- Institute of Synthetic Biology, Biomedical Center, Guangdong Province Key Laboratory of Improved Variety Reproduction in Aquatic Economic Animals and South China Sea Bio-Resource Exploitation and Utilization Collaborative Innovation Center, School of Life Sciences, Sun Yat-sen University, Guangzhou, China
α-Pinene is a natural and active monoterpene, which is widely used as a flavoring agent and in fragrances, pharmaceuticals, and biofuels. Although it has been successfully produced by genetically engineered microorganisms, the production level of pinene is much lower than that of hemiterpene (isoprene) and sesquiterpenes (farnesene) to date. We first improved pinene tolerance to 2.0% and pinene production by adaptive laboratory evolution after atmospheric and room temperature plasma (ARTP) mutagenesis and overexpression of the efflux pump to obtain the pinene tolerant strain Escherichia coli YZFP, which is resistant to fosmidomycin. Through error-prone PCR and DNA shuffling, we isolated an Abies grandis geranyl pyrophosphate synthase variant that outperformed the wild-type enzyme. To balance the expression of multiple genes, a tunable intergenic region (TIGR) was inserted between A. grandis GPPSD90G/L175P and Pinus taeda Pt1Q457L. In an effort to improve the production, an E. coli-E. coli modular co-culture system was engineered to modularize the heterologous mevalonate (MEV) pathway and the TIGR-mediated gene cluster of A. grandis GPPSD90G/L175P and P. taeda Pt1Q457L. Specifically, the MEV pathway and the TIGR-mediated gene cluster were integrated into the chromosome of the pinene tolerance strain E. coli YZFP and then evolved to a higher gene copy number by chemically induced chromosomal evolution, respectively. The best E. coli-E. coli co-culture system of fermentation was found to improve pinene production by 1.9-fold compared to the mono-culture approach. The E. coli-E. coli modular co-culture system of whole-cell biocatalysis further improved pinene production to 166.5 mg/L.
Introduction
α-Pinene is a natural and active monoterpene, which is widely used in flavorings, fragrances, insecticides, pharmaceuticals, and fine chemicals (Breitmaier, 2006; Behr and Johnen, 2009; Kirby and Keasling, 2009; Gandini and Lacerda, 2015). It was recently produced as a candidate renewable jet fuel due to its favorable energy content, cold weather properties, and high octane/cetane numbers (George et al., 2015). The main source of pinene is turpentine, a by-product of the wood pulp industry (Behr and Johnen, 2009). However, this extraction from plants is tedious and inefficient and requires substantial expenditure of natural resources due to low content (Chang and Keasling, 2006). Therefore, there is much interest in developing biotechnologies for pinene production from renewable resources by engineering microorganisms. Similar to other monoterpenes, α-pinenes are biosynthesized from the C5 intermediates isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) via geranyl diphosphate synthase (GPPS). The head-to-tail condensation produces geranyl diphosphate (GPP, C10), which is, in turn, cyclized by pinene synthase (PS) to produce either α- or β-pinene. Escherichia coli (Yang et al., 2013; Sarria et al., 2014; Tashiro et al., 2016) and Corynebacterium glutamicum (Kang et al., 2014) have been engineered to produce α-pinene. α-Pinene (5.4 mg/L) has been produced in engineered E. coli through the introduction of a heterologous mevalonate (MEV) pathway and α-pinene synthase (Pt30) from Pinus taeda (Yang et al., 2013). The combinatorial expression of Abies grandis GGPS-PS fusion proteins enhanced pinene production (32 mg/L) in E. coli (Sarria et al., 2014). The directed evolution of α-pinene synthase (Pt1) from P. taeda increased α-pinene productivity. E. coli plasmid-expressing the evolved α-pinene synthase (Pt1Q457L) from P. taeda, MEV pathway enzymes, IPP isomerase and A. grandis GGPS produced the highest levels of pinene (140 mg/L) in a flask culture to date (Tashiro et al., 2016). The coexpression of native 1-deoxy-d-xylulose-5-phosphate synthase (Dxs) and isopentenyl diphosphate isomerase (Idi) with P. taeda PS and A. grandis GPPS in C. glutamicum yielded a pinene level of 27 μg/g cell dry weight (Kang et al., 2014).
However, the production level of pinene is much lower than that of hemiterpene (isoprene) (Whited et al., 2010) and sesquiterpenes (farnesene) (Zhu et al., 2014) to date. Pinene is highly toxic to E. coli. E. coli growth is inhibited by 0.5% pinene (Dunlop et al., 2011). The inherent tolerance of E. coli may limit the production potential. It was demonstrated that increasing the tolerance of E. coli by overexpressing the efflux pump AcrBDFa (YP_692684) from Alcanivorax borkumensis significantly enhanced limonene production (Dunlop et al., 2011). Another reason for the lower yield may be that PS has a lower expression level and/or lower enzymatic activity in E. coli. Thus, we first combined tolerance engineering with directed evolution of the enzyme to improve pinene production in E. coli.
Recently, there has emerged a new modular co-culture engineering approach for engineering microorganisms. Modular co-culture engineering approaches divide a complete biosynthetic pathway into separate serial modules, which are introduced into different strains to accommodate individual modules for achieving designed biosynthesis (Zhang and Wang, 2016). The advantages of using modular co-culture engineering include the following: (1) reducing the metabolic burden on each host strain; (2) providing diversified cellular environments for functional expression of the different pathway genes; (3) reducing the undesired interference of different pathways; (4) easily balancing the biosynthetic pathway between individual pathway modules by simply changing the strain-to-strain ratio; (5) high-efficiency utilization of complex materials containing multiple active substrates; and (6) supporting the plug-and-play biosynthesis of various target products (Zhang and Wang, 2016). Thus, modular co-culture engineering was also used to enhance pinene production in E. coli.
Materials and Methods
Strains, Plasmids, and Primers
The bacterial strains and plasmids used in this study are listed in Table 1. The primers used in this study are listed in Supplementary Table 1.
Genetic Methods
pMEVI was derived from pJBEI-6409 (Alonso-Gutierrez et al., 2013), which was obtained from Addgene. pJBEI-6409 contains six genes of the MEV pathway (atoB from E. coli, HMGS, and HMGR from Staphylococcus aureus and MK, PMK, and PMD from Saccharomyces cerevisiae, idi from E. coli, GPPS from A. grandis, and limonene synthase gene (LS) from Mentha spicata). The GPPS-LS gene cluster was removed from pJBEI-6409 to obtain pMEVI. The fusion gene cluster of the codon-optimized GPPS and PS from A. grandis with a (GSG)2 linker was synthesized by Suzhou GENEWIZ, Inc. (Suzhou, China) and ligated into pQE30 to obtain pQE-GPPS-L-PS. The GPPS-PS gene cluster from pQE-GPPS-L-PS was inserted into the BamHI/XhoI sites of pMEVI to obtain pMEVIGPS. The evolved codon-optimized Pt1 (Pt1Q457L) from P. taeda was synthesized by Suzhou GENEWIZ, Inc. (Suzhou, China) and ligated into pQE30 to obtain pQE-Pt1Q457L. The PS gene of pQE-GPPS-L-PSDNAshuffling was replaced with the Pt1Q457L gene to obtain pQE-GPPSMUT-L-Pt1Q457L.
The acrB and acrAB were amplified from E. coli and inserted into pZEABP to obtain pZEA-acrB and pZEA-acrAB, respectively. The P. putida KT2440 ttgB, P. putida KT244 mexF, and A. borkumensis acrBDFa were amplified from pBbA5K-EPL11, pBbA5K-EPL14, and pBbA5K-EPL95 and inserted into pZEABP to obtain pZEA-ttgB, pZEA-mexF, and pZEA-acrBDFa, respectively.
The TIGR-mediated GPPSMUT-Pt1Q457L gene cluster was cut from pQE-GPPSMUT-TIGR-Pt1Q457L with EcoRI/HindIII and then cloned into EcoRI/HindIII-digested pHKKF3T5b to obtain pHKKF3T5b-GPPSMUT-TIGR-Pt1Q457L. The MEVI operon was cut from pMEVI with EcoRI/XhoI and then cloned into EcoRI/SalI-digested pP21KF3T5b to obtain pP21KF3T5b-MEVI. Chromosomal integration was carried out by direct transformation as described by Chen et al. (2013). Chemically induced chromosomal evolution (CIChE) of the above construct was carried out by subculturing the resulting strains in 5 mL Super Optimal Broth (SOB) medium with increasing concentrations of triclosan in 15 mL culture tubes, as described by Chen et al. (2013). The strains were grown to the stationary phase in 1 μM triclosan for pP21KF3T5b-GPPSMUT-TIGR-Pt1Q457L or 0.25 μM for pHKKF3T5b-MEVI. Fifty milliliters of the culture were subcultured into a new culture tube, in which the triclosan concentration was doubled to 132 or 32 μM and allowed to grow to the stationary phase. The process was repeated until the desired concentration was reached. The recA gene of the CIChE strain was then deleted by the markerless deletion approach using the isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible ccdB as a counter-selectable marker (Wei et al., 2016).
Gene replacement of the native promoter of E. coli acrAB and the integration of ttgB from P. putida KT2440 were carried out by the CRISPR-Cas method as described by Jiang et al. (2015). To enhance specificity and reduce off-target effects, the cas9 on pCas (Jiang et al., 2015) was site-directed mutated into cas9(K848A/K1003A/R1060A) as described as Slaymaker et al. (2016) to obtain pCas*. To easily assemble the sgRNA sequence using the BglBrick standard method, the BglII site in the sgRNA plasmid pTargetF was first removed, and then a BglII site was added in the front of EcoRI site to obtain the sgRNA plasmid pTargetB.
Adaptive Laboratory Evolution for Improving Pinene Tolerance
A 1-mL culture of logarithmic phase E. coli was collected by centrifugation, washed twice with saline, and diluted to a cell concentration of 106 to 107 with physiological saline. Then, atmospheric and room temperature plasma (ARTP) mutagenesis was performed using an ARTP mutation system (ARTP-IIS, Tmaxtree Biotechnology Co, Ltd, Wuxi, China) with the following parameters: (1) the radio frequency power input was 100 W; (2) the flow of pure helium was 10 standard liters per min; (3) the distance between the plasma torch nozzle exit and the slide was 2 mm; and (4) the different treatment times were selected (10, 20, 40, 60, 80, 100, and 120 s). Ten microliters of the aforementioned cell dilution were evenly scattered on the slide and subjected to ARTP mutagenesis. After treatment, the slide was washed with LB medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl), transferred to 5 mL of LB medium with 0.5% pinene in a 15 mL falcon tube, and cultivated at 30 °C and 200 rpm for 24 h. The cultures were serially passed into fresh medium (initial OD600 of 0.2) daily. Continuously repeating this transfer procedure at 0.5% pinene until OD600 at 24 h did not increase further, the culture was then sequentially transferred to a pinene concentration of 1.0%, 1.5% and 2.0%. The cultures were frozen and stored at −80°C at every pinene concentration.
The cultures of 2.0% pinene stored at −80°C were transferred by the IPP/FPP sensor plasmid pPrstA-GFP. Single colonies were inoculated in individual wells of a 48 deep-well microplate (4.6 mL) containing 600 μL of LB medium and incubated at 30°C and 200 rpm for 24 h on a Multitron shaker (Infors). The cells were harvested by centrifugation at 14000 × g for 2 min and then resuspended with 0.6 mL (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4). Then, 200 μL of the bacterial culture was transferred into a 96-well plate in which the OD600 and fluorescence were read with the excitation at 485 nm and emission at 528 nm using a SynergyNeo2 multi-mode reader (SynergyNeo2, BioTek, USA).
Generating Random Mutagenesis Libraries Using Error-Prone PCR and Screening
The random mutagenesis libraries of the fusion gene cluster of AgGPPS-AgPS after optimization of the first 18 codons using the 6AA method (Boë et al., 2016) were constructed through error-prone PCR. The gene cluster of AgGPPS-AgPS was amplified from pQE-GPPS6AA-L-PS using the primers EcoRI-GPPS/HindIII-PS. The error-prone PCR reaction mixture consisted of 5 mM MgCl2, 0.3 mM MnCl2, 0.2 mM each of dATP and dGTP, 1 mM each of dCTP and dTTP and Tag DNA polymerase. The PCR product was digested by EcoRI/HindIII, ligated into the EcoRI/HindIII sites of pQE30, and then transferred into the lycopene-producing strain E. coli LYCOP to generate the mutant library.
The mutant library was plated on LB agar with ampicillin and IPTG. The plates were incubated at 30°C overnight. The mutant plasmid was isolated from the whiter colony and then transferred into component E. coli BW25113 (PT5-dxs, pMEVI). The pinene productions of them were analyzed in a shake flask.
Generating Random Mutagenesis Libraries Using DNA Shuffling and Screening
DNA shuffling experiments were performed by the following steps: parental template preparation, DNase I digestion, primer-less PCR and PCR with primers. The mutant plasmids from the 7 colonies resulted from error-prone PCR and were used as the template to amplify the gene cluster fragments with the primers EcoRI-GPPS/HindIII-PS. Following purification, 2 μg of the eight PCR products was mixed and treated with 0.02 U of DNaseI in 100 μL of the 10 × DNaseI buffer on ice for 2 min and terminated by the loading buffer containing SDS. The purified fragments of 50–300 bp were used in the primer-less PCR reactions to reassemble into full-length genes. The primer-less PCR reaction mixture contained 0.5 mM each dNTP, 10 × Taq buffer and 0.5 μL Taq DNA polymerase (Takara). The PCR reaction conditions were as follows: 95°C for 1 min, 35 cycles of 94°C for 30 s, 45°C for 30 s, 72°C for 3 min, and final incubation at 72°C for 8 min. The PCR products with the correct size were purified and subjected to PCR amplification using the same conditions with the primers EcoRI-GPPS/HindIII-PS. Finally, the mutated PCR products of the full-length gene were digested by EcoRI/HindIII, ligated into the EcoRI/HindIII sites of pQE30, and transferred into the lycopene-producing strain E. coli LYCOP to generate the mutant library.
The mutant library was plated on LB agar with ampicillin and IPTG. The plates were incubated at 30°C overnight. Single colonies with a whiter color were inoculated in individual wells of a 48 deep-well microplate (4.6 mL) containing 1 mL of LB medium and incubated at 30°C and 200 rpm on a Multitron shaker (Infors). After 8 h, the cultures were induced with 1 mM IPTG and overlaid with 20% dodecane to trap pinene. After induction, the cultures were incubated at 30°C and 200 rpm for 48 h. The pinene concentration in individual wells was assayed using the concentrated sulfuric acid method as follows. One hundred microliters of the dodecane layer were mixed with 200 μL sulfuric acid, then inoculated for 5 min in boiling water, and the absorbance of the reaction solution at 450 nm was determined using a spectrophotometer (Shimadzu, Japan).
Creating TIGR Libraries and Screening
TIGRs were synthesized using PCR to assemble the oligonucleotides into chimeric DNA sequences as described by Pfleger et al. (2006) and Li et al. (2015). Briefly, 40 mmols of an equimolar oligonucleotide (A, B, C, and D in Supplemental Table 1) mixture was added to a mixture containing 2.5 units of Primer Star DNA Polymerase (Takara, Dalian, China). The assembly was conducted over 35 cycles of PCR for 10 s at 98°C, 30 s at 72°C, and 20 + 5 s/cycle at 72°C. The assembly products were purified using a nucleotide removal column and amplified using the end-specific primers TIGRs-F(X)/TIGRs-R(A) and then cloned into the SacI/SalI sites of pQE-GPPSMUT-Pt1 Q457L to obtain the plasmid libraries pQE-GPPSMUT-TIGRs-Pt1Q457L. The plasmid libraries were transferred into component E. coli BW25113 (PT5-dxs, pMEVI) to generate the mutant library.
The TIGR library was plated on LB agar with ampicillin. The plates were incubated at 30°C overnight. Single colonies were inoculated in individual wells of a 48 deep-well microplate (4.6 mL) containing 1 mL of LB medium and incubated at 30°C and 200 rpm on a Multitron shaker (Infors). After 8 h, the cultures were induced with 1 mM IPTG and overlaid with 20% dodecane to trap pinene. After induction, the cultures were incubated at 30°C and 200 rpm for 48 h. The pinene concentration in individual wells was assayed using the above concentrated sulfuric acid method.
Pinene Biosynthesis in Shake Flasks
For pinene fermentation production, a single colony was inoculated into 5 mL of LB medium in a falcon tube, which was cultured overnight at 37°C. The overnight seed culture was then inoculated into 50 mL of SBMSN medium with a starting OD600 of 0.1. SBMSN medium (pH 7.0) containing the following (g/L): sucrose 20, peptone 12, yeast extract 24, KH2PO4 1.7, K2HPO4 211.42, MgCl2·6H2O 1, ammonium oxalate 1.42, and Tween-80 2. The main cultures were then incubated at 37°C and 200 rpm until an OD600 of 0.8 was reached. Then, the cultures were induced with 1 mM IPTG and overlaid with 20% dodecane to trap pinene. After induction, the cultures were incubated at 30°C and 130 rpm for 72 h.
Co-culture of E. coli PINE and MEVI for Pinene Production
E. coli PINE and MEVI cells were first separately grown in 5 mL SBMSN medium in a falcon tube at 37°C overnight. The overnight culture was inoculated into 50 mL of SBMSN medium with a starting OD600 of 0.1 and incubated at 37°C and 200 rpm until an OD600 of approximately 6.0 was reached. The cultures were then incubated at 20°C and 200 rpm for 16 h. For pinene biosynthesis using co-cultures, the E. coli PINE culture and the desired amount of the E. coli MEVI culture were inoculated into the 30 mL SBMSN medium with a starting OD600 of 0.1. The mixed culture was culture at 37°C and 200 rpm until an OD600 of 0.8 was reached. Then, the cultures were overlaid with 20% dodecane to trap pinene, and were incubated at 30°C and 130 rpm for 72 h.
Whole-Cell Biocatalysis for Pinene Production
A single colony of E. coli PINE and MEVI was separately inoculated into 5 mL of SBMSN medium in a falcon tube, which was cultured overnight at 37°C. The overnight cultures were then inoculated into 50 mL SBMSN medium with a starting OD600 of 0.1. The cultures were then incubated at 37°C and 200 rpm until an OD600 of approximately 6.0 was reached. Then, the cultures were incubated at 20°C and 200 rpm for 16 h. Finally, the E. coli PINE culture was mixed with the E. coli MEVI culture at the inoculation ratio of 2:1. The mixed cells were harvested by centrifugation (6000 × g at 4°C) and washed twice with cooled phosphate buffer (0.1 M, pH 7.0).
For biocatalysis, the above cells were resuspended in 10 mL phosphate buffer (0.1 M, pH 7.0) containing 20 g/L of sucrose, 10 mM MgCl2 and 5 mM MnCl2 to form the cell suspension (OD600 = 30). The reaction mixture was overlaid with 20% dodecane. The catalysis was performed for 28 h at 30°C and 130 rpm.
GC Analysis
Five hundred microliters of the dodecane layer was placed in a 1.5-mL microcentrifuge tube and centrifuged at 25,000 × g for 1 min, and 50 μL of dodecane was diluted in 450 μL of ethyl acetate spiked with the internal standard limonene (10 μg/L). The samples were analyzed by GC-FID by using a standard curve of α-pinene (Sigma Aldrich). The GC-FID (Techcomp GC7900, Techcomp Ltd, China) was used with a TM-5 column (30 m × 0.32 mm × 0.50 μm). The inlet temperature was set to 300°C, with the flow at 1 mL/min, the oven at 50°C for 30 s, ramp at 4°C/min to 70°C, and ramp at 25°C/min to 240°C.
Quantitative Real-Time PCR (qRT-PCR)
The total RNA from E. coli cells grown for 24 h in shake flasks was isolated using an RNA extraction kit (Dongsheng Biotech, Guangzhou, China), following the manufacturer's instructions. The first-strand cDNA was synthesized using an All-in-One™ First-Strand cDNA Synthesis kit (GeneCopoeia, Guangzhou, China). The qRT-PCR was perfor1med with the All-in-One™ qPCR Mix kit (GeneCopoeia) on an iCycler iQ5 Real Time PCR system (Bio-Rad Laboratories, California, USA). The template was 100 ng of cDNA. The PCR conditions were as follows: 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s, and extension at 72°C for 15 s. The primers for qRT-PCR are presented in Supplementary Table 1. The data were analyzed by the 2−ΔΔCt method described by Livak and Schmittgen (2001) and normalized by cysG gene expression.
Gene copy numbers were measured by qPCR on genomic DNA isolated from the appropriate CIChE strains. qPCR was performed as described above. The primers QPt1F/QPt1R and QHF/QHR (Supplementary Table 1) were used to measure the copy number of Pt1 and HMGS, respectively.
Statistical Analysis
All experiments were conducted in triplicate, and the data were averaged and presented as the means ± standard deviation. One-way analysis of variance followed by Tukey's test was used to determine significant differences using the OriginPro (version 7.5) package. Statistical significance was defined as p < 0.05.
Results
Tolerance Engineering to Improve Pinene Production
To improve pinene tolerance, E. coli cells harboring pPrstA-GFP were treated with ARTP and then serially transferred into LB medium supplemented with increased concentrations of pinene of 0.5, 1.0, 1.5, and 2.0%. The culture was transferred daily. After the adaptive evolution at 2.0% pinene, the culture was streaked on LB plates for isolated colonies. It has been demonstrated that the IPP/FPP sensor plasmid pPrstA-GFP has been successfully used to test the intracellular IPP/FPP concentration and to screen the library with higher IPP/FPP concentrations (Dahl et al., 2013; Shen et al., 2016). Thus, we also used it to screen the library. Of the 670 clones, 14 strains with higher fluorescence strength (Supplementary Figure 1) were selected for further shake flask analysis. As shown in Figure 1, E. coli YZ-3 produced the highest level of pinene (7.3 ± 0.2 mg/L), which was 31% higher than the starting strain E. coli BW25113 (PT5-dxs).
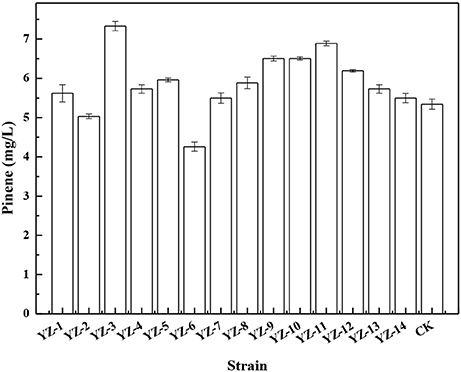
Figure 1. Pinene production by the selected adaptive laboratory evolution strains harboring pMEVIGPS. E. coli BW25113 (PT5-dxs, pMEVIGPS) was set as the control strain (CK). The data represent the means of three replicates and error bars represent standard deviations.
To improve pinene production, we investigated effects of efflux pumps on pinene production. Dunlop et al. (2011) reported that expressing some efflux pumps significantly improved pinene tolerance. Thus, we tested whether pumps that improved pinene tolerance also enhanced its production. As shown in Figure 2A, expressing native AcrB, AcrAB, or TtgB (NP_743544) from Pseudomonas putida KT2440 in E. coli YZ-3 using plasmid resulted in increased pinene production. However, expressing A. borkumensis AcrBDFa (YP_692684) or P. putida KT2440 MexF (NP_745564) from did not improve pinene production. Therefore, we first replaced the native promoter of E. coli YZ-3 acrAB operon with the strong P37 promoter to obtain E. coli YZ-3-A, resulting in an increase in pinene production to 8.1 ± 0.2 mg/L from 7.3 ± 0.2 mg/L (Figure 2B). Then, we integrated the ttgB from P. putida KT2440 under the control of the P37 promoter in E. coli YZ-3-A to obtain E. coli YZ-3-A-T. The modification further improved pinene production to 9.1 ± 0.2 mg/L (Figure 2B). These results indicate that overexpressing some efflux pumps (E. coli acrAB and Pseudomonas putida KT2440 ttgB), whcih improved pinene tolerance, also enhanced its production.
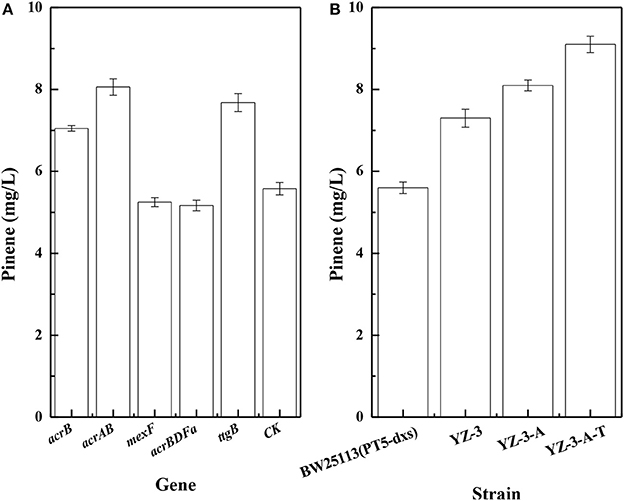
Figure 2. Effect of overexpression of efflux pumps on pinene production. (A) Plasmid-expression in E. coli YZ-3 (pMEVIGPS). E. coli YZ-3 (pMEVIGPS, pZEABP) was set as the control strain (CK); (B) Chromosomal-expression in E. coli harboring pMEVIGPS. The data represent the means of three replicates and error bars represent standard deviations.
To further improve pinene production, we isolated a mutant resistant to an inhibitor of biosynthetic pathway after ARTP mutagenesis. Isolating a mutant resistant to an inhibitor of biosysnthetic pathway is a common strategy used for strain improvement. In E. coli, the important precursors IPP and DMAPP are produced by the 1-deoxy-D-xylulose-5-phosphate (DXP) pathway. Fosmidomycin is the DXP pathway inhibitor that inhibits 1-deoxy-D-xylulose-5-phosphate reductoisomerase (Dxr) and methylerythritol phosphate cytidyltransferase (IspD) of the DXP pathway (Zhang et al., 2011). Genes involved in the DXP pathway are essential for E. coli growth. The wild-type E. coli YZ-3-A-T can grow in the presence of 2% pinene (Supplementary Figure 2A), but does not grow in the presence of 35 μM fosmidomycin (Supplementary Figure 2B). After ARTP mutagenesis, cells grow well in the presence of 35 μM fosmidomycin (Supplementary Figure 2C). Overexpression of dxr or ispD in E. coli improved the fosmidomycin tolerance (Zhang et al., 2011). This indicates that the fosmidomycin resistant mutants may show higher level of Dxr and IspD. Screening the fosmidomycin resistant mutants will increase the probability to obtain a mutant with higher IPP flux. Thus, to increase the probability to obtain a mutant with higher IPP flux, we screened the fosmidomycin resistant mutants using the IPP/FPP sensor. E. coli YZ-3-A-T cells harboring pPrstA-GFP were treated with ARTP. After ARTP mutagenesis, the cells were transferred into the LB medium supplemented with 35 μM fosmidomycin and 2.0% pinene. A total of 720 clones were screened for analyzing fluorescence strength in deep-well microplate cultures (Supplementary Figure 3). Twenty-one strains with higher fluorescence strength were selected for further shake flask analysis. As shown in Figure 3, Strain No. 19, which was denoted as E. coli YZFP, produced the highest level of pinene, which reached 9.9 ± 0.1 mg/L. In fact, our quantitative real-time PCR analysis also demonstrates that the dxs, dxr and ispD of the DXP pathway in E. coli YZFP showed higher transcription level than the wild-type strain (Data not shown, will be published in another paper).
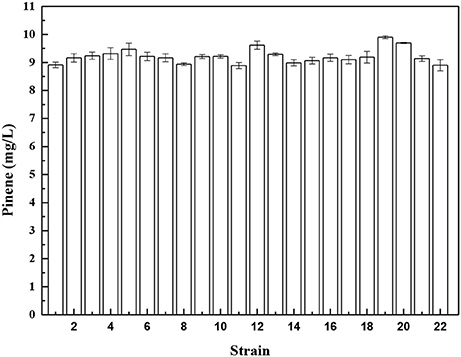
Figure 3. Pinene production of the selected mutants resistant to fosmidomycin harboring pMEVIGPS. E. coli BW25113 (PT5-dxs, pMEVIGPS) (strain No. 22) was set as the control strain. The data represent the means of three replicates and error bars represent standard deviations.
To characterize the pinene tolerance, the growth of the above strains were compared in different concentrations of pinene. Figure 4A shows the growths of these strains in the presence of 2% pinene. The starting strain did not grow well in the presence of 2% pinene. The above engineered strains did grow well in the presence of 2% pinene. The maximum cell densities of the three engineered strains were similar. The growth rate of E. coli YZFP was higher than that of the other engineered strains. These results indicate that the engineered strains have higher pinene tolerance than the starting strain. However, the maximum cell densities of the three engineered strains were lower than that of the starting strain in the absence of pinene (Figure 4B). The reason may be that the three engineered strains produced higher level of IPP than the starting strain. IPP is toxicity to E. coli. We also investigated the genetic stability of E. coli YZFP. The strain can also grow well in the presence of 2% pinene and the level of pinene production remained constant after 20 rounds of subculturing in absence of selective pressure (data not shown).

Figure 4. Growth of the selected tolerance strains in the presence of 2% pinene (A) and in the absence of pinene (B). E. coli BW25113 (PT5-dxs) (▾), E. coli YZ-3 (▴), E. coli YZ-3-A-T (•), and E. coli YZFP (■). The data represent the means of three replicates and error bars represent standard deviations.
Evolution Engineering to Improve Pinene Production
The lower expression level and/or lower enzymatic activity of GPPS and PS in E. coli may result in the lower yield of pinene production. Sarria et al. (2014) compared GPPSs and PSs from A. grandis and P. taeda and found that the combination of GPPS and PS from A. grandis was most suitable for pinene production. Thus, we first optimized the first 48 nucleotide sequences of A. grandis GPPS with the 6AA method to increase the expression level of the A. grandis GPPS-PS gene cluster in E. coli. The 6AA method substitutes all Arg, Asp, Gln, Glu, His, and Ile codons with the synonymous codon having the highest single-variable logistic regression slope (CGT, GAT, CAA, GAA, CAT, and ATT, respectively), while the other 14 amino acids were not changed from the wild-type gene sequence (Boë et al., 2016). The 6AA optimization increased pinene production from 5.6 ± 0.1 mg/L to 6.4 ± 0.3 mg/L (Table 2).

Table 2. Effect of evolution engineering on pinene production in Escherichia coli BW25113 (PT5-dxs, pMEVI).
Because pinene shares the same 5-carbon precursors IPP and DMAPP with carotenoids, a lycopene-producing strain E. coli LYCOP (Chen et al., 2013) was used to screen the error-prone PCR mutant libraries of the GPPS-PS cluster from A. grandis after 6AA optimization. The higher the activity of the GPPS-PS cluster, the lower the intracellular precursor levels for lycopene biosynthesis, thereby reducing the pigmentation of the E. coli. Of approximately 1 500 colonies, 7 colonies with a whiter color were observed. The mutant plasmids were isolated from the 7 colonies and then were co-transferred with the MEV pathway plasmid pMEVI into E. coli BW25113 (PT5-dxs). The pinene productions of them were analyzed in a shake flask, and the results are presented in Figure 5A. The strains harboring the mutant gene cluster produced higher pinene by 7.8–85.7% than that with the wild-type gene cluster. To increase the gene cluster activity, the 7 mutant gene clusters were used for DNA shuffling.
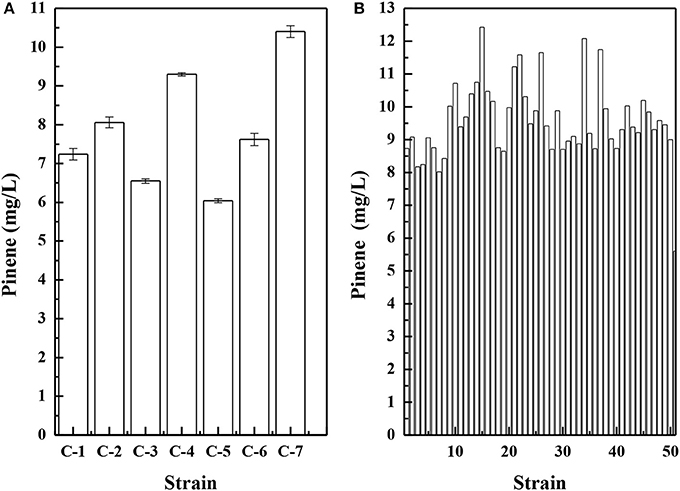
Figure 5. Pinene production by E. coli BW25113 (PT5-dxs, pMEVI) harboring mutant gene clusters from error-prone PCR (A) and by E. coli LYCOP harboring mutant gene clusters from DNA shuffling (B). Pinene concentrations were measured using the GC-FID (A) and the concentrated sulfuric acid (B) methods. The data represent the means of three replicates and error bars represent standard deviations.
Because the colonies of E. coli LYCOP harboring the above mutant gene cluster became a faint color, it is difficult to discriminate these colonies by using the above carotenoid-based method. A more sensitive and quantitative screening method is needed. It is known that monoterpene can hydrate readily in the presence of acid catalysts, such as H2SO4 (Robles-Dutenhefner et al., 2001). As a result, the initial reaction solutions turn yellow and then brown. After reaction with concentrated sulfuric acid in boiling water for 5 min, it was observed that the color of the reaction solution become darker as the pinene concentration increases and the absorbance at 450 nm is linearly related with pinene concentration (Supplementary Figure 4). Thus, the concentrated sulfuric acid method can quantitatively predict pinene concentrations.
After DNA shuffling, the mutant plasmids were transferred into E. coli LYCOP harboring pMEVI. Fifty colonies with a whiter color were used for assays of pinene production in a shake flask using the concentrated sulfuric acid method. The results are presented in Figure 5B. E. coli LYCOP harboring the mutant gene cluster produced higher pinene (6.5–10.1 mg/L) than those with the wild-type gene cluster. The mutant plasmid with the highest pinene production was isolated from strain No. 15 and then was co-transferred with pMEVI into E. coli BW25113 (PT5-dxs). E. coli BW25113 (PT5-dxs) harboring the mutant plasmid and pMEVI produced 12.4 ± 0.2 mg/L of pinene (Table 2). The mutant plasmid with the highest pinene production was then sequenced. The two amino acid mutants (D90G and L175P) were observed in the CDS of GPPS from A. grandis. No mutant in the CDS of PS from A. grandis was observed. It has been reported that (-)-α-pinene synthase (Pt1) from P. taeda has the lowest Km for GPP among the known PSs (Phillips et al., 2003). Tashiro et al. engineered an E. coli with the highest yield of pinene so far using the pinene synthase mutant (Pt1Q457L) from P. taeda (Tashiro et al., 2016). Thus, replacing A. grandis PS in the mutant plasmid with P. taeda pinene synthase mutant gene (Pt1Q457L) yielded pQE30-GPPSmut-L-Pt1Q457L. E. coli BW25113 (PT5-dxs) harboring pQE30-GPPSmut-L-Pt1Q457L produced a higher level of pinene (15.2 ± 0.2 mg/L) than those harboring pQE30-GPPSmut-L-AgPS, which achieved 12.4 ± 0.2 mg/L (Table 2).
The unbalanced expression of multiple genes may overburden the cell and cause accumulation of toxic metabolic intermediates, resulting in reduced product titers. Pfleger et al. (2006) developed a combinatorial engineering approach for coordinating the expression of cascade enzymes. For this purpose, libraries of tunable intergenic regions (TIGRs) are generated that encode mRNAs with diverse secondary structures with RNase cleavage sites. The TIGR approach was applied to balance the gene expression of the MEV pathway using the TIGR approach, resulting in a 7-fold increase in mevalonate production. Moreover, our previous paper demonstrated that the TIGR approach was more efficient compared to protein fusion for coordinating expression (Li et al., 2015). Thus, we constructed a library of TIGRs to balance the expression of A. grandis GPPSD90G/L175P and P. taeda Pt1Q457L. The library of TIGRS was inserted between GPPSD90G/L175P and P. taeda Pt1Q457L to yield a series of operons. The functional operons from the libraries were screened by using the concentrated sulfuric acid method. A total of 768 colonies were used for the assay of pinene production in deep-well microplate cultures using the concentrated sulfuric acid method (Supplementary Figure 5). Forty-three strains with higher OD450 were selected for further shake flask analysis. As shown in Figure 6, strain No. 6 produced the highest level of pinene (17.6 ± 0.2 mg/L). Thus, the TIGR-mediated plasmid was recovered from strain No. 6 and sequenced (Supplementary Table 2). We also retransformed the plasmid back to the host strain E. coli BW25113 (PT5-dxs) and checked the pinene production. The resulting strain produced the same level of pinene (17.9 ± 0.1 mg/L), indicating that the pinene production improvement is the result of TIGR-mediated optimization.
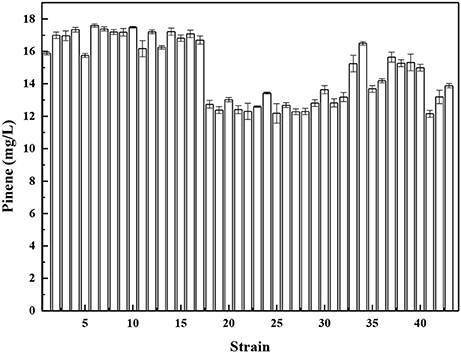
Figure 6. Pinene production by E. coli BW25113 (PT5-dxs, pMEVI) harboring the selected TIGR-mediated gene cluster. The data represent the means of three replicates and error bars represent standard deviations.
Modular Co-culture Engineering to Improve Pinene Production
To take advantage of emerging co-culture engineering approaches to improve overall pinene biosynthesis in E. coli, the complete biosynthetic pathway was divided into the following two modules: the upstream module of the MEV pathway and the downstream module of the TIGR-mediated gene cluster of A. grandis GPPSMut and P. taeda Pt1MUT (Figure 7). The two modules were integrated into the chromosome of the pinene tolerance strain E. coli YZFP and then then evolved to a higher gene copy number by triclosan induction, respectively.
Figure 8A shows the results of pinene production in CIChE strains of the TIGR-mediated gene cluster of A. grandis GPPSMut and P. taeda Pt1MUT without the MEV pathway. The maximum pinene production was obtained by the CIChE strains resistant to 32 μM triclosan. Thus, the recA gene of the CIChE strain resistant to 32 μM triclosan was deleted to obtain E. coli PINE. We determined the GPPS-Pt1 gene copy number in E. coli PINE. The copy number reached approximately 60 in the CIChE strain, which is the equivalent copy number of a high copy plasmid. Figure 8B shows the results of IPP/FPP concentration of the CIChE strains of the MEV pathway measured by the IPP/FPP sensor (pPrstA-GFP). As shown in Figure 8B, the maximum IPP/FPP production was obtained by the CIChE strains resistant to 0.5 μM triclosan. Thus, the recA gene of the CIChE strain resistant to 0.5 μM triclosan was deleted to obtain E. coli MEVI. We also determined the MEV pathway gene copy number in E. coli MEVI. The copy number reached approximately 4 in E. coli MEVI.
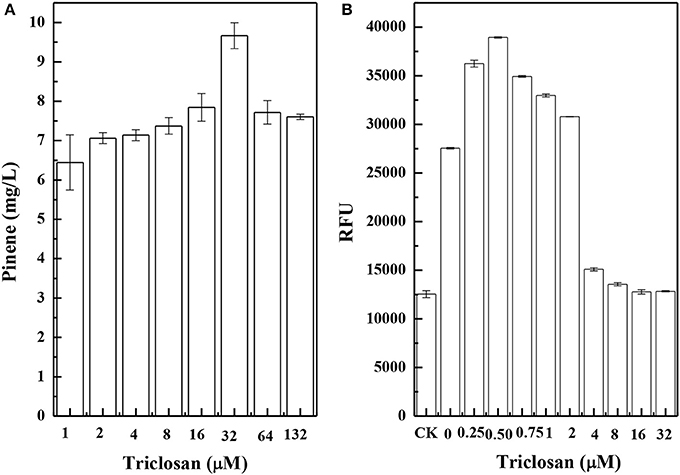
Figure 8. Pinene production of chemically induced chromosomal evolution (CIChE) strains of the GPPS-Pt1 cluster without the MEV pathway (A) and the MEV pathway (B) at different triclosan concentrations. The data represent the means of three replicates and error bars represent standard deviations.
Zhou et al. (2015) demonstrate that the modular co-culture engineering can be applicable to isoprenoids because their scaffold moleculars can generally permeate membranes. To demonstrate IPP can also cross cell membranes, we cultured E. coli (pPrstA-GFP) with the cell-free culture broth of E. coli MEVI and measured fluorescence strength. After addition of the cell-free culture broth of E. coli MEVI, E. coli (pPrstA-GFP) showed higher fluorescence strength (Supplementary Figure 6). Moreover, the E. coli MEVI: PINE co-culture produced higher level of pinene than E. coli PINE (Figure 9). These results indicate that IPP produced by E. coli MEVI diffused into E. coli PINE and was subsequently converted into pinene. We then optimized the E. coli MEVI: PINE co-culture system to further improve pinene production. To this end, different inoculation ratios between E. coli MEVI and PINE were investigated. As shown in Figure 9, the highest pinene production of 64.9 ± 0.9 mg/L was achieved when E. coli MEVI and PINE were inoculated at a ratio of 1:2. Compared with the mono-culture strategy using E. coli PINE harboring pMEVI, the pinene production was increased by 1.9-fold (from 22.3 ± 0.2 mg/L to 64.9 ± 0.9 mg/L). To test if all of IPP produced by E. coli MEVI were converted into pinene by E. coli PINE, we measured the IPP concentration in the broth of the co-culture and the E. coli MEVI after 28 h using the IPP sensor plasmid. The results showed that about 57.8% of IPP were converted by E. coli PINE (Supplementary Table 3). Thus, we introduced pQE-GPPSMUT-TIGR-Pt1Q457L to overexpress the pinene biosynthetic pathway and checked the pinene production. The co-culture system after introducing the pinene biosynthetic pathway into E. coli MEVI produced higher level of pinene (60.2 ± 0.2 mg/L) than the control strain (52.1 ± 0.1 mg/L) harboring the empty plasmid (Supplementary Table 4). The result also demonstrates that not all of IPP can be converted in the co-culture system.
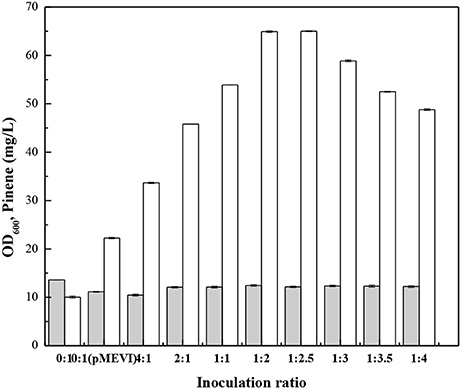
Figure 9. Effect of the inoculation ratio of E. coli PINE and MEVI on pinene production in the co-culture system. OD600 (Gray bars), Pinene concentration (White bars). 0:1, only E. coli PINE; 0:1 (pMEVI), only E. coli PINE (pMEVI); others, the E. coli MEVI: PINE co-culture system with different inoculation ratio. The data represent the means of three replicates and error bars represent standard deviations.
Biotechnological approaches for chemicals production can be broadly classified into fermentation and biocatalysis. In biocatalysis, cell growth and production phase are separated. In comparison to the fermentation bioprocess, whole-cell biocatalysis is an attractive method due to its great efficiency and relative simplification of downstream processing (Lin and Tao, 2017). The whole-cell biocatalysis processes comprise the following two stages: growth and conversion of the substrates. After the cells are cultured, they are harvested and washes with a buffer solution and suspended in the buffer for biocatalysis. Thus, the E. coli-E. coli modular co-culture system of whole-cell biocatalysis was used to further enhance pinene production. As shown in Figure 10, the highest pinene production of 166.5 ± 0.3 mg/L was achieved by the whole-cell biocatalyst after 28 h. The pinene titer obtained by the whole-cell biocatalysis was 2.6-fold higher than that produced by the fermentation process.
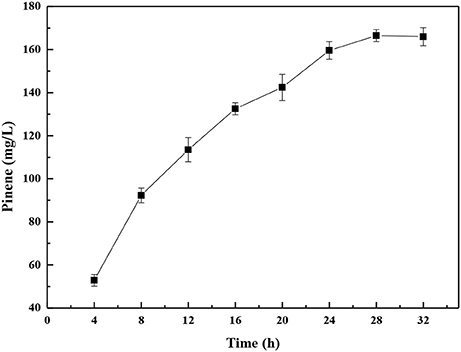
Figure 10. Time course of pinene production by the modular co-culture system of the whole-cell biocatalyst. The data represent the means of three replicates and error bars represent standard deviations.
Discussion
It has been reported that E. coli growth is inhibited by 0.5% pinene (Dunlop et al., 2011). We first improved pinene tolerance from 0.5 to 2.0% and pinene production by adaptive laboratory evolution after ARTP mutagenesis. In fact, improvements in tolerance are not sufficient to guarantee an increase production. Our results also demonstrate this point. Overexpreesion of A. borkumensis acrBDFa or P. putida KT2440 mexF that improved pinene tolerance did not improved pinene production (Figure 2A). To obtain a mutant with higher level of pinene production, we used the IPP/FPP sensor pPrstA-GFP to screen the mutants tolerant to 2% pinene. Tolerance engineering has also successfully been used to improve the production of limonene (Dunlop et al., 2011), amorphadiene (Zhang et al., 2016), olefin (Mingardon et al., 2015), n-octane (Foo and Leong 2013). Although the level of pinene production reported in literatures did not inhibit growth, higher tolerance is beneficial to pinene production. Thus, the 2% pinene tolerant strain E. coli was used the parent strain in this study. To further improve pinene production, we then expressed the efflux pumps in the pinene tolerant strain E. coli and subsequently selected a mutant resistant to fosmidomycin after ARTP mutagenesis. The pinene tolerant strain E. coli YZFP with higher level of pinene production was obtained through a two-step screening process. There is no directed evidence to prove the improved pinene production is the result of improved pinene tolerance.
Our study demonstrates that the overexpression of some efflux pumps improved pinene tolerance and production. Many groups also reported that overexpression of efflux pumps enhanced biofuel tolerance. Dunlop et al. (2011) reported that the overexpression of efflux pumps, such as A. borkumensis AcrBDFa, P. putida KT2440 MexF, P. putida KT2440 TtgB or E. coli AcrB, enhanced pinene tolerance. However, they did not investigate the effects of the pumps on pinene production. Our results demonstrate that overexpression of E. coli AcrAB and P. putida KT2440 TtgB enhanced pinene production (Figure 2). Overexpression of A. borkumensis AcrBDFa or P. putida KT2440 MexF did not improved pinene production (Figure 2A). However, Dunlop et al. (2011) reported that overexpression of A. borkumensis AcrBDFa enhanced limonene tolerance and yield. Overexpression of tolC together with ABC family transporters (macAB) or MFS family transporters (emrAB or emrKY) was found to improve amorphadiene titer by more than 3-fold (Zhang et al., 2016). Overexpression of the native and evolved acrB improved olefin tolerance and production (Mingardon et al., 2015). Evolved AcrB variants with improved tolerance to pinene and n-octane have also been reported by Foo and Leong (2013). Taken together with these previous studies, our results show that a combination of the adaptive laboratory evolution with overexpression of some efflux pumps can improve pinene tolerance and production.
In this study, we reported a high-throughput screening method, which is known as the concentrated sulfuric acid method, for recombinant E. coli that overproduce pinene. We successfully applied the concentrated sulfuric acid method to screen the DNA shuffling library of the GPPS-PS gene cluster and the library of the TIGR-mediated GPPS-Pt1 gene cluster. Because limonene has the same properties as pinene, the concentrated sulfuric acid method can also be used to screen mutants for limonene production. Although the carotenoid-based method has been successfully used to screen isoprene synthase variants (Emmerstorfer-Augustin et al., 2016), the carotenoid-based method has a limitation when the colony has a faint color.
GPPS and PS have been identified as a major limiting factor in pinene production (Yang et al., 2013; Sarria et al., 2014; Tashiro et al., 2016). After directed evolution of the A. grandis GPPS-PS gene cluster using error-prone PCR and DNA shuffling, pinene production was increased by 1.2-fold (Table 2). Two amino acid mutants were observed in the CDS of A. grandis GPPS. However, no mutant was observed in the CDS of A. grandis PS. Tashiro et al. evolved P. taeda Pt1 and constructed a recombinant E. coli with the highest pinene yield reported in literatures using the evolved variant (Tashiro et al., 2016). Using the A. grandis GPPSMut-P. taeda Pt1MUT gene cluster resulted in an increase in pinene production by 22.6% compared to using the A. grandis GPPSMut-PS gene cluster (Table 2).
GPPS and PS are inhibited by their substrate (GPP) or product (pinene) (Sarria et al., 2014). To overcome GPPS inhibition by GPP, GPPS was fused to PS, resulting in improved pinene production (Sarria et al., 2014; Tashiro et al., 2016). Our previous paper demonstrated that the TIGR approach was more efficient compared to protein fusion for coordinating expression (Li et al., 2015). This study shows that using the TIGR-mediated gene cluster led to an increase in pinene production by 15.8% compared with the fused gene cluster (Table 2).
In the present study, an E. coli-E. coli co-culture system was engineered to modularize the MEV and heterologous biosynthetic pathway. The MEV pathway and heterologous biosynthetic pathway (the A. grandis GPPSMut-P. taeda Pt1MUT gene cluster) was engineered in the pinene tolerance strain E. coli YZFP, respectively. The best co-culture system was found to improve pinene production by 1.9-fold compared to the mono-culture system. The modular co-culture can distribute the metabolic burden and allow for modular optimization by simply changing the strain-to-strain ratio. The E. coli-E. coli modular co-culture system has been successfully used to improve 3-amino-benzoic acid (Zhang and Stephanopoulos, 2016), flavonoid (Jones et al., 2016), muconic acid (Zhang et al., 2015), and perillyl acetate (Willrodt et al., 2015), etc. In fact, the critical issue for modular co-culture engineering is the mass transfer of the pathway intermediate (IPP). It has been demonstrated that isoprenoids scaffold molecules can cross cell membranes (Zhou et al., 2015). Our results also demonstrate that IPP can cross cell membranes and secreted to the extracellular medium (Supplementary Figure 6 and Figure 9). Moreover, our results showed that the pinene tolerance strain E. coli YZFP (pPrstA-GFP) had higher fluorescence strength than the parent strain harboring pPrstA-GFP after addition the cell-free broth of E. coli MEVI (Supplementary Figure 6), indicating that E. coli YZFP shows greater membrane permeability than the parent strain. Our results demonstrate that there were still some IPP not to be converted into pinene by E. coli PINE (Supplementary Tables 3, 4). Moreover, Overexpression of the pinene biosynthetic pathway in E. coli MEVI enhanced pinene production in the E. coli MEVI-E. coli PINE co-culture system (Supplementary Table 4). However, overexpression of the pinene biosynthetic pathway in E. coli PINE did not enhance pinene production (Supplementary Table 4). Increasing the inoculation ratio of E. coli PINE and E. coli MEVI from 2:1 to 2.5:1 or 3:1 did not enhanced pinene production (Figure 9). These results indicate that the IPP transportation may be a key factor for further improving pinene production. Transporter engineering strategies have successfully been used to enhance the secretion of the pathway intermediates, improving production (Boyarskiy and Tullman-Ercek, 2015; Kell et al., 2015; Zhang et al., 2015). Thus, appropriate metabolite transporters engineering strategies may be used to further improve pinene production of the E. coli-E. coli co-culture system.
This study also demonstrated that whole-cell biocatalysis further improved pinene production by 1.6-fold compared to the fermentation process. The whole-cell biocatalysis has also successfully been used in many biotechnological production (Tao et al., 2011; Lin et al., 2015; Kogure et al., 2016; Chen et al., 2017; Lin and Tao, 2017). Kogure et al. (2016) also reported that the significantly higher shikimate productivity (141.3 g/L) was achieved by the whole-cell biocatalysis compared to that (78.8 g/L) achieved by the fed-batch fermentation accompanying cell growth. The pinene production improvement may be resulted from higher cell density (OD600 of 30) and the growth-arrested cells used in the whole-cell biocatalysis.
Conclusions
Pinene tolerance and production were first improved via adaptive laboratory evolution and efflux pump overexpression. Through error-prone PCR and DNA shuffling, a GPPS variant was screened, which outperformed the wild-type enzyme. To balance the expression of multiple genes, a TIGR was inserted between A. grandis GPPSD90G/L175P and P. taeda Pt1Q457L. To construct an E. coli-E. coli co-culture system to modularize the MEV and heterologous biosynthetic pathway, the MEV pathway and heterologous biosynthetic pathway (the A. grandis GPPSMut-P. taeda Pt1MUT gene cluster) was integrated into the chromosome of the pinene tolerance strain E. coli YZFP and then evolved to a higher gene copy number by CIChE, respectively. The E. coli-E. coli modular co-culture system of whole-cell biocatalysis resulted in the highest pinene production of 166.5 mg/L. Our results demonstrate that the E. coli-E. coli modular co-culture system of the whole-cell biocatalysis is a promising approach for the production of pinene.
Author Contributions
F-XN performed all of the experimental works. XH and Y-QW performed the pinene assay. J-ZL designed the study and wrote the manuscript. All the authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant NO. 21276289), the Natural Science Foundation of Guangdong Province (NO. 2015A030311036), the Project of the Scientific and Technical Program of Guangdong Province (NO. 2015A010107004) and the Project of the Scientific and Technical Program of Guangzhou (NO. 201607010028).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01623/full#supplementary-material
References
Alonso-Gutierrez, J., Chan, R., Batth, T. S., Adams, P. D., Keasling, J. D., Petzold, C. J., et al. (2013). Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 19, 33–41. doi: 10.1016/j.ymben.2013.05.004
Behr, A., and Johnen, L. (2009). Myrcene as a natural base chemical in sustainable chemistry: a critical review. ChemSusChem 2, 1072–1095. doi: 10.1002/cssc.200900186
Boë, G., Letso, R., Neely, H., Price, W. N., Wong, K. H., Su, M., et al. (2016). Codon influence on protein expression in E. coli correlates with mRNA levels. Nature 529, 358–363. doi: 10.1038/nature16509
Boyarskiy, S., and Tullman-Ercek, D. (2015). Getting pumped: membrane efflux transporters for enhanced biomolecule production. Curr. Opin. Chem. Biol. 28, 15–19. doi: 10.1016/j.cbpa.2015.05.019
Chang, M. C., and Keasling, J. D. (2006). Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol. 2, 674–681. doi: 10.1038/nchembio836
Chen, K., Pang, Y., Zhang, B., Feng, J., Xu, S., Wang, X., et al. (2017). Process optimization for enhancing production of cis-4-hydroxy-L-proline by engineered Escherichia coli. Microb. Cell Fact. 16:210. doi: 10.1186/s12934-017-0821-7
Chen, Y. Y., Shen, H. J., Cui, Y. Y., Chen, S. G., Weng, Z. M., Zhao, M., et al. (2013). Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol. 13:6. doi: 10.1186/1472-6750-13-6
Dahl, R. H., Zhang, F., Alonso-Gutierrez, J., Baidoo, E., Batth, T. S., Redding-Johanson, A. M., et al. (2013). Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 31, 1039–1046. doi: 10.1038/nbt.2689
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Dunlop, M. J., Dossani, Z. Y., Szmidt, H. L., Chu, H. C., Lee, T. S., Keasling, J. D., et al. (2011). Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 7:487. doi: 10.1038/msb.2011.21
Emmerstorfer-Augustin, A., Moser, S., and Pichler, H. (2016). Screening for improved isoprenoid biosynthesis in microorganisms. J. Biotechnol. 235, 112–120. doi: 10.1016/j.jbiotec.2016.03.051
Foo, J. L., and Leong, S. S. (2013). Directed evolution of an E. coli inner membrane transporter for improved efflux of biofuel molecules. Biotechnol. Biofuels 6:81. doi: 10.1186/1754-6834-6-81
Gandini, A., and Lacerda, T. M. (2015). From monomers to polymers from renewable resources: recent advances. Prog. Polym. Sci. 48, 1–39. doi: 10.1016/j.progpolymsci.2014.11.002
George, K. W., Alonso-Gutierrez, J., Keasling, J. D., and Lee, T. S. (2015). Isoprenoid drugs, biofuels, and chemicals-artemisinin, farnesene, and beyond. Biotechnol. Isoprenoids 148, 355–389. doi: 10.1007/10_2014_288
Jiang, Y., Chen, B., Duan, C., Sun, B., Yang, J., and Yang, S. (2015). Multigene editing in the Escherichia coli genome via the crispr-cas9 system. Appl. Environ. Microbiol. 81, 2506–2514. doi: 10.1128/Aem.04023-14
Jones, J. A., Vernacchio, V. R., Sinkoe, A. L., Collins, S. M., Ibrahim, M. H. A., Lachance, D. M., et al. (2016). Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab. Eng. 35, 55–63. doi: 10.1016/j.ymben.2016.01.006
Kang, M. K., Eom, J. H., Kim, Y., Um, Y., and Woo, H. M. (2014). Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum. Biotechnol. Lett. 36, 2069–2077. doi: 10.1007/s10529-014-1578-2
Kell, D. B., Swainston, N., Pir, P., and Oliver, S. G. (2015). Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol. 33, 237–246. doi: 10.1016/j.tibtech.2015.02.001
Kirby, J., and Keasling, J. D. (2009). Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu. Rev. Plant Biol. 60, 335–355. doi: 10.1146/annurev.arplant.043008.091955
Kogure, T., Kubota, T., Suda, M., Hiraga, K., and Inui, M. (2016). Metabolic engineering of Corynebacterium glutamicum for shikimate overproduction by growth-arrested cell reaction. Metab. Eng. 38, 204–216. doi: 10.1016/j.ymben.2016.08.005
Li, X. R., Tian, G. Q., Shen, H. J., and Liu, J. Z. (2015). Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 42, 627–636. doi: 10.1007/s10295-014-1565-6
Lin, B., and Tao, Y. (2017). Whole-cell biocatalysts by design. Microb. Cell Fact. 16:106. doi: 10.1186/s12934-017-0724-7
Lin, B., Fan, K., Zhao, J., Ji, J., Wu, L., Yang, K., et al. (2015). Reconstitution of TCA cycle with DAOCS to engineer Escherichia coli into an efficient whole cell catalyst of penicillin G. Proc. Natl. Acad. Sci. U. S. A. 112, 9855–9859. doi: 10.1073/pnas.1502866112
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mingardon, F., Clement, C., Hirano, K., Nhan, M., Luning, E. G., Chanal, A., et al. (2015). Improving olefin tolerance and production in E. coli using native and evolved AcrB. Biotechnol. Bioeng. 112, 879–888. doi: 10.1002/bit.25511
Pfleger, B. F., Pitera, D. J. D., Smolke, C., and Keasling, J. D. (2006). Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat. Biotechnol. 24, 1027–1032. doi: 10.1038/nbt1226
Phillips, M. A., Wildung, M. R., Williams, D. C., Hyatt, D. C., and Croteau, R. (2003). cDNA isolation, functional expression, and characterization of (+)-alpha-pinene synthase and (-)-alpha-pinene synthase from loblolly pine (Pinus taeda): stereocontrol in pinene biosynthesis. Arch. Biochem. Biophys. 411, 267–276. doi: 10.1016/S0003-9861(02)00746-4
Robles-Dutenhefner, P. A., da Silva, K. A., Siddiqui, M. R. H., Kozhevnikov, I. V., and Gusevskaya, E. V. (2001). Hydration and acetoxylation of monoterpenes catalyzed by heteropoly acid. J. Mol. Catalysis Chem. 175, 33–42. doi: 10.1016/S1381-1169(01)00217-5
Sarria, S., Wong, B., Martin, H. G., Keasling, J. D., and Peralta-Yahya, P. (2014). Microbial synthesis of pinene. ACS Synth. Biol. 3, 466–475. doi: 10.1021/sb4001382
Shen, H. J., Cheng, B. Y., Zhang, Y. M., Tang, L., Li, Z., Bu, Y. F., et al. (2016). Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 38, 180–190. doi: 10.1016/j.ymben.2016.07.012
Slaymaker, I. M., Gao, L., Zetsche, B., Scott, D. A., Yan, W. X., and Zhang, F. (2016). Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88. doi: 10.1126/science.aad5227
Tao, F., Zhang, Y., Ma, C., and Xu, P. (2011). One-pot bio-synthesis: N-acetyl-D-neuraminic acid production by a powerful engineered whole-cell catalyst. Sci. Rep. 1:142. doi: 10.1038/srep00142
Tashiro, M., Kiyota, H., Kawai-Noma, S., Saito, K., Ikeuchi, M., Iijima, Y., et al. (2016). Bacterial production of pinene by a laboratory-evolved pinene-synthase. ACS Synth. Biol. 5, 1011–1020. doi: 10.1021/acssynbio.6b00140
Wei, T., Cheng, B. Y., and Liu, J. Z. (2016). Genome engineering Escherichia coli for L-DOPA overproduction from glucose. Sci. Rep. 6:30080. doi: 10.1038/srep30080
Weng, Z. M., Wang, Y., and Liu, J. Z. (2012). Overproduction of lycopene by metabolic engineering Escherichia coli. Bioprocess 2, 51–57. doi: 10.4236/bp.2012.22009
Whited, G. M., Feher, F. J., Benko, D. A., Cervin, M. A., Chotani, G. K., McAuliffe, J. C., et al. (2010). Development of a gas-phase bioprocess for isoprene monomer production using metabolic pathway engineering. Indus. Biotechnol. 6, 152–163.
Willrodt, C., Hoschek, A., Buhler, B., Schmid, A., and Julsing, M. K. (2015). Coupling limonene formation and oxyfunctionalization by mixed-culture resting cell fermentation. Biotechnol. Bioeng. 112, 1738–1750. doi: 10.1002/bit.25592
Yang, J. M., Nie, Q. J., Ren, M., Feng, H. R., Jiang, X. L., Zheng, Y. N., et al. (2013). Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels 6:60. doi: 10.1186/1754-6834-6-60
Zhang, B. C., Watts, K. M., Hodge, D., Kemp, L. M., Hunstad, D. A., Hicks, L. M., et al. (2011). A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50, 3570–3577. doi: 10.1021/bi200113y
Zhang, C. Q., Chen, X. X., Stephanopoulos, G., and Too, H. P. (2016). Efflux transporter engineering markedly improves amorphadiene production in Escherichia coli. Biotechnol. Bioeng. 113, 1755–1763. doi: 10.1002/bit.25943
Zhang, H., and Stephanopoulos, G. (2016). Co-culture engineering for microbial biosynthesis of 3-amino-benzoic acid in Escherichia coli. Biotechnol. J. 11, 981–987. doi: 10.1002/biot.201600013
Zhang, H. R., and Wang, X. N. (2016). Modular co-culture engineering, a new approach for metabolic engineering. Metab. Eng. 37, 114–121. doi: 10.1016/j.ymben.2016.05.007
Zhang, H. R., Pereira, B., Li, Z. J., and Stephanopoulos, G. (2015). Engineering Escherichia coli coculture systems for the production of biochemical products. Proc. Natl. Acad. Sci. U. S. A. 112, 8266–8271. doi: 10.1073/pnas.1506781112
Zhou, K., Qiao, K. J., Edgar, S., and Stephanopoulos, G. (2015). Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 33, 377–U157. doi: 10.1038/nbt.3095
Keywords: pinene biosynthesis, Escherichia coli, tolerance engineering, directed evolution, chemically induced chromosomal evolution, modular co-culture
Citation: Niu F-X, He X, Wu Y-Q and Liu J-Z (2018) Enhancing Production of Pinene in Escherichia coli by Using a Combination of Tolerance, Evolution, and Modular Co-culture Engineering. Front. Microbiol. 9:1623. doi: 10.3389/fmicb.2018.01623
Received: 30 April 2018; Accepted: 28 June 2018;
Published: 31 July 2018.
Edited by:
Dipesh Dhakal, Sun Moon University, South KoreaReviewed by:
Shuai Qian, Rice University, United StatesVlada B. Urlacher, Heinrich Heine Universität Düsseldorf, Germany
Copyright © 2018 Niu, He, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Zhong Liu, bHNzbGp6QG1haWwuc3lzdS5lZHUuY24=
 Fu-Xing Niu
Fu-Xing Niu Jian-Zhong Liu
Jian-Zhong Liu