- 1Redox Biology & Proteomics Laboratory, Department of Zoology, Ravenshaw University, Cuttack, India
- 2Center of Excellence in Environment & Public Health, Ravenshaw University, Cuttack, India
Cancer remains the leading cause of death worldwide. Despite decades of continuous research, limitations persist in existing therapeutic approaches. Conventional strategies such as surgery, chemotherapy, and radiotherapy, though advanced, face challenges including poor bioavailability, toxic side effects, inadequate targeting of cancer cells, and limited survival benefits. The major issue lies in the inability of improved drug formulations to effectively reach cancer cells. Emerging approaches such as photodynamic therapy (PDT) and immunotherapy have shown greater promise, offering reduced side effects and higher treatment efficiency compared to traditional methods. Various natural and synthetic nanocarriers, including exosomes, liposomes, solid lipid nanoparticles (SLNs) and micelles have been explored as drug delivery vehicles in these therapies. Among them, exosomes, being natural secretory vesicles, have shown unique potential as independent delivery systems. However, challenges and limitations remain in their application for precise cancer targeting. A combinational strategy, integrating exosomes with other lipid-based drug delivery systems (LBDDS), while preserving their intrinsic properties and engineering their surface to carry photosensitizers (PS) or immune modulators, could overcome these barriers. Such well-designed natural cargos may enhance therapeutic efficacy, modulate the tumor microenvironment, and address current shortcomings in cancer therapy. This review highlights the individual applications of PDT and immunotherapy using exosomes and LBDDS, and explores their potential synergistic use for more effective and targeted cancer treatment.
1 Introduction
Cancer remains the leading cause of death worldwide, significantly impacting human health and well-being. While traditional treatment strategies, such as surgery, chemotherapy, and radiotherapy, have advanced over the years, they often come with severe side effects, poor tumour targeting, and limited survival rates (1). These limitations have driven the exploration of more modern treatment approaches, including photodynamic therapy (PDT) and immunotherapy, both of which show considerable promise in enhancing treatment efficacy while minimizing adverse effects.
Photodynamic therapy (PDT) has made notable progress, particularly in treating solid tumours. Photodynamic therapy (PDT) involves the administration of a photosensitizer followed by localized irradiation with light of a specific wavelength, generating reactive oxygen species (ROS) that selectively induce cytotoxicity in targeted cells (2–5). These ROS cause apoptosis, DNA damage, and immune responses at the tumour site. However, the clinical application of PDT faces several obstacles, such as poor solubility, aggregation, and off-target effects of PSs. Therefore, developing efficient and safe drug delivery platforms is crucial (6). Despite its potential, PDT is still challenged by off-target effects, necessitating the development of optimized delivery systems that ensure precise tumour targeting while minimizing unwanted impacts.
Immunotherapy, which harnesses the body’s immune system, often in combination with monoclonal antibodies, has emerged as a promising cancer treatment. By stimulating the immune response, immunotherapy aims to target and eliminate cancer cells. However, its therapeutic potential is limited by challenges such as off-target delivery, immune tolerance induction, and immune evasion by tumours (7, 8). Additionally, the hypoxic and immunosuppressive characteristics of the tumour microenvironment (TME) further reduce the efficacy of these therapies (8). The TME’s ability to induce immune tolerance and evade immune surveillance poses significant barriers to effective immunotherapy, making it imperative to overcome these obstacles to improve cancer treatment outcomes.
Exosomes, naturally occurring extracellular vesicles (EVs) with liposome-like bilayer structures, have shown great promise in cancer therapy due to their prolonged circulation time, immune system evasion, and tumour-homing capabilities. These properties make exosomes ideal candidates for targeted drug delivery (6). Moreover, exosome-based cancer immunotherapy has emerged as a promising strategy to combat the immunosuppressive TME, engage immune checkpoint blockades, and deliver cancer vaccines (9).
In recent years, biomimetic drug delivery systems (BDDSs), such as lipid-based nanocarriers (liposomes, nano-emulsions, solid lipid nanoparticles, nanostructured lipid carriers, and lipid-polymer hybrid nanoparticles), have attracted significant attention for their ability to deliver therapeutic agents including PSs with greater precision and efficiency. Lipid-based systems are particularly appealing due to their enhanced biocompatibility, solubility, and permeability (10, 11). These systems improve the bioavailability of hydrophobic and lipophilic drugs, making them versatile in delivering both hydrophobic and hydrophilic compounds (12, 13). Despite these advancements, precision targeting of tumour cells while minimizing systemic side effects remains an active area of research. Although preclinical studies have demonstrated the promise of exosome and lipid-based systems, their clinical translation is limited by challenges related to production, scalability, and stability. Addressing these challenges is crucial for unlocking the full potential of these systems in clinical cancer treatment.
This review explores the application of exosomes and lipid-based systems for the delivery of therapeutic agents in PDT and immunotherapy. It examines the potential advantages of these innovative drug delivery platforms, identifies current limitations, and outlines promising future directions to overcome these challenges in cancer treatment.
2 Exosomes as drug delivery vehicles
Exosomes are a specific type of nanosized extracellular lipid bilayer membrane vesicles secreted by almost all cell types and play a pivotal role in intercellular communication (Figure 1) (9, 14). In 1990s from immunological studies by Raposo et al. (1996) the role of exosomes in adaptive immunity was established by demonstrating secretion of exosomes B lymphocytes which are capable of antigen presentation to T cells, carrying functional MHC class II molecules (15). Shortly thereafter, another report by Zitvogel et al. (1998) exhibited that dendritic cell-derived exosomes could prime cytotoxic T lymphocytes and eradicate established murine tumors, thereby introducing exosomes as a novel platform for cancer immunotherapy (16). Building on these discoveries of exosomes, their therapeutic potential as a drug delivery vehicle was later established by Alvarez-Erviti et al. (2011), who commenced targeted exosome engineering to deliver siRNA systemically across the blood–brain barrier. All together, these pioneering studies laid the foundation for the broad exploration of exosomes in immunotherapy, oncology, and nanomedicine (17).
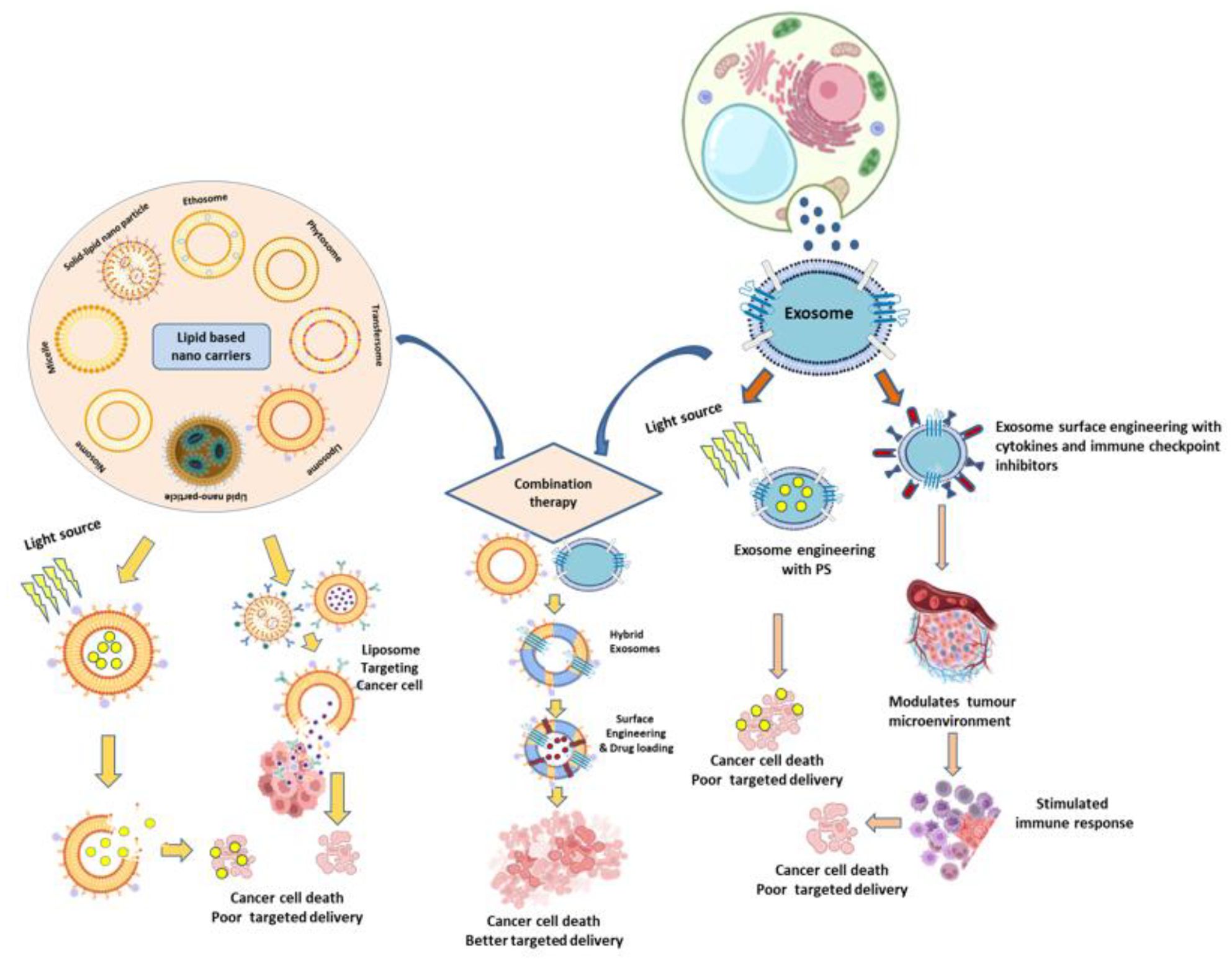
Figure 1. Schematic representation of combined exosome and lipid-based nanocarrier strategies for cancer therapy, integrating photodynamic therapy (PDT) and immunotherapy to achieve improved therapeutic efficacy compared to conventional treatments.
2.1 Biogenesis and characteristics
Exosomes, typically ranging in size from ~30 to 150 nm, are characterized by their ability to encapsulate various biological molecules, including nucleic acids and proteins, within their lumen or lipid bilayer. These vesicles are released from cells under both physiological and pathological conditions to facilitate cell-to-cell communication and enable cargo transport both in vivo and in vitro. The biogenesis of exosomes occurs either constitutively or can be induced by external stimuli, originating from endosomal compartments. Endosomes give rise to three primary types of vesicular structures: macrovesicles (50–1000 nm in diameter), which are shed from the cell membrane through membrane remodelling and outward blebbing; apoptotic blebs (100–5000 nm), which emerge from dying cells during the final stages of apoptosis; and exosomes (30–150 nm, though size can vary across studies), which are released from multi-vesicular bodies (18).
2.2 Exosomes in photodynamic therapy
Photodynamic therapy (PDT) employs photosensitizers (PSs) that, when activated by a specific wavelength of light in the presence of oxygen (O2), produce cytotoxic free radicals and ROS to destroy cancer cells (19, 20). However, free PSs in PDT often face limitations such as poor water solubility, photostability, aggregation, and off-target effects, which restrict their clinical applications (21, 22). Exosomes offer a promising solution by enhancing PS delivery to tumour cells, improving accumulation, and reducing systemic toxicity (23). Additionally, novel PDT strategies with PS agents can be developed via aggregation-induced emission (AIE) properties through ROS generation and tumour-targeted phototherapy (24, 25). Studies have demonstrated that the acidic tumour microenvironment, combined with laser irradiation and exosome-based carriers such as PMA/Au BSA@Ce6 or ChiP-Exo, can significantly enhance PDT efficacy via dual-stage light-directed subcellular destruction (26). The Exo-PMA/Au-BSA@Ce6 system is an advanced, exosome-based nanoplatform that elegantly combines enhanced tumor-targeted delivery, real-time fluorescence imaging, and potent photodynamic therapy. PMA/Au-BSA@Ce6 nanoparticles accommodate an amphiphilic polymer (PMA), ultra-small gold nanoparticles (Au), bovine serum albumin (BSA), and the photosensitizer chlorin e6 (Ce6) which were loaded into urinary exosomes via an instant electroporation technique, creating the hybrid Exo-PMA/Au-BSA@Ce6 nanovehicles. This nanoparticle structures got collapsed and released inside cancer cells under 633nm laser irradiation and acidic condition, producing considerable singlet oxygen, effectively inhibiting growth of tumor cells (27). Although photosensitizer-induced reactive oxygen species (ROS) are cytotoxic, their short lifespan and limited diffusion restrict the overall anti-tumor efficacy. To overcome this limitation, Zhao et al. (2021) developed a nucleus-targeted exosome engineered with a chimeric peptide (ChiP-Exo) to enhance photodynamic therapy (PDT). Using a dual-stage light strategy, they achieved sequential plasma membrane and nuclear degradation in cancer cells. This approach enabled in situ ROS activation at the nuclear level, leading to effective nuclei disruption, inhibition of tumor growth, and reduced systemic toxicity (28). ChiP-Exo’s plasma membrane-targeted PDT, for example, can degrade membrane structures, induce photochemical internalization (PCI), and promote lysosomal escape (26). Natural PSs like hypericin from Hypericum perforatum have also been investigated; to address their poor solubility, high lipophilicity, instability, and production cost, H. perforatum-derived exosome-like nanovesicles (HPDENs) have been introduced as an innovative PS platform for PDT (26). Furthermore, encapsulation within exosomes protects PSs from degradation, improving photostability and therapeutic efficiency (23). While exogenous nanocarriers such as hyaluronic acid, polydopamine, and chitosan have been explored to encapsulate PSs, they often face immune system clearance (26), whereas engineered exosomes loaded with PSs demonstrate low immunogenicity, high biocompatibility, and enhanced blood circulation, thereby improving PDT performance (23). To overcome challenges such as poor tumour targeting and limited tissue penetration of light, orchestrated nanoplatforms of indocyanine green (ICG) have been developed to improve bioavailability and tumour specificity in PDT (29). For instance, a novel bio-nanoplatform was developed by integrating edible ginger-derived exosome-like nanoparticles (GDNPs) with the photosensitizer indocyanine green (ICG), forming GDNPs@ICG. These nanoparticles were internalized by tumor cells through a lipid-dependent pathway. Upon 808 nm near-infrared (NIR) laser irradiation, GDNPs@ICG generated high levels of ROS, malondialdehyde (MDA), and local hyperthermia within the tumor, leading to lipid peroxidation and endoplasmic reticulum (ER) stress, thereby enhancing the efficacy of photo-mediated breast tumor therapy. Expression analyses of biomarkers such as CD31, N-cadherin, IL-6, IFN-γ, CD8, p16, p21, and p53 further demonstrated that GDNPs@ICG effectively reduced angiogenesis, suppressed metastasis, activated anti-tumor immune responses, and promoted tumor cell senescence (30). In another study, melanoma-derived exosomes were employed to design perfluorocarbon (PFC)-based drug nanocarriers co-loaded with ICG and camptothecin (CPT) (ICFESs), enabling targeted photochemotherapy (31). Similarly, a combinational therapeutic strategy was reported using tumor exosome-based nanoparticles co-formulated with ICG and the tyrosine kinase inhibitor gefitinib (IG@EXOs). This approach demonstrated enhanced antitumor efficacy against oral squamous cell carcinoma (OSCC) through synergistic phototherapy and molecularly targeted therapy (32).
Additionally, the synthesis of organic PSs capable of generating ROS from intrinsic non-photosensitizer fluorophores upon light irradiation is an emerging approach for effective cancer treatment (33). Addressing melanin interference in PDT, coordination-driven assembly of Ir (III) complex PSs with Fe (III) ions into nanopolymers, camouflaged with exosomes, has been shown to eradicate melanoma tumours and inhibit metastasis formation in mouse models (34).
2.3 Exosomes in immunotherapy
Immunotherapy leverages the immune system to selectively eradicate cancer cells and offers advantages over conventional treatments, which often damage healthy tissues and promote drug resistance (35, 36). Exosome-based immunotherapy is emerging as a promising alternative due to its ability to deliver tumour-associated antigens, immune checkpoint inhibitors (ICIs), and immunomodulatory molecules with high specificity and low immunogenicity (9) (Figure 2). Nasopharyngeal carcinoma (NPC), a malignancy prevalent in Southeast Asia, is often diagnosed late and exhibits high recurrence and metastatic rates, compounded by resistance to chemo-radiotherapy and limited responses to immune checkpoint inhibitors due to T cell exhaustion and an immunosuppressive tumor microenvironment (TME). Exosomes, bilayered vesicles of 30–150 nm, play crucial roles in cell–cell communication within the TME, and tumor-derived exosomes (TEX) in NPC have been linked to angiogenesis, metastasis, and therapeutic resistance, though their role in immune evasion remains underexplored; importantly, their detectability in body fluids highlights their potential as biomarkers for early diagnosis and prognostication (37). Beyond NPC, exosomes are increasingly investigated as therapeutic platforms, such as in genetically engineered tumor cell-derived exosomes co-delivering endogenous tumor antigens and immunostimulatory CpG DNA, which enhanced dendritic cell activation and elicited robust antitumor immunity in murine melanoma models (38). Similarly, their unique lipid–protein composition and natural role in genetic material transport position exosomes as promising low-toxicity, high-efficiency vectors for gene therapy, although further work is required to optimize targeting and cargo loading (39). Moreover, innovations such as dendritic cell-mimicking nanovaccines (HybridDC), engineered with tumor-associated exosomes, costimulatory molecules, and CCR7, have demonstrated superior antigen delivery, improved lymph node targeting, and synergy with immune checkpoint blockade in glioma models, underscoring the potential of exosome-based strategies to reshape the immune landscape and enhance personalized cancer immunotherapies (40). Engineered exosomes, such as GEMINI-Exos armed with anti-CD3, anti-EGFR, PD-1, and OX40L, have demonstrated significant inhibition of triple-negative breast cancer in mice (41), while surface modifications like PEGylation or CD47 overexpression enhance circulation and tumour targeting (26). SMART-Exos displaying bispecific antibodies (anti-CD3/anti-EGFR or anti-CD3/anti-HER2) enable simultaneous T cell activation and redirection toward tumour cells, and CD40L-expressing exosomes further boost dendritic cell maturation and cytokine secretion (42). By overcoming tumour immune escape mechanisms and enabling precise modulation of the tumour microenvironment, exosome-based strategies including antigen delivery, immune checkpoint blockade, and TME normalization hold transformative potential for next-generation cancer immunotherapy (Figure 1).
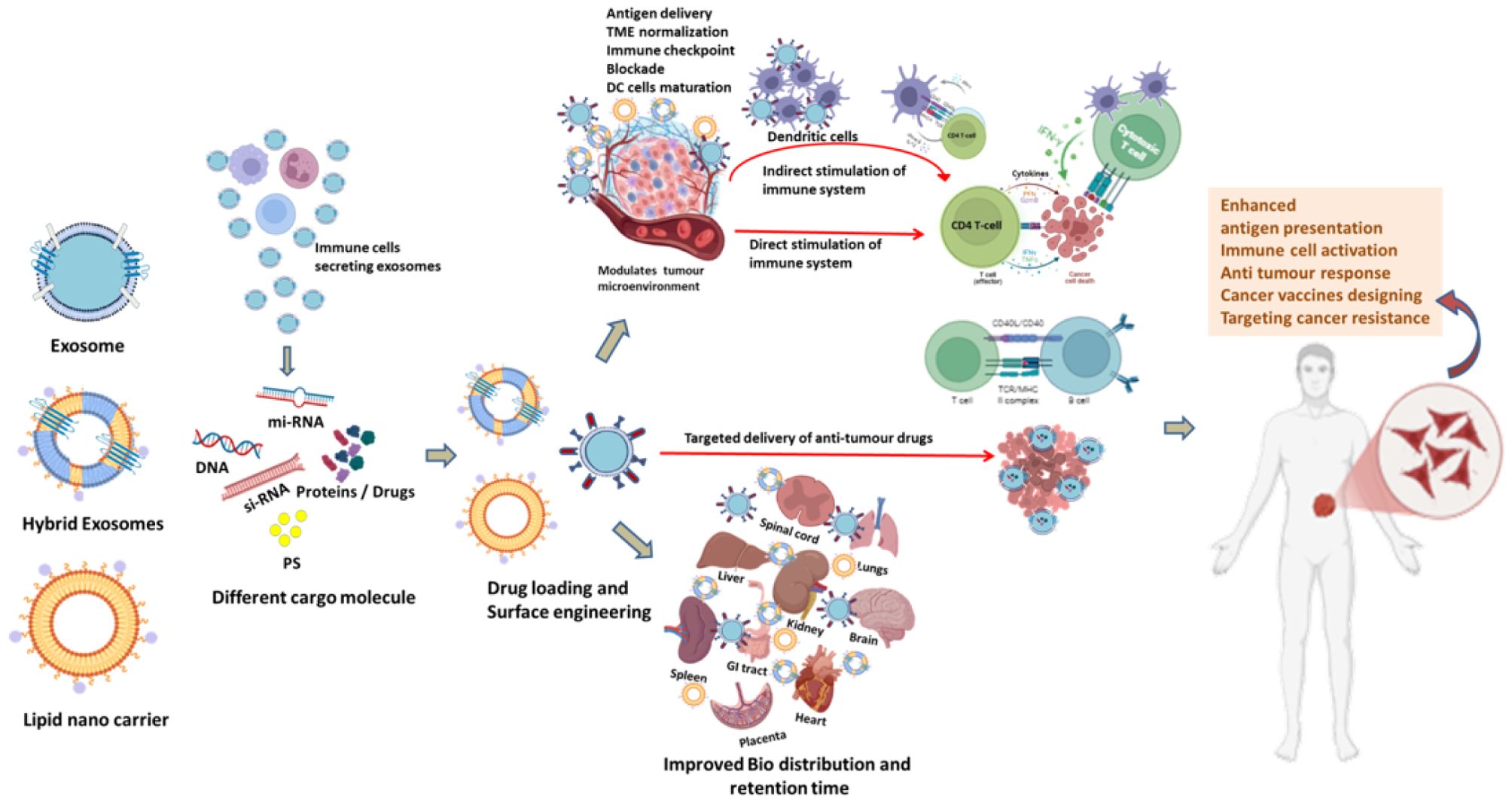
Figure 2. Schematic representation of improvement in Bio-distribution of exosomes in different human body organs and immune cell activation of exosomes and Lipid nano carriers directly, indirectly or targeted type to elicit specific immune response modulating TME to target cancer cells in cancer immunotherapy. DC (Dendritic cell), (TME) Tumour micro-environment.
2.4 Engineering exosomes for PDT and immunotherapy
2.4.1 Advantages of engineered exosomes & its potential for PDT & immunotherapy
Engineered exosomes combine unique biological and physicochemical properties that make them highly attractive for targeted cancer therapy. Their nanoscale size (30–150 nm) facilitates deep penetration into tumor tissue via the enhanced permeability and retention (EPR) effect, while the native lipid bilayer provides structural stability and protects encapsulated cargo from enzymatic degradation during systemic circulation (43). Compared with synthetic nanocarriers, exosomes display low immunogenicity and high biocompatibility, thereby minimizing the risk of adverse immune reactions (44) (Figure 3) (Table 1).
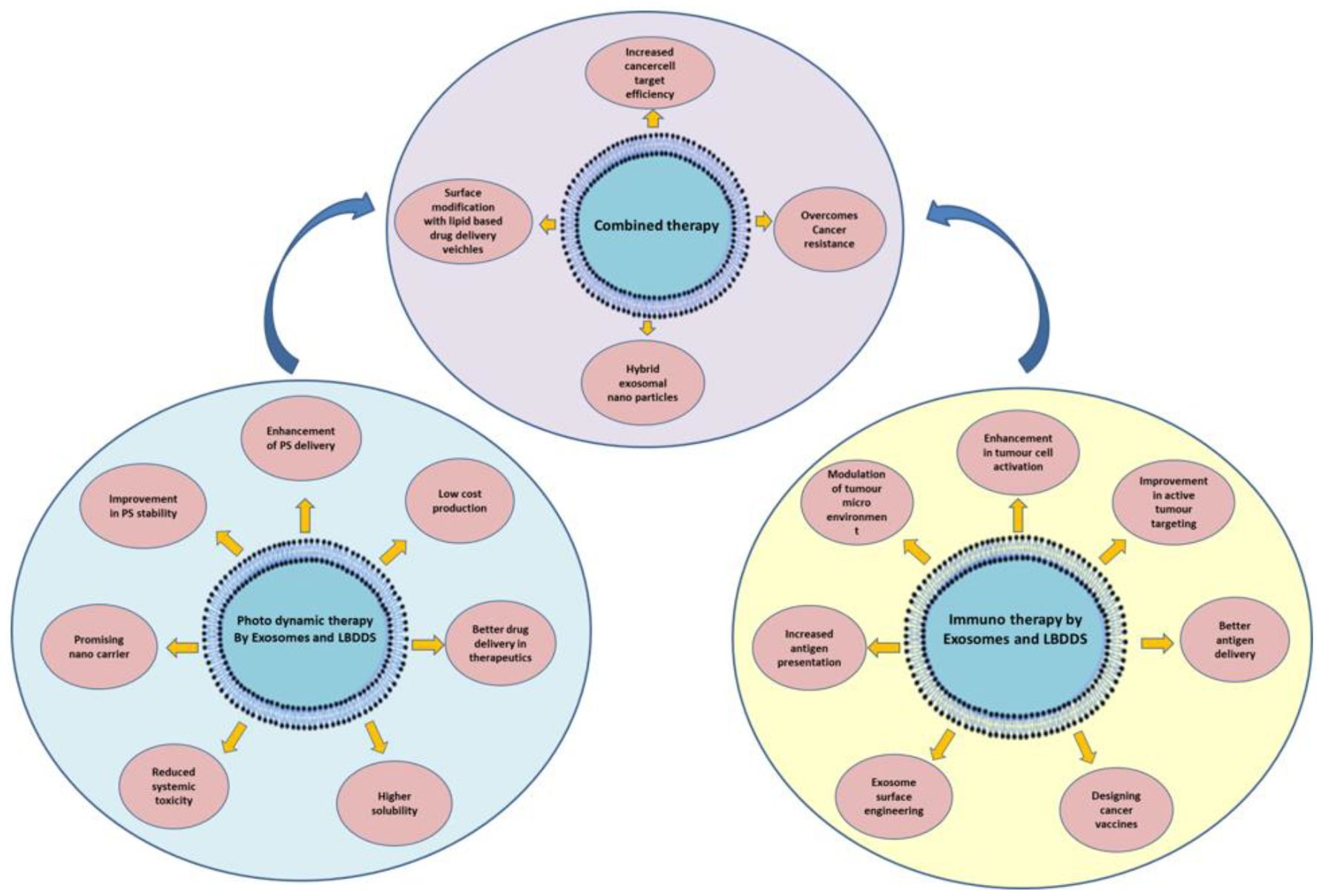
Figure 3. Combining the advantages of exosome and lipid-based nanocarriers in photodynamic therapy (PDT) and immunotherapy to enhance cancer cell targeting.
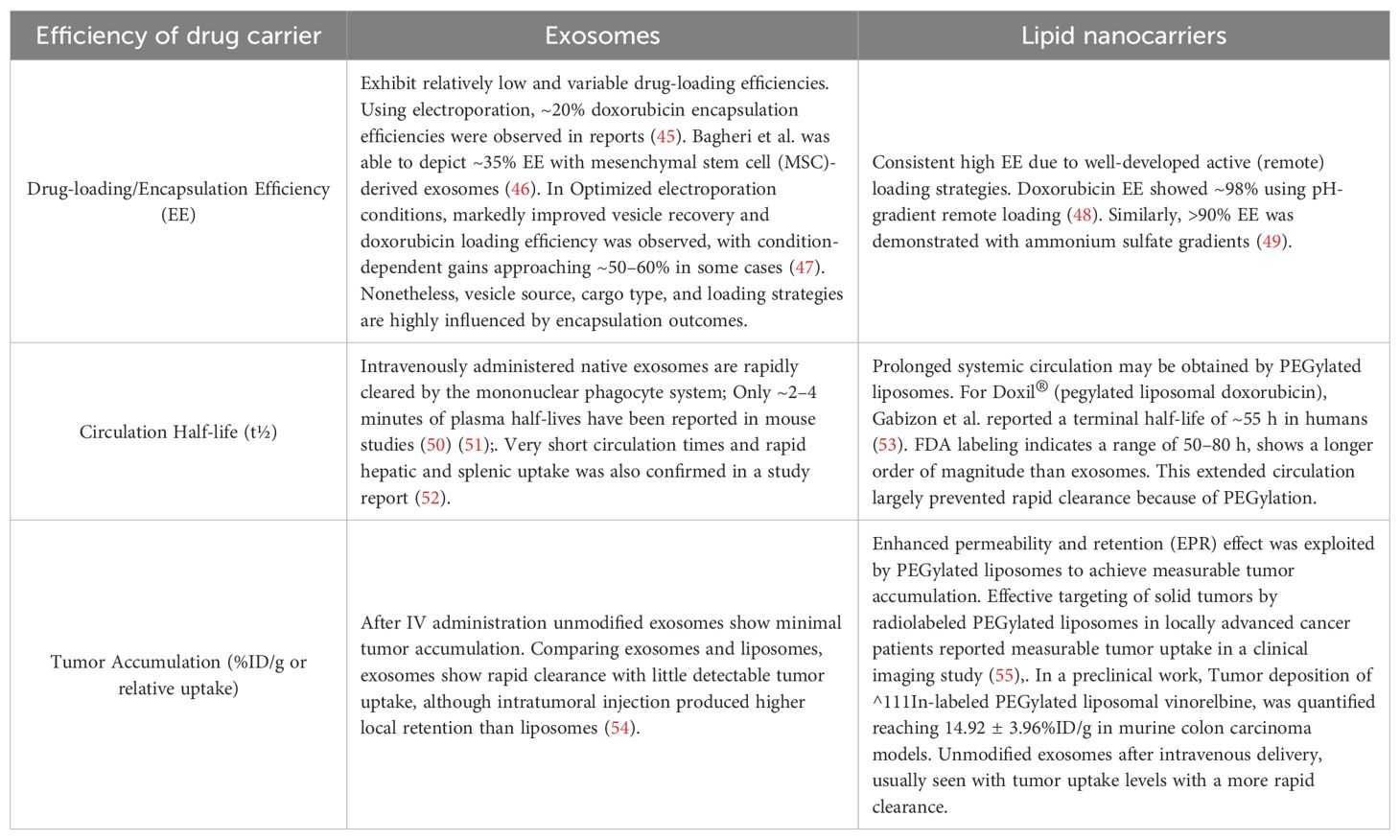
Table 1. Comparision of therapeutic potential characteristics between exosomes and lipid nano-carriers.
A key advantage lies in their intrinsic homing capability, dictated by protein and lipid signatures inherited from donor cells. This property enables selective accumulation in tumors and other specific tissues without extensive chemical modification (9). Precision targeting can be further enhanced through surface engineering strategies such as ligand conjugation, genetic fusion of targeting motifs, or chemical modification, which improve tissue specificity, help bypass biological barriers, and minimize off-target effects (56).
Engineered exosomes also support multimodal therapeutic applications. Their membranes can simultaneously incorporate imaging agents and therapeutic cargo, enabling real-time biodistribution tracking alongside treatment delivery. This multifunctionality supports theranostic approaches, where diagnosis and therapy are combined within a single platform (14). Moreover, exosomes can accommodate a wide spectrum of hydrophilic, hydrophobic, and macromolecular therapeutics including nucleic acids, proteins, and chemotherapeutics providing versatility across oncology, immunotherapy, and regenerative medicine (Figure 1; Figure 2).
Over the past decade, significant progress has been made in designing exosomes for targeted cancer therapy with diverse payloads such as siRNA (57), miRNA (58), and chemotherapeutic agents (59). Their stability protects cargo from enzymatic degradation, while their innate homing and biocompatibility ensure safety and tolerability in vivo (43, 44). For instance, exosomes from breast cancer cells engineered to overexpress miR-134 suppressed Hsp90, inhibited invasion and migration, and enhanced sensitivity to anti-Hsp90 drugs. Similarly, endothelial cell-derived exosomes enriched with miR-503 reduced tumor cell proliferation and invasion in vitro (56). THP-1 macrophage–derived exosomes transfected with miR-143, when intravenously administered to colon cancer-bearing mice, elevated miR-143 expression in tumors, kidneys, and serum, resulting in significant tumor growth inhibition (43, 60–62).
Beyond miRNA delivery, genetically engineered exosomes have also been explored as immunotherapeutic platforms. Streptavidin-lactadherin (SAV-LA) expressing exosomes from B16BL6 melanoma cells, functionalized with biotinylated CpG DNA (CpG-SAV-exo), effectively activated dendritic cells, enhanced antigen presentation, and produced stronger antitumor responses than exosomes or CpG DNA alone (38). Similarly, PD1-engineered exosomes co-loaded with the immune adjuvant imiquimod (PD1-Imi Exo) demonstrated potential in augmenting checkpoint blockade therapy (63). Artificial exosomes derived from Siglec-10 engineered M1 macrophages, formulated into a hydrogel encapsulating the efferocytosis inhibitor MRX-2843, reprogrammed macrophage polarization and efferocytosis when combined with X-ray radiation, thereby enhancing phagocytosis, antigen presentation, and robust antitumor immunity in ovarian cancer (64).
The method of drug loading is another critical determinant of therapeutic efficacy. Cargo hydrophilicity, lipophilicity, molecular weight, membrane integrity, and vesicle stability collectively influence loading efficiency and release kinetics (65). For example, studies on milk-derived exosomes show that hydrophilic drugs achieve significantly higher loading rates (33–65%) compared to hydrophobic drugs (13–22%) [Milk-derived exosomes as a promising vehicle for oral delivery of hydrophilic biomacromolecule drugs]. Doxorubicin encapsulation efficiency varies by species and method, with goat-derived exosomes exhibiting favorable biphasic release profiles (66). Similarly, engineered exosomes demonstrated superior loading of hydrophilic porphyrins via saponin-assisted incubation and hypotonic dialysis achieving up to 11-fold higher efficiency whereas hydrophobic porphyrins consistently showed poor incorporation (67) (Table 1).
Engineered exosomes also serve as potent vehicles for apoptosis-inducing agents. TRAIL-loaded exosomes from mesenchymal stem cells (MSCs) have demonstrated strong cytotoxic activity against lung, pleural mesothelioma, renal, breast, and neuroblastoma cell lines (68, 69). Likewise, HEK293T-derived exosomes engineered to express Lamp2B fused with the IL-3 receptor, overexpressed in chronic myeloid leukemia (CML), have been loaded with imatinib or BCR-ABL siRNA. These IL3-Lamp2B (IL3L) exosomes showed enhanced tumor targeting and therapeutic efficacy in preclinical models (62, 69).
In immunotherapy, engineered exosomes are emerging as modular vaccine platforms. One strategy fused the ovalbumin antigen to the lipid-binding C1C2 domain of lactadherin, displaying the antigen on the exosome surface. When used as a DNA vaccine, this design elicited robust antigen-specific CD4+ and CD8+ T-cell responses, effectively suppressing tumor progression in fibrosarcoma, melanoma, and thymoma models. Drug-loaded exosomes modified with targeting ligands have also shown selective accumulation in tumors following intravenous administration, where doxorubicin- and imatinib-loaded constructs inhibited tumor growth without systemic toxicity (45, 69, 70).
Genetic engineering further expands the utility of exosomes as gene delivery systems. Ohno et al. demonstrated efficient delivery of let-7a miRNA to malignant cells by fusing platelet-derived growth factor with the GE11 peptide, thereby enhancing binding to EGFR-positive tumors and correcting miRNA dysregulation (56, 71).
Taken together, engineered exosomes offer a highly versatile platform for precision drug delivery, PDT, and immunotherapy. Their biocompatibility, stability, and ability to integrate diagnostic and therapeutic functions position them at the forefront of next-generation cancer therapeutics (Table 1).
2.4.2 Key preclinical and clinical studies
A growing body of preclinical data underscores the translational promise of engineered exosomes in oncology. TRAIL-loaded MSC-derived exosomes have demonstrated potent induction of apoptosis in lung, breast, renal, neuroblastoma, and mesothelioma cell lines, with significant tumour regression observed in xenograft models (68). In hematologic malignancies, IL3-Lamp2B–engineered exosomes loaded with imatinib or BCR-ABL siRNA selectively targeted chronic myeloid leukemia cells, reducing tumour burden without overt systemic toxicity (69).
In solid tumours, GE11 peptide modified exosomes successfully delivered let-7a miRNA to epidermal growth factor receptor positive breast cancer cells, restoring tumour-suppressor miRNA levels and reducing proliferation in vitro and in vivo (71). Similarly, exosomes loaded with doxorubicin and modified with tumour-homing peptides showed preferential tumour accumulation, significant growth inhibition, and minimal cardiotoxicity compared to free drug administration (70).
A clinical pilot trial report explained plasma-derived exosomes from head and neck cancer patients undergoing PDT reflecting dynamic EMT-to-epithelial transitions, positioning them as potential biomarkers of therapeutic response (72). ROS-sensitive PEGylated exosomes for chemo-PDT could be applied as a preclinical advancement to engineered exosomes (73). Other studies include oral milk exosomes for brain-targeted PDT (74), and tumor-derived exosome hybrids co-loaded with photosensitizers and drugs for synergistic PDT chemotherapy (31). Together, these findings underscore exosomes as both biomarkers and multifunctional PDT carriers with high translational potential.
Early-stage clinical investigations are also underway. A first-in-human Phase I trial (NCT03608631) assessed Participants received mesenchymal stromal cells-derived exosomes with KrasG12D siRNA IV over 15–20 minutes on days 1, 4, and 10 with treatment repeatation in every 14 days for up to 3 courses in the absence of disease progression or unacceptable toxicity. Participants who responded were continued 3 additional courses (75). Another trial (NCT01159288) explored autologous dendritic cell–derived exosomes loaded with tumour antigens as a personalized cancer vaccine for non-small cell lung cancer, reporting induction of tumour-specific T-cell responses (76). An immunotherapy was developed by Gustave Roussy and Curie institutes involving metronomic cyclophosphamide (mCTX) followed by vaccinations with tumor antigen-loaded dendritic cell-derived exosomes (Dex). mCTX inhibits Treg (regulatory T-cells) functions restoring T and NK cell effector functions and Dex are able to activate the innate and adaptive immunity. The median progression-free survival (PFS) in patients responding or stabilized after 4 chemotherapy cycles ranges from 2 to 2.8 months. They proposed a maintenance immunotherapy in 47 advanced unresectable NSCLC patients responding or stabilized after induction chemotherapy with Dex-based treatment to improve PFS rate at 4 months in these patients (76).
Collectively, these studies illustrate the versatility, safety, and therapeutic potential of engineered exosomes, laying a robust foundation for their translation into precision oncology and next-generation immunotherapies.
3 Lipid-based nanocarriers
Lipid-based nanocarriers are nanoscale delivery platforms composed of biocompatible lipid components designed to encapsulate and transport therapeutic agents with enhanced pharmacokinetic and pharmacodynamic profiles such as stability, solubility, and targeted delivery profiles, while minimizing off-target effects (77, 78). Owing to their structural versatility, these systems can encapsulate both hydrophilic and hydrophobic molecules, enhance drug solubility, protect labile compounds from degradation, and enable targeted delivery, thereby reducing systemic toxicity (79, 80) They have been extensively utilized in cancer therapy, gene delivery, vaccine formulations, and other biomedical applications (13, 81). Lipid based drug delivery system is broadly classified into 3 types, namely, (i) emulsion type, (ii) vesicular system and (iii) lipid particulate system (82). Liposomes, solid lipid nanoparticles (SLNs) (11, 83), and nanostructured lipid carriers (NLCs), alongside a wider array of lipid-based drug delivery systems (LBDDS) such as lipospheres, lipid drug conjugate nanoparticles (LDCs), self-emulsifying formulations (SEFs), Pickering emulsions, dry emulsions, micro- and nano-emulsions, solidified reverse micellar solution (SRMS) tablets, herbosomes (84), Phytosomes (85) cryptosomes (86), niosomes (87, 88), ethosomes (89) bilosomes (90), and transferosomes (91) were some of the modulated form of the basic LBDDS catagory. These formulations employ diverse excipients such as triglyceride oils, mixed glycerides, lipophilic and hydrophilic surfactants, and water-soluble components, allowing high flexibility in drug formulation design (13, 81). Each of these nanocarriers offers unique advantages in drug loading, release kinetics, and stability (92, 93).
Liposomes are spherical vesicles composed of one or more phospholipid bilayers are widely used for delivering anticancer drugs, nucleic acids, and vaccine antigens (78, 94). SLNs, consisting of a solid lipid matrix stabilized by surfactants, provide high drug entrapment efficiency and controlled release (92). NLCs, the second generation of SLNs, incorporate a blend of solid and liquid lipids, improving payload capacity and preventing drug expulsion during storage (93, 95). Advances in lipid composition engineering, PEGylation, ligand-mediated targeting, and stimuli-responsive designs have further improved their therapeutic precision and clinical applicability (96, 97).
Lipid-based nanocarriers provide multiple advantages, including biocompatibility, ease of chemical modification, high physical stability, and the capacity to carry both hydrophilic and hydrophobic drugs (81, 98). They address key pharmaceutical challenges such as the poor solubility and limited bioavailability of hydrophobic drugs, while enabling fine-tuning for disease indication, administration route, stability, and therapeutic efficacy. Clinically, lipid-based formulations are widely deployed for topical, oral, pulmonary, and parenteral delivery with minimal systemic toxicity, in part by altering drug biodistribution to avoid sensitive organs. Liposomes, for instance, have been adapted to carry anti-tumour and antimicrobial agents, chelating agents, peptide hormones, enzymes, proteins, vaccines, and genetic material (98). Notably, lipid nanoparticles represent the first nanomedicine delivery system to achieve widespread clinical translation, successfully delivering anti-cancer, anti-fungal, and antibiotic drugs, as well as gene therapies and anti-inflammatory agents (78).Specifically PDT and immunotherapy based therapeutic approaches maximally employs Liposomes, Micelles, SLNs and LNPs for successful applications.
Specialized systems such as cochleates formed via precipitation of negatively charged lipids with cations have shown promise for targeted delivery applications (99). Despite these advances, key developmental challenges remain, particularly related to the diversity of encapsulated cargo and the lack of standardized characterization methods, which complicate stability assessment, classification, and regulatory approval pathways (91).
3.1 Formulation techniques and functionalization of lipid-based drug delivery systems
Formulation techniques and functionalization strategies are central to optimizing the therapeutic performance of LBDDS, aiming to maximize stability, enhance bioavailability, enable controlled release, and achieve targeted delivery while minimizing adverse effects (91). Functionalization focuses on surface modification to improve biodistribution, facilitate tissue-specific targeting, and enhance biological interactions.
3.1.1 Formulation techniques
The formulation types of LBDDS are also categorized into 4 types (i,e, Type I, Type II, Type III and Type IV). Type I formulation consists of oils without surfactants (e.g., tri-, di-, and monoglycerides), Type II bears oils and water insoluble surfactants, Type III contains oils, surfactants, and cosolvents (both water-insoluble and water-soluble excipients) and Type IV can be prepared with water-soluble surfactants and co-solvents (91). The process of formation of these LBDDS are explained briefly here as this is beyond the scope of this manuscript.
Liposome Formation: Liposomes self-assemble from phospholipids in aqueous environments to form bilayer vesicles capable of encapsulating active pharmaceutical ingredients. Common preparation methods include film hydration, solvent evaporation, and reverse-phase evaporation (77).
Solid Lipid Nanoparticles (SLNs): Produced by emulsifying a solid lipid in a liquid lipid or aqueous phase under high shear, SLNs offer stable encapsulation for hydrophilic and hydrophobic drugs (92).
Micelle Formation: Amphiphilic surfactants or block copolymers self-assemble into micelles in aqueous media, enabling solubilization of poorly water-soluble drugs and improving their pharmacokinetic profiles (100).
Nanoemulsions and Microemulsions: Generated by emulsifying oils and surfactants in water under high shear, these systems produce stable nanoscale droplets that enhance solubility and stability of hydrophobic drugs (101).
Nanostructured Lipid Carriers (NLCs): Formulated by blending solid and liquid lipids, NLCs improve drug loading capacity and release profiles over SLNs, making them particularly suitable for lipophilic drugs (102).
3.1.2 Functionalization strategies
Functionalization of lipid based nanocarriers are mainly achieved in the following ways.
Surface Coating: Functionalizing nanocarriers with biocompatible polymers such as polyethylene glycol (PEG) extends circulation time by reducing recognition and clearance by the mononuclear phagocyte system (103).
Targeted Functionalization: Conjugation of specific ligands or antibodies (e.g., folate, transferrin) to nanocarrier surfaces enables receptor-mediated uptake in tumour cells or inflamed tissues, improving specificity (104).
pH-Responsive and Enzyme-Responsive Functionalization: Engineering nanocarriers to release their payload in acidic tumour microenvironments or in the presence of specific enzymes allows spatially controlled drug release (105).
Dual or Multi-Functionalization: Combining multiple targeting moieties or therapeutic agents on a single nanocarrier platform enables combination therapy or multi-modal drug delivery with enhanced efficacy (106).
These formulation and functionalization strategies collectively enable the development of next-generation lipid-based delivery systems that offer improved therapeutic index, reduced systemic toxicity, and high precision in treating complex diseases, particularly cancer (107).
3.2 Applications of lipid-based nanocarriers in photodynamic therapy
The therapeutic efficacy of PDT is often constrained by poor solubility, rapid clearance, and low tumour selectivity of photosensitizers (108). Lipid-based nanocarriers have been employed to overcome these limitations by improving photosensitizer stability, enhancing tumour accumulation via the enhanced permeability and retention (EPR) effect, and enabling co-delivery of chemotherapeutics or immune modulators for synergistic effects (Table 2) (109, 110).
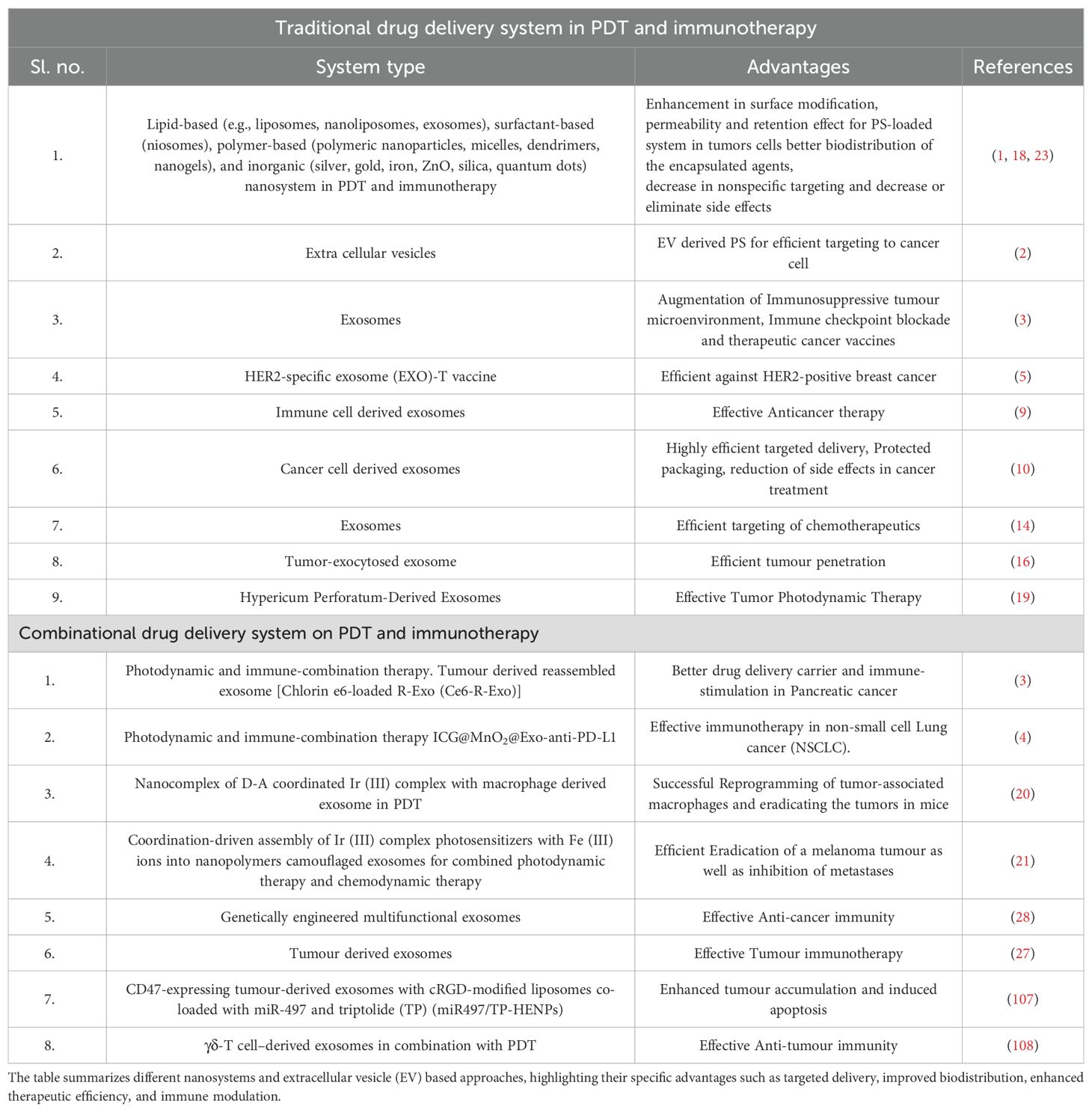
Table 2. Overview of traditional and combinational drug delivery systems in photodynamic therapy (PDT) and immunotherapy.
3.2.1 Liposomes
Liposomes have been extensively investigated for PDT applications, with formulations such as liposomal zinc phthalocyanine and verteporfin demonstrating improved pharmacokinetics and enhanced tumour phototoxicity (111, 112). A comprehensive review highlighted the role of liposomal formulations differing in size, composition, and surface modification (e.g., folate conjugation) in enhancing tumour targeting, reducing off-target toxicity, and improving PDT efficacy with photosensitizers such as chlorin e6, phthalocyanines, and porphyrins (113). Liposomal temoporfin (Foslip) demonstrated improved pharmacokinetics, enhanced tumour uptake, and reduced prolonged skin photosensitivity in preclinical and clinical evaluation, addressing a major limitation of conventional PDT (114). Similarly, a liposomal benzoporphyrin derivative monoacid ring A (BPD-MA, marketed as Visudyne) exhibited controlled biodistribution and an improved safety profile compared to free drug. Lipid-anchored BPD-liposome combinations achieved significantly greater PDT efficacy at lower light doses compared to either formulation alone.
3.2.2 Solid lipid nanoparticles
SLNs represent another important lipid-based drug delivery system for PDT. For example, SLNs loaded with aluminum phthalocyanine chloride modulated immunogenic cell death in melanoma models (115). Hypericin (Hy), a natural phenanthroperylenequinone photosensitizer from Hypericum perforatum, shows therapeutic potential but suffers from hydrophobicity. Encapsulation into SLNs (<200 nm, ultrasonication-prepared) achieved high entrapment efficiency, enhanced photostability, and improved drug loading (116). Thermoresponsive solid lipid nanoparticles with non-covalently bound temoporfin (T-SLNP) exhibited faster accumulation kinetics and higher phototoxicity in vitro, and biodegradable nanosystems (<50 nm) based on polymer-surfactant stabilized T-SLNPs demonstrated improved in vivo anticancer efficacy compared with commercial temoporfin formulations, along with controlled release and superior biocompatibility (117). SLNs have also improved the solubility and PDT efficacy of photosensitizers such as SLN-AlPc, MPPa-loaded SLNs (115, 118) and verteporfin (119).
3.2.3 Polymeric micelles
Micelles have also been widely explored for PDT. Thermosensitive mPEG-b-p(HPMAm-Lac2) micelles efficiently encapsulated hydrophobic Si(sol)2Pc photosensitizers, demonstrating high loading efficacy, controlled release, and strong photocytotoxicity (120). Polymeric micelles help address poor water solubility of many photosensitizers (121). For instance, DSPE-PEG2000 micelles trapped BODIPY3, yielding BODIPY3-PEG3 nanocomplexes with excellent solubility and stability in aqueous media (122). Micelles further extend circulation time by avoiding rapid recognition by proteins and macrophages (121). Encapsulation polymers include pluronics, PEG–lipid conjugates, and pH-sensitive systems such as poly(N-isopropylacrylamide) or polyion complex (PIC) micelles. Notably, imidazole-bearing ^1O2-responsive polymeric micelles allowed light-triggered on-demand delivery of photosensitizers, demonstrating stability during systemic circulation via ionic crosslinking (123).
3.2.4 Nanostructured lipid carriers
NLCs have been used to increase drug-loading efficiency of hydrophobic photosensitizers such as curcumin and hypericin, thereby improving bioavailability and ROS generation (116, 124). A topical NLC formulation of 5-ALA for basal-cell carcinoma enhanced skin penetration and PDT effect (125). Targeted NLC approaches, such as Angiopep-2-modified Ce6-NLCs, demonstrated BBB penetration and enhanced PDT efficacy in glioblastoma (126). Natural lipid nanoparticles (LNPs) loaded with aluminum phthalocyanine showed significant therapeutic potential for melanoma PDT (127).
Targeted Lipid-Based Systems: Ligand-targeted liposomal PDT agents, including folate-conjugated formulations, selectively accumulated in cancer cells overexpressing folate receptors, thereby enhancing therapeutic specificity (128). This strategy exemplifies how lipid carriers can be engineered for precision targeting in PDT.
3.3 Applications of lipid-based nanocarriers in immunotherapy
Cancer immunotherapy seeks to harness the host immune system to recognize and eradicate malignant cells, using strategies such as immune checkpoint blockade, cancer vaccines, and adoptive T cell transfer (129, 130). Lipid-based nanocarriers have emerged as promising delivery platforms for immunotherapeutic agents, as they can encapsulate antigens, adjuvants, and immunomodulatory drugs, facilitate co-delivery to antigen-presenting cells (APCs), and modulate immune responses through controlled release and targeting (131, 132) (Figure 2).
3.3.1 Liposomes
Liposomes have been widely explored in immunotherapy. They can deliver tumor-associated antigens (TAAs) along with Toll-like receptor (TLR) agonists to dendritic cells, eliciting robust antigen-specific cytotoxic T lymphocyte (CTL) responses (133). PEGylated and pH-sensitive liposomes enable efficient cytosolic delivery of nucleic acid vaccines (mRNA/DNA), thereby improving antigen expression and immunogenicity (133, 134). Gao et al. designed immune agonist-anchoring liposomes to co-deliver IL-2 and an anti-CD137 antibody, which promoted tumor infiltration of CD8+ T cells, enhanced cytokine and granzyme secretion, and elicited strong antitumor responses while reducing systemic toxicity (135). Another formulation, ILP (34A-PEG-ILP), conjugated to antibodies at the distal PEG end, demonstrated superior targeting efficiency to lung endothelial cells and tumour tissue compared with conventional liposomes (136). Liposomes are being developed to address challenges in cancer immunotherapy by enhancing vaccine efficacy through improved antigen delivery, normalizing the tumor microenvironment, modulating signaling pathways, and serving in combination regimens with chemotherapy, radiotherapy, and phototherapy (137). In addition, highly pH-sensitive polymer-modified liposomes prepared by surface modification of phospholipid vesicles with 3-methylglutarylated poly(glycidol)—facilitated endosomal escape and cytosolic delivery of antigenic molecules, proving effective in inducing antigen-specific immune responses (138). Archaeosomes (liposomes derived from archaeal lipids) present another innovative approach, capable of activating dendritic cells and enhancing adjuvant responses. Furthermore, liposomes have been integrated into multimodal strategies, combining photodynamic therapy (PDT) and photothermal therapy (PTT), to potentiate antitumor immunity (135).
3.3.2 Micelles
Polymeric micelles provide another versatile lipid-based nanocarrier system in immunotherapy. PEG-polyglutamate micelles encapsulating IL-2 showed prolonged circulation and enhanced dendritic cell (DC) vaccine efficacy in tumor-bearing mice, leading to strong CTL responses. Similarly, micelles co-loaded with doxorubicin (DOX) and IL-12 plasmid DNA significantly outperformed single-agent formulations in inhibiting tumor growth. Micelles have also been engineered to deliver macrophage colony-stimulating factor (M-CSF), inducing T cell–mediated antitumor immunity, while SART3 peptide-loaded micelles promoted CTL and NK cell activity, along with enhanced DC infiltration into tumors. PEG-PLL-PLLeu micelles co-delivering STAT3 siRNA and ovalbumin upregulated DC activation markers (CD86, CD40) and IL-12 production, further boosting immune responses. Indoximod-based micelles co-loaded with DOX improved therapeutic efficacy by simultaneously inhibiting immunosuppressive pathways and augmenting chemotherapy. SLNs and NLCs have also been employed for the delivery of immune checkpoint inhibitors such as anti-PD-1 peptides and siRNAs, improving their stability and tumour accumulation (139) Other strategies have employed PEG-PE micelles as adjuvant carriers (e.g., MPLA for TLR signaling), or combination micelles (e.g., tranilast-, epirubicin-, or Doxil-based micelles) to enhance T cell infiltration and establish durable immunological memory in resistant cancers (140, 141). Advanced micelle platforms include IDO-responsive tryptophan-polymer micelles that disassemble in tumor cells to release IDO inhibitors, thereby recruiting effector T cells (142). Another self-assembled micelle system combined immunomodulators (epigallocatechin gallate palmitate and metformin) with DOX and immune checkpoint inhibitors to reduce PD-L1 expression and reshape the tumor microenvironment (143). Mannose-modified micelles have been optimized for DC targeting and vaccine delivery, with mixed micelles co-delivering ovalbumin and TLR-7 agonists showing robust antigen-specific humoral and cellular immunity (144). Additionally, inorganic nanovaccine micelles incorporating zinc-doped iron oxide nanoparticles successfully co-delivered peptide antigens and TLR3 agonists, stimulating potent immune responses (145).
3.3.3 Solid lipid nanoparticles
SLNs have demonstrated significant promise in immunotherapy. SLN-AlPc formulations retained the activity of the hydrophobic photosensitizer aluminum phthalocyanine in aqueous media, inducing immunogenic cell death (ICD) and activating DCs in melanoma models (115). Cationic SLNs (cSLNs) have proven effective vaccine adjuvants, enhancing antigen uptake, BMDC activation, and memory immune responses in models of inactivated foot-and-mouth disease virus (146). Beyond vaccines, cSLNs have been used to encapsulate anticancer agents and proteins for improved in vitro and in vivo efficacy (81). P18 N PI ME-loaded SLNs demonstrated sustained release and improved PDT outcomes in cancer models (147), while SLNs also provided controlled release of immune suppressants such as MMF (148). Chitosan-coated AmB-SLNs enhanced macrophage cytokine responses (TNF-α, IL-12) (149), and actarit-loaded SLNs improved splenic targeting and retention in vivo (150). Collectively, these findings highlight SLNs as multifunctional carriers for peptides, proteins, small molecules, and vaccines (151). Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have also been employed for the delivery of immune checkpoint inhibitors such as anti-PD-1 peptides and siRNAs, enhancing their stability and tumor accumulation (139).
3.3.4 Emerging concepts
Lipid-based micelles have also been adapted for anti-inflammatory roles, such as polymeric micelles carrying a Ru (CO)3Cl (amino acidate) segment for CO release, which attenuated LPS-induced monocyte inflammation (152). Recent studies highlight the integration of lipid-based nanocarriers with immune-stimulating PDT, termed photo-immunotherapy, where PDT-induced ICD is leveraged alongside nanocarrier-mediated delivery of immune adjuvants to amplify antitumor immune responses (153, 154). Such combined approaches represent a frontier in nanomedicine-driven immuno-oncology.
4 Combined applications of exosomes and LBDDS in PDT and immunotherapy
Exosomes and LBDDS possess bioactive cargos of proteins, nucleic acids, and lipids that naturally facilitate intercellular communication with intrinsic stability, low immunogenicity, biocompatibility, and efficient membrane penetration, making them attractive drug delivery systems (155). Many engineering strategies originally developed combined application for liposomes and exosomes such as sonication, extrusion, freeze thaw cycles, and microfluidic methods have been adapted for exosomes, improving their therapeutic potential.
4.1 Exosome–lipid hybrids in PDT and immunotherapy
Hybrid exosome–lipid formulations combine the biological advantages of exosomes with the tunable properties of lipid nanocarriers, improving drug loading, stability, targeting, and intracellular delivery. A notable example is the loading of indocyanine green (ICG) into hollow manganese dioxide (MnO2) nanospheres followed by encapsulation in PD-L1 monoclonal antibody–reprogrammed exosomes (ICG@MnO2@Exo-anti-PD-L1). This platform modulated the tumour microenvironment (TME) in non-small cell lung cancer by enabling synergistic PDT and immunotherapy: acidic pH triggered controlled anti-PD-L1 release, while MnO2 catalyzed H2O2-to-O2 conversion, alleviating hypoxia and enhancing T-cell activation (Table 2) (8). Photoimmunotherapy approaches have also leveraged γδ-T cell–derived exosomes in combination with PDT to potentiate antitumour immunity (156).
Exosomal lipid composition determined by parental cell type and physiological state—affects membrane curvature, cargo protection, and stability, making lipidomic profiling a potential diagnostic and therapeutic tool in oncology (155). Hybridization strategies such as fusing exosomal and endosomal membranes with pH-sensitive fusogenic peptides, introducing cationic lipids, or applying lipid extruders have enhanced cytosolic delivery of therapeutic cargos (157). For example, folate-modified lipid nano-assemblies (FD9R) combined with tumour-derived exosome inhibition and IRF3 silencing demonstrated synergy with immune checkpoint blockade in a murine breast cancer model (158). Similarly, incorporating the cationic lipid-sensitive endosomolytic peptide L17E into exosome-based systems promoted efficient cytosolic release of RNA therapeutics (159).
In hepatocellular carcinoma (HCC) models, a hybrid adipocyte-derived exosome platform co-assembled a ROS-cleavable docetaxel prodrug (DSTG) and a lipid-conjugated photosensitizer (PPLA) into lipid cores (HEMPs and NEMPs), which were encapsulated within exosome membranes. These hybrids exhibited significantly greater uptake efficiency in HCC cells compared with lipid-only nanoparticles (160).
4.2 Integration of exosomes with lipid-based systems
The integration of exosomes with lipid-based drug delivery systems (LBDDS) has enabled multifunctional platforms for PDT and immunotherapy. Methods such as fusion with fusogenic liposomes or assembly of lipid-enriched exosomal cargo using lipid extruders yield potent hybrid transport vehicles with synergistic therapeutic benefits (161, 162). Lipid nanoparticles can also be integrated onto exosome surfaces to improve targeting and delivery (162), while lipids themselves facilitate exosome biogenesis, secretion, and fusion with the multivesicular body (MVB) membrane. Lipid-rich exosomes, particularly those derived from the central nervous system, contain 1.5–3-fold higher ceramide (Cer), phosphatidylserine (PS), cholesterol, and sphingomyelin (SM) than other exosome types, reflecting parental cell origin and supporting cargo loading, endocytosis, macropinocytosis, and phagocytosis (163).
4.3 Bioinspired hybrid platforms
An important example of bioinspired design is the fusion of CD47-expressing tumour-derived exosomes with cRGD-modified liposomes co-loaded with miR-497 and triptolide (TP), producing hybrid nanoparticles (miR497/TP-HENPs) that markedly enhanced tumour accumulation and induced apoptosis (164).
Together, exosome lipid hybrids provide multifunctional delivery systems that unite natural biocompatibility with synthetic flexibility, enabling synergistic PDT and immunotherapy. Such approaches not only improve drug loading and targeting but also harness exosomal lipid biology to modulate tumour–immune interactions, marking them as promising candidates for next-generation cancer nanotherapeutics.
5 Therapeutic outlook and future directions
Hybrid exosomes combining lipid-based drug delivery systems (LBDDS) with native exosomes create drug carriers enriched with exogenous lipids while retaining the intrinsic biological properties of exosomes. Liposomes contribute chemical versatility, ease of large-scale production, extended shelf life, and circulation stability, while exosomes provide inherent biocompatibility, natural targeting ligands, and complex bioactive cargo (165). This synergistic integration holds promise for advancing clinical nanomedicine by delivering high drug payloads with precise tumour targeting, controlled release, stability under physiological stress, and minimal immunogenicity. The collective evidence strongly supports the potential of LBDDS exosome hybrids as transformative platforms for cancer therapy, including PDT and immunotherapy.
Although lipid-based nanocarriers have shown considerable success in PDT and immunotherapy, future development should focus on multifunctional hybrid platforms that integrate imaging, therapy, and immune modulation in a single nanosystem. In PDT, innovations such as NIR-responsive lipid carriers with enhanced photostability and tissue penetration, coupled with oxygen-generating or hypoxia-responsive elements, could overcome tumour microenvironment constraints (2, 166). In immunotherapy, the next generation of lipid nanocarriers may incorporate personalized tumour antigens and immune adjuvants with precision targeting ligands for dendritic cells or T cells, boosting antigen presentation and immune activation (133).
The integration of bioinformatics and AI-driven lipid formulation design could optimize nanocarrier composition and payload combinations for patient-specific therapies. Addressing challenges in scalable manufacturing, long-term stability, and regulatory harmonization will be critical for clinical adoption. Additionally, theranostic lipid-based nanocarriers capable of both therapy and real-time monitoring via incorporated imaging agents represent a promising direction for precision oncology.
6 Epilogue
The convergence of lipid-based nanocarrier technology and exosome-mediated delivery presents a compelling pathway toward next-generation targeted therapeutics. Lipid-based nanocarriers offer structural flexibility, high payload capacity, and facile surface modification, making them well-suited for applications in photodynamic therapy (PDT) and immunotherapy. Exosomes, in contrast, provide innate targeting, biocompatibility, and the ability to cross physiological barriers while evading immune clearance (167). Hybrid approaches such as synthetic lipid exosome chimeras or bioinspired lipid nanoparticles engineered to mimic exosomal properties promise to integrate the precision of synthetic nanocarriers with the natural communication networks of biological vesicles. These innovations hold potential to enhance biodistribution, therapeutic index, and patient outcomes.
Despite this promise, translating exosome-based PDT and immunotherapy into the clinic remains challenging. Preclinical data support their ability to simultaneously target tumors and sustain immune activation; however, regulatory uncertainties, the lack of standardized potency assays, and the complexity of reproducible large-scale manufacturing present significant barriers. Addressing these challenges will require proactive engagement with regulatory agencies, the development of scalable bioprocessing platforms, and rigorous quality control frameworks to ensure product consistency and safety. Furthermore, clinical trial designs that incorporate immune biomarkers and clearly demonstrate added value beyond conventional PDT or immunotherapy will be critical to establishing clinical relevance.
Ultimately, the successful clinical translation of hybrid exosome lipid systems will depend on aligning scientific innovation with pragmatic solutions to regulatory and manufacturing hurdles. By doing so, these platforms could advance from promising laboratory concepts to viable, multimodal cancer therapeutics that drive the next era of personalized medicine.
Author contributions
AS: Data curation, Methodology, Writing – review & editing, Investigation, Writing – original draft. SJ: Conceptualization, Validation, Writing – review & editing, Supervision, Formal analysis, Data curation. LS: Conceptualization, Supervision, Writing – review & editing, Data curation, Project administration, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Infrastructure support to the Centre of Excellence in Environment and Public Health by Higher Education Department, Government of Odisha (Grant No.: 26913/HED/HE-PTC-WB-02–17), Post-doctoral fellowship to SRJ from Indian Council of Medical Research (ICMR), Government of India (Grant No.: 2021–10784/CMB-BMS) is gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Esfahani FN, Karimi S, Jalilian Z, Alavi M, Aziz B, Alhagh Charkhat Gorgich E, et al. Functionalized and theranostic lipidic and tocosomal drug delivery systems: potentials and limitations in cancer photodynamic therapy. Adv Pharm Bull. (2024) 14:524–36. doi: 10.34172/apb.2024.038
2. Lucky SS, Soo KC, and Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. (2015) 115:1990–2042. doi: 10.1021/cr5004198
3. Parihar A, Dube A, and Gupta PK. Conjugation of chlorin p6 to histamine enhances its cellular uptake and phototoxicity in oral cancer cells. Cancer Chemother Pharmacol. (2011) 68:359–69. doi: 10.1007/s00280-010-1492-9
4. Parihar A, Dube A, and Gupta PK. Photodynamic treatment of oral squamous cell carcinoma in hamster cheek pouchs model using chlorin p6-histamine conjugates. Photodiagn Photodyn Ther. (2013) 10:79–86. doi: 10.1016/j.pdpdt.2012.05.005
5. Parihar A, Shrivastava R, and Dube A. Interaction of Cp6-his and Cp6 with bovine serum albumin and liver microsomes: spectroscopic and molecular docking studies. J Photochem Photobiol. (2021) 5:100013. doi: 10.1016/j.jpap.2020.100013
6. Lv B, Huang J, Lin X, He Y, Liu C, Zhang J, et al. Extracellular vesicles: an advanced delivery platform for photosensitizers in tumor photodynamic therapy. Chem Eng J. (2024) 498:155438. doi: 10.1016/j.cej.2024.155438
7. Jang Y, Kim H, Lee H, Kim CH, Park JH, Kim C, et al. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J Control Release. (2021) 330:293–304. doi: 10.1016/j.jconrel.2020.12.039
8. Guo J, Xu X, Li M, Gong M, Cheng Y, and Xie H. Reprogramming exosomes for immunity-remodeled photodynamic therapy against non-small cell lung cancer. Bioact Mater. (2024) 39:206–23. doi: 10.1016/j.bioactmat.2024.05.030
9. Zhang H, Wang Y, Sun P, Zhang Z, Cao W, and Li X. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol. (2023) 13:1093607. doi: 10.3389/fimmu.2022.1093607
10. Kumar R, Awasthi A, Verma A, Dubey A, and Pandey V. Lipid based nanocarriers: production techniques, concepts, and commercialization aspect. J Drug Delivery Sci Technol. (2022) 74:103526. doi: 10.1016/j.jddst.2022.103526
11. Mehta M, Satija S, Singh B, Chellappan DK, and Dua K. Lipid-based nanoparticles for drug/gene delivery: an overview of the production techniques and difficulties encountered in their industrial development. ACS Mater Au. (2023) 3:600–19. doi: 10.1021/acsmaterialsau.3c00032
12. Prabhakar P, Mishra P, Sharma A, and Singh RK. Trends and advances in liposome formulation technology with an emphasis on ensuring safety and quality in food and drug applications. Food Biosci. (2025) 69:106913. doi: 10.1016/j.fbio.2025.106913
13. Fang Z, Ding Y, Xue Z, Li P, Li J, and Li F. Roles of exosomes as drug delivery systems in cancer immunotherapy: a mini-review. Discov Oncol. (2022) 13:74. doi: 10.1007/s12672-022-00539-5
14. Hosseinpouri A, Saberi MJ, Yazdansetad Z, Arabpour Z, and Zarei-Behjani Z. Exosomes from cancer cells: innovative approach for targeted cancer treatment. Regener Eng Transl Med. (2025) 4:1–14. doi: 10.1007/s40883-025-00393-1
15. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. (1996) 183:1161–72. doi: 10.1084/jem.183.3.1161
16. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. (1998) 4:594–600. doi: 10.1038/nm0598-594
17. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, and Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. (2011) 29:341–5. doi: 10.1038/nbt.1807
18. Sen S, Xavier J, Kumar N, Ahmad MZ, and Ranjan OP. Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech. (2023) 13:101. doi: 10.1007/s13205-023-03521-2
19. Baskaran R, Lee J, and Yang SG. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. (2018) 22:25. doi: 10.1186/s40824-018-0140-z
20. Kibria G, Ramos EK, Wan Y, Gius DR, and Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. (2018) 15:3625–33. doi: 10.1021/acs.molpharmaceut.8b00277
21. Huis in’t Veld RV, Heuts J, Ma S, Cruz LJ, Ossendorp FA, and Jager MJ. Current challenges and opportunities of photodynamic therapy against cancer. Pharmaceutics. (2023) 15:330. doi: 10.3390/pharmaceutics15020330
22. Palakurthi SS, Shah B, Kapre S, Charbe N, Immanuel S, Pasham S, et al. comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. (2024) 6:5803–26. doi: 10.1039/d4na00501e
23. Liu Y, Wang L, Kong F, Liu T, and Liu H. An immunomodulatory photosensitizer-mediated photodynamic therapy synergizes with PD-L1 blockade against metastatic triple-negative breast cancer. Front Pharmacol. (2025) 16:1651165. doi: 10.3389/fphar.2025.1651165
24. Zhu D, Duo Y, Suo M, Zhao Y, Xia L, Zheng Z, et al. Tumor-exocytosed exosome/aggregation-induced emission luminogen hybrid nanovesicles facilitate efficient tumor penetration and photodynamic therapy. Angew Chem. (2020) 132:13940–7. doi: 10.1002/ange.202003672
25. Pradhan A, Behera D, Jena SR, Soren S, Parhi P, and Samanta L. NIR-triggered SrSnO3-photosynthesized nanocatalyst: a potent anticancer agent against triple negative breast cancer cells. J Mol Struct. (2023) 1294:136513. doi: 10.1016/j.molstruc.2023.136513
26. Dao A, Kushwaha R, Kumar A, Huang H, and Banerjee S. Engineered exosomes as a photosensitizer delivery platform for cancer photodynamic therapy. ChemMedChem. (2022) 17:e202200119. doi: 10.1002/cmdc.202200119
27. Pan S, Pei L, Zhang A, Zhang Y, Zhang C, Huang M, et al. Passion fruit-like exosome-PMA/Au-BSA@Ce6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials. (2020) 230:119606. doi: 10.1016/j.biomaterials.2019.119606
28. Zhao Y, Liu P, Tan H, Chen X, Wang Q, and Chen T. Exosomes as smart nanoplatforms for diagnosis and therapy of cancer. Front Oncol. (2021) 11:743189. doi: 10.3389/fonc.2021.743189
29. Ma X, Chen N, Zeng P, He Y, Zhang T, Lu Y, et al. Hypericum perforatum-derived exosomes-like nanovesicles: a novel natural photosensitizer for effective tumor photodynamic therapy. Int J Nanomedicine. (2025) 20:1529–41. doi: 10.2147/IJN.S510339
30. Guo Z, Li G, Shen L, Pan J, Dou D, Gong Y, et al. Ginger-derived exosome-like nanoparticles loaded with indocyanine green enhances phototherapy efficacy for breast cancer. Int J Nanomedicine. (2025) 20:1147–69. doi: 10.2147/IJN.S478435
31. Lee YH and Huang CY. Engineered perfluorochemical cancer-derived exosomes loaded with indocyanine green and camptothecin provide targeted photochemotherapy for effective cancer treatment. Int J Nanomedicine. (2025) 20:327–42. doi: 10.2147/IJN.S505458
32. Li M, Yin S, Xu A, Kang L, Ma Z, Liu F, et al. Synergistic phototherapy-molecular targeted therapy combined with tumor exosome nanoparticles for oral squamous cell carcinoma treatment. Pharmaceutics. (2024) 16:33. doi: 10.3390/pharmaceutics16010033
33. Kang T, Wu X, Wang F, Shi Y, Wei F, Dong M, et al. Ir(III)-based photosensitizer-loaded M1 macrophage exosomes for synergistic photodynamic therapy. Adv Funct Mater. (2024) 34:2408142. doi: 10.1002/adfm.202408142
34. Feng T, Tang Z, Karges J, Shen J, Jin C, Chen Y, et al. Exosome camouflaged coordination-assembled iridium (III) photosensitizers for apoptosis–autophagy–ferroptosis induced combination therapy against melanoma. Biomaterials. (2023) 301:122212. doi: 10.1016/j.biomaterials.2023.122212
35. Huang Z, Wei G, Zeng Z, Huang Y, Huang L, Shen Y, et al. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics. (2019) 9:5542–57. doi: 10.7150/thno.35343
36. Xie J, Zheng Z, Tuo L, Deng X, Tang H, Peng C, et al. Recent advances in exosome-based immunotherapy applied to cancer. Front Immunol. (2023) 14:1296857. doi: 10.3389/fimmu.2023.1296857
37. Chak PT, Kam NW, Choi TH, Dai W, and Kwong DLW. Unfolding the complexity of exosome–cellular interactions on tumour immunity and their clinical prospects in nasopharyngeal carcinoma. Cancers. (2024) 16:919. doi: 10.3390/cancers16050919
38. Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, and Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. (2016) 111:55–65. doi: 10.1016/j.biomaterials.2016.09.031
39. Rezaie J, Ajezi S, Avci ÇB, Karimipour M, Geranmayeh MH, Nourazarian A, et al. Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol. (2018) 55:3372–93. doi: 10.1007/s12035-017-0582-7
40. Wang W, Zou C, Liu X, He L, Cao Z, Zhu M, et al. Biomimetic dendritic cell-based nanovaccines for reprogramming the immune microenvironment to boost tumor immunotherapy. ACS Nano. (2024) 18:34063–76. doi: 10.1021/acsnano.4c09653
41. Cheng Q, Dai Z, Smbatyan G, Epstein AL, Lenz HJ, and Zhang Y. Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes. Mol Ther. (2022) 30:3066–73. doi: 10.1016/j.ymthe.2022.06.013
42. Kugeratski FG and Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. (2021) 288:10–35. doi: 10.1111/febs.15558
43. Yadav D and Malviya R. Exploring potential of exosomes drug delivery system in the treatment of cancer: advances and prospective. Med Drug Discov. (2023) 20:100163. doi: 10.1016/j.medidd.2023.100163
44. Gutierrez-Millan C, Calvo Díaz C, Lanao JM, and Colino CI. Advances in exosomes-based drug delivery systems. Macromol Biosci. (2021) 21:2000269. doi: 10.1002/mabi.202000269
45. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. (2014) 35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083
46. Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, and Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. (2020) 261:118369. doi: 10.1016/j.lfs.2020.118369
47. Lennaárd AJ, Mamand DR, Wiklander RJ, El Andaloussi S, and Wiklander OP. Optimised electroporation for loading of extracellular vesicles with doxorubicin. Pharmaceutics. (2021) 14:38. doi: 10.3390/pharmaceutics14010038
48. Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta Biomembr. (2005) 1718:22–31. doi: 10.1016/j.bbamem.2005.10.011
49. Viguera AR, González-Mañas JM, Taneva S, and Goñi FM. Early and delayed stages in the solubilization of purple membrane by a polyoxyethylenic surfactant. Biochim Biophys Acta Biomembr. (1994) 1196:76–80. doi: 10.1016/0005-2736(94)90297-6
50. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. (2013) 165:77–84. doi: 10.1016/j.jbiotec.2013.03.013
51. Morishita M, Takahashi Y, Nishikawa M, and Takakura Y. Pharmacokinetics of exosomes—an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J Pharm Sci. (2017) 106:2265–9. doi: 10.1016/j.xphs.2017.02.030
52. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, and Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. (2015) 199:145–55. doi: 10.1016/j.jconrel.2014.12.013
53. Davis SS and Illum L. Absorption enhancers for nasal drug delivery. Clin Pharmacokinet. (2003) 42:1107–28. doi: 10.2165/00003088-200342130-00003
54. Wong AD, Ye M, Ulmschneider MB, and Searson PC. Quantitative analysis of the enhanced permeation and retention (EPR) effect. PloS One. (2015) 10:e0123461. doi: 10.1371/journal.pone.0123461
55. Chow TH, Lin YY, Hwang JJ, Wang HE, Tseng YL, Pang VF, et al. Therapeutic efficacy evaluation of 111In-labeled PEGylated liposomal vinorelbine in murine colon carcinoma. J Control Release. (2009) 137:136–43. doi: 10.1016/j.jconrel.2009.03.015
56. Lin Y, Lu Y, and Li X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J Drug Targeting. (2020) 28:129–41. doi: 10.1080/1061186X.2019.1641508
57. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. (2017) 546:498–503. doi: 10.1038/nature22341
58. Zhang H, Wu J, Wu J, Fan Q, Zhou J, Wu J, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnol. (2019) 17:29. doi: 10.1186/s12951-019-0461-7
59. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, et al. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. (2019) 86:1–14. doi: 10.1016/j.actbio.2018.12.045
60. Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. (2015) 6:10253–68. doi: 10.18632/oncotarget.3520
61. Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, et al. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. (2010) 17:398–408. doi: 10.1038/cgt.2009.88
62. Gilligan KE and Dwyer RM. Engineering exosomes for cancer therapy. Int J Mol Sci. (2017) 18:1122. doi: 10.3390/ijms18061122
63. Li P, Xie Y, Wang J, Bao C, Duan J, Liu Y, et al. Gene engineered exosome reverses T cell exhaustion in cancer immunotherapy. Bioact Mater. (2024) 34:466–81. doi: 10.1016/j.bioactmat.2024.01.008
64. Li Q, Song Q, Zhao Z, Lin Y, Cheng Y, Karin N, et al. Genetically engineered artificial exosome-constructed hydrogel for ovarian cancer therapy. ACS Nano. (2023) 17:10376–92. doi: 10.1021/acsnano.3c00804
65. Mkhobongo B, Chandran R, and Abrahamse H. The role of melanoma cell-derived exosomes (MTEX) and photodynamic therapy (PDT) within a tumor microenvironment. Int J Mol Sci. (2021) 22:9726. doi: 10.3390/ijms22189726
66. Ahmed F, Tamma M, Pathigadapa U, Reddanna P, and Yenuganti VR. Drug loading and functional efficacy of cow, buffalo, and goat milk-derived exosomes: a comparative study. Mol Pharm. (2022) 19:763–74. doi: 10.1021/acs.molpharmaceut.1c00182
67. Fuhrmann G, Serio A, Mazo M, Nair R, and Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. (2015) 205:35–44. doi: 10.1016/j.jconrel.2014.11.029
68. Yuan Z, Kolluri KK, Gowers KH, and Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. (2017) 6:1265291. doi: 10.1080/20013078.2017.1265291
69. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3 receptor-targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. (2017) 7:1333–45. doi: 10.7150/thno.17092
70. Gomari H, Forouzandeh Moghadam M, and Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. (2018) 11:5753–62. doi: 10.2147/OTT.S173110
71. Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. (2013) 21:185–91. doi: 10.1038/mt.2012.180
72. Chulpanova DS, Kitaeva KV, James V, Rizvanov AA, Solovyeva VV, et al. Therapeutic prospects of extracellular vesicles in cancer treatment. Front Immunol. (2018) 9:1534. doi: 10.3389/fimmu.2018.01534
73. Wan Z, Gan X, Mei R, Du J, Fan W, Wei M, et al. ROS triggered local delivery of stealth exosomes to tumors for enhanced chemo/photodynamic therapy. J Nanobiotechnol. (2022) 20:385. doi: 10.1186/s12951-022-01591-7
74. Choi J, Jang H, Park D, Park B, Jang Y, Lee S, et al. Oral delivery of photoresponsive milk-derived exosomes for the local therapy of glioblastoma. ACS Mater Lett. (2024) 6:4019–27. doi: 10.1021/acsmaterialslett.4c01122
75. Surana R, LeBleu VS, Lee JJ, Smaglo BG, Zhao D, Lee MS, et al. Phase I study of mesenchymal stem cell (MSC)-derived exosomes with KRASG12D siRNA in patients with metastatic pancreatic cancer harboring a KRASG12D mutation. J Clin Oncol. (2022) 40:TPS633. doi: 10.1200/JCO.2022.40.4_suppl.TPS633
76. Viaud S, Ploix S, Lapierre V, Théry C, Commere PH, Tramalloni D, et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-γ. J Immunother. (2011) 34:65–75. doi: 10.1097/CJI.0b013e3181fe535b
77. Torchilin VP. Multifunctional pharmaceutical nanocarriers: Development of the concept. In: Torchilin VP, editor. Multifunctional Pharmaceutical Nanocarriers. Springer, New York, NY (2008). p. 1–32. doi: 10.1007/978-0-387-76554-9_1
78. Allen TM and Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Delivery Rev. (2013) 65:36–48. doi: 10.1016/j.addr.2012.09.037
79. Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit Rev Ther Drug Carrier Syst. (2009) 26:523–80. doi: 10.1615/CritRevTherDrugCarrierSyst.v26.i6.10
80. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. (2015) 6:286. doi: 10.3389/fphar.2015.00286
81. Chime SA and Onyishi IV. Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. (2013) 7:3034–59. doi: 10.5897/AJPPX2013.0004
82. Shrestha H, Bala R, and Arora S. Lipid-based drug delivery systems. J Pharm. (2014) 2014:801820. doi: 10.1155/2014/801820
83. Kim SJ, Puranik N, Yadav D, Jin JO, and Lee PC. Lipid nanocarrier-based drug delivery systems: therapeutic advances in the treatment of lung cancer. Int J Nanomedicine. (2023) 18:2659–76. doi: 10.2147/IJN.S406415
84. Tufail SSM, Prakash KJ, and Wadood KSA. Formulation and characterization of herbosome: A review. Int J Res Pharm Allied Sci. (2025) 4:167–73. doi: 10.71431/IJRPAS.2025.4514
85. Kalaivani P and Kamaraj R. Phytosome technology: A novel breakthrough for the health challenges. Cureus. (2024) 16:e68180. doi: 10.7759/cureus.68180
86. Kumar S, Antal S, Kumar D, Tanwar R, and Mor A. Insights into novel promising drug delivery system–cryptosomes. Res J Pharm Technol. (2024) 17:5666–70. doi: 10.52711/0974-360X.2024.00863
87. Fadaei MS, Fadaei MR, Kheirieh AE, Rahmanian-Devin P, Dabbaghi MM, Tavallaei KN, et al. Niosome as a promising tool for increasing the effectiveness of anti-inflammatory compounds. EXCLI J. (2024) 23:212–23. doi: 10.17179/excli2023-6868
88. Rezaei H, Iranbakhsh A, Sepahi AA, Mirzaie A, Larijani K, et al. Formulation, preparation of niosome loaded zinc oxide nanoparticles and biological activities. Sci Rep. (2024) 14:16692. doi: 10.1038/s41598-024-67509-5
89. Chauhan N, Vasava P, Khan SL, Siddiqui FA, Islam F, Chopra H, et al. Ethosomes: A novel drug carrier. Ann Med Surg. (2022) 82:104595. doi: 10.1016/j.amsu.2022.104595
90. Gupta DK, Ahad A, Waheed A, Aqil M, Al-Jenoobi FI, and Al-Mohizea AM. Bilosomes: a novel platform for drug delivery. In: Systems of Nanovesicular Drug Delivery. London, United Kingdom: Academic Press (2022). p. 293–309. doi: 10.1016/B978-0-323-91864-0.00004-8
91. Rajan R, Jose S, Mukund VPB, and Vasudevan DT. Transferosomes – A vesicular transdermal delivery system for enhanced drug permeation. J Adv Pharm Technol Res. (2011) 2:138–43. doi: 10.4103/2231-4040.85524
92. Mehnert W and Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Delivery Rev. (2012) 64:83–101. doi: 10.1016/j.addr.2012.09.021
93. Müller RH, Shegokar R, Gohla S, and Keck CM. Nanocrystals: production, cellular drug delivery, current and future products. In: Intracellular delivery: Fundamentals and applications. Springer Netherlands, Dordrecht (2011). p. 411–32. doi: 10.1007/978-94-007-1248-5_15
94. Immordino ML, Dosio F, and Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. (2006) 1:297–315. doi: 10.2147/DIJN.1.S633
95. Beloqui A, Memvanga PB, Coco R, Reimondez-Troitino S, Alhouayek M, Muccioli GG, et al. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids Surf B Biointerfaces. (2016) 143:327–35. doi: 10.1016/j.colsurfb.2016.03.038
96. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. (2018) 16:71. doi: 10.1186/s12951-018-0392-8
97. Bulbake U, Doppalapudi S, Kommineni N, and Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. (2017) 9:12. doi: 10.3390/pharmaceutics9020012
98. Gu L, Sun R, Wang W, and Xia Q. Nanostructured lipid carriers for the encapsulation of phloretin: Preparation and in vitro characterization studies. Chem Phys Lipids. (2022) 242:105150. doi: 10.1016/j.chemphyslip.2021.105150
99. Zarif L, Graybill TL, Gounder M, Gao J, and Demirjian J. Cochleates: new lipid-based drug delivery system. J Liposome Res. (2000) 10:523–38. doi: 10.3109/08982100009031116
100. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. (2013) 8:102. doi: 10.1186/1556-276X-8-102
101. Solans C, Izquierdo P, Nolla J, Azemar N, and Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. (2005) 10:102–10. doi: 10.1016/j.cocis.2005.06.004
102. Müller RH, Radtke M, and Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. (2002) 242:121–28. doi: 10.1016/S0378-5173(02)00180-1
103. Owens DE and Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. (2006) 307:93–102. doi: 10.1016/j.ijpharm.2005.10.010
104. Danhier F, Feron O, and Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. (2010) 148:135–46. doi: 10.1016/j.jconrel.2010.08.027
105. Bae Y and Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release. (2011) 153:198–205. doi: 10.1016/j.jconrel.2011.06.001
106. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, and Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. (2007) 2:751–60. doi: 10.1038/nnano.2007.387
107. Koo H, Huh MS, Ryu JH, Lee DE, Sun IC, Choi K, et al. Nanoprobes for biomedical imaging in living systems. Nano Today. (2011) 6:204–20. doi: 10.1016/j.nantod.2011.02.007
108. Abrahamse H and Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. (2016) 473:347–64. doi: 10.1042/BJ20150942
109. Yoon I, Li JZ, and Shim YK. Advance in photosensitizers and light delivery for photodynamic therapy. Clin Endosc. (2013) 46:7–23. doi: 10.5946/ce.2013.46.1.7
110. Zhao Z, Shi S, Huang Y, Tang S, and Chen X. Simultaneous photodynamic and photothermal therapy using photosensitizer-functionalized Pd nanosheets by single continuous wave laser. ACS Appl Mater Interfaces. (2014) 6:8878–85. doi: 10.1021/am501608c
111. Allison RR and Moghissi K. Photosensitizers in clinical PDT. Photodiagn Photodyn Ther. (2004) 1:27–42. doi: 10.1016/S1572-1000(04)00007-9
112. Kou J, Dou D, and Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget. (2017) 8:81591–603. doi: 10.18632/oncotarget.20189
113. Fahmy SA, Azzazy HME-S, and Schaefer J. Liposome photosensitizer formulations for effective cancer photodynamic therapy. Pharmaceutics. (2021) 13:1345. doi: 10.3390/pharmaceutics13091345
114. Yakavets I, Millard M, Lamy L, François A, Scheglmann D, Wiehe A, et al. Matryoshka-type liposomes offer the improved delivery of temoporfin to tumor spheroids. Cancers. (2019) 11:1366. doi: 10.3390/cancers11091366
115. Simões MM, Paiva KL, de Souza IF, Mello VC, Martins da Silva IG, Souza PE, et al. The potential of photodynamic therapy using solid lipid nanoparticles with aluminum phthalocyanine chloride as a nanocarrier for modulating immunogenic cell death in murine melanoma. vitro Pharm. (2024) 16:941. doi: 10.3390/pharmaceutics16070941
116. Lima AM, Dal Pizzol C, Monteiro FB, Creczynski-Pasa TB, Andrade GP, Ribeiro AO, et al. Hypericin encapsulated in solid lipid nanoparticles: phototoxicity and photodynamic efficiency. J Photochem Photobiol B. (2013) 125:146–54. doi: 10.1016/j.jphotobiol.2013.05.010
117. Brezaniova I, Hruby M, Kralova J, Kral V, Cernochova Z, Cernoch P, et al. Temoporfin-loaded 1-tetradecanol-based thermoresponsive solid lipid nanoparticles for photodynamic therapy. J Control Release. (2016) 241:34–44. doi: 10.1016/j.jconrel.2016.09.009
118. Yeo S, Lee TH, Kim MJ, Shim YK, Yoon I, Song YK, et al. Improved anticancer efficacy of methyl pyropheophorbide-a–incorporated solid lipid nanoparticles in photodynamic therapy. Sci Rep. (2023) 13:7391. doi: 10.1038/s41598-023-34265-x
119. Mendes T, Granja A, and Reis S. Lipid nanoparticles as efficient verteporfin nanocarriers for photodynamic therapy of cancer. Mater Adv. (2024) 5:1137–46. doi: 10.1039/D3MA00797A
120. Rijcken CJ, Hofman JW, van Zeeland F, Hennink WE, and van Nostrum CF. Photosensitiser-loaded biodegradable polymeric micelles: preparation, characterisation and in vitro PDT efficacy. J Control Release. (2007) 124:144–53. doi: 10.1016/j.jconrel.2007.09.002
121. van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Delivery Rev. (2004) 56:9–16. doi: 10.1016/j.addr.2003.07.013
122. Li M, Li X, Cao Z, Wu Y, Chen JA, Gao J, et al. Mitochondria-targeting BODIPY-loaded micelles as novel class of photosensitizer for photodynamic therapy. Eur J Med Chem. (2018) 157:599–609. doi: 10.1016/j.ejmech.2018.08.024
123. Li X, Gao M, Xin K, Zhang L, Ding D, Kong D, et al. Singlet oxygen-responsive micelles for enhanced photodynamic therapy. J Control Release. (2017) 260:12–21. doi: 10.1016/j.jconrel.2017.05.025
124. Chen Y, Pan L, Jiang M, Li D, and Jin L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Deliv. (2016) 23:1383–92. doi: 10.3109/10717544.2015.1049719
125. Qidwai A, Khan S, Md S, Fazil M, Baboota S, Narang JK, et al. Nanostructured lipid carrier in photodynamic therapy for the treatment of basal-cell carcinoma. Drug Deliv. (2016) 23:1476–85. doi: 10.3109/10717544.2016.1165310
126. Pucci C, De Pasquale D, Degl'Innocenti A, Montorsi M, Desii A, Pero M, et al. Chlorin e6-loaded nanostructured lipid carriers targeted by Angiopep-2: Advancing photodynamic therapy in glioblastoma. Adv Healthc Mater. (2025) 14:2402823. doi: 10.1002/adhm.202402823
127. Mello VC, Araújo VH, de Paiva KL, Simões MM, Marques DC, da Silva Costa NR, et al. Development of new natural lipid-based nanoparticles loaded with aluminum-phthalocyanine for photodynamic therapy against melanoma. Nanomaterials. (2022) 12:3547. doi: 10.3390/nano12203547
128. Moret F, Scheglmann D, and Reddi E. Folate-targeted PEGylated liposomes improve the selectivity of PDT with meta-tetra(hydroxyphenyl)chlorin (m-THPC). Photochem Photobiol Sci. (2013) 12:823–34. doi: 10.1039/C3PP25384H
129. Mellman I, Coukos G, and Dranoff G. Cancer immunotherapy comes of age. Nature. (2011) 480:480–89. doi: 10.1038/nature10673
130. Chen DS and Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
131. Zhang B, Wu Q, Zhou YL, Guo X, Ge J, and Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. (2018) 63:292–98. doi: 10.1016/j.intimp.2018.08.014
132. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, and Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. (2017) 16:489–96. doi: 10.1038/nmat4822
133. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. (2016) 534:396–401. doi: 10.1038/nature18300
134. Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. (2017) 17:1326–35. doi: 10.1021/acs.nanolett.6b03329
135. Gao A, Hu XL, Saeed M, Chen BF, Li YP, and Yu HJ. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol Sin. (2019) 40:1129–37. doi: 10.1038/s41401-019-0281-1
136. Olusanya TO, Haj Ahmad RR, Ibegbu DM, Smith JR, and Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. (2018) 23:907. doi: 10.3390/molecules23040907
137. Gu Z, Da Silva CG, van der Maaden K, Ossendorp F, and Cruz LJ. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics. (2020) 12:1054. doi: 10.3390/pharmaceutics12111054
138. Yuba E, Harada A, Sakanishi Y, Watarai S, and Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. (2013) 34:3042–52. doi: 10.1016/j.biomaterials.2012.12.031
139. Xiao Y, Chen J, Zhou H, Zeng X, Ruan Z, Pu Z, et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat Commun. (2022) 13:758. doi: 10.1038/s41467-022-28279-8
140. Wan Z, Zheng R, Moharil P, Liu Y, Chen J, Sun R, et al. Polymeric micelles in cancer immunotherapy. Molecules. (2021) 26:1220. doi: 10.3390/molecules26051220
141. Panagi M, Mpekris F, Chen P, Voutouri C, Nakagawa Y, Martin JD, et al. Polymeric micelles effectively reprogram the tumor microenvironment to potentiate nano-immunotherapy in mouse breast cancer models. Nat Commun. (2022) 13:7165. doi: 10.1038/s41467-022-34744-1
142. Park J, Nah Y, and Kim WJ. IDO-triggered swellable polymeric micelles for IDO inhibition and targeted cancer immunotherapy. J Control Release. (2023) 363:496–506. doi: 10.1016/j.jconrel.2023.09.050
143. Zhu H, Ma K, Ruan R, Yang C, Yan A, Li J, et al. Tumor-targeted self-assembled micelles reducing PD-L1 expression combined with ICIs to enhance chemo-immunotherapy of TNBC. Chin Chem Lett. (2024) 35:108536. doi: 10.1016/j.cclet.2023.108536
144. Cheng L, Yu J, Hao T, Wang W, Wei M, and Li G. Advances in polymeric micelles: responsive and targeting approaches for cancer immunotherapy in the tumor microenvironment. Pharmaceutics. (2023) 15:2622. doi: 10.3390/pharmaceutics15112622
145. Mi Y, Hagan CT IV, Vincent BG, and Wang AZ. Emerging nano-/microapproaches for cancer immunotherapy. Adv Sci. (2019) 6:1801847. doi: 10.1002/advs.201801847
146. Li S, Yang Y, Lin X, Li Z, Ma G, Su Z, et al. Biocompatible cationic solid lipid nanoparticles as adjuvants effectively improve humoral and T cell immune response of foot and mouth disease vaccines. Vaccine. (2020) 38:2478–86. doi: 10.1016/j.vaccine.2020.02.004
147. Yeo S, Wu H, Yoon I, Kim HS, Song YK, and Lee WK. Enhanced photodynamic therapy efficacy through solid lipid nanoparticle of purpurin-18-N-propylimide methyl ester for cancer treatment. Int J Mol Sci. (2024) 25:10382. doi: 10.3390/ijms251910382
148. Iqbal A, Zaman M, Wahab Amjad M, Adnan S, Abdul Ghafoor Raja M, Haider Rizvi SF, et al. Solid lipid nanoparticles of mycophenolate mofetil: an attempt to control the release of an immunosuppressant. Int J Nanomedicine. (2020) 15:5603–12. doi: 10.2147/IJN.S255636
149. Jain V, Gupta A, Pawar VK, Asthana S, Jaiswal AK, Dube A, et al. Chitosan-assisted immunotherapy for intervention of experimental leishmaniasis via amphotericin B-loaded solid lipid nanoparticles. Appl Biochem Biotechnol. (2014) 174:1309–30. doi: 10.1007/s12010-014-1084-y
150. Ye J, Wang Q, Zhou X, and Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm. (2008) 352:273–79. doi: 10.1016/j.ijpharm.2007.10.014
151. Almeida AJ and Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Delivery Rev. (2007) 59:478–90. doi: 10.1016/j.addr.2007.04.007
152. Hasegawa U, van der Vlies AJ, Simeoni E, Wandrey C, and Hubbell JA. Carbon monoxide-releasing micelles for immunotherapy. J Am Chem Soc. (2010) 132:18273–80. doi: 10.1021/ja1075025
153. Chen Q, Xu L, Liang C, Wang C, Peng R, and Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. (2016) 7:13193. doi: 10.1038/ncomms13193
154. Wang Y, Wang M, Wu HX, and Xu RH. Advancing to the era of cancer immunotherapy. Cancer Commun. (2021) 41:803–29. doi: 10.1002/cac2.12178
155. Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, and Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. (2022) 16:17802–46. doi: 10.1021/acsnano.2c08774
156. Gao Y, Liu J, Wu M, Zhang Y, Wang M, Lyu Q, et al. Photosensitive hybrid γδ-T exosomes for targeted cancer photoimmunotherapy. ACS Nano. (2025) 19:4251–68. doi: 10.1021/acsnano.4c11024
157. Nakase I and Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. (2015) 5:10112. doi: 10.1038/srep10112
158. Kim J, Lee Y, Kang Y, Kim D, Lee H, Han S, et al. Dual-delivery of exosome inhibitor and immune-activating gene via lipid nano-assemblies for tumor immune evasion inhibition. J Control Release. (2025) 381:113569. doi: 10.1101/gr.089417.108
159. Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. (2020) 10:7889–905. doi: 10.7150/thno.45028
160. Liu X, Chen Y, Zhang Y, Chen Q, Chen Y, Qiu X, et al. Hybrid adipocyte-derived exosome nano platform for potent chemo-phototherapy in targeted hepatocellular carcinoma. J Control Release. (2024) 370:168–81. doi: 10.1016/j.jconrel.2024.04.031
161. Golchin A, Shams F, Basiri A, Ranjbarvan P, Kiani S, Sarkhosh-Inanlou R, et al. Combination therapy of stem cell-derived exosomes and biomaterials in the wound healing. Stem Cell Rev Rep. (2022) 18:1892–911. doi: 10.1007/s12015-021-10309-5
162. Kim H, Jang H, Cho H, Choi J, Hwang KY, Choi Y, et al. Recent advances in exosome-based drug delivery for cancer therapy. Cancers. (2021) 13:4435. doi: 10.3390/cancers13174435
163. Skotland T, Llorente A, and Sandvig K. Lipids in extracellular vesicles: what can be learned about membrane structure and function? Cold Spring Harb Perspect Biol. (2023) 15:a041415. doi: 10.1101/cshperspect.a041415
164. Li L, Liu H, Xu H, Li Y, Zhang L, Meng Q, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR-497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. (2022) 20:50. doi: 10.1186/s12951-022-01264-5
165. Moholkar DN, Shinde K, Deshmukh S, Dandekar S, Pawar S, Naik J, et al. Advances in lipid-based carriers for cancer therapeutics: liposomes, exosomes and hybrid exosomes. Cancer Lett. (2023) 565:216–20. doi: 10.1016/j.canlet.2023.216220
166. Liu P, Wang Y, Zhang H, Zhou M, Wang X, Zhang J, et al. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J Mater Chem B. (2019) 7:6924–33. doi: 10.1039/C9TB01573F
Keywords: exosomes, nanocarriers, photodynamic therapy, immunotherapy and cancer, liposomes
Citation: Swain A, Jena SR and Samanta L (2025) Smart nanocarriers for cancer: harnessing exosomes and lipid systems in photodynamic and immunotherapy. Front. Immunol. 16:1687953. doi: 10.3389/fimmu.2025.1687953
Received: 18 August 2025; Accepted: 29 September 2025;
Published: 17 October 2025.
Edited by:
Paromita Sarbadhikary, Faculty of Health Sciences, University of Johannesburg, South AfricaReviewed by:
Arpana Parihar, Advanced Materials and Processes Research Institute (CSIR), IndiaChanda Bhandari, The University of Texas at Dallas, United States
Copyright © 2025 Swain, Jena and Samanta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luna Samanta, bHNhbWFudGFAcmF2ZW5zaGF3dW5pdmVyc2l0eS5hYy5pbg==; Soumya Ranjan Jena, c3JqZW5hQHJhdmVuc2hhd3VuaXZlcnNpdHkuYWMuaW4=
 Amrita Swain
Amrita Swain Soumya Ranjan Jena
Soumya Ranjan Jena Luna Samanta
Luna Samanta