- 1School of Clinical Medicine, University of Cambridge, Cambridge, United Kingdom
- 2Department of Neurology, Mount Sinai Hospital, New York, NY, United States
- 3Division of Academic Neurosurgery, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
Introduction: Spinal cord injury (SCI) is a traumatic injury resulting in significant life-changing disability. Elucidating the molecular processes associated with SCI may help to design novel therapeutics targeted at improving patient outcomes. Current pharmacological candidates include histone deacetylase (HDAC) inhibitors, whose anti-inflammatory properties are postulated to be of value in SCI. The objective was to synthesise the impact of HDAC inhibitors on neurobehavioural outcomes in preclinical studies of traumatic and non-traumatic SCI and to evaluate the suitability of HDAC inhibitors for clinical trials in patients with SCI.
Methods: The review was prospectively registered with PROSPERO (CRD42023477882) and conducted following PRISMA 2020 guidelines. MEDLINE and Embase were searched. Studies of animal models of traumatic or non-traumatic SCI evaluating the effect of HDAC inhibition on neurobehavioural outcomes were eligible for inclusion. Risk of bias was assessed using the SYRCLE checklist. Screening, data-extraction and risk of bias assessments were completed in duplicate.
Results: Of 10,549 studies identified, 42 studies met inclusion criteria. Animal models were rats (n=28), mice (n=13) and rabbits (n=1). SCI models included spinal cord contusion (n=24), epidural compression (n=2), vascular clip compression (n=6), hemisection (n=5), ischaemia/reperfusion injury (n=4) and dorsolateral funiculus crush (n=1). Valproate was the most frequently studied HDAC inhibitor (n=20), followed by 4-phenylbutyrate (4-PBA; n=7) and RGFP966 (n=3). Trichostatin A, tubastatin A, entinostat, PCI-34051, scriptaid, CI-994, TMP269, vorinostat, 3-TYP, SW-100 and ACY1215 were each evaluated in a single study. Three studies used the sirtuin-1 (HDAC class III) inhibitor EX527 administered with an activator molecule: melatonin (n=1), MLN4924 (n=1) and oxymatrine (n=1). Locomotor function was assessed in 98% (41/42) of studies, with improvement in locomotor outcome reported in 73% (30/41). Pain and anxiety were evaluated in one study, in which significant improvement was demonstrated.
Conclusion: HDAC inhibitors are associated with functional motor recovery and improved anxiety and pain scores in preclinical models of SCI. However, the results should be interpreted with caution as risk of bias of included studies was unclear. These results support further investigation of HDAC inhibitors in preclinical studies before translation into clinical trials.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023477882.
Introduction
Spinal cord injury (SCI) is a significant public health problem with an estimated 20.6 million individuals affected worldwide and a global incidence of approximately 0.9 million cases each year (1). Currently available treatments have limited efficacy and aim to optimise quality of life rather than reverse the injury (2, 3). Following the acute management phase, care focuses on avoidance of complications and on rehabilitation. This continues for many years after the initial injury. There is therefore an unmet need for better treatments for SCI.
Pathophysiological classifications divide SCI into primary, secondary and chronic phases. Primary injury results from mechanical damage from the initial impact force; secondary injury follows and is divided into acute and subacute phases. The acute phase includes pro-apoptotic signalling leading to cellular dysfunction, death and increased inflammatory cytokine signalling, including tumour necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β). This promotes macrophage, neutrophil and lymphocyte recruitment, potentiating the inflammatory response. In the subacute phase, cell death follows intracellular Ca2+ dysregulation, glutamate excitotoxicity and free radical release, which hinder neuronal regeneration. Cellular processes in the secondary phase of SCI constitute potential targets for histone deacetylase (HDAC) inhibition (2, 4–6).
HDACs are enzymes that catalyse the removal of acetyl groups from lysine residues of histone and non-histone proteins (7). Removal of acetyl groups from N-terminal tails of histone proteins leads to a more condensed chromatin structure and decreased gene expression (8). HDACs can be divided into four classes (I, II, III, IV) (9–11). Many HDAC inhibitors are pan-inhibitors that target multiple HDACs in class I and II, such as trichostatin A, vorinostat and valproate. More selective HDAC inhibitors include class I inhibitors romidepsin, RGFP966 and entinostat and class III (sirtuin) inhibitors sirtinol, AK-7, splitomicin and nicotinamide (12). Moreover, there are inhibitors that are highly selective for specific HDACs, for example HDAC6-selective inhibitors SW-100 and tubastatin A.
Multiple studies have demonstrated the potential of HDAC inhibitors to interact with molecular pathways important in the mechanisms of SCI (6, 13–17). For example, increased HDAC activity has been detected in nuclear extracts from peripheral blood mononuclear cells after SCI (13) and the neuroprotective properties of HDAC inhibitors have been demonstrated in a mouse model of traumatic brain injury, with increased preservation of myelinated axons and improved neuronal conduction (17). Moreover, in lipopolysaccharide-stimulated macrophages, trichostatin A has been found to reduce the expression of pro-inflammatory cytokines IL-6, TNF-α and IL-1β and increase expression of the immunosuppressive cytokine IL-10 (14). The anti-inflammatory and neuroprotective effects of pan-HDAC inhibitors such as trichostatin A, givinostat, and scriptaid have also been demonstrated across in vitro, animal and human studies (6, 14–16).
HDAC inhibitors therefore appear to be promising candidates for adjuvant treatment in SCI. The aim of this systematic review was to study the impact of HDAC inhibitors on neurobehavioural outcomes in preclinical studies of traumatic and non-traumatic SCI and assess potential suitability for clinical trials in SCI patients.
Methods
Study design
The systematic review was prospectively registered with PROSPERO (CRD42023477882) and conducted adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) guidelines (18).
Eligibility criteria
Inclusion criteria
● Animal study
● English language
● Spinal cord injury
● Use of any HDAC inhibitor
● Assessment of any neurobehavioural outcome
Exclusion criteria
● Review or meta-analysis
● Editorial
● Letter
● Correction
● Conference abstract
● Full text unavailable
Additional details on the inclusion/exclusion criteria are presented in Supplementary Table 1.
Information sources
MEDLINE and Embase were searched from inception to 14th April 2025. MEDLINE and Embase searches were performed using the Ovid platform (Ovid Technologies, New York, USA).
Search strategy
Initial scoping searches were performed to refine the review question. The final search strategy was developed over several iterations to maximise the sensitivity (Supplementary Table 2). Search sensitivity was assessed using a list of eight articles known to meet inclusion criteria, with all studies successfully captured.
Selection process
Deduplication was performed using EndNote 21.5 (Clarivate, Philadelphia, United States). Before title and abstract screening, pilot screening of 100 studies was conducted to ensure concordance between screeners. Title and abstract screening was completed by two blinded researchers (NJ, CC) using Rayyan (Rayyan Systems, Cambridge, United States). Disagreements were resolved by discussion. Full text screening was conducted in duplicate by two blinded researchers (NJ, CC). Reasons for exclusions of full-texts are presented in Supplementary Table 3.
Data collection
Data extraction was performed in duplicate by two authors (NJ and CC) in Excel (Microsoft, Washington, United States) using a piloted extraction table.
Data items
Extracted data points included: author, year, study location, study characteristics, sample characteristics, injury model, intervention, neurobehavioural outcomes, time of assessment, relevant statistical analysis and key findings (Supplementary Table 4). We included studies that assessed neurobehavioural outcomes, which are defined as outcomes assessing motor and/or sensory function. Neurobehavioural outcomes of interest included, but were not limited to, locomotor function measured using the Basso, Beattie and Bresnahan (BBB) locomotor scale or the Basso Mouse Scale (BMS), forelimb grip strength and assessments of pain and anxiety. We excluded studies which exclusively assessed non-neurobehavioural outcomes such as electrophysiological measures, autonomic function and histological analysis.
Risk of bias assessment
To assess the risk of bias of included studies, the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) checklist was used (19). The assessment was conducted in duplicate by two blinded and independent researchers (Supplementary Table 5). All disagreements were resolved through discussion.
Synthesis methods
Meta-analysis was not feasible due to the heterogeneity in SCI model design, HDAC therapy administration and outcome measurements. Therefore, a narrative synthesis following the Synthesis Without Meta-analysis (SWiM) reporting guidelines was conducted (20). The SWiM checklist is provided in Supplementary Table 6.
Neurobehavioural outcomes were grouped into locomotor, pain and anxiety. For each included study, the differences between the intervention and control group were reported, including statistical tests where available. Due to the diversity of data, the results were transformed into a standardised metric of direction of effect (improvement/deterioration/no effect or conflicting findings) presented in the form of a table and harvest plots (21–23).
Results
Study selection
A total of 10,549 records were identified from database searching; 42 studies were included in the final review (Figure 1).

Figure 1. PRISMA flow diagram of study selection (73). After deduplication, 1412 duplicates were removed, leaving 9137 unique studies. Following title and abstract screening, 8996 studies were removed. The remaining 139 studies were included in full text screening. Full texts were not available for 6 studies. A further 91 studies that did not meet eligibility criteria were excluded.
Study characteristics
The majority of studies (67%, 28/42) used rat models of SCI (6, 24–50) with Sprague-Dawley rats being most frequently used (Figure 2 (24–33, 37, 40–49);. Male animals were used in 50% (21/42) of studies (6, 25, 27, 31, 33, 38–43, 45, 47, 48, 50–56), female animals in 40% (17/42) of studies (24, 26, 28, 29, 32, 34–37, 44, 46, 49, 57–61) and 10% (4/42) of studies did not report the sex of animals used (30, 62–64). Most studies (76%, 32/42) reported the age of animals used (6, 25, 28, 29, 31–33, 36–38, 40–47, 49–59, 61, 63, 64). The majority (81%, 34/42) of studies investigated SCI at the thoracic level (6, 25–32, 34, 35, 37, 38, 40–46, 49–59, 61, 62, 64).
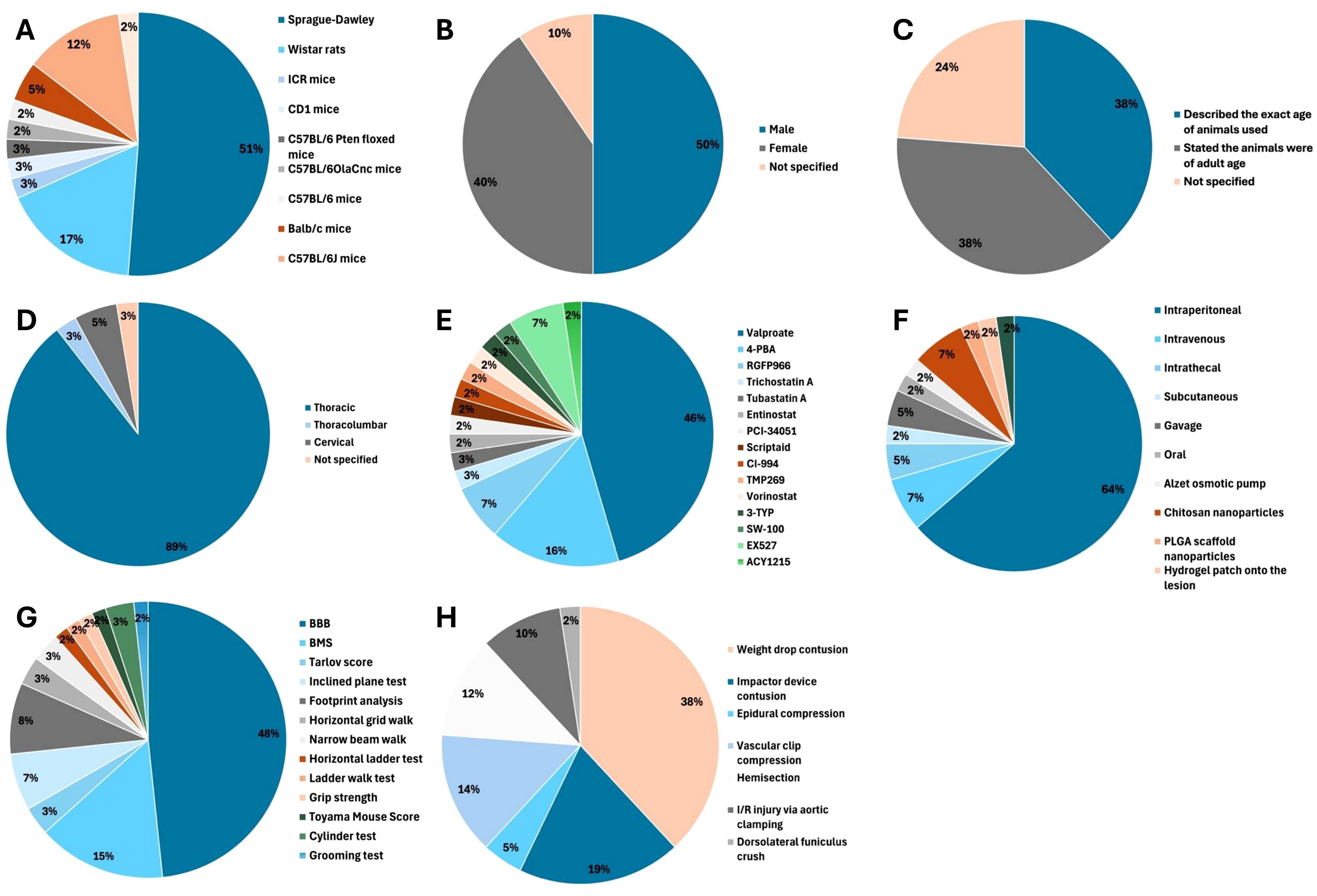
Figure 2. Of 42 included studies, 28 (67%) used rat models (6, 24–47), 13 (31%) used mice (51–59, 61–64) and 1 (2%) study used Japanese white rabbits (60). (A) Animal model. (B) Sex. (C) Age. (D) Level of spinal cord injury. (E) HDAC inhibitor used. (F) Mode of HDAC inhibitor administration. (G) Locomotor function test. (H) SCI model.
A total of 15 different HDAC inhibitors were used, with valproate (VPA) the most commonly evaluated (48%, 20/42) (6, 24, 26, 28, 30–32, 34–42, 45, 50, 51, 57). The time of first administration varied from immediately from before SCI (33, 47, 58, 60), to immediately after SCI (28–31, 35, 38, 44, 46, 54) to seven days later (51). Dose, duration of administration and total duration of treatment varied between studies (Supplementary Table 4).
Neurobehavioural outcome assessment
A variety of neurobehavioural outcome measures were used (Supplementary Table 7). Locomotor function was evaluated in 41 (98%, 41/42) studies; pain and anxiety were evaluated in one (2%, 1/42) study (56).
Thirteen different scoring systems of locomotor function were used (Figure 2). The Basso, Beattie, Bresnahan (BBB) locomotor score was employed in 29 (69%, 29/42) studies (6, 24–47, 49–51, 54) and the Basso Mouse Scale (BMS) was used in 9 (21%, 9/42) studies (52, 53, 55, 57–59, 61, 62, 64).
Assessments of pain were made using the von Frey hairs test (56) and the thermal paw withdrawal latency test (56). Four tests of anxiety were used: the elevated plus maze test (56), the novelty suppressed feeding test (56), the forced swimming test (56) and the open field test (56). The time of neurobehavioural outcome assessment ranged from 15 minutes (56) to 13 weeks after administration of an HDAC inhibitor (51).
Effect of HDAC inhibition on locomotor function
Improvement in locomotor function was observed in studies using BBB, BMS, footprint analysis, grid walk test, grip strength, inclined plane test, narrow beam test, Tarlov score, Toyama mouse score (TMS) and grooming test (Figure 3).
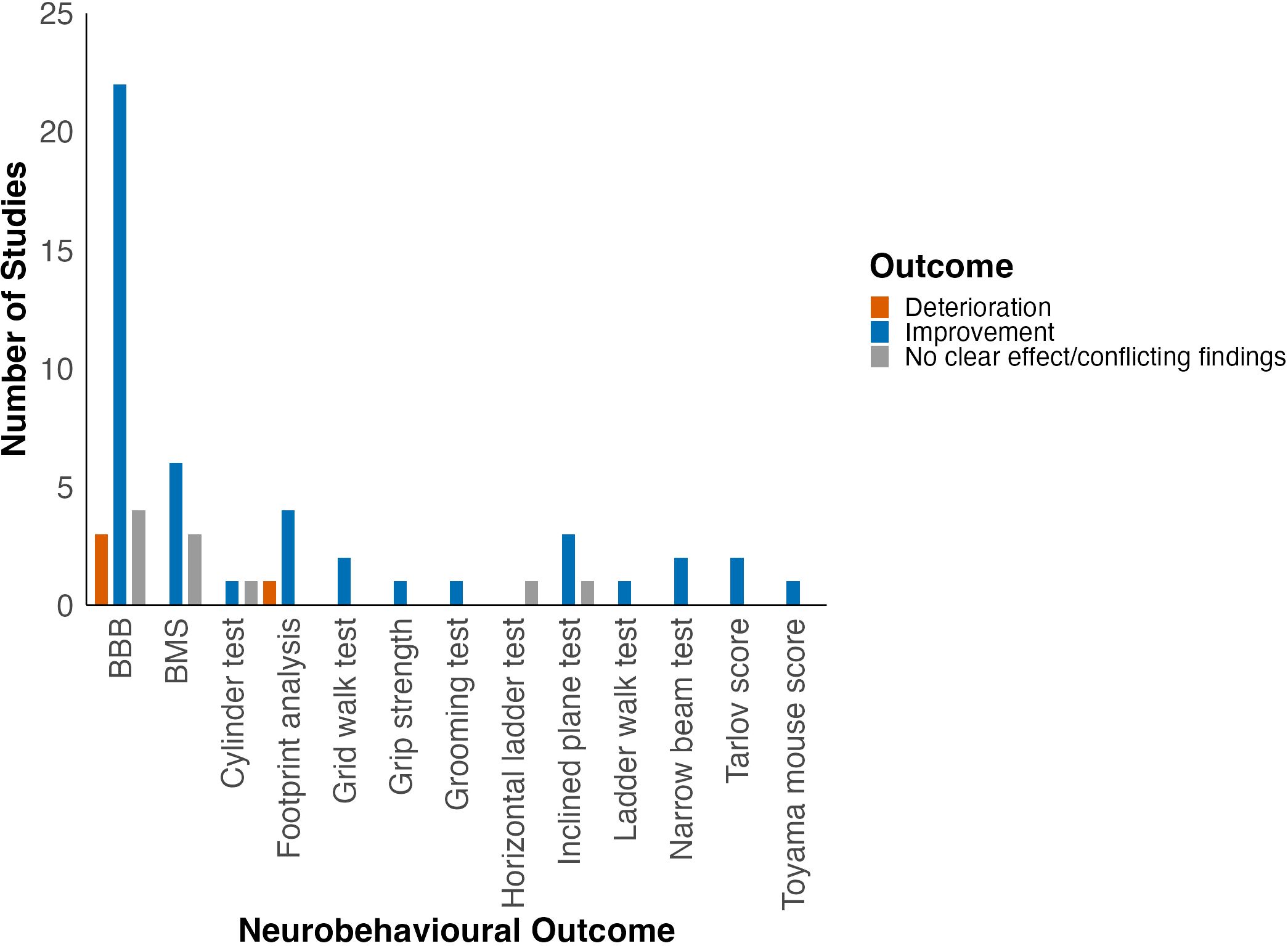
Figure 3. Effect of HDAC inhibition on locomotor function grouped by locomotor outcome. Adapted from Bhatti et al. (2021) (22).
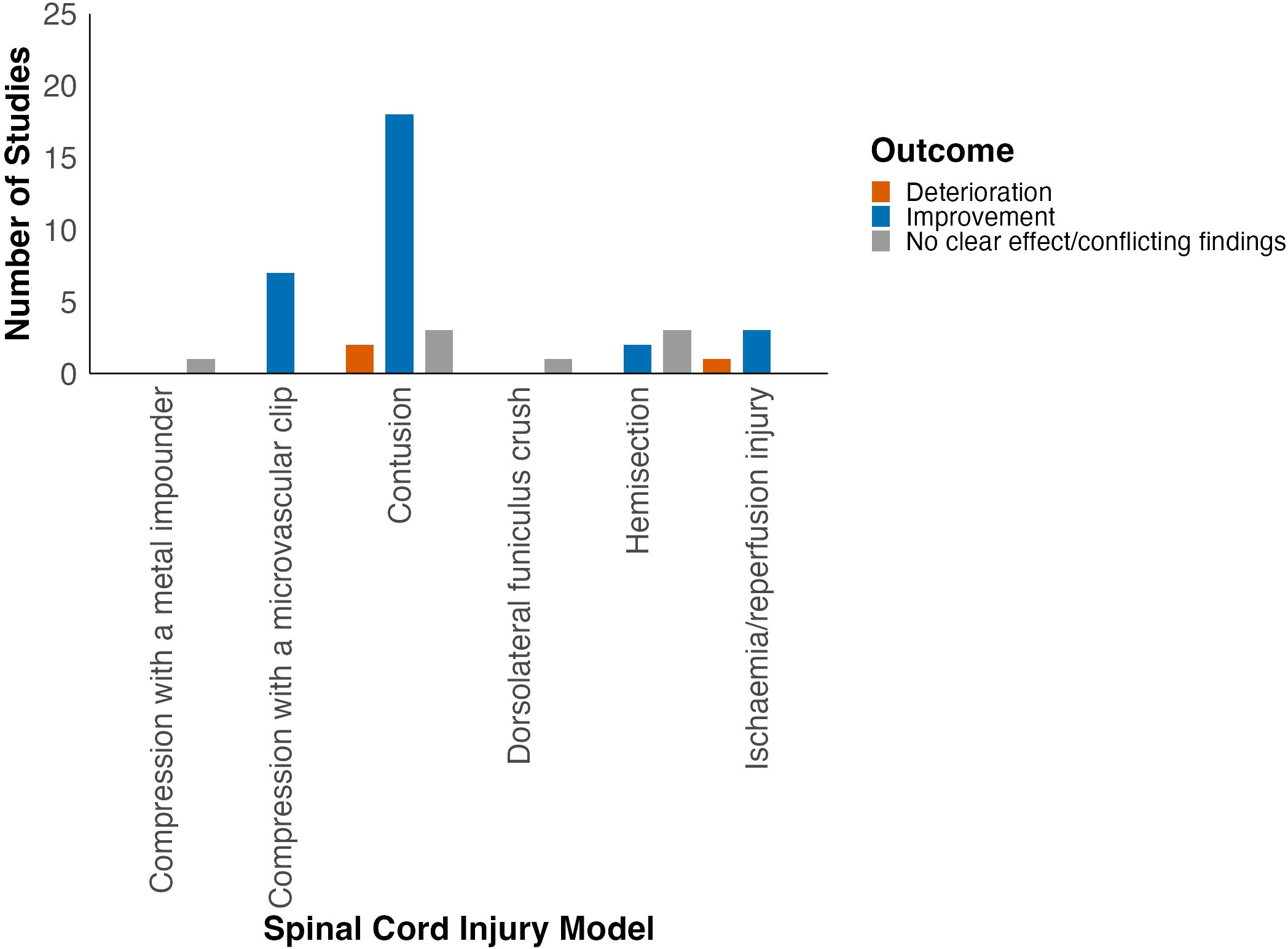
Locomotor function was assessed in six different SCI models (Supplementary Table 8). Most studies used the contusion model of traumatic SCI (57%, 24/42; Figure 2). Improvement in locomotor outcomes appeared most consistent amongst studies using the compression (88%, 7/8) and contusion SCI models (78%, 18/23; Figure 4, Table 1). Studies using ischaemia/reperfusion injury models also predominantly reported improvement in locomotor outcomes (75%, 3/4). On the contrary, spinal cord hemisection studies predominantly reported no effect of HDAC inhibition on neurobehavioural outcomes.
When grouped by HDAC inhibitor class, most studies evaluated pan-HDAC (valproate, 4-PBA, trichostatin A, scriptaid) and class I HDAC inhibitors (RGFP966, entinostat, PCI-34051, CI-994). The most consistent improvement in neurobehavioural outcomes was demonstrated for class IIb HDAC inhibitors (tubastatin A, SW-100, ACY1215; 100%, 3/3), followed by pan-HDAC inhibitors (79%, 23/29) and class I HDAC inhibitors (67%, 4/6; Table 2). Moreover, when grouped by HDAC inhibitor used, improvement in locomotor function was seen in studies using 4-PBA, VPA, RGFP966, CI-994, SW-100, entinostat, tubastatin A and ACY1215 (Figure 5).
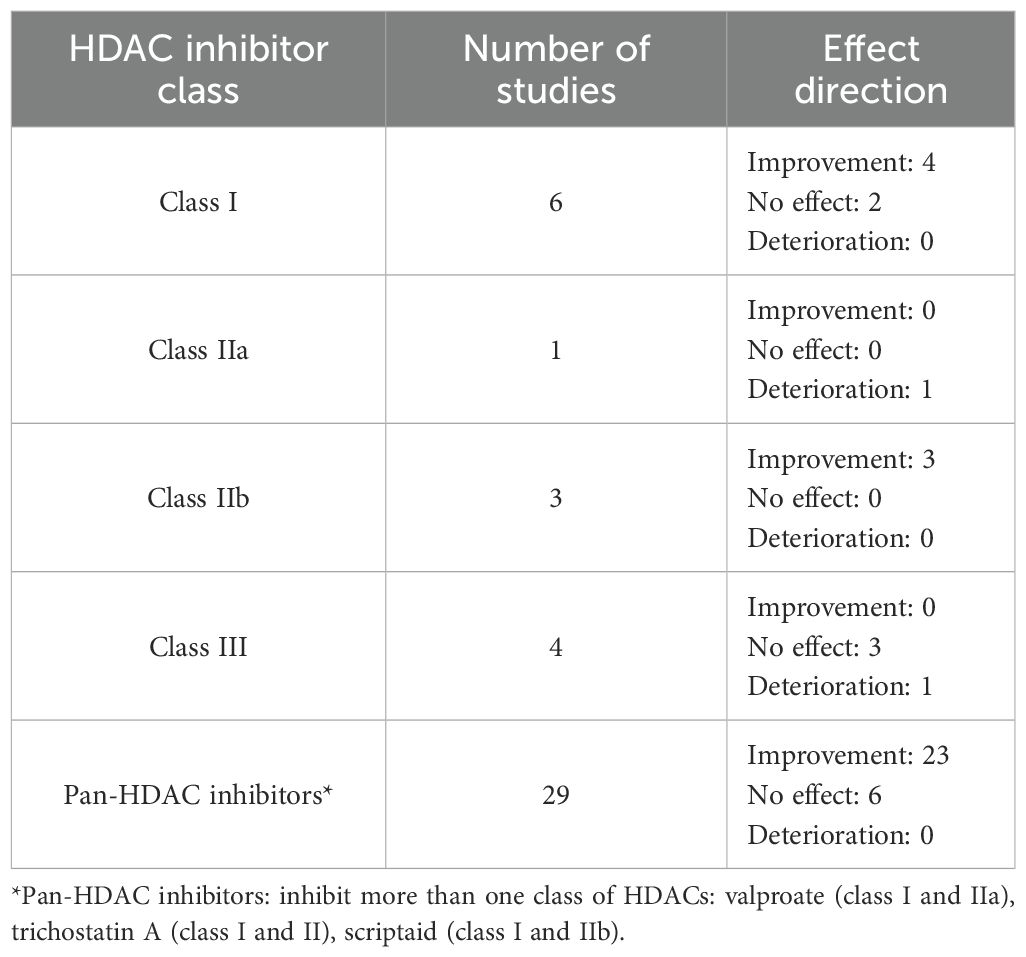
Table 2. Summary of locomotor outcomes in included studies by HDAC class. No class IV-selective HDAC inhibitors were used in the included studies.
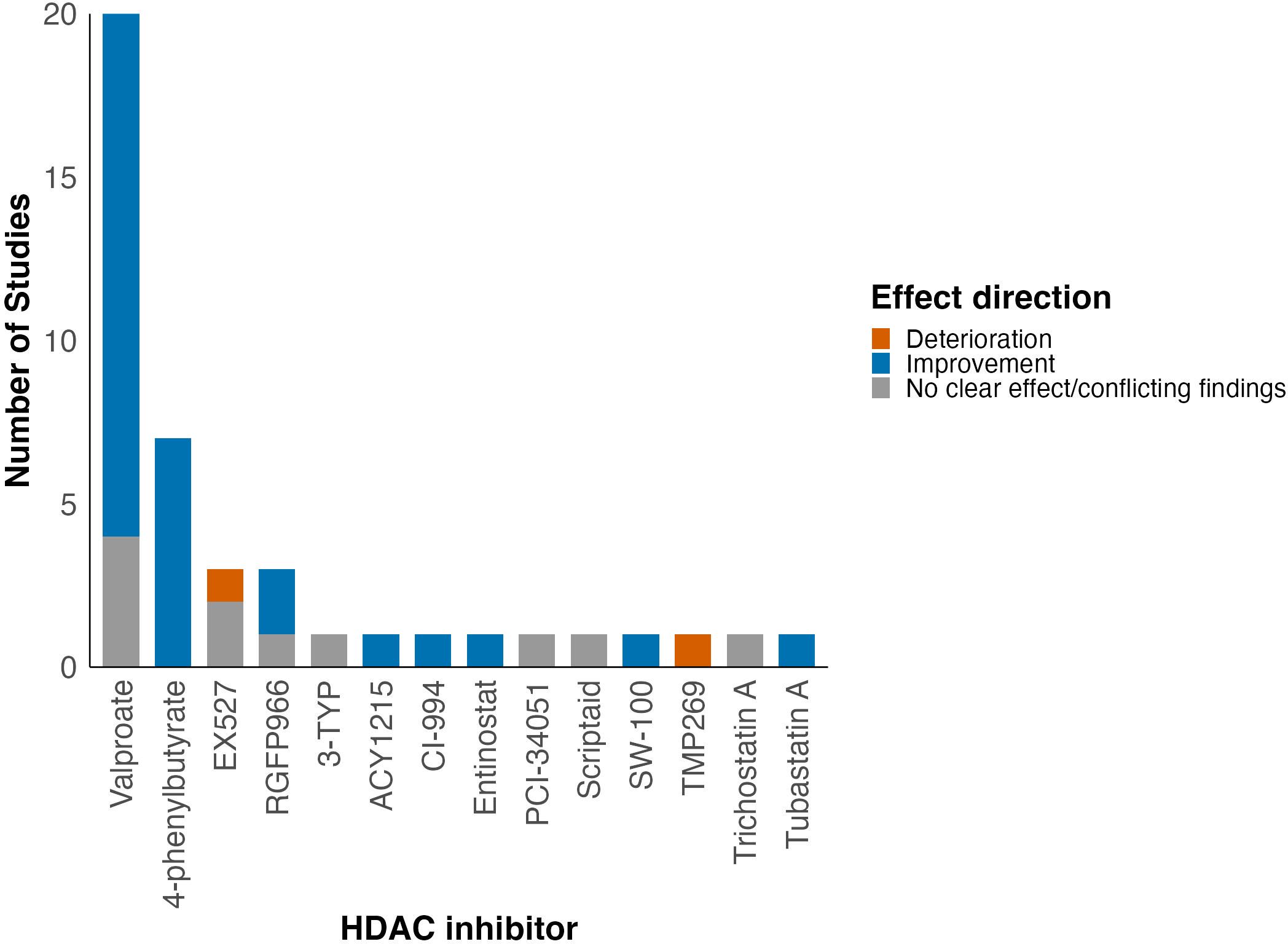
Figure 5. Summary of effect of HDAC inhibition on locomotor function grouped by HDAC inhibitor assessed in each study. A more detailed summary table grouped by HDAC inhibitor class can be found in the Supplementary Table 9.
Valproate
Administration of VPA was associated with improved neurobehavioural outcomes in 80% (16/20) of studies (6, 24, 26, 28, 30–32, 34–37, 39–42, 45). However, four studies reported no significant difference in functional outcomes between treatment and control groups at any time point (38, 50, 51, 57).
Abetmatsu et al. (2010) assessed VPA treatment alone and in combination with a neural stem cell transplant and identified conflicting findings (51). Mice that received neural stem cell treatment alone and those that received it in combination with VPA showed significant improvement in locomotor function compared to untreated SCI mice (51). However, mice treated with VPA alone showed no improvement in locomotor function (BBB score) compared to untreated SCI mice. Conflicting results were also seen when assessing the combinatorial efficacy of VPA delivered on chitosan nanoparticles compared to VPA administered alone. In one study, after treatment with VPA on chitosan nanoparticles, BBB scores were significantly improved (40). However, VPA or chitosan nanoparticle treatment alone resulted in no improvement in BBB score at any time point assessed (40). In the second study, BBB scores were significantly increased in the VPA and chitosan nanoparticles group and in the VPA-only group (41).
4-phenylbutyrate
Improvement in neurobehavioural outcomes was observed in all seven studies using 4-PBA (29, 33, 43, 44, 48, 53, 60). For example, 4-PBA treatment was associated with significantly improved locomotor function (BBB score) in rats after ischaemia/reperfusion SCI both alone and when used simultaneously with xenon postconditioning (33). Moreover, Zhou et al. (2016) reported 4-PBA treatment administered immediately after SCI followed by daily administration for two weeks significantly improved BBB score 6–14 days after injury in rats following vascular clip compression SCI (44). Furthermore, Lanza et al. (2019) reported a significant improvement in BMS score in mice treated with 4-PBA following SCI (53). Additionally, He et al. (2017) demonstrated the maximum angle at which an animal can maintain its grip on an inclined plane was significantly increased in diabetic rats with SCI who were treated with 4-PBA (29).
Class I HDAC inhibitors (RGFP966, entinostat, CI-994, PCI-34051)
RGFP966 was used in three studies with two (67%) demonstrating improvement in locomotor scores including BBB, BMS and TMS following contusional SCI in mice and rats compared to untreated SCI animals (25, 59). Another study by Sanchez et al. (2018) used a hemisection SCI model and showed no difference in hindlimb movements (BMS scores) between mice treated with RGFP966 and the untreated SCI group (61).
One study of entinostat demonstrated improvement in locomotor function assessed using BMS score and forelimb grip strength in mice following a compression SCI (52). Another study in which CI-994 was administered once daily for 14 days after induction of a dorsal hemisection SCI demonstrated overall improvement in several measures of locomotor function including BMS score, narrow beam walk test, horizontal grid walk test and ladder walk test in treated mice (64).
In contrast, Hendrix et al. (2020) administered PCI-34051 to mice following spinal cord hemisection and found no effect of treatment on locomotor recovery assessed using the BMS score (57).
Class IIb inhibitors (tubastatin A, SW-100, ACY1215)
Zheng et al. (2020) demonstrated improvement in BMS score and footprint patterns in mice treated with tubastatin A compared to untreated mice, suggesting improvement in hindlimb weakness after SCI at 28 days after injury (55). Another study evaluating SW-100 demonstrated improved locomotor function (BMS score) after administration of SW-100 alongside miR-34a-5p inhibitor delivered in exosomes following a contusion SCI (62). A study by Dai et al. (2024) found that treatment with ACY1215 led to significant improvement in locomotor scores (BBB) in SCI rats compared to untreated SCI rats (49).
Class III inhibitors (EX527, 3-TYP)
All studies using EX527 used a sirtuin activator compound to investigate whether activation of the sirtuin pathway may have beneficial effects in improving the locomotor function following SCI. In all three studies, addition of EX527 which is a sirtuin 1 inhibitor led to reduction in BBB scores reflecting poorer motor function compared to the SCI + sirtuin activator alone group (27, 46, 47).
In a study assessing 3-TYP in mice, BMS score in the treated group was not significantly different from that of the untreated-SCI mice (58).
Other HDAC inhibitors (trichostatin A, scriptaid, TMP269)
Trichostatin A (class I and II HDAC inhibitor) treatment was associated with age-dependent opposite effects in mice after SCI: in older animals it was associated with significantly higher foot slip cumulative error score in a horizontal ladder test corresponding to poor locomotor function, whilst the opposite was observed in young mice (63).
Studies using scriptaid (class I and IIb HDAC inhibitor) in mice following hemisection SCI demonstrated no difference in functional outcomes between treated and control groups (61). However, one study of mice treated with TMP269 (class IIa HDAC inhibitor) showed reduced hindlimb movement compared with vehicle-treated group that was maintained for up to 6 weeks after injury (54).
Effect of HDAC inhibition on pain and anxiety
One study assessed the effects of vorinostat on pain and anxiety following contusion SCI. It used two outcome measures for pain: the von Frey filament test and the thermal paw withdrawal latency test and four outcome measures for anxiety: the elevated plus maze test, the novelty suppressed feeding test, the forced swimming test and the open field test. Both tests for pain demonstrated significant improvement after HDAC inhibitor treatment. In the assessment of anxiety behaviours, none of the tests used reached statistical significance but they all demonstrated direction of effect favouring vorinostat treatment (56).
Risk of bias assessments
Only one (2%, 1/42) study adequately generated and applied the allocation sequence. A total of 62% (26/42) of studies reported baseline characteristics. The allocation sequence was adequately concealed in 5% (2/42) of studies. None of the included studies reported whether animals were randomly housed during the experiment. One (2%, 1/42) study reported the investigators were blinded. None of the studies reported if the animals were selected at random for the outcome assessment. The outcome assessor was blinded in 69% (29/42) of studies. Incomplete outcome data were adequately addressed in 74% (31/42) of studies. Selective outcome reporting was noted in 12% (5/42) of studies. A total of 19% (8/42) studies chose insufficient control groups which may have contributed to selection bias. Overall, the risk of bias was therefore unclear (Supplementary Table 5).
Discussion
Summary of main findings
The aim of this review was to synthesise the evidence on the effect of HDAC inhibitors on neurobehavioural outcomes in preclinical models of SCI. We found that the majority of class I, class IIb and pan-HDAC inhibitors were associated with beneficial effects on neurobehavioural outcomes in animal models of SCI. The only exceptions were trichostatin A, scriptaid and PCI-34051 which had unclear effect on locomotor function, and TMP269 which was associated with poorer functional recovery after SCI. Class III inhibitors appeared to have no effect or be associated with poorer locomotor function following SCI.
Differences in HDAC inhibitor mechanisms of action and heterogeneity in neurobehavioural outcomes
Class I and IIb HDAC inhibitors appeared to exhibit the most consistent neuroprotective effects following SCI. Their mechanisms of action are similar and include reduction of inflammatory and apoptotic signalling and promotion of autophagy through increased microtubular transport and decreased neuronal endoplasmic reticular stress at the site of SCI (Supplementary Table 10) 6, 36, 60).
For example, Zheng et al. (2020) studied class IIb HDAC inhibitors and found that HDAC6 expression increases at the SCI site and is associated with impaired autophagy and increased neuronal apoptosis. Inhibition of HDAC6 appears to increase tubulin acetylation, supporting motor protein recruitment and retrograde transport in neurones. This is particularly important for autophagy (55), with failure associated with increased neuronal apoptosis. Class IIb HDAC inhibitors may therefore promote neuronal survival following SCI (65).
Class I HDAC inhibitors appear to have significant anti-inflammatory effects. For example, HDAC3 was found to significantly contribute to SCI pathogenesis, particularly through its role in activation of the inflammatory response (6). Inhibiting class I HDACs, especially HDAC3, was found to reduce microglial activation and restrict production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) (6). An increase in sirtuin 1 expression is associated with HDAC3 inhibition (25). This is consistent with the findings from our review that inhibition of class III HDACs (of which sirtuin 1 is an example) had either no effect or was associated with poorer functional recovery following SCI (25, 66). Moreover, Sanchez et al. (2018) demonstrated that HDAC3 inhibition promotes a shift from the M1-like macrophage phenotype to anti-inflammatory, pro-regenerative M2-like phenotype further supporting the role of class I HDACs in inflammation following SCI (61).
In addition, valproate, which predominantly inhibits class I HDACs, has been associated with increased expression of BDNF and GDNF neurotrophic factors in vitro, promoting neuroregeneration and counterbalancing the inhibitory environment for neuronal growth driven by Nogo-A (34, 67).
Therefore, HDAC3 (class I HDAC) and HDAC6 (class IIb HDAC) appear particularly promising targets in the context of SCI treatment and more studies of selective HDAC3 (e.g. RGFP966) and HDAC6 (e.g. SW-100, ACY1215) inhibitors are needed to elucidate their mechanism and efficacy in improving neurological function following SCI.
Interestingly, HDAC5 inhibition seems to have an important role in regulation of pain following SCI. The mechanism behind this effect may be related to reduction in Nav1.7 channel expression following targeted protein degradation (56). As Nav1.7 channels are known to play an important role in nociception, HDAC5 inhibition represents a promising research avenue, even beyond spinal cord injury (56).
In contrast, class IIa HDAC inhibition (e.g. TMP269) appears to promote inflammation at the injury site, an effect associated with a shift in macrophage polarisation towards the M1-type (54). This may explain why the HDAC inhibitors which target both class I and class IIa/IIb HDACs (VPA, trichostatin A) do not always appear to be associated with definitive or significant improvement in functional outcomes following SCI in included studies.
Similarly to class IIa inhibition, class III HDAC inhibition increases oxidative damage in neurones, promotes apoptosis and reduces autophagy following SCI. However, activators of class III HDACs (melatonin, MLN4924, oxymatrine) improve functional outcomes following SCI (25, 27, 46, 47, 68).
Opportunities for translational clinical trials
To date, no clinical trials assessing effects of HDAC inhibitors on functional outcomes following SCI have been conducted. The only clinical trial assessing an HDAC inhibitor in patients after SCI was conducted by Drewes et al. (1994) and assessed the effects of valproate treatment on chronic central pain after SCI (69).
Overall, class I and IIb HDAC inhibitors appear to have beneficial effects on locomotor function, pain and anxiety after SCI in animals suggesting that HDAC inhibition may have potential to improve patient outcomes in clinical trials. The review identified VPA and 4-PBA as having the most favourable neurobehavioural outcomes amongst the reviewed studies with 80% of studies using VPA and 100% of studies using 4-PBA reporting improvement in neurobehavioural outcomes making them the best candidates for further studies. However, due to the high heterogeneity and unclear risk of bias observed in those studies, more preclinical evidence is required. Additionally, it may be useful to assess the effects of other FDA-approved HDAC inhibitors (Supplementary Table 11) on neurobehavioural outcomes in animal models of SCI, given that the toxicity profiles of these drugs are already well-understood, which may simplify translation of preclinical evidence into future clinical trials.
Limitations
Firstly, limited reporting, scored using the SYRCLE risk of bias assessments, affects certainty about the quality of the results of included studies. This limits certainty of conclusions. Selection bias, performance bias and detection bias related questions, including adequate generation of the allocation sequence, baseline characteristics, blinding of caregivers and investigators and details on housing of animals were poorly reported. Therefore, risk of bias was unclear. Lack of adequate reporting appears to be an issue with many preclinical studies and is thought to be related to a historical lack of strict reporting requirements for animal studies. A systematic review by Bhatti et al. (2021) advocated for the widespread use of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines to improve the quality of evidence from preclinical studies (22, 70).
In addition, the included studies are highly heterogenous. There is significant variability in SCI models used, timing and route of administration of HDAC inhibitors. There were also significant differences in the severity and mechanism of SCI between models. For example, it is recognised that in the contusion model there may be axonal sparing, which can be falsely interpreted as neuronal regeneration at the lesion site. On the other hand, hemisectional SCI should not cause axonal sparing at the lesion site and any observed regeneration can be more confidently attributed to effects of the studied treatment (71).
The selectivity of HDAC inhibitors differs amongst the drugs included in the review. The current evidence suggests that the main HDACs that should be targeted in SCI are HDAC3 and HDAC6. The most studied inhibitor in context of SCI was valproate. This targets both class I and class II HDACs, which may explain contradictory results in some of the studies.
Furthermore, certain methods of HDAC inhibitor delivery may be difficult to implement in the clinical environment. For example, intrathecal delivery is technically challenging and carries higher risks of infection and neurological toxicity compared to other methods of administration (72).
Future directions
To generate more robust and translatable evidence, there is a need for larger, well-reported preclinical studies of HDAC inhibitors. Secondly, standardised SCI models for testing HDAC inhibitors may help to alleviate heterogeneity observed within each HDAC inhibitor group studied. Moreover, standardisation of the dose, time and route of administration is also important. In addition, there is a need for more mechanistic studies of HDAC3 and HDAC6 inhibitors which have significant potential for SCI treatment and are limited by few studies of their neurobehavioural effects and mechanism of action compared to 4-PBA and valproate.
Conclusion
Class I and class IIb HDAC inhibitors are associated with functional locomotor recovery and improved pain and anxiety scores in preclinical models of SCI. By contrast, class III HDAC inhibitors and class IIa HDAC inhibitors are associated with either no effect or deterioration in functional recovery after SCI. However, due to unclear risk of bias in all included studies and high heterogeneity amongst study characteristics, the results should be interpreted with caution. Nevertheless, these findings may be helpful in recognising promising targets for future translational research, including HDAC3 and HDAC6.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
NJ: Visualization, Formal analysis, Conceptualization, Data curation, Writing – review & editing, Methodology, Writing – original draft, Investigation. CC: Formal analysis, Data curation, Validation, Investigation, Writing – review & editing. JB: Conceptualization, Methodology, Writing – review & editing, Supervision, Writing – original draft. RC: Methodology, Visualization, Supervision, Writing – review & editing. BD: Supervision, Writing – review & editing. MK: Writing – review & editing, Supervision. OM: Validation, Supervision, Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We gratefully acknowledge Isla Kuhn (IK) from the Medical Library at the School of Clinical Medicine University of Cambridge for the help with designing the search strategy. MK gratefully acknowledges support by the Cambridge NIHR Brain Injury MedTech Cooperative. ODM is supported by a Clinical Research Training Fellowship funded by the Cancer Research UK Clinical Academic Training Programme (SEBCATP-2024/100008) and supported by the Cancer Research UK Cambridge Centre (C9685/A25117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1690997/full#supplementary-material
References
1. Safdarian M, Trinka E, Rahimi-Movaghar V, Thomschewski A, Aali A, Abady GG, et al. Global, regional, and national burden of spinal cord injury, 1990&x2013;2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2023) 22:1026–47. doi: 10.1016/S1474-4422(23)00287-9
2. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. (2017) 3:17018. doi: 10.1038/nrdp.2017.18
3. Batchelor PE, Wills TE, Skeers P, Battistuzzo CR, Macleod MR, Howells DW, et al. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PloS One. (2013) 8:e72659. doi: 10.1371/journal.pone.0072659
4. Milhorat TH, Capocelli AL Jr, Anzil AP, Kotzen RM, and Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. (1995) 82:802–812. doi: 10.3171/jns.1995.82.5.0802
5. McKeon RJ, Schreiber RC, Rudge JS, and Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. (1991) 11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991
6. Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J Neuroinflammation. (2018) 15:150. doi: 10.1186/s12974-018-1193-6
7. Gregoretti IV, Lee YM, and Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. (2004) 338:17–31. doi: 10.1016/j.jmb.2004.02.006
8. Huber LC, Stanczyk J, Jüngel A, and Gay S. Epigenetics in inflammatory rheumatic diseases. Arthritis Rheum. (2007) 56:3523–3531. doi: 10.1002/art.22948
9. Yang XJ and Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. (2008) 9:206–218. doi: 10.1038/nrm2346
10. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, and van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. (2003) 370:737–749. doi: 10.1042/BJ20021321
11. Sauve AA, Wolberger C, Schramm VL, and Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. (2006) 75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500
12. Didonna A and Opal P. The promise and perils of HDAC inhibitors in neurodegeneration. Ann Clin Trans Neurol. (2015) 2:79–101. doi: 10.1002/acn3.147
13. Ma YD, Fang J, Liu H, and Zhou L. Increased HDAC3 and decreased miRNA-130a expression in PBMCs through recruitment HDAC3 in patients with spinal cord injuries. Int J Clin Exp Pathol. (2015) 8:1682–1689.
14. Han SB and Lee JK. Anti-inflammatory effect of Trichostatin-A on murine bone marrow-derived macrophages. Arch Pharm Res. (2009) 32:613–624. doi: 10.1007/s12272-009-1418-4
15. Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. (2006) 176:5015–5022. doi: 10.4049/jimmunol.176.8.5015
16. Vojinovic J, Damjanov N, D'Urzo C, Furlan A, Susic G, Pasic S, et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. (2011) 63:1452–1458. doi: 10.1002/art.30238
17. Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci U S A. (2015) 112:2853–2858. doi: 10.1073/pnas.1501441112
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
19. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, and Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodology. (2014) 14:43. doi: 10.1186/1471-2288-14-43
20. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. (2020) 368:l6890. doi: 10.1136/bmj.l6890
21. Boon MA-O and Thomson H. The effect direction plot revisited: Application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res Synth Methods. (2021) 12:29–33. doi: 10.1002/jrsm.1458
22. Bhatti FI, Mowforth OD, Butler MB, Bhatti AI, Adeeko S, Akhbari M, et al. Systematic review of the impact of cannabinoids on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury. Spinal Cord. (2021) 59:1221–39. doi: 10.1038/s41393-021-00680-y
23. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, and Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane (2024). Available online at: www.cochrane.org/handbook.
24. Abdanipour A, Schluesener HJ, and Tiraihi T. Effects of valproic acid, a histone deacetylase inhibitor, on improvement of locomotor function in rat spinal cord injury based on epigenetic science. Iran. (2012) 16:90–100. doi: 10.6091/ibj.1060.2012
25. Chen S, Ye J, Wu G, Shi J, Li X, Chen X, et al. Histone deacetylase 3 inhibition ameliorates microglia-mediated neuro-inflammation via the SIRT1/nrf2 pathway after traumatic spinal cord injury. Neurorehabil Neural Repair. (2023) 37:503–18. doi: 10.1177/15459683231183716
26. Darvishi M, Tiraihi T, Mesbah-Namin SA, Delshad A, and Taheri T. Decreased GFAP expression and improved functional recovery in contused spinal cord of rats following valproic acid therapy. Neurochemical Res. (2014) 39:2319–33. doi: 10.1007/s11064-014-1429-5
27. Gao K, Niu J, and Dang X. Neuroprotection of melatonin on spinal cord injury by activating autophagy and inhibiting apoptosis via SIRT1/AMPK signaling pathway. Biotechnol Lett. (2020) 42:2059–69. doi: 10.1007/s10529-020-02939-5
28. Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB, et al. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci Bull. (2013) 29:484–92. doi: 10.1007/s12264-013-1355-6
29. He Z, Zou S, Yin J, Gao Z, Liu Y, Wu Y, et al. Inhibition of endoplasmic reticulum stress preserves the integrity of blood-spinal cord barrier in diabetic rats subjected to spinal cord injury. Sci Rep. (2017) 7:7661. doi: 10.1038/s41598-017-08052-4
30. Jafarimanesh MA, Ai J, Shojaei S, Khonakdar HA, Darbemamieh G, and Shirian S. Sustained release of valproic acid loaded on chitosan nanoparticles within hybrid of alginate/chitosan hydrogel with/without stem cells in regeneration of spinal cord injury. Prog. (2023) 12:75–86. doi: 10.1007/s40204-022-00209-3
31. Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, and Yune TY. Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochemistry. (2012) 121:818–29. doi: 10.1111/j.1471-4159.2012.07731.x
32. Lu WH, Wang CY, Chen PS, Wang JW, Chuang DM, Yang CS, et al. Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia. J Neurosci Res. (2013) 91:694–705. doi: 10.1002/jnr.23200
33. Luo L, Wang Y, Tong J, Li L, Zhu Y, and Jin M. Xenon postconditioning attenuates neuronal injury after spinal cord ischemia/reperfusion injury by targeting endoplasmic reticulum stress-associated apoptosis. Neurosurg Rev. (2023) 46:213. doi: 10.1007/s10143-023-02125-x
34. Lv L, Han X, Sun Y, Wang X, and Dong Q. Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro. Exp Neurol. (2012) 233:783–90. doi: 10.1016/j.expneurol.2011.11.042
35. Lv L, Sun Y, Han X, Xu CC, Tang YP, and Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. (2011) 1396:60–8. doi: 10.1016/j.brainres.2011.03.040
36. Mardi A, Biglar A, Nejatbakhsh R, and Abdanipour A. Valproic acid ameliorates locomotor function in the rat model of contusion via alteration of mst1, bcl-2, and nrf2 gene expression. Iran. (2021) 25:303–7. doi: 10.52547/ibj.25.4.303
37. Penas C, Verdu E, Asensio-Pinilla E, Guzman-Lenis MS, Herrando-Grabulosa M, Navarro X, et al. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. (2011) 178:33–44. doi: 10.1016/j.neuroscience.2011.01.012
38. Reis KP, Sperling LE, Teixeira C, Sommer L, Colombo M, Koester LS, et al. VPA/PLGA microfibers produced by coaxial electrospinning for the treatment of central nervous system injury. Braz J Med Biol Res. (2020) 53:e8993. doi: 10.1590/1414-431x20208993
39. Ulas M and Argadal OG. Trace element, antioxidant and oxidant levels in spinal cord injury: different perspective on the effects of valproic acid. Eur Rev Med Pharmacol Sci. (2023) 27:3892–905. doi: 10.26355/eurrev_202305_32295
40. Wang D, Wang K, Liu Z, Wang Z, and Wu H. Valproic acid-labeled chitosan nanoparticles promote recovery of neuronal injury after spinal cord injury. Aging (Albany NY). (2020) 12:8953–67. doi: 10.18632/aging.103125
41. Wang D, Wang K, Liu Z, Wang Z, and Wu H. Valproic acid labeled chitosan nanoparticles promote the proliferation and differentiation of neural stem cells after spinal cord injury. Neurotox Res. (2021) 39:456–66. doi: 10.1007/s12640-020-00304-y
42. Yu SH, Cho DC, Kim KT, Nam KH, Cho HJ, and Sung JK. The neuroprotective effect of treatment of valproic Acid in acute spinal cord injury. J. (2012) 51:191–8. doi: 10.3340/jkns.2012.51.4.191
43. Zhang H, Piao M, Guo M, Meng L, and Yu H. MicroRNA-211-5p attenuates spinal cord injury via targeting of activating transcription factor 6. Tissue Cell. (2021) 68:101459. doi: 10.1016/j.tice.2020.101459
44. Zhou Y, Ye L, Zheng B, Zhu S, Shi H, Zhang H, et al. Phenylbutyrate prevents disruption of blood-spinal cord barrier by inhibiting endoplasmic reticulum stress after spinal cord injury. Am J Transl Res. (2016) 8:1864–75.
45. Chu W, Yuan J, Huang L, Xiang X, Zhu H, Chen F, et al. Valproic acid arrests proliferation but promotes neuronal differentiation of adult spinal NSPCs from SCI rats. Neurochemical Res. (2015) 40:1472–86. doi: 10.1007/s11064-015-1618-x
46. Li J, Cao Y, Li LN, Chu X, Wang YS, Cai JJ, et al. Neuroprotective effects of oxymatrine via triggering autophagy and inhibiting apoptosis following spinal cord injury in rats. Mol Neurobiol. (2023) 60:4450–71. doi: 10.1007/s12035-023-03364-1
47. Yu S, Xie L, Liu Z, Li C, and Liang Y. MLN4924 Exerts a Neuroprotective Effect against Oxidative Stress via Sirt1 in Spinal Cord Ischemia-Reperfusion Injury. Oxid Med Cell Longev. (2019) 2019:7283639. doi: 10.1155/2019/7283639
48. Cui Y, Cen Q, Feng J, Wei J, Wang L, Chang C, et al. Sodium butyrate alleviates spinal cord injury via inhibition of NLRP3/Caspase-1/GSDMD-mediated pyroptosis. Metab Brain Disease. (2025) 40:157. doi: 10.1007/s11011-025-01589-8
49. Dai C, Wang X, Liu R, Gao W, Zhang H, Yin Z, et al. ACY1215 exerts anti-inflammatory effects by inhibition of NF-kappaB and STAT3 signaling pathway to repair spinal cord injury. Biol Pharm Bulletin. (2024) 47:1734–45. doi: 10.1248/bpb.b23-00603
50. Kalimullina T, Sachdeva R, Pawar K, Cao S, Marwaha A, Liu J, et al. Neuroprotective agents ineffective in mitigating autonomic dysreflexia following experimental spinal cord injury. Exp neurology. (2024) 382:114993. doi: 10.1016/j.expneurol.2024.114993
51. Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. (2010) 120:3255–66. doi: 10.1172/JCI42957
52. Dai C, Liu B, Peng B, Qu B, Lin J, Peng B, et al. Entinostat improves motor function and neuronal damage via downregulating NLRP3 inflammasome activation after spinal cord injury. Front Pharmacol. (2021) 12:774539. doi: 10.3389/fphar.2021.774539
53. Lanza M, Campolo M, Casili G, Filippone A, Paterniti I, Cuzzocrea S, et al. Sodium butyrate exerts neuroprotective effects in spinal cord injury. Mol Neurobiol. (2019) 56:3937–47. doi: 10.1007/s12035-018-1347-7
54. Qi X and Wang P. Class IIa HDACs inhibitor TMP269 promotes M1 polarization of macrophages after spinal cord injury. J Cell Biochem. (2018) 119:3081–90. doi: 10.1002/jcb.26446
55. Zheng Z, Zhou Y, Ye L, Lu Q, Zhang K, Zhang J, et al. Histone deacetylase 6 inhibition restores autophagic flux to promote functional recovery after spinal cord injury. Exp Neurology. (2020) 324:113138. doi: 10.1016/j.expneurol.2019.113138
56. Wang C, Chen R, Zhu X, and Zhang X. Suberoylanilide hydroxamic acid ameliorates pain sensitization in central neuropathic pain after spinal cord injury via the HDAC5/NEDD4/SCN9A axis. Neurochemical Res. (2023) 48:2436–50. doi: 10.1007/s11064-023-03913-z
57. Hendrix S, Sanchez S, Ventriglia E, and Lemmens S. HDAC8 Inhibition Reduces Lesional Iba-1+ Cell Infiltration after Spinal Cord Injury without Effects on Functional Recovery. Int. (2020) 21:25. doi: 10.3390/ijms21124539
58. Jiang D, Yang X, Ge M, Hu H, Xu C, Wen S, et al. Zinc defends against Parthanatos and promotes functional recovery after spinal cord injury through SIRT3-mediated anti-oxidative stress and mitophagy. CNS Neurosci Ther. (2023) 29:2857–72. doi: 10.1111/cns.14222
59. Kuboyama T, Wahane S, Huang Y, Zhou X, Wong JK, Koemeter-Cox A, et al. HDAC3 inhibition ameliorates spinal cord injury by immunomodulation. Sci Rep. (2017) 7:8641. doi: 10.1038/s41598-017-08535-4
60. Mizukami T, Orihashi K, Herlambang B, Takahashi S, Hamaishi M, Okada K, et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J Vasc Surg. (2010) 52:1580–6. doi: 10.1016/j.jvs.2010.06.172
61. Sanchez S, Lemmens S, Baeten P, Sommer D, Dooley D, Hendrix S, et al. HDAC3 inhibition promotes alternative activation of macrophages but does not affect functional recovery after spinal cord injury. Exp Neurobiology. (2018) 27:437–52. doi: 10.5607/en.2018.27.5.437
62. Qin T, Li C, Xu Y, Qin Y, Jin Y, He R, et al. Local delivery of EGFR+NSCs-derived exosomes promotes neural regeneration post spinal cord injury via miR-34a-5p/HDAC6 pathway. Bioactive Materials. (2024) 33:424–43. doi: 10.1016/j.bioactmat.2023.11.013
63. Seira O, Wang W, Lee S, Roskams J, and Tetzlaff W. HDAC inhibition leads to age-dependent opposite regenerative effect upon PTEN deletion in rubrospinal axons after SCI. Neurobiol Aging. (2020) 90:99–109. doi: 10.1016/j.neurobiolaging.2020.02.006
64. Zhang S, Fujita Y, Matsuzaki R, and Yamashita T. Class i histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury article/13/51/64/60/38/77. Cell Death Disease. (2018) 9:460. doi: 10.1038/s41419-018-0543-8
65. Sakamoto K, Ozaki T, Ko Y-C, Tsai C-F, Gong Y, Morozumi M, et al. Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nat Chem Biol. (2019) 15:699–709. doi: 10.1038/s41589-019-0274-x
66. Yang R, Song C, Chen J, Zhou L, Jiang X, Cao X, et al. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine. (2020) 69:153211. doi: 10.1016/j.phymed.2020.153211
67. Wu X, Chen Ps Fau - Dallas S, Dallas S Fau - Wilson B, Wilson B Fau - Block ML, Block Ml Fau - Wang C-C, Wang Cc Fau - Kinyamu H, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. (2008) 11:1123–1134. doi: 10.1017/S1461145708009024
68. Jiang T, Qin T, Gao P, Tao Z, Wang X, Wu M, et al. SIRT1 attenuates blood-spinal cord barrier disruption after spinal cord injury by deacetylating p66Shc. Redox Biol. (2023) 60:102615. doi: 10.1016/j.redox.2023.102615
69. Drewes AM, Andreasen A, and Poulsen LH. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia. (1994) 32:565–569. doi: 10.1038/sc.1994.89
70. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. (2020) 18:e3000410. doi: 10.1371/journal.pbio.3000410
71. Lee D-H and Lee JK. Animal models of axon regeneration after spinal cord injury. Neurosci Bulletin. (2013) 29:436–44. doi: 10.1007/s12264-013-1365-4
72. Delhaas EM and Huygen F. Complications associated with intrathecal drug delivery systems. BJA Educ. (2020) 20:51–57. doi: 10.1016/j.bjae.2019.11.002
Keywords: spinal cord injury, histone deacetylase inhibitors, preclinical study, valproate, 4-PBA
Citation: Jagodzinska NM, Cole C, Brannigan J, Chintapalli R, Davies BM, Kotter MR and Mowforth OD (2025) The impact of histone deacetylase inhibition on neurobehavioural outcomes in preclinical models of traumatic and non-traumatic spinal cord injury: a systematic review. Front. Immunol. 16:1690997. doi: 10.3389/fimmu.2025.1690997
Received: 27 August 2025; Accepted: 27 October 2025;
Published: 07 November 2025.
Edited by:
Guillem Paniagua Soriano, Principe Felipe Research Center (CIPF), SpainReviewed by:
Zhigang Chang, National Center of Gerontology, ChinaMohammadhossein Vazirizadeh-Mahabadi, Iran University of Medical Sciences, Iran
Copyright © 2025 Jagodzinska, Cole, Brannigan, Chintapalli, Davies, Kotter and Mowforth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oliver D. Mowforth, b2xpdmVyLm1vd2ZvcnRoQG5ocy5uZXQ=
 Natalia M. Jagodzinska
Natalia M. Jagodzinska Caleb Cole
Caleb Cole Jamie Brannigan
Jamie Brannigan Renuka Chintapalli
Renuka Chintapalli Benjamin M. Davies
Benjamin M. Davies Mark R. Kotter3
Mark R. Kotter3 Oliver D. Mowforth
Oliver D. Mowforth