Abstract
Background:
The kappa free light chain (κ-FLC) index is a sensitive marker of intrathecal immunoglobulin (Ig) synthesis and is increasingly used in cerebrospinal fluid (CSF) analysis of patients with suspected multiple sclerosis (MS). The relative contribution of the different Ig isotypes to intrathecal κ-FLC production remains unclear.
Methods:
We retrospectively analysed CSF data from patients with a first demyelinating event suggestive of MS enrolled in studies at the Medical Universities of Innsbruck and Vienna. Of all included patients, results on Ig and κ-FLC concentrations in CSF and serum were available. Linear regression analysis was used to assess the impact of Ig intrathecal fractions (IF) on κ-FLC index.
Results:
A total of 188 patients with a median age of 31 (25-39) years and a predominantly female sex distribution (62%) were included. The κ-FLC index was significantly higher in patients with isolated intrathecal IgG synthesis [32.5 (17.7-81.0); n=130] compared to patients without intrathecal immunoglobulin production [3.0 (2.0-5.9); p<0.001; n=18] and was further elevated in patients with both intrathecal IgG and IgM synthesis [68.4 (48.4-120.6); n=29]. Both IgG and IgM IF independently contributed to the κ-FLC index in linear regression analysis, with IgG IF having approximately 3.5 times the effect size of IgM IF. Exploratory analysis of the contribution of IgA IF to κ-FLC index revealed qualitatively the same results.
Conclusion:
Increase of κ-FLC index in patients with MS is predominantly due to an intrathecal IgG synthesis, while the contribution of intrathecal IgM is less frequent and quantitatively low.
Introduction
The synthesis of immunoglobulins (Ig) within the intrathecal compartment is a hallmark of multiple sclerosis (MS) (1–3) and other inflammatory neurological diseases (4). Different methods are available to detect intrathecally produced Ig. These include the quantitative measurement of IgA, IgM and IgG in paired cerebrospinal fluid (CSF) and serum samples followed by the calculation of the intrathecal fraction (IF) (5, 6), as well as the qualitative detection of CSF-restricted IgG oligoclonal bands (OCB) (7). In recent years, the quantification of kappa free light chains (κ-FLC) in CSF and serum followed by the calculation of the κ-FLC index has been introduced into routine CSF diagnostics (8). κ-FLC are produced by plasma cells in excess to intact immunoglobulins and also accumulate in the intrathecal compartment during inflammatory diseases of the central nervous system (CNS) (9).
While determination of the Ig IF shows only moderate diagnostic sensitivity (4), the detection of CSF-restricted OCB is technically demanding, time-consuming and exclusively reflects intrathecal IgG synthesis. The κ-FLC index overcomes the weaknesses of these two approaches. Measurement of κ-FLC is easy, fast, cost-effective and reliable (9) and the κ-FLC index shows a high diagnostic sensitivity and specificity comparable to OCB (10). Furthermore, the κ-FLC index increases not only in case of intrathecal IgG but also in case of intrathecal IgM and/or IgA synthesis (11, 12).
However, the relative contribution of intrathecal synthesis of the different Ig isotypes, i.e., of IgG, IgA and IgM, to an intrathecal κ-FLC synthesis, i.e., to an increase of κ-FLC index, in patients with MS is not known, which is why we performed the present study.
Methods
Patients and samples
We included patients from observational studies at the Medical University of Innsbruck [Risk Assessment in Multiple Sclerosis by Cerebrospinal Fluid Free Light Chains (RIMSC) (13) and Biomarker Risk Assessment in Multiple Sclerosis (BRAMS)] and the Medical University of Vienna [Austrian Multiple Sclerosis Cohort (AMSC)] (14). For this study, in patients with a first demyelinating event of the CNS suggestive of MS, lumbar puncture (LP) and determination of Ig IF as well as of the κ-FLC index were necessary for inclusion. All patients were treatment-naïve at the time of LP. Details on inclusion of patients are given in Supplementary Figure S1. Diagnosis of MS was made using the 2017 revised McDonald criteria (1).
All CSF samples were collected by LP. Serum samples were collected concomitantly within 30 minutes via venipuncture. Samples were centrifuged at 2000 g for 10 minutes at room temperature before measurement (15).
Routine cerebrospinal fluid analysis
White blood cell (WBC) and red blood cell (RBC) counts were determined using Fuchs-Rosenthal chamber (MUI) (4), or automated by Sysmex confirmed by visual counting using Fuchs-Rosenthal chamber if counts were ≥5/μL (MUV).
Immunoglobulin concentrations were determined by nephelometry. Intrathecal synthesis of IgG, IgM and IgA was calculated using the Auer & Hegen formulae as previously published (5). Detection of OCB was performed by isoelectric focusing and subsequent immunoblotting using IgG-specific antibody staining at both centres as previously described (7).
Determination of albumin and k-free light chains
Albumin and κ-FLC in CSF and serum were measured by nephelometry (Atellica; Siemens, Erlangen, Germany) using the N Albumin and N Latex FLC kappa assay (16, 17), respectively, according to the manufacturer’s instructions.
Calculation of the κ-FLC Index
The κ-FLC index was calculated using the following formula:
A κ-FLC index ≥6.1 was considered positive (10).
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and continuous variables as median, 25th, 75th percentile and range as appropriate. For group comparisons, the Mann-Whitney-U test and Kruskal-Wallis test were applied. Bonferroni correction was done for multiple testing. Pearson correlation coefficient (r) was used for correlation analysis. Linear regression model was performed to evaluate the influence of the IF of the different Ig isotypes (continuous) on the κ-FLC index. The distribution of the κ-FLC index is highly right-skewed (Supplementary Figure S2). Furthermore, the impact of change of IgG IF (or IgM IF) on the κ-FLC index is not constant over the full range of possible κ-FLC index values (Supplementary Figure S3). Therefore, a log-transformation of k-FLC index is appropriate in linear regression.
A p-value <0.05 was considered statistically significant. In regression analyses, according to the clear one-sided hypotheses, i.e. higher κ-FLC index in case of intrathecal IgG, IgA and/or IgM synthesis (12, 13), one-sided hypothesis testing was used and, thus, one-sided p-values are shown. The unidirectional relationship between κ-FLC and Ig is based on a clear biological mechanism: Plasma cells produce intact Ig and in excess κ-FLC, i.e., in case of intrathecal plasma cell activity both Ig and κ-FLC accumulate in the intrathecal compartment (9). Therefore, the parameters that quantitatively capture intrathecal Ig and κ-FLC synthesis, i.e., IF Ig (4) as well as κ-FLC index (2, 3), increase in case intrathecal plasma cell activity.
All statistical analyses were performed in R (18).
Ethics statement
The study was approved by the Ethics committees of the Medical Universities of Innsbruck (approval number: 1050/2023 and 1244/2019) and Vienna (approval number: 1368/2023). Informed consent was obtained from all participants. We adhered to the declaration of Helsinki and national regulations during all study procedures.
Results
A total of 188 patients at a median age of 31 (25–39) years showing a female predominance (62%) were included into this study. One hundred seventy-seven (94%) patients were diagnosed as relapsing-remitting MS (RRMS), while the remaining patients were considered as clinically isolated syndrome. The κ-FLC index ranged from 1.3 to 508.8 and was elevated in 167 (89%) of patients. While 18 (10%) patients had no intrathecal IgG synthesis (OCB negative), 130 (69%) had isolated intrathecal IgG synthesis (OCB positive) and 29 (15%) patients had intrathecal IgG and IgM synthesis. Only 2 (1%) patients had intrathecal IgG and IgA synthesis. Further details on demographics, clinical characteristics and CSF findings are shown in Table 1.
Table 1
| Demographics | |
|---|---|
| Age (years) | 31 (25–39) |
| Sex (female) | 116 (62) |
| Clinical characteristics | |
| RRMS (according 2017 McDonald criteria) | 177 (94) |
| Clinically isolated syndrome | 11 (6) |
| CSF findings | |
| WBC (/μL) | 7 (3-13) |
| RBC (/μL) | 0 (0-1) |
| Total protein (mg/dL) | 39 (32-52) |
| CSF albumin (mg/dL) | 19.2 (15.6-27.3) |
| Serum albumin (mg/dL) | 4280 (4000-4520) |
| Qalb (×10-3) | 4.7 (3.6-6.3) |
| IgG IF (%) >0 | 112 (59.6) |
| IgG IF (%)* | 28.9 (13.9-48.9) |
| IgM IF (%) >0 | 38 (20.2) |
| IgM IF (%)* | 37.9 (23.6-61.3) |
| IgA IF (%) >0 | 11 (5.9) |
| IgA IF (%)* | 8.8 (4.7-41.8) |
| OCB positive, n (%) | 170 (90.4) |
| CSF κ-FLC (mg/dL) | 0.2 (0.1-0.5) |
| Serum κ-FLC (mg/dL) | 1.2 (0.9-1.5) |
| Qκ-FLC (×10-3) | 17.6 (7.8-40.5) |
| κ-FLC index | 35.8 (15.2-89.2) |
| κ-FLC index ≥ 6.1 | 167 (88.8) |
Demographics, clinical characteristics and CSF findings.
Data are given as median (25th-75th percentile) and n (%), as appropriate. * Given only for patients with Ig IF >0%.
WBC, white blood count; RBC, red blood cell count; CSF, cerebrospinal fluid; Qalb, CSF/serum albumin ratio; Ig, immunoglobulin; IF, intrathecal fraction; RRMS, relapsing-remitting multiple sclerosis; OCB, oligoclonal bands; FLC, free light chain; Qκ-FLC, CSF/serum κ-FLC ratio.
The κ-FLC index was statistically significantly higher in patients with isolated intrathecal IgG synthesis [32.5 (17.7-81.0)] as well as in patients with intrathecal IgG and IgM synthesis [68.4 (48.4-120.6] compared to patients with no intrathecal Ig synthesis [3.0 (2.0-5.9), both p<0.001, Figure 1]. The percentage IF of IgG was higher in patients with IgG and IgM synthesis compared to patients with isolated IgG synthesis (Supplementary Figure S4). Regarding the impact of intrathecal IgA synthesis, we refrained from group-wise statistical comparisons due to the small number of patients. A descriptive depiction is given in Supplementary Figures S5, S6. Qualitatively, the findings were similar.
Figure 1

κ-FLC index according to the presence of intrathecal synthesis of different immunoglobulin isotypes. No Ig synthesis = OCB negativity and IgA IF ≤0 and IgM IF ≤0. IgG synthesis = OCB positivity. IgG + IgM synthesis = OCB positivity and IgM IF >0%. FLC, free light chain; Ig, immunoglobulin.
Multivariable linear regression was performed to identify the relative independent contribution of intrathecal IgG synthesis and IgM synthesis to elevation of the κ-FLC index. Overall, the κ-FLC index (ln) increased with the % IgG IF (β=0.041, p<0.001) and with the % IgM IF (β=0.005, p=0.051, Table 2A, Supplementary Table S1). Similarly, in the subgroup of patients with both positive IgG as well as IgM IF, linear regression revealed qualitatively the same results, i.e. the κ-FLC index (ln) increased with % IgG IF (β=0.026, p<0.001) and % IgM IF (β=0.008, p=0.023) (Table 2B; Figure 2; Supplementary Table S1). Furthermore, there was no correlation between IF IgG and IF IgM (Supplementary Figure S7).
Table 2
| (A) | ||||
|---|---|---|---|---|
| ln (κ-FLC index) | Estimate | Standard error | P-value | |
| One-sided | Two-sided | |||
| IgG IF (per % increase) | 0.041 | 0.003 | <0.001 | <0.001 |
| IgM IF (per % increase) | 0.005 | 0.003 | 0.051 | 0.101 |
| (B) | ||||
|---|---|---|---|---|
| ln (κ-FLC index) | Estimate | Standard error | P-value | |
| One-sided | Two-sided | |||
| IgG IF (per % increase) | 0.026 | 0.004 | <0.001 | <0.001 |
| IgM IF (per % increase) | 0.008 | 0.004 | 0.023 | 0.045 |
Multivariable linear regression analyses identifying the contribution of intrathecal IgG and IgM synthesis to the increase of κ-FLC index.
2.R = 0.586, VIF = 1.1.
2.R = 0.593, VIF = 1.0.
Linear regression analyses to evaluate the influence of the IgG and IgM IF on κ-FLC index using (A) the whole patient cohort and (B) the subgroup of patients positive for both IgG and IgM IF (>0%).
Due to one-sided hypothesis testing, one-sided p-values are given.
FLC, free light chain; Ig, immunoglobulin; IF, intrathecal fraction.
Figure 2
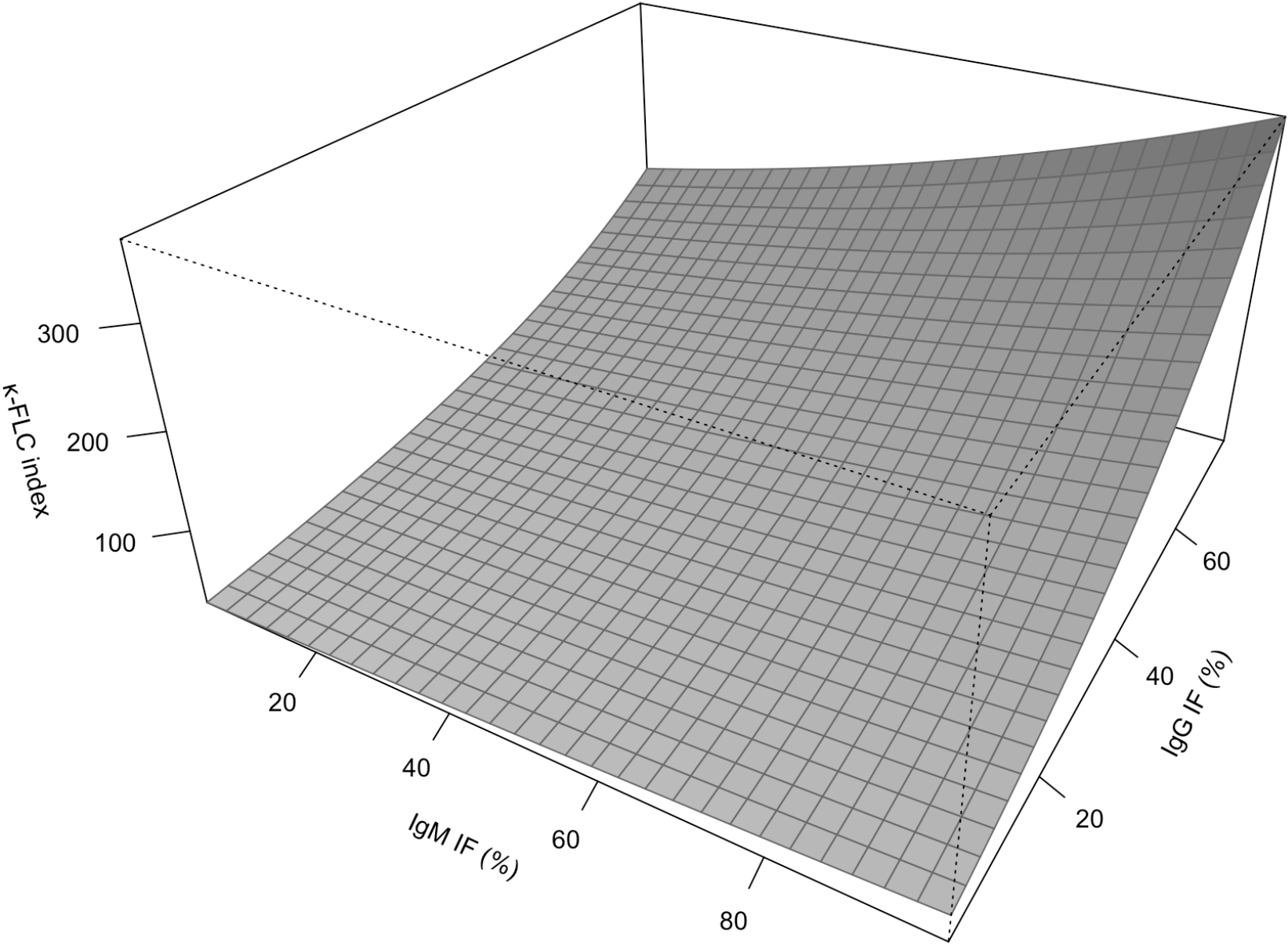
Contribution of intrathecal IgG and IgM synthesis to κ-FLC index according to multivariable model. FLC, free light chain; Ig, immunoglobulin; IF, intrathecal fraction.
Discussion
Here, we investigated to what extent the intrathecal synthesis of different immunoglobulin isotypes contributes to an intrathecal κ-FLC synthesis. We made two main observations: in patients with a first demyelinating event suggestive of MS, i) intrathecal IgG synthesis was most frequently observed (OCB: 90%, elevated IgG IF: 60% compared to elevated IgA IF and IgM IF in 6% and 20%, respectively), ii) quantitatively, intrathecal IgG synthesis is the main contributor to an increase in κ-FLC index, while the contribution of intrathecal IgM synthesis (by approximately 3.5-fold) is lower.
Plasma cells secrete intact immunoglobulins, along with excess light chains which circulate freely in body fluids including CSF (8–10). The production of FLC is not isotype-specific, that is, it is not possible to determine whether a given kappa light chain derives from an IgG-, IgA-, or IgM-producing plasma cell (19). To understand the contribution of specific immunoglobulin isotypes to κ-FLC index, it was necessary first to quantitatively assess the intrathecal production of individual immunoglobulin isotypes, and by calculating the IF of IgG, IgA and IgM (5) and correlating them with κ-FLC index values, conclusions can be made about each isotype’s relative contribution to κ-FLC index.
It is well established that intrathecal IgG and IgM production plays a significant role in MS pathophysiology (20). The detection of clonal IgG expansion in CNS – as measured qualitatively by OCB - has been a part of MS diagnostic criteria since 1983 (21). Given the high concordance and near-interchangeability of OCB detection and the κ-FLC index in MS diagnostics (8, 10), it is expected that intrathecal IgG synthesis is the predominant contributor to elevated κ-FLC index values.
With regard to IgM, previous studies highlighted a role for intrathecal IgM synthesis in MS, as it was linked to unfavourable disease course (22–24). In line with previous findings (25) we observed intrathecal IgM synthesis in 20% of patients, however, we also observed that IgM synthesis is quantitively less important. Given that the κ-FLC index holds prognostic value for MS disease course, predicting time to relapse (11), new brain MRI activity (26) and cognitive deterioration (27), it seems that the amount of general immune activation is of importance rather than a specific Ig isotype.
Intrathecal IgA synthesis was only present in 6% of patients. Due to the small number of patients with an intrathecal IgA synthesis, we could not reliably assess the relative contribution to κ-FLC index. It is known that an isolated intrathecal IgA synthesis can lead to increase in κ-FLC index (11). In MS, however, it occurs infrequently, confirming previous findings (approximately 10%) (25) and its involvement in MS is controversial and poorly understood (28). Nevertheless, we observed qualitatively the same pattern as with IgM, i.e. in case of an additional intrathecal IgA synthesis, there was also a stronger intrathecal IgG synthesis, thus, probably explaining the higher κ-FLC index. Again, we have to clearly state that role of IgA could not be confirmed in this study, as we did not have enough statistical power to reach statistical significance in the analyses.
There are some limitations of the study. First, it was a retrospective analysis with all its inherent restrictions. Second, the sample size is relatively small. Third, we included only patients with a CNS demyelinating event suggestive of MS. While this is a strength in terms of studying treatment-naïve patients (e.g., as a potential confounding effect of immune treatment on κ-FLC index levels (29) are eliminated), the contribution of different Ig isotypes to the κ-FLC index might shift in later, more chronic stages of the disease. Furthermore, our findings cannot be generalized to other inflammatory neurological diseases. This is subject to further research.
In summary, our study demonstrates that intrathecal IgG synthesis is the main contributor to intrathecal κ-FLC synthesis, i.e. increased κ-FLC index values, in patients with MS. This further substantiates the high agreement between κ-FLC index and OCB (30, 31).
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committees of the Medical Universities of Innsbruck (approval number: 1050/2023 and 1244/2019) and Vienna (approval number: 1368/2023). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MS: Writing – original draft, Formal analysis, Project administration, Data curation, Conceptualization, Writing – review & editing, Investigation. KB: Project administration, Writing – review & editing, Formal analysis, Conceptualization, Writing – original draft, Data curation. MA: Writing – review & editing, Writing – original draft. FP: Writing – original draft, Writing – review & editing. FF: Writing – original draft, Writing – review & editing. NK: Writing – review & editing, Writing – original draft. FD: Writing – original draft, Writing – review & editing. GB: Writing – original draft, Writing – review & editing. JW: Formal analysis, Writing – review & editing, Writing – original draft. HH: Resources, Conceptualization, Methodology, Investigation, Validation, Visualization, Writing – review & editing, Supervision, Formal analysis, Writing – original draft, Data curation, Project administration, Funding acquisition.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
MS has participated in meetings sponsored by or received travel grants from Novartis, Sanofi-Genzyme and Amgen. KB has participated in meetings sponsored by and received travel funding or speaker honoraria from Roche, Teva, Merck, Biogen, Sanofi and Novartis. He is associate editor of Frontiers in Immunology/Neurology, Section Multiple Sclerosis and Neuroimmunology and member of the editorial board of Neurology International. MA has received speaker honoraria and/or travel grants from Biogen, Merck, Novartis, and Sanofi Genzyme, Horizon Therapeutics/Amgen and Zentiva. FP has participated in meetings sponsored by, received honoraria lectures, advisory boards, consultations, or travel funding from Bayer, Biogen, Celgene BMS, Merck, Novartis, Sanofi-Genzyme, Teva, and Roche. Her institution has received research grants from Roche. FF Has received speaker honoraria and travel funding from Novartis. NK has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS. FD has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Merck, Novartis, Roche, and Sanofi-Genzyme. His institution received scientific grants from Biogen and Sanofi-Genzyme. GB has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, BMS, Heidelberg Engineering, Janssen, Lilly, Medwhizz, Merck, Neuraxpharm, Novartis, Roche, Sanofi, Teva and Zeiss, and received honoraria for consulting Adivo Associates, Biogen, BMS, Janssen, Merck, Novartis, Roche, Sanofi and Teva. He has received unrestricted research grants from BMS, Merck and Novartis. He serves on the Executive Committee of the European Committee for Treatment and Research in Multiple Sclerosis ECTRIMS and the Board of Directors of the International Multiple Sclerosis VisualSystem Consortium IMSVISUAL. HH has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, Amgen, Bayer, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as consultant for Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi-Genzyme, and Teva. His institution has received research grants from Alexion, Amgen, Novartis and Roche.
The remaining author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors KB, HH declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2026.1747659/full#supplementary-material
References
1
Thompson AJ Banwell BL Barkhof F Carroll WM Coetzee T Comi G et al . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
2
Deisenhammer F Hegen H Arrambide G Banwell BL Coetzee T Gnanapavan S et al . Positive cerebrospinal fluid in the 2024 McDonald criteria for multiple sclerosis. eBioMedicine. (2025) 120:105905. doi: 10.1016/j.ebiom.2025.105905
3
Montalban X Lebrun-Frénay C Oh J Arrambide G Moccia M Amato MP et al . Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. (2025) 24:850–65. doi: 10.1016/S1474-4422(25)00270-4
4
Deisenhammer F Bartos A Egg R Gilhus NE Giovannoni G Rauer S et al . Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. (2006) 13:913–22. doi: 10.1111/j.1468-1331.2006.01493.x
5
Auer M Hegen H Zeileis A Deisenhammer F . Quantitation of intrathecal immunoglobulin synthesis - a new empirical formula. Eur J Neurol. (2016) 23:713–21. doi: 10.1111/ene.12924
6
Reiber H . Flow rate of cerebrospinal fluid (CSF) — A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. (1994) 122:189–203. doi: 10.1016/0022-510X(94)90298-4
7
Freedman MS Thompson EJ Deisenhammer F Giovannoni G Grimsley G Keir G et al . Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch Neurol. (2005) 62:865–70. doi: 10.1001/archneur.62.6.865
8
Hegen H Arrambide G Gnanapavan S Kaplan B Khalil M Saadeh R et al . Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Mult. Scler. J. (2023) 29:182–195. doi: 10.1177/13524585221134217
9
Hegen H Berek K Deisenhammer F . Cerebrospinal fluid kappa free light chains as biomarker in multiple sclerosis-from diagnosis to prediction of disease activity. Wien Med Wochenschr 1946. (2022) 172:337–45. doi: 10.1007/s10354-022-00912-7
10
Hegen H Walde J Berek K Arrambide G Gnanapavan S Kaplan B et al . Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult Scler Houndmills Basingstoke Engl. (2023) 29:169–81. doi: 10.1177/13524585221134213
11
Hannich MJ Dressel A Budde K Petersmann A Nauck M Süße M . Kappa free light chains in the context of blood contamination, and other igA- and igM-related cerebrospinal fluid disease pattern. Cells. (2021) 10:616. doi: 10.3390/cells10030616
12
Hegen H Milosavljevic D Schnabl C Manowiecka A Walde J Deisenhammer F et al . Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin Chem Lab Med. (2018) 56:1383–91. doi: 10.1515/cclm-2018-0028
13
Berek K Bsteh G Auer M Di Pauli F Grams A Milosavljevic D et al . Kappa-free light chains in CSF predict early multiple sclerosis disease activity. Neurol Neuroimmunol Neuroinflammation. (2021) 8:e1005. doi: 10.1212/NXI.0000000000001005
14
Bsteh G Föttinger F Ponleitner M Berek K Pauli FD Heschl B et al . The Austrian MS Database and the Austrian MS Cohort – a national effort towards data harmonization and prospective data collection. medRxiv. (2025). doi: 10.1101/2025.02.17.25322422v1
15
Teunissen CE Petzold A Bennett JL Berven FS Brundin L Comabella M et al . A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. (2009) 73:1914–22. doi: 10.1212/WNL.0b013e3181c47cc2
16
Hoedemakers RMJ Pruijt JFM Hol S Teunissen E Martens H Stam P et al . Clinical comparison of new monoclonal antibody-based nephelometric assays for free light chain kappa and lambda to polyclonal antibody-based assays and immunofixation electrophoresis. Clin Chem Lab Med. (2011) 50:489–95. doi: 10.1515/cclm-2011-0568
17
Velthuis HT Knop I Stam P van den Broek M Bos HK Hol S et al . N Latex FLC - new monoclonal high-performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med. (2011) 49:1323–32. doi: 10.1515/CCLM.2011.624
18
RStudio Team . RStudio: Integrated Development for R. Boston, MA: RStudio, PBC (2020). Available online at: http://www.rstudio.com/ (Accessed November 26, 2024).
19
Nakano T Matsui M Inoue I Awata T Katayama S Murakoshi T . Free immunoglobulin light chain: Its biology and implications in diseases. Clin Chim Acta Int J Clin Chem. (2011) 412:843–9. doi: 10.1016/j.cca.2011.03.007
20
Yu X Graner M Kennedy PGE Liu Y . The role of antibodies in the pathogenesis of multiple sclerosis. Front Neurol. (2020) 11:533388. doi: 10.3389/fneur.2020.533388
21
Poser CM Paty DW Scheinberg L McDonald WI Davis FA Ebers GC et al . New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol. (1983) 13:227–31. doi: 10.1002/ana.410130302
22
Villar LM Casanova B Ouamara N Comabella M Jalili F Leppert D et al . Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol. (2014) 76:231–40. doi: 10.1002/ana.24190
23
Sola P Mandrioli J Simone AM Ferraro D Bedin R Annecca R et al . Primary progressive versus relapsing-onset multiple sclerosis: presence and prognostic value of cerebrospinal fluid oligoclonal IgM. Mult Scler Houndmills Basingstoke Engl. (2011) 17:303–11. doi: 10.1177/1352458510386996
24
Masi F Al Qudsi S Visigalli D Zardini E Capello E Dicembre LP et al . Oligoclonal IgM band patterns in multiple sclerosis: A two-center study. J Neuroimmunol. (2025) 404:578622. doi: 10.1016/j.jneuroim.2025.578622
25
Berek K Bsteh G Auer M Di Pauli F Zinganell A Berger T et al . Cerebrospinal fluid findings in 541 patients with clinically isolated syndrome and multiple sclerosis: A monocentric study. Front Immunol. (2021) 12:675307. doi: 10.3389/fimmu.2021.675307
26
Berek K Schmidauer M Bsteh G Auer M Barket R Berger T et al . Kappa free light chain index predicts long-term disease activity and disability accrual in multiple sclerosis. Mult Scler J. (2025) 16:13524585251344807. doi: 10.1177/13524585251344807
27
Rosenstein I Axelsson M Novakova L Rasch S Blennow K Zetterberg H et al . High levels of kappa free light chain synthesis predict cognitive decline in relapsing-remitting multiple sclerosis. Front Immunol. (2023) 14:1106028/full. doi: 10.3389/fimmu.2023.1106028/full
28
Woof JM Kerr MA . The function of immunoglobulin A in immunity. J Pathol. (2006) 208:270–282. doi: 10.1002/path.1877
29
Duell F Asplund Högelin K Vlad B Neidhart S Khademi M Jelcic I et al . Kappa free light chain index correlates with prognostic biomarkers in multiple sclerosis and decreases slowly following treatment. Eur J Neurol. (2025) 32:e70291. doi: 10.1111/ene.70291
30
Arrambide G Espejo C Carbonell-Mirabent P Dieli-Crimi R Rodríguez-Barranco M Castillo M et al . The kappa free light chain index and oligoclonal bands have a similar role in the McDonald criteria. Brain J Neurol. (2022) 145:3931–42. doi: 10.1093/brain/awac220
31
Hegen H Berek K Cavalla P Christiansen M Emeršič A Di Filippo M et al . Diagnostic value of kappa free light chain index in patients with primary progressive multiple sclerosis - a multicentre study. Front Immunol. (2023) 14:1327947. doi: 10.3389/fimmu.2023.1327947
Summary
Keywords
biomarker, crebrospinal fluid, diagnostic, IgG, IgM, Kappa (κ) FLC, multiple sclerosis
Citation
Schmidauer M, Berek K, Auer M, Di Pauli F, Föttinger F, Krajnc N, Deisenhammer F, Bsteh G, Walde J and Hegen H (2026) Kappa free light chain index in CSF diagnostics: the impact of different immunoglobulin isotypes. Front. Immunol. 17:1747659. doi: 10.3389/fimmu.2026.1747659
Received
16 November 2025
Revised
27 January 2026
Accepted
31 January 2026
Published
17 February 2026
Volume
17 - 2026
Edited by
Antonio Bertolotto, Koelliker Hospital, Italy
Reviewed by
Massimiliano Castellazzi, University of Ferrara, Italy
Massimo Pieri, University of Rome Tor Vergata, Italy
Updates
Copyright
© 2026 Schmidauer, Berek, Auer, Di Pauli, Föttinger, Krajnc, Deisenhammer, Bsteh, Walde and Hegen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harald Hegen, harald.hegen@i-med.ac.at
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.