- 1Department of Soil Microbiology and Symbiotic Systems, Estación Experimental del Zaidín, Agencia Estatal Consejo Superior de Investigaciones Científicas, Granada, Spain
- 2Department of Agricultural Sciences, National University of Huancavelica, Huancavelica, Peru
The bacterial endophytic communities residing within roots of maize (Zea mays L.) plants cultivated by a sustainable management in soils from the Quechua maize belt (Peruvian Andes) were examined using tags pyrosequencing spanning the V4 and V5 hypervariable regions of the 16S rRNA. Across four replicate libraries, two corresponding to sequences of endophytic bacteria from long time maize-cultivated soils and the other two obtained from fallow soils, 793 bacterial sequences were found that grouped into 188 bacterial operational taxonomic units (OTUs, 97% genetic similarity). The numbers of OTUs in the libraries from the maize-cultivated soils were significantly higher than those found in the libraries from fallow soils. A mean of 30 genera were found in the fallow soil libraries and 47 were in those from the maize-cultivated soils. Both alpha and beta diversity indexes showed clear differences between bacterial endophytic populations from plants with different soil cultivation history and that the soils cultivated for long time requires a higher diversity of endophytes. The number of sequences corresponding to main genera Sphingomonas, Herbaspirillum, Bradyrhizobium and Methylophilus in the maize-cultivated libraries were statistically more abundant than those from the fallow soils. Sequences of genera Dyella and Sreptococcus were significantly more abundant in the libraries from the fallow soils. Relative abundance of genera Burkholderia, candidatus Glomeribacter, Staphylococcus, Variovorax, Bacillus and Chitinophaga were similar among libraries. A canonical correspondence analysis of the relative abundance of the main genera showed that the four libraries distributed in two clearly separated groups. Our results suggest that cultivation history is an important driver of endophytic colonization of maize and that after a long time of cultivation of the soil the maize plants need to increase the richness of the bacterial endophytes communities.
Introduction
Chacras are small (200–10,000 m2) plots in the Quechua region of the Peruvian Andes where maize, pea, wheat, potatoes and other vegetables and cereals are cultivated by the native peasants. Commodities that they use for their own consumption and for trade in local and regional markets. Genetic and archeological data indicate that after domestication in Mexico about 8,700 years before the present (cal. BP) (Piperno et al., 2009; Van Heerwaarden et al., 2011; Grobman et al., 2012), maize spread in other Mexican regions and into south of Mexico, reaching the southern Andean highlands by 4,000 before the present (Perry et al., 2006). Since then maize is the staple diet of the Quechua natives who continue growing it as their ancestors did. Mostly without chemical fertilization, no pesticide application and without irrigation and yet chacras maintain a sustainable production for years.
Bacterial endophytes have been defined as microorganisms that could be isolated from surface-sterilized plant tissues and do not visibly harm host plants (Petrini, 1991; Hallmann et al., 1997; Schulz and Boyle, 2006). It is now considered that endophytism is a universal phenomenon (Kobayashi and Palumbo, 2000) and is likely that all plants harbor endophyte bacteria (Rosenblueth and Martínez-Romero, 2006; Ryan et al., 2008; Compant et al., 2010; Dudeja and Giri, 2014). Endophytes have been involved in plant growth promotion, biological control of plant pathogens, isolation of compounds of pharmaceutical or biotechnological interest (reviewed in Schulz, 2006; Weyens et al., 2009; Li et al., 2012; Malfanova et al., 2013; Hardoim et al., 2015; Ma et al., 2016; Vejan et al., 2016; Sharma et al., 2017).
Previous studies have analyzed bacterial taxa associated with maize. Most of the work has been done by using culture-dependent methods (Rai et al., 2007; Rijavec et al., 2007; Pereira et al., 2009, 2011; Ikeda et al., 2013; Celador-Lera et al., 2016; Menéndez et al., 2016; Sandhya et al., 2017) or assessed bacterial diversity independently of culture approaches (Schmalenberger and Tebbe, 2003; Herschkovitz et al., 2005a,b; Pereira et al., 2011; Correa-Galeote et al., 2016; Liu et al., 2017). The high-throughput pyrosequencing technology introduced by 454 Life Science (Margulies et al., 2005; Rothberg and Leamon, 2008) has been used to assess diversity in cultivar-specific bacterial endophyte communities in potato roots (Manter et al., 2010), leaf vegetables (Jackson et al., 2013), the spermosphere and phyllosphere of spinach (López-Velasco et al., 2013), tomato leaves (Romero et al., 2014), grapevine leaves and stems (Yousaf et al., 2014) and roots and shoots of cucumber (Eevers et al., 2016).
Most of the works has been done toward understand the effect of an engineered varieties of maize in the bacterial diversity in maize rhizosphere (Schmalenberger and Tebbe, 2003; Ikeda et al., 2013; Liu et al., 2017) or to analyse the changes in the rhizosphere of maize after the application of an inoculant (Herschkovitz et al., 2005a,b; Sanguin et al., 2006a,b; Alves et al., 2015). In recent years, sustainability agriculture methods have also been considered as great potential source of new information and perspective in agriculture and food systems (Wezel et al., 2011). Bacterial diversity is central to ecosystem sustainability and soil biological function, for which the role of roots is especially important (Sanguin et al., 2006b). However, the characterization of bacterial endophyte community of agroecology systems as is the Quechua practices has been poorly analyzed.
In this work we have analyzed the bacterial endophytes that inhabit the roots of maize plants grown under a different management history in the Quechua region by pyrosequencing the V4 and V5 hypervariable regions of the 16S rRNA gene. Our hypothesis was that the soil cultivation history plays a pivotal role of structuring the endophytic bacterial communities of maize plants.
Materials and Methods
Site Description and Root Sampling
Maize (Zea mays L.) plants were grown at 4 chacras located inside the same farm field (Figure S1A) near Allpas (12° 50′ 27″ S, 74° 34′ 14″ W, at 3,537 m above sea level), a village in the province of Acobamba (Huancavelica, Peru), following the traditional agricultural practices of the Quechua natives. The lateral roots (~2 mm diameter, 2–3 cm long) of the maize plants (morphotype Qarway) were harvested 120 days after sowing. At the sampling time two of the four chacras had been cultivated with maize for at least 5 years (MC soil) and the other two were under fallow conditions before the maize sowing (F soil) for at least 5 years (Figure S1B). For each chacra, roots were sampled from plants grown at three different sites (four plants per site), pooled together, washed with sterile tap water to remove attached soil and stored at −20°C until further processing. Physicochemical analyses of the four chacras indicated they have identical soil characteristics with a sandy loam texture (64.0% sand, 30.0% silt, 6% clay), pH 5.74, 1.6% organic C and 0.11% total N (Table S1).
Surface Sterilization of Maize Roots and Isolation of Endophytes
Unfrozen roots were surface-sterilized as indicated by Liu et al. (2017). Essentially, roots were immersed in 70% ethanol for 3 min, washed with fresh sodium hypochlorite solution (2.5% available Cl−) for 5 min, rinsed with 70% ethanol for 30 s and finally washed thoroughly with sterile distilled water. To confirm that the sterilization process was successful, small pieces of roots were cut and placed on Petri dishes containing yeast extract-mannitol (YEM) medium (Vincent, 1970). The plates were examined for bacterial growth after incubation at 30°C for 12 days. Maize roots that were not contaminated as detected by culture-dependent sterility test were used for further experiments.
Extraction of DNA From Maize Roots
DNA was extracted from 250 mg of unfrozen tissue as previously indicated (Correa-Galeote et al., 2013). Essentially, after thoroughly cutting with an sterile scalpel, samples were homogenized in 1 ml of extraction buffer containing 100 mM Tris (pH 8.0), 100 mM EDTA, 100 mM NaCl, 1% (w/v) polyvinylpyrrolidone and 2% (w/v) sodium dodecyl sulfate using a 2-ml mini-bead-beater tube containing 0.5 and 0.1 g of 106-μm- and 2-mm-diameter glass beads, respectively, for 60 s at 27 Hz. Cell debris was eliminated by centrifugation (14,000 rpm for 5 min at 4°C). Proteins were removed by treatment with 5 M sodium acetate. After treatment for 12 h with ice-cold isopropanol, nucleic acids were precipitated by centrifugation (14,000 rpm for 30 min at 4°C), washed with 70% ice-cold ethanol, recentrifruged (14,000 rpm for 15 min at 4°C) and air-dried for 30 min. Finally, DNA was purified using GeneClean columns (Qiagen). Quality and size of DNA were checked by electrophoresis on 1% agarose and quantified by spectrophotometry at 260 nm using a Nanodrop spectrophotometer (NanoDrop ND1000).
Amplification and Pyrosequencing of DNA From Maize Roots
Polymerase chain reaction (PCR) amplification of the hypervariable V4-V5 regions of the 16S rRNA gene was performed over each individual DNA extraction from roots of maize plants grown in F and MC soils using universal primers U519F and U926R (Baker et al., 2003) joined to a multiplex identifier sequence (Binladen et al., 2007; Parameswaran et al., 2007). For each sample, amplicons were generated in several replicate PCRs using mixtures (25 μl) that contained 25 pmol of each primer, 1.8 mM MgCl2, 0.2 mM dNTPs, 1 × the corresponding Taq buffer, 1 U of Taq Master (5 Prime, USA) and 10 ng of the DNA template. The PCR program consisted of an initial denaturation step at 94°C for 4 min, 25 cycles of denaturation at 94°C for 15 s, primer annealing at 55°C for 45 s and extension at 72°C for 1 min, followed by a final step of heating at 72°C for 10 min. Amplicons of the same treatment were pooled together to reduce per-PCR variability and purified using the ultracentrifugal filters Ultracel-100 K membranes (Amicon) according to the manufacturer's instructions. After quantification by Nanodrop ND1000 and visualization of the DNA by agarose electrophoresis, the samples were combined in equimolar amounts and pyrosequenced in a Roche Genome Sequencer FLX system using 454 Titanium chemistry at LifeSequencing S.L. (Valencia, Spain).
Taxonomic Assignment of Sequence Reads and Diversity Indexes
Raw sequences were processed through the Ribosomal Database Project (RDP) pyrosequencing pipeline (http://pyro.cme.msu.edu) release 11 (Cole et al., 2014). Sequences were trimmed for primers, filtered and assigned to four libraries (F1G, F2G, MC1G and MC2G) according to their tags. Sequences shorter than 150 base pair, with quality scores <20 or containing any unresolved nucleotides were removed from the dataset. Chimeras were identified using the Uchime tool from FunGene database (Edgar et al., 2011) and removed from the dataset. Sequences were aligned using the SILVA-based bacterial reference alignment in the MOTHUR program (Schloss and Westcott, 2011). Aligned sequences were clustered into operational taxonomic units (OTUs) defined at 97% similarity cutoff using MOTHUR and their relative abundances calculated. The number of sequences in each OTU was employed to calculate the Good's coverage index, which is considered a relative measure of how well the sequences obtained represent the entire populations (Hughes and Bohannan, 2004). Taxonomic assignation of the sequences was performed using Geneious (Biomatters). Shannon (H′) and Simpson (S′) diversity indexes and Jaccard indexes (Jclass and Jabund) were used to analyze the alpha- and beta-diversity, respectively (Chao et al., 2005).
Statistical Analyses
Relative abundances of the main genera and values of the diversity index were compared using the Student t-test in the XLSTAT software (Addinsoft). Multivariate techniques were used to analyze the relative abundance of endophytes using PC-ORD (McCune et al., 2002). A canonical correspondence analysis (CCA) was built to study differences in composition of dominant endophyte genera in roots from plants grown in F and MC soils.
Accession Numbers
Pyrosequencing reads are deposited in GenBank under accession numbers KT764133 to KT764925.
Results
A total of 38,443 sequences were obtained from the four 16S rDNA samples sent to pyrosequencing, of which 11,278 were retained after filtering and removing chimeras. The mean number of total retained sequences per library was 2,819, ranging from 1,770 to 3,718. Average length of retained sequences was 374 ± 5 base pair (mean ± SD). Using the MOTHUR program all the sequences aligned correctly in the expected position of the 16S rDNA sequence of Escherichia coli and were grouped at 97% similarity in 244 distinct OTUs. A representative sequence from each OTU was sent to NCBI for identification, after which 10,485 sequences were removed as they were identified as Streptophyta-related sequences. The remaining 793 sequences grouped into 188 bacterial OTUs, of which 17 were supported by 10 or more reads and 91 corresponded to singletons (Table S2). Values of the Good's coverage index were higher than 68% for all the samples. The number of OTUs in libraries F1G and F2G were 48 and 53, respectively, significantly lower than those of 88 and 112 found in libraries MC1G and MC2G (Table 1). The Shannon index for OTUs in F1G and F2G showed similar values, 3.32 and 3.62, respectively, that were statistically lower than those of 4.04 and 4.02 for OTUs in MC1G and MC2G, respectively. On the other hand, values of the Simpson index for the OTUS for the four libraries varied between 0.23 and 0.32, and no significant differences were found among them (Table 1). The Jaccard index for Jclass and Jabund also showed that the degree of similarity between libraries MC1G and MC2G was higher than that between F1G and F2G (Table 2). The number of shared genera between pair to pair libraries is shown in Table 2.
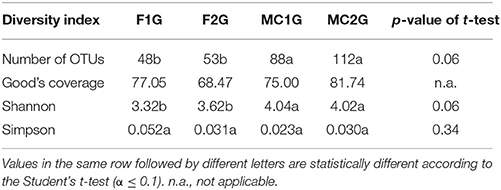
Table 1. Number of OTUs, values of Good's coverage index and Shannon and Simpson biodiversity index of bacterial endophytes from roots of maize plants grown in fallow (F1 and F2) and maize-cultivated (MC1 and MC2) soils.
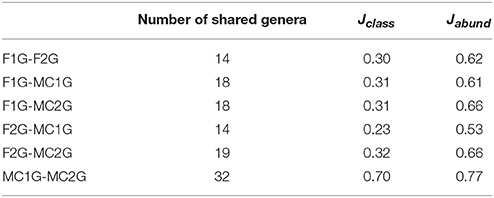
Table 2. Number of shared genera between clone libraries, and Jaccard similarity index using genera presence/absence (Jclass) and relative abundances (Jabund) of the bacterial endophyte communities from roots of maize plants grown in fallow (F1 and F2) and maize-cultivated (MC1 and MC2) soils.
Unclassified sequences were 14 (11.48%) and 15 (13.51%) for libraries F1G and F2G, respectively, and 28 (13.73%) and 64 (17.98%) for MC1G and MC2G, respectively (Table 3, Figure 1). The remaining sequences distributed into 6 and 7 phyla for F1G and F2G, respectively, and 7 and 8 for MC1G and MC2G, respectively (Table 3). Phyla Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, Acidobacteria, Chloroflexi and Cyanobacteria (in decreasing abundance) were found in each one of the four libraries, Deinococcus-Thermus and Gemmatimonadetes were detected only in libraries FG and Verrucomicrobia was found exclusively in endophytes from libraries MCG (Figure 1). The number of classes, orders, families and genera are also shown in Table 3.
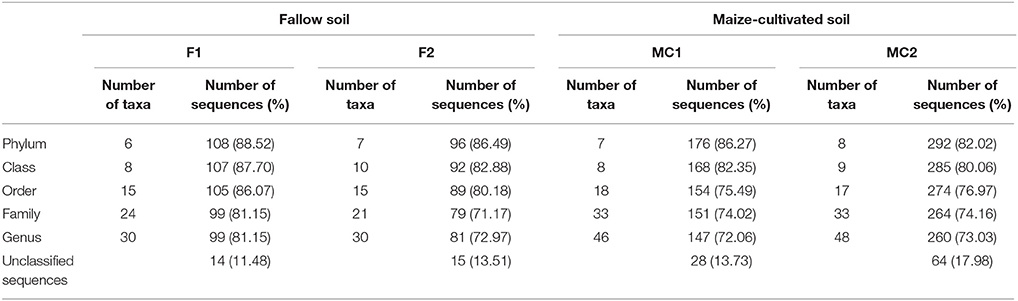
Table 3. Number of taxa and distribution of sequences (%) of bacterial endophytes in roots of maize plants grown in fallow (F1 and F2) and maize-cultivated (MC1 and MC2) soils.
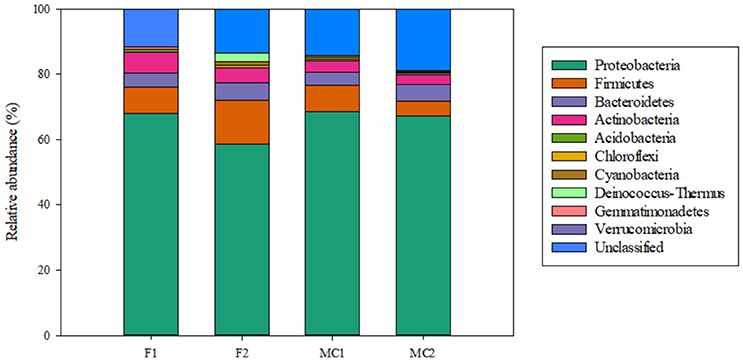
Figure 1. Relative abundance of bacterial endophytes from roots of maize plants grown in fallow (F1 and F2) and maize-cultivated (MC1 and MC2) soils.
The total 188 OTUs distributed into 82 different genera (Table S2), 12 of which showed a relative abundance higher than 1% and represented the 55% of the total endophytes. Altogether, these genera were (in decreasing order of abundance) Sphingomonas, Burkholderia, Candidatus Glomeribacter, Dyella, Herbaspirillum, Bradyrhizobium, Staphylococcus, Methylophilus, Variovorax, Streptococcus, Bacillus and Chitinophaga (Table S2). The number of sequences corresponding to genera Sphingomonas, Herbaspirillum, Bradyrhizobium and Methylophilus in libraries MCG were statistically (α ≤ 0.1) more abundant than those in the F libraries, and sequences of genera Dyella and Sreptococcus were significantly more abundant in the F libraries (Figure 2). Relative abundance of genera Burkholderia, candidatus Glomeribacter, Staphylococcus, Variovorax, Bacillus and Chitinophaga were similar among libraries (Figure 2).
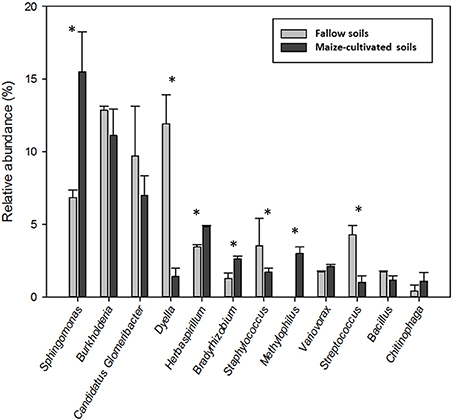
Figure 2. Relative abundance of the 12 main genera found in roots of maize plants grown in fallow and maize-cultivated soils. *Indicates statistically significant differences according to the Student's t-test (α ≤ 0.1).
A CCA sample ordination based on the relative abundance of the 12 main genera mentioned above showed that they distributed in two clearly separated groups (Figure 3). The two CCA axes explained 93% of the total variance and revealed that cultivation history of the soil was responsible for the grouping of the libraries along the axis 1 (canonical coefficient 1.10).
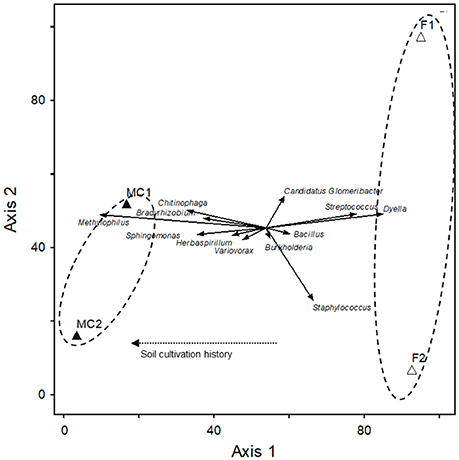
Figure 3. Canonical correspondence analysis (CCA) of the 12 main genera found in roots of maize plants grown in fallow and maize-cultivated soils. Solid arrows represent vector scores for the different genera. Open and closed triangles represent the axes 1 and 2 scores for the main genera found in fallow (F1 and F2) and maize-cultivated (MC1 and MC2) soils, respectively. The dashed arrow represents the biplot vector for cultivation history of the soil.
Discussion
One of the most successful soil management techniques in agricultural land is the use of fallow periods (Costa et al., 2015), this work is a first approach to understand the role of the soil cultivation history in the bacterial diversity of the endophytic bacteria of maize plants cultivated under sustainably practices. Using 454 next generation sequencing we assessed the composition and abundance of endophytic communities inside roots of amilaceous maize plants grown under fallow and maize-cultivated conditions in Andean chacras. Pyrosequencing revealed an unprecedented number of bacterial endophytes as compared with those of the genera found in previous studies based on culture-dependent and culture-independent methods (McInroy and Kloepper, 1995; Chelius and Triplett, 2001; Rai et al., 2007; Pereira et al., 2011; Ikeda et al., 2013; Sandhya et al., 2017). Altogether, a 15.26% of the total sequences found inside roots corresponded to unclassified bacteria, which indicates the presence of hitherto uncultured bacterial groups. Nevertheless, despite the resolving power of pyrosequencing to detect phylogenetic groups, genera Pantoea, Klebsiella and Erwinia found by other authors (Pereira et al., 2011; Montañez et al., 2012; Ikeda et al., 2013; Liu et al., 2017) after sequencing of the 16S rRNA gene of endophytes isolated from roots of different maize genotypes were not detected in our libraries. This could be due to qualitative differences in endophytic colonization (Ikeda et al., 2013).
A variety of bacteria have been reported to be endophytic, among them mostly Proteobacteria, but also Firmicutes, Actinobacteria and Bacteroidetes (reviewed in Rosenblueth and Martínez-Romero, 2006; Bulgarelli et al., 2013; Malfanova et al., 2013; Hardoim et al., 2015; Liu et al., 2017). In our study, regardless of the cultivation history of the soil, members of phylum Proteobacteria were the most abundant followed by those of Firmicutes, Bacteroidetes and Actinobacteria.
Number of OTUs and Shannon index values were statistically higher for libraries MC1G and MC2G than those for libraries FG1 and FG2. However, although the Jclass and Jabund indexes were also higher for the MC communities, the bacterial endophytic communities within the plant roots from MC soils were more similar. Eleven out the 12 main genera were present in both MC and F soils and four of them (Sphingomonas, Herbaspirillum, Bradyrhizobium and Methylophilus) had increased relative abundance in the MC soils in comparison with that in the F soils. These results, together with those of the clone libraries diversity, indicate that plant cultivation history could have a pivotal role responsible for selection of roots endophytes from rhizospheric bacterial reservoirs. Also, these results could indicated that the maize plant growth in soils cultivated for long time requires a higher diversity of endophytes than the plants grown in a soil under a fallow time due that the natural resources of the soil are depleted after 5 years of cultivation. For example, excessive cultivation can wreck the structure of soil by reducing the capacity of holding enough moisture for growing plants (FAO, 1994) and also has been demonstrate that after 3 years of cultivation organic C, N and P declined about a 25% (Bowman et al., 1990). According to Wood et al. (2017), the fallow period is a key determinant of vegetation and soil dynamics as this period renew soil fertility, biomass and biodiversity. Therefore, after a long-time cultivation the maize plants needs a higher presence of endophytes to minimize the depletion of the soil resources.
Bacterial endophytes have been shown to modulate plant growth and development through N2 fixation, solubilization of insoluble phosphorus, production of siderophores, production of phytohormones, lowering of ethylene concentration, production of antibiotics and antifungal metabolites and inducing systemic resistance (Somers et al., 2004; Hardoim et al., 2008, 2015; Ahemad and Kibret, 2014; Vejan et al., 2016). Some genera in this study have been shown to be diazotrophic bacteria (Sphingomonas, Burkholderia, candidatus Glomeribacter, Herbaspirillum, Bradyrhizobium and Bacillus), others solubilize inorganic phosphorus (Sphingomonas, Burkholderia, Herbaspirillum, Bradyrhizobium, Staphylococcus, Methylophilus, Variovorax, Streptococcus, Bacillus and Chitinophaga), are siderophore (Sphingomonas, Burkholderia, Herbaspirillum, Bradyrhizobium, Staphylococcus, Methylophilus, Variovorax, Streptococcus, Bacillus and Chitinophaga) or indole acetic acid (Sphingomonas, Burkholderia, Dyella, Herbaspirillum, Bradyrhizobium, Staphylococcus, Methylophilus, Variovorax, Bacillus and Chitinophaga) producers, have 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity (Sphingomonas, Burkholderia, Dyella, Herbaspirillum, Bradyrhizobium, Staphylococcus, Variovorax and Bacillus) and are involved in biocontrol activity (Sphingomonas, Burkholderia, candidatus Glomeribacter, Herbaspirillum, Methylophilus, Variovorax, Bacillus and Chitinophaga; see Table S3).
To our knowledge, 6 of the 12 main genera in this study (Bradyrhizobium, Variovorax, Chitinophaga, candidatus Glomeribacter, Dyella and Streptococcus) have not been reported as endophytes of amilaceous maize. It should be noted, that the bacteria reported as maize endophytes for the first time could present biotechnological implications as is the case of the formulation of new microbial inoculants. The presence of Streptococcus, Dyella and Staphylococcus is intriguing as they are well-known human pathogens; these three genera have been detected in maize seeds, roots of blackberry, grapevine shoots, apple and orange fresh fruits (Liu et al., 2013; Phukon et al., 2013; Pinto et al., 2014; Yousaf et al., 2014; Contreras et al., 2016) and they were reported as the dominant endophytes of legumes (Boine et al., 2008; Becerra-Castro et al., 2011). Moreover, recent works suggest that pathogenic bacteria are common inhabitants of the interior of plants (Szilagyi-Zecchin et al., 2014; Blain et al., 2017; Sandhya et al., 2017).
There is to note, out of the 12 main endophytic genera here described, the genera Candidatus Glomeribacter, Dyella, Herbaspirillum and Streptococcus were not found in a previous work (Correa-Galeote et al., 2016) that describe the rizhospherics communities of these chacras and therefore the mechanisms of how these bacteria arrive to the interior of the maize roots is still unclear.
The plant host genotype (Ding et al., 2013), soil type (Rasche et al., 2006; Bulgarelli et al., 2013) and environmental soil conditions (Lundberg et al., 2012) among other factors shape bacterial community composition. Because seeds of amilaceous maize used for planting were the same and the environmental conditions, including soil type, soil psychochemical properties and irrigation, were very much alike for the four chacras used in this study, our results suggest that soil cultivation history could be a main factor controlling colonization of the internal root tissues of the plants.
Taken together our results lend support to the suggestion that cultivation history is an important driver of endophytic colonization of maize and that after a long time of cultivation of the soil the maize plants there grown need to increase the richness of the bacterial endophytes communities. Also, these results point to the importance of the fallow period of the traditional and sustainable Quechua agriculture methods in the maintenance of the soil fertility of Peruvian soils.
As a caveat, since richness of bacteria colonizing maize roots was based on pyrosequencing, it is not known whether the detection of bacteria based on DNA signature alone represent active microbes that are interacting with the host plant. Further experiments should be made in order to isolate the main endophytes described in this work and also analyze their role in the development of the maize host plant cultivated under a sustainable method as is the traditional Quechua agriculture.
Author Contributions
DC-G made substantial contributions to the conception and design of the work, acquisition, analysis and interpretation of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. EB made substantial contributions to the conception or design of the work, interpretation of data, revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. GA made substantial contributions to the conception or design of the work, revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by ERDF-cofinanced grant PE2012-AGR1968 from Consejería de Economía, Innovación y Ciencia (Junta de Andalucía, Spain) and the CSIC-sponsored I-COOP Agrofood project 2014CD0013.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00484/full#supplementary-material
References
Ahemad, M., and Kibret, M. (2014). Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. JKSUES 26, 1–20. doi: 10.1016/j.jksus.2013.05.001
Alves, G. C., Videira, S. S., Urquiaga, S., and Reis, V. M. (2015). Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil 387, 307–321. doi: 10.1007/s11104-014-2295-2
Baker, G. C., Smith, J. J., and Cowan, D. A. (2003). Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 55, 541–555. doi: 10.1016/j.mimet.2003.08.009
Becerra-Castro, C., Kidd, P. S., Prieto-Fernández, Á., Weyens, N., Acea, M. J., and Vangronsveld, J. (2011). Endophytic and rhizoplane bacteria associated with Cytisus striatus growing on hexachlorocyclohexane-contaminated soil: isolation and characterisation. Plant Soil 340, 413–433. doi: 10.1007/s11104-010-0613-x
Binladen, J., Gilbert, M. T., Bollback, J. P., Panitz, F., Bendixen, C., Nielsen, R., et al. (2007). The use of coded PCR primers enables high-throughput sequencing of multiple homolog amplification products by 454 parallel sequencing. PLoS ONE 2:e197. doi: 10.1371/journal.pone.0000197
Blain, N. P., Helgason, B. L., and Germida, J. J. (2017). Endophytic root bacteria associated with the natural vegetation growing at the hydrocarbon-contaminated Bitumount provincial historic site. Can. J. Microbiol. 63, 502–515. doi: 10.1139/cjm-2017-0039
Boine, B., Naujoks, G., and Stauber, T. (2008). Investigations on influencing plant-associated bacteria in tissue cultures of black locust (Robinia pseudoacacia L.). Plant Cell Tissue Organ Cult. 94, 219–223. doi: 10.1007/s11240-008-9395-8
Bowman, R. A., Reeder, J. D., and Lober, R. W. (1990). Changes in soil properties in a central plains rangeland soil after 3, 20, and 60 years of cultivation. Soil Sci. 150, 851–857. doi: 10.1097/00010694-199012000-00004
Bulgarelli, D., Schlaeppi, K., Spaepen, S., van Themaat, E. V. L., and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
Celador-Lera, L., Menéndez, E., Flores-Félix, J. D., Mateos, P. F., and Rivas, R. (2016). “Analysis of the PGPB potential of bacterial endophytes associated with maize,” in Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction, eds F. González-Andrés and E. James (Berlin: Springer), 23–35.
Chao, A., Chazdon, R. L., Colwell, R. K., and Shen, T. J. (2005). A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159. doi: 10.1111/j.1461-0248.2004.00707.x
Chelius, M. K., and Triplett, E. W. (2001). The diversity of Archaea and bacteria in association with the roots of Zea mays L. Microb. Ecol. 41, 252–263. doi: 10.1007/s002480000087
Cole, J. R., Wang, Q., Fish, J. A., Chai, B., McGarrell, D. M., Sun, Y., et al. (2014). Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642. doi: 10.1093/nar/gkt1244
Compant, S., Van Der Heijden, M. G., and Sessitsch, A. (2010). Climate change effects on beneficial plant–microorganism interactions. FEMS. Microbiol. Ecol. 73, 197–214. doi: 10.1111/j.1574-6941.2010.00900.x
Contreras, M., Loeza, P. D., Villegas, J., Farias, R., and Santoyo, G. (2016). A glimpse of the endophytic bacterial diversity in roots of blackberry plants (Rubus fruticosus). Genet. Mol. Res. 15:gmr.15038542. doi: 10.4238/gmr.15038542
Correa-Galeote, D., Bedmar, E. J., Fernández-González, A. J., Fernández-López, M., and Arone, G. J. (2016). Bacterial communities in the rhizosphere of amilaceous maize (Zea mays L.) as assessed by pyrosequencing. Fron. Plant. Sci. 7:1016. doi: 10.3389/fpls.2016.01016
Correa-Galeote, D., Tortosa, G., and Bedmar, E. J. (2013). Determination of denitrification genes abundance in environmental samples. Metagenomics 2:235702. doi: 10.4303/mg/235702
Costa, J. L., Aparicio, V., and Cerdà, A. (2015). Soil physical quality changes under different management systems after 10 years in the Argentine humid pampa. Solid Earth 6, 361–371. doi: 10.5194/se-6-361-2015
Ding, T., Palmer, M. W., and Melcher, U. (2013). Community terminal restriction fragment length polymorphisms reveal insights into the diversity and dynamics of leaf endophytic bacteria. BMC Microbiol. 13:1. doi: 10.1186/1471-2180-13-1
Dudeja, S. S., and Giri, R. (2014). Beneficial properties, colonisation, establishment and molecular diversity of endophytic bacteria in legumes and non-legumes. Afr. J. Microbiol. Res. 8, 1562–1572. doi: 10.5897/AJMR2013.6541
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Eevers, N., Beckers, B., de Beeck, M. O., White, J. C., Vangronsveld, J., and Weyens, N. (2016). Comparison between cultivated and total bacterial communities associated with Cucurbita pepo using cultivation-dependent techniques and 454 pyrosequencing. Syst. Appl. Microbiol. 39, 58–66. doi: 10.1016/j.syapm.2015.11.001
FAO (1994). Land Degradation in South Asia: Its Severity, Causes and Effects Upon the People. Roma: World Soil Resources Reports.
Grobman, A., Bonavia, D., Dillehay, T. D., Piperno, D. R., Iriarte, J., and Holst, I. (2012). Preceramic maize from Paredones and Huaca Prieta, Peru. Proc. Natl. Acad. Sci. U.S.A. 109, 1755–1759. doi: 10.1073/pnas.1120270109
Hallmann, J., Quadt-Hallmann, A., Mahaffee, W. F., and Kloepper, J. W. (1997). Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43, 895–914. doi: 10.1139/m97-131
Hardoim, P. R., van Overbeek, L. S., Berg, G., Pirttilä, A. M., Compant, S., Campisano, A., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79, 293–320. doi: 10.1128/MMBR.00050-14
Hardoim, P. R., van Overbeek, L. S., and van Elsas, J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16, 463–471. doi: 10.1016/j.tim.2008.07.008
Herschkovitz, Y., Lerner, A., Davidov, Y., Okon, Y., and Jurkevitch, E. (2005a). Azospirillum brasilense does not affect population structure of specific rhizobacterial communities of inoculated maize (Zea mays). Environ. Microbiol. 7, 1847–1852. doi: 10.1111/j.1462-2920.2005.00926.x
Herschkovitz, Y., Lerner, A., Davidov, Y., Rothballer, M., Hartmann, A., Okon, Y., et al. (2005b). Inoculation with the plant-growth-promoting rhizobacterium Azospirillum brasilense causes little disturbance in the rhizosphere and rhizoplane of maize (Zea mays). Microb. Ecol. 50, 277–288. doi: 10.1007/s00248-004-0148-x
Hughes, J. B., and Bohannan, B. J. M. (2004). “Application of ecological diversity statistics in microbial ecology,” in Molecular Microbial Ecology Manual, Section 7, eds G. A. Kowalchuk, F. J. de Bruijn, I. M. Head, A. D. Akkermans, and J. D. van Elsas (Berlin: Springer), 3223–3246.
Ikeda, A. C., Bassani, L. L., Adamoski, D., Stringari, D., Cordeiro, V. K., Glienke, C., et al. (2013). Morphological and genetic characterization of endophytic bacteria isolated from roots of different maize genotypes. Microb. Ecol. 65, 154–160. doi: 10.1007/s00248-012-0104-0
Jackson, C. R., Randolph, K. C., Osborn, S. L., and Tyler, H. L. (2013). Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 13:274. doi: 10.1186/1471-2180-13-274
Kobayashi, D. Y., and Palumbo, J. D. (2000). “Bacterial endophytes and their effects on plants and uses in agriculture,” in Microbial Endophytes, eds C. W. Bacon and J. F. White (New York, NY: Marcel Dekker Inc.), 199–233.
Li, H. Y., Wei, D. Q., Shen, M., and Zhou, Z. P. (2012). Endophytes and their role in phytoremediation. Fungal Divers. 54, 11–18. doi: 10.1007/s13225-012-0165-x
Liu, Y., Wang, R., Li, Y., Cao, Y., Chen, C., Qiu, C., et al. (2017). High-throughput sequencing-based analysis of the composition and diversity of endophytic bacterial community in seeds of “Beijing” hybrid maize planted in China. Plant Growth Regul. 81, 317–324. doi: 10.1007/s10725-016-0208-5
Liu, Y., Zuo, S., Zou, Y., Wang, J., and Song, W. (2013). Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L., Nongda108) at different growth stages. Ann. Microbiol. 63, 71–79. doi: 10.1007/s13213-012-0446-3
López-Velasco, G., Carder, P. A., Welbaum, G. E., and Ponder, M. A. (2013). Diversity of the spinach (Spinacia oleracea) spermosphere and phyllosphere bacterial communities. FEMS Microbiol. Lett. 346, 146–154. doi: 10.1111/1574-6968.12216
Lundberg, D. S., Lebeis, S. L., Herrera-Paredes, S., Yourstone, S., Gehring, J., Malfatti, S., et al. (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488, 86–90. doi: 10.1038/nature11237
Ma, Y., Rajkumar, M., Zhang, C., and Freitas, H. (2016). Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manage. 174, 14–25. doi: 10.1016/j.jenvman.2016.02.047
Malfanova, N., Lugtenberg, B., and Berg, G. (2013). “Bacterial endophytes: who and where, and what are they doing there?,” in Molecular Microbial Ecology of the Rhizosphere Volume I, ed F. J. de Brujin (New York, NY: Wiley-Blackwell), 393–403.
Manter, D. K., Delgado, J. A., Holm, D. G., and Stong, R. A. (2010). Pyrosequencing reveals a highly diverse and cultivar-specific bacterial endophyte community in potato roots. Microb. Ecol. 60, 157–166. doi: 10.1007/s00248-010-9658-x
Margulies, M., Egholm, M., Altman, W. E., Attiya, S., Bader, J. S., Bemben, L. A., et al. (2005). Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380. doi: 10.1038/nature03959
McCune, B., Grace, J. B., and Urban, D. L. (2002). Analysis of Ecological Communities, Vol. 28. Gleneden Beach, OR: MjM Software Design.
McInroy, J. A., and Kloepper, J. W. (1995). Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 173, 337–342. doi: 10.1007/BF00011472
Menéndez, E., Ramirez-Bahena, M. H., Peix, A., Tejedor, C., Mulas, R., González-Andrés, F., et al. (2016). “Analysis of cultivable endophytic bacteria in roots of maize in a soil from León province in mainland Spain,” in Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction, eds F. González-Andrés and E. James (Berlin: Springer), 45–53.
Montañez, A., Blanco, A. R., Barlocco, C., Beracochea, M., and Sicardi, M. (2012). Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil Ecol. 58, 21–28. doi: 10.1016/j.apsoil.2012.02.009
Parameswaran, P., Jalili, R., Tao, L., Shokralla, S., Gharizadeh, B., Ronaghi, M., et al. (2007). A pyrosequencing-tailored nucleotide barcode design unveils opportunities for large-scale sample multiplexing. Nucleic Acids Res. 35:e130. doi: 10.1093/nar/gkm760
Pereira, C. J., Pereira, E. J. G., Cordeiro, E. M. G., Della Lucia, T. M. C., Tótola, M. R., and Guedes, R. N. C. (2009). Organophosphate resistance in the maize weevil Sitophilus zeamais: magnitude and behavior. Crop Prot. 28, 168–173. doi: 10.1016/j.cropro.2008.10.001
Pereira, P., Ibáñez, F., Rosenblueth, M., Etcheverry, M., and Martínez-Romero, E. (2011). Analysis of the bacterial diversity associated with the roots of maize (Zea mays L.) through culture-dependent and culture-independent methods. ISRN Ecol. 2011:938546. doi: 10.5402/2011/938546
Perry, L., Sandweiss, D. H., Piperno, D. R., Rademaker, K., Malpass, M. A., Umire, A., et al. (2006). Early maize agriculture and interzonal interaction in southern Peru. Nature 440, 76–79. doi: 10.1038/nature04294
Petrini, O. (1991). “Fungal endophytes of tree leaves,” in Microbial Ecology of Leaves, eds J. H. Andrews and S. S. Hirano (New York, NY: Springer-Verlag Inc.), 179–197.
Phukon, M., Sahu, P., Srinath, R., Nithya, A., and Babu, S. (2013). Unusual occurrence of Staphylococcus warneri as endophyte in fresh fruits along with usual Bacillus spp. J. Food Saf. 33, 102–106. doi: 10.1111/jfs.12028
Pinto, C., Pinho, D., Sousa, S., Pinheiro, M., Egas, C., and Gomes, A. C. (2014). Unravelling the diversity of grapevine microbiome. PLoS ONE 9:e85622. doi: 10.1371/journal.pone.0085622
Piperno, D. R., Ranere, A. J., Holst, I., Iriarte, J., and Dickau, R. (2009). Starch grain and phytolith evidence for early ninth millennium BP maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. U.S.A. 106, 5019–5024. doi: 10.1073/pnas.0812525106
Rai, R., Dash, P. K., Prasanna, B. M., and Singh, A. (2007). Endophytic bacterial flora in the stem tissue of a tropical maize (Zea mays L.) genotype: isolation, identification and enumeration. World J. Microbiol. Biotechnol. 23, 853–858. doi: 10.1007/s11274-006-9309-z
Rasche, F., Velvis, H., Zachow, C., Berg, G., van Elsas, J. D., and Sessitsch, A. (2006). Impact of transgenic potatoes expressing anti-bacterial agents on bacterial endophytes is comparable with the effects of plant genotype, soil type and pathogen infection. J. Appl. Ecol. 43, 555–566. doi: 10.1111/j.1365-2664.2006.01169.x
Rijavec, T., Lapanje, A., Dermastia, M., and Rupnik, M. (2007). Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 53, 802–808. doi: 10.1139/W07-048
Romero, F. M., Marina, M., and Pieckenstain, F. L. (2014). The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol. Lett. 351, 187–194. doi: 10.1111/1574-6968.12377
Rosenblueth, M., and Martínez-Romero, E. (2006). Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 19, 827–837. doi: 10.1094/MPMI-19-0827
Rothberg, J. M., and Leamon, J. H. (2008). The development and impact of 454 sequencing. Nat. Biotechnol. 26, 1117–1124. doi: 10.1038/nbt1485
Ryan, R. P., Germaine, K., Franks, A., Ryan, D. J., and Dowling, D. N. (2008). Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278, 1–9. doi: 10.1111/j.1574-6968.2007.00918.x
Sandhya, V., Shrivastava, M., Ali, S. Z., and Prasad, V. S. S. K. (2017). Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ. Agric. Sci. 43, 22–34. doi: 10.3103/S1068367417010165
Sanguin, H., Herrera, A., Oger-Desfeux, C., Dechesne, A., Simonet, P., Navarro, E., et al. (2006a). Development and validation of a prototype 16S rRNA-based taxonomic microarray for Alphaproteobacteria. Environ. Microbiol. 8, 289–307. doi: 10.1111/j.1462-2920.2005.00895.x
Sanguin, H., Remenant, B., Dechesne, A., Thioulouse, J., Vogel, T. M., Nesme, X., et al. (2006b). Potential of a 16S rRNA-based taxonomic microarray for analyzing the rhizosphere effects of maize on Agrobacterium spp. and bacterial communities. Appl. Environ. Microbiol. 72, 4302–4312. doi: 10.1128/AEM.02686-05
Schloss, P. D., and Westcott, S. L. (2011). Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 77, 3219–3226. doi: 10.1128/AEM.02810-10
Schmalenberger, A., and Tebbe, C. C. (2003). Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies. Mol. Ecol. 12, 251–262. doi: 10.1046/j.1365-294X.2003.01716.x
Schulz, B. (2006). “Mutualistic interactions with fungal root endophytes,” in Microbial Root Endophytes, eds B. Schulz, C. Boyle, and T. N. Sieber (Berlin: Springer), 261–279.
Schulz, B., and Boyle, C. (2006). “What are endophytes?,” in Microbial Root Endophytes, eds B. Schulz, C. Boyle, and T. N. Sieber (Berlin: Springer), 1–13.
Sharma, I. P., Chandra, S., Kumar, N., and Chandra, D. (2017). “PGPR: heart of soil and their role in soil fertility,” in Agriculturally Important Microbes for Sustainable Agriculture Volume I, eds V. S. Meena, P. K. Mishra, J. K. Bisht, and A. Pattanayak (Singapore: Springer), 51–67. doi: 10.1007/978-981-10-5589-8_3
Somers, E., Vanderleyden, J., and Srinivasan, M. (2004). Rhizosphere bacterial signaling: a love parade beneath our feet. Crit. Rev. Microbiol. 30, 205–240. doi: 10.1080/10408410490468786
Szilagyi-Zecchin, V. J., Ikeda, A. C., Hungria, M., Adamoski, D., Kava-Cordeiro, V., Glienke, C., et al. (2014). Identification and characterization of endophytic bacteria from corn (Zea mays L.) roots with biotechnological potential in agriculture. AMB Exp. 4:26. doi: 10.1186/s13568-014-0026-y
Van Heerwaarden, J., Doebley, J., Briggs, W. H., Glaubitz, J. C., Goodman, M. M., Gonzalez, J. D. J. S., et al. (2011). Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc. Natl. Acad. Sci. U.S.A. 108, 1088–1092. doi: 10.1073/pnas.1013011108
Vejan, P., Abdullah, R., Khadiran, T., Ismail, S., and Nasrulhaq Boyce, A. (2016). Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21:573. doi: 10.3390/molecules21050573
Vincent, J. M. (1970). A Manual for the Practical Study of the Root-Nodule Bacteria. Hoboken, NJ: Wiley-Blackwell.
Weyens, N., van der Lelie, D., Taghavi, S., Newman, L., and Vangronsveld, J. (2009). Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 27, 591–598. doi: 10.1016/j.tibtech.2009.07.006
Wezel, A., Bellon, S., Doré, T., Francis, C., Vallod, D., and David, C. (2011). “Agroecology as a science, a movement and a practice,” in Sustainable Agriculture, Vol. 2, eds E. Lichtfouse, M. Hamelin, M. Navarrete, and P. Debaeke (Amsterdam: Springer), 27–43.
Wood, S. L., Rhemtulla, J. M., and Coomes, O. T. (2017). Cropping history trumps fallow duration in long-term soil and vegetation dynamics of shifting cultivation systems. Ecol. Appl. 27, 519–531. doi: 10.1002/eap.1462
Keywords: biodiversity, endophytes, maize, Quechua region, 16S rRNA, pyrosequencing, PGPR bacteria
Citation: Correa-Galeote D, Bedmar EJ and Arone GJ (2018) Maize Endophytic Bacterial Diversity as Affected by Soil Cultivation History. Front. Microbiol. 9:484. doi: 10.3389/fmicb.2018.00484
Received: 10 October 2017; Accepted: 28 February 2018;
Published: 16 March 2018.
Edited by:
Diana Elizabeth Marco, National Scientific Council (CONICET), ArgentinaReviewed by:
Ravindra Soni, Indira Gandhi Agricultural University, IndiaJosé A. Herrera-Cervera, University of Granada, Spain
Copyright © 2018 Correa-Galeote, Bedmar and Arone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Correa-Galeote, Y29ycmVhQGNjZy51bmFtLm14
 David Correa-Galeote
David Correa-Galeote Eulogio J. Bedmar
Eulogio J. Bedmar Gregorio J. Arone
Gregorio J. Arone