Abstract
The core mechanism of acupuncture therapy lies in converting local mechanical stimulation into systemic physiological regulatory effects. Building on this concept, this review highlights the central role of macrophages in this mechanotransduction event, mainly occurring in the acupoint(s). During acupuncture, the practitioner’s manipulation techniques, such as lifting-thrusting and twisting, cause significant mechanical stress at an acupoint through the entanglement and traction of collagen fibers through the acupuncture needle. This physical signal is transmitted through the extracellular matrix (ECM) and delivered to the mechanosensitive cells, such as fibroblasts and macrophages. Concurrently, while fibroblasts receive the mechanical stimuli, they also release alarmin proteins, such as interleukin-33 (IL-33), to further regulate macrophages’ activities. As a key mechanical sensing and effect unit, macrophages perceive mechanical signals through multiple pathways, including Piezo1, transient receptor potential vanilloid 4 (TRPV4) mechanically sensitive channels, the integrin family of mechanotransduction receptors, and podosomes on the cell body. These pathways promptly initiate intracellular Ca2 fluctuations and promote the Yes−associated protein (YAP) and transcriptional co−activator with PDZ−binding motif (TAZ) for their nuclear translocation, as well as induce other mechanisms, generating a cascade reaction to activate macrophages. After activation, macrophages effectively recruit neutrophils and monocytes by coordinating the chemokine network and dominate the resolution of inflammation and the initiation of tissue repair via dynamic polarization between M1 and M2 phenotypes. Additionally, they regulate T cell-mediated adaptive immune responses through antigen presentation and other means, and collaborate with fibroblasts to promote the remodeling and repair of the ECM. This article focuses on providing a systematic perspective on the cellular and molecular basis of acupuncture initiation through the response of macrophages to acupuncture signals and their regulation of the immune network.
1 Introduction
Acupuncture is a treasure of Chinese medicine. With a history of application spanning thousands of years, it has been recognized in 196 countries and regions around the world for its remarkable therapeutic effects and has become one of the most influential forms of traditional medical therapies (1). In 2010, UNESCO also included acupuncture in the Representative List of the Intangible Cultural Heritage of Humanity (2). Currently, acupuncture in clinical practice mainly falls into two types: manual acupuncture and electroacupuncture. Although both types rely on needle insertion into the acupoint, they differ significantly in their mechanisms of action, operational modes, and clinical application (3, 4). Manual acupuncture, the mainstream form of traditional acupuncture therapy, depends on the operator’s manual techniques to exert therapeutic effects. Its core mechanism lies in applying direct, fine mechanical stimulation to the subcutaneous tissue of the acupoint area through techniques such as twisting and lifting the filiform needle, thereby altering the structure and physical properties of the local ECM. These mechanical signals can further be transmitted to the cells attaching on the ECM, thereby regulating those cells’ arrangement, morphology, and functional state, and giving rise to the changes in the microenvironment. Ultimately, these effects lead to the modulation of immune regulation and neural conduction. On the other hand, electroacupuncture, a modern acupuncture method derived from manual acupuncture, enhances the stimulation to the subcutaneous tissue of the acupoint area by connecting the needle handle to an electrode, hence applying controllable current to the acupoint, affecting nerve fibers, muscle tissues, and other electrically sensitive cells. The application of current alters the membrane potentials of neuron cells and activates the voltage-gated ion channels on their membranes, thereby interfering with the conduction and integration of nerve impulses. Although both methods have their unique features, the focus of this study lies in understanding the initiation mechanism of hand acupuncture therapy.
A large amount of clinical evidence in various diseases has affirmed the therapeutic effect of acupuncture; the modern biological mechanism of the sustained immunomodulatory effect after needle withdrawal remains to be systematically clarified. In recent years, progress in cell mechanics has provided a new perspective for understanding the effects of acupuncture. The mechanical stresses involved in acupuncture manipulation, such as lifting-thrusting and twisting, can cause local tissue deformation at the acupoint, activate the resident cells in the ECM, and induce a series of biochemical responses. However, which cells sense mechanical signals at the early stage of acupuncture, participate in initiating the immune cascade reaction, and mediate immune regulation remain key scientific questions.
Based on the current understanding of cell mechanics, fibroblasts and macrophages may be the first line of cells change in their spatial distribution and dynamics in the complex cellular network within the acupoint area to directly respond to the mechanical stimulation of acupuncture (Figure 1). Fibroblasts, as the main mechanical sensing units in connective tissue, undergo deformation and displacement when the needle wraps around and pulls on collagen fibers, directly receiving mechanical stimulation, initiating dynamic signal transduction, and triggering early biological responses. Macrophages, as resident immune cells in the acupoint area, also sense mechanical stress in the early stage of needling, participating in the rapid transduction of signals and the initiation of immune responses.
Figure 1
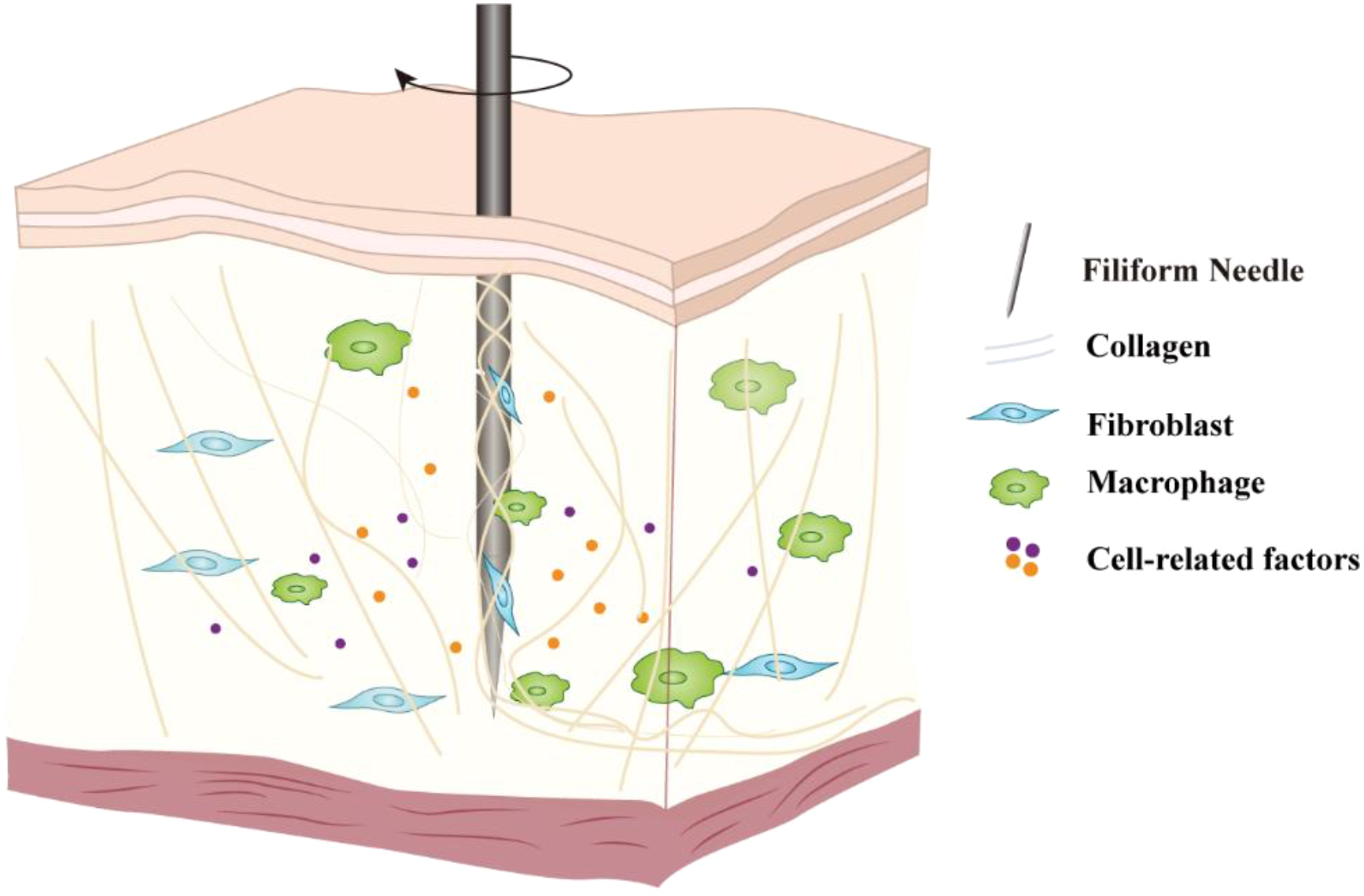
Schematic diagram of the cellular response initiated by acupuncture. The mechanical signals generated by the lifting-thrusting and twisting operations of the needle cause changes in the extracellular matrix structure at the acupoint, which are then perceived by core mechanosensitive cells such as fibroblasts and macrophages, triggering intracellular signal transduction and the secretion of biological factors, thereby amplifying the acupuncture signal.
This review focuses on discussing the dynamic role of macrophages in the process of initiating acupuncture signals, systematically sorting out how they perceive acupuncture stimulation through mechanically sensitive channels, how they collaborate with fibroblasts and other cells to construct an immune microenvironment in the acupoint area, and how they guide the recruitment and functional polarization of subsequent immune cells. The aim is to reveal the dynamic role of macrophages in the acupuncture effect, explain the biological basis of acupunctural immune regulation, and provide theoretical support for the occurrence of long-term clinical efficacy.
2 Acupuncture mechanics initiates cellular responses through ECM mediation
2.1 Foundation of acupoint structure and mechanical transmission
As the key action sites for acupuncture treatment, the structural characteristics of acupoint tissues provide the morphological basis for the initiation and transduction of acupuncture signals. Acupoints are three-dimensional functional units mainly composed of multiple layers of connective tissues, including the epidermis, dermis, subcutaneous loose connective tissue layer, and fascial network layer, with the muscle layer beneath. Additionally, complex tissue structures, such as nerve and vascular bundles that traverse and distribute within acupoints (4). Among them, connective tissue plays a crucial role in the mechanical signal pathway and response (5). The dermis is composed of dense connective tissue, rich in cells involved in immune mechanisms such as fibroblasts, mast cells, macrophages, and dendritic cells (6). Furthermore, it is distributed with multiple neural cell-riched mechanorecepto (such as free nerve endings, Pacinian corpuscles, and Meissner’s corpuscles), constituting the primary stratification for mechanical stimulus perception. Meanwhile, the subcutaneous loose connective tissue layer and fascial network layer provide a structural basis for the transmission of mechanical signals. The matrix components within them, such as collagen fibers, elastic fibers, and hyaluronic acid, together form a microenvironment rich in mechanical properties, directly influencing cell behavior and signal propagation.
2.2 Extracellular matrix-mediated mechanical signal transduction
The ECM is a three-dimensional dynamic network constructed by collagen fibers composed of collagen, elastin, glycosaminoglycans, proteoglycans, and glycoproteins such as fibronectin and laminin (7). Besides providing structural support and elasticity for mesenchymal tissues, it also participates in the regulation of tissue homeostasis through continuous remodeling (8). During acupuncture, the needle entangles with collagen fibers, resulting in mechanical coupling, causing tissue deformation and tension changes, thereby altering the mechanical microenvironment of the ECM. Studies have shown that macrophages migrate only through the mechanical traction of the ECM by micro-needles but without the presence of biochemical chemoattractants, confirming that dynamic mechanical signals themselves can form chemotaxis (9).
In recent years, our growing understanding of cellular mechanics has provided us with the opportunity to further investigate the transduction mechanisms of acupuncture signals. Quantitative observations by ultrasound elastography show that when rotating the filiform needle at the acupuncture point, the mechanical coupling effect between the needle and the tissue is significantly enhanced, resulting in the displacement amplitude of the local tissue caused by the lifting and thrusting movement of the needle increasing to several times that without rotation (10). Moreover, the tissue deformation induced by the needling technique not only occurs during the process of needle insertion and withdrawal, but also causes the tissue’s elastic retraction during the needle body’s movement. Acupuncture, in essence, constitutes a highly dynamic mechanical stimulation, wherein the signals propagate through the fibrous network within connective tissue in the form of “vibration waves,” achieving a transmission velocity up to three times that of neural conduction (4). This phenomenon provides theoretical support for explaining the rapid effects of acupuncture: mechanical waves can be rapidly transmitted to distant sites, and through the interaction between the matrix and cells, cells sensitive to mechanical forces release specific biochemical molecules at their sites, further activating other types of cells in the surrounding area, forming a cascade response of the cell population in the acupoint area to acupuncture.
3 Fibroblast-mediated mechanical signal transduction
3.1 Mechanical sensing and morphological responses of fibroblasts
Fibroblasts not only serve as the primary cells responsible for secreting the ECM, but also adhere to the ECM, enabling them to directly receive and be activated by mechanical signals from acupuncture (11). As such, they constitute the preferential responsive cells to mechanical stimulation induced by acupuncture (5). In vitro cellular studies have demonstrated that mechanical stretch stimulation can induce rapid and significant morphological responses in fibroblasts (5). Animal experiments have also demonstrated that the mechanical forces generated by acupuncture significantly alter the morphology and arrangement of fibroblasts in the acupoint region, resulting in spindle-shaped cytoskeletons with denser alignment, increased cross-sectional area, and interconnected cytoskeletal structures with adjacent cells, forming filamentous or reticular networks (12). In vitro experiments have shown that a 25% stretching (lasting from 10 minutes to 2 hours) can cause fibroblasts to increase their cell body perimeter and cross-sectional area in a time-dependent manner (13). More convincingly, within seconds to minutes after the acupuncture manipulation, fibroblasts actively respond to tissue stretching deformation through dynamic cytoskeleton remodeling. This rapid cytoskeleton remodeling enables the cross-sectional area of fibroblasts within the tissue plane to expand several times, demonstrating their high mechanical sensitivity (13). Therefore, fibroblasts convert mechanical stimuli into biological signals through signal transduction, thereby altering the microenvironment of the acupuncture area and influencing the activities of other cells, playing a crucial role in the initiation of the acupuncture effect.
3.2 Paracrine signaling and immune recruitment by fibroblasts
Fibroblasts activated by mechanical signals transmitted through the ECM are capable of secreting various chemokines and pro-inflammatory factors, thereby recruiting immune cells such as macrophages to cluster in the lesion area, laying the foundation for subsequent immune regulation. For instance, following mechanical stimulation via acupuncture, fibroblasts may release cytokines such as prostaglandin E2 (PGE2), C-C motif chemokine ligand 2 (CCL2), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) (14), which attract immune cells, including macrophages, thereby participating in tissue repair and immune responses. In addition to those chemokines, IL-33 is a cytokine that dynamically distributes between the nucleus and cytoplasm of cells and can be secreted by living cells through a non-classical pathway under mechanical stimulation. In vitro experiments have shown that the level of extracellular IL-33 significantly increases in skin fibroblasts after periodic biaxial stretching stimulation (15). As suppression of tumorigenicity 2 (ST2) serves as the receptor for IL-33, the transduction of IL-33 signaling is contingent upon the expression of ST2 (16). Transwell migration assays have demonstrated that IL-33 exerts a dose-dependent chemotactic effect on RAW264.7 cells through the expression of ST2 (17), suggesting that macrophage recruitment may depend on the fibroblasts’ IL-33 release during acupuncture. Furthermore, fibroblast contraction generates mechanical forces, which also propagate through the fibrous ECM, providing long-range physical signals to macrophages and directing their migration toward the force source (9).
In summary, current in vitro models have provided valuable clues for the explanation of the acupuncture initiation mechanisms, but the in vivo evidence that can directly verify these mechanisms is still missing. Moreover, the specific mechanisms by which fibroblasts affect macrophage function are mostly hypothetical at present and urgently need to be confirmed through further studies.
4 Mechanical sensing and functional regulation of macrophages
Macrophages, as a type of immune cell sensitive to mechanical signals, perceive mechanical stimuli through their adhesion to the ECM and adjust their migratory behavior accordingly (18). In vitro studies have shown that in three-dimensional (3D) cultures, both the fiber structure and the ECM stiffness can regulate the migratory patterns of macrophages: in dense Matrigel or collagen gels, macrophages migrate in an amoeboid pattern, while in loose fibrous collagen gels with larger fiber gaps, they migrate in an interstitial cell pattern. Moreover, individual macrophages can dynamically switch between the two migration modes in response to changes in the matrix architecture (19, 20). In addition, matrix stiffness also affects the cells’ ability to modulate the matrix architecture (such as pore size and rigidity), thus indirectly altering the cell migratory patterns. Interestingly, this phenomenon is not an isolated event; for example, CD4+ T cells also follow similar migration rules (21). Therefore, the rheological properties of the ECM play a crucial role in the migration of immune cells.
As an exogenous mechanical intervention, the core physical effect of acupuncture lies in the instantaneous and sustained remodeling of the physical and chemical microenvironment of the ECM through the mechanical coupling between the needle and the tissue. This remodeled mechanical microenvironment can be perceived by highly mechanosensitive macrophages in the tissue through multiple mechanisms in a coordinated manner (22, 23), including integrin-mediated, cytoskeleton-mediated, and podosome-mediated mechanical signal transduction, as well as the rapid activation of mechanically sensitive ion channels such as Piezo1/TRPV4. By converting physical signals into intracellular biochemical signals and integrating them through complex signaling networks, it ultimately drives the process of M1/M2 phenotypic polarization, directional migration, changes in phagocytic activity, and tissue repair function of macrophages, thus playing a core role in the initiation, amplification, and maintenance of the acupuncture effect.
4.1 Mechanical sensing pathways of macrophages during acupuncture
4.1.1 Integrin-mediated adhesion and mechanotransduction
While acupuncture produces mechanical stimuli in the acupoint area, the integrins on the macrophages’ cell membrane may be activated and trigger the “ECM-integrin-cytoskeleton-nucleus” mechanotransduction cascade (24) (Figure 2). In this process, integrins aggregate and activate in response to the increase in external force. The activated integrins can recruit and activate focal adhesion kinase (FAK) and Src family kinases, forming a FAK-Src signaling complex. This complex then phosphorylates the downstream adaptor protein Paxillin, providing anchor sites for the recruitment of structural proteins such as Vinculin and Talin, ultimately promoting the maturation of focal adhesions and enhancing their roles as a stable anchorage site of the actin cytoskeleton (25). While the Ras homolog family member A (RhoA) is activated through mitogen, its downstream protein Rho−associated protein kinase (ROCK) drives myosin to generate a strong contractile force along the actin cytoskeletal network, directly exerting mechanical traction on the nucleus. On one hand, this contractile force inhibits the Hippo pathway, preventing the degradation of the transcriptional coactivators YAP/TAZ. On the other hand, it promotes the entry of YAP/TAZ into the nucleus, which initiates transcriptional reprogramming and ultimately alters the function of macrophages.
Figure 2
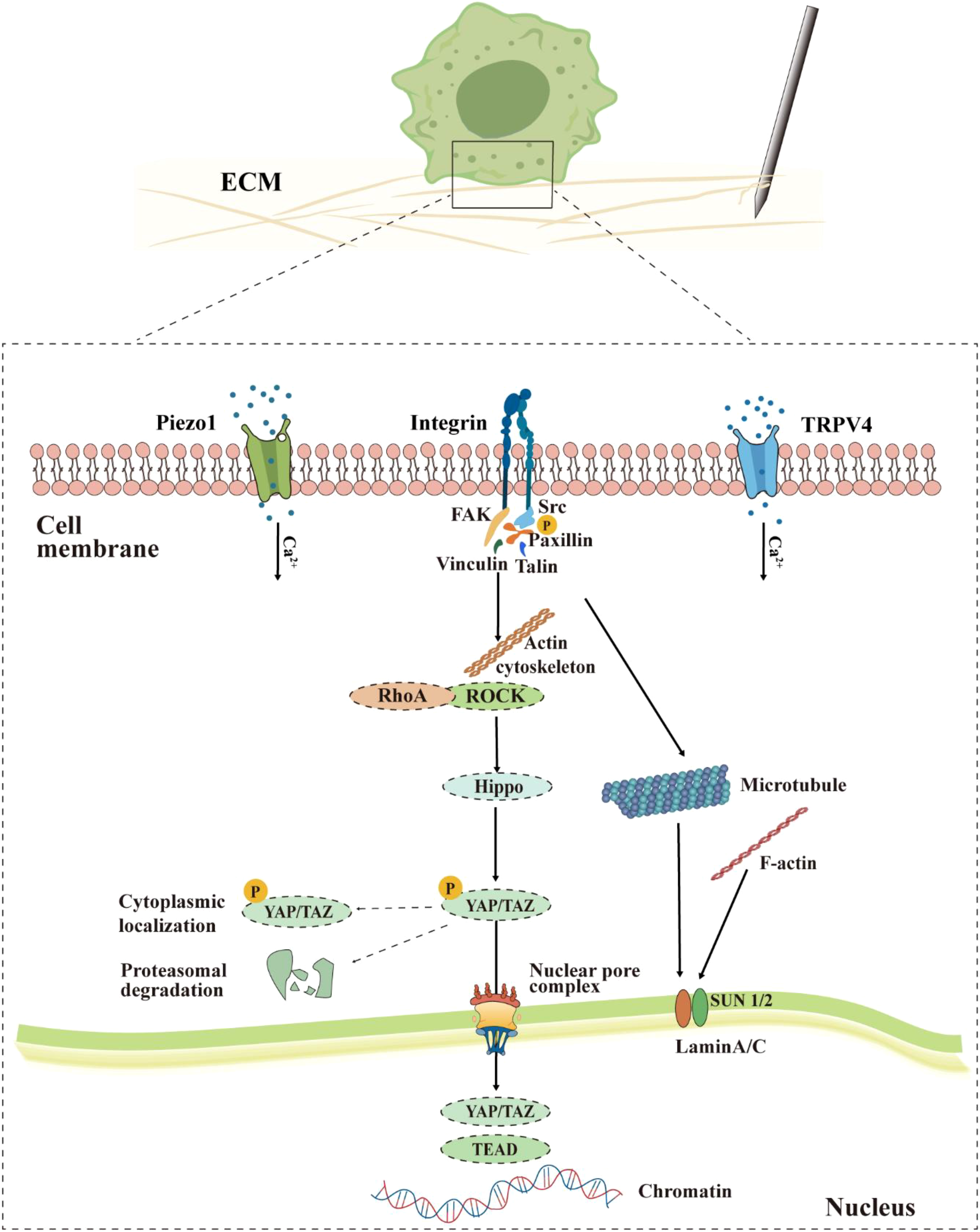
Proposed model of macrophage mechanotransduction during acupuncture. Mechanical stimulation is perceived through integrins, Piezo1/TRPV4 channels, activating downstream signals such as FAK, RhoA/ROCK, and causing dynamic reorganization of the cytoskeleton (actin, microtubules). This process inhibits the retention and degradation of YAP/TAZ in the cytoplasm, promoting their passage through the nuclear pore complex into the nucleus. Within the nucleus, YAP/TAZ interacts with chromatin, while nuclear lamina proteins such as Lamin A/C respond to mechanical signals, jointly mediating the functional reprogramming of macrophages.
Integrins are transmembrane heterodimeric receptors composed of α and β subunits, serving as the main connectors between the ECM and the cytoskeleton, and playing a crucial role in cell-ECM adhesion and mechanical signal transduction (26). Macrophages may precisely sense the mechanical stimuli from the ECM through adhesion molecules such as integrin (27), converting external mechanical signals into internal biochemical signals, thereby precisely regulating the functions of macrophages, such as adhesion, migration, phagocytosis, and inflammatory responses. Integrin-mediated adhesion can promote the formation of foot processes in macrophages. These structures are not only essential for their migration but are also regarded as potential mechanical sensing units due to their tight connection with the cytoskeleton (28–30).
At the functional level, integrin-mediated adhesion promotes the reorganization and dynamic adjustment of the actin cytoskeleton, which is the structural basis for the mechanical sensing and response of macrophages (27). For instance, the expression levels of β2 and β3 integrins are directly associated with the adhesion and migration capabilities of macrophages, and the inhibition of these integrins can significantly weaken these functions (31–33). In the regulation of phagocytosis, blocking αvβ3 integrin completely inhibits the phagocytosis of fibronectin-coated particles (34). Additionally, integrin signaling possesses a negative regulatory effect on excessive inflammatory activation of macrophages, whereas its absence enhances the production of TNF-α under inflammatory stimulation (35).
Integrin-sensed mechanical signals drive actin cytoskeleton reorganization and enhance cell contractility by activating the RhoA/ROCK pathway. The resulting mechanical tension inhibits the activity of the Hippo pathway downstream kinases LATS1/2, blocking the phosphorylation and cytoplasmic retention of YAP/TAZ. YAP/TAZ undergo nuclear translocation and bind to transcription factors such as TEAD to activate the transcription of target genes that regulate cell growth and polarization (36–39) (Figure 2). YAP and TAZ act as key effectors of the Hippo pathway and play a crucial role in the intranuclear transduction of mechanical and cytoskeletal signals (36–38). Their cellular localization is strictly regulated by the mechanical properties of the cell microenvironment. Under conditions of high tension, large cell spreading area, or hard matrix, YAP/TAZ enter the nucleus and activate transcription; while under conditions of soft matrix, small cell area, or high cell density, YAP/TAZ are phosphorylated and retained in the cytoplasm or degraded (36). Twelve hours of mechanical stretching can significantly increase the expression levels of YAP (by approximately 3.2 times) and its upstream regulatory protein Wnt5a (by 2.3 times) in macrophages and promote YAP’s nuclear translocation. Additionally, cyclic mechanical stretching loads can further induce YAP nuclear localization. These evidences support that YAP plays a certain role while macrophages suffer mechanical stimuli (40). During acupuncture, Lamin A/C in macrophages may also sense mechanical signals from the ECM that are transmitted through the integrin-mediated actin-cytoskeleton. Lamin A/C is the main structural protein of the nuclear lamina and serves as an important mechanical sensor within the nucleus. It accurately conveys external mechanical information to the interior of the nucleus, regulates chromatin structure and gene expression, and thus plays an irreplaceable role in maintaining cell morphology, migration, differentiation, and mechanical adaptability (41, 42). Changes in the expression of Lamin A/C can also directly affect the physiological functions of macrophages. Under compressive force stimulation, the levels of SUN1/SUN2 proteins in cells decrease, accompanied by a transient downregulation of Lamin A/C. This downregulation enhances the nuclear localization of yes-associated protein 1 (YAP1), thereby amplifying the effects of mechanical force-induced inflammation and proliferation inhibition (43). Under LPS stimulation, the mRNA and protein levels of Lamin A/C in macrophages show reversible downregulation, accompanied by upregulation of pro-inflammatory gene expression and increased cytokine secretion (44). Inhibition of the activities of cyclin-dependent kinase 1 (CDK1) and Caspase-6 can reduce the production of pro-inflammatory factors IL-6 and TNF-α; while blocking the degradation of Lamin A/C can alleviate the pro-inflammatory response, suggesting its significant role in inflammation regulation (44). Therefore, Lamin A/C constitutes an important bridge connecting extracellular mechanical stimuli with pro-inflammatory gene expression within the nucleus, and its dynamic changes may be the core molecular switch determining whether macrophages maintain a steady state or enter a pro-inflammatory state.
4.1.2 Cytoskeleton-dependent non-classical mechanical sensing mechanism
The cytoskeleton is a highly organized filamentous network within the cells, playing a core role in cell morphology, migration, division, and material transport, and other fundamental life activities. In eukaryotes, the cytoskeleton is composed of three types of cytoskeletal polymers, namely actin filaments, intermediate filaments, and microtubules, as well as three motor protein families, namely myosin, kinesin, and dynein (45, 46). In macrophages, the dynamic reorganization of the cytoskeleton directly participates in regulating their polarized phenotype, ECM degradation, directional migration, and phagocytic activity. Meanwhile, a series of immune responses serves as the chemical stimuli for achieving macrophage recognition, chemotaxis, targeted recruitment, and functional execution (47).
Recent studies have further revealed that macrophages can directly respond to the mechanical properties of the ECM through a non-classical, integrin-independent mechanical sensing mechanism. This process is mediated by the dynamic remodeling of the cytoskeleton during migration, whereas the migration pattern is similar to that of amoeboid movement, which is referred to as “amoeboid-like mechanical sensing” (48). This mechanism involves the direct regulation of the expression of key tissue repair genes, including Fizz1, and does not require the traditional focal adhesion-integrin pathway. In addition, the dynamic changes of the cytoskeleton can also integrate biochemical signals from the microenvironment. For instance, colony-stimulating factor 1 (CSF1) released by activated fibroblasts can induce cytoskeletal remodeling similar to that in high-stiffness ECM and ultimately precisely control the expression program of mechanically sensitive genes by regulating chromatin accessibility, achieving the synergistic integration of mechanical and biochemical signals at the transcriptional level (48).
During acupuncture, the mechanical force generated by the movement of the needle can directly regulate the function of macrophages through the cytoskeleton by altering the physical properties of the local ECM. Meanwhile, CSF1 released by activated fibroblasts can also act on macrophages, and the two work together to initiate and adjust the tissue repair process in response to acupuncture.
4.1.3 Rapid responses mediated by mechanosensitive channels
Piezo1, a calcium-permeable mechanosensitive channel highly expressed in macrophages, serves as a “molecular bridge” coupling physical stimuli with immune responses. In vitro experiments have confirmed that the mRNA expression of Piezo1 in the post-acupoint area is significantly upregulated after acupuncture (49). This channel can rapidly open under the action of tissue stretching, shearing, or pressure changes caused by acupuncture, mediating the influx of extracellular Ca²+ and initiating calcium-dependent signal cascades at the millisecond level (50). The Piezo1-mediated mechanosensory pathway, along with integrin-dependent signaling, is incorporated into the mechanotransduction model (Figure 2), thereby regulating macrophage polarization, phagocytosis, and migration, as well as the secretion of cytokine profiles (51). Additionally, Piezo1 is highly expressed in bone marrow-derived macrophages and participates in regulating multiple functions, including Toll-like receptor 4 (TLR4) signaling, phagocytosis, and bactericidal activity (51). The activation of Piezo1 can promote the activation of calcium-dependent protease Calpain and protein kinase C (PKC), thereby enhancing integrin activation and indirectly influencing macrophage functions (52). Matrix stiffening can also upregulate Piezo1 expression, enabling it to synergize with integrins to activate their common downstream mechanical signaling effector YAP. Activated YAP, as a key regulator of macrophage polarization, can promote their polarization towards the M1 phenotype while inhibiting the M2 phenotype, thus modulating the role of macrophages in immune responses (53, 54). Given the above mechanism, it is reasonable to infer that the mechanical force applied by acupuncture may also be perceived by the Piezo1 and other mechanically sensitive channels of the macrophages in the acupoint area, and trigger a series of similar mechanical signal transduction and functional remodeling.
In addition to Piezo1, macrophages also express multiple TRP family channels, including TRPV2, TRPV4, TRPC6, and TRPM7, which play significant roles in macrophage polarization, inflammatory activation, and phagocytic function (55–60). Among these, TRPV4 is recognized as the primary mechanosensitive TRP channel in macrophages (56). TRPV4 responds to mechanical stimuli such as interstitial fluid flow and changes in substrate stiffness (61). Compared with macrophages cultured on softer substrates, those cultured on harder substrates show significantly enhanced phagocytic ability and intracellular calcium influx after LPS stimulation, and this substrate stiffness effect disappears when TRPV4 is inhibited by drugs or its expression is reduced (56). Hence, TRPV4 works in concert with Piezo1 to enhance macrophage sensitivity to complex mechanical environments by modulating membrane potential and calcium signaling (56, 61–65). Current research has found that acupuncture can activate TRPV channels (especially TRPV4, which is highly expressed in acupoints), mediate the release of ATP in the acupoint area, and thereby produce an analgesic effect of acupuncture (61, 63). However, it is still unknown which specific cells are activated. Additionally, Acupoint Catgut Embedding (ACE), as a novel form of acupuncture, can increase intracellular Ca2+ concentration and the number of macrophages by activating TRPV4 (62).
Recent studies indicate that fluid shear stress activates Piezo1, triggering an initial calcium signal that subsequently leads to TRPV4 channel opening and sustained Ca2+ elevation (66, 67). The synergistic mechanism of Piezo1 and TRPV4 has not yet been directly confirmed in macrophages or in vivo models of acupuncture. However, given that both are key mechanosensitive ion channels and are involved in the regulation of mechanically induced calcium signals in various cell types, we speculate that, in the complex mechanical environment imposed by acupuncture, these two channels may jointly participate in the integration and decoding of mechanical signals through functional synergy. Therefore, Piezo1 and TRPV4 may not only independently mediate rapid mechanical perception and calcium responses, but also potentially form a fine and sensitive mechanical perception network through functional coupling, thereby playing a regulatory role in the immune modulation and tissue repair processes of acupuncture.
In summary, this section integrates the current research on macrophage mechanosensing and proposes a comprehensive model of acupuncture mechanical signal transduction (summarized in Figure 2). It should be noted that several key mechanisms involved in this model have been partially verified in different research systems: For instances, the “ECM-integrin-cytoskeleton-nucleus” mechanical transduction pathway and downstream processes such as YAP/TAZ nuclear translocation have been widely explored in cell mechanics research; integrin-independent non-classical mechanosensing mechanisms have also been revealed; the mechanical sensitivity of Piezo1/TRPV4 channels in macrophages and their mediation of rapid Ca2+ responses have been supported by in vitro experimental results. However, whether these synergistic effects reported in other cell types are also present in macrophages remains unclear. Although in vitro studies suggest potential associations among these pathways, the spatiotemporal activation of these molecular events and their functional coupling in the context of in vivo acupuncture models still lack systematic evaluation. Future research should leverage live cell imaging acquired in specific gene knockout models and other means to dissect these details.
In essence, the inference here is essentially a reasonable hypothesis based on cross-disciplinary integration, aiming to provide a clear and testable research framework and theoretical basis for exploring the mechanisms specific to the acupuncture context.
4.1.4 Mechanical sensing by podosomes
During the process of acupuncture, the mechanical force applied by the needle causes changes in the mechanical properties of the ECM. Macrophages are likely to detect such alterations directly via podosomes, thereby initiating subsequent mechanotransduction processes.
While adherent cells attach to the ECM through integrins, they recruit adhesion-related proteins to form focal adhesions at the cell membrane, which in turn mediate cytoskeletal remodeling and mechanical signal transduction. In macrophages, this process is primarily carried out by specialized structures known as podosomes. Although similar in protein composition to focal adhesions, the macrophage podosomes exhibit distinct structural morphology and dynamic behavior from those in other cell types (28, 68). Podosomes in macrophages not only participate in cell adhesion but also sense the stiffness and topography of 3D matrices, enabling bidirectional mechanical stress transmission between the intracellular and extracellular environments, and possess the ability to degrade the ECM (28, 69–71).
Located at the interface between the cell and the matrix, this structure is a microfilament-rich, spot-like, and with a typical three-layer architecture of “core-ring-cap”: the core is composed of branched actin filaments, surrounded by a ring formed by adhesion proteins (such as talin, Paxillin, and vinculin), and the top is covered by crosslinked and bundled actin filaments to form a cap-like structure. This unique three-dimensional organization provides it with highly sensitive mechanical perception functions (69, 72, 73) (Figure 3).
Figure 3
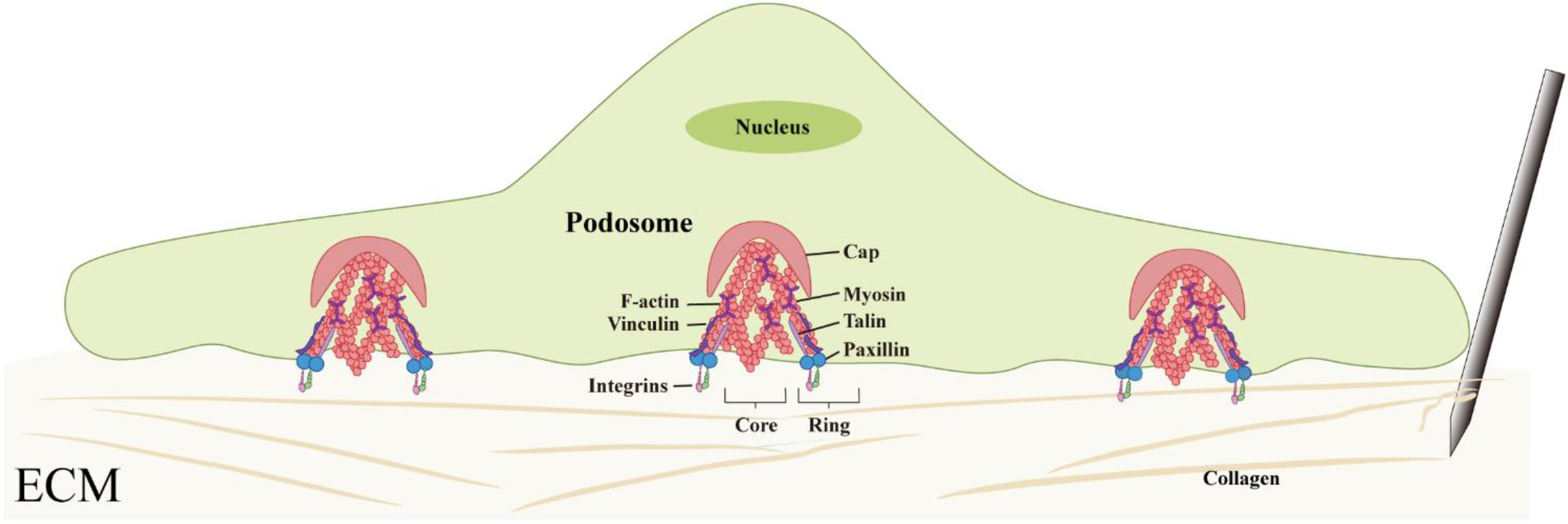
Structural organization and mechanosensory function of macrophage podosomes. Podosomes exhibit a characteristic core–ring–cap architecture composed of actin filaments and adhesion proteins, enabling macrophages to sense matrix stiffness and transmit mechanical forces.
The mechanosensory capacity of podosomes is established through the sustained polymerization of actin filaments combined with myosin-mediated contraction. The detection of activated phosphorylated myosin light chain (pMLC) within and between macrophage podosomes confirms their contractile activity (70). Additionally, in the ring structure of pseudopod bodies, there are mechanically sensitive proteins such as talin and p130Cas that can undergo conformational changes under tension, allowing signal modules such as the binding sites of vinculin or the phosphorylation sites of Src kinase to open after being subjected to force (74, 75). Under the synergistic action of other proteins such as p130Cas, talin, paxillin, and zyxin, mechanical stimuli can be converted into intracellular biochemical signals, completing the transformation from mechanical perception to signal response (70).In summary, the pseudopod body with its characterised “core-ring-cap” ultrastructure, potentially enables macrophages to sensitively detect the mechanical changes in the ECM caused by acupuncture. This structure is believed to not only participate in cell adhesion and migration but also play a role in the perception and transduction of mechanical signals, suggesting that it may be an important structural base for macrophages to achieve mechanosensing (Figure 3).
4.2 M1/M2 polarization and temporal regulation
As the mechanical stimulation from acupuncture ceases, the biochemical reactions it triggers still undergo a cascade amplification, resulting in a lag effect. Therefore, after acupuncture, the subsequent biochemical reactions still influence macrophage behaviors, including self-chemotaxis, phagocytic activity, antigen presentation, and polarization, laying a functional foundation for regulating other immune cells.
In a mouse model of acute myocardial infarction, after transplanting the infarcted heart into another mouse, the original immune cells (monocytes) in the damaged area of the heart rapidly decreased, while monocytes from the recipient (host) were recruited at an unexpectedly fast rate: 6 hours after transplantation, recipient-derived monocytes accounted for 20 ± 1% of the monocytes in the infarcted area; this proportion increased to 40 ± 3% at 12 hours; and reached 60 ± 0.5% after 24 hours. Hence, the newly recruited monocytes quickly showed a transient residence characteristic in the infarcted myocardial tissue (about 20 hours), maintaining the homeostasis of the local monocyte/macrophage pool in the injury area through this rapid turnover pattern (76). During acupuncture, the immune cells initially recruited by mechanical signals may follow the same rule, and their subsequent M1/M2 phenotypic polarization is a precise functional adjustment and adaptation to the microenvironment of the acupoint area based on this type of dynamic turnover.
Acupuncture may also promote inflammation clearance and tissue repair by regulating the M1/M2 polarization phenotype of macrophages, providing a “function-oriented” guidance for the regulation of other immune cells. Existing research shows that mechanical stress has a bidirectional or even multi-directional effect on macrophage polarization, which is related to the magnitude of the force and other specific factors in the environment. For instance, 12 hours after acupuncture, the phenotype of macrophages changed: in the sepsis model treated with acupuncture, small intestinal macrophages were observed to shift from the pro-inflammatory M1 phenotype to the anti-inflammatory/repair M2 phenotype through immunofluorescence 12 hours after treatment (77). In addition, acupuncture alleviates pain and inflammatory responses by promoting the M1/M2 phenotypic conversion of macrophages and increasing the concentration of IL-10 in muscles (78).
In terms of temporal sequence, the response of skin macrophages to mechanical stress has distinct and dynamic characteristics. Under normal skin conditions, the number of M2-type macrophagesis higher than that of M1-type macrophages (79). However, tissue-resident macrophages release chemokines shortly after being stimulated by acupuncture and then adapt to the changes in the acupoint environment through phenotypic changes over a long period of time. Meanwhile, in the early stage, fibroblasts release pro-inflammatory factors such as IL-6 in response to mechanical stimulation (14). Acupuncture, as a sterile inflammatory operation, damages-associated molecular patterns (DAMPs) released during the acupuncture procedure induce M1 polarization in both tissue-resident macrophages and newly recruited monocytes (80, 81). This M1 polarization leads to the production of mediators such as IL-1β, IL-6, TNF-α, and reactive oxygen species (ROS), nitric oxide (NO), which contribute to tissue repair and regeneration by effectively clearing pathogens and necrotic tissue (82, 83). As the repair process progresses, the concentration of PGE2 in the acupoint area increases (mainly due to the release of fibroblasts under the stimulation of acupuncture), which in turn drives the transformation of macrophages and monocytes into M2 type (14). These transformed cells promote ECM reconstruction, angiogenesis, and tissue regeneration by secreting factors such as TGF-β, IL-10, and VEGF-A (82–84). The phenotypic conversion of macrophages is precisely regulated by microenvironmental signals, but the specific molecular mechanisms and tissue-specific subtype differentiation pathways still need further research.
5 Macrophages’ integrated regulation of the acupoint immune microenvironment
After perceiving the mechanical signals of acupuncture, macrophages engaged in adjusting the microenvironment of the acupoint area by altering the chemokine network, leading to the remodeling of the ECM, and transforming other immune cells, thereby transforming the initial mechanical stimulation into a highly regulated immune repair process.
5.1 Synergistic regulation of immune cell recruitment and tissue microenvironment remodeling
In the chemokine network, macrophages act as rapid response units, releasing chemokines such as CXCL1 and CCL2 upon activation of the Piezo1 channel, which respectively recruit neutrophils, monocytes and T cells, initiating the early immune response; while fibroblasts serve as “location markers”, secreting cytokines such as CCL19, CCL21, CXCL12, IL-33 and IL-7 under steady state conditions, maintaining the positioning and functional continuity of immune cells (15, 85–88). Both of them form a positive feedback loop through factors such as CSF1-PDGF and, under the regulation of signaling pathways such as NF-κB and STAT, coordinate the orderly transition of immune cells from recruitment to repair (85, 86). Macrophages rapidly recruit monocytes from the bone marrow and spleen by releasing chemokines such as CCL2 (89), and these monocytes differentiate into macrophages or dendritic cells in the lesion area (90), promptly replenishing the local immune cell count and performing functions such as phagocytosis, antigen presentation, inflammatory regulation, and tissue repair (90–93). In addition, macrophages also enhance the immune defense capacity of the acupoint area by secreting cytokines such as CXCL8 and G-CSF, in collaboration with neutrophils (94, 95) (Figure 4). During the ECM remodeling process, fibroblasts respond to mechanical and inflammatory signals, synthesize and deposit ECM components such as collagen (87, 88), and release prostaglandin E2 (PGE2) to promote tissue repair and angiogenesis (14). Macrophages promote the migration of immune cells to the injury site by decomposing the ECM, clearing pathogens, apoptotic cells, and ECM degradation products, and provide a stable microenvironment for tissue repair (95). These two cells work in concert to reconstruct the ECM (87). On one hand, macrophages secrete TGF-β1 to induce fibroblasts-to-myofibroblasts transformation, promoting collagen deposition and tissue repair (82, 96); while the M1/M2 polarization phenotypes cooperatively promote angiogenesis and ECM reconstruction through differentially secreting angiogenic factors and matrix remodeling proteins, such as matrix metalloproteinase-9 (MMP-9) (97). On the other hand, fibroblasts maintain the homeostasis of ECM metabolism by regulating the balance of matrix metalloproteinases (MMPs), tissue inhibitors of MMPs (TIMPs) (88). During the resolution phase of inflammation, macrophages promote the repair process by phagocytosing apoptotic neutrophils and regulating IL-10 and IL-12 (98, 99). If this coordinated mechanism is imbalanced, it can lead to abnormal deposition or degradation of the ECM, thereby inducing pathological outcomes such as fibrosis.
Figure 4
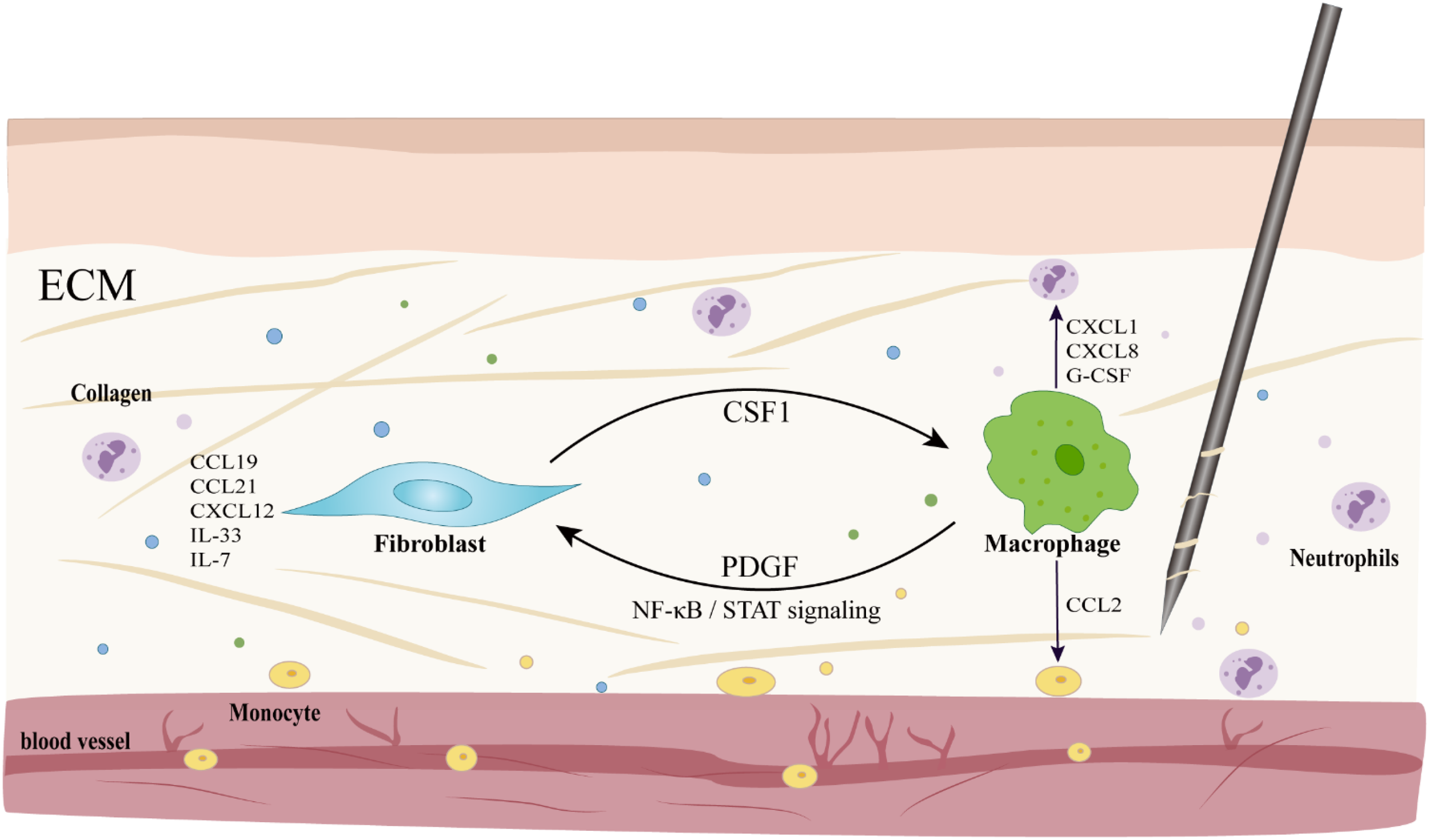
Macrophage-coordinated immune cell recruitment and intercellular communication in the microenvironment of acupoints. Activated macrophages release chemokines such as CXCL1 and CCL2 to recruit neutrophils and monocytes, respectively; meanwhile, fibroblasts secrete factors like IL-33 to exert chemotactic effects and act as “location markers”. The two form a positive feedback through molecules such as CSF1-PDGF, and under the regulation of signaling pathways such as NF-κB/STAT, they jointly maintain the immune homeostasis of the local microenvironment.
In coordinating innate immune cells, as the main scavenger of senescent and apoptotic neutrophils, macrophages acquire powerful neutrophilic antibacterial molecules, thereby enhancing their own antibacterial capacity (100). The transformation of local macrophages from the early inflammatory M1 phenotype to the later repetitive M2 phenotype at the injury site depends on their phagocytic effect on apoptotic neutrophils. Apoptotic neutrophils release “eat-me” signals, which induce macrophages to upregulate the expression of phagocytosis-related genes and release repair-related cytokines, such as IL-10 and TGF-β (99). The efficient collaboration between macrophages and neutrophils significantly accelerates the resolution of inflammation and the restoration of tissue homeostasis, laying a solid foundation for the sustained effect of acupuncture.
Macrophages act as rapid response units, initiating the recruitment of immune cells by releasing chemokines such as CXCL1 and CCL2. They form a synergy with fibroblasts, which serve as “location markers”, through a cytokine network and positive feedback loop, jointly regulating the progression from inflammation to repair. The interaction between the two in cell communication and ECM remodeling constitutes the potential cellular basis of the acupuncture effect. However, the current understanding mainly stems from in vitro experiments and extended inferences from other disease models. The dynamic response patterns, interaction details, and exact contributions of these two types of cells under acupuncture-specific stimulation, as well as their roles in the overall effect, still require rigorous in vivo acupuncture research for empirical validation.
5.2 Regulation of adaptive immunity
Macrophages are not only the core effector cells of innate immunity but also the key hub connecting innate and adaptive immunity. They mainly regulate T-cell responses precisely through antigen presentation and cytokine secretion, thereby guiding the acupuncture-induced local inflammation towards immune homeostasis and tissue repair.
In terms of antigen presentation, macrophages are important antigen-presenting cells (APCs) (101), presenting antigen peptides via MHC class I and MHC class II molecules on their surface, providing the essential “first signal” for T cell activation (101, 102). The T cell receptor (TCR) on the T cell membrane recognizes the pMHC complex on the surface of APCs, with CD4+ T cells mainly recognizing antigens presented by MHC class II molecules, while CD8+ T cells mainly recognize antigens presented by MHC class I molecules (101–103). Beyond TCR binding to pMHC, full T cell activation also requires co-stimulatory signals (the “second signal”). This signal is typically provided by the binding of co-stimulatory molecules on APCs, such as CD80/CD86, to the CD28 receptor on T cells (101–103). Under the combined action of both signals, naive T cells become activated and can further differentiate into distinct effector subsets with specific functions, such as helper T cells (including Th1 and Th2) or regulatory T cells (Treg), under the regulation of specific cytokine environments (104) (Figure 5). Activated Th1 cells secrete large amounts of IFN-γ, further promoting macrophage polarization toward the pro-inflammatory M1 phenotype and enhancing their phagocytic and bactericidal capabilities. Conversely, Th2 cells further promote the polarization of macrophages to the repair-oriented M2 phenotype by secreting factors such as IL-4 and IL-13, participating in tissue repair and other processes. Meanwhile, Treg cells effectively suppress excessive inflammatory responses by secreting inhibitory cytokines such as IL-10 and TGF-β, maintaining immune homeostasis and self-tolerance in the body (105–107). Additionally, macrophages can also indirectly enhance the efficiency and specificity of antigen presentation by transferring pre-processed antigen fragments to dendritic cells, promoting cross-presentation and preventing the spread of immune responses to non-target tissues (108).
Figure 5
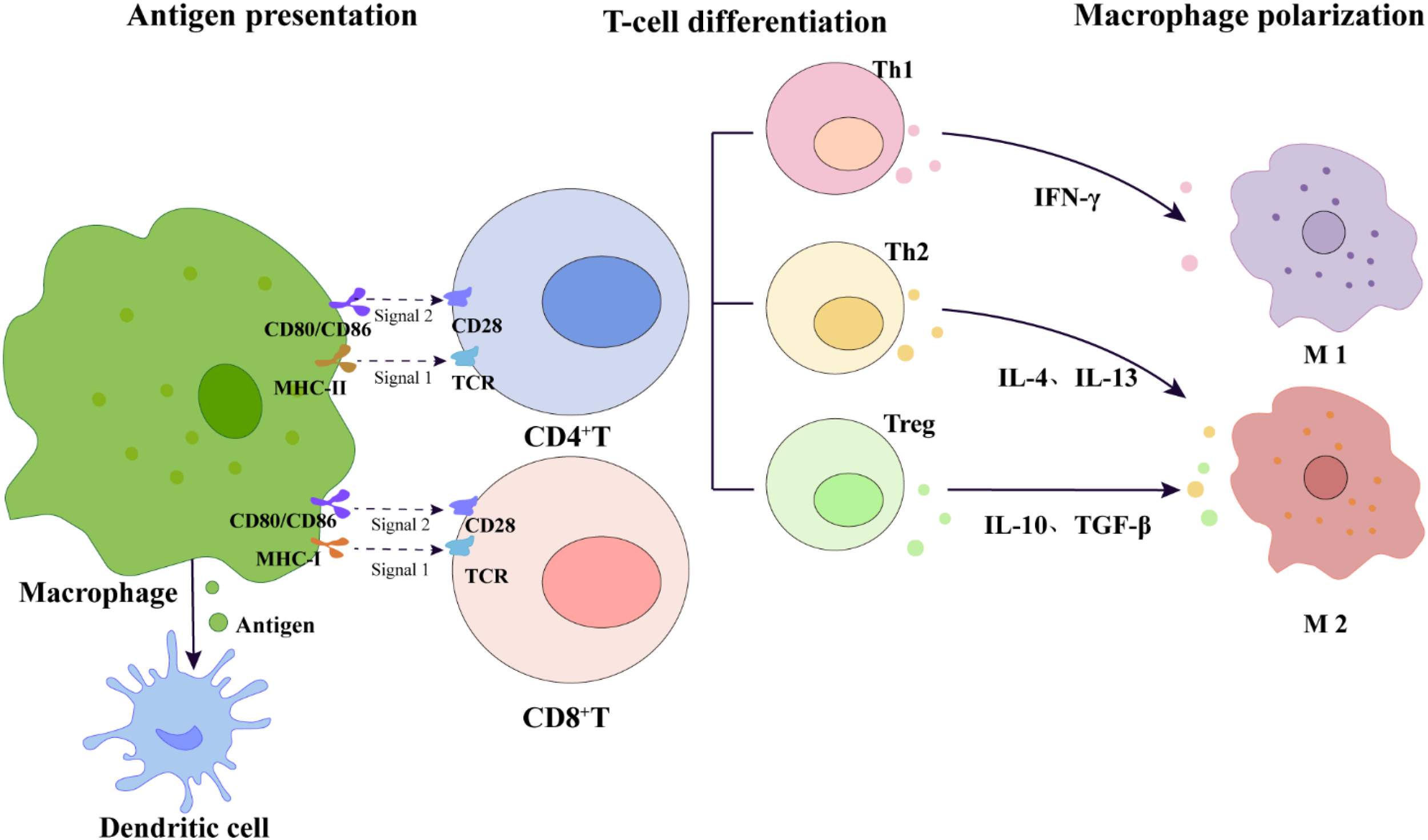
Macrophage-mediated T-cell dual-signal activation and subsequent cytokine-driven differentiation and macrophage polarization. This schematic diagram illustrates the dual-signal activation model of T cells: Signal 1 is the presentation of antigen peptides to the T cell receptor (TCR) by MHC class I and MHC class II molecules on the surface of macrophages; Signal 2 is transmitted through the co-stimulatory interaction between CD80/CD86 on the surface of macrophages and CD28 on the surface of T cells. Activated CD4+T cells further differentiate into different effector subgroups, including Th1 (secreting IFN-γ), Th2 (secreting IL-4 and IL-13), and Treg (secreting IL-10 and TGF-β), which feed back to regulate the polarization of macrophages to M1 or M2 phenotypes, thereby maintaining overall immune homeostasis. The diagram also indicates the pathway by which macrophages transfer antigens to dendritic cells to support cross-presentation, jointly participating in the homeostatic regulation after immune activation induced by acupuncture.
Macrophages activate T cells through antigen presentation and co-stimulatory signals, while activated T cells feed back to regulate the polarization state of macrophages by secreting specific cytokines, forming a bidirectional regulatory network (Figure 5). This finely interlocked mechanism is of great significance for translating the local immune response triggered by acupuncture into microenvironmental repair. However, the spatiotemporal dynamic changes of this network under acupuncture stimulation, the sequence of key molecular events, and their specific pathways of action in the overall effect still need to be systematically clarified through the construction and utilization of in vivo research models specific to acupuncture.
6 Summary and prospect
This review systematically demonstrates that there exists a complete mechanical-biological signal transduction pathway for the initiation of acupuncture. This pathway is initiated by the mechanical force generated through the manipulation of filiform needles, which alters the mechanical microenvironment of the ECM. Macrophages and fibroblasts act as core sensors and respond in a coordinated manner, thereby completing the transduction and amplification from physical stimulation to biochemical signals. Macrophages, as the core hub, sense stress signals through various pathways such as Piezo1/TRPV4 mechanically sensitive channels, integrins, and podosomes, and promptly initiate a cascade of intracellular Ca2+ fluctuations and YAP/TAZ nuclear translocation responses. Furthermore, they recruit neutrophils and monocytes by secreting chemokines (such as CCL2, CXCL1, etc), initiating innate immunity. Their function then shifts from rapid recruitment to fine regulation. By means of the dynamic polarization of M1/M2 phenotypes and the secretion of factors such as TGF-β and IL-10, it not only guides the differentiation of monocytes into repair-type macrophages but also bridges and activates T cells through antigen presentation, thus establishing a complete “innate-adaptive immune” regulatory network. Meanwhile, macrophages and fibroblasts collaborate precisely in the remodeling of the ECM. The former mediates degradation and clearance, whereas the latter governs synthesis and deposition. Together, they optimize the mechanical microenvironment of the lacunae, driving local immune homeostasis and tissue repair.
Looking ahead, research on mechanical signal transduction and immune regulation in acupuncture still faces several key challenges. Current studies mostly employ single or static mechanical stimulation models, which are unable to reflect the dynamic interaction of multiple parameters, such as intensity, frequency, duration, and technique during clinical acupuncture, as well as their overall regulation of macrophage function and the immune network. Moreover, there is a significant gap between the parameter settings of existing in vitro mechanical models and actual acupuncture operations, which hinders the in-depth explanation of related mechanisms and their clinical translation potential.
The realization of acupuncture effects relies on the coordinated actions of various cells in the acupoint area, but the specific mechanisms are not yet fully understood. For instance, how mechanically activated fibroblasts regulate the recruitment and polarization of macrophages through chemokine signals such as IL-33; what molecular dialogues between fibroblasts and macrophages coordinate the regulation of ECM remodeling and the immune microenvironment; how macrophages activate T cells through antigen presentation, and how activated T cells, in turn, regulate macrophage function through cytokine feedback to jointly maintain immune homeostasis. These key links urgently need to be systematically verified through acupuncture-specific in vivo models. At the same time, the temporal activation sequence, cooperative mechanisms, and their impact on the final immune outcome of multiple mechanical sensing pathways, such as Piezo1/TRPV4 channels and integrin-cytoskeleton-nuclear signaling axes during the acupuncture process, also need to be further systematically clarified.
Therefore, future research should establish a standardized mechanical stimulation system for acupuncture that is closer to clinical reality, and integrate in vivo models with live imaging techniques to analyze the dynamic impact of mechanical input on immune responses from a spatiotemporal perspective. The focus should be on building acupuncture-specific in vivo research systems, integrating cell-specific intervention and real-time dynamic observation techniques, to systematically reveal the integration mechanism of mechanical signals in the microenvironment of the acupoint area and their precise regulatory rules on immune cell behavior and tissue repair programs, thereby providing a solid scientific basis for the modernization and precision of acupuncture therapy.
Statements
Author contributions
WS: Conceptualization, Writing – original draft. RW: Investigation, Methodology, Writing – review & editing. XX: Investigation, Methodology, Writing – review & editing. PZ: Visualization, Writing – review & editing. JZ: Visualization, Writing – review & editing. YT: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was financially supported by the National Key R&D Program of China (NO. 2022YFC3500405 to YDT), Taishan Scholar Foundation of Shandong Province (NO. tstp20221125 to YDT), China Postdoctoral Science Foundation (NO. 2024MM761884 to XQX), and Shandong Province Traditional Chinese Medicine Science and Technology Project (NO. M20241713 to XQX).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Zhang SQ Li JC . An introduction to traditional chinese medicine, including acupuncture. Anatomical Rec (Hoboken NJ: 2007). (2021) 304:2359–64. doi: 10.1002/ar.24782
2
Obringer F . Chinese medicine and the enticement of heritage status. China perspectives (2010) 3:15–22. doi: 10.4000/chinaperspectives.5621
3
Langevin HM Schnyer R MacPherson H Davis R Harris RE Napadow V et al . Manual and electrical needle stimulation in acupuncture research: pitfalls and challenges of heterogeneity. J Altern complementary Med (New York NY). (2015) 21:113–28. doi: 10.1089/acm.2014.0186
4
Yunshan L Chengli X Peiming Z Haocheng Q Xudong L Liming L . Integrative research on the mechanisms of acupuncture mechanics and interdisciplinary innovation. Biomed Eng Online. (2025) 24:30. doi: 10.1186/s12938-025-01357-w
5
Abbott RD Koptiuch C Iatridis JC Howe AK Badger GJ Langevin HM . Stress and matrix-responsive cytoskeletal remodeling in fibroblasts. J Cell Physiol. (2013) 228:50–7. doi: 10.1002/jcp.24102
6
Nguyen AV Soulika AM . The dynamics of the skin’s immune system. Int J Mol Sci. (2019) 20:1811. doi: 10.3390/ijms20081811
7
Kai F Drain AP Weaver VM . The extracellular matrix modulates the metastatic journey. Dev Cell. (2019) 49:332–46. doi: 10.1016/j.devcel.2019.03.026
8
Bonnans C Chou J Werb Z . Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. (2014) 15:786–801. doi: 10.1038/nrm3904
9
Pakshir P Alizadehgiashi M Wong B Coelho NM Chen X Gong Z et al . Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat Commun. (2019) 10:1850. doi: 10.1038/s41467-019-09709-6
10
Langevin HM Konofagou EE Badger GJ Churchill DL Fox JR Ophir J et al . Tissue displacements during acupuncture using ultrasound elastography techniques. Ultrasound Med Biol. (2004) 30:1173–83. doi: 10.1016/j.ultrasmedbio.2004.07.010
11
Qu F Cui Y Zeng J Zhang M Qiu S Huang X et al . Acupuncture induces adenosine in fibroblasts through energy metabolism and promotes proliferation by activating mapk signaling pathway via adenosine(3) receptor. J Cell Physiol. (2020) 235:2441–51. doi: 10.1002/jcp.29148
12
Liu N Zhao Z Zhou Q Zhang X Wang Y Huang S et al . The roles of skin fibroblasts at local acupoints in chrono-acupuncture. Pain Res Manage. (2020) 2020:3731510. doi: 10.1155/2020/3731510
13
Langevin HM Bouffard NA Badger GJ Iatridis JC Howe AK . Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. (2005) 288:C747–56. doi: 10.1152/ajpcell.00420.2004
14
Bo C Yongfen L Jin C Lingmei F Xiaofang Y Lin F . A comparative study on the release of pge2 and ll -6 by taking static pressure on fibroblast cells gained from the rat “Zusanli”and its adjacent areas. Chin J Acupunct Moxibust. (2007) 02):135–40.
15
Kakkar R Hei H Dobner S Lee RT . Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. (2012) 287:6941–8. doi: 10.1074/jbc.M111.298703
16
Schmitz J Owyang A Oldham E Song Y Murphy E McClanahan TK et al . Il-33, an interleukin-1-like cytokine that signals via the il-1 receptor-related protein st2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23:479–90. doi: 10.1016/j.immuni.2005.09.015
17
Xu R Pan Y Zheng K Chen M Yin C Hu Q et al . Il-33/st2 induces macrophage-dependent ros production and trpa1 activation that mediate pain-like responses by skin incision in mice. Theranostics. (2024) 14:5281–302. doi: 10.7150/thno.97856
18
Yamada KM Sixt M . Mechanisms of 3d cell migration. Nat Rev Mol Cell Biol. (2019) 20:738–52. doi: 10.1038/s41580-019-0172-9
19
Maridonneau-Parini I . Control of macrophage 3d migration: A therapeutic challenge to limit tissue infiltration. Immunol Rev. (2014) 262:216–31. doi: 10.1111/imr.12214
20
Cougoule C Van Goethem E Le Cabec V Lafouresse F Dupré L Mehraj V et al . Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3d environments. Eur J Cell Biol. (2012) 91:938–49. doi: 10.1016/j.ejcb.2012.07.002
21
Overstreet MG Gaylo A Angermann BR Hughson A Hyun YM Lambert K et al . Inflammation-induced interstitial migration of effector cd4+ T cells is dependent on integrin Av. Nat Immunol. (2013) 14:949–58. doi: 10.1038/ni.2682
22
Gruber EJ Leifer CA . Molecular regulation of tlr signaling in health and disease: mechano-regulation of macrophages and tlr signaling. Innate Immun. (2020) 26:15–25. doi: 10.1177/1753425919838322
23
Zhang X Kim TH Thauland TJ Li H Majedi FS Ly C et al . Unraveling the mechanobiology of immune cells. Curr Opin Biotechnol. (2020) 66:236–45. doi: 10.1016/j.copbio.2020.09.004
24
Jiaqi S Juan L Jun L . Advances in mechanotransduction pathway of macrophage. J Med Biomechanics. (2023) 38:408–14. doi: 10.16156/j.1004-7220.2023.02.030
25
Parsons JT Horwitz AR Schwartz MA . Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. (2010) 11:633–43. doi: 10.1038/nrm2957
26
Pang X He X Qiu Z Zhang H Xie R Liu Z et al . Targeting integrin pathways: mechanisms and advances in therapy. Signal transduction targeted Ther. (2023) 8:1. doi: 10.1038/s41392-022-01259-6
27
Tamkun JW DeSimone DW Fonda D Patel RS Buck C Horwitz AF et al . Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. (1986) 46:271–82. doi: 10.1016/0092-8674(86)90744-0
28
Collin O Na S Chowdhury F Hong M Shin ME Wang F et al . Self-organized podosomes are dynamic mechanosensors. Curr biology: CB. (2008) 18:1288–94. doi: 10.1016/j.cub.2008.07.046
29
Meli VS Veerasubramanian PK Atcha H Reitz Z Downing TL Liu WF . Biophysical regulation of macrophages in health and disease. J leukocyte Biol. (2019) 106:283–99. doi: 10.1002/jlb.Mr0318-126r
30
Van Goethem E Poincloux R Gauffre F Maridonneau-Parini I Le Cabec V . Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol (Baltimore Md: 1950). (2010) 184:1049–61. doi: 10.4049/jimmunol.0902223
31
Weerasinghe D McHugh KP Ross FP Brown EJ Gisler RH Imhof BA . A role for the alphavbeta3 integrin in the transmigration of monocytes. J Cell Biol. (1998) 142:595–607. doi: 10.1083/jcb.142.2.595
32
Yakubenko VP Belevych N Mishchuk D Schurin A Lam SC Ugarova TP . The role of integrin alpha D beta2 (Cd11d/cd18) in monocyte/macrophage migration. Exp Cell Res. (2008) 314:2569–78. doi: 10.1016/j.yexcr.2008.05.016
33
Féréol S Fodil R Labat B Galiacy S Laurent VM Louis B et al . Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskeleton. (2006) 63:321–40. doi: 10.1002/cm.20130
34
Blystone SD Graham IL Lindberg FP Brown EJ . Integrin alpha V beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol. (1994) 127:1129–37. doi: 10.1083/jcb.127.4.1129
35
Han C Jin J Xu S Liu H Li N Cao X . Integrin cd11b negatively regulates tlr-triggered inflammatory responses by activating syk and promoting degradation of myd88 and trif via cbl-B. Nat Immunol. (2010) 11:734–42. doi: 10.1038/ni.1908
36
Halder G Dupont S Piccolo S . Transduction of mechanical and cytoskeletal cues by yap and taz. Nat Rev Mol Cell Biol. (2012) 13:591–600. doi: 10.1038/nrm3416
37
Elosegui-Artola A Andreu I Beedle AEM Lezamiz A Uroz M Kosmalska AJ et al . Force triggers yap nuclear entry by regulating transport across nuclear pores. Cell. (2017) 171:1397–410.e14. doi: 10.1016/j.cell.2017.10.008
38
Meng Z Moroishi T Guan KL . Mechanisms of hippo pathway regulation. Genes Dev. (2016) 30:1–17. doi: 10.1101/gad.274027.115
39
Saraswathibhatla A Indana D Chaudhuri O . Cell-extracellular matrix mechanotransduction in 3d. Nat Rev Mol Cell Biol. (2023) 24:495–516. doi: 10.1038/s41580-023-00583-1
40
Dong L Song Y Zhang Y Zhao W Wang C Lin H et al . Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. (2021) 236:6376–90. doi: 10.1002/jcp.30312
41
Dubik N Mai S . Lamin a/C: function in normal and tumor cells. Cancers. (2020) 12:123688. doi: 10.3390/cancers12123688
42
Jabre S Cherchame E Pinzón N Lemerle E Bitoun M Coirault C . Lamin a/C protects chromatin accessibility during mechanical loading in human skeletal muscle. Cell communication signaling: CCS. (2025) 23:452. doi: 10.1186/s12964-025-02437-z
43
Wang Y Ruf S Wang L Heimerl T Bange G Groeger S . The dual roles of lamin a/C in macrophage mechanotransduction. Cell proliferation. (2025) 58:e13794. doi: 10.1111/cpr.13794
44
Mehl JL Earle A Lammerding J Mhlanga M Vogel V Jain N . Blockage of lamin-a/C loss diminishes the pro-inflammatory macrophage response. iScience. (2022) 25:105528. doi: 10.1016/j.isci.2022.105528
45
Pollard TD Goldman RD . Overview of the cytoskeleton from an evolutionary perspective. Cold Spring Harbor Perspect Biol. (2018) 10:a030288. doi: 10.1101/cshperspect.a030288
46
Fletcher DA Mullins RD . Cell mechanics and the cytoskeleton. Nature. (2010) 463:485–92. doi: 10.1038/nature08908
47
Mei J Huang X Fan C Fang J Jiu Y . Cytoskeleton network participates in the anti-infection responses of macrophage. BioEssays: News Rev molecular Cell Dev Biol. (2023) 45:e2200225. doi: 10.1002/bies.202200225
48
Meizlish ML Kimura Y Pope SD Matta R Kim C Philip NH et al . Mechanosensing regulates tissue repair program in macrophages. Sci Adv. (2024) 10:eadk6906. doi: 10.1126/sciadv.adk6906
49
Chen Z Yao K Wang X Liu Y Du S Wang S et al . Acupuncture promotes muscle cells atp metabolism in st36 acupoint local exerting effect by activating trpv1/camkii/ampk/pgc1α Signaling pathway. Chin Med. (2025) 20:112. doi: 10.1186/s13020-025-01169-z
50
Young MN Sindoni MJ Lewis AH Zauscher S Grandl J . The energetics of rapid cellular mechanotransduction. Proc Natl Acad Sci United States America. (2023) 120:e2215747120. doi: 10.1073/pnas.2215747120
51
Geng J Shi Y Zhang J Yang B Wang P Yuan W et al . Tlr4 signalling via piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun. (2021) 12:3519. doi: 10.1038/s41467-021-23683-y
52
Aglialoro F Hofsink N Hofman M Brandhorst N van den Akker E . Inside out integrin activation mediated by piezo1 signaling in erythroblasts. Front Physiol. (2020) 11:958. doi: 10.3389/fphys.2020.00958
53
Meli VS Atcha H Veerasubramanian PK Nagalla RR Luu TU Chen EY et al . Yap-mediated mechanotransduction tunes the macrophage inflammatory response. Sci Adv. (2020) 6:eabb8471. doi: 10.1126/sciadv.abb8471
54
Mei F Guo Y Wang Y Zhou Y Heng BC Xie M et al . Matrix stiffness regulates macrophage polarisation via the piezo1-yap signalling axis. Cell proliferation. (2024) 57:e13640. doi: 10.1111/cpr.13640
55
Dutta B Goswami R Rahaman SO . Trpv4 plays a role in matrix stiffness-induced macrophage polarization. Front Immunol. (2020) 11:570195. doi: 10.3389/fimmu.2020.570195
56
Scheraga RG Abraham S Niese KA Southern BD Grove LM Hite RD et al . Trpv4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J Immunol (Baltimore Md: 1950). (2016) 196:428–36. doi: 10.4049/jimmunol.1501688
57
Schappe MS Szteyn K Stremska ME Mendu SK Downs TK Seegren PV et al . Chanzyme trpm7 mediates the ca(2+) influx essential for lipopolysaccharide-induced toll-like receptor 4 endocytosis and macrophage activation. Immunity. (2018) 48:59–74.e5. doi: 10.1016/j.immuni.2017.11.026
58
Li L Wei C Cai S Fang L . Trpm7 modulates macrophage polarization by stat1/stat6 pathways in raw264.7 cells. Biochem Biophys Res Commun. (2020) 533:692–7. doi: 10.1016/j.bbrc.2020.10.062
59
Link TM Park U Vonakis BM Raben DM Soloski MJ Caterina MJ . Trpv2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. (2010) 11:232–9. doi: 10.1038/ni.1842
60
Riazanski V Gabdoulkhakova AG Boynton LS Eguchi RR Deriy LV Hogarth DK et al . Trpc6 channel translocation into phagosomal membrane augments phagosomal function. Proc Natl Acad Sci United States America. (2015) 112:E6486–95. doi: 10.1073/pnas.1518966112
61
Luo D Liu L Zhang HM Zhou YD Zhou MF Li JX et al . Relationship between acupuncture and transient receptor potential vanilloid: current and future directions. Front Mol Neurosci. (2022) 15:817738. doi: 10.3389/fnmol.2022.817738
62
Hou X Liang X Lu Y Zhang Q Wang Y Xu M et al . Investigation of local stimulation effects of embedding pgla at zusanli (St36) acupoint in rats based on trpv2 and trpv4 ion channels. Front Neurosci. (2024) 18:1469142. doi: 10.3389/fnins.2024.1469142
63
Zheng Y Zuo W Shen D Cui K Huang M Zhang D et al . Mechanosensitive trpv4 channel-induced extracellular atp accumulation at the acupoint mediates acupuncture analgesia of ankle arthritis in rats. Life (Basel Switzerland). (2021) 11:513. doi: 10.3390/life11060513
64
Babaniamansour P Jacho D Rabino A Garcia-Mata R Yildirim-Ayan E . Synergetic role of trpv4 inhibitor and mechanical loading on reducing inflammation. Front Immunol. (2024) 15:1456042. doi: 10.3389/fimmu.2024.1456042
65
Wilson HM . Modulation of macrophages by biophysical cues in health and beyond. Discov Immunol. (2023) 2:kyad013. doi: 10.1093/discim/kyad013
66
Tomilin V Stavniichuk A Pyrshev K Kordysh M Zaika O Pochynyuk O . Piezo1 and trpv4 complementary contribute to [Ca2+]I homeostasis in collecting duct cells. Physiology. (2023) 38:S1. doi: 10.1152/physiol.2023.38.S1.5729575
67
Swain SM Liddle RA . Piezo1 acts upstream of trpv4 to induce pathological changes in endothelial cells due to shear stress. J Biol Chem. (2021) 296:100171. doi: 10.1074/jbc
68
Van Goethem E Guiet R Balor S Charrière GM Poincloux R Labrousse A et al . Macrophage podosomes go 3d. Eur J Cell Biol. (2011) 90:224–36. doi: 10.1016/j.ejcb.2010.07.011
69
Linder S Cervero P Eddy R Condeelis J . Mechanisms and roles of podosomes and invadopodia. Nat Rev Mol Cell Biol. (2023) 24:86–106. doi: 10.1038/s41580-022-00530-6
70
Linder S Wiesner C . Feel the force: podosomes in mechanosensing. Exp Cell Res. (2016) 343:67–72. doi: 10.1016/j.yexcr.2015.11.026
71
Jain N Moeller J Vogel V . Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu Rev Biomed Eng. (2019) 21:267–97. doi: 10.1146/annurev-bioeng-062117-121224
72
van den Dries K Linder S Maridonneau-Parini I Poincloux R . Probing the mechanical landscape - new insights into podosome architecture and mechanics. J Cell Sci. (2019) 132:jcs236828. doi: 10.1242/jcs.236828
73
Hu F Zhu D Dong H Zhang P Xing F Li W et al . Super-resolution microscopy reveals nanoscale architecture and regulation of podosome clusters in primary macrophages. iScience. (2022) 25:105514. doi: 10.1016/j.isci.2022.105514
74
Lakkakorpi PT Nakamura I Nagy RM Parsons JT Rodan GA Duong LT . Stable association of pyk2 and P130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. (1999) 274:4900–7. doi: 10.1074/jbc.274.8.4900
75
Zambonin-Zallone A Teti A Grano M Rubinacci A Abbadini M Gaboli M et al . Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: A beta 3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp Cell Res. (1989) 182:645–52. doi: 10.1016/0014-4827(89)90266-8
76
Leuschner F Rauch PJ Ueno T Gorbatov R Marinelli B Lee WW et al . Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. (2012) 209:123–37. doi: 10.1084/jem.20111009
77
Xu X Huang X Xiao L Wang J Yang X Wu Y . Mechanism of electro-acupuncture in alleviating intestinal injury in septic mice via polyamine-related M2-macrophage polarization. Front Immunol. (2024) 15:1373876. doi: 10.3389/fimmu.2024.1373876
78
da Silva MD Bobinski F Sato KL Kolker SJ Sluka KA Santos AR . Il-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. (2015) 51:19–31. doi: 10.1007/s12035-014-8790-x
79
Hongfei D Xi H Xianhui L Yanbiao Z Xuyang W Bing W et al . Placenta-derived mesenchymal stem cells in promoting acute skin wound healing in rats. Chin J Tissue Eng Res. (2024) 28:2047–53.
80
Zindel J Kubes P . Damps, pamps, and lamps in immunity and sterile inflammation. Annu Rev Pathol. (2020) 15:493–518. doi: 10.1146/annurev-pathmechdis-012419-032847
81
Zhao X Chen J Sun H Zhang Y Zou D . New insights into fibrosis from the ecm degradation perspective: the macrophage-mmp-ecm interaction. Cell bioscience. (2022) 12:117. doi: 10.1186/s13578-022-00856-w
82
Mantovani A Biswas SK Galdiero MR Sica A Locati M . Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. (2013) 229:176–85. doi: 10.1002/path.4133
83
Guan F Wang R Yi Z Luo P Liu W Xie Y et al . Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets. Signal transduction targeted Ther. (2025) 10:93. doi: 10.1038/s41392-025-02124-y
84
Shapouri-Moghaddam A Mohammadian S Vazini H Taghadosi M Esmaeili SA Mardani F et al . Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
85
Link A Vogt TK Favre S Britschgi MR Acha-Orbea H Hinz B et al . Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. (2007) 8:1255–65. doi: 10.1038/ni1513
86
Buechler MB Fu W Turley SJ . Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity. (2021) 54:903–15. doi: 10.1016/j.immuni.2021.04.021
87
Franklin RA . Fibroblasts and macrophages: collaborators in tissue homeostasis. Immunol Rev. (2021) 302:86–103. doi: 10.1111/imr.12989
88
Galati A Gallo RL Cavagnero KJ . Multifaceted roles of dermal fibroblasts in skin health and disease. Dermatologic surgery: Off Publ Am Soc Dermatologic Surg. (2025) 51:S5–s11. doi: 10.1097/dss.0000000000004668
89
Serbina NV Pamer EG . Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor ccr2. Nat Immunol. (2006) 7:311–7. doi: 10.1038/ni1309
90
Cheong C Matos I Choi JH Dandamudi DB Shrestha E Longhi MP et al . Microbial stimulation fully differentiates monocytes to dc-sign/cd209(+) dendritic cells for immune T cell areas. Cell. (2010) 143:416–29. doi: 10.1016/j.cell.2010.09.039
91
Gordon S Taylor PR . Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733
92
Geissmann F Manz MG Jung S Sieweke MH Merad M Ley K . Development of monocytes, macrophages, and dendritic cells. Sci (New York NY). (2010) 327:656–61. doi: 10.1126/science.1178331
93
Robbins CS Swirski FK . The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life sciences: CMLS. (2010) 67:2685–93. doi: 10.1007/s00018-010-0375-x
94
Simon HU . Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. (2003) 193:101–10. doi: 10.1034/j.1600-065x.2003.00038.x
95
Wynn TA Vannella KM . Macrophages in tissue repair, regeneration, and fibrosis. Immunity. (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
96
Feng F Liu M Pan L Wu J Wang C Yang L et al . Biomechanical regulatory factors and therapeutic targets in keloid fibrosis. Front Pharmacol. (2022) 13:906212. doi: 10.3389/fphar.2022.906212
97
Spiller KL Anfang RR Spiller KJ Ng J Nakazawa KR Daulton JW et al . The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. (2014) 35:4477–88. doi: 10.1016/j.biomaterials.2014.02.012
98
Persson YA Blomgran-Julinder R Rahman S Zheng L Stendahl O . Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes infection. (2008) 10:233–40. doi: 10.1016/j.micinf.2007.11.007
99
He W Yan L Hu D Hao J Liou YC Luo G . Neutrophil heterogeneity and plasticity: unveiling the multifaceted roles in health and disease. MedComm. (2025) 6:e70063. doi: 10.1002/mco2.70063
100
Savill JS Wyllie AH Henson JE Walport MJ Henson PM Haslett C . Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. (1989) 83:865–75. doi: 10.1172/jci113970
101
Brancewicz J Wójcik N Sarnowska Z Robak J Król M . The multifaceted role of macrophages in biology and diseases. Int J Mol Sci. (2025) 26:2107. doi: 10.3390/ijms26052107
102
Acuña-Castillo C Escobar A García-Gómez M Bachelet VC Huidobro-Toro JP Sauma D et al . P2x7 receptor in dendritic cells and macrophages: implications in antigen presentation and T lymphocyte activation. Int J Mol Sci. (2024) 25:2495. doi: 10.3390/ijms25052495
103
Kohlgruber AC Dezfulian MH Sie BM Wang CI Kula T Laserson U et al . High-throughput discovery of mhc class I- and ii-restricted T cell epitopes using synthetic cellular circuits. Nat Biotechnol. (2025) 43:623–34. doi: 10.1038/s41587-024-02248-6
104
Bour-Jordan H Bluestone JA . Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. (2009) 229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x
105
Josefowicz SZ Lu LF Rudensky AY . Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
106
Chen S Saeed A Liu Q Jiang Q Xu H Xiao GG et al . Macrophages in immunoregulation and therapeutics. Signal transduction targeted Ther. (2023) 8:207. doi: 10.1038/s41392-023-01452-1
107
Gordon S Martinez FO . Alternative activation of macrophages: mechanism and functions. Immunity. (2010) 32:593–604. doi: 10.1016/j.immuni.2010.05.007
108
Xu Y Liu Y Yang C Kang L Wang M Hu J et al . Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance cd4(+) T-cell responses. Immunology. (2016) 149:157–71. doi: 10.1111/imm.12630
Summary
Keywords
acupuncture initiation, immune regulation, macrophages, mechanotransduction, signal amplification
Citation
Sheng W, Wang R, Xue X, Zhao P, Zhang J and Tseng Y (2026) From mechanosensing to immune regulation: mechanisms of acupuncture signal initiation and amplification mediated by macrophages. Front. Immunol. 17:1744045. doi: 10.3389/fimmu.2026.1744045
Received
11 November 2025
Revised
17 January 2026
Accepted
27 January 2026
Published
16 February 2026
Volume
17 - 2026
Edited by
Shan-hui Hsu, National Taiwan University, Taiwan
Reviewed by
Jun Zhu, Chengdu University of Traditional Chinese Medicine, China
Chuech Yu Lin, Tzu Chi University, Taiwan
Updates
Copyright
© 2026 Sheng, Wang, Xue, Zhao, Zhang and Tseng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiider Tseng, ytseng@sdutcm.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.