- Laboratory of Plant and Environmental Biotechnology, Department of Biochemistry and Biotechnology, University of Thessaly, Larissa, Greece
Auxotrophy to amino acids and vitamins is a common feature in the bacterial world shaping microbial communities through cross-feeding relations. The amino acid auxotrophy of pollutant-degrading bacteria could hamper their bioremediation potential, however, the underlying mechanisms of auxotrophy remain unexplored. We employed genome sequence-based metabolic reconstruction to identify potential mechanisms driving the amino acid auxotrophy of a Sphingomonas haloaromaticamans strain degrading the fungicide ortho-phenylphenol (OPP) and provided further verification for the identified mechanisms via in vitro bacterial assays. The analysis identified potential gaps in the biosynthesis of isoleucine, phenylalanine and tyrosine, while methionine biosynthesis was potentially effective, relying though in the presence of B12. Supplementation of the bacterium with the four amino acids in all possible combinations rescued its degrading capacity only with methionine. Genome sequence-based metabolic reconstruction and analysis suggested that the bacterium was incapable of de novo biosynthesis of B12 (missing genes for the construction of the corrin ring) but carried a complete salvage pathway for corrinoids uptake from the environment, transmembrane transportation and biosynthesis of B12. In line with this the bacterium maintained its degrading capacity and growth when supplied with environmentally relevant B12 concentrations (i.e., 0.1 ng ml–1). Using genome-based metabolic reconstruction and in vitro testing we unraveled the mechanism driving the auxotrophy of a pesticide-degrading S. haloaromaticamans. Further studies will investigate the corrinoids preferences of S. haloaromaticamans for optimum growth and OPP degradation.
Introduction
Auxotrophy is the inability of an organism to synthesize a particular biomolecule which is necessary for its growth. Auxotrophic bacterial mutants are known to evolve spontaneously upon long-term growth in an environment rich in biomolecules leading bacteria to genetic drift and loss of the corresponding biosynthetic pathways (Van der Kaaij et al., 2014). Amino acid auxotrophy is a common feature of bacterial genomes which have been evolutionary optimized to reduce the metabolic burden stemming from the production of energetically costly amino acids (i.e., phenylalanine, tyrosine, and methionine) (Mee et al., 2014). Instead their biosynthesis is assigned to a few bacteria leading to the establishment of a mutualistic trade among bacteria to ensure access to essential biomolecules (D’Souza et al., 2014; Pande et al., 2015). Vitamin auxotrophy constitutes another frequent bacterial phenotype in aquatic ecosystems, terrestrial ecosystems, and the human gut. Comparative genomics showed that each of the eight vitamins was produced by 45–60% of the gut bacteria suggesting that exchange of vitamins amongst human gut bacteria enables the survival of members that could not synthesize any of these co-factors (Magnúsdóttir et al., 2015). Similarly, Gomez-Consarnau et al. (2018) showed that vitamins synthesis and exchange by bacteria leads to a mosaic of metabolic interdependencies which contribute to the shaping of microbial communities in marine ecosystems. For instance, Rhodobacterales dominated the expression of vitamin-B12 synthesis, but relied on Flavobacteria for the production and supply of vitamin B7. Price et al. (2018) challenged experimentally the value of comparative genomic studies (i.e., their data showed that auxotrophy in bacteria is not as common as claimed by comparative genomic studies) and suggested that their findings should be always verified experimentally.
Amino acids auxotrophy has been reported in several pesticide-degrading bacteria. First, Sørensen et al. (2001) noted that an isoproturon-degrading Sphingomonas strain SRS2 was able to maintain its degrading capacity only under external supplementation with casamino acids (CA); a mixture of all 20 essential amino acids. Follow up studies revealed that strain SRS2 required external supply of methionine or the presence of another bacterium in the culture (Sørensen et al., 2002), which was probably able to provide to strain SRS2 the missing nutrient factors. The need for external supply of amino acids to maintain their degrading activity and growth on organic pollutants is widespread among sphingomonads with examples of a Sphingomonas xenophaga degrading 1-amino-4-bromoanthraquinone-2-sulfonic acid (Lu et al., 2015), a Sphingobium sp. degrading MCPA (Onneby et al., 2014), and a Sphingomonas sp. degrading triclosan (Hay et al., 2001). This has been assumed to be the result of incomplete amino acid biosynthetic pathways, however, the exact underlying mechanism remains unknown.
Vitamin B12 (cyanocobalamin) and its methyl and adenosyl derivatives, are essential co-factors of several key enzymes like methionine synthase, ribonucleotide reductase, diol dehydratase, and ethanolamine ammonia lyase (Rodionov et al., 2003). Its biosynthesis is the most energetically costly pathway and is restricted to a number of bacteria and archaea (Roth et al., 1996). A comparative genomic analysis showed that nearly 90% of the 11,000 bacterial genomes examined had at least one and max 15 B12-dependent enzymatic families, yet only 37% carried the full B12 biosynthetic pathway (Shelton et al., 2019), reinforcing the key role of B12 in the synergism among microbial populations. Such B12-based symbiotic relationships have been described between algae and bacteria (Croft et al., 2005; Romine et al., 2017) or between bacteria (Men et al., 2012) shaping microbial communities in marine water systems (Heal et al., 2017) and the human gut (Degnan et al., 2014).
We recently isolated a Sphingomonas haloaromaticamans strain which was able to degrade the fungicide ortho-phenylphenol (OPP) only when supplemented with CA or co-cultivated with other bacteria (Perruchon et al., 2016). We aimed to further explore the mechanism driving this auxotrophy of the S. haloaromaticamans strain to amino acids. The hypothesis initially tested was that S. haloaromaticamans has one or more incomplete amino acid biosynthesis pathways, hence the need for external supply of CA. To verify this hypothesis, genome-based metabolic reconstruction of the 20 amino acids biosynthesis in S. haloaromaticamans identified possible gaps in the biosynthesis of three amino acids, in addition to methionine whose biosynthetic pathway was complete but relied on the de novo biosynthesis of B12. The latter was needed as a co-factor of methionine synthase since the bacterium did not carry a B12-indepenedent isofunctional enzyme. In vitro assays identified methionine as the amino acid imposing the auxotrophy of S. haloaromaticamans and genome sequence-based metabolic reconstruction suggested that the bacterium was unable to synthesize B12. Further in vitro studies with a range of B12 concentrations verified its key role in the auxotrophy of S. haloaromaticamans, which could cover its B12 requirements probably by uptake and utilization of B12 through the corrinoids salvage pathway present in its genome.
Materials and Methods
Bacterial Strain, Growth Conditions, and Chemicals
The strain S. haloaromaticamans used in the current study was isolated from soil of a wastewater disposal site and it was able to degrade the fungicide OPP only when supplemented with CA (Perruchon et al., 2016). The bacterium was routinely cultivated in minimal salts media supplemented with nitrogen (MSMN), OPP (30–50 mg L–1) and CA in a shaking incubator at 27°C in the dark. MSMN preparation and OPP chromatographic analysis was as described by Perruchon et al. (2016), while bacterial growth was determined by measurement of the optical density at 600 nm (OD600). Inoculation of flasks was performed by fresh bacterial cells grown at the mid-log phase, pelleted by centrifugation, washed three times with sterile ddH2O and resuspended with MSMN to an OD600 of 0.1.
L-methionine, L-isoleucine, L-tyrosine, L-phenylalanine, L-homoserine, O-succinyl-L-homoserine, L-cystathionine, L-homocysteine and cyanocobalamin (synonym to vitamin B12 in the manuscript), were purchased by Sigma-Aldrich (Taufkirchen, Germany) and they were used for the preparation of aqueous solutions (0.05 mM). These were filter sterilized and used for the preparation of MSMN containing amino acids, B12 and intermediates of methionine biosynthesis at the desired concentrations.
Bioinformatic and Phylogenetic Analysis
Genome Sequence-Based Metabolic Reconstruction of Amino Acids and B12 Biosynthesis in Sphingomonas haloaromaticamans
Genomic analysis of S. haloaromaticamans has been described by Perruchon et al. (2017) and the assembled and annotated genome is available at DDBJ/ENA/GenBank under the accession number MIPT00000000. The genome annotation data were used for obtaining the Enzyme Commission (EC) numbers and reconstruct the biosynthetic pathways of all amino acids and B12 using the Pathway Tools software v19.0 (Karp et al., 2015) bundled with the EcoCyc (Karp et al., 2014), BioCyc and MetaCyc (Caspi et al., 2016) databases. Missing genes were searched against the genome translated open reading frames (ORFs) after downloading the associated protein sequences of the RefSeq database of the National Center for Biotechnology Information (NCBI) using the basic local alignment search tool (BLAST) v2.7.1+ (Camacho et al., 2009).
B12 Transporters and Riboswitches Identification in the Genome of Sphingomonas haloaromaticamans
The genome of S. haloaromaticamans was searched for TonB-dependent transporters (TBDT) and associated energy transducing ABC transporters involved in the uptake and translocation of corrinoids. Beyond the original annotation described in Perruchon et al. (2017), a second NCBI RefSeq based annotation was performed with BLAST. We further looked for cobalamin-associated riboswitches, acting as regulatory elements of B12 uptake, using the Riboswitch scanner web-based tool (Mukherjee and Sengupta, 2016).
Phylogenetic Analysis of MetB/Z
Closely related sequences to MetB/Z found to be encoded in the genome of S. haloaromaticamans were retrieved from NCBI with BLASTv2.2.27+ and were clustered with Cdhit v4.6 (Li et al., 2001) to reduce sequence redundancy. The sequences were then aligned with Muscle v3.8.31 (Edgar, 2004). Improperly aligned and uninformative alignment blocks were removed using Gblocks v0.91b (Talavera and Castresana, 2007). The remaining concatenated alignment blocks were subjected to maximum likelihood phylogenies with the RAxML software v8.1.24 (Stamatakis, 2014) and 1000 bootstrap replicates using the best model according to ProtTest v3.4 (Abascal et al., 2005) and the associated Akaike information criterion values. Tree visualization was performed using the APE v3.5 (Paradis et al., 2004) and Phangorn v2.0.4 (Schliep, 2011) R v3.3.118 software packages (R Core Team, 2015).
Degradation of Ortho-Phenylphenol by Sphingomonas haloaromaticamans Externally Supplied With Amino Acids
Triplicate 40-ml cultures of MSMN + OPP (50 mg L–1) were supplemented with CA (0.15 g L–1, CA) or methionine (3.6 mg L–1, M), isoleucine (6.75 mg L–1, I), tyrosine (2.7 mg L–1, T), and phenylalanine (5.7 mg L–1, P) added individually and in all possible combinations. These concentrations were selected based on the concentrations of the given amino acids in the CA. Triplicate MSMN + OPP (50 mg L–1) samples not supplemented with any amino acids were also included for comparative purposes. All the above cultures were inoculated with S. haloaromaticamansas described above. Immediately after inoculation and at regular intervals thereafter aliquots (0.5 ml) of each culture were removed and analyzed for residues of OPP. The degradation of OPP was also measured in triplicate flasks containing MSMN + OPP (50 mg L–1) which were not inoculated to serve as abiotic controls.
Degradation of Ortho-Phenylphenol by Sphingomonas haloaromaticamans Externally Supplied With B12 and Methionine Precursors
Triplicate 40-ml cultures of MSMN + OPP (30 mg L–1) were supplemented with appropriate amounts of aqueous solutions of 0.05 mM of B12, methionine and its precursors homoserine, O-succinyl-homoserine, cystathionine and homocysteine. In the case of homocysteine, the ultimate precursor in the biosynthesis of methionine, three further cultures were co-supplemented with B12 to evaluate if its addition rescues the OPP degradation capacity of the bacterium. Triplicate cultures of MSMN + OPP (30 mg L–1) not supplemented with methionine, B12 or any methionine precursor were also included for comparative purposes. All these cultures were inoculated with S. haloaromaticamans as described above. Immediately after inoculation and at regular intervals thereafter aliquots (0.5 ml) of each culture were removed and analyzed for OPP. The degradation of OPP was also measured in triplicate MSMN + OPP (30 mg L–1) not inoculated with the bacterium to serve as abiotic controls.
Degradation of Ortho-Phenylphenol by Sphingomonas haloaromaticamans Supplied With a Range of B12 Concentrations
Triplicate 40-ml cultures of MSMN + OPP (25 mg L–1) were supplemented with various amounts of different aqueous solutions of B12 (1, 100 and 10000 mg L–1) aiming to final concentrations of 0.1, 0.5, 1, 5, 10, 50, 100, 1000, and 10000 ng ml–1 in the medium. Triplicate cultures of MSMN + OPP (30 mg L–1) not supplemented with B12 were also included for comparative purposes. All cultures were inoculated with S. haloaromaticamans as described above. Immediately after inoculation and at regular intervals thereafter aliquots (0.5 ml) of each culture were removed and analyzed for OPP. The degradation of the fungicide was also measured in triplicate MSMN + OPP which were not inoculated to serve as abiotic controls. In parallel, we determined the growth of the bacterium along the degradation of OPP as described above.
Results and Discussion
Genome Sequence-Based Metabolic Reconstruction of the Biosynthesis of Amino Acids in Sphingomonas haloaromaticamans
The first objective of the study was to identify, based on the draft genome of S. haloaromaticamans, biosynthetic pathways of amino acids which were potentially incomplete. We noted possible gaps in the biosynthetic pathways of phenylalanine, tyrosine and isoleucine (Figure 1 and Supplementary Table S1). The biosynthetic pathway of phenylalanine and tyrosine was lacking the enzyme prephenate transaminase (EC 2.6.1.79) (Figure 1). This is commonly found in arogenate-competent bacteria, like α-proteobacteria, and is responsible for the transamination of prephenate to arogenate which is then used for the biosynthesis of tyrosine or phenylalanine (Graindorge et al., 2014). Alternatively, the biosynthesis of tyrosine and phenylalanine proceeds via the transformation of prephenate to 4-hydroxyphenyl pyruvate or phenyl pyruvate, which are then transaminated, with glutamate as amino group donor, to tyrosine or phenylalanine, respectively (Ikeda, 2006). Genes for these pathways were not found in the genome of S. haloaromaticamans. Instead we identified two copies of the hisC gene encoding an imidazole acetole phosphate aminotransferase, which has been reported before in another α-proteobacterium (Zymomonas mobilis) to act also as tyrosine transaminase and phenylalanine transaminase (Gu et al., 1995). This might indicate that the tyrosine, phenylalanine biosynthetic pathway is not incomplete in S. haloaromaticamans, however, both amino acids were included in our in vitro tests for completeness.
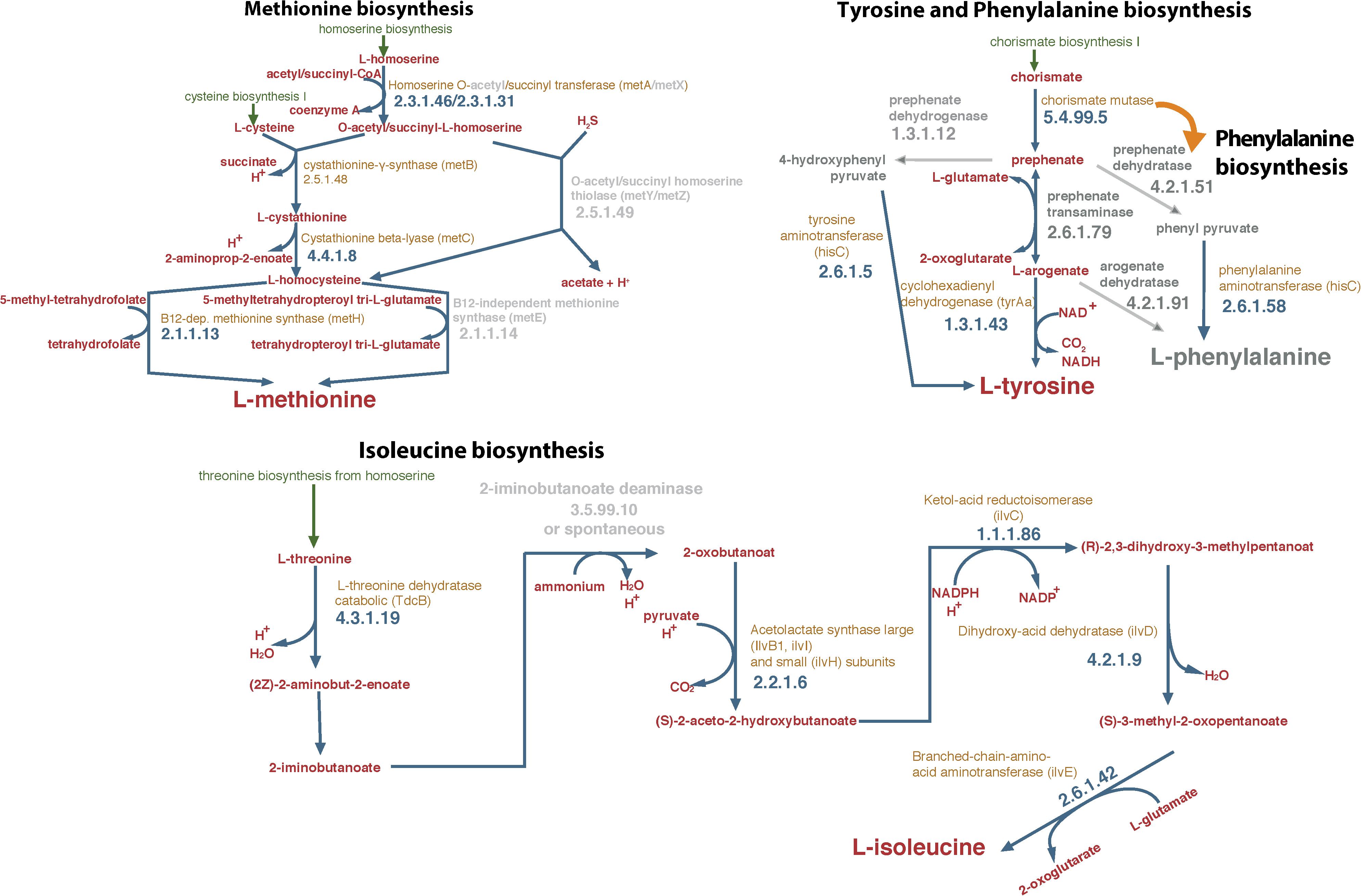
Figure 1. Reconstruction of the biosynthetic pathways of methionine, isoleucine, tyrosine, and phenylalanine of Sphingomonas haloaromaticamans based on its genome analysis and assemblage with the Pathway Tools software suit v18.5 (Karp et al., 2015) and the associated database. Gray color arrows indicate the absence of the enzyme from the draft genome of S. haloaromaticamans. Gene annotation evidence according to BioCyc and RefSeq database hits is provided in Supplementary Table S1.
No gene encoding 2-iminobutanoate deaminase (EC.3.5.99.10), an enzyme with potential role in the biosynthesis of isoleucine was detected in the draft genome of S. haloaromaticamans (Figure 1). The transformation of threonine to 2-oxobutanoate was originally considered an one-step process controlled by threonine ammonia lyase (tdcB) (Risso et al., 2008). It is now suggested that this metabolic step is a two-step process involving the formation of two intermediates [(2Z)-2-aminobut-2-enoate and 2-iminobutanoate] before final formation of 2-oxobutanoate. Lambreht et al. (2012) proposed that 2-iminobutanoate deaminase accelerates the transformation of 2-iminobutanoate to 2-oxobutanoate, a reaction which could occur spontaneously but its acceleration could protect cells from the reactive intermediates. This indicates that isoleucine biosynthesis in S. haloaromaticamans might not be incomplete but it was included in the following in vitro assays for completeness.
In contrast to the above three amino acids, the genome of S. haloaromaticamans carries the necessary genes for the biosynthesis of methionine (Figure 1 and Supplementary Table S1). However, functional annotation of genes in the pathway required clarification. We detected metA encoding homoserine-O-succinyl transferase (EC 2.3.1.46), which drives the transformation of homoserine to O-succinyl-homoserine. This is transformed to homocysteine via transsulfuration or sulfhydrylation, depending on the S source, cysteine, or sulfide, respectively (Hwang et al., 2002). The former is a two-step process involving metB and metC encoding cystathionine-γ-synthase (EC 2.5.1.48) and cystathionine-β-lyase (EC 4.4.1.8), respectively. The latter is controlled by O-succinyl homoserine sulfhydrylase (EC 4.2.99.9) encoded by metZ (Ferla and Patrick, 2014). A gene showing high homology both to metB and metZ was detected in the genome of S. haloaromaticamans. The enzymes encoded by these two genes belong to the same cystathionine gamma-synthase (CGS) evolutionary family (Gophna et al., 2005) and could function either as cystathione-γ-synthase or as O-succinyl homoserine sulfhydrylase depending on the source of S (Hacham et al., 2003). Phylogenetic analysis showed that the relevant gene clustered with metB genes of various Sphingomonas strains (Supplementary Figure S1) indicating a probable function as cystathionine-γ-synthase. The final step of methionine biosynthesis, the transformation of homocysteine to methionine, is controlled by two isoforms of methionine synthase; a cobalamin-independent (MetE; 5-methyltetrahydropteroyltri-L-glutamate:L-homocysteine S-methyltransferase, EC.2.1.1.14) and its cobalamin-dependent isoform (MetH, EC.2.1.1.13) (Gonzalez et al., 1996), only the latter detected in the genome of S. haloaromaticamans. In most bacteria, metE and metH coexists, however, several bacteria possess only one of these two genes (Shelton et al., 2019). The widespread B12 auxotrophy of algae has been attributed to the evolutionary loss of MetE (Helliwell et al., 2011). Hence, the presence of only metH in the genome of S. haloaromaticamans might be a limiting factor in the biosynthesis of methionine, for this reason it was included in our in vitro studies.
Methionine External Supply Rescues the Degradation Phenotype of Sphingomonas haloaromaticamans
Sphingomonas haloaromaticamans was able to effectively degrade OPP in 7 days in the presence of CA and in all treatments where methionine was provided either alone or in combination with other amino acids (Figure 2). In contrast, limited or no degradation of OPP was observed in the non-inoculated cultures and in the S. haloaromaticamans cultures supplemented with the other three amino acids and their combinations. Methionine is the less abundant amino acid in bacterial proteins (Pasamontes and Garcia-Vallve, 2006) but plays an important role in the initiation of translation, has a structural role in hydrophobic cores of proteins, is involved in stabilizing interactions with aromatic amino acids in 1/3 of all known protein structures (Valley et al., 2012), and it is the key component of the cofactor S-adenosyl-methionine which constitutes the main cellular carrier of methyl groups (Chiang et al., 1996). Our findings are in agreement with previous studies which have reported an auxotrophy of other pesticide-degrading sphingomonads to methionine (Sørensen et al., 2002; Onneby et al., 2014). However, none of the above studies looked further into the mechanism driving the reported auxotrophy. In light of the genome-based metabolic analysis of the methionine pathway the observed auxotrophy of S. haloaromaticamans to methionine could be the result of the absence of a complete B12 biosynthetic pathway in the studied bacterium.
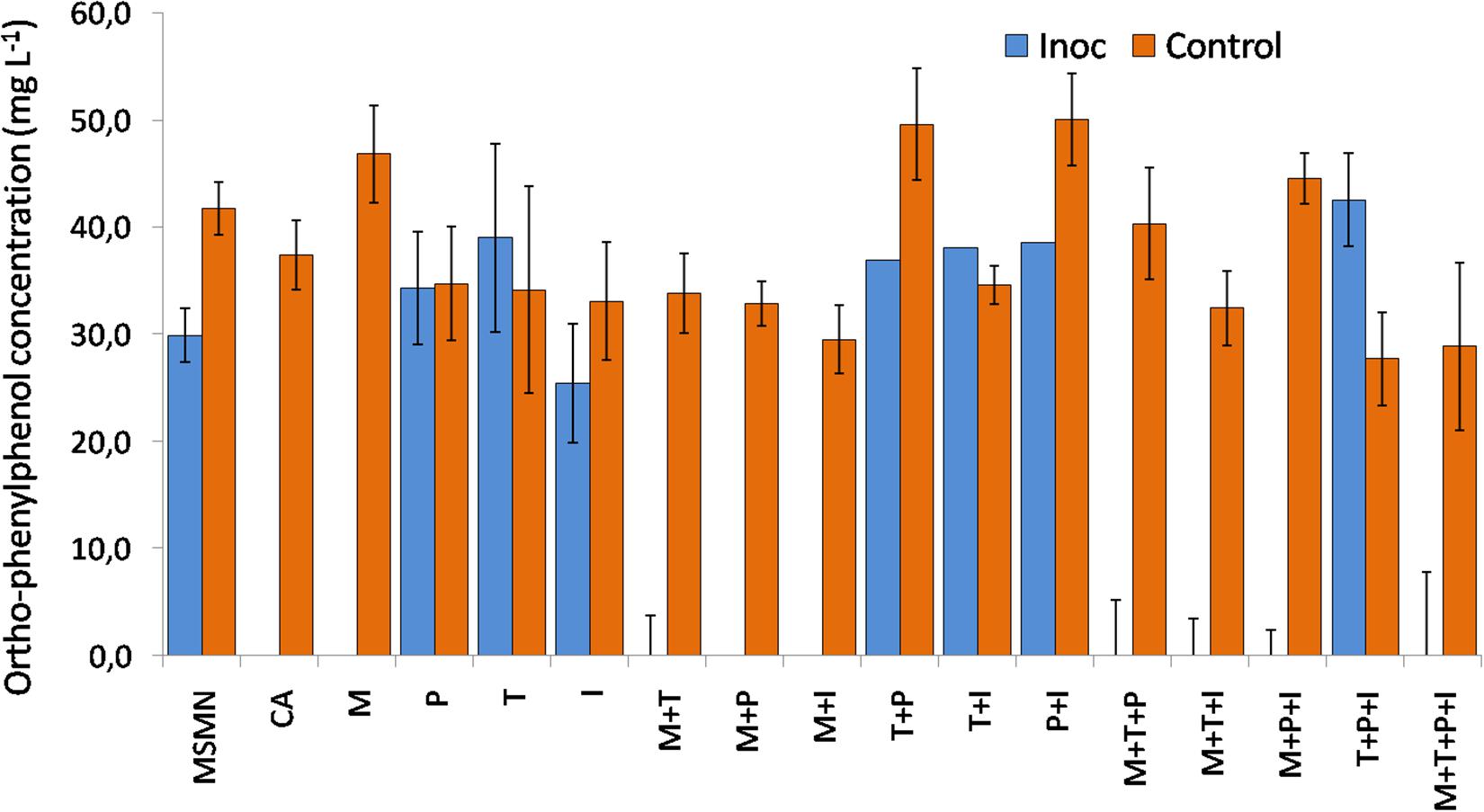
Figure 2. Degradation of OPP 48 h after inoculation (Inoc) with S. haloaromaticamans in duplicate cultures of a selective minimal medium (MSMN) supplemented with CA (0.15 g L–1, CA) or methionine (3.6 mg L–1, M), isoleucine (6.75 mg L–1, I), tyrosine (2.7 mg L–1, T), and phenylalanine (5.7 mg L–1, P) added individually and in all possible combinations. The degradation of OPP was also determined in duplicate inoculated cultures of MSMN without any amino acid supplementation (MSMN) and in corresponding non-inoculated controls (Control).
Genome Sequence-Based Metabolic Reconstruction of the Biosynthesis of B12 in Sphingomonas haloaromaticamans
As a next step we reconstructed the biosynthetic pathway of B12 in S. haloaromaticamans to investigate its potency to synthesize the co-factor. The bacterial genome lacked all the genes for the biosynthesis of the corrin ring of B12 (cobIZGJMFKLH) (Figure 3 and Supplementary Table S2). Instead it carries nearly all genes of the lower pathway (cobABNSTWOCQDPUVCZ) from the conversion of hydrogenobyrinate to its a,c-diamide derivative through Co2+ chelation to adenosyl-cobalamin (Raux et al., 2000).
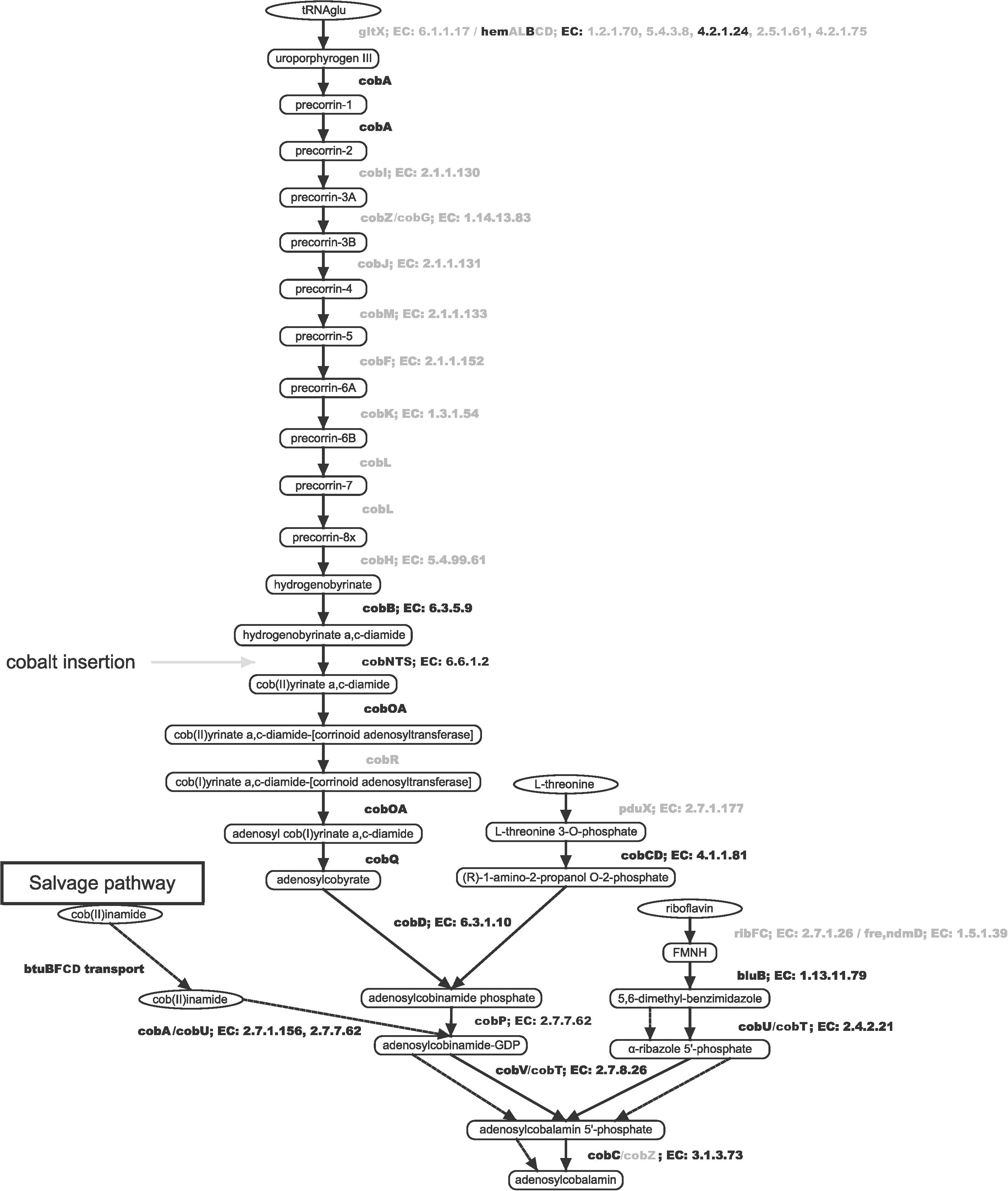
Figure 3. Reconstruction of the biosynthetic pathway of B12 in S. haloaromaticamans based on its genome analysis and assemblage with the Pathway Tools software suit v18.5 (Karp et al., 2015). Enzyme names indicated with black or gray color were either detected or not detected in the genome of S. haloaromaticamans, respectively. Dashed arrows denote the steps of the cobalamin salvage pathway. Gene annotation evidence according to BioCyc and RefSeq database hits is provided in Supplementary Table S2.
The absence of a de novo biosynthetic pathway of B12 in S. haloaromaticamans genome and its demonstrated absolute requirement for B12-dependent methionine synthesis, led us to speculate that the bacterium should possess a high affinity system for uptake and translocation of corrinoids. The bacterial genome was searched for genes encoding for energy-dependent uptake of B12. We detected 88 genes with potential role in the energy-dependent uptake of various biomolecules including B12: 43 were annotated to code for TonB-dependent receptors (outer membrane transportation system), 20 coded for the TonB/ExpB/ExpD complex components (inner membrane transportation system), 21 genes coded for genes with a potential role in the regulatory system of the TonB-dependent uptake (20 comprising the FecI/FecRand one being the iron uptake repressor “fur” gene), and 4 genes were screened due to either their vicinity to TBDT or potential association (due to their annotation or their content) with riboswitches (Supplementary Table S3). Sphingomonadales genomes are known to be rich in TBDT [i.e., Sphingomonas wittichii RW1 carried 134 TBDTs (Tang et al., 2012)], in line with their high abundance in the genome of S. haloaromaticamans. From the 43 TonB-dependent receptors (i) 16 were annotated as putative B12 outer membrane receptors (BtuB) (Chimento et al., 2003), showing best sequence matches to the previously published S. haloaromaticamans strain A175 DSM13477 genome (Wittich et al., 2007) (ii) seven were annotated as colicin receptors (CirA) (Ferguson et al., 2002), and (iii) the rest as ferripyoverdine (FpvA), ferric pseudobactin (PupA) (Adams et al., 2006), ferrichrome iron (FhuA) (Locher et al., 1998) and various siderophore receptors (Noinaj et al., 2010). Considering the very close structures of the several TBDT, including B12/colicin/siderophore transporters, their sequence-based substrate determination should be considered with caution (Koster, 2001; Rodionov et al., 2003).
From these TonB-dependent receptors only three fulfill the stringent criterium of Degnan et al. (2014) in order to be assigned a cobalamin transporter function, “to be located downstream of riboswitches.” These are RNA elements that change conformation upon binding of a specific molecule affecting negatively or positively the transcription and translation of downstream genes including B12 uptake and biosynthesis (Montange and Batey, 2008). In silico screening of the genome for regulatory riboswitches identified three of them residing in the 5′ region of TBDT (Figure 4). The first (position of putative riboswitch: 1309712-1309955) was located upstream of a region encoding BtuB, a protein of the HoxN family known to encode high affinity Co+2/Ni+2 transporter genes (Komeda et al., 1997), cobW, cobN, and cobO, respectively, encoding proteins involved in Co chelation, cobalt chelatase subunit N and a corrinoid adenosyltransferase (Rodionov et al., 2003), all involved in the biosynthesis of B12, and BtuF, the substrate-binding component of the ABC transporter system for B12 uptake. Such a gene organization has been reported in several proteobacteria where hoxN and cobW are located immediately upstream of cobN and the whole locus is flanked upstream with a riboswitch (Rodionov et al., 2003). T he second (position 4162841-4163031) and the third riboswitches (position 4166483-4166691) were closely localized. The former is located upstream of bluB, a hypothetical protein and fecD (e-value1e-718, 78% identities and 86% positives with the Sphingopyxis granuli btuC, AMG76476.1), hmuV (e-value8e-97, 66% identities and 79% positives with the Sphingopyxis sp. strain C-1 btuD, GAO77867.1) genes encoding, respectively, a permease and an ATP-binding protein forming a btuCD-like ABC transporter component of the cobalamin transportation system. BluB belongs to the flavin destructase family proteins and controls the transformation of FMN2 to 5,6-dimethylbenzimidazole (Taga et al., 2007), which is the lower axial ligand of B12, and the preferred ligand in the cobamides of most bacteria (Hazra et al., 2013). Considering the key role of bluB in the biosynthesis or remodeling of salvaged corrinoids by bacteria it is not surprising that its translation might be regulated by B12-associated riboswitches. The third riboswitch is located in the 5′ region of btuB. In all other cases no riboswitches or other regulatory systems were detected. This could be due to missing alignment profiles from the databases which are necessary for identifying riboswitches in sphingomonads, or, given that cobalamin is a well-defined structure, maybe riboswitches do not exist as a regulatory mechanism. In the latter case, a different regulatory system or even a housekeeping role of the associated transporters could be possible.
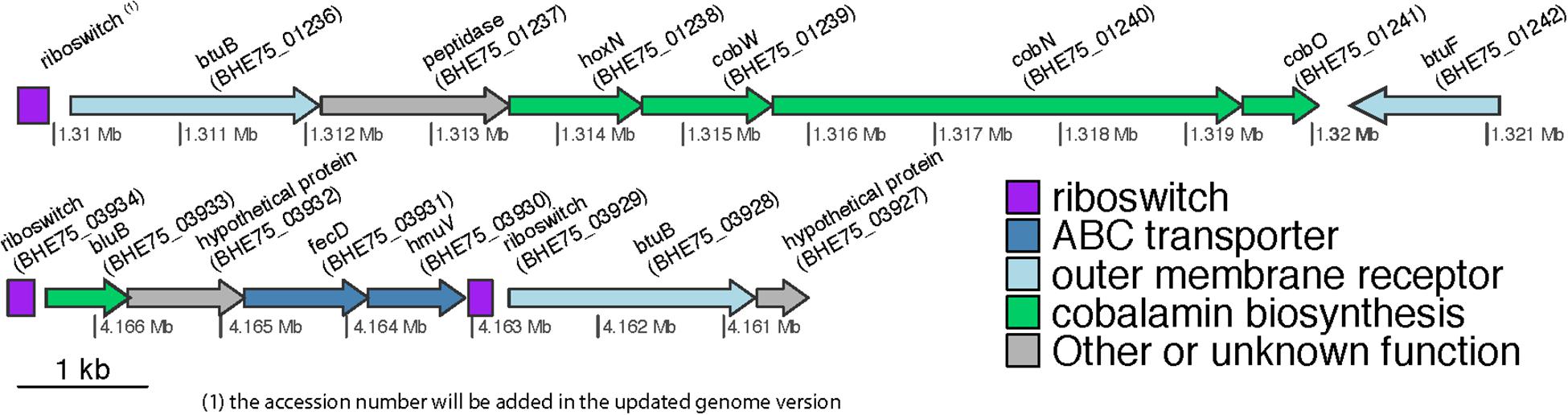
Figure 4. Genetic organization of the genomic regions in S. haloaromaticamans downstream of the three riboswitches associated with B12-biosynthesis. Genes annotation btuB: TonB-dependent corrinoid transporter; hoxN, high affinity Co+2/Ni+2 transporter; cobW, cobalt-chelation associated protein; cobN, cobalt chelatase subunit N; cobO, corrinoid adenosyltransferase; btuF, substrate-binding component of the ABC cobalamin transporter system; bluB, 5,6-dimethylbenzimidazole synthase; fecD, permease of a btuCD-like ABC cobalamin transporter system; hmuV, ATP-binding subunit of a butCD-like ABC cobalamin transporter system.
Overall, our in silico analysis of the biosynthetic pathway of B12 suggests that S. haloaromaticamans is not able to biosynthesize B12 and meet its cellular needs for the methionine biosynthesis. However, its genome is enriched in receptors for transportation of corrinoids across its outer membrane, operating the energetically inexpensive salvage pathway to serve its needs in the primary cellular metabolism.
B12 and Not Methionine Precursors Rescues the Degradation Capacity of Sphingomonas haloaromaticamans
Based on the genome sequence-based metabolic reconstruction evidence which suggested that S. haloaromaticamans was not capable of de novo biosynthesis of B12, we evaluated in vitro whether the supplementation of methionine precursors and/or B12 will rescue the degradation capacity of S. haloaromaticamans. The bacterium maintained its degrading capacity against OPP when supplemented with methionine (Figure 5B) and B12 (Figure 5C). However, it failed to degrade OPP when it was not supplemented with any of the above (Figure 5A) or supplemented with intermediates of methionine biosynthesis (Figures 5D–G) unless B12 was also co-provided as in the case of homocysteine (Figure 5G). These results verified genome-based metabolic reconstruction findings that B12 is the main driver of the auxotrophy of S. haloaromaticamans to methionine.
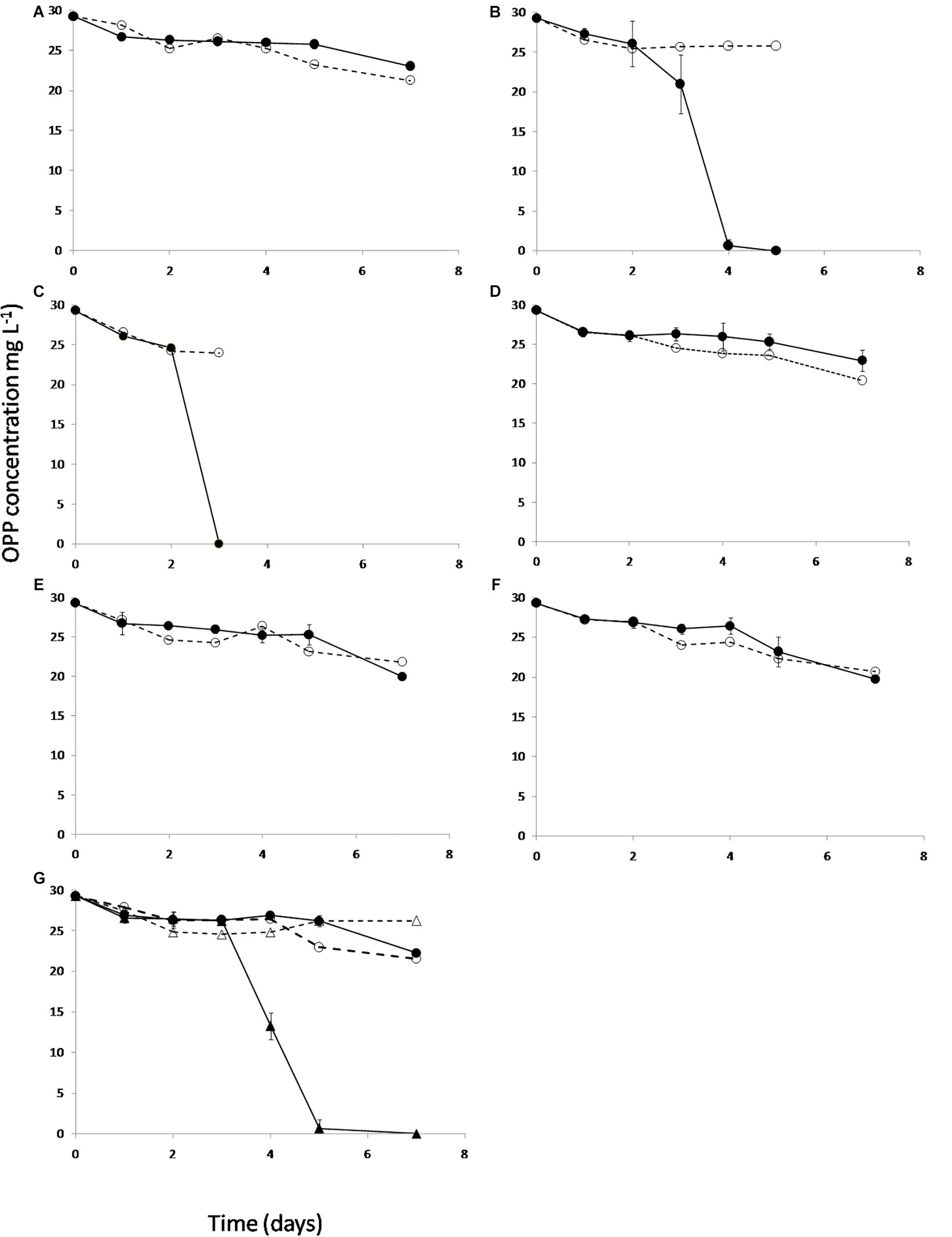
Figure 5. The degradation of OPP by S. haloaromaticamans in MSMN (•, solid lines) without any supplementation (A) and with supplementation of methionine (B), B12 (C), and of various intermediates of the methionine biosynthetic pathway like homoserine (D), O-succinyl homoserine (E), cystathionine (F), and homocysteine [plus (▲) or minus B12 (•)] (G). Each value is the mean of triplicates ± the standard deviation of the mean. In all treatments the degradation of OPP in non-inoculated cultures was also determined (∘, △ dashed lines).
The Degradation and Growth of Sphingomonas haloaromaticamans Supplied With a Range of B12 Concentrations
We finally tested the minimum concentrations of B12 provided to S. haloaromaticamans to avert its auxotrophy to methionine and degrade OPP. The bacterium was able to degrade OPP even when supplied with 0.1 ng ml–1 of B12 (Figure 6A), which is close to the concentration levels of B12 and corrinoids found in fresh water (2–6 ng L–1) (Kurata, 1986), soil solution (5 μg L–1) (Mozafar and Oertli, 1992), and wastewaters (0.05–5.2 mg L–1) (Selimoglu et al., 2015). In addition, all concentrations of B12 supported similar growth rates of S. haloaromaticamans (Figure 6B). As expected, no growth and degradation of OPP was observed when the bacterium was not supplemented with B12.
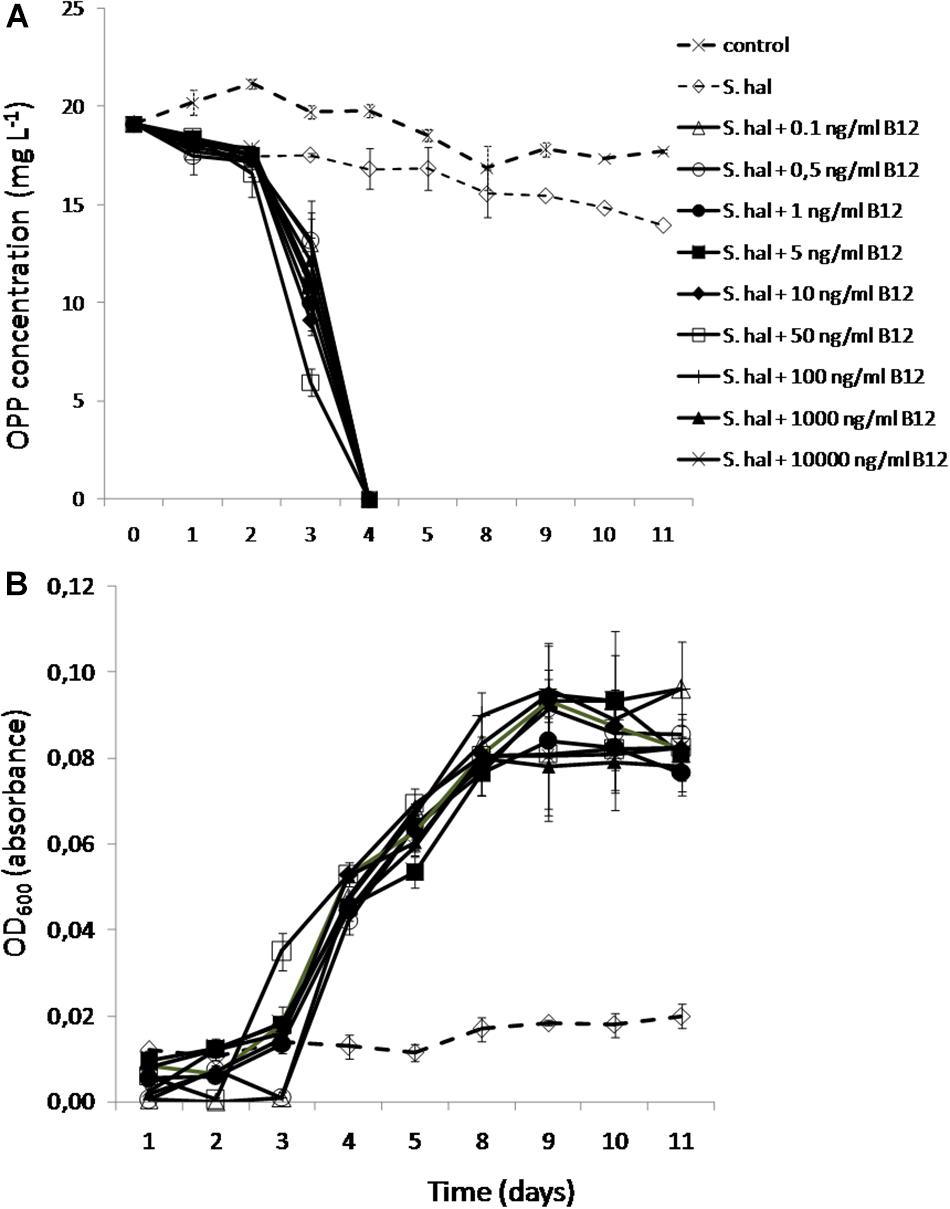
Figure 6. The degradation of OPP (A) and the growth of S. haloaromaticamans (B) in MSMN supplemented with 0, 0.1, 0.5, 1, 5, 10, 50, 100, 1000, and 10000 ng ml–1 of B12. The degradation of OPP in non-inoculated controls was also determined (control). Each value is the mean of triplicates ± the standard deviation.
Conclusion
A combination of genome-based metabolic reconstruction and in vitro tests demonstrated that the auxotrophy of a fungicide-degrading S. haloaromaticamans strain was driven by the lack of a MetE B12-independent cobalamine synthase and the absence of a de novo B12 biosynthetic pathway. The bacterium could maintain its degradation and growth capacity in the presence of environmentally relevant concentrations of B12 suggesting that its application in natural and engineered systems would not be hampered by its auxotrophy to B12, in line with the presence in its genome of several potential B12 outer membrane receptors and a near complete cobalamine salvage pathway. Further studies will focus on (a) the corrinoids preferences of S. haloaromaticamans for optimum growth and OPP degradation using cross-feeding studies with corrinoid-producing bacteria (Mesorhizobium loti) and external supply of selected corrinoids (b) the role of the riboswitches detected on the regulation of cobalamin biosynthesis and corrinoids uptake.
Data Availability Statement
The datasets generated for this study can be found in the DDBJ/ENA/GenBank accession number MIPT00000000.
Author Contributions
CP isolated the microorganism, performed the in vitro experiment, and drafted the manuscript. SV performed the bioinformatic analysis and genome-based reconstruction, and helped in drafting of the manuscript. EP performed the in vitro experiment and reviewed the manuscript. DK had the experimental idea, supervised and planned the experiments, and revised the final manuscript.
Funding
This work was funded by the projects (i) “Bioremediatomics – The Microbial Detoxification of Pesticides From the Fruit-Packaging Industry: Using Omics in Bioremediation” (Grant No. 4781) which is implemented under the “ARISTEIA” Action of the “Operational Programme Education and Lifelong Learning” and co-funded by the European Social Fund (ESF) and National Resources, Greece, and (ii) “OMIC-ENGINE-Synthetic Biology: From Omics Technologies to Genomic Engineering” (MIS 5002636) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure,” funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund). SV was supported by the MSCA-IF-H2020 project EMIGRATE (Grant Agreement No. 749463).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03009/full#supplementary-material
FIGURE S1 | Phylogenetic analysis of the metB/Z gene encoding cystathione γ-synthase/O-succinyl homoserine sulfhydrylase detected in the genome of Sphingomonas haloaromaticamans.
TABLE S1 | The functional annotation of the genes participating the biosynthetic pathways of methionine, isoleucine, tyrosine, and phenylalanine in the genome of the Sphingomonas haloaromaticamans strain. Annotation was performed using UniProt and RefSeq BLAST searches and verified further through Pfam searches.
TABLE S2 | The functional annotation of the genes participating in cobalamin biosynthetic pathways in the Sphingomonas haloaromaticamans strain. Annotation was performed using UniProt and RefSeq BLAST searches and verified further through Pfam searches.
TABLE S3 | The functional annotation of the genes associated directly or indirectly with the energy-dependent uptake of various biomolecules (i.e., cobinamides, siderophores, colicin etc.). Annotation was performed using UniProt and RefSeq BLAST searches and verified further through Pfam searches. Purple color denotes genes with TonB-dependent transporter function, orange color denotes genes with energy transducing role (ABC transporters), blue color denotes genes with regulatory function in B12 transportation and gray color denotes other genes with indirect association with B12 biosynthesis or transportation.
References
Abascal, F., Zardoya, R., and Posada, D. (2005). ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104–2105. doi: 10.1093/bioinformatics/bti263
Adams, H., Zeder-Lutz, G., Schalk, I., Pattus, F., and Celia, H. (2006). Interaction of TonB with the outer membrane receptor FpvA of Pseudomonas aeruginosa. J. Bacteriol. 188, 5752–5761. doi: 10.1128/jb.00435-06
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al. (2009). BLAST+: architecture and applications. BMC Bioinf. 10:421. doi: 10.1186/1471-2105-10-421
Caspi, R., Billington, R., Ferrer, L., Foerster, H., Fulcher, C. A., Keseler, I. M., et al. (2016). The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 44, D471–D480. doi: 10.1093/nar/gkv1164
Chiang, P. K., Gordon, R. K., Tal, J., Zeng, G. C., Doctor, B. P., Pardhasaradhi, K., et al. (1996). S-Adenosylmethionine and methylation. FASEB J. 10, 471–480.
Chimento, D. P., Mohanty, A. K., Kadner, R. J., and Wiener, M. C. (2003). Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10, 394–401. doi: 10.1038/nsb914
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J., and Smith, A. G. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93. doi: 10.1038/nature04056
Degnan, P. H., Barry, N. A., Mok, K. C., Taga, M. E., and Goodman, A. L. (2014). Human gut microbes use multiple transporters to distinguish vitamin B12 analogs and compete in the gut. Cell Host Microbe 15, 47–57. doi: 10.1016/j.chom.2013.12.007
D’Souza, G., Waschina, S., Pande, S., Bohl, K., Kaleta, C., and Kost, C. (2014). Less is more: selective advantages can explain the prevalent loss of biosynthetic genes in bacteria. Evolution 68, 2559–2570. doi: 10.1111/evo.12468
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Ferguson, A. D., Chakraborty, R., Smith, B. S., Esser, L., van der Helm, D., and Deisenhofer, J. (2002). Structural basis of gating by the outer membrane transporter FecA. Science 295, 1715–1719. doi: 10.1126/science.1067313
Ferla, M. P., and Patrick, W. M. (2014). Bacterial methionine biosynthesis. Microbiology 160, 1571–1584. doi: 10.1099/mic.0.077826-0
Gomez-Consarnau, L., Sachdeva, R., Gifford, S. M., Cutter, L. S., Fuhrman, J. A., Sanudo-Wilhelmy, S. A., et al. (2018). Mosaic patterns of B-vitamin synthesis and utilization in a natural marine microbial community. Environ. Microbiol. 20, 2809–2823. doi: 10.1111/1462-2920.14133
Gonzalez, J. C., Peariso, K., Penner-Hahn, J. E., and Matthews, R. G. (1996). Cobalamin-independent methionine synthase from Escherichia coli: a zinc metalloenzyme. Biochemistry 35, 12228–12234. doi: 10.1021/bi9615452
Gophna, U., Bapteste, E., Doolittle, W. F., Biran, D., and Ron, E. Z. (2005). Evolutionary plasticity of methionine biosynthesis. Gene 355, 48–57. doi: 10.1016/j.gene.2005.05.028
Graindorge, M., Guistini, C., Kraut, A., Moyet, L., Curien, G., and Matringe, M. (2014). Three different classes of aminotransferases evolved prephenate aminotransferase functionality in arogenate-competent microorganisms. J. Biol. Chem. 289, 3198–3208. doi: 10.1074/jbc.M113.486480
Gu, W., Zhao, G., Eddy, C., and Jensen, R. A. (1995). Imidazole acetol phosphate aminotransferase in Zymomonas mobilis: molecular genetic, biochemical, and evolutionary analyses. J. Bacteriol. 177, 1576–1584. doi: 10.1128/jb.177.6.1576-1584.1995
Hacham, Y., Gophna, U., and Amir, R. (2003). In vivo analysis of various substrates utilized by cystathionine γ-synthase and O-acetylhomoserine sulfhydrylase in methionine biosynthesis. Molec. Biol. Evol. 20, 1513–1520. doi: 10.1093/molbev/msg169
Hay, A. G., Dees, P. M., and Sayler, G. M. (2001). Growth of a bacterial consortium on triclosan. FEMS Microbiol. Ecol. 36, 105–112. doi: 10.1016/s0168-6496(01)00127-1
Hazra, A. B., Tran, J. L. A., Crofts, T. S., and Taga, M. E. (2013). Analysis of substrate specificity in CobT homologs reveals widespread preference for DMB, the lower axial ligand of vitamin B12. Chem. Biol. 20, 1275–1285. doi: 10.1016/j.chembiol.2013.08.007
Heal, K. R., Qin, W., Ribalet, F., Bertagnolli, A. D., Coyote-Maestras, W., Hmelo, L. R., et al. (2017). Two distinct pools of B12 analogs reveal community interdependencies in the ocean. PNAS 114, 364–369. doi: 10.1073/pnas.1608462114
Helliwell, K. E., Wheeler, G. L., Leptos, K. C., Goldstein, R. E., and Smith, A. G. (2011). Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Biol. Evol. 28, 2921–2933. doi: 10.1093/molbev/msr124
Hwang, B.-J., Yeom, H.-I., Kim, Y., and Lee, H.-S. (2002). Corynebacterium glutamicum utilizes both trans-sulfuration and direct sulfhydrylation pathways for methionine biosynthesis. J. Bacteriol. 184, 1277–1286. doi: 10.1128/jb.184.5.1277-1286.2002
Ikeda, M. (2006). Towards bacterial strains overproducing L-tryptophan and other aromatics by metabolic engineering. Appl. Microbiol. Biotechn. 69, 615–626. doi: 10.1007/s00253-005-0252-y
Karp, P., Weaver, D., Paley, S., Fulcher, C., Kubo, A., Kothari, A., et al. (2014). The EcoCyc Database. EcoSal Plus. 41, D605–D612. doi: 10.1128/ecosalplus.ESP-0009-2013
Karp, P. D., Latendresse, M., Paley, S. M., Krummenacker, M., Ong, Q. D., Billington, R., et al. (2015). Pathway Tools version 19.0 update: software for pathway/genome informatics and systems biology. Brief Bioinform. 17, 877–890. doi: 10.1093/bib/bbv079.2015
Komeda, H., Kobayashi, M., and Shimizu, S. (1997). A novel transporter involved in cobalt uptake. Proc. Natl. Acad. Sci. U.S.A. 94, 36–41. doi: 10.1073/pnas.94.1.36
Koster, W. (2001). ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152, 291–301. doi: 10.1016/s0923-2508(01)01200-1
Kurata, A. (1986). Chrysophytes: Aspects and Problems, eds J. Kristiansen, and R. A. Andersen, (Cambridge: Cambridge Univ. Press), 185–196.
Lambreht, J. A., Flynn, J. M., and Downs, D. M. (2012). Conserved YjgF protein family deaminates reactive enamine/imine intermediates of pyridoxal 5-phosphate (PLP)-dependent enzyme reactions. J. Biol. Chem. 287, 3454–3461. doi: 10.1074/jbc.M111.304477
Li, W., Jaroszewski, L., and Godzik, A. (2001). Clustering of highly homologous sequences to reduce the size of large protein databases. Bioinformatics 17, 282–283. doi: 10.1093/bioinformatics/17.3.282
Locher, K. P., Rees, B., Koebnik, R., Mitschler, A., Moulinier, L., Rosenbusch, J. P., et al. (1998). Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95, 771–778. doi: 10.1016/s0092-8674(00)81700-6
Lu, H., Guan, X., Wang, J., Zhou, J., and Zhang, H. (2015). Enhanced bio-decolorization of 1-amino-4-bromoanthraquinone-2-sulfonic acid by Sphingomonas xenophaga with nutrient amendment. J. Environ. Sci. 27, 124–130. doi: 10.1016/j.jes.2014.05.041
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V., and Thiele, I. (2015). Systematic genome assessment of B-vitamin biosynthesis suggests co-operation amonggut microbes. Front. Genet. 6:148. doi: 10.3389/fgene.2015.00148
Mee, M. T., Collins, J. J., Church, G. M., and Wang, H. H. (2014). Syntrophic exchange in synthetic microbial communities. Proc. Natl. Acad. Sci. 111, E2149–E2156. doi: 10.1073/pnas.1405641111
Men, Y., Feil, H., VerBerkmoes, N. C., Shah, M. B., Johnson, D. R., Lee, P. K. H., et al. (2012). Sustainable syntrophic growth of Dehalococcoides ethenogenes strain 195 with Desulfovibrio vulgaris Hildenborough and Methanobacterium congolense: global transcriptomic and proteomic analyses. ISME J. 6, 410–421. doi: 10.1038/ismej.2011.111
Montange, R. K., and Batey, R. T. (2008). Riboswitches: emerging themes in RNA structure and function. Annu. Rev. Biophys. 37, 117–133. doi: 10.1146/annurev.biophys.37.032807.130000
Mozafar, A., and Oertli, J. J. (1992). Uptake of microbially -produced vitamin (B12) by soybean roots. Plant Soil 139, 23–30. doi: 10.1007/bf00012838
Mukherjee, S., and Sengupta, S. (2016). Riboswitch Scanner: an efficient pHMM-based web-server to detect riboswitches in genomic sequences. Bioinformatics 32, 776–778. doi: 10.1093/bioinformatics/btv640
Noinaj, N., Guillier, M., Barnard, T. J., and Buchanan, S. K. (2010). TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64, 43–60. doi: 10.1146/annurev.micro.112408.134247
Onneby, K., Hakansson, S., Pizzul, L., and Stenstrom, J. (2014). Reduced leaching of the herbicide MCPA after bioaugmentation with a formulated and stored Sphingobium sp. Biodegradation 25, 291–300. doi: 10.1007/s10532-013-9660-3
Pande, S., Shitut, S., Freund, L., Westermann, M., Bertels, F., Colesie, C., et al. (2015). Metabolic cross-feeding via intercellular nanotubes among bacteria. Nature Commun. 6, 6238–6251. doi: 10.1038/ncomms7238
Paradis, E., Claude, J., and Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Pasamontes, A., and Garcia-Vallve, S. (2006). Use of a multi-way method to analyze the amino acid composition of a conserved group of orthologous proteins in prokaryotes. BMC Bioinf. 7:257. doi: 10.1186/1471-2105-7-257
Perruchon, C., Patsioura, V., Vasileiadis, S., and Karpouzas, D. G. (2016). Isolation and characterisation of a Sphingomonas strain able to degrade the fungicide ortho-phenylphenol. Pest Manag. Sci. 72, 113–124. doi: 10.1002/ps.3970
Perruchon, C., Vasileiadis, S., Rousidou, C., Papadopoulou, E. S., Tanou, G., Samiotaki, M., et al. (2017). Metabolic pathway and cell adaptation mechanisms revealed through genomic, proteomic and transcription analysis of a Sphingomonas haloaromaticamans strain degrading ortho-phenylphenol. Sci. Rep. 7:6449. doi: 10.1038/s41598-017-06727-6
Price, M. N., Zane, G. M., Kuehl, J. V., Melnyk, R. A., Wall, J. D., Deutschbauer, A. M., et al. (2018). Filling gaps in bacterial amino acid biosynthesis pathways with high-throughput genetics. PLoS Genet. 14:e1007147. doi: 10.1371/journal.pgen.1007147
R Core Team. (2015). R: A Language and Environment for Statistical Computing, Reference Index Version 3.2.2. 2015. Vienna: R Core Team.
Raux, E., Schubert, H. L., and Warren, M. J. (2000). Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum. Cell. Moll. Life Sci. 57, 1880–1893. doi: 10.1007/pl00000670
Risso, C., Van Dien, S. J., Orloff, A., Lovley, D. R., and Coppi, M. V. (2008). Elucidation of an alternative isoleucine biosynthesis pathway in Geobacter sulforreducens. J. Bacteriol. 190, 2266–2274. doi: 10.1128/jb.01841-07
Rodionov, D., Vitreschak, A. G., Mironov, A. A., and Gelfand, M. S. (2003). Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem. 278, 41148–41159. doi: 10.1074/jbc.m305837200
Romine, M. F., Rodionov, D. A., Maezato, Y., Osterman, A. L., and Nelson, W. C. (2017). Underlying mechanisms for syntrophic metabolism of essential enzyme cofactors in microbial communities. ISME J. 11, 1434–1446. doi: 10.1038/ismej.2017.2
Roth, J. R., Lawrence, J. G., and Bobik, T. A. (1996). Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 50, 137–181. doi: 10.1146/annurev.micro.50.1.137
Schliep, K. P. (2011). Phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593. doi: 10.1093/bioinformatics/btq706
Selimoglu, F., Obek, E., Karatas, F., Arslan, E. I., and Tatar, S. Y. (2015). Determination of amounts of some vitamin B groups indomestic wastewater treatment plants. Turk. J. Sci. Technol. 10, 1–5.
Shelton, A., Seth, E. C., Mok, K. C., Han, A. W., Jackson, S. N., Haft, D. R., et al. (2019). Uneven distribution of cobamide biosynthesis and dependence in bacteria predicted by comparative genomics. ISME J. 13, 789–804. doi: 10.1038/s41396-018-0304-9
Sørensen, S. R., Ronen, Z., and Aamand, J. (2001). Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl. Environ. Microbiol. 67, 5403–5409. doi: 10.1128/aem.67.12.5403-5409.2001
Sørensen, S. R., Ronen, Z., and Aamand, J. (2002). Growth in co-culture stimulates metabolism of the phenylurea herbicide isoproturon by Sphingomonas sp strain SRS2. Appl. Environ. Microbiol. 68, 3478–3485. doi: 10.1128/aem.68.7.3478-3485.2002
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Taga, M. E., Larsen, N. A., Howard-Jones, A. R., Walsh, C. T., and Walker, G. C. (2007). BluB canibalizes flavin to form the lower ligand of vitamin B12. Nature 446, 449–453. doi: 10.1038/nature05611
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577. doi: 10.1080/10635150701472164
Tang, K., Jiao, N., Liu, K., Zhang, Y., and Li, S. (2012). Distribution and functions of TonB-dependent transporters in marine bacteria and environments: implications for dissolved organic matter utilization. PLoS One 7:e41204. doi: 10.1371/journal.pone.0041204
Valley, C. C., Cembran, A., Perlmutter, J. D., Lewis, A. K., Labello, N. P., Gao, J., et al. (2012). The methionine-aromatic motif plays a unique role in stabilizing protein structure. J. Biol. Chem. 287, 34979–34991. doi: 10.1074/jbc.M112.374504
Van der Kaaij, H., Desiere, F., Mollet, B., and Germond, J.-E. (2014). L-Alanine auxotrophy of Lactobacillus johnsonii as demonstrated by physiological, genomic, and gene complementation approaches. Appl. Environ. Microbiol. 70, 1869–1873. doi: 10.1128/aem.70.3.1869-1873.2004
Keywords: Sphingomonas haloaromaticamans, methionine, ortho-phenylphenol, genome-based metabolic reconstruction, B12 auxotrophy
Citation: Perruchon C, Vasileiadis S, Papadopoulou ES and Karpouzas DG (2020) Genome-Based Metabolic Reconstruction Unravels the Key Role of B12 in Methionine Auxotrophy of an Ortho-Phenylphenol-Degrading Sphingomonas haloaromaticamans. Front. Microbiol. 10:3009. doi: 10.3389/fmicb.2019.03009
Received: 14 September 2019; Accepted: 16 December 2019;
Published: 10 January 2020.
Edited by:
Dimitris G. Hatzinikolaou, National and Kapodistrian University of Athens, GreeceReviewed by:
Dmitry A. Rodionov, Sanford Burnham Prebys Medical Discovery Institute, United StatesYujie Men, University of California, Riverside, United States
Copyright © 2020 Perruchon, Vasileiadis, Papadopoulou and Karpouzas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios G. Karpouzas, ZGthcnBvdXphc0BiaW8udXRoLmdy
†These authors have contributed equally to this work
 Chiara Perruchon
Chiara Perruchon Sotirios Vasileiadis
Sotirios Vasileiadis Evangelia S. Papadopoulou
Evangelia S. Papadopoulou Dimitrios G. Karpouzas
Dimitrios G. Karpouzas