- 1College of Food Science, South China Agricultural University, Guangzhou, China
- 2Guangdong Institute of Microbiology Guangdong Academy of Sciences, State Key Laboratory of Applied Microbiology Southern China, Guangdong Provincial Key Laboratory of Microbial Culture Collection and Application, Guangdong Open Laboratory of Applied Microbiology, Guangzhou, China
Salmonella remains the leading cause of reported bacterial foodborne disease in China. Meat products are recognized as one of the major sources of human salmonellosis; however, there is a lack of comprehensive, quantitative data concerning Salmonella contamination of these foods. Therefore, the objectives of this study were to investigate the prevalence, bacterial load, and antimicrobial resistance profiles of various Salmonella serovars in retail meat across the whole of China. Between July 2011 and June 2016, a total of 807 retail meat samples were collected, covering most provincial capitals in China. Overall, 159 (19.7%) samples tested positive for Salmonella. The highest contamination rate occurred in pork (37.3%, n = 287), followed by beef (16.1%, n = 161), mutton (10.9%, n = 92), dumplings (6.6%, n = 212), and smoked pork (3.6%, n = 55). Most probable number (MPN) analysis revealed that contamination was mainly in the range of 0.3–10 MPN/g among those samples testing positive using this method (n = 83), with eight samples exceeding 110 MPN/g. Among the 456 Salmonella enterica subsp. enterica isolates obtained in this study, 29 serovars and 33 multilocus sequence typing patterns were identified, with S. Derby, S. Typhimurium, S. London, S. Rissen, S. 1,4,[5],12:i:-, S. Weltevreden, and S. Enteritidis being the most prevalent. Among the 218 non-duplicate isolates, 181 (83.0%) were resistant to at least one class of antimicrobials and 128 (58.7%) were resistant to at least three classes. High rates of resistance were observed for tetracycline (65.6%), ampicillin (45.4%), trimethoprim-sulfamethoxazole (40.8%), streptomycin (40.4%), and nalidixic acid (35.8%), with the seven most prevalent serovars, except S. Weltevreden, showing higher rates of resistance and multidrug resistance compared with the less dominant serovars. Of note, all S. Indiana isolates exhibited resistance to extended-spectrum cephalosporins (including ceftriaxone and cefepime), ciprofloxacin, and multiple other classes of antibiotics. Further, two S. 1,4,[5],12:i:- isolates showed resistance to imipenem. This study provides systematic and comprehensive data on the prevalence and antimicrobial resistance profiles of various Salmonella serovars isolated from meat products in China, indicating their potential risk to public health.
Introduction
Foodborne diseases caused by Salmonella are an important public health concern. Globally, 94 million cases of gastroenteritis and 155,000 deaths are attributed to Salmonella each year (Majowicz et al., 2010; Deng et al., 2012). Although more than 2,600 Salmonella enterica serovars have been reported (Achtman et al., 2012), the majority of infections are caused by a limited number of serovars, which may vary from country to country and over time (Hendriksen et al., 2011; Van et al., 2012). S. enterica subsp. enterica serovars Enteritidis and Typhimurium are the most commonly reported serovars associated with human salmonellosis cases worldwide (Hendriksen et al., 2011). S. 1,4,[5],12:i:- is a monophasic variant of S. Typhimurium and has recently been recognized as a novel serovar and an emerging cause of infection (Yang et al., 2015). Different serovars are associated with different disease potentials (Achtman et al., 2012), making serotype determination particularly important for epidemiological surveillance and disease assessment.
China has a high incidence of salmonellosis (Cui et al., 2009; Deng et al., 2012), with meat products recognized as a significant source of human infection (Yang et al., 2010; Deng et al., 2012). Correspondingly, high rates of Salmonella contamination of retail meats such as pork, beef, and mutton have been reported in several cities and provinces in China (Yan et al., 2010; Yang et al., 2010; Li et al., 2014). However, previous surveillance reports from China are based on sampling carried out in only one or a small number of cities, limiting the applicability of the data. As such, there is a lack of comprehensive data concerning Salmonella contamination of retail meat products across China as a whole. In particular, studies carrying out direct enumeration of Salmonella from retail meat products in China are limited. Thus, the levels of Salmonella contamination of meat products and their potential risk to public health have never been evaluated.
In addition, the increasing prevalence of multidrug resistance (MDR) among Salmonella isolates is a global concern. Moreover, emerging resistance to extended-spectrum cephalosporins and fluoroquinolones is of extreme importance to public health, as these classes of antibiotics are vital to the management of human cases of salmonellosis (Lunguya et al., 2013). Therefore, in the current study, samples of meat products were examined to provide scientific data for the quantitative assessment of the risks of Salmonella to public health. Samples were collected from retail markets in China and assessed to determine the prevalence and contamination rates of Salmonella. The resulting Salmonella isolates were also characterized to determine their serotypes, genotypes, and antimicrobial resistance profiles.
Materials and Methods
Sample Collection
Between July 2011 and June 2016, 807 meat samples were collected, including pork (n = 287), beef (n = 161), mutton (n = 92), dumplings (n = 212), and smoked pork (n = 55). The sampled meat products were collected from three types of retail stores: supermarkets, fairs, and farmers’ markets, which covered most of the capital cities of the different provinces of China, including Hong Kong and Macao, resulting in a large geographic spread (Supplementary Table S1 and Supplementary Figure S1). Each sample was weighed, labeled, and placed in a separate sterile bag before being immediately transported to the laboratory in an icebox.
Detection and Enumeration of Salmonella
All of the samples were subjected to qualitative and quantitative analysis for Salmonella. Qualitative detection was performed as described in National Food Safety Standard GB 4789.4-2010 for the microbiological examination of Salmonella (National Food Safety Standards of China). Briefly, 25 g of homogenized samples were added to 225 ml of buffered peptone water (BPW) (Huankai, Guangzhou, China) and incubated overnight at 37°C. Then, 1 ml aliquots of cultures were incubated in 10 ml of selenite cystine broth (SC) (Huankai) at 37°C and 10 ml of tetrathionate brilliant green broth (TTB) at 42°C for 24 h. Loopfuls of SC and TTB cultures were streaked onto xylose-lysine-tergitol 4 (XLT4) selective agar plates (Difco, Detroit, MI, United States) and chromogenic Salmonella agar plates (Huankai), then incubated at 37°C for 24 h. Presumptive colonies were picked from each plate, stabbed into a triple sugar iron slant (Huankai), and incubated at 37°C for 24 h. Isolates with typical Salmonella phenotypes were further confirmed using API 20E test strips (bioMerieux, Marcy-l’Etoile, France).
The enumeration of Salmonella in the samples was determined using the three-tube most probable number (MPN) method. For the MPN method, 25 g of homogenized samples were mixed with 225 ml of BPW (Huankai). Then, 10 ml of this mixture was added to three empty tubes, and transferring in triplicate 1 ml of the mixture into three tubes containing 9 ml of BPW followed by making 10-fold dilution. Salmonella detection of each tube was the same with qualitative method. The MPN value was determined on the basis of the number of positive tube(s) in each of the three sets using the MPN table.
Serotyping and Multilocus Sequence Typing (MLST)
All confirmed Salmonella isolates were serotyped by slide agglutination using commercial O and H antisera (Tianrun Bio-Pharmaceutical, Ningbo, China, and S&A Reagents Lab, Bangkok, Thailand) according to the manufacturer’s instructions. The isolates were then further characterized by MLST. MLST was performed using seven housekeeping genes (aroC, dnaN, hemD, hisD, purE, sucA, thrA) with the amplification conditions and primers described on the MLST website1, while sequence types (ST) were assigned according to the MLST database available from the same site. Cluster analysis was performed using BioNumerics 7.6 software (Applied Maths, Sint-Martens-Latem, Belgium), while a minimum spanning tree generated from the allelic profiles of the isolates was produced.
Antimicrobial Susceptibility Testing
Salmonella isolates were evaluated for antimicrobial resistance using the Kirby–Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines (Clinical and Laboratory Standards Institute [Clsi], 2018). Susceptibility to the following 22 antibiotics was tested: ampicillin, amoxicillin-clavulanic acid, cefazolin, cefoxitin, ceftriaxone, ceftazidime, cefotaxime, ceftiofur, cefepime, aztreonam, imipenem, gentamicin, kanamycin, amikacin, streptomycin, tetracycline, ciprofloxacin, enrofloxacin, nalidixic acid, trimethoprim-sulfamethoxazole, chloramphenicol, and florfenicol (Oxoid, Basingstoke, United Kingdom).
Results
Prevalence and Enumeration of Salmonella in Meat Products Collected From Retail Markets
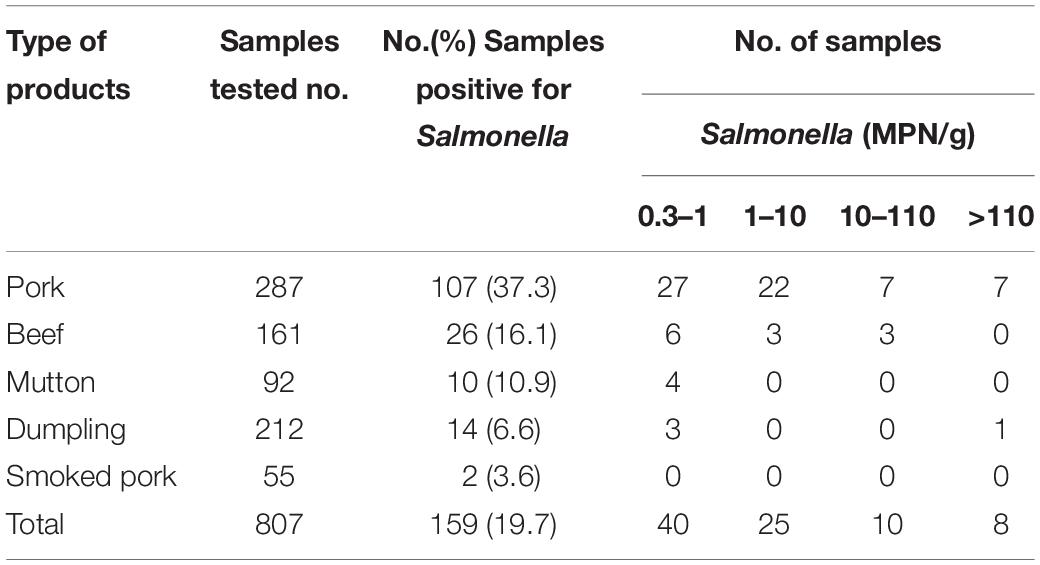
Out of the 807 samples, 159 (19.7%) were positive for Salmonella. Pork had the highest prevalence (37.3%, 107/287) of Salmonella contamination, followed by beef (16.1%, 26/161), mutton (10.9%, 10/92), dumplings (6.6%, 14/212), and smoked pork (3.6%, 2/55). Of the 83 samples that tested positive using the MPN method, 40 (48.2%) had a contamination level of less than 1 MPN/g, while 25 samples (30.1%) were in the range of 1–10 MPN/g. Ten samples (12.0%) reached 10 MPN/g and eight samples (9.6%) exceeded 110 MPN/g (Table 1).
Serotyping and MLST of Salmonella
A total of 456 Salmonella isolates were recovered from the 159 positive samples. Based on serotyping and MLST analyses, 29 distinct serovars and 33 STs were identified among the 456 Salmonella isolates (Table 2). One isolate belonging to each serotype and ST was selected from each positive sample for further analysis. Thus, 218 non-duplicate isolates were selected from among the 456 Salmonella isolates.
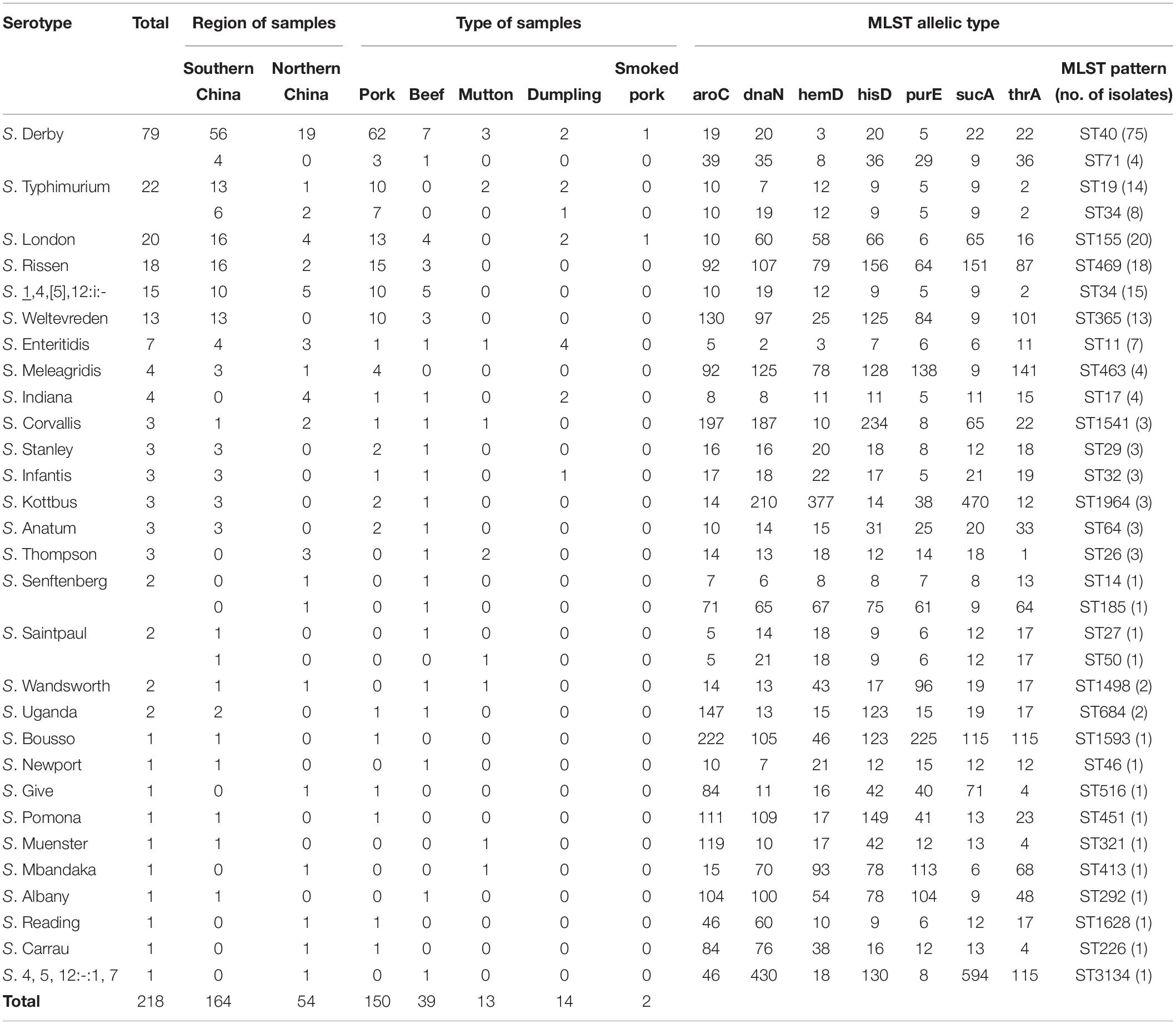
Table 2. Distribution of Salmonella serovars and multilocus sequence typing patterns of isolates from retail meat and meat products from China.
The three most commonly isolated serovars were S. Derby (36.2%), S. Typhimurium (10.1%), and S. London (9.2%), followed by S. Rissen (8.3%), S. 1,4,[5],12:i:- (6.9%), S. Weltevreden (6.0%), and S. Enteritidis (3.2%). Notably, two different serovars were simultaneously identified in 31 samples, three different serovars were detected in four samples, four different serovars were detected in four samples, and five different serovars were detected in two samples. S. Derby in combination with S. Typhimurium was the predominant (18.6%, 8/43) co-contamination pattern.
A minimum spanning tree based on the concatenated sequences of the seven genes used for MLST analysis revealed the relationships between the 218 Salmonella isolates. The STs of the Salmonella isolates were then further analyzed relative to serovar and sample type (Figures 1A,B). Among the serovars represented by more than two isolates, only S. Derby, S. Typhimurium, S. Senftenberg, and S. Saintpaul showed multiple MLST patterns (Table 2 and Figures 1A,B). Further, only ST34 was associated with multiple Salmonella serovars, including eight S. Typhimurium isolates and 15 S. 1,4,[5],12:i:- isolates.
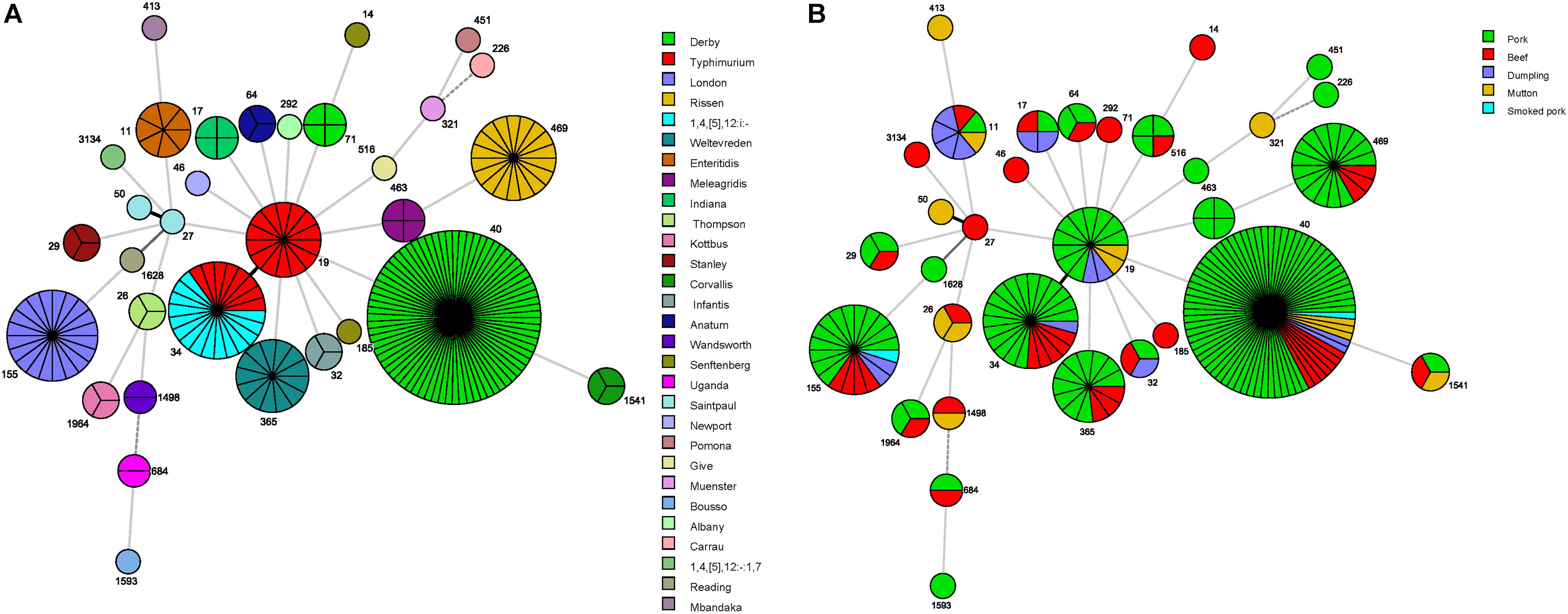
Figure 1. Minimum spanning tree based on multilocus sequence typing data for the 218 Salmonella isolates examined in this study. Each circle represents one ST, subdivided into one sector per isolate, flanked by the ST number in small print. The size of circle is related to the number of strains within this ST. The colors in the circles in (A) represent the serovars, and the colors in the circles in (B) represent the food sources.
The serovars were distributed across the different food sources, indicating a high degree of genetic diversity among Salmonella strains in China. Overall, the pork meat products contained isolates displaying the greatest ST diversity. Only S. Derby was recovered from all five types of meat product (Table 2 and Figures 1A,B) and from all cities sampled in this study (data not shown). Importantly, source-specific differences in the most frequently detected serovars were observed, as follows: pork (S. Derby, S. Typhimurium, S. Rissen); beef (S. Derby, S. 1,4,[5],12:i:-, S. London); mutton (S. Derby, S. Typhimurium, S. Thompson); and dumplings (S. Enteritidis, S. Typhimurium) (Table 2).
Antimicrobial Susceptibility Profiles
As shown in Table 3, among the 218 isolates, only 12 (5.5%) were susceptible to all tested antimicrobials. Overall, 181 isolates (83.0%) were resistant to at least one class of antimicrobials and 128 isolates (58.7%) were resistant to at least three classes. A high prevalence of resistance was observed for tetracycline (65.6%), ampicillin (45.4%), trimethoprim-sulfamethoxazole (40.8%), streptomycin (40.4%), and nalidixic acid (35.8%). In addition, 16.1% of isolates showed resistance to ciprofloxacin, with S. Derby accounting for the majority of these isolates (20/35), while a high proportion of isolates (34.9%) showed intermediate resistance to this antibiotic. Resistance to third- and fourth-generation cephalosporins ranged from 4.6 to 11.0% among the Salmonella isolates. Overall, 5.5% (12/218) and 4.6% (10/218) of the Salmonella isolates were resistant to ceftriaxone and cefotaxime, respectively, with S. Indiana (four isolates) and S. Infantis (two isolates) being the most commonly resistant serovars. Another 11.0% (24/218) of isolates were resistant to ceftazidime, with S. Derby (six isolates) and S. Enteritidis (five isolates) showing the highest rates of resistance. Of note, 6.4% (14/218) of isolates were resistant to cefepime, with S. Indiana (four isolates) and S. London, S. Enteritidis, S. Meleagridis, and S. Infantis (two isolates each) representing the most commonly resistant serovars.
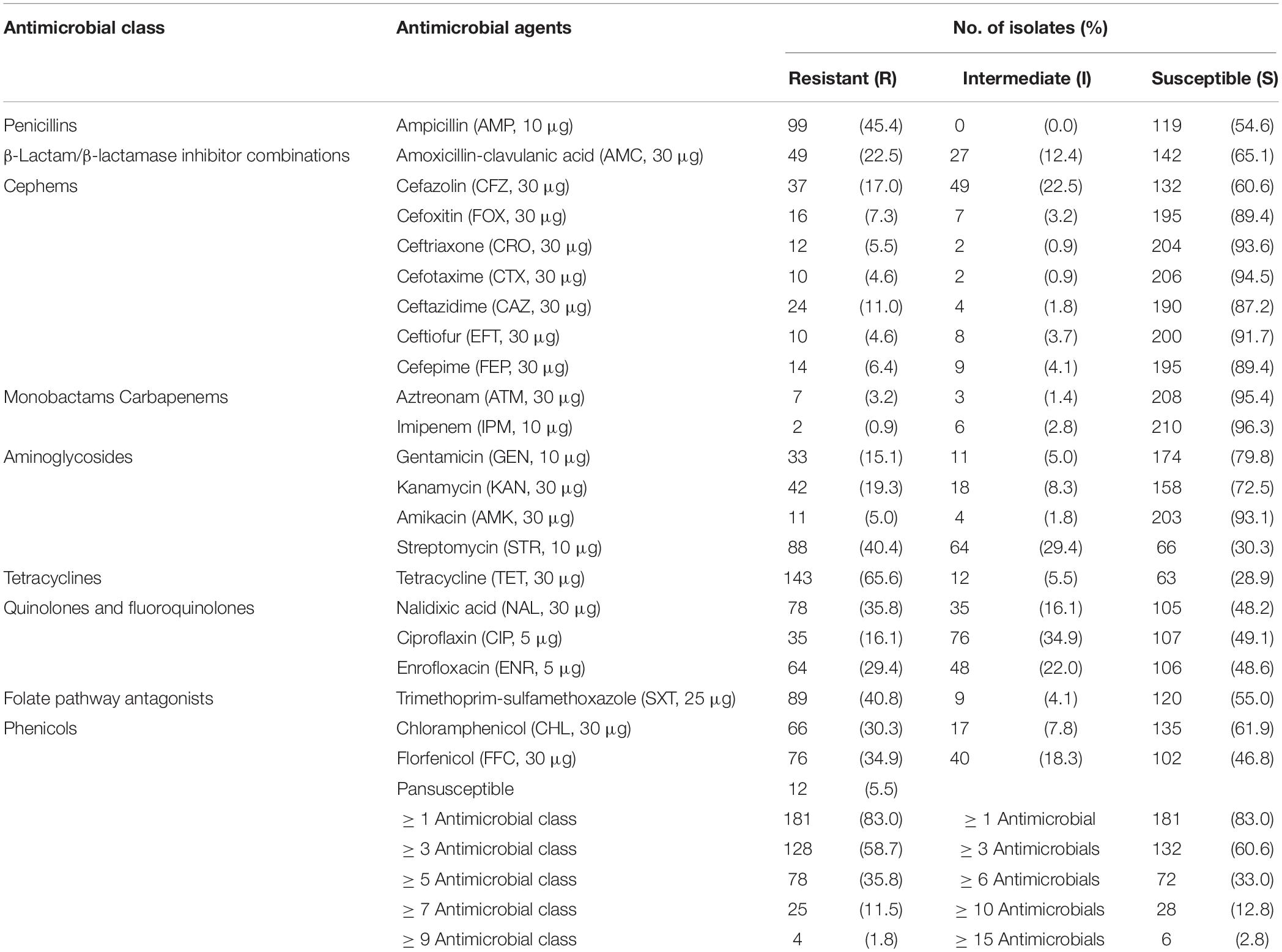
Table 3. Results of antimicrobial susceptibility testing of Salmonella isolates obtained in the present study.
In addition, all four S. Indiana isolates and one S. Thompson isolate were identified as being co-resistant to ceftriaxone and ciprofloxacin. These isolates were also resistant to multiple other antimicrobial agents. Of particular concern, three S. Indiana isolates showed resistance to all classes of antibiotics tested in the current study, except imipenem. Only two isolates were resistant to imipenem, both of which were S. 1,4,[5],12:i:-.
Of the Salmonella serovars identified in the present study, S. Derby, S. Typhimurium, S. London, S. Rissen, S. 1,4,[5],12:i:-, and S. Enteritidis had the highest rates of antimicrobial resistance and MDR, while the S. Weltevreden isolates were generally quite susceptible to antibiotics (Table 4).
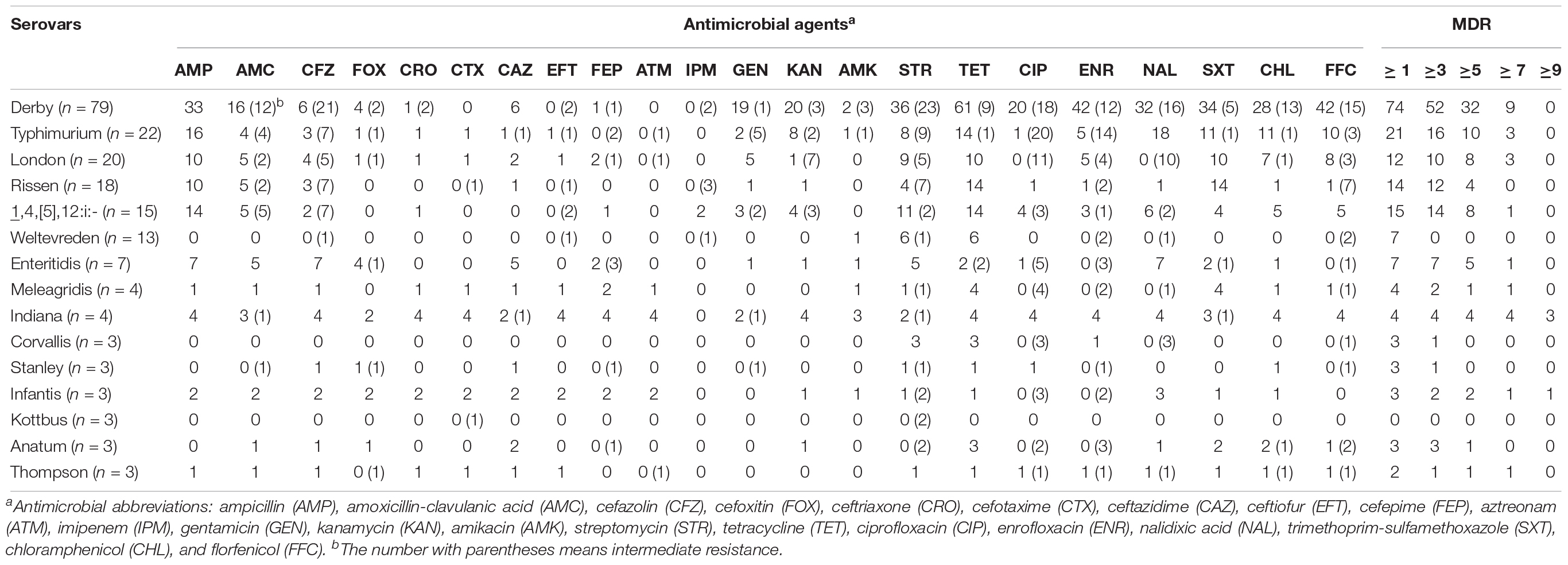
Table 4. Resistance profiles of the top 15 Salmonella serotypes isolated from retail meat and meat products from China.
Discussion
Prevalence of Salmonella
This study showed that the overall prevalence rate of Salmonella was 19.7% (159/807) for meat products. The isolation rates observed in the current study are similar to those of a previous study in Shaanxi Province, China (Yang et al., 2010). However, the current rates are higher than those obtained in Jiangsu Province, eastern China, where only 14.1% (154/1096) of pork samples were Salmonella-positive (Li et al., 2014). Interestingly, a study from Hebei Province, China, reported much higher rates of Salmonella contamination of beef and mutton (33.3% for each) compared with pork (26.7%) (Yan et al., 2010). These disparities are likely the result of the different geographic locations of the sampling sites. However, in the current study, samples were collected from 39 cities across China, including most provincial capitals as well as Hong Kong and Macao. To our knowledge, this was the most comprehensive countrywide study of Salmonella isolates recovered from meat products in China. Therefore, the resulting data is more comprehensive and representative of China as a whole and will be hugely beneficial for future risk assessment.
Compared with studies conducted in other countries, the prevalence of Salmonella contamination of meat products in the current study was lower than the 82% recorded for beef and 93% for pork in Laos (Boonmar et al., 2013). However, the current rates are much higher than those recorded in Canada, where Salmonella prevalence rates of only 2.0% and 0% were determined for pork and beef, respectively (Aslam et al., 2012).
Salmonella Serotypes
S. Derby and S. Typhimurium were the most prevalent serovars identified in the current study, accounting for 46.3% of all strains. Both serovars were mostly isolated from pork. The prevalence of different Salmonella serovars in meat products has been investigated in many areas of China. S. Derby followed by S. Typhimurium were the two most prevalent serovars among Salmonella isolated from retail pork, beef, and lamb samples in Shaanxi Province (Yang et al., 2010) and from pork in Henan (Yang et al., 2013) and Jiangsu (Li et al., 2014) Provinces. While all of these studies focused on a single province, the results were similar to our nationwide data, suggesting that S. Derby, followed by S. Typhimurium, may be the predominant serovars in livestock-derived meat products across China.
In contrast, studies conducted in different countries have found that other serovars are more common in livestock-derived meat products. A study by Thai et al. (2012) showed that S. Anatum was the most common serovar recovered from pork in Vietnam, while in Portugal, S. I 4,[5],12:i:- was the predominant serovar in food products of swine and bovine origin (Clemente et al., 2013). However, a high prevalence of S. Derby and S. Typhimurium was also detected in these two studies (Thai et al., 2012; Clemente et al., 2013). According to a report by Ma̧ka et al. (2014), S. Enteritidis was the predominant serotype isolated from pork and beef in Poland, and although S. Typhimurium was also isolated, no S. Derby isolates were detected. Overall, S. Derby and S. Typhimurium are reportedly the most common serovars associated with human infection worldwide (Cui et al., 2009; Greig and Ravel, 2009; Deng et al., 2012). Therefore, the high prevalence of these serovars in the current study indicates a significant risk to consumers.
Other serovars that were repeatedly recovered in the present study included S. London, S. Rissen, S. 1,4,[5],12:i:-, S. Weltevreden, and S. Enteritidis. To the best of our knowledge, S. London, S. Rissen, and S. Weltevreden have rarely been reported in meat by previous studies in China (Yang et al., 2010, 2013; Li et al., 2013, 2014). This suggests that these serovars may be becoming more frequent contaminants of food products in China and should therefore be considered a public health concern.
S. 1,4,[5],12:i:-, lacking the phase 2 flagellar antigen has recently been recognized as a monophasic variant of S. Typhimurium. Although rarely identified prior to the mid-1990s, the number of human salmonellosis cases caused by S. 1,4,[5],12:i:- has increased rapidly in recent years (Yang et al., 2015). Since 2009, S. 1,4,[5],12:i:- has also ranked among the four most frequently identified serovars causing human salmonellosis in China (Deng et al., 2012). The prevalence of S. 1,4,[5],12:i:- in the current study reminds us that it is critical to monitor the emergence and prevalence of different Salmonella serotypes to better control salmonellosis.
S. Enteritidis is frequently identified worldwide and is one of the most common serovars associated with human salmonellosis (Greig and Ravel, 2009; Hendriksen et al., 2011). This serovar also accounted for 3.2% of the isolates recovered from meat products in the current study. Of note, S. Enteritidis was the predominant serovar isolated from dumplings, indicating poor hygiene practices during dumpling preparation.
Worryingly, most of the Salmonella serovars identified in the present study are recognized as frequent causes of human salmonellosis in China (Cui et al., 2009; Deng et al., 2012). Thus, the dissemination of these serovars amongst meat products in China is the likely source of human infections.
Antimicrobial Susceptibility
Among the isolates recovered in this study, rates of resistance to the various classes of antimicrobial agents ranged from 0.9–65.6%, with resistance to traditional antimicrobial agents such as tetracycline, ampicillin, trimethoprim-sulfamethoxazole, streptomycin, and nalidixic acid being most frequently observed. These results agreed with previous reports from China (Yang et al., 2013; Li et al., 2014), Thailand (Wannaprasat et al., 2011), and Vietnam (Thai et al., 2012). In comparison, rates of resistance among Salmonella isolates from meat and dairy products in Egypt were significantly higher, with 95.7 and 91.5% of isolates showing resistance to ampicillin and streptomycin, respectively (Ahmed et al., 2014). In the present study, the highest rates of antimicrobial resistance were recorded for tetracycline (65.6%), which is one of the most widely used antimicrobials in feed additives in livestock farming in China and other countries. The high prevalence of antimicrobial resistance observed here shows the detrimental impact of the uncontrolled use of these antimicrobials for prophylaxis and growth promotion, as well as in medicine, in China.
Multidrug resistance is defined as resistance to at least three classes of antimicrobial agents (Ahmed et al., 2014; Michael and Schwarz, 2016). In total, 128 (58.7%) Salmonella isolates showing a MDR phenotype were detected in the current study, a much higher frequency than has been reported by other studies carried out in China (Yang et al., 2013; Li et al., 2014). These results highlight the enormous challenges associated with the treatment of Salmonella infections in humans and animals, and further legislation regarding the prudent use of antimicrobials should be implemented by the authorities in China.
As critically important antibiotics in human medicine, extended-spectrum cephalosporins (e.g., ceftriaxone) are the drugs of choice to treat very young patients, while fluoroquinolones (e.g., ciprofloxacin) have been recommended for the treatment of Salmonella infections in adults or in cases caused by strains showing extended-spectrum cephalosporin resistance. Resistance to these antibiotics was observed in the current study. Of particular concern was the detection of ciprofloxacin and ceftriaxone co-resistance in all four of the S. Indiana isolates and one S. Thompson isolate, which poses a significant risk to public health. More seriously, three of the S. Indiana isolates showed resistance to all classes of antibiotics tested, except imipenem. Multidrug-resistant S. Indiana isolates have been detected previously in China, with most isolated from food-producing animals and humans (Lai et al., 2013; Bai et al., 2015, 2016; Gong et al., 2016). However, to our knowledge, this is the first report of S. Indiana isolated from retail meat products exhibiting resistance to extended-spectrum cephalosporins (including ceftriaxone and cefepime), ciprofloxacin, and multiple other antimicrobials in China. These strains are of significant clinical concern because they are unlikely to be adequately controlled by commonly used antibiotics.
Carbapenems (e.g., imipenem) are advocated for the treatment of infections caused by extended-spectrum β-lactamase- and/or AmpC β-lactamase-producing Enterobacteriaceae. Unfortunately, the emergence of resistance to carbapenems among Enterobacteriaceae has become a global concern. However, the prevalence of imipenem resistance among foodborne Salmonella strains in China has not been evaluated (Yan et al., 2010; Yang et al., 2010; Li et al., 2014). Although only two S. 1,4,[5],12:i:- isolates showed imipenem resistance in the current study, the dissemination of these strains is a very real threat to public health.
Conclusion
In summary, Salmonella contamination was common in retail meat products in China. In addition to S. Typhimurium and S. Enteritidis, the two most prevalent Salmonella serovars worldwide, numerous other serovars associated with human salmonellosis were identified in the food samples. Moreover, serovar-specific analysis showed that among the dominant serovars, S. Derby, S. Typhimurium, S. London, S. Rissen, S. 1,4,[5],12:i:-, and S. Enteritidis had much higher rates of antimicrobial resistance and MDR, whereas S. Weltevreden was generally quite susceptible to all antimicrobial agents. Of note, S. Indiana isolates were characterized by their resistance to several extended-spectrum cephalosporins (including ceftriaxone and cefepime), ciprofloxacin, and multiple other antimicrobials, while two S. 1,4,[5],12:i:- isolates showed resistance to imipenem. These findings may lead to a greater understanding of the prevalence, load, serotype distribution, genetic diversity, and antimicrobial resistance of Salmonella in retail meat products in China. Such data provides support for the development of new approaches to control Salmonella infection and antimicrobial resistance.
Data Availability
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
XY, QW, and JZ conceived and designed the experiments. XY, JH, and LC performed the experiments. XY, SW, HZ, JW, and JZ analyzed the data. MC, HW, QG, and XW contributed reagents, materials, and analysis tools.
Funding
This work was supported by the National Key R&D Program of China (2017YFC1601200 and 2017YFC1601203), the National Natural Science Foundation of China (No. 31801656), the Natural Science Foundation of Guangdong Province (2018A030310480), and GDAS’ Special Project of Science and Technology Development (2017GDASCX-0201).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02121/full#supplementary-material
FIGURE S1 | Map of China showing the sampling locations (provinces and cities) of the current study.
TABLE S1 | The sampling sites and time of the current study.
Footnotes
References
Achtman, M., Wain, J., Weill, F. X., Nair, S., Zhou, Z., Sangal, V., et al. (2012). Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 8:e1002776. doi: 10.1371/journal.ppat.1002776
Ahmed, A. M., Shimamoto, T., and Shimamoto, T. (2014). Characterization of integrons and resistance genes in multidrug-resistant Salmonella enterica isolated from meat and dairy products in Egypt. Int. J. Food Microbiol. 189, 39–44. doi: 10.1016/j.ijfoodmicro.2014.07.031
Aslam, M., Checkley, S., Avery, B., Chalmers, G., Bohaychuk, V., Gensler, G., et al. (2012). Phenotypic and genetic characterization of antimicrobial resistance in Salmonella serovars isolated from retail meats in Alberta, Canada. Food Microbiol. 32, 110–117. doi: 10.1016/j.fm.2012.04.017
Bai, L., Lan, R., Zhang, X., Cui, S., Xu, J., Guo, Y., et al. (2015). Prevalence of Salmonella isolates from chicken and pig slaughterhouses and emergence of ciprofloxacin and cefotaxime co-resistant S. enterica serovar Indiana in Henan, China. PLoS One 10:e0144532. doi: 10.1371/journal.pone.0144532
Bai, L., Zhao, J., Gan, X., Wang, J., Zhang, X., Cui, S., et al. (2016). Emergence and diversity of Salmonella enterica serovar Indiana isolates with concurrent resistance to ciprofloxacin and cefotaxime from food-producing animals and patients in China. Antimicrob. Agents Chemother. 60, 3365–3371. doi: 10.1128/AAC.02849-15
Boonmar, S., Morita, Y., Pulsrikarn, C., Chaichana, P., Pornruagwong, S., Chaunchom, S., et al. (2013). Salmonella prevalence in meat at retail markets in Pakse, Champasak Province, Laos, and antimicrobial susceptibility of isolates. J. Glob. Antimicrob. Resist. 1, 157–161. doi: 10.1016/j.jgar.2013.05.001
Clemente, L., Manageiro, V., Ferreira, E., Jones-Dias, D., Correia, I., Themudo, P., et al. (2013). Occurrence of extended-spectrum β-lactamases among isolates of Salmonella enterica subsp. enterica from food-producing animals and food products, in Portugal. Int. J. Food Microbiol. 167, 221–228. doi: 10.1016/j.ijfoodmicro.2013.08.009
Clinical and Laboratory Standards Institute, [Clsi] (2018). Performance Standards for Antimicrobial Susceptibility Testing. 28th Edn. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
Cui, S., Li, J., Sun, Z., Hu, C., Jin, S., Li, F., et al. (2009). Characterization of Salmonella enterica isolates from infants and toddlers in Wuhan, China. J. Antimicrob. Chemother. 63, 87–94. doi: 10.1093/jac/dkn452
Deng, X., Ran, L., Wu, S., Ke, B., He, D., Yang, X., et al. (2012). Laboratory-based surveillance of non-typhoidal Salmonella infections in Guangdong Province, China. Foodborne Pathog. Dis. 9, 305–312. doi: 10.1089/fpd.2011.1008
Gong, J., Wang, C., Shi, S., Bao, H., Zhu, C., Kelly, P., et al. (2016). Highly drug-resistant Salmonella enterica serovar Indiana clinical isolates recovered from broilers and poultry workers with diarrhea in China. Antimicrob. Agents Chemother. 60, 1943–1947. doi: 10.1128/AAC.03009-15
Greig, J. D., and Ravel, A. (2009). Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 130, 77–87. doi: 10.1016/j.ijfoodmicro.2008.12.031
Hendriksen, R. S., Vieira, A. R., Karlsmose, S., Lo Fo Wong, D. M., Jensen, A. B., Wegener, H. C., et al. (2011). Global monitoring of Salmonella serovar distribution from the world health organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 8, 887–900. doi: 10.1089/fpd.2010.0787
Lai, J., Wang, Y., Shen, J., Li, R., Han, J., Foley, S. L., et al. (2013). Unique class 1 integron and multiple resistance genes co-located on IncHI2 plasmid is associated with the emerging multidrug resistance of Salmonella Indiana isolated from chicken in China. Foodborne Pathog. Dis. 10, 581–588. doi: 10.1089/fpd.2012.1455
Li, R., Lai, J., Wang, Y., Liu, S., Li, Y., Liu, K., et al. (2013). Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. Int. J. Food Microbiol. 163, 14–18. doi: 10.1016/j.ijfoodmicro.2013.01.020
Li, Y. C., Pan, Z. M., Kang, X. L., Geng, S. Z., Liu, Z. Y., Cai, Y. Q., et al. (2014). Prevalence, characteristics, and antimicrobial resistance patterns of Salmonella in retail pork in Jiangsu province, eastern China. J. Food Prot. 77, 236–245. doi: 10.4315/0362-028X.JFP-13-269
Lunguya, O., Lejon, V., Phoba, M. F., Bertrand, S., Vanhoof, R., Glupczynski, Y., et al. (2013). Antimicrobial resistance in invasive non-typhoid Salmonella from the democratic republic of the congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl. Trop. Dis. 7:e2103. doi: 10.1371/journal.pntd.0002103
Majowicz, S. E., Musto, J., Scallan, E., Angulo, F. J., Kirk, M., O’Brien, S. J., et al. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50, 882–889. doi: 10.1086/650733
Ma̧ka, L., Mackiw, E., Sciezynska, H., Pawłowska, K., and Popowska, M. (2014). Antimicrobial susceptibility of Salmonella strains isolated from retail meat products in Poland between 2008 and 2012. Food Control 36, 199–204. doi: 10.1016/j.foodcont.2013.08.025
Michael, G. B., and Schwarz, S. (2016). Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin. Microbiol. Infect. 22, 968–974. doi: 10.1016/j.cmi.2016.07.033
Thai, T. H., Hirai, T., Lan, N. T., and Yamaguchi, R. (2012). Antibiotic resistance profiles of Salmonella serovars isolated from retail pork and chicken meat in North Vietnam. Int. J. Food Microbiol. 156, 147–151. doi: 10.1016/j.ijfoodmicro.2012.03.016
Van, T. T., Nguyen, H. N., Smooker, P. M., and Coloe, P. J. (2012). The antibiotic resistance characteristics of non-typhoidal Salmonella enterica isolated from food-producing animals, retail meat and humans in South East Asia. Int. J. Food Microbiol. 154, 98–106. doi: 10.1016/j.ijfoodmicro.2011.12.032
Wannaprasat, W., Padungtod, P., and Chuanchuen, R. (2011). Class 1 integrons and virulence genes in Salmonella enterica isolates from pork and humans. Int. J. Antimicrob. Agents. 37, 457–461. doi: 10.1016/j.ijantimicag.2010.12.001
Yan, H., Li, L., Alam, M. J., Shinoda, S., Miyoshi, S., and Shi, L. (2010). Prevalence and antimicrobial resistance of Salmonella in retail foods in northern China. Int. J. Food Microbiol. 143, 230–234. doi: 10.1016/j.ijfoodmicro.2010.07.034
Yang, B., Qiao, L., Zhang, X., Cui, Y., Xia, X., Cui, S., et al. (2013). Serotyping, antimicrobial susceptibility, pulse field gel electrophoresis analysis of Salmonella isolates from retail foods in henan province, China. Food Control 32, 228–235. doi: 10.1016/j.foodcont.2012.11.022
Yang, B., Qu, D., Zhang, X., Shen, J., Cui, S., Shi, Y., et al. (2010). Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int. J. Food Microbiol. 141, 63–72. doi: 10.1016/j.ijfoodmicro.2010.04.015
Keywords: Salmonella, serovar, meat, prevalence, enumeration, antimicrobial resistance
Citation: Yang X, Wu Q, Zhang J, Huang J, Chen L, Wu S, Zeng H, Wang J, Chen M, Wu H, Gu Q and Wei X (2019) Prevalence, Bacterial Load, and Antimicrobial Resistance of Salmonella Serovars Isolated From Retail Meat and Meat Products in China. Front. Microbiol. 10:2121. doi: 10.3389/fmicb.2019.02121
Received: 11 April 2019; Accepted: 28 August 2019;
Published: 24 September 2019.
Edited by:
Julio Parra-Flores, University of the Bío Bío, ChileReviewed by:
Alejandro Castillo, Texas A&M University, United StatesMarisa Cardoso, Federal University of Rio Grande do Sul, Brazil
Yajaira Esquivel Hernandez, Universidad Autónoma de Querétaro, Mexico
Copyright © 2019 Yang, Wu, Zhang, Huang, Chen, Wu, Zeng, Wang, Chen, Wu, Gu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingping Wu, d3VxcDIwM0AxNjMuY29t
 Xiaojuan Yang1,2
Xiaojuan Yang1,2 Qingping Wu
Qingping Wu