- 1Department of Critical Care Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Anesthesia and Respiratory Critical Medicine, Jinan, Shandong, China
- 2School of Clinical and Basic Medical Sciences, Shandong First Medical University& Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 3Key Laboratory of Endocrine Glucose & Lipids Metabolism and Brain Aging, Ministry of Education; Department of Endocrinology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Sepsis induced acute lung injury/acute respiratory distress syndrome (ALI/ARDS) remains a devastating complication of sepsis, marked by uncontrolled pulmonary inflammation, alveolar–capillary barrier disruption, and high mortality. Despite advances in supportive care, no targeted medicines are currently available. Ferroptosis is an iron-dependent and non-apoptotic form of cell death characterized by the iron induced accumulation of lipid reactive oxygen species (ROS). Emerging evidence indicates that ferroptosis is involved in the progression of sepsis induced ALI/ARDS, although the mechanism of action of ferroptosis in sepsis induced ALI/ARDS is still poorly understood. This mini-review summarizes the mechanism of ferroptosis action on sepsis induced ALI/ARDS, with particular focus on immune dysregulation, endothelial/epithelial dysfunction, and oxidative stress. We highlight key molecular pathways, including glutathione peroxidase 4 (GPX4) inactivation, iron metabolism disruption, and lipid peroxidation cascades, supported by both preclinical studies and emerging clinical correlates. Furthermore, discuss the potential therapeutic approaches currently used to treat ARDS. This review also discusses major challenges to clinical translation and highlights further directions for the treatment and prevention of sepsis induced ALI/ARDS.
1 Introduction
Sepsis, a prevalent critical condition in clinics, continues to be the leading cause of death from infections and a global healthcare issue. Among the organs susceptible to the harmful effects of sepsis, the lungs are notably the most frequently affected. Consequently, patients with sepsis are predisposed to developing acute lung injury (ALI), and in severe cases, acute respiratory distress syndrome (ARDS) with a high mortality rate, exceeding 30% (1, 2). Despite significant advance in the understanding and management of ALI/ARDS, there remains a substantial lack of drugs capable of effectively treating ALI/ARDS induced by sepsis due to limited research on its underling mechanism.
Ferroptosis was proposed by Dixon (3) in 2012, driven by iron-dependent phospholipid peroxidation, is regulated by multiple cellular metabolic pathways, including redox homeostasis, iron handling, and metabolism of amino acids, lipids and sugars, in addition to various signaling pathways relevant to disease. Numerous organ injuries and degenerative pathologies are driven by ferroptosis, such as neurodegenerative diseases (4), endocrine diseases and cancer (5, 6). In recent years, the role of ferroptosis in sepsis induced ALI/ARDS has received more research attention (7, 8). The research on the role of ferroptosis in sepsis induced ALI/ARDS has progressed rapidly. Studies has highlighted the importance of immune dysfunction, oxidative stress, and barrier disruption in its pathogenesis. This mini-review integrates the latest (2020–2025) advances in understanding ferroptosis in sepsis-induced ALI/ARDS, emphasizing the role of ferroptosis and the potential therapeutic strategies by targeting ferroptosis. Moreover, we discuss its significance in the treatment of sepsis-induced ALI/ARDS and provide new directions for the treatment and prevention of sepsis-induced ALI/ARDS by targeting ferroptosis.
2 Ferroptosis in sepsis-induced ALI/ARDS
2.1 Molecular mechanism of ferroptosis
Intracellular labile iron (Fe²+) is central to ferroptosis, catalyzing the Fenton reaction to generate hydroxyl radicals (•OH), which initiate lipid peroxidation (9). Thus, ferroptosis fundamentally reflects a breakdown of redox homeostasis. Iron balance is tightly regulated by uptake, export, and storage. Transferrin receptor 1 (TfR1) mediates iron entry, and its upregulation in sepsis-induced ARDS correlates with ferroptosis markers (10). Conversely, ferroportin (FPN), the only known iron exporter, is downregulated in sepsis due to hepcidin overexpression, trapping iron intracellularly (11). In septic lung injury, ferroptosis amplifies tissue damage through iron-dependent lipid peroxidation in alveolar epithelial cells. Recent studies reveal that sepsis-induced hypoxia upregulates hypoxia-inducible factor-1α (HIF-1α), which increases cellular iron uptake via transferrin receptor 1 (TfR1). Of course, hepcidin expression requires synergistic activation by IL-6 (inflammatory pathway) and BMP6 (iron-sensing pathway), which exhibits elevated in sepsis-induced ARDS patients (12). Moreover, Ferritin, which sequesters iron, undergoes degradation via ferritinophagy (a selective autophagy process) in sepsis-induced ARDS. Ferritinophagy mediated by Nuclear receptor coactivator 4 (NCOA4), which first precisely recognizes and binds to ferritin and recruits and directs the autophagy machinery to enclose ferritin into autophagosomes, ultimately transporting it to lysosomes for degradation, thereby releasing the free iron for cellular use (13). In addition, ferroptosis activators such as erastin and RSL3 increase intracellular iron accumulation by impairing antioxidant systems (14) (Figure 1). The glutathione (GSH)-GPX4 axis is the primary defense against ferroptosis. GPX4 reduces LPO to non-toxic alcohols by using GSH, but this system is compromised in sepsis-induced ARDS. System Xc- (a cystine/glutamate antiporter) supplies cystine for GSH synthesis. In sepsis, TNF-α and IL-1β downregulate its subunit SLC7A11, reducing GSH levels by 40%–60% in lung tissues (15, 16). Concurrently, sepsis depletes GSH by downregulating the xCT transporter, impairing GPX4 activity (61). This dual hit iron overload + antioxidant failure explains the prominence of ferroptosis in septic lungs. While these mechanisms are increasingly well-characterized, key questions remain regarding cell-type specific responses and the temporal sequence of these interactions during sepsis progression. The above results suggest that ferroptosis is involved in the pathogenesis of lung injury, which will provide a new theoretical basis for the clinical treatment of ARDS. However, no clinical studies have examined the association of these ferroptosis indicators with severity and prognosis of ARDS.
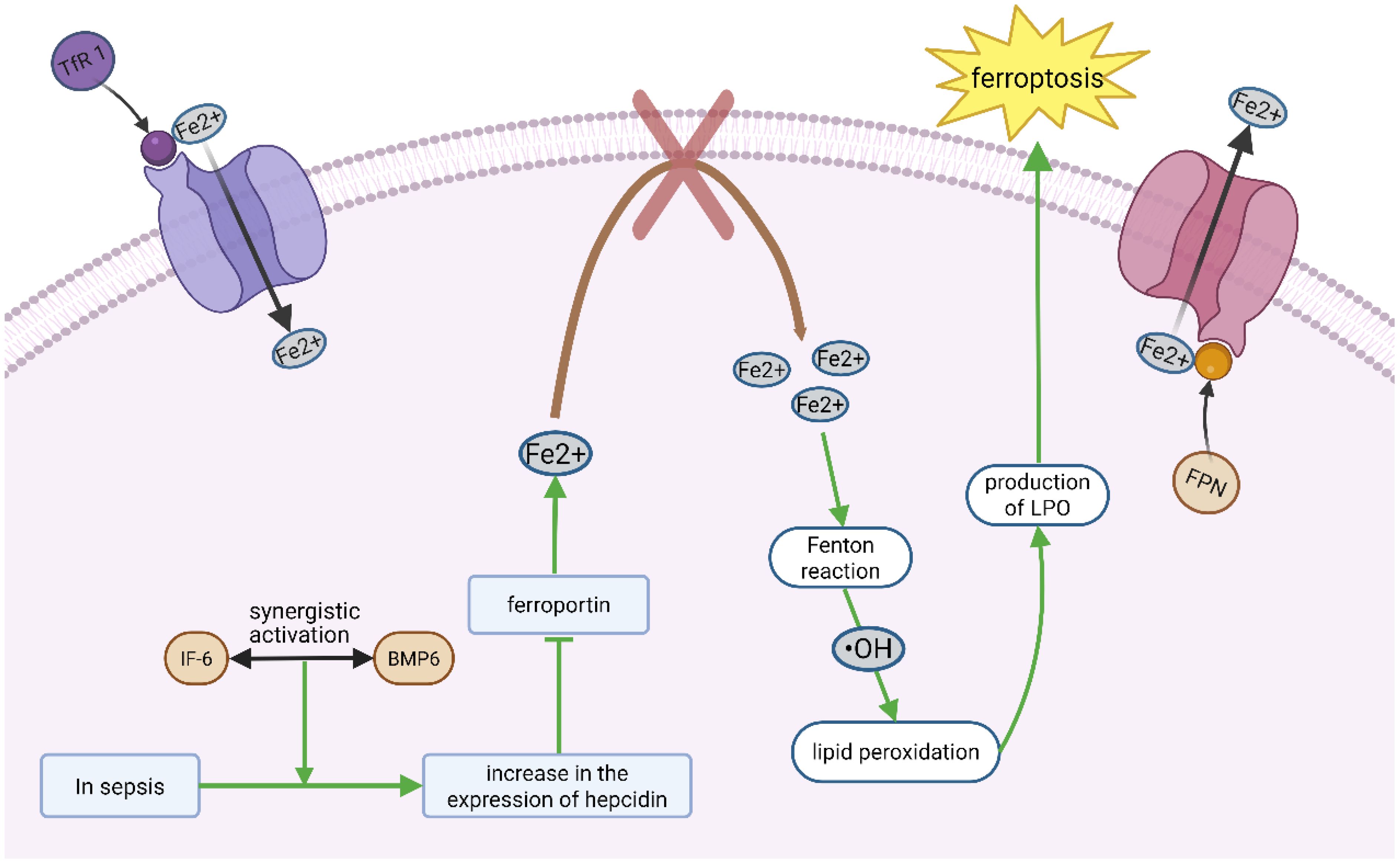
Figure 1. Ferroptosis mainly involves the disruption of redox homeostasis. Transferrin receptor 1 (TfR 1) mediates iron entry, whereas iron transporter (FPN) mediates iron output. Under sepsis conditions, the synergistic activation of IL-6 (inflammatory pathway) and Bmp6 (iron-sensitive pathway) triggers an increased expression of hepcidin, which inhibits the function of the iron export protein ferroportin. As a result, iron is not properly released from the lung cells, causing iron accumulation within the cells. The accumulated free iron generates highly reactive radicals through the Fenton reaction, which initiate and catalyze chain reactions of lipid peroxidation, producing a large amount of lipid peroxides (LPO), which then directly damages the cellular lipid membrane and becomes a significant factor triggering iron-induced cell death.
2.2 Endothelial/epithelial cell damage
Sepsis-induced ARDS releases abundant inflammatory mediators that disrupt the structural integrity of the alveolar–capillary endothelial barrier, causing neutrophil infiltration (NI) and diffuse pulmonary edema (DPE) (17, 18). Macrophage-derived extracellular vesicles promote endothelial ferroptosis, further compromising barrier integrity (19). Through LPO-mediated membrane injury, ferroptosis damages tight junctions, increasing alveolar-capillary permeability. Notably, single-cell RNA sequencing identified endothelial ferroptosis as an early sepsis-induced ARDS event, preceding edema formation (20). Extracellular vesicles also play protective roles: for example, ADSC-derived exosomal miR-125b-5p mitigates inflammation-induced pulmonary microvascular endothelial ferroptosis in sepsis-induced ARDS by regulating Keap1/Nrf2/GPX4 expression (21). Type II alveolar epithelial cells (AECs), rich in polyunsaturated fatty acids (PUFAs), are particularly vulnerable to ferroptosis. Their loss impairs surfactant protein B production, worsening hypoxemia (22). Activating the Nrf2/PHB2 pathway in type II AECs reduces mitochondrial injury caused by sepsis in mice, thereby promoting the survival of type II AECs and preventing progression to ALI (23). Additionally, neutrophil extracellular traps (NETs) exacerbate sepsis-induced ARDS by inducing ferroptosis in alveolar epithelial cells (24). (Figure 2). Above data suggests that ferroptosis induced endothelial and epithelial cell ferroptosis damage play an important role in sepsis induced ALI/ARDS, protecting against its ferroptosis will supply potential therapeutic strategy for this disease.
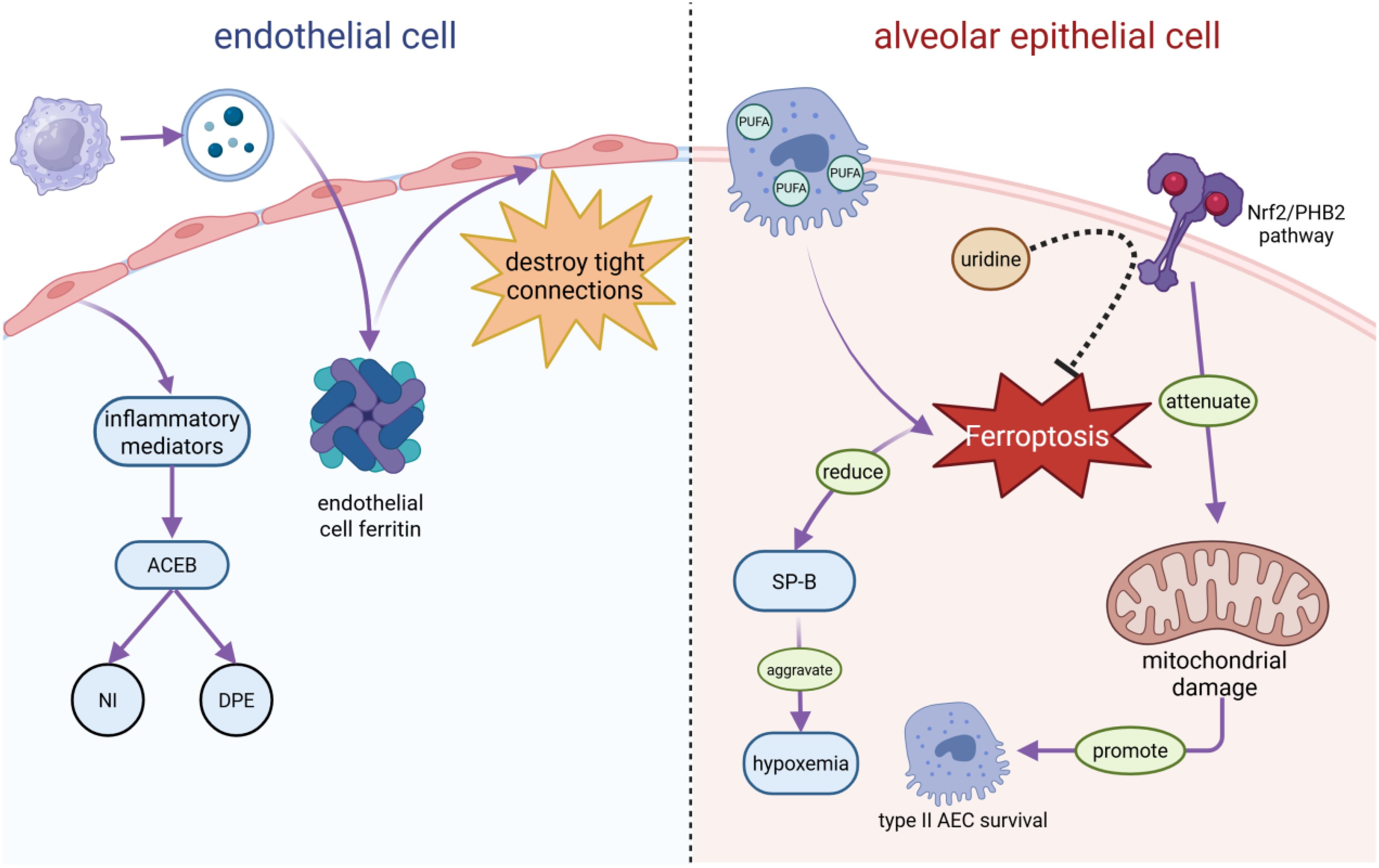
Figure 2. S-ALI causes endothelial and epithelial cell damage. In sepsis-induced ARDS, endothelial cells secrete inflammatory mediators that disrupt the alveolar-capillary endothelial barrier (ACEB), triggering neutrophil infiltration (NI) and diffuse pulmonary edema (DPE). Meanwhile, macrophage-derived extracellular vesicles induce an increase in endothelial cell ferritin, which, in turn, destroys tight connections and increases the alveolar capillary permeability. In alveolar epithelial cells (AECs), type II AECs are prone to iron apoptosis owing to their abundance of polyunsaturated fatty acids (PUFAs), which reduce surfactant protein B (SP-B) and aggravate hypoxemia. The activation of its Nrf2/PHB2 pathway attenuates mitochondrial damage, promotes type II AEC survival, and prevents progression to ALI in sepsis mice. In addition, uridine can inhibit iron apoptosis in macrophages by activating the Nrf2-signaling pathway.
2.3 Immune regulation
Ferroptosis is closely intertwined with immune regulation. Ferritin degradation promotes the release of damage-associated molecular patterns, including HMGB1 and ATP, which in turn activate multifunctional nanozyme systems such as MET-CMS@FeTA (MCMSFT) and the NLRP3 inflammasome, while also driving macrophage polarization (25). From 2021–2023, studies have revealed that ferroptosis-derived LPO directly activates NLRP3, triggering IL-1β and IL-18 secretion, which further enhances ferroptosis through SLC7A11 downregulation (7, 26, 27). Also, pro-inflammatory signals (TNF-α/IL-1β) activate core signaling pathways (mainly NF-κB/MAPK), jointly increasing cellular sensitivity to ferroptosis at multiple levels. The main targets include: downregulation of GSH synthesis genes, upregulation of ACSL4/LPCAT3, and regulation of iron storage and uptake (28).
In septic mice, dysregulated inflammation can cause widespread damage to alveolar epithelial and microvascular endothelial cells, resulting in pulmonary edema and excessive neutrophil infiltration. Sepsis disrupt immune homeostasis, with pulmonary macrophages differentiating into distinct subtypes to regulate inflammation at different stages. Moreover, previous research has shown that mediating the Nrf2 pathway can inhibits ferroptosis in macrophages, thereby exerting a protective effect against sepsis-induced ARDS (24). Moreover, Ferrostatin-1, the inhibitor of ferroptosis, was found to rescue the downregulation of ferroptosis markers including cysteine/glutamate transporter (SLC7A11) and GPX4 in sepsis induced ALI/ARDS (29). Also, STING promotes sepsis-induced ALI/ARDS by inducing macrophage ferroptosis in a cGAS- and interferon-independent manner. Mechanistically, Q237, E316, and S322 in the CBD domain of STING are critical binding sites for the interaction with the coiled-coil domain of NCOA4. Their interaction not only triggers ferritinophagy-mediated ferroptosis, but also maintains the stability of STING dimers leading to enhanced inflammatory response, and reduces the nuclear localization of NCOA4, which impairs the transcription factor coregulator function of NCOA4 (30). Additionally, ferroptotic cell debris skews macrophages toward an M1 phenotype, establishing a proinflammatory feed-forward loop (31). M2 macrophages inhibit ferroptosis in themselves and surrounding cells by secreting substances such as cysteine and lactoferrin, and by utilizing their inherent iron metabolism reprogramming and antioxidant programs (32). Inflammatory mediators generated during sepsis also increase intracellular ROS and oxidative metabolites, further exacerbating lung injury. Itaconate, a metabolite produced during inflammatory macrophage activation, inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced ALI/ARDS (33). Similarly, uridine can activate Nrf2 signaling to suppress macrophage ferroptosis (34). Therefore, macrophage ferroptosis plays a crucial role in sepsis-induced ALI, and the inhibition of macrophage ferroptosis may serve as a novel potential therapeutic strategy for ALI/ARDS. Further animal experiments and clinical studies are needed to verify these points. The regulation of the macrophage inflammation by inhibiting ferroptosis to thereby alleviate ARDS may also be a new therapeutic strategy.
2.4 Clinical correlation
Emerging clinical evidence supports a link between ferroptosis and sepsis-induced ALI/ARDS severity. Plasma LPO markers such as malondialdehyde and 4-hydroxynonenal (4-HNE), along with ferritin, are elevated in sepsis-induced ALI/ARDS patients and correlate with mortality (35). Reduced GPX4 activity and SLC7A11 expression in bronchoalveolar lavage (BAL) cells predict poor clinical outcomes (36), and GPX4 itself has been proposed as a biomarker of ferroptosis (37). Additionally, iNOS-derived nitric oxide in sepsis S-nitrosylates GPX4, inhibiting its activity. A 2025 study demonstrated that iNOS knockout preserved GPX4 function and reduced ferroptosis in sepsis-induced ALI/ARDS (38). Prostaglandin-Endoperoxide Synthase 2 (PTGS2), as an emerging comprehensive biomarker, has an increase that is a sensitive and quantifiable readout of ferroptosis (3). A 2025 study showed that the PRMT1/EGR1/GLS2 signaling axis drives ferroptosis in sepsis-induced ALI/ARDS. Monitoring the expression levels of PRMT1, EGR1, and GLS2 may provide clues for identifying ferroptosis (39). In addition, GSH, as an important intracellular antioxidant, significantly decreases during ferroptosis (40). Increasingly, ferroptosis (“iron death”) is being recognized as a therapeutic target in S-ARDS. Inhibition of ferroptosis significantly alleviates lung tissue injury by modulating pathways such as GPX4 and FSP1. Thus, ferroptosis inhibitors are emerging as promising therapeutic candidates for sepsis induced ALI/ARDS.
However, the current research has key limitations: most mechanistic studies rely on mouse models, but there are differences in iron metabolism pathways between mice and humans (for example, human TfR1 has a broader tissue expression profile), resulting in the clinical translational value of some targets (such as ACSL4) still needing to be validated. In addition, most studies have not directly focused on the relationship between ferroptosis and ALI/ARDS. Therefore, the direct relationship between them needs to be explored, together with more treatments targeting ferroptosis.
3 Therapeutic strategies targeting ferroptosis in sepsis-induced ALI/ARDS
Recent preclinical studies (2020–2025) have validated multiple approaches to inhibit ferroptosis in sepsis-induced ALI/ARDS, many of which exert immunomodulatory effects.
3.1 Radical-trapping antioxidants
Ferrostatin-1 (Fer-1) reduces ferroptosis by scavenging lipid-free radicals (8). Its more stable analog liproxstatin-1 (Lip-1) preserves ~55% of alveolar–capillary barrier integrity in LPS-induced sepsis-induced ALI/ARDS (41). A next-generation ferroptosis inhibitor, SRS-11-94, demonstrated 10-fold higher potency and fewer off-target effects than Fer-1 in human alveolar epithelial cultures (42). Nanotechnology-based approaches are also emerging: DQB@C nanosystems alleviate oxidative stress and inflammation in lung cells by upregulating Slc 7a 11/xCT and downregulating Cox 2, thereby regulating ferroptosis (43). Chalcone reduces pulmonary edema by maintaining the integrity of pulmonary vascular endothelial cells and alveolar epithelial cells due to its antioxidant properties and ability to scavenge oxygen-free radicals (44). Irisin treatment also reduces sepsis-induced lung damage and the levels of oxidative stress-related indicators such as ROS and Fe²+ (45). Multi-mechanism synergistic interventions indicate that sepsis-induced ALI/ARDS is a complex pathological process involving multiple mechanisms and may become a more effective strategy. In the future, drugs may be combined with different mechanisms to more comprehensively inhibit lung injury through synergy.
3.2 Iron chelators
Deferoxamine (DFO) binds unstable iron and reduces iron levels in BAL fluid by 45% when delivered via nebulization in septic rats, effectively alleviating pulmonary edema without causing systemic iron deficiency (46). In multimicrobial sepsis, oral deralarose lowered the levels of ferroptosis markers, such as ACSL4 and 4-HNE, and improved the survival of model animals (47, 48). Targeted iron chelators further improve the intervention effect, such as liposome-encapsulated DFO can be specifically accumulated in lung tissues and its efficacy is 3-fold higher than that of free DFO (49). Moreover, AUF1 inhibits ferroptosis by upregulating NRF2 and downregulating ATF3, thereby reducing sepsis-induced ALI/ARDS (50). Therefore, local targeted iron metabolism is better for avoiding systemic side effects. Optimizing the dosage form of iron chelators can significantly improve the efficacy, indicating that targeted delivery can enhance the specificity and efficiency of intervention.
3.3 GPX4 activation and GSH restoration
Selenium (Se), a cofactor of GPX4, restores its activity; supplementation at 0.5 mg/kg increased GPX4 activity by 70% and reduced LPO in sepsis-induced ALI/ARDS mice (51, 52). N-acetylcysteine, a GSH precursor, reduced BAL fluid LPO by 50% and improved respiratory compliance in septic pigs at medium-to-high doses (53). Zyloxadin also boosted GSH levels in human lung endothelial cells exposed to septic plasma, reducing ferroptosis (54, 55).
3.4 Combination therapies
The combination of ferroptosis inhibitors with classic anti-inflammatory/antioxidant agents, such as ferroptosis inhibitors (Fer-1/Lip-1) combined with glucocorticoids, can simultaneously block the feedback loop of ‘inflammation promoting ferroptosis’ and ‘ferroptosis exacerbating inflammation,’ achieving synergistic protection (56). In addition, the combination of an iron chelator (deferoxamine, DFO) with glutathione precursors can simultaneously address the two fundamental problems of ‘iron overload’ and ‘antioxidant system failure’ (57). Combining ferroptosis inhibitors with pyroptosis inhibitors can comprehensively alleviate tissue damage and inflammatory responses driven by both ferroptosis and pyroptosis (58).
3.5 Novel drugs
Dipyridamole inhibits adenosine uptake, activates bypass signaling, and enhances cystine uptake and glutathione synthesis, thereby effectively suppressing ferroptosis (59). Zileuton reduced LPO by 40% in sepsis-induced ALI/ARDS, with additive effects when combined with Fer-1 (60). Yes-associated protein 1 (YAP1), a Hippo pathway regulator, modulates ferroptosis-related genes and mitigates sepsis-induced ALI/ARDS (10). Srg3 knockdown promotes M2 macrophage polarization, significantly improving sepsis-induced ALI/ARDS outcomes in rats (61). Hydroxychloroquine reduces lung iron accumulation by disrupting the acidic environment of lysosomes, inhibiting the degradation of ferritin and the recycling of iron, and sequestering iron in an inert storage form (62, 63). YAP1, Srg3, and others are involved in ferroptosis regulation, suggesting that ferroptosis-targeted interventions extend beyond traditional iron metabolism and antioxidant frameworks, encompassing signaling pathway cross-talk and immune cell phenotype regulation.
4 Conclusions and prospects
Ferroptosis has emerged as a pivotal driver of sepsis-induced ALI/ARDS, with recent studies (2020–2025) clarifying its molecular interplay with immune dysfunction, oxidative stress, and barrier disruption. While ferroptosis-targeted therapies have shown strong preclinical potential against sepsis-induced ALI/ARDS, their clinical translation faces key challenges. The first is the issue of biomarker recognition, which currently lacks validated sepsis-induced ALI/ARDS-specific ferroptosis biomarkers, and GPX4 activity in plasma acylcarnitine and BAL fluid cells is a promising candidate (64, 65). Second, the drug specificity is insufficient, and current ferroptosis inhibitors (RTAs, iron chelators) may affect non-ferroptotic pathways (66, 67). Furthermore, the timing of intervention is critical, as the peak window for ferroptosis is 6–24 h after sepsis onset, and delayed treatment reduces efficacy (68). At the same time, precise drug dosage control is required to avoid side effects, and safer formulations are needed (69, 70). Nonetheless, combination therapies, including those integrating immunotherapy, hold significant promise (71, 72). Future research should prioritize developing reliable biomarkers, optimizing drug delivery systems, and validating combination therapies. Targeting ferroptosis represents a transformative strategy to improve outcomes and reduce mortality in sepsis-induced ALI/ARDS, addressing a long-standing unmet clinical need.
Author contributions
LW: Writing – original draft. WZ: Data curation, Formal Analysis, Investigation, Visualization, Writing – review & editing. XF: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China 82073911 (to X.F), Taishan Scholar Foundation of Shandong Province Tsqn202211220 (to X.F) and Shandong Province Natural Science Foundation ZR2025MS1431 (to X.F) and the Joint Innovation Team for Clinical & Basic Research (202409).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qiao X, Yin J, Zheng Z, Li L, and Feng X. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: pathogenesis and therapeutic implications. Cell Commun Signal. (2024) 22:241. doi: 10.1186/s12964-024-01620-y
2. Meyer NJ, Gattinoni L, and Calfee CS. Acute respiratory distress syndrome. Lancet. (2021) 398:622–37. doi: 10.1016/S0140-6736(21)00439-6
3. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. (2012) 149:1060–72. doi: 10.1016/j.cell.2012.03.042
4. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. (2017) 171:273–85. doi: 10.1016/j.cell.2017.09.021
5. Jiang X, Stockwell BR, and Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. (2021) 22:266–82. doi: 10.1038/s41580-020-00324-8
6. Wang S, Liu Z, Geng J, Li L, and Feng X. An overview of ferroptosis in non-alcoholic fatty liver disease. BioMed Pharmacother. (2022) 153:113374. doi: 10.1016/j.biopha.2022.113374
7. Huang Y, Xu W, and Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. (2021) 18:2114–27. doi: 10.1038/s41423-021-00740-6
8. Zhang H, Wang Y, Wang S, Xue X, Huang K, Xu D, et al. Tangeretin alleviates sepsis-induced acute lung injury by inhibiting ferroptosis of macrophage via Nrf2 signaling pathway. Chin Med. (2025) 20:11. doi: 10.1186/s13020-025-01063-8
9. Chen X, Yu C, Kang R, and Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. (2020) 8:590226. doi: 10.3389/fcell.2020.590226
10. Zhang J, Zheng Y, Wang Y, Wang J, Sang A, Song X, et al. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front Immunol. (2022) 13:884362. doi: 10.3389/fimmu.2022.884362
11. Ganz T. Hepcidin in iron metabolism. Curr Opin Hematol. (2004) 11:251–4. doi: 10.1097/00062752-200407000-00004
12. Frost JN, Tan TK, Abbas M, Wideman SK, Bonadonna M, Stoffel NU, et al. Hepcidin-mediated hypoferremia disrupts immune responses to vaccination and infection. Med. (2021) 2:164–179.e12. doi: 10.1016/j.medj.2020.10.004
13. Ohshima T, Yamamoto H, Sakamaki Y, Saito C, and Mizushima N. NCOA4 drives ferritin phase separation to facilitate macroferritinophagy and microferritinophagy. J Cell Biol. (2022) 221. doi: 10.1083/jcb.202203102
14. Tang D, Chen X, Kang R, and Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1
15. Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. (2015) 520:57–62. doi: 10.1038/nature14344
16. Xu J, Tao L, Jiang L, Lai J, Hu J, Tang Z, et al. Moderate hypothermia alleviates sepsis-associated acute lung injury by suppressing ferroptosis induced by excessive inflammation and oxidative stress via the keap1/GSK3β/nrf2/GPX4 signaling pathway. J Inflammation Res. (2024) 17:7687–704. doi: 10.2147/JIR.S491885
17. Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. (2021) 11:316–29. doi: 10.7150/thno.49713
18. Xia W, Pan Z, Zhang H, Zhou Q, and Liu Y. Inhibition of ERRα Aggravates sepsis-induced acute lung injury in rats via provoking inflammation and oxidative stress. Oxid Med Cell Longev. (2020) 2020:2048632. doi: 10.1155/2020/2048632
19. Li Z, Bu Y, Wang C, Yu Y, Han L, Liu C, et al. Genetic and multi-omics insights into monocyte pantothenate-mediated protection in acute respiratory distress syndrome. J Cell Mol Med. (2025) 29:e70812. doi: 10.1111/jcmm.70812
20. Yao J, Zhang Y, Li M, Sun Z, Liu T, Zhao M, et al. Single-cell RNA-seq reveals the promoting role of ferroptosis tendency during lung adenocarcinoma EMT progression. Front Cell Dev Biol. (2021) 9:822315. doi: 10.3389/fcell.2021.822315
21. Shen K, Wang X, Wang Y, Jia Y, Zhang Y, Wang K, et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. (2023) 62:102655. doi: 10.1016/j.redox.2023.102655
22. Velotti F, Costantini L, and Merendino N. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) for immunomodulation in COVID-19 related acute respiratory distress syndrome (ARDS). J Clin Med. (2022) 12. doi: 10.3390/jcm12010304
23. Jin SH, Sun JJ, Liu G, Shen LJ, Weng Y, Li JY, et al. Nrf2/PHB2 alleviates mitochondrial damage and protects against Staphylococcus aureus-induced acute lung injury. MedComm (2020). (2023) 4:e448. doi: 10.1002/mco2.448
24. Zhang H, Liu J, Zhou Y, Qu M, Wang Y, Guo K, et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int J Biol Sci. (2022) 18:3337–57. doi: 10.7150/ijbs.69141
25. Zhang H, Lv J, Wu H, He Y, Li M, Wu C, et al. Endogenous/exogenous dual-responsive nanozyme for photothermally enhanced ferroptosis-immune reciprocal synergistic tumor therapy. Sci Adv. (2025) 11:eadq3870. doi: 10.1126/sciadv.adq3870
26. Shi X, Tan S, and Tan S. NLRP3 inflammasome in sepsis (Review). Mol Med Rep. (2021) 24. doi: 10.3892/mmr.2021.12153
27. Wang Z, Li Y, Ye Y, Zhu H, Zhang J, Wang H, et al. NLRP3 inflammasome deficiency attenuates cerebral ischemia-reperfusion injury by inhibiting ferroptosis. Brain Res Bull. (2023) 193:37–46. doi: 10.1016/j.brainresbull.2022.11.016
28. Wang W, Xu R, Zhao H, Xiong Y, and He P. CircEXOC5 promotes ferroptosis by enhancing ACSL4 mRNA stability via binding to PTBP1 in sepsis-induced acute lung injury. Immunobiology. (2022) 227:152219. doi: 10.1016/j.imbio.2022.152219
29. Liu P, Feng Y, Li H, Chen X, Wang G, Xu S, et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett. (2020) 25:10. doi: 10.1186/s11658-020-00205-0
30. Wu J, Liu Q, Zhang X, Tan M, Li X, Liu P, et al. The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages. Cell Death Dis. (2022) 13:653. doi: 10.1038/s41419-022-05115-x
31. Wang Z and Wang Z. The role of macrophages polarization in sepsis-induced acute lung injury. Front Immunol. (2023) 14:1209438. doi: 10.3389/fimmu.2023.1209438
32. Chen X, Wang J, Yang P, Liu HY, Zhong S, Lu C, et al. SENP3 sensitizes macrophages to ferroptosis via de-SUMOylation of FSP1. Redox Biol. (2024) 75:103267. doi: 10.1016/j.redox.2024.103267
33. He R, Liu B, Xiong R, Geng B, Meng H, Lin W, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. (2022) 8:43. doi: 10.1038/s41420-021-00807-3
34. Lai K, Song C, Gao M, Deng Y, Lu Z, Li N, et al. Uridine alleviates sepsis-induced acute lung injury by inhibiting ferroptosis of macrophage. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24065093
35. Mehta P, Samanta RJ, Wick K, Coll RC, Mawhinney T, Mcaleavey PG, et al. Elevated ferritin, mediated by IL-18 is associated with systemic inflammation and mortality in acute respiratory distress syndrome (ARDS). Thorax. (2024) 79:227–35. doi: 10.1136/thorax-2023-220292
36. Li Q, Peng F, Yan X, Chen Y, Zhou J, Wu S, et al. Inhibition of SLC7A11-GPX4 signal pathway is involved in aconitine-induced ferroptosis in vivo and in vitro. J Ethnopharmacol. (2023) 303:116029. doi: 10.1016/j.jep.2022.116029
37. Kounatidis D, Vallianou NG, Psallida S, Panagopoulos F, Margellou E, Tsilingiris D, et al. Sepsis-associated acute kidney injury: where are we now? Med (Kaunas). (2024) 60. doi: 10.3390/medicina60030434
38. Wang ZY, Zeng T, Wang X, Zhuo X, Zheng JW, Zhu L, et al. Targeting inducible nitric oxide synthase with 1400W mitigates septic acute lung injury through inhibiting SLC7A11/GPX4 mediated ferroptosis. Nitric Oxide. (2025) 158:4–10. doi: 10.1016/j.niox.2025.05.008
39. Lin CK, Chen ZW, Lin YH, Yang CT, Shi CS, Lin CM, et al. MCC950 alleviates fat embolism-induced acute respiratory distress syndrome through dual modulation of NLRP3 inflammasome and ERK pathways. Int J Mol Sci. (2025) 26. doi: 10.3390/ijms26157571
40. Bu Y, Li Z, Wang C, Yu Y, Liu C, Sun Y, et al. Anemoside B4 targets RAGE to attenuate ferroptosis in sepsis-induced acute lung injury. Front Pharmacol. (2025) 16:1590797. doi: 10.3389/fphar.2025.1590797
41. Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. (2017) 3:232–43. doi: 10.1021/acscentsci.7b00028
42. Devisscher L, Van Coillie S, Hofmans S, Van Rompaey D, Goossens K, Meul E, et al. Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy. J Med Chem. (2018) 61:10126–40. doi: 10.1021/acs.jmedchem.8b01299
43. Wang Y, Han Q, Liu L, Wang S, Li Y, Qian Z, et al. Natural hydrogen gas and engineered microalgae prevent acute lung injury in sepsis. Mater Today Bio. (2024) 28:101247. doi: 10.1016/j.mtbio.2024.101247
44. Shen YZ, Luo B, Zhang Q, Hu L, Hu YC, and Chen MH. Exploration potential sepsis-ferroptosis mechanisms through the use of CETSA technology and network pharmacology. Sci Rep. (2025) 15:13527. doi: 10.1038/s41598-025-95451-7
45. Zhang F, Wang J, Wang W, Chen G, Tang H, and Zhai X. The therapeutic potential of irisin in alleviating acute lung injury via inflammation and ferroptosis modulation. J Int Med Res. (2025) 53:3000605251340338. doi: 10.1177/03000605251340338
46. Hu J, Cheng M, Jiang C, Liu L, He Z, Liu L, et al. Deferoxamine mitigates ferroptosis and inflammation in hippocampal neurons after subarachnoid hemorrhage by activating the nrf2/TXNRD1 axis. Mol Neurobiol. (2024) 61:1044–60. doi: 10.1007/s12035-023-03525-2
47. Wu Y, Ran L, Yang Y, Gao X, Peng M, Liu S, et al. Deferasirox alleviates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. (2023) 314:121312. doi: 10.1016/j.lfs.2022.121312
48. Zhang L, Luo YL, Xiang Y, Bai XY, Qiang RR, Zhang X, et al. Ferroptosis inhibitors: past, present and future. Front Pharmacol. (2024) 15:1407335. doi: 10.3389/fphar.2024.1407335
49. Wang Y, Wang W, Zhang Y, Fleishman JS, and Wang H. Targeting ferroptosis offers therapy choice in sepsis-associated acute lung injury. Eur J Med Chem. (2025) 283:117152. doi: 10.1016/j.ejmech.2024.117152
50. Wang Y, Chen D, Xie H, Jia M, Sun X, Peng F, et al. AUF1 protects against ferroptosis to alleviate sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell Mol Life Sci. (2022) 79:228. doi: 10.1007/s00018-022-04248-8
51. Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. (2018) 172:409–422.e21. doi: 10.1016/j.cell.2017.11.048
52. Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. (2019) 177:1262–1279.e25. doi: 10.1016/j.cell.2019.03.032
53. Galicia-Moreno M, Monroy-Ramirez HC, Caloca-Camarena F, Arceo-Orozco S, Muriel P, Sandoval-Rodriguez A, et al. A new opportunity for N-acetylcysteine. An outline of its classic antioxidant effects and its pharmacological potential as an epigenetic modulator in liver diseases treatment. Naunyn Schmiedebergs Arch Pharmacol. (2025) 398:2365–86. doi: 10.1007/s00210-024-03539-0
54. Koppula P, Zhuang L, and Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. (2021) 12:599–620. doi: 10.1007/s13238-020-00789-5
55. Gao N, Liu XY, Chen J, Hu TP, Wang Y, and Zhang GQ. Menaquinone-4 alleviates sepsis-associated acute lung injury via activating SIRT3-p53/SLC7A11 pathway. J Inflammation Res. (2024) 17:7675–85. doi: 10.2147/JIR.S486984
56. Xiong M, Luo R, Zhang Z, Liu P, Peng Q, Xu F, et al. IL-27 regulates macrophage ferroptosis by inhibiting the Nrf2/HO1 signaling pathway in sepsis-induced ARDS. Inflammation Res. (2025) 74:39. doi: 10.1007/s00011-024-01986-2
57. Deng RM, Huang G, Wang T, and Zhou J. Regulated programmed cell death in sepsis associated acute lung injury: From pathogenesis to therapy. Int Immunopharmacol. (2025) 148:114111. doi: 10.1016/j.intimp.2025.114111
58. Gu X, Wei S, Chen B, Zhang W, and Zheng S. Development and validation of a combined ferroptosis- and pyroptosis-related gene signatures for the prediction of clinical outcomes in lung adenocarcinoma. Am J Cancer Res. (2022) 12:3870–91.
59. Chen X, Shen J, Jiang X, Pan M, Chang S, Li J, et al. Characterization of dipyridamole as a novel ferroptosis inhibitor and its therapeutic potential in acute respiratory distress syndrome management. Theranostics. (2024) 14:6947–68. doi: 10.7150/thno.102318
60. Hu WM, Liu SQ, Zhu KF, Li W, Yang ZJ, Yang Q, et al. The ALOX5 inhibitor Zileuton regulates tumor-associated macrophage M2 polarization by JAK/STAT and inhibits pancreatic cancer invasion and metastasis. Int Immunopharmacol. (2023) 121:110505. doi: 10.1016/j.intimp.2023.110505
61. Ling X, Wei S, Ling D, Cao S, Chang R, Wang Q, et al. Irf7 regulates the expression of Srg3 and ferroptosis axis aggravated sepsis-induced acute lung injury. Cell Mol Biol Lett. (2023) 28:91. doi: 10.1186/s11658-023-00495-0
62. Xu J, Yang KC, Go NE, Colborne S, Ho CJ, Hosseini-Beheshti E, et al. Chloroquine treatment induces secretion of autophagy-related proteins and inclusion of Atg8-family proteins in distinct extracellular vesicle populations. Autophagy. (2022) 18:2547–60. doi: 10.1080/15548627.2022.2039535
63. Bhatt V, Lan T, Wang W, Kong J, Lopes EC, Wang J, et al. Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors. Cell Death Dis. (2023) 14:61. doi: 10.1038/s41419-023-05592-8
64. Arjmand B, Dehghanbanadaki H, Yoosefi M, Rezaei N, Mohammadi Fateh S, Ghodssi-Ghassemabadi R, et al. Association of plasma acylcarnitines and amino acids with hypertension: A nationwide metabolomics study. PloS One. (2023) 18:e0279835. doi: 10.1371/journal.pone.0279835
65. Woo MS, Mayer C, Binkle-Ladisch L, Sonner JK, Rosenkranz SC, Shaposhnykov A, et al. STING orchestrates the neuronal inflammatory stress response in multiple sclerosis. Cell. (2024) 187:4043–4060.e30. doi: 10.1016/j.cell.2024.05.031
66. Chen X, Kang R, Kroemer G, and Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. (2021) 18:280–96. doi: 10.1038/s41571-020-00462-0
67. Wang H, Cheng Y, Mao C, Liu S, Xiao D, Huang J, et al. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. (2021) 29:2185–208. doi: 10.1016/j.ymthe.2021.03.022
68. Zeng F, Nijiati S, Tang L, Ye J, Zhou Z, and Chen X. Ferroptosis detection: from approaches to applications. Angew Chem Int Ed Engl. (2023) 62:e202300379. doi: 10.1002/anie.202300379
69. Hamilton JL, Imran Ul-Haq M, Abbina S, Kalathottukaren MT, Lai BF, Hatef A, et al. In vivo efficacy, toxicity and biodistribution of ultra-long circulating desferrioxamine based polymeric iron chelator. Biomaterials. (2016) 102:58–71. doi: 10.1016/j.biomaterials.2016.06.019
70. Guo Y, Lu C, Hu K, Cai C, and Wang W. Ferroptosis in cardiovascular diseases: current status, challenges, and future perspectives. Biomolecules. (2022) 12. doi: 10.3390/biom12030390
71. Wang J, Yang S, Jing G, Wang Q, Zeng C, Song X, et al. Inhibition of ferroptosis protects sepsis-associated encephalopathy. Cytokine. (2023) 161:156078. doi: 10.1016/j.cyto.2022.156078
Keywords: ferroptosis, sepsis induced acute lung injury/acute respiratory distress syndrome, therapeutic strategy, immune regulation, oxidative stress
Citation: Wang L, Zhang W and Feng X (2025) Ferroptosis in sepsis induced acute lung injury/acute respiratory distress syndrome (ALI/ARDS): a potential therapeutic strategy. Front. Immunol. 16:1689155. doi: 10.3389/fimmu.2025.1689155
Received: 20 August 2025; Accepted: 13 November 2025; Revised: 10 November 2025;
Published: 26 November 2025.
Edited by:
Priscilla Brebi, University of La Frontera, ChileReviewed by:
Lulong Bo, Navy Medical University, ChinaCopyright © 2025 Wang, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujing Feng, eGpmZW5nQHNkZm11LmVkdS5jbg==
†These authors share the first authorship
 Linlin Wang1,2†
Linlin Wang1,2† Xiujing Feng
Xiujing Feng