- 1Department of Translational Research and Precision Medicine, Cyprus Cancer Research Institute (CCRI), Nicosia, Cyprus
- 2Medical School, University of Cyprus, Nicosia, Cyprus
- 3Department of Medical Oncology, Bank of Cyprus Oncology Centre, Nicosia, Cyprus
Osteosarcoma (OS) and rhabdomyosarcoma (RMS) are the most prevalent pediatric sarcoma subtypes of the bones and soft tissues respectively. The lack in targeted treatment approaches alongside the generally dismal prognosis in the metastatic setting render the discovery of novel therapeutic modalities for these diseases a pressing need. Chimeric antigen receptor (CAR)-therapy has emerged as an innovative strategy for cancer management with marked success in the treatment of hematological malignancies. The specific approach employs genetic engineering to redirect the specificity of immune cells, primarily T cells, through the exogenous expression of fully synthetic receptors, eventually arming them with the capacity to recognize tumor associated antigens (TAA). CAR-based treatment for OS and RMS has been under investigation in pre-clinical studies over the past few years, while the first promising results from a clinical trial have recently been published. However, the so far limited efficacy of CAR-therapy in solid tumors due to various constraining factors, such as poor CAR-T cell trafficking to the tumor, minimal tumor infiltration and reduced in vivo persistence, still needs to be properly addressed. In this mini review we focus on the most recent CAR-therapy strategies explored in OS and RMS while we briefly review the evolution of CARs through the years and highlight existing challenges in the CAR field.
1 Introduction
Osteosarcoma (OS) and rhabdomyosarcoma (RMS) represent the predominant bone and soft tissue sarcomas respectively, affecting children (1, 2). Although with the current therapeutic armamentarium consisting mainly of surgery, radiotherapy and chemotherapy localized OS and RMS can achieve complete remission, prognosis for metastatic and high-risk patients remains poor (3, 4). In addition, the relapse rate for treated localized disease is still considerably high. There is therefore an urgent need for the identification of novel targeted therapeutic approaches for more efficient management and durable antitumor responses. Chimeric antigen receptor (CAR)-therapy, an immunotherapeutic modality that has exhibited promising results in the treatment of hematological malignancies (5, 6), has posed as an attractive approach for the management of sarcomas including OS and RMS (7, 8). CAR-therapy, a form of adoptive cell therapy (ACT), employs genetically engineered T cells to recognize tumor cell surface antigens, providing a targeted and potentially long-lasting antitumor effect (9, 10). Isolated primary T cells are engineered ex vivo to express synthetic CARs that recognize tumor-associated antigens (TAAs), triggering T cell activation and antitumor responses (Figure 1A). Despite considerable advances in the CAR-therapy field, CAR application in the solid tumor context has proven challenging. The diminished capacity of the CAR-engineered cells to traffic to and infiltrate the tumors, the poor performance and/or persistence of the cells in vivo, the hostile tumor microenvironment (TME), as well as the TAA loss or expression heterogeneity are hurdles pending resolution (11, 12). This mini review summarizes recent advances in the field of OS and RMS CAR-therapy, while taking a glance at the CAR evolution through the years and pointing out challenges yet to be addressed.
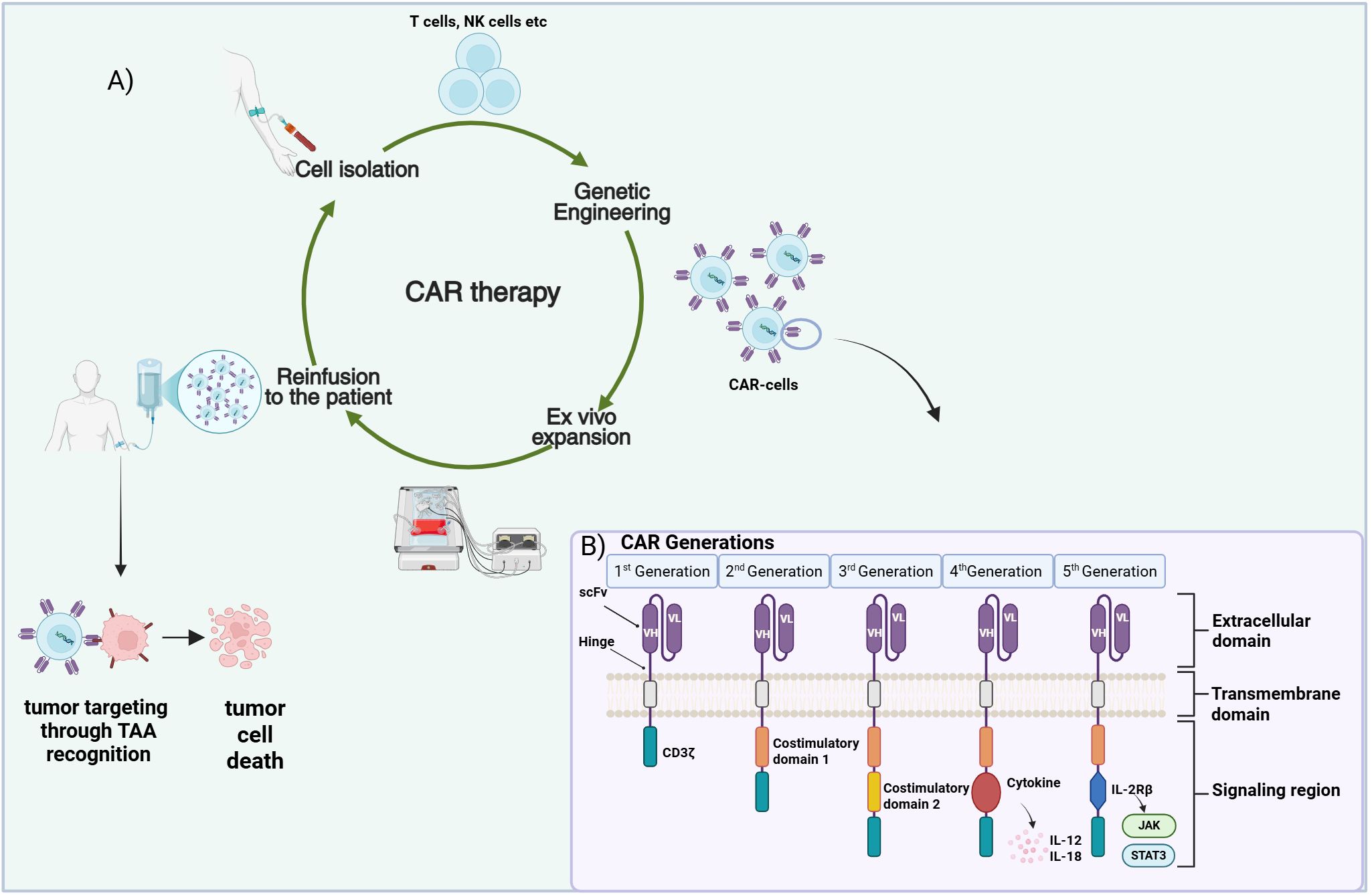
Figure 1. CAR-therapy principle and CAR structure. (A) Presentation of the CAR-based therapeutic approach. Immune cells (T, NK) are isolated from patient’s blood and are genetically engineered to express a CAR specific to an OS/RMS tumor associated antigen (TAA). Subsequent infusion of the expanded CAR-T cells into the patient leads to their trafficking and homing to the cancerous tissues where they recognize and attack the tumor cells. (B) The five different CAR generations designed up to date. A single chain variable fragment (scFv) consisting of the variable heavy (VH) and the variable light chains (VL) derived from an antibody represents the extracellular antigen binding domain of the CAR. A transmembrane domain is used to anchor the construct into the membrane. Various intracellular signaling domain combinations are employed for signal transmission inside the cell. Figure created in BioRender. Stavrou, M. (2025) https://BioRender.com/bh7uggd.
2 Chimeric antigen receptors: structure and evolution through the years
A CAR is a modular construct consisting of four major components: a) an extracellular domain, commonly in the form of an antibody derived single-chain variable fragment (scFv)—responsible for antigen recognition, b) a hinge—flexible element enabling appropriate CAR orientation for antigen binding, c) a transmembrane domain—as membrane anchor and d) intracellular signaling domain(s)—for signal transmission (13, 14). Since their introduction CARs have evolved through five generations (Figure 1B) with every new CAR iteration seeking to further improve the activation, antitumor capacity, tumor homing/infiltration and in vivo expansion/persistence of the CAR-bearing T cells, thereby reinforcing their role as efficient cancer treatment tools (15). First-generation CARs incorporated the CD3ζ chain as the CAR signaling domain (16). T cells expressing these CARs resulted in limited antitumor activity and in vivo persistence when employed in clinical trials (17, 18) prompting the inclusion of co-stimulatory signaling in the CAR construct for higher efficacy. Introduction of a single co-stimulatory domain in the CAR gave rise to the second-generation CARs. Incorporation of CD28 or 4-1BB (CD137) costimulatory proteins in tandem with CD3ζ resulted in superior proliferative potential and cytotoxic activity and enhanced CAR-T cell persistence (19, 20). Taking it a step forward, third-generation CARs integrated two or more co-stimulatory domains, such as CD28, 4-1BB, OX40 assuming that distinct costimulatory proteins with different features could complement each other, leading to superior responses (21–23). The concept of adding a third signal, further to CD3ζ and co-stimulation, yielded cytokine-supplemented fourth-generation CARs with the hope of superior potency against solid tumors (14, 24). T cells modified with fourth-generation CARs are known as TRUCK-T cells, T cells Redirected for Universal Cytokine-mediated Killing (25). Fourth-generation CAR constructs are equipped with one or more pro-inflammatory cytokine genes, like Interleukin-12 (IL-12) or Interleukin-18 (IL-18) which are under the regulation of the nuclear factor for activated T cells (NFAT) responsive cassette. Cytokine secretion, induced upon T cell activation, provides an additional signal that not only improves CAR-T cell efficacy, but also triggers the innate immunity and possibly modulates the tumor microenvironment (TME), eventually evoking more robust and prolonged antitumor responses (24, 25). Fifth-generation CARs, the most recent CAR design integrate an additional membrane receptor to the construct; truncated cytoplasmatic domain of the Interleukin-receptor 2β (IL-2Rβ) was the first to be explored (26). This addition allows binding of the Signal Transducer and Activator of Transcription 3 (STAT3), leading to the subsequent activation of the Janus Kinase-STAT (JAK-STAT) signaling pathway (26, 27). The synergistic effect of the three signaling components: i) CD3ζ–initiating activation, ii) costimulatory molecules–enhancing activation/proliferation and iii) IL-2R–inducing JAK-STAT3/5 pathway supporting T cell survival can promote more vigorous and long-lasting immune responses. Apart from the evolution in the design of the CAR per se, other strategies have also been employed to further improve the efficacy, safety and wider applicability of the CAR-therapy. Several of them such as, generation of dual-CAR T cells, introduction of chemokine receptor genes or suicide genes in the CAR-cassette are outlined in the next section reviewing current CAR-based approaches in OS and RMS.
3 CAR approaches for the treatment of osteosarcoma and rhabdomyosarcoma
3.1 CAR-T against osteosarcoma
Various strategies have been explored for osteosarcoma CAR treatment ranging from the use of second and third generation CARs targeting certain TAAs to the use of CARs incorporating elements such as chemokine receptor or interleukin genes. Given that efficient CAR-T cell tumor trafficking and penetration are crucial for an effective response, interventions to improve CAR-T cell homing by means of incorporating chemokine receptor genes emerged as a promising approach. In addition, the incorporation of interleukin genes holds the promise to further potentiate the activation, proliferation and survival of the CAR-T cells. The concept of a switchable universal CAR was also explored as an attractive off-the-shelf versatile tool for OS treatment, solely relying on the development of appropriate adaptor molecules for its function.
B7-H3 presents an attractive target for osteosarcoma CAR-therapy owing to its overexpression in the malignant tissues and minimal expression in healthy tissues (28, 29). Majzner et al. first explored the potential of second generation B7-H3-4-1BB CAR-T cells in a highly metastatic xenograft OS model, demonstrating regression of the established tumors while reporting the dependence of CAR activity to high target antigen levels (30). In a separate study, MGA271 anti-human B7-H3 antibody with proved cross-reactivity against the B7-H3 canine counterpart was employed for the generation of canine CAR-T cells (31). MGA271-CAR-T cells with either CD28 or 4-1BB co-stimulation were efficient at killing canine OS spheroids in vitro and they were safely transferred into healthy canine subjects in vivo (31). T cells expressing a third-generation B7-H3-CAR incorporating both the 4-1BB and CD28 costimulatory domains demonstrated excellent killing capacity against target-positive OS cell lines in vitro (32). Significant anti-tumor effect was exhibited in an OS patient-derived xenograft (PDX) mouse model with profound tumor growth inhibition achieved with either a high (1X107) or a low (5X106) CAR-T cell dose (32). Talbot et al. tested C-X-C chemokine receptor type 2 (CXCR2) and 6 (CXCR6) within their B7-H3-CAR cassette to target OS (33). Chemokine receptor inclusion enhanced CAR-T cell migration towards target cells in vitro. Similarly, improved homing and expansion at the tumor sites were observed for the CXCR-expressing CAR-T cells in xenograft models and a pulmonary metastasis model, accompanied by augmentation of antitumor activity in the latter (33). In another study, canine T cells expressing a dual B7-H3-CAR/CXCR2 construct exhibited specific canine OS-target killing and elevated cytokine production in vitro (34). In canine xenograft models, the abovementioned cells demonstrated increased persistence and successful tumor growth inhibition in all mice (34). Based on Natural Killer Group 2, Member D (NKG2D) ligand detection on several malignant cells, including OS cells, CD45RA- memory T cells were engineered to express a second generation NKG2D-CAR to target OS (35). NKG2D-CAR-T cells were characterized by amplified in vitro cytotoxic potential along with improved ability to control tumor growth and prolong survival in an orthotopic OS mouse model (35). To further boost infiltration and persistence of NKG2D-CAR-T cells, Hui et al. combined their CAR with interleukin-7 (IL-7)—previously reported to prevent CAR-T cell exhaustion via the regulation of metabolic pathways (36)—and CXCR5 (C5) (37). A substantial increase in C5/IL7-NKG2D-CAR-T cell activation, cytokine release and degranulation were detected in vitro compared to conventional NKG2D-CAR-T cells. Elevated T cell survival and proliferation in vivo, translated to greater antitumor activity and was correlated with a profound elevation in phosphorylated STAT5 (37). The interesting approach of a switchable universal CAR was investigated by Hidalgo et al. (38). The system utilizes an anti- Fluorescein Isothiocyanate (FITC) CAR whose activation depends on the presence of a FITC-conjugated adaptor molecule—a monoclonal antibody (mAb) specific to the targeted TAA. TAA binding by the adaptor molecule mediates the anti-FITC-CAR-T cell trafficking to the tumor. In vitro, anti-FITC CAR-T cells along with administration of B7-H3-specific FITC-labeled mAb established strong tumoricidal effects against B7-H3 positive OS cells, which could be further amplified when FITC-labeled mAbs targeting different tumor antigens were combined (38).
3.2 CAR-T against rhabdomyosarcoma
The efforts for successful application of CAR therapy in RMS are currently coordinated towards the identification and targeting of appropriate TAAs, the optimization of CAR domains to enhance the activity and persistence of the cells and the investigation of means to mitigate antigen heterogeneity/loss. Strategies have been employed utilizing either bicistronic CAR constructs or dual CAR-transduced cells simultaneously targeting two antigens to counteract the effect of antigen loss. Moreover, considerations regarding the safety of the CAR constructs have led to the validation of suicide gene systems as part of the CAR cassette. These are of great value as they facilitate the conditional ablation of CAR-T cells in vivo, thereby alleviating any treatment-related adverse events such as cytokine release syndrome (CRS), neurotoxicity or other on-target off-tumor toxicities.
Overexpression of receptor tyrosine kinase fibroblast growth factor receptor 4 (FGFR4) was reported in RMS tumors prompting its use as a target antigen (39, 40). Sullivan et al. introduced a novel CAR design targeting the proximal FGFR4 domain, improving cytotoxicity against RMS in vitro (41). Although no efficacy against orthotopic RMS tumors was initially observed, combination therapy with anti-myeloid drugs enabled successful orthotopic tumor clearance potentially via circumventing the immunosuppressive TME (41). A second-generation FGFR4-4-1BBζ-CAR was described by Tian et al. for the treatment of RMS (42). The produced CAR-T cells exhibited robust target-selective antitumor responses in vitro. Potent in vivo responses were reported in two metastatic mouse models marked by significantly reduced tumor burden, increased survival probability and good persistence of the transferred CAR-expressing cells (42). Similarly, CAR-T cells efficiently infiltrated the tumor and controlled its growth in two orthotopic mouse models (42). Replacement of the CD8 hinge and transmembrane domain and the 4-1BB costimulatory domain of the abovementioned FGFR4-CAR with those of CD28 augmented the CAR anti-tumor efficacy, nonetheless, at the expense of the CAR-T cell in vivo persistence (43). The subsequent design of a bicistronic construct encompassing an FGFR4-CD28 and a B7-H3-4-1BB CAR resulted in superior cytotoxicity, increased T cell differentiation and prolonged in vivo persistence. Most importantly, CAR-T cells expressing the bicistronic CAR could overcome the heterogeneous TAA expression exerting consistent cytotoxicity irrespectively of the presence or absence of either target antigen (43). On a similar note, Timpanaro et al. generated dual CAR-T cells expressing both a B7-H3-CAR and an FGFR4-CAR (44). Dual CAR-T and B7-H3-CAR-T cells were equally efficient at eradicating orthotopic RMS tumors in mice when B7-H3 was expressed above a critical threshold. Contrarily, low B7-H3 levels were not permissive of tumor control by either T cell type (44). The study suggested careful consideration in the CAR design and selection of target combination when targeting tumors with low antigen expression. Xiao et al. employed, a second generation FGFR4-4-1BBζ CAR approach for RMS treatment combined with the inducible caspase 9 (iCasp9) suicide construct (45). Administration of the small molecule dimerizer, AP20187, enabling the subsequent caspase activation efficiently induced apoptosis in vitro accompanied by reduced antitumor toxicity and cytokine production (45). Evaluation of the murine version of the CAR, (m)FGFR4-CAR, in a syngeneic mouse model demonstrated tumor growth control with no evident off-tumor toxicity (45). OS and alveolar RMS are characterized by high GD2 expression. T cells transduced with a third-generation GD2.CAR-CD28.4–1BBζ incorporating the iCasp9 gene presented robust in vitro antitumor responses highly dependent on GD2 expression levels (46). In a metastatic embryonal RMS xenograft mouse model GD2-CAR-T cells efficiently eradicated tumor cells and prolonged survival. Nevertheless, the marked variability in the antitumor responses in two different OS orthotopic models, suggested the implication of the TME on shaping the CAR-T cell induced responses; myeloid-derived suppressor cells (MDSCs) were identified as key influencers in this context (46).
3.3 Other immune cells explored in OS and RMS CAR-treatment
CAR-engineered natural killer (NK) cells represent a promising alternative to CAR-T cells owing to their inherent cytotoxic capacities and their superior safety profile characterized by reduced risk for CRS and Graft versus Host Disease (GvHD). NK cells redirected against the ehprin-type-A Receptor 2 (EphA2), overexpressed in a spectrum of pediatric sarcomas including OS and RMS have demonstrated encouraging antitumor effects (47). In RMS and OS orthotopic in vivo models, the EphA2-CAR-expressing cells were efficient at suppressing tumor growth, inducing a substantial survival benefit. Notably the EphA2-CAR constructed by this group carried specific chemical modifications at the mRNA level, namely, the replacement of uridine by the N1-pseudomethyluridine (m1ψ) and the addition of 10% adenosine 5’-(α-thio)-triphosphate (ATPαS) a modified form of ATP to enhance EphA2-CAR expression and mRNA stability respectively (47). Epidermal growth factor receptor (EGFR) is commonly overexpressed in RMS, potentially correlating with an increased likelihood of resistance to treatment and disease recurrence. In vitro assessment of EGFR-CAR expressing primary NK cells demonstrated augmented cytotoxic potential compared to their non-transduced counterparts against two-dimensional RMS cell line cultures and tumor spheroids (48). EGFR-CAR cells also exhibited robust cytotoxicity against chemotherapy resistant RMS cell lines and patient derived tumor cells. Although reduced tumor infiltration ability was initially observed in vivo, this improved considerably upon combination of CAR-therapy with radiotherapy; likely attributed to irradiation-induced changes in the chemokine levels and subsequent TME modulation (48). Cytokine-induced killer (CIK) cells are a heterogenous cell population possessing features from both the T and the NK cells. While preserving the characteristics of the adaptive T cell-mediated immunity, these cells are non-major histocompatibility complex (MHC)-restricted, alleviating the risk of GvHD in the allogeneic setting. CAR-CIK cells targeting ERBB2 (HER2) exhibited superiority compared to T cells in eliminating RMS cell lines and primary tumor cells (49). An equally vigorous antitumor activity was reported for the ErbB2-CAR-T and the ErbB2-CAR-CIK cells impeding metastasis and prolonging survival in an in vivo metastatic RMS xenograft model (49).
4 Current clinical trials assessing CAR-therapy for OS and RMS
Clinical trials are underway for the evaluation of CAR-therapy for sarcomas. Focusing on different approaches these trials aim at enhancing antitumor efficacy and persistence while retaining safety and tolerability. Current approaches involve CAR-T cell dose optimization and validation of different infusion schedules, combination of CAR-T cell therapy with lymphodepleting regimens—to achieve better engraftment or with immune checkpoint inhibitors (ICI)—to overcome the immunosuppressive TME. The concept of dual CAR transduced cells targeting more than one antigen is also under investigation. Encouraging results were reported from HEROS2.0 clinical trial. The phase-I trial assessed the safety of either a dose of 1X108 total T cells/m2 or 1X108 CAR+ T cells/m2 following patient lymphodepletion to treat advanced sarcomas (50). Out of fourteen total enrollments for the study, 8 were OS cases and 4 were RMS. A good overall safety profile was reported with half of the patients receiving CAR-T cells presenting disease stabilization or remission. Notably, multiple infusions of the CAR-T cell product were required to achieve marked T cell expansion in the patients (50). A boy with metastatic RMS demonstrated complete remission (CR) for a 6 month period before relapsing and reenrolling into the study (51). A second CR was achieved after further treatment which was ongoing at over 6 years up to the reporting time (50). The safety of HER2-CAR T cell treatment combined with the ICIs pembrolizumab or nivolumab in lymphodepleted patients is the primary aim of the currently recruiting HEROS3.0 clinical trial (NCT04995003). B7-H3-CAR engineered T cells are also part of various sarcoma clinical trials. A currently ongoing clinical trial further to the evaluation of B7-H3-CAR-T cell safety, is additionally testing a combination of B7-H3-CAR and CD19-CAR T cells aiming to exploit the antigen-presenting nature of CD19+ B cells to enhance CAR-T cell expansion and persistence (NCT04483778). The same trial is also set to explore the feasibility of combining CAR treatment with pembrolizumab. More trials looking into alternative target antigens, different CAR designs and/or combination with other treatment regimens are presented in Table 1.
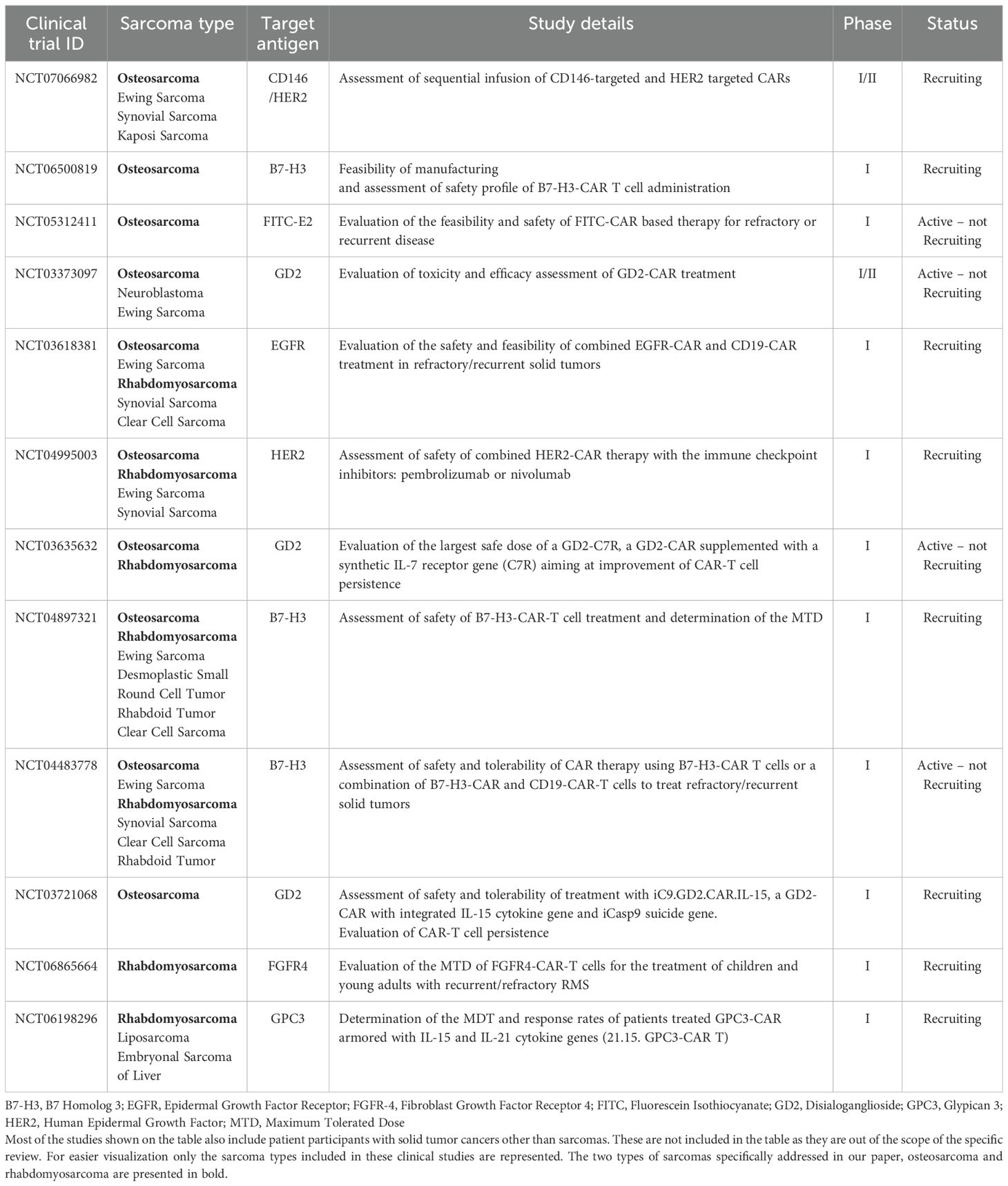
Table 1. Active or currently recruiting clinical trials assessing CAR-therapy in osteosarcoma and rhabdomyosarcoma.
5 Concluding remarks
CAR-therapy has shown encouraging results in pre-clinical studies of osteosarcoma and rhabdomyosarcoma, and clinical trials are underway to further assess this therapeutic approach. Although several formidable challenges remain to be addressed for the wide application of CAR-therapy in solid tumors, the continuous expansion of the field holds a great promise for the future. Novel CAR designs integrating combinations of signaling domains, cytokines/cytokine receptors have enhanced the antitumor capacity and persistence of the CAR-engineered cells. Multi-targeted CAR-T cell approaches (bispecific CARs, dual-CAR cells), whereby more than one TAAs are targeted could potentially ameliorate antigen loss/expression heterogeneity (52). Other strategies, including the use of logic gates (53) to mitigate TAA heterogeneity and reduce healthy tissue CAR-reactivity, or the introduction of tunable CARs allowing the on-demand CAR activity downregulation are also areas of active research (54). Immune cells with intrinsic cytotoxic capacity and non-MHC-restricted nature, such as NK and CIK cells are actively tested as promising T cell alternatives, hoping to enhance the antitumor potential and enable CAR-therapy application in the allogeneic context. Furthermore, CAR-therapy in conjunction with other treatment modalities including chemotherapy, radiotherapy and ICIs is extensively explored as preliminary data supports synergistic effects of the combined therapeutic entities. Studies focusing on better understanding the TME in OS and RMS are ongoing and could provide additional tools for mitigating challenges related to CAR treatment. A single-cell RNA study in OS showed that regulatory T cells (Tregs) dominate the TME driving immune evasion via CXCL12/CXCR4 and TGFB1 signaling, highlighting targets to enhance CAR-T cell infiltration (55). In RMS, single-cell profiling revealed enrichment of M2-like macrophages and NECTIN3–TIGIT interactions driving T cell dysfunction, suggesting that modulating myeloid polarization or TIGIT signaling could improve CAR-T efficacy (56). Considering a solid tumor as a complex ecosystem rather than an isolated mass and attempting to modulate the TME in parallel to the CAR-therapy could be the key to the improvement of the CAR-induced antitumor responses.
Author contributions
MS: Conceptualization, Writing – original draft, Writing – review & editing. TN: Writing – original draft, Writing – review & editing. MG: Writing – original draft, Writing – review & editing. AC: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACT, Adoptive Cell Therapy; ATPαS-5’, (α-thio)-triphosphate; B7-H3, B7 Homolog 3; CAR, Chimeric Antigen Receptor; CIK, Cytokine Induced; CRS, Cytokine Release syndrome; CXCR2, C-X-C Chemokine Receptor type 2; CXCR5, C-X-C Chemokine Receptor type 5; CXCR6, C-X-C Chemokine Receptor type 6; EGFR, Epidermal Growth Factor; EphA2, ephrin type-A Receptor 2; FGFR4, Fibroblast Growth Factor Receptor 4; FITC, Fluorescein Isothiocyanate; G-CSF, Granulocyte Colony-stimulating Factor; GD2, Disialoganglioside; GvHD, Graft versus Host Disease; HER2, Human Epidermal Growth Factor; ICI, Immune Checkpoint Inhibitor; IL-7, Interleukin-7; IL-12, Interleukin-12; IL-18, Interleukin-18; IL2Rβ, Interleukin-receptor 2β; JAK, Janus Kinase; m1ψ, N1-pseudomethyluridine; mAb, monoclonal Antibody; MDSC, Myeloid-derived Suppressor Cell; MHC, Major Histocompatibility Complex; NFAT, Nuclear Factor for Activated T cells; NK, Natural Killer; NKG2D, Natural Killer Group 2, Member D; OS, Osteosarcoma; PDX, Patient Derived Xenograft; RMS, Rhabdomyosarcoma; ScFv, single chain variable fragment; STAT, Signal Transducer and Activator of Transcription; TAA, Tumor Associated Antigen; TME, Tumor Microenvironment; Treg, Regulatory T cell; TRUCKs, T cells Redirected for Universal Cytokine-mediated Killing; VH, variable heavy; VL, variable light.
References
1. Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Primers. (2022) 8(1):77. doi: 10.1038/s41572-022-00409-y
2. Chen S, Kelsey AM, and Rudzinski ER. Rhabdomyosarcoma in children and young adults. Virchows Arch. (2025) 486:101–16. doi: 10.1007/s00428-024-03961-y
3. Pilavaki P, Gahanbani Ardakani A, Gikas P, and Constantinidou A. Osteosarcoma: current concepts and evolutions in management principles. J Clin Med. (2023) 12(8):2785. doi: 10.3390/jcm12082785
4. Zarrabi A, Perrin D, Kavoosi M, Sommer M, Sezen S, Mehrbod P, et al. Rhabdomyosarcoma: current therapy, challenges, and future approaches to treatment strategies. Cancers. (2023) 15(4):5269. doi: 10.3390/cancers15215269
5. Lu J and Jiang G. The journey of CAR-T therapy in hematological Malignancies. Mol Cancer. (2022) 21:194. doi: 10.1186/s12943-022-01663-0
6. Zhang X, Zhu L, Zhang H, Chen S, and Xiao Y. CAR-T cell therapy in hematological Malignancies: current opportunities and challenges. Front Immunol. (2022) 13:927153. doi: 10.3389/fimmu.2022.927153
7. Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, and Duan Z. Chimeric antigen receptor T (CAR-T) cell immunotherapy for sarcomas: from mechanisms to potential clinical applications. Cancer Treat Rev. (2020) 82:101934. doi: 10.1016/j.ctrv.2019.101934
8. Guzman G, Reed MR, Bielamowicz K, Koss B, and Rodriguez A. CAR-T therapies in solid tumors: opportunities and challenges. Curr Oncol Rep. (2023) 25:479–89. doi: 10.1007/s11912-023-01380-x
9. De Marco RC, Monzo HJ, and Ojala PM. CAR T cell therapy: A versatile living drug. Int J Mol Sci. (2023) 24(7):6300. doi: 10.3390/ijms24076300
10. Mitra A, Barua A, Huang L, Ganguly S, Feng Q, and He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. (2023) 14:1188049. doi: 10.3389/fimmu.2023.1188049
11. Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. (2024) 21:47–66. doi: 10.1038/s41571-023-00832-4
12. Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. (2023) 22:20. doi: 10.1186/s12943-023-01723-z
13. Jayaraman J, Mellody MP, Hou AJ, Desai RP, Fung AW, Pham AHT, et al. CAR-T design: elements and their synergistic function. EBioMedicine. (2020) 58:102931. doi: 10.1016/j.ebiom.2020.102931
14. Uscanga-Palomeque AC, Chavez-Escamilla AK, Alvizo-Baez CA, Saavedra-Alonso S, Terrazas-Armendariz LD, Tamez-Guerra RS, et al. CAR-T cell therapy: from the shop to cancer therapy. Int J Mol Sci. (2023) 24(21):15688. doi: 10.3390/ijms242115688
15. Sterner RC and Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11:69. doi: 10.1038/s41408-021-00459-7
16. Sadelain M, Rivière I, and Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. (2003) 3:35–45. doi: 10.1038/nrc971
17. Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase ix: first clinical experience. J Clin Oncol. (2006) 24:e20–2. doi: 10.1200/jco.2006.05.9964
18. Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. (2006) 12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183
19. Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. (2011) 121:1822–6. doi: 10.1172/JCI46110
20. Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1bb signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. (2004) 18:676–84. doi: 10.1038/sj.leu.2403302
21. Zhong XS, Matsushita M, Plotkin J, Riviere I, and Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment pi3kinase/AKT/bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. (2010) 18:413–20. doi: 10.1038/mt.2009.210
22. Guercio M, Orlando D, Di Cecca S, Sinibaldi M, Boffa I, Caruso S, et al. CD28.OX40 co-stimulatory combination is associated with long in vivo persistence and high activity of CAR.CD30 T-cells. Haematologica. (2021) 106:987–99. doi: 10.3324/haematol.2019.231183
23. Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol Ther. (2018) 26:2727–37. doi: 10.1016/j.ymthe.2018.09.009
24. Tang L, Pan S, Wei X, Xu X, and Wei Q. Arming CAR-T cells with cytokines and more: innovations in the fourth-generation CAR-T development. Mol Ther. (2023) 31:3146–62. doi: 10.1016/j.ymthe.2023.09.021
25. Chmielewski M and Abken H. Trucks, the fourth-generation CAR T cells: current developments and clinical translation. Adv Cell Gene Ther. (2020) 3:e84. doi: 10.1002/acg2.84
26. Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. (2018) 24:352–9. doi: 10.1038/nm.4478
27. Smirnov S, Mateikovich P, Samochernykh K, and Shlyakhto E. Recent advances on CAR-T signaling pave the way for prolonged persistence and new modalities in clinic. Front Immunol. (2024) 15:1335424. doi: 10.3389/fimmu.2024.1335424
28. Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PloS One. (2013) 8:e70689. doi: 10.1371/journal.pone.0070689
29. Lynch MM, Al-Marayaty R, Obeidin F, Alexiev BA, Chen EY, Viveiros P, et al. B7-H3 is widely expressed in soft tissue sarcomas. BMC Cancer. (2024) 24:1336. doi: 10.1186/s12885-024-13061-4
30. Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. (2019) 25:2560–74. doi: 10.1158/1078-0432.CCR-18-0432
31. Zhang S, Black RG, Kohli K, Hayes BJ, Miller C, Koehne A, et al. B7-H3 specific CAR T cells for the naturally occurring, spontaneous canine sarcoma model. Mol Cancer Ther. (2022) 21:999–1009. doi: 10.1158/1535-7163.MCT-21-0726
32. Zhang Q, Zhang Z, Liu G, Li D, Gu Z, Zhang L, et al. B7-H3 targeted CAR-T cells show highly efficient anti-tumor function against osteosarcoma both in vitro and in vivo. BMC Cancer. (2022) 22:1124. doi: 10.1186/s12885-022-10229-8
33. Talbot LJ, Chabot A, Ross AB, Beckett A, Nguyen P, Fleming A, et al. Redirecting B7-H3.Car T cells to chemokines expressed in osteosarcoma enhances homing and antitumor activity in preclinical models. Clin Cancer Res. (2024) 30:4434–49. doi: 10.1158/1078-0432.CCR-23-3298
34. Cao JW, Lake J, Impastato R, Chow L, Perez L, Chubb L, et al. Targeting osteosarcoma with canine B7-H3 CAR T cells and impact of CXCR2 co-expression on functional activity. Cancer Immunol Immunother. (2024) 73:77. doi: 10.1007/s00262-024-03642-4
35. Fernandez L, Metais JY, Escudero A, Vela M, Valentin J, Vallcorba I, et al. Memory T cells expressing an NKG2D-CAR efficiently target osteosarcoma cells. Clin Cancer Res. (2017) 23:5824–35. doi: 10.1158/1078-0432.CCR-17-0075
36. Li L, Li Q, Yan ZX, Sheng LS, Fu D, Xu P, et al. Transgenic expression of IL-7 regulates CAR-T cell metabolism and enhances in vivo persistence against tumor cells. Sci Rep. (2022) 12:12506. doi: 10.1038/s41598-022-16616-2
37. Hui X, Farooq MA, Chen Y, Ajmal I, Ren Y, Xue M, et al. A novel strategy of co-expressing CXCR5 and IL-7 enhances CAR-T cell effectiveness in osteosarcoma. Front Immunol. (2024) 15:1462076. doi: 10.3389/fimmu.2024.1462076
38. Hidalgo L, Somovilla-Crespo B, Garcia-Rodriguez P, Morales-Molina A, Rodriguez-Milla MA, and Garcia-Castro J. Switchable CAR T cell strategy against osteosarcoma. Cancer Immunol Immunother. (2023) 72:2623–33. doi: 10.1007/s00262-023-03437-z
39. Khan J, Wei JS, Ringnér M, Saal LH, Ladanyi M, Westermann F, et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat Med. (2001) 7:673–9. doi: 10.1038/89044
40. Taylor JG6, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. (2009) 119:3395–407. doi: 10.1172/JCI39703
41. Sullivan PM, Kumar R, Li W, Hoglund V, Wang L, Zhang Y, et al. FGFR4-targeted chimeric antigen receptors combined with anti-myeloid polypharmacy effectively treat orthotopic rhabdomyosarcoma. Mol Cancer Ther. (2022) 21:1608–21. doi: 10.1158/1535-7163.MCT-22-0059
42. Tian M, Wei JS, Shivaprasad N, Highfill SL, Gryder BE, Milewski D, et al. Preclinical development of a chimeric antigen receptor T cell therapy targeting FGFR4 in rhabdomyosarcoma. Cell Rep Med. (2023) 4:101212. doi: 10.1016/j.xcrm.2023.101212
43. Tian M, Wei JS, Cheuk AT, Milewski D, Zhang Z, Kim YY, et al. CAR T-cells targeting FGFR4 and CD276 simultaneously show potent antitumor effect against childhood rhabdomyosarcoma. Nat Commun. (2024) 15:6222. doi: 10.1038/s41467-024-50251-x
44. Timpanaro A, Piccand C, Dzhumashev D, Anton-Joseph S, Robbi A, Moser J, et al. CD276-CAR T cells and dual-CAR T cells targeting CD276/FGFR4 promote rhabdomyosarcoma clearance in orthotopic mouse models. J Exp Clin Cancer Res. (2023) 42:293. doi: 10.1186/s13046-023-02838-3
45. Xiao W, Xu L, Wang J, Yu K, Xu B, Que Y, et al. FGFR4-specific CAR-T cells with inducible caspase-9 suicide gene as an approach to treat rhabdomyosarcoma. Cancer Gene Ther. (2024) 31:1571–84. doi: 10.1038/s41417-024-00823-2
46. Pezzella M, Quintarelli C, Quadraccia MC, Sarcinelli A, Manni S, Iaffaldano L, et al. Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells. J Hematol Oncol. (2024) 17:127. doi: 10.1186/s13045-024-01641-7
47. Lam PY, Omer N, Wong JKM, Tu C, Alim L, Rossi GR, et al. Enhancement of anti-sarcoma immunity by NK cells engineered with mRNA for expression of a EphA2-targeted CAR. Clin Transl Med. (2025) 15:e70140. doi: 10.1002/ctm2.70140
48. Reindl LM, Jalili L, Bexte T, Harenkamp S, Thul S, Hehlgans S, et al. Precision targeting of rhabdomyosarcoma by combining primary car NK cells and radiotherapy. J Immunother Cancer. (2025) 13(7):e011330. doi: 10.1136/jitc-2024-011330
49. Moser LM, Heim C, KosChade SE, Wendel P, Bozkurt S, Harenkamp S, et al. CAR-CIK vs. CAR-T: benchmarking novel cytokine-induced killer cells as solid tumor immunotherapy in ErbB2+ Rhabdomyosarcoma. Front Immunol. (2025) 16:1485817. doi: 10.3389/fimmu.2025.1485817
50. Hegde M, Navai S, DeRenzo C, Joseph SK, Sanber K, Wu M, et al. Autologous HER2-specific CAR T cells after lymphodepletion for advanced sarcoma: A phase 1 trial. Nat Cancer. (2024) 5:880–94. doi: 10.1038/s43018-024-00749-6
51. Hegde M, Joseph SK, Pashankar F, DeRenzo C, Sanber K, Navai S, et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun. (2020) 11:3549. doi: 10.1038/s41467-020-17175-8
52. Gómez-Melero S, Hassouneh F, Vallejo-Bermúdez IM, Agüera-Morales E, Solana R, and Caballero-Villarraso J. Tandem car-T cell therapy: recent advances and current challenges. Front Immunol. (2025) 16:1546172. doi: 10.3389/fimmu.2025.1546172
53. Hamieh M, Mansilla-Soto J, Riviere I, and Sadelain M. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov. (2023) 13:829–43. doi: 10.1158/2159-8290.CD-23-0101
54. Cheng J, Liu M, and Zhang J. Intelligent tunable CAR-T cell therapy leads the new trend. Synth Syst Biotechnol. (2023) 8:606–9. doi: 10.1016/j.synbio.2023.09.001
55. Cheng D, Zhang Z, Mi Z, Tao W, Liu D, Fu J, et al. Deciphering the heterogeneity and immunosuppressive function of regulatory T cells in osteosarcoma using single-cell RNA transcriptome. Comput Biol Med. (2023) 165:107417. doi: 10.1016/j.compbiomed.2023.107417
Keywords: cancer, osteosarcoma, rhabdomyosarcoma, chimeric antigen receptor, targeted therapy
Citation: Stavrou M, Nicolaou T, Georgalli M and Constantinidou A (2025) Current landscape of CAR-therapy for osteosarcoma and rhabdomyosarcoma. Front. Immunol. 16:1696830. doi: 10.3389/fimmu.2025.1696830
Received: 30 September 2025; Accepted: 14 November 2025; Revised: 07 November 2025;
Published: 27 November 2025.
Edited by:
Sujith K Joseph, Baylor College of Medicine, United StatesReviewed by:
Michele Bernasconi, University Children’s Hospital Bern, SwitzerlandMichelle Choe, Fred Hutchinson Cancer Center, United States
Copyright © 2025 Stavrou, Nicolaou, Georgalli and Constantinidou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anastasia Constantinidou, Y29uc3RhbnRpbmlkb3UuYW5hc3Rhc2lhQHVjeS5hYy5jeQ==
 Maria Stavrou
Maria Stavrou Tatiana Nicolaou
Tatiana Nicolaou Maria Georgalli
Maria Georgalli Anastasia Constantinidou
Anastasia Constantinidou