. (2022) 7:117. doi: 10.1038/s41392-022-00951-x
58. Kanaya N, Kuroda S, Kakiuchi Y, Kumon K, Tsumura T, Hashimoto M, et al. Immune modulation by telomerase-specific oncolytic adenovirus synergistically enhances antitumor efficacy with anti-PD1 antibody. Mol Ther. (2020) 28:794–804. doi: 10.1016/j.ymthe.2020.01.003
59. Groeneveldt C, Kinderman P, van den Wollenberg DJM, van den Oever RL, Middelburg J, Mustafa DAM, et al. Preconditioning of the tumor microenvironment with oncolytic reovirus converts CD3-bispecific antibody treatment into effective immunotherapy. J Immunother Cancer. (2020) 8:e001191. doi: 10.1136/jitc-2020-001191
60. Zhong Y, Le H, Zhang X, Dai Y, Guo F, Ran X, et al. Identification of restrictive molecules involved in oncolytic virotherapy using genome-wide CRISPR screening. J Hematol Oncol. (2024) 17:36. doi: 10.1186/s13045-024-01554-5
61. Mullins-Dansereau V, Petrazzo G, Geoffroy K, Béland D, and Bourgeois-Daigneault MC. Pre-surgical oncolytic virotherapy improves breast cancer outcomes. Oncoimmunology. (2019) 8:e1655363. doi: 10.1080/2162402X.2019.1655363
62. Flores EB, Aksoy BA, and Bartee E. Initial dose of oncolytic myxoma virus programs durable antitumor immunity independent of in vivo viral replication. J Immunother Cancer. (2020) 8:e000804. doi: 10.1136/jitc-2020-000804
63. Nguyen HM, Bommareddy PK, Silk AW, and Saha D. Optimal timing of PD-1 blockade in combination with oncolytic virus therapy. Semin Cancer Biol. (2022) 86:971–80. doi: 10.1016/j.semcancer.2021.05.019
64. Roulstone V, Mansfield D, Harris RJ, Twigger K, White C, de Bono J, et al. Antiviral antibody responses to systemic administration of an oncolytic RNA virus: the impact of standard concomitant anticancer chemotherapies. J Immunother Cancer. (2021) 9:e002673. doi: 10.1136/jitc-2021-002673
65. Chen WY, Chen YL, Lin HW, Chang CF, Huang BS, Sun WZ, et al. Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and DAMPs. Cancer Lett. (2021) 523:149–61. doi: 10.1016/j.canlet.2021.09.040
66. Gilchrist VH, Jémus-Gonzalez E, Said A, and Alain T. Kinase inhibitors with viral oncolysis: Unmasking pharmacoviral approaches for cancer therapy. Cytokine Growth Factor Rev. (2020) 56:83–93. doi: 10.1016/j.cytogfr.2020.07.008
67. Crespo-Rodriguez E, Bergerhoff K, Bozhanova G, Foo S, Patin EC, Whittock H, et al. Combining BRAF inhibition with oncolytic herpes simplex virus enhances the immune-mediated antitumor therapy of BRAF-mutant thyroid cancer. J Immunother Cancer. (2020) 8:e000698. doi: 10.1136/jitc-2020-000698
68. Murphy SA, Mapes NJ Jr., Dua D, and Kaur B. Histone modifiers at the crossroads of oncolytic and oncogenic viruses. Mol Ther. (2022) 30:2153–62. doi: 10.1016/j.ymthe.2022.02.006
69. Hicks KC, Knudson KM, Lee KL, Hamilton DH, Hodge JW, Figg WD, et al. Cooperative immune-mediated mechanisms of the HDAC inhibitor entinostat, an IL15 superagonist, and a cancer vaccine effectively synergize as a novel cancer therapy. Clin Cancer Res. (2020) 26:704–16. doi: 10.1158/1078-0432.CCR-19-0727
70. Zhang J, Liu Y, Tan J, Zhang Y, Wong CW, Lin Z, et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene. (2021) 40:4783–95. doi: 10.1038/s41388-021-01869-4
71. Berry JTL, Muñoz LE, Rodríguez Stewart RM, Selvaraj P, and Mainou BA. Doxorubicin conjugation to reovirus improves oncolytic efficacy in triple-negative breast cancer. Mol Ther Oncolytics. (2020) 18:556–72. doi: 10.1016/j.omto.2020.08.008
72. Soliman H, Hogue D, Han H, Mooney B, Costa R, Lee MC, et al. A phase I trial of talimogene laherparepvec in combination with neoadjuvant chemotherapy for the treatment of nonmetastatic triple-negative breast cancer. Clin Cancer Res. (2021) 27:1012–8. doi: 10.1158/1078-0432.CCR-20-3105
73. Soliman H, Hogue D, Han H, Mooney B, Costa R, Lee MC, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat Med. (2023) 29:450–7. doi: 10.1038/s41591-023-02309-4
74. Deng X, Shen Y, Yi M, Zhang C, Zhao B, Zhong G, et al. Combination of novel oncolytic herpesvirus with paclitaxel as an efficient strategy for breast cancer therapy. J Med Virol. (2023) 95:e28768. doi: 10.1002/jmv.28768
75. Béguin J, Foloppe J, Maurey C, Laloy E, Hortelano J, Nourtier V, et al. Preclinical evaluation of the oncolytic vaccinia virus TG6002 by translational research on canine breast cancer. Mol Ther Oncolytics. (2020) 19:57–66. doi: 10.1016/j.omto.2020.08.020
76. Kuo YT, Liu CH, Wong SH, Pan YC, and Lin LT. Small molecules baicalein and cinnamaldehyde are potentiators of measles virus-induced breast cancer oncolysis. Phytomedicine. (2021) 89:153611. doi: 10.1016/j.phymed.2021.153611
77. Arulanandam R, Taha Z, Garcia V, Selman M, Chen A, Varette O, et al. The strategic combination of trastuzumab emtansine with oncolytic rhabdoviruses leads to therapeutic synergy. Commun Biol. (2020) 3:254. doi: 10.1038/s42003-020-0972-7
78. Hecht JR, Raman SS, Chan A, Kalinsky K, Baurain JF, Jimenez MM, et al. Phase Ib study of talimogene laherparepvec in combination with atezolizumab in patients with triple negative breast cancer and colorectal cancer with liver metastases. ESMO Open. (2023) 8:100884. doi: 10.1016/j.esmoop.2023.100884
79. Dyer A, Frost S, Fisher KD, and Seymour LW. The role of cancer metabolism in defining the success of oncolytic viro-immunotherapy. Cytokine Growth Factor Rev. (2020) 56:115–23. doi: 10.1016/j.cytogfr.2020.07.006
80. Roy DG, Geoffroy K, Marguerie M, Khan ST, Martin NT, Kmiecik J, et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nat Commun. (2021) 12:2626. doi: 10.1038/s41467-021-22929-z
81. Naik S and Russell L. The 13(th) International Oncolytic Virus Conference: Powerful payloads gain clinical momentum. Mol Ther. (2022) 30:1361–3. doi: 10.1016/j.ymthe.2022.03.010
Keywords: breast cancer, oncolytic virus, tumor microenvironment, cancer vaccines, combination therapy
Citation: Xianshu K, Zhonghua L, Junyu D, Qing P, Li Z, Feiyue Z and Xuqing S (2025) Harnessing oncolytic viruses to overcome immunosuppression in breast cancer: from mechanisms to clinical translation. Front. Immunol. 16:1687190. doi: 10.3389/fimmu.2025.1687190
Received: 17 August 2025; Accepted: 29 August 2025;
Published: 10 September 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Mohammed Alahmadi, King Fahad Hospital-Medina, Saudi ArabiaCopyright © 2025 Xianshu, Zhonghua, Junyu, Qing, Li, Feiyue and Xuqing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Li, ZHJlYW1tYWtlci1saUBvdXRsb29rLmNvbQ==; Zhang Feiyue, ZmVpeXVlMDExOEAxMjYuY29t; Su Xuqing, c3V4dXFpbmdAeWVhaC5uZXQ=
 Kong Xianshu1
Kong Xianshu1 Zhang Li
Zhang Li