- 1Department of Parasitology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon – Accra, Ghana
- 2Neglected Tropical Diseases Programme, Ghana Health Service, Accra, Ghana
- 3Neglected Tropical Diseases Support Centre, Taskforce for Global Health, Atlanta, GA, United States
- 4Department of Epidemiology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon – Accra, Ghana
Many lymphatic filariasis (LF) endemic countries, including Ghana, have successfully implemented mass drug administration (MDA) and made significant progress towards the elimination of the disease as a public health problem. Unfortunately, the existence of individuals who seldom or never take part in MDA pose a threat to this success, as they may serve as reservoirs of infection, re-infecting their communities. In this study we implemented strategies to identify and treat these individuals, while also assessing their level of infection, to inform programme actions. The study was undertaken in the Ahanta West hotspot district in Ghana, which has received more than 17 rounds of MDA. Through the community registers used in recording participation in MDAs, we identified and offered treatment to individuals who were ineligible or inadvertently missed the last MDA in April 2021 (Engage and Treat – E&T), or testing using the filariasis test strip followed by treatment to community members who for various reasons chose not to participate in the last MDA (Test and Treat – T&T). During the study, 23,879 individuals ranging from 5 to 98 years were reached, of whom 78% were not captured in the MDA register. Among the E&T group, 75.06% willingly received and swallowed the treatment drugs. The remaining 24.94% were offered testing followed by a re-engagement to receive the drug in the T&T group. Overall, 22,830 (95.61%) of participants were treated by either strategy. Of the participants in the T&T group, 516 (8.66%; 95% CI= 7.96 – 9.41) were positive by the FTS. The highest antigen prevalence was detected among children 5 to 10 years, with 16.59% (95% CI= 12.02 – 22.06) and 22.54% (95% CI= 17.11 – 28.74) among females and males, respectively. Mapping of the data revealed that most infections are in a few select communities. Of the 516 FTS positives, 27.33% reportedly missed MDA once, 18.41% missed MDA twice and 54.26% missed all of the last three MDAs. The main reasons for missing MDA included absence (25.49%), travel (21.24%), being unaware of MDA (20.27%), refusals to take the drug (10.65%), illnesses (7.07%) and fear of adverse events (6.13%). This study demonstrates that greater sensitization and engagement strategies, with a test and treat strategy reserved for the most hesitant individuals, could significantly increase the number of individuals who receive treatment and therefore help districts reach their elimination targets by reducing the remaining reservoir or infection. NTD programmes require new tools to help them identify, engage and treat these individuals, as part of their overall monitoring and evaluation strategy.
Introduction
The WHO 2030 NTD roadmap has set ambitious targets for the elimination of lymphatic filariasis (LF) in 58 out of 72 endemic countries (1). Through the Global Programme to Eliminate Lymphatic Filariasis (GPELF), established in the year 2000, significant achievements have been made. Of 72 endemic countries, 11 (two in Africa), have been validated for elimination as a public health problem, 10 have stopped mass drug administration (MDA) and are under surveillance, 46 are implementing MDA, and 5 are yet to start MDA (2). The main aim adopted by the GPELF includes the treatment of an entire endemic population using a combination of ivermectin, diethylcarbamazine and albendazole, depending on co-endemicity with other diseases such as onchocerciasis and loaisis. The second aim relates to morbidity management and disability prevention.
MDA for LF elimination in Ghana started in 2001, reaching 100% national coverage by 2006. By 2014, 69/98 endemic districts passed the first transmission assessment survey (TAS) and stopped MDA (3). As of 2017, there were 17 districts that had still not passed the TAS, and thus could not stop MDA (4). That number decreased to 11 districts in 2021 (GHS-NTD Programme 2021, unpublished). Several challenges, including inaccurate reported data, socio-cultural norms, a weak health system, and disease vectors have been encountered in these districts that impact the ability to attain the recommended 65% treatment coverage (5–8). These challenges point to the existence of individuals who never or seldom participate in MDA and who may serve as reservoirs for infection in the communities. To address these challenges, various initiatives are being explored to improve treatment coverage (9–11). In this paper, we present results from a strategy targeting individuals identified via LF MDA treatment registers who seldom, or never, take part in MDA and encouraging them to swallow the treatment through an ‘engage and treat’ (E&T) or ‘test and treat’ (T&T) approach.
Materials and methods
Methodology
Study sites
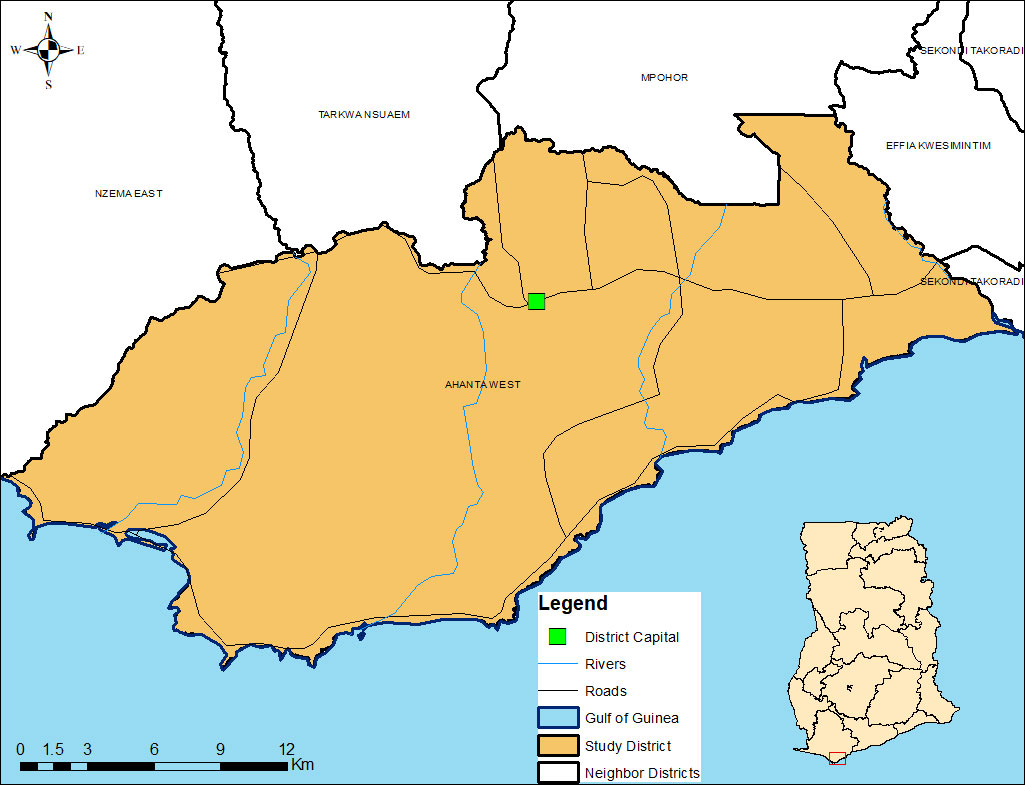
The Ahanta West District, the study site chosen for its identification as a “hotspot”, is located in the Western Region of Ghana (Figure 1). The main economic activities are agriculture and fishing. It is within the wet evergreen ecological zone of Ghana.
Aside from the main administrative capital, communities in the districts are predominantly rural. The region has one of the highest levels of LF endemicity in the country (12, 13) and has had more than 15 rounds of yearly treatment, without interrupting transmission. In the Ahanta West district, the persistent transmission of LF has been reported following several rounds of MDA (14) and previous studies also indicated inaccuracies in the treatment coverage data reported (6). The district is made of 120 communities (112 of which are rural). In terms of health accessibility, the district has one hospital, four health centres, three clinics, 12 Community-based Health Planning and Services (CHPS) compounds and 100 community health outreach points. Epidemiological surveys in the district, in 2017, indicated an antigen and microfilaria prevalence of 10.6% and 1.6%, respectively (14). Rural communities were selected for this study because urban areas tend to be very densely populated with large mobile populations. Additionally, drug distribution in urban areas poses a major challenge for programs involved in the elimination of LF and requires specific strategies tailored for the different identifiable groups rather than a uniform distribution strategy. As a proof-of-concept study, it was therefore easier to implement in the rural areas.
Project design
Every community involved in MDA in Ghana has a paper-based register detailing the names, ages, and house numbers of every community member. Based on these registers, CDDs distribute albendazole and ivermectin, noting in the register if an individual has received treatment and the number of tablets of each drug received. At the end of the MDA, the registers are kept at the closest health facility to a given community in the district. As part of this study, the registers were reviewed, following the 2021 MDA, to enumerate all non-treated individuals after the MDA. Through these registers, individuals who failed to receive treatment in the community were identified, provided with information about the drug administration, and engaged to receive treatment or tested by community health nurses (CHNs) using the Filariasis Test Strip (FTS), and if positive for W. bancrofti infection, provided with treatment. The participant identification and flowchart is presented in Figure 2.
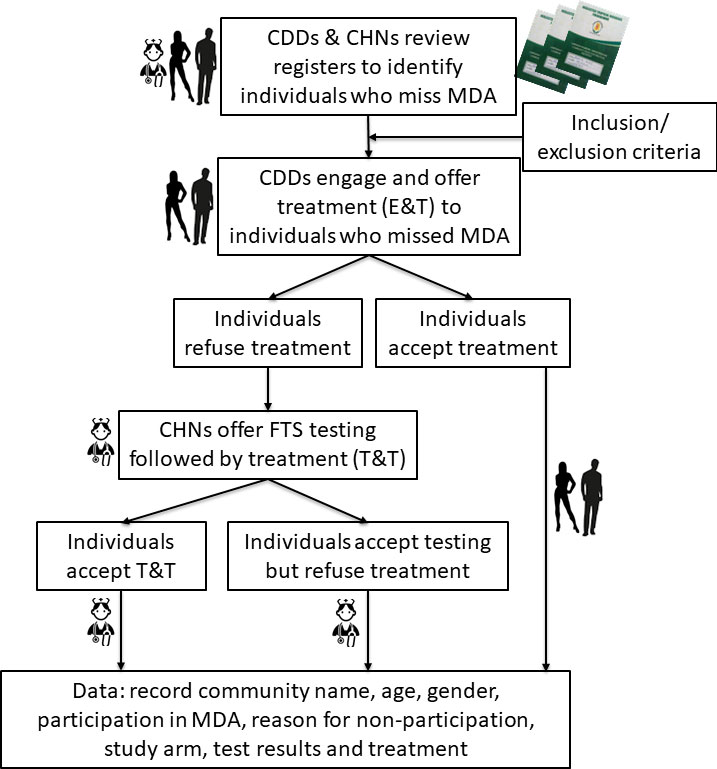
Figure 2 Study flowchart for participant identification and assignment to study arms. CDD, Community drug distributor; CHN, Community health nurse; E&T, Engage & treat; T&T, Test & treat; FTS, Filariasis test strip.
Participants
The following inclusion and exclusion criteria were considered in identifying individuals in the district who never or seldom participate in MDA.
Inclusion criteria:
● No participation in the previous year MDA
● Residence in a rural community in the district
● Aged 5 years and above
● Ability to provide informed consent
Exclusion criteria:
● Residency in urban areas
● Children less than 5 years
● Individuals who are sick or bedridden
● Pregnant women
● Breastfeeding mothers
Sample size
The study was undertaken in the rural population of the Ahanta West District. The population of the district is approximately 106,000 inhabitants, located in 120 communities. Thirty percent of the population is urban, the remaining 70% are considered rural (https://www.wrcc.gov.gh/en/mmda-s/districts/ahanta-west). About 7.5% of the population are children below the age of five. Surveys in 2017 indicate that about 31% of the population does not recall participating in previous MDAs (5). Given this information, it is estimated that approximately 21,300 people living in rural areas in the district did not participate in the MDA and therefore represent the target population for this study. This equals an average of 254 individuals to be targeted in each community.
Sensitization activities
Following an assessment of the MDA non-compliance challenges in the district using questionnaires and focus group discussions (Supplementary File S1–S3), the study team conducted sensitization training for community health nurses (CHNs) and community drug distributors (CDDs) in the project communities. Subsequently, a team comprising of the district disease control officer (DCO), the CHNs and CDDs informed the village chief, notable figures, and local leaders of the study and the team’s presence. The leaders were encouraged to announce the project in local community meetings. In addition, CHNs were encouraged to disseminate the information during outreach programmes, inviting individuals who seldom or never take part in MDA to get tested and treated if results were positive. Radio broadcasts, community announcement systems, and village meetings were also used to inform communities about the study.
The information from the qualitative and quantitative assessments were used to develop a poster to inform the communities about the causes and prevention of LF. The posters were prepared in English, Twi, and Ahanta (the major languages in the district). These were first field tested in two communities to understand the views of the community members on the appropriateness of the tools. Comments received from the community members were used to improve the poster before they were printed for distribution. The posters were hung in communities and health centers.
Implementation of strategies
Based on earlier studies (15), individuals identified in the registers and found to have missed the most recent MDA were placed into one of two arms: Engage and Treat (E&T) or Test and Treat (T&T). However, at the beginning of the study, it was observed that the CDDs reported data for untreated individuals in the community who were not captured in the registers. The identification of these were done through the untreated people in the registers, who reported knowledge of other individuals who also did not take part in the MDA. This led to a snowballing identification of other individuals not in the registers. The registers in each community were thoroughly reviewed by the CDDs (with random checks by the study team during monitoring visits) to ensure the individuals identified as not being the registers were not captured in the same registers under different households. All treatments (using ivermectin and albendazole) and drug supplies in this study were deployed following procedures established by the NTD Programme.
Engage and treat
Individuals enrolled in the E&T strategy included those who were ill during the MDA, missed treatment for no reason, were away during the MDA, or were unaware of treatment (i.e., not reached). The MDA register makes it possible to identify these individuals, through the status definitions provided in the register.
Test and treat
Individuals enrolled in the T&T strategy included those who plainly refused MDA or those who cited fears of adverse events (AE) (i.e., refusals). Although refusals are recorded in the registers, the reasons for the refusal are not. As such, CDDs probed to understand the reasons for the refusal. As part of the E&T strategy, individuals refusing treatment were encouraged to get tested by a CHN using the FTS, thus effectively enrolled in the T&T study arm. If found to be positive, they were further engaged to receive treatment, using sensitization guidelines developed as part of this study.
Process evaluation
There was continuous monitoring and evaluation of all processes to determine whether the study interventions were faithfully implemented. Bi-weekly visits were conducted by the study team to collect the data and address any challenges reported by the CDDs and CHNs. A WhatsApp group was also created where CDDs and CHNs reported any challenge, and the study team addressed the challenges and provided information as required. This constant monitoring helped to identify challenges and implement corrective measures quickly. At the end of the study participants selected in different communities in the district were surveyed by the study team, using data collection tools developed for the study (Supplementary File S4).
Data management and analysis
The geographic coordinates of all communities were taken and entered into a Microsoft Excel database. Data collection forms containing the month, community, number of individuals identified who seldom or never take part in MDA, reasons for missing MDA, strategy adopted (E&T or T&T), number subsequently treated, number tested and number of FTS positives were collected monthly.
The results are presented below in tables and graphs, with percentages of the different indicators assessed. The prevalence of infection was estimated using the MedCalc Software (Version 18.6), with 95% confidence intervals. The site-specific datasets were overlaid with geo-referenced community maps of the district using ArcGIS Software (Version 10.4.1). Kriging, or Gaussian process regression, analysis was used to identify hotspots in the district where more inputs into E&T or T&T will be required.
Ethical approval
Ethical approval for the study was obtained from the NMIMR Institutional Review Board (CPN 021/19-20). Informed consent for night blood collection was obtained from adults and from parents or guardians of individuals less than 18 years old. For children 12 years and above, assent to participate in the study was also collected.
Results
A total of 26,934 individuals were reached during the study, higher than the estimated sample size. However, following data cleaning, only the information for 23,879 participants has been analyzed and is being presented. The reasons for excluding the data for 3,055 participants relate to missing information and alterations to procedures at the start of the study following the early process evaluations. For example, when implementation started in June, it was discovered that people listed in and outside the MDA registers were being captured by the CDDs. The data tools were therefore updated to enable us to capture this information. As such, the data for June is not captured in this analysis since we are unable to determine which participants were present in the register or not. Similarly, it was noted early in the study that CDDs were also including children below 5 years of age in the study and treatment, contrary to the inclusion/exclusion criteria. They were subsequently informed to strictly proceed per the NTDP guidelines. Thus, these data were also taken out.
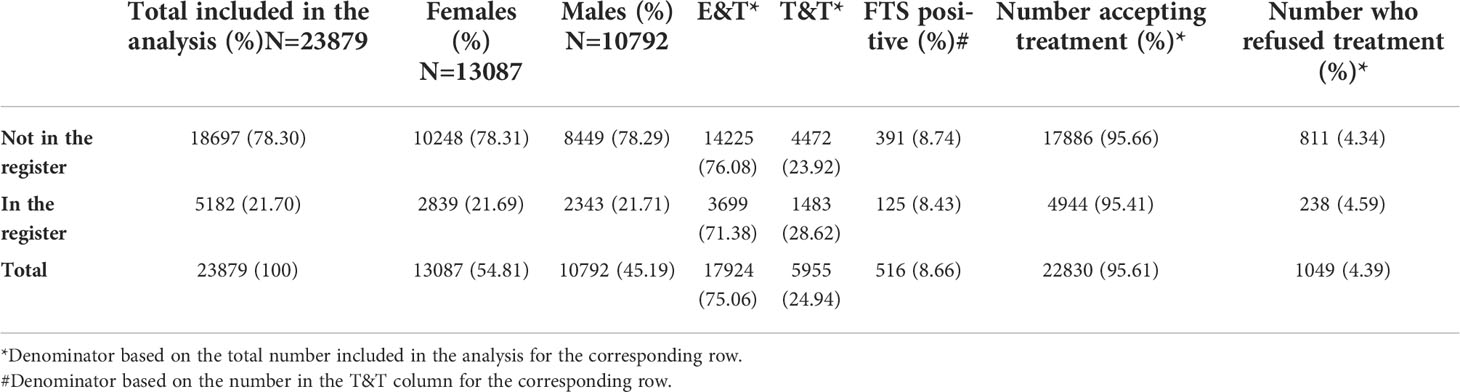
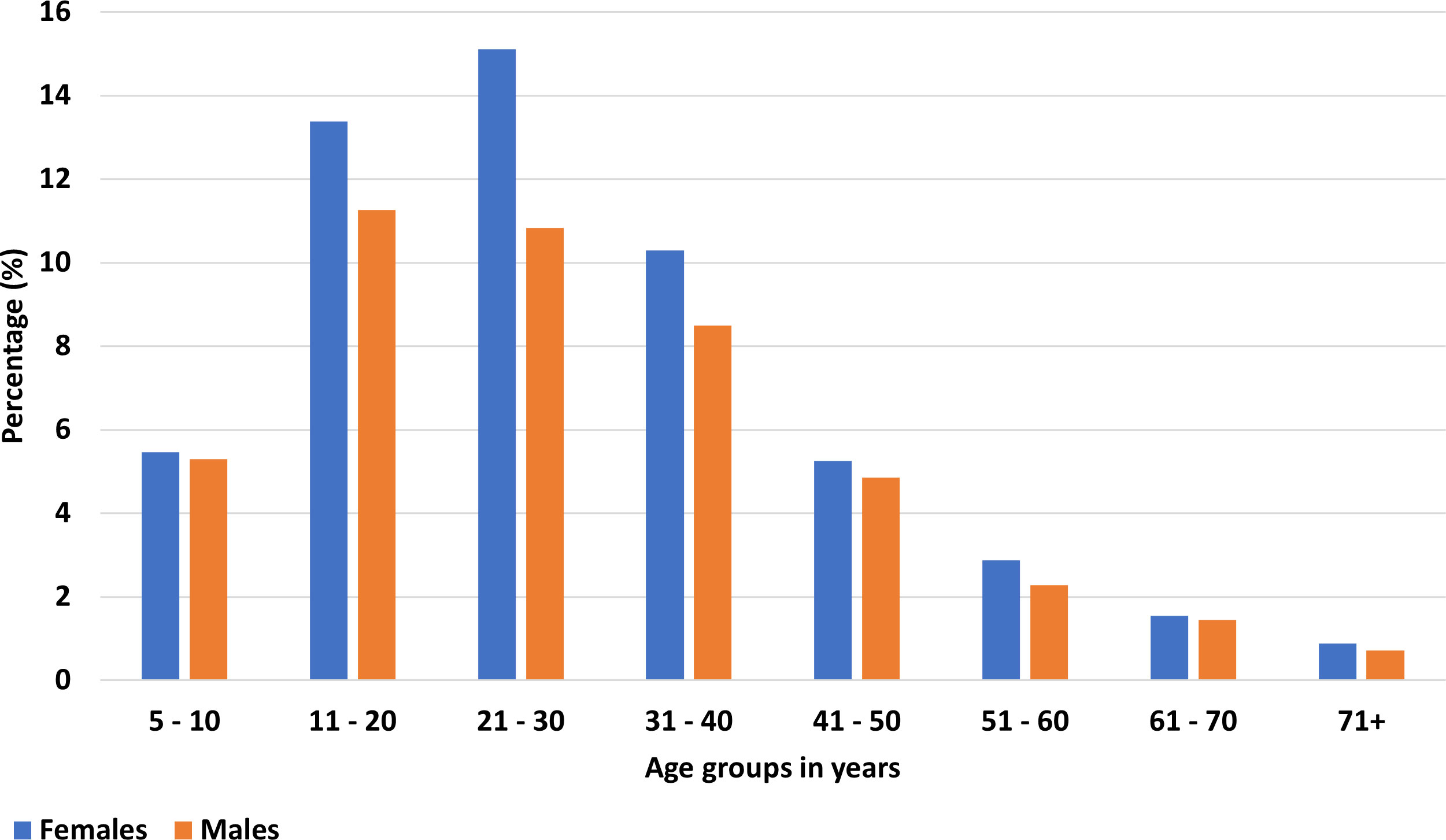
The participants’ ages ranged from 5 to 98 years (mean= 28.61; median= 26). 13,087 (54.81%) were females and 10,792 (45.19%) were males (Table 1). 18,697 (78.30%) were not captured in the register compared to 5,182 (21.70%) that were in the register. Of all the 23,879 participants, 17,924 (75.06%) were in the E&T group and the remaining 5,955 (24.94%) in the T&T group. Of the participants in the T&T group, 516 (8.66%; 95% CI= 7.96 – 9.41) tested positive by the FTS. Regardless of their study arm, 22,830 (95.61%) of participants were treated. The remaining 1,049 (4.39) in either E&T or T&T group refused treatment (Table 1), including 3 individuals who tested positive by the FTS. The majority of participants were in the 11 to 40 years age group (Figure 3).
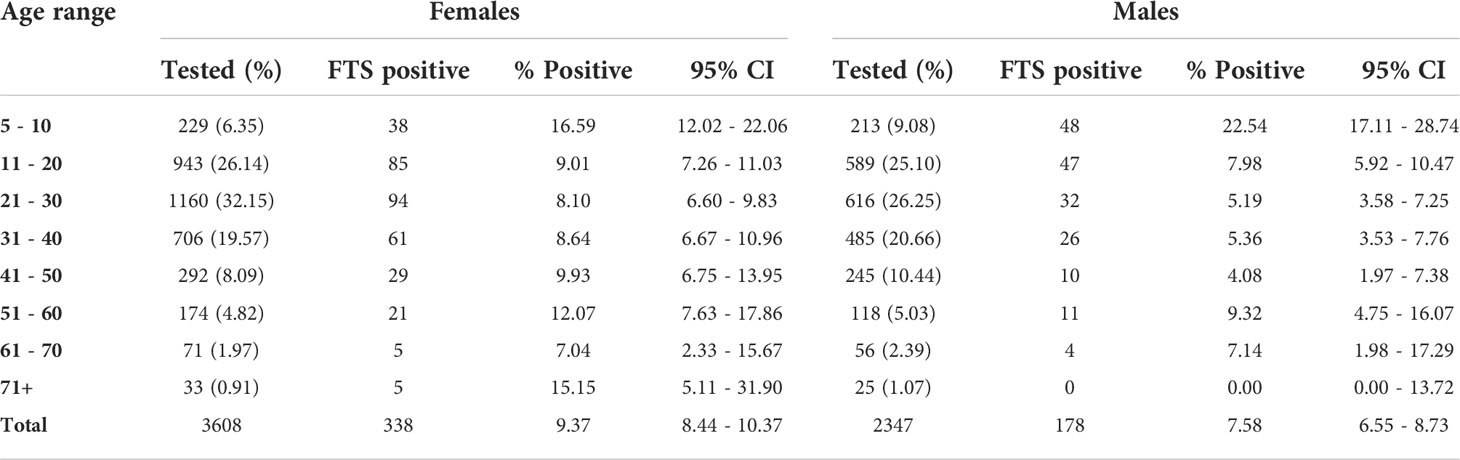
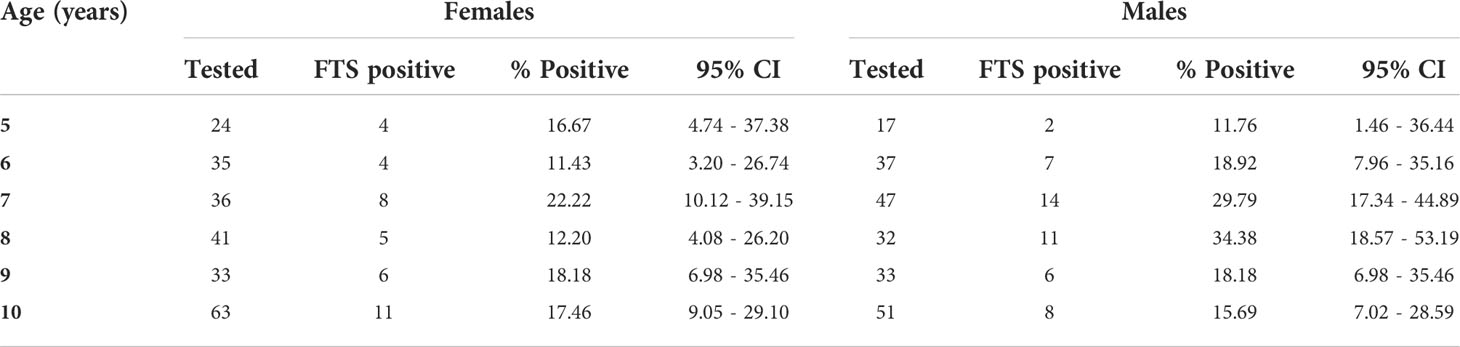
Overall, 338 (9.37%; 95% CI= 8.44 – 10.37) females and 178 (7.58%; 95% CI= 6.55 – 8.73) males in the T&T group were positive by the FTS (Table 2). Surprisingly, the highest antigen prevalence was detected among children 5 to 10 years, with 16.59% (95% CI= 12.02 – 22.06) and 22.54% (95% CI= 17.11 – 28.74) among females and males, respectively (Table 3). Plotting the coordinates of the communities and the LF prevalence revealed most infections are in few communities (Figure 4). Table 4 shows the FTS prevalence in the positive communities.
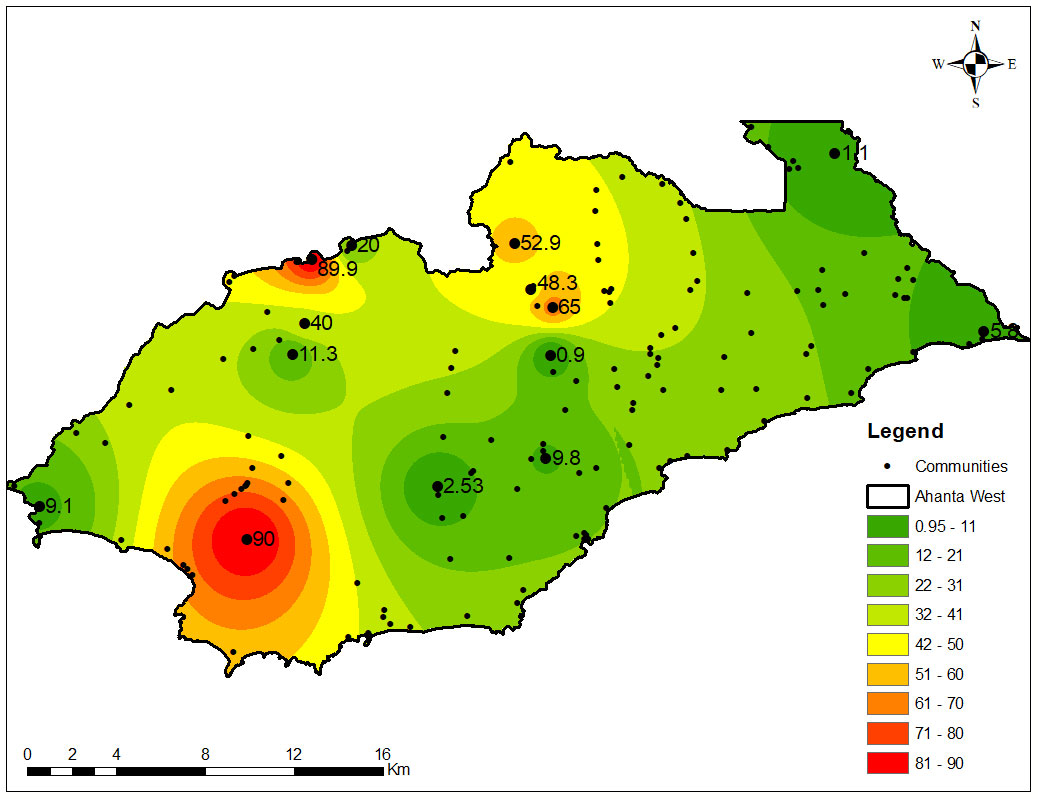
Figure 4 Distribution of LF antigen prevalence among individuals who seldom or never take part in MDA. The values on the map show the point prevalence for specific communities. The map shows hotspots of infection among study participants, tested for W. bancrofti using the FTS, and represents areas in the district where efforts will need to be focused for successful interruption of transmission and cessation of MDA.
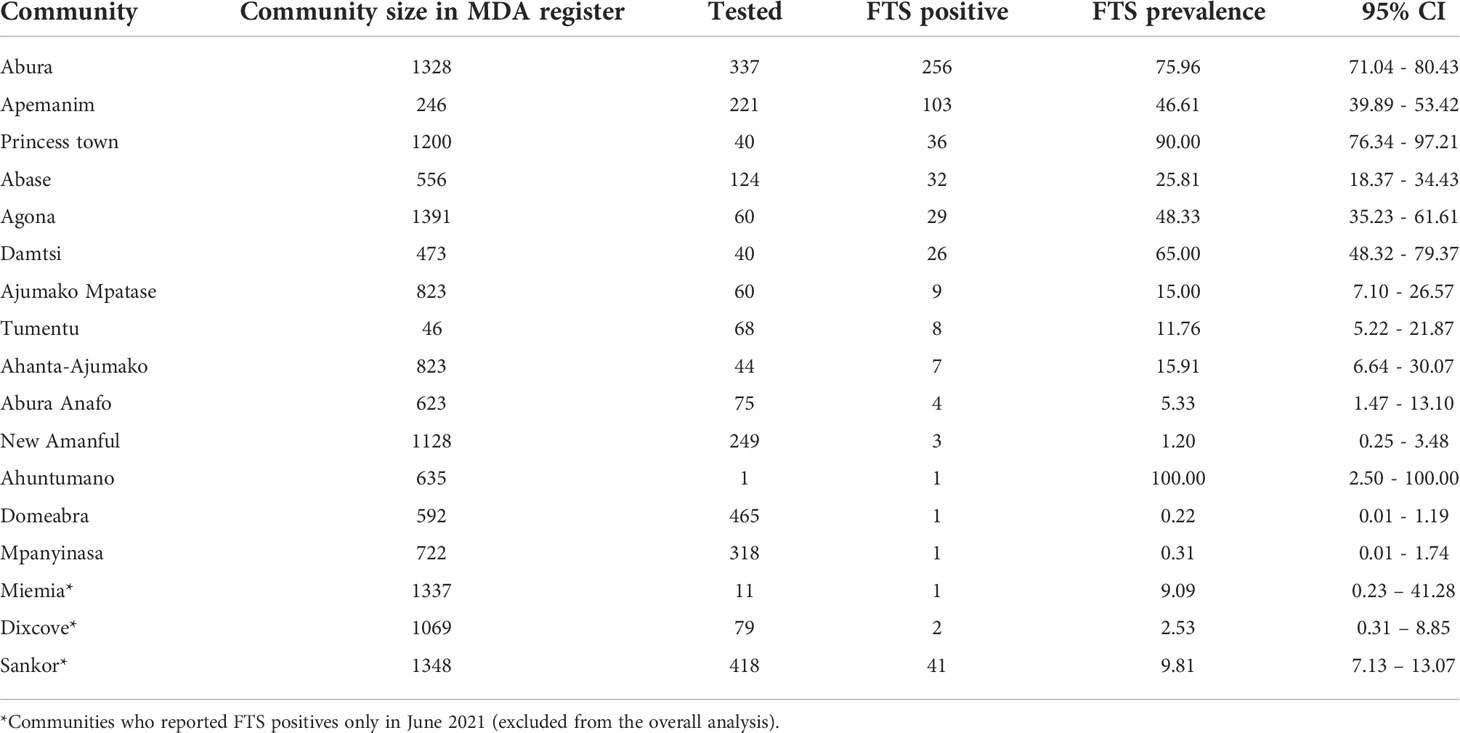
Table 4 FTS prevalence in communities with infection. One hundred (100) communities had zero FTS prevalence within the reporting period (July – October 2021) of the study.
The reasons reported by study participants for missing any MDA included being under height – in children (1.83%), breastfeeding (2.39%), pregnancy (4.61%), fear of adverse events (6.13%), illnesses (7.07%), refusals to take the drug (10.65%), being unaware of the date and time of MDA (20.27%), travel (21.24%) and absence (25.49%). Other reasons for missing MDA included alcoholism (1), religion (1), not considering MDA as important (1), never heard of MDA [completely unaware of MDA] (3) and being in school (16). Among the study participants from either treatment arm, 10,710 (44.83%) reportedly missed MDA once in the last 5 years, while 6,967 (29.16%); 6,061 (25.37%); 25 (0.1%) and 126 (0.25%) reported missing MDA, 2, 3, 4 and 5 or more times, respectively. Of the 516 FTS positives, 141 reportedly missed MDA once, 95 missed MDA twice and 380 missed MDA in all the last three MDAs. Table 5 below shows the prevalence among tested individuals who missed MDA for various reasons.
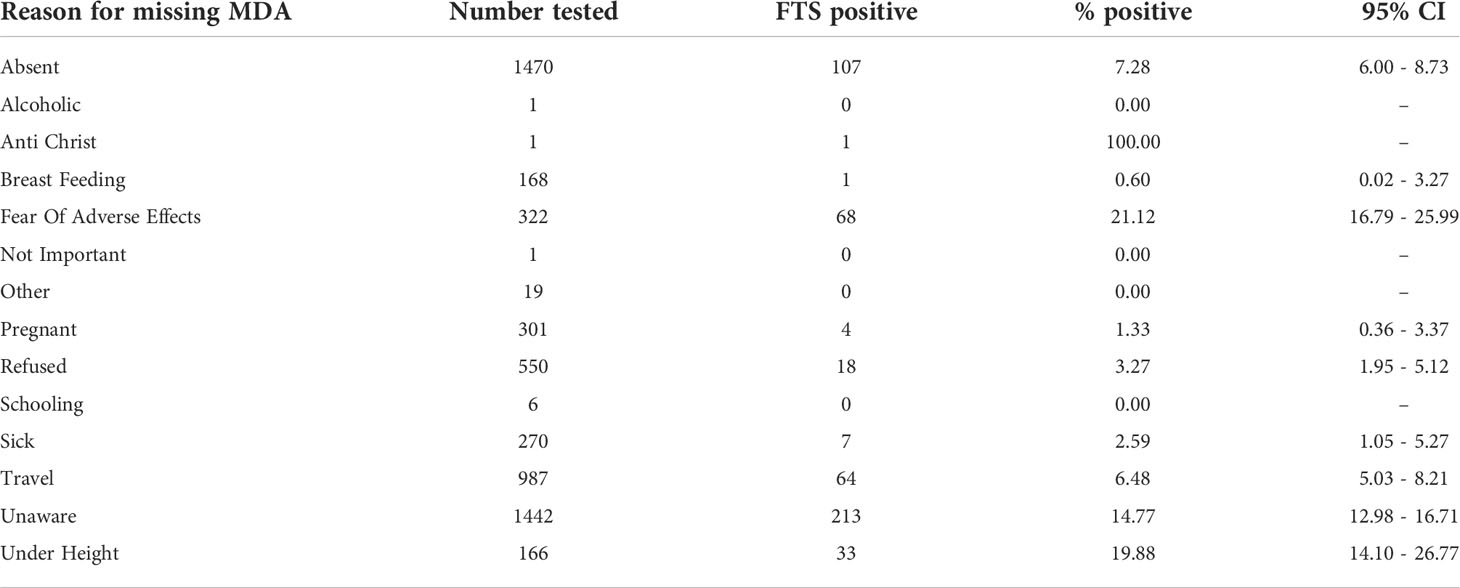
Table 5 LF antigen prevalence among participants in the T&T group who missed MDA for different reasons.
Process evaluation
Characteristics of participants
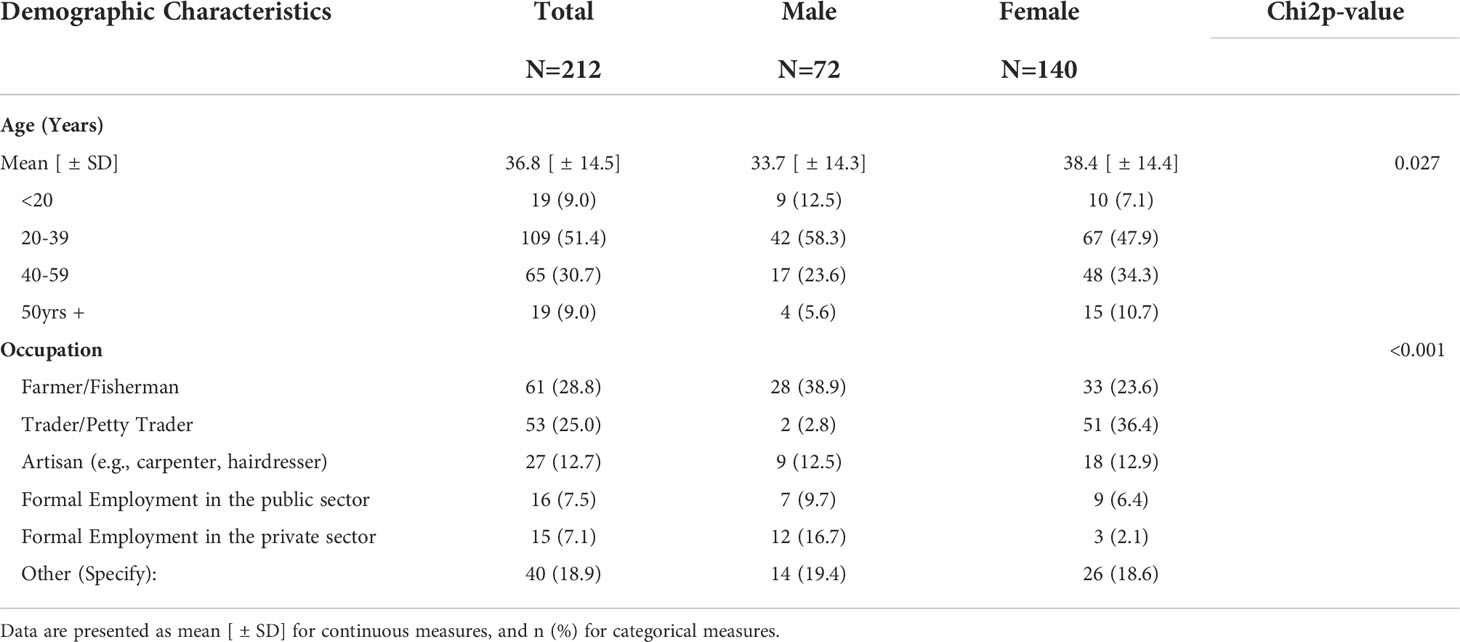
Table 6 shows the demographics of individuals who participated in the process evaluation. Seventy-two (34%) males and 140 (66%) females were among the 212 randomly selected beneficiaries. The average reported age is 37 years old. Males were younger (33.7 years ±14.3 years) than females (38.4 years ±14.4). Most participants are employed and engaged in farming/fishing (29%) and trading (25%). Gender differences in age and occupation were observed to be statistically significant.
Reach and treatment received
Table 7 shows the characteristics of individuals who received different services through this study. In all, 97% of targeted recipients received treatment. Individuals under the age of 20, those between the ages of 40 and 59, and those working in the private sector and farming/fishing/petty commerce participated more actively in the intervention. However, no statistically significant differences were found across demographic factors.
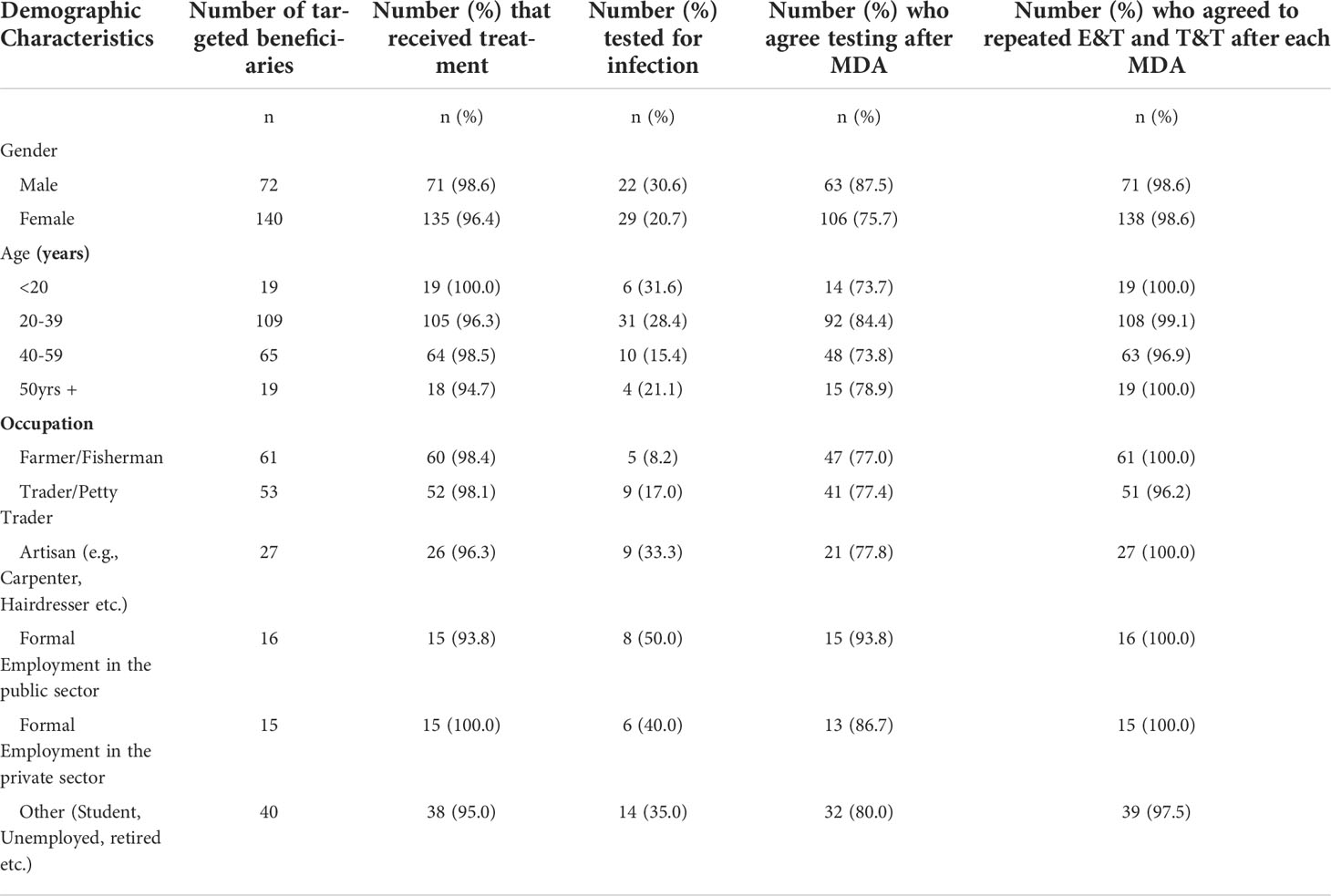
Table 7 Characteristics of study participants enrolled in the process evaluation, by services received.
In terms of utilization of testing services, termed in this project as T&T, only 24% of study participants assessed in the process evaluation were tested. Males accepted testing at a higher rate (30.6%) than females (20.7%).
Around 80% of participants agreed to the provision of testing for all individuals who missed MDA to assess their infection status. A strong majority of participants (98.6%) responded that E&T and T&T should be repeated after each mass drug administration in the study district.
Individuals who received the drugs without being tested perceived the drug distribution process during the E&T as being fair (52.7%), while only 20.0% of individuals who opted for T&T perceived the process as being fair to everyone.
CDDs perspectives on the implementation process of activities and services
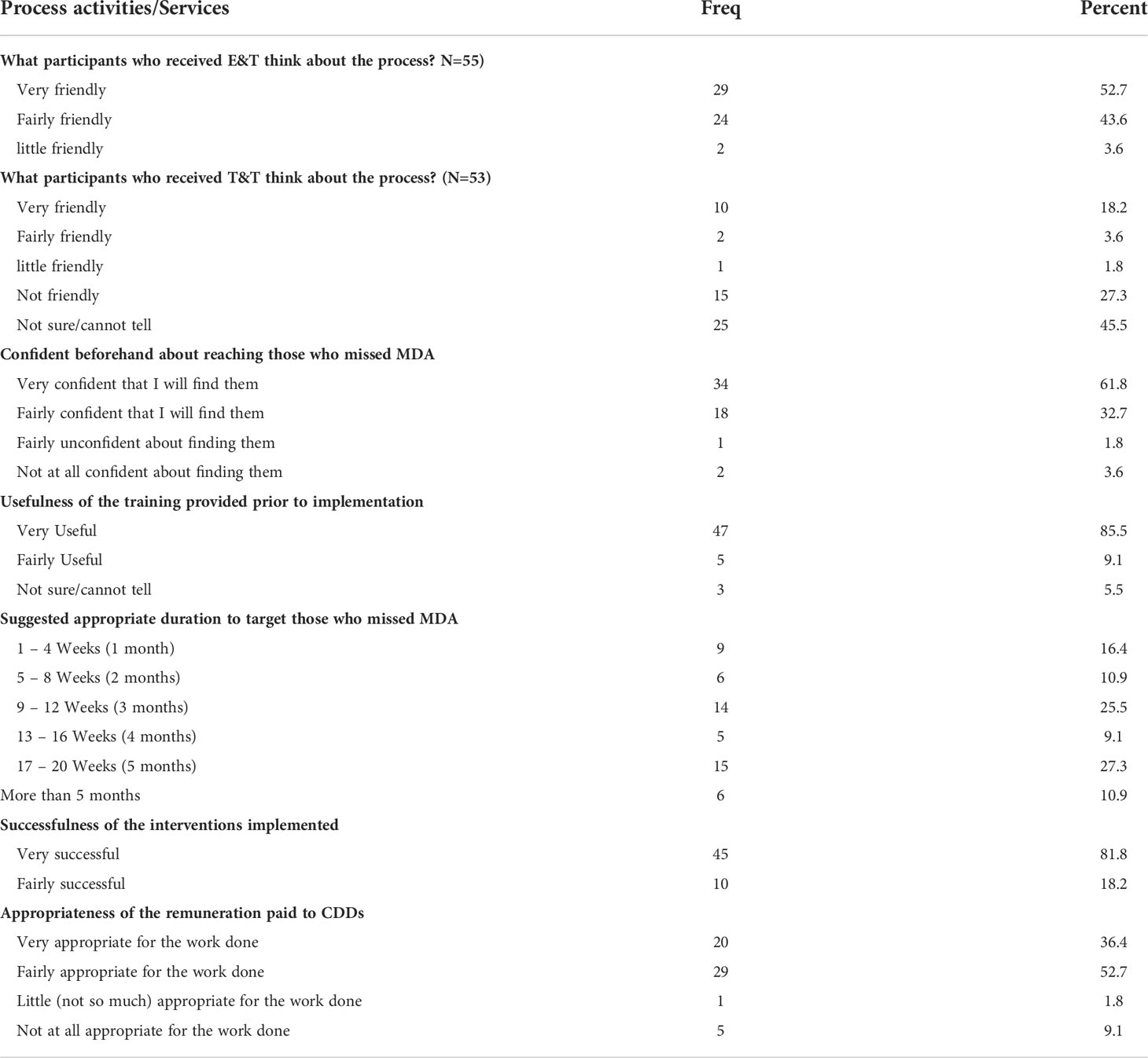
Table 8 displays the perspectives of community drug distributors (CDDs) on the implementation activities/services to reduce lymphatic filariasis transmission in the study district. In total, 55 CDDs were interviewed using a structured questionnaire.
Community distributors reported that individuals who participated in the E&T intervention perceived the process as very friendly (52.7%) and fairly friendly (43.6%). Thus, an overwhelming majority of 96.3% found the process to be friendly. However, CDDs reported that individuals who participated in the T&T intervention perceived the process as very friendly (18.2%) and fairly friendly (3.6%), while (27.3%) indicated that it was not friendly; the remaining 45.5% were unsure or could not tell if the process was friendly.
More than half of the CDDs (67.3%) indicated that all persons who missed MDA should be offered testing to see if they have an infection before treating them. A substantial majority of the CDDs (96.4%) reported that the process of E&T and T&T must be repeated after subsequent MDA so that people who missed treatment could be reached. They believed that this would help to break the persistent transmission in the study district.
The majority (86%) of CDDs reported that the training offered them prior to the implementation of E&T and T&T interventions was adequate and successful. 61.8% of the CDDs were confident in reaching out to individuals who seldom or never take part in MDA using the educational poster, which was part of their training package. 61.9% of the CDDs recommended a time of 12 to 20 weeks to be set aside after every MDA round to target the individuals who missed MDA, with 8 out of 10 CDDs considering the E&T and T&T activities implemented to be very successful. 76.4% of the CDDs considered the remuneration paid to them during the implementation of the E&T and T&T interventions as appropriate.
Health workers’ perspectives on the implementation process activities/services
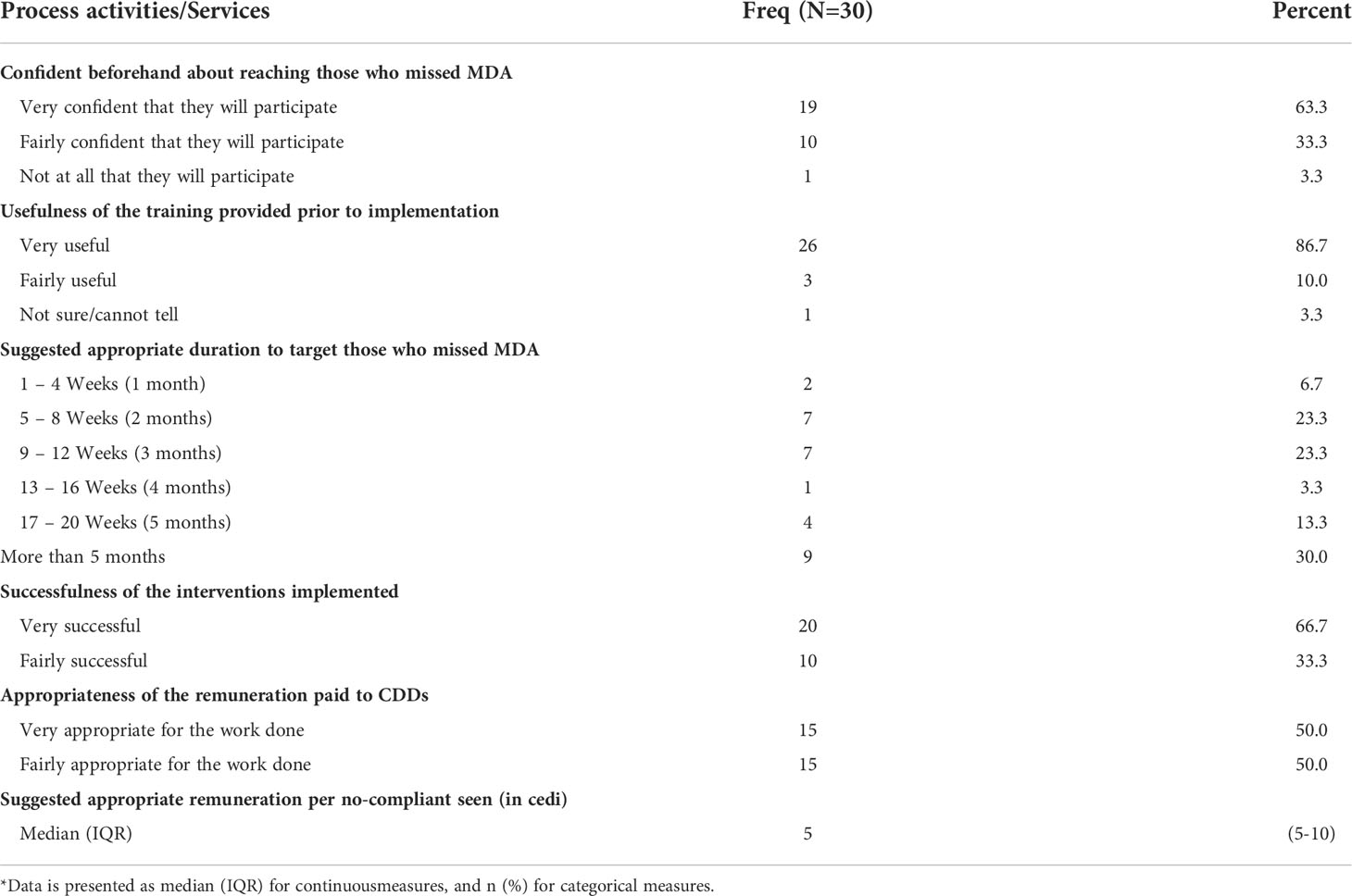
We explored health workers’ (nurses and disease control officers) perceptions on the implementation process of the E&T and T&T activities/services. Table 9 summarizes the responses of the 30 health workers interviewed, using a semi-structured questionnaire. 97% of them agreed that anyone who missed the MDA should be tested to determine their infection status. Again, a substantial majority (97%) recommended that E&T and T&T should be repeated after every subsequent MDA.
A majority (87%) of the health workers indicated that the training offered them prior to the implementation of the E&T and T&T interventions was adequate and successful with 63% of the health workers reporting feeling very confident in reaching out to individuals who miss MDA in their respective communities. Only one of the 30 health workers considered the training to be of no use to her. She intimated in a follow up discussion that she did not see anything new about the training because she has been conducting malaria RDT test, which is like the FTS training provided. More than half of them (63%) believed that the adequate time to target those who miss MDA was 8 to 20 weeks.
Majority (87%) thought the remuneration was appropriate for nurses and community drug distributors and when they were asked to suggest an appropriate amount, they have suggested an amount between 5 and 10 Ghana cedis (0.66 – 1.33 US$) with a median of 5 Ghana cedis (IQR: 5-10) per each person reached.
Discussions
Despite the many achievements of the GPELF, there are endgame challenges hampering the attainment of the LF elimination goals in many settings. This study set out to explore novel strategies of dealing with MDA non-compliance. We implemented an E&T and T&T approach to address non-compliance based on participants not being reached (i.e., those who were ill during the MDA, missed treatment for no reason, were away during the MDA, or were unaware of treatment) or refusing MDA (as a result of fear of AEs or low risk perceptions). Participants were identified through the LF MDA registers and subsequent snowball identification of those not in the registers.
In this study, we have shown an 8% infection prevalence among individuals who seldom or never take part in MDA. Based on this data and the knowledge that the same, willing individuals participate in all community-based interventions, it is likely that a proportion of people who are never-treated may exist in other districts, even those that have passed TAS. The impact of these groups on the long-term resurgence of the disease is currently unknown, although mathematical models show that while low-level prevalence can be maintained for a long period after MDA and the risk of resurgence is low, recrudescence can occur after the recommended post-intervention surveillance period of 4–6 years (16). In a district in Indonesia, post MDA surveillance indicated microfilariae prevalence of 5% in adults (42% of which did not participate in MDA) (17). The current recommendations of 2% antigenemia or 1% microfilaria prevalence to proceed to TAS (18), may therefore only be relevant in the “willing” population. Thus, new techniques, indicators and thresholds for quantifying the number of individuals who never or seldom participate in MDA should be added to epidemiological monitoring surveys to minimize the risk that a reservoir of infection remains that could jeopardize elimination gains.
While high antigen prevalence in children 5 – 10 years is a clear indication of ongoing transmission in the study communities, the reason for this high level of infection could be due to poor vector control activities. In earlier studies in the same district, poor vector control and bednet usage was observed (19). Further the higher antigenemia prevalence in 6 – 7-year-old males than females, compared to the general trend of a higher prevalence in females compared to males across age groups in the study is unknown but could be due to the demographic characteristic among this age group. Similarly, it is also not clear why males accepted testing at a higher rate (30.6%) compared to females (20.7%). These age and gender differences in the study warrant the need for further assessments towards the development of strategies to improve the acceptance of treatment and control interventions among these groups.
Current M&E decisions for LF (20) are based on population average prevalence and not meant to identify individuals who are positive. The M&E approach also assumes that the target population is randomly sampled; however, if some people actively refuse both treatment and testing, then the results will be biased. Further, current surveillance approaches follow a vertical system with a team of 3 to 5 people from the national programme moving from district to district. The associated running costs (fuel, vehicle hire and maintenance, and personnel cost) of NTD programme M&E activities may therefore prevent the team from spending enough time in each districts for effective surveys. Further, participation in programme surveys is voluntary and individuals assessed are those always likely to take part in MDA. On the other hand, the approach in this study relies on using health personnel stationed in the district and targeting individuals who otherwise want nothing to do with the MDA. Our results indicate that the surveillance strategies (18) to decide on when districts can implement TAS and stop treatment can be significantly improved. Further, LF being a focal disease, improved monitoring, evaluation and surveillance strategies are required compared to the selection of two sentinel/spot check sites to represent an entire implementation unit for pre-TAS.
Over 95% of the participants in our study who missed the previous MDA accepted the treatment. The study was very well accepted by both participants, CHNs/CDDs and health workers, the majority of whom recommended that this be repeated after every MDA. It was observed in the field that many participants in the T&T group accepted the treatment, including those who tested negative. A follow-up with some of the participants indicated that they felt more comfortable receiving the treatment from the CHNs compared to the CDDs. This may indicate the need for more purposeful, targeted measures to addressing MDA non-compliance. This finding indicates that the use of health professionals for community health initiatives may improve MDA coverage. It should however be noted that despite the efforts of the CDD and CHNs around 4% of individuals refused participation and acceptance of the treatment drugs and remain an important population to consider when addressing the challenges with MDA non-compliance.
The main lesson learned from our initial attempt to reduce MDA non-compliance is that unexpected findings occur. As such, it is important to have a method to assess implementation progress on an ongoing basis in order to identify barriers and quickly correct them through an iterative evaluation (21). Per the Ghana NTD programme guidelines, MDA registers must be updated annually immediately prior to MDA. These registers provide spaces to record the names and ages of household members and their participation status for five consecutive years of MDA. In the SENTINEL study, community drug distributors (CDDs) were asked to compile the list of all individuals who missed the 2021 MDA. A follow up to these individuals’ homes was then undertaken to sensitize and encourage them to receive treatment. During the implementation of the study, it was observed that a significant number of individuals who miss MDA in the district were not captured in the treatment register. A thorough assessment by the CDDs was then undertaken to ensure that these individuals were not listed under different households in the community. A follow-up assessment of some of these individuals indicated that they believed their names were in the previous and current registers. These findings indicate three major challenges to the MDA programme. First, the LF MDA registers were not up to date, which leads to a potential to miss pockets of population, thus resulting in inaccurate coverage estimates (22). Secondly, this could represent a lapse in the NTD programme activities, which were either rushed due to the pressure put on NTD programmes by donors to use funds and report quickly and/or CDDs failing to update registers. Thirdly, those who are not listed in the register may include migrant populations, seasonal workers and those whose name was in previous registers.
Another important observation was that many of the individuals not in the registers were identified through a snowballing effect where individuals in the register who missed MDA pointed to other individuals who also did not participate in the MDA. This finding is very important as it indicates the role of social networks in the decision to take part in MDA activities (23), and the use of snowball sampling in other settings and studies aiming to identify individuals who seldom or never take part in MDA.
LF MDA activities in Ghana take place over a period of two weeks. Challenges in implementing MDA within such a short period of time have earlier been identified in Ghana and elsewhere (7, 24). The findings from this study indicate that more time will be needed by CDDs if treatment coverage is to be improved and a majority of community members reached during the exercise. Another identified barrier to MDA participation is that community members are not involved in scheduling timeframes for MDA. Furthermore, the timing is not uniform. In Ghana, MDA activities have been known to take place from April to November, based on factors such as funding availability or other planned activities by the NTD programme. A primary reason for missing MDA included travel. For maximum participation, community representatives should be consulted when scheduling MDA date and times in order to maximize the impact. While MDAs are influenced by donor programmes and fiscal timelines, data reporting needs and drug requests schedules, it is becoming evident that the stakeholders supporting MDA in endemic countries need to be flexible and interventions should be customized to local conditions. Of course, this is not to imply that activities should be left entirely to the discretion of communities, but rather to plan these at a time when the desired coverage can be achieved or maximized.
Other activities, such as National Immunization Days, are better funded than NTD programmes and therefore take precedence over MDA activities (7). Integration needs not be only at the level of the NTDs, but across the health system (25). Given such challenges, a national planning team with the task to harmonize and integrate activities and funding as possible is suggested.
For the elimination of LF as a public health problem, a coverage of 65% in endemic implementation units is recommended (18). However, this benchmark does not take into consideration the individuals who are part of this benchmark. In other words, if the same 65% of population in the district consistently take part in MDA, the impact of the remaining 35% is overlooked. Such benchmarks may tend to impede progress towards disease elimination, especially when programmes cease to put in more effort towards activities once the benchmark target is met. Again, the importance of people missing from the registers needs to be determined but may have serious implications on the accuracy of coverage estimates. This is, therefore, a rational to establish modalities for monitoring and reporting the proportion of the population who seldom or never take part in MDA.
This study clearly indicates the need for tailored approaches in addressing the challenges to MDA non-compliance (26). In this study, a formative study was undertaken to identify the barriers to MDA compliance. Based on the results, a poster was designed and translated into relevant local languages to provide information to the community members, as well as guide the CDDs and CHNs in addressing the questions relating to MDA. We believe, the tailored behavior change communication strategies adopted in this study led to high levels of engagement and treatment acceptability, and proved useful in engaging individuals who seldom or never take part in MDA activities.
Limitations
There were several challenges and limitations to the study. First, the corrections implemented after the first month of the study led to a number of individuals including FTS positives being excluded from the results presented herein. Secondly, the data on those who missed MDA but refused participation in the study was not captured (following the inclusion/exclusion criteria) and is a missed opportunity to better understand the proportion of individuals who completely reject all public health activities. Another missed opportunity was the failure to capture the household information and coordinates, that would have enabled correlation analysis between children and adults who missed/refused MDA, and more detailed analysis of case distribution at the community level. A network analysis (27) of individuals missing/refusing MDA was also not possible as the link between individuals was not captured. Data on migration habits of participants, proximity to urban areas, average community and district compliance over the years were also not collected to enable an analysis of the factors responsible for infections in few communities.
A further limitation was that the LF MDA registers in Ghana are designed to capture information for five years. Thus, information captured on individuals’ participation in MDA was restricted and does not effectively enable the determination of those who never participated in any MDA since the start of the control activities. For those not in the registers, they were asked how many rounds of MDAs they participated in or missed, noting that there may be recall bias.
In terms of the test results, while the batch of FTS used was tested using positive control blood samples and materials prior to distribution, the testing in the field by the CHNs was unsupervised. The CHNs are trained in conducting malaria testing and it is assumed that the additional training on the use of the FTS was adequate to enable them carry out the test. All tests were considered positive if the test and control bands were visible, irrespective of their intensity. Samples of FTS positive and negative tests were examined and cross-checked with recorded results during monitoring visits by the study team. However, invalid test results (if any) were not recorded. Attempts to collect night blood samples for microscopy were not successful, as most blood samples collected by the CHNs in the vacutainers provided were not properly mixed and thus coagulated by the time they were received by the study team.
Conclusion
In conclusion, the findings from this study indicate the presence a significant number of individuals in the district who not participating in the MDA. The persistent infection among individuals who do not participate in MDA may jeopardize elimination efforts. Greater sensitization and engagement strategies, with a Test and Treat strategy reserved for the most hesitant individuals, could significantly increase the number of individuals who receive treatment and therefore help districts reach their elimination targets. Improved epidemiological monitoring surveys to identify these individuals are needed. Further, a cost effectiveness analysis of these strategies that will inform their implementation as stand-alone activities or integration into MDA activities is required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DS, CA, DB, KG, EL conceived the study. KG, EL provided funding. DS, JO, CA collected the data. OA, JOp, KA-M provided oversight and treatment drugs. DS, JS, CA analyzed the data. DS wrote the first draft. All authors reviewed and approved the final manuscript.
Funding
This work received financial support from the Coalition for Operational Research on Neglected Tropical Diseases, which is funded at The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation, by the United States Agency for International Development through its Neglected Tropical Diseases Program, and with UK aid from the British people.
Acknowledgments
We are grateful to Dr. Sellase Pi-Bansa for her support during the formative and training stages of the study. We are also grateful to all study communities and participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.953094/full#supplementary-material
References
1. World Health Organization. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030 (2020). Available at: https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs–NTD-Roadmap.pdf.
2. World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2019. Wkly Epidemiol Rec (2020) 95:509–24. Available at: https://www.who.int/publications/i/item/who-wer9543
3. Biritwum N-K, de Souza DK, Marfo B, Odoom S, Alomatu B, Asiedu O, et al. Fifteen years of programme implementation for the elimination of lymphatic filariasis in Ghana: Impact of MDA on immunoparasitological indicators. PloS Negl Trop Dis (2017) 11(3):e0005280. doi: 10.1371/journal.pntd.0005280
4. Biritwum N-K, Yikpotey P, Marfo BK, Odoom S, Mensah EO, Asiedu O, et al. Persistent “hotspots” of lymphatic filariasis microfilaraemia despite 14 years of mass drug administration in Ghana. Trans R Soc Trop Med Hyg (2016) 110:690–5. doi: 10.1093/trstmh/trx007
5. Ahorlu CSK, Koka E, Adu-Amankwah S, Otchere J, De Souza DK. Community perspectives on persistent transmission of lymphatic filariasis in three hotspot districts in Ghana after 15 rounds of mass drug administration: A qualitative assessment. BMC Public Health (2018) 18. doi: 10.1186/s12889-018-5157-7
6. de Souza DK, Yirenkyi E, Otchere J, Biritwum N-K, Ameme DK, Sackey S, et al. Assessing lymphatic filariasis data quality in endemic communities in Ghana, using the neglected tropical diseases data quality assessment tool for preventive chemotherapy. PloS Negl Trop Dis (2016) 10. doi: 10.1371/journal.pntd.0004590
7. Biritwum N-K, Garshong B, Alomatu B, de Souza DK, Gyapong M. Improving drug delivery strategies for lymphatic filariasis elimination in urban areas in Ghana. PloS Negl Trop Dis (2017) 11. doi: 10.1371/journal.pntd.0005619
8. Pi-Bansa S, Osei JHN, Frempong KK, Elhassan E, Akuoko OK, Agyemang D, et al. Potential factors influencing lymphatic filariasis transmission in “hotspot” and “control” areas in Ghana: The importance of vectors. Infect Dis Poverty (2019) 8. doi: 10.1186/s40249-019-0520-1
9. Manyeh AK, Ibisomi L, Baiden F, Chirwa T, Ramaswamy R. Using intervention mapping to design and implement quality improvement strategies towards elimination of lymphatic filariasis in northern Ghana. PloS Negl Trop Dis (2019) 13:e0007267. doi: 10.1371/journal.pntd.0007267
10. Manyeh AK, Ibisomi L, Ramaswamy R, Baiden F, Chirwa T. Exploring factors affecting quality implementation of lymphatic filariasis mass drug administration in bole and central gonja districts in northern Ghana. PloS Negl Trop Dis (2020) 14:e0007009. doi: 10.1371/journal.pntd.0007009
11. Manyeh AK, Chirwa T, Ramaswamy R, Baiden F, Ibisomi L. Evaluating context-specific evidence-based quality improvement intervention on lymphatic filariasis mass drug administration in northern Ghana using the RE-AIM framework. Trop Med Health (2021) 49:16. doi: 10.1186/s41182-021-00305-3
12. Gyapong JO, Adjei S, Sackey SO. Descriptive epidemiology of lymphatic filariasis in Ghana. Trans R Soc Trop Med Hyg (1996) 90:26–30. doi: 10.1016/s0035-9203(96)90466-6
13. Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, et al. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol (2002) 96:695–705. doi: 10.1179/000349802125001735
14. de Souza DK, Otchere J, Ahorlu CS, Adu-Amankwah S, Larbi IA, Dumashie E, et al. Low microfilaremia levels in three districts in coastal Ghana with at least 16 years of mass drug administration and persistent transmission of lymphatic filariasis. Trop Med Infect Dis (2018) 3:4–15. doi: 10.3390/tropicalmed3040105
15. de Souza DK, Gass K, Otchere J, Htet YM, Asiedu O, Marfo B, et al. Review of MDA registers for lymphatic filariasis: Findings, and potential uses in addressing the endgame elimination challenges. PloS Negl Trop Dis (2020) 14:e0008306. doi: 10.1371/journal.pntd.0008306
16. Prada JM, Davis EL, Touloupou P, Stolk WA, Kontoroupis P, Smith ME, et al. Elimination or resurgence: Modelling lymphatic filariasis after reaching the 1% microfilaremia prevalence threshold. J Infect Dis (2020) 221:S503–9. doi: 10.1093/infdis/jiz647
17. Santoso, Yahya, Supranelfy Y, Suryaningtyas NH, Taviv Y, Yenni A, et al. Risk of recrudescence of lymphatic filariasis after post-MDA surveillance in brugia malayi endemic belitung district, Indonesia. Korean J Parasitol (2020) 58:627–34. doi: 10.3347/kjp.2020.58.6.627
18. World Health Organization. Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphatic filariasis: A manual for national elimination programmes. Geneva PP - Geneva: World Health Organization (2011). Available at: https://apps.who.int/iris/handle/10665/44580.
19. Pi-Bansa S, Osei JHN, Kartey-Attipoe WD, Elhassan E, Agyemang D, Otoo S, et al. Assessing the presence of wuchereria bancrofti infections in vectors using xenomonitoring in lymphatic filariasis endemic districts in Ghana. Trop Med Infect Dis (2019) 4:1–13. doi: 10.3390/tropicalmed4010049
20. Gounoue-Kamkumo R, Nana-Djeunga HC, Bopda J, Akame J, Tarini A, Kamgno J. Loss of sensitivity of immunochromatographic test (ICT) for lymphatic filariasis diagnosis in low prevalence settings: consequence in the monitoring and evaluation procedures. BMC Infect Dis (2015) 15:579. doi: 10.1186/s12879-015-1317-x
21. Stetler CB, Legro MW, Wallace CM, Bowman C, Guihan M, Hagedorn H, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med (2006) 21 Suppl 2:S1–8. doi: 10.1111/j.1525-1497.2006.00355.x
22. Kamara W, Zoerhoff KL, Toubali EH, Hodges MH, Bisanzio D, Chowdhury D, et al. Are census data accurate for estimating coverage of a lymphatic filariasis MDA campaign? results of a survey in Sierra Leone. PloS One (2019) 14:e0224422–e0224422. doi: 10.1371/journal.pone.0224422
23. Chami GF, Molyneux DH, Kontoleon AA, Dunne DW. Exploring network theory for mass drug administration. Trends Parasitol (2013) 29:370–9. doi: 10.1016/j.pt.2013.04.005
24. Njomo DW, Kimani BW, Kibe LW, Okoyo C, Omondi WP, Sultani HM. Implementation challenges and opportunities for improved mass treatment uptake for lymphatic filariasis elimination: Perceptions and experiences of community drug distributors of coastal Kenya. PloS Negl Trop Dis (2020) 14:e0009012–e0009012. doi: 10.1371/journal.pntd.0009012
25. Banda GT, Deribe K, Davey G. How can we better integrate the prevention, treatment, control and elimination of neglected tropical diseases with other health interventions? a systematic review. BMJ Glob Heal (2021) 6:e006968. doi: 10.1136/bmjgh-2021-006968
26. Krentel A, Fischer PU, Weil GJ. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PloS Negl Trop Dis (2013) 7:e2447. doi: 10.1371/journal.pntd.0002447
Keywords: lymphatic filariasis, mass drug administration (MDA), non – compliance never-treated, Ghana, test and treat strategy, engage and treat strategy
Citation: de Souza DK, Otchere J, Sumboh JG, Asiedu O, Opare J, Asemanyi-Mensah K, Boakye DA, Gass KM, Long EF and Ahorlu CS (2022) Finding and eliminating the reservoirs: Engage and treat, and test and treat strategies for lymphatic filariasis programs to overcome endgame challenges. Front. Trop. Dis 3:953094. doi: 10.3389/fitd.2022.953094
Received: 25 May 2022; Accepted: 04 July 2022;
Published: 11 August 2022.
Edited by:
Alexander Yaw Debrah, Kwame Nkrumah University of Science and Technology, GhanaReviewed by:
Henry Kanyi, Kenya Medical Research Institute (KEMRI), KenyaDenis Sereno, Institut de Recherche Pour le Développement (IRD), France
Jubin Osei-Mensah, Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR), Ghana
Copyright © 2022 de Souza, Otchere, Sumboh, Asiedu, Opare, Asemanyi-Mensah, Boakye, Gass, Long and Ahorlu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dziedzom K. de Souza, ZGRlc291emFAbm9ndWNoaS51Zy5lZHUuZ2g=
 Dziedzom K. de Souza
Dziedzom K. de Souza Joseph Otchere1
Joseph Otchere1 Joseph Opare
Joseph Opare Daniel A. Boakye
Daniel A. Boakye Katherine M. Gass
Katherine M. Gass