Abstract
Background:
ABO-incompatible living donor kidney transplantation (ABOi-LDKT) offers a potential solution to organ shortages, but its safety and economic impact in China remain uncertain. This study evaluated short- to mid-term outcomes of ABOi-LDKT in a Chinese population by integrating evidence from a meta-analysis and a single-center cohort.
Methods:
We performed a meta-analysis of 18 studies including 15,611 kidney transplant recipients to compare graft and patient survival between ABOi-LDKT and ABO-compatible LDKT (ABOc-LDKT). In parallel, we conducted a retrospective single-center cohort study of 41 ABOi-LDKT and 132 ABOc-LDKT recipients transplanted between 2021 and 2022. Outcomes included patient and graft survival, renal function, postoperative complications, infections, delayed graft function (DGF), acute rejection, and hospitalization costs.
Results:
The meta-analysis showed lower 1-year graft survival and 3-year patient survival in ABOi-LDKT than in ABOc-LDKT. In contrast, our single-center cohort demonstrated comparable 1- and 3-year patient and graft survival between groups. No significant differences were observed in surgical complications, infections, DGF, or acute rejection. ABOi-LDKT recipients showed better renal function at two weeks post-transplant, with no sustained differences thereafter. Hospitalization costs were higher in the ABOi-LDKT group, reflecting additional desensitization procedures.
Conclusion:
In this Chinese single-center cohort, ABOi-LDKT achieved short- to mid-term clinical outcomes comparable to ABOc-LDKT despite higher upfront costs. When combined with contemporary desensitization protocols, ABOi-LDKT appears to be a safe and feasible strategy to expand access to living donor kidney transplantation in settings with severe organ shortages.
1 Introduction
Kidney transplantation remains the gold standard for end-stage renal disease (ESRD), yet the scarcity of compatible organs severely restricts its application (1–3). ABO-incompatible living donor kidney transplantation (ABOi-LDKT) has emerged as a critical strategy to expand the donor pool, with reports suggesting it can increase living donation by up to 30% (4). Historically, the “viability” of ABOi-LDKT was challenged by high rates of hyperacute rejection. However, the evolution of desensitization protocols—transitioning from splenectomy to Rituximab-based regimens combined with plasmapheresis (PE)—has revolutionized outcomes, enabling graft survival rates comparable to ABO-compatible living donor kidney transplantation (ABOc-LDKT) (5, 6).
Despite these advances, the broad viability of ABOi-LDKT in developing regions remains debated, centering on three critical pillars: effectiveness (successful antibody depletion and prevention of rebound), safety (balancing desensitization against infection risks), and economy (the cost-benefit ratio of pre-conditioning). In clinical practice, heterogeneous desensitization protocols regarding Rituximab dosing and target isoagglutinin titers have led to varying outcomes, particularly concerning infection-related mortality and antibody-mediated rejection (AMR) (7, 8). Furthermore, the economic burden of desensitization in self-pay or mixed-payer healthcare systems poses a barrier to widespread adoption.
To comprehensively assess the viability of ABOi-LDKT, this study adopts a dual approach. We integrated a systematic meta-analysis of Asian cohorts to establish regional benchmarks for safety and efficacy. Concurrently, we conducted a single-center retrospective study to rigorously validate an individualized desensitization protocol. Specifically, we focused on the effectiveness of CD19+ B-cell targeted Rituximab dosing in reducing isoagglutinin titers, the safety of stratified infection prophylaxis in mitigating perioperative complications, and the economic implications of these interventions. This study aims to provide robust evidence that ABOi-LDKT is a viable, safe, and cost-effective therapeutic option for expanding renal transplantation access in China.
2 Material and method
2.1 Search strategy and selection criteria
A systematic literature search was conducted across international and Chinese databases, including Web of Science, Embase, PubMed, Google Scholar, CNKI, Baidu Scholar, and Wanfang. The search window spanned from January 2001 to April 2024. Keywords and Medical Subject Headings (MeSH) included “kidney transplantation,” “ABOi,” “renal transplantation,” “ABO incompatible,” “ABOi-rTx,” “ABOi-KT,” and “ABOi-LDKT”.
Study eligibility was determined using the PICO framework to ensure relevance and homogeneity:
Population (P): Asian patient populations undergoing living donor kidney transplantation.
Intervention (I): ABOi-LDKT utilizing a Rituximab-based desensitization protocol.
Comparator (C): ABOc-LDKT.
Outcomes (O): Clinical outcomes including patient and graft survival with a minimum follow-up duration of 1 year, providing sufficient data for extraction.
2.2 Data extraction and quality assessment
Data were extracted independently by two investigators using a standardized form. Extracted variables included lead author, publication year, follow-up duration, dialysis history, sample sizes, and event counts (death/graft loss) at 1, 3, and 5 years. Quality assessment was performed using the Newcastle-Ottawa Scale (NOS). (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Any discrepancies in data extraction or quality scoring were resolved through discussion or by consulting a third senior investigator.
2.3 Meta-analysis statistical methods
Meta-analysis was performed using Stata 12.0 (StataCorp, College Station, TX, USA). Pooled Odds Ratios (ORs) with 95% Confidence Intervals (CIs) were calculated for graft and patient survival. Subgroup analyses evaluated complications including Cytomegalovirus (CMV)/BK virus infection and acute rejection. Heterogeneity was assessed via the Cochrane Q test and I2 statistic. To account for potential between-study variance, a random-effects model was applied if significant heterogeneity was observed (P < 0.1 or I2 > 50%); otherwise, a fixed-effects model was utilized. Publication bias was assessed using Begg’s test and funnel plots, and sensitivity analysis was conducted to verify result stability.
2.4 Inclusion criteria for the receiver in clinical analysis
Recipients in both groups were required to meet the following criteria: (1) follow-up duration of >1 year in our outpatient department; (2) ≤3 human leukocyte antigen (HLA) mismatches; (3) complement-dependent cytotoxicity (CDC) <10%; (4) negative reactivity for class I and class II antibodies; (5) first kidney transplant from a direct blood relative who had provided written informed consent for altruistic donation; (6) adult recipients; and (7) implantation of the graft in the right iliac fossa. Recipients with left iliac fossa implantation (n = 15) were excluded a priori to reduce surgical and technical heterogeneity (e.g., vascular length and angle, ureteral course, and wound exposure), as these factors could influence early non-immunologic complications in our small cohort.
2.5 Desensitization protocol (ABOi-LDKT group only)
The experimental group received desensitization therapy based on the Technical Operating Specifications for ABO-Incompatible Living Donor Kidney Transplantation in China (2019) (7). An individualized Rituximab regimen was administered based on preoperative CD19+ B-cell counts: patients with >15% received 200 mg at 4 weeks, 100 mg at 2 weeks, and 100 mg at 1 day preoperatively; those with 10–15% received 100 mg at these three time points; and those with <10% received 100 mg at 4 and 2 weeks preoperatively. Isoagglutinin titers (IgM and IgG) were monitored at initial typing, before and after pharmacotherapy, before and after PE, and on postoperative days 2, 5, and 14. The target titer was <1:16. If titers exceeded 1:16, PE or Double Filtration Plasmapheresis (DFPP) was performed using albumin or fresh frozen plasma as replacement fluids until the target was met.
2.6 Immunosuppression and perioperative management
While the ABOc-LDKT group did not receive immunosuppressants before surgery, the ABOi-LDKT group initiated triple therapy 1–2 weeks preoperatively, consisting of Tacrolimus (0.08–0.1 mg/kg/day, target serum trough 10–12 ng/ml), Mycophenolate Mofetil (MMF, 1.0–1.5 g/day, target AUC 30–60 ng/ml), and Prednisone (20 mg/day). Induction therapy for all patients included Antithymocyte Globulin (ATG, 25 mg on days 0, 1, and 2) and methylprednisolone sodium succinate (500 mg IV on days 0, 1, and 2). Postoperatively, methylprednisolone was tapered daily to 120 mg, 80 mg, and 40 mg on days 3, 4, and 5, respectively, and switched to oral prednisone (10 mg/day) on day 6. MMF (1.0–1.5 g/day) was resumed on the evening of surgery, and oral Tacrolimus (initial dose 0.1–0.2 mg/kg/day) was started on postoperative day 3, adjusted to maintain therapeutic levels.
2.7 Perioperative management: infection prophylaxis
Routine antibacterial prophylaxis utilizing Mezlocillin sodium/Sulbactam sodium was administered from postoperative day (POD) 0 to 12 targeting Gram-negative bacteria; coverage for fungi or Gram-positive bacteria was reserved for culture-positive cases derived from donor perfusion or drainage fluid analysis. Prophylaxis against Pneumocystis jirovecii pneumonia was initiated on POD 7 with Sulfamethoxazole-Trimethoprim (SMZ-TMP) (200/40 mg daily) and continued for 6 months. Ganciclovir was administered for CMV prophylaxis from POD 7 to month 3, with dosages strictly stratified by estimated glomerular filtration rate (eGFR): (1) eGFR >70 mL/min: 1000 mg PO three times daily (tid); (2) eGFR 50–69 mL/min: 500 mg PO tid; (3) eGFR 25–49 mL/min: 500 mg PO twice daily (bid); (4) eGFR 10–24 mL/min: 500 mg PO once daily (qd); (5) eGFR <10 mL/min: 500 mg PO every other day (qod).
2.8 Clinical data collection and outcome definitions
Comprehensive clinical data were systematically collected for recipients, including demographics, preoperative history, dialysis modality, and perioperative metrics such as hospital stay and costs. Specific surveillance outcomes included the incidence of severe pulmonary and urinary tract infection (UTI) requiring re-hospitalization within 1 year post-transplantation, while acute rejection episodes and surgery-related complications were monitored during the perioperative period. Follow-up assessments tracked patient and graft survival at 7 days, 14 days, 1 month, 6 months, and 1 year post-transplantation. Key outcomes were defined as follows: delayed graft function (DGF) was defined as the requirement for dialysis within the first week post-transplantation; Acute Rejection was suspected based on clinical indicators and confirmed by percutaneous renal biopsy according to the Banff classification; Surgical complications were categorized as ureteral stenosis, anastomotic hemorrhage, or wound infection/dehiscence; and postoperative renal function was assessed using the CKD-EPI creatinine equation (race-free) reported as eGFR(mL/min/1.73 m²).
Donor demographics and baseline characteristics were recorded following the recipient protocol. Preoperative donor glomerular filtration rate (GFR) was measured via renal dynamic scintigraphy using the Gates camera method with 99mTc-DTPA. Net injected counts were obtained by syringe pre/post counting, and regions of interest (ROIs) were drawn over each kidney with background subtraction. Attenuation was corrected using Tonnesen depths (YL = 13.2×W/H+0.7; YR = 13.3×W/H+0.7; μ = 0.153 cm−1), and the 2–3-min window provided bilateral uptake (%). Total GFR was calculated as GFRtotal (mL/min) = 9.813×uptake (%) − 6.825, with single-kidney GFR = GFRtotal × split function; values were BSA-standardized to 1.73 m².
2.9 Statistical analysis
Data analyses were performed using R v4.2.2 (R Foundation, Vienna, Austria) and GraphPad Prism v9.0 (GraphPad Software, San Diego, CA, USA). Continuous variables were expressed as mean ± SD or median (IQR) and tested for normality using the Kolmogorov–Smirnov test (P < 0.05 indicated non-normal distribution). For group comparisons, normally distributed continuous variables were analyzed using the unpaired Student’s t-test, while non-normally distributed variables were assessed using the Mann–Whitney U test. To evaluate longitudinal within-group changes in serum creatinine (Scr) and eGFR at different follow-up time points, paired t-tests were employed. Categorical data were compared using the Chi-square test or Fisher’s exact test as appropriate. Survival models were constructed using the “survival” and “survminer” R packages to generate Kaplan–Meier curves, with differences assessed via the log-rank test. While propensity score methods were considered to balance baseline covariates, the modest sample size of the ABOi-LDKT cohort after prespecified exclusions would have led to unstable estimates. Therefore, we focused on strict eligibility criteria and standardized perioperative management to minimize major confounding in this exploratory cohort. A two-tailed P-value < 0.05 was considered statistically significant.
3 Results
3.1 Meta-analysis results
The selection process used in this study is illustrated in Figure 1A. A total of 18 papers were included in this meta-analysis, consisting of 17 in English (6, 9–24) and 1 in Chinese (8). All studies were published between January 1, 2001, and April 1, 2024, involving 15,611 patients (Figure 1a). Table 1 summarizes the detailed information on the studies included in this meta-analysis. All included papers were scored 5 or higher on the NOS scale.
Figure 1

Flowchart for the current study. (a) Process of the literate selection of the meta-analysis. (b) Process of the collection of clinical samples.
Table 1
| Author | Country | Year | ABOc-LDKT | ABOi-LDKT | Research type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Follow-up time | Mean age | BMI | Dialysis time | n | Follow-up time | Mean age | BMI | Dialysis time | ||||
| Tanabe et al. (6) | Japan | 2009 | 55 | NA | 38.9 ± 12.5 | 20.8 ± 2.6 | 43 (16-86) | 24 | NA | 43.0 ± 13.5 | 21.4 ± 2.4 | 27 (19-66) | RCT |
| Jeon et al. (9) | South Korea | 2010 | 61 | NA | NA | NA | NA | 22 | NA | 45 (33-61) | NA | NA | RCT |
| Fuchinoue et al. (10) | Japan | 2011 | 280 | 36.4 ± 19.57 | 43.5 ± 13.7 | NA | 39.7 (0–304.8) | 50 | 36.4 ± 19.6 | 52.6 ± 12.1 | NA | 31.2 (0.7–184.6) | RCT |
| Kohei et al. (11) | Japan | 2012 | 83 | NA | 40.4 ± 12.6 | NA | 44 (16–86) | 57 | NA | 44.0 ± 14.8 | NA | 26 (19–57) | RCT |
| Hwang et al. (12) | South Korea | 2013 | 138 | 31 | 42.8 ± 11.2 | 22.9 ± 3.6 | 19.8 ± 32.1 (n=103) | 35 | 31 | 44.1 ± 9.2 | 22.9 ± 3.6 | 27.2 ± 35.3 (n=25) | RCT |
| Takahashi et al. (13) | Japan | 2013 | 6716 | NA | NA | NA | NA | 1427 | NA | NA | NA | NA | RCT |
| Ashimine et al. (14) | Japan | 2014 | 228 | 36 | 40.0 ± 15.1 | NA | NA | 30 | 36 | 49.0 ± 15.1 | NA | NA | RCT |
| Shin et al. (15) | South Korea | 2015 | 396 | 46 (1-65) | 43 (7-73) | 22.2 (12.3-39.7) | 7 (0-207) | 73 | 39(2-63) | 44 (20-64) | 22.8 (16.1-31.0) | 10 (0-252) | RCT |
| Okumi et al. second area (16) | Japan | 2016 | 333 | 56 (31-83) | 45.2 ± 13.9 | NA | 31 (12-68) | 144 | 48(31-72) | 47.8 ± 13.1 | NA | 26 (14-57) | RCT |
| Park et al. (17) | South Korea | 2016 | 21 | 15.2 ± 7.7 | 50.9 ± 8.4 | NA | 21.4 ± 39.7 | 11 | 14.5 ± 8.0 | 49.0 ± 6.5 | NA | 38.5 ± 48.8 | RCT |
| Ko et al. (18) | South Korea | 2017 | 1541 | NA | 42.6 ± 13 | NA | 18.6 ± 34.1 | 248 | NA | 44.2 ± 12.4 | NA | 19.6 ± 36.2 | RCT |
| Song et al. (19) | South Korea | 2017 | 121 | 41.1 ± 16.2 | 44.1 ± 12.5 | NA | 18.9 ± 39.1 | 95 | 38.0 ± 14.7 | 44.2 ± 13.54 | NA | 15.2 ± 30.7 | RCT |
| Hamano et al. (20) | Japan | 2020 | 70 | 74.4 ± 44.4 | 43.0 ± 14.0 | NA | 18.0 ± 36.0 | 37 | 61.2 ± 38.4 | 47.0 ± 13.0 | NA | 31.2 ± 69.6 | RCT |
| Jiang et al. (8) | China | 2020 | 779 | NA | 32.8 ± 5.7 | 22.2 ± 3.8 | 23.4 ± 16.7 | 342 | NA | 34.5 ± 7.4 | 24.6 ± 4.3 | 23.4 ± 16.7 | RCT |
| Ko et al. (21) | South Korea | 2020 | 1190 | NA | 45.9 ± 12.0 | 25.0 ± 7.5 | 20.9 ± 37.9 | 358 | NA | 47.3 ± 11.6 | 23.5 ± 9.1 | 20.2 ± 33.2 | RCT |
| Wang et al. (22) | China | 2020 | 96 | 29.8 (2-52) | 29.0 (15-53) | 20.2 (14.7-31.6) | 12 (0-108) | 48 | 27.6(4-51.6) | 30 (9-53) | 20.4 (14.8-32.6) | 12 (0-96) | RCT |
| Kim et al. (23) | South Korea | 2021 | 222 | NA | 63.9 ± 3.5 | 23.4 ± 3.1 | 3 (1-13) | 80 | NA | 63.7 ± 3.2 | 24.9 ± 9.1 | 2 (1-17) | RCT |
| Prabhakar et al. (24) | India | 2021 | 100 | 27.2 ± 20.6 | 40.8 (17-68) | NA | 5.5 (0-12) | 100 | 25.9 ± 20.5 | 40.9 (17-61) | NA | 5.77 (0-12) | RCT |
Summary of included cohorts: ABO-compatible and ABO-incompatible.
Data are presented as mean ± SD or median (IQR) as indicated in the table. Abbreviations: ABOi-LDKT, ABO-incompatible living donor kidney transplantation; ABOc-LDKT, ABO-compatible living donor kidney transplantation. RCT, randomized controlled trial.
In the analysis of graft survival rates, 14 studies were included for the 1-year survival rate, 11 for the 3-year survival rate, and 10 for the 5-year survival rate. As shown in Table 2 and Figure 2, the 1-year graft survival rate for the ABOi-LDKT group was lower than that for the ABOc-LDKT group (Pz < 0.01, OR = 0.518, CI = 0.386 - 0.695). However, there were no significant differences in the 3-year (Pz = 0.330, OR = 1.307, CI = 0.763 - 2.240) and 5-year (Pz = 0.193, OR = 0.623, CI = 0.305 - 1.270) survival rates between the two groups. In addition, the ABOi-LDKT group had a lower patient survival rate at 3 years (Pz = 0.021, OR = 0.552, CI = 0.333 - 0.914), but not at 1 year (Pz = 0.860, OR = 0.969, CI = 0.679 - 1.381) or 5 years (Pz = 0.187, OR = 1.199, CI = 0.916 - 1.569). In the subgroup analysis of postoperative complication risks, Cytomegalovirus (CMV) infection, BK virus infection, UTI, and postoperative acute rejection were investigated. No significant differences were observed between these groups, as shown by the forest plots in Figure 3.
Table 2
| Outcome | Number of studies (N) | OR (95%Cl) | Pz | PH | Model selection |
|---|---|---|---|---|---|
| Graft Survival Rate | |||||
| 1-year | 15 | 0.518 (0.386-0.695) | <0.01 | 0.987 | Fixed |
| 3-year | 11 | 1.307 (0.763-2.240) | 0.330 | 0.508 | Fixed |
| 5-year | 10 | 0.623 (0.305-1.270) | 0.193 | 0.001 | Random |
| Patient Survival Rate | |||||
| 1-year | 15 | 0.969 (0.679-1.381) | 0.860 | 0.586 | Fixed |
| 3-year | 14 | 0.552 (0.333-0.914) | 0.021 | 0.52 | Fixed |
| 5-year | 9 | 1.199 (0.916-1.569) | 0.187 | 0.645 | Fixed |
| Complications | |||||
| Cytomegalovirus | 13 | 1.002 (0.837-1.199) | 0.982 | 0.243 | Fixed |
| BK virus | 7 | 1.121 (0.885-1.421) | 0.344 | 0.133 | Fixed |
| Urinary Infection | 5 | 0.759 (0.483-1.194) | 0.233 | 0.068 | Random |
| Acute Rejection | 18 | 1.207 (0.925-1.574) | 0.166 | 0.095 | Random |
Meta-analysis results for patient survival rate, graft survival rate, and postoperative complications.
This table summarizes the pooled estimates for patient survival rates, graft survival rates, and postoperative complications based on data from included studies. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported for each time point.
Figure 2

Meta-analysis results for patient and graft survival rates at 1, 3, and 5 years. (a–c) Patient survival rate for 1, 3, 5 years respectively. (d–f) Graft survival rate for 1, 3, 5 years respectively. Odds Ratios (ORs) with 95% confidence intervals (CIs) are shown. Significant differences are indicated where p < 0.05.
Figure 3

Subgroup analysis of the meta-analysis results. (a) BK virus-related outcomes. (b) CMV-related outcomes. (c) Acute rejection- related outcomes. (d) Urinary tract infection-related outcomes. Hazard ratios (HRs) with 95% confidence intervals (CIs) are shown. Significant differences are indicated where p < 0.05.
Sensitivity analyses were performed using a leave-one-out strategy, sequentially excluding individual studies to evaluate the robustness of the pooled results. The recalculated pooled effect sizes (HRs/ORs) and corresponding 95% confidence intervals (CIs) remained consistent, confirming that no single study disproportionately influenced the overall findings (Supplementary Table S1; Supplementary Figure S1). Furthermore, publication bias was assessed through visual inspection of funnel plots and Begg’s rank correlation test. As shown in Supplementary Figure S2 and Supplementary Table S2, the symmetrical distribution of the funnel plots, corroborated by the non-significant results of Begg’s test (P > 0.05), indicates the absence of substantial publication bias among the included studies.
3.2 Clinical study result
3.2.1 Baseline characteristics of patients in the two groups
From January 1, 2021, to December 31, 2022, a total of 188 living donor kidney transplant recipients were screened at our center. In accordance with the prespecified eligibility criteria, 15 recipients who received grafts in the left iliac fossa were excluded to maintain technical homogeneity. The final cohort comprised 173 patients, including 41 in the ABOi-LDKT group and 132 in the ABOc-LDKT group. The screening process is summarized in Figure 1b. There were no statistically significant differences in sex, age, height, weight, body mass index (BMI), or donor GFR between the two donor and recipient groups. Baseline characteristics are summarized in Table 3 and Supplementary Figure S3.
Table 3
| Characteristics | ABOi-LDKT (n = 41) | ABOc-LDKT (n = 132) | P |
|---|---|---|---|
| Doner gender (n) | 0.683 | ||
| Male | 9 | 35 | |
| Female | 32 | 97 | |
| Doner age (y) | 57.07 ± 7.63 | 56.73 ± 7.03 | 0.754 |
| Doner BMI (kg/m2) | 24.47 ± 2.88 | 24.17 ± 3.55 | 0.447 |
| Donor kidney GFR by renography (mL/min) | 36.25 ± 9.60 | 35.26 ± 8.12 | 0.565 |
| Recipients Gender (n) | 0.301 | ||
| Male | 28 | 102 | |
| Female | 13 | 30 | |
| Recipient age (y) | 33.61 ± 7.57 | 34.55 ± 12.87 | 0.974 |
| Recipient BMI (kg/m2) | 21.13 ± 2.81 | 22.16 ± 3.65 | 0.064 |
| Preoperative Hypertension Status (n) | 27 | 66 | 0.106 |
| Preoperative Hypertension Duration (mo) | 49.44 ± 52.21 | 33.41 ± 30.73 | 0.237 |
| Preoperative Dialysis Status (n) | 0.550 | ||
| Hemodialysis | 28 | 89 | |
| Peritoneal Dialysis | 5 | 24 | |
| None | 8 | 19 | |
| Preoperative Dialysis Duration (mo) | 25.61 ± 27.25 | 24.81 ± 31.25 | 0.796 |
The basic information of donors and recipients in ABOi-LDKT and ABOc-LDKT.
Donor kidney GFR by renography: measured preoperatively by renal dynamic scintigraphy with ^99mTc-DTPA using the Gates camera method; values are reported as mL/min and represent the whole-kidney GFR of the donated side. Time units: durations are expressed in months (mo) unless otherwise indicated; years (y) are used where specified. Data are presented as mean ± SD as indicated in the table. Abbreviations: BMI, Body Mass Index, values are reported as kg/m2; ABOi-LDKT, ABO-incompatible living donor kidney transplantation; ABOc-LDKT, ABO-compatible living donor kidney transplantation.
3.2.2 Clinical data analysis results of the two groups of patients
All patients were followed until June 1, 2025. No significant differences were observed in patient or graft survival at 1 and 3 years between the ABOi-LDKT and ABOc-LDKT groups (Figures 4a, b and Table 4). Longitudinal renal function assessment revealed that ABOi recipients achieved superior eGFR at 2 weeks post-transplantation (P<0.05), likely attributable to the preconditioning apheresis. However, this difference was transient, with no significant disparities in eGFR or Scr levels observed at 1 month, 6 months, 1 year, or 3 years (Figure 5; Supplementary Table S3). Consistent with these findings, the analysis of SCr levels demonstrated similar recovery trajectories between the two groups, with no significant differences observed during the long-term follow-up period (Supplementary Figure S4; Supplementary Table S3).
Figure 4

Survival outcomes of ABOi-LDKT and ABOc-LDKT groups. (a) Kaplan–Meier survival curves for patient survival in the two groups. (b) Kaplan–Meier survival curves for graft survival in the two groups.
Figure 5
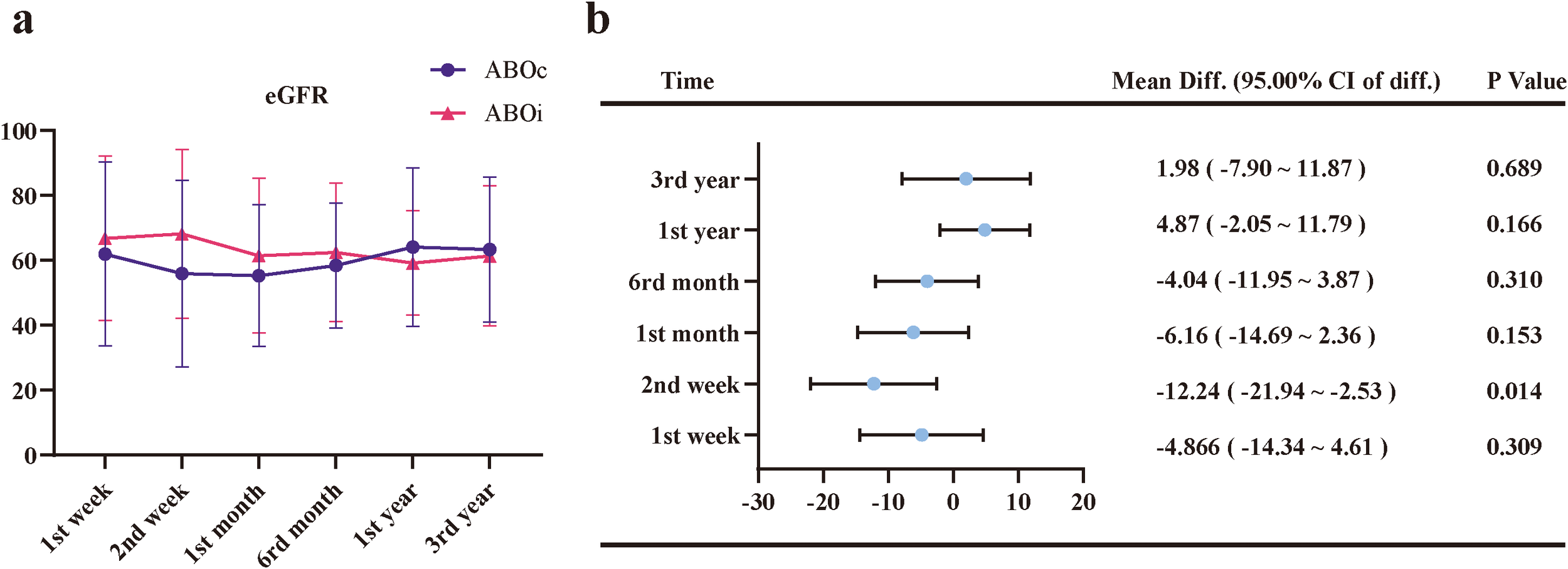
Longitudinal changes in estimated glomerular filtration rate (eGFR) and corresponding mean differences between the ABOi and ABOc groups. (a) Trends in eGFR levels at 1 week, 2 weeks, 1 month, 6 months, 1 year, and 3 years post-transplantation. (b) Forest plot showing mean differences (Mean Diff.) and 95.00% confidence intervals of the difference (95.00% CI of diff.) for eGFR between the ABOi and ABOc groups at each time point. Error bars represent standard deviations. A statistically significant difference was observed at 2 weeks post-transplantation (p < 0.05), while no significant differences were found at other time points.
Table 4
| Clinical Outcomes | ABOi-LDKT | ABOc-LDKT | P |
|---|---|---|---|
| Number | 41 | 132 | |
| Postoperative Hospital Stay (Day) | 22.27 ± 7.10 | 22.20 ± 9.30 | 0.955 |
| In-patient Care Spending (thousand CNY) | 127.13 ± 41.21 | 91.34 ± 38.74 | <0.001 |
| Delayed Graft Function | 2.44% | 3.03% | 1.000 |
| Pulmonary Infection | 34.15% | 20.45% | 0.092 |
| Urinary Tract Infection | 4.88% | 3.79% | 0.670 |
| Surgical Complication | 14.63% | 8.33% | 0.240 |
| Grafts Survival 1st year | 95.12% | 94.70% | 1.000 |
| Grafts Survival 3rd year | 92.68% | 92.42% | 1.000 |
| Patients Survival 1st year | 97.56% | 96.21% | 1.000 |
| Patients Survival 3rd year | 97.56% | 93.94% | 0.692 |
The clinical data analysis results of the two groups of patients.
Infection data represent episodes of severe infection requiring re-hospitalization within 1 year post-transplantation.
AMR occurred in only one patient (2.4%) in the ABOi-LDKT group on postoperative day 7. This patient presented with oliguria and a rebound in isoagglutinin titers but responded well to rescue therapy (PE and pulse steroids) with full functional recovery. The absence of rejection in the ABOc group and the rapid reversal of the single AMR case in the ABOi group underscore the efficacy of the immunosuppression protocols employed.
There were no statistically significant differences in the incidence of surgical complications (14.6% vs. 8.3%, P = 0.240). Specific complications in the ABOi group included ureteral stenosis (n=1), perinephric effusion (n=1), anastomotic bleeding (n=1), and wound dehiscence (n=3). All patients were successfully discharged following active treatment. Infection surveillance showed a numerically higher rate of pulmonary infection in the ABOi group (34.1% vs. 20.5%) and comparable rates of UTI (4.9% vs. 3.8%), though neither reached statistical significance. This relatively high incidence of pulmonary infection may be partly attributed to the overlap of the study period with the COVID-19 pandemic, as well as the strict criterion of recording only infections necessitating re-hospitalization. While the length of postoperative hospital stay was similar between groups (P = 0.955), total hospitalization costs were significantly higher for ABOi recipients (P<0.001), reflecting the additional resource utilization associated with desensitization therapy (Table 4).
3.2.3 Immunological outcomes and regimen efficacy
All 41 ABOi recipients underwent an individualized desensitization regimen tailored to their baseline immunological risk. The median number of pre-conditioning PE/DFPP sessions required was 2 (range: 1–12). This protocol demonstrated robust effectiveness: the median baseline isoagglutinin titers were 1:16 (IQR 1:8–1:32) for IgM and 1:8 (IQR 1:2–1:64) for IgG. Immediately prior to transplantation, median titers were significantly reduced to 1:4 (IQR 1:2–1:4) for IgM and 1:4 (IQR 1:2–1:8) for IgG (P<0.001), with 100% of patients achieving the target titer of ≤1:16. Postoperatively, antibody levels remained stable; the median titers for both IgM and IgG at postoperative days 3, 7, and 14 maintained at low levels (≤1:2), as shown in Figure 6, indicating minimal antibody rebound.
Figure 6

Effectiveness of the desensitization protocol and longitudinal dynamics of isoagglutinin titers. The graph illustrates the longitudinal changes in median IgM (blue solid line) and IgG (red dashed line) isoagglutinin titers for the ABOi cohort (n=41) at five key time points: baseline, pre-operation (Pre-op), and postoperative days (POD) 3, 7, and 14. Data points represent the median titer, and error bars represent the interquartile range (IQR). To ensure visual clarity, data points for IgM and IgG at the same time intervals are slightly offset. The horizontal dotted line at 1:16 indicates the clinical safety threshold for transplantation. Following the individualized desensitization regimen, median titers for both IgM and IgG significantly declined from baseline to pre-operation (P<0.001) and remained consistently low (≤1:2) throughout the early postoperative period, demonstrating effective antibody depletion and the absence of significant rebound.
4 Discussion
Despite the established status of ABOi-LDKT globally, its application in Asian populations has been hindered by concerns regarding the balance between aggressive desensitization and the risks of infection or rejection. This study aimed to comprehensively evaluate the “viability” of ABOi-LDKT in the Chinese setting through a dual approach: a systematic meta-analysis of Asian cohorts and a rigorous single-center validation. Our meta-analysis revealed that while ABOi-LDKT carries a historically higher risk of early graft loss and patient mortality compared to ABOc-LDKT transplants, our single-center experience demonstrates that these risks can be effectively mitigated. By implementing an individualized Rituximab-based desensitization protocol and stratified infection prophylaxis, we achieved 1-year and 3-year patient and graft survival rates comparable to ABOc-LDKT. These findings suggest that the “viability” of ABOi-LDKT is not an inherent fixity but is contingent upon the effectiveness of antibody depletion, the safety of perioperative management, and the acceptance of its economic trade-offs.
The primary determinant of ABOi-LDKT effectiveness is the successful depletion of isoagglutinins to prevent hyperacute rejection while fostering immunological accommodation. Historically, splenectomy was the standard for B-cell reduction, but it carried significant morbidity (6, 13). Our study validates the efficacy of a contemporary, spleen-preserving regimen utilizing low-dose Rituximab tailored to preoperative CD19+ B-cell counts. This “individualized” strategy proved highly effective: All of ABOi recipients maintained isoagglutinin titers below the safety threshold of 1:16 without needing rescue splenectomy. Notably, our single-center cohort reported a very low incidence of biopsy-proven acute rejection, with only one case (2.4%) of AMR. This case, characterized by a transient rebound of titers, was successfully reversed with plasma exchange, supporting the theory of “accommodation”—whereby the graft develops resistance to humoral injury over time despite the recurrence of antibodies (25). Furthermore, the observation that ABOi recipients exhibited superior eGFR at 2 weeks post-transplantation suggests that effective pre-conditioning, particularly PE, may remove inflammatory cytokines and mitigate early ischemia-reperfusion injury. Thus, our data confirm that modern desensitization protocols can effectively overcome the immunological barrier of blood type incompatibility.
Safety concerns, particularly infection-related mortality, have long been the Achilles’ heel of ABOi-LDKT. Our meta-analysis indicated significantly lower 3-year patient survival in pooled Asian cohorts, a finding consistent with USRDS data where infection remains a leading cause of death in desensitized patients (26, 27). However, our single-center results diverge positively from this trend, showing no significant difference in survival or severe infectious complications between ABOi and ABOc groups. We attribute this safety profile to our “Standardized Perioperative Management” protocol. Unlike earlier eras where “blanket” immunosuppression was common, we employed a risk-stratified approach. Specifically, Ganciclovir dosing for CMV prophylaxis was strictly adjusted based on postoperative eGFR (5-tier stratification), and Sulfamethoxazole-Trimethoprim was routinely initiated for Pneumocystis pneumonia prophylaxis. Although the incidence of pulmonary infection was numerically higher in the ABOi group (34.1% vs. 20.5%), reflecting the inevitable impact of enhanced immunosuppression, the lack of statistical significance and the absence of infection-related mortality highlight that these risks are manageable. This underscores that the safety of ABOi-LDKT relies less on reducing immunosuppression intensity and more on vigilant surveillance and preemptive antimicrobial strategy.
In the context of developing nations, the economic viability of ABOi-LDKT is as critical as its clinical success. Critics often point to the high upfront costs as a barrier. Indeed, our analysis confirmed that hospitalization costs for ABOi-LDKT were significantly higher ($127.1k vs. $91.3k CNY), driven largely by the specialized requirements of PE, adsorbers, and desensitization pharmacotherapy (28). However, this “sticker shock” must be contextualized within the broader health-economic landscape. The cost of maintaining a patient on hemodialysis for 2–3 years frequently exceeds the one-time premium of ABOi transplantation, not to mention the profound disparity in quality of life and societal reintegration (3, 29). Therefore, while ABOi-LDKT presents a higher initial financial threshold, it remains a cost-effective long-term investment for healthcare systems and a life-altering opportunity for patients stranded on waitlists.
This study has limitations inherent to its design. The single-center retrospective nature and modest sample size of the ABOi cohort precluded the use of propensity score matching, potentially introducing selection bias. Additionally, while we focused on short- to mid-term outcomes (up to 3 years), long-term complications such as chronic antibody-mediated rejection and late graft fibrosis require extended follow-up to fully assess. Finally, the heterogeneity of desensitization protocols in the included meta-analysis studies may affect the generalizability of the pooled risk estimates.
5 Conclusion
In conclusion, by integrating a meta-analysis of Asian cohorts with single-center clinical validation, this study confirms the viability of ABOi-LDKT for Chinese patients. Within the constraints of sample size and follow-up duration, we demonstrated that an individualized desensitization protocol combined with stratified infection prophylaxis can achieve 1-year and 3-year patient and graft survival rates comparable to ABOc-LDKT. Although ABOi-LDKT incurs higher upfront hospitalization costs, the effective depletion of isoagglutinins successfully minimized early acute rejection risks and supported favorable graft function recovery without increasing severe infectious complications. These findings provide robust, context-specific evidence that ABOi-LDKT is a clinically feasible and safe strategy to expand the living donor pool in China. Future large-scale, multicenter studies with extended follow-up are warranted to validate long-term outcomes and further optimize the cost-effectiveness of these protocols.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a retrospective, non-interventional study using only de-identified data extracted from routine clinical medical records, and involved no direct contact with patients or any change to their management, the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (approval No. PJ24-11-34) formally waived the requirement for written informed consent in accordance with national regulations and the Declaration of Helsinki.
Author contributions
YC: Formal analysis, Writing – original draft. JP: Writing – original draft, Formal analysis. DL: Writing – review & editing, Data curation. KW: Software, Writing – original draft. HD: Writing – original draft, Software. FZ: Validation, Writing – original draft. WW: Writing – review & editing, Data curation. PL: Writing – original draft, Formal analysis. JZ: Conceptualization, Writing – review & editing. GL: Investigation, Validation, Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the Outstanding Scientific Research and Innovation Team for Male Genitourinary Diseases in Anhui Provincial Universities (2022AH010071).
Acknowledgments
We thank Prof. Jialin Meng, and Dr. Qintao Ge from the Department of Urology, The First Affiliated Hospital of Anhui Medical University, for their valuable assistance with the statistical analysis. The authors used ChatGPT, a language model developed by OpenAI, to assist with language editing and stylistic refinement of the manuscript. All content was reviewed and approved by the authors to ensure accuracy and scientific integrity.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. Generative AI (ChatGPT, OpenAI) was used only to assist with language editing and improving the clarity of the manuscript. All scientific content, study design, data collection, analysis, interpretation, and final conclusions were generated, checked, and approved by the authors, who remain fully responsible for the work.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2026.1747411/full#supplementary-material
Supplementary Figure 1Sensitivity analysis results from the meta-analysis. (a) Patient survival rate at 1st year post-transplantation. (b) Graft survival rate at 1st year post-transplantation. (c) Patient survival rate at 3rd year post-transplantation. (d) Graft survival rate at 3rd year post-transplantation. (e) Patient survival rate at 5th year post-transplantation. (f) Graft survival rate at 5th year post-transplantation. (g) Outcomes related to BK virus infection. (h) Outcomes related to Cytomegalovirus (CMV) infection. (i) Outcomes related to acute rejection. (j) Outcomes related to urinary tract infection (UTI). Each panel presents the results of the sensitivity analysis for the corresponding outcome, showing pooled hazard ratios (HRs) with 95% confidence intervals (CIs). Significant differences are indicated where p < 0.05.
Supplementary Figure 2Funnel plot results from the meta-analysis. (a) Patient survival rate at 1st year post-transplantation. (b) Graft survival rate at 1st year post-transplantation. (c) Patient survival rate at 3rd year post-transplantation. (d) Graft survival rate at 3rd year post-transplantation. (e) Patient survival rate at 5th year post-transplantation. (f) Graft survival rate at 5th year post-transplantation. (g) BK virus infection outcomes. (h) Cytomegalovirus (CMV) infection outcomes. (i) Acute rejection outcomes. (j) Urinary tract infection (UTI) outcomes. Each panel represents a funnel plot showing the distribution of effect sizes for the corresponding outcome. The symmetry of the plots suggests the absence of publication bias. Significant differences are indicated where p < 0.05.
Supplementary Figure 3Baseline demographic and clinical characteristics of donors and recipients in the ABOi-LDKT and ABOc-LDKT groups.
Supplementary Figure 4Longitudinal changes in serum creatinine (Scr) levels and corresponding mean differences between the ABOi and ABOc groups. (a) Trends in Scr levels at 1 week, 2 weeks, 1 month, 6 months, 1 year, and 3 years post-transplantation. (b) Forest plot showing mean differences (Mean Diff.) and 95.00% confidence intervals of the difference (95.00% CI of diff.) for Scr between the ABOi and ABOc groups at each time point. Error bars represent standard deviations. Consistent with the eGFR findings, no clinically significant long-term differences were observed between the two groups.
References
1
Hill NR Fatoba ST Oke JL Hirst JA O'Callaghan CA Lasserson DS et al . Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PloS One. (2016) 11:e0158765. doi: 10.1371/journal.pone.0158765
2
Plantinga LC Johansen K Crews DC Shahinian VB Robinson BM Saran R et al . Association of CKD with disability in the United States. Am J Kidney Dis. (2011) 57:212–27. doi: 10.1053/j.ajkd.2010.08.016
3
Jian-feng Y Cheng Q Hong-tao L . Effect of hemodialysis, peritoneal dialysis and kidney transplantation on life quality in patients with end-stage renal disease and influencing factors. BME & Clin Med. (2020) 24:310–4. doi: 10.13339/j.cnki.sglc.20200415.002
4
Lo P Sharma A Craig JC Wyburn K Lim W Chapman JR et al . Preconditioning therapy in ABO-incompatible living kidney transplantation: A systematic review and meta-analysis. Transplantation. (2016) 100:933–42. doi: 10.1097/TP.0000000000000933
5
Alexandre GP Squifflet JP De Bruyere M Latinne D Reding R Gianello P et al . Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. (1987) 19:4538–42.
6
Tanabe K Ishida H Shimizu T Omoto K Shirakawa H Tokumoto T . Evaluation of two different preconditioning regimens for ABO-incompatible living kidney donor transplantation. A comparison of splenectomy vs. rituximab-treated non-splenectomy preconditioning regimens. Contrib Nephrol. (2009) 162:61–74. doi: 10.1159/000170813
7
Yi W Hongtao J . The technical operating specifications for ABO-incompatible living donor kidney transplantation in China(2019). Kidney Transplantation. (2019) 10:533–9.
8
Jiang H Li T Ren K Xiaohua Y Yi W Shanbin Z et al . A multicenter study of living kidney transplantation from ABO-incompatible relatives. Chin J Organ Transplantation. (2020) 41:259–64.
9
Jeon BJ Kim IG Seong YK Han BH . Analysis of the results of ABO-incompatible kidney transplantation: in comparison with ABO-compatible kidney transplantation. Korean J Urol. (2010) 51:863–9. doi: 10.4111/kju.2010.51.12.863
10
Fuchinoue S Ishii Y Sawada T Murakami T Iwadoh K Sannomiya A et al . The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. (2011) 91:853–7. doi: 10.1097/TP.0b013e31820f08e8
11
Kohei N Hirai T Omoto K Ishida H Tanabe K . Chronic antibody-mediated rejection is reduced by targeting B-cell immunity during an introductory period. Am J Transplant. (2012) 12:469–76. doi: 10.1111/j.1600-6143.2011.03830.x
12
Hwang JK Kim YK Kim JM Chung BH Choi BS Yang CW et al . Comparative analysis of ABO-incompatible living donor kidney transplantation with ABO-compatible grafts: a single-center experience in Korea. Transplant Proc. (2013) 45:2931–6. doi: 10.1016/j.transproceed.2013.08.038
13
Takahashi K Saito K . ABO-incompatible kidney transplantation. Transplant Rev (Orlando. (2013) 27:1–8. doi: 10.1016/j.trre.2012.07.003
14
Ashimine S Watarai Y Yamamoto T Hiramitsu T Tsujita M Nanmoku K et al . Neither pre-transplant rituximab nor splenectomy affects de novo HLA antibody production after renal transplantation. Kidney Int. (2014) 85:425–30. doi: 10.1038/ki.2013.291
15
Shin E Kwon SW Yang WS Baeck C Yu H Cho H et al . Long-term outcomes of ABO-incompatible living donor kidney transplantation: A comparative analysis. Transplant Proc. (2015) 47:1720–6. doi: 10.1016/j.transproceed.2015.05.026
16
Okumi M Toki D Nozaki T Shimizu T Shirakawa H Omoto K et al . ABO-incompatible living kidney transplants: evolution of outcomes and immunosuppressive management. Am J Transplant. (2016) 16:886–96. doi: 10.1111/ajt.13502
17
Park WY Kang SS Park SB Park UJ Kim HT Cho WH et al . Comparison of clinical outcomes between ABO-compatible and ABO-incompatible spousal donor kidney transplantation. Kidney Res Clin Pract. (2016) 35:50–4. doi: 10.1016/j.krcp.2015.11.001
18
Ko EJ Yu JH Yang CW Chung BH Korean Organ Transplantation Registry Study G . Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: a nationwide cohort study. Transpl Int. (2017) 30:1215–25. doi: 10.1111/tri.12979
19
Song SH Lee J Kim BS Kim S Lee JG Jeong HJ et al . Successful launch of an ABO-incompatible kidney transplantation program to overcome the shortage of compatible living donors: experience at a single center. Clin Nephrol. (2017) 88:117–23. doi: 10.5414/CN109114
20
Hamano I Hatakeyama S Fujita T Murakami R Hamaya T Togashi K et al . Outcome of ABO blood type-incompatible living-related donor kidney transplantation under a contemporary immunosuppression strategy in Japan. Transplant Proc. (2020) 52:1700–4. doi: 10.1016/j.transproceed.2020.01.152
21
Ko Y Kim JY Kim SH Kim DH Lim SJ Shin S et al . Acute rejection and infectious complications in ABO- and HLA-incompatible kidney transplantations. Ann Transplant. (2020) 25:e927420. doi: 10.12659/AOT.927420
22
Wang XD Liu JP Fan Y Song TR Shi YY Li YM et al . Individualized preconditioning for ABO-incompatible living-donor kidney transplantation: an initial report of 48 cases from China. Ann Transplant. (2020) 25:e920224. doi: 10.12659/AOT.920224
23
Kim DG Lee J Kim MS Kwon OJ Jung CW Lee KW et al . Outcomes of ABO-incompatible kidney transplantation in older patients: a national cohort study. Transpl Int. (2021) 34:290–301. doi: 10.1111/tri.13794
24
Prabhakar A Gang S Hegde U Konnur A Patel H Rajapurkar M . Kidney transplantation with ABO-incompatible donors: A comparison with matched ABO compatible donor transplants. Indian J Nephrol. (2021) 31:358–64. doi: 10.4103/ijn.IJN_206_20
25
Park WD Grande JP Ninova D Nath KA Platt JL Gloor JM et al . Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. (2003) 3:952–60. doi: 10.1034/j.1600-6143.2003.00179.x
26
Jackson KR Motter JD Bae S Kernodle A Long JJ Werbel W et al . Characterizing the landscape and impact of infections following kidney transplantation. Am J Transplant. (2021) 21:198–207. doi: 10.1111/ajt.16106
27
Lentine KL Axelrod D Klein C Simpkins C Xiao H Schnitzler MA et al . Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation. (2014) 98:54–65. doi: 10.1097/TP.0000000000000029
28
Schwartz J Stegall MD Kremers WK Gloor J . Complications, resource utilization, and cost of ABO-incompatible living donor kidney transplantation. Transplantation. (2006) 82:155–63. doi: 10.1097/01.tp.0000226152.13584.ae
29
Tyden G . Cost effectiveness of ABO-incompatible kidney transplantations. Transplantation. (2006) 82:166–7. doi: 10.1097/01.tp.0000226104.73438.5e
Summary
Keywords
ABO-incompatible living donor kidney transplantation, desensitization, graft survival, meta-analysis, postoperative outcomes
Citation
Chen Y, Pan J, Li D, Wang K, Ding H, Zhang F, Wang W, Li P, Zhong J and Liao G (2026) The viability of ABO-incompatible kidney transplants: a single-center cohort in China. Front. Immunol. 17:1747411. doi: 10.3389/fimmu.2026.1747411
Received
16 November 2025
Revised
17 January 2026
Accepted
26 January 2026
Published
17 February 2026
Volume
17 - 2026
Edited by
Cheng Yang, Fudan University, China
Reviewed by
Xianding Wang, Sichuan University, China
Jina Wang, Fudan University, China
Updates
Copyright
© 2026 Chen, Pan, Li, Wang, Ding, Zhang, Wang, Li, Zhong and Liao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiyi Liao, liaoguiyi@ahmu.edu.cn; Jinbiao Zhong, zhongjinbiao@ahmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.