Abstract
For decades, sex disparities have been acknowledged to influence immune regulation in health and disease throughout the life span, and contribute to variations in epidemiology, pathophysiology, manifestation, progression, and therapeutic response in multiple disorders. However, the underlying cellular and molecular mechanisms governing these disparities remain understudied. This review articulates the effects of sex as critical regulators of the major causes of morbidity and mortality in diseases. We summarize the key factors driving sex differences, including sexual hormones and sex chromosomes, as well as elaborate how these factors influence physiology and disease, especially by modulating the function and fate of immune cells. Our aim is to disentangle the intricacies of sexually-differentiated immune responses within physiological and pathological contexts, thereby establishing the groundwork for precision medicine approaches customized to sex-specific requirements in clinical settings.
1 Introduction
Historically, the diagnosis, prevention, and treatment of disease has originated from research mostly conducted on male cells, male animals, as well as men (1). This is probably due to the safety consideration for women of childbearing age, and their offspring, which are always excluded from most clinical trials. Therefore, on the assumption that female and male cells and/or animals were biologically identical, medical research has concentrated on male physiology and evidence-based medicine depends on clinical studies done primarily in men. However, the neglect of sex disparities is sometimes harmful to human health. For example, in the United States, ten prescription drugs were withdrawn from usage between 1997 and 2001, among which eight had been proved to pose more risk for women than men (2). As a result, sex-based biology and medicine should be viewed as a core consideration in medical study and translational science, rather than a research area of interest.
Actually, accumulating evidence has revealed intricate differences in disease prevalence, severity, and outcomes between men and women (3). It has been noticed that males often present more intense inflammatory responses (4, 5), while females tend to exhibit a higher incidence of chronic autoimmune diseases (6, 7). Specifically, acute respiratory distress syndrome (ARDS) patients show male predominance (females accounting for 28%-38%) and consistently exhibit a higher annual mortality from ARDS compared to women (5, 8). Except for infants under 9 years old, women are less commonly affected by ARDS across all age groups, demonstrating a protective effect of sex disparity extending from puberty to post-menopause (5, 9). The recent coronavirus disease 2019 pandemic also underscore the sex difference in the outcomes, with men showing an elevated risk for severe infection and increased mortality rather than women (10). Conversely, over 50% of patients with autoimmune diseases (e.g., systemic lupus erythematosus [SLE] and Sjogren’s syndrome) are women, and these diseases are among the third most common disease categories (7). Furthermore, sex differences also exist in leukemia, particularly in acute myeloid leukemia, with females exhibiting lower incidence and mortality rates, along with lower healthcare expenditures (11, 12). Regrettably, most of the current understandings center on the epidemiological and clinical differences, with the underlying mechanisms remaining largely unexplored.
Efforts are being made to elucidate the factors contributing to sexual dimorphism in health and disease. Among them, sex hormones and chromosomal differences are thought to play significant roles. Study has pointed out that the zenith of rheumatoid arthritis onset in female aligns with menopause, characterized by a precipitous decline in estrogen level, implying an intricate link between sex hormones and disease etiology (13). Another representative example is bronchial asthma. Serum testosterone levels in both males and females are inversely correlated with the incidence (14, 15); while fluctuations in levels of estradiol and progesterone during the menstrual cycle are associated with the worsened symptoms in female patients (16–18). However, our understanding of key mechanisms shaping the sex dimorphism, especially in functional reprogramming of immune cells, remains surprisingly sparse (6, 19, 20). This review proposes a critical shift from descriptive cataloguing of immune sexual dimorphism toward a mechanistic, cell-type-resolved understanding. We uniquely systematically map sex differences across the immune continuum, linking hormonal and chromosomal drivers to functional outcomes; decipher the underlying molecular circuits (e.g., receptor signaling, transcription factors) that enact sex-specific functions in physiology and disease; and integrate the modulating effects of age, menopause, and environmental exposures. By synthesizing these layered insights, we provide a translational framework to rationally guide the development of sex-tailored immunotherapies and advance precision medicine.
2 Classification, distribution, function, and signaling pathways of sex-determining system
Sex-determining system linking to physiological differences is primarily divided into sexual hormones and sex chromosomes. The former mainly includes estrogen, progestogen, and androgen, all of which are steroid hormones synthesized from cholesterol. The initial step of the synthesis involves the conversion of cholesterol to pregnenolone by cytochrome P450 side-chain cleavage enzyme 11A1 on mitochondrial membrane. Pregnenolone subsequently follows two pathways: one is transformed into progesterone via 3β-hydroxysteroid dehydrogenase; the other is first converted to 17α-hydroxypregnenolone and then to dehydroepiandrosterone (DHEA) by cytochrome P450 17A1. DHEA can be further turned into androstenedione, which is then converted to testosterone and aromatized into estrogen by aromatase (21, 22). Meanwhile, sex chromosomes, X and Y, are essential in determining sex disparities: the Y chromosome, with sex-determining Region Y (SRY) gene, triggers male development and differentiation (23) (Figure 1).
Figure 1

Schematic diagram of the sex hormone pathway. Signaling pathways of estrogen, progesterone, and androgen, including their interactions with receptors and subsequent biological functions. E2, Estradiol; ER, Estrogen receptor; GPR, G protein-coupled receptor; ERE, Estrogen response element; P, Progesterone; PR, progesterone receptor; mPR, membrane-progesterone receptor; T, Testosterone; DT, Dihydrotestosterone; AR, Androgen receptor; ARE, Androgen response element.
2.1 Estrogen
Estrogen is a widely studied female hormone and exists in three forms: estrone, estradiol, and estriol. Among them, estradiol is the most potent and prevalent in premenopausal women, playing a crucial role in the development of reproductive organs and maintenance of tissue homeostasis. Besides, estradiol and estrone can be interconverted by 17β-hydroxysteroid dehydrogenase, and estriol is primarily produced from the other two during pregnancy (24). Estrogen predominantly derives from ovary, corpus luteum, and placenta during reproductive periods; while is generated from kidneys, adipose tissue, skin, and brain in pre-puberty or post-menopause stage. In females, estrogen level fluctuates significantly across different physiological periods, with estradiol peaking during the menstrual cycle, estriol rising during pregnancy, and overall estrogen level declining during menopause, transitioning from ovarian to peripheral tissue production (25). Interestingly, males maintain relatively consistent level of estrogen throughout their adult life, while post-menopausal women have less estrogen than men of the same age (26).
Estrogen exerts its effects via the classic nuclear receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), as well as novel membrane-associated receptors, G protein-coupled receptor 30, Gαq protein-coupled estrogen receptor, and ER-X. Notably, it has been reported that 88% of the human peripheral blood monocyte population is positive for ER (using a monoclonal antibody that recognizes both isoforms), providing direct evidence for ER expression in a key immune cell subset and supporting estrogen’s modulatory role in monocyte function. ERα is mainly expressed in uterus, liver, kidney, mammary gland, and adipose tissue, while ERβ can be found in lung, colon, cardiovascular system, and central nervous system (27, 28). For membrane-associated receptors, the former predominantly exists in adrenal medulla, renal pelvis, and ovary (28). Gαq-ER, though still in the early stage of research with its tissue distribution not yet fully established, is involved in rapid signal transduction via Gαq protein activation (29), while ER-X is known for its high-affinity binding in tissues like brain and uterus, particularly during postnatal development. Estrogen performs biological functions through different initiated pathways. Within the nuclear-initiated, estrogen interacts with ERα or ERβ, leading to translocation of the activated receptors from cell membrane to nucleus. The ERs then recruit transcriptional complexes and other co-regulatory factors to specific DNA sequences known as estrogen responsive elements, thereby modulating target gene transcription (30). This genomic action often involves the formation of ER homodimers or heterodimers on DNA. In addition, ERs also orchestrate a cascade of rapid estrogen responses through membrane-initiated signaling, far outpacing the above transcriptional process (31). These non-genomic signals are mediated by membrane-associated receptors and can rapidly activate pathways such as extracellular signal-regulated kinase)/mitogen-activated protein kinase, protein kinase C (elevating intracellular Ca2+), JNK/phosphatidylinositol 3 kinase, and cAMP-dependent transcription, which regulate critical cellular processes including apoptosis. At the receptor level, ERα and ERβ exhibit similar affinities for estradiol, whereas ERβ can antagonize and negatively regulate the function of ERα (32). ERα and ERβ are able to form homodimers or heterodimers within the cell nucleus; besides, ERα can also form heterodimers with androgen receptor (AR), thereby modulating the transcriptional activity (33).
Notably, estrogen modulates the functions of specific immune cell subsets (neutrophils, macrophages, CD4+ T cells) through these ER-mediated signaling pathways by regulating key transcription factors including peroxisome proliferator-activated receptor gamma (PPAR-γ); shaping cell-type dependent cytokine profiles such as promoting the secretion of interleukin (IL) -4, IL-6, and interferon gamma (IFN-γ) while inhibiting anti-inflammatory IL-10 and regulating IL-17A; and modulating the expression of surface receptors including C-X-C chemokine receptor type 2 (CXCR2), integrin alpha M, and toll-like receptor (TLR) 4. Detail mechanisms of ER-mediated sex-specific regulation in immune cells are elaborated subsequently.
2.2 Progesterone
Progesterone, also the principal female hormone, is integral to various physiological processes, including embryogenesis, puberty, menstrual cycle, and pregnancy. While primarily synthesized in the ovaries, a small amount of progesterone is also produced by the brain and adipose tissue. During reproductive years, progesterone fluctuates significantly, peaking in the luteal phase of menstrual cycle and rising substantially during pregnancy when the placenta takes over its production from the corpus luteum. After menopause, progesterone level drops sharply, with limited production continuing in peripheral tissues like adrenal glands. Beyond its reproductive role, progesterone is critical for maintaining tissue homeostasis and modulating the immune system, particularly preventing fetal rejection during pregnancy (34).
Functionally, progesterone exerts its effects by binding to specific receptors: progesterone receptor (PR) and, in some contexts, glucocorticoid receptor (GR). The PR is encoded by progesterone receptor gene, located on chromosome 11q22. PR-A and PR-B are two main isoforms of nuclear PR, which differ in structure and function. PR-B generally acts as a positive regulator of progesterone, while PR-A can modulate and, in some cases, antagonize the effects of PR-B (35). These receptors, upon binding to progesterone, translocate to the nucleus and interact with specific progesterone response elements on DNA, influencing the transcription of genes related to cell proliferation, differentiation, and maintenance of the reproductive tissues (34). In addition, progesterone also signals through membrane PRs (mPRs), such as mPRα and mPRβ, which mediate rapid, non-genomic actions. The receptors activate G-proteins, initiate intracellular signaling cascades, and affect key cellular processes like smooth muscle contraction, immune regulation, and apoptosis (36). For immune regulation, progesterone acts on macrophages and regulatory T cells (Tregs) via PR-mediated pathways, which involves inhibiting nuclear factor kappa-B (NF-κB) and upregulating forkhead box P3 (FOXP3) at the transcription factor level; reducing the release of pro-inflammatory cytokines including IL-6 and tumor necrosis factor-α while enhancing anti-inflammatory IL-10; and suppressing the formation of neutrophil extracellular traps (NETs) by neutrophils. Sex-biased immunomodulation of progesterone on cells is later discussed.
2.3 Androgen
Androgens are a group of hydrophobic steroid hormones including testosterone, androstenedione, dihydrotestosterone and DHEA, and play an important role in maintaining male characteristics and physiological functions (37). Of these, testosterone is the most important in men, since it ensures the ability to produce sperm and participation in sexual activity. In addition, testosterone can also affect men’s physical strength, brain function, and heart health, which is equally important for women and helps maintain their overall health and sexual desire. In mammals, androgens are mainly synthesized and secreted by interstitial cells of the testis, with small amounts also produced by adrenal cortex and ovary. The level of androgen reaches peak during adolescence, and gradually decreases with age, especially over the age of 50, when its production declines by about one-third (37).
AR is a member of the ligand-activated transcription factor superfamily and mediates the effects of androgen. Among the hormones, only testosterone and dihydrotestosterone can bind to AR with high specificity and affinity, with the latter having approximately four times higher affinity than the former (38). Androgen exerts functions through both genomic and non-genomic pathways. Within the former, conformational change in the AR induced by ligand binding leads to the translocation of the receptor from cytoplasm to nucleus. The AR then acts as a homodimer and directly interacts with DNA at the androgen response element in the target gene regulatory region. In contrast, the non-genomic response occurs efficiently, and independently of gene transcription or protein synthesis, involving interactions with cell membrane-associated signaling molecules such as membrane receptors, ion channels or cytoplasmic regulatory proteins (38). Specifically, testosterone (the major androgen) mediates sex-biased immune cell functions in natural killer (NK) cells, CD8+ T cells, and neutrophils via AR-dependent genomic and non-genomic pathways, which involves suppressing interferon regulatory factor 8, activating forkhead box O1, and modulating T-Box transcription factor 21 at the transcription factor level; inhibiting the secretion of pro-inflammatory cytokines such as IL-1β and type I interferon (IFN)-γ; and regulating surface receptor expression by upregulating programmed death-ligand 1 (PD-L1) and downregulating natural killer group 2D. AR-dependent sex-specific functions in NK, CD8+ T cells, and neutrophils are systematically detailed thereafter.
It is noteworthy that, multiple sex hormone receptors (ERα/β, PR-A/B, and AR) all belong to the nuclear receptor superfamily and share common structural domains: the C-terminal ligand-binding domain, the variable N-terminal with a transcriptional activation function domain, the central DNA-binding domain, and the hinge region. The second transcriptional activation function domain is located within the C-terminal ligand-binding domain region and acts as an additional transcriptional activation area upon ligand binding (39). These structural similarities enable interactions between various sex hormones at multiple levels.
2.4 Sex chromosome
Sex chromosomes, comprising X and Y chromosomes, genetically determine the sex disparities from reproductive organs to the appearances/behaviors of adult individuals. The differentiation between X and Y chromosomes arises from their distinct evolutionary paths. X chromosome is larger and contains more amounts of genes, many of which are essential for basic cellular functions and immune responses (40). In contrast, the Y chromosome is smaller, expressing fewer genes, which are primarily related to male sex determination and spermatogenesis. Sex chromosomes can be classified into gonadal and non-gonadal actions. Due to the previous discussion on the effects of sex hormones, we mainly focus on the non-gonadal actions of sex chromosomes here.
2.4.1 X chromosome
X chromosome plays a critical role in numerous physiological and immunological processes except for the influence on sex determination. In somatic cells of XX individuals, one X chromosome undergoes transcriptional silencing, ensuring that most X-linked genes are expressed equally from the single active X chromosome in both XX and XY cells. However, several genes (up to 20%) escape the inactivation, resulting in their more expression in XX cells compared to XY cells. Notable examples include TLR, histone demethylases lysine demethylase 5C and 6A, the translation initiation factor eukaryotic translation initiation factor 2 subunit 3 X-linked, and RNA helicase DEAD-box helicase 3 X-linked. Specifically, Tlr7 located on X chromosome is crucial for recognizing viral RNA and initiating antiviral response. Overexpression of TLR7 and TLR8 in females can lead to stronger immune responses and a higher prevalence of autoimmune diseases (40), like systemic lupus erythematosus (41). Another case is that patients with systemic sclerosis exhibit a higher ratio of X chromosome inactivation and decreased regulatory T cell activity (42). Meanwhile, significant difference in susceptibility to various infectious and inflammatory diseases has been revealed between prepubertal boys and girls, underscoring the critical role of X chromosome (43, 44).
2.4.2 Y chromosome
Y chromosome is essential for male sex differentiation and also participates in various physiological processes, though its function is more restricted compared to X chromosome due to lower gene content. The most important action of Y chromosome is driven by SRY gene, triggering the development of testes in males. Interestingly, SRY can also be expressed in brain, particularly in catecholaminergic cells and neurons, suggesting a modulatory role in physiological conditions, such as hypertension (45). Notably, the limited number of genes on Y chromosome has made it challenging to investigate its non-gonadal effects. Study on XY mice with distinct Y chromosome variants have demonstrated significant variations in the severity of autoimmune diseases, highlighting the function of Y chromosome in immune responses (46). Y chromosome has also been implicated in modulating CD4+ T cells, further illustrating its contribution to the sex dimorphism in immunology (47).
Collectively, sex hormones act via specific receptors and signaling axes, while sex chromosomes provide genetic underpinnings for sex disparities—these factors together shape immune cell sexual dimorphism, as detailed in Section 3.
3 Sexual dimorphism of different cell types in physiological and pathological conditions
The immune system serves as a critical regulator of host physiology, necessitating specialized control across different tissues, ages, and sexes. This tailored regulation is believed to explain the variations in disease incidence, tropism and severity between male and female. This section examines the sex-specific biological factors that may contribute to differences in physiological and pathological conditions. It is noteworthy that, while environmental and social factors, such as occupational hazards, habits and social stresses, can influence these discrepancies, our focus is mainly on studies at cellular and in vivo levels.
3.1 Neutrophil
Polymorphonuclear leukocytes (neutrophils) are the predominant type of leukocytes in peripheral blood and known to play a key role in the defense of pathogen invasion and external stimuli. As frontline immune responder, neutrophils are rapidly recruited to the sites of infection, and combat invading pathogens via multiple mechanisms, such as phagocytosis, degranulation, secretion of reactive oxygen species (ROS), and formation of NETs. However, excessive accumulation and dysfunction of these cells lead to abnormal inflammatory process and may ultimately determine patient outcomes (48). Meanwhile, neutrophils expresses both ER and AR, which largely affect its quantity, function, and fate (49). This aspect warrants further investigation for deeper insight in sex-specific differentiation of neutrophils (Figure 2) (Table 1).
Figure 2
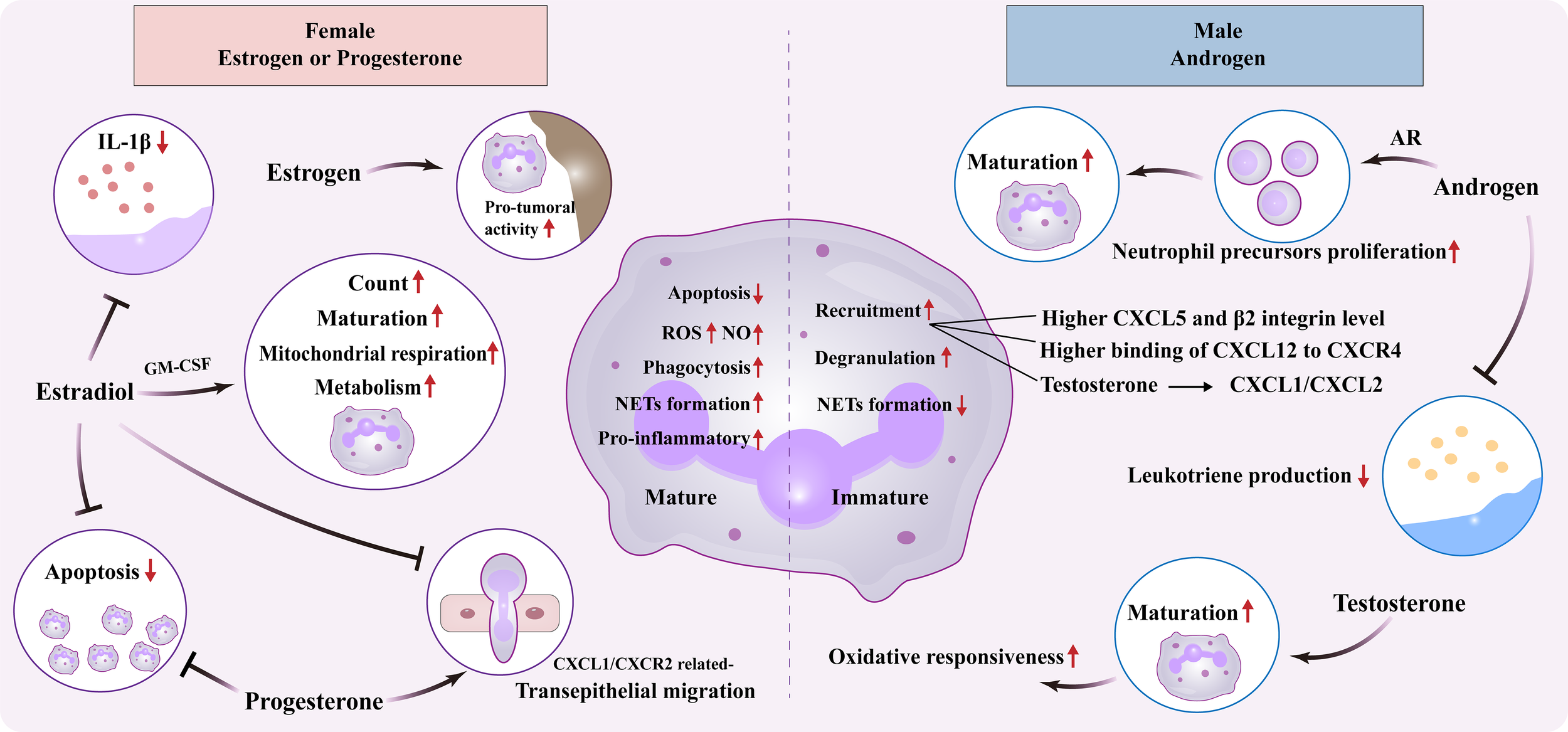
Sexual dimorphism of neutrophil. Effect of female estrogens or progesterones and male androgens on neutrophil biology, including maturation, apoptosis, metabolism, and inflammatory/tumor activity. ER, Estrogen receptor; AR, Androgen receptor; GM-CSF, Granulocyte-macrophage colony-stimulating factor; ROS, Reactive oxygen species; NO, Nitric oxide; IL, Interleukin; CXCL, Chemokine C-X-C motif ligand; CXCR, C-X-C chemokine receptor; NETs, Neutrophil Extracellular Traps.
Table 1
| Etiology | Model/Source | Effects | References |
|---|---|---|---|
| Physiology | Human blood neutrophil | Immature profile with diminished NETs formation in male | Blazkova et al., 2017 (50) |
| Physiology | Human blood neutrophil | Mature profile with activated IFN pathway and proinflammatory response in female, while increased mitochondrial mass and respiration in male | Gupta et al., 2020 (51) |
| Systemic juvenile idiopathic arthritis | Human blood neutrophil | Immature signature in female with suppressed therapeutic responsiveness of anti-IL-1R | Prada-Medina et al., 2020 (52) |
| Influenza vaccine | Human blood neutrophil | E2 triggers GM-CSF production and neutrophil maturation | Furman et al., 2014 (54) |
| Physiology | AR -/- mouse | Androgen-AR promotes precursor proliferation and neutrophil maturation | Chuang et al., 2009 (55) |
| Peritonitis and pleurisy | Rat, T cell-deficient mouse | Increased neutrophil recruitment and exacerbated inflammatory damage in male | Scotland et al., 2011 (57) |
| Ischemia/reperfusion | Rat, mouse, human skin blisters | Increased neutrophil recruitment in male | Madalli et al., 2015 (58) |
| Inhalation lung injury | Mouse | Increased recruitment and activation of neutrophil in male | Chatterjee et al., 2023 (59) |
| Post-weaning | Mouse | Estrogen/ERα/CXCR2 signaling governs neutrophil activity, thus fostering inflammation and adipocyte repopulation | Lim et al., 2020 (60) |
| C. albicans vaginal infection | Cxcr2 −/− mouse | E2 disrupts while progesterone promotes neutrophil transepithelial migration | Lasarte et al., 2016 (61) |
| Bacterial prostatitis | Mouse | Testosterone increases CXCL1/CXCL2 expression and neutrophil recruitment with an N2-like phenotype | Scalerandi et al., 2018 (62) |
| E. coli endotoxin | Rat | Higher capacity for endotoxin phagocytosis in reproductive-age female | Spitzer & Zhang, 1996 (63) |
| Staphylococcus aureus infection | Mouse | Enhanced ROS production and bactericidal capacity in female | Pokhrel et al., 2020 (64) |
| Myocardial infarction | Mouse | Tend to generate nitric oxide, ROS and phagocytosis to remove necrotic tissue in female | DeLeon-Pennell et al., 2018 (65) |
| Sepsis | Mouse | Females exhibit a propensity for NETosis, whereas males display a predilection for elastase degranulation | Lu et al., 2021 (66) |
| Acute colitis | Rabbit | Elevated pro-inflammatory prostaglandin in male | LeDuc & Nast, 1990 (67) |
| Physiology | Human blood neutrophil | Androgen inhibits leukotriene production in male | Pergola et al., 2011 (68) |
| Spontaneous lupus | Mouse | Estrogen increases neutrophil count and activity | Dai et al., 2017 (69) |
| Aneurysm rupture | Mouse | Estrogen promotes NETosis and subsequent aneurysm rupture | Patel et al., 2023 (70) |
| Pregnancy | Human blood neutrophil | Progesterone suppresses NETs formation | Giaglis et al., 2016 (71) |
| Pregnancy | Human blood neutrophil | Delayed apoptosis with physiological neutrophilia in pregnancy female | Tulchinsky & Hobel, 1973 (75); Watson et al., 1999 (76) |
| Physiology | Human blood neutrophil | Decreased spontaneous apoptosis in female of reproductive age; estradiol and progesterone decrease neutrophils apoptosis | Molloy et al., 2003 (77) |
| Psoriatic inflammation | Mouse | E2 inhibits IL-1β generation and IL-17A-mediated psoriatic inflammation | Adachi et al., 2022 (72) |
| Mammary tumor | Mouse | Estrogen promotes tumor growth by upregulating the pro-tumoral activity of neutrophil | Lim & Lin, 2021 (78) |
| Mammary tumor | Mouse | Estrogen fosters pro-tumoral microenvironment | Chung et al., 2017 (73) |
| Melanoma tumor | Mouse | Impaired maturation and oxidative responsiveness of neutrophil in both female and castrated male | Markman et al., 2020 (74) |
Sex difference of neutrophil.
NETs, Neutrophil Extracellular Traps; IFN, Interferon; IL, Interleukin; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; ER, Estrogen Receptor; AR, Androgen Receptor; CXCL, C-X-C Motif Chemokine Ligand; Cxcr2:C-X-C Chemokine Receptor 2; ROS, Reactive Oxygen Species; E2, Estradiol.
3.1.1 Neutrophil maturation
Neutrophil maturation, encompassing a multi-stage process from myelocyte to segmented neutrophil, is critical for immune defense. There are opposite opinions about its difference between men and women. On study reported an increased immature-like forms of neutrophil in healthy adult males compared to females of the same age, as manifested by the diminished capacity in NETs formation upon distinct stimuli (50). Another research also demonstrated that healthy young females possessed a mature neutrophil profile characterized by activated IFN pathway and proinflammatory responses, while males exhibited an immature-like genotype in circulating neutrophils (51). However, in patients with systemic juvenile idiopathic arthritis, peripheral neutrophils from females exhibit controversially higher immature signatures, which affects the therapeutic responsiveness of anti- IL-1R (52).
The underlying mechanism may be related to a key modulator of neutrophil maturation, granulocyte-macrophage colony-stimulating factor (GM-CSF), which was observed to be higher in young adult females than males, followed by diminishing after menopause (53). In addition, estradiol is capable of triggering GM-CSF production, thus influencing neutrophil maturation (54). Notably, neutrophil maturation shows conflicting sex biases: healthy adult males exhibit more immature phenotypes, while females with systemic juvenile idiopathic arthritis present higher immature signatures, likely due to disease-induced inflammatory cues overriding basal sex hormone regulation. Yet, this opinion requires further validation. Meanwhile, androgens also wield significance in shaping neutrophil maturation. Mice lacking ARs unveiled neutropenia owing to reduced proliferation of the precursor cells, ultimately compromising the host defense against microbial infections (55). While recent evidence pointed that, during urinary tract infection, androgen exposure raises the proportion of immature neutrophils in the kidney to ~50-60% (vs. <25% in controls). Selective deletion of ARs in myeloid cells largely rescues this defect, reducing immature neutrophils to ~25-30% (56).
3.1.2 Neutrophil recruitment
Prior studies have identified certain patterns of sex differences in neutrophil recruitment or migration. In comparison with females, male rodents exhibited increased neutrophil recruitment and exacerbated inflammatory damage upon acute exposure to bacteria or other irritants (57). This sex-based disparity in neutrophil kinetics could be explained by both more production of chemokine C-X-C motif ligand 5 (CXCL5) in the circulation, tissue, bone marrow and higher expression of β2 integrin on neutrophils of males but not females in the animal model of ischemia/reperfusion (58). In a model of inhalation lung injury, Cl2 gas exposure also induced higher susceptibility and mortality in male mice than females, along with increased recruitment and activation of neutrophils. The differential binding of CXCL12 to its receptor, CXCR4, might be responsible for the sexual dimorphism in neutrophil migration and immune response (59).
When it comes to the specific mechanism, sex hormones exert distinct regulatory effects on neutrophil recruitment. In the study of mammary involution post-weaning, estrogen was uncovered to govern neutrophil activity in mammary tissue via ERα-mediated CXCR2 signaling, thus fostering inflammation and adipocyte repopulation (60). In addition, estradiol could disrupt while progesterone promoted the CXCL1/CXCR2 axis, followed by coordinating neutrophil transepithelial migration into the vagina (61). As for male hormones, testosterone was able to increase the recruitment of neutrophils to infection sites by upregulating expression of CXCL1/CXCL2. However, these neutrophils display an N2-like phenotype characterized by reduced efficiency in bacterial killing and heightened expression of immunomodulatory molecules like IL-10 and transforming growth factor-1 (62).
3.1.3 Neutrophil function
Sex-dependent performance of neutrophil function has long been a subject of interest and is likely closely associated with certain diseases. During the battle to pathogens, neutrophils from reproductive-age female rats exhibited a greater capacity for endotoxin phagocytosis compared to age-matched male rats of the same strain, as well as those before or after reproductive age (63). Female mice manifested elevated serum levels of complement C3 and higher expression of complement receptor 3 on the neutrophils, thus enhancing their ROS production and killing capacity upon Staphylococcus aureus challenge (64). Another study indicated that female neutrophils effectively removed necrotic tissue following myocardial infarction via generating nitric oxide and ROS, as well as phagocytosis, protecting from collateral damage incurred by degranulation of proteolytic enzymes (65). Additionally, female mice tended to use NETosis, which forms a net outside the cell to capture pathogens (66). Comparatively, male neutrophils have their own characters. In male post-myocardial infarction, neutrophils eliminated necrotic tissue mainly through CD36-dependent degranulation of matrix metalloproteinase-9, leading to more excessive damage to the myocardium compared to the females (65). Similarly, during the antiviral immunity, neutrophils of male mice also exerted a killing effect through degranulation of elastase, but could be more prone to aging (66). As important pro-inflammatory mediators derived from arachidonic acid, prostaglandin is demonstrated to be elevated in males, while leukotriene is increased in females (67). Wherein, male neutrophils display a stronger ability to generate prostaglandin, likely due to heightened cyclooxygenase-2 expression linked to increased nuclear factor kappa-B activation during acute inflammation compared with the females (67). Moreover, androgens have been observed to inhibit leukotriene production in human neutrophils, resulting in its lower level in males (68). Collectively, these findings on neutrophil function may explain the differences in manifestations and clinical outcomes between men and women in certain diseases, like infection.
The regulation of neutrophil function by estrogen and its impact on diseases have been subject to ongoing debate. Male neutrophils exhibited increased mitochondrial mass and respiration, akin to earlier forms of neutrophils/myeloid cells. However, when treated with estradiol, these traits were reversed, suggesting the discrepancy in neutrophil bioenergetics between male and female (51). Meanwhile, estrogen administration dramatically increases the count and activity of neutrophils in the spleen of C57BL/6 mice, which might link to the potential promotion of spontaneous lupus (69). Intracranial aneurysm is more prevalent in women than men, while estrogen can protect against its progression to rupture via NETs formation (70), while progesterone has been shown to have a suppressive effect on NETs formation (71). Besides, estradiol could inhibit IL-1β generation from neutrophils and macrophages, which subsequently suppressed IL-17A-mediated psoriatic inflammation in the mouse model (72). The modulatory action of estrogen on neutrophil function appears to involve in tumor development. During the mammary involution, estrogen was reported to increase the expression of pro-tumoral cytokines/chemokines, and tissue-remodeling enzymes in the neutrophils, which created a pro-tumoral microenvironment (73). In addition, almost two-thirds of melanoma deaths are men. Researcher have discovered impaired maturation and oxidative responsiveness of neutrophils in both female and castrated male mice. This was correlated with increased melanoma tumor burden within the lungs and could be reversed by administering a physiological level of testosterone (74).
3.1.4 Neutrophil fate
It has been noticed by previous studies that neutrophils in women at full-term pregnancy demonstrate a remarkable delay in apoptosis along with physiological neutrophilia (75, 76). Thereby raises the interest of Molloy’s team in investigating the effect of female hormones on the fate and function of neutrophils (77). They validate a decreased spontaneous apoptosis in women of reproductive age compared to men, which can be further delayed by estradiol and progesterone at physiologic doses. The regulatory action of female sex steroids relies on reduced release of cytochrome c from the mitochondria and subsequent changes in caspase cleavage and activity. Conversely, prolonged estrogen treatment in tumor-bearing mice significantly hampers the output from bone marrow, shortens the life span, and upregulates the pro-tumoral activity of neutrophils, thus promoting the growth of mammary tumor (78).
Collectively, estrogen regulates neutrophil recruitment and maturation via ERα/CXCR2 and GM-CSF-dependent pathways, while androgens impair neutrophil functional maturation through AR signaling; progesterone suppresses NETs formation, together shaping the sex-specific functional bias of neutrophils. These sex-dependent differences (e.g., enhanced phagocytosis and NETosis in females, immature phenotype and elastase degranulation in males) underlie the higher prevalence of autoimmune diseases in females and increased susceptibility to severe infections (e.g., ARDS, novel coronavirus pneumonia) and melanoma in males.
3.2 Eosinophil and basophil
Eosinophils orchestrate allergic response, anti-parasitic defense, and inflammatory modulation, whereas basophils initiate allergic cascades through histamine and inflammatory mediators release. Despite their immunological importance, research on sex differences in these granulocytes remains limited, with mechanistic insights particularly lacking (Table 2).
Table 2
| Cell | Etiology | Model/Source | Effects | References |
|---|---|---|---|---|
| NK | Myocarditis | Mouse | Estrogen inhibits cardiac IFN-γ+ NK cell infiltration in female | Zhou et al., 2018 (124) |
| Parkinson | Mouse | Heightened NK cell receptor expression and IFN-γ production in young female | Menees et al., 2021 (125) | |
| Prostate cancer | Human PCa cell line | Dihydrotestosterone reduces the cytotoxicity of NK cells | Tang et al., 2022 (126) | |
| Bladder cancer | Human BCa cell lines, human NK cell line | Anti-androgen therapy or AR depletion enhances the tumor-killing efficacy of NK cells | Liu et al., 2022 (127) | |
| Macrophage | Q fever | Human peripheral macrophage | Estrogen enhances inflammatory profile of macrophages while testosterone fosters the anti-inflammatory response | Gay et al., 2021 (129) |
| Peritonitis and pleurisy | Rat, mouse | E2 or progesterone enhances the phagocytic activity of peritoneal macrophage | Chao et al., 1996 (130); Scotland et al., 2011 (57) | |
| Atherosclerosis | Human, Ldlr-/- mouse | Estrogen-ERα inhibits TLR4 signaling activation and macrophage-associated inflammation | Meng et al., 2023 (131) | |
| Pregnancy loss | Human, mouse | E2 negatively modulates TLR4-induced M1 polarization and increases Th2 immune response | Lou et al., 2023 (132) | |
| Lipopolysaccharide infection | Human peripheral macrophage | Allopregnanolone inhibits TLR4/TLR7 activation and chemokine/cytokine generation in female | Balan et al., 2022 (133) | |
| Non-alcoholic steatohepatitis | Mouse | Estrogen-ERα decreases the recruitment and activation of M1 macrophage | Shu et al., 2022 (134) | |
| Postmenopausal chronic stress | Rat, mouse | E2 promotes macrophage polarization from M1 to M2 phenotype | Hou et al., 2021 (135) | |
| Asthma | Mouse | Estrogen-ERα enhances IL-4-induced M2 polarization | Bang et al., 2011 (137); Keselman et al., 2017 (136) | |
| Asthma | Human peripheral macrophage | Stronger IL-4 responsiveness with increased monocyte recruitment and M2 polarization in female | Becerra-Díaz et al., 2021 (138) | |
| Streptococcus agalactiae pneumonia | Mouse | Higher level of chromosome X-linked miR-223-3p which negatively correlates with M1 phenotype in female | Deny et al., 2022 (141) | |
| Eosinophil | Asthma | Mouse | Estrogen-ERα amplifies Th2 cell polarization and exacerbates eosinophilic inflammation | Lauzon-Joset et al.,2020 (81); Cephus et al., 2021(80) |
| Breast tumor | Mouse, mouse breast cancer line | Estrogen-ERα suppresses tumoral and circulating eosinophils, promoting tumor growth | Artham et al., 2024(82) | |
| Basophil | Idiopathic anaphylaxis | Human | Null effect of estrogen/progesterone on basophil histamine release | Slater et al., 1987(83) |
| Allergic disease | Human | Androgen modulates basophil functionality and suppresses Th2 cytokine production | Grobe et al., 2020(84) |
Sex difference of NK, macrophage, eosinophil and basophil.
NK, Natural Killer cell; IFN, Interferon; PCa, Prostate Cancer; Bca, Breast Cancer; AR, Androgen Receptor; ER, Estrogen Receptor; E2, Estradiol; TLR, Toll - like receptor; IL, Interleukin; Th2, T helper 2.
Pioneering observation in 1955 reveals sexually dimorphic eosinophil fluctuations in guinea pigs during physiological cycles, suggesting sexual hormonal modulation of the generation, maturation, and survival of eosinophils (79). Subsequent rodent studies identify estrogen’s regulatory capacity: it amplifies T helper 2 (Th2) cell polarization in allergic airways by enhancing IL-33 release from epithelial cells, thereby exacerbating eosinophilic inflammation via group 2 innate lymphoid cell activation (80, 81). Paradoxically, estrogen exhibits tumor-promoting effects in breast cancer models by suppressing tumoral and circulating eosinophils, which can be reversed with ERα blockade (82). This aforementioned differences in estrogen’s effects on eosinophils may stem from tumor microenvironment-specific signaling pathways or cell subset heterogeneity.
Basophil regulation displays contrasting hormonal dynamics. Clinical studies in idiopathic anaphylaxis patients demonstrate null effects of estrogen/progesterone on basophil histamine release (83). Conversely, androgens modulate basophil functionality indirectly via peripheral blood mononuclear cell-derived soluble factors, suppressing Th2 cytokine production and potentially explaining male-female disparities in allergic disease prevalence (84). In short, estrogen modulates eosinophil function via ERα-mediated IL-33/Th2 axis activation, while androgens indirectly regulate basophil functionality through suppressing Th2 cytokine production; progesterone shows no significant effect on basophil histamine release. This hormonal regulation explains the female predominance in allergic asthma (linked to eosinophilic inflammation) and the tumor-promoting effect of estrogen in breast cancer (via eosinophil suppression), as well as sex differences in the prevalence of allergic diseases.
3.3 T cell
T cells, the critical component of adaptive immune system, play an important role in immune surveillance and pathogen elimination through their diverse subtypes, including CD4+ T cells (T helper cells (Th) and Tregs and CD8+ cytotoxic T cells. Studies have shed light on potential sex-based disparities in T cells, which participate in the development and progression of various diseases (16, 85, 86). Mechanistically, T cell’s state and function can be largely regulated by estrogen or androgen signaling (87, 88), and even X/Y chromosome factors, affecting immune responses in physiological and pathological conditions (89, 90). Therefore, understanding the sex divergence of T cells is paramount for developing effective therapies for distinct diseases (Figure 3) (Table 3).
Figure 3

Sexual dimorphism of T cell. Sex hormones on various T-cell types, including T helper cells, CD8+ T cells, regulatory T cells, and NKT cells. Th, T helper cell; ER, Estrogen receptor; AR, Androgen receptor; PPAR-γ, Peroxisome proliferator-activated receptor gamma; PD-1/PD-L1, Programmed death receptor 1/programmed cell death 1 ligand 1; Treg, Regulatory T cell; FOXP3, Forkhead box P3; IL, Interleukin; NKT, Natural killer T cell.
Table 3
| Cell | Etiology | Model/Source | Effects | References |
|---|---|---|---|---|
| Th | Asthma | Human peripheral T cell | Estrogen increases type 2 cytokine production and reduces Th2 cell apoptosis | Vijeyakumaran et al., 2023 (16) |
| Bone loss | Mouse | Estrogen deficiency enhances antigen presentation and T cell activation | Cenci et al., 2003 (92) | |
| Cerebral aneurysm rupture | Mouse | Estrogen deficiency intensifies circulating Th17 cells and IL-17A levels | Hoh et al., 2018 (93) | |
| Autoimmune disease | CD4-PPARγKO mice | PPARγ restrains Th17 differentiation in male while regulates Th1, Th2, and Th17 differentiation in female | Park et al., 2016 (94) | |
| Colitis | CD4-creERαfl/fl mice, T cell-deficient mouse | ERα deletion reduces T cell proliferation and activation | Mohammad et al., 2018 (95) | |
| Collagen-induce arthritis | Mouse | E2-ERα mitigates the migration of Th17 cells | Andersson et al., 2015 (96) | |
| Autoimmune encephalomyelitis | Mouse | E2-ERα limits Th17 cell differentiation | Garnier et al., 2018 (97) | |
| Asthma | Mouse | Dihydrotestosterone decreases while AR depletion aggravates Th2 inflammation | Ejima et al., 2022 (98) | |
| Physiology | Human peripheral T cell | Androgen increases Th1 responses in male | Girón-González et al., 2000 (100) | |
| Treg | Autoimmune encephalomyelitis |
Mouse | E2 promotes immune tolerance via expanding the Treg compartment | Polanczyk et al., 2004 (101) |
| Obesity | Mouse | E2 modulates Treg localization in adipose tissue | Ishikawa et al., 2020 (102) | |
| Autoimmune encephalomyelitis | Mouse | Estrogen elevates FOXP3 level and immunosuppressive activity of Treg | Polanczyk et al., 2005 (103) | |
| Ileitis | Erα -/-, Erβ-/- mouse | ERβ knockout impairs Treg differentiation | Goodman et al., 2020 (104) | |
| Pneumococcal pneumonia | Mouse | E2-ERβ axis augments Tregs number with intensified immunosuppressive effect | Xiong et al., 2021 (105) | |
| Allergic airway inflammation | Mouse | AR enhances the stability and suppressive function of Tregs and protects against type 2 inflammation | Gandhi et al., 2022 (106) | |
| Adipose tissue inflammation | Mouse | Androgen recruits and locally expands Treg cell number in adipose tissue | Vasanthakumar et al., 2020 (107) | |
| CD8+ T | Bladder carcinogenesis | Mouse | Androgen-AR promotes dysfunction and exhaustion of CD8+ T cell | Kwon et al., 2022 (109) |
| Prostate cancer | Human, mouse | Blocking AR restores CD8+ T cell function in male | Guan et al., 2022 (110); Yang et al., 2022 (111) | |
| Non-small cell lung cancer | Human, mouse | Androgen-AR attenuates NF-ĸB-associated anti-tumor response in T cells in male | Zhang et al., 2023 (112) | |
| Glioblastoma | Mouse | More functionally exhausted CD8+ T cells in male due to Kdm6a escape from the X-inactivation | Lee et al., 2023 (113) | |
| Coxsackievirus B3 infection | Mouse | Increased activated CD8+ T cells in female | Dhalech et al., 2023 (114) | |
| NKT | Immunological hyperactivity | Mouse | Estrogen regulates IL-4 and IFNγ production in iNKT cells and participates in Th1/Th2 differentiation | Gourdy et al., 2005 (118) |
| Non-obese diabetes | Jα18 −/− mouse | E2 reinstates the immunomodulatory function of iNKT cell | Gourdy et al., 2016 (119) | |
| Steatohepatitis | Mouse | NKT cell depletion exacerbates leukocyte infiltration and inflammatory liver injury in male | Cuño-Gómiz et al., 2023 (120) | |
| Amebic liver abscess | Mouse | Testosterone inhibits NKT cell activation | Lotter et al., 2013 (121) | |
| Influenza A virus infection | Mouse | Expansion of iNKT cell population with boosted inflammation in female | Humeniuk et al., 2023 (122) |
Sex difference of T cells.
Th, T helper cell; IFN, Interferon; IL, Interleukin; E-cadherin, Epithelial cadherin; PPAR-γ, Peroxisome proliferator-activated receptor gamma; ER, Estrogen Receptor; Treg, Regulatory T cell; FOXP3, Forkhead box P3; AR, Androgen Receptor; NKT, Natural Killer T cell; E2, Estradiol.
3.3.1 Th cell
Th cells are a pivotal subset of CD4+ T cells that orchestrate immune responses by providing essential signals to other immune cells. These cells differentiate into various subtypes, divided into Th1, Th2, Th17 and etc., each defined by distinct cytokine profiles and function (91). Among various sex factors that affect Th cells, estrogen has been extensively studied and was found to increase type 2 cytokine production and reduce Th2 cell apoptosis, leading to the airway inflammation in severe asthma (16). Otherwise, estrogen deficiency is found to upregulate IFN-γ-induced class II trans-activator, enhance antigen presentation and T cell activation, resulting in bone loss (92). Estrogen shortage also increases the risk of cerebral aneurysm rupture by intensifying levels of circulating Th17 cells and IL-17A levels, as well as downregulating E-cadherin expression (93). Peroxisome proliferator-activated receptor gamma has recently been highlighted a key modulator of sexual differences in adaptive immunity and autoimmune diseases. It only restrains Th17 differentiation in male T cells while regulates the differentiation of Th1, Th2, and Th17 in female T cells according to distinct estrogen levels (94). To further investigate the underlying mechanism, researcher have constructed T cell-specific ERα knockout mice and found that ERα deletion reduced T cell proliferation and activation, contributing to their decreased pathogenicity in a murine model of colitis (95). In collagen-induced experimental arthritis, estradiol treatment potently mitigates its severity via regulating the migration of Th17 cells in an ERα-dependent manner (96). Meanwhile, estradiol-ERα axis in FOXP3neg CD4+ T cells limit Th17 cell differentiation, thus inhibiting the development of autoimmune encephalomyelitis in the mouse model (97). Furthermore, androgen signaling also plays a significant role in the sex-based regulation of Th cells. Scholars have discovered that dihydrotestosterone administration could decrease while AR depletion in T cells aggravated Th2 inflammation by inducing the expression of dual specificity phosphatase-2 in a murine model of acute allergic asthma (98). Other studies also pointed that androgen can increase Th1 responses in males (99, 100). The evidence may also explain the sex bias of asthma post adolescence. Notably, estrogen’s effect on Th17 differentiation differs by disease context: it restrains Th17 in autoimmune encephalomyelitis but promotes Th17 in cerebral aneurysm rupture, likely due to disease type and local estrogen concentration, reflecting the context-dependent nature of sex hormone-immune crosstalk.
3.3.2 Tregs
Tregs are a specialized subset of CD4+ T cells and play a critical role in maintaining immune homeostasis and preventing autoimmunity. Exogenous estradiol has been shown to promote immune tolerance via expanding the Treg compartment (101). It can also modulate chemokine signals associated with Treg localization and mitigate obesity-related chronic inflammation (102). Additionally, CD4+CD25+ T cells from pregnant and estrogen-treated mice exhibit elevated FOXP3 level and enhanced suppressive activity, controlling abnormal inflammatory responses (103). Compared with males, female patients with Crohn’s disease exhibit decreased ERβ expression on T cells in both the ileal mucosa and peripheral blood. ERβ-deficient mice display impaired Treg differentiation followed by a female-specific exacerbation of intestinal inflammation (104). In male mice with severe pneumococcal pneumonia, estradiol-ERβ axis alleviates lung inflammation and tissue injury by augmenting the number of Tregs with their intensified immunosuppressive effect (105). Studies have also highlighted the effects of AR signaling on Tregs. It is capable of enhancing the stability and suppressive function of Tregs via IL-33/suppression of tumorigenicity 2 (ST2) pathway, thereby safeguarding against the progression of allergen-induced type 2 inflammation (106). Recently, sexual dimorphism of Tregs in the visceral adipose tissue has been uncovered and is determined in a sex hormone-dependent manner. Androgen actively recruits and locally expands Treg cell numbers, which regulates immune responses and limits adipose tissue inflammation (107).
3.3.3 CD8+ T cell
CD8+ T cells, also known as cytotoxic T lymphocytes, are another crucial subtype of T cells primarily responsible for identifying and eliminating cancerous or infected cells (108). So far, numerous studies have focused on sexual disparities in development and progression of certain nonreproductive system cancers, with the underlying mechanisms remain enigmatic. In preclinical cancer models, androgen-AR axis is proved to promote CD8+ T cell dysfunction and contribute to the male bias in the frequency of progenitor exhausted CD8+ T cells in the tumor microenvironment (109). Other studies also concentrate on the regulation of androgen signaling on T cell exhaustion and indicate that blocking AR could restore CD8+ T cell function and enhance responsiveness to anti-programmed death receptor 1 (PD-1)/PD-L1 therapy in males (110, 111). Likewise, activation of androgen-AR signaling pathway attenuates nuclear factor kappa-B-associated anti-tumor response in T cells and anti-PD-1 effects in male mice, which can be reversed through either pharmacological intervention or surgical castration (112). Another noteworthy study has revealed a worse prognosis in male glioblastoma patients than females, attributing to more tendency of functionally exhausted CD8+ T cells in the male. Mechanistically, the gene Kdm6a that escape from X-inactivation, rather than sex hormone, plays a role in the sexual dimorphism of T cell-mediated anti-tumor immunity (113). In addition, sex bias in CD8+ T cells is also observed in other diseases. Coxsackievirus B3 triggers the expansion of antigen-experienced CD8+ T cells with an activated phenotype in female mice, whereas male mice exhibit no significant changes in T cell-related immune responses (114). Beyond viral infections, sex differences in CD8+ T cells also contribute to chronic pain, a condition predominantly affecting women: Studies indicate that in females, Pannexin-1 channel-expressing microglia and T cells differentially induce mechanical hyperalgesia, with female-derived CD8+ T cells showing a greater tendency to release leptin, as pro-inflammatory adipokine, further enhances pain sensitivity (115). This heightened immunity in females facilitates more effective pathogen elimination but also a predisposition to autoimmune diseases.
3.3.4 Natural killer T cell
Natural killer T cell (NKT) represents a special subpopulation of T cells with both T cell receptors and NK cell receptors on the surface (116, 117). These cells can rapidly release numerous cytokines and play a bridging role between the innate and adaptive immunity. Currently, attentions have been paid on sex differences of NKT cells in several physiological and pathological conditions. Estrogens have the ability to regulate the production of IL-4 and IFNγ in invariant NKT (iNKT) cells, which participate in the regulation of Th1/Th2 differentiation (118). The subsequent research has revealed that estradiol could reinstate the immunomodulatory function of iNKT cells in non-obese diabetic mice, and might be beneficial to insulin secretion in type 1 diabetes (119). Other study demonstrates that male steatohepatitis mice lacking NKT cells experience exacerbated infiltration of neutrophil and macrophage, resulting in more severe inflammatory liver injury compared to females with the known protection provided by estrogens (120). Amebic liver abscess has shown strong preferences for adult males. By use of the mouse model, researcher have discovered that testosterone inhibited the activation of NKT cells, manifested by IFN-γ generation, thereby promoting development of the abscesses (121). Contrarily, influenza A virus infection leads to higher mortality in women of reproductive age. In the infectious murine model, female mice exhibit a sex-dependent expansion of iNKT cell populations in the lung and liver, along with boosted inflammatory responses compared with males, although the underlying mechanism remains unclear (122).
Together, estrogen governs Th cell polarization and Treg expansion via ERα/PPAR-γ signaling, androgen promotes CD8+ T cell exhaustion through AR/PD-L1 axis, and both hormones regulate NKT cell cytokine secretion (IL-4/IFN-γ), collectively mediating sex dimorphism in T cell subsets. These mechanisms contribute to female-biased autoimmune diseases (e.g., SLE, rheumatoid arthritis) driven by Th17 overactivation, reduced anti-tumor immunotherapy efficacy in males (due to CD8+ T cell exhaustion), and sex differences in viral infection outcomes (e.g., Coxsackievirus B3) and chronic pain.
3.4 NK cell
NK cells are innate lymphocytes that primarily exhibit cytotoxic effects by producing perforin, granzyme and IFN-γ in response to pathogens and malignant cells (123). However, research on their sexual dimorphism remains limited. Based on the observation that Coxsackievirus B3-associated myocarditis predominantly affects males, Zhou et al. explore that estrogen inhibits the expression of Th1-specific T-box transcription factor and the infiltration of cardiac IFN-γ+ NK cells, thereby preventing myocarditis in female (124). In Parkinson’s mice, NK cell from young female presents a higher expression of its receptors and IFN-γ production, which becomes less pronounced with aging (125). Androgens can also influence the function of NK cells. Elevated level of dihydrotestosterone reduces the cytotoxicity of NK cells, leading to their diminished ability to eliminate castration-resistant prostate cancer cells through the AR/PD-L1 signaling pathway (126). Furthermore, Liu et al. demonstrate that targeting AR, via either antiandrogen therapy or AR depletion, could enhance the tumor-killing efficacy of NK cells due to the decreased PD-L1 expression in bladder cancer (127) (Figure 4) (Table 2). Therefore, in NK cell, estrogen inhibits IFN-γ+ NK cell infiltration via suppressing Th1-specific transcription factors, while androgens reduce NK cell cytotoxicity through AR/PD-L1 signaling, leading to sex-specific NK cell functional differences. These differences explain the male predominance in Coxsackievirus B3-induced myocarditis and castration-resistant prostate cancer, as well as the potential of anti-androgen therapy to enhance NK cell-mediated tumor killing in bladder cancer.
Figure 4

Sexual dimorphism of other immune cells. The effects of sex hormones on various immune cells along with the differences in immune responses associated with biological sex. Th, T helper cell;ER, Estrogen receptor; AR, Androgen receptor;NK, Natural killer cell; PD-1/PD-L1, Programmed death receptor 1/programmed cell death 1 ligand 1; M, Macrophage; IFN, Type I interferon; TLR, Toll-like receptor; IL, Interleukin; DC, Dendritic cell; ILC2, Group 2 innate lymphoid cell; HMGB1, high mobility group box 1; HIF, hypoxia-inducible factor; BCR, B cell receptor; TLR, Toll-like receptor.
3.5 Macrophage
Macrophages are versatile immune cells with diverse functions, mainly including eliminating pathogens, presenting antigens, and producing cytokines. They can polarize into pro-inflammatory (M1) or anti-inflammatory (M2) state, adapting to environmental signals to regulate immune response, tissue repair, and homeostasis (128). The regulation of sex-determining factors on macrophage function is diverse and complex. In infectious diseases, Q fever influences more males than females, despite a similar level of Coxiella burnetii exposure. It has been reported that the protective role of estrogen is achieved by enhancing the inflammatory profile of macrophages, whereas testosterone might affect disease progression via fostering an anti-inflammatory response (129). As one of the most important antibacterial mechanisms, phagocytic activity is markedly diminished in peritoneal macrophages from female mice subjected to ovariectomy compared with the sham group (57), while can be enhanced through treatment with estradiol or progesterone (130).
However, in inflammatory reactions, the situation is completely different. Estrogen-ERα axis exerts inhibitory action on the activation of TLR4 signaling and macrophage-associated inflammation, thereby promoting the stability of atherosclerotic plaques post-menopause (131). Another study claims that estradiol negatively modulates TLR4-induced M1 polarization and increases Th2 immune responses by activating serum-glucocorticoid regulated kinase 1 at the maternal-fetal interface, thus avoiding recurrent pregnancy loss (132). Besides, the anti-inflammatory role of allopregnanolone has also been identified in human macrophages via the inhibitory effects on both TLR4 and TLR7 activation, which lead to cytokine/chemokine reduction, particularly in female donors (133). When fed with high fat diet, ovariectomy increases the risk of non-alcoholic fatty liver disease in female mice. This is probably due to the inhibited recruitment and activation of M1 macrophages by estrogen-ERα signaling via the downregulation of CCR2 (134). In a mouse model of postmenopausal chronic stress, estradiol supplementation promotes the polarization of M1 to M2 macrophages in a β2-adrenoceptor-dependent manner, therefore facilitating the resolution of myocardial inflammation (135). Asthma is a chronic Th2 inflammation of the lung that affects more women than men in adulthood, with alveolar macrophage emerging as predominant mediator of the allergic inflammation. Estrogen-ERα axis is demonstrated to enhance IL-4-induced M2 polarization in asthmatic mice of both sexes challenged by ovalbumin (136, 137). Other findings also highlight the stronger IL-4 responsiveness in macrophages from female asthma patients than the males, leading to increased monocyte recruitment and M2 polarization in this disease (138). Sexually dimorphism is also prominent in pulmonary fibrosis, 5-hydroxytryptamine receptor 2C+alveolar macrophages specifically secrete angiopoietin-like protein 4, which mediates macrophage metabolic reprogramming, promoting lipolysis and inhibiting glycolysis, thereby suppressing grancalcin expression, reducing its accumulation in the lungs of female mice, and alleviating the pathological progression of pulmonary fibrosis (139). Notably, external stimuli like chemotherapy also trigger sex-specific macrophage responses: female macrophages upregulate intercellular adhesion molecule-1 more rapidly upon exposure, enhancing pro-inflammatory M1 polarization and release of pro-nociceptive factors and amplifying macrophage-peripheral nociceptor crosstalk, accelerating nociceptor activation and earlier onset of chemotherapy-induced neuropathic pain hallmarks (e.g., mechanical allodynia) in females (140). Further studies are needed to determine how macrophage polarization is specifically instructed by the pathological state (infection vs. autoimmunity) and local cytokine signals.
It is noteworthy that sex bias in macrophages not only attributes to sex hormones but may also be due to the chromosomes. Deny et al. discover that female macrophages exhibit higher level of chromosome X-linked microRNA-223-3p (miR-223-3p), which negatively correlates with pro-inflammatory M1 phenotype in Streptococcus agalactiae-associated pneumonia (141) (Figure 4) (Table 2).
Collectively, estrogen regulates macrophage polarization (M1/M2) via ERα/TLR4 and serum-glucocorticoid regulated kinase 1 pathways, testosterone fosters anti-inflammatory macrophage responses, and X-chromosome-linked miR-223-3p in females negatively correlates with M1 phenotype, jointly mediating sex bias in macrophage function. These regulatory effects underlie sex differences in Q fever susceptibility (male predominance), postmenopausal atherosclerotic plaque stability, asthma severity (female predominance), and non-alcoholic fatty liver disease risk.
3.6 Dendritic cell
Dendritic cells (DCs) are antigen-presenting cells and pivotal for initiating immune responses and maintaining immune tolerance (142). The action of sex-dependent DC functions in health and disease is complicated. Research has shown that ERα S-glutathionylation mediated by glutathione S-transferase Pi controls the differentiation and metabolic function of mouse dendritic cells (143). The estradiol-ERα signaling contributes to the strong sex bias on the capacity of plasmacytoid DC to produce IFN-1 in response to TLR7 (144, 145). Thus, targeting the ER signaling pathway in plasmacytoid DCs exhibits inhibitory effects on pathogenic generation of IFN-1 and autoantibodies, thereby preventing the progression of lupus-like syndrome in the initial stage of SLE (146, 147). The sexual dimorphism of DC function also appears in psoriasis, autoimmune encephalomyelitis and asthma. Selective ERα agonist enhances IL-23 secretion in DCs under LPS stimulation and aggravates pruritic responses in a psoriasis mouse model initiated by imiquimod (148). In encephalomyelitis, ERα signaling suppresses the neurotoxic function and maturation status of DC, which in turn impacts IL-12 and IFN-β production and ultimately modulates disease progression (149). With regard to asthma, estradiol elevates Th2-oriented CD103+ DCs in the bronchial lymph node, which may explain the higher prevalence and severity in female patients compared with the males (150). However, the activated IFN-1 signaling in bone marrow-derived DCs from 7-day-old female mice can also be protective in respiratory syncytial virus-related pneumonia, suggesting the diversity of sex dimorphism in DCs (151) (Figure 4) (Table 4). Jointly, estradiol-ERα signaling modulates plasmacytoid DC IFN-1 production and CD103+ DC Th2 polarization, while ERα S-glutathionylation controls DC differentiation and metabolic function, contributing to sex-specific DC-mediated immune responses. This sex dimorphism explains the female predominance in SLE (linked to pathogenic IFN-1 production by plasmacytoid DCs), psoriasis, and adult asthma, as well as the protective role of DC IFN-1 signaling in female respiratory syncytial virus-related pneumonia.
Table 4
| Cell | Etiology | Model/Source | Effects | References |
|---|---|---|---|---|
| DC | Lipopolysaccharide infection | Mouse | ERα S-glutathionylation controls DC differentiation and metabolism | Zhang et al., 2018 (143) |
| Immune-related disease | Human, mouse | E2-ERα signaling contributes to the strong sex bias on the pathogenicity of plasmacytoid DC | Meier et al., 2009 (145); Seillet et al., 2012 (144) | |
| SLE | Mouse | ER signaling elevates pathogenic generation of IFN-1 and auto-antibodies in plasmacytoid DC | Rowland et al., 2014 (147); Sisirak et al., 2014 (146) | |
| Psoriasis | Mouse | ERα agonist enhances IL-23 secretion in DC under LPS stimulation | Iwano et al., 2020 (148) | |
| Encephalomyelitis | Mouse | ERα signaling suppresses neurotoxic DCs’ function and maturation | Khaw et al., 2023 (149) | |
| Asthma | Mouse | E2 elevates Th2-oriented CD103+ DCs in the bronchial lymph node in female | Masuda et al., 2018 (150) | |
| RSV-related pneumonia | Mouse | Activated IFN-1 signaling in bone marrow-derived DC is protective in female | Malinczak et al., 2023 (151) | |
| ILC2 | Asthma | Human | Elevated level of circulating and sputum ILC2s in female | Aw et al., 2019 (154); Cephus et al., 2017 (153) |
| Asthma | Human, mouse | Higher levels of ILC2s and Th2 cytokines in female mice; testosterone reduces the proliferation of ILC2s and their production of type 2 cytokines | Wang et al., 2020 (155) | |
| Asthma | Mouse | Heightened type 2 inflammation with increased eosinophil influx and expansion of ILC2 in female | Zhao et al., 2019 (156) | |
| Asthma | Mouse | AR signaling limits the expansion, maturation and function of ILC2 | Laffont et al., 2017 (157) | |
| Skin immunity | ILC2-deficient transgenic mouse | Androgen reduces ILC2 and the accumulation and activation of DC | Chi et al., 2024 (158) | |
| B cell | Immune-related disease | Mouse | E2 reduce B cell apoptosis/activation | Grimaldi et al., 2002 (162) |
| Immune-related disease | Mouse | E2-ERα signaling increase capacity for class-switch recombination | Hill et al., 2011 (166) | |
| Physiology | Mouse | testosterone tend to exert immunosuppressive effects on B cells | Zhao R, et al. 2020 (167); Wilhelmson et al. 2018 (168) | |
| SLE | Human peripheral B cell | Escape of X-inactivation leads to dual-allelic expression of TLR7 in B cells and monocytes, resulting in cell-intrinsic hyperresponsiveness | Souyris et al., 2018 (169) | |
| Physiology | GM and K562 cell lines | Loss of XIST-mediated silencing in B cells leads to expansion of atypical B cells | Yu et al., 2021 (170) |
Sex difference of DC, ILC2 and B cell.
E2, Estradiol; AR, Androgen Receptor; ER, Estrogen Receptor; IFN, Interferon; IL, Interleukin; ILC2, Type 2 Innate Lymphoid Cell; DC, Dendritic Cell; SLE, Systemic Lupus Erythematosus; RSV, Respiratory Syncytial Virus; TLR, Toll-like receptor; XIST, X-inactive specific transcript.
3.7 Group 2 innate lymphoid cell
Group 2 innate lymphoid cells (ILC2s) are a unique subpopulation of immune cells with lymphocyte morphology but do not express lineage markers. ILC2s possess strong immune regulatory functions on allergic inflammation and tissue repair (152). Sexual dimorphism can be observed in ILC2s, though the conclusion is controversial. These conflicting ILC2 findings (elevated vs. no difference in asthma) may stem from asthma severity, sample source (circulating vs. sputum), or hormonal status (reproductive vs. post-menopause). Cephus et al. (153) claimed an elevated level of circulating ILC2s in women with moderate to severe asthma, while Aw et al. (154) observed higher sputum ILC2 numbers in female mild asthmatics. Yet, Wang et al. (155) reported no significant sex-based difference in the numbers of ILC2s in patients with asthma or allergic rhinitis (155). In asthmatic mouse model initiated by ovalbumin+IL-33, the females exhibit heightened type 2 inflammation, along with increased eosinophil influx and expansion of ILC2s, which may be attributed to greater IL-13 production compared to the males (156). Consistent with this finding, Wang et al. affirm higher levels of ILC2s and Th2 cytokines in female mice with the induction of IL-33 compared to the males. They further find a crucial protective role of testosterone in this allergic disease model (155). Mechanistically, AR signaling hinders the expansion and maturation of ILC2 and limits their responsiveness to IL-33 in type 2 airway inflammation (157). A more recent study published in Science try to unveil the mechanism underlying the sexual immune dimorphism: androgens negatively regulate ILC2 and the accumulation and activation of DCs in the skin, leading to the reduced tissue immunity in males (158) (Figure 4) (Table 4). In conjunction, androgen-AR signaling restrains ILC2 expansion, maturation, and responsiveness to IL-33, while female mice exhibit heightened ILC2-mediated type 2 inflammation, leading to sex differences in ILC2 function. These differences contribute to the higher prevalence and severity of asthma in females, as well as reduced tissue immunity in males (mediated by androgen-dependent ILC2 suppression).
3.8 B cell
B cells are central to humoral immunity, primarily functioning through antigen presentation, cytokine secretion, and antibody production. Significant sexual dimorphism exists in B cell biology, with females generally mounting stronger humoral responses than males. This is evidenced by higher baseline levels of immunoglobulins and more robust antibody production after vaccination or infection (159, 160). The enhanced B cell activity in females is a double-edged sword, contributing to both superior antiviral protection and a markedly higher susceptibility to systemic autoimmune diseases like SLE, where pathogenic autoantibodies are central (161).
The mechanisms are multifaceted, involving both hormonal and genetic pathways. At the hormonal level, estrogen promotes B cell activation and antibody diversification. It alters thresholds for B cell apoptosis and activation and has been shown to protect purified B cells from B cell receptor-mediated apoptosis (162). Concurrently, in vitro studies demonstrate that estrogen can significantly enhance antibody production, increasing the output of immunoglobulin G (IgG) and IgM from human B cells by more than two-fold (163). The hormone drives antibody diversification primarily through ER, which binds directly to the immunoglobulin heavy chain locus to participate in the three-dimensional DNA looping required for class-switch recombination, thereby influencing antibody isotype selection. This genomic action is specific to ERα, which is uniquely capable of disrupting B cell tolerance and permitting the survival of high-affinity autoreactive clones, directly linking this mechanism to female-biased autoimmunity (164–166). In contrast, testosterone tends to exert immunosuppressive effects, such as inhibiting germinal center formation (167, 168).
At the genetic level, contributions to the female bias in autoimmunity include incomplete X-chromosome inactivation, leading to biallelic expression of immune-related genes like Tlr7 in a subset of female immune cells. This results in a higher gene dosage and enhanced cellular responsiveness, such as lowered activation thresholds in B cells (169). Crucially, the maintenance of X-inactivation itself is actively regulated in B cells. Recent work shows that a B cell-specific X-inactive specific transcript complex enforces this silencing, and its dysfunction results in the expansion of pathogenic atypical B cells, directly linking the stability of X-inactivation to autoimmune B cell responses (170). These genetic mechanisms provide an explanation for the female bias in autoimmunity that is independent of, but potentially synergistic with, sex hormone effects. Additionally, B cells exhibit sex-based disparities in tumor immunity, and the regulation of B cell malignancy by sex hormones has been comprehensively reviewed elsewhere (159).
4 Modulatory effects of regulatory variables on sexual dimorphism of immune cells
4.1 Age, menopause and andropause
Previous study pointed that women consistently generate at least twice the amount of influenza vaccine-induced antibodies as men. Intriguingly, age acts as a key modifier of this sexual dimorphism. Immune and inflammatory responses are more robust in boys prior to puberty, with this advantage shifting to adult women post-puberty (171). Age-related hormonal shifts occur in both sexes: females experience reduced estrogen and progesterone, while males see declining testosterone. Such age-related hormonal decline during menopause and andropause directly reshapes immune cell functions by modulating sex hormone signaling (171–174). In detail, for menopause (female), postmenopausal estrogen drops by 60%-80%, reducing bone marrow GM-CSF secretion, impairing neutrophil maturation (25% lower mature proportion) and bacterial phagocytosis (52). Additionally, B and T cell counts decline (with fewer circulating lymphocytes vs. younger women), pro-inflammatory cytokines (IL-1β, IL-6, tumor necrosis factor-α) are markedly elevated, and T cell apoptosis increases following natural or surgical menopause. Estrogen-containing hormone replacement therapy in postmenopausal women improves immune function by increasing circulating B cell counts and reducing baseline pro-inflammatory cytokine levels, relative to non- hormone replacement therapy users. For andropause (male), studies indicate that low testosterone impairs CD4+T cell counts, Th1 responses, and Treg function; however, the impact of testosterone replacement therapy on immunity in aged men remains unreported (175–177).
4.2 Environmental exposures
Environmental factors serve as another critical modulator that can amplify or alter inherent immune sexual dimorphisms by interfering with sex hormone signaling pathways. The core mechanism involves environmental pollutants acting as endocrine disruptors, which can competitively bind to sex hormone receptors or affect their expression, thereby dysregulating normal hormone-immune communication (178). For instance, cigarette smoke may suppress the expression of ERα in lung tissue, attenuating the estrogen-mediated enhancement of neutrophil phagocytic function and consequently reducing the antibacterial capacity in female smokers. This is supported by clinical research showing that smoking causes a statistically significant decrease in phagocytic activity specifically in female subjects, impairing host defense functions (179, 180). Similarly, pollutants like PM2.5 are implicated in potentiating the inhibitory effects of androgens on immune cells such as NK cells, which may contribute to a gender-differential susceptibility to diseases like lung cancer (179). Animal studies reveal that the inflammatory response to particulate matter differs fundamentally by sex: exposed male mice show markers linked to acute inflammation (e.g., IL-1β, cyclooxygenase-2), whereas females exhibit increases in mediators like IL-17 that may promote chronic inflammation and tissue remodeling. Further evidence demonstrates that exposure to environmental stressors—including chlorine inhalation injury or prenatal stress, can lead to significant sex-based differences in immune cell migration, activation, and inflammatory responses (59, 178, 181).
4.3 Lifestyle interventions
Lifestyle factors are increasingly recognized as potential modulators of sexual dimorphism in immune responses, with growing evidence revealing sex-specific nuances in immune cell responses to these interventions beyond shared regulatory goals. For instance, dietary patterns such as high-fat diets can activate pathways that preferentially enhance estrogen-mediated M2 polarization of macrophages in females, contributing to differential metabolic disease susceptibility (182, 183). Similarly, the immunomodulatory effects of physical activity exhibit distinct sexual dimorphism (184). In males, aerobic exercise can elevate testosterone, which acts directly on CD4+T lymphocytes to increase the production of the anti-inflammatory cytokine IL-10 (185). Concurrently, exercise boosts Tregs’ frequency and suppressive capacity with post-pubertal males typically having more abundant, suppressive circulating Tregs than females (186, 187). Meanwhile, males show greater immune sensitivity to stress, due to androgens activating corticotropin-releasing hormone neurons expressing androgen receptors, enhancing hypothalamic-pituitary-adrenal axis activity and glucocorticoid release, which in turn triggers more intense apoptosis of immune cells like thymic T cells (188). These findings underscore that the impact of common lifestyle factors, though broadly beneficial, is precisely fine-tuned by biological sex at the level of specific immune cell subsets and functions.
5 Concluding remarks
For decades, sex disparities exist and affect health and disease, yet underlying mechanisms are largely understudied, including the synergistic regulatory effects between sex hormones and X/Y chromosome genes which remain to be deciphered. Our review undertakes a comprehensive synthesis, focusing on sex as a pivotal regulator in both physiological processes and pathological states. We expound upon various factors, including hormones and chromosomes, and dissect their profound impacts on physiology and diseases through immune cells. The aim is to elucidate sex - specific responses, thereby paving the way for the implementation of sex - tailored precision medicine. The aforementioned research also provides clinically transferable insights, as using sex hormones to modulate neutrophil functions and apoptosis may impede disease progression; or combining sex hormones with immune checkpoint inhibitors may enhance the anti-tumor abilities of cells, such as CD8+T and Tregs (as a typical example of translating hormone-immune axis mechanisms into clinical applications). Further exploration of sex dimorphism in emerging immune cell subsets, such as ILC1/ILC3 and γδT cells identified in recent years, will fill critical gaps in understanding sex-biased immunity and disease.
Although research efforts have increasingly illuminated the underlying mechanisms, further advancements in depth and methodology are essential. We anticipate novel studies should consider four key directions: Firstly, as current understanding of sex differences is shallow, with the rapid progress of life sciences and biotechnology, new techniques like single-cell RNA sequencing and clustered regularly interspaced short palindromic repeats-Cas9 gene editing can seek a breakthrough. The former helps explore cell characteristics related to sex differences, and the latter enables precise gene modifications in sex-related processes. Secondly, targeting hormone-immune axes for precision medicine. Regarding the application of sex hormones or agonists and inhibitors of their signaling pathways, the main problem is the overabundance of drug targets, resulting in significant side effects. Hence, it is crucial to develop targeted approaches like nano-delivery systems, which can proficiently modulate sex-specific cellular responses and minimize the incidence of adverse side effects. Thirdly, clinical studies should integrate hormonal fluctuations across key life stages (125) (as adolescence, reproductive, menopause) and consider ‘gender’ identity when assessing treatment responses. This will help tailor interventions to the specific needs of diverse patient populations, ensuring more precise and effective care. Lastly, policy and clinical practice must address the unique health needs of women, particularly in light of sex-specific disease outcomes. Efforts should also focus on raising awareness about sex differences in medical care, using research findings to guide policy decisions and reduce health disparities. By pursuing these directions, we can enhance our understanding of sex differences in cells, health and disease, ultimately leading to more personalized and equitable healthcare strategies.
Statements
Author contributions
XL: Writing – original draft, Investigation. DW: Investigation, Writing – original draft, Visualization. JG: Visualization, Software, Writing – original draft. FX: Visualization, Writing – original draft. ZL: Writing – original draft, Software. WM: Writing – original draft, Validation. KW: Writing – review & editing, Validation, Funding acquisition. YG: Conceptualization, Writing – review & editing, Visualization. QL: Writing – review & editing, Conceptualization, Funding acquisition. WG: Funding acquisition, Writing – review & editing, Project administration.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by National Natural Science Foundation of China (grant number: 82570119), the Natural Science Foundation of Shanghai (grant number: 25ZR1402443), Healthcare Talents Youth Program of Shanghai Pudong New Area (grant number: 2025PDWSYCQN-02) for Wei Gao; National Natural Science Foundation of China (grant number: 82270116), Shanghai East Hospital Key Discipline Funding of Respiratory Medicine (grant number: 2024-DFZD-002) for Qiang Li.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AR, Androgen Receptor; Bca, Breast Cancer; CXCL, C-X-C Motif Chemokine Ligand; CXCR, C-X-C chemokine receptor; DC, Dendritic Cell; DHEA, Dehydroepiandrosterone; E2, Estradiol; ER, Estrogen Receptor; FOXP3, Forkhead box P3; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; GR, Glucocorticoid Receptor; IFN, Interferon; IL, Interleukin; ILC2, Group 2 innate lymphoid cell; M, Macrophage; NETs, Neutrophil Extracellular Traps; NK, Natural killer cell; NKT, Natural killer T cell; PD-1, Programmed death receptor 1; PD-L1, Programmed cell death 1 ligand 1; PPAR-γ, Peroxisome proliferator-activated receptor gamma; PR, Progesterone Receptor; ROS, Reactive oxygen species; SRY, Sex-determining Region Y; Th, T helper cell; TLR, Toll-like receptor; Treg, Regulatory T cell.
References
1
Clayton JA . Studying both sexes: a guiding principle for biomedicine. FASEB J. (2016) 30:519–24. doi: 10.1096/fj.15-279554
2
Drug safety: most drugs withdrawn in recent years had greater health risks for women. Available online at: https://www.gao.gov/products/gao-01-286r (Accessed December 9, 2024).
3
Merdji H Long MT Ostermann M Herridge M Myatra SN De Rosa S et al . Sex and gender differences in intensive care medicine. Intensive Care Med. (2023) 49:1155–67. doi: 10.1007/s00134-023-07194-6
4
Attaway AH Scheraga RG Bhimraj A Biehl M Hatipoglu U . Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ. (2021) 372:n436. doi: 10.1136/bmj.n436
5
Moss M Mannino DM . Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996). Crit Care Med. (2002) 30:1679–85. doi: 10.1097/00003246-200208000-00001
6
Klein SL Flanagan KL . Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
7
Dou DR Zhao Y Belk JA Zhao Y Casey KM Chen DC et al . Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. (2024) 187:733–749.e716. doi: 10.1016/j.cell.2023.12.037
8
Gorman EA O’Kane CM McAuley DF . Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. (2022) 400:1157–70. doi: 10.1016/S0140-6736(22)01439-8
9
Erfinanda L Ravindran K Kohse F Gallo K Preissner R Walther T et al . Oestrogen-mediated upregulation of the Mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. Eur Respir J. (2021) 57:2000921. doi: 10.1183/13993003.00921-2020
10
Scully EP Haverfield J Ursin RL Tannenbaum C Klein SL . Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. (2020) 20:442–7. doi: 10.1038/s41577-020-0348-8
11
Stabellini N Tomlinson B Cullen J Shanahan J Waite K Montero AJ et al . Sex differences in adults with acute myeloid leukemia and the impact of sex on overall survival. Cancer Med. (2023) 12:6711–21. doi: 10.1002/cam4.5461
12
Tettero JM Cloos J Bullinger L . Acute myeloid leukemia: does sex matter? Leukemia. (2024) 38:2329–31. doi: 10.1038/s41375-024-02435-z
13
Goemaere S Ackerman C Goethals K De Keyser F van der Straeten C Verbruggen G et al . Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J Rheumatol. (1990) 17:1620–2.
14
Marozkina N Zein J DeBoer MD Logan L Veri L Ross K et al . Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: A pilot study. Pulm Ther. (2019) 5:213–20. doi: 10.1007/s41030-019-00101-9
15
Zein JG McManus JM Sharifi N Erzurum SC Marozkina N Lahm T et al . Benefits of airway androgen receptor expression in human asthma. Am J Respir Crit Care Med. (2021) 204:285–93. doi: 10.1164/rccm.202009-3720OC
16
Vijeyakumaran M Jawhri MA Fortunato J Solomon L Shrestha Palikhe N Vliagoftis H et al . Dual activation of estrogen receptor alpha and glucocorticoid receptor upregulate CRTh2-mediated type 2 inflammation; mechanism driving asthma severity in women? Allergy. (2023) 78:767–79. doi: 10.1111/all.15543
17
Zein JG Erzurum SC . Asthma is different in women. Curr Allergy Asthma Rep. (2015) 15:28. doi: 10.1007/s11882-015-0528-y
18
DeBoer MD Phillips BR Mauger DT Zein J Erzurum SC Fitzpatrick AM et al . Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med. (2018) 18:58. doi: 10.1186/s12890-018-0612-x
19
Fink AL Engle K Ursin RL Tang WY Klein SL . Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U.S.A. (2018) 115:12477–82. doi: 10.1073/pnas.1805268115
20
Chen W Mempel M Traidl-Hofmann C Al Khusaei S Ring J . Gender aspects in skin diseases. J Eur Acad Dermatol Venereol. (2010) 24:1378–85. doi: 10.1111/j.1468-3083.2010.03668.x
21
Payne AH Hales DB . Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. (2004) 25:947–70. doi: 10.1210/er.2003-0030
22
Miller WL . Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. (2013) 379:62–73. doi: 10.1016/j.mce.2013.04.014
23
Skaletsky H Kuroda-Kawaguchi T Minx PJ Cordum HS Hillier L Brown LG et al . The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. (2003) 423:825–37. doi: 10.1038/nature01722
24
Nelson LR Bulun SE . Estrogen production and action. J Am Acad Dermatol. (2001) 45:S116–124. doi: 10.1067/mjd.2001.117432
25
Sherman BM West JH Korenman SG . The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. (1976) 42:629–36. doi: 10.1210/jcem-42-4-629
26
Khosla S Melton LJ 3rd Atkinson EJ O’Fallon WM . Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. (2001) 86:3555–61. doi: 10.1210/jcem.86.8.7736
27
Hutson DD Gurrala R Ogola BO Zimmerman MA Mostany R Satou R et al . Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ. (2019) 10:4. doi: 10.1186/s13293-019-0219-9
28
Girgert R Emons G Gründker C . Estrogen signaling in ERα-negative breast cancer: ERβ and GPER. Front Endocrinol (Lausanne). (2018) 9:781. doi: 10.3389/fendo.2018.00781
29
Alencar AKN Swan KF Pridjian G Lindsey SH Bayer CL . Connecting G protein-coupled estrogen receptor biomolecular mechanisms with the pathophysiology of preeclampsia: a review. Reprod Biol Endocrinol. (2023) 21:60. doi: 10.1186/s12958-023-01112-7
30
Hall JM Couse JF Korach KS . The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. (2001) 276:36869–72. doi: 10.1074/jbc.R100029200
31
Chimento A Sirianni R Delalande C Silandre D Bois C Andò S et al . 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Mol Cell Endocrinol. (2010) 320:136–44. doi: 10.1016/j.mce.2010.01.035
32
Zhang Z Teng CT . Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. (2000) 275:20837–46. doi: 10.1074/jbc.M001880200
33
Bennett NC Gardiner RA Hooper JD Johnson DW Gobe GC . Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. (2010) 42:813–27. doi: 10.1016/j.biocel.2009.11.013
34
Vaisbuch E Erez O Romero R . Physiology of progesterone. In: CarpHJA, editor. Progestogens in obstetrics and gynecology. Springer International Publishing, Cham (2021). p. 1–29.
35
Linowiecka K Urbanowska-Domańska O Guz J Foksiński M . The potential influence of breast cancer estrogen receptors’ distribution on active DNA demethylation. Contemp Oncol (Pozn). (2019) 23:74–80. doi: 10.5114/wo.2019.85200
36
Kasubuchi M Watanabe K Hirano K Inoue D Li X Terasawa K et al . Membrane progesterone receptor beta (mPRβ/Paqr8) promotes progesterone-dependent neurite outgrowth in PC12 neuronal cells via non-G protein-coupled receptor (GPCR) signaling. Sci Rep. (2017) 7:5168. doi: 10.1038/s41598-017-05423-9
37
Naamneh Elzenaty R du Toit T Flück CE . Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab. (2022) 36:101665. doi: 10.1016/j.beem.2022.101665
38
Pihlajamaa P Sahu B Jänne OA . Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. (2015) 36:357–84. doi: 10.1210/er.2015-1034
39
McKenna NJ Lanz RB O’Malley BW . Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. (1999) 20:321–44. doi: 10.1210/edrv.20.3.0366
40
Libert C Dejager L Pinheiro I . The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. (2010) 10:594–604. doi: 10.1038/nri2815
41
Umiker BR Andersson S Fernandez L Korgaokar P Larbi A Pilichowska M et al . Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur J Immunol. (2014) 44:1503–16. doi: 10.1002/eji.201344283
42
Broen JC Wolvers-Tettero IL Geurts-van Bon L Vonk MC Coenen MJ Lafyatis R et al . Skewed X chromosomal inactivation impacts T regulatory cell function in systemic sclerosis. Ann Rheum Dis. (2010) 69:2213–6. doi: 10.1136/ard.2010.129999
43
Casimir GJ Mulier S Hanssens L Zylberberg K Duchateau J . Gender differences in inflammatory markers in children. Shock. (2010) 33:258–62. doi: 10.1097/SHK.0b013e3181b2b36b
44
Muenchhoff M Goulder PJR . Sex differences in pediatric infectious diseases. J Infect Dis. (2014) 209:S120–6. doi: 10.1093/infdis/jiu232
45
Loke H Harley V Lee J . Biological factors underlying sex differences in neurological disorders. Int J Biochem Cell Biol. (2015) 65:139–50. doi: 10.1016/j.biocel.2015.05.024
46
Case LK Teuscher C . Y genetic variation and phenotypic diversity in health and disease. Biol Sex Differ. (2015) 6:6. doi: 10.1186/s13293-015-0024-z
47
Case LK Wall EH Dragon JA Saligrama N Krementsov DN Moussawi M et al . The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. (2013) 23:1474–85. doi: 10.1101/gr.156703.113
48
Burn GL Foti A Marsman G Patel DF Zychlinsky A . The neutrophil. Immunity. (2021) 54:1377–91. doi: 10.1016/j.immuni.2021.06.006
49
Blesson C . (2011).
50
Blazkova J Gupta S Liu Y Gaudilliere B Ganio EA Bolen CR et al . Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol. (2017) 198:2479–88. doi: 10.4049/jimmunol.1601855
51
Gupta S Nakabo S Blanco LP O’Neil LJ Wigerblad G Goel RR et al . Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci U.S.A. (2020) 117:16481–91. doi: 10.1073/pnas.2003603117
52
Prada-Medina CA Peron JPS Nakaya HI . Immature neutrophil signature associated with the sexual dimorphism of systemic juvenile idiopathic arthritis. J Leukoc Biol. (2020) 108:1319–27. doi: 10.1002/JLB.6MA0720-015RR
53
Robertson SA Mayrhofer G Seamark RF . Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol Reprod. (1996) 54:183–96. doi: 10.1095/biolreprod54.1.183
54
Furman D Hejblum BP Simon N Jojic V Dekker CL Thiébaut R et al . Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U.S.A. (2014) 111:869–74. doi: 10.1073/pnas.1321060111
55
Chuang KH Altuwaijri S Li G Lai JJ Chu CY Lai KP et al . Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med. (2009) 206:1181–99. doi: 10.1084/jem.20082521
56
Hreha TN Collins CA Cole EB Jin RJ Hunstad DA . Androgen exposure impairs neutrophil maturation and function within the infected kidney. mBio. (2024) 15:e0317023. doi: 10.1128/mbio.03170-23
57
Scotland RS Stables MJ Madalli S Watson P Gilroy DW . Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. (2011) 118:5918–27. doi: 10.1182/blood-2011-03-340281
58
Madalli S Beyrau M Whiteford J Duchene J Singh Nandhra I Patel NS et al . Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol Sex Differ. (2015) 6:27. doi: 10.1186/s13293-015-0047-5
59
Chatterjee T Lewis TL Arora I Gryshyna AE Underwood L Masjoan Juncos JX et al . Sex-based disparities in leukocyte migration and activation in response to inhalation lung injury: role of SDF-1/CXCR4 signaling. Cells. (2023) 12:1719. doi: 10.3390/cells12131719
60
Lim CL Or YZ Ong Z Chung HH Hayashi H Shrestha S et al . Estrogen exacerbates mammary involution through neutrophil-dependent and -independent mechanism. Elife. (2020) 9:e57274. doi: 10.7554/eLife.57274
61
Lasarte S Samaniego R Salinas-Muñoz L Guia-Gonzalez MA Weiss LA Mercader E et al . Sex hormones coordinate neutrophil immunity in the vagina by controlling chemokine gradients. J Infect Dis. (2016) 213:476–84. doi: 10.1093/infdis/jiv402
62
Scalerandi MV Peinetti N Leimgruber C Cuello Rubio MM Nicola JP Menezes GB et al . Inefficient N2-like neutrophils are promoted by androgens during infection. Front Immunol. (2018) 9:1980. doi: 10.3389/fimmu.2018.01980
63
Spitzer JA Zhang P . Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation. (1996) 20:485–98. doi: 10.1007/BF01487041
64
Pokhrel S Triplett KD Daly SM Joyner JA Sharma G Hathaway HJ et al . Complement receptor 3 contributes to the sexual dimorphism in neutrophil killing of staphylococcus aureus. J Immunol. (2020) 205:1593–600. doi: 10.4049/jimmunol.2000545
65
DeLeon-Pennell KY Mouton AJ Ero OK Ma Y Padmanabhan Iyer R Flynn ER et al . LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. (2018) 113:40. doi: 10.1007/s00395-018-0699-5
66
Lu RJ Taylor S Contrepois K Kim M Bravo JI Ellenberger M et al . Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex- and age-related functional regulation. Nat Aging. (2021) 1:715–33. doi: 10.1038/s43587-021-00086-8
67
LeDuc LE Nast CC . Chemotactic peptide-induced acute colitis in rabbits. Gastroenterology. (1990) 98:929–35. doi: 10.1016/0016-5085(90)90017-U
68
Pergola C Rogge A Dodt G Northoff H Weinigel C Barz D et al . Testosterone suppresses phospholipase D, causing sex differences in leukotriene biosynthesis in human monocytes. FASEB J. (2011) 25:3377–87. doi: 10.1096/fj.11-182758
69
Dai R Cowan C Heid B Khan D Liang Z Pham CT et al . Neutrophils and neutrophil serine proteases are increased in the spleens of estrogen-treated C57BL/6 mice and several strains of spontaneous lupus-prone mice. PloS One. (2017) 12:e0172105. doi: 10.1371/journal.pone.0172105
70
Patel D Dodd WS Lucke-Wold B Chowdhury MAB Hosaka K Hoh BL . Neutrophils: novel contributors to estrogen-dependent intracranial aneurysm rupture via neutrophil extracellular traps. J Am Heart Assoc. (2023) 12:e029917. doi: 10.1161/JAHA.123.029917
71
Giaglis S Stoikou M Sur Chowdhury C Schaefer G Grimolizzi F Rossi SW et al . Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the pro-NETotic effect of estrogen and G-CSF. Front Immunol. (2016) 7:565. doi: 10.3389/fimmu.2016.00565
72
Adachi A Honda T Egawa G Kanameishi S Takimoto R Miyake T et al . Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol. (2022) 150:909–919.e908. doi: 10.1016/j.jaci.2022.03.028
73
Chung HH Or YZ Shrestha S Loh JT Lim CL Ong Z et al . Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution. Sci Rep. (2017) 7:46485. doi: 10.1038/srep46485
74
Markman JL Porritt RA Wakita D Lane ME Martinon D Noval Rivas M et al . Loss of testosterone impairs anti-tumor neutrophil function. Nat Commun. (2020) 11:1613. doi: 10.1038/s41467-020-15397-4
75
Tulchinsky D Hobel CJ . Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17 alpha-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. Am J Obstet Gynecol. (1973) 117:884–93. doi: 10.1016/0002-9378(73)90057-4
76
Watson RW O’Neill A Brannigan AE Coffey R Marshall JC Brady HR et al . Regulation of Fas antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett. (1999) 453:67–71. doi: 10.1016/S0014-5793(99)00688-2
77
Molloy EJ O’Neill AJ Grantham JJ Sheridan-Pereira M Fitzpatrick JM Webb DW et al . Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. (2003) 102:2653–9. doi: 10.1182/blood-2003-02-0649
78
Lim CL Lin VC . Estrogen markedly reduces circulating low-density neutrophils and enhances pro-tumoral gene expression in neutrophil of tumour-bearing mice. BMC Cancer. (2021) 21:1017. doi: 10.1186/s12885-021-08751-2
79
Schweizer M . A study of the sex difference in the number of circulating eosinophils in the Guinea pig. Endocrinology. (1955) 56:693–8. doi: 10.1210/endo-56-6-693
80
Cephus JY Gandhi VD Shah R Brooke Davis J Fuseini H Yung JA et al . Estrogen receptor-α signaling increases allergen-induced IL-33 release and airway inflammation. Allergy. (2021) 76:255–68. doi: 10.1111/all.14491
81
Lauzon-Joset JF Mincham KT Abad AP Short BP Holt PG Strickland DH et al . Oestrogen amplifies pre-existing atopy-associated Th2 bias in an experimental asthma model. Clin Exp Allergy. (2020) 50:391–400. doi: 10.1111/cea.13544
82
Artham S Juras PK Goyal A Chakraborty P Byemerwa J Liu S et al . Estrogen signaling suppresses tumor-associated tissue eosinophilia to promote breast tumor growth. Sci Adv. (2024) 10:eadp2442. doi: 10.1126/sciadv.adp2442
83
Slater JE Kaliner M . Effects of sex hormones on basophil histamine release in recurrent idiopathic anaphylaxis. J Allergy Clin Immunol. (1987) 80:285–90. doi: 10.1016/0091-6749(87)90033-9
84
Grobe W Peng W Allam JP Yu CF Novak N . Androgens have an anti-inflammatory effect on human basophils. vitro. Allergy. (2020) 75:992–4. doi: 10.1111/all.14131
85
Dakup PP Porter KI Little AA Zhang H Gaddameedhi S . Sex differences in the association between tumor growth and T cell response in a melanoma mouse model. Cancer Immunol Immunother. (2020) 69:2157–62. doi: 10.1007/s00262-020-02643-3
86
Staples JE Gasiewicz TA Fiore NC Lubahn DB Korach KS Silverstone AE . Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. (1999) 163:4168–74. doi: 10.4049/jimmunol.163.8.4168
87
Zhu ML Bakhru P Conley B Nelson JS Free M Martin A et al . Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. (2016) 7:11350. doi: 10.1038/ncomms11350
88
Dragin N Bismuth J Cizeron-Clairac G Biferi MG Berthault C Serraf A et al . Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. (2016) 126:1525–37. doi: 10.1172/JCI81894
89
Roden AC Moser MT Tri SD Mercader M Kuntz SM Dong H et al . Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. (2004) 173:6098–108. doi: 10.4049/jimmunol.173.10.6098
90
Fairweather D Beetler DJ McCabe EJ Lieberman SM . Mechanisms underlying sex differences in autoimmunity. J Clin Invest. (2024) 134(18):e180076. doi: 10.1172/JCI180076
91
Sun L Su Y Jiao A Wang X Zhang B . T cells in health and disease. Signal Transduct Target Ther. (2023) 8:235. doi: 10.1038/s41392-023-01471-y
92
Cenci S Toraldo G Weitzmann MN Roggia C Gao Y Qian WP et al . Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U.S.A. (2003) 100:10405–10. doi: 10.1073/pnas.1533207100
93
Hoh BL Rojas K Lin L Fazal HZ Hourani S Nowicki KW et al . Estrogen deficiency promotes cerebral aneurysm rupture by upregulation of th17 cells and interleukin-17A which downregulates E-cadherin. J Am Heart Assoc. (2018) 7:e008863. doi: 10.1161/JAHA.118.008863
94
Park HJ Park HS Lee JU Bothwell AL Choi JM . Gender-specific differences in PPARγ regulation of follicular helper T cell responses with estrogen. Sci Rep. (2016) 6:28495. doi: 10.1038/srep28495
95
Mohammad I Starskaia I Nagy T Guo J Yatkin E Väänänen K et al . Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. (2018) 11(526):eaap9415. doi: 10.1126/scisignal.aap9415
96
Andersson A Stubelius A Karlsson MN Engdahl C Erlandsson M Grahnemo L et al . Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res Ther. (2015) 17:32. doi: 10.1186/s13075-015-0548-y
97
Garnier L Laffont S Lélu K Yogev N Waisman A Guéry JC . Estrogen signaling in bystander foxp3(neg) CD4(+) T cells suppresses cognate th17 differentiation in trans and protects from central nervous system autoimmunity. J Immunol. (2018) 201:3218–28. doi: 10.4049/jimmunol.1800417
98
Ejima A Abe S Shimba A Sato S Uehata T Tani-Ichi S et al . Androgens alleviate allergic airway inflammation by suppressing cytokine production in th2 cells. J Immunol. (2022) 209:1083–94. doi: 10.4049/jimmunol.2200294
99
Sciarra F Campolo F Franceschini E Carlomagno F Venneri MA . Gender-specific impact of sex hormones on the immune system. Int J Mol Sci. (2023) 24(7):6302. doi: 10.3390/ijms24076302
100
Girón-González JA Moral FJ Elvira J García-Gil D Guerrero F Gavilán I et al . Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. (2000) 143:31–6. doi: 10.1530/eje.0.1430031
101
Polanczyk MJ Carson BD Subramanian S Afentoulis M Vandenbark AA Ziegler SF et al . Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. (2004) 173:2227–30. doi: 10.4049/jimmunol.173.4.2227
102
Ishikawa A Wada T Nishimura S Ito T Okekawa A Onogi Y et al . Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PloS One. (2020) 15:e0230885. doi: 10.1371/journal.pone.0230885
103
Polanczyk MJ Hopke C Huan J Vandenbark AA Offner H . Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. (2005) 170:85–92. doi: 10.1016/j.jneuroim.2005.08.023
104
Goodman WA Bedoyan SM Havran HL Richardson B Cameron MJ Pizarro TT . Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc Natl Acad Sci U.S.A. (2020) 117:17166–76. doi: 10.1073/pnas.2002266117
105
Xiong Y Zhong Q Palmer T Benner A Wang L Suresh K et al . Estradiol resolves pneumonia via ERβ in regulatory T cells. JCI Insight. (2021) 6(3):e133251. doi: 10.1172/jci.insight.133251
106
Gandhi VD Cephus JY Norlander AE Chowdhury NU Zhang J Ceneviva ZJ et al . Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J Clin Invest. (2022) 132(4):e153397. doi: 10.1172/JCI153397
107
Vasanthakumar A Chisanga D Blume J Gloury R Britt K Henstridge DC et al . Sex-specific adipose tissue imprinting of regulatory T cells. Nature. (2020) 579:581–5. doi: 10.1038/s41586-020-2040-3
108
Kang JH Zappasodi R . Modulating Treg stability to improve cancer immunotherapy. Trends Cancer. (2023) 9:911–27. doi: 10.1016/j.trecan.2023.07.015
109
Kwon H Schafer JM Song NJ Kaneko S Li A Xiao T et al . Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci Immunol. (2022) 7:eabq2630. doi: 10.1126/sciimmunol.abq2630
110
Guan X Polesso F Wang C Sehrawat A Hawkins RM Murray SE et al . Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. (2022) 606:791–6. doi: 10.1038/s41586-022-04522-6
111
Yang C Jin J Yang Y Sun H Wu L Shen M et al . Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity. (2022) 55:1268–1283.e1269. doi: 10.1016/j.immuni.2022.05.012
112
Zhang X Cheng L Gao C Chen J Liao S Zheng Y et al . Androgen signaling contributes to sex differences in cancer by inhibiting NF-κB activation in T cells and suppressing antitumor immunity. Cancer Res. (2023) 83:906–21. doi: 10.1158/0008-5472.CAN-22-2405
113
Lee J Nicosia M Hong ES Silver DJ Li C Bayik D et al . Sex-Biased T-cell exhaustion drives differential immune responses in glioblastoma. Cancer Discov. (2023) 13:2090–105. doi: 10.1158/2159-8290.CD-22-0869
114
Dhalech AH Condotta SA Pattnaik A Corn C Richer MJ Robinson CM . Coxsackievirus B3 elicits a sex-specific CD8+ T cell response which protects female mice. PloS Pathog. (2023) 19:e1011465. doi: 10.1371/journal.ppat.1011465
115
Fan CY McAllister BB Stokes-Heck S Harding EK Pereira de Vasconcelos A Mah LK et al . Divergent sex-specific pannexin-1 mechanisms in microglia and T cells underlie neuropathic pain. Neuron. (2025) 113:896–911.e899. doi: 10.1016/j.neuron.2025.01.005
116
Crosby CM Kronenberg M . Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. (2018) 18:559–74. doi: 10.1038/s41577-018-0034-2
117
Fowlkes BJ Kruisbeek AM Ton-That H Weston MA Coligan JE Schwartz RH et al . A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. (1987) 329:251–4. doi: 10.1038/329251a0
118
Gourdy P Araujo LM Zhu R Garmy-Susini B Diem S Laurell H et al . Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood. (2005) 105:2415–20. doi: 10.1182/blood-2004-07-2819
119
Gourdy P Bourgeois EA Levescot A Pham L Riant E Ahui ML et al . Estrogen therapy delays autoimmune diabetes and promotes the protective efficiency of natural killer T-Cell activation in female nonobese diabetic mice. Endocrinology. (2016) 157:258–67. doi: 10.1210/en.2015-1313
120
Cuño-Gómiz C de Gregorio E Tutusaus A Rider P Andrés-Sánchez N Colell A et al . Sex-based differences in natural killer T cell-mediated protection against diet-induced steatohepatitis in Balb/c mice. Biol Sex Differ. (2023) 14:85. doi: 10.1186/s13293-023-00569-w
121
Lotter H Helk E Bernin H Jacobs T Prehn C Adamski J et al . Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PloS One. (2013) 8:e55694. doi: 10.1371/journal.pone.0055694
122
Humeniuk P Barrett A Axelsson H Corciulo C Drevinge C Pons ADC et al . Profiling of innate and adaptive immune cells during influenza virus infection reveals sex bias in invariant natural killer T (iNKT) cells. Immun Inflammation Dis. (2023) 11:e837. doi: 10.1002/iid3.837
123
Vivier E Rebuffet L Narni-Mancinelli E Cornen S Igarashi RY Fantin VR . Natural killer cell therapies. Nature. (2024) 626:727–36. doi: 10.1038/s41586-023-06945-1
124
Zhou N Yue Y Xiong S . Sex hormone contributes to sexually dimorphic susceptibility in CVB3-induced viral myocarditis via modulating IFN-γ(+) NK cell production. Can J Cardiol. (2018) 34:492–501. doi: 10.1016/j.cjca.2018.01.002
125
Menees KB Earls RH Chung J Jernigan J Filipov NM Carpenter JM et al . Sex- and age-dependent alterations of splenic immune cell profile and NK cell phenotypes and function in C57BL/6J mice. Immun Ageing. (2021) 18:3. doi: 10.1186/s12979-021-00214-3
126
Tang M Sun Y Huang CP Chen L Liu B You B et al . High dose androgen suppresses natural killer cytotoxicity of castration-resistant prostate cancer cells via altering AR/circFKBP5/miRNA-513a-5p/PD-L1 signals. Cell Death Dis. (2022) 13:746. doi: 10.1038/s41419-022-04956-w
127
Liu Q You B Meng J Huang CP Dong G Wang R et al . Targeting the androgen receptor to enhance NK cell killing efficacy in bladder cancer by modulating ADAR2/circ_0001005/PD-L1 signaling. Cancer Gene Ther. (2022) 29:1988–2000. doi: 10.1038/s41417-022-00506-w
128
Park MD Silvin A Ginhoux F Merad M . Macrophages in health and disease. Cell. (2022) 185:4259–79. doi: 10.1016/j.cell.2022.10.007
129
Gay L Melenotte C Lopez A Desnues B Raoult D Leone M et al . Impact of sex hormones on macrophage responses to coxiella burnetii. Front Immunol. (2021) 12:705088. doi: 10.3389/fimmu.2021.705088
130
Chao TC Phuangsab A Van Alten PJ Walter RJ . Steroid sex hormones and macrophage function: regulation of chemiluminescence and phagocytosis. Am J Reprod Immunol. (1996) 35:106–13. doi: 10.1111/j.1600-0897.1996.tb00015.x
131
Meng Q Bi Y Feng H Ding X Zhang S Chen Q et al . Activation of estrogen receptor α inhibits TLR4 signaling in macrophages and alleviates the instability of atherosclerotic plaques in the postmenopausal stage. Int Immunopharmacol. (2023) 116:109825. doi: 10.1016/j.intimp.2023.109825
132
Lou Y Fu Z Tian Y Hu M Wang Q Zhou Y et al . Estrogen-sensitive activation of SGK1 induces M2 macrophages with anti-inflammatory properties and a Th2 response at the maternal-fetal interface. Reprod Biol Endocrinol. (2023) 21:50. doi: 10.1186/s12958-023-01102-9
133
Balan I Aurelian L Williams KS Campbell B Meeker RB Morrow AL . Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: Sex differences and structural requirements. Front Immunol. (2022) 13:940095. doi: 10.3389/fimmu.2022.940095
134
Shu Z Zhang G Zhu X Xiong W . Estrogen receptor α mediated M1/M2 macrophages polarization plays a critical role in NASH of female mice. Biochem Biophys Res Commun. (2022) 596:63–70. doi: 10.1016/j.bbrc.2022.01.085
135
Hou H Adzika GK Wu Q Ma T Ma Y Geng J et al . Estrogen attenuates chronic stress-Induced cardiomyopathy by adaptively regulating macrophage polarizations via β(2)-Adrenergic receptor modulation. Front Cell Dev Biol. (2021) 9:737003. doi: 10.3389/fcell.2021.737003
136
Keselman A Fang X White PB Heller NM . Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol. (2017) 199:1573–83. doi: 10.4049/jimmunol.1601975
137
Bang BR Chun E Shim EJ Lee HS Lee SY Cho SH et al . Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp Mol Med. (2011) 43:275–80. doi: 10.3858/emm.2011.43.5.028
138
Becerra-Díaz M Lerner AD Yu DH Thiboutot JP Liu MC Yarmus LB et al . Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. (2021) 360:104252. doi: 10.1016/j.cellimm.2020.104252
139
Xiang P Wang L Feng X Guo Q Xie G Sheng L et al . Sexual dimorphism of lung immune-regulatory units imprint biased pulmonary fibrosis. Cell Mol Immunol. (2025) 22:743–59. doi: 10.1038/s41423-025-01293-8
140
Chen L Zou X Liu CC Yan P Deng J Wang C et al . Earlier onset of chemotherapy-induced neuropathic pain in females by ICAM-1-mediated accumulation of perivascular macrophages. Sci Adv. (2025) 11:eadu2159. doi: 10.1126/sciadv.adu2159
141
Deny M Arroba Nuñez LA Romano M Denis O Casimir G Chamekh M . Sex difference in innate inflammatory response and macrophage polarization in Streptococcus agalactiae-induced pneumonia and potential role of microRNA-223-3p. Sci Rep. (2022) 12:17126. doi: 10.1038/s41598-022-21587-5
142
De Leeuw E Hammad H . The role of dendritic cells in respiratory viral infection. Eur Respir Rev. (2024) 33:230250. doi: 10.1183/16000617.0250-2023
143
Zhang J Ye ZW Chen W Manevich Y Mehrotra S Ball L et al . S-Glutathionylation of estrogen receptor α affects dendritic cell function. J Biol Chem. (2018) 293:4366–80. doi: 10.1074/jbc.M117.814327
144
Seillet C Laffont S Trémollières F Rouquié N Ribot C Arnal JF et al . The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood. (2012) 119:454–64. doi: 10.1182/blood-2011-08-371831
145
Meier A Chang JJ Chan ES Pollard RB Sidhu HK Kulkarni S et al . Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. (2009) 15:955–9. doi: 10.1038/nm.2004
146
Sisirak V Ganguly D Lewis KL Couillault C Tanaka L Bolland S et al . Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med. (2014) 211:1969–76. doi: 10.1084/jem.20132522
147
Rowland SL Riggs JM Gilfillan S Bugatti M Vermi W Kolbeck R et al . Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. (2014) 211:1977–91. doi: 10.1084/jem.20132620
148
Iwano R Iwashita N Takagi Y Fukuyama T . Estrogen receptor α activation aggravates imiquimod-induced psoriasis-like dermatitis in mice by enhancing dendritic cell interleukin-23 secretion. J Appl Toxicol. (2020) 40:1353–61. doi: 10.1002/jat.3988
149
Khaw YM Anwar S Zhou J Kawano T Lin PC Otero A et al . Estrogen receptor alpha signaling in dendritic cells modulates autoimmune disease phenotype in mice. EMBO Rep. (2023) 24:e54228. doi: 10.15252/embr.202154228
150
Masuda C Miyasaka T Kawakami K Inokuchi J Kawano T Dobashi-Okuyama K et al . Sex-based differences in CD103(+) dendritic cells promote female-predominant Th2 cytokine production during allergic asthma. Clin Exp Allergy. (2018) 48:379–93. doi: 10.1111/cea.13081
151
Malinczak CA Fonseca W Mire MM Parolia A Chinnaiyan A Rasky AJ et al . Sex-associated early-life viral innate immune response is transcriptionally associated with chromatin remodeling of type-I IFN-inducible genes. Mucosal Immunol. (2023) 16:578–92. doi: 10.1016/j.mucimm.2023.06.002
152
Licona-Limón P Kim LK Palm NW Flavell RA . TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. (2013) 14:536–42. doi: 10.1038/ni.2617
153
Cephus JY Stier MT Fuseini H Yung JA Toki S Bloodworth MH et al . Testosterone attenuates group 2 innate lymphoid cell-Mediated airway inflammation. Cell Rep. (2017) 21:2487–99. doi: 10.1016/j.celrep.2017.10.110
154
Aw M Nair P Salter B Chen R Machida K Inman M et al . Effect of sex on group 2 innate lymphoid cells in the airways of mild and severe asthmatics. Allergy. (2019) 74:1397–400. doi: 10.1111/all.13742
155
Wang C Xu ZB Peng YQ Zhang HY Yu QN Guo YB et al . Sex differences in group 2 innate lymphoid cell-dominant allergic airway inflammation. Mol Immunol. (2020) 128:89–97. doi: 10.1016/j.molimm.2020.09.019
156
Zhao H Moarbes V Gaudreault V Shan J Aldossary H Cyr L et al . Sex differences in IL-33-induced STAT6-dependent type 2 airway inflammation. Front Immunol. (2019) 10:859. doi: 10.3389/fimmu.2019.00859
157
Laffont S Blanquart E Savignac M Cénac C Laverny G Metzger D et al . Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. (2017) 214:1581–92. doi: 10.1084/jem.20161807
158
Chi L Liu C Gribonika I Gschwend J Corral D Han SJ et al . Sexual dimorphism in skin immunity is mediated by an androgen-ILC2-dendritic cell axis. Science. (2024) 384:eadk6200. doi: 10.1126/science.adk6200
159
Tao X Wang Y Xiang B Hu D Xiong W Liao W et al . Sex bias in tumor immunity: insights from immune cells. Theranostics. (2025) 15:5045–72. doi: 10.7150/thno.106465
160
Peckham H Webb K Rosser EC Butler G Ciurtin C . Gender-diverse inclusion in immunological research: benefits to science and health. Front Med (Lausanne). (2022) 9:909789. doi: 10.3389/fmed.2022.909789
161
Forsyth KS Jiwrajka N Lovell CD Toothacre NE Anguera MC . The conneXion between sex and immune responses. Nat Rev Immunol. (2024) 24:487–502. doi: 10.1038/s41577-024-00996-9
162
Grimaldi CM Cleary J Dagtas AS Moussai D Diamond B . Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. (2002) 109:1625–33. doi: 10.1172/JCI0214873
163
Kanda N Tsuchida T Tamaki K . Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. (1999) 42:328–37. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#
164
Asaba J Bandyopadhyay M Kindy M Dasgupta S . Estrogen receptor signal in regulation of B cell activation during diverse immune responses. Int J Biochem Cell Biol. (2015) 68:42–7. doi: 10.1016/j.biocel.2015.08.012
165
Jones BG Penkert RR Xu B Fan Y Neale G Gearhart PJ et al . Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol Immunol. (2016) 77:97–102. doi: 10.1016/j.molimm.2016.07.015
166
Hill L Jeganathan V Chinnasamy P Grimaldi C Diamond B . Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Mol Med. (2011) 17:211–20. doi: 10.2119/molmed.2010.00172
167
Zhao R Chen X Ma W Zhang J Guo J Zhong X et al . A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature. (2020) 577:416–20. doi: 10.1038/s41586-019-1873-0
168
Wilhelmson AS Lantero Rodriguez M Stubelius A Fogelstrand P Johansson I Buechler MB et al . Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat Commun. (2018) 9:2067. doi: 10.1038/s41467-018-04408-0
169
Souyris M Cenac C Azar P Daviaud D Canivet A Grunenwald S et al . TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. (2018) 3(19):eaap8855. doi: 10.1126/sciimmunol.aap8855
170
Yu B Qi Y Li R Shi Q Satpathy AT Chang HY . B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell. (2021) 184:1790–1803.e1717. doi: 10.1016/j.cell.2021.02.015
171
Gubbels Bupp MR Potluri T Fink AL Klein SL . The confluence of sex hormones and aging on immunity. Front Immunol. (2018) 9:1269. doi: 10.3389/fimmu.2018.01269
172
Shibata T Sakamoto J Osaka Y Neyatani N Fujita S Oka Y et al . Myeloperoxidase in blood neutrophils during normal and abnormal menstrual cycles in women of reproductive age. Int J Lab Hematol. (2017) 39:169–74. doi: 10.1111/ijlh.12599
173
Perheentupa A Huhtaniemi I . Aging of the human ovary and testis. Mol Cell Endocrinol. (2009) 299:2–13. doi: 10.1016/j.mce.2008.11.004
174
Bain J . Testosterone and the aging male: to treat or not to treat? Maturitas. (2010) 66:16–22. doi: 10.1016/j.maturitas.2010.01.009
175
Deguchi K Kamada M Irahara M Maegawa M Yamamoto S Ohmoto Y et al . Postmenopausal changes in production of type 1 and type 2 cytokines and the effects of hormone replacement therapy. Menopause. (2001) 8:266–73. doi: 10.1097/00042192-200107000-00008
176
Giglio T Imro MA Filaci G Scudeletti M Puppo F De Cecco L et al . Immune cell circulating subsets are affected by gonadal function. Life Sci. (1994) 54:1305–12. doi: 10.1016/0024-3205(94)00508-7
177
Kamada M Irahara M Maegawa M Yasui T Yamano S Yamada M et al . B cell subsets in postmenopausal women and the effect of hormone replacement therapy. Maturitas. (2001) 37:173–9. doi: 10.1016/S0378-5122(00)00180-8
178
Sanchez SS Sillé FCM . Sex-specific effects of environmental pollutants on pulmonary immune responses. Curr Opin Physiol. (2025) 43:100813. doi: 10.1016/j.cophys.2025.100813
179
Sharma S Gibbons A Saphire EO . Sex differences in tissue-specific immunity and immunology. Science. (2025) 389:599–603. doi: 10.1126/science.adx4381
180
Sato J Takahashi I Umeda T Matsuzaka M Danjyo K Tsuya R et al . Effect of alcohol drinking and cigarette smoking on neutrophil functions in adults. Luminescence. (2011) 26:557–64. doi: 10.1002/bio.1270
181
Yoshizaki K Brito JM Silva LF Lino-Dos-Santos-Franco A Frias DP RCR ES et al . The effects of particulate matter on inflammation of respiratory system: Differences between male and female. Sci Total Environ. (2017) 586:284–95. doi: 10.1016/j.scitotenv.2017.01.221
182
Vick LV Rosario S Riess JW Canter RJ Mukherjee S Monjazeb AM et al . Potential roles of sex-linked differences in obesity and cancer immunotherapy: revisiting the obesity paradox. NPJ Metab Health Dis. (2024) 2:5. doi: 10.1038/s44324-024-00007-4
183
Xing E Billi AC Gudjonsson JE . Sex bias and autoimmune diseases. J Invest Dermatol. (2022) 142:857–66. doi: 10.1016/j.jid.2021.06.008
184
Gillum TL Kuennen MR Schneider S Moseley P . A review of sex differences in immune function after aerobic exercise. Exerc Immunol Rev. (2011) 17:104–21.
185
Liva SM Voskuhl RR . Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. (2001) 167:2060–7. doi: 10.4049/jimmunol.167.4.2060
186
Robinson GA Peng J Peckham H Butler G Pineda-Torra I Ciurtin C et al . Investigating sex differences in T regulatory cells from cisgender and transgender healthy individuals and patients with autoimmune inflammatory disease: a cross-sectional study. Lancet Rheumatol. (2022) 4:e710–24. doi: 10.1016/S2665-9913(22)00198-9
187
Blank M Israeli D Shoenfeld Y . Exercise, autoimmune diseases and T-regulatory cells. J Autoimmun. (2024) 149:103317. doi: 10.1016/j.jaut.2024.103317
188
Meng Y Li Y Gu H Chen Z Cui X Wang X . Androgen receptors in corticotropin-releasing hormone neurons mediate the sexual dimorphism in restraint-induced thymic atrophy. Proc Natl Acad Sci U.S.A. (2025) 122:e2426107122. doi: 10.1073/pnas.2426107122
Summary
Keywords
disease, immune cells, physiology, sex disparity, sexual hormone
Citation
Liao X, Wu D, Gao J, Xie F, Li Z, Wang M, Wang K, Gao Y, Li Q and Gao W (2026) Sex differences in immune cells: mechanistic perspectives and clinical correlates in physiology and disease. Front. Immunol. 17:1650527. doi: 10.3389/fimmu.2026.1650527
Received
18 January 2026
Revised
26 January 2026
Accepted
31 January 2026
Published
17 February 2026
Volume
17 - 2026
Edited by
Fahmida Alam, Independent University, Bangladesh
Reviewed by
Xuefang Cao, University of Maryland, United States
Perry Ayn Mayson Maza, Baylor College of Medicine, United States
Dingchuan Peng, Capital Medical University, China
Updates
Copyright
© 2026 Liao, Wu, Gao, Xie, Li, Wang, Wang, Gao, Li and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Gao, grace19881118@126.com; Qiang Li, liqressh1962@163.com; Yixuan Gao, gaoyixua@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.