Abstract
In type 1 diabetes (T1D), chronic autoimmune responses lead to the destruction of β-cells in pancreatic islets. As more of the β-cell mass is destroyed, the disease progresses, resulting in insulin deficiency. Recent discoveries uncovering the mechanisms behind the autoimmune attack on β-cells have allowed for a better understanding of the development of the disease and categorizing it into stages of progression. Further, FDA approval of the first drug for the prevention of T1D demonstrated the potential for early intervention therapies. Meanwhile, for patients whose β-cell mass is fully destroyed, islet transplantation has been shown to achieve long-term insulin independence. However, this procedure requires lifelong immunosuppression, which can increase the risk of infections and malignancies. Here, we will review recent advances in immunomodulation approaches for the prevention of type 1 diabetes and strategies for islet cell replacement. First, we introduce the pathogenesis of T1D and the stages of the disease that require different immunomodulatory approaches. Then, we will discuss current immunotherapies for the prevention of T1D, highlighting strategies such as antigen-specific, immune blockade, and cell-based therapies, which aim to stop autoimmune attack for patients at early stages of T1D. Afterwards, we discuss advancement for islet replacement approaches, highlighting islet engineering, device encapsulation, and immunomodulatory biomaterials, which aim to prolong the survival of transplanted islets and minimize the need for immunosuppression. We expect this review to provide a comprehensive understanding of current advances in immunomodulatory therapies for T1D.
1 Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease in which insulin-producing islet β-cells are destroyed by the host immune system, causing the lifelong need for exogenous insulin supply. T1D affects more than 9.5 million people worldwide, and that number is expected to grow to between 13.5 and 17.4 million individuals by 2040 (1, 2). Additionally, the incidence of T1D is increasing in both youth and older adults (3, 4). For instance, T1D incidence among youth aged 10–24 years has grown from 7.78 in 1990 to 11.07 in 2019 per 100,000 individuals (3). At the same time, several studies have demonstrated a peak in T1D incidence among older adults (4). Of the 503,000 newly diagnosed cases of T1D in 2024, 56% were adults, further emphasizing the increasing incidence of T1D in that population (5).
T1D development tends to occur in individuals with a genetic predisposition triggered by environmental or immunological events. Genetic predisposition is determined by specific combinations of genes in the human leukocyte antigen (HLA) region, which affect how T cells recognize and tolerate foreign and self-molecules (6). It is also influenced by polymorphism in genes encoding protein tyrosine phosphatase (PTPN22) (7), cytotoxic T lymphocyte-associated antigen 4 (CTLA4) (8, 9), IL-2 receptor subunit a (IL2Ra) (10) and other genes involved in regulating immune responses. Additionally, polymorphism in insulin (INS) leads to the escape of autoreactive T cells from the thymus (11, 12). These genetic factors play a crucial role in shaping the immune response.
T1D development is closely linked with β-cells mass destruction, and the disease progresses as more β-cells are lost (Figure 1A) (13, 14). For earlier stages of T1D, where most of the β-cells’ mass is still intact, there is an opportunity window for immunotherapies to prevent further autoimmune attack and destruction of β-cells (15). For advanced T1D patients, where β-cell mass is severely reduced, maintaining normoglycemia with insulin is challenging (16). The recent FDA approval of Lantidra, the first allogeneic islet therapy for T1D, established islet transplantation as a viable option for some patients, enabling tighter blood glucose control (17). This was a significant milestone for the field as it was the first cell therapy approved for the treatment of T1D. However, this procedure requires extensive immunosuppression to prevent islet graft rejection. The heavy immunosuppressive regimen puts the patient at risk of infections, malignancies, worsening islet graft function, and organ damage (18–20). This highlights the urgent need for novel interventions to prevent autoimmune attack on β-cells and delay disease progression. In parallel, innovative strategies must be developed to support the long-term survival of transplanted islets in advanced-stage patients without systemic immunosuppression.
Figure 1

The immunomodulatory strategies as Type 1 diabetes progresses. (A) Type 1 diabetes progresses as the β-cells mass is lost. There are several stages of T1D: Stage 1 is characterized by two or more autoantibodies; Stage 2 includes two or more autoantibodies along with β-cell dysfunction: Stage 3 corresponds to clinical T1D; and Stage 4, which is long-standing T1D. (B) Approaches for the prevention of the onset and β-cell replacement as the disease progresses. (i) Antigen-specific strategies utilizing autoantigens have been investigated for individuals at stage 1 or at risk of T1D. These approaches present antigens in a non-inflammatory environment to shift the phenotype of autoreactive T cells by anergy or tolerizing them to become type 1 regulatory cells. These approaches rely on tolerogenic dendritic cells. Hence, nanoparticles that can shift dendritic cell phenotype to become tolerogenic are often utilized for antigen delivery. (ii) While for stage 2 & 3 T1D patients, immune blockade approaches targeting T cells, antigen-presenting cells, and proinflammatory cytokines could be promising to prevent autoimmune attack on β-cells. (iii) Additionally, cell-based therapies utilizing regulatory T cells and tolerogenic dendritic cells have the potential to inhibit the activity of autoreactive effector T cells in stages 2 & 3 of the disease. (iv-vi) For patients at the later stage of T1D, β-cell replacement utilizing engineered islets, encapsulation in biomaterials, and utilizing immunomodulatory biomaterials to enhance islet graft survival have been explored. (iv) Islets have been engineered to downregulate MHC class and overexpress CD47 to prevent their recognition and destruction by the immune system. (v) Alternatively, encapsulation of islets can mitigate immune clearance, which has been investigated in the clinic. (vi) Biomaterials incorporating chemically modified coating, immunomodulatory agents (including cytokines, chemokines, checkpoint ligands), and immunomodulatory drugs have been explored for long-term graft protection. NP, nanoparticle; tDC, tolerogenic dendritic cells; Tr1, type 1 regulatory T cell; Grzm B, granzyme B; Teff, effector T cell; Treg, regulatory T cell; MHC, major histocompatibility complex.
This review aims to provide a comprehensive overview of immunomodulatory approaches across all stages of T1D progression. First, we will discuss prevention strategies, such as antigen-specific, immune blockade, and cell-based therapies, which aim to protect endogenous β-cells. Then we will review the strategies for islet replacement, which are necessary at the later stages when majority of β-cells mass is destroyed, including utilizing engineered islets, islet encapsulation in macro or micro device and utilizing immunomodulatory biomaterials for long-term survival graft islets (Figure 1B). These approaches are inherently linked, as advances in immune modulation directly impact the success and durability of β-cell replacement therapies by preventing graft rejection and recurrent autoimmunity. Overall, we expect this review to provide an extensive summary of the latest advancements and remaining gaps in the field of immunomodulation for prevention and cure for T1D.
2 Immunological mechanism of T1D
Although the immune mechanisms leading to β-cell autoimmunity are heterogeneous, T1D is believed to be a T-cell-mediated autoimmune disease. The defects in both central and peripheral tolerance lead to the generation of autoreactive T cells (12, 21). In central tolerance, naïve T cells travel from the bone marrow to the thymus, undergoing positive and negative selection to develop into CD8 or CD4 cells. T cells acquire their T-cell receptor (TCR) after the positive selection stage, where they demonstrate that they recognize MHC molecules. This is followed by negative selection, where a broad array of peripheral tissue self-antigens is displayed by medullary thymic epithelial cells (mTECs) to deplete self-reactive T cells from the pool, which demonstrate high-affinity TCR binding to MHC presenting self-antigen (22). In the case of T1D, various parameters can influence the efficiency of negative selection. For instance, low expression of insulin due to the polymorphism at the INS gene promoter results in defective negative selection of insulin-reactive T cells. At the same time, expression of the PTPN22 gene leads to diminished TCR signaling and consequent reduction of apoptosis of autoreactive T cells (23, 24).
Once autoreactive T cells reach the periphery, dendritic cells in the pancreatic draining lymph nodes serve as antigen-presenting cells (APCs) and present fragments of islet molecules from damaged β-cells to these T cells. Additionally, these dendritic cells secrete cytokines interleukin (IL)-12 and -15, enhancing the expression of costimulatory molecules and activating the autoreactive T cells (11, 25). Activated autoreactive T cells then differentiate into effector T cells and migrate to the islets through the circulation. A small population of autoreactive T cells exists in the pancreatic lymph node as stem-like T cells continuously sustaining anti-islet immunity. This is important because effector T cells have a short lifespan and do not persist once they reach the islet niche (12, 25, 26). Upon reaching the islets, the autoreactive effector T cells initiate insulitis, an infiltration of immune cells into the pancreatic islets, marking immune-mediated β-cells for destruction. During this process, autoreactive effector CD8 cells destroy β-cells by secreting IFNγ and granzyme B (Figure 2), while CD4 cells contribute by secreting proinflammatory cytokines (21, 26–30). Although immune cells play an essential role in the destruction of β-cells, recent findings also suggest that β-cells themselves are prone to self-destruction due to their susceptibility to stress and limited self-defense (21). This suggests that escaped autoreactive T cells, in tandem with susceptible β-cells, may lead to autoimmunity in T1D.
Figure 2
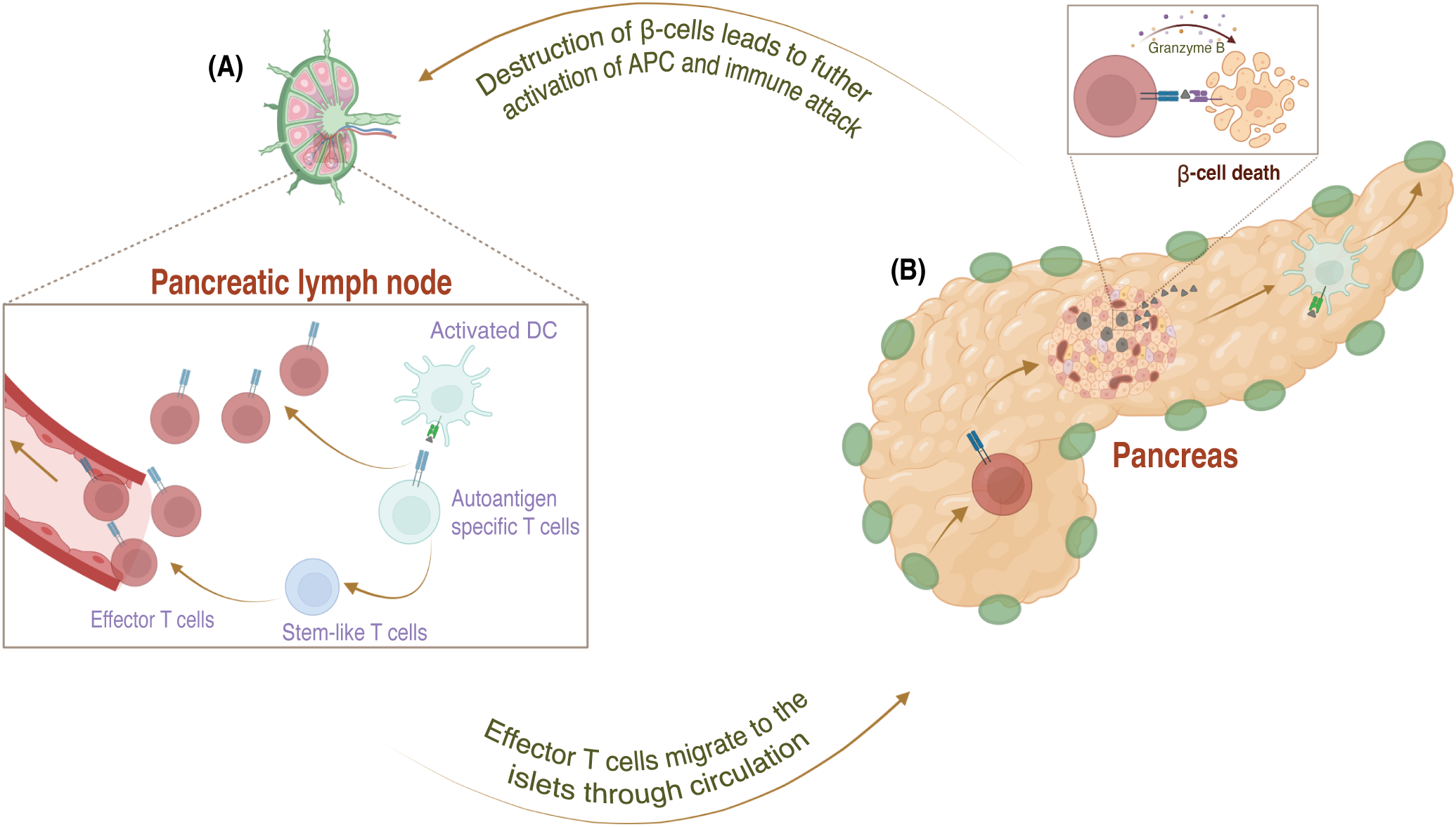
The mechanism of pathogenesis in Type 1 diabetes in the periphery. (A) In the pancreatic lymph node, APCs activate these autoreactive T cells, which escape negative selection, by presenting antigens from damaged β-cells and providing a co-stimulatory signal via secretion of proinflammatory cytokines. Activated autoreactive T cells differentiate into effector cells and migrate to the pancreatic islets through the bloodstream. (B) In the pancreas, effector T cells attack the β-cells by secreting interferon (IFN)-γ and granzyme (B) The initial destruction of β-cells leads to a vicious cycle that fuels the immune attack and speeds up the destruction of even more β-cells.
3 Prevention approaches for T1D
Recent advancements in early genetic and antibody-based screening, coupled with an enhanced understanding of the pathogenesis of disease, have significantly accelerated the progression in T1D prevention efforts (31, 32). Consequently, the ability to identify at-risk patients allowed for the development and evaluation of stage-specific prevention strategies. For instance, antigen-specific strategies have been explored for patients at the early stage of T1D to alter autoreactive T-cell response. This approach relies on utilizing antigens presented in a tolerogenic context to silence autoreactive T cells (33). For individuals at stage 2 and stage 3, approaches such as immune blockade and cell-based immunotherapies have been pursued. The immune blockade approaches encompass monoclonal antibodies that target T cells, antigen-presenting cells, or proinflammatory cytokines, thereby preventing these immune components from driving disease progression. Meanwhile, cell-based therapies involve engineering immune cells, (e.g., Tregs, dendritic cells) immunomodulatory stromal cells (e.g., MSCs) or platelets to enhance their tolerogenic function to prevent autoimmune attack (Figure 1B). Together, these approaches reflect a shift toward precision prevention in T1D, emphasizing the importance of tailoring immunotherapeutic interventions to the underlying immunological landscape and disease stage. However, for individuals in stage 4, the research focus shifts towards islet replacement strategies. A major barrier in this field has been the reliance on systemic immunosuppression, which carries significant risks. To address this, next-generation therapies have been developed to achieve localized immunomodulation.
3.1 Antigen-specific
As preclinical models have allowed a greater understanding of T1D disease mechanisms, antigen-specific strategies have become a point of interest. By presenting the autoantigens in a tolerogenic environment through controlled delivery of the antigens themselves or manipulation of immune cells, antigen-specific aims to combat autoimmune responses. Many antigen-specific strategies are discussed in this review, from antigen-specific regulatory T cells to tolerogenic dendritic cells pulsed with personalized peptides. This section focuses on autoantigens, the most notable being insulin, which has seen much exploration in clinical trials (34).
Autoantigens, or self-antigens, are produced within the body and are recognized as threats to the immune systems of patients with autoimmune diseases. The most prominent autoantigens used to test for T1D, which are now targeted in clinical trials for T1D prevention, are insulin, proinsulin, and glutamic acid decarboxylase (GAD65) (Table 1) (35). In recent years, efforts have mainly been centered on assessing whether insulin administration can affect immune tolerance in high-risk, younger patients as a preventative measure for T1D development. Studies in NOD mice revealed that insulin can achieve tolerance by elevating anti-inflammatory cytokine levels and Tregs (77). NOD mice, widely regarded as the gold standard preclinical model for Type 1 Diabetes, exhibit disease pathogenesis that closely mirrors human T1D, making them invaluable for studying autoimmunity and evaluating therapeutic strategies (78, 79). In 2015, Bonafacio et al. demonstrated that high oral insulin doses in children were safe, causing no hypoglycemia, and induced a protective immune profile characterized by an elevated ratio of regulatory T cells to IFN- γ-expressing cells (80). Since then, studies have found that oral insulin is safe in both children and adults, with the most recent clinical study finding oral insulin to reduce blood glucose levels in adults (36, 41). However, the efficacy of oral insulin as a standalone treatment requires further exploration, as it has shown mixed results across clinical trials. For instance, in the trial involving infants at risk of T1D, oral insulin was ineffective in preventing the development of islet autoantibodies (37). While in another trial, oral insulin was shown to be effective only in a subset of patients with high levels of IA-2 autoantibodies (38). Currently, another clinical trial is investigating a different insulin administration route (intramuscular), utilizing insulin B chain with MAS-1 adjuvant (39).
Table 1
| Target | Treatment name(s) | Progress | Limitations | Status | References, Clinicaltrials.gov identifier |
|---|---|---|---|---|---|
| Insulin | Oral insulin | Safety objective reached | Treatment not associated with immune response | Phase 2 completed 2017 Phase 2 completed 2024 |
(35–38), NCT02547519 NCT00419562 NCT03364868 |
| Insulin B chain | MER3101 | Results not posted | Results not posted | Phase 1 in progress | (39), NCT03624062 |
| Proinsulin | Gold nanoparticles conjugated with proinsulin peptide | Safe and well tolerated | Small number of participants | Phase 1 completed 2019 | (40), NCT02837094 |
| GAD65 | GAD-alum with Vitamin D/D3 supplementation | Preserves C-peptide levels; | Efficacious in genetically selected group | Phase 2 trials completed 2021 | (41), NCT03345004 |
| CD3 on T cells | Teplizumab (anti-CD3 monoclonal antibody) | Delayed T1D progression in stage 2 patients | Most patients experience adverse effects | Teplizumab FDA approved (2022) | (42–44), NCT03875729, NCT01030861 |
| CD2 on T cells; inhibits T cell stimulation with CD58 | Alefacept (LFA-3-Ig) | Insulin secretion preserved long-term in 30% of patients | β-cell function not preserved | Phase 2 trial terminated | (45, 46), NCT00965458 |
| T-cell surface markers | Anti-thymocyte globulin (ATG) | Preserves β-cell function; CD4 exhaustion | Prevented T1D in only 50% of high-risk individuals | Phase 2 completed 2018 | (47–52), NCT02215200 |
| CD2 on T cells and NK cells | Siplizumab (monoclonal antibody) | Results not posted | Results not posted | Phase 2 terminated | (53, 54), NCT05574335, NCT06025110 |
| B7; inhibit co-stimulation of T cells through interaction of B7 with CD28 | Abatacept (CTLA4-Ig) | Preserved insulin secretion and reduced follicular helper T cell population | Did not delay T1D progression, adverse effects | Phase 2 completed 2022 | (55–59), NCT00505375, NCT01773707 |
| CD40 on antigen presenting cells | Frexalimab (anti-CD40L) | Results not posted | Results not posted | Phase 2 trial recruiting | (60) NCT06111586 |
| CD20 on B cells | Autologous Tregs + Rituximab (anti-CD20 monoclonal antibody) | Delayed C-peptide level reduction | Adverse effects, did not delay T1D progression | Phase 1/2 trial completed 2022 | (61, 62), NCT00279305, NCT06688331 |
| TNF- α | Golimumab (anti-TNF-α monoclonal antibody) | Long-term benefits, lower reduction in C-peptide | Small number of eligible patients | Phase 2 trials completed 2021 | (63, 64), NCT02846545, NCT02846545 |
| TNF- α | Etanercept (TNF-α inhibitor), GAD-alum, Vitamin D | Feasible and well-tolerated | No effect on β-cell function | Phase 2 completed 2019 | (65), NCT02464033 |
| IL-6 | Tocilizumab (IL-6 receptor inhibitor) | reduced T cell IL-6R signaling | Did not slow β-cell loss of function | Phase 2 completed 2020 | (66), NCT02293837 |
| IL-8 | Ladarixin (IL-8 receptor inhibitor) | Some differences in HbA1C levels | No effect on β-cell function, no change in C-peptide levels | Phase 2 completed 2019 | (67), NCT02814838 |
| GLP-1R | Liraglutide + Tregs | Results not posted | Results not posted | Results not posted | (68), NCT03011021 |
| IL-21, GLP-1R | NN-8828 (anti-IL-21), liraglutide (GLP-1 RA) | Changes in C-peptide and HbA1C levels | Therapeutic effects did not last after treatment stopped | Phase 2 completed 2019 | (69), NCT02443155 |
| IL-12 and IL-23 | Ustekinumab (a monoclonal antibody targeting the p40 subunit) | Well tolerated; higher C-peptide levels | Delayed efficacy | Phase 2 completed 2021; Phase 2/3 in progress | (70) ISRCTN 14274380, NCT03941132 |
| Tregs | Autologous polyclonal Tregs + low dose IL-2 | Expands exogenously administered Tregs | Off-target effects | Phase 1 completed 2021 | (71), NCT02772679 |
| Tregs | Polyclonal expanded Tregs | Safe | Did not preserve C-peptide levels | Phase 2 completed 2020 | (72), NCT02691247 |
| CD4 T cells | PIpepTolDC (Tolerogenic DCs pulsed with islet antigen) | Long-term reduction in T-cell autoreactivity | Assessment pending on if β-cell function is preserved | Phase 1 in progress | (73, 74), NCT04590872 |
| CD8 T cells | AVT001 (Autologous Dendritic Cells) | Less decline from normal C-peptide levels | No significant change in HbA1c and insulin dose | Phase 1/2 completed in 2023 | (75), NCT03895996 |
| Inflammatory immune response | Mesenchymal Stem Cells | Increased Treg population, more anti-inflammatory cytokines, improved HbA1C levels | Limited patients meet eligibility criteria | Status unknown, but likely that Phase 1/2 trial completed in 2020 | (76), NCT04078308 |
Current therapies in clinical trials for the prevention of the onset of diabetes.
Another molecule of interest is proinsulin, the insulin precursor. Proinsulin has been proven effective in preserving β-cell function, but its positive immunomodulatory effects are not uniform across patients (35, 81, 82). Recently, Tatovic et al. tested the safety of gold nanoparticles conjugated with proinsulin peptide. Using microneedles as a delivery system, this method was safe in patients, which may provide a possibility to further optimize proinsulin in clinical trials, using the anti-inflammatory properties of gold (40). On the preclinical front, proinsulin has been combined with other immunomodulatory therapies like IL-10 or anti-CD3 to enhance efficacy in vivo. These efforts aim to address the challenge with current treatment efficacy being dependent on disease duration at the time of administration and continuous oral administration of proinsulin (83).
GAD65 is another autoantigen critical in T1D disease progression and a potential marker of impaired β-cell function (84). GAD combined with aluminum hydroxide, GAD-alum, has been tested in clinical trials. Salami et al. used GAD-alum in healthy children at risk for diabetes, finding that the treatment was associated with lower levels of cytotoxic and autoreactive T cells long-term. However, the trial was terminated early, leading to a small cohort size (85). Ludvigsson et al. combined intra-lymphatic administration of GAD-alum with vitamin D, an immunomodulatory agent, preserving C-peptide levels, a biomarker for insulin production, in patients of a specific haplotype (86). Another well-established autoantigen involved in T1D pathogenesis is insulinoma-associated protein 2 (IA-2). Its presence has been implicated as a good predictor of T1D, but not much work has been done to assess IA-2’s utility for T1D treatment (87). Oral delivery of the intracellular domain of IA-2 using bacterium-like particles in NOD mice prevented T1D onset (88). Zn-T8 has been explored as a diagnostic tool for T1D, but not as a treatment (89, 90).
More recently discovered autoantigens are islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) and chromogranin A (ChGA). IGRP-specific T cells are upregulated in T1D patients (91). Similarly, changes in ChGA concentration can indicate T1D disease progression (92, 93). In NOD mice, a liposome targeting IGRP delayed T1D onset by lessening the activity of autoreactive T cells (94). ChGA-targeted therapies have not been tested in clinical trials, but ChGA deficiency has been shown to reduce T cell autoreactivity and protect mice from T1D (95). In NOD mice, Jamison et al. tested nanoparticles containing an insulin-ChgA hybrid peptide, which prevented T1D by impairing T cells’ ability to produce proinflammatory cytokines; an increase in Tregs and a decrease in effector T cells (Teffs) was observed. Recent work has identified other potential autoantigen targets involved in T1D that may become more prominent in the future, including toll-like receptor 4 (TLR4), Tetraspanin, prolyl-4-hydroxylase β subunit (P4Hb), insulin gene enhancer protein (ISL-1), and GRP78 (35, 83, 96–101).
These tolerance-inducing strategies, using autoantigens, seem the most highly tested therapeutic among those discussed in this review. However, challenges persist in this area. Namely, recent clinical trials have primarily served as safety checkpoints, with limited efficacy for patient outcomes. Doses, combinatorial regimes, and administration routes must be refined to improve effectiveness. Further, due to the vast array of immune patterns in T1D patients, it is plausible that certain autoantigens are effective in specific patient groups, rather than serving as a universal cure, presenting a screening barrier for widespread adoption (34). The delivery route remains critical, with ongoing trials investigating intravenous, intranasal, oral, and intralymphatic administration. Further research is needed to standardize delivery methods and optimize antigen-specific therapies for different patient subgroups (102). In this area, therapies targeting immune cells may provide an alternate route.
3.2 Immune blockade
Immune blockade strategies are among the most prominent efforts to prevent autoimmune-mediated β-cell destruction. These approaches target essential T-cell activation, co-stimulation, and inflammatory signaling pathways to preserve β-cell function. This section explores the mechanisms of action currently targeted under investigation in clinical trials and the challenges limiting the wide use of these therapies.
3.2.1 T-cell blockade
Several therapies targeting T cells have been investigated to prevent the progression of T1D (Table 1). The most notable one is teplizumab, an Fc receptor, non-binding humanized anti-CD3 monoclonal antibody, which received FDA approval in 2022. This was a significant milestone since it was a first drug approved for preventing the progression of T1D in patients at stage 2 of the disease (Figure 3A) (42). Treatment with teplizumab comprises a 14-day daily intravenous (IV) course, which has been shown to delay the progression of the disease in 43% of patients for 48.4 months in the phase 2 trial (43). However, due to the nature of the systemic administration of the treatment, multiple adverse events were observed in patients treated with teplizumab. An integrated analysis from five clinical trials revealed that most patients (99.5%) who received the teplizumab course experienced at least one adverse effect associated with the treatment (44). Although adverse events were generally transient, the response to teplizumab among stage 2 patients was heterogeneous (103).
Figure 3

Mechanism of action for promising immunotherapies in clinical trials targeting T cells, APCs, and cytokines for preventing Type 1 Diabetes. (A-D) T cell blockade therapies aim to suppress autoreactive T cells. (A) Anti-CD3 depletes the T cells by binding to CD3 on T cells and preventing their activation. (B) LFA3-Ig antibody prevents T cell activation by targeting CD2 and blocking its interaction with CD58, a critical co-stimulatory signal. (C) Anti-thymocyte globulin (ATG) broadly depletes T cells by binding to multiple surface markers, including CD2, CD3, and CD25, etc. (D) CTLA4-Ig inhibits T cell activation by binding to B7, preventing its interaction with the co-stimulatory receptor CD28. (E-F) APC blockade strategies focus on disrupting the activation of antigen-presenting cells. (E) Anti-CD40L blocks the CD40L-CD40 interaction, halting the activation of APCs and subsequent inflammatory signaling. (F) Anti-CD20 targets CD20 on B cells, inducing their apoptosis and reducing autoantibody production. (G-I) To counteract the pro-inflammatory environment of T1D, cytokine blockade therapies target key inflammatory pathways. (G) TNF-α inhibitors behave like TNF receptor 2 or directly block TNF-α, suppressing this pro-inflammatory cytokine’s effects. (H) IL-21 inhibitor neutralizes the pro-inflammatory signaling of this cytokine (I) while anti-12p40/IL23p40 targets the p40 subunit shared by IL-12 and IL-23.
Other therapies targeting the activation of T cells via costimulatory and co-inhibitory signals in new-onset T1D patients, such as LFA-3-Ig, anti-thymocyte globulin (ATG), CTLA4-Ig, have been investigated in clinical trials and showed transient protection of β-cells. For instance, a phase 2 trial in patients with recent-onset T1D treated with LFA-3-Ig fusion protein (alefacept), which binds to CD2 and inhibits T cell co-stimulation with CD58, demonstrated insulin production preserved in 30% of subjects for up to two years post-treatment. LFA-3-Ig was administered via intramuscular injections weekly for two 12-weeks, separated by a 12-week interval. Despite promising results, the trial was terminated due to the discontinuation of production by the drug manufacturer, and enrollment was reduced to 49 participants. (Figure 3B) (45, 104). The peripheral blood mononuclear cells (PBMCs) from trial participants revealed that alefacept allowed for the preservation of β-cells by exhausting CD8 T cells. However, the higher numbers or frequencies of islet antigen–reactive CD4+ T cells were associated with poor response to alefacept, suggesting that dosing may be inadequate to eliminate islet antigen-reactive CD4+ T cells in specific individuals (45, 46). While the clinical trials with LFA-3-Ig were terminated, CTLA4-Ig and anti-thymocyte globulin (ATG) are still under investigation (32).
ATG broadly depletes the T cells by recognizing their surface markers, such as TCR/CD3, CD4, CD8, CD25, CD45, HLA class I, and HLA-DR, and binding to them, which leads to T cell killing via complement activation, antibody-dependent cell-mediated cytotoxicity, or activation-induced cell death (Figure 3C) (47, 48). Although the FDA has approved ATG to prevent renal allograft rejection (49), its efficacy in delaying the progression of type 1 diabetes is still under investigation. A phase 2 trial by TrialNet demonstrated that a 2-day course of treatment with low-dose ATG-infused IV preserved β-cell function for one year in new-onset stage T1D patients (50). Responders to the treatment revealed CD4 exhaustion and temporarily elevated levels of IL-6, CXCL10, and TNF-α two weeks posttreatment (51). The most recent study with children at stage 2 of T1D treated with a 2-day course of a low dose of ATG demonstrated that three subjects (50%) remained diabetes free after 1.5, 3, and 4 years of follow-up, while the other three developed stage 3 within 1–2 months after therapy (52). Siplizumab, a humanized anti-CD2 monoclonal antibody, has also shown depletion of Teffs and promotion of Tregs in vitro (105). Siplizumab was investigated in clinical trials for patients with new-onset T1D; however, the trial was terminated early due to the company’s decision (53). Another dose-finding clinical trial with Siplizumab in patients recently diagnosed with T1D was terminated as well due to excessive lymphodepletion (54).
Humanized CTLA4-Ig fusion protein is a cytotoxic T lymphocyte-associated antigen four fused to modified human immunoglobulin G and is another widely studied treatment for preventing the onset of T1D (106). CTLA-4 binds to B7 molecules on antigen-presenting cells (APCs), thereby inhibiting T cell activation and proliferation by blocking the critical B7-CD28 costimulatory signaling pathway(Figure 3D) (107).CTLA4-Ig, abatacept, is already FDA-approved for treating prophylaxis of acute graft versus host disease (108) and rheumatoid arthritis (109); however, similar to ATG, its efficacy for delaying the onset of T1D is still being explored. A recent phase 2 placebo-controlled clinical trial on stage 1 T1D patients who received monthly abatacept IV infusion for 12 months showed that the treatment preserved insulin secretion and reduced the frequency of a subset of ICOS+ follicular helper T (Tfh) cells, which previously was reported to be an indication of successful clinical response in T1D patients (55, 56). Inducible co-stimulatory molecule (ICOS) plays a critical role in positively regulating T cell function, and its elevated expression is linked to an increase in circulating peripheral helper T cells (Tph) (57, 58). However, abatacept infusions did not delay the progression of the disease (56). Previously, abatacept has demonstrated promise in stage 3 T1D by delaying the loss of insulin production by 9.6 months when administered over two years. However, its systemic delivery has been associated with infusion-related adverse effects in 22% of patients, highlighting the need for safer and more targeted approaches for immunotherapy (59).
3.2.2 Antigen-presenting cell blockade
Antigen-presenting cells are also targeted to prevent the progression of T1D, as they participate in the development of the disease by priming autoreactive β-cell-pecific T cells and promoting the activation of autoantibody secretors (110–112). Additionally, APCs can lead to islet graft recognition and subsequent rejection (113–115). One of the currently explored APC blockade therapies is the anti-CD40 ligand (CD40L) antibody (Figure 3E). CD40L expressed on activated T cells binds to CD40, which is strongly expressed on APCs, such as dendritic cells and B cells. CD40 and CD40L interaction leads to inflammatory responses such as B cell activation and differentiation, production of antibodies and proinflammatory cytokines, and T cell clonal expansion (116–118). Although anti-CD40 therapies have also been extensively investigated, they are less efficacious than anti-CD40L targeting approaches in graft rejection prevention (119). For instance, anti-CD40 has been investigated in clinical trials by Novartis (120). Still, the clinical trial was discontinued at phase 2 because it was less efficacious compared to the established regimen for kidney transplant (121). While CD40L targeting therapies have shown promising results in clinical trials for the treatment of autoimmune diseases such as Sjögren’s disease (122), systemic lupus erythematosus (123), and multiple sclerosis (124), it still needs to be explored more in the context of T1D.
Recently, anti-CD40L antibody (frexalimab) has been under investigation in phase 2 clinical trials for preserving endogenous insulin production in newly onset T1D patients (60). Additionally, anti-CD40L has been explored for islet graft rejection mitigation. For instance, preclinical work by Anwar et al. demonstrated an engineered anti-CD40L antibody (tegoprubart) that reduces the risk of thromboembolic events for promoting islet survival in diabetic nonhuman primates (NHPs). Among two NHPs that received the islets and tegoprubart, one demonstrated long-term graft survival for 182 days and increased Treg frequency, while the other one rejected the graft after 2 weeks; however, it showed partial graft function after the second islet transplantation (118). All these promising studies with anti-CD40L lead to an ongoing clinical trial by Eledon in collaboration with the University of Chicago Transplant Institute for evaluating anti-CD40L to prevent rejection of transplanted islets (125).
Another commonly explored therapy for APC blockade is anti-CD20. CD20 is mainly expressed on the surface of B cells (Figure 3F) (126). Hence, anti-CD20 is called B-cell-depleting therapy (61). Previously, a phase 2 clinical trial with rituximab, an anti-CD20 monoclonal antibody, revealed that four infusions of rituximab over 1 month in recent-onset T1D patients delayed the reduction in C-peptide by 8.2 months (61). This encouraged another clinical trial in combination with autologous expanded Tregs to improve efficacy. However, although the combination with Tregs was better than the Treg alone group, the treatment did not significantly delay the T1D progression (62). Additionally, like in the previous study, there were significant adverse events, 93% in the first study (127) and 80% in the combination study (62), associated with the treatment.
3.2.3 Cytokine blockade
Cytokines play a complex role in T1D pathogenesis, and inflammatory cytokines have been associated with the pathogenesis of T1D as they can exacerbate the condition (128, 129). Several cytokines, such as IFN-γ, IL-1β, tumor necrosis factor (TNF)-α (130–132), IL-6 (133), IL-8 (134), IL-12 (135), IL-21 (136), IL-23 (137) have been implicated in β-cell destruction. Several anti-cytokine therapies have been under investigation in clinical trials to address the negative effects of these pro-inflammatory cytokines (Table 1). One such therapy is anti-TNF-α, a monoclonal antibody (golimumab) that demonstrated a 43% lower reduction in C-peptide levels compared to control after 52 weeks of biweekly subcutaneous injections in phase 2 clinical trials in new-onset T1D youth (63). A 2-year follow-up study showed similar trends (64). However, 23% of the golimumab group experienced adverse events associated with the treatment (63). Another TNF-α-blocking therapy is etanercept, which binds to TNFα by acting like its receptor (Figure 3G) (138). The pilot trial, which used a combination of etanercept, GAD-alum, and vitamin D, showed that the treatment was well tolerated but failed to delay the progression of T1D in patients with recent onset (65). IL-6 receptor inhibitor (tocilizumab) is another recently investigated cytokine blockade therapy for new-onset T1D patients. A phase 2 trial with tocilizumab administered IV monthly for 7 months did not delay the loss of β-cells function or change frequencies of CD4 or Treg subsets (66). Another phase 2 clinical trial evaluating the IL-8 receptor inhibitor (ladarixin) administered twice daily in three cycles of two weeks on and two weeks off, in newly diagnosed T1D patients, failed to demonstrate efficacy in delaying disease progression (67). Both studies showed no adverse events associated with the treatment.
Another phase 2 trial evaluated the efficacy of the combination of anti-IL-21 antibody (Figure 3I) (NN-8828) and glucagon-like peptide-1 receptor agonist (GLP-1 RA), liraglutide, in adults with recent onset of T1D. Liraglutide was utilized as a GLP-1 RA, which has been shown to be effective in improving glycemic control in T1D patients (139). Anti-IL-21 was administered intravenously every six weeks, while liraglutide was given via subcutaneous injections once daily for 54 weeks. This combination resulted in 48% higher C-peptide levels and a 34% reduction in hypoglycemia in the treatment group after 54 weeks; however, these effects did not last after treatment was discontinued. Additionally, no changes in immune cells were observed (69). A liraglutide and ex vivo expanded Treg combination therapy is currently being assessed in a Phase 1/2 clinical trial. Ustekinumab, a monoclonal antibody targeting the p40 subunit shared by IL-12 and IL-23, was recently investigated in adolescents with recent-onset T1D (Figure 3H). Subcutaneous injections of ustekinumab at weeks 0, 4, and then every 8 weeks until week 44 resulted in 49% higher C-peptide levels than controls at 52 weeks and were well tolerated in patients. While these results are promising, the delayed efficacy of ustekinumab and anti-IL21 compared to less specific T-cell blockade therapies raises concerns about their ability to delay disease progression effectively before the complete loss of β-cell function (70).
Overall, immune blockade strategies have been shown to be efficacious in delaying diabetes onset in some patients with T1D. Furthermore, FDA approval of teplizumab furthered interest in targeting immune players involved in autoimmune attack against β-cells. Building on this momentum, other T cell and APC blockade monoclonal antibodies, initially developed for promoting graft tolerance, are now being repurposed to be investigated for T1D prevention. However, several limitations are associated with these approaches, such as systemic side effects, limited longevity of tolerance, and increased risk of immune-related complications. Although blockades of pro-inflammatory cytokines are currently being explored as an alternative, more targeted approach, the results from clinical trials show that inhibiting these proinflammatory signals may not be enough for robust and sustained control of autoimmunity.
Importantly, most clinical trials evaluate these therapeutics for patients at stage 3 of the disease, potentially missing the opportunity window for preserving more β-cell function. This highlights the need for more advanced and precise screening to identify at-risk individuals earlier and evaluate these interventions for earlier stages of disease. Looking ahead, utilizing regenerative approaches in combination with developing more targeted delivery methods may allow for safer, more durable, and more effective prevention of diabetes progression.
3.3 Cell-based therapies
Cell-based immune therapies have also emerged as a promising treatment for T1D. Therapies in this area seek to target cells involved in T1D pathogenesis and use them to induce tolerance in host immune systems. Tregs and tolerogenic dendritic cells (tDCs) have garnered particular attention in this area. Recent clinical studies have proven the safety of autologous Tregs in human patients (Table 1). However, a strong and desirable immune response was not generated through this method alone (71, 140). Combinatorial regimens and engineered Tregs are being tested in preclinical models to improve efficacy and tolerance. Similarly, tDCs exhibit significant immunoregulatory functions, promoting Treg induction and inhibiting proinflammatory responses. Clinical trials have proven the safety of autologous tDCs, with a current phase 1 clinical trial for T1D assessing a vaccine made of host dendritic cells and a proinsulin protein. Current efforts similarly focus on improving tDC efficacy through genetic engineering or combinatorial regimens in preclinical and clinical trials. Outside of tDCs and Tregs, other cell types such as platelets and MSCs have also been explored for localized immunomodulation. This section reviews recent efforts in cell-based therapies, including insights from clinical trials, engineering efforts, and combinatorial regimens, while addressing remaining challenges in translating these approaches to the clinic.
3.3.1 Regulatory T cell therapy
T cells play a significant role in the inflammation and progression of T1D as cytotoxic T cells destroy pancreatic β-cells. An imbalance between effector T cells and regulatory T cells has also been observed in early T1D development before disease onset (141). Tregs can suppress autoreactive T cells, a critical T1D onset and progression component. For this reason, a depleted Treg population has been implicated in T1D pathogenesis (142, 143). Due to the anti-inflammatory nature of Tregs and their role in autoimmunity, they have been of interest for immunomodulatory therapies (144, 145). While Tregs are also explored for prolonging islet grafts, this section will mostly discuss their application in T1D prevention, where their therapeutic application has been more extensively pursued. Despite their therapeutic promise, Tregs can be hard to isolate from blood and other tissues, making it difficult for T-reg-based therapies to be viably used. Therefore, many therapies focus on engineering effector T cells into Tregs ex vivo to prevent the onset of T1D. Although Tregs are also explored for prolonging islet transplantation survival in clinical trials, this section will mostly discuss prevention effects as more efforts are done in this front.
Clinical trials have primarily been conducted with autologous polyclonal Tregs, which have been well tolerated in patients under a standard immunosuppressive regimen, supporting the safety of Treg therapies (140). Combining Treg therapies with low-dose IL-2 has also been tested in clinical trials. Low-dose IL-2 has been shown to induce an anti-inflammatory gene expression profile in T1D, selectively expand Tregs, and reduce the frequency of IL-21+ T cells (146). In a phase 1 trial by Bluestone et. al, T1D patients were treated with polyclonal Tregs and low-dose IL-2. The IL-2 increased endogenous Tregs within patients’ peripheral blood but also had an off-target effect and expanded cytotoxic cells. Although the Treg infusion was deemed safe, these authors stressed the importance of developing Treg therapies that are more specific to the patient Treg population and avoid off-target effects (71). This previous work can better inform future clinical trials of engineered Tregs and combinatorial regimens. In NOD mouse models, Tregs have also been used with other immunosuppressive agents such as cytokines, antibodies, and standard immunosuppressants to improve engraftment of donor Tregs (147, 148). Further, preclinical work in NOD mice has developed Treg engineering through chimeric antigen receptor technology and genetic engineering.
Chimeric antigen receptor (CAR) technology is a relatively new method to create Tregs for autoimmune diseases like T1D. Engineered Tregs using CARs allow for the redirection of Treg specificity towards specific targets, which is of special interest as antigen-specific Tregs have been more effective in preventing autoimmune disease onset than polyclonal Tregs (144, 149, 150). Using CAR Tregs to suppress the autoreactivity in T1D can help improve the efficacy of Treg-based therapies (151). Tenspolde et. al, for example, used CARs to change T cell specificity towards insulin and FOXP3 transduction to redirect Teffs to Tregs. Although the generated Tregs did not prevent diabetes progression in NOD mice, they were still detectable after 4 months and mimicked the activity of natural Tregs. Their method was the first recorded instance in literature of using CAR technology to convert Teffs to Tregs for T1D (152). The MHC class II peptide complex has also been a target for CAR Treg technology. This is mainly due to previous work proving that Tregs can regulate immunosuppression by depleting MHC class II peptides (153, 154). Spanier et. al, for example, created the first T cell receptor-like CAR specific for an MHC class II peptide using a monoclonal antibody. In a NOD mouse model, T1D was suppressed entirely when treated with a 3:1 Treg/BDC2.5 T cell ratio (149). Zhang et al. showed that a single infusion of CAR T cells, MHC class II showed antigen-specific Tregs induced through this method, exert a greater protective effect than nonspecific polyclonal Tregs (155, 156). CAR T-regs have been used in clinical trials with some success, primarily as a therapy for solid tumors. While the targets for T1D CAR therapies are different and these previous trials utilize effector T cells rather than regulatory T cells, these examples show that CAR T cell therapy has been proven safe in humans and can likely be adapted for T1D treatment (157, 158).
Engineering gene expression is another method to engineer Treg specificity and T-reg mediated immunoregulation. In this area, many efforts focus on regulating Forkhead box protein 3 (FOXP3) expression, which is essential for Treg suppressive activity (159–161). Most recent studies in this area have used homology-directed repair (HDR) based gene editing to alter the expression of FOXP3. Gene editing through this method has been used to create stable and high expression of FOXP3 in primary human CD4+ T cells, which displayed T-reg-like properties long-term and suppressed polyclonal islet-specific T cells from individuals with T1D and prevented diabetes onset in a T1D mouse model (162, 163). Uenishi et al. used similarly engineered Tregs enriched and expanded with rapamycin to prevent T1D in NOD mice, showing that engineered Tregs can be combined with standard immunosuppressants and enhance T1D outcomes (164). Outside of FOXP3 expression regulation, Rafiqi et al. inhibited the eukaryotic initiation factor 5a (eIF5a), which is overexpressed in T1D patients and promotes a proinflammatory environment, as well as a key pathway in T cell maturation. A Treg phenotype was induced when this method was used on isolated human effector T cells. While these Tregs remain untested in vivo, the group has previously shown that this inhibition is safe in humanized mouse models and on islets in vitro (165, 166).
Clinical trials with Tregs have primarily served to verify the safety of autologous polyclonal Tregs in T1D patients. The focus of preclinical models has been engineering Tregs to enhance their specificity or manufacturing Tregs from Teffs cells. Current clinical trials have allowed for some testing of how autologous polyclonal Tregs are tolerated in patients, potential side effects, doses, and effective administration routes may vary for engineered Tregs. More broadly, concerns also include storage and complex manufacturing processes. In addition, Treg exhaustion can be a further point of concern that limits the efficacy of this therapy (167).
3.3.2 Dendritic cell therapy
Dendritic cells (DCs) are another portion of the immune system being assessed to help form a method of regulating T1D. While there are DCs that tend to be more pro-inflammatory and have been implicated in the pathogenesis of T1D, there are other DCs more associated with immunosuppressive capacities, referred to as tolerogenic dendritic cells (tDCs) (168, 169). Tolerogenic dendritic cells are an immature to semi-mature form of dendritic cells and are functionally distinct from fully mature, pro-inflammatory DCs. Compared to proinflammatory DCs, they exhibit low expression of MHC class II and co-stimulatory molecules such as CD80, CD86, and CD40. Additionally, they are characterized by high expression of IL10 and TGF-β, as well as expression of PD-L1 and PD-L2 (170, 171).They can trigger immunosuppressive activity by influencing T cell populations, increasing Treg activity, and suppressing effector T cells (172–174). Further, tDCs are essential in regulating immune activity, and T1D onset has been associated with fewer tDCs in the pancreatic area (168, 175, 176). Therefore, therapies involving engineered tDCs present a promising avenue to T1D therapies.
Autologous DCs’ safety has previously been evaluated in humans, and current efforts in clinical trials focus on improving the therapy’s efficacy (177). The AVT001 autologous DC therapy targets the Q/E CD8+ Treg pathway, which is defective in many people with type 1 diabetes. This treatment was recently completed in a phase 1/2 clinical trial intended to evaluate the safety of the treatment in patients. Treatment was associated with less decline from normal C-peptide levels, but other indicators like HbA1c and insulin did not demonstrate significant change from baseline (75). However, these results indicate preservation of insulin secretion and show that DC therapies can be viable as T1D treatments. Another clinical trial, in phase 1, utilized vaccines with tDCs pulsed with a proinsulin peptide (73). After verifying this method’s safety, it could selectively stimulate Tregs and reduce effector T cell populations in vivo. This reduction was preserved even two years after treatment, and improved glycemic control was observed in eight of nine patients. These promising results suggest that this method can alter the immune system towards autoantigen-specific tolerance (74).
Preclinical work in NOD mice aims to further enhance the capabilities of tDCs to prevent the onset of T1D. For example, Passeri et al. created tDCs by genetically engineering monocytes with lentiviral vectors co-encoding for immunodominant antigen-derived peptides and IL-10. In two T1D mouse models, the engineered DCs completely prevented disease development in 38% and 45% of recipients by inducing Tregs and downregulating Teffs (178). Further, human dendritic cells engineered with this method were able to inhibit T effector cell responses and promote Treg populations in celiac disease patients in vitro (178). These results support the versatility of this method and its potential viability in clinical trials.
Current work in clinical trials has shown the safety and impact of autologous tDCs on the immune system of T1D patients. However, none of these trials exhibit high efficacy for T1D treatment. Current preclinical trials focus on engineering tDCs to improve effectiveness, with some promising results. However, barriers remain for adoption in clinical trials regarding doses, administration routes, and potential immunogenicity of engineered tDCs in humans.
3.3.3 MSCs therapies
Other immune cells have also been explored to prevent the onset of T1D. MSCs, for example, have been a cell chassis of interest due to their immunomodulatory properties. MSCs have also been investigated in clinical trials as a treatment modality for T1D. Carlsson et. al’s 2014 study first verified the safety of MSCs in T1D patients, and more recent work has been focused on evaluating long-term safety and benefits of this method (179). In a phase 1/2 clinical trial by Izadi et. al, each patient received two doses of bone marrow-derived MSCs and was followed up with for one-year post-transplantation. MSC transplantation was safe long-term and shifted serum cytokine populations from proinflammatory to anti-inflammatory, emphasizing MSCs’ immunomodulatory effects (76). To provide more mechanistic analysis, Wu et al.’s preclinical study found that umbilical cord-derived MSCs helped improve C-peptide levels by inhibiting T cell proliferation (180). MSCs induce immune tolerance by suppressing autoimmune attack from effector T cells and shifting the immune environment to a more anti-inflammatory phenotype (e.g., through secretion of cytokines). Other studies have also noted MSCs’ ability to promote the generation and expansion of regulatory T cells and M2-like macrophages (181). MSCs have also been explored for islet transplantation. For instance, a study by Wang et al. demonstrated the use of engineered MSCs to express PD-L1 and CTLA4-Ig and co-transplanted them with islets in a T1D mouse model. Without systemic immunosuppression, the allograft survived for up to 100 days and had reduced infiltration by T-effector cells with a higher Treg presence (182). However, despite preliminary promising results, there is still a need for more effective and complete immunomodulation, which is the subject of exploration in many preclinical models. As with other therapies, patient-specific characteristics like lifestyle or autoantigens can further affect treatment efficacy, presenting an additional consideration when creating MSC-based therapies (183). Another consideration is poor MSC survival during transplantation due to host immune system attack, requiring long-term immunosuppression, and oxidative stress, among other factors (184).
3.3.4 Platelet cell therapies
Another cell population of interest is platelets. Zhang et al. engineered platelets to express programmed death ligand 1 (PD-L1), a commonly explored immunomodulatory checkpoint ligand, achieving long-term reversal of new diabetes onset in 58% of 10-week-old NOD mice treated with ten IV injections of the modified platelets. Notably, these engineered platelets released platelet microparticles (PMPs), which exhibited enhanced infiltration into the pancreas due to their smaller size. Additionally, mice treated with the engineered platelets showed reduced pancreas-infiltrating CD8+ T cells and increased T regs within the pancreas (185). More recently, Zhang et al. bioengineered platelets to produce higher-than-normal amounts of immune checkpoint ligands, including PD-L1, PD-L2, B and T lymphocyte attenuator (BTLA), and Galectin-9 (Gal-9). With this technique, the amount and activity of pancreas-infiltrating T cells were reduced with an increase in Tregs and macrophage polarization towards the M2-like phenotype (186). Despite these results, the therapeutic potential of platelets is limited by their short lifespan, requiring frequent administration. Additionally, platelets may face targeting specificity and stability challenges, which could reduce their efficacy and increase off-target effects (187).
Many cells are involved in T1D and can be harnessed for cell-based therapies. We have discussed MSCs and platelets, including their work in clinical and preclinical models and their drawbacks. While MSCs have immunomodulatory capabilities, poor cell survival presents a drawback. For platelets, their short lifespan limits clinical applicability. Similar disadvantages must be considered with these and other cell types potentially employed in this space.
4 Cell replacement therapies
Despite significant advances in insulin therapy and glucose monitoring technologies, Type 1 diabetes (T1D) remains a lifelong disease characterized by progressive β-cell loss, glycemic instability, and a substantial risk of acute and chronic complications. Even with intensive insulin management, many patients experience hypoglycemia unawareness, glycemic variability, and reduced quality of life, highlighting the fundamental limitation of exogenous insulin in replicating the dynamic, glucose-responsive function of endogenous β-cells. As a result, restoring functional β-cell mass remains the only strategy with the potential to provide durable physiological glycemic control. However, current β-cell replacement approaches are restricted to a small subset of patients with severe disease, require lifelong immunosuppression, and face significant barriers related to graft survival, immune rejection, donor availability, and long-term durability. These limitations underscore the urgent need to improve β-cell replacement therapies beyond existing clinical protocols (188–190).
Allogeneic islet transplantation replenishes the lack of functional β-cells in patients with T1D. The Edmonton protocol, which is the main procedure for transplanting donor human islets by infusion into the hepatic portal vein of the recipient, played a revolutionary role in the islet transplantation field since the early 2000s. Long-term follow-ups in patients receiving human islets have shown promising results in terms of better insulin independence for up to 20 years after transplantation (191–194). As a result, the U.S. Food and Drug Administration approved Lantidra by CellTrans, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for treating type 1 diabetes. Lantidra is approved for the treatment of adults with type 1 diabetes who are suffering from severe hypoglycemia. The procedure involves administering 5000 islet equivalents/kg body weight via transhepatic access into the hepatic portal vein. 63% of the patients met the efficacy endpoint determined by HbA1c levels ≤6.5% and absence of severe hypoglycemic events (SHEs) through 1 year after last transplant (195). However, some critical challenges still need to be solved, such as a regulatory framework, like manufacturing consistency, or long-term efficacy. Additionally, transplanted islets are susceptible to instances of blood-mediated inflammatory reactions and rejection, which could lead to their early destruction. Graft rejection can occur through direct recognition, where host T cells identify donor antigens, or indirect recognition, where host antigen-presenting cells (APCs) present donor islet antigens to host T cells alongside costimulatory signals. Alloantigen-specific T cells are activated in both scenarios, destroying donor islets by CD8+ T cells (196–198).
Another point of consideration is that selecting an appropriate transplantation site for donor islets can significantly affect the efficacy of the transplanted cells. The most common transplantation site is the intrahepatic vein due to its accessibility and favorable blood supply. It allows fast diffusion of oxygen and nutrients to the cells and insulin into the blood system. However, this site also poses several challenges, such as instant blood-mediated inflammatory reaction (IBMIR), which leads to immediate loss of a significant portion of transplanted islets, reducing the overall efficacy of the procedure (199). IBMIR is caused by direct exposure of islets to the bloodstream, triggering pro-inflammatory cytokines to be released, which is followed by the activation and recruitment of innate immune cells, enhancing the inflammation and destruction of islets (200). Additionally, transplantation into the intrahepatic vein requires immunosuppressants as the liver’s immune environment can contribute to alloimmune and autoimmune responses against the transplanted islets. Another implantation site is the subcutaneous space, which is also easily accessible but has a relatively poor blood supply, which can impair islet survival and function (201). Finally, transplanting islets into the mesenteric fat offers a better vascularized environment than subcutaneous tissue. Nonetheless, this approach is still under investigation, and long-term outcomes remain uncertain (201).
In contrast, transplantation under the kidney capsule is widely used as a preclinical benchmark site, as it provides a confined and well-vascularized environment that supports robust islet engraftment and facilitates mechanistic studies of revascularization and immune responses. Nevertheless, the kidney subcapsular space has important limitations for Type 1 diabetes therapy, including poor clinical translatability, limited volume, a lack of physiological insulin drainage into the portal circulation, and continued susceptibility to autoimmune and alloimmune attacks in the absence of immunomodulation (202).
Cell therapies have been developed to overcome some of the limitations of using donor islets. One such example is Vertex’s ongoing phase 3 clinical trial using stem cell-derived islets to achieve glycemic correction in patients with T1D (Table 2). This trial investigates zimislecel (formerly known as VX-880), which involves the delivery of the insulin-producing cells via infusion into the hepatic (liver) portal vein. This approach demonstrated insulin independence in 10 out of 12 participants for 1 year (210). However, this platform still requires ongoing immunosuppression, using tacrolimus and mycophenolate mofetil (MMF) to suppress T-cell activation and inhibit lymphocyte proliferation, respectively, and therefore to ensure the immune cells do not attack transplanted cells. The need for immunosuppression drugs for life comes with several risks, such as developing kidney diseases (218, 219), infections (220), cancer (221), or β-cells toxicity-related issues (19). These issues highlight the need to develop novel approaches that can improve islet transplantation.
Table 2
| Therapy type | Target/ additional information | Treatment name(s) | Progress | Limitations | Current status | Reference, clinicaltrials.gov identifier |
|---|---|---|---|---|---|---|
| Encapsulation | Engrafted pancreatic endoderm cells become islets | VC-02 (stem cell-derived pancreatic endoderm cells) | Safe, positive C-peptide levels in some patients | Only 35% of initial islet mass survived | Phase 1/2 completed 2023 | (203), NCT03163511 |
| Encapsulation | Subcutaneous implant, without immunosuppression and with oxygen supply | Human islets in Beta Air device | Results not posted | Results not posted | Phase 1/2 in progress | (204), NCT02064309 |
| Implantation Device | Abdominal wall pre-vascularized device for 3 weeks with immunosuppression regimen | Sernova Cell Pouch | Stimulated C-peptide levels for 9 months; persistent graft function; reduced HbA1C and insulin dosage | Currently only 7 patients tested; preliminary results; invasive surgery | Phase 1/2 recruiting | (205), NCT03513939 |
| Implantation Device | Subcutaneous abdominal implantation of pre-vascularized device for 22–130 days before islet transplantation | Sernova Cell Pouch | Results not posted | Results not posted | Phase 1/2 terminated | (206), NCT01652911 |
| Implantation Device | Perforated device to deliver cells | VCTX210A, VCTX211 (devices to deliver genetically engineered allogeneic pancreatic endoderm cells) | Results not posted | Results not posted | Phase 1 completed (VCTX210A); Phase 1/2 in progress (VCTX211) | (207, 208), NCT05210530, NCT05565248 |
| Implantation Device | Immunoprotective device that does not require additional immunosuppression | VX-264 (encapsulated stem cell derived islet therapy) | Trial stopped early due to lack of efficacy | Lack of efficacy led to termination | Phase 1/2 terminated (update posted 2025) | (209), NCT05791201 |
| Stem Cell Derived Therapy | Hepatic Portal Vein infusion | Zimislecel (VX-880) | 12 participants treated; restored endogenous insulin secretion; 92% insulin reduction; 10/12 insulin-independent at 1 year; HbA1c <7% and >70% time in range | Requires immunosuppression; durability beyond 1 year still uncertain | Phase 3 recruiting following successful Phase 1/2 | (210, 211), NCT04786262 |
| Stem Cell Derived Therapy | Intramuscular transplantation | Hypoimmune islets | First-in-human treated July 2025; C-peptide detected; insulin production confirmed; HbA1c improved; effect ongoing at 6 months | N=1; short follow-up; donor islet supply constraints; long-term safety unknown | Phase 1 recruiting | (194, 212) NCT06239636 |
| Monoclonal antibody | CD40 on antigen presenting cells | Tegoprubart (anti-CD40L) | Promising results in preclinical model | Results not posted | Phase 1/2 trial recruiting | (125), NCT06305286 |
| MSC + Islets | Transplantation of islets and MSCs in T1D patients | Allograft islets and autograft MSCs | Results not posted | Results not posted | Current status unknown | (213), NCT00646724 |
| Polyclonal Tregs | CD4+ T cells | Ex vivo-selected, and ex vivo-expanded autologous polyTregs combined with islet transplantation | Results not posted | Results not posted | Phase 1 in progress | (214), NCT03444064 |
| Islet + Treg cells | Tregs from either donor or recipient administered with islets | Clinical Islet Transplantation with Apheresis, Isolation and Reintroduction of Recipient Regulatory T Cells | Results not posted | Results not posted | Phase 1 in progress | (215), NCT05973734 |
| Polyclonal Tregs | CD4+ T cells | cePolyTregs (cryopreserved PolyTregs) | Results not posted | Results not posted | Phase 1 in progress | (216), NCT05349591 |
| Encapsulation/Xenotransplantation | Encapsulated porcine islets + device for patients with established T1D and severe hypoglycemia | OPF-310 | Phase I/II trial began; testing safety & dose (6000 or 12,000 IEQ/kg) | Early stage; unknown long-term safety, immune reaction, encapsulation device durability; supply/scaling of xenogeneic islets | Phase I/II recruiting/ongoing | NCT06575426 (217) |
Strategies for islet replacement.
4.1 Islet engineering for enhanced immune evasion
In addition, to address some of the previously mentioned issues, several groups are trying to engineer islets to enhance immune evasion while keeping the functionality of donor islets. One of these strategies is the use of hypoimmune islets, which are genetically engineered pancreatic islets designed to evade immune system detection and rejection. The use of hypoimmune islets allows for the elimination of lifelong immunosuppression while achieving glycemic correction. Hu et al. developed human allogeneic gene-engineered hypoimmune islets, which showed glycemic correction in pre-clinical trials. The authors achieved hypoimmunity by eliminating MHC class I and II expression by knocking out the genes β2-microglobulin (B2M) and class II major histocompatibility complex transactivator (CIITA), respectively, using CRISPR-Cas9 gene editing techniques. In addition, they overexpressed CD47 to prevent phagocytosis by macrophages and natural killer (NK) cell-mediated destruction. The findings demonstrate that the hypoimmune (HIP) islets evaded rejection and restored normal blood glucose in allogeneic humanized mice, autoimmune NOD mice, and STZ-induced diabetic mice for at least 30 days (222). In addition, this same group applied their HIP modifications to induced pluripotent stem cells (iPSCs) and tested their survival in a fully immunocompetent, allogeneic rhesus macaque model without immunosuppression. The key findings showed that HIP-iPSCs survived for over 16 weeks after transplantation, significantly longer than unmodified iPSCs, which were rapidly rejected. These HIP cells successfully differentiated into multiple cell types without triggering immune activation, confirming their ability to evade both innate and adaptive immune responses (223). Additionally, the same team also similarly engineered primary pancreatic islets derived from rhesus macaques to create “hypoimmune pseudo-islets”. The authors implanted the engineered cells into a fully immunocompetent diabetic cynomolgus monkey without administering immunosuppressive drugs and showed significant improvements in glucose regulation by observing an initial increase and later stabilization in serum C-peptide levels correlated with controlled blood glucose levels (224). The technology derived from these findings is currently undergoing phase 1 clinical trials as the first in-human safety study of hypoimmune pancreatic islet transplantation in adult subjects with type 1 diabetes (Table 2) (225).
On the other hand, Parent et al. followed a different approach by modifying human pluripotent stem cells (hPSCs) into insulin-producing cells to minimize the immune system’s recognition and rejection of these cells, by deleting specific HLA genes responsible for presenting antigens to immune cells. The authors removed the highly polymorphic HLA-A, HLA-B, and HLA-C genes, except for retaining the common HLA-A2 allele and the less variable HLA-E, HLA-F, and HLA-G genes. By using humanized mouse models, the researchers demonstrated that these genetically altered islet cells experienced significantly reduced NK cell activity and diminished T-cell-mediated alloimmune responses while not impairing the cells’ ability to function as insulin-producing β-cells (226). Similarly, Gerace et al. genetically engineered human stem cell-derived islets (SC-islets) to express PD-L1 and HLA-E, two immune-regulatory molecules that inhibit T cell and NK cell responses. Using CRISPR-Cas9, the authors knocked out HLA class I molecules to reduce allogeneic recognition while overexpressing PD-L1 to engage inhibitory PD-1 receptors on T cells. The engineered islets were encapsulated in a retrievable device and implanted into immunocompetent mice. These modifications allowed the islets to evade immune rejection and maintain function without systemic immunosuppression.
4.2 Islet encapsulation in micro or macro devices to evade immune clearance
Encapsulation of islets in a biomaterial has previously been shown to be promising in preclinical studies for the long-term survival of transplanted islets by providing an immune-isolative layer to avoid direct graft recognition without immunosuppression (203, 227–231) (203, 228–232) (203, 227–230, 233). Additionally, microencapsulated stem-cell-derived β-cells have been evaluated in clinical trials. Shapiro et al. initially developed a macro-device made of a multi-layer platform made of expanded polytetrafluoroethylene with engineered portals and an external polyester mesh that provides stability. Pancreatic endoderm cells (PEC) were then housed within the macro-encapsulation device, which provided immune protection from the host (VC-02) (Table 2) (203). This platform was tested in a phase 1/2 clinical trial for T1D and demonstrated positive C-peptide levels in 35,3% of patients as early as 6 months post-implant. The device was implanted subcutaneously under the immunosuppression regimen initiated with ATG and maintained with, mycophenolate mofetil and tacrolimus, common immunosuppression drugs to avoid transplant rejection. This clinical trial showed the potential of microencapsulation devices to house β-cells for immune protection and correction of blood glucose levels in type 1 diabetes patients. Despite promising outcomes, analysis of retrieved devices revealed that only 35% of the initial islet mass survived, a significantly lower percentage compared to preclinical studies. This discrepancy may be attributed to a more robust foreign body response in humans, among other contributing factors (231). These findings suggest that current immune isolation strategies, such as microencapsulation and standard immunosuppression regimens, are insufficient to fully mitigate the foreign body response, thereby limiting the long-term survival of transplanted islets. The implantation of foreign materials triggers a cascade of responses that leads to inflammation and recruitment of the immune cells at the site of the implant. This process results in a dense fibrous capsule formation around the device. This fibrotic barrier hinders the exchange of essential nutrients and oxygen, as well as the diffusion of insulin, ultimately compromising the viability of implanted islets and limiting the functionality and longevity of the implant (232, 234, 235).
In parallel, Sernova Biotherapeutics has developed a promising encapsulation platform known as the Cell Pouch™ System, which is an implantable, vascularizing device engineered to house and support insulin-producing cells. So far, for the ongoing phase 1/2 clinical trial at the University of Chicago, the Cell Pouch™ has shown encouraging results in patients with type 1 diabetes who suffer from hypoglycemia unawareness (Table 2). In Cohort A, which involved the implantation of the 8-channel Cell Pouch followed by donor islet transplantation, all six patients achieved sustained insulin independence, with durations ranging from 5.5 months to over four years. The first patient maintained insulin independence for more than 50 months, with HbA1c levels consistently within the non-diabetic range (≤6.5%). Histological analyses confirmed the presence of well-vascularized and functioning islets, producing insulin, glucagon, and somatostatin, even five years post-transplant. In the newer Cohort B, featuring an upgraded 10-channel Cell Pouch with 50% more islet capacity, preliminary data from seven patients show continued production of C-peptide and improved HbA1c levels, suggesting effective and durable islet function. The device has been well-tolerated across all participants, with no significant safety concerns reported, further supporting the potential of the Cell Pouch as a long-term, scalable therapy for restoring natural insulin production in T1D patients (236).
On the other hand, to further enhance the clinical translatability of encapsulation platforms, another approach utilizing oxygen-generating or supplementing technologies is explored. This strategy allows for the survival of high-density encapsulated islets, which can be beneficial for reducing implant size. For instance, Evron et al. demonstrated a device housing encapsulated islets within an alginate protected by an outer polytetrafluoroethylene (PTFE) membrane, thereby shielding them from host immune responses. These encapsulated islets were supplied with oxygen through the adjacent chamber, which was replenished daily through an external port. This device allowed for the survival of encapsulated allogeneic islets at a surface density of 4,800 islet equivalents/cm3 for more than 7 months in diabetic rats (237). This device is currently investigated in clinical trials (238). Another study by Wang et al. utilized an oxygen-generating device that recycles carbon dioxide generated by islets and converts it into oxygen via lithium peroxide particulates. Here, islets were encapsulated in alginate hydrogel and connected via a hollow silicon tube to the gas tank containing the PFC-immersed lithium oxide formulation. This approach allowed for the survival of rat islets in the diabetic mouse model for over three months (239). Krishnan et al. developed a different oxygen-generating method using electrochemical water splitting by proton-exchange membrane electrolysis to achieve functional survival of encapsulated rat islets at ~1,000 islets/cm2, when implanted in the subcutaneous space of diabetic mice for over 1 month (240). A different approach by Pham et al. utilized body moisture via electrolysis to support a high density of 60,000 IEQ/mL islets. This device allowed for survival of allogeneic islets for up to 3 months in a diabetic rat model (241).
4.3 Immunomodulatory biomaterials
Building on the promise of encapsulation devices, immunomodulatory biomaterials are being developed to enhance graft survival, minimize immune rejection, and avoid systemic toxicity associated with traditional immunosuppression. These materials can create a localized immune-privileged site through foreign body evading-chemical modifications, sustained release of cytokines or chemokines, presentation of checkpoint ligands, or localized delivery of immunosuppressive drugs. This section outlines the diverse strategies used to modulate the local immune environment, their application in preclinical studies, and challenges that remain for durable and scalable translation for curing T1D.
4.3.1 Chemically modified biomaterial for evading foreign body response
To avoid the use of immunosuppressants and allow for low immune recognition during islet transplantation, research has focused on chemical modifications to materials that reduce immune recognition (Figure 4A). For example, Vegas et al. encapsulated human embryonic-derived β-cells with triazole-thiomorpholine dioxide (TMTD) modified alginate and implanted them in the intraperitoneal space of C57BL/6J mice, which allowed for 174 days of normoglycemia (227). The same approach has been demonstrated in a non-human primate by implanting encapsulated allogeneic islets in the omental bursa of macaques, where islets remained viable for up to 4 months (229). Zwitterions are another class of molecules that have been extensively studied for their antifouling properties (242–244) and, more recently, for their use as a biomaterial chemical modification for preventing the foreign body response. Zwitterions prevent fibrosis by forming a strongly hydrated, charge-balanced surface that resists protein adsorption and immune cell adhesion. This blocks the activation of macrophages and fibroblasts, interrupting the fibrotic cascade at its source. Complementing these passive surface modification strategies, next-generation drug-releasing systems have been developed to actively achieve localized immunomodulation. For instance, co-encapsulation strategies utilizing crystallization-analyzed drug-eluting materials can provide sustained, low-dose release of anti-inflammatory agents for months to years. This approach has demonstrated prolonged islet graft survival while maintaining systemic drug levels below toxicity thresholds, effectively mitigating the risks of off-target effects (245). In addition, Liu et al. achieved long-term glycemic control for 200 days in STZ-treated diabetic mice using zwitterionic sulfobetaine-modified alginate and xenogeneic islets (246). Building on their success with fibrosis-evading zwitterions, they further enhanced their device by incorporating polyurethane for improved mechanical strength (247). Rat islets suspended in alginate and encapsulated within a polyurethane-zwitterion polymer and implanted in the IP space of C57BL/6J mice for six months demonstrated a reduction in (17.3% & 21.1%) insulin and fibrinogen adherence, compared to a non-modified control (113). TMTD modification was directly compared with zwitterionic material modification by coating a xenogeneic cell encapsulation device with zwitterionic coatings, poly(phosphorylcholine), poly(carbxybetaine), poly(sulfobetaine), or TMTD coating tetrahydropyran phenyl triazole and implanting in STZ-C57BL/6 mice (248). TMTD devices allowed for longer normoglycemia (60 days vs 4 weeks) and less surface collagen content at 4 weeks compared to zwitterionic-modified devices.
Figure 4

Immunomodulatory biomaterial strategies to prolong islet graft survival. (A) Chemical modification of the biomaterials can allow for prolonged evasion of immune recognition and subsequent fibrosis of the implant. (B-C) Biologics can be conjugated to a biomaterial or encapsulated within the material for direct presentation or localized release to immune cells in the vicinity. (B) Incorporation of anti-inflammatory cytokines such as IL-33, TGF-β, and CXCL12 within the biomaterial device can allow for a localized shift of T cell phenotype to Treg phenotype to suppress effector T cells. (C) While checkpoint ligands presented on the biomaterial can allow for inhibition of T cell activity by leading to their anergy or Fas-mediated death. (D) Additionally, polymer micro/nanoparticles can be co-delivered with islets for the release of immunosuppressants locally to the islet engraftment site. Mechanistically, these strategies primarily aim to limit cell adherence and inflammatory cell proliferation and coax cells in the surrounding area towards an anti-inflammatory lineage. For instance, delivery of Dex-PLGA microparticles leads to an M2-like shift in macrophages while nanoparticle delivery downregulates CD80/86 in dendritic cells, which can lead to suppression of immune responses. Treg, regulatory T cell; RAPA-PS: rapamycin polymersome, RAPA-NP: rapamycin eluting nanoparticle, Dex-PLGA: dexamethasone eluting PLGA micelle.
4.3.2 Localized delivery or coating with anti-inflammatory cytokines and chemokines
Another strategy to reduce immune response for islet transplantation is the use of immunomodulatory molecules like cytokines and chemokines, which can be incorporated into microdevices to create a localized tolerogenic environment that supports encapsulated islets (Figure 4B). By selecting biomaterials with varying diffusion rates, the release of these molecules can be controlled and sustained over time, ensuring prolonged therapeutic effects through material biodegradation (249). For example, Chen et al. incorporated the chemokine stromal cell-derived factor-1α (CXCL12), which can repel effector T cells while recruiting Tregs into alginate microcapsules to support long-term allo- and xenoislet transplantation without systemic immune suppression. The authors investigated two distinct incorporation methods (1): direct surface coating (adsorption) of CXCL12 onto the islet surface, and (2) matrix co-encapsulation, where soluble CXCL12 was homogenously mixed into the alginate hydrogel precursor prior to crosslinking abreat concentrations ranging from 100 ng/ml to 1 µg/ml. The co-encapsulation of CXCL12 and mouse islets into microcapsules led to delayed rejection of both allogeneic and xenogeneic islets transplanted into the peritoneal cavity of diabetic C57/BL6 mice, enabling a linear release of CXCL12 over 25 days (250). Similarly, Alagpulinsa et al. encapsulated stem-cell derived β-cells with CXCL12 and showed that a concentration of 2.0 µg/ml CXCL12 can enhance long-term glucose responsiveness and glycemic correction in STZ-induced diabetic mice for 150 days. Correction of blood glucose levels was facilitated by preventing foreign body response and prolonging cell viability when encapsulating high concentrations of CXCL12 (251). CXCL12 has also been used in NHP models. Sremac et al. incorporated this chemokine into alginate microbeads to evaluate the post-implantation immunological and histopathological changes over 6 months. Implantation of 400,000 alginate beads with CXCL12 showed a lower basal activation state for cytokine levels than the NHP without CXCL12. Additionally, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-18, IL-5, and IL-6 showed significant variation in concentration between different time points for the CXCL12 and control groups. Overall, these results provided support for the utility of CXCL12 in immunomodulation for these types of alginate microbeads (252). In addition to CXCL12, other immunomodulatory factors have also been combined with biomaterials to extend their release over time.
Transforming growth factor-β1 (TGF-β1) has previously been co-delivered with islets within microporous poly(lactide-co-glycolide) (PLG) scaffolds, showing controlled release of the protein over 30 days. The addition of TGF-β1 showed delayed graft failure when transplanting allogeneic islets from Balb/c mice into diabetic C57BL/6 mice (253). Another study by Neshat et al. demonstrated artificial APCs that are made of microparticles (700 +/- 200nm) of poly(lactic-co-glycolic acid/poly(beta-amino ester)) (PLGA/PBAE) with anti-CD3 and anti-CD28 antibodies conjugated and TGF-β loaded within for co-transplantation with islets to activate T\reg cells local to the transplant site. Subcutaneous injection of allogeneic islets and artificial APCs in STZ-induced C57BL/6 mice as a proof of concept revealed increased FOXP3 (Treg) expression 3 days after implantation and improved glucose correction compared to controls within 20 days (254). Though long-term glucose correction was not demonstrated, the artificial APCs can be optimized and delivered in combination with other strategies for improved graft survival. Another study demonstrated that the use of IL-33, an M2-polarizing cytokine, was added to PLG scaffolds, which allowed a delay in early engraftment of allogeneic islets and diabetes reversal compared to control scaffolds. Further research showed that IL-33 presence in the allogeneic model enriched counts of beneficial Treg cells, shifting their percentage in the population of total CD4+ cells from 40% to 75%, while also decreasing graft-rejecting CD8+ cells (255).
4.3.3 Localized checkpoint ligand presentation or release
Another widely explored strategy for creating a localized tolerogenic environment using biomaterials involves the presentation or controlled release of checkpoint ligands from micro- or macro-devices, which engage inhibitory receptors (such as PD-1 or CTLA-4) on T cells to suppress their activation, promote regulatory T cell induction, and ultimately establish immune tolerance at the implantation site (Figure 4C). One notable example is the NICHE (Neovascularized Implantable Cell Homing and Encapsulation) device developed by Paez-Mayorga et al. This macro-device features a dual reservoir system housing vascularizing mesenchymal stem cells (MSCs) and rat islets, while the outer reservoir contains CTLA-Ig and anti-lymphocyte serum (analog of rat ATG) for local delivery was implanted in the subcutaneous space of STZ diabetic eight-week-old male Lewis rats. In combination with rat islets, the NICHE device maintained normoglycemia for over 175 days, requiring periodic refilling of the drug reservoir on days 0, 28, and 56 (230). These results are obtained due to the favorable immune profile displayed by the treatment, exhibiting a significantly reduced number of cytotoxic T cells and macrophages, as well as pro-inflammatory cell types. Moreover, in NHP biocompatibility studies, the authors showed a 4-fold decrease in infiltrating leukocytes when the NICHE device was present. In summary, this macro-device platform shows promising results in modulating the immune system for islet transplantation; however, re-administration of both islets and local immunosuppressants was still needed to achieve the exhibited results (230). This technology has been licensed by NanoGland LLC and is in the initial stage of the FDA regulatory pipeline (256).
CTLA4-Ig has also been incorporated into other biomaterial platforms for localized delivery. For instance, Kuppan et al. developed CTLA4-Ig-loaded microparticles to induce long-term survival and tolerance of murine islet allografts by co-transplanting them with PLGA microparticles loaded with cyclosporine A (CsA) and a short course of CTLA4-Ig. CsA is a potent immunosuppressive drug used to prevent allograft rejection in solid organ transplantation and to treat autoimmune diseases. Co-delivery of BALB/c mice islets with CsA PLGA microparticles and CTL4-Ig into C57BL/6 mice led to about 55% of the recipients maintaining allograft function for 214 days post-transplant. Further study of proinflammatory cytokine profiles at 7 days posttransplant showed IL-1β, IL-6, INF-γ, and TNF-α expression reduced in both recipients treated by CsA microparticles and those treated by CsA microparticles and CTLA4-Ig compared to islet-alone recipients (257). In contrast, Barra et al. conducted a study showcasing the efficacy of non-toxic poly(N-vinylpyrrolidone) (PVPON) coated with antioxidant tannic acid (TA) and CTLA4-Ig, used to encapsulate NOD.Rag islets and implanted into the kidney capsule of C57BL/6 mice. The PVPON/TA/CTLA4-Ig encapsulated islets demonstrated remarkable graft survival exceeding 125 days. Furthermore, this approach effectively reduced proinflammatory macrophage signaling via diminished STAT1 activity, suppressed effector T cell populations, and promoted an increase in Tregs and anergic CD4+ cells, highlighting its potential to modulate immune responses favorably (258).
Programmed death ligand 1 (PD-L1) is another checkpoint ligand that is commonly incorporated within biomaterials for localized tolerance. PD-L1 binds to the inhibitory receptor PD-1 expressed at the surface of activated T cells. This interaction suppresses T effector function, playing a crucial role in maintaining immune homeostasis and promoting tolerance (259, 260). Several studies on T1D patients reveal abnormalities in the PD-1/PD-L1 pathway, suggesting it is essential for maintaining tolerance within the pancreas (259). Coronel et al. employed microgels made of four-armed poly (ethylene glycol) PEG macromers conjugated to streptavidin-PD-L1 (SA-PD-L1), which were co-transplanted with BALB/c mouse islets from in the epididymal fat pads of C57BL/6 STZ-induced mice. A low-dose 15-day course of rapamycin, which is an immunosuppressive drug that inhibits the mTOR pathway to directly inhibit T cell proliferation (261), was given to some groups of mice to mitigate an immune attack on the graft during the early post-transplantation period. The group of mice that received SA-PD-L1 microgels only demonstrated that 22% of grafts survived, with mice staying normoglycemic for 50 days. While, the mice that received a course of rapamycin, in addition, showed that around 60% of grafts survived for over 100 days (262). Gene expression analysis revealed an increase in regulatory factors FOXP3, Jun, Egr3, Ccr4, and PD-1, and flow cytometry showed an increase in Treg cells in response to SA-PD-L1 microgels. Further, PD-L1 microgels were shown to affect innate immunity by increasing macrophages at the transplant site. In this study, epididymal fat pads were selected as the implantation site due to their similarity to the murine equivalent of the omentum (263). The omentum has been demonstrated to be a superior site for islet transplantation, as it supports significantly better revascularization compared to the kidney capsule (264). In addition, Colonel et al. evaluated the combination of PD-L1 presenting microgels with a short course of the immunosuppressant rapamycin for the prevention of islet rejection in autoimmune NOD mice. Results showed significant extended islet graft survival due to microgels, with approximately 60% of recipients maintaining normoglycemia for over 100 days. Furthermore, the therapy promoted a tolerogenic immune environment at the graft site. There was an increase in regulatory T cells (CD4+CD25+FoxP3+) and a rise in exhausted cytotoxic T cell populations, which are associated with reduced immune-mediated graft destruction (265).
Fas ligand (FasL) is another commonly studied checkpoint ligand in this context. FasL binds to Fas on activated T cells, inducing apoptosis (266), and is essential for eliminating autoreactive lymphocytes (267). Previously, the same team that developed SA-PD-L1 microgels demonstrated using the same platform with FasL for the long-term survival of allogeneic islets with a short course of rapamycin in the kidney capsule of STZ-induced C57BL/6 mice for more than 200 days (266). This success inspired further studies in diabetic NHPs, where SA-FasL microgels were co-transplanted with allogeneic islets into the omentum under transient rapamycin treatment. These NHPs maintained normoglycemia for over six months. The omentum was selected as the implantation site to avoid thrombosis risks associated with portal vein delivery of microgels and islets, further demonstrating its translational potential (268). Li et al. conducted another study utilizing SA-FasL, but for modifying PLG scaffolds encapsulating β-cells derived from human pluripotent stem cells. These scaffolds were implanted into the epididymal fat pad of NSG mice and retrieved for analysis on days 4, 7, and 14 post-transplantations. The results showed significantly reduced infiltration of neutrophils and macrophages in comparison to the control group, highlighting the immune-modulatory potential of this approach (269).
4.3.4 Localized immunomodulatory agent delivery
Another approach to prevent the rejection of transplanted islets involves the use of immunomodulatory agents, which help regulate the immune system’s response to donor islets. To minimize the side effects associated with systemic immunosuppression, these agents have been incorporated into biomaterial delivery platforms, allowing for localized administration (Figure 4D). Kuppan et al. were able to fabricate dexamethasone (Dex) -eluting PLGA micelles to increase immune protection in a murine islet allograft model. Dex is a corticosteroid that has the ability to promote the conversion of macrophages into their more favorable anti-inflammatory M2-like phenotype (270). Dex PLGA micelles were characterized to have the cumulative release of dexamethasone at different concentrations for over 30 days. When combined with CTLA4-Ig and transplanted islets into a diabetic mouse model, 80% (8 of 10) of the recipients maintained allograft function for 60 days posttransplant, doubling the long-term normoglycemia prevalence compared to CTLA4-Ig monotherapy recipients (271).
Moreover, biomaterials can be integrated with immunomodulatory agents to reduce the immune response when transplanting β-cells for T1D. Burke et al. loaded rapamycin into poly(ethylene glycol)-b-poly(propylene sulfide)) (PEG-b-PPS) polymersomes (rPSs). Authors were able to achieve encapsulation efficiency over 55%, which, when implanted in the subcutaneous (SC) space of C57BL/6 mice it led to a potent immunomodulation response compared to unloaded polymersomes. Further studies analyzed mouse organs (blood, liver, spleen and lymph nodes) after implantation of rapamycin-loaded rPSs at different time points, showing downregulation of CD80 and CD86 coreceptors in DCs in addition to a significant decrease in CD4 expression. These immunosuppressive abilities enabled a reduction in the effective drug dosage needed to achieve normoglycemia in STZ diabetic C57BL6 mice for 100 days by receiving 200 IEQs from BALB/c mice into the liver via the portal vein (261). Similarly, Nguyen et al. combined MSCs with rapamycin-releasing PLGA microparticles (RAP-MPs) to prevent immune rejection of islet xenografts in diabetic C57BL/6 mice. When transplanting 400 rat IEQs with and without the RAP-MPs spheroids into the liver capsules of STZ-induced C57BL/6 mice, recipients administered with 100 ng of RAP (HS100) within the spheroids led to normoglycemia for up to 60 days. Delivery of HS100 spheroids significantly decreased the levels of pro-inflammatory cytokines such as IL-2, IL-3, IL-6, IFN-γ and TNF-α in the serum. Additionally, flow cytometry data from retrieved lymph nodes showed that delivery of HS100 spheroids significantly reduces the generation of both effector memory T cells and central memory cells (272).
In summary, both hypo-immune islets and encapsulated islets are being studied to improve islet transplantation outcomes and achieve normoglycemia in type 1 diabetes patients. The use of hypoimmune cells offers advantages over encapsulation by eliminating diffusion barriers between the cells and the native environment, allowing for faster glucose sensing and insulin release by the transplanted cells. Without encapsulation, nutrients, oxygen, and waste can freely exchange with the bloodstream, leading to quicker metabolic responses. Additionally, hypoimmune cells can be infused into the hepatic portal vein, eliminating the need for surgical or invasive procedures. However, gene editing techniques pose potential risks, including tumorigenicity, which remains a safety concern. In contrast, the use of encapsulation devices creates a barrier that slows nutrient and oxygen exchange, requiring additional technological advancements to maintain cell viability, such as oxygenation strategies or vascularization enhancements, as well as long-term evasion of foreign body response. Despite this limitation, encapsulation devices provide other advantages such as higher localization of the transplanted cells, potentially reducing the loss of cells associated with cell infusions into the hepatic portal vein (200).
These benefits motivated significant preclinical work to incorporate immunomodulatory agents within biomaterials for prolonging graft survival while minimizing the need for systemic immunosuppression. Although localized delivery of immunomodulators, within the encapsulated device or co-delivered along with naked islets, is promising in extending the survival of transplanted islets in vivo, they often need complementary systemic immunosuppressors to be used in tandem for long-term results. This can further complicate the treatment protocol. Moreover, most of these delivery systems require the replenishment of immunomodulatory agents. These limitations can hinder the clinical translatability of these strategies, adding to the complexity and cost of manufacturing.
5 Conclusion and outlook
Recent progress in understanding the immune mechanisms behind the pathogenesis of type 1 diabetes has accelerated the development of preventative therapies. These efforts led to FDA approval of the first disease-modifying drug and increased clinical interest in immunotherapies for the prevention. One notable example is that immune blockade therapies have been shown to be efficacious in clinical trials. However, systemic administration and broad immune targeting often lead to severe adverse effects, limiting their use as early-stage interventions, when their use could be most effective. Alternatively, antigen-specific and cell-based immunotherapies are explored as more targeted options, but they have shown limited efficacy. Antigen-specific strategies also have demonstrated variability in patient response and delivery limitations, while cell-based therapies have been shown to exhibit off-target effects and face challenges in isolation and expansion. These advances show the promise and complexity of designing efficacious and targeted interventions for disease prevention.
In parallel, β-cell replacement therapies are being developed to restore endogenous insulin production for patients at the late stage of the disease. Clinical milestones such as FDA approval of allogeneic islet transplantation and ongoing phase 3 clinical trials with stem cell-derived islets have demonstrated the potential of these solutions. Yet, their continued dependence on systemic immunosuppression poses safety and durability limits their broad application. To address this, several immunomodulatory approaches are being explored to create an immune-privileged environment around transplanted islets. However, these systems either lack long-term efficacy, still require systemic immunosuppression, or frequently necessitate the replenishment of immunomodulatory agents. All these factors exacerbate manufacturing complexity and cost. Additionally, the foreign body response to biomaterial-based platforms, limited vascularization, and inadequate oxygen supply at some implantation sites compromise islet viability and functionality. Moreover, clinical trials have also revealed a significantly lower islet survival rate than preclinical models, reflecting a gap between experimental findings and real-world outcomes.
In summary, while substantial progress has been made in immunomodulation strategies and β-cell replacement, there are still significant obstacles. Advancing these therapies requires addressing issues such as immune specificity, long-term efficacy, and clinical scalability, which will be critical for translating these promising approaches into effective and widely accessible treatments for T1D.
Statements
Author contributions
DV: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. PD: Visualization, Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. OV: Conceptualization, Supervision, Visualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Breakthrough T1D (3-SRA-2022-1255-S-B and 3-SRA-2024-1557-S-B to OV).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ogle GD Wang F Haynes A Gregory GA King TW Deng K et al . Global type 1 diabetes prevalence, incidence, and mortality estimates 2025: Results from the International diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res Clin Pract. (2025) 225:112277. doi: 10.1016/j.diabres.2025.112277
2
Gregory GA Robinson TIG Linklater SE Wang F Colagiuri S de Beaufort C et al . Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. (2022) 10:741–60. doi: 10.1016/S2213-8587(22)00218-2
3
Gong B Yang W Xing Y Lai Y Shan Z . Global, regional, and national burden of type 1 diabetes in adolescents and young adults. Pediatr Res. (2025) 97:568–76. doi: 10.1038/s41390-024-03107-5
4
Tomic D Harding JL Jenkins AJ Shaw JE Magliano DJ . The epidemiology of type 1 diabetes mellitus in older adults. Nat Rev Endocrinol. (2025) 21:92–104. doi: 10.1038/s41574-024-01046-z
5
IDF Diabetes Atlas . Diabetes atlas (2025). Available online at: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (Accessed December 12, 2025).
6
Redondo MJ Onengut-Gumuscu S Gaulton KJ . Genetics of type 1 diabetes. In: LawrenceJMCasagrandeSSHermanWHWexlerDJCefaluWT, editors. Diabetes in america. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, Bethesda (MD (2023). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK597411/.
7
Tizaoui K Shin JI Jeong GH Yang JW Park S Kim JH et al . Genetic polymorphism of PTPN22 in autoimmune diseases: A comprehensive review. Med (Mex). (2022) 58:1034. doi: 10.3390/medicina58081034
8
Alnek K Tagoma A Metsküla K Talja I Janson H Mandel M et al . Comparison of immunological and immunogenetic markers in recent-onset type 1 diabetes among children and adults. Sci Rep. (2025) 15:15491. doi: 10.1038/s41598-025-99664-8
9
Pang H Luo S Huang G Li X Xie Z Zhou Z . The Association of CTLA-4 rs231775 and rs3087243 Polymorphisms with Latent Autoimmune Diabetes in Adults: A Meta-Analysis. Biochem Genet. (2022) 60:1222–35. doi: 10.1007/s10528-021-10152-w
10
Tang W Cui D Jiang L Zhao L Qian W Long SA et al . Association of common polymorphisms in the IL2RA gene with type 1 diabetes: evidence of 32,646 individuals from 10 independent studies. J Cell Mol Med. (2015) 19:2481–8. doi: 10.1111/jcmm.12642
11
Herold KC Delong T Perdigoto AL Biru N Brusko TM Walker LSK . The immunology of type 1 diabetes. Nat Rev Immunol. (2024) 24:435–51. doi: 10.1038/s41577-023-00985-4
12
Ilonen J Lempainen J Veijola R . The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. (2019) 15:635–50. doi: 10.1038/s41574-019-0254-y
13
Sims EK Besser REJ Dayan C Geno Rasmussen C Greenbaum C Griffin KJ et al . Screening for type 1 diabetes in the general population: A status report and perspective. Diabetes. (2022) 71:610–23. doi: 10.2337/dbi20-0054
14
American Diabetes Association Professional Practice Committee . 2. Diagnosis and classification of diabetes: standards of care in diabetes—2025. Diabetes Care. (2024) 48:S27–49. doi: 10.2337/dc25-S002
15
Bluestone JA Buckner JH Herold KC . Immunotherapy: Building a bridge to a cure for type 1 diabetes. Science. (2021) 373:510–6. doi: 10.1126/science.abh1654
16
Perkins BA Sherr JL Mathieu C . Type 1 diabetes glycemic management: Insulin therapy, glucose monitoring, and automation. Science. (2021) 373:522–7. doi: 10.1126/science.abg4502
17
Mullard A . 2023 FDA approvals. Nat Rev Drug Discov. (2024) 23:88–95. doi: 10.1038/d41573-024-00001-x
18
Qin T Smink AM De Vos P . Enhancing longevity of immunoisolated pancreatic islet grafts by modifying both the intracapsular and extracapsular environment. Acta Biomater. (2023) 167:38–53. doi: 10.1016/j.actbio.2023.06.038
19
Dai C Walker JT Shostak A Padgett A Spears E Wisniewski S et al . Tacrolimus- and sirolimus-induced human β cell dysfunction is reversible and preventable. JCI Insight. (2020) 5:e130770. doi: 10.1172/jci.insight.130770
20
Kabakchieva P Assyov Y Gerasoudis S Vasilev G Peshevska-Sekulovska M Sekulovski M et al . Islet transplantation-immunological challenges and current perspectives. World J Transplant. (2023) 13:107–21. doi: 10.5500/wjt.v13.i4.107
21
Roep BO Thomaidou S Van Tienhoven R Zaldumbide A . Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system)? Nat Rev Endocrinol. (2021) 17:150–61. doi: 10.1038/s41574-020-00443-4
22
Ashby KM Hogquist KA . A guide to thymic selection of T cells. Nat Rev Immunol. (2024) 24:103–17. doi: 10.1038/s41577-023-00911-8
23
Khosravi-Maharlooei M Madley R Borsotti C Ferreira LMR Sharp RC Brehm MA et al . Modeling human T1D-associated autoimmune processes. Mol Metab. (2022) 56:101417. doi: 10.1016/j.molmet.2021.101417
24
Clark M Kroger CJ Tisch RM . Type 1 diabetes: A chronic anti-self-inflammatory response. Front Immunol. (2017) 8:1898. doi: 10.3389/fimmu.2017.01898
25
Sun F Yang CL Wang FX Rong SJ Luo JH Lu WY et al . Pancreatic draining lymph nodes (PLNs) serve as a pathogenic hub contributing to the development of type 1 diabetes. Cell Biosci. (2023) 13:156. doi: 10.1186/s13578-023-01110-7
26
Gearty SV Dündar F Zumbo P Espinosa-Carrasco G Shakiba M Sanchez-Rivera FJ et al . An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature. (2022) 602:156–61. doi: 10.1038/s41586-021-04248-x
27
Varela-Calvino R Calviño-Sampedro C Gómez-Touriño I Cordero OJ . Apportioning blame: autoreactive CD4+ and CD8+ T cells in type 1 diabetes. Arch Immunol Ther Exp (Warsz). (2017) 65:275–84. doi: 10.1007/s00005-016-0452-4
28
Yang K Zhang Y Ding J Li Z Zhang H Zou F . Autoimmune CD8+ T cells in type 1 diabetes: from single-cell RNA sequencing to T-cell receptor redirection. Front Endocrinol. (2024) 15:1377322/full. doi: 10.3389/fendo.2024.1377322/full
29
Espinosa-Carrasco G Le Saout C Fontanaud P Stratmann T Mollard P Schaeffer M et al . CD4+ T helper cells play a key role in maintaining diabetogenic CD8+ T cell function in the pancreas. Front Immunol. (2018) 8:2001/full. doi: 10.3389/fimmu.2017.02001/full
30
Krishnamurthy B Lacorcia M Kay TWH Thomas HE Mannering SI . Monitoring immunomodulation strategies in type 1 diabetes. Front Immunol. (2023) 14:1206874. doi: 10.3389/fimmu.2023.1206874
31
Narayan K Mikler K Maguire A Craig ME Bell K . The current landscape for screening and monitoring of early-stage type 1 diabetes. J Paediatr Child Health. (2025) 61:676–84. doi: 10.1111/jpc.70016
32
Jacobsen LM Schatz DA Herold KC Skyler JS . Prevention of type 1 diabetes. In: LawrenceJMCasagrandeSSHermanWHWexlerDJCefaluWT, editors. Diabetes in america. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, Bethesda (MD (2023). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK604182/.
33
Buckner JH . Antigen-specific immunotherapies for autoimmune disease. Nat Rev Rheumatol. (2025) 21:88–97. doi: 10.1038/s41584-024-01201-w
34
Ludvigsson J . Autoantigen treatment in type 1 diabetes: unsolved questions on how to select autoantigen and administration route. Int J Mol Sci. (2020) 21:1598. doi: 10.3390/ijms21051598
35
Rodriguez-Fernandez S Almenara-Fuentes L Perna-Barrull D Barneda B Vives-Pi M . A century later, still fighting back: antigen-specific immunotherapies for type 1 diabetes. Immunol Cell Biol. (2021) 99:461–74. doi: 10.1111/imcb.12439
36
Assfalg R Knoop J Hoffman KL Pfirrmann M Zapardiel-Gonzalo JM Hofelich A et al . Oral insulin immunotherapy in children at risk for type 1 diabetes in a randomised controlled trial. Diabetologia. (2021) 64:1079–92. doi: 10.1007/s00125-020-05376-1
37
Ziegler AG Achenbach P Weiß A Berner R Casteels K Larsson HE et al . Efficacy of once-daily, high-dose, oral insulin immunotherapy in children genetically at risk for type 1 diabetes (POInT): a European, randomised, placebo-controlled, primary prevention trial. Lancet. (2025) 406:2564–76. doi: 10.1016/S0140-6736(25)01726-X
38
Zhao LP Papadopoulos GK Skyler JS Parikh HM Kwok WW Bondinas GP et al . Oral insulin delay of stage 3 type 1 diabetes revisited in HLA DR4-DQ8 participants in the trialNet oral insulin prevention trial (TN07). Diabetes Care. (2024) 47:1608–16. doi: 10.2337/dc24-0573
39
University of colorado, denver. MER3101: MAS-1 adjuvanted antigen-specific immunotherapeutic for prevention and treatment of type 1 diabetes. (2024). Available online at: https://clinicaltrials.gov/study/NCT03624062. Report No.: NCT03624062.
40
Tatovic D McAteer MA Barry J Barrientos A Rodríguez Terradillos K Perera I et al . Safety of the use of gold nanoparticles conjugated with proinsulin peptide and administered by hollow microneedles as an immunotherapy in type 1 diabetes. Immunother Adv. (2022) 2:ltac002. doi: 10.1093/immadv/ltac002
41
Latres E Plum-Moerschel L Murugesan SMN Lou O Panda J Marwah A et al . An open-label, multiple ascending dose trial of orally administered insulin Tregopil in patients with type 1 diabetes mellitus to evaluate its pharmacokinetics, pharmacodynamics, safety and tolerability. Diabetes Obes Metab. (2025) 27:3154–64. doi: 10.1111/dom.16327
42
Ramos EL Dayan CM Chatenoud L Sumnik Z Simmons KM Szypowska A et al . Teplizumab and β-cell function in newly diagnosed type 1 diabetes. N Engl J Med. (2023) 389:2151–61. doi: 10.1056/NEJMoa2308743
43
Herold KC Bundy BN Long SA Bluestone JA DiMeglio LA Dufort MJ et al . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. (2019) 381:603–13. doi: 10.1056/NEJMoa1902226
44
Herold KC Gitelman SE Gottlieb PA Knecht LA Raymond R Ramos EL . Teplizumab: A disease-modifying therapy for type 1 diabetes that preserves β-cell function. Diabetes Care. (2023) 46:1848–56. doi: 10.2337/dc23-0675
45
Diggins KE Serti E Muir V Rosasco M Lu T Balmas E et al . Exhausted-like CD8+ T cell phenotypes linked to C-peptide preservation in alefacept-treated T1D subjects. JCI Insight. (2021) 6:e142680. doi: 10.1172/jci.insight.142680
46
Balmas E Chen J Hu AK DeBerg HA Rosasco MG Gersuk VH et al . Islet-autoreactive CD4+ T cells are linked with response to alefacept in type 1 diabetes. JCI Insight. (2023) 8:e167881. doi: 10.1172/jci.insight.167881
47
Acharya S Lama S Kanigicherla DA . Anti-thymocyte globulin for treatment of T-cell-mediated allograft rejection. World J Transplant. (2023) 13:299–308. doi: 10.5500/wjt.v13.i6.299
48
Mohty M . Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. (2007) 21:1387–94. doi: 10.1038/sj.leu.2404683
49
Alloway RR Woodle ES Abramowicz D Segev DL Castan R Ilsley JN et al . Rabbit anti-thymocyte globulin for the prevention of acute rejection in kidney transplantation. Am J Transplant. (2019) 19:2252–61. doi: 10.1111/ajt.15342
50
Haller MJ Long SA Blanchfield JL Schatz DA Skyler JS Krischer JP et al . Low-dose anti-thymocyte globulin preserves C-peptide, reduces hbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes. (2019) 68:1267–76. doi: 10.2337/db19-0057
51
Jacobsen LM Diggins K Blanchfield L McNichols J Perry DJ Brant J et al . Responders to low-dose ATG induce CD4+ T cell exhaustion in type 1 diabetes. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.161812
52
Foster TP Jacobsen LM Bruggeman B Salmon C Hosford J Chen A et al . Low-dose antithymocyte globulin: A pragmatic approach to treating stage 2 type 1 diabetes. Diabetes Care. (2023) 47:285–9. doi: 10.2337/dc23-1750
53
ITB-med LLC. A 12-month, randomized, single-blind, placebo-controlled exposure-response study of TCD601 (Siplizumab) in new onset type 1 diabetes patients (STRIDE). (2024).
54
National Institute of Allergy and Infectious Diseases (NIAID) . A T cell phenotype signature driven dose finding study with siplizumab in type 1 diabetes mellitus (ITN095AI). (2024).
55
Edner NM Heuts F Thomas N Wang CJ Petersone L Kenefeck R et al . Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nat Immunol. (2020) 21:1244–55. doi: 10.1038/s41590-020-0744-z
56
Russell WE Bundy BN Anderson MS Cooney LA Gitelman SE Goland RS et al . Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: A randomized, double-masked, controlled trial. Diabetes Care. (2023) 46:1005–13. doi: 10.2337/dc22-2200
57
Rudd CE Schneider H . Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. (2003) 3:544–56. doi: 10.1038/nri1131
58
Kim T Martínez-Bonet M Wang Q Hackert N Sparks JA Baglaenko Y et al . Non-coding autoimmune risk variant defines role for ICOS in T peripheral helper cell development. Nat Commun. (2024) 15:2150. doi: 10.1038/s41467-024-46457-8
59
Orban T Bundy B Becker DJ DiMeglio LA Gitelman SE Goland R et al . Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. (2011) 378:412–9. doi: 10.1016/S0140-6736(11)60886-6
60
Mj H Rotthaeuser B Cherkas A Coudert M Labard P Baccara-Dinet M et al . 2021-LB: FABULINUS—A randomized, controlled trial with frexalimAB, to assess endogenous insulin secretion in new-onset stage 3 type 1 diabetes adults and adolescents. Diabetes. (2024) 73:2021–LB. doi: 10.2337/db24-2021-LB
61
Pescovitz MD Greenbaum CJ Bundy B Becker DJ Gitelman SE Goland R et al . B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. (2014) 37:453–9. doi: 10.2337/dc13-0626
62
Zieliński M Żalińska M Iwaszkiewicz-Grześ D Gliwiński M Hennig M Jaźwińska-Curyłło A et al . Combined therapy with CD4+CD25highCD127– T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: Randomized phase I/II trial. Diabetes Obes Metab. (2022) 24:1534–43. doi: 10.1111/dom.14723
63
Quattrin T Haller MJ Steck AK Felner EI Li Y Xia Y et al . Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. (2020) 383:2007–17. doi: 10.1056/NEJMoa2006136
64
Rigby MR Hayes B Li Y Vercruysse F Hedrick JA Quattrin T . Two-year follow-up from the T1GER study: continued off-therapy metabolic improvements in children and young adults with new-onset T1D treated with golimumab and characterization of responders. Diabetes Care. (2023) 46:561–9. doi: 10.2337/dc22-0908
65
Ludvigsson J Routray I Vigård T Hanås R Rathsman B Carlsson A et al . Combined Etanercept, GAD-alum and vitamin D treatment: an open pilot trial to preserve beta cell function in recent onset type 1 diabetes. Diabetes Metab Res Rev. (2021) 37:e3440. doi: 10.1002/dmrr.3440
66
Greenbaum CJ Serti E Lambert K Weiner LJ Kanaparthi S Lord S et al . IL-6 receptor blockade does not slow β cell loss in new-onset type 1 diabetes. JCI Insight. (2021) 6:e150074. doi: 10.1172/jci.insight.150074
67
Piemonti L Keymeulen B Gillard P Linn T Bosi E Rose L et al . Ladarixin, an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset type 1 diabetes: A multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2022) 24:1840–9. doi: 10.1111/dom.14770
68
Zhou Z . Phase 1&x2F; phase 2 study of the therapeutic effect of ex-vivo expanded umbilical cord blood regulatory T cells with liraglutide on autoimmune diabetes. (2023). Available online at: https://clinicaltrials.gov/study/NCT03011021 (Accessed December 24 2025).
69
Von Herrath M Bain SC Bode B Clausen JO Coppieters K Gaysina L et al . Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. (2021) 9:212–24. doi: 10.1016/S2213-8587(21)00019-X
70
Tatovic D Marwaha A Taylor P Hanna SJ Carter K Cheung WY et al . Ustekinumab for type 1 diabetes in adolescents: a multicenter, double-blind, randomized phase 2 trial. Nat Med. (2024) 30:2657–66. doi: 10.1038/s41591-024-03115-2
71
Dong S Hiam-Galvez KJ Mowery CT Herold KC Gitelman SE Esensten JH et al . The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight. (2021) 6:e147474. doi: 10.1172/jci.insight.147474
72
Bender C Wiedeman AE Hu A Ylescupidez A Sietsema WK Herold KC et al . A phase 2 randomized trial with autologous polyclonal expanded regulatory T cells in children with new-onset type 1 diabetes. Sci Transl Med. (2024) 16:eadn2404. doi: 10.1126/scitranslmed.adn2404
73
Nikolic T Zwaginga JJ Uitbeijerse BS Woittiez NJ DE Koning EJ Aanstoot HJ et al . Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide—for type 1 diabetes. Lancet Diabetes Endocrinol. (2020) 8:470–2. doi: 10.1016/S2213-8587(20)30104-2
74
Nikolic T Suwandi JS Wesselius J Laban S Joosten AM Sonneveld P et al . Tolerogenic dendritic cells pulsed with islet antigen induce long-term reduction in T-cell autoreactivity in type 1 diabetes patients. Front Immunol. (2022) 13:1054968/full. doi: 10.3389/fimmu.2022.1054968/full
75
Gaglia JL Daley HL Bryant NK Ritz J Dong T Skyler JS et al . Novel autologous dendritic cell therapy AVT001 for type 1 diabetes. NEJM Evid. (2024) 3:EVIDoa2300238. doi: 10.1056/EVIDoa2300238
76
Izadi M Sadr Hashemi Nejad A Moazenchi M Masoumi S Rabbani A Kompani F et al . Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. (2022) 13:264. doi: 10.1186/s13287-022-02941-w
77
Zhang L Londono P Yu L Grimes S Blackburn P Gottlieb P et al . MAS-1 adjuvant immunotherapy generates robust Th2 type and regulatory immune responses providing long-term protection from diabetes in late-stage pre-diabetic NOD mice. Autoimmunity. (2014) 47:341–50. doi: 10.3109/08916934.2014.910768
78
Aubin AM Lombard-Vadnais F Collin R Aliesky HA McLachlan SM Lesage S . The NOD mouse beyond autoimmune diabetes. Front Immunol. (2022) 13:874769. doi: 10.3389/fimmu.2022.874769
79
Chen D Thayer TC Wen L Wong FS . Mouse models of autoimmune diabetes: the nonobese diabetic (NOD) mouse. Methods Mol Biol Clifton NJ. (2020) 2128:87–92. doi: 10.1007/978-1-0716-0385-7_6
80
Bonifacio E Ziegler AG Klingensmith G Schober E Bingley PJ Rottenkolber M et al . Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the pre-POINT randomized clinical trial. JAMA. (2015) 313:1541–9. doi: 10.1001/jama.2015.2928
81
Alhadj Ali M Liu YF Arif S Tatovic D Shariff H Gibson VB et al . Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. (2017) 9:eaaf7779. doi: 10.1126/scitranslmed.3006103
82
Roep BO Solvason N Gottlieb PA Abreu JRF Harrison LC Eisenbarth GS et al . Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci Transl Med. (2013) 5:191ra82–191ra82. doi: 10.1126/scitranslmed.3006103
83
Cook DP Cunha JPMCM Martens PJ Sassi G Mancarella F Ventriglia G et al . Intestinal Delivery of Proinsulin and IL-10 via Lactococcus lactis Combined With Low-Dose Anti-CD3 Restores Tolerance Outside the Window of Acute Type 1 Diabetes Diagnosis. Front Immunol. (2020) 11:1103/full. doi: 10.3389/fimmu.2020.01103/full
84
Aulanni’am A Wuragil DK Susanto H Herawati A Nugroho YM Laili Fajri WN et al . The early detection of type 1 diabetes mellitus and latent autoimmune diabetes in adults (LADA) through rapid test reverse-flow immunochromatography for glutamic acid decarboxylase 65 kDa (GAD65). Heliyon. (2022) 8:e08695. doi: 10.1016/j.heliyon.2021.e08695
85
Salami F Spiliopoulos L Maziarz M Lundgren M Brundin C Bennet R et al . Long-term GAD-alum treatment effect on different T-cell subpopulations in healthy children positive for multiple beta cell autoantibodies. J Immunol Res. (2022) 2022:3532685. doi: 10.1155/2022/3532685
86
Ludvigsson J Sumnik Z Pelikanova T Nattero Chavez L Lundberg E Rica I et al . Intralymphatic glutamic acid decarboxylase with vitamin D supplementation in recent-onset type 1 diabetes: A double-blind, randomized, placebo-controlled phase IIb trial. Diabetes Care. (2021) 44:1604–12. doi: 10.2337/dc21-0318
87
Acevedo-Calado MJ Pietropaolo SL Morran MP Schnell S Vonberg AD Verge CF et al . Autoantibodies directed toward a novel IA-2 variant protein enhance prediction of type 1 diabetes. Diabetes. (2019) 68:1819–29. doi: 10.2337/db18-1351
88
Mao R Yang M Yang R Chen Y Diao E Zhang T et al . Oral delivery of the intracellular domain of the insulinoma-associated protein 2 (IA-2ic) by bacterium-like particles (BLPs) prevents type 1 diabetes mellitus in NOD mice. Drug Deliv. (2022) 29:925–36. doi: 10.1080/10717544.2022.2053760
89
Fuentes-Cantero S González-Rodríguez C Rodríguez-Chacón C Galvan-Toribio R Hermosín-Escudero J Pérez-Pérez A et al . Study of the diagnostic efficiency of anti-ZnT8 autoantibodies for type 1 diabetes in pediatric patients. Lab Med. (2024) 55:299–303. doi: 10.1093/labmed/lmad079
90
de Andrade LJ de Oliveira GCM Rocha RM Bittencourt AMV David TM de Oliveira LM . Evaluating the diagnostic accuracy of anti- zinc transporter 8 antibodies in type 1 diabetes: A systematic review and meta-analysis. medRxiv. (2024). doi: 10.1101/2024.06.07.24308627v1
91
Chujo D Kawabe A Matsushita M Tsutsumi C Haseda F Imagawa A et al . Fulminant type 1 diabetes patients display high frequencies of IGRP-specific type 1 CD8+ T cells. Clin Immunol. (2021) 233:108893. doi: 10.1016/j.clim.2021.108893
92
Ochocińska A Wysocka-Mincewicz M Świderska J Cukrowska B . Selected serum markers associated with pathogenesis and clinical course of type 1 diabetes in pediatric patients—The effect of disease duration. J Clin Med. (2023) 12:2151. doi: 10.3390/jcm12062151
93
Xu G Grimes TD Grayson TB Chen J Thielen LA Tse HM et al . Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat Commun. (2022) 13:1159. doi: 10.1038/s41467-022-28826-3
94
Buckle I Loaiza Naranjo JD Bergot AS Zhang V Talekar M Steptoe RJ et al . Tolerance induction by liposomes targeting a single CD8 epitope IGRP206–214 in a model of type 1 diabetes is impeded by co-targeting a CD4+ islet epitope. Immunol Cell Biol. (2021) 100:33–48. doi: 10.1111/imcb.12506
95
Srivastava N Hu H Vomund AN Peterson OJ Baker RL Haskins K et al . Chromogranin A deficiency confers protection from autoimmune diabetes via multiple mechanisms. Diabetes. (2021) 70:2860–70. doi: 10.2337/db21-0513
96
Yang ML Connolly SE Gee RJ Lam TT Kanyo J Peng J et al . Carbonyl posttranslational modification associated with early-onset type 1 diabetes autoimmunity. Diabetes. (2022) 71:1979–93. doi: 10.2337/db21-0989
97
Azoury ME Samassa F Buitinga M Nigi L Brusco N Callebaut A et al . CD8+ T cells variably recognize native versus citrullinated GRP78 epitopes in type 1 diabetes. Diabetes. (2021) 70:2879–91. doi: 10.2337/db21-0259
98
Ediger BN Du A Liu J Hunter CS Walp ER Schug J et al . Islet-1 is essential for pancreatic β-cell function. Diabetes. (2014) 63:4206–17. doi: 10.2337/db14-0096
99
Shi X Huang G Wang Y Liu Z Deng C Li X et al . Tetraspanin 7 autoantibodies predict progressive decline of beta cell function in individuals with LADA. Diabetologia. (2019) 62:399–407. doi: 10.1007/s00125-018-4799-4
100
Purcell AW Sechi S DiLorenzo TP . The evolving landscape of autoantigen discovery and characterization in type 1 diabetes. Diabetes. (2019) 68:879–86. doi: 10.2337/dbi18-0066
101
Wang Y Wang JW Li Y Tian XH Feng XS Zhang SC et al . Bone marrow-derived mesenchymal stem cells improve rat islet graft revascularization by upregulating ISL1. Stem Cells. (2021) 39:1033–48. doi: 10.1002/stem.3378
102
Roep BO Wheeler DCS Peakman M . Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. (2019) 7:65–74. doi: 10.1016/S2213-8587(18)30109-8
103
Galderisi A Sims EK Evans-Molina C Petrelli A Cuthbertson D Nathan BM et al . Trajectory of beta cell function and insulin clearance in stage 2 type 1 diabetes: natural history and response to teplizumab. Diabetologia. (2025) 68:646–61. doi: 10.1007/s00125-024-06323-0
104
Rigby MR DiMeglio LA Rendell MS Felner EI Dostou JM Gitelman SE et al . Targeting effector memory T cells with alefacept in new onset type 1 diabetes: 12 month results from the T1DAL study. Lancet Diabetes Endocrinol. (2013) 1:284–94. doi: 10.1016/S2213-8587(13)70111-6
105
Podestà MA Binder C Sellberg F DeWolf S Shonts B Ho SH et al . Siplizumab selectively depletes effector memory T-cells and promotes a relative expansion of alloreactive regulatory T-cells in vitro. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2020) 20:88–100. doi: 10.1111/ajt.15533
106
Miura Y Isogai S Maeda S Kanazawa S . CTLA-4-Ig internalizes CD80 in fibroblast-like synoviocytes from chronic inflammatory arthritis mouse model. Sci Rep. (2022) 12. doi: 10.1038/s41598-022-20694-7
107
Murphy KM Weaver C Janeway C . Janeway’s immunobiology. Garland Sci. (2017), 904.
108
Raghunandan S Gorfinkel L Graiser M Bratrude B Suessmuth Y Gillespie S et al . Abatacept for the prevention of GVHD in patients receiving mismatched unrelated transplants: a real-world analysis. Blood Adv. (2023) 7:4395–9. doi: 10.1182/bloodadvances.2023010225
109
Liu M Yu Y Hu S . A review on applications of abatacept in systemic rheumatic diseases. Int Immunopharmacol. (2021) 96:107612. doi: 10.1016/j.intimp.2021.107612
110
Smith MJ Simmons KM Cambier JC . B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. (2017) 13:712–20. doi: 10.1038/nrneph.2017.138
111
Creusot RJ Postigo-Fernandez J Teteloshvili N . Altered function of antigen-presenting cells in type 1 diabetes: A challenge for antigen-specific immunotherapy? Diabetes. (2018) 67:1481–94. doi: 10.2337/db17-1564
112
Li Y Sun F Yue TT Wang FX Yang CL Luo JH et al . Revisiting the antigen-presenting function of β Cells in T1D pathogenesis. Front Immunol. (2021) 12:690783. doi: 10.3389/fimmu.2021.690783
113
Lin CM Gill RG . Direct and indirect allograft recognition: pathways dictating graft rejection mechanisms. Curr Opin Organ Transplant. (2016) 21:40–4. doi: 10.1097/MOT.0000000000000263
114
Ricordi C Ildstad ST Starzl TE . Induction of pancreatic islet graft acceptance: the role of antigen presenting cells. Transplant Sci. (1992) 2:34–8.
115
Wolf LA Coulombe M Gill RG . Donor antigen-presenting cell-independent rejection of islet xenografts. Transplantation. (1995) 60:1164–70. doi: 10.1097/00007890-199511270-00018
116
Elgueta R Benson MJ de Vries VC Wasiuk A Guo Y Noelle RJ . Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. (2009) 229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x
117
Maxwell JR Campbell JD Kim CH Vella AT . CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion1. J Immunol. (1999) 162:2024–34. doi: 10.4049/jimmunol.162.4.2024
118
Anwar IJ Berman DM DeLaura I Gao Q Willman MA Miller A et al . The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates. Sci Transl Med. (2023) 15:eadf6376. doi: 10.1126/scitranslmed.adf6376
119
Perrin S Magill M . The inhibition of CD40/CD154 costimulatory signaling in the prevention of renal transplant rejection in nonhuman primates: A systematic review and meta analysis. Front Immunol. (2022) 13:861471. doi: 10.3389/fimmu.2022.861471
120
Novartis . Novartis announces discontinuation of CIRRUS-1 study of CFZ533 (iscalimab) in kidney transplant patients. Available online at: https://www.novartis.com/news/novartis-announces-discontinuation-cirrus-1-study-cfz533-iscalimab-kidney-transplant-patients (Accessed December 12, 2025).
121
Manjunatha TA Chng R Yau WP . Efficacy and safety of tacrolimus-based maintenance regimens in de novo kidney transplant recipients: A systematic review and network meta-analysis of randomized controlled trials. Ann Transplant. (2021) 26:e933588–1-e933588-22. doi: 10.12659/AOT.933588
122
St. Clair EW Baer AN Ng WF Noaiseh G Baldini C Tarrant TK et al . CD40 ligand antagonist dazodalibep in Sjögren’s disease: a randomized, double-blinded, placebo-controlled, phase 2 trial. Nat Med. (2024) 30:1583–92. doi: 10.1038/s41591-024-03009-3
123
Furie RA Bruce IN Dörner T Leon MG Leszczyński P Urowitz M et al . Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology. (2021) 60:5397–407. doi: 10.1093/rheumatology/keab381
124
Carvalho T . Anti-CD40L antibody frexalimab slows new brain lesions in multiple sclerosis. Nat Med. (2023) 29:1882–3. doi: 10.1038/d41591-023-00060-4
125
Eledon announces clinical progress with tegoprubart in the prevention of transplant rejection. Eledon Pharmaceuticals, Inc. (2024). Available online at: https://ir.eledon.com/news-releases/news-release-details/eledon-announces-clinical-progress-tegoprubart-prevention (Accessed November 24, 2025).
126
Kläsener K Jellusova J Andrieux G Salzer U Böhler C Steiner SN et al . CD20 as a gatekeeper of the resting state of human B cells. Proc Natl Acad Sci. (2021) 118:e2021342118. doi: 10.1073/pnas.2021342118
127
Pescovitz MD Greenbaum CJ Krause-Steinrauf H Becker DJ Gitelman SE Goland R et al . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. (2009) 361:2143–52. doi: 10.1056/NEJMoa0904452
128
Lu J Liu J Li L Lan Y Liang Y . Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunol. (2020) 9:e1122. doi: 10.1002/cti2.1122
129
Syed Khaja AS Binsaleh NK Beg MMA Ashfaq F Khan MI Almutairi MG et al . Clinical importance of cytokine (IL-6, IL-8, and IL-10) and vitamin D levels among patients with Type-1 diabetes. Sci Rep. (2024) 14:24225. doi: 10.1038/s41598-024-73737-6
130
Eizirik DL Sammeth M Bouckenooghe T Bottu G Sisino G Igoillo-Esteve M et al . The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PloS Genet. (2012) 8:e1002552. doi: 10.1371/journal.pgen.1002552
131
Ramos-Rodríguez M Raurell-Vila H Colli ML Alvelos MI Subirana-Granés M Juan-Mateu J et al . The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat Genet. (2019) 51:1588–95. doi: 10.1038/s41588-019-0524-6
132
Nakayasu ES Syed F Tersey SA Gritsenko MA Mitchell HD Chan CY et al . Comprehensive proteomics analysis of stressed human islets identifies GDF15 as a target for type 1 diabetes intervention. Cell Metab. (2020) 31:363–374.e6. doi: 10.1016/j.cmet.2019.12.005
133
Hundhausen C Roth A Whalen E Chen J Schneider A Long SA et al . Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med. (2016) 8:356ra119–356ra119. doi: 10.1126/scitranslmed.aad9943
134
Alhamar G Fallucca S Pieralice S Valente L Pozzilli P . 1492-P: IL-8/CXCL8 may identify a new type 1 diabetes endotype. Diabetes. (2023) 72:1492–P. doi: 10.2337/db23-1492-P
135
Luo J Ning T Li X Jiang T Tan S Ma D . Targeting IL-12 family cytokines: A potential strategy for type 1 and type 2 diabetes mellitus. BioMed Pharmacother. (2024) 170:115958. doi: 10.1016/j.biopha.2023.115958
136
Van Belle TL Nierkens S Arens R von Herrath MG . Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. (2012) 36:1060–72. doi: 10.1016/j.immuni.2012.04.005
137
Robertson CC Inshaw JRJ Onengut-Gumuscu S Chen WM Santa Cruz DF Yang H et al . Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat Genet. (2021) 53:962–71. doi: 10.1038/s41588-021-00880-5
138
Rao A Quinn LM Narendran P . Long-term TNF-alpha therapy for preserving beta cell function in new onset type 1 diabetes: a case report. Clin Diabetes Endocrinol. (2024) 10:26. doi: 10.1186/s40842-024-00185-6
139
Guyton J Jeon M Brooks A . Glucagon-like peptide 1 receptor agonists in type 1 diabetes mellitus. Am J Health Syst Pharm. (2019) 76:1739–48. doi: 10.1093/ajhp/zxz179
140
Bergström M Yao M Müller M Korsgren O Zur-Mühlen B Lundgren T . Autologous regulatory T cells in clinical intraportal allogenic pancreatic islet transplantation. Transpl Int. (2021) 34:2816–23. doi: 10.1111/tri.14163
141
Mitchell AM Alkanani AA McDaniel KA Pyle L Waugh K Steck AK et al . T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc Natl Acad Sci. (2021) 118. doi: 10.1073/pnas.2019129118
142
Bettini M Bettini ML . Function, failure, and the future potential of tregs in type 1 diabetes. Diabetes. (2021) 70:1211–9. doi: 10.2337/dbi18-0058
143
ElEssawy B Li XC . Type 1 diabetes and T regulatory cells. Pharmacol Res. (2015) 98:22–30. doi: 10.1016/j.phrs.2015.04.009
144
Raffin C Vo LT Bluestone JA . Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol. (2020) 20:158–72. doi: 10.1038/s41577-019-0232-6
145
Ferreira LMR Muller YD Bluestone JA Tang Q . Next-generation regulatory T cell therapy. Nat Rev Drug Discov. (2019) 18:749–69. doi: 10.1038/s41573-019-0041-4
146
Zhang JY Hamey F Trzupek D Mickunas M Lee M Godfrey L et al . Low-dose IL-2 reduces IL-21+ T cell frequency and induces anti-inflammatory gene expression in type 1 diabetes. Nat Commun. (2022) 13:7324. doi: 10.1038/s41467-022-34162-3
147
De Groot AS Skowron G White JR Boyle C Richard G Serreze D et al . Therapeutic administration of Tregitope-Human Albumin Fusion with Insulin Peptides to promote Antigen-Specific Adaptive Tolerance Induction. Sci Rep. (2019) 9:16103. doi: 10.1038/s41598-019-52331-1
148
Cabello-Kindelan C MacKey S Sands A Rodriguez J Vazquez C Pugliese A et al . Immunomodulation followed by antigen-specific treg infusion controls islet autoimmunity. Diabetes. (2020) 69:215–27. doi: 10.2337/db19-0061
149
Spanier JA Fung V Wardell CM Alkhatib MH Chen Y Swanson LA et al . Tregs with an MHC class II peptide–specific chimeric antigen receptor prevent autoimmune diabetes in mice. J Clin Invest. (2023) 133. doi: 10.1172/JCI168601
150
De Paula Pohl A Schmidt A Zhang AH Maldonado T Königs C Scott DW . Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell Immunol. (2020) 358:104222. doi: 10.1016/j.cellimm.2020.104222
151
Mohammadi V Maleki AJ Nazari M Siahmansouri A Moradi A Elahi R et al . Chimeric antigen receptor (CAR)-based cell therapy for type 1 diabetes mellitus (T1DM); current progress and future approaches. Stem Cell Rev Rep. (2024) 20:585–600. doi: 10.1007/s12015-023-10668-1
152
Tenspolde M Zimmermann K Weber LC Hapke M Lieber M Dywicki J et al . Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J Autoimmun. (2019) 103:102289. doi: 10.1016/j.jaut.2019.05.017
153
Ben-Skowronek I Sieniawska J Pach E Wrobel W Skowronek A Tomczyk Z et al . Potential therapeutic application of regulatory T cells in diabetes mellitus type 1. Int J Mol Sci. (2022) 23:390. doi: 10.3390/ijms23010390
154
Akkaya B Oya Y Akkaya M Al Souz J Holstein AH Kamenyeva O et al . Regulatory T cells mediate specific suppression by depleting peptide–MHC class II from dendritic cells. Nat Immunol. (2019) 20:218–31. doi: 10.1038/s41590-018-0280-2
155
Zhang L Sosinowski T Cox AR Cepeda JR Sekhar NS Hartig SM et al . Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. J Autoimmun. (2019) 96:50–8. doi: 10.1016/j.jaut.2018.08.004
156
Obarorakpor N Patel D Boyarov R Amarsaikhan N Cepeda JR Eastes D et al . Regulatory T cells targeting a pathogenic MHC class II: Insulin peptide epitope postpone spontaneous autoimmune diabetes. Front Immunol [Internet]. (2023) 14:1207108/full. doi: 10.3389/fimmu.2023.1207108/full
157
Chavez JC Bachmeier C Kharfan-Dabaja MA . CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. (2019) 10:2040620719841581. doi: 10.1177/2040620719841581
158
Patel U Abernathy J Savani BN Oluwole O Sengsayadeth S Dholaria B . CAR T cell therapy in solid tumors: A review of current clinical trials. eJHaem. (2022) 3:24–31. doi: 10.1002/jha2.356
159
Konopacki C Pritykin Y Rubtsov Y Leslie CS Rudensky AY . Transcription factor Foxp1 regulates Foxp3 chromatin binding and coordinates regulatory T cell function. Nat Immunol. (2019) 20:232–42. doi: 10.1038/s41590-018-0291-z
160
Mertowska P Mertowski S Podgajna M Grywalska E . The importance of the transcription factor foxp3 in the development of primary immunodeficiencies. J Clin Med. (2022) 11:947–7. doi: 10.3390/jcm11040947
161
Deng G Song X Fujimoto S Piccirillo CA Nagai Y Greene MI . Foxp3 post-translational modifications and treg suppressive activity. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.02486
162
Honaker Y Hubbard N Xiang Y Fisher L Hagin D Sommer K et al . Gene editing to induce FOXP3 expression in human CD4 + T cells leads to a stable regulatory phenotype and function. Sci Transl Med. (2020) 12. doi: 10.1126/scitranslmed.aay6422
163
Yang SJ Singh AK Drow T Tappen T Honaker Y Barahmand-pour-Whitman F et al . Pancreatic islet-specific engineered T regs exhibit robust antigen-specific and bystander immune suppression in type 1 diabetes models. Sci Transl Med. (2022) 14. doi: 10.1126/scitranslmed.abn1716
164
Uenishi GI Repic M Yam JY Landuyt A Saikumar-Lakshmi P Guo T et al . GNTI-122: an autologous antigen-specific engineered Treg cell therapy for type 1 diabetes. JCI Insight. (2024) 9. doi: 10.1172/jci.insight.171844
165
Rafiqi SI Aldasouqi A Paparodis RD Dewan S Farooqi A Faisal S et al . Conversion of T effector cells into T regulatory cells in type 1 diabetes/latent autoimmune diabetes of adults by inhibiting eIF5A and notch pathways. ImmunoTargets Ther. (2025) 14:205–26. doi: 10.2147/ITT.S504555
166
Imam S Prathibha R Dar P Almotah K Al-Khudhair A Abdul-Moiz S et al . eIF5A inhibition influences T cell dynamics in the pancreatic microenvironment of the humanized mouse model of Type 1 Diabetes. Sci Rep. (2019) 9. doi: 10.1038/s41598-018-38341-5
167
Huang Q Zhu J . Regulatory T cell-based therapy in type 1 diabetes: Latest breakthroughs and evidence. Int Immunopharmacol. (2024) 140:112724. doi: 10.1016/j.intimp.2024.112724
168
Giannoukakis N . Tolerogenic dendritic cells in type 1 diabetes: no longer a concept. Front Immunol. (2023) 14:1212641/full. doi: 10.3389/fimmu.2023.1212641/full
169
Iberg CA Hawiger D . Natural and induced tolerogenic dendritic cells. J Immunol. (2020) 204:733–44. doi: 10.4049/jimmunol.1901121
170
Zhuang Q Cai H Cao Q Li Z Liu S Ming Y . Tolerogenic dendritic cells: the pearl of immunotherapy in organ transplantation. Front Immunol. (2020) 11:552988. doi: 10.3389/fimmu.2020.552988
171
Morante-Palacios O Fondelli F Ballestar E Martínez-Cáceres EM . Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol. (2021) 42:59–75. doi: 10.1016/j.it.2020.11.001
172
Nam JH Lee JH Choi SY Jung NC Song JY Seo HG et al . Functional ambivalence of dendritic cells: tolerogenicity and immunogenicity. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22094430
173
Choo EH Lee JH Park EH Park HE Jung NC Kim TH et al . Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation. (2017) 135:1444–57. doi: 10.1161/CIRCULATIONAHA.116.023106
174
Byun SH Lee JH Jung NC Choi HJ Song JY Seo HG et al . Rosiglitazone-mediated dendritic cells ameliorate collagen-induced arthritis in mice. Biochem Pharmacol. (2016) :115:85–93. doi: 10.1016/j.bcp.2016.05.009
175
Welzen-Coppens JMC van Helden-Meeuwsen CG Leenen PJM Drexhage HA Versnel MA . Reduced numbers of dendritic cells with a tolerogenic phenotype in the prediabetic pancreas of NOD mice. J Leukoc Biol. (2012) 92:1207–13. doi: 10.1189/jlb.0312168
176
Cifuentes-Rius A Desai A Yuen D Johnston APR Voelcker NH . Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat Nanotechnol. (2021) 16:37–46. doi: 10.1038/s41565-020-00810-2
177
Giannoukakis N Phillips B Finegold D Harnaha J Trucco M Phase I . (Safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. (2011) 34:2026–32. doi: 10.2337/dc11-0472
178
Passeri L Andolfi G Bassi V Russo F Giacomini G Laudisa C et al . Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J Autoimmun. (2023) 138:103051. doi: 10.1016/j.jaut.2023.103051
179
Carlsson PO Schwarcz E Korsgren O Le Blanc K . Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. (2015) 64:587–92. doi: 10.2337/db14-0656
180
Wu C Liu W Liu Y Xu T Li M Li X et al . Human umbilical cord mesenchymal stem cell-derived TGFBI attenuates streptozotocin-induced type 1 diabetes mellitus by inhibiting T-cell proliferation. Hum Cell. (2023) 36:997–1010. doi: 10.1007/s13577-023-00868-9
181
Zeinhom A Fadallah SA Mahmoud M . Human mesenchymal stem/stromal cell based-therapy in diabetes mellitus: experimental and clinical perspectives. Stem Cell Res Ther. (2024) 15:384. doi: 10.1186/s13287-024-03974-z
182
Wang X Wang K Yu M Velluto D Hong X Wang B et al . Engineered immunomodulatory accessory cells improve experimental allogeneic islet transplantation without immunosuppression. Sci Adv. (2022) 8:eabn0071. doi: 10.1126/sciadv.abn0071
183
Jayasinghe M Prathiraja O Perera PB Jena R Silva MS Weerawarna PSH et al . The role of mesenchymal stem cells in the treatment of type 1 diabetes. Cureus. (2022) 14:e27337. doi: 10.7759/cureus.27337
184
Yu S Yu S Liu H Liao N Liu X . Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. (2023) 14:235. doi: 10.1186/s13287-023-03476-4
185
Zhang X Kang Y Wang J Yan J Chen Q Cheng H et al . Engineered PD-L1-expressing platelets reverse new-onset type 1 diabetes. Adv Mater Deerfield Beach Fla. (2020) 32:e1907692. doi: 10.1002/adma.201907692
186
Ma Y Meng Y Lin Z Chen Y Lan T Yang Z et al . Bioengineering platelets presenting PD-L1, galectin-9 and BTLA to ameliorate type 1 diabetes. Ma - Advanced Sci. (2025) 12. doi: 10.1002/advs.202501139
187
Xia L . Engineered platelets for clinical application: a step closer. Blood. (2022) 140:2314–5. doi: 10.1182/blood.2022018291
188
Pepper AR Bruni A Shapiro AMJ . Clinical islet transplantation: is the future finally now. Curr Opin Organ Transplant. (2018) 23:428–39. doi: 10.1097/MOT.0000000000000546
189
Holt RIG DeVries JH Hess-Fischl A Hirsch IB Kirkman MS Klupa T et al . The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2021) 64:2609–52. doi: 10.1007/s00125-021-05568-3
190
Langlois A Pinget M Kessler L Bouzakri K . Islet transplantation: current limitations and challenges for successful outcomes. Cells. (2024) 13:1783. doi: 10.3390/cells13211783
191
Markmann JF Rickels MR Eggerman TL Bridges ND Lafontant DE Qidwai J et al . Phase 3 trial of human islet-after-kidney transplantation in type 1 diabetes. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2021) 21:1477–92. doi: 10.1111/ajt.16174
192
Hering BJ Clarke WR Bridges ND Eggerman TL Alejandro R Bellin MD et al . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. (2016) 39:1230–40. doi: 10.2337/dc15-1988
193
Marfil-Garza BA Imes S Verhoeff K Hefler J Lam A Dajani K et al . Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. (2022) 10:519–32. doi: 10.1016/S2213-8587(22)00114-0
194
Chetboun M Drumez E Ballou C Maanaoui M Payne E Barton F et al . Association between primary graft function and 5-year outcomes of islet allogeneic transplantation in type 1 diabetes: a retrospective, multicentre, observational cohort study in 1210 patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. (2023) 11:391–401. doi: 10.1016/S2213-8587(23)00082-7
195
Rios P Mcgarrigle Jj Lamonica G Li Y Cook J Joshi I et al . 35-OR: lantidra, first FDA-approved cellular therapy to treat type 1 diabetes. Diabetes. (2024) 73:35–OR. doi: 10.2337/db24-35-OR
196
Wang X Brown NK Wang B Shariati K Wang K Fuchs S et al . Local immunomodulatory strategies to prevent allo-rejection in transplantation of insulin-producing cells. Adv Sci. (2021) 8:2003708. doi: 10.1002/advs.202003708
197
Lansberry TR Stabler CL . Immunoprotection of cellular transplants for autoimmune type 1 diabetes through local drug delivery. Adv Drug Delivery Rev. (2024) 206:115179. doi: 10.1016/j.addr.2024.115179
198
Kuwabara R Hu S Smink AM Orive G Lakey JRT de Vos P . Applying immunomodulation to promote longevity of immunoisolated pancreatic islet grafts. Tissue Eng Part B Rev. (2022) 28:129–40. doi: 10.1089/ten.teb.2020.0326
199
Shapiro AMJ . Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabetes Stud RDS. (2012) 9:385–406. doi: 10.1900/RDS.2012.9.385
200
Naziruddin B Iwahashi S Kanak MA Takita M Itoh T Levy MF . Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. (2014) 14:428–37. doi: 10.1111/ajt.12558
201
Wang Q Huang Y Liu L Zhao X Sun Y Mao X et al . Pancreatic islet transplantation: current advances and challenges. Front Immunol. (2024) 15:1391504/full. doi: 10.3389/fimmu.2024.1391504/full
202
Hering BJ Rickels MR Bellin MD Millman JR Tomei AA García AJ et al . Advances in cell replacement therapies for diabetes. Diabetes. (2025) 74:1068–77. doi: 10.2337/db25-0037
203
Shapiro AMJ Thompson D Donner TW Bellin MD Hsueh W Pettus J et al . Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med. (2021) 2. doi: 10.1016/j.xcrm.2021.100466
204
Carlsson PO . An open label, pilot investigation, to assess the safety and efficacy of transplantation of macro-encapsulated human islets within the bioartificial pancreas beta-air in patients with type 1 diabetes mellitus. (2023). Available online at: https://clinicaltrials.gov/study/NCT02064309 (Accessed December 24 2025).
205
Bachul P Generette GS Perez-Gutierrez A Borek P Wang LJ Golab K et al . 307.5: modified approach allowed for improved islet allotransplantation into pre-vascularized sernova cell pouchTM device - preliminary results of the phase I/II clinical trial at university of chicago. Transplantation. (2021) 105:S25. doi: 10.1097/01.tp.0000886884.76423.6d
206
A Phase I/ II Study of the Safety and Efficacy of Sernova's Cell PouchTM for Therapeutic Islet Transplantation. University of alberta. A phase I&x2F;II study of the safety and efficacy of sernova&x27;s cell pouchTM for therapeutic islet transplantation. (2022). Available online at: https://clinicaltrials.gov/study/NCT01652911.
207
CRISPR Therapeutics AG . An open-label, first-in-human study evaluating the safety and tolerability of VCTX210A combination product in subjects with type 1 diabetes mellitus (T1D). (2023). Available online at: https://clinicaltrials.gov/study/NCT05210530 (Accessed December 24 2025).
208
CRISPR Therapeutics AG . An open-label, first-in-human study evaluating the safety, tolerability, and efficacy of VCTX211 combination product in subjects with type 1 diabetes mellitus (T1D). (2024). Available online at: https://clinicaltrials.gov/study/NCT05565248 (Accessed November 21, 2025).
209
A Safety, Tolerability, and Efficacy Study of VX-264 in Participants With Type 1 Diabetes. Vertex pharmaceuticals incorporated. A phase 1&x2F;2 study to evaluate the safety, tolerability, and efficacy of VX-264 in subjects with type 1 diabetes mellitus. (2024). Available online at: https://clinicaltrials.gov/study/NCT05791201.
210
Vertex pharmaceuticals incorporated. A phase 1&x2F;2 study to evaluate the safety, tolerability, and efficacy of VX-880 in subjects who have type 1 diabetes mellitus with impaired hypoglycemic awareness and severe hypoglycemia. (2024). Available online at: https://clinicaltrials.gov/study/NCT04786262.
211
Vertex announces program updates for type 1 diabetes portfolio | Vertex pharmaceuticals. Available online at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-program-updates-type-1-diabetes-portfolio.
212
Sana biotech’s T1D trial presents early data. Available online at: https://www.thejdca.org/publications/report-library/archived-reports/2025-reports/cell-protection-without-immunosuppression-sana-biotechs-t1d-trial-presents-early-data.html.
213
HOSPITAL FG. Cotransplantation of islet and mesenchymal stem cell in type 1 diabetic patients. (2011). Available online at: https://clinicaltrials.gov/study/NCT00646724.
214
University of alberta. Polyclonal regulatory T cell (PolyTreg) immunotherapy in islet transplantation. (2024). Available online at: https://clinicaltrials.gov/study/NCT03444064.
215
Busque S . Clinical islet transplantation with apheresis, isolation and reintroduction of recipient regulatory T cells or administration of deceased donor vertebral bone marrow in type 1 diabetes. (2023). Available online at: https://clinicaltrials.gov/study/NCT05973734 (Accessed November 24, 2025).
216
Study details | CePolyTregs in islet transplantation. (2025). Available online at: https://clinicaltrials.gov/study/NCT05349591?term=NCT05349591&rank=1 (Accessed November 24, 2025).
217
Study details | NCT06575426 | A study to investigate safety and effectiveness of porcine pancreatic cells (OPF-310) in patients with type 1 diabetes mellitus. (2025). Available online at: https://clinicaltrials.gov/study/NCT06575426 (Accessed November 21, 2025).
218
Kim JH Lee YH Lim BJ Jeong HJ Kim PK Shin JI . Influence of cyclosporine A on glomerular growth and the effect of mizoribine and losartan on cyclosporine nephrotoxicity in young rats. Sci Rep. (2016) 6:22374. doi: 10.1038/srep22374
219
Naesens M Kuypers DRJ Sarwal M . Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol CJASN. (2009) 4:481–508. doi: 10.2215/CJN.04800908
220
Higdon LE Tan JC Maltzman JS . Infection, rejection, and the connection. Transplantation. (2023) 107:584–95. doi: 10.1097/TP.0000000000004297
221
Rama I Grinyó JM . Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. (2010) 6:511–9. doi: 10.1038/nrneph.2010.102
222
Hu X Gattis C Olroyd AG Friera AM White K Young CY et al . Human hypoimmune primary pancreatic islets avoid rejection and autoimmunity and alleviate diabetes in allogeneic humanized mice. Sci Trans Med. (2023) 15. doi: 10.1126/scitranslmed.adg5794?utm_source=chatgpt.com
223
Hu X White K Olroyd AG DeJesus R Dominguez AA Dowdle WE et al . Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat Biotechnol. (2023) 42:413–23. doi: 10.1038/s41587-023-01784-x
224
Hu X White K Young C Olroyd AG Kievit P Connolly AJ et al . Hypoimmune islets achieve insulin independence after allogeneic transplantation in a fully immunocompetent non-human primate. Cell Stem Cell. (2024) 31:334–40. doi: 10.1016/j.stem.2024.02.001
225
Study details | First-in-human safety study of hypoimmune pancreatic islet transplantation in adult subjects with type 1 diabetes. (2024). Available online at: https://clinicaltrials.gov/study/NCT06239636?cond=NCT06239636&rank=1 (Accessed November 24, 2025).
226
Parent AV Faleo G Chavez J Saxton M Berrios DI Kerper NR et al . Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. (2021) 36. doi: 10.1016/j.celrep.2021.109538
227
Vegas AJ Veiseh O Gürtler M Millman JR Pagliuca FW Bader AR et al . Long-term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat Med. (2016) 22:306–11. doi: 10.1038/nm.4030
228
Veiseh O Doloff JC Ma M Vegas AJ Tam HH Bader AR et al . Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. (2015) 14:643–51. doi: 10.1038/nmat4290
229
Bochenek MA Veiseh O Vegas AJ McGarrigle JJ Qi M Marchese E et al . Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat BioMed Eng. (2018) 2:810. doi: 10.1038/s41551-018-0275-1
230
Paez-Mayorga J Campa-Carranza JN Capuani S Hernandez N Liu HC Chua CYX et al . Implantable niche with local immunosuppression for islet allotransplantation achieves type 1 diabetes reversal in rats. Nat Commun. (2022) 13:7951. doi: 10.1038/s41467-022-35629-z
231
Keymeulen B De Groot K Jacobs-Tulleneers-Thevissen D Thompson DM Bellin MD Kroon EJ et al . Encapsulated stem cell–derived β cells exert glucose control in patients with type 1 diabetes. Nat Biotechnol. (2024) 42:1507–14. doi: 10.1038/s41587-023-02055-5
232
Kharbikar BN Chendke GS Desai TA . Modulating the foreign body response of implants for diabetes treatment. Adv Drug Delivery Rev. (2021) 174:87–113. doi: 10.1016/j.addr.2021.01.011
233
Lim F Sun AM . Microencapsulated islets as bioartificial endocrine pancreas. Science. (1980) 210:908–10. doi: 10.1126/science.6776628
234
Veiseh O Vegas AJ . Domesticating the foreign body response: Recent advances and applications. Adv Drug Delivery Rev. (2019) 144:148–61. doi: 10.1016/j.addr.2019.08.010
235
Overcoming immune hurdles to implant longevity. Nat BioMed Eng. (2024) 8:1191–2. doi: 10.1038/s41551-024-01276-6
236
Sernova announces additional patient insulin independent and cohort 2 enrollment complete in ongoing phase I/II clinical trial for treatment of type 1 diabetes. sernova.com (2024). Available online at: https://sernova.com/press_releases/sernova-announces-additional-patient-insulin-independent-and-cohort-2-enrollment-complete-in-ongoing-phase-i-ii-clinical-trial-for-treatment-of-type-1-diabetes/.
237
Evron Y Colton CK Ludwig B Weir GC Zimermann B Maimon S et al . Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. (2018) 8:6508. doi: 10.1038/s41598-018-23862-w
238
Carlsson PO . An open label, pilot investigation, to assess the safety and efficacy of transplantation of macro-encapsulated human islets within the bioartificial pancreas beta-air in patients with type 1 diabetes mellitus. (2024). Available online at: https://clinicaltrials.gov/study/NCT02064309 (Accessed December 24 2025).
239
Wang LH Ernst AU Flanders JA Liu W Wang X Datta AK et al . An inverse-breathing encapsulation system for cell delivery. Sci Adv [Internet]. (2021). doi: 10.1126/sciadv.abd5835
240
Krishnan SR Liu C Bochenek MA Bose S Khatib N Walters B et al . A wireless, battery-free device enables oxygen generation and immune protection of therapeutic xenotransplants in vivo. Proc Natl Acad Sci. (2023) 120. doi: 10.1073/pnas.2311707120
241
Pham TT Tran PL Tempelman LA Stone SG Piccirillo C Li A et al . A continuously oxygenated macroencapsulation system enables high-density packing and delivery of insulin-secreting cells. Nat Commun. (2025) 16:7199. doi: 10.1038/s41467-025-62271-2
242
Jiang S Cao Z . Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. (2010) 22:920–32. doi: 10.1002/adma.200901407
243
Ladd J Zhang Z Chen S Hower JC Jiang S . Zwitterionic polymers exhibiting high resistance to nonspecific protein adsorption from human serum and plasma. Biomacromolecules. (2008) 9:1357–61. doi: 10.1021/bm701301s
244
Goda T Konno T Takai M Moro T Ishihara K . Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials. (2006) 27:5151–60. doi: 10.1016/j.biomaterials.2006.05.046
245
Farah S Doloff JC Müller P Sadraei A Han HJ Olafson K et al . Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat Mater. (2019) 18:892–904. doi: 10.1038/s41563-019-0377-5
246
Liu Q Chiu A Wang LH An D Zhong M Smink AM et al . Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat Commun. (2019) 10:5262. doi: 10.1038/s41467-019-13238-7
247
Liu Q Wang X Chiu A Liu W Fuchs S Wang B et al . A zwitterionic polyurethane nanoporous device with low foreign-body response for islet encapsulation. Adv Mater Deerfield Beach Fla. (2021) 33:e2102852. doi: 10.1002/adma.202102852
248
Bose S Volpatti LR Thiono D Yesilyurt V McGladrigan C Tang Y et al . A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells. Nat BioMed Eng. (2020) 4:814–26. doi: 10.1038/s41551-020-0538-5
249
Mndlovu H Kumar P du Toit LC Choonara YE . A review of biomaterial degradation assessment approaches employed in the biomedical field. NPJ Mater Degrad. (2024) 8:1–19. doi: 10.1038/s41529-024-00487-1
250
Chen T Yuan J Duncanson S Hibert ML Kodish BC Mylavaganam G et al . Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am J Transplant. (2015) 15:618–27. doi: 10.1111/ajt.13049
251
Alagpulinsa DA Cao JJL Driscoll RK Sîrbulescu RF Penson MFE Sremac M et al . Alginate-microencapsulation of human stem cell-derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2019) 19:1930–40. doi: 10.1111/ajt.15308
252
Sremac M Lei J Penson MFE Schuetz C Lakey JRT Papas KK et al . Preliminary studies of the impact of CXCL12 on the foreign body reaction to pancreatic islets microencapsulated in alginate in nonhuman primates. Transplant Direct. (2019) 5:e447. doi: 10.1097/TXD.0000000000000890
253
Liu JMH Zhang J Zhang X Hlavaty KA Ricci CF Leonard JN et al . Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials. (2016) 80:11–9. doi: 10.1016/j.biomaterials.2015.11.065
254
Neshat SY Bauer SJ Rhodes KR Quiroz VM Wong VW Lowmaster SM et al . Improvement of islet engrafts via treg induction using immunomodulating polymeric tolerogenic microparticles. ACS Biomater Sci Eng. (2023) 9:3522–34. doi: 10.1021/acsbiomaterials.3c00329
255
Liu JMH Zhang X Joe S Luo X Shea LD . Evaluation of biomaterial scaffold delivery of IL-33 as a localized immunomodulatory agent to support cell transplantation in adipose tissue. J Immunol Regener Med. (2018) 1:1–12. doi: 10.1016/j.regen.2018.01.003
256
Paez-Mayorga J Lukin I Emerich D De Vos P Orive G Grattoni A . Emerging strategies for beta cell transplantation to treat diabetes. Trends Pharmacol Sci. (2022) 43:221–33. doi: 10.1016/j.tips.2021.11.007
257
Kuppan P Wong J Kelly S Lin J Worton J Castro C et al . Long-term survival and induction of operational tolerance to murine islet allografts by co-transplanting cyclosporine A microparticles and CTLA4-ig. Pharmaceutics. (2023) 15:2201. doi: 10.3390/pharmaceutics15092201
258
Barra JM Kozlovskaya V Burnette KS Banerjee RR Fraker CA Kharlampieva E et al . Localized CTLA-4-Ig and antioxidant islet encapsulation alters macrophage STAT1 signaling and induces regulatory and anergic T cells to enhance allograft survival. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2023) 23:498–511. doi: 10.1016/j.ajt.2023.01.007
259
Falcone M Fousteri G . Role of the PD-1/PD-L1 dyad in the maintenance of pancreatic immune tolerance for prevention of type 1 diabetes. Front Endocrinol. (2020) 11. doi: 10.3389/fendo.2020.00569
260
Lin X Kang K Chen P Zeng Z Li G Xiong W et al . Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. (2024) 23:108. doi: 10.1186/s12943-024-02023-w
261
Burke JA Zhang X Bobbala S Frey MA Bohorquez Fuentes C Freire Haddad H et al . Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat Nanotechnol. (2022) 17:319–30. doi: 10.1038/s41565-021-01048-2
262
Coronel MM Martin KE Hunckler MD Barber G O’Neill EB Medina JD et al . Immunotherapy via PD-L1–presenting biomaterials leads to long-term islet graft survival. Sci Adv. (2020) 6:eaba5573. doi: 10.1126/sciadv.aba5573
263
Weaver JD Headen DM Aquart J Johnson CT Shea LD Shirwan H et al . Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv. (2017) 3:e1700184. doi: 10.1126/sciadv.1700184
264
Addison P Fatakhova K Rodriguez Rilo HL . Considerations for an alternative site of islet cell transplantation. J Diabetes Sci Technol. (2020) 14:338–44. doi: 10.1177/1932296819868495
265
Coronel MM Linderman SW Martin KE Hunckler MD Medina JD Barber G et al . Delayed graft rejection in autoimmune islet transplantation via biomaterial immunotherapy. Am J Transplant. (2023) 23:1709–22. doi: 10.1016/j.ajt.2023.07.023
266
Headen DM Woodward KB Coronel MM Shrestha P Weaver JD Zhao H et al . Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat Mater. (2018) 17:732–9. doi: 10.1038/s41563-018-0099-0
267
Strasser A Jost PJ Nagata S . The many roles of FAS receptor signaling in the immune system. Immunity. (2009) 30:180–92. doi: 10.1016/j.immuni.2009.01.001
268
Lei J Coronel MM Yolcu ES Deng H Grimany-Nuno O Hunckler MD et al . FasL microgels induce immune acceptance of islet allografts in nonhuman primates. Sci Adv. (2022) 8:9881. Available online at: https://www.science.org.
269
Li F Crumley K Bealer E King JL Saito E Grimany-Nuno O et al . Fas ligand-modified scaffolds protect stem cell derived β-cells by modulating immune cell numbers and polarization. ACS Appl Mater Interfaces. (2023) 15:50549–59. doi: 10.1021/acsami.2c12939
270
Stabler CL Li Y Stewart JM Keselowsky BG . Engineering immunomodulatory biomaterials for type 1 diabetes. Nat Rev Mater. (2019) 4:429. doi: 10.1038/s41578-019-0112-5
271
Kuppan P Kelly S Polishevska K Hojanepesov O Seeberger K Korbutt GS et al . Co-localized immune protection using dexamethasone-eluting micelles in a murine islet allograft model. Am J Transplant. (2020) 20:714–25. doi: 10.1111/ajt.15662
272
Nguyen TT Pham DV Park J Phung CD Nepal MR Pandit M et al . Engineering of hybrid spheroids of mesenchymal stem cells and drug depots for immunomodulating effect in islet xenotransplantation. Sci Adv. (2022) 24. doi: 10.1126/sciadv.abn8614
Summary
Keywords
immune modulation, immunotherapies, islet transplantation, prevention of type 1 diabetes, type 1 diabetes
Citation
Vohidova D, Desai P, Moreno Lozano A and Veiseh O (2026) Modulating immune response for the prevention and treatment of type 1 diabetes. Front. Immunol. 17:1715863. doi: 10.3389/fimmu.2026.1715863
Received
29 September 2025
Revised
11 January 2026
Accepted
26 January 2026
Published
16 February 2026
Volume
17 - 2026
Edited by
Lonnie Shea, University of Michigan, United States
Reviewed by
Lauren Higdon, Immune Tolerance Network, United States
Joshua C. Doloff, Johns Hopkins University, United States
Victor Quiroz, Johns Hopkins University, in collaboration with reviewer JD
Updates
Copyright
© 2026 Vohidova, Desai, Moreno Lozano and Veiseh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omid Veiseh, omid.veiseh@rice.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.