- 1Department of Respiratory and Critical Care Medicine, Funan County People’s Hospital, Fuyang, Anhui, China
- 2Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 3Institute of Respiratory Diseases, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 4Thoracic Surgery Department, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
Osteopontin, a phosphorylated glycoprotein, is highly expressed in lung tissues and is elevated in inflammatory diseases. However, its role in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) remains unclear. A total of 281 AECOPD patients, 89 stable COPD (SCOPD) cases, and 89 healthy volunteers were enrolled in this prospective cohort according to the inclusion and exclusion criteria. Demographic information and clinical features were obtained from electronic medical records systems. Fasting venous blood was collected on the day of admission, and baseline serum osteopontin was measured using an enzyme-linked immunosorbent assay. The primary endpoints—death, frequency of acute exacerbations, and hospital length of stay—were evaluated through a follow-up study. Baseline serum osteopontin levels were higher in AECOPD patients compared to SCOPD patients and healthy volunteers. Linear regression analysis revealed positive associations between serum osteopontin and severity scores in AECOPD patients, and an inverse correlation of serum osteopontin and pulmonary function in SCOPD patients. In addition, baseline serum osteopontin levels were elevated in AECOPD patients with poorer prognosis. Logistic regression analysis indicated that serum osteopontin was positively correlated with the risks of death and acute exacerbations in the first year. Receiver operating characteristic (ROC) curve analysis suggested that the predictive ability of serum osteopontin for poor prognosis was comparable to that of the COPD Assessment Test (CAT) score and superior to the modified Medical Research Council (mMRC) score among AECOPD patients. Our findings indicate that baseline serum osteopontin is positively associated with severity scores and adverse clinical outcomes, highlighting its potential value as a surrogate prognostic biomarker in AECOPD patients.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic lung disease characterized by partially reversible airflow limitation, persistent inflammation, and abnormal respiratory symptoms (1, 2). The global prevalence of COPD is estimated at approximately 12.16% (3), with a slightly higher incidence in men than in women (4). At present, COPD ranks as the fourth most common chronic disease (5). Its high prevalence, associated disability, and mortality impose a substantial economic burden on both individuals and society (6). The pathological mechanism of COPD is complex; inhalation of harmful particles, gases, outdoor, and both outdoor and indoor air pollution can contribute to COPD through various mechanisms (7–9). Therefore, early disease detection and prognosis prediction are essential for identifying individuals at higher risk of progressing to an acute exacerbation of COPD (AECOPD).
Increasing studies have investigated potential biomarkers for estimating the prognosis in COPD patients. Currently, attention has been paid to osteopontin, which is a phosphorylated glycoprotein and highly expressed in the bone and many tissues (10). It is known that osteopontin is implicated in a number of physiopathological processes, including inflammation, bone homeostasis, cell survival and adhesion, and immune response (11, 12). Osteopontin plays important roles in many diseases, including rheumatoid arthritis, osteoarthritis, fibrosis, obesity, diabetes, neuroinflammation, and cancers (13–16). Further studies have also shown that osteopontin is expressed in various cell types, such as osteoblasts, fibroblasts, macrophages, neutrophils, lymphoid cells, and epithelial cells (17–19).
Osteopontin is also produced in lung tissues and is strongly correlated with various pulmonary diseases. Under physiological conditions, osteopontin is expressed at low levels in macrophages, bronchioles, fibroblasts, club cells, and endothelial cells of the lungs. On the contrary, cell stress and inflammation activation increase osteopontin expression (20–22). In pulmonary fibrosis, osteopontin expression is elevated in macrophages, fibroblasts, and epithelial cells (23, 24). Increased osteopontin contributes to pulmonary fibrosis through epithelial–mesenchymal transition, and osteopontin knockdown significantly alleviates bleomycin-evoked pulmonary fibrosis in mice (24). In addition, osteopontin is increased in the macrophages of patients with coronavirus disease 2019 (25). It has been observed that osteopontin expression is higher in senescent pulmonary vascular cells of mice with pulmonary hypertension than in young mice (26). Moreover, osteopontin levels in sputum supernatant are upregulated in COPD patients compared with healthy subjects (27). Animal experiments have also shown increased osteopontin expression in the lung tissues of COPD mice (28). These findings underscore the significant role of osteopontin in COPD.
However, the exact role of osteopontin in AECOPD remains unclear. No evidence has yet demonstrated a correlation between serum osteopontin and prognosis in AECOPD patients. In the current study, serum osteopontin concentrations were measured in AECOPD patients, healthy volunteers, and stable COPD (SCOPD) cases. The relationship between serum osteopontin and disease progression was also explored in AECOPD patients. Our results provide the first evidence that serum osteopontin expression may reflect disease severity in AECOPD.
2 Materials and methods
2.1 Subjects
AECOPD patients were selected from the Anhui COPD cohort (AHCC), which was described in previous studies from our laboratory (29, 30). AECOPD patients were recruited at the time of diagnosis in the Department of Respiratory Medicine from three tertiary hospitals in Anhui Province. The follow-up investigation was conducted 2 years later. All AECOPD patients had a confirmed COPD diagnosis consistent with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (31). All included AECOPD patients, who had no other respiratory diseases, were over 18 years old and voluntarily participated in the follow-up study. On the day of admission, fasting venous blood samples were collected from AECOPD patients before treatment. In addition, to evaluate serum osteopontin expression, age- and sex-matched healthy participants and SCOPD patients were enrolled. The hospital stay following the acute exacerbation was recorded. Follow-up assessments were conducted every 3 months after the first exacerbation, during which hospitalization duration, exacerbations, and deaths were recorded. Patients who experienced two or more exacerbations per year were classified as having frequent exacerbations (32). Moreover, patients whose hospitalization lasted more than 13 days (third quartile) in the first year were categorized as prolonged hospital stays (33). Based on the inclusion and exclusion criteria, 350 AECOPD patients were initially selected from the Anhui COPD cohort. As shown in the flow diagram of recruitment and follow-up, 281 eligible AECOPD cases were ultimately included in the current clinical investigation (Supplementary Figure S1).
2.2 Data collection
On the first day of admission, demographic data were obtained using a questionnaire. All COPD patients and healthy volunteers underwent medical history and physical examination. Routine blood tests were performed, and biochemical indicators were collected, including liver function, renal function, and inflammatory cytokines. In addition, pulmonary function was assessed in SCOPD patients by a professional nurse in accordance with the American Thoracic Society guidelines (34). Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were recorded. SCOPD patients were categorized into four groups based on GOLD grades: I (FEV1% ≥ 80%), II (50% ≤ FEV1% < 80%), III (30% ≤ FEV1% < 50%), and IV (FEV1% < 30%). On admission, the symptoms and severity of AECOPD patients were assessed using the COPD Assessment Test (CAT) score (35). Based on the CAT score, AECOPD patients were classified into four groups: low (CAT score < 10), medium (10 ≤ CAT score < 20), high (20 ≤ CAT score < 30), and very high (30 ≤ CAT score < 40). Moreover, the degree of breathlessness was evaluated using the modified Medical Research Council (mMRC) score (36). Based on mMRC scores, AECOPD patients were classified into five subgroups: 0 (breathlessness only during vigorous exercise); 1 (expiratory dyspnea when walking quickly on level ground or climbing a gentle slope); 2 (walking slower than peer or needing to stop when climbing a gentle slope); 3 (needing breath after walking 100 m or several minutes on level ground); 4 (severe breathlessness at rest, or when dressing or undressing).
2.3 Enzyme-linked immunosorbent assay
Fasting blood samples were collected from all participants at 6:00 in the morning. All samples were kept at room temperature for 2 h, then centrifuged at 3,500 rpm for 10 mins. The resulting serum samples were extracted and stored in − 80°C until analysis. Serum osteopontin levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (CSB-E08392h, CUSABIO, http://www.cusabio.cn/) following the manufacturer’s standardized protocols (37, 38). Briefly, serum samples were diluted and added to the assay plate. The biotin-conjugated antibody was added and incubated at 37°C for 1.5 h. After washing, HRP-avidin was added to each well and incubated for an additional 1.5 h. The TMB substrate was then added, and the reaction was stopped by adding the stop solution. Lastly, absorbance was measured at 450 nm using a microplate reader.
2.4 Statistics analysis
The normality of the data was assessed using the Kolmogorov–Smirnov test, with a p-value greater than 0.05 considered indicative of a normal distribution. Normally distributed data are presented as mean ± standard deviation and compared using one-way analysis of variance (ANOVA). Nonnormally distributed data are expressed as median and analyzed using the Wilcoxon rank-sum test. Categorical variables are presented as counts (percentages), and differences between groups were assessed using a nonparametric test. In addition, the relationships between baseline serum osteopontin expression and CAT, mRMC, and pulmonary function parameters were examined using Pearson correlation and linear regression analyses. To evaluate the robustness of the statistical models, stratified analyses were performed according to age, gender, and smoking status. Based on serum osteopontin tertiles, AECOPD patients were categorized into three groups: low, serum osteopontin ≤ 1.118 ng/mL; medium, serum osteopontin 1.118~1.579 ng/mL; and high, serum osteopontin ≥ 1.579 ng/mL. The association of serum osteopontin expression and poor prognosis was evaluated using logistic regression analysis. The predictive capacity for major in-hospital clinical outcomes was assessed with a receiver operating characteristic (ROC) curve. Predictive performance was expressed as the area under the curve (AUC). A p-value less than 0.05 was considered statistically significant.
3 Results
3.1 Clinical data and demographic features
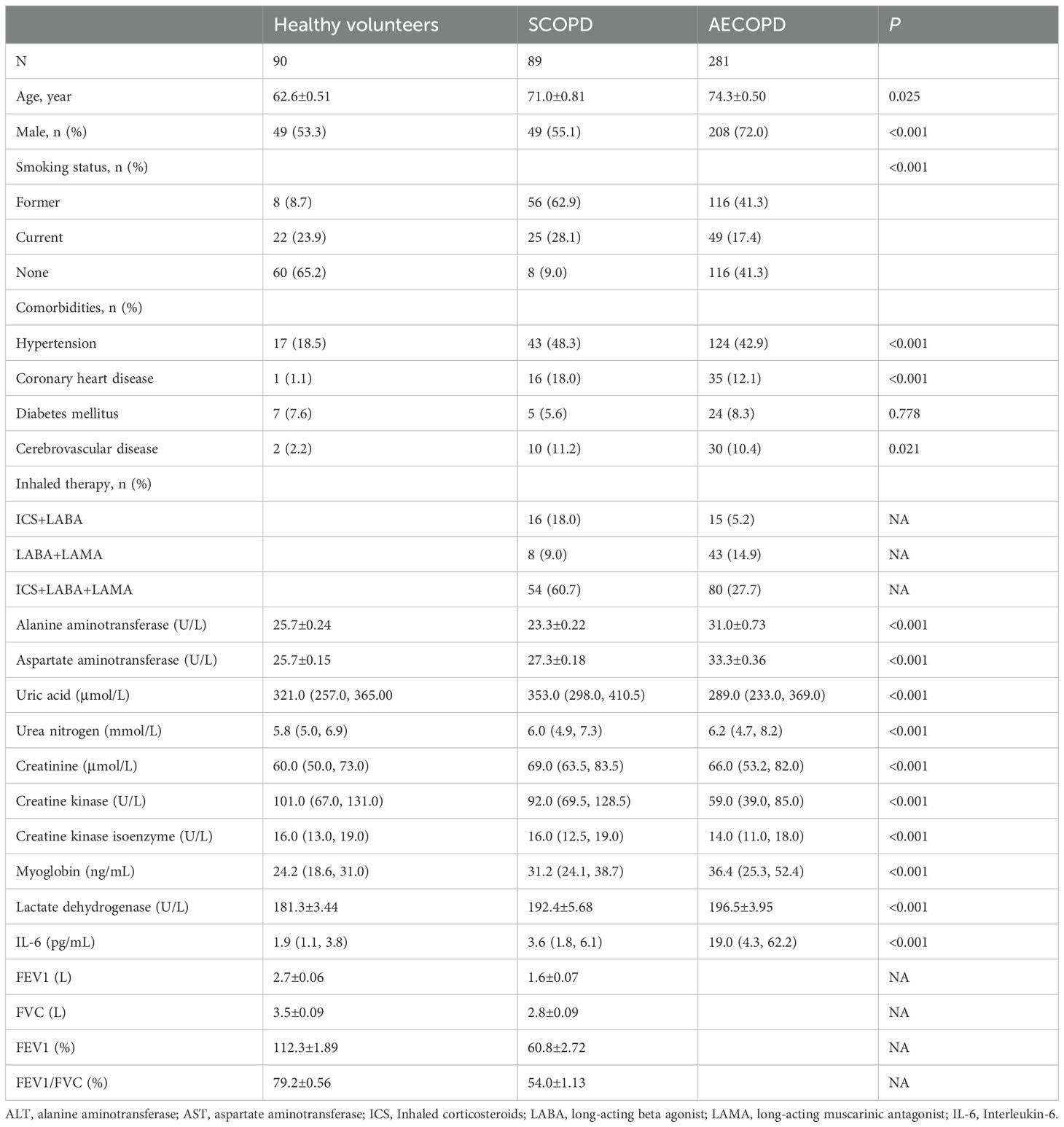
In this investigation, 90 healthy volunteers, 89 SCOPD patients, and 281 AECOPD patients were enrolled. Ages were higher in SCOPD and AECOPD patients than in healthy volunteers (Table 1). Significant differences were observed among the three groups in men’s smoking status, hypertension, coronary heart disease, and cerebrovascular disease (Table 1). In addition, several clinical parameters—including ALT, AST, uric acid, urea nitrogen, creatinine, creatine kinase, creatine kinase isoenzyme, myoglobin, lactate dehydrogenase, and interleukin-6 (IL-6)—differed significantly among the groups (Table 1).
3.2 Serum osteopontin levels in SCOPD and AECOPD patients with varying disease severity
Serum osteopontin expression was elevated in patients with SCOPD and COPD compared to healthy volunteers and was highest in AECOPD patients (Figure 1A). In AECOPD patients, serum osteopontin levels were compared across different severity scores and were found to increase progressively with higher CAT and mMRC scores (Figures 1B, C). Moreover, in SCOPD patients, serum osteopontin levels were assessed according to GOLD stages, showing that higher GOLD stages were associated with higher serum osteopontin levels (Figure 1D).
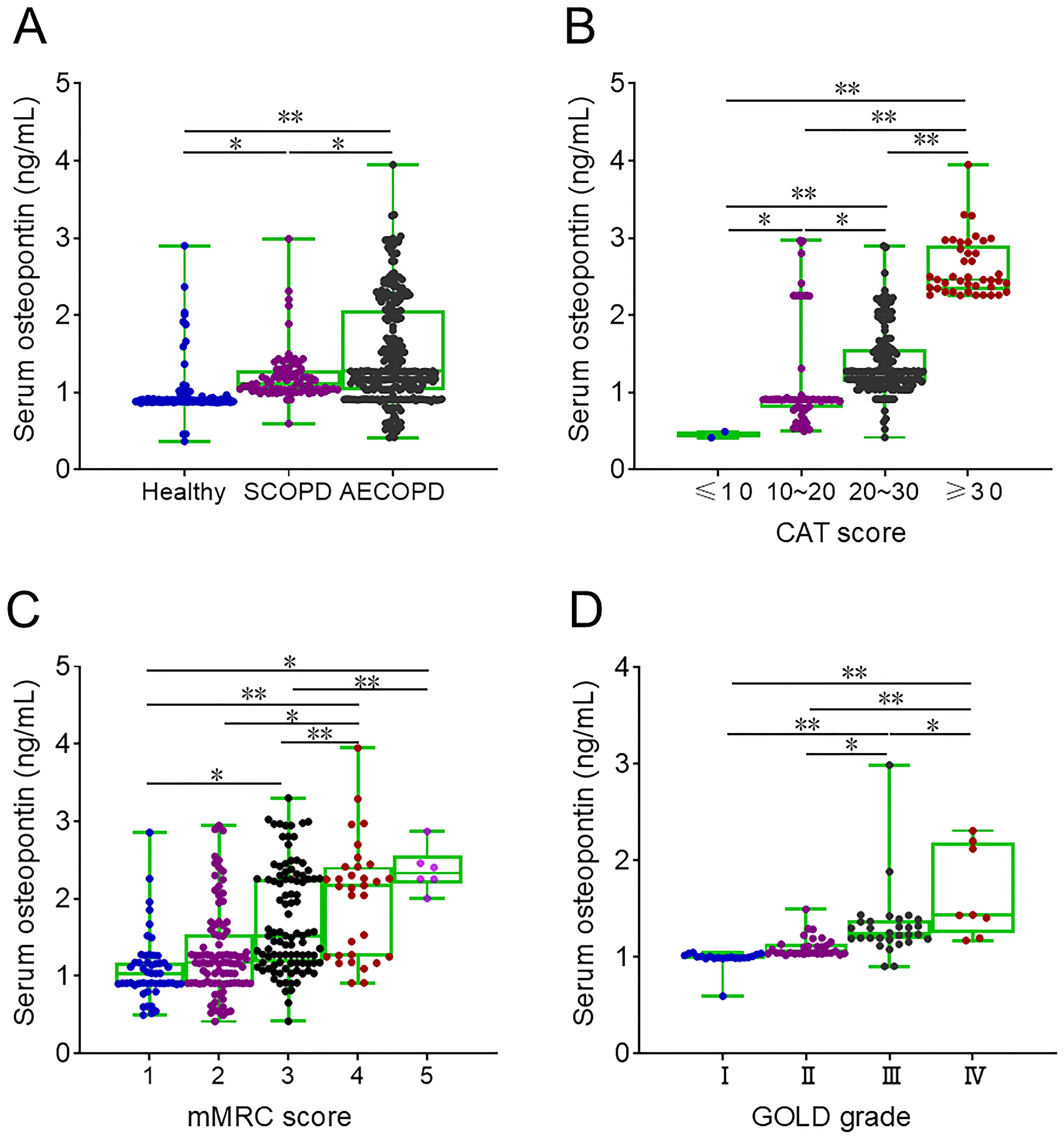
Figure 1. Baseline serum osteopontin expression in AECOPD patients. (A–D) Serum samples were collected, and baseline serum osteopontin expression was determined by ELISA. Differences in serum osteopontin levels were then compared. (A) Baseline serum osteopontin expression in healthy volunteers, SCOPD patients, and AECOPD patients. (B) Baseline serum osteopontin expression in AECOPD patients with distinct CAT scores. (C) Baseline serum osteopontin expression in AECOPD patients with distinct mMRC scores. (D) Baseline serum osteopontin expression in SCOPD patients with distinct GOLD grades. *p < 0.05; **p < 0.01.
3.3 Association between serum osteopontin expression on the day of admission and severity
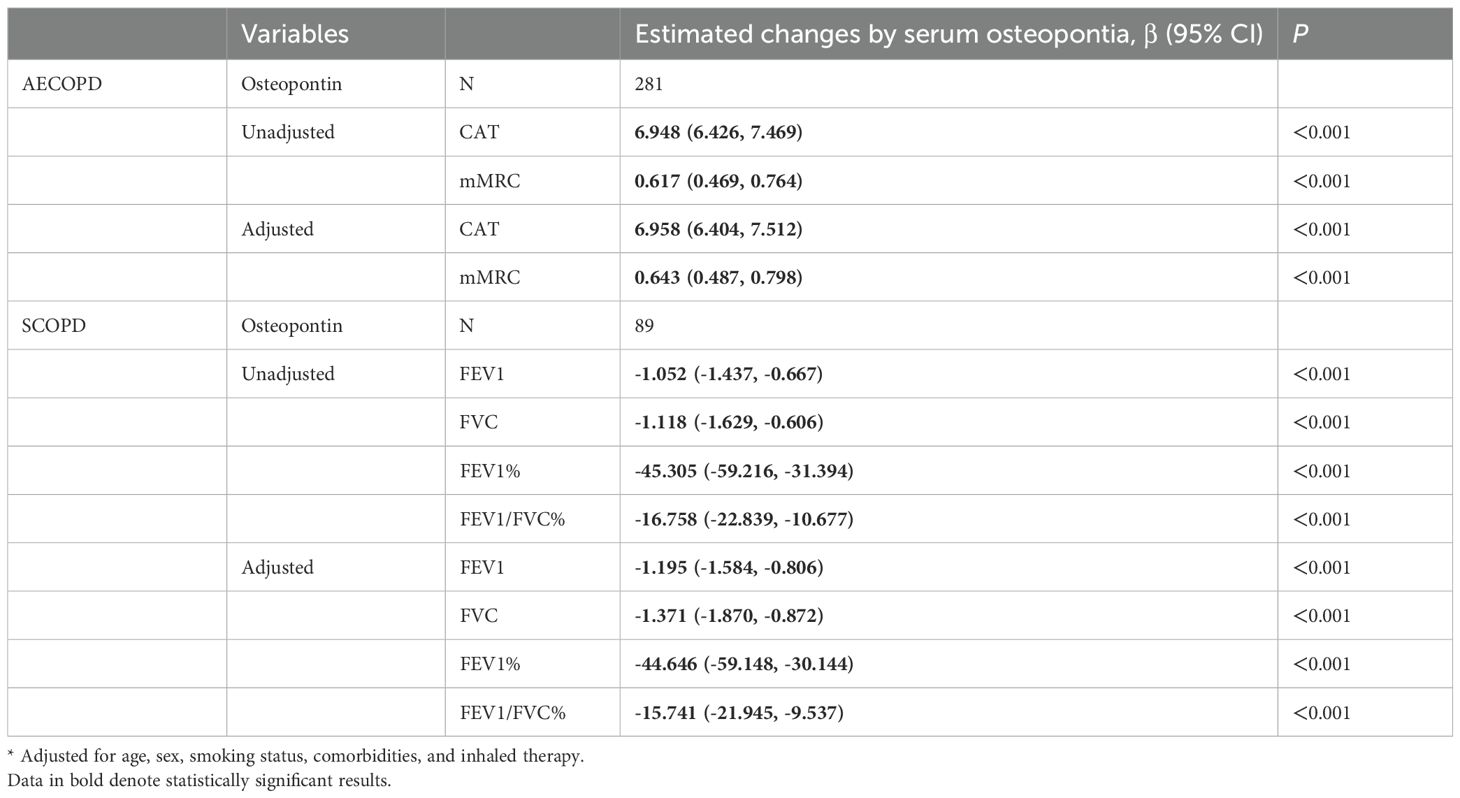
The correlations between serum osteopontin concentration on the day of admission and severity scores were evaluated by Pearson correlation analysis among AECOPD patients. As shown in Figures 2A, B, serum osteopontin expression was positively associated with CAT (r = 0.438; p < 0.01) and mMRC (r = 0.844; p < 0.01) scores among AECOPD patients. In addition, Pearson correlation analysis confirmed inverse relationships between serum osteopontin expression on the day of admission and FEV1/FVC% (r = − 0.506; p < 0.01), FEV1% (r = − 0.570; p < 0.01), FEV1 (r = − 0.503; p < 0.01), and FVC (r = − 0.422; p < 0.01) in SCOPD patients (Figures 2C–F). The correlations of serum osteopontin expression with severity scores and pulmonary function indicators were further evaluated using linear regression analysis. Univariate linear regression analysis revealed positive correlations between serum osteopontin expression and CAT (β = 6.948; 95% CI: 6.426–7.469) as well as mMRC (β = 0.617; 95% CI: 0.469–0.764) in AECOPD patients (Table 2). Moreover, each 1 ng/mL increase in baseline serum osteopontin was significantly associated with reductions of 1.052, 1.118, 45.305, and 16.758 in FEV1, FVC, FEV1%, and FEV1/FVC%, respectively, in SCOPD patients (Table 2). Several confounding variables, such as age, sex, smoking status, comorbidities, and inhaled therapy, were adjusted. Multivariate linear regression further validated the abovementioned relationships (Table 2). To further evaluate the relationship between serum osteopontin and severity, stratified analysis was conducted in different subgroups. The results indicated that serum osteopontin remained positively associated with the severity scores of AECOPD cases (Supplementary Table S1) and inversely correlated with pulmonary function parameters in COPD patients (Supplementary Table S2). Age, sex, and smoking status had a weak influence on the association of serum osteopontin and severity.
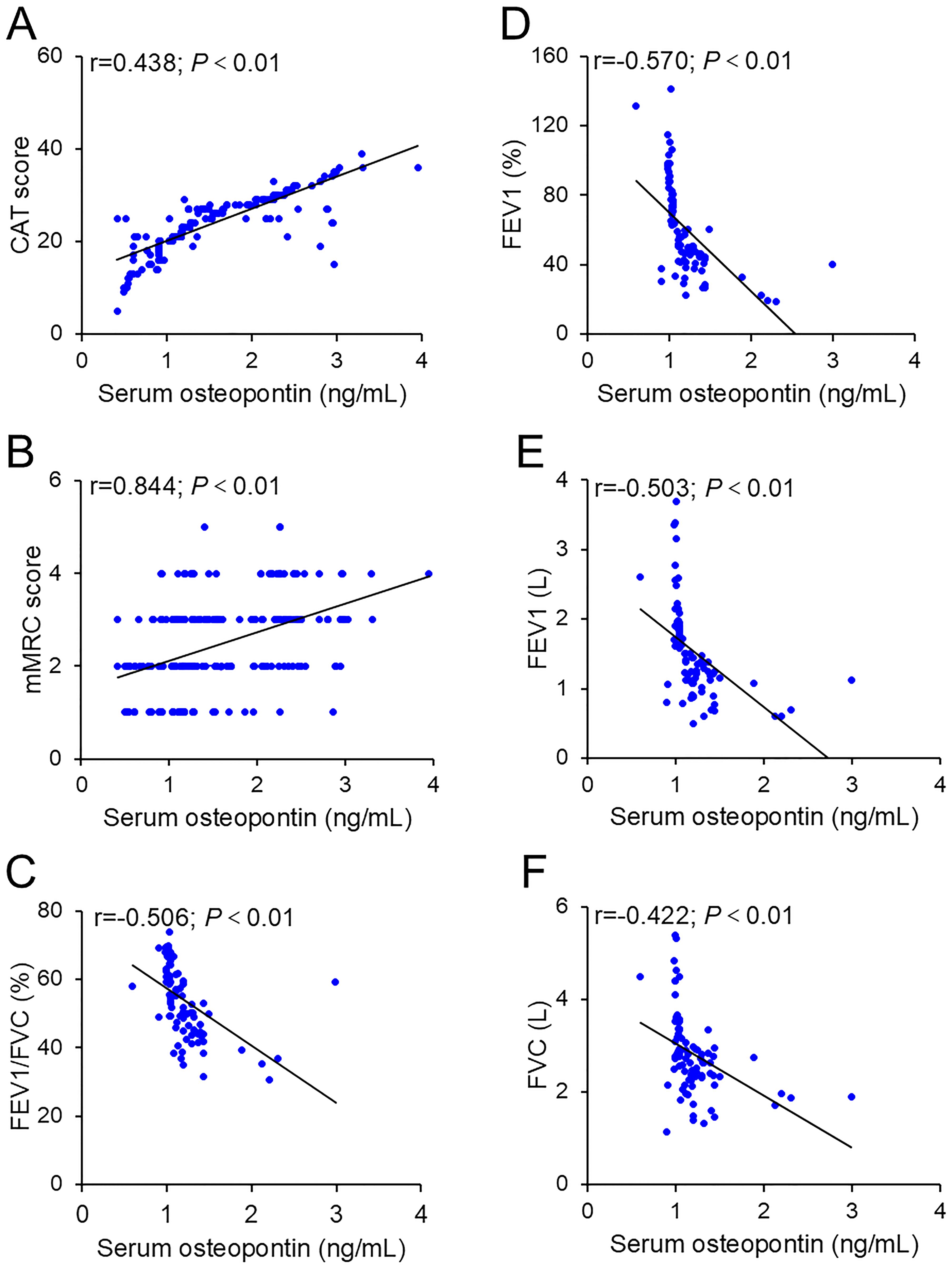
Figure 2. Correlation between baseline serum osteopontin expression and severity. (A, B) Correlations between serum osteopontin and CAT scores (A) or mMRC scores (B) were estimated using Pearson correlation analysis. (C–F) Associations between serum osteopontin and pulmonary function parameters were also evaluated using Pearson correlation analysis, including FEV1/FVC% (C), FEV1% (D), FEV1 (E), and FVC (F).
3.4 Serum expressions of osteopontin in AECOPD patients with different prognoses
The level of serum osteopontin on the day of admission was elevated in deceased AECOPD patients compared to those who survived (Figure 3A). The baseline serum osteopontin expression was then compared between AECOPD patients with and without frequent exacerbations. The data revealed that serum osteopontin levels on the day of admission were higher in AECOPD patients who experienced frequent exacerbations compared to those with infrequent exacerbations in both the first and second years (Figures 3B, C). Moreover, no significant difference in baseline serum osteopontin was observed between AECOPD patients with and without prolonged hospital stays (Figure 3D).
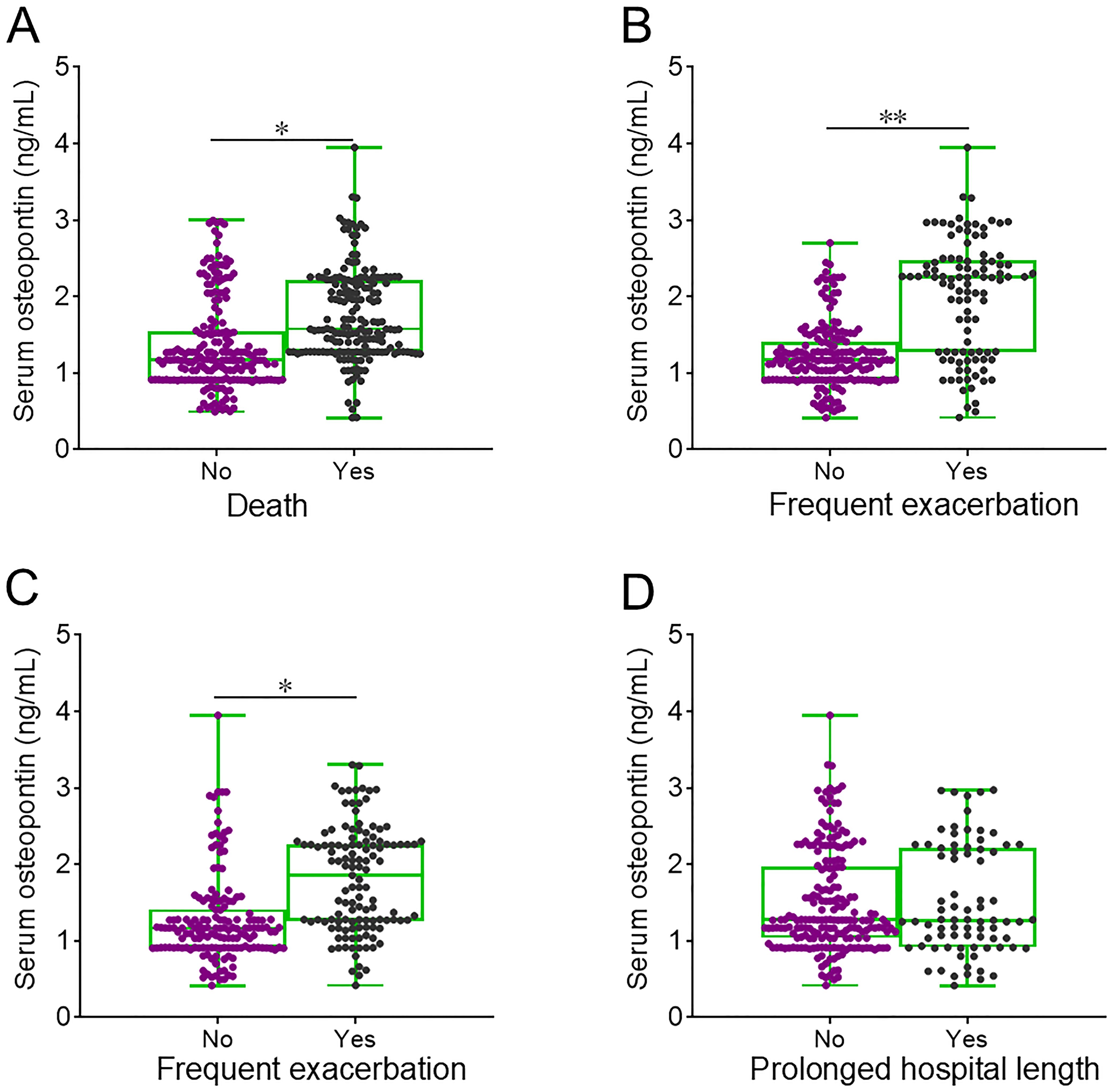
Figure 3. Baseline serum osteopontin expression in AECOPD patients with different clinical outcomes. (A–D) Serum osteopontin expression was compared on the day of admission among AECOPD patients who experienced different clinical outcomes: death (A), frequent exacerbation in the first year (B), frequent exacerbation in the second year (C), and prolonged hospital stay (D). *p < 0.05; **p < 0.01.
3.5 Correlation between serum osteopontin expression on the day of admission and prognosis
The number of cases who experienced death at the follow-up stage was 13 (13.8%), 26 (27.7%), and 45 (48.4%) in the low, medium, and high groups, respectively (Table 3). Compared to the low group, univariate logistic regression analysis indicated that the relative risk of death was increased in medium (RR = 2.382; 95% CI: 1.137–4.992) and high (RR = 5.841; 95% CI: 2.863–11.917) groups (Table 3). Additionally, the numbers of acute exacerbation in the first year were 42 (44.7%), 55 (58.5%), and 88 (94.6%), while the cases of acute exacerbation in the second year were 43 (45.7%), 63 (67.0%), and 76 (81.7%) in the low, medium, and high groups, respectively (Table 3). Univariate logistic regression analysis showed that serum osteopontin concentration on the day of admission was positively correlated with the risk of acute exacerbations in the first and second years among AECOPD cases. After controlling for several confounding variables, multivariate logistic regression analysis still confirmed that high serum osteopontin concentration increased the risk of death and acute exacerbations in the first year of AECOPD patients (Table 3). However, no significant association was found between serum osteopontin expression and prolonged hospital stay in AECOPD patients (Table 3).
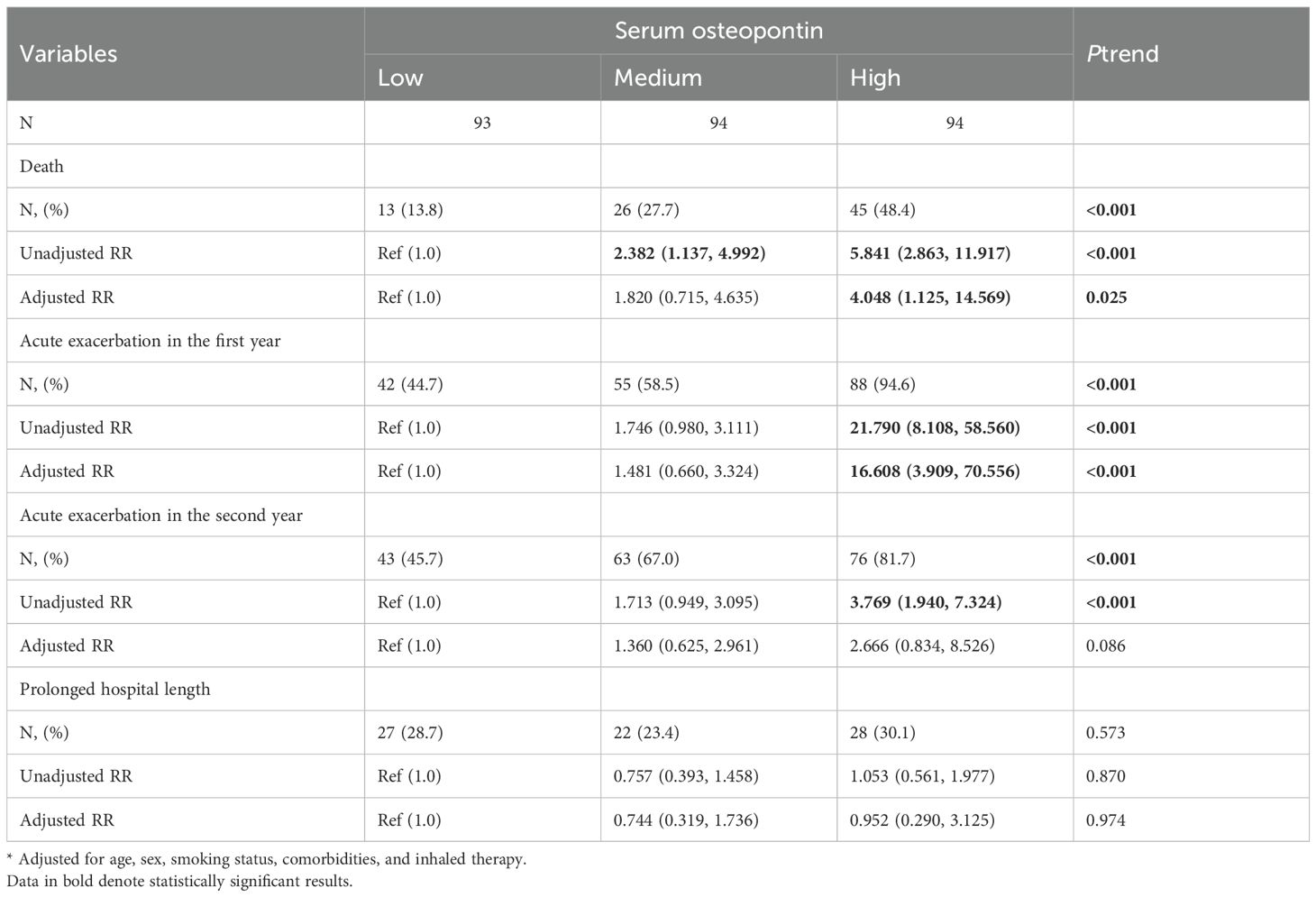
Table 3.. Associations between serum osteopontin and prognostic outcomes in AECOPD patients at follow-up stage.
3.6 Predictive value of serum osteopontin expression for adverse prognosis in AECOPD patients
The predictive values of serum osteopontin concentration and severity scores for in-hospital adverse prognosis were analyzed via ROC among AECOPD patients. As shown in Figure 4A, the AUCs for death were as follows: serum osteopontin, 0.701; CAT score, 0.687; and mMRC score, 0.574, indicating that the predictive power of serum osteopontin and CAT score for death was higher compared with the mMRC score within 2 years among AECOPD patients. Moreover, the predictive power of serum osteopontin concentration (AUC = 0.752) for acute exacerbation in the first year was comparable to that of the CAT score (AUC = 0.739), and higher than that of the mMRC score (AUC = 0.608) (Figure 4B). Additionally, there was no significant difference in AUC for acute exacerbation in the second year between serum osteopontin expression (AUC = 0.624) and the CAT score (AUC = 0.644), while the predictive capacity was reduced in the mMRC score (AUC = 0.531) among AECOPD patients (Figure 4C).
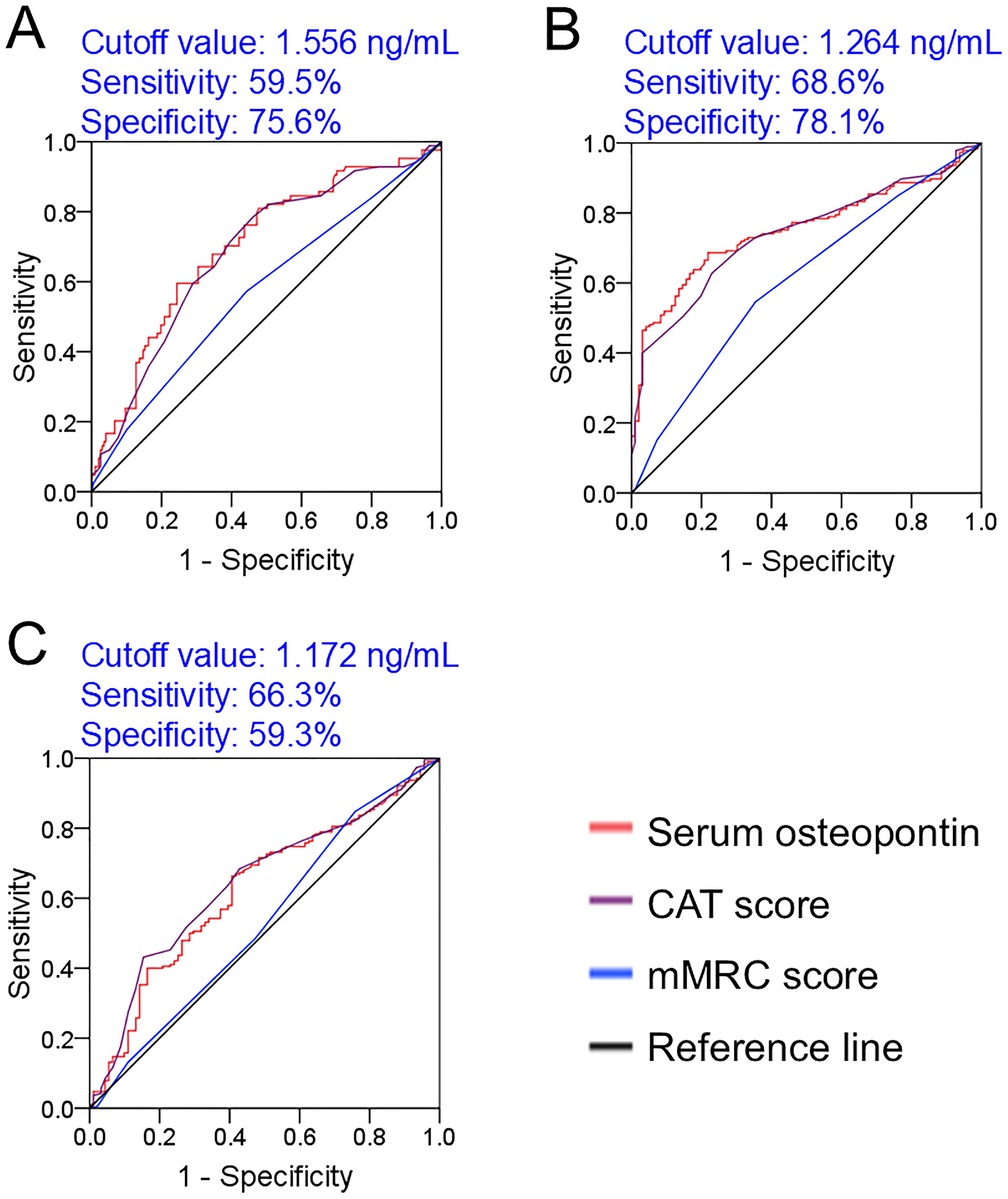
Figure 4. Predictive power of baseline serum osteopontin expression for poor prognosis in AECOPD patients. (A–C) ROC curves were used to estimate the predictive capacity of baseline serum osteopontin for adverse clinical outcomes. (A) Prediction of death. (B) Frequent exacerbation in the first year. (C) Frequent exacerbation in the second year.
4 Discussion
This study primarily measured serum osteopontin levels on the day of admission and analyzed their relationship with disease severity and prognosis in AECOPD patients. Our findings revealed, for the first time, that upregulated serum osteopontin expression is associated with increased clinical severity and adverse outcomes in AECOPD patients over a 2-year period.
Osteopontin, a phosphorylated glycoprotein, is highly expressed in lung tissue and is present in various cell types, including fibroblasts, macrophages, neutrophils, lymphocytes, and epithelial cells (10, 17–19). Numerous studies have demonstrated that osteopontin plays key roles in the pathophysiology of pulmonary diseases. Under normal conditions, osteopontin is expressed in most cells (20–22). Pulmonary osteopontin expression is increased in both the mouse model and patients with lung adenocarcinoma (39). Moreover, pulmonary osteopontin levels are upregulated in macrophages and epithelial cells of patients with fibrosis (23, 24). In sepsis-incurred acute lung injury, both osteopontin mRNA and protein levels are elevated in mouse lungs (40). Pulmonary osteopontin expression is also increased in patients with COPD (41). These findings suggest a vital role for osteopontin in respiratory diseases. However, the precise relationships between serum osteopontin expression, disease severity, and prognosis have rarely been investigated in AECOPD cases. Our current projects found that serum osteopontin expression on the day of admission was elevated in AECOPD patients compared to SCOPD patients and healthy volunteers. Among AECOPD patients, serum osteopontin levels were higher in those with elevated CAT and mMRC scores. Correlative analysis revealed that serum osteopontin expression was inversely associated with pulmonary function in SCOPD patients and positively correlated with CAT and mMRC scores in AECOPD patients. These findings confirm that serum osteopontin expression on the day of admission is positively related to clinical severity in AECOPD patients.
Increasing evidence suggests that osteopontin expression is associated with the prognosis of various diseases. A recent study reported that osteopontin expression in macrophages is positively correlated with cancer stage and tumor grade in patients with head and neck squamous cell carcinoma (42). Overexpression of the osteopontin gene has been linked to shorter overall survival and progression-free interval (43). In addition, elevated plasma osteopontin levels are associated with higher mortality in sepsis patients (44). Based on these findings, we speculated that serum osteopontin expression on the day of admission may be associated with an increased risk of poor outcomes in AECOPD patients. We found that serum osteopontin on the day of admission was elevated in deceased cases and in patients with frequent exacerbations. Logistic regression analysis further confirmed the positive association of baseline serum osteopontin expression with both mortality and the number of exacerbations. ROC curve analysis showed that the predictive power of serum osteopontin for poor prognosis was similar to that of the CAT score and higher than that of the mMRC score. These data indicate that serum osteopontin expression on the day of admission is positively correlated with in-hospital poor prognosis in AECOPD patients.
Our study demonstrated that serum osteopontin levels were positively correlated with disease severity and poor prognosis in AECOPD patients. However, the precise mechanism by which osteopontin influences prognosis in these patients remains unclear. Previous studies have shown that osteopontin can activate nuclear factor-κ B (NF-κB), a key transcription factor that regulates downstream target genes, including proinflammatory cytokines and chemokines, thereby triggering inflammatory cascades and promoting COPD progression (45). Dysregulated inflammation is recognized as a central mechanism in the initiation and progression of COPD (46). Inflammatory cytokines not only activate immune responses but also alter the levels of eosinophils, lymphocytes, and neutrophils in the human body. An abnormal inflammatory state can result in alveolar damage and airway stenosis, as well as trigger acute exacerbations and worsen prognosis in COPD patients (47–49). In addition, several studies have confirmed that osteopontin is a major component of the senescence-associated secretory phenotype (SASP), can promote tissue remodeling, and regulate fibroblast function (26, 50). Emerging evidence highlights the pivotal role of cellular senescence in the progression of COPD (51). Senescent cells exacerbate inflammation, promote malignant transformation, alter the local microenvironment, and impair pulmonary structure and function (52). Thus, these findings suggest that elevated osteopontin may contribute to adverse COPD progression, at least in part through mechanisms involving inflammation and cellular senescence.
Although this investigation provides new insights into the role of osteopontin in COPD progression, several limitations should be acknowledged. First, this was a single-center study; more AECOPD patients should be recruited from multiple hospitals. Second, as a clinical epidemiological study, it only explored the association between serum osteopontin and AECOPD, and the precise mechanism underlying serum osteopontin upregulation remains unclear. Only animal and cellular experiments can reveal the underlying molecular mechanisms. Third, this study analyzed only the correlation between serum osteopontin and AECOPD patients. In reality, many other inflammatory cytokines and components of the SASP are simultaneously elevated in AECOPD cases. The potential confounding effects of these factors on the relationship between serum osteopontin and prognosis cannot be fully excluded. Epidemiological research can generally estimate only one or a few factors at a time. Therefore, to some extent, a new mixed forecasting model may help address this issue.
Clinically, the diagnosis of AECOPD primarily relies on medical history and clinical manifestations. Acute exacerbations are the main cause of death and progressive decline in pulmonary function. By improving early detection and risk assessment in AECOPD patients, clinicians can better identify those at risk of poor clinical outcomes (53). However, the similarities between AECOPD and other pulmonary diseases make diagnosis challenging. In addition, in the stable and early stages, COPD symptoms are often mild and can be easily overlooked by both patients and clinicians. Thus, earlier diagnosis, timely assessment, and therapeutic interventions can help improve prognosis and reduce mortality (54). Clinical examinations, pulmonary function tests, and severity score evaluations all play important roles in identifying the risk of acute exacerbations. However, the predictive power of these assessments may be limited by complex indicators and equipment constraints. Single, readily available biomarkers can help compensate for these limitations. Serum osteopontin is easily and inexpensively measured, with diagnostic performance comparable to the CAT score and superior to the mMRC score. Therefore, measuring serum osteopontin may provide a valuable tool for enhancing diagnostic accuracy and predicting poor prognosis in AECOPD patients.
5 Conclusion
In summary, by comparing the baseline expression of serum osteopontin in AECOPD, SCOPD, and healthy volunteers, we found that serum osteopontin is significantly upregulated in AECOPD and SCOPD patients compared to healthy volunteers, particularly in AECOPD patients. Additionally, baseline serum osteopontin expression is positively correlated with disease severity and may serve as a prognostic biomarker for poor clinical outcomes in AECOPD patients during hospitalization. These findings highlight the potential clinical utility of serum osteopontin in diagnosis and prognosis assessment for AECOPD patients. Nevertheless, further studies are needed to explore the association of these findings with patient phenotypes and the underlying mechanisms driving osteopontin elevation.
Data availability statement
Publicly available datasets were analyzed in this study. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This human study was approved by Ethics Committee of Second Affiliated Hospital of Anhui Medical University - approval: YX2021-146. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LF: Conceptualization, Project administration, Writing – review & editing, Writing – original draft, Funding acquisition, Resources. K-SM: Writing – original draft, Data curation, Conceptualization, Methodology, Project administration, Formal Analysis, Investigation. L-NL: Methodology, Conceptualization, Data curation, Investigation, Writing – original draft, Formal Analysis, Project administration. Y-CM: Formal Analysis, Writing – original draft, Methodology, Investigation, Conceptualization, Project administration, Data curation. R-LF: Writing – original draft, Project administration. GC: Project administration, Writing – original draft. HZ: Resources, Conceptualization, Writing – review & editing, Funding acquisition, Writing – original draft, Project administration. K-XQ: Conceptualization, Project administration, Resources, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82270071), the Research Funds of the Center for Big Data and Population Health of IHM (JKS2022007, JKS2023010), and the University Natural Science Research Project of Anhui Province (2023AH030117).
Acknowledgments
We would like to extend our heartfelt thanks to all patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1708595/full#supplementary-material
References
1. López-Campos JL, Tan W, and Soriano JB. Global burden of COPD. Respirology. (2016) 21:14–23. doi: 10.1111/resp.12660
2. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
3. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. (2022) 10:447–58. doi: 10.1016/S2213-2600(21)00511-7
4. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. (2015) 5:20415. doi: 10.7189/jogh.05.020415
5. Boers E, Barrett M, Su JG, Benjafield AV, Sinha S, Kaye L, et al. Global burden of chronic obstructive pulmonary disease through 2050. JAMA Netw Open. (2023) 6:e2346598. doi: 10.1001/jamanetworkopen.2023.46598
6. Chen S, Kuhn M, Prettner K, Yu F, Yang T, Bärnighausen T, et al. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: a health-augmented macroeconomic modelling study. Lancet Glob Health. (2023) 11:e1183–93. doi: 10.1016/S2214-109X(23)00217-6
7. Lange P, Ahmed E, and Lahmar ZM. Natural history and mechanisms of COPD. Respirology. (2021) 26:298–321. doi: 10.1111/resp.14007
8. Decramer M, Janssens W, and Miravitlles M. Chronic obstructive pulmonary disease. Lancet. (2012) 379:1341–51. doi: 10.1016/S0140-6736(11)60968-9
9. Wang RR, Chen DL, Wei M, Li SR, Zhou P, Sun J, et al. Histone lactylation-induced premature senescence contributes to 1-nitropyrene-Induced chronic obstructive pulmonary disease. Redox Biol. (2025) 84:103703. doi: 10.1016/j.redox.2025.103703
10. Martín-Márquez BT, Sandoval-García F, Corona-Meraz FI, Martínez-García EA, Sánchez-Hernández PE, Salazar-Páramo M, et al. Osteopontin: A bone-derived protein involved in rheumatoid arthritis and osteoarthritis immunopathology. Biomolecules. (2023) 13:502. doi: 10.3390/biom13030502
11. Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. (2014) 18:883–95. doi: 10.1517/14728222.2014.925447
12. Kahles F, Findeisen HM, and Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. (2014) 3:384–93. doi: 10.1016/j.molmet.2014.03.004
13. Al-Dalahmah O, Lam M, McInvale JJ, Qu W, Nguyen T, Mun JY, et al. Osteopontin drives neuroinflammation and cell loss in MAPT-N279K frontotemporal dementia patient neurons. Cell Stem Cell. (2024) 31:676–3.e10. doi: 10.1016/j.stem.2024.03.013
14. Tang Z, Xia Z, Wang X, and Liu Y. The critical role of osteopontin (OPN) in fibrotic diseases. Cytokine Growth Factor Rev. (2023) 74:86–99. doi: 10.1016/j.cytogfr.2023.08.007
15. Wang Y, Zhang W, Yang Y, Qin J, Wang R, Wang S, et al. Osteopontin deficiency promotes cartilaginous endplate degeneration by enhancing the NF-κB signaling to recruit macrophages and activate the NLRP3 inflammasome. Bone Res. (2024) 12:53. doi: 10.1038/s41413-024-00355-3
16. Kumari A, Kashyap D, and Garg VK. Osteopontin in cancer. Adv Clin Chem. (2024) 118:87–110. doi: 10.1016/bs.acc.2023.11.002
17. Icer MA and Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. (2023) 59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003
18. Lamort AS, Giopanou I, Psallidas I, and Stathopoulos GT. Osteopontin as a link between inflammation and cancer: the thorax in the spotlight. Cells. (2023) 8:815. doi: 10.3390/cells8080815
19. Inoue M and Shinohara ML. Intracellular osteopontin (iOPN) and immunity. Immunol Res. (2011) 49:160–72. doi: 10.1007/s12026-010-8179-5
20. Serlin DM, Kuang PP, Subramanian M, O’Regan A, Li X, Berman JS, et al. Interleukin-1beta induces osteopontin expression in pulmonary fibroblasts. J Cell Biochem. (2023) 97:519–29. doi: 10.1002/jcb.20661
21. Tsukui T, Ueha S, Abe J, Hashimoto S, Shichino S, Shimaoka T, et al. Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J Pathol. (2023) 183:758–73. doi: 10.1016/j.ajpath.2013.06.005
22. Kato A, Okura T, Hamada C, Miyoshi S, Katayama H, Higaki J, et al. Cell stress induces upregulation of osteopontin via the ERK pathway in type II alveolar epithelial cells. PloS One. (2023) 9:e100106. doi: 10.1371/journal.pone.0100106
23. Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J. (2019) 54:1802441. doi: 10.1183/13993003.02441-2018
24. Ji J, Zheng S, Liu Y, Xie T, Zhu X, Nie Y, et al. Increased expression of OPN contributes to idiopathic pulmonary fibrosis and indicates a poor prognosis. J Transl Med. (2023) 21:640. doi: 10.1186/s12967-023-04279-0
25. Lee JTH, Barnett SN, Roberts K, Ashwin H, Milross L, Cho JW, et al. Integrated histopathology, spatial and single cell transcriptomics resolve cellular drivers of early and late alveolar damage in COVID-19. Nat Commun. (2025) 16:1979. doi: 10.1038/s41467-025-56473-x
26. Saker M, Lipskaia L, Marcos E, Abid S, Parpaleix A, Houssaini A, et al. Osteopontin, a key mediator expressed by senescent pulmonary vascular cells in pulmonary hypertension. Arterioscler Thromb Vasc Biol. (2016) 36:1879–90. doi: 10.1161/ATVBAHA.116.307839
27. Papaporfyriou A, Loukides S, Kostikas K, Simoes DCM, Papatheodorou G, Konstantellou E, et al. Increased levels of osteopontin in sputum supernatant in patients with COPD. Chest. (2014) 146:951–8. doi: 10.1378/chest.13-2440
28. Huang J, Zhou X, Xu Y, Yu C, Zhang H, Qiu J, et al. Shen Qi Wan regulates OPN/CD44/PI3K pathway to improve airway inflammation in COPD: Network pharmacology, bioinformatics, and experimental validation. Int Immunopharmacol. (2025) 144:113624. doi: 10.1016/j.intimp.2024.113624
29. Zheng L, Jiang YL, Fei J, Cao P, Zhang C, Xie GF, et al. Circulatory cadmium positively correlates with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. Ecotoxicol Environ Saf. (2021) 215:112164. doi: 10.1016/j.ecoenv.2021.112164
30. Sun J, Deng YP, Xu J, Zhu FM, He QY, Tang MM, et al. Association of blood cadmium concentration with chronic obstructive pulmonary disease progression: a prospective cohort study. Respir Res. (2024) 25:91. doi: 10.1186/s12931-024-02726-0
31. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. (2016) 374:1811–21. doi: 10.1056/NEJMoa1505971
32. Kim C, Ko Y, Kim SH, Yoo HJ, Lee JS, Rhee CK, et al. Urinary desmosine is associated with emphysema severity and frequent exacerbation in patients with COPD. Respirology. (2018) 23:176–81. doi: 10.1111/resp.13170
33. Wang Y, Fei J, Xu J, Cheng ZY, Ma YC, Wu JH, et al. Associations of the serum KL-6 with severity and prognosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Lung. (2024) 202:245–55. doi: 10.1007/s00408-024-00702-5
34. Standardization of Spirometry. 1994 update. American thoracic society. Am J Respir Crit Care Med. (1995) 152:1107–36. doi: 10.1164/ajrccm.152.3.7663792
35. Jones P, Harding G, Wiklund I, Berry P, and Leidy N. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J. (2009) 18:208–15. doi: 10.4104/pcrj.2009.00053
36. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, and Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. (1999) 54:581–6. doi: 10.1136/thx.54.7.581
37. Wang Y, Jiang Y, Xie M, Qi B, Pu K, Du W, et al. Cross-sectional and longitudinal associations of serum LRG1 with severity and prognosis among adult community-acquired pneumonia patients. J Inflammation Res. (2024) 17:7951–62. doi: 10.2147/JIR.S485932
38. Du Y, Chu CM, Zhuo D, and Ning JZ. The inhibition of TRIM35-mediated TIGAR ubiquitination enhances mitochondrial fusion and alleviates renal ischemia-reperfusion injury. Int J Biol Macromol. (2022) 209:725–36. doi: 10.1016/j.ijbiomac.2022.04.054
39. Giopanou I, Kanellakis NI, Giannou AD, Lilis I, Marazioti A, Spella M, et al. Osteopontin drives KRAS-mutant lung adenocarcinoma. Carcinogenesis. (2020) 41:1134–44. doi: 10.1093/carcin/bgz190
40. Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, et al. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care. (2015) 19:53. doi: 10.1186/s13054-015-0782-3
41. Ali MN, Mori M, Mertens TCJ, Siddhuraj P, Erjefält JS, Önnerfjord P, et al. Osteopontin expression in small airway epithelium in copd is dependent on differentiation and confined to subsets of cells. Sci Rep. (2019) 9:15566. doi: 10.1038/s41598-019-52208-3
42. Liu C, Wu K, Li C, Zhang Z, Zhai P, Guo H, et al. SPP1+ macrophages promote head and neck squamous cell carcinoma progression by secreting TNF-α and IL-1β. J Exp Clin Cancer Res. (2024) 43:332. doi: 10.1186/s13046-024-03255-w
43. Zhang Z, Liu B, Lin Z, Mei L, Chen R, and Li Z. SPP1 could be an immunological and prognostic biomarker: From pan-cancer comprehensive analysis to osteosarcoma validation. FASEB J. (2024) 38:e23783. doi: 10.1096/fj.202400622RR
44. Shen YZ, Xiong W, Hu YC, and Zhong W. SPP1 is a plasma biomarker associated with the diagnosis and prediction of prognosis in sepsis. Sci Rep. (2024) 14:27205. doi: 10.1038/s41598-024-78420-4
45. Abdelnaby R, Sonbol YT, Dardeer KT, ELgenidy A, Ebrahim MA, Elmenawi KA, et al. Could Osteopontin be a useful biomarker in the diagnosis and severity assessment of osteoarthritis? A systematic review and meta-analysis of recent evidence. Clin Immunol. (2023) 246:109187. doi: 10.1016/j.clim.2022.109187
46. Li L, Wang Y, Gao W, Yuan C, Zhang S, Zhou H, et al. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract-induced inflammation in chronic obstructive pulmonary disease. J Biol Chem. (2015) 290:27890–900. doi: 10.1074/jbc.M115.655431
47. Zhang Y, Tan X, Hu S, Cui Z, and Chen W. Relationship between systemic immune-inflammation index and risk of respiratory failure and death in COPD: A retrospective cohort study based on the MIMIC-IV database. Int J Chron Obstruct Pulmon Dis. (2024) 19:459–73. doi: 10.2147/COPD.S446364
48. David B, Bafadhel M, Koenderman L, and De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. (2021) 76:188–95. doi: 10.1136/thoraxjnl-2020-215167
49. Cao K, Miao X, and Chen X. Association of inflammation and nutrition-based indicators with chronic obstructive pulmonary disease and mortality. J Health Popul Nutr. (2024) 43:209. doi: 10.1186/s41043-024-00709-x
50. Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation. (2018) 138:809–22. doi: 10.1161/CIRCULATIONAHA.117.031358
51. Zhan Y, Huang Q, Deng Z, Chen S, Yang R, Zhang J, et al. DNA hypomethylation-mediated upregulation of GADD45B facilitates airway inflammation and epithelial cell senescence in COPD. J Adv Res. (2025) 68:201–14. doi: 10.1016/j.jare.2024.02.005
52. Kumar M, Seeger W, and Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. (2014) 51:323–33. doi: 10.1165/rcmb.2013-0382PS
53. Xu Z, Li F, Xin Y, Wang Y, and Wang Y. Prognostic risk prediction model for patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD): a systematic review and meta-analysis. Respir Res. (2024) 25:410. doi: 10.1186/s12931-024-03033-4
54. Cai C, Zeng W, Wang H, and Ren S. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and monocyte-to-lymphocyte ratio (MLR) as biomarkers in diagnosis evaluation of acute exacerbation of chronic obstructive pulmonary disease: A retrospective, observational study. Int J Chron Obstruct Pulmon Dis. (2024) 19:933–43. doi: 10.2147/COPD.S452444
Keywords: COPD, osteopontin, death, acute exacerbation, cohort study
Citation: Ma K-S, Li L-N, Ma Y-C, Fan R-L, Chen G, Zhao H, Qu K-X and Fu L (2025) Serum osteopontin as a prognostic biomarker in acute exacerbations of chronic obstructive pulmonary disease. Front. Immunol. 16:1708595. doi: 10.3389/fimmu.2025.1708595
Received: 19 September 2025; Accepted: 27 October 2025;
Published: 11 November 2025.
Edited by:
Rodolfo P. Vieira, Centro Universitário UniEvangélica, BrazilReviewed by:
Stelvio Tonello, University of Eastern Piedmont, ItalySelda Günaydin, Giresun University, Türkiye
Copyright © 2025 Ma, Li, Ma, Fan, Chen, Zhao, Qu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Fu, ZnVsaW5kZXZAMTI2LmNvbQ==; Kai-Xin Qu, MjU0NDIyODc4MEBxcS5jb20=; Hui Zhao, emhhb2h1aWNoZW54aUAxMjYuY29t
†These authors have contributed equally to this work
 Kai-Shu Ma1†
Kai-Shu Ma1† Hui Zhao
Hui Zhao Lin Fu
Lin Fu