- 1Department of Biological Sciences, Xi’an Jiaotong-Liverpool University, Suzhou, China
- 2Department for Evolutionary Ecology, Institute for Organismic and Molecular Evolution, Johannes Gutenberg University Mainz, Mainz, Germany
The negative effects of honey bee parasitic mites and deformed wing virus (DWV) on honey bee and colony health have been well characterized. However, the relationship between DWV and mites, particularly viral replication inside the mites, remains unclear. Furthermore, the physiological outcomes of honey bee immune responses stimulated by DWV and the mite to the host (honey bee) and perhaps the pathogen/parasite (DWV/mite) are not yet understood. To answer these questions, we studied the tripartite interactions between the honey bee, Tropilaelaps mercedesae, and DWV as the model. T. mercedesae functioned as a vector for DWV without supporting active viral replication. Thus, DWV negligibly affected mite fitness. Mite infestation induced mRNA expression of antimicrobial peptides (AMPs), Defensin-1 and Hymenoptaecin, which correlated with DWV copy number in honey bee pupae and mite feeding, respectively. Feeding T. mercedesae with fruit fly S2 cells heterologously expressing honey bee Hymenoptaecin significantly downregulated mite Vitellogenin expression, indicating that the honey bee AMP manipulates mite reproduction upon feeding on bee. Our results provide insights into the mechanism of DWV transmission by the honey bee parasitic mite to the host, and the novel role of AMP in defending against mite infestation.
Introduction
Large-scale loss of managed honey bee colonies has been recently reported across the globe (Goulson et al., 2015). Since pollination by honey bees is vital for maintaining ecosystems and the production of many crops (Klein et al., 2007; Aizen and Harder, 2009), prevention of honey bee colony losses has become a major focus in both apiculture and agriculture. Colony losses have often been associated with the ectoparasitic mites Varroa destructor and Tropilaelaps mercedesae, which feed on honey bees and transmit honey bee viruses, particularly deformed wing virus (DWV) to the host (de Miranda and Genersch, 2010; Rosenkranz et al., 2010; Chantawannakul et al., 2018). In the absence of mites, DWV copy numbers remain low in honey bees without specific symptoms (covert infection). However, DWV levels associated with honey bees are dramatically increased in mite infested colonies (Shen et al., 2005; Forsgren et al., 2009; Khongphinitbunjong et al., 2015; Wu et al., 2017). These honey bees often show multiple symptoms (overt infection), which include the death of pupae, deformed wings, shortened abdomen, and reduced lifespan (Yue and Genersch, 2005; Tentcheva et al., 2006; de Miranda and Genersch, 2010; Rosenkranz et al., 2010). Winter colony loss is strongly correlated with the presence of DWV and V. destructor (Highfield et al., 2009; Nazzi and Le Conte, 2016).
Although the impacts of DWV and V. destructor on individual honey bees and colonies are well characterized, the actual relationship between DWV and honey bee mite is not yet understood. Several studies have suggested that DWV replicates in V. destructor and that more virulent DWV strains are amplified for transmission to honey bees (Martin et al., 2012; Ryabov et al., 2014). However, the results of other studies are inconsistent with this view (Erban et al., 2015; Dong et al., 2017; Posada-Florez et al., 2019). Thus, it is important to address this issue to uncover the mechanism by which mites function as vectors for DWV.
DWV copy numbers in mites can exceed 106 (Wu et al., 2017; Posada-Florez et al., 2019). Thus, DWV could have significant effects on mite physiology. Previous studies have reported that DWV infection and/or V. destructor infestation induce honey bee immune responses that include synthesis of antimicrobial peptides (AMPs) (Gregorc et al., 2012; Kuster et al., 2014). However, their effects on the host (honey bee), pathogen (DWV), and parasite (mite) are still uncharacterized. AMPs were originally identified as short positively-charged peptides that inhibit the viability of bacteria and fungi (Bahar and Ren, 2013; Hanson and Lemaitre, 2019). Since AMPs are induced under various conditions, their physiological functions could be more diverse and remain to be tested.
In this study, we first examined whether T. mercedesae functions as a bona fide vector for DWV, and then characterized the effects of DWV on mites to understand the precise relationship. We also examined the immune responses of honey bee pupae to T. mercedesae infestation and found that Hymenoptaecin down-regulates the mite Vitellogenin (Vg) gene. Using these results, we discuss the mechanisms of DWV transmission by the honey bee parasitic mites, as well as how the host (honey bee) defends against the parasite (mite) by suppressing reproduction.
Materials and Methods
Artificial Infestation of Honey Bee Pupae With Mites
A. mellifera colonies were obtained from local beekeepers and maintained at Xi’an Jiaotong-Liverpool University. Honey bee worker pupae (n = 33) with white eyes were sampled from the mite-free colony by opening the capped brood cells. Adult female mites (n = 15) were collected from another colony heavily infested with T. mercedesae as above. The average copy number of DWV in the mite infested pupae was 6.2 × 107. A single pupa and mite were put inside a gelatin capsule. As the control, the remaining pupae (n = 18) were individually incubated without the mite. The capsules were inserted to a tube rack vertically positioned in an incubator at 33°C with 70% relative humidity for a week (Egekwu et al., 2018).
Isolation of Total RNA and RT-PCR
Head was first dissected from each pupa and total RNA was extracted from the individual pupal heads and mites using TRI Reagent® (Sigma-Aldrich) according to the manufacturer’s instruction. Glycogen (1 μg) was added to facilitate isopropanol precipitation of the mite RNA sample. Reverse transcription (RT) reaction was carried out using 1 μL of total RNA, random primer (TOYOBO), ReverTra Ace (TOYOBO), and RNase inhibitor (Beyotime). RNase H (Beyotime) was then added to digest RNA in RNA/cDNA heteroduplex after cDNA synthesis. DWV in the honey bee and mite samples was detected by RT-PCR using DWV #1 primers (Supplementary Table S1) and the cycling condition of 2 min at 94°C followed by 32 cycles of 10 sec at 98°C, 20 sec at 55°C, and 30 sec at 68°C. The PCR products were analyzed by 2% agarose gel. A. mellifera and T. mercedesae EF-1α mRNAs (Supplementary Table S1) were used as the positive controls to verify successful RT.
Sequencing of RT-PCR Products
PCR products obtained by above RT-PCR were purified by QIAquick PCR Purification Kit (QIAGEN) according to the manufacturer’s instruction, and then directly sequenced by Sanger method using the PCR primers.
Analysis of DWV, Honey Bee AMP, and Mite Vg mRNAs by qRT-PCR
DWV copy number was determined by qRT-PCR using a HieffTM ® qRT-PCR SYBR Green Master Mix (Low Rox Plus, Yesen) and DWV #2 primers (Supplementary Table S1). To prepare a standard curve for DWV, PCR product obtained by above primers was purified and the copy number was determined by a formula below.
6.6 × 1011 ng/mol is the average molecular mass of one base pair and 6.02 × 1023 copies/mol is Avogadro’s number. We conducted qPCR using 101–109 copy number of the PCR product and then plotted the Ct values against the log values of copy numbers. DWV copy number in the sample was determined using the standard curve. The amount of cDNA added to each qPCR reaction was normalized using either A. mellifera or T. mercedesae 18S rRNA as the endogenous reference (Supplementary Table S1).
The relative amounts of Hymenoptaecin, Defensin-1, and T. mercedesae Vg mRNAs in the samples were measured by the ΔΔCt method. The primers for Hymenoptaecin, Defensin-1, and five T. mercedesae Vgs are listed in Supplementary Table S1. A. mellifera or T. mercedesae 18S rRNA was used as the endogenous reference.
Effects of Introducing Wound on DWV Copy Numbers in Honey Bee Pupae
The mite-free honey bee pupae with pink eyes were sampled from T. mercedesae-infested colony. A small physical wound was introduced to the thorax (n = 6) with a sterilized microliter syringe (GAOGE) and control pupae (n = 7) were untreated. All pupae were then individually put in a gelatin capsule and incubated for 37 h and DWV copy numbers in the individual pupal heads were measured as above.
Raising Antibodies Against VP1 and RdRP of DWV
The P-domain of VP1 (amino acid 748-901 of DWV polyprotein) was PCR amplified using 5′-NdeI-P-domain and 3′-XhoI-P-domain primers (Supplementary Table S1). The PCR amplicon was digested with NdeI (NEB) and XhoI (NEB). The part of RdRP (amino acid 2563–2797 of DWV polyprotein) was also PCR amplified using 5′-KpnI-RdRP and 3′-Hind III-RdRP primers (Supplementary Table S1). The PCR product was digested with KpnI (NEB) and Hind III (NEB). The restriction enzyme digested DNA fragments were purified and then cloned to the corresponding sites in pCold-I vector (TAKARA) followed by transformation to E. coli BL21.
The transformed E. coli was grown in LB medium containing ampillicin (0.1 mg/mL) at 37°C until the A600 reached to approximately 0.5, and then the culture was cooled down and added with isopropyl-thio-galactoside at the final concentration of 0.5 mM to induce the protein expression at 15°C overnight. E. coli was suspended with 100 mL of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 1 mM DTT, and protease inhibitors at pH 8.0). The cell suspension was then sonicated for 45 min (30 sec sonication with 3 min interval) at amplitude 100 on ice using Q700 sonicator (Qsonica). After centrifugation, the supernatant was collected and incubated with His-tag Protein Purification Resin (Beyotime) at 4°C for 2 h. The resin was then washed five times with 10 mL of washing buffer (50 mM NaH2PO4, 300 mM NaCl, and 2 mM imidazole, pH 8.0), and the bound protein was eluted six times with 1 mL then twice with 5 mL of elution buffer (50 mM NaH2PO4, 500 mM NaCl, and 250 mM imidazole, pH 8.0). The purified proteins were dialyzed against PBS at 4°C overnight, and then concentrated using Vivaspin® 6 polyethersulfone 10 kDa (Sartorius). Concentrations of the purified proteins were measured using BCA protein assay kit (Beyotime) according to the manufacturer’s instruction. Purification of the RdRP peptide was carried out using above buffers containing 0.1% sarcosyl to increase the protein solubility. The purified proteins were delivered to a company (GeneScript-Nanjing) to obtain the affinity purified rabbit polyclonal antibodies.
SDS-PAGE and Western Blot
The proteins were suspended with the sample buffer (2% SDS, 10% glycerol, 10% β-mercaptoethanol, 0.25% bromophenol blue, and 50 mM Tris-HCl, pH 6.8), and then heated at 99°C for 5 min followed by applying to 12% SDS-PAGE gel. After electrophoresis, the gel was treated with the staining buffer (0.25% Coomassie brilliant blue G-250, 40% methanol, and 10% acetic acid), and then washed with the destaining buffer (40% methanol and 10% acetic acid). The bands were visualized and analyzed using ChemiDocTM MP imaging system and Image LabTM touch software (BIO-RAD).
Honey bee pupal heads and Tropilaelaps mites were individually homogenized with 300 and 50 μL of sample buffer, respectively. Drosophila S2 cells in 12-well plate were lysed with 200 μL of sample buffer. All samples were heated as above and centrifuged, and then the supernatants were applied to 10 (for honey bee and mite lysates) or 15% (for S2 cell lysates) SDS-PAGE gel. After electrophoresis, proteins in the gel were transferred to a pure nitrocellulose blotting membrane (Pall® Life Sciences). The membrane with proteins was first blocked with PBST (0.1% Tween-20/PBS) containing 5% BSA at room temperature for 1 h, and then incubated with 1000-fold diluted primary antibody (anti-VP1 antibody, anti-RdRP antibody, or anti-His tag antibody) in above buffer at 4°C overnight. The membrane was washed with PBST three times for 5 min each, and then incubated with 10,000-fold diluted IRDye® 680RD donkey anti-rabbit IgG (H + L) (LI-COR Biosciences) in PBST containing 5% skim milk at room temperature for 1.5 h. The membrane was washed as above, and then visualized using Odyssey Imaging System (LI-COR Biosciences).
Analysis of Mite Transcriptomes by RNA-Seq
Individual Tropilaelaps mites were tested for DWV by RT-PCR as above and separated to two groups with either high (High_A and High_B) or low (Low_A and Low_B) DWV level. Each group was made of total RNAs prepared from ten mites and sequenced at BGI (Shenzhen, China) using Illumina HiSeq 4000 platform. After sequencing, the raw data were filtered to remove the adaptor sequences, contamination, and low-quality reads by BGI. The Quality control (QC) was further analyzed using FastQC. All RNA-seq data are available in SRA database with the accession #: PRJNA608093.
The reference genome and annotated genes of T. mercedesae were first acquired from NCBI1, and then used for building the index by Hisat2–build indexer (Kim et al., 2015). The generated index files were used to align the clean reads of four RNA-seq samples to the reference genome. Subsequently, SAM file outputs from the previous step were sorted using SAMtools (Li et al., 2009). HTSeq-count (Anders et al., 2015) was further applied to obtain the raw read counts for downstream analysis of identifying the differentially expressed genes (DEGs) between the mites with low and high DWV loads in R (V3.4.3) based Bioconductor edgeR package (V3.20.9) (Robinson et al., 2010). The DEGs were cut-off by a False Discovery Rate (FDR) at 0.05.
Immunostaining of the Mite Thin Sections
Tropilaelaps mites collected from the hive were washed with bleach and PBS, and then fixed with 4% paraformaldehyde/PBS at 4°C for 24 h with gentle shaking. The fixed samples were stored in methanol at −20°C and then converted into absolute n-butanol followed by embedding in Technovit 8100 (Kulzer, Wehrheim, Germany). On the rotation microtome (Leica RM 2245), 8 μm thick serial sections were prepared with glass knives and placed into water drops on silanized slides. The sections were dried for 30 min at 50°C. The above thin sections were washed five times with PBS, and then treated with 2 mg/mL pepsin in 0.9% NaCl, pH 2.0 at 37°C for 10 min. They were then washed three times with PBS, three times with PT (PBS containing 0.1% Triton-X 100), and once with PBS. The sections were blocked with PBS containing 3% BSA and 1% normal goat serum at 4°C overnight and then they were alternatively incubated with 1,000-fold diluted anti-VP1 antibody and the pre-immune serum in above buffer at 4°C overnight. After washing eight times with PBS, the sections were incubated with 1,000-fold diluted Goat anti-rabbit IgG (H + L) SuperclonalTM secondary antibody, Alexa Fluor 555 (Thermo Scientific) at room temperature for 1.5 h. After washing seven times with PBS, the sections were incubated with 0.5 μg/mL DAPI (Beyotime) at room temperature for 15 min. Following the final wash with PBS, the sections were mounted with Antifade mounting medium (Beyotime). Each wash was conducted for 10 min. The immunostained sections above were observed using a confocal microscope, LSM 880 (Zeiss) with the TileScan method. ImageJ was used for the image analysis.
Phylogenetic Trees of the DWV Isolates and Mite Vgs
The representative RNA sequences of DWV isolates from the honey bee pupae and mites were aligned using MUSCLE (Edgar, 2004), and then Tamura 3-parameter + G was selected as the best-fit substitution model for constructing the phylogeny. The condensed phylogenetic tree was constructed using the maximum likelihood method and a bootstrap value of 1,000 replicates with MEGA7 (Kumar et al., 2016). Amino acid sequences of all T. mercedesae and V. destructor Vgs were retrieved from NCBI and the phylogenetic tree was constructed as above except Jones-Taylor-Thornton + G + F was used as the best model.
Codon Usage Analysis
Codon usage tables to show the codon frequencies per 1000 codons for DWV, A. mellifera, and T. mercedesae were obtained using HIVE-CUTs (Athey et al., 2017). To compare the codon frequencies between DWV and either A. mellifera or T. mercedesae, three stop codons, and codons for methionine and tryptophan were omitted from the analysis.
Establishing Drosophila S2 Cells to Stably Express Hymenoptaecin
Hymenoptaecin cDNA was obtained by RT-PCR using honey bee RT and Hymenoptaecin #1 primers (Supplementary Table S1). The PCR amplicon was digested with EcoRI and AgeI and cloned to the same sites in pAc5.1/V5-His B (Thermo Fisher Scientific) to express the His-tagged protein. S2 cells in 12-well plate were transfected with either 2 μg of Hymenoptaecin expression vector or empty vector and 10 μL of HilyMax (DOJINDO) for 24 h. After replacing the medium, the cells were cultured for 24 h, and then analyzed by western blot using rabbit polyclonal anti-His tag antibody (BBI). After confirming the protein expression, the untagged version of Hymenoptaecin cDNA was PCR amplified using Hymenoptaecin #2 primers (Supplementary Table S1) and digested with XhoI and EcoRV followed by inserting to the same sites in pMK33/pMtHy (Koelle et al., 1991). S2 cells were transfected with this expression vector or empty pMK33/pMtHy as above and the stable transfectants were first selected by 0.1 mg/mL hygromycin for 10 days, and then the concentration was increased to 0.3 mg/mL. The hygromycin-resistant S2 cells (2 × 107) were harvested during the logarithmic growth phase and suspended with 0.3 mL of Grace medium followed by sonication and store at −20°C.
Feeding Mites With S2 Cell Extracts
Feeding Tropilaelaps mites was carried out in an inverted flat-cap 2 mL microcentrifuge tube. The bottom of the tube was cut and plugged with cotton to provide ventilation and the cap contained a small piece of a sterile cotton ball. S2 cell extracts prepared as above together with 2% Royal blue (50 μL) were dispensed onto the cotton and then 10 starved mites were added prior to closing the tube. The assay tubes were placed in an incubator at 33°C with 90% relative humidity for 24 h under dark. The fed and survived mites were collected and then five Vg mRNAs were analyzed by qRT-PCR.
Statistical Analysis
Statistical analyses were performed with BellCurve for Excel (Social Survey Research Information Co., Ltd.) and no data point was excluded. To compare the statistical difference between two groups, we used Brunner–Munzel test, a non-parametric method applicable to samples with unequal variance. To test correlation between two factors, Pearson correlation test was used. All data presented were from representative independent experiments. The applied statistical tests and P-values are also described in figure legends.
Results
Role of T. mercedesae as a Vector for DWV
To directly test whether T. mercedesae functions as a vector to transmit DWV to honey bees, we individually incubated white-eyes worker pupae with T. mercedesae. The heads of test pupae (the ones artificially infested by the mites) contained higher DWV copy numbers than those of the control pupae (Figure 1A). We also found a positive correlation between DWV copy number in pupae and infesting mites (Figure 1B). Sanger sequencing of the PCR amplicons (Supplementary Figure S1) revealed that ten pupa-mite pairs were infected by a single variant of DWV. Two pairs were infected by multiple variants in both pupae and mites. Two pairs were infected by single and multiple variants in pupae and mites, respectively. One pair was infected by single and multiple variants in mite and pupa, respectively (Supplementary Table S2). A phylogenetic tree was constructed using DWV sequences obtained from test pupae, infesting mites, as well as the control pupae. In case multiple DWV variants are present in pupae and mites, only samples in which we could identify the dominant variants were analyzed. All 18 control pupae were infected by multiple variants at very low level and the eight samples (Bee-C2, 6, 9, 10, 11, 14, 15, and 16) contained the dominant DWV variant, which was identical to the one present in seven test pupae (Bee-1, 4, 7, 8, 9, 12, and 14) and five infesting mites (Mite-1, 7, 8, 9, and 12). Three pupa-mite pairs (Bee/Mite-3, 13, and 15) contained the same variant. Mite-4 and Mite-14 shared the same variant, which was different from the one present in the infested pupae (Bee-4 and Bee-14). These two pairs represent the example that pupa and the infesting mite do not share the same DWV variant. As shown in Figure 1C, six pupa-mite pairs (Bee/Mite-3, 5, 10, 11, 13, and 15) out of 15 pairs were infected by four variants that were different from the ones present in the control pupae (Fisher’s exact test; P < 0.005). These results demonstrated that these variants were transferred from the infesting mites derived from a colony that was different from the one in which all pupae were sampled. To test whether T. mercedesae also enhances replication of the endogenous DWV by inflicting injury, we induced wounds at the pupal thorax with a sterile needle and compared the copy numbers of DWV in the heads of wounded and control (untreated) pupae. The copy numbers were higher in the wounded pupae than in the control pupae (Figure 1D), suggesting that wound induction alone is sufficient to stimulate replication of the endogenous DWV in honey bee pupae.
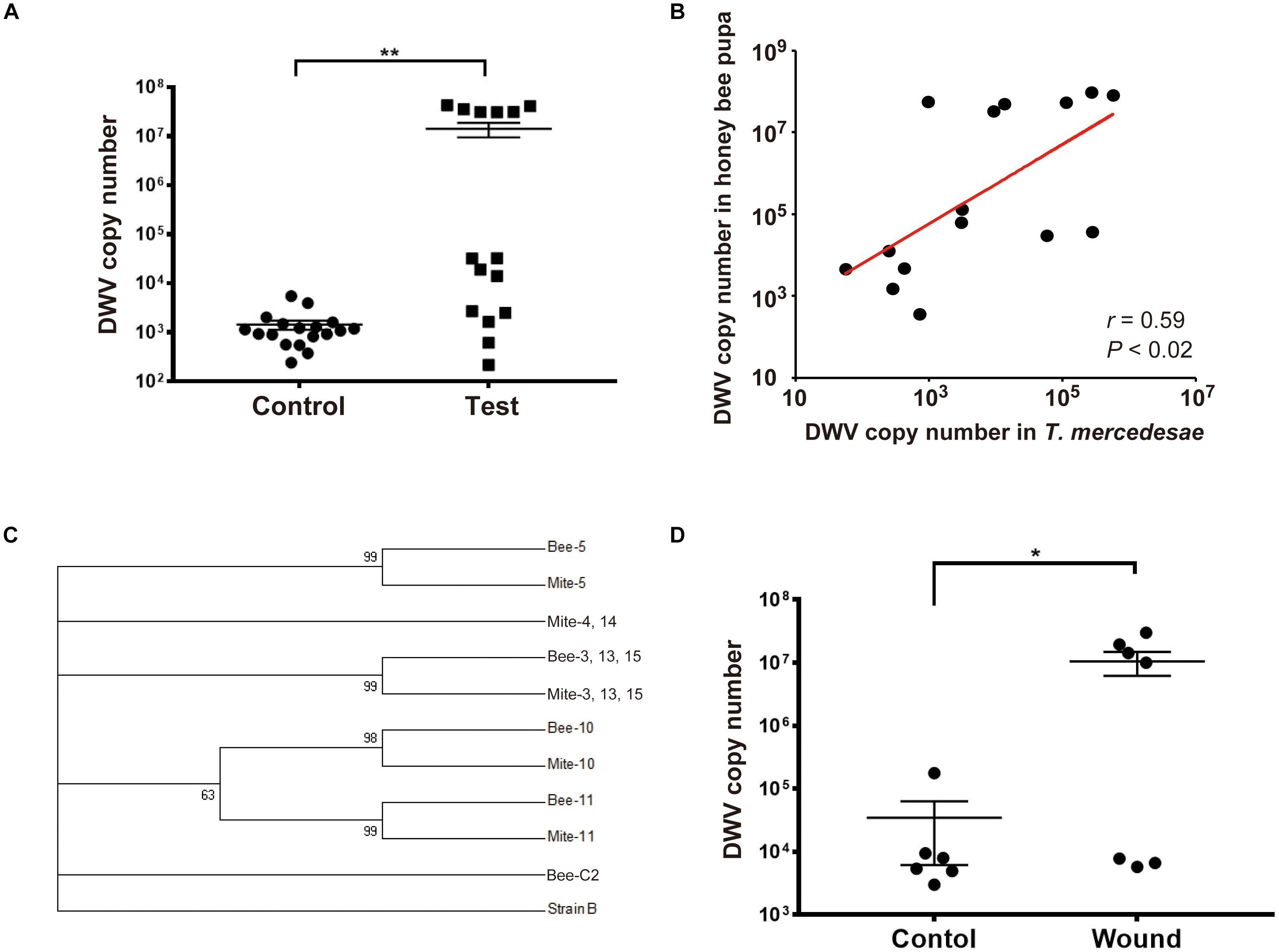
Figure 1. T. mercedesae functions as a vector for DWV. (A) DWV copy numbers in the heads of honey bee pupae artificially infested by T. mercedesae (Test, n = 15) and the ones without mites (Control, n = 18). Mean values with error bars (± SEM) are indicated. Two groups are statistically different by Brunner–Munzel test (**P < 1.3E-06). (B) DWV copy numbers in individual honey bee pupae and the infesting T. mercedesae are plotted on the Y- and X-axis, respectively. The red line represents a fitted curve and the Pearson correlation value and P-value are also shown. (C) Maximum-likelihood condensed phylogeny of DWV isolates from honey bee pupae (Bee) and T. mercedesae (Mite) was constructed based on partial VP2 and VP1, and full VP4 sequences. Sequence of Bee-C2 (control pupa) sample is identical to those of other seven control pupae (Bee-C6, 9, 10, 11, 14, 15, and 16), seven test pupae (Bee-1, 4, 7, 8, 9, 12, and 14) and five infesting mites (Mite-1, 7, 8, 9, and 12). Bootstrap values are shown at the corresponding node of each branch and DWV type B strain was used as an outgroup. (D) DWV copy numbers in wounded (Wound, n = 7) and untreated (Control, n = 6) honey bee pupae. Mean values with error bars (± SEM) are indicated. Two groups are statistically different by Brunner–Munzel test (*P < 0.041).
Impact of DWV on T. mercedesae
To examine the effects of DWV on T. mercedesae, we compared the gene expression profiles of mites carrying either high or low DWV copy numbers by RNA-seq. The average mapping rates of RNA-seq reads derived from the mites with high and low DWV loads to T. mercedesae genomes were 38.7 and 83.0%, respectively, and those to the DWV genome were 22.8 and 0.05%, respectively (Supplementary Table S3). We identified a few DEGs between mites with low and high DWV loads (Supplementary Table S4). The high DWV load had little effect on the transcriptome profile of T. mercedesae, suggesting that DWV does not actively infect/replicate the mite cells. To test this hypothesis, we analyzed the protein extracts of individual honey bee pupal heads and Tropilaelaps mites to detect VP1 (capsid protein, structural protein) and RdRP (RNA dependent RNA polymerase, non-structural protein) of DWV. As shown in Figure 2A, two bands of 90 and 53 kDa were specifically detected by anti-RdRP antibody, suggesting that the large and small bands represent the precursor of RdRP fused with 3C-protease (3C-Pro) and the matured RdRP, respectively. The cleavage between 3C-Pro and RdRP appears to be rate-limiting for DWV, similar to other picornaviruses (Jiang et al., 2014). RdRP and the precursor were detected in the four VP1-positive, but not one VP1-negative honey bee pupal heads. However, these proteins were absent in the four Tropilaelaps mites, irrespective of the presence of VP1 (Figure 2A). In total, RdRP was detected in seven VP1-positive honey bee pupae but not in eight VP1-positive mites. These results indicated that DWV infects and actively replicates in the honey bee pupal head, but not the mite cells. Lack of active synthesis of DWV protein in Tropilaelaps mites was also supported by their large differences in codon usage. The codon usage of DWV is apparently adapted to that of the original host, A. mellifera, as previously reported (Chantawannakul and Cutler, 2008), but quite different from that of mite (r = 0.046, P < 0.73) (Figures 2B,C). To uncover the major sites for localization of DWV in Tropilaelaps mite, transverse thin sections of mites were immunostained by anti-VP1 antibody. DWV was primarily localized in the lumen of the entire midgut as large dense spheres (Figure 3), consistent with the lack of active viral replication. There was no specific staining with the pre-immune serum (Supplementary Figure S2).
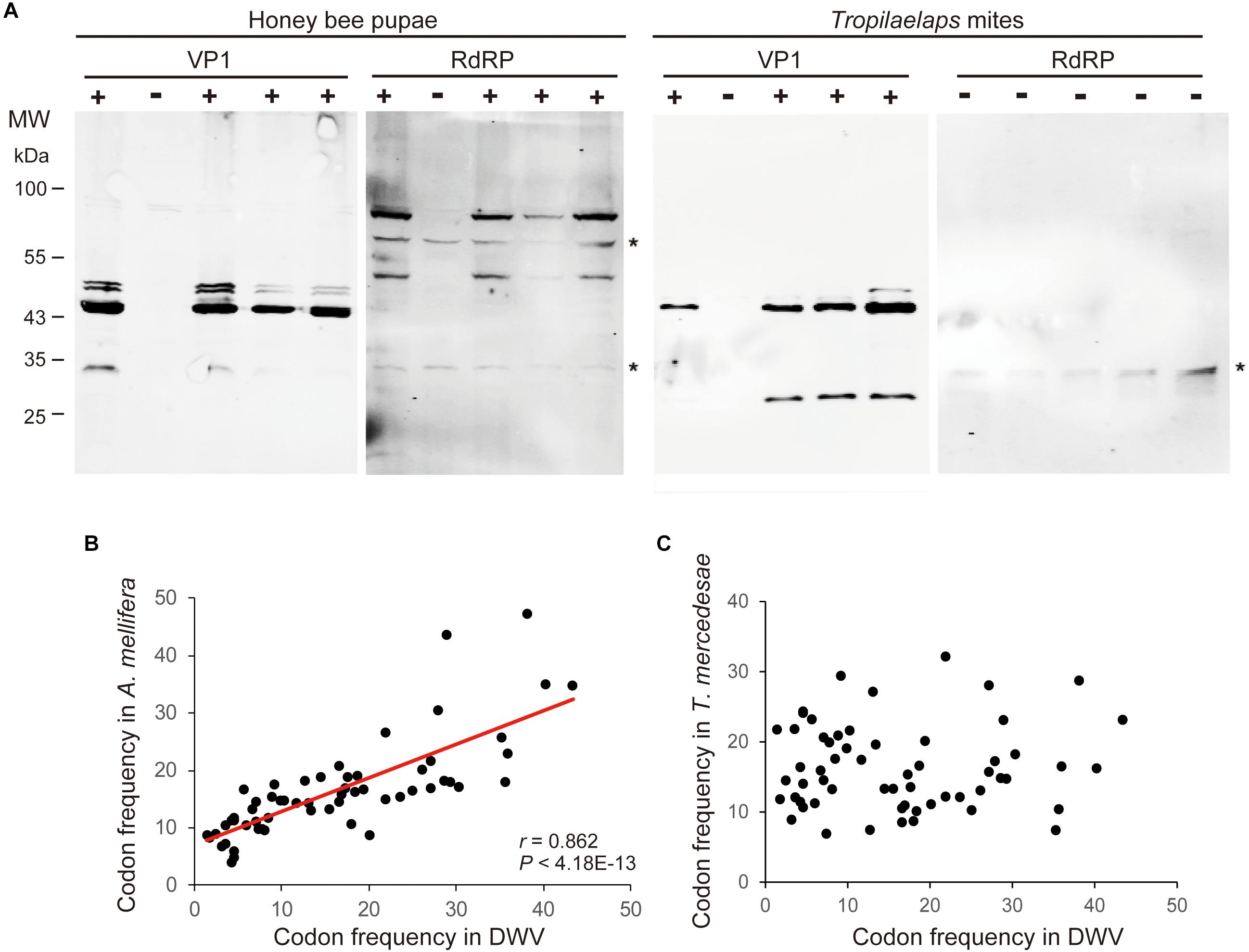
Figure 2. Lack of active replication of DWV in T. mercedesae. (A) Detection of DWV VP1 and RdRP in the lysates of individual honey bee pupae and Tropilaelaps mites by western blot. A major 47 kDa band was detected in VP1-posiitive (+) but not negative (−) pupa and mite; however, RdRP (90 and 53 kDa bands) was only present in the VP1-positive pupa. The asterisks represent non-specific proteins cross reacted with anti-RdRP antibody. The size of protein molecular weight marker (MW) is at the left. (B) Frequencies of each codon out of 1,000 codons in DWV and A. mellifera genomes are plotted on the X- and Y-axis, respectively. The red line represents a fitted curve and the Pearson correlation value and P-value are also shown. (C) Frequencies of each codon out of 1,000 codons in DWV and T. mercedesae genomes are plotted on the X- and Y-axis, respectively.
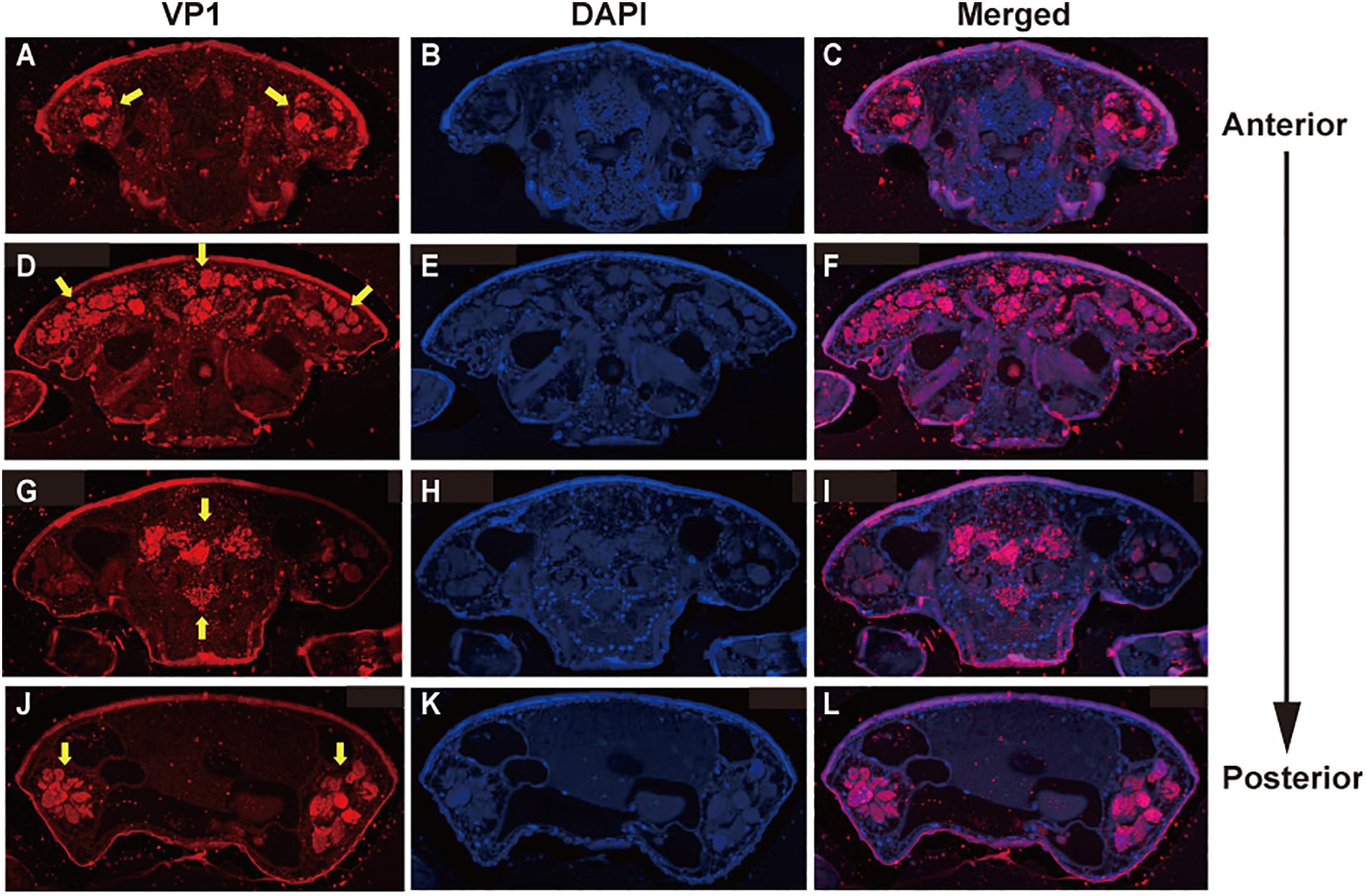
Figure 3. Localization of DWV in T. mercedesae. Transverse sections of T. mercedesae were immunostained by anti-VP1 antibody (VP1: A,D,G,J) as well as DAPI (B,E,H,K). The merged images (Merged: C,F,I,L) are also shown. VP1-positive large dense spheres are indicated by yellow arrows. The dorsal side of mite is up and the anterior to posterior direction of mite body is also shown.
Hymenoptaecin and Defensin-1 mRNAs Are Induced in Honey Bee Pupae by Infestation of T. mercedesae
To understand the effects of T. mercedesae infestation on honey bees, we quantified mRNAs of two AMPs, Hymenoptaecin and Defensin-1, in the above pupae artificially infested with T. mercedesae and the control (uninfested) pupae. We focused on Defensin-1 and Hymenoptaecin because they are induced in pupae by V. destructor infestation (Kuster et al., 2014) and under the control of the Toll and Imd pathways, respectively (Schlüns and Crozier, 2007; Lourenço et al., 2018). Thus, they represent the downstream effectors activated by Toll and Imd pathways. As shown in Figures 4A,B, both Hymenoptaecin and Defensin-1 mRNAs were increased by mite infestation. However, there was no significant correlation between the amounts of Hymenoptaecin and Defensin-1 mRNAs expressed in individual mite infested pupae (r = −0.08, P < 0.78; Figure 4C), suggesting that these AMPs were induced by different mechanisms during mite infestation. We then tested the correlation of DWV copy numbers and the amounts of either Hymenoptaecin or Defensin-1 mRNA in both control and test pupae. The amount of Defensin-1, but not Hymenoptaecin (r = 0.141, P < 0.45) mRNA, was positively correlated with DWV copy number (Figure 4D), suggesting that Defensin-1 is induced by DWV infection and replication. We next measured the relative amounts of honey bee 18S rRNA in the individual mites to determine the degree of feeding of honey bee cells in fat body (Ramsey et al., 2019) and hemolymph. A positive correlation was evident between the ingestion of honey bee cells by the mite and the amount of Hymenoptaecin mRNA, but there was no correlation for Defensin-1 mRNA (r = 0.4648, P < 0.83; Figure 4E).
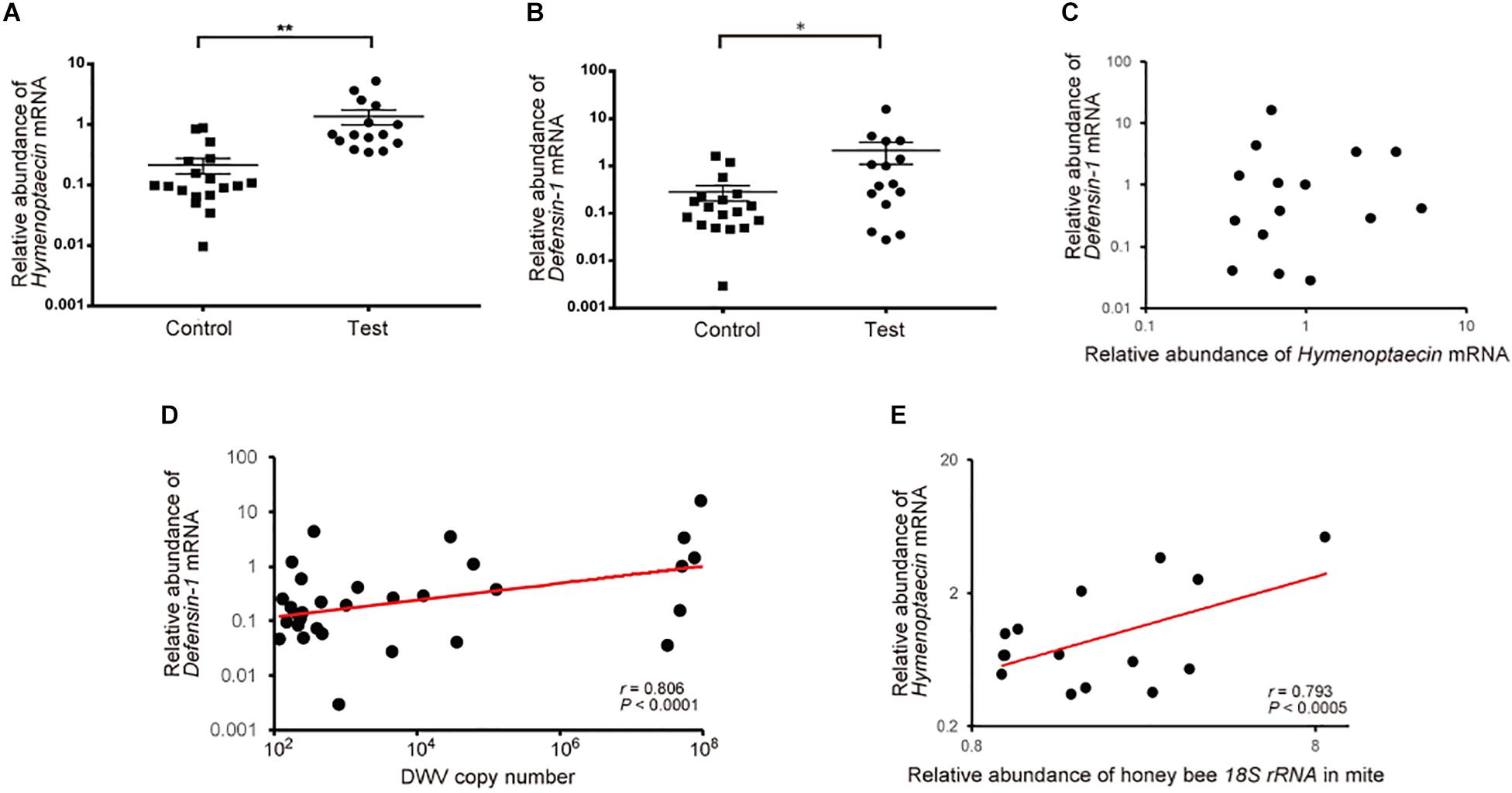
Figure 4. Increase of Hymenoptaecin and Defensin-1 mRNAs by T. mercedesae infestation. Relative amounts of Hymenoptaecin (A) and Defensin-1 (B) mRNAs in honey bee pupae artificially infested by T. mercedesae (Test, n = 15) and the ones without mites (Control, n = 18). Mean values ± SEM (error bars) are shown. Brunner–Munzel test was used for the statistical analysis (Hymenoptaecin: **P < 3.1E-08; Defensin-1: *P < 0.042). (C) Relative amounts of Hymenoptaecin and Defensin-1 mRNAs in individual honey bee pupae are plotted on the X- and Y-axis, respectively. (D) DWV copy number and relative amount of Defensin-1 mRNA in individual honey bee pupae are plotted on the X- and Y-axis, respectively. The red line represents a fitted curve and the Pearson correlation value and P-value are also shown. (E) Relative amounts of Hymenoptaecin mRNA in individual honey bee pupae and honey bee 18S rRNA in the infesting mites are plotted on the Y- and X-axis, respectively. The red line represents a fitted curve and the Pearson correlation value and P-value are also shown.
Hymenoptaecin Down-Regulates T. mercedesae Vg Gene
Since immune effector molecules, such as AMPs, are induced in honey bee pupae by Tropilaelaps mite infestation, the molecules could affect the physiology of the pupa, but also the mite, by feeding on the fat body and other tissues. Vg mRNA decreases with high load of DWV in Tropilaelaps mite by the transcriptome analysis (Supplementary Table S4). Five Vg related genes were identified in T. mercedesae genome (Accession numbers: OQR67440.1, OQR68606.1, OQR72029.1, OQR72561.1, and OQR79705.1). Based on the phylogenetic tree of V. destructor and T. mercedesae Vgs, OQR72561.1, OQR68606.1, and OQR79705.1 appeared to be T. mercedesae orthologs of V. destructor Vg-1, Vg-2, and XP_022657753.1, respectively. OQR72029.1 and OQR67440.1 were related to V. destructor Vg-1 and Vg-2, respectively (Supplementary Figure S3). We tested the correlation between the amounts of the five Vg mRNAs in T. mercedesae and either Hymenoptaecin or Defensin-1 mRNA in mite infested pupae. There was no correlation between Defensin-1 mRNA and any of the five Vg mRNAs (Supplementary Table S5). However, a negative correlation was evident between Hymenoptaecin mRNA and TmVg-1 or TmVg-2, but not between other Vg mRNAs (Figures 5A,B and Supplementary Table S5). These results suggest that expression of T. mercedesae Vg genes is downregulated by honey bee Hymenoptaecin. To test this hypothesis, we first established Drosophila melanogaster S2 cells expressing honey bee Hymenoptaecin (Supplementary Figure S4), and then fed T. mercedesae with the extracts of S2 cells with or without Hymenoptaecin as previously described for V. destructor (Cabrera et al., 2017). The amounts of the five Vg mRNAs were measured in individual mites and then compared. The experiments were repeated twice and the percentages of fed/survived mites were 35 (mean) ± 7.1 (SD) % for both treatments. Consistent with the above results, TmVg-2 mRNA was significantly reduced in mites fed with the extract of S2 cells expressing Hymenoptaecin (Figure 5C).
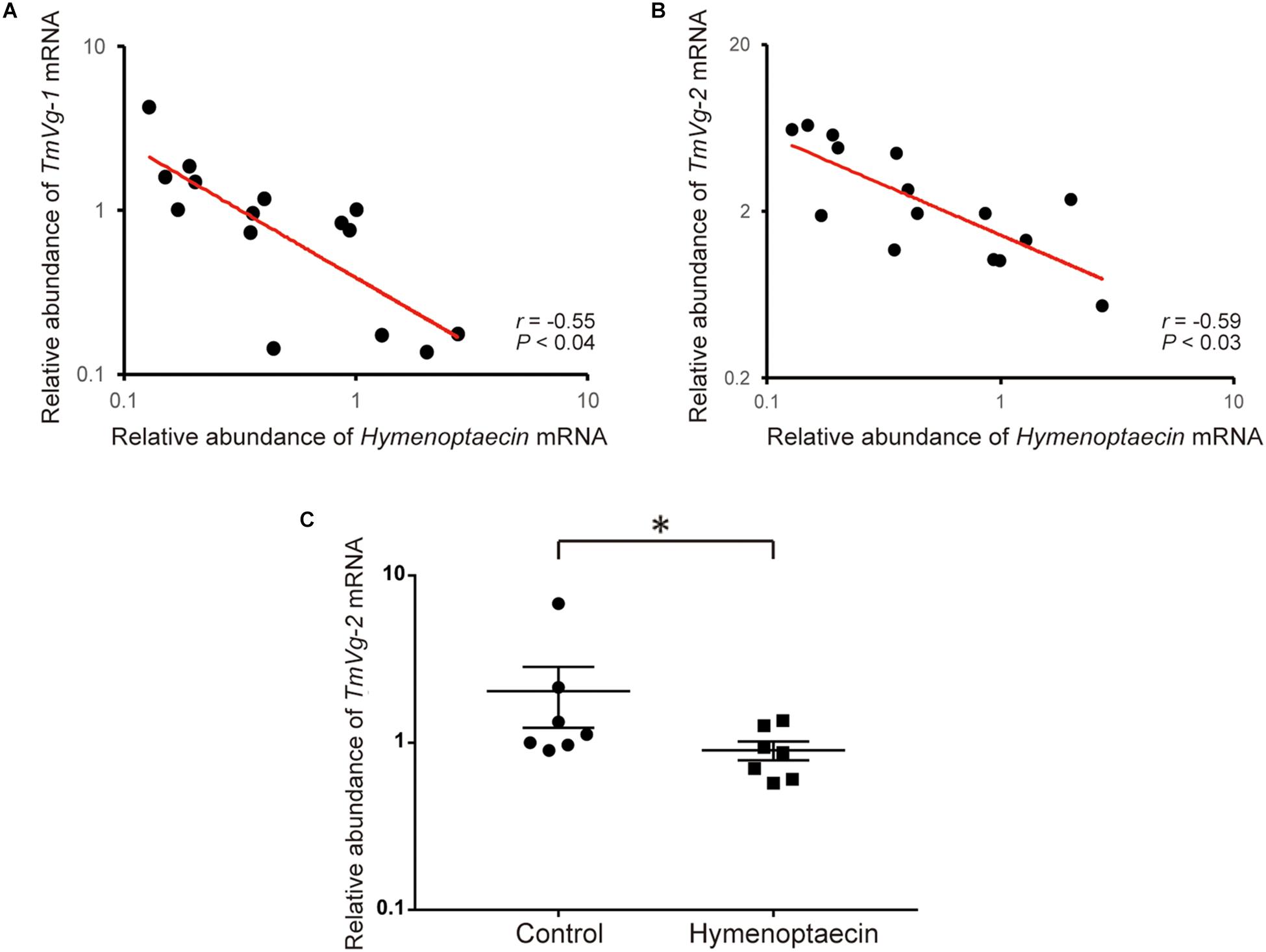
Figure 5. Hymenoptaecin down-regulates T. mercedesae Vg gene. Relative amounts of Hymenoptaecin mRNA in individual honey bee pupae and TmVg-1 (A) or TmVg-2 mRNA (B) in the infesting mites are plotted on the X- and X-axis, respectively. The red line represents a fitted curve and the Pearson correlation value and P-value are also shown. (C) Relative amounts of TmVg-2 mRNA in individual mites fed with the extracts of S2 cells expressing Hymenoptaecin or wild type S2 cells (Control). Mean values ± SEM (error bars) are shown. Brunner–Munzel test was used for the statistical analysis (∗P < 0.025).
Discussion
T. mercedesae Functions as a Vector for DWV Without Active Replication Inside Mite
Our results show that the artificial infestation of honey bee pupae with T. mercedesae increases DWV copy number in pupae, but also transfers the variant present in the mite to the pupa. These results demonstrate that T. mercedesae functions as a bona fide vector for DWV and also stimulates replication of the endogenous DWV in honey bee pupae. These properties are similar to those of V. destructor (Kuster et al., 2014). Replication of the endogenous DWV can be induced by wounds caused by mite feeding. Consistent with previous reports for both T. mercedesae and V. destructor (Wu et al., 2017; Posada-Florez et al., 2019), the copy number of DWV could exceed 106. However, we found that DWV had little effect on the transcriptome of T. mercedesae. Thus, DWV does not appear to significantly affect the fitness of mites. In fact, most DWV is present in the midgut lumen of mite, as previously reported with V. destructor (Zhang et al., 2007; Santillan-Galicia et al., 2008). Furthermore, DWV RdRP was not detected in the mites with high DWV loads by western blotting. These results demonstrate that most of the DWV in the mite is derived from infested honey bee pupae and does not actively replicate inside the mite cells. Furthermore, the large difference in codon usage between DWV and T. mercedesae is unlikely to support the active translation of DWV proteins. Proteomic analysis of T. mercedesae and V. destructor also failed to detect the non-structural proteins of honey bee viruses (Erban et al., 2015; Dong et al., 2017). Previous studies have suggested that the presence of a negative strand of DWV genome RNA in V. destructor is evidence of viral replication (Ongus et al., 2004; Yue and Genersch, 2005). However, this was recently questioned (Posada-Florez et al., 2019). Therefore, our results do not support that the mites actively amplify the specific variant of DWV. The six pupa-mite pairs (Bee/Mite-3, 5, 10, 11, 13, and 15) contained four variants originally derived from the mites, indicating that they were specifically amplified in the honey bee over the endogenous variants. These results suggest that there is a mechanism to amplify the specific variant introduced by mite in honey bee. Since T. mercedesae is capable of transferring the associated variant to honey bee pupae, DWV must be present in the mite salivary gland. Although we did not detect DWV in the salivary gland by immunostaining of the sections, DWV has been previously detected in the salivary gland of V. destructor using mass spectrometry (Zhang and Han, 2019). The migration mechanism of DWV from the midgut to the salivary gland in the mites could be common in Arthropod-borne viruses, such as dengue virus and Zika virus in mosquitos (Cui et al., 2019). It is also possible that selection of DWV variants may occur during migration from the midgut to the salivary gland. The mechanism of DWV transmission by honey bee mites as well as the virus/mite relationship could also help to understand the mechanisms of tick-borne viral diseases, for example, Tick-borne meningoencephalitis (Mansfield et al., 2009), and mosquito-borne virus diseases.
Downregulation of Honey Bee Mite Reproduction by Host Immune Effector
Consistent with previous reports for V. destructor (Gregorc et al., 2012; Kuster et al., 2014), we found that Defensin-1 and Hymenoptaecin mRNAs were induced by Tropilaelapse mite infestation, but by different mechanisms. Our results demonstrate that Defensin-1 and Hymenoptaecin are induced by DWV replication and mite feeding, respectively. Since Defensin-1 and Hymenoptaecin were suggested to be under the control of the Toll and Imd pathways, respectively (Schlüns and Crozier, 2007; Lourenço et al., 2018), these immune signaling pathways would be independently activated by the above events. We also demonstrated a negative correlation between Hymenoptaecin mRNA and TmVg-1 or TmVg-2 mRNA, and further demonstrated that Hymenoptaecin in S2 cell extract down-regulates expression of TmVg-2 mRNA. Vg is a precursor for the major yolk protein vitellin, providing essential nutrients for the embryo (Tufail and Takeda, 2008). Thus, Vg and Vitellogenin receptor are essential for the reproduction of mites, such as Panonychus citri (Ali et al., 2017). Accordingly, in V. destructor, high levels of VdVg-1 and VdVg-2 mRNAs are present at the oviposition stage (Cabrera Cordon et al., 2013; McAfee et al., 2017). A previous study also reported a negative association between the reproductive capability of V. destructor and honey bee immune gene mRNAs, such as Relish, PGRP-S1, and Hymenoptaecin (Kuster et al., 2014). Hymenoptaecin is an AMP that was originally identified as a small positively-charged peptide capable of killing microorganisms by targeting the negatively charged membranes (Casteels et al., 1993). However, the physiological roles of AMPs were not precisely characterized, and recent studies have revealed that they are also critical for tumor elimination, brain function, neurodegeneration, and aging (Hanson and Lemaitre, 2019). Since the Imd pathway is involved in processes that include viral defense, resistance to dessication, resistance to oxygen stress, and autophagy (Zhai et al., 2018), AMPs as well as the other downstream effectors could be involved in these processes. Thus, it is possible that Hymenoptaecin affects the mite tissue(s) to decrease the amount of Vg mRNA. Nevertheless, the mechanism of downregulation as well as the tissues synthesizing Vg remains to be elucidated. Hymenoptaecin can modulate specific cell signaling pathways in mite cells by indirect activation of receptors by displacing ligands, altering membrane microdomains, or directly acting as an alternate ligand. Given the present observation that Hymenoptaecin expressed in S2 cells failed to repress TmVg-1 mRNA, it could be under the control of other downstream effectors of the Imd pathway. Hymenoptaecin induced by mite feeding could exert negative feedback on mite reproduction by repressing Vg synthesis. In fact, fecundity of both T. mercedesae and V. destructor is low (<3 and <7, respectively per reproductive cycle) and significant fractions of non-reproductive mites have also been described (Nazzi and Le Conte, 2016; Chantawannakul et al., 2018). This equilibrium between the host (honey bee) and parasite (mite) could be established by the interaction between Hymenoptaecin and mite Vg. Our study should help further explorations of the novel physiological roles of AMPs in honey bees and other insects.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.
Author Contributions
TK conceived and designed the research strategy. YW, QL, and BW performed the research. YW, MK, and TK analyzed the data and drafted and edited the manuscript.
Funding
This work was supported by Jinji Lake Double Hundred Talents Programme and XJTLU Research Development Fund (RDF-15-01-25) to TK.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Gongjie Wu, Nihao Sun, and Meng Yuan for their contributions to conduct the experiments. We also thank Dr. Ferdinand Kappes for his help to prepare the mite sections. This manuscript has been released as a pre-print at bioRxiv (Wu et al., 2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01037/full#supplementary-material
Footnotes
References
Aizen, M. A., and Harder, L. D. (2009). The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915–918. doi: 10.1016/j.cub.2009.03.071
Ali, M. W., Zhang, Z. Y., Xia, S., and Zhang, H. (2017). Biofunctional analysis of Vitellogenin and Vitellogenin receptor in citrus red mites, Panonychus citri by RNA interference. Sci. Rep. 7:16123. doi: 10.1038/s41598-017-16331-3
Anders, S., Pyl, P. T., and Huber, W. (2015). HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. doi: 10.1093/bioinformatics/btu638
Athey, J., Alexaki, A., Osipova, E., Rostovtsev, A., Santana-Quintero, L. V., Katneni, U., et al. (2017). A new and updated resource for codon usage tables. BMC Bioinformatics 18:391. doi: 10.1186/s12859-017-1793-7
Cabrera, A. R., Shirk, P. D., and Teal, P. E. A. (2017). A feeding protocol for delivery of agents to assess development in Varroa mites. PLoS One 12:e0176097. doi: 10.1371/journal.pone.0176097
Cabrera Cordon, A. R., Shirk, P. D., Duehl, A. J., Evans, J. D., and Teal, P. E. (2013). Variable induction of vitellogenin genes in the varroa mite, Varroa destructor (Anderson & Trueman), by the honeybee, Apis mellifera L, host and its environment. Insect. Mol. Biol. 22, 88–103. doi: 10.1111/imb.12006
Casteels, P., Ampe, C., Jacobs, F., and Tempst, P. (1993). Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J. Biol. Chem. 268, 7044–7054.
Chantawannakul, P., and Cutler, R. W. (2008). Convergent host-parasite codon usage between honeybee and bee associated viral genomes. J. Invertebr. Pathol. 98, 206–210. doi: 10.1016/j.jip.2008.02.016
Chantawannakul, P., Ramsey, S., vanEngelsdorp, D., Khongphinitbunjong, K., and Phokasem, P. (2018). Tropilaelaps mite: an emerging threat to European honey bee. Curr. Opin. Insect. Sci. 26, 69–75. doi: 10.1016/j.cois.2018.01.012
Cui, Y., Grant, D. G., Lin, J., Yu, X., and Franz, A. W. E. (2019). Zika Virus Dissemination from the midgut of Aedes aegypti is facilitated by bloodmeal-mediated structural modification of the midgut basal lamina. Viruses 11:1056. doi: 10.3390/v11111056
de Miranda, J. R., and Genersch, E. (2010). Deformed wing virus. J. Invertebr. Pathol. 103, S48–S61. doi: 10.1016/j.jip.2009.06.012
Dong, X., Armstrong, S. D., Xia, D., Makepeace, B. L., Darby, A. C., and Kadowaki, T. J. G. (2017). Draft genome of the honey bee ectoparasitic mite, Tropilaelaps mercedesae, is shaped by the parasitic life history. Gigascience 6:gix008. doi: 10.1093/gigascience/gix008
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Egekwu, N. I., Posada, F., Sonenshine, D. E., and Cook, S. (2018). Using an in vitro system for maintaining Varroa destructor mites on Apis mellifera pupae as hosts: studies of mite longevity and feeding behavior. Exp. Appl. Acarol. 74, 301–315. doi: 10.1007/s10493-018-0236-0
Erban, T., Harant, K., Hubalek, M., Vitamvas, P., Kamler, M., Poltronieri, P., et al. (2015). In-depth proteomic analysis of Varroa destructor: detection of DWV-complex, ABPV, VdMLV and honeybee proteins in the mite. Sci. Rep. 5, 1–16. doi: 10.1038/srep13907
Forsgren, E., De Miranda, J. R., Isaksson, M., Wei, S., Fries, I. J. E., and Acarology, A. (2009). Deformed wing virus associated with Tropilaelaps mercedesae infesting European honey bees (Apis mellifera). Exp. Appl. Acarol. 47, 87–97. doi: 10.1007/s10493-008-9204-4
Goulson, D., Nicholls, E., Botías, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. doi: 10.1126/science.1255957
Gregorc, A., Evans, J. D., Scharf, M., and Ellis, J. D. (2012). Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect. Physiol. 58, 1042–1049. doi: 10.1016/j.jinsphys.2012.03.015
Hanson, M. A., and Lemaitre, B. (2019). New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 62, 22–30. doi: 10.1016/j.coi.2019.11.008
Highfield, A. C., El Nagar, A., Mackinder, L. C., Noël, L. M., Hall, M. J., Martin, S. J., et al. (2009). Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 75, 7212–7220. doi: 10.1128/AEM.02227-09
Jiang, P., Liu, Y., Ma, H. C., Paul, A. V., and Wimmer, E. (2014). Picornavirus morphogenesis. Microbiol. Mol. Biol. Rev. 78, 418–437.
Khongphinitbunjong, K., De Guzman, L. I., Tarver, M. R., Rinderer, T. E., and Chantawannakul, P. (2015). Interactions of Tropilaelaps mercedesae, honey bee viruses and immune response in Apis mellifera. J. Apic. Res. 54, 40–47.
Kim, D., Langmead, B., and Salzberg, S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12:357. doi: 10.1038/nmeth.3317
Klein, A. M., Vaissière, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Koelle, M. R., Talbot, W. S., Segraves, W. A., Bender, M. T., Cherbas, P., and Hogness, D. S. (1991). The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 67, 59–77. doi: 10.1016/0092-8674(91)90572-g
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Kuster, R. D., Boncristiani, H. F., and Rueppell, O. (2014). Immunogene and viral transcript dynamics during parasitic Varroa destructor mite infection of developing honey bee (Apis mellifera) pupae. J. Exp. Biol. 217(Pt 10), 1710–1718. doi: 10.1242/jeb.097766
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Lourenço, A. P., Florecki, M. M., Simões, Z. L. P., and Evans, J. D. (2018). Silencing of Apis mellifera dorsal genes reveals their role in expression of the antimicrobial peptide defensin-1. Insect. Mol. Biol. 27, 577–589. doi: 10.1111/imb.12498
Mansfield, K. L., Johnson, N., Phipps, L. P., Stephenson, J. R., Fooks, A. R., and Solomon, T. (2009). Tick-borne encephalitis virus - a review of an emerging zoonosis. J. Gen. Virol. 90(Pt 8), 1781–1794. doi: 10.1099/vir.0.011437-0
Martin, S., Highfield, A., Brettell, L., Villalobos, E., Budge, G., Powell, M., et al. (2012). Global honey bee viral landscape altered by a parasitic mite. Science 336, 1304–1306. doi: 10.1126/science.1220941
McAfee, A., Chan, Q. W. T., Evans, J., and Foster, L. J. (2017). A Varroa destructor protein atlas reveals molecular underpinnings of developmental transitions and sexual differentiation. Mol. Cell Proteom. 16, 2125–2137. doi: 10.1074/mcp.RA117.000104
Nazzi, F., and Le Conte, Y. (2016). Ecology of Varroa destructor, the major ectoparasite of the western honey bee. Apis mellifera. Annu. Rev. Entomol. 61, 417–432. doi: 10.1146/annurev-ento-010715-023731
Ongus, J. R., Peters, D., Bonmatin, J.-M., Bengsch, E., Vlak, J. M., and van Oers, M. M. (2004). Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 85, 3747–3755. doi: 10.1099/vir.0.80470-0
Posada-Florez, F., Childers, A. K., Heerman, M. C., Egekwu, N. I., Cook, S. C., Chen, Y., et al. (2019). Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci. Rep. 2:12445. doi: 10.1038/s41598-019-47447-3
Ramsey, S. D., Ochoa, R., Bauchan, G., Gulbronson, C., Mowery, J. D., Cohen, A., et al. (2019). feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. U.S.A. 116, 1792–1801. doi: 10.1073/pnas.1818371116
Robinson, M. D., McCarthy, D. J., and Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. doi: 10.1093/bioinformatics/btp616
Rosenkranz, P., Aumeier, P., and Ziegelmann, B. (2010). Biology and control of Varroa destructor. J. Invertebr. Pathol. 103, S96–S119. doi: 10.1016/j.jip.2009.07.016
Ryabov, E. V., Wood, G. R., Fannon, J. M., Moore, J. D., Bull, J. C., Chandler, D., et al. (2014). A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 10:e1004230. doi: 10.1371/journal.ppat.1004230
Santillan-Galicia, M. T., Carzaniga, R., Ball, B. V., and Alderson, P. G. (2008). Immunolocalization of deformed wing virus particles within the mite Varroa destructor. J. Gen. Virol. 89, 1685–1689. doi: 10.1099/vir.0.83223-0
Schlüns, H., and Crozier, R. H. (2007). Relish regulates expression of antimicrobial peptide genes in the honeybee, Apis mellifera, shown by RNA interference. Insect. Mol. Biol. 16, 753–759. doi: 10.1111/j.1365-2583.2007.00768.x
Shen, M., Yang, X., Cox-Foster, D., and Cui, L. (2005). The role of varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 342, 141–149. doi: 10.1016/j.virol.2005.07.012
Tentcheva, D., Gauthier, L., Bagny, L., Fievet, J., Dainat, B., Cousserans, F., et al. (2006). Comparative analysis of deformed wing virus (DWV) RNA in Apis mellifera and Varroa destructor. Apidologie 37, 41–50. doi: 10.1051/apido:2005057
Tufail, M., and Takeda, M. (2008). Molecular characteristics of insect vitellogenins. J. Insect. Physiol. 54, 1447–1458.
Wu, Y., Dong, X., and Kadowaki, T. (2017). Characterization of the Copy Number and Variants of Deformed Wing Virus (DWV) in the pairs of honey bee pupa and infesting. Front. Microbiol. 8:1558. doi: 10.3389/fmicb.2017.01558
Wu, Y., Liu, Q., Weiss, B., Kaltenpoth, M., and Kadowaki, T. (2020). Honey bee suppresses the parasitic mite Vitellogenin by antimicrobial peptide. bioRxiv [Preprint] doi: 10.1101/2020.02.29.970913v1
Yue, C., and Genersch, E. (2005). RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86(Pt 12), 3419–3424. doi: 10.1099/vir.0.81401-0
Zhai, Z., Huang, X., and Yin, Y. (2018). Beyond immunity: the Imd pathway as a coordinator of host defense, organismal physiology and behavior. Dev. Comp. Immunol. 83, 51–59. doi: 10.1016/j.dci.2017.11.008
Zhang, Q., Ongus, J. R., Boot, W. J., Calis, J., Bonmatin, J.-M., Bengsch, E., et al. (2007). Detection and localisation of picorna-like virus particles in tissues of Varroa destructor, an ectoparasite of the honey bee. Apis mellifera. J. Invertebr. Pathol. 96, 97–105. doi: 10.1016/j.jip.2007.03.019
Keywords: host-parasite/pathogen interaction, vector-pathogen interaction, honey bee, parasitic mite, deformed wing virus, antimicrobial peptide, Vitellogenin
Citation: Wu Y, Liu Q, Weiss B, Kaltenpoth M and Kadowaki T (2020) Honey Bee Suppresses the Parasitic Mite Vitellogenin by Antimicrobial Peptide. Front. Microbiol. 11:1037. doi: 10.3389/fmicb.2020.01037
Received: 25 March 2020; Accepted: 27 April 2020;
Published: 25 May 2020.
Edited by:
Akio Adachi, Kansai Medical University, JapanReviewed by:
Nor Chejanovsky, Agricultural Research Organization (ARO), IsraelMarcelo Lisandro Signorini, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Eugene Ryabov, United States Department of Agriculture (USDA), United States
Copyright © 2020 Wu, Liu, Weiss, Kaltenpoth and Kadowaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatsuhiko Kadowaki, VGF0c3VoaWtvLkthZG93YWtpQHhqdGx1LmVkdS5jbg==
 Yunfei Wu
Yunfei Wu Qiushi Liu
Qiushi Liu Benjamin Weiss
Benjamin Weiss Martin Kaltenpoth
Martin Kaltenpoth Tatsuhiko Kadowaki
Tatsuhiko Kadowaki