- 1Department of Urology, Peking University Third Hospital, Beijing, China
- 2Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education
Background: Presurgical molecular therapy (PMT) including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) showed various outcomes for renal cell carcinoma (RCC) with tumor thrombus (TT). We aimed to evaluate the impact of PMT on Mayo level or TT height and the treatment-related adverse events (AEs).
Methods: A systematic literature search was conducted in PubMed, Embase, Cochrane Library, and Web of Science up to June 2023 to identify relevant studies investigating the impact of PMT on RCC patients with TT. The literature investigating the impact of PMT on RCC patients with venous TT, whether followed by surgery or not, was included.
Results: Overall, 184 patients were enrolled in this study. 30.7% (95% CI, 17.6–43.8%, I2 = 79%, p<0.01) patients experienced a decrease in TT levels after receiving PMT, while only 1.5% (95% CI, 0–0.044%, I2 = 0%, p=0.98) exhibited an increase in TT levels. An average decrease of 15.2mm (95% CI, 22.4–8.0, I2 = 77%, p<0.01) of TT in 117 patients was observed after PMT. The most common AEs was hypertension (49.9%, 95% CI, 27.1–77.7, I2 = 88%, p<0.01), diarrhea (20.2%, 95% CI, 2.7–37.6, I2 = 83%, p<0.01), fatigue (25.3%, 95% CI, 6.1–44.4, I2 = 84%, p<0.01) and hand-foot syndrome (25.5%, 95% CI, 5.6–45.5, I2 = 86%, p<0.01).
Conclusion: PMT is available to assist in lowering the TT level in RCC patients aiming to simply the surgical procedures, particularly in patients with Mayo grade 3/4. The frequency and severity of AEs during PMT are tolerable.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD420234399128.
Introduction
Renal cell carcinoma (RCC) constitutes approximately 3% of all cancers, ranking third among urinary system tumors (1, 2). RCC exhibits a distinct biologic propensity for vascular invasion, with 4-15% of cases developing renal vein or inferior vena cava (IVC) tumor thrombus (TT), and 30-50% of these patients experience distant metastasis (3–5). Radical nephrectomy and thrombectomy (RNAT) remains the standard treatment for RCC with TT, improving the prognosis to 40%-65% in 5-year cancer-specific survival (6). Nevertheless, the surgical procedures, particularly TT resection and IVC reconstruction, carry a high risk of surgical morbidity and mortality and its rates escalate with increasing TT level (7–10). Therefore, reducing TT level to simplify surgical procedures and mitigate perioperative risk represent a critical clinical need (11, 12). Presurgical molecular therapy (PMT) including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) has shown the potential to reduce TT level, making it possible to reduce surgical difficulty. Cost et al. conducted the initial retrospective study on PMT for RCC with TT and observed that sunitinib positively impacts TT regression (13). However, the clinical significance and relevance remain unclear. Subsequent investigations suffer from limitations including small sample sizes, single-center designs, and heterogeneous populations, leading to divergent conclusions, rendering this a contentious clinical issue (14–17). Presently, the inaugural prospective study, NAXIVA, demonstrates that 37.5% patients experienced a decrease in TT grade, and 75% exhibited a reduction in TT height following standardized axitinib therapy (18). Nevertheless, there is still a lack of large-scale clinical studies to verify the efficacy and safety of PMT. Therefore, given the mixed early evidence for PMT, a systematic synthesis of existing data is urgently needed to clarify the impact of PMT on TT downstaging, as well as its safety profile. This study conducted a systematic review and meta-analysis to comprehensively evaluate the effect of PMT on Mayo level and height of TT in RCC patients, with the aim to provide evidence to guide clinical decision-making for this high-risk cohort.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria. The review protocol for this study was registered on PROSPERO (CRD420234399128).
Search strategy
We conducted a systematic literature search in PubMed, Embase, Cochrane Library, and Web of Science up to June 2023 to identify relevant studies investigating the impact of PMT on RCC patients with TT. To ensure the transparency and rigor of the study design in line with the PRISMA guidelines, the systematic review and meta-analysis was structured by the PICO (Population, Intervention, Comparison, Outcome) framework. Population (P): Patients with pathologically confirmed RCC with TT. Intervention (I): PMT as the experimental intervention, including TKIs (e.g., sunitinib, sorafenib, axitinib, pazopanib), ICIs (e.g., nivolumab, ipilimumab, pembrolizumab, avelumab) or both. PMT was administered preoperatively, with regimens (drug type, dosage, and treatment cycle) clearly documented in the included studies. Comparison (C): Given the scarcity of standard treatment of PMT for RCC with TT, the present analysis focused on the intra-intervention effect of PMT, namely changes in TT indicators before and after PMT rather than a direct head-to-head comparison. This approach aligns with the core objective of evaluating whether PMT can alter TT status, which is consistent with the exploratory nature of current research in this field. Outcome (O): The primary outcomes include Mayo grade and TT height, which is based on their clinical relevance to surgical management and prognosis of RCC with TT. Mayo grade directly determines surgical complexity and perioperative risk. The key metrics for this outcome included the proportion of patients with TT grade downstaging and grade upstaging. TT height is a continuous outcome measured in millimeters (mm), representing the maximum longitudinal length of TT in the venous system (assessed via imaging before and after PMT). Decrease of TT height can simplify surgical dissection and reduce the need for complex IVC reconstruction, thereby lowering perioperative morbidity. The key metric for this outcome was the average change in TT height before and after PMT. Secondary Outcome: Treatment-related adverse events (AEs) of PMT to assess the safety of PMT.
Separate searches were performed using population ((renal cell carcinoma, renal cell cancer, renal tumor, kidney cancer, kidney carcinoma, renal neoplasm, kidney neoplasm) and (tumor thrombus, tumor thrombosis, tumor embolus, inferior vena cava thrombus, venous tumor thrombus, venous thrombus)), intervention (neoadjuvant therapy, presurgical therapy, target molecular therapy, tyrosine kinase inhibitor, immunotherapy, sunitinib, sorafenib or pazopanib, axitinib, cabozantinib, temsirolimus, lapatinib, pembrolizumab, nivolumab, Ipilimumab, bevacizumab). Furthermore, we examined the identified original papers, reviews, meta-analyses, and comments that were included in the references from the pertinent research.
Inclusion criteria and study eligibility
The present study enrolled patients diagnosed with RCC and venous TT who underwent PMT involving TKIs, ICIs, or both. The inclusion criteria encompassed literature investigating the impact of PMT on RCC patients with venous TT, whether followed by surgery or not. Exclusion criteria comprised the following: (1) fundamental research studies; (2) studies only concentrating on RCC without TT; (3) non-original articles (such as reviews, editorials, comments, letters, editorials, systematic reviews, and meta-analysis); (4) gray literature (e.g., thesis, abstracts only); (5) studies lacking data on TT changes after PMT. In order to maintain the homogeneity of the cases included in the study, we strictly screened the literature according to the following inclusion criteria: (1) studies must explicitly confirm RCC diagnosis via pathological examination; (2) venous TT must be verified by imaging modalities including computed tomography, magnetic resonance imaging, or contrast-enhanced ultrasound; (3) drug type, dosage, and treatment cycle of PMT must be clearly documented; (4) post-PMT changes in TT (Mayo grade or height) must be available for assessment. In cases where multiple studies examined the same variable at the same endpoint, the data were merged. The most illuminating study was chosen with the biggest sample size when different studies within the same patient cohort reported the same characteristic. Two authors (K.C. and L.Z.) independently reviewed titles and abstracts, resolving any disagreements through discussion with senior authors (Z.L).
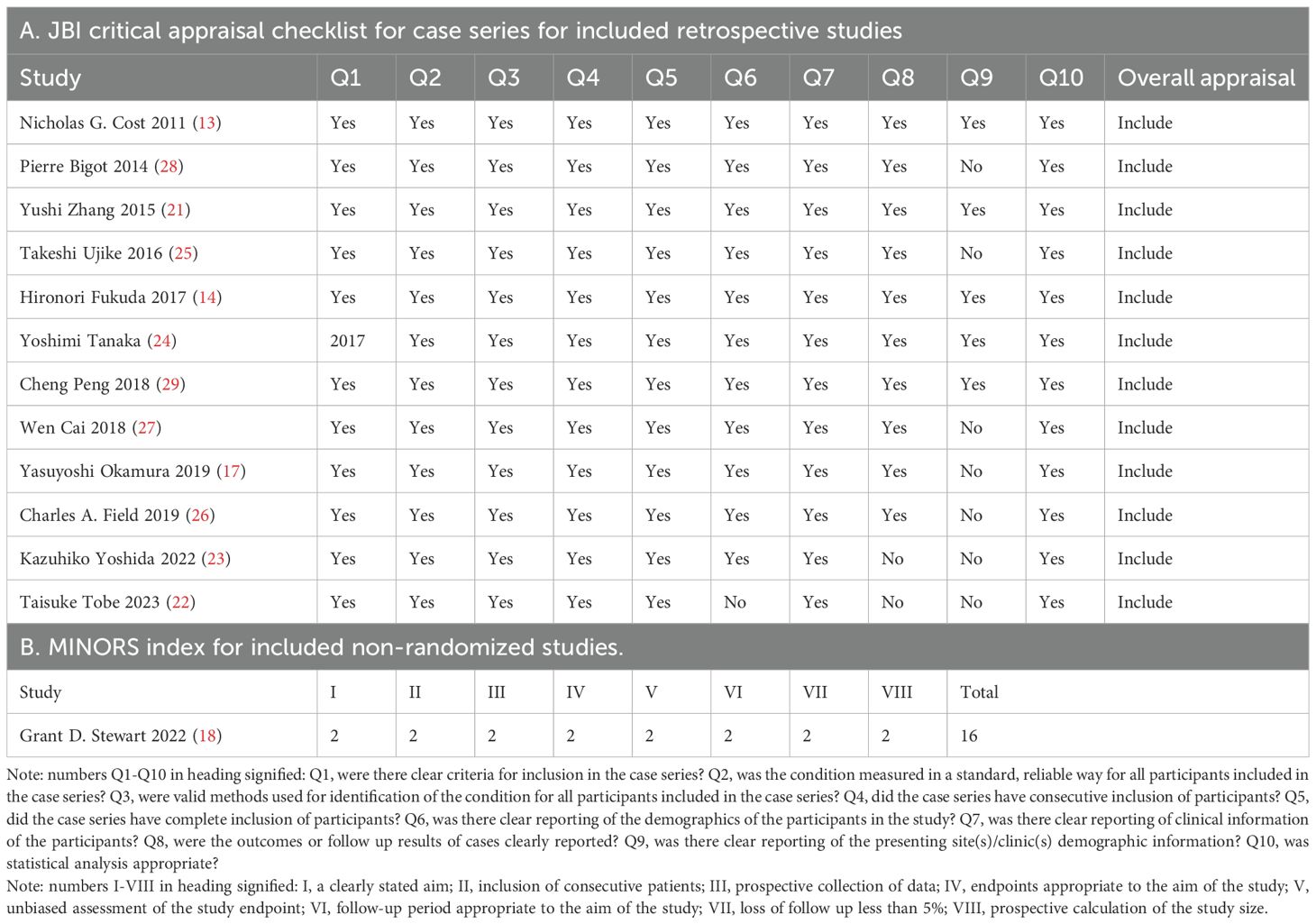
Quality assessment
The quality of the included prospective studies was assessed using the Methodological Evaluation Metrics for Non-Randomized Controlled Trials (MINORS) (19). Twelve assessment indications make up MINORS, and each one has a score range of 0 to 2. 0 indicates that there is no data reported. 1 indicates that there is data reported, but not enough details. 2 indicates that there is enough information in the data report. The retrospective studies without comparison group were assessed by JBI Critical Appraisal Checklist for Case Series (20).
Data extraction
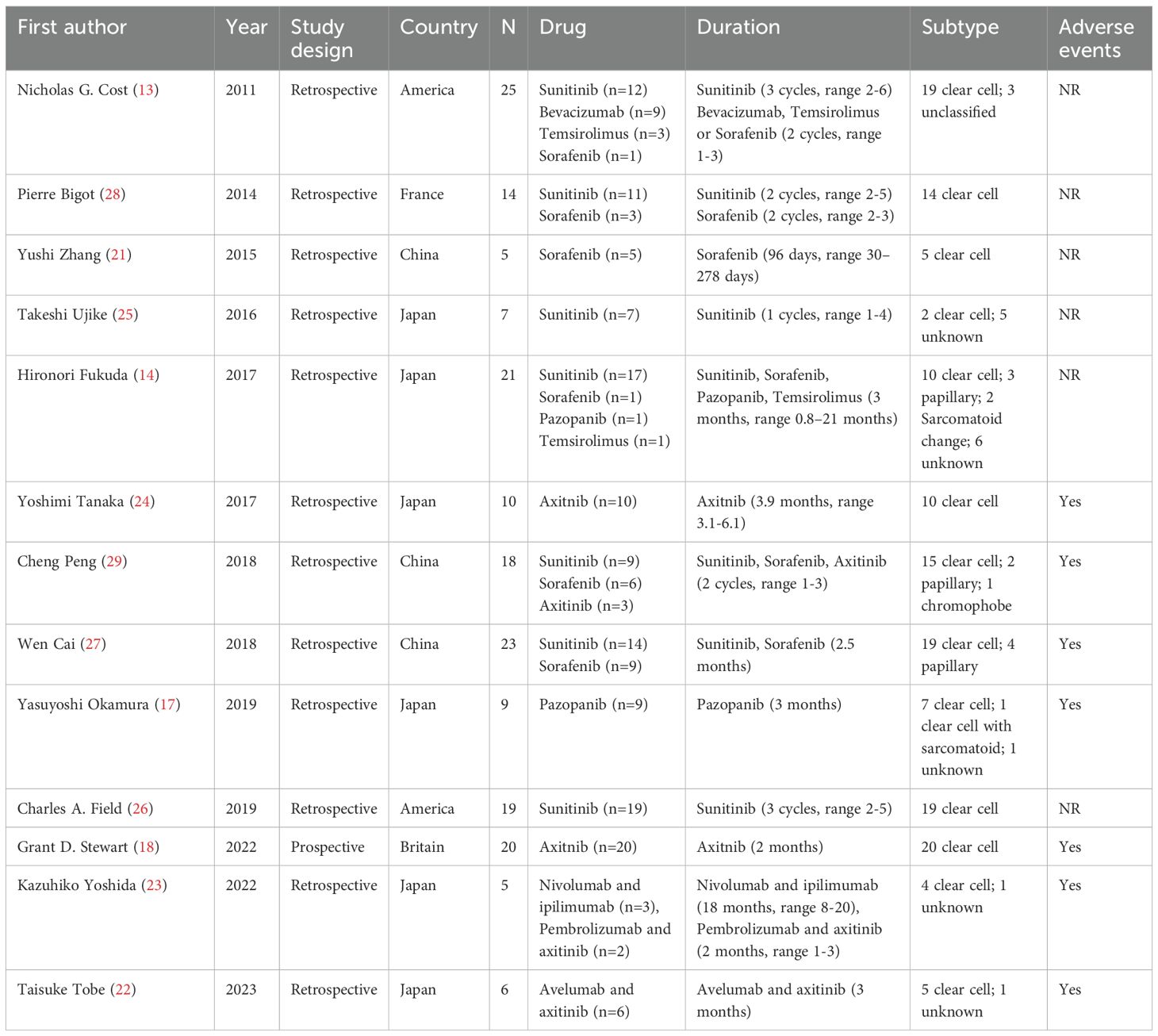
The selection of studies was carried out independently by two investigators (K.C. and L.Z.), and any discrepancies between the two would be discussed jointly by the third author (Z.L.). The following details were noted about the characteristics of the included studies: authors, year, study design, country, sample size, therapeutic drug, therapeutic period, number of patients, age, reported endpoints, and AEs. While original data were hardly accessed, the data was extracted from the histogram or line chart by software Engauge Digitizer version 10.8.
Statistical analysis
The statistical methodology employed in this study involved the random-effect model or the fixed-effect model after double arcsine conversion. The effect size for all combined results was expressed using 95% confidence intervals (CI) with upper and lower limits. To assess statistical heterogeneity, we utilized both the Cochrane Q statistic and the I2 statistic. Specifically, if the p-value from the Cochran Q test was less than 0.05 or the I2 statistic exceeded 50%, significant heterogeneity among the literature was present, and we employed a random-effect model. Otherwise, a fixed-effect model was used. Additionally, we conducted sensitivity analysis by systematically excluding each individual study to evaluate the stability of our findings. All statistical analyses were conducted by R software (version 3.2.2, Mac).
Results
Study selection and characteristics
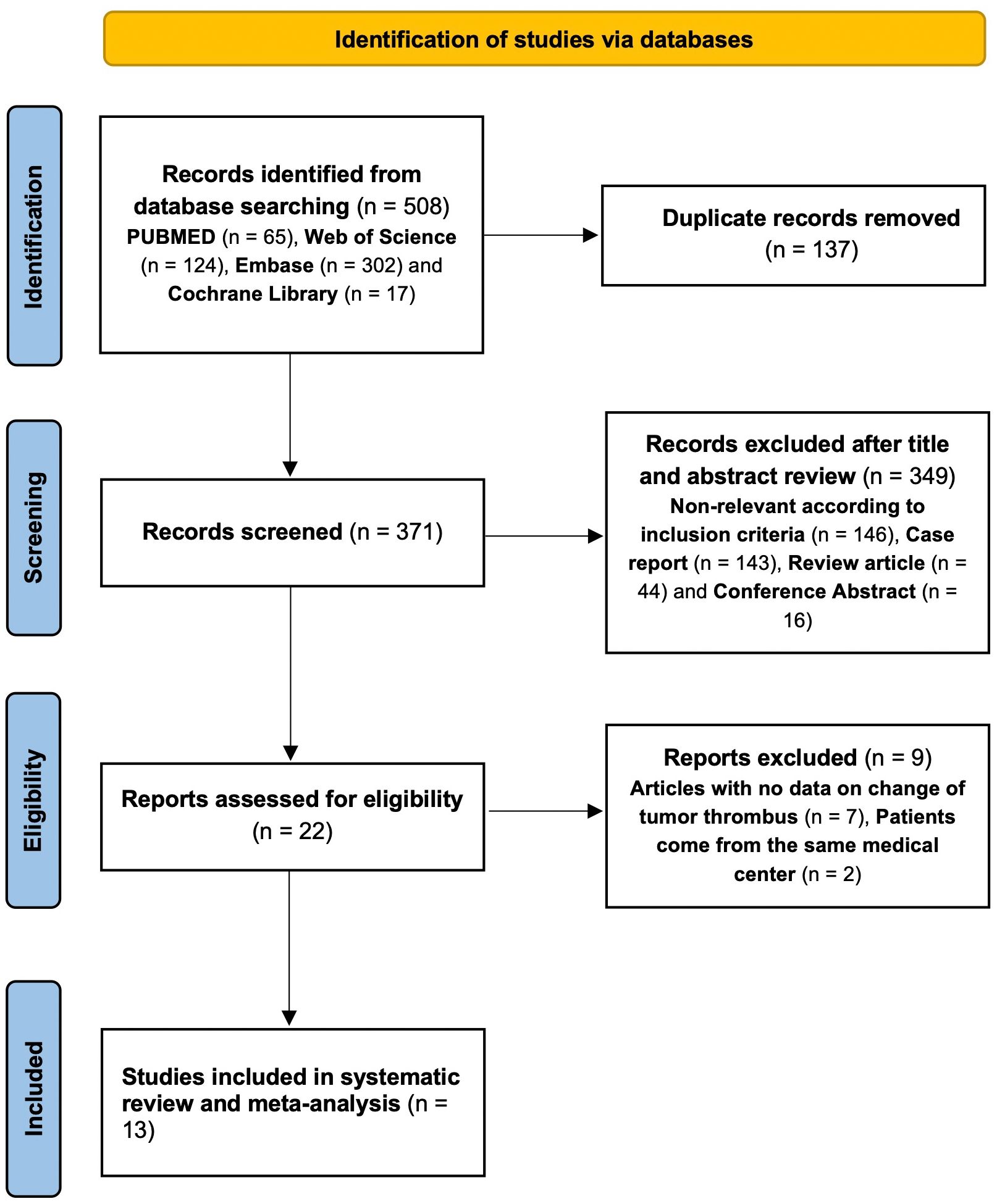
The initial search yielded 508 relevant references in PubMed (n=65), Web of science (n=124), Embase (n=302) and the Cochrane Library (n=17). After removing duplicate studies and conducting thorough screenings of titles, abstracts, and full-texts, 13 studies were ultimately included in our analysis. These comprised 1 prospective study (18) and 12 retrospective studies (13, 14, 17, 21–29), involving a total of 184 patients. Detailed study characteristics are presented in Table 1. Among the 13 studies, 11 focused on targeted therapy and 2 investigated a combination of targeted therapy and immunotherapy. The study selection process is shown in Figure 1.
Quality assessment
The quality assessment results of all included articles are acceptable for the present meta-analysis (Table 2).
Tumor thrombus response
Thirteen studies provided data of TT levels in RCC patients after receiving PMT. Among 184 patients, 30.7% (95% CI, 17.6–43.8%, I2 = 79%, p<0.01, Figure 2A) patients experienced a decrease in TT levels after receiving PMT, while only 1.5% (95% CI, 0–0.044%, I2 = 0%, p=0.98, Figure 2B) exhibited an increase in TT levels. Additionally, we specifically analyzed patients with Mayo 3/4 TT. Among these patients, 48.8% (95% CI, 27.7%–69.8%, I2 = 77%, p<0.01, Figure 2C) experienced a decrease in TT level, while only 1.5% (95% CI, 0–8.7%, I2 = 0%, p=0.99, Figure 2D) exhibited an increase. Further analysis of the changes in TT levels in the above studies showed that the average TT level decreased by 0.28 grade (95% CI, 0–0.044%, I2 = 0%, p=0.98) (Figure 2E). Sensitivity analysis of reduced tumor thrombus levels is shown in Figure 3 and other sensitivity analysis about TT level changes were shown in Supplementary Figure 1.
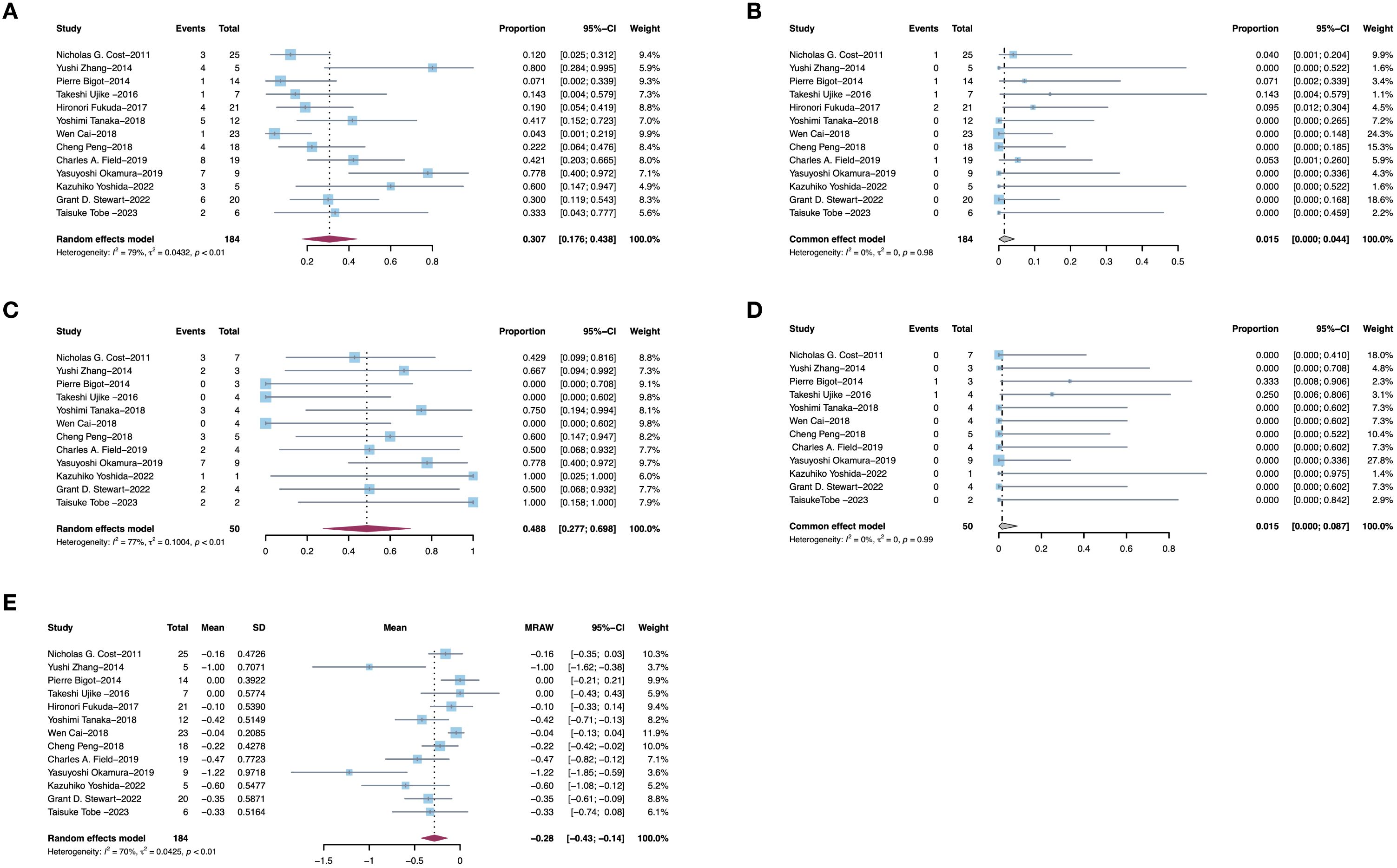
Figure 2. Pooled estimates of efficacies of presurgical molecular therapy on Mayo grade for renal cell carcinoma with tumor thrombus. (A) Downstage Mayo grade. (B) Upstage Mayo grade. (C) Downstage Mayo grade 3/4. (D) Upstage Mayo grade 3/4. (E) Average Mayo grade changes.
![Forest plots titled A and B display studies with corresponding proportions, confidence intervals, P-values, Tau2, Tau, and I2 values. Each plot includes a list of omitted studies with years, showing differing effects on the results. Both feature random effects models, indicated by diamonds at the bottom, showing pooled estimates with confidence intervals. Plot A indicates a proportion of 0.31 with a confidence interval of [0.18; 0.44] and plot B shows 0.49 [0.28; 0.70].](https://www.frontiersin.org/files/Articles/1705494/fimmu-16-1705494-HTML/image_m/fimmu-16-1705494-g003.jpg)
Figure 3. Sensitivity analysis of reduced tumor thrombus levels. (A) Downstage Mayo grade of all patients. (B) Downstage Mayo grade of patients with Mayo grade 3/4.
12 studies simultaneously assessed changes in TT height following PMT. Among these patients, 73.2% (95% CI, 57.6%–88.9%, I2 = 86%, p<0.01, Figure 4A) experienced a decrease in TT height and 10.3% (95% CI, 3.2%–17.4%, I2 = 86%, p<0.01, Figure 4B) experienced an increase in TT height. Among the above studies, 10 reported absolute height changes of TT, with an average decrease of 15.2mm (95% CI, 22.4–8.0, I2 = 77%, p<0.01, Figure 4C) in 117 patients after PMT. Sensitivity analysis about TT height changes were shown in Supplementary Figure 2.
![Forest plots labeled A, B, and C display data from various studies. Plots A and B present the number of events and total participants for each study, along with the corresponding proportion, confidence interval (CI), and weight. Plot C presents the mean, standard deviation, raw mean (MRAW), confidence interval (CI), and weight.Plot A used the Random Effects model to assess the proportion of Decrease in thrombus height.. The overall pooled proportion is 0.732, 95% CI is [0.576,0.889],.Plot B used the Random Effects model to assess the proportion of Increase in thrombus height. The overall pooled proportion is 0.103, 95% CI is [0.032, 0.174]. Plot C used the Random Effects model to assess Average thrombus height changes. The overall MRAW is -15.18, 95% CI is [-22.39, -7.96].](https://www.frontiersin.org/files/Articles/1705494/fimmu-16-1705494-HTML/image_m/fimmu-16-1705494-g004.jpg)
Figure 4. Pooled estimates of efficacies of presurgical molecular therapy on thrombus height for renal cell carcinoma with tumor thrombus. (A) Decrease in thrombus height. (B) Increase in thrombus height. (C) Average thrombus height changes.
Adverse events
AEs reported in the included literature include hypertension, hypothyroidism, fatigue, hand-foot syndrome, diarrhea, nausea, leucopenia, anemia, thrombocytopenia, liver function abnormalities, mucositis and anorexia. The most common AEs was hypertension (49.9%, 95% CI, 27.1–77.7, I2 = 88%, p<0.01, Figure 5A), diarrhea (20.2%, 95% CI, 2.7–37.6, I2 = 83%, p<0.01, Figure 5B), fatigue (25.3%, 95% CI, 6.1–44.4, I2 = 84%, p<0.01, Figure 5C) and hand-foot syndrome (25.5%, 95% CI, 5.6–45.5, I2 = 86%, p<0.01, Figure 5D). Sensitivity analysis about AEs were shown in Supplementary Figurel 3.
![Four forest plots labeled A, B, C, and D depict study results with confidence intervals and weights. Four forest plots labeled A toD display meta-analysis of study data. All plots present the number of events and total participants for each study, along with the corresponding proportion, confidence interval (CI), and weight. Plot A used the Random Effects model to assess the proportion of hypertension.. The overall pooled proportion is 0.494, 95% CI is [0.271,0.717]. Plot B used the Random Effects model to assess the proportion of diarrhea. The overall pooled proportion is 0.202, 95% CI is [0.027, 0.376]. Plot B used the Random Effects model to assess the proportion of fatigue. The overall pooled proportion is 0.253, 95% CI is [0.061, 0.444]. Plot B used the Random Effects model to assess the proportion of hand-foot syndrome. The overall pooled proportion is 0.255, 95% CI is [0.056, 0.455].](https://www.frontiersin.org/files/Articles/1705494/fimmu-16-1705494-HTML/image_m/fimmu-16-1705494-g005.jpg)
Figure 5. Pooled estimates of adverse effects of presurgical molecular therapy on for renal cell carcinoma with tumor thrombus. (A) hypertension. (B) diarrhea. (C) fatigue. (D) hand-foot syndrome.
Discussion
A well-documented characteristic of RCC is its propensity for vascular invasion, which manifests as TT in the renal vein or IVC in 4-15% of cases (2, 6). In cases of non-metastatic RCC with vascular invasion, RNAT represents the standard treatment, which significantly raises the 5-year cancer-specific survival rate (30, 31). However, the considerable technical complexity of RNAT, particularly for high-level TT, especially Mayo 3/4 TT, carries a substantial risk of perioperative morbidity and mortality (32–34). The complication rate demonstrated considerable variation depending on the TT level, with reported ranges of 12.4% to 46.9% (9, 34). Especially, the morbidity rate escalates with the level of TT, reaching 10-40% in Mayo 4 patients (35). Consequently, PMT to reduce TT level and facilitate less complex surgery has emerged as a potential strategy (36–38). The first prospective study, NAXIVA, evaluated the efficacy of neoadjuvant therapy in RCC with TT, which demonstrated that PMT effectively reduced TT levels, thereby facilitating surgical complexity (18, 39). Furthermore, several retrospective studies have explored the feasibility of PMT in RCC with TT, however, their conclusions exhibit substantial heterogeneity, primarily attributable to the study design (23, 26, 29, 39). Consequently, the synthesis of current study data to confirm the function of PMT in RCC with TT has vital clinical value, which provides preliminary evidence for subsequent clinical studies.
The efficacy of PMT in RCC with TT is subject to be controversial, as evidenced by heterogeneous study outcomes (15, 16, 40). Overall, retrospective investigations have produced inconsistent findings, with TT level reductions reported in 7.1% to 41.7% of patients (26, 28, 38). Cost et al. documented a 44% decline in TT height and a 48% reduction in primary lesions. However, the median TT height decrease was below 1 cm, and only 12% of patients achieved the outcome of lowering TT level (13). Bigot et al. further illustrated this variability, with merely 1 of 14 patients showing a TT reduction from Mayo 2 to 1, while one patient experienced TT escalation from Mayo 3 to 4, impeding surgical resection (28). Conversely, more optimistic data emerge from other studies. Karakiewicz et al. initially reported sunitinib induced TT reduction that simplified surgery and more case reports demonstrated consistent results (39). In addition, more case series studies have shown that PMT can effectively reduce TT levels and simplify surgical procedures. Peng et al. reinforced these positive trends, observing significant TT size decreases in 61.1% of patients, including 60% of those with Mayo 3/4 TT (29). Supporting this, the prospective study, NAXIVA, demonstrated marked TT height reductions with preoperative axitinib, resulting in altered surgical approaches for 41.1% of cases (18). As ICIs are gradually applied to RCC, some clinical studies have reported more positive results. Studies based on ICIs have demonstrated a decrease in TT level in 33.3% to 60% of patients, suggesting that future PMT strategies incorporating ICIs may represent a more promising therapeutic approach (23).
Through a systematic review, we executed a comprehensive meta-analysis aiming to derive new insights into molecular therapeutics for RCC with TT. This analysis included 13 single-arm studies comprising 184 patients. Of the 184 participants, 30.7% demonstrated a decrease in TT Mayo level after PMT, with a mean reduction of 0.28 in TT grade. These findings provide preliminary evidence for the feasibility of PMT in reducing TT levels and facilitating surgical procedures. Owing to substantial heterogeneity across studies, sensitivity analysis was applied to test the robustness of conclusions. In sensitivity analysis, after successive exclusion, the fluctuation range of the combined effect of TT reduction ratio is 26%-33%. It is important to note that the application of PMT has been constrained by the highly invasive nature of TT and TKIs or ICIs might not effectively restrain tumor progression (41–43). Nevertheless, our results showed that merely 1.5% of patients had elevated TT levels during therapy. Therefore, the principal apprehension that TT progression under PMT could exacerbate surgical challenges and worsen outcomes is mitigated. Subgroup analysis concentrated on RCC patients with Mayo 3/4 TT, who face elevated perioperative risks (30, 44–46). In this subgroup, 48.8% exhibited a decrease in TT level, compared to only 1.5% with an increase, indicating that PMT is particularly applicable to cases with high Mayo level (32, 47–49). Furthermore, as surgical complexity correlates not only with Mayo level but also TT height, we analyzed this outcome separately. Overall, 73.2% of analyzed patients had a reduction in TT height, versus 10.3% with an increase. Additional analyses revealed an average TT height decrease of 15.2 mm after PMT. Therefore, these data preliminarily demonstrate the feasibility of PMT in reducing TT levels.
The management of AEs is also very important during PMT (50–52), so this outcome is also analyzed. The results showed that the common AEs were hypertension, diarrhea, fatigue, and hand-foot syndrome. Although the incidence of hypertension is high, most patients experience relief after drug treatment. The incidence of AEs including diarrhea, fatigue, hand-foot syndrome and other AEs is relatively low, and very few patients stop treatment due to serious AEs. This helps to improve the application of PMT in RCC patients with TT (53, 54).
In addition, with the exploration of ICIs, therapy based on ICIs for preoperative intervention seems feasible, however, although some studies have reported the effect of combination therapy, the current level of evidence is insufficient due to its small sample size (23, 50). In this study, the response rate of PMT based on ICIs was higher than that based on TKIs. Despite the potential for a greater reduction rate among ICIs treated patients relative to targeted therapy (44.9% vs 29.1%), the small case numbers restrict the generalizability of this result. However, it suggests that ICIs could potentially benefit more patients in this field. Consequently, more extensive clinical research is needed to verify the effectiveness and safety of ICIs.
This study provides preliminary evidence for PMT in RCC patients with TT to reduce the difficulty of surgery, but there are some limitations. First of all, most of the literatures included in this study were retrospective studies with small individual sample sizes, and the total sample size of the meta-analysis (184 patients) remains relatively small. This is mainly attributed to the low incidence of venous TT in RC, resulting in a limited number of eligible cases in clinical practice. Secondly, the present analysis combined data from patients receiving TKIs, ICIs, or combinations, considering that the mechanisms of TKIs and ICIs are not the same, which may obscure potential differences in efficacy and safety between different PMT regimens. At the same time, the effects of different drugs are not compared, because there is no consensus on the current PMT for such patients. Finally, due to the absence of follow-up results from most studies, it is unclear whether PMT improves outcomes. Future research should prioritize multicenter, prospective studies with larger sample sizes to verify the efficacy and safety of PMT in RCC patients with venous TT. As more clinical data accumulate (especially data from phase II/III clinical trials), updated meta-analyses with expanded sample sizes can be conducted to further validate or refine the conclusions of this preliminary study.
Conclusion
PMT is available to assist in lowering the TT level in RCC patients through exploring the literature and analyzing data from the preceding 20 years, particularly in patients with Mayo grade 3/4. This conclusion is a preliminary extrapolation given the presence of selection bias and other confounding factors in the included literature, which gives the base to design a prospective study. Further conclusions would be facilitated by including more clinical studies with large scale and conducting an exploration of drug categories.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
KC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. ZL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. LG: Conceptualization, Formal Analysis, Investigation, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. LY: Conceptualization, Data curation, Formal Analysis, Investigation, Resources, Software, Visualization, Writing – review & editing. SZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research received Natural Science Foundation of China (No. 81972381, No. 82273389 and No. 82173385).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1705494/full#supplementary-material
Supplementary FIGURE 1 | Sensitivity analysis of reduced tumor thrombus levels. (A) Upstage Mayo grade. (B) Upstage Mayo grade 3/4. (C) Average Mayo grade changes.
Supplementary FIGURE 2 | Sensitivity analysis of changes of tumor thrombus. (A) Decrease in thrombus height. (B) Increase in thrombus height. (C) Average thrombus height changes.
Supplementary FIGURE 3 | Sensitivity analysis of adverse effects. (A) hypertension. (B) diarrhea. (C) fatigue. (D) hand-foot syndrome.
References
1. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. (2018) 103:356–87. doi: 10.1016/j.ejca.2018.07.005
2. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. (2022) 82:399–410. doi: 10.1016/j.eururo.2022.03.006
3. Blute ML, Leibovich BC, Lohse CM, Cheville JC, and Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. (2004) 94:33–41. doi: 10.1111/j.1464-410X.2004.04897.x
4. Abel EJ, Wood CG, Eickstaedt N, Fang JE, Kenney P, Bagrodia A, et al. Preoperative pulmonary embolism does not predict poor postoperative outcomes in patients with renal cell carcinoma and venous thrombus. J Urol. (2013) 190:452–7. doi: 10.1016/j.juro.2013.02.033
5. Wagner B, Patard JJ, Méjean A, Bensalah K, Verhoest G, Zigeuner R, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol. (2009) 55:452–9. doi: 10.1016/j.eururo.2008.07.053
6. Al Otaibi M, Abou Youssif T, Alkhaldi A, Sircar K, Kassouf W, Aprikian A, et al. Renal cell carcinoma with inferior vena caval extention: impact of tumour extent on surgical outcome. BJU Int. (2009) 104:1467–70. doi: 10.1111/j.1464-410X.2009.08575.x
7. Karnes RJ and Blute ML. Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol. (2008) 5:329–39. doi: 10.1038/ncpuro1122
8. Chen K, Liu Z, Li Y, Zhao X, Zhang Y, Bi H, et al. Long-term outcomes after cytoreductive nephrectomy and thrombectomy of patients with metastatic renal cell carcinoma with venous tumor thrombus: a retrospective study from a large Chinese center. World J Surg Oncol. (2023) 21:170. doi: 10.1186/s12957-023-03048-z
9. Chen K, Liu Z, Li Y, Zhao X, Wang G, Tian X, et al. Prevention, incidence, and risk factors of chyle leak after radical nephrectomy and thrombectomy. Cancer Med. (2024) 13:e6858. doi: 10.1002/cam4.6858
10. Miyake H, Sugiyama T, Aki R, Matsushita Y, Tamura K, Motoyama D, et al. Oncological outcomes after cytoreductive nephrectomy for patients with metastatic renal cell carcinoma with inferior vena caval tumor thrombus. Int J Clin Oncol. (2018) 23:553–8. doi: 10.1007/s10147-017-1232-9
11. Ghoreifi A, Vaishampayan U, Yin M, Psutka SP, and Djaladat H. Immune checkpoint inhibitor therapy before nephrectomy for locally advanced and metastatic renal cell carcinoma: A review. JAMA Oncol. (2024) 10:240–8. doi: 10.1001/jamaoncol.2023.5269
12. Bindayi A, Hamilton ZA, McDonald ML, Yim K, Millard F, McKay RR, et al. Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol. (2018) 36:31–7. doi: 10.1016/j.urolonc.2017.07.015
13. Cost NG, Delacroix SE Jr., Sleeper JP, Smith PJ, Youssef RF, Chapin BF, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. (2011) 59:912–8. doi: 10.1016/j.eururo.2011.02.032
14. Fukuda H, Kondo T, Takagi T, Iizuka J, Nagashima Y, and Tanabe K. Limited benefit of targeted molecular therapy for inferior vena cava thrombus associated with renal cell carcinoma. Int J Clin Oncol. (2017) 22:767–73. doi: 10.1007/s10147-017-1119-9
15. Harshman LC, Srinivas S, Kamaya A, and Chung BI. Laparoscopic radical nephrectomy after shrinkage of a caval tumor thrombus with sunitinib. Nat Rev Urol. (2009) 6:338–43. doi: 10.1038/nrurol.2009.84
16. Kroeger N, Gajda M, Zanow J, Petersen I, Settmacher U, Wunderlich H, et al. Downsizing a tumor thrombus of advanced renal cell carcinoma with neoadjuvant systemic therapy and resulting histopathological effects. Urol Int. (2010) 84:479–84. doi: 10.1159/000296301
17. Okamura Y, Terakawa T, Sakamoto M, Bando Y, Suzuki K, Hara T, et al. Presurgical pazopanib improves surgical outcomes for renal cell carcinoma with high-level IVC tumor thrombosis. In Vivo. (2019) 33:2013–9. doi: 10.21873/invivo.11698
18. Stewart GD, Welsh SJ, Ursprung S, Gallagher FA, Jones JO, Shields J, et al. A Phase II study of neoadjuvant axitinib for reducing the extent of venous tumour thrombus in clear cell renal cell cancer with venous invasion (NAXIVA). Br J Cancer. (2022) 127:1051–60. doi: 10.1038/s41416-022-01883-7
19. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, and Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
20. Hou K, Yu Z, Jia Y, Fang H, Shao S, Huang L, et al. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: A single-arm meta-analysis. Crit Rev Oncol Hematol. (2020) 152:103010. doi: 10.1016/j.critrevonc.2020.103010
21. Zhang Y, Li Y, Deng J, Ji Z, Yu H, and Li H. Sorafenib neoadjuvant therapy in the treatment of high risk renal cell carcinoma. PloS One. (2015) 10:e0115896. doi: 10.1371/journal.pone.0115896
22. Tobe T, Terakawa T, Hara T, Ueki H, Shiraishi Y, Wakita N, et al. The efficacy of presurgical therapy with avelumab and axitinib for renal cell carcinoma with inferior vena cava tumor thrombus. Clin Genitourin Cancer. (2023) 21:613.e1–.e6. doi: 10.1016/j.clgc.2023.04.010
23. Yoshida K, Hata K, Iizuka J, Kondo T, Ishihara H, Ishida H, et al. Immune checkpoint inhibitor combination therapy for renal cell carcinomas with concomitant inferior vena cava thrombi. In Vivo. (2022) 36:1030–4. doi: 10.21873/invivo.12798
24. Tanaka Y, Hatakeyama S, Hosogoe S, Tanaka T, Hamano I, Kusaka A, et al. Presurgical axitinib therapy increases fibrotic reactions within tumor thrombus in renal cell carcinoma with thrombus extending to the inferior vena cava. Int J Clin Oncol. (2018) 23:134–41. doi: 10.1007/s10147-017-1169-z
25. Ujike T, Uemura M, Kawashima A, Nagahara A, Fujita K, Miyagawa Y, et al. Clinical and histopathological effects of presurgical treatment with sunitinib for renal cell carcinoma with inferior vena cava tumor thrombus at a single institution. Anticancer Drugs. (2016) 27:1038–43. doi: 10.1097/cad.0000000000000422
26. Field CA, Cotta BH, Jimenez J, Lane BR, Yim K, Lee HJ, et al. Neoadjuvant sunitinib decreases inferior vena caval thrombus size and is associated with improved oncologic outcomes: A multicenter comparative analysis. Clin Genitourin Cancer. (2019) 17:e505–e12. doi: 10.1016/j.clgc.2019.01.013
27. Cai W, Huang J, Yuan Y, Hu X, Li M, Kong W, et al. Sunitinib or sorafenib as neoadjuvant therapy may not improve the survival outcomes of renal cell carcinoma with tumor thrombus. Urol Int. (2018) 101:391–9. doi: 10.1159/000492723
28. Bigot P, Fardoun T, Bernhard JC, Xylinas E, Berger J, Rouprêt M, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol. (2014) 32:109–14. doi: 10.1007/s00345-013-1088-1
29. Peng C, Gu L, Wang L, Huang Q, Wang B, Guo G, et al. Role of presurgical targeted molecular therapy in renal cell carcinoma with an inferior vena cava tumor thrombus. Onco Targets Ther. (2018) 11:1997–2005. doi: 10.2147/ott.S158114
30. Beksac AT, Shah QN, Paulucci DJ, Lo JZ, Okhawere KE, Elbakry AA, et al. Trends and outcomes in contemporary management renal cell carcinoma and vena cava thrombus. Urol Oncol. (2019) 37:576.e17–.e23. doi: 10.1016/j.urolonc.2019.05.010
31. Almatari AL, Sathe A, Wideman L, Dewan CA, Vaughan JP, Bennie IC, et al. Renal cell carcinoma with tumor thrombus: A review of relevant anatomy and surgical techniques for the general urologist. Urol Oncol. (2023) 41:153–65. doi: 10.1016/j.urolonc.2022.11.021
32. Wang B, Li H, Ma X, Zhang X, Gu L, Li X, et al. Robot-assisted laparoscopic inferior vena cava thrombectomy: different sides require different techniques. Eur Urol. (2016) 69:1112–9. doi: 10.1016/j.eururo.2015.12.001
33. Dason S, Mohebali J, Blute ML, and Salari K. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Urol Clin North Am. (2023) 50:261–84. doi: 10.1016/j.ucl.2023.01.007
34. Gold S, Taylor J, and Margulis V. Renal cell carcinoma with inferior vena cava thrombus: did we make progress in oncologic outcomes and complications? Curr Opin Urol. (2023) 33:142–6. doi: 10.1097/mou.0000000000001069
35. Parekh DJ, Cookson MS, Chapman W, Harrell F Jr., Wells N, Chang SS, et al. Renal cell carcinoma with renal vein and inferior vena caval involvement: clinicopathological features, surgical techniques and outcomes. J Urol. (2005) 173:1897–902. doi: 10.1097/01.ju.0000158459.42658.95
36. Robert G, Gabbay G, Bram R, Wallerand H, Deminière C, Cornelis F, et al. Case study of the month. Complete histologic remission after sunitinib neoadjuvant therapy in T3b renal cell carcinoma. Eur Urol. (2009) 55:1477–80. doi: 10.1016/j.eururo.2008.12.036
37. Shuch B, Riggs SB, LaRochelle JC, Kabbinavar FF, Avakian R, Pantuck AJ, et al. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int. (2008) 102:692–6. doi: 10.1111/j.1464-410X.2008.07660.x
38. Di Silverio F, Sciarra A, Parente U, Andrea A, Von Heland M, Panebianco V, et al. Neoadjuvant therapy with sorafenib in advanced renal cell carcinoma with vena cava extension submitted to radical nephrectomy. Urol Int. (2008) 80:451–3. doi: 10.1159/000132708
39. Karakiewicz PI, Suardi N, Jeldres C, Audet P, Ghosn P, Patard JJ, et al. Neoadjuvant sutent induction therapy may effectively down-stage renal cell carcinoma atrial thrombi. Eur Urol. (2008) 53:845–8. doi: 10.1016/j.eururo.2007.11.006
40. Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. (2022) 128:2085–97. doi: 10.1002/cncr.34180
41. Gu L, Li H, Wang Z, Wang B, Huang Q, Lyu X, et al. A systematic review and meta-analysis of clinicopathologic factors linked to oncologic outcomes for renal cell carcinoma with tumor thrombus treated by radical nephrectomy with thrombectomy. Cancer Treat Rev. (2018) 69:112–20. doi: 10.1016/j.ctrv.2018.06.014
42. Agochukwu N and Shuch B. Clinical management of renal cell carcinoma with venous tumor thrombus. World J Urol. (2014) 32:581–9. doi: 10.1007/s00345-014-1276-7
43. Sandberg M, Bissette R, Waggener K, Ciancio G, and Rodriguez AR. Reviewing the current state of renal cell carcinoma with a tumor thrombus: epidemiology, pathophysiology, metastasis, and systemic therapy. Curr Urol Rep. (2025) 26:71. doi: 10.1007/s11934-025-01301-4
44. Keranmu A, Wang M, Li Y, Yang F, Wahafu W, Chen D, et al. Feasibility of single position laparoscopic radical nephrectomy and tumor thrombectomy for left renal cell carcinoma with high-risk Mayo grade 0 and 1 tumor thrombus. BMC Urol. (2021) 21:181. doi: 10.1186/s12894-021-00924-2
45. Tian X, Hong P, Liu Z, Huang Y, Wang G, Hou X, et al. En bloc retroperitoneal laparoscopic radical nephrectomy with inferior vena cava thrombectomy for renal cell carcinoma with level 0 to II venous tumor thrombus: A single-center experience. Cancer. (2020) 126 Suppl 9:2073–8. doi: 10.1002/cncr.32747
46. Chopra S, Simone G, Metcalfe C, de Castro Abreu AL, Nabhani J, Ferriero M, et al. Robot-assisted level II-III inferior vena cava tumor thrombectomy: step-by-step technique and 1-year outcomes. Eur Urol. (2017) 72:267–74. doi: 10.1016/j.eururo.2016.08.066
47. Faria-Costa G, Freitas R, Braga I, Alzamora MA, Magalhães S, Carvalho J, et al. Renal cell carcinoma with venous tumor thrombus: 15 years of experience in an oncology center. J Clin Med. (2024) 13. doi: 10.3390/jcm13206260
48. Vinzant NJ, Christensen JM, Smith MM, Leibovich BC, and Mauermann WJ. Perioperative outcomes for radical nephrectomy and level III-IV inferior vena cava tumor thrombectomy in patients with renal cell carcinoma. J Cardiothorac Vasc Anesth. (2022) 36:3093–100. doi: 10.1053/j.jvca.2022.04.023
49. Nooromid MJ, Ju MH, Havelka GE, Kozlowski JM, Kundu SD, and Eskandari MK. Fifteen-year experience with renal cell carcinoma with associated venous tumor thrombus. Surgery. (2016) 160:915–23. doi: 10.1016/j.surg.2016.06.029
50. Karam JA, Msaouel P, Haymaker CL, Matin SF, Campbell MT, Zurita AJ, et al. Phase II trial of neoadjuvant sitravatinib plus nivolumab in patients undergoing nephrectomy for locally advanced clear cell renal cell carcinoma. Nat Commun. (2023) 14:2684. doi: 10.1038/s41467-023-38342-7
51. Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, and Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: A 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. (2021) 79:635–54. doi: 10.1016/j.eururo.2020.07.003
52. Qin Q, Tachibana I, Margulis V, Cadeddu JA, and Zhang T. A review of neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Cancers (Basel). (2025) 17. doi: 10.3390/cancers17020312
53. Blackmur JP, van der Mijn JCK, Warren AY, Browning L, Burgers F, Hirsch MS, et al. Assessing pathological response to neoadjuvant therapy in renal cell carcinoma: a systematic review and guidelines for sampling and reporting standards from the International Neoadjuvant Kidney Cancer Consortium. Lancet Oncol. (2025) 26:e536–e46. doi: 10.1016/s1470-2045(25)00345-6
Keywords: renal cell carcinoma, tumor thrombus, presurgical molecular therapy, radical nephrectomy and thrombectomy, tyrosine kinase inhibitors
Citation: Chen K, Zhuo L, Liu Z, Ge L, Yu L and Zhang S (2025) Presurgical molecular therapy for renal cell carcinoma with venous tumor thrombus: a systematic review and meta-analysis. Front. Immunol. 16:1705494. doi: 10.3389/fimmu.2025.1705494
Received: 15 September 2025; Accepted: 07 November 2025; Revised: 05 November 2025;
Published: 28 November 2025.
Edited by:
Giandomenico Roviello, University of Firenze, ItalyReviewed by:
Francesco Pierantoni, Veneto Institute of Oncology (IRCCS), ItalyFederica Cosso, University of Florence, Italy
Copyright © 2025 Chen, Zhuo, Liu, Ge, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shudong Zhang, emhhbmdzaHVkb25nQGJqbXUuZWR1LmNu
†Present address: Lin Zhuo, Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, China
‡These authors have contributed equally to this work
 Kewei Chen
Kewei Chen Lin Zhuo
Lin Zhuo Zhuo Liu1‡
Zhuo Liu1‡ Shudong Zhang
Shudong Zhang