- 1Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, China
- 2Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Fudan University, Shanghai, China
- 3Suzhou Institute of Systems Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Suzhou, China
- 4Department of Clinical Laboratory, Shanghai Corps Hospital, Chinese People’s Armed Police Forces, Shanghai, China
Nemonoxacin, a newly developed non-fluorinated quinolone (NFQ), selectively inhibits bacterial DNA topoisomerase activity. However, its activities against Mycoplasmas have rarely been studied to date. Herein, the activities of nemonoxacin were evaluated against clinical isolates of 50 Mycoplasma pneumoniae, 20 Mycoplasma hominis, and 77 Ureaplasma spp., and they were compared to fluoroquinolones, tetracyclines, and macrolides. Nemonoxacin MICs (μg/ml) ranged from 0.03 to 0.25 for M. pneumoniae, 0.25 to 8 for M. hominis, and 0.06 to >16 for Ureaplasma spp., and all of the ranges are similar to those of fluoroquinolones. The activity of nemonoxacin against Mycoplasmas was not affected by resistance to macrolides in the strains tested, but it seems to have the same resistant mechanism as fluoroquinolones. In addition, minimum bactericidal concentrations (MBC) of nemonoxacin to M. pneumoniae were within two dilutions of the MIC values, indicating a bactericidal effect on M. pneumoniae. Nemonoxacin merits further study for treating infections caused by these organisms.
Introduction
Nemonoxacin, a newly developed non-fluorinated quinolone (NFQ), has showed broad-spectrum activity against Gram-positive, Gram-negative, and atypical pathogens in vitro and is generally safe and well tolerated in vivo (Chung et al., 2010). It has rapid oral absorption with a peak serum concentration (Cmax) attained in 1–2 h and an elimination half-life of 12.83–18.56 h. The binding of nemonoxacin to serum proteins is approximately 16%, which is lower than any currently existing quinolone on the market (Lai et al., 2014). Nemonoxacin has more than 95% bioavailability, which is similar to levofloxacin and moxifloxacin (File, 2010; Wilson and Macklin-Doherty, 2012). In October 2016, the China Food and Drug Administration approved nemonoxacin malate capsules as a clinical drug for the treatment of community-acquired pneumonia (CAP) in adults. Although clinical trials demonstrated that nemonoxacin had favorable bacteriological success against atypical pathogens, there are no available data on the activity of nemonoxacin against Mycoplasma pneumoniae, Ureaplasma spp., and Mycoplasma hominis, which necessitates exploration (van Rensburg et al., 2010; Huang et al., 2015).
Mycoplasma pneumoniae is a common microorganism found in respiratory tract infections in children and adults and is also responsible for many extrapulmonary manifestations, such as dermatological disorders, central nervous system diseases, and thrombotic thrombocytopenic purpura, which can range in severity from mild to life-threatening (Waites et al., 2017b). Ureaplasma spp. and M. hominis, are frequently found in human urogenital symptomatic patients and healthy individuals (Horner et al., 2018). Ureaplasma spp. can be grouped into Ureaplasma parvum (biovar 1) and Ureaplasma urealyticum (biovar 2), whose DNA homology is less than 60%. U. parvum is more common than U. urealyticum, when isolated from clinical specimens, but both biovars may occur simultaneously in some patients (Fanrong et al., 1999; Waites et al., 2005). In men, M. hominis and U. parvum are not evidently related to diseases, but U. urealyticum is associated with non-gonococcal urethritis cases, but there is no link to infertility. In women, there is no adequate evidence that these three organisms can result in cervicitis, urethritis, inflammatory vulvovaginitis, or infertility (Horner et al., 2018). In addition to urogenital diseases, Ureaplasma spp. is also associated with adverse pregnancy outcomes and newborn diseases, such as chronic lung disease and retinopathy of prematurity (Kokkayil and Dhawan, 2015).
Macrolides, tetracyclines, and fluoroquinolones are three common antibiotic families against M. pneumoniae, Ureaplasma spp., and M. hominis. Acquired macrolide resistance to M. pneumoniae strains in clinical settings has emerged worldwide and is currently complicating treatment, especially in Asia (Waites et al., 2014). Mutations at various positions in 23S rRNA and insertions or deletions in ribosomal proteins L4 and L22 can cause macrolide resistance of M. pneumoniae (Okazaki et al., 2001; Matsuoka et al., 2004). An increasing prevalence of tetracycline and fluoroquinolone resistance of Ureaplasma spp. and M. hominis has been reported in Europe and North America, whereas it is rare in China (Xiao et al., 2012; Beeton and Spiller, 2016; Meygret et al., 2018). Additionally, Ureaplasma spp. resistant rates against macrolides are very low (Schneider et al., 2015; Fernandez et al., 2016). Acquired high-level tetracyclines resistance is related to the presence of the tet(M) gene, while acquired fluoroquinolones resistance is associated with mutations in the quinolone resistance-determining regions (QRDRs) of the gyrA, gyrB, parC, and parE genes (Bebear et al., 2003; Beeton and Spiller, 2016). Here, the aim of this study was to assess the susceptibility of M. pneumoniae, Ureaplasma spp., and M. hominis to in vitro nemonoxacin and other drugs, including 16-membered macrolide. In addition, the resistance mechanisms of resistant isolates were also evaluated.
Materials and Methods
Mycoplasmas Clinical Strains Culture
1. Mycoplasma pneumoniae: Fifty M. pneumoniae clinical strains were originally obtained from Shanghai Children’s Hospital between November 2017 and August 2018 and were stored in the strain bank at the Institute of Antibiotics, Huashan Hospital at −80°C. M. pneumoniae culturing was performed as described previously (Dorigo-Zetsma et al., 1999). All of the strains were identified by colony morphology and PCR assays. PCR amplification of the 16S rRNA genes was performed to identify M. pneumoniae using 5′-GCCACCCTCGGGGGCAGTCAG-3′ and 5′-GAGTCGGGATTCCCCGCGGAGG-3′ primers as previously described (Ieven et al., 1996).
2. Mycoplasma hominis: Twenty M. hominis clinical strains were originally obtained from Shanghai Corps Hospital of Chinese People’s Armed Police between 2013 and 2018 and stored in the strain bank at the Institute of Antibiotics, Huashan Hospital at –80°C. M. hominis culturing was performed as described previously (Novosad et al., 2017). All strains were identified by colony morphology.
3. Ureaplasma spp.: Seventy-seven Ureaplasma spp. clinical strains were originally obtained from Shanghai Corps Hospital of Chinese People’s Armed Police between 2013 and 2018 and stored in the strain bank at the Institute of Antibiotics, Huashan Hospital at –80°C. Ureaplasma spp. culturing was performed as described previously (Xiao et al., 2010). All strains were identified by colony morphology and PCR assays. Confirmation of Ureaplasma spp. was determined by amplification of the Ureaplasma-specific urease gene (a 430-bp DNA product) using Blanchard et al. (1993) U4 and U5 primers. After confirmation, isolates were further separated into species based on PCR primers (UM-1), as published by Teng et al. (1994). A region of the multiple-banded antigen (MBA) was amplified and sequenced. The UM-1 primer sets for U. parvum yielded a 403-bp product, and there was a 448-bp product for U. urealyticum.
The study was approved by the Ethics Committee of Huashan Hospital, Fudan University, China. All strains we used in this study were obtained from biological samples from the strain bank at the Institute of Antibiotics, Huashan Hospital, Shanghai. These samples were obtained during routine clinical testing, and then they were stored in the strain bank. The ethics committee of Huashan Hospital authorized our study, and written informed consent was not required. This study did not harm the rights, benefits and health of the subjects, and the privacy and personal identity information of the subjects has been and will be protected.
All reference strains, including ATCC 29342 (M. pneumoniae), ATCC 23114 (M. hominis), ATCC33699 (U. urealyticum), and ATCC27815 (U. parvum) were purchased from China’s Antubio (603658.SH).
Test for Mycoplasmas Sensitivity to Antibiotics
In this study, 11 antimicrobials were used, including: Nemonoxacin (manufactured by Zhejiang Medicine Co., Ltd., Zhejiang, China), macrolides (erythromycin, roxithromycin, azithromycin, and josamycin), tetracyclines (tetracycline, minocycline, and doxycycline), and fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin) (Dalian Meilun Biotech Co., China). All agents were obtained in powdered form of known purity and diluted according to their respective manufacturer’s instructions. The broth microdilution method was performed and the minimal inhibitory concentration (MIC) was defined according to Clinical and Laboratory Standards Institutes (Waites et al., 2011).
The minimum bactericidal concentrations (MBCs) for nemonoxacin was determined as previously described (Waites et al., 2017a), which included 11 M. pneumoniae isolates, 5 M. hominis isolates, and 5 Ureaplasma spp. isolates. The positive and negative controls consisted of tetracycline (bacteriostatic) and moxifloxacin (bactericidal), respectively. When the MBC was ≥3 dilutions (eightfold) greater than the MIC, the drug was considered bacteriostatic. MBCs that were ≤2 dilutions (fourfold) greater than the MIC were considered bactericidal.
Mutation Point Detection
For M. pneumoniae, the total DNA was extracted by manual nucleic acid extraction (Qiagen QI Amp DNA Mini Kit, Valencia, CA, United States). PCR assays for 23S rRNA were performed to amplify and sequence the full length of the 23S rRNA fragment, as described previously (Liu et al., 2009). In addition, the L4 and L22 ribosomal protein genes were amplified and sequenced (Pereyre et al., 2004).
For M. hominis, antibiotic susceptibility testing of the M. hominis strains have been previously described (Bebear et al., 1997). Total DNA of resistant strains was used as a template in a PCR to amplify the QRDRs of the gyrA, gyrB, parC, and parE (Bébéar et al., 1998).
For Ureaplasma spp., DNA was extracted by manual nucleic acid extraction (Qiagen QI Amp DNA Mini Kit, Valencia, CA, United States). For U. parvum, primers for gyrA-1, gyrA-2, gyrB-3, gyrB-4, parC-5, parC-6, parE-7, and parE-8 were used to amplify the gyrA, gyrB, parC, and parE genes, respectively. For U. urealyticum, primers for the gyrA, parC, and parE genes were the same as the primers used for U. parvum, whereas primers for gyrB-F-UuUp and gyrB-R-UuUp were designed to amplify gyrB, as described previously (Meygret et al., 2018). Primers for TetMF and TetMR were used to verify the existence of tet(M) in tetracycline-resistant strains. All gene sites and protein residues were numbered according to M. pneumonia, M. hominis, or Ureaplasma spp.
Results
Nemonoxacin Was Active Against M. pneumoniae
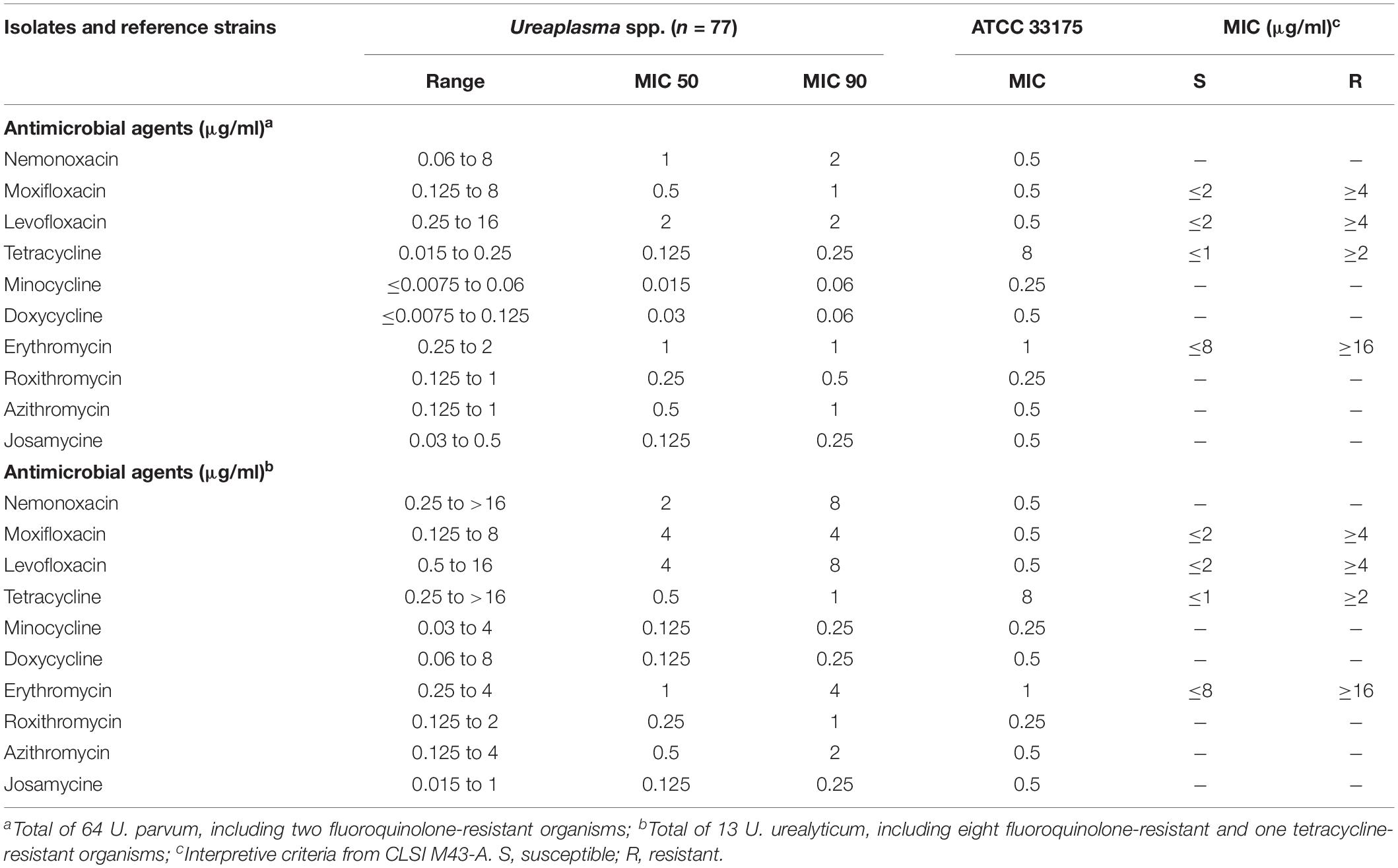
Here, we detected the nemonoxacin MIC in 50 M. pneumoniae isolates, which suggested that nemonoxacin was active in vitro against all 50 clinical isolates of M. pneumoniae. Nemonoxacin and moxifloxacin had the lowest MIC50 and MIC90 values (0.125 and 0.25 μg/ml, respectively) among all 11 drugs tested against M. pneumoniae, and their dilutions were twofold lower than those of the tetracyclines and levofloxacin, with all nemonoxacin MICs of ≤0.25 μg/ml (Table 1). We found that 46 of the 50 isolates were macrolides-resistant organisms and had A to G mutation at position 2,063 in their 23S rRNA gene compared with the M. pneumoniae reference strain. No mutations in ribosomal proteins L4 and L22 were found. These organisms had elevated MICs for erythromycin, roxithromycin, and azithromycin, ranging from 4 to >128 μg/ml, while the nemonoxacin MICs were 0.06 to 0.25 μg/ml. Azithromycin possessed lower MIC50 and MIC90 levels (8 and 32 μg/mL, respectively) than those of erythromycin and roxithromycin. Additionally, the 16-member macrolide josamycin had lower MICs than the 14- and 15-member macrolides. All 50 M. pneumoniae isolates were susceptible to the tetracyclines (tetracycline, doxycycline, and minocycline) and fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin) we tested. An exhaustive MIC distribution of the 11 antibiotics against M. pneumoniae is shown in Supplementary Table 1. Taken together, these results suggest that nemonoxacin and moxifloxacin were active against M. pneumoniae and had lower MIC levels than other antibiotics.
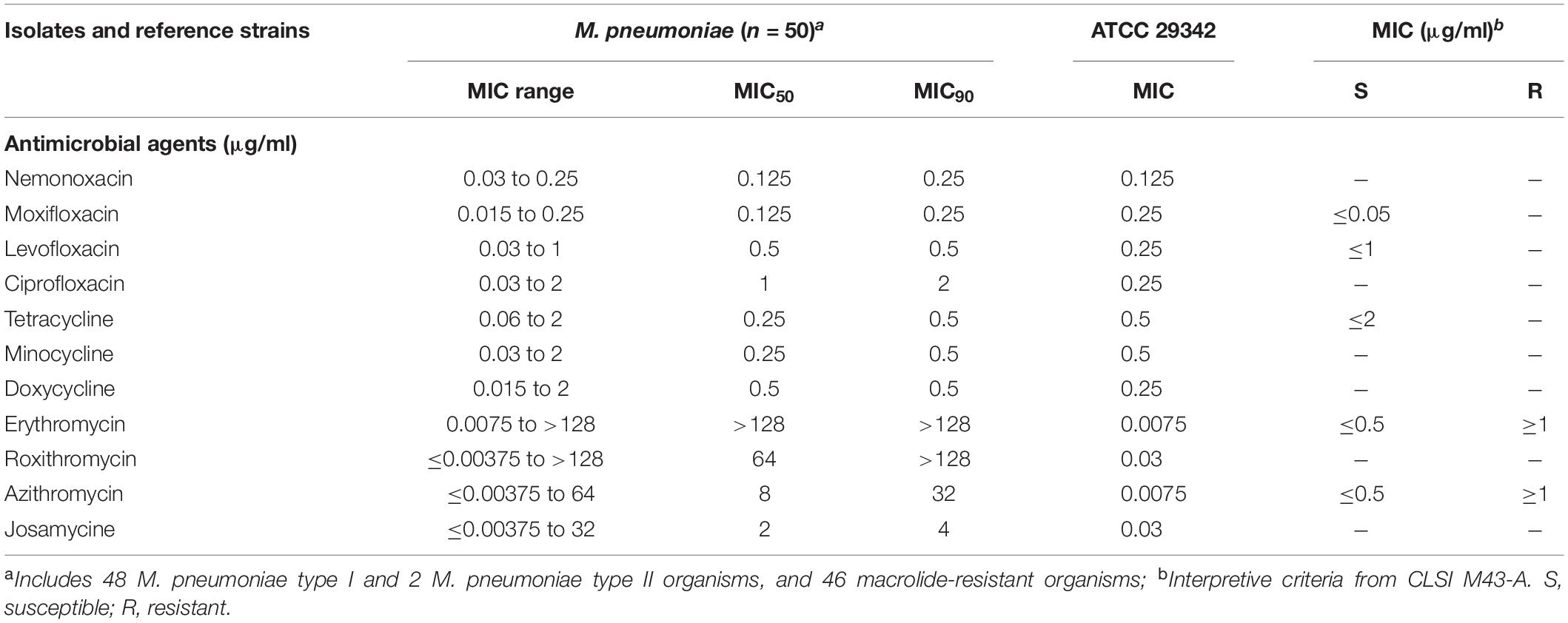
Table 1. Minimal inhibitory concentration summary of nemonoxacin and comparator agents against M. pneumoniae.
The Activity of Nemonoxacin Against M. hominis Is Similar to That of Fluoroquinolones
The nemonoxacin MICs against 20 M. hominis clinical strains isolated from urogenital specimens ranged from 0.25 to 8 μg/ml and had an MIC50 of 2 μg/ml, making nemonoxacin less active in vitro than the tested tetracyclines (Table 2). Due to the intrinsic resistance of M. hominis to 14- and 15-membered macrolides, the MICs of erythromycin, roxithromycin, and azithromycin were >128 μg/ml. However, josamycin, as a 16-membered macrolide, had lower MIC50 and MIC90 levels than the fluoroquinolones and nemonoxacin, but the levels were higher than those of the tetracyclines. In these drugs, the resistance breakpoints of josamycin, ciprofloxacin, doxycycline, and minocycline were not defined. A MIC distribution of the 11 antibiotics against M. hominis is shown in Supplementary Table 2. No tetracycline-resistant organisms were found, but 16 out of the 20 M. hominis isolates were fluoroquinolone-resistant organisms. The tetracyclines, especially doxycycline and minocycline, had the lowest MIC50 and MIC90 values among the 11 drugs tested against M. hominis.
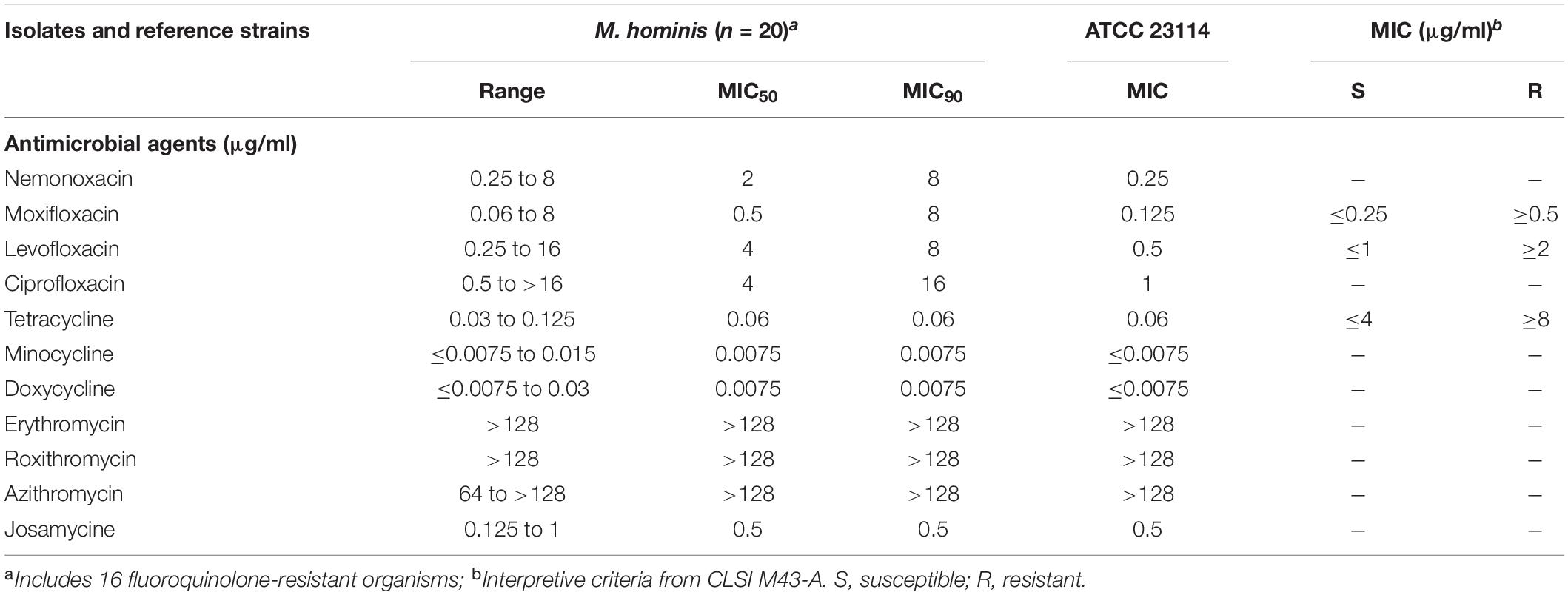
Table 2. Minimal inhibitory concentration summary of nemonoxacin and comparator agents against M. hominis.
By sequencing gyrA, gyrB, parC, and parE, some gene point mutations and amino acid changes were found (Table 3). These mutations were mainly located at position 458 (C-T) and 606 (T-C) for gyrA, 272 (G-T) for parC, which lead to a substitution of Ser-153 by Leu in the gyrA protein, and Ser-91 by Ile in the parC protein. Only one strain had a gene point mutation and amino acid changes in the parE protein (Pro445Ser, C1333T). In gyrA and parC, there are many synonymous mutations apart from only one point mutation corresponding to one amino acid substitution. Collectively, these results suggest that quinolone resistance determining region mutations, such as gyrA and parC, may be related to elevated MICs of nemonoxacin to M. hominis.
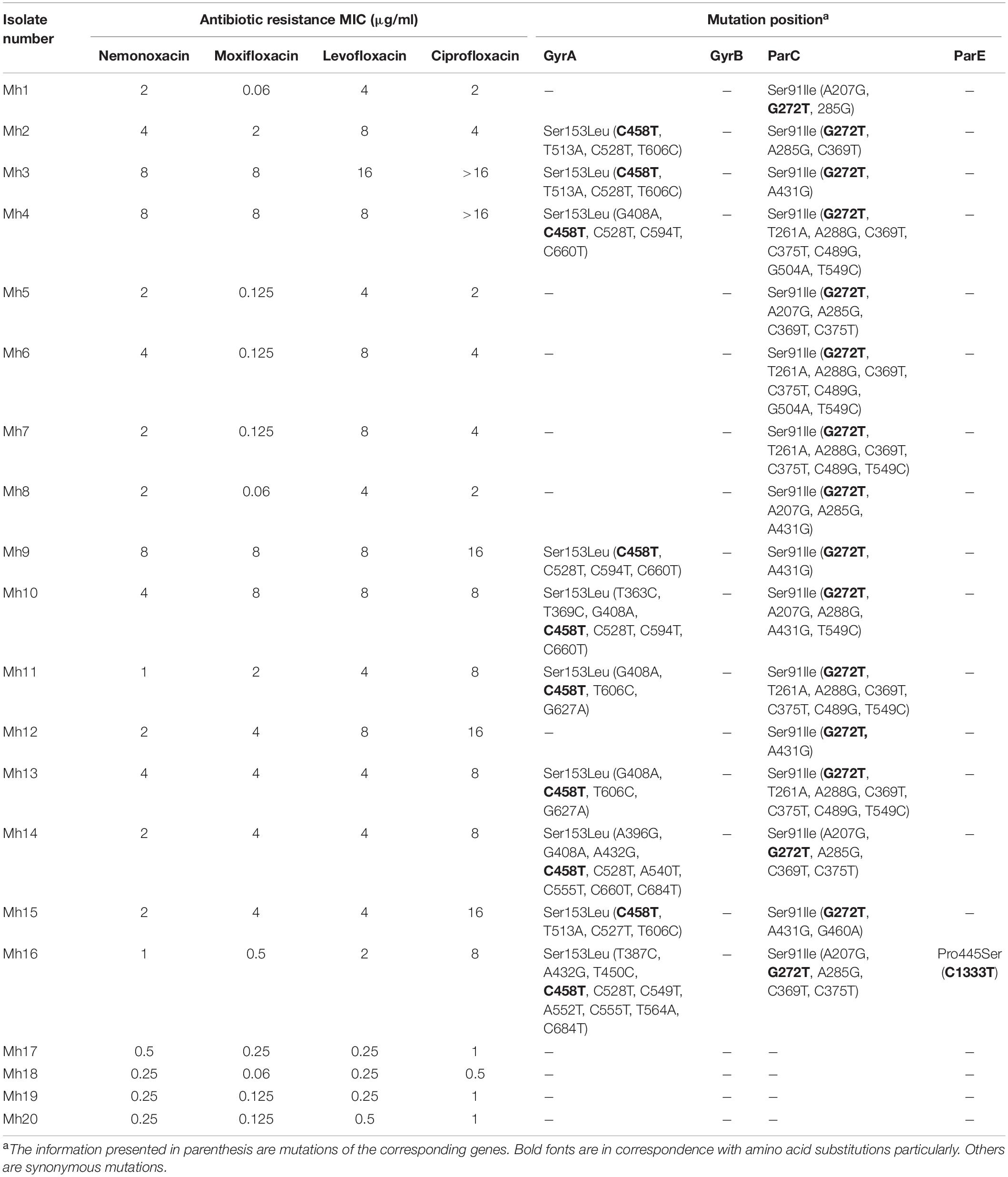
Table 3. Gene point mutation and amino acid changes in GyrA, GyrB, ParC, and ParE in the Mh clinical isolates.
MICs of Nemonoxacin for Ureaplasma spp.
Of the 77 Ureaplasma spp., 64 isolates were U. parvum (83.12%), and 13 were U. urealyticum (16.88%). All isolates were sensitive to erythromycin and had lower MIC50 and MIC90 values for azithromycin, roxithromycin, and josamycine compared with the fluoroquinolones. Only one tetracycline resistant isolate (tetracycline MIC > 16 μg/ml) with tet(M) was found, while the tet(M) element was not detected in the other tetracycline susceptible isolates.
In all the U. parvum isolates, the nemonoxacin MICs (MIC50 = 1 μg/ml, MIC90 = 2 μg/ml) were higher than any of the other comparator agents, except levofloxacin (Table 4). There were 2 U. parvum strains resistant to fluoroquinolones, whose nemonoxacin MICs were 4 and 8 μg/ml. One mutation in gryB (C1384T, Pro153Ser) was found in only one U. parvum resistant isolate (levofloxacin MIC = 16 μg/ml, moxifloxacin MIC = 4 μg/ml). Another U. parvum isolate (levofloxacin MIC = 4 μg/ml) showed fluoroquinolone resistance, but it still retained all wild-type sequences.
Meanwhile, we also collected eight fluoroquinolone-resistant isolates from among the 13 U. urealyticum strains; one was also resistant to the tetracyclines with tet(M). The nemonoxacin MICs of the eight fluoroquinolone-resistant isolates ranged from 2 to >16 μg/ml, while moxifloxacin and levofloxacin were 1–8 and 4–16 μg/ml, respectively. Seven fluoroquinolone-resistant isolates revealed the presence of the same mutation at position 248 (C-T) for parC, which leads to a substitution of Ser-83 by Leu in the parC protein. Another fluoroquinolone-resistant isolate existed, which had gene mutations at position 248 (C-T) and 310 (G-A) with a substitution of Ser-83 by Leu in the parC.
A detailed MIC distribution of 10 antibiotics against Ureaplasma spp. is shown in Supplementary Table 3.
Nemonoxacin Is Bactericidal Against M. pneumoniae
In this study, the MBCs of nemonoxacin for M. pneumoniae were within twofold dilutions of the MIC, which indicates that it is bactericidal against M. pneumoniae. However, all MBCs for M. hominis and Ureaplasma spp. were three or more dilutions greater than their corresponding MICs, indicating a bacteriostatic effect.
Discussion
A fluorine atom at position C-6 and various substitutions on the basic bicyclic ring structure produce fluoroquinolones, namely, norfloxacin, levofloxacin, ciprofloxacin, and many other drugs (Kocsis et al., 2016). Adding a fluorine at the sixth position can greatly improve the activity of fluoroquinolones compared with the former quinolones. Ciprofloxacin, levofloxacin, and moxifloxacin are currently the most commonly prescribed fluoroquinolones in medical practice, but their use is limited due to toxic side effects and an increasing number of resistant pathogens (Huang et al., 2015; Kocsis et al., 2016). Nemonoxacin may have increased antibacterial effectiveness and decreased frequency of toxic adverse effects due to its C-8-methoxy substituent on the quinolone ring and the lack of a fluorine residue (Barry et al., 2001; Andersson and MacGowan, 2003). In our research, nemonoxacin and moxifloxacin exhibited more potent antibacterial activities against M. pneumoniae than other fluoroquinolones, which may be attributed to their 8-methoxy structures that enable the targeting of both topoisomerases II (i.e., DNA gyrase) and IV (Siegert et al., 2000; Guo et al., 2012; Lai et al., 2014).
For many years, the empirical treatments of choice for M. pneumoniae infections were macrolides, especially for children, in whom tetracyclines and fluoroquinolones were limited because of possible toxicities (Waites et al., 2017b). Unfortunately, 92% (46/50) of macrolide resistance rates were detected in our study, which is similar to our previous study and other data from Asia. In the last 10 years, the macrolide resistance rates of M. pneumoniae have not changed in Shanghai, while in Europe and the United States, the resistance prevalence is substantially lower than that in Asia (Morozumi et al., 2008; Liu et al., 2009; Waites et al., 2017b). In addition, all macrolides resistant isolates in our study only had the one same mutation in 23S rRNA gene (A2063G), which is slightly different from previous studies that showed another mutation in the 2,064 position of the 23S rRNA gene (Pereyre et al., 2007; Morozumi et al., 2008; Xin et al., 2009). Josamycine, a 16-membered macrolide, differs from 14-membered macrolides by having extended C-5 sugar units, which can slow down the formation of the first peptide bond and inhibit formation of any further bonds. Methylation of A2058 (Escherichia coli numbering) in 23S rRNA is the classical resistance mechanism of 14-membered macrolides, but it does not have such a dramatic effect on 16-membered macrolides (Arsic et al., 2018). Indeed, josamycine had lower MICs for M. pneumoniae than did the 14-membered macrolides in our results. Until now, resistance to tetracyclines and fluoroquinolones in M. pneumoniae have never been reported (Waites et al., 2017b). No resistant strains to tetracyclines and fluoroquinolones were detected in our collected M. pneumoniae isolates. Between the macrolide sensitive and resistant strains, there was no evident difference in the nemonoxacin MICs. Therefore, mutations related to macrolides resistance may not influence the activity of nemonoxacin.
The 20 M. hominis isolates were not resistant to the tetracyclines we tested and had low josamycine MICs; however, 80% (16/20) of the isolates were resistant to the fluoroquinolones we tested. There are many previous studies that showed susceptibility of M. hominis to tetracyclines and fluoroquinolones, but few data support susceptibility to josamycine. In Korea, similarly, M. hominis isolates were susceptible to doxycycline and tetracycline (100 and 94.12%, respectively) (Choi et al., 2018). In France, Meygret et al. (2018) reported the resistance rate of M. hominis to tetracycline was 14.8% from 2010 to 2015, and Degrange et al. (2008) found the rate was 18.75% from 1999 to 2002. Almost all fluoroquinolone resistance in M. hominis was caused by gyrA and/or parC mutations in our research. In comparison with a parC mutation alone, it is interesting that an additional mutation in gyrA seems to increase the quinolone resistance by at least twofold, particularly for moxifloxacin and ciprofloxacin, which occurred in Meygret’s and Bebear’s articles as well (Bebear et al., 2000; Meygret et al., 2018). Like the Ureaplasma spp. strains, the higher nemonoxacin MICs in the fluoroquinolone-resistant strains may have gained resistance via mutations of the QRDRs, whereas those isolates without the mutations were susceptible.
In this study, we found only one U. parvum isolate that was resistant to tetracycline with tet(M), and no macrolide-resistant Ureaplasma spp. strains. In Switzerland, low rates of non-susceptibility were observed for clarithromycin (4.9%), erythromycin (1.9%), and azithromycin (1%), and there were no isolates that showed a reduced susceptibility to josamycine and tetracyclines, according to the IST 2 kit (Schneider et al., 2015). In the United States, all 250 Ureaplasma isolates detected were not resistant to erythromycin and had parallel MICs to azithromycin by broth microdilution, while one U. parvum isolate was resistant to tetracycline as well (Fernandez et al., 2016). The slight inconsistencies between various research studies might be due to different methodologies and interpretation criteria of susceptibility. Beeton et al. (2009b) found the tetracycline-susceptible strain (HPA6) was tetM positive, meanwhile Fernandez et al. (2016) also reported that one isolate with tet(M) in 10 U. parvum isolates was susceptible. However, no tet(M) element was found in the tetracycline-susceptible isolates in our study, which is in contrast to those mentioned above.
Regarding the detection rate, the fluoroquinolone-resistant rate and the resistance mechanism, a comparison of U. urealyticum with U. parvum highlights obvious differences in our results. Of the 77 Ureaplasma spp. strains we observed, we detected 13 U. urealyticum (16.88%) and 64 U. parvum (83.12%) strains. In other research studies, U. urealyticum was also isolated much less frequently than U. parvum from patients (Beeton et al., 2009b; Fernández et al., 2016). However, around a 61% (8/13) resistance rate of U. urealyticum to fluoroquinolones is far higher than U. parvum’s 3% (2/64) resistance rate. Of the fluoroquinolone-resistant Ureaplasma spp. strains, the portion of U. urealyticum was 80% (8/10). All fluoroquinolone-resistant U. urealyticum had a parC Ser83Leu mutation alone, whereas a gyrB Pro153Ser mutation was found in one of two fluoroquinolone-resistant U. parvum isolates. As previous studies, we also found that about 80% U. urealyticum was detected and the ParC Ser83Leu mutation was largely found in all fluoroquinolone-resistant Ureaplasma spp. (Beeton et al., 2009a). Compared with fluoroquinolone-susceptible isolates, the nemonoxacin MICs of fluoroquinolone-resistant strains had higher values in our results. Therefore, the mutations related to fluoroquinolones resistance in Ureaplasma spp. could affect the activity of nemonoxacin, which is similar to Streptococcus pneumoniae, E. coli, and methicillin-resistant Staphylococcus aureus (Lauderdale et al., 2010).
In summary, this report highlights the superior activity of nemonoxacin and moxifloxacin against M. pneumoniae in vitro in comparison with Ureaplasma spp. and M. hominis. For macrolide-resistant M. pneumoniae infections, 16-membered macrolides may be a great choice for children. According to different antibiotic susceptibilities, identifying U. parvum or U. urealyticum in clinical isolates could help clinicians choose appropriate drugs for treatment. For U. urealyticum infections especially, fluoroquinolones are not recommended because of their high resistance rate. Additionally, 16-membered macrolide and tetracycline can be used as treatments for M. hominis infections in China.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
The strains, we used in this study, obtained from the biological sample and strains bank of the Institute of Antibiotics, Huashan Hospital, Shanghai. They come from the normal clinical testing. And then, they were stored in the strains bank. The ethics committee of Huashan Hospital authorized our study and written informed consent is not required. This study would not do harm to rights, benefits, and health of the subjects, and the privacy and personal identity information of the subjects will be protected.
Author Contributions
YL and NW contributed to the design and implementation of the study. NW, WL, and YZ performed the experimental studies and analysis. All authors aided in the interpretation and outcomes of the experimental analyses, contributed to the revisions and editing of the manuscript, and read and approved the final version of the manuscript for submission. NW and WL wrote the first draft of the manuscript. YL and YZ extensively edited the subsequent drafts of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC81772235), the Natural Science Foundation of Shanghai (13ZR1449700), and the Shanghai Pujiang Talent Program (17PJD004).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01890/full#supplementary-material
References
Andersson, M. I., and MacGowan, A. P. (2003). Development of the quinolones. J. Antimicrob. Chemother. 51(Suppl.1), 1–11. doi: 10.1093/jac/dkg212
Arsic, B., Barber, J., Cikos, A., Mladenovic, M., Stankovic, N., and Novak, P. (2018). 16-membered macrolide antibiotics: a review. Int. J. Antimicrob. Agents 51, 283–298. doi: 10.1016/j.ijantimicag.2017.05.020
Barry, A. L., Fuchs, P. C., and Brown, S. D. (2001). In vitro activities of three nonfluorinated quinolones against representative bacterial isolates. Antimicrob. Agents Chemother. 45, 1923–1927. doi: 10.1128/aac.45.6.1923-1927.2001
Bebear, C., Renaudin, H., Charron, A., Gruson, D., Lefrancois, M., and Bebear, C. (2000). In Vitro activity of trovafloxacin compared to those of five antimicrobials against mycoplasmas includingmycoplasma hominis and Ureaplasma urealyticumfluoroquinolone-resistant isolates that have been genetically characterized. Antimicrob. Agents Chemother. 44, 2557–2560. doi: 10.1128/AAC.44.9.2557-2560.2000
Bebear, C. M., Bove, J. M., Bebear, C., and Renaudin, J. (1997). Characterization of Mycoplasma hominis mutations involved in resistance to fluoroquinolones. Antimicrob. Agents Chemother. 41, 269–273. doi: 10.1128/AAC.41.2.269
Bébéar, C. M., Renaudin, H., Charron, A., Bové, J. M., Bébéar, C., and Renaudin, J. (1998). Alterations in topoisomerase IV and DNA gyrase in quinolone-resistant mutants of Mycoplasma hominis obtained in vitro. Antimicrob. Agents Chemother. 42, 2304–2311. doi: 10.1128/AAC.42.9.2304
Bebear, C. M., Renaudin, H., Charron, A., Clerc, M., Pereyre, S., and Bebear, C. (2003). DNA gyrase and topoisomerase IV mutations in clinical isolates of Ureaplasma spp. and Mycoplasma hominis resistant to fluoroquinolones. Antimicrob. Agents Chemother. 47, 3323–3325. doi: 10.1128/AAC.47.10.3323-3325.2003
Beeton, M. L., Chalker, V. J., Kotecha, S., and Spiller, O. B. (2009a). Comparison of full gyrA, gyrB, parC and parE gene sequences between all Ureaplasma parvum and Ureaplasma urealyticum serovars to separate true fluoroquinolone antibiotic resistance mutations from non-resistance polymorphism. J. Antimicrob. Chemother. 64, 529–538. doi: 10.1093/jac/dkp218
Beeton, M. L., Chalker, V. J., Maxwell, N. C., Kotecha, S., and Spiller, O. B. (2009b). Concurrent titration and determination of antibiotic resistance in ureaplasma species with identification of novel point mutations in genes associated with resistance. Antimicrob. Agents Chemother. 53, 2020–2027. doi: 10.1128/AAC.01349-08-1348
Beeton, M. L., and Spiller, O. B. (2016). Antibiotic resistance among Ureaplasma spp. isolates: cause for concern? J. Antimicrob. Chemother. 72, 330–337. doi: 10.1093/jac/w425
Blanchard, A., Hentschel, J., Duffy, L., Baldus, K., and Cassell, G. H. (1993). Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin. Infect. Dis. 17, 148–153. doi: 10.1093/clinids/17.Supplement_1.S148
Choi, J. B., Lee, S.-J., Lee, M.-K., Lee, S.-J., Park, D. C., Kim, H. Y., et al. (2018). Prevalence and antimicrobial susceptibility of Ureaplasma spp. and Mycoplasma hominis in asymptomatic individuals in Korea. Microb. Drug Resist. 24, 1391–1396. doi: 10.1089/mdr.2017.0431
Chung, D. T., Tsai, C.-Y., Chen, S.-J., Chang, L.-W., King, C.-H. R., Hsu, C.-H., et al. (2010). Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob. Agents Chemother. 54, 411–417. doi: 10.1128/AAC.00683-09-689
Degrange, S., Renaudin, H., Charron, A., Bebear, C., and Bebear, C. M. (2008). Tetracycline resistance in Ureaplasma spp. and Mycoplasma hominis: prevalence in Bordeaux, France, from 1999 to 2002 and description of two tet(M)-positive isolates of M. hominis susceptible to tetracyclines. Antimicrob. Agents Chemother. 52, 742–744. doi: 10.1128/AAC.00960-07-967
Dorigo-Zetsma, J. W., Zaat, S. A., Wertheim-van Dillen, P. M., Spanjaard, L., Rijntjes, J., van Waveren, G., et al. (1999). Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J. Clin. Microbiol. 37, 14–17.
Fanrong, K., James, G., Zhenfang, M., Gordon, S., Wang, B., and Gilbert, G. L. (1999). Phylogenetic analysis of Ureaplasma urealyticum – support for the establishment of a new species, Ureaplasma parvum. Microbiol. Soc. 49(Pt 4), 1879–1889. doi: 10.1099/00207713-49-4-1879
Fernandez, J., Karau, M. J., Cunningham, S. A., Greenwood-Quaintance, K. E., and Patel, R. (2016). Antimicrobial susceptibility and clonality of clinical ureaplasma isolates in the United States. Antimicrob. Agents Chemother. 60, 4793–4798. doi: 10.1128/AAC.00671-616
Fernández, J., Karau, M. J., Cunningham, S. A., Greenwood-Quaintance, K. E., and Patel, R. J. (2016). Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob. Agents Chemother. 60, 4793–4798. doi: 10.1128/AAC.00671-616
File, T. M. (2010). Levofloxacin in the treatment of community acquired pneumonia. Expert Rev. Anti Infect. Ther. 4, 725–742. doi: 10.1586/14787210.4.5.725
Guo, B., Wu, X., Zhang, Y., Shi, Y., Yu, J., Cao, G., et al. (2012). Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clin. Drug Investig. 32, 475–486. doi: 10.2165/11632780-000000000-00000
Horner, P., Donders, G., Cusini, M., Gomberg, M., Jensen, J., Unemo, M., et al. (2018). Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?–a position statement from the European STI guidelines editorial board. J. Eur. Acad. Dermatol. 32, 1845–1851. doi: 10.1111/jdv.15146
Huang, C. H., Lai, C. C., Chen, Y.-H., and Hsueh, P. R. (2015). The potential role of nemonoxacin for treatment of common infections. Expert Opin. Pharmacol. 16, 263–270. doi: 10.1517/14656566.2015.978288
Ieven, M., Ursi, D., Van Bever, H., Quint, W., Niesters, H. G., and Goossens, H. (1996). Detection of Mycoplasma pneumoniae by two polymerase chain reactions and role of M. pneumoniae in acute respiratory tract infections in pediatric patients. J. Infect. Dis. 173, 1445–1452. doi: 10.1093/infdis/173.6.1445
Kocsis, B., Domokos, J., and Szabo, D. (2016). Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 15, 34–34. doi: 10.1186/s12941-016-0150-154
Kokkayil, P., and Dhawan, B. (2015). Ureaplasma: current perspectives. Indian J. Med. Microbial. 33, 205–214. doi: 10.4103/0255-0857.154850
Lai, C.-C., Lee, K.-Y., Lin, S.-W., Chen, Y.-H., Kuo, H.-Y., Hung, C.-C., et al. (2014). Nemonoxacin (TG-873870) for treatment of community-acquired pneumonia. Expert Rev. Anti Infect. Ther. 12, 401–417. doi: 10.1586/14787210.2014.894881
Lauderdale, T.-L., Shiau, Y.-R., Lai, J.-F., Chen, H.-C., and King, C.-H. R. (2010). Comparative in vitro activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrob. Agents Chemother. 54, 1338–1342. doi: 10.1128/AAC.01197-09-1199
Liu, Y., Ye, X., Zhang, H., Xu, X., Li, W., Zhu, D., et al. (2009). Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob. Agents Chemother. 53, 2160–2162. doi: 10.1128/AAC.01684-08-1688
Matsuoka, M., Narita, M., Okazaki, N., Ohya, H., Yamazaki, T., Ouchi, K., et al. (2004). Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob. Agents Chemother. 48, 4624–4630. doi: 10.1128/AAC.48.12.4624-4630.2004
Meygret, A., Le Roy, C., Renaudin, H., Bébéar, C., and Pereyre, S. (2018). Tetracycline and fluoroquinolone resistance in clinical Ureaplasma spp. and Mycoplasma hominis isolates in France between 2010 and 2015. J. Antimicrob. Chemother. 73, 2696–2703. doi: 10.1093/jac/dky238
Morozumi, M., Iwata, S., Hasegawa, K., Chiba, N., Takayanagi, R., Matsubara, K., et al. (2008). Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob. Agents Chemother. 52, 348–350. doi: 10.1128/AAC.00779-07-777
Novosad, S. A., Basavaraju, S. V., Annambhotla, P., Mohr, M., Halpin, A. L., Foy, L., et al. (2017). Mycoplasma hominis infections transmitted through amniotic tissue product. Clin. Infect. Dis. 65, 1152–1158. doi: 10.1093/cid/cix507
Okazaki, N., Narita, M., Yamada, S., Izumikawa, K., Umetsu, M., Kenri, T., et al. (2001). Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol. Immunol. 45, 617–620. doi: 10.1111/j.1348-0421.2001.tb01293.x
Pereyre, S., Charron, A., Renaudin, H., Bebear, C., and Bebear, C. M. (2007). First report of macrolide-resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. J. Clin. Microbiol. 45, 3534–3539. doi: 10.1128/JCM.01345-07-1347
Pereyre, S., Guyot, C., Renaudin, H., Charron, A., Bebear, C., Bebear, C. M., et al. (2004). In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 48, 460–465. doi: 10.1128/AAC.48.2.460-465.2004
Schneider, S. C., Tinguely, R., Droz, S., Hilty, M., Dona, V., Bodmer, T., et al. (2015). Antibiotic susceptibility and sequence type distribution of ureaplasma species isolated from genital samples in Switzerland. Antimicrob. Agents Chemother. 59, 6026–6031. doi: 10.1128/AAC.00895-815
Siegert, R., Gehanno, P., Nikolaidis, P., Bagger-Sjoback, D., Ibanez, J. M., Hampel, B., et al. (2000). A comparison of the safety and efficacy of moxifloxacin (BAY 12-8039) and cefuroxime axetil in the treatment of acute bacterial sinusitis in adults. Respir. Med. 94, 337–344. doi: 10.1053/rmed.1999.0769
Teng, L. J., Zheng, X., Glass, J. I., Watson, H. L., Tsai, J., and Cassell, G. H. (1994). Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J. Clin. Microbiol. 32, 1464–1469.
van Rensburg, D. J., Perng, R. P., Mitha, I. H., Bester, A. J., Kasumba, J., Wu, R.-G., et al. (2010). Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob. Agents Chemother. 54, 4098–4106. doi: 10.1128/AAC.00295-210
Waites, K. B., Bade, D. J., Bébéar, C., Brown, S. D., Davidson, M. K., Duffy, L. B., et al. (2011). Methods for Antimicrobial Susceptibility Testing of Human Mycoplasmas. Approved Guideline M43-A. Wayne, PA: Clinical and Laboratory Standards Institute.
Waites, K. B., Crabb, D. M., Duffy, L. B., Jensen, J. S., Liu, Y., and Paukner, S. (2017a). In Vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob. Agents Chemother. 61, e2008–e2016. doi: 10.1128/AAC.02008-16
Waites, K. B., Xiao, L., Liu, Y., Balish, M. F., and Atkinson, T. P. (2017b). Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microb. Rev. 30, 747–807. doi: 10.1128/CMR.00114-116
Waites, K. B., Katz, B., and Schelonka, R. L. (2005). Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18, 757–789. doi: 10.1128/CMR.18.4.757-789.2005
Waites, K. B., Lysnyansky, I., and Bébéar, C. M. (2014). “Emerging antimicrobial resistance in mycoplasmas of humans and animals,” in Mollicutes: Molecular Biology and Pathogenesis, eds G. F. Browning and C. Citti (Norfolk, VA: Caister Academic Press).
Wilson, R., and Macklin-Doherty, A. (2012). The use of moxifloxacin for acute exacerbations of chronic obstructive pulmonary disease and chronic bronchitis. Expert Rev. Respir. Med. 6, 481–492. doi: 10.1586/ers.12.50
Xiao, L., Crabb, D. M., Duffy, L. B., Paralanov, V., Glass, J. I., and Waites, K. B. (2012). Chromosomal mutations responsible for fluoroquinolone resistance in Ureaplasma species in the United States. Antimicrob. Agents Chemother. 12, 2780–2783. doi: 10.1128/AAC.06342-6311
Xiao, L., Glass, J. I., Paralanov, V., Yooseph, S., Cassell, G. H., Duffy, L. B., et al. (2010). Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J. Clin. Microbiol. 48, 2715–2723. doi: 10.1128/JCM.01877-09-1879
Keywords: nemonoxacin, Mycoplasma, Ureaplasma, antimicrobial resistance, MICs
Citation: Wang N, Liu W, Zhou Y and Liu Y (2019) In vitro Activities of Nemonoxacin and Other Antimicrobial Agents Against Human Mycoplasma and Ureaplasmas Isolates and Their Defined Resistance Mechanisms. Front. Microbiol. 10:1890. doi: 10.3389/fmicb.2019.01890
Received: 09 May 2019; Accepted: 31 July 2019;
Published: 13 August 2019.
Edited by:
Florence Tardy, National Agency for Sanitary Safety of Food, Environment and Labor (ANSES), FranceReviewed by:
Sabine Pereyre, Université de Bordeaux, FranceRoger Dumke, Dresden University of Technology, Germany
Copyright © 2019 Wang, Liu, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, bGl1eWFuZ0BmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
 Na Wang
Na Wang Wancheng Liu
Wancheng Liu Yunheng Zhou
Yunheng Zhou Yang Liu
Yang Liu