- 1Pathology and Molecular Biology Laboratory, Gonçalo Moniz Institute (IGM), Oswaldo Cruz Foundation (Fiocruz), Salvador, BA, Brazil
- 2Clinical Analysis Laboratory, Professor Edgard Santos University Hospital, Federal University of Bahia (UFBA), Salvador, BA, Brazil
- 3School of Biomedicine, Bahiana School of Medicine and Public Health, Salvador, BA, Brazil
- 4School of Medicine, UFBA, Salvador, BA, Brazil
- 5Experimental Pathology Laboratory, IGM, Fiocruz, Salvador, BA, Brazil
- 6School of Pharmacy, UFBA, Salvador, BA, Brazil
- 7Health Science Institute, UFBA, Salvador, BA, Brazil
- 8School of Public Health, Yale University, New Haven, CT, United States
Background: Recently, different arboviruses became endemic in Brazil mostly causing acute febrile illnesses, however, neurological manifestations have also been reported. This study aimed to investigate which viruses were involved in the meningitis etiology and the contribution of the circulating arboviruses in Salvador, Bahia, Brazil.
Methods: From June 2014 to February 2016, 170 patients with suspected viral meningitis were identified in Couto Maia Hospital, Salvador-BA, Brazil. Their CSF samples were investigated for possible viral etiology by reverse transcription-PCR (RT-PCR) for different arboviruses: DENV, ZIKV and CHIKV; and for the EV; and by PCR for the HHV1-5 complex (HSV1-2, VZV, EBV and CMV). Also, ELISA was carried out in a subgroup of remaining samples for detection of DENV IgM and NS1 antigen, CHIKV IgM and ZIKV IgM.
Results: Thirty-seven patients were PCR or ELISA positive for at least one of the studied viruses (overall positivity 21.8%). EV was the agent most frequently detected (10 cases; 27.0%), along with all four DENV serotypes (10 cases; 27.0%); followed by CHIKV (6 cases; 16.2%), ZIKV (6 cases; 16.2%), and Varicella zoster virus (VZV) (1 case; 2.7%). Four cases (10.8%) presented viral co-infection detected: DENV1 + CHIKV, DENV1 + EV, DENV4 + ZIKV, and CHIKV + ZIKV. Arboviruses (DENV, CHIKV and ZIKV) accounted for the great majority of cases (26 cases; 70.3%) of all single and co-infections: DENV has been the most frequently detected arbovirus (13 cases; 35.1%). Among non-arboviral meningitis, the most common etiology was the EV (11 cases; 29.7%).
Conclusions: Arboviruses accounted for the majority of identified viruses among patients with suspected viral meningitis. In areas where they are endemic it is crucial to increase viral surveillance and consider them in the differential diagnosis of meningitis.
Introduction
After the introduction of vaccines against the causative agents of bacterial meningitis in Brazil between 1999 and 2010, the relative importance of viral meningitis in the country increased. In Brazil, between 2010 and 2020, there were 186,766 reported cases of meningitis nationwide, 85,202 (45.7%) of which were considered to have a viral etiology. Alone, the state of Bahia, located in the country’s Northeast Region, accounted for 9,014 cases of meningitis, of which 4,307 (47.8%) were suspected viral meningitis (1). Even though many cases are not reported, the actual burden of viral meningitis is probably much more significant (2).
Classically, viral meningitis is associated with non-polio enterovirus (EV). Viruses from Herpesviridae family (HHV) have been implicated in meningitis and encephalitis within immunocompromised individuals (3, 4). Viral meningitis is characterized by acute onset of fever, headache, photophobia, and neck stiffness, often accompanied by nausea and vomiting.
Dengue virus (DENV), Chikungunya virus (CHIKV), and Zika virus (ZIKV) are arthropod-borne viruses (arboviruses) transmitted by mosquitoes from the genus Aedes that cause mostly acute fever illnesses. Arboviral infections have become more frequent in the last decades due to urbanization, deforestation, and other anthropogenic effects, imposing a considerable burden on the Brazilian population, with yet unmet solutions (5).
In the last three decades, DENV caused the greatest public health problems in Brazil, with continuous reintroductions that were responsible for the maintenance of the virus in the country and the introduction of new lineages and co-circulation of the four DENV serotypes (6). CHIKV was first documented in Brazil in 2014 (7), followed by ZIKV in 2015 (8). Since then, the northeastern regions of Brazil where Salvador, the capital of Bahia, is located has been experienced the co-circulation of those three arboviruses in a hyperendemic manner (9, 10). The city of Salvador is a large urban setting with a population of approximately 3 million inhabitants with a long history of bacterial meningitis. Aseptic causes were previously reported and included non-polio enterovirus and leptospirosis but no arbovirus or other infectious agents were found (11).
Despite their well-known relevance in acute febrile illnesses, presenting non-specific signs and symptoms (fever, myalgia and arthralgia), DENV, CHIKV, ZIKV and other arboviruses have also been associated with neurological manifestations over the last years (12–14). ZIKV is notorious due to its range of neurological manifestations in fetuses and newborns. Still, it has also been associated with outcomes in adults with meningoencephalitis, myelitis, and Guillain-Barré syndrome (GBS) (15).
Therefore, the inclusion of arboviruses in the differential diagnosis of central nervous system (CNS) infections has been considered increasingly important, especially in endemic regions.
This study aimed to investigate which viruses are involved in the meningitis etiology and the contribution of arboviruses as the putative cause of the illness in Salvador, Bahia, Brazil.
Materials and methods
Study design, site and patients
From June 2014 to February 2016, a cross-sectional surveillance study was conducted involving patients with suspected viral meningitis seen at Couto Maia Hospital, a public reference hospital for infectious diseases in Salvador, the capital of the state of Bahia-Brazil. All patients who underwent lumbar puncture as part of routine diagnosis and met the following inclusion/exclusion criteria were invited to participate in the study: patients whose cerebrospinal fluid (CSF) presented a predominance of mononuclear leukocytes or ≤100 polymorphonuclear leukocytes, as well as were a negative test for bacteria in CSF. The present study was approved by Fiocruz-BA institutional review board (protocol no. 613.123). All participants or their legal guardians, in the case of children and patients lacking mental capacity, provided written informed consent.
Data and sample collection
Demographic, medical, and epidemiological background, as well as clinical and laboratory data, were collected through interviews and medical chart reviews. REDCap software (Vanderbilt University, USA) was used for data management.
A total of 170 patients with suspected viral meningitis were enrolled. The mean patient age was 22.2 years old (median = 18 years old, range: 1 month to 73 years old). Neither gender was predominant, as 50.6% (86/170) of the sample was female versus 49.4% (84/170) male. Most individuals were of African descent (88.0% self-reported black or brown skin color) and lived in Salvador or surrounding municipalities (Salvador Metropolitan Area, 83.4%, Table 1).
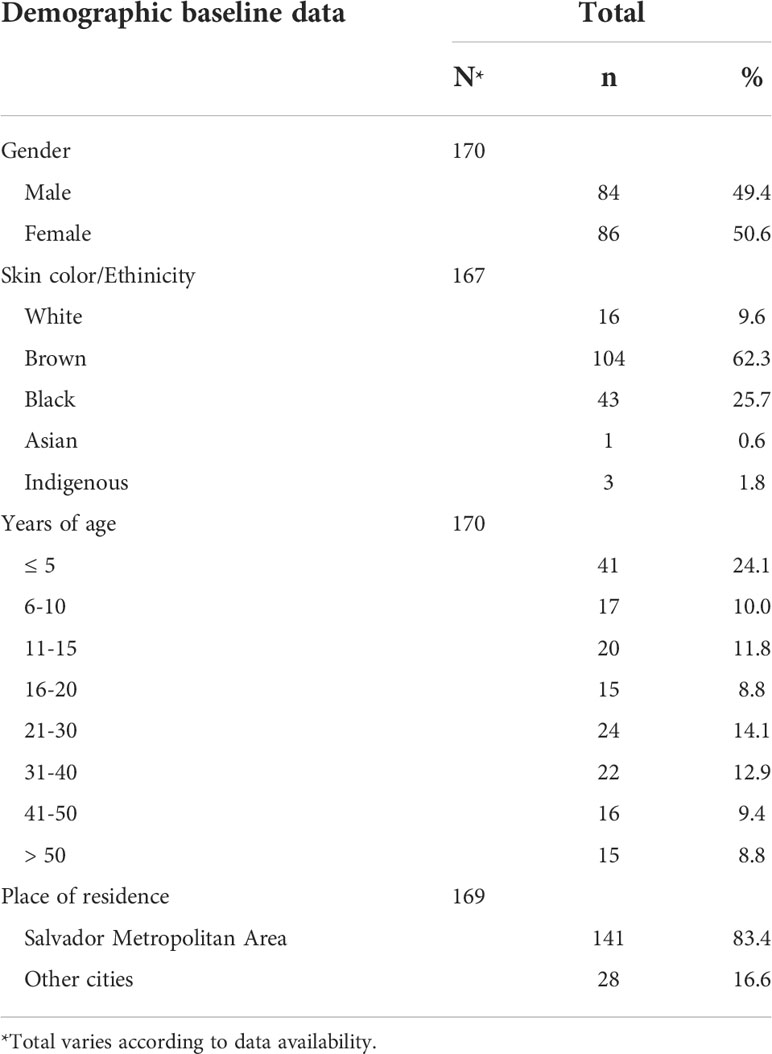
Table 1 Demographic baseline data from patients with suspected viral meningitis attended Couto Maia Hospital, between July 2014 and February 2016, Salvador (BA), Brazil.
CSF samples from all 170 subjects were collected, but the volume varied considerably among participants. Within 2 h after lumbar puncture, all samples were aliquoted and stored immediately at -20°C freezers. Transportation to Fiocruz-BA was carried out in dry ice weekly for definitive storage at -70°C freezers until use. Aliquots for molecular testing were not thawed more than once prior to analysis to avoid RNA degradation.
Laboratory diagnosis
CSF study
Routine laboratory testing of CSF samples as performed by hospital laboratory personnel consisted of total and differential cell counts, glucose and protein level determination, direct microscopy of CSF Gram, Ziehl-Neelsen and India-ink bacteria staining and culturing to detect bacteria, fungi, and Mycobacterium tuberculosis.
Molecular testing
EV, DENV, CHIKV, and ZIKV RNA genomes were extracted using a QIAamp® Viral RNA Mini kit (QIAGEN, USA) from 140 µL of CSF, while HHV DNA was extracted using QIAamp® MinElute Virus Spin kit (QIAGEN, USA) from 200 µL of CSF; both procedures were conducted in accordance with the manufacturer’s instructions. All samples were extracted at least twice, and the extraction was repeated when necessary. RNA templates were reverse transcribed into cDNA using random primers (Invitrogen, USA) and Sensiscript reverse transcriptase (Qiagen, USA) as per manufacturer´s directions. cDNA and DNA templates were amplified by PCR using Top Taq Master Mix (QIAGEN, USA) and previously described virus-specific primers were utilized for DENV1-4 (designed by Lanciotti, Calisher (16), for CHIKV (designed by Edwards, Welch (17), for ZIKV (designed by Balm, Lee (18), for EV (designed by Santos, Burlandy (19) and for HHV1-5 (designed by Markoulatos, Georgopoulou (20). To the most extent, we used singleplex PCR (except for DENV and HHV, which were multiplex), so there were many PCR reactions per sample. PCR conditions were replicated as described by the authors cited above, and results were analyzed by electrophoresis on a 3% agarose gel.
Immunodiagnostic testing
Due to the low volume of some samples, immunodiagnostic tests were performed in a subgroup of 107 remaining CSF using Dengue IgM Capture ELISA (Abbott, USA) and Panbio Dengue Early ELISA (Alere, USA) for determining DENV IgM and the NS1 antigen, respectively, and EuroImmun Chikungunya virus IgM ELISA (Perkin-Elmer, Germany) in accordance with the respective manufacturer’s instructions. In addition, 98 CSF samples that were available at the time were also tested for ZIKV IgM following the CDC Zika MAC-ELISA protocol (21). For a test to be valid, the optical density from the ratio of the positive and negative (P/N) controls must be greater than or equal to 2.0. Since the CSF was not submitted alongside a patient-matched serum, only specimens with P/N greater than or equal to 3.0 were reported as presumptive ZIKV IgM positive.
Statistical analysis
Statistical analyses were performed using SPSS v.21 (22) and STATA v.10.0 (23) software. All events of interest were reported as proportions. Prevalence ratios (PR) were used as a measure of associations. To compare proportions, the Chi-square test or Fisher’s exact test were used, and P-values < 0.05 were considered significant.
Results
Viral infection positivity
Thirty-seven patients tested positive for at least one of the studied viruses (overall positivity 21.8%) under PCR or ELISA. Of all tested samples, 17.1% (29/170) were PCR positive and 9.3% (10/107) were ELISA positive. We did not obtain positive results for the same virus in both methodologies. However, for two coinfections the viruses were both detected by PCR and for the other two coinfections, one virus was detected by PCR and the other by ELISA (Table 2).
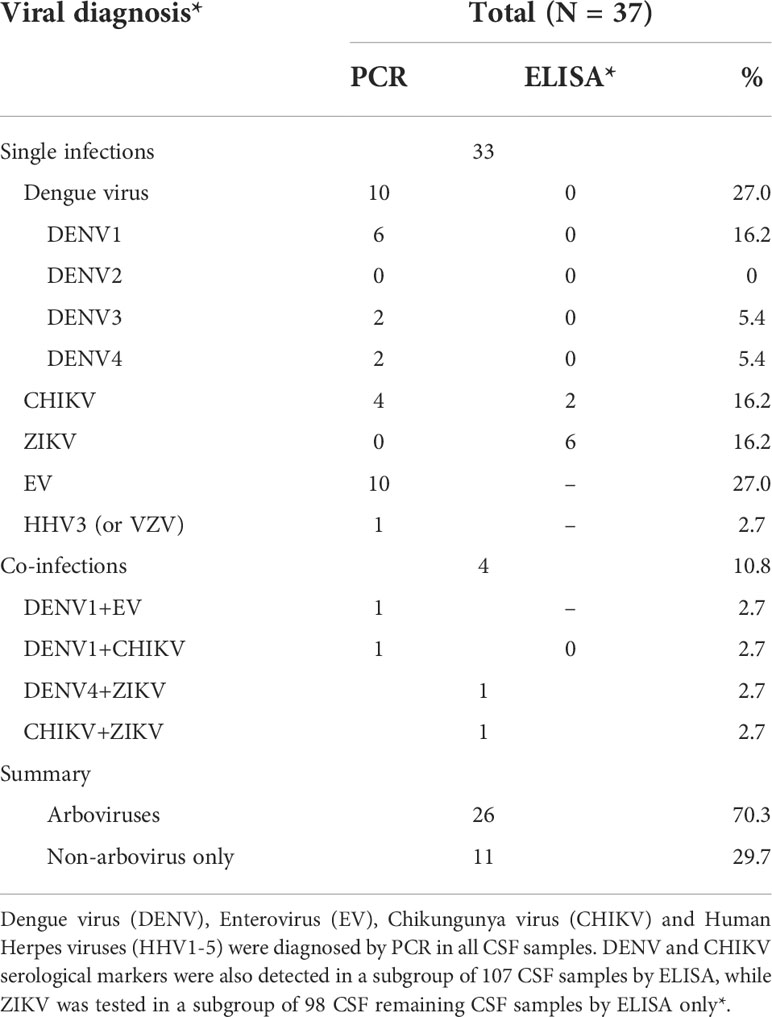
Table 2 Viral diagnosis from patients with suspected viral meningitis who attended Couto Maia Hospital, between July 2014 and February 2016, Salvador (BA), Brazil.
EV was the agent most frequently detected (10 cases; 27.0%), along with all four DENV serotypes (10 cases; 27.0%); followed by CHIKV (6 cases; 16.2%) and ZIKV (6 cases; 16.2%) and Varicella zoster virus (VZV) (1 case; 2.7%). Four cases (10.8%) presented viral co-infection detected: DENV1 + CHIKV, DENV1 + EV, DENV4 + ZIKV, and CHIKV + ZIKV (Table 2).
Arboviruses (DENV, CHIK and ZIKV) accounted for the great majority of cases (26 cases; 70.3%) of all single and co-infections. DENV was the most frequently detected arbovirus (13 cases; 35.1%): 8 (21.6%) were DENV1 serotype, while 2 (5.4%) were DENV3, and 3 (8.1%) were DENV4. CHIKV was detected in 8 (21.6%) cases, but no case of DENV2 and ZIKV was identified by PCR. All 107 remaining samples tested negative both for DENV IgM and the NS1 antigen by ELISA. Only 98 samples were submitted to ZIKV IgM testing, of which 8 (21.6%) demonstrated ELISA positivity.
Non-arbovirus infections were mostly represented by EV (11 cases; 29.7%). Only one (2.7%) sample tested positive for Varicella zoster virus (VZV, also known as HHV3). No cases of HSV1-2 (HHV1-2), EBV (HHV4) or CMV (HHV5) were detected (Table 2).
Epidemiological surveillance
The temporal distribution of the number of confirmed and suspected viral meningitis cases is shown in Figure 1. There was no clear seasonal pattern for any of the studied viruses. DENV1 was detected exclusively in 2014 alone or in coinfection with EV and CHIKV. On the other hand, DENV3 and DENV 4 were detected after 2015. First two CHIKV cases were detected in August 2014. ZIKV cases were detected from May through December 2015.
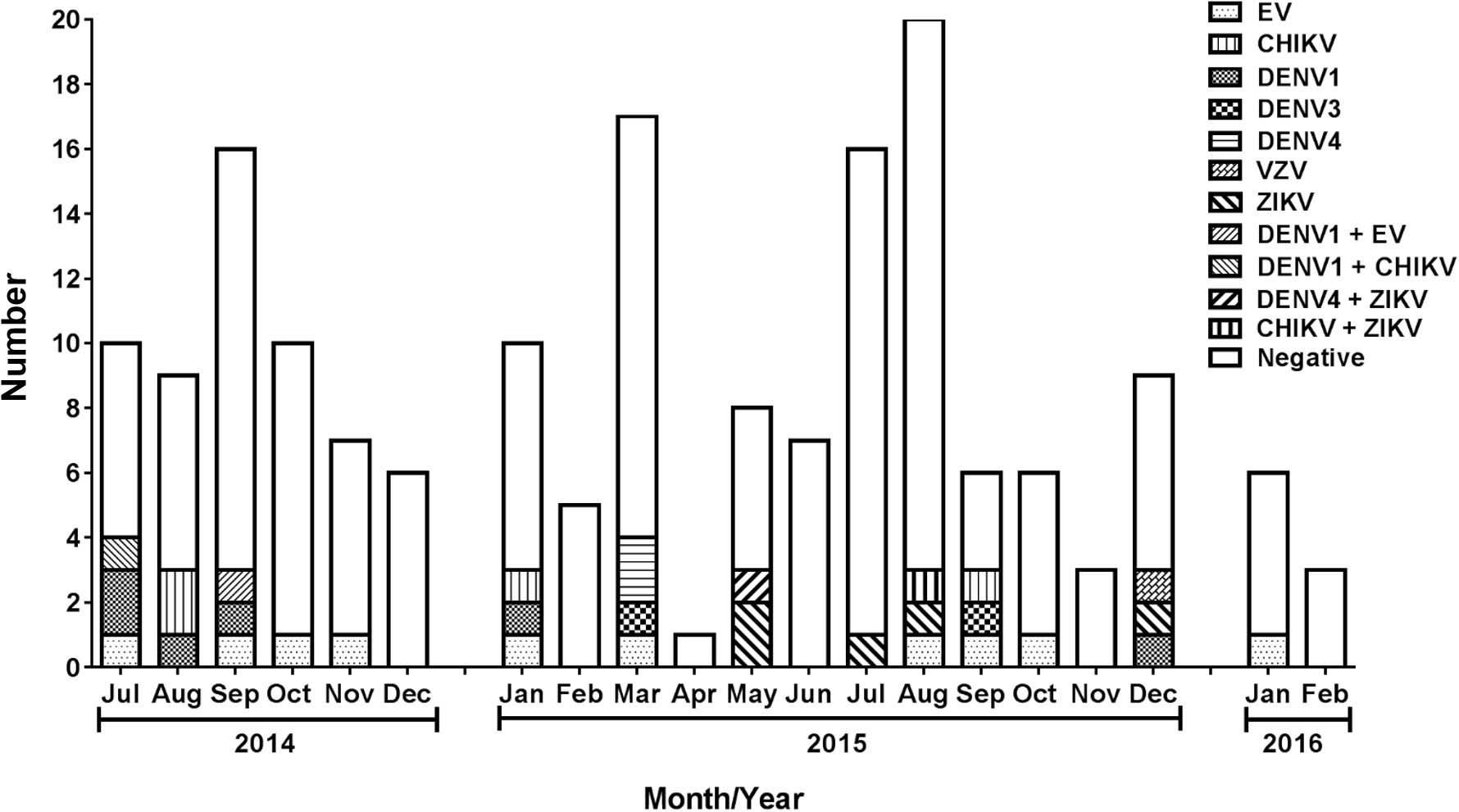
Figure 1 Number of suspected viral meningitis cases included in the study among patients who attended Couto Maia Hospital, between July 2014 and February 2016, Salvador (BA), Brazil.
CHIKV cases were detected in one patient from Amargosa (a city located about 240 km from Salvador and about 150 km from Feira de Santana) and another from Salvador. The only ZIKV case detected outside Salvador Metropolitan Area was from Itaberaba (a city located about 300 km from Salvador). VZV was detected in Salvador Metropolitan Area.
Clinical and hospital course
Among all suspected cases, fever (80.5%) was the most frequently reported sign and symptom upon attendance, followed by vomiting (53.8%), neck pain (50.0%) and neck rigidity (32.0%). Considering the neurological manifestations, headache (80.4%) was the main symptom, followed by somnolence (14.4%), seizures (8.2%) or altered state of consciousness (6.6%). Other frequent signs and symptoms were myalgia (25.4%), arthralgia (24.3%) and retro-orbital pain (21.1%). The same profile was observed among confirmed cases, except for neck rigidity, which was a little higher in this group (32.0% vs 44.1%, Supplementary Table 1).
Most suspected cases (57.9%) were referred from other health care units and 63.7% of them sought medical attention ≤5 days after initial symptoms. Despite clinical presentation and suspicion of viral meningitis, only 36.9% (62/168) of patients were hospitalized for 10.8 days on average (ranging from 1 to 54 days). All confirmed cases presented no complications and were discharged without sequelae, whereas three negative cases presented with sequelae (data not shown).
CSF samples from most suspected patients were deemed “normal”: colorless (95.2%), limpid (79.0%), presenting a cell count ≤5 cells/mm³ (60.8%) with predominance of mononuclear leukocytes (97.0%). CSF glucose and protein were within the normal range, 69.5% and 68.3%, respectively (data not shown). For confirmed cases, we observed the same pattern, excluding cell count ≤5 cells/mm³, which was less frequent (46.9%) (data not shown).
When data is stratified by etiological agent, gender varies widely. Arboviruses were frequently detected among adults older than 15 years old while EV were confirmed mostly among younger patients. Fever and headache remained the most frequent clinical sign and symptoms throughout all agents. Myalgia was slightly more frequent among CHIKV cases and co-infected individuals, while arthralgia was frequent among both CHIKV and ZIKV cases (Table 3).
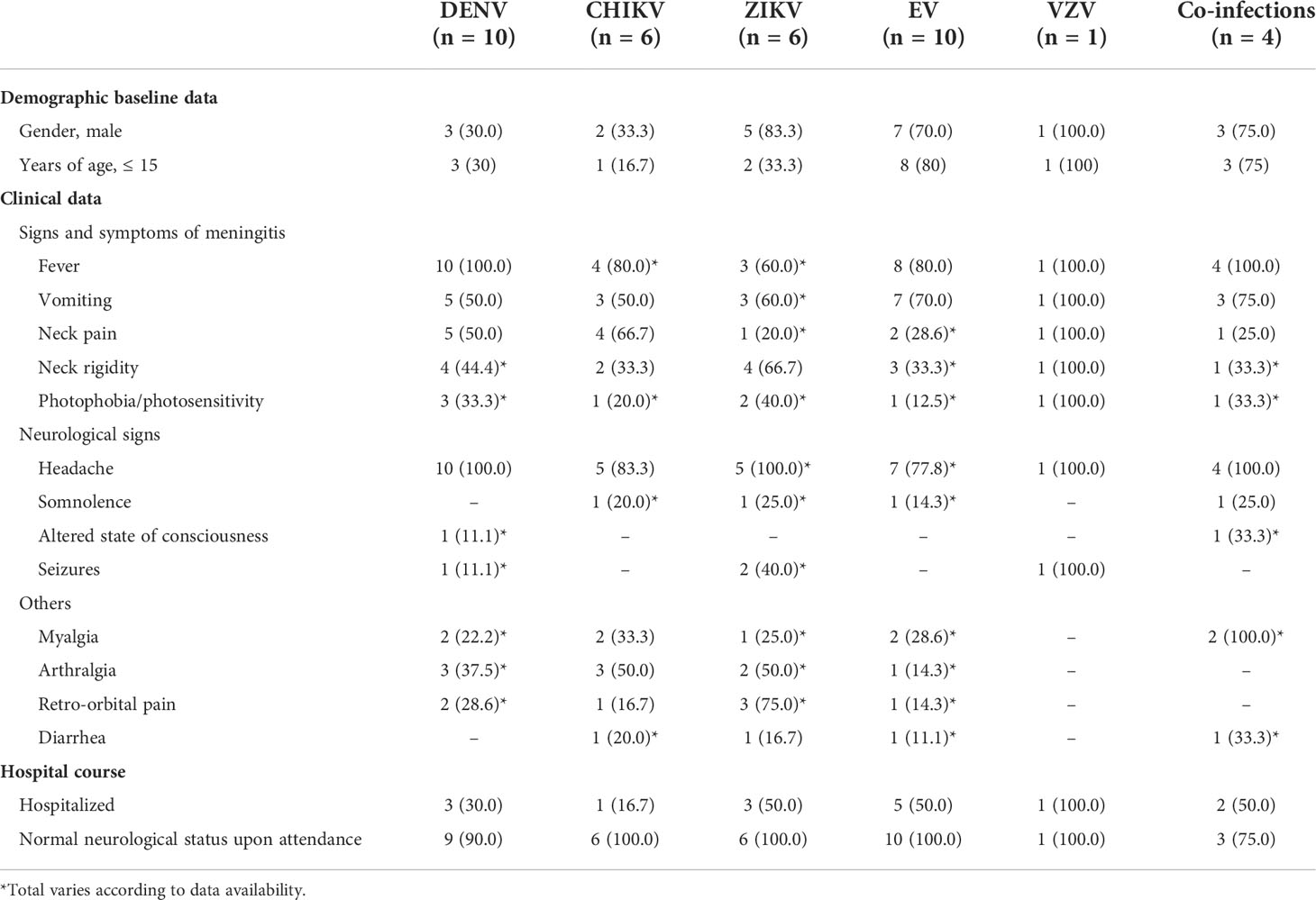
Table 3 Data stratified by etiological agent from patients with confirmed viral meningitis who attended Couto Maia Hospital, between July 2014 and February 2016, Salvador (BA), Brazil.
Prevalence ratio analysis and the risk of viral meningitis
Overall positivity of viral meningitis was not associated with any medical background, clinical or hospital course characteristics, except neck rigidity and a CSF sample presenting a turbid aspect. Arboviruses, as a group, were associated with neck rigidity only. A CSF sample presenting turbid aspect and more than 5 cells/mm³ were statistically significant variables within the ZIKV cases. Being ≤15 years of age, a CSF sample presenting a turbid aspect and more than 100 cells/mm³ were statistically significant variables within the EV cases. There was no association between the studied characteristics and DENV, CHIKV or VZV (Supplementary Table 2).
Discussion
This study aimed to investigate which viruses were involved in the meningitis etiology and the contribution of arboviruses as a cause of the illness in Salvador, Bahia, Brazil. Despite Brazil having a well-organized network of public health laboratories for surveillance and diagnosis, molecular methods have been available for a limited number of agents, such as virus-causing chronic diseases (HIV, HCV, and HBV) and arboviruses in the context of acute fever illnesses. Thus, viral meningitis remains barely identified.
Although classically associated with acute febrile illnesses, the inclusion of arboviruses in the differential diagnosis of CNS infections had been considered increasingly important throughout the years, especially in endemic regions where they have been associated with neurological manifestations (12–14). The mechanisms underlying potential viral persistence and the contribution of neuroinflammation to CNS pathophysiology are unclear. However, arboviral infections may have long-lasting effects on the nervous system because of the direct interaction of viruses with cells of the brain, or, indirectly, because of the neuroinflammatory status found associated with the infections (24).
Indeed, arboviruses were responsible for 70.3% of all confirmed cases, including DENV, CHIKV and ZIKV. DENV was the most prevalent virus identified, accounting for 35.1% of the positive cases. DENV-1 was the most frequently reported, which is also a reflection of DENV serotype circulation in Brazil (25). Similar to our results, Acevedo, Waggoner (12) found that DENV was responsible for 31.2% of positive cases. It’s been reported that DENV meningitis caused may occur without the signs and symptoms classically assigned to DENV cases (14), and only about one-third of our DENV cases presented classical symptoms, such as myalgia, arthralgia and retro-orbital pain (data not shown).
Even though EV is classically the major virus causing meningitis (3, 4), it was the second most prevalent virus in the present study, accounting for 29.7% of the positive cases. In the literature, it is responsible for 32.6% to 70.1% of viral meningitis cases (13, 26, 27). In a study carried out in the same reference hospital as the present study, Silva, Tanajura (11) had a detection rate of 44.6% (50/112), and EV accounted for 84% of that positivity [19]. In their study, CSF and/or stool culture were available, as well as PCR, but they did not detect any arbovirus.
de Crom, van Furth (28) analyzed meningitis cases caused by EV in the absence of CSF pleocytosis. The authors concluded that especially in young children, the absence of pleocytosis does not justify the EV exclusion from the differential diagnosis. This conclusion supports our decision of including patients who presented a CSF cell count of any value in case of mononuclear leukocyte predominance, and it also supports the results presented in this paper, in which 47.1% of CSF from patients with detectable virus presented ≤5 cells/mm³. Viral meningitis should be considered even when CSF cell count is under the established normal range cutoff of 5 cells/mm³.
CHIKV accounted for 21.6% of the positive cases. CHIKV was first identified in Bahia in an outbreak in the city of Feira de Santana (about 120 Km from Salvador) in September 2014, but the epidemiologic investigations suggested that the index case-patient went to an emergency health unit in May (7, 29, 30). In this study, the first two CHIKV cases were detected in August 2014, one from the city of Amargosa (about 150 km from Feira de Santana) and the other from Salvador. These findings suggest that CHIKV could be quickly transmitted to other municipalities farther than 100 Km from Feira de Santana.
ZIKV accounted for 21.6% of confirmed cases, and its presence in the CNS has already been described by several authors, including cases of meningitis/encephalitis (12, 31). This detection of this virus in the CSF coincides with the same period of its identification in Brazil, in early 2015 (32). The detection in Itaberaba (about 300 km from Salvador) may be related to the high flow of people to and from this touristic area. False-negative results can arise from specimen collection conducted outside the ZIKV IgM antibody production timeframe (typically up to 4 days and after 12 weeks post-onset of symptoms) (33). On the other hand, the most common cause of false-positive results is cross-reactivity with IgM specific for other flaviviruses such as DENV. Since no sample was DENV positive, one can assume that those were truly ZIKV positive samples.
We reported 4 cases of co-infection (10.8% among confirmed cases): DENV1+EV, DENV1+CHIKV, DENV4+ZIKV and CHIKV+ZIKV. Taraphdar, Sarkar (34) also reported a co-infection of DENV+CHIKV in acute febrile illnesses. Chahar, Bharaj (35) also showed that in areas where both viruses co-circulate, DENV and CHIKV can be transmitted together, with a co-infection rate of 8.7%, and we showed that different arboviruses can also be transmitted together. Acevedo, Waggoner (12) identified a high rate of co-infections in the CSF, 75.0% (9/12). Reports of DENV+EV co-infection have not been found up to this moment. There are no data describing the impact of co-infections in the CNS accounting for the severity of the clinical presentation or its outcome.
The detection rate based on the PCR positivity, alone, was low (17.1%), but similar to the rates described elsewhere, which vary from 14.8% to 62.9% (13, 26, 36). This can be explained, partially, by the patients’ timing to seek medical attention. We prioritized molecular testing because of its sensitivity in the acute phase. Due to the low volume of some samples, immunodiagnostics needed to be performed on the leftover CSF. Even though some samples were ELISA positive (9.3%), one could question the appropriateness of the CSF for serological testing. Nowadays, a wide range of biological fluids such as serum, plasma, blood, saliva, and CSF has been used for the identification of viral infection both by molecular and immunological tests (33, 37). At the time of the collection of these samples, we did not speculate a priori on the importance of arboviruses, so only CSF samples were available. For a better diagnostic algorithm, it would be more reliable to have results based on paired serum samples and, additionally, follow-up samples for confirmatory testing (38). Another issue is the eventual cross-reactivity between ZIKV and DENV which could lead to false-positive results (39). Thus, the interpretation of the immunological results based only on CSF samples must be done carefully, which we could consider a limitation of this study.
Only a few variables were statistically different between the confirmed cases. This suggests that meningitis cases of viral etiology are usually mild, but, nonetheless, should be investigated. Although not statistically significant, antibiotic use was more reported among negative cases, which could suggest those were false-negative cases of bacterial meningitis, undiagnosed due to therapy prior to sample collection. Further tests would be necessary to confirm that hypothesis.
Although EV is, classically, the most common virus causing meningitis and it was associated with some cases in this study, arboviruses accounted for more than 60% of the identified viruses. DENV, CHIKV, ZIKV, EV and VZV are associated with widely varied neurological manifestations making diagnosis challenging. In arboviruses endemic areas it is crucial to increase viral surveillance and consider them in the differential diagnosis of meningitis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by IGM Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
JR and MR conceived of the presented idea and established the hospital cooperation. LM; LB; VR; LV-B and CA performed the data collection that included the interview and medical chart review. PB and LN ran the serological analysis for DENV and CHIKV, and CS and IS confirmed CHIKV results and added the serological evaluation of ZIKV. TD reviewed the literature and defined the molecular methods we would use and validated the initial PCR methods for all viruses (EV, DENV, CHIKV, ZIKV and HHV group). Along with TD; LT and GC performed the PCR reactions and gel electrophoresis. As part of TD doctoral degree training, she developed the database and data entry and applied all the statistical methods to analyze and synthesize the study data. She also was responsible for writing the draft of this paper. GR; IS and LS verified the analytical methods. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Programa Pesquisa para o SUS [Grant number: SUS0011/2014, edital PPSUS-2013-II-chamada-versão-final-22.11.13] Brazilian National Council for Scientific and Technological Development (CNPq) [Grant number: 311365/2021-3], and Programa de Apoio a Núcleos de Excelência [Grant number: PNX0017/2009, edital n° 20/2009 PRONEX].
Acknowledgments
The authors thank Rita de Cassia Palma Cunha Lima and Theomira Mauadie de Azevedo Carmo for their initial contribution to protocol validation of enterovirus detection. Special thank you to Dr. Ronald Blanton for reviewing the paper and for all the suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.1023588/full#supplementary-material
References
1. SINAN. Doenças e agravos de notificação - de 2007 em diante. Brasília-DF, Brazil: Meningite. Brazilian Health Minitry (2022). Available at: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def.
2. Emmerick ICM, Campos MR, Schramm J, da Silva RS, Costa M. Estimativas corrigidas de casos de meningite, brasil 2008-2009. Epidemiologia e Serviços Saúde. (2014) 23:215–26. doi: 10.5123/S1679-49742014000200003
3. Logan SA, MacMahon E. Viral meningitis. Bmj (2008) 336(7634):36–40. doi: 10.1136/bmj.39409.673657.AE
4. Ramachandran TS. Aseptic meningitis: Medscape (2017). Available at: https://emedicine.medscape.com/article/1169489-overview?pa=CeEy3i5yW%2F8VyxMdu%2F%2FWH9AKBqE0wML%2FSvpORkUvHRCYYRW1x7XvmO99VZL7cFtqDX4FKQtzrkK9DRbGCq38P1aycSibeA0Q%2FJsWK%2BpGHzs%3D.
5. Zanotto PMA, Leite LCC. The challenges imposed by dengue, zika, and chikungunya to Brazil. Front Immunol (2018) 9:1964. doi: 10.3389/fimmu.2018.01964
6. Villabona-Arenas CJ, de Oliveira JL, Capra Cde S, Balarini K, Loureiro M, Fonseca CR, et al. Detection of four dengue serotypes suggests rise in hyperendemicity in urban centers of Brazil. PloS Negl Trop diseases. (2014) 8(2):e2620. doi: 10.1371/journal.pntd.0002620
7. Nunes MR, Faria NR, de Vasconcelos JM, Golding N, Kraemer MU, de Oliveira LF, et al. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med (2015) 13:102. doi: 10.1186/s12916-015-0348-x
8. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of zika virus in Brazil. Memorias do Instituto Oswaldo Cruz. (2015) 110(4):569–72. doi: 10.1590/0074-02760150192
9. Cardoso CW, Kikuti M, Prates AP, Paploski IA, Tauro LB, Silva MM, et al. Unrecognized emergence of chikungunya virus during a zika virus outbreak in Salvador, Brazil. PloS Negl Trop diseases. (2017) 11(1):e0005334. doi: 10.1371/journal.pntd.0005334
10. Silva MMO, Tauro LB, Kikuti M, Anjos RO, Santos VC, Goncalves TSF, et al. Concomitant transmission of dengue, chikungunya, and zika viruses in Brazil: Clinical and epidemiological findings from surveillance for acute febrile illness. Clin Infect diseases: An Off Publ Infect Dis Soc America. (2019) 69(8):1353–9. doi: 10.1093/cid/ciy1083
11. Silva HR, Tanajura GM, Tavares-Neto J, Gomes M, Linhares A, Vasconcelos PFC, et al. Síndrome da meningite asséptica por enterovírus e leptospira sp em crianças de Salvador, bahia. Rev Soc Bras Med Trop (2002) 35:159–65. doi: 10.1590/S0037-86822002000200006
12. Acevedo N, Waggoner J, Rodriguez M, Rivera L, Landivar J, Pinsky B, et al. Zika virus, chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, guayaquil, Ecuador. Front Microbiol (2017) 8:42. doi: 10.3389/fmicb.2017.00042
13. de Oliveira DB, Candiani TM, Franco-Luiz AP, Almeida GM, Abrahao JS, Rios M, et al. Etiological agents of viral meningitis in children from a dengue-endemic area, southeast region of Brazil. J Neurol Sci (2017) 375:390–4. doi: 10.1016/j.jns.2017.02.025
14. Marinho PE, Bretas de Oliveira D, Candiani TM, Crispim AP, Alvarenga PP, Castro FC, et al. Meningitis associated with simultaneous infection by multiple dengue virus serotypes in children, Brazil. Emerg Infect Dis (2017) 23(1):115–8. doi: 10.3201/eid2301.160817
15. Leon LL, Lima RG, Boffi LC, Bindilatti RN, Garlipp CR, Costa SCB, et al. Arbovirus, herpesvirus, and enterovirus associated with neurological syndromes in adult patients of a university hospital, 2017-2018. Rev da Sociedade Bras Medicina Tropical. (2021) 54:e0127. doi: 10.1590/0037-8682-0127-2021
16. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol (1992) 30(3):545–51. doi: 10.1128/jcm.30.3.545-551.1992
17. Edwards CJ, Welch SR, Chamberlain J, Hewson R, Tolley H, Cane PA, et al. Molecular diagnosis and analysis of chikungunya virus. J Clin Virol (2007) 39(4):271–5. doi: 10.1016/j.jcv.2007.05.008
18. Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, Tang JW. A diagnostic polymerase chain reaction assay for zika virus. J Med Virol (2012) 84(9):1501–5. doi: 10.1002/jmv.23241
19. Santos G, Burlandy FM, Costa E, Silva E. Direct detection of enterovirus genome in cell-culture negative cerebrospinal fluid from aseptic meningitis cases in Brazil. Virus Rev Res (2012) 17(1-2):39–43.
20. Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-Kremastinou J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol (2001) 39(12):4426–32. doi: 10.1128/JCM.39.12.4426-4432.2001
21. CDC. Centers for Disease Control and Prevention. Zika MAC-ELISA. instructions for use (2018). Available at: https://www.cdc.gov/zika/pdfs/non-eua-zika-mac-elisa-protocol.pdf.
24. Cle M, Eldin P, Briant L, Lannuzel A, Simonin Y, Van de Perre P, et al. Neurocognitive impacts of arbovirus infections. J Neuroinflammation. (2020) 17(1):233. doi: 10.1186/s12974-020-01904-3
25. SVS. Secretaria de Vigilância em Saúde. Monitoramento dos casos de dengue e febre de chikungunya até a semana epidemiológica 20, 2015 Vol. 15. Brasília-DF, Brazil: Ministério da Saúde (2015). Available at: http://portalarquivos2.saude.gov.br/images/pdf/2015/junho/30/Monitoramento-dos-casos-de-dengue-e-febre-de-chikungunya-20.pdf.
26. Ai J, Xie Z, Liu G, Chen Z, Yang Y, Li Y, et al. Etiology and prognosis of acute viral encephalitis and meningitis in Chinese children: A multicentre prospective study. BMC Infect Dis (2017) 17(1):494. doi: 10.1186/s12879-017-2572-9
27. Bastos MS, Lessa N, Naveca FG, Monte RL, Braga WS, Figueiredo LT, et al. Detection of herpesvirus, enterovirus, and arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J Med Virol (2014) 86(9):1522–7. doi: 10.1002/jmv.23953
28. de Crom SC, van Furth MA, Peeters MF, Rossen JW, Obihara CC. Characteristics of pediatric patients with enterovirus meningitis and no cerebral fluid pleocytosis. Eur J Pediatr (2012) 171(5):795–800. doi: 10.1007/s00431-011-1626-z
29. Azevedo Rdo S, Oliveira CS, Vasconcelos PF. Chikungunya risk for Brazil. Rev Saúde Pública (2015) 49:58. doi: 10.1590/S0034-8910.2015049006219
30. Teixeira MG, Andrade AM, Costa Mda C, Castro JN, Oliveira FL, Goes CS, et al. East/Central/South African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis (2015) 21(5):906–7. doi: 10.3201/eid2105.141727
31. Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, et al. Zika virus associated with meningoencephalitis. New Engl J Med (2016) 374(16):1595–6. doi: 10.1056/NEJMc1602964
32. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, bahia, Brazil. Emerg Infect Dis (2015) 21(10):1885–6. doi: 10.3201/eid2110.150847
33. Lee WT, Wong SJ, Kulas KE, Dupuis AP 2nd, Payne AF, Kramer LD, et al. Development of zika virus serological testing strategies in new York state. J Clin Microbiol (2018) 56(3):e01591–17. doi: 10.1128/JCM.01591-17
34. Taraphdar D, Sarkar A, Mukhopadhyay BB, Chatterjee S. A comparative study of clinical features between monotypic and dual infection cases with chikungunya virus and dengue virus in West Bengal, India. Am J Trop Med Hyg (2012) 86(4):720–3. doi: 10.4269/ajtmh.2012.11-0704
35. Chahar HS, Bharaj P, Dar L, Guleria R, Kabra SK, Broor S. Co-Infections with chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis (2009) 15(7):1077–80. doi: 10.3201/eid1507.080638
36. Dupuis M, Hull R, Wang H, Nattanmai S, Glasheen B, Fusco H, et al. Molecular detection of viral causes of encephalitis and meningitis in New York state. J Med Virol (2011) 83(12):2172–81. doi: 10.1002/jmv.22169
37. Gorchakov R, Berry RM, Patel SM, El Sahly HM, Ronca SE, Murray KO. Optimizing PCR detection of zika virus from various body fluids. Am J Trop Med hygiene. (2019) 100(2):427–33. doi: 10.4269/ajtmh.18-0755
38. Atmar RL. Immunological detection and characterization. In: Kaslow RA, Stanberry LR, Le Duc JW, editors. Viral infections of humans: Epidemiology and control, vol. p . Boston, MA: Springer US (2014). p. 47–62.
Keywords: viral meningitis, arboviruses, molecular diagnosis, serological diagnosis, case series
Citation: Dias TT, Tauro LB, Macêdo LEN, Brito LO, Ribeiro VHO, Santos CS, Jacob-Nascimento LC, Vilas-Boas LS, Amado C, Barbosa PS, Reis JN, Campos GS, Ribeiro GS, Siqueira IC, Silva LK and Reis MG (2022) The emergence of arboviruses changes the profile of viral meningitis in Salvador, Bahia: A case series. Front. Trop. Dis. 3:1023588. doi: 10.3389/fitd.2022.1023588
Received: 19 August 2022; Accepted: 13 October 2022;
Published: 02 November 2022.
Edited by:
Amit Sinha, New England Biolabs, United StatesReviewed by:
Antonio C. R. Vallinoto, Federal University of Pará, BrazilHugo Carrillo-Ng, Nutrition Research Institute (IIN), Peru
Copyright © 2022 Dias, Tauro, Macêdo, Brito, Ribeiro, Santos, Jacob-Nascimento, Vilas-Boas, Amado, Barbosa, Reis, Campos, Ribeiro, Siqueira, Silva and Reis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitermayer G. Reis, bWl0ZXJtYXllci5yZWlzQGZpb2NydXouYnI=
†Present address: Laura B. Tauro, Subtropical Biology Institute CONICET-UNaM, Puerto Iguazú, Misiones, Argentina
 Tamiris T. Dias1,2
Tamiris T. Dias1,2 Laura B. Tauro
Laura B. Tauro Leile C. Jacob-Nascimento
Leile C. Jacob-Nascimento Caio Amado
Caio Amado Joice N. Reis
Joice N. Reis Gubio Soares Campos
Gubio Soares Campos Guilherme S. Ribeiro
Guilherme S. Ribeiro Isadora C. Siqueira
Isadora C. Siqueira Luciano K. Silva
Luciano K. Silva Mitermayer G. Reis
Mitermayer G. Reis