- 1School of Life Sciences and Engineering, Southwest University of Science and Technology, Mianyang, China
- 2National Co-Innovation Center for Nuclear Waste Disposal and Environmental Safety, Southwest University of Science and Technology, Mianyang, China
- 3State Key Laboratory of NBC Protection for Civilian, Beijing, China
- 4Sichuan Institute of Atomic Energy, Chengdu, China
- 5Institute of Biochemistry, University of Balochistan, Quetta, Pakistan
This study investigated the influence of heavy metals on bacterial community structure in a uranium mine. Soils from three differently polluted ditches (Yangchang ditch, Zhongchang ditch, and Sulimutang ditche) were collected from Zoige County, Sichuan province, China. Soil physicochemical properties and heavy metal concentrations were measured. Differences between bacterial communities were investigated using the high-throughput sequencing of the 16S rRNA genes. The obtained results demonstrated that bacterial richness index (Chao and Ace) were similar among three ditches, while the highest bacterial diversity index was detected in the severely contaminated soils. The compositions of bacterial communities varied among three examined sites, but Proteobacteria and Acidobacteria were abundant in all samples. Redundancy analysis revealed that soil organic matter, Cr and pH were the three major factors altering the bacterial community structure. Pearson correlation analysis indicated that the most significant correlations were observed between the contents of non-residual Cr and the abundances of bacterial genera, including Thiobacillus, Nitrospira, and other 10 genera. Among them, the abundances of Sphingomonas and Pseudomonas were significant and positively correlated with the concentrations of non-residual U and As. The results highlighted the factors influencing the bacterial community in uranium mines and contributed a better understanding of the effects of heavy metals on bacterial community structure by considering the fraction of heavy metals.
Introduction
With the rapid development of nuclear technology, uranium mining, and smelting activities have increased. Activities associated with mining have generated enormous waste materials containing uranium and other toxic metals (Ilton et al., 2008). Uranium is considered one of the most hazardous pollutants among metals, due to its chemical and radiological toxicities (Qi et al., 2019). Uranium occurs environmentally in two oxidation states: the oxidized form U (VI) and the reduced form U (IV). The strong oxidizing power of U (VI) shows a predominance of chemical toxicity. Oxidative DNA damage causes long-term genotoxic effects in the form of mutagenesis, carcinogenesis, and other pathologies (Gudkov et al., 2016). It is of great importance to study the influence of uranium pollution on soil ecological environment.
Microorganisms are always considered the sensitive indicators of environmental stress (Giller et al., 2009; Bano et al., 2018). In the soil ecosystem, microorganisms play important roles in energy flow, nutrient cycling, and organic matter recycling (Chodak et al., 2013). They may act as a nutrient source in soils and participate in the humification processes, degradation of pollutants, and maintenance of soil structure (Verstraete and Top, 1999; Preston et al., 2001). A well-functioning microbial community is a precondition for soil fertility. Research on microbial communities in uranium mines will not only help in understanding the habituating features of microbes in heavy metals and radioactive environments but will also elaborate the possibilities of bioremediation of uranium contaminated soil (Chen et al., 2012). Mondani et al. (2011) studied the effect of uranium on bacterial communities and the results indicated that sequences related to iron-reducing bacteria and iron-oxidizing species are specific to uraniferous samples. Kenarova et al. (2014) found that the physiological diversity of bacteria was site specific in three abandoned mines. An investigation of uranium mill tailings revealed that Proteobacteria was the most dominant flora (Yan and Luo, 2015). These reports help us to understand microbial community structures under the stress of a uranium mine. The existing information in literature about the effect of heavy metals and uranium on soil microorganisms is limited. Meanwhile, the total contents of heavy metals may not necessarily be an indicator reflecting adverse effects on microbiomes, because the toxic effects are dependent not only dependent on the intrinsic toxicity and concentration of contaminant but also on geochemical phases in which it is present in soil (Olaniran et al., 2017). The geochemical forms of heavy metals are varied in natural environment, including exchangeable, carbonate, Fe–Mn bound, organic, and residual fractions (Tessier et al., 1979; Neto et al., 2016; Palleiro et al., 2016). These forms are directly related to the interactions process, bioavailability, transfer behaviors and potential toxicity of many metals (Najamuddin et al., 2016). Exchangeable forms can be easily absorbed by microorganisms, while carbonate, Fe–Mn bound and organic fractions can be translated into activated forms as a result of changes in some conditions of soil environment, like pH, salinity, and redox potential (Ma et al., 2016; Huang et al., 2017). In contrast, residual fractions appear to be biologically inactive since they are embedded into the crystalline lattices of the soil clay (Palleiro et al., 2016). Estimations of heavy metal contents in various geochemical fractions are helpful for evaluating the effects of heavy metals on microorganisms.
In recent years, the next-generation sequencing techniques have widely been used for investigating the complexity of microbiomes (Kuppusamy et al., 2016; Li X. et al., 2017; Narendrula-Kotha and Nkongolo, 2017), such as Illumina sequencing of 16S rRNA amplicons can provide much higher resolution approaches studying the phylogenetic composition of microbial communities and obtaining an enormous number of sequences in a short time (Fierer et al., 2012). This method is very useful for exploring the relationships between contents of heavy metals and diversity and abundances of soil microbial communities. In this work we investigated the structure of indigenous bacterial community in uranium mine area using the 16S rRNA gene amplicon sequencing. We collected samples from contaminated fields located in Zoige County in Sichuan Province of China. Soils at these sites were polluted, mostly with uranium and heavy metals, as a result of the exploitation of mining. The main objectives of the study were to characterize the diversity and composition of bacterial communities in uranium mine and to evaluate the relationships between the contents of contaminants and bacterial community structures. This research can provide a deep understanding of microbiomes revealed by high-throughput sequencing in polymetallic and uranium contaminated soils.
Materials and Methods
Sampling of Soils Contaminated With Uranium and Heavy Metals
The significant part of Zoige uranium mine is located in Zoige County in Sichuan province and the rest of mine extends to the east, to Diebu County in Gansu province of China. The ore belt is developed from west to east and is accompanied by heavy metals. The total length of the ore belt is approximately 50 km, with a width of approximately 10 km wide. It consists of mine deposits of different sizes. This mining was explored during 1970–1990s and has been abandoned from approximately 20 years (Yang et al., 2018). Soils of the region have been seriously polluted by numerous metals due to mining activities. The samples were collected from the Zoige mine field (102°45′–102°46′E, 34°12′N–34°13′N) in April 2014 along with the Yangchang (DY1, DY2, and DY3), Zhongchang (DZ1, DZ2, and DZ3), and Sulimutang (DS1, DS2, and DS3) ditches. DZ represents the sample collected from a site close to the center of the mine. DY was a mineral-leaching area. DS was far away region from mine, which were slightly contaminated with heavy metals. The elevation of the mine is approximately 3300–3700 m; DZ and DS have the highest and lowest average elevation, respectively. All the samples were collected and labeled according to their flow direction of flow from upstream to downstream. The sampling points were first mapped (Geographic Information System location), and a total of nine points were selected (Figure 1). Top soil from the 5–20 cm layer was collected. Sampling at the 2 m2 area of each site and each sample was comprised of five subsamples. The subsamples were well-mixed after removing large stones and plant residues. The soil samples were sealed in plastic bags, immediately moved to the laboratory in cooling boxes. These samples were separated into two parts. One part was stored in −80°C freezer for molecular analysis. And the other part was stored at 4°C for chemical analysis.
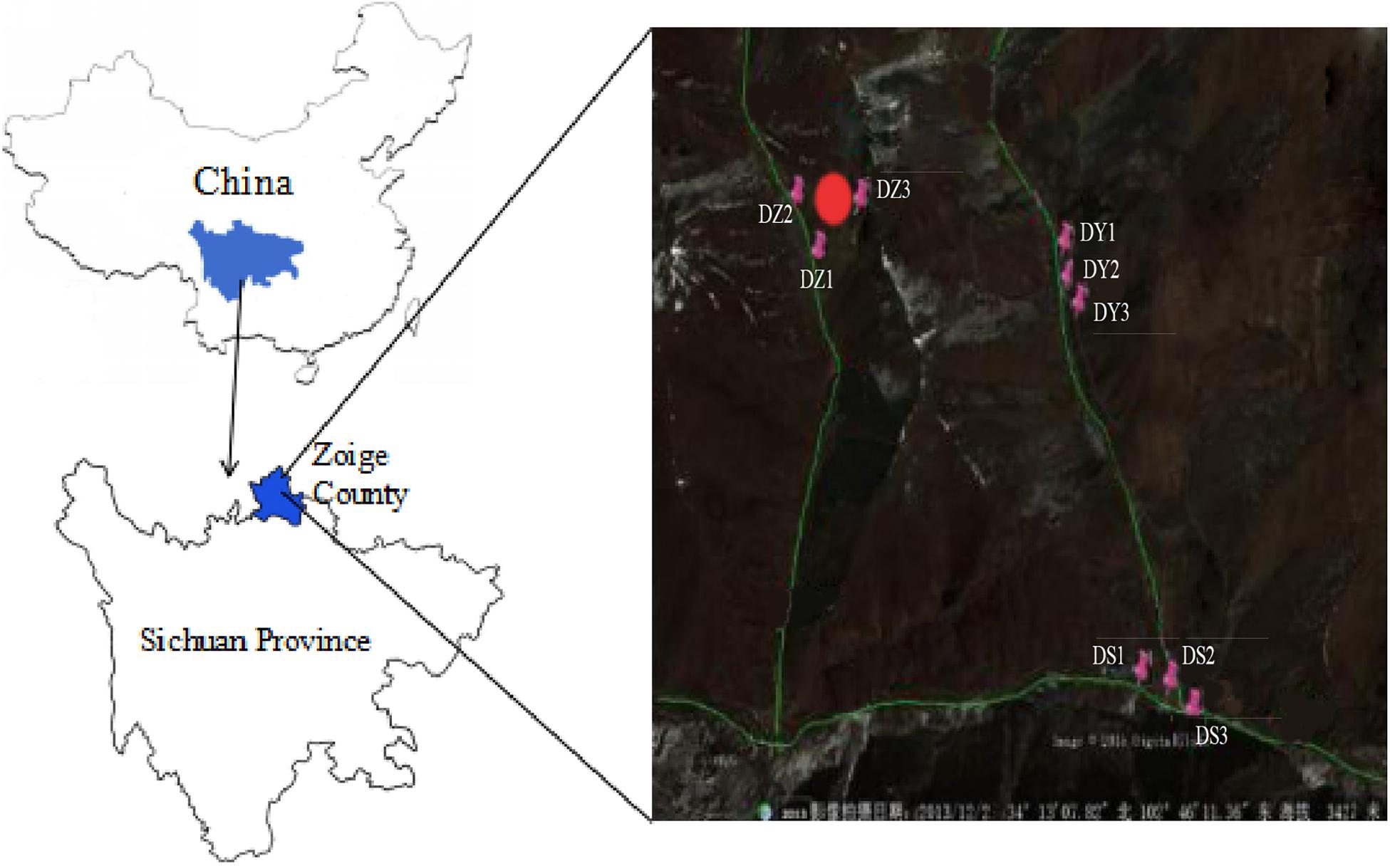
Figure 1. Location of samples in the mine area. “DZ,” soil samples in ditch Z; “DY,” soil samples in ditch Y; “DS,” soil samples in ditch S. The red mark represents the sampling sites. The three green lines represent the three ditches, with DZ on the left, DY on the right, and DS at the bottom. The direction of flow was from top to bottom and left to right (©2019 satellite map).
Total Content and Speciation of Uranium and Heavy Metals in Soils
Soil moisture content was dried at 105°C for 24 h. Soil pH was determined using a calibrated pH meter (soil/solution ratio of 1:2.5). Soil organic matter (SOM) was tested by the K2Cr2O7 colorimetric method. The five-step sequential extraction method was conducted to determine the speciations of heavy metals in soil samples (Tessier et al., 1979). Five fractions were determined including exchangeable, carbonate, Fe–Mn bound, organic and residual fractions. Details of extraction process can be found in several references (Tessier et al., 1979; Buccolieri et al., 2004; Xiao et al., 2015). The contents of heavy metals in extracted solution were analyzed by the inductively coupled plasma atomic emission spectrometry (ICP-AES, ICAP6500, Thermo Scientific). Total contents of heavy metals were also determined by ICP-AES after soil samples were digested in the HClO4-HNO3-HF mixture in Teflon tubes.
Heavy metals are the natural component of soil. It is very important to distinguish between the natural background values and anthropogenic inputs. To evaluate the soil contamination rate, the geo-accumulation index Igeo) can be applied. This index was used to assess the presence and intensity of anthropogenic contamination deposition on soil. It can be calculated according to the following formula:
Cn is the measured content of metal n, and Bn is the geological background value of metal n. Factor 1.5 is the background matrix correlation factor due to lithospheric effects. The background value for As, Cr, Mo, Sr, V, Zn, and U is 11, 61, 2.0, 166, 82, 74, and 3.0 mg kg–1, respectively (Chen et al., 1991). The Igeo of each heavy metal can be classified as: unpolluted (Igeo ≤ 0); weakly polluted (0 < Igeo ≤ 1); moderately polluted (1 < Igeo ≤ 2); moderately to heavily polluted (2 < Igeo ≤ 3); heavily polluted (3 < Igeo ≤ 4); heavily to extremely polluted (4 < Igeo ≤ 5); extremely polluted (Igeo > 5) (Muller, 1981).
DNA Extraction, PCR Amplification, and Sequencing
The total microbial DNA was extracted from 0.5 g soil using a PowerSoil® DNA Isolation kit (Omega Bio-tek, Inc., Doraville, GA, United States) according to the manufacturer’s instructions. The extracted DNA was analyzed by 1% agar gel electrophoresis and used as a template for amplification. The average concentration of DNA in nine soil samples was 74.2 ng/l. The universal bacterial primer 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 338R (5′-TGCTG CCTCCCGTAGGAGT-3′) (Ravel et al., 2011) were used to amplify the hypervariable fragments (V1–V3) of the 16S rRNA. PCR amplification was performed using TransGen AP221-02:TransStart FastPfu DNA Polymerase (TransGen Biotech, Beijing, China) on an ABI GeneAmp® 9700 system, in triplicate assays. The PCR system included 4 μl 5× Buffer, 2 μl 2.5 mM dNTPs, 5 μM of each forward and reverse primer, 0.2 μl rTaq Polymerase 0.2 μl Bovine Serum Albumin, 10 ng of template DNA was added in ddH2O tp 20 μl. PCR procedure was set as: an initial denaturing step at 95°C for 3 min, followed by 35 cycles at 95°C (30 s), 55°C (30 s), 72°C (45 s) and a final extension step at 72°C for 10 min. Amplicons were extracted from 2% agarose gels and purified with an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) according to the manufacturer instructions and quantified using QuantiFluorTM -ST (Promega, United States). The sequencing library was constructed using mixture of 150 ng of each recovered PCR product. Sequencing were conducted on an Illumina Miseq platform by the Majorbio Bio-Pharm Technology, Co., Ltd. (Shanghai, China). The raw reads had been submitted to the NCBI and the accession numbers of sequences for DY1, DY2, DY3, DZ1, DZ2, DZ3, DS1, DS2, and DS3 was SRR9611297, SRR9611293, SRR9611298, SRR9611292, SRR9611295, SRR9603206, SRR9611294, SRR9611299, and SRR9611296.
Data Processing and Statistical Analyses
The raw data (FASTQ files) were quality-filtered using QIIME (version 1.9.1) (Caporaso et al., 2010) based on the following criteria: (a) 300 bp reads were truncated at any site with an average quality score < 20 over a 50 bp sliding window, and the truncated reads below 50 bp were discarded; (b) the exact barcodes were matched, two-nucleotide mismatch in primer matching and reads containing ambiguous base calls were removed and (c) only sequences with overlaps longer than 10 bp were merged together. Reads that could not be merged were discarded.
The filtered sequences were clustered into operational taxonomic units (OTUs) at 97% sequence similarity cut-off using Usearch (Version 7.1) (Edgar, 2010), and representative sequences were selected after the removal of putative chimeras. The most abundant sequence within each OTU was selected to represent the respective OTU in subsequent assays. The taxonomic identity of each phylotype was determined using RDP Classifier (Cole et al., 2009) with a 70% bootstrap score; the database selected was Silva (Release 128). Additionally, the richness indices Chao and ACE were evaluated with Mothur software (version 1.30.1) (Kozich et al., 2013). The Shannon and Simpson diversity index were also measured with alpha diversity analyses.
Detrended correspondence analysis (DCA) was used to identify the models. The results showed a linear model according to the length of the first axis (1.729). Based on the results of DCA, RDA was carried to determine which environmental factors exert significant impact on bacterial community structure, which were performed using Canoco software (version 4.5, for Windows). Hierarchical clustering analysis based on Bray-Curtis dissimilarity (Bray and Curtis, 1957) was performed by QIIME. One-way ANOVA analyses and Tukey test were applied to identify the significant differences in soil heavy metal content and diversity index among soil samples. Pearson correlation analysis was conducted to investigate correlation between heavy metals content and species richness (genus level) using SPSS version 17.0.
Results
Content of Uranium and Heavy Metals in Soil
Soil pH, moisture content (MC), SOM and concentration of heavy metals are shown in Table 1. The contents of SOM and MC in DS were significantly higher than those in DY and DZ. Soil pH was the highest in DS, but no significant difference was observed among three sites. Igeo was taken into consideration to assess the pollution level of heavy metals in this region, which takes the geochemical background value as a reference. Figure 2 shows that the Igeo value for Cr and Mo in all soil samples of DY, DZ, and DS were classified into moderately polluted category. Comparatively, V and Zn in all soils showed “weakly polluted” level according to the Igeo values. All negative Igeo values for Sr were obtained in soils, indicating an “unpolluted” level. For As and U, the pollution level was varied in different ditch. In DS, soil samples were moderately polluted by these two kinds of heavy metals. However, Igeo of As and U was higher than 2.0 in DY, indicating that soils in this ditch was moderately to heavily polluted by As and U. Considerably, DZ was heavily polluted by U according to Igeo value. Generally, the pollution levels in DY and DZ were relatively more serious than DS. The pollution level of determined metals followed the sequence As ≈ U > Mo > Cr > Zn > V > Sr.
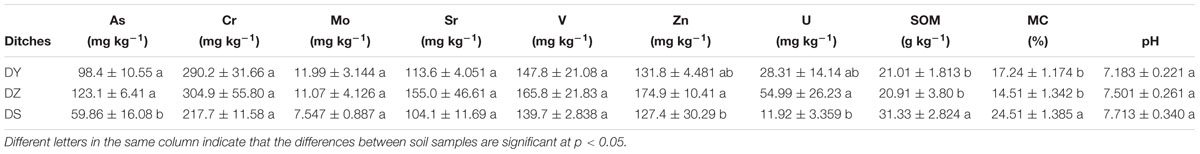
Table 1. Mean values of uranium and heavy metals contents in three soil samples collected from the same ditch (mean ± SE).
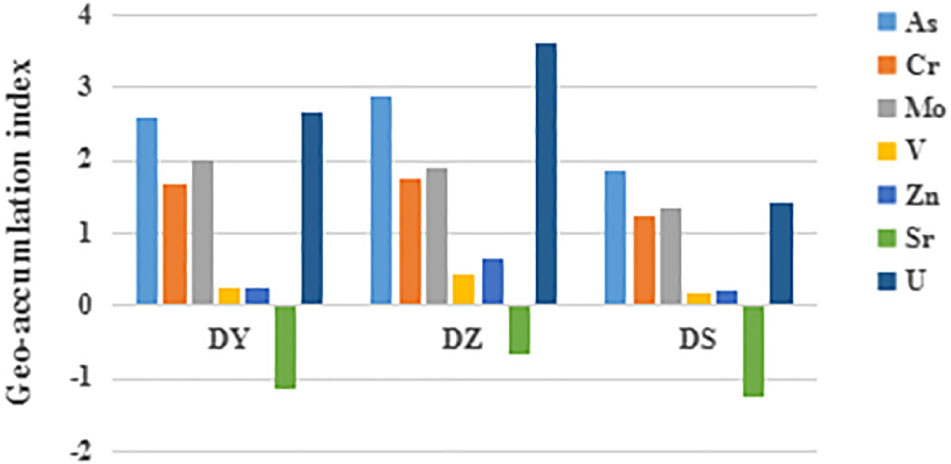
Figure 2. Geo-accumulation index (Igeo) of uranium and heavy metals in soil samples from different ditches. DY: Igeo of DY; DZ: Igeo of DZ; DS: Igeo of DS.
A well-established sequential extraction method (Tessier et al., 1979) was performed to investigate the fractional distribution of heavy metals in soil samples. The values of heavy metals pertaining to various geochemical phases of each site are shown in Figure 3 On average, all the studied heavy metals showed dominant levels in residual fractions, with mean value of 86.06, 71.37, 91.3, 75.93, 77.89, and 87.99% of the mean total concentration for As, Cr, Mo, V, Zn, and U, respectively. For Mo, V and U, the carbonate fraction was the second abundant form, accounting for 2.96, 6.72, and 3.64% of the mean total concentration, respectively. The mean proportions of Mo, V, and U in the exchangeable fraction were 2.59, 4.97, and 2.88%, while 1.67, 6.22, and 3.14% in the Fe–Mn bound fraction and 1.48, 6.16, and 2.34% in the organic fraction. The exchangeable was the most abundant form of non-residual Cr (8.9%). For As and Zn, Fe–Mn bound fraction was the most predominant form of the non-residual fraction.
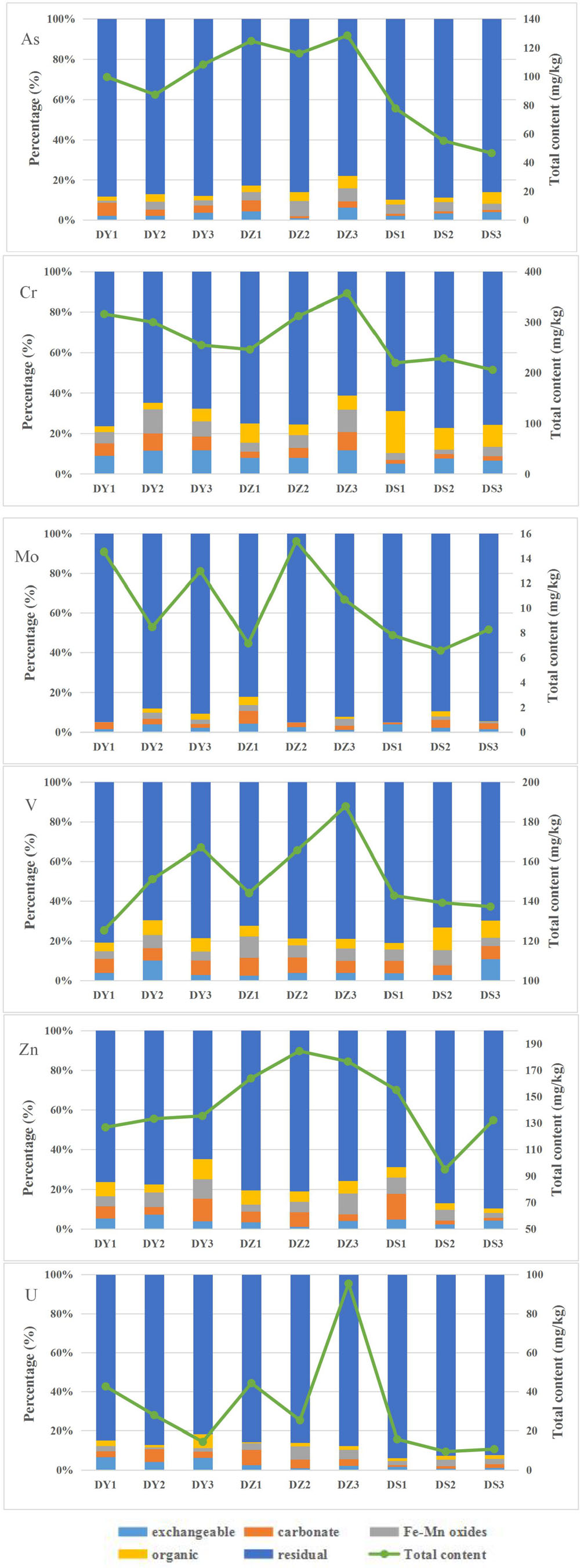
Figure 3. Uranium and heavy metals total contents and their distributions in different geochemical phases of the nine soil samples from the Zoige uranium mine, China.
Bacterial Community Structure
A total of 79363 high-quality 16S rRNA gene reads were obtained for all nine samples, with an average of 8818 (ranging from 5591 to 12111) reads per community. In total, 8384 OTUs were identified. The rarefaction curve (Supplementary Figure S1) reached saturation, indicating the sequencing depths were reasonable for further analysis. Bacterial alpha diversity was further estimated based on 97% OTUs. As shown in Table 2, the OTU number showed slight change in three areas. The Chao1 estimator and ACE were used to estimate the amounts of OTU, considering the number of singletons per sample, since they may suggest additional undetected phylotypes (Chao, 1984). Both parameters further confirmed the order of bacterial richness (OTU) and were similar among three ditches. However, alpha diversity indexes, including Simpson and Shannon diversity index were found in significant differences between DS and the other two sites. The highest diversity was found in DY, with indices ranging from 6.09 to 6.15, while the diversity index in DS was the lowest, with indices ranging from 5.78 to 5.84.
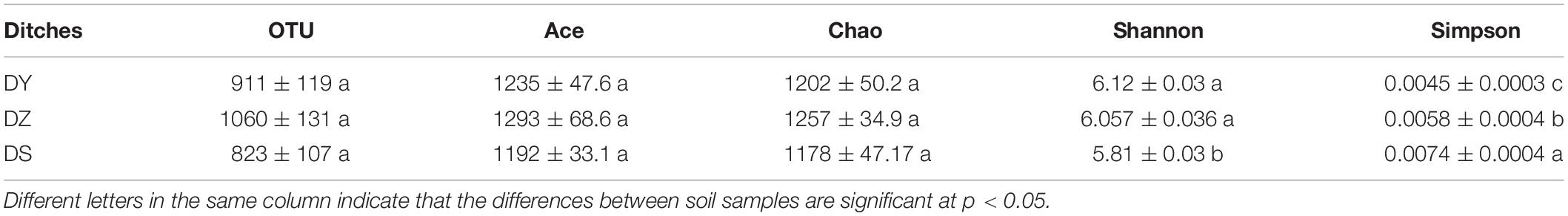
Table 2. Bacterial diversity indices and estimated bacterial OTUs of soil samples collected from three ditches.
Taxonomic information at the phylum level is shown in Figure 4. Among all identified bacterial phylum in the studied regions, Proteobacteria and Acidobacteria were two major phyla. The remaining dominant community was composed of Actinobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Gemmatimonadetes, and Nitrospirae. These eight phyla totally accounted for a high proportion (93.54–96.2%) in soil bacterial communities. The mean percentage of Proteobacteria in soils were much higher than other phyla, which also had the similar distribution pattern in three ditches (47.75% in DY, 46.89% in DZ, and 47.84% in DS). The site-specific trend influenced bacterial distribution. Gemmatimonadetes (3.90% in DY, 3.69% in DZ, and 3.06% in DS) and Bacteroidetes (6.93% in DY, 4.77% in DZ, and 3.52% in DS) were more abundant in DY, but Nitrospirae (1.79% in DY, 4.32% in DZ, and 4.01% in DS) and Firmicutes (2.14% in DY, 3.57% in DZ, and 0.83% in DS) were more abundant in DZ. The highest proportion of Chloroflexi (4.84% in DY, 7.01% in DZ, and 10.83% in DS) was in DS. In addition, the abundance of Phyla Actinobacteria (14.98% in DY, 9.24% in DZ, and 3.88% in DS) was significantly higher in DY than in DS, while Acidobacteria (12.50% in DY, 15.23% in DZ, and 21.11% in DS) was significantly lower in DY and DZ than DS.
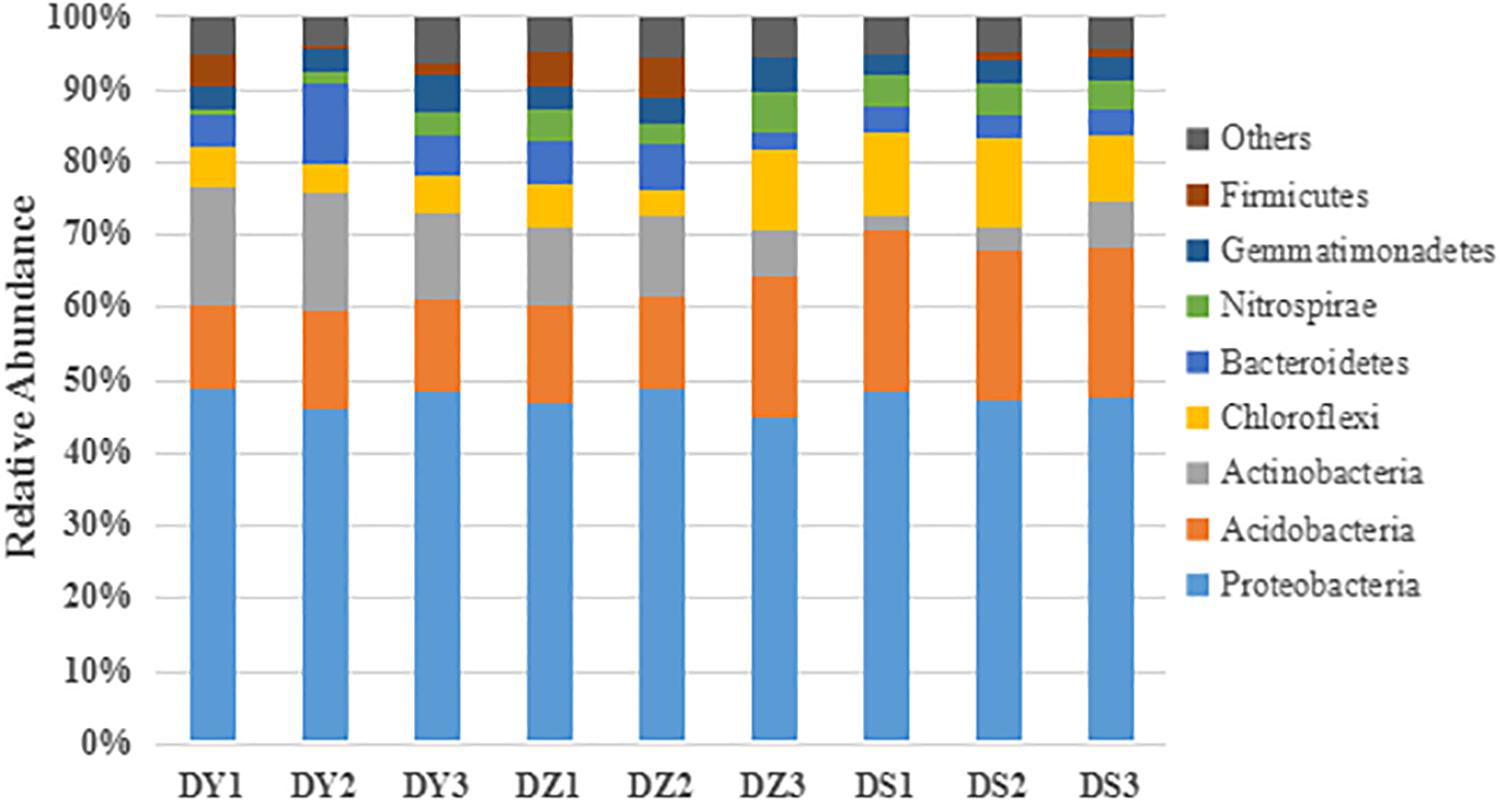
Figure 4. Taxonomic composition of the dominant bacteria at the phylum level in different soil samples. Others represent the relative abundance of the phyla outside the eight phyla.
At the genus level, most of genera showed different abundance in different ditch (Figure 5). Thiobacillus, Nitrospira, and Sphingomonas were dominant in all soil samples, in which these bacteria accounted for 10.06, 8.28, and 4.37% of the total community in DY, DZ, and DS respectively. In DZ, the specifically dominant members were Bacillus and Pseudomonas, while DY was specifically dominated by Variovorax. Moreover, Arthrobacter (1.59% DY, 1.13% DZ, and 0.12% DS), Streptomyces (1.7% DY, 1.3% DZ, and 0.27% DS), Bradyrhizobium (2.89% DY, 2.37% DZ, and 0.34% DS) and Rhizomicrobium (1.97% DY, 1.51% DZ, and 0.43% DS) were more abundant in DY and DZ than those in DS. However, Ralstonia and Psedulabrys were predominated in DS, totally accounting for 13.41% of the total community.
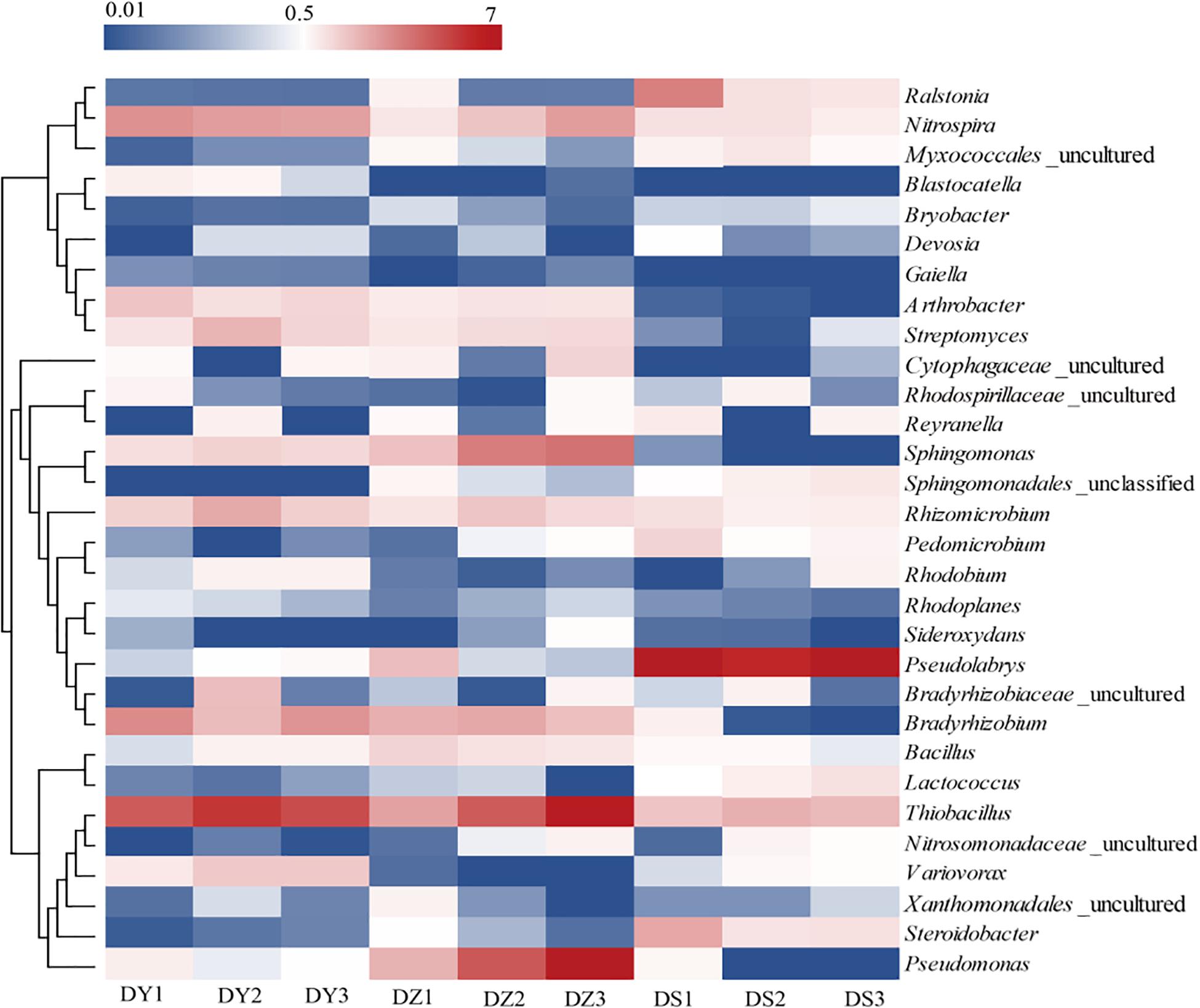
Figure 5. Heatmap analysis of the dominant genera in different soil samples. Changes in bacterial community compositions was depicted by the color intensity ranged from 0.01 to 7 (blue to red).
Relationship Between Bacterial Community Structures and Concentrations of Uranium and Heavy Metals
Using RDA we revealed a clear relation between bacterial community structure and environmental factors (Figure 6). The length of the arrow representing each parameter is directly proportional to the influence of environmental factors on bacterial community structure. The first two axes of the RDA explained 70.8 and 15.9% of the total variation, respectively. Furthermore, the first dimension (RDA1) was driven by SOM, Cr and pH, while the RDA2 was driven by As and U. The effect of SOM, Cr, and pH on the bacterial communities were significant (p < 0.05), indicating that three parameters were the principle factors influencing the bacterial communities. However, the other factors had no obvious effect on bacterial communities. The Pearson correlation was performed to analyze correlations between environmental parameters and bacterial abundance (Figure 7). The abundances of Thiobacillus, Nitrospira, Sphingomonas, Pseudomonas, Streptomyces, Arthrobacter, Gaiella, and Sideroxydans were significant and positively correlated with the concentrations of non-residual Cr, while the abundances of Bryobacter, Lactococcus, and Rhodoplanes negatively correlated with concentrations of the same metals. The abundances of Sphingomonas and Pseudomonas were significant and positively correlated with the concentrations of non-residual U and As. The abundance of Bradyrhizobium, Lactococcus, Sphingomonadales_unclassified and Myxococcales_uncultured were positively correlated with SOM and MC, while Lactococcus was negatively correlated with non-residual Cr, U, and Zn. In addition, Ralstonia, Bryobacter, Pseudolabrys, and Steroidobacter were positively correlated with pH, while Thiobacillus, Nitrospira, Arthrobacter, and Gaiella were negatively correlated with it.
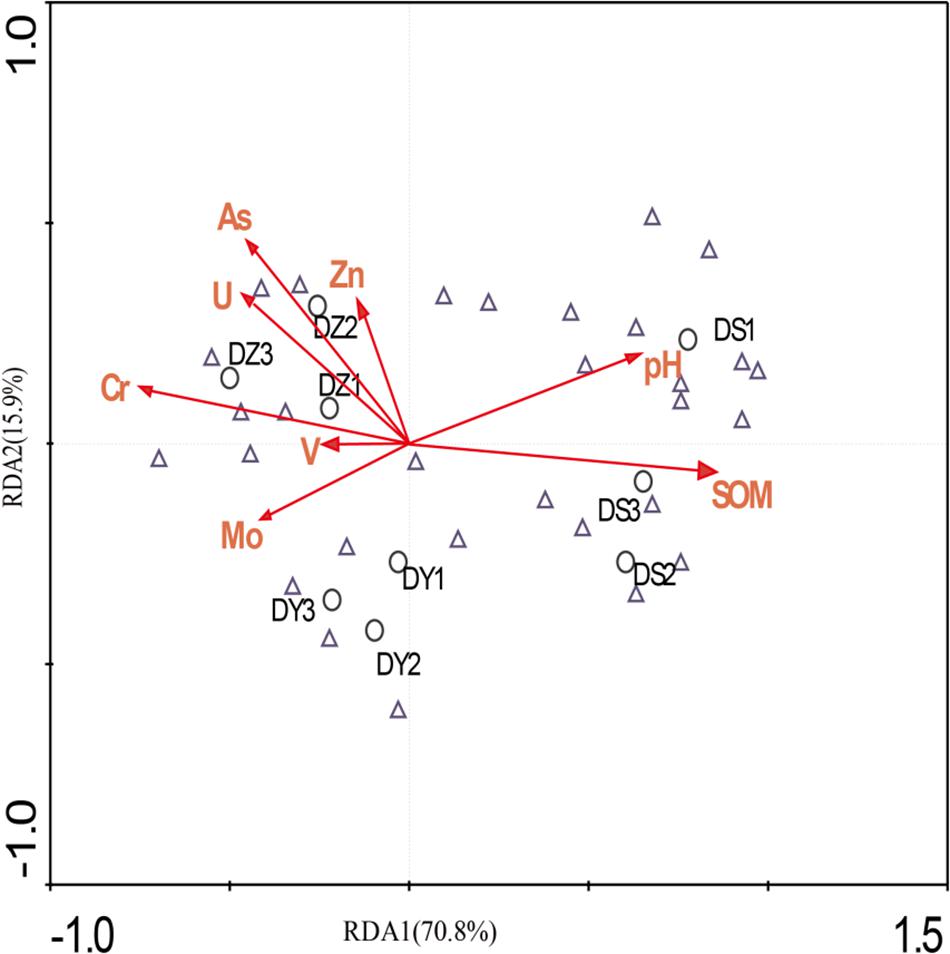
Figure 6. Redundancy analysis of bacterial data and environmental parameters. Samples and species are represented as circles and triangles, respectively. Arrows indicate the directions and magnitudes of heavy metals associated with bacterial community structures.
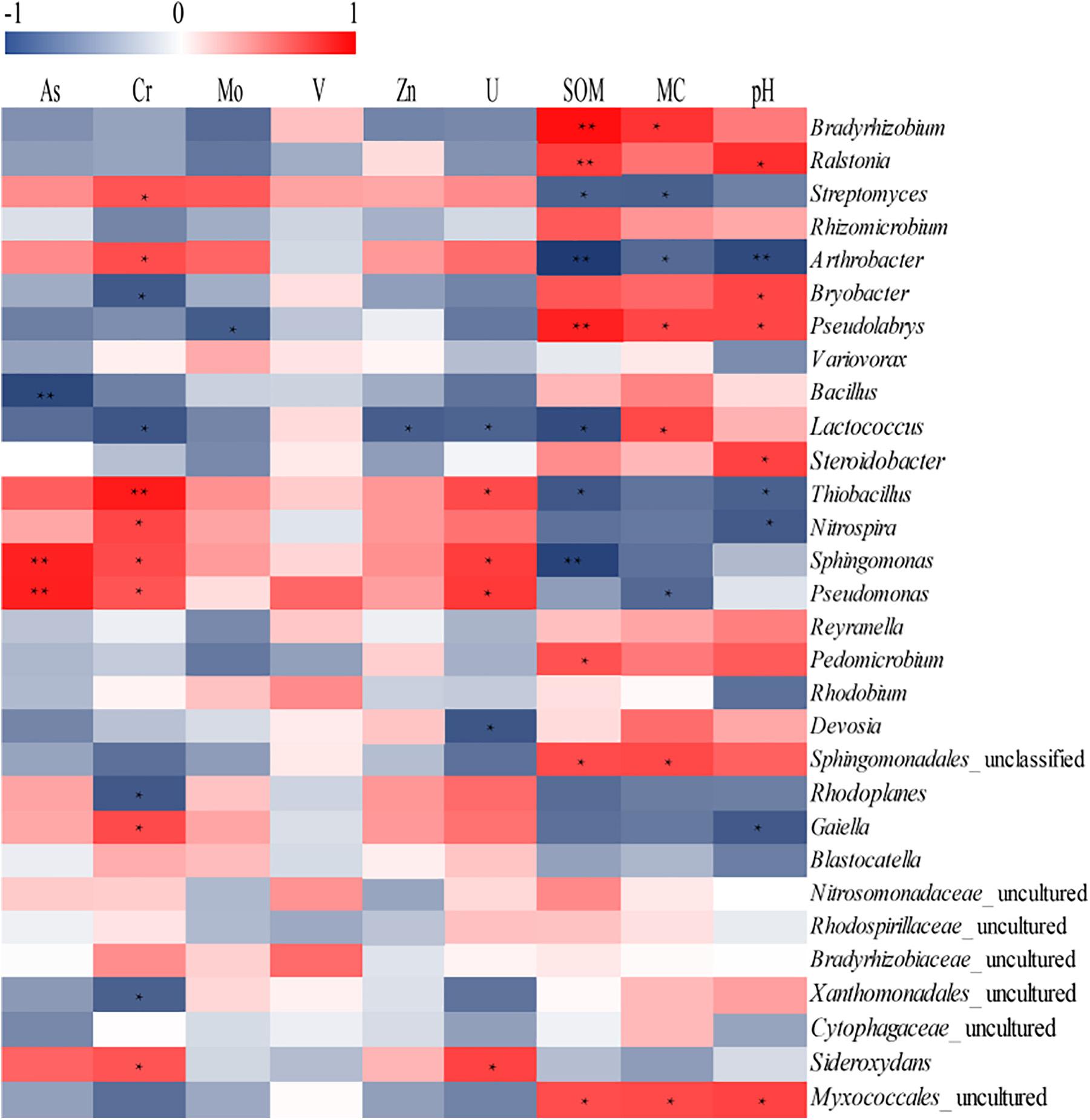
Figure 7. Analysis of correlation between environmental parameters and dominant genera richness. The corresponding values of the heat map is the Pearson correlation coefficient. The value of “r” is between –1 and 1, r < 0 is a negative correlation, r > 0 is a positive correlation, values marked * indicate a significance test at p < 0.05, and values marked ∗∗ indicate a significance test at p < 0.01.
Discussion
Uranium and Heavy Metals Distribution and Speciation in All Soil Samples
The SOM and MC among three sites decreased as follows: DS > DY > DZ, while the content of heavy metals showed an opposite trend. This may be because the DZ site was close to the center of the polluted area than DS site. The DY site was situated in a mineral-leaching area, where numerous heavy metals could get into soils. Heavy metal contamination in this area originated mainly from the mining activities. The industrial activities were able to constrain the accumulation of organic matter in soil. The MC was highest in DS, which might attribute to the highest content of SOM in DS which could increase soil porosity, thus improving its ability to retain water (Wu et al., 2013).
The value of Igeo indicated that all three ditches were most seriously polluted by U and As, followed by Mo, Cr, Zn, and V. Sr in all cases did not exceed the background levels. However, Igeo was calculated based on the total heavy metal concentration, although the toxicity of heavy metals to soil microorganisms are directly dependent from the presence of specific chemical forms in which metals are present in soil (Kot and Namiesñik, 2000; Wang et al., 2007b; Ke et al., 2017). The exchangeable and carbonate fractions can be easily leached in soil and exist in a free form, which allows them to be directly absorbed by microorganisms. An increase of oxidizing conditions in soil can dissolve Fe–Mn bound and the organic matter fractions, transforming these fractions into active forms (Arenas-Lago et al., 2016). Metals of the residual fraction are embedded into the crystalline lattices of the soil clay, which mainly originated from geogenic sources and appear to be rather inactive (Islam et al., 2015). Studies of metal extraction from solution by aquatic organisms verified the high availability of free metal ions (Luoma, 1983). Based on the previous researches, the metals which exist in non-residual forms, are more likely utilized by organisms and may relate to their concentrations in microorganisms (Roosa et al., 2016; Rosado et al., 2016; Yıldırım and Tokalıoğlu, 2016). Consequently, the potential bioavailability of heavy metals is mainly determined by proportions in non-residual fraction.
The distribution of five forms of heavy metals in DY, DZ, and DS were varied. In DY, the exchangeable fraction showed the biggest proportion (6.27% in DY1, 8.9% in DY2, and 6.58% in DY3) among non-residual fractions, which was significantly higher than those of the organic form (3.77% in DY1, 4.23% in DY2, and 4.41% in DY3). Inversely, the organic fraction was the major component of the non-residual fraction in DS, which accounted for 9.76% in DS1, 8.44% in DS2, and 7.46% in DS3, respectively. Compared with the other two ditches, Fe–Mn bound was the most abundant forms of the non-residual fraction in DZ. Generally, the percentage of the non-residual fractions in each ditch followed the order: DY > DZ > DS, indicating that soils in DY and DZ might have produced more negative effects on microorganisms.
Diversity and Compositions of Bacterial Community
The high-throughput sequencing is a powerful and precise tool for exploring diversity of complex bacterial communities. The popularity of this method is ever growing (Guo et al., 2017; He et al., 2017). In the present study, the high-throughput sequencing was used to evaluate the diversity and abundance of bacterial communities in soils contaminated with heavy metals as a result of mining. The OTU numbers showed a slightly change among ditches with different levels of pollution. Similarly, to our results, Li X. et al. (2017) reported similar OTU numbers of microbial communities in long-term moderate and severe heavy-metal contamination. However, the diversity of soil bacteria (Shannon) in the studied soils showed significant change. Some researchers have concluded that high concentrations of heavy metals exert a significant impact on soil bacterial community and reduce the values of Shannon’s indexes (Jose et al., 2011). However, a growing metal stress in soils may result in changes on microbial diversity, which relies on the initial conditions of the system and can either decrease or increase (Giller et al., 2009). In our research, the highest bacterial diversity was found in DY. Soils from this site was severely contaminated with heavy metals. It is possible that the highest bacterial diversity in severed-contaminated areas was a result of development of the acquired tolerance after adaptation to metal stress, which in turn can lead to an increase in bacterial diversity. Adaption in the bacterial community was reported for metal-contaminated soils, where bacterial richness remained high in long-term Cr-, Zn-, and Hg- polluted soil compared to unpolluted soils (Bourceret et al., 2016; Frossard et al., 2018).
Proteobacteria, the predominant phyla in all nine soil samples, was insensitive to heavy metal pollution. Multiple reports suggest that Proteobacteria constitute the main phylum in environments with high concentrations of heavy metal, such as uranium mine soil (Sar et al., 2007), arsenic mine sediments (Halte et al., 2011), heavy metal-polluted river, sediments, soils (Serkebaeva et al., 2013; Yin et al., 2015; Li X. et al., 2017) and electronic waste region with heavy metal pollution (Wu et al., 2017). The strong adaptability of Proteobacteria to heavy metals can be possibly explained by their complex lifestyles and use of the various energy sources (Bouskill et al., 2010). Janssen (2006) studied the microbial community in unpolluted soil which was different from those revealed in our soils in which Verrucomicrobia and Planctomycetes were dominant in normal soil but were not found in our research. Long term heavy metal pollution have resulted in a remarkable changes in bacterial communities structure of soils examined in current research compared to those of previous studied unpolluted soils (Janssen, 2006). Changes in the bacterial community may be attributed to the already occurred selection of stress tolerant groups of microorganisms, while, at the same time, number of sensitive to stress bacterial species was reduced.
Further taxonomic classification at the genus level revealed more detailed information on the bacterial community structure. Thiobacillus, Sphingomonas, and Nitrospira were prevailing genera in all soil samples. Species from these genera are known to be predominant in metal-contaminated environments and can resist heavy metal toxicity (Li X. F. et al., 2015; Nitzsche et al., 2015; Guo et al., 2017). It was reported that Thiobacillus species were often found in bioleaching systems that contain various heavy metals (Fowler and Crundwell, 1998; Li X. et al., 2017). Other studies have demonstrated that Nitrospira and Sphingomonas can degrade a variety of toxic organic compounds (Baraniecki et al., 2002; Song et al., 2015) which could co-exist with heavy metals at contaminated sites. Considerably, the dominance of particular groups of microorganisms might be attributed to their tolerance to high concentrations of heavy metals.
In addition, several genera were predominant in DY and DZ, including Arthrobacter, Streptomyces, Bradyrhizobium, and Rhizomicrobium. According to previous reports, species in these genera exhibited various responses to heavy metals (Akobe et al., 2007; Rastogi et al., 2010). For instance, the exopolysaccharides in Arthrobacter sp. carry the ability to remove Cr (VI) without adjusting pH (Li Y. M. et al., 2015). Nies (1999) found that Streptomyces could precipitate and agglomerate colloidal suspensions in water by producing the flocculating active metabolite and releasing it to the extracellular environment. This metabolite can mitigate the toxicity of heavy metals. The ability of Bradyrhizobium to absorb metal ions has been reported (Cao et al., 2017). On the other hand, Guo et al. (2017) reported that species of Rhizomicrobium are heavy metal sensitive, since their abundance in soil negatively correlate with content of heavy metals. Some microorganisms that are tolerant to heavy metal can slowly and constantly repair the quality of soil by reducing the valences of heavy metal ions or changing contents of heavy metals, and therefore heavy-metal-sensitive microorganisms can survive in the presence of such microbes. However, this hypothesis about microbial ‘cooperation’ still requires conformation. Pseudomonas was the most predominant genus in DZ3 and is known as an extremophilic bacterium with strong adaptability. It is one of the most diversified bacterial genera broadly used in the field of environmental pollution control and biotechnology (Lustman et al., 2015; Yin et al., 2016; Feng et al., 2018; Singh et al., 2019). Some recent studies have reported that the Pseudomonas exert impact on the toxicity of heavy metals by redox (Rastogi et al., 2010; Cao et al., 2017). Moreover, Choudhary and Sar (2011) demonstrated that Pseudomonas could accumulate uranium within the cell envelope while maintaining its viability, indicating that the predominance abundance of Pseudomonas in DZ3 might be related to higher uranium concentration.
Overall, heavy metal tolerance involved different mechanisms including, biosorption, bioaccumulation, precipitation by extracellular polymeric substances and redox transformations. These dominant genera which could tolerate heavy metal toxicity and grow in contaminated soil have the potential for bioremediation of the heavy metal contaminated soil.
Relationships Between Environmental Parameters and Bacterial Community Structures
Several researchers suggested that uranium had a great influence on soil bacterial communities. Rastogi et al. (2010) found that high toxic metal levels exerted high pressure on indigenous microbial communities in uranium mine. Sar et al. (2007) revealed that microbial communities were shifted after long-term uranium pollution and around 20% of clone sequences may represent new organisms adapted this pollution environment. In this study, uranium contamination was the most serious in the studied area, but uranium had little effect on bacterial communities compared with Cr and As. In natural environment, uranium mainly exists in U (VI) which is less toxic to bacteria. Moreover, Lixiviation experiments conducted by Mondani et al. (2011) indicate that 0.1% of uranium was water soluble in nature uranium-rich soils, which provided further evidence that the content of uranium to which bacteria are really exposed. The soil was highly polluted with uranium (95.37 mg kg–1) in the sampling site, though the non-residual fraction was only 1.9%. Therefore, the low impact may be attributed to the low bioavailable uranium content. Furthermore, microbes can potentially affect uranium migration by various processes as well. Microorganisms that inhabited in radionuclide contaminated soil were well-adapted to this harsh conditions (Satchanska et al., 2004; Pollmann et al., 2005), since they played an important role in the mobility of U through making altered chemical complexes (Merroun et al., 2006; Beazley et al., 2007) and redox reactions (Beller, 2005).
Some authors revealed that heavy metal pollution did not exert a significant impact on soil bacterial community (Roy et al., 2004; Zhang et al., 2013). In this study, SOM and pH were the dominant factors influencing bacterial communities. Soil organic matter was of vital importance for the ecological function of soil. It stimulated microbial growth, since it is the main source of energy and nutrients for soil microbiota (Ondrasek et al., 2019). The significant relationships between SOM and microorganisms could be observed. Moreover, SOM participated in the making chemical complexes and chelation of heavy metals, which could affect the migration and transformation of heavy metals. Kirkham (2006) suggested that SOM was one of the most important factors influencing the bioavailability of heavy metals in soil. Study performed by Chodak et al. (2013) concluded that the effect of heavy metals on soil microorganisms was weaker compared with the effects of soil pH. Soil pH was known to affect mobility and thus bioavailability of heavy metals, since it could transform inactive forms of heavy metals into active forms (McBride et al., 1997; Zhang et al., 2017). The weaker influence of heavy metals on microbial communities compared with these environmental factors might due to the mitigation of the toxic effect of metal by environment (Nannipieri et al., 2011), or by the low-level of metal biological availability (Wang et al., 2007a). Specifically, Cr was the key metal related to the regulation of the bacterial community in the studied area. Our results are in line with Li B. X. et al. (2017) who reported that Cr content was the major metal affecting soil bacterial community. Cr is widely distributed in some soil environments as a result of its extensive use in various industrial production, such as electrofacing, corrosion control, and leather-tanning processes. Unlike other heavy metals, Cr exert discrepant effects in different chemical forms and therefore it is deemed to be a prime example among other elements. The trivalent chromite [Cr (III)] and hexavalent chromate [Cr (VI)] are the most stable valence states of Cr in various forms and therefore they are predominant in the natural environment (Elzinga and Cirmo, 2010). The compounds of Cr (III) are extremely stable in soil since it entirely precipitates. In contrast, Cr (VI) is highly unstable and remain immobilized in soils (Kabata-Pendias, 2010). Therefore, microbial-induced conversion of Cr (VI) to Cr (III) leads to a reduction in toxicity, which may be a result of the precipitation of various Cr (III) chemical forms. Moreover, a toxicity has been observed even in soils containing very low contents of Cr (VI) (<1 mg kg–1) (Martí et al., 2013). Min et al. (2017) reported that total Cr content affiliated with Cr (VI) was the major factor for the difference of soil bacterial communities. Similar results can be found in works of He et al. (2016). The findings of these studies supported the different impact of Cr, indicating that the influence of Cr is more dependent on its occurrence state. In our cases, the percentage of non-residual Cr was relatively high, suggesting that more Cr were bioavailable in studied area, and the results compared well with those of previous studies.
Based on 16S rRNA high throughput sequencing, we provided a detailed insight into bacterial communities associated with uranium mine. Bacterial community displayed higher diversity in DY and DZ than those in DS, while bacterial richness was not significant different among three ditches. SOM, Cr and pH were the key factors that had the great impact on microbiota, other were less important. More correlations between non-residual Cr and dominant bacterial abundance were observed. Among dominant genera, the abundances of Sphingomonas and Pseudomonas were significant and positively correlated with the concentrations of non-residual Cr, U, and As. Compared with total content of heavy metal, soil physicochemical properties and non-residual heavy metal could impact a more important role in understanding bacterial communities interaction with environments.
Data Availability
The raw reads had been submitted to NCBI and the accession numbers of sequences for DY1, DY2, DY3, DZ1, DZ2, DZ3, DS1, DS2 and DS3 are SRR9611297, SRR9611293, SRR9611298, SRR9611292, SRR9611295, SRR9603206, SRR9611294, SRR9611299, and SRR9611296.
Author Contributions
QZ collected the samples from uranium mine. SX and QZ was responsible for conceiving the idea of this study. SX carried out the analyses reported here, involved in the writing and finalizing of the manuscript and all presented data. XC supervised and directed the entire project and obtained funds for carrying out these studies. HC detected the chemical elements. ML, FD, and IA revised the manuscript. All authors read and approved the final manuscript.
Funding
The current study was supported by State Key Laboratory of NBC Protection for Civilian (Grant No. JK2017A06308), the Major State Development Program of China (973 Program, No. 2014CB846003), Sichuan Province Foundation (Grant No. 18YYJC0927), National Defense Science and Technology Foundation of China (Grant No. 16ZG6101), Fundamental Science on Nuclear Wastes and Environmental Safety Laboratory Program (Grant No. 15yyhk05).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01867/full#supplementary-material
References
Akobe, D. M., Mills, H. J., and Kostka, J. E. (2007). Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS. Microbiol. Ecol. 59, 95–107. doi: 10.1111/j.1574-6941.2006.00203.x
Arenas-Lago, D., Andrade, M. L., Vega, F. A., and Singh, B. R. (2016). TOF-SIMS and FE-SEM/EDS to verify the heavy metal fractionation in serpentinite quarry soils. Catena 136, 30–43. doi: 10.1016/j.catena.2015.03.005
Bano, A., Hussain, J., Akbar, A., Mehmood, K., Anwar, M., Hasni, M. S., et al. (2018). Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 199, 218–222. doi: 10.1016/j.chemosphere.2018.02.043
Baraniecki, C. A., Aislabie, J., and Foght, J. M. (2002). Characterization of Sphingomonas sp. Ant 17, an aromatic hydrocarbon-degrading bacterium isolated from antarctic soil. Microb. Ecol. 43, 44–54. doi: 10.1007/s00248-001-1019-3
Beazley, M. J., Martinez, R. J., Sobecky, P. A., Webb, S. M., and Taillefert, M. (2007). Uranium biomineralization as a result of bacterial phosphatase activity: insight from bacterial isolates from a contaminated subsurface. Environ. Sci. Technol. 41, 5701–5707. doi: 10.1021/es070567g
Beller, H. (2005). Anaerobic, nitrate-dependent oxidation of U(IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl. Environ. Microbiol. 71, 2170–2174. doi: 10.1128/aem.71.4.2170-2174.2005
Bourceret, A., Cébron, A., Tisserant, E., Poupin, P., Bauda, P., Beguiristain, T., et al. (2016). The bacterial and fungal diversity of an aged PAH-and heavy metal-contaminated soil is affected by plant cover and edaphic parameters. Microb. Ecol. 71, 711–724. doi: 10.1007/s00248-015-0682-8
Bouskill, N. J., Barker-Finkel, J., Galloway, T. S., Handy, R. D., and Ford, T. E. (2010). Temporal bacterial diversity associated with metal-contaminated river sediments. Ecotoxicology 19, 317–328. doi: 10.1007/s10646-009-0414-2
Bray, J. R., and Curtis, J. T. (1957). An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349. doi: 10.2307/1942268
Buccolieri, A., Buccolieri, G., Cardellicchio, N., Dell’Atti, A., Di Leo, A., Maci, A., et al. (2004). Distribution and speciation of metals in surface sediments of Taranto Gulf (Inoian Sea, Southern Italy). Ann. Chim. 94, 469–478. doi: 10.1002/adic.200490060
Cao, X. L., Diao, M. H., Zhang, B. G., Liu, H., Wang, S., and Yang, M. (2017). Spatial distribution of vanadium and microbial community responses in surface soil of Panzhihua mining and smelting area, China. Chemosphere 183, 9–17. doi: 10.1016/j.chemosphere.2017.05.092
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336.
Chao, A. (1984). Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270.
Chen, J. S., Wei, F. S., Zheng, C. J., Wu, Y. Y., and Adriano, D. C. (1991). Background concentrations of elements in soils of China. Water Soil Air Poll. 5, 699–712. doi: 10.1007/bf00282934
Chen, Z., Cheng, Y., Pan, D., Wu, Z., Li, B., Pan, X., et al. (2012). Diversity of microbial community in Shihongtan sandstone-type uranium deposits, Xinjiang, China. Geomicrobiol. J. 29, 255–263. doi: 10.1080/01490451.2011.598604
Chodak, M., Gołêbiewski, M., Morawska-Płoskonka, J., Kuduk, K., and Nikliñska, M. (2013). Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil. Ecol. 64, 7–14. doi: 10.1016/j.apsoil.2012.11.004
Choudhary, S., and Sar, P. (2011). Uranium biomineralization by a metal resistant Pseudomonas aeruginosa strain isolated from contaminated mine waste. J. Hazard. Mater. 186, 336–343. doi: 10.1016/j.jhazmat.2010.11.004
Cole, J. R., Wang, Q., Cardenas, E., Fish, J., Chai, B., and Farris, R. J. (2009). The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145. doi: 10.1093/nar/gkn879
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Elzinga, E. J., and Cirmo, A. (2010). Application of sequential extractions and X-ray absorption spectroscopy to determine the speciation of chromium in Northern New Jersey marsh soils developed in chromite ore processing residue (COPR). J. Hazard. Mater. 183, 145–154. doi: 10.1016/j.jhazmat.2010.06.130
Feng, T., Wu, J., and Chai, K. (2018). Effect of Pseudomonas sp. on the degradation of aluminum/epoxy coating in seawater. J. Mol. Liq. 263, 248–254. doi: 10.1016/j.molliq.2018.04.103
Fierer, N., Leff, J. W., Adams, B. J., Nielsen, U. N., Bates, S. T., Lauber, C. L., et al. (2012). Cross-biome metagenomic analyses of soil microbial communities and their functional arributes. Proc. Natl. Acad. Sci. U.S.A. 109, 21390–21395. doi: 10.1073/pnas.1215210110
Fowler, T. A., and Crundwell, F. K. (1998). Leaching of zinc sulfide by Thiobacillus ferrooxidans: experiments with a controlled redox potential indicate No direct bacterial mechanism. Appl. Environ. Microbiol. 64, 3570–3575.
Frossard, A., Donhauser, J., and Mestrot, A. (2018). Long- and short-term effects of mercury pollution on the soil microbiome. Soil Biol. Biochem. 120, 191–199. doi: 10.1016/j.soilbio.2018.01.028
Giller, K. E., Witter, E., and McGrath, S. P. (2009). Heavy metals and soil microbes. Soil Biol. Biochem. 41, 2031–2037. doi: 10.1016/j.soilbio.2009.04.026
Gudkov, S. V., Chernikov, A. V., and Bruskov, V. I. (2016). Chemical and radiological toxicity of uranium compounds. Russ. J. Gen. Chem. 86, 1531–1538. doi: 10.1134/s1070363216060517
Guo, H. H., Nasir, M., Lv, J. L., Dai, Y. C., and Gao, J. K. (2017). Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotox. Environ. Saf. 144, 300–306. doi: 10.1016/j.ecoenv.2017.06.048
Halte, D., Cordi, A., Gribaldo, S., Gallien, S., Goulhen-Chollet, F., Heinrich, S. A., et al. (2011). Taxonomic and functional prokaryote diversity in mildly arsenic-contaminated sediments. Res. Microbiol. 162, 877–887. doi: 10.1016/j.resmic.2011.06.001
He, H. D., Li, W. H., Yu, R. Q., and Ye, Z. H. (2017). Illumina-based analysis of bulk and rhizosphere soil bacterial communities in paddy fields under mixed heavy metal contamination. Pedosphere 27, 569–578. doi: 10.1016/s1002-0160(17)60352-7
He, Z. G., Hu, Y. T., Yin, Z., Hu, Y. H., and Zhong, H. (2016). Microbial diversity of chromium-contaminated soils and characterization of six chromium-removing bacteria. Environ. Manage. 57, 1319–1328. doi: 10.1007/s00267-016-0675-5
Huang, J. H., Yuan, F., Zeng, G. M., Li, X., Gu, Y. L., Shi, L. X., et al. (2017). Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 173, 199–206. doi: 10.1016/j.chemosphere.2016.12.137
Ilton, E. S., Qafoku, N. P., Liu, C., Moore, D. A., and Zachara, J. M. (2008). Advective removal of intraparticle uranium from contaminated vadose zone sediments, Handford, U.S. Environ. Sci. Technol. 42, 1565–1571. doi: 10.1021/es071113m
Islam, M. S., Ahmed, M. K., Raknuzzaman, M., Habibullah-Al-Mamun, M., and Masunaga, S. (2015). Metal speciation in sediment and their bioaccumulation in fish species of three urban rivers in Bangladesh. Arch. Environ. Contam. Toxicol. 68, 92–106. doi: 10.1007/s00244-014-0079-6
Janssen, P. H. (2006). Identifying the dominant soil bacterial taxa in libraries of 16s rRNA and 16s rRNA genes. Appl. Environ. Microbiol. 72, 1719–1728. doi: 10.1128/aem.72.3.1719-1728.2006
Jose, J., Giridhar, R., Anas, A., Bharathi, P. A. L., and Nair, S. (2011). Heavy metal pollution exerts reduction/adaptation in the diversity and enzyme expression profile of heterotrophic bacteria in cochin estuary. India. Environ. Pollut. 159, 2775–2780. doi: 10.1016/j.envpol.2011.05.009
Ke, X., Gui, S., Huang, H., Zhang, H., Wang, C., and Guo, W. (2017). Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere 175, 473–481. doi: 10.1016/j.chemosphere.2017.02.029
Kenarova, A., Radeva, G., Traykov, I., and Boteva, S. (2014). Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotox. Environ. Saf. 100, 226–232. doi: 10.1016/j.ecoenv.2013.11.012
Kirkham, M. B. (2006). Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137, 19–32. doi: 10.1016/j.geoderma.2006.08.024
Kot, A., and Namiesñik, J. (2000). The role of speciation in analytical chemistry. TRAC Trends Anal. Chem. 19, 69–79. doi: 10.1016/s0165-9936(99)00195-8
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K., and Schloss, P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Kuppusamy, S., Thavamani, P., Megharaj, M., Venkateswarlu, K., Lee, Y. B., and Naidu, R. (2016). Pyrosequencing analysis of bacterial diversity in soils contaminated long-term with PAHs and heavy metals: implications to bioremediation. J. Hazard. Mater. 317, 169–179. doi: 10.1016/j.jhazmat.2016.05.066
Li, X., Meng, D. L., Li, J., Yin, H. Q., Liu, H. W., Liu, X. D., et al. (2017). Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 231, 908–917. doi: 10.1016/j.envpol.2017.08.057
Li, B. X., Bao, Y. X., Xu, Y. N., Xie, S. G., and Huang, J. (2017). Vertical distribution of microbial communities in soils contaminated by chromium and perfluoroalkyl substances. Sci. Total. Environ. 599-600, 156–164. doi: 10.1016/j.scitotenv.2017.04.241
Li, X. F., You, F., Bond, P. L., and Huang, L. B. (2015). Establishing microbial diversity and functions in weathered and neutral Cu–Pb–Zn tailings with native soil addition. Geoderma 247–248, 108–116. doi: 10.1016/j.geoderma.2015.02.010
Li, Y. M., Li, Q., Yang, F. Y., Bao, J., Hu, Z. H., Zhu, W. W., et al. (2015). Chromium (VI) detoxification by oxidation and flocculation of exopolysaccharides from Arthrobacter sp. B4. Int. J. Biol. Macromol. 81, 235–240. doi: 10.1016/j.ijbiomac.2015.07.013
Luoma, S. N. (1983). Bioavailability of trace metals to aquatic to organisms-a review. Sci. Total. Environ. 28, 1–22. doi: 10.1016/s0048-9697(83)80004-7
Lustman, L. J. R., Tribelli, P. M., Ibarra, J. G., Catone, M. V., and Venero, E. C. S. (2015). Genome sequence analysis of Pseudomonas extremaustralis provides new insights into environmental adaptability and extreme conditions resistance. Extremophiles 19, 207–220. doi: 10.1007/s00792-014-0700-7
Ma, X., Zuo, H., Tian, M. J., Zhang, L. Y., Meng, J., Zhou, X. N., et al. (2016). Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144, 264–272. doi: 10.1016/j.chemosphere.2015.08.026
Martí, E., Sierra, J., Cáliz, J., Montserrat, G., Vila, X., Garau, M. A., et al. (2013). Ecotoxicity of Cr, Cd, and Pb on two mediterranean soils. Arch. Environ. Contam. Toxicol. 64, 377–387. doi: 10.1007/s00244-012-9841-9
McBride, M., Sauve, S., and Hendershot, W. (1997). Solubility control of Cu, Zn Cd and Pb in contaminated soils. Eur. J. Soil Sci. 48, 337–346. doi: 10.1111/j.1365-2389.1997.tb00554.x
Merroun, M. L., Nedelkova, M., Rossberg, A., Hennig, C., and Selenska-Pobell, S. (2006). Interaction mechanisms of uranium with bacterial strains isolated from extreme habitats. Radiochim. Acta 94, 723–729.
Min, X. B., Wang, Y. Y., Chai, L. Y., Yang, Z. H., and Liao, Q. (2017). High-resolution analyses reveal structural diversity patterns of microbial communities in chromite ore processing residue (COPR) contaminated soils. Chemosphere 183, 266–276. doi: 10.1016/j.chemosphere.2017.05.105
Mondani, L., Benzerara, K., Carriere, M., Christen, R., Mamindy-Pajany, Y., Fevrier, L., et al. (2011). Influence of uranium on bacterial communities: a comparison of natural uranium-rich soils with control. PLoS One 6:e25771. doi: 10.1371/journal.pone.0025771
Muller, G. (1981). The heavy metal pollution of the sediments of Neckars and its tributary:a stocktaking. Chemiker-Zeitung. 105, 157–164.
Najamuddin, P. T., Sanusi, H. S., and Nurjaya, I. W. (2016). Seasonal distribution and geochemical fractionation of heavy metals from surface sediment in a tropical estuary of Jeneberang River, Indonesia. Mar. Pollut. Bull. 111, 456–462. doi: 10.1016/j.marpolbul.2016.06.106
Nannipieri, P., Giagnoni, L., Landi, L., and Renella, G. (2011). “Role of phosphatase enzymes in soils,” in Phosphorus in Action Biological Processes in Soil Phosphorus Cycling, Series: Soil Biology, Vol. 26, eds E. K. Bunemann, A. Oberson, and E. Frossard (Berlin: Springer-Verlag), 2015–2044.
Narendrula-Kotha, R. K., and Nkongolo, K. (2017). Bacterial and fungal community structure and diversity in a mining region under long-term metal exposure revealed by metagenomics sequencing. Ecol. Genet. Genomics 2, 13–24. doi: 10.1016/j.egg.2016.11.001
Neto, J. A. B., Rangel, C. M. A., and Fonseca, E. M. D. (2016). Concentrations and physicochemical speciation of heavy metals in urban runoff sediment from S~ ao Goncalo-Rio de Janeiro/Brazil. Environ. Earth. Sci. 75:1209.
Nies, D. H. (1999). Microbial heavy -metal resistance. Appl. Microbiol. Biotechnol. 51, 730–750. doi: 10.1007/s002530051457
Nitzsche, K. S., Weigold, P., Lösekann-Behrens, T., Kappler, A., and Behrens, S. (2015). Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam. Chemosphere 138, 47–59. doi: 10.1016/j.chemosphere.2015.05.032
Olaniran, A. O., Balgobind, A., Kumar, A., and Pillay, B. (2017). Treatment additives reduced arsenic and cadmium bioavailability and increased 1,2-dichloroethane biodegradation and microbial enzyme activities in co-contaminated soil. J. Soil Sediment. 17, 2019–2029. doi: 10.1007/s11368-017-1683-7
Ondrasek, G., Begic, H. B., Zovko, M., Filipovic, L., Merino-Gergichevich, C., Savic, R., et al. (2019). Biogeochemistry of soil organic matter in agroecosystems & environmental implications. Sci. Total Environ. 658, 1559–1573. doi: 10.1016/j.scitotenv.2018.12.243
Palleiro, L., Patinha, C., Rodríguez-Blanco, M. L., Taboada-Castro, M. M., and Taboada-Castro, M. T. (2016). Metal fractionation in topsoils and bed sediments in the Mero River rural basin: bioavailability and relationship with soil and sediment properties. Catena 144, 34–44. doi: 10.1016/j.catena.2016.04.019
Pollmann, K., Raff, J., Schnopfeil, M., Radeva, G., and Selenska-Pobell, S. (2005). Novel surface layer protein genes in Bacillus sphaericus associated with unusual insertion elements. Microbiology 151, 2961–2973. doi: 10.1099/mic.0.28201-0
Preston, S., Wirth, S., Ritz, K., Griffiths, B. S., and Young, I. M. (2001). The role played by microorganisms in the biogenesis of soil cracks: importance of substrate quantity and quality. Soil. Biol. Biochem. 33, 1851–1858. doi: 10.1016/s0038-0717(01)00113-4
Qi, X., Hao, X. C., Chen, X. M., Xiao, S. Q., Chen, S. L., Luo, X. G., et al. (2019). Integrated phytoremediation system for uranium-contaminated soils by adding a plant growth promoting bacterial mixture and mowing grass. J. Soil Sediment. 19, 1799–1808. doi: 10.1007/s11368-018-2182-1
Rastogi, G., Osman, S., and Vaishampayan, P. A. (2010). Microbial diversity in uranium mining-impacted soils as revealed by high-density 16S microarray and clone library. Microb. Ecol. 59, 94–108. doi: 10.1007/s00248-009-9598-5
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., and Forney, L. J. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4680–4687.
Roosa, S., Prygiel, E., Lesven, L., Wattiez, R., Gillan, D., Ferrari, B. J. D., et al. (2016). On the bioavailability of trace metals in surface sediments: a combined geochemical and biological approach. Environ. Sci. Pollut. Res. 23, 10679–10692. doi: 10.1007/s11356-016-6198-z
Rosado, D., Usero, J., and Morillo, J. (2016). Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 153, 10–17. doi: 10.1016/j.chemosphere.2016.03.040
Roy, S., Bhattacharyya, P., and Ghosh, A. K. (2004). Influence of toxic metals on activity of acid and alkaline phosphatase enzymes in metal-contaminated landfill soils. Aust. J. Soil Res. 42, 339–344.
Sar, P., Dhal, P. K., Islam, E., and Kazy, S. K. (2007). Molecular analysis of microbial community structure and diversity in contaminated and non contaminated sites of uranium mine area at Jaduguda, India. Adv. Mater. Res. 21, 413–416. doi: 10.4028/www.scientific.net/amr.20-21.413
Satchanska, C., Golovinsky, E., and Selenskapobell, S. (2004). Bacterial diversity in a soil sample from uranium mining waste pile as estimated via a culture-independent 16S rDNA approach. Inorganica Chim. Acta 2004, 48–52.
Serkebaeva, Y. M., Kim, Y., Liesack, W., and Dedysh, S. N. (2013). Pyrosequencing-based assessment of the bacteria diversity in surface and subsurface peat layers of a northern wetland, with focus on poorly studied phyla and candidate divisions. PLoS One 8:e63994. doi: 10.1371/journal.pone.0063994
Singh, S. K., Singh, P. P., Gupta, A., Singh, A. K., and Keshri, J. (2019). “Chapter Twelve-tolerance of heavy metal toxicity using PGPR strains of Pseudomonas species,” in PGPR Amelioration in Sustainable Agriculture, eds A. Kishore Singh, A. Kumar, and P. Kumar Singh (Sawston: Woodhead Publishing), 239–252. doi: 10.1016/b978-0-12-815879-1.00012-4
Song, M. K., Luo, C. L., Li, F. B., Jiang, L. F., Wang, Y., Zhang, D. Y., et al. (2015). Anaerobic degradation of polychlorinated biphenyls (PCBs) and polychlorinated biphenyls ethers (PBDEs) and microbial community dynamics of electronic wastecontaminated soil. Sci. Total Environ. 523, 426–433. doi: 10.1016/j.scitotenv.2014.09.045
Tessier, A., Campbell, P. G. C., and Bisson, M. (1979). Sequntial extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844–851. doi: 10.1021/ac50043a017
Verstraete, W., and Top, E. M. (1999). Soil clean-up: lessons to remember. Int. Biodeteriorat. Biodegrad. 43, 147–153. doi: 10.1016/s0964-8305(99)00043-8
Wang, Y. P., Shi, J., Wang, H., Lin, Q., Chen, X. C., and Chen, Y. X. (2007a). The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a cropper smelter. Ecotox. Environ. Saf. 67, 75–81. doi: 10.1016/j.ecoenv.2006.03.007
Wang, Y. P., Shi, J. Y., Lin, Q., and Chen, X. C. (2007b). Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J. Environ. Sci. 19, 848–853. doi: 10.1016/s1001-0742(07)60141-7
Wu, H., Zeng, G., and Liang, J. (2013). Changes of soil microbial biomass and bacterial community structure in Dongting Lake: impacts of 50,000 dams of Yangtze River. Ecol. Eng. 57, 72–78. doi: 10.1016/j.ecoleng.2013.04.038
Wu, W. C., Dong, C. X., Wu, J. H., Liu, X. W., Wu, Y. X., Chen, X. B., et al. (2017). Ecological effects of soil properties and metal concentrations on the composition and diversity of microbial communities associated with land use patterns in an electronic waste recycling region. Sci. Total Environ. 601-602, 57–65. doi: 10.1016/j.scitotenv.2017.05.165
Xiao, R., Bai, J. H., Lu, Q. Q., Zhao, Q. Q., Gao, Z. Q., Wen, X. J., et al. (2015). Fractionation, transfer, and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronosequence of reclamation in an estuary of China. Sci. Total Environ. 517, 66–75. doi: 10.1016/j.scitotenv.2015.02.052
Yan, X., and Luo, X. G. (2015). Radionuclides distribution, properties, and microbial diversity of soilsin uranium mill tailings from southeastern China. J. Environ. Radioactiv. 139, 85–90. doi: 10.1016/j.jenvrad.2014.09.019
Yang, G. F., Dong, M., Liu, X., Nie, M., Zong, C., Peng, H., et al. (2018). Interactive effect of radioactive and heavy-metal contamination on soil enzyme activity in a former uranium mine. Polish. J. Environ. Stud. 27, 1343–1351. doi: 10.15244/pjoes/76182
Yıldırım, G., and Tokalıoğlu, Ş (2016). Heavy metal speciation in various grain sizes of industrially contaminated street dust using multivariate statistical analysis. Ecotoxicol. Environ. Saf. 124, 369–376. doi: 10.1016/j.ecoenv.2015.11.006
Yin, H., Ni, J., and Ren, Y. (2015). An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci. Rep. 5:14266. doi: 10.1038/srep14266
Yin, K., Lv, M., Wang, Q., Wu, Y., Liao, C., Zhang, W., et al. (2016). Simultaneous bioremediation and biodetection of mercury ion through surface display of carboxylesterase E2 from Pseudomonas aeruginosa PA1. Water Res. 103, 383–390. doi: 10.1016/j.watres.2016.07.053
Zhang, G., Bai, J., and Xiao, R. (2017). Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamation-affected rivers of the Pearl River Estuary, China. Chemosphere 184, 278–288. doi: 10.1016/j.chemosphere.2017.05.155
Keywords: uranium mine, heavy metal pollution, chromium, bacterial community, high-throughput sequencing
Citation: Xiao S, Zhang Q, Chen X, Dong F, Chen H, Liu M and Ali I (2019) Speciation Distribution of Heavy Metals in Uranium Mining Impacted Soils and Impact on Bacterial Community Revealed by High-Throughput Sequencing. Front. Microbiol. 10:1867. doi: 10.3389/fmicb.2019.01867
Received: 16 April 2019; Accepted: 29 July 2019;
Published: 13 August 2019.
Edited by:
Edgardo Donati, National University of La Plata, ArgentinaReviewed by:
Sangeeta Choudhary, Banasthali University, IndiaGalina Simeonova Radeva, Institute of Molecular Biology (BAS), Bulgaria
Copyright © 2019 Xiao, Zhang, Chen, Dong, Chen, Liu and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Chen, Y3hteGtkQDE2My5jb20=; Imran Ali, aW1yYW5hbGlzaGVpa0BnbWFpbC5jb20=
 Shiqi Xiao
Shiqi Xiao Qian Zhang1,2
Qian Zhang1,2 Xiaoming Chen
Xiaoming Chen Imran Ali
Imran Ali