- 1Department of Pathogen Biology, Provincial Laboratories of Pathogen Biology and Zoonoses Anhui, School of Basic Medicine, Anhui Medical University, Hefei, China
- 2Department of Clinical Laboratory, The Second Hospital of Hefei, Hefei, China
- 3The Key Laboratory of Oral Disease Research of Anhui, College and Hospital of Stomatology, Anhui Medical University, Hefei, China
- 4Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Imbalance of Th1 and Th2 response at the maternal–fetal interface is considered as a radical event in the pathogenesis of immunity-related pregnant diseases. It has been demonstrated that the ROP16I, a rhoptry protein of Toxoplasma gondii, and the viable parasite with ROP16I may induce M2 macrophage polarization in host innate immunity and may be involved in the adverse pregnant outcomes. However, the mechanisms by which T. gondii-derived effectors subvert the immune tolerance in the pathology of pregnancy remain unclear. Here, we constructed the RH strain with ROP16I deletion (RHΔrop16) to explore the pathogenesis of abnormal pregnancy. We found that C57BL/6 mice infected with RHΔrop16 exhibited the increased resorption of fetuses and more severe adverse pathology of placentae at the early phase of gestation, as compared to the mice infected with RH wild type (RH WT) parasite. Additionally, RHΔrop16 strain infection significantly promoted M1 macrophage phenotypes of CD80 and CD86, and decreased CD206 expression of M2 macrophages, with upregulation of the iNOS and downregulation of the Arg-1 expression in placental homogenates. Simultaneously, the pro-inflammatory cytokines of IL-12 and TNF-α were elevated whereas the anti-inflammatory cytokine of TGF-β1 was dampened. Moreover, the p38α mitogen-activated protein kinase (p38α MAPK) was notably phosphorylated in placental macrophages infected with both RHΔrop16 and RH WT strains compared with the control. Taken together, our findings indicated that ROP16I deletion of type I RH strain may cause exacerbated adverse pregnant outcomes, which is attributable to subversion of the maternal immune tolerance due to the increased pro-inflammatory cytokines in the pregnant animals. The results also suggest that ROP16I might be a protective factor and other T. gondii-derived molecules might be involved in the M1–Th1 biased pathological process in aberrant pregnancy at the early phase of gestation.
Introduction
Toxoplasma gondii is an opportunistic food-borne protozoon with an extraordinarily broad host range of all warm-blooded animals, including humans (Montoya and Liesenfeld, 2004; Leng et al., 2009). Humans and animals are infected by ingesting food that contains cysts or water that is contaminated with oocysts of T. gondii (Black and Boothroyd, 2000; Yarovinsky, 2014). It is estimated that over one billion people are chronically infected with this parasite worldwide, although the data of regional investigations vary considerably (Montoya and Liesenfeld, 2004; Dubey, 2009). T. gondii infection is usually asymptomatic in immunocompetent individuals but may result in severe consequences in immunocompromised people (e.g., patients with AIDS, organ transplantation, or cancers) (Nissapatorn, 2009; Luma et al., 2013; Schmidt et al., 2013). Importantly, vertical transmission of T. gondii via placenta may cause abortion, constituting a serious threat to humans and leading to great loss of livestock production (Montoya and Remington, 2008; Hide et al., 2009). Initial infection of women with T. gondii during pregnancy, particularly in the first trimester, may cause miscarriage and preterm birth and increase the susceptibility of fetuses to toxoplasmosis resulting in hydrocephaly, microcephaly, intracranial calcification, and even loss of life (Pfaff et al., 2007; Robbins et al., 2012). The variability of disease severity in infected host is linked to the genetic structure of T. gondii strains and to the exposure burden of the parasite (Saeij et al., 2006; Melo et al., 2011; Hunter and Sibley, 2012; Shwab et al., 2014).
Toxoplasma gondii isolates from Europe and North America mostly belong to types I (RH and GT1), II (PRU and ME49), and III (CTG) (Lehmann et al., 2006; Shwab et al., 2014), but those from China present a dramatic difference in genetic structure and virulence, termed as type Chinese 1 (Wang et al., 2013). Studies have revealed that the majority of T. gondii isolates causing congenital toxoplasmosis in Europeans possess the feature of type II phenotype whereas type I strains are the most prevalent in Spanish people (Fuentes et al., 2001).
Macrophages can be roughly categorized into two types: classically activated macrophage (M1) and alternatively activated macrophage (M2). As antigen-presenting cells, macrophages have cross-talk between innate immunity and adaptive immunity. T. gondii parasite was found to preferentially invade macrophage/DC lineage cells during in vivo infection (Courret et al., 2006). Decidual immune cell populations approximately consist of 20% of macrophages that play a critical part in maintaining normal pregnancy. It has been well recognized that M2 macrophages are responsible for sustaining the normal microenvironment of pregnancy at the maternal–fetal interface (Nagamatsu and Schust, 2010). Actually, any subversion of M1/M2 macrophage balance may lead to pregnant disorders, such as pregnant loss, premature birth, or fetal growth restriction (Renaud and Graham, 2008; Brown et al., 2014).
Toxoplasma gondii establishes the long-lasting infection in host and has the developed sophisticated ways to manipulate host immunity. For instance, the parasite delivers effector proteins, which are released from the contents of rhoptries, micronemes, and dense granules of T. gondii, into host cells (Jensen et al., 2011; Rosowski et al., 2011; Hunter and Sibley, 2012). The secreted rhoptry proteins (ROPs) and dense granule proteins (GRAs) are characterized to hijack cell-signaling pathways of the host cells, which manipulate host immune response, and therefore determine parasite virulence and infection consequences (Jensen et al., 2011; Hakimi et al., 2017). Interestingly, ROP16 (the member of ROPs) gets injected into the host cell nucleus where it activates signal transducer and activator of transcription Stat3 and Stat6, initiating the signaling cascade of host cells. ROP16I of type I strains, rather than ROP16II of type II strains, decides ROP16 kinase activity on Stat3 and Stat6 (Yamamoto et al., 2009). Phosphorylation of Stat3 and Stat6 triggers anti-inflammatory response and M2 macrophage activation, which plays a crucial role in modulating host immunity at the early phase and is associated with the consequences of infection at the late phase. Contrarily, the GRA15II directly activates the NF-κB of the host cells, promotes its rapid nuclear transcription, and drives the macrophage to M1 polarization (Rosowski et al., 2011). ROP16I and GRA15II can work independently of pattern recognition receptors to achieve M1 or M2 polarization (Jensen et al., 2011). Intriguingly, the WH3 strain of type Chinese 1, a predominant genotype in China, carries both key effectors: ROP16I and GRA15II. Our previous study showed that the WH3Δrop16 strain with GRA15II background of type Chinese 1 evoked the Th1- and Th17-biased response, leading to subversion of immune tolerance at the maternal–fetus interface and adverse pregnant outcomes (Wang et al., 2018). Recent studies have shown that RHΔrop16 infection in mice may cause serious ocular toxoplasmosis in the immune-privileged microenvironment, which is far away from the proliferation sites of the parasite, suggesting that the RH strain, with ROP16I deficiency, remains a high pathogenesis of severe retinopathy in animal model (Rochet et al., 2019).
It has been known that vertical transmission of viable T. gondii parasite is a crucial route in aberrant pregnancy. However, we tried to identify the parasite by Toxo-DNA detection and bioassay in the maternal placental tissues with positive antibodies against T. gondii and abnormal pregnancies, but failed (data not published). Previous studies revealed that pregnant mice of IL-10 deletion infected with T. gondii resulted in a worse pregnancy than the control (Lao et al., 2015). Additionally, adoptive transfer of Treg cells to mice could improve adverse pregnancies of the animals (Liu et al., 2014). All of these investigations strongly suggest that the imbalance of immune tolerance at the maternal–fetal interface, in addition to direct parasite invasion, might be involved in the occurrence of abnormal pregnant consequences. To characterize the role of ROP16I molecule in the pathogenicity of virulent type I strain, we generated T. gondii RHΔrop16 utilizing CRISPR/Cas9 technology to decipher the possible influence of ROP16I-deficient parasite infection on and the pathophysiology in the abnormal pregnancies. Our results uncovered that infection of mice with RHΔrop16 strain cause exacerbated adverse pregnant outcomes in which type I dominant response and enhanced pro-inflammatory cytokine expression were present in placenta tissues. The data provide further evidence that ROP16I plays a potentially protective role in maintaining physiological immune tolerance during pregnancy, and other parasite-derived effectors might be associated with the M1–Th1 biased response at the maternal–fetal interface and the adverse pregnant outcomes.
Materials and Methods
Parasite Collection
Toxoplasma gondii RH WT and RHΔrop16 tachyzoites were cultured in human foreskin fibroblast (HFF) cells.
T. gondii Infection in Pregnant Mice
The 8- to 10-week-old male and 6- to 8-week-old female C57BL/6 mice were raised for 1 week after purchase to adapt to the animal center environment with normal feeding and drinking at room temperature. The female and male mice were caged overnight at a 2:1 ratio. The female mice were examined for the vaginal plug as gestation day 0 (GD0). All pregnant mice were randomly divided into three groups: control group, RH WT group, and RHΔrop16 group, with seven mice in each. All of the experimental procedures were performed in accordance with the Institutional Review Board of AMU Institute of Biomedicine AMU (permit No: AMU26-081108). The studies were performed in licensed Biosafety II Laboratory. The RH WT and RHΔrop16 viable tachyzoites were harvested from HFF cells and counted with an Improved Neubauer counting board and diluted to 2 × 103/ml. On gestation day 8 (GD8), mice of RH WT and RHΔrop16 groups were intraperitoneally injected with 200 μl of PBS containing 400 wild type and Δrop16 tachyzoites, respectively. The control animals were only given equal volume of PBS solution. On GD14, the animals were euthanized, blood was taken, and placentae, fetuses, uterus, and spleens were aseptically removed. The fetuses and placentae were photographed and weighed, and the number of resorbed fetuses was calculated. The resorptivity was determined by the small size, hemorrhagic appearance, and necrotic placenta and embryos. The ratio of the resorption to the total fetuses was recorded as a percentage of fetal loss. The sera were obtained by centrifugation (4,000 rpm, 10 min, 4°C) for ELISA detection.
Pathology of Placental Tissues
Mice were randomly selected into three groups, with seven in each, for placental fixation with 4% paraformaldehyde, paraffin-embedded sections and hematoxylin–eosin staining. The hyperemia of placentae was observed by histopathological analyses. All mice in each group were photographed, with five photos for sections of each animal.
Collection of Macrophages From Uterus and Placental Tissues
The uterus and placental tissues were quickly excised in a culture dish after removal, followed by digestion with 0.5% BSA, 5 mg/ml Collagenase IV, and HEPES (10 mM) (St. Louis, MO, United States) in RPMI 1640 solution (Montreal, QC, Canada), and slightly shaken at 37°C for 30 min at 150 rpm. Subsequently, the suspended cells were filtered through sterile nylon mesh. Macrophages were obtained by density gradient centrifugation at 25/50% Percoll (GE Healthcare Life Sciences).
Isolation of Control Placental Macrophages and Infection of T. gondii in vitro
Macrophages in uterus and placental tissues from control pregnant mice were harvested according to the methods previously described and 2 × 106 cells/well were cultured in 12-well plates. Macrophages were infected with 2 × 106 RH WT and RHΔrop16 parasites, and the control group remained uninfected. The cells were cultured in an incubator at 37°C for 24 h to obtain macrophages. The macrophages were subjected to detection of the mRNA and protein expression of iNOS and Arg-1 and examination of phosphorylation of p38α mitogen-activated protein kinase (p38α MAPK).
Western Blotting
Placental tissues of all mice and macrophages infected with the parasite were lysed using protein lysate (RIPA: PMSF = 100: 1), followed by centrifugation at 12,000 × g for 15 min at 4°C. The denatured proteins were separated by 10% separation gel in SDS-PAGE and transferred to NC membrane followed by blocking in 5% of milk on a shaker for 90 min. The NC membrane was incubated with primary antibodies against β-actin, Arg-1, and iNOS for placental tissues and additional incubation of p-p38α MAPK (Abcam, United Kingdom) for the macrophages infected with the parasite (both had 1:5000 dilution) at 4°C overnight. The anti-β-actin was used as the control. The NC membrane was washed with TBST three times, followed by the horseradish peroxidase-labeled secondary antibody incubation with slight shaking for 90 min. The specific bands were visualized by enhanced chemiluminescence. All experimental data were analyzed by ImageJ 1.46 software.
Measurement of Macrophage Polarization by Flow Cytometry
Macrophages were obtained from the uterus and placental tissues of all mice and 2 × 106 cells with 5 μl of mouse sera were added to each tube and let stand at 4°C for 30 min. The cells were labeled with anti-F4/80-BV421, anti-CD80-PE/cy5, anti-CD86-PE, and anti-CD206-AF647 (New York, BD, United States) at 4°C for 30 min in the dark. The cell surface markers were fixed after washing twice by PBS. All labeled samples were quickly detected by flow cytometry (FCM).
Isolation and Culture of Splenocytes
The spleen tissues were taken out from the pregnant mice of three groups, and half of the tissues were mashed on a sterile nylon mesh to obtain spleen cells. Simultaneously, 2–3 ml of 1 × ACK Lysis buffer was added to the spleen cells in a tube for disrupting erythrocytes. Finally, the lysis was stopped with PBS, and the erythrocyte debris was removed by centrifugation at 2,500 rpm for 5 min, and the spleen cells were isolated and cultured at 37°C for 5 h under stimulation with ionomycin (1 mg/ml) and PMA (20 ng/ml) (St. Louis, MO, United States), and then centrifuged (4,000 rpm × 10 min at 4°C) to obtain supernatants for ELISA detection. The remaining spleen tissues were stored at −80°C for RNA extraction.
Real-Time PCR for Detection of Cytokines
Total RNAs were extracted from spleens, placentae, and macrophages infected with the parasite and then reversed into cDNAs according to the Takara Kit instructions (Takara, Japan). Quantitative detection of IL-12, IL-10, TNF-α, TGF-β1, iNOS, and Arg-1 of placentae was performed with SYBR-Green Premix Ex Taq kit (Takara, Japan). Spleen tissues were only detected for IL-12, IL-10, TNF-α, and TGF-β1. The parasite-infected macrophages were detected for iNOS and Arg-1. The primers used in the qRT-PCR are listed in Table 1. The measurements were completed with Roche Applied Science Light Cycler TM 480 instrument. The results were normalized by β-actin and the results were calculated using the 2–ΔΔCt method.
ELISA for Cytokines
The placental tissues were weighed and ultrasonicated in the ratio of 1 mg/10 μl PBS (at 3-s intervals) and centrifuged at 12,000 × g at 4°C for 10 min. The supernatants were extracted and cytokines of TNF-α (Cusabio, Houston, TX, United States) and IL-12 (Elabscience, Wuhan, China) in the supernatants of placental homogenates, spleen cell culture, and sera were detected by ELISA. The examinations of all specimens were performed three times and the OD values were measured at a wavelength of 450 nm. The concentration of TNF-α and IL-12 was calculated by plotting a standard curve according to the manufacturer’s instruction.
Statistical Analysis
The data were presented as the mean ± SD and were statistically analyzed by one-way ANOVA after precheck for normal distribution and homogeneity of variances, and p < 0.05 or p < 0.01 indicated statistical significance. Analyses of experimental data and graphic production were obtained by GraphPad Prism Software.
Results
RHΔrop16 Strain Infection Caused Severe Abnormal Pregnancy
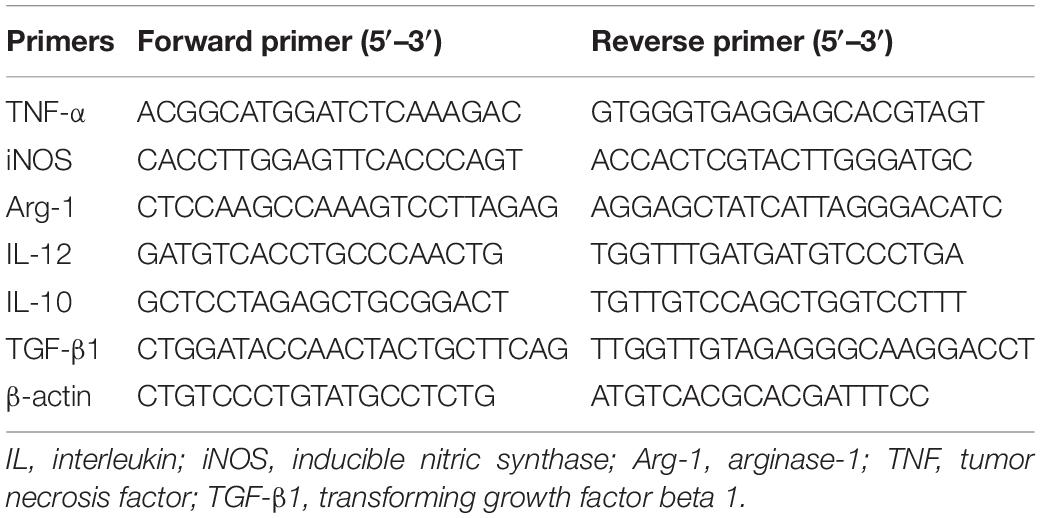
Pregnancy outcomes were examined at GD14 (i.e., 6 days post-infection). The pregnant mice infected with RHΔrop16 strain showed a clinical manifestation of wilting and arching, accompanied with aggravated fetal resorption and placental hemorrhage when compared with the RH WT group (Figures 1A,B). The H&E staining of placenta tissues exhibited more severe hyperemia in the RHΔrop16-infected group than that in the RH WT group (Figure 1C). The weights of placentae and fetuses in mice of the RHΔrop16-infected group were notably reduced compared with the RH WT group. Accordingly, the resorptivity of the fetuses in the RHΔrop16-infected group significantly increased (Figure 1D).
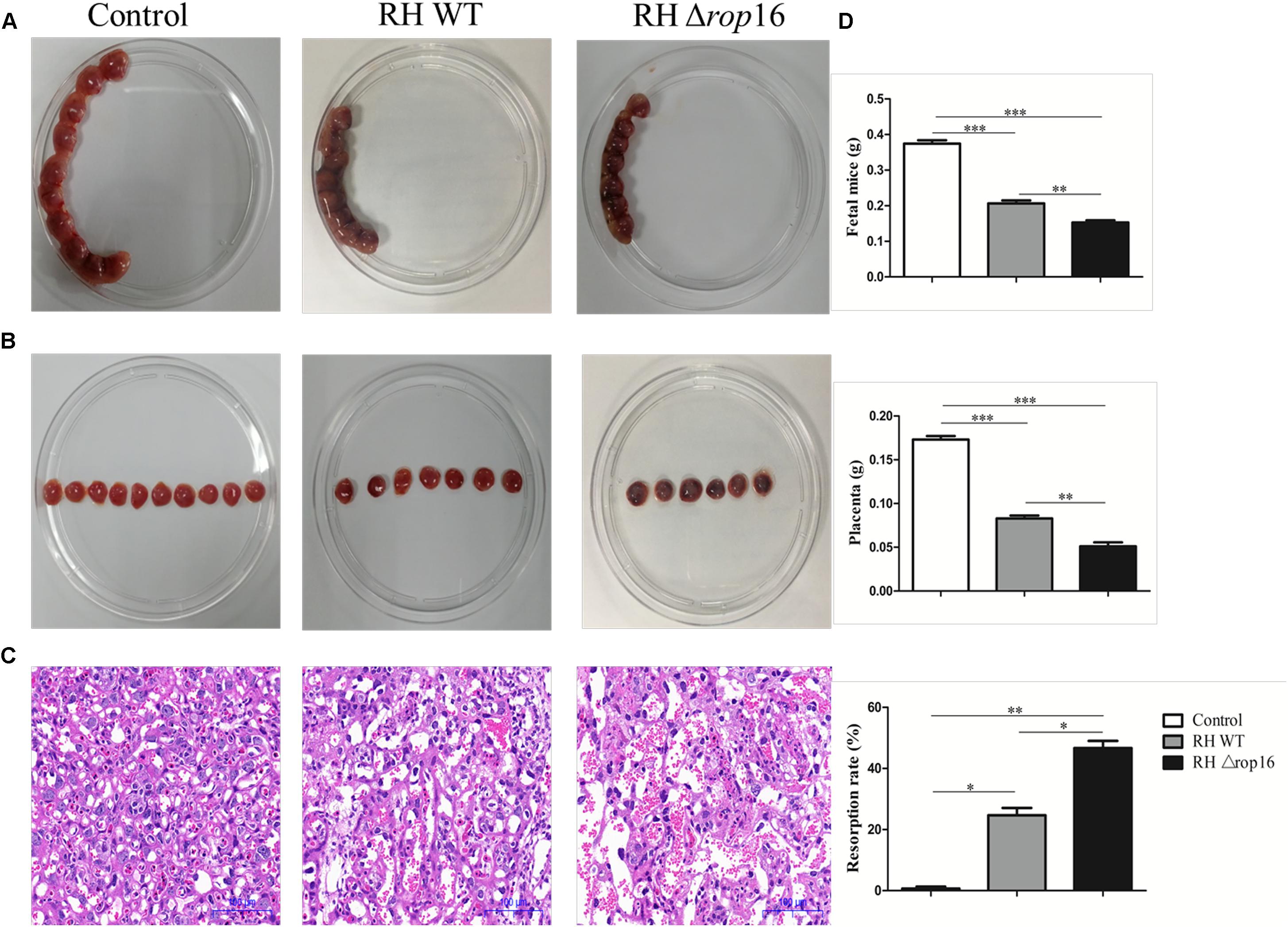
Figure 1. The occurrence of adverse pregnancy outcomes caused by Toxoplasma gondii infection. Fetuses and placentae of the control mice developed normally. The less number of fetuses and the more serious hemorrhage of placentae were seen in the mice of the RHΔrop16-infected group when compared with the RH WT group (A,B). H&E staining showed deteriorated hyperemia of placentae caused by RHΔrop16 strain infection (C). The weights of fetuses and placentae and fetus resorption of each group were measured after 6 days of infection. The ratio of resorption to total fetuses was calculated as the percentage of fetal loss (D). Each group contained seven mice. The results were presented as mean ± SD and were analyzed by one-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Expression of iNOS, Arg-1, and p-p38α MAPK Varied in Placental Macrophages Infected With RHΔrop16 Strain in vitro
To examine the direct impact of T. gondii ROP16I on the bias of macrophages, we isolated primary macrophages from placental and uterine tissues of control pregnant mice. The cells were infected with RH WT and RHΔrop16 parasites, and cultured for 24 hr. The data showed that the expression of iNOS was significantly elevated while that of Arg-1 was diminished in the macrophages with RHΔrop16 infection when tested by qRT-PCR (Figure 2A) and Western blotting (Figure 2B). No significant difference of p-p38α MAPK expression, however, was noted between RH WT and RHΔrop16-infected macrophages by Western blotting analysis (Figure 2B and Supplementary Figure S1).
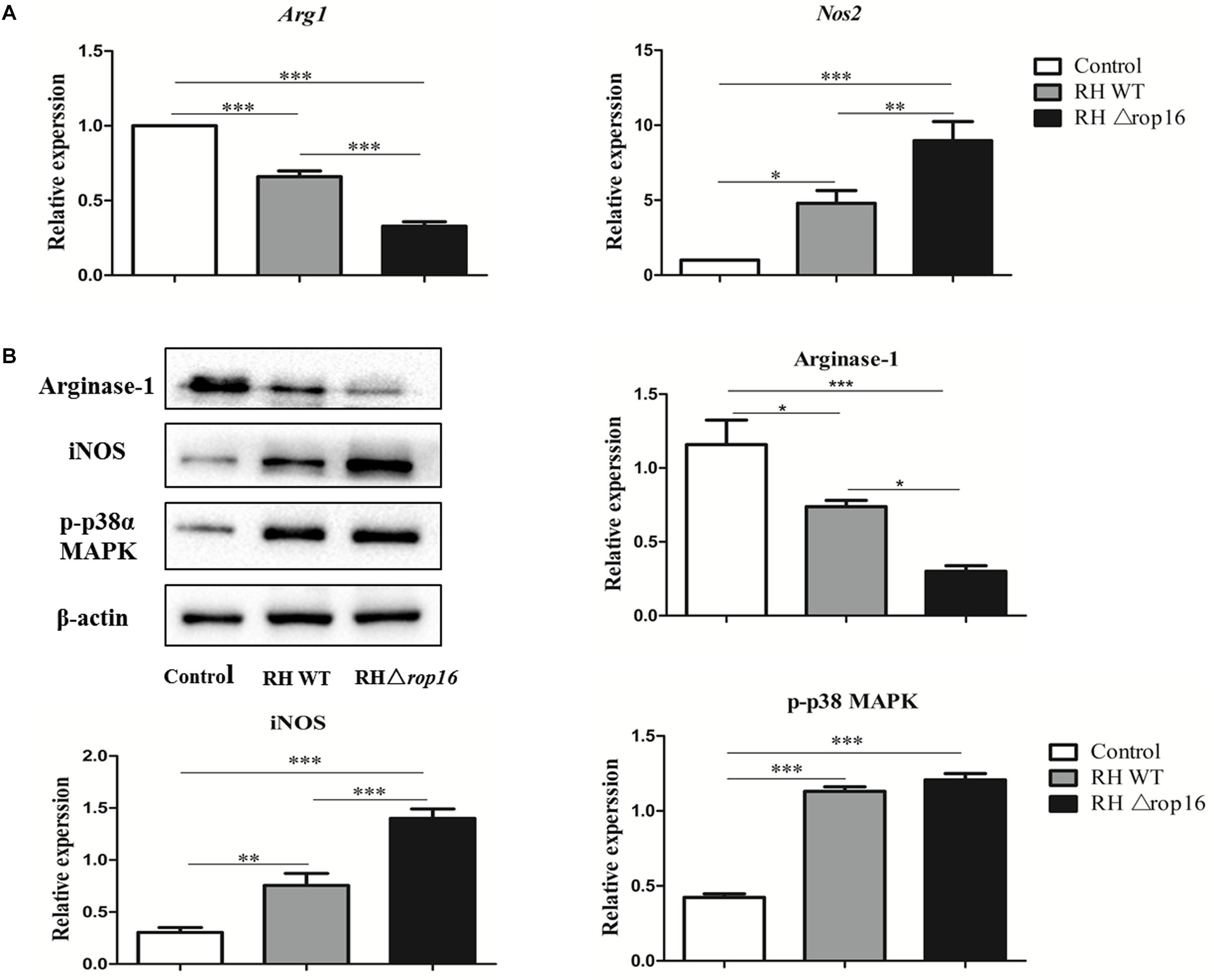
Figure 2. In vitro expression of Arg-1, iNOS, and p-p38α MAPK of placental macrophages from control pregnant mice following T. gondii infection. Placental macrophages of control pregnant mice were collected and infected with RH WT and RHΔrop16 in vitro for 24 h. The mRNA and protein expressions of Arg-1 and iNOS of macrophages were examined by qRT-PCR (A) and Western blotting (B). Phosphorylation of p38α MAPK was detected by Western blotting (B). Each group contained seven mice. The results were given as mean ± SD and were analyzed by one-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
RHΔrop16 Strain Infection Modulated Synthesis of iNOS and Arg-1 in Placental Tissues in vivo
Expression of iNOS and Arg-1 in placental tissues in mice of three groups was assayed by qRT-PCR (Figure 3A) and Western blotting (Figure 3B). As shown in Figure 3, the upregulated expression of iNOS mRNAs and downregulated expression of Arg-1 mRNAs were observed in the RHΔrop16-infected group, when compared to the RH WT group. Correspondingly, the protein level of iNOS was remarkably elevated but Arg-1 was reduced when detected by Western blotting in RHΔrop16-inoculated mice.
Figure 3. In vivo expression of Arg-1 and iNOS in placental tissues. Placental tissues were removed from three experimental groups. The mRNA and protein expressions of Arg-1 and iNOS of placental tissues were examined. The relative mRNA expressions of Arg-1 and iNOS were detected in placental tissues of mice infected with RHΔrop16 strain (A). The corresponding results of protein expressions were also noted with Western blotting (B). The results were presented as mean ± SD obtained from seven mice in each group and were analyzed by one-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
The Phenotypes of Mouse Placental Macrophages Altered Following RH Δrop16 Strain Infection
Density gradient centrifugation was used to isolate placental macrophages for detection of polarization of the macrophages in placental tissues of mice infected with RHΔrop16 strain. The surface markers of CD86 and CD80 on M1 macrophages and CD206 on M2 cells were detected by FCM. The results showed that RHΔrop16 infection induced higher expression of CD86 (Figure 4A) and CD80 (Figure 4B) but lower expression of CD206 (Figure 4C) on the surface of placental macrophages, when compared to the RH WT infection group.
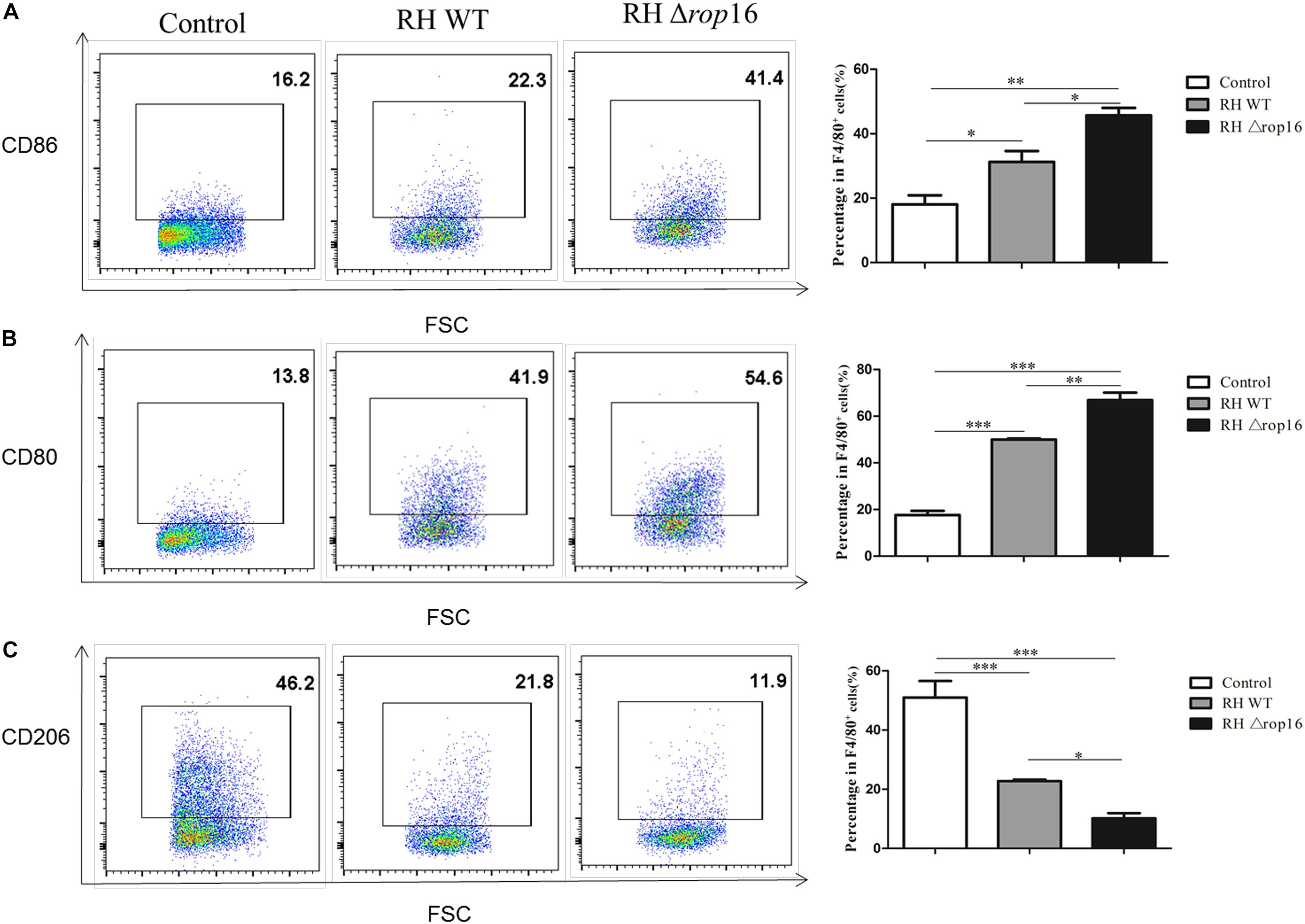
Figure 4. Expression of CD86, CD80, and CD206 markers at the maternal–fetal interface. Placental macrophages were removed from three experimental groups. A high expression of CD206 but low expression of CD80 and CD86 of macrophages were noted in control pregnant mice by flow cytometry, while RHΔrop16 parasite infection stimulated high-level expressions of CD86 (A) and CD80 (B), and low level of CD206 (C) in the placental macrophages of pregnant mice. Experimental and control group each contain seven mice. The results were given as mean ± SD and were analyzed by one-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
RHΔrop16 Infection Evoked Th1-Biased Response in Spleens, Placentae, and Sera
Tissues of placentae and spleens were collected from three groups, and the mRNA expression of IL-12, TNF-α, IL-10, and TGF-β1 was measured by qRT-PCR. The results showed that the mRNA expression of IL-12 and TNF-α in the spleens (Figure 5A) and placentae (Figure 5D) were highly transcribed, but expression of TGF-β1 mRNA was dramatically dampened (Figures 5B,E) in the animals of RHΔrop16-inoculated mice, when compared to the RH WT infection group. Unexpectedly, the level of IL-10 expression in the spleens (Figure 5B) and placentae (Figure 5E) was significantly elevated in either of the two infected groups compared to the control. However, the ratio of IL-10/IL-12 as well as IL-10/TNF-α in the spleens (Figure 5C) and placentae (Figure 5F) of the RHΔrop16-infected mice remained low in comparison to the control mice. Additionally, the ELISA assay revealed the significantly increased levels of pro-inflammatory cytokines of IL-12 and TNF-α in the supernatants of splenocytes and placental homogenates, and sera as well, in RHΔrop16-infected mice (Figures 6A–C).
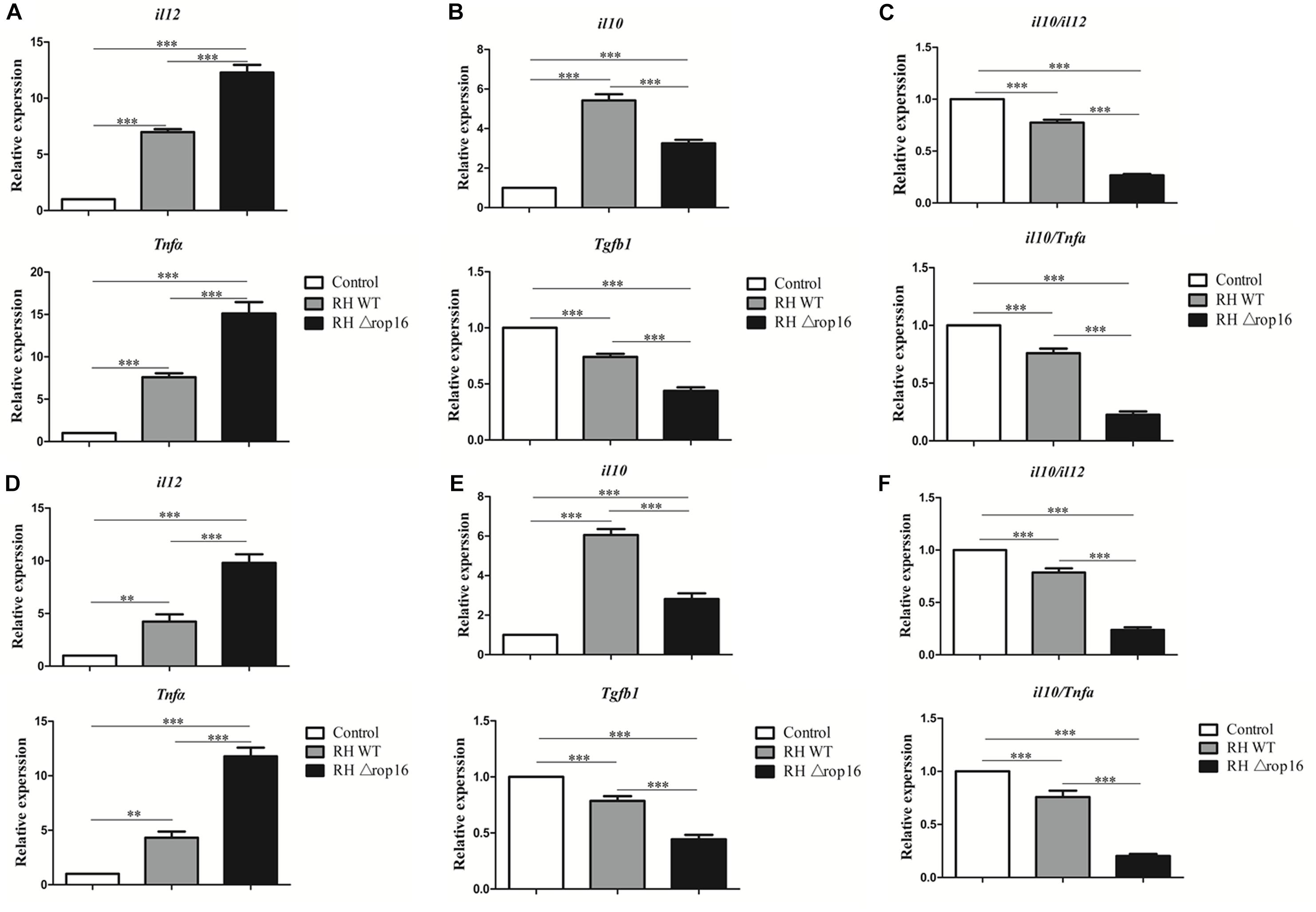
Figure 5. Expression of IL-12, TNF-α, IL-10, and TGF-β1 in spleens and placentae of mice with T. gondii infection. Spleen tissues and placentae were taken from three groups of experimental mice. The mRNA transcriptions of pro-inflammatory cytokines of IL-12 and TNF-α and anti-inflammatory cytokine of IL-10 and TGF-β1 were examined in spleen tissues (A,B) and placentae (D,E). The ratio of mRNA transcriptions of IL-10 to IL-12 and IL-10 to TNF-α was calculated in spleen tissues (C) and placentae (F). Each sample was performed in triplicate. The results were presented as mean ± SD from seven mice of each group (∗∗p < 0.01, ∗∗∗p < 0.001).
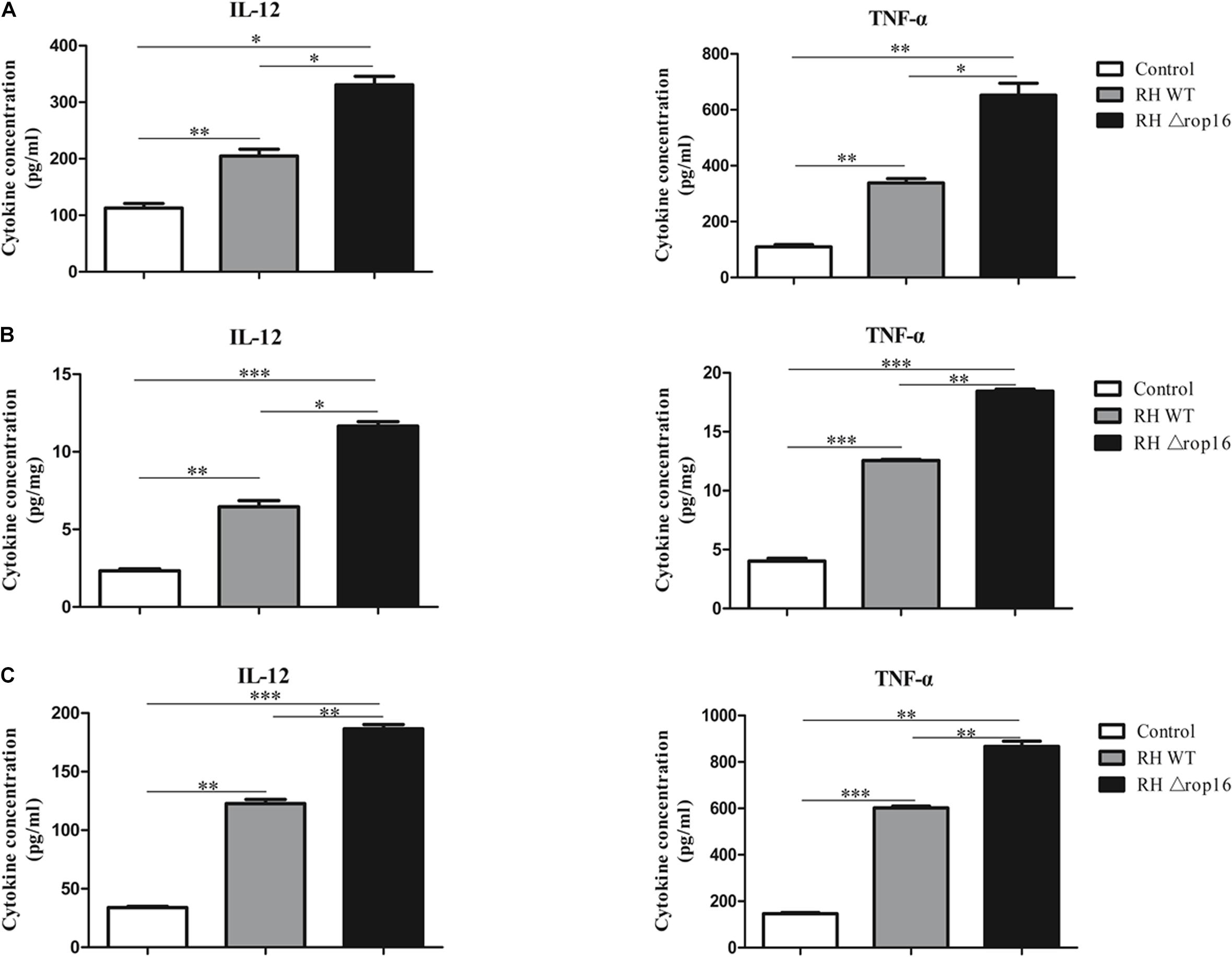
Figure 6. IL-12 and TNF-α expression in splenocytes, placental tissues, and sera. The supernatants of splenocytes and placental homogenates were taken from three experimental groups. The sera were obtained by centrifugation (4,000 rpm, 10 min, 4°C) from the three groups. Expressions of IL-12 and TNF-α in the supernatants of splenocytes (pg/ml) (A), placental homogenates (pg/mg) (B), and sera (pg/ml) of pregnant mice (C) were evaluated with ELISA. Each sample was performed in triplicate. Each group contained seven animals. The results were presented as mean ± SD (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Discussion
It has been known that numerous immune cells contribute to maintaining the equilibrium state of the maternal–fetal immune interface. Macrophages are the first line of defense in innate immunity. During normal pregnancy, macrophages also perform functions such as angiogenesis, tissue repair, and immune tolerance (Mor and Abrahams, 2003). Alternatively activated macrophages (M2 macrophages), uterine/decidual NK cells, as well as Tregs are crucial cell populations that contribute to the immune tolerance at the maternal–fetal interface and keep the fundamental physiological processes of pregnancy by secreting large amounts of cytokines and chemokines (Lidstrom et al., 2003; Kalkunte et al., 2008; Co et al., 2013; Gaynor and Colucci, 2017). A growing body of evidence indicated that pregnancy failure induced by T. gondii infection is associated with the altered maternal immunity (Zhang et al., 2012; Zhao et al., 2017). It has been reported that the LILRB4 expression of decidual macrophages decreased after infection with T. gondii, which induced polarization of classically activated macrophages (M1 macrophages) and inhibited the immune bias of M2 direction, leading to adverse pregnancy outcomes (Li et al., 2017). In addition, trophoblast cells are the only cells that are in direct contact with the maternal immune system and play an important part in embryo implantation and maternal–fetal immune tolerance. Apoptosis of trophoblast cells induced by inflammation may directly impact the microenvironment of maternal–fetal interface, resulting in abnormal pregnant outcomes such as miscarriage (Reister et al., 2001). Additional investigations showed that T. gondii infection induced the activation of macrophages and their secretion of a variety of pro-inflammatory factors through oxidative stress-mediated apoptosis of placental trophoblasts, more likely leading to adverse pregnancy events (Aschkenazi et al., 2002; Neale et al., 2003; Liu et al., 2013; Xu et al., 2015). Overall, any peripheral or local infection in the maternal side during the first trimester is prone to cause serious adverse pregnancy outcomes.
Toxoplasma gondii effectors of ROP16I and GRA15II are responsible for inducing macrophage polarizations and play a pivotal role in host innate immunity and directly affect the consequences of infection or disease. During invasion into host cells, T. gondii injects the rhoptry protein ROP16 into the host cytoplasm, which subsequently localizes to the parasitophorous vacuole membrane (PVM). We previously found that deletion of ROP16I of either RH strain of type I or WH3 strain of type Chinese 1 did not affect the virulence of the parasite to mice (Wang et al., 2018), although ROP16 is believed as one of polymorphic and strain-dependent virulence factors (Saeij et al., 2006). Studies have shown that ROP16I, rather than ROP16II, serves as a central regulator of parasite replication and transmission at early phase of infection and drives the macrophages to M2 polarization. Contrarily, GRA15II, one of the key molecules secreted by dense granules of type II strains, evokes strong M1 macrophage polarization by directly activating NF-κB and facilitating a high expression of IL-12 and iNOS that are involved in the subsequent Th1 predominant immune response and the pro-inflammatory reaction (Jensen et al., 2011; Rosowski et al., 2011). We previously demonstrated that T. gondii strains of type Chinese 1, distinct from the strains of archetypal I, II, and III strains prevalent in the other continents of the world, carry both ROP16I and GRA15II alleles. Deletion of ROP16I of the Chinese 1 WH3Δrop16, with GRA15II genetic background, resulted in remarkably adverse pregnancy outcomes in mice (Wang et al., 2018). However, the pathogenic feature of the RHΔrop16 strain of type I, with neither ROP16I nor GRA15II background, in T. gondii-induced aberrant pregnancy remains unknown. Thus, we examined the pathology of adverse pregnant outcomes caused by ROP16I-deficient T. gondii (RHΔrop16) and the mechanisms by which the parasite infection subverts the immune tolerance at the maternal–fetal interface in the murine model. We noted that all of the mice infected with T. gondii had clinical manifestations of wilting and arching; however, the RHΔrop16 strain caused the exacerbated abnormal pregnancy outcomes when compared with the RH WT strain, as evidenced by the lower number and reduced weights of placentae and fetuses, hemorrhage of placentae, and a high rate of fetal resorption. A previous study showed that deficiency of ROP16I of the RH strain did not affect proliferation of the tachyzoites (Butcher et al., 2011), but no parasites were seen in the damaged placenta tissues of RHΔrop16-infected mice (data not shown), suggesting that the pathology of placentae might be attributable to the biased M1–Th1 immune response following infection. Additionally, our data indicated that IL-12 and TNF-α were significantly increased whereas TGF-β1 was decreased in the RHΔrop16-infected animals; the ratios of IL-10/IL-12 and IL-10/TNF-α of the RHΔrop16-infected mice declined significantly compared to those of the RH WT group, indicative of the Th1-polarized response in RHΔrop16 infection. Moreover, placental macrophages tended to be driven to M1 skewing after 6 days of T. gondii infection. The results suggest that the pro-inflammatory factors in the placentae induced by RHΔrop16 infection might be involved in the aberrant pregnancy of mice, which is attributable to the deletion of ROP16I of the RH strain.
Theoretically, any negative impact of infections on immune tolerance at the maternal–fetal microenvironment may lead to abnormal pregnancy. It has been demonstrated that T. gondii type II strains are frequently found in human infection of Europeans (Wujcicka et al., 2014) and are responsible for pregnant failure (Liu et al., 2018), which might be related to GRA15II-induced M1–Th1 dominant response. Genetic structures of T. gondii in South America are complex, and these non-archetypal strains may cause serious consequences of pregnancies (Rajendran et al., 2012). It is speculated that multiple parasite-derived molecules, in addition to the direct invasion of parasites, may contribute to the adverse pregnant consequences. We here noted that mice infected with the RH strain with rop16 deletion caused deteriorated pregnant outcomes, suggesting a protective influence of ROP16I on normal pregnancy. Similar reports were given by Jensen et al. (2011) that ROP16I might have an ameliorative effect on T. gondii-induced ileitis. Additionally, our studies strongly suggested that other T. gondii effectors, besides GRA15II, might be involved in the pathology process of pregnancy. This speculation is supported by the previous investigations that demonstrated that GRA24, a member of GRAs family with 542 amino acids similar to GRA15II of T. gondii in function, can bypass the classical MKK3/MKK6 and MKK4 pathways to directly bind to the p38α MAPK, leading to activation and nuclear translocation of the host kinase and increasing IL-12 secretion and M1 macrophages polarization (Kim et al., 2005; Braun et al., 2013). Further approaches are needed to identify the effectors of T. gondii that induce IL-12 production and M1 polarization in the occurrence of abnormal pregnancy.
Taken together, the RH strain of type I T. gondii with ROP16I deletion may cause severe abnormal pregnant outcomes in mice, with the feature of M1 macrophage phenotype and Th1 dominant response in system and placental tissues, suggesting that ROP16I might be a protective factor and other parasite-derived molecules may be involved in the pathology process of pregnancy failure.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
Ethical permission was obtained from the Institutional Review Board of AMU Institute of Biomedicine AMU (Permit No. AMU26–081108).
Author Contributions
JS, WW, and WC elaborated and designed the study, and drafted the manuscript. WC, CW, and TX performed the experiments. QL analyzed the data. All authors have read and approved the final version of the manuscript.
Funding
This work was funded by the Natural Science Foundation of Anhui Educational Committee (No. KJ2015A330), the Science and Education Foundation of the Second Hospital of Hefei (No. 2019-45-44), and the National Natural Science Foundation of China (No. 81471983).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03151/full#supplementary-material
FIGURE S1 | The expression of β-actin (40KD), Arg-1(36KD), and p-p38 MAPK (40KD) of placental macrophages infected with the parasite were examined by Western blotting.
References
Aschkenazi, S., Straszewski, S., Verwer, K. M., Foellmer, H., Rutherford, T., and Mor, G. (2002). Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol. Reprod. 66, 1853–1861. doi: 10.1095/biolreprod66.6.1853
Black, M. W., and Boothroyd, J. C. (2000). Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64, 607–623. doi: 10.1128/mmbr.64.3.607-623.2000
Braun, L., Brenier-Pinchart, M. P., Yogavel, M., Curt-Varesano, A., Curt-Bertini, R. L., Hussain, T., et al. (2013). A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med. 210, 2071–2086. doi: 10.1084/jem.20130103
Brown, M. B., von Chamier, M., Allam, A. B., and Reyes, L. (2014). M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 5:606. doi: 10.3389/fimmu.2014.00606
Butcher, B. A., Fox, B. A., Rommereim, L. M., Kim, S. G., Maurer, K. J., Yarovinsky, F., et al. (2011). Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 7:e1002236. doi: 10.1371/journal.ppat.1002236
Co, E. C., Gormley, M., Kapidzic, M., Rosen, D. B., Scott, M. A., Stolp, H. A., et al. (2013). Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 88:155. doi: 10.1095/biolreprod.112.099465
Courret, N., Darche, S., Sonigo, P., Milon, G., Buzoni-Gatel, D., and Tardieux, I. (2006). CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316. doi: 10.1182/blood-2005-02-0666
Dubey, J. P. (2009). Toxoplasmosis in sheep–the last 20 years. Vet. Parasitol. 163, 1–14. doi: 10.1016/j.vetpar.2009.02.026
Fuentes, I., Rubio, J. M., Ramirez, C., and Alvar, J. (2001). Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J. Clin. Microbiol. 39, 1566–1570. doi: 10.1128/JCM.39.4.1566-1570.2001
Gaynor, L. M., and Colucci, F. (2017). Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front. Immunol. 8:467. doi: 10.3389/fimmu.2017.00467
Hakimi, M. A., Olias, P., and Sibley, L. D. (2017). Toxoplasma effectors targeting host signaling and transcription. Clin. Microbiol. Rev. 30, 615–645. doi: 10.1128/CMR.00005-17
Hide, G., Morley, E. K., Hughes, J. M., Gerwash, O., Elmahaishi, M. S., Elmahaishi, K. H., et al. (2009). Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology 136, 1877–1885. doi: 10.1017/S0031182009990941
Hunter, C. A., and Sibley, L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778. doi: 10.1038/nrmicro2858
Jensen, K. D., Wang, Y., Wojno, E. D., Shastri, A. J., Hu, K., Cornel, L., et al. (2011). Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9, 472–483. doi: 10.1016/j.chom.2011.04.015
Kalkunte, S., Chichester, C. O., Gotsch, F., Sentman, C. L., Romero, R., and Sharma, S. (2008). Evolution of non-cytotoxic uterine natural killer cells. Am. J. Reprod. Immunol. 59, 425–432. doi: 10.1111/j.1600-0897.2008.00595.x
Kim, L., Del Rio, L., Butcher, B. A., Mogensen, T. H., Paludan, S. R., Flavell, R. A., et al. (2005). p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 174, 4178–4184. doi: 10.4049/jimmunol.174.7.4178
Lao, K., Zhao, M., Li, Z., Liu, X., Zhang, H., Jiang, Y., et al. (2015). IL-10 regulate decidual Tregs apoptosis contributing to the abnormal pregnancy with Toxoplasma gondii infection. Microb. Pathog. 89, 210–216. doi: 10.1016/j.micpath.2015.10.002
Lehmann, T., Marcet, P. L., Graham, D. H., Dahl, E. R., and Dubey, J. P. (2006). Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 103, 11423–11428. doi: 10.1073/pnas.0601438103
Leng, J., Butcher, B. A., and Denkers, E. Y. (2009). Dysregulation of macrophage signal transduction by Toxoplasma gondii: past progress and recent advances. Parasite Immunol. 31, 717–728. doi: 10.1111/j.1365-3024.2009.01122.x
Li, Z., Zhao, M., Li, T., Zheng, J., Liu, X., Jiang, Y., et al. (2017). Decidual macrophage functional polarization during abnormal pregnancy due to Toxoplasma gondii: role for LILRB4. Front. Immunol. 8:1013. doi: 10.3389/fimmu.2017.01013
Lidstrom, C., Matthiesen, L., Berg, G., Sharma, S., Ernerudh, J., and Ekerfelt, C. (2003). Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am. J. Reprod. Immunol. 50, 444–452. doi: 10.1046/j.8755-8920.2003.00112.x
Liu, T., Zhang, Q., Liu, L., Xu, X., Chen, H., Wang, H., et al. (2013). Trophoblast apoptosis through polarization of macrophages induced by Chinese Toxoplasma gondii isolates with different virulence in pregnant mice. Parasitol Res. 112, 3019–3027. doi: 10.1007/s00436-013-3475-3
Liu, X., Jiang, M., Ren, L., Zhang, A., Zhao, M., Zhang, H., et al. (2018). Decidual macrophage M1 polarization contributes to adverse pregnancy induced by Toxoplasma gondii PRU strain infection. Microb. Pathog. 124, 183–190. doi: 10.1016/j.micpath.2018.08.043
Liu, Y., Zhao, M., Xu, X., Liu, X., Zhang, H., Jiang, Y., et al. (2014). Adoptive transfer of Treg cells counters adverse effects of Toxoplasma gondii infection on pregnancy. J. Infect. Dis. 210, 1435–1443. doi: 10.1093/infdis/jiu265
Luma, H. N., Tchaleu, B. C., Temfack, E., Doualla, M. S., Ndenga, D. P., Mapoure, Y. N., et al. (2013). HIV-associated central nervous system disease in patients admitted at the Douala General Hospital between 2004 and 2009: a retrospective study. AIDS Res. Treat. 2013:709810. doi: 10.1155/2013/709810
Melo, M. B., Jensen, K. D., and Saeij, J. P. (2011). Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 27, 487–495. doi: 10.1016/j.pt.2011.08.001
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Montoya, J. G., and Remington, J. S. (2008). Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47, 554–566. doi: 10.1086/590149
Mor, G., and Abrahams, V. M. (2003). Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol. 1:119. doi: 10.1186/1477-7827-1-119
Nagamatsu, T., and Schust, D. J. (2010). The contribution of macrophages to normal and pathological pregnancies. Am. J. Reprod. Immunol. 63, 460–471. doi: 10.1111/j.1600-0897.2010.00813.x
Neale, D., Demasio, K., Illuzi, J., Chaiworapongsa, T., Romero, R., and Mor, G. (2003). Maternal serum of women with pre-eclampsia reduces trophoblast cell viability: evidence for an increased sensitivity to Fas-mediated apoptosis. J. Matern. Fetal Neonatal Med. 13, 39–44. doi: 10.1080/jmf.13.1.39.44
Nissapatorn, V. (2009). Toxoplasmosis in HIV/AIDS: a living legacy. Southeast Asian J. Trop. Med. Public Health 40, 1158–1178.
Pfaff, A. W., Abou-Bacar, A., Letscher-Bru, V., Villard, O., Senegas, A., Mousli, M., et al. (2007). Cellular and molecular physiopathology of congenital toxoplasmosis: the dual role of IFN-gamma. Parasitology 134, 1895–1902. doi: 10.1017/S0031182007000200
Rajendran, C., Su, C., and Dubey, J. P. (2012). Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol. 12, 359–368. doi: 10.1016/j.meegid.2011.12.010
Reister, F., Frank, H. G., Kingdom, J. C., Heyl, W., Kaufmann, P., Rath, W., et al. (2001). Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab. Invest. 81, 1143–1152. doi: 10.1038/labinvest.3780326
Renaud, S. J., and Graham, C. H. (2008). The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Invest. 37, 535–564. doi: 10.1080/08820130802191375
Robbins, J. R., Zeldovich, V. B., Poukchanski, A., Boothroyd, J. C., and Bakardjiev, A. I. (2012). Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 80, 418–428. doi: 10.1128/IAI.05899-11
Rochet, E., Argy, N., Greigert, V., Brunet, J., Sabou, M., Marcellin, L., et al. (2019). Type I ROP16 regulates retinal inflammatory responses during ocular toxoplasmosis. PLoS One 14:e0214310. doi: 10.1371/journal.pone.0214310
Rosowski, E. E., Lu, D., Julien, L., Rodda, L., Gaiser, R. A., Jensen, K. D., et al. (2011). Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208, 195–212. doi: 10.1084/jem.20100717
Saeij, J. P., Boyle, J. P., Coller, S., Taylor, S., Sibley, L. D., Brooke-Powell, E. T., et al. (2006). Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314, 1780–1783. doi: 10.1126/science.1133690
Schmidt, M., Sonneville, R., Schnell, D., Bige, N., Hamidfar, R., Mongardon, N., et al. (2013). Clinical features and outcomes in patients with disseminated toxoplasmosis admitted to intensive care: a multicenter study. Clin. Infect. Dis. 57, 1535–1541. doi: 10.1093/cid/cit557
Shwab, E. K., Zhu, X. Q., Majumdar, D., Pena, H. F., Gennari, S. M., Dubey, J. P., et al. (2014). Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 141, 453–461. doi: 10.1017/S0031182013001844
Wang, C., Cheng, W., Yu, Q., Xing, T., Chen, S., Liu, L., et al. (2018). Toxoplasma Chinese 1 strain of WH3Δrop16I/III/gra15II genetic background contributes to abnormal pregnant outcomes in murine model. Front. Immunol. 9:1222. doi: 10.3389/fimmu.2018.01222
Wang, L., Chen, H., Liu, D., Huo, X., Gao, J., Song, X., et al. (2013). Genotypes andmouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS One 8:e53483. doi: 10.1371/journal.pone.0053483
Wujcicka, W., Wilczyński, J., and Nowakowska, D. (2014). Do the placental barrier, parasite genotype and Toll-like receptor polymorphisms contribute to the course of primary infection with various Toxoplasma gondii genotypes in pregnant women? Eur. J. Clin. Microbiol. Infect. Dis. 33, 703–709. doi: 10.1007/s10096-013-2017-3
Xu, X., He, L., Zhang, A., Li, Q., Hu, W., Chen, H., et al. (2015). Toxoplasma gondii isolate with genotype Chinese 1 triggers trophoblast apoptosis through oxidative stress and mitochondrial dysfunction in mice. Exp. Parasitol. 154, 51–61. doi: 10.1016/j.exppara.2015.04.008
Yamamoto, M., Standley, D. M., Takashima, S., Saiga, H., Okuyama, M., Kayama, H., et al. (2009). A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 206, 2747–2760. doi: 10.1084/jem.20091703
Yarovinsky, F. (2014). Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 14, 109–121. doi: 10.1038/nri3598
Zhang, H., Hu, X., Liu, X., Zhang, R., Fu, Q., and Xu, X. (2012). The Treg/Th17 imbalance in Toxoplasma gondii-infected pregnant mice. Am. J. Reprod. Immunol. 67, 112–121. doi: 10.1111/j.1600-0897.2011.01065.x
Keywords: Toxoplasma gondii, ROP16, macrophage, immune tolerance, adverse pregnant outcome
Citation: Cui W, Wang C, Luo Q, Xing T, Shen J and Wang W (2020) Toxoplasma gondii ROP16I Deletion: The Exacerbated Impact on Adverse Pregnant Outcomes in Mice. Front. Microbiol. 10:3151. doi: 10.3389/fmicb.2019.03151
Received: 30 August 2019; Accepted: 29 December 2019;
Published: 31 January 2020.
Edited by:
Martin Mueller, University Hospital of Bern, SwitzerlandReviewed by:
Jichun Zhou, Zhejiang University, ChinaAngelica Oliveira Gomes, Universidade Federal do Triângulo Mineiro, Brazil
Copyright © 2020 Cui, Wang, Luo, Xing, Shen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jilong Shen, c2hlbmppbG9uZzUzQDEyNi5jb20=; Wei Wang, YXlkd3dlaUAxNjMuY29t
†These authors have contributed equally to this work
 Wen Cui
Wen Cui Cong Wang
Cong Wang Qingli Luo
Qingli Luo Tian Xing
Tian Xing Jilong Shen
Jilong Shen Wei Wang
Wei Wang