- 1Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa
- 2Saselamani Pharmacy, Saselamani, South Africa
- 3Department of Pharmacy, Faculty of Medicine, University of Banja Luka, Banja Luka, Republic of Srpska, Bosnia and Herzegovina
- 4School of Health Sciences, University of Manchester, Manchester, United Kingdom
- 5Department of Pharmacology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 6Centre for Neonatal and Paediatric Infection, Institute for Infection and Immunity, City St. George’s, University of London, London, United Kingdom
- 7Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 8South African Vaccination and Immunisation Centre, Sefako Makgatho Health Sciences University, Pretoria, South Africa
Background: Antimicrobial resistance (AMR) is an appreciable threat to public health, especially among low- and middle-income countries (LMICs), exacerbated by high levels of inappropriate prescribing and dispensing of antibiotics in these countries. There have been variable levels of dispensing of antibiotics without a prescription among community pharmacies in South Africa. Given the importance of community pharmacies, especially in rural South Africa, there is a need to assess their knowledge and attitudes towards antibiotics, AMR, and antibiotic stewardship. This was the aim of this study.
Methods: A previously piloted questionnaire was administered to pharmacy personnel currently operating community pharmacies in a rural province in South Africa, where dispensing of antibiotics without a prescription is likely to be greatest. The questionnaire included key knowledge questions regarding antibiotics and AMR, as well as ways to reduce AMR. Community pharmacies were divided into three categories: Independent, chain, and franchise pharmacies.
Results: A total of 128 pharmacies participated (75.7%), with independent pharmacies representing the majority (60.9%). A total of 313 completed questionnaires were returned (78.3% response rate), including responses from 106 pharmacists (33.9%) and 207 pharmacist assistants (66.1%). Overall, there was very good knowledge among both community pharmacists and pharmacist assistants concerning antibiotics and AMR. However, there was a significant misconception regarding the potential role of antibiotics in relieving pain. Encouragingly, attitudes regarding the risks associated with obtaining antibiotics without a prescription among both community pharmacists and pharmacist assistants were high. There was also strong agreement among both community pharmacists and pharmacist assistants for potential solutions to AMR.
Conclusion: Overall, the findings showed that most pharmacists and pharmacist assistants in this rural province demonstrated a strong understanding of the effectiveness of antibiotics in bacterial infections and their lack of effectiveness to treat viral infections. They also demonstrated considerable knowledge regarding the risks associated with the inappropriate dispensing of antibiotics without a prescription, as well as ways to address rising AMR rates.
1 Introduction
Antimicrobial resistance (AMR) is an increasing global threat in view of appreciable and growing morbidity, mortality, and costs, becoming the next pandemic unless multiple activities are urgently undertaken (1–7). The greatest burden of AMR is currently in low- and middle-income countries (LMICs), which include sub-Saharan African countries (8–11). AMR is driven by the misuse of antibiotics, including appreciable purchasing of antibiotics without a prescription for essentially self-limiting infections, which is particularly prevalent in LMICs, including sub-Saharan Africa (10, 12–20). Coupled with this are concerns regarding hygiene and sanitation, diagnostic facilities, and low vaccine uptake where effective vaccines are available (11, 21–24).
Ongoing global activities to reduce AMR include the World Health Organization Global Action Plan instigated in 2015 (GAP) (25–28), with the GAP subsequently translated into National Action Plans (29–32). South Africa has implemented its NAP (33), with key elements including the monitoring of antibiotic utilization patterns alongside a number of agreed activities to reduce AMR (34). This includes ongoing monitoring of the goals and objectives of the NAP combined with active surveillance of AMR patterns as well as instigating/monitoring antimicrobial stewardship (AMS) activities across sectors (35–41).
High rates of self-purchasing of antibiotics in sub-Saharan Africa are enhanced by the convenience and ready availability of antibiotics among community pharmacists and drug sellers, as well as lower costs when factoring in co-payments with seeing prescribers in primary healthcare (PHC) centers alongside associated travel costs and long waiting times, which are important considerations especially in rural areas (13, 16, 42–46). As a result, rates of purchasing antibiotics without a prescription have reached up to 100% in community pharmacies in some African countries (13, 47). Such activities are enhanced by concerns regarding the knowledge of community pharmacists and their assistants across Africa about antibiotics, AMR, and AMS (Table 1), coupled with pressure from patients for community pharmacists to dispense antibiotics for often self-limiting conditions (48–51).
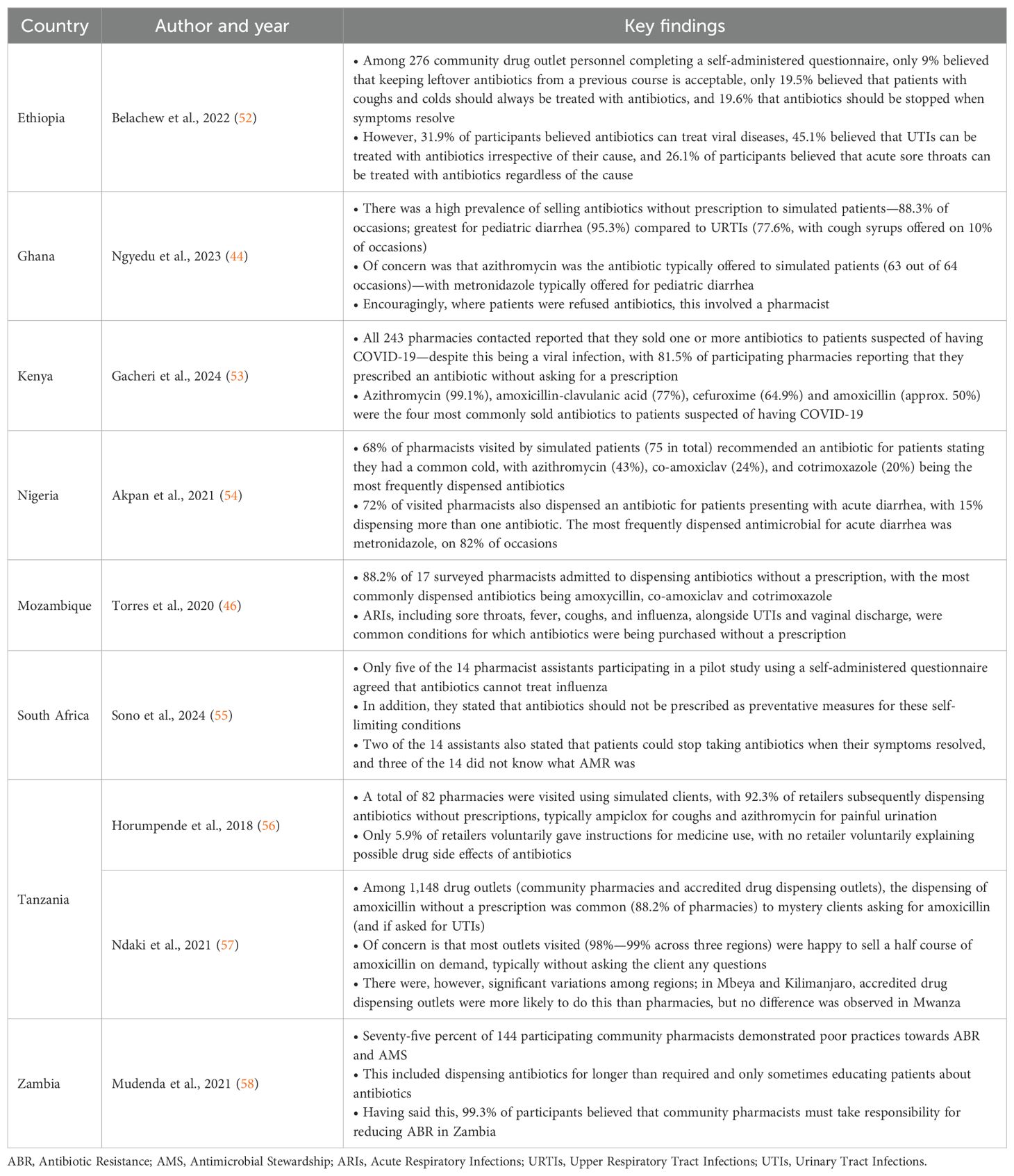
Table 1. Examples of concerns with knowledge, attitude and practices regarding antibiotics and AMR among community pharmacy personnel across Africa.
There is a variable situation in South Africa regarding the purchasing of antibiotics without a prescription, as seen in the studies by Anstey Watkins et al. (2016) and Do et al. (2021) conducted in rural South Africa where there was very limited or no purchasing of antibiotics without a prescription (59, 60). However, Mokwele et al. (2022) did not find this, reporting that antibiotics were sold without a prescription primarily among privately-owned independent pharmacies (61). Sono et al. (2024) had similar findings to Mokwele et al. (2022) when surveying both community pharmacy personnel and patients in recent pilot studies (55, 61, 62) as well as in the main study, primarily among independent pharmacies in a rural province (63). Such activities occur despite South African legislation prohibiting the purchasing of antibiotics without a prescription in the country (13, 55).
We previously reported that community pharmacists and pharmacist assistants almost always or mostly (approximately 98%) offered symptomatic relief before dispensing antibiotics without a prescription to patients presenting with self-limiting infectious disease symptoms, including acute respiratory infections, with approximately half of them never suggesting antibiotics for self-limiting infectious diseases (63). Considering this, there is a need to further assess their knowledge and attitudes toward antibiotic use, as community pharmacists and pharmacist assistants play an increasingly important role in providing healthcare to patients within the South African healthcare system, as emphasized during the COVID-19 pandemic (49, 64–66). Community pharmacists have also begun to audit prescribers’ prescriptions and provide feedback to prescribers to improve the future use of antibiotics, which can be built upon (67). This is essential given an appreciable increase in the utilization of antibiotics generally in the public healthcare system in South Africa in recent years, as well as specifically antibiotics from the WHO Watch list with their greater resistance potential (68–70). Watch antibiotics constituted 52% of total antibiotic use in the public sector in 2022, up from 21% previously (68), with rises in the utilization of WHO Watch antibiotics also seen in other LMICs (70).
Consequently, the principal objective of this study was to determine the knowledge and attitudes of community pharmacists and pharmacist assistants regarding the use of antibiotics in this rural South African province. Subsequently, we assessed their agreement and support for potentially different policies to reduce AMR in South Africa. The results, combined with the findings we previously reported (63), can be used to suggest potential activities to reduce the inappropriate dispensing of antibiotics in South Africa and across Africa, building on recent suggestions among LMICs (18, 49, 50). This is particularly important in South Africa, given concerns regarding the rising utilization of Watch and Reserve antibiotics in the country, coupled with rising AMR rates, leading to renewed calls on the government to implement additional policies in line with the National Action Plan to reduce AMR (71).
2 Materials and methods
The methodology has been previously described in detail (63). Consequently, more limited details are provided in this paper.
2.1 Study setting, population, and study design
A rural province was selected for this study because the extent of purchasing antibiotics without a prescription in South Africa is likely to be greatest in this setting, given the appreciable differences seen in the extent of purchasing antibiotics without a prescription in previous studies (18, 55, 59–61). A descriptive survey methodology was used to collect data directly from community pharmacists and pharmacist assistants, with community pharmacies divided into three categories: chain, franchise, and independent pharmacies. Chain pharmacies include ‘Clicks,’ ‘Dischem,’ and ‘Medirite’ at Checkers (supermarket), which are owned by corporate entities. Franchise pharmacies are independently owned by franchisees. However, they operate under a brand name and include for instance ‘The Local Choice,’ ‘Link,’ and ‘Van Heerden.’ Independent pharmacies are standalone pharmacies that do not have any ties to a particular brand or group (63).
All community pharmacies in this province were targeted for this study, except for the 11 pharmacies included in the pilot study, as reported previously (55, 63).
Pharmacist assistants in South Africa work under the supervision of registered pharmacists to process prescriptions and deal with patients. They undergo a training program at selected Universities in South Africa, with the program consisting of two levels: basic and post-basic, with further on-the-job training (72). The courses are accredited by the South African Pharmacy Council (SAPC), with the basic Pharmacist Assistant Course (NQF Level 3) spanning 12 months (full-time) or up to 18 months (part-time). The content covers pharmaceutical principles, dispensing techniques, ethical practices, and inventory management. The duration of the Post Basic Pharmacist Assistant Course (NQF Level 4) typically spans an additional 12–18 months (part-time). This course delves deeper into pharmacology, patient care, chronic disease management, and clinical support. In addition, more advanced input into dispensing techniques is provided (73). Pharmacist assistants are principally certified. Pharmacists are university-trained with their degree accredited by the SAPC. They can either be employed as pharmacists or practice as the responsible pharmacist on the premises.
2.2 Data collection
Four trained data collectors, including TMM and MTM, collected the data. On the day of data collection, the pharmacist in charge of the pharmacy was approached with an invitation for all pharmacists and pharmacist assistants present in the pharmacy at that time to participate in the study. The rationale for the study was explained to those present, with a request to complete the questionnaire independently.
Each questionnaire was accompanied by a consent form that participants were requested to sign prior to completing the questionnaire. Participants were instructed to place the signed consent forms in a sealed box within the pharmacy. Pharmacists and pharmacist assistants were similarly requested to place their completed anonymized questionnaires in a provided sealed envelope, which was then placed in a separate sealed collection box in the pharmacy. Both sealed boxes were collected at the end of the day or the following day, depending on the logistical feasibility during data collection.
2.3 Data collection instrument
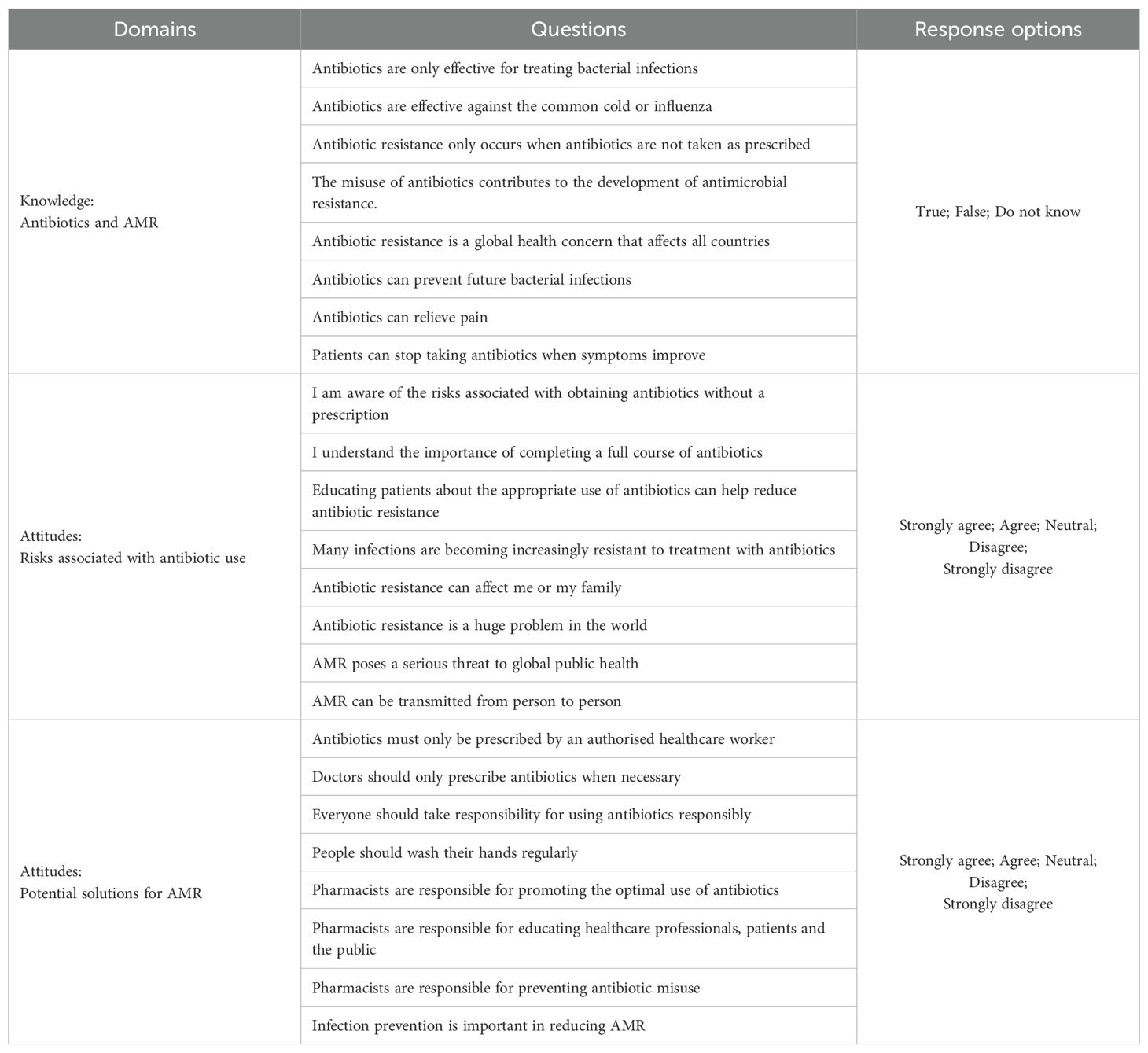
We previously described the development of the self-administered questionnaire (Supplementary Table 1) and reported the pilot study in detail (55). Briefly, the initial questionnaire was based on the published literature, coupled with the considerable knowledge of the co-authors in this area, to cover three principal domains (13, 74–76). The subsequent pilot study ensured that the revised final questionnaire was relevant, comprehensive, understandable, and appropriately structured (55). This was important for meeting the overall objectives of the study. The pilot study confirmed that the questionnaire for the main study could be completed within 10 minutes to enhance participation (55). For the knowledge and attitude domains of the questionnaire, which are reported in this study, key variables related to the knowledge of antibiotics and AMR, as well as attitudes towards the risks associated with antibiotic use. Potential solutions to help reduce AMR in the country were also included in the final questionnaire, with potential health authority activities based on previous publications combined with the considerable knowledge of the co-authors. We have used this approach before in previous studies (12, 13, 15, 29, 49).
Table 2 shows the questions included in the knowledge and attitude domains with respective response options, i.e., ‘True,’ ‘False,’ or ‘Do not know’ for knowledge questions and a 5-point Likert scale ranging from ‘Strongly agree’ to ‘Strongly disagree’ for the attitude domains (77–80). The final questions, divided into three components, were deliberately direct to reduce the chances of ambiguity.
This combined approach, incorporating questionnaire development based on the published literature combined with input from key personnel knowledgeable in the subject area and subsequently tested in a pilot study, is based on the approach used in multiple published studies in this subject area (46, 52, 53, 58, 80, 81).
2.4 Data management and analysis
Data entry was performed using Microsoft Excel™, with the initial entry completed by TMM. To ensure the reliability of data entry, a second individual verified the data accuracy by double-checking the entered data. Prior to analysis, the dataset was subjected to cleaning and coding procedures to standardize the format of variables, address missing values and correct any inconsistencies.
Following these procedures, the final dataset was imported into Jamovi (https://www.jamovi.org/) for statistical analysis. Descriptive statistics included frequencies and percentages for categorical variables and means with standard deviation (SD) for age. For the purpose of summarizing knowledge scores and undertaking statistical analyses, responses were categorized as ‘good knowledge’ and ‘poor knowledge.’ Attitude responses were combined and categorized into two categories: ‘positive attitudes’ (strongly agree; agree) and ‘negative attitudes’ (strongly disagree; disagree; neutral). The Fischers exact test was used to investigate differences in knowledge and attitudes between pharmacists and pharmacist assistants, with p <0.05 considered as statistically significant.
2.5 Ethical considerations
Ethical approval for this study was obtained from the Sefako Makgatho University Research Ethics Committee. Since no data were collected from public health facilities, the National Department of Health was not approached for permission to collect data. Participation in the study was entirely voluntary, with potential participants being informed that they could withdraw at any time without providing a reason for their decision. Before participating in the study, each participant provided written informed consent. To ensure anonymity, signed consent forms were placed in a sealed box separate from the survey responses.
All collected information was treated as confidential, with data stored securely in a password-protected computer and backed up in a cloud service, accessible only to the principal investigator (TMM). Data will be kept securely for 5 years following the publication of the study results and will then be destroyed in accordance with university policies.
3 Results
3.1 Response rate and sample
As reported previously (63), 17 of the 186 pharmacies (9.1%) in this rural province were nonoperational at the time of data collection, leaving 169 operational pharmacies. Among the 169 operational pharmacies, 41 (24.3%) declined to participate for various reasons. The reasons included time constraints, the need for owner approval before participation, or the absence of qualified personnel to complete the questionnaire. This resulted in a 75.7% participation rate at the pharmacy level (128 of 169 operational pharmacies). These were principally independent pharmacies (60.9%, n = 78), with chain and franchise pharmacies accounting for 21.9% (n = 28) and 17.1% (n = 22), respectively, of all pharmacies surveyed.
Overall, 400 questionnaires were distributed among the participating pharmacies, of which 313 completed questionnaires were returned, yielding a 78.3% response rate at the individual questionnaire level. The participants included 106 pharmacists (33.9%) and 207 pharmacist assistants (66.1%). Pharmacist assistants were the sole participants in a minority of chain and franchise pharmacies; however, they were the sole pharmacy personnel present in 37.2% of independent pharmacies.
3.2 Sociodemographic information
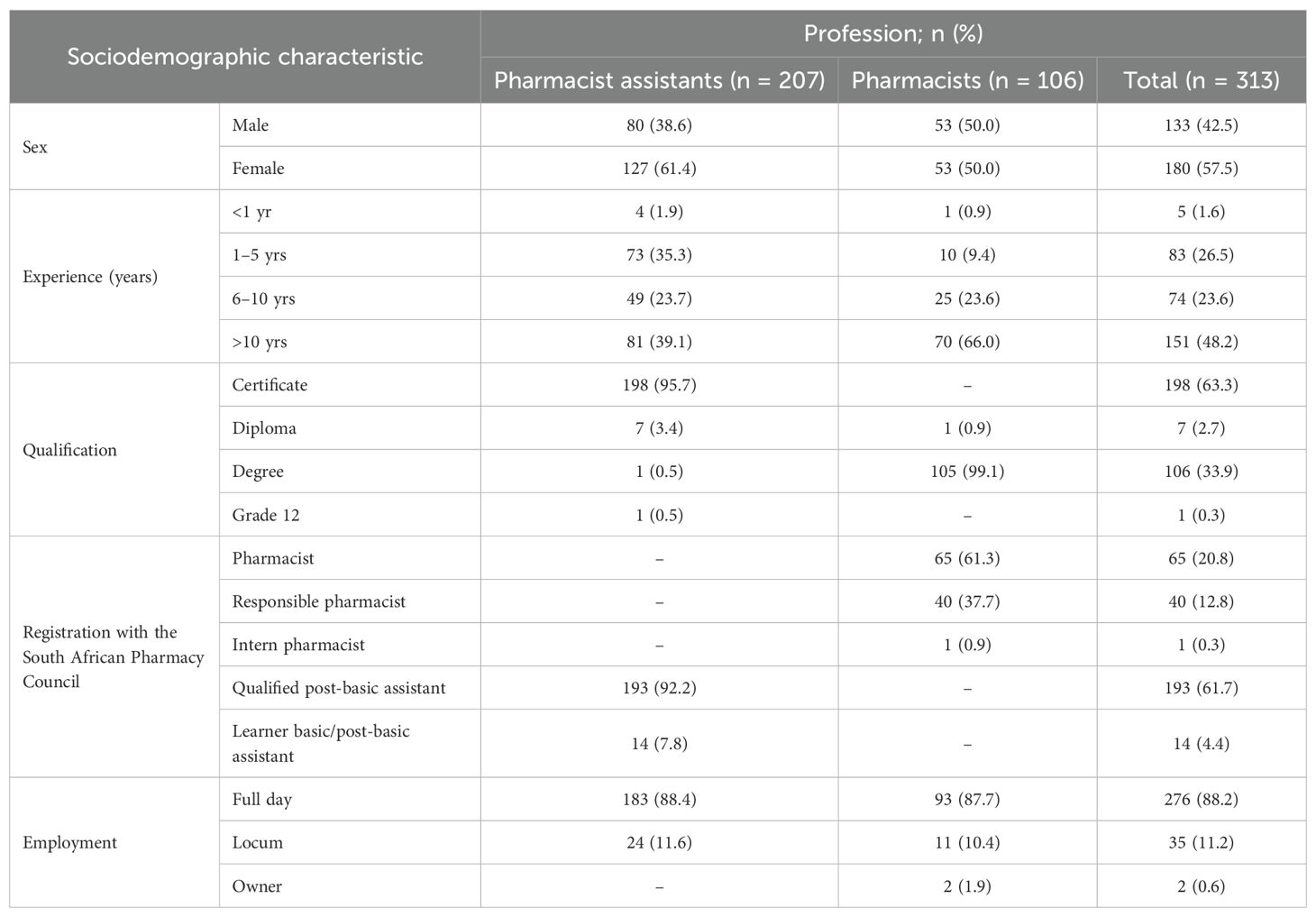
The mean age of the participants in this study was 36.7 (SD: 7.9) years, with the mean age of pharmacist assistants [35.0; SD: 6.1)] being younger than pharmacists [39.9; SD: 9.7)]. There were equal numbers of male and female pharmacists participating in the study, with a higher number of female (127, 61.4%) than male pharmacist assistants (Table 3). Most participants had more than 10 years of experience, with pharmacist assistants typically having only a certificate (95.7%). All participants were registered with SAPC as either pharmacists, post-basic pharmacist assistants, or learner pharmacist assistants, with most employed on a full-time basis (88.2%).
3.3 Knowledge of antibiotics and AMR
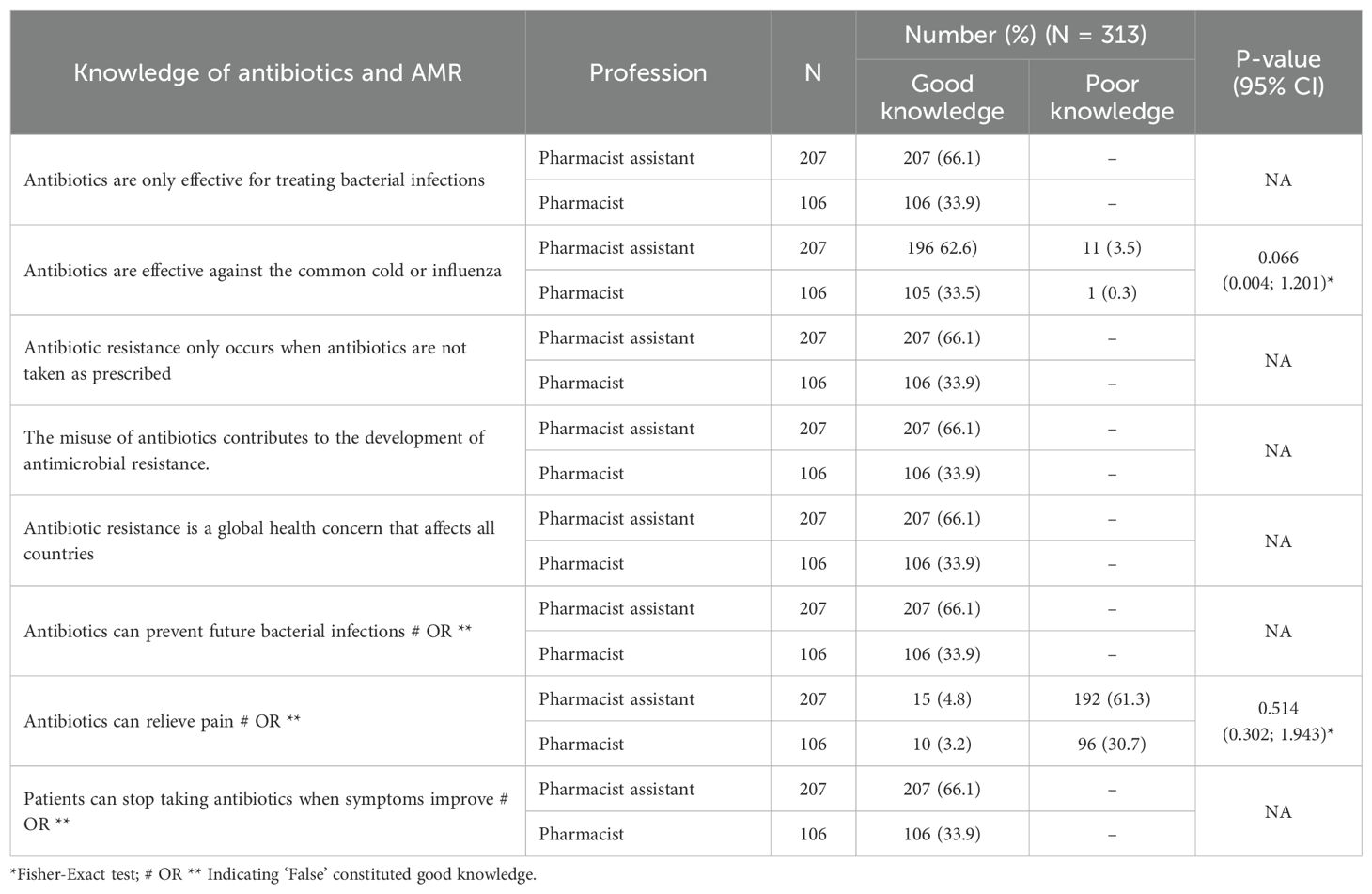
This study assessed the knowledge of pharmacists and pharmacist assistants regarding antibiotics, their use, and their effectiveness. Overall, there was very good knowledge among both community pharmacists and pharmacist assistants concerning these subjects, with no statistically significant difference between the two groups (Table 4). Consequently, no further analyses, including those of potential predictors of misconceptions, were performed.
However, there was a misconception regarding the statement ‘Antibiotics can relieve pain,’ where only 3.2% of participating pharmacists and 3.2% of pharmacist assistants answered this correctly. This indicates a notable misunderstanding of this question, given the high level of correct answers for the other statements and all community pharmacists in the pilot study answering this correctly (55).
3.4 Attitudes toward the risks associated with obtaining antibiotics and potential solutions of antibiotic resistance
Overall, the attitudes of both community pharmacists and pharmacist assistants regarding the risks associated with obtaining antibiotics without a prescription were positive, with no statistically significant differences between the two groups (Table 5). Similarly, there was a strong agreement, with no statistically significant differences identified, among community pharmacists and pharmacist assistants for potential solutions to AMR (Table 5). Consequently, no further analyses were performed.
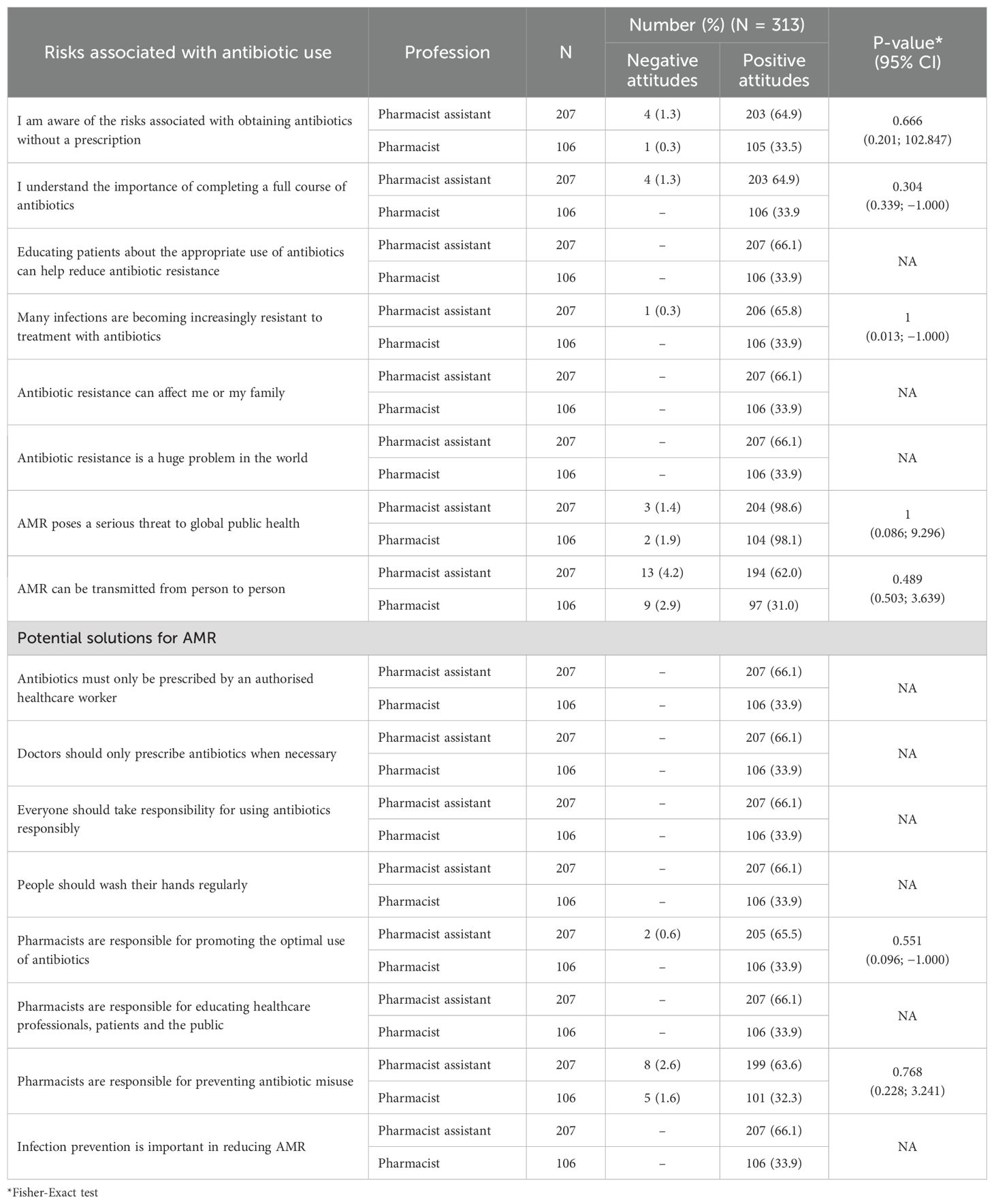
Table 5. Attitudes toward the risks associated with antibiotic use and potential solutions for AMR among pharmacy personnel stratified by profession.
4 Discussion and suggested activities
The study revealed that most participating pharmacists and pharmacist assistants demonstrated a strong understanding of the effectiveness of antibiotics against bacterial infections, coupled with their lack of effectiveness against self-limiting infections, such as colds and influenza (Table 5). This was an improvement from the pilot study, where only 5 of the 14 participating pharmacist assistants agreed that antibiotics cannot treat influenza and that antibiotics should not be prescribed as preventative measures (55); however, similar to the situation among pharmacists. In addition, 100% of the participating pharmacists and pharmacist assistants correctly stated that antibiotics cannot prevent future infections. This is unlike the situation in several African countries (Table 1). The improved knowledge among pharmacist assistants may reflect the outcome of discussions between participating pharmacists and assistants; however, further research is needed before we can make any conclusions with certainty.
Encouragingly, all the surveyed pharmacists and pharmacist assistants believed that antibiotics should not be stopped when patients feel better, which again was an improvement among pharmacist assistants from the pilot (55). This is welcomed since patients in several African countries where affordability is a key issue have stated in several studies that they stop taking antibiotics when they feel better and are happy to share antibiotics with friends and family members to save money (49, 50). This suggests a strong foundation for responsible antibiotic use among pharmacy professionals in this rural province in South Africa, although further research is needed to confirm this. Our findings may also help explain why in the first study of Maluleke et al. (2025) community pharmacists and pharmacist assistants almost always or mostly offered (approximately 98%) symptomatic relief before dispensing antibiotics without a prescription to patients presenting with self-limiting infectious disease symptoms, including acute respiratory infections (63). In addition, approximately half never suggested antibiotics for self-limiting infectious diseases.
However, there was concern that only 9.4% of participating pharmacists and 7.2% of pharmacist assistants correctly answered the question relating to antibiotics being indicated to relieve pain. This may have been a misunderstanding, with participants thinking about infections that cause pain, such as tooth infections, with pain alleviated by antibiotics. However, this needs further research before we can say anything with certainty, especially as all seven community pharmacists in the pilot study answered this question correctly (55).
Encouragingly, both pharmacists and pharmacist assistants had considerable knowledge of the risks associated with obtaining antibiotics without a prescription. This is welcomed and suggests that pharmacy professionals surveyed acknowledge the increasing threat of AMR and the necessity of proactive interventions, including not dispensing antibiotics inappropriately without a prescription, to reduce AMR. Despite this knowledge, this practice still occurred, albeit to a limited extent, among nearly all participating independent pharmacies in this rural province (63). We have seen these differences between knowledge and practice among LMICs (49), which need to be addressed in the future. This includes investigating patients directly as they exit community pharmacies in rural provinces. This is because community pharmacy personnel may underestimate the extent of self-purchasing without a prescription, especially if such activities are illegal (82).
It was reassuring to see that pharmacists and pharmacist assistants recognized their responsibility in promoting the optimal use of antibiotics to members of the public presenting with infectious diseases. This builds on their appreciable knowledge in this area as evidenced by their correct responses. This is to be welcomed, with pharmacists and their assistants recognized as key personnel in healthcare delivery, who are often the first HCPs that patients visit in LMICs with their infectious diseases, as well as other conditions, especially with viral infections such as coughs, colds, and influenza (12, 49, 83–87).
Several activities are suggested to improve future antibiotic use among community pharmacists and pharmacist assistants in this rural province (Box 1), with applicability throughout South Africa and beyond. This is in addition to implementing ASPs to further improve antibiotic use, building on experiences in other countries (Table 6).
Box 1. Suggested activities to improve antibiotic use among community pharmacists and pharmacist assistants.
• Targeted education and training:
o Develop workshops and training sessions for community pharmacists and pharmacist assistants that specifically address potential misconceptions about antibiotics, including their role in pain relief and viral infections. This is particularly important to help them manage inappropriate requests from patients or carers for antibiotics to treat self-limiting conditions such as coughs, colds, and influenza, as well as misconceptions that antibiotics can provide pain relief, which is clearly not the case (49, 50). This approach has worked well in other countries (Table 6).
o Implement structured continuing education programs on antimicrobial stewardship to ensure that pharmacists and pharmacist assistants fully understand their responsibilities in improving the future use of antibiotics in South Africa, given current concerns. This includes the WHO AWaRe system and related guidance given concerns with the appreciable availability and dispensing of antibiotics from the WHO Watch list seen among community pharmacies across LMICs (49, 88–91), with increasing use of antibiotics from the WHO Watch list in the public healthcare system in South Africa in recent years (68).
o Trained community pharmacists can also support audits of prescribers to improve their antibiotic use, building on ongoing examples in South Africa (92).
• Public awareness campaigns:
o Support patient education programs in community pharmacies that focus on the importance of completing antibiotic courses and avoiding inappropriate self-medication (48, 49).
o Distribute informative materials in pharmacies and healthcare settings on AMR prevention strategies to promote appropriate antibiotic use among patients.
• Policy and regulatory srengthening:
o Advocate for stricter regulations on antibiotic dispensing, to enhance compliance with prescribing requirements. This can include more frequent inspections and potentially fines for pharmacy personnel, including responsible pharmacists and pharmacy owners, for selling antibiotics without a prescription (Table 6). However, if fines for community pharmacy personnel are too low, they may have limited impact as seen in Vietnam (93). Conversely, if fines are excessive, they may lead to the closure of community pharmacies and greater reliance on the informal sector, which is not ideal due to increased inappropriate dispensing of antibiotics, including substandard and falsified antibiotics (50, 94). Any potential fines though must be balanced against concerns with current excessive prescribing of antibiotics in primary care across South Africa including for self-limiting infections such as URTIs (Supplementary Table 2). This practice was not seen among community pharmacists and pharmacist assistants (63). Alongside this, instigating the Medicines and Related Substances Act Section 22A(15) in recent years has seen the scope of pharmacists activities increasing with pharmacists' prescribing via Primary Care Drug Therapy (PCDT) expanded for specific primary care conditions, including infectious syndromes governed by the Primary Health Care Standard Treatment Guidelines (95). The next step includes a regulatory pilot for pharmacist-initiated dispensing of selected antibiotics such as single-dose fosfomycin for uncomplicated urinary tract infections in women. This mirrors the structured, evidence-based protocols operationalized in the UK’s Pharmacy First service. Current informal sales of antibiotics when requested without a prescription whilst illegal in South Africa reflect consumer needs alongside health system barriers. Consequently, a formalized, pharmacist-led access route with stewardship controls provides a pragmatic, safer alternative that also aligns with equity and national health priorities. These prescribing models must though entail explicit protocols, patient eligibility checklists, and robust post-dispensing surveillance, to ensure their safe use and minimise AMR risk.
o In countries such as the United Kingdom, pharmacists can dispense antibiotics for agreed infections, serving as an exemplar for the authorities in South Africa (13) especially given concerns with current excessive prescribing of antibiotics among prescribers in primary healthcare clinics in South Africa including for self-limiting infections such as URTIs (Supplementary Table 2).
• Enhance monitoring systems/IT infrastructure
o Seek to introduce IT systems to track antibiotics through the supply chain and reduce inappropriate antibiotic sales across all community pharmacies, particularly in rural areas. Such practices will also help limit the circulation of counterfeit antibiotics, which is a concern across Africa, although less so in South Africa (50, 94).
o Introducing IT systems will also support real-time monitoring of antibiotic use, allowing for the implementation of agreed quality indicators based on the WHO AWaRe classification to improve future use (96).
• Collaborative stewardship Initiatives:
o Strengthen interprofessional collaboration among pharmacists, other healthcare professionals, and government public health officials to ensure responsible antibiotic prescribing.
o Encourage pharmacies to engage in community outreach programs that reinforce responsible antibiotic use and infection prevention. South Africa can draw on global experiences including England’s Pharmacy First and successes from pharmacists involved in stewardship initiatives (Table 6) to inform the creation of a legal pathway for pharmacists prescribing and dispensing specified antibiotics for defined indications, e.g. fosfomycin for acute uncomplicated cystitis representing a high-impact, low-risk pilot situation given current concerns with high levels of inappropriate prescribing of antibiotics in primary care (Supplementary Table 2).
NB: AMR = Antimicrobial resistance; AWaRe: Access, Watch and Reserve; LMICs = Low- and Middle-Income Countries; URTIs = Upper Respiratory Tract Infections.
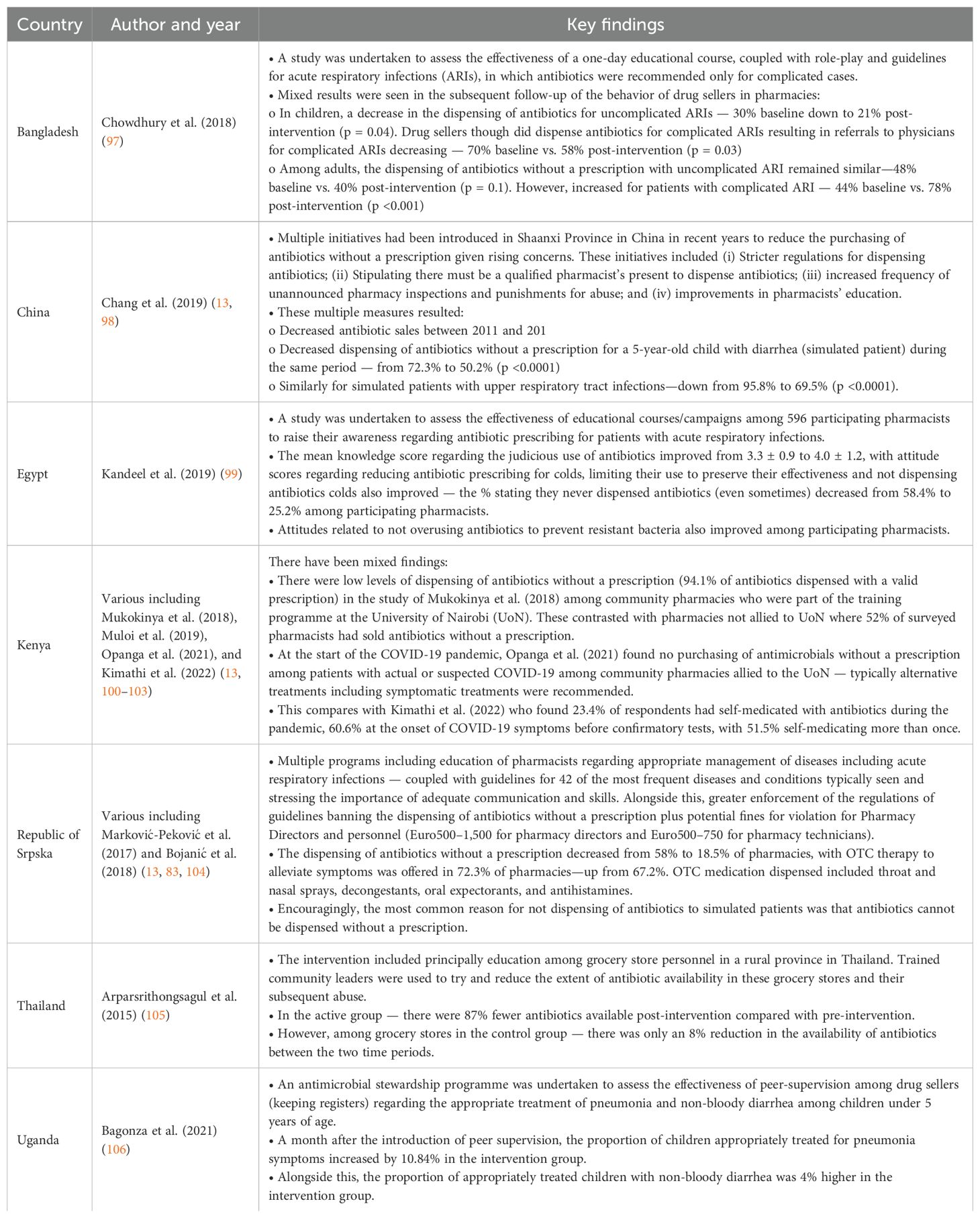
Table 6. Antimicrobial Stewardship Programmes among community pharmacies and drug stores across LMICs including the effectiveness of educational campaigns.
We acknowledge several limitations of this study. Firstly, not all community pharmacists and their assistants in this rural province of South Africa participated in the study. However, 75.7% of the community pharmacies approached participated, adding to the robustness of the findings. Secondly, we where unable to verify the accuracy of the replies from the pharmacists or pharmacist assistants for their accuracy. This is an issue given some differences observed between the pilot and main study, including knowledge-related responses and answers to the question regarding antibiotics and pain relief. However, this limitation is common in self-administered questionnaires. In addition, we did not question pharmacists or their assistants directly, which may itself have introduced bias. Despite these limitations, we are confident in the findings and their implications for improving antibiotic utilization in this rural province and throughout South Africa.
5 Conclusion
We believe this study provides valuable insights into the knowledge and perceptions of community pharmacists and pharmacist assistants regarding antibiotics, AMR, and AMS in a rural province of South Africa. Overall, the findings showed that most pharmacists and pharmacist assistants in this rural province demonstrated a strong understanding of the effectiveness of antibiotics in treating bacterial infections and their lack of effectiveness in treating viral infections. The participating pharmacists and pharmacist assistants also demonstrated considerable knowledge of the risks associated with inappropriate dispensing of antibiotics without a prescription, as well as strategies to address rising AMR rates. However, some of the replies raised concerns. The next stages of the research will include assessing the situation among patients, including their experience with purchasing antibiotics without a prescription, as well as their knowledge and attitudes toward antibiotics, AMR, and AMS. The combined findings can be used to develop pertinent future polices for all key stakeholder groups in South Africa and across Africa to improve antibiotic dispensing and reduce AMR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Sefako Makgatho University Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TM: Investigation, Data curation, Methodology, Writing – review & editing, Conceptualization, Validation, Formal analysis, Visualization, Writing – original draft. MM: Writing – review & editing, Validation, Investigation, Formal analysis, Methodology, Data curation. AG: Conceptualization, Validation, Methodology, Data curation, Writing – review & editing. SC: Data curation, Validation, Methodology, Conceptualization, Investigation, Formal analysis, Writing – review & editing. VM-P: Methodology, Writing – review & editing, Validation, Formal analysis, Conceptualization, Data curation. NS: Data curation, Investigation, Conceptualization, Methodology, Writing – review & editing, Formal analysis. NR: Validation, Data curation, Methodology, Writing – review & editing, Conceptualization. BG: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Validation, Writing - original draft, Writing – review & editing. JM: Methodology, Formal analysis, Visualization, Investigation, Data curation, Supervision, Validation, Writing – review & editing, Conceptualization.
Funding
The authors declare financial support was received for the research and/or publication of this article. South African National Research Foundation (NRF) Grants Reference: MND210917640292UID and Reference: SRUG200509520910; Grant No: 129365.
Acknowledgments
We sincerely thank Veronica Mboweni and Ntwanano Eulander Sono for their assistance with the data collection for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors BG.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2025.1637843/full#supplementary-material
References
1. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
2. Naghavi M, Vollset SE, Ikuta KS, Swetschinski LR, Gray AP, Wool EE, et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet. (2024) 404:1199–226. doi: 10.1016/S0140-6736(24)01867-1
3. Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributa ble deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. (2019) 19:56–66. doi: 10.1016/S1473-3099(18)30605-4
4. Gautam A. Antimicrobial resistance: the next probable pandemic. JNMA J Nepal Med Assoc. (2022) 60:225–8. doi: 10.31729/jnma.7174
6. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. (2019) 12:3903–10. doi: 10.2147/IDR.S234610
7. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol. (2019) 17:3. doi: 10.1038/s41579-018-0125-x
8. Lewnard JA, Charani E, Gleason A, Hsu LY, Khan WA, Karkey A, et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis. Lancet. (2024) 403:2439–54. doi: 10.1016/S0140-6736(24)00862-6
9. Antimicrobial Resistance Collaborators. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Health. (2024) 12:e201–e16. doi: 10.1016/S2214-109X(23)00539-9
10. Totaro V, Guido G, Cotugno S, De Vita E, Asaduzzaman M, Patti G, et al. Antimicrobial resistance in sub-saharan africa: A comprehensive landscape review. Am J Trop Med hygiene. (2025) 113:253–63. doi: 10.4269/ajtmh.25-0035
11. Lubanga AF, Bwanali AN, Kambiri F, Harawa G, Mudenda S, Mpinganjira SL, et al. Tackling antimicrobial resistance in sub-Saharan Africa: challenges and opportunities for implementing the new people-centered WHO guidelines. Expert Rev Anti Infect Ther. (2024) 22:379–86. doi: 10.1080/14787210.2024.2362270
12. Godman B, Haque M, McKimm J, Abu Bakar M, Sneddon J, Wale J, et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin. (2020) 36:301–27. doi: 10.1080/03007995.2019.1700947
13. Sono TM, Yeika E, Cook A, Kalungia A, Opanga SA, Acolatse JEE, et al. Current rates of purchasing of antibiotics without a prescription across sub-Saharan Africa; rationale and potential programmes to reduce inappropriate dispensing and resistance. Expert Rev Anti Infect Ther. (2023) 21:1025–55. doi: 10.1080/14787210.2023.2259106
14. Llor C and Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. (2014) 5:229–41. doi: 10.1177/2042098614554919
15. Godman B, Egwuenu A, Haque M, Malande OO, Schellack N, Kumar S, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. (2021) 11:528. doi: 10.3390/life11060528
16. Torres NF, Chibi B, Kuupiel D, Solomon VP, Mashamba-Thompson TP, and Middleton LE. The use of non-prescribed antibiotics; prevalence estimates in low-and-middle-income countries. A systematic Rev meta-analysis. Arch Public Health. (2021) 79:2. doi: 10.1186/s13690-020-00517-9
17. Auta A, Hadi MA, Oga E, Adewuyi EO, Abdu-Aguye SN, Adeloye D, et al. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J Infect. (2019) 78:8–18. doi: 10.1016/j.jinf.2018.07.001
18. Sono TM, Markovic-Pekovic V, and Godman B. Effective programmes to reduce inappropriate dispensing of antibiotics in community pharmacies especially in developing countries. Adv Hum Biol. (2024) 14:1–4. doi: 10.4103/aihb.aihb_128_23
19. Yeika EV, Ingelbeen B, Kemah BL, Wirsiy FS, Fomengia JN, and van der Sande MAB. Comparative assessment of the prevalence, practices and factors associated with self-medication with antibiotics in Africa. Trop Med Int Health. (2021) 26:862–81. doi: 10.1111/tmi.13600
20. Ndaki PM, Mushi MF, Mwanga JR, Konje ET, Mugassa S, Manyiri MW, et al. Non-prescribed antibiotic dispensing practices for symptoms of urinary tract infection in community pharmacies and accredited drug dispensing outlets in Tanzania: a simulated clients approach. BMC Prim Care. (2022) 23:287. doi: 10.1186/s12875-022-01905-6
21. Essack S. Water, sanitation and hygiene in national action plans for antimicrobial resistance. Bull World Health Organ. (2021) 99:606–8. doi: 10.2471/BLT.20.284232
22. Collignon P, Beggs JJ, Walsh TR, Gandra S, and Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. (2018) 2:e398–405. doi: 10.1016/S2542-5196(18)30186-4
23. Ljungqvist G, van Kessel R, Mossialos E, Saint V, Schmidt J, Mafi A, et al. Mapping socioeconomic factors driving antimicrobial resistance in humans: An umbrella review. One Health. (2025) 20:100986. doi: 10.1016/j.onehlt.2025.100986
24. Laxminarayan R, Impalli I, Rangarajan R, Cohn J, Ramjeet K, Trainor BW, et al. Expanding antibiotic, vaccine, and diagnostics development and access to tackle antimicrobial resistance. Lancet. (2024) 403:2534–50. doi: 10.1016/S0140-6736(24)00878-X
25. WHO. Global action plan on antimicrobial resistance (2016). Available online at: https://www.who.int/publications/i/item/9789241509763 (Accessed May 9 2025).
26. OECD Health Policy Studies. Stemming the superbug tide (2018). Available online at: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html (Accessed May 10, 2025).
27. The Fleming Fund. Fleming Fund Online AMR Course: new modules (2021). Available online at: https://www.flemingfund.org/publications/fleming-fund-online-amr-course/ (Accessed May 10, 2025).
28. Sneddon J, Drummond F, Guise T, Gilchrist M, and Jenkins DR. Accreditation of antimicrobial stewardship programmes: addressing a global need to tackle antimicrobial resistance. JAC-Antimicrobial Resistance. (2024) 6:dlae007. doi: 10.1093/jacamr/dlae007
29. Godman B, Egwuenu A, Wesangula E, Schellack N, Kalungia AC, Tiroyakgosi C, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Safety. (2022) 21:1089–111. doi: 10.1080/14740338.2022.2106368
30. Sariola S, Butcher A, Cañada JA, Aïkpé M, and Compaore A. Closing the GAP in antimicrobial resistance policy in Benin and Burkina Faso. mSystems. (2022) 7:e0015022. doi: 10.1128/msystems.00150-22
31. Pallett SJ, Charani E, Hawkins L, Mazzella A, Anton-Vazquez V, Banerjee R, et al. National action plans for antimicrobial resistance and variations in surveillance data platforms. Bull World Health Organ. (2023) 101:501–12f. doi: 10.2471/BLT.22.289403
32. Mukoko J, Wesangula E, Gitonga N, Kusu N, Odhiambo C, Tanui E, et al. Kenya’s National Action Plan on antimicrobial resistance: measuring implementation progress. Frontiers. Trop Dis. (2025) 6:1540713. doi: 10.3389/fitd.2025.1540713
33. Department of Health Republic of South Africa. South african antimicrobial resistance national strategy framework; A one health approach - 2017 – 2024 (017). Available online at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-03/AMR%20National%20Action%20Plan%202018%20-%202024.pdf (Accessed May 9, 2025).
34. WHO implementation handbook for national action plans on antimicrobial resistance: guidance for the human health sector (2022). Available online at: https://www.who.int/publications/i/item/9789240041981 (Accessed May 10, 2025).
35. Mendelson M, Brink A, Gouws J, Mbelle N, Naidoo V, Pople T, et al. The One Health stewardship of colistin as an antibiotic of last resort for human health in South Africa. Lancet Infect Dis. (2018) 18:e288–e94. doi: 10.1016/S1473-3099(18)30119-1
36. Department of Health Republic of South Africa. Surveillance for antimicrobial resistance (2020). Available online at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-03/Guide%20to%20access%20the%20National%20AMR%20Surveillance%20Dashboard.pdf (Accessed May 9, 2025).
37. Departments of Health and Agriculture. Forestry and fisheries for the republic of South Africa: antimicrobial resistance national strategy framework (2017 – 2024). Available online at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-03/AMR%20National%20Action%20Plan%202018%20-%202024.pdf (Accessed May 10, 2025).
38. Engler D, Meyer JC, Schellack N, Kurdi A, and Godman B. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: are we there yet? J Chemother. (2021) 33:21–31. doi: 10.1080/1120009X.2020.1789389
39. Department of Health Republic of South Africa. Practical manual for implementation of the national infection prevention and control strategic framework (2020). Available online at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-04/Practical%20Manual%20for%20implementation%20of%20the%20National%20IPC%20Strategic%20Framework%20March%202020.pdf (Accessed May 9, 2025).
40. Engler D, Meyer JC, Schellack N, Kurdi A, and Godman B. Antimicrobial stewardship activities in public healthcare facilities in South Africa: A baseline for future direction. Antibiotics. (2021) 10:996. doi: 10.3390/antibiotics10080996
41. Department of Health Republic of South Africa. Become an antibiotic guardian (2022). Available online at: https://antibioticguardian.com/south-africa/ (Accessed May 10, 2025).
42. Loosli K, Davis A, Muwonge A, and Lembo T. Addressing antimicrobial resistance by improving access and quality of care-A review of the literature from East Africa. PloS Negl Trop Dis. (2021) 15:e0009529. doi: 10.1371/journal.pntd.0009529
43. Demissie F, Ereso K, and Paulos G. Self-medication practice with antibiotics and its associated factors among community of bule-hora town, south west Ethiopia. Drug Healthc Patient Saf. (2022) 14:9–18. doi: 10.2147/DHPS.S325150
44. Ngyedu EK, Acolatse J, Akafity G, Incoom R, Rauf A, Seaton RA, et al. Selling antibiotics without prescriptions among community pharmacies and drug outlets: a simulated client study from Ghana. Expert Rev Anti-infective Ther. (2023) 21:1373–82. doi: 10.1080/14787210.2023.2283037
45. Simon B and Kazaura M. Prevalence and factors associated with parents self-medicating under-fives with antibiotics in bagamoyo district council, Tanzania: a cross-sectional study. Patient Prefer Adherence. (2020) 14:1445–53. doi: 10.2147/PPA.S263517
46. Torres NF, Solomon VP, and Middleton LE. Identifying the commonly used antibiotics for self-medication in urban Mozambique: a qualitative study. BMJ Open. (2020) 10:e041323. doi: 10.1136/bmjopen-2020-041323
47. Kalungia AC, Burger J, Godman B, Costa JO, and Simuwelu C. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev Anti Infect Ther. (2016) 14:1215–23. doi: 10.1080/14787210.2016.1227702
48. Ramdas N, Meyer JC, Schellack N, Godman B, Turawa EB, and Campbell SM. Knowledge, attitudes, motivations and expectations regarding antimicrobial use among community members seeking care at the primary healthcare level: a scoping review protocol. BMJ Open. (2025) 15:e088769. doi: 10.1136/bmjopen-2024-088769
49. Saleem Z, Moore CE, Kalungia AC, Schellack N, Ogunleye O, Chigome A, et al. Status and implications of the knowledge, attitudes and practices towards AWaRe antibiotic use, resistance and stewardship among low- and middle-income countries. JAC-Antimicrobial Resistance. (2025) 7:dlaf033. doi: 10.1093/jacamr/dlaf033
50. Saleem Z, Mekonnen BA, Orubu ES, Islam MA, Nguyen TTP, Ubaka CM, et al. Current access, availability and use of antibiotics in primary care among key low- and middle-income countries and the policy implications. Expert Rev Anti Infect Ther. (2025), 1–42. doi: 10.1080/14787210.2025.2477198
51. Ramdas N, Biyela T, Thema M, Sibanda M, Sono TM, Campbell SM, et al. Patient knowledge, attitudes and behaviors related to antimicrobial use in South African primary healthcare settings: development and testing of the CAMUS and its implications. Front Trop Diseases. (2025) 6:1569076. doi: 10.3389/fitd.2025.1569076
52. Belachew SA, Hall L, and Selvey LA. Community drug retail outlet staff’s knowledge, attitudes and practices towards non-prescription antibiotics use and antibiotic resistance in the Amhara region, Ethiopia with a focus on non-urban towns. Antimicrob Resist Infect Control. (2022) 11:64. doi: 10.1186/s13756-022-01102-1
53. Gacheri J, Hamilton KA, Munywoki P, Wakahiu S, Kiambi K, Fèvre EM, et al. Antibiotic prescribing practices in community and clinical settings during the COVID-19 pandemic in Nairobi, Kenya. PloS Glob Public Health. (2024) 4:e0003046. doi: 10.1371/journal.pgph.0003046
54. Akpan RM, Udoh EI, Akpan SE, and Ozuluoha CC. Community pharmacists’ management of self-limiting infections: a simulation study in Akwa Ibom State, South-South Nigeria. Afr Health Sci. (2021) 21:576–84. doi: 10.4314/ahs.v21i2.12
55. Sono TM, Maluleke MT, Jelić AG, Campbell S, Marković-Peković V, Schellack N, et al. Potential strategies to limit inappropriate purchasing of antibiotics without a prescription in a rural province in South Africa: pilot study and the implications. Adv Hum Biol. (2024) 14:60–7. doi: 10.4103/aihb.aihb_127_23
56. Horumpende PG, Sonda TB, van Zwetselaar M, Antony ML, Tenu FF, Mwanziva CE, et al. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PloS One. (2018) 13:e0207465. doi: 10.1371/journal.pone.0207465
57. Ndaki PM, Mushi MF, Mwanga JR, Konje ET, Ntinginya NE, Mmbaga BT, et al. Dispensing Antibiotics without Prescription at Community Pharmacies and Accredited Drug Dispensing Outlets in Tanzania: A Cross-Sectional Study. Antibiotics. (2021) 10:1025. doi: 10.3390/antibiotics10081025
58. Mudenda S, Hankombo M, Saleem Z, Sadiq MJ, Banda M, Munkombwe D, et al. Knowledge, attitude, and practices of community pharmacists on antibiotic resistance and antimicrobial stewardship in lusaka, Zambia. J BioMed Res Environ Sci. (2021) 2:1005–14. doi: 10.37871/jbres1343
59. Anstey Watkins J, Wagner F, Xavier Gómez-Olivé F, Wertheim H, Sankoh O, and Kinsman J. Rural South African community perceptions of antibiotic access and use: qualitative evidence from a health and demographic surveillance system site. Am J Trop Med Hyg. (2019) 100:1378–90. doi: 10.4269/ajtmh.18-0171
60. Do NTT, Vu HTL, Nguyen CTK, Punpuing S, Khan WA, Gyapong M, et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health. (2021) 9:e610–e9. doi: 10.1016/S2214-109X(21)00024-3
61. Mokwele RN, Schellack N, Bronkhorst E, Brink AJ, Schweickerdt L, and Godman B. Using mystery shoppers to determine practices pertaining to antibiotic dispensing without a prescription among community pharmacies in South Africa—a pilot survey. JAC-Antimicrobial Resistance. (2022) 4:dlab196. doi: 10.1093/jacamr/dlab196
62. Sono TM, Maluleke MT, Ramdas N, Jelic AG, Campbell S, Markovic-Pekovic V, et al. Pilot study to evaluate the feasibility of a patient questionnaire for the purpose of investigating the extent of purchasing antibiotics without a prescription in a rural province in South Africa: rationale and implications. Adv Hum Biol. (2024) 14:138–47. doi: 10.4103/aihb.aihb_140_23
63. Maluleke TM, Maluke MT, Jelić AG, Campbell SM, Marković-Peković V, Schellack N, et al. Estimated extent of purchasing of antibiotics without a prescription from community pharmacies in a rural province in South Africa and the implications. Front Trop Dis. (2025) 6. doi: 10.3389/fitd.2025.1637362/abstract
64. Schellack N, Coetzee M, Schellack G, Gijzelaar M, Hassim Z, Milne M, et al. COVID-19: guidelines for pharmacists in South Africa. S Afr J Infect Dis. (2020) 35:206. doi: 10.4102/sajid.v35i1.206
65. Ogunleye OO, Basu D, Mueller D, Sneddon J, Seaton RA, Yinka-Ogunleye AF, et al. Response to the novel corona virus (COVID-19) pandemic across africa: successes, challenges, and implications for the future. Front Pharmacol. (2020) 11:1205. doi: 10.3389/fphar.2020.01205
66. Bachoolall R and Suleman F. Community pharmacists’ perceptions and experiences of medicine shortages in disruptive situations: a qualitative study. Int J Clin Pharm. (2025) 47:210–7. doi: 10.1007/s11096-024-01799-7
67. Van Hecke PO, Adegoke DY, von Pressentin PK, Namane PM, Mendelson PM, Butler PC, et al. Impact of pharmacist-prescriber partnerships to track antibiotic prescribing in publicly funded primary care in the Cape Town Metropole, South Africa: an implementation study. Int J Infect Diseases. (2025) 152:107626. doi: 10.1016/j.ijid.2024.107626
68. Department of Health and Republic of South Africa. Surveillance for antimicrobial resistance and consumption of antibiotics in South Africa 2018-2022 (2024). Available online at: https://www.nicd.ac.za/wp-content/uploads/2024/04/South-African-AMR-Surveillance-Report-2022.pdf (Accessed May 10, 2025).
69. Sulis G, Sayood S, Katukoori S, Bollam N, George I, Yaeger LH, et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: a systematic review and meta-analysis. Clin Microbiol Infect. (2022) 28:1193–202. doi: 10.1016/j.cmi.2022.03.014
70. Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis. (2021) 21:107–15. doi: 10.1016/S1473-3099(20)30332-7
71. Mendelson M, van Vuuren M, Govind C, Brink AJ, Schellack N, du Plessis NM, et al. Urgent need to reinstate a national action plan and scientific advisory body on antimicrobial resistance in South Africa (2025). Available online at: https://groundup.org.za/media/uploads/documents/open_letter_to_minister_motsoaledi_final-20250619.pdf (Accessed July 1, 2025).
72. Unisasapplication. Where to study pharmacist assistant course in South Africa (2025). Available online at: https://unisasapplication.co.za/where-to-study-pharmacist-assistant-course-in-south-africa/ (Accessed May 10, 2025).
73. Dakarai K. Top 3 pharmacy assistant courses in South Africa (2024). Available online at: https://coursesouthafrica.co.za/pharmacy-assistant-courses-in-south-africa/ (Accessed May 10, 2025).
74. Alduraibi RK and Altowayan WM. A cross-sectional survey: knowledge, attitudes, and practices of self-medication in medical and pharmacy students. BMC Health Serv Res. (2022) 22:352. doi: 10.1186/s12913-022-07704-0
75. Raees I, Atif HM, Aslam S, Mustafa ZU, Meyer JC, Hayat K, et al. Understanding of final year medical, pharmacy and nursing students in Pakistan towards antibiotic use, antimicrobial resistance and stewardship: findings and implications. Antibiotics. (2023) 12:135. doi: 10.3390/antibiotics12010135
76. Zawahir S and Lekamwasam. S, aslani P. A. cross-sectional Natl survey Community Pharm staff: Knowledge antibiotic provision. PloS One. (2019) 14:e0215484. doi: 10.1371/journal.pone.0215484
77. Orubu ESF, Albeik S, Ching C, Hussein R, Mousa A, Horino M, et al. A survey assessing antimicrobial prescribing at united nations relief and works agency primary health care centers in Jordan. Am J Trop Med Hyg. (2022) 107:474–83. doi: 10.4269/ajtmh.22-0042
78. Kandasamy G, Sivanandy P, Almaghaslah D, Khobrani M, Chinnadhurai M, Vasudevan R, et al. Knowledge, attitude, perception and practice of antibiotics usage among the pharmacy students. Int J Clin Pract. (2020) 74:e13599. doi: 10.1111/ijcp.13599
79. Christanti JV, Setiadi AP, Wibowo YI, Presley B, Halim SV, Setiawan E, et al. A cross-sectional assessment of Indonesian female health cadres’ knowledge and attitude towards antibiotics. J Infect Dev Ctries. (2021) 15:1453–61. doi: 10.3855/jidc.14325
80. Mudenda S, Bangara F, Sitali L, and Banda M. Knowledge, Attitude, and practices on antibiotic resistance among pharmacists at the university teaching hospitals in lusaka, Zambia. J Harmonized Res Pharmacy. (2019) 8:12–24.
81. Mate I, Come CE, Gonçalves MP, Cliff J, and Gudo ES. Knowledge, attitudes and practices regarding antibiotic use in Maputo City, Mozambique. PloS One. (2019) 14:e0221452. doi: 10.1371/journal.pone.0221452
82. Edessa D, Assefa N, Dessie Y, Asefa F, Dinsa G, and Oljira L. Non-prescribed antibiotic use for children at community levels in low- and middle-income countries: a systematic review and meta-analysis. J Pharm Policy Pract. (2022) 15:57. doi: 10.1186/s40545-022-00454-8
83. Marković-Peković V, Grubiša N, Burger J, Bojanić L, and Godman B. Initiatives to reduce nonprescription sales and dispensing of antibiotics: findings and implications. J Res Pharm Pract. (2017) 6:120–5. doi: 10.4103/jrpp.JRPP_17_12
84. Gentilini A, Kasonde L, and Babar ZU. Expanding access to NCD services via community retail pharmacies in LMICs: a systematic review of the literature. J Pharm Policy Pract. (2025) 18:2462450. doi: 10.1080/20523211.2025.2462450
85. Babar ZU. Ten recommendations to improve pharmacy practice in low and middle-income countries (LMICs). J Pharm Policy Pract. (2021) 14:6. doi: 10.1186/s40545-020-00288-2
86. Cadogan CA and Hughes CM. On the frontline against COVID-19: Community pharmacists’ contribution during a public health crisis. Res Soc Adm Pharm. (2021) 17:2032–5. doi: 10.1016/j.sapharm.2020.03.015
87. Hedima EW, Adeyemi MS, and Ikunaiye NY. Community Pharmacists: On the frontline of health service against COVID-19 in LMICs. Res Soc Adm Pharm. (2021) 17:1964–6. doi: 10.1016/j.sapharm.2020.04.013
88. Sharland M, Zanichelli V, Ombajo LA, Bazira J, Cappello B, Chitatanga R, et al. The WHO essential medicines list AWaRe book: from a list to a quality improvement system. Clin Microbiol Infect. (2022) 28:1533–5. doi: 10.1016/j.cmi.2022.08.009
89. Zanichelli V, Sharland M, Cappello B, Moja L, Getahun H, Pessoa-Silva C, et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ. (2023) 101:290–6. doi: 10.2471/BLT.22.288614
90. Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. (2019) 19:1278–80. doi: 10.1016/S1473-3099(19)30532-8
91. Abdullah S, Zikria S, Brian G, Khurshid HF, Abdul H, et al. Surge of branded generics and antimicrobial resistance: analyzing the antibiotic market dynamics in Pakistan through the WHO essential medicines and AWaRe lens. In: Expert review of anti-infective therapy. (2025) 23:513–21.
92. Van Hecke O, Adegoke Y, Allwood M, von Pressentin K, Namane M, Butler C, et al. Impact of pharmacist-prescriber partnerships to track antibiotic prescribing in publicly funded primary care in the Cape Town metropole, South Africa: An implementation study. South Afr Med J. (2024) 114:e1914. doi: 10.7196/SAMJ.2024.v114i12.1914
93. Nguyen TTP, Do TX, Nguyen HA, Nguyen CTT, Meyer JC, Godman B, et al. A national survey of dispensing practice and customer knowledge on antibiotic use in Vietnam and the implications. Antibiotics. (2022) 11:1091. doi: 10.3390/antibiotics11081091
94. Asrade Mekonnen B, Getie Yizengaw M, and Chanie Worku M. Prevalence of substandard, falsified, unlicensed and unregistered medicine and its associated factors in Africa: a systematic review. J Pharm Policy Pract. (2024) 17:2375267. doi: 10.1080/20523211.2024.2375267
95. Tromp M, Truter I, and du Toit J. Primary care drug therapy pharmacists in South Africa: Practice settings and conditions treated. Explor Res Clin Soc Pharm. (2023) 12:100352. doi: 10.1016/j.rcsop.2023.100352
96. Funiciello E, Lorenzetti G, Cook A, Goelen J, Moore CE, Campbell SM, et al. Identifying AWaRe indicators for appropriate antibiotic use: a narrative review. J Antimicrob Chemother. (2024) 79:3063–77. doi: 10.1093/jac/dkae370
97. Chowdhury F, Sturm-Ramirez K, Mamun AA, Iuliano AD, Chisti MJ, Ahmed M, et al. Effectiveness of an educational intervention to improve antibiotic dispensing practices for acute respiratory illness among drug sellers in pharmacies, a pilot study in Bangladesh. BMC Health Serv Res. (2018) 18:676. doi: 10.1186/s12913-018-3486-y
98. Chang J, Xu S, Zhu S, Li Z, Yu J, Zhang Y, et al. Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: a mixed cross-sectional and longitudinal study. Lancet Infect Dis. (2019) 19:1345–54. doi: 10.1016/S1473-3099(19)30324-X
99. Kandeel A, Palms DL, Afifi S, Kandeel Y, Etman A, Hicks LA, et al. An educational intervention to promote appropriate antibiotic use for acute respiratory infections in a district in Egypt- pilot study. BMC Public Health. (2019) 19:498. doi: 10.1186/s12889-019-6779-0
100. Mukokinya MMA, Opanga S, Oluka M, and Godman B. Dispensing of antimicrobials in Kenya: A cross-sectional pilot study and its implications. J Res Pharm Pract. (2018) 7:77–82. doi: 10.4103/jrpp.JRPP_17_88
101. Opanga S, Rizvi N, Wamaitha A, Abebrese Sefah I, and Godman BB. Availability of medicines in community pharmacy to manage patients with COVID-19 in Kenya; pilot study and implications. Sch Acad J Pharm. (2021) 3:36–42. doi: 10.36347/sajp.2021.v10i03.001
102. Kimathi G, Kiarie J, Njarambah L, Onditi J, and Ojakaa D. A cross-sectional study of antimicrobial use among self-medicating COVID-19 cases in Nyeri County, Kenya. Antimicrob Resist Infect Control. (2022) 11:111. doi: 10.1186/s13756-022-01150-7
103. Muloi D, Fèvre EM, Bettridge J, Rono R, Ong’are D, Hassell JM, et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J Glob Health. (2019) 9:010412. doi: 10.7189/jogh.09.020412
104. Bojanić L, Marković-Peković V, Škrbić R, Stojaković N, Ðermanović M, Bojanić J, et al. Recent initiatives in the republic of srpska to enhance appropriate use of antibiotics in ambulatory care; their influence and implications. Front Pharmacol. (2018) 9:442. doi: 10.3389/fphar.2018.00442
105. Arparsrithongsagul S, Kulsomboon V, and Zuckerman IH. Multidisciplinary perspective intervention with community involvement to decrease antibiotic sales in village groceries in Thailand. Asia Pac J Public Health. (2015) 27:Np2480–8. doi: 10.1177/1010539513479968
Keywords: community pharmacists, antibiotics, knowledge, attitudes, practices, antimicrobial resistance, antimicrobial stewardship, South Africa
Citation: Maluleke TM, Maluleke MT, Jelić AG, Campbell SM, Marković-Peković V, Schellack N, Ramdas N, Godman B and Meyer JC (2025) Current knowledge and attitudes toward antibiotic use among community pharmacy personnel in a rural province in South Africa and the implications. Front. Trop. Dis. 6:1637843. doi: 10.3389/fitd.2025.1637843
Received: 29 May 2025; Accepted: 29 August 2025;
Published: 02 October 2025.
Edited by:
Sylvia Opanga, University of Nairobi, KenyaReviewed by:
Shoaib Ahmad, Punjab Medical College, PakistanGayathri Govindaraju, Rutgers, The State University of New Jersey, United States
Copyright © 2025 Maluleke, Maluleke, Jelić, Campbell, Marković-Peković, Schellack, Ramdas, Godman and Meyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Godman, YnJpYW4uZ29kbWFuQHNtdS5hYy56YQ==
†ORCID: Tiyani Milta Maluleke, orcid.org/0000-0001-6437-7198
Ana Golić Jelić, orcid.org/0000-0001-6883-4739
Stephen M. Campbell, orcid.org/0000-0002-2328-4136
Vanda Marković-Peković, orcid.org/0000-0001-8963-5720
Natalie Schellack, orcid.org/0000-0001-9690-6285
Brian Godman, orcid.org/0000-0001-6539-6972
Johanna C. Meyer, orcid.org/0000-0003-0462-5713
Nishana Ramdas, orcid.org/0000-0002-9534-2972
 Tiyani Milta Maluleke1,2†
Tiyani Milta Maluleke1,2† Ana Golić Jelić
Ana Golić Jelić Vanda Marković-Peković
Vanda Marković-Peković Natalie Schellack
Natalie Schellack Nishana Ramdas
Nishana Ramdas Brian Godman
Brian Godman Johanna C. Meyer
Johanna C. Meyer