- 1Department of Biochemistry and Molecular Biology, School of Medicine, Jiangsu University, Zhenjiang, China
- 2Department of Forensic Medicine, School of Medicine, Jiangsu University, Zhenjiang, China
- 3Department of Laboratory Diagnosis, Changhai Hospital, The Second Military Medical University, Shanghai, China
Antisense RNAs from complementary strands of protein coding genes regulate the expression of genes involved in many cellular processes. Using deep sequencing analysis of the Salmonella enterica serovar Typhi (S. Typhi) transcriptome, a novel antisense RNA encoded on the strand complementary to the rpoH gene was revealed. In this study, the molecular features of this antisense RNA were assessed using northern blotting and rapid amplification of cDNA ends. The 3,508 nt sequence of RNA was identified as the antisense RNA of the rpoH gene and was named ArpH. ArpH was found to attenuate the invasion of HeLa cells by S. Typhi by regulating the expression of SPI-1 genes. In an rpoH mutant strain, the invasive capacity of S. Typhi was increased, whereas overexpression of ArpH positively regulates rpoH mRNA levels. Results of this study suggest that the cis-encoded antisense RNA ArpH is likely to affect the invasive capacity of S. Typhi by regulating the expression of rpoH.
Introduction
Salmonella enterica serovar Typhi (S. Typhi) is an important Gram-negative pathogenic bacterium that can cause symptoms ranging from mild diarrhea to serious systemic infections, such as typhoid fever, owing to many virulence genes (Dougan and Baker, 2014). Concentrated areas of virulence genes, called Salmonella pathogenicity islands (SPI), are located on the bacterial chromosome, plasmids, or phages (Matsui et al., 2008). The majority of virulence genes are located on SPIs on the chromosome. Virulence genes protect bacteria from damage caused by the host immune system. However, S. Typhi also interacts with the host and changes the host cell environment. The ability of S. Typhi to invade and survive in non-phagocytic cells is an indication of its pathogenicity. These pathogenic mechanisms have been linked to SPIs (Nieto et al., 2016).
Pathogens can quickly adapt to changing circumstances by regulating expression of their virulence genes during the process of infection. Many non-coding RNAs (ncRNAs) involved in complex regulatory mechanisms have been identified. Research has demonstrated that ncRNAs are involved in transcriptional and post-transcriptional regulation via the formation of complexes with DNA or proteins through base pairing interactions (Kaikkonen et al., 2011; Ghosal et al., 2013). Previously, ncRNA was considered to be transcriptional noise that was not involved in protein coding; however, a growing number of studies have confirmed that ncRNA can play a role in regulating the expression of many genes in bacteria, including ABC transport system (Miyakoshi et al., 2015), quorum sensing (Bardill and Hammer, 2012), oxidative stress (Gerstle et al., 2012), acid resistance (Aiso et al., 2011), and virulence genes. ncRNAs are classified as either trans-encoded or cis-encoded based on their position in the genome. Trans-encoded ncRNAs are located between protein coding genes and, because they are located at distant genomic locations, are only partially complementary to their target mRNAs and act via incomplete base pairing. Cis-encoded ncRNAs are located within protein-coding genes and, because they are located in the same region, are fully complementary to their target mRNAs (Storz et al., 2011).
We previously conducted deep RNA sequencing analysis of the transcriptome and identified several novel cis-encoded antisense RNAs (Dadzie et al., 2013, 2014). A 1164-nt transcript partially encoded by the minus strand of rpoH was expressed. The maximum expression of the transcript is from 1887 nt downstream from the start codon of yhhK and overlaps the 192-nt region upstream from the rpoH gene start codon, as shown by bioinformatic prediction (Figure 1A). RpoH activates expression of the hfq gene, which encodes an RNA-binding regulatory protein. RpoH also regulates the expression of genes involved in adaptations to environmental stresses, such as thermal stress (Martinez-Salazar et al., 2009; López-Leal et al., 2016). However, the full length and function of the antisense RNA of rpoH is not yet known. In the present study, we described the identification and characterization of a novel antisense RNA encoded by the strand complementary to the rpoH gene sequence that we named ArpH. We demonstrated that ArpH is likely to affect the invasive capacity of S. Typhi by regulating the expression of rpoH.
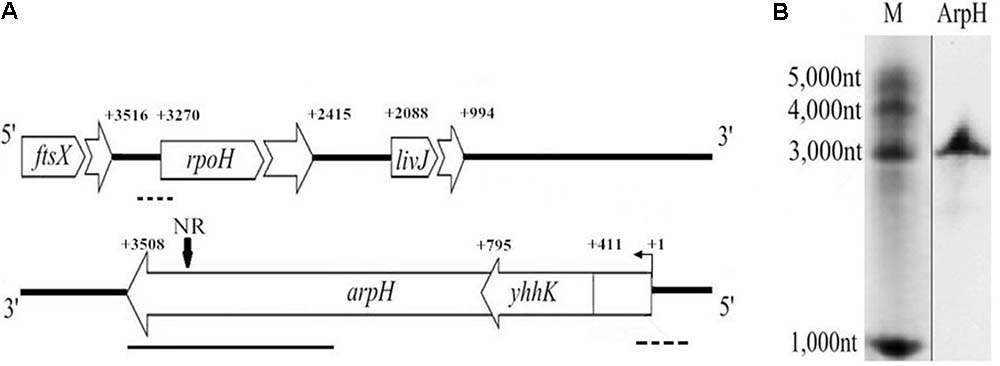
FIGURE 1. Genomic location and northern blotting analysis of arpH. (A) Schematic representation of the genomic location of ftsX, rpoH, livJ, yhhK, and arpH genes. NR represents the specific probe used in the northern blot. Dotted line, a deleted fragment in the rpoH or arpH mutants; bold line, a 1164 bp fragment used for plasmid pBAD-arpH construction; bent arrow, transcription start site; +1, experimentally determined transcription start site of arpH; +number, gene transcription start or termination site. (B) ArpH was detected in S. Typhi by northern blotting analysis using an arpH-specific digoxigenin-labeled cDNA probe.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions
The strains and plasmids used in this study are listed in Table 1. All strains were grown at 37°C in Luria-Bertani (LB) medium supplemented with 100 μg/mL ampicillin or 50 μg/mL kanamycin when required.
Strain Construction
The oligonucleotides used in this study are listed in Table 2. To construct the arpH mutant strain, primer pairs F1A/F1B and F2A/F2B were used to amplify fragments F1 (467 bp) and F2 (409 bp), which are located 355 nt upstream and 80 nt downstream of the arpH gene transcriptional start site, respectively. F1 and F2 were used as templates for a second PCR using primers F1A/F2B to obtain a single 876 bp fragment. BamHI sites were added to the 5′-ends of primers F1A and F2B. The 876 bp fragment was then inserted into the BamHI site of the suicide plasmid pGMB151, carrying the sucrose-sensitivity gene sacB. pGMB151 containing the insert was electroporated into the S. Typhi wild-type strain. Putative arpH mutants were cultured on LB plates containing sucrose. Colonies were screened for inserts by PCR with primers F1A/F2B and by DNA sequencing and mutants were named ΔarpH (Huang et al., 2004).
An rpoH mutant was constructed using the lambda Red recombinase method (Datsenko and Wanner, 2000). A kanamycin resistance (kan) gene was amplified from the pET-28a plasmid using primers rpoHF1A/F1B. The kan sequences in the amplified fragment were flanked with 50 bp sequences complementary to either end of the rpoH promoter region. The deletion fragment is located 171 nt upstream and 60 nt downstream of the rpoH gene transcriptional start site. Electrocompetent cells containing the plasmid pKD46 were transformed with the purified PCR product. The transformants were cultured on LB plates containing kanamycin. rpoH mutant strains were screened by PCR using the primers rpoHF2A/F2B and rpoHF2A/F1B and the mutants named ΔrpoH.
Plasmid Construction
To construct the plasmid pBAD-arpH, primers PA and PB were used to amplify a 1,182 bp fragment of arpH, which consists of 1164bp transcript and 18bp of restriction fragment (Figure 1A). The PCR product was cloned into an NcoI/HindIII-digested pBAD/Myc-His A vector (Invitrogen). The wild-type S. Typhi and ΔrpoH strains were transformed with the recombinant vector pBAD-arpH or the pBAD plasmid alone using electroporation to generate the strains WT-pBAD-arpH, WT-pBAD, ΔrpoH-pBAD-arpH, and ΔrpoH-pBAD. RNase E and RNase III mutants were electroporated with pBAD-arpH to obtain Δrne-arpH and Δrnc-arpH (Dadzie et al., 2013).
RNA Extraction
Salmonella enterica serovar Typhi grown overnight in LB broth was diluted 1:100 and grown at 37°C with shaking at 250 rpm. Total RNA was extracted at OD600 = 0.8 using the RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol for assessment of the presence of ArpH. For overexpression analysis, S. Typhi and ΔrpoH carrying pBAD or pBAD-arpH plasmids were grown in LB broth until the OD600 reached 0.4 and induced by the addition of 0.2% L-arabinose. The cells were harvested by centrifugation at 10,000 × g at 4°C. Total RNA was then extracted using TRIzol Reagent (Invitrogen) followed by treatment with RNase-free DNase I (Takara). RNA was quantified using spectrophotometer at 260 nm.
5′- and 3′-RACE Analysis
We performed 5′ rapid amplification of cDNA ends (5′-RACE) experiments as previously described (Zhang et al., 2015). A total of 10 units of calf intestine alkaline phosphatase (TakaRa) was added to 5 μg total RNA for 60 min at 50°C. One unit of tobacco acid pyrophosphatase (TakaRa) was then added for 60 min at 37°C. Total RNA was ligated using T4 RNA ligase (TakaRa) for 60 min at 16°C. cDNA was synthesized using M-MLV reverse transcriptase (TakaRa) according to the manufacturer’s instructions with an antisense-specific primer (5′RACE RT). The first round of PCR amplification was performed using a 5′-RACE adaptor-specific primer (5′RACE outer primer) and an arpH-specific primer (5′RACE GSP1). A second round of PCR amplification was performed using the primers 5′RACE inner primer and 5′RACE GSP2. The PCR products were extracted, subcloned, and sequenced to confirm the transcription initiation site of the arpH sequence.
3′-RACE was carried out using a SMARTer RACE kit (TakaRa) according to the manufacturer’s instructions. Total RNA was treated with calf intestine alkaline phosphatase (TakaRa) and ligated to the 5′-phosphorylated 3′-RACE adaptor (3′RACE adaptor). Reverse transcription was performed using an arpH-specific primer (3′RACE RT) and an adaptor-specific primer (3′RACE adaptor primer) complementary to the 3′RACE adaptor. The outer and inner PCR reactions were performed using the 3′-RACE adaptor primer and arpH-specific primers (3′RACE GSP1 and 3′RACE GSP2). PCR amplification, cloning, and sequencing were performed as described for 5′-RACE.
Northern Blotting Analysis
To detect ArpH, an arpH-specific cDNA probe was used, which was located from 130 nt to 183 nt downstream of the rpoH gene initiation codon on the complementary strand (Figure 1). A total of 10–20 μg of total RNA was separated on a 6% polyacrylamide gel containing 7 M urea and electroblotted onto HybondTM N+ membranes (GE Healthcare). The cDNA probes were synthesized using a DIG Northern Starter kit (Roche). Membranes were prehybridized in Rapid-Hyb buffer (GE Healthcare). The digoxigenin-labeled riboprobes were hybridized overnight at 42°C with the prehybridized membranes. Membranes were washed as previously described (Zhang et al., 2015). Riboprobed membranes were exposed to KODAK x-ray film at -70°C.
Quantitative Real-Time PCR
Equal amounts of RNA were reverse transcribed into cDNA using random primers (Takara). The primer pairs used for quantitative real-time PCR (qRT-PCR) analysis are shown in Table 2. Reactions were monitored using a C1000 Thermal Cycler (Bio-Rad) and transcript levels were normalized to 5S ribosomal RNA using the 2-ΔΔCt method (Bader et al., 2003). Each experiment was performed according to the manufacturers’ protocols in triplicate.
Growth Curves
Strain growth was determined by measuring the OD600 using a BioPhotometer (Eppendorf). WT-pBAD and WT-pBAD-arpH strains were grown overnight in LB broth containing 100 mg/mL ampicillin, diluted 1:100, and incubated at 37°C with shaking at 250 rpm with the addition of 0.2% L-arabinose. To monitor the growth rate, cells were cultured in LB to an OD600 of 0.4 under acid stress (HCl, pH 4.5), oxidative stress (1 mM H2O2), or osmotic stress (0.3 M NaCl) conditions. The absorbance was measured at 1 h intervals for 24 h and growth curves were determined from biological triplicates.
Microarray Analysis
For gene expression profiling experiments, single colonies of WT-pBAD and WT-pBAD-arpH grown overnight in LB broth containing 100 mg/mL ampicillin were diluted 1:100 and incubated at 37°C with shaking at 250 rpm with 0.2% L-arabinose. Cells were then cultured in LB to an OD600 of 0.4 and treated with 1 mM of H2O2 for 4 h. Total RNA was extracted using the RNeasy Mini kit (Invitrogen) according to the manufacturer’s protocol. Samples were harvested by centrifugation at 10,000 × g at 4°C. Total RNA was isolated using TRIzol Reagent (Invitrogen) followed by treatment with RNase-free DNase I (Takara). A total of 25 μg RNA was used as the template for cDNA synthesis. Genomic microarray analysis of S. Typhi was performed as described previously (Sethi et al., 2010; Du et al., 2011). The microarray was analyzed by comparing spot intensities of WT-pBAD and WT-pBAD-arpH strains (Zhang et al., 2009).
Epithelial Cell Invasion Assay
HeLa cells (2 × 105) were seeded into 24-well tissue culture plates containing RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum and incubated at 37°C in 5% CO2 for 16 h. S. Typhi cultures were grown to an OD600 of 0.4. The bacterial cells were washed thoroughly with phosphate-buffered saline at a multiplicity of infection of 20. The monolayers were either lysed with Triton X-100 to evaluate the level of adherence (T0) or incubated for a further 3 h in medium containing 100 μg/mL gentamicin to eliminate extracellular bacteria and to assess the level of invasion (T90). The invasiveness of S. Typhi was previously demonstrated by our laboratory (Gong et al., 2015).
Statistical Analysis
Results are presented as mean ± SD. Significant differences between groups were assessed using Student’s t-tests or analysis of variance using SPSS software (SPSS Inc.). Differences were considered significant at P < 0.05.
Results
Identification and Expression of ArpH
Recently, several new ncRNAs were identified by deep sequencing analysis of the S. Typhi genome (Dadzie et al., 2013, 2014; Gong et al., 2015; Zhang et al., 2015). The RNA-seq analysis of S. Typhi under stress conditions showed a 1164-nt transcript partially encoded by the minus strand of rpoH, extending from 192 nt upstream to 972 nt downstream from the start codon of rpoH (Figure 1A). In the current study, total RNA was extracted from a wild-type S. Typhi strain and hybridized with an arpH-specific digoxigenin-labeled cDNA probe to confirm the existence of ArpH. We found that the arpH fragment was approximately 3,000 nt (Figure 1B). 5′-RACE was then performed to map the 5′-end of the arpH sequence, which was found to be 411 nt upstream of the yhhK initiation codon (Figure 1A). 3′-RACE revealed that the 3′-end of the arpH sequence was 238 nt upstream of the rpoH initiation codon on the complementary strand. The results of RACE analyses indicated that the total length of the arpH sequence is 3508 nt.
We monitored the levels of ArpH over time and under different stress conditions using northern blotting analysis and qRT-PCR. Total RNA was harvested from wild-type S. Typhi grown to an OD600 of 0.3, 0.8, 1.3, or 2.0, representing the lag phase through the stationary phase (Figure 2A). The expression of ArpH was also measured by qRT-PCR (Figure 2B). To examine levels of ArpH under stress conditions, we simulated the environment that Salmonella encounters upon invasion or within macrophages. Total RNA was extracted after cells were subjected to acid, osmotic, or oxidative stress. Northern blotting and qRT-PCR demonstrated that ArpH levels were lower when S. Typhi was exposed to acidic conditions and higher when exposed to oxidative conditions compared to S. Typhi grown under normal conditions (Figures 3A,B). These results indicate that ArpH is expressed in S. Typhi and that its expression changes in response to stress conditions.
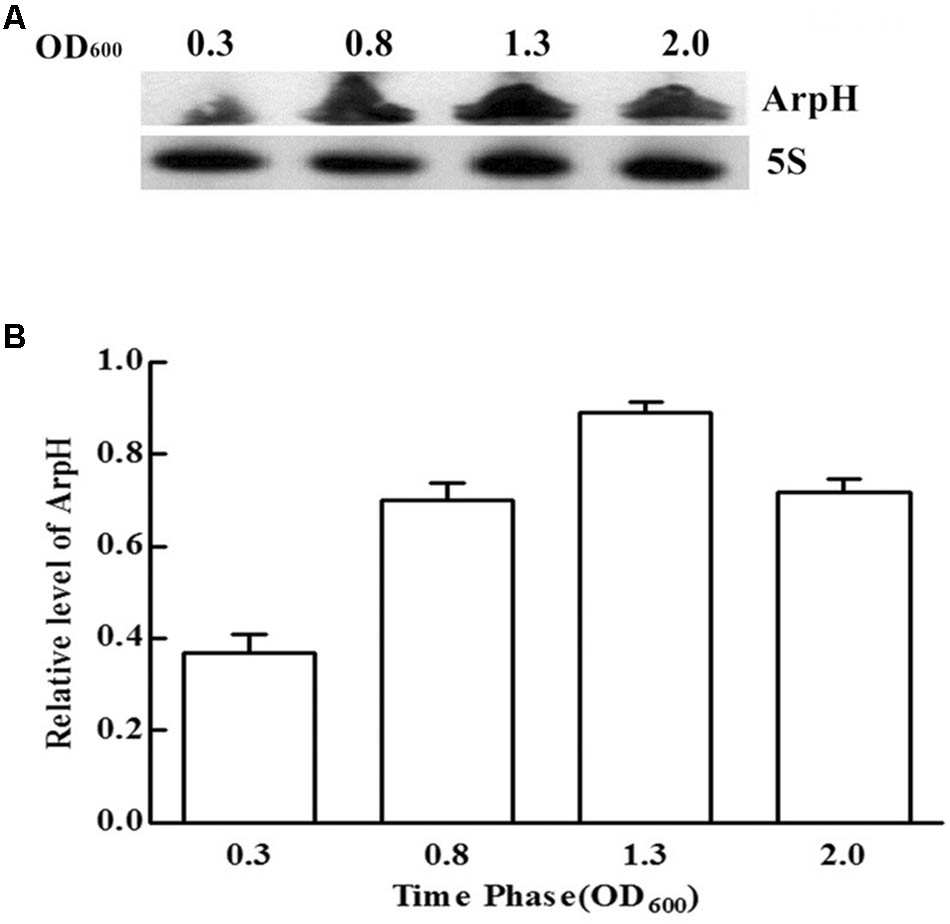
FIGURE 2. ArpH expression levels in S. Typhi grown to different OD600 values. (A) Total RNA was harvested at an OD600 of 0.3, 0.8, 1.3, or 2.0. An arpH-probe was used for northern blotting analysis. The 5S rRNA was used as the control. (B) The levels of ArpH in S. Typhi harvested at an OD600 of 0.3, 0.8, 1.3, or 2.0 were measured using qRT-PCR and normalized to 5S rRNA.
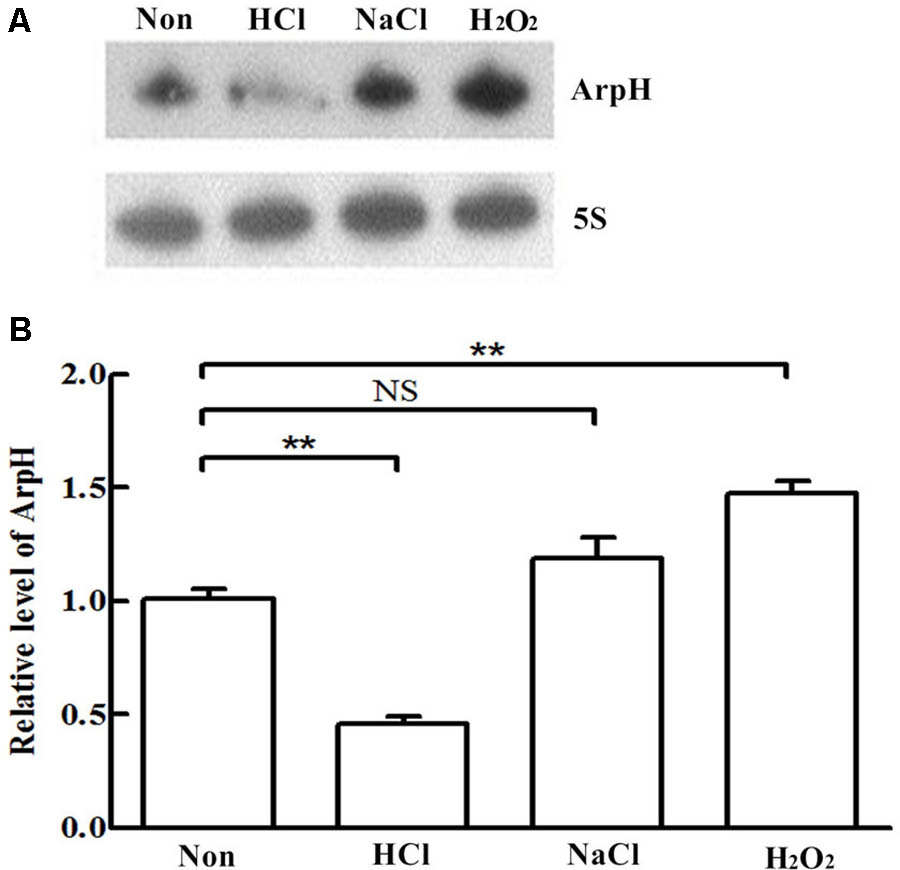
FIGURE 3. ArpH expression under stress conditions. (A) Northern blotting analysis and (B) qRT-PCR analysis of total RNA extracted from wild-type S. Typhi cultured in LB to an OD600 of 0.4 and subjected for 30 min to acid stress (HCl), osmotic stress (NaCl), or oxidative stress (H2O2). The 5S rRNA was used as the internal reference. ∗∗P < 0.01. NS, not statistically significant.
Effect of ArpH Overexpression on rpoH mRNA Levels
To examine the effect of ArpH on its putative mRNA target rpoH, we investigated expression levels of rpoH mRNA using qRT-PCR after overexpressing the partial arpH sequence from the arabinose-inducible recombinant plasmid WT-pBAD-arpH. The partial overexpression of the arpH did not affect the expression of the yhhK, livJ, and rpoH gene, which were estimated with qRT-PCR (data not shown). The relative level of ArpH in WT-pBAD-arpH strain was significantly higher than the WT-pBAD strain by qRT-PCR (data not shown), which were consistent with our expectation. In contrast to WT-pBAD alone, the mRNA level of rpoH increased gradually within 20 min of ArpH overexpression (Figures 4A,B). These results indicate that ArpH positively regulates rpoH mRNA levels.
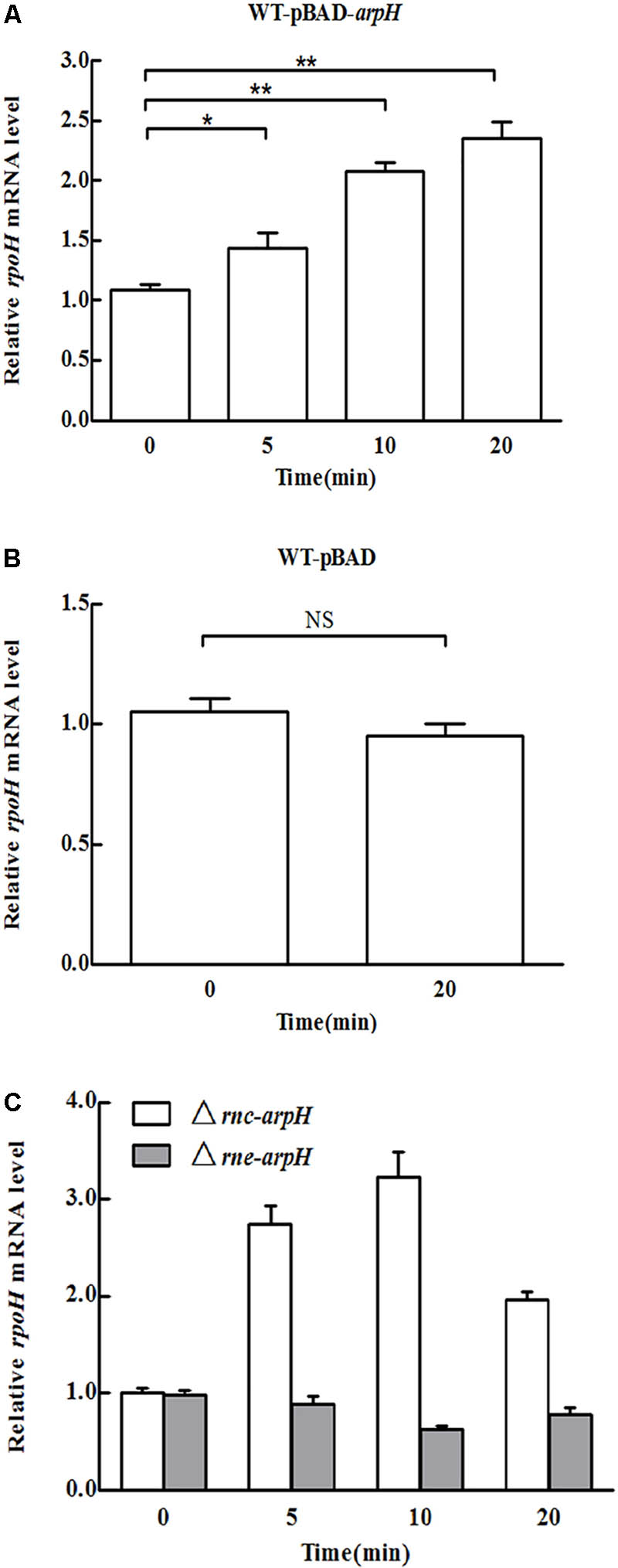
FIGURE 4. Overexpression of ArpH. mRNA levels of rpoH in (A) WT-pBAD-arpH, (B) WT-pBAD, and (C) Δrnc-arpH and Δrne-arpH strains were measured using qRT-PCR. Total RNA was harvested from strains grown to an OD600 of 0.4 after 0, 5, 10, or 20 min of induction with 0.2% L-arabinose. ∗P < 0.05, ∗∗P < 0.01. NS, not statistically significant.
RNase E and RNase III are the main endoribonucleases that cleave antisense RNA-induced target mRNA (Saramago et al., 2014). RNase E cuts RNA at a number of single-stranded regions, whereas RNase III cleaves double-stranded RNA (Arraiano et al., 2010). To determine the effect of ArpH overexpression on rpoH mRNA levels in RNase E and RNase III mutants, we constructed Δrne-arpH and Δrnc-arpH strains. The mRNA expression of rpoH was sixfold higher in RNase III mutants than in the control strains (Figure 4C), whereas the levels were relatively similar in RNase E mutants, indicating that RNase III may play a more significant role in the coupled degradation of the ArpH/RpoH duplex.
Effect of ArpH Overexpression on S. Typhi Growth
We investigated the growth of WT-pBAD and WT-pBAD-arpH strains over a 24 h period. Figure 5A shows that the growth curves of the two strains were similar during the lag and stationary phases; however, during the logarithmic phase, the WT-pBAD-arpH strain grew slightly faster than the WT-pBAD strain. Growth curves were also constructed for the WT-pBAD and WT-pBAD-arpH strains under different growth conditions. The growth curves for WT-pBAD and WT-pBAD-arpH strains were similar under osmotic stress. However, the growth of the WT-pBAD-arpH strain was enhanced under oxidative stress during the early logarithmic phase and overexpression of ArpH resulted in significant reduction of bacterial growth under acid stress during the late logarithmic phase (Figures 5B–D). Additionally, the growth curves of the WT-pBAD and WT-pBAD-arpH strains were similar under thermal stress (data not shown).
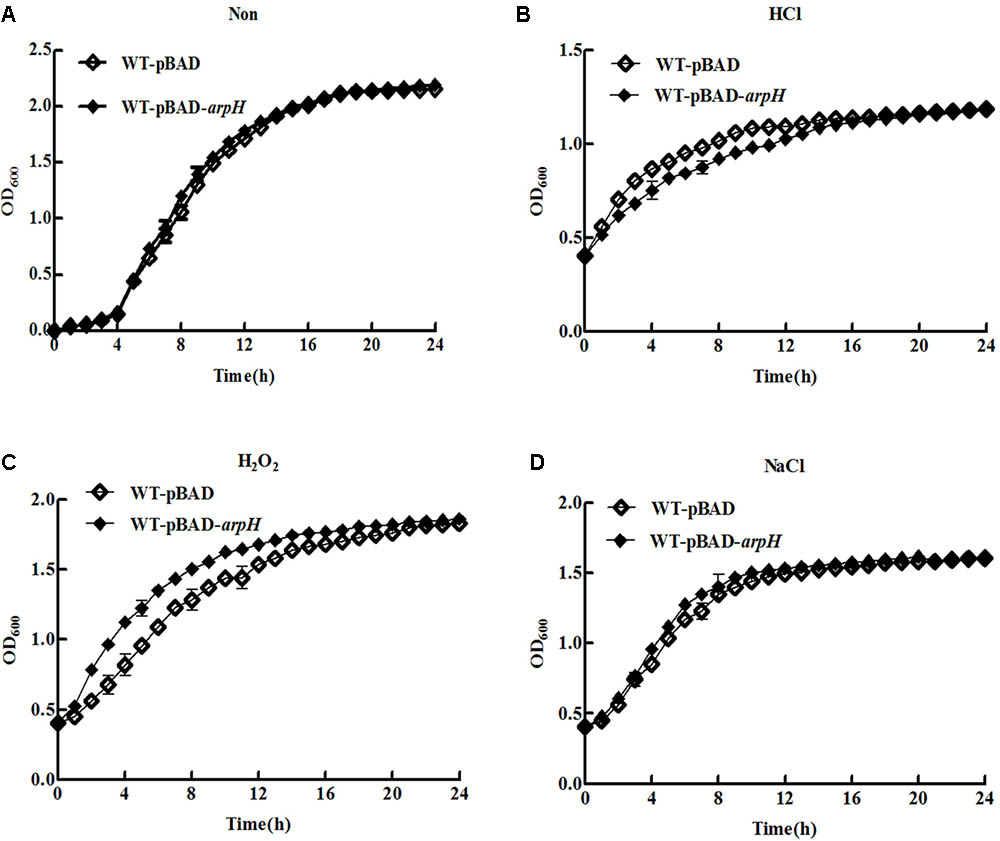
FIGURE 5. Growth curves of WT-pBAD and WT-pBAD-arpH strains. Single colonies of WT-pBAD and WT-pBAD-arpH were cultured overnight at 37°C with shaking, diluted 1:100 in LB medium, and induced with 0.2% L-arabinose. (A) Growth curves of strains grown under normal conditions were generated by measuring the OD600 at 1 h intervals. To assess growth under stress conditions, cells were cultured in LB to an OD600 of 0.4 under stress conditions including (B) acid stress (HCl, pH 4.5), (C) oxidative stress (H2O2, 1 mM), and (D) osmotic stress (NaCl, 0.3 M). Growth curves were generated by measuring the OD600 at 1 h intervals.
Analysis of Genes Regulated by ArpH
To investigate the effect of ArpH overexpression in oxidative stress conditions, a WT-pBAD-arpH strain was created. The wild-type S. Typhi strain was transformed with the recombinant vector pBAD-arpH or the pBAD plasmid by electroporation. The gene expression profile of WT-pBAD-arpH was assessed using a whole genome microarray to investigate the influence of ArpH overexpression in S. Typhi under oxidative stress conditions (Supplementary Material). Microarray results showed differential expression of genes involved in SPI-1 and invasion, flagellar biosynthesis, and virulence between the WT-pBAD-arpH and WT-pBAD strains. SPI-1 and invasion-associated genes, including prgHIK, iagA, sipCDA, invFGA, spaKINM, and tviACDE, were downregulated in the WT-pBAD-arpH strain. Metabolism-associated genes, including astB, msyB, glpD, argD, and sdhDC, were also downregulated in the WT-pBAD-arpH strain. Expression of flagellum-associated genes, such as flgBCDEF, fliACJHLSZ, and flhDC, were not significantly different in the WT-pBAD-arpH strain. However, the superoxide dismutase gene sodB and the oxygen-regulated invasion gene orgA were upregulated in the WT-pBAD-arpH strain. Using qRT-PCR, we confirmed the microarray results by comparing the expression of the genes sodB, orgA, prgH, and sipA between the WT-pBAD-arpH and WT-pBAD strains (Figure 6). These findings suggested that ArpH plays a role in the antioxidant defenses of S. Typhi under oxidative stress conditions (Bang et al., 2005).
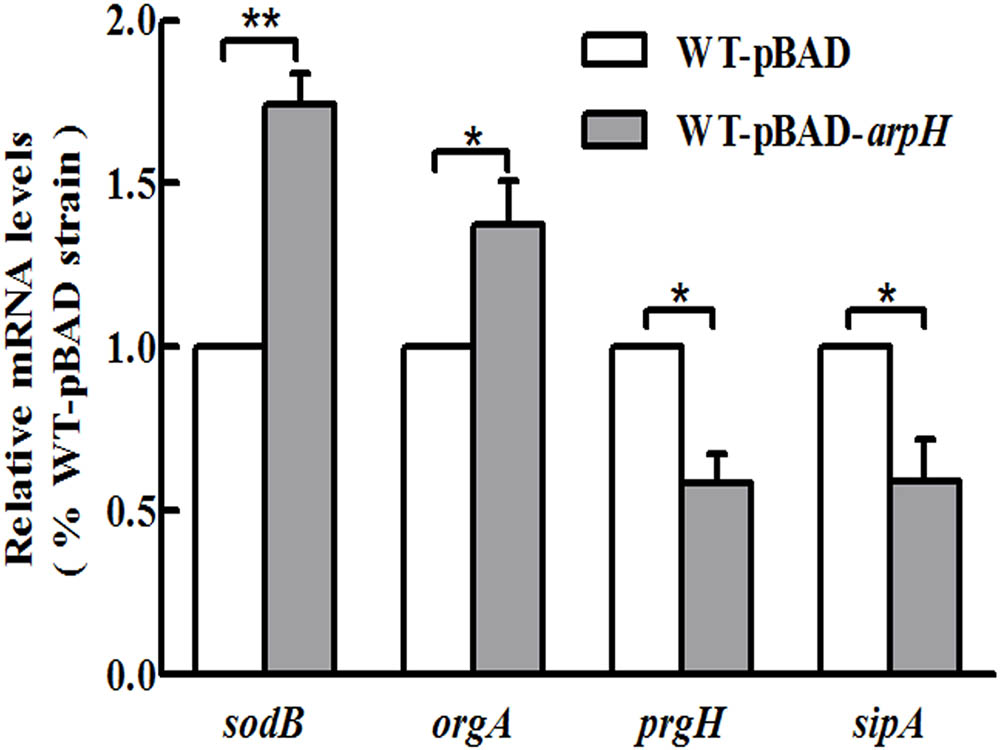
FIGURE 6. Gene expression levels of sodB, orgA, prgH, and sipA. Single colonies of WT-pBAD and WT-pBAD-arpH strains were cultured overnight at 37°C with shaking, diluted 1:100 in LB medium, and induced by 0.2% L-arabinose. Cells were cultured in LB to an OD600 of 0.4 and treated with 1 mM of H2O2 for 4 h. Total RNA was harvested and the relative mRNA levels of sodB, orgA, prgH, and sipA were measured by qRT-PCR. The 5S rRNA was used as the internal reference. ∗P < 0.05, ∗∗P < 0.01.
arpH Deletion Enhances the Invasion of Epithelial Cells by S. Typhi
We constructed an arpH mutant in which the 435-nt arpH fragment was disrupted, whereas the SD box and the ORF structure of yhhK, livJ, and rpoH were retained intact. The deletion of the arpH did not affect the expression of the yhhK, livJ, and rpoH gene, which were estimated with qRT-PCR (data not shown), and the results were consistent with our expectation. An rpoH deletion mutant was also constructed using the lambda Red recombinase method. Because the arpH-encoding region overlaps the rpoH gene and the arpH might influence RpoH function, we investigated the function of rpoH in this study. A rpoH mutant was constructed, in which the deleted region was only the promoter region of rpoH. The deletion of the rpoH did not affect the expression of the arpH gene, which was estimated with qRT-PCR (data not shown), and found to be consistent with our expectation. To examine the possibility that ArpH may play a role in the invasion of epithelial cells by Salmonella, we examined the invasion efficiency of wild-type, ΔarpH, and ΔrpoH strains into HeLa cells. To confirm these findings, we used qRT-PCR to measure the mRNA levels of the SPI-1 and invasion-associated genes prgH, sipA, and invF (Figure 7A). From the results shown in Figure 7B, we concluded that the invasion efficiency of the ΔarpH strain was significantly higher than that of the wild-type strain. Taken together, these findings indicate that ArpH on RpoH might negatively regulate the invasion of S. Typhi into the intestinal epithelium.
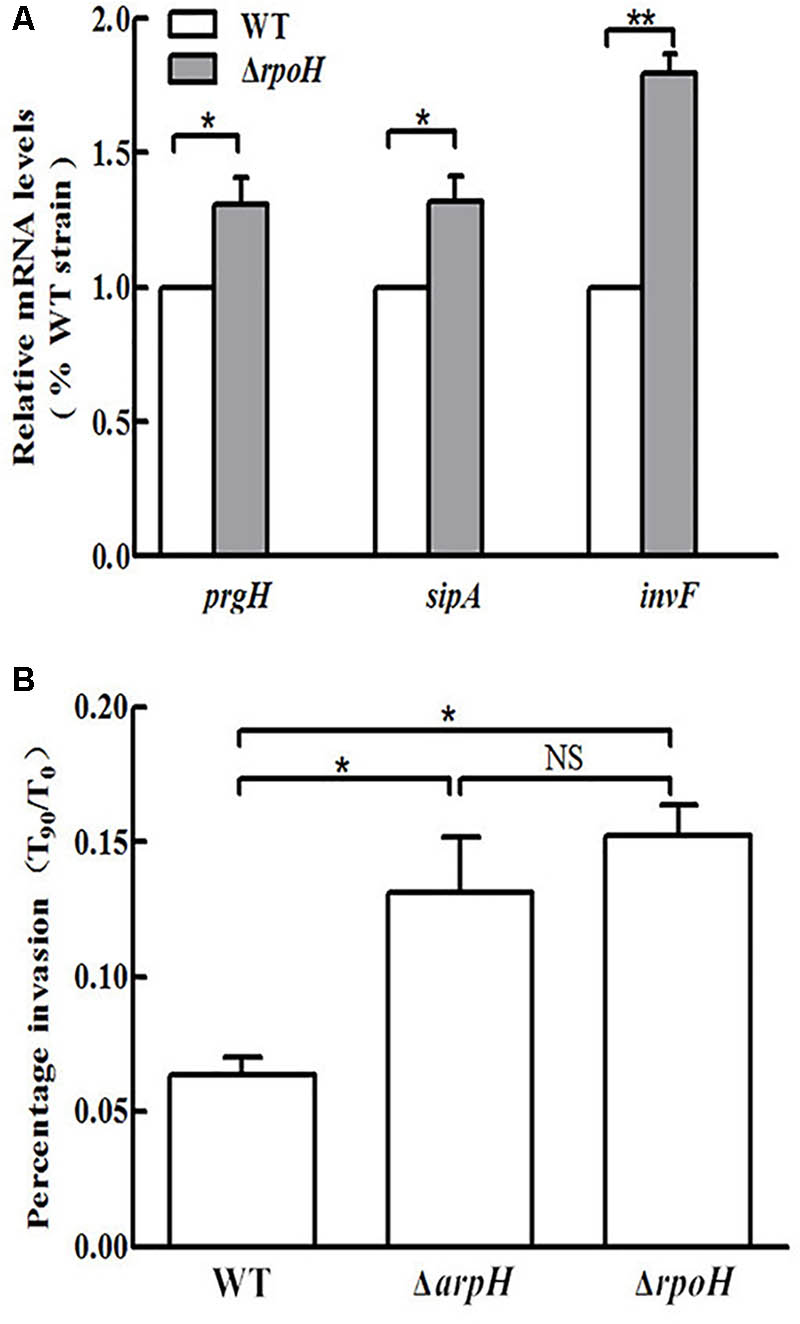
FIGURE 7. Invasion of HeLa cells by wild-type, ΔarpH, and ΔrpoH strains. (A) Total RNA was harvested and the relative mRNA levels of the invasion-associated genes prgH, sipA, and invF were measured in wild-type (WT) and ΔrpoH strains using qRT-PCR. The 5S rRNA was used as the internal reference. (B) The invasiveness of S. Typhi into HeLa cells was measured by counting the number of bacteria at 0 min (T0) and after 90 min (T90). The capacity of S. Typhi to invade HeLa cells in vitro was determined by the ratio of T90 to T0. The data are shown as mean values and standard deviations of three experiments. ∗P < 0.05, ∗∗P < 0.01. NS, not statistically significant.
rpoH Deletion Upregulates Expression of Invasion-Associated Genes
Transcriptome analysis of the rpoH mutant was carried out using a whole genome microarray. Significant upregulation of the invasion-associated genes prgH, sipA, and invF was observed in the ΔrpoH strain. In contrast, no significant increase in the expression levels of these genes was observed in the wild-type strain. To confirm the findings of the microarray experiment, we used qRT-PCR to measure the expression of the prgH, sipA, and invF genes (Figure 7A). The HeLa cell invasion assay demonstrated that the invasion capacity of the ΔrpoH strain was dramatically enhanced compared to the wild-type strain. This result is consistent with previous literature (Matsui et al., 2008). However, the results of the invasion assay demonstrated that there was no significant difference in invasion capacity between the ΔarpH and ΔrpoH mutants (Figure 7B). Taken together, these findings suggest that ArpH might downregulate the invasiveness of S. Typhi by affecting the expression of rpoH.
Effect of ArpH Overexpression on Invasiveness of S. Typhi
In order to further study the mechanism by which ArpH affects the invasiveness of S. Typhi, the wild-type S. Typhi and ΔrpoH strains were transformed with the recombinant vector pBAD-arpH or the pBAD plasmid alone using electroporation to generate the strains WT-pBAD-arpH, WT-pBAD, ΔrpoH-pBAD-arpH, and ΔrpoH-pBAD. We then used qRT-PCR to measure the mRNA levels of the invasion-associated gene invF and examined the invasion efficiency of these strains into HeLa cells. We concluded that the mRNA levels of invF and invasion efficiency of the WT-pBAD-arpH strain were lower than that of the WT-pBAD strain. However, when ArpH was overexpressed in the rpoH mutant, mRNA levels of invF and invasion into HeLa cells were not significantly different from the rpoH mutant alone (Figure 8). These results seem to indicate that the effect of ArpH on the invasiveness of S. Typhi may be due solely to its effects on rpoH. Additionally, the difference of twofold in the HeLa cell invasion assay may be statistically calculated to be significant, but in reality from a biological perspective it is probably almost insignificant. Therefore, the effect of ArpH on the invasiveness of S. Typhi needs further research to exclude experimental variation.
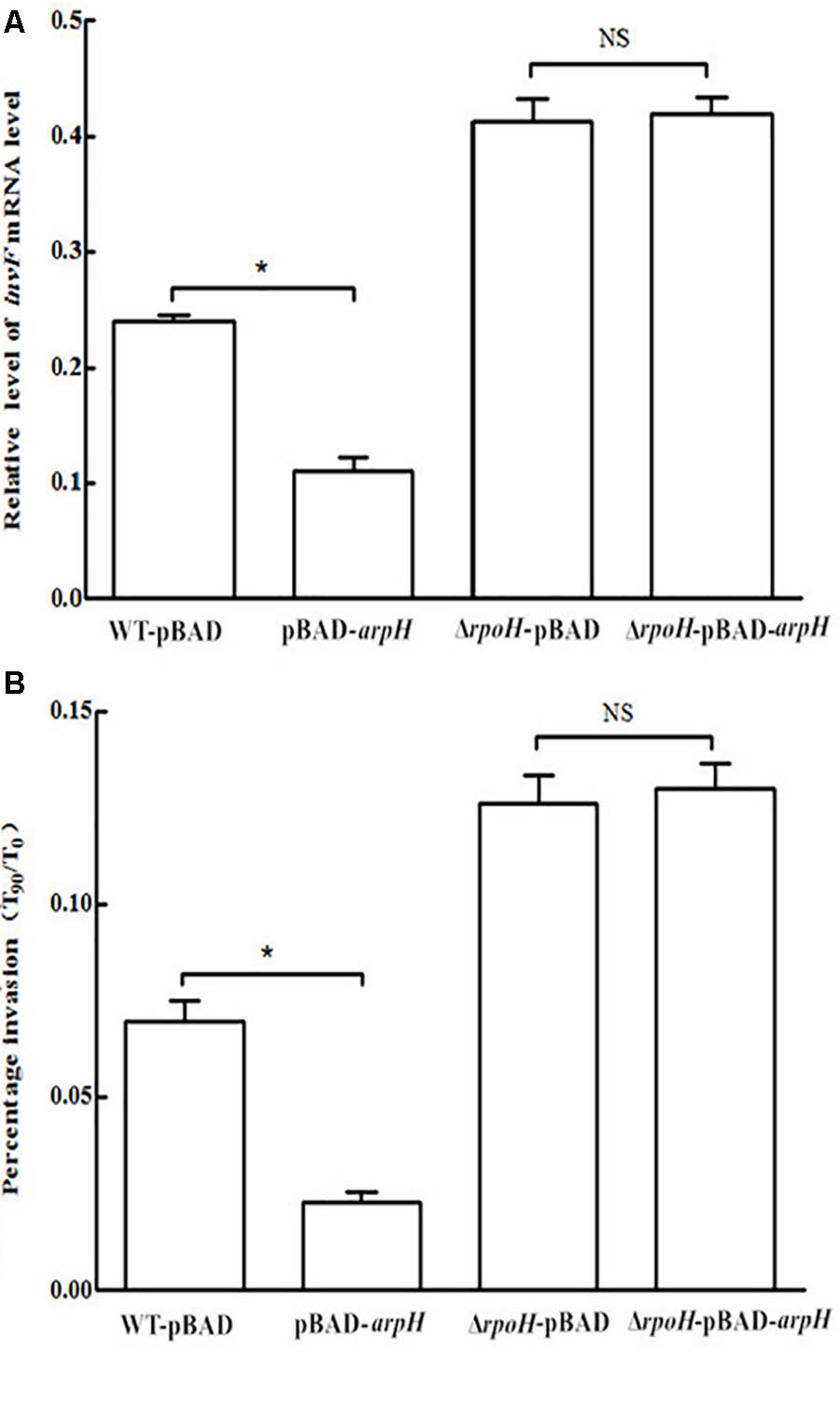
FIGURE 8. Effect of ArpH overexpression on mRNA levels of invF and invasion efficiency of rpoH mutants. (A) The mRNA levels of the invasion-associated gene invF were measured by qRT-PCR. The 5S rRNA was used as the internal reference. (B) Comparison of the invasion efficiencies of WT-pBAD-arpH, WT-pBAD, ΔrpoH-pBAD, and ΔrpoH-pBAD-arpH into HeLa cells. Invasion of HeLa cells was measured by counting the number of bacteria at 0 min (T0) and after 90 min (T90). The capacity of S. Typhi to invade HeLa cells in vitro was determined by the ratio of T90 to T0. The data are shown as mean values and standard deviations of three experiments. ∗P < 0.05. NS, not statistically significant.
Discussion
Many ncRNAs are involved in regulating the expression of genes involved in different biological processes through a variety of mechanisms (Loh et al., 2012; Chew et al., 2018). We applied deep sequencing analysis to discover ncRNAs in bacteria, including those involved in the invasion of epithelial cells by Salmonella (Dadzie et al., 2013, 2014; Gong et al., 2015; Zhang et al., 2015). Recently, novel ncRNAs associated with the response to stress were identified (Gong et al., 2011, 2015). By combining the findings of northern blotting and RACE analyses, we identified the cis-encoded ncRNA ArpH, which is complementary to rpoH mRNA. The full-length antisense RNA transcript was 3,508 nt from 411 nt upstream of the yhhK gene initiation codon to 238 nt upstream of the rpoH gene initiation codon on the complementary strand (Figure 1). No open reading frame region longer than 150 nt or Shine–Dalgarno sequence except for yhhK was identified in the entire sequence. Therefore, we believe that ArpH is a new ncRNA. It is worth mentioning that the yhhK gene might play an ancillary role in pantothenate biosynthesis in S. enterica (Stuecker et al., 2012). Because the arpH-encoding region overlaps the yhhK gene and the arpH might influence YhhK function. We constructed ArpH deletion mutant and overexpression strains away from yhhK coding region, which were estimated with qRT-PCR (Supplementary Figures S1, S2). In addition, the livJ gene is a pseudogene in S. Typhi.
We performed northern blotting and qRT-PCR to determine the expression of ArpH in S. Typhi. Peak expression of ArpH occurred in the late logarithmic phase (Figure 2). ArpH expression was reduced in acid stress conditions and increased in oxidative stress conditions (Figure 3). Based on these results, we investigated the effect of ArpH expression on bacterial growth. Growth curves of wild-type and ArpH overexpressing strains were similar during the lag and stationary phases. However, during the logarithmic phase, when the levels of endogenous ArpH transcripts were highest, the overexpression strain grew slightly more quickly than the wild-type strain (Figure 5A). Growth curves were also generated for WT-pBAD and WT-pBAD-arpH strains under different growth conditions. The growth curves of both strains were similar under osmotic stress. However, the growth of the WT-pBAD-arpH strain was enhanced under oxidative stress during the early logarithmic phase and overexpression of ArpH resulted in significant reduction of bacterial growth under acid stress during the late logarithmic phase (Figures 5B–D), indicating that the expression of ArpH enhances growth under oxidative stress conditions (Bang et al., 2005; Nuss et al., 2009; Remes et al., 2017). These findings are in accordance with the observation that, in general, ncRNAs are activated in response to different environmental conditions and that they function to help cells adapt to stress (Waters and Storz, 2009).
ncRNAs can regulate the translation of target genes by altering the stability of mRNAs (Ayupe and Reis, 2017). To clarify the effect of ArpH on its putative mRNA target rpoH, we investigated the expression levels of rpoH mRNA using qRT-PCR after overexpressing the partial arpH sequence from the arabinose-inducible recombinant plasmid WT-pBAD-arpH. rpoH mRNA levels increased gradually within 20 min of ArpH overexpression compared with WT-pBAD (Figures 4A,B). These results indicate that overexpression of ArpH positively regulates rpoH mRNA levels. To further determine the effect of ArpH overexpression on rpoH mRNA levels, we constructed RNase E and RNase III mutants. Expression levels of rpoH mRNA increased significantly in RNase III mutants (Figure 4C), whereas levels were relatively similar in RNase E mutants, indicating that RNase III may play a more significant role in the coupled degradation of the ArpH/RpoH duplex (Nicholson, 2014). Although some antisense RNAs inhibit target sense mRNA expression, many sense-antisense RNA pairs promote coordinated expression, suggesting that antisense RNAs may be involved in regulating the stability of cis-encoded mRNAs (Chinni et al., 2010; Lee and Groisman, 2010).
Microarray experiments showed that genes involved in SPI-1 and invasion, flagellar biosynthesis, and virulence were differentially expressed between WT-pBAD-arpH and WT-pBAD strains under oxidative stress conditions (see Supplementary Material). The superoxide dismutase gene sodB and the oxygen-regulated invasion gene orgA were upregulated in the WT-pBAD-arpH strain (Figure 6). The orgA gene is involved in promoting cellular invasion of the pathogen. Previously published work has indicated that the prgH, -I, -J, and -K genes are transcribed from a promoter distinct from that used by the gene immediately downstream, orgA (Klein et al., 2000). However, its exact role in virulence is still unclear mainly due to difficulties in understanding its complex regulation. Previous studies have been consistent regarding oxygen regulation of orgA (Russell et al., 2004). The reason may be that orgA is mainly regulated by oxidative stress. This suggests that ArpH may be involved in regulating the anti-oxygenation of S. Typhi under oxidative stress conditions (Glaeser et al., 2011). These findings are in agreement with the fact that the WT-pBAD-arpH strain grew more quickly than the WT-pBAD strain under oxidative stress conditions. Taken together, these data suggested that ArpH played a role in the antioxidant defenses of S. Typhi under oxidative stress conditions. These results are consistent with literature (Bang et al., 2005; Matsui et al., 2008). The microarray data revealed that the expression of flagellum-associated genes, such as flgBCDEF, fliACJHLSZ, and flhDC, were not significantly different in the WT-pBAD-arpH strain (see Supplementary Material). This indicates that ArpH does not participate in the regulation of S. Typhi motility. In addition, no significant changes were observed in the motility phenotypes of the WT-pBAD-arpH or WT-pBAD strains in motility agar (data not shown), which is in agreement with the microarray data.
A type III secretion system encoded by SPI-1 mediates the invasion of epithelial cells by Salmonella (Hensel, 2004). The deletion of ArpH increased bacterial invasion compared to wild-type cells (Figure 7). And the mRNA levels of invF and invasion efficiency of the WT-pBAD-arpH strain were lower than that of the WT-pBAD strain (Figure 8). This corresponds to the microarray results, which showed that invasion-associated genes, including prgH and sipA, were downregulated when ArpH was overexpressed (Figure 6). Our research is the first to demonstrate that ArpH is a player in host cell invasion by S. Typhi. It was previously reported that RpoH regulates virulence in many bacteria, including Salmonella (Bang et al., 2005; Delory et al., 2006; Grall et al., 2009; De la Cruz et al., 2016). Additionally, RpoH negatively regulates SPI-1 expression (Matsui et al., 2008). Due to the increase in expression of invasion-related genes, and in bacterial invasion observed in both arpH and rpoH deletion mutants, we hypothesize that ArpH may affect Salmonella invasiveness by acting on rpoH (Figure 7). When ArpH was overexpressed in rpoH mutants, the mRNA levels of invF and the invasion efficiency into HeLa epithelial cells were not significantly different from wild-type cells (Figure 8). These results seem to indicate that the effect of ArpH on the invasiveness of S. Typhi may be due solely to rpoH. We hypothesize that the mechanism by which ArpH affects Salmonella invasiveness is through the activation of rpoH expression (Bang et al., 2005; Matsui et al., 2008). However, while rpoH may have a small effect on the invasion phenotype, there are several confusing details about how arpH alters transcription of invasion genes, therefore, further detailed research is needed.
Conclusion
The full-length arpH sequence was found to be 3,508 nt located 411 nt upstream of the yhhK initiation codon and 238 nt upstream of the rpoH initiation codon on the complementary strand. ArpH is likely to affect the expression of rpoH in cis at the transcriptional level.
Author Contributions
CX and XH conceived and designed the experiments. CX, XL, XZ, and JL performed the experiments. CX, XL, and XH analyzed the data. CX, SX, and XL contributed reagents, materials, and analysis tools. CX and XH wrote the manuscript.
Funding
The research was supported by the National Natural Science Foundation of China (81371780 and 31670131), the Research Innovation Program for Postgraduates of Jiangsu Province (CXLX13_692).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00978/full#supplementary-material
References
Aiso, T., Murata, M., and Gamou, S. (2011). Transcription of an antisense RNA of a gadE mRNA is regulated by GadE, the central activator of the acid resistance system in Escherichia coli. Genes Cells 16, 670–680. doi: 10.1111/j.1365-2443.2011.01516.x
Arraiano, C. M., Andrade, J. M., Domingues, S., Guinote, I. B., Malecki, M., Matos, R. G., et al. (2010). The critical role of RNA processing and degradation in the control of gene expression. FEMS Microbiol. Rev. 34, 883–923. doi: 10.1111/j.1574-6976.2010.00242.x
Ayupe, A. C., and Reis, E. M. (2017). Evaluating the stability of mRNAs and noncoding RNAs. Methods Mol. Biol. 1468, 139–153. doi: 10.1007/978-1-4939-4035-6_11
Bader, M. W., Navarre, W. W., Shiau, W., Nikaido, H., Frye, J. G., McClelland, M., et al. (2003). Regulation of Salmonella Typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50, 219–230. doi: 10.1046/j.1365-2958.2003.03675.x
Bang, I. S., Frye, J. G., McClelland, M., Velayudhan, J., and Fang, F. C. (2005). Alternative sigma factor interactions in Salmonella: σE and σH promote antioxidant defences by enhancing σS levels. Mol. Microbiol. 56, 811–823. doi: 10.1111/j.1365-2958.2005.04580.x
Bardill, J. P., and Hammer, B. K. (2012). Non-coding sRNAs regulate virulence in the bacterial pathogen Vibrio cholerae. RNA Biol. 9, 392–401. doi: 10.4161/rna.19975
Chew, C. L., Conos, S. A., Unal, B., and Tergaonkar, V. (2018). Noncoding RNAs: master regulators of inflammatory signaling. Trends Mol. Med 24, 66–84. doi: 10.1016/j.molmed.2017.11.003
Chinni, S. V., Raabe, C. A., Zakaria, R., Randau, G., Hoe, C. H., Zemann, A., et al. (2010). Experimental identification and characterization of 97 novel ncRNA candidates in Salmonella enterica serovar Typhi. Nucleic Acids Res. 38, 5893–5908. doi: 10.1093/nar/gkq281
Dadzie, I., Ni, B., Gong, M., Ying, Z., Zhang, H., Sheng, X., et al. (2014). Identification and characterization of a cis antisense RNA of the parC gene encoding DNA topoisomerase IV of Salmonella enterica serovar Typhi. Res. Microbiol. 165, 439–446. doi: 10.1016/j.resmic.2014.05.032
Dadzie, I., Xu, S., Ni, B., Zhang, X., Zhang, H., Sheng, X., et al. (2013). Identification and characterization of a cis-encoded antisense RNA associated with the replication process of Salmonella enterica serovar Typhi. PLoS One 8:e61308. doi: 10.1371/journal.pone.0061308
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
De la Cruz, M. A., Morgan, J. K., Ares, M. A., Yáñez-Santos, J. A., Riordan, J. T., and Girón, J. A. (2016). The two-component system CpxRA negatively regulates the locus of enterocyte effacement of enterohemorrhagic Escherichia coli involving σ(32) and Lon protease. Front. Cell Infect. Microbiol. 6:11. doi: 10.3389/fcimb.2016.00011
Delory, M., Hallez, R., Letesson, J. J., and De Bolle, X. (2006). An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M. J. Bacteriol. 188, 7707–7710. doi: 10.1128/JB.00644-06
Dougan, G., and Baker, S. (2014). Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol. 68, 317–336. doi: 10.1146/annurev-micro-091313-103739
Du, H., Wang, M., Luo, Z., Ni, B., Wang, F., Meng, Y., et al. (2011). Coregulation of gene expression by sigma factors RpoE and RpoS in Salmonella enterica serovar Typhi during hyperosmotic stress. Curr. Microbiol. 62, 1483–1489. doi: 10.1007/s00284-011-9890-8
Gerstle, K., Klätschke, K., Hahn, U., and Piganeau, N. (2012). The small RNA RybA regulates key-genes in the biosynthesis of aromatic amino acids under peroxide stress in E. coli. RNA Biol. 9, 458–468. doi: 10.4161/rna.19065
Ghosal, S., Das, S., and Chakrabarti, J. (2013). Long noncoding RNAs: new players in the molecular mechanism for maintenance and differentiation of pluripotent stem cells. Stem Cells Dev. 22, 2240–2253. doi: 10.1089/scd.2013.0014
Glaeser, J., Nuss, A. M., Berghoff, B. A., and Klug, G. (2011). Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 58, 141–173. doi: 10.1016/B978-0-12-381043-4.00004-0
Gong, H., Vu, G. P., Bai, Y., Chan, E., Wu, R., Yang, E., et al. (2011). A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors. PLoS Pathog. 7:e1002120. doi: 10.1371/journal.ppat.1002120
Gong, M., Xu, S., Jin, Y., Zhang, Y., Dadzie, I., Zhang, X., et al. (2015). 5′-UTR of malS increases the invasive capacity of Salmonella enterica serovar Typhi by influencing the expression of bax. Future Microbiol. 10, 941–954. doi: 10.2217/fmb.15.12
Grall, N., Livny, J., Waldor, M., Barel, M., Charbit, A., and Meibom, K. L. (2009). Pivotal role of the Francisella tularensis heat-shock sigma factor RpoH. Microbiology 155, 2560–2572. doi: 10.1099/mic.0.029058-0
Hensel, M. (2004). Evolution of pathogenicity islands of Salmonella enterica. Int. J. Med. Microbiol. 294, 95–102. doi: 10.1016/j.ijmm.2004.06.025
Huang, X., Phung Le, V., Dejsirilert, S., Tishyadhigama, P., Li, Y., Liu, H., et al. (2004). Cloning and characterization of the gene encoding the z66 antigen of Salmonella enterica serovar Typhi. FEMS Microbiol. Lett. 234, 239–246. doi: 10.1111/j.1574-6968.2004.tb09539.x
Kaikkonen, M. U., Lam, M. T., and Glass, C. K. (2011). Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 90, 430–440. doi: 10.1093/cvr/cvr097
Klein, J. R., Fahlen, T. F., and Jones, B. D. (2000). Transcriptional organization and function of invasion genes within Salmonella enterica serovar Typhimurium pathogenicity island 1, including the prgH, prgI, prgJ, prgK, orgA, orgB, and orgC genes. Infect. Immun. 68, 3368–3376. doi: 10.1128/IAI.68.6.3368-3376.2000
Lee, E. J., and Groisman, E. A. (2010). An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol. Microbiol. 76, 1020–1033. doi: 10.1111/j.1365-2958.2010.07161.x
Loh, E., Memarpour, F., Vaitkevicius, K., Kallipolitis, B. H., Johansson, J., and Sondén, B. (2012). An unstructured 5’-coding region of the prfA mRNA is required for efficient translation. Nucleic Acids Res. 40, 1818–1827. doi: 10.1093/nar/gkr850
López-Leal, G., Cevallos, M. A., and Castillo-Ramírez, S. (2016). Evolution of a sigma factor: an all-in-one of gene duplication, horizontal gene transfer, purifying selection, and promoter differentiation. Front. Microbiol. 7:581. doi: 10.3389/fmicb.2016.00581
Martinez-Salazar, J. M., Sandoval-Calderon, M., Guo, X., Castillo-Ramirez, S., Reyes, A., Loza, M. G., et al. (2009). The Rhizobium etli RpoH1 and RpoH2 sigma factors are involved in different stress responses. Microbiology 155, 386–397. doi: 10.1099/mic.0.021428-0
Matsui, M., Takaya, A., and Yamamoto, T. (2008). σ32-mediated negative regulation of Salmonella pathogenicity island 1 expression. J. Bacteriol. 190, 6636–6645. doi: 10.1128/JB.00744-08
Miyakoshi, M., Chao, Y., and Vogel, J. (2015). Cross talk between ABC transporter mRNAs via a target mRNA-derived sponge of the GcvB small RNA. EMBO J. 34, 1478–1492. doi: 10.15252/embj.201490546
Nicholson, A. W. (2014). Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip. Rev. RNA 5, 31–48. doi: 10.1002/wrna.1195
Nieto, P. A., Pardo-Roa, C., Salazar-Echegarai, F. J., Tobar, H. E., Coronado-Arrázola, I., Riedel, C. A., et al. (2016). New insights about excisable pathogenicity islands in Salmonella and their contribution to virulence. Microbes. Infect. 18, 302–309. doi: 10.1016/j.micinf.2016.02.001
Nuss, A. M., Glaeser, J., and Klug, G. (2009). RpoH(II) activates oxidative-stress defense systems and is controlled by RpoE in the singlet oxygen-dependent response in Rhodobacter sphaeroides. J. Bacteriol. 191, 220–230. doi: 10.1128/JB.00925-08
Remes, B., Rische-Grahl, T., Müller, K. M. H., Förstner, K. U., Yu, S. H., Weber, L., et al. (2017). An RpoHI-dependent response promotes outgrowth after extended stationary phase in the alphaproteobacterium Rhodobacter sphaeroides. J. Bacteriol 199, e249–e217. doi: 10.1128/JB.00249-17
Russell, D. A., Dooley, J. S., and Haylock, R. W. (2004). The steady-state orgA specific mRNA levels in Salmonella enterica serovar Typhimurium are repressed by oxygen during logarithmic growth phase but not early-stationary phase. FEMS Microbiol. Lett. 236, 65–72. doi: 10.1016/j.femsle.2004.05.025
Saramago, M., Bárria, C., Dos Santos, R. F., Silva, I. J., Pobre, V., Domingues, S., et al. (2014). The role of RNases in the regulation of small RNAs. Curr. Opin. Microbiol. 18, 105–115. doi: 10.1016/j.mib.2014.02.009
Sethi, D., Kumar, A., Gandhi, R. P., Kumar, P., and Gupta, K. C. (2010). New protocol for oligonucleotide microarray fabrication using SU-8-coated glass microslides. Bioconjug. Chem. 21, 1703–1708. doi: 10.1021/bc100262n
Storz, G., Vogel, J., and Wassarman, K. M. (2011). Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 43, 880–891. doi: 10.1016/j.molcel.2011.08.022
Stuecker, T. N., Hodge, K. M., and Escalante-Semerena, J. C. (2012). The missing link in coenzyme A biosynthesis: PanM (formerly YhhK), a yeast GCN5 acetyltransferase homologue triggers aspartate decarboxylase (PanD) maturation in Salmonella enterica. Mol. Microbiol. 84, 608–619. doi: 10.1111/j.1365-2958.2012.08046.x
Waters, L. S., and Storz, G. (2009). Regulatory RNAs in bacteria. Cell 136, 615–628. doi: 10.1016/j.cell.2009.01.043
Zhang, H., Sheng, X., Xu, S., Gao, Y., Du, H., Li, J., et al. (2009). Global transcriptional response of Salmonella enterica serovar Typhi to anti-z66 antiserum. FEMS Microbiol. Lett. 298, 51–55. doi: 10.1111/j.1574-6968.2009.01692.x
Keywords: Salmonella enterica serovar Typhi, antisense RNA, ArpH, rpoH, invasion
Citation: Xiong C, Li X, Liu J, Zhao X, Xu S and Huang X (2018) Identification and Characterization of a Cis Antisense RNA of the rpoH Gene of Salmonella enterica Serovar Typhi. Front. Microbiol. 9:978. doi: 10.3389/fmicb.2018.00978
Received: 01 February 2018; Accepted: 25 April 2018;
Published: 15 May 2018.
Edited by:
Jonathan Michael Plett, Western Sydney University, AustraliaReviewed by:
Bradley D. Jones, University of Iowa, United StatesOlga Soutourina, UMR9198 Institut de Biologie Intégrative de la Cellule (I2BC), France
Copyright © 2018 Xiong, Li, Liu, Zhao, Xu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxiang Huang, aHV4aW54QHVqcy5lZHUuY24=
 Changyan Xiong
Changyan Xiong Xuejiao Li1,3
Xuejiao Li1,3 Xinxiang Huang
Xinxiang Huang