- 1Department of Global Health and Infection, Brighton and Sussex Medical School, Brighton, United Kingdom
- 2Centre for Infection and Antimicrobial Research, Brighton and Sussex Medical School., Brighton, United Kingdom
- 3Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, Trondheim, Norway
- 4Bacterial and Viral Diseases Research Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
- 5Center for Equity in Global Surgery, and Department of Pharmacology, University of Global Health Equity, Kigali, Rwanda
- 6Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 7Centre for Inflammation Research, Institute for Regeneration and Repair, University of Edinburgh, Edinburgh, United Kingdom
- 8Department of Clinical and Chemical Pathology, Galala University, Suez, Egypt
- 9Faculty of Pharmacy, Ahram Canadian University, Cairo, Egypt
- 10Department of Microbiology and Immunology, Egyptian Drug Authority, Cairo, Egypt
- 11Department of Clinical Pathology, National Cancer Institute, Cairo, Egypt
- 12Department of Microbiology, College of Medicine, Qassim University, Qassim, Saudi Arabia
- 13Department of International Relations, University of Sussex, Brighton, United Kingdom
- 14Division of Antimicrobial Resistance, Federal Ministry of Health, Khartoum, Sudan
- 15Institute for Medical Microbiology, Immunology and Hygiene, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- 16German Centre for Infection Research Deutschen Zentrum für Infektionsforschung (DZIF), Partner Site Bonn-Cologne, Cologne, Germany
- 17Institute of Global Health and Human Ecology, The American University in Cairo, Cairo, Egypt
- 18Department of Medical Laboratories Sciences, College of Applied Health Sciences, A’Sharqiyah University, Ibra, Oman
- 19Bioscience Research Institute, Khartoum, Sudan
Introduction: Antibiotic resistance (ABR) is a global issue that requires a One Health approach, addressing human, animal, and environmental health sectors together. The PRESAR network aims to reduce the emergence and spread of ABR in the Nile Valley, seeks to fill research gaps in ABR. This scoping review (ScR) focuses on mapping the existing literature and data on ABR in Egypt, Ethiopia, and Sudan, specifically examining the application of the One Health approach and reviewing the national action plans (NAPs) of these countries.
Methods: The ScR was conducted using the PCC framework (population, concept, and context) and incorporated relevant keywords and MeSH terms in: Medline, Scopus, and Web of Knowledge. Two reviewers per sector (animal, environment/agriculture, human) screened articles, including peer-reviewed studies published in English across all years. Studies on non-bacterial or mycobacterial infections, and systematic reviews, were excluded. Data such as infection type, sample source, and observed resistance were recorded. The NAPs were compared with the WHO Global Action Plan (GAP) to assess similarities and differences.
Results: The review included 492 articles from Ethiopia, 331 from Egypt, and 31 from Sudan. Preliminary findings show that multidrug-resistant (MDR) S. aureus and K. pneumoniae dominate human infections, while Staphylococcus spp. and ESBL-producing Enterobacterales are more prevalent in animal and environmental sectors. There was large variability in diagnostic methodologies used across the sectors and countries, which in turn may lead to discrepancies in identification of bacteria at the species level and thereby inaccurate epidemiological data on prevalence and burden. While NAPs are generally aligned with the GAP, variations exist in areas like process ownership, research capacity, and funding.
Discussion: The review underscores the need for more research in non-human sectors and highlights the importance of One Health in tackling ABR. We strongly advocate for a unified and strategic approach among local stakeholders, scientists, and international agencies to prioritize and fund research, aiming for a sustainable reduction in antibiotic resistance.
1 Introduction
Antibiotic resistance (ABR) is one of the greatest threats to global health, causing increased morbidity, mortality, and requiring urgent attention to limit the spread of ABR pathogens. The true magnitude and global burden of ABR is difficult to establish, however a review on Antimicrobial Resistance commissioned in 2016 by the UK government estimated antimicrobial resistance (including ABR) to become the leading cause of death for >10 million people by 2050 (1). Low- and Middle-Income Countries (LMICs) bear the highest rates due to weak health systems, limited diagnostic laboratory infrastructure to support robust surveillance, and unregulated and improper use of antibiotics (2). Murray et al. used predictive statistical models linking ABR to an estimated 4.95 million deaths in 2019, with the most significant rates observed in Sub-Saharan Africa (3).
The One Health approach integrates human, animal, and environmental health sectors to collaboratively address and mitigate the spread of antibiotic resistance and other health threats. ABR in LMICs is often investigated as a medical problem, where other dimensions contributing to the problem are often overlooked, including food production through agricultural and veterinary practices, human migration via population displacement and settlement, as well as the interconnectedness of the human, animal and environmental sectors (One Health framework) all contributing to the global spread of ABR (4). In response to the need for a global, coordinated, multi-sectorial response to ABR, The One Health Platform to Reduce the Emergence and Spread of Antibiotic Resistance in the Nile Valley (PRESAR) network was established in 2020. It is a collaborative, multidisciplinary network aiming to address ABR research gaps in Egypt, Ethiopia, and Sudan, to support locally generated evidence-based research and policies.
The Nile Valley (Sudan, Egypt, and Ethiopia) has a high population density (5). The extensive population movement and displacement in the Nile Valley, settlement and expansion of urbanization into agriculture and animal grazing areas, and the intensive animal husbandry systems raise the risk of resistant pathogens in the region. Nevertheless, current efforts and research to control ABR are focused primarily on hospital and human-based settings, with much of the burden in other sectors (i.e., veterinary, and environmental sectors) overlooked and not systematically studied. Most antibiotics used in the human sector are also used for animals as prophylaxis (or similar drugs within each class), treatment of disease, and growth promotion, the consequent selective pressure of which leading to the selection and potential transfer of resistance determinants across different sectors (6, 7). Antimicrobial use as growth promoter for animals is 4x greater than the use of antimicrobials in humans (8). ABR pathogens can circulate in populations of humans and animals, through food, water, and the environment, which is further facilitated by trade and travel/movement of both humans and animal. In Egypt, Sudan, and Ethiopia, AMR is driven by high rates of resistance among key pathogens such as Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus (1–3). The economic, cultural and epidemiological diversity within the Nile Valley countries is therefore likely to result in local and regional distinct patterns of infections and resistance rates, necessitating the need to understand the local data, infrastructure, capacity and resources, to implement interventions that are locally applicable to control the spread of resistance regionally and globally.
In the absence of robust national surveillance networks and data in LMICs, research gaps and fragmented data is a recognizable problem in many LMICs, despite the prevalence of ABR across the One Health sectors (2). Upon the establishment of PRESAR, we aimed to generate locally relevant research pertinent to the three countries, and collate published research outputs to provide a comprehensive overview of the prevalence of infectious bacteria and rates of ABR in the Nile Valley countries (Egypt, Ethiopia, and Sudan) across One Health sectors in order to identify the gaps and future direction for research strategy based on local data.
Furthermore, individual countries have their own National Action Plan (NAP) based on the World Health Organisation (WHO) Global Action Plan (GAP). This review will discuss how likely they are to reflect the context and priorities in each country. The scoping review will allow us to map the current literature and overall examine how ABR research across One Health is undertaken in these three countries.
2 Methods
A scoping review (ScR) was chosen as the best suited summation for the study objectives, as it provides a broader research framework that is explanatory and descriptive (4, 5) allowing the mapping of evidence, identifying main concepts, theories, sources and knowledge gaps (6). The PRISMA-ScR template was used to guide the protocol, reporting, and synthesis of evidence (Supplementary File 1) (6). The population, concept, context (PCC) framework was used with relevant keywords and MeSH terms (Supplementary File 2 with detailed search strategy). The population was defined as: “human”, OR “animal”, OR “environment”, OR “agriculture”; the concept defined as: Antibiotic resistant bacterial infection; and context defined as: Egypt, OR Sudan, OR Ethiopia. The search was carried out on three databases: Medline/PubMed, Scopus, and Web of Knowledge, and concluded in January 2024.
The inclusion criteria included all peer-reviewed full text studies containing data on ABR bacteria (including clinical infections and surveillance studies), in English, with no year limitation. The exclusion criteria included papers with no access to full text, studies not in English, and which included data non-bacterial pathogens and/or mycobacteria, and systematic reviews. References cited in systematic reviews were screened to identify any primary research that could be included in the current ScR.
Two independent reviewers were assigned to each sector in each country (i.e two for the human sector in Egypt, Sudan and Ethiopia, respectively). All the literature searches were carried out by the two independent reviewers and cross-checked, per sector, per country. Any discrepancies between reviewers were discussed to reach a consensus, and resolved by a third reviewer if necessary. Initial screening was of title and abstract as per the eligibility criteria. The included articles were agreed upon by the two reviewers, and data was extracted on a pre-defined data collection sheet using Microsoft Excel (Supplementary File 3). The data charting process was 1) On article information: country/regions, type of study, sample size and study setting; 2) Type of sample, infection (if recorded), and 3) clinical phenotypes: sample source, bacteria identification method, bacterial species, resistance rates & mechanisms (if listed), whether colonization is defined or not, and whether antibiotic use is listed in the study (yes/no, did not include specific data).
Data was analysed by descriptive analysis using Microsoft Excel (2021) and SPSS Statistics (IBM SPSS Statistics 28.0.0.0). Bacterial nomenclature follows standard conventions with genus names abbreviated after first mention (e.g., Escherichia coli subsequently as E. coli). Resistance rates are reported as percentages with denominators: X% (n/N).Antibiotic resistance rates were grouped by antibiotic class (Supplementary Table 1). For that, we recorded and calculated the average resistance rates based on the total number of samples listed for a more accurate representation.
The National Action Plans were compared to the Global Action Plan (GAP), and key data were collected on process owners, timeline, implementation strategy and budget using Microsoft Excel.
3 Results
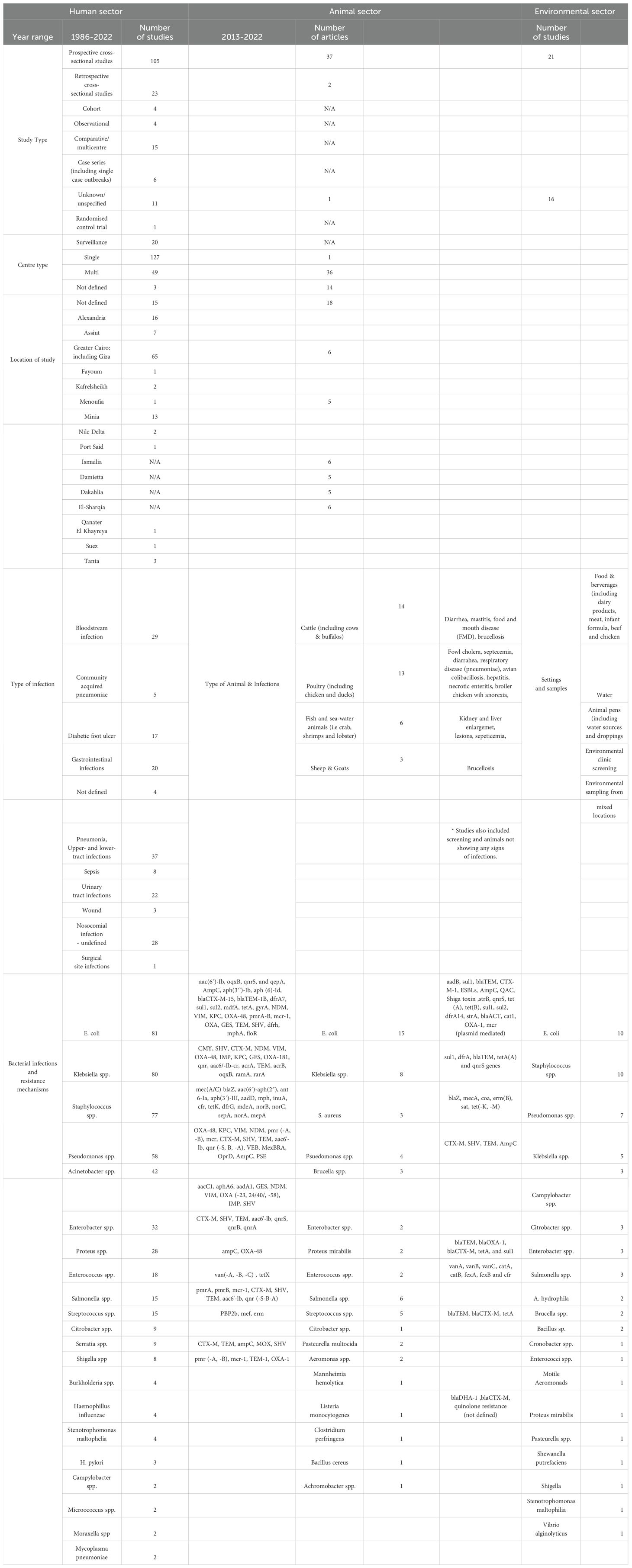
The details of the flow diagram for data collection and extraction are presented in Figure 1. The initial search yielded 5,129 articles across the three countries (Egypt: 1,773; Ethiopia: 4,483; Sudan: 873). After screening and eligibility assessment, 854 articles were included (Egypt: 258; Ethiopia: 396; Sudan: 35). The distribution of studies by sector and methodology is summarized in Table 1. In Sudan no studies were included from the environmental/agricultural settings. Reasons for exclusion are listed in the flow diagram (Figure 1).
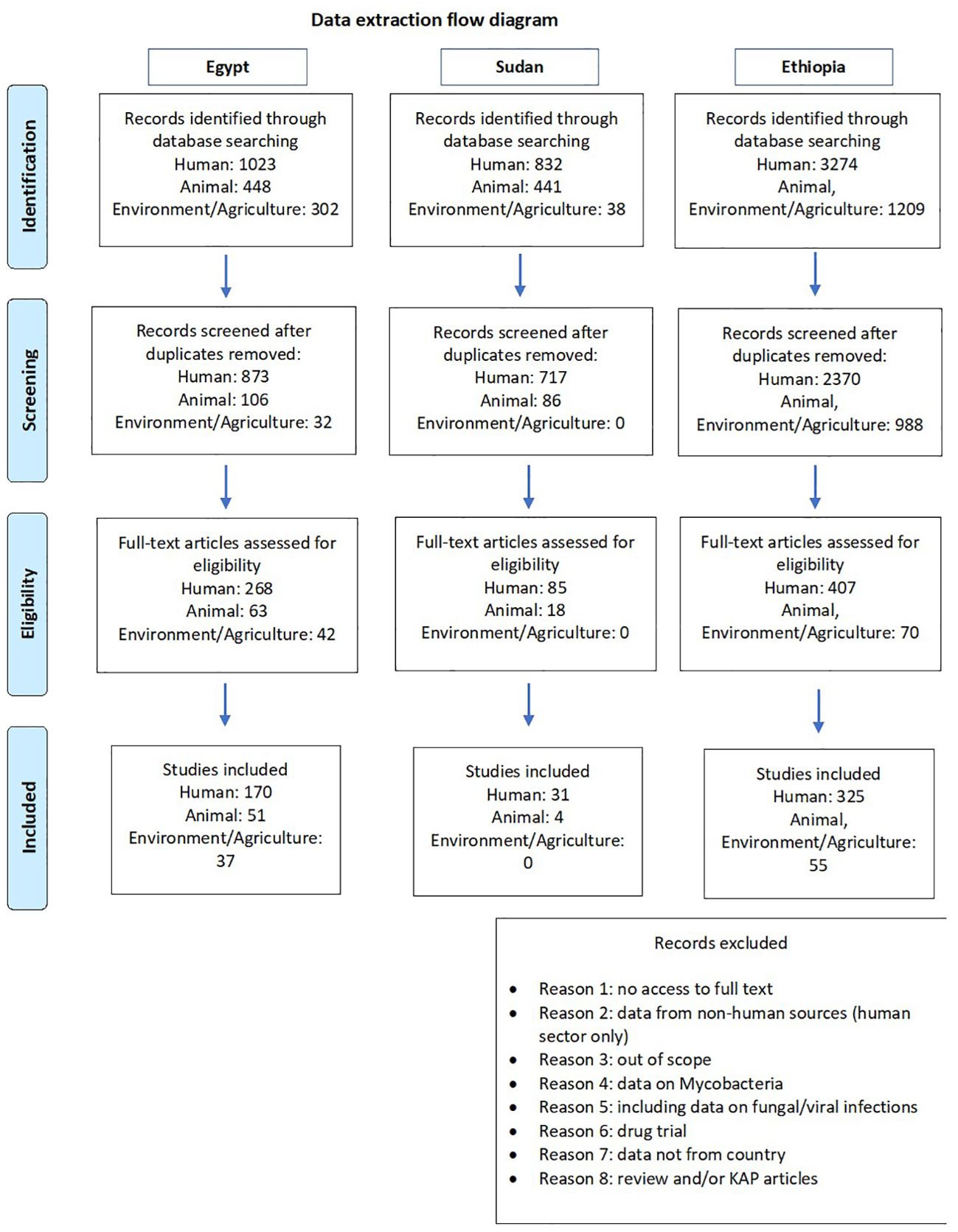
Figure 1. Flow diagram for the data collection for all the countries. Based on PRISMA 2009 Flow diagram.
3.1 Egypt
3.1.1 Human sector
A total of 1023 articles were identified in the initial search, of which a final number of 170 were included in the study, the earliest being from 1986 (Supplementary File 4). As shown in Table 1, the most common methodology identified was prospective studies (n=105), followed by retrospective studies (n=23). The dataset also includes 20 surveillance studies, 4 cohort studies, 4 observational studies, and 11 studies in which the methodology was either ‘Not defined’ or ‘Unknown’. Additionally, a case study approach is mentioned twice.
Cairo was the most frequently cited city, with a total of 65 studies, followed by Mansoura (n=28 studies), Alexandria (n=16 studies), Minia (n=13 studies), Zagazig (n=9 studies), Assuit (n=7 studies), Tanta and Ismailia each had 3 studies three. Additional locations like Qena, Qanater El Khayreya, Nile Delta, Kafr El Sheikh, Suez, Fayoum, Port Said, and Menoufia were mentioned less frequently, each ranging from one to three studies. This distribution highlights a concentration of studies across various cities in Egypt, with a clear emphasis on Cairo, Alexandria and Mansoura. Figure 2 represents the distribution of the studies in Egypt for the 3 sectors.
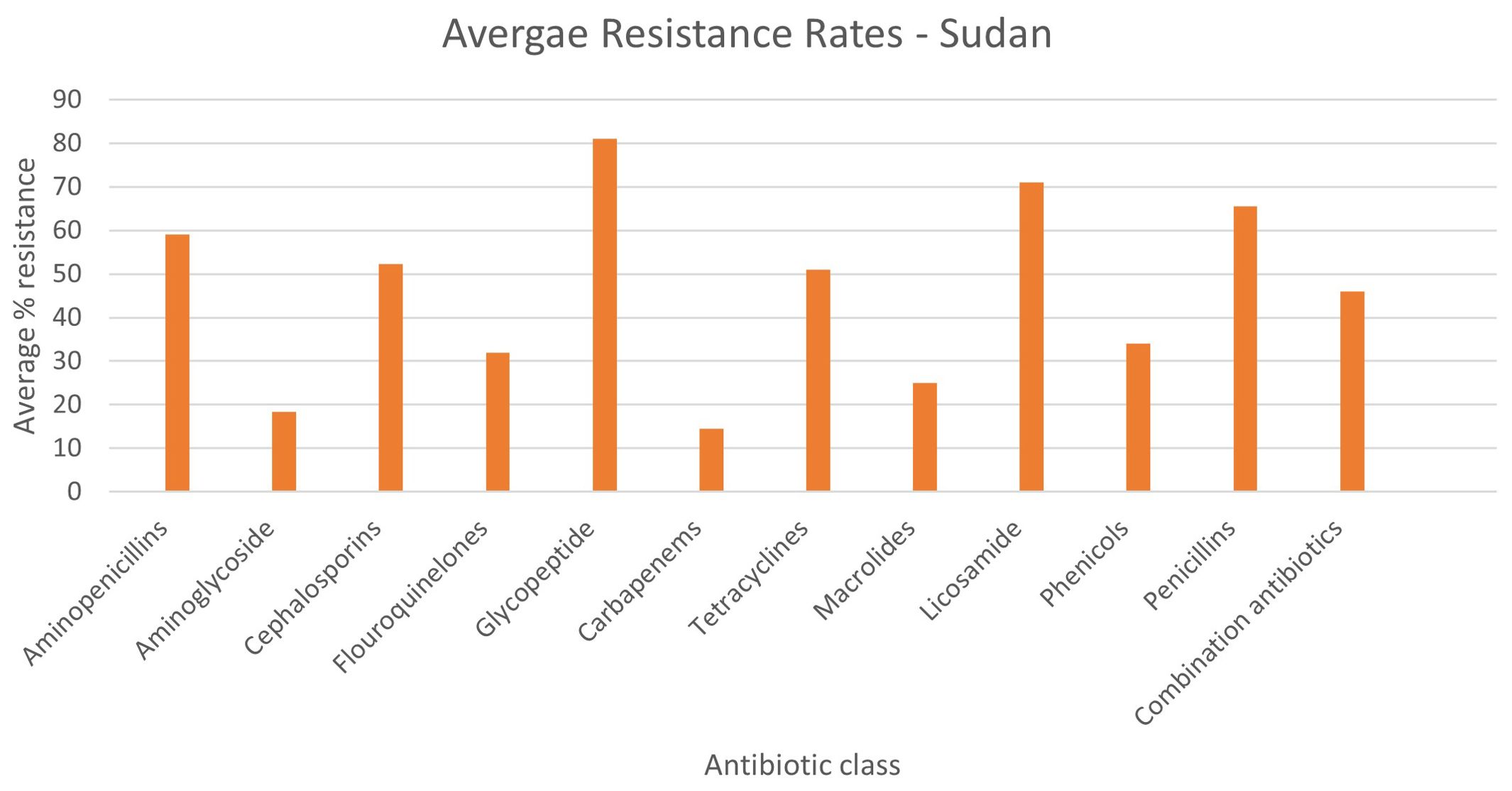
Figure 2. Distribution of studies in Egypt. Scale is based on the number of articles found in each region.
There is a notable distribution between single-centre and multi-centre locations, with more prevalent single-centre (n=127) than multi-centre studies (n=49). Additionally, the category ‘Unknown’ is mentioned three times, indicating instances where the nature of the centre (single or multiple) was not specified or was unclear in the data provided. Table 1 summarizes the study description.
The dataset encompasses a comprehensive range of diagnostic methods, sample types, and infection sites across various medical conditions. Various diagnostic methods were used for clinical specimen, including automated and semi-automated machines (Microscan and Vitek), as well as standard biochemical methods and Gram staining. Antimicrobial susceptibility testing (AST) was conducted either phenotypically via disk diffusion or using semi-automated platforms. A combination of phenotypic and genotypic methods (such as PCR) were utilized for mixed sample types, including nasopharyngeal specimens, as well as for targeted screening of resistance factors. Diagnostic samples include sputum, endotracheal aspirate, blood, wound exudate, burn exudate, urine, nasal and diarrheal swabs, pleural tissue specimens, bronchoalveolar lavage (BAL), various swabs from surgical sites, urine for Urinary Tract infections, stool for gastroenteritis, and other diverse types depending on the infection and clinical requirements (Table 1). Most of the studies only conducted genomic investigations on a subset of pathogens rather than on the whole collection reported. In some cases, such as the study by Ghaith et al., 2020, the overall prevalence of bacteria causing neonatal sepsis was presented, yet the detailed genomic investigation was focused on Klebsiella pneumoniae isolates only (7). Another study by Ghaith et al. (2019), assessed faecal colonization by carbapenemase-producing Enterobacteriaceae (CRE) among children admitted to Cairo University paediatric intensive care units over a period of 6 months. This study focussed only on screening of resistance genes and did not report any resistance and MIC rates (8).
Table 1 and Figure 3 summarises the most common bacterial species and resistance mechanisms reported in the literature from Egypt. The most commonly reported bacteria from the human sector is E. coli (n=81), followed by Klebsiella spp. (n=80), Staphylococcus spp. (n=77), and Pseudomonas spp. (n=58). Due to the discrepancy in diagnostic methods used in the literature, we have summarised the outputs as bacterial species rather than at the genus level in Table 1.
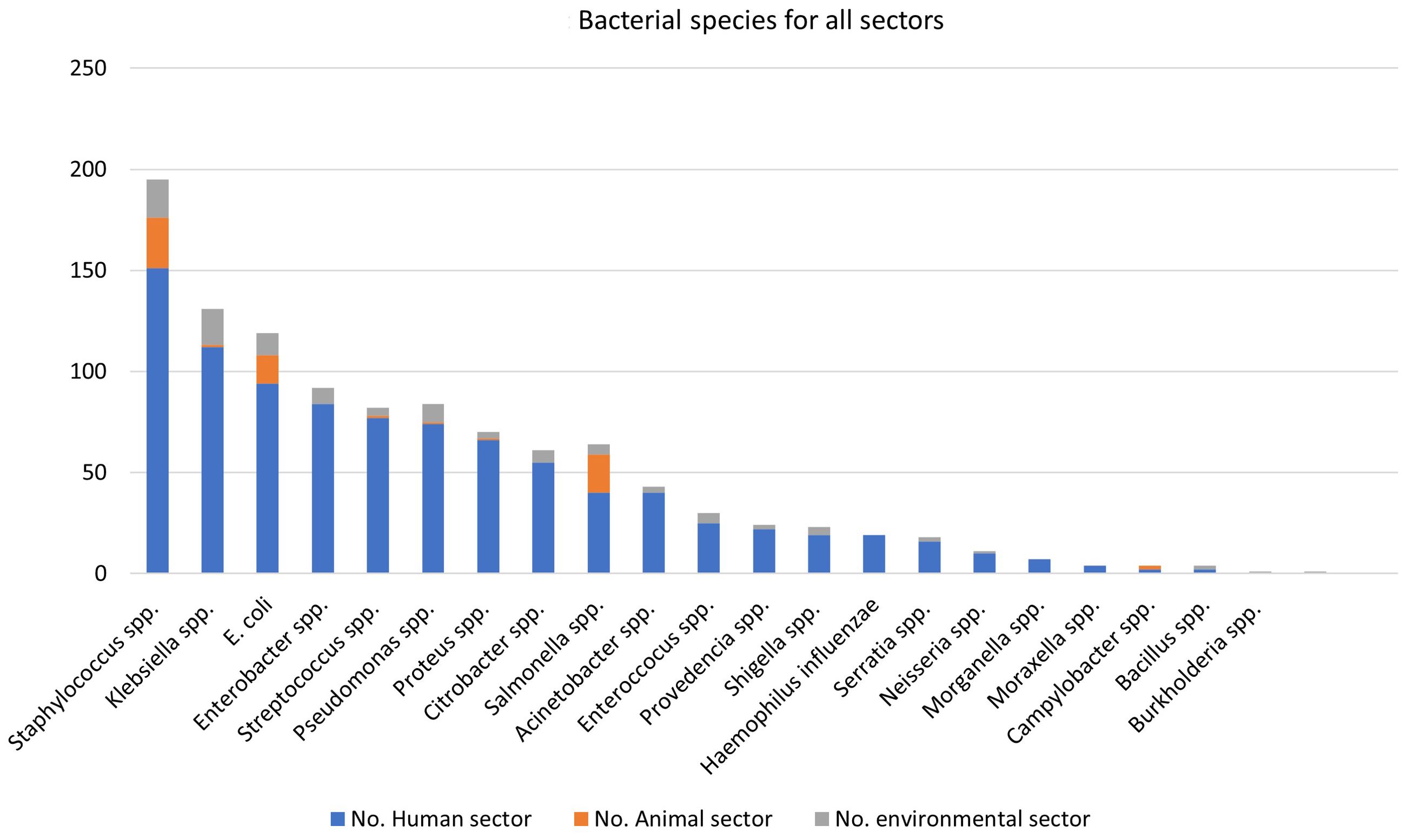
Figure 3. Distribution of bacterial species per sector – Egypt. The figure represents the number of articles for the specified bacterial species. Some articles contained information on multiple bacterial species.
Figure 4 and Supplementary Table 1 shows the average resistance rates to different antibiotic classes, whereby the highest resistance rates were reported to aminopenicillins is at 99%, followed by 86% for cephalosporins, 68% and 67% to aminoglycosides and tetracyclines, respectively. The average resistance for macrolides is 59%, 58% for fluoroquinolones, 56% for licosamides, 55% for combination antibiotics (i.e trimethoprim-sulfamethoxazole, piperacillin/tazobactam), and 41% for carbapenems. The lowest average resistance rates were reported for glycopeptides at 17%. Multiple resistance mechanisms were reported, most commonly for E. coli, Klebsiella spp. and Pseudomonas spp. (Table 1). Genetic screening of resistance mechanisms for Gram-positive bacteria was less common, and focussed mainly on mecA in Methicillin Resistant Staphylococcus aureus (MRSA) or vanA/B in Enterococcus spp. A total of 64 papers either did not define or investigate specific resistance mechanisms of the bacterial species reported.
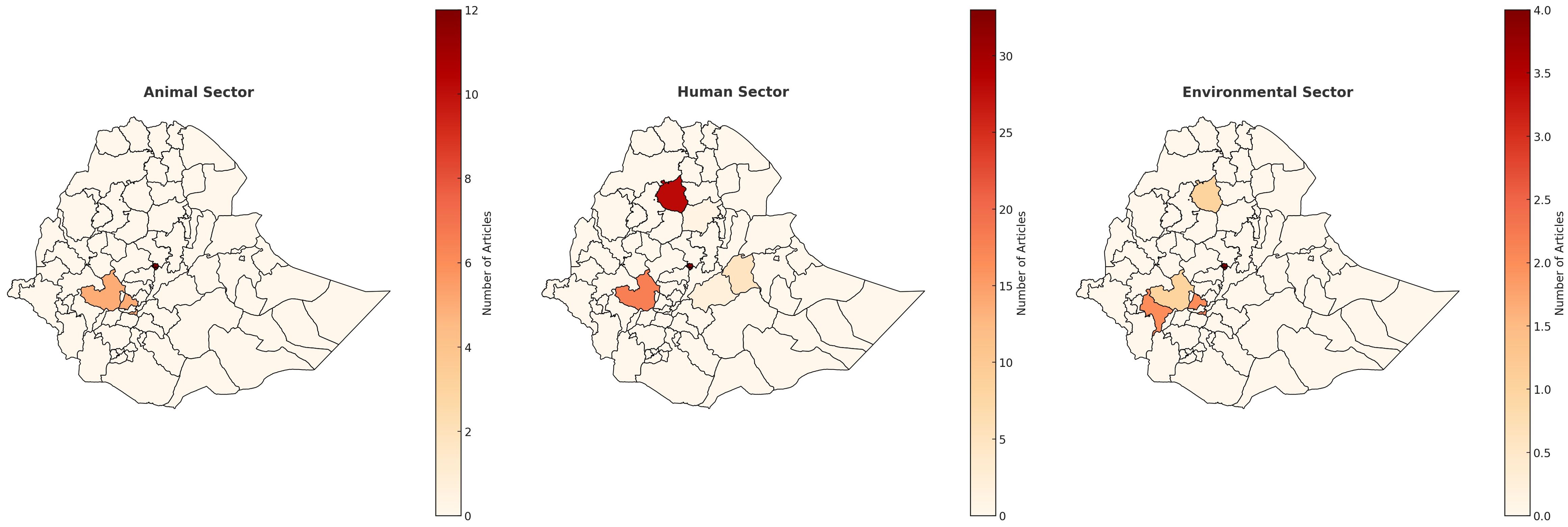
Figure 4. Average rates of resistance - Egypt. This summarizes the average antibiotic resistance rates for antibiotic classes for the 3 sectors. Antibiotic resistance rates were grouped by antibiotic class (Supplementary Table S1). For that, we recorded and calculated the average resistance rates based on the total number of samples listed for a more accurate representation.
3.1.2 Animal sector
A total of 448 articles were identified in the initial search, of which a final number of 51 were included in the study. Prospective cross-sectional studies were the most common study design conducted (n=32), and year ranges from 2016-2022. As seen in Figure 2 of the distribution of the study sites, the majority of the studies were conducted in Giza (n=6 studies), Ismailia (n=6 studies), and El-Sharqia (n=6 studies), followed by Dakalia (n=5 studies), Damietta (n=5 studies) and Menoufia (n=5 studies). Only one study was from a single centre, whereas the 36 were multi-centre, and 14 did not define the specific study site (Table 1).
All the studies included were from screening samples and identified phenotypically only. Cattle was the most common animal studied (n=14), followed by chicken (n=13). Other animals included were fish (n=6), goats (n=1), and sheep (n=2). Samples were taken from milk, faeces, blood, rectal swabs and soft tissue samples. Specific studies on cattle included milk swabs from lactating cows to identify mastitis infections (9).
E. coli was the most common species reported in the literature (n=14). Staphylococcus spp. was identified only three times (Table 1, Figure 3). Additionally, Streptococcus spp. and Pseudomonas spp. are frequently reported in the literature. The most frequently reported multi-drug resistant organism include E. coli, Salmonella spp., and Brucella spp., with E. coli being the most common pathogen across the dataset. As shown in Figure 4, the highest average resistance in the animal sector is to cephalosporins at 94%, followed by penicillins at 89%, aminopencillins is at 73%, macrolides at 71%, and tetracycline resistance at 58%. Lower average resistance rates were reported for fluoroquinolones and glycopeptides at 38%, 41% for lincosamide, and 45% average resistance to combination antibiotics (i.e trimethoprim-sulfamethoxazole, piperacillin/tazobactam). The lowest average antibiotic resistance rates were observed for aminoglycosides at 28%, and carbapenems at 13%. Extended Spectrum Beta-Lactamase (ESBL) enzymes in Gram negative bacteria were the most reported, including TEM and CTX-M.
3.1.3 Environmental/agriculture sector
A total of 302 articles were identified in the initial search, of which a final number of 37 were included in the review. Prospective cross-sectional studies were the most common study design conducted (n=21), and year ranges from 2007-2021. As seen in Figure 2 the majority of the studies were conducted Mansoura (n=7), followed by Sharqia (n=5), Cairo (n=4), Minya (n=3), Beni- Suef (n=2) and Kafr-El Sheikh (n=2). Of these studies, different sites were taken into consideration and included: milk samples (n=4), local supermarkets and butchers (n=2), water (n=3), poultry workers’ hands: (n=1), poultry farms (n=1), fish farms (n=4), chicken and beef meat (n=1), camel carcass (n=1), uncooked beef patty (n=1), fresh chicken meat (n=1 studies) and dental clinics (n=1).
In the compilation of sample types across various studies, a diverse array of specimens was identified. The term “Screening” appears once in the list, indicating a general type of study or sample collection. Milk was a common sample in food safety and microbiological studies (n=4 studies). This category encompasses a range of dairy products, including raw market milk, bulk tank milk, different types of cheeses, and small scale-produced ice cream. Poultry workers’ hands were noted as a specific sample once. Similarly, the combination of litter, bird droppings, and water samples were mentioned once (n=1 study), reflecting environmental sampling in avian habitats. Samples of water and hand swabs were also tallied once (n=1). The analysis included raw beef and chicken (n=1 study), while chicken carcasses, drumsticks, chicken gizzards, and chicken livers were mentioned twice. Fish samples, specifically tilapia and mullet, were recorded in addition to chicken breast fillets, luncheon meat, and chicken nuggets (n=1 study). Tap and well water samples were also counted once, a critical component in water quality studies. Samples related to meat industry, included camel meat and slaughterhouse workers (n=1 study), raw milk samples and cheese (n=1), and a variety of meat and dairy products (n=1). Additionally, samples from butchers’ shops, supermarkets, human faeces, various dairy products, and different types of water and sludge were included. Livestock drinking water samples from animal pens (n=1 study) and workers’ hand swabs and faeces (n=1) were also noted. Environmental surfaces within dental clinics (n=1 study) and whole milk powder (n=1) rounded out the diverse array of samples. Further, the list included samples from floors, walls, bed linens, water taps, and toilet seats (n=1 study), offering insight into the diversity of environmental sampling in healthcare and public settings.
In analysing the frequency of bacterial species mentioned across various studies, we observed a diverse array of pathogens: Pseudomonas aeruginosa, a common opportunistic bacterium (n=4), K. pneumoniae, another notable pathogen especially in nosocomial infections (n=3), and E. coli (n=7). Pseudomonas otitidis, Bacillus sp., Pseudomonas mendocina, Stenotrophomonas maltophilia, and Pseudomonas stutzeri, each mentioned once. Aeromonas hydrophila was identified twice, signifying its relevance in the environmental samples studied. The Shiga toxin-producing E. coli (STEC), a significant concern for food safety, was listed once. Brucella melitensis and Brucella abortus, both associated with brucellosis, were each mentioned once (10). Staphylococcus spp. incluidng S. aureus, known for its clinical significance and potential for antibiotic resistance, was noted five times. Instances of Salmonella were also recorded, which is critical due to its role in foodborne illnesses. The count for Citrobacter spp, including C. koseri and C. freundii, along with various other individual species, each mentioned once. Salmonella enterica serovar Typhimurium and a ciprofloxacin-resistant strain of Salmonella enterica subsp. enterica serotype Kentucky ST198 were each noted once. Campylobacter spp., including C. coli, C. jejuni, were cited, emphasizing their importance in gastrointestinal infection. Shigella spp., known for causing dysentery, was also noted once. Enterobacter hormaechei and Bacillus cereus, important in clinical and food microbiology, were each mentioned once one. PVL-Positive S.aureus and Cronobacter, were also mentioned once.
3.2 Ethiopia
3.2.1 Human sector
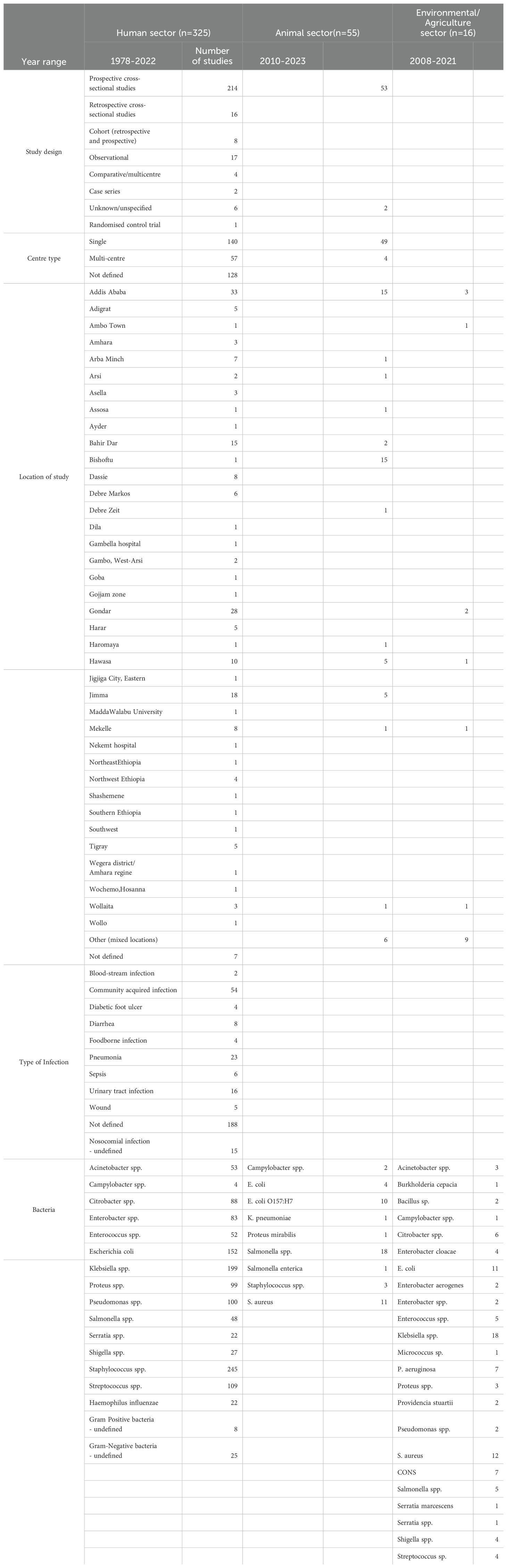
A total of 3274 articles were identified in the initial search, of which 325 were included in the study (Figure 1). Prospective cross-sectional studies were the most common study design conducted (n=214), and year ranges from 1978-2022 (Table 2). As seen in Figure 5, the majority of the studies (n=33 studies) were conducted in Addis Ababa (Central region of Ethiopia), followed by Gondar (n=28) in the North-West region of Ethiopia. One hundred and thirty-four studies were conducted in single-sites, 53 studies were multi-centre studies, and 128 studies did not define the specific study sites. (Table 2).
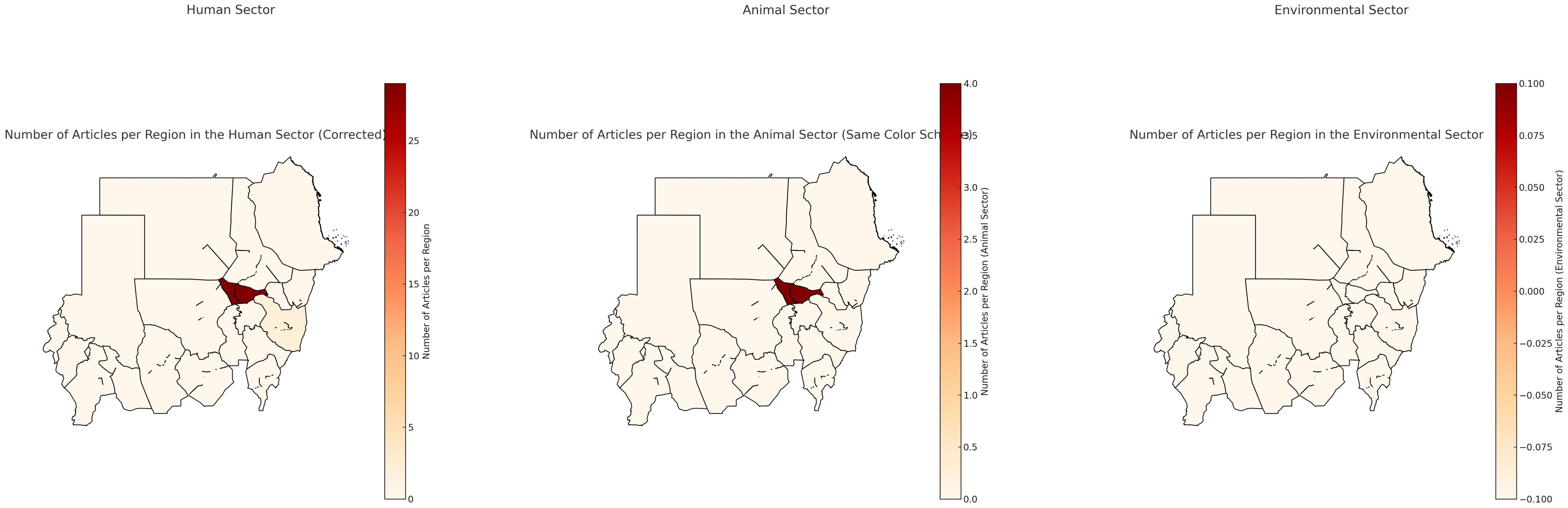
Figure 5. Ethiopia map of outputs for all sectors. Scale is based on the number of articles found in each region.
Samples that were reported in the literature are represented in Table 2 where urinary tract (urine and urethral swab samples) and blood samples were the most reported (17.4% and 17.2%, respectively), followed by skin and soft tissue samples (from wounds, pus, ulcers and abscess) in 13.1% of the literature. Thirty-two studies (6.7%) did not specify specific samples/infections.
Bacterial species identification was performed phenotypically using conventional microbiological and biochemical methods in 192 studies, whereas 45 studies used combination of phenotypic and genotypic methods. Two hundred and twelve studies were hospital-based (in- and out-patients, surgery, and ICU). The average number of samples included in the studies was 127, with a maximum of 1072 samples from a 10 year retrospective study on ABR bacteria causing otitis media (11), and minimum of six samples in a study investigating the prevalence of Salmonella typhi in febrile patients (12).
We noted that the majority of studies were conducted on a variety of samples rather than focused on a specific sample set or bacterial infection. For example, a 10-year retrospective study was conducted on antimicrobial resistance trends of bacterial uropathogens at the University of Gondar Hospital (13). The more focused studies were either on meningitis, identifying Streptococcus pneumoniae from cerebrospinal fluid samples (14), or food-borne Salmonella spp. infections isolated from stool samples (15, 16).
Staphylococcus spp. was the most commonly reported bacterial genus reported in the literature (n=354, out of which 146 were S. aureus), followed by Klebsiella spp. (n=199, of which 91 were K. pneumoniae). E. coli, Streptococcus spp. and Pseudomonas spp. were also prevalent in the literature (Table 2, Figure 6).
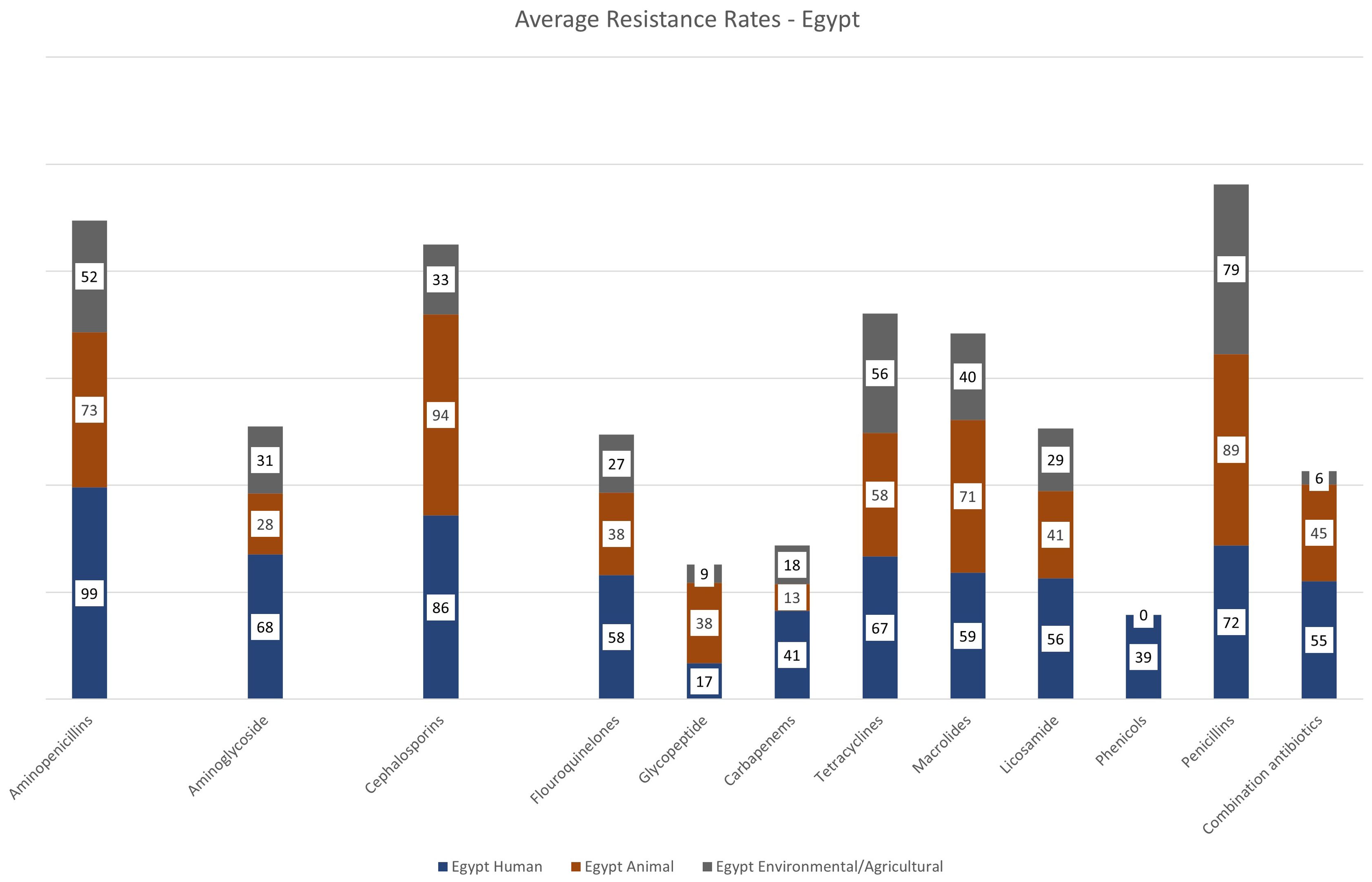
Figure 6. Distribution of bacterial species per sector – Ethiopia. The figure represents the number of articles for the specified bacterial species. Some articles contained information on multiple bacterial species.
As seen in Figure 7 and Supplementary Table 1, antimicrobial resistance is high against the majority of antibiotic classes. The highest rates of resistance were observed for aminopenicillins (average 76% for combined percentages for ampicillin, amoxicillin and amoxicillin/clavulanic acid). Cephalosporin resistance (1st to 4th generation antibiotics) was an average of 51%. Tetracycline resistance was 59%. Resistance aminoglycosides, glycopeptides and carbapenems is estimated at 24%, 22% and 20%, respectively.
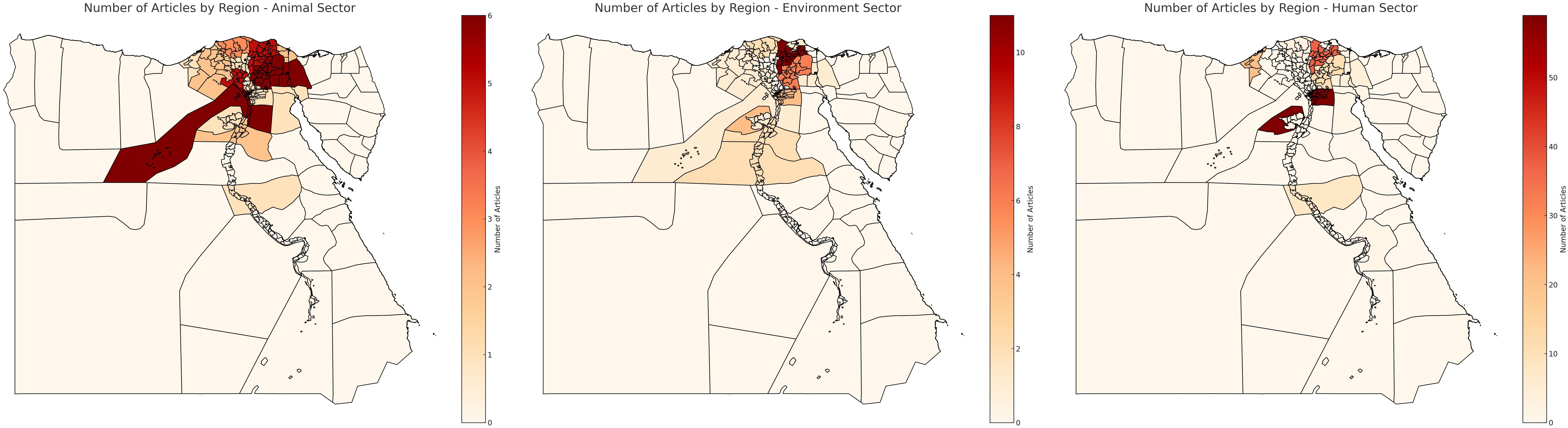
Figure 7. Average rates of resistance – Ethiopia. This summarizes the average antibiotic resistance rates for antibiotic classes for the 3 sectors. Antibiotic resistance rates were grouped by antibiotic class (Supplementary Table S1). For that, we recorded and calculated the average resistance rates based on the total number of samples listed for a more accurate representation.
3.2.2 Animal sector
A total of 44 articles were included in the scoping review that covered ABR in the animal sector in Ethiopia. They were all prospective cross-sectional studies, and year ranges from 2010-2023. Thirty-nine of the studies were from single sites, and three were multi-centre studies. Figure 5 represents the locations of the studies, where 24 were conducted in Addis Ababa and the Central region (Bishoftu, Debre Zeit, Holota, Sebeta, Oromia Regions), and a few in the North (n=4 studies), South (n=7studies), and West (n=5 studies).
All the studies included were from screening samples and identified phenotypically only. Cattle was the most common animal studied (n=26 studies), followed by chicken (n=12 studies). Other animals included poultry, goats and sheep (Table 2). Samples were taken were faecal/droppings, cattle carcasses, and rectal swabs. Specific studies on cattle included milk and udder swabs from lactating cows to identify mastitis infections. Eggs and cloacae swab of chicken were also investigated for prevalence of Salmonella spp (17).
Salmonella spp. was the most commonly reported bacterial species in the animal sector from Ethiopia (n=18 articles), followed by E. coli (n=14), of which O157:H7 represented 10/14 studies. Staphylococcus spp (including S. aureus) was reported in 14 articles. Other bacteria reported in the literature include Campylobacter spp. (n=2), K. pneumoniae (n=1), and Proteus mirabilis (n=1) (Table 2, Figure 6).
Detailed resistance rates are presented in Figure 7. Penicillin resistance was high in the animal sector in Ethiopia at 93%, followed by aminopenicillin and macrolide resistance both at 64%. Cephalosporin resistance was at 50%, similar to glycopeptide resistance at 49%. No carbapenem resistance is reported in the literature from the animal sector in Ethiopia.
3.2.3 Environment/agriculture sector
Sixteen prospective cross-sectional studies were included from the Environmental and/or Agricultural sectors from Ethiopia (n=15 studies from Environment, n=1 Agriculture), with year ranges from 2008–2021 with only one study being a multi-centre study, and the rest conducted in single centres, the majority of which in the Central Region of Ethiopia (Addis Ababa & Ambo Town, n=4 studies and n=3 studies respectively) (Figure 5, Table 2).
Only one study was conducted in the agricultural sector, from samples of lettuce and green pepper. Studies in the environmental sector were from hospital environments/surfaces and air samples in nine studies, where are the rest of the studies included samples from wastewater, river water & sewage samples.
The agricultural screening study included 161 lettuce and green pepper samples and revealed that 97% of the lettuce and 58% of the green pepper samples had Enterobacteraceae counts of ≥ log 5 colony-forming unit per gram (cfu/g), with Salmonella and Shigella spp isolated from 40% of the samples (18).
From environmental swabs and samples in hospital environments (air and surface samples), various organisms were reported, including Klebsiella spp (n=18, including six K. pneumoniae), E. coli (n=11 studies), and S. aureus (n=12 studies). Other species such as Acinetobacter spp., Bacillus spp., Enterococcus spp., and Pseudomonas spp. are less commonly reported (Table 2). Data from river water samples indicated the presence of C. freundii, E. coli, K. pneumoniae and Klebsiella oxytoca (19). In a study by Mekengo et al., 2021 on bacteria recovered from the sewage systems of health institutions found in Hawassa, all sewage samples (n = 27) examined in the study contained potential pathogenic bacteria, the most prevalent of which was E. coli, Shigella spp., Pseudomonas spp., Salmonella spp. (n = 25, 19.4%), S. aureus, and Klebsiella spp (20).
Resistance to aminopenicillins was the highest reported resistance in the environmental and agricultural sectors in Ethiopia at 71% across the literature, followed by cephalosporin and tetracycline resistance at 64% and 57%, respectively (Figure 7). Glycopeptide resistance was an average of 46%, and macrolide resistance was at 43%. Carbapenem resistance was at an average of 20%.
Articles contained information on bacteria in food mainly sampling from street foods such as white lupin, donat, bambolino, sambusa, raw cow milk, juices and raw meat samples from knives and cutting boards (21–25). White lupin is considered as an economical and nutritious food and is particularly popular in Ethiopia (21). In this study, E. coli, Salmonella spp., and Shigella spp. were found in 40 samples from street vendors in Bahir Dar in Northwestern Ethiopia. The study by Eromo et al., 2016 determined the bacteriological quality of ready-to-eat foods (such as local bread, raw fish, chilli, avocado and cooked potatoes) sold on streets in Hawassa City, and found that 31% of the 72 food samples showed total colony counts ranging from 1.7x105 to 6.7x106 cfu/g which is beyond the acceptable limits set for microbiological quality of ready-to-eat foods (22). The bacteria identified included E. coli, Salmonella spp. and vancomycin resistant S. aureus. Antibiotic resistance rates reported in the literature is highest for ampicillin, amoxicillin and tetracycline at an average of 79.5%, 77.2%, and 63.4% respectively. The bacteria identified included E. coli, Salmonella spp. and vancomycin resistant S. aureus. Antibiotic resistance rates reported in the literature is highest for ampicillin, amoxicillin and tetracycline at an average of 79.5%, 77.2%, and 63.4% respectively.
3.3 Sudan
3.3.1 Human sector
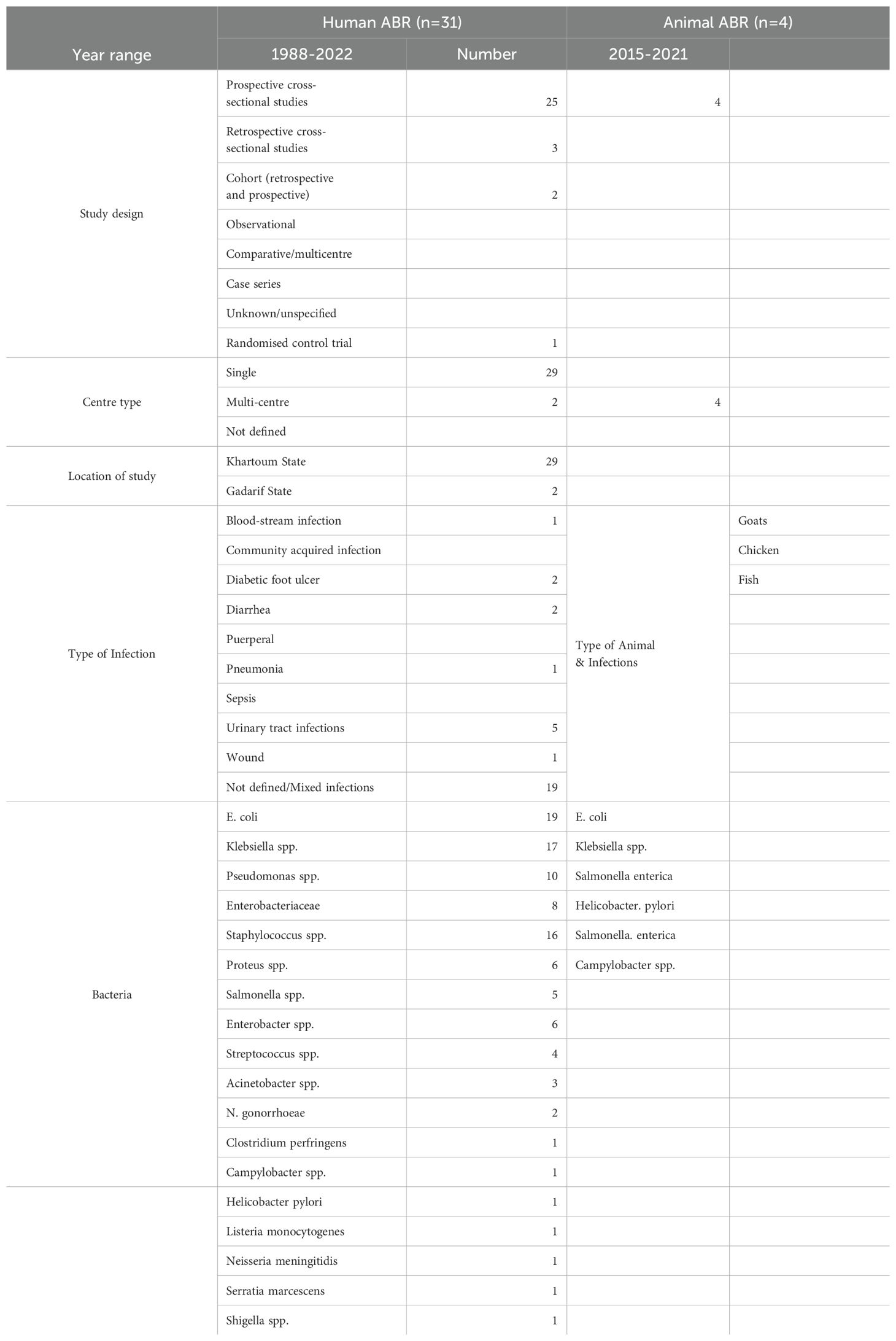
The databases search yielded 832 articles, of which 85 were assessed for eligibility by the initial screening, and subsequently a total of 31 articles were included in the review (Figure 1). Cross-sectional studies were the most common study type (n=24 studies), with year ranges from 1988 to 2022. Most of the studies were conducted in Khartoum, the capital city of Sudan, except two studies were conducted in Gadarif State in the Eastern region of Sudan (Figure 8). Studies were mostly conducted in single centres (n=29 studies), and 2 studies were from multiple centres.
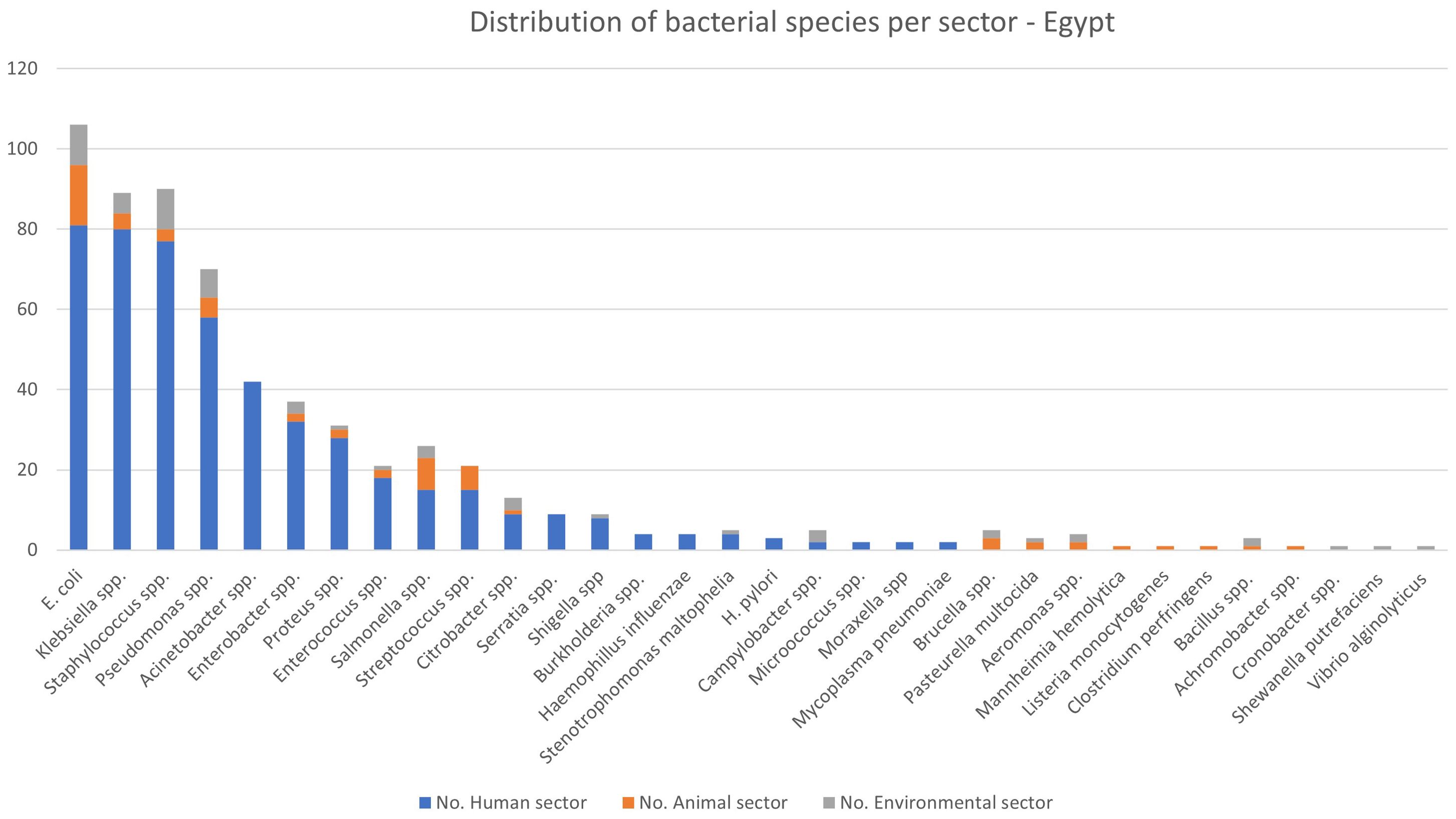
Figure 8. Distribution of studies in Sudan. Scale is based on number or articles from each sector and region.
Studies were mostly conducted from diagnostic samples in 24 articles, and seven from screening samples. Bacterial identification was conducted phenotypically only in 19 studies, whereas 12 studies relied on both phenotypic and genotypic identification of bacterial species. Mixed nosocomial infections accounted for the majority of the studies included (n=19 studies), followed by urinary tract infections (UTI) in five studies, and other specific studies on diabetes (n=2 studies), gastrointestinal and diarrhoeal diseases (n=2 studies), neonatal sepsis (n=1 study), wound (n=1 study), puerperal (n=1 study), and respiratory (n=1 study) infections. The minimum number of samples in the studies were five samples (26), and the maximum was 1451 respiratory samples from a retrospective study from 2009-2015 (27). With the exception of the disease/infection specific studies noted above, most of the studies contained mixed samples (i.e., blood, urine, stool, wound).
E. coli and Klebsiella spp. were the most commonly reported bacteria in the literature from the human sector in Sudan (n=17 reports for E. coli, n=17 Klebsiella spp. including 14 reports of K. pneumonia, 1 K. oxytoca), 16 S. aureus (including three reports of MRSA), and 10 Pseudomonas spp. (of which eight were P. aeruginosa). Only 10 studies were focused on single species, whereas the remaining 21 included data on multiple bacterial species (Table 3). Resistance rates were only reported in 10 studies, with the highest rates being for penicillin and amoxicillin (86%), followed by vancomycin resistance at 81%, 3rd generation cephalosporins at 67.7% and 2nd generation cephalosporins at 62% resistance (Supplementary Table S1, Figure 9). Overall aminoglycoside (amikacin, gentamicin and tobramycin) resistance was 18.3%, the highest of which was gentamicin resistance at 29%. Ciprofloxacin resistance was also high at 47%, in contrast to carbapenem resistance which was an average of 14.5% for imipenem and meropenem. Some studies conducted genomic characterisation of resistance and includes, bla-NDM, bla-OXA-48 in K. pneumoniae (28, 29), bla-IMP, -TEM, -VEB, -OXA-1, -DHA, -VIM, -GIM, -KPC, and -GES in P. aeruginosa (30), and in A. baumannii: bla-OXA-23, bla-CTX-M-1, and bla-GES (31).
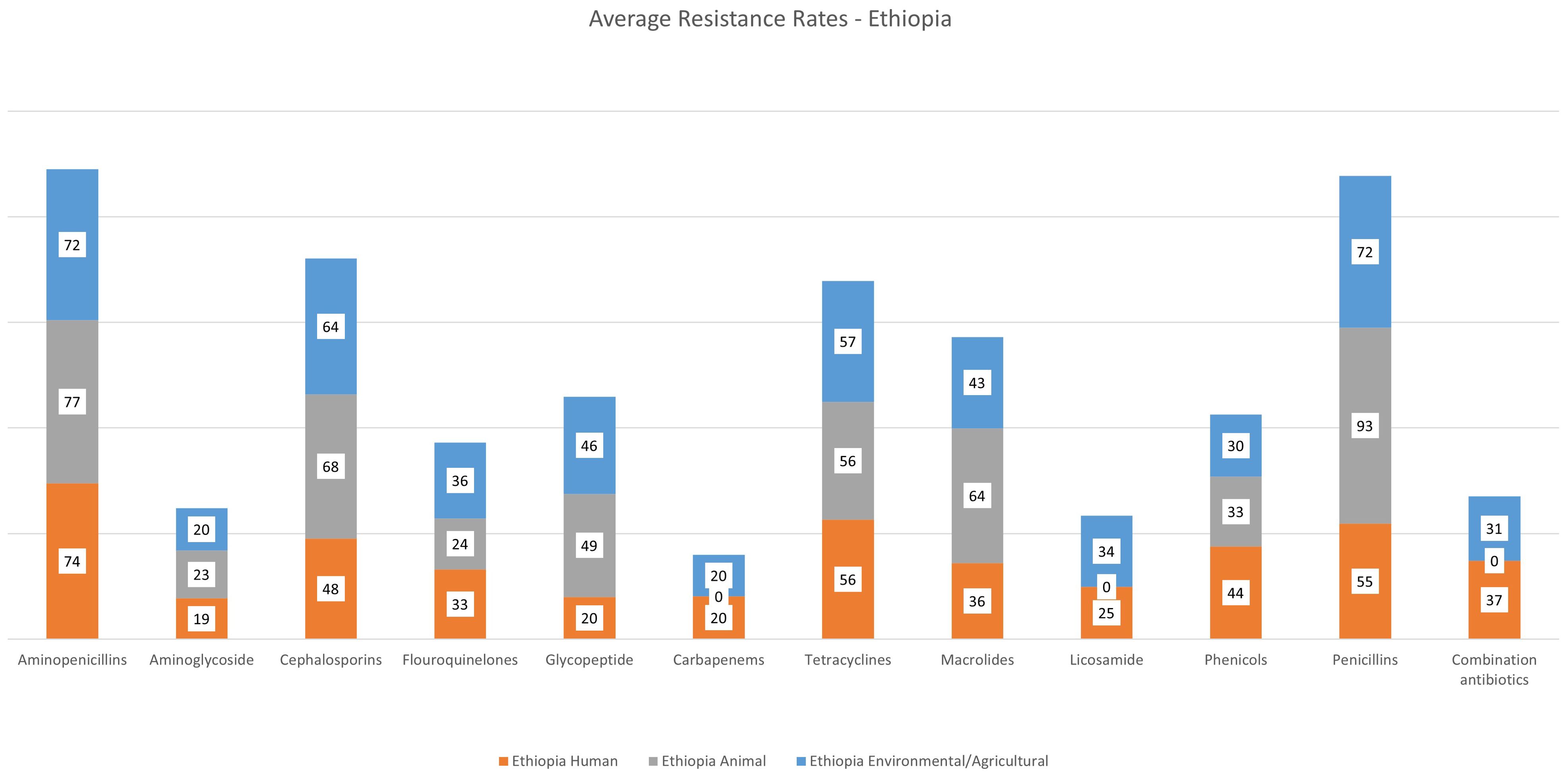
Figure 9. Average rates of resistance – Sudan. Human data only represented, No data was available from other sectors.
3.3.2 Animal sector
A total of four studies were included in the review, all of which were cross-sectional multi-centre studies in Khartoum from 2015–2021 included. Studies were conducted on animals such as goats, chicken, fish, as well as milk from goats and cows (32–35) (Table 3). The studies reported one bacterial species and three genera: Helicobacter pylori, S. enterica, Campylobacter spp., and Listeria spp. No data was available on resistance rates.
No studies were included in the environmental or agricultural sectors from Sudan.
3.4 Analysis of the national action plans
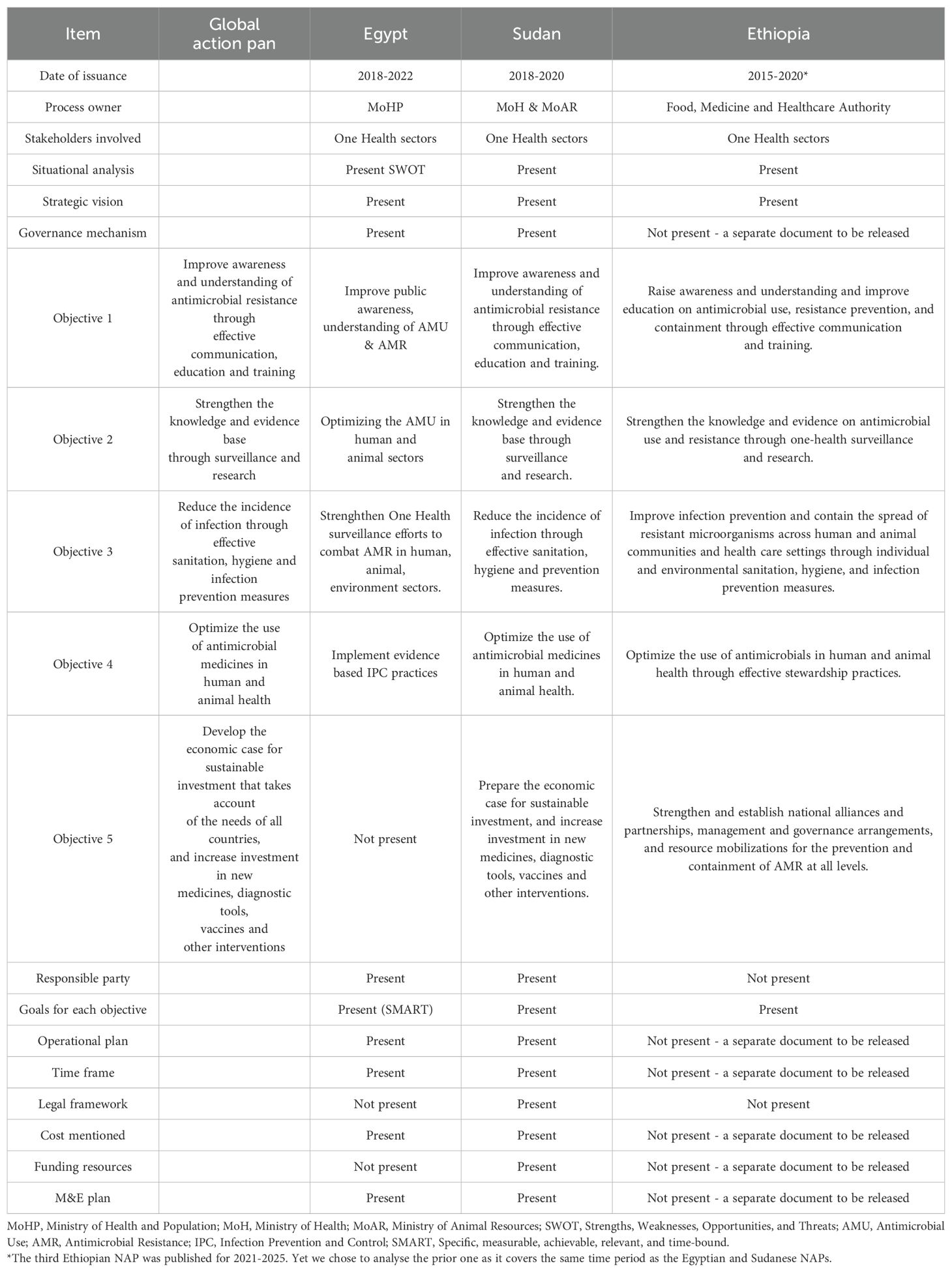
Table 4 summarises the individual AMR NAPs of Egypt, Sudan and Ethiopia which reflect distinct strategies shaped by each country’s healthcare context and resource availability. Egypt’s NAP focuses on enhancing surveillance systems for AMR and antimicrobial use, coupled with extensive public awareness campaigns aimed at educating both the public and healthcare professionals. The plan emphasizes stricter regulations on antibiotic distribution and delineates specific roles for various government ministries, fostering partnerships with international organizations to support implementation. Sudan’s NAP prioritizes ensuring access to quality medications and improving laboratory capacity for AMR testing. It adopts a multi-sectoral approach, recognizing the need for collaboration across human health, veterinary health, and agriculture sectors. However, Sudan faces significant challenges due to limited resources and infrastructure, which hinder effective implementation and coordination among involved agencies. This is further augmented by the ongoing conflict since April 2023. Ethiopia’s NAP presents a comprehensive strategy encompassing surveillance, rational drug use, and infection prevention. It highlights the importance of strengthening regulatory frameworks and engaging community health workers to promote awareness about AMR. Ethiopia’s plan is particularly detailed in its approach to infection control, aligning AMR efforts with broader health policies.
4 Discussion
This review aimed to capture the range of published literature and data on antibiotic resistance in the three Nile Valley countries: Egypt, Sudan and Ethiopia, using the PCC framework. The literature review encompassed the One Health sectors: human, animal, environmental and agricultural to highlight trends and ongoing research, which could be used to plan future research projects and support policies to reduce the incidence and burden of ABR.
As expected, there was significantly more output from the human sector across the countries as seen in the flow diagram (Figure 1), which illustrates the emphasis on ABR in the human sector. In Sudan, for example, only four articles met the study inclusion criteria from the animal sector, and none from the environmental/agricultural sector. In Egypt, the human sector included 170 articles in comparison with 37 from the environmental/agricultural sector.
Overall, prospective, cross-sectional, single-centre studies were also more common, in addition to a noteworthy occurrence of studies being conducted in larger cities (Figures 2, 5, 8). Studies in the human sector were centred in major cities with significant medical infrastructure, such as Cairo and Alexandria and Addis Ababa in Egypt and Ethiopia, respectively, suggesting a geographic bias in research concentration. It was a similar finding in Sudan, with only two studies conducted outside of Khartoum. These findings show that research studies are commonly conducted in larger cities with Higher Education settings and infrastructure, suggesting bias in local/regional prevalence, as well as an incomplete epidemiological picture of the true burden of infections and ABR.
Across the three countries, research in the human sector was focused on a broad array of infections and bacterial species, rather than focusing on specific diseases. This highlights a need for targeted disease-specific studies to deepen our understanding of specific ABR patterns. Few publications were focused on a specific diseases, such as causative agents for meningitis or ocular infections (14, 36), or on bacteria, such as the molecular epidemiology and detailed investigations (including antimicrobial resistance and/or virulence mechanisms) (29, 31, 37, 38). Most studies were more general and descriptive. Nonetheless, despite regional differences in diseases and epidemiology, the ESKAPE organisms dominated the literature across the three countries, which emphasizes the global prevalence and problem that these pathogens present, and the urgent need for more research, surveillance, and epidemiological investigations to assess their true burden and to aid in their control to break the transmission chain.
Similarly, for the animal sector, studies were primarily conducted in cities with large animal husbandries; in the Nile Delta region of Egypt (El-Sharqia, Damietta and Dakahlia), and the central region of Ethiopia. Interestingly, no data was available from Ethiopia prior to 2010, indicating more interest and awareness of researchers to investigate the noticeable rise of ABR incidence in the animal sector. All studies were from screening samples, and bacterial identification was only performed phenotypically. Several articles highlighted public health concerns such as Salmonella spp. and Brucella spp. (causing Brucellosis) due to their zoonotic potential (39) and indicating a significant challenge in treating bacterial infections in the animal sector due to the high prevalence of ABR. El-Kenany (2018) reported the prevalence of Salmonella serotypes at 13.5% broiler chicken farms in Egypt highlighting the risk of zoonotic spread of ciprofloxacin and azithromycin resistant-Salmonella spp. from Egyptian poultry through the food chain (40). Another study reported the emergence of a linezolid sensitive-vancomycin resistant Enterococci recovered from poultry in Egypt, suggesting that poultry could be potential vectors for transmission of multidrug resistant Enterococcus posing a public health risk. This highlights the magnitude of ABR across One Health and the need for coordinated epidemiological studies using genomics to confirm the true bacterial transmission links within One Health sectors. A study on the link between community- (CA), hospital- (HA), and livestock- acquired (LA) MRSA in Egypt using antibiogram typing, molecular characteristics and antimicrobial resistance and virulence genes’ profiles, revealed that HA‐MRSA strains tended to be multidrug resistant and less virulent than both LA‐ and CA‐MRSA strains. CA‐MRSA strains had a high genetic homology with some HA‐ and LA‐MRSA strains, yet, no similarity was observed between HA‐ and LA‐MRSA strains (41).
This review highlights a very important element in the identification of infections and resistance. There was large variability in diagnostic methodologies used across the sectors and countries, which in turn may lead to discrepancies in identification of bacteria at the species level and thereby inaccurate epidemiological data on prevalence and burden. Of the studies included in the review, 62% used only phenotypic identification, 28% combined phenotypic and genotypic methods, and 10% did not specify their methodology. This heterogeneity was most pronounced in Sudan (71% phenotypic only) compared to Egypt (58%) and Ethiopia (59%). For example, a study in Sudan reported > 40% of K. pneumoniae cases were misidentified using conventional phenotypical testing methods, highlighting the urgent need for adopting more reliable diagnostic methodologies across the region (42). As previously noted, it is difficult to make comparisons between Salmonella surveillance surveys conducted in different countries as the prevalence of Salmonella serovars varies regionally, and isolation rates depend upon the country, sampling, and laboratory identification methodology used (43). This variability in identification reflects the local variability in capacity for diagnosis and research infrastructure. Akal and Andualem (2018) assessed the capacity of 42 health facilities in the Afar Regional State in Ethiopia and found suboptimal materials and equipment available in diagnostic facilities, which is one of the major needs for providing quality healthcare (44). Limited hospital microbiology diagnostic capacity was noted as one of the challenges in the implementation of a sustainable surveillance system in resource-limited countries, represented in the nonavailability of essential supplies, high turnover of staff, and limited availability of external quality assurance programs (45). Gaps in infrastructure undermines efforts to tackle diseases, and hampers preventative measures (46). Additional challenges arise when considering incorporating molecular and genomic studies, however there’s evidence that researchers are increasingly incorporating these methods in more recent studies, particularly within the last five years, and especially since the COVID pandemic (42). We recognise the difficulties in incorporating novel diagnostic methodologies due to constraints on local resources, however we urge local stakeholders to standardise methodology where possible and rely on gold-standard identification and antibiotic susceptibility testing methodology in order to avoid delayed outbreak recognition, inappropriate empirical therapy, and overestimation of resistance rates.
The studies from the environmental sector represented a large diversity in terms of settings, ranging from animal farms, dairy products, and animal carcasses, but also hospital environments. We note that multiple publications report bacteria and resistance rates from hospital environments (i.e surfaces), which may therefore reflect nosocomial transmissions, rather than true representation of potential environmental reservoirs of resistant bacteria on surfaces. A systematic review by Asfaw et al. (2020) assessing ABR pathogens in wastewater reported overall MDR prevalence was 34.2% among bacteria isolated from treated and final effluents in Southern Ethiopia (47). Other studies from food sources reveals high incidence of potentially pathogenic bacteria and ABR genes, posing important public health hazards that warrants active surveillance of food, agricultural and environmental potential as reservoirs for ABR genes. Furthermore, environmental food samples such as from workers’ hand highlights the human element in food contamination studies (48). High resistance rates to commonly used antibiotics like cephalosporins, penicillins, and macrolides were observed, posing substantial public health risks as well as the presence of resistant bacteria in food and water. The high resistance rates observed in environmental samples indicate that ABR is not confined to clinical settings but is pervasive across various sectors which can lead to increased transmission of resistant pathogens through food and water, complicating efforts to control infections. The lack of environmental data from Sudan reveals a critical gap that must be addressed to fully understand the ABR landscape in the Nile Valley. The variability in diagnostic methodologies and lack of standardized practices further exacerbate the challenge, potentially leading to inaccurate epidemiological data. Addressing these issues requires a coordinated, multisectoral approach, emphasizing the need for robust surveillance, standardized diagnostics, and stringent regulations. Enhancing collaboration among stakeholders and investing in research and infrastructure are crucial steps to mitigate the impact of ABR and safeguard public health.
To advance research on antibiotic resistance (ABR) in the Nile Valley, it is crucial to expand geographic coverage by increasing surveillance efforts in rural and remote areas, ensuring a comprehensive picture of environmental reservoirs. Enhancing sample diversity to include soil, air, and various water sources will provide a better understanding of transmission pathways, as well as methodology for identification and processing (i.e metagenomics) which may pose as a significant hindering factor given the high cost and expertise required (49). Therefore, investing in research and innovation to understand resistance mechanisms, develop new diagnostic tools, and explore alternative treatments will be vital for combating ABR effectively.
Data was particularly scarce from Sudan (n=31 and n=4 from the human and animal sectors, respectively). The lack of environmental data was particularly alarming. However we noted the incorporation of genomic methodologies per total number, and several studies containing information on genetic screening of antibiotic resistance determinants (31, 50). Five studies from Sudan were excluded from the review because they were focused on whole-genome sequencing of individual bacterial strains, and therefore out of scope for this review. These studies revealed the genetic basis of resistance to multiple antibiotic classes (51, 52). Despite the limited data, ABR appears to be prevalent in Sudan. Azab et al. (2021) conducted a study on comparing Extended-spectrum beta-lactamase (ESBL) rates in Egypt, Sudan and Saudi Arabia, which revealed that overall prevalence of E. coli, K. pneumoniae and P. aeruginosa was higher in Sudan (40%, 17.5% and 42.5%), in comparison to rates from Egypt (33.3%, 14.8% and 25.9%), respectively (53). The highest frequency of MDR isolates was detected in Sudan at 97.5%. The burden of disease in Sudan is affected by poverty and is complicated by geography, politics, and recently, armed conflict (46).
Overall antibiotic resistance rates were high for all three countries (Figures 4, 7, 8). Average aminopencillin resistance was considerably high in Egypt: 99% in the human sector, 73% in the animal sector, and 52% in the environmental/agricultural sector. Average cephalosporin and macrolide resistance was higher in the animal vs the human sector in Egypt (94% vs 86%, and 71% vs 59%), respectively. Noticeably, average carbapenem resistance rates are still low in the animal sector (13%). Similar trends are observed in data from Ethiopia, with average cephalosporin, macrolide and glycopeptide resistance higher in the animal sector (68%, 64% and 49%) vs the human sector (48%, 36%, and 20%), respectively. Carbapenem resistance is still relatively low in the human sector, at an average of 20%, and no carbapenem resistance was reported from the animal sector. It is however important to note that there were large discrepancies in the literature, particularly in the number of publications and sample, including susceptibility testing in each sector. In order to reduce reporting bias, the study team collected resistance data based on the total number of isolates investigated in the individual studies and averaged the rates accordingly. Susceptibility testing was only conducted on a subset of the isolates in a number of studies (187 studies lacked complete resistance data), and multiple methods (i.e phenotypic disk diffusion assays, semi-automated machine) were reported in the literature further highlighting the lack of standardised methodology, and possible misrepresentation of the local epidemiology and resistance rates. We cannot exclude possible over-estimation of ABR due to the emphasis on research and publications on ABR-pathogens and infections (54). Nonetheless, the literature indicates high rates of resistance to multiple classes of antibiotics, and thereby the urgent need for implementation of antimicrobial stewardship and infection control policies across the One Health sectors. The alarming resistance rates in the animal sector call for immediate policy interventions, including stricter regulations on the use of antimicrobials as prophylactics and growth promoters in livestock. In a multi-country study – including Ethiopia – high awareness of ABR by prescribers (both in the human and animal sectors) was noted, however, this awareness did not translate to hesitation to use antibiotics (55). On the other hand, the study found evidence of escalation of antibiotic choice due to the lack of information and data on local resistance patterns and epidemiology. Farmers, especially animal producers supply many antimicrobials by mixing them with feed and/or water to their animals which yields the prevalence of AMR in food animals was higher than in non-food animals (54, 56).
With regards to the NAPs, all three countries emphasize the importance of multi-sectoral collaboration, recognizing that AMR requires a One Health approach involving health, agriculture, and environmental sectors. They share a commitment to strengthening surveillance systems for AMR and antibiotic use, alongside public awareness campaigns to educate citizens and healthcare providers about responsible antibiotic use. However, differences emerge in their implementation capacities: Egypt benefits from stronger institutional frameworks and resources, allowing for more robust execution of its plan, while Sudan and Ethiopia face greater challenges due to limited infrastructure and funding. Ethiopia’s NAP is particularly comprehensive in its focus on infection prevention and control measures, reflecting a proactive public health strategy. Furthermore, Egypt emphasizes stringent regulatory measures for antibiotic distribution, whereas Sudan and Ethiopia encounter enforcement challenges. Lastly, Ethiopia highlights community engagement through local health workers, a focus less pronounced in the other two countries’ plans. Addressing these variations is crucial for developing effective, tailored strategies in the fight against antimicrobial resistance. All three countries recognize the necessity of a coordinated, multi-sectoral approach to combat AMR in their NAPs, however they differ in implementation capacity and focus areas. Egypt benefits from a stronger institutional framework, while Sudan and Ethiopia face resource constraints that require additional support. Egypt`s NAP lacked any goal related to research and investment for sustainable implementation, while those for Ethiopia and Sudan did tackle this.
In addition to the NAPs, there have been local and regional efforts across Africa to raise awareness on the increasing threat of AMR in the community and healthcare settings, such as enrolment in the WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) and targeted training to improve antibiotic stewardship, such as the case in Ethiopia in collaboration with the Africa Centre for Disease Control in Ethiopia (57, 58). In Egypt, some localized campaigns also report improved public understanding of antibiotic misuse (59). These initiatives reflect a broader push across Africa to raise awareness, improve prescribing practices, and preserve the effectiveness of antibiotics.
5 Study limitations
The current ScR aimed to provide a comprehensive overview of the available data, rates and epidemiology of ABR within One Health in Egypt, Sudan and Ethiopia. However, we acknowledge several limitations in the study. Firstly, due to the nature of the scoping review, the data reported herein are non-standardised, and therefore might be under- or over-estimating bacterial and resistance rates. Due to the differences in number, methodology and quality of outputs across the countries, we have not been able to compare the data across countries and draw firm conclusions, accordingly. Secondly, the study focused only on published literature in the abovementioned databases, and we therefore acknowledge that we may have missed data from grey literature (such as PhD theses, unpublished data, or studies conducted and published in the native language). While our data shed significant light on ABR from a One Health perspective, the non-standardized nature of the collected data and potential publication bias could limit the accuracy and applicability of our findings.
6 Conclusion
In conclusion, ESKAPE pathogens are prevalent in the Nile Valley countries, across all the sectors, and are associated with high antibiotic resistance rates. Carbapenem resistance in K. pneumoniae reached 41% in Egypt, 20% in Ethiopia, and 14.5% in Sudan. Methicillin-resistant S. aureus exceeded 60% prevalence in human sectors across all countries. Aminopenicillin resistance was widespread across all countries: Egypt: 99%, Ethiopia: 76%, Sudan: 86%. These rates significantly exceed WHO critical thresholds, demanding immediate intervention. Bacterial infections and the associated resistance patterns are most accurately captured in incidence studies, which we acknowledge are resource-intensive, and therefore not easily applicable in resource-poor settings. However, these kinds of data are more reliable and better representative of the actual burden of bacterial infections and ABR locally. We noted over-representation of data from the human sector, and more incorporation of genetic and genomics in bacterial identification and outputs investigating the molecular epidemiology and genetics of resistance. This further highlights the human-clinical focus of ABR research, but also the lack of interdisciplinary collaboration and emphasis on the One Health framework. Each country’s NAP strategy reflects its unique challenges and opportunities, emphasizing the need for tailored solutions in the fight against AMR. We strongly advocate for a unified and strategic approach among local stakeholders, scientists, and international agencies to prioritize and fund AMR research, aiming for a sustainable reduction in antibiotic resistance. Robust epidemiological data is essential in planning relevant policies and guidelines to limit the spread of ABR locally and globally.
7 Future direction
Future efforts to combat antibiotic resistance (ABR) in Egypt, Sudan, and Ethiopia should prioritize expanding One Health surveillance, particularly in the underrepresented animal and environmental sectors, while ensuring geographic diversity in research to capture rural and regional trends. Strengthening laboratory capacity through standardized diagnostic methodologies and integrating genomic tools will improve surveillance accuracy. Effective implementation of National Action Plans (NAPs) requires stronger regulatory enforcement, increased funding, and multisectoral collaboration. Policy reforms should focus on antimicrobial stewardship, stricter regulations in agriculture, and responsible antibiotic use education. Finally, international partnerships and community engagement are essential for sustainable ABR mitigation and public health improvement. To address gaps identified in the current review effectively, it is essential to focus on capacity building by investing in laboratory infrastructure, training personnel, and securing funding for sustained harmonized surveillance efforts. Community engagement is crucial for raising awareness about antibiotic resistance and promoting responsible antibiotic use across all the sectors. Mechanisms for cross-sectoral coordination should be established to facilitate collaboration among human, animal, and environmental health sectors, ensuring a One Health approach to combating antibiotic resistance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LA-H: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. AN: Formal Analysis, Investigation, Visualization, Writing – review & editing. GB: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review & editing. BY: Data curation, Investigation, Writing – review & editing. DB: Data curation, Investigation, Writing – review & editing. KD: Data curation, Investigation, Writing – review & editing. MW: Data curation, Investigation, Writing – review & editing. TD-S: Data curation, Investigation, Writing – review & editing. AF: Data curation, Investigation, Writing – review & editing. MZ: Data curation, Investigation, Writing – review & editing. RE: Data curation, Investigation, Writing – review & editing. HE-M: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. NE-A: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AR-M: Investigation, Methodology, Writing – review & editing. SA: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. PH: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. AM: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – review & editing. PN: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. EO: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MM: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The PRESAR network was funded by the Academy of Medical Sciences Global Challenges Research Fund Networking Grant GCRFNGR6\1598 awarded to MM and LA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2025.1629274/full#supplementary-material
References
1. Gemeda BA, Assefa A, Jaleta MB, Amenu K, and Wieland B. Antimicrobial resistance in Ethiopia: A systematic review and meta-analysis of prevalence in foods, food handlers, animals, and the environment. One Heal. (2021) 13:100286. doi: 10.1016/j.onehlt.2021.100286
2. Raouf M, Ghazal T, Kassem M, Agamya A, and Amer A. Surveillance of surgical-site infections and antimicrobial resistance patterns in a tertiary hospital in Alexandria, Egypt. J Infect Dev Ctries. (2020) 14:277–83. doi: 10.3855/jidc.12124
3. Elbadawi HS, Elhag KM, Mahgoub E, Altayb HN, and Abdel Hamid MM. Antimicrobial resistance surveillance among gram-negative bacterial isolates from patients in hospitals in Khartoum State, Sudan [version 1; peer review: Awaiting peer review. F1000Research. (2019) 8. doi: 10.12688%2FF1000RESEARCH.17744.1&partnerID=40&md5=1f079287a5eb7b17f7521887dfeb49df
4. Khalil H, Peters MD, Tricco AC, Pollock D, Alexander L, McInerney P, et al. Conducting high quality scoping reviews-challenges and solutions. J Clin Epidemiol. (2021) 130:156–60. doi: 10.1016/j.jclinepi.2020.10.009
5. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, and Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
6. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist SECTION. Ann Intern Med. (2018) 169:11–2. Available online at: http://www.prisma-statement.org/Extensions/ScopingReviews.
7. Ghaith DM, Zafer MM, Said HM, Elanwary S, Elsaban S, Al-Agamy MH, et al. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. (2020) 39:583–91. doi: 10.1007/s10096-019-03761-2
8. Ghaith DM, Mohamed ZK, Farahat MG, Aboulkasem Shahin W, and Mohamed HO. Colonization of intestinal microbiota with carbapenemase-producing Enterobacteriaceae in paediatric intensive care units in Cairo, Egypt. Arab J Gastroenterol Off Publ Pan-Arab Assoc Gastroenterol. (2019) 20:19–22. doi: 10.1016/j.ajg.2019.01.002
9. Selim A, Kelis K, AlKahtani MDF, Albohairy FM, and Attia KA. Prevalence, antimicrobial susceptibilities and risk factors of Methicillin resistant Staphylococcus aureus (MRSA) in dairy bovines. BMC Vet Res. (2022) 18:293. doi: 10.1186%2Fs12917-022-03389-z&partnerID=40&md5=91be7ebb8b38a377259f2953a3072578
10. Elsayed MSAE, Awad A, Tarabees R, and Marzouk A. Virulence repertoire and antimicrobial resistance profile of Shiga toxin-producing E.coli isolated from sheep and goat farms from Al-Buhayra Egypt. Pak Vet J. (2018) 38:429–33. doi: 10.29261%2Fpakvetj%2F2018.082&partnerID=40&md5=cfe4739ad5d712f245955e140c50f01b
11. Argaw-Denboba A, Abejew AA, and Mekonnen AG. Antibiotic-resistant bacteria are major threats of otitis media in wollo area, northeastern Ethiopia: A ten-year retrospective analysis. Int J Microbiol. (2016) 2016:8724671. doi: 10.1155/2016/8724671
12. Wabeto W, Abraham Y, and Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Heal Popul Nutr. (2017) 36:52. doi: 10.1186/s41043-017-0131-z
13. Kasew D, Desalegn B, Aynalem M, Tila S, Diriba D, Afework B, et al. Antimicrobial resistance trend of bacterial uropathogens at the university of Gondar comprehensive specialized hospital, northwest Ethiopia: A 10 years retrospective study. PloS One. (2022) 17(4):e0266878. doi: 10.1371/journal.pone.0266878
14. Assegu Fenta D, Lemma K, Tadele H, Tilahun BT, and Derese B. Antimicrobial sensitivity profile and bacterial isolates among suspected pyogenic meningitis patients attending at Hawassa University Hospital: Cross-sectional study. BMC Microbiol. (2020) 20(1):153. doi: 10.1186%2Fs12866-020-01808-5&partnerID=40&md5=71e3f245460201cf04aff29046eb4a7f
15. Zelalem A, Abegaz K, Kebede A, Terefe Y, and Vipham JL. Investigation on Salmonella enterica, Escherichia coli, and coliforms in beef from Ethiopian abattoirs: A potential risk of meat safety. Food Sci Nutr. (2022) 10:1714–24. doi: 10.1002/fsn3.2752
16. Kebede R, Alemayehu H, Medhin G, and Eguale T. Nontyphoidal salmonella and their antimicrobial susceptibility among diarrheic patients attending private hospitals in addis ababa, Ethiopia. BioMed Res Int. (2021) 2021:6177741. doi: 10.1155/2021/6177741
17. Tadese ND, Gebremedhi EZ, Moges F, Borana BM, Marami LM, Sarba EJ, et al. Occurrence and antibiogram of escherichia coli O157: H7 in raw beef and hygienic practices in abattoir and retailer shops in ambo town, Ethiopia. Vet Med Int. (2021) 2021:8846592. doi: 10.1155/2021/8846592
18. Guchi B and Ashenafi M. Microbial load, prevalence and antibiograms of Salmonella and Shigella in lettuce and green peppers. Ethiop J Health Sci. (2011) 20(1):41–8. doi: 10.4314/ejhs.v20i1.69431
19. Belachew T, Mihret A, Legesse T, Million Y, and Desta K. High level of drug resistance by gram-negative bacteria from selected sewage polluted urban rivers in Addis Ababa, Ethiopia. BMC Res Notes. (2018) 11:1–6. doi: 10.1186/s13104-018-3622-0
20. Mekengo BM, Hussein S, and Ali MM. Distribution and antimicrobial resistance profile of bacteria recovered from sewage system of health institutions found in Hawassa, Sidama Regional State, Ethiopia: A descriptive study. SAGE Open Med. (2021) 9:20503121211039097. doi: 10.1177/20503121211039097
21. Kibret M and Tadesse M. The bacteriological safety and antimicrobial susceptibility of bacteria isolated from street-vended white lupin (Lupinus albus) in Bahir Dar, Ethiopia. Ethiop J Health Sci. (2013) 23:19–26.
22. Eromo T, Tassew H, Daka D, and Kibru G. Bacteriological quality of street foods and antimicrobial resistance of isolates in hawassa, Ethiopia. Ethiop J Health Sci. (2016) 26:533–42. doi: 10.4314/ejhs.v26i6.5
23. Ejo M, Garedew L, Alebachew Z, and Worku W. Prevalence and antimicrobial resistance of salmonella isolated from animal-origin food items in gondar, Ethiopia. BioMed Res Int. (2016) 2016:4290506. doi: 10.1155/2016/4290506
24. Amare A, Worku T, Ashagirie B, Adugna M, Getaneh A, and Dagnew M. Bacteriological profile, antimicrobial susceptibility patterns of the isolates among street vended foods and hygienic practice of vendors in Gondar town, Northwest Ethiopia: A cross sectional study. BMC Microbiol. (2019) 19:1–9. doi: 10.1186/s12866-019-1509-4
25. Worku M, Getie M, Moges F, and Mehari AG. Extended-spectrum beta-lactamase- and carbapenemase-producing enterobacteriaceae family of bacteria from diarrheal stool samples in northwest Ethiopia. Interdiscip Perspect Infect Dis. (2022) 2022:7905350. doi: 10.1155/2022/7905350
26. Bayoumi MA and Hamid OM. The emergence of carbapenem resistant enterobacteriaceae producing GIM-1 and SIM-1 clinical isolates in khartoum-Sudan. Infect Drug Resist. (2022) 15:2679–84. doi: 10.2147/IDR.S365983
27. Ahmed SMA-Z, Abdelrahman SS, Saad DM, Osman IS, Osman MG, and Khalil EAG. Etiological trends and patterns of antimicrobial resistance in respiratory infections. Open Microbiol J. (2018) 12:34–40. doi: 10.2174/1874285801812010034
28. Osman EA, El-Amin NI, Al-Hassan LL, and Mukhtar M. Multiclonal spread of Klebsiella pneumoniae across hospitals in Khartoum, Sudan. J Glob Antimicrob Resist. (2021) 24:241–5. doi: 10.1016/j.jgar.2020.12.004
29. Osman EA, Yokoyama M, Altayb HN, Cantillon D, Wille J, Seifert H, et al. Klebsiella pneumonia in Sudan: multidrug resistance, polyclonal dissemination, and virulence. Antibiotics. (2023) 12(2):233. doi: 10.3390/antibiotics12020233
30. Omer THS, Mustafa SAM, and Mohamed SOO. Extended Spectrum beta-Lactamase-Mediated Resistance and Antibiogram of Pseudomonas aeruginosa Isolates from Patients Attending Two Public Hospitals in Khartoum, Sudan. Int J Microbiol. (2020) 2020:2313504. doi: 10.1155/2020/2313504
31. Al-Hassan L, Elbadawi H, Osman E, Ali S, Elhag K, Cantillon D, et al. Molecular epidemiology of carbapenem-resistant acinetobacter baumannii from khartoum state, Sudan. Front Microbiol. (2021) 12. doi: 10.3389/fmicb.2021.628736
32. Elbrissi A, Sabeil YA, Khalifa KA, Enan K, Khair OM, and El Hussein AM. Isolation, identification and differentiation of Campylobacter spp. using multiplex PCR assay from goats in Khartoum State, Sudan. Trop Anim Health Prod. (2017) 49:575–81. doi: 10.1007/s11250-017-1231-x
33. El Hag MMA, El Zubeir IEM, and Mustafa NEM. Prevalence of Listeria species in dairy farms in Khartoum State (Sudan). Food Control. (2021) 123:107699. doi: 10.1016/j.foodcont.2020.107699
34. Osman EY, El-Eragi AMS, Musa AM, El-Magboul SB, Rahman MBA, and Abdo AE. Detection of Helicobacter pylori glmM gene in bovine milk using Nested polymerase chain reaction. Vet World. (2015) 8:913–7. doi: 10.14202/vetworld.2015.913-917
35. Elmadiena MMAN, El Hussein AA, Muckle CA, Cole L, Wilkie E, Mistry K, et al. Antimicrobial susceptibility and multi-drug resistance of Salmonella enterica subspecies enterica serovars in Sudan. Trop Anim Health Prod. (2013) 45:1113–8. doi: 10.1007/s11250-012-0334-7
36. Belyhun Y, Moges F, Endris M, Asmare B, Amare B, Bekele D, et al. Ocular bacterial infections and antibiotic resistance patterns in patients attending Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. (2018) 11(1):597. doi: 10.1186%2Fs13104-018-3705-y&partnerID=40&md5=f9599d7b2552b6f1cc9b9ddffe407792
37. Abdeen EE, Mousa WS, Abdelsalam SY, Heikal HS, Shawish RR, Nooruzzaman M, et al. Prevalence and characterization of coagulase positive staphylococci from food products and human specimens in Egypt. Antibiotics-Basel. (2021) 10(1):75. doi: 10.3390/antibiotics10010075
38. Enany S, Zakeer S, Diab AA, Bakry U, and Sayed AA. Whole genome sequencing of Klebsiella pneumoniae clinical isolates sequence type 627 isolated from Egyptian patients. PloS One. (2022) 17(3):e0265884. doi: 10.1371/journal.pone.0265884
39. Abd El-Ghany WA. Salmonellosis: A food borne zoonotic and public health disease in Egypt. J Infect Dev Ctries. (2020) 14:674–8. doi: 10.3855/jidc.12739
40. Elkenany RM, Eladl AH, and El-Shafei RA. Genetic characterisation of class 1 integrons among multidrug-resistant Salmonella serotypes in broiler chicken farms. J Glob Antimicrob Resist. (2018) 14:202–8. doi: 10.1016%2Fj.jgar.2018.04.009&partnerID=40&md5=66eed7f4179cbfc77ffeec3c30d81364
41. Abd El-Hamid MI, Bendary MM, Merwad AMA, Elsohaby I, Ghaith DM, and Alshareef WA. What is behind phylogenetic analysis of hospital-, community- and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound Emerg Dis. (2019) 66:1506–17. doi: 10.1016/j.jgar.2018.04.009
42. Osman EA, El-Amin N, Adrees EAE, Al-Hassan L, and Mukhtar M. Comparing conventional, biochemical and genotypic methods for accurate identification of Klebsiella pneumoniae in Sudan. Access Microbiol. (2019) 66(4):1506–17. doi: 10.1111/tbed.13170
43. El Hussein AA, Mohy-Eldin HS, Elmadiena MMN, and El Siddig MA. Prevalence, detection and antimicrobial resistance pattern of salmonella in Sudan. In: Annous BA and Gurtler JB, editors. Salmonella - distribution, adaptation, control measures and molecular technologies (2012). p. 51–80.
44. Genet Akal C and Andualem T. A cross-sectional study to assess capacity of health facility laboratories in zone one of afar regional state, Ethiopia. J Trop Med. (2018) 2018. doi: 10.1155/2018/9274127
45. Talaat M, El-Shokry M, El-Kholy J, Ismail G, Kotb S, Hafez S, et al. National surveillance of health care–associated infections in Egypt: Developing a sustainable program in a resource-limited country. Am J Infect Control. (2016) 44:1296–301. doi: 10.1016/j.ajic.2016.04.212
46. Charani E, Cunnington AJ, Yousif AHA, Ahmed MS, Ahmed AEM, Babiker S, et al. In transition: current health challenges and priorities in Sudan. BMJ Glob Heal. (2019) 4(11):1296–301. doi: 10.1136/bmjgh-2019-001723
47. Asfaw T, Genetu D, and Shenkute D. High burden of antibiotic-resistant bacteria from wastewater in Ethiopia: A systematic review. Risk Manag Healthc Policy. (2020) 13:3003–11. doi: 10.1186/s12941-025-00799-3
48. Abu El-Hammed W, Soufy H, El-Shemy A, Nasr SM, Dessouky MI, Adel WA, et al. Prevalence and Molecular Characterization of Extended-Spectrum beta-Lactamases and AmpC beta-lactamase-Producing Enterobacteriaceae among Human, Cattle, and Poultry. Adv Anim Vet Sci. (2021) 9:54359–77. doi: 10.1136/bmjgh-2019-001723
49. Forbes JD, Knox NC, Ronholm J, Pagotto F, and Reimer A. Metagenomics: The next culture-independent game changer. Front Microbiol. (2017) 8:1–21. doi: 10.3389/fmicb.2017.01069
50. Adam MA and Elhag WI. Prevalence of metallo–lactamase acquired genes among carbapenems susceptible and resistant Gram-negative clinical isolates using multiplex PCR, Khartoum hospitals, Khartoum Sudan. BMC Infect Dis. (2018) 18(1):54359–77. doi: 10.1186/s12879-018-3581-z
51. Mohamed SB, Hassan M, Munir A, Kambal S, Abdalla NI, Hamad A, et al. Whole-genome sequence of acinetobacter baumannii strain NUBRI-A, isolated from a hospitalized patient in khartoum, Sudan. Microbiol Resour Announc. (2019)8:9–10. doi: 10.1128/mra.00542-19
52. Mohamed SB, Kambal S, Munir A, Abdalla N, Hassan M, Hamad A, et al. First whole-genome sequence of a highly resistant klebsiella pneumoniae sequence type 14 strain isolated from Sudan. Microbiol Resour Announc. (2019) 8:14–5. doi: 10.1128/mra.00552-19
53. Farag MMS. Distribution of extended-spectrum β -lactamase gram-negative pathogens collected from three different countries. (2021).
54. de Kraker MEA, Stewardson AJ, and Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PloS Med. (2016) 13:1–6. doi: 10.1371/journal.pmed.1002184
55. Pearson M and Chandler C. Knowing antimicrobial resistance in practice: a multi-country qualitative study with human and animal healthcare professionals. Glob Health Action. (2019) 12:1599560. doi: 10.1080/16549716.2019.1599560
56. Gebeyehu DT, Bekele D, Mulate B, Gugsa G, and Tintagu T. Knowledge, attitude and practice of animal producers towards antimicrobial use and antimicrobial resistance in Oromia zone, north eastern Ethiopia. PloS One. (2021) 16(11):1–6. doi: 10.1371/journal.pone.0251596
57. Kariuki S, Kering K, Wairimu C, Onsare R, and Mbae C. Antimicrobial resistance rates and surveillance in sub-saharan africa: where are we now? Infect Drug Resist. (2022) 15:3589–609. doi: 10.1371/journal.pone.0251596
58. Osman EA, Omer SA, Elmubarak RMA, Abdelnabi M, Abdelgadir S, Ahmed DG, et al. Antibiotic resistance in Sudan: assessing the knowledge and practices of healthcare workers in Khartoum. JAC-Antimicrobial Resist. (2024) 6:1–7. doi: 10.1093/jacamr/dlae049
Keywords: antibiotic resistance, Africa, One Health, national action plan, Nile Valley
Citation: Al-Hassan L, Nowbuth AA, Beyene GT, Yeshitela B, Berhe DF, Desta K, Wolde M, Dorai-Schneiders T, Farouk A, Zafer M, ElShimy R, El-Mahallawy H, El-Amin N, Roemer-Mahler A, Aizzeldin S, Higgins PG, Moghith A, Nahar P, Osman EA and Mukhtar M (2025) A scoping review of antibiotic resistance through a One Health lens. Insights from the Nile Valley: Egypt, Sudan, and Ethiopia. Front. Trop. Dis. 6:1629274. doi: 10.3389/fitd.2025.1629274
Received: 15 May 2025; Accepted: 11 July 2025;
Published: 14 August 2025.
Edited by:
Sylvia Opanga, University of Nairobi, KenyaReviewed by:
Amira Awad Moawad, Friedrich Loeffler Institut, GermanyGayathri Govindaraju, Rutgers, The State University of New Jersey, United States
Copyright © 2025 Al-Hassan, Nowbuth, Beyene, Yeshitela, Berhe, Desta, Wolde, Dorai-Schneiders, Farouk, Zafer, ElShimy, El-Mahallawy, El-Amin, Roemer-Mahler, Aizzeldin, Higgins, Moghith, Nahar, Osman and Mukhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Einas A. Osman, ZWluYXMub3NtYW5AYXN1LmVkdS5vbQ==
 Leena Al-Hassan
Leena Al-Hassan Anya A. Nowbuth3
Anya A. Nowbuth3 Getachew Tesfaye Beyene
Getachew Tesfaye Beyene Biruk Yeshitela
Biruk Yeshitela Derbew Fikadu Berhe
Derbew Fikadu Berhe Mistire Wolde
Mistire Wolde Thamarai Dorai-Schneiders
Thamarai Dorai-Schneiders Amira Farouk
Amira Farouk Mai Zafer
Mai Zafer Rana ElShimy
Rana ElShimy Hadir El-Mahallawy
Hadir El-Mahallawy Nagwa El-Amin
Nagwa El-Amin Anne Roemer-Mahler
Anne Roemer-Mahler Paul G. Higgins
Paul G. Higgins Aalaa Moghith
Aalaa Moghith Papreen Nahar
Papreen Nahar Einas A. Osman
Einas A. Osman