Abstract
Introduction:
Intrauterine adhesion (IUA) is characterized by endometrial fibrosis with partial or complete obliteration of the uterine cavity due to adhesion of the uterine wall. Currently, there is lack of effective strategies to address IUA and a strategy that can resolve endometrial fibrosis is needed. Human endometrial mesenchymal stem cells (H-EMSCs) and macrophages (mø) both reside in endometrial tissues and are important for endometrial repair. However, whether co-delivery of H-EMSCs and mø using a biocompatible biomaterial platform could address endometrial fibrosis and enhance repair remains unknown.
Methods:
This study developed a H-EMSCs-mø co-delivery system using an electrospun polycaprolactone-hyaluronic acid (PCL-HA) membrane and established a rat endometrial damage model. The effects of the co-delivery system on endometrial tissue fibrosis, M1 and M2 mø phenotypic marker modulation, and the pro-inflammatory (TNF-α) and anti-inflammatory (IL-10) factor production were investigated. The mechanisms involved in the interactions between H-EMSCs and mø were also delineated. All data were analyzed using analysis of variance with Tukey’s test for pair-wise comparisons or an independent samples t-test where appropriate.
Results:
In the rat endometrial damage model, H-EMSCs and mø co-delivery system (PCL-HA/H-E/mø) could significantly reduce endometrial fibrosis at day 14 after implantation vs. PCL-HA alone or H-EMSCs single-delivery (PCL-HA/H-E). In addition, PCL-HA/H-E/mø supported M1 (CD80, CD86) to M2-type mø phenotypic marker change and enhanced MSC marker (CD90) expression at days 3, 7 and 14, when compared to PCL-HA and PCL-HA/H-E groups. Moreover, PCL-HA/H-E/mø system had lower TNF-α gene expression but higher IL-10 gene and protein expression at day 7 post-implantation, suggesting an overall change from a more pro-inflammatory to a more wound-healing endometrial tissue microenvironment. In probing the mechanisms underlying the anti-fibrotic effect of the PCL-HA/H-E/mø system, it was found that IL-10 could have significantly reduced endometrial tissue fibrosis.
Discussions:
Co-delivery system reduced fibrosis and supported an M1-type to M2-type overall change of the mø phenotypes in the endometrial tissue damage model. IL-10 was one of the important factors in inhibiting endometrial fibrosis in the co-delivery system. This study provided significant insights into using a co-delivery system PCL-HA/H-E/mø, to effectively alleviate endometrial fibrosis and potentially for a more effective treatment of IUA.
Graphical Abstract

1 Background
Intrauterine adhesion (IUA), which is typically a uterine manifestation of Asherman’s syndrome, is characterized by endometrial fibrosis, with partial or complete obliteration of the uterine cavity due to adhesions of the uterine wall (1). IUA can be caused by trauma, infection and repeated curettage (2), and is a leading cause of uterine infertility worldwide (3). At present, the gold-standard clinical treatment for IUA is transcervical resection of adhesion (TCRA), in which an intrauterine device and balloon are inserted to expand the narrow uterine cavity (4). However, TCRA can have low effectiveness, and the reoccurrence rate post-surgery is up to 62.5% (5). The root cause for such a high reoccurrence rate is that TCRA relies on the endometrium’s self-repair capacity, instead of fundamentally addressing the underlying fibrotic pathology (6). IUA treatment would therefore benefit from a strategy to prevent fibrosis and facilitate the natural repair of the damaged endometrium.
Human endometrial mesenchymal stem cells (H-EMSCs) have self-renewal, immunomodulation capabilities and can promote new endometrial tissue formation and increase endometrial thickness after menstruation (7, 8). In other work, it has been found that monocyte-derived macrophages (mø) play a crucial role in endometrial repair and regeneration after injury (9). Mø are well-acknowledged to be key regulators of inflammation, fibrosis, tissue repair and regeneration (10). Specifically, mø can remove endometrial tissue debris through phagocytosis and produce a number of pro-inflammatory and anti-inflammatory cytokines, and growth factors to regulate the micro-environment, thereby contributing to the repair and regeneration of the endometrial tissues (11). Mø are classified into many differentiated phenotypes which fall under two major categories, each with their own multitude of sub-phenotypes: the classically activated mø sub-types (M1) and alternatively activated mø subtypes (M2) (12). Mø can also influence the survival, proliferation and migration of MSCs. Specifically, M2 mø can enhance bone marrow MSCs growth, proliferation and engraftment (13), while M1 mø can inhibit bone marrow MSCs proliferation and induce their apoptosis (14). On the other hand, MSCs can regulate mø via cell-cell contact or paracrine signaling mechanisms (15, 16). For example, the exosomes derived from umbilical-cord and placenta derived MSCs could induce the polarization of M1 pro-inflammatory mø towards the M2 anti-inflammatory phenotype mø, which enhanced the endometrial tissue repair (17, 18).
Direct injection of MSCs has been used for endometrial tissue repair (19, 20), but the benefit is limited due to a low survival rate and short retention time of the transplanted MSCs (21). Biomaterials could be designed as carriers for MSCs to address those issues associated with cell injection, as biomaterials can mimic the morphology of the natural extracellular matrix (ECM) and provide a proper microenvironment for cell attachment and proliferation (22). However, using biomaterials as a vehicle to deliver cells for treating endometrial tissue fibrosis and IUA has not been well explored. Polycaprolactone (PCL) is an organic polymer that has strong mechanical properties, good biocompatibility and is biodegradable over defined periods (23). Hyaluronic acid (HA) is an ECM-derived linear anionic polysaccharide, and has demonstrated excellent hydrophilicity, biocompatibility, biodegradability and non-immunoreactivity (24). In a previous study carried by our group, a PCL-HA electrospun membrane was fabricated to be morphologically similar to the native ECM, with ultrafine continuous fibers, high surface to volume ratio and high porosity (25). This PCL-HA scaffold supported H-EMSCs’ attachment, proliferation, enhanced H-EMSCs’ expression of wound-healing genes IL-10, VEGFA, TGF-β but suppressed their expression of tissue inflammation gene IL-6 (25). In addition, PCL-HA also supported MSC markers CD90 and Meflin expression of the seeded H-EMSCs (25). All of our previous findings have established PCL-HA as a model biomaterial platform for endometrial tissue repair and IUA treatment.
In this study, considering the fact that both H-EMSCs and mø play important roles in endogenous endometrial regeneration, we investigated the co-delivery of H-EMSCs and mø on PCL-HA membranes for treating endometrial fibrosis and IUA. The work also delineated some of the mechanisms involved in the interactions between H-EMSCs and mø when co-delivered with PCL-HA.
2 Methods
All chemicals of this work were purchased from Solarbio Life Sciences and used as is unless stated otherwise.
2.1 Isolation and culture of H-EMSCs
Human endometrial tissue pieces (released into menstrual blood) were obtained from the Yuhuangding Hospital of Yantai (ethics approval number: 2023-043). 15 ml PBS (P1020, Solarbio) containing 1% penicillin/streptomycin (PB180120, Procell) was added to 50 ml centrifuge tubes, and human endometrial tissue samples were collected in the tubes and transported to the processing lab on ice. The endometrial tissue was rinsed with PBS, cut into 1 mm³ pieces and digested with collagenase type I (1 mg/ml, BS163, Biosharp) for 60 minutes at 37°C in a constant temperature oscillator (80 r/min). DMEM/F12 complete medium (PM150312, Procell, containing 10% FBS (164210, Procell) and 1% penicillin/streptomycin) was used to terminate the digestion. The cells were centrifuged at 1000 r/min for 5 min and the cell pellet was re-suspended with DMEM/F12 medium and seeded into T75 TCPS culture flasks to obtain P1 (passage 1) H-EMSCs. Culture medium was changed 24h immediately after seeding and then changed every 2–3 days in culture. When H-EMSCs reached 80%-90% confluency, they were passaged using a ratio of 1:2. P4 (passage four) H-EMSCs were used for all the experiments in this study, in accordance with previous reported studies (26–28). The clinical information of patients who donated endometrial tissue samples of the study can be found in the Supplementary Table 1 below. Characterization of the isolated H-EMSCs including (morphology, cell doubling time, flow cytometry for CD90, CD73 and CD45, colony forming capability, adipogenic, osteogenic and chondrogenic differentiation) was already performed in a previous study carried by our group (25), so it was not repeated in the current study.
2.2 Fabrication of PCL-HA electrospun membranes
PCL-HA electrospun membranes were fabricated according to our previously established methods (25). Specifically, PCL: HA (80:20) were added to 10 mL hexafluoro-isopropanol and the solution was magnetically stirred for 12h to obtain transparent spinning solutions. The solution was poured into a syringe and the electrospinner (Ne300, Inovenso Inc.) was set to have a flow rate of 1.5 mL/h, a spinning voltage of 12.6 kV and a spinning distance of 20 cm. After electrospinning, the PCL-HA electrospun membranes (2.5 cm×0.5 cm) were dried in a vacuum oven for 48h to remove residual solvent and then stored in a desiccator in dark until use. Characterization studies of the PCL-HA electrospun membranes include scanning electron microscopy, fiber diameter, material porosity % and degradation rate, as well as the material biocompatibility (the viability, proliferation and metabolic activities of the H-EMSCs) were already carried in our previous study (25), so those were not repeated in the current study.
2.3 H-EMSCs mono-delivery and H-EMSCs/Mø co-delivery via PCL-HA membrane
PCL-HA electrospun membranes were placed into tissue culture discs and sterilized with 70% ethanol (overnight) and then PBS was added to remove all residual ethanol. 1×106 H-EMSCs suspended in 50 μl or 1×106 H-EMSCs+0.25×106 Mø [isolated from human peripheral blood based on our lab’s previous protocol (29, 30)] suspended in 50 μl were seeded onto the PCL-HA electrospun membranes for the H-EMSCs monoculture and H-EMSCs/Mø co-culture conditions, respectively. The cell seeding density and the H-EMSCs:Mø ratio in the co-culture condition were determined according to our group’s previously published studies (31, 32). The cell-seeded PCL-HA electrospun membranes were implanted into the rat endometrial damage model 24h post-cell seeding.
2.4 Establishing the rat endometrial damage model and implantation of PCL-HA patches
Female Sprague-Dawley (SD) rats (220–250 g, 8–10 weeks old) were purchased from Jinan Pengyue Biotechnology Co., Ltd. The rats were maintained at 22 °C with 12 h/12 h light and dark cycle and served with adequate food and water before the experiments. Ninety estrus female SD rats were divided into five groups: Sham operation group, natural repair group (NR), PCL-HA group, PCL-HA/H-E group and PCL-HA/H-E/mø group, with three time points (day 3, day 7, and day 14, 6 rats/time point) for each group. All rats were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg), shaved in the middle and lower abdominal wall, and sprayed with 75% ethanol to disinfect the operation area. Under the conditions of aseptic operation, the rats’ abdominal wall was cut layer by layer, to fully expose the bilateral uterine horn, and a small incision was made near the fallopian tube of the uterus. A 16G syringe needle was inserted to scratch the uterine wall 8–10 times until the uterine surface became rough and bleeding, to establish the physical endometrial damage model. After flushing with PBS, the PCL-HA, PCL-HA/H-E and PCL-HA/H-E/mø patches were implanted to the surface of the damaged endometrium and it was confirmed that the patches were tightly adhered to the damaged endometrium, then the uterine wall incision was closed with 6–0 absorbable sutures (Supplementary Figure 1).
For the IL-10 inhibition experiments, 18 female SD rats in estrus were divided into the following three groups, with 6 rats in each group: PCL-HA/H-E group: PCL-HA electrospun membrane (1×106 H-EMSCs) was transplanted; PCL-HA/H-E/mø group: PCL-HA electrospun membrane (1×106 H-EMSCs + 0.25×106 mø) was transplanted; PCL-HA/H-E/mø/IL-10 inhibitor group: PCL-HA electrospun membrane (1×106 H-EMSCs and 0.25×106 mø) was implanted, IL-10 inhibitor (Ossirene (HY-101019, MCE), 3 mg/kg) was injected into the rat uterine cavity at the time of implantation.
2.5 Collection of endometrial tissue samples and preparation of paraffin and frozen sections
At 3, 7 and 14 days, the rats from each group were anesthetized, and the endometrial tissue samples were collected. Specifically, a longitudinal incision was made along the uterine segment and then it was spread out flat. Then, the endometrial layer and the underlying muscularis were separated gently according to the junctions between the two. The endometrial tissue samples were washed with PBS and fixed with 4% paraformaldehyde (BL539A, Biosharp) at 4 °C for 48h. For the IL-10 inhibition experiment, the samples were taken at day 7. Subsequently, paraffin and frozen sections were prepared for the histology (Masson staining) and immunofluorescence staining experiments were carried out. For qRT-PCR experiments, the endometrial tissues were collected and stored in liquid nitrogen before use.
For the paraffin section preparation, the fixed endometrial tissues were cut into 3 mm-long segments, dehydrated and embedded with the following process: 75% ethanol (2h) →85% ethanol (2h) →95% ethanol (1h x2) →100% ethanol (1h x2) →xylene (30 min x2) →wax (1h) →embedding. The endometrial tissue samples were cut to a thickness of 5 μm using a microtome (Leica Biosystems Inc). Deparaffinization was achieved using the following procedure: xylene (15 min x2) →100% ethanol (10 min x2) →95% ethanol (5 min x2) →85% ethanol (5 min) →75% ethanol (5 min) →distilled water (1 min).
For the frozen section preparation, the fixed tissue samples were dehydrated in 30% sucrose for 48h. Then, tissue-Tek O.C.T. frozen section embedding medium (4583, Sakura Finetek Japan Co., Ltd.) was used to embed the endometrial tissue samples. Thermo Scientific™ CryoStar™ NX50 Cryostat was used to obtain frozen tissue sections with a 10 μm thickness.
2.6 Masson’s Trichrome staining
Masson’s Trichrome staining kit (G1340, Solarbio) was used to stain the endometrial tissue sections according to the manufacturer’s instructions. After staining with the dye solution, the tissue sections were rapidly dehydrated with 95% ethanol (3s) and 100% ethanol (10s x3), treated with xylene (1 min x3) and sealed with neutral balsam. Endometrial tissue sections were observed under inverted histology microscope (Zeiss). The quantification of the Masson’s Trichrome staining area was performed using the “Area” analysis tool in Image J software (Version 1.54).
2.7 Immunofluorescence
The frozen sections were soaked in PBS for 10 min to remove the tissue-Tek O.C.T. frozen section embedding medium. 10% goat serum (SL038, Solarbio) was used for blocking (room temperature, 1h) and the sections were treated with primary antibody solution at 4 °C for overnight. The next day, the tissue sections were incubated at room temperature for 1h and washed with PBS (5 min x3). The sections were then treated with secondary antibody solutions for 1h in the dark at room temperature. Cell nuclei were counter-stained with DAPI (10 μg/ml, C0065, Solarbio) (room temperature, 10 min in the dark) and anti-fade mountant (S2100, Solarbio) was added to all tissue sections before they were imaged with a Nikon upright fluorescence microscope. The following lists the primary and secondary antibodies used: rabbit polyclonal to α-SMA (1:200, YT5053, Immunoway); mouse monoclonal to CD80 (1:200, sc-376012, Santa Cruz Biotechnology); rabbit polyclonal to CD86 (1:200, YT7823, Immunoway); rabbit monoclonal to CD163 (1:200, AB182422, Abcam); rabbit polyclonal to CD206 (1:200, YT5640, Immunoway); mouse monoclonal to CD90 (1:200, 66766-1-lg, Proteintech); rabbit polyclonal to IL-10 (1:200, YT5138, Immunoway); AlexaFluor® 568 goat anti-rabbit IgG (1:200, A-11011, Thermo scientific) and AlexaFluor® 488 goat anti-mouse IgG (1:200, A28175, Thermo scientific). Immunofluorescence image analysis was performed using Image J software (Version 1.54) according to our previously established protocols (31, 32). It should be noted that the immunofluorescence image quantification is based on positive staining area (%) of the proteins, which does not distinguish cell number from protein expression level. However, in this study, the cell distribution was generally uniform in the tissue sections, so cell number should not significantly affect the relative protein expression levels in the different conditions.
2.8 qRT-PCR
mRNA was extracted from the cells or endometrial tissues using the Trizol (R401-01, Vazyme) method. mRNA quality and quantity were checked with NanoDrop™ 1000 Spectrophotometer (Thermo Scientific), and the samples were stored in -80 °C freezer. cDNA was synthesized using the reverse transcription kit (R233-01, Vazyme), according to the manufacturer’s instructions. The obtained cDNA samples were diluted 10 times with nuclease free water and used for qRT-PCR. Reaction mixtures containing 10 μl 2 x ChamQ SYBR qPCR Master Mix (Q311-02, Vazyme), 0.4 μl forward primer (10 μM), 0.4 μl reverse primer (10 μM), 0.7 μl cDNA sample, 8.5 μl ddH2O were prepared. The qPCR reaction was performed with Roche LightCycler™ Real-Time PCR Detection System, using the following protocol: Pre-incubation: 95 °C for 10 min, Amplification (40 cycles): 95 °C for 10 s, 60 °C for 30 s, Melting curve: 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. The data was analyzed using the comparative 2-ΔΔCt method. The forward and reverse primer sequences of the genes can be found in the following Table 1.
Table 1
| Gene Name | Primer Sequences |
|---|---|
| rat-α-SMA | Forward (5’–3’): CGTGACTACTGCTGAGCGTGA Reverse (5’–3’): TGCCCATCAGGCAGTTCGTAG |
| rat-CD90 | Forward (5’–3’): CACTCTCCTGCTTTCAGTCTTGC Reverse (5’–3’): GGCTGAACTCATGCTGGATGG |
| rat-TNF-α | Forward (5’–3’): CCCAAATGGGCTCCCTCTCAT Reverse (5’–3’): TTGGTGGTTTGCTACGACGTG |
| rat-IL-10 | Forward (5’–3’): CAGACCCACATGCTCCGAGA Reverse (5’–3’): CAAGGCTTGGCAACCCAAGTA |
The forward and reverse primer sequences of the genes (rat-α-SMA, rat-CD90, rat-TNF-α, rat-IL-10).
For the IL-10 inhibition experiment, the cells included the following groups: Control group: 5×104 RFs (CRL-1764, ATCC) treated with DMEM/F12 complete medium; PCL-HA/H-E group: 5×104 RFs treated with cell culture supernatant of H-EMSCs seeded on PCL-HA; PCL-HA/H-E/mø group: 5×104 RFs treated with cell culture supernatant of H-EMSCs/macrophages co-seeded on PCL-HA; PCL-HA/H-E/mø/IL-10 inhibitor group: 5×104 RFs treated with cell culture supernatant of H-EMSCs/macrophages co-seeded on PCL-HA and IL-10 inhibitor Ossirene (1μg/mL, according to manufacturer’s instructions). The cells were treated for 24h before mRNA extraction.
2.9 ELISA
A longitudinal incision was made along the uterine segment and then it was spread out flat. Then, the endometrial layer and the underlying muscularis were separated gently according to the junctions between the two. About 0.5g of endometrial tissue samples were collected and washed with pre-cooled PBS to remove blood, minced with ophthalmic scissors, homogenized using a tissue homogenizer and then the tissue samples were transferred to a 1.5ml centrifuge tube containing 200μl PBS, and centrifuged at 11200 rpm for 15 min at 4°C and stored on ice. The protein concentrations of the samples were measured using the BCA kit (PC0020, Solarbio) according to the manufacturer’s instructions. The absorbance was measured at 562 nm wavelength using a spectrometer (SpectraMax M2, Molecular Devices, LLC), and the protein concentrations of the samples were determined and recorded.
For the IL-10 quantification of the endometrial tissue homogenates, Rat IL-10 ELISA Kit (EK310/2, Lianke) was used according to the manufacturer’s instructions. Specifically, 100 μl diluted standard or sample solution was added to each test well and 100 μl sample diluents were added to the blank wells. Then, 50 μl of the detection antibody solution was added to each well and the plate was sealed and incubated at room temperature for 2h. After incubation, the plate was washed with 300 μl wash buffer/well for 6 times, and 100 μl of streptavidin-HRP was added to each well and incubated for 45 min. Finally, the plate was washed with 300 μl wash buffer/well for 6 times, and 100 μl of HRP substrate (TMB) was added to each well and incubated at room temperature in the dark for 15 min. 100 μl of stop solution was added to each well to terminate the reaction, and the absorbance was taken at 450 nm wavelength using SpectraMax M2 immediately.
2.10 Data analysis
All data were analyzed using SPSS 22.0 software (SPSS Inc., Chicago, IL) by analysis of variance (ANOVA) with Tukey’s test for pair-wise comparisons or an independent samples t-test, where appropriate. All experiments were repeated at least three times with at least three samples each time (N = 3, n=3) unless stated otherwise. Data are represented as mean ± S.E.M, p < 0.05 indicated statistical significance.
3 Results
This study established a rat endometrial damage model and probed the effects of a PCL-HA electrospun membrane-based co-delivery system containing H-EMSCs and mø, on resolving endometrial tissue fibrosis and promoting endometrial tissue repair. In addition, the regulation of the co-delivery system (PCL-HA/H-E/mø) on M1/M2 mø marker-expression shifts, the MSC marker expression as well as the generation of the proinflammatory and anti-inflammatory factors within the local endometrial tissue micro-environment was investigated. Finally, a prominent signaling pathway underlying the significant reduction of endometrial fibrosis by the PCL-HA/H-E/mø co-delivery system was discovered.
3.1 PCL-HA/H-E/mø co-delivery system reduced endometrial tissue fibrosis
As can be seen in Figures 1A, B, comparing the NR (damaged group without any treatment, stands for normal repair) condition with Sham, NR showed significantly higher fibrosis (blue area). Therefore, the endometrial damage animal model was validated. In addition, the implantation of H-EMSCs and mø co-delivery system (PCL-HA/H-E/mø) into the endometrial damage model showed greater effectiveness in reducing endometrium tissue fibrosis at day 7 vs. Sham, NR, PCL-HA alone or H-EMSCs mono-delivery system (PCL-HA, PCL-HA/H-E) (Figures 1A, B). At day 14, after implantation, the PCL-HA/H-E/mø repairing group had the smallest endometrial tissue fibrotic area vs. NR, PCL-HA, and the PCL-HA/H-E group (Figures 1A, B). Importantly, it was found that the endometrium fibrotic area of the PCL-HA/H-E/mø group was reduced to a level that was similar to the Sham group at day 14 (Figures 1A, B).
Figure 1

Masson staining of Sham, normal repair (NR), PCL-HA membrane alone, PCL-HA membrane with H-EMSCs mono-delivery (PCL-HA/H-E), PCL-HA membrane with H-EMSCs and mø co-delivery (PCL-HA/H-E/mø) groups, at day 3, 7 and 14. α-SMA gene and protein expression of the rat endometrial tissues in the NR, PCL-HA, PCL-HA/H-E, PCL-HA/H-E/mø groups, at day 3, 7 and 14. (A) Representative Masson images of the rat uterine tissues. Scale bar=110 µm. (B) Quantification of the endometrium fibrotic area (%). *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ###p < 0.001 vs. NR. &&p < 0.01, &&&p < 0.001 vs. Sham. (C) α-SMA gene expression. *p < 0.001. #p < 0.001 vs. NR. (D) Representative images of α-SMA immunostaining of the uterine tissues. The nuclei were stained in blue while α-SMA was stained in red. Scale bar=;100 µm. (E) Quantification of the α-SMA immunostaining area (%). *p < 0.05, ***p < 0.001. #p < 0.05, ###p < 0.001 vs. NR.
Since α-SMA is a well-acknowledged marker for endometrial tissue fibrosis (33, 34), the effects of PCL-HA/H-E/mø co-delivery system on the α-SMA gene and protein expression in the endometrium were also evaluated. It was found that the PCL-HA/H-E/mø treatment group had lower α-SMA gene expression in the endometrial damage tissue vs. the NR, PCL-HA, PCL-HA/H-E groups (Figure 1C), consistent with reduced tissue fibrosis (Figures 1A, B). The endometrial damage tissue α-SMA protein expression profile also matched well with the α-SMA gene expression data, indicating lower endometrial tissue fibrosis in the PCL-HA/H-E/mø condition (Figures 1D, E). Also, since the endometrium and the underlying muscularis were separated in the tissue processing procedures, the observed α-SMA gene and protein expression can be confirmed to be associated with the endometrial tissue, but not the muscularis.
3.2 PCL-HA/H-E/mø co-delivery system regulated M1/M2 mø marker-expression shifts
Previous studies have shown that M1-type mø can enhance the inflammatory tissue microenvironment and can initiate tissue fibrosis, while M2 mø phenotypes are typically associated with tissue repair and wound-healing (35). Since the PCL-HA/H-E/mø treatment group significantly reduced fibrosis in the endometrial damage model, markers of the M1 (CD80, CD86) and M2 (CD163, CD206) mø were investigated in the different treatment groups (Figures 2, 3). It was found that the expressions of M1-type (CD80, CD86) mø markers in the endometrial tissues were significantly lower in the PCL-HA/H-E/mø group vs. the NR, PCL-HA, PCL-HA/H-E groups at day 3, 7 and 14 time points (Figure 2). However, it was observed that the expression levels of M2-type (CD163, CD206) mø markers, in the endometrial tissues, were the opposite trend to the M1-type marker expression (Figure 3). Both CD163 and CD206 expression in the endometrial tissues were the highest for the PCL-HA/H-E/mø group at days 3, 7 and 14 when compared to the NR, PCL-HA and PCL-HA/H-E treatment groups (Figure 3).
Figure 2

CD80 and CD86 immunostaining of rat uterine tissues of the NR, PCL-HA, PCL-HA/H-E and PCL-HA/H-E/mø groups at day 3, 7 and 14. (A) Representative images of CD80 immunostaining of the uterine tissues. The nuclei were stained in blue while CD80 was stained in green. Scale bar=100 µm. (B) Representative images of CD86 immunostaining of the uterine tissues. The nuclei were stained in blue while CD86 was stained in red. Scale bar=100 µm. (C) Quantification of the CD80 immunostaining area (%). *p < 0.05, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NR. (D) Quantification of the CD86 immunostaining area (%). *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NR.
Figure 3

CD163 and CD206 immunostaining of rat uterine tissues of the NR, PCL-HA, PCL-HA/H-E and PCL-HA/H-E/mø groups at day 3, 7 and 14. (A) Representative images of CD163 immunostaining of the uterine tissues. The nuclei were stained in blue while CD163 was stained in red. Scale bar=100 µm. (B) Representative images of CD206 immunostaining of the uterine tissues. The nuclei were stained in blue while CD206 was stained in red. Scale bar=100 µm. (C) Quantification of the CD163 immunostaining area (%). *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NR. (D) Quantification of the CD206 immunostaining area (%). *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ###p < 0.001 vs. NR.
3.3 PCL-HA/H-E/mø co-delivery system regulated CD90, IL-10 and TNF-α expression within the endometrial tissues
The endometrial mesenchymal stem cell marker CD90, anti-inflammatory marker IL-10, as well as proinflammatory marker TNF-α expressions within the endometrial tissues of the different treatment groups, were assessed at days 3, 7 and 14 (Figures 4, 5). It was observed that the mesenchymal stem cell marker CD90 gene expression was higher in the endometrial tissue that was implanted with PCL-HA/H-E/mø when compared with the PCL-HA/H-E, PCL-HA and NR groups at days 7 and 14 (Figure 4A). Similarly, the PCL-HA/H-E/mø group showed higher CD90 protein expression for immunostaining data, within the endometrial tissues vs. NR, PCL-HA, PCL-HA/H-E treatment groups at days 3, 7 and 14 (Figures 4C, E). The results of Figures 4A, C, E suggested that the implantation of PCL-HA/H-E/mø co-delivery system appeared to be enhancing H-EMSCs proliferation or was effectively recruiting more H-EMSCs from the local tissues during the repairing process of the damaged endometrial tissues.
Figure 4

CD90, IL-10 gene expression and CD90, IL-10 immunostaining of rat uterine tissues of the NR, PCL-HA, PCL-HA/H-E and PCL-HA/H-E/mø groups at day 3, 7 and 14. (A) CD90 gene expression of the rat endometrial tissues. *p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NR. (B) IL-10 gene expression of the rat endometrial tissues. *p < 0.05, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001vs. NR. (C) Representative images of CD90 immunostaining of the uterine tissues. The nuclei were stained in blue while CD90 was stained in green. Scale bar=100 µm. (D) Representative images of IL-10 immunostaining of the uterine tissues. The nuclei were stained in blue while IL-10 was stained in red. Scale bar=100 µm. (E) Quantification of the CD90 immunostaining area (%). *p < 0.05, **p < 0.01, ***p < 0.001. ###p < 0.001vs. NR. (F) Quantification of the IL-10 immunostaining area (%). *p < 0.05, ***p < 0.001. #p < 0.05, ###p < 0.001 vs. NR.
Figure 5
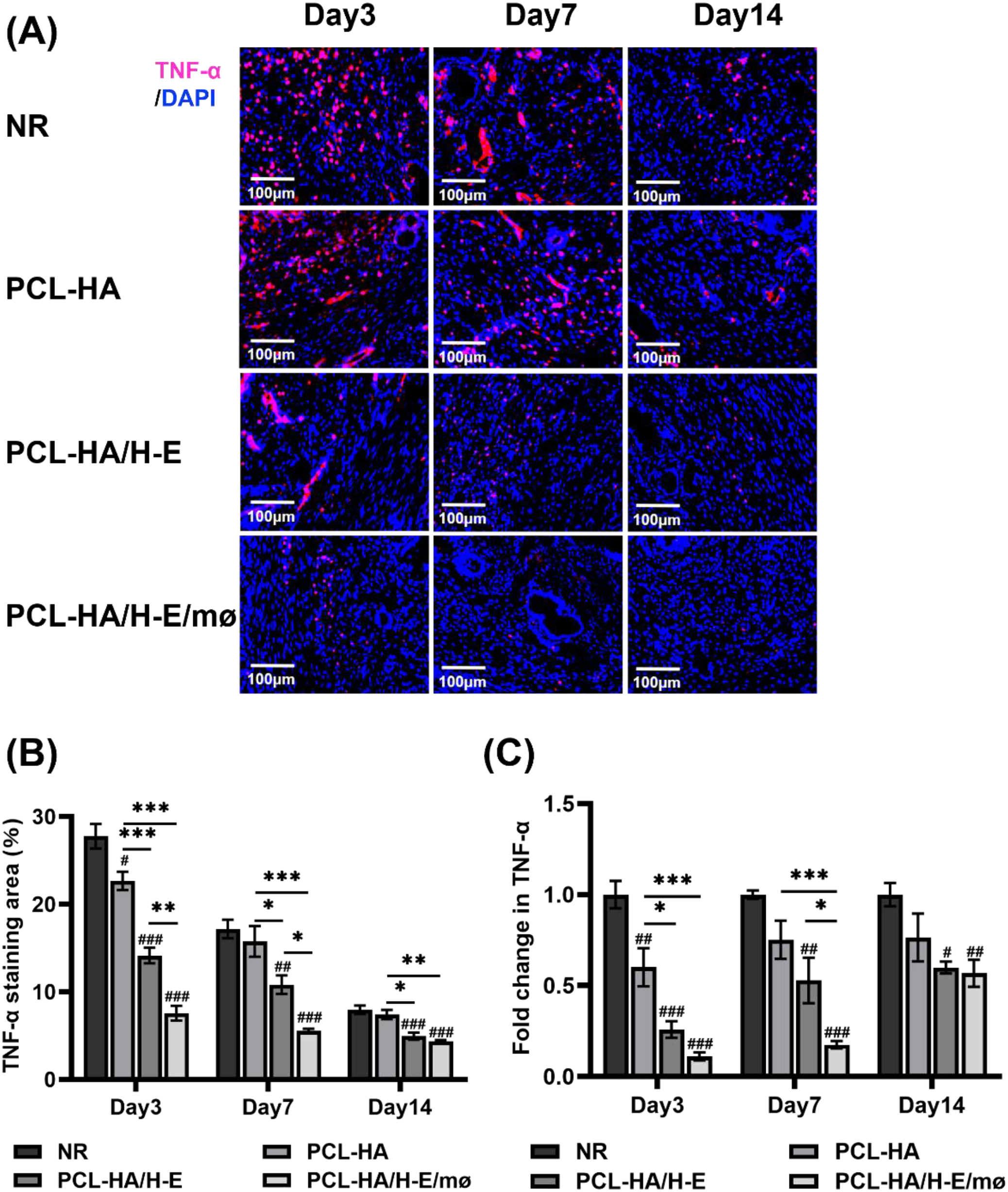
TNF-α immunostaining and TNF-α gene expression of rat uterine tissues of the NR, PCL-HA, PCL-HA/H-E and PCL-HA/H-E/mø groups at day 3, 7 and 14. (A) Representative images of TNF-α immunostaining of the uterine tissues. The nuclei were stained in blue while TNF-α was stained in red. Scale bar=100 µm. (B) Quantification of the TNF-α immunostaining area (%).*p < 0.05, **p < 0.01, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. NR. (C) TNF-α gene expression of the rat endometrial tissues. *p < 0.05, ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001vs. NR.
When investigating IL-10 and TNF-α expressions within the endometrial tissues of the different treatment groups (Figures 4B, D, F, 5), it was found that the co-delivery treatment group showed higher IL-10 gene expression level vs. NR, PCL-HA, PCL-HA/H-E at all time points (day 3, 7 and 14) (Figure 4B). Additionally, the IL-10 protein expression quantification matched the gene expression data, showing that the co-delivery group significantly upregulated the IL-10 protein expression in the endometrial tissue vs. all other treatment groups at days 3, 7 and 14 (Figures 4D, F). On the other hand, the gene and protein expression pattern of IL-10 for the different treatment groups displayed the opposite trends to that of TNF-α. Specifically, it was demonstrated that the PCL-HA/H-E/mø group had lower TNF-α gene (day 7 post-implantation) and protein expression (day 3, 7 post-implantation) vs. NR, PCL-HA, PCL-HA/H-E (Figure 5). At day 3, the co-delivery group showed lower TNF-α gene expression vs. NR and PCL-HA, however, no significant differences were detected between mono-delivery and co-delivery (Figure 5C).
3.4 IL-10 may inhibit α-SMA expression and endometrial tissue fibrosis
Previous studies have shown that IL-10 can inhibit skin, lung, kidney and cardiac tissue fibrosis by inhibiting fibrosis-associated miRNAs (miRNA-21, -145, and -208) and decreasing collagen I and III production (36–38). Based on the results obtained in sections 3.1-3.3, it appeared that the PCL-HA/H-E/mø co-delivery had enhanced both IL-10 gene and protein expression, while reducing the endometrial tissue fibrosis. As a result, further experiments were designed to investigate the relationship between the IL-10 gene and protein upregulation in the co-delivery treatment group and the decreased α-SMA expression and endometrial tissue fibrosis (Figures 6–8). As can be seen in Figures 6A-E, when the RFs cultured in vitro were treated with cell culture supernatants obtained from PCL-HA/H-E/mø co-seeding condition (7-day culture period), their IL-10 gene expression level was significantly increased when compared with the PCL-HA/H-E mono-seeding condition, or the control group. On the other side, the RFs treated with the co-seeding medium showed lower α-SMA gene expression vs. the mono-seeding or control groups (Figure 6F), which was the exact opposite of the IL-10 gene expression profile.
Figure 6

Assessment of (A, B) Representative images of rat RFs and immunostaining of (C) Vimentin (+staining) and (D) CD31 (-staining). Scale bar =100 µm. (E) IL-10 and (F) α-SMA expression of the RFs treated without (control) and with cell culture supernatant collected from PCL-HA/H-E or PCL-HA/H-E/mø. ***p < 0.001. ###p < 0.001 vs. Control. (G) IL-10 and (H) α-SMA expression of the RFs treated with cell culture supernatant collected from PCL-HA/H-E, PCL-HA/H-E/mø and PCL-HA/H-E/mø with IL-10 inhibitor (PCL-HA/H-E/mø/IL-10 inhibitor). ***p < 0.001. ###p < 0.001 vs. PCL-HA/H-E group.
Figure 7

IL-10, α-SMA gene and protein expression of rat uterine tissues of the PCL-HA/H-E, PCL-HA/H-E/mø and PCL-HA/H-E/mø/IL-10 inhibitor groups at day 7. (A) IL-10 gene expression. (B) Quantification of IL-10 protein concentration (pg/ml). (C) Representative images of IL-10 immunostaining. Cell nuclei stained in blue while IL-10 stained in red. Scale bar=100 µm. (D) Quantification of IL-10 staining area (%). (E) α-SMA gene expression. (F) Representative images of α-SMA immunostaining. Cell nuclei stained in blue while α-SMA stained in red. Scale bar=100 µm. (G) Quantification of α-SMA staining area (%). ***p < 0.001. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. PCL-HA/H-E group.
Figure 8

Masson staining of rat uterine tissues of the following groups: PCL-HA/H-E, PCL-HA/H-E/mø and PCL-HA/H-E/mø/IL-10 inhibitor, at day 7. (A) Representative Masson images of the rat uterine tissues of the different treatment groups at day 7. Scale bar=110 µm. (B) Quantification of the endometrium fibrotic area (%). ***p < 0.001. ##p < 0.01, ###p < 0.001 vs. PCL-HA/H-E group.
Since increased IL-10 gene expression was associated with decreased α-SMA expression, IL-10 inhibition studies were undertaken to test the hypothesis that IL-10 played significant role in down-regulating α-SMA expression and reducing endometrial tissue fibrosis. As can be seen in Figure 6G, when the RFs were treated with cell culture supernatant from the co-delivery condition plus the IL-10 inhibitor (PCL-HA/H-E/mø/IL-10 inhibitor), IL-10 gene expression of RFs was dramatically decreased to a level that was lower than the mono-delivery condition and the no inhibitor control. On the contrary, the RFs of the PCL-HA/H-E/mø/IL-10 inhibitor group showed increased α-SMA gene expression when compared to the no inhibitor and mono-delivery condition (Figure 6H).
For the in vivo IL-10 inhibition experiments, it was found that with the addition of the IL-10 inhibitor, the co-delivery treatment group showed lower IL-10 gene (Figure 7A) and protein expression (Figures 7B–D) in the endometrial tissues, when compared with the mono-delivery and co-delivery without IL-10 inhibitor groups, suggesting successful IL-10 inhibition in the in vivo endometrial damage model. The successful IL-10 inhibition in the endometrial damage model caused an increased α-SMA gene and protein expression, when compared with the co-delivery and mono-delivery conditions (Figures 7E–G).
Finally, Masson staining of rat uterine tissues (Figure 8) confirmed that adding IL-10 inhibitors in the co-delivery condition can interfere with the reduction of endometrial fibrosis. Hence, the IL-10 inhibition studies confirmed that the PCL-HA/H-E/mø co-delivery system exerted its effect on reducing endometrial tissue fibrosis (inhibiting the α-SMA expression) via the upregulation of the anti-inflammatory marker IL-10 expression.
4 Discussion
The endometrium, which consists of basal and functional layers, is a highly regenerative tissue that undergoes monthly growth, differentiation, and shedding during women’s reproductive age (39). Endometrial tissue injury and abnormal endometrial tissue repair are amongst the most important causes of female infertility (39). IUA is usually caused by curettage and iatrogenic injury, and its essence is endometrial fibrosis, which most often associates with persistent inflammation (40). At present, the different clinical interventions focus primarily on TCRA procedures or the injection of HA gel to treat IUA (1, 41). These interventions have had limited improvement on reproductive outcomes because the intrauterine devices cannot fundamentally prevent endometrial tissue fibrosis from re-occurring and often need to be removed, and HA gel has a short retention time in the uterus (41).
In recent years, different types of biomaterial scaffolds, pro-wound-healing/anti-fibrotic therapeutic factors, and MSC therapies have been investigated as more effective strategies to address IUA (42–44). Biomaterial nanofiber membranes constructed by electrospinning technology can have a three-dimensional structure mimicking natural ECM, with high surface area/volume ratio, good porosity, and strong mechanical properties, which can provide a suitable microenvironment for cell growth, proliferation, and the loading of bioactive factors (45, 46). The physical, chemical and biological properties of the nanofibrous electrospun scaffolds can be adjusted by changing the electrospinning parameters, for instance, the addition of natural ECM components can further improve the biocompatibility of the electrospun scaffolds with the local cells and ECM proteins (45). Additionally, it has been found that MSCs derived from various tissues, such as bone marrow, adipose tissue and placenta/umbilical cord, can migrate to areas of tissue injury, release anti-inflammatory cytokines and promote immunomodulation and wound healing (47, 48). Notably, bone marrow MSCs have been shown to promote M2-type polarization of mø phenotypes to enhance tissue repair and remodeling via direct cell-cell contact and the secretion of soluble factors (49). In a previous study, our group fabricated electrospun PCL-HA synthetic-natural composite membranes and they showed good structural and biochemical properties and exerted great potential to support H-EMSCs’ adhesion and proliferation (25). Additionally, H-EMSCs seeded on PCL-HA membranes showed self-renewal and low immunogenicity characteristics and could have the potential for endometrial tissue repair and regeneration (25).
In this study, considering the fact that both H-EMSCs and mø could play important roles in endogenous endometrial regeneration, we investigated the co-delivery of H-EMSCs and mø via PCL-HA membranes for treating endometrial fibrosis and IUA. It was observed that the PCL-HA electrospun membrane with H-EMSCs and mø co-seeding significantly reduced the endometrial fibrosis, enhanced M2 anti-inflammatory macrophage marker expression in the endometrial tissue injury model, when compared to the normal repair, PCL-HA membrane alone and PCL-HA with H-EMSCs mono-seeding groups. This current study suggested that the biocompatible PCL-HA electrospun membrane, when co-seeded with H-EMSCs and mø rather than just H-EMSCs, could have great potential in preventing endometrial fibrosis and promoting more natural endometrial tissue repair.
Using MSCs to educate mø to enhance tissue repair and regeneration has been reported previously in different tissues of the body. For instance, it was found that bone marrow derived-MSCs that were in direct contact with M1 pro-inflammatory decidual mø could promote their change to the M2 anti-inflammatory skew, by enhancing TNF-stimulated gene-6 (TSG-6) production and CD200 expression by the MSCs, in an abortion model (15). In addition, in a mouse IUA model, it was found that human umbilical MSCs pre-conditioned with IL-1β, TNF-α and IFN-γ promoted anti-inflammatory M2-type mø polarization, decreased endometrial tissue inflammation and fibrosis, and improved the immune microenvironment of endometrial regeneration by downregulating the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway (2). The interactions between MSCs and mø have not only attracted attention in the repair and regeneration of uterine tissues but also been investigated in sepsis and spinal cord injury models (50, 51). In the sepsis disease model, it was found that bone marrow MSCs produced prostaglandin E2 (PGE2), which bound to EP2 and EP4 receptors on the mø’s surfaces to induce anti-inflammatory cytokine IL-10 expression, to relieve sepsis symptoms (52). Additionally, bone marrow MSCs enhanced the polarization of M2-type mø phenotypes in a spinal cord injury model and promoted effective spinal tissue repair, indicating that the bone marrow MSCs were critical for balancing the M1/M2 polarization states of the mø for normal spinal tissue repair (51). More interestingly, it was also found that bone marrow MSCs can promote M2 mø polarization by changing the metabolic status through a PGE2-dependent mechanism (53).
The observations of the current study agreed well with those previous findings, demonstrating that the endometrial co-delivery system, fabricated by co-seeding H-EMSCs and mø onto PCL-HA composite electrospun membranes, promoted the overall change of a more M1-type to a more M2-type mø phenotypes, upregulated anti-inflammatory cytokine IL-10 expression, downregulated pro-inflammatory factor TNF-α expression. What is particularly important and a novel finding for the literature here, is that at the dose and ratio of cells used for delivering the two cell types on a foreign biomaterial, a pro-inflammatory to anti-inflammatory endometrial tissue microenvironment change was achieved in a manner that appeared to have contributed to the inhibition of endometrial fibrosis rather than yielding a classical foreign body fibrotic response. The presence of the biomaterial enabled the co-delivery which appeared to be important since the co-delivery patch induced more CD90 gene and protein expression in the damaged endometrial tissue vs. other conditions, including that of the H-EMSCs alone. Such findings indicated that when mø were co-delivered in proximity with the H-EMSCs via PCL-HA, the retention of the seeded H-EMSCs or recruitment of the H-EMSCs reduced fibrosis and enabled enhanced repair of the native endometrial tissue. This aligned well with the roles that M2 type mø play, in promoting tissue repair by MSCs (13, 54). However, it should be noted that macrophage polarization in vivo is more dynamic than a strict M1/M2 dichotomy (55). Specifically, macrophages are only broadly categorized into two primary functional types: classically activated (M1) macrophages and alternatively activated (M2) macrophages. The M1 type is proinflammatory, specialized in pathogen and tumor clearance through the robust production of pro-inflammatory cytokines (56). The M2 type encompasses several subtypes, including the wound-healing (M2a) and regulatory (M2b, M2c) macrophages, which are involved in tissue repair and immune modulation (56). However, studies have revealed a significant overlap in vivo, where macrophages within the same tissue microenvironment can co-express markers characteristic of both M1 and M2 states (55, 57). Therefore, macrophages are best understood not as rigid, discrete populations, but as a plastic continuum of functional states.
It was of critical importance in this work to show the nature of the anti-fibrotic pathway associated with the delivery vehicle and its payload of cells, as PCL is well known to induce chronic foreign body responses (58), which would negate the outcomes that the authors were seeking to overcome. In fact, the PCL-HA controls in this work showed greater fibrosis when not carrying the co-seeded cells. However, in this particular processed form and combination of the co-seeded PCL-HA patch, the authors observed a significantly reduced fibrosis in the endometrial tissue damaged model. HA is a natural linear anionic polysaccharide derived from native ECM, and has good hydrophilicity, biocompatibility and non-immunoreactivity (24). Previous studies showed that HA can modulate skin tissue inflammation and wound-healing by binding to fibrinogen to activate the clotting pathways, inhibiting neutrophil migration to decrease inflammation, and stimulating the secretion of matrix metalloproteinases for promoting angiogenesis (59–61). Our previous study also revealed that H-EMSCs cultured on PCL-HA showed decreased IL-6 gene expression and increased IL-10, VEGFA, TGF-β gene expression vs. PCL-SF and PCL (25). The unique physiochemical and biological characteristics of this specific PCL-HA formulation might have contributed to the attenuated fibrosis observed in this study.
According to the literature, IL-10 has oftentimes been considered as an anti-inflammatory cytokine that has anti-fibrotic properties in tissue repair and remodeling (62). Consequently, the study investigated whether more IL-10 production in the co-delivery condition could be contributing to the reduced endometrial fibrosis. With successful blocking of IL-10 in the in vitro and in vivo experiments, it was found that the fibrotic marker α-SMA expression was significantly increased at both the gene and protein levels. Additionally, the Masson staining of the endometrial tissues for the IL-10 inhibitor group also showed dense collagenous fibrotic tissue formation. These findings provided substantial support that a key mechanistic pathway associated with the anti-fibrotic effects of PCL-HA based co-delivery of H-EMSCs and mø, was related partially to IL-10 released in the local endometrial tissue micro-environment. This finding agreed well with anti-fibrotic studies in the repairing of other tissue types. For example, viral vector-mediated IL-10 gene transfer into skin wounds led to regenerative healing, and the ECM formed was both morphologically and biomechanically indifferent when compared with that of the unwounded skin tissue (63). In addition, it was also found that the application of IL-10 in a myocardial infarction model suppressed pro-inflammatory cytokine production, inhibited inflammatory cell infiltration and reduced myocardial tissue fibrosis (64). Similarly, it is noted in the literature that a deficiency in IL-10 could aggravate kidney inflammation and fibrosis in a unilateral ureteral obstruction mouse model (65). Although IL-10 is implicated in the anti-fibrotic effect of the co-delivery system, the cellular source of IL-10 in vivo remains unclear (macrophages vs. H-EMSCs vs. other cells), future studies will identify the cellular source of IL-10 in vivo by combining genetic tools with advanced immunofluorescence-based cytokine tracing techniques. Additionally, fibrosis evaluation in this study is based on Masson staining and α-SMA expression, next-step studies could further examine the expression of collagen-specific markers in the different conditions to compare with the Masson staining and α-SMA expression data.
Further, it should however be noted that IL-10 likely is not alone responsible for the enhanced repair and may only be one of the factors that the co-delivery was able to activate towards the inhibition of the endometrial fibrosis due to the close proximity of the cells, which enabled effective chemokine communication. Future studies will be investigating other prominent signaling molecules and pathways (e.g., IGFBP3, IL-17A, RhoA/ROCK1/MYL9 (66–68)) involved in down-regulating endometrial fibrosis, using the PCL-HA based H-EMSCs-mø co-delivery system.
5 Conclusions
In summary, this study established a rat endometrial damage model and probed the application of co-delivery of H-EMSCs and mø, via a PCL-HA patch-based system. It was found that the co-delivery system could significantly reduce fibrosis in the endometrial tissue damage model. The co-delivery patch supported an overall M1-type to M2-type change of the mø phenotypes and increased MSC numbers in the endometrial tissue. It appeared that IL-10 played an important role in reducing endometrial fibrosis, mediated by the H-EMSCs and mø co-seeded PCL-HA patch. The study provides significant insights into using an H-EMSCs-mø co-delivery system for effectively alleviating endometrial fibrosis and IUA in the future.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics committee of Binzhou medical university. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Ethics committee of Binzhou medical university. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JA: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. SL: Data curation, Formal analysis, Investigation, Writing – original draft. TM: Formal analysis, Writing – review & editing. YC: Formal analysis, Writing – review & editing. JS: Supervision, Writing – review & editing. WW: Conceptualization, Resources, Writing – review & editing. PM: Conceptualization, Resources, Writing – review & editing. XZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the following grants: the Taishan Scholars Youth Project (grant number: tsqn202103111), the Natural Science Foundation of Shandong Province-Youth Foundation (grant number: ZR2021QC034), Yantai Provincial Talent Matching Funds (grant number: 1007-10073802) and Overseas High-level Talent Workstation Fund of Shandong Province (grant number: 2023119005).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2026.1750456/full#supplementary-material
Supplementary Figure 1Rat endometrial injury model and implantation of PCL-HA based endometrial patches. (A–C) After anesthesia, the rat abdomen was dissected to expose the uterine horn. (D, E) Creating the endometrial lesion in the rat uterus, with a sterile needle. (F–H) Implanting the PCL-HA based endometrial patches onto the endometrial lesion area. (J) Closing the wound with absorbable sutures.
Abbreviations
IUA, Intrauterine adhesion; H-EMSCs, Human endometrial mesenchymal stem cells; Mø, Macrophages; PCL, Polycaprolactone; HA, Hyaluronic acid; TCRA, Transcervical resection of adhesion; SD, Sprague-Dawley; NR, Natural repair group; ANOVA, Analysis of variance; TSG-6, TNF-stimulated gene-6; JAK/STAT, Janus kinase/signal transducers and activators of transcription; PGE2, Prostaglandin E2.
References
1
MaJZhanHLiWZhangLYunFWuRet al. Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater Res. (2021) 25:40. doi: 10.1186/s40824-021-00242-6
2
YaoSZhouZWangLLvHLiuDZhuQet al. Targeting endometrial inflammation in intrauterine adhesion ameliorates endometrial fibrosis by priming MSCs to secrete C1INH. iScience. (2023) 26:107201. doi: 10.1016/j.isci.2023.107201
3
LeeWLLiuCHChengMChangWHLiuWMWangPH. Focus on the primary prevention of intrauterine adhesions: Current concept and vision. Int J Mol Sci. (2021) 22:5175. doi: 10.3390/ijms22105175
4
SunYChenXQianZCaoLZhanSHuangL. Estradiol and intrauterine device treatment for moderate and severe intrauterine adhesions after transcervical resection. BMC Women Health. (2022) 22:357. doi: 10.1186/s12905-022-01940-6
5
ChenYLiuLLuoYChenMHuanYFangR. Effects of aspirin and intrauterine balloon on endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: A prospective cohort study. BioMed Res Int. (2017) 2017:8526104. doi: 10.1155/2017/8526104
6
LinYLiYChenPZhangYSunJSunXet al. Exosome-based regimen rescues endometrial fibrosis in intrauterine adhesions via targeting clinical fibrosis biomarkers. Stem Cells Transl Med. (2023) 12:154–68. doi: 10.1093/stcltm/szad007
7
CenJZhangYBaiYMaSZhangCJinLet al. Research progress of stem cell therapy for endometrial injury. Mater Today Bio. (2022) 16:100389. doi: 10.1016/j.mtbio.2022.100389
8
ZuoWXieBLiCYanYZhangYLiuWet al. The clinical applications of endometrial mesenchymal stem cells. Biopreserv Biobank. (2018) 16:158–64. doi: 10.1089/bio.2017.0057
9
BrownEMartínez-AguilarRMaybinJAGibsonDA. Endometrial macrophages in health and disease. Int Rev Cell Mol Biol. (2022) 367:183–208. doi: 10.1016/bs.ircmb.2022.03.011
10
WynnTAVannellaKM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
11
FengDLiYZhengHWangYDengJLiuTet al. IL-4-induced M2 macrophages inhibit fibrosis of endometrial stromal cells. Reprod Biol. (2024) 24:100852. doi: 10.1016/j.repbio.2023.100852
12
MassENimmerjahnFKierdorfKSchlitzerA. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat Rev Immunol. (2023) 23:563–79. doi: 10.1038/s41577-023-00848-y
13
Maldonado-LasunciónIO’neillNUmlandOVerhaagenJOudegaM. Macrophage-derived inflammation induces a transcriptome makeover in mesenchymal stromal cells enhancing their potential for tissue repair. Int J Mol Sci. (2021) 22:781. doi: 10.3390/ijms22020781
14
XiaYHeXTXuXYTianBMAnYChenFM. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ. (2020) 2020:e8970. doi: 10.7717/peerj.8970
15
LiYZhangDXuLDongLZhengJLinYet al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. (2019) 16:908–20. doi: 10.1038/s41423-019-0204-6
16
SaldañaLVallésGBensiamarFManceboFJGarcía-ReyEVilaboaN. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci Rep. (2017) 7:14618. doi: 10.1038/s41598-017-15217-8
17
XinLLinXZhouFLiCWangXYuHet al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. (2020) 113:252–66. doi: 10.1016/j.actbio.2020.06.029
18
LiuHZhangXZhangMZhangSLiJZhangYet al. Mesenchymal stem cell derived exosomes repair uterine injury by targeting transforming growth factor-β Signaling. ACS Nano. (2024) 18:3509–19. doi: 10.1021/acsnano.3c10884
19
LvQWangLLuoXChenX. Adult stem cells in endometrial regeneration: Molecular insights and clinical applications. Mol Reprod Dev. (2021) 88:379–94. doi: 10.1002/mrd.23476
20
WangZXiaLChengJLiuJZhuQCuiCet al. Combination therapy of bone marrow mesenchymal stem cell transplantation and electroacupuncture for the repair of intrauterine adhesions in rats: mechanisms and functional recovery. Reprod Sci. (2024) 31:2318–30. doi: 10.1007/s43032-024-01465-3
21
GaoYWuGXuYZhaoDZhengL. Stem cell-based therapy for asherman syndrome: promises and challenges. Cell Transplant. (2021) 30:9636897211020734. doi: 10.1177/09636897211020734
22
ZhangFKingMW. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv Healthc Mater. (2020) 9:e1901358. doi: 10.1002/adhm.201901358
23
AzariAGolchinAMaymandMMMansouriFArdeshirylajimiA. Electrospun polycaprolactone nanofibers: current research and applications in biomedical application. Adv Pharm Bull. (2022) 12:658–72. doi: 10.34172/apb.2022.070
24
KotlaNGMohd IsaILLarrañagaAMaddiboyinaBSwamySKSivaramanGet al. Hyaluronic acid-based bioconjugate systems, scaffolds, and their therapeutic potential. Adv Healthc Mater. (2023) 12:e2203104. doi: 10.1002/adhm.202203104
25
AnJMaTWangQZhangJSanterreJPWangWet al. Defining optimal electrospun membranes to enhance biological activities of human endometrial MSCs. Front Bioeng Biotechnol. (2025) 13:1551791. Available online at: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1551791 (Accessed February 2, 2026).
26
RahimipourMJafarabadiMSalehniaM. In vitro implantation model using human endometrial SUSD2+ mesenchymal stem cells and myometrial smooth muscle cells. Cell J. (2021) 23:154–63. doi: 10.22074/cellj.2021.6979
27
ZhangJSongHFanXHeSYinWPengZet al. Optimizing human endometrial mesenchymal stem cells for maximal induction of angiogenesis. Mol Cell Biochem. (2023) 478:1191–204. doi: 10.1007/s11010-022-04572-4
28
AleahmadMBozorgmehrMNikooSGhanavatinejadAShokriMRMontazeriSet al. Endometrial mesenchymal stem/stromal cells: The Enigma to code messages for generation of functionally active regulatory T cells. Stem Cell Res Ther. (2021) 12:536. doi: 10.1186/s13287-021-02603-3
29
BoyntonELWaddellJMeekELabowRSEdwardsVSanterreJP. The effect of polyethylene particle chemistry on human monocyte-macrophage function in vitro. J BioMed Mater Res. (2000) 52:239–45. doi: 10.1002/1097-4636(200011)52:2<239::AID-JBM1>3.0.CO;2-R
30
DinnesDLMSanterreJPLabowRS. Influence of biodegradable and non-biodegradable material surfaces on the differentiation of human monocyte-derived macrophages. Differentiation. (2008) 76:232–44. doi: 10.1111/j.1432-0436.2007.00221.x
31
ZhangXBattistonKGLabowRSSimmonsCASanterreJP. Generating favorable growth factor and protease release profiles to enable extracellular matrix accumulation within an in vitro tissue engineering environment. Acta Biomater. (2017) 54:81–94. doi: 10.1016/j.actbio.2017.02.041
32
ZhangXSimmonsCAPaul SanterreJ. Paracrine signaling from monocytes enables desirable extracellular matrix accumulation and temporally appropriate phenotype of vascular smooth muscle cell-like cells derived from adipose stromal cells. Acta Biomater. (2020) 103:129–41. doi: 10.1016/j.actbio.2019.12.006
33
XuCBaoMFanXHuangJZhuCXiaW. EndMT: New findings on the origin of myofibroblasts in endometrial fibrosis of intrauterine adhesions. Reprod Biol Endocrinol. (2022) 20:9. doi: 10.1186/s12958-022-00887-5
34
ViganoPCandianiMMonnoAGiacominiEVercelliniPSomiglianaE. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reproduct. (2018) 33:347–52. doi: 10.1093/humrep/dex354
35
SpillerKLKohTJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Delivery Rev. (2017) 122:74–83. doi: 10.1016/j.addr.2017.05.010
36
ShiJHGuanHShiSCaiWXBaiXZHuXLet al. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res. (2013) 305:341–52. doi: 10.1007/s00403-013-1314-0
37
SteenEHWangXBalajiSButteMJBollykyPLKeswaniSG. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New Rochelle). (2020) 9:184–98. doi: 10.1089/wound.2019.1032
38
VermaSKGarikipatiVNSKrishnamurthyPSchumacherSMGrisantiLACiminiMet al. Interleukin-10 inhibits bone marrow fibroblast progenitor cell-mediated cardiac fibrosis in pressure-overloaded myocardium. Circulation. (2017) 136:940–53. doi: 10.1161/CIRCULATIONAHA.117.027889
39
VilellaFWangWMorenoIQuakeSRSimonC. Understanding the human endometrium in the 21st century. Am J Obstet Gynecol. (2021) 225:1–2. doi: 10.1016/j.ajog.2021.04.224
40
EmingrMHalajMMalčákMHanáčekJ. Prevention of intrauterine adhesions. Ceska Gynekol. (2023) 88:210–3. doi: 10.48095/cccg2023210
41
KouLJiangXXiaoSZhaoYZYaoQChenR. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J Controlled Rel. (2020) 318:25–37. doi: 10.1016/j.jconrel.2019.12.007
42
WuMZhangQShangLDuanP. Microfluidics-derived hierarchical microparticles for the delivery of dienogest for localized endometriosis therapy. Acta Biomater. (2024) 178:257–64. doi: 10.1016/j.actbio.2024.02.017
43
YanCWangXWangQLiHSongHZhouJet al. A novel conductive polypyrrole-chitosan hydrogel containing human endometrial mesenchymal stem cell-derived exosomes facilitated sustained release for cardiac repair. Adv Healthc Mater. (2024) 13:e2304207. doi: 10.1002/adhm.202304207
44
CaoYQiJWangJChenLWangYLongYet al. Injectable “Homing-like” Bioactive short-fibers for endometrial repair and efficient live births. Adv Sci. (2024) 11:e2306507. doi: 10.1002/advs.202306507
45
MaurmannNFrançaFSGirónJPrankeP. Cell electrospinning: a review of materials and methodologies for biofabrication. Adv Biol. (2023) 7:e2300058. doi: 10.1002/adbi.202300058
46
LiuZRamakrishnaSLiuX. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. (2020) 4:030901. doi: 10.1063/5.0012309
47
ShiYWangYLiQLiuKHouJShaoCet al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
48
HuSDaiYXinLZhengXYeZZhangSet al. Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels–Alder click reaction for the treatment of intrauterine adhesions. Acta Biomater. (2024) 177:77–90. doi: 10.1016/j.actbio.2024.02.001
49
KimJHemattiP. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. (2009) 37:1445–53. doi: 10.1016/j.exphem.2009.09.004
50
XingJWangRCuiFSongLMaQXuH. Role of the regulation of mesenchymal stem cells on macrophages in sepsis. Int J Immunopathol Pharmacol. (2023) 37:3946320221150722. doi: 10.1177/03946320221150722
51
FuSPWuXCYangRLZhaoDZChengJQianHet al. The role and mechanisms of mesenchymal stem cells regulating macrophage plasticity in spinal cord injury. Biomed Pharmacother. (2023) 168:115632. doi: 10.1016/j.biopha.2023.115632
52
NémethKLeelahavanichkulAYuenPSTMayerBParmeleeADoiKet al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. (2009) 15:42–9. doi: 10.1038/nm.1905
53
VasandanABJahnaviSShashankCPrasadPKumarAJyothi PrasannaS. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2 -dependent mechanism. Sci Rep. (2016) 6:38308. doi: 10.1038/srep38308
54
FreytesDOKangJWMarcos-CamposIVunjak-NovakovicG. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. (2013) 114:220–9. doi: 10.1002/jcb.24357
55
MosserDMEdwardsJP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
56
Shapouri-MoghaddamAMohammadianSVaziniHTaghadosiMEsmaeiliSAMardaniFet al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
57
GuilliamsMSvedbergFR. Does tissue imprinting restrict macrophage plasticity? Nat Immunol. (2021) 22:118–27. doi: 10.1038/s41590-020-00849-2
58
SiddiquiNAsawaSBirruBBaadheRRaoS. PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol. (2018) 60:506–32. doi: 10.1007/s12033-018-0084-5
59
KawanoYPatruleaVSubletEBorchardGIyodaTKageyamaRet al. Wound healing promotion by hyaluronic acid: Effect of molecular weight on gene expression and in vivo wound closure. Pharmaceuticals. (2021) 14:301. doi: 10.3390/ph14040301
60
FrenkelJS. The role of hyaluronan in wound healing. Int Wound J. (2014) 11:159–63. doi: 10.1111/j.1742-481X.2012.01057.x
61
KaulAShortWDKeswaniSGWangX. Immunologic roles of hyaluronan in dermal wound healing. Biomolecules. (2021) 11:1234. doi: 10.3390/biom11081234
62
ShiJLiJGuanHCaiWBaiXFangXet al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PloS One. (2014) 9:e98228. doi: 10.1371/journal.pone.0098228
63
BalajiSWangXKingALeLDBhattacharyaSSMolesCMet al. Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. FASEB J. (2017) 31:868–81. doi: 10.1096/fj.201600856R
64
KrishnamurthyPRajasinghJLambersEQinGLosordoDWKishoreR. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. (2009) 104:e9–18. doi: 10.1161/CIRCRESAHA.108.188243
65
JinYLiuRXieJXiongHHeJCChenN. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest. (2013) 93:801–11. doi: 10.1038/labinvest.2013.64
66
ZhuYBaoMWangTAiXQiuDWangC. Novel therapeutic targets, including IGFBP3, of umbilical cord mesenchymal stem-cell-conditioned medium in intrauterine adhesion. Biol Open. (2024) 13:bio060141. doi: 10.1242/bio.060141
67
CuiXXiaoHCPanW. The predictive value of serum IL-17A and IL-6 expression in postoperative recurrence in patients with intrauterine adhesion. Am J Reprod Immunol. (2024) 91:e13808. doi: 10.1111/aji.13808
68
QinXZengBSoorannaSRLiM. LAMB3 promotes myofibrogenesis and cytoskeletal reorganization in endometrial stromal cells via the rhoA/ROCK1/MYL9 pathway. Cell Biochem Biophys. (2024) 82:127–37. doi: 10.1007/s12013-023-01186-5
Summary
Keywords
co-delivery, electrospun patch, endometrial fibrosis, human endometrial mesenchymal stem cells, immune-regulation, macrophages
Citation
An J, Li S, Ma T, Chen Y, Santerre JP, Wang W, Ma P and Zhang X (2026) Co-delivery of endometrial mesenchymal stem cells and macrophages with an electrospun patch suppresses endometrial fibrosis via IL-10 related signaling. Front. Immunol. 17:1750456. doi: 10.3389/fimmu.2026.1750456
Received
20 November 2025
Revised
31 January 2026
Accepted
02 February 2026
Published
19 February 2026
Volume
17 - 2026
Edited by
Silvia Gregori, San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), Italy
Reviewed by
Giada Amodio, San Raffaele Hospital (IRCCS), Italy
Marie Carbonnel, Hôpital Foch, France
Updates
Copyright
© 2026 An, Li, Ma, Chen, Santerre, Wang, Ma and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenshuang Wang, jesusloveyouandme@163.com; Xiaoqing Zhang, xqz@bzmc.edu.cn; Peng Ma, 894508645@qq.com
†These authors have contributed equally to this work
ORCID: Xiaoqing Zhang, orcid.org/0009-0006-1491-9773
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.