Abstract
The rumen is a complex ecosystem composed of anaerobic bacteria, protozoa, fungi, methanogenic archaea and phages. These microbes interact closely to breakdown plant material that cannot be digested by humans, whilst providing metabolic energy to the host and, in the case of archaea, producing methane. Consequently, ruminants produce meat and milk, which are rich in high-quality protein, vitamins and minerals, and therefore contribute to food security. As the world population is predicted to reach approximately 9.7 billion by 2050, an increase in ruminant production to satisfy global protein demand is necessary, despite limited land availability, and whilst ensuring environmental impact is minimized. Although challenging, these goals can be met, but depend on our understanding of the rumen microbiome. Attempts to manipulate the rumen microbiome to benefit global agricultural challenges have been ongoing for decades with limited success, mostly due to the lack of a detailed understanding of this microbiome and our limited ability to culture most of these microbes outside the rumen. The potential to manipulate the rumen microbiome and meet global livestock challenges through animal breeding and introduction of dietary interventions during early life have recently emerged as promising new technologies. Our inability to phenotype ruminants in a high-throughput manner has also hampered progress, although the recent increase in “omic” data may allow further development of mathematical models and rumen microbial gene biomarkers as proxies. Advances in computational tools, high-throughput sequencing technologies and cultivation-independent “omics” approaches continue to revolutionize our understanding of the rumen microbiome. This will ultimately provide the knowledge framework needed to solve current and future ruminant livestock challenges.
Global agricultural challenges
There are currently 7.5 billion humans on the planet, and the world Hunger Map estimates that 795 million people (over 10%) do not have access to sufficient food (WFP, 2015). Whilst some models predict the world population to peak at 9.7 billion in 2050, others estimate a population of 11.2 billion in 2100 (United Nations, 2015). To meet an increasing demand for food, the Food and Agriculture Organization of the United Nations (FAO) predicts that total agricultural production (including crops and animals) will need to be 60% higher than in 2005. With animal protein demand rising at a proportionally faster rate, estimates suggest that global meat and milk production will have to increase by 76 and 63%, respectively (Alexandratos and Bruinsma, 2012).
This extensive population growth, coupled with an increased consumption of ruminant products by developing countries, will add to the strain on the availability of safe and nutritious ruminant products. Due to land constraints, the number of pastured ruminants cannot increase and therefore efforts should be directed toward increasing production efficiency. Indeed, efficient utilization of feed by the rumen microbiome results in enhanced nutrient availability to the host, and thus improved production efficiency is central to ensuring food security. Feed for ruminants typically accounts for 60–70% of total expenditure in beef production (Karisa et al., 2014; Fouhse et al., 2017), whilst requiring substantial land mass for plant growth. Residual feed intake (RFI), which is the difference between the predicted (based on energy demands) and actual intake, has been proposed as a more meaningful measure for calculating feed efficiency (Berry and Crowley, 2012; Shabat et al., 2016). RFI values of 1.45 (high RFI) and −1.64 kg/day (low RFI) have been noted for crossbred steers (with 0 being the expected and values <0 inferring that the animal has greater feed efficiency than expected), resulting in high RFI animals requiring approximately 1,000 kg more feed/annum than low RFI animals to achieve the same production parameters (Fouhse et al., 2017). Therefore, understanding the underlying mechanisms for RFI, particularly with respect to the involvement of the rumen microbiome, could aid efficiency and sustainability of ruminant production (Mizrahi, 2011).
Ruminant livestock production has been estimated to be responsible for approximately 14% of anthropogenic methane, a potent greenhouse gas (GHG), released annually into the atmosphere due to the activity of rumen methanogens (Gerber et al., 2013). The released methane, produced by rumen methanogens, is a major problem for the environment, but also a great concern to livestock production as around 2–8% of the dietary energy can be lost to methane (CH4) production (IPCC, 2006); values as high as 12% have been reported for low quality feeds (Johnson and Johnson, 1995). Nonetheless, reductions in methane emissions do not always result in a redirection of energy, leading to enhanced animal production. For example, 3-nitrooxypropanol (3-NOP) has been shown to reduce methane emissions by up to 30% (Hristov et al., 2015; Jayanegara et al., 2018). However, a meta-analysis of all available animal data following supplementation with 3-NOP only shows modest increases in animal production, possibly due to decreased volatile fatty acid (VFA) produced from breakdown of cellulose and increased H2 production; both processes requiring energy input (Jayanegara et al., 2018).
The rumen microbiome is also pivotal to nitrogen (N) use efficiency due to its role in proteolysis and catabolism of amino acids, resulting in microbial N, which contributes 60–90% of protein absorbed at the duodenum (Wallace et al., 1997). Ruminant N use efficiency also needs to be improved to optimize production and reduce the environmental footprint of the industry as ruminants excrete approximately 70% of ingested N (Macrae and Ulyatt, 1974; Dewhurst et al., 1996; Edwards et al., 2008; Kingston-Smith et al., 2008, 2010). Once in soil, a portion of the N can be converted by bacteria into N2O, a GHG with a 298-fold greater global warming potential than CO2 (Hristov et al., 2013).
In summary, the rumen microbiome is central to addressing the grand challenges facing agriculture globally. A better understanding of the roles played by the constituent microbes is central to the development of advanced methods to manipulate the rumen microbiome in a manner that improves ruminant production whilst reducing environmental impact (Yáñez-Ruiz et al., 2015).
The rumen microbiome
The rumen is a complex, dynamic ecosystem composed of mainly anaerobic bacteria, protozoa, anaerobic fungi, methanogenic archaea and phages. These microbes interact with each other and have a symbiotic relationship with the host, providing energy from the breakdown of plant cell wall carbohydrates that are largely inedible by humans (Mizrahi, 2013). Recently, it has also been hypothesized that these microbes display niche specialization in terms of nutrient utilization and they also engineer the rumen ecosystem in terms of subsequent microbial colonization and nutrient utilization (Pereira and Berry, 2017; Shaani et al., 2018). As a consequence of their highly evolved rumen microbiome, ruminants provide human-edible nutritious foods derived from marginal land, without competing with food crop production (Kingston-Smith et al., 2010).
Rumen bacteria
The seminal work of Robert Hungate, the father of rumen microbiology, resulted in many of the culture technologies for anaerobic bacteria that are still widely used throughout the world (Hungate, 1966). These cultivation techniques enabled researchers to show that the rumen bacteria are the most abundant and diverse group of microorganisms in the rumen ecosystem. As a whole, they possess a multitude of enzymatic activities (i.e., amylases, cellulases, proteases, lipases) that carry out digestion of starch, plant cell walls, proteins and lipids in the rumen. Whilst there have been significant technological advancements during the last decade, the function of the rumen bacteria and their interactions with other members of the rumen microbiome is still poorly understood and consequently there are only a few examples where direct manipulation of the composition of this community has generated beneficial outcomes.
One of these successes relates to Leucaena leucocephala, which is a leguminous plant, that is high in protein and used as a ruminant feed in tropical countries. Nonetheless, the plant also produces toxins, causing salivation, live weight losses and generally poor animal performance. L. leucocephala contains the toxin mimosine which is converted in the rumen to 4-hydroxy-4(H)-pyridone (DHP), an effective goitrogen (Wallace, 2008) The rumen microbiomes of Hawaiian goats were shown to be tolerant to L. leucocephala (Jones and Megarrity, 1986) and further investigations revealed that these goats possessed a bacterium, Synergistes jonesii which was capable of degrading DHP. This is a unique example whereby understanding the role of the rumen bacteria transformed livestock nutrition, as S. jonesii is now used as an inoculum in many tropical countries as means of counteracting DHP toxicity (Wallace, 2008).
Rumen archaea
The archaeal domain in the rumen is composed largely of methanogenic archaea from the phylum Euryarchaeota. These methanogens are responsible for methane production in the rumen, which is then eructed and released to the environment. Methane is produced primarily via the hydrogenotrophic pathway (Figure 1) as a result of the reduction of CO2, and less so through the utilization of methyl groups (methylotrophic pathway), or even less commonly from acetate (acetoclastic pathway; Morgavi et al., 2010; Tapio et al., 2017). Hydrogenotrophic methanogens include Methanobrevibacter (Mbb.), which is sub-divided into the SMT clade (Mbb. smithii, Mbb. gottschalki, Mbb. millerae, and Mbb. thaurei) or the RO clade (Mbb. ruminantium and Mbb. Olleyae; Tapio et al., 2017). Methylotrophic methanogens are less abundant and include Methanosarcinales, Methanosphaera, and Methanomassiliicoccaceae. Recently, methylotrophic methanogens and their functionality were found to be highly enriched in young ruminants whilst being less abundant and showing decreased functionality in mature animals (Friedman et al., 2017). Nonetheless, some published data show that Methanomassiliicoccaceae can represent approximately 50–70% of the rumen archaea (Huang et al., 2016; Wang P. et al., 2016). The Methanosarcinales can also produce methane via the acetoclastic pathway (Morgavi et al., 2010). Whilst methanogenesis has major implications for the environment, it serves an important purpose of elimination fermentative hydrogen from the rumen (Wright and Klieve, 2011). Strategies to reduce methane emissions must therefore take into account the need to remove excess hydrogen rom the rumen.
Figure 1
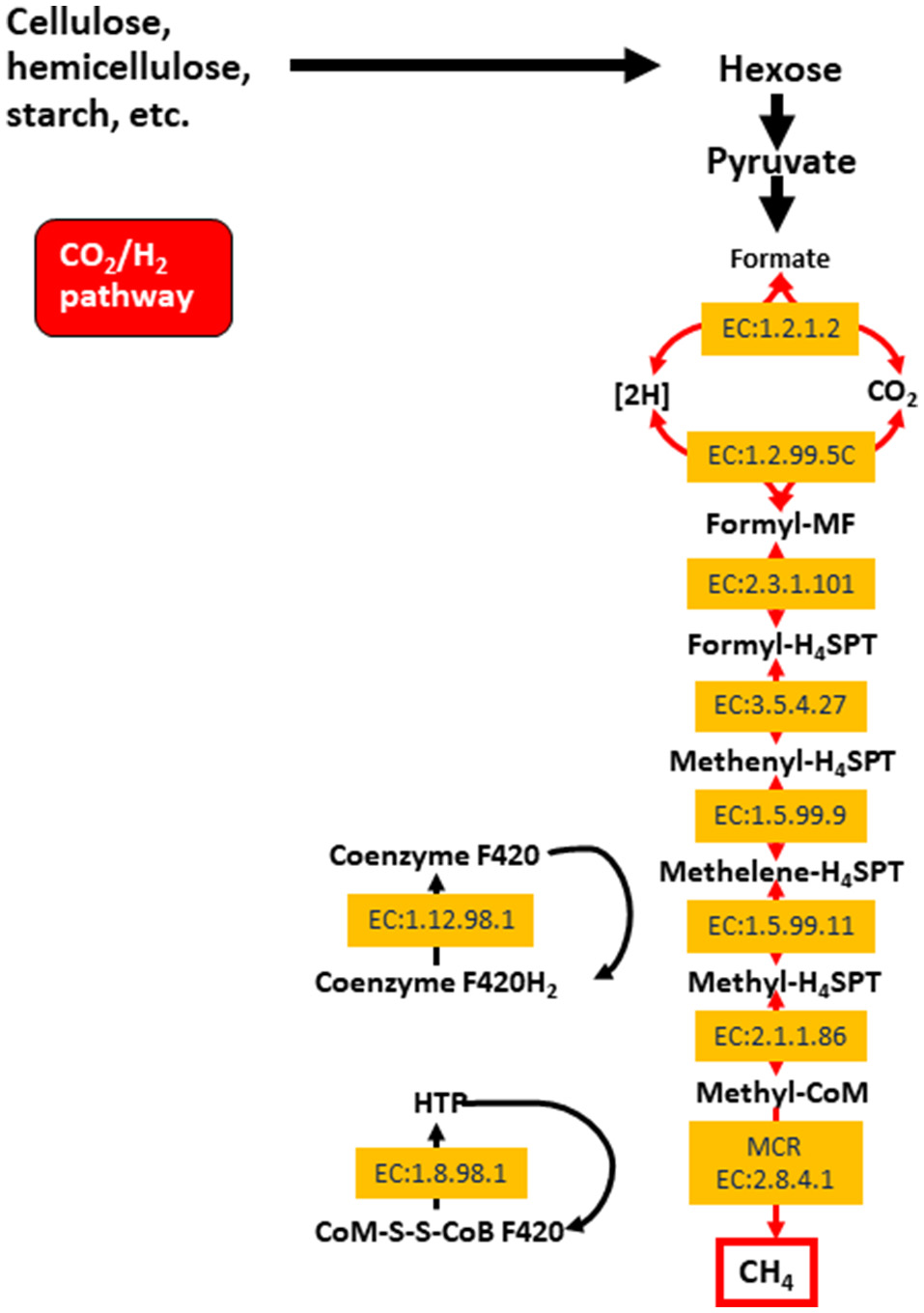
The hydrogenotrophic methane production pathway including enzyme classifications (EC) for enzyme involved in the process. Reproduced from Shi et al. (2014).
Bacteriophage
Lytic phages were first isolated from rumen fluid and the bacterial genera Serratia and Streptococcus as far back as 1966 (Adams et al., 1966). Whilst much research ensued to isolate phage in the 1970s and 1980s, only those with potential biotechnological applications were further characterized and retained in culture collections (Gilbert and Klieve, 2015). Recently, Gilbert et al. (2017) isolated and obtained complete genome sequences for lytic phages, belonging to the order Caudovirales, capable of infecting Bacteroides, Ruminococcus, and Streptococcus. Whilst it is known that phage alter the ecology and evolution of microbial communities (Koskella and Brockhurst, 2014), the effects of phage on the rumen microbiome remains to be determined.
Rumen protozoa
Whilst the rumen bacteria are the most numerate, the rumen protozoa represent a large proportion of the microbial biomass within the rumen (approximately 20% and up to 50% in some conditions) due to their cell volume. Rumen protozoa were first described by Gruby and Delafond in 1843 (Gruby and Delafond, 1843) and, along with fungi, make up the rumen eukaryote members of the microbiota (Williams and Coleman, 1997; Newbold et al., 2015). Ciliates dominate in the rumen, with flagellates such as Trichomonas sp., Monocecromonas sp. and Chilomastix sp. occasionally seen, but in much lower densities (Williams and Coleman, 1997). Ruminants commonly harbor distinct protozoal populations from birth, with only minor changes in diversity throughout life, although the abundances of species fluctuate with changes in diet (Williams and Coleman, 1997). For example, Dastrychia and Entodinium were shown to be the predominant genera in rumen fluid taken from dairy cows and Dastrychia has been shown to be more predominant in the rumen fluid taken from cows fed corn stover as compared with those fed alfalfa hay and corn silage (Zhang et al., 2015). Protozoal populations in the rumen have also been categorized as A-type (characterized by an abundance of Polyplastron multivesiculatum), B-type (characterized by an abundance of Epidinium caudatum or Eudiplodinium maggii), O-type (characterized by an abundance of Entodinum, Dasytrycha, and Isotricha), or lastly K-type (characterized by an abundance of Elytroplastron bubali; Kittelmann and Janssen, 2011).
The contribution of protozoa to rumen fermentation remains controversial. It is known that protozoa can be removed from the rumen, a process known as defaunation, and the animal will still survive (Williams and Coleman, 1992; Newbold et al., 2015). A recent meta-analysis used 23 in vivo defaunation studies in an effort to determine the function of rumen protozoa (Newbold et al., 2015). Based on their analysis, Newbold and colleagues found evidence that the removal of protozoa from the rumen caused a decrease in organic matter degradation, especially of neutral and acid detergent fiber. This confirmed the original data of Williams and Coleman (1992) that some of the rumen protozoa (i.e., Epidinium, Polyplastron and Entodinium spp.) possess fibrolytic activity. Indeed, light microscopy of rumen contents clearly shows that Epidinium spp. are strongly associated with plant cells and are capable of scavenging plant chloroplasts, which are rich in protein and lipids (Huws et al., 2009, 2012; Figure 2). In addition to their capacity to degrade fiber, protozoa have been linked to methanogenesis as defaunation reduces methane output by approximately 11% (Hegarty, 1999; Morgavi et al., 2010; Newbold et al., 2015). This is likely due to the fact that rumen protozoal hydrogenosomes produce H2, which then serves as a substrate for methanogens to reduce CO2 to methane via the hydrogenotrophic pathway (Vogels et al., 1980; Belanche et al., 2014). This suggests that removal of protozoa may be a strategy to reduce production of methane by ruminants. However, rumen protozoa vary substantially in their contributions to plant degradation and methane production. For example, Epidinium spp. contribute substantially to plant degradation (Huws et al., 2009) and generally holotrichs support methanogens and methanogenesis (Belanche et al., 2014). As a consequence, a strategy which eliminates all protozoa may not be the best approach, nonetheless, elimination of a sub-group of protozoa is a major challenge which currently is technologically challenging.
Figure 2
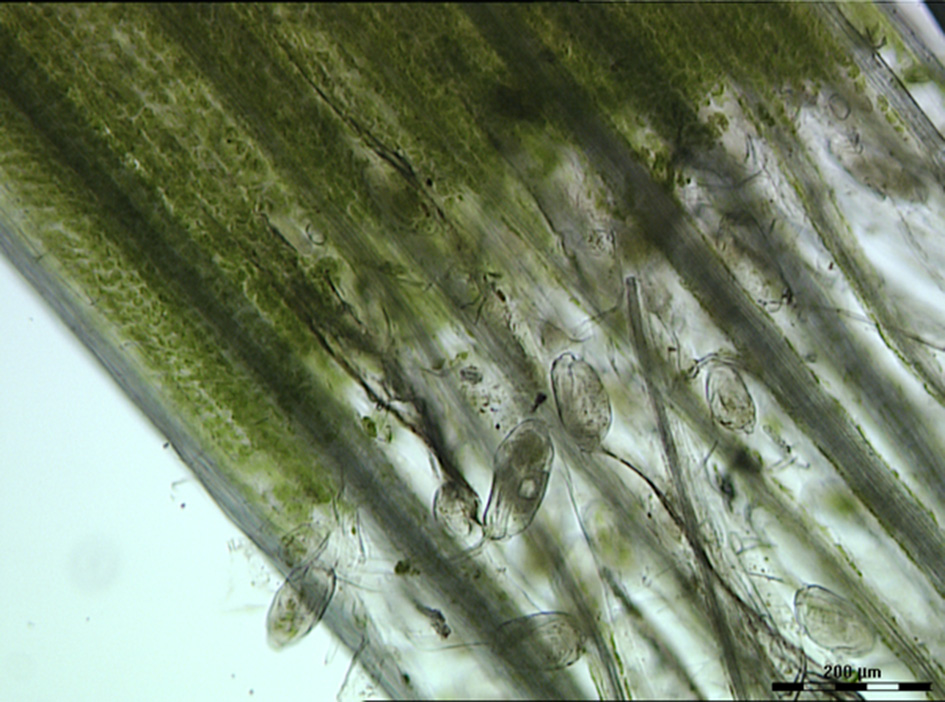
Light microscopy image of rumen contents taken from a ruminant possessing B-type protozoal diversity and showing close interactions of Epidinium spp. with fresh perennial ryegrass. Scale bar: 200 μM.
Rumen fungi
The flagellated zoospores of anaerobic fungi (Neocallimastigomycetes) were first observed in the early 1900's. However, it was not until the 1970's that their true identity was confirmed (Orpin, 1974, 1977a). To date, nine anaerobic fungal genera have been characterized with many other uncultivated taxa known to exist (Koetschan et al., 2014; Edwards et al., 2017; Paul et al., 2018). Paul et al. (2018) attempted to get consensus on the diversity of anaerobic fungi inhabiting the guts of herbivores and concluded that among the cultured genera, Piromyces was the most represented with Buwchfawromyces being the least represented in sequence data obtained from the Genbank database. Paul et al. (2018) also suggest that possibly another 25 new genera exist in the guts of herbivores, which remain uncharacterized. Irrespective, anaerobic fungi are among the most potent fiber-degrading organisms in the known biological world, primarily due to their efficient and extensive set of enzymes for the degradation of plant structural polymers (Solomon et al., 2016). Furthermore, their rhizoids have the ability to physically penetrate plant structural barriers (Orpin, 1977a,b). The latter ability benefits other rumen microbes by increasing the plant cell surface area available for colonization. Rumen fungi also possess amylolytic (Gordon and Phillips, 1998) and proteolytic activity (Gruninger et al., 2014).
The activity of anaerobic fungi is enhanced by methanogenic archaea (Cheng et al., 2009), which are known to physically attach to anaerobic fungal biomass. Anaerobic fungi are clearly beneficial, and have been shown to improve feed intake, feed digestibility, feed efficiency, daily weight gain and milk production (Lee et al., 2000; Dey et al., 2004; Paul et al., 2004; Tripathi et al., 2007; Saxena et al., 2010; Puniya et al., 2015). Chitin measurements (Rezaeian et al., 2004) and rRNA transcript abundance (Elekwachi et al., 2017) indicate that anaerobic fungi represent 10–20% of the rumen microbiome. However, like protozoa, they are not routinely studied despite suitable cultivation independent tools being available (Edwards et al., 2017).
Despite the importance of the rumen eukaryotes, our understanding of their function is far less than that of rumen bacteria. Beyond the study of their fiber degrading enzymes, much of the activity and metabolism of anaerobic fungi remains unknown, particularly due to the limited annotation of the multiple genome sequences and transcriptomes now available (Edwards et al., 2017). As with protozoa, key challenges include their cultivation, lack of genomic information, and lack of consensus on best practices to analyse sequence data (Ishaq et al., 2017). Thus, there are still many challenges which need to be overcome to enable a comprehensive understanding of the rumen microbiome as a whole.
Importance of the biofilm phenotype and membrane vesicle production to host nutrient availability
Similar to most other microbiomes in nature, the rumen microbiome is dominated by microbes existing within biofilms, which are defined as a consortia of microbes attached to a surface, encased in a self-produced extracellular polymeric matrix (EPS; Figure 3; Cheng et al., 1979; Cheng and Costerton, 1980; Mcallister et al., 1994; Huws et al., 2013, 2014, 2016; Zhao et al., 2018). The biofilm phenotype has many advantages, including the concentration of digestive enzymes within the EPS in proximity to the substrate, an arrangement that enables effective hydrolysis of plant material within the rumen (Minato et al., 1966; Wolin et al., 1997; Michalet-Doreau et al., 2001; Leng, 2014). The EPS is also rich in DNA, protein, and lipids, which possibly play a role in biofilm stability, whilst also being a source of nutrients for the ruminant following its out-flow from the rumen to the lower digestive tract (Shukla and Rao, 2017; Sugimoto et al., 2018). Whilst protein concentration within EPS is greater than within the attached bacteria, very little consideration has been given to this structure in terms of contribution to the nutrition of the host.
Figure 3
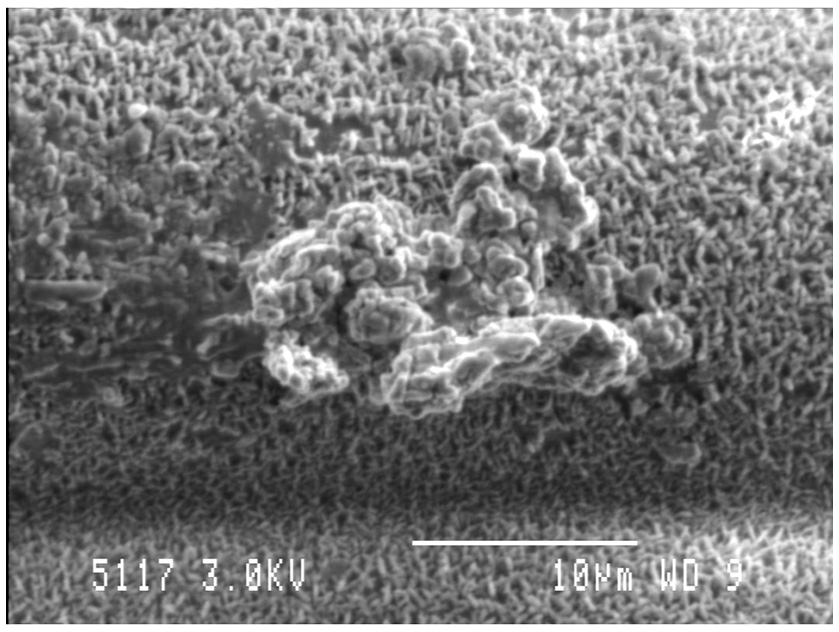
Biofilm community on the adaxial surface of fresh perennial following in vitro incubation in the presence of rumen fluid as outlined in Huws et al. (2014). Scale bar: 10 μM.
Membrane vesicles are often blebbed from the bacterial cell membrane, that extend into the EPS. Numerous bacterial pure culture studies have shown that bacteria are adept at producing membrane vesicles (Schooling and Beveridge, 2006). These membrane vesicles are packed with DNA, proteins and lipids (Schooling et al., 2009) and likely promote biofilm stability. These membrane vesicles have been recently observed in the rumen bacterium Fibrobacter succinogenes (Arntzen et al., 2017). These membrane vesicles can contain high concentrations of glycosyl hydrolases, allowing F. succinogenes to effectively degrade plant cellulose (Arntzen et al., 2017). Also, Prevotella ruminocola is suggested to produce membrane vesicles, but their role in plant degradation remains to be defined (Huws, personal communication; Figure 4).
Figure 4
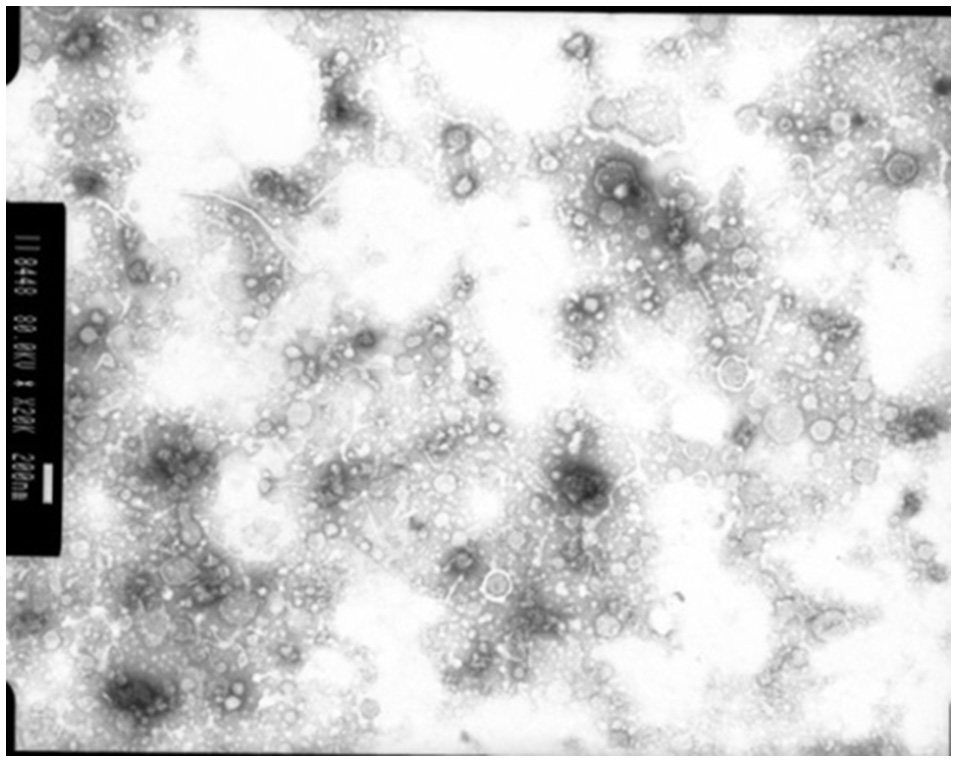
Membrane vesicles isolated from Prevotella ruminocola incubated in vitro in Hungate tubes. Scale bar: 200 nM.
Untangling the influence of diet on the rumen microbiome and consequently host phenotype
Adult animals
A recent global comparison study of the rumen microbiome in 742 samples across 32 species from various geographical locations (Henderson et al., 2015), identified that 30 of the most abundant bacterial groups were present in over 90% of the samples. Members of the methanogen clades Methanobrevibacter gottschalkii and Methanobrevibacter ruminantium were found in nearly all samples and accounted for 74% of the archaea. The consistency of common microbes across a wide variety of ruminants led Henderson et al. (2015) to conclude that global evolutionary pressures selected for common microbial components within the fermentatative microbiomes. This reasons with Darwin's theory of natural selection, considering a natural diet high in forage is common amongst ruminants. This study also concluded that the composition of the rumen microbiome was mainly driven by the diet (Henderson et al., 2015). Indeed, dietary interventions have been historically used to improve upon ruminant phenotypes due to their influence on the rumen microbiome (Table 1).
Table 1
| Dietary intervention | Effects | Mode of action | References |
|---|---|---|---|
| High quality forage | Improve milk fat content and quality | Favor fibrolytic microbes and microbial diversity (resilience) | Couvreur et al., 2006 |
| High concentrate diet | Increase animal productivity | Favor propionate production and decrease microbial diversity | Fernando et al., 2010; Belanche et al., 2012 |
| Antibiotics | Increase productivity and decrease rumen acidosis | Favor propionate-producers | Schelling, 1984 |
| Red Clover | Increase productivity | Protein complexing with polyphenol oxidase allowing more protein to by-pass rumen | Broderick, 1995; Lee, 2014; Hart et al., 2016 |
| Bicarbonate | Prevention rumen acidosis | Limit the rumen pH depression and the negative impact on rumen microbes | Keunen et al., 2003; González et al., 2012 |
| Probiotics (Yeast) | Prevention rumen acidosis and increase feed efficiency | Oxygen scavenging | Newbold et al., 1996; Desnoyers et al., 2009 |
| Essential oils | Prevention rumen acidosis | Shift in the microbial community | Calsamiglia et al., 2007; Macheboeuf et al., 2008 |
| Tannins | Improve the flow of digestible rumen by-pass protein | Formation of phenol-dietary protein complexes | Mcsweeney et al., 2001; Patra, 2010 |
| Saponins | Methane inhibition and increased microbial protein synthesis | Antiprotozoal effect | Patra and Saxena, 2009b; Ramos-Morales et al., 2017 |
| Unsaturated fats | Methane inhibition | Antiprotozoal effect | Martin et al., 2010 |
| Methane analogs | Methane inhibition | Inhibition of rumen methanogens | Knight et al., 2011; Abecia et al., 2012; Hristov et al., 2015 |
Examples of dietary interventions that have been used to modulate the rumen microbiome.
Among these dietary interventions, the modification of the forage:concentrate ratio is the most studied (Fernando et al., 2010). Ruminants have traditionally been fed high forage diets to decrease feeding costs, and to avoid competition with plant sources that can be used as food for humans. Moreover, a linear relationship has been noted between the proportion of fresh grass within the diet, and milk fat composition and butter properties in dairy cattle (Couvreur et al., 2006). In particular, fresh grass in comparison to grass hay promotes an accelerated feed colonization by rumen microbes and subsequent digestion (Belanche et al., 2017). Furthermore, microbial protein synthesis is increased and methane emissions lowered (Belanche et al., 2016). However, most of the intensive ruminant production systems, particularly beef feedlot systems, use high-grain diets to maximize growth rates and feed efficiency. Supplementation of the diet with easily digestible carbohydrates minimizes the negative effects of dietary protein shortage (Belanche et al., 2012) and promotes a modification of the rumen microbiome (Fernando et al., 2010), due to a simplification of the rumen microbial community. As a result, animals fed high-grain diets tend to have lower bacterial diversity and lower concentrations of fibrolytic microbes (i.e., protozoa and anaerobic fungi), which are generally associated with lower rumen proteolysis and ultimately higher feed efficiency (Belanche et al., 2012). Moreover, high grain diet was shown to affect the composition of the rumen methanogenic community via its effect on the rumen redox potential, with a specific effect on the Methanomicrobiales order (Friedman et al., 2017). However, this strategy often leads to a decrease in rumen pH due to high VFA and lactate accumulation and ultimately to digestive disorders (rumen acidosis with lactate accumulation occurring in severe cases only) and energy spilling reactions (Russell and Strobel, 1993). To prevent lactic acidosis, antibiotics such as ionophores, which select against Gram positive bacteria that produce lactate are often included in high-grain diets. However, globally antibiotics and growth promoters have been drastically reduced in livestock production, with a ban enforced in the EU (Russell and Houlihan, 2003). Novel cost-effective strategies to modulate rumen microbial fermentation need to be identified.
Feeding red clover to ruminants results in increased nitrogen efficiency due to the fact that it possesses the enzyme polyphenol oxidase (PPO; Broderick, 1995; Lee, 2014). PPO is a copper metallo-protein that, in the presence of oxygen, catalyzes the oxidation of endogenous phenols to quinones (Lee et al., 2004). PPO protects plant protein from ruminal degradation, allowing intact protein to by-pass to the abomasum. The mechanism of protein protection seems to be related to the deactivation of plant proteases by the PPO enzyme as well as PPO mediated protein-quinone binding (Mayer and Harel, 1979; Lee, 2014). PPO is located in the chloroplast and until recently the substrate for activating PPO was thought to exist only in the plant vacuole. Recent data now indicates that PPO preferentially protects proteins within chloroplasts, suggesting that there are also PPO-activating substrates within chloroplasts (Hart et al., 2016; Boeckx et al., 2017). It is also known that feeding red clover silage alters rumen microbial diversity compared with a perennial ryegrass silage-based diet, which contributes to changes seen in animal phenotype when red clover is fed (Huws et al., 2010).
Sodium bicarbonate and yeast (Saccharomyces cerevisiae) supplementation both have shown some success in preventing sub-acute acidosis (SARA; Keunen et al., 2003; González et al., 2012; Ishaq et al., 2017). Due to its oxygen scavenging activity in the rumen (Newbold et al., 1996), S. cerevisiae can increase the density of fibrolytic bacteria and hence feed efficiency (Desnoyers et al., 2009). Ishaq et al. (2017) also showed that dairy cows with diet-induced SARA had a higher abundance of rumen fungi and lower abundances of rumen protozoa compared with healthy cows. Ishaq et al. (2017) then fed active dry yeast to the dairy cows with induced SARA and noted an increase in pH and rumen protozoal abundance. Moreover, in recent years, a variety of plant bioactive compounds, including saponins, essential oils, tannins and flavonoids have also been evaluated for their ability to modulate rumen microbial fermentation (Patra and Saxena, 2009a,b). Essential oils have been proven to slow down starch and protein degradation, decreasing the risk of acidosis while causing minor reductions in rumen methanogenesis (Calsamiglia et al., 2007). However, their application has been limited because of their adverse effects on fiber digestion and rumen fermentation (Macheboeuf et al., 2008). Phenolic compounds such as condensed and hydrolysable tannins can also have anti-nutritional effects due to their interaction with enzymes and their antimicrobial properties. However, if fed at the right level, it is well established that tannins protect dietary protein from degradation in the rumen without significantly affecting the efficiency of carbohydrate digestion (Mcsweeney et al., 2001). Additionally, it has been suggested that tannins may also decrease methanogenesis by inhibition of rumen protozoa, methanogens and, to a lesser extent, hydrogen-producing microbes (Patra, 2010). Saponins, a group of plant secondary compounds derived mainly from Yucca shidigera and Quillaja saponaria, have also shown potential for modifying rumen fermentation primarily through the inhibition of protozoa. Furthermore, saponins can decrease methane production by selectively targeting certain groups of rumen protozoa, methanogens, fungi and bacteria (Patra and Saxena, 2009b). However, the antiprotozoal effect of saponins is transitory as when saponins are deglycosylated to sapogenins by rumen microorganisms, they become inactive (Newbold et al., 1997). This presents a challenge for the practical application use of saponins in ruminant nutrition (Ramos-Morales et al., 2017).
One of the most promising compounds for reducing ruminal methanogenesis is 3-NOP, which is an analog of the methyl-coenzyme M subunit of the nickel enzyme methyl-coenzyme M reductase in rumen archaea. This enzyme catalyzes the last step of methane-forming reactions (Duin et al., 2016) and its inhibition can result in a reduction in rumen methanogenesis (up to 30%) without negative effects to the animal (Hristov et al., 2015). Nonetheless, benefits for ruminant production are comparatively low, likely due to high H2 accumulation (Jayanegara et al., 2018). However, a recent study has shown that benefits for animal productivity could be enhanced (Martinez-Fernandez et al., 2017). Supplementation of phloroglucinol together with 3-NOP promotes capture of excess hydrogen from methanogenesis and generates valuable metabolites for the host (Martinez-Fernandez et al., 2017). The addition of acetogenic rumen bacteria to remove excess hydrogen has also been widely suggested as an effective intervention which may work in combination with 3-NOP (Wright and Klieve, 2011)
In summary, more multidisciplinary studies are needed to uncover the mode of action of these nutritional interventions and their true potential to modulate the rumen microbiome under farm conditions. Furthermore, data shows that in mature ruminants, dietary changes can be short-lived. Instead, interventions in the early life of the ruminant may offer a better longer-term strategy to improve animal phenotype.
Early-life
In contrast to the developed rumen, where a stable and resilient microbial community is established, during the development of the rumen after birth a succession of different microbial groups colonize and start occupying the different ecological niches. The instability occurring during this period potentially allows for manipulation to assemble a specific community composition that persist later in life for better health and productivity within a given production system (Yáñez-Ruiz et al., 2015).
At birth, ruminants display a non-developed reticulo-rumen. Until the system is fully matured, they function as monogastrics, whereby the milk fed is not digested in the rumen but flows to the abomasum via an esophageal groove (Church, 1988). Colonization of the developing rumen begins immediately after birth and progresses through the first few months of life until a stable community establishes (Jami et al., 2013). The dynamics of the gut microbial community establishment in young ruminants occurs in three successive steps (Rey et al., 2014; Abecia et al., 2017): (i) initial colonization (0–2 days post-partum) originated from a combination of sources such as microbiota of mother's vagina, skin, colostrum and microbes within the environment (Van Nimwegen et al., 2011; Yeoman et al., 2018); (ii) transitional stage (3–15 days) during the transition from colostrum to milk, and iii) maturation stage in which solid feed intake progressively increases and the distribution of main bacterial phyla and other microbial groups is comparable to that in adult animals. It is important to note that although the rumen microbiome establishes before intake of solid feeds, the type of feed consumed plays a significant role in shaping the established rumen microbiome. Hence, the early phases of solid feed intake represents a window of opportunity to modulate the composition of the initial colonizers of the different ecological niches in the rumen according to dietary and management strategies (Yáñez-Ruiz et al., 2015). Indeed, the use of probiotics, such as lactic acid bacteria, in early life to mitigate incidence of digestive and respiratory diseases has shown promise (Timmerman et al., 2005; Signorini et al., 2012). Yáñez-Ruiz et al. (2010) also reported that feeding forage vs. concentrate around weaning modifies the bacterial population colonizing the rumen of lambs and that the effect persists over 4 months. It is also known that feeding concentrate in early life stimulates the development of the epithelium, while feeding high fiber diets can stimulate development of rumen muscularization and volume (Zitnan et al., 1998). Nonetheless, little is known regarding the impact of management practices, such as milk intake, delayed weaning etc. on early-life programming of the rumen microbiome and its implications for ruminant productivity.
Another factor that promotes differences in rumen colonization is the presence of the dam and the associated increase in the availability of microorganisms in the environment. This can allow earlier (and different) inoculation of microbes in the digestive tract of naturally raised newborns as compared to those fed milk replacer and kept in isolation (Abecia et al., 2017). Direct contact with the mother offers a constant source of microbes through the mouth, feces, skin and milk (Yeoman et al., 2018), sources that are not available for calves raised in isolation on milk replacer. This explains the greater number of Operational Taxonomic Units (OTUs) and bacterial diversity observed in naturally reared calves. Another distinctive feature between natural and artificial rearing systems is the near absence of protozoa in the rumen of artificially reared calves, as protozoa can only be inoculated in the rumen by direct contact with the dam or other mature animals through saliva (Abecia et al., 2014). A relatively recent study by Ishaq et al. (2015) showed that exposure of neonate lambs to the dam for 1 week followed by subsequent separation was enough to ensure the establishment of a stable rumen protozoal population for their lifetime.
Nutritional interventions in early-life may include (i) the direct inoculation of specific microorganisms or (ii) the use of additives that prevent or facilitate the colonization of some microbial groups. Feeding live microorganisms to ruminants is not a novel concept and extensive work has been published on the use of “direct-fed microbials” (DFM; Martin and Nisbet, 1992; Jeyanathan et al., 2014). The effect of supplementing S. cerevisiae on rumen development and growth performance in neonatal dairy calves has also been evaluated (Lesmeister et al., 2004). Although yeast cultures are widely used in ruminant nutrition, the concept of applying them in the diet of pre-ruminants deserves further assessment, especially in terms of their long term effects on the microbiome (Alugongo et al., 2017). A different approach that uses compounds to inhibit the establishment of certain microbial groups or favor the development of others is also now starting to attract attention. It has been shown that application of bromochloromethane (BCM) to young goat kids modified archaeal colonization of the rumen, and was linked to a reduction in methane emission of around 50%, with the effects persisting for 3 months after weaning (Abecia et al., 2013, 2014).
Despite some promising results from early-life dietary interventions, the ecological dynamics underpinning the microbial colonization, the most effective window of time for intervention and the long-term implications have yet to be identified.
Untangling the influence of host genomics on the rumen microbiome and consequently host phenotype
Consistent with human twin heritability studies (Goodrich et al., 2016), it is reasonable to hypothesize that animals possessing similar genomes should have more similar rumen microbiomes. Evidence of the influence of the host on the rumen microbiome was first postulated by Weimer et al. (2010) who found that after near total exchange of the rumen contents between cows, individuals restored their bacterial composition back to pre-exchange conditions, which also returned rumen pH and volatile fatty acid (VFA) concentration to pre-exchange values. Furthermore, in another near-total rumen content exchange between high- and low-efficiency Holstein cows, Weimer et al. (2017) demonstrated the hosts ability to return the rumen bacterial community to the original status, whilst linking the rumen microbiome to milk production efficiency.
Whilst Henderson et al. (2015) postulated that diet was the main driver for rumen microbiome composition, they also identified some differences in the relative abundance of certain bacterial populations across ruminant species. Similarly, when the microbiome of water buffalo (Bubalus bubalis) and Jersey cows were compared under comparable feeding conditions variations in bacterial, protozoa and methanogen populations were found between the two species (Iqbal et al., 2018), suggesting that the rumen microbiome is controlled, to a certain extent, by the genetics of the host. In a beef cattle experiment, Roehe et al. (2016) ranked beef sire progeny groups based on relative archaeal abundance and reported that group ranking remained consistent overall and within diet, suggesting that archaeal abundance in ruminal digesta is also, in part under host genetic control. Using sire progeny groups in dairy cattle, further evidence of genetic control was documented by the discovery that 22 bacterial OTUs, exhibited a heritability estimate of 0.7 or greater in dairy cattle (Sasson et al., 2017). In addition, these heritable OTUs were found to be correlated with traits such as DMI (dry matter intake) and RFI. Pinares-Patiño et al. (2011) and Pinares-Patiño et al. (2013) demonstrated that methane production is also regulated by host genetics in sheep and that selection of low methane emitting animals by genotyping is possible.
Nonetheless, De Mulder et al. (2018), stated that the differences in rumen microbiome composition may be due to other factors other than host genomics, including early life events and the fact that some breeds of cattle, such as Belgian Blue cattle, have a higher rate of cesarean section birth. The host immune system also likely plays an influential role on the rumen microbiome. For example, secretory immunoglobulin A (SIgA), which favors commensal bacteria in the gut (Gutzeit et al., 2014), has been shown to coat rumen bacteria (Fouhse et al., 2017) and control the host's recognition of certain microbial species. In addition, the rumen epithelium plays an important role in both nutrient uptake and immunity. The physiology of the rumen has also been highlighted as a potential factor that influences the rumen microbiome. For example, differences in rumen and camelid foregut volume, physiology as well as feeding frequencies, was suggested as a reason for the proportionally higher abundance of unclassified Veillonellaceae in camelids, deer and sheep compared to cattle (Henderson et al., 2015). In addition, methane yield is associated with retention time in the rumen (Pinares-Patiño et al., 2003) correlating increased passage rate in the rumen with reduced methane yield. Janssen (2010) provides a thorough review of these studies which in essence show that increased passage rate leads to less feed being fermented in the rumen and subsequently less substrate is available for methanogenesis (Tapio et al., 2017). It has also been demonstrated that both a shorter rumen retention time and a smaller rumen result in reduced methane yield (Goopy et al., 2014). Additionally, variation in the rumination behavior of animals can influence particle retention time (Mcsweeney et al., 1989). Therefore, genetic influence of the host on rumen passage rate is likely to be one host factor that influences the rumen microbiome, but other factors should also be considered (Pinares-Patiño et al., 2013).
Whilst there is increased evidence that host genetics has an influential role on the microbial population residing in the rumen (Tapio et al., 2017), our current understanding of the extent of this influence and the underlying mechanisms (Sasson et al., 2017) remains incomplete, although a region on chromosome 6 was recently associated with Actinobacteria, Euryarchaeota, and Fibrobacteres densities (Golder et al., 2018).
Contributions of the lower gastrointestinal tract microbiomes to ruminant phenotype
Typically, scientists have focussed their attention on understanding the rumen in order to deliver upon global livestock challenges. However, the lower gastrointestinal (GI) tract microbiomes also play an important role, particularly in early life (Meale et al., 2017). The microbial composition of the post-ruminal gastrointestinal tract is shaped by pH, gut motility, redox potential, and host secretions present in different compartments of the digestive tract. Most microbes flowing from the rumen into the abomasum are lysed by the low pH and enzymatic activity within the organ. As a consequence of the harsh environmental conditions prevailing in the abomasum and at the beginning of the small intestine, microbial numbers and diversity plummet by several orders of magnitude in the abomasum, duodenum and jejunum as compared to the rumen (Frey et al., 2010; He et al., 2018; Yeoman et al., 2018). From the ileum onwards, including caecum, colon and feces, favorable fermentation conditions are present again and microbial density and phylogenetic diversity increase to a level comparable to that of the rumen (Frey et al., 2010; De Oliveira et al., 2013; Popova et al., 2017; He et al., 2018; Yeoman et al., 2018).
The post-ruminal microbial community is composed predominantly of bacteria, but methanogenic archaea and anaerobic fungi have been described (Davies et al., 1993), although the later phylogentic group has not been targeted intensively with high-throughput sequencing techniques. There are significant difference in the microbial community assemblage depending on the region of the GI tract (i.e., rumen vs. post-rumen; Mao et al., 2015; Bergmann, 2017; Zeng et al., 2017; Yeoman et al., 2018), and the post rumen microbiota differ further between the small (duodenum, jejunum, and ileum) and the large (cecum, colon, and rectum) intestine (Mao et al., 2015; Bergmann, 2017; Wang et al., 2017; Yeoman et al., 2018). In general terms, compared to the rumen, the proportion of Bacteroidetes decrease and that of Firmicutes and Proteobacteria increase. Prevotella, Bacteroides, Ruminococcus, Treponema, and Desulfovibrio genera were detected in all segments of the GI tract of ruminant animals, while Fibrobacter was only present in the foregut (Zeng et al., 2017). Prevotella, Bacteroides, Ruminococcus, Faecalibacterium, Roseburia and Clostridium are consistently identified in fecal samples from ruminants and are considered part of the core microbiota (Dowd et al., 2008; Durso et al., 2012). As for the rumen, the rectal microbiota shows important inter-individual variation (Durso et al., 2010) and are affected by diet (Shanks et al., 2011).
The mucosa-associated microbial community is also an important modulator of immunological function and health (Malmuthuge et al., 2015). Mucosa-associated communities differ from those associated with luminal contents; and also vary among intestinal regions (Malmuthuge et al., 2014; Mao et al., 2015; Yeoman et al., 2018). Potential pathogens such as Escherichia, Shigella, Salmonella and Treponema spp. are most frequently found in the mucosa-associated bacterial microbiota (Mao et al., 2015; Song et al., 2018). Recently, differences in both the mucosa-associated microbiota of the rectoanal junction and fecal microbiota of cattle have been shown to influence the shedding of the human pathogen Escherichia coli O157 in cattle feces (Stenkamp-Strahm et al., 2018; Wang et al., 2018).
The role of the intestinal microbiota in feed degradation appears to be less important than that of the rumen (Al-Masaudi et al., 2017). Its main function has been suggested to be related to animal health and cross-talk interaction with the animal host (Lyte et al., 2018), although work in this area is only in its nascent phase and these aspects need further investigation. Notwithstanding, it is highlighted that feces and samples from the intestines cannot be used as proxies of rumen function on a microbiome biomarker level (Tapio et al., 2016). Nonetheless, concentrations of the compound archaeol in feces has been shown to correlate with methane emissions in cattle (Mccartney et al., 2014).
Developing microbiome biomarkers for prediction of ruminant phenotype
The sheer size of the rumen (12–15% of body mass) and connectedness with the vascular, respiratory and immune systems mean that it is well-placed to both affect, and be affected by, animal function. There is a growing number of examples where the interaction between host and intestinal microbial metabolism can be used to explain, or act as a biomarker for, complex traits such as nutrient efficiency, responses to stressors such as disease and adverse environments, as well as to predict animal behavior.
Nucleic acids have long been used as biomarkers for rumen microbial processes. Early work focussed on rumen microbial protein synthesis and RNA (Mcallan and Smith, 1969), while purine bases (Zinn and Owens, 1986) were also used as biomarkers for microbial (protein) synthesis in studies with intestinally cannulated animals. More recent attempts to develop non-invasive biomarker approaches to estimate microbial protein synthesis have used urinary metabolites derived from microbial purines (allantoin and uric acid; Chen et al., 1990). Recent advances in analytical technologies and bioinformatics have now greatly expanded our capacity to investigate the role of the rumen and its microbiome in complex traits by studying the composition of microbial DNA and RNA (metataxonomics, metagenomics and metatranscriptomics), as well as microbial metabolites in blood or urine (metabolomics). In terms of metataxonomics, microbial correlations to feed efficiency and/or methane production in ruminants, using rRNA genes or the Methyl coenzyme M reductase (mcrA) gene in methanogens are difficult to interpret, due to the confounding factors such as animal type, feed, and rumen sample processing and analysis (see Metataxonomy section). Recent studies suggest that methanogen diversity, and not density, is critically important to methane output, with more diversity being associated with higher emissions (Janssen and Kirs, 2008; Carberry et al., 2014). However, most studies involve a small number of animals, making it difficult to clearly confirm the link between methanogen diversity and methane emissions (Morgavi et al., 2010). When investigating the rumen bacterial associations with methane production, density of Sharpea has been shown to be significantly lower in low methane emitting animals (Kamke et al., 2016). Positive correlations between Eubacterium sp. and reduced feed efficiency were also reported by Hernandez-Sanabria et al. (2012). Jami et al. (2014) also reported a positive correlation between RFI and the uncultured rumen bacterium RF39, whereas Shabat et al. (2016), suggested that an increase in the acrylate pathway coded by Megasphaera elsdenii and Coprococcus catus in the rumen may increase feed efficiency and reduce methane. It has been suggested also that the ratio of bacteria:archaea reflects methane output from the animal with positive correlations reported in a few studies (Wallace et al., 2014; Auffret et al., 2017b), but results are not consistent (Tapio et al., 2017). Recent data also suggest that the rumen microbiome of feed efficient ruminants is less diverse than their inefficient counterparts (Shabat et al., 2016; Li and Guan, 2017). The microbial diversity within the rumen offers the animal resilience from dietary related perturbations, such as acidosis. Therefore, care must be taken to ensure that breeding for increased feed efficiency in ruminants does not negatively impact resilience of the microbiome and increase the susceptibility of the host to digestive diseases. Irrespective, metataxonomic data is highly variable due largely to the differences in techniques employed across published datasets (see Metataxonomy section) and animal variation. As such the use of gene biomarkers using metagenomics and/or metranscriptomic approaches may be more useful given that rumen microbes possess genes coding for a high level of functional redundancy (Edwards et al., 2008; Weimer, 2015).
Recent work using metagenomics and/or metatranscriptomics has confirmed significant relationships between the abundances of key rumen microbial genes and feed efficiency (Roehe et al., 2016; Shabat et al., 2016; Li and Guan, 2017) and/or methane production (Roehe et al., 2016). Due to the vastness of these datasets it is difficult to compare and investigate whether studies commonly find consensus genes that would serve as good global biomarkers in their correlation studies. Microbial gene correlations with RFI, data from Shabat et al. (2016) and Li and Guan (2017) showed some consensus as both showed that genes involved in amino acid metabolism were less abundant in feed efficient animals (Table 2). These data corresponds with observations that feed efficient animals excrete less urinary ammonia suggesting better rumen nitrogen use efficiency (Bach et al., 2005; Broderick and Reynal, 2009). Consensus of other genes across the three published datasets were not found (Table 3). Likewise, genes correlating to methane emissions show very little consensus amongst the five papers investigated. Nevertheless, in four datasets the methyl coenzyme reductase enzyme, which is involved in the last step of the hydrogenotrophic methane pathway (Figure 1), showed the most correlation to methane. The lack of consensus across experiments, whilst perhaps being biologically correct, likely also reflects the challenges associated with comparing of datasets from different animals, variation in diet, as well as differences in sampling method, sample preparations and data interpretation. Clearly more comparative large datasets are required to develop microbiome based biomarkers for estimation of RFI and methane output. Alongside this is the need to obtain samples that are representative of the rumen microbiome in a non-invasive manner. Recently it was suggested that the oral microbiome of ruminants reflects the microbial diversity seen in the rumen (Tapio et al., 2016), raising the possibility that buccal swabbing may be used as a proxy for rumen samples.
Table 2
| KEGG number | Description | E.C number | Pathway | Published papers |
|---|---|---|---|---|
| K00075 | UDP-N-acetylmuramate dehydrogenase | EC:1.3.1.98 | Amino acid sugar and nucleotide sugar metabolism; Peptidoglycan biosynthesis; Metabolic pathways | Roehe et al., 2016 |
| K00121 | S-(hydroxymethyl)glutathione dehydrogenase/alcohol dehydrogenase | EC:1.1.1.284 or 1.1.1.1 | Glycolysis/Gluconeogenesis; Fatty acid degradation; Tyrosine metabolism; Chloroalkane and chloroalkene degradation; Naphthalene degradation; Methane metabolism; Retinol metabolism; Metabolism of xenobiotics by cytochrome P450; Drug metabolism–cytochrome P450; Metabolic pathways; Biosynthesis of secondary metabolites; Microbial metabolism in diverse environments; Biosynthesis of antibiotics; Carbon metabolism; Degradation of aromatic compounds; Chemical carcinogenesis | Li and Guan, 2017 |
| K00179 | Indolepyruvate ferredoxin oxidoreductase, alpha subunit | EC:1.2.7.8 | - | Roehe et al., 2016 |
| K00230 | Menaquinone-dependent protoporphyrinogen oxidase | EC:1.3.5.3 | Porphyrin and chlorophyll metabolism; Metabolic pathways; Biosynthesis of secondary metabolites | Li and Guan, 2017 |
| K00240 | Succinate dehydrogenase/fumarate reductase, iron-sulfur subunit | EC:1.3.5.1 or 1.3.5.4 | Citrate cycle (TCA cycle); Oxidative phosphorylation; Butanoate metabolism; Carbon fixation pathways in prokaryotes; Metabolic pathways; Biosynthesis of secondary metabolites; Microbial metabolism in diverse environments; Biosynthesis of antibiotics; Carbon metabolism | Li and Guan, 2017 |
| K00260 | Glutamate dehydrogenase | EC:1.4.1.2 | Arginine biosynthesis; Alanine, aspartate and glutamate metabolism; Taurine and hypotaurine metabolism; Nitrogen metabolism; Metabolic pathways | Shabat et al., 2016 |
| K00270 | Phenylalanine dehydrogenase | EC:1.4.1.20 | Tyrosine metabolism; Phenylalanine metabolism; Phenylalanine, tyrosine and tryptophan biosynthesis; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics | Shabat et al., 2016; Li and Guan, 2017 |
| K00278 | L-aspartate oxidase | EC:1.4.3.16 | Alanine, aspartate and glutamate metabolism; Nicotinate and nicotinamide metabolism; Metabolic pathways | Roehe et al., 2016 |
| K00281 | Glycine dehydrogenase | EC:1.4.4.2 | Glycine, serine and threonine metabolism; Glyoxylate and dicarboxylate metabolism; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics; Carbon metabolism | Li and Guan, 2017 |
| K00290 | Saccharopine dehydrogenase (NAD+, L-lysine forming) | EC:1.5.1.7 | Lysine biosynthesis; Lysine degradation; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics; Biosynthesis of amino acids | Shabat et al., 2016 |
| K00315 | Dimethylglycine dehydrogenase | EC:1.5.8.4 | Glycine, serine and threonine metabolism; Metabolic pathways | Li and Guan, 2017 |
| K00330 | NADH-quinone oxidoreductase subunit A | EC:1.6.5.3 | Oxidative phosphorylation; Metabolic pathways | Shabat et al., 2016 |
| K00340 | NADH-quinone oxidoreductase subunit K | EC:1.6.5.3 | as above | Shabat et al., 2016; Li and Guan, 2017 |
| K00350 | Na+-transporting NADH:ubiquinone oxidoreductase subunit E | EC:1.6.5.8 | - | Shabat et al., 2016; Li and Guan, 2017 |
| K00360 | Assimilatory nitrate reductase electron transfer subunit | EC:1.7.99.- | Nitrogen metabolism; Microbial metabolism in diverse environments | Shabat et al., 2016 |
| K00362 | Nitrite reductase (NADH) large subunit | EC:1.7.1.15 | Nitrogen metabolism; Microbial metabolism in diverse environments | Li and Guan, 2017 |
| K00375 | GntR family transcriptional regulator/MocR family aminotransferase | - | - | Roehe et al., 2016 |
| K00380 | Sulfite reductase (NADPH) flavoprotein alpha-component | EC:1.8.1.2 | Sulfur metabolism; Metabolic pathways; Microbial metabolism in diverse environments | Shabat et al., 2016 |
| K00394 | Adenylylsulfate reductase, subunit A | EC:1.8.99.2 | Sulfur metabolism; Metabolic pathways; Microbial metabolism in diverse environments | Roehe et al., 2016 |
| K00400 | Methyl coenzyme M reductase system, component A2 | - | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments | Shabat et al., 2016 |
| K00471 | Gamma-butyrobetaine dioxygenase | EC:1.14.11.1 | Lysine degradation | Li and Guan, 2017 |
| K00480 | Salicylate hydroxylase | EC:1.14.13.1 | Dioxin degradation; Polycyclic aromatic hydrocarbon degradation; Naphthalene degradation; Metabolic pathways; Microbial metabolism in diverse environments; Degradation of aromatic compounds | Li and Guan, 2017 |
| K00520 | Mercuric reductase | EC:1.16.1.1 | - | Li and Guan, 2017 |
| K00521 | Ferric-chelate reductase | EC:1.16.1.7 | - | Li and Guan, 2017 |
| K00633 | Galactoside O-acetyltransferase | EC:2.3.1.18 | - | Li and Guan, 2017 |
| K00670 | N-alpha-acetyltransferase 30 | EC:2.3.1.256 | - | Li and Guan, 2017 |
| K00680 | Uncharacterized N-acetyltransferase | EC:2.3.1.- | - | Shabat et al., 2016 |
| K00730 | Oligosaccharyl transferase complex subunit OST4 | - | N-Glycan biosynthesis; Various types of N-glycan biosynthesis; Metabolic pathways; Protein processing in endoplasmic reticulum | Li and Guan, 2017 |
| K00766 | Anthranilate phosphoribosyltransferase | EC:2.4.2.18 | Phenylalanine, tyrosine and tryptophan biosynthesis; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics; Biosynthesis of amino acids | Roehe et al., 2016 |
| K00770 | 1,4-beta-D-xylan synthase | EC:2.4.2.24 | Amino sugar and nucleotide sugar metabolism; Metabolic pathways | Li and Guan, 2017 |
| K00785 | Beta-galactosamide-alpha-2,3-sialyltransferase | EC:2.4.99.- | - | Li and Guan, 2017 |
| K00790 | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | EC:2.5.1.7 | Amino sugar and nucleotide sugar metabolism; Metabolic pathways; Peptidoglycan biosynthesis | Li and Guan, 2017 |
| K00860 | Adenylylsulfate kinase | EC:2.7.1.25 | Purine metabolism; Sulfur metabolism; Metabolic pathways; Microbial metabolism in diverse environments | Li and Guan, 2017 |
| K00868 | Pyridoxine kinase | EC:2.7.1.35 | Vitamin B6 metabolism; Metabolic pathways | Roehe et al., 2016 |
| K00900 | 6-Phosphofructo-2-kinase | EC:2.7.1.105 | Fructose and mannose metabolism | Li and Guan, 2017 |
| K00908 | Calcium/calmodulin-dependent protein kinase kinase 1 | EC:2.7.11.17 | Alcoholism | Li and Guan, 2017 |
| K00920 | 1-Phosphatidylinositol-5-phosphate 4-kinase | EC:2.7.1.149 | Inositol phosphate metabolism; Phosphatidylinositol signaling system; Regulation of actin cytoskeleton | Li and Guan, 2017 |
| K00941 | Hydroxymethylpyrimidine/phosphomethylpyrimidine kinase | EC:2.7.1.49 or 2.7.4.7 | Thiamine metabolism; Metabolic pathways | Roehe et al., 2016 |
| K00956 | Sulfate adenylyltransferase subunit 1 | EC:2.7.7.4 | Purine metabolism; Monobactam biosynthesis; Selenocompound metabolism; Sulfur metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Biosynthesis of antibiotics | Roehe et al., 2016 |
| K00974 | tRNA nucleotidyltransferase (CCA-adding enzyme) | EC:2.7.7.72 or 3.1.3.- 3.1.4.- | RNA transport | Roehe et al., 2016 |
| K01051 | Pectinesterase | EC:3.1.1.11 | Pentose and glucuronate interconversions; Metabolic pathways | Li and Guan, 2017 |
| K01055 | 3-Oxoadipate enol-lactonase | EC:3.1.1.24 | Benzoate degradation; Metabolic pathways; Microbial metabolism in diverse environments; Degradation of aromatic compounds | Li and Guan, 2017 |
| K01104 | Protein-tyrorsine phosphatase | EC:3.1.3.48 | - | Roehe et al., 2016 |
| K01129 | dGTPase | EC:3.1.5.1 | Purine metabolism | Roehe et al., 2016 |
| K01195 | Beta-glucuronidase | EC:3.2.1.31 | Pentose and glucuronate interconversions; Metabolic pathways; Glycosaminoglycan degradation; Porphyrin and chlorophyll metabolism; Flavone and flavonol biosynthesis; Drug metabolism–other enzymes; Biosynthesis of secondary metabolites; Lysosome | Roehe et al., 2016 |
| K01269 | Aminopeptidase | EC:3.4.11.- | - | Roehe et al., 2016 |
| K01358 | ATP-dependent Clp protease, protease subunit | EC:3.4.21.92 | Cell cycle–Caulobacter; Longevity regulating pathway–worm | Roehe et al., 2016 |
| K01493 | dCMP deaminase | EC:3.5.4.12 | Pyrimidine metabolism; Metabolic pathways | Roehe et al., 2016 |
| K01613 | Phosphatidylserine decarboxylase | EC:4.1.1.65 | Glycerophospholipid metabolism; Metabolic pathways; Biosynthesis of secondary metabolites | Roehe et al., 2016 |
| K01784 | UDP-glucose 4-epimerase | EC:5.1.3.2 | Galactose metabolism; Amino sugar and nucleotide sugar metabolism; Metabolic pathways | Roehe et al., 2016 |
| K01814 | Phosphoribosylformimino-5-aminoimidazole carboxamide ribotide isomerase | EC:5.3.1.16 | Histidine metabolism; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of amino acids | Roehe et al., 2016 |
| K01818 | L-fucose/D-arabinose isomerase | EC:5.3.1.25 or 5.3.1.3 | Fructose and mannose metabolism; Microbial metabolism in diverse environments | Roehe et al., 2016 |
| K01876 | Aspartyl-tRNA synthetase | EC:6.1.1.12 | Aminoacyl-tRNA biosynthesis | Roehe et al., 2016 |
| K01924 | UDP-N-acetylmuramate–alanine ligase | EC:6.3.2.8 | D-Glutamine and D-glutamate metabolism; Peptidoglycan biosynthesis; Metabolic pathways | Roehe et al., 2016 |
| K01928 | UDP-N-acetylmuramoyl-L-alanyl-D-glutamate−2,6-diaminopimelate ligase | EC:6.3.2.13 | Lysine biosynthesis; Peptidoglycan biosynthesis | Roehe et al., 2016 |
| K02006 | Obalt/nickel transport system ATP-binding protein | - | ABC transporters | Roehe et al., 2016 |
| K02008 | Cobalt/nickel transport system permease protein | - | ABC transporters | Roehe et al., 2016 |
| K02030 | Polar amino acid transport system substrate-binding protein | - | - | Li and Guan, 2017 |
| K02313 | Chromosomal replication initiator protein | - | Two-component system; Cell cycle–Caulobacter | Roehe et al., 2016 |
| K02343 | DNA polymerae III gamma/tau | EC:2.7.7.7 | Purine metabolism; Pyrimidine metabolism; Metabolic pathways; DNA replication; Mismatch repair; Homologous recombination | Roehe et al., 2016 |
| K02377 | GDP-L-fucose synthase | EC:1.1.1.271 | Fructose and mannose metabolism; Amino sugar and nucleotide sugar metabolism; Metabolic pathways | Roehe et al., 2016 |
| K02907 | Large subunit ribosomal protein L30 | - | Ribosome | Roehe et al., 2016 |
| K03111 | Single-strand DNA binding protein | - | DNA replication; Mismatch repair; Homologous recombination | Roehe et al., 2016 |
| K03410 | Chemotaxis protein CheC | - | Bacterial chemotaxis | Li and Guan, 2017 |
| K03426 | NAD+ diphosphatase | EC:3.6.1.22 | Nicotinate and nicotinamide metabolism; Metabolic pathways; Peroxisome | Roehe et al., 2016 |
| K03458 | Nucleobase:cation symporter-2, NCS2 family | - | - | Roehe et al., 2016 |
| K03501 | 16S rRNA (guanine527-N7)-methyltransferase | EC:2.1.1.170 | - | Roehe et al., 2016 |
| K03581 | Exodeoxyribonuclease V alpha subunit | EC:3.1.11.5 | Homologous recombination | Roehe et al., 2016 |
| K03615 | Electron transport complex protein RnfC | - | - | Roehe et al., 2016 |
| K03631 | DNA repair protein RecN (Recombination protein N) | - | - | Roehe et al., 2016 |
| K03657 | DNA helicase II/ATP-dependent DNA helicase PcrA | EC:3.6.4.12 | Nucleotide excision repair; Mismatch repair | Roehe et al., 2016 |
| K03694 | ATP-dependent Clp protease ATP-binding subunit ClpA | - | - | Roehe et al., 2016 |
| K04112 | Benzoyl-CoA reductase subunit C | EC:1.3.7.8 | Benzoate degradation; Metabolic pathways; Microbial metabolism in diverse environments; Degradation of aromatic compounds | Li and Guan, 2017 |
| K04130 | Muscarinic acetylcholine receptor M2 | - | Calcium signaling pathway; cAMP signaling pathway; Neuroactive ligand-receptor interaction; PI3K-Akt signaling pathway; Cholinergic synapse; Regulation of actin cytoskeleton | Li and Guan, 2017 |
| K04517 | Prephenate dehydrogenase | EC:1.3.1.12 | Phenylalanine, tyrosine and tryptophan biosynthesis; Novobiocin biosynthesis; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics; Biosynthesis of amino acids | Roehe et al., 2016 |
| K04974 | Transient receptor potential cation channel subfamily V member 5 | - | Parathyroid hormone synthesis, secretion and action; Endocrine and other factor-regulated calcium reabsorption | Shabat et al., 2016 |
| K08483 | Phosphotransferase system, enzyme I, PtsI | EC:2.7.3.9 | Phosphotransferase system (PTS) | Roehe et al., 2016 |
| K08602 | Oligoendopeptidase F | EC:3.4.24.- | - | Roehe et al., 2016 |
| K09811 | Cell division transport system permease protein | - | ABC transporters | Roehe et al., 2016 |
| K11752 | Diaminohydroxyphosphoribosylaminopyrimidine deaminase/5-amino-6-(5-phosphoribosylamino)uracil reductase | EC:3.5.4.26 or 1.1.1.193 | Riboflavin metabolism; Metabolic pathways; Biosynthesis of secondary metabolites; Quorum sensing | Roehe et al., 2016 |
| K13542 | Uroporphyrinogen III methyltransferase/synthas | EC:2.1.1.107 or 4.2.1.75 | Porphyrin and chlorophyll metabolism; Metabolic pathways; Biosynthesis of secondary metabolites; Microbial metabolism in diverse environments | Roehe et al., 2016 |
Potential gene biomarkers indicative of feed efficiency in ruminants.
For simplicity only genes from Shabat et al. (2016) with significant correlation to amino acid metabolism are shown. Genes that lacked function annotation were removed. Genes identified consistently across experiments and therefore representing the most promising marker genes are highlighted in gray.
Table 3
| KEGG number | Description | E.C number | Pathway | Published papers |
|---|---|---|---|---|
| K00123 | Formate dehydrogenase, alpha subunit | EC:1.17.1.9 | Glyoxylate and dicarboxylate metabolism; Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism | Wallace et al., 2015; Roehe et al., 2016; Auffret et al., 2017b |
| K00125 | Formate dehydrogenase, beta subunit | EC:1.17.98.3 or 1.8.98.6 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism | Roehe et al., 2016 |
| K00150 | Glyceraldehyde-3-phosphate dehydrogenase [NAD(P)] | EC:1.2.1.59 | Glycolysis/Gluconeogenesis; Carbon fixation in photosynthetic organisms; Metabolic pathways; Biosynthesis of secondary metabolites; Microbial metabolism in diverse environments; Biosynthesis of antibiotics; Carbon metabolism; Biosynthesis of amino acids | Auffret et al., 2017b |
| K00169 | Pyruvate ferredoxin oxidoreductase, alpha subunit | EC:1.2.7.1 | Glycolsis/Gluconeogenesis; Citrate cycle (TCA); Pyruvate metabolism; Nitrotoluene degradation; Propanoate degradation; Butanoate degradation; Methane metabolism; Carbon fixation in proaryotes; Metabolic pathways; Microbial metabolism in diverses environments; Biosynthesis of antibiotics; Carbon metabolism | Roehe et al., 2016; Auffret et al., 2017b |
| K00170 | Pyruvate ferredoxin oxidoreductase, beta subunit | EC:1.2.7.1 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K00200 | Foormylmethanofuran dehydrogenase subunit A | EC:1.2.7.12 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Biosynthessis of antibiotics; Carbon metabolism | Wallace et al., 2015; Roehe et al., 2016; Auffret et al., 2017b |
| K00201 | Formylmethanofuran dehydrogenase subunit B | EC:1.2.7.12 | As above | Wallace et al., 2015; Roehe et al., 2016; Auffret et al., 2017b |
| K00203 | Formylmethanofuran dehydrogenase subunit D | EC:1.2.7.12 | As above | Auffret et al., 2017b |
| K00205 | Formylmethanofuran dehydrogenase subunit F | EC:1.2.7.12 | As above | Roehe et al., 2016 |
| K00311 | Electron-transferring-flavoprotein dehydrogenase | EC:1.5.51 | One carbon pool by folate; Carbon fixation pathways in prokaryotes; Metabolic pathways; Microbial metabolism in diverse environments | Kamke et al., 2016 |
| K00323 | NAD(P) transhydrogenase | EC:1.6.1.2 | Nicotinate and nicotinamide metabolism; Metabolic pathways | Kamke et al., 2016* |
| K00399 | Methyl coenzyme M reductase alpha subunit | EC:2.8.4.1 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism | Shi et al., 2014; Wallace et al., 2015; Kamke et al., 2016; Roehe et al., 2016; Auffret et al., 2017b |
| K00400 | Methyl coenzyme M reductase system, component A2 | EC:2.8.4.1 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K00401 | Methyl coenzyme M reductase system, beta subunit | EC:2.8.4.1 | As above | Shi et al., 2014; Wallace et al., 2015 |
| K00402 | Methyl coenzyme M reductase system, gamma subunit | EC:2.8.4.1 | As above | Shi et al., 2014; Auffret et al., 2017b |
| K00441 | Coenzyme F420 hydrogenase beta subunit | EC:1.12.98.1 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments | Roehe et al., 2016 |
| K00539 | Oxidoreducatase | EC: 1.97.1.- | - | Kamke et al., 2016 |
| K00577 | Tetrahydromethanopterin S-methyltransferase subunit A | EC:2.11.86 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Biosynthessis of antibiotics; Carbon metabolism | Roehe et al., 2016 |
| K00580 | Tetrahydromethanopterin S-methyltransferase subunit D | EC:2.11.86 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K00581 | Tetrahydromethanopterin S-methyltransferase subunit E | EC:2.11.86 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K00584 | Tetrahydromethanopterin S-methyltransferase subunit H | EC:2.11.86 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K00666 | Fatty-acyl-CoA synthase | EC:6.2.1.- | - | Kamke et al., 2016 |
| K00672 | Formylmethanofuran-tetrahydromethanopterin N-formyltransferase | EC:2.3.1.101 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Biosynthessis of antibiotics; Carbon metabolism | Roehe et al., 2016; Auffret et al., 2017b |
| K00758 | Thymidine phosphorylase | EC:2.4.2.4 | Pyrimidine metabolism; Drug metabolism–other enzuymes; Metabolic pathways; Bladder cancer | Kamke et al., 2016 (DNA and RNA) |
| K00814 | Alanine transaminase | EC: 2.6.12 | Arginie biosynthesis; Alanine, aspartate and glutamate metabolism; Carbon fixation in photosynthetic organisms; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism; 2-oxocarboxylic acid metabolism; Biosynthesis of amino acids | Kamke et al., 2016* |
| K00827 | Alanine-glyoxylate transaminase/(R)-3-amino-2-methylpropionate-pyruvate transaminase | EC:2.6.1.44 or EC:2.6.1.40 | Metabolic pathways; Biosynthesis of secondary metabolites; Alanine, aspartate and glutamate metabolism; Glycine, serine and threonine metabolism; Cysteine and methionine metabolism; Valine, leucine and isoleucine degradation | Kamke et al., 2016 |
| K00953 | FAD synthetase | EC:2.7.7.2 | Riboflavin metabolism; Metabolic pathways; Biosynthesis of secondary metabolites | Kamke et al., 2016 |
| K01160 | Crossover junction endodeoxyribonuclease RusA | EC:3.1.22.4- | - | Kamke et al., 2016 |
| K01342 | Subtilisin | EC:3.4.21.62 | Quorum sensing | Kamke et al., 2016 |
| K01479 | Formiminoglutamase | EC:3.5.3.8 | Histidine metabolism | Shi et al., 2014; Kamke et al., 2016* |
| K01499 | Methenyltetrahydromethanopterin cyclohydrolase | EC:3.5.4.27 | As above | Roehe et al., 2016; Auffret et al., 2017b |
| K01631 | 2-Dehydro-3-deoxyphosphogalactonate aldolase | EC:4.1.2.21 | Galactose metabolism; Metabolic pathways | Kamke et al., 2016 |
| K01673 | Carbonic anhydrase | EC:4.2.11 | Nitrogen metabolism | Auffret et al., 2017b |
| K01792 | Glucose-6-phosphate 1-epimerase | EC:5.1.3.15 | Glycolysis/Gluconeogenesis; Metabolic pathways; Biosynthesis of secondary metabolites; Microbial metabolism in diverse environments; Biosynthesis of antibiotics | Kamke et al., 2016 |
| K01846 | Methylaspartate mutase | EC:5.4.99.1 | C5-Branched dibasic acid metabolism; Purine metabolism | Shi et al., 2014 |
| K01846 | Methylaspartate mutase | EC:5.4.99.1 | Carbon metabolism; Glyoxylate and dicarboxylate metabolism; C5-Branched dibasic acid metabolism; Metabolic pathways | Kamke et al., 2016 |
| K01912 | Phenylacetate-CoA ligase | EC:6.2.1.30 | Phenylalanine metabolism; Microbial metabolism in diverse environments; Biofilm formation–Vibrio cholerae | Kamke et al., 2016 |
| K01913 | Trans-2-methyl-5-isopropylhexa-2,5-dienoate-CoA ligase | - | - | Kamke et al., 2016 |
| K01959 | Pyruvate carboxylase subunit A | EC:6.4.1.1 | Citrate cycle (TCA cycle); Pyruvate metabolism; Carbon fixation pathways in prokaryotes; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism; Biosynthesis of amino acids | Auffret et al., 2017b |
| K02117 | V-type H+-transporting ATPase subunit A | EC:3.6.3.14 or EC:3.6.3.15 | Oxidative phosphorylation; Metabolic pathways | Wallace et al., 2015; Auffret et al., 2017b |
| K02118 | V-type H+-transporting ATPase subunit B | EC:3.6.3.14 or EC:3.6.3.15 | Oxidative phosphorylation; Metabolic pathways | Wallace et al., 2015; Auffret et al., 2017b |
| K02319 | DNA polymerase | EC:2.7.7.7 | Pyrimidine metabolism | Shi et al., 2014) |
| K02319 | DNA polymerase I | EC:2.7.7.7 | Metabolic pathways; Purine metabolism; Pyrimidine metabolism; DNA replication | Kamke et al., 2016 |
| K02674 | Type IV pilus assembly protein PilY1 | - | - | Kamke et al., 2016 |
| K02683 | DNA primase | EC:2.7.7.- | DNA replication | Kamke et al., 2016 |
| K02856 | L-rhamnose-H+ transport protein | - | - | Kamke et al., 2016 |
| K03045 | DNA-directed RNA polymerase subunit B″ | EC:2.7.7.6 | Purine metabolism; Pyrimidine metabolism; Metabolic pathways; RNA polymerase | Kamke et al., 2016* |
| K03053 | DNA-directed RNA polymerase subunit H | EC:2.7.7.6 | Purine metabolism; Pyrimidine metabolism; Metabolic pathways; RNA polymerase | Kamke et al., 2016* |
| K03124 | Transcription initiation factor TFIIB | - | Basal transcription factors; Epstein-Barr virus infection; Viral carcinogenesis | Kamke et al., 2016* |
| K03167 | DNA topoisomerase VI subunit B | EC:5.99.1.3 | - | Kamke et al., 2016* |
| K03219 | Type III secretion protein SctC | - | Bacterial secretion system | Kamke et al., 2016 |
| K03222 | Type III secretion protein SctJ | - | as above | Kamke et al., 2016 |
| K03223 | Type III secretion protein SctL | - | as above | Kamke et al., 2016 |
| K03224 | ATP synthase in type III secretion protein SctN | EC:3.6.3.14 | as above | Kamke et al., 2016 |
| K03226 | Type III secretion protein SctR | - | as above | Kamke et al., 2016 |
| K03227 | Type III secretion protein SctS | - | as above | Kamke et al., 2016 |
| K03228 | Type III secretion protein SctT | - | as above | Kamke et al., 2016 |
| K03229 | Type III secretion protein SctU | - | as above | Kamke et al., 2016 |
| K03230 | Type III secretion protein SctV | - | as above | Kamke et al., 2016 |
| K03579 | ATP-dependent helicase HrpB | EC:3.6.4.13 | - | Kamke et al., 2016 |
| K04058 | Type III secretion protein SctW | - | Bacterial secretion system | Kamke et al., 2016 |
| K04795 | Fibrillarin-like pre-rRNA processing protein | - | - | Kamke et al., 2016* |
| K04801 | Replication factor C small subunit | - | - | Kamke et al., 2016* |
| K04835 | Methylaspartate ammonia-lyase | EC:4.3.1.2 | C5-Branched dibasic acid metabolism | Shi et al., 2014 |
| K04857 | Voltage-dependent calcium channel L type alpha-1S | - | MAPK signaling pathway; Calcium signaling pathway; cGMP-PKG signaling pathway; cAMP signaling pathway; Cardiac muscle contraction; Adrenergic signaling in cardiomyocytes; Vascular smooth muscle contraction; Retrograde endocannabinoid signaling; Cholinergic synapse; Serotonergic synapse; GABAergic synapse; Insulin secretion; GnRH signaling pathway; Oxytocin signaling pathway; Renin secretion; Aldosterone synthesis and secretion; Cortisol synthesis and secretion; Cushing's syndrome; Alzheimer's disease; Hypertrophic cardiomyopathy (HCM); Arrhythmogenic right ventricular cardiomyopathy (ARVC); Dilated cardiomyopathy (DCM) | Kamke et al., 2016* |
| K04874 | Potassium voltage-gated channel Shaker-related subfamily A member 1 | - | - | Kamke et al., 2016 |
| K05830 | Acetylornithine/LysW-gamma-L-lysine aminotransferase | EC:2.6.1.11 | Arginine biosynthesis; Lysine biosynthesis; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics; 2-Oxocarboxylic acid metabolism; Biosynthesis of amino acids | Kamke et al., 2016 |
| K06863 | (Beta)-D-ribofuranosyl 5′-monophosphate synthetase | EC:6.3.4.- | Purine metabolism | Shi et al., 2014 |
| K06863 | 5-Formaminoimidazole-4-carboxamide-1-(beta)-D-ribofuranosyl 5′-monophosphate synthetase | EC:6.3.4.23 | Purine metabolism; Metabolic pathways; Biosynthesis of secondary metabolites; Biosynthesis of antibiotics | Kamke et al., 2016* |
| K06907 | Phage tail sheath protein FI | - | - | Kamke et al., 2016* |
| K06927 | Diphthine-ammonia ligase | EC:6.3.1.14 | - | Kamke et al., 2016* |
| K06937 | 7,8-dihydro-6-hydroxymethylpterin dimethyltransferase | EC:2.1.1.- | - | Auffret et al., 2017b |
| K07249 | Retinal dehydrogenase | EC:1.2.1.36 | Retinol metabolism; Metabolic pathways | Kamke et al., 2016 |
| K07283 | Putative salt-induced outer membrane protein | - | - | Kamke et al., 2016 |
| K07318 | Adenine-specific DNA-methyltransferase | EC:2.1.1.72 | - | Kamke et al., 2016* |
| K07463 | Archaea-specific RecJ-like exonuclease | - | - | Kamke et al., 2016* |
| K07558 | tRNA nucleotidyltransferase (CCA-adding enzyme) | EC:2.7.7.72 | - | Kamke et al., 2016* |
| K07569 | RNA-binding protein | - | - | Kamke et al., 2016* |
| K07732 | Riboflavin kinase, archaea type [EC:2.7.1.161] | EC:2.7.1.161 | Riboflavin metabolism; Metabolic pathways | Kamke et al., 2016* |
| K07796 | Cu(I)/Ag(I) efflux system outer membrane protein CusC/SilC | - | - | Kamke et al., 2016 |
| K08605 | Coccolysin [EC:3.4.24.30] | EC:3.4.24.30 | Quorum sensing | Kamke et al., 2016 |
| K08635 | Membrane metallo-endopeptidase-like 1 | - | - | Kamke et al., 2016 |
| K08636 | Phosphate-regulating neutral endopeptidase | EC:3.4.24.- | - | Kamke et al., 2016 |
| K08641 | D-alanyl-D-alanine dipeptidase [EC:3.4.13.22] | EC:3.4.13.22 | Vancomycin resistance; Two-component system | Kamke et al., 2016 |
| K09482 | Glutamyl-tRNA (Gln) amidotransferase subunit D | EC:6.3.5.7 | Aminoacyl-tRNA biosynthesis | Shi et al., 2014 |
| K09482 | Glutamyl-tRNA(Gln) amidotransferase subunit D | EC:6.3.5.7 | Aminoacyl-tRNA biosynthesis; Metabolic pathways | Kamke et al., 2016* |
| K09610 | Endothelin-converting enzyme-like 1 | EC:3.4.24.- | - | Kamke et al., 2016 |
| K10060 | C-type lectin domain family 4 member F | - | - | Kamke et al., 2016 |
| K10639 | E3 ubiquitin-protein ligase CCNP1IP1 | EC:6.3.2.19 | - | Kamke et al., 2016 |
| K10725 | Archaeal cell division control protein 6 | - | - | Kamke et al., 2016* |
| K10896 | Fanconi anemia group M protein | - | Fanconi anemia pathway | Kamke et al., 2016* |
| K11382 | MFS transporter, OPA family, phosphoglycerate transporter protein | - | Two-component system | Kamke et al., 2016 |
| K11404 | Histone deacetylase 3 | EC:3.5.1.98 | Thyroid hormone signaling pathway; Alcoholism; Human papillomavirus infection; Viral carcinogenesis | Kamke et al., 2016 |
| K11900 | Type VI secretion system protein ImpC | - | Biofilm formation–Pseudomonas aeruginosa | Kamke et al., 2016 |
| K12204 | Defect in organelle trafficking protein DotC | - | - | Kamke et al., 2016 |
| K12206 | Intracellular multiplication protein IcmB | - | - | Kamke et al., 2016 |
| K12217 | Intracellular multiplication protein IcmO | - | - | Kamke et al., 2016 |
| K12221 | Intracellular multiplication protein IcmS | - | - | Kamke et al., 2016 |
| K12434 | Polyketide synthase 7 | - | - | Kamke et al., 2016 |
| K12448 | UDP-arabinose 4-epimerase | EC:5.1.3.5 | Amino sugar and nucleotide sugar metabolism; Metabolic pathways | Kamke et al., 2016 |
| K12739 | Peptidyl-prolyl cis-trans isomerase-like 6 | EC:5.2.1.8 | - | Kamke et al., 2016* |
| K13085 | Phosphatidylinositol-4,5-bisphosphate 4-phosphatase | EC:3.1.3.78 | Bacterial invasion of epithelial cells; Shigellosis; Salmonella infection | Kamke et al., 2016 |
| K13600 | Chlorophyllide a oxygenase | EC:1.14.13.122 | Porphyrin and chlorophyll metabolism; Metabolic pathways; Biosynthesis of secondary metabolites | Kamke et al., 2016 |
| K13670 | Putative glycosyltransferase | EC:2.4.-.- | - | Kamke et al., 2016 |
| K13812 | Bifunctional enzyme Fae/Hps | EC:4.2.1.147 or 4.1.2.43 | Pentose phosphate pathway; Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism; Biosynthessis of amino acids | Roehe et al., 2016; Auffret et al., 2017b |
| K13886 | Coronin-1B | - | - | |
| K13893 | Microcin C transport system substrate-binding protein | - | ABC transporters | Kamke et al., 2016 |
| K14123 | Energy-converting hydrogenase B N | - | - | Roehe et al., 2016 |
| K14128 | F420-non-reducing hydrogenase subunit G | EC:1.12.99.- or EC:1.8.98.5 | Methane metabolism; Metabolic pathways; Microbial metabolism in diverse environments; Carbon metabolism | Roehe et al., 2016; Auffret et al., 2017b |
| K14275 | D-xylonate dehydratase | EC:4.2.1.82 | Pentose and glucuronate interconversions | Kamke et al., 2016 |
| K14324 | Histone deacetylase complex subunit SAP18 | - | RNA transport; mRNA surveillance pathway | Kamke et al., 2016 |
| K14333 | 2,3-dihydroxybenzoate decarboxylase | EC:4.1.1.46 | Benzoate degradation; Aminobenzoate degradation | Shi et al., 2014 |
| K14333 | 2,3-dihydroxybenzoate decarboxylase | EC:4.1.1.46 | Benzoate degradation; Aminobenzoate degradation; Microbial metabolism in diverse environments | Kamke et al., 2016 |
| K14414 | Transcriptional regulatory protein RtcR | - | - | Kamke et al., 2016 |
| K14426 | Solute carrier family 12 (sodium/chloride transporter), member 3 | - | - | Kamke et al., 2016 |
| K14429 | Solute carrier family 12 (potassium/chloride transporters), member 9 | - | - | Kamke et al., 2016 |
| K14995 | Solute carrier family 38 (sodium-coupled neutral amino acid transporter), member 9 | - | mTOR signaling pathway | Kamke et al., 2016* |
Potential gene biomarkers indicative of methane production from ruminants.
Data are from metagenomics sequences unless marked with an asterisk.
Indicates metatranscriptomic datas. Genes that lacked functional annotation were removed. Genes identified consistently across experiments and therefore representing the most promising marker genes are highlighted in gray.
Prospects for enhancing rumen microbiome understanding and animal phenotype predictions via mathematical modeling
Mathematical models can be used to integrate our understanding of feed, intake, digestion and passage rates on the resulting energy available to the microbiome and ultimately the host. The development of rumen models has been deployed mainly via the consolidation of four model structures (Molly, Karoline, Cornell, and Dijkstra models) that have been improved over the years to enhance the understanding of rumen function (Mills et al., 2014; Huhtanen et al., 2015; Van Amburgh et al., 2015; Gregorini et al., 2016). These models represent relevant aspects that determine the nutritional and emission responses for a given diet but do not attempt to provide a detailed description of the microbiota or its function (Ellis et al., 2008). This gap between the available omics data of the rumen microbiome and the models needs to be bridged to improve our understanding of rumen function (Bannink et al., 2016; Muñoz-Tamayo et al., 2016). To make these model applications possible, rumen modeling should embrace the framework of genome-scale metabolic models (GEMs). The basis of a GEM is the stoichiometry matrix that links metabolites and biochemical reactions that the microbe is able to perform as a result of its genetic potential. The stoichiometry matrix is organism-specific and results from a genome-scale network reconstruction obtained by a protocol that includes functional genome annotation, curation of a draft reconstruction of metabolic reactions and finally translation of the reconstructed network into a computational model (GEM). The full process capitalizes on high-throughput network-wide and bibliomic data (Feist et al., 2009), and on dedicated software (Henry et al., 2010; Aite et al., 2018). The construction of a rumen microbiome GEM will need to address central questions that remain to be elucidated due to the early stage of microbial community modeling (Zengler and Palsson, 2012). One of these key questions is how microbial species, their metabolic networks, and interspecies interactions should be represented (Biggs et al., 2015). Once this question is elucidated, a plethora of constraint-based reconstruction and analysis (COBRA) methods can be deployed to investigate genotype–phenotype relationships (Lewis et al., 2012).
The COBRA methods rely on the principle that microbial metabolism is bound by constraints that include thermodynamics, substrate and enzyme availability. These methods mainly operate under steady-state. The most popular COBRA method is flux balance analysis (FBA; Varma et al., 1993; Varma and Palsson, 1994), which looks at finding the network reaction fluxes that optimize a regulatory condition (e.g., microbial growth). Overall, COBRA approaches provide rational tools for metabolic engineering. The number of applications is broad and includes the development of tools for (i) studying interactions among different microbial groups, i.e., protozoa, fungi, archaea, bacteria and viruses or bacteriophages, (ii) developing selective cultivation strategies for as yet uncultured rumen microbes (Pope et al., 2011), (iii) designing methane mitigation strategies by exploiting the metabolic networks of genome-sequenced rumen archaea (Leahy et al., 2010; Pope et al., 2011), and (iv) developing prediction tools that exploit microbiome biomarkers for fiber hydrolysis (Dai et al., 2015; Comtet-Marre et al., 2017, 2018) and methane production (Popova et al., 2013; Shi et al., 2014; Auffret et al., 2017b).
Clearly rumen GEMs must be further integrated into whole rumen digestion models to provide a system-level picture of the dynamic interplay between the diet, the animal host and the rumen microbiota. Central to this task and for the development of novel strategies to enhance ruminant production and reduce environmental impact is the need for data sharing and collaboration. The co-authors of this paper are all members of the Global Research Alliance's Rumen Microbial Genomics Network, which is set up to allow global collaborations and data sharing for this very purpose. This integration task is far from trivial due to multiple time scales, among other aspects such as parameter identifiability (Muñoz-Tamayo et al., 2018). Moreover, since COBRA approaches mainly operates at steady-state, dynamic frameworks (Mahadevan et al., 2002; Baroukh et al., 2014) will need to be adapted to account for the dynamic fluctuations within the rumen environment. A great challenge is to deploy different model structures, capitalizing on “omics” data, and responding to different goals varying from supporting livestock management within a precision farming context to guiding microbial programming strategies.
Technological advances to further our understanding of the rumen microbiome
Genomics/culturomics
Large culture collections are incredibly powerful as the organisms in the collection can be studied both in vitro and in vivo. However, they may also be limited to, or biased toward, strains that are easy to culture, highly abundant organisms and organisms which are of specific interest to research. Since the seminal work of Robert Hungate published in his book “The rumen and its microbes” in 1966 (Hungate, 1966), technologies have rapidly advanced. The foundational work of Robert Hungate formed the backbone of the Hungate1000 project (Seshadri et al., 2018) led by AgResearch, New Zealand and formed a major project within the Global Research Alliance's Rumen Microbial Genomics Network. It's aims were to sequence 1,000 cultured rumen microbial genomes to aid our understanding of the rumen microbiome. The Hungate1000 project recently finished having sequenced 420 representatives of rumen microbes (mainly bacteria), and thus providing a major tool for the community (Seshadri et al., 2018).
However, many of the rumen bacteria remain uncultured and uncharacterized, with genomic information on the rumen eukaroytes being especially sparse in the Hungate1000 genomes as a result of the challenges of sequencing the A-T rich genomes of these microbes. Our ability to culture rumen bacteria has improved in recent years through the development of culture media (Kenters et al., 2011). A recent study by Poelaert et al. (2018) showed that reducing agents were not required to culture all rumen bacteria and that when removed they resulted in higher microbial diversity. Techniques such as dilution to extinction have also improved our ability to culture bacteria in many ecosystems, including the rumen (Kenters et al., 2011). Another method, which has had success for culturing marine bacteria, is the microdroplet encapsulation technique (Zengler et al., 2002). This involves using a version of the natural environment by using a dilution to extinction technique, followed by encapsulation in a gel and suspending microdroplets in a column. The medium from the environment in which the bacterium was isolated can then be flowed through to provide nutrients for growth (Stewart, 2012). Indeed, there are many technologies emerging that should be investigated for their ability to culture the as yet unculturable rumen bacteria.
Metataxonomy and inference of function
The onset of next generation sequencing resulted in an explosion in publications exploring the metataxonomy of the rumen microbiome under differing parameters. Although these studies are of great value, interpretation of the data generated across different publications remains a challenge. Differences among studies exist with respect to DNA extraction, primers and cycling parameters, as well as downstream computational analysis, resulting in conflicting data (Yu and Morrisson, 2004a,b; Edwards et al., 2007; Huws et al., 2007; Kim et al., 2011; Ishaq and Wright, 2014; Vaidya et al., 2018). With respect to DNA extraction Yu and Morrisson (2004a) evaluated three extraction techniques (a modified phenol-free bead-beating method (referred to as repeated bead beating plus column (RBB + C) method, FastDNA SPIN Kit (MP Biomedicals, California) and the QIAamp DNA Stool Mini Kit (Qiagen, Germany). They concluded that the RBC + C method yielded more DNA and that bead beating was crucial. Vaidya et al. (2018) further tested 4 DNA extraction methods (Repeated bead beating (RBB) developed by Yu and Morrisson (2004a), phenol dependent bead beating (PBB), Fast SPIN DNA kit for soil (MP Biomedicals, California), and the PQIAmini) using both rumen fluid and fibrous rumen samples. The authors concluded that each method was effective but gave different results, for example PBB extracted DNA extracted resulted in higher abundances of Ruminococcaceae compared with abundances obtained using the FDSS method, whereas abundances of Fibrobacteraceae was lower compared with the RBB method. They conclude that each method has advantages and disadvantages which need to be considered based on sample type, but bead beating is critical. Further downstream many authors have investigated the importance of primer choice for metataxonomic investigations of the rumen microbiome (Yu and Morrisson, 2004b; Edwards et al., 2007; Huws et al., 2007; Kim et al., 2011; Ishaq and Wright, 2014). Yu and Morrisson (2004b) investigated primer choice in terms of diversity observed on denaturing gradient gel electrophoresis (DGGE) gels and concluded that primers targeting the V3 region were the best. Huws et al. (2007) and Edwards et al. (2007), however showed that the V3 primers were non-specific to bacteria and could amplify plant chloroplastic DNA as well as archaeal 16S rDNA and protozoal 18S rDNA sequences. The amplification of plant chloroplast sequences is a substantial issue for samples taken from animals fed fresh forage (these are often assigned taxonomically as cyanobacteria), with an abundance of intact chloroplast DNA being present in the rumen, and often masks microbial sequences (Edwards et al., 2007). This is the case when investigated using DGGE and NGS based sequencing (Huws, personal communication). Nonetheless, if animals are not fed fresh forage and primers which are more broad are required to cover a greater proportion of the microbes as a whole is required then the V3 region is perhaps a justifiable choice. However, Huws et al. (2007) concluded that for specific bacterial 16S rRNA V6-V8 primers were more appropriate. Edwards et al. (2007) also developed a primer pair based on the V6-V8 region which reduce the amplification of chloroplastic DNA. Nonetheless, the amplicon size obtained using the Edwards et al. (2017) primers are often too large for effective sequencing, therefore the reverse primer has been changed to enable avoidance of chloroplast identification and production of a smaller amplicon for NGS sequencing (Belanche et al., 2017). The annealing temperatures and number of cycles used for PCR are also clearly going to bias results somewhat. Nonetheless, using a basic set of standardized protocols may not be possible, due to the differing hypotheses and the complex nature of the ecosystem (i.e., host animal), however ensuring data accuracy by using internal standards represents one approach to ensure that comparisons among datasets are valid. Pollock et al. (2018) attempted to describe the guidelines and consensus best practices for metataxonomic studies and concluded that bead beating is critical for DNA extraction as is the use of internal standards for metataxonomic studies amongst other recommendations. Also the construction of rumen microbiome databases to aid accurate taxonomical assignment, such as RIM-DB (for methanogens; Seedorf et al., 2014), the ureC database (ureolytic bacteria; Jin et al., 2017), and AF-RefSeq (anaerobic fungi; Paul et al., 2018) drastically improve our ability to monitor rumen microbial diversity.
Irrespective, metataxonomic rDNA data have provided insights into the composition of the rumen microbiome under differing parameters, but these techniques are limited in terms of providing insight into microbial function. Nonetheless, due to their low cost, these techniques are the most published and will continue to be important in microbiome research for the near future. Software to predict microbial function from metataxonomic data, such as PICRUSt (Langille et al., 2013), has been applied to many different ecosystems, including the rumen. Although this approach saves on the cost associated with more thorough and accurate shotgun metagenomic analysis, it has limitations in accurately represent microbiome function (Wilkinson et al., 2018). The accuracy of PICRUSt prediction, originally intended for human microbiota data, has recently been tested for the rumen microbiome using datasets with 16S rDNA data and accompanying metagenomics or metatranscriptomic datasets (Wilkinson et al., 2018). The data shows poor correlation of predicted function with the actual function seen within the metagenomics/metatranscriptomic datasets (Wilkinson et al., 2018). Wilkinson et al. (2018), developed CowPI an improved 16S rDNA inference platform for the rumen which is based on PICRUSt but uses the Hungate1000 genomes as the searchable genomes (http://www.cowpi.org/). Other platforms allowing inference of function from 16S rDNA data have also been developed, such as Tax4Fun (Aßhauer et al., 2015) and PanFP (Jun et al., 2015), and have been proposed to provide more accurate functional annotations than PICRUSt (Koo et al., 2017). However, the ability of these programs to predict the function of the rumen microbiome has not been investigated. Regardless, inferring metabolic function from phylogenetic data allows the scientific community to obtain retrospective value from these datasets in order to understand the rumen microbiome in light of global agricultural challenges.
Metagenomics
The benefits of metagenomics include the ability to assemble whole- and fragmented-genomes, predict genes, map enzymes and pathways, discover new enzymes and pathways, and quantify the abundance of functional genomic elements across and between samples. Shotgun metagenomics was first applied to the rumen in order to discover novel biomass degrading enzymes from switchgrass-associated microbes (Hess et al., 2011). Subsequently, metagenomics has been used to study many aspects of rumen microbiology, including methane emissions in cattle (Wallace et al., 2015) and sheep (Shi et al., 2014), biomarkers to predict ruminal methanogenesis (Auffret et al., 2017b), the effect of feed-conversion-ratio, and breed and host genetics on the composition of the rumen microbiome (Roehe et al., 2016), nutrient acquisition (Mayorga et al., 2016; Rubino et al., 2017), and effects of diet (Auffret et al., 2017a,b), and investigate impact of feed additives (Thomas et al., 2017) on the abundance of antimicrobial-resistance genes. The rumen also remains a source of valuable bioactives for the biotechnology industry, and metagenomics is a key tool for such bioprospecting (Oyama et al., 2017; Roumpeka et al., 2017). More recently, metagenomic sequences have also resulted in an enhanced understanding of niche specialization within rumen bacteria (Rubino et al., 2017). Rubino et al. (2017) showed that, within metagenome sequences from 14 silage-fed cows, that the genus Prevotella possessed higher levels of glycosyl hydrolase (GH) isoforms relating specifically to the degradation of hemicellulose, whilst Clostridium contained higher levels of GH isoforms for enzymes specifically involved in cellulose degradation. Their data suggests that isoform diversity maintains selective advantage and niche specialization within these genera.
Binning genomes from metagenomes
Another major advancement in understanding the capacity of rumen bacteria has been our increased ability to bin genomes from metagenomes. Assembly binning refers to the construction of complete or near complete microbial genomes directly from metagenomic sequencing data, and was first achieved by Tyson et al. (2004) from an acidophilic biofilm. Hess et al. (2011) were the first to apply this to ruminants, assembling 15 draft microbial genomes from the switchgrass associated microbiome of cattle. Subsequently, Svartstrom et al. (2017) assembled 99 microbial genomes from the moose rumen, Stewart et al. (2018) assembled 913 microbial genomes from the rumen of cattle, and Parks et al. (2017) assembled over 8,000 novel microbial genomes from 1,500 public datasets, some of which originated from the rumen. Traditional metagenomic binning takes an in silico approach whereby metagenomic assembled contigs are clustered by base-composition and abundance across multiple datasets—the hypothesis being that contigs from the same organism will follow a very similar abundance profile across multiple samples, and will have a roughly similar base composition. The success of such binning procedures is validated by investigating the number of single-copy core-genome genes within each bin, as implemented by software such as CheckM (Parks et al., 2015). More recently, physical methods of metagenomic binning such as the use of Hi-C have been published (Beitel et al., 2014). In Hi-C experiments, parts of the chromosome that are in contact with one another inside the cell are cross-linked using formaldehyde; cells are then lysed, the DNA is fragmented using a restriction enzyme, followed by random ligation, amplification and sequencing. Each pair of paired-end reads therefore comes from two separate fragments of the same original chromosome, and that information can be used to collate assembled contigs into genomes. Hi-C binning has been used effectively on human feces (Press et al., 2017) as well as in ruminants (Stewart et al., 2018).
Metatranscriptomics
Metatranscriptomics involves the profiling of community-wide expressed genes (mRNA), and is often termed RNA-seq. Whilst metagenomics allows us to evaluate diversity and the potential functional capacity of a microbiome, metatranscriptomes provide insight into the actual function of microbiomes via gene expression. Due to the abundance of rRNA, metatranscriptomics requires either very deep sequencing to obtain sufficient mRNA sequences coupled with computational binning of the rRNA genes (these can also be useful for metataxonomics) or use of kits to deplete rRNA pre-sequencing. Deep sequencing is of course expensive and the kits used to remove rRNA for metranscriptomics of the rumen microbiome, have varying degrees of success (Huws personal communication). These kits are also bespoke for the removal of bacterial or eukaryotic rRNA, and thus for the complex rumen microbiome, a variety of kits are required to remove prokaryotic and eukaryotic rRNA. This is both costly and laborious, with the time required likely resulting in partial RNA degradation, which will ultimately bias down-stream analysis. Nonetheless Comtet-Marre et al. (2017) developed a bespoke rRNA kit which was effective in removing rumen microbial rRNA, providing a potential solution for future experiments.
Despite these developments, it has also been shown that the correlation between mRNA and protein levels can be weak and variable, possibly due to post-transcriptional modifications (Greenbaum et al., 2003; Csárdi et al., 2015). Ribosome profiling (riboseq) has been developed as a direct method to quantify and characterize translation (Ingolia et al., 2009). Riboseq takes advantage of the fact that during translation, the ribosome protects around 30 nucleotides of the mRNA from nuclease activity. High-throughput sequencing of these ribosome protected fragments offers a precise record of the number and location of the ribosomes at the time translation ceases. Mapping the position of the ribosome-protected fragments is indicative of the translated regions within the transcriptome. Nonetheless, whilst the use of this technique on pure cultures has been effective, the development of the technique (MetaRibo-Seq) at a metatranscriptomic level is in its infancy and still requires validation for the rumen micobiome.
Metaproteomics
Metaproteomics falls between the established DNA and RNA sequencing and metabolomics procedures as an approach to characterize the functional activity of the microbial community. While still an emerging technology, the concept was introduced by Wilmes and Bond (2004), who used 2D PAGE methods to separate and identify proteins from a complex sample extracted from waste water treatment. As a concept, it has some theoretical advantages over RNA sequencing methods in that the half-life of proteins can be significantly longer than RNA transcripts. Therefore, if the data represent a “snapshot” of microbial activity at a single time point, then identification of the proteins arguably will provide a more accurate picture than sequencing mRNA. Moreover, as individual proteins can be identified by their amino acid sequence, function can still be linked to taxa using protein sequence alignment tools e.g., UniPept (Mesuere et al., 2018).
The 2D PAGE methodology involves using a pH gradient firstly to separate proteins in one dimension based on their isoelectric point. The proteins are then subject to gel electrophoresis to separate them by size. This results in a spot pattern representing the metaproteome. Individual spots can then be excised, digested and the resulting peptides identified using mass spectrometry. This method has been used to identify proteins in waste water samples (Abram et al., 2009) soils, sediments (Benndorf et al., 2009; Chourey et al., 2010), the rhizosphere (Wu et al., 2011) and human feces (Klaassens et al., 2007). However, when applied to rumen digesta samples this approach revealed a major shortcoming (Snelling and Wallace, 2017), as the rumen contains high levels of plant secondary compounds, such as tannins and other phenolics that complex with the proteins, and interfere with protein extraction and purification (Snelling and Wallace, 2017). Snelling and Wallace (2017) reported that repeated wash steps and microfiltration were not effective in removing the contaminants, which prevent accurate protein quantitation and obscure spot patterns in gels. Humic acid is also highly abundant in soil and feces, which cause similar implications for recovery for good metaproteomic data in these systems. One possible solution to this problem is to use acid precipitation to separate peptides (Qian and Hettich, 2017). The authors concluded that sample quality was a key factor, with best results obtained from fresh digesta or samples with high microbial protein content relative to contaminants. Despite these limitations, Snelling and Wallace (2017) did identify proteins directly associated with the functional activity of the rumen microbial community from 2D PAGE spots. Abundant structural proteins were identified including actin, alpha and beta tubulin, and axonemal isoforms dynein light chain, which are all involved in the locomotion of ciliates. Among prokaryotic proteins were enzymes from the Phyla Firmicutes and Bacteroidetes involved in central metabolism. Archaeal proteins were also found in surprisingly high abundance considering the relatively small proportion of the microbial community that this group occupies in the rumen. This may be a reflection of the persistence of archaeal proteins after the original transcripts are degraded. These archaeal proteins, were identified as key enzymes involved in the synthesis of methane. This finding was particularly relevant to the efforts to understand the mechanisms behind methane production.
As a consequence of these technical challenges, very few studies have attempted to characterize the rumen microbial community using metaproteomics. However, in recent years, the development of next generation mass spectrometers and accompanying software have provided the means to identify proteins en masse in an approach to analogous to shotgun DNA sequencing. Using this technique Deusch and Seifert (2015) described over 2,000 proteins associated with the rumen microbial community. Research recently conducted by Hart et al. (2018) explored the potential to develop a metaproteomic approach to analyse the rumen that allowed comparison of data with meta-transcriptomic information. Although in its infancy, the meta-proteomic methodology did allow for the identification of members of the protein families that were associated with the transcriptome of the rumen microbiome (Hart and Kingston-Smith, Personal Communication). The development of software for meta-proteome analysis such as Meta-proteome Analyzer (Muth et al., 2015) can also aid in the analysis and interpretation of meta-protein data. As it stands the method shows great potential and is a complement to other omics technologies to determine the functionality of the rumen microbiome.
Metabolomics
Like the field of proteomics, the study of metabolomics within the rumen is also in its infancy. Metabolomics can be defined as the comprehensive (qualitative and quantitative) analysis of metabolites by gathering as much metabolic information as possible from an organism or biological system (Yi et al., 2016). Metabolomics focuses mainly on low molecular mass molecules (<1,000 Da), which can be related to the functional status of the organism (Bundy et al., 2009). The main challenges for analyzing these metabolites include their chemical complexity and heterogeneity. Sample preparation for metabolomics can be as simple as a liquid-liquid extraction procedure, but using assertive methods is key to ensure effective metabolite extraction (Patejko et al., 2017). Various analytical instruments can be used for metabolomics, differing mainly on sensitivity and coverage. To date, the majority of rumen metabolome studies have used liquid chromatography-mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR), with the latter the most used due to reliability and absolute quantification (Goldansaz et al., 2017). There is growing interest for LC-MS due to recent advances in instrument sensitivity, high processing capacity, data analysis and the development of data repositories where the community can curate large data sets have brought interest to tandem mass spectrometry (MS). Furthermore, with the introduction of the online tool Global Natural Products Social Molecular Networking, a crowd-sourced knowledge repository and analysis infrastructure, whereby MS/MS spectra can be clustered based on spectral similarity data, and greatly improving data interpretation (Wang M. et al., 2016).
Usually, two approaches can be used for metabolic investigations: targeted and untargeted analysis. The targeted analysis focuses on examination of a group of known metabolites, usually for hypothesis-driven studies (Patti et al., 2012), whereas untargeted analysis evaluates large numbers of compounds. One of the main advantages of using an untargeted approach is the prospection of novel compounds and metabolic pathways (Patti et al., 2012). To date, most studies regarding the rumen metabolome used the targeted approach and had an interest in the effect of diets on the rumen metabolome (Ametaj et al., 2010; Saleem et al., 2012, 2013; Zhao et al., 2014; Zhang et al., 2015; Mao et al., 2016, 2017; Zhang R. et al., 2017; Do Prado et al., 2018; O'callaghan et al., 2018). Using a combination of several metabolomics platforms, the pioneer study by Saleem et al. (2013) demonstrated that the rumen metabolome is not as simple as previously anticipated. Indeed, 246 compounds were reported as part of the rumen metabolome, including: phospholipids, inorganic ions, gases, amino acids, dicarboxylic acids, fatty acids, volatile fatty acids, glycerides, carbohydrate and cholesterol esters (Saleem et al., 2013). As part of this study Saleem et al. (2013) also set up a searchable rumen metabolome database to improve metabolite assignments in the rumen (www.rumendb.ca). Recent studies have mainly explored how rumen metabolites are affected by different levels of roughages and concentrate. For example, there is alteration to organic acids, amino acids, amines, sugars and nucleosides/nucleotides when cows were fed diets low on concentrate (40%, DM basis) compared to high concentrate (70%, DM basis; Zhang R. et al., 2017). In another study using dairy cows fed diets varying in roughage to concentrate ratio (80:20, 60:40, 40:60, and 20:80) ruminal amino acids, lipids, organic acids, and carbohydrates were affected (Zhang J. et al., 2017).
Metabolites of microbial origin are also precursors of ruminant products (e.g., meat and milk), which might suggest biochemical insights into the role played by rumen-diet interactions (Saleem et al., 2013; Sun et al., 2015). Also, metabolites such as phosphatidylcholine have been suggested as biomarkers for protozoa abundance (Saleem et al., 2012). Finally, the rumen fluid metabolome can be used to identify potential differences in rumen function. Recently, Artegoitia et al. (2017) reported 33 metabolites differing in cattle having high or low average daily gain. Kingston-Smith et al. (2013), using Fourier Transform Infra-Red (FT-IR) spectroscopy-based metabolite profiling, also showed that it was possible to discriminate differences in the rumen plant-microbe interactome when three different cultivars of perennial ryegrass were used as substrate. This suggests that FT-IR could be used as an approach to improve forages for livestock production (Kingston-Smith et al., 2013). However, there are few studies to date combining both sequencing and metabolomics to provide a more comprehensive analysis of the rumen system that would contribute information regarding metabolic expression of the genetic potential of the microbiota (Mao et al., 2016; Zhang J. et al., 2017; O'callaghan et al., 2018).
The rumen environment is composed of a myriad of molecules of microbial, plant and animal origins. Currently, studies have focused on characterizing how the ruminal environment is affected by diet. There is an opportunity to extend this to explore microbial interactions and how ruminal microbes cope with stressors. Concentrating on exploring the ecological foundations of the rumen microbiota might deliver an improved comprehension of the rumen, novel compounds and unexplored pathways.
Data integration for enhanced understanding of the rumen microbiome
Each of the previously described technological approaches provide novel and fascinating insights into aspects of the structure, function and/or activity of the rumen microbiome. However, if these different types of information were to be integrated into a model that describes microbial activity at all levels recorded, our understanding would be further enhanced. At the simplest level we can use the central dogma of molecular biology that describes the manner in which genetic information is transcribed and translated to proteins (DNA = > RNA = > Protein) to allow the “functional potential” in whole sequenced genomes from cultured rumen microbes, metagenomically assembled genomes (MAGs) or de novo assembled metagenomes to be linked to the actively transcribed genes captured using whole transcriptomes from cultured microbes, or meta-transcriptomic data from rumen samples. This level of data integration has provided some novel insights missed by either approach alone, such as by Shi et al. (2014) who demonstrated that differences in methane production were not due to differences in the abundances of the genes responsible, but rather a result of their differential expression. It would not have been possible to make this distinction without integration of both types of data. Similarly, the comparison of proteomic data to transcriptomic or genomic data from the same samples can allow better protein identification (using tools such as MASCOT) and provide results that are more relevant to the rumen environment (Hart et al., 2018).
This, however, is only scratching the surface of what is possible. For example, online databases, such as the Kyoto Encyclopedia of Genes and Genomes (KEGG; Kanehisa et al., 2017) and BioCyc (Caspi et al., 2016), which provide collections of characterized metabolic pathways and software tools for exploring them, have been used to provide a better understanding of rumen microbial activity in a variety of contexts (Hess et al., 2011; Shi et al., 2014; Rubino et al., 2017; Seshadri et al., 2018). However, many of these databases are targeted toward human pathogens or model aerobic organisms, such as Escherichia coli, and do not necessarily reflect the functions important to the facultative anaerobic lifestyle of rumen microbes. With nearly 500 genomes from rumen organisms currently available, an opportunity exists to generate rumen-specific databases of known and predicted metabolic functions, similar to INTERMINE (Kalderimis et al., 2014) although this will likely require a parallel concerted effort to generate better phenotypic information for these cultured organisms in vitro and in vivo.
The ultimate goal is to be able to combine information from all types of “omics” data, and provide a true “systems” overview of the rumen microbiome. Progress toward achieving this continues for a few other model microbiomes, where the goal has been to identify interactions in order to interpret biological data and ultimately model in silico how microbial communities behave (Bittner et al., 2010; Faust and Raes, 2012). This is generally accomplished through three tasks: firstly by identifying the “scaffold” of interactions between organisms (Faust and Raes, 2012; Friedman and Alm, 2012); secondly, decomposing these interactions into “important components” (Lee and Tzou, 2009); and thirdly, carrying out cellular-systems modeling and analysis (Nobu et al., 2015; Mcgeachie et al., 2016). It is easy to see how such an approach could also be integrated with the mathematical models of the rumen environment described earlier.
Network-based approaches are likely to provide the best hope of integrating all this information by allowing a complete overview of the rumen microbiome as they are capable of capturing the fundamental properties of the microbial ecosystem including taxonomy (Barberan et al., 2012), functional similarity (Martiny et al., 2015), metabolic processes (Shlomi et al., 2008), co-expression (Zhang and Horvath, 2005) and gene-sharing (Smillie et al., 2011). Network-based approaches have proven successful for generating insight into the functional and genetic potential of microbial communities, bringing clarity to the complex history of microbes, providing tools that allow analyses of mosaic sequences (Adai et al., 2004), and identify genomes harboring sequences of multiple origins (Lima-Mendez et al., 2008; Fondi and Fani, 2010; Kloesges et al., 2011; Halary et al., 2013). Network-based approaches also provide a framework where genetic diversity can be compared and quantified, even when analyzing highly divergent sequences (Bhattacharya et al., 2013). We are only in the early stages of applying these approaches to the rumen microbiome, and while network scaffolds for specific animal studies have been generated (Roehe et al., 2016), large-scale, integrative models have yet to be developed.
Conclusions
The terminology of microbiome is often misused within the rumen context as there is a tendency to focus on the study of the rumen bacteria alone, without consideration of the eukaryotes. This undoubtedly limits our ability to understand the rumen microbiome, as these groups of microbes interact with each other, consequently affecting production and the environmental impact of ruminants (Table 4). Also, little emphasis is given to understanding ecological interactions, niche specialization and consequences of the biofilm phenotype and membrane vesicles on animal phenotype. An increased fundamental understanding of the rumen microbiome as well as that of the lower GI tract of ruminants is essential to develop novel approaches for improving livestock production and reducing environmental impact. Irrespective, much emphasis has been given to using plant/additive/supplement strategies to manipulate the rumen microbiome in a manner that improves efficiency of animal production. Although, these have had limited success when applied to the mature animal, recent data show that ruminant breeding programs coupled with a defined dietary management protocols from birth may be the best ways to achieve lifelong animal phenotype benefits with limited financial and labor input. Further research is required to assess the role of the host genome on the rumen microbiome and animal phenotype on a global geographic scale (Table 4). Likewise, application of these innovative techniques to early life ruminant nutrition is in its infancy, and a global effort is required to define the best practices in early life which will differ based on geography (Table 4). Improving our ability to measure phenotype using rumen microbiome gene based biomarkers may also allow high throughput phenotype predictions (Table 4). Irrespective, the ruminant sector has made major strides to improving animal phenotype, through understanding the rumen microbiome, and further advances will no doubt be accomplished, especially given the exponential advances in “omic” technologies.
Table 4
|
Outstanding questions.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
SH, DM, MP, RM-T, SW, IT, HS, JE, SK, GA, and CC acknowledge the support of ERA-net gas co-fund for funding (Project name: RumenPredict). SH, HM and CC acknowledge support from BBSRC (BBL/L026716/1 and BBL/L026716/2) and a British Council Newton Institutional Links funding (Grant 172629373). IM acknowledges funding from the European Research Council under the European Union's Horizon 2020 research and innovation program (Grant 640384). JE acknowledges funding from an EU H2020 Marie Curie Fellowship (706899). CC, AK-S, and EH were supported by the Biotechnology and Biological Sciences Research Council (Grants BBS/OS/GC/000011B and BBS/E/W/0012843D). CN and OM acknowledge the support of the British Council Newton Institutional Links funding (Grant 216425215). SRUC receives financial support from the Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS). RD and RR acknowledge financial support from the Biotechnology and Biological Sciences Research Council (BBSRC BB/N01720X/1). DY-R and AB acknowledge funding from MINECO, Spain (Grant AGL2017-86938-R). GS acknowledges funding from the U.S. Department of Agriculture National Institute of Food and Agriculture foundational (Grant 2015-67015-23246). EP acknowledges funding from CNPq (Grant 401590/2014-3). All authors are also members of the Global Research Alliance Rumen Microbial Genomics network.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Abecia L. Jimenez E. Martinez-Fernandez G. Martin-Garcia A. I. Ramos-Morales E. Pinloche E. et al . (2017). Natural and artificial feeding management before weaning promote different rumen microbial colonization but not differences in gene expression levels at the rumen epithelium of newborn goats. PLoS ONE12:e0182235. 10.1371/journal.pone.0182235
2
Abecia L. Martín-García A. I. Martínez G. Newbold C. J. Yáñez-Ruiz D. R. (2013). Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning1. J. Anim. Sci.91, 4832–4840. 10.1017/S1751731113001699
3
Abecia L. Ramos-Morales E. Martínez-Fernandez G. Arco A. Martín-García A. I. Newbold C. J. et al . (2014). Feeding management in early life influences microbial colonisation and fermentation in the rumen of newborn goat kids. Anim. Prod. Sci.54, 1449–1454. 10.1071/AN14337
4
Abecia L. Toral P. G. Martin-Garcia A. I. Martinez G. Tomkins N. W. Molina-Alcaide E. et al . (2012). Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. J. Dairy Sci.95, 2027–2036. 10.3168/jds.2011-4831
5
Abram F. Gunnigle E. O'flaherty V. (2009). Optimisation of protein extraction and 2-DE for metaproteomics of microbial communities from anaerobic wastewater treatment biofilms. Electrophoresis30, 4149–4151. 10.1002/elps.200900474
6
Adai A. T. Date S. V. Wieland S. Marcotte E. M. (2004). LGL: creating a map of protein function with an algorithm for visualizing very large biological networks. J. Mol. Biol.340, 179–190. 10.1016/j.jmb.2004.04.047
7
Adams J. C. Gazaway J. A. Brailsford M. D. Hartman P. A. Jacobson N. L. (1966). Isolation of bacteriophages from the bovine rumen. Experientia22, 717–718. 10.1007/BF01901335
8
Aite M. Chevallier M. Frioux C. Trottier C. Got J. Cortés M. P. et al . (2018). Traceability, reproducibility and wiki-exploration for “à-la-carte” reconstructions of genome-scale metabolic models. Plos. Comput. Biol.14:e1006146. 10.1371/journal.pcbi.1006146
9
Alexandratos N. Bruinsma J. (2012). World Agriculture Towards 2030/2050: The 2012 Revision, FAO, Food and Agriculture Organization of the United Nations.
10
Al-Masaudi S. El Kaoutari A. Drula E. Al-Mehdar H. Redwan E. M. Lombard V. et al . (2017). A metagenomics investigation of carbohydrate-active enzymes along the gastrointestinal tract of Saudi sheep. Front. Microbiol.8:666. 10.3389/fmicb.2017.00666
11
Alugongo G. M. Xiao J. Wu Z. Li S. Wang Y. Cao Z. (2017). Review: utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol.8:34. 10.1186/s40104-017-0165-5
12
Ametaj B. N. Zebeli Q. Saleem F. Psychogios N. Lewis M. J. Dunn S. M. et al . (2010). Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolism6, 583–594. 10.1007/s11306-010-0227-6
13
Arntzen M. Ø. Várnai A. Mackie R. I. Eljsink V. G. H. Pope P. B. (2017). Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environ. Microbiol.19, 2701–2714. 10.1111/1462-2920.13770
14
Artegoitia V. M. Foote A. P. Lewis R. M. Freetly H. C. (2017). Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Sci. Rep.7:2864. 10.1038/s41598-017-02856-0
15
Aßhauer K. P. Wemheuer B. Daniel R. Meinicke P. (2015). Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics31, 2882–2884. 10.1093/bioinformatics/btv287
16
Auffret M. D. Dewhurst R. J. Duthie C. A. Rooke J. A. John Wallace R. Freeman T. C. et al . (2017a). The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome5:159. 10.1186/s40168-017-0378-z
17
Auffret M. D. Stewart R. Dewhurst R. J. Duthie C. A. Rooke J. A. Wallace R. J. et al . (2017b). Identification, comparison, and validation of robust rumen microbial biomarkers for methane emissions using diverse Bos Taurus breeds and basal diets. Front. Microbiol.8:2642. 10.3389/fmicb.2017.02642
18
Bach A. Calsamiglia S. Stern M. D. (2005). Nitrogen metabolism in the rumen. J. Dairy Sci.88, E9–E21. 10.3168/jds.S0022-0302(05)73133-7
19
Bannink A. Van Lingen H. J. Ellis J. L. France J. Dijkstra J. (2016). The contribution of mathematical modeling to understanding dynamic aspects of rumen metabolism. Front. Microbiol.7:1820. 10.3389/fmicb.2016.01820
20
Barberan A. Bates S. T. Casamayor E. O. Fierer N. (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J.6, 343–351. 10.1038/ismej.2011.119
21
Baroukh C. Muñoz-Tamayo R. Steyer J.-P. Bernard O. (2014). DRUM: A new framework for metabolic modeling under non-balanced growth. Application to the carbon metabolism of unicellular microalgae. PLoS ONE9:e104499. 10.1371/journal.pone.0104499
22
Beitel C. W. Froenicke L. Lang J. M. Korf I. F. Michelmore R. W. Eisen J. A. et al . (2014). Strain- and plasmid-level deconvolution of a synthetic metagenome by sequencing proximity ligation products. PeerJ2:e415. 10.7717/peerj.415
23
Belanche A. De La Fuente G. Newbold C. J. (2014). Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol.90, 663–677. 10.1111/1574-6941.12423
24
Belanche A. Doreau M. Edwards J. E. Moorby J. M. Pinloche E. Newbold C. J. (2012). Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J. Nutr.142, 1684–1692. 10.3945/jn.112.159574
25
Belanche A. Kingston-Smith A. Newbold C. J. (2016). An integrated multi-omics approach reveals the effects of supplementing grass or grass hay with vitamin E on the rumen microbiome and its function. Front. Microbiol.7:905. 10.3389/fmicb.2016.00905
26
Belanche A. Newbold C. J. Lin W. Stevens P. R. Kingston-Smith A. H. (2017). A systems biology approach reveals differences in the dynamics of colonization and degradation of grass vs. hay by rumen microbes with minor effects of vitamin E supplementation. Front. Microbiol.8:1456. 10.3389/fmicb.2017.01456
27
Benndorf D. Vogt C. Jehmlich N. Schmidt Y. Thomas H. Woffendin G. et al . (2009). Improving protein extraction and separation methods for investigating the metaproteome of anaerobic benzene communities within sediments. Biodegradation20, 737–750. 10.1007/s10532-009-9261-3
28
Bergmann G. T. (2017). Microbial community composition along the digestive tract in forage- and grain-fed bison. BMC Vet. Rese.13:253. 10.1186/s12917-017-1161-x
29
Berry D. P. Crowley J. J. (2012). Residual intake and body weight gain: a new measure of efficiency in growing cattle. J. Anim. Sci.90, 109–115. 10.2527/jas.2011-4245
30
Bhattacharya D. Price D. C. Bicep C. Bapteste E. Sarwade M. Rajah V. D. et al . (2013). Identification of a marine cyanophage in a protist single-cell metagenome assembly. J. Phycol.49, 207–212. 10.1111/jpy.12028
31
Biggs M. B. Medlock G. L. Kolling G. L. Papin J. A. (2015). Metabolic network modeling of microbial communities. Wiley interdisciplinary reviews. Sys. Biol. Med.7, 317–334. 10.1002/wsbm.1308
32
Bittner L. Halary S. Payri C. Cruaud C. De Reviers B. Lopez P. et al . (2010). Some considerations for analyzing biodiversity using integrative metagenomics and gene networks. Biol. Direct.5:47. 10.1186/1745-6150-5-47
33
Boeckx T. Winters A. Webb K. J. Kingston-Smith A. H. (2017). Detection of potential chloroplastic substrates for polyphenol oxidase suggests a role in undamaged leaves. Front. Plant. Sci.8:237. 10.3389/fpls.2017.00237
34
Broderick G. A. (1995). Desirable characteristics of forage legumes for improving protein utilization in ruminants. J. Anim. Sci.73, 2760–2773.
35
Broderick G. A. Reynal S. M. (2009). Effect of source of rumen-degraded protein on production and ruminal metabolism in lactating dairy cows. J. Dairy Sci.92, 2822–2834. 10.3168/jds.2008-1865
36
Bundy J. G. Davey M. P. Viant M. R. (2009). Environmental metabolomics: a critical review and future perspectives. Metabolism5:3. 10.1007/s11306-008-0152-0
37
Calsamiglia S. Busquet M. Cardozo P. Castillejos L. Ferret A. (2007). Invited review: essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci.90, 2580–2595. 10.3168/jds.2006-644
38
Carberry C. A. Waters S. M. Kenny D. A. Creevey C. J. (2014). Rumen methanogenic genotypes differ in abundance according to host residual feed intake phenotype and diet type. Environ. Microbiol. 80:2039. 10.1128/AEM.03131-13
39
Caspi R. Billington R. Ferrer L. Foerster H. Fulcher C. A. Keseler I. M. et al . (2016). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nuc. Acid. Res.44, D471–D480. 10.1093/nar/gkr1014
40
Chen X. B. Hovell F. D. Orskov E. R. (1990). Excretion of purine derivatives by ruminants: recycling of allantoin into the rumen via saliva and its fate in the gut. Br. J. Nutr.63, 197–205. 10.1079/BJN19900107
41
Cheng K.-J. Costerton J. W. (1980). Adherent rumen bacteria—their role in the digestion of plant material, urea and epithelial cells, in Digestive Physiology and Metabolism in Ruminants: Proceedings of the 5th International Symposium on Ruminant Physiology, held at Clermont—Ferrand, on 3rd−7th September, 1979. eds RuckebuschY.ThivendP. (Dordrecht: Springer Netherlands), 227–250.
42
Cheng K. J. Mccowan R. P. Costerton J. W. (1979). Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am. J. Clin. Nutr.32, 139–148. 10.1093/ajcn/32.1.139
43
Cheng Y. F. Mao S. Y. Liu J. X. Zhu W. Y. (2009). Molecular diversity analysis of rumen methanogenic archaea from goat in eastern China by DGGE methods using different primer pairs. Lett. Appl. Microbiol.48, 585–592. 10.1111/j.1472-765X.2009.02583.x
44
Chourey K. Jansson J. Verberkmoes N. Shah M. Chavarria K. L. Tom L. M. et al . (2010). Direct cellular lysis/protein extraction protocol for soil metaproteomics. J. Proteome Res.9, 6615–6622. 10.1021/pr100787q
45
Church D. C. (1988). The Ruminant Animal, Digestive Physiology and Nutrition. Englewood Cliff, NJ: Prentice Hill Inc.
46
Comtet-Marre S. Chaucheyras-Durand F. Bouzid O. Mosoni P. Bayat A. R. Peyret P. et al . (2018). FibroChip, a functional DNA microarray to monitor cellulolytic and hemicellulolytic activities of rumen microbiota. Front. Microbiol.9:215. 10.3389/fmicb.2018.00215
47
Comtet-Marre S. Parisot N. Lepercq P. Chaucheyras-Durand F. Mosoni P. Peyretaillade E. et al . (2017). Metatranscriptomics reveals the active bacterial and eukaryotic fibrolytic communities in the rumen of dairy cow fed a mixed diet. Front. Microbiol.8:67. 10.3389/fmicb.2017.00067
48
Couvreur S. Hurtaud C. Lopez C. Delaby L. Peyraud J.-L. (2006). The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. J. Dairy Sci.89, 1956–1969. 10.3168/jds.S0022-0302(06)72263-9
49
Csárdi G. Franks A. Choi D. S. Airoldi E. M. Drummond D. A. (2015). Accounting for experimental noise reveals that mRNA levels, amplified by post-transcriptional processes, largely determine steady-state protein levels in yeast. PLoS Genet.11:e1005206. 10.1371/journal.pgen.1005206
50
Dai X. Tian Y. Li J. Su X. Wang X. Zhao S. et al . (2015). Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol.81, 1375–1386. 10.1128/AEM.03682-14
51
Davies D. R. Theodorou M. K. Lawrence M. I. G. Trinci A. P. J. (1993). Distribution of anaerobic fungi in the digestive tract of cattle and their survival in faeces. J. Gen. Microbiol.139, 1395–1400. 10.1099/00221287-139-6-1395
52
De Mulder T. Peiren N. Vandaele L. Ruttink T. De Campeneere S. Van De Wiele T. et al . (2018). Impact of breed on the rumen microbial community composition and methane emission of Holstein Friesian and Belgian Blue heifers. Livestock Sci.207, 38–44. 10.1016/j.livsci.2017.11.009
53
De Oliveira M. N. V. Jewell K. A. Freitas F. S. Benjamin L. A. Tótola M. R. Borges A. C. et al . (2013). Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol.164, 307–314. 10.1016/j.vetmic.2013.02.013
54
Desnoyers M. Giger-Reverdin S. Bertin G. Duvaux-Ponter C. Sauvant D. (2009). Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci.92, 1620–1632. 10.3168/jds.2008-1414
55
Deusch S. Seifert J. (2015). Catching the tip of the iceberg–evaluation of sample preparation protocols for metaproteomic studies of the rumen microbiota. Proteomics15, 3590–3595. 10.1002/pmic.201400556
56
Dewhurst R. J. Mitton A. M. Offer N. W. Thomas C. (1996). Effects of the composition of grass silages on milk production and nitrogen utilization by dairy cows. Anim. Sci.62, 25–34. 10.1017/S1357729800014272
57
Dey A. Sehgal J. P. Puniya A. K. Singh K. (2004). Influence of an anaerobic fungal culture (Orpinomyces sp.) administration on growth rate, ruminal fermentation and nutrient digestion in calves. Asian-Australas. J. Anim. Sci.17, 820–824. 10.5713/ajas.2004.820
58
Do Prado R. M. Porto C. Nunes E. De Aguiar C. L. Pilau E. J. (2018). Metabolomics and agriculture: what can be done?mSystems3, e00156–e00117. 10.1128/mSystems.00156-17
59
Dowd S. E. Callaway T. R. Wolcott R. D. Sun Y. Mckeehan T. Hagevoort R. G. et al . (2008). Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol.8:125. 10.1186/1471-2180-8-125
60
Duin E. C. Wagner T. Shima S. Prakash D. Cronin B. Yáñez-Ruiz D. R. et al . (2016). Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Nat. Acad. Sci. U.S.A.113, 6172–6177. 10.1073/pnas.1600298113
61
Durso L. Harhay G. Smith T. Bono J. Desantis T. Harhay D. et al . (2010). Animal-to-animal variation in fecal microbial diversity among beef cattle. Appl. Environ. Microbiol.76, 4858–4920. 10.1128/AEM.00207-10
62
Durso L. Wells J. Harhay G. Rice W. Kuehn L. Bono J. et al . (2012). Comparison of bacterial communities in faeces of beef cattle fed diets containing corn and wet distillers' grain with solubles. Lett. Appl.Microbiol.55, 109–114. 10.1111/j.1472-765X.2012.03265.x
63
Edwards J. E. Forster R. J. Callaghan T. M. Dollhofer V. Dagar S. S. Cheng Y. et al . (2017). PCR and omics based techniques to study the diversity, ecology and biology of anaerobic fungi: Insights, challenges and opportunities. Front. Microbiol.8:1657. 10.3389/fmicb.2017.01657
64
Edwards J. E. Huws S. A. Kim E. J. Kingston-Smith A. H. (2007). Characterization of the dynamics of initial bacterial colonization of nonconserved forage in the bovine rumen. FEMS Microbiol. Ecol.62, 323–335. 10.1111/j.1574-6941.2007.00392.x
65
Edwards J. E. Huws S. A. Kim E. J. Lee M. R. Kingston-Smith A. H. Scollan N. D. (2008). Advances in microbial ecosystem concepts and their consequences for ruminant agriculture. Animal2, 653–660. 10.1017/S1751731108002164
66
Elekwachi C. O. Wang Z. Wu X. Rabee A. Forster R. J. (2017). Total rRNA-seq analysis gives insight into bacterial, fungal, protozoal and archaeal communities in the rumen using an optimized RNA isolation method. Front. Microbiol.8:1814. 10.3389/fmicb.2017.01814
67
Ellis J. L. Dijkstra J. Kebreab E. Bannink A. Odongo N. E. Mcbride B. W. et al . (2008). Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle. J. Agri. Sci.146, 213–233. 10.1017/S0021859608007752
68
Faust K. Raes J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol.10, 538–550. 10.1038/nrmicro2832
69
Feist A. M. Herrgård M. J. Thiele I. Reed J. L. Palsson B. Ø. (2009). Reconstruction of biochemical networks in microbial organisms. Nat. Rev. Microbiol.7, 129–143. 10.1038/nrmicro1949
70
Fernando S. C. Purvis H. T. Najar F. Z. Sukharnikov L. O. Krehbiel C. R. Nagaraja T. G. et al . (2010). Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol.76, 7482–7490. 10.1128/AEM.00388-10
71
Fondi M. Fani R. (2010). The horizontal flow of the plasmid resistome: clues from inter-generic similarity networks. Environ. Microbiol.12, 3228–3242. 10.1111/j.1462-2920.2010.02295.x
72
Fouhse J. M. Smiegielski L. Tuplin M. Guan L. L. Willing B. P. (2017). Host immune selection of rumen bacteria through salivary secretory IgA. Front. Microbiol.8:848. 10.3389/fmicb.2017.00848
73
Frey J. C. Pell A. N. Berthiaume R. Lapierre H. Lee S. Ha J. K. et al . (2010). Comparative studies of microbial populations in the rumen, duodenum, ileum and faeces of lactating dairy cows. J. Appl. Microbiol.108, 1982–1993. 10.1111/j.1365-2672.2009.04602.x
74
Friedman J. Alm E. J. (2012). Inferring correlation networks from genomic survey data. PLoS Comput. Biol.8:e1002687. 10.1371/journal.pcbi.1002687
75
Friedman N. Elie J. Itzhak M. (2017). Compositional and functional dynamics of the bovine rumen methanogenic community across different developmental stages. Environ. Microbiol.19, 3365–3373. 10.1111/1462-2920.13846
76
Gerber P. J. Steinfeld H. Henderson B. Mottet A. Opio C. Dijkman J. et al . (2013). Tackling Climate Change Through Livestock – A Global Assessment of Emissions and Mitigation Opportunities. Rome: Food and Agriculture Organization of the United Nations (FAO).
77
Gilbert R. A. Kelly W. J. Altermann E. Leahy S. C. Minchin C. Ouwerkerk D. et al . (2017). Toward understanding phage: host interactions in the rumen; complete genome sequences of lytic phages infecting rumen bacteria. Front. Microbiol.8:2340. 10.3389/fmicb.2017.02340
78
Gilbert R. A. Klieve A. V. (2015). Ruminal viruses (bacteriophages, archaeaphages), in Rumen Microbiology: From Evolution to Revolution. eds PuniyaA. K.SinghR.KamraD. N. (New Delhi: Springer India), 121–141.
79
Goldansaz S. A. Guo A. C. Sajed T. Steele M. A. Plastow G. S. Wishart D. S. (2017). Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE12:e0177675. 10.1371/journal.pone.0177675
80
Golder H. M. Thomson J. M. Denman S. E. Mcsweeney C. S. Lean I. J. (2018). Genetic markers are associated with the ruminal microbiome and metabolome in grain and sugar challenged dairy heifers. Front. Genet. 9:62. 10.3389/fgene.2018.00062
81
González L. Manteca X. Calsamiglia S. Schwartzkopf-Genswein K. Ferret A. (2012). Ruminal acidosis in feedlot cattle: Interplay between feed ingredients, rumen function and feeding behavior (a review). Anim. Feed. Sci. Technol.172, 66–79. 10.1016/j.anifeedsci.2011.12.009
82
Goodrich J. K. Davenport E. R. Waters J. L. Clark A. G. Ley R. E. (2016). Cross-species comparisons of host genetic associations with the microbiome. Science352, 532–535. 10.1126/science.aad9379
83
Goopy J. P. Donaldson A. Hegarty R. Vercoe P. E. Haynes F. Barnett M. et al . (2014). Low-methane yield sheep have smaller rumens and shorter rumen retention time. Brit. J. Nutr.111, 578–585. 10.1017/S0007114513002936
84
Gordon G. L. Phillips M. W. (1998). The role of anaerobic gut fungi in ruminants. Nutr. Res. Rev.11, 133–168. 10.1079/NRR19980009
85
Greenbaum D. Colangelo C. Williams K. Gerstein M. (2003). Comparing protein abundance and mRNA expression levels on a genomic scale. Genome. Biol.4:117. 10.1186/gb-2003-4-9-117
86
Gregorini P. Beukes P. C. Dalley D. Romera A. J. (2016). Development of an improved representation of the rumen igesta outflow in a mechanistic an dynamic model of a dairy cow, Molly. Ecol. Model.313, 293–306. 10.1016/j.ecolmodel.2015.06.042
87
Gruby D. Delafond H. M. O. (1843). Recherches ser des animalcules se de veloppant en grand nombre dans l'estomac et dans les intestins, pedant la digestion des animaux herbivores et carnivores. Compt. Rend. Acad. Sci.17, 1304–1308.
88
Gruninger R. J. Puniya A. K. Callaghan T. M. Edwards J. E. Youssef N. Dagar S. S. et al . (2014). Anaerobic fungi (phylum Neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol. Ecol.90, 1–17. 10.1111/1574-6941.12383
89
Gutzeit C. Magri G. Cerutti A. (2014). Intestinal IgA production and its role in host-microbe interaction. Immun. Rev.260, 76–85. 10.1111/imr.12189
90
Halary S. Mcinerney J. O. Lopez P. Bapteste E. (2013). EGN: a wizard for construction of gene and genome similarity networks. BMC Evol. Biol.13:146. 10.1186/1471-2148-13-146
91
Hart E. H. Creevey C. J. Hitch T. Kingston-Smith A. H. (2018). Meta-proteomics of rumen microbiota indicates niche compartmentalisation and functional dominance in a limited number of metabolic pathways between abundant bacteria. Sci. Rep.8:10504. 10.1038/s41598-018-28827-7
92
Hart E. H. Onime L. A. Davies T. E. Morphew R. M. Kingston-Smith A. H. (2016). The effects of PPO activity on the proteome of ingested red clover and implications for improving the nutrition of grazing cattle. J. Proteomics141, 67–76. 10.1016/j.jprot.2016.04.023
93
He J. Yi L. Hai L. Ming L. Gao W. Ji R. (2018). Characterizing the bacterial microbiota in different gastrointestinal tract segments of the Bactrian camel. Sci. Rep.8:654. 10.1038/s41598-017-18298-7
94
Hegarty R. S. (1999). Reducing rumen methane emissions through elimination of rumen protozoa. Aus. J. Agri. Res.50, 1321–1328. 10.1071/AR99008
95
Henderson G. Cox F. Ganesh S. Jonker A. Young W. Abecia L. et al . (2015). Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep.5:14567. 10.1038/srep14567
96
Henry C. S. Dejongh M. Best A. A. Frybarger P. M. Linsay B. Stevens R. L. (2010). High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat. Biotech.28, 977–U922. 10.1038/nbt.1672
97
Hernandez-Sanabria E. Goonewardene L. A. Wang Z. Durunna O. N. Moore S. S. Guan L. L. (2012). Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl. Environ. Microbiol.78, 1203–1214. 10.1128/AEM.05114-11
98
Hess M. Sczyrba A. Egan R. Kim T. W. Chokhawala H. Schroth G. et al . (2011). Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science331, 463–467. 10.1126/science.1200387
99
Hristov A. N. Oh J. Giallongo F. Frederick T. W. Harper M. T. Weeks H. L. et al . (2015). An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Nat. Acad. Sci.U.S.A.112, 10663–10668. 10.1073/pnas.1504124112
100
Hristov A. N. Oh J. Lee C. Meinen R. Montes F. Ott T. et al . (2013). Mitigation of greenhouse gas emissions in livestock production. A review of options for non-CO2 emissions. eds GerberP. J.HendersonB.MakkarH. P. S.Rome: FAO Animal production and Health Paper No 177; FAO. 226.
101
Huang X. D. Martinez-Fernandez G. Padmanabha J. Long R. Denman S. E. Mcsweeney C. S. (2016). Methanogen diversity in indigenous and introduced ruminant species on the Tibetan plateau. Archaea2016:5916067. 10.1155/2016/5916067
102
Huhtanen P. Cabezas-Garcia E. H. Utsumi S. Zimmerman S. (2015). Nordic dairy cow model Karoline in predicting methane emissions: 1. Model description and sensitivity analysis. Livestock Sci.178, 71–80. 10.1016/j.livsci.2015.05.009
103
Hungate R. E. (1966). The Rumen and its Microbes. New York, NY: Academic Press.
104
Huws S. A. Edwards J. E. Creevey C. J. Rees Stevens P. Lin W. Girdwod S. E. et al . (2016). Temporal dynamics of metabolically active rumen bacteria colonizing fresh perennial ryegrass. FEMS Microbiol. Ecol.92:fiv137. 10.1093/femsec/fiv137
105
Huws S. A. Edwards J. E. Kim E. J. Scollan N. D. (2007). Specificity and sensitivity of eubacterial primers utilized for molecular profiling of bacteria within complex microbial ecosystems. J. Microbiol. Meth.70, 565–569. 10.1016/j.mimet.2007.06.013
106
Huws S. A. Kim E. J. Kingston-Smith A. H. Lee M. R. Muetzel S. M. Cookson A. R. et al . (2009). Rumen protozoa are rich in polyunsaturated fatty acids due to the ingestion of chloroplasts. FEMS Microbiol. Ecol.69, 461–471. 10.1111/j.1574-6941.2009.00717.x
107
Huws S. A. Lee M. R. Kingston-Smith A. H. Kim E. J. Scott M. B. Tweed J. K. et al . (2012). Ruminal protozoal contribution to the duodenal flow of fatty acids following feeding of steers on forages differing in chloroplast content. Brit. J. Nutr.108, 2207–2214. 10.1017/S0007114512000335
108
Huws S. A. Lee M. R. Muetzel S. M. Scott M. B. Wallace R. J. Scollan N. D. (2010). Forage type and fish oil cause shifts in rumen bacterial diversity. FEMS Microbiol. Ecol.73, 396–407. 10.1111/j.1574-6941.2010.00892.x
109
Huws S. A. Mayorga O. L. Theodorou M. K. Kim E. J. Cookson A. Newbold J. C. et al . (2014). Differential colonization of plant parts by the rumen microbiota is likely to be due to different forage chemistries. J. Microb. Biochem. Technol.6, 80–86. 10.4172/1948-5948.1000126
110
Huws S. A. Mayorga O. L. Theodorou M. K. Onime L. A. Kim E. J. Cookson A. H. et al . (2013). Successional colonization of perennial ryegrass by rumen bacteria. Lett. Appl. Microbiol.56, 186–196. 10.1111/lam.12033
111
Ingolia N. T. Ghaemmaghami S. Newman J. R. S. Weissman J. S. (2009). Genome-Wide Analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science324, 218–223. 10.1126/science.1168978
112
IPCC (Ed.) (2006). Guidelines for National Greenhouse Inventories, vol 4. Agriculture, forestry and other land use. Japan, Institute for Global Environmental Strategies (IGES)
113
Iqbal M. W. Zhang Q. Yang Y. Li L. Zou C. Huang C. et al . (2018). Comparative study of rumen fermentation and microbial community differences between water buffalo and Jersey cows under similar feeding conditions. J. Appl. Anim. Res.46, 740–748. 10.1080/09712119.2017.1394859
114
Ishaq S. L. AlZahal O. Wlker N. McBride B. (2017). An investigation into rumen fungal and protozoal diversity in the three rumen fractions, during high fiber or grain-induced sub-acute acidosis conditions, with or without active dry yeast supplementation. Front. Microbiol.8:1943. 10.3389/fmicb.2017.01943
115
Ishaq S. L. Kim C. J. Reis D. Wright A. D. (2015). Fibrolytic bacteria isolated from the rumen of North Amerian moose (Alces alces) and their use as a probiotic in neonatal lambs. PLoS ONE10:e0144804. 10.1371/journal.pone.0144804
116
Ishaq S. L. Wright A. D. (2014). Design and validation of four new primers for next-generation sequencing to target the 18S rRNA genes of gastrointestinal ciliate protozoa. Appl Environ. Microbiol.80, 5515–5521. 10.1128/AEM.01644-14
117
Jami E. Israel A. Kotser A. Mizrahi I. (2013). Exploring the bovine rumen bacterial community from birth to adulthood. ISMEJ.7:1069. 10.1038/ismej.2013.2
118
Jami E. White B. A. Mizrahi I. (2014). Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE9:e85423. 10.1371/journal.pone.0085423
119
Janssen P. H. (2010). Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed. Sci. Technol.160, 1–22. 10.1016/j.anifeedsci.2010.07.002
120
Janssen P. H. Kirs M. (2008). Structure of the archaeal community of the rumen. Appl. Environ. Microbiol. 74, 3619–2066. 10.1128/AEM.02812-07
121
Jayanegara A. Sarwono K. A. Kondo M. Matsui H. Ridla M. Laconi E. B. et al . (2018). Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: a meta-analysis. Ital. J. Anim. Sci.17, 650–656. 10.1080/1828051X.2017.1404945
122
Jeyanathan J. Martin C. Morgavi D. P. (2014). The use of direct-fed microbials for mitigation of ruminant methane emissions: a review. Animal8, 250–261. 10.1017/S1751731113002085
123
Jin D. Zhao S. Zheng N. Bu D. Beckers Y. Denman S. E. et al . (2017). Differences in ureolytic bacterial composition between the rumen digesta and rumen wall based on ureC gene classification. Front. Microbiol.8:385. 10.3389/fmicb.2017.00385
124
Johnson K. A. Johnson D. E. (1995). Methane emissions from cattle. J. Anim. Sci.73, 2483–2492. 10.2527/1995.7382483x
125
Jones R. J. Megarrity R. G. (1986). Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome toxicity of Leucaena. Aus. Vet. J.63, 259–262. 10.1111/j.1751-0813.1986.tb02990.x
126
Jun S.-R. Robeson M. S. Hauser L. J. Schadt C. W. Gorin A. A. (2015). PanFP: pangenome-based functional profiles for microbial communities. BMC Res. Notes.8:479. 10.1186/s13104-015-1462-8
127
Kalderimis A. Lyne R. Butano D. Contrino S. Lyne M. Heimbach J. et al . (2014). InterMine: extensive web services for modern biology. Nuc. Acid. Res.42, W468–472. 10.1093/nar/gku301
128
Kamke J. Kittelmann S. Soni P. Li Y. Tavendale M. Ganesh S. et al . (2016). Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome4:56. 10.1186/s40168-016-0201-2
129
Kanehisa M. Furumichi M. Tanabe M. Sato Y. Morishima K. (2017). KEGG: new perspectives on genomes, pathways, diseases and drugs. Nuc. Acid. Res.45, D353–D361. 10.1093/nar/gkw1092
130
Karisa B. Moore S. Plastow G. (2014). Analysis of biological networks and biological pathways associated with residual feed intake in beef cattle. Anim. Sci. J.85, 374–387. 10.1111/asj.12159
131
Kenters N. Henderson G. Jeyanathan J. Kittelmann S. Janssen P. H. (2011). Isolation of previously uncultured rumen bacteria by dilution to extinction using a new liquid culture medium. J. Microbiol. Meth.84, 52–60. 10.1016/j.mimet.2010.10.011
132
Keunen J. Plaizier J. Kyriazakis I. Duffield T. Widowski T. Lindinger M. et al . (2003). Effects of subacute ruminal acidosis on free-choice intake of sodium bicarbonate in lactating dairy cows. J. Dairy. Sci.86, 954–957. 10.3168/jds.S0022-0302(03)73678-9
133
Kim M. Morrison M. Yu Z. (2011). Evaluation of dfferent partial 16S rRNA gene sequence regions for phylogenetic analysis of microbiomes. J. Microbiol. Meth.84, 81–87. 10.1016/j.mimet.2010.10.020
134
Kingston-Smith A. H. Davies T. E. Edwards J. E. Theodorou M. K. (2008). From plants to animals; the role of plant cell death in ruminant herbivores. J. Exp. Bot.59, 521–532. 10.1093/jxb/erm326
135
Kingston-Smith A. H. Davies T. E. Rees Stevens P. Mur L. A. J. (2013). Comparative metabolite fingerprinting of the rumen system during colonisation of three forage grass (Lolium perenne L.) varieties. PLoS ONE8:e82801. 10.1371/journal.pone.0082801
136
Kingston-Smith A. H. Edwards J. E. Huws S. A. Kim E. J. Abberton M. (2010). Plant-based strategies towards minimising ‘livestock's long shadow’. Proc. Nutr. Soc.69, 613–620. 10.1017/S0029665110001953
137
Kittelmann S. Janssen P. H. (2011). Characterization of rumen ciliate community composition in domestic sheep, deer, and cattle, feeding on varying diets, by means of PCR-DGGE and clone libraries. FEMS Microbiol. Ecol.75, 468–481. 10.1111/j.1574-6941.2010.01022.x
138
Klaassens E. S. De Vos W. M. Vaughan E. E. (2007). Metaproteomics approach to study the functionality of the microbiota in the human infant gastrointestinal tract. Appl. Environ. Microbiol.73, 1388–1392. 10.1128/AEM.01921-06
139
Kloesges T. Popa O. Martin W. Dagan T. (2011). Networks of gene sharing among 329 proteobacterial genomes reveal differences in lateral gene transfer frequency at different phylogenetic depths. Mol. Biol. Evol.28, 1057–1074. 10.1093/molbev/msq297
140
Knight T. Ronimus R. Dey D. Tootill C. Naylor G. Evans P. et al . (2011). Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle. Anim. Feed Sci. Technol.166, 101–112. 10.1016/j.anifeedsci.2011.04.059
141
Koetschan C. Kittelmann S. Lu J. Al-Halbouni D. Jarvis G. N. Muller T. et al . (2014). Internal transcribed spacer 1 secondary structure analysis reveals a common core throughout the anaerobic fungi (Neocallimastigomycota). PLoS ONE9:e91928. 10.1371/journal.pone.0091928
142
Koo H. Mojib N. Hakim J. A. Hawes I. Tanabe Y. Andersen D. T. et al . (2017). Microbial communities and their predicted metabolic functions in growth laminae of a unique large conical mat from Lake Untersee, East Antarctica. Front. Microbiol.8:1347. 10.3389/fmicb.2017.01347
143
Koskella B. Brockhurst M. A. (2014). Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev.38, 916–931. 10.1111/1574-6976.12072
144
Langille M. G. Zaneveld J. Caporaso J. G. Mcdonald D. Knights D. Reyes J. A. et al . (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol.31, 814–821. 10.1038/nbt.2676
145
Leahy S. C. Kelly W. J. Altermann E. Ronimus R. S. Yeoman C. J. Pacheco D. M. et al . (2010). The genome sequence of the rumen methanogen Methanobrevibacter ruminantium reveals new possibilities for controlling ruminant methane emissions. PLoS ONE5:e8926. 10.1371/journal.pone.0008926
146
Lee M. R. F. (2014). Forage polyphenol oxidase and ruminant livestock nutrition. Front. Plant Sci.5:694. 10.3389/fpls.2014.00694
147
Lee M. R. F. Winters A. L. Scollan N. D. Dewhurst R. J. Theodorou M. K. Minchin F. R. (2004). Plant-mediated lipolysis and proteolysis in red clover with different polyphenol oxidase activities. J. Sci. Food Agri.84, 1639–1645. 10.1002/jsfa.1854
148
Lee S. S. Ha J. K. Cheng K. J. (2000). Influence of an anaerobic fungal culture administration on in vivo ruminal fermentation and nutrient digestion. Anim. Feed Sci. Technol.88, 201–217. 10.1016/S0377-8401(00)00216-9
149
Lee W. P. Tzou W. S. (2009). Computational methods for discovering gene networks from expression data. Brief. Bioinform.10, 408–423. 10.1093/bib/bbp028
150
Leng R. A. (2014). Interactions between microbial consortia in biofilms: a paradigm shift in rumen microbial ecology and enteric methane mitigation. Anim. Prod. Sci.54, 519–543. 10.1071/AN13381
151
Lesmeister K. E. Heinrichs A. J. Gabler M. T. (2004). Effects of supplemental yeast (Saccharomyces cerevisiae) culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J. Dairy Sci.87, 1832–1839. 10.3168/jds.S0022-0302(04)73340-8
152
Lewis N. E. Nagarajan H. Palsson B. O. (2012). Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol.10, 291–305. 10.1038/nrmicro2737
153
Li F. Guan L. L. (2017). Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol.83:9. 10.1128/AEM.00061-17
154
Lima-Mendez G. Van Helden J. Toussaint A. Leplae R. (2008). Reticulate representation of evolutionary and functional relationships between phage genomes. Mol. Biol. Evol.25, 762–777. 10.1093/molbev/msn023
155
Lyte M. Villageli,ú D. N. Crooker B. A. Brown D. R. (2018). Symposium review: microbial endocrinology—why the integration of microbes, epithelial cells, and neurochemical signals in the digestive tract matters to ruminant health. J. Dairy Sci.101, 5619–5628. 10.3168/jds.2017-13589
156
Macheboeuf D. Morgavi D. Papon Y. Mousset J.-L. Arturo-Schaan M. (2008). Dose–response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol.145, 335–350. 10.1016/j.anifeedsci.2007.05.044
157
Macrae J. C. Ulyatt M. J. (1974). Quantitative digestion of fresh herbage by sheep: II. The sites of digestion of some nitrogenous constituents. J. Agri. Sci.82, 309–319. 10.1017/S0021859600059207
158
Mahadevan R. Edwards J. S. Doyle F. J. (2002). Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J.83, 1331–1340. 10.1016/S0006-3495(02)73903-9
159
Malmuthuge N. Griebel P. J. Guan L. L. (2014). Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl. Environ. Microbiol.80, 2021–2028. 10.1128/AEM.03864-13
160
Malmuthuge N. Griebel P. J. Guan L. L. (2015). The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci.2:36. 10.3389/fvets.2015.00036
161
Mao S. Wenjie H. Junhua L. Ruiyang Z. Weiyun Z. (2017). In vitro effects of sodium bicarbonate buffer on rumen fermentation, levels of lipopolysaccharide and biogenic amine, and composition of rumen microbiota. J. Sci. Food. Agri.97, 1276–1285. 10.1002/jsfa.7861
162
Mao S. Zhang M. Liu J. Zhu W. (2015). Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci. Rep.5:16116. 10.1038/srep16116
163
Mao S. Y. Wen-Jie H. Wei-Yun Z. (2016). Microbiome–metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol.18, 525–541. 10.1111/1462-2920.12724
164
Martin C. Morgavi D. Doreau M. (2010). Methane mitigation in ruminants: from microbe to the farm scale. Animal4, 351–365. 10.1017/S1751731109990620
165
Martin S. A. Nisbet D. J. (1992). Effect of direct-fed microbials on rumen microbial fermentation. J. Dairy Sci.75, 1736–1744. 10.3168/jds.S0022-0302(92)77932-6
166
Martinez-Fernandez G. Denman S. E. Cheung J. Mcsweeney C. S. (2017). Phloroglucinol degradation in the rumen promotes the capture of excess hydrogen generated from methanogenesis inhibition. Front. Microbiol.8:1871. 10.3389/fmicb.2017.01871
167
Martiny J. B. Jones S. E. Lennon J. T. Martiny A. C. (2015). Microbiomes in light of traits: a phylogenetic perspective. Science350:6261. 10.1126/science.aac9323
168
Mayer A. M. Harel E. (1979). Polyphenol oxidases in plants. Phytochem.18, 193–215. 10.1016/0031-9422(79)80057-6
169
Mayorga O. L. Kingston-Smith A. H. Kim E. J. Allison G. G. Wilkinson T. J. Hegarty M. J. et al . (2016). Temporal metagenomic and metabolomic characterization of fresh perennial ryegrass degradation by rumen bacteria. Front. Microbiol.7:1854. 10.3389/fmicb.2016.01854
170
Mcallan A. B. Smith R. H. (1969). Nucleic acid metabolism in the ruminant: determination of nucleic acids in digesta. Brit. J. Nutr.23, 671–682. 10.1079/BJN19690075
171
Mcallister T. A. Bae H. D. Jones G. A. Cheng K. J. (1994). Microbial attachment and feed digestion in the rumen. J. Anim. Sci.72, 3004–3018.
172
Mccartney C. A. Dewhurst R. J. Bull I. D. (2014). Changes in the ratio of tetraether to diether lipids in cattle feces in response to altered dietary ratio of grass silage and concentrates. J. Anim. Sci.92, 4095–4098. 10.2527/jas.2014-7929
173
Mcgeachie M. J. Sordillo J. E. Gibson T. Weinstock G. M. Liu Y. Y. Gold D. R. et al . (2016). Longitudinal prediction of the infant gut microbiome with dynamic bayesian networks. Sci. Rep.6:20359. 10.1038/srep20359
174
Mcsweeney C. S. Kennedy P. M. John A. (1989). Reticulo-ruminal motility in cattle (Bos indicus) and water buffaloes (Bubalus bubalis) fed a low quality roughage diet. Comp. Biochem. Physiol. A. Comp. Physiol.94, 635–638.
175
Mcsweeney C. S. Palmer B. Mcneill D. M. Krause D. O. (2001). Microbial interactions with tannins: nutritional consequences for ruminants. Anim. Feed Sci. Technol.91, 83–93. 10.1016/S0377-8401(01)00232-2
176
Meale S. J. Li S. C. Azevedo P. Derakhshani H. Devries T. J. Plaizier J. C. et al . (2017). Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Sci. Rep.7:198. 10.1038/s41598-017-00223-7
177
Mesuere B. Van Der Jeugt F. Willems T. Naessens T. Devreese B. Martens L. et al . (2018). High-throughput metaproteomics data analysis with Unipept: a tutorial. J. Proteomics171, 11–22. 10.1016/j.jprot.2017.05.022
178
Michalet-Doreau B. Fernandez I. Peyron C. Millet L. Fonty G. (2001). Fibrolytic activities and cellulolytic bacterial community structure in the solid and liquid phases of rumen contents. Reprod. Nutr. Dev.41, 187–194. 10.1051/rnd:2001122
179
Mills J. A. Crompton L. A. Ellis J. L. Dijkstra J. Bannink A. Hook S. et al . (2014). A dynamic mechanistic model of lactic acid metabolism in the rumen. J. Dairy Sci.97, 2398–2414. 10.3168/jds.2013-7582
180
Minato H. Endo A. Ootomo Y. Uemura T. (1966). Ecological treatise on the rumen fermentation II. The amylolytic and cellulolytic activities of the fractionated bacterial portions attached to the rumen solids. J. Gen. Appl. Microbiol.12, 53–69.
181
Mizrahi I. (2011). The role of the rumen microbiota in determining the feed efficiency of dairy cows, in Beneficial Microorganisms in Multicellular Life Forms. eds RosenbergE.GophnaU. (Berlin; Heidelberg: Springer Science & Business Media), 203–210.
182
Mizrahi I. (2013). Rumen Symbioses, in The Prokaryotes: Prokaryotic Biology and Symbiotic Associations. eds RosenbergE.DeLongE. F.LoryS.StackebrandtE.ThompsonF. (Berlin, Heidelberg: Springer Berlin Heidelberg), 533–544.
183
Morgavi D. P. Forano E. Martin C. Newbold C. J. (2010). Microbial ecosystem and methanogenesis in ruminants. Animal4, 1024–1036. 10.1017/S1751731110000546
184
Muñoz-Tamayo R. Giger-Reverdin S. Sauvant D. (2016). Mechanistic modelling of in vitro fermentation by rumen microbiota. Anim. Feed. Sci. Technol.220, 1–21. 10.1016/j.anifeedsci.2016.07.005
185
Muñoz-Tamayo R. Puillet L. Daniel J. B. Sauvant D. Martin O. Taghipoor M. et al . (2018). Review: To be or not to be an identifiable model. Is this a relevant question in animal science modelling?Animal12, 701–712. 10.1017/S1751731117002774
186
Muth T. Behne A. Heyer R. Kohrs F. Benndorf D. Hoffmann M. et al . (2015). The metaproteomeanalyzer: a powerful open-Source software suite for metaproteomics data analysis and interpretation. J. Proteome. Res.14, 1557–1565. 10.1021/pr501246w
187
Newbold C. El Hassan S. Wang J. Ortega M. Wallace R. (1997). Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Brit. J. Nutr.78, 237–249.
188
Newbold C. J. De La Fuente G. Belanche A. Ramos-Morales E. Mcewan N. R. (2015). The role of ciliate protozoa in the rumen. Front. Microbiol.6:1313. 10.3389/fmicb.2015.01313
189
Newbold C. J. Wallace R. Mcintosh F. (1996). Mode of action of the yeast Saccharomyces cerevisiae as a feed additive for ruminants. Brit. J. Nutr.76, 249–226. 10.1079/BJN19960029
190
Nobu M. K. Narihiro T. Rinke C. Kamagata Y. Tringe S. G. Woyke T. et al . (2015). Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor. ISME J.9, 1710–1722. 10.1038/ismej.2014.256
191
O'callaghan T. Vázquez-Fresno R. Serra-Cayuela A. Dong E. Mandal R. Hennessy D. et al . (2018). Pasture feeding changes the bovine rumen and milk metabolome. Metabolites.8:27. 10.3390/metabo8020027
192
Orpin C. G. (1974). The rumen flagellate Callimastix frontalis: does sequestration occur?J. Gen. Microbiol.84, 395–398.
193
Orpin C. G. (1977a). The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 99, 107–117.
194
Orpin C. G. (1977b). Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J Gen Microbiol. 98, 423–430.
195
Oyama L. B. Girdwood S. E. Cookson A. R. Fernandez-Fuentes N. Prive F. Vallin H. E. et al . (2017). The rumen microbiome: an underexplored resource for novel antimicrobial discovery. NPJ Biofilms Microbiomes 3;33. 10.1038/s41522-017-0042-1
196
Parks D. H. Imelfort M. Skennerton C. T. Hugenholtz P. Tyson G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res.25, 1043–1055. 10.1101/gr.186072.114
197
Parks D. H. Rinke C. Chuvochina M. Chaumeil P. A. Woodcroft B. J. Evans P. N. et al . (2017). Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol.2, 1533–1542. 10.1038/s41564-017-0012-7
198
Patejko M. Jacyna J. Markuszewski M. J. (2017). Sample preparation procedures utilized in microbial metabolomics: an overview. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci.1043, 150–157. 10.1016/j.jchromb.2016.09.029
199
Patra A. K. (2010). Meta-analyses of effects of phytochemicals on digestibility and rumen fermentation characteristics associated with methanogenesis. J. Sci. Food Agri.90, 2700–2708. 10.1002/jsfa.4143
200
Patra A. K. Saxena J. (2009a). Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Anton. Van Leeuwen.96, 363–375. 10.1007/s10482-009-9364-1
201
Patra A. K. Saxena J. (2009b). The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev.22, 204–219. 10.1017/S0954422409990163
202
Patti G. J. Yanes O. Siuzdak G. (2012). Metabolomics: the apogee of the omic triology. Nat. Rev. Mol. Cell Biol.13, 263–269. 10.1038/nrm3314
203
Paul S. S. Bu D. Xu J. Hyde K. D. Yu Z. (2018). A phylogenetic census of global diversity of gut anaerobic fungi and a new taxonomic framework. Fung. Divers.89, 253–266. 10.107/s13225-018-0396-6
204
Paul S. S. Kamra D. N. Sastry V. R. B. Sahu N. P. Agarwal N. (2004). Effect of administration of an anaerobic gut fungus isolated from wild blue bull (Boselaphus tragocamelus) to buffaloes (Bubalus bubalis) on in vivo ruminal fermentation and digestion of nutrients. Anim. Feed Sci. Technol.115, 143–157. 10.1016/j.anifeedsci.2004.01.010
205
Pereira F. C. Berry D. (2017). Microbial nutrient niches in the gut. Environ. Microbiol.19, 1366–1378. 10.1111/1462-2920.13659
206
Pinares-Patiño C. S. Ebrahimi S. H. McEwan J. C. Dodds K. G. Clark H. Luo D. (2011). Is rumen retention time implicated in sheep differences in methane emission?Proc. N. Z. Soc. Anim. Product.71, Invercargill219–222.
207
Pinares-Patiño C. S. Hickey S. M. Young E. A. Dodds K. G. Maclean S. Molano G. et al . (2013). Heritability estimates of methane emissions from sheep. Animal7, 316–321. 10.1017/S1751731113000864
208
Pinares-Patiño C. S. Ulyatt M. J. Lassey K. R. Barry T. N. Holmes C. W. (2003). Rumen function and digestion parameters associated with differences between sheep in methane emissions when fed chaffed lucerne hay. J. Agri. Sci.140, 205–214. 10.1017/S0021859603003046
209
Poelaert C. Nollevaux G. Boudry C. Taminiau B. Nezer C. Daube G. et al . (2018). Reducing agent can be omitted in the incubation medium of the batch in vitro fermentation model of the pig intestines. Animal12, 1154–1164. 10.1017/S1751731117002749
210
Pollock J. Glendinning L. Wisedchanwet T. Watson M. (2018). The madness of microbiome: attempting to find consensus ‘Best practice’ for 16S microbiome studies. Appl. Environ. Microbiol.84, e02627–e02617. 10.1128/AEM.02627-17
211
Pope P. B. Smith W. Denman S. E. Tringe S. G. Barry K. Hugenholtz P. et al . (2011). Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies. Science333:646. 10.1126/science.1205760
212
Popova M. Mcgovern E. Mccabe M. S. Martin C. Doreau M. Arbre M. et al . (2017). The structural and functional capacity of ruminal and cecal microbiota in growing cattle was unaffected by dietary supplementation of linseed oil and nitrate. Front. Microbiol.8:937. 10.3389/fmicb.2017.00937
213
Popova M. Morgavi D. P. Martin C. (2013). Methanogens and methanogenesis in the rumens and ceca of lambs fed two different high-grain-content diets. Appl. Environ. Microbiol.79, 1777–1786. 10.1128/AEM.03115-12
214
Press M. O. Wiser A. H. Kronenberg Z. N. Langford K. W. Shakya M. Lo C.-C. et al . (2017). Hi-C deconvolution of a human gut microbiome yields high-quality draft genomes and reveals plasmid-genome interactions. bioRxiv. 10.1101/198713
215
Puniya A. K. Salem A. Z. M. Kumar S. Dagar S. S. Griffith G. W. Puniya M. et al . (2015). Role of live microbial feed supplements with reference to anaerobic fungi in ruminant productivity: a review. J. Integr. Agri.14, 550–560. 10.1016/S2095-3119(14)60837-6
216
Qian C. Hettich R. L. (2017). Optimized extraction method to remove humic acid interferences from soil samples prior to microbial proteome measurements. J. Proteome Res.16, 2537–2546. 10.1021/acs.jproteome.7b00103
217
Ramos-Morales E. De La Fuente G. Duval S. Wehrli C. Bouillon M. Lahmann M. et al . (2017). Antiprotozoal effect of saponins in the rumen can be enhanced by chemical modifications in their structure. Front. Microbiol.8:399. 10.3389/fmicb.2017.00399
218
Rey M. Enjalbert F. Combes S. Cauquil L. Bouchez O. Montelis V. (2014). Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol.116, 245–257. 10.1111/jam.12405
219
Rezaeian M. Beakes G. W. Parker D. S. (2004). Distribution and estimation of anaerobic zoosporic fungi along the digestive tracts of sheep. Mycol. Res.108, 1227–1233.
220
Roehe R. Dewhurst R. J. Duthie C. A. Rooke J. A. Mckain N. Ross D. W. et al . (2016). Bovine host genetic variation influences rumen microbial methane production with best selection criterion for low methane emitting and efficiently feed converting hosts based on metagenomic gene aqbundance. PloS Genet.12:20. 10.1371/journal.pgen.1005846
221
Roumpeka D. D. Wallace R. J. Escalettes F. Fotheringham I. Watson M. (2017). A Review of bioinformatics tools for bio-prospecting from metagenomic sequence data. Front. Genet.8:23. 10.3389/fgene.2017.00023
222
Rubino F. Carberry C. Waters S. M. Kenny D. Mccabe M. S. Creevey C. J. (2017). Divergent functional isoforms drive niche specialisation for nutrient acquisition and use in rumen microbiome. ISME J.11:1510. 10.1038/ismej.2016.172
223
Russell J. B. Houlihan A. J. (2003). Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev.27, 65–74. 10.1016/S0168-6445(03)00019-6
224
Russell J. B. Strobel H. J. (1993). Microbial energetics, in Quantitative Aspects of Ruminant Digestion and Metabolism, eds FobesJ. M.FranceJ. (Wallingford: CAB International).
225
Saleem F. Ametaj B. N. Bouatra S. Mandal R. Zebeli Q. Dunn S. M. et al . (2012). A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J. Dairy Sci.95, 6606–6623. 10.3168/jds.2012-5403
226
Saleem F. Bouatra S. Guo A. C. Psychogios N. Mandal R. Dunn S. M. et al . (2013). The bovine ruminal fluid metabolome. Metabolomics9, 360–378. 10.1007/s11306-012-0458-9
227
Sasson G. Ben-Shabat S. K. Seroussi E. Doron-Faigenboim A. Shterzer N. Yaacoby S. et al . (2017). Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow's capacity to harvest energy from it's feed. Mbio8:12. 10.1128/mBio.00703-17
228
Saxena S. Sehgal J. P. Puniya A. K. Singh K. (2010). Effect of administration of rumen fungi on production performance of lactating buffaloes. Benef. Microbes1, 183–188. 10.3920/BM2009.0018
229
Schelling G. T. (1984). Monensin mode of action in the rumen. J. Anim. Sci.58, 1518–1527.
230
Schooling S. R. Beveridge T. J. (2006). Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol.188, 5945–5957. 10.1128/JB.00257-06
231
Schooling S. R. Hubley A. Beveridge T. J. (2009). Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol.191, 4097–4102. 10.1128/JB.00717-08
232
Seedorf H. Kittelmann S. Henerson G. Janssen P. H. (2014). RIM-DB: a taxonomic framework for community structure analysis of methanogenic archaea from the rumen and other intestinal environments. Peer J.2:e494. 10.7717/peerj.494
233
Seshadri R. Leahy S. C. Attwood G. T. Teh K. H. Lambie S. C. Cookson A. L. et al . (2018). Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotech.36:359. 10.1038/nbt.4110
234
Shaani Y. Zehavi T. Eyal S. Miron J. Mizrahi I. (2018). Microbiome niche modification drives diurnal rumen community assembly, overpowering individual variability and diet effects. ISME J. 10.1038/s41396-018-0203-0. [Epub ahead of print].
235
Shabat S. K. Sasson G. Doron-Faigenboim A. Durman T. Yaacoby S. Berg Miller M. E. et al . (2016). Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J.10, 2958–2972. 10.1038/ismej.2016.62
236
Shanks O. C. Kelty C. A. Archibeque S. Jenkins M. Newton R. J. Mclellan S. L. et al . (2011). Community structures of fecal bacteria in cattle from different animal feeding operations. Appl. Environ. Microbiol.77, 2992–3001. 10.1128/AEM.02988-10
237
Shi W. Moon C. D. Leahy S. C. Kang D. Froula J. Kittelmann S. et al . (2014). Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Res.24, 1517–1525. 10.1101/gr.168245.113
238
Shlomi T. Cabili M. N. Herrgard M. J. Palsson B. O. Ruppin E. (2008). Network-based prediction of human tissue-specific metabolism. Nat. Biotechnol.26, 1003–1010. 10.1038/nbt.1487
239
Shukla S. K. Rao T. S. (2017). Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian J. Med. Res.146, S1–S8. 10.4103/ijmr.IJMR_410_15
240
Signorini M. L. Soto L. P. Zbrun M. V. Sequeira G. J. Rosmini M. R. Frizzo L. S. (2012). Impact of probiotic administration on the health and fecal microbiota of young calves: a meta-analysis of randomized controlled trials of lactic acid bacteria. Res. Vet. Sci.93, 250–258. 10.1016/j.rvsc.2011.05.001
241
Smillie C. S. Smith M. B. Friedman J. Cordero O. X. David L. A. Alm E. J. (2011). Ecology drives a global network of gene exchange connecting the human microbiome. Nature480, 241–244. 10.1038/nature10571
242
Snelling T. J. Wallace R. J. (2017). The rumen microbial metaproteome as revealed by SDS-PAGE. BMC Microbiol.17:9. 10.1186/s12866-016-0917-y
243
Solomon K. V. Haitjema C. H. Henske J. K. Gilmore S. P. Borges-Rivera D. Lipzen A. et al . (2016). Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science351, 1192–1195. 10.1126/science.aad1431
244
Song Y. Malmuthuge N. Steele M. A. Guan L. L. (2018). Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol. Ecol.94:fix179. 10.1093/femsec/fix179
245
Stenkamp-Strahm C. Mcconnel C. Magzamen S. Abdo Z. Reynolds S. (2018). Associations between Escherichia coli O157 shedding and the faecal microbiota of dairy cows. J. Appl. Microbiol.124, 881–898. 10.1111/jam.13679
246
Stewart E. J. (2012). Growing Unculturable Bacteria. J. Bacteriol.194, 4151–4160. 10.1128/JB.00345-12
247
Stewart R. D. Auffret M. D. Warr A. Wiser A. H. Press M. O. Langford K. W. et al . (2018). Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Comm.9:870. 10.1038/s41467-018-03317-6
248
Sugimoto S. Sato F. Miyakawa R. Chiba A. Onodera S. Hori S. et al . (2018). Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep.8:2254. 10.1038/s41598-018-20485-z
249
Sun H.-Z. Wang D.-M. Wang B. Wang J.-K. Liu H.-Y. Guan L. L. et al . (2015). Metabolomics of four biofluids from dairy cows: potential biomarkers for milk production and quality. J. Proteome Res.14, 1287–1298. 10.1021/pr501305g
250
Svartstrom O. Alneberg J. Terrapon N. Lombard V. De Bruijn I. Malmsten J. et al . (2017). Ninety-nine de novo assembled genomes from the moose (Alces alces) rumen microbiome provide new insights into microbial plant biomass degradation. ISME J.11, 2538–2551. 10.1038/ismej.2017.108
251
Tapio I. Shingfield K. J. Mckain N. Bonin A. Fischer D. Bayat A. R. et al . (2016). Oral Samples as non-invasive proxies for assessing the composition of the rumen microbial community. PLoS ONE11:e0151220. 10.1371/journal.pone.0151220
252
Tapio I. Snelling T. J. Strozzi F. Wallace R. J. (2017). The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol.8:11. 10.1186/s40104-017-0141-0
253
Thomas M. Webb M. Ghimire S. Blair A. Olson K. Fenske G. J. et al . (2017). Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep.7:12257. 10.1038/s41598-017-12481-6
254
Timmerman H. M. Mulder L. Everts H. van Espen D. C. van der Wal E. Klaassen G. et al . (2005). Health and growth of veal calves fed milk replacers with or without probiotics. J. Dairy Sci.88, 2154–2165. 10.3168/jds.S0022-0302(05)72891-5
255
Tripathi V. K. Sehgal J. P. Puniya A. K. Singh K. (2007). Effect of administration of anaerobic fungi isolated from cattle and wild blue bull (Boselaphus tragocamelus) on growth rate and fibre utilization in buffalo calves. Arch. Anim. Nutr.61, 416–423. 10.1080/17450390701556759
256
Tyson G. W. Chapman J. Hugenholtz P. Allen E. E. Ram R. J. Richardson P. M. et al . (2004). Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature428, 37–43. 10.1038/nature02340
257
United Nations (2015). World Population Prospects. Available online at: http://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html
258
Vaidya J. D. van den Bogert B. Edwards J. E. Boekhorst J. van Gastelen S. Saccenti E. et al . (2018). The effect of DNA extraction methods on observed microbial communities from fibrous and liquid rumen fractions of dairy cows. Front. Microbiol.9:92. 10.3389/fmicb.2018.00092
259
Van Amburgh M. E. Collao-Saenz E. A. Higgs R. J. Ross D. A. Recktenwald E. B. Raffrenato E. et al . (2015). The cornell net carbohydrate and protein system: updates to the model and evaluation of version 6.5. J. Dairy Sci.98, 6361–6380. 10.3168/jds.2015-9378
260
Van Nimwegen F. A. Penders J. Stobberingh E. E. Postma D. S. Koppelman G. H. Kerkhof M. et al . (2011). Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol.128, 948–955.e943. 10.1016/j.jaci.2011.07.027
261
Varma A. Boesch B. W. Palsson B. O. (1993). Stoichiometric interpretation of Escherichia coli glucose catabolism under various oxygenation rates. Appl. Environ. Microbiol.59, 2465–2473.
262
Varma A. Palsson B. O. (1994). Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl. Environ. Microbiol.60, 3724–3731.
263
Vogels G. D. Hoppe W. F. Stumm C. K. (1980). Association of methanogenic bacteria with rumen ciliates. Appl. Environ. Microbiol.40, 608–612.
264
Wallace R. J. (2008). Gut microbiology – broad genetic diversity, yet specific metabolic niches. Animal2, 661–668. 10.1017/S1751731108001687
265
Wallace R. J. Onodera R. Cotta M. A. (1997). Metabolism of nitrogen-containing compounds, in The rumen microbial ecosystem, eds HobsonP. N.StewartC. S. (New York, NY: Chapman & Hall), 283–328.
266
Wallace R. J. Rooke J. A. Duthie C.-A. Hyslop J. J. Ross D. W. Mckain N. et al . (2014). Archaeal abundance in post-mortem ruminal digesta may help predict methane emissions from beef cattle. Sci. Rep.4:5892. 10.1038/srep05892
267
Wallace R. J. Rooke J. A. Mckain N. Duthie C.-A. Hyslop J. J. Ross D. W. et al . (2015). The rumen microbial metagenome associated with high methane production in cattle. BMC Genomics.16:839. 10.1186/s12864-015-2032-0
268
Wang J. Fan H. Han Y. Zhao J. Z. Zhou Z. J. (2017). Characterization of the microbial communities along the gastrointestinal tract of sheep by 454 pyrosequencing analysis. Asian-Aus. J. Anim. Sci.30, 100–110. 10.5713/ajas.16.0166
269
Wang M. Carver J. J. Phelan V. V. Sanchez L. M. Garg N. Peng Y. et al . (2016). Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol.34:828. 10.1038/nbt.3597
270
Wang O. Mcallister T. A. Plastow G. Stanford K. Selinger B. Guan L. L. (2018). Interactions of the hindgut mucosa-associated microbiome with its host regulate shedding of Escherichia coli O157:H7 by cattle. Appl. Environ. Microbiol.84:e01738–17. 10.1128/AEM.01738-17
271
Wang P. Zhao S. Wang X. Zhang Y. Zheng N. Wang J. (2016). Ruminal methanogen community in dairy cows fed agricultural residues of corn stover, rapeseed, and cottonseed meals. J. Agric. Food Chem.64, 5439–5445. 10.1021/acs.jafc.6b00708
272
Weimer P. J. (2015). Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front. Microbiol.6:296. 10.3389/fmicb.2015.00296
273
Weimer P. J. Cox M. S. Vieira De Paula T. Lin M. Hall M. B. Suen G. (2017). Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci.100, 7165–7182. 10.3168/jds.2017-12746
274
Weimer P. J. Stevenson D. M. Mantovani H. C. Man S. L. (2010). Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. J. Dairy Sci.93, 5902–5912. 10.3168/jds.2010-3500
275
WFP (2015). World Food Programme Hunger Map. Rome, Italy.
276
Wilkinson T. J. Huws S. A. Edwards J. E. Kingston-Smith A. H. Siu-Ting K. Hughes M. et al . (2018). CowPI: a rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol.9:1095. 10.3389/fmicb.2018.01095
277
Williams A. G. Coleman G. S. (1992). The Rumen Protozoa. New York, NY: Springer-Verlag.
278
Williams A. G. Coleman G. S. (1997). The rumen protozoa, in The Rumen Microbial Ecosystem, eds HobsonP. N.StewartC. S. (Dordrecht: Springer).
279
Wilmes P. Bond P. L. (2004). The application of two-dimensional polyacrylamide gel electrophoresis and downstream analyses to a mixed community of prokaryotic microorganisms. Environ. Microbiol.6, 911–920. 10.1111/j.1462-2920.2004.00687.x
280
Wolin M. J. Miller T. L. Stewart C. S. (1997). Microbe-microbe interactions, in The Rumen Microbial Ecosystem, eds HobsonP. N.StewartC. S. (Dordrecht: Springer), 467–491.
281
Wright A-D. G. Klieve A. V. (2011). Does the complexity of the rumen microbial ecology preclude methane mitigation. Anim. Feed Sci. Technol.166–167, 248–253. 10.1016/j.anifeedsci.2011.04.015
282
Wu L. Wang H. Zhang Z. Lin R. Zhang Z. Lin W. (2011). Comparative metaproteomic analysis on consecutively rehmannia glutinosa-monocultured rhizosphere soil. PLoS ONE.6:e20611. 10.1371/journal.pone.0020611
283
Yáñez-Ruiz D. R. Abecia L. Newbold C. J. (2015). Manipulating rumen microbiome and fermentation through interventions during early life: a review. Front. Microbiol.6:1133. 10.3389/fmicb.2015.01133
284
Yáñez-Ruiz D. R. Macías B. Pinloche E. Newbold C. J. (2010). The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiol. Ecol.72, 272–278. 10.1111/j.1574-6941.2010.00852.x
285
Yeoman C. J. Ishaq S. L. Bichi E. Olivo S. K. Lowe J. Aldridge B. M. (2018). Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci. Rep.8:3197. 10.1038/s41598-018-21440-8
286
Yi L. Shi S. Wang Y. Huang W. Xia Z.-A. Xing Z. et al . (2016). Serum metabolic profiling reveals altered metabolic pathways in patients with post-traumatic cognitive impairments. Sci. Rep.6:21320. 10.1038/srep21320
287
Yu Z. Morrisson M. (2004a). Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol.70, 4800–4806. 10.1128/AEM.70.8.4800-4806.2004
288
Yu Z. Morrisson M. (2004b). Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques36, 808–812. 10.2144/04365ST04
289
Zeng Y. Zeng D. Ni X. Zhu H. Jian P. Zhou Y. et al . (2017). Microbial community compositions in the gastrointestinal tract of Chinese Mongolian sheep using Illumina MiSeq sequencing revealed high microbial diversity. AMB Express.7:75. 10.1186/s13568-017-0378-1
290
Zengler K. Palsson B. O. (2012). A road map for the development of community systems (CoSy) biology. Nat. Rev. Microbiol.10:366. 10.1038/nrmicro2763
291
Zengler K. Toledo G. Rappe M. Elkins J. Mathur E. J. Short J. M. et al . (2002). Cultivating the uncultured. Proc. Natl. Acad. Sci. U.S.A.99, 15681–15686. 10.1073/pnas.252630999
292
Zhang B. Horvath S. (2005). A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4:17. 10.2202/1544-6115.1128
293
Zhang J. Shi H. Wang Y. Li S. Cao Z. Ji S. et al . (2017). Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front. Microbiol.8:2206. 10.3389/fmicb.2017.02206
294
Zhang J. Zhao S. Zhang Sun P. Bu D. Wang J. (2015). New primers targetting full-length ciliate 18S rRNA genes and evaluation of dietary effect on rumen ciliate diversity in dairy cows. Curr. Microbiol.71, 650–657. 10.1007/s00284-015-0898-3
295
Zhang R. Zhu W. Jiang L. Mao S. (2017). Comparative metabolome analysis of ruminal changes in Holstein dairy cows fed low- or high-concentrate diets. Metabolomics13:74. 10.1007/s11306-017-1204-0
296
Zhao S. Li G. Zheng N. Wang J. Yu Z. (2018). Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresour. Technol.253, 244–251. 10.1016/j.biortech.2018.01.024
297
Zhao S. Zhao J. Bu D. Sun P. Wang J. Dong Z. (2014). Metabolomics analysis reveals large effect of roughage tpes on rumen microbial metabolic profilein dairy cows. Lett. Appl. Microbiol.59, 79–85. 10.1111/lam.12247
298
Zinn R. A. Owens F. N. (1986). A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Can. J. Anim. Sci.66, 157–166. 10.4141/cjas86-017
299
Zitnan R. Voigt J. Schonhusen U. Wegner J. Kokardova M. Hagemeister H. et al . (1998). Influence of dietary concentrate to forage ratio on the development of rumen mucosa in calves. Arch. Tierernahr.51, 279–291.
Summary
Keywords
rumen, microbiome, host, diet, production, methane, omics
Citation
Huws SA, Creevey CJ, Oyama LB, Mizrahi I, Denman SE, Popova M, Muñoz-Tamayo R, Forano E, Waters SM, Hess M, Tapio I, Smidt H, Krizsan SJ, Yáñez-Ruiz DR, Belanche A, Guan L, Gruninger RJ, McAllister TA, Newbold CJ, Roehe R, Dewhurst RJ, Snelling TJ, Watson M, Suen G, Hart EH, Kingston-Smith AH, Scollan ND, do Prado RM, Pilau EJ, Mantovani HC, Attwood GT, Edwards JE, McEwan NR, Morrisson S, Mayorga OL, Elliott C and Morgavi DP (2018) Addressing Global Ruminant Agricultural Challenges Through Understanding the Rumen Microbiome: Past, Present, and Future. Front. Microbiol. 9:2161. doi: 10.3389/fmicb.2018.02161
Received
31 May 2018
Accepted
23 August 2018
Published
25 September 2018
Volume
9 - 2018
Edited by
Zhongtang Yu, The Ohio State University, United States
Reviewed by
Shengguo Zhao, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, China; Suzanne Lynn Ishaq, University of Oregon, United States
Updates
Copyright
© 2018 Huws, Creevey, Oyama, Mizrahi, Denman, Popova, Muñoz-Tamayo, Forano, Waters, Hess, Tapio, Smidt, Krizsan, Yáñez-Ruiz, Belanche, Guan, Gruninger, McAllister, Newbold, Roehe, Dewhurst, Snelling, Watson, Suen, Hart, Kingston-Smith, Scollan, do Prado, Pilau, Mantovani, Attwood, Edwards, McEwan, Morrisson, Mayorga, Elliott and Morgavi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharon A. Huws s.huws@qub.ac.uk
This article was submitted to Microbial Symbioses, a section of the journal Frontiers in Microbiology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.