- 1Institut de Recherche en Biologie Végétale, Université de Montréal and Jardin Botanique de Montréal, Montreal, QC, Canada
- 2Quebec Research and Development Centre, Agriculture and Agri-Food Canada, Quebec City, QC, Canada
- 3Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, Lethbridge, AB, Canada
- 4Lacombe Research and Development Centre, Agriculture and Agri-Food Canada, Lacombe, AB, Canada
- 5AgroBiosciences, Mohammed VI Polytechnic University, Ben Guerir, Morocco
The rhizosphere hosts a complex web of prokaryotes interacting with one another that may modulate crucial functions related to plant growth and health. Identifying the key factors structuring the prokaryotic community of the plant rhizosphere is a necessary step toward the enhancement of plant production and crop yield with beneficial associative microorganisms. We used a long-term field experiment conducted at three locations in the Canadian prairies to verify that: (1) the level of cropping system diversity influences the α- and β-diversity of the prokaryotic community of canola (Brassica napus) rhizosphere; (2) the canola rhizosphere community has a stable prokaryotic core; and (3) some highly connected taxa of this community fit the description of hub-taxa. We sampled the rhizosphere of canola grown in monoculture, in a 2-phase rotation (canola-wheat), in a 3-phase rotation (pea-barley-canola), and in a highly diversified 6-phase rotation, five and eight years after cropping system establishment. We detected only one core bacterial Amplicon Sequence Variant (ASV) in the prokaryotic component of the microbiota of canola rhizosphere, a hub taxon identified as cf. Pseudarthrobacter sp. This ASV was also the only hub taxon found in the networks of interactions present in both years and at all three sites. We highlight a cohort of bacteria and archaea that were always connected with the core taxon in the network analyses.
Introduction
A plant in its natural environment coexists with myriads of archaea, bacteria, fungi, as well as with other unicellular eukaryotic microorganisms that constitute its microbiota. The rhizosphere is a hotspot of microbial interactions between species that have various ecological functions. These microbial communities are particularly important for plant health as they influence its development and its productivity (Barriuso et al., 2008; Bulgarelli et al., 2013; Bakker et al., 2014). Throughout their life, plant roots exude compounds creating the rhizosphere environment (Bais et al., 2006). Spatial and temporal variation in rhizodeposition allows plants to shape their rhizosphere microbial communities to their benefit (Tkacz et al., 2015; Pii et al., 2016; Eisenhauer et al., 2017).
Plant rhizosphere can host mutualistic microbes such as mycorrhiza or plant growth promoting bacteria (PGPB) that facilitate nutrient uptake, mitigate abiotic stress, and prevent root infection by pathogens (Barriuso et al., 2008; Farina et al., 2012; Fincheira and Quiroz, 2018). Plant-microbe and microbe-microbe interactions are diverse. Plants live in symbiotic and commensal relationships with numerous organisms, but they must also face pathogenic attacks (Hajishengallis et al., 2012). Rhizosphere organisms may influence each other, thus forming a complex web of interactions. For example, we know that mycorrhizal fungi have their own bacterial microbiota (Bianciotto et al., 2003; Iffis et al., 2014, 2017). These bacteria can be endophytic or form biofilm at the surface of the hyphae and can facilitate symbiosis formation in plants (Fitter and Garbaye, 1994; Iffis et al., 2014; Taktek et al., 2017).
Since the last decade, new generation sequencing (NGS) improved our access to microbial genetic information leading to significant advances in microbial ecology. This technological improvement lead to new ways of analyzing plant microbial communities (Duffy et al., 2007; Bulgarelli et al., 2013; Mendes et al., 2015). Now, we can identify with confidence the factors shaping the microbial communities of the rhizosphere (Kuramae et al., 2011; Agler et al., 2016). The microbiome of the rhizosphere is extremely large and diverse. To summarize this complexity, we can divide it into pools of microbes based on their functions or occurrence (Ridout and Newcombe, 2016). In a given community, microbial taxa are likely to be favored by their host plant throughout its existence (Rout, 2014). These taxa are expected to be always part of the plant microbiota at a defined time t, regardless of environmental conditions. According to Vandenkoornhuyse et al. (2015), the taxa always present in association with the plant forms the core microbiome and have preferential interaction with their host. The definition of a pool of microorganisms always present at t time in the plant microbiota is appropriate for most ecological studies concerning the plant microbiota as they mostly rely on a single sampling time. However, it was necessary to consider temporal variation in our definition of the core microbiota, and this is what we did in this study.
The interactions between microbes in the plant rhizosphere remains largely obscure. Next Generation Sequencing techniques can provide information on the abundance of the taxa interacting in a microbiome, but cannot reveal the biochemistry of interacting microbes in the ecosystem. That is why computational approaches aiming at identifying the nature of the links between the variations in the abundance of microbial taxa were developed as a complement to NGS (Ings et al., 2009; Deng et al., 2012; van der Heijden and Hartmann, 2016). Network analysis allows us to identify microbial taxa that are functionally linked to others within the microbiome. Highly connected microorganisms may have a greater impact on plants and ecosystem functioning than others, because they theoretically interact with many partners and antagonists; these highly interacting species are named hub taxa (Agler et al., 2016). Interactions occurring in microbial communities are known to be complex and difficult to retrieve with usual statistical methods (Kurtz et al., 2015). However, the information provided by NGS can be processed through network analysis to identify cohorts represented by hub taxa. Simplifying the study of complex microbiome, Taktek et al. (2017) showed taxa that recruit organisms beneficial to the host plant, but hub taxa could also be pathogens. Some hub taxa in the human microbiome can articulate infection by consortia of pathogens (Hajishengallis et al., 2012). As pathogens can affect the plant microbiome, pathogenic hub taxa may occur in the rhizosphere. The hub taxa are a useful concept and help to understand the ecology of the root and rhizosphere ecosystems, which could lead to the development of applications in crop plant root systems.
Canola was shown to possess a specific bacterial component of the core microbiota conserved across the Canadian prairie (Lay et al., 2018). Floc’h et al. (2020) reported the temporal stability of the fungal component of the core microbiota in canola rhizosphere, despite considerable changes in the plant rhizosphere microbiota across years. In the present study, we aimed to test if the bacterial component of the canola microbiota has a similar pattern of temporal variation. We investigated the temporal stability of the bacterial component of the core canola rhizosphere microbiota in order to ascertain whether a persistent bacterial component exists. Another aim was to determine if the canola rhizosphere harbors bacterial hub taxa, and to visualize the variation between years in the structure of interactions among the bacteria living in the canola rhizosphere microbiota. We sought to identify a universal bacterial component of the core microbiota in the rhizosphere of a plant species, specifically canola grown over the years under a range of climatic conditions and biological environments. We thus used a gradient of crop diversification levels to create variation in the biological environment of rhizosphere soil and examine over two years what in the bacterial component of the canola microbiota is invariable: the core microbiota. Canola is a crop of economical importance for Canada. It is also a good model plant to study the rhizosphere microbiome as canola produce antimicrobial isocyanates (Zheng et al., 2014) leading to simpler microbial communities in its rhizosphere (Rumberger and Marschner, 2003).
Materials and Methods
Three sites located in three pedoclimatic zones of the canola-producing area of western Canada were used. Two sites were in Alberta, specifically in Lacombe (lat. 52.5°N, long. 113.7°W) and Lethbridge (lat. 49.7°N, long. 112.8°W), and the third site was in Scott, Saskatchewan (lat. 52.4°N, long 108.8°W). The soil in Lethbridge is a Brown Chernozem with a silty loam texture, while the Dark Brown Chernozems have a loamy texture at the Scott site and a clay loam texture at Lacombe.
Plots of a larger long-term crop rotation experiment initiated in 2008 were used for this study. Site description, experimental design and sampling methods are described in details in Floc’h et al. (2020). This study had a complete randomized block design replicated at three geographic sites, each with 4 blocks and 4 crop rotation treatments, and we collected samples from the Roundup Ready (RR) canola phase of the crop rotations on 2 years, in 2013 and 2016. The four treatments were four levels of cropping system diversification: (1) monoculture of RR canola, (2) wheat-RR canola, (3) pea-barley-RR canola, and (4) lentil-wheat-Liberty Link canola-pea-barley-RR canola (Table 1). Crops were grown according to best management practices. Information on crop management is described in Harker et al. (2015).
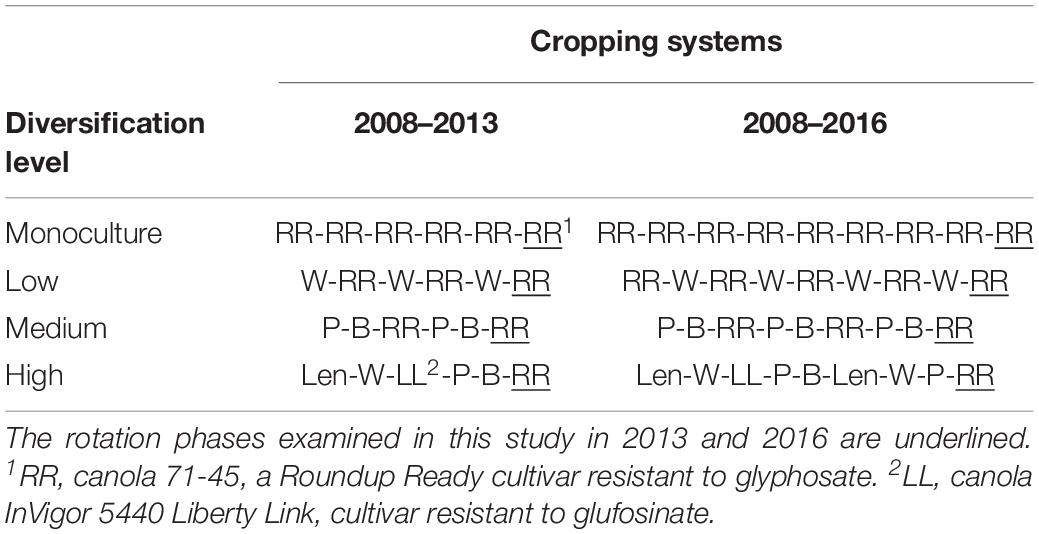
Table 1. Selected treatments from a long-term experiment established in 2008 at three different sites in the Canadian prairies (Harker et al., 2015).
Rhizosphere samples were collected during the fourth week of July in 2013 and 2016, which corresponds to the end of canola flowering period. Three to four plants randomly selected within each plot were uprooted with a shovel. The shoots were removed and roots were placed in plastic bags and brought to the laboratory on ice in a cooler. About 5 g of rhizosphere soil per plot was collected by gently brushing the roots. The samples were kept at 4°C before being shipped on ice to Lethbridge, Alberta, where they were preserved at -80°C until DNA extraction.
DNA Extraction and Amplification
DNA extraction was conducted as described in Floc’h et al. (2020). We constructed amplicon libraries for bacterial 16S rRNA gene sequences by using target-specific PCR primers attached to Illumina overhang sequences for NextEra library preparation. The primer pairs were GTGCCAGCMGCCGCGGTAA (515F-Illu) and GGACTACHVGGGTWTCTAAT (806R-Illu). This primer set was selected because it is used by the Earth Microbiome Project.1 Two PCR reactions were performed to prepare the amplicon library. In the first PCR reaction, the V4 hypervariable region of prokaryotic 16S RNA genes was amplified using primers previously described (515F and 806R). The PCR reaction was performed in a 25-μL reaction mixture containing 1 μL of template DNA, 1 × PCR-buffer (Qiagen, Germantown, MD, United States), 1.8 mM MgCl2, 1.25 μL of 5% dimethylsulfoxide (DMSO), 0.2 mM dNTP, 0.5 U Taq DNA polymerase (Roche, Branford, CT, United States), and 0.6 μM of each primer. The 5′ ends of the forward and reverse primers were tagged with CS1 (ACACTGACGACATGGTTCTACA) and CS2 (TACGGTAGCAGAGACTTGGTCT), respectively, which were used as anchors for the PCR reaction. The conditions to amplify the prokaryotic 16S rRNA fragments consisted of an initial denaturation at 94°C for 2 min, 33 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s and elongation at 72°C for 30 s, followed by a final elongation at 72°C for 7 min.
The second PCR reaction was used to add barcodes to each sample and the Illumina sequencing adapters. This PCR reaction was performed in a 20-μl reaction mixture, containing 1 × PCR-buffer (Qiagen, Germantown, MD, United States), 1.8 mM MgCl2, 1 μl of 5% DMSO, 0.2 mM dNTP, 0.5 U Taq DNA polymerase (Roche, Branford, CT, United States), 2 μM of NextEra XT index primers (Illumina Inc., San Diego, CA, United States), and 1 μL of 1/150 dilution of the first PCR products. The PCR conditions were as follows: initial denaturation at 95°C for 10 min, 15 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and elongation at 72°C for 1 min followed by a final elongation at 72°C for 3 min. After the second amplification, PCR products were quantified using Quant-iTTM PicoGreen® dsDNA Assay Kit (Life Technologies, Canada) and the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (D-Mark, Canada). The amplicon library was purified using calibrated AMPure XP beads (Agencourt, United States), and the average size and quantity of each library were assessed on the LabChip GX (Perkin Elmer, United States) instrument. The library was then sequenced on Illumina MiSeq using the paired-end 250 protocol at Génome Québec Innovation Centre at McGill University (Montreal, Canada).
ASV Determination and Bioinformatic Pipeline
The bioinformatic pipeline used for the processing of our 16S rRNA gene sequences from 2013 and 2016 was DADA2 v1.8 (Callahan et al., 2016). We first used Cutadapt 1.13 to remove the primer part of the 16S rRNA gene sequences. Then, we excluded the sequences with less than 200 bp as the base quality of the sequences tended to diminish below that threshold in our data with the command “filterAndTrim” with a “maxEE” score of 2, “trunQ” score of 2 and “minLen” argument set to 50. Then, we calculated the error rate using the machine learning algorithm implemented in DADA2 with the command “learnErrors.” As the error rate was satisfying according to developer’s recommendations, we merged the forward and reverse sequences using the command “mergePairs.” Afterward, the Amplicon Sequence Variant (ASV) table was calculated and the chimeras eliminated using the command “makeSequenceTable,” resulting in a sequence length ranging from 250 to 253 nucleotides. ASVs were then identified using the naïve Bayesian classifier method on the databases SILVA and RDP, and the identity of ASVs of interest was verified manually using BLAST on the NCBI nt database. With the taxonomic resolution of the 16S RNA gene, it is generally not possible to identify a bacterium at the species level. Thus, the identifications at species level presented here must be consider with caution despite they perfectly match (100% similarity and coverage) the reference sequences of NCBI.
The MiSeq sequencing data generated as part of this work are publicly available on Zenodo.2
Data Processing and Statistical Analyses
We first wanted to assess the variation occurring in canola rhizosphere caused by the crop diversification systems. The dataset was standardized by randomly subsampling the read data from each sample to the lowest number of reads (13 241) encountered for a sample, using the function “rrarefy” of the vegan package v.2.4.6 in R v. 3.4.3, before calculating Chao1 (Chao, 1984), Shannon and Simpson’s α-diversity indices using the same package.
The significance of crop diversification effect on α-diversity indices was tested by analysis of variance (ANOVA) one year at a time, combining sites and blocks in one random effect with 12 blocks (four blocks per each of the three sites), and comparisons between treatment means were made with Tukey’s post-hoc tests using the R package agricolae v1.3.1 (Mendiburu, 2015). The effect of crop diversification on bacterial community structure was assessed by permutational multivariate analysis of variance (PERMANOVA) (Anderson, 2001), considering 12 blocks (four blocks per each of the three sites), using the function “adonis” of the vegan package v 2.4.6 (Oksanen et al., 2019) in R v3.4.3, and the entire (non-subsampled) set of relative abundance data. The blocked multi-response permutation procedure (BMRPP) was used for pairwise comparison of community structure under the different crop diversification treatments. using Šidák correction for pairwise comparison in the R package “RVAideMemoire” v0.9 (Hervé, 2015).
After determining the impact of crop diversification on canola rhizosphere, we aimed at identifying its universal bacterial component of the core microbiota and hub taxa. We defined the core microbiota as the set of organisms that are present in the microbiota at all sites and plots at t and t+1. To assess the interactions among bacterial taxa in the microbiota, we created a co-occurrence network using the package SPIEC-EASI v 1.0.6 in R 3.4.3 (Kurtz et al., 2015). The analysis was conducted over all bacterial rhizosphere communities of each year. The input data consisted in the matrix of the raw abundance of ASVs of one year of sampling. We first filtered the dataset to remove the ASVs with a frequency less than 20%. The SPIEC-EASI run was done with the algorithm “mb” with the lambda min ratio set at 10–2 and 50 repetitions. We then imported the networks in Cytoscape 3.7.1 (Smoot et al., 2011) for plotting and used the “organic” layout to draw the network. Edges where defined as co-occurrences or mutual exclusion regarding the positives or negatives values of inverse covariance linking the nodes. Betweenness centrality, defined as the fraction of the shortest path between all other nodes in the network containing the given node, and degree score, highlight central nodes and provide information about network architecture. A score of betweenness centrality and degree of connectivity greater than the score of 95% of the network taxa could suggest participation in multipartite interactions in the community and allow us to flag the highly connected taxa as hub-taxa. Hub-taxa were defined as the nodes possessing a score of betweenness centrality > 0.40 and a degree score > 10.
Spearman’s correlations between abundance of hub-taxa and of their cohorts with canola yield were computed on R 3.4.3.
Results
Taxonomic Affiliation of the Bacterial Component of the Canola Rhizosphere Microbiota
Our bioinformatic pipeline retrieved 2 175 992 reads from the 96 samples, that were assigned to 10 385 ASVs. Read number per sample ranged from 10 938 to 60 896. The ASVs belong mostly to four bacterial phyla that did not vary substantially in abundance in the two years of study: Proteobacteria (25%), Actinobacteria (22.5%), Acidobacteria (16%), and Chloroflexi (13%) (Figure 1). Rarefaction curves indicated that read abundances were close to saturation for all the samples (Supplementary Figure S2).
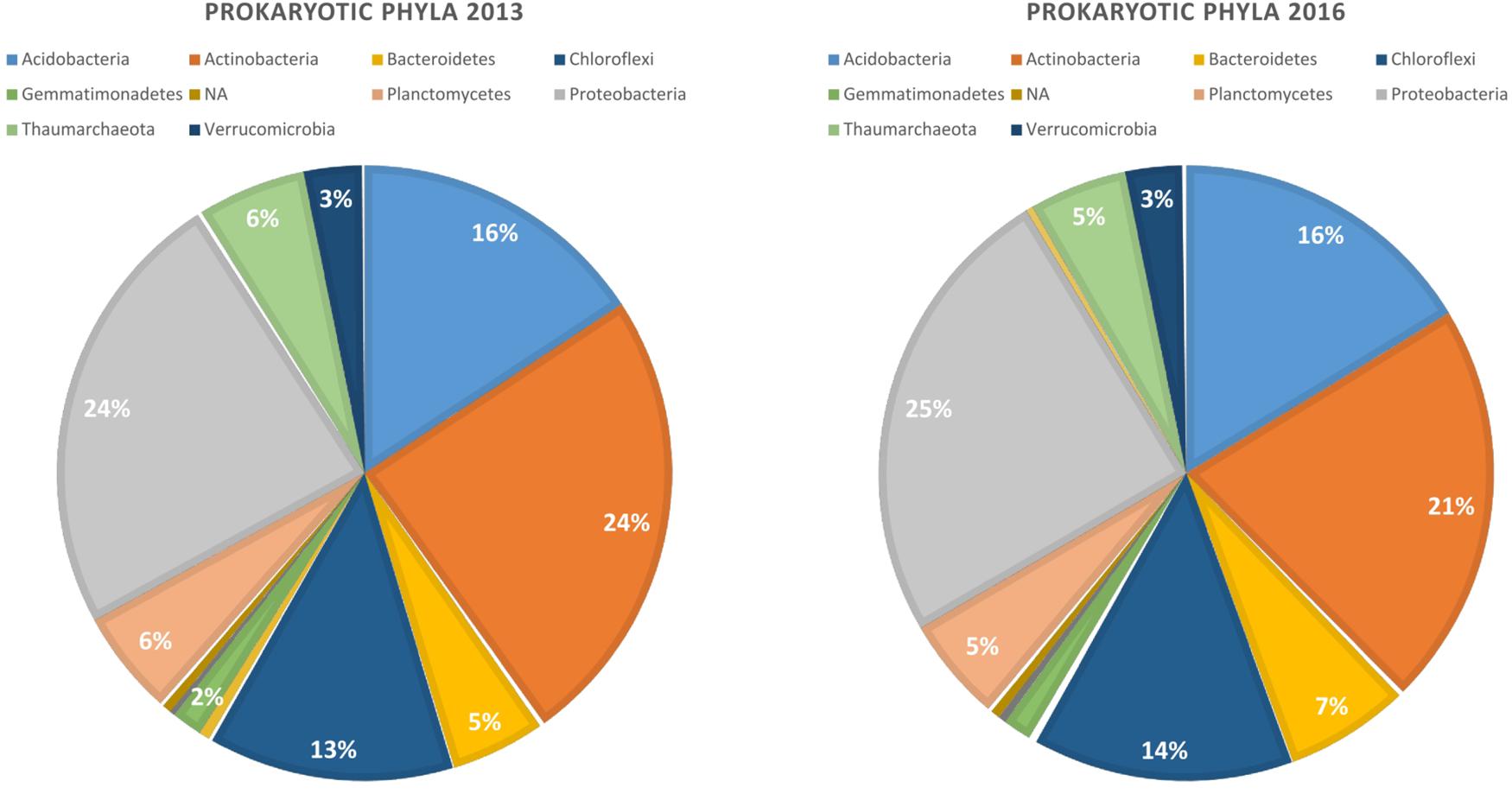
Figure 1. Relative abundance of dominant bacterial phyla in the rhizosphere of canola in 2013 and 2016.
Effect of Treatments on Communities
Crop diversification had no significant influence on α-diversity indices (Table 2) or on the structure of bacterial communities of canola rhizosphere in 2013 (Table 3). On the other hand, crop diversification significantly affected the community structure of canola rhizosphere in 2016 by PERMANOVA (P = 0.047), where the rhizosphere communities of canola in monoculture and in the highly diversified system were structurally different according to the BMRPP test (Table 3). PCoA analyses showed a clear segregation of prokaryotic communities by site, but did not show clear patterns between diversification levels either in 2013 and 2016 (Supplementary Figures S3, S4). Since the prokaryotic communities segregated per site, additional PERMANOVA were conducted to assess whether, within each site, the crop diversification level has an effect on the microbiota structure. Results did not show any differential effect of crop diversification per site and year (Table 3). Indicator species analysis revealed ASVs significantly associated with crop diversifications. In 2013, the highest level of crop diversification had the highest number of indicator species (15), whereas monoculture had nine and the diversification treatment with wheat and canola had only one (Table 4). No indicator species was found in association with the medium crop diversification level in 2013. In 2016, monoculture showed the highest number of indicator species with 26 ASVs, the low crop diversification had four and the medium diversification had one. No indicator species were found in association with the highest level of crop diversification in 2016. ASV108 (cf. Thermomicrobiales sp.) was and indicator species of the monoculture in 2013 and 2016; it is also the only indicator species to be found in both years of sampling.
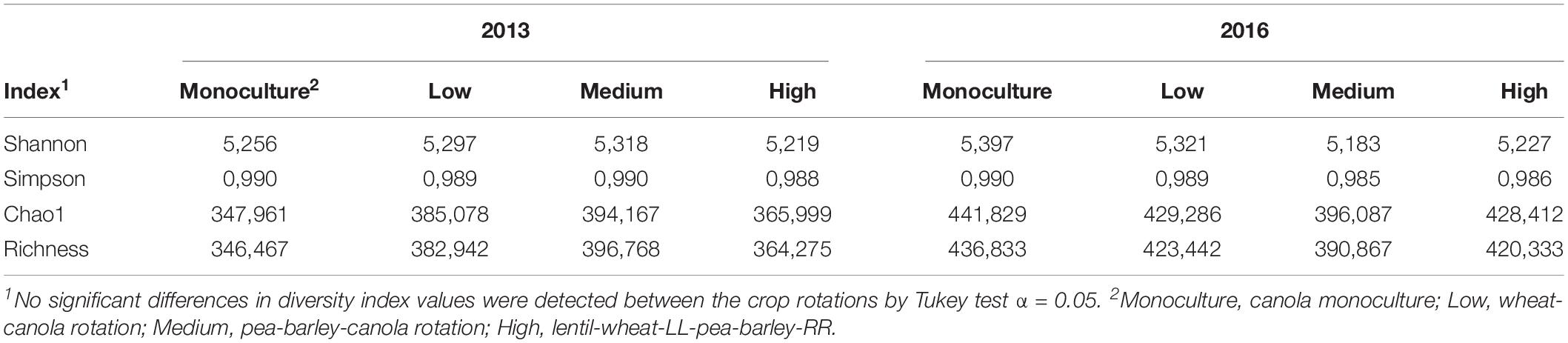
Table 2. Mean values of bacterial α-diversity indices in the rhizosphere of canola under different crop diversification levels, in 2013 and 2016.
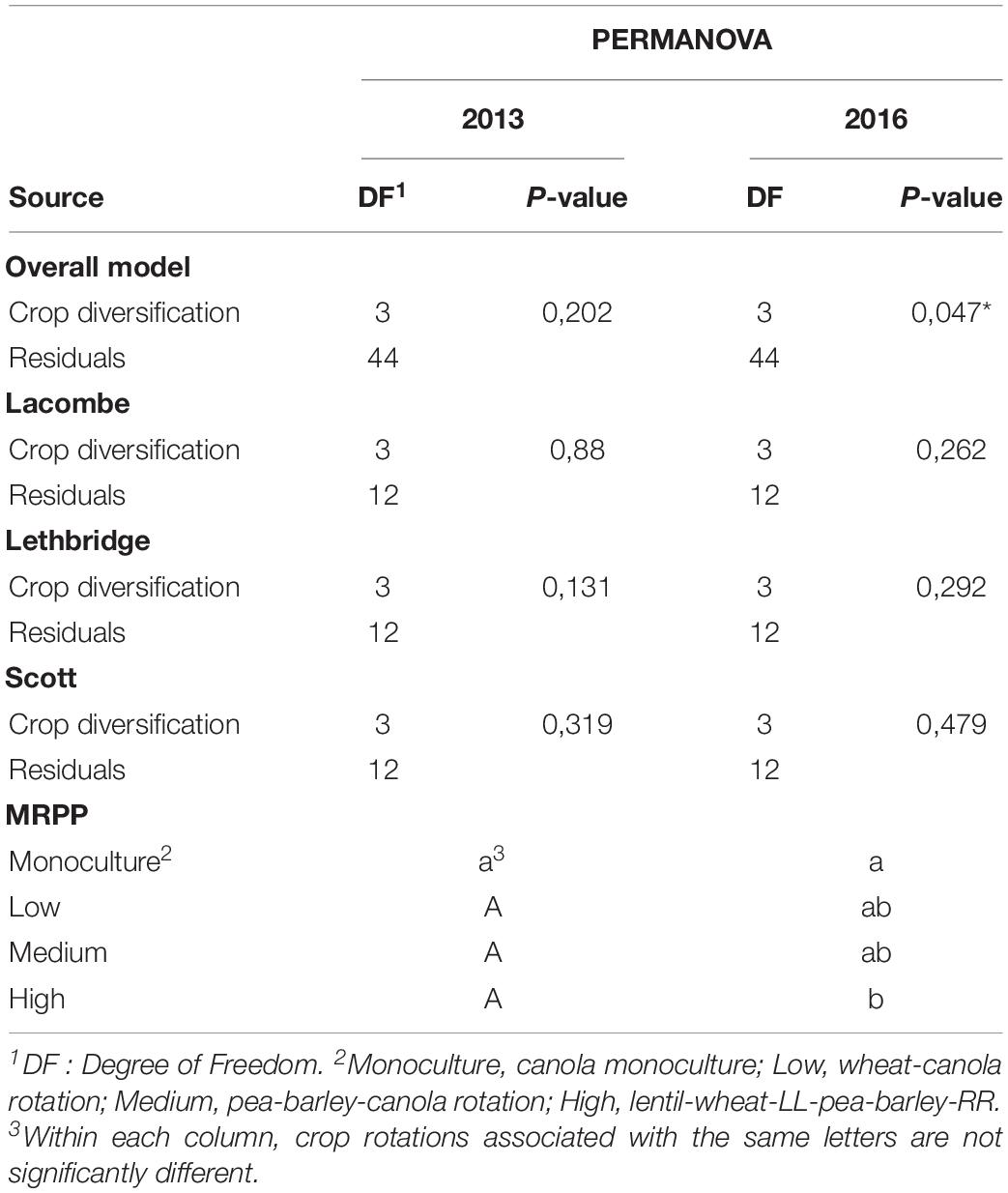
Table 3. Effects of crop diversification on the structure of the bacterial community in the canola rhizosphere, in 2013 and 2016, according to PERMANOVA (α = 0.05, n = 12), and significant differences between the structure of bacterial communities per crop diversification level according to Blocked Multi-Response Permutation Procedures (BMRPP) with Šidák correction for two-way comparisons (α = 0.035, n = 12).
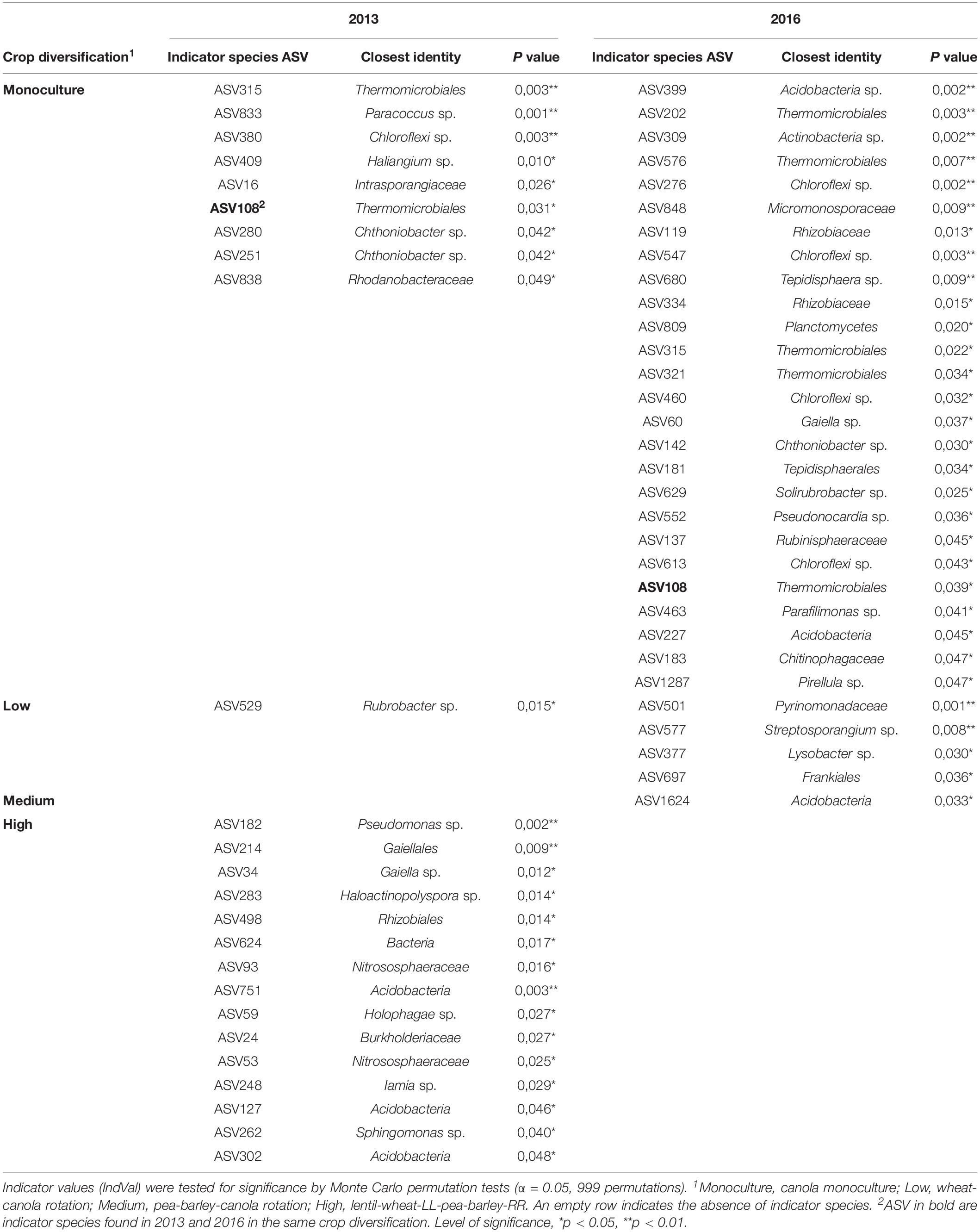
Table 4. Indicator species analysis of the prokaryotic ASV residing in the rhizosphere of canola in response to cropping diversification treatment in 2013 and 2016.
Core Bacterial Component of the Canola Rhizosphere Microbiota
Only one bacterial ASV remained present across all the samples in every crop rotation and both years: ASV1. ASV1 was identified as cf. Pseudarthrobacter sp. according to SILVA and RDP databases and was the most abundant bacterial ASV in the canola rhizosphere in both years of the study. Its relative abundance ranged from 3.4% of the bacterial community in 2013 to 2.6% in 2016 and was not influenced by cropping system diversification.
Network Analysis of the Bacterial Component of the Microbiota
A network composed of 47 ASVs and 56 edges was found in 2013 (Figure 2). This network was modular and included 13 mutual exclusions and 43 co-occurrences between bacterial taxa. A module was organized around ASV12 (cf. Acidobacteria sp.) which shared 5 co-occurrences and 2 mutual exclusions. Another module was organized around ASV1 (cf. Pseudarthrobacter sp.) which shared 9 co-occurrences and 3 mutual exclusions with other bacterial taxa. In 2016, the interaction network between bacteria was more complex than in 2013, with 51 ASVs and 83 edges (Figure 3). The network showed no modularity but was organized on ASV1 which shared 10 co-occurrences and 3 mutual exclusions with the other members of the network. Taxonomical affiliation of the ASVs of the networks in 2013 and 2016 can be found in Supplementary Tables S1, S2.
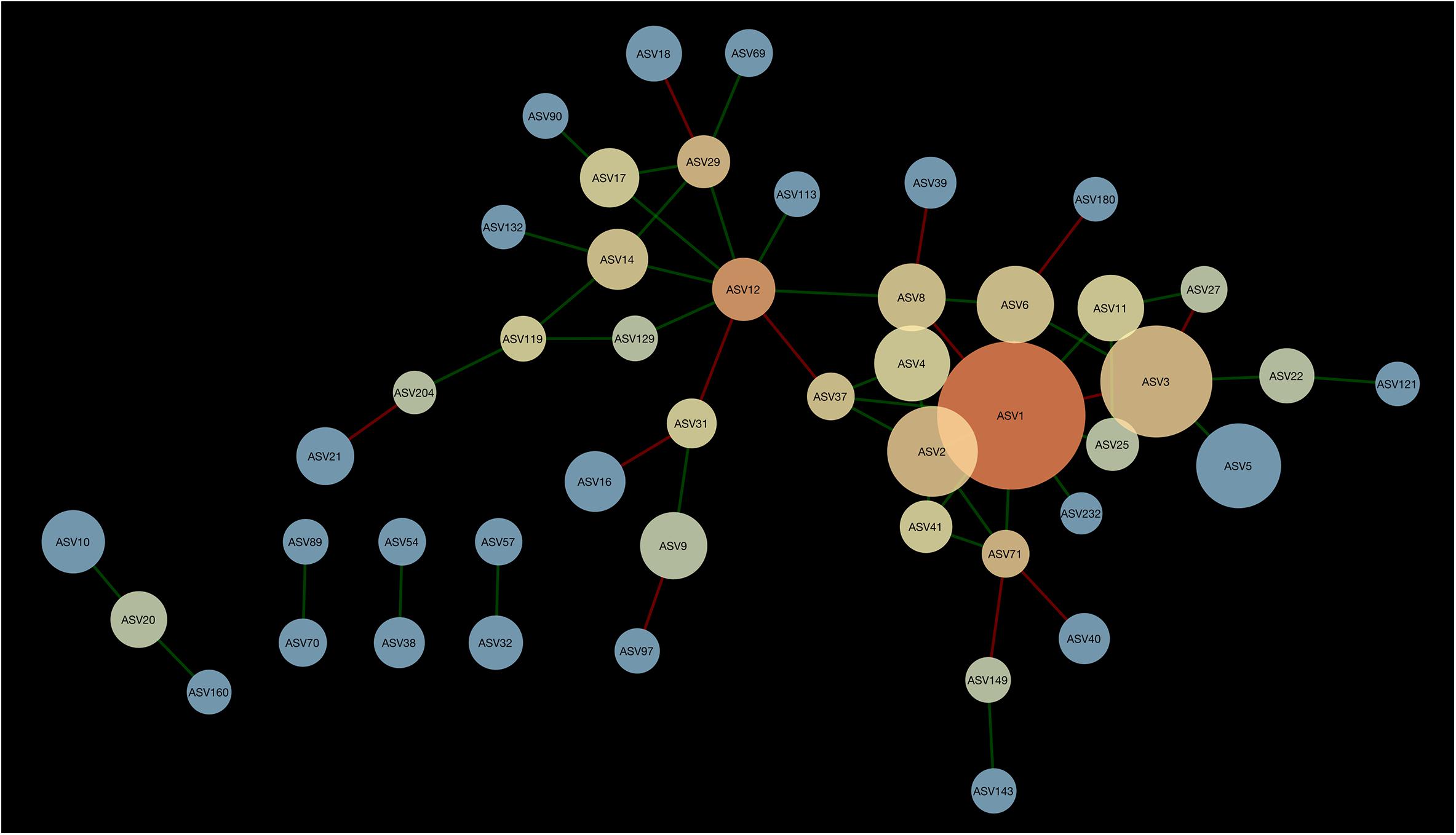
Figure 2. Network of interactions between bacteria forming the microbiome of canola rhizosphere in 2013. Dot size is proportional to the relative abundance of ASV, and shades indicate the degree of betweenness centrality: ASVs with warm colors are more connected with the other members of the network than the cold colored ones. Green edges indicate positive relationships and red edges, negative relationships.
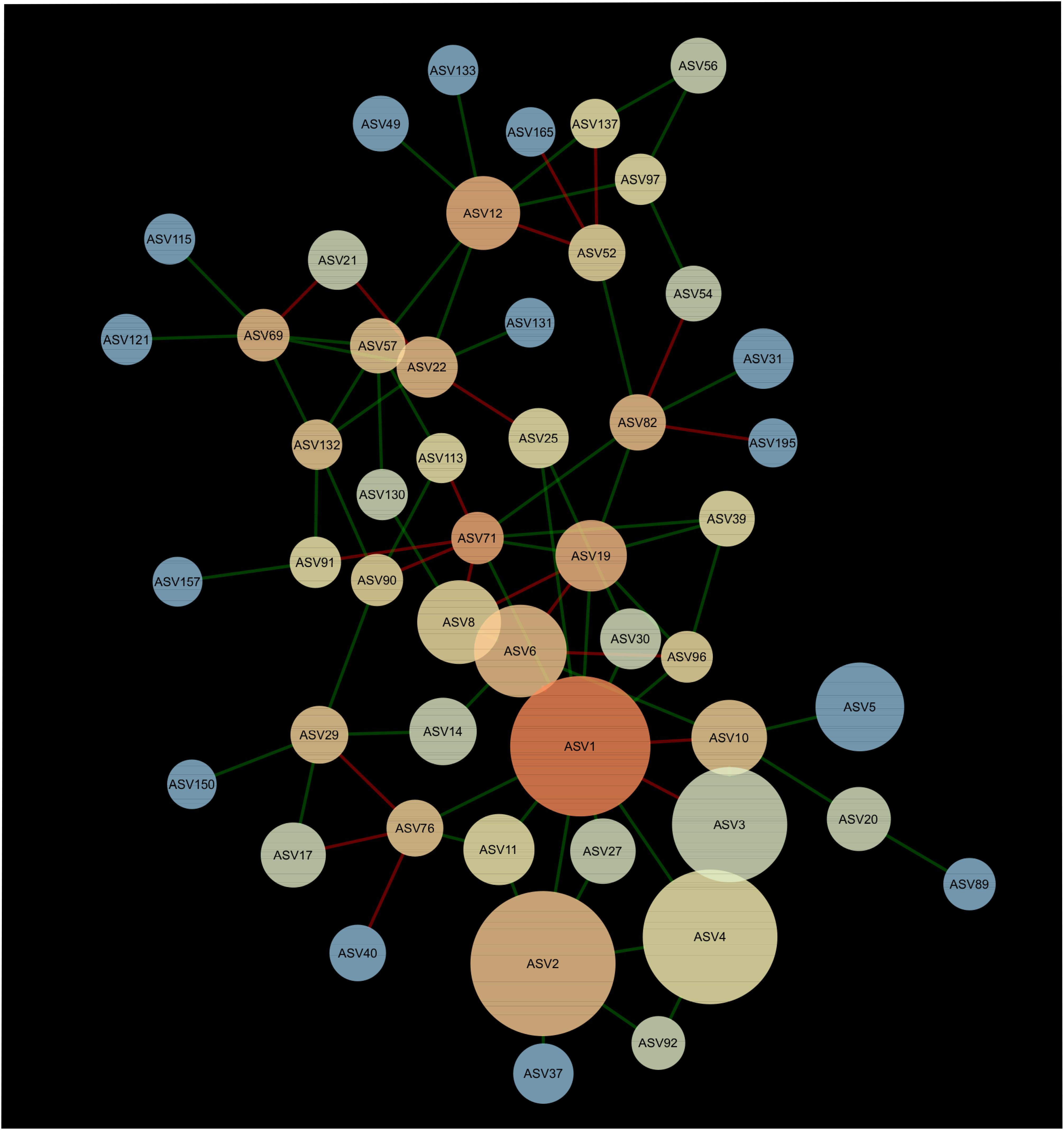
Figure 3. Network of interactions between the bacteria forming the microbiome of canola rhizosphere in 2016. Dot size is proportional to relative abundance of ASV, and shades indicate the degree of betweenness centrality: ASVs with warm colors are more connected with the other members of the network than the cold colored ones. Green edges indicate positive relationships and red edges, negative relationships.
There was one ASV identified as hub-taxa in 2013 and in 2016, ASV1 (cf. Pseudarthrobacter sp.), that was also the only relatively abundant member of the bacterial component of the canola rhizosphere microbiota. In 2013, ASV1 had a score of betweenness centrality of 0.44 and a degree score of 11, and in 2016 a score of betweenness centrality of 0.44 and a degree score of 13. No other ASV of the networks had values of betweenness centrality and degree score above the threshold of 95% as it was the case for ASV1. We were able to identify a cohort of bacterial taxa that were connected with ASV1 in 2013 and in 2016 (Table 5). The types of interaction between ASV1 and its cohort members were consistent and stable through years. In particular, ASV1 was always positively linked with ASV2 (cf. Yersinia sp.), ASV4 (cf. Stenotrophomonas sp.), ASV11 (cf. Stenotrophomonas sp.), ASV25 (cf. Candidatus Nitrosocosmicus sp.) and ASV71 (cf. Paenarthrobacter sp.), and negatively linked with ASV3 (cf. Nitrosophaeraceae sp.) and ASV6 (cf. Chloroflexi KD4-96).
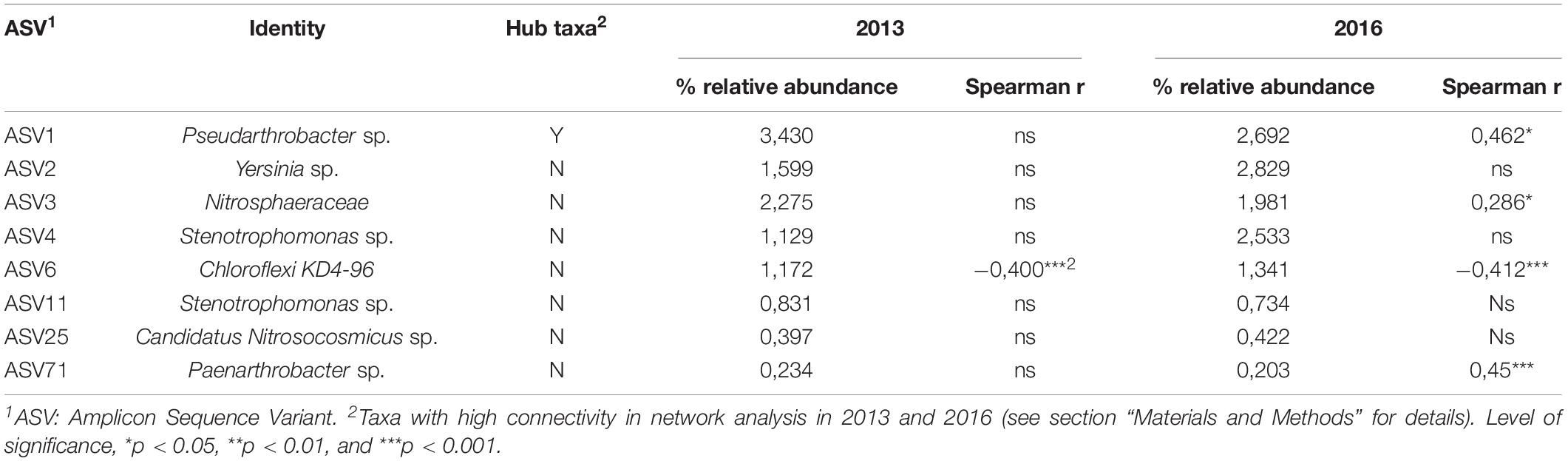
Table 5. Spearman’s correlation between the hub taxa ASV1 and its cohort members with canola yield (N = 48) in 2013 and 2016.
Correlation Between ASV1 and Its Cohort Members, and Canola Yield
Spearman’s correlations were used to assess the relationship between ASV1 and its cohort members and canola yield in each years (Table 5). ASV1 and most of its cohort members were not related to canola yield in 2013, only ASV6 showed a moderate negative correlation (R = -0.40, P = 0.0149) with canola grain yield, according to Ratner (2009). However, in 2016, ASV1 was positively correlated with canola yield (R = 0.46, P = 0.001), as it was the case for ASV3 (R = 0.23, P = 0.05) and ASV71 (R = 0.45, P = 0.0012). ASV6 remained negatively correlated with canola yield (R = -0.41, P = 0.003).
Discussion
We validated that a core bacterial component of the canola rhizosphere microbiota cannot only be stable across pedoclimatic zones but also through years. This core bacterial component was formed of only one taxon, ASV1 identified as cf. Pseudarthrobacter sp., which was also identified as a hub taxon and had a cohort of seven bacterial taxa with stable relationships across the two years of the study.
ASV1, cf. Pseudarthrobacter sp.
ASV1 was the only bacterial member that fit the definition of a core microbiota member that was detected in the canola rhizosphere and it was the most abundant ASV in both years of sampling. With our current sequencing technology (Illumina MIseq), it is likely that prokaryotes can go unseen if their abundance is low in a sample. ASV1 was the only bacterial core member identified, but it is probable that other less abundant prokaryotic members of this core microbiota were undetected. Furthermore, 16S rRNA gene sequences obtained with Illumina MiSeq technology do not have enough taxonomic resolution to distinguish between closely related species and uncertainty exists: ASV1 matches with 100% identity with at least 100 Arthrobacter and Pseudarthrobacter sequences in NCBI database. Arthrobacter is a genus of gram-positive bacteria from the Micrococcaceae family that was subdivided in several other genera like Pseudarthrobacter (Busse, 2016). This genus includes mainly soil bacterial species (Busse, 2016). Arthrobacter is also a genus with many species known as PGPB (Chan and Katznelson, 1961; Manzanera et al., 2015; Ullah and Bano, 2015; Aviles-Garcia et al., 2016; Fincheira and Quiroz, 2018) colonizing the roots and rhizosphere of a large spectrum of agricultural crops, such as rice or tomato. Lay et al. (2018) reported a member of canola rhizosphere core microbiota identified as Arthrobacter that shared 100% identity with ASV1 in similar sites of the Canadian Prairies in 2014. They also reported that their Arthrobacter was positively correlated with canola yield as it was the case here with ASV1 in 2016. Furthermore, an Arthrobacter sp. was previously shown to increase canola yield and acts as PGPB (Kloepper, 1988). This genus was reported as a highly competitive and fast growing bacteria in canola rhizosphere (Tkacz et al., 2015). Lay et al. (2018) also reported the presence of Arthrobacter sp. in wheat and pea rhizospheres in rotation with canola, but in smaller proportions than in canola rhizosphere. That omnipresence and abundance of ASV1 (cf. Pseudarthrobacter sp.) in all our plots suggest a selection by canola and highlight this taxon as a good PGPB candidate.
Variations in Bacterial Microbiota
Bacterial communities are known to be sensitive to changes in abiotic factors such as pH and humidity, or nutrient availability (Norman and Barrett, 2016; Wan et al., 2020). As plants actively control their rhizosphere microbiota through root exudates (Bais et al., 2006; Eisenhauer et al., 2017), we expected important differences in the bacterial communities of our crop diversification treatments. This was not the case. In 2013, no effect of crop rotation on bacterial community structure was detected and in 2016, the only significant difference was between the two extreme treatments, i.e., canola monoculture and the highest level of crop diversification, and the difference was marginally significant (P = 0.047). Indicator species analysis showed those two crop diversification treatments as the ones that had the highest number of indicator species. It is possible that the number of indicator species (26) of the monoculture in 2016 with a dominance of Chloroflexi (Table 4) could be the source of the difference in community structure, with the highest level of crop diversification with the BMRPP, even if no significant differences was found in 2013 between those two crop diversification treatments. Long lasting effect of agricultural management such as crop rotation were reported in the literature (Buckley and Schmidt, 2001). In the Brazilian Amazon for example, crop management seems to have a significant impact on microbial community structure (Jesus et al., 2009). For temperate environments, our results are consistent with Jesus et al. (2016) who did not find any influence of crop rotation on soil microbial communities in Michigan.
In our study, we examined the bacterial community in the canola rhizosphere, a component of the microbiota that is principally influenced by canola root exudates (Rumberger and Marschner, 2003), mitigating the effects of other crops in the rotation systems. We do not know if the crop diversification levels influenced the bulk soil bacterial communities. However, our results showed that canola recruited similar bacterial communities between all crop diversification levels in 2013. Even if most of the microbes in the rhizosphere are probably selected by the plant from its surrounding soil, it is also possible that a part of the canola rhizosphere microbiota can be inherited maternally with the seed microbiome as it is known to be the case for a wide range of plants (Shade et al., 2017). That could explain the similarities of canola rhizosphere community structure in systems with different levels of diversification. It is also possible that the bacterial communities in our diversified system were not host-specific, but colonize the roots of all crop species used in rotation, as it was reported by Lay et al. (2018). They found that the bacterial microbiota of canola rhizosphere was more similar to the one found in pea than the one found in wheat rhizosphere. But here, we did not find significant difference in community structure between the low, medium and high crop diversification in 2013 and only a slightly significant difference in 2016, suggesting that rotation crops have a limited influence on the bacterial communities of canola rhizosphere. Thus, we can consider the influence of abiotic variation on bacterial community in our study. A previous study showed that soil type and the frequency of rainfall have stronger effects on the microbial community of canola rhizosphere than crop rotations (Schlatter et al., 2019). Floc’h et al. (2020) also found a large variation in fungal rhizosphere community structure that was linked with difference in water availability in canola rhizosphere. In the present study, the experimental plots and sampling times were the same as those used in Floc’h et al. (2020). But the difference in precipitation between years (Supplementary Figure S2) did not affect the stability of the bacterial community structure observed in 2013 and 2016, contrarily to what was found for the fungal community in Floc’h et al. (2020). This stability is noteworthy. Bacterial interactions in canola rhizosphere microbiota also showed stability through years, here.
Interactions in the Bacterial Component of the Microbiota
Using the same rhizosphere soil samples, Floc’h et al. (2020) reported drastic changes between years in the dynamics of fungal interactions in the microbiota of canola rhizosphere. In the present work, if the complexity of the interaction network changed between the two years of sampling, the pool of bacteria forming its nucleus remained the same. The hotspot of interaction was always articulated around ASV1 (Pseudarthrobacter sp.). ASV1 was the only core bacterial member of the microbiota of canola rhizosphere and the only hub taxa detected with network analysis for both years of the present study. The fungal hub taxa in canola rhizosphere were subject to change between the years of the study, but it was not the case for bacterial hub taxa.
For both year of sampling, ASV1 was interacting with seven other taxa: ASV2 (cf. Yersinia sp.), ASV3 (cf. Nitrososphaeraceae sp.), ASV4 (cf. Stenotrophomonas sp.), ASV6 (cf. Chloroflexi KD4-96), ASV11 (cf. Stenotrophomonas sp.), ASV25 (cf. Candidatus Nitrosocosmicus sp.) and ASV71 (cf. Paenarthrobacter sp). The persistence of these interactions across time suggests a close interaction of ASV1 with these other members of the community. The fact that ASV6 was negatively linked with ASV1 and negatively correlated with canola yield raises interest. This phylum is associated with several agricultural plants like potato (Ýnceoğlu et al., 2011), lettuce (Cardinale et al., 2015) or maize (Peiffer et al., 2013) and was found in a large spectrum of soil ecosystems including forest, grassland, and tundra ecosystems (Fierer et al., 2012). Chloroflexi appears as characteristic of the rhizosphere of canola monoculture: 3 of 9 ASVs in 2013 and 9 of 26 ASVs were identified as indicator species in 2016 (Table 4). Monoculture of canola was found to have lower yield values across time and favour accumulation of microbial pathogenic taxa in soil (Hummel et al., 2009; Harker et al., 2015). Chloroflexi have been reported in the canola rhizosphere previously, but there was no mention of Chloroflexi species being pathogenic to canola (Gkarmiri et al., 2017). Correlations do no indicate that there is a causal relationship between the abundance of the different bacterial ASVs and canola yield. Correlations may point to bacteria that benefit from higher canola growth, or to a condition favorable to both canola and these bacteria, rather than an effect of the bacteria on plant productivity. However, the correlation values can be used as an index for identifying potential bacterial ASV of interest for the enhancement of canola production, since the bacteria directly beneficial to canola would be among those showing positive correlation with yield. It is possible that ASV6 could be commensal of canola fungal pathogens or of other microbes that are favored by monoculture (Floc’h et al., 2020), or pathogenic itself. Tests of pathogenicity should be made, or cross-kingdom network interactions studies conducted to verify the occurrence of ASV6 with pathogenic microbes.
In the cohort of taxa associated with ASV1, two other taxa were positively correlated with canola yield in 2016: ASV3 and ASV71. ASV71 was identified as Arthrobacter, so it is phylogenetically closely related to ASV1, and could be a potential PGPB with ASV1 (Manzanera et al., 2015; Ullah and Bano, 2015; Pereira et al., 2019). ASV3 is an archaea identified as a member of the Nitrososphaeraceae family that was poorly correlated with canola yield. Little information about this family is available. The presence of Nitrososphaeraceae was previously reported by Gkarmiri et al. (2017), and Lay et al. (2018) found core microbiota members of canola rhizosphere that were genetically close to Nitrocosmicus spp. Another study mentioned Nitrososphaeraceae as a microbial taxa retrieved from spacecraft surfaces (La Duc et al., 2012). This family appears to be widely distributed in the environment. As hub taxa can have very strong influence on the whole microbiota and on plant performance, ASV1 and its cohort members could be important. These bacteria should be isolated and tested under controlled conditions in structured experiments to examine their potential PGPB activity or pathogenic behavior on canola.
Conclusion
In this work, we have shown that the bacterial component of the core microbiota of canola rhizosphere is stable across years despite dissimilarity in precipitations. We identified the single core bacterial ASV in the microbiota of canola rhizosphere as cf. Pseudarthrobacter sp. In both years of the study, this single bacterial core microbiota member was a hub taxon in stable association with a cohort of bacteria. Chloroflexi were somewhat typical of canola monoculture, but the influence of crop diversification level on bacterial community structure, was only marginal, showing that the bacterial component of the microbiota of canola rhizosphere is more stable than its fungal component. This study provides information about bacterial and archaeal species in canola rhizosphere that could be important for future enhancement of canola production through microbiota manipulation or development of new cohorts for bio-inoculants.
Data Availability Statement
The MiSeq sequencing data generated as part of this work are publicly available on Zenodo (https://zenodo.org/record/3626047#.XisHASZOmV4).
Author Contributions
J-BF and CH designed and performed the experiment. MS-A, CH, and MH supervised the project. NL, KH, CH, and MS-A provided the material and analytic tools. J-BF analyzed the data. J-BF, MS-A, and CH wrote the manuscript. All authors revised and approved the manuscript.
Funding
This study received funding from the following sources: the Agricultural Bioproducts Innovation Program (ABIP), the Canola Agronomic Research Program (CARP), the Agriculture and Agri-Food Canada Canola Cluster Initiative, the Alberta Canola Producers Commission, the Saskatchewan Canola Development Commission, the Manitoba Canola Growers Association, the Canola Council of Canada, the Western Grains Research Foundation, and the Natural Sciences and Engineering Research Council of Canada.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Mario Laterrière for his assistance with the bioinformatics and thank the technical staff of the AAFC research centers in Lacombe, Lethbridge, and Scott for carrying out the sampling and providing useful advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01587/full#supplementary-material
FIGURE S1 | Rarefaction curves for each rhizosphere soil sample, showing the relationship between the number of ASVs and the abundance of 16S rRNA gene sequences reads, in the 2013 and 2016 dataset.
FIGURE S2 | Cumulative precipitation proportion at each site in 2013 and 2016.
FIGURE S3 | PCOA analysis of prokaryotic communities of canola rhizosphere using Hellinger distances in 2013.
FIGURE S4 | PCOA analysis of prokaryotic communities of canola rhizosphere using Hellinger distances in 2016.
Footnotes
- ^ http://www.earthmicrobiome.org/emp-standard-protocols/16s/
- ^ https://zenodo.org/record/3626047#.XisHASZOmV4
References
Agler, M. T., Ruhe, J., Kroll, S., Morhenn, C., Kim, S.-T., Weigel, D., et al. (2016). Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 14:e1002352. doi: 10.1371/journal.pbio.1002352
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Aviles-Garcia, M. E., Flores-Cortez, I., Hernández-Soberano, C., Santoyo, G., and Valencia-Cantero, E. (2016). La rizobacteria promotora del crecimiento vegetal Arthrobacter agilis UMCV2 coloniza endofíticamente a Medicago truncatula. Rev. Argent. Microbiol. 48, 342–346. doi: 10.1016/j.ram.2016.07.004
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Bakker, M. G., Schlatter, D. C., Otto-Hanson, L., and Kinkel, L. L. (2014). Diffuse symbioses: roles of plant-plant, plant-microbe and microbe-microbe interactions in structuring the soil microbiome. Mol. Ecol. 23, 1571–1583. doi: 10.1111/mec.12571
Barriuso, J., Solano, B. R., and Gutiérrez Mañero, F. J. (2008). Protection against pathogen and salt stress by four plant growth-promoting rhizobacteria isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 98, 666–672. doi: 10.1094/PHYTO-98-6-0666
Bianciotto, V., Lumini, E., Bonfante, P., and Vandamme, P. (2003). ‘Candidatus glomeribacter gigasporarum’ gen. nov., sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. Int. J. Syst. Evol. Microbiol. 53, 121–124. doi: 10.1099/ijs.0.02382-0
Buckley, D. H., and Schmidt, T. M. (2001). The structure of microbial communities in soil and the lasting impact of cultivation. Microb. Ecol. 42, 11–21. doi: 10.1007/s002480000108
Bulgarelli, D., Schlaeppi, K., Spaepen, S., van Themaat, E. V. L., and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
Busse, H.-J. (2016). Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibacter gen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int. J. Syst. Evol. Microbiol. 66, 9–37. doi: 10.1099/ijsem.0.000702
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Cardinale, M., Grube, M., Erlacher, A., Quehenberger, J., and Berg, G. (2015). Bacterial networks and co-occurrence relationships in the lettuce root microbiota. Environ. Microbiol. 17, 239–252. doi: 10.1111/1462-2920.12686
Chan, E. C. S., and Katznelson, H. (1961). Growth interactions of Arthrobacter globiformis and Pseudomonas Sp. in relation to the rhizosphere effect. Can. J. Microbiol. 7, 759–767. doi: 10.1139/m61-090
Chao, A. (1984). Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270.
Deng, Y., Jiang, Y.-H., Yang, Y., He, Z., Luo, F., and Zhou, J. (2012). Molecular ecological network analyses. BMC Bioinform. 13:113. doi: 10.1186/1471-2105-13-113
Duffy, J. E., Cardinale, B. J., France, K. E., McIntyre, P. B., Thébault, E., and Loreau, M. (2007). The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538. doi: 10.1111/j.1461-0248.2007.01037.x
Eisenhauer, N., Lanoue, A., Strecker, T., Scheu, S., Steinauer, K., Thakur, M. P., et al. (2017). Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 7:44641. doi: 10.1038/srep44641
Farina, R., Beneduzi, A., Ambrosini, A., de Campos, S. B., Lisboa, B. B., Wendisch, V., et al. (2012). Diversity of plant growth-promoting rhizobacteria communities associated with the stages of canola growth. Appl. Soil Ecol. 55, 44–52. doi: 10.1016/j.apsoil.2011.12.011
Fierer, N., Lauber, C. L., Ramirez, K. S., Zaneveld, J., Bradford, M. A., and Knight, R. (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6, 1007–1017. doi: 10.1038/ismej.2011.159
Fincheira, P., and Quiroz, A. (2018). Microbial volatiles as plant growth inducers. Microbiol. Res. 208, 63–75. doi: 10.1016/j.micres.2018.01.002
Fitter, A. H., and Garbaye, J. (1994). Interactions between mycorrhizal fungi and other soil organisms. Plant Soil 159, 123–132. doi: 10.1007/BF00000101
Floc’h, J.-B., Hamel, C., Harker, K. N., and St-Arnaud, M. (2020). Fungal communities of the canola rhizosphere: keystone species and substantial between-year variation of the rhizosphere microbiome. Microb. Ecol. doi: 10.1007/s00248-019-01475-8
Gkarmiri, K., Mahmood, S., Ekblad, A., Alström, S., Högberg, N., and Finlay, R. (2017). Identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Appl. Environ. Microbiol. 83:e01938-17. doi: 10.1128/AEM.01938-17
Hajishengallis, G., Darveau, R. P., and Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725. doi: 10.1038/nrmicro2873
Harker, K. N., O’Donovan, J. T., Turkington, T. K., Blackshaw, R. E., Lupwayi, N. Z., Smith, E. G., et al. (2015). Canola rotation frequency impacts canola yield and associated pest species. Can. J. Plant Sci. 95, 9–20. doi: 10.4141/cjps-2014-289
Hervé, M. (2015). RVAideMemoire: Diverse Basic Statistical and Graphical Functions. R Package Version 0.9-45-2. Available online at: http://CRAN.R-project.org/package=RVAideMemoire doi: 10.4141/cjps-2014-289 (accessed November 16, 2019).
Hummel, J. D., Dosdall, L. M., Clayton, G. W., Turkington, T. K., Lupwayi, N. Z., Harker, K. N., et al. (2009). Canola–wheat intercrops for improved agronomic performance and integrated pest management. Agron. J. 101, 1190–1197. doi: 10.2134/agronj2009.0032
Iffis, B., St-Arnaud, M., and Hijri, M. (2014). Bacteria associated with arbuscular mycorrhizal fungi within roots of plants growing in a soil highly contaminated with aliphatic and aromatic petroleum hydrocarbons. FEMS Microbiol. Lett. 358, 44–54. doi: 10.1111/1574-6968.12533
Iffis, B., St-Arnaud, M., and Hijri, M. (2017). Petroleum contamination and plant identity influence soil and root microbial communities while AMF spores retrieved from the same plants possess markedly different communities. Front. Plant Sci. 8:1381. doi: 10.3389/fpls.2017.01381
Ýnceoğlu, Ö, Al-Soud, W. A., Salles, J. F., Semenov, A. V., and van Elsas, J. D. (2011). Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS One 6:e23321. doi: 10.1371/journal.pone.0023321
Ings, T. C., Montoya, J. M., Bascompte, J., Blüthgen, N., Brown, L., Dormann, C. F., et al. (2009). Review: ecological networks – beyond food webs. J. Anim. Ecol. 78, 253–269. doi: 10.1111/j.1365-2656.2008.01460.x
Jesus, E., da, C., Liang, C., Quensen, J. F., Susilawati, E., Jackson, R. D., et al. (2016). Influence of corn, switchgrass, and prairie cropping systems on soil microbial communities in the upper Midwest of the United States. GCB Bioenerg. 8, 481–494. doi: 10.1111/gcbb.12289
Jesus, E. D. C., Marsh, T. L., Tiedje, J. M., and Moreira, F. M. S. (2009). Changes in land use alter the structure of bacterial communities in Western Amazon soils. ISME J. 3, 1004–1011. doi: 10.1038/ismej.2009.47
Kloepper, J. W. (1988). Plant growth-promoting rhizobacteria on canola (Rapeseed). Plant Dis. 72:42. doi: 10.1094/PD-72-0042
Kuramae, E., Gamper, H., van Veen, J., and Kowalchuk, G. (2011). Soil and plant factors driving the community of soil-borne microorganisms across chronosequences of secondary succession of chalk grasslands with a neutral pH. FEMS Microbiol. Ecol. 77, 285–294. doi: 10.1111/j.1574-6941.2011.01110.x
Kurtz, Z. D., Müller, C. L., Miraldi, E. R., Littman, D. R., Blaser, M. J., and Bonneau, R. A. (2015). Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 11:e1004226. doi: 10.1371/journal.pcbi.1004226
La Duc, M. T., Vaishampayan, P., Nilsson, H. R., Torok, T., and Venkateswaran, K. (2012). Pyrosequencing-derived bacterial, archaeal, and fungal diversity of spacecraft hardware destined for Mars. Appl. Environ. Microbiol. 78, 5912–5922. doi: 10.1128/AEM.01435-12
Lay, C.-Y., Bell, T. H., Hamel, C., Harker, K. N., Mohr, R., Greer, C. W., et al. (2018). Canola root–associated microbiomes in the Canadian prairies. Front. Microbiol. 9:1188. doi: 10.3389/fmicb.2018.01188
Manzanera, M., Narváez-Reinaldo, J. J., García-Fontana, C., Vílchez, J. I., and González-López, J. (2015). Genome sequence of Arthrobacter koreensis 5J12A, a plant growth-promoting and desiccation-tolerant strain. Genome Announc. 3:e00648-15. doi: 10.1128/genomeA.00648-15
Mendes, L. W., Tsai, S. M., Navarrete, A. A., de Hollander, M., van Veen, J. A., and Kuramae, E. E. (2015). Soil-borne microbiome: linking diversity to function. Microb. Ecol. 70, 255–265. doi: 10.1007/s00248-014-0559-2
Mendiburu, F. D. (2015). agricolae: Statistical Procedures for Agricultural Research. Available online at: http://CRAN.R-project.org/package=agricolae (accessed November 16, 2019).
Norman, J. S., and Barrett, J. E. (2016). Substrate availability drives spatial patterns in richness of ammonia-oxidizing bacteria and archaea in temperate forest soils. Soil Biol. Biochem. 94, 169–172. doi: 10.1016/j.soilbio.2015.11.015
Oksanen, J., Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2019). Package ‘Vegan’. Available online at: https://cran.ism.ac.jp/web/packages/vegan/vegan.pdf (accessed November 16, 2019).
Peiffer, J. A., Spor, A., Koren, O., Jin, Z., Tringe, S. G., Dangl, J. L., et al. (2013). Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. U.S.A. 110, 6548–6553. doi: 10.1073/pnas.1302837110
Pereira, L. B., Andrade, G. S., Meneghin, S. P., Vicentini, R., and Ottoboni, L. M. M. (2019). Prospecting plant growth-promoting bacteria isolated from the rhizosphere of sugarcane under drought stress. Curr. Microbiol. 76, 1345–1354. doi: 10.1007/s00284-019-01749-x
Pii, Y., Borruso, L., Brusetti, L., Crecchio, C., Cesco, S., and Mimmo, T. (2016). The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 99, 39–48. doi: 10.1016/j.plaphy.2015.12.002
Ratner, B. (2009). The correlation coefficient: its values range between +1/-1, or do they? J. Target Meas. Anal. Mark. 17, 139–142. doi: 10.1057/jt.2009.5
Ridout, M., and Newcombe, G. (2016). Disease suppression in winter wheat from novel symbiosis with forest fungi. Fungal Ecol. 20, 40–48. doi: 10.1016/j.funeco.2015.10.005
Rout, M. E. (2014). The plant microbiome. Adv. Bot. Res. 69, 279–309. doi: 10.1016/B978-0-12-417163-3.00011-1
Rumberger, A., and Marschner, P. (2003). 2-Phenylethylisothiocyanate concentration and microbial community composition in the rhizosphere of canola. Soil Biol. Biochem. 35, 445–452. doi: 10.1016/S0038-0717(02)00296-1
Schlatter, D. C., Hansen, J. C., Schillinger, W. F., Sullivan, T. S., and Paulitz, T. C. (2019). Common and unique rhizosphere microbial communities of wheat and canola in a semiarid Mediterranean environment. Appl. Soil Ecol. 144, 170–181. doi: 10.1016/j.apsoil.2019.07.010
Shade, A., Jacques, M.-A., and Barret, M. (2017). Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 37, 15–22. doi: 10.1016/j.mib.2017.03.010
Smoot, M. E., Ono, K., Ruscheinski, J., Wang, P.-L., and Ideker, T. (2011). Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432. doi: 10.1093/bioinformatics/btq675
Taktek, S., St-Arnaud, M., Piché, Y., Fortin, J. A., and Antoun, H. (2017). Igneous phosphate rock solubilization by biofilm-forming mycorrhizobacteria and hyphobacteria associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza 27, 13–22. doi: 10.1007/s00572-016-0726-z
Tkacz, A., Cheema, J., Chandra, G., Grant, A., and Poole, P. S. (2015). Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 9, 2349–2359. doi: 10.1038/ismej.2015.41
Ullah, S., and Bano, A. (2015). Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 61, 307–313. doi: 10.1139/cjm-2014-0668
van der Heijden, M. G. A., and Hartmann, M. (2016). Networking in the plant microbiome. PLoS Biol. 14:e1002378. doi: 10.1371/journal.pbio.1002378
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Van, A. L., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Wan, W., Tan, J., Wang, Y., Qin, Y., He, H., Wu, H., et al. (2020). Responses of the rhizosphere bacterial community in acidic crop soil to pH: changes in diversity, composition, interaction, and function. Sci. Total Environ. 700:134418. doi: 10.1016/j.scitotenv.2019.134418
Keywords: bacteria, archaea, microbial ecology, agroecosystem, crop rotations, Brassica napus
Citation: Floc’h J-B, Hamel C, Lupwayi N, Harker KN, Hijri M and St-Arnaud M (2020) Bacterial Communities of the Canola Rhizosphere: Network Analysis Reveals a Core Bacterium Shaping Microbial Interactions. Front. Microbiol. 11:1587. doi: 10.3389/fmicb.2020.01587
Received: 21 January 2020; Accepted: 17 June 2020;
Published: 10 July 2020.
Edited by:
Soumitra Paul Chowdhury, Helmholtz Zentrum München, GermanyReviewed by:
Massimiliano Cardinale, University of Salento, ItalyGuo-chun Ding, China Agricultural University, China
Ederson Da Conceicao Jesus, Brazilian Agricultural Research Corporation (EMBRAPA), Brazil
Copyright © 2020 Floc’h, Hamel, Lupwayi, Harker, Hijri and St-Arnaud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc St-Arnaud, bWFyYy5zdC1hcm5hdWRAdW1vbnRyZWFsLmNh
 Jean-Baptiste Floc’h
Jean-Baptiste Floc’h Chantal Hamel
Chantal Hamel Newton Lupwayi3
Newton Lupwayi3 Mohamed Hijri
Mohamed Hijri Marc St-Arnaud
Marc St-Arnaud