- 1The First School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Hematology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
This article reports a rare case of a patient with POEMS syndrome who developed secondary myelodysplastic syndrome (MDS) two years after undergoing autologous stem cell transplantation (ASCT). The patient was initially misdiagnosed with chronic inflammatory demyelinating polyneuropathy (CIDP) due to symptoms of limb numbness and weakness. Two years later, the diagnosis was corrected to POEMS syndrome. After induction therapy with the lenalidomide-dexamethasone (RD) regimen, ASCT is performed and partial remission is achieved. And lenalidomide was used for maintenance therapy. Over a year later, he was infected with SARS-CoV-2 and subsequently developed pancytopenia. Bone marrow routine revealed increased myeloblasts with multilineage dysplasia, and next-generation sequencing (NGS) found a TP53 mutation, leading to the diagnosis of secondary MDS. The pathogenesis of secondary MDS in POEMS syndrome is discussed from three aspects: cytotoxic therapy, genetic predisposition, and SARS-CoV-2 infection. This case underscores the importance of prolonged surveillance for secondary myeloid neoplasms (sMN) in POEMS patients and suggests that early genomic profiling and individualized treatment may improve outcomes.
1 Introduction
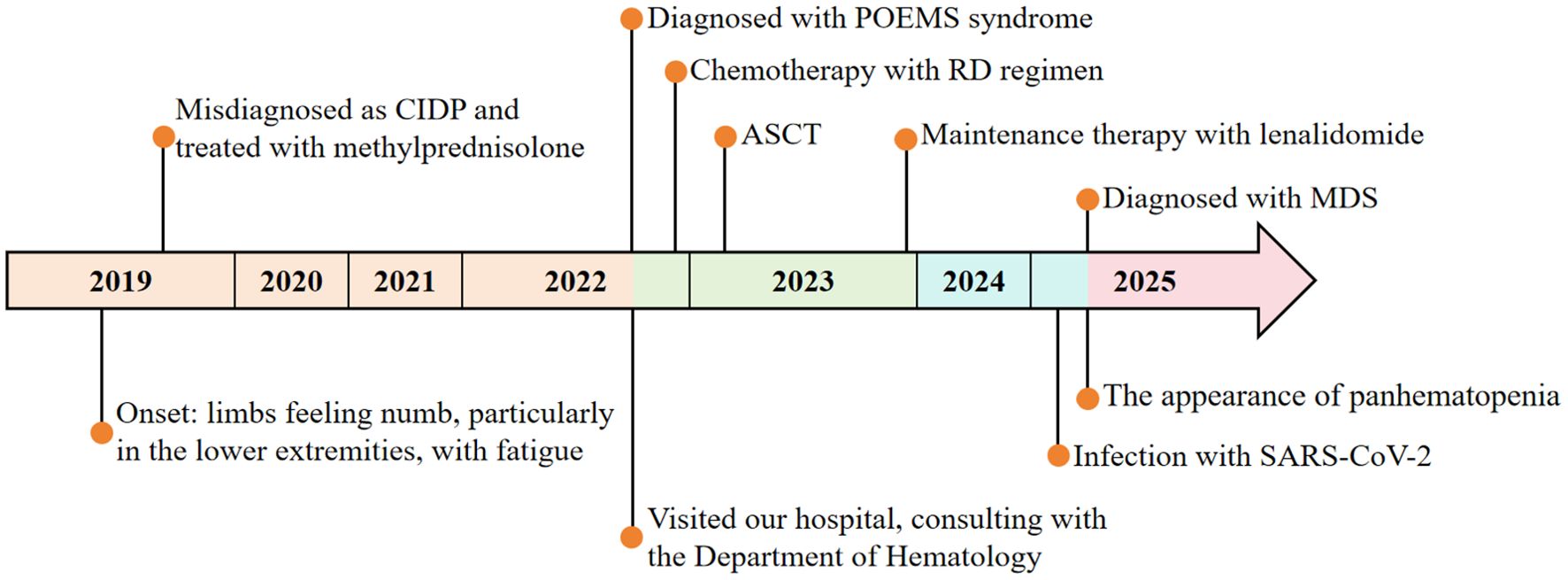
POEMS syndrome is a rare paraneoplastic syndrome caused by abnormal proliferation of plasma cells, characterized primarily by polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes. Its name is derived from the initial letters of these features (1). Although combined chemotherapy and autologous stem cell transplantation (ASCT) have markedly improved long-term survival (2), secondary myeloid neoplasms (sMN) are gaining attention as a rare late complication. This case reports a patient with POEMS syndrome who developed secondary myelodysplastic syndrome (MDS) two years after ASCT, which provides clinical evidence for the potential association between POEMS syndrome and MDS, enhances understanding of its complex complications, and offers a reference for expanding research on disease mechanisms and clinical management. The clinical course is summarized below (Figure 1).
2 Case description
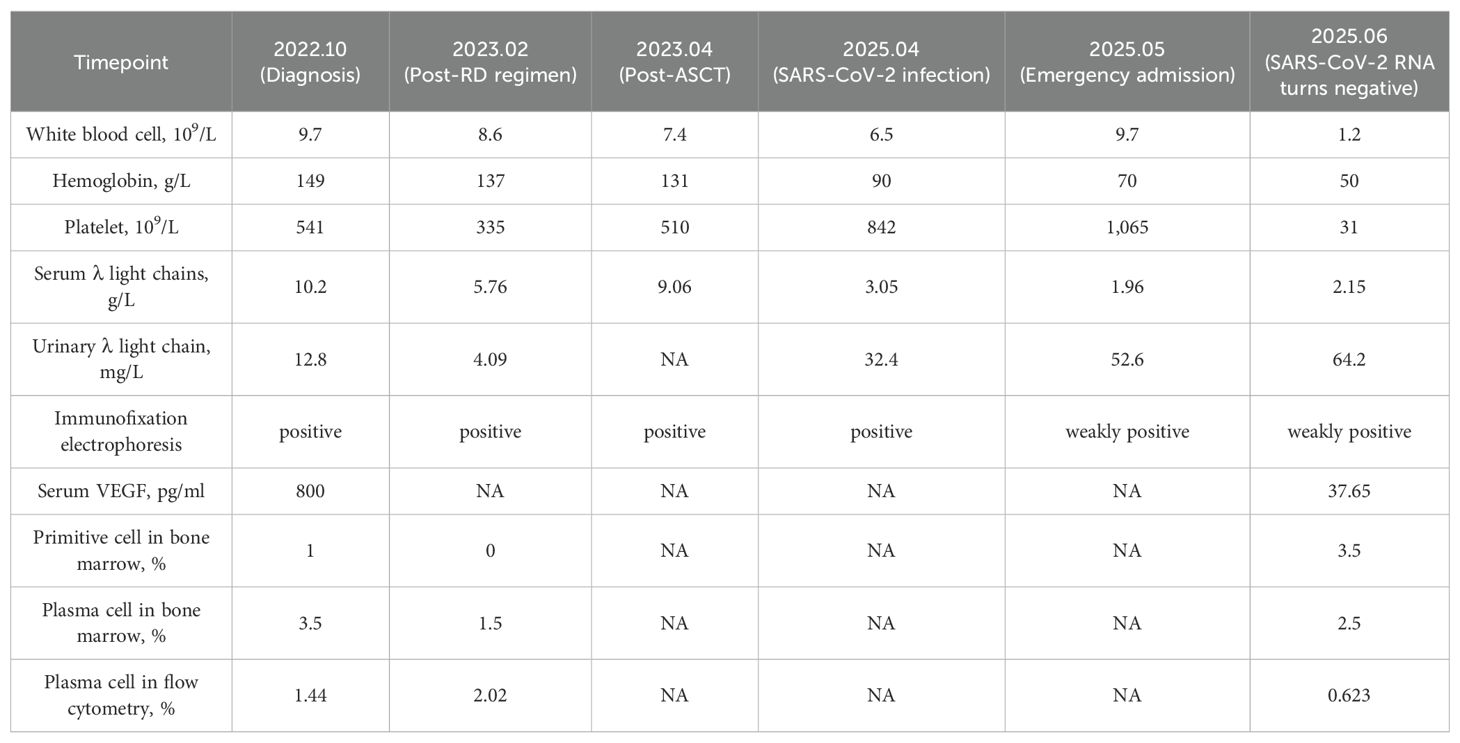
In October 2022, a 51-year-old male presented to Zhejiang Provincial Hospital of Traditional Chinese Medicine with recurrent numbness and weakness of all four limbs for over two years. In 2019, evaluation at the First Affiliated Hospital of Zhejiang University had included lumbar puncture, revealing cerebrospinal fluid protein elevated to 1.06 g/L, and electromyography (EMG) demonstrating motor fiber damage of peripheral nerves in both upper and lower limbs, predominantly in the lower limbs. On the basis of these findings, chronic inflammatory demyelinating polyneuropathy (CIDP) was diagnosed. The symptoms were improved significantly after treatment with methylprednisolone, but following four months of dose reduction, his condition recurred, presenting with bilateral lower limb weakness accompanied by burning pain in the soles, limiting his ambulation to 300 meters. Symptoms were attenuated by methylprednisolone escalation and exacerbated by dose reduction. On October 28, 2022, the patient visited the Department of Neurology at our hospital. Upon routine examination, he discovered an increase in light chains. After consulting with the Department of Hematology, relevant laboratory and imaging data are presented in Table 1, Figure 2 and the following records.
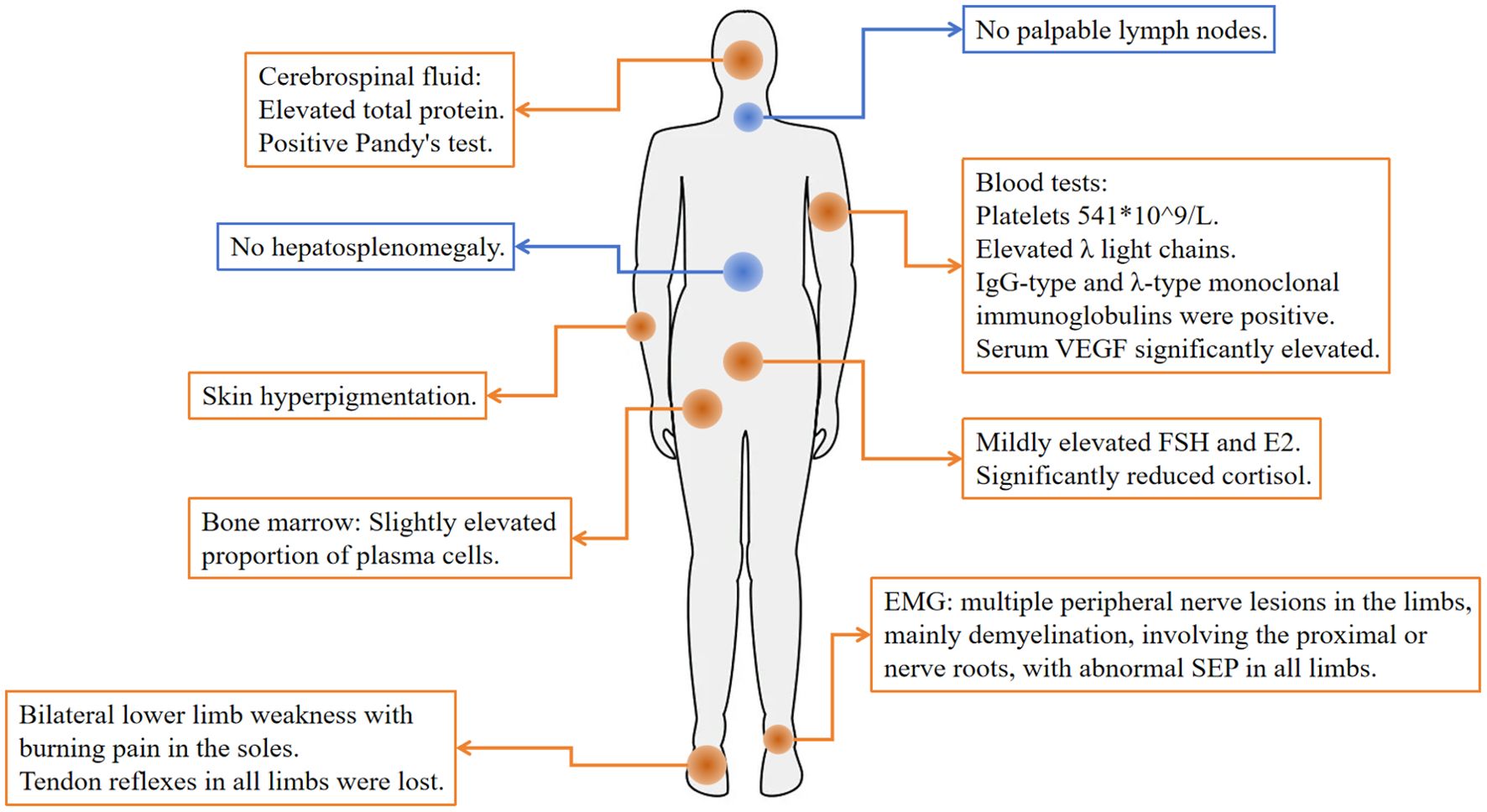
Figure 2. Physical examination and abnormal tests at diagnosis. Orange represents abnormal, and blue represents normal.
Physical examination: The patient exhibited skin hyperpigmentation without palpable lymph nodes or hepatosplenomegaly. Tendon reflexes were absent in all limbs, but muscle strength and tone were normal, bilateral deep and superficial sensation were intact, and no pathological reflexes were elicited. Relevant laboratory tests: Blood routine: white blood cells 9.7×109/L, hemoglobin 149 g/L, platelets 541×109/L. Biochemistry: creatinine 87 μmol/L, globulin 26.4 g/L, AST 13 U/L, ALT 15 U/L. Both serum and urine λ light chains were elevated (10.20 g/L and 12.8 mg/L), and immunofixation electrophoresis demonstrated IgG-λ monoclonal bands. Serum vascular endothelial growth factor (VEGF) was significantly elevated (800 pg/mL). Follicle-stimulating hormone (FSH) and estradiol (E2) were modestly elevated, whereas cortisol was significantly reduced (66.7 nmol/L at 8:00 AM). Adrenocorticotropic hormone (ACTH) and thyroid hormones remained within the normal range. Cerebrospinal fluid analysis showed elevated total protein (0.687 g/L), a positive Pandy test, and a normal white blood cell count. EMG showed multifocal, predominantly demyelinating, peripheral nerve lesions affecting proximal segments and nerve roots, and somatosensory evoked potentials (SEP) were abnormal in all limbs. Bone marrow routine showed a slightly elevated proportion of plasma cells (3.5%-4.5%), with preserved trilineage hematopoiesis (Figures 3A, B). Flow cytometry revealed abnormal plasma cells accounting for about 1.44% of nucleated cells. Cytogenetics yielded a normal 46, XY karyotype. Epstein-Barr virus (EBV) DNA and Cytomegalovirus (CMV) DNA were not detected. Ultrasonography of the thyroid, lymph nodes, liver, gallbladder, pancreas, spleen, and kidneys disclosed no abnormalities.
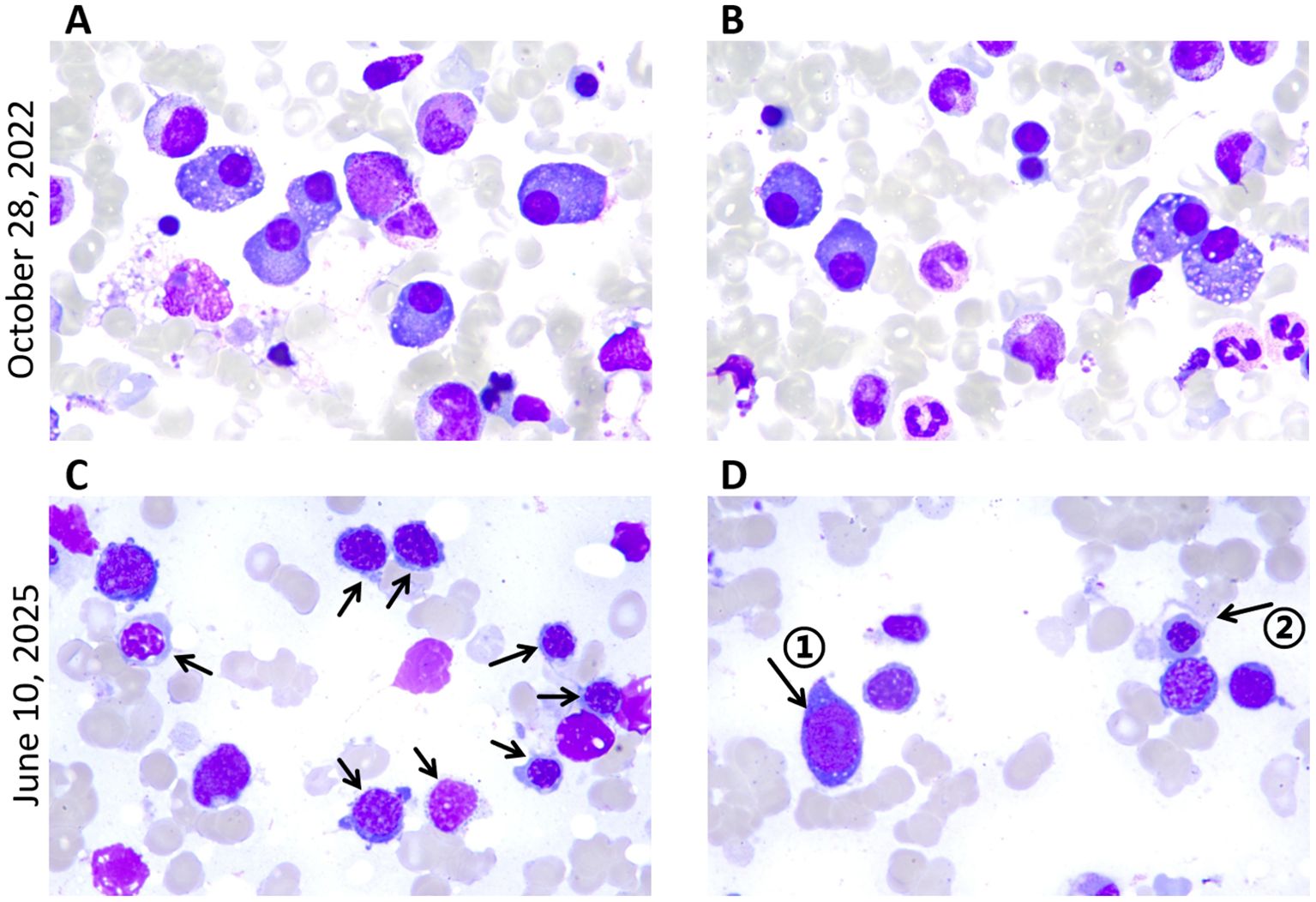
Figure 3. Bone marrow smears at diagnosis of POEMS syndrome and MDS. Wright-Giemsa staining of bone marrow smear from posterior superior iliac spine (magnification, x1,000); (A, B) High proportion of plasma cells. (C) Erythroid dysplasia: relatively high proportion of erythroblasts, with active erythroid hyperplasia and chromatin condensation. Arrows denote abnormal erythroblasts. (D) Arrow ① denotes the primitive cell, and arrow ② denotes megaloblast-like cells.
According to the 2014 consensus of the International Myeloma Working Group (IMWG), the patient was diagnosed with POEMS syndrome (3, 4) and classified as intermediate-risk in prognosis stratification (5). After one cycle of RD regimen (lenalidomide 25 mg qd×21d + dexamethasone 45 mg qd×d1-4, d9-12, d17-20), ASCT was scheduled. Mobilization was performed with cyclophosphamide 2.8 g×2d plus G-CSF starting February 6, 2023. On February 20, 198 mL of peripheral blood stem cells were collected, including 3.83×108/kg nucleated cells, 1.76×108/kg mononuclear cells, and 4.24×106/kg CD34+ cells. On March 20, 2023, pre-transplant conditioning consisted of melphalan 350 mg×1d, and autologous cells were reinfused on March 22, with successful engraftment. After transplantation, persistent plantar numbness was noted, with monoclonal IgG-λ bands remaining detectable by immunofixation (Table 1). Because only a partial response (PR) had been achieved, lenalidomide 25 mg daily was initiated as maintenance starting December 28, 2023 (6).
In April 2025, the patient appeared with fever and diarrhea, tested positive for SARS-CoV-2 RNA, and experienced recurrent fever despite antiviral treatment. On May 8, 2025, the patient presented to our emergency department. The blood routine showed white blood cells 9.7×109/L, hemoglobin 70 g/L, platelets 1065×109/L, and CRP 59.01 mg/L. SARS-CoV-2 RNA remained positive, with EBV DNA, CMV DNA, and other virological tests negative. Chest CT revealed bibasilar infiltrates with bilateral pleural effusion. After inpatient antiviral therapy, the lung infection significantly improved and the virus was cleared. However, on June 7, pancytopenia was detected: white blood cells 1.2×109/L, hemoglobin 50 g/L, and platelets 31×109/L (Supplementary Table S1, Supplementary Figure S1). Bone marrow routine revealed 3.5% myeloblasts with multilineage dysplasia (Table 1; Figures 3C, D). Flow cytometry showed abnormal plasma cells accounting for about 0.623% of nucleated cells. Cytogenetics yielded a 46, XY karyotype, and next-generation sequencing (NGS) indicated a TP53 mutation at 79.91% variant allele frequency. Based on these findings, a diagnosis of myelodysplastic syndrome with multilineage dysplasia and low blasts (MDS-LB) was established (7) and classified as intermediate-risk according to IPSS-R (8). Subsequently, the patient underwent haploidentical allogeneic hematopoietic stem cell transplantation (allo-HSCT) and achieved successful engraftment.
3 Discussion
POEMS syndrome is a disease caused by abnormal proliferation of plasma cells, with a chronic progression. The 5-year progression-free survival (PFS) and overall survival (OS) rates are 58% and 78%, respectively (9). However, its diverse symptoms often lead to misdiagnosis. For instance, early peripheral neuropathy is often misdiagnosed as CIDP or Guillain-Barré syndrome (GBS) (10, 11). As a result, definitive diagnosis is normally delayed by 12–16 months, during which multi-organ damage and irreversible neurologic deficits may develop, even compromising survival (12, 13). In this case, CIDP was initially diagnosed in the Department of Neurology, and the correct diagnosis was not established until 2 years later.
The pathogenesis of POEMS syndrome remains incompletely understood. Although VEGF is the cytokine most strongly associated with disease activity (14), responses to anti-VEGF therapy suggest that it acts as a downstream mediator rather than the primary driver (15, 16). B-cell dysregulation is also a key factor in disease initiation. At present, anti-plasma-cell therapy is the main treatment, including ASCT, lenalidomide plus dexamethasone (RD regimen), bortezomib plus dexamethasone (BD regimen), etc., with complete response (CR) rates all exceeding 40% (2, 17, 18). Among them, ASCT shows superior efficacy in improving neuropathy, inducing more durable M-protein and VEGF responses, and prolonging PFS and OS (2).
MDS is a group of malignant clonal myeloid diseases characterized by ineffective hematopoiesis, peripheral blood cytopenia, and a high risk of progression to acute myeloid leukemia (AML) (19). It is classified as either primary or secondary. Secondary MDS accounts for 10-15% of cases (20), primarily resulting from prior exposure to cytotoxic drugs, ionizing radiation, chemical toxins, viral infections, autoimmune diseases, or malignant tumors etc (21). Therapy-related MDS and MDS arising after aplastic anemia are the most frequently recognized subtypes (22).
MDS secondary to POEMS syndrome is rarely reported, as the two disorders are assigned to distinct hematopoietic lineages (myeloid and plasma cell (B-cell)) respectively. How both lineages become clonally involved in the same patient remains unresolved. The potential reasons are discussed below under the headings of cytotoxic therapy, genetic predisposition, and viral infection.
3.1 Cytotoxic therapy
Due to the scarcity of sMN reports in POEMS syndrome, analysis can be conducted through homologous plasma cell disorders such as multiple myeloma (MM), Waldenström’s macroglobulinemia (WM), and monoclonal gammopathy of undetermined significance (MGUS). They are all associated with abnormally proliferating monoclonal plasma cells and produce M proteins. In MM, the incidence of secondary primary hematologic malignancies (SPHM) approaches 7%, while therapy-related myeloid neoplasms (t-MN) occur in approximately 3% (23–27). Within their treatment regimens, alkylating agents (e.g., cyclophosphamide, melphalan) and lenalidomide are considered as drivers of secondary MDS.
A study indicated that secondary acute lymphoblastic leukemia (ALL) is more frequently observed in MM patients who have not undergone ASCT, whereas sMN predominates among transplant recipients (28). The incidence of secondary MDS/AML after ASCT was approximately 1-2%, with a median interval from auto-HCT to MDS/AML diagnosis of 58.5 months (range 6.2–206.5 months) (29, 30). As early as 1970, Kyle et al. proposed that melphalan might play a role in the pathogenesis of acute leukemia (31). Current research consistently indicates that cyclophosphamide and high-dose melphalan used in pre-transplant conditioning induce mutational accumulation in myeloid cells, thereby increasing the risk of SPHM (25, 26, 32, 33). Moreover, melphalan is recognized to possess greater mutagenic potential than other alkylating agents (23).
Lenalidomide is also associated with a risk of inducing secondary malignancies (28, 34). In 2012, McCarthy et al. demonstrated that lenalidomide significantly increased the risk of secondary malignancies in MM patients after ASCT (35). A meta-analysis of seven RCTs by Palumbo et al. found an increased 5-year cumulative incidence of SPHM in lenalidomide-treated patients (3.1% vs. 1.4%) (36), with t-MN accounting for the majority at approximately 3% (37, 38). Additional studies have shown that lenalidomide significantly increases the TP53 mutation rate in t-MN patients (39–41). TP53 is a critical tumor suppressor gene, repairing DNA damage and promoting apoptosis of malignant cells. Roughly 10% of MDS patients harbor functionally deficient TP53 mutations (42). Additionally, t-MN often involves multiple genetic defects, resulting in shorter survival and poorer prognosis compared to primary malignancies, with a median overall survival of only 11.8 months from t-MN diagnosis (30, 43, 44). These findings suggest that genetic testing should be integrated into treatment planning for POEMS patients, given the potential risk of secondary malignancies.
3.2 Genetic predisposition
Whether sMN is attributable solely to exposure factors such as chemotherapy and radiotherapy is still a controversy. After studying secondary malignancies in plasma cell disorders, researchers found that a large number of myeloid neoplasms are diagnosed concurrently with or shortly after MM (45). Given that t-MN typically exhibits a latency period of 5–7 years (41), this finding supports the existence of an intrinsic predisposition to sMN in plasma cell disorders. Another argument is that the risk of secondary AML/MDS is increased by 11.51-fold in MM, and 8-fold in WM, despite the fact that they have divergent therapeutic approaches (46). Furthermore, MGUS without standard therapies still remains a 2.4-fold increase in the risk of developing MDS, with a median latency period of 14.4 months (47, 48). Notably, M-protein levels >1.5 g/dL confer a higher risk than those below the threshold, implicating elevated paraprotein burden as a risk factor (48). These studies collectively indicate that there are treatment-unrelated mechanisms in the progression from plasma cell disorders to myeloid neoplasms.
It has been proposed that abnormal myeloid clones already exist at the time of diagnosis or before maintenance therapy, and that continuous cytotoxic treatment selectively expands them (49–51). Mutations including TP53, TET2, DNMT3A, and ASXL1 are frequently detected before treatment, among which TP53 and TET2 showed significant expansion after sMN development, which are not only related to the pathogenesis of myeloid neoplasms, but also portend a poor prognosis (52). In this case, a high TP53 mutation had been detected at 79.91% upon MDS diagnosis. Unfortunately, the genetic testing was not performed at the time of POEMS syndrome diagnosis, so the timing of the mutation cannot be ascertained.
3.3 Viral infection
Multiple microbial pathogens, primarily viruses, are implicated in the pathogenesis of hematologic malignancies (53). In this case, SARS-CoV-2 infection was tested before prolonged pancytopenia, prompting speculation that the virus may have acted as an additional driver of MDS.
SARS-CoV-2 is reported to affect multiple organs, including the hematopoietic system, with both quantitative and qualitative abnormalities observed (54). Abnormalities in peripheral blood cell counts include anemia, leukopenia/leukocytosis, thrombocytopenia/thrombocytosis, etc (55). Morphologic changes mainly occur in leukocytes and platelets, including changes typically observed in MDS, such as dysplastic neutrophils and giant platelets (56). Normally, primitive cells account for 1-5% of nucleated cells in bone marrow, and they are generally absent from peripheral blood. However, there was a report of 2% primitive cells detected in the peripheral blood of COVID-19 patients (54). Nevertheless, most of these abnormalities can recover after viral clearance, and are not regarded as definitive evidence that SARS-CoV-2 directly induces MDS.
A kidney transplant recipient had been reported that developed EBV and CMV viremia following SARS-CoV-2 infection, and was diagnosed with MDS six months later (57). The authors propose that immune dysregulation induced by SARS-CoV-2 promoted clonal expansion and MDS progression, or that MDS was triggered by the reactivation of EBV or CMV. Although the immune mechanisms triggered by SARS-CoV-2 remain incompletely understood, heterogeneous activation of CD8+ or CD4+ T-cell pathways is documented, yet the selection process is unclear (58). The activated immune system produces dysregulated cytokines, thereby creating a bone marrow microenvironment conducive to clonal expansion (59, 60). Multiple viruses are implicated in the development of MDS, including CMV (61), human T-cell lymphotropic virus type 1 (HTLV-1) (62), parvovirus B19 (63), and human herpesvirus 6 (HHV-6) (64). Among them, reactivation of herpes viruses, including EBV, CMV, and HHV-6, is frequently observed in COVID-19 patients (65). An additional report describes a 30-year-old man in whom MDS was diagnosed concurrently with SARS-CoV-2 infection (66), but the causal link remains to be verified.
4 Conclusion
This case describes a rare clinical course of MDS secondary to POEMS syndrome after ASCT, and discusses potential reasons for the lineage transformation, including cytotoxic therapy, genetic predisposition, and SARS-CoV-2 infection. In the future, multicenter clinical studies are required to further clarify the epidemiological characteristics and risk factors for MDS secondary to POEMS syndrome, which will help improve the long-term management of patients with POEMS syndrome and may also inform prevention strategies for myeloid neoplasms secondary to other plasma cell disorders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ML: Writing – original draft. ND: Writing – review & editing. ZT: Writing – review & editing. BY: Writing – review & editing. JC: Writing – review & editing. SL: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the “Jianbing Lingyan + X” Science and Technology Program of Zhejiang Province (Grant No. 2024C03190).
Acknowledgments
The authors gratefully acknowledge the patient for allowing us to publish the clinical case, and the medical staff of the Department of Hematology, The First Affiliated Hospital of Zhejiang Chinese Medical University, for their meticulous patient care and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1711447/full#supplementary-material
References
1. Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, and Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Med (Baltimore). (1980) 59:311–22. doi: 10.1097/00005792-198007000-00006
2. Zhao H, Huang X-F, Gao X-M, Cai H, Zhang L, Feng J, et al. What is the best first-line treatment for POEMS syndrome: autologous transplantation, melphalan and dexamethasone, or lenalidomide and dexamethasone? Leukemia. (2019) 33:1023–9. doi: 10.1038/s41375-019-0391-2
4. Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. (2014) 32:587–600. doi: 10.1200/JCO.2013.48.7934
5. Wang C, Huang X-F, Cai Q-Q, Cao X-X, Duan M-H, Cai H, et al. Prognostic study for overall survival in patients with newly diagnosed POEMS syndrome. Leukemia. (2017) 31:100–6. doi: 10.1038/leu.2016.168
6. Gao X-M, Li A-A, Zhao H, Shen K-N, and Li J. Long-term outcomes of newly diagnosed POEMS syndrome patients who received first-line lenalidomide-based therapy. Haematologica. (2024) 109:3776–80. doi: 10.3324/haematol.2024.285282
7. Greenberg PL, Stone RM, Abaza Y, Al-Kali A, Anand S, Ball B, et al. NCCN guidelines® Insights: myelodysplastic syndromes, version 2.2025. J Natl Compr Canc Netw. (2025) 23:66–75. doi: 10.6004/jnccn.2025.0013
8. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. (2012) 120:2454–65. doi: 10.1182/blood-2012-03-420489
9. Kourelis TV, Buadi FK, Gertz MA, Lacy MQ, Kumar SK, Kapoor P, et al. Risk factors for and outcomes of patients with POEMS syndrome who experience progression after first-line treatment. Leukemia. (2016) 30:1079–85. doi: 10.1038/leu.2015.344
10. Plasma Cell Disease Group, Chinese Society of Hematology, Chinese Medical Association, and Chinese Myeloma Committee-Chinese Hematology Association. Chinese expert consensus on the diagnosis and treatment of POEMS syndrome (2025). Zhonghua Xue Ye Xue Za Zhi. (2025) 46:389–96. doi: 10.3760/cma.j.cn121090-20241219-00578
11. Dispenzieri A. POEMS syndrome: Update on diagnosis, risk-stratification, and management. Am J Hematol. (2023) 98:1934–50. doi: 10.1002/ajh.27081
12. Keddie S, Foldes D, Caimari F, Baldeweg SE, Bomsztyk J, Ziff OJ, et al. Clinical characteristics, risk factors, and outcomes of POEMS syndrome: A longitudinal cohort study. Neurology. (2020) 95:e268–79. doi: 10.1212/WNL.0000000000009940
13. Lee K, Kourelis T, Tschautscher M, Warsame R, Buadi F, Gertz M, et al. Capillary leak phenotype as a major cause of death in patients with POEMS syndrome. Leukemia. (2025) 39:703–9. doi: 10.1038/s41375-024-02489-z
14. Wang C, Huang X-F, Cai Q-Q, Cao X-X, Cai H, Zhou D, et al. Remarkable expression of vascular endothelial growth factor in bone marrow plasma cells of patients with POEMS syndrome. Leuk Res. (2016) 50:78–84. doi: 10.1016/j.leukres.2016.09.017
15. Sekiguchi Y, Misawa S, Shibuya K, Nasu S, Mitsuma S, Iwai Y, et al. Ambiguous effects of anti-VEGF monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol Neurosurg Psychiatry. (2013) 84:1346–8. doi: 10.1136/jnnp-2012-304874
16. Cerri F, Falzone YM, Riva N, and Quattrini A. An update on the diagnosis and management of the polyneuropathy of POEMS syndrome. J Neurol. (2019) 266:258–67. doi: 10.1007/s00415-018-9068-4
17. Li J, Huang X-F, Cai Q-Q, Wang C, Cai H, Zhao H, et al. A prospective phase II study of low dose lenalidomide plus dexamethasone in patients with newly diagnosed polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome. Am J Hematol. (2018) 93:803–9. doi: 10.1002/ajh.25100
18. He H, Fu W, Du J, Jiang H, and Hou J. Successful treatment of newly diagnosed POEMS syndrome with reduced-dose bortezomib based regimen. Br J Haematol. (2018) 181:126–8. doi: 10.1111/bjh.14497
19. Chinese Society of Hematology, Chinese Medical Association. Expert consensus on diagnosis and treatment of myelodysplastic syndrome (2014). Zhonghua Xue Ye Xue Za Zhi. (2014) 35:1042–8. doi: 10.3760/cma.j.issn.0253-2727.2014.11.023
20. Adès L, Itzykson R, and Fenaux P. Myelodysplastic syndromes. Lancet. (2014) 383:2239–52. doi: 10.1016/S0140-6736(13)61901-7
21. Calvete O, Mestre J, Jerez A, and Solé F. The secondary myelodysplastic neoplasms (MDS) jigsaw. Cancers (Basel). (2023) 15:1483. doi: 10.3390/cancers15051483
22. Thalambedu N, Mohan Lal B, Harbaugh B, Alapat DV, Gaddam M, Gentille Sanchez CG, et al. Unveiling myelodysplastic syndromes: exploring pathogenic mechanisms and therapeutic advances. Cancers (Basel). (2025) 17:508. doi: 10.3390/cancers17030508
23. Poh C, Keegan T, and Rosenberg AS. Second primary Malignancies in multiple myeloma: A review. Blood Rev. (2021) 46:100757. doi: 10.1016/j.blre.2020.100757
24. Usmani SZ, Sawyer J, Rosenthal A, Cottler-Fox M, Epstein J, Yaccoby S, et al. Risk factors for MDS and acute leukemia following total therapy 2 and 3 for multiple myeloma. Blood. (2013) 121:4753–7. doi: 10.1182/blood-2012-11-466961
25. Nadiminti K, Sidiqi MH, Meleveedu K, Alkhateeb HB, Hogan WJ, Litzow M, et al. Characteristics and outcomes of therapy-related myeloid neoplasms following autologous stem cell transplantation for multiple myeloma. Blood Cancer J. (2021) 11:63. doi: 10.1038/s41408-021-00454-y
26. Radivoyevitch T, Dean RM, Shaw BE, Brazauskas R, Tecca HR, Molenaar RJ, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome after autotransplants for lymphomas and plasma cell myeloma. Leuk Res. (2018) 74:130–6. doi: 10.1016/j.leukres.2018.07.016
27. Dong N, Ye B, and Liu S. Investigating additional Malignancy rates and prognostic factors in multiple myeloma patients: a Surveillance, Epidemiology, and End Results (SEER) database retrospective cohort study. Transl Cancer Res. (2025) 14:2192–206. doi: 10.21037/tcr-24-1721
28. Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. (2022) 387:132–47. doi: 10.1056/NEJMoa2204925
29. Krishnan AY, Mei M, Sun C-L, Thomas SH, Teh JB, Kang T, et al. Second primary Malignancies after autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. (2013) 19:260–5. doi: 10.1016/j.bbmt.2012.09.023
30. Yalniz FF, Greenbaum U, Pasvolsky O, Milton DR, Kanagal-Shamanna R, Ramdial J, et al. Characteristics and outcomes of patients with multiple myeloma who developed therapy-related acute myeloid leukemia and myelodysplastic syndrome after autologous cell transplantation. Transplant Cell Ther. (2024) 30:205. doi: 10.1016/j.jtct.2023.06.015
31. Kyle RA, Pierre RV, and Bayrd ED. Multiple myeloma and acute myelomonocytic leukemia. N Engl J Med. (1970) 283:1121–5. doi: 10.1056/NEJM197011192832101
32. Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. (2020) 52:1219–26. doi: 10.1038/s41588-020-00710-0
33. Gaddam SJ, Grewal US, Thevuthasan S, and Ramadas P. Therapy-related myeloid neoplasms: a real-world pharmacovigilance analysis (1970-2023). Ann Hematol. (2024) 103:6051–2. doi: 10.1007/s00277-024-05985-5
34. Geyer MB, Shaffer BC, Bhatnagar B, Mims AS, Klein V, Dilip D, et al. Lenalidomide-associated B-cell ALL: clinical and pathologic correlates and sensitivity to lenalidomide withdrawal. Blood Adv. (2023) 7:3087–98. doi: 10.1182/bloodadvances.2022009212
35. McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. (2012) 366:1770–81. doi: 10.1056/NEJMoa1114083
36. Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary Malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. (2014) 15:333–42. doi: 10.1016/S1470-2045(13)70609-0
37. Fernández-Caballero M, Salmerón D, Dolores Chirlaque M, Chen-Liang TH, Hurtado AM, García Malo MD, et al. Increasing therapy-related myeloid neoplasms in multiple myeloma. Eur J Clin Invest. (2019) 49:e13050. doi: 10.1111/eci.13050
38. Pemmaraju N, Shah D, Kantarjian H, Orlowski RZ, Nogueras González GM, Baladandayuthapani V, et al. Characteristics and outcomes of patients with multiple myeloma who develop therapy-related myelodysplastic syndrome, chronic myelomonocytic leukemia, or acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2015) 15:110–4. doi: 10.1016/j.clml.2014.07.001
39. Sperling AS, Guerra VA, Kennedy JA, Yan Y, Hsu JI, Wang F, et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood. (2022) 140:1753–63. doi: 10.1182/blood.2021014956
40. Singhal D, Kutyna MM, Hahn CN, Shah MV, and Hiwase DK. Therapy-related myeloid neoplasms: complex interactions among cytotoxic therapies, genetic factors, and aberrant microenvironment. Blood Cancer Discov. (2024) 5:400–16. doi: 10.1158/2643-3230.BCD-24-0103
41. Shah MV, Arber DA, and Hiwase DK. TP53 -mutated myeloid neoplasms: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. (2025) 100 Suppl 4:88–115. doi: 10.1002/ajh.27655
42. Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, et al. TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discov. (2022) 12:2516–29. doi: 10.1158/2159-8290.CD-22-0332
43. Jelloul FZ, Quesada AE, Yang RK, Li S, Wang W, Xu J, et al. Clinicopathologic features of therapy-related myeloid neoplasms in patients with myeloma in the era of novel therapies. Mod Pathol. (2023) 36:100166. doi: 10.1016/j.modpat.2023.100166
44. Costa A, Pilo F, Pettinau M, Piras E, Targhetta C, Rojas R, et al. Impact of primary cancer history and molecular landscape in therapy-related myeloid neoplasms. Front Oncol. (2025) 15:1563990. doi: 10.3389/fonc.2025.1563990
45. Castillo JJ and Gertz MA. Secondary Malignancies in patients with multiple myeloma, Waldenström macroglobulinemia and monoclonal gammopathy of undetermined significance. Leuk Lymphoma. (2017) 58:773–80. doi: 10.1080/10428194.2016.1217527
46. Varettoni M, Tedeschi A, Arcaini L, Pascutto C, Vismara E, Orlandi E, et al. Risk of second cancers in Waldenström macroglobulinemia. Ann Oncol. (2012) 23:411–5. doi: 10.1093/annonc/mdr119
47. Roeker LE, Larson DR, Kyle RA, Kumar S, Dispenzieri A, and Rajkumar SV. Risk of acute leukemia and myelodysplastic syndromes in patients with monoclonal gammopathy of undetermined significance (MGUS): a population-based study of 17 315 patients. Leukemia. (2013) 27:1391–3. doi: 10.1038/leu.2013.34
48. Mailankody S, Pfeiffer RM, Kristinsson SY, Korde N, Bjorkholm M, Goldin LR, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood. (2011) 118:4086–92. doi: 10.1182/blood-2011-05-355743
49. Maia C, Puig N, Cedena M-T, Goicoechea I, Valdes-Mas R, Vazquez I, et al. Biological and clinical significance of dysplastic hematopoiesis in patients with newly diagnosed multiple myeloma. Blood. (2020) 135:2375–87. doi: 10.1182/blood.2019003382
50. Escure G, Fournier E, Saade C, Issa LHB, Arib I, Tilmont R, et al. Small myeloid subclones are present at diagnosis of multiple myeloma in patients who develop secondary myelodysplastic syndromes. Haematologica. (2024) 109:1289–92. doi: 10.3324/haematol.2023.284050
51. Cooperrider JH, Karaoglu DA, Kubicki T, Jiang CR, Postich E, Shimamoto K, et al. Evolution of clonal hematopoiesis on and off lenalidomide maintenance for multiple myeloma. Leukemia. (2025) 39:2285–8. doi: 10.1038/s41375-025-02707-2
52. Guarnera L, Pascale MR, Hajrullaj H, Cristiano A, Mallegni F, Onorato A, et al. The role of clonal progression leading to the development of therapy-related myeloid neoplasms. Ann Hematol. (2024) 103:3507–17. doi: 10.1007/s00277-024-05803-y
53. Sadrzadeh H, Abtahi SM, and Fathi AT. Infectious pathogens and hematologic Malignancy. Discov Med. (2012) 14:421–33.
54. Lüke F, Orsó E, Kirsten J, Poeck H, Grube M, Wolff D, et al. Coronavirus disease 2019 induces multi-lineage, morphologic changes in peripheral blood cells. EJHaem. (2020) 1:376–83. doi: 10.1002/jha2.44
55. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
56. Ahnach M, Ousti F, Nejjari S, Houssaini MS, and Dini N. Peripheral blood smear findings in COVID-19. Turk J Haematol. (2020) 37:310–02. doi: 10.4274/tjh.galenos.2020.2020.0262
57. Juric I, Katalinic L, Furic-Cunko V, and Basic-Jukic N. Myelodysplastic syndrome in a kidney transplant recipient after SARS-CoV-2 infection: can SARS-CoV-2 induce myelodysplastic syndrome? Int Urol Nephrol. (2022) 54:1775–6. doi: 10.1007/s11255-021-03069-1
58. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. (2020) 369:eabc8511. doi: 10.1126/science.abc8511
59. Raza A. Hypothesis: myelodysplastic syndromes may have a viral etiology. Int J Hematol. (1998) 68:245–56. doi: 10.1016/s0925-5710(98)00051-6
60. Kristinsson SY, Björkholm M, Hultcrantz M, Derolf ÅR, Landgren O, and Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. (2011) 29:2897–903. doi: 10.1200/JCO.2011.34.8540
61. Raza A. Initial transforming event in myelodysplastic syndromes may be viral: case for cytomegalovirus. Med Oncol. (1998) 15:165–73. doi: 10.1007/BF02821935
62. Karlic H, Möstl M, Mucke H, Pavlova B, Pfeilstöcker M, and Heinz R. Association of human T-cell leukemia virus and myelodysplastic syndrome in a central European population. Cancer Res. (1997) 57:4718–21.
63. Urban C, Lackner H, Müller E, Benesch M, Strenger V, Sovinz P, et al. Stem cell transplantation in 6 children with parvovirus B19- induced severe aplastic anaemia or myelodysplastic syndrome. Klin Padiatr. (2011) 223:332–4. doi: 10.1055/s-0031-1287839
64. Kagialis-Girard S, Durand B, Mialou V, Pagès M-P, Galambrun C, Bertrand Y, et al. Human herpesvirus 6 infection and transient acquired myelodysplasia in children. Pediatr Blood Cancer. (2006) 47:543–8. doi: 10.1002/pbc.20667
65. Simonnet A, Engelmann I, Moreau A-S, Garcia B, Six S, El Kalioubie A, et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. (2021) 51:296–9. doi: 10.1016/j.idnow.2021.01.005
Keywords: POEMS syndrome, myelodysplastic syndrome, autologous stem cell transplantation, secondary myeloid neoplasms, TP53 mutation, SARS-CoV-2, case report
Citation: Le M, Dong N, Tan Z, Ye B, Chen J and Liu S (2025) Case Report: Secondary myelodysplastic syndrome following autologous stem cell transplantation in a patient with POEMS syndrome. Front. Immunol. 16:1711447. doi: 10.3389/fimmu.2025.1711447
Received: 23 September 2025; Accepted: 31 October 2025;
Published: 18 November 2025.
Edited by:
Zheng Zhong, University of Michigan, United StatesReviewed by:
Chongjian Gao, University of Michigan, United StatesYaozhong Liu, University of Michigan, United States
Copyright © 2025 Le, Dong, Tan, Ye, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyan Liu, Y2F0bWk3OTAzQDE2My5jb20=
 Miaoya Le
Miaoya Le Nanxi Dong
Nanxi Dong Zhengwei Tan
Zhengwei Tan Baodong Ye
Baodong Ye Junfa Chen2
Junfa Chen2 Shuyan Liu
Shuyan Liu