Abstract
The mucosal immune system represents the largest and most significant component of the immune network, providing critical defense against infectious pathogens at mucosal surfaces. Mucosal surfaces include the oronasal cavities, ocular surface, gastrointestinal tract, respiratory tract, and reproductive tract. Mucosal tissue-resident memory (TRM) CD4+ and CD8+ TRM cells serve as sentinels and critical mediators of adaptive mucosal immunity, continuously trafficking to mucosal tissues to surveil and clear invading pathogens. The development of mucosal CD4+ and CD8+ TRM cells is regulated by mechanisms distinct from those governing circulating effector memory (TEM) and central memory (TCM) T cells. Current models suggest that the generation, retention, and expansion of CD4+ and CD8+ TRM cells within mucosal tissues are coordinated by mucosa-specific chemokines and adhesion molecules, thereby facilitating their selective homing and retention at mucosal surfaces. Among the 48 known chemokines, CXCL17, CCL25, CCL28, and CXCL14 have emerged as major key mucosal-specific chemokines that orchestrate mucosal CD4+ and CD8+ TRM cell responses. This review (1) describes the roles of these four major mucosal chemokines in shaping TRM cell-mediated immunity against mucosal pathogens, with a focus on herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), two infectious pathogens of the ocular and genital mucosae and (2) discuss harnessing these mucosal chemokine–receptor axes to develop a tissue-targeted Prime/Pull/Keep (PPK) herpes vaccine and immunotherapeutic strategies.
1 Introduction
Mucosal tissues serve as critical frontline barriers, continually exposed to a wide array of infectious pathogens (1). The mucosal immune system, the most significant component of the overall immune network, safeguards these vulnerable sites, including the oral, nasal, ocular, gastrointestinal, respiratory, and female reproductive tracts, the principal portals of pathogen entry (2).
It is now well established that a distinct subset of non-recirculating tissue-resident memory T cells (TRM) arises and persists within peripheral non-lymphoid mucosal tissues (3). These mucosal TRM cells are phenotypically and functionally distinct from conventional circulating memory T cells, including effector memory (TEM) and central memory (TCM) subsets, which transit through the bloodstream and secondary lymphoid and non-lymphoid tissues (4–6). The developmental pathways regulating the generation, retention, and expansion of CD4+ and CD8+ TRM cells within mucosal tissues are mechanistically distinct from those that guide conventional TEM and TCM populations (7–10).
Current paradigms suggest that the tissue-specific trafficking of memory and effector CD4+ and CD8+ T cells to mucosal sites is governed by the coordinated expression of adhesion molecules and epithelial-derived chemokines, often in a tissue-restricted manner (11–19) (Table 1). The identification of epithelial-expressed chemokines, such as CCL25 in the small intestine and CCL28 in the respiratory and genital tracts, underscores the pivotal role of chemokines in directing T cell localization at mucosal surfaces under both homeostatic and inflammatory conditions (20, 35–39). This chemokine-mediated trafficking, known as lymphocyte homing, enables the selective accumulation of distinct TRM subsets within mucosal tissues. Thus, chemokine-driven positioning of both antigen-presenting cells (APCs) and T cells is fundamental to the development of TRM-mediated immune surveillance and protection against mucosal infections.
Table 1
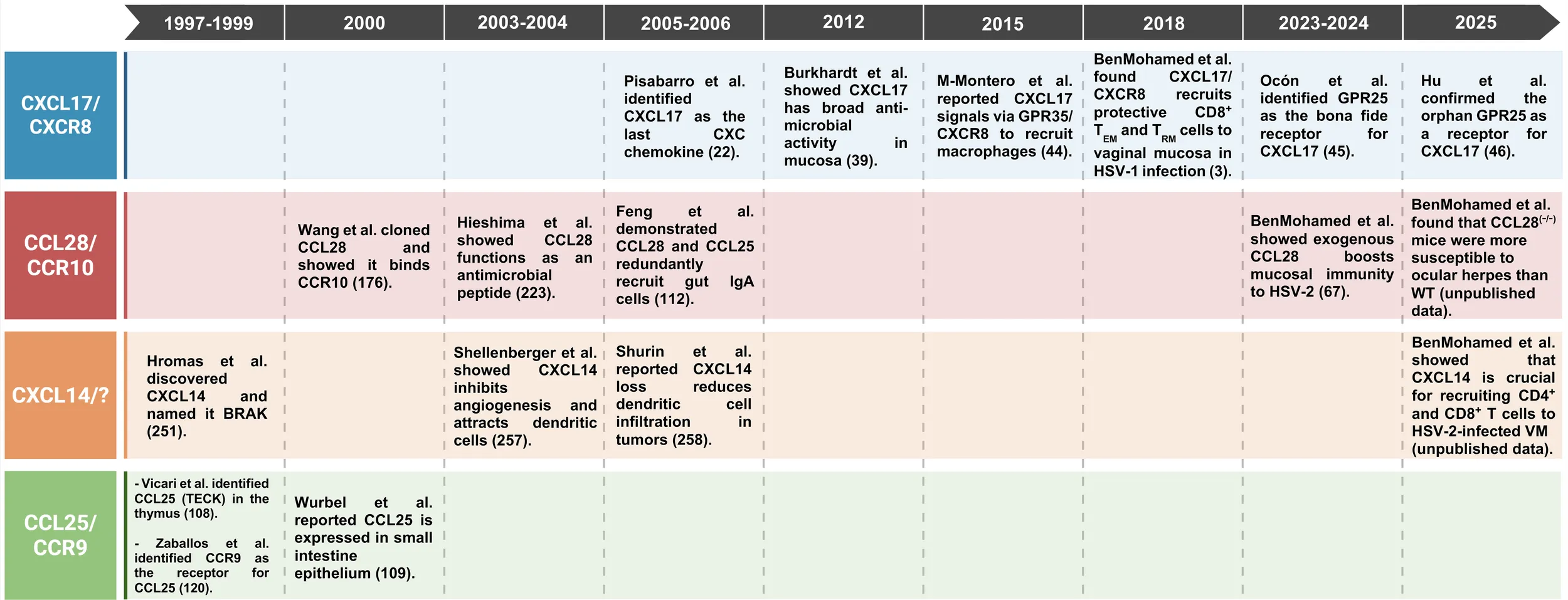
|
Timeline highlighting key advances in the study of chemokines CXCL17, CXCL14, CCL28, and CCL25, along with their receptors.
Chemokines constitute a family of approximately 50 small, secreted polypeptides characterized by chemotactic activity (40, 41) and classified into four subfamilies (C, CC, CXC, and CX3C) based on conserved cysteine motifs (42). Inflammatory chemokines predominantly regulate immune responses during infection and injury, whereas homeostatic chemokines orchestrate immune cell migration during tissue maintenance and immune homeostasis. By binding to G protein-coupled receptors on leukocytes, chemokines direct the trafficking of various immune cell subsets, including CD4+ and CD8+ T cells, across both lymphoid and non-lymphoid compartments (40, 43, 44). While several chemokines, such as CCL5, CXCL9, CXCL10, CXCL11, and CCL18, have well-characterized roles in non-mucosal T-cell recruitment (45–49), mucosa-associated chemokines, including CXCL17, CCL25, CCL28, and CXCL14, have emerged as key regulators of T-cell immunity at mucosal surfaces under both steady-state and inflammatory conditions. However, the specific mechanisms by which these epithelial-derived chemokines influence the development, localization, and maintenance of mucosal TRM populations remain incompletely understood and are likely distinct from those governing conventional TEM and TCM subsets.
The TRM cells reside permanently within non-lymphoid tissues, such as mucosal surfaces, and do not recirculate through the blood. They are identified by markers such as CD69 and CD103, which facilitate tissue retention and residence. TRM cells serve as frontline sentinels, capable of rapid responses to local antigen re-exposure, thereby providing immediate protective immunity and reinforcing barrier defense (50–52). (2) The TCM cells efficiently circulate between the blood and secondary lymphoid organs (SLOs), such as lymph nodes and spleen. They are characterized by expression of CCR7 and CD62L, which support homing to SLOs. Upon antigen challenge, TCM cells proliferate and give rise to effector T cells, bolstering the immune response throughout the body (21); and (3) The TEM cells circulate through non-lymphoid tissues (NLTs) and blood, lacking lymphoid homing markers (CCR7, CD62L). TEM cells are poised for rapid effector function and can respond promptly to pathogens in peripheral tissues, serving a surveillance role across different organ systems (53). Chemokines play a critical role in the development and localization of the T cell memory subsets described above (54). Chemokines such as CXCL9, CXCL10, and CCL25 promote the recruitment, retention, and compartmentalization of TRM cells within barrier tissues by inducing the expression of specific homing and retention molecules (e.g., CD69, CD103). These chemokine-mediated signals help guide memory precursor T cells to mucosal sites where they can differentiate into TRM cells and sustain long-term local immunity. Additionally, chemokines regulate the migration patterns of TCM and TEM cell subsets, ensuring efficient immune surveillance and the establishment of robust immunological memory at mucosal barriers (54).”
In this review, we focus on the physiological roles of the major mucosal-specific chemokines CXCL17, CCL25, CCL28, and CXCL14 in shaping CD4+ and CD8+ TRM cell-mediated immunity to mucosal infectious diseases (Figure 1 and Table 1). The phylogenetic analysis of all these mucosal chemokines (CXCL14, CXCL17, CCL25, CCL28) and their receptors (GPR35, GPR25, CXCR4, CCR9, CCR10, and CCR3) revealed, notably, a smaller genetic distance among the mucosal chemokines, indicating an evolutionary relationship among them (Figure 2).
Figure 1
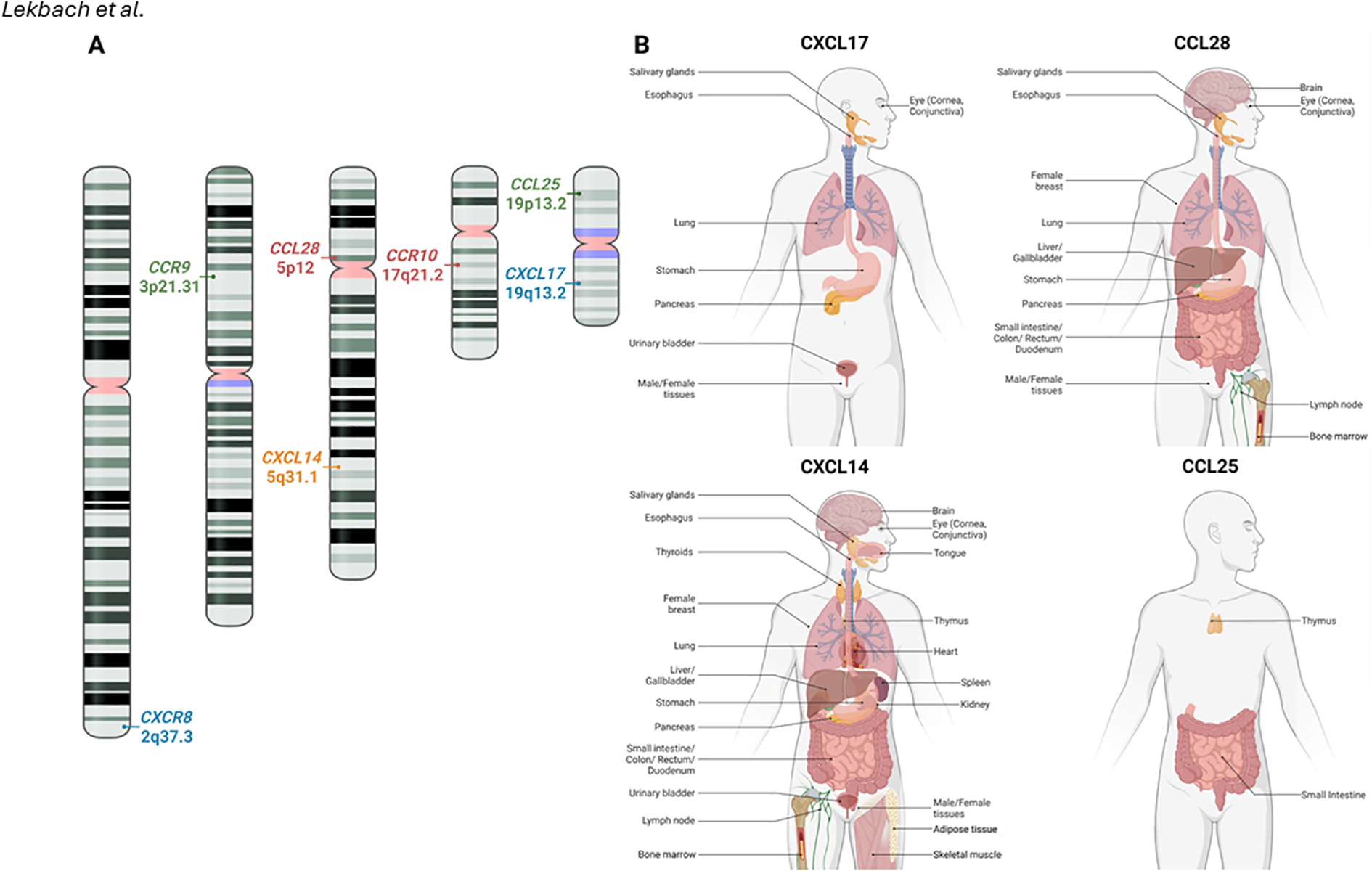
Mucosal chemokines chromosomal location and tissue expression in humans: (A) The chromosomal positions of chemokine genes CXCL17, CCL28, CXCL14, and CCL25, along with their respective receptors, are illustrated on human chromosomes. (B) The expression of CXCL17, CCL28, CXCL14, and CCL25 in human tissues. Data was retrieved from the Human Protein Atlas (www.proteinatlas.org). The receptor for CXCL14 has yet to be identified and created with BioRender.com.
Figure 2

Evolutionary comparison between different mucosal chemokines and their receptors: (A) Genetic distances based on Maximum Composite Likelihood model among the mucosal chemokines (CXCL14, CXCL17, CCL25, CCL28) and their receptors (GPR35, GPR25, CXCR4, CCR9, CCR10, and CCR3). Nucleotide sequences were specific to human for these mucosal chemokines and their receptors. In comparison to CXCL14, results indicate less genetic distance (indicating genetic similarity) among other mucosal chemokines CCL28 (3.201), CXCL17 (2.531), and CCL25 (2.964) respectively. (B) Phylogenetic analysis performed to evaluate evolutionary relationship among the mucosal chemokines and their receptors using the UPGMA method. The optimal tree is shown. The tree is drawn to scale, with branch lengths (next to the branches) in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. All ambiguous positions were removed for each sequence pair (pairwise deletion). There was a total of 56106 positions in the final dataset for the nucleotide sequences of the 10 mucosal chemokine ligands and receptors. Evolutionary analyses were conducted in MEGA11.
Furthermore, we discuss emerging strategies targeting chemokine–receptor axes to enhance mucosal vaccine efficacy and develop novel immunotherapeutic approaches against HSV-1 and HSV-2 infections.
2 The mucosal chemokine (C-X-C motif) ligand 17
2.1 The CXCL17 chemokine is primarily expressed by mucosal tissues of the oral cavity, gastrointestinal, respiratory, and genital tracts
The mucosal chemokine CXCL17 was first characterized in 2006 by Pisabarro et al. (Genentech Inc., San Francisco, CA) as a monocyte-attracting chemokine (Table 1) (37). CXCL17 is also referred to as Vascular Endothelial Correlated Chemokine (VCC-1) or VEGF Co-regulated Chemokine 1, and as Dendritic Cell and Monocyte Chemokine-like Protein (DMC) (37, 38). The CXCL17 gene is located on chromosome 19 at position 19q13.2 (Figure 1A). It consists of four exons that encode a 119-amino-acid propeptide, with an approximate molecular mass of ~13.8 kDa in humans and ~13.6 kDa in mice (37, 38). CXCL17 is constitutively expressed in the oral mucosal tissues, as well as the gastrointestinal, respiratory, and genital tracts (Figure 1B) (22, 55, 56). Additionally, CXCL17 has been implicated in regulating infection and inflammation in the gastrointestinal, respiratory, and female reproductive systems (23). Notably, it exhibits broad antimicrobial activity at mucosal surfaces (24). Nevertheless, the precise mechanisms by which CXCL17 mediates mucosal protection against invading pathogens remain largely unclear.
In 2022, Shabgah et al. reviewed the role of the chemokine CXCL17 in infection and inflammation (23). CXCL17 plays a crucial role in maintaining homeostasis across various mucosal tissues by regulating myeloid cell recruitment, promoting angiogenesis, and controlling microbial populations (23). Their findings confirmed that CXCL17: (1) under homeostatic conditions, exhibits anti-inflammatory, antibacterial, and chemotactic activities; (2) under pathological conditions such as malignancies, promotes angiogenesis, metastasis and cellular proliferation; (3) possesses anti-tumor properties; (3) is implicated in the pathogenesis of diseases including idiopathic pulmonary fibrosis, multiple sclerosis, asthma, and systemic sclerosis; and (5) may serve as a biomarker for disease diagnosis and prognosis via its dysregulation.
In 2024, Dominguez-Lopez et al. reported significant upregulation of CXCL17 expression in conjunctival and corneal mucosal epithelial cells in patients with dry eye disease (57). These findings suggest that corneal and conjunctival epithelial cells within the ocular mucosal immune system (OMIS) may serve as a source of CXCL17 during ocular infections and inflammatory responses (57–59).
2.2 CXCR8 (GPR35), GPR25, and CXCR4 are receptors of CXCL17 chemokine
Back in 2010, Fallarini et al. were the first to report on the expression of the orphan G protein-coupled receptor (GPR35) on human iNKT cells (60). Later, in 2015, Maravillas-Montero et al. confirmed that CXCL17 signals through GPR35, which was later renamed CXCR8 (61). Several studies have suggested that CXCR8 may have both pro-inflammatory and anti-inflammatory effects (60, 61). More recently, GPR25 has been identified as a receptor for CXCL17 by multiple groups (62, 63).
In 2018, Pease et al. reported the existence of another CXCL17 receptor, CXCR4 (20). Similarly, in 2018, Hill et al. confirmed that CXCR4 is the other receptor for CXCL17, in addition to CXCR8 (20, 35, 64). In 2023, Peace et al. demonstrated that the C-terminal fragments of CXCL17 readily bound glycosaminoglycans (GAGs) and may serve as prototypic inhibitors of CXCR4 function (20, 41, 65). Later, in 2024, Hill et al. confirmed that CXCL17 was an allosteric endogenous inhibitor of CXCR4, an effect that was mimicked by GAGs, surfen, and protamine sulfate (20, 35, 64). Disruption of putative GAG binding domains in CXCL17 prevented CXCR4 binding (20, 35, 64).
A characteristic feature of chemokines is their similar three-dimensional structure, four conserved cysteines that form two disulfide bonds, and binding to glycosaminoglycans (GAGs). Both disulfides are essential for receptor activation, and GAG binding is critical for the directed migration of leukocytes. However, CXCL17 has only three conserved cysteines (instead of four) and thus may not adopt the typical chemokine structure. It also exhibits a distinct distribution of positively charged residues, suggesting a novel GAG-binding mechanism. Another unusual feature of CXCL17 is that it is active only at high concentrations, with optimal activity at concentrations up to 1000-fold higher than those typically observed for most chemokines (µM vs. nM). Below, we review and discuss how CXCL17 regulates the homing of neutrophils, monocytes/macrophages, dendritic cells, and T cells to mucosal tissues (20, 35–39).
2.3 CXCL17 is a pro-inflammatory chemokine that promotes early neutrophil recruitment to inflamed mucosal tissues
Identifying the underlying mechanism of CXCL17’s activity in homeostatic, inflammatory, and pathological situations holds promise for the development of novel treatment strategies. CXCL17 has been implicated in several human pathologies and inflammatory diseases (39, 41, 56, 66–68). More recently, in 2024, Lowry et al. demonstrated that, compared to their littermate control wild-type (WT) mice, CXCL17(-/-) deficient mice were significantly impaired in peritoneal neutrophil recruitment following lipopolysaccharide (LPS) challenge (69). The CXCL17(-/-) deficient mice showed: (1) dysregulated levels of inflammatory mediators CXCL1, CXCR2, and IL6, all of which directly impact neutrophil recruitment (69); and (2) no difference in monocyte recruitment following LPS-challenge (69). The study confirmed that the CXCL17 pro-inflammatory chemokine plays a crucial role in early inflammatory responses by promoting neutrophil recruitment to the lungs (69).
2.4 CXCL17 promotes the recruitment of dendritic cells and macrophages to inflamed mucosal tissues of the respiratory tract
In 2014, Burkhardt et al. demonstrated in a mouse model that CXCL17 is a central chemotactic mediator for macrophage recruitment into the lungs (70). Compared with WT mice, Cxcl17(-/-)mice displayed a significant reduction in the frequency of macrophages in their lungs (70). In addition, the authors detected a concurrent increase in a new population of macrophage-like cells in the lungs of CXCL17(-/-) deficient mice, characterized by an F4/80(+)CD11c(mid) phenotype (70). Later, in 2019, Hernandez-Ruiz et al. confirmed that CXCL17(-/-) deficient mice displayed lower frequencies of macrophages and dendritic cells in the lungs. In 2021, Choreño-Parra et al. reported that CXCL17 production increased in the lungs of mice infected with M. tuberculosis, and that mice treated with recombinant CXCL17 showed increased accumulation of lung myeloid cells (71). Taken together, these results suggest that CXCL17 is a chemoattractant for both macrophages and dendritic cells in mucosal tissues of the respiratory tract.
Since CXCL17 facilitates the recruitment of both macrophages and dendritic cells, two primary antigen-presenting cells (APCs), into mucosal tissues (20, 35–39), we will review how CXCL17 regulates the trafficking and localization of CD4+ and CD8+ T cells to combat invading mucosal pathogens (Figure 3).
Figure 3
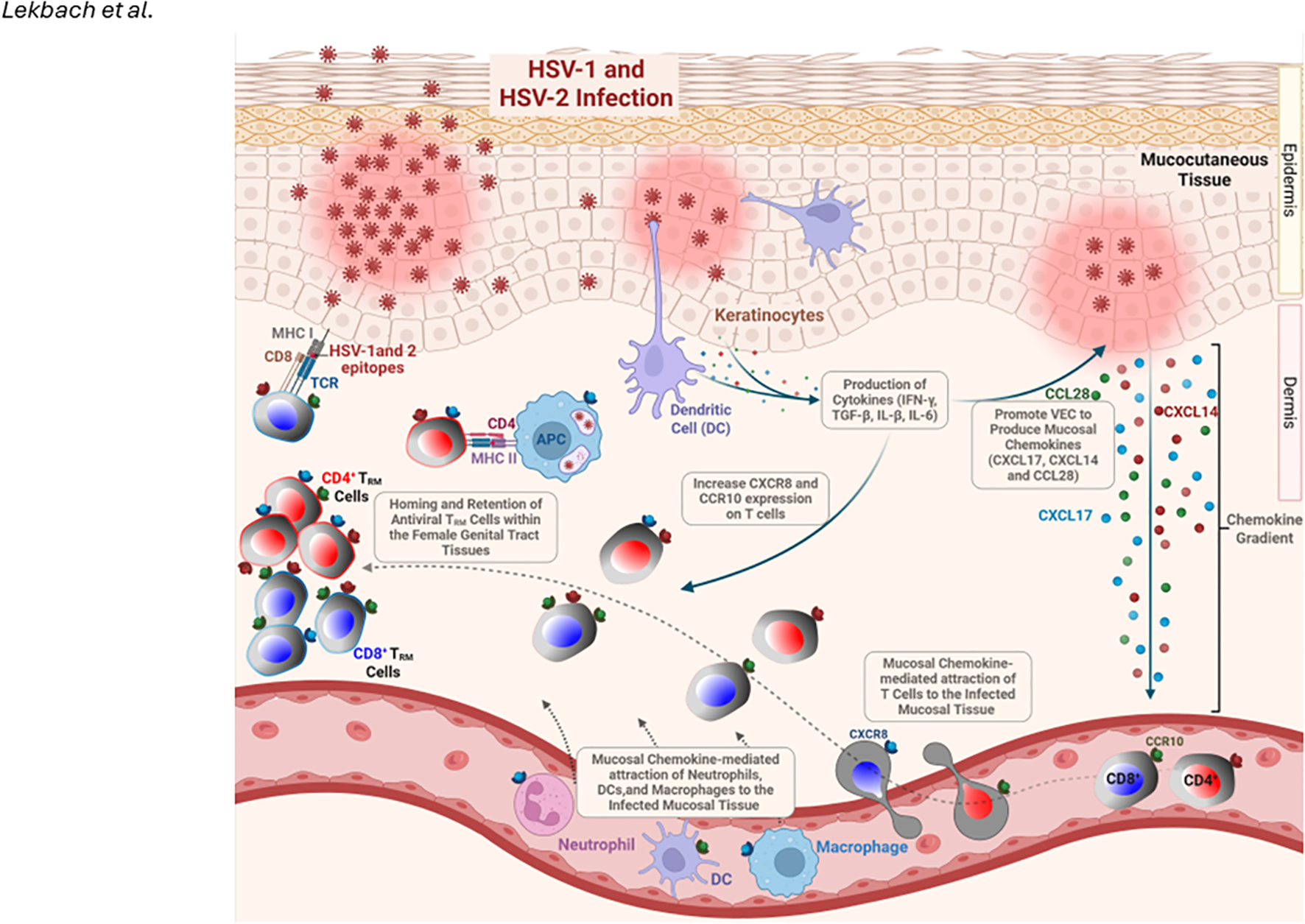
Role of Mucosal Chemokines in T Cell Homing and Retention in the Mucocutaneous Tissue During HSV-1/2 Infection: HSV-1/2 infection of vaginal epithelial cells (VECs) and keratinocytes triggers the production of inflammatory cytokines (IFN-γ, TGF-β, IL-1β, IL-6), which promote them to: (1) produce mucosal chemokines CXCL17, CXCL14, and CCL28, and (2) upregulate CXCR8 and CCR10 expression on T cells. These chemokines facilitate the homing and retention of antiviral tissue-resident memory (TRM) cells in the genital tract while also recruiting dendritic cells, neutrophils, and macrophages to the site of infection. Created with BioRender.com.
2.5 The CXCL17/CXCR8 chemokine/receptor pair regulates effector and regulatory CD4+ and CD8+ T cells trafficking and localization into inflamed and infected mucosal tissues
Studies have shown that GPR35 activation can stimulate the production of pro-inflammatory cytokines and facilitate the movement of immune cells, including effector CD4+ and CD8+ TRM cells, towards inflammatory and infected mucosal tissues of gastrointestinal tract, respiratory tract, and female genital tract (28, 60, 72–80). Conversely, other investigations have suggested that GPR35 possesses anti-inflammatory properties in mucosal tissues by inhibiting the generation of inflammatory mediators and promoting the differentiation of regulatory T cells (Treg cells) (28, 60, 72–82).
Later, in 2018, we reported that CXCL17 is involved in the recruitment of CD8+ T cells that mediate protective immunity against sexually transmitted herpes infection (4, 83). We used herpes simplex virus type 1 (HSV-1) as a model of a genital viral pathogen. We selected HSV-1 because (i) it is widely present in more than 3.7 billion people worldwide and (ii) it is becoming an increasingly common cause of genital infection, mainly in developed countries (84). We found that intravaginal HSV-1 infection of WT B6 mice increased the frequency of HSV-specific IFN-γ-producing cytotoxic CXCR8+CD8+ T cells in the female genital tract, and that this recruitment was associated with protection against genital herpes infection and disease. In contrast, when compared to WT B6 mice, following HSV-1 infection, the female genital tract of CXCL17(-/-) deficient mice developed significantly fewer HSV-specific functional IFN-gamma-producing cytotoxic CXCR8+CD8+ TRM cells. It generates more exhausted VISTA+TIGIT+CD8+ T cells that fail to control genital herpes infection (4, 83). These observations strongly suggest that the CXCL17/CXCR8 axis is a major regulator of female genital tract-resident CD103highCD8+ TRM cell responses, which lead to the clearance of genital herpes infection (4, 83). Whether the increase in the number of CXCR8+CD8+ T cells and CCR10+CD8+ T cells is due to increased recruitment, formation, retention, and/or expansion of these cells within the female genital tract remains to be determined.
In 2019, Hernandez-Ruiz et al. reported that CXCL17 is also a chemoattractant that suppresses myeloid cell chemoattraction and recruits regulatory T cells (85). Compared with WT littermate mice, CXCL17(-/-) mice developed more severe disease in a T-cell-dependent model of experimental autoimmune encephalomyelitis (EAE) (85). After immunization with myelin oligodendrocyte peptide, only 44% of CXCL17(-/-) deficient mice were still alive vs. 90% for WT mice. During EAE, CXCL17(-/-) mice exhibited reduced myeloid cell infiltration into the CNS and higher serum levels of inflammatory cytokines (85). In 2021, Choreño-Parra et al. reported that, in humans, lower serum CXCL17 levels are observed among active pulmonary TB patients than among subjects with latent TB infection and healthy controls, suggesting a protective role (71).
These reports suggest that CXCL17 may regulate the development of protective mucosal CD4+ and CD8+ TRM cells at steady state and during inflammatory and infectious conditions (Figure 3).
2.6 Role of CXCL17/CXCR8 chemokine/receptor axis in the development of genital tract mucosal CD4+ and CD8+ TRM cells against genital herpes
The genital tract mucosal surface represents one entry route of herpes simplex virus type 1 and/or type 2 (HSV-1 and HSV-2) (86, 87). Herpes infection is widespread in human populations, with a higher incidence in women than in men (84, 88–91). HSV viruses cause initial infections in the mucocutaneous tissues of the mouth, lips, genital tract, nose, or eyes (92). Most HSV-seropositive women are asymptomatic (ASYMP) (93–96). They do not experience any recurrent herpetic genital disease even though the virus spontaneously reactivates from latency and sheds multiple times each year in their vaginal secretions (89, 90, 97, 98). In contrast, a small proportion of HSV-seropositive women are symptomatic (SYMP) and experience endless recurrences of herpetic disease, usually multiple times a year (86, 87), often requiring continuous antiviral therapy (i.e., acyclovir and derivatives).
Mucosa-resident memory TRM cell subsets are phenotypically and functionally distinct from conventional TEM and TCM cell subsets that circulate through the blood to access lymphoid and non-lymphoid tissues (4–6, 99–101). The mechanisms that regulate the generation, retention, and expansion of genital tract mucosal CD4+ and CD8+ TRM cells are distinct from those regulating conventional circulating TEM and TCM cells (7–10). Direct experiments in animal models (102–104) and indirect evidence in humans (105, 106) suggest that the induction of robust, polyfunctional TRM cells residing within the vaginal submucosal tissues contributes to the successful control of herpes infection in the female genital tract (107). However, the female genital tract tissues appear to be immunologically restricted and remain “a closed immunological compartment, “ resistant to the homing of peripheral T cells that may originate from the draining lymph nodes and circulation (108, 109). Genital herpes infection of vaginal epithelial cells (VECs) likely triggered the production of inflammatory cytokines, such as IFN-gamma TGF-beta, IL-1beta, and IL-6, which promotes VECs: (1) to produce mucosal chemokines, such as CXCL17 and CCL28, and (2) increased CXCR8 expression on T cells contributing to homing and retention of antiviral TRM cells within the female genital tract tissues (Figure 3) (108, 109).
Specifically, CD8+ TRM cells mediate protection against genital herpes in both human and mouse models (90, 103, 110–114). CD8+ TRM cells are maintained in the dermal-epidermal junction of the female genital tract (32, 115). We recently demonstrated the role of CXCL17, a mucosal chemokine, in the development of anti-herpes CD8+ T cell memory (TRM) cells within the female genital tract (4). We showed that following intravaginal HSV-1 infection of WT B6 mice, the CXCL17 production is strongly induced in the female genital tract, and CXCL17 local expression paralleled an increase in both the number and effector function (IFN-gamma-production and CD107a/b degranulation) of HSV-specific CXCR8+CD8+ TRM cells associated with protection against genital herpes infection and disease (4). In contrast, HSV-1 infection of CXCL17(-/-) deficient mice resulted in an increased number of female genital tract-resident exhausted VISTA+TIGIT+CD8+ TRM cells, associated with a failure to contain genital herpes infection and disease (4). These results were the first to suggest a crucial role of the CXCL17/CXCR8 chemokine axis in the development of protective female genital tract mucosal CD8+ TRM cells (4). Besides effector CD8+ T cells, CD4+ T helper cells appeared to indirectly control the migration of CD8+ T cells through the secretion of IFN-gamma and local induction of chemokine secretion in the infected female genital tract tissue (34). We recently reported that the CXCL17 chemokine plays a role in CD4+ T cell immunity in the female genital tract (4, 83, 116). A recent study also showed that chemokines secreted by a local macrophage network maintained vaginal TRM cells in memory lymphocyte clusters (MLCs) independently of circulating memory T cells and dendritic cells (51).
In 2018, we reported that CXCR8 is constitutively expressed in female genital tract-derived B cells, T cells, dendritic cells (DCs), and monocytes/macrophages (4). CXCR8 expression on these cells increased following HSV-2 genital herpes infection (4). Moreover, both the frequency of female genital tract-resident CXCR8+CD8+ T cells and the expression level of CXCR8 were significantly increased following the intravaginal administration of exogenous CXCL10 in HSV-2-infected mice (4). These results suggest that, although the CXCL10/CXCR3 axis contributes to the recruitment of CD8+ T cells into the female genital tract, their retention within the mucosal epithelium for an extended period is controlled by the CXCL17:CXCR8 axis (4).
Therapeutic manipulation of the immune system, referred to as immunotherapy, is an attractive strategy for addressing genital herpes disease, HSV-1 and HSV-2 shedding, and ultimately reducing HSV-1 and HSV-2 transmission in the community (93–96). For successful herpes immunotherapy to occur, one must first identify the immune mechanisms underlying the apparent protection observed in seropositive asymptomatic women, who appear to contain infection and disease immunologically. Understanding the mechanisms by which CD8+ TRM cells develop, maintain, and expand in the female genital tract tissue will have a significant impact not only on genital herpes but also on other sexually transmitted infectious pathogens (STIs).
3 The mucosal chemokine (C-C motif) ligand 25 in human health and disease
3.1 The CCL25 chemokine is primarily expressed by mucosal tissues of the nasal cavity, gastrointestinal tract, and mammary glands
Herpes simplex virus (HSV) can infect the gastrointestinal tract, leading to conditions such as herpes gastritis, herpes proctitis, herpes esophagitis, and herpes colitis, which are rare but serious health concerns in immunocompromised patients (27, 117, 118). While the CCL25 mucosal chemokine is highly expressed in gut-associated lymphoid tissues (GALT), its role in gut immunity and immunopathology in response to herpes gastritis, herpes proctitis, herpes esophagitis, and herpes colitis remains to be fully determined.
The mucosal chemokine CCL25 belongs to the CC chemokine family, which is also known as TECK (Thymus-Expressed Chemokine). Vicari et al. first reported in 1997 that CCL25 is a thymic dendritic cell-specific CC chemokine involved in T-cell development (Table 1) (119). The CCL25 chemokine was first identified in the thymus of mice and mapped to chromosome 8. The primary source of CCL25 in the thymus was thymic dendritic cells and mucosal epithelial cells (Figure 1B) (120). In contrast, bone marrow-derived dendritic cells do not express CCL25. Later, in 1998, the human CCL25 gene (SCYA25) was mapped to chromosome 19 (Figure 1A) (121). Human CCL25 is produced as a 151-amino acid protein precursor (121). In humans, mice, and pigs, CCL25 plays a crucial role in the segregation and compartmentalization of the mucosal immune system by recruiting B and T cells to specific mucosal locations (121, 122). CCL25 attracts an overlapping subpopulation of IgA-secreting B cells, which are concentrated in the small intestine and its draining lymphoid tissues (33, 123–126).
Meurens et al. reported the cloning and the sequencing of ovine CCL25 and the subsequent assessment of its mRNA expression by quantitative PCR in several tissues, including thymus, gut-associated lymphoid tissue, and mammary gland, from young and adult sheep and in the fetal lamb during the development of the immune system (127–129). CCL25 mRNA is highly expressed in the thymus and gut. These results are consistent with observations in mice, humans, and other species (127–129). In fetuses, CCL25 mRNA is expressed early in the thymus, small intestine, and nasal mucosa. Furthermore, their expression increased towards the end of gestation (127–129). Consequently, CCL25 may play a crucial role in the lymphocyte colonization of fetal tissues, thereby facilitating the development of a functional immune system (127–129).
3.2 The CCL25 chemokine binds to CCR9 receptor expressed on specific subsets of mucosal-resident CD4+ and CD8+T cells
The CCR9 is a G protein-coupled receptor and the main receptor for CCL25 chemokine. Zaballos et al. first reported that the receptor for the CCL25 ligand is C-C chemokine receptor type 9 (CCR9) (130). In 2015, Kim et al. discovered CCR9-mediated signaling through beta-catenin and confirmed CCL25 as a CCR9 antagonist (131). The CCR9 receptor for CCL25 was later confirmed in 2000 by Gosling et al., who reported that it is expressed on dendritic cells (DCs) and T cells (132). CCR9 was also expressed on subsets of mucosal CD4+ and CD8+ T cells, gamma-delta T cells, plasmacytoid dendritic cells (pDCs), IgA plasma blasts, IgA plasma cells, and intraepithelial lymphocytes (IELs) from the mucosal tissues cited above (133, 134). CCR9 is specifically co-expressed on a subset of gut-homing B and T cells expressing the integrin α4β7, immunoglobulin A (IgA)- secreting B cells from the gastrointestinal tract. CCR9 drives the migration of these B and T cell subsets to gradients of its cognate ligand, the CCL25 chemokine (133, 134). CCR9 expression defines a subset of peripheral blood T cells with a mucosal phenotype and a Th1 or T-regulatory 1 cytokine profile (135–139). The CCR9 receptor equips these T cells to respond to CCL25 (140). Notably, CCR9 appears to be predominantly expressed on CD8+ T cells but less so on CD4+ T cell subsets, thereby imposing distinct tissue tropisms on CD4+ and CD8+ T cells in various mucosal tissues.
3.3 Role of CCL25/CCR9 chemokine/receptor axis in the development of mucosal tissues-resident CD4+ and CD8+ T cells
Naive CD4+ and CD8+ T cells require activation in lymphoid tissues before differentiating into effector or memory T cells capable of trafficking to nonlymphoid mucosal tissues (141, 142). Distinct non-recirculating TRM cell subsets are developed and retained within peripheral non-lymphoid mucosal tissues (4–6, 99–101, 143). These mucosa-resident TRM cells are phenotypically and functionally distinct from conventional memory T cells, which are defined as TEM and TCM cells that circulate in the blood and access lymphoid and non-lymphoid tissues (4–6, 99–101, 143). The mechanisms that regulate the generation, retention, and expansion of mucosal CD4+ and CD8+ TRM cells within the mucosal tissues are distinct from those regulating conventional circulating TEM and TCM cells (7–10). While there are well-established T-cell-attracting chemokines (i.e., CCL5, CXCL9, CXCL10, CXCL11, and CCL18) (45–49), several mucosal chemokines also play a role in shaping mucosal T cell immunity (92, 144–159). Tissue-selective trafficking of memory and effector CD4+ and CD8+ T cells is mediated by distinct combinations of adhesion molecules and chemokines (20, 35–39). The role of CCR9-CCL25 in immune cell migration, including effector and regulatory T cells, is well studied in both homeostasis and disease (133).
3.3.1 Role of the CCL25/CCR9 axis in the development of gut-resident T cells
The discovery of the related epithelial-expressed CCL25 chemokine in the small intestine and other diverse mucosal sites highlights an essential role for CCL25 in controlling memory and effector CD4+ and CD8+ T cells trafficking and localization in the intestine. Constitutively expressed epithelial chemokines may help determine the character of local T-cell responses and contribute to the organization of the gut immune system. CD4+ and CD8+ T cells primed by intestinal dendritic cells (DC) express the gut-homing receptors CCR9 and alpha4beta7 (α4β7), which recognize CCL25 and mucosal address cell-adhesion molecule-1 in the intestine, promoting the development of regional immunity (160–165).
The role of CCL25, a mucosal chemokine, in trafficking T cells into the gastrointestinal tract and shaping intestinal immunity was reported in 2002 by Kunkel, Campbell, and Butcher (166). Staton et al. later reported, in 2004 and 2006, that CD8+ recent thymic emigrants (RTEs) migrated directly into the small intestine and that CCR9, CCL25, and α4β7 integrin were all required for gut entry of these CD8+ RTEs (141, 142). After antigen-driven T cell receptor stimulation, the intestinal CD8+ RTEs proliferated and acquired a specific surface phenotype resembling that of intraepithelial T cells. These CD8+ RTEs efficiently populated the gut of lymphotoxin-alpha-deficient mice, which lack lymphoid organs (141, 142). These studies challenged the traditional concept of naive CD4+ and CD8+ T cell trafficking, suggesting that RTEs may play a role in maintaining a specific and diverse CD4+ and CD8+ T cell repertoire at mucosal surfaces (141, 142).
CCR9 overexpression was detrimental to the proper tissue distribution and terminal differentiation of CD4+ T cells in the gut epithelium (140). A recent study demonstrated that the expression of CCR9 interferes with tissue trafficking and differentiation of CD4+ T cells in the small intestine intraepithelial tissues (140). Specifically, the differentiation of small intestine epithelial-resident CD4+ T cells into immunoregulatory CD4+CD8αα+ T cells was impaired by the overexpression of CCR9 and conversely increased by the genetic deletion of CCR9 (140). The study revealed a previously unappreciated role for CCR9 in the tissue homeostasis and effector function of CD4+ T cells in the gut (140). More recently, in 2022, Li et al. reported that the chemokine receptor CCR9 suppresses the differentiation of CD4+CD8 α (+) intraepithelial T cells in the gut (140). In a mouse model, the recombinant CCL25 protein exhibited chemotactic activity for activated macrophages, dendritic cells, and thymocytes (140). CCR9 ameliorates inflammation in a T cell-mediated mouse colitis model (122).
Pathak et al. also demonstrated the role of the CCL25/CCR9 chemokine ligand/receptor pair in mucosal APC and T cell differentiation (133). Using a dextran sodium sulfate (DSS)-induced gut inflammation model, they demonstrated that CCR9(+) dendritic cells (DCs), specifically CD11b(-) CD103(+) DCs, were significantly increased in the GALT compared to control WT mice (133). These CCR9(+) DCs express lower levels of MHC II and CD86 molecules and possess regulatory surface markers, including FasL and latency-associated peptide (LAP), in the GALT. CCR9 signaling in DCs drives the differentiation of Foxp3+ Tregs and suppresses the allergic IgE response in the gut (133, 134). In the presence of CCL25, CCR9(+) DCs promoted in vitro differentiation of Foxp3(+) regulatory CD4(+) T cells (Tregs) (133). CCL25-induced differentiation of Tregs was due to intrinsic signaling in the DCs but not through CD4(+) T cells, which was driven by the production of thymic stromal lymphopoietin (TSLP) rather than IL-10 (133). Furthermore, the adoptive transfer of CCR9(+) DCs in WT C57BL/6 mice promoted Tregs and reduced Th17 cells in the GALT, thereby suppressing the OVA-specific gut-allergic response (133).
A 2022 study in a zebrafish model reported that CCR9+ T cells are recruited to a band in the lamina propria next to the muscular mucosa in which frequent CCL25-expressing cells are present. CCR9+ T cells interact with APCs for several minutes in a process mediated by Connexin 43 (167). This type of interaction was observed in both homeostasis and inflammation states, with the interaction being longer and more frequent during inflammation (167). The study suggested that the mucosal immune response in the intestinal mucosa is organized and organized into specific regions with specialized microenvironments and functions (167).
3.3.2 Role of CCL25 chemokine in the development of genital and gastrointestinal tract-resident protective CCR9+β7+ T cells
The location and manner of antigen-presenting cells (APCs) and T cells within the mucosal tissue are essential in the defense against mucosal infectious pathogens.
In 2008, Reinhart et al. measured chemokine and cytokine mRNA levels in multiple lymphoid tissue compartments from SIV-infected vs. uninfected cynomolgus macaques (Macaca fascicularis) (168). It was found that CCL25 mRNA levels in SIV-infected lymphoid tissues were decreased, and that CCL25 levels were negatively correlated with the numbers of proliferating and apoptotic cells (168). In vitro analyses revealed that CCL25 could reduce SIV-induced apoptosis (168). These findings suggest that increased apoptosis in lymphoid tissues, resulting from reduced levels of the anti-apoptotic chemokine CCL25, may contribute to the loss of immune function following pathogenic SIV infection (168).
In 2011, Cromwell et al. reported that SIV-specific CD8+ T cells are enriched in the female genital tract of rhesus macaques and express receptors for inflammatory chemokines, including CXCR3, CCR5, and CCR9 (169). Cromwell et al. found that the frequency of SIV-specific CD8+ T cells was 3- to 30-fold higher in genital mucosal tissues compared to peripheral blood (169). SIV-specific CD8+ T cells in genital mucosal tissues expressed high levels of CXCR3 and CCR5, chemokine receptors commonly expressed on memory T cells that home to inflamed tissues (169). Cells expressing CXCR3 colocalized with its chemokine ligand CXCL9 in the vaginal lamina propria (169).
In 2012, Mavigner et al. investigated the trafficking of CD4+ T cells expressing the gut-homing receptors CCR9 and integrin α4β7 in HIV-infected individuals and found that many of these T cells remained in circulation rather than repopulating the small intestinal mucosa (170). This is likely because the expression of CCL25, the ligand of CCR9, was lower in the small intestine of HIV-infected individuals (170). The defective homing of CCR9+β7+CD4+ T cells, a T cell subset with a critical role in mucosal immune defense, into the gut of HIV-infected individuals correlated with high plasma concentrations of markers of mucosal damage, microbial translocation, and systemic T cell activation (170). These results suggest that alterations in CCR9+β7+CD4+ T cell homing to the gut prevented efficient mucosal immune reconstitution in HIV-infected individuals (170).
In 2019, Marelli-Berg et al. reported that the CCR9 receptor signals during naïve T cell priming and promotes the differentiation of α4β+7 IFN-γ-producing memory CD4+ T cell subset (171). These CCR9+β7+CD4+ T cells display a TRM molecular signature, which is preferentially localized to the gastrointestinal tract and associated lymphoid tissue (171).
3.4 CCL25 chemokine immunoadjuvant to improve vaccines against infectious pathogens
Early in 2012, Kathuria et al. demonstrated the generation of antigen-specific T cells following systemic immunization with a DNA vaccine encoding CCL25 chemokine as immunoadjuvant (172).
In 2020, using a Chemokine-Adjuvanted Plasmid DNA expressing CCL25, Aldon et al. demonstrated in mice enhanced both splenic and intestinal, vaginal Ag-specific antibodies and T-cells primed by intramuscular immunization (173–176). This indicates that CCL25, a genetic chemokine immunoadjuvant, enhances vaccine Antigen-Specific humoral and cellular responses and induces homing to the gastrointestinal and female genital tract mucosae (173–176).
Similarly, in 2020, Hsu et al. demonstrated that parenteral administration of a virus-like particle-based vaccine formulated with CCL25 chemokine as an immunoadjuvant induced protective systemic and mucosal immune responses (144). This suggests that VLPs formulated with the CCL25 immunoadjuvant may serve as a potential vaccine strategy to protect against enteric viral infections.
More recently, in 2020, McKay et al. investigated the programming of T and B cells to home to the gastrointestinal and female genital tracts using genetic chemokine adjuvants (173). BALB/c mice were primed intramuscularly with plasmid DNA encoding a model Ag HIV-1 Env gp140 and selected chemokines/cytokines and boosted intravaginally with gp140 recombinant protein (173). CCL25 enhanced splenic and vaginal Ag-specific T cell responses, whereas CCL28 increased the levels of specific T cells only in the female genital tract (173). The levels of Ab were modulated in the systemic circulation, as well as the vaginal vault and intestinal lumen, with CCL20 playing a central role (173).
3.4.1 Targeting the CCL25/CCR9 axis as therapies for inflammatory and infectious diseases
CCL25/CCR9 chemokine axis and ulcerative colitis: Ulcerative colitis patients display increased numbers of circulating pro-inflammatory monocyte human leukocyte antigen-DR [HLA-DRhi] monocytes expressing high levels of the gut-homing CCR9 receptor of CCL25 and tumor necrosis factor [TNF]- α. In 2016, Trivedi et al. demonstrated that intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity (177, 178). CCL25 expression is upregulated in active colitis and correlates strongly with the burden of inflammatory bowel disease. Pathogenic CCR9+ T-cells undergo adhesion to stimulated hepatic endothelium more readily than CCR9− T-cells. Accordingly, several clinical trials have been conducted to block the CCL25/CCR9 chemokine axis in the treatment of patients with various disease conditions (25, 179).
In 2017, Eberhardson et al. reported the results of a randomized, double-masked, placebo-controlled trial of CCR9-targeted leukapheresis treatment in patients with ulcerative colitis (179). Patients with ulcerative colitis were treated every second day with leukapheresis using either a CCL25 column or a placebo column for five sessions (179). This clinical trial aimed to evaluate the removal of circulating CCR9-expressing monocytes via leukapheresis in patients with moderate-to-severe ulcerative colitis, focusing on safety, tolerability, and immunological responses. Patients with ulcerative colitis were treated every second day with leukapheresis during five sessions with a CCL25 column or a placebo column. This clinical induction trial, utilizing CCL25-tailored leukapheresis, demonstrates the safe and effective removal of activated monocytes with a clinical effect in patients with ulcerative colitis (25, 179). Later, in 2018, Igaki et al. demonstrated MLN3126, an antagonist of the CCL25 chemokine receptor (122).
The receptor CCR9 and its ligand, CCL25, also play essential roles in gut inflammation and autoimmune colitis (133). Gut mucosa-homing DCs express CCR9 and are predominantly localized in the gut lining and thymus (133, 134). CCR9+ DCs are implicated in regulating gut inflammation and food allergyin the gut (133, 134). The differential interaction of CCR9+ DCs with T cells in secondary lymphoid tissues and mucosal sites provides crucial insights into immune regulation (133, 134). The phenotypes, distributions, and interactions of CCR9+ DCs have recently been reviewed by Pathak et al., who elucidate the functions and roles of CCR9+ DCs in inflammation (133, 134). In 2007, Saruta et al. demonstrated that CCR9(+) T cells in Crohn’s disease are pro-inflammatory, supporting the rationale for using CCR9 antagonists in the treatment of human Crohn’s disease (136).
CCL25/CCR9 chemokine axis and Inflammatory bowel disease: The CCL25/CCR9 chemokine ligand-receptor pair has been reported to play a crucial role in small bowel immunity and inflammation (136). The CCR9/CCL25 axis contributes to the maintenance of mucosal T cell immunity and pathogenesis of inflammatory bowel disease (IBD) through the recruitment of pathogenic CCR9+ T cells into the gut mucosa (122). More recently, adoptive transfer of CCR9(+) DCs in B6 mice promoted Tregs but reduced the Th17 cells in the GALT and suppressed the OVA-specific gut-allergic response; Pathak et al. suggested CCR9(+) DCs have a regulatory function and may provide a new cellular therapeutic strategy to control gut inflammation and allergic immune reaction (133).
CCL25/CCR9 chemokine axis and Crohn’s disease: CCL25 is also necessary for the attraction and generation of B cells to the small intestine, lamina propria, and intraepithelial regions (180). Decreased circulating CCR9+CD4+ T helper cells are also associated with elevated levels of the. CCL25 chemokine in the salivary glands of patients with Sjögren’s syndrome, which facilitates their concerted migration (181). A randomized clinical trial using vercirnon, an oral CCR9 antagonist, versus placebo as induction therapy in active Crohn’s disease has been reported (182).
CCL25/CCR9 chemokine axis and COVID-19 disease: There is a consensus regarding the chemokine profile among COVID-19 patients, in which the variety of CXC and CC chemokines, as well as their association with COVID-19 vaccination, are major contributors to the immunopathology post-SARS-CoV-2 infection and remain important targets for therapy (42). The CC and CXC chemokines, in particular, appeared to contribute to the severity of COVID-19 disease (see Table 1 and reference (42)). In late 2022, Khalid et al. sequenced pooled peripheral blood mononuclear cell (PBMC) transcriptomes from SARS-CoV-2 patients with moderate and critical clinical outcomes to identify novel host receptors and biomarkers that could inform the development of translational nanomedicines and vaccine therapies (183). In 2021, Su et al. provided evidence that the ORF7a protein induced the NF-κB-dictated production of pro-inflammatory cytokines and chemokines, including significant upregulation of CCL25 (among many other chemokines) in severely ill COVID-19 patients (184). The ORF7a protein is then proposed as a target to minimize uncontrolled inflammation in COVID-19 patients (184). The hyperproduction of chemokines in lung mucosal tissue significantly worsens the prognosis of COVID-19 disease. In 2022, Karimabad et al. reviewed the roles of CXC, CC, and C chemokines in the pathogenesis of COVID-19 and their use as surrogates of COVID-19 vaccine-induced innate and adaptive Immunity (42).
4 The mucosal chemokine (C-C motif) ligand 28 in human health and disease
The chemokine (C-C motif) ligand 28 (CCL28), is one of the most widely expressed mucosal CC chemokine, also known as mucosal-associated epithelial chemokine (MEC), CCK1 and SCYA28, discovered back in 2000 by Wang et al. (Table 1) (185). CCL28 is a dual homeostatic/inflammatory chemokine that plays a role in mucosal immunity, as a chemoattractant for immune cells expressing CCR10 (known as GPR2) and CCR3 receptors, and as a broad-spectrum antimicrobial protein (33, 125, 129, 185–192).
The human CCL28 gene is located on chromosome 5 at 5p12 (Figure 1A) and is transcribed into an RNA transcript of 373 nucleotides, comprising four exons (185, 192, 193). The gene encodes a 127-amino-acid CCL28 protein that includes a 22-amino-acid N-terminal signal peptide. The human CCL28 shares 76% nucleotide identity and 83% amino acid similarity with the mouse CCL28 (185, 192, 193). CCL28 is constitutively expressed in thymic dendritic cells and mucosal epithelial cells in the salivary glands, bronchial epithelium, and the digestive tract, and its expression is induced in the mammary glands. CCL28 is also expressed by columnar epithelial cells in the gut and lungs (Figure 1B).
In 2006 and 2007, Meurens et al. reported that, like mouse and human CCL28 and CCR10, the ovine CCL28 and CCR10 are expressed in porcine mucosal digestive tract tissues (127–129). The ovine CCL28 and CCR10 were cloned and sequenced, and their mRNA expression was subsequently assessed by q-PCR in several tissues, including thymus, gut-associated lymphoid tissue, and mammary gland, from young and adult sheep and in the fetal lamb during the development of the immune system (127–129). CCL28 mRNA was confirmed to be highly expressed in the large intestine, trachea, tonsils, and mammary glands, especially at the end of gestation (127–129). These results are consistent with previous observations in other species, suggesting similar roles for the CCL28 chemokine. In fetuses, mRNA for CCL28 and its receptor, CCR10, is expressed early in the thymus and mucosal tissues, including the small intestine and nasal mucosa (127–129). Furthermore, their expression increased towards the end of gestation. Consequently, CCL28 plays a vital role in the T cell colonization of fetal tissues, enabling the development of a functional immune system (127–129).
Knowledge of cellular and molecular immunology of the ocular mucosa-associated lymphoid tissue (OMIS) may aid in understanding ocular pathologies and in designing more effective immunization strategies to induce local anti-pathogen CD4+ and CD8+ TRM cells, thereby protecting against ocular pathogens (58, 59). We have previously described OMIS and elucidated the structure and function of the humoral and cellular immune systems that protect the ocular mucosa (58, 59). A 2024 report by Dominguez-Lopez et al. showed that the gene expression of CCL28 was significantly upregulated in the conjunctiva of patients with dry eyes (57). CCL28 expression correlated positively with symptomatology, corneal staining, heat sensitivity threshold, and dendritic cell density (57). These results suggest that corneal and conjunctival epithelial cells could be a source of CCL28 on the ocular surface and that CCL28 might be involved in dry eye pathogenesis (57). Ocular mucosal immunoprophylactic and immunotherapeutic vaccine strategies have been evaluated to control the many pathogens that infect the ocular mucosa (58, 59). Topical ocular delivery of subunit adjuvanted vaccines against ocular herpes HSV-1 infection has been described (59, 194–197). Future challenges and issues related to the ocular mucosal delivery of molecularly defined subunit vaccines include how to generate efficient ocular mucosal CD4+ and CD8+ TRM cells to combat invading ocular pathogens.
4.1 CCR10 and CCR3 are both C-C receptors of CCL28 chemokine
CCL28 is a β- or CC chemokine that is involved in host immunity through the interactions with its receptors CCR10 and CCR3. CCL28 chemokine is expressed by columnar epithelial cells in the gut, lung, breast, and salivary glands. One or both receptors are expressed by B and T cells, eosinophils, mast cells, and inflammatory cells, and drive their mucosal homing (33, 124, 125, 190, 198–201). The CCR10 Receptor, originally called orphan receptor GPR2, is a receptor for CCL28 (124, 125, 132, 190, 200, 202). CCR10 is most highly expressed in eosinophils and basophils but is also expressed in Th1 and Th2 cells and airway epithelial cells. The CCR10 receptor has been implicated in skin inflammation and is involved in the recruitment of regulatory T cells (Tregs) into mucosal layers (188, 203–215). The CCR10/CCL28 axis influences the initiation and progression of mucosal inflammation in asthma by regulating the recruitment and retention of leukocyte populations in healthy and inflamed genital tracts.
The CCR receptor CCR3 is also a receptor for CCL28 that attracts B and T cells to mucosal tissues (26, 190, 216–226). Thus, CCR3 contributes to allergic reactions. CCR3 is also known as CD193 (26, 190, 219–226).
4.2 The CCL28/CCR10 chemokine ligand/receptor pair and the development of mucosal tissues-resident CD4+ and CD8+ T cells
The role of CCL28 chemokine in T cell trafficking and intestinal immunity was reported back in 2002 by Kunkel, Campbell, and Butcher (166). The CCL28 chemokine regulates the chemotaxis of cells that express the chemokine receptors CCR3 and CCR10. CCL28 is expressed by columnar epithelial cells in the gut, lung, breast, and salivary glands. It drives the mucosal homing of T cells expressing CCR10 and the migration of eosinophils expressing CCR3 (33, 125, 190). This chemokine is constitutively expressed in the colon. Still, its levels can be increased by pro-inflammatory cytokines and certain bacterial products, implying a role in effector cell recruitment to sites of epithelial injury (227–231). CCL28 has also been implicated in the migration of IgA-expressing cells to the mammary gland, salivary gland, intestine, and other mucosal tissues (227–231). It has also been shown to be a potential antimicrobial agent effective against specific pathogens, including Gram-negative and Gram-positive bacteria, as well as the fungus Candida albicans (227–231).
In humans, mice, and pigs, CCL28 plays a crucial role in the segregation and compartmentalization of the mucosal immune system by recruiting B and T cells expressing CCR10 to specific locations (125, 147, 188, 232–236), facilitating the migration of eosinophils expressing CCR3 (190, 237). CCL28 plays a crucial role in the migration of IgA-expressing cells to the mammary glands (238), salivary glands, intestines (123), and other mucosal tissues (33). In 2006, Eksteen et al. demonstrated that epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. The authors suggested that CXCR3 promotes the recruitment of Tregs to inflamed tissues, and CCR10 allows them to respond to CCL28 secreted by epithelial cells, resulting in the accumulation of tissue-resident CCR10+ Tregs at mucosal surfaces. An indispensable role for CCL28 and its receptor, CCR10, in the accumulation of IgA antibody-secreting B cells in the lactating mammary gland was reported by Wilson and Butcher in 2004 (238, 239). In 2008, Morteau et al. reported an essential role for the chemokine receptor CCR10 in the accumulation of IgA antibody-secreting cells (239).
In 2022, Terefe et al. confirmed that CCR10 is involved in the trafficking, recruitment, and infiltration of T cells into epithelia, such as the skin, through its interactions with the chemokines CCL27 and CCL28 (232). Furthermore, CCL28 binds to the chemokine receptor CCR3. CCR3 and/or CCR10 receptors are expressed by T cells that are implicated in the pathogenesis of asthma.
Lazarus et al. demonstrate that the mucosae-associated CCL28 chemokine is selectively chemotactic for IgA-secreting B cells (ASC): CCL28 attracts IgA- but not IgG- or IgM-producing ASC from both intestinal and non-intestinal lymphoid and effector tissues, including the intestines, lungs, and lymph nodes draining the bronchopulmonary tree and oral cavity (33, 123–126). These findings suggest a broad and unifying role for CCL28 in the physiology of the mucosal IgA immune system (33, 123–126).
4.3 Role of CCL28/CCR10 axis in the development of CD4+ and CD8+ TRM cells against genital herpes
Indirect evidence in humans (105, 106) and direct experiments in animal models (102–104) suggest that successful control of herpes infection is associated with induction of robust and polyfunctional TRM cells that reside within the vaginal sub-mucosal tissues (107). However, the female genital tract tissues appear to be immunologically restricted and remain “a closed immunological compartment, “ resistant to the homing of T cells that may originate from the draining lymph nodes and circulation (108, 109). Genital herpes infection of VECs likely triggered the production of inflammatory cytokines, such as IFN-gamma, TGF-beta, IL-1beta, and IL-6, which promote VECs: (1) to produce CCL28, and (2) increased CCR10 expression on local T cells contributing to homing and their retention of even more TRM cells within the female genital tract tissues (Figure 3; Table 2) (108, 109). Moreover, following genital herpes infection, increased T-cell production, which attracts CXCL9 and CXCL10 chemokines, likely contributes to the recruitment of additional TRM cells within the female genital tract tissues (Figure 4) (108, 109).
Table 2
| Chemokine | Receptor | Expression in mucosal tissues | Stage of TRM cell differentiation | Contribution to TRM cell biology | References |
|---|---|---|---|---|---|
| CCL25 | CCR9 | Thymus, small intestine epithelium, and GALT mucosal epithelia | Priming/Homing stage of effector and memory T cells (e.g., in GALT-homing and imprinting). |
Imprints GALT-homing (e.g., α4β7+CCR9+) of activated T cells and promotes differentiation of GALT-resident TRM-like cells. The CCL25-CCR9 ligand receptor interaction and signaling yields IFN-γ+ TRM cells that preferentially reside in GALT. | PMID: 41012114, PMID: 30863398, PMID: 14592943, PMID: 38632596, PMID: 12393847, PMID: 39695803, PMID: 28207301 and PMID: 21300065 |
| CCL28 | CCR10 (and CCR3) | Salivary and mammary glands, respiratory tract (i.e., trachea, bronchus), colon, and female reproductive tract (i.e., uterus, cervix, vagina) mucosal epithelia | Recruitment and Retention of effector and memory T cells |
Provides a recruitment and homeostatic chemotactic gradient for CCR10+ memory CD4+ and CD8+ T cells at mucosal epithelia. Enhances antiviral immunity by mobilizing CCR10+ effector memory CD8+ cells into the genital (vaginal) mucosa (protecting against HSV). | PMID: 41012114, PMID: 22684736, PMID: 38307549, PMID: 37222480, PMID: 36859428, PMID: 36155126, and PMID: 28207301, PMID: 33910988 |
| CXCL17 | CXCR8 (also known as GPR35) | Upper gastrointestinal tract (i.e., tongue, esophagus, stomach) and respiratory mucosa (i.e., trachea, lung) mucosal epithelia |
Homing of effector and memory T cells | Recruits myeloid cells and memory T cells. CXCL17 attracts CXCR8+ T cells to mucosal tissues. Notably, CXCL17 drives CXCR8+CD8+ TEff/Mem and TRM cells into the vaginal mucosa, enhancing protection against genital HSV in mice (via GPR35/CXCR8). | PMID: 41012114, PMID: 37210967, PMID: 33670758, PMID: 33515898, PMID: 29068046, PMID: 30860634, PMID: 28207301 and PMID: 29549178 |
| CXCL14 | No confirmed Receptor for CXCR4) |
Skin, oral mucosa, gut (i.e., stomach and intestines), lung, kidney, and mammary gland mucosal epithelia | Homeostatic and Maintenance of effector and memory T cells |
Has broad antimicrobial activity and by modulating innate cell recruitment and acting as a CXCR4 inhibitor, CXCL14 influences the niches that support TRM survival and function in mucosal sites. High epithelial expression of CXCL14 sustains TRM through tissue homeostasis and antimicrobial defense. | PMID: 41012114, PMID: 39931761, PMID: 39095323; PMID: 36012586, PMID: 31417179, PMID: 28382159, and PMID: 28207301 |
Mucosal chemokines in TRM cell biology.
Figure 4
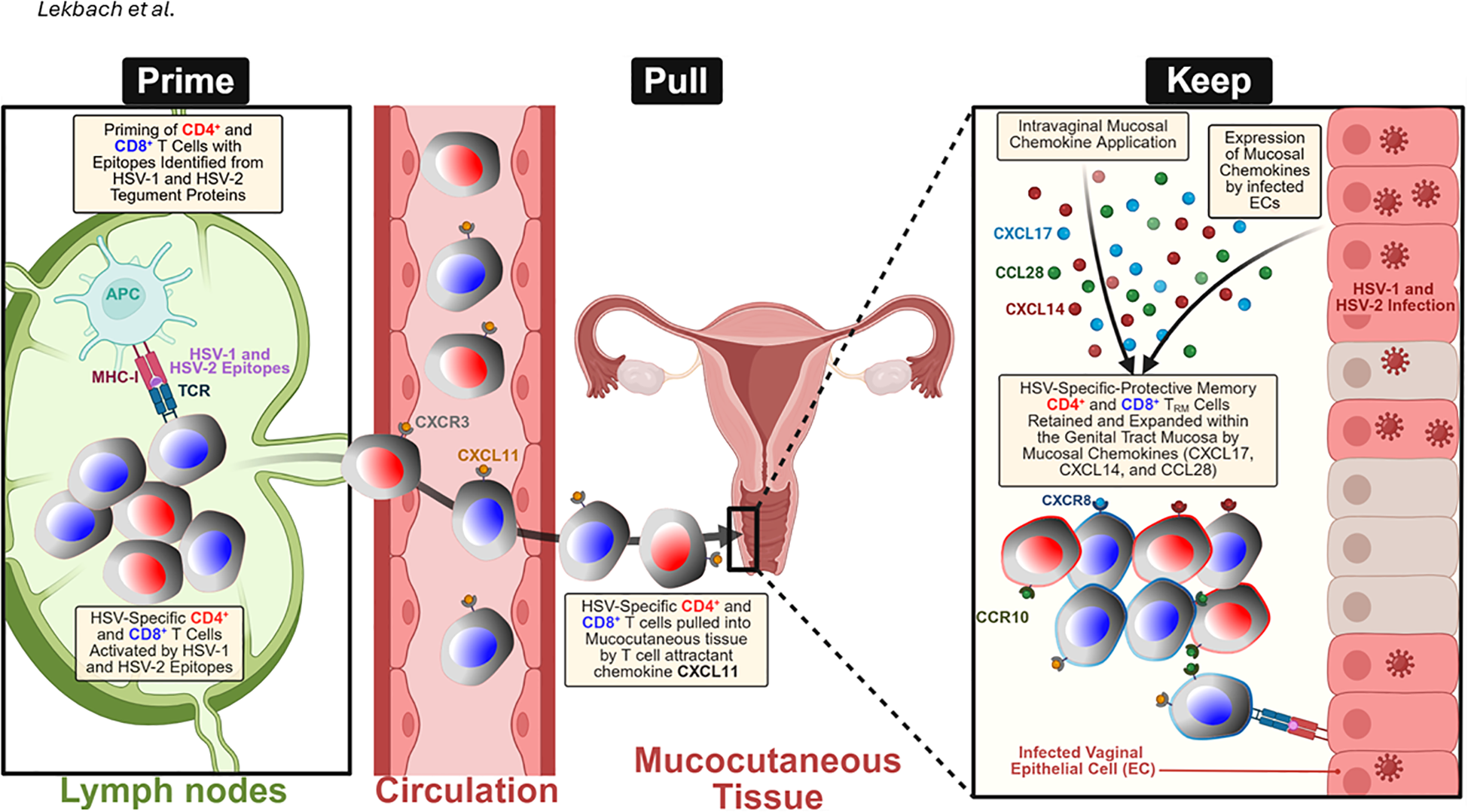
“Prime-Pull-Keep” (PPK) strategy for generating and maintaining HSV-2-specific tissue-resident memory T (TRM) cells in the mucocutaneous tissue: During the Prime phase, antigen-presenting cells (APCs) in the lymph nodes present HSV-1 and two tegument protein-derived epitopes to naïve CD4⁺ and CD8⁺ T cells, triggering their activation and expansion. During the Pull phase, activated HSV-specific T cells expressing CXCR3 are recruited from the circulation into the infected tissue by the T-cell-attracting chemokine CXCL11. During the Keep phase, the administration of mucosal chemokines, including CXCL14, CXCL17, and CCL28, alongside endogenous chemokine expression by HSV-1- or HSV-2-infected vaginal epithelial cells (VECs) and keratocytes, facilitates the retention and expansion of CD4⁺ and CD8⁺ TRM cells within the genital tract. These effects are mediated through chemokine receptors, such as CCR10 and CXCR8, which promote long-term mucosal immune protection. Created with BioRender.com.
We recently performed bulk RNA sequencing of herpes-specific CD8+ T cells isolated from the peripheral blood mononuclear cells (PBMC) of women infected with HSV who were either symptomatic (SYMP) or asymptomatic (ASYMP) (83). Our analysis revealed distinct regulation of the chemokine pathway and significantly increased expression of the CC28 chemokine receptor, CCR10, in ASYMP compared with SYMP herpes-infected women. We further confirmed this result by flow cytometry analysis of immune cells from PBMCs of SYMP and ASYMP HSV-infected women (83). Thus, we detected increased CCR10 expression on HSV-specific CD8+ T cells in ASYMP compared with SYMP women, both at the transcriptional and translational levels (83). We also explored the role of the mucosal chemokine CCL28/CCR10 axis in protection against genital herpes infection and disease in mouse models (83). We used SYMP and ASYMP mice infected intravaginally with HSV. We found that an increased expression of CCL28 chemokine in the VM was associated with protection in ASYMP mice but not in SYMP mice. We further confirmed increased expression of the chemokine CCL28 in the VM of HSV-2-infected ASYMP mice using Western blotting and immunohistochemistry (83). Moreover, we found that the number of female genital tract CCR10+CD8+ T cells increased following intravaginal administration of the chemokine CXCL10 in HSV-2-infected mice (83). The corresponding increase in CCR10-expressing genital tract mucosal-resident memory T cells was demonstrated by flow cytometry, further suggesting a critical role for the mucosal chemokine CCL28/CCR10 axis in protective T-cell immunity against genital herpes (83).
The immune profile of cells in the VM of infected mice showed that CCL28(-/-) mice had fewer CCR10-expressing CD8+ and CD4+ T cells, as well as a lower frequency of CCR10+CD44+ memory CD8+ T cells, compared with WT mice (83). The role of the CCL28/CCR10 chemokine axis in the mobilization of IgA-secreting cells in the mucosa is well established in the literature (83). To further elucidate the role of CCL28 and its receptor in humoral immunity during genital herpes infection, we investigated CCR10 expression on B cells in the VM. Interestingly, most memory B cells in the VM of these mice expressed the chemokine receptor CCR10. There was also a decrease in the frequency of CD27+B220+ memory B cells in these CCL28(-/-) mice (83). The increased frequency of CCR10-expressing CD8+ T cells in asymptomatic individuals with herpes may suggest an association between the mucosal chemokine CCL28 and protection against herpes infection. Thus, the mucosal chemokine CCL28 mediates protection from disease severity by mobilizing both CCR10+CD44+ memory CD8+ T cells and CCR10+B220+CD27+ memory B cells to the VM (83) (Table 2).
We recently generated novel knockout (KO) mice for the CCL28 chemokine and the CCR10 receptor and are currently studying their phenotypes upon infection with virulent and non-virulent strains of HSV-1 and HSV-2.
4.4 Targeting the CCL28/CCR10 chemokine/receptor axis to improve vaccines and immunotherapies against herpetic diseases
During the last 20 years, only a single vaccine strategy (adjuvanted recombinant HSV glycoprotein D (gD), with or without gB) has been tested and retested in clinical trials (91). Despite inducing potent HSV-specific neutralizing antibodies, this strategy failed to meet the primary endpoint of reducing herpes disease (240). These failures emphasize the need to induce T-cell-mediated immunity (241). Following the resolution of viral infections, a long-lived memory CD8+ T cell subset that protects against secondary (2°) infections is generated (242–246). This memory CD8+ T cell subset is heterogeneous but can be divided into three major subsets: (1) effector memory CD8+ T cells (CD8+ TEM cells); (2) central memory CD8+ T cells (CD8+ TCM cells); and (3) CD8+ T cells (CD8+ TRM cells) (108). The three significant memory subpopulations of T cells differ in their phenotypes, functions, and anatomical distributions. TCM cells are CD62LhighCCR7highCD103low. TEM cells are CD62LlowCCR7lowCD103low. TRM cells are CD62LlowCCR7lowCD103highCD11ahighCD69high (108, 247, 248). CD8+ TRM cells are found in the female genital tract and offer protection in mouse models of genital herpes (113). CD8+ TEM cells are also found in the dermal-epidermal junction in the female genital tract (32, 115). Once formed, TRM cells do not re-enter the circulation and play an essential role in locally guarding mucosal tissues against secondary (2°) infections. However, the precise mechanisms by which non-circulating mucosa-resident memory CD8+ TRM cells are formed, maintained, and expanded remain incompletely understood. In a recent study, we found that a high frequency of CD8+ TRM cells is retained in the female genital tract of HSV-infected asymptomatic mice compared to symptomatic mice and that this is associated with CCL28 mucosal chemokine production. Specifically, we demonstrated that higher frequencies of CCL28-dependent antiviral CD8+ TRM cells in the female genital tract are a key mediator of protection against genital herpes, consistent with previous reports (90, 103, 110–112, 115, 249). Since the primary cell target of HSV-2 is VECs, the key to achieving anti-herpes mucosal immunity is likely to boost the frequencies of HSV-specific CD8+ TRM cells in the female genital tract that can expand locally and persist in the long term. CD8+ TRM cells persist long-term in tissues and are often located at the epithelial borders of mucosal tissues (29, 250–253). However, little information exists on the mechanisms regulating the formation, retention, and expansion of vaginal-mucosa-resident CD8+ TRM cells. This report is the first to show that CCL28/CCR10 chemokine axis-mediated signals may be required for high frequencies of female genital tract antiviral CD8+ TRM cells. It remains to determine the mechanism of expansion and long-term retention of these CD8+ TRM cells within the female genital tract. Such knowledge would inform the design of innovative vaccines that induce CD8+ TRM cell-mediated protection against genital herpes. Collectively, this knowledge could significantly enhance our understanding of mucosal immunity and provide a unique opportunity to develop a robust, long-lasting genital herpes vaccine, with a substantial impact on the epidemiology of this disease. To our knowledge, our study represents the first in-depth analysis of the role of the CCL28/CCR10 chemokine axis in anti-herpes T- and B-cell responses in the VM during HSV-2 infection (83) (Table 2). We demonstrated that following intravaginal HSV-2 re-infection of B6 mice, high production of the CCL28 chemokine in the VM was associated with increased infiltration of CCR10+CD44+ memory CD8+ T cells and CD27+B220+ memory B cells in the VM (83) (Table 2). Our findings could further aid in immunotherapeutic approaches for genital herpes. In 2020, Aldon et al. demonstrated that the use of Chemokine-Adjuvanted Plasmid DNA expressing CCL28 increased the levels of specific T cells only in the female genital tract (173–176). This indicates that CCL28, a genetic chemokine immunoadjuvant, enhances vaccine Antigen-Specific humoral and cellular responses and induces homing to the female genital mucosa (173–176).
In 2021, Hu et al. demonstrated that CCL28 facilitated HSV-2 gD-induced protective systemic immunity against genital herpes (233). They showed that CCL28 enhanced robust antibody responses to the HSV-2 gD ectodomain (gD-306aa) and to Th1- and Th2-like responses that were recalled after the HSV-2 challenge. Interestingly, as expected, the mucosal chemokine CCL28 appeared to be more effective than CCL19 in promoting gD-specific immune responses and in promoting T-cell migration to secondary lymphoid tissues. Notably, both CCL19 and CCL28 significantly facilitated gD-induced protective mucosal immune responses in the genital tract. The findings collectively highlight the potential of the mucosal chemokine CCL28, in combination with gD, as a strategy for controlling HSV-2 infection. Recently, Huppler et al. demonstrated that CCL28 is also a potent therapeutic agent for Oropharyngeal Candidiasis (254).
In 2022, Terefe et al. confirmed that CCR10 is implicated in the trafficking, recruitment, and infiltration of T cells into epithelia via ligation by the chemokines CCL27 and CCL28 (232).
The above reports support the use of mucosal chemokines as an immune adjuvant to augment parenteral subunit vaccine-induced B- and T-cell immunity at mucosal surfaces (Table 2).
5 The mucosal chemokine (C-X-C motif) ligand 14 in human health and disease
The mucosal chemokine CXCL14 is a small cytokine belonging to the CXC chemokine family known as breast and kidney-expressed chemokine (BRAK chemokines), as reported back in 1999 by Hromas et al. (Table 1) (30, 255). The gene for CXCL14 contains four exons and is located on chromosome 5q31.1 in humans (Figure 1A) (30, 255). Mature CXCL14 has many of the conserved features of the CXC chemokine subfamily but has some differences, such as a shorter N-terminus and five extra amino acids in the region between its third and fourth cysteines (30, 255). Between 2006 and 2008, Meuter and Moser described the constitutive expression of CXCL14 in healthy human and murine epithelial tissues (31, 256, 257). CXCL14 is constitutively expressed in several normal tissues, including the brain, breast, cervix, lung, kidney, and skin, where its cellular source is thought to be fibroblasts (Figure 1B) (258).
CXCL14, a relatively novel chemokine, is a non-ELR (glutamic acid-leucine-arginine) chemokine with a broad spectrum of biological activities. Squamous epithelia, in particular, express high levels of CXCL14, supporting the notion that CXCL14 plays a homeostatic role in the skin. CXCL14 chemokine is chemotactic for monocytes in the presence of prostaglandin E2 (PGE2), an inflammatory mediator (258). It is also a potent chemoattractant and activator of dendritic cells (259–261) and activated natural killer (NK) cells (262–264). CXCL14 also inhibits angiogenesis, possibly by blocking endothelial cell chemotaxis (262).
A 2024 report by Dominguez-Lopez et al. showed that CXCL14 gene expression was significantly upregulated in conjunctival samples from patients with dry eyes (57). CXCL14 expression correlated positively with age, ocular pain, conjunctival staining, tactile sensitivity, and image reflectivity (57). These results suggest that corneal and conjunctival epithelial cells could be a source of CXCL14 on the ocular surface (57). CXCL14 has an atypical, yet highly conserved, primary structure characterized by a short N-terminus and high sequence identity between human and mouse. Proinflammatory chemokine CXCL14 activates MAS-related G protein-coupled receptor MRGPRX2 and its putative mouse ortholog MRGPRB2.
There is no widely accepted specific receptor for CXCL14 (265–273). One study predicted potential receptors using a protein interaction network database (STRING), suggesting that CXCR4, CXCR3, CXCR2, CCR2, CCR1, CXCR5, CCR7, CXCR1, and CCR5 may interact closely with CXCL14 and could be potential receptors (271, 274). Other studies suggested that ACKR2, CXCR4, GPR25, and GPR182 (271, 274, 275) are potential receptors for CXCL14.
5.1 Role of CXCL14 chemokine axis in the development of CD4+ and CD8+ TRM cells
Although CXCL14 induces chemotaxis of monocytic cells at high concentrations, its physiological role in leukocyte trafficking remains elusive (276, 277). CXCL14 primarily regulates T cell migration but also plays a distinct role in antimicrobial immunity and is proposed to combat bacteria at the earliest stage of infection, well before the establishment of inflammation (31).
In 2016, Pyeon et al. demonstrated the protective role of the CXCL14 chemokine in preventing human papillomavirus (HPV) infection, which can lead to cancer, through a mechanism that induces NK, CD4+,and CD8+ T cells (278–283). Conversely, immune evasion is demonstrated by HPVs, which suppress antitumor immune responses through epigenetic downregulation of CXCL14 expression (282). Pyeon et al. then analyzed gene-expression changes across all known chemokines and their receptors using our global gene-expression datasets from human HPV-positive and -negative head and neck cancer and cervical tissue specimens at different disease stages (282). While many proinflammatory chemokines are upregulated throughout cancer progression, CXCL14 is markedly downregulated in HPV-positive cancers in humans (282). Restoration of CXCL14 expression in HPV-positive mouse oropharyngeal carcinoma cells clears tumors in immunocompetent syngeneic mice but not in Rag1-deficient mice (282).
Furthermore, CXCL14 re-expression significantly increases the infiltration of natural killer (NK) cells, CD4+ T cells, and CD8+ T cells into the tumor-draining lymph nodes in vivo (282). In vitro transwell migration assays show that CXCL14 re-expression induces chemotaxis of NK, CD4+, and CD8+ T cells (282). Later, in 2019, the Pyeon et al. group demonstrated that CXCL14 suppresses human papillomavirus-associated head and neck cancer through antigen-specific CD8+ T-cell responses by upregulating MHC-I expression (279, 280). In 2024, based on the above strong results, Pyeon et al. proposed targeting the CXCL14 chemokine signaling pathway as a practical approach for cancer immunotherapy to halt HPV-cancer progression (278).
Given its roles in NK, CD4+, and CD8+ T-cell homing to tissues, CXCL14 stands out as a promising candidate for novel cell immunotherapies (Figure 3). However, it remains to be determined: the molecular mechanism that regulates CXCL14-mediated activity; how to deliver CXCL14; the appropriate dose; and which combinations with existing approved therapies may enhance NK, CD4+, and CD8+ T cell responses. We recently generated knockout (KO) mice for the CXCL14 chemokine and are currently studying their phenotype upon infection with HSV-1 and HSV-2. Preliminary, unpublished data suggest that CXCL14 plays a significant role in the homing of CD4+ and CD8+ T cells to peripheral tissues. In CXCL14(⁻/⁻) mice intravaginally infected with HSV-2, we observed increased disease severity and mortality, accompanied by reduced infiltration of CD4+ and CD8+ T cells at the site of infection and elevated viral loads.
In addition to CXCL14, in 2023-2024, Han et al. reported that CXCL13, primarily produced by CD4+ T cells, is an important chemokine involved in the recruitment of CXCR5-expressing naïve B and T follicular helper cells to the lungs ( (284) and Abstract AAI, 2024).
In summary, the initial differentiation of TRM cells is primarily driven by local tissue signals, including cytokines such as TGF-β, IL-15, and IL-33, as well as transcription factors like Hobit and BLIMP-1 (285, 286). The chemokines listed here do not primarily generate the TRM lineage itself but create the necessary microenvironment for their homing and subsequent survival/retention. (285, 286). (1) Homing: CCL25 and CCL28 act as crucial “pull” signals, expressed constitutively by epithelial cells, guiding circulating T cell precursors to specific mucosal sites (e.g., small intestine for CCL25) where they can differentiate into TRM cells (285, 286). CXCL17 also functions as a chemoattractant for T cells and antigen-presenting cells (APCs) to mucosal tissues. (2) Maintenance: Once established, these chemokines, along with adhesion molecules such as CD103 (which binds to E-cadherin) and CD69 (which prevents S1P-mediated egress), contribute to the long-term residency and survival of TRM cells in their respective niches (285, 286). The continuous presence of the chemokines acts as a “keep” signal, retaining cells locally. (3) Reactivation: Upon local antigen re-encounter, pre-existing TRM cells are rapidly reactivated (e.g., secreting IFN-γ \ and TNF-α) to control infection and recruit circulating immune cells, but the specific role of these four chemokines in the reactivation process itself is less defined compared to their roles in homing and maintenance (285). The expression profile during inflammation may change (e.g., elevated CCL28/CXCL17 in dry eye or infection), but their primary role remains homing/retention (285, 286).
6 The prime/pull/keep immunotherapy for genital herpes
Despite decades of effort, an effective vaccine or immunotherapy for genital herpes remains elusive. Traditional approaches, including inactivated “killed” virus, live-attenuated, replication-defective, and subunit glycoprotein vaccines, have largely failed to prevent recurrent HSV-2 disease (287). For example, a glycoprotein D subunit vaccine with adjuvant (aluminum salt) induced neutralizing antibodies but provided only modest and inconsistent protection in clinical trials. These challenges underscore the need for a new strategy that elicits robust T-cell immunity, rather than relying solely on antibodies. Researchers have observed that individuals with better control of genital herpes have robust HSV-2-specific CD4+ and CD8+ T cells residing in the vaginal mucosa (VM, the site of recurrent lesions) and their dorsal root ganglia (DRG, the site of viral latency) (288). An ideal therapeutic vaccine should therefore induce such TRM cells at both the central neuronal immunity in DRG and the peripheral epithelial immunity in VM. However, memory T cells do not circulate freely into immune-restrictive sites, such as the VM, skin, or lung airways, at steady state; instead, their trafficking is governed by chemokine gradients induced by local infection or inflammation (52). Chemokines such as CXCL9 and CXCL10, which act through CXCR3, and mucosal chemokines such as CXCL17, CXCL14, and CCL28 have been shown to recruit and retain functional memory T cells within mucosal tissues (289). Building on this principle, the Prime/Pull/Keep (PPK) strategy represents this paradigm shift: first, prime the host with key HSV antigens to generate a broad T-cell response, then pull those T cells into infected tissues using chemokine cues, and finally keep them on site for long-term protection (Figure 4). In HSV-2-infected guinea pigs, we demonstrated that intravaginal administration of a neurotropic AAV8 vector encoding CXCL11 significantly enhanced the infiltration of functional CXCR3+ CD4+ and CD8+ TRM and TEM cells in both the DRG and VM, resulting in a substantial reduction in viral shedding and lesion recurrence (290). Further incorporation of mucosal chemokines, such as CCL28 and CXCL17, has demonstrated additive benefits, facilitating the long-term retention of CCR10+ and CXCR8+ memory T cells within the VM (4, 83) (Table 3). By establishing sustained antiviral T-cell immunity in both central and peripheral target tissues, the PPK strategy represents a promising paradigm shift in the development of effective immunotherapeutics against genital herpes. We used Guinea pigs because consensus among herpes scientists is that the guinea pig model, which develops spontaneous viral reactivation and human-like recurrent genital herpes disease, is the gold standard and a widely used small-animal model for preclinical testing of vaccine candidates translatable to humans (99, 290–300).
Table 3
| Chemokine | Genital mucosa findings (HSV-1/2) | Ocular mucosa findings (HSV-1/2) | Key reference (2022–2025) |
|---|---|---|---|
| CCL25 | Primarily a gut-associated chemokine (CCR9 ligand) expressed in small intestinal and thymic epithelium. No significant findings in genital HSV – it is not known to be induced or required in vaginal HSV infection (mucosal immunity to HSV-2 does not appear to involve CCL25). | No data in ocular HSV. (CCL25 is tissue-specific to the gastrointestinal tract; it is not typically expressed in ocular mucosa) |
Meurens et al., 2006 – J. Interferon Cytokine Res. (CCL25 in mucosal immunity context); Smith et al., 2022 – Front. Immunol. (notes major mucosal chemokines and need for further study in HSV). |
| CCL28 | Protective role: Homeostatically produced in vaginal mucosa. Elevated in asymptomatic HSV-infected individuals and mice, correlating with higher HSV-specific effector memory CCR10+ CD8+ T cells and memory B cells in vaginal tissue. CCL28-CCR10 axis mobilizes antiviral lymphocytes to infection site; CCL28knockout mice have increased susceptibility to genital HSV-2 and reduced mucosal memory T/B cell infiltration. | Not studied in ocular HSV. (Ocular surface epithelial cells can secrete CCL28 during inflammation, but its role in HSV-1 ocular infection is undetermined. Excess chemokine in eye may contribute to immunopathology rather than protection.) | Dhanushkodi et al., 2023 – J. Immunol. 211(1):118–129 (demonstrates CCL28’s role in recruiting CCR10+ T and B cells for genital HSV protection; DOI: 10.4049/jimmunol.2300093). |
| CXCL17 | Protective role: Constitutively present in vaginal mucosa and induced by HSV. Higher CXCL17 levels observed in protected mice after intravaginal HSV exposure, accompanied by increased CXCR8+ CD8+ effector memory and CD103^+ tissue-resident T cells in the vaginal mucosa. In CXCL17 knockout mice, genital HSV-1 infection leads to fewer tissue CD8 T cells, more viral replication, and more latency in ganglia, indicating CXCL17 is crucial for mobilizing protective CD8+ T cells to the genital mucosa. | Not studied in ocular HSV. (No published evidence of CXCL17’s role in HSV-1 eye infections. Ocular herpes lesions are driven by an intense chemokine/cytokine storm causing inflammation, so any CXCL17 effect is uncharacterized. It has been suggested as a mucosal immunomodulator, but no direct data in the eye.) |
Srivastava et al., 2018 – J. Immunol. 200(8):2915–2926 (CXCL17 mediates recruitment of CXCR8+ CD8 TEM and TRM, protecting against vaginal HSV; DOI: 10.4049/jimmunol.1701474). Smith et al., 2022 – Front. Immunol. (review, notes chemokine CXCL17 in HSV mucosal immunity). |
| CXCL14 | Constitutively expressed in mucosal epithelium; specific role in genital HSV infection remains unclear (no direct studies). Proposed to participate in basal immune surveillance but not confirmed in HSV-2 models. | Upregulated on ocular surface under inflammatory stress; no HSV-specific data. Ocular infection triggers broad chemokine release that mainly contributes to tissue damage rather than protection. | Dhanushkodi et al., 2023 – J. Immunol. (211(1):118–129, discusses CXCL14 as a mucosal chemokine; DOI: 10.4049/jimmunol.2300093). |
Role of mucosal chemokine in HSV-1/2 infections.
7 Targeted PPK immunotherapy for ocular herpes
HSV-1 establishes lifelong latency in the trigeminal ganglion (TG) and is responsible for recurrent ocular herpes (301). Current antiviral therapies offer only temporary suppression, and vaccine efforts, such as adjuvanted glycoprotein subunit vaccines, have failed to prevent recurrence, highlighting the need for innovative strategies that elicit local antiviral immunity (91). The PPK approach represents a targeted immunotherapeutic solution designed to restore and sustain TRM cells at sites of latency and reactivation. Recently, we tested a novel PPK therapeutic vaccine in an HLA-A*0201 transgenic rabbit model of ocular herpes (302). The vaccine combined HSV-1 CD8+ and CD4+ T-cell epitopes (prime) with the chemokine CXCL11 (pull) to recruit antiviral T cells into the TG, and IL-2/IL-15 (keep) to support their local maintenance. This strategy significantly increased TG-infiltrating CD8+ T cells and reduced both corneal disease and viral shedding, highlighting the potential of tissue-targeted immunotherapy to control HSV-1 reactivation. Moreover, mucosal chemokines such as CCL28, CXCL14, and CXCL17 are expressed in the cornea under homeostatic conditions (57). They could be incorporated into the PPK strategy to help retain antiviral memory T cells within infected ocular tissues, reinforcing local immune surveillance (Figure 5). By directing and retaining effector and memory T cells precisely at sites of HSV-1 persistence, the PPK strategy offers a promising path toward durable control of ocular herpes reactivation.
Figure 5
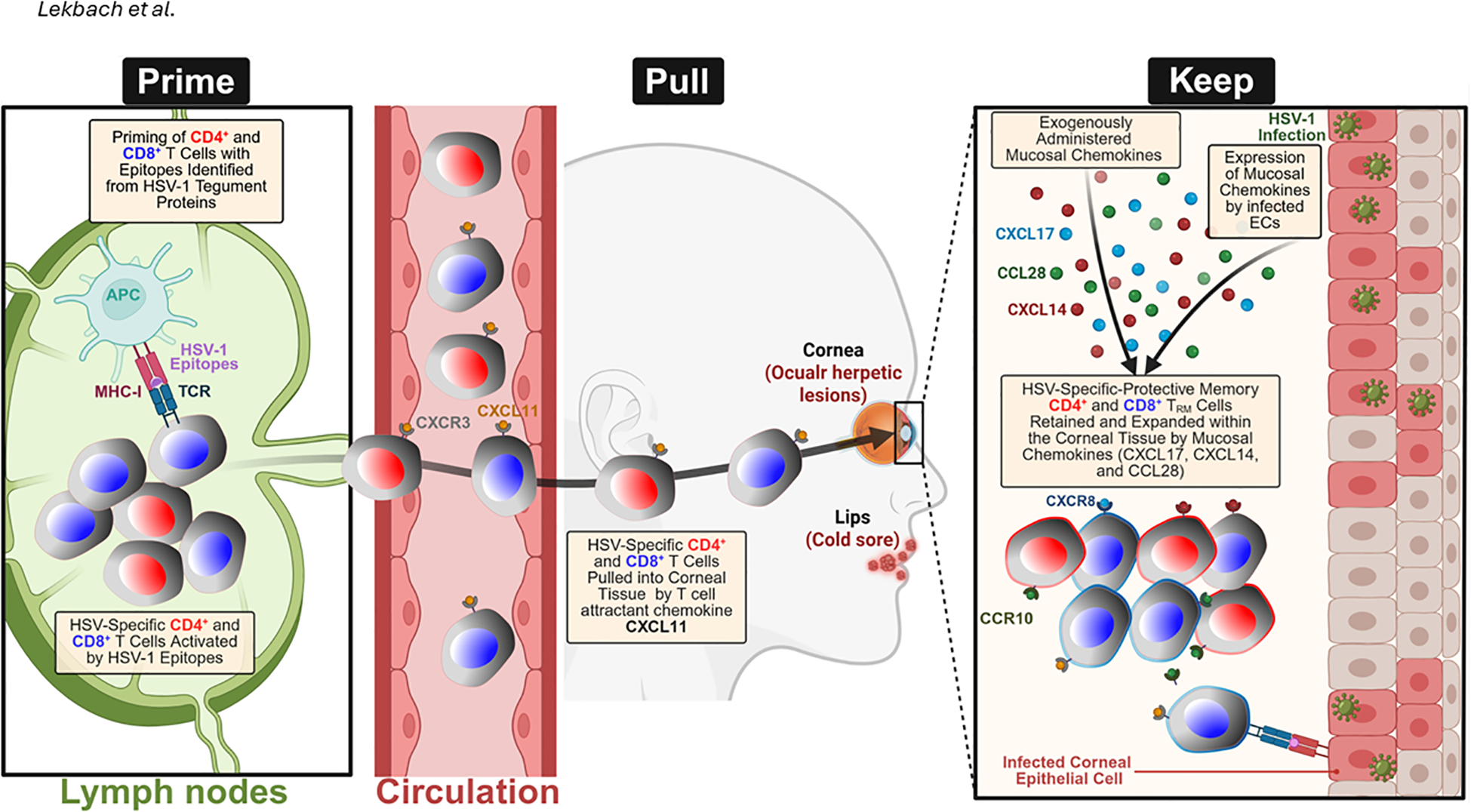
Distinct phases of the “Prime-Pull-Keep” (PPK) approach facilitate the generation and maintenance of HSV-1-specific T cell receptor (TRM) cells in the corneal mucosa: Initially, in the Prime phase, naïve CD4⁺ and CD8⁺ T cells are activated in regional lymph nodes upon recognition of HSV-1 antigens presented by antigen-presenting cells (APCs). The Pull phase involves CXCL11-mediated recruitment of circulating HSV-1-specific T cells, which express CXCR3, into the infected corneal tissue. During the Keep phase, chemokines such as CXCL14, CXCL17, and CCL28, produced locally by infected corneal epithelial cells or administered exogenously, enhance the persistence and expansion of TRM cells in the cornea. This retention is directed by chemokine receptors, including CXCR8 and CCR10, which support a robust and localized antiviral immune defense. Created with BioRender.com.
Statements
Author contributions
LB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. SP: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SK: Data curation, Methodology, Writing – original draft, Writing – review & editing. KH-D: Conceptualization, Writing – original draft, Writing – review & editing. JD: Writing – original draft, Writing – review & editing. AQ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. Studies of this report were supported by Public Health Service Research grants AI158060, AI150091, AI143348, AI147499, AI143326, AI138764, AI124911, and AI110902 from the National Institutes of Allergy and Infectious Diseases (NIAID) to LB and by R43AI174383 to TechImmune, LLC. The author(s) declared that this work received funding fromTechImmune LLC. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
Author LB was employed by company TechImmune, LLC. LB has an equity interest in TechImmune, LLC., a company that may potentially benefit from the research results and serves on the company’s Scientific Advisory Board. LB’s relationship with TechImmune, LLC., has been reviewed and approved by the University of California, Irvine in accordance with its conflict-ofinterest policies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) AC and SP declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Hernandez-Ruiz M Zlotnik A . Mucosal chemokines. J Interferon Cytokine Res. (2017) 37:62–70. doi: 10.1089/jir.2016.0076
2
Zhou X Wu Y Zhu Z Lu C Zhang C Zeng L et al . Mucosal immune response in biology, disease prevention and treatment. Signal Transduction Targeted Ther. (2025) 10:7. doi: 10.1038/s41392-024-02043-4
3
Park SL Painter MM Manne S Alcalde V McLaughlin M Sullivan MA et al . Divergent ontogeny of Tissue Resident Memory and Tissue Resident Exhausted CD8(+) T cells underlies distinct functional potential. bioRxiv. (2025). doi: 10.1101/2025.08.08.669213
4
Srivastava R Hernandez-Ruiz M Khan AA Fouladi MA Kim GJ Ly VT et al . CXCL17 chemokine-dependent mobilization of CXCR8(+)CD8(+) effector memory and tissue-resident memory T cells in the vaginal mucosa is associated with protection against genital herpes. J Immunol. (2018) 200:2915–26. doi: 10.4049/jimmunol.1701474
5
Roy S Coulon PG Srivastava R Vahed H Kim GJ Walia SS et al . Blockade of LAG-3 immune checkpoint combined with therapeutic vaccination restore the function of tissue-resident anti-viral CD8(+) T cells and protect against recurrent ocular herpes simplex infection and disease. Front Immunol. (2018) 9:2922. doi: 10.3389/fimmu.2018.02922
6
Khan AA Srivastava R Chentoufi AA Kritzer E Chilukuri S Garg S et al . Bolstering the number and function of HSV-1-specific CD8(+) effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J Immunol. (2017) 199:186–203. doi: 10.4049/jimmunol.1700145
7
Rout N . Enhanced th1/th17 functions of CD161+ CD8+ T cells in mucosal tissues of rhesus macaques. PLoS One. (2016) 11:e0157407. doi: 10.1371/journal.pone.0157407
8
Sowell RT Rogozinska M Nelson CE Vezys V Marzo AL . Cutting edge: generation of effector cells that localize to mucosal tissues and form resident memory CD8 T cells is controlled by mTOR. J Immunol. (2014) 193:2067–71. doi: 10.4049/jimmunol.1400074
9
Sowell RT Goldufsky JW Rogozinska M Quiles Z Cao Y Castillo EF et al . IL-15 complexes induce migration of resting memory CD8 T cells into mucosal tissues. J Immunol. (2017) 199:2536–46. doi: 10.4049/jimmunol.1501638
10
Coffin SE Clark SL Bos NA Brubaker JO Offit PA . Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J Immunol. (1999) 163:3064–70. doi: 10.4049/jimmunol.163.6.3064
11
Openshaw P . T cells take the inside track. Nat Med. (2006) 12:614–5. doi: 10.1038/nm0606-614
12
Taghavi S Campbell A Engelhardt D Duchesne J Shaheen F Pociask D et al . Dimethyl malonate protects the lung in a murine model of acute respiratory distress syndrome. J Trauma Acute Care Surg. (2024) 96:386–93. doi: 10.1097/TA.0000000000004184
13
Kell SA Kachura MA Renn A Traquina P Coffman RL Campbell JD . Preclinical development of the TLR9 agonist DV281 as an inhaled aerosolized immunotherapeutic for lung cancer: Pharmacological profile in mice, non-human primates, and human primary cells. Int Immunopharmacol. (2019) 66:296–308. doi: 10.1016/j.intimp.2018.11.019
14
Zemans RL Briones N Campbell M McClendon J Young SK Suzuki T et al . Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci U.S.A. (2011) 108:15990–5.
15
Beane J Vick J Schembri F Anderlind C Gower A Campbell J et al . Characterizing the impact of smoking and lung cancer on the airway transcriptome using RNA-Seq. Cancer Prev Res (Phila). (2011) 4:803–17. doi: 10.1158/1940-6207.CAPR-11-0212
16
Morgan AJ Guillen C Symon FA Birring SS Campbell JJ Wardlaw AJ . CXCR6 identifies a putative population of retained human lung T cells characterised by co-expression of activation markers. Immunobiology. (2008) 213:599–608. doi: 10.1016/j.imbio.2008.01.005
17
Thomson L Blaylock MG Sexton DW Campbell A Walsh GM . Cetirizine and levocetirizine inhibit eotaxin-induced eosinophil transendothelial migration through human dermal or lung microvascular endothelial cells. Clin Exp Allergy. (2002) 32:1187–92. doi: 10.1046/j.1365-2745.2002.01444.x
18
Campbell JJ Brightling CE Symon FA Qin S Murphy KE Hodge M et al . Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. (2001) 166:2842–8. doi: 10.4049/jimmunol.166.4.2842
19
Bhatia M Brady M Zagorski J Christmas SE Campbell F Neoptolemos JP et al . Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. (2000) 47:838–44. doi: 10.1136/gut.47.6.838
20
Binti Mohd Amir NAS Mackenzie AE Jenkins L Boustani K Hillier MC Tsuchiya T et al . Evidence for the existence of a CXCL17 receptor distinct from GPR35. J Immunol. (2018) 201:714–24. doi: 10.4049/jimmunol.1700884
21
Takamura S . Divergence of tissue-memory T cells: distribution and function-based classification. Cold Spring Harb Perspect Biol. (2020) 12. doi: 10.1101/cshperspect.a037762
22
Guo YJ Zhou YJ Yang XL Shao ZM Ou ZL . The role and clinical significance of the CXCL17-CXCR8 (GPR35) axis in breast cancer. Biochem Biophys Res Commun. (2017) 493:1159–67. doi: 10.1016/j.bbrc.2017.09.113
23
Gowhari Shabgah A Jadidi-Niaragh F Ebrahimzadeh F Mohammadi H Askari E Pahlavani N et al . A comprehensive review of chemokine CXC17 (VCC1) in cancer, infection, and inflammation. Cell Biol Int. (2022) 46:1557–70. doi: 10.1002/cbin.11846
24
Burkhardt AM Tai KP Flores-Guiterrez JP Vilches-Cisneros N Kamdar K Barbosa-Quintana O et al . CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol. (2012) 188:6399–406. doi: 10.4049/jimmunol.1102903
25
Eberhardson M Marits P Jones M Jones P Karlen P Karlsson M et al . Treatment of inflammatory bowel disease by chemokine receptor-targeted leukapheresis. Clin Immunol. (2013) 149:73–82. doi: 10.1016/j.clim.2013.05.021
26
Kitaura M Nakajima T Imai T Harada S Combadiere C Tiffany HL et al . Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. (1996) 271:7725–30. doi: 10.1074/jbc.271.13.7725
27
Khoury-Hanold W Yordy B Kong P Kong Y Ge W Szigeti-Buck K et al . Viral spread to enteric neurons links genital HSV-1 infection to toxic megacolon and lethality. Cell Host Microbe. (2016) 19:788–99. doi: 10.1016/j.chom.2016.05.008
28
Melhem H Kaya B Kaymak T Wuggenig P Flint E Roux J et al . Epithelial GPR35 protects from Citrobacter rodentium infection by preserving goblet cells and mucosal barrier integrity. Mucosal Immunol. (2022) 15:443–58. doi: 10.1038/s41385-022-00494-y
29
Mackay LK Gebhardt T . Tissue-resident memory T cells: local guards of the thymus. Eur J Immunol. (2013) 43:2259–62. doi: 10.1002/eji.201343930
30
Hromas R Broxmeyer HE Kim C Nakshatri H Christopherson K Azam M et al . Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus Malignant cells. Biochem Biophys Res Commun. (1999) 255:703–6. doi: 10.1006/bbrc.1999.0257
31
Maerki C Meuter S Liebi M Muhlemann K Frederick MJ Yawalkar N et al . Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol. (2009) 182:507–14. doi: 10.4049/jimmunol.182.1.507
32
Peng T Zhu J Phasouk K Koelle DM Wald A Corey L . An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol. (2012) 86:10587–96. doi: 10.1128/JVI.01237-12
33
Lazarus NH Kunkel EJ Johnston B Wilson E Youngman KR Butcher EC . A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. (2003) 170:3799–805. doi: 10.4049/jimmunol.170.7.3799
34
Nakanishi Y Lu B Gerard C Iwasaki A . CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. (2009) 462:510–3. doi: 10.1038/nature08511
35
Ding J Hillig C White CW Fernandopulle NA Anderton H Kern JS et al . CXCL17 induces activation of human mast cells via MRGPRX2. Allergy. (2024). doi: 10.1111/all.16036
36
Ahmed T Tripathi AK Ahmed RS Das S Suke SG Pathak R et al . Endosulfan-induced apoptosis and glutathione depletion in human peripheral blood mononuclear cells: Attenuation by N-acetylcysteine. J Biochem Mol Toxicol. (2008) 22:299–304. doi: 10.1002/jbt.20240
37
Pisabarro MT Leung B Kwong M Corpuz R Frantz GD Chiang N et al . Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol. (2006) 176:2069–73. doi: 10.4049/jimmunol.176.4.2069
38
Weinstein EJ Head R Griggs DW Sun D Evans RJ Swearingen ML et al . VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. (2006) 350:74–81. doi: 10.1016/j.bbrc.2006.08.194
39
Choreno-Parra JA Thirunavukkarasu S Zuniga J Khader SA . The protective and pathogenic roles of CXCL17 in human health and disease: Potential in respiratory medicine. Cytokine Growth Factor Rev. (2020) 53:53–62. doi: 10.1016/j.cytogfr.2020.04.004
40
Zlotnik A Yoshie O . The chemokine superfamily revisited. Immunity. (2012) 36:705–16. doi: 10.1016/j.immuni.2012.05.008
41
Giblin SP Pease JE . What defines a chemokine? - The curious case of CXCL17. Cytokine. (2023) 168:156224. doi: 10.1016/j.cyto.2023.156224
42
Karimabad MN Hassanshahi G Kounis NG Mplani V Roditis P Gogos C et al . and C in the pathogenesis of COVID-19 disease and as surrogates of vaccine-induced innate and adaptive protective responses. Vaccines (Basel) 10. (2022). doi: 10.3390/vaccines10081299
43
Moser B Wolf M Walz A Loetscher P . Chemokines: multiple levels of leukocyte migration control. Trends Immunol. (2004) 25:75–84. doi: 10.1016/j.it.2003.12.005
44
Russo RC Quesniaux VFJ Ryffel B . Homeostatic chemokines as putative therapeutic targets in idiopathic pulmonary fibrosis. Trends Immunol. (2023) 44:1014–30. doi: 10.1016/j.it.2023.10.003
45
Borges TJ Abarzua P Gassen RB Kollar B Lima-Filho M Aoyama BT et al . T cell-attracting CCL18 chemokine is a dominant rejection signal during limb transplantation. Cell Rep Med. (2022) 3:100559. doi: 10.1016/j.xcrm.2022.100559
46
Gunaltay S Kumawat AK Nyhlin N Bohr J Tysk C Hultgren O et al . Enhanced levels of chemokines and their receptors in the colon of microscopic colitis patients indicate mixed immune cell recruitment. Mediators Inflammation. (2015) 2015:132458. doi: 10.1155/2015/132458
47
Dhanushkodi NR Prakash S Quadiri A Zayou L Srivastava R Shaik AM et al . Antiviral and anti-inflammatory therapeutic effect of RAGE-ig protein against multiple SARS-coV-2 variants of concern demonstrated in K18-hACE2 mouse and Syrian golden hamster models. J Immunol. (2024) 212:576–85. doi: 10.4049/jimmunol.2300392
48
Hauser AE Debes GF Arce S Cassese G Hamann A Radbruch A et al . Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. (2002) 169:1277–82. doi: 10.4049/jimmunol.169.3.1277
49
Meyer M Hensbergen PJ van der Raaij-Helmer EM Brandacher G Margreiter R Heufler C et al . Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol. (2001) 31:2521–7. doi: 10.1002/1521-4141(200108)31:8<2521::AID-IMMU2521>3.0.CO;2-Q
50
Shin H Kumamoto Y Gopinath S Iwasaki A . CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun. (2016) 7:13346. doi: 10.1038/ncomms13346
51
Iijima N Iwasaki A . T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. (2014) 346:93–8. doi: 10.1126/science.1257530
52
Shin H Iwasaki A . Tissue-resident memory T cells. Immunol Rev. (2013) 255:165–81. doi: 10.1111/imr.12087
53
Rahimi RA Luster AD . Redefining memory T cell subsets. Trends Immunol. (2020) 41:645–8. doi: 10.1016/j.it.2020.06.003
54
Lange J Rivera-Ballesteros O Buggert M . Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol. (2022) 15:389–97. doi: 10.1038/s41385-021-00467-7
55
Zhang L Lu X Xu Y La X Tian J Li A et al . Tumor-associated macrophages confer colorectal cancer 5-fluorouracil resistance by promoting MRP1 membrane translocation via an intercellular CXCL17/CXCL22-CCR4-ATF6-GRP78 axis. Cell Death Dis. (2023) 14:582. doi: 10.1038/s41419-023-06108-0
56
Xiao S Xie W Zhou L . Mucosal chemokine CXCL17: What is known and not known. Scand J Immunol. (2021) 93:e12965. doi: 10.1111/sji.12965
57
Dominguez-Lopez A Blanco-Vazquez M Calderon-Garcia AA Garcia-Vazquez C Gonzalez-Garcia MJ Calonge M et al . Analysis of the mucosal chemokines CCL28, CXCL14, and CXCL17 in dry eye disease: An in vitro and clinical investigation. Exp Eye Res. (2024) 241:109854. doi: 10.1016/j.exer.2024.109854
58
Chentoufi AA Dasgupta G Nesburn AB Bettahi I Binder NR Choudhury ZS et al . Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol. (2010) 17:342–53. doi: 10.1128/CVI.00347-09
59
Nesburn AB Bettahi I Zhang X Zhu X Chamberlain W Afifi RE et al . Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf. (2006) 4:178–87. doi: 10.1016/S1542-0124(12)70164-7
60
Fallarini S Magliulo L Paoletti T de Lalla C Lombardi G . Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun. (2010) 398:420–5. doi: 10.1016/j.bbrc.2010.06.091
61
Maravillas-Montero JL Burkhardt AM Hevezi PA Carnevale CD Smit MJ Zlotnik A . Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol. (2015) 194:29–33. doi: 10.4049/jimmunol.1401704
62
Ocón B Xiang M Bi Y Tan S Brulois K Ayesha A et al . A lymphocyte chemoaffinity axis for lung, non-intestinal mucosae and CNS. Nature. (2024) 635:736–45. doi: 10.1038/s41586-024-08043-2
63
Hu W-F Yu J Wang J-J Sun R-J Zheng Y-S Zhang T et al . Identification of orphan GPR25 as a receptor for the chemokine CXCL17. FEBS J. (2025).
64
White CW Platt S Kilpatrick LE Dale N Abhayawardana RS Dekkers S et al . CXCL17 is an allosteric inhibitor of CXCR4 through a mechanism of action involving glycosaminoglycans. Sci Signal. (2024) 17:eabl3758. doi: 10.1126/scisignal.abl3758
65
Giblin SP Ranawana S Hassibi S Birchenough HL Mincham KT Snelgrove RJ et al . CXCL17 binds efficaciously to glycosaminoglycans with the potential to modulate chemokine signaling. Front Immunol. (2023) 14:1254697. doi: 10.3389/fimmu.2023.1254697
66
Denisov SS . CXCL17: the black sheep in the chemokine flock. Front Immunol. (2021) 12:712897. doi: 10.3389/fimmu.2021.712897
67
Hashemi SF Khorramdelazad H . The cryptic role of CXCL17/CXCR8 axis in the pathogenesis of cancers: a review of the latest evidence. J Cell Commun Signal. (2023) 17:409–22. doi: 10.1007/s12079-022-00699-7
68
Ullah A Zhao J Li J Singla RK Shen B . Involvement of CXC chemokines (CXCL1-CXCL17) in gastric cancer: Prognosis and therapeutic molecules. Life Sci. (2024) 336:122277. doi: 10.1016/j.lfs.2023.122277
69
Lowry E Chellappa RC Penaranda B Sawant KV Wakamiya M Garofalo RP et al . Cxcl17 is a proinflammatory chemokine and promotes neutrophil trafficking. J Leukoc Biol. (2024). doi: 10.1093/jleuko/qiae028
70
Burkhardt AM Maravillas-Montero JL Carnevale CD Vilches-Cisneros N Flores JP Hevezi PA et al . CXCL17 is a major chemotactic factor for lung macrophages. J Immunol. (2014) 193:1468–74. doi: 10.4049/jimmunol.1400551
71
Choreno-Parra JA Dunlap MD Swanson R Jimenez-Alvarez LA Munoz-Torrico M Guzman-Beltran S et al . CXCL17 Is Dispensable during Hypervirulent Mycobacterium tuberculosis HN878 Infection in Mice. Immunohorizons. (2021) 5:752–9. doi: 10.4049/immunohorizons.2100048
72
McCully ML Ladell K Andrews R Jones RE Miners KL Roger L et al . CCR8 expression defines tissue-resident memory T cells in human skin. J Immunol. (2018) 200:1639–50. doi: 10.4049/jimmunol.1701377
73
Pham TH Kim EN Trang NM Jeong GS . Gallic acid induces osteoblast differentiation and alleviates inflammatory response through GPR35/GSK3beta/beta-catenin signaling pathway in human periodontal ligament cells. J Periodontal Res. (2024) 59:204–19. doi: 10.1111/jre.13208
74
Otkur W Liu X Chen H Li S Ling T Lin H et al . GPR35 antagonist CID-2745687 attenuates anchorage-independent cell growth by inhibiting YAP/TAZ activity in colorectal cancer cells. Front Pharmacol. (2023) 14:1126119. doi: 10.3389/fphar.2023.1126119
75
De Giovanni M Dang EV Chen KY An J Madhani HD Cyster JG . Platelets and mast cells promote pathogenic eosinophil recruitment during invasive fungal infection via the 5-HIAA-GPR35 ligand-receptor system. Immunity. (2023) 56:1548–1560.e5. doi: 10.1016/j.immuni.2023.05.006
76
De Giovanni M Chen H Li X Cyster JG . GPR35 and mediators from platelets and mast cells in neutrophil migration and inflammation. Immunol Rev. (2023) 317:187–202.
77
Baumgartner R Casagrande FB Mikkelsen RB Berg M Polyzos KA Forteza MJ et al . Disruption of GPR35 signaling in bone marrow-derived cells does not influence vascular inflammation and atherosclerosis in hyperlipidemic mice. Metabolites. (2021) 11. doi: 10.3390/metabo11070411
78
Wang W Han T Tong W Zhao J Qiu X . Overexpression of GPR35 confers drug resistance in NSCLC cells by beta-arrestin/Akt signaling. Onco Targets Ther. (2018) 11:6249–57. doi: 10.2147/OTT.S175606
79
Park SJ Lee SJ Nam SY Im DS . GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17-induced migration stimulatory response in THP-1 cells; is GPR35 a receptor for CXCL17? Br J Pharmacol. (2018) 175:154–61. doi: 10.1111/bph.14082
80
Barsheshet Y Wildbaum G Levy E Vitenshtein A Akinseye C Griggs J et al . CCR8(+)FOXp3(+) T(reg) cells as master drivers of immune regulation. Proc Natl Acad Sci U.S.A. (2017) 114:6086–91.
81
Liu L Rangan L Vanalken N Kong Q Schlenner S De Jonghe S et al . Development of a cellular model to study CCR8 signaling in tumor-infiltrating regulatory T cells. Cancer Immunol Immunother. (2024) 73:11. doi: 10.1007/s00262-023-03607-z
82
Ueyama A Nogami W Nashiki K Haruna M Miwa H Hagiwara M et al . Immunotherapy targeting CCR8+ Regulatory T cells induces antitumor effects via dramatic changes to the intratumor CD8+ T cell profile. J Immunol. (2023) 211:673–82. doi: 10.4049/jimmunol.2300067
83
Dhanushkodi NR Prakash S Quadiri A Zayou L Srivastava R Tran J et al . Mucosal CCL28 Chemokine Improves Protection against Genital Herpes through Mobilization of Antiviral Effector Memory CCR10+CD44+ CD62L-CD8+ T Cells and Memory CCR10+B220+CD27+ B Cells into the Infected Vaginal Mucosa. J Immunol. (2023) 211:118–29. doi: 10.4049/jimmunol.2300093
84
Samandary S Kridane-Miledi H Sandoval JS Choudhury Z Langa-Vives F Spencer D et al . Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol. (2014) 75:715–29. doi: 10.1016/j.humimm.2014.04.016
85
Hernandez-Ruiz M Othy S Herrera C Nguyen HT Arrevillaga-Boni G Catalan-Dibene J et al . Cxcl17(-/-) mice develop exacerbated disease in a T cell-dependent autoimmune model. J Leukoc Biol. (2019) 105:1027–39.
86
Dasgupta G BenMohamed L . Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. (2011) 29:5824–36. doi: 10.1016/j.vaccine.2011.06.083
87
Chentoufi AA Binder NR Berka N Durand G Nguyen A Bettahi I et al . Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. (2008) 82:11792–802. doi: 10.1128/JVI.00692-08
88
Dervillez X Qureshi H Chentoufi AA Khan AA Kritzer E Yu DC et al . Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol. (2013) 191:5124–38. doi: 10.4049/jimmunol.1301415
89
Chentoufi AA Zhang X Lamberth K Dasgupta G Bettahi I Nguyen A et al . HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. (2008) 180:426–37. doi: 10.4049/jimmunol.180.1.426
90
Zhang X Dervillez X Chentoufi AA Badakhshan T Bettahi I Benmohamed L . Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol. (2012) 189:4496–509. doi: 10.4049/jimmunol.1201121
91
Kuo T Wang C Badakhshan T Chilukuri S BenMohamed L . The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine. (2014) 32:6733–45. doi: 10.1016/j.vaccine.2014.10.002
92
Smith JB Herbert JJ Truong NR Cunningham AL . Cytokines and chemokines: The vital role they play in herpes simplex virus mucosal immunology. Front Immunol. (2022) 13:936235. doi: 10.3389/fimmu.2022.936235
93
Long D Skoberne M Gierahn TM Larson S Price JA Clemens V et al . Identification of novel virus-specific antigens by CD4(+) and CD8(+) T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology. (2014) 464-465:296–311. doi: 10.1016/j.virol.2014.07.018
94
Hosken N McGowan P Meier A Koelle DM Sleath P Wagener F et al . Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. (2006) 80:5509–15. doi: 10.1128/JVI.02659-05
95
Dasgupta G Chentoufi AA Kalantari M Falatoonzadeh P Chun S Lim CH et al . Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol. (2012) 86:4358–69. doi: 10.1128/JVI.07107-11
96
Kalantari-Dehaghi M Chun S Chentoufi AA Pablo J Liang L Dasgupta G et al . Discovery of potential diagnostic and vaccine antigens in herpes simplex virus 1 and 2 by proteome-wide antibody profiling. J Virol. (2012) 86:4328–39. doi: 10.1128/JVI.05194-11
97
Chentoufi AA Dasgupta G Christensen ND Hu J Choudhury ZS Azeem A et al . (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. (2010) 184:2561–71. doi: 10.4049/jimmunol.0902322
98
Langenberg AG Corey L Ashley RL Leong WP Straus SE . A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. New Engl J Med. (1999) 341:1432–8. doi: 10.1056/NEJM199911043411904
99
Srivastava R Roy S Coulon PG Vahed H Prakash S Dhanushkodi N et al . Therapeutic mucosal vaccination of herpes simplex virus 2-infected Guinea pigs with ribonucleotide reductase 2 (RR2) protein boosts antiviral neutralizing antibodies and local tissue-resident CD4(+) and CD8(+) TRM cells associated with protection against recurrent genital herpes. J Virol. (2019) 93. doi: 10.1128/JVI.02309-18
100
Roy S Coulon PG Srivastava R Vahed H Kim GJ Walia SS et al . Blockade of LAG-3 immune checkpoint combined with therapeutic vaccination restore the function of tissue-resident anti-viral CD8+ T cells and protect against recurrent ocular herpes simplex infection and disease. Front In Immunol. (2018) 9:2922. doi: 10.3389/fimmu.2018.02922
101
Roy S Coulon PG Prakash S Srivastava R Geertsema R Dhanushkodi N et al . Blockade of PD-1 and LAG-3 immune checkpoints combined with vaccination restore the function of anti-viral tissue-resident CD8(+) TRM cells and reduce ocular herpes simplex infection and disease in HLA transgenic rabbits. J Virol. (2019). doi: 10.1128/JVI.00827-19
102
King NJ Parr EL Parr MB . Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. (1998) 160:1173–80. doi: 10.4049/jimmunol.160.3.1173
103
Zhang X Chentoufi AA Dasgupta G Nesburn AB Wu M Zhu X et al . A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. (2009) 2:129–43. doi: 10.1038/mi.2008.81
104
Zhao X Deak E Soderberg K Linehan M Spezzano D Zhu J et al . Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. (2003) 197:153–62. doi: 10.1084/jem.20021109
105
Koelle DM Posavad CM Barnum GR Johnson ML Frank JM Corey L . Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. (1998) 101:1500–8. doi: 10.1172/JCI1758
106
Posavad CM Koelle DM Shaughnessy MF Corey L . Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U.S.A. (1997) 94:10289–94.
107
Jing L Haas J Chong TM Bruckner JJ Dann GC Dong L et al . Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. (2012). doi: 10.1172/JCI65722
108
Gebhardt T Whitney PG Zaid A Mackay LK Brooks AG Heath WR et al . Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. (2011) 477:216–9. doi: 10.1038/nature10339
109
Mueller SN Gebhardt T Carbone FR Heath WR . Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. (2013) 31:137–61. doi: 10.1146/annurev-immunol-032712-095954
110
Schiffer JT Abu-Raddad L Mark KE Zhu J Selke S Koelle DM et al . Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U.S.A. (2010) 107:18973–8.
111
Zhu J Hladik F Woodward A Klock A Peng T Johnston C et al . Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. (2009) 15:886–92. doi: 10.1038/nm.2006
112
Zhu J Koelle DM Cao J Vazquez J Huang ML Hladik F et al . Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. (2007) 204:595–603. doi: 10.1084/jem.20061792
113
Tang VA Rosenthal KL . Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol. (2010) 87:39–44. doi: 10.1016/j.jri.2010.06.155
114
Zhu J Peng T Johnston C Phasouk K Kask AS Klock A et al . Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. (2013) 497:494–7. doi: 10.1038/nature12110
115
Zhu J Peng T Johnston C Phasouk K Kask AS Klock A et al . Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. (2013) 497:494–7. doi: 10.1038/nature12110
116
Chentoufi AA Dhanushkodi NR Srivastava R Prakash S Coulon PA Zayou L et al . Combinatorial herpes simplex vaccine strategies: from bedside to bench and back. Front Immunol. (2022) 13:849515. doi: 10.3389/fimmu.2022.849515
117
Lavery EA Coyle WJ . Herpes simplex virus and the alimentary tract. Curr Gastroenterol Rep. (2008) 10:417–23. doi: 10.1007/s11894-008-0078-8
118
Colemont LJ Pen JH Pelckmans PA Degryse HR Pattyn SR Van Maercke YM . Herpes simplex virus type 1 colitis: an unusual cause of diarrhea. Am J Gastroenterol. (1990) 85:1182–5.
119
Vicari AP Figueroa DJ Hedrick JA Foster JS Singh KP Menon S et al . TECK: A novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. (1997) 7:291–301. doi: 10.1016/S1074-7613(00)80531-2
120
Wurbel M-A Philippe J-M Nguyen C Victorero G Freeman T Wooding P et al . The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. (2000) 30:262–71. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0
121
Nomiyama H Amano K Kusuda J Imai T Miura R Yoshie O et al . The human CC chemokine TECK (SCYA25) maps to chromosome 19p13.2. Genomics. (1998) 51:311–2. doi: 10.1006/geno.1998.5352
122
Igaki K Komoike Y Nakamura Y Watanabe T Yamasaki M Fleming P et al . MLN3126, an antagonist of the chemokine receptor CCR9, ameliorates inflammation in a T cell mediated mouse colitis model. Int Immunopharmacol. (2018) 60:160–9. doi: 10.1016/j.intimp.2018.04.049
123
Feng N Jaimes MC Lazarus NH Monak D Zhang C Butcher EC et al . Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J Immunol. (2006) 176:5749–59. doi: 10.4049/jimmunol.176.10.5749
124
Jaimes MC Rojas OL Kunkel EJ Lazarus NH Soler D Butcher EC et al . Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J Virol. (2004) 78:10967–76. doi: 10.1128/JVI.78.20.10967-10976.2004
125
Kunkel EJ Kim CH Lazarus NH Vierra MA Soler D Bowman EP et al . CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. (2003) 111:1001–10. doi: 10.1172/JCI17244
126
Bowman EP Kuklin NA Youngman KR Lazarus NH Kunkel EJ Pan J et al . The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med. (2002) 195:269–75. doi: 10.1084/jem.20010670
127
Meurens F Whale J Brownlie R Dybvig T Thompson DR Gerdts V . Expression of mucosal chemokines TECK/CCL25 and MEC/CCL28 during fetal development of the ovine mucosal immune system. Immunology. (2007) 120:544–55. doi: 10.1111/j.1365-2567.2006.02532.x
128
Meurens F Berri M Siggers RH Willing BP Salmon H Van Kessel AG et al . Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors. PLoS One. (2007) 2:e677. doi: 10.1371/journal.pone.0000677
129
Meurens F Berri M Whale J Dybvig T Strom S Thompson D et al . Expression of TECK/CCL25 and MEC/CCL28 chemokines and their respective receptors CCR9 and CCR10 in porcine mucosal tissues. Vet Immunol Immunopathol. (2006) 113:313–27. doi: 10.1016/j.vetimm.2006.05.014
130
Zaballos A Gutierrez J Varona R Ardavin C Marquez G . Cutting edge: identification of the orphan chemokine receptor GPR-9–6 as CCR9, the receptor for the chemokine TECK. J Immunol. (1999) 162:5671–5. doi: 10.4049/jimmunol.162.10.5671
131
Lee S Heinrich EL Li L Lu J Choi AH Levy RA et al . CCR9-mediated signaling through beta-catenin and identification of a novel CCR9 antagonist. Mol Oncol. (2015) 9:1599–611. doi: 10.1016/j.molonc.2015.04.012
132
Gosling J Dairaghi DJ Wang Y Hanley M Talbot D Miao Z et al . Cutting edge: identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. J Immunol. (2000) 164:2851–6. doi: 10.4049/jimmunol.164.6.2851
133
Pathak M Padghan P Halder N Shilpi Kulkarni N Sonar SA et al . CCR9 signaling in dendritic cells drives the differentiation of Foxp3(+) Tregs and suppresses the allergic IgE response in the gut. Eur J Immunol. (2020) 50:404–17. doi: 10.1002/eji.201948327
134
Pathak M Lal G . The regulatory function of CCR9(+) dendritic cells in inflammation and autoimmunity. Front Immunol. (2020) 11:536326. doi: 10.3389/fimmu.2020.536326
135
Apostolaki M Manoloukos M Roulis M Wurbel MA Muller W Papadakis KA et al . Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn’s disease. Gastroenterology. (2008) 134:2025–35. doi: 10.1053/j.gastro.2008.02.085
136
Saruta M Yu QT Avanesyan A Fleshner PR Targan SR Papadakis KA . Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. (2007) 178:3293–300. doi: 10.4049/jimmunol.178.5.3293
137
Papadakis KA Landers C Prehn J Kouroumalis EA Moreno ST Gutierrez-Ramos JC et al . CC chemokine receptor 9 expression defines a subset of peripheral blood lymphocytes with mucosal T cell phenotype and Th1 or T-regulatory 1 cytokine profile. J Immunol. (2003) 171:159–65. doi: 10.4049/jimmunol.171.1.159
138
Papadakis KA Prehn J Moreno ST Cheng L Kouroumalis EA Deem R et al . CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. (2001) 121:246–54. doi: 10.1053/gast.2001.27154
139
Papadakis KA Prehn J Nelson V Cheng L Binder SW Ponath PD et al . The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. (2000) 165:5069–76. doi: 10.4049/jimmunol.165.9.5069
140
Li C Kim HK Prakhar P Luo S Crossman A Ligons DL et al . Chemokine receptor CCR9 suppresses the differentiation of CD4(+)CD8alphaalpha(+) intraepithelial T cells in the gut. Mucosal Immunol. (2022) 15:882–95. doi: 10.1038/s41385-022-00540-9
141
Staton TL Habtezion A Winslow MM Sato T Love PE Butcher EC . CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. (2006) 7:482–8. doi: 10.1038/ni1319
142
Staton TL Johnston B Butcher EC Campbell DJ . Murine CD8+ recent thymic emigrants are alphaE integrin-positive and CC chemokine ligand 25 responsive. J Immunol. (2004) 172:7282–8. doi: 10.4049/jimmunol.172.12.7282
143
Srivastava R Roy S Coulon PG Vahed H Parkash S Dhanushkodi N et al . Therapeutic mucosal vaccination of herpes simplex virus 2-infected Guinea pigs with ribonucleotide reductase 2 (RR2) protein boosts antiviral neutralizing antibodies and local tissue-resident CD4(+) and CD8(+) TRM cells associated with protection against recurrent genital herpes. J Virol. (2019) 93. doi: 10.1128/JVI.02309-18
144
Hsu CW Chang MH Chang HW Wu TY Chang YC . Parenterally administered porcine epidemic diarrhea virus-like particle-based vaccine formulated with CCL25/28 chemokines induces systemic and mucosal immune protectivity in pigs. Viruses. (2020) 12. doi: 10.3390/v12101122
145
Groom JR . Chemokines in cellular positioning and human disease. Immunol Cell Biol. (2015) 93:328–9. doi: 10.1038/icb.2015.27
146
Ying S O’Connor B Ratoff J Meng Q Fang C Cousins D et al . Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. (2008) 181:2790–8. doi: 10.4049/jimmunol.181.4.2790
147
Hermsen JL Gomez FE Maeshima Y Sano Y Kang W Kudsk KA . Decreased enteral stimulation alters mucosal immune chemokines. Jpen. (2008) 32:36–44. doi: 10.1177/014860710803200136
148
Di Carlo E Magnasco S D’Antuono T Tenaglia R Sorrentino C . The prostate-associated lymphoid tissue (PALT) is linked to the expression of homing chemokines CXCL13 and CCL21. Prostate. (2007) 67:1070–80.
149
Bermejo-Martin JF Garcia-Arevalo MC De Lejarazu RO Ardura J Eiros JM Alonso A et al . Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw. (2007) 18:162–7.
150
Suresh P Wanchu A . Chemokines and chemokine receptors in HIV infection: role in pathogenesis and therapeutics. J Postgrad Med. (2006) 52:210–7.
151
Ying S O’Connor B Ratoff J Meng Q Mallett K Cousins D et al . Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. (2005) 174:8183–90. doi: 10.4049/jimmunol.174.12.8183
152
Takahata Y Takada H Nomura A Nakayama H Ohshima K Hara T . Detection of interferon-gamma-inducible chemokines in human milk. Acta Paediatr. (2003) 92:659–65. doi: 10.1111/j.1651-2227.2003.tb00595.x
153
Hirose J Kawashima H Yoshie O Tashiro K Miyasaka M . Versican interacts with chemokines and modulates cellular responses. J Biol Chem. (2001) 276:5228–34. doi: 10.1074/jbc.M007542200
154
Eo SK Lee S Chun S Rouse BT . Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J Virol. (2001) 75:569–78. doi: 10.1128/JVI.75.2.569-578.2001
155
Eck M Schmausser B Scheller K Toksoy A Kraus M Menzel T et al . CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. (2000) 122:192–9. doi: 10.1046/j.1365-2249.2000.01374.x
156
Aubertin AM Le Grand R Wang Y Beyer C Tao L Neildez O et al . Generation of CD8+ T cell-generated suppressor factor and beta-chemokines by targeted iliac lymph node immunization in rhesus monkeys challenged with SHIV-89. 6P by rectal route. AIDS Res Hum Retroviruses. (2000) 16:381–92. doi: 10.1089/088922200309269
157
Saavedra M Taylor B Lukacs N Fidel P . Local production of chemokines during experimental vaginal candidiasis. Infection Immun. (1999) 67:5820–6. doi: 10.1128/IAI.67.11.5820-5826.1999
158
Yamaoka Y Kita M Kodama T Sawai N Tanahashi T Kashima K et al . Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. (1998) 42:609–17. doi: 10.1136/gut.42.5.609
159
Cuff CA Schwartz J Bergman CM Russell KS Bender JR Ruddle NH . Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. (1998) 161:6853–60. doi: 10.4049/jimmunol.161.12.6853
160
Desanti GE Jenkinson WE Parnell SM Boudil A Gautreau-Rolland L Eksteen B et al . Clonal analysis reveals uniformity in the molecular profile and lineage potential of CCR9(+) and CCR9(-) thymus-settling progenitors. J Immunol. (2011) 186:5227–35. doi: 10.4049/jimmunol.1002686
161
Eksteen B Adams DH . GSK-1605786, a selective small-molecule antagonist of the CCR9 chemokine receptor for the treatment of Crohn’s disease. IDrugs. (2010) 13:472–81.
162
Withers DR Jaensson E Gaspal F McConnell FM Eksteen B Anderson G et al . The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J Immunol. (2009) 183:5079–84. doi: 10.4049/jimmunol.0901514
163
Eksteen B Mora JR Haughton EL Henderson NC Lee-Turner L Villablanca EJ et al . Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. (2009) 137:320–9. doi: 10.1053/j.gastro.2009.02.046
164
Jaensson E Uronen-Hansson H Pabst O Eksteen B Tian J Coombes JL et al . Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. (2008) 205:2139–49. doi: 10.1084/jem.20080414
165
Eksteen B Grant AJ Miles A Curbishley SM Lalor PF Hubscher SG et al . Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. (2004) 200:1511–7. doi: 10.1084/jem.20041035
166
Kunkel EJ Butcher EC . Chemokines and the tissue-specific migration of lymphocytes. Immunity. (2002) 16:1–4. doi: 10.1016/S1074-7613(01)00261-8
167
Aghaallaei N Agarwal R Benjaminsen J Lust K Bajoghli B Wittbrodt J et al . Antigen-presenting cells and T cells interact in a specific area of the intestinal mucosa defined by the ccl25-ccr9 axis in medaka. Front Immunol. (2022) 13:812899. doi: 10.3389/fimmu.2022.812899
168
Qin S Sui Y Murphey-Corb MA Reinhart TA . Association between decreased CXCL12 and CCL25 expression and increased apoptosis in lymphoid tissues of cynomolgus macaques during SIV infection. J Med primatology. (2008) 37 Suppl 2:46–54. doi: 10.1111/j.1600-0684.2008.00327.x
169
Cromwell MA Carville A Mansfield K Klumpp S Westmoreland SV Lackner AA et al . SIV-specific CD8+ T cells are enriched in female genital mucosa of rhesus macaques and express receptors for inflammatory chemokines. Am J Reprod Immunol. (2011) 65:242–7. doi: 10.1111/j.1600-0897.2010.00966.x
170
Mavigner M Cazabat M Dubois M L’Faqihi FE Requena M Pasquier C et al . Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. (2012) 122:62–9. doi: 10.1172/JCI59011
171
Fu H Jangani M Parmar A Wang G Coe D Spear S et al . A subset of CCL25-induced gut-homing T cells affects intestinal immunity to infection and cancer. Front Immunol. (2019) 10:271. doi: 10.3389/fimmu.2019.00271
172
Kathuria N Kraynyak KA Carnathan D Betts M Weiner DB Kutzler MA . Generation of antigen-specific immunity following systemic immunization with DNA vaccine encoding CCL25 chemokine immunoadjuvant. Hum Vaccin Immunother. (2012) 8:1607–19. doi: 10.4161/hv.22574
173
Aldon Y Kratochvil S Shattock RJ McKay PF . Chemokine-adjuvanted plasmid DNA induces homing of antigen-specific and non-antigen-specific B and T cells to the intestinal and genital mucosae. J Immunol. (2020) 204:903–13. doi: 10.4049/jimmunol.1901184
174
King DF McKay PF Mann JF Jones CB Shattock RJ . Plasmid DNA vaccine co-immunisation modulates cellular and humoral immune responses induced by intranasal inoculation in mice. PLoS One. (2015) 10:e0141557. doi: 10.1371/journal.pone.0141557
175
Sumida SM McKay PF Truitt DM Kishko MG Arthur JC Seaman MS et al . Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J Clin Invest. (2004) 114:1334–42. doi: 10.1172/JCI200422608
176
Barouch DH McKay PF Sumida SM Santra S Jackson SS Gorgone DA et al . Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J Virol. (2003) 77:8729–35. doi: 10.1128/JVI.77.16.8729-8735.2003
177
Trivedi PJ Schmidt C Bruns T . Letter: the therapeutic potential of targeting CCL25/CCR9 in colonic inflammatory bowel disease - reading between the lines. Aliment Pharmacol Ther. (2016) 44:307–8. doi: 10.1111/apt.13679
178
Trivedi PJ Bruns T Ward S Mai M Schmidt C Hirschfield GM et al . Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J Autoimmun. (2016) 68:98–104. doi: 10.1016/j.jaut.2016.01.001
179
Eberhardson M Karlen P Linton L Jones P Lindberg A Kostalla MJ et al . Randomised, double-blind, placebo-controlled trial of CCR9-targeted leukapheresis treatment of ulcerative colitis patients. J Crohns Colitis. (2017) 11:534–42.
180
Calabrese GC Luczak EN Roux ME . Importance of CCL25 in the attraction of T cells and the role of IL-7 on the signaling pathways in intestinal epithelial cells. Immunobiology. (2008) 214:403–9. doi: 10.1016/j.imbio.2008.12.008
181
Blokland SLM Kislat A Homey B Smithson GM Kruize AA Radstake T et al . Decreased circulating CXCR3 + CCR9+T helper cells are associated with elevated levels of their ligands CXCL10 and CCL25 in the salivary gland of patients with Sjogren’s syndrome to facilitate their concerted migration. Scand J Immunol. (2020) 91:e12852. doi: 10.1111/sji.12852
182
Feagan BG Sandborn WJ D’Haens G Lee SD Allez M Fedorak RN et al . Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn’s disease. Aliment Pharmacol Ther. (2015) 42:1170–81. doi: 10.1111/apt.13398
183
Khalid Z Huan M Sohail Raza M Abbas M Naz Z Kombe Kombe AJ et al . Identification of novel therapeutic candidates against SARS-coV-2 infections: an application of RNA sequencing toward mRNA based nanotherapeutics. Front Microbiol. (2022) 13:901848. doi: 10.3389/fmicb.2022.901848
184
Su CM Wang L Yoo D . Activation of NF-kappaB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep. (2021) 11:13464. doi: 10.1038/s41598-021-92941-2
185
Wang W Soto H Oldham ER Buchanan ME Homey B Catron D et al . Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J Biol Chem. (2000) 275:22313–23. doi: 10.1074/jbc.M001461200
186
Castelletti E Lo Caputo S Kuhn L Borelli M Gajardo J Sinkala M et al . The mucosae-associated epithelial chemokine (MEC/CCL28) modulates immunity in HIV infection. PLoS One. (2007) 2:e969. doi: 10.1371/journal.pone.0000969
187
Cha HR Ko HJ Kim ED Chang SY Seo SU Cuburu N et al . Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. J Immunol. (2011) 187:3044–52. doi: 10.4049/jimmunol.1100402
188
Eksteen B Miles A Curbishley SM Tselepis C Grant AJ Walker LS et al . Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol. (2006) 177:593–603. doi: 10.4049/jimmunol.177.1.593
189
O’Gorman MT Jatoi NA Lane SJ Mahon BP . IL-1beta and TNF-alpha induce increased expression of CCL28 by airway epithelial cells via an NFkappaB-dependent pathway. Cell Immunol. (2005) 238:87–96.
190
Pan J Kunkel EJ Gosslar U Lazarus N Langdon P Broadwell K et al . A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. (2000) 165:2943–9. doi: 10.4049/jimmunol.165.6.2943
191
Xiong N Fu Y Hu S Xia M Yang J . CCR10 and its ligands in regulation of epithelial immunity and diseases. Protein Cell. (2012) 3:571–80. doi: 10.1007/s13238-012-2927-3
192
Homey B Wang W Soto H Buchanan ME Wiesenborn A Catron D et al . Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol. (2000) 164:3465–70. doi: 10.4049/jimmunol.164.7.3465
193
Allen TM O’Connor DH Jing P Dzuris JL Mothe BR Vogel TU et al . Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. (2000) 407:386–90. doi: 10.1038/35030124
194
Khan AA Srivastava R Chentoufi AA Geertsema R Thai NT Dasgupta G et al . Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells. J Virol. (2015) 89:6619–32. doi: 10.1128/JVI.00788-15
195
Dasgupta G Chentoufi AA Nesburn AB Wechsler SL BenMohamed L . New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines. (2009) 8:1023–35. doi: 10.1586/erv.09.60
196
Nesburn AB Bettahi I Dasgupta G Chentoufi AA Zhang X You S et al . Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol. (2007) 81:7647–61. doi: 10.1128/JVI.00294-07
197
Nesburn AB Ramos TV Zhu X Asgarzadeh H Nguyen V BenMohamed L . Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. (2005) 23:873–83. doi: 10.1016/j.vaccine.2004.08.019
198
Rodriguez MW Paquet AC Yang YH Erle DJ . Differential gene expression by integrin beta 7+ and beta 7- memory T helper cells. BMC Immunol. (2004) 5:13. doi: 10.1186/1471-2172-5-13
199
Wright N Hidalgo A Rodriguez-Frade JM Soriano SF Mellado M Parmo-Cabanas M et al . The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J Immunol. (2002) 168:5268–77. doi: 10.4049/jimmunol.168.10.5268
200
Kunkel EJ Campbell DJ Butcher EC . Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. (2003) 10:313–23. doi: 10.1038/sj.mn.7800196
201
John AE Thomas MS Berlin AA Lukacs NW . Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am J Pathol. (2005) 166:345–53. doi: 10.1016/S0002-9440(10)62258-4
202
Sisirak V Vey N Vanbervliet B Duhen T Puisieux I Homey B et al . CCR6/CCR10-mediated plasmacytoid dendritic cell recruitment to inflamed epithelia after instruction in lymphoid tissues. Blood. (2011) 118:5130–40. doi: 10.1182/blood-2010-07-295626
203
Stroukov W Mastronicola D Albany CJ Catak Z Lombardi G Scotta C . OMIP-090: A 20-parameter flow cytometry panel for rapid analysis of cell diversity and homing capacity in human conventional and regulatory T cells. Cytometry A. (2023) 103:362–7. doi: 10.1002/cyto.a.24720
204
Qiu C Yang L Liu S Zhang C Zhang Q Jin Z . Interleukin-35 dampens T helper 22 phenotype shift in CD4(+)CD25(+)CD127(dim/-) regulatory T cells in primary biliary cholangitis. Int Immunopharmacol. (2023) 24:109751. doi: 10.1016/j.intimp.2023.109751
205
Alexander MR Dale BL Smart CD Elijovich F Wogsland CE Lima SM et al . Immune profiling reveals decreases in circulating regulatory and exhausted T cells in human hypertension. JACC Basic Transl Sci. (2023) 8:319–36. doi: 10.1016/j.jacbts.2022.09.007
206
Chen FY Geng CA Chou CK Zheng JB Yang Y Wang YF et al . Distepharinamide, a novel dimeric proaporphine alkaloid from Diploclisia glaucescens, inhibits the differentiation and proliferative expansion of CD4(+)Foxp3(+) regulatory T cells. Phytomedicine. (2022) 107:154482. doi: 10.1016/j.phymed.2022.154482
207
Kim J Lee J Kim HJ Kameyama N Nazarian R Der E et al . Single-cell transcriptomics applied to emigrating cells from psoriasis elucidate pathogenic versus regulatory immune cell subsets. J Allergy Clin Immunol. (2021) 148:1281–92. doi: 10.1016/j.jaci.2021.04.021
208
Wang P Qi X Xu G Liu J Guo J Li X et al . CCL28 promotes locomotor recovery after spinal cord injury via recruiting regulatory T cells. Aging (Albany NY). (2019) 11:7402–15. doi: 10.18632/aging.102239
209
Ohue Y Nishikawa H . Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. (2019) 110:2080–9. doi: 10.1111/cas.14069
210
Zhang YY Wang AX Xu L Shen N Zhu J Tu CX . Characteristics of peripheral blood CD4+CD25+ regulatory T cells and related cytokines in severe atopic dermatitis. Eur J Dermatol. (2016) 26:240–6. doi: 10.1684/ejd.2015.2709
211
Gorman S Geldenhuys S Judge M Weeden CE Waithman J Hart PH . Dietary vitamin D increases percentages and function of regulatory T cells in the skin-draining lymph nodes and suppresses dermal inflammation. J Immunol Res. (2016) 2016:1426503. doi: 10.1155/2016/1426503
212
Fu Y Yang J Xiong N . Cutting edge: skin CCR10+ CD8+ T cells support resident regulatory T cells through the B7.2/receptor axis to regulate local immune homeostasis and response. J Immunol. (2016) 196:4859–64. doi: 10.4049/jimmunol.1502662
213
Ahn JB Kang SA Kim DH Yu HS . Activation and Recruitment of Regulatory T Cells via Chemokine Receptor Activation in Trichinella spiralis-Infected Mice. Korean J Parasitol. (2016) 54:163–71. doi: 10.3347/kjp.2016.54.2.163
214
Xia M Hu S Fu Y Jin W Yi Q Matsui Y et al . CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J Allergy Clin Immunol. (2014) 134:634–644.e10. doi: 10.1016/j.jaci.2014.03.010
215
Khoo AL Koenen HJ Michels M Ooms S Bosch M Netea MG et al . High-dose vitamin D3 supplementation is a requisite for modulation of skin-homing markers on regulatory T cells in HIV-infected patients. AIDS Res Hum Retroviruses. (2013) 29:299–306. doi: 10.1089/aid.2012.0051
216
Kim CH Qu CK Hangoc G Cooper S Anzai N Feng GS et al . Abnormal chemokine-induced responses of immature and mature hematopoietic cells from motheaten mice implicate the protein tyrosine phosphatase SHP-1 in chemokine responses. J Exp Med. (1999) 190:681–90. doi: 10.1084/jem.190.5.681
217
Kim CH Broxmeyer HE . Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. (1999) 65:6–15. doi: 10.1002/jlb.65.1.6
218
Youn BS Zhang SM Lee EK Park DH Broxmeyer HE Murphy PM et al . Molecular cloning of leukotactin-1: a novel human beta-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J Immunol. (1997) 159:5201–5. doi: 10.4049/jimmunol.159.11.5201
219
Sato H Nakajima N Hasegawa G Kawata Y Sato Y Suzuki K et al . Immunohistochemical differentiation of eosinophilic esophageal myositis from eosinophilic esophagitis. J Gastroenterol Hepatol. (2017) 32:106–13. doi: 10.1111/jgh.13466
220
Suzaki Y Hamada K Nomi T Ito T Sho M Kai Y et al . A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. (2008) 31:783–9. doi: 10.1183/09031936.00111507
221
Nakajima H Fukuda K Doi Y Sugino M Kimura F Hanafusa T et al . Expression of TH1/TH2-related chemokine receptors on peripheral T cells and correlation with clinical disease activity in patients with multiple sclerosis. Eur Neurol. (2004) 52:162–8. doi: 10.1159/000081856
222
Fukagawa K Okada N Fujishima H Nakajima T Tsubota K Takano Y et al . CC-chemokine receptor 3: a possible target in treatment of allergy-related corneal ulcer. Invest Ophthalmol Vis Sci. (2002) 43:58–62.
223
Terada N Hamano N Kim WJ Hirai K Nakajima T Yamada H et al . The kinetics of allergen-induced eotaxin level in nasal lavage fluid: its key role in eosinophil recruitment in nasal mucosa. Am J Respir Crit Care Med. (2001) 164:575–9. doi: 10.1164/ajrccm.164.4.2009046
224
Kitaura M Suzuki N Imai T Takagi S Suzuki R Nakajima T et al . Molecular cloning of a novel human CC chemokine (Eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem. (1999) 274:27975–80. doi: 10.1074/jbc.274.39.27975
225
Kato H Tsuchiya N Izumi S Miyamasu M Nakajima T Kawasaki H et al . New variations of human CC-chemokine receptors CCR3 and CCR4. Genes Immun. (1999) 1:97–104. doi: 10.1038/sj.gene.6363638
226
Zheng S Yang B Li L Chen M Zhang L Chi W et al . CRTAM promotes antitumor immune response in triple negative breast cancer by enhancing CD8+ T cell infiltration. Int Immunopharmacol. (2024) 129:111625. doi: 10.1016/j.intimp.2024.111625
227
Harasawa H Yamada Y Hieshima K Jin Z Nakayama T Yoshie O et al . Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk Lymphoma. (2006) 47:2163–73. doi: 10.1080/10428190600775599
228
Hieshima K Kawasaki Y Hanamoto H Nakayama T Nagakubo D Kanamaru A et al . CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. (2004) 173:3668–75. doi: 10.4049/jimmunol.173.6.3668
229
Hanamoto H Nakayama T Miyazato H Takegawa S Hieshima K Tatsumi Y et al . Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin’s disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol. (2004) 164:997–1006. doi: 10.1016/S0002-9440(10)63187-2
230
Nakayama T Hieshima K Izawa D Tatsumi Y Kanamaru A Yoshie O . Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. (2003) 170:1136–40. doi: 10.4049/jimmunol.170.3.1136
231
Hieshima K Ohtani H Shibano M Izawa D Nakayama T Kawasaki Y et al . CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. (2003) 170:1452–61. doi: 10.4049/jimmunol.170.3.1452
232
Mergia Terefe E Catalan Opulencia MJ Rakhshani A Ansari MJ Sergeevna SE Awadh SA et al . Roles of CCR10/CCL27-CCL28 axis in tumour development: mechanisms, diagnostic and therapeutic approaches, and perspectives. Expert Rev Mol Med. (2022) 24:e37.
233
Yan Y Hu K Fu M Deng X Luo S Tong L et al . CCL19 and CCL28 assist herpes simplex virus 2 glycoprotein D to induce protective systemic immunity against genital viral challenge. mSphere. (2021) 6. doi: 10.1128/mSphere.00058-21
234
Muthuswamy RV Sundstrom P Borjesson L Gustavsson B Quiding-Jarbrink M . Impaired migration of IgA-secreting cells to colon adenocarcinomas. Cancer Immunol Immunother. (2013) 62:989–97. doi: 10.1007/s00262-013-1410-1
235
Sundstrom P Lundin SB Nilsson LA Quiding-Jarbrink M . Human IgA-secreting cells induced by intestinal, but not systemic, immunization respond to CCL25 (TECK) and CCL28 (MEC). Eur J Immunol. (2008) 38:3327–38.
236
Hansson M Hermansson M Svensson H Elfvin A Hansson LE Johnsson E et al . CCL28 is increased in human Helicobacter pylori-induced gastritis and mediates recruitment of gastric immunoglobulin A-secreting cells. Infect Immun. (2008) 76:3304–11. doi: 10.1128/IAI.00041-08
237
Weber KS Grone HJ Rocken M Klier C Gu S Wank R et al . Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur J Immunol. (2001) 31:2458–66. doi: 10.1002/1521-4141(200108)31:8<2458::AID-IMMU2458>3.0.CO;2-L
238
Wilson E Butcher EC . CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. (2004) 200:805–9. doi: 10.1084/jem.20041069
239
Morteau O Gerard C Lu B Ghiran S Rits M Fujiwara Y et al . An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J Immunol. (2008) 181:6309–15. doi: 10.4049/jimmunol.181.9.6309
240
Belshe PB Leone PA Bernstein DI Wald A Levin MJ Stapleton JT et al . Efficacy results of a trial of a herpes simplex vaccine. New Engl J Med. (2012) 366:34–43. doi: 10.1056/NEJMoa1103151
241
Knipe DM Corey L Cohen JI Deal CD . Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Next Generation Herpes Simplex Virus Vaccines. Vaccine. (2014) 32:1561–2. doi: 10.1016/j.vaccine.2014.01.052
242
Ariotti S Hogenbirk MA Dijkgraaf FE Visser LL Hoekstra ME Song JY et al . T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. (2014) 346:101–5. doi: 10.1126/science.1254803
243
Mackay LK Rahimpour A Ma JZ Collins N Stock AT Hafon ML et al . The developmental pathway for CD103CD8 tissue-resident memory T cells of skin. Nat Immunol. (2013). doi: 10.1038/ni.2744
244
Mackay LK Rahimpour A Ma JZ Collins N Stock AT Hafon ML et al . The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. (2013) 14:1294–301. doi: 10.1038/ni.2744
245
Wakim LM Woodward-Davis A Liu R Hu Y Villadangos J Smyth G et al . The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. (2012) 189:3462–71. doi: 10.4049/jimmunol.1201305
246
Gebhardt T Mackay LK . Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol. (2012) 3:340. doi: 10.3389/fimmu.2012.00340
247
Mackay LK Wakim L van Vliet CJ Jones CM Mueller SN Bannard O et al . Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol. (2012) 188:2173–8. doi: 10.4049/jimmunol.1102719
248
Mackay LK Stock AT Ma JZ Jones CM Kent SJ Mueller SN et al . Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U.S.A. (2012) 109:7037–42.
249
Zhu J Peng T Johnston C Phasouk K Kask AS Klock A et al . Immune surveillance by CD8alphaalpha skin-resident T cells in human herpes virus infection. Nature. (2013). doi: 10.1038/nature12110
250
Laidlaw BJ Zhang N Marshall HD Staron MM Guan T Hu Y et al . CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. (2014) 41:633–45. doi: 10.1016/j.immuni.2014.09.007
251
Purwar R Campbell J Murphy G Richards WG Clark RA Kupper TS . Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. (2011) 6:e16245.
252
Sathaliyawala T Kubota M Yudanin N Turner D Camp P Thome JJ et al . Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. (2013) 38:187–97. doi: 10.1016/j.immuni.2012.09.020
253
Wu T Hu Y Lee YT Bouchard KR Benechet A Khanna K et al . Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. (2014) 95:215–24. doi: 10.1189/jlb.0313180
254
He J Thomas MA de Anda J Lee MW Van Why E Simpson P et al . Chemokine CCL28 is a potent therapeutic agent for oropharyngeal candidiasis. Antimicrob Agents Chemother. (2020) 64. doi: 10.1128/AAC.00210-20
255
Starnes T Rasila KK Robertson MJ Brahmi Z Dahl R Christopherson K et al . The chemokine CXCL14 (BRAK) stimulates activated NK cell migration: implications for the downregulation of CXCL14 in Malignancy. Exp Hematol. (2006) 34:1101–5. doi: 10.1016/j.exphem.2006.05.015
256
Meuter S Moser B . Constitutive expression of CXCL14 in healthy human and murine epithelial tissues. Cytokine. (2008) 44:248–55. doi: 10.1016/j.cyto.2008.08.009
257
Meuter S Schaerli P Roos RS Brandau O Bosl MR von Andrian UH et al . Murine CXCL14 is dispensable for dendritic cell function and localization within peripheral tissues. Mol Cell Biol. (2007) 27:983–92. doi: 10.1128/MCB.01648-06
258
Kurth I Willimann K Schaerli P Hunziker T Clark-Lewis I Moser B . Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med. (2001) 194:855–61. doi: 10.1084/jem.194.6.855
259
Nitzsche B Rong WW Goede A Hoffmann B Scarpa F Kuebler WM et al . Coalescent angiogenesis-evidence for a novel concept of vascular network maturation. Angiogenesis. (2022) 25:35–45. doi: 10.1007/s10456-021-09824-3
260
Shellenberger TD Wang M Gujrati M Jayakumar A Strieter RM Burdick MD et al . BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. (2004) 64:8262–70. doi: 10.1158/0008-5472.CAN-04-2056
261
Shurin GV Ferris R Tourkova IL Perez L Lokshin A Balkir L et al . Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo1. J Immunol. (2005) 174:5490–8. doi: 10.4049/jimmunol.174.9.5490
262
Flamme I Frolich T Risau W . Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. (1997) 173:206–10. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C
263
Risau W Flamme I . Vasculogenesis. Annu Rev Cell Dev Biol. (1995) 11:73–91. doi: 10.1146/annurev.cb.11.110195.000445
264
Flamme I Breier G Risau W . Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. (1995) 169:699–712. doi: 10.1006/dbio.1995.1180
265
Zhang Y Jin Y Li J Yan Y Wang T Wang X et al . CXCL14 as a key regulator of neuronal development: insights from its receptor and multi-omics analysis. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25031651
266
Miyajima R Tanegashima K Naruse N Denda M Hara T Otaka A . Identification of low-density lipoprotein receptor-related protein 1 as a CXCL14 receptor using chemically synthesized tetrafunctional probes. ACS Chem Biol. (2024) 19:551–62. doi: 10.1021/acschembio.3c00717
267
Al Hamwi G Namasivayam V Buschbell B Gedschold R Golz S Muller CE . Proinflammatory chemokine CXCL14 activates MAS-related G protein-coupled receptor MRGPRX2 and its putative mouse ortholog MRGPRB2. Commun Biol. (2024) 7:52. doi: 10.1038/s42003-023-05739-5
268
Kouzeli A Collins PJ Metzemaekers M Meyrath M Szpakowska M Artinger M et al . CXCL14 preferentially synergizes with homeostatic chemokine receptor systems. Front Immunol. (2020) 11:561404. doi: 10.3389/fimmu.2020.561404
269
Atanes P Hawkes RG Olaniru OE Ruz-Maldonado I Amisten S Persaud SJ . CXCL14 inhibits insulin secretion independently of CXCR4 or CXCR7 receptor activation or cAMP inhibition. Cell Physiol Biochem. (2019) 52:879–92. doi: 10.33594/000000061
270
Tanegashima K Takahashi R Nuriya H Iwase R Naruse N Tsuji K et al . CXCL14 acts as a specific carrier of cpG DNA into dendritic cells and activates toll-like receptor 9-mediated adaptive immunity. EBioMedicine. (2017) 24:247–56. doi: 10.1016/j.ebiom.2017.09.012
271
Tang L Chen X Hou J Wei X . CXCL14 in prostate cancer: complex interactions in the tumor microenvironment and future prospects. J Transl Med. (2025) 23:9. doi: 10.1186/s12967-024-06022-9
272
Wu T Yang W Sun A Wei Z Lin Q . The role of CXC chemokines in cancer progression. Cancers (Basel). (2022) 15. doi: 10.3390/cancers15010167
273
Spaks A . Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. (2017) 9:S164–71. doi: 10.21037/jtd.2017.03.61
274
Feng D Shi X Zhang F Xiong Q Wei Q Yang L . Mitochondria dysfunction-mediated molecular subtypes and gene prognostic index for prostate cancer patients undergoing radical prostatectomy or radiotherapy. Front Oncol. (2022) 12:858479. doi: 10.3389/fonc.2022.858479
275
Sjoberg E Meyrath M Milde L Herrera M Lovrot J Hagerstrand D et al . A novel ACKR2-dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res. (2019) 25:3702–17. doi: 10.1158/1078-0432.CCR-18-1294
276
Dai C Basilico P Cremona TP Collins P Moser B Benarafa C et al . CXCL14 displays antimicrobial activity against respiratory tract bacteria and contributes to clearance of Streptococcus pneumoniae pulmonary infection. J Immunol. (2015) 194:5980–9. doi: 10.4049/jimmunol.1402634
277
Benarafa C Wolf M . CXCL14: the Swiss army knife chemokine. Oncotarget. (2015) 6:34065–6. doi: 10.18632/oncotarget.6040
278
Giacobbi NS Mullapudi S Nabors H Pyeon D . The chemokine CXCL14 as a potential immunotherapeutic agent for cancer therapy. Viruses. (2024) 16. doi: 10.3390/v16020302
279
Westrich JA Vermeer DW Colbert PL Spanos WC Pyeon D . The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol Carcinog. (2020) 59:794–806. doi: 10.1002/mc.23188
280
Westrich JA Vermeer DW Silva A Bonney S Berger JN Cicchini L et al . CXCL14 suppresses human papillomavirus-associated head and neck cancer through antigen-specific CD8(+) T-cell responses by upregulating MHC-I expression. Oncogene. (2019) 38:7166–80. doi: 10.1038/s41388-019-0911-6
281
Cicchini L Blumhagen RZ Westrich JA Myers ME Warren CJ Siska C et al . High-risk human papillomavirus E7 alters host DNA methylome and represses HLA-E expression in human keratinocytes. Sci Rep. (2017) 7:3633. doi: 10.1038/s41598-017-03295-7
282
Cicchini L Westrich JA Xu T Vermeer DW Berger JN Clambey ET et al . Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio. (2016) 7. doi: 10.1128/mBio.00270-16
283
den Boon JA Pyeon D Wang SS Horswill M Schiffman M Sherman M et al . Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci U.S.A. (2015) 112:E3255–64.
284
Han D Lee AY Kim T Choi JY Cho MY Song A et al . Microenvironmental network of clonal CXCL13+CD4+ T cells and Tregs in pemphigus chronic blisters. J Clin Invest. (2023) 133. doi: 10.1172/JCI166357
285
Quadiri A Lekbach Y Elfatimi E Prakash S Vahed H Karan S et al . The path towards effective long-lasting tissue-targeted prime/pull/keep herpes simplex therapeutic vaccines. Vaccines (Basel). (2025) 13. doi: 10.3390/vaccines13090908
286
Takamura S . Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol. (2018) 9:1214. doi: 10.3389/fimmu.2018.01214
287
Us D . Herpes simplex virus vaccine studies: from past to present. Mikrobiyol Bul. (2006) 40:413–33.
288
Koelle DM Dong L Jing L Laing KJ Zhu J Jin L et al . HSV-2-specific human female reproductive tract tissue resident memory T cells recognize diverse HSV antigens. Front Immunol. (2022) 13:867962. doi: 10.3389/fimmu.2022.867962
289
Zhang M Deng X Guan X Geng L Fu M Zhang B et al . Herpes simplex virus type 2 infection-induced expression of CXCR3 ligands promotes CD4+ T cell migration and is regulated by the viral immediate-early protein ICP4. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.02932
290
Quadiri A Prakash S Dhanushkodi NR Singer M Zayou L Shaik AM et al . Therapeutic prime/pull vaccination of HSV-2-infected Guinea pigs with the ribonucleotide reductase 2 (RR2) protein and CXCL11 chemokine boosts antiviral local tissue-resident and effector memory CD4(+) and CD8(+) T cells and protects against recurrent genital herpes. J Virol. (2024) 98:e0159623. doi: 10.1128/jvi.01596-23
291
Quadiri A Prakash S Vahed H Tadros JM Sun M Hormi-Carver KK et al . Therapeutic mucosal vaccination of herpes simplex virus type 2 infected Guinea pigs with an adenovirus-based vaccine expressing the ribonucleotide reductase 2 and glycoprotein D induces local tissue-resident CD4+ and CD8+ TRM cells associated with protection against recurrent genital herpes. Front Immunol. (2025) 16:1568258. doi: 10.3389/fimmu.2025.1568258
292
Awasthi S Lubinski JM Shaw CE Barrett SM Cai M Wang F et al . Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in Guinea pigs compared to immunization with gD alone. J Virol. (2011) 85:10472–86. doi: 10.1128/JVI.00849-11
293
Awasthi S Balliet JW Flynn JA Lubinski JM Shaw CE DiStefano DJ et al . Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive Guinea pigs. J Virol. (2014) 88:2000–10. doi: 10.1128/JVI.03163-13
294
Hook LM Cairns TM Awasthi S Brooks BD Ditto NT Eisenberg RJ et al . Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in Guinea pigs. PLoS Pathog. (2018) 14:e1007095. doi: 10.1371/journal.ppat.1007095
295
Egan K Hook LM Naughton A Friedman HM Awasthi S . Herpes simplex virus type 2 trivalent protein vaccine containing glycoproteins C, D and E protects Guinea pigs against HSV-1 genital infection. Hum Vaccin Immunother. (2020) 16:2109–13. doi: 10.1080/21645515.2020.1749509
296
Chentoufi AA Kritzer E Tran MV Dasgupta G Lim CH Yu DC et al . The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol. (2011) 85:9127–38. doi: 10.1128/JVI.00587-11
297
Chentoufi AA Dervillez X Dasgupta G Nguyen C Kabbara WK Jiang X et al . The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol. (2012) 25(3). doi: 10.1089/vim.2011.0091
298
Bernstein DI Cardin RD Bravo FJ Awasthi S Lu P Pullum DA et al . Successful application of prime and pull strategy for a therapeutic HSV vaccine. NPJ Vaccines. (2019) 4:33. doi: 10.1038/s41541-019-0129-1
299
Hussain MT Stanfield BA Bernstein DI . Small animal models to study herpes simplex virus infections. Viruses. (2024) 16. doi: 10.3390/v16071037
300
Bernstein DI Pullum DA Cardin RD Bravo FJ Dixon DA Kousoulas KG . The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the Guinea pig model of genital herpes. Vaccine. (2019) 37:61–8. doi: 10.1016/j.vaccine.2018.11.042
301
Lee S Ives AM Bertke AS . Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol. (2015) 89:8383–91. doi: 10.1128/JVI.00468-15
302
Chentoufi AA Prakash S Vahed H Karan S Quadiri A Nesburn AB et al . A tissue-targeted prime/pull/keep therapeutic herpes simplex virus vaccine protects against recurrent ocular herpes infection and disease in HLA-A*0201 transgenic rabbits. J Virol. (2025) 99:e00135–25. doi: 10.1128/jvi.00135-25
Summary
Keywords
CCL25, CCL28, cellular immunity, chemokines, CXCL14, CXCL17, memory CD4+ and CD8+ T cells, tissue-resident
Citation
Lekbach Y, Chentoufi AA, Prakash S, Karan S, Quadiri A, Hormi-Carver KK, Dorotta JC and BenMohamed L (2026) Role of mucosal chemokines in the development of tissue-resident CD4+ and CD8+ TRM cells to fend off herpes simplex infections. Front. Immunol. 17:1661640. doi: 10.3389/fimmu.2026.1661640
Received
08 July 2025
Revised
25 December 2025
Accepted
19 January 2026
Published
19 February 2026
Volume
17 - 2026
Edited by
Cong Sun, Sun Yat-sen University Cancer Center (SYSUCC), China
Reviewed by
Luis Alberto Sanchez Vargas, Universidad del Papaloapan, Mexico
K.A.S.N Shanaka, The Ohio State University, United States
Updates
Copyright
© 2026 Lekbach, Chentoufi, Prakash, Karan, Quadiri, Hormi-Carver, Dorotta and BenMohamed.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lbachir BenMohamed, Lbenmoha@uci.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.