- 1Clinic for Urology, Pediatric Urology and Andrology, Justus-Liebig University Giessen, Giessen, Germany
- 2Department of Urology, Technical University of Munich, Munich, Germany
- 3Institute for Infection and Immunity, St George’s, University of London, London, United Kingdom
- 4Helperby Therapeutics Ltd., London, United Kingdom
Antimicrobial susceptibility testing (AST) performed according to defined guidelines is important to identify resistance and to predict the clinical success or failure of specific antibiotic therapy. However, these guidelines do not cover all physiological conditions that can have a tremendous impact on in vivo resistance. In this study, we tested the susceptibility of thirteen mcr-1-positive Escherichia coli strains against colistin, one of the last resort antibiotics for treating multi-drug resistant pathogens, in media recommended for ASTs as well as – physiologically more relevant – in human serum and artificial urine (AU). Minimal inhibitory concentration (MIC) values in heat-inactivated human serum were similar to those in cation-adjusted Mueller-Hinton broth (CAMHB), but reduced in native serum for almost all strains that could grow in this media. In AU MIC values for mcr-1 positive E. coli were increased significantly up to 16-fold compared to that in CAMBH, which did not apply to the colistin-susceptible E. coli strains tested. Although different growth media could affect the MIC of colistin alone, their impact on the synergistic effect of the combination with the antiviral drug azidothymidine was minimal. The higher divalent cation concentration combined with acidic pH values is most likely responsible for the increased MIC values of the mcr-1 harboring E. coli strains tested against colistin in AU compared to that in CAMHB. Antimicrobial susceptibility screening procedures for colistin using CAMHB only could lead to an underestimation of resistance under different physiological conditions. Therefore, not only pharmacokinetic but also pharmacodynamic studies in urine are as important as in serum or plasma.
Introduction
Gram-negative bacteria, especially Enterobacteriaceae are the major cause of community and hospital acquired urinary tract infections (UTI). Due to a worldwide increasing number of multidrug-resistant (MDR) Gram-negative bacteria combined with a lack of development of new antibiotics, physicians are turning to older antibacterials to treat antibiotic-resistant infections. Thus, polymyxins like colistin, which was discarded because of toxicity concerns, are now seen as last line defense (World Health Organization [WHO], 2011).
Unfortunately, appreciable emergence of resistance to colistin has been shown recently (Paterson and Harris, 2016). Modifications of the lipid A moiety of lipopolysaccharide (LPS), which result in an inefficient binding of colistin, can be attributed either to a chromosome-encoded machinery or to the plasmid-transferred mobilized colistin resistance (mcr-1) gene (Baron et al., 2016; Cannatelli et al., 2016; Lui et al., 2016). MCR-1 is a member of the phosphoethanolamine transferase enzyme family catalyzing the addition of phosphoethanolamine to lipid A (Lui et al., 2016). Since the development of new antibiotics is decreasing, it is mandatory to find solutions to break the resistance to or enhance the antimicrobial activity of older antibiotics by co-administration of appropriate drugs, otherwise not used for antibiotic therapy. Recently, we have demonstrated that colistin in combination with the antiviral drug azidothymidine enhanced the colistin activity against colistin-resistant Enterobacteriaceae (Hu et al., 2018; Loose et al., 2018).
Antimicrobial susceptibility testing (AST) is important to predict the clinical outcome of an antibiotic therapy against particular pathogenic microorganisms. To ensure the reproducibility of the results, these tests are performed using guidelines established by the Clinical and Laboratory Standard Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These guidelines recommend AST using cation adjusted Mueller-Hinton broth (CAMHB) as growth medium and define a bacterial inoculum of ∼5 × 105 CFU/mL (Clinical and Laboratory Standards Institute, 1999, 2015). However, the susceptibility of bacteria to antibiotics are influenced by many factors such as bacterial inoculum size, composition of the medium (e.g., pH, ion concentrations) and host factors (e.g., serum factors) (Yang et al., 2014; Li et al., 2017). A recent study showed an increase of colistin Minimal inhibitory concentration (MIC) values for colistin-resistant strains by enhancing the calcium concentration in CAMHB (Gwozdzinski et al., 2018). But so far no data of MIC determinations of colistin in physiologically more relevant media such as urine or human serum have been published.
In this study we determined the minimal inhibitory and bactericidal concentrations (MIC/MBC) of colistin using different media, including artificial urine (AU) and human serum, with different inoculum size against mcr-1-positive as well as colistin-susceptible E. coli strains. In addition, we investigated whether these different media and inoculum sizes also affect the synergistic effect of azidothymidine on colistin antimicrobial activity.
Materials and Methods
Bacterial Strains
Thirteen clinical Escherichia coli isolates harboring the colistin resistance gene mcr-1 as well as twelve colistin-susceptible E. coli strains (one reference strains, E. coli ATCC25922 and eleven clinical isolates) were included in this study (Supplementary Table S1).
Determination of Minimal Inhibitory and Bactericidal Concentrations (MIC/MBC)
Minimal inhibitory concentration (MIC) and Minimal inhibitory bactericidal concentrations (MBC) determinations were performed according to the CLSI- and EUCAST-Standards (Clinical and Laboratory Standards Institute, 1999, 2015; Clinical laboratory testing, 2006). Broth microdilution assay was used for determination of the MIC of colistin sulfate (Sigma-Aldrich, Darmstadt, Germany) in CAMHB (Sigma-Aldrich), IsoSensitest broth (ISB) (Oxoid, Wesel, Germany), native and heat-inactivated (30 min at 56°C) human serum (Sigma-Aldrich), as well as AU, which contains [g/L] CaCl2 – 0.49, MgCl2 × 6H2O – 0.65, NaCl2 – 4.6, Na2SO4 – 2.3, Na2 citrate 2H2O – 0.65, Na2C2O4 – 0.02, KH2PO4 – 2.8, KCl – 1.6, NH4Cl – 1.0, Urea – 25.0, gelatine – 5.0 and Tryptone soya broth – 10.0; pH 6.1 (Stickler et al., 1999). Two final inocula were used: 105CFU/mL and 106 CFU/mL, confirmed by plating, ranged from 3.4–8.5 × 105 CFU/mL and from 1–5 × 106 CFU/mL, respectively. The MIC was defined as the lowest concentration inhibiting visible growth (OD600 < 0.1) after incubation at 37°C for 20 ± 2 h. For determination of MBCs, in a second step 3 μl were transferred onto IsoSensitest agar supplemented with 5% blood (Oxoid) using a one-time inoculator (Dr. Brinkmann Floramed, Nürtingen, Germany). The plates were incubated overnight at 37°C. The number of colonies subsequently grown was used to determine the bactericidal endpoint. MBC was defined as a >99.9% (>3-log) reduction of the initially inoculated colony counts (0.01% threshold for inoculum 105/106 CFU/mL: 1–2/10–12 colonies). MIC/MBC determinations were performed at least three times.
For chelating divalent cations in CAMHB and in AU 1 mM EDTA (ethylenediaminetetraacetic acid; Sigma-Aldrich) was added 30 min prior to colistin sulfate addition.
Checkerboard Assays
A two-dimensional, two-agent broth microdilution checkerboard titration method was used to study the interaction between colistin sulfate and azidothymidine (AZT; Sigma-Aldrich) (Eliopoulos and Moellering, 1996). The final inoculum, confirmed by plating, ranged from 3.1–6.5 × 105 CFU/mL or 1.0–5.3 × 106 CFU/mL. After 20 ± 2 h of incubation at 37°C, the MIC and MBC of azidothymidine and colistin sulfate were determined. Checkerboard determinations were performed only once.
Interactions between azidothymidine and colistin sulfate were then evaluated using the fractional inhibitory/bactericidal concentrations indices (ΣFIC/ΣFBC) calculated as the sum of the FIC or FBC as follows:
FIC – MIC of the substance in combination/MIC of the substance alone.
Correlation between ΣFIC/ΣFBC and the effect of the combination according to EUCAST definition (European Committee for Antimicrobial Susceptibility Testing [EUCAST] of the European Society of Clinical Microbiology and Infectious Diseases [ESCMID], 2000): Synergy − ≤ 0.5, additive >0.5–1, indifference >1 to < 2 and antagonism ≥2.
Statistical Analysis
MIC and MBC values are reported as median values. High off-scale MIC results were converted to the next highest concentration and low off-scale results were left unchanged for comparisons. Concentration data were transformed to compensate for the doubling dilution series by log2 transformation prior to statistical analysis. Differences between MIC or MBC values were identified following analysis of variance (ANOVA) and post hoc analysis of significance for each of the variables. MIC/MBCs within ±1 log2 dilution (two-fold) were regarded as identical. Statistical calculations were performed by using Microsoft excel 2016.
Results
Minimal Inhibitory and Bactericidal Concentrations
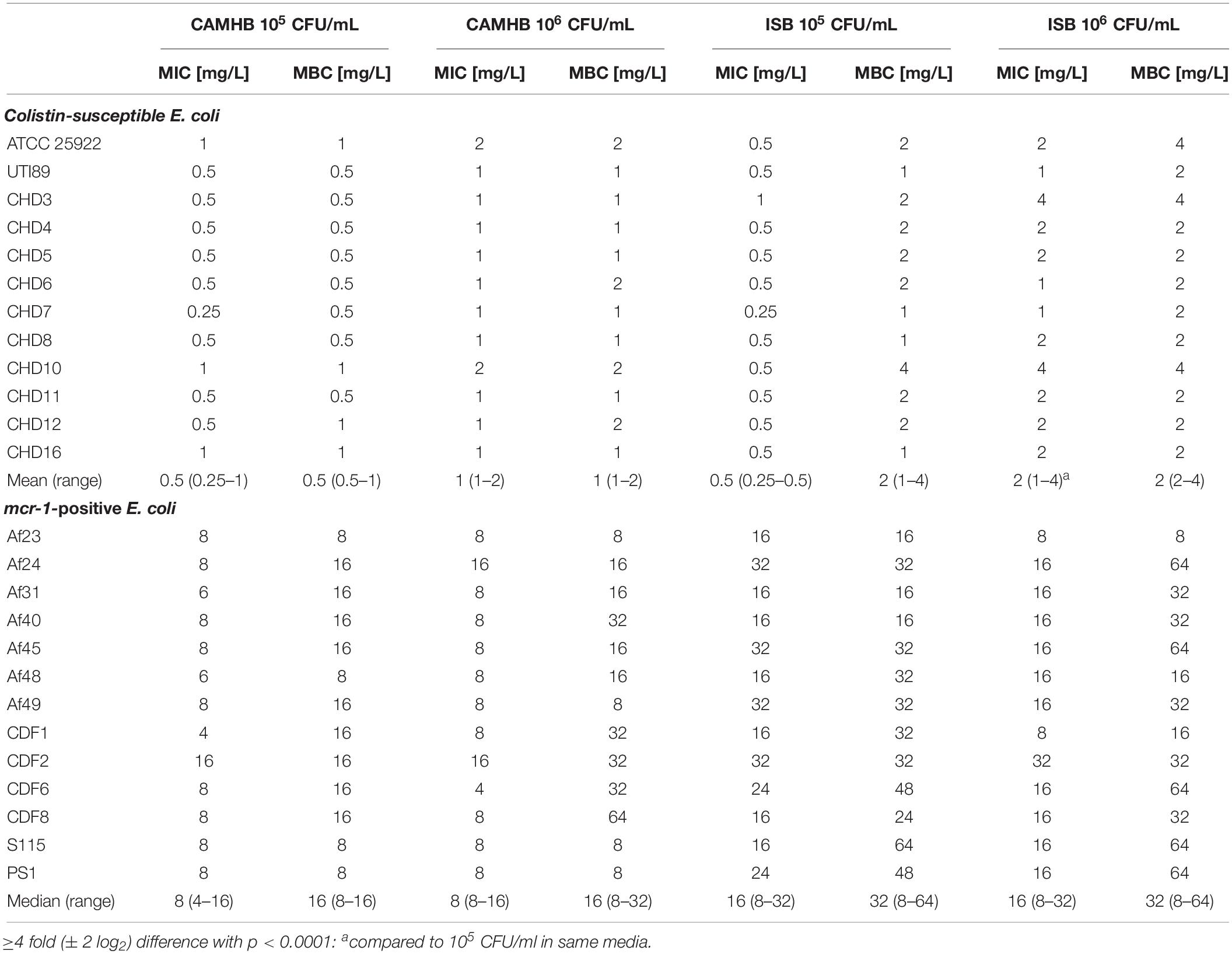
The median of MIC determinations of colistin performed according to EUCAST and CLSI guidelines in CAMHB with an inoculum of 105CFU/mL (Clinical and Laboratory Standards Institute, 1999, 2015; Clinical laboratory testing, 2006) revealed a range between 4–16 mg/L for the mcr-1-positive strains classifying them as colistin-resistant according to the EUCAST definition (there are no CLSI criteria for colistin and Enterobacteriaceae) (The European Committee on Antimicrobial Susceptibility Testing, 2017). Colistin MIC values for the non-mcr-1 strains ranged from 0.25–1 mg/L classifying them as colistin-susceptible (Table 1). The median MBC values in CAMHB with an inoculum of 105 CFU/mL hardly differ to the obtained MIC values for colistin-resistant as well as –susceptible strains (Table 1). Using IsoSensitest broth (ISB), another traditional medium used for antimicrobial testing, showed an slight colistin MIC increase for the colistin-resistant, but not the susceptible, strains. Since patients with symptomatic UTI usually show bacterial loads greater than 105CFU/mL and since inoculum size can affect MIC values of antibiotics, we also performed MIC/MBC determinations with inocula of 106 CFU/mL. There were no differences between median MIC and MBC values for the mcr-1-positive strains between using inoculum of 105 and 106 CFU/mL. In contrast, increasing inoculum led to increased MIC values in ISB for the colistin-susceptible strains (Table 1).
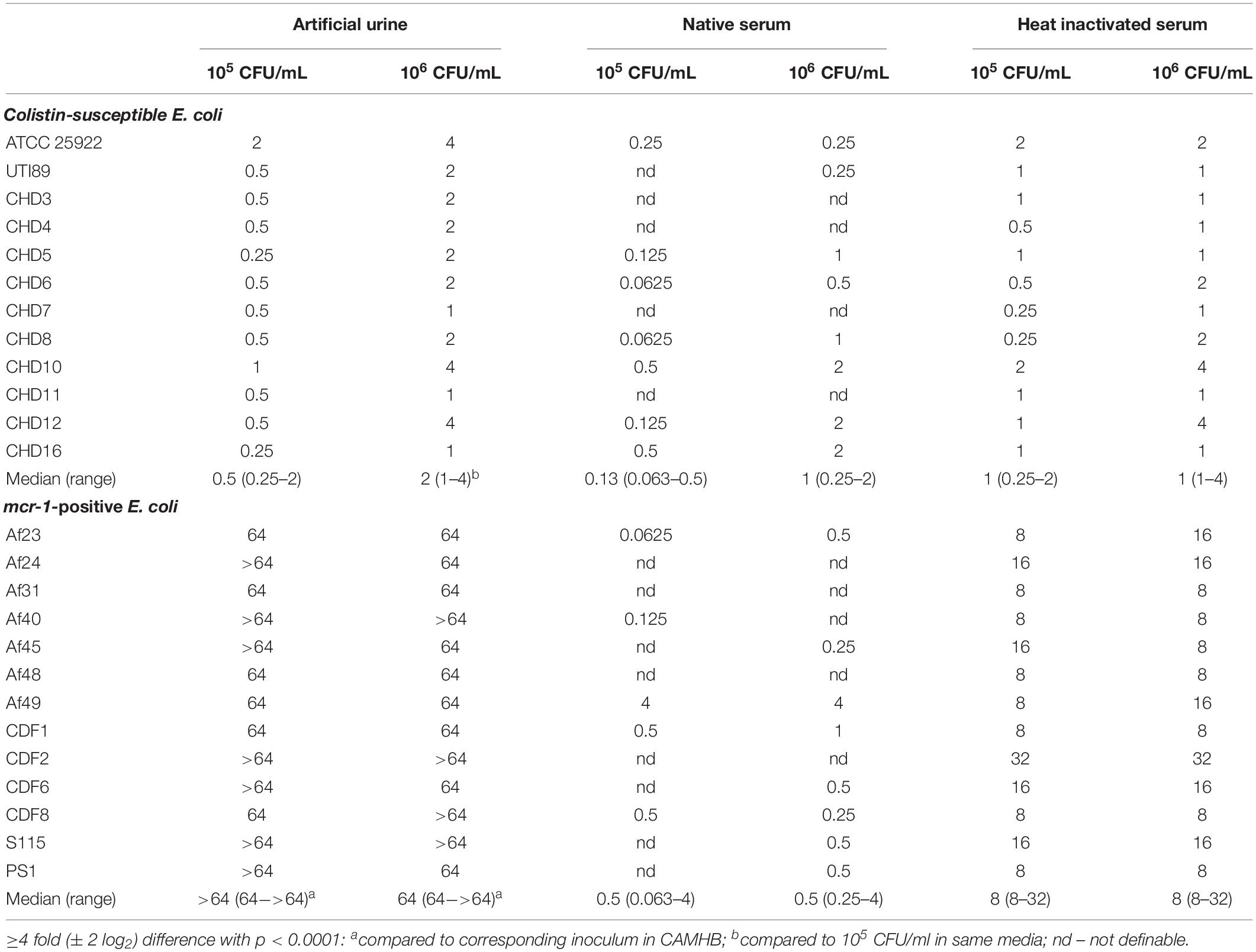
Since all E. coli strains used were isolated mostly from blood and urine, we were interested to determine MIC values under these physiological conditions. Therefore, AU and human serum were used. The MIC values for the mcr-1-positive strains in AU were significantly increased up to16 fold (4 log2) compared to CAMHB for both inocula tested. In contrast, for colistin-susceptible E. coli strains the MIC values remained almost unchanged in AU compared to CAMHB (Table 2). Median MIC values in heated serum were almost equal to those in CAMHB for all strains tested. Furthermore increasing the inoculum size had no effect on the MIC values in AU and heated serum for the mcr-1 positive E. coli strains tested, but resulted in a slight but significant increase of the MIC values of the colistin-susceptible strains in AU (Table 2). Not all of the strains used were able to grow constantly in native human serum. Therefore, MIC determinations in native serum were performed with lower number of strains. Median MIC values of the mcr-1 containing E. coli (except for E. coli Af49), growing in native human serum, were clearly decreased as compared to those in CAMHB. Only the MIC value for E. coli Af49 was comparable in native serum (4 mg/L) to that in CAMHB (8 mg/L). In contrast, MIC values were only slightly reduced in native serum for colistin-susceptible strains with the lower inocula (Table 2).
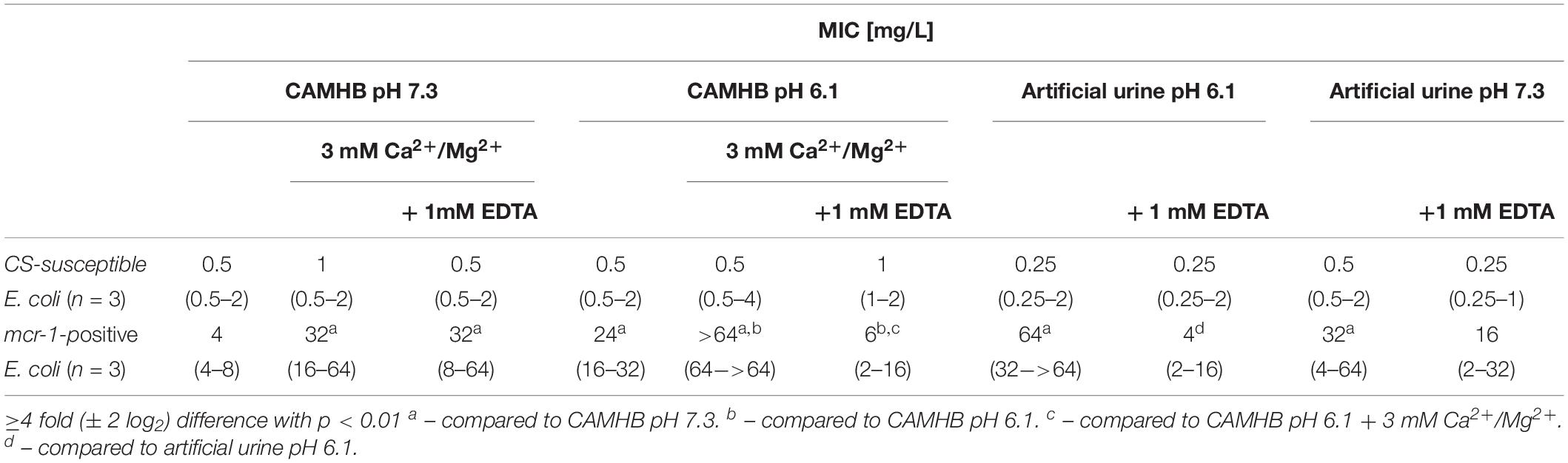
The E. coli strains tested showed a similar growth rate in AU compared to CAMHB (Supplementary Figure S1). Thus, a slower bacterial growth due to a lack of nutrients in the AU can be ruled out as a reason for the different MIC values. Since divalent cations are known to influence the activity of colistin (D’Amato et al., 1975; Gwozdzinski et al., 2018), we were interested in whether the increased MIC values in AU resulted from higher concentrations of Ca2+/Mg2+ (4.4/3.2 mM vs. 0.5/0.4 mM) compared to CAMHB. Supplementation of CAMHB with Ca2+ and Mg2+ to a final concentration of 3 mM increased the median MIC values eight-fold for all three mcr-1-positive strains tested (Table 3). In addition, adding of 1 mM EDTA to chelate divalent cations had no effect in CAMHB but in AU it diminished MICs 16-fold reaching values equal to CAMHB for mcr-1-positive strains. In contrast, cation-supplementation did not affect MIC values for the colistin-susceptible E. coli tested and EDTA addition led to no reduction of MIC values (Table 3). Furthermore, we determined the effect of difference in pH between AU (pH 6.1) and CAMHB (pH 7.3) by adjusting the pH to 7.3 and 6.1, respectively. For the mcr-1-positive strains, reducing the pH of CAMHB increased the MIC values, which was further enhanced by cation supplementation and was abolished by adding of EDTA comparable to AU. Changing of the pH or chelating cations by adding EDTA in AU did not affect MIC values of colistin for the colistin-susceptible E. coli strains (Table 3).
Checkerboard Assays to Determine the Combination Effect of Colistin and Azidothymidine
Recently we showed that a combination of colistin with the antiviral drug azidothymidine, which has also antibacterial properties (Elwell et al., 1987), was synergistic for the treatment of colistin-resistant E. coli (Hu et al., 2018; Loose et al., 2018). Since FIC indices calculations are based on MIC values measured by microbroth dilutions in CAMHB with an inoculum 105 CFU/mL (Hu et al., 2018), we were interested to ask whether using a higher inoculum or different growth media could have an influence on the synergistic activity between colistin and azidothymidine. Using CAMHB and an inoculum of 105 CFU/mL, the combination with azidothymidine reduced the colistin MIC50/MBC50 value for the mcr-1-positive strains by four-fold. Furthermore, based on the ΣFICmin values the combination of colistin with azidothymidine was synergistic for 46% and additive for the remaining strains tested. The results were similar to the previous study (Hu et al., 2018). Based on the ΣFBCmin values synergistic activities were shown even for 69% of the tested strains (Table 4). Also with an increased inoculum and/or in ISB and AU, the MIC50 values of colistin were reduced by two to four-fold and the MBC50 values even by four to eight-fold with the combination of azidothymidine and colistin as compared to colistin alone (Table 4). Based on ΣFICmin/ΣFBCmin, however, the combination of colistin with azidothymidine was only slightly less synergistic in CAMHB/ISB with an inoculum of 106 CFU/ml as compared to an inoculum of 105 CFU/mL in CAMHB.
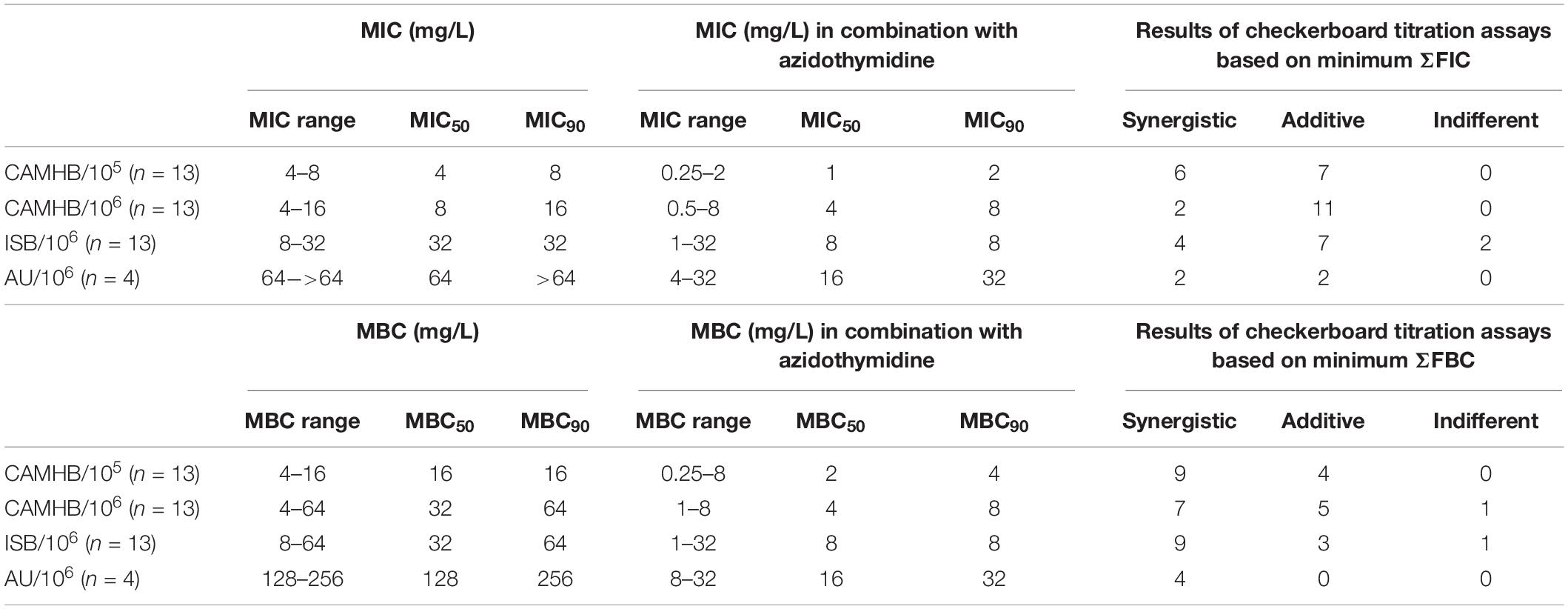
Table 4. MIC and MBC range. MIC/MBC50 and MIC/MBC90 values for colistin before and after combination with azidothymidine in different media/with different inoculum.
Discussion
According to EUCAST and CLSI guidelines, our study demonstrated that all the mcr-1-positive E. coli strains tested are resistant to colistin as confirmed in previous studies (Poirel et al., 2016; Hu et al., 2018; Loose et al., 2018). We showed for the first time, that MIC values for the mcr-1-positive strains, but not colistin-susceptible strains, significantly increased up to 16-fold in AU compared to CMHB. This increased resistance depends most likely on the cationic concentrations and pH of the medium.
It is well known that increasing concentrations of divalent cations antagonize the activity of colistin (D’Amato et al., 1975). In addition our data showed that higher concentrations of divalent cations in AU increased the MIC values of mcr-1 positive colistin-resistant strains. Since MIC values of colistin-susceptible strains were not affected, it can be assumed that increased cation concentrations do not generally inhibit the antimicrobial activity of colistin. Recently, Gwozdzinski et al. showed an increase of colistin MIC values in calcium enhanced (5 mM) CAMHB compared to normal CAMHB for mcr-1 harboring colistin-resistant E. coli, but not colistin-susceptible Enterobacteriaceae strains. Furthermore, they showed that MIC values increased in calcium enhanced media after a colistin-susceptible strain was transformed with different mcr-1-containing plasmids (Gwozdzinski et al., 2018). This supports our assumption that increased cation concentrations influence the mcr-1 mediated colistin resistance. Despite the relatively small number of strains used in this study, the mcr-1 positive E. coli strains represent a heterogeneous group including different plasmids, sequence types and source of isolation (Supplementary Table S1). Irrespective of these differences, each individual mcr-1 positive strain tested, but none of the colistin-susceptible strains, showed a marked MIC increase of 6–16 fold in AU compared to CAMHB, leading to the assumption that the calcium-induced mcr-1 mediated colistin resistance is independent of the different plasmids groups or sequence types. This was also indicated by Gwozdzinski et al. (2018). Since plasmid-encoded mcr-1 polymyxin resistant has also been identified in other Enterobacteriaceae as Klebsiella pneumoniae and Enterobacter cloacae, a calcium-induced resistance could also be assumed for these strains. However, this must be further investigated, as well as a possible cation- and pH-dependence of the other plasmid-borne colistin resistance genes, such as mcr-2, mcr-3, and mcr-4.
Resistance of the mcr-1-positive strains is mediated by 4′-phosphoethanolamine modification of the lipid A on LPS masking the negatively charged phosphate groups on the bacterial surface, which are involved in interactions with colistin. This modification is catalyzed by the MCR-1 enzyme (Pristovsek and Kidric, 1999; Lui et al., 2016). On the one hand, divalent cations may act as cofactors during lipid A modification as for a Ca2+-induced phosphoethanolamine transferase in E. coli (Kanipes et al., 2000). On the other hand, increased cation levels could result in positively charged modified LPS. Furthermore, acidification of CAMHB hand in hand with increasing cation concentrations resulted in increased MIC values for the mcr-1-positive strains. Thus, the lower pH of the AU could additionally promote the reduction of the net negative charge of lipid A, effectively diminishing the electrostatic colistin-bacteria interaction. In addition, low pH may enhance the phosphoethanolamine addition to lipid A as was shown for Cronobacter sakazakii (Liu et al., 2016). Concentrations of colistin, necessary to eliminate mcr-1-positive strains, are much higher under physiologically relevant conditions in AU. Assumptions that antibiotic concentrations reached in urine are high enough to eradicate even resistant pathogens are often based only on pharmacokinetic, but not pharmacodynamic results using MIC values obtained in CAMHB only. Pharmacokinetic parameters, like AUC (area under the concentration-time curve)/MIC and Cmax (maximum concentration of the drug in urine)/MIC ratios will often be much less favorable under physiologically relevant conditions. Therefore, not only pharmacokinetic but also pharmacodynamic studies in urine are as important as in serum or plasma.
Antibiotic susceptibility increases notably in native serum for almost all strains, including those that are able to grow in serum. Serum contains more than thirty proteins of the complement system, which is the first line of defense and is essential for a rapid elimination of invading pathogens. An activated complement cascade triggers the formation of a ring-structured pore (the membrane-attack complex), which increases the permeability of the bacterial membrane (Walport, 2011). Weakening the bacterial membrane integrity by the complement system, even without killing the bacteria, could enhance the effectiveness of colistin. Thus, lower concentrations of colistin are usually necessary to kill bacteria in native serum. On the other hand, lipid A modifications to evade complement system could encourage the binding of colistin leading to an increased antimicrobial activity. For instance, one strategy to evade the attack of the complement system and thus being able to grow in serum is a switch to less acylated lipid A structures in the LPS (Matsuura, 2013). Coincidently, it was shown that K. pneumoniae strains with under-acylated lipid A are more susceptive to polymyxin B binding (Velkov et al., 2013). Susceptibility of colistin in heat inactivated human serum, by which the complement system is destroyed, did not differ from those in CAMHB.
Results of our checkerboard testing confirmed the recent findings (Hu et al., 2018), namely that the combination of colistin with azidothymidine can reduce the MIC of colistin by about 2 to 32 fold for mcr-1-positive E. coli in CAMHB. The antibacterial activity of azidothymidine can be traced back to the inhibition of the replicative DNA-synthesis after incorporation of azidothymidine-triphosphate (Olivero, 2016). The membrane permeabilization by colistin (Katz et al., 2003) could increase the entry of azidothymidine into the bacterial cells. The beneficial effect of this combination is even more pronounced when testing the effect for the MBCs. Therefore, additional evaluation of MBC data could be an advantage to show actual bacterial killing. Although the increase of the inoculum size or changing the growth media to ISB or AU affected the MIC of colistin alone, they only had a small influence on the synergistic effect of the colistin and azidothymidine combination. It should be noted, however, that the colistin MICs/MBCs in AU, even when reduced by the combination with azidothymidine, are still higher in AU than those of colistin alone in CAMHB.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
ML performed all the experiments and wrote the manuscript, with close cooperation and supervision of FW and considering critical comments from all coauthors. KN, YH, and AC made substantial contributions to the conception and design, and all authors contributed to analysis and interpretation of the data. YH and AC were the co-inventors of the antibiotic resistance breaker technology, and in particular of the combination of azidothymidine and colistin/CMS (patents issued), first to test this combination against highly resistant Enterobacteriaceae (AAC 2018, pii: AAC.01630-18. 10.1128/AAC.01630-18), and originated the concept and performed the background work upon which this work is based. YH and AC revised the manuscript critically for important intellectual content and gave final approval of the revised version of the manuscript.
Funding
This study originated from and was supported by the Helperby Therapeutics-Group Ltd., London, United Kingdom.
Conflict of Interest
ML reports grants from Helperby Therapeutics Ltd., during the conduct of the study. KN reports personal fees from Adamed, personal fees from Allecra, personal fees from Apogepha, personal fees from Bionorica, personal fees from Enteris Biopharma, personal fees from Galenus, personal fees from GlaxoSmithKline, personal fees from Hermes, personal fees from Leo, personal fees from Medice, personal fees from MerLion, personal fees from MSD SHARP& DOHME, personal fees from Paratek, personal fees from Roche, personal fees from Rosen, personal fees from Saxonia, personal fees from Vifor, outside the submitted work. AC as Chief Scientific officer of Helperby Therapeutics Ltd., was involved in designing the study as well as interpreting the data. FW reports personal fees and other from Achaogen, personal fees from AstraZeneca, personal fees from Bionorica, other from Enteris BioPharma, other from Helperby Therapeutics Ltd., personal fees from Janssen, personal fees from LeoPharma, personal fees from MerLion, personal fees from MSD, personal fees from OM Pharma/Vifor Pharma, personal fees from Pfizer, personal fees from RosenPharma, personal fees and other from Shionogi, personal fees from VenatoRx, personal fees from GSK, outside the submitted work. YH reports grants and other from Helperby Therapeutics Ltd., during the conduct of the study. In addition, YH and AC have a patent “Zidovudine combination therapies for treating microbial infections” issued.
Acknowledgments
The mcr-1-positive E. coli strains were kindly provided by the Patrice Nordmann, University of Fribourg.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00054/full#supplementary-material
References
Baron, S., Hadjadj, L., Rolain, J. M., and Olaitan, A. O. (2016). Molecular mechanism of polymxin resistance: knows and unknows. Int. J. Antimicrob. Agents 48, 583–591. doi: 10.1093/jac/dky134
Cannatelli, A., D’Andrea, M. M., Giani, T., Di Pilato, V., Arena, F., Ambretti, S., et al. (2016). In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivating of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 57, 5521–5526. doi: 10.1128/aac.01480-13
Clinical and Laboratory Standards Institute (1999). Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26-A. Wayne, PA: CLSI.
Clinical and Laboratory Standards Institute (2015). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M07-A10. CLSI: Wayne, PA.
Clinical laboratory testing, (2006). Clinical Laboratory Testing and In Vitro Diagnostic Test Systems – Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices – Part 1: Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases (ISO 20776-1:2006); German version EN ISO 20776-1.
D’Amato, R. F., Thornsberry, C., Baker, C. N., and Kirven, L. A. (1975). Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin, polymyxin B and carbenicillin. Antimicrob. Agents Chemother. 7, 596–600. doi: 10.1128/aac.7.5.596
Eliopoulos, G. M., and Moellering, R. C. Jr. (1996). “Antimicrobial combinations,” in Antibiotics in Laboratory Medicine, 4th Edn, ed. V. Lorian, (Baltimore, MD: The Williams & Wilkins Co.), 330–396.
Elwell, L. P., Ferone, R., Freeman, G. A., Fyfe, J. A., Hill, J. A., Ray, P. H., et al. (1987). Antibacterial activity and mechanism of action of 3′-azido-3′-deoxythymidine (BW A509U). Antimicrob. Agents Chemother. 31, 274–280. doi: 10.1128/aac.31.2.274
European Committee for Antimicrobial Susceptibility Testing [EUCAST] of the European Society of Clinical Microbiology and Infectious Diseases [ESCMID] (2000). EUCAST definitive document E. Def 1.2, May 2000: terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 6, 503–508. doi: 10.1046/j.1469-0691.2000.00149.x
Gwozdzinski, K., Azarderakhsh, S., Imirzalioglu, C., Falgenhauer, L., and Chakrabrty, T. (2018). An improved medium for colistin susceptibility testing. J. Clin. Microbiol. 56:e1950-17. doi: 10.1128/JCM.01950-17
Hu, Y., Liu, Y., and Coates, A. (2018). Azidothymidine produces synergistic activity in combination with colistin against antibiotic-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 29:AAC.1630-18. doi: 10.1128/AAC.01630-18
Kanipes, M. I., Lin, S., Cotter, R. J., and Raetz, C. R. (2000). Ca2+-induced Phosphoethanolamine Transfer to the Outer 3-Deoxy-D-manno-octulosonic Acid Moiety of Escherichia coli Lipopolysaccharide. A novel membrane enzyme dependent upon phosphatidyl-ethanolamine. J. Biol. Chem. 276, 1156–1163. doi: 10.1074/jbc.m009019200
Katz, M., Tsubery, H., Kolusheva, S., Shames, A., Fridkin, M., and Jelinek, R. (2003). Lipid binding and membrane penetration of polymyxin B derivatives studied in a biomimetic vesicle system. Biochem. J. 375, 405–413. doi: 10.1042/bj2003078
Li, J., Xie, S., Ahmed, S., Wang, F., Gu, Y., Zhang, C., et al. (2017). Antimicrobial activity and resistance: influencing factors. Front. Pharmacol. 8:364. doi: 10.3389/fphar.2017.00364
Liu, L., Li, Y., Wang, X., and Guo, W. (2016). A phosphoethanolamine transferase specific for the 4′-phosphate residue of Cronobacter sakazakii lipid A. J. Appl. Microbiol. 121, 11444–11456.
Loose, M., Naber, K., Hu, Y., Coates, A., and Wagenlehner, A. (2018). Serum bactericidal activity of colistin and azidothymidine combinations against mcr-1 positive colistin-resistant Escherichia coli. Int. J. Antimicrob. Agents. 52, 783–789. doi: 10.1016/j.ijantimicag.2018.08.01
Lui, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Matsuura, M. (2013). Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 4:109. doi: 10.3389/fimmu.2013.00109
Olivero, O. A. (2016). Mechanism of genotoxiciy of nucleoside reverse transcriptase inhibitors. Environ. Mol. Mutagen. 48, 215–223.
Paterson, D. L., and Harris, P. N. (2016). Colistin resistance: a major breach in our last line defence. Lancet Infect. Dis. 16, 132–132.
Poirel, L., Kieffer, N., Brink, A., Coetze, J., Jayol, A., and Nordmann, P. (2016). Genetic features of MCR-1-producing Colistin-resistant Escherichia coli isolates in South Africa. Antimicrob. Agents Chemother. 60, 4394–4397. doi: 10.1128/AAC.00444-16
Pristovsek, P., and Kidric, J. (1999). Solution structure of polymyxin B and E and effect of binding to lipopolysaccharide: an NMR and molecular modelling study. J. Med. Chem. 42, 4604–4613. doi: 10.1021/jm991031b
Stickler, D. J., Morris, N. S., and Winters, C. (1999). Simple physical model to study formation and physiology of biofilms on urethral catheters. Methods Enzymol. 310, 494–501. doi: 10.1016/s0076-6879(99)10037-5
The European Committee on Antimicrobial Susceptibility Testing (2017). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.1.
Velkov, T., Soon, R. L., Chong, P. L., Huang, J. X., Cooper, M. A., Azad, M. A., et al. (2013). Molecular basis for the increased polymyxin susceptibility of Klebsiella pneumoniae strains with under-acylated lipid A. Innate Immun. 19, 265–277. doi: 10.1177/1753425912459092
World Health Organization [WHO] (2011). Critically Important Antimicrobials for Human Medicine, 3rd Edn. Geneva: World Health Organization.
Keywords: colistin resistance, colistin, MIC/MBC, influence pH and cations, azidothymidine
Citation: Loose M, Naber KG, Coates A, Wagenlehner FME and Hu Y (2020) Effect of Different Media on the Bactericidal Activity of Colistin and on the Synergistic Combination With Azidothymidine Against mcr-1-Positive Colistin-Resistant Escherichia coli. Front. Microbiol. 11:54. doi: 10.3389/fmicb.2020.00054
Received: 12 April 2019; Accepted: 13 January 2020;
Published: 29 January 2020.
Edited by:
Jack Wong, The Chinese University of Hong Kong, ChinaReviewed by:
Hong Du, Soochow University, ChinaMaria Bagattini, University of Naples Federico II, Italy
Copyright © 2020 Loose, Naber, Coates, Wagenlehner and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Loose, TWFyaWEuTG9vc2VAY2hpcnUubWVkLnVuaS1naWVzc2VuLmRl
 Maria Loose
Maria Loose Kurt G. Naber2
Kurt G. Naber2 Yanmin Hu
Yanmin Hu