Abstract
Bronchiectasis is a complex and heterogeneous disease, and our current understanding of its pathophysiological mechanisms remains in its infancy. Currently, few specifically approved treatments for non-cystic fibrosis bronchiectasis are applied globally, and most guideline-recommended therapies have limited evidence of efficacy. Consequently, the development of new treatment methods is particularly urgent. To gain insight into new drugs under clinical development for adults with bronchiectasis, we conducted a review of the scientific literature and clinical trial registries. In this review, we discuss in detail the mechanisms and potential effects of these emerging therapies, which focus on their anti-inflammatory effects, enhancement of mucociliary clearance, and anti-infectious effects. Clinical research has revealed significant variability among patients, suggesting the need for a multidisciplinary and comprehensive approach. Future efforts should focus on patient stratification using biomarkers and clinical assessments, with an emphasis on precision medicine. Furthermore, it is essential to develop new antibiotics, innovative neutrophil-targeted therapies, and effective mucus-clearing agents to alleviate patient disease burden and minimize the risk of exacerbations.
1 Introduction
In the last two decades, the occurrence and frequency of bronchiectasis have notably increased. In the UK, it affects more than 500 people per 100,000 people (1). Comparable or increased rates have been documented in the United States (2), Singapore (3), Germany (4), China (5), and Spain (6) (Figure 1). This could be attributed to improvements in diagnostic methods and criteria. Typically, we assess such diseases on the basis of their underlying pathobiological mechanisms and/or response to treatment. Over the past decade, international registries and clinical research collaborations focused on bronchiectasis in adults, adolescents, and children have been established (7–9). These initiatives enhance awareness of the disease and promote research efforts, including the investigation of new treatments and translational science. Consequently, they have led to significant progress in elevating the profile of bronchiectasis. The clinical efficacy of long-term antibiotic and macrolide therapy in reducing disease progression has been well established (10). International guidelines have contributed to the stratification of exacerbations that may benefit from antibiotic treatment and provided management guidance (11). Additionally, novel treatments, including anti-inflammatory drugs that inhibit dipeptidyl peptidase-1 (DPP-1), are emerging (12). Cystic fibrosis transmembrane conductance regulator (CFTR) correction therapy has also demonstrated promising results in mucus clearance in CF, but they have not been extensively studied in non-CF bronchiectasis (13, 14). Despite the advancements made over the past decade, numerous ongoing clinical studies have aimed to further elucidate the fundamental principles of bronchiectasis management. In this review, we summarize these clinical studies. Current areas of investigation include the exploration of new drugs, the utilization of large registry databases to characterize endotypes and phenotypes, and the examination of alternative treatments. Future clinical trials may be essential for advancing bronchiectasis treatment by more precisely targeting patient subgroups that are most likely to benefit from specific therapies.
Figure 1

The frequency of bronchiectasis in the overall population based on existing data. Studies from these nations are listed in the corresponding boxes. The data represent the number of individuals impacted by the disease per 100,000 people. The blue and pink symbols denote males and females, respectively.
2 Pathobiologic mechanisms
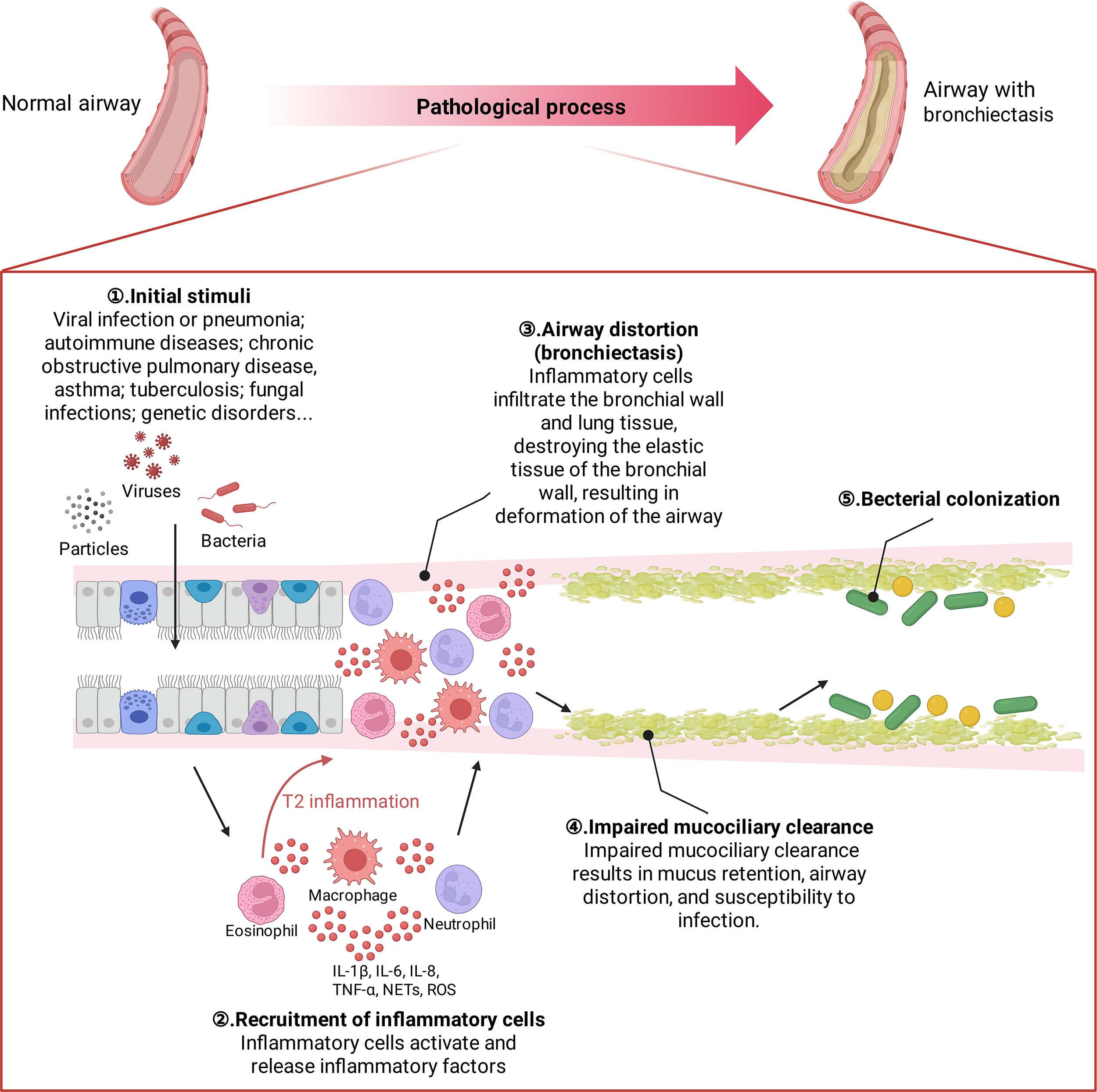
Bronchiectasis is a heterogeneous disease. Multiple stimuli play a role in the progression of bronchiectasis, ultimately leading to a vicious cycle of airway remodeling and dilation (15, 16). Initial injury can lead to inflammation, airway disruption and dysfunction, structural disease, and infection. Once this developmental cycle is entered, it progressively worsens and surpasses local and systemic host defense mechanisms. Mucociliary dysfunction leads to impaired mucus clearance, which ultimately results in airway distortion, mucus retention and bacterial colonization. infection causes the recruitment of neutrophils to the bronchial lumen and the infiltration of neutrophils, CD4+ T lymphocytes, and macrophages into the bronchial wall and lung tissue; inflammatory cells activate and release leukotriene B4, TNF-α, IL-8, IL-10, endothelin-1, etc.; hence bronchiectasis has often been viewed as having Th1 inflammatory patterns. Inflammatory factors and inflammatory mediators further promote the infiltration of inflammatory cells, causing the “cascade effect” of inflammation. Neutrophil elastase appears to be a key marker. It is now, however, recognized that approximately 20% of patients with bronchiectasis also have significantly elevated sputum eosinophils and Th2 markers (17) (Figure 2).
Figure 2
Pathological developmental process of bronchiectasis. The major pathological changes in the bronchiectating airway include mucus hypersecretion and/or blockage, bacterial colonization (including biofilm formation), mucociliary dysfunction, smooth muscle proliferation, and infiltration of inflammatory cells.
Initial stimulus factors vary from person to person; however, in nearly 40% of people, the cause is unknown (18, 19). Postinfectious bronchiectasis (such as after severe respiratory viral infection or pneumonia) is the most common type. Other causes include autoimmune diseases, chronic obstructive pulmonary disease (COPD), asthma, tuberculosis, fungal infections and genetic disorders (20). Genetic disorders encompass a range of conditions, such as cystic fibrosis, Mounier–Kuhn syndrome, primary ciliary dyskinesia and alpha-1 antitrypsin deficiency. Within the realm of autoimmune diseases, patients may suffer from rheumatoid arthritis, Sjögren’s syndrome, or inflammatory bowel disease; thus, these patients may also present to rheumatologists and gastroenterologists. Furthermore, patients with immune deficiency syndromes, particularly those with common variable immunodeficiency or human immunodeficiency virus infection, may also be at risk for developing bronchiectasis (21). Sequencing the genomes of individuals suffering from idiopathic bronchiectasis might provide a deeper understanding of disease development and reveal possibilities for early intervention.
3 Treatment strategy for bronchiectasis
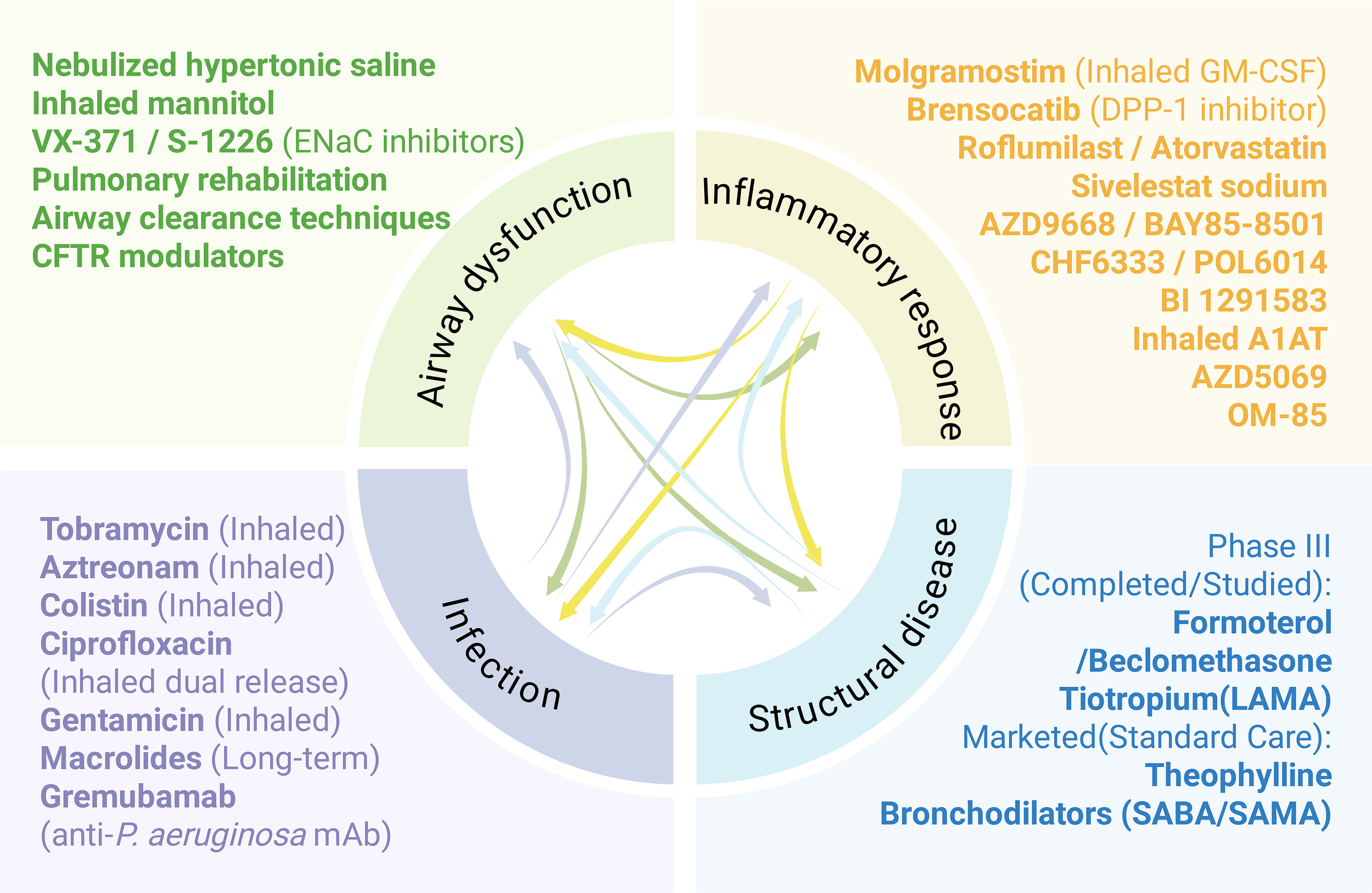
The symptoms associated with bronchiectasis are complex and varied, necessitating a holistic and individualized treatment approach. Given that recurrent respiratory infections, ongoing inflammation, damage to the airways and mucociliary dysfunction represent the four primary elements of the detrimental cycle observed in individuals with bronchiectasis, each pathophysiological step influences the others. This process can be more effectively illustrated via the concept of vortex (16) (Figure 3). The vortex model may elucidate why a single treatment, such as antibiotics or anti-inflammatory drugs, yields only modest improvements in the clinical outcomes of bronchiectasis. For example, rather than interrupting the vicious cycle that could be anticipated to halt disease progression, antibiotics focus mainly on a single element of the cycle, suggesting that inflammation and lung injury might result or persist due to additional triggers. Pharmacological interventions targeting numerous pathways could be effective for the treatment of bronchiectasis. Expectorants work on the mucus present on the surface of airways, increasing osmotic pressure, promoting mucus hydration, and facilitating expectoration (22). Antibiotics can reduce the bacterial load in airways or assist in eliminating pathogenic bacteria such as Pseudomonas aeruginosa, thereby inhibiting airway inflammation (23). Additionally, anti-inflammatory drugs can mitigate inflammation by targeting neutrophils and potentially eosinophils (Figure 3). The following sections introduce the latest advancements in drugs that target various pathological mechanisms.
Figure 3
The drug development pipeline for bronchiectasis is categorized by various aspects of the ‘vicious spiral’ model of pathogenesis.
3.1 Anti-inflammatory drugs
The primary cell type involved in the inflammatory process of bronchiectasis is neutrophils, although eosinophils, lymphocytes and macrophages also contribute significantly to the disease (24, 25). Patients with bronchiectasis exhibit a chronic inflammatory reaction in the airways characterized by the accumulation of neutrophils and the subsequent secretion of various inflammatory mediators (26). Upon activation, many neutrophils release a range of proteases, reactive oxygen species, cytokines, and other mediators, leading to significantly elevated levels of inflammatory mediators in the sputum of these patients (27–29). Owing to the significant link between neutrophilic inflammation and bacterial infections, focusing on neutrophils has become a potential treatment strategy for individuals who suffer from recurrent bronchiectasis episodes; this method is presently being explored in clinical studies. Additionally, various methods for suppressing inflammation through immune regulation have yielded positive outcomes. A summary of these treatments is provided below.
3.1.1 Targeting neutrophils
Neutrophil elastase (NE) has the potential to serve as a direct biomarker for neutrophilic inflammation in predicting the risk of future exacerbations (24), although it is not yet widely utilized in daily clinical practice. As a major protease with significant hydrolytic activity, NE plays a crucial role in the progression of bronchiectasis. Studies have confirmed that NE is overexpressed in bronchiectasis, representing a key risk factor for the development of structural lung damage and decreased lung function (30). Additionally, NE is associated with airway mucus hypersecretion and impaired mucociliary clearance (31), both of which are critical risk factors for bacterial colonization and contribute to the foundation of chronic inflammation (32). NE plays a crucial role in the onset and progression of bronchiectasis through various pathways. Even a single NE inhibition strategy can impact the entire complex protease network. As a result, NE is considered a potential therapeutic target, and several NE inhibitors have been developed. Sivelestat sodium is the only NE inhibitor currently approved for clinical use and is utilized primarily in patients with COVID-19 in China (33). However, there is a notable lack of relevant research concerning its application in bronchiectasis. AZD9668, an oral NE inhibitor, has undergone a phase II trial that failed to demonstrate a significant difference in sputum NE activity between the experimental and observation groups post-treatment. Nonetheless, the study indicated that lung function in the experimental group improved somewhat, and sputum inflammatory marker levels tended to decrease (34). BAY85-8501, which is highly selective and specific for NE, did not result in any adverse reactions during the phase I trial. In the phase IIa clinical trial, two groups of bronchiectasis patients were administered either a placebo or BAY85-8501. The findings revealed no significant changes in sputum NE activity or lung function between the two groups following treatment; however, blood NE activity in the treatment group decreased significantly (35). Researchers have hypothesized that the oral administration method may lead to insufficient drug concentrations in the lungs, contributing to low clinical efficacy. Consequently, they developed an inhaled NE inhibitor. CHF6333 is an NE inhibitor delivered as a dry powder inhaler and offers opportunity to assess the importance of higher lung concentrations than serum. In vitro experiments have demonstrated that CHF6333 exhibits high selectivity for NE (36). A phase Ib trial of CHF6333 in patients with cystic fibrosis and bronchiectasis concluded in 2021; however, no data are currently available (NCT04010799: 2019-07-08; US Clinical Trials Registry). Additionally, a new clinical trial is currently recruiting participants (NCT06166056). POL6014 is a novel inhaled inhibitor that has undergone phase I trials, which indicated that it is safe, well tolerated, and achieves high drug concentrations in the lungs. Furthermore, it has been shown to inhibit NE activity in sputum from patients with cystic fibrosis following a single administration (37).
Serendex has developed a nebulizer containing Molgramostim, a non-glycosylated form of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF). While most therapies aim to downregulate neutrophils to prevent protease damage, inhaled GM-CSF aims to restore or enhance neutrophil bactericidal function, which may be beneficial for clearing persistent infections like NTM A phase 1 randomized, double-blind, placebo-controlled clinical trial (NCT02468908) involving healthy adults has been completed in the UK. This trial evaluated the safety and tolerability of both single ascending doses and multiple ascending doses of Molgramostim in humans. The results indicated that inhaled Molgramostim was well tolerated by the subjects, demonstrated minimal absorption into the bloodstream, and significantly affected white blood cell counts within the normal range. The most commonly reported adverse event was cough. Patients with bronchiectasis are particularly susceptible to non-tuberculous mycobacterial pulmonary disease (NTM-PD). Additionally, an open-label, single arm pilot trial (OPTIMA) was designed to assess the efficacy and safety of inhaled GM-CSF (Molgramostim nebulized solution) in treating NTM-PD in adults with non-CF bronchiectasis (38). Overall, the study found that the study drug was safe and reasonably well tolerated, suggesting that treatment with inhaled Molgramostim may represent a promising therapeutic option.
Although these data have not fully demonstrated the exact efficacy of NE inhibitors in the treatment of bronchiectasis, researchers continue to explore and optimize the therapeutic strategies for NE inhibitors, including the development of inhaled NE inhibitors to increase the concentration of the drug in the lungs and thus improve the clinical efficacy. Currently, the development of multifunctional drugs that not only block the signaling pathways associated with NE but also possess anti-protease and anti-inflammatory properties and promote airway repair is considered a promising avenue for future research.
3.1.2 Cathepsin C inhibition
A different strategy, as opposed to the conventional inhibition of NE, includes the use of medications that focus on the activation of serine proteases within the bone marrow. Cathepsin C (CTSC), which is also referred to as DPP-1, functions as a lysosomal cysteine protease that plays a crucial role in the maturation of neutrophils. This enzyme is responsible for the cleavage of several key proteins, including NE, cathepsin G, and proteinase 3, during the activation process of neutrophils. As neutrophils mature, the activated serine proteases that they produce are subsequently packaged into granules within these cells. Once mature neutrophils enter the systemic circulation, they are able to carry out their functions in the immune response (39). Consequently, the implementation of CTSC inhibitors has the potential to impede the activity of neutrophil serine proteases within airways, thereby possibly influencing inflammatory responses in these regions. As of June 2020, the U.S. FDA has designated brensocatib, a CTSC inhibitor, as a breakthrough therapy for adult non-cystic fibrosis bronchiectasis. Brensocatib, an oral reversible DPP-1 inhibitor, indirectly inhibits NE activity by blocking neutrophil serine protease activation. A phase II clinical trial for bronchiectasis revealed that patients receiving oral treatment with varying doses of brensocatib experienced a significant reduction in sputum NE activity, as well as an extended time to the first exacerbation, suggesting that brensocatib may offer clinical benefits in reducing inflammation and preventing exacerbations (12). The drug has completed the Phase III development for the treatment of bronchiectasis (NCT04594369). The Phase 3 ASPEN trial results, published in the New England Journal of Medicine in 2025, demonstrated that brensocatib significantly reduced the annual rate of pulmonary exacerbations and prolonged the time to the first exacerbation compared to placebo (40). BI 1291583 is a novel, highly effective and selective DPP-1 inhibitor that is currently being investigated as a potential disease-modifying therapy in patients with bronchiectasis (41). Results from recent phase II trials showed that treatment with BI 1291583 can alleviate deterioration in adult patients with bronchiectasis (NCT05238675) (42). Following these promising results, several additional trials are now in progress (NCT05865886, NCT05846230). HSK31858 Is an oral, reversible DPP1 inhibitor developed by Haisco, being tested for the treatment of noncystic fibrosis bronchiectasis. HSK31858 demonstrated good safety and tolerability in phase I clinical trials (NCT05663593) and dose dependently suppressed whole blood NE activity. The phase II of the results have not yet been fully made public and it is currently in phase III clinical trials (NCT06660992). In addition, there are several other DPP-1 inhibitors currently in development. Overall, these DPP-1 inhibitors, which act by blocking the maturation and activation of neutrophil serine proteases within the bone marrow, are in different stages of clinical trials. Most have yielded promising results, offering potential new systemic options for reducing the protease burden in bronchiectasis.
Distinct from the upstream mechanism of DPP-1 inhibition, alpha 1-antitrypsin (A1AT, also known as alpha 1-protease inhibitor) functions as a direct, competitive inhibitor of neutrophil elastase (NE). It is currently being investigated for its potential to directly neutralize active proteases in the airways, mitigate inflammation, and potentially enhance neutrophil function (NCT05582798). This direct protease-inhibition strategy provides a complementary approach to targeting the inflammatory cascade in patients with bronchiectasis.
3.1.3 Inhibiting chemotaxis
A different strategy to alleviate neutrophilic inflammation focuses on directly reducing the influx of neutrophils into affected tissues. The chemokine receptor CXCR2 is pivotal in this process, as its activation by chemokines like CXCL1 and CXCL8 facilitates neutrophil migration. Research has demonstrated that CXCR2 antagonism, as exemplified by the oral CXCR2 antagonist AZD5069 in a clinical trial targeting CXCR1, effectively reduces neutrophil recruitment to the lungs without impairing their immune functions. Specifically, in a study with bronchiectasis patients receiving AZD5069 at 80 mg twice daily for 28 days, a significant 69% reduction in sputum neutrophil counts was observed compared to a placebo control group (43). While exacerbations were reported in both groups without significant difference, four cases of treatment discontinuation due to infection (one pneumonia and three bronchiectasis exacerbations) were noted in the AZD5069 group. Additional studies with CXCR2 antagonists in healthy volunteers and nonhuman primates demonstrated a rise in in blood cytokines, including CXCL1, CXCL8 and CXCR2 ligands, post-treatment, though the clinical significance of this increase is unclear. Furthermore, the 28-day treatment duration may be insufficient to assess all clinically significant effects, highlighting the need for larger-scale studies. Notably, other CXCR2-targeting compounds did not yield positive results in clinical trials (NCT03250689). Alternatively, Roflumilast, a phosphodiesterase 4 (PDE-4) inhibitor, reduces PDE-4 production, suppresses neutrophil chemotaxis and migration, and promotes cell apoptosis for anti-inflammatory effects (44). Currently, its anti-inflammatory effect in stable noncystic bronchial fibrosis is being evaluated in a phase II trial for 12 weeks (NCT04322929) in single-arm, open label, study of 42 patients; no results are available yet.
3.1.4 Targeting eosinophils
Approximately 20% of patients with bronchiectasis, excluding those with asthma, allergic bronchopulmonary aspergillosis, or chronic sinusitis with nasal polyps, exhibit eosinophilia (defined as > 3% or 300/μL), a condition referred to as eosinophilic bronchiectasis (45). Notably, eosinophilic inflammation in bronchiectasis does not imply the absence of neutrophil inflammation (46). The precise role of eosinophils in these patients remains unclear. It is hypothesized that eosinophils may possess bactericidal and antiviral properties against the pathogenic microorganisms commonly associated with bronchiectasis. This study revealed a correlation between blood eosinophil levels and the microbiome. Elevated eosinophil levels are associated with accelerated progression of Pseudomonas aeruginosa infection, whereas low eosinophil levels are predictive of severe bronchiectasis and an increased risk of mortality (45), thereby establishing new indicators for disease subtypes. Patients diagnosed with eosinophilic bronchiectasis frequently require inhaled corticosteroids (47); however, the role of Th2-dominant (eosinophilic) inflammation is becoming increasingly recognized (45). Consequently, other anti-Th2 drug treatments have demonstrated greater efficacy (48). In addition, benralizumab is an interleukin-5 receptor-directed cytolytic monoclonal antibody that induces direct, rapid, and massive eosinophil depletion through antibody-dependent cytotoxic activity (49). Retrospective studies have shown a certain relieving effect of benralizumab on bronchiectasis (50).
3.1.5 Vaccine
Commonly used injectable vaccines include the influenza vaccine and the pneumococcal vaccine. Influenza vaccination can prevent secondary lung infections caused by the influenza virus. Although there is a lack of robust specific evidence regarding the benefits of the influenza vaccine for patients with bronchiectasis, indirect evidence suggests that annual influenza vaccination can reduce mortality and morbidity and reduce healthcare costs in high-risk groups (51). A randomized controlled study demonstrated that the administration of the 23-valent pneumococcal vaccine can decrease the frequency of acute exacerbations (52). Furthermore, a study conducted by Japanese researchers involving 105 patients with chronic respiratory diseases, including 20 patients with bronchiectasis, revealed that vaccination with both the influenza vaccine and the 23-valent pneumococcal vaccine can prevent lower respiratory tract infections in these patients (53). Consequently, the bronchiectasis guidelines from the United Kingdom and Saudi Arabia, along with the expert consensus in China, recommend vaccination for patients to mitigate the onset of bronchiectasis (54, 55).
3.1.6 Bacterial lysate
The bacterial lysate OM-85 BV is an immunomodulator widely utilized to prevent acute attacks or exacerbations of COPD, chronic bronchitis, respiratory infections, chronic sinusitis, and other related conditions (56). The primary components of OM-85 BV include freeze-dried lysates from eight of the most prevalent pathogenic bacteria associated with respiratory tract infections, namely, Haemophilus influenzae, Diplococcus pneumoniae, Klebsiella pneumoniae, Klebsiella odorifera, Staphylococcus aureus, Viridans streptococci, Streptococcus pyogenes, and Neisseria catarrhalis. Currently, small-sample observational studies have confirmed the preventive effects of Panfusu in patients with bronchiectasis. Furthermore, a double-blind, placebo-controlled randomized clinical trial assessing the efficacy of OM-85 BV in preventing acute attacks in Chinese patients with bronchiectasis is underway (NCT01968421). The results of this study aim to substantiate its preventive effects on acute bronchiectasis attacks through evidence-based medicine (57).
3.1.7 Statins
Recent studies have confirmed the immunomodulatory effects of statins (58). Notably, high-dose atorvastatin has been shown to significantly reduce cough in bronchiectasis patients who do not have a Pseudomonas aeruginosa infection (59). Bedi et al. conducted a double-blind, crossover randomized controlled trial demonstrating that atorvastatin can alleviate patient symptoms by reducing systemic inflammation in individuals with bronchiectasis infected by Pseudomonas aeruginosa (60). (NCT01299194). However, this study had several limitations, including low statistical power for secondary efficacy endpoints, a short study duration, and a lack of matching between the two groups. Despite these limitations, the results support the potential benefits of statins as anti-inflammatory agents in patients with chronic bronchiectasis infections. Nonetheless, longer and larger multicenter studies are necessary to further validate these findings.
3.2 Anti-infectious drugs
Pseudomonas aeruginosa (PA) is one of the primary pathogenic bacteria affecting bronchiectasis patients, and its infection or colonization significantly impacts both the quality of life and prognosis of these patients (61–63). Research indicates that PA infection elevates the risk of all-cause mortality and disease progression and exacerbates psychological distress and cough symptoms (64–66). Several studies have revealed that patients with PA infection experience more extensive damage to their lung lobes and have poorer lung function (67–69). Prospective cohort studies have demonstrated that these patients face a significantly heightened risk of hospitalization (70). PA is adept at forming biofilms and is highly resistant to carbapenem antibiotics. Furthermore, multidrug resistance is prevalent, which complicates treatment efforts.
Since 2000, numerous studies have investigated the clinical application of inhaled antibiotics in patients experiencing frequent exacerbations of bronchiectasis. These studies typically focus on bronchiectasis associated with the long-term use of inhaled antibiotics in the treatment of cystic fibrosis. However, the overall results of these studies have not met expectations. Specifically, while inhaled gentamicin and inhaled colistin have demonstrated potential, clinical trials involving inhaled tobramycin, aztreonam, and ciprofloxacin (both dry powder and liposomal formulations) have failed to achieve the predefined study endpoints (71–77). These unsatisfactory outcomes may stem from limitations in study design, the implementation of cyclic treatment regimens, or the inherent heterogeneity of bronchiectasis itself (78). To date, none of these inhaled antibiotic treatments have received formal approval from regulatory agencies, despite some indications of clinical promise.
Retrospective studies on Pseudomonas aeruginosa have reported initial eradication rates of bronchiectasis via various regimens, including oral, inhaled, and intravenous antibiotics, ranging from 52% to 80%. However, these eradication rates decline significantly after one year, underscoring the importance of timely recognition and clinical intervention (79–81). It remains unclear whether this longitudinal decrease in eradication success reflects treatment-related suppression of infection or whether reinfection occurs following successful eradication. Notably, patient groups with higher bacterial loads have exhibited more favorable responses to inhaled antibiotic therapy (82). Consequently, the British Thoracic Society and the European Respiratory Society recommend in their guidelines that long-term inhaled antibiotic treatment should be considered for patients with chronic Pseudomonas aeruginosa infections who experience three or more exacerbations per year (83, 84). A small randomized controlled trial involving 35 patients further corroborated the effectiveness of this strategy; two weeks of intravenous ceftazidime or tobramycin was administered, followed by three months of aerosolized tobramycin. One year later, bacterial culture results were consistently negative in 55% of patients (12). However, the efficacy of this approach against Pseudomonas aeruginosa and other emerging pathogens requires further validation.
When bronchiectasis deteriorates, targeted systemic antibiotics are typically introduced, and the choice of administration route (oral or intravenous) must consider the severity of the current condition, the resistance profile of the pathogen, and the potential side effects associated with the antibiotic. International guidelines categorize exacerbations that may benefit from antibiotic treatment and provide management recommendations (11); however, the optimal duration of treatment remains inadequately defined, with a 14-day course generally suggested. Nonetheless, there is ongoing debate regarding treatment duration, particularly for adult patients involved in intravenous antibiotic trials, and the advantages and disadvantages of 7-day versus 14-day treatment regimens warrant further investigation (85). The National Institute for Health and Care Research’s SBIVA study (ISRCTN14057228) will assess the feasibility of shorter treatment durations across the UK.
For patients experiencing significant daily symptoms and frequent attacks (three or more per year), it is particularly important to implement additional treatment strategies aimed at improving quality of life and preventing further lung damage. Meta-analyses indicate that the use of macrolide antibiotics can reduce the frequency of attacks, extend the time to the next attack, and increase patients’ overall quality of life (86). Although the precise mechanism of action remains incompletely understood, macrolides may exert their effects by inhibiting quorum sensing in Pseudomonas aeruginosa (87). Commonly employed dosing regimens include azithromycin 500 mg three times per week or 250 mg daily, with studies demonstrating that macrolides are generally safe and associated with acceptable side effects over a one-year period (88). Furthermore, the BOAT trial (Bronchiectasis: Optimizing Azithromycin prevention Treatment to reduce exacerbations; ISRCTN59922389), a pragmatic phase IV randomized controlled trial, is currently investigating the optimization of long-term azithromycin treatment to further refine prevention strategies for exacerbations. Nonetheless, owing to the risks of drug resistance and potential gastrointestinal, cardiac, and auditory side effects, macrolides should be administered with caution, particularly in patients with nontuberculous mycobacterial infections or when such infections have not been conclusively ruled out.
In summary, when considering long-term macrolide therapy or inhaled antibiotic therapy, it is essential to comprehensively evaluate individual patient disease characteristics, drug contraindications, adverse reactions, and other relevant factors to optimize therapeutic outcomes.
3.3 Airway clearance therapies
Under normal circumstances, airway mucus is secreted by submucosal glands, goblet cells, and airway epithelial cells, with water being the primary component. Although the mucoprotein (MUC) content is low, it plays a crucial role in the viscoelasticity of mucus. During bronchiectasis, persistent inflammation in the airways occurs, leading to the stimulation of goblet cell differentiation and proliferation by inflammatory cells and mediators, which is often accompanied by submucosal gland hypertrophy. The expression of MUC5AC and MUC5B is upregulated, with MUC5AC influencing the thickness of the mucus gel layer and viscosity affecting the consistency of the mucus sol layer. The hypersecretion of airway mucus is common throughout disease progression and significantly impacts patient prognosis (18, 89–92). In patients with bronchiectasis, airway mucus becomes highly concentrated, suggesting that intervention in mucus hypersecretion may represent a viable target for drug treatment. Airway clearance therapies encompass nonpharmacological strategies, mucus-activating treatments, and pulmonary rehabilitation combined with exercise. The primary objective of these interventions is to mobilize secretions, thereby reducing coughing and dyspnea while preventing further airway damage. Some of the relevant treatments are summarized below.
3.3.1 Drug treatment strategy
Published guidelines identify airway clearance as a critical treatment for bronchiectasis, although the supporting evidence remains relatively weak (83, 84, 93). Research on epithelial dysfunction in bronchiectasis is limited compared with that in cystic fibrosis, where CFTR corrector therapy has shown promising results (94). CFTR mutations are controversial noncystic fibrosis bronchiectasis, with studies showing varying frequencies of these mutations (95–97). However, CFTR dysfunction may play a role in some cases, making CFTR correction a potential treatment option (98). Inhibitors of the epithelial sodium channel (ENaC) represent a potential therapeutic strategy to facilitate mucus rehydration by hindering excessive sodium absorption. While the rationale for ENaC inhibition has been extensively explored in cystic fibrosis (CF) due to its role in CFTR-dependent secretion, it is critical to distinguish these mechanisms from the pathophysiological environment of non-CF bronchiectasis. Clinical evidence in the bronchiectasis population remains limited; for instance, a phase II clinical trial of the ENaC inhibitor VX-371 failed to demonstrate significant improvements in the percentage of predicted forced expiratory volume (ppFEV1), although the treatment was generally well-tolerated. Similar challenges in demonstrating clear clinical benefits have been observed with other agents like BI 1265162, contributing to ongoing debates regarding ENaC as a viable therapeutic target in these populations. A major breakthrough in the management of airway clearance was provided by the CLEAR trial (2025) (99). This landmark study evaluated the efficacy of hypertonic saline versus carbocisteine specifically in patients with bronchiectasis; however, the results demonstrated no significant benefit in exacerbation reduction or quality of life with either the systemic mucolytic or nebulized hypertonic saline. By providing high-quality comparative data for non-CF cohorts, the CLEAR trial underscores the necessity of moving toward evidence-based, precision airway clearance therapies tailored specifically for the bronchiectasis population.
In addition to CFTR and ENaC-related treatments, several novel therapies are currently under investigation. ARINA-1 is a new nebulized product composed of ascorbic acid, glutathione, and bicarbonate that operates through a mechanism independent of CFTR, offering a potential new treatment for mucus clearance disorders in cystic fibrosis (100). Recently, a phase IIa clinical trial of ARINA-1 demonstrated positive results in adult subjects with noncystic fibrosis bronchiectasis (NCFBE) characterized by excess mucus and cough (NCT05495243). Hypertonic saline delivered via nebulization serves as a mucoactive therapy and has shown promising advantages for individuals diagnosed with bronchiectasis. In a limited-scale investigation, researchers reported that daily nebulization of a 7% saline solution led to improvements in lung function and quality of life (101). Furthermore, inhaled dry powder mannitol is utilized in the treatment of cystic fibrosis and has been shown to enhance ciliary clearance, although its precise mechanism of action remains unclear (102, 103). Another innovative biophysical therapeutic agent, S-1226, employs carbon dioxide (CO2)-rich air in combination with aerosolized perfluorocarbons (PFOB) to promote airway dilation, enhance mucus clearance, and improve blood oxygenation. Preliminary efficacy results indicate that S-1226 may positively impact the management of cystic fibrosis-related lung disease; however, further clinical trials are necessary to validate these findings (NCT03903913). Finally, CSL-787 is a nebulized immunoglobulin formulation currently in development for the treatment of noncystic fibrosis bronchiectasis (NCT04643587). The emergence of these new therapies offers renewed hope for the management of bronchiectasis and cystic fibrosis.
3.3.2 Nonpharmacological methods
Nonpharmacological methods of airway clearance include active recirculation breathing techniques, autogenic drainage (which involves a controlled expiration rate and depth to facilitate secretion mobilization), slow expiration with the glottis open in the lateral decubitus position, and the application of positive expiratory pressure. Additionally, oscillation devices and high-frequency chest wall oscillation are utilized (21). The advantages of these treatments lie in their independence for patients, as they are generally safe and straightforward to learn (104). Observational studies focusing on pulmonary rehabilitation and exercise programs carried out in both Australia and Italy have demonstrated significant improvements in various physiological measurements, including the 6-minute walk distance and quality of life related to health (105, 106).
4 Bronchodilators
Some patients with bronchiectasis present clinically with airflow limitation or dyspnea due to structural damage (107). Current guidelines from the European Respiratory Society and the British Thoracic Society recommend trials of bronchodilator therapy (83, 84), although robust evidence supporting their efficacy is lacking. The effectiveness of theophylline as a bronchodilator remains unproven. A completed Phase 4 clinical trial evaluated the safety and clinical effectiveness of theophylline for treating bronchiectasis that is not related to cystic fibrosis; however, the findings have not yet been released (NCT01684683). Consequently, there is currently no evidence to support or refute the use of theophylline in treating bronchiectasis. Studies have indicated that other bronchodilator treatments can enhance lung function (108); for example, the combination therapy of formoterol and budesonide can significantly alleviate symptoms and enhance the quality of life for patients (109). A two-way crossover trial indicated that tiotropium improved forced expiratory volume in 1 second, although it did not lead to a reduction in exacerbation frequency (110). Additionally, a Phase III clinical trial evaluating the combination of formoterol and beclomethasone in the treatment of bronchiectasis is currently recruiting participants (NCT03846570). While the sample sizes of existing trials are relatively small, they provide preliminary evidence of the benefits of bronchodilators in improving lung function and alleviating dyspnea. However, it remains unclear how effective they are in diminishing the occurrence of exacerbations and improving quality of life. Future studies are needed to better identify minimal clinically important differences in key lung function variables and phenotypic characteristics that predict treatment response. The difficulty of establishing the efficacy of bronchodilators in this heterogeneous population is further exemplified by the premature termination of a recent major clinical trial (111). This termination underscores the ongoing challenges in recruitment and the potential lack of clinical benefit for unselected patients, reinforcing the necessity for precision medicine to identify specific phenotypes that may respond to bronchodilator therapy.
5 Conclusion
While some progress has been made in the research and treatment strategies for bronchiectasis, no officially licensed specific treatment has yet been developed. To address this gap, it is imperative to pursue breakthroughs through an increased number of randomized controlled trials. Specifically, further investigations are needed to identify which patient groups benefit from physical chest clearance, mucus-modulating treatments, and regimens such as inhaled corticosteroids, long-acting beta-agonists, anticholinergics, and anti-inflammatory and antiinfective treatments. This will allow for more precise targeting of patient groups and optimization of the effectiveness of clinical trials. Additionally, further exploration and research are necessary to determine the optimal route of administration and duration of these treatments.
5.1 Search strategy
Literature was systematically searched in databases including PubMed and international clinical trial registries (Controlled-Trials.com and ClinicalTrials.gov) from their inception through December 2025. This review focused on articles related to bronchiectasis regarding incidence, therapy, drugs, healthcare, etiologies, and pathobiological mechanisms, excluding those without radiological confirmation. Only adult studies were considered, except for occasional pediatric data in high-risk population studies.
Statements
Author contributions
LY: Writing – original draft. H-rZ: Methodology, Investigation, Writing – original draft. X-hJ: Writing – original draft. JD: Writing – review & editing, Writing – original draft. TJ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was funded by the 2025 Zhejiang Provincial College Student Science and Technology Innovation Program (Xinmiao Talent Program) (No. 2025R480A021), and the 2024 Basic Research Special Fund of Jinhua Central Hospital (No. JY2024-6-04), the Key Discipline of Respiratory and Critical Care Medicine of Jinhua City (No. JYZDXK-2023-10).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
QuintJKMillettERJoshiMNavaratnamVThomasSLHurstJRet al. Changes in the incidence, prevalence and mortality of bronchiectasis in the uk from 2004 to 2013: A population-based cohort study. Eur Respir J. (2016) 47:186–93. doi: 10.1183/13993003.01033-2015
2
HenkleEChanBCurtisJRAksamitTRDaleyCLWinthropKL. Characteristics and health-care utilization history of patients with bronchiectasis in us medicare enrollees with prescription drug plans, 2006 to 2014. Chest. (2018) 154:1311–20. doi: 10.1016/j.chest.2018.07.014
3
PhuaHPLimWYGanesanGYoongJTanKBAbisheganadenJAet al. Epidemiology and economic burden of bronchiectasis requiring hospitalisation in Singapore. ERJ Open Res. (2021) 7(4):00334–2021. doi: 10.1183/23120541.00334-2021
4
RingshausenFCRademacherJPinkIde RouxAHicksteinLPlonerTet al. Increasing bronchiectasis prevalence in Germany, 2009-2017: A population-based cohort study. Eur Respir J. (2019) 54(6):1900499. doi: 10.1183/13993003.00499-2019
5
LinJLXuJFQuJM. Bronchiectasis in China. Ann Am Thorac Soc. (2016) 13:609–16. doi: 10.1513/AnnalsATS.201511-740PS
6
MonteagudoMRodriguez-BlancoTBarrechegurenMSimonetPMiravitllesM. Prevalence and incidence of bronchiectasis in catalonia, Spain: A population-based study. Respir Med. (2016) 121:26–31. doi: 10.1016/j.rmed.2016.10.014
7
ChalmersJDAlibertiSPolverinoEVendrellMCrichtonMLoebingerMet al. The embarc european bronchiectasis registry: Protocol for an international observational study. ERJ Open Res. (2016) 2(1):00081–2015. doi: 10.1183/23120541.00081-2015
8
ChangABBoydJBushAGrimwoodKHillATPowellZet al. Children's bronchiectasis education advocacy and research network (child-bear-net): An ers clinical research collaboration on improving outcomes of children and adolescents with bronchiectasis. Eur Respir J. (2021) 58(4):2101657. doi: 10.1183/13993003.01657-2021
9
MazulovOHillATMarchantJ. Developments and priorities in bronchiectasis research. Lancet Respir Med. (2023) 11:669–70. doi: 10.1016/S2213-2600(23)00258-8
10
KellyCChalmersJDCrossinghamIRelphNFelixLMEvansDJet al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Rev. (2018) 3:CD012406. doi: 10.1002/14651858.CD012406.pub2
11
ChangABZacharasiewiczAGoyalVBoydJAlexopoulouEAlibertiSet al. European respiratory society statement for defining respiratory exacerbations in children and adolescents with bronchiectasis for clinical trials. Eur Respir J. (2022) 60(5):2200300. doi: 10.1183/13993003.00300-2022
12
ChalmersJDHaworthCSMeterskyMLLoebingerMRBlasiFSibilaOet al. Phase 2 trial of the dpp-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. (2020) 383:2127–37. doi: 10.1056/NEJMoa2021713
13
JihKYLinWYSohmaYHwangTC. Cftr potentiators: From bench to bedside. Curr Opin Pharmacol. (2017) 34:98–104. doi: 10.1016/j.coph.2017.09.015
14
MorrisonCBShafferKMArabaKCMarkovetzMRWykoffJAQuinneyNLet al. Treatment of cystic fibrosis airway cells with cftr modulators reverses aberrant mucus properties via hydration. Eur Respir J. (2022) 59(2):2100185. doi: 10.1183/13993003.00185-2021
15
ColePJ. Inflammation: A two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl. (1986) 147:6–15.
16
FlumePAChalmersJDOlivierKN. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet. (2018) 392:880–90. doi: 10.1016/S0140-6736(18)31767-7
17
BushAFlotoRA. Pathophysiology, causes and genetics of paediatric and adult bronchiectasis. Respirology. (2019) 24:1053–62. doi: 10.1111/resp.13509
18
ChalmersJDChangABChotirmallSHDharRMcShanePJ. Bronchiectasis. Nat Rev Dis Primers. (2018) 4:45. doi: 10.1038/s41572-018-0042-3
19
ChandrasekaranRMac AogáinMChalmersJDElbornSJChotirmallSH. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC pulmonary Med. (2018) 18:1–14. doi: 10.1186/s12890-018-0638-0
20
The Lancet Respiratory Medicine. New developments in bronchiectasis. Lancet Respir Med. (2023) 11:755. doi: 10.1016/S2213-2600(23)00304-1
21
O'DonnellAE. Bronchiectasis - a clinical review. N Engl J Med. (2022) 387:533–45. doi: 10.1056/NEJMra2202819
22
WilkinsonMSugumarKMilanSJHartACrockettACrossinghamI. Mucolytics for bronchiectasis. Cochrane Database Syst Rev. (2014) 2014:CD001289. doi: 10.1002/14651858.CD001289.pub2
23
Suarez-CuartinGHernandez-ArgudoMPereaLSibilaO. Long-term antibiotics in bronchiectasis. Semin Respir Crit Care Med. (2021) 42:606–15. doi: 10.1055/s-0041-1730945
24
ChalmersJDMoffittKLSuarez-CuartinGSibilaOFinchSFurrieEet al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. (2017) 195:1384–93. doi: 10.1164/rccm.201605-1027OC
25
ChoiHRyuSKeirHRGiamYHDickerAJPereaLet al. Inflammatory molecular endotypes in bronchiectasis: A european multicenter cohort study. Am J Respir Crit Care Med. (2023) 208:1166–76. doi: 10.1164/rccm.202303-0499OC
26
ChalmersJDElbornSGreeneCM. Basic, translational and clinical aspects of bronchiectasis in adults. Eur Respir Rev. (2023) 32(168):230015. doi: 10.1183/16000617.0015-2023
27
AngrillJAgustiCDe CelisRFilellaXRanoAElenaMet al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. (2001) 164:1628–32. doi: 10.1164/ajrccm.164.9.2105083
28
ChalmersJDHillAT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol. (2013) 55:27–34. doi: 10.1016/j.molimm.2012.09.011
29
KeirHRChalmersJD. Pathophysiology of bronchiectasis. Semin Respir Crit Care Med. (2021) 42:499–512. doi: 10.1055/s-0041-1730891
30
VoynowJAShinbashiM. Neutrophil elastase and chronic lung disease. Biomolecules. (2021) 11(8):1065. doi: 10.3390/biom11081065
31
OrianoMAmatiFGramegnaADe SoyzaAManteroMSibilaOet al. Protease-antiprotease imbalance in bronchiectasis. Int J Mol Sci. (2021) 22(11):5996. doi: 10.3390/ijms22115996
32
DomonHTeraoY. The role of neutrophils and neutrophil elastase in pneumococcal pneumonia. Front Cell Infect Microbiol. (2021) 11:615959. doi: 10.3389/fcimb.2021.615959
33
ZhangHZengJLiJGongHChenMLiQet al. Sivelestat sodium attenuates acute lung injury by inhibiting jnk/nf-kappab and activating nrf2/ho-1 signaling pathways. Biomol BioMed. (2023) 23:457–70. doi: 10.17305/bb.2022.8549
34
FazleenAWilkinsonT. The emerging role of proteases in α1-antitrypsin deficiency and beyond. ERJ Open Res. (2021) 7(4):00494–2021. doi: 10.1183/23120541.00494-2021
35
WatzHNagelschmitzJKirstenAPedersenFvan der MeyDSchwersSet al. Safety and efficacy of the human neutrophil elastase inhibitor bay 85–8501 for the treatment of non-cystic fibrosis bronchiectasis: A randomized controlled trial. Pulm Pharmacol Ther. (2019) 56:86–93. doi: 10.1016/j.pupt.2019.03.009
36
CrocettiLQuinnMTSchepetkinIAGiovannoniMP. A patenting perspective on human neutrophil elastase (hne) inhibitors (2014-2018) and their therapeutic applications. Expert Opin Ther Pat. (2019) 29:555–78. doi: 10.1080/13543776.2019.1630379
37
BarthPBruijnzeelPWachASellier KesslerOHooftmanLZimmermannJet al. Single dose escalation studies with inhaled pol6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J Cyst Fibros. (2020) 19:299–304. doi: 10.1016/j.jcf.2019.08.020
38
ThomsonRMLoebingerMRBurkeAJMorganLCWatererGWGanslandtC. Optima: An open-label, noncomparative pilot trial of inhaled molgramostim in pulmonary nontuberculous mycobacterial infection. Ann Am Thorac Soc. (2024) 21:568–76. doi: 10.1513/AnnalsATS.202306-532OC
39
GuarinoCHamonYCroixCLamortASDallet-ChoisySMarchand-AdamSet al. Prolonged pharmacological inhibition of cathepsin c results in elimination of neutrophil serine proteases. Biochem Pharmacol. (2017) 131:52–67. doi: 10.1016/j.bcp.2017.02.009
40
ChalmersJDBurgelP-RDaleyCLDe SoyzaAHaworthCSMaugerDet al. Phase 3 trial of the dpp-1 inhibitor brensocatib in bronchiectasis. New Engl J Med. (2025) 392:1569–81. doi: 10.1056/NEJMoa2411664
41
KreideweissSSchanzleGSchnappGVintonyakVGrundlMA. Bi 1291583: A novel selective inhibitor of cathepsin c with superior in vivo profile for the treatment of bronchiectasis. Inflammation Res. (2023) 72:1709–17. doi: 10.1007/s00011-023-01774-4
42
ChalmersJDShteinbergMMallMAO'DonnellAEWatzHGuptaAet al. Cathepsin c (dipeptidyl peptidase 1) inhibition in adults with bronchiectasis: Airleaf(r), a phase ii randomised, double-blind, placebo-controlled, dose-finding study. Eur Respir J. (2024) 65:2401551. doi: 10.1183/13993003.01551-2024
43
De SoyzaAPavordIElbornJSSmithDWrayHPuuMet al. A randomised, placebo-controlled study of the cxcr2 antagonist azd5069 in bronchiectasis. Eur Respir J. (2015) 46:1021–32. doi: 10.1183/13993003.00148-2015
44
KawamatawongT. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J Thorac Dis. (2017) 9:1144–54. doi: 10.21037/jtd.2017.03.116
45
ShoemarkAShteinbergMDe SoyzaAHaworthCSRichardsonHGaoYet al. Characterization of eosinophilic bronchiectasis: A european multicohort study. Am J Respir Crit Care Med. (2022) 205:894–902. doi: 10.1164/rccm.202108-1889OC
46
PollockJGoeminnePC. Eosinophils in bronchiectasis: A u-turn for bronchiectasis management. CHEST. (2023) 164:561–63. doi: 10.1016/j.chest.2023.05.016
47
AlibertiSSotgiuGBlasiFSaderiLPosadasTMartinez GarciaMA. Blood eosinophils predict inhaled fluticasone response in bronchiectasis. Eur Respir J. (2020) 56(2):2000453. doi: 10.1183/13993003.00453-2020
48
GuanWJOsculloGHeMZXuDYGomez-OlivasJDMartinez-GarciaMA. Significance and potential role of eosinophils in non-cystic fibrosis bronchiectasis. J Allergy Clin Immunol Pract. (2023) 11:1089–99. doi: 10.1016/j.jaip.2022.10.027
49
CrinerGJCelliBRBrightlingCEAgustiAPapiASinghDet al. Benralizumab for the prevention of copd exacerbations. N Engl J Med. (2019) 381:1023–34. doi: 10.1056/NEJMoa1905248
50
CampisiRNolascoSPelaiaCImpellizzeriPD'AmatoMPortacciAet al. Benralizumab effectiveness in severe eosinophilic asthma with co-presence of bronchiectasis: A real-world multicentre observational study. J Clin Med. (2023) 12(12):3953. doi: 10.3390/jcm12123953
51
McDonnellMJWardCLordanJLRutherfordRM. Non-cystic fibrosis bronchiectasis. QJM. (2013) 106:709–15. doi: 10.1093/qjmed/hct109
52
ChangCCSingletonRJMorrisPSChangAB. Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database Syst Rev. (2009) 2009:CD006316. doi: 10.1002/14651858.CD006316.pub3
53
SumitaniMTochinoYKamimoriTFujiwaraHFujikawaT. Additive inoculation of influenza vaccine and 23-valent pneumococcal polysaccharide vaccine to prevent lower respiratory tract infections in chronic respiratory disease patients. Intern Med. (2008) 47:1189–97. doi: 10.2169/internalmedicine.47.0799
54
Al-JahdaliHAlshimemeriAMobeireekAAlbannaASAl ShirawiNNWaliSet al. The saudi thoracic society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis. Ann Thorac Med. (2017) 12:135–61. doi: 10.4103/atm.ATM_171_17
55
PasteurMCBiltonDHillATBritish Thoracic Society. British thoracic society guideline for non-cf bronchiectasis. Thorax. (2010) 65 Suppl 1:i1–58. doi: 10.1136/thx.2010.136119
56
SolerMMutterleinRCozmaGSwiss-GermanOMSG. Double-blind study of om-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration. (2007) 74:26–32. doi: 10.1159/000093933
57
GaoJGaoXKongL. To investigate the prevention of om-85 on bronchiectasis exacerbations (iprobe) in chinese patients: Study protocol for a randomized controlled trial. Trials. (2014) 15:150. doi: 10.1186/1745-6215-15-150
58
KwakBMulhauptFMyitSMachF. Statins as a newly recognized type of immunomodulator. Nat Med. (2000) 6:1399–402. doi: 10.1038/82219
59
MandalPChalmersJDGrahamCHarleyCSidhuMKDohertyCet al. Atorvastatin as a stable treatment in bronchiectasis: A randomised controlled trial. Lancet Respir Med. (2014) 2:455–63. doi: 10.1016/S2213-2600(14)70050-5
60
BediPChalmersJDGrahamCClarkeADonaldsonSDohertyCet al. A randomized controlled trial of atorvastatin in patients with bronchiectasis infected with pseudomonas aeruginosa: A proof of concept study. Chest. (2017) 152:368–78. doi: 10.1016/j.chest.2017.05.017
61
WangHJiXBMaoBLiCWLuHWXuJF. Pseudomonas aeruginosa isolation in patients with non-cystic fibrosis bronchiectasis: A retrospective study. BMJ Open. (2018) 8:e014613. doi: 10.1136/bmjopen-2016-014613
62
GuanWJGaoYHXuGLinZYTangYLiHMet al. Aetiology of bronchiectasis in guangzhou, southern China. Respirology. (2015) 20:739–48. doi: 10.1111/resp.12528
63
ZhouYYWangYHHeSQWangWYWangXYLiDSet al. Gender differences in clinical characteristics of patients with non-cystic fibrosis bronchiectasis in different age groups in northern China. Clin Respir J. (2023) 17:311–19. doi: 10.1111/crj.13596
64
ChalmersJDAlibertiSFilonenkoAShteinbergMGoeminnePCHillATet al. Characterization of the "frequent exacerbator phenotype" in bronchiectasis. Am J Respir Crit Care Med. (2018) 197:1410–20. doi: 10.1164/rccm.201711-2202OC
65
AraujoDShteinbergMAlibertiSGoeminnePCHillATFardonTCet al. The independent contribution of pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J. (2018) 51(2):1701953. doi: 10.1183/13993003.01953-2017
66
FinchSMcDonnellMJAbo-LeyahHAlibertiSChalmersJD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. (2015) 12:1602–11. doi: 10.1513/AnnalsATS.201506-333OC
67
KwokWCHoJCMTamTCCIpMSMLamDCL. Risk factors for pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir Res. (2021) 22:132. doi: 10.1186/s12931-021-01729-5
68
DaviesGWellsAUDoffmanSWatanabeSWilsonR. The effect of pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J. (2006) 28:974–9. doi: 10.1183/09031936.06.00074605
69
Martinez-GarciaMAOsculloGPosadasTZaldivarEVillaCDobarganesYet al. Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin Microbiol Infect. (2021) 27:428–34. doi: 10.1016/j.cmi.2020.04.007
70
WangRDingSLeiCYangDLuoH. The contribution of pseudomonas aeruginosa infection to clinical outcomes in bronchiectasis: A prospective cohort study. Ann Med. (2021) 53:459–69. doi: 10.1080/07853890.2021.1900594
71
MurrayMPGovanJRDohertyCJSimpsonAJWilkinsonTSChalmersJDet al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. (2011) 183:491–9. doi: 10.1164/rccm.201005-0756OC
72
HaworthCSFowerakerJEWilkinsonPKenyonRFBiltonD. Inhaled colistin in patients with bronchiectasis and chronic pseudomonas aeruginosa infection. Am J Respir Crit Care Med. (2014) 189:975–82. doi: 10.1164/rccm.201312-2208OC
73
BarkerAFCouchLFielSBGotfriedMHIlowiteJMeyerKCet al. Tobramycin solution for inhalation reduces sputum pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. (2000) 162:481–5. doi: 10.1164/ajrccm.162.2.9910086
74
BarkerAFO'DonnellAEFlumePThompsonPJRuziJDde GraciaJet al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (air-bx1 and air-bx2): Two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med. (2014) 2:738–49. doi: 10.1016/S2213-2600(14)70165-1
75
AksamitTDe SoyzaABandelTJCriolloMElbornJSOperschallEet al. Respire 2: A phase iii placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. (2018) 51(1):1702053. doi: 10.1183/13993003.02053-2017
76
De SoyzaAAksamitTBandelTJCriolloMElbornJSOperschallEet al. Respire 1: A phase iii placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. (2018) 51(1):1702052. doi: 10.1183/13993003.02052-2017
77
HaworthCSBiltonDChalmersJDDavisAMFroehlichJGondaIet al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with pseudomonas aeruginosa (orbit-3 and orbit-4): Two phase 3, randomised controlled trials. Lancet Respir Med. (2019) 7:213–26. doi: 10.1016/S2213-2600(18)30427-2
78
SpencerSDonovanTChalmersJDMathioudakisAGMcDonnellMJTsangAet al. Intermittent prophylactic antibiotics for bronchiectasis. Cochrane Database Syst Rev. (2022) 1:CD013254. doi: 10.1002/14651858.CD013254.pub2
79
PietersABakkerMHoekRASAltenburgJvan WestreenenMAertsJet al. Predicting factors for chronic colonization of pseudomonas aeruginosa in bronchiectasis. Eur J Clin Microbiol Infect Dis. (2019) 38:2299–304. doi: 10.1007/s10096-019-03675-z
80
WhiteLMirraniGGroverMRollasonJMalinASuntharalingamJ. Outcomes of pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med. (2012) 106:356–60. doi: 10.1016/j.rmed.2011.11.018
81
VallieresETumeltyKTunneyMMHannahRHewittOElbornJSet al. Efficacy of pseudomonas aeruginosa eradication regimens in bronchiectasis. Eur Respir J. (2017) 49(4):1600851. doi: 10.1183/13993003.00851-2016
82
SibilaOLasernaEShoemarkAKeirHRFinchSRodrigo-TroyanoAet al. Airway bacterial load and inhaled antibiotic response in bronchiectasis. Am J Respir Crit Care Med. (2019) 200:33–41. doi: 10.1164/rccm.201809-1651OC
83
HillATSullivanALChalmersJDDe SoyzaAElbornSJFlotoARet al. British thoracic society guideline for bronchiectasis in adults. Thorax. (2019) 74:1–69. doi: 10.1136/thoraxjnl-2018-212463
84
PolverinoEGoeminnePCMcDonnellMJAlibertiSMarshallSELoebingerMRet al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. (2017) 50(3):1700629. doi: 10.1183/13993003.00629-2017
85
BediPCartlidgeMKZhangYTurnbullKDonaldsonSClarkeAet al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: A proof-of-concept randomised controlled trial. Eur Respir J. (2021) 58(6):2004388. doi: 10.1183/13993003.04388-2020
86
ChalmersJDBoersmaWLonerganMJayaramLCrichtonMLKaralusNet al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: An individual participant data meta-analysis. Lancet Respir Med. (2019) 7:845–54. doi: 10.1016/S2213-2600(19)30191-2
87
BurrLDRogersGBChenACHamiltonBRPoolGFTaylorSLet al. Macrolide treatment inhibits pseudomonas aeruginosa quorum sensing in non-cystic fibrosis bronchiectasis. An analysis from the bronchiectasis and low-dose erythromycin study trial. Ann Am Thorac Soc. (2016) 13:1697–703. doi: 10.1513/AnnalsATS.201601-044OC
88
HillAT. Macrolides for clinically significant bronchiectasis in adults: Who should receive this treatment? Chest. (2016) 150:1187–93. doi: 10.1016/j.chest.2016.08.1451
89
FirthJ. Respiratory medicine: bronchiectasis. Clin Med. (2019) 19:64–7. doi: 10.7861/clinmedicine.19-1-64
90
RamseyKAChenACHRadicioniGLourieRMartinMBroomfieldAet al. Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. (2020) 201:661–70. doi: 10.1164/rccm.201906-1219OC
91
LeeALBurgeATHollandAE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. (2015) 2015:CD008351. doi: 10.1002/14651858.CD008351.pub3
92
MuñozGde GraciaJBuxóMAlvarezAVendrellM. Long-term benefits of airway clearance in bronchiectasis: A randomised placebo-controlled trial. Eur Respir J. (2018) 51(1):1701926. doi: 10.1183/13993003.01926-2017
93
Martinez-GarciaMAMaizLOlveiraCGironRMde la RosaDBlancoMet al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol (Engl Ed). (2018) 54:79–87. doi: 10.1016/j.arbres.2017.07.015
94
Taylor-CousarJLRobinsonPDShteinbergMDowneyDG. Cftr modulator therapy: Transforming the landscape of clinical care in cystic fibrosis. Lancet. (2023) 402:1171–84. doi: 10.1016/S0140-6736(23)01609-4
95
CasalsTDe-GraciaJGallegoMDorcaJRodriguez-SanchonBRamosMDet al. Bronchiectasis in adult patients: An expression of heterozygosity for cftr gene mutations? Clin Genet. (2004) 65:490–5. doi: 10.1111/j.0009-9163.2004.00265.x
96
BienvenuTSermet-GaudelusIBurgelPRHubertDCrestaniBBassinetLet al. Cystic fibrosis transmembrane conductance regulator channel dysfunction in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. (2010) 181:1078–84. doi: 10.1164/rccm.200909-1434OC
97
KingPTFreezerNJHolmesPWHoldsworthSRForshawKSartDD. Role of cftr mutations in adult bronchiectasis. Thorax. (2004) 59:357–8. doi: 10.1136/thx.2003.020263
98
LambertJARajuSVTangLPMcNicholasCMLiYCourvilleCAet al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. (2014) 50:549–58. doi: 10.1165/rcmb.2013-0228OC
99
BradleyJMO'NeillBMcAuleyDFChalmersJDDe SoyzaAHillATet al. Hypertonic saline or carbocisteine in bronchiectasis. New Engl J Med. (2025) 393(16):1565–77. doi: 10.1056/NEJMoa2510095
100
AdewaleATFalk LibbyEFuLLenzieABoitetERBirketSEet al. Novel therapy of bicarbonate, glutathione, and ascorbic acid improves cystic fibrosis mucus transport. Am J Respir Cell Mol Biol. (2020) 63:362–73. doi: 10.1165/rcmb.2019-0287OC
101
KellettFRobertNM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med. (2011) 105:1831–5. doi: 10.1016/j.rmed.2011.07.019
102
NevittSJThorntonJMurrayCSDwyerT. Inhaled mannitol for cystic fibrosis. Cochrane Database Syst Rev. (2020) 5:CD008649. doi: 10.1002/14651858.CD008649.pub4
103
FlumePAAmelinaEDainesCLCharltonBLeadbetterJGuasconiAet al. Efficacy and safety of inhaled dry-powder mannitol in adults with cystic fibrosis: An international, randomized controlled study. J Cyst Fibros. (2021) 20:1003–09. doi: 10.1016/j.jcf.2021.02.011
104
O'NeillKO'DonnellAEBradleyJM. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology. (2019) 24:227–37. doi: 10.1111/resp.13459
105
OngHKLeeALHillCJHollandAEDenehyL. Effects of pulmonary rehabilitation in bronchiectasis: A retrospective study. Chron Respir Dis. (2011) 8:21–30. doi: 10.1177/1479972310391282
106
ZaniniAAielloMAdamoDCherubinoFZampognaESotgiuGet al. Effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: A retrospective analysis of clinical and functional predictors of efficacy. Respiration. (2015) 89:525–33. doi: 10.1159/000380771
107
ChalmersJDGoeminnePAlibertiSMcDonnellMJLonniSDavidsonJet al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. (2014) 189:576–85. doi: 10.1164/rccm.201309-1575OC
108
LeeSYLeeJSLeeSWOhY-M. Effects of treatment with long-acting muscarinic antagonists (lama) and long-acting beta-agonists (laba) on lung function improvement in patients with bronchiectasis: An observational study. J Thorac Dis. (2020) 13:169–77. doi: 10.21037/jtd-20-1282
109
Martinez-GarciaMASoler-CatalunaJJCatalan-SerraPRoman-SanchezPTorderaMP. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest. (2012) 141:461–68. doi: 10.1378/chest.11-0180
110
JayaramLVandalACChangCLLewisCTongCTufferyCet al. Tiotropium treatment for bronchiectasis: A randomised, placebo-controlled, crossover trial. Eur Respir J. (2022) 59(6):2102184. doi: 10.1183/13993003.02184-2021
111
WilsonNMortonMHomerTKonkothABJoyceRKershawAet al. Dual bronchodilators in bronchiectasis study: A randomised controlled trial. ERJ Open Res. (2025) 11(3):01079–2024. doi: 10.1183/23120541.01079-2024
Summary
Keywords
anti-infectious drugs, anti-inflammatory drugs, bronchiectasis, bronchodilators, therapy
Citation
Yu L, Zhu H-r, Jin X-h, Ding J and Jin T (2026) Therapies for bronchiectasis: mechanisms and potential effects. Front. Immunol. 17:1735598. doi: 10.3389/fimmu.2026.1735598
Received
30 October 2025
Revised
03 February 2026
Accepted
04 February 2026
Published
18 February 2026
Volume
17 - 2026
Edited by
Diego Kauffmann-Guerrero, LMU Munich University Hospital, Germany
Reviewed by
Anthony De Soyza, Newcastle University, United Kingdom
Susanne Nährig, LMU Munich University Hospital, Germany
Updates
Copyright
© 2026 Yu, Zhu, Jin, Ding and Jin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Jin, 784343509@qq.com; Jin Ding, jhdingjin@zju.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.