- 1Rheumatology Unit, Department of Medicine-DIMED, University of Padova, Padua, Italy
- 2Rheumatology Unit, Department of Clinical and Biological Sciences, University of Torino, Turin, Italy
A 55-year-old woman was diagnosed with anti-melanoma differentiation-associated gene 5 (anti-MDA5) positive dermatomyositis (DM) in 2020, having low grade fever, weight loss, arthritis in small joint of hands, erythematous-desquamative lesions on hands, cuticle dystrophy and severe skin ulcerations. Firstly, she was treated with cyclosporine (CsA), soon discontinued due to gastrointestinal intolerance. She was subsequently treated with steroid pulses, hydroxychloroquine (HCQ) and mycophenolate (MMF), without improvement. In March 2021 she started therapy with intravenous immunoglobulins (IVIG) and prostanoids, leading to ulcer improvement, but stopped due to gastrointestinal intolerance. A chest high resolution computed tomography (HRCT) done in June 2021 showed interstitial lung disease (ILD). In September 2021 rituximab (RTX) was stopped at the first infusion due to gastrointestinal intolerance. From January 2022 the patient also started to walk with difficulty due to development of deep asthenia. Therapy with various Jak inhibitors was started (first tofacitinib, then baricitinib, finally upadacitinib), leading to improvement of cutaneous ulcers, but stopped every time after a few months due to gastrointestinal intolerance and dizziness. In August 2023 during hospitalization a spirometry showed reduction of diffusion capacity of the lung for the carbon monoxide (DLCO). In June 2024, in consideration of poor disease control and refractoriness of the disease (loss of appetite and weight, worsening of asthenia which forced the patient into a wheelchair, persistence of polyarthritis, skin ulcers, alopecia, Gottron’s signs, radiological progression of ILD), she was hospitalized again and Anifrolumab (ANI) was started in July 2024 (300 mg IV every four weeks). After four infusions the patient reported improved appetite with significant weight gain, resolution of arthritis and disappearance of cutaneous ulcers, Gottron’s sign and alopecia. In February 2025, after seven ANI infusions, a HRCT demonstrated a significant radiological improvement of the ILD compared to 2024, and spirometry showed significant improvement of DLCO compared to 2023. In this period, no adverse effects were observed from the new therapy. After twelve total infusions, constitutional, articular and cutaneous involvement remained in good control. This case suggests the potential efficacy of ANI in refractory anti-MDA5-positive DM not only on skin manifestation, but also on articular and lung involvement.
1 Introduction
Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of autoimmune diseases characterized by inflammatory involvement primarily of skeletal muscle. They can also affect many other organs such as skin, joints, lung and heart. DM is a subset of IIM typically characterized by muscle and skin inflammation (1). Myositis specific and associated autoantibodies can be detected in most of the patients affected by IIM, such as TIF1-γ, NXP-2, Mi-2, MDA5 and SAE, usually identifying a typical clinical phenotype (2).
Anti-MDA5 positive DM is a distinct clinical subtype of DM characterized by autoantibodies targeting MDA5, most often associated with hypo or amyopathic features, severe cutaneous manifestations including skin ulcers and a high risk of rapidly-progressive interstitial lung disease (RP-ILD) development (3, 4). The pathogenesis involves a combination of innate immune activation, autoimmunity and vasculopathy, but it is believed that the type I interferon (IFN) pathway plays a central role, as its signature has been shown to be upregulated in anti-MDA5 + DM (5). A triggering event, frequently a viral infection, occurs in a genetically predisposed individual leading to excessive MDA5 production, its mislocalization within cells, tissue injury and a loss of immune tolerance. Once anti-MDA5 antibodies are generated, they lead to inflammation and tissue damage, potentially initiating a cytokine storm (6). In this context, elevated IFN levels could contribute to blood vessel damage through toxic effects on the endothelium (7). The body’s attempt to repair the ongoing damage and cope with ischemia may then lead to the recruitment of macrophages, the development of fibrosis, and ultimately irreversible organ damage (8).
Anti-MDA5 DM treatment is individualized in consideration of the heterogeneity of clinical manifestations and their severity. Steroids typically are the first line of therapy, in refractory cases followed by immunomodulants or immunosuppressants such as hydroxychloroquine (HCQ), methotrexate (MTX), azathioprine (AZA), micophenolate mofetil (MMF), calcineurin inhibitors (CNIs), JAK inhibitors (JAK-i), intravenous immunoglobulin (IVIG) and rituximab (RTX) (9, 10). Most severe cases, especially due to RP-ILD, are treated with steroid pulses, plasma exchange therapy (PEX) and cyclophosphamide (CYC), eventually in association with CNIs (11, 12).
ANI is a human monoclonal antibody to type I IFN receptor subunit 1 currently used in patients with SLE, due to the interferogenic signature involved in its pathogenesis (13). Therefore, it is a promising therapy for interferogenic manifestations of anti-MDA5 + DM such as cutaneous, articular and pulmonary involvement. Specifically, it could be effective in treating the most severe manifestations of this subset of DM such as cutaneous ulcers, polyarthritis and ILD, maybe also the RP-ILD.
In this paper we describe a patient affected by anti-MDA5 + DM with cutaneous, articular and pulmonary involvement refractory or intolerant to multiple immunosuppressive therapies successfully treated with ANI.
2 Case description
A 55-year-old Caucasian female patient, non-smoker, with no past medical history nor familiarity for autoimmune and rheumatologic diseases, was diagnosed with anti-MDA5-positive DM in July 2020. The disease started in February 2020 with Gottron’s papules on hands and erythematous rash on the neck and arms, followed by hand arthralgias, fever (maximum body temperature of 38.2 °C) and progressive weight loss. At the time complete immunological panel was performed, showing negativity of anti-nuclear antibodies (ANA), anti-extractable nuclear antigens antibodies (anti-ENA), rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPAs), and positivity of anti-MDA5 antibodies. The only relevant result in routine blood exams was a slight polyclonal hypergammaglobulinemia with a concomitant mild increase of serum immunoglobulin G (IgG). Complete blood count (CBC), inflammatory markers, muscle enzymes, transaminases, proteins, albumin, IgM, IgA, creatinine and urine exam were in normal range.
In July 2020 she started prednisone 50 mg daily (1 mg/kg/day) with mild improvement. In the same period a capillaroscopy was performed, showing a pattern of a specific microangiopathy. Suspecting an amyopathic DM, at the end of July 2020 she was hospitalized. During her hospitalization many examinations were performed. Joint ultrasound (US) confirmed metacarpophalangeal (MCP) and proximal interphalangeal (PIP) arthritis. Chest X-ray, mammography and abdomen US showed no significant abnormalities. On 4th August 2020 she self-discharged, at that time she was taking prednisone 25 mg daily (0,5 mg/kg/day). In the same month she performed HRCT that showed no ILD, lymphadenopathies nor pleural effusion. In October 2020 she started therapy with CsA, discontinued shortly after due to gastrointestinal discomfort. In November 2020 she was treated with steroid pulses (methylprednisolone 250 mg daily for three days) without significant improvement on the cutaneous and articular manifestations. Furthermore, in the same period cutaneous ulcers appeared on hands, elbows, shoulders and heels (Figure 1), therefore in December 2020 she started therapy with MMF 3 g daily and HCQ 300 mg daily. As the patient developed oral ulcers, MMF was temporarily reduced to 2 g daily. Since March 2021, in consideration of persistent cutaneous and articular manifestations, monthly IVIG dosed at 0,4 mg/kg and prostanoids infusions were added, leading to ulcers mild improvement. Unfortunately, both IVIG and prostanoids were stopped in June 2021 due to post-infusion headache and gastrointestinal intolerance. Concomitantly, a new HRCT showed the development of mild ILD. In September 2021 she was treated with RTX, but the first infusion was prematurely stopped due to malaise and gastrointestinal intolerance. Therefore, in January 2022 she started therapy with JAK-inhibitor (tofacitinib) that led to significant ulcers improvement; this drug was stopped in August 2022 for hypotension and gastrointestinal intolerance. In consideration of the good response to JAK-inhibitors, baricitinib was started, with stabilization of cutaneous manifestation, however nausea, headache and vertigo occurred and the drug was stopped after few weeks with regression of the symptoms. Due to recurrent gastrointestinal disorders, onset of loss of appetite with progressive weight loss and persistence of polyarthritis, in August 2023 the patient was admitted to our Rheumatology department. To evaluate her gastrointestinal symptoms, she carried out numerous investigations such as an abdominal ultrasound, an abdomen CT scan, an EGDS and an MR cholangiography, which ruled out possible organic causes. She also performed a gastroenterological evaluation, which confirmed attribution of the symptoms to the medications the patient was taking at the time, as no other causes were identified. During hospitalization, hand polyarthritis was confirmed by clinical and US evaluation. She also performed spirometry that showed severe DLCO reduction (27%).

Figure 1. Cutaneous involvement before ANI (left side) and correspondent localizations after ANI (right side).
Subsequently, she was treated with steroid therapy (oral prednisone 10 mg/day) with transient benefit on articular and constitutional symptoms and in October 2023 another JAK-inhibitor was started (upadacitinib), without significant improvement of cutaneous ulcers and arthritis and with persistence of gastrointestinal discomfort. Therefore, the drug was stopped after few months.
In June 2024, in consideration of poor disease control and refractoriness (loss of appetite and weight, deep asthenia with forced ambulation in a wheelchair, persistence of hand polyarthritis with swollen joint count -TJC- of 18 and swollen joint count -SJC- of 15, skin ulcers and alopecia with cutaneous dermatomyositis disease area and severity index -CDASI- 5), she was hospitalized again. During her stay HRCT showed radiological progression of ILD. Therefore, she started therapy with ANI (300 mg IV every four weeks), firstly administered in July 2024 (Figure 2). At this time, she was taking 15 mg per day of prednisone, and the steroid decalage was started. No other disease modifying anti-rheumatic drugs (DMARDs) were prescribed concomitantly. After four infusions, the patient reported improvement of asthenia with recovery of walking (no more need of wheelchair), increased appetite with subsequent 6 kg weight gain (from 38 to 44 kg), marked improvement of arthritis (TJC: 2, SJC: 0) and disappearing of cutaneous manifestations, including ulcers (Figure 1) (CDASI 0). At this time, the patient was assuming 5 mg per day of prednisone, in further progressive decalage.
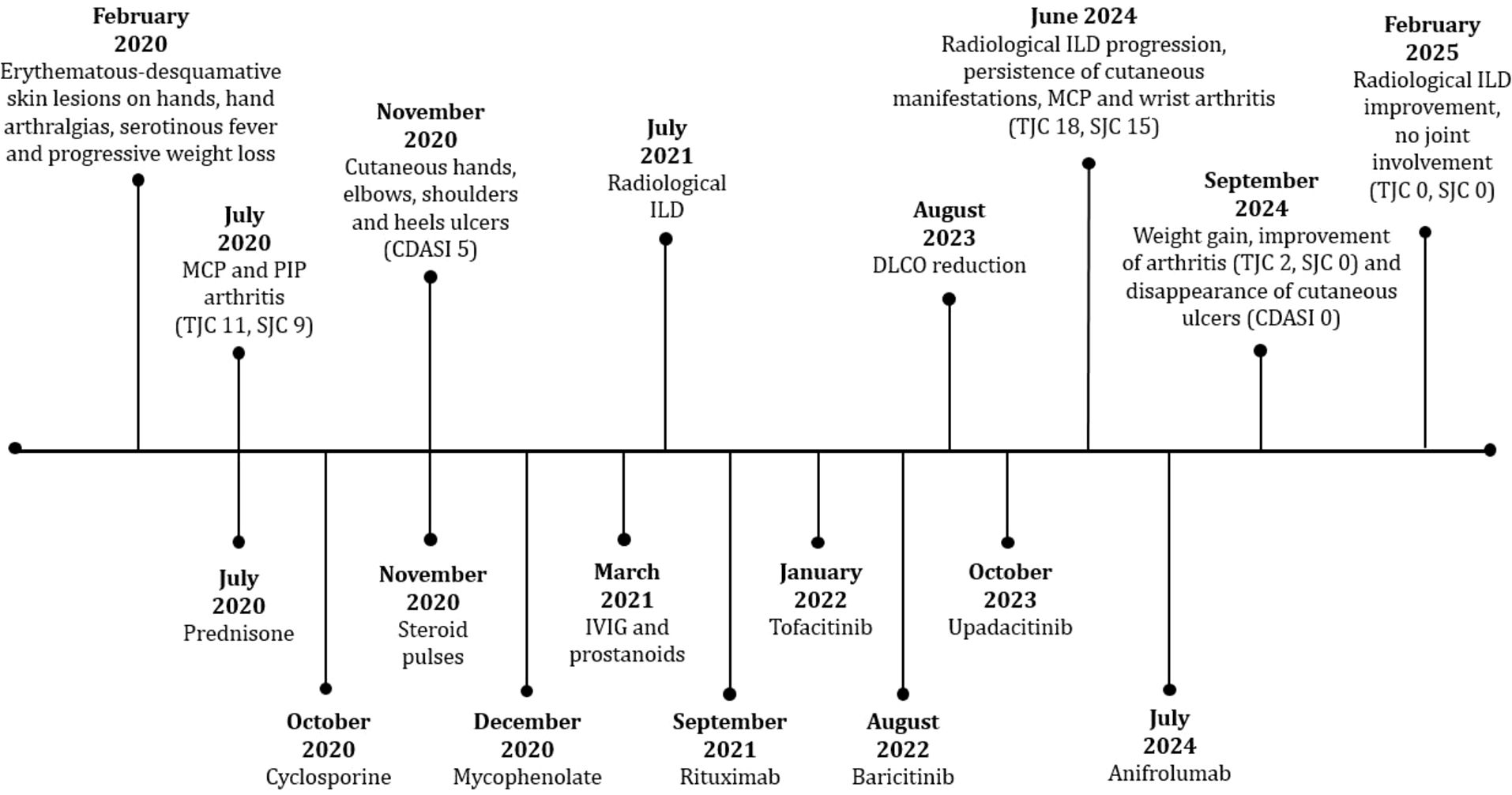
Figure 2. Timeline showing manifestation of the disease and corresponding treatments from diagnosis to starting of therapy.
Furthermore, in February 2025 the patient repeated HRCT and the case was discussed in a multidisciplinary medical team with dedicated pneumologists and radiologists, showing a significant improvement of the ILD compared to 2024 (Figure 3), and spirometry showing significant improvement of DLCO (47%) compared to 2023. In Table 1 we summarized main clinical, radiological and functional data across different years of follow-up.
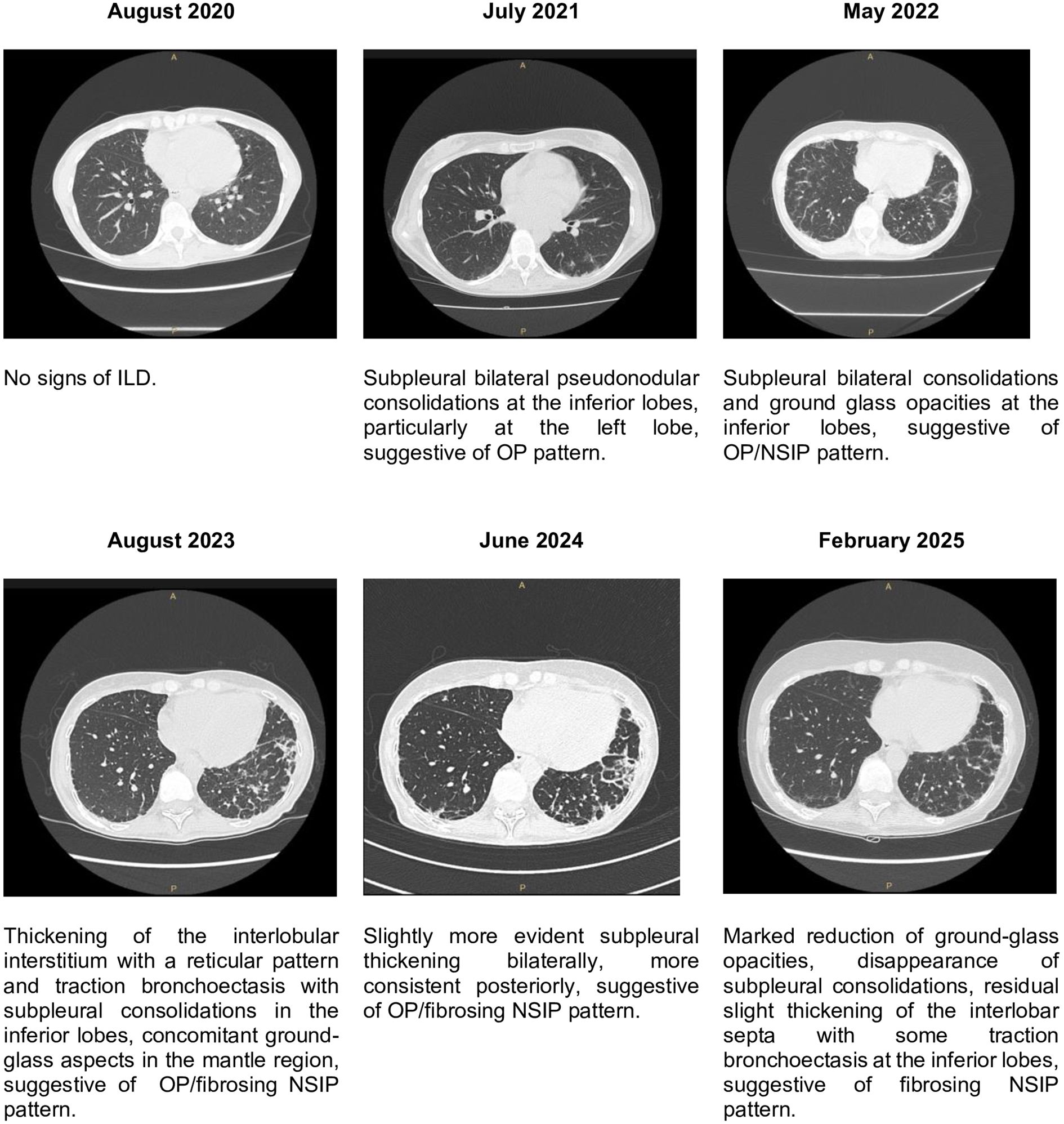
Figure 3. Images and radiological reports of lungs HRCT, showing the more important findings and the predominant ILD pattern.
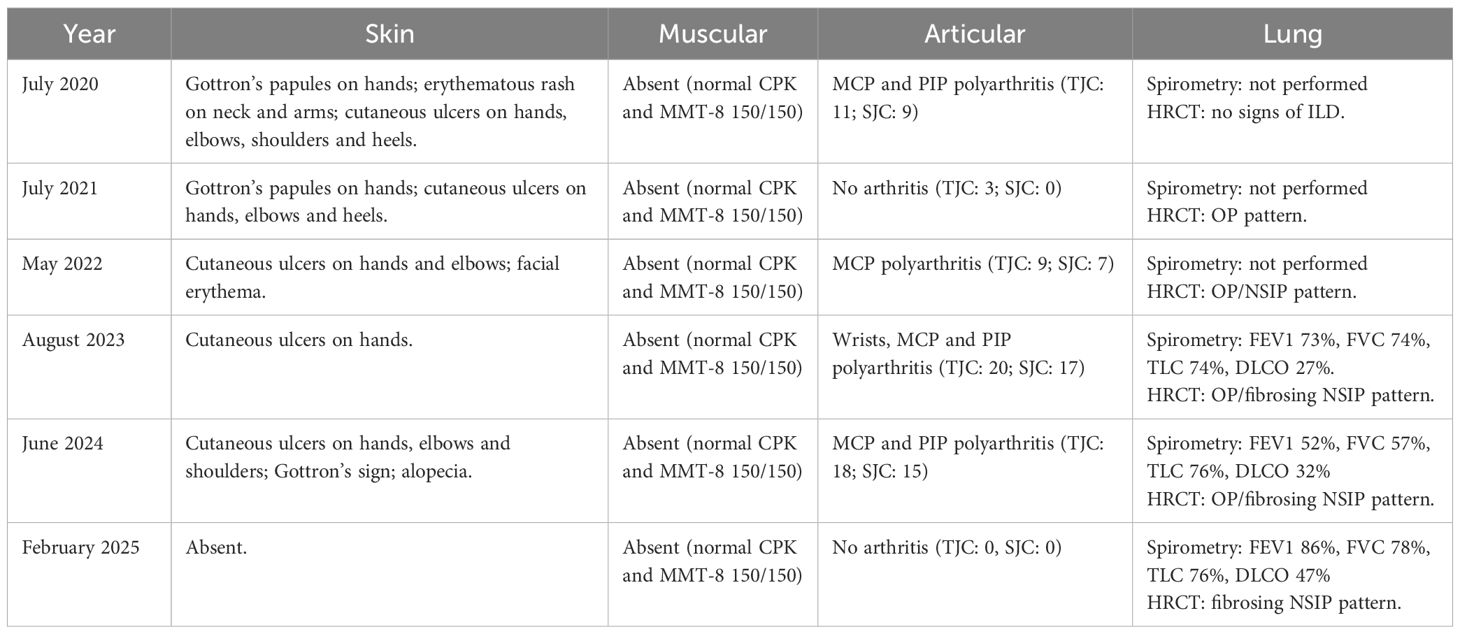
Table 1. clinical, radiological and functional data during follow-up, sorted by type of involvement.
In this period of time, no adverse effects were observed from the new therapy. In July 2025, after a total of twelve ANI infusions, steroid was completely discontinued. In September 2025, after fourteen total infusions, constitutional, articular and cutaneous involvement persisted in good control, with absence of arthritis (TJC: 0, SJC: 0) and CDASI 0.
3 Diagnosis, therapy, follow-up, outcomes
From the diagnosis the patient was evaluated with regular ambulatory follow-up, six-monthly in periods of clinical stability and three-monthly in periods of poor disease control.
For each medical visit the patient repeated periodical laboratory exams, including complete blood count (CBC), C-reactive protein (CRP), creatine phospho-kinase (CPK), myoglobin, aldolase, lactate dehydrogenase (LAD), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine and urine exam.
Disease activity was assessed differently for each organ involvement.
Cutaneous involvement was assessed by clinical evaluation, using CDASI. After starting in July 2024, a significant cutaneous improvement was observed, as shown by CDASI reduction from 5 (Gottron’s sign, skin ulcerations and alopecia) to 0.
Articular involvement was evaluated clinically (TJC and SJC) and radiologically, with musculoskeletal US and conventional X-ray. After starting ANI, joint involvement improved as documented by complete disappearance of arthralgia and arthritis, consequently TJC decreased from 18 to 0 and SJC from 15 to 0.
Lung involvement was assessed by clinical evaluation, periodical global spirometry with DLCO and HRCT. Clinically she never experienced exertional dyspnoea neither dry cough nor other respiratory symptoms. In August 2020 HRCT showed no signs of ILD. In June 2021 HRCT showed initial ILD with organizing pneumonia (OP) pattern at inferior lobes. Subsequent HRCT performed in 2022, 2023 and 2024 showed slow ILD progression during years. In August 2023 spirometry showed mild reduction of lung volumes (FEV1 73%, FVC 74%, TLC 74%) and severe reduction of DLCO (27%). In March 2024 spirometry worsened with moderate volume reduction (FEV1 52%, FVC 57%, TLC 76%) and stability of DLCO (32%). In January 2025, after 6 infusions of ANI spirometry demonstrated stability of lung volumes (FEV1 86%, FVC 78%, TLC 76%) and significant improvement of DLCO (47%).
In February 2025 the last HRCT showed a significant ILD improvement compared to the previous one of 2024, confirmed by a multidisciplinary medical team with dedicated pneumologists and radiologists. We report that in August 2023, during the hospitalization in our ward, an echocardiogram was performed showing a concentric left ventricle remodeling and a mild reduction of ejection fraction (EF, 43%) in absence of altered chamber volumes, indirect signs of increased pressure in the pulmonary circulation (sPAP 23 mmHg) and pericardial effusion. No cardiac symptoms were reported at the time and cardiac enzymes (troponin I, NT-proBNP) were normal. A cardiologic evaluation suggested the execution of coronary CT and cardiac MRI, however the patient refused further investigations. In June 2024 another echocardiogram showed stability of EF (43%) and of the remaining echocardiographic findings.
Muscle involvement was evaluated clinically by performing manual muscle test 8 (MMT-8), that always resulted normal (150/150), and serologically with myocytolysis enzymes (CK, myoglobin, aldolase, LDH, AST, ALT), that always resulted in normal range. Moreover, the patient never reported muscle weakness.
4 Discussion and literature review
Based on our research, this is the first case report showing efficacy of ANI not only in skin manifestations, but also in lung and articular involvement in IIMs. Furthermore, to the best of our knowledge there are no data on the efficacy of in anti-MDA5+ DM.
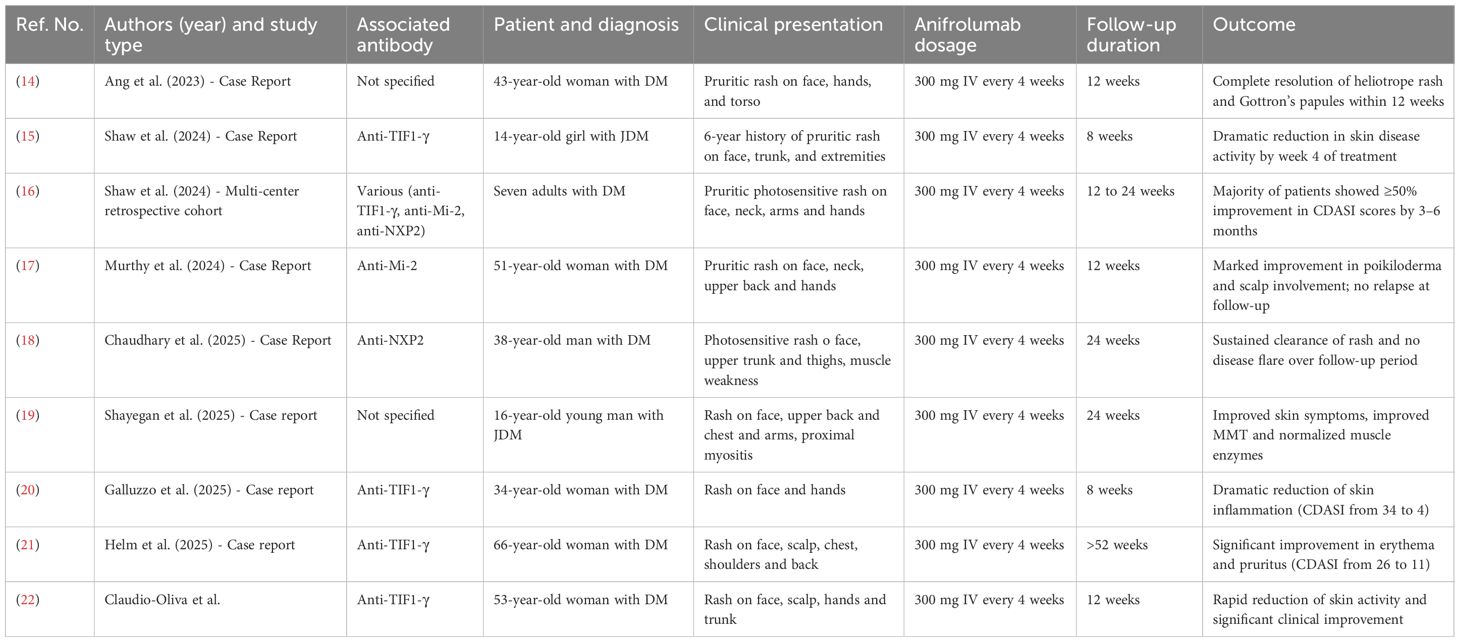
Some case reports (14–19) have been published about the effectiveness of ANI in treating refractory cutaneous and muscular involvement of DM, as summarized in Table 2.
Of these patients, three had juvenile DM (15, 19) while the others had adult-onset DM (16–18, 20); two patients had paraneoplastic DM (14, 22). Of the six cases that reported autoantibodies, four had anti-TIF1-γ autoantibody (15, 20–22), one had anti-Mi2 autoantibody (17) and one had anti-NXP2 autoantibody (18), while none of them had anti-MDA5-positive autoantibodies. All patients were treated with 300 mg of ANI intravenously every four weeks, and none of them reported adverse effects related to ANI during the follow-up period.
All case reports described the efficacy of ANI in DM as mainly limited to cutaneous manifestations with significant reduction of CDASI, when reported, only one case also described improvement in muscle involvement (19). In contrast, in our anti-MDA5–positive DM patient ANI demonstrated broader efficacy, significantly reducing arthritis and interstitial lung disease and improving skin disease not only in classical erythematous manifestations but also in ulcers, thus extending the spectrum of observed therapeutic benefits. Notably, in our patient marked improvement of refractory ulcers, polyarthritis and lung involvement was achieved soon after starting of ANI and in the same period no other therapeutic changes were made and the patient was in treatment only with ANI. For these reasons, it is reasonable to conclude that these improvements were achieved by ANI. Limitations arise from the nature of case report, which shows a single clinical case. To demonstrate the efficacy of anifrolumab in IIMs, we are awaiting the outcome of the ongoing randomized controlled phase III trial (23).
5 Patient perspective
After ANI initiation the patient’s quality of life significantly improved, regaining autonomy in walking and leaving the wheelchair. It has already been 14 months since the start of treatment and she is satisfied since until now it was the only treatment both effective and well tolerated among her medical history.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FP: Writing – review & editing, Conceptualization, Data curation, Writing – original draft, Investigation. LB: Data curation, Writing – review & editing, Investigation. MB: Writing – review & editing. ADG: Writing – review & editing. BM: Data curation, Writing – review & editing, Investigation. RD: Writing – review & editing. MZ: Writing – review & editing. EZ: Writing – review & editing. MG: Writing – review & editing. AD: Supervision, Writing – review & editing. LI: Data curation, Supervision, Writing – review & editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Prime. (2021) 7:86. doi: 10.1038/s41572-021-00321-x
2. Ghirardello A, Gatto M, Franco C, Zanatta E, Padoan R, Ienna L, et al. Detection of myositis autoantibodies by multi-analytic immunoassays in a large multicenter cohort of patients with definite idiopathic inflammatory myopathies. Diagn (Basel). (2023) 13:3080. doi: 10.3390/diagnostics13193080
3. Fuzzi E, Gatto M, Zen M, Franco C, Zanatta E, Ghirardello A, et al. Anti-MDA5 dermatomyositis: an update from bench to bedside. Curr Opin Rheumatol. (2022) 34:365–73. doi: 10.1097/BOR.0000000000000908
4. Zanatta E, Cocconcelli E, Castelli G, Giraudo C, Fraia AS, De Zorzi E, et al. Interstitial lung disease with and without progressive fibrosing phenotype in patients with idiopathic inflammatory myopathies: data from a large multicentric cohort. RMD Open. (2023) 9:e003121. doi: 10.1136/rmdopen-2023-003121
5. Gasparotto M, Franco C, Zanatta E, Ghirardello A, Zen M, Iaccarino L, et al. The interferon in idiopathic inflammatory myopathies: Different signatures and new therapeutic perspectives. A Literat Rev Autoimmun Rev. (2023) 22:103334. doi: 10.1016/j.autrev.2023.103334
6. Lu X, Peng Q, and Wang G. Anti-MDA5 antibody-positive dermatomyositis: pathogenesis and clinical progress. Nat Rev Rheumatol. (2024) 20:48–62. doi: 10.1038/s41584-023-01054-9
7. He C, Chen J, Luo X, and Yan B. Evaluation of biomarkers related to endothelial dysfunction: proof of vasculopathy in anti-melanoma differentiation-associated gene 5 dermatomyositis. Clin Exp Rheumatol. (2021) 39:151–7. doi: 10.55563/clinexprheumatol/ubov8b
8. Horiike Y, Suzuki Y, Fujisawa T, Yasui H, Karayama M, Hozumi H, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatol (Oxford). (2019) 58:2143–52. doi: 10.1093/rheumatology/kez185
9. Gamba A, Depascale R, Zanatta E, Ienna L, Cruciani C, Gatto M, et al. Effectiveness and safety of low dose Rituximab as remission-maintenance treatment for patients with refractory idiopathic inflammatory myopathies: results of a retrospective study from a monocentric cohort. Clin Rheumatol. (2024) 43:3167–74. doi: 10.1007/s10067-024-07079-z
10. Nalotto L, Iaccarino L, Zen M, Gatto M, Borella E, Domenighetti M, et al. Rituximab in refractory idiopathic inflammatory myopathies and antisynthetase syndrome: personal experience and review of the literature. Immunol Res. (2013) 56:362–70. doi: 10.1007/s12026-013-8408-9
11. Fujisawa T. Management of myositis-associated interstitial lung disease. Med (Kaunas). (2021) 57:347. doi: 10.3390/medicina57040347
12. Shih PC, Lee YH, Huo AP, and Leong PY. Advance in management of anti-MDA5 antibody-positive dermatomyositis: Conquering therapeutic obstacles. Int J Rheum Dis. (2024) 27:e15401. doi: 10.1111/1756-185X.15401
13. Siebeler R, de Winther MPJ, and Hoeksema MA. The regulatory landscape of macrophage interferon signaling in inflammation. J Allergy Clin Immunol. (2023) 152:326–37. doi: 10.1016/j.jaci.2023.04.022
14. Ang PS, Ezenwa E, Ko K, and Hoffman MD. Refractory dermatomyositis responsive to anifrolumab. JAAD Case Rep. (2023) 43:27–9. doi: 10.1016/j.jdcr.2023.10.023
15. Shaw KS, Reusch DB, Castillo RL, Hashemi KB, Sundel R, Dedeoglu F, et al. Rapid improvement in recalcitrant cutaneous juvenile dermatomyositis with anifrolumab treatment. JAMA Dermatol. (2024) 160:237–8. doi: 10.1001/jamadermatol.2023.4744
16. Shaw KS, Hashemi KB, Castillo RL, Rainone E, Ho AW, Kahn PJ, et al. Anifrolumab in recalcitrant cutaneous dermatomyositis: A multicenter retrospective cohort study. J Am Acad Dermatol. (2024) 91:1217–9. doi: 10.1016/j.jaad.2024.07.1491
17. Srinivasa Murthy M and Haikal A. A Novel Treatment for Skin Manifestations in Dermatomyositis: A Case Report. Cureus. (2024) 16:e66704. doi: 10.7759/cureus.66704
18. Chaudhary S, Baumer M, Upton L, Ong S, Smith K, West D, et al. Recalcitrant dermatomyositis treated with anifrolumab. ACR Open Rheumatol. (2025) 7:e11792. doi: 10.1002/acr2.11792
19. Shayegan LH, Shaw KS, Wieschhoff GG, Ezeh N, Kim YJ, Shahriari N, et al. Improvement in dermatomyositis-associated muscle disease with anifrolumab. Br J Dermatol. (2025) 192:1126–8. doi: 10.1093/bjd/ljaf064
20. Galluzzo C, Pipitone N, Chiapparoli I, and Salvarani C. Successful therapy of refractory dermatomyositis skin rash with anifrolumab. Clin Exp Rheumatol. (2025). doi: 10.55563/clinexprheumatol/haftre
21. Helm MF, Maglakelidze N, Shamloul N, and Foulke G. Successful treatment of refractory cutaneous dermatomyositis with anifrolumab. Int J Dermatol. (2025) 64:1661–3. doi: 10.1111/ijd.17675
22. Claudio-Oliva A, Viedma-Martínez M, Gallo-Pineda G, Prieto-Rozados M, Villegas-Romero I, Jiménez-Gallo D, et al. Anifrolumab use in refractory dermatomyositis: a case report and literature review. Clin Exp Dermatol. (2025) 50:874–6. doi: 10.1093/ced/llae476
23. AstraZeneca. A multicenter, parallel-group, double-blind, 2-arm, phase III study to investigate the efficacy and safety of anifrolumab administered as subcutaneous injection and added to standard of care compared with placebo added to standard of care in adult participants with idiopathic inflammatory myopathies (Polymyositis and dermatomyositis) (2025). Available online at: https://clinicaltrials.gov/study/NCT06455449. Report No.: NCT06455449. Disponibile su. (Accessed October 31, 2025).
Keywords: anti-MDA5, anifrolumab, ILD, skin ulcers, case report
Citation: Pettorossi F, Brischigliaro L, Bracalenti M, Di Gregorio A, Moccaldi B, Depascale R, Zen M, Zanatta E, Gatto M, Doria A and Iaccarino L (2025) Efficacy of anifrolumab on skin, joint and lung involvement in anti-MDA5 positive dermatomyositis: a case report. Front. Immunol. 16:1717766. doi: 10.3389/fimmu.2025.1717766
Received: 02 October 2025; Accepted: 17 November 2025; Revised: 31 October 2025;
Published: 27 November 2025.
Edited by:
Rosaria Talarico, Azienda Ospedaliero Universitaria Pisana, ItalyReviewed by:
Tsuneyasu Yoshida, Kyoto University, JapanMaja Stojanovic, University of Belgrade, Serbia
Copyright © 2025 Pettorossi, Brischigliaro, Bracalenti, Di Gregorio, Moccaldi, Depascale, Zen, Zanatta, Gatto, Doria and Iaccarino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Iaccarino, bHVjYS5pYWNjYXJpbm9AdW5pcGQuaXQ=
 Federico Pettorossi1
Federico Pettorossi1 Laura Brischigliaro
Laura Brischigliaro Margherita Zen
Margherita Zen Elisabetta Zanatta
Elisabetta Zanatta Mariele Gatto
Mariele Gatto Andrea Doria
Andrea Doria Luca Iaccarino
Luca Iaccarino